Abstract
Background
Laparoscopic adrenalectomy is an accepted treatment worldwide for adrenal gland disease in adults. The transperitoneal approach is more common. The retroperitoneal approach may be preferred, to avoid entering the peritoneum, but no clear advantage has been demonstrated so far.
Objectives
To assess the effects of laparoscopic transperitoneal adrenalectomy (LTPA) versus laparoscopic retroperitoneal adrenalectomy (LRPA) for adrenal tumours in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, ICTRP Search Portal, and ClinicalTrials.gov to 3 April 2018. We applied no language restrictions.
Selection criteria
Two review authors independently scanned the abstract, title, or both sections of every record retrieved to identify randomised controlled trials (RCTs) on laparoscopic adrenalectomy for preoperatively assessed adrenal tumours. Participants were affected by corticoid and medullary, benign and malignant, functional and silent tumours or masses of the adrenal gland, which were assessed by both laboratory and imaging studies.
Data collection and analysis
Two review authors independently extracted data, assessed trials for risk of bias, and evaluated overall study quality using GRADE criteria. We calculated the risk ratio (RR) for dichotomous outcomes, or the mean difference (MD) for continuous variables, and corresponding 95% confidence interval (CI). We primarily used a random‐effects model for pooling data.
Main results
We examined 1069 publications, scrutinized 42 full‐text publications or records, and included five RCTs. Altogether, 244 participants entered the five trials; 127 participants were randomised to retroperitoneal adrenalectomy and 117 participants to transperitoneal adrenalectomy. Two trials had a follow‐up of nine months, and three trials a follow‐up of 31 to 70 months. Most participants were women, and the average age was around 40 years. Three trials reported all‐cause mortality; in two trials, there were no deaths, and in one trial with six years of follow‐up, four participants died in the LRPA group and one participant in the LTPA group (164 participants; low‐certainty evidence). The trials did not report all‐cause morbidity. Therefore, we analysed early and late morbidity, and included specific adverse events under these outcome measures. The results were inconclusive between LRPA and LTPA for early morbidity (usually reported within 30 to 60 days after surgery; RR 0.56, 95% CI 0.27 to 1.16; P = 0.12; 5 trials, 244 participants; very low‐certainty evidence). Nine out of 127 participants (7.1%) in the LRPA group, compared with 16 out of 117 participants (13.7%) in the LTPA group experienced an adverse event. Participants in the LRPA group may have a lower risk of developing late morbidity (reported as latest available follow‐up; RR 0.12, 95% CI 0.01 to 0.92; P = 0.04; 3 trials, 146 participants; very low‐quality evidence). None of the 78 participants in the LRPA group, compared with 7 of the 68 participants (10.3%) in the LTPA group experienced an adverse event.
None of the trials reported health‐related quality of life. The results were inconclusive for socioeconomic effects, assessed as time to return to normal activities and length of hospital stay, between the intervention and comparator groups (very low‐certainty evidence). Participants who had LRPA may have had an earlier start on oral fluid or food intake (MD ‐8.6 hr, 95% CI ‐13.5 to ‐3.7; P = 0.0006; 2 trials, 89 participants), and ambulation (MD ‐5.4 hr, 95% CI ‐6.8 to ‐4.0 hr; P < 0.0001; 2 trials, 89 participants) than those in the LTPA groups. Postoperative and operative parameters (duration of surgery, operative blood loss, conversion to open surgery) showed inconclusive results between the intervention and comparator groups.
Authors' conclusions
The body of evidence on laparoscopic retroperitoneal adrenalectomy compared with laparoscopic transperitoneal adrenalectomy is limited. Late morbidity might be reduced following laparoscopic retroperitoneal adrenalectomy, but we are uncertain about this effect because of very low‐quality evidence. The effects on other key outcomes, such as all‐cause mortality, early morbidity, socioeconomic effects, and operative and postoperative parameters are uncertain. LRPA might show a shorter time to oral fluid or food intake and time to ambulation, but we are uncertain whether this finding can be replicated. New long‐term RCTs investigating additional data, such as health‐related quality of life, surgeons' level of experience, treatment volume of surgical centres, and details on techniques used are needed.
Plain language summary
Transperitoneal versus retroperitoneal laparoscopic adrenalectomy for adrenal tumours in adults
Review question
What are the effects of laparoscopic retroperitoneal adrenalectomy compared with laparoscopic transperitoneal adrenalectomy in adults?
Background
The adrenal glands are found above the kidneys, and produce several hormones, such as adrenaline, aldosterone, and cortisol. A tumour of the adrenal gland may be benign or cancerous, and is often found during routine examinations, such as ultrasonography of the belly (abdomen). The surgical removal of one or both adrenal glands (adrenalectomy) is usually recommended if the adrenal mass is more than 4 cm in diameter, if the mass enlarges by 1 cm or more during the period of observation, or if evidence of autonomous hormonal secretion develops.
There are several techniques to remove an adrenal gland. Nowadays, surgeons most often use keyhole surgery (laparoscopic surgery) instead of open surgery, using small cuts in the belly to introduce special surgical instruments and a small camera (laparoscope) with light. Laparoscopic transperitoneal adrenalectomy uses a cut through the belly that includes cutting a special membrane inside the belly (peritoneum) to expose the adrenal gland. Laparoscopic retroperitoneal adrenalectomy approaches the adrenal gland from the back, without cutting the peritoneum. Advocates of the latter technique have proposed better results, like shorter operative time, less blood loss, less postoperative pain, and shorter hospital stay.
Study characteristics
We found five randomised controlled trials (clinical trials where people are randomly put into one of two or more treatment groups) with 244 participants. A total of 127 participants were randomised to retroperitoneal adrenalectomy and 117 participants to transperitoneal adrenalectomy. Two studies had an observation period after surgery of nine months. Three studies observed their participants for 31 to 70 months. Most participants were women, and the average age was around 40 years.
This evidence is up to date as of April 2018.
Key results
In the short‐term period after surgery, no deaths were reported for either adrenalectomy technique. One study with a six‐year observation period, reported that out of 164 participants, four participants from the retroperitoneal adrenalectomy group died, and one participant from the transperitoneal adrenalectomy group died. We compared early poor health (morbidity), reported after 30 to 60 days, and late morbidity, reported at the longest observation time after surgery. Early morbidity was comparable between the two techniques, but late morbidity might be lower following retroperitoneal adrenalectomy (none out of 78 participants) than following transperitoneal adrenalectomy (7 out of 68 participants). No study reported on health‐related quality of life. Time to return to normal activities, length of hospital stay, duration of surgery, operative blood loss, and a change to open surgery were comparable between the two techniques. Time to oral fluid or food intake and time getting out of bed and engaging in light activity seemed a couple of hours shorter following retroperitoneal adrenalectomy (on average 8.6 hours) compared to transperitoneal adrenalectomy (on average 5.4 hours).
Certainty of the evidence
We are uncertain which adrenalectomy technique is best, mainly because of the small number of studies, small number of participants, and some systematic errors in the majority of our analysed studies. New studies should especially investigate health‐related quality of life. Surgeons' level of experience and treatment volume of surgical centres might also influence results.
Summary of findings
Summary of findings for the main comparison. Laparoscopic retroperitoneal adrenalectomy compared to laparoscopic transperitoneal adrenalectomy for adrenal tumours in adults.
| Laparoscopic retroperitoneal adrenalectomy compared to laparoscopic transperitoneal adrenalectomy for adrenal tumours in adults | ||||||
|
Patient: adults with adrenal tumours Settings: inpatients Intervention: laparoscopic retroperitoneal adrenalectomy (LRPA) Comparison: laparoscopic transperitoneal adrenalectomy (LTPA) | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments |
| LTPA | LRPA | |||||
|
All‐cause mortality Follow‐up: median 31 months |
See comment | 164 (3) | ⊕⊕⊝⊝ lowa | 3 trials reported on mortality: 2 reported no deaths; in 1 trial, 4 participants died in the LRPA group and 1 participant in the LTPA group (6‐year follow‐up) | ||
|
Early morbidity (any complication during the first 30 to 60 days following surgery) Follow‐up: 60 days |
137 per 1000 | 77 per 1000 (37 to 159) | RR 0.56 (0.27 to 1.16) | 244 (5) | ⊕⊝⊝⊝ very lowb | ‐ |
|
Late morbidity (any complication following surgery at latest available follow‐up) Follow‐up: median 31 months |
103 per 1000 | 12 per 1000 (1 to 95) | RR 0.12 (0.01 to 0.92) | 146 (3) | ⊕⊝⊝⊝ very lowb | ‐ |
| Health‐related quality of life | Not reported | |||||
|
Socioeconomic effects
a. Time to return to normal activities b. Time to ambulation c. Length of hospital stay Follow‐up: median 31 months |
a. The mean number of days to return to normal activities across control groups (range 12.5 days to 32.9 days) b. The mean number of hours to ambulation across control groups (range 11.5 hr to 40.8 hr) c. The mean number of days of hospital stay across control groups (range 1.0 day to 6.0 days) |
a. The mean number of days to return to normal activities in the LRPA groups were 1.3 days shorter (5.4 days shorter to 2.8 days longer) b. The mean number of hours to ambulation in the LRPA groups were 5.4 hr shorter (6.8 hr shorter to 4.0 hr shorter) c. The mean number of days of hospital stay in the LRPA groups were 0.4 days shorter (1.2 days shorter to 0.4 days longer) |
‐ | a. 102 (3) b. 89 (2) c. 250 (5) | a, b, and c ⊕⊝⊝⊝ very lowc |
a. normal activities had unclear definitions |
| *The basis for the assumed risk (e.g. the mean control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of LRPA (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; hr: hour(s) | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by two levels because of serious imprecision (low number of participants and trials) ‐ see Appendix 15 bDowngraded by three levels because of high risk of performance and detection bias and serious imprecision ‐ see Appendix 15 cDowngraded by one level because of unclear risk of performance and detection bias, and by two levels because of (serious) imprecision (low to moderate number of participants and trials) ‐ see Appendix 15
Background
Description of the condition
Tumours or masses of the adrenal gland are quite common and usually unilateral. They may originate from the adrenal corticoid or the adrenal medulla of the gland. They are categorised as either functional (hormone‐secreting) or silent (non hormone‐secreting). Moreover, as any tumoral tissue, they may be benign or malignant. The majority of adrenocortical tumours are benign, non‐functioning adenomas, which are discovered incidentally on abdominal imaging studies. This is usually called an adrenal 'incidentaloma'. Hormone‐secreting adrenocortical tumours are generally discovered because their symptoms cause Cushing's syndrome (showing enhanced secretion of adrenocorticotropic hormone, primary aldosteronism, or much less commonly, virilization). Carcinomas of the adrenal corticoid are quite rare, but can be aggressive. More rarely, tumours arise from the medulla of the gland. These are called phaeochromocytomas. Phaeochromocytomas are catecholamine‐secreting tumours that arise from chromaffin cells of the adrenal medulla. They may be benign or malignant.
Description of the intervention
Adrenalectomy is usually recommended if the adrenal mass is 4 cm or larger in diameter, if the mass enlarges by 1 cm or more during the period of observation, or if evidence of autonomous hormonal secretion develops (Young 2007). Open transperitoneal adrenalectomy was the gold standard of treatment for adrenal disease until 1992, when laparoscopic adrenalectomy was first described as a transperitoneal technique (Gagner 1992).
Laparoscopic transperitoneal adrenalectomy (LTPA) is performed under general anaesthesia; the individual is placed in lateral decubitus position, with the affected side elevated around 60°, usually with the help of a bean bag. The ipsilateral arm is generally supported by a metal L‐shaped support that is secured to the table. Three to five transperitoneal ports are positioned through the anterior abdominal wall. After the adrenal gland space is exposed, the retroperitoneum is incised, and the gland dissected. On the left side, the descending colon is reflected medially; the adrenal vein is divided with bipolar diathermy or cutting between clips, and dissection is carried out, starting just superior to the renal vein, until the adrenal gland is freed. On the right side, the liver must be retracted, but the colon rarely requires mobilisation. The peritoneum is incised along the lateral aspect of the inferior vena cava, down to the superior edge of the renal vein. The short right adrenal vein is identified, and divided between clips. When the dissection of the gland is completed at the lateral side, the specimen is bagged and removed.
Laparoscopic retroperitoneal adrenalectomy (LRPA) was introduced in 1995 (Mercan 1995). In this technique, the surgeon approaches the adrenal gland directly through the retroperitoneal space, without breaching the peritoneum. Individuals are placed in the lateral decubitus position, and the table is flexed in order to expand the operating space between the 12th rib and the iliac crest. To obtain retroperitoneal access, a 1 cm skin incision is made below the 12th rib, and a space is created below the fascia by careful finger dissection. On the left side, the Gerota's fascia is incised around the superior aspect of the kidney, and dissection is continued medially along the renal vein, until the main adrenal vein is encountered and divided between clips, until mobilisation is completed. On the right side, the investing fascial layer is opened transversely along the upper renal pole until the inferior vena cava is identified; dissection continues superiorly along its lateral edge to the right adrenal vein, which is divided between clips, completing mobilisation of the gland.
Adverse effects of the intervention
Intraoperative bleeding may occur in 1% to 4% of cases, depending on the techniques used (Constantinides 2012). Small liver injuries can occur during retraction or adrenal dissection on the right, which might be controlled by bipolar coagulation, simple compression, or placement of fibrin material to achieve haemostasis. Rarely, the surgeon may have to convert to open surgery. Vascular injuries, especially to the inferior vena cava, comprise almost 7% of all complications, and are the leading cause for conversion (Corcione 2001). Small injuries may be compressed and treated with coagulant agents; increasing the intra‐abdominal pressure helps to control bleeding. If bleeding continues, laparoscopic suturing is an option, but only if the surgeon has sufficient experience and skills; otherwise, the surgeon should convert to open surgery. Inadvertent injuries of the diaphragm causing pneumothorax may occur occasionally, requiring direct diaphragm suture closure (Naito 1995). A chest tube placement is rarely required initially, although it may be required later if a significant pneumothorax develops. Injury to the spleen and the left side of the pancreas have also been reported (Greco 2011; Terachi 2000).
How the intervention might work
Numerous trials have shown the safety and feasibility of laparoscopy since its introduction in 1992. Several benefits were seen compared to open procedures: a decreased hospital stay, faster recovery, decreased pain and narcotic use, and fewer complications (Assalia 2004). Minimally invasive adrenalectomy is now considered the standard treatment for benign adrenal masses (Jacobs 1997). Laparoscopic retroperitoneal adrenalectomy was first performed and described in 1995 (Mercan 1995). By directly entering the retroperitoneal space, and not breaching the peritoneum, this technique showed the potential to result in a shorter operative time, less blood loss, less postoperative pain, and shorter hospital stay (Constantinides 2012). Despite reports of favourable results for minimally invasive adrenalectomy techniques, using either the transperitoneal or retroperitoneal route, only a few trials have compared the two techniques. They showed no superiority of either technique. However, most trials have been limited by a small sample size and a single‐institution design.
Why it is important to do this review
The intrinsic difficulties of laparoscopic transperitoneal adrenalectomy limited the diffusion of the technique, which was performed in less than 20% of cases until 2006 (Murphy 2010). In the following years, demonstration of the general advantages of laparoscopy, such as reduced blood loss, shorter hospital stay, and faster return to normal activity, together with accumulated experience, reversed the trend in favour of a laparoscopic technique (Guazzoni 1995). Today, laparoscopic transperitoneal adrenalectomy has become the most widely used procedure for people with benign adrenal disease.
Introduced in 1995, laparoscopic retroperitoneal adrenalectomy was proposed as a good alternative in selected cases, and surgeons started to use it more frequently (Mercan 1995). Potential advantages were the same; shorter operative time, less postoperative pain, and shorter hospital stay (Constantinides 2012).Some trials have compared the outcomes of laparoscopic transperitoneal adrenalectomy and laparoscopic retroperitoneal adrenalectomy, but their results are inconclusive because of their design and inclusion criteria. Several meta‐analyses have compared laparoscopic transperitoneal and retroperitoneal adrenalectomy (Chen 2013; Constantinides 2012; Nigri 2013). However, these analyses were not fully reliable, because they included a small number of both observational and interventional trials, and heterogeneous trial populations. Recent randomised trials were not included, as they were published prior to the literature searches (Barczynski 2014; Mohammadi‐Fallah 2013). Finally, the review authors did not adequately assess the risk of bias. Therefore, our systematic review will try to establish a reliable body of evidence of relevant outcomes in people undergoing either laparoscopic transperitoneal adrenalectomy or laparoscopic retroperitoneal adrenalectomy.
Objectives
To assess the effects of laparoscopic transperitoneal adrenalectomy versus laparoscopic retroperitoneal adrenalectomy for adrenal tumours in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs).
Types of participants
We included data from adults (older than 16 years) who underwent laparoscopic transperitoneal or laparoscopic retroperitoneal adrenalectomy for preoperatively assessed adrenal tumours.
