Abstract
Background
Glutamine is a non‐essential amino acid which is abundant in the healthy human body. There are studies reporting that plasma glutamine levels are reduced in patients with critical illness or following major surgery, suggesting that glutamine may be a conditionally essential amino acid in situations of extreme stress. In the past decade, several clinical trials examining the effects of glutamine supplementation in patients with critical illness or receiving surgery have been done, and the systematic review of this clinical evidence has suggested that glutamine supplementation may reduce infection and mortality rates in patients with critical illness. However, two recent large‐scale randomized clinical trials did not find any beneficial effects of glutamine supplementation in patients with critical illness.
Objectives
The objective of this review was to:
1. assess the effects of glutamine supplementation in critically ill adults and in adults after major surgery on infection rate, mortality and other clinically relevant outcomes;
2. investigate potential heterogeneity across different patient groups and different routes for providing nutrition.
Search methods
We searched the Cochrane Anaesthesia Review Group (CARG) Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 5); MEDLINE (1950 to May 2013); EMBASE (1980 to May 2013) and Web of Science (1945 to May 2013).
Selection criteria
We included controlled clinical trials with random or quasi‐random allocation that examined glutamine supplementation versus no supplementation or placebo in adults with a critical illness or undergoing elective major surgery. We excluded cross‐over trials.
Data collection and analysis
Two authors independently extracted the relevant information from each included study using a standardized data extraction form. For infectious complications and mortality and morbidity outcomes we used risk ratio (RR) as the summary measure with the 95% confidence interval (CI). We calculated, where appropriate, the number needed to treat to benefit (NNTB) and the number needed to treat to harm (NNTH). We presented continuous data as the difference between means (MD) with the 95% CI.
Main results
Our search identified 1999 titles, of which 53 trials (57 articles) fulfilled our inclusion criteria. The 53 included studies enrolled a total of 4671 participants with critical illness or undergoing elective major surgery. We analysed seven domains of potential risk of bias. In 10 studies the risk of bias was evaluated as low in all of the domains. Thirty‐three trials (2303 patients) provided data on nosocomial infectious complications; pooling of these data suggested that glutamine supplementation reduced the infectious complications rate in adults with critical illness or undergoing elective major surgery (RR 0.79, 95% CI 0.71 to 0.87, P < 0.00001, I² = 8%, moderate quality evidence). Thirty‐six studies reported short‐term (hospital or less than one month) mortality. The combined rate of mortality from these studies was not statistically different between the groups receiving glutamine supplement and those receiving no supplement (RR 0.89, 95% CI 0.78 to 1.02, P = 0.10, I² = 22%, low quality evidence). Eleven studies reported long‐term (more than six months) mortality; meta‐analysis of these studies (2277 participants) yielded a RR of 1.00 (95% CI 0.89 to 1.12, P = 0.94, I² = 30%, moderate quality evidence). Subgroup analysis of infectious complications and mortality outcomes did not find any statistically significant differences between the predefined groups. Hospital length of stay was reported in 36 studies. We found that the length of hospital stay was shorter in the intervention group than in the control group (MD ‐3.46 days, 95% CI ‐4.61 to ‐2.32, P < 0.0001, I² = 63%, low quality evidence). Slightly prolonged intensive care unit (ICU) stay was found in the glutamine supplemented group from 22 studies (2285 participants) (MD 0.18 days, 95% CI 0.07 to 0.29, P = 0.002, I² = 11%, moderate quality evidence). Days on mechanical ventilation (14 studies, 1297 participants) was found to be slightly shorter in the intervention group than in the control group (MD ‐ 0.69 days, 95% CI ‐1.37 to ‐0.02, P = 0.04, I² = 18%, moderate quality evidence). There was no clear evidence of a difference between the groups for side effects and quality of life, however results were imprecise for serious adverse events and few studies reported on quality of life. Sensitivity analysis including only low risk of bias studies found that glutamine supplementation had beneficial effects in reducing the length of hospital stay (MD ‐2.9 days, 95% CI ‐5.3 to ‐0.5, P = 0.02, I² = 58%, eight studies) while there was no statistically significant difference between the groups for all of the other outcomes.
Authors' conclusions
This review found moderate evidence that glutamine supplementation reduced the infection rate and days on mechanical ventilation, and low quality evidence that glutamine supplementation reduced length of hospital stay in critically ill or surgical patients. It seems to have little or no effect on the risk of mortality and length of ICU stay, however. The effects on the risk of serious side effects were imprecise. The strength of evidence in this review was impaired by a high risk of overall bias, suspected publication bias, and moderate to substantial heterogeneity within the included studies.
Plain language summary
Giving glutamine supplements to critically ill adults
Glutamine is a non‐essential amino acid which is abundant in the healthy human body. There are studies reporting that muscle and plasma glutamine levels are reduced in patients with critical illness, or following major surgery, suggesting that the body's demand for glutamine is increased in these situations. In the past decade, several clinical trials have examined the effects of glutamine supplementation in patients with critical illness or receiving surgery, and a systematic review of this clinical evidence suggested that giving glutamine to these patients may reduce infection and mortality rates. However, two recent large‐scale clinical trials, published in 2011 and 2013, did not find any beneficial effects of glutamine supplementation in patients with critical illness.
In this review, we searched the available literature until May 2013 and included studies which compared the outcomes with glutamine supplementation and without in adults with a critical illness or undergoing elective major surgery. We included 57 articles from 53 randomized controlled studies in this review. These 53 studies enrolled a total of 4671 patients with critical illness or undergoing elective major surgery. The risk of mortality, length of intensive care unit stay and the incidence of side effects were not significantly different between those given glutamine and those who were not. However, our findings showed that the risk of infectious complications in patients who were given glutamine was 79% of the risk for those who were not. In other words, 12 patients need to be supplemented with glutamine to prevent one case of infection. This result needs to be interpreted cautiously as the quality of evidence for infectious complications was moderate. The funnel plot for this outcome suggested that smaller studies with outcomes favouring non‐supplemented patients have not been published, and further research is likely to have an impact on the estimate of effect for this outcome. The findings from this review also suggested that the average length of hospital stay in critically ill or surgical patients supplemented with glutamine was 3.46 days shorter than for patients without glutamine supplementation. This result should also be treated with care as there was substantial heterogeneity between these studies. We found in this review that the mean number of days on mechanical ventilation was 0.69 days shorter in patients with glutamine supplementation than for patients without glutamine supplementation.
Summary of findings
Summary of findings for the main comparison. Glutamine‐supplemented nutrition compared to non‐supplemented nutrition for critically ill patients.
| Glutamine‐supplemented nutrition compared to non‐supplemented nutrition for critically ill patients | ||||||
| Patient or population: critically ill patients or receiving major surgery Settings: inpatient Intervention: glutamine‐supplemented nutrition | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Glutamine‐supplemented nutrition | |||||
| Infectious complications | 399 per 1000 | 315 per 1000 (283 to 347) | RR 0.79 (0.71 to 0.87) | 2303 (33 studies) | ⊕⊕⊕⊝ moderate1 | |
| Short‐term mortality (within hospital stay or closest to one month) | 197 per 1000 | 175 per 1000 (154 to 201) | RR 0.89 (0.78 to 1.02) | 3454 (36 studies) | ⊕⊕⊝⊝ low1,2 | |
| Long‐term mortality (closest to six month) | 328 per 1000 | 328 per 1000 (292 to 367) | RR 1 (0.89 to 1.12) | 2277 (11 studies) | ⊕⊕⊕⊝ moderate2 | |
| Length of hospital stay | The mean length of hospital stay ranged across control groups from 9 to 73 days | The mean length of hospital stay in the intervention groups was 3.46 lower (4.61 to 2.32 lower) | 2963 (36 studies) | ⊕⊕⊝⊝ low2,3 | ||
| Length of ICU stay | The mean length of ICU stay ranged across control groups from 0.5 to 28.38 days | The mean length of ICU stay in the intervention groups was 0.18 higher (0.07 to 0.29 higher) | 2284 (22 studies) | ⊕⊕⊕⊝ moderate2 | ||
| Days on mechanical ventilation | The mean days on mechanical ventilation ranged across control groups from 4.47 to 22 days | The mean days on mechanical ventilation in the intervention groups was 0.69 lower (1.37 to 0.02 lower) | 1297 (14 studies) | ⊕⊕⊕⊝ moderate2 | ||
| Serious adverse events | 40 per 1000 | 56 per 1000 (31 to 103) | RR 1.4 (0.77 to 2.57) | 856 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Publication bias is suspected as the funnel plot for this outcome suggested that smaller studies with outcomes favouring non‐supplemented patients were lacking 2 The proportion of high risk of bias trials in this outcome was higher than 30%, the potential limitations are likely to reduce confidence in the estimate of effect 3 There was substantial variability in effect estimates (I² = 63%) 4 The 95% CI of the pooled estimate was not narrow enough for a confident judgment of the effect size
Background
Description of the condition
Critically ill adults can be defined as a group of patients with a life‐threatening medical or surgical status who need treatment in an intensive care unit (ICU) (Mizock 2010). They are vulnerable to nosocomial infections as severe illness may bring about immunofunctional deficiency (O'Leary 1996). In response to a severe condition the local inflammatory reaction of the body develops into systemic inflammation. Immunosuppression may be induced by the corresponding compensatory anti‐inflammatory response, which may increase the risk of infection and organ failure in critically ill patients (Mizock 2010; Tschoeke 2007). Specific nutrients for the immune system, such as glutamine, can be administered enterally or parenterally to impact favourably on the immune response and thus reduce the complications associated with critical illness (Mizock 2010; Novak 2002).
Description of the intervention
Glutamine is a type of free amino acid which is abundant in the body. It accounts for approximately 30% of the plasma free amino acids. Most glutamine in the body is synthesized by skeletal muscle, while the brain and the lung synthesize the remainder (Tjader 2007). Although glutamine is non‐essential in the diet of healthy human beings, endogenous glutamine biosynthesis may be insufficient to meet the needs of patients in situations of extreme stress (Bongers 2007). In patients undergoing surgery or suffering trauma or sepsis the levels of free glutamine in muscle are decreased (Gamrin 1996; Vinnars 1975). Plasma glutamine levels are also reduced in patients with critical illness or following major surgery (Melis 2004; Parry‐Billings 1992). Clinical evidence has revealed that glutamine supplementation in critical illness may reduce infection and mortality rates (Novak 2002).
Glutamine supplementation can be used to prevent a new infection in non‐infected patients or a subsequent infection in critically ill patients who have already had an infection. The latter situation is a far greyer area since the immune dysfunction associated with repeated infections and how these affect subsequent outcomes are far less clearly understood. In this systematic review we will focus on the effect of glutamine in preventing new infective complications in non‐infected participants (Andrews 2011).
How the intervention might work
There are many potential mechanisms which could account for the benefit of glutamine supplementation in critically ill patients. First, there is a depletion of free glutamine and a shortage in relation to needs under conditions of severe metabolic stress such that exogenous glutamine supplementation is required in order to maintain normal concentrations of plasma glutamine (Tjader 2007). Second, both in vitro and in vivo studies have suggested that glutamine could induce the expression of heat shock proteins (HSPs), especially HSP‐70 (Kim 2013). Hsp‐70 expression could protect cells against cytotoxic mediators and reduce organ injury and mortality in critical illness settings (Roth 2008). In critically ill patients it was reported that glutamine supplementation significantly increased serum HSP‐70 and the magnitude of the HSP‐70 enhancement in the glutamine group was correlated with improved clinical outcomes, further indicating that glutamine may play a protective role by inducing the expression of HSPs (Ziegler 2005). Third, clinical trials revealed that glutamine supplementation attenuated hyperglycaemia and insulin resistance in ICU patients (Bakalar 2006; Déchelotte 2006) and it seems reasonable to speculate that glutamine may exert its effects through regulating glucose metabolism in the critically ill. Fourth, glutamine supplementation in patients has been shown to maintain the gastrointestinal structure (Melis 2004), improve nitrogen balance (Hammarqvist 1989), elevate immune cell function (Ogle 1994) and reduce the production of proinflammatory cytokines (O'Riordain 1996). Fifth, glutamine is a precursor for glutathione and could play its role as an antioxidant by producing glutathione (Melis 2004).
Why it is important to do this review
Although glutamine may play an important role in the treatment of critically ill patients in some countries (such as the UK) enteral or parenteral supplementation with glutamine has not been widely adopted for patients with critical illness or those who have had surgery (Avenell 2006) due to a lack of good quality randomized controlled trials (RCTs). Also, when dealing with nutritional deficiency any effect on patient outcome is dependent on the prior and current states of nutrition and health, and the consequences of a deficiency depends on how well the patient's own endogenous system can meet that need. Some of the recently published, large sample size studies have tested the influence of glutamine supplementation given only for a few days on short‐term and long‐term outcomes (Andrews 2011; Déchelotte 2006); few have studied long‐term glutamine supplementation. Therefore, it is necessary to investigate and summarize the potential heterogeneity in the design of these studies and assess any association with their results.
Furthermore, there are differences in the use of exogenous glutamine by parenteral or enteral administration. There is controversy as to whether both total parenteral and enteral glutamine supplementation can reduce infection in patients with critical illness (Avenell 2009). Some studies have assessed the influence of risk of bias in the RCTs that have compared parenteral or enteral nutrition with no nutritional intervention in critically ill patients (Koretz 2007a; Koretz 2007b; Koretz 2007c). The results show that in the trials with lower risk of bias parenteral nutrition appeared to cause infection, and the alleged benefits of enteral nutrition may actually have been due to bias. Therefore, even if the conclusion of our review is that the addition of glutamine results in fewer infections, we will need to interpret the results cautiously. Still, the inflammatory response accompanying critical illness was far more intense and complex than the response elective surgical patients had experienced (Avenell 2009). Thus, the effects of glutamine supplementation may differ between patients undergoing surgery and patients who are critically ill. Like in the previous systematic reviews (Avenell 2009; Novak 2002) we planned to examine the treatment effects of glutamine supplementation in surgical patients and critically ill patients separately. As heterogeneity may exist between critically ill patients and surgical patients the combined results of these two populations in our review must be interpreted cautiously.
Lastly, large sample multicentre RCTs examining the role of glutamine in critical illness have recently been completed (Andrews 2011; Heyland 2013). Therefore a rigorous and objective systematic review is needed to evaluate the role of glutamine supplementation in preventing infections in critically ill adults.
Objectives
The objective of this review was to:
determine the effects of glutamine supplementation in critically ill adults and in adults after major surgery on infection rate, mortality and other clinically relevant outcomes;
investigate potential heterogeneity across different patient groups and different routes for providing nutrition.
Methods
Criteria for considering studies for this review
Types of studies
We included full reports of controlled clinical trials with random or quasi‐random allocation. We excluded cross‐over trials.
Types of participants
We included studies in adults with a critical illness or undergoing elective major surgery. We excluded participants receiving prophylactic supplementation of glutamine before surgery.
We included studies reporting mixed groups of participants (for example, combined data from critically ill and medical patients). We planned to treat these as separate groups in subgroup analyses (for example, medical, surgical, mixed).
Types of interventions
We examined glutamine supplementation delivered by the parenteral or enteral route versus no supplementation or placebo. The nutritional regime was determined by each of the included trials and the glutamine was given in addition to a standard nutritional regime.
Types of outcome measures
Primary outcomes
Number of infectious complications (as defined in each of the included studies)
Mortality (measured at the follow‐up times closest to one and six months)
Secondary outcomes
Length of hospital stay
Length of ICU stay
Days on mechanical ventilation
Possible side effects, as described by the authors
Quality of life
With secondary outcomes 1, 2 and 3, for patients who died before discharge death was considered as discharge.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Anaesthesia Review Group (CARG) Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 5), see Appendix 1; MEDLINE via OvidSP (1950 to May 2013), see Appendix 2; EMBASE via OvidSP (1980 to May 2013), see Appendix 3; and Web of Science (1945 to May 2013), see Appendix 4.
We used the following text words: glutam* or dipeptide* or L‐glutamin*.
We developed a specific search strategy for each database based on that developed for MEDLINE. We did not apply any language restriction.
Searching other resources
Two authors (WFX, LQY) independently handsearched the following intensive care and nutrition journals (up to April 2013) as advised by the reviewers. These are available in our library:
Clinical Nutrition;
Critical Care Medicine;
Intensive Care Medicine;
Proceedings of the Nutrition Society;
Journal of Parenteral and Enteral Nutrition;
Chest;
Journal of Orthomolecular Medicine.
Two authors (YMS, ZJL) searched the references of all identified trials and previous reviews.
One author (KMT) searched the following websites for ongoing clinical trials and unpublished studies (up to June 2013):
Data collection and analysis
Selection of studies
Two authors (KMT, XQL) independently screened the abstracts of all studies identified by the search strategy. We obtained the full texts of articles that seemed to meet our inclusion criteria and independently re‐assessed them. We resolved any disagreements by discussion until a consensus was achieved.
Data extraction and management
Two authors (KMT, XQL) independently extracted the relevant information from each included study using a standardized data extraction form. We resolved any disagreements by discussion. One author (KMT) entered data into Review Manager software (RevMan 5.2) and re‐checked the data for accuracy. If we needed further information to enable us to reach a decision then we attempted to contact the study authors.
Assessment of risk of bias in included studies
Based on the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), two authors (LQY and ZJL) assessed the risk of bias for each included trial by using the domain‐based evaluation. We assessed the following seven domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. We also gave reasons for our judgements. Three authors (LQY, ZJL and WFY) resolved disagreements by reviewing the data together.
We judged an included trial to be a trial with low risk of bias if the risk of bias was evaluated as 'low' in all of the domains. If the risk of bias was judged as 'high', then we listed the trial as having a high risk of bias. Those trials that we judged 'unclear' were listed as having an unclear risk of bias. Using the content of the included studies and the 'risk of bias' tables, we presented the quality of the evidence using the GRADE approach, giving particular attention to the limitations of study design and heterogeneity of results.
Measures of treatment effect
For dichotomous data, we used the risk ratio (RR) as the summary measure. We calculated the number needed to treat to benefit (NNTB) and the number needed to treat to harm (NNTH) where appropriate. We presented continuous data as the difference between means. Data reported as median and range were converted into mean (standard deviation) before meta‐analysis by using the formula from Hozo 2005, and the estimated standard deviation was calculated from the interquartile range based on the assumption that the width of the interquartile range will be approximately 1.35 standard deviations (Handbook 2011).
Unit of analysis issues
The unit of analysis was the participating adult in individually randomized trials.
Dealing with missing data
We contacted the authors of the included trials to obtain relevant missing data. If data were obtained from the trial authors, then we performed an intention‐to‐treat (ITT) analysis. We investigated attrition, for example from dropouts, losses to follow‐up and withdrawals. We critically appraised issues of missing data according to the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Assessment of heterogeneity
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1. We also specifically examined heterogeneity with the I2 statistic, quantifying inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis, where an I2 statistic of 50% and more indicates a substantial level of inconsistency (Handbook 2011). When statistical heterogeneity was found, we attempted to determine the potential reasons for it by examining the individual trials and the subgroup characteristics.
We assessed the statistical heterogeneity of any included studies that were assessed as having clinical diversity (for example, different types of patients, enteral or parenteral glutamine supplementation) and methodological diversity.
Assessment of reporting biases
We tried to minimize the impact of publication bias through a thorough review of all the published data. As the review included more than 10 studies, we assessed publication bias and small study effects in a qualitative manner using a funnel plot. If significant asymmetry was found in the funnel plot, we reported it in our results and in the summary of findings table.
Data synthesis
On the basis that no substantial clinical heterogeneity was found between trials, we performed the analysis using RevMan software (RevMan 5.2). We performed meta‐analysis of the included trials by using a fixed‐effect model if the P value of the heterogeneity test was more than 0.1, otherwise we used a random effects model. We expressed dichotomous data as the risk ratio (RR) with the 95% confidence interval (CI). We calculated the NNTB for the infectious complications outcome by combining the overall RR with an estimate of the prevalence of the event in the control groups of the trials. We analysed continuous outcomes according to the difference in the mean treatment effects and the 95% CI. We assumed a P value of < 0.05 to be of statistical significance.