Diagnostic criteria
Corticoid and medullary, benign and malignant, functional and silent tumours, or masses of the adrenal gland were assessed by both laboratory and imaging studies.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
Laparoscopic retroperitoneal adrenalectomy (LRPA), defined as any technique approaching the adrenal gland directly through the retroperitoneal space, and not breaching the peritoneum.
Comparator
Laparoscopic transperitoneal adrenalectomy (LTPA), defined as any technique approaching the adrenal gland directly through the abdominal wall and peritoneal sac.
Concomitant interventions had to be the same in the intervention and comparator groups to establish fair comparisons.
Types of outcome measures
Primary outcomes
All‐cause mortality
Early and late morbidity
Secondary outcomes
-
Operative parameters
Duration of surgery
Intraoperative bleeding
Operative blood loss
Conversion to open surgery
-
Postoperative parameters
Time to oral fluid or food intake
Chest infection or pleural effusion
Abdominal abscess
Health‐related quality of life
Socioeconomic effects
Method of outcome measurement
All‐cause mortality: defined as death from any cause.
Early and late morbidity: defined as any deviation from the regular postoperative course (e.g. chest infection, liver injury, splenic injury, vascular injury, pneumothorax or haemothorax, massive subcutaneous emphysema, surgical access site herniation).
Duration of surgery: defined as the duration of general anaesthesia.
Intraoperative bleeding: defined as the occurrence of blood loss greater than 200 mL.
Operative blood loss: defined as the quantity of blood loss in millilitres (mL).
Conversion to open surgery: defined as any technical failure requiring a larger skin incision before the complete dissection of the gland.
Time to oral fluid or food intake: defined as the time to oral intake of fluids or food.
Chest infection or pleural effusion: defined as any diagnosis of infection that affected the lungs (either larger or smaller air sacs), or the accumulation of fluid in the pleural space.
Abdominal abscess: defined as "a pus‐containing confined structure or collection, localised in abdominal cavity" (Schein 2010).
Health‐related quality of life: evaluated by a validated instrument such as the 36‐item Short‐Form Health Survey (SF‐36).
Socioeconomic effects: such as length of hospital stay, time to return to normal activities, time to ambulation (time to autonomous walking), and time to return to work.
Timing of outcome measurement
All‐cause mortality, early and late morbidity, socioeconomic effects: measured within 30 to 60 days after surgery and overall, during the entire follow‐up provided.
Duration of surgery: measured at the end of general anaesthesia.
Operative blood loss, intraoperative bleeding, conversion to open surgery, splenic or liver injury, vascular injury: measured at the end of surgery.
Time to oral fluid or food intake: measured within one week after surgery.
Socioeconomic effects, pneumothorax or haemothorax, chest infection, pleural effusion: measured within 30 to 60 days after surgery.
Health‐related quality of life: measured within the first week, at 30 days after surgery, and overall, during the entire follow‐up provided.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date; we placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Register of Studies Online (searched 3 April 2018).
MEDLINE Ovid Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and MEDLINE (1946 to 3 April 2018).
Embase Ovid (1974 to 2015 Week 13). RCTs indexed in Embase are now prospectively added to CENTRAL via a highly sensitive screening process (CENTRAL creation details).
ClinicalTrials.gov (searched 3 April 2018).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Protal (apps.who.int/trialsearch/; searched 3 April 2018).
For details on search strategies, see Appendix 1. After submitting the review for editorial approval, the Cochrane Metabolic and Endocrine Disorders (CMED) Group's Information Specialist updated the literature search and send the results to the review authors. Had we identified new trials for inclusion, we would have evaluated them, incorporated the findings into our review, and resubmitted the updated review (Beller 2013).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, (systematic) reviews, meta‐analyses, and health technology assessment reports. In addition, we contacted authors of included trials in an attempt to identify trials we may have missed.
Data collection and analysis
Selection of studies
Two review authors (AA, RC) independently scanned the abstract, title, or both, of every record retrieved, to determine which trials should be assessed further. We investigated all potentially relevant articles as full text. We resolved any discrepancies through consensus, or by consulting with a third review author (GC). When we could not resolve a disagreement, we added the trial as a 'study awaiting classification', and contacted the trial authors for clarification. We present an adapted PRISMA flow diagram showing the process of trial selection (Liberati 2009).
Data extraction and management
For the trials that fulfilled our inclusion criteria, two review authors (AA, RC) independently extracted key participant and intervention characteristics. We reported data on efficacy outcomes and adverse events using standard data extraction templates as supplied by the CMED Group. We resolved any disagreements by discussion, or if required, by consulting a third review author (RP). For details see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15.
1. Overview of trial populations.
| Trial ID (design) | Intervention and comparator | Sample sizea | Screened/eligible (N) | Randomised (N) [number of adrenalectomies] | Analysed (N) | Finishing trial, short‐term follow‐up (N) | Randomised finishing study (%) | Follow‐up timeb |
|
Chai 2017 (parallel RCT) |
I: posterior retroperitoneoscopic adrenalectomy | " ... sample size ... was calculated for a 5% two‐sided significance and 80% power on the basis of mean operative time (80.4 minutes for LRPA and 96 minutes for LTPA) and standard deviation of operative time (12.71 minutes for LRPA and 28.32 minutes for LTPA), which were obtained from a pilot study (data not shown), resulting in 33 patients per group that was divided by 0.864. The final sample size was 42 patients in each group, allowing for a 5% dropout rate" | 84b | 41 | 41 | 41 | 100 | 31 months |
| C: transperitoneal adrenalectomy | 42 | 42 | 42 | 100 | ||||
| total: | 83 | 83 | 83 | 100 | ||||
|
Barczynski 2014c (parallel RCT) |
I: posterior retroperitoneoscopic adrenalectomy | Under the assumption of a 20% reduction in duration of surgery, it was calculated that 24 participants would be required in each treatment arm to give the study a power of 90%; anticipating a 25% loss to follow‐up, 32 participants per arm were required in the trial | 88 | 33 | 33 | 30 | 91 | 5 years |
| C: transperitoneal adrenalectomy | 32 | 32 | 30 | 94 | ||||
| total: | 65 | 65 | 60 | 92 | ||||
|
Fernández‐Cruz 1996 (parallel RCT) |
I: retroperitoneal adrenalectomy | ‐ | ‐ | 8 (11) | 8 | 8 | 100 | 9 months |
| C: transperitoneal adrenalectomy | 7 (10) | 7 | 7 | 100 | ||||
| total: | 15 | 15 | 15 | 100 | ||||
|
Mohammadi‐Fallah 2013 (parallel RCT) |
I: retroperitoneal adrenalectomy | ‐ | ‐ | 13 | 12d | 13 | 100 | 9 months |
| C: transperitoneal adrenalectomy | 11 | 11 | 11 | 100 | ||||
| total: | 24 | 23 | 24 | 100 | ||||
|
Rubinstein 2005e (parallel RCT) |
I: retroperitoneal adrenalectomy | ‐ | ‐ | 32 | 32 | 32 | 100 | 6 years |
| C: transperitoneal adrenalectomy | 25 | 25 | 25 | 100 | ||||
| total: | 57 | 57 | 57 | 100 | ||||
| Grand total | All interventions | 127 | ||||||
| All comparators | 117 | |||||||
| All interventions and comparators | 244 | |||||||
‐ denotes not reported
aFollow‐up under randomised conditions until end of trial ( (= duration of intervention + follow‐up post‐intervention or identical to duration of intervention); extended follow‐up refers to follow‐up of participants once the original trial was terminated, as specified in the power calculation bOne participant refused follow‐up (a total of 83 participants were eligible for analysis) c61 participants completed the 5‐year follow‐up, 4 participants lost to long‐term follow‐up (retroperitoneoscopic adrenalectomy: 1 withdrew from trial, 2 unable to communicate; transperitoneal adrenalectomy: 1 unable to communicate) dOne participant with open conversion because of "failure to progress" e52 participants finished the 6‐year follow‐up
C: comparator; I: intervention; ITT: intention‐to‐treat; LTPA: laparoscopic transperitoneal adrenalectomy; LRPA: posterior retroperitoneoscopic adrenalectomy ; N/A: not applicable; RCT: randomised controlled trial
We provided information, including the trial identification number, about potentially relevant ongoing trials in the Characteristics of ongoing studies table, and in the Matrix of trial endpoints (publications and trial documents), in Appendix 7. We tried to find the protocol for each included trial. If successful, we reported primary, secondary, and other outcomes, and compared them with the data provided in publications, in Appendix 7.
We sent an email request to all authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 14 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from them, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary trial, we maximised our yield of information by collating all available data, and using the most complete dataset, aggregated across all known publications.
Assessment of risk of bias in included studies
Two review authors (AA, RC) independently assessed the risk of bias of each included trial. We resolved any disagreements by consensus, or by consultation with a third review author (GC).
We used the Cochrane 'Risk of bias' assessment tool, and assigned assessments of low, high, or unclear risk of bias (Higgins 2017; Appendix 2; Appendix 3). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions, according to the criteria and associated categorisations contained therein(Higgins 2017).
Summary assessment of risk of bias
We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure.
We distinguished between self‐reported, investigator‐assessed, and adjudicated outcome measures.
We defined the following endpoints as self‐reported outcomes.
Health‐related quality of life
We defined the following endpoints as investigator‐assessed outcomes.
All‐cause mortality
Early and late morbidity
Operative and postoperative parameters
Socioeconomic effects
Risk of bias for a trial across outcomes
Some risk of bias domains, such as selection bias (sequence generation and allocation sequence concealment), affect the risk of bias across all outcome measures in a trial. In cases of high risk of selection bias, we marked all endpoints investigated in the associated trial as being at high risk. Otherwise, we did not perform a summary assessment of the risk of bias across all outcomes for a trial.
Risk of bias for an outcome within a trial and across domains
We assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both trial‐level entries and outcome‐specific entries). We considered low risk of bias to denote a low risk of bias for all domains, unclear risk to denote an unclear risk of bias for one or more domains, and high risk to denote a high risk of bias for one or more domains.
Risk of bias for an outcome across trials and across domains
These are the main summary assessments that we incorporated into our judgments about the quality of evidence in the 'Summary of findings' tables. We defined outcomes as at low risk of bias when most information came from trials at low risk of bias, unclear risk when most information came from trials at low or unclear risk of bias, and high risk when a sufficient proportion of information came from trials at high risk of bias.
Measures of treatment effect
When at least two trials were available for a comparison and a given outcome, we expressed dichotomous data as risk ratios (RRs) or risk differences (RD) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) or standardised mean differences (SMDs) with 95% CIs. We expressed time‐to‐event data as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
We had planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials, and multiple observations for the same outcome.
Dealing with missing data
If possible, we obtained missing data from trial authors, and carefully evaluated important numerical data, such as screened and randomised participants, as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates (e.g. drop‐outs, losses to follow‐up, withdrawals), and we critically appraised issues of missing data and imputation methods (e.g. last observation carried forward).
Where means and standard deviations (SDs) for outcomes were not reported, and we had not received the needed information from trial authors, we imputed these values by estimating the mean and variance from the median, range, and the size of the sample (Hozo 2005), or by assuming the standard deviation of the missing outcome to be the average of the standard deviations from trials where this information was reported.
We had planned to investigate the impact of imputation on meta‐analyses, by means of sensitivity analysis.
Assessment of heterogeneity
In the event of substantial clinical, methodological, or statistical heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) through visual inspection of the forest plots, and by using a standard Chi² test with a significance level of α = 0.1 (Deeks 2017). In view of the low power of this test, we also considered the I² statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003).
Had we found heterogeneity, we would have attempted to determine possible reasons for it, by examining individual trial and subgroup characteristics.
Assessment of reporting biases
Because there were fewer than 10 trials investigating any outcome, we did not construct a funnel plot to assess small‐study effects. Therefore, we interpreted results carefully (Sterne 2011).
Data synthesis
Even if there was good evidence for homogeneous effects across trials, we summarised primarily low risk of bias data using a random‐effects model because such models are more appropriate in medical decision‐making contexts, especially when there are rare events (Ades 2005; DerSimonian 1986; Fleiss 1991; Shuster 2007). We interpreted random‐effects meta‐analyses with consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). We performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and had intended to carry out subgroup analyses to investigate the interactions (Altman 2003).
Tumour size: < 6 cm versus ≥ 6 cm.
Previous abdominal surgery: yes or no.
Body mass index (BMI): < 30 kg/m² versus ≥ 30 kg/m².
However, there were not enough data to carry out these analyses.
Sensitivity analysis
We had intended to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes, by restricting analysis to the following.
Published trials.
Effect of risk of bias, as specified in the Assessment of risk of bias in included studies section.
Very long or large trials, to establish the extent to which they dominate the results.
Using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country.
However, there were too few trials to carry out these analyses.
We tested the robustness of the results by repeating the analysis using different measures of effect size (RR, OR, etc.), and different statistical models (fixed‐effect and random‐effects models).
Certainty of the evidence
We assessed the overall quality of the evidence for each outcome specified below, according to the GRADE approach, which takes into account issues related to both internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity, such as directness of results. Two review authors (AA, RC) independently rated the quality for each outcome. We resolved any differences in assessment by discussion, or consulting a third review author (NN).
We also established an appendix entitled 'Checklist to aid consistency and reproducibility of GRADE assessments' to help with standardisation of our 'Summary of findings' table results (Meader 2014). Alternatively, we planned to use GRADEpro GDT software, and present evidence profile tables as an appendix (GRADEpro GDT 2015). We presented results for the outcomes as described in the Types of outcome measures section. If we were unable to complete a meta‐analysis, we presented the results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the quality of the evidence using footnotes, and made comments to aid the reader's understanding of the Cochrane Review, where necessary.
Summary of findings table
We presented a summary of the evidence in a 'Summary of findings' table. This provided key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies. It also includes the number of participants and trials addressing each important outcome, and a rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017), using Review Manager 5 table editor (RevMan 2014). We reported the following outcomes, listed according to priority.
All‐cause mortality
Early morbidity
Late morbidity
Health‐related quality of life
Socioeconomic effects
Results
Description of studies
For a detailed description of trials, see the 'Characteristics of included studies', 'Characteristics of excluded studies' and 'Characteristics of ongoing studies' sections.
Results of the search
Our comprehensive literature searches identified a total of 1229 records. There were 1069 records after removing duplicates. From these, we identified 42 full‐text articles and records for further examination. We excluded the other publications on the basis of titles or abstracts, because they did not meet the inclusion criteria, or because the trials were not relevant to the review objectives. See Figure 1 for the amended PRISMA trial flow diagram. After screening the full‐text of the selected publications, five trials (seven publications) met the inclusion criteria. All trials were published in English.
1.
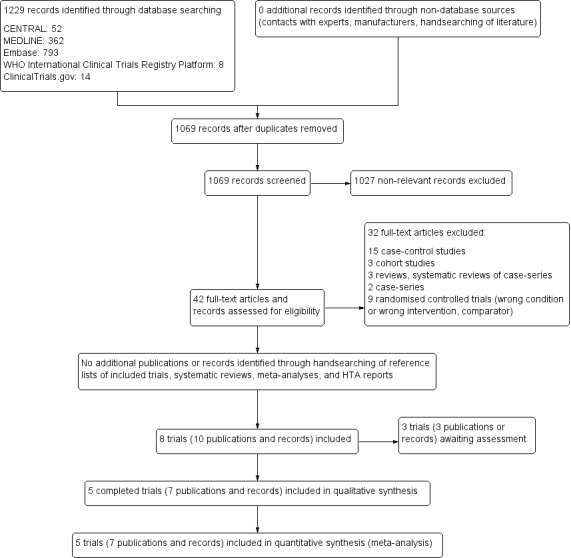
Study flow diagram
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies; Appendix 4; Appendix 5; Appendix 6; Appendix 7). The following is a succinct overview.
Source of data
Using the literature search strategy, we identified 1229 publications: 52 in CENTRAL, 362 in MEDLINE, 793 in Embase, 8 in the WHO ICTRP, and 14 in ClinicalTrials.gov. After removal of duplicates, we had 1069 publications to screen.
Comparisons
One trial included six bilateral and nine monolateral adrenalectomies, performed according to the two techniques, laparoscopic retroperitoneal adrenalectomy (LRPA) or laparoscopic transperitoneal adrenalectomy (LTPA), assigned in a randomised fashion (Fernández‐Cruz 1996). All participants were included in the analysis for all‐cause mortality, all‐cause morbidity, adverse events, and postoperative parameters (time periods, unwanted effects). For operative parameters (duration of surgery, operative blood loss, intraoperative bleeding, and conversion to open surgery), post‐operative parameters (pneumothorax, haemothorax, chest infection, pleural effusion, splenic injury, abdominal abscess), and socioeconomic effects, only participants undergoing monolateral procedures were taken into consideration. All the other trials compared only monolateral adrenalectomies performed either by LRPA or LTPA, assigned in a randomised fashion.
Overview of trial populations
A total of 244 individuals participated in the five trials, 127 participants were randomised to LRPA, and 117 to LTPA. Almost all randomised participants finished the trials. Five participants were lost at follow‐up before the five‐year deadline in Barczynski 2014. Only two trials reported sample size calculation (Barczynski 2014; Chai 2017). The number of recruited participants ranged from 15 (Fernández‐Cruz 1996), to 65 (Barczynski 2014).