Subgroup analysis and investigation of heterogeneity
As the treatment effect of glutamine supplementation may be different in surgical patients compared with critically ill patients, and it may also be affected by different routes of administration and different disease status, we performed subgroup analysis for the primary outcomes by:
elective surgical patients and critically ill patients,
enteral glutamine supplementation only and parenteral glutamine supplementation only,
patients with malignant and non‐malignant disease.
Sensitivity analysis
We performed sensitivity analysis for trials with a low risk of bias, for all the outcomes. We compared random‐effects and fixed‐effect estimates for each outcome variable. In the case of any missing data, we planned to use the best case, worst case for imputation of missing data. We excluded and included any study that appeared to have a large effect size (often the largest or earliest study) in order to assess its impact on the meta‐analysis.
Summary of findings
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with the following specific outcomes in our review:
number of infectious complications;
mortality (mortality closest to at one and six months);
length of hospital stay;
length of ICU stay;
days on mechanical ventilation;
serious adverse events.
We constructed a 'summary of findings' (SoF) table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence takes into consideration within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
We identified a total of 1999 citations. Through reading the titles and then abstracts, we obtained the full texts of 89 citations that were potentially eligible for inclusion in our review. Of those 89 citations, 25 did not meet our inclusion criteria and were excluded for the reasons described in the table Characteristics of excluded studies. Six ongoing clinical trials were identified through searching the websites clinicaltrials.gov and controlled‐trials.com (Characteristics of ongoing studies). One study (Hallay 2002) is waiting assessment as we were unable to obtain the full text. Finally, we included 57 articles from 53 studies (four articles were secondary publications) in this review (Figure 1).
1.
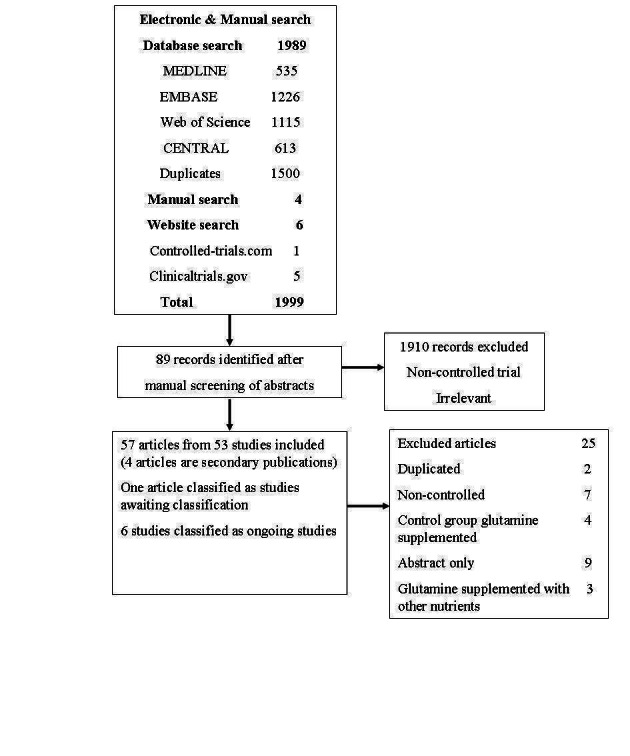
Study flow diagram.
Included studies
These 53 studies enrolled a total of 4671 patients. All participants were adults with a critical illness or undergoing elective major surgery; they were allocated to either a glutamine supplementation group or control group. All studies reported at least one of the outcomes which we had predefined in our protocol. The studies fell broadly into the following groups.
Studies in intensive care unit (ICU) patients
We included 19 studies in this category. Of these 19 studies, 12 included participants admitted to ICU with varying diagnoses (Andrews 2011; Çekmen 2011; Déchelotte 2006; Goeters 2002; Grau 2011; Griffiths 1997; Luo 2008; Pérez‐Bárcena 2008; Tjäder 2004; Tremel 1994; Wernerman 2011; Zhang 2007). Two studies included trauma patients (Eroglu 2009; Pérez‐Bárcena 2010). One study included patients with systemic inflammatory response syndrome (Conejero 2002). One study included aged patients (Chai 2006). One study included patients with multiple organ dysfunction syndrome (Tian 2006). One study included patients requiring mechanical ventilation (Hall 2003), and a further study included mechanically ventilated adult patients with two or more failed organs (Heyland 2013).
Studies in surgical patients
We included 18 studies in this category. Of these 18 studies, 14 included patients receiving abdominal surgery (Dong 2008; Fuentes‐Orozco 2004; Jiang 1999; Kłek 2005; Kumar 2007; Lin 2002; Lu 2011; Morlion 1998; Neri 2001; O'Riordain 1994; Qiu 2009; Spittler 2001; Yao 2007; Zhu 2000). Two studies included patients receiving thoracic or abdominal surgery (Estívariz 2008; Mertes 2000). One study included patients undergoing aortic abdominal aneurysm reconstruction (Karwowska 2001). One study included patients receiving laryngectomy (Wang 2006).
Studies in burns patients
Eight trials investigating the impact of glutamine supplementation in burns patients were included in the review (Cucereanu‐Badica 2013; Garrel 2003; Pattanshetti 2009; Peng 2004; Wischmeyer 2001; Zhou 2002; Zhou 2003; Zhou 2004).
Studies in patients with acute pancreatitis
Seven trials were included in this category (Fuentes‐Orozco 2008; Hajdú 2012; He 2004; Ockenga 2002; Sahin 2007; Yang 2008; Zhao 2013).
The remaining one study included patients both from ICU and other departments (Powell‐Tuck 1999).
Excluded studies
We excluded 25 clinical studies for the reasons described in the Characteristics of excluded studies.
Studies awaiting classification
One study that examined the effects of enteral glutamine supplementation in patients receiving subtotal oesophagectomy for malignancy (Hallay 2002) is waiting classification as we were unable to obtain the full text (see Characteristics of studies awaiting classification).
Ongoing studies
Six ongoing studies enrolling trauma, ICU, burns and acute pancreatitis participants were identified (see Characteristics of ongoing studies).
Risk of bias in included studies
See: Characteristics of included studies; Figure 2; Figure 3.
2.
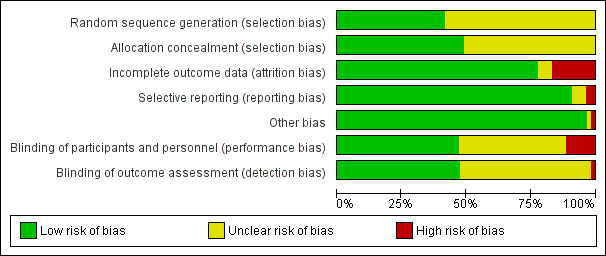
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We analysed seven domains of potential risk of bias for the included studies; 42, 48 and 51 studies were rated at low risk of attrition bias, selective reporting bias and other bias respectively. Eighteen studies were rated at low risk of selection bias. We rated 25 studies each at low risk of performance bias and detection bias. Blinding of participants and personnel and incomplete outcome data were rated at high risk in six and nine included studies respectively.
Allocation
Eighteen studies clearly described the process for adequate generation of a randomized sequence and concealment of allocation and were classified as low risk of bias. Random sequence generation or allocation concealment was rated as having an unclear risk of bias in the remaining 35 studies due to not reporting sequence generation or allocation details. No study was rated as high risk of bias (Figure 3).
Blinding
Performance bias
Six studies were at high risk for performance bias. In four studies patients were not blinded (Kumar 2007; Pattanshetti 2009; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010). One study was performed in an unblinded fashion (Goeters 2002) and in another study treatment was not blinded for the attending physicians (Tjäder 2004). Twenty‐two studies gave insufficient information to assess performance bias and 25 studies were at low risk for performance bias as blinding of participants and key study personnel was ensured in these studies (Figure 2; Figure 3).
Detection bias
One open randomized trial was rated as high risk for detection bias (Goeters 2002) and 27 included studies were rated at an unclear risk of detection bias (Figure 2; Figure 3).
Incomplete outcome data
In nine studies the number of patients withdrawn after randomization was high (4 to 49) and the studies were judged as having a high risk for attrition bias (Conejero 2002; Estívariz 2008; Garrel 2003; Goeters 2002; Hajdú 2012; Kłek 2005; Luo 2008; Mertes 2000; Yang 2008). Two studies were rated at unclear risk: one study planned to exclude the patients who died within 72 hours of admission but did not report the number of patients excluded (Pattanshetti 2009), another study withdrew five patients without giving reasons (Wernerman 2011). For the remaining 42 studies the risk of attrition bias was low (Figure 2; Figure 3).
Selective reporting
We judged two studies as high risk for reporting bias. One study did not report the length of hospital stay outcome completely (Qiu 2009), the other study did not report the length of ICU stay and all‐cause six‐month mortality outcomes completely (Wernerman 2011). We were, therefore, unable to enter these two studies in a meta‐analysis. Three studies were judged as having an unclear risk for reporting bias. One study only reported the average length of stay and mortality outcomes in the subgroup of patients treated for longer than nine days, and whether this was pre‐specified was unclear (Goeters 2002). Two other studies either did not report baseline infection associated blood test results (Pattanshetti 2009) or did not report organ function tests (Zhu 2000) and were therefore judged at unclear risk. The remaining 48 studies were free from selective reporting bias (Figure 2; Figure 3).
Other potential sources of bias
One study had baseline bias and was rated at high risk. This was because the patients in the treatment group were younger than those in the control group (Kumar 2007). In another study, patients that were clinically accepted for parenteral nutrition were included in the trial, it was unclear whether the patients were all critically ill. This study was rated at unclear risk (Powell‐Tuck 1999). No other significant potential sources of bias were identified in the remaining trials (Figure 2; Figure 3).
Effects of interventions
See: Table 1
See: summary of findings for the main comparison glutamine supplementation for critically ill adults, Table 1.
Infectious complications
Thirty‐three trials (including 2303 patients) provided data on the nosocomial infectious complications (see Characteristics of included studies). The absolute risk for infectious complications was 39.9% (458/1148) in the control group and 31.8% (367/1155) in the intervention group. Pooling of these data suggested that glutamine supplementation reduced the infectious complications rate in adults with critical illness or undergoing elective major surgery (RR 0.79, 95% CI 0.71 to 0.87, P < 0.00001, I² = 8%) (Analysis 1.1). By setting the assumed control risk at 40%, we calculated the number needed to treat for an additional beneficial outcome as 12. The funnel plot for this outcome was asymmetrical, suggesting that smaller studies with outcomes favouring non‐supplemented patients appeared to be lacking. One newly published large sample, randomized blinded trial (Heyland 2013) provided data on the infectious outcome in glutamine supplementation patients together with patients on antioxidant supplementation. We were told by the author that the detailed infectious incidence in glutamine supplementation patients will be published in the future (Heyland 2013 [pers comm]). This study was not included in the analysis. We also used the trim and fill method for this outcome to identify and correct for funnel plot asymmetry arising from publication bias. The method suggested that 13 studies are missing. Using a fixed‐effect model (inverse variance method) the original point estimate (RR) and 95% CI for the combined studies was 0.84 (95% CI 0.80 to 0.92). Using trim and fill the imputed point estimate was 0.89 (95% CI 0.81 to 0.98). Using the random‐effects model the RR was 0.83 (95% CI 0.75 to 0.92). Using trim and fill the imputed point estimate was 0.87 (95% CI 0.77 to 0.99). These results suggested that the combined results were not changed after adding the missing studies.
1.1. Analysis.
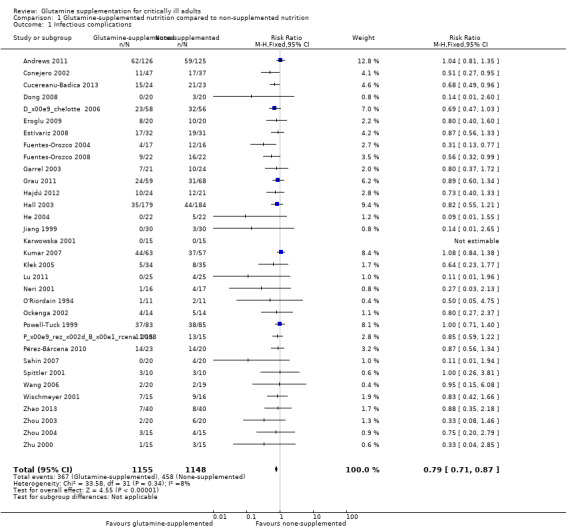
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 1 Infectious complications.
Sensitivity analysis showed that the risk of infectious complications in the low risk of bias studies (Andrews 2011; Déchelotte 2006; Dong 2008; Grau 2011; Hall 2003; Zhou 2003; Zhou 2004) was not different between the groups (RR 0.85, 95% CI 0.72 to 1.01, P = 0.06, I² = 9%). The difference in combined effect size between the main analysis and the sensitivity analysis could be explained by the reduced sample size but more importantly the change in RR from 0.79 in the main analysis to 0.85 in the sensitivity analysis suggested that the effect size could have been partly explained by within study biases.
Infectious complications: elective surgery patients versus critically ill patients
Eleven trials (Dong 2008; Estívariz 2008; Jiang 1999; Karwowska 2001; Kłek 2005; Lu 2011; Neri 2001; O'Riordain 1994; Spittler 2001; Wang 2006; Zhu 2000) included 456 patients who were enrolled for elective surgery. The pooled RR was 0.59 (95% CI 0.41 to 0.86, P = 0.005, I² = 0%). Twenty‐two trials (Andrews 2011; Conejero 2002; Cucereanu‐Badica 2013; Déchelotte 2006; Eroglu 2009; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Grau 2011; Hajdú 2012; Hall 2003; He 2004; Kumar 2007; Ockenga 2002; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Powell‐Tuck 1999; Sahin 2007; Wischmeyer 2001; Zhao 2013; Zhou 2003; Zhou 2004) that enrolled 1847 critically ill patients were pooled to give a RR of 0.82 (95% CI 0.73 to 0.91, P = 0.001, I² = 18%) (Analysis 2.1).
2.1. Analysis.
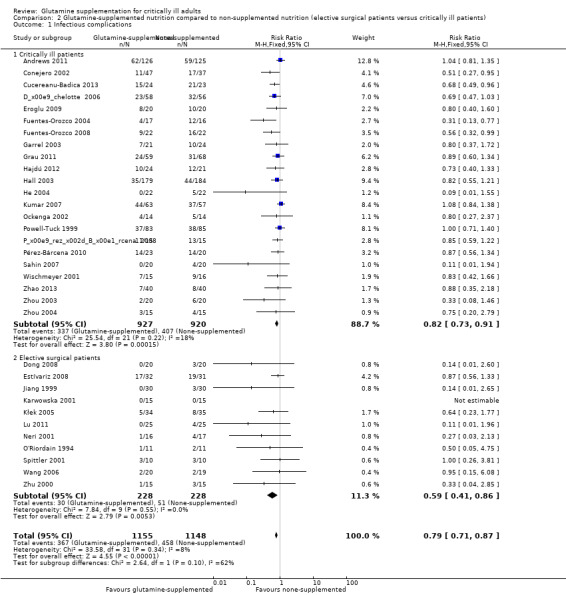
Comparison 2 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (elective surgical patients versus critically ill patients), Outcome 1 Infectious complications.
Infectious complications: enteral glutamine supplementation versus parenteral glutamine supplementation
Twenty‐eight studies (Andrews 2011; Cucereanu‐Badica 2013; Déchelotte 2006; Dong 2008; Eroglu 2009; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Grau 2011; Hajdú 2012; He 2004; Jiang 1999; Karwowska 2001; Kłek 2005; Lu 2011; Neri 2001; Ockenga 2002; O'Riordain 1994; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Powell‐Tuck 1999; Sahin 2007; Spittler 2001; Wang 2006; Wischmeyer 2001; Zhao 2013; Zhou 2004; Zhu 2000) examined the effects of parenteral glutamine supplementation on infection. Meta‐analysis showed a lower risk of infectious complications in patients receiving parenteral glutamine supplementation than those without glutamine supplementation (RR 0.78, 95% CI 0.69 to 0.87, P < 0.00001, I² = 0%). Five studies (Conejero 2002; Garrel 2003; Hall 2003; Kumar 2007; Zhou 2003) tested the effects of enteral glutamine supplementation on the infection rate. There was no significant difference between the treatment and control groups (RR 0.83, 95% CI 0.67 to 1.02, P = 0.07, I² = 50%) (Analysis 3.1). The test for subgroup differences was not statistically significant (P = 0.61).
3.1. Analysis.
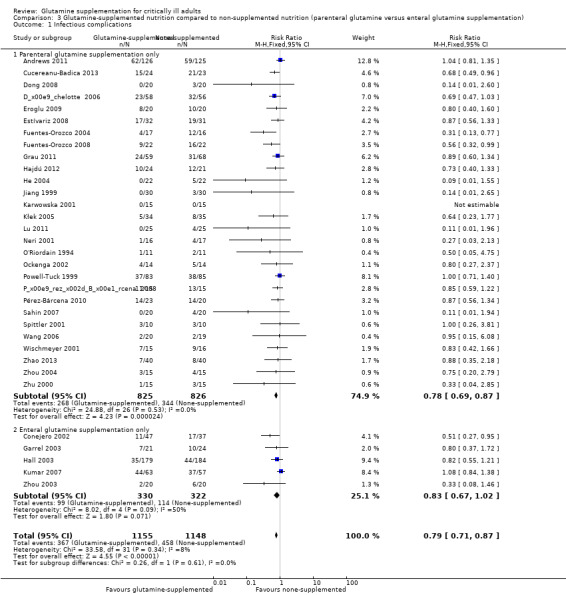
Comparison 3 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (parenteral glutamine versus enteral glutamine supplementation), Outcome 1 Infectious complications.
Infectious complications: patients with malignant disease versus patients with non‐malignant disease
Six studies (Kłek 2005; Lu 2011; Neri 2001; O'Riordain 1994; Wang 2006; Zhu 2000) included surgical patients with malignant disease and the pooled RR was 0.45 (95% CI 0.23 to 0.90, P = 0.02, I² = 0%). Seventeen studies (Conejero 2002; Cucereanu‐Badica 2013; Eroglu 2009; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Hajdú 2012; He 2004; Karwowska 2001; Ockenga 2002; Pérez‐Bárcena 2010; Sahin 2007; Wischmeyer 2001; Zhao 2013; Zhou 2003; Zhou 2004) enrolled patients with non‐malignant disease. The pooled analysis showed that the risk of infectious complications was lower in the glutamine supplemented patients than in the patients that did not receive the supplement (RR 0.66, 95% CI 0.55 to 0.78, P < 0.00001, I² = 0%). The remaining 10 studies (Andrews 2011; Déchelotte 2006; Dong 2008; Grau 2011; Hall 2003; Jiang 1999; Kumar 2007; Pérez‐Bárcena 2008; Powell‐Tuck 1999; Spittler 2001) enrolled patients with uncertain or mixed disease. The pooled RR was 0.91 (95% CI 0.80 to 1.03, P = 0.15, I² = 0%) (Analysis 4.1).
4.1. Analysis.
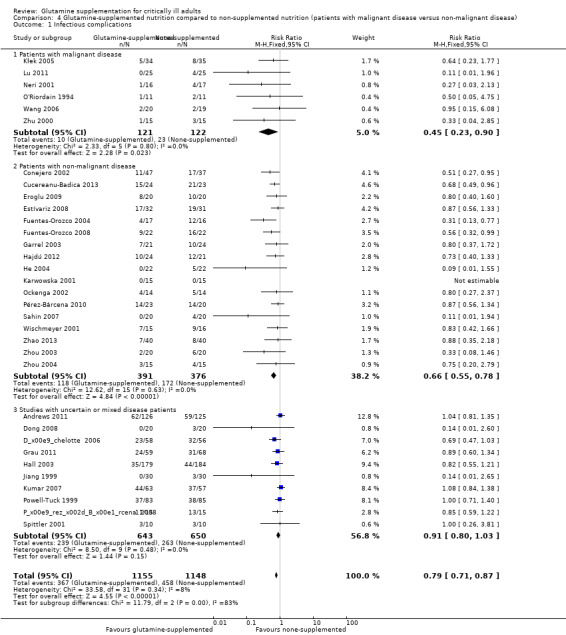
Comparison 4 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (patients with malignant disease versus non‐malignant disease), Outcome 1 Infectious complications.
Mortality
Thirty‐seven studies (3494 patients) reported on mortality. Of these 37 studies, 36 (Andrews 2011; Çekmen 2011; Chai 2006; Conejero 2002; Cucereanu‐Badica 2013; Déchelotte 2006; Dong 2008; Eroglu 2009; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Goeters 2002; Grau 2011; Griffiths 1997; Hajdú 2012; Hall 2003; He 2004; Heyland 2013; Karwowska 2001; Kumar 2007; Lin 2002; Neri 2001; Ockenga 2002; Pattanshetti 2009; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Powell‐Tuck 1999; Qiu 2009; Sahin 2007; Tian 2006; Wang 2006; Wernerman 2011; Wischmeyer 2001; Yang 2008; Zhao 2013) reported short‐term (hospital or closest to one month) mortality. The combined rate of mortality from these studies was not statistically different between the group which received a glutamine supplement and the group which did not (RR 0.89, 95% CI 0.78 to 1.02, P = 0.10, I² = 22%) (Analysis 1.2). Eleven studies (Andrews 2011; Déchelotte 2006; Goeters 2002; Grau 2011; Griffiths 1997; Hall 2003; Heyland 2013; Powell‐Tuck 1999; Qiu 2009; Tjäder 2004; Wernerman 2011) reported long‐term (closest to six months) mortality. Meta‐analysis of these 11 studies yielded an RR of 1.00 (95% CI 0.89 to 1.12, P = 0.94, I² = 30%) (Analysis 1.3). The funnel plot for the short‐term mortality outcome was asymmetrical, suggesting that smaller studies with outcomes favouring non‐supplemented patients appeared to be lacking; while the funnel plot for the long‐term mortality outcome was symmetrical. The trim and fill method suggested that 10 studies were missing for the short‐term mortality outcome, and results indicated that the combined results were not changed after filling in these missing studies. Using the fixed‐effect model (inverse variance method) the original point estimate (RR) for the combined studies was 0.93 (95% CI 0.81 to 1.07). Using the trim and fill method the imputed point estimate was 1.00 (95% CI 0.87 to 1.14). Using the random‐effects model the RR for the combined studies was 0.83 (95% CI 0.69 to 1.00). Using trim and fill the imputed RR was 0.93 (95% CI 0.76 to 1.15).
1.2. Analysis.
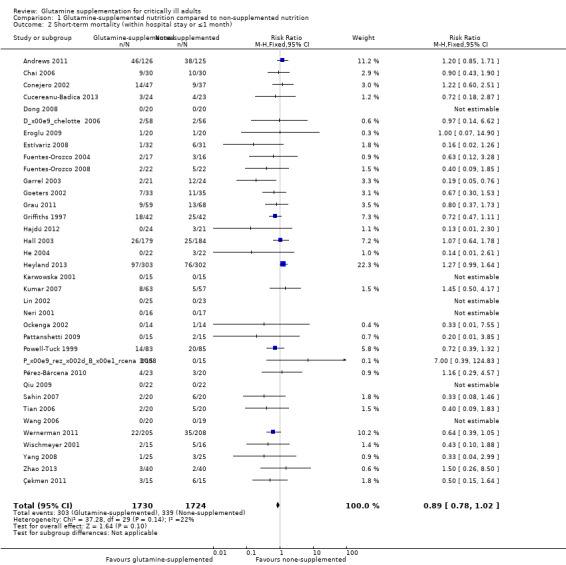
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 2 Short‐term mortality (within hospital stay or ≤1 month).
1.3. Analysis.
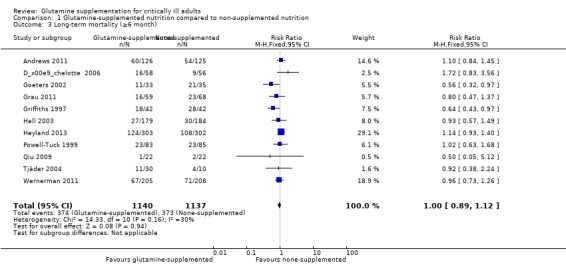
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 3 Long‐term mortality (≥6 month).
Sensitivity analysis showed that both the short‐ and long‐term mortality rates in low risk of bias studies were not different between the groups (RR 1.10, 95% CI 0.93 to 1.30, P = 0.29, I² = 21%; RR 0.99, 95% CI 0.80 to 1.23, P = 0.96, I² = 46% respectively).
Short‐term mortality: elective surgery patients versus critically ill patients
Twenty‐nine studies (Andrews 2011; Çekmen 2011; Chai 2006; Conejero 2002; Cucereanu‐Badica 2013; Déchelotte 2006; Eroglu 2009; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Goeters 2002; Grau 2011; Griffiths 1997; Hajdú 2012; Hall 2003; He 2004; Heyland 2013; Kumar 2007; Ockenga 2002; Pattanshetti 2009; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Powell‐Tuck 1999; Sahin 2007; Tian 2006; Wernerman 2011; Wischmeyer 2001; Yang 2008; Zhao 2013) enrolling 3157 critically ill patients reported short‐term mortality. The pooled RR was 0.91 (95% CI 0.79 to 1.04, P = 0.15, I² = 19%). The mortality outcome was reported in seven studies (Dong 2008; Estívariz 2008; Karwowska 2001; Lin 2002; Neri 2001; Qiu 2009; Wang 2006) enrolling patients for elective surgery. No deaths occurred during the study period in six of the seven studies so the data could not be combined (Analysis 2.2).
2.2. Analysis.
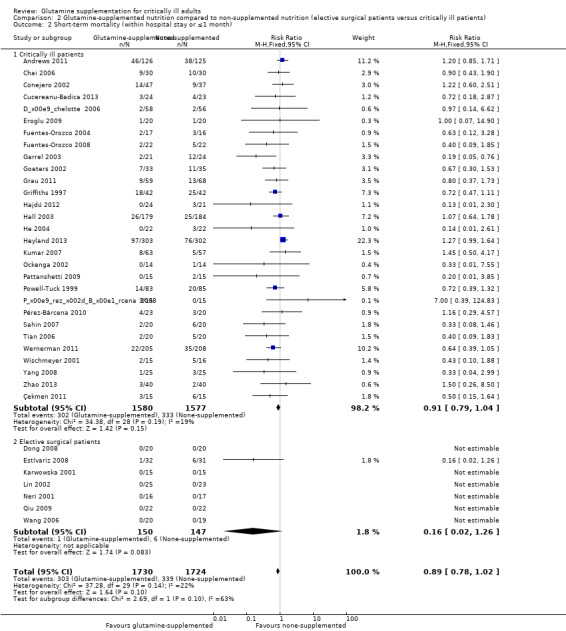
Comparison 2 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (elective surgical patients versus critically ill patients), Outcome 2 Short‐term mortality (within hospital stay or ≤1 month).
Short‐term mortality: enteral glutamine supplementation versus parenteral glutamine supplementation
Five clinical trials (Conejero 2002; Garrel 2003; Hall 2003; Kumar 2007; Pattanshetti 2009) examined the effects of enteral glutamine supplementation on short‐term mortality. Pooling of these studies suggested no difference in mortality rate between the intervention and control groups (RR 0.91, 95% CI 0.64 to 1.30, P = 0.61, I² = 48%). Thirty trials (Andrews 2011; Çekmen 2011; Chai 2006; Cucereanu‐Badica 2013; Déchelotte 2006; Dong 2008; Eroglu 2009; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Goeters 2002; Grau 2011; Griffiths 1997; Hajdú 2012; He 2004; Karwowska 2001; Lin 2002; Neri 2001; Ockenga 2002; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Powell‐Tuck 1999; Qiu 2009; Sahin 2007; Tian 2006; Wang 2006; Wernerman 2011; Wischmeyer 2001; Yang 2008; Zhao 2013) used parenterally supplemented glutamine. We pooled the data on the 2207 patients enrolled in these studies, which yielded a RR of 0.75 (95% CI 0.63 to 0.90, P = 0.002, I² = 0%) (Analysis 3.2). Only one study (Heyland 2013) supplemented glutamine both parenterally and enterally but this study could not be entered into a subgroup analysis.
3.2. Analysis.
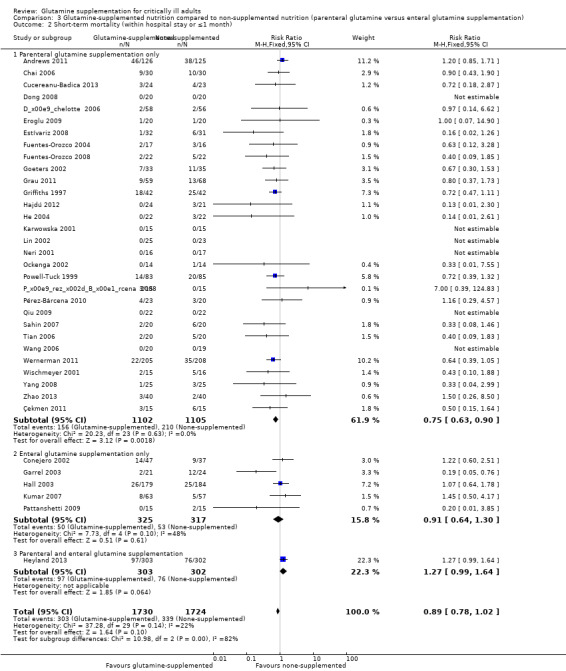
Comparison 3 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (parenteral glutamine versus enteral glutamine supplementation), Outcome 2 Short‐term mortality (within hospital stay or ≤1 month).
Short‐term mortality: patients with malignant disease versus patients with non‐malignant disease
Three studies (Lin 2002; Neri 2001; Wang 2006) included 120 surgical patients with malignant disease. No deaths occurred during the study period in these three studies, so the RR could not be estimated. Nineteen studies (Chai 2006; Conejero 2002; Cucereanu‐Badica 2013; Eroglu 2009; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Griffiths 1997; Hajdú 2012; He 2004; Karwowska 2001; Ockenga 2002; Pattanshetti 2009; Pérez‐Bárcena 2010; Sahin 2007; Wischmeyer 2001; Yang 2008; Zhao 2013) enrolled 921 patients with non‐malignant disease. The pooled analysis of these studies showed a lower mortality risk for participants receiving glutamine supplementation compared with those receiving placebo (RR 0.61, 95% CI 0.46 to 0.79, P = 0.002, I² = 0%). The remaining 14 studies (Andrews 2011; Çekmen 2011; Déchelotte 2006; Dong 2008; Goeters 2002; Grau 2011; Hall 2003; Heyland 2013; Kumar 2007; Pérez‐Bárcena 2008; Powell‐Tuck 1999; Qiu 2009; Tian 2006; Wernerman 2011) enrolled 2413 patients with uncertain or mixed disease, and the pooled RR was 1.02 (95% CI 0.87 to 1.20, P = 0.78, I² = 26%) (Analysis 4.2).
4.2. Analysis.
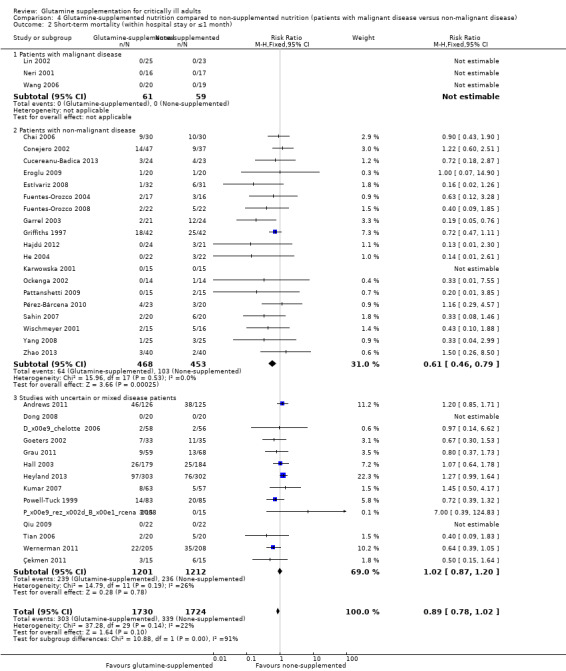
Comparison 4 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (patients with malignant disease versus non‐malignant disease), Outcome 2 Short‐term mortality (within hospital stay or ≤1 month).
Long‐term mortality
Data on 124 patients with non‐malignant disease from two studies comparing nutrition with glutamine supplementation to nutrition without glutamine supplementation (Griffiths 1997; Tjäder 2004) were pooled to give a RR of 0.69 (95% CI 0.48 to 1.00, P = 0.05, I² = 0%). The remaining subgroup analysis did not find a mortality difference between the groups (Analysis 2.3; Analysis 3.3; Analysis 4.3).
2.3. Analysis.
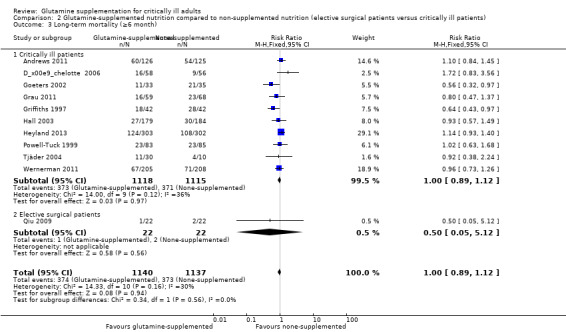
Comparison 2 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (elective surgical patients versus critically ill patients), Outcome 3 Long‐term mortality (≥6 month).
3.3. Analysis.
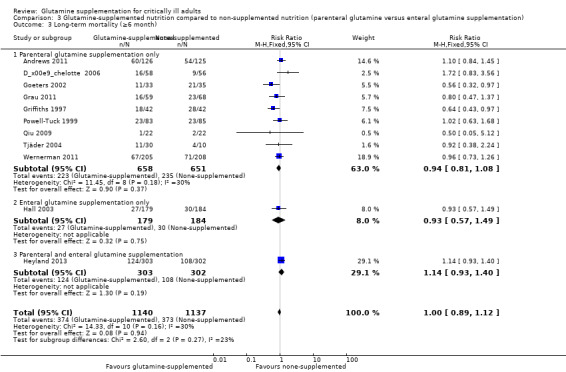
Comparison 3 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (parenteral glutamine versus enteral glutamine supplementation), Outcome 3 Long‐term mortality (≥6 month).
4.3. Analysis.
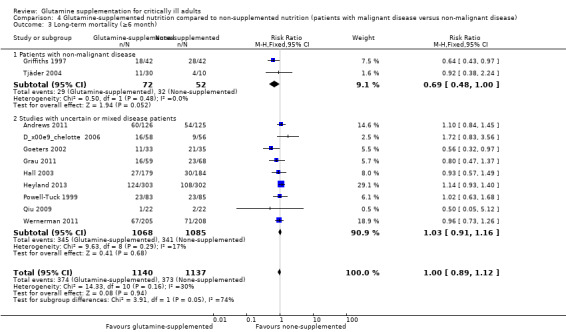
Comparison 4 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (patients with malignant disease versus non‐malignant disease), Outcome 3 Long‐term mortality (≥6 month).
Length of hospital stay
Hospital length of stay was reported in 36 studies (see Characteristics of included studies). Of these 36 studies, 25 (Cucereanu‐Badica 2013; Dong 2008; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Goeters 2002; He 2004; Jiang 1999; Karwowska 2001; Mertes 2000; Morlion 1998; Neri 2001; Pattanshetti 2009; Peng 2004; Pérez‐Bárcena 2008; Sahin 2007; Spittler 2001; Wang 2006; Yang 2008; Yao 2007; Zhao 2013; Zhou 2002; Zhou 2003; Zhou 2004) reported the outcome as mean (standard deviation or standard error of mean). Pooled analysis of these studies showed that the length of hospital stay was shorter in the glutamine supplementation group than in the control group (mean difference (MD) ‐4.14, 95% CI ‐5.46 to ‐2.81, P < 0.0001, I² = 70%). In the remaining 11 studies (Andrews 2011; Déchelotte 2006; Grau 2011; Hajdú 2012; Hall 2003; Heyland 2013; Kumar 2007; Kłek 2005; Ockenga 2002; Pérez‐Bárcena 2010; Powell‐Tuck 1999) the data were reported as the median (interquartile range) or median (range). The median (range) in each study was converted into mean (standard deviation) by using the formula from Hozo 2005, while the estimated standard deviation was calculated from the interquartile range based on the assumption that the width of the interquartile range was approximately 1.35 standard deviations (Handbook 2011). All 36 studies were included in the meta‐analysis after conversion and the results suggested that the length of hospital stay was shorter in the intervention group than in the control group (MD ‐3.46, 95% CI ‐4.61 to ‐2.32, P < 0.0001, I² = 63%) (Analysis 1.4). The funnel plot for this outcome was symmetrical.
1.4. Analysis.
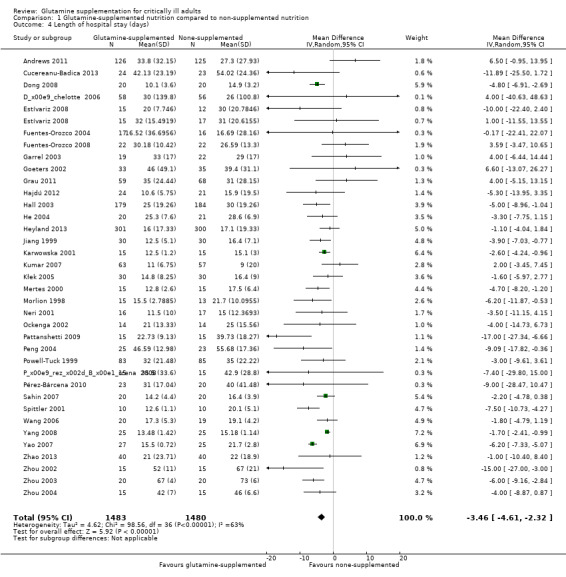
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 4 Length of hospital stay (days).
Sensitivity analysis, including eight low risk of bias studies (Andrews 2011; Déchelotte 2006; Dong 2008; Grau 2011; Hall 2003; Heyland 2013; Zhou 2003; Zhou 2004), also showed that glutamine supplementation had beneficial effects in reducing the length of hospital stay (MD ‐2.9, 95% CI ‐5.3 to ‐0.5, P = 0.02, I² = 58%).
The substantial heterogeneity between studies for this outcome may have been because these studies included participants with different categories of disease. Hence we did the following subgroup analysis according to types of diseases. Among the 36 trials, seven trials included burns patients (Cucereanu‐Badica 2013; Garrel 2003; Pattanshetti 2009; Peng 2004; Zhou 2002; Zhou 2003; Zhou 2004) and seven trials included acute pancreatitis patients (Fuentes‐Orozco 2008; Hajdú 2012; He 2004; Ockenga 2002; Sahin 2007; Yang 2008; Zhao 2013). Meta‐analysis of these two subgroups suggested beneficial effects of glutamine in reducing the length of hospital stay, with mild to moderate heterogeneity between studies (MD ‐7.24, 95% CI ‐11.28 to ‐3.21, P = 0.0004, I² = 49% for burn patients; MD ‐1.75, 95% CI ‐2.42 to ‐1.08, P < 0.00001, I² = 0% for acute pancreatitis patients). However, combining the data from nine trials that included critically ill patients other than burns and acute pancreatitis patients (Andrews 2011; Déchelotte 2006; Goeters 2002; Grau 2011; Hall 2003; Heyland 2013; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Powell‐Tuck 1999) revealed that there was no difference between the treatment and control groups, with moderate heterogeneity between studies (MD ‐1.16, 95% CI ‐4.05 to 1.72, P = 0.43, I² = 25%). A meta‐analysis of 13 trials including surgical patients (Dong 2008; Estívariz 2008; Fuentes‐Orozco 2004; Jiang 1999; Karwowska 2001; Kumar 2007; Kłek 2005; Mertes 2000; Morlion 1998; Neri 2001; Spittler 2001; Wang 2006; Yao 2007) showed that there was still substantial heterogeneity among these studies (MD ‐4.04, 95% CI ‐5.44 to ‐2.64, P < 0.00001, I² = 56%). This may have been because patients received different types of surgery in these studies.
Length of intensive care unit (ICU) stay
We analysed 22 studies (see Characteristics of included studies) which reported the length of stay in ICU. The data were combined using the fixed‐effect model and revealed slightly prolonged ICU stay in the glutamine supplemented group (MD 0.18, 95% CI 0.07 to 0.29, P = 0.002, I² = 11%) (Analysis 1.5). Using the random‐effects model there was no significant difference in ICU days between the treatment and control groups (MD ‐0.12, 95% CI ‐0.62 to 0.37, P = 0.63, I² = 11%). The funnel plot for this meta‐analysis was symmetrical.