Trial design
Trials were randomised controlled trials (RCTs), but only one declared a parallel group superiority design (Barczynski 2014). All trials were monocentric. Only one trial declared it was double‐blinded, for participants and personnel (Barczynski 2014). One trial did not declare the time of recruitment (Fernández‐Cruz 1996), while the four others reported recruitment between 1997 and 2012. The mean duration of the intervention ranged between 51 (Barczynski 2014) and 128 minutes (Mohammadi‐Fallah 2013) in the LRPA group, and 60 (Chai 2017) and 130 minutes (Rubinstein 2005) in the LTPA group. The mean duration of follow‐up ranged from 9 (Fernández‐Cruz 1996; Mohammadi‐Fallah 2013) to 59 months (Rubinstein 2005). No trials reported a run‐in period. No trials were terminated early.
Settings
One trial was conducted in Spain (Fernández‐Cruz 1996), one in Ohio, United States (Rubinstein 2005), one in Iran (Mohammadi‐Fallah 2013), one in Poland (Barczynski 2014) and one in South Korea (Chai 2017) Four of the trials were conducted at academic institutions (Barczynski 2014; Chai 2017; Fernández‐Cruz 1996; Mohammadi‐Fallah 2013). No trial was performed in an outpatient setting.
Participants
Participants required an adrenalectomy for a variety of diseases, including aldosterone‐producing adenomas, phaeochromocytoma, Cushing’s disease, Cushing’s adenoma, non‐functional adenomas, metastasis, adrenal carcinoma, and leiomyosarcoma. Eigthy‐nine (36%) participants came from low‐ and middle‐income countries, such as Iran and Poland (Barczynski 2014; Mohammadi‐Fallah 2013). No trial provided information about ethnic groups. No trial provided information about the duration of the preoperative condition or disease. Surgical procedures were most commonly performed in women (67%). The mean age of participants ranged from 42.2 years (Mohammadi‐Fallah 2013) to 57.5 years (Rubinstein 2005) in the LRPA group, and from 39.9 years (Fernández‐Cruz 1996) to 57.0 years (Rubinstein 2005) in the LTPA group. One hundred and eight (44%) procedures were on the right side, 130 (53%) were on the left, and six (3%) were bilateral adrenalectomies. Four trials reported the BMI: Barczynski 2014 reported a mean of 27.6 kg/m² in the LRPA group compared with 27.3 kg/m² in the LTPA group; Rubinstein 2005 reported a median of 30.4 kg/m² in the LRPA group compared with 29.1 kg/m² in the LTPA group; Mohammadi‐Fallah 2013 noted a mean 27.5 kg/m² in the LRPA group compared with 26.7 kg/m² in the LTPA group; Chai 2017 reported a mean of 23.6 kg/m² in the LRPA group compared with 24.2 kg/m² in the LTPA group. Only one trial reported on comorbidities (Chai 2017).
The main exclusion criteria were: a history of major abdominal surgery, planned bilateral adrenal surgery, adrenal tumour larger than 6 cm or 7 cm in diameter, and age less than 16 years or 18 years. Some trials excluded participants with suspected adrenocortical cancer or metastasis to the adrenal gland, some trials excluded participants with a BMI higher than 40 kg/m², and some trials excluded participants with an indication for bilateral adrenalectomy, which was an inclusion criterion in one trial (Fernández‐Cruz 1996).
Diagnosis
Corticoid and medullary, benign and malignant, functional and silent tumours, or masses of the adrenal gland were assessed by both laboratory and imaging studies. One trial reported a complete endocrinological work‐up before surgery, without details (individuals referred by an endocrinologist (Mohammadi‐Fallah 2013)); one did not report diagnostic procedures (Rubinstein 2005).
Laparoscopic retroperitoneal adrenalectomy was compared with laparoscopic transperitoneal adrenalectomy, performed with either a lateral approach or an anterior approach.
No trial reported treatment before the start of the trial.
Outcomes
Barczynski 2014 and Chai 2017 explicitly stated primary and secondary endpoints in the publication. The defined primary outcome was duration of surgery.
Excluded studies
We excluded 32 trials after careful evaluation of the full publication and record. The main reason for exclusion was that trials did not compare laparoscopic retroperitoneal adrenalectomy with laparoscopic transperitoneal adrenalectomy. There was one retrospective cohort study, with participants who underwent single incision laparoscopic surgery (Barbaros 2014).
Risk of bias in included studies
For details on the risk of bias of the included trials see Characteristics of included studies. For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials see Figure 2 and Figure 3. We investigated performance bias, detection bias, and attrition bias on an outcome level.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some studies)
3.
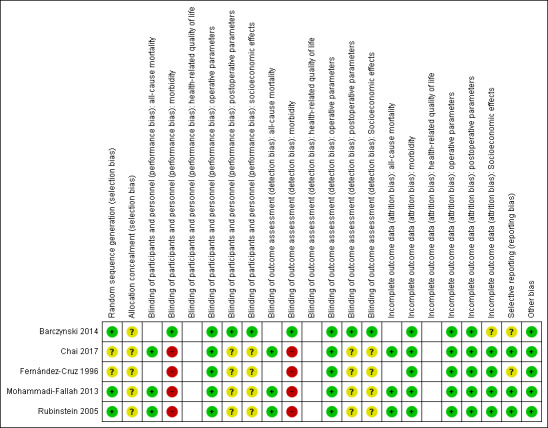
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome)
Allocation
Two trials reported unclear methods for random sequence generation, and we rated them as unclear risk of bias (Chai 2017; Fernández‐Cruz 1996). No trial described the method of allocation concealment adequately.
Blinding
Only one trial explicitly reported that it blinded the participants and personnel, describing the methods used to achieve blinding (Barczynski 2014). This trial also provided adequate details about blinding the outcome assessors.
Incomplete outcome data
One trial described the numbers of participants who discontinued the trial; four participants were lost to follow‐up (Barczynski 2014). No publication mentioned the use of an intention‐to‐treat analysis. None of the included trials provided detailed descriptions of participants’ withdrawals or reasons underpinning them. No trial had attrition rates that would probably have had an impact on the effect estimates.
Selective reporting
According to the Outcome Reporting Bias In Trials (ORBIT) classification, none of the included trials had a high risk of reporting bias.
Other potential sources of bias
We did not detect any additional risk of bias.
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics, see Appendix 5 and Appendix 6.
Primary outcomes
All‐cause mortality
Three trials provided data on deaths from any cause (Chai 2017; Mohammadi‐Fallah 2013; Rubinstein 2005). Two trials reported no deaths, in Rubinstein 2005 four participants died in the LRPA group, and one participant in the LTPA group within six years of follow‐up (low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 1 All‐cause mortality.
Early and late morbidity
Because all‐cause morbidity was not reported in the trials, we analysed early and late morbidity, and included specific adverse events (e.g. liver injury, splenic injury, vascular injury, pneumothorax or haemothorax, massive subcutaneous emphysema, surgical access site herniation) under these outcome measures.
For early morbidity (usually reported within 30 to 60 days after surgery), the results were inconclusive between the two techniques (risk ratio (RR) 0.56, 95% confidence interval (CI) 0.27 to 1.16; P = 0.12; 5 trials, 244 participants; very low‐certainty evidence; Analysis 1.2). Nine out of 127 participants (7.1%) in the LRPA group, compared with 16 out of 117 participants (13.7%) in the LTPA group, experienced specific adverse events (superficial wound infection, dehiscent wound necessitating closure, massive subcutaneous emphysema, haematoma, fever, transient hypoaesthesia of the abdominal wall, postoperative ileus, atrial fibrillation, tachyarrhythmia requiring medication, systolic blood pressure more than 200 mmHg, pneumonia, noninfectious diarrhoea, urinary retention and urinary tract infection).
1.2. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 2 Early and late morbidity.
LRPA resulted in less late morbidity, measured at the latest available follow‐up, than LTPA (RR of 0.12, 95% CI 0.01 to 0.92; P = 0.04; 3 trials, 146 participants; very low‐quality evidence; Analysis 1.2). None of the 78 participants in the LRPA group, compared with 7 of the 68 participants (10.3%) in the LTPA group experienced events, such as incisional hernia.
Secondary outcomes
Operative parameters
Duration of surgery
All trials reported this outcome. The mean difference (MD) between LRPA and LTPA duration of surgery was inconclusive (MD 0.7 min; 95% CI ‐20.0 to 21.3; P = 0.95; 5 trials, 250 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 3 Operative parameters: duration of surgery (min).
Intraoperative bleeding.
No trials measured this outcome.
Operative blood loss
All trials reported this outcome. The mean difference between LRPA and LTPA blood loss was inconclusive (MD ‐13 mL, 95% CI ‐32 to 5; P = 0.17; 5 trials, 250 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 4 Operative parameters: blood loss (mL).
Conversion to open surgery
The risk of converting to open surgery was inconclusive between LRPA and LTPA (RR 1.72, 95% CI 0.31 to 9.62; P = 0.54; 4 trials, 228 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 5 Operative parameters: conversion to open surgery.
Postoperative parameters
Time to oral fluid or food intake
Those who had a LRPA were able to take oral fluids or food earlier than those who had a LTPA (MD ‐8.6 hours, 95% CI ‐13.5 to ‐3.7; P = 0.0006; 2 trials, 89 participants; Analysis 1.6).
1.6. Analysis.
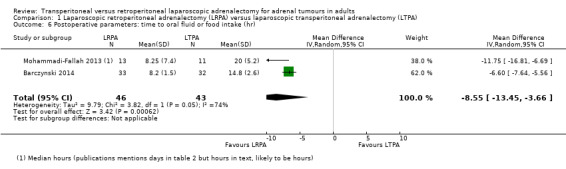
Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 6 Postoperative parameters: time to oral fluid or food intake (hr).
Time to ambulation
Those who had a LRPA were able to ambulate more quickly than those who had a LTPA (MD ‐5.4 hours, 95% CI ‐6.8 to ‐4; P < 0.0001; 2 trials, 89 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 7 Postoperative parameters: time to ambulation (hr).
Chest infection or pleural effusion
Barczynski 2014 reported this outcome in one participant per group (Analysis 1.8). In Mohammadi‐Fallah 2013, no participant had this outcome.
1.8. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 8 Postoperative parameters: chest infection, abdominal abscess.
Abdominal abscess
Barczynski 2014 reported this outcome in one participant in the LTPA group (Analysis 1.8). In Mohammadi‐Fallah 2013, no participant had this outcome.
Health‐related quality of life
No trial reported health‐related quality of life.
Socioeconomic effects
Time to return to normal activities
The results were inconclusive between the two techniques for the time it took participants to return to normal activities, which were ambiguously defined (MD ‐1.3 days, 95% CI ‐5.4 to 2.8; P = 0.52; 3 trials, 102 participants; very low‐quality evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1 Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA), Outcome 9 Socioeconomic effects.
Length of hospital stay
There were inconclusive results for length of hospital stay between the two techniques (MD ‐0.36 days, 95% CI ‐1.16 to 0.44; P = 0.37; 5 trials, 250 participants; Analysis 1.9).
Subgroup analyses
We did not perform subgroups analyses because there were not enough trials to estimate effects in various subgroups.
Sensitivity analyses
We performed sensitivity analyses by examining each outcome using fixed‐effects and random‐effects models (data not shown). There were inconclusive differences between models. Similarly, we examined how varying the effect size statistic, using odds ratio or risk ratio, influenced trial outcomes (data not shown). Again, we found inconclusive differences between effect size measures.
Assessment of reporting bias
We did not draw funnel plots due to limited number of trials (N = 5).
Trials awaiting classification
We found three RCTs comparing LRPA and LTPA, which we categorised as 'awaiting classification'. One completed trial was registered in ClinicalTrials.gov (NCT02618694) without results and no associated publication, and two trials were only published as conference abstracts (Abou 2016; Grubnik 2016).
Discussion
Summary of main results
A systematic review of the literature identified five randomised controlled trials (RCTs) comparing the effects of laparoscopic retroperitoneal adrenalectomy (LRPA) with laparoscopic transperitoneal adrenalectomy (LTPA) for adrenal tumours in adults (Barczynski 2014; Chai 2017; Fernández‐Cruz 1996; Mohammadi‐Fallah 2013; Rubinstein 2005). The five trials showed some heterogeneity in surgical approaches.
Of our key, or primary, outcome measures, no events were reported for all‐cause mortality, while one trial reported few events for late morbidity. Contrary to early morbidity, LRPA reported fewer late morbidity events than LTPA, but we are uncertain about this result due to very low‐certainty evidence.
Of our secondary outcomes, the results were inconclusive between groups in the analysis of operative parameters (duration of surgery, operative blood loss, conversion to open surgery). For our postoperative parameter time to oral fluid or food intake, we noted an advantage of LRPA, but only two trials reported on this outcome. No trials reported on health‐related quality of life.
Overall completeness and applicability of evidence
There was some heterogeneity regarding the surgical techniques used by the different trial authors, especially in indications for surgery. No trial evaluated the experience level and skills of the surgeons.
Quality of the evidence
We evaluated the certainty of the evidence using the GRADE approach, which also considers risk of bias. No trial was at low risk of selection bias, because there was insufficient information about allocation concealment. We downgraded the certainty of the evidence for all‐cause mortality, because of the small number of trials and low number of events. Blinding of participants and personnel was difficult; only Barczynski 2014 attempted blinding of trial participants and personnel, but it is likely that the blinding could have been broken in the postsurgical period. For early and late morbidity, we downgraded the evidence to very low quality due to the small number of trials and low number of participants. As the certainty of the evidence varied from low to very low, readers should interpret the results with caution.
Potential biases in the review process
Despite extensive search efforts, we might have overlooked unpublished data, especially regarding the Asian literature. Information, such as the experience of the surgeon performing the adrenalectomy would have been essential for the interpretation of the results, but was not available.
Agreements and disagreements with other studies or reviews
A recent systematic review, based on retrospective studies, found that many studies showed that LRPA was superior, or at least comparable to LTPA, in operation time, pain score, blood loss, hospitalisation, complications rates, and return to normal activity (Conzo 2016). However LTPA appeared to be as safe as LRPA, with a similar low morbidity rate. These results are in line with the results of two previous systematic reviews and meta‐analyses that reported comparable outcomes between the two techniques (Constantinides 2012; Nigri 2013). Our results, based on five RCTs, showed that long‐term morbidity, time to ambulation, and time to oral fluid or food intake might be reduced following laparoscopic retroperitoneal adrenalectomy.
Authors' conclusions
Implications for practice.
The body of evidence on laparoscopic retroperitoneal adrenalectomy compared with laparoscopic transperitoneal adrenalectomy is limited. Very low‐quality evidence indicates that for relatively small lesions (less than 6 cm to 7 cm), late morbidity might be reduced following the retroperitoneal approach. While no conclusive differences were observed between intervention and comparator groups in the analysis of operative parameters, the analysis of some postoperative parameters, such as the time to oral fluid of food intake and the time to ambulation, may show an advantage for the laparoscopic retroperitoneal adrenalectomy technique. All results have to be interpreted with caution, due to risk of bias, small sample sizes, and low number of events. The other findings of our review could only partially address our objectives. In particular, the included trials did not adequately address or report on health‐related quality of life.
Implications for research.
Long follow‐up periods were reported in only two of our included trials, which limits the body of evidence. No trial was powered to analyse all‐cause mortality, and none of the trials investigated health‐related quality of life. Important data, such as the treatment volume of the surgical centres and the surgeon's level of experience were not reported, although they may play an important role in operative and postoperative outcomes. New trials reporting on these aspects are required.
Notes
We have based parts of the background and methods sections, the appendices, additional tables, and Figures 1 to 3 of this review on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
The review was substantially edited by the Co‐ordinating Editor of the Cochrane Metabolic and Endocrine Disorders (CMED) Group, Bernd Richter. Gudrun Paletta, Assistant Managing Editor of the CMED Group, assisted us during the review and revision of the manuscript. The CMED Group's Information Specialist, Maria‐Inti Metzendorf, developed and performed the search.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
| 1. ((transperiton* or retroperiton* or laparoscop* or endoscop*) ADJ7 adrenalectom*):TI,AB,KY 2. MESH DESCRIPTOR Adrenalectomy 3. MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 4. #2 AND #3 5. #1 OR #4 |
| MEDLINE Ovid |
| 1. ((transperiton* or retroperiton* or laparoscop* or endoscop*) adj6 adrenalectom*).tw.