1.5. Analysis.
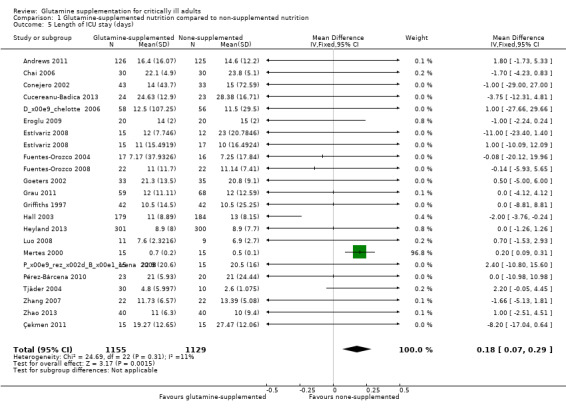
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 5 Length of ICU stay (days).
Of the 22 studies, eight studies (Andrews 2011; Conejero 2002; Déchelotte 2006; Grau 2011; Griffiths 1997; Hall 2003; Heyland 2013; Pérez‐Bárcena 2010) reported the days in ICU as the median (range) or median (interquartile range). They were changed into mean (standard deviation) as described above (Measures of treatment effect). A sensitivity analysis, excluding these studies by using the fixed‐effect model, reported the same result (MD 0.19, 95% CI 0.08 to 0.30, P = 0.001, I² = 22%). A sensitivity analysis including seven low risk of bias studies (Andrews 2011; Déchelotte 2006; Grau 2011; Griffiths 1997; Hall 2003; Heyland 2013; Çekmen 2011) and using the fixed‐effect model or the random‐effects model showed that glutamine supplementation had no effect on reducing the length of ICU stay (MD ‐0.54, 95% CI ‐1.48 to 0.4, P = 0.26, I² = 25%; MD ‐0.6, 95% CI ‐1.98 to 0.79, P = 0.40, I² = 25%, respectively).
Days on mechanical ventilation
Eleven studies with 455 patients (Chai 2006; Cucereanu‐Badica 2013; Eroglu 2009; Estívariz 2008; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; Garrel 2003; Heyland 2013; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Zhang 2007) reported this outcome as the mean (standard deviation). The combined MD of these studies was ‐1.54 days (95% CI ‐2.44 to ‐0.65, P = 0.0008, I² = 0%) suggesting that glutamine supplementation reduced the number of days on mechanical ventilation in critically ill and surgical patients. In three studies (Déchelotte 2006; Grau 2011; Heyland 2013) the original data were reported as the median (interquartile ranges) or median (range). These studies were included in the meta‐analysis after they were converted into the mean (standard deviation) and the combined MD of all 14 studies was ‐0.69 days (95% CI ‐1.37 to ‐0.02, P = 0.04, I² = 18%) (Analysis 1.6). The funnel plot for this outcome was symmetrical, suggesting no evidence of publication bias for this outcome.
1.6. Analysis.
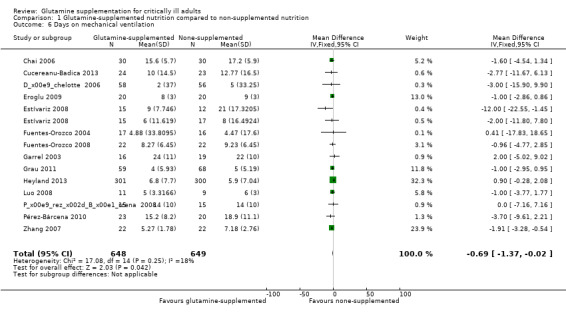
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 6 Days on mechanical ventilation.
Sensitivity analysis including three low risk of bias studies (Déchelotte 2006; Grau 2011; Heyland 2013) showed that glutamine supplementation did not reduce the number of days on mechanical ventilation (MD 0.37, 95% CI ‐0.64 to 1.38, P = 0.47, I² = 32%).
Side effects
Eleven studies (Estívariz 2008; Çekmen 2011; Goeters 2002; Grau 2011; Lin 2002; Morlion 1998; Neri 2001; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Yao 2007; Zhu 2000) reported that glutamine supplementation was well tolerated and no adverse events related to the administration of glutamine were observed. In two large sample, clinical trials (Andrews 2011; Heyland 2013) with 856 patients the serious adverse events outcome was reported and the pooled analysis of these two studies revealed that there was no significant difference in the risk of serious adverse events between the intervention and control groups (RR 1.40, 95% CI 0.77 to 2.57, P = 0.27, I² = 0%) (Analysis 1.7). In one trial (Heyland 2013) it was also found that the frequency of high urea levels (> 50 mmol/L) was higher among patients who received glutamine than for those that did not (13.4% versus 4.0%, P < 0.001). However, meta‐analysis of biological markers of liver and kidney function, such as alanine aminotransferase (Analysis 1.8), aspartate aminotransferase (Analysis 1.9) and creatinine (Analysis 1.10), did not find any difference between the groups during the treatment period. A further three studies reported on side effects. Two studies (Conejero 2002; Hall 2003) that supplemented glutamine enterally in critical ill patients reported gastrointestinal complications. The incidence of gastrointestinal complications was similar between the two groups. One study (Déchelotte 2006) of parenterally supplemented glutamine for ICU patients found that the number of adverse events per patient was significantly lower in the alanyl‐glutamine group than in the placebo group (2.0 versus 2.8, P < 0 .01).
1.7. Analysis.
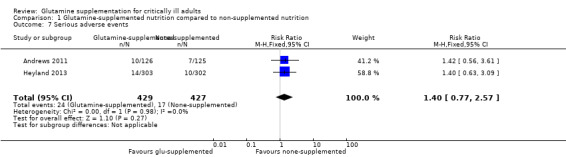
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 7 Serious adverse events.
1.8. Analysis.
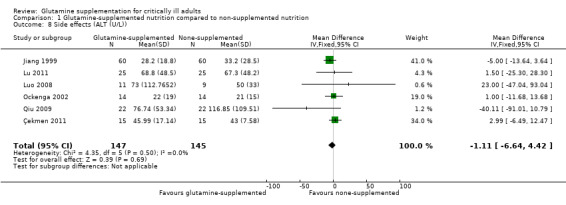
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 8 Side effects (ALT (U/L)).
1.9. Analysis.
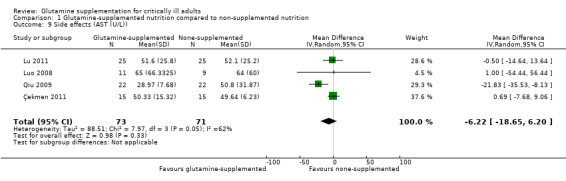
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 9 Side effects (AST (U/L)).
1.10. Analysis.
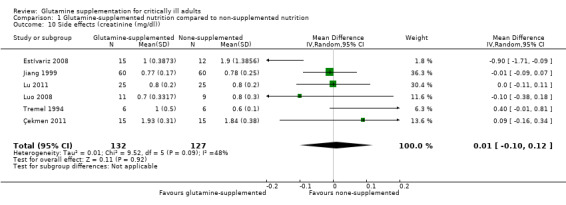
Comparison 1 Glutamine‐supplemented nutrition compared to non‐supplemented nutrition, Outcome 10 Side effects (creatinine (mg/dl)).
Quality of life
This outcome was recorded in four studies. One study (Griffiths 1997) used the Whiston Health Questionnaire (Jones 1993) to assess patients' pre‐morbid health status and found that the morbidity of the participants at six months was similar between the glutamine supplemented and control groups. One study (Hall 2003) described the functional status of surviving patients at six months, and no differences were detected between the two groups. In one study (Powell‐Tuck 1999) patients were asked to score their mood, sleep, energy, appetite, pain, and mobilization on a 10‐point scale with 0 = 'perfect' and 10 = 'as bad as it can be'. No statistical difference was found between the intervention and control groups at the end of the treatment. In another study (Andrews 2011) quality of life measures (SF‐12 and EQ‐5D questionnaires) were collected at three and six months by post; the results have not been published.
Discussion
The results of our meta‐analysis provided moderate evidence that glutamine supplementation could reduce the infection rate and days on mechanical ventilation, and low quality evidence that glutamine supplementation reduced length of hospital stay in critically ill or surgical participants. However, the differences in risk of mortality, length of ICU stay and quality of life were not significant between the intervention and control groups. According to the results of predefined subgroup analyses, our review also suggests that the effects of glutamine supplementation in reducing the risk of infectious complications and mortality were not different between elective surgical patients and critically ill patients, patients receiving enteral supplementation and parenteral supplementation, and patients with malignant and non‐malignant diseases respectively.
Enteral nutrition is the preferred way of feeding the critically ill patient (Kreymann 2006), however our review suggested that parenteral glutamine had the same effect in reducing the risk of infectious complications and mortality in critically ill patients. Considering the biologically plausible reasons for this result, there is a clinical study suggesting that the intestinal absorption of glutamine was impaired during critically ill conditions (Salloum 1991). Still, in a clinical trial in which glutamine was enterally supplemented at a dose of 26 g/day in burns patients it was found that there was no difference in plasma glutamine levels between the supplemented and non‐supplemented groups, suggesting that most of the glutamine that is administered is consumed by the gut. The immune system outside the gut would not have been affected by the glutamine supplementation (Garrel 2003).
Several meta‐analyses had been undertaken to examine the effects of perioperative glutamine supplementation in surgical patients (Wang 2010; Yue 2013; Zheng 2006). These meta‐analyses all found that perioperative glutamine supplementation is safe in reducing the morbidity of postoperative infectious complications in surgical patients. In this review we have included 18 studies on surgical patients. For the short‐ and long‐term mortality outcomes, deaths were reported in only one trial for each (Estívariz 2008; Qiu 2009). This prohibited further analysis. For infectious complications, our subgroup analysis revealed that glutamine supplementation had beneficial effects in reducing the risk of infection in both critically ill and surgical participants. This result suggests that participants with critical illness or undergoing elective major surgery may have similar glutamine deficiencies. Further research focusing on the different effects of glutamine supplementation in these two groups of patients may not be required.
In this systematic review we also predefined a subgroup analysis to compare the treatment effects of glutamine in participants with malignant and non‐malignant disease. Cancer patients included in this meta‐analysis were all surgical patients, hence we found that the results in cancer patients were the same as those in surgical patients. Still, the studies that we included mostly focused on non‐oncology or mixed disease patients. Few trials have focused on oncology patients but their results showed an infection benefit in this population. No conclusions can be drawn on the differences in risk of infection and death between oncology and non‐oncology patients given the limited data available. There is still a lack of large, quality trials that focus on oncology patients.
Summary of main results
This meta‐analysis found moderate evidence that glutamine supplementation could reduce the infection rate and days on mechanical ventilation in critically ill or surgical participants. However, it seems to have little or no effect on the risk of mortality and length of ICU stay. Although there is substantial heterogeneity within the included studies, our review also suggests that glutamine supplementation may reduce the length of hospital stay in these participants (Table 1).
Overall completeness and applicability of evidence
We investigated 53 controlled clinical trials that met our predefined inclusion criteria for the participants, intervention and outcomes. The pre‐specified objectives and outcomes of the review are sufficiently addressed, except for the quality of life outcome. Quality of life was only descriptively analysed in this review as there were few included studies that reported on this outcome, and they used different measurement scales.
In recent guidelines on nutrition support, parenteral supplementation with glutamine was supported in critically ill patients, while enteral glutamine supplementation in surgical or critically ill patients was not recommended because of lack of evidence (Clinical Evaluation Research Unit 2013; Kreymann 2006; Singer 2009). Our review further classified and analysed this evidence and will provide guidance for clinical nutrition therapy.
Quality of the evidence
We identified 10 low risk of bias studies (Andrews 2011; Çekmen 2011; Déchelotte 2006; Dong 2008; Grau 2011; Griffiths 1997; Hall 2003; Heyland 2013; Zhou 2003; Zhou 2004); 16 high risk of bias studies (Conejero 2002; Estívariz 2008; Garrel 2003; Goeters 2002; Hajdú 2012; Kłek 2005; Kumar 2007; Luo 2008; Mertes 2000; Pattanshetti 2009; Pérez‐Bárcena 2008; Pérez‐Bárcena 2010; Qiu 2009; Tjäder 2004; Wernerman 2011; Yang 2008); and 27 studies with an unclear risk of bias (Chai 2006; Cucereanu‐Badica 2013; Eroglu 2009; Fuentes‐Orozco 2004; Fuentes‐Orozco 2008; He 2004; Jiang 1999; Karwowska 2001; Lin 2002; Lu 2011; Morlion 1998; Neri 2001; Ockenga 2002; O'Riordain 1994; Peng 2004; Powell‐Tuck 1999; Sahin 2007; Spittler 2001; Tian 2006; Tremel 1994; Wang 2006; Wischmeyer 2001; Yao 2007; Zhang 2007; Zhao 2013; Zhou 2002; Zhu 2000).
Potential biases in the review process
Firstly, the risk of overall bias in our review is probably high as four‐fifths of the included studies were judged as having unclear or high risk of bias (Figure 2; Figure 3). Secondly, we converted continuous outcomes that were reported as the median (range) or median (interquartile range) into the mean (SD), this will introduce bias in the final results of the meta‐analysis. Thirdly, although efforts were made to retrieve all relevant trials, there is still one article that is awaiting classification (Hallay 2002). Fourthly, the meta‐analysis of infection and short‐term mortality outcomes included studies with no events in one arm and excluded studies where there were no events in both arms, this may introduce bias in the combined treatment effect estimates. Fifthly, funnel plots for infection and short‐term mortality suggest that smaller studies with outcomes favouring non‐supplemented patients appear to be lacking, our analysis is therefore at risk of publication bias. Finally, we could not include the detailed infectious complications results from Heyland's study (Heyland 2013), and there is time lag between the search and publication of the review dates; they are likely to introduce bias into the review process.
Agreements and disagreements with other studies or reviews
The results of our review are comparable to previous meta‐analyses (Avenell 2006; Avenell 2009; Bollhalder 2013; Novak 2002). There are several differences between our review and the other published reviews. First, we included recently published RCTs (Andrews 2011; Heyland 2013) and more clinical trials were analysed with the rigorous Cochrane methods in our review. Second, we added side effects and quality of life as our secondary outcomes. Third, we undertook subgroup analysis to investigate the heterogeneity between patients with malignant and non‐malignant disease.
In this review we observed a significant effect on infection complications, which is in contrast with the results from two recently published large‐scale, high quality RCTs (Andrews 2011; Heyland 2013) that did not find any beneficial effects of glutamine supplementation for reducing the rates of mortality and infection complications in critically ill patients. On the one hand, this may have been due to the fact that we could not include in this review the detailed infectious complications reported in the Heyland study (Heyland 2013). We will include this study in the updated review when the results are published. On the other hand, the uniqueness and limitations of these two studies should be noted. Heyland's study (Heyland 2013) was distinct from other traditional glutamine trials supplementing complete parenteral nutrition in that it administered glutamine (and antioxidants) independent of any nutrition support. The average nutritional delivery for the entire study period was about 50% of the prescribed delivery, so not meeting the prescribed patient needs. In addition, more then 30% of patients in the Heyland study were found to have baseline renal failure at admission, which universally was an exclusion criterion in other traditional nutrition‐based glutamine trials. Further, all patients that were enrolled were in multi‐system organ failure at enrolment, which was an exclusion criterion in most of the previous trials using parenteral glutamine as a supplement to parenteral nutrition. Hence the Heyland study may indicate that glutamine should not be supplemented in patients early in the acute phase of critical illness, when patients are shown to have multiple organ failure or to be in unresuscitated shock requiring significant vasopressor support; and patients with renal failure who are not receiving dialysis should receive supplemental glutamine cautiously. In the Andrews trial (Andrews 2011) concerns including lack of availability of some key data regarding missing values, dropouts, protocol violations and complete follow‐up data have been raised. The actual values for the glutamine doses based on body weight have not been published and this should be noted.
Authors' conclusions
Implications for practice.
There is moderate evidence that glutamine supplementation could reduce the infection rate and days on mechanical ventilation, and low quality evidence that glutamine supplementation reduced the length of hospital stay for critically ill or surgical participants. However, it has no effect on the risk of mortality, length of ICU stay and risk of side effects in these participants. Subgroup analysis of infectious complications and mortality outcomes did not find any statistically significant difference between the predefined groups (elective surgical patients and critically ill patients, patients with enteral glutamine supplementation only and parenteral glutamine supplementation only, patients with malignant and non‐malignant disease).
Implications for research.
The strength of evidence in our review was reduced as there was a high risk of overall bias, suspected publication bias and moderate to substantial heterogeneity within the included studies. Future clinical trials on glutamine supplementation should be conducted strictly in accordance with the principles advised by the Consolidated Standards of Reporting Trials Statement (CONSORT) to minimize the overall risk of bias in trials. Regarding the heterogeneity between the included studies, it is possible that glutamine's beneficial effects correlate with the types and severity of the disease. Hence future high quality RCTs should focus on the differences in the glutamine supplementation effects in critically ill patients with different characteristics. In this review no conclusions have been drawn on the difference in risk of infection and death between oncology and non‐oncology patients as very few trials have focused on this subject as a primary aim. Oncology patients are theoretically more likely to be glutamine deficient and thus large, quality trials that focus on oncology patients are still needed.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We thank Dr Chun‐bo Li for his help with statistics and advice on the methods section. We also would like to thank Jane Cracknell (Managing Editor of CARG), Harald Herkner (content and statistical editor), Ronald L Koretz, Richard D Griffiths, Daren Heyland (peer reviewers) and Ann Fonfa (consumer) for their help and editorial advice during the preparation of the protocol for the systematic review.