2. Adrenalectomy/ and exp Laparoscopy/
3. 1 or 2 [4.‐13. Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version, without "drug therapy.fs."] 4. randomized controlled trial.pt. 5. controlled clinical trial.pt. 6. randomi?ed.ab. 7. placebo.ab. 8. randomly.ab. 9. trial.ab. 10. groups.ab. 11. or/4‐10 12. exp animals/ not humans/ 13. 11 not 12 14. 3 and 13 |
| Embase Ovid |
| 1. ((transperiton* or retroperiton* or laparoscop* or endoscop*) adj6 adrenalectom*).tw. 2. adrenalectomy/ and exp laparoscopy/ 3. 1 or 2 [4:Wong 2006"sound treatment studies" filter – BS version] 4. random*.tw. or clinical trial*.mp. or exp health care quality/ 5. 3 and 4 [6.‐9.TSC Portal filter for exclusion of animal references] 6. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 7. human/ or normal human/ or human cell/ 8. 6 and 7 9. 6 not 8 10. 5 not 9 11. limit 10 to embase |
| ClinicalTrials.gov (Basic Search) |
| (transperitoneal OR retroperitoneal OR laparoscopic OR endoscopic OR retroperitoneoscopic) AND (adrenalectomy OR adrenalectomies) |
| ICTRP Search Portal (Standard Search) |
| transperiton* AND adrenalectom* OR retroperiton* AND adrenalectom* OR laparoscop* AND adrenalectom* OR endoscop* AND adrenalectom* |
Appendix 2. 'Risk of bias' assessment
| 'Risk of bias' domains |
|
1. Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included trial, we will describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
2. Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment) For each included trial, we will describe the method used to conceal allocation to interventions prior to assignment, and we will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will also evaluate trial baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgment for selection bias (Corbett 2014). Chance imbalances may also affect judgments on the risk of attrition bias. In the case of unadjusted analyses, we will distinguish between trials that we rate as being at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials that we judge as being at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We will reclassify judgements of unclear, low, or high risk of selection bias as specified in Appendix 5. 3. Blinding of participants and trial personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) We will evaluate the risk of detection bias separately for each outcome (Hróbjartsson 2013). We will note whether endpoints were self‐reported, investigator‐assessed, or adjudicated outcome measures (see below).
4. Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment We will evaluate the risk of detection bias separately for each outcome (Hróbjartsson 2013). We will note whether endpoints were self‐reported, investigator‐assessed, or adjudicated outcome measures (see below).
5. Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data) For each included trial or each outcome, or both, we will describe the completeness of data, including attrition and exclusions from the analyses. We will state whether the trial reported attrition and exclusions, and report the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention and comparator groups). We will also note if the trial reported the reasons for attrition or exclusion, and whether missing data were balanced across groups, or were related to outcomes. We will consider the implications of missing outcome data per outcome, such as high dropout rates (e.g. above 15%), or disparate attrition rates (e.g. difference of 10% or more between trial arms).
6. Selective reporting (reporting bias due to selective outcome reporting) We will assess outcome reporting bias by integrating the results of the appendix 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Mathieu 2009), with those of the appendix 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis will form the basis for the judgement of selective reporting.
7. Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for trials that reported unadjusted analyses: comparison of results obtained using method details alone with results using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | Risk of bias judgement using methods reporting | Information gained from trial characteristics data | Risk of bias using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly randomised or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias, compared with using methods reporting alone. bImbalance identified that appears likely to be due to chance. cDetails for the remaining important prognostic variables are not reported. | |||
Appendix 4. Description of interventions
| Trial ID | Intervention | Comparator | Adrenalectomy |
| Chai 2017 | Retroperitoneal adrenalectomy | Transperitoneal adrenalectomy | Unilateral adrenalectomies |
| Barczynski 2014 | Retroperitoneal adrenalectomy | Transperitoneal adrenalectomy | Unilateral total adrenalectomies |
| Mohammadi‐Fallah 2013 | Retroperitoneal adrenalectomy | Transperitoneal adrenalectomy | Unilateral adrenalectomies |
| Rubinstein 2005 | Retroperitoneal adrenalectomy | Transperitoneal adrenalectomy | Unilateral adrenalectomies |
| Fernández‐Cruz 1996 | Retroperitoneal adrenalectomy | Transperitoneal adrenalectomy | Unilateral and bilateral adrenalectomies |
Appendix 5. Baseline characteristics (I)
| Trial ID | Intervention and comparator | Duration of follow‐up | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) | Duration of disease (mean years (SD)) |
| Chai 2017 | I: retroperitoneal adrenalectomy | 31.3 months | Individuals with adrenal gland adenomas | 2012 to 2016 | South Korea | Seoul National University Hospital, Seoul | ‐ | ‐ |
| C: transperitoneal adrenalectomy | ‐ | ‐ | ||||||
| Barczynski 2014 | I: retroperitoneal adrenalectomy | 1, 12, 24, 36, 48, and 60 months after surgery | Individuals with adrenal tumours | 2006 to 2008 | Poland | Medical College, Jagiellonian University, Krakow | ‐ | ‐ |
| C: transperitoneal adrenalectomy | ||||||||
| Mohammadi‐Fallah 2013 | I: retroperitoneal adrenalectomy | Mean follow‐up 9 months | Individuals with surgical adrenal disease | 2008 to 2011 | Iran | Imam Medical Center, Urmia University | ‐ | ‐ |
| C: transperitoneal adrenalectomy | ||||||||
| Rubinstein 2005 | I: retroperitoneal adrenalectomy | Mean follow‐up 5.9 years | Individuals with surgical adrenal disease | 1997 to 1999 | USA | Glickman Urological Institute, Cleveland | ‐ | ‐ |
| C: transperitoneal adrenalectomy | ||||||||
| Fernández‐Cruz 1996 | I: retroperitoneal adrenalectomy | Participants with total bilateral adrenalectomy: mean follow‐up 9.2 months (SD 6.1) | Individuals with Cushing's syndrome (including Cushing's disease and Cushing's adenoma) | ‐ | Spain | Hospìtal Clìnic, University of Barcelona | ‐ | ‐ |
| C: transperitoneal adrenalectomy | ||||||||
| ‐ denotes not reported C: comparator; I: intervention; SD: standard deviation | ||||||||
Appendix 6. Baseline characteristics (II)
| Trial ID | Intervention and comparator | Sex (female %) | Age (mean years (SD), or as reported) | BMI (mean kg/m² (SD), or as reported) | Tumour size (mm) and preoperative diagnosis (number) | Previous abdominal surgery | Lateral location (right side/left side) | Comorbidities |
| Chai 2017 | I: retroperitoneal adrenalectomy | 63 | 46.4 (11.0) | 23.6 (3.0) | Mean 30 (SD 13) Preoperative diagnosis: Aldosteronoma: 16 Cushing syndrome: 10 Phaeochromocytoma: 7 Nonfunctioning tumour: 8 |
Yes (29%) | 18/23 | Hypertension: 66% |
| C: transperitoneal adrenalectomy | 67 | 48.0 (11.4) | 24.2 (3.3) | Mean 29 (14) Preoperative diagnosis: Aldosteronoma: 20 Cushing syndrome: 7 Phaeochromocytoma: 8 Nonfunctioning tumour: 7 |
Yes (26%) | 18/24 | Hypertension: 76% | |
| Barczynski 2014 | I: retroperitoneal adrenalectomy | 76 | 47.9 | 27.6 | Mean 39 (10 to 70) Preoperative diagnosis: Aldosteronoma: 7 Glucocorticoid adrenal adenoma: 4 Phaeochromocytoma: 8 Nonfunctioning tumour: 14 |
Yes (not major) | 16/17 | ‐ |
| C: transperitoneal adrenalectomy | 72 | 46.6 | 27.3 | Mean 40 (10 to 65) Preoperative diagnosis: Aldosteronoma: 7 Glucocorticoid adrenal adenoma: 3 Phaeochromocytoma: 7 Nonfunctioning tumour: 15 |
Yes (not major) | 15/17 | ‐ | |
| Mohammadi‐Fallah 2013 | I: retroperitoneal adrenalectomy | 62 | 42.2 (median) | 27.5 (median) | Median 26 (IQR 20 to 50) Preoperative diagnosis: Aldosteronoma: 1 Phaeochromocytoma: 2 Cushing's syndrome: 4 Nonfunctioning tumour: 6 |
Yes (not major) | 8/5 | ‐ |
| C: transperitoneal adrenalectomy | 55 | 42.9 (median) | 26.7 (median) | Median 29 (IQR 20 to 50) Preoperative diagnosis: Aldosteronoma: 2 Phaeochromocytoma: 2 Cushing's syndrome: 3 Nonfunctioning tumour: 4 |
Yes (not major) | 6/5 | ‐ | |
| Rubinstein 2005 | I: retroperitoneal adrenalectomy | 59 | 57.5 (median) | 30.4 (median) | Median 26 (IQR 17 to 49) Preoperative diagnosis: Aldosteronoma: 10 Not specified: 15 Phaeochromocytoma: 2 Cushing's syndrome: 3 Metastasis: 1 Adrenal carcinoma: 1 |
Yes (not major) | 9/23 | ‐ |
| C: transperitoneal adrenalectomy | 48 | 57 (median) | 29.1 (median) | Median 27 (IQR 16 to 42) Preoperative diagnosis: Aldosteronoma: 10 Not specified: 5 Phaeochromocytoma: 7 Cushing's syndrome: 2 Metastasis: 1 Adrenal carcinoma: 0 |
Yes (not major) | 12/13 | ‐ | |
| Fernández‐Cruz 1996 | I: retroperitoneal adrenalectomy | 73 | 49.9 (17.8) | ‐ | Preoperative diagnosis: 5 adenoma, tumour size: 51 (38 to 75) 6 hyperplasia, , tumour size: 54 (35 to 79) | ‐ | Adenoma: 2/3 Hyperplastic glands: 3/3 | ‐ |
| C: transperitoneal adrenalectomy | 71 | 39.9 (18.4) | ‐ | Preoperative diagnosis: 4 adenoma, tumour size: 49 (25 to 65) 6 hyperplasia, tumour size: 58 (40 to 72) | ‐ | Adenoma: 1/3 Hyperplastic glands: 3/3 | ‐ | |
| ‐ denotes not reported BMI: body mass index; C: comparator; I: intervention; IQR: interquartile range; SD: standard deviation | ||||||||
Appendix 7. Matrix of trial endpoints (publications and trial documents)
| Study ID | Endpoints quoted in trial document(s)a | Trial results available in trial register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c |
| Chai 2017 |
Source:NCT01676025 Primary outcome measure(s): operation time (participants will be followed until the first visit at outpatient clinic after discharge, an expected average of 3 weeks) |
No | Primary outcome measure(s): operative time | Primary outcome measure(s): operative time |
| Secondary outcome measure(s): pain sensation after surgery (pain score will be described daily during hospitalisation, and also at outpatient clinic after discharge, an expected average of 3 weeks); recovery of bowel movement (participants will be followed for the duration of hospital stay, an expected average of 5 days); wound complication (participants will be followed until the first visit at outpatient clinic after discharge, an expected average of 3 weeks); blood loss during operation (participants will be followed until the first visit at outpatient clinic after discharge, an expected average of 3 weeks); intraoperative haemodynamic status (participants will be followed until the first visit at outpatient clinic after discharge, an expected average of 3 weeks; severe hypertension (systolic blood pressure > 200 mmHg), severe hypotension (systolic blood pressure < 90 mmHg), tachycardia (heart rate > 110/min), bradycardia (heart rate < 50/min) | Secondary outcome measure(s): blood loss, intraoperative haemodynamic stability, postoperative pain, recovery of bowel movement, and complication rates | Secondary outcome measure(s): blood loss, intraoperative haemodynamic stability, postoperative pain, recovery of bowel movement, complication rates | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Barczynski 2014 |
Source:NCT01959711 Primary outcome measure(s): duration of surgery (time frame: intraoperatively) |
No | Primary outcome measure(s): duration of surgery | Primary outcome measure(s): duration of surgery |
| Secondary outcome measure(s): postoperative recovery including postoperative pain, length of hospital stay, time to oral intake, time to ambulation; blood loss (all: participants were followed for the duration of hospital stay, an expected average of 7 days); postoperative complications (up to 5 years after surgery) including: pneumothorax or haemothorax, surgical emphysema, chest infection, visceral injury, peritonitis or abscess, wound infection, neuralgia, and surgical access site herniation | Secondary outcome measure(s): intraoperative blood loss, conversion rate, postoperative pain, prevalence of shoulder‐tip pain, additional analgesia requests, nausea and vomiting, time to oral intake, time to ambulation, length of hospital stay, total cost of the operation, postoperative complications (including long‐term surgical access site herniation and need for hernia repair), and in cases of active tumours, also biochemical and clinical cure rate during 5‐year follow‐up | Secondary outcome measure(s): blood loss, conversion rate, postoperative recovery, morbidity, costs | ||
| Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | Other outcome measure(s): ‐ | ||
| Mohammadi‐Fallah 2013 | N/T | Primary outcome measure(s): convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): blood loss, operative time, open conversion, analgesic requirement dose, oral intake, ambulation, hospital stay, postoperative pain | Other outcome measure(s): ‐ | |||
| Rubinstein 2005 | N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): operative time, estimated blood loss, specimen weight, number of open conversions, analgesic requirement, days to oral intake, days to ambulation, days of hospital stay, complications, median convalescence, number of deaths | Other outcome measure(s): operative time, estimated blood loss, specimen weight, number of open conversions, analgesic requirement, days to oral intake, days to ambulation, days of hospital stay; complications, median convalescence, number of deaths | |||
| Fernández‐Cruz 1996 | N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): paCO2, arterial pH, operating time, intraoperative bleeding, need for blood transfusion, analgesic dose requirements, hospital stay, time to achieve normal activity, complications | Other outcome measure(s): paCO2, arterial pH, operating time, intraoperative bleeding, need for blood transfusion, analgesic dose requirements, hospital stay, time to achieve normal activity, complications | |||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers, and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers) bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents, or multiple reports of a primary trial) cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures EMA: European Medicines Agency; FDA: Food and Drug Administration (US); N/T: no trial document available | ||||
Appendix 8. High risk of outcome reporting bias according to ORBIT classification
| Trial ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Chai 2017 | N/A | ||||
| Barczynski 2014 | N/A | ||||
| Mohammadi‐Fallah 2013 | N/A | ||||
| Rubinstein 2005 | N/A | ||||
| Fernández‐Cruz 1996 | N/A | ||||
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed, but only reports that result was not significant (Classification A, table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report states that outcome was analysed, but no results reported (Classification D, table 2, Kirkham 2010)
cClear that outcome was measured, but not necessarily analysed; judgement says likely to have been analysed, but not reported because of non‐significant results (Classification E, table 2, Kirkham 2010)
dUnclear whether the outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed, but not reported on the basis of non‐significant results (Classification G, table 2, Kirkham 2010) N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 9. Definition of endpoint measurement
| Trial ID | All‐cause mortality | Morbidity | Health‐related quality of life | Operative parameters | Postoperative parameters |
| Chai 2017 | N/D | N/D | N/I | "Operative time was calculated from the first incision to the final stitch" "Amount of blood loss was calculated by the volume of suction and the weight of the gauze" |
"Postoperative pain was evaluated using a VAS with a range from 0 (no pain) to 10 (worst pain). VAS was checked 3 to 5 times daily, and expressed as a daily mean value" |
| Barczynski 2014 | N/R | "Surgical complications were classified according to the Dindo‐Clavien classification" | N/I | "Duration of surgery was calculated from skin incision to skin closure. Intraoperative blood loss was calculated on the basis of hematocrit assessment in the saline fluid used for irrigation in relation to the blood hematocrit" | "Pain intensity was assessed using the visual analog scale (VAS) at 4, 8, 12, 24, and 48 hours postoperatively" |
| Mohammadi‐Fallah 2013 | Deaths | Late complications, such as portal‐site hernia | N/I | N/R | "...the convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs" |
| Rubinstein 2005 | "Mortality due to various unrelated causes ... during the 6‐year follow‐up" | N/D | N/I | N/R | "Convalescence was defined as the period needed for complete recovery from the physical aftereffects of surgery" |
| Fernández‐Cruz 1996 | N/R | N/D | N/I | "The following parameters were evaluated in each patient with retroperitoneal and with transperitoneal approaches: operative time, estimated blood loss, length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity" | N/R |
| N/D: not defined; N/I: not investigated; N/R: not reported; VAS: visual analogue scale | |||||
Appendix 10. Definition of endpoint measurement
| Trial ID | Severe/serious adverse events | Socioeconomic effects |
| Chai 2017 | N/D | N/D |
| Barczynski 2014 | N/D | N/D |
| Mohammadi‐Fallah 2013 | N/D | "...The convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs" |
| Rubinstein 2005 | N/D | "Convalescence was defined as the period needed for complete recovery from the physical aftereffects of surgery" |
| Fernández‐Cruz 1996 | N/D | The following parameters were evaluated in each participant with retroperitoneal and with transperitoneal approaches: operative time, estimated blood loss, length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity |
| N/D: not defined | ||
Appendix 11. Adverse events (I)
| Trial ID | Intervention and comparator | Participants included in analysis (N) | Deaths (N) | Deaths (%) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with at least one severe or serious adverse event (N) | Participants with at least one severe or serious adverse event (%) |
| Chai 2017 | I: posterior retroperitoneoscopic adrenalectomy | 41 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 42 | 0 | 0 | ‐ | ‐ | 1 | 2.4 | |
| Barczynski 2014 | I: posterior retroperitoneoscopic adrenalectomy | 33 | ‐ | ‐ | 6 | 18.2 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 32 | ‐ | ‐ | 15 | 46.8 | ‐ | ‐ | |
| Mohammadi‐Fallah 2013 | I: retroperitoneal adrenalectomy | 12 | 0 | 0 | 1 | 8.3 | 0 | 0 |
| C: transperitoneal adrenalectomy | 11 | 0 | 0 | 1 | 9.1 | 0 | 0 | |
| Rubinstein 2005 | I: retroperitoneal adrenalectomy | 32 | 4 | 12.5 | 1 | 3.1 | _ | ‐ |
| C: transperitoneal adrenalectomy | 25 | 1 | 4 | 2 | 8 | ‐ | ‐ | |
| Fernández‐Cruz 1996 | I: retroperitoneal adrenalectomy | 8 | ‐ | ‐ | 0 | 0 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 7 | ‐ | ‐ | 2 | 28.6 | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | ||||||||
Appendix 12. Adverse events (II)
| Trial ID | Intervention and comparator | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) |
| Chai 2017 | I: retroperitoneal adrenalectomy | 41 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 42 | ‐ | ‐ | |
| Barczynski 2014 | I: retroperitoneal adrenalectomy | 33 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 32 | ‐ | ‐ | |
| Mohammadi‐Fallah 2013 | I: retroperitoneal adrenalectomy | 12 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 11 | ‐ | ‐ | |
| Rubinstein 2005 | I: retroperitoneal adrenalectomy | 32 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 25 | ‐ | ‐ | |
| Fernández‐Cruz 1996 | I: retroperitoneal adrenalectomy | 8 | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 7 | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | ||||
Appendix 13. Adverse events (III)
| Trial ID | Intervention and comparator | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Chai 2017 | I: retroperitoneal adrenalectomy | 41 | Surgical site herniation | 0 | 0 |
| C: transperitoneal adrenalectomy | 42 | Surgical site herniation | 0 | 0 | |
| Barczynski 2014 | I: posterior retroperitoneoscopic adrenalectomy | 33 | (1) Massive subcutaneous emphysema (2) Surgical access site herniation |
(1) 2 (2) 0 |
(1) 6.2 (2) 0 |
| C: transperitoneal adrenalectomy | 32 | (1) Massive subcutaneous emphysema (2) Surgical access site herniation |
(1) 1 (2) 5 |
(1) 3.1 (2) 15.5 |
|
| Mohammadi‐Fallah 2013 | I: retroperitoneal adrenalectomy | 12 | ‐ | ‐ | |
| C: transperitoneal adrenalectomy | 11 | ‐ | ‐ | ||
| Rubinstein 2005 | I: retroperitoneal adrenalectomy | 32 | Port site hernia | 0 | 0 |
| C: transperitoneal adrenalectomy | 25 | Port site hernia | 2 | 8 | |
| Fernández‐Cruz 1996 | I: retroperitoneal adrenalectomy | 8 | ‐ | ‐ | ‐ |
| C: transperitoneal adrenalectomy | 7 | ‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | |||||
Appendix 14. Survey of study investigators providing information on included trials
| Trial ID | Date trial author was contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Chai 2017 | Will be contacted for the next review update | |||
| Barczynski 2014 | 26 May 2015 by email, and 17 September 2015 by regular mail | No reply | N/A | N/A |
| Mohammadi‐Fallah 2013 | No email address available, sent written message by regular mail on 26 May 2015 and 17 September 2015 | No reply | N/A | N/A |
| Rubinstein 2005 | 26 May 2015 by email, and 17 September 2015 by regular mail | No reply | N/A | N/A |
| Fernández‐Cruz 1996 | 26 May 2015 by email, and 17 September 2015 by regular mail | No reply | N/A | N/A |
| N/A: not applicable | ||||
Appendix 15. Checklist to aid consistency and reproducibility of GRADE assessments
| (1) All‐cause mortality | (2) Early morbidity | (3) Late morbidity | (4) Health‐related quality of life | (5) Socioeconomic effects (time to return to normal activities, time to ambulation, length of hospital stay) | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Not reported | Yes |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | ||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | No (↓) | No (↓) | Unclear | ||
| Was there blinding of outcome assessment (i.e. no potential for detection bias), or was outcome measurement not likely to be influenced by lack of blinding? | Yes | No (↓) | No (↓) | Unclear | ||
| Was an objective outcome used? | Yes | Unclear | Unclear | Unclear | ||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | ||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Unclear | Unclear | Yes | ||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | ||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | ||
| Inconsistencyb | Point estimates did not vary widely? | N/A | Yes | Yes | Yes | |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | Substantial | Substantial | Substantial, Substantial. Some | ||
| Was the direction of effect consistent? | N/A | Yes | Yes | Yes, Yes, No | ||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | N/A | Moderate | Low | Moderate, Low, High (↓) | ||
| Was the test for heterogeneity statistically significant (P < 0.1)? | N/A | Not statistically significant | Not statistically significant | Statistically significant (↓) | ||
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | ||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Unclear | ||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | ||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | ||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | No (↓) | Yes | No (↓), Yes, No (↓) | |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | Low (↓) | Low (↓) to intermediate | ||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Intermediate | Small (↓) | Small (↓), Small (↓), Moderate | ||
| Was the outcome a common event (e.g. occurs more than 1/100)? | No | Yes | Yes | N/A | ||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | |
| Was grey literature searched? | Yes | Yes | Yes | Yes | ||
| Were no restrictions applied to trial selection on the basis of language? | Yes | Yes | Yes | Yes | ||
| There was no industry influence on studies included in the review? | Unclear | Unclear | Unclear | Unclear | ||
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | ||
| There was no discrepancy in findings between published and unpublished trials? | N/A | N/A | N/A | N/A | ||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis, rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity is based on I² cWhen judging the width of the confidence interval, it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry, and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); N/A: not applicable | ||||||
Data and analyses
Comparison 1. Laparoscopic retroperitoneal adrenalectomy (LRPA) versus laparoscopic transperitoneal adrenalectomy (LTPA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Early and late morbidity | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Early morbidity | 5 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.27, 1.16] |
| 2.2 Late morbidity | 3 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 0.92] |
| 3 Operative parameters: duration of surgery (min) | 5 | 250 | Mean Difference (IV, Random, 95% CI) | 0.68 [‐19.94, 21.29] |
| 4 Operative parameters: blood loss (mL) | 5 | 250 | Mean Difference (IV, Random, 95% CI) | ‐13.06 [‐31.55, 5.44] |
| 5 Operative parameters: conversion to open surgery | 4 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.31, 9.62] |
| 6 Postoperative parameters: time to oral fluid or food intake (hr) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | ‐8.55 [‐13.45, ‐3.66] |
| 7 Postoperative parameters: time to ambulation (hr) | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐5.41 [‐6.77, ‐4.04] |
| 7.1 Time to ambulation (hr) | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐5.41 [‐6.77, ‐4.04] |
| 8 Postoperative parameters: chest infection, abdominal abscess | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Chest infection/pleural effusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Abdominal abscess | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Socioeconomic effects | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Time to return to normal activities (days) | 3 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐5.43, 2.76] |
| 9.2 Length of hospital stay (days) | 5 | 250 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.16, 0.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barczynski 2014.
| Methods | Design: parallel randomised controlled clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: planned unilateral adrenal surgery for a benign tumour up to 7 cm in diameter, nonfunctioning adrenal tumours between 4 cm and 7 cm, or smaller lesion, which was progressively enlarging Exclusion criteria: diffuse peritonitis in history, major abdominal surgery in history, planned bilateral adrenal surgery, adrenal tumour more than 7 cm in diameter, suspected adrenocortical cancer, metastasis to adrenal gland, previous adrenal surgery, pregnancy or lactation, age less than 18 or more than 80 years, American Society of Anesthesiologists fitness grade IV, and inability to comply with the follow‐up protocol. Diagnostic criteria: abdominal spiral computed tomography (CT) and hormonal activity (urinary metoxycatecholamines, diurnal cortisol, dexamethasone suppression test, ACTH, DHEAS, blood ions, serum aldosterone concentration and serum renin activity). |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: not declared Retroperitoneal laparoscopic adrenalectomy operations were performed using the Walz’s technique (individuals are positioned on a rectangular support with bent hip joints at a 90‐degree angle to maximally open the space between the 12th rib and iliac crest. Three trocars are used for both right and left procedures, which are placed just under the 12th rib, with direct palpation and finger guidance after the dorsal lumbar fascia is digitally perforated. Balloon trocar in place in the middle port, retroperitoneal space insufflation with 20 to 30 mmHg of CO2. The dissection of the space is completed with blunt dissection of the area underneath the diaphragm, and the fatty tissue above the superior border of the kidney. Dissection of the adrenal gland starts inferiorly in a plane close to the kidney surface. Identification and ligation of the adrenal vein in a medial or inferomedial position with either clips or a haemostatic device. Mobilisation of the gland is completed by dissecting laterally between the diaphragm and the psoas; the superior attachments are divided last). Transperitoneal laparoscopic adrenalectomy operations were performed employing the Gagner’s technique (individuals are positioned at a 60 to 90 degree angle with tumour side up, and the table is flexed to maximally open the space between the tip of the 12th rib and the iliac crest. 3 ports for left adrenalectomy and 4 ports for right adrenalectomy (the fourth port is used for liver retraction). During left adrenalectomy, procedural steps include taking down the splenic flexure of the colon, freeing the splenic ligaments to mobilize the spleen and rotate it medially, and dissecting in the avascular plane between the tail of the pancreas and kidney, and controlling and dividing the adrenal vein as it enters the left renal vein. During right adrenalectomy, procedural steps include mobilisation of the right triangular ligament of the liver, a hockey stick incision between the retroperitoneal attachments of the right lobe of the liver and the lateral border of the IVC, dissection of the lateral edge of the IVC, and taking the right adrenal vein at the takeoff from the IVC. Superior retraction of the liver must be maintained by the assistant throughout the case to aid exposure of the right adrenal. Mobilisation of the gland follows a superior‐lateral to medial‐inferior progression unless surgeon preference is for taking the adrenal vein early, in which case, an inferior to superior and medial to lateral mobilisation of the gland is preferred). CO2 insufflation pressures are 12 mmHg. Intraoperative dissection and haemostasis was achieved in both groups by the ultrasonic harmonic shears (LCSC5 or ACE; Ultracision; Ethicon EndoSurgery, Somerville, NJ). In both groups, the mobilised adrenal gland was removed through the trocar port with a latex endocath bag (Endocatch, USSC Norwalk, CT). In selected cases, the skin incision was extended to allow for removing the intact surgical material (morcellation was not used). The same fascia closure technique was used for laparoscopic retroperitoneal adrenalectomy (LRPA) and laparoscopic transperitoneal adrenalectomy (LTPA) operations using interrupted absorbable sutures and a BERCI Facial Closing Instrument (STORZ, Tutlingen, Germany) for all 10 mm trocar sites, whereas 5 mm trocar sites were not closed. Drainage of the wounds was not used. |
|
| Outcomes |
Composite outcome measures reported: none Primary endpoint: duration of surgery Secondary endpoints: intraoperative blood loss, conversion rate, postoperative pain, prevalence of shoulder tip pain, additional analgesia requests, nausea and vomiting, time to oral intake, time to ambulation, length of hospital stay, total cost of the operation, postoperative complications (including long‐term surgical access site herniation and need for hernia repair), and in cases of active tumours, biochemical and clinical cure rate during 5‐year follow‐up |
|
| Study details |
Run‐in period: none Trial terminated early: no Trials register identifier:NCT01959711 |
|
| Publication details |
Language of publication: English Commercial funding, non‐commercial funding, other funding: not declared Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The aim of this study was to evaluate the results of PRA versus LTLA in patients with unilateral benign adrenal tumours up to 7 cm in diameter. It was hypothesized that PRA may be superior to LTLA for both short‐term and long‐term outcomes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "The randomization sequence was generated by a computer. Sequencing was based on permuted blocks of 2 and 3 to balance the number of participants in the treatment groups" |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Information on the type of intervention remained in consecutively numbered and sealed envelopes that were stored in the operating theatre. An envelope containing the allocation was added to the patient’s file in the operating room. The envelope was opened and the surgeon performed the assigned intervention." Comment: questionable whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) morbidity | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes assessing clinical investigators knew the relevant group assignment. Both the abdomen and lumbar region on the side of the operation were covered with an opaque, large, and air‐permeable but water‐resistant dressing to conceal the site of the surgical access for the initial 3 postsurgery days" Comment: blinding of trial participants and personnel attempted, but likely that the blinding could have been broken after the 3 postsurgery days |
| Blinding of participants and personnel (performance bias) operative parameters | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes assessing clinical investigators knew the relevant group assignment. Both the abdomen and lumbar region on the side of the operation were covered with an opaque, large, and air‐permeable but water‐resistant dressing to conceal the site of the surgical access for the initial 3 postsurgery days" Comment: blinding of trial participants and personnel attempted but not in the operating room; outcome measure not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) postoperative parameters | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes assessing clinical investigators knew the relevant group assignment. Both the abdomen and lumbar region on the side of the operation were covered with an opaque, large, and air‐permeable but water‐resistant dressing to conceal the site of the surgical access for the initial 3 postsurgery days" Comment: blinding of trial participants and personnel attempted, but likely that the blinding could have been broken after the 3 postsurgery days; outcome measure not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes assessing clinical investigators knew the relevant group assignment. Both the abdomen and lumbar region on the side of the operation were covered with an opaque, large, and air‐permeable but water‐resistant dressing to conceal the site of the surgical access for the initial 3 postsurgery days" Comment: blinding of trial participants and personnel attempted, but likely that the blinding could have been broken after the 3 postsurgery days |
| Blinding of outcome assessment (detection bias) morbidity | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes‐assessing clinical investigators knew the relevant group assignment" Quote from publication: "The following data were collected by independent clinical investigators during the trial: duration of surgery, intraoperative blood loss, conversion rate, postoperative pain, prevalence of shoulder‐tip pain, additional analgesia requests, nausea and vomiting, time to oral intake of clear liquids and solid diet, time to ambulation, length and cost of hospital stay, morbidity, biochemical laboratory values, and clinical disease recovery data" Comment: blinding of outcome assessment attempted, but likely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) operative parameters | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes‐assessing clinical investigators knew the relevant group assignment" Quote from publication: "The following data were collected by independent clinical investigators during the trial: duration of surgery, intraoperative blood loss, conversion rate, postoperative pain, prevalence of shoulder‐tip pain, additional analgesia requests, nausea and vomiting, time to oral intake of clear liquids and solid diet, time to ambulation, length and cost of hospital stay, morbidity, biochemical laboratory values, and clinical disease recovery data" Comment: blinding of outcome assessment attempted, but likely that the blinding could have been broken; outcome measure not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) postoperative parameters | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes‐assessing clinical investigators knew the relevant group assignment" Quote from publication: "The following data were collected by independent clinical investigators during the trial: duration of surgery, intraoperative blood loss, conversion rate, postoperative pain, prevalence of shoulder‐tip pain, additional analgesia requests, nausea and vomiting, time to oral intake of clear liquids and solid diet, time to ambulation, length and cost of hospital stay, morbidity, biochemical laboratory values, and clinical disease recovery data" Comment: blinding of outcome assessment attempted, but likely that the blinding could have been broken; outcome measure not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Socioeconomic effects | Low risk |
Quote from publication: "Neither of the patients, nurses, or outcomes‐assessing clinical investigators knew the relevant group assignment" Quote from publication: "The following data were collected by independent clinical investigators during the trial: duration of surgery, intraoperative blood loss, conversion rate, postoperative pain, prevalence of shoulder‐tip pain, additional analgesia requests, nausea and vomiting, time to oral intake of clear liquids and solid diet, time to ambulation, length and cost of hospital stay, morbidity, biochemical laboratory values, and clinical disease recovery data" Comment: blinding of outcome assessment attempted, but likely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) morbidity | Low risk |
Quote from publication: "65 patients with complete short‐term follow‐up (33 in the PRA and 32 in the LTLA group) and 61 patients who were followed‐up for 5 years (30 in the PRA and 31 in the LTLA group)" Comment: low attrition rate |
| Incomplete outcome data (attrition bias) operative parameters | Low risk |
Quote from publication: "65 patients with complete short‐term follow‐up (33 in the PRA and 32 in the LTLA group), and 61 patients who were followed up for 5 years (30 in the PRA and 31 in the LTLA group)" Comment: low attrition rate |
| Incomplete outcome data (attrition bias) postoperative parameters | Low risk |
Quote from publication: "65 patients with complete short‐term follow‐up (33 in the PRA and 32 in the LTLA group), and 61 patients who were followed up for 5 years (30 in the PRA and 31 in the LTLA group)" Comment: low attrition rate |
| Incomplete outcome data (attrition bias) Socioeconomic effects | Unclear risk |
Quote from publication: "65 patients with complete short‐term follow‐up (33 in the PRA and 32 in the LTLA group), and 61 patients who were followed up for 5 years (30 in the PRA and 31 in the LTLA group)" Comment: low attrition rate |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary outcome reported as stated. All secondary outcomes are reported except blood loss. In the protocol of trial, the outcome was blood loss for the duration of hospital stay, but the article reported only the intraoperative blood loss. In the article, the outcomes pneumothorax or haemothorax, chest infection, visceral injury, peritonitis or abscess, and neuralgia, as reported in the protocol of the trial, were not mentioned. Deaths were not mentioned. |
| Other bias | Low risk | Comment: none detected |
Chai 2017.