We would like to thank Harald Herkner (content editor), Cathal Walsh (statistical editor), Thomas Bongers, Philip C Calder, Paul Wischmeyer (peer reviewers) and Robert Wyllie (consumer referee) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Glutamine explode all trees #2 MeSH descriptor Dipeptides explode all trees #3 glutamin* or dipeptid* or L?glutamin* #4 (#1 OR #2 OR #3) #5 MeSH descriptor Infection explode all trees #6 MeSH descriptor Critical Illness explode all trees #7 (critical* near ill*):ti,ab or supplement* or infect*:ti,ab #8 (#5 OR #6 OR #7) #9 (#4 AND #8)
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. (glutamin* or dipeptid* or L?glutamin*).mp. or exp Glutamine/ or Dipeptides/ 2. exp Infection/ or exp Critical Illness/ or (critical* adj3 ill*).ti,ab. or supplement*.mp. or infect*.ti,ab. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. EMBASE (OvidSP) search strategy
1 (glutamin* or dipeptid* or l?glutamin*).ti,ab. or glutamine/ or dipeptide/ 2 infection/ or critical illness/ or (critical* adj3 ill*).ti,ab. or supplement*.mp. or infect*.ti,ab. 3 1 and 2 4 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 5 3 and 4
Appendix 4. ISI Web of Science search strategy
#1 TS=(glutamin* or dipeptid* or L?glutamin*) #2 TS=(critical* SAME ill*) or TS=supplement* or TI=infect* #3 TS=(random* or (trial* SAME (clinical or controlled))) or TS=(prospective or multicenter or placebo*) or TS=((blind* or mask*) SAME (single or double or triple or treble)) #4 #1 or #2 or #3
Appendix 5. Study characteristics, quality assessment and data extraction form
Study characteristics
| Trial characteristics | |
| First author/year | |
| Journal/conference proceedings | |
| Single centre/multicentre | |
| RCT/quasi/CCT | |
| Participants | |
| Location | |
| Period of study | |
| Age (mean, median, range, etc.) | |
| Disease status/type, etc. | |
| Inclusion criteria | |
| Exclusion criteria | |
| Interventions | |
| Timing of intervention | |
| Groups | |
| Assessed | |
| Outcomes | |
| Length of follow‐up | |
| Main outcomes | |
| Other outcomes | |
| Other | |
Methodological quality assessment
| Random sequence generation (selection bias) | |
| State here reasons for grading | Risk of bias |
| |
Low |
| High | |
| Unclear | |
| Allocation concealment (selection bias) | |
| State here reasons for grading | Risk of bias |
| Low | |
| High | |
| Unclear | |
| Incomplete outcome data (attrition bias) | |
| State here reasons for grading | Risk of bias |
| Low | |
| High | |
| Unclear | |
| Selective reporting (reporting bias) | |
| State here reasons for grading | Risk of bias |
| Low | |
| High | |
| Unclear | |
| Blinding of participants and personnel (performance bias) | |
| State here reasons for grading | Risk of bias |
| Low | |
| High | |
| Unclear | |
| Blinding of outcome assessment (detection bias) | |
| State here reasons for grading | Risk of bias |
| Low | |
| High | |
| Unclear | |
| Other bias | |
| State here reasons for grading | Risk of bias |
| Low | |
| High | |
| Unclear | |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
| Other | |
Data extraction form
| Outcomes relevant to your review | Reported in paper | Subgroups | |
| Primary outcomes | 1. Critically ill patients 2. Surgical patients 3. Enteral glutamine supplementation 4. Parenteral glutamine supplementation 5. Patients with malignant disease 6. Patients with non‐malignant disease |
||
| Outcome 1: Number of infectious complications | Yes/No | ||
| Outcome 2: Mortality | Yes/No | ||
| Secondary outcomes | |||
| Outcome 3: Length of stay in hospital | Yes/No | ||
| Outcome 4: Length of ICU stay | Yes/No | ||
| Outcome 5: Days on mechanical ventilation | Yes/No | ||
| Outcome 6: Side effects | Yes/No | ||
| Outcome 7: Quality of life outcome | Yes/No | ||
| Other | |||
| For continuous data | |||
| Outcomes | Intervention group | Control group | Details if outcome only described in text |
| N/Mean (SD) | N/Mean (SD) | ||
| Outcome 3: Length of stay in hospital | |||
| Outcome 4: Length of ICU stay | |||
| Outcome 5: Days on mechanical ventilation | |||
| For dichotomous data | |||
| Outcomes | Intervention group | Control group | Details if outcome only described in text |
| n/N | n/N | ||
| Outcome 1: Number of infectious complications | |||
| Outcome 2: Mortality | |||
| Outcome 6: Side effects | |||
| Outcome 7: Quality of life outcome | |||
| Other information which you feel is relevant to the results | |||
Data and analyses
Comparison 1. Glutamine‐supplemented nutrition compared to non‐supplemented nutrition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infectious complications | 33 | 2303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.71, 0.87] |
| 2 Short‐term mortality (within hospital stay or ≤1 month) | 36 | 3454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.02] |
| 3 Long‐term mortality (≥6 month) | 11 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 4 Length of hospital stay (days) | 36 | 2963 | Mean Difference (IV, Random, 95% CI) | ‐3.46 [‐4.61, ‐2.32] |
| 5 Length of ICU stay (days) | 22 | 2284 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.07, 0.29] |
| 6 Days on mechanical ventilation | 14 | 1297 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.37, ‐0.02] |
| 7 Serious adverse events | 2 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.77, 2.57] |
| 8 Side effects (ALT (U/L)) | 6 | 292 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐6.64, 4.42] |
| 9 Side effects (AST (U/L)) | 4 | 144 | Mean Difference (IV, Random, 95% CI) | ‐6.22 [‐18.65, 6.20] |
| 10 Side effects (creatinine (mg/dl)) | 6 | 259 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
Comparison 2. Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (elective surgical patients versus critically ill patients).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infectious complications | 33 | 2303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.71, 0.87] |
| 1.1 Critically ill patients | 22 | 1847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.73, 0.91] |
| 1.2 Elective surgical patients | 11 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.86] |
| 2 Short‐term mortality (within hospital stay or ≤1 month) | 36 | 3454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.02] |
| 2.1 Critically ill patients | 29 | 3157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 2.2 Elective surgical patients | 7 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.26] |
| 3 Long‐term mortality (≥6 month) | 11 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 3.1 Critically ill patients | 10 | 2233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 3.2 Elective surgical patients | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.12] |
Comparison 3. Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (parenteral glutamine versus enteral glutamine supplementation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infectious complications | 33 | 2303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.71, 0.87] |
| 1.1 Parenteral glutamine supplementation only | 28 | 1651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.69, 0.87] |
| 1.2 Enteral glutamine supplementation only | 5 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.02] |
| 2 Short‐term mortality (within hospital stay or ≤1 month) | 36 | 3454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.02] |
| 2.1 Parenteral glutamine supplementation only | 30 | 2207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.63, 0.90] |
| 2.2 Enteral glutamine supplementation only | 5 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.30] |
| 2.3 Parenteral and enteral glutamine supplementation | 1 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.99, 1.64] |
| 3 Long‐term mortality (≥6 month) | 11 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 3.1 Parenteral glutamine supplementation only | 9 | 1309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.08] |
| 3.2 Enteral glutamine supplementation only | 1 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.49] |
| 3.3 Parenteral and enteral glutamine supplementation | 1 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.93, 1.40] |
Comparison 4. Glutamine‐supplemented nutrition compared to non‐supplemented nutrition (patients with malignant disease versus non‐malignant disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infectious complications | 33 | 2303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.71, 0.87] |
| 1.1 Patients with malignant disease | 6 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.90] |
| 1.2 Patients with non‐malignant disease | 17 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.78] |
| 1.3 Studies with uncertain or mixed disease patients | 10 | 1293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 2 Short‐term mortality (within hospital stay or ≤1 month) | 36 | 3454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.02] |
| 2.1 Patients with malignant disease | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Patients with non‐malignant disease | 19 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.79] |
| 2.3 Studies with uncertain or mixed disease patients | 14 | 2413 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 3 Long‐term mortality (≥6 month) | 11 | 2277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 3.1 Patients with non‐malignant disease | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 1.00] |
| 3.2 Studies with uncertain or mixed disease patients | 9 | 2153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrews 2011.
| Methods | Randomized, double‐blinded, factorial, controlled trial | |
| Participants | 502 adults in intensive care units and high dependency units for ≥48 hours, with gastrointestinal failure and requiring parenteral nutrition | |
| Interventions | Parenteral glutamine (20.2 g/day) or selenium (500 μg/day), or both, for up to seven days. Group A (n=125) standard formulation (12.5 g nitrogen, 2000 kcal) Group B (n=126) glutamine formulation (12.5 g nitrogen (including 20.2 g glutamine), 2000 kcal) Group C (n=127) standard formulation with addition of 500 μg selenium Group D (n=124) glutamine formulation with addition of 500 μg selenium |
|
| Outcomes | 1. Primary outcomes were participants with new infections in the first 14 days, and mortality 2. Secondary outcomes included critical care unit and acute hospital lengths of stay, days of antibiotic use, and modified SOFA (sepsis related organ failure assessment) score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A remote telephone computer system generated random allocation to one of four possible groups with minimisation on trial centre, age (<65 or ≥65 years), sex, patient group (medical or surgical, including trauma), and nutritional status (undernourished, normal, or obese by subjective clinical assessment) |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, clinicians, and investigators were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Chai 2006.
| Methods | Prospective randomized and controlled clinical study | |
| Participants | 90 critically ill aged patients | |
| Interventions | Subjects were divided into 3 groups Group A (standard nutrition support, n=30) Group B (standard nutrition support + 10%Gln 100 ml/d, n=30), Group C (standard nutrition support + GIn 100 ml/d+rhGH 10 U/d, n=30), for up to 14 days |
|
| Outcomes | 1. Albumin,pre‐albumin, C‐reactive protein (CRP) immunoglobulin G (IgG ), T‐cell subsets, CD14 human leukocyte antigen (locus) DR (CD14 HLA‐DR), and total lymphocytes 2. The changes in acute physiology and chronic health evaluation (APACHE), multiple organ dysfunction syndrome (MODS) scores, the duration of intensive care unit (ICU) stay and mechanical ventilation, and 28‐day survival rate |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | Subjects were randomly divided into 3 groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Conejero 2002.
| Methods | Prospective, randomized, single‐blind, multicentre trial | |
| Participants | 84 patients with systemic inflammatory response syndrome of any etiology | |
| Interventions | Subjects were randomly allocated to receive a glutamine‐enriched enteral diet or a control diet without glutamine for at least 7 days | |
| Outcomes | 1. Nosocomial infections rate during ICU stay or after 28 d of treatment 2. ICU mortality and ICU length of stay, intestinal permeability, APACHE II score, and multiple organ failure also were recorded |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers table, with stratification according to centres |
| Allocation concealment (selection bias) | Low risk | A sequence of 12 numbered, opaque, sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eight patients died or were moved to other hospitals in the first 48 hours after admission and were not eligible for analysis |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators remained blinded to treatment group for the diagnosis of nosocomial infection, statistical analysis, and the final number of patients recruited until the end of the study |
Cucereanu‐Badica 2013.
| Methods | Randomized, double‐blind, placebo‐controlled study | |
| Participants | 59 severely burned patients | |
| Interventions | Patients from the study group received glutamine 0.5 g/kgbw/day for at least 24 consecutive days. The patients from the control group received an isonitrogenous standard amino acid solution in continuous infusion during the same period of the day | |
| Outcomes | 1. Infectious complications 2. Ventilation‐dependent days, the ICU stay and hospital stay, morbidity and mortality 3. Ammonia and liver enzymes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | Patients were participants at the study being randomized in 2 groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients included in the study were assigned in a double‐blind manner to one of the two groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
Dong 2008.
| Methods | Controlled clinical study | |
| Participants | 40 patients after total gastrectomy | |
| Interventions | Subjects were divided into the study group (Ala‐Gln 0.5 g/kg/day, n = 20) or control group (n = 20) for 6 day | |
| Outcomes | 1. Infectious complications,mortality and hospitalization duration 2. Plasma levels of CD4 and CD4/CD8, tumour necrosis factor alpha, soluble interleukin‐2 receptor, C‐reactive protein |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
Déchelotte 2006.
| Methods | Prospective, double‐blind, controlled, randomized trial | |
| Participants | 114 ICU patients admitted for multiple trauma, complicated surgery, or pancreatitis | |
| Interventions | Patients receive isocaloric isonitrogenous TPN via a central venous catheter providing 37.5 kcal and 1.5 g amino acids/kg/day at least 5 days Ala‐Gln group, 58 patients supplemented with either L‐alanyl‐L‐glutamine dipeptide (0.5 g/kg/day) Control group, 56 patients supplemented with L‐alanine L‐proline |
|
| Outcomes | 1.Primary outcome variable was the occurrence of a major complication over the duration of parenteral nutrition feeding ICU and total hospital, the TISS score 2.Secondary variables were length of stay (LOS) and 6‐month mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study medications were randomly assigned to the patient numbers at the study sites and were randomized on a 1:1 basis, by means of a computer program |
| Allocation concealment (selection bias) | Low risk | The randomization envelopes were held by the pharmacist and were assigned in strict chronological ascending order within each treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 of 114 patients (6, Ala‐Gln; 4, control) only received 4 days of TPN because of adverse events or other reasons were included in the Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All patients, investigators, and coworkers were unaware of treatment allocation and remained blinded to the treatment allocation until the final statistical evaluation was completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Eroglu 2009.
| Methods | Randomized controlled trial | |
| Participants | 40 adult patients with severe trauma according to the Injury Severity Score >20 | |
| Interventions | The patients were assigned to 2 groups: Group G received 0.5 g/kg/d of alanyl‐glutamine dipeptide supplementation IV, and Group C received a control solution without alanyl‐glutamine for 7 days |
|
| Outcomes | 1. Total plasma glutathione levels 2. Number of days on mechanical ventilation, total ICU length of stay, 1 month mortality rate, and complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to two groups using sealed, shuffled, numbered, and opaque envelopes |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Estívariz 2008.
| Methods | Randomized double‐blinded controlled study | |
| Participants | 63 adult patients requiring parenteral nutrition and SICU care after surgery for pancreatic necrosis, cardiac, vascular, or colonic surgery | |
| Interventions | All subjects received isocaloric/isonitrogenous parenteral nutrition plus: 31 subjects providing 1.5 g/kg/d standard glutamine free amino acids (STD‐PN) 32 subjects providing 1.0 g/kg/d standard amino acids + 0.5 g/kg/d glutamine dipeptide (GLN‐PN) for at least 5 days |
|
| Outcomes | Primary endpoint: 1. Total number of new nosocomial infections diagnosed during the entire hospitalization after initiation of study PN and the presumed causative micro‐organisms Secondary endpoints: 1. The site of infection and the number of patients affected 2. Number of patients with multiple hospital infections 3. Days on mechanical ventilation after entry 4. Development of acute respiratory distress syndrome (ARDS) after entry 5. SICU and hospital LOS 6. Hospital mortality 7. Antibiotic days during the hospitalization 8. Metabolic endpoints:blood glucose, serum creatinine, total bilirubin, alkaline phosphatase, and transaminase concentrations, maximal blood ammonia concentration, change in lymphocyte subset number, change in plasma GLN and glutamate (GLU) concentrations, D‐xylose absorption |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Low risk | The subjects were block‐randomized to 2 groups by the EUH Investigational Drug Service research pharmacist on the basis of type of operation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 subjects (2 control STD‐PN and 2 GLN‐PN‐treated) excluded from the main outcome analysis because they did not receive the required 5 days of study PN |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ''Double blind", the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No details available, as an interim analysis was performed in a blinded fashion by the General Clinical Research Center statistician (GAC) on the initial 30 subjects,the outcome measurement is not likely to be influenced by lack of blinding |
Fuentes‐Orozco 2004.
| Methods | Randomized, double‐blind, controlled clinical trial | |
| Participants | 33 patients with diagnosis of secondary peritonitis who required TPN were enrolled | |
| Interventions | Two TPN formulae were isonitrogenous and isocaloric, which commenced the morning after surgery and ran continuously for 10 consecutive days 1. Control group (n=16) received standard TPN 2. Treatment group (n=17) was given L‐alanyl‐L‐glutamine, 0.40 g/kg/d |
|
| Outcomes | Infectious morbidity, hospital and intensive care unit (ICU) stays, mortality, nitrogen balance, leukocytes, lymphocytes, subpopulations CD4 and CD8, immunoglobulin A (IgA), total proteins, and albumin were evaluated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients satisfying the enrolment criteria received an ascending serial number in the order of their enrollment.Based on the serial number, the patients were assigned to one of the study groups |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The preparation of double‐blind solutions was conducted with the help of the pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators involved with the analysis, the patients, and their relatives remained blind to the randomization until the 10‐day follow‐up had been completed for all subjects |
Fuentes‐Orozco 2008.
| Methods | Randomized double‐blinded controlled study | |
| Participants | 44 patients with severe acute pancreatitis were admitted | |
| Interventions | 2 groups received isocaloric/isonitrogenous parenteral nutrition for 10 days The control group (n=22) received standard PN, 30 kcal/kg per day The treatment group (n=22) was given standard PN plus 0.4 g/kg of L‐glutamine per day |
|
| Outcomes | Primary efficacy variables: 1. Infectious morbidity 2. Nitrogen balance 3. Counts of leucocytes, lymphocytes and CD4 and CD8 subpopulation, and levels of IgA,total protein, albumin, CRP, IL‐6, and IL‐10 Secondary efficacy variables: 1. Durations of hospital stay 2. ICU stay 3. Ventilatory support 4. The incidence of mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients satisfying the enrolment criteria received a serial number allocated in ascending order of enrolment, based on the serial number, the patients were assigned to one of the study groups |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The preparation of double‐blind solutions was conducted with the help of the pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators involved with the analysis, the patients, and their relatives remained blind to the randomization until the 10‐day follow‐up had been completed for all subjects |
Garrel 2003.
| Methods | Double‐blinded, randomized clinical trial | |
| Participants | 45 adults with severe burns | |
| Interventions | Patients were randomized to receive either glutamine (26 g/day) or an isonitrogenous control mixture until complete healing occurred | |
| Outcomes | 1. Length of care, incidence of positive blood culture, and mortality were recorded 2. Phagocytosis by circulating polymorphonuclear cells was measured every 3 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table with stratification of burn severity |
| Allocation concealment (selection bias) | Low risk | Patients admitted to our burns centre were assigned to one of two groups according to a concealed randomization table, so that the study investigators were not aware in advance to which group the patients would be assigned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 45 randomized patients, 3 died within 72 hrs and 1 could not receive enteral nutrition and amino acid supplements for the first 10 days were excluded from the analyses, with the exception of analysis of mortality rate |
| Selective reporting (reporting bias) | Low risk | Reported all predefined outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blind to patients, trial conductors and assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Goeters 2002.
| Methods | Prospective, open randomized trial | |
| Participants | 144 critically ill patients with indications for parenteral nutrition and an expected stay on intensive care unit for >5 days | |
| Interventions | Patients were randomized to receive either standard parenteral nutrition or supplemented parenteral nutrition with L‐alanyl‐L‐glutamine (0.3 g·kg/body weight/day) | |
| Outcomes | Average length of stay in the intensive care unit and hospital and the mortality in the intensive care unit and within 30 days and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients admitted to the ICU were randomized to receive standard parenteral nutritional support or an Ala‐Gln‐supplemented treatment |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The patients not completing nutrition protocol for 5 days (49 of 144 patients) were excluded from analysis due to secondary exclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | Only reported average length of stay and mortality in patients with EN >9 days |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | An open study |
Grau 2011.
| Methods | Multicentre, prospective, double‐blind, randomized trial | |
| Participants | 127 patients with Acute Physiology and Chronic Health Evaluation II score >12 and requiring parenteral nutrition for 5‐9 days | |
| Interventions | The study group received 0.5 g/kg/d of glutamine dipeptide and the control total parenteral nutrition group a similar amount of amino acids | |
| Outcomes | 1. The primary outcome was the incidence of nosocomial infections acquired throughout the stay in ICU in patients who received at least 4 days of treatment 2. Other secondary endpoints were: days of stay in the ICU and hospital, overall all‐cause mortality during the ICU stay, and 6‐month mortality. Hyperglycaemia and insulin needs were also analysed to assess the effect of glutamine on insulin resistance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized to receive either Ala‐Gln TPN or control TPN. Allocation to treatment was done with a computer generated random numbers table with a minimization program and two stratification levels |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five patients were lost after randomization, intention‐to‐treat analyses were performed |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Insufficient information for judgement, but the outcome is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators remained blinded until the end of the study for treatment group, diagnosis of nosocomial infection, statistical analysis, and the final number of patients recruited |
Griffiths 1997.
| Methods | Prospective, block‐randomized, double‐blind treatment study design | |
| Participants | 84 critically ill adult patients requiring nutritional support received PN only if enteral nutrition was contraindicated or unsuccessful | |
| Interventions | Gin‐containing parenteral nutrition (25g L‐Gln/day, n=42) compared with an isonitrogenous, isoenergetic control feed (n=42) | |
| Outcomes | Morbidity, mortality, and cost at 6 months postintervention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by members of the pharmacy department, who were blind to the clinical aspects of the study |
| Allocation concealment (selection bias) | Low risk | Patients were block‐randomized into treatment and control groups by a closed‐envelope technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators involved with analysis, the patients, and their relatives remained blind to the randomization code until the 6 month post‐ICU follow‐up had been completed for all subjects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Hajdú 2012.
| Methods | Prospective randomized clinical study | |
| Participants | 61 patients with severe acute pancreatitis | |
| Interventions | Glutamine was given 0.5 g/kg/day intravenously, while the control group received normal amino acid solution in the same quantity for 7 days | |
| Outcomes | Primary endpoints: the rate of pancreas‐specific infectious complications and organ failure Secondary endpoints: the necessity for radiological and surgical interventions, length of hospital stay and mortality rate |
|
| Notes | Article in Hungarian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled randomisation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 patients after randomization were lost for different reasons, ITT analysis was not applied |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and study personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
Hall 2003.
| Methods | Two‐armed, triple‐blind clinical trial | |
| Participants | 363 adults admitted to the ICU and received enteral nutritional support (median duration=10 days) | |
| Interventions | The intervention solution contained 20 g/L glutamine and the control solution was isojoulic and isonitrogenous | |
| Outcomes | Main study endpoints were: (a) death within 6 months of discharge from ICU (b) severe sepsis incidence Secondary endpoints were: 1. the length of hospital stay, 2. positive microbiological cultures, 3. the use of antimicrobial agents, 4. the functional status of surviving patients at 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Concealment was achieved by placing the group allocation into opaque and serially numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, attending staff, research nurse were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
He 2004.