| Methods | Design: parallel randomised controlled clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria (from ClinicalTrials.gov):
Exclusion criteria (from ClinicalTrials.gov):
Diagnostic criteria: adrenal protocol computed tomography (CT) was routinely performed before surgery. Biochemical marker screening tests were performed for primary aldosteronism (plasma renin, aldosterone), Cushing syndrome (overnight 1 mg dexamethasone suppression test, serum cortisol, and urine cortisol), and phaeochromocytoma (24‐hour urine dopamine, epinephrine, metanephrine, norepinephrine, normetanephrine, and VMA). If primary aldosteronism was suspected, adrenal venous sampling was routinely performed for lateralisation, and the patient was prescribed spironolactone for at least 4 weeks preoperatively. If the blood pressure remained uncontrolled, the spironolactone dose was increased, and additional medications were added until systolic and diastolic blood pressures were maintained below 150 mmHg and 100 mmHg, respectively. In phaeochromocytoma patients, an alpha‐blocker (doxazosin) was prescribed for at least 4 weeks preoperatively. A beta‐blocker and a calcium channel blocker were added in a step‐wise manner, until systolic and diastolic blood pressures were maintained below 150 mmHg and 100 mmHg, respectively |
|
| Interventions |
Number of trial centres: 1 (performed by a single surgeon at Seoul National University Hospital. The surgeon had performed 55 cases of laparoscopic transperitoneal adrenalectomies and 29 cases of posterior transperitoneal adrenalectomies before the start of the trial) Treatment before trial: not reported Retroperitoneal laparoscopic adrenalectomy: the patient was intubated in a patient bed and then turned prone when moved onto the operating table. The procedure was performed with the patient in the prone jackknife position with a moderately bent hip joint. The first incision was made, 2 cm in length, at the lower tip of the 12th rib. The retroperitoneal space was entered under direct vision and widened using the surgeon’s index finger. Medial and lateral incisions, 5 mm in length, were made just lateral to the erector spinae muscles, and at the lower tip of the 11th rib, respectively. Trocars were introduced through the medial and lateral incisions under the guidance of the index finger, inserted through the first incision. A 12 mm balloon trocar was then inserted through the first incision. The medial port was used as a camera port. Operative procedures were performed as described by Walz et al. Arterial lines were routinely used in all patients and central venous lines were used in pheochromocytoma patients Transperitoneal laparoscopic adrenalectomy: the patient was placed in the lateral decubitus position, affected side up, on a vacuum bean bag. Then, the operative bed was flexed at the waist to 80°. The operative procedures were performed as described in the literature, with the exception that 3 ports were used on the left side and four on the right |
|
| Outcomes |
Composite outcome measures reported: no Primary endpoint: operative time Secondary endpoints: blood loss, intraoperative haemodynamic stability, postoperative pain, recovery of bowel movement, complication rates |
|
| Study details |
Run‐in period: none Trial terminated early: no Trials register identifier:NCT01676025 |
|
| Publication details |
Language of publication: English Commercial funding, non‐commercial funding, other funding: not reported Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "... compare the surgical outcomes of LTPA and LRPA in patients with adrenal gland adenomas" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Web‐based randomization"; "... patients were randomized to either the LTPA group or the LRPA group in a 1:1 ratio by randomized block design, with block sizes of 4 and 6. The type of intervention was determined on preoperative day 1" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) all‐cause mortality | Low risk |
Quote from publication: "... the participants in this study were not blinded as to which procedure was performed" Comment: outcome measure not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) morbidity | High risk |
Quote from publication: "... the participants in this study were not blinded as to which procedure was performed" Comment: outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) operative parameters | Low risk |
Quote from publication: "... the participants in this study were not blinded as to which procedure was performed" Comment: outcome measure not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) postoperative parameters | Unclear risk |
Quote from publication: "... the participants in this study were not blinded as to which procedure was performed" Comment: outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Unclear risk |
Quote from publication: "... the participants in this study were not blinded as to which procedure was performed" Comment: outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality | Low risk | Comment: open label trial, outcome not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) morbidity | High risk | Comment: open label trial, outcome potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) operative parameters | Low risk | Comment: open label trial, outcome not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) postoperative parameters | Unclear risk | Comment: open label trial, outcome potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Socioeconomic effects | Unclear risk | Comment: open label trial, outcome potentially influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause mortality | Low risk | Comment: only 1 participant refused follow‐up |
| Incomplete outcome data (attrition bias) morbidity | Low risk | Comment: only 1 participant refused follow‐up |
| Incomplete outcome data (attrition bias) operative parameters | Low risk | Comment: only 1 participant refused follow‐up |
| Incomplete outcome data (attrition bias) postoperative parameters | Low risk | Comment: only 1 participant refused follow‐up |
| Incomplete outcome data (attrition bias) Socioeconomic effects | Low risk | Comment: only 1 participant refused follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: no substantial differences between information in ClinicalTrials.gov and the publication (see Appendix 7) |
| Other bias | Low risk | Comment: none detected |
Fernández‐Cruz 1996.
| Methods | Design: parallel randomised controlled clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: Cushing's syndrome, Cushing disease, Cushing adenoma, age > 16 yrs, informed consent Exclusion criteria: none declared Diagnostic criteria: elevated plasma cortisol levels with loss of diurnal rhythm, low‐dose dexamethasone suppression test positive, urinary excretion of 17‐hydroxycorticosteroids and 17‐ketosteroid, plasma adrenocorticotropic hormone measured by radioimmunoassay; in some individuals, petrosal venous sinus catheterization was performed; computed tomography scan was used to localise the adrenal tumour in individuals with suspected Cushing's adenoma |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: none declared Flank approach in lateral decubitus position was used in all the participants Retroperitoneal laparoscopic adrenalectomy: 1.5 cm incision midaxillary line below the 12th rib, Muscular layer was split to allow the passage of the finger, and create a retroperitoneal space, insertion of a trocar with an inflated balloon tip. The balloon‐tipped trocar was withdrawn and a 10 mm Hasson cannula was inserted, insufflation of the space with C02 gas at 12 mmHg. 30° optics insertion and three retroperitoneal 10 mm trocars placement respectively below the costal margin, above the iliac crest, and dorsally under direct vision. For right adrenalectomy, the dissection along the vena cava between the renal vein and the liver identified for transparency through the peritoneal wall, which was retracted medially; meticulous dissection separated the adrenal gland from the attachments to the vena cava, and all vascular elements were divided between two clips. For left adrenalectomy, adrenal gland visualisation, dissection of the infero‐medial border of the gland, localisation of the renal vein. At this point, the adrenal veins come to view, where they are transected with two clips. The superior adrenal vessels are left in the final dissection for ligature with clips. Transperitoneal laparoscopic adrenalectomy: CO2 insufflation in the subcostal area with a Veress needle at up to 12 mmHg. Placement of one trocar (10 mm) in the right or left subcostal area at the level of the anterior axillary line and three more (10 mm) on each side were inserted under direct vision in the flank, under the 12th rib, and dorsally. 30° optics inserted. Right adrenal gland was first exposed with sectioning of the right triangular ligament of the liver and retraction of the right lobe of the liver medially. The vena cava was exposed, and the renal vein was identified as a landmark; the dissection then proceeded in a cephalad direction, visualising the adrenal veins that issue directly from the vena cava in a parallel manner. All vessels were sealed with two clips. Operative exposure of the left adrenal gland started by reflection of the splenic flexure of the colon downward and division of the avascular splenocolic ligament. The spleen was mobilised by means of a lateral peritoneal incision up to the superior border, allowing mobilisation of the distal pancreas for good exposure of the Gerota's fascia. Exposition for clipping and division of the left adrenal vein entering the superior part of the renal vein were performed. The superior pole of the gland was dissected, and the left adrenal arteries were secured with clips and transected. After bilateral adrenalectomy or right or left adrenalectomy for Cushing's adenoma, a sterile plastic bag was used for adrenal or tumour removal through the anterior trocar site |
|
| Outcomes |
Composite outcome measures reported: none No primary or secondary outcome stated The following parameters were reported: operative time, estimated blood loss, length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity |
|
| Study details |
Run‐in period: none Trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial funding, non‐commercial funding, other funding: not declared Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The aim of this study is to evaluate, in a prospective, randomised study, the feasibility, safety, and outcome of the retroperitoneoscopic approach compared with the laparoscopic transperitoneal approach in patients with Cushing's syndrome" | |
| Notes | Possible imprecision of the results due to unclear determination of sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "patients were randomised to have laparoscopic surgery performed with the transperitoneal technique or the retroperitoneal approach" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "patients were randomised to have laparoscopic surgery performed with the transperitoneal technique or the retroperitoneal approach" Comment: insufficient information about the allocation concealment |
| Blinding of participants and personnel (performance bias) morbidity | High risk | Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) operative parameters | Low risk |
Quote from publication: "The following parameters were reported: operative time, estimated blood loss, [...]". Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) postoperative parameters | Unclear risk |
Quote from publication: "The following parameters were reported: [...] length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity". Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Unclear risk | Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) morbidity | High risk | Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) operative parameters | Low risk |
Quote from publication: "The following parameters were reported: operative time, estimated blood loss, [...]". Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) postoperative parameters | Unclear risk |
Quote from publication: "The following parameters were reported: [...] length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity". Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Socioeconomic effects | Unclear risk | Comment: no information, outcome measure potentially influenced by lack of blinding |
| Incomplete outcome data (attrition bias) morbidity | Low risk | Comment: no missing data |
| Incomplete outcome data (attrition bias) operative parameters | Low risk |
Quote from publication: "The following parameters were reported: operative time, estimated blood loss, [...]". Comment: no missing data |
| Incomplete outcome data (attrition bias) postoperative parameters | Low risk |
Quote from publication: "The following parameters were reported: [...] length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity". Comment: no missing data |
| Incomplete outcome data (attrition bias) Socioeconomic effects | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: all parameters stated in the methods section were reported in the results section (operative time, estimated blood loss, length of hospital stay, postoperative analgesic requirements, and the time needed to achieve normal activity) |
| Other bias | Low risk | Comment: none detected |
Mohammadi‐Fallah 2013.
| Methods | Design: parallel randomised controlled clinical trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: surgical adrenal disease Exclusion criteria: morbid obesity (BMI > 40), previous major abdominal surgery, clinical suspicion of malignancy, tumour size > 6 cm, bilateral adrenalectomy Diagnostic criteria: complete endocrinological work‐up before surgery (individuals referred by an endocrinologist) |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: none declared Retroperitoneal laparoscopic adrenalectomy: 15 mm incision under the tip of the 12th rib; underlying muscle and fasciae division with cautery; surgeon's index finger inserted through the incision to create a plane between posterior Gerota's fascia and Psoas fascia; trocar placement; blunt dissection under direct vision using the laparoscope; insufflation of the retroperitoneum; 5 mm trocar placement at the angle of paraspinal muscle at the origin of the 12th rib; 10 mm trocar placement about two finger widths above the iliac crest near the anterior superior iliac spine; dissection, clipping and division of adrenals artery and vein; blunt division of the remaining attachments to the kidney; endoscopic bag placement and specimen extraction; operative field examination for bleeding with a insufflation pressure down to 5 mmHg Transperitoneal laparoscopic adrenalectomy: insufflation of the abdomen; four trocar placement along the costal margin; line of Toldt incision; medial mobilisation of the left or right colon; (Kocher manoeuvre on the right side to dissect away the duodenum; dissection, clipping and division of adrenal artery and vein; blunt division of the remaining attachments to the kidney; endoscopic bag placement and specimen extraction; operative field examination for bleeding with a insufflation pressure down to 5 mmHg) |
|
| Outcomes |
Composite outcome measures reported: none Primary outcome: convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs Other outcomes: blood loss, operative time, open conversion, analgesic (tramadol) requirement dose, oral intake, ambulation, hospital stay, postoperative pain |
|
| Study details |
Run‐in period: none Trial terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding: Urmia University of Medical Sciences Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "We report here our prospective, randomised comparison of TLA versus RLA in 24 patients with medium‐term follow‐up" | |
| Notes | Possible imprecision of the results due to unclear determination of sample size. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were prospectively randomised by a computer‐generated program" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) all‐cause mortality | Low risk | Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) morbidity | High risk | Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) operative parameters | Low risk |
Quote from publication: "The primary surgeon was informed about the preselected laparoscopic approach for each individual patient in the operating room, just before the surgery. Intraoperative data were documented by the surgeon in the operating room immediately at the end of the procedure, using a previously designed data sheet. Information that was analysed included [...] intraoperative outcomes." Comment: outcome measure unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) postoperative parameters | Unclear risk |
Quote from publication: "The primary surgeon was informed about the preselected laparoscopic approach for each individual patient in the operating room, just before the surgery. Intraoperative data were documented by the surgeon in the operating room immediately at the end of the procedure, using a previously designed data sheet. Information that was analysed included [...] postoperative outcomes, and pathological adrenal features." Comment: outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Unclear risk |
Quote from publication: "The primary outcome was the convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs." Comment: outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality | Low risk |
Quote from publication: "Intraoperative data were documented by the surgeon in the operating room, immediately at the end of the procedure, using a previously designed data sheet" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) morbidity | High risk |
Quote from publication: "Intraoperative data were documented by the surgeon in the operating room immediately at the end of the procedure using a previously designed data sheet" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) operative parameters | Low risk |
Quote from publication: "Intraoperative data were documented by the surgeon in the operating room, immediately at the end of the procedure, using a previously designed data sheet" Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) postoperative parameters | Unclear risk |
Quote from publication: "Intraoperative data were documented by the surgeon in the operating room, immediately at the end of the procedure, using a previously designed data sheet" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Socioeconomic effects | Unclear risk |
Quote from publication: "The primary outcome was the convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs." Comment: no information, outcome measure potentially influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause mortality | Low risk | Comment: outcome evaluated in each individual |
| Incomplete outcome data (attrition bias) morbidity | Low risk | Comment: outcome evaluated in each individual |
| Incomplete outcome data (attrition bias) operative parameters | Low risk | Comment: outcome evaluated in each individual |
| Incomplete outcome data (attrition bias) postoperative parameters | Low risk | Comment: outcome evaluated in each individual |
| Incomplete outcome data (attrition bias) Socioeconomic effects | Low risk |
Quote from publication: "The primary outcome was the convalescence period, defined as the period needed for complete recovery from the physical aftereffects of surgery and return to normal personal jobs." Comment: outcome evaluated in each individual |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol was not available; all parameters stated in the methods section were reported in the result section |
| Other bias | Low risk | Comment: none detected |
Rubinstein 2005.
| Methods | Design: parallel randomised controlled clinical trial, randomisation ratio: 1:1 | |
| Participants |
Inclusion criteria: surgical adrenal disease Exclusion criteria: age > 80 yrs, BMI > 40, bilateral adrenalectomy, significant prior abdominal surgery in the quadrant of interest Diagnostic criteria: not declared |
|
| Interventions |
Number of trial centres:1 Treatment before trial: not declared Retroperitoneal laparoscopic adrenalectomy: individual positioned in lateral decubitus (full flank), the operating table was then flexed; horizontal 1.5 cm to 2 cm transverse skin incision just below the tip of the last (12th) rib; dissection with the index fingertip to create a space for balloon dilator; balloon dilatation to allow direct access to the adrenal gland; a 10 mm blunt‐tip Hasson's trocar was placed as the primary port; the CO2 pneumoretroperitoneum was established (15 mmHg), and a 30° 10 mm laparoscope was inserted; usually two, and rarely three, secondary ports were placed (the anterior port was inserted near the anterior axillary line at least 3 cm cephalad to the iliac crest; a posterior port was inserted at the junction of the lateral border of the erector spinae muscle with the undersurface of the 12th rib; rarely, a fourth port (2 mm) may be required at the level of the primary port in the anterior axillary line for retraction of the adrenal gland and kidney in an anterior direction). Transperitoneal laparoscopic adrenalectomy: individual positioning in a semilateral position with extension of the lateral abdominal wall of the affected side; four 10 mm trocars were placed for a left adrenalectomy; on the right side, a 5 mm trocar was added to lift up the liver near the xiphoid process; the first trocar was inserted lateral to the umbilicus by the open laparoscopic procedure under direct vision to prevent accidental injury to the bowels or large vessels; pneumoperitoneum creation with carbon dioxide gas at 14 mmHg to 15 mmHg for insertion of the other trocars; the pressure was reduced to 8 mmHg to 10 mmHg at the beginning of the intraperitoneal procedure after the insertions were complete; on the left side, the peritoneum was incised on the white line of Toldt lateral to the descending colon, which was then retracted medially above the anterior leaf of Gerota's fascia; Gerota's fascia was opened just above the left renal vein to expose the inferior adrenal vein and adrenal gland; the inferior adrenal vein was divided between clips, and the fatty tissue and remaining vessels around the adrenal gland were then divided between clips by electro cauterization or scissors; division of a few superior adrenal veins to free the adrenal gland; on the right side, the adrenal gland usually appeared through the peritoneum when the liver was lifted up; the hepatocolic ligament and peritoneum were incised to expose the anterior surface of the gland, which then was dissected inferiorly, laterally posteriorly, and finally medially, with division of the inferior adrenal vessels; the middle adrenal vein was excised next from the inferior vena cava; then the narrow superior adrenal vessels were excised; the adrenal gland was trapped in an Endopouch® (Ethicon, Cincinnati, OH) and removed from the abdominal cavity through the largest port; the first trocar port made in the open laparoscopic procedure (port A) was closed in two layers: the anterior rectal sheath and the skin; at the other port sites, the skin and subcutaneous layer were closed in a single layer. |
|
| Outcomes |
Composite outcome measures reported: no Outcome reported: median operative time, median estimated blood loss, median specimen weight, number of open conversion; median analgesic requirement, median days to oral intake, median days to ambulation, median days hospital stay; complications, median convalescence, number of deaths |
|
| Study details |
Run‐in period: none Trial terminated early: none |
|
| Publication details |
Language of publication: English Commercial funding, non‐commercial funding, other funding: not declared Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "Report our prospective, randomised, single institution comparison of transperitoneal vs retroperitoneal laparoscopic adrenalectomy in 57 consecutive patients with intermediate‐term follow‐up" | |
| Notes | Possible imprecision of the results due to unclear determination of sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were prospectively randomised by a computer‐generated program" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) all‐cause mortality | Low risk |
Quote from publication: "The primary surgeon was informed about the preselected laparoscopic approach for each individual patient in the operating suite, immediately prior to positioning the patient for surgery" Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) morbidity | High risk |
Quote from publication: "The primary surgeon was informed about the preselected laparoscopic approach for each individual patient in the operating suite, immediately prior to positioning the patient for surgery" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) operative parameters | Low risk |
Quote from publication: "The primary surgeon was informed about the preselected laparoscopic approach for each individual patient in the operating suite, immediately prior to positioning the patient for surgery ... Intraoperative data were documented by the primary surgeon in the operating room,, immediately at the end of the procedure, using a statistically validated data sheet. All data were prospectively maintained in a computerised database with Institutional Review Board approval ... Information analysed included [...] intraoperative and postoperative outcomes." Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) postoperative parameters | Unclear risk |
Quote from publication: "The primary surgeon was informed about the preselected laparoscopic approach for each individual patient in the operating suite, immediately prior to positioning the patient for surgery ... Intraoperative data were documented by the primary surgeon in the operating room, immediately at the end of the procedure, using a statistically validated data sheet. All data were prospectively maintained in a computerised database with Institutional Review Board approval ... Information analysed included [...] intraoperative and postoperative outcomes." Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) socioeconomic effects | Unclear risk | Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) all‐cause mortality | Low risk |
Quote from publication: "Intraoperative data were documented by the primary surgeon in the operating room, immediately at the end of the procedure, using a validated data sheet" Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) morbidity | High risk |
Quote from publication: "Intraoperative data were documented by the primary surgeon in the operating room, immediately at the end of the procedure, using a validated data sheet" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) operative parameters | Low risk |
Quote from publication: "Intraoperative data were documented by the primary surgeon in the operating room, immediately at the end of the procedure, using a validated data sheet" Comment: no information, outcome measure unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) postoperative parameters | Unclear risk |
Quote from publication: "Intraoperative data were documented by the primary surgeon in the operating room, immediately at the end of the procedure, using a validated data sheet" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Socioeconomic effects | Unclear risk |
Quote from publication: "Intraoperative data were documented by the primary surgeon in the operating room, immediately at the end of the procedure, using a validated data sheet" Comment: no information, outcome measure potentially influenced by lack of blinding |
| Incomplete outcome data (attrition bias) all‐cause mortality | Low risk | Comment: during the 6‐year follow‐up, 5 participants died (1 in the transperitoneal, and 4 in the retroperitoneal group) |
| Incomplete outcome data (attrition bias) morbidity | Low risk | Comment. during the 6‐year follow‐up, 5 participants died (1 in the transperitoneal, and 4 in the retroperitoneal group) |
| Incomplete outcome data (attrition bias) operative parameters | Low risk | Comment. during the 6‐year follow‐up, 5 participants died (1 in the transperitoneal, and 4 in the retroperitoneal group) |
| Incomplete outcome data (attrition bias) postoperative parameters | Low risk | Comment: during the 6‐year follow‐up, 5 participants died (1 in the transperitoneal, and 4 in the retroperitoneal group) |
| Incomplete outcome data (attrition bias) Socioeconomic effects | Low risk | Comment: during the 6‐year follow‐up, 5 participants died (1 in the transperitoneal, and 4 in the retroperitoneal group) |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol was not available. All parameters stated in the methods section were reported in the results section |
| Other bias | Low risk | Comment: none reported |
Note: where the judgement is 'Unclear' and the description is blank, the trial did not report that particular outcome.
ACTH: adrenocorticotropic hormone AST: aspartate aminotransferase ALT: alanine transaminase BMI: body mass index CT: computed tomography DHEAS: dehydroepiandrosterone sulfate IVC: inferior vena cava LT(L)A: lateral transperitoneal (laparoscopic) adrenalectomy PRA: posterior retroperitoneoscopic adrenalectomy RLA: retroperitoneal laparoscopic adrenalectomy TLA: transperitoneal laparoscopic adrenalectomy VMA: vanillylmandelic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barbaros 2014 | A retrospective cohort study on participants who underwent single incision laparoscopic surgery |
| Bonjer 1997 | A case‐control study comparing open adrenalectomy with laparoscopic retroperitoneal adrenalectomy and laparoscopic transperitoneal adrenalectomy |
| Bradley 2013 | Systematic review of case‐series studies including participants affected by adrenal metastases and treated by surgery with different techniques |
| Desai 2005 | A randomised controlled trial on participants who underwent transperitoneal or retroperitoneal laparoscopic radical nephrectomy for renal tumour |
| Fu 2011 | A randomised multicentric trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma |
| Gockel 2005 | A case‐control study comparing laparoscopic retroperitoneal adrenalectomy with laparoscopic transperitoneal adrenalectomy |
| Hallfeldt 2003 | A case‐control study comparing laparoscopic retroperitoneal adrenalectomy with laparoscopic transperitoneal adrenalectomy |
| Henry 2002 | A retrospective cohort study on participants who underwent laparoscopic transperitoneal adrenalectomy |
| Hsu 2002 | A case‐control study comparing participants undergoing bilateral adrenalectomy with either laparoscopic retroperitoneal or laparoscopic transperitoneal technique |
| Khaira 2004 | A randomised, double‐blind, placebo controlled trial in which the port sites and hand assist incision were infiltrated with bupivacaine or placebo prior to surgery ‐‐ consisting of transperitoneal laparoscopic renal and adrenal surgery |
| Lee 2012 | A case‐control study comparing participants undergoing open, robotic, and laparoscopic adrenalectomy with both transperitoneal and retroperitoneal techniques |
| Lezoche 2002 | A case‐control study comparing participants undergoing lateral laparoscopic, endoscopic retroperitoneal, or anterior laparoscopic adrenalectomy at three different institutions |
| Lezoche 2008 | A randomised trial comparing participants undergoing the lateral approach and the anterior submesocolic access carrying out left laparoscopic adrenalectomy |
| Li 2003 | A case‐control study comparing participants undergoing open or laparoscopic adrenalectomy with either transperitoneal or retroperitoneal technique for the treatment of adrenal tumours |
| Liao 2001 | A case‐control study comparing participants undergoing open or laparoscopic adrenalectomy with either transperitoneal or retroperitoneal technique, for the treatment of adrenal tumours |
| Morino 2004 | A randomised trial comparing participants undergoing laparoscopic adrenalectomy and robot‐assisted adrenalectomy |
| Naya 2002 | A case‐control study comparing participants undergoing laparoscopic adrenalectomy with either transperitoneal or retroperitoneal technique, for the treatment of adrenal tumours |
| Ramacciato 2008 | A case‐control study comparing participants undergoing laparoscopic adrenalectomy with either transperitoneal or retroperitoneal technique, for the treatment of adrenal tumours |
| Ramacciato 2011 | A case‐control study comparing participants undergoing laparoscopic adrenalectomy with either transperitoneal or retroperitoneal technique, for the treatment of adrenal tumours |
| Sood 2006 | A randomised trial comparing participants undergoing laparoscopic transperitoneal adrenalectomy with either an intraabdominal pressure of 15 mmHg or 8 mmHg to10 mmHg |
| Suzuki 2001 | A case‐control study comparing participants undergoing anterior, lateral, and retroperitoneal laparoscopic adrenalectomy |
| Tai 2006 | A case‐control study comparing participants undergoing transperitoneal and retroperitoneal laparoscopic adrenalectomy |
| Takeda 2000 | A case‐control study comparing participants undergoing transperitoneal and retroperitoneal laparoscopic adrenalectomy |
| Tiberio 2008 | A randomised trial evaluating cardiovascular instability during open or laparoscopic adrenalectomy for pheochromocytoma |
| Todorov 2007 | A case‐control study comparing participants undergoing transperitoneal and retroperitoneal laparoscopic adrenalectomy |
| Utsumi 2014 | A case‐series of participants with primary aldosteronism who were treated by unilateral laparoscopic adrenalectomy |
| Wang 2012 | A randomised trial comparing cost‐effectiveness of three strategies (adrenalectomy, surveillance, or no follow‐up) in participants with a nonfunctional, 4‐cm adrenal incidentaloma with no radiographic suspicion of adrenocortical carcinoma |
| Wu 2013 | A randomised trial comparing catecholamines levels in participants undergoing transperitoneal or retroperitoneal for pheochromocytoma, randomly assigned to dissection after ligation or dissection before ligation of the adrenal vein |
| Yang 2013 | A case‐series assessing safety and feasibility of a staged laparoscopic training program for beginners with no experience of open technique, to perform laparoscopic adrenalectomy |
| Zacharias 2006 | Review on different techniques of adrenalectomy and case‐series presentation on participants undergoing transperitoneal laparoscopic adrenalectomy |
| Zhang 2009 | A case‐series assessing safety and feasibility of a staged laparoscopic training program for beginners with no experience of open technique, to perform laparoscopic adrenalectomy |
| Zografos 2009 | Review on laparoscopic surgery techniques for malignant adrenal tumours |
Characteristics of studies awaiting assessment [ordered by study ID]
Abou 2016.
| Methods | Randomised controlled trial |
| Participants | Adults with any benign adrenal masses without local or distant invasion, and children with neuroblastoma; posterior retroperitoneoscopic adrenalectomy 7 participants, anterior transperitoneal adrenalectomy 6 participants |
| Interventions | Posterior retroperitoneoscopic adrenalectomy compared with anterior transperitoneal adrenalectomy |
| Outcomes | Operative time, estimated blood loss, postoperative hospital stay, rate of complications |
| Study details | Trial was performed in the Alexandria University, Egypt |
| Publication details | Conference abstract |
| Stated aim of study | Comparison of the perioperative short‐term outcomes of posterior retroperitoneoscopic adrenalectomy versus anterior transperitoneal adrenalectomy |
| Notes | Publication not yet available |
Grubnik 2016.
| Methods | Randomised controlled trial |
| Participants | 68 participants with tumours of the left adrenal gland; laparoscopic adrenalectomy 35 participants, retroperitoneoscopic adrenalectomy 33 participants |
| Interventions | Laparoscopic adrenalectomy versus retroperitoneoscopic adrenalectomy for left adrenalectomies |
| Outcomes | Conversion rate, operative time, time to first oral intake, analgesic requirements, length of hospital stay, postoperative complications |
| Study details | Trial was performed in the Odessa National Medical University, Ukraine; trial period 2010 to 2015 |
| Publication details | Conference abstract |
| Stated aim of study | Comparison of the results of laparoscopic adrenalectomy versus retroperitoneoscopic adrenalectomy for left adrenalectomies |
| Notes | Publication not yet available |
NCT02618694.
| Methods | Parallel randomised controlled trial, double‐blind (participant, investigator) |
| Participants | Participants with functioning or nonfunctioning adrenal adenoma, secondary metastatic adrenal mass, adrenal hyperplasia. Exclusion criteria: cardiovascular disease, pregnancy, locally advanced malignant disease, regional lymph node involvement, vascular malignant invasion, malignant uncontrolled hypertension with phaeochromocytoma, need for other simultaneous surgical intervention at the same session |
| Interventions | Transperitoneal laparoscopic adrenalectomy versus posterior retroperitoneoscopic adrenalectomy |
| Outcomes | Primary: mean operative time (1 year), mean amount of intraoperative blood loss (1 year), mean days of postoperative hospital stay (1.5 years), rate of complications (1.5 years) Secondary: mean of postoperative pain score (1 year), mean of scar cosmetic assessment score (1 year) |
| Study details | Trial started in April 2016 and was completed in December 2016; performed in the Alexandra Main University Hospital, Egypt (collaborators: Suez Canal University, Ismailia) |
| Publication details | Registered trial in ClincalTrials.gov |
| Stated aim of study | To assess the safety and efficacy of the posterior retroperitoneoscopic adrenalectomy in comparison to the anterior transperitoneal laparoscopic approach |
| Notes | Publication not yet available; ClinicalTrial.gov‐ID: NCT02618694 (responsible party: Ahmed Mohamed Bakr Arabi, Suez Canal University) |
Differences between protocol and review
All‐cause morbidity was not reported in the included trials. Therefore, we changed this outcome to early and late morbidity, and included specific adverse events (e.g. liver injury, splenic injury, vascular injury, pneumothorax or haemothorax, massive subcutaneous emphysema, surgical access site herniation) under these outcome measures, depending on when the outcome was measured (early morbidity 30 to 60 days post surgery, and late morbidity at latest available follow‐up). Consequently, we changed our primary outcomes from 'all‐cause mortality, all‐cause morbidity, adverse events' to 'all‐cause mortality, early morbidity, late morbidity'. We also adapted our 'Summary of finding' table from '1. All‐cause mortality, 2. All‐cause morbidity, 3. Adverse events, 4. Health‐related quality of life, 5. Socioeconomic effects' to '1. All‐cause mortality, 2. Early morbidity, 3. Late morbidity, 4. Health‐related quality of life, 5. Socioeconomic effects'.
We redefined operative blood loss from the maximum decrease in haemoglobin, to quantities of blood loss in millilitres (mL) measured at the end of surgery, as the decrease in haemoglobin was not reported.
Contributions of authors
All review authors read and approved the final review draft.
Alberto Arezzo (AA): protocol drafting, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Alberto Bullano (AB): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Giovanni G Cochetti (GGC): protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Roberto Cirocchi (RC): protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Justus J Randolph (JR): protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Ettore E Mearini (EM): protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Andrea Evangelista (AE): data analysis, data interpretation, review of drafts and future review updates.
Giovannino Ciccone (GC): data analysis, data interpretation, review of drafts and future review updates.
Hendrick Jaap Bonjer (HJB): data extraction, data analysis, data interpretation, review of drafts and future review updates.
Mario Morino (MM): data extraction, data analysis, data interpretation, review of drafts and future review updates.
Sources of support
Internal sources
none, Other.
External sources
No sources of support supplied
Declarations of interest
Alberto Arezzo: none known.
Alberto Bullano: none known.
Giovanni G Cochetti: none known.
Roberto Cirocchi: none known.
Justus J Randolph: none known.
Ettore E Mearini: none known.
Andrea Evangelista: none known.
Giovannino Ciccone: none known.
Hendrick Jaap Bonjer: none known.
Mario Morino: none known.
New
References
References to studies included in this review
Barczynski 2014 {published data only}
- Barczynski M, Konturek A, Nowak W. Randomized clinical trial of posterior retroperitoneoscopic adrenalectomy versus lateral transperitoneal laparoscopic adrenalectomy with a 5‐year follow‐up. Annals of Surgery 2014;260:740‐7. [PUBMED: 25243546] [DOI] [PubMed] [Google Scholar]
- NCT01959711. Randomized clinical trial of posterior retroperitoneoscopic adrenalectomy versus lateral laparoscopic adrenalectomy. clinicaltrials.gov/ct2/show/NCT01959711 (first posted 10 October 2013).
Chai 2017 {published data only}
- Chai YJ, Yu HW, Song RY, Kim SJ, Choi JY, Lee KE. Lateral transperitoneal adrenalectomy versus posterior retroperitoneoscopic adrenalectomy for benign adrenal gland disease: randomized controlled trial at a single tertiary medical center. Annals of Surgery 2017;20:20. [DOI] [PubMed] [Google Scholar]
- NCT01676025. Comparison between posterior retroperitoneoscopic adrenalectomy and laparoscopic adrenalectomy. clinicaltrials.gov/ct2/show/NCT01676025 (first posted 30 August 2012).