| Methods | Clinical study | |
| Participants | 78 patients with serious acute pancreatitis | |
| Interventions | 1. The patients in group I (n = 23) were treated by the traditional conservative therapy 2. Combined with traditional conservative therapy, 21 patients in group II and 20 cases in group III were treated by isocaloric (104.5 kJ/kg/d) and isonitrogenous (0.2 g/kg/d) TPN, and in group III with additional glutamine dipeptide supplementation (alanyl‐glutamine 0.4 g/kg/d) for at least 2 weeks |
|
| Outcomes | The concentration of serum albumin, recovery time of blood amylase, the incidence of complications, mortality, length of hospital stay (LOS) and body mass of the patients in each group were monitored | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly divided into three therapeutic groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three patients (one from group II, and two from group III) were excluded from analysis due to medical reasons |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Heyland 2013.
| Methods | Multicentre, blinded 2 by 2 factorial randomized controlled trial | |
| Participants | 1223 critically ill adults in 40 intensive care units who had multiorgan failure and were receiving mechanical ventilation | |
| Interventions | Group 1 received glutamine supplementation (0.35g/kg/day intravenously and 30 g of glutamine, per day given enterally) Group 2 received 500 μg of selenium intravenously plus the vitamins and minerals enterally Group 3 received both antioxidants and glutamine supplementation Group 4 received placebo intravenously plus placebo enterally for a maximum of 28 days |
|
| Outcomes | Primary outcome was 28‐day mortality Secondary outcomes included length of ICU stay, development of infectious complications, multiple organ dysfunction using sequential organ failure assessment scores, duration of mechanical ventilation, hospital length of stay, antibiotic use, and survival up to 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A centralized randomization system used to allocate patients to study treatments |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple‐blinded |
Jiang 1999.
| Methods | Randomized, double‐blind, controlled, parallel multicentre clinical trial | |
| Participants | Patients undergoing major abdominal surgery | |
| Interventions | The study group (n= 30) received Ala‐Gln 0.50 g/kg per day,while control group (n= 30) received isonitrogenous (0.20 g/kg body wt per day) and isocaloric (30 kcal/kg body wt per day) parenteral nutrition without glutamine supplementation for 6 days | |
| Outcomes | 1. Clinical chemistry variables, plasma amino acids profile, nitrogen balance, intestinal permeability (lactulose/mannitol ratio (L/M ratio)) 2. Hospital stay and infection rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization table from each centre was prepared by randomization software |
| Allocation concealment (selection bias) | Low risk | Patients satisfying the enrolment criteria received an ascending serial number in the order of their enrolment. Based on this number, the patients were assigned to one of the two study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Karwowska 2001.
| Methods | Clinical study | |
| Participants | 30 well‐nourished male patients undergoing elective aortic abdominal aneurysm reconstruction | |
| Interventions | Patients were randomly assigned to a control group (group I) or an experimental group (group II) in which glutamine‐enriched PN was used. Those in group I received standard amino‐acid solution, and those in group II received 0.202 g of glutamine per kilogram per day for 10 days | |
| Outcomes | 1. Daily nitrogen balance 2. Lymphocyte subpopulations and immunoglobulin (A, M, and G) concentrations in peripheral blood on postoperative day 1 and day 11 3. Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly assigned to a control group (group I) or an experimental group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Kumar 2007.
| Methods | Prospective, interventional, observer‐blind, randomized clinical trial | |
| Participants | 120 patients with peritonitis or abdominal trauma | |
| Interventions | Patients were randomized to receive either enteral glutamine 45 g/day for 5 days in addition to standard care (n=63; group A) or standard care alone (n=57; group B) | |
| Outcomes | 1. Serum malondialdehyde and glutamine levels 2. Infectious complications 3. Survival rate and duration of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized using a computer generated randomization, stratified for nature of disease (peritonitis or trauma) into two groups |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | High risk | Baseline bias: group A patients were younger than group B patients (P=0.036) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only observer‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Observer‐blind |
Kłek 2005.
| Methods | Prospective clinical trial | |
| Participants | 105 patients operated on for gastric carcinoma | |
| Interventions | During the postoperative period, patients were randomly allocated to one of three groups: standard PN (A), PN + glutamine (B), and PN + omega‐3‐FA (C) | |
| Outcomes | 1. Labotory blood test (serum concentration of albumin and prealbumin/total lymphocyte count/SGOT/SGTP/creatinine and urea) 2. Number and type of complications 3. Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly allocated to one of three groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 of 105 patients (5 from Group A, 4 from Group B, 6 from Group C) were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Lin 2002.
| Methods | Prospective, randomized double‐blind clinical trial | |
| Participants | 48 patients with major abdominal surgery | |
| Interventions | The control group (n= 23) received 1.5 g amino acid/kg/d. Patients of the test group (Ala‐Gln group, n=25) received 0.972 g amino acid/kg/d supplemented with 0.417 g/kg/d L‐Alanyl‐L‐Glutamine which provide 0.28 g/kg/d glutamine for 6 days | |
| Outcomes | 1. Cumulative nitrogen balance 2. Plasma glutamine concentration 3. Urine 3‐methylhistidine concentrations 4. Blood T lymphocyte, CD4, CD8 cell and total lymphocyte count 5. Mortality, adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | These patients were randomly allocated to either a test group or a control group.Test and control solutions were packed at the same size and packaging materials have the same appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Lu 2011.
| Methods | Randomized and double‐blind trial | |
| Participants | 50 GI cancer patients received postoperative 7 days of isocaloric and isonitrogenous TPN after operation | |
| Interventions | The control group received 1.5 g/kg/day amino acids solution The test group received 1.2 g/kg/day amino acids solution, and supplemented with 0.3 g/kg/day glutamine |
|
| Outcomes | 1. Routine hematological and biochemical measurements 2. Nutritional assessment, inflammation‐related cytokines 3. Postoperation complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly divided to receive either glutamine‐enriched TPN or standard TPN |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
Luo 2008.
| Methods | Randomized, double‐blind, placebo‐controlled study | |
| Participants | 44 medical and surgical ICU patients | |
| Interventions | During the 8‐day study period, all subjects received standard polymeric tube feeds plus intravenous (iv) or enteral Gln dipeptide supplementation or placebo IV‐AG group: continuous 24‐hour central venous infusion of 20% L‐Ala‐L‐Gln dipeptide at 0.5 g/kg/day EN‐AG group: iv normal saline (placebo) plus enteral Ala‐Gln powder mixed in the daily tube feedings at 0.5 g/kg/day Placebo group: intravenous 15% Clinisol® at 0.5 g/kg/day |
|
| Outcomes | 1. Blood concentrations of amino acids, IGF‐1 and IGF‐BP3 2. Antioxidant capacity 3. Peripheral lymphocytes and lymphocyte subsets 4. Plasma GSH concentrations 5. Intestinal permeability 6. Nitrogen balance 7. Serial illness severity scoring |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization format (generated by the GCRC research statistician) |
| Allocation concealment (selection bias) | Unclear risk | Eligible subjects were assigned by the research pharmacist in a double‐blind, block‐randomization format to one of three study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 subjects were excluded from the analysis (6 randomised to the control group, 3 to the IV AG group, and 3 to the EN‐AG group) |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study subjects and all other research and clinical personnel were blinded to the randomization |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Mertes 2000.
| Methods | Double‐blind, randomized, controlled trial | |
| Participants | 37 patients following major abdominal surgery receiving TPN | |
| Interventions | Subjects receiving isonitrogenous isoenergetic TPN with or without alanyl‐glutamine supplementation (0.5 g/kg BW/day) over a 5‐day period | |
| Outcomes | Nitrogen balance, selected biochemical parameters and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | 50 patients were randomized |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 patients had been excluded in the final evaluation |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
Morlion 1998.
| Methods | Randomized, double‐blind, controlled study | |
| Participants | 28 patients undergoing elective abdominal surgery | |
| Interventions | 2 groups receive isonitrogenous (0.24 g nitrogen/kg/day) and isoenergetic (29 kcal/122 kJ/kg/day) TPN over 5 days. Controls received 1.5 g of amino acids/kg/day, and the test group received 1.2 g of amino acids and 0.3 g of L‐Alanyl‐L‐Glutamine (Ala‐Gin)/kg/day | |
| Outcomes | 1. Nitrogen balances, plasma Gln concentration, lymphocyte recovery 2. Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly allocated to either a test group or a control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
Neri 2001.
| Methods | Randomized, controlled study | |
| Participants | 33 Patients after major abdominal surgery | |
| Interventions | Subjects receive Gln‐supplemented TPN (0.3 g/kg of body weight per day, study group, n= 16) or an isocaloric, isonitrogenous TPN, without Gln (control group, n= 17) for more than a week | |
| Outcomes | 1. Nitrogen balance and lymphocyte count 2. Incidence of surgical infections and hospital mortality 3. Mean length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Some 33 consecutive patients were randomized, stratified for disease, to receive Gln‐supplemented TPN (study group) or an isocaloric, isonitrogenous TPN, without Gln (control group) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All clinicians were blind to randomization, except the study co‐ordinator (AN) who did not take part in clinical decisions on therapy and discharge of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
O'Riordain 1994.
| Methods | Randomized clinical study | |
| Participants | 22 patients undergoing colorectal resection | |
| Interventions | Patients were randomized to receive conventional TPN (0.2 g nitrogen/kg/d) or an isonitrogenous/isocaloric regimen with 0.18 g of glutamine/kg/d from days 1 to 6 postoperatively | |
| Outcomes | 1.T‐cell DNA synthesis and interleukin (IL)‐2 production and peripheral blood mononuclear cell IL‐6 and tumour necrosis factor (TNF) production were measured in vitro preoperatively and on days 1 and 6 postoperatively 2. Urinary nitrogen excretion and nitrogen balance, infection complication |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomized to receive either conventional TPN (group I) or glutamine‐supplemented TPN (group II) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinicians responsible for patient management were blinded to which TPN regimen was used |
Ockenga 2002.
| Methods | Randomized, controlled study | |
| Participants | 28 patients with acute pancreatitis | |
| Interventions | Subjects received either a standard TPN with 1.5 g/kg body weight protein or an isonitrogenous, isocaloricTPN which contains 0.3 g/kg L‐alanine‐L‐glutamine. TPN was administered for at least 1 week | |
| Outcomes | 1. Nutritional and inflammatory parameters 2. Infectious complications 3. Length of TPN, length of hospital stay (LOS) and cost of TPN |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all predefined outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The patients, nurses and the physicians responsible for all clinical decisions were unaware of who was belonging to which group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Pattanshetti 2009.
| Methods | Randomized controlled trial | |
| Participants | 30 patients with burns | |
| Interventions | Two groups with 15 patients in each, the study (glutamine enteral supplemented, 0.5 g/kg/day) and control group | |
| Outcomes | Incidence of positive blood culture, wound culture, total leucocyte count, hospital‐stay and mortality was recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patient was assigned to one of the two groups according to a randomisation table |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Mortality analysis excluding which occurs within 72 hours of admission |
| Selective reporting (reporting bias) | Unclear risk | The baseline infection associated blood and urine test were not reported |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not a double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurers of study outcomes were unaware of allocation of each patient to study or control group |
Peng 2004.
| Methods | Clinical study | |
| Participants | 48 severely burned patients | |
| Interventions | Subjects were randomly divided into two groups: burn control group (B group, 23 patients) and glutamine‐treated group (Gln group, 25 patients). Glutamine granules 0.5 g/kg were supplied orally for 14 days in Gln group, and the same dosage of placebo were given for 14 days in B group | |
| Outcomes | 1. The plasma level of glutamine, endotoxin and the activity of diamine oxidase (DAO), as well as intestinal mucosal permeability were determined 2. Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Patients were randomly divided into glutamine group and burn control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Powell‐Tuck 1999.
| Methods | Randomized, double‐blind, controlled trial | |
| Participants | 170 patients clinically accepted for parenteral nutrition | |
| Interventions | Patient was randomized to receive or not to receive 20 g glutamine included in the parenteral feed as part of the overall estimated nitrogen requirement for a median of 8 days | |
| Outcomes | Primary outcome: 1.Mortality, length of hospital stay Secondary outcome: 1. Comparison of cause of hospital death between groups 2. Septic complication rates 3. Perceived morbidity, 'quality of life' scores 4. Plasma amino acid concentrations |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement |
| Allocation concealment (selection bias) | Low risk | Randomization was carried out in the pharmacy using sealed envelopes such that the assigning pharmacist could not know the group to which the patient would be randomised prior to the decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Unclear risk | Patients from surgery or ICU department were included, whether they were critically ill was uncertain |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, clinical carers, investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Pérez‐Bárcena 2008.
| Methods | Prospective, randomized, single‐blind study | |
| Participants | 30 adult patients admitted to the ICU and received PN as part of their treatment,of these patients, 16 were admitted for trauma, 6 after surgery, and 8 for a medical pathology | |
| Interventions | 1. 15 subjects in treatment group received basic PN solution plus a daily glutamine supplement of 0.35 g/kg of N2‐L‐alanyl‐L‐glutamine for 5d 2. 15 subjects in control group received a supplemental volume of the basic PN solution for 5 d to achieve an isocaloric/isonitrogenous formula with the treatment group |
|
| Outcomes | 1. Analysis of CD14, TLRs expressions in peripheral blood monocytes 2. Infection incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection was based on a computer generated list that assigned patients to groups consecutively |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Pérez‐Bárcena 2010.
| Methods | Prospective, randomized and single‐blind study | |
| Participants | Trauma patients admitted to the intensive care unit (ICU) at a university third‐level hospital between 18 and 75 years (inclusive) with moderate to severe trauma | |
| Interventions | The study group (n= 23 )received a daily glutamine supplement of 0.35 g/kg weight as N2‐L‐Alanyl‐LGlutamine (0.5 g/kg/d; Dipeptiven Fresenius Kabi España) during five days The control group (n = 20 patients) received a supplemental volume of the basic TPN solution to achieve an isocaloric and isonitrogenous formula with the study group |
|
| Outcomes | 1. TLRs expression in peripheral blood monocytes, 2. complications and outcome of patients | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection was based on a computer generated list that assigned patients to groups consecutively |
| Allocation concealment (selection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Qiu 2009.
| Methods | Randomized, controlled clinical trial | |
| Participants | 65 patients with the diagnosis of end‐stage liver disease or hepatic cellular carcinoma admitted for orthotopic liver transplantation | |
| Interventions | Patients were randomly divided into 3 groups: Diet group (n=21) TPN group (n=22), and Gln group (0.35g/kg/day, n=22) Patients in the TPN and Gln groups received isocaloric and isonitrogenous TPN for 7 days |
|
| Outcomes | 1. Nutritional assessment, liver function assessment, serum immunoglobulins 2. Clinical outcomes: hospital mortality, mean postoperative hospital stay, and 1‐year survival |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly assigned to receive either routine treatment and oral diet without additional nutritional support therapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | High risk | Length of hospital stay are reported incompletely so that it can not be entered in a meta‐analysis |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Sahin 2007.
| Methods | Controlled clinical study | |
| Participants | 40 patients with acute pancreatitis who had Ranson's score between 2 and 4 | |
| Interventions | Patients received either standard TPN (control group) or TPN with glutamine (treatment group, 0.3 g/kg/day) for 15 days | |
| Outcomes | 1. Nutritional and inflammatory parameters 2. Mortality, length of TPN and length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly assigned to two groups as control and treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Spittler 2001.
| Methods | Controlled clinical trial | |
| Participants | 30 patients undergoing major abdominal surgery | |
| Interventions | Patients were randomly allocated to receive either 1500ml Vamin (control) or an isonitrogenic formulation containing Vamin and 500ml glycyl‐glutamine (35 g GLN; 0.5g/kg BW) (GLY‐GLN), or Vamin and 500ml alanyl‐glutamine (35 g GLN; 0.5 g/kg BW) (ALA‐GLN) as a continuous infusion over 48 h postoperatively | |
| Outcomes | 1. Primary endpoint of the study the expression of HLA‐DR on peripheral blood monocytes 2. Plasma amino acid levels, white blood cell count, immune status of peripheral leukocytes, TNF‐a release following ex vivo LPS stimulation and plasma cytokine production 3. Complications, length of hospital stay (days) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were preoperatively randomized to one of three groups of 10 patients each |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Tian 2006.
| Methods | Randomized controlled study | |
| Participants | 40 patients with MODS in the intensive care unit | |
| Interventions | Group A (n=20) was given routine TPN, and group B (n=20) was given extra glutamine 0.27 g/kg/d (dipeptiven 0.4 g/kg/d) for 7 days | |
| Outcomes | 1. The activity of plasma DAO and D‐lactate content 2. The TPN treatment time and the mortality rate |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Tjäder 2004.
| Methods | A prospective, blinded, randomized study | |
| Participants | 40 ICU patients with multiple organ failure who were expected to stay in the unit for more than five days | |
| Interventions | Patients received 0, 0.28, 0.57 or 0.86 g of glutamine per kg bodyweight per day intravenously for five days as part of an isocaloric, isonitrogenous and isovolumetric diet | |
| Outcomes | 1. Plasma glutamine concentration, free muscle glutamine concentration 2. Length of ICU stay, 6‐month outcome |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by the closed envelope system after inclusion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded for attending physicians |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Tremel 1994.
| Methods | Clinical trial | |
| Participants | 12 intensive care unit patients | |
| Interventions | Patients were randomized to receive isonitrogenous and isoenergetic parenteral nutrition over 9 days The control group received a conventional amino acid solution (1.5 g amino acids/kg/day), and the test group received a complete amino acid solution containing the dipeptide L‐alanyl‐L‐glutamine (0.3g/kg/day) |
|
| Outcomes | 1. D‐xylose test 2. Nutrition assessment, liver and kidney function test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were uniformly randomized to an experimental group and a control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Wang 2006.
| Methods | Clinical trial | |
| Participants | 39 patients following laryngectomy | |
| Interventions | Subjects were randomly assigned to receive parenteral nutrition containing glutamine‐bipeptide (Gln group, n=20) or general PN (PN group, n=19) for 6 days | |
| Outcomes | 1. Plasma level of glutamine, endotoxin, IgG/IgA/CD3/CIM/CD4/CD8 2. Infectious complications, hospital stay and hospitalization expense |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personal ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
Wernerman 2011.
| Methods | Multi‐centre randomized clinical trial | |
| Participants | Patients (n=418) given nutrition by an enteral and/or a parenteral route with the aim of providing full nutrition were included within 72 h after ICU admission | |
| Interventions | Glutamine was supplemented as iv L‐alanyl‐L‐glutamine, 0.283 g glutamine/kg body weight/24 h for the entire ICU stay. Placebo was iv saline infusion in identical bottles | |
| Outcomes | The predefined primary endpoint was the change in the SOFA score from day 1 to day 7 Predefined secondary endpoints were: (1) change in the SOFA score from day 1 to day 10, (2) length of ICU stay, (3) ICU mortality and (4) all‐cause 6‐month mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data were available for 5 patients without reason |
| Selective reporting (reporting bias) | High risk | Two outcomes (length of ICU stay and all‐cause 6‐month mortality) are reported incompletely so that they cannot be entered in the meta‐analysis |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personal ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
Wischmeyer 2001.
| Methods | Prospective, double‐blind, randomized trial | |
| Participants | 31 severe burns patients with total burn surface area of 25% to 90% and presence of full‐thickness burns | |
| Interventions | Either intravenous glutamine or an isonitrogenous control amino acid solution was administered as a continuous infusion during burns intensive care unit stay | |
| Outcomes | 1. Positive blood cultures and antibiotic use 2. Mortality, length of stay in the burns ICU, and total hospital length of stay 3. Laboratory tests (transferrin, prealbumin, C‐reactive protein, ammonia and liver enzymes) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization process |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomized to receive either intravenous GLN or an isonitrogenous control amino acid solution |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five of 31 patients excluded after randomization Misssing outcome data balanced in numbers across groups, with similar reasons for missing data |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, patients, and burns centre staff were blinded to randomization |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Yang 2008.
| Methods | Clinical trial | |
| Participants | 61 patients with severe pancreatitis | |
| Interventions | Subjects were randomized equally into 2 groups and received standard total parenteral nutrition (TPN, n=30) with intravenous infusion of Gln or normal saline (control, n=31) for 1 week | |
| Outcomes | 1. The plasma level of glutamine 2. Serum IL‐8, TNF‐alpha, and heat shock protein 70 3. The length of hospital stay, mortality |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomized equally into 2 groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing patients (GLN (n=5) and control group (n=6)) were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | No selecting reporting |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Yao 2007.
| Methods | Clinical study | |
| Participants | 52 patients undergoing abdominal surgery | |
| Interventions | Subjects were randomly divided into two groups to receive isonitrogenous and isoenergetic TPN over 5 days. The control group received 1.5 g/kg/d of amino acids, and the study group received 1.2 g/kg/d of amino acids and 0.3 g/kg/d of L‐alanyl‐L‐glutamine | |
| Outcomes | 1. Plasma free amino acid, IgG, IgM, IgA, CD3, CD4, and CD4/CD8 were counted and the generation of cys‐teinyl‐leukotrienes from polymorphonuclear neutrophil granulocytes 2. Length of hospital stay |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly divided into two groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Zhang 2007.
| Methods | Controlled clinical study | |
| Participants | 44 patients requiring parenteral nutrition for more than 7 days,admitted to emergency intensive care unit and neurosurgical intensive care unit | |
| Interventions | Participants were randomly divided into two groups, one was the control group, the other was the Gln treatment group (each n=22). Patients in both groups received PN and EN. In addition, glutamine 0.4g/kg per day was given to patients of Gln treatment group in PN for 7 days | |
| Outcomes | 1. Serum HSP70, GIn concentrations were measured at admission and 7 days after the nutritional supplementation 2. Clinical outcomes: length of mechanical ventilation, the length of stay in intensive care unit, the incidence of liver and kidney dysfunction before and after treatment |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly divided into two groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
Zhao 2013.
| Methods | Randomized clinical trial | |
| Participants | 120 patients with severe acute pancreatitis were enrolled | |
| Interventions | Patients were resuscitated with normal saline (NS group), combination of normal saline and hydroxyethyl starch (SH group) or combination of normal saline, hydroxyethyl starch and glutamine (SHG group) | |
| Outcomes | 1. Complications and outcomes 2. Intra‐abdominal pressure 3. Serum TNF‐α, IL‐8 and C‐reactive protein (CRP), urine lactulose/mannitol (L/M) ratio, serum concentration of endotoxin |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement |
| Allocation concealment (selection bias) | Unclear risk | The patients were randomly divided into three groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information for judgement |
Zhou 2002.
| Methods | Clinical trial | |
| Participants | 30 burns patients with TBSA of 30‐70% and degree burn area more than 20% | |
| Interventions | Subjects randomly divided into control and study groups. Glutamine dipeptide powder in dose of 0.5 g/kg/day was given orally in bolus to those patients in study group during 1‐12 postburn days | |
| Outcomes | 1. Plasma levels of glutamine/endotoxin 2. Wound healing rate at 30 PBD and total hospital stay days |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Patients randomly divided into control and study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
Zhou 2003.
| Methods | Randomized double‐blind, controlled clinical trial | |
| Participants | 41 thermally injured patients | |
| Interventions | One group received Gln‐enriched EN (0.35g/kg BW/day) and the other group received the standard EN for 12 days | |
| Outcomes | 1. Plasma glutamine concentration, endotoxin concentration 2. Intestinal permeability was determined by L/M absorption technique 3. Wound infections, body weight, length of hospital stay, total cost |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table retrieved from software |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient who died during the trial was excluded from analysis |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, care providers, and lab worker were blinded from the group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Zhou 2004.
| Methods | Randomized, double‐blind, controlled clinical trial | |
| Participants | 30 patients with severe burns | |
| Interventions | Subjects received parenteral nutrition with (0.35 g glutamine/kg/d, test group) or without (control group) glutamine dipeptide supplementation for 12 days | |
| Outcomes | 1. Plasma glutamine, serum endotoxin concentrations, and the lactulose/mannitol absorption ratio 2. Survival rate of skin graft, wound healing time, infection rate, length of hospital stay and total costs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random tables were retrieved from software |
| Allocation concealment (selection bias) | Low risk | Each patient had a series number as well as an envelope for randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Reported all predefined outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians, nursing staff and laboratory workers were not aware of the procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Zhu 2000.
| Methods | Randomized double‐blind trial | |
| Participants | 30 patients above 60 years old undergoing gastro‐intestinal operation | |
| Interventions | The study group received alanyl‐glutamine 0.5 g/kg/d, control group received impact isocaloric parenteral nutrition for 6 days | |
| Outcomes | 1. Plasma amino acids profile, nitrogen balance, intestinal permeability 2. Clinical prognosis, infection, adverse effects |
|
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | The results of organ function tests were reported incompletely |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detailed information |
Çekmen 2011.
| Methods | Randomized, prospective, controlled, double‐blind study | |
| Participants | 30 patients admitted to the ICU and requiring TPN for more than 5 days | |
| Interventions | Patients received either Gln‐supplemented TPN (containing L‐alanyl‐L‐glutamine dipeptide; 0.5 g/kg per day; n=15) or standard Gln‐free TPN (control group n=15) | |
| Outcomes | Time of TPN, APACHE II score, type of diagnosis, biochemistry, nutritional, immune parameters, ICU length of stay, ICU mortality, and in‐hospital mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | The randomization envelopes were held by the pharmacist and were assigned in strict chronological ascending order within each treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported all outcome data |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes |
| Other bias | Low risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All patients, investigators and coworkers were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boelens 2002 | The control group was supplemented with glutamine |
| Boelens 2003 | The study is duplicated of Houdijk 1998 |
| Brantley 2000 | Abstract only |
| Duska 2008a | Improper control setting; the control group without intravenous placebo |
| Duska 2008b | Improper control setting; the control group without intravenous placebo |
| Griffiths 2002 | The study is duplicated of Griffiths 1997 |
| Grigoryev 2010 | Abstract only |
| Gurnani 2012 | Patients in the study group received omega 3 fatty acids and glutamine supplementation |
| Heyland 2007 | Improper control setting;The control group received no IV placebo |
| Houdijk 1998 | The control group was supplemented with glutamine |
| Jiang 2007 | Improper control setting; the study group received enteral nutrition, while control group received parenteral nutrition |
| Jones 1999 | The control group was supplemented with glutamine |
| Juan 2012 | Abstract only |
| Koksal 2011 | Abstract only |
| Liu 2012 | Glutamine was supplemented with arginine |
| Mangano 1993 | Abstract only |
| McQuiggan 2008 | Improper control setting; Glutamine group received immune‐enhancing diet (IED) plus Gln supplementation, while control group received IED plus whey protein supplement |
| Nitta 2001 | Abstract only |
| Ozgultekin 2008 | the group I patients only received standardized enteral nutrition, while the Group III patients received standard enteral nutrition plus parenteral glutamine supplement, it can not be conclude from this study that the difference in outcome was due to glutamine supplementation treatment or IV infusion treatment, hence we excluded this study from analysis |
| Palmese 2006 | Glutamine supplemented with other nutrients: intravenous glutamine administration with FOS‐enriched enteral nutrition vs. isocaloric isonitrogenous enteral nutrition without FOS and intravenous glutamine |
| Schulman 2005 | The control group was supplemented with glutamine |
| Xiangdong 2012 | Abstract only |
| Xue 2008 | Improper control setting; early glutamine supplementation group was compared with late glutamine supplementation group |
| Ziegler 2004 | Abstract only |
| Ziegler 2012 | Abstract only |
Characteristics of studies awaiting assessment [ordered by study ID]
Hallay 2002.
| Methods | Randomized controlled trial |
| Participants | Patients treated with subtotal oesophagectomy |
| Interventions | Patients with glutamine‐rich Stresson Multi Fibre diet (23 patients) and patients with Nutrison Multi Fibre glutamine‐poor diet (13 patients) |
| Outcomes | Levels of prealbumin, retinol‐binding protein, immunoglobulins and outcome of patients |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Al Balushi 2010.
| Trial name or title | Effect of Intravenous GLutamine Supplementation IN Trauma Patients Receiving Enteral Nutrition Study Protocol (GLINT Study): A Prospective, Blinded, Randomized, Placebo‐controlled Clinical Trial |
| Methods | Prospective, blinded, randomized, placebo‐controlled clinical trial |
| Participants | Critically ill patients with multiple trauma |
| Interventions | Participants receive either 0.5 g/kg/day intravenous undiluted alanyl‐glutamine or intravenous placebo by continuous infusion (24 h/day) |
| Outcomes | 1. The change in daily total Sequential Organ Failure Assessment Score (SOFA) each day over 10 days 2. The change in daily total SOFA plotted each day over 10 days, where we will compare the regression slope between the two arms of the study 3. The change in daily total SOFA on the last day of treatment as a measure of severity of organ dysfunction 4. Number of infections that are documented during intensive care unit stay 5. Number of deaths occurring on or before day 60 6. Length of stay in intensive care unit (Time frame: At discharge from intensive care unit) (Designated as safety issue: No) 7. Length of stay in hospital. 8. Number of days on mechanical ventilation 9. Number of days of antibiotic use during intensive care unit stay |
| Starting date | March 2011 |
| Contact information | ruqaiya.albalushi@uqconnect.edu.au |
| Notes |
Pérez‐Barcena 2010.
| Trial name or title | Effectiveness of Dipeptide N (2)‐L‐Alanyl‐L‐Glutamine in Trauma ICU Patients: Pilot, Prospective, Randomized and Double Blind Study |
| Methods | prospective, randomized and double‐blind study |
| Participants | Trauma ICU patients |
| Interventions | The patients who fulfil the inclusion criteria will receive either glutamine or placebo, independently of the type on nutrition. Glutamine will be administered as a pharmaconutrient at 0.5 g/kg/day during 5 days as a continuous perfusion |
| Outcomes | 1. Number of infections 2. ICU mortality 3. Safety of endovenous administration |
| Starting date | October 2010 |
| Contact information | juan.perez@ssib.es |
| Notes |
Rastmanesh 2010.
| Trial name or title | Iranian Intensive Care Unit (ICU) Glutamine Study |
| Methods | Double‐blinded placebo‐controlled block randomized study |
| Participants | Trauma patients in ICU |
| Interventions | Dietary supplement: Glutamine 0.5 g/kg/day Dietary supplement: Glutamine 1 g/kg/day Dietary supplement: Usual enteral nutrition |
| Outcomes | 1. Infection probability score 2. Systemic inflammatory response syndrome 3. Length of stay 4. Mechanical ventilation |
| Starting date | October 2010 |
| Contact information | Dr Reza Rastmanesh, Shahid Beheshti University of Medical Sciences |
| Notes |
Talukdar 2012.
| Trial name or title | Efficacy of Enteral Glutamine Supplementation in Patients With Predicted Severe Acute Pancreatitis‐ A Double‐Blinded Randomized Controlled Trial |
| Methods | Randomized controlled trial |
| Participants | Patients with acute pancreatitis |
| Interventions | Dietary supplement: Enteral glutamine, dosage of glutamine: 0.57 g/kg, ˜ 30 g/day = 3 sachets Dietary supplement: Placebo, similar appearing nutritional supplement without glutamine |
| Outcomes | 1. Development of infected (peri)pancreatic necrosis 2. Mortality 3. Change in levels of HS‐CRP 4. Change in mucosal permeability 5. Change in level of oxidative stress 6. Change in cytokine levels in serum |
| Starting date | January 2012 |
| Contact information | rup_talukdar@yahoo.com |
| Notes |
Wischmeyer 2010.
| Trial name or title | The RE‐ENERGIZE study: a RandomizEd trial of ENtERal Glutamine to minimIZE thermal injury |
| Methods | Multicentre randomized double‐blind controlled trial |
| Participants | Patients with severe thermal burn injuries |
| Interventions | Glutamine group: Patients will receive glutamine (L‐glutamine) powder mixed with water through their feeding tube, every 4 hours or three times a day if given orally, for a total of 0.5 g/kg/day. The glutamine powder will be supplied in pre‐packaged aliquots of 5 grams and will be delivered to the ICU in blinded sachets and will be mixed in with water at the bedside by the patient's nurse Control group: Patients will receive maltodextrin (placebo) mixed with water instead of glutamine |
| Outcomes | 1. Death: Hospital mortality recorded until complete healing, defined as 7 days after the last grafting procedure 2. Six‐month mortality 3. Incidence of infections 4. Length of care (defined as length of healing): defined as 7 days after the last grafting operation 5. Length of mechanical ventilation: number of days on ventilator 6. Clinical status in the ICU: APACHE score at entry and SOFA score every day during the ICU stay until the discontinuation of mechanical ventilation. These scores are derived from the clinical and biological monitoring of the patients in the ICU and are standardised. All data needed for the calculation of these scores will be transferred to our electronic database and calculated automatically 7. Length of time in the ICU |
| Starting date | 01/06/2010 |
| Contact information | paul.wischmeyer@ucdenver.edu |
| Notes |
Ziegler 2013.
| Trial name or title | A Randomized Controlled Trial of Glutamine Dipeptide in Severe Trauma (GLND Trauma) |
| Methods | Randomized controlled trial |
| Participants | Critically ill patients with trauma |
| Interventions | Drug: Glutamine dipeptide 0.5 gm/kg/day will be administered as a continuous infusion over 24 hours for up to 7 days or until ICU discharge Other: Placebo of normal saline of equivalent volume to the experimental drug infusion administered as a continuous infusion over 24 hours for up to 7 days or until ICU discharge |
| Outcomes | 1. Mortality 2. Change of insulin resistance by euglycaemic hyperinsulinaemic clamp |
| Starting date | February 2013 |
| Contact information | Addison K May, MD FACS FCCM 615‐936‐7188 |
| Notes |
Differences between protocol and review
Types of studies: we removed "We will also include cluster‐randomized studies", as we did not find any cluster randomized trial.
Types of outcome measures: we added an additional outcome "days on mechanical ventilation" to the list of secondary outcomes. This is because we found that many clinical trials reported this outcome and this outcome could also reflect the treatment effects of glutamine supplementation on the respiratory system.
Handsearch journals: There was a typo in the protocol, "Journal of Nutrition and Orthomolecular Medicine" has been changed to "Journal of Orthomolecular Medicine".
Measures of treatment effect: We had not introduced the method for converting the median (range or interquartile range) to the mean (standard deviation) in the protocol. We amended it by referring to the formulas from Cochrane Handbook 2011 and Hozo 2005.
Unit of analysis: the sentence "the adult unit (or sub‐unit) for cluster randomized trials" had been removed, as we did not include any cluster randomized trial.
Reference: Heyland 2006 in the protocol had been changed into Heyland 2013, as the results of this clinical trial had been published.
Summary of findings tables: we added the days on mechanical ventilation outcome to the summary of findings table in the review, and deleted the quality of life outcome from the protocol, as there were few included studies reporting this outcome and these results can not be meta‐analysed.