Fernández‐Cruz 1996 {published data only}
- Fernández‐Cruz L, Saenz A, Benarroch G, Astudillo E, Taura P, Sabater L. Laparoscopic unilateral and bilateral adrenalectomy for Cushing's syndrome. Transperitoneal and retroperitoneal approaches. Annals of Surgery 1996;224:727‐34. [PUBMED: 8968227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi‐Fallah 2013 {published data only}
- Mohammadi‐Fallah MR, Mehdizadeh A, Badalzadeh A, Izadseresht B, Dadkhah N, Barbod A. Comparison of transperitoneal versus retroperitoneal laparoscopic adrenalectomy in a prospective randomized study. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2013;23:342‐6. [PUBMED: 23573882] [DOI] [PubMed] [Google Scholar]
Rubinstein 2005 {published data only}
- Rubinstein M, Gill IS, Aron M, Kilciler M, Meraney AM, Finelli A, et al. Prospective, randomized comparison of transperitoneal versus retroperitoneal laparoscopic adrenalectomy. Journal of Urology 2005;174:442‐5. [PUBMED: 16006861] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barbaros 2014 {published data only}
- Barbaros U, Aksakal N, Ozkurt E, Tukenmez M, Agcaoglu O, Kilic B. Evaluation of clinical results of single‐incision laparoscopic surgeries in general surgery. Surgical Endoscopy and Other Interventional Techniques 2014;28:S80. [Google Scholar]
Bonjer 1997 {published data only}
- Bonjer HJ, Lange JF, Kazemier G, Herder WW, Steyerberg EW, Bruining HA. Comparison of three techniques for adrenalectomy. The British Journal of Surgery 1997;84(5):679‐82. [PUBMED: 9171764] [PubMed] [Google Scholar]
Bradley 2013 {published data only}
- Bradley JF, Williams KB, Walters AL, Wormer BA, Dacey KT, Sing RF. Is there a presence of a: 'July effect' for minimally invasive surgery fellows for solid organ surgery?. Surgical Endoscopy and Other Interventional Techniques 2013;27:S480. [Google Scholar]
Desai 2005 {published data only}
- Desai MM, Strzempkowski B, Matin SF, Steinberg AP, Ng C, Meraney AM, et al. Prospective randomized comparison of transperitoneal versus retroperitoneal laparoscopic radical nephrectomy. The Journal of Urology 2005;173(1):38‐41. [PUBMED: 15592021] [DOI] [PubMed] [Google Scholar]
Fu 2011 {published data only}
- Fu B, Zhang X, Wang GX, Lang B, Ma X, Li HZ. Long‐term results of a prospective, randomized trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma. Journal of Urology 2011;185:1578‐82. [DOI] [PubMed] [Google Scholar]
Gockel 2005 {published data only}
- Gockel I, Vetter G, Heintz A, Junginger T. Endoscopic adrenalectomy for pheochromocytoma: difference between the transperitoneal and retroperitoneal approaches in terms of the operative course. Surgical Endoscopy 2005;19(8):1086‐92. [PUBMED: 16021380] [DOI] [PubMed] [Google Scholar]
Hallfeldt 2003 {published data only}
- Hallfeldt KK, Mussack T, Trupka A, Hohenbleicher F, Schmidbauer S. Laparoscopic lateral adrenalectomy versus open posterior adrenalectomy for the treatment of benign adrenal tumors. Surgical Endoscopy 2003;17(2):264‐7. [PUBMED: 12399875] [DOI] [PubMed] [Google Scholar]
Henry 2002 {published data only}
- Henry JF, Sebag F, Iacobone M, Mirallie E. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World Journal of Surgery 2002;26(8):1043‐7. [PUBMED: 12045859] [DOI] [PubMed] [Google Scholar]
Hsu 2002 {published data only}
- Hsu TH, Gill IS. Bilateral laparoscopic adrenalectomy: retroperitoneal and transperitoneal approaches. Urology 2002;59(2):184‐9. [PUBMED: 11834382] [DOI] [PubMed] [Google Scholar]
Khaira 2004 {published data only}
- Khaira HS, Wolf JS Jr. Intraoperative local anesthesia decreases postoperative parenteral opioid requirements for transperitoneal laparoscopic renal and adrenal surgery: a randomized, double‐blind, placebo controlled investigation. Journal of Urology 2004;172:1422‐6. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee CR, Walz MK, Park S, Park JH, Jeong JS, Lee SH, et al. A comparative study of the transperitoneal and posterior retroperitoneal approaches for laparoscopic adrenalectomy for adrenal tumors. Annals of Surgical Oncology 2012;19(8):2629‐34. [PUBMED: 22526902] [DOI] [PubMed] [Google Scholar]
Lezoche 2002 {published data only}
- Lezoche E, Guerrieri M, Feliciotti F, Paganini AM, Perretta S, Baldarelli M, et al. Anterior, lateral, and posterior retroperitoneal approaches in endoscopic adrenalectomy. Surgical Endoscopy 2002;16(1):96‐9. [PUBMED: 11961614] [DOI] [PubMed] [Google Scholar]
Lezoche 2008 {published data only}
- Lezoche E, Guerrieri M, Crosta F, Lezoche G, Baldarelli M, Campagnacci R. Flank approach versus anterior sub‐mesocolic access in left laparoscopic adrenalectomy: a prospective randomized study. Surgical Endoscopy 2008;22:2373‐8. [DOI] [PubMed] [Google Scholar]
Li 2003 {published data only}
- Li ZY, Long HM, Gu MX, Yang XQ, Yin M, Yang YQ, et al. Comparative study on transperitoneal retroperitoneal laparoscopic adrenalectomy and open adrenalectomy for adrenal tumours. Zhonghua Wai Ke Za Zhi (Chinese Journal of Surgery) 2003;41(8):617‐9. [PUBMED: 14505540] [PubMed] [Google Scholar]
Liao 2001 {published data only}
- Liao CH, Chen J, Chueh SC, Tu YP, Chen SC, Yuan RH. Effectiveness of transperitoneal and trans‐retroperitoneal laparoscopic adrenalectomy versus open adrenalectomy. Journal of the Formosan Medical Association 2001;100(3):186‐91. [PUBMED: 11393114] [PubMed] [Google Scholar]
Morino 2004 {published data only}
- Morino M, Benincà G, Giraudo G, Genio GM, Rebecchi F, Garrone C. Robot‐assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surgical Endoscopy 2004;18:1742‐6. [DOI] [PubMed] [Google Scholar]
Naya 2002 {published data only}
- Naya Y, Nagata M, Ichikawa T, Amakasu M, Omura M, Nishikawa T, et al. Laparoscopic adrenalectomy: comparison of transperitoneal and retroperitoneal approaches. British Journal of Urology International 2002;90(3):199‐204. [PUBMED: 12133053] [DOI] [PubMed] [Google Scholar]
Ramacciato 2008 {published data only}
- Ramacciato G, Nigri G, Santo V, Piccoli M, Pansadoro V, Buniva P, et al. Minimally invasive adrenalectomy: transperitoneal vs. retroperitoneal approach [Surrenalectomia mininvasiva: confonto tra approccio transperitoneale e retroperitoneale]. Chirurgia Italiana 2008;60(1):15‐22. [PUBMED: 18389743] [PubMed] [Google Scholar]
Ramacciato 2011 {published data only}
- Ramacciato G, Nigri GR, Petrucciani N, Santo V, Piccoli M, Buniva P, et al. Minimally invasive adrenalectomy: a multicenter comparison of transperitoneal and retroperitoneal approaches. The American Surgeon 2011;77(4):409‐16. [PUBMED: 21679547] [PubMed] [Google Scholar]
Sood 2006 {published data only}
- Sood J, Jayaraman L, Kumra VP, Chowbey PK. Laparoscopic approach to pheochromocytoma: is a lower intra‐abdominal pressure helpful?. Anesthesia and Analgesia 2006;102:637‐41. [DOI] [PubMed] [Google Scholar]
Suzuki 2001 {published data only}
- Suzuki K, Kageyama S, Hirano Y, Ushiyama T, Rajamahanty S, Fujita K. Comparison of 3 surgical approaches to laparoscopic adrenalectomy: a non‐randomized, background matched analysis. The Journal of Urology 2001;166(2):437‐43. [PUBMED: 11458043] [PubMed] [Google Scholar]
Tai 2006 {published data only}
- Tai CK, Li SK, Hou SM, Fan CW, Fung TC, Wah MK. Laparoscopic adrenalectomy: comparison of lateral transperitoneal and lateral retroperitoneal approaches. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2006;16(3):141‐5. [PUBMED: 16804455] [DOI] [PubMed] [Google Scholar]
Takeda 2000 {published data only}
- Takeda M. Laparoscopic adrenalectomy: transperitoneal vs retroperitoneal approaches. Biomedicine & Pharmacotherapy 2000;54 Suppl 1:207s‐10s. [PUBMED: 10915026] [DOI] [PubMed] [Google Scholar]
Tiberio 2008 {published data only}
- Tiberio GA, Baiocchi GL, Arru L, Agabiti Rosei C, Ponti S, Matheis A, et al. Prospective randomized comparison of laparoscopic versus open adrenalectomy for sporadic pheochromocytoma. Surgical Endoscopy 2008;22:1435‐9. [DOI] [PubMed] [Google Scholar]
Todorov 2007 {published data only}
- Todorov G, Lukanova T. Retroperitoneal endoscopic adrenalectomy vs. conventional adrenalectomy in treatment of benign adrenal lesions ‐‐ comparative analysis. Acta Chirurgica Iugoslavica 2007;54(2):45‐8. [PUBMED: 18044315] [DOI] [PubMed] [Google Scholar]
Utsumi 2014 {published data only}
- Utsumi T, Kamiya N, Endo T, Yano M, Kamijima S, Kawamura K. Development of a novel nomogram to predict hypertension cure after laparoscopic adrenalectomy in patients with primary aldosteronism. World Journal of Surgery 2014;38:2640‐4. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang TS, Cheung K, Roman SA, Sosa JA. A cost‐effectiveness analysis of adrenalectomy for nonfunctional adrenal incidentalomas: is there a size threshold for resection?. Surgery 2012;152:1125‐32. [DOI] [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu G, Zhang B, Yu C, Gao L, Gao Y, Huang Y, et al. Effect of early adrenal vein ligation on blood pressure and catecholeamine fluctuation during laparoscopic adrenalectomy for pheochromocytoma. Urology 2013;82:606‐11. [DOI] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang Q, Du J, Zhao ZH, Chen XS, Zhou L, Yao X. Is laboratory training essential for beginners in learning laparoscopic adrenalectomy?. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2013;23:184‐8. [DOI] [PubMed] [Google Scholar]
Zacharias 2006 {published data only}
- Zacharias M, Haese A, Jurczok A, Stolzenburg JU, Fornara P. Transperitoneal laparoscopic adrenalectomy: outline of the preoperative management, surgical approach, and outcome. European Urology 2006;49(3):448‐59. [PUBMED: 16481096] [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang X, Wang B, Ma X, Zhang G, Shi T, Ju Z, et al. Laparoscopic adrenalectomy for beginners without open counterpart experience: initial results under staged training. Urology 2009;73:1061‐5. [DOI] [PubMed] [Google Scholar]
Zografos 2009 {published data only}
- Zografos GN, Vasiliadis G, Farfaras AN, Aggeli C, Digalakis M. Laparoscopic surgery for malignant adrenal tumors. Journal of the Society of Laparoendoscopic Surgeons 2009;13:196‐202. [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abou 2016 {published data only}
- Abou Youssif T, Badawy H, Assem A, Bakr A, Shaaban S. Posterior retroperitoneoscopic approach versus transperitoneal laparoscopic approach in management of adrenal tumors: a randomized comparative study. Journal of Endourology 2016;30:A40. [Google Scholar]
Grubnik 2016 {published data only}
- Grubnik VV, Burlak OS, Ilyashenko VV, Grubni VV. Comparison of retroperitoneoscopic and laparoscopic left adrenalectomy: a randomized controlled trial. Surgical Endoscopy and Other Interventional Techniques 2016;30:S21. [Google Scholar]
NCT02618694 {published data only}
- NCT02618694. Efficacy and safety of posterior retroperitoneoscopic adrenalectomy: a comparative study. clinicaltrials.gov/ct2/show/NCT02618694 (last accessed 12 April 2018) (first posted 1 December 2015).
Additional references
Ades 2005
- Ades AE, Lu G, Higgins JP. The interpretation of random‐effects meta‐analysis in decision models. Medical Decision Making 2005;25(6):646‐54. [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Assalia 2004
- Assalia A, Gagner M. Laparoscopic adrenalectomy. British Journal of Surgery 2004;91:1259–74. [DOI] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. [DOI] [PubMed] [Google Scholar]
CENTRAL creation details
- CENTRAL creation details. www.cochranelibrary.com/central/central‐creation (accessed 21 August 2018).
Chen 2013
- Chen W, Li F, Chen D. Retroperitoneal versus transperitoneal laparoscopic adrenalectomy in adrenal tumour: a meta‐analysis. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2013;23:121‐7. [DOI] [PubMed] [Google Scholar]
Constantinides 2012
- Constantinides VA, Christakis I, Touska P, Palazzo FF. Systematic review and meta‐analysis of retroperitoneoscopic versus laparoscopic adrenalectomy. British Journal of Surgery 2012;99:1639–48. [DOI] [PubMed] [Google Scholar]
Conzo 2016
- Conzo G, Tartaglia E, Gambardella C, Esposito D, Sciascia V, Mauriello C, et al. Minimally invasive approach for adrenal lesions: systematic review of laparoscopic versus retroperitoneoscopic adrenalectomy and assessment of risk factors for complications. International Journal of Surgery 2016;Suppl 1:S118‐23. [DOI] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79‐85. [DOI] [PubMed] [Google Scholar]
Corcione 2001
- Corcione F, Esposito C, Cuccurullo D, Settembre A, Fusco F, Bianco A. Vena cava injury: a serious complication during right adrenalectomy. Surgical Endoscopy 2001;15:218. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Fleiss 1991
- Fleiss JL, Gross AJ. Meta‐analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. Journal Clinical Epidemiology 1991;44(2):127‐39. [DOI] [PubMed] [Google Scholar]
Gagner 1992
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. New England Journal of Medicine 1992;327(14):1033. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 25 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greco 2011
- Greco F, Hoda MR, Rassweiler J, Fahlenkamp D, Neisius DA, Kutta A. Laparoscopic adrenalectomy in urological centres ‐ the experience of the German Laparoscopic Working Group. British Journal of Urology International 2011;108:1646–51. [DOI] [PubMed] [Google Scholar]
Guazzoni 1995
- Guazzoni G, Montorsi F, Bocciardi A. Transperitoneal laparoscopic versus open adrenalectomy for benign hyperfunctioning adrenal tumours: a comparative study. Journal of Urology 1995;153:1597–600. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of The Royal Statistical Society. Series A 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and non‐blinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 1997
- Jacobs JK, Goldstein RE, Geer RJ. Laparoscopic adrenalectomy: a new standard of care. Annals of Surgery 1997;225:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. The Journal of the American Medical Association 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercan 1995
- Mercan S, Seven R, Ozarmagan S, Tezelman S. Endoscopic retroperitoneal adrenalectomy. Surgery 1995;118:1071–6. [DOI] [PubMed] [Google Scholar]
Murphy 2010
- Murphy MM, Witkowski ER, Ng SC. Trends in adrenalectomy: a recent national review. Surgical Endoscopy and Other Interventional Techniques 2010;24:2518–26. [DOI] [PubMed] [Google Scholar]
Naito 1995
- Naito S, Uozumi J, Shimura H, Ichimiya H, Tanaka M, Kumazawa J. Laparoscopic adrenalectomy: review of 14 cases and comparison with open adrenalectomy. Journal of Endourology 1995;9:491‐5. [DOI] [PubMed] [Google Scholar]
Nigri 2013
- Nigri G, Rosman AS, Petrucciani N. Meta‐analysis of trials comparing laparoscopic transperitoneal and retroperitoneal adrenalectomy. Surgery 2013;153:111–9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Schein 2010
- Schein M. Intra‐abdominal abscesses. In: Schein M, Rogers P, Assalia A editor(s). Schein's Common Sense Emergency Abdominal Surgery. Berlin: Springer, 2010. [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Shuster 2007
- Shuster JJ, Jones LS, Salmon DA. Fixed vs random effects meta‐analysis in rare event studies: the rosiglitazone link with myocardial infarction and cardiac death. Statistical Medicine 2007;26(24):4375‐85. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Terachi 2000
- Terachi T, Yoshida O, Matsuda T, Orikasa S, Chiba Y, Takahashi K. Complications of laparoscopic and retroperitoneoscopic adrenalectomies in 370 cases in Japan: a multi‐institutional study. Biomedicine & Pharmacotherapy 2000;54(Suppl 1):211s–4s. [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Young 2007
- Young WF Jr. The incidentally discovered adrenal mass. New England Journal of Medicine 2007;356:601‐10. [DOI] [PubMed] [Google Scholar]