Contributions of authors
Kun‐Ming Tao (KMT), Xiao‐Qian Li (XQL), Li‐Qun Yang (LQY), Wei‐Feng Yu (WFY), Zhi‐Jie Lu (ZJL), Yu‐Ming Sun (YMS), Fei‐Xiang Wu (WFX)
Conceiving the review: KMT, WFY
Co‐ordinating the review: WFY
Undertaking manual searches: WFX, LQY, YMS, ZJL
Screening search results: KMT, XQL
Organizing retrieval of papers: KMT
Screening retrieved papers against inclusion criteria: LQY, KMT, WFY
Appraising quality of papers: LQY, ZJL, WFY
Abstracting data from papers: FXW, LQY
Writing to authors of papers for additional information: KMT, XQL
Providing additional data about papers: KMT
Obtaining and screening data on unpublished studies: FXW, LQY
Data management for the review: KMT, XQL
Entering data into Review Manager (RevMan 5.2): KMT, XQL
RevMan statistical data: KMT, XQL
Other statistical analysis not using RevMan: KMT, XQL
Interpretation of data: KMT, WFY
Statistical inferences: KMT, XQL, WFY
Writing the review: KMT, XQL, WFY
Guarantor for the review (one author): WFY
Person responsible for reading and checking review before submission: KMT, WFY
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Kun‐Ming Tao: none known
Xiao‐Qian Li: none known
Li‐Qun Yang: none known
Wei‐Feng Yu: none known
Zhi‐Jie Lu: none known
Yu‐Ming Sun: none known
Fei‐Xiang Wu: none known
Edited (no change to conclusions)
References
References to studies included in this review
Andrews 2011 {published data only}
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;17(342):d1542. [PUBMED: 21415104] [DOI] [PubMed] [Google Scholar]
Çekmen 2011 {published data only}
- Cekmen N, AydIn A, Erdemli Ö. The impact of L‐alanyl‐L‐glutamine dipeptide supplemented total parenteral nutrition on clinical outcome in critically patients. European e‐Journal of Clinical Nutrition and Metabolism 2011;6:e64‐7. [DOI: 10.1016/j.eclnm.2011.02.001] [DOI] [Google Scholar]
Chai 2006 {published data only}
- Cai GL, Yan J, Yu YH, Zhang ZC, Gong SJ, Dai HW, et al. Influence of glutamine and growth hormone intensified nutrition support on immunomodulation in critically ill elderly patients. Chinese Critical Care Medicine 2006;18(10):595‐8. [PubMed] [Google Scholar]
Conejero 2002 {published data only}
- Conejero R, Bonet A, Grau T, Esteban A, Mesejo A, Montejo JC, et al. Effect of a glutamine‐enriched enteral diet on intestinal permeability and infectious morbidity at 28 days in critically ill patients with systemic inflammatory response syndrome: a randomized, single‐blind, prospective, multicenter study. Nutrition 2002;18(9):716‐21. [PUBMED: 12297203] [DOI] [PubMed] [Google Scholar]
Cucereanu‐Badica 2013 {published data only}
- Cucereanu‐Badica I, Luca I, Negres S, Grintescu I, Lascar I. The effects of intravenous glutamine supplementation in severely burned, multiple traumatized patients. Farmacia 61;1:212‐9. [http://www.revistafarmacia.ro/201301/art‐19‐2013‐1‐cucereanu%20212‐219.pdf] [Google Scholar]
Déchelotte 2006 {published data only}
- Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, et al. L‐alanyl‐L‐glutamine dipeptide‐supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double‐blind, multicenter study. Critical Care Medicine 2006;34(3):598‐604. [PUBMED: 16505644] [DOI] [PubMed] [Google Scholar]
Dong 2008 {published data only}
- Dong GL, Kang ZH, Liu XN, Ji G, Wang CY, Wan Y, et al. Effect of alanyl‐glutamine on the clinical outcome of patients after total gastrectomy. Chinese Journal of Clinical Nutrition 2008;16(2):70‐3. [EMBASE: 2008262444] [Google Scholar]
Eroglu 2009 {published data only}
- Eroglu A. The effect of intravenous alanyl‐glutamine supplementation on plasma glutathione levels in intensive care unit trauma patients receiving enteral nutrition: the results of a randomized controlled trial. Anesthesia and Analgesia 2009;109(2):502‐5. [PUBMED: 19608826] [DOI] [PubMed] [Google Scholar]
Estívariz 2008 {published data only}
- Estívariz CF, Griffith DP, Luo M, Szeszycki EE, Bazargan N, Dave N, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. Journal of Parenteral and Enteral Nutrition 2008;32(4):389‐402. [PUBMED: 18596310] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez‐Estivariz C, Griffith DP, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Medicine 2005;31(8):1079‐86. [PUBMED: 15973519] [DOI] [PubMed] [Google Scholar]
Fuentes‐Orozco 2004 {published data only}
- Fuentes‐Orozco C, Anaya‐Prado R, González‐Ojeda A, Arenas‐Márquez H, Cabrera‐Pivaral C, Cervantes‐Guevara G, et al. L‐alanyl‐L‐glutamine‐supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clinical Nutrition 2004;23(1):13‐21. [PUBMED: 14757388] [DOI] [PubMed] [Google Scholar]
Fuentes‐Orozco 2008 {published data only}
- Fuentes‐Orozco C, Cervantes‐Guevara G, Muciño‐Hernández I, López‐Ortega A, Ambriz‐González G, Gutiérrez‐de‐la‐Rosa JL, et al. L‐alanyl‐L‐glutamine‐supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. Journal of Parenteral and Enteral Nutrition 2008;32(4):403‐11. [PUBMED: 18596311] [DOI] [PubMed] [Google Scholar]
Garrel 2003 {published data only}
- Garrel D, Patenaude J, Nedelec B, Samson L, Dorais J, Champoux J, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Critical Care Medicine 2003;31(10):2444‐9. [PUBMED: 14530749] [DOI] [PubMed] [Google Scholar]
Goeters 2002 {published data only}
- Goeters C, Wenn A, Mertes N, Wempe C, Aken H, Stehle P, et al. Parenteral L‐alanyl‐L‐glutamine improves 6‐month outcome in critically ill patients. Critical Care Medicine 2002;30(9):2032‐7. [PUBMED: 12352037] [DOI] [PubMed] [Google Scholar]
Grau 2011 {published data only}
- Grau T, Bonet A, Miñambres E, Piñeiro L, Irles JA, Robles A, et al. The effect of L‐alanyl‐L‐glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Critical Care Medicine 2011;39(6):1263‐8. [PUBMED: 21336131] [DOI] [PubMed] [Google Scholar]
Griffiths 1997 {published data only}
- Griffiths RD, Jones C, Palmer TE. Six‐month outcome of critically ill patients given glutamine‐supplemented parenteral nutrition. Nutrition 1997;13(4):295‐302. [PUBMED: 9178278] [PubMed] [Google Scholar]
Hajdú 2012 {published data only}
- Hajdú N, Belágyi T, Issekutz A, Bartek P, Gartner B, Oláh A. Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis ‐a prospective randomized clinical study. Magyar Sebeszet 2012;65(2):44‐51. [PUBMED: 22512878] [DOI] [PubMed] [Google Scholar]
Hall 2003 {published data only}
- Hall JC, Dobb G, Hall J, DeSousa R, Brennan L, McCauley R. A clinical trial evaluating enteral glutamine in critically ill patients. American Journal of Clinical Nutrition 2002;75:415S. [Google Scholar]
- Hall JC, Dobb G, Hall J, Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Medicine 2003;29(10):1710‐6. [PUBMED: 12923621] [DOI] [PubMed] [Google Scholar]
He 2004 {published data only}
- He XL, Ma QJ, Lu JG, Chu YK, Du XL. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP). Clinical Nutrition Supplements 2004;1(1):43‐7. [DOI: 10.1016/j.clnu.2004.07.011] [DOI] [Google Scholar]
Heyland 2013 {published data only}
- Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. New England Journal of Medicine 2013;368(16):1489‐97. [PUBMED: 23594003] [DOI] [PubMed] [Google Scholar]
Jiang 1999 {published data only}
- Jian ZM, Cao JD, Zhu XG, Zhao WX, Yu JC, Ma EL, et al. The impact of alanyl‐glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double‐blind, controlled study of 120 patients. Journal of Parenteral and Enteral Nutrition 1999;23(5 Suppl):S62‐6. [PUBMED: 10483898] [DOI] [PubMed] [Google Scholar]
Karwowska 2001 {published data only}
- Karwowska KA, Dworacki G, Trybus M, Zeromski J, Szulc R. Influence of glutamine‐enriched parenteral nutrition on nitrogen balance and immunologic status in patients undergoing elective aortic aneurysm repair. Nutrition 2001;17(6):475‐8. [PUBMED: 11399407] [DOI] [PubMed] [Google Scholar]
Kumar 2007 {published data only}
- Kumar S, Kumar R, Sharma SB, Jain BK. Effect of oral glutamine administration on oxidative stress, morbidity and mortality in critically ill surgical patients. Indian Journal of Gastroenterology 2007;26(2):70‐3. [PUBMED: 17558069] [PubMed] [Google Scholar]
Kłek 2005 {published data only}
- Kłek S, Kulig J, Szczepanik AM, Jedrys J, Kołodziejczyk P. The clinical value of parenteral immunonutrition in surgical patients. Acta Chirurgica Belgica 2005;105(2):175‐9. [PUBMED: 15906909] [PubMed] [Google Scholar]
Lin 2002 {published data only}
- Lin MT, Kung SP, Yeh SL, Lin C, Lin TH, Chen KH, et al. The effect of glutamine‐supplemented total parenteral nutrition on nitrogen economy depends on severity of diseases in surgical patients. Clinical Nutrition 2002;21(3):213‐8. [PUBMED: 12127929] [DOI] [PubMed] [Google Scholar]
Lu 2011 {published data only}
- Lu CY, Shih YL, Sun LC, Chuang JF, Ma CJ, Chen FM, et al. The inflammatory modulation effect of glutamine‐enriched total parenteral nutrition in postoperative gastrointestinal cancer patients. The American Surgeon 2011;77(1):59‐64. [PUBMED: 21396307] [PubMed] [Google Scholar]
Luo 2008 {published data only}
- Luo M, Bazargan N, Griffith DP, Estívariz CF, Leader LM, Easley KA, et al. Metabolic effects of enteral versus parenteral alanyl‐glutamine dipeptide administration in critically ill patients receiving enteral feeding: a pilot study. Clinical Nutrition 2008;27(2):297‐306. [PUBMED: 18258342] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertes 2000 {published data only}
- Mertes N, Schulzki C, Goeters C, Winde G, Benzing S, Kuhn KS, et al. Cost containment through L‐alanyl‐L‐glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective randomized double‐blind controlled study. Clinical Nutrition 2000;19(6):395‐401. [PUBMED: 11104589] [DOI] [PubMed] [Google Scholar]
Morlion 1998 {published data only}
- Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, et al. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double‐blind, controlled study. Annals of Surgery 1998;227(2):302‐8. [PUBMED: 9488531 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neri 2001 {published data only}
- Neri A, Mariani F, Piccolomini A, Testa M, Vuolo G, Cosmo L. Glutamine‐supplemented total parenteral nutrition in major abdominal surgery. Nutrition 2001;17(11‐12):968‐9. [PUBMED: 11744350] [DOI] [PubMed] [Google Scholar]
O'Riordain 1994 {published data only}
- O'Riordain MG, Fearon KC, Ross JA, Rogers P, Falconer JS, Bartolo DC, et al. Glutamine‐supplemented total parenteral nutrition enhances T‐lymphocyte response in surgical patients undergoing colorectal resection. Annals of Surgery 1994;220(2):212‐21. [PUBMED: 8053744 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ockenga 2002 {published data only}
- Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine‐enriched total parenteral nutrition in patients with acute pancreatitis. Clinical Nutrition 2002;21(5):409‐16. [PUBMED: 12381339] [DOI] [PubMed] [Google Scholar]
Pattanshetti 2009 {published data only}
- Pattanshetti VM, Powar RS, Godhi AS, Metgud SC. Enteral glutamine supplementation reducing infectious morbidity in burns patients: a randomised controlled trial. Indian Journal of Surgery 2009;71(4):193‐7. [PUBMED: 23133153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2004 {published data only}
- Peng X, Yan H, You Z, Wang P, Wang S. Clinical and protein metabolic efficacy of glutamine granules‐supplemented enteral nutrition in severely burned patients. Burns 2005;31(3):342‐6. [PUBMED: 15774291] [DOI] [PubMed] [Google Scholar]
- Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 2004;30(2):135‐9. [PUBMED: 15019120] [DOI] [PubMed] [Google Scholar]
- Peng X, Yan H, You Z, Wang P, Wang S. Glutamine granule‐supplemented enteral nutrition maintains immunological function in severely burned patients. Burns 2006;32(5):589‐93. [PUBMED: 16725264] [DOI] [PubMed] [Google Scholar]
Pérez‐Bárcena 2008 {published data only}
- Pérez‐Bárcena J, Regueiro V, Marsé P, Raurich JM, Rodríguez A, Ibáñez J, et al. Glutamine as a modulator of the immune system of critical care patients: effect on Toll‐like receptor expression. A preliminary study. Nutrition 2008;24(6):522‐7. [PUBMED: 18367379] [DOI] [PubMed] [Google Scholar]
Pérez‐Bárcena 2010 {published data only}
- Pérez‐Bárcena J, Crespí C, Regueiro V, Marsé P, Raurich JM, Ibáñez J, et al. Lack of effect of glutamine administration to boost the innate immune system response in trauma patients in the intensive care unit. Critical Care 2010;14(6):R233. [PUBMED: 21184675] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell‐Tuck 1999 {published data only}
- Powell‐Tuck J, Jamieson CP, Bettany GE, Obeid O, Fawcett HV, Archer C, et al. A double blind, randomised, controlled trial of glutamine supplementation in parenteral nutrition. Gut 1999;45(1):82‐8. [PUBMED: 10369709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2009 {published data only}
- Qiu Y, Zhu X, Wang W, Xu Q, Ding Y. Nutrition support with glutamine dipeptide in patients undergoing liver transplantation. Transplantation Proceedings 2009;41(10):4232‐7. [PUBMED: 20005375] [DOI] [PubMed] [Google Scholar]
Sahin 2007 {published data only}
- Sahin H, Mercanligil SM, Inanç N, Ok E. Effects of glutamine‐enriched total parenteral nutrition on acute pancreatitis. European Journal of Clinical Nutrition 2007;61(12):1429‐34. [PUBMED: 17311061] [DOI] [PubMed] [Google Scholar]
Spittler 2001 {published data only}
- Spittler A, Sautner T, Gornikiewicz A, Manhart N, Oehler R, Bergmann M, et al. Postoperative glycyl‐glutamine infusion reduces immunosuppression: partial prevention of the surgery induced decrease in HLA‐DR expression on monocytes. Clinical Nutrition 2001;20(1):37‐42. [PUBMED: 11161542] [DOI] [PubMed] [Google Scholar]
Tian 2006 {published data only}
- Tian H, Wang KF, Wu TJ. Effect of total parenteral nutrition with supplementation of glutamine on the plasma diamine oxidase activity and D‐lactate content in patients with multiple organ dysfunction syndrome. Chinese Critical Care Medicine 2006;18(10):616‐8. [PubMed] [Google Scholar]
Tjäder 2004 {published data only}
- Tjäder I, Rooyackers O, Forsberg AM, Vesali RF, Garlick PJ, Wernerman J. Effects on skeletal muscle of intravenous glutamine supplementation to ICU patients. Intensive Care Medicine 2004;30(2):266‐75. [PUBMED: 14722645] [DOI] [PubMed] [Google Scholar]
Tremel 1994 {published data only}
- Tremel H, Kienle B, Weilemann LS, Stehle P, Fürst P. Glutamine dipeptide‐supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology 1994;107(6):1595‐601. [PUBMED: 7958669] [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang XY, Huang WN, Wang LY, Yang Y, Liu F, Xiao LY, et al. Effects of glutamine on plasma endotoxin and immune function in postoperative patients with laryngeal carcinoma. Chinese Journal of Clinical Nutrition 2006;14(4):222‐6. [Google Scholar]
Wernerman 2011 {published data only}
- Wernerman J, Kirketeig T, Andersson B, Berthelson H, Ersson A, Friberg H, et al. Scandinavian glutamine trial: a pragmatic multi‐centre randomised clinical trial of intensive care unit patients. Acta Anaesthesiologica Scandinavica 2011;55(7):812‐8. [PUBMED: 21658010] [DOI] [PubMed] [Google Scholar]
Wischmeyer 2001 {published data only}
- Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, et al. Glutamine administration reduces Gram‐negative bacteremia in severely burned patients: a prospective, randomized, double‐blind trial versus isonitrogenous control. Critical Care Medicine 2001;29(11):2075‐80. [PUBMED: 11700398] [DOI] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang SQ, Xu JG, Li LT, Xu J. Efect of glutamine on serum interleukin‐8 and tumor necrosis factor‐α levels in patients with severe pancreatitis. Journal of Southern Medical University 2008;28(1):129‐31. [PubMed] [Google Scholar]
Yao 2007 {published data only}
- Yao GQ, Zhu X, Bo SN, Wang HX, Lin Y. Efect of glutamine dipeptide on nitrogen balance and immune function in patients after abdominal surgery. Chinese Journal of Clinical Nutrition 2007;15(4):223‐7. [Google Scholar]
Zhang 2007 {published data only}
- Zhang Z, Qin HD, Ni HB, Xu Y, Wu HR, Cheng H, et al. Effect of early enriched parenteral alanyl‐glutamine on heat shock protein 70 (HSP70) expression in critical patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19(8):481‐4. [PUBMED: 17708846] [PubMed] [Google Scholar]
Zhao 2013 {published data only}
- Zhao G, Zhang JG, Wu HS, Tao J, Qin Q, Deng SC, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World Journal Gastroenterology 2013;19(13):2044‐52. [PUBMED: 23599623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2002 {published data only}
- Zhou Y, Sun Y, Jiang Z, He G, Yang N. The effects of glutamine dipeptide on the improvement of endotoxemia in severely burned patients. Zhonghua Shao Shang Za Zhi 2002;18(6):343‐5. [PUBMED: 12641984] [PubMed] [Google Scholar]
Zhou 2003 {published data only}
- Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double‐blind, controlled clinical trial. Journal of Parenteral and Enteral Nutrition 2003;27(4):241‐5. [PUBMED: 12903886 ] [DOI] [PubMed] [Google Scholar]
Zhou 2004 {published data only}
- Zhou YP, Jiang ZM, Sun YH, He GZ, Shu H. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: a randomized, double‐blind, controlled clinical trial. Clinical Nutrition Supplements 2004;1(1):55‐60. [DOI: 10.1016/j.clnu.2004.07.012] [DOI] [Google Scholar]
Zhu 2000 {published data only}
- Zhu M, Tang D, Zhao X, Cao J, Wei J, Chen Y, et al. Impact of glutamine of gut permeability and clinical prognosis on the aging patients undergoing gastric‐intestinal operation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2000;22(5):425‐7. [PUBMED: 12903420] [PubMed] [Google Scholar]
References to studies excluded from this review
Boelens 2002 {published data only}
- Boelens PG, Houdijk AP, Fonk JC, Nijveldt RJ, Ferwerda CC, Blomberg‐Van Der Flier BM, et al. Glutamine‐enriched enteral nutrition increases HLA‐DR expression on monocytes of trauma patients. Journal of Nutrition 2002;132(9):2580‐6. [PUBMED: 12221212] [DOI] [PubMed] [Google Scholar]
Boelens 2003 {published data only}
- Boelens PG, Houdijk AP, Thouars HN, Teerlink T, Engeland MI, Haarman HJ, et al. Plasma taurine concentrations increase after enteral glutamine supplementation in trauma patients and stressed rats. American Journal of Clinical Nutrition 2003;77(1):250‐6. [PUBMED: 12499349] [DOI] [PubMed] [Google Scholar]
Brantley 2000 {published data only}
- Brantley S, Pierce J. Effects of enteral glutamine on trauma patients. Nutrition in Clinical Practice 2000;15:S13. [http://ncp.sagepub.com/content/15/3/S11.full.pdf+html] [Google Scholar]
Duska 2008a {published data only}
- Duska F, Fric M, Waldauf P, Pazout J, Andel M, Mokrejs P, et al. Frequent intravenous pulses of growth hormone together with glutamine supplementation in prolonged critical illness after multiple trauma: effects on nitrogen balance, insulin resistance, and substrate oxidation. Critical Care Medicine 2008;36(6):1707‐13. [PUBMED: 18496372] [DOI] [PubMed] [Google Scholar]
Duska 2008b {published data only}
- Duska F, Fric M, Pazout J, Waldauf P, Tůma P, Pachl J. Frequent intravenous pulses of growth hormone together with alanylglutamine supplementation in prolonged critical illness after multiple trauma: effects on glucose control, plasma IGF‐I and glutamine. Growth Hormone & IGF Research 2008;18(1):82‐7. [PUBMED: 17709266] [DOI] [PubMed] [Google Scholar]
Griffiths 2002 {published data only}
- Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine‐supplemented parenteral nutrition on acquired infection. Nutrition 2002;18(7‐8):546‐52. [PUBMED: 12093428] [DOI] [PubMed] [Google Scholar]
Grigoryev 2010 {published data only}
- Grigoryev EG. Therapy of compartmentalization of the proinflammatory and anti‐inflammatory cytokines in sepsis. Critical Care 2010;14 Suppl 1:P566. [DOI: 10.1186/cc8798] [DOI] [Google Scholar]
Gurnani 2012 {published data only}
- Gurnani A, Agarwal A. Immunonutrition and correlation with biomarkers in critically ill an Indian perspective. Clinical Nutrition, Supplement 2012;7(1):144. [Google Scholar]
Heyland 2007 {published data only}
- Heyland DK, Dhaliwalm R, Day A, Drover J, Cote H, Wischmeyer P. Optimizing the dose of glutamine dipeptides and antioxidants in critically ill patients: a phase I dose‐finding study. Journal of Parenteral and Enteral Nutrition 2007;31(2):109‐18. [PUBMED: 17308251] [DOI] [PubMed] [Google Scholar]
Houdijk 1998 {published data only}
- Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, et al. Randomised trial of glutamine‐enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 1998;352(9130):772‐6. [PUBMED: 9737282] [DOI] [PubMed] [Google Scholar]
Jiang 2007 {published data only}
- Jiang H, Li B, Yan LN, Lu SC, Wen TF, Zhao JC, et al. Effect of Intravenous glutamine‐dipeptide fortified enteral nutrition on clinical outcomes in patients after liver transplantation: A prospective randomized controlled study. Chinese Journal of Clinical Nutrition 2007;15(1):21‐5. [Google Scholar]
Jones 1999 {published data only}
- Jones C, Palmer TE, Griffiths RD. Randomized clinical outcome study of critically ill patients given glutamine‐supplemented enteral nutrition. Nutrition 1999;15(2):108‐15. [PUBMED: 9990574] [DOI] [PubMed] [Google Scholar]
Juan 2012 {published data only}
- Juan M, Mesejo A, Serrano A, Corcobado C, Bueno A, Yuste L, et al. Comparison of three enteral formulas to glycemic and infectious control in critically ill patients under mechanical ventilation: High‐protein, high‐protein disease‐specific and disease‐specific diet supplemented with glutamine. Intensive Care Medicine 2012;38:S122. [Google Scholar]
Koksal 2011 {published data only}
- Koksal G, Karaoren G, Akarcay H, Karabulut E, Tunali Y, Vehid S, et al. Comparison of the effects of intravenous, enteral and enteral + intravenous supply of glutamine on malnutrition in sepsis. 31st International Symposium on Intensive Care and Emergency Medicine; 2011; Brussels, Belgium. Critical Care 15. S137.
Liu 2012 {published data only}
- Liu H, Ling W, Shen ZY, Jin X, Cao H. Clinical application of immune‐enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. Journal of Digestive Diseases 2012;13(8):401‐6. [PUBMED: 22788925] [DOI] [PubMed] [Google Scholar]
Mangano 1993 {published data only}
- Mangano A, Panza N, Paganini N, Croce G, Cassone E, Trevisan M, et al. Effects of parenteral glutamine supplementation on the nutritional status of patients with radical cystectomy in neoplasia. Acta Urologica Italica 1993;7 Suppl 2:119‐20. [EMBASE: 1994073349] [Google Scholar]
McQuiggan 2008 {published data only}
- McQuiggan M, Kozar R, Sailors RM, Ahn C, McKinley B, Moore F. Enteral glutamine during active shock resuscitation is safe and enhances tolerance of enteral feeding. Journal of Parenteral and Enteral Nutrition 2008;32(1):28‐35. [PUBMED: 18165444] [DOI] [PubMed] [Google Scholar]
Nitta 2001 {published data only}
- Nitta H, Ikeda K, Aoki K. Effects of perioperative great amount of glutamine supplementation by enteral route on amino acids metabolism. Journal of Parenteral and Enteral Nutrition 2001;25:S22. [http://pen.sagepub.com/content/25/1/S1.full.pdf+html] [Google Scholar]
Ozgultekin 2008 {published data only}
- Ozgultekin A, Turan G, Durmus Y, Dincer E, Akgun N. Comparison of the efficacy of parenteral glutamine and branched‐chain amino acid solutions given as extra supplements in parallel to the enteral nutrition in head trauma. e‐SPEN, the European e‐Journal of Clinical Nutrition and Metabolism 2008;3(5):e211‐6. [DOI: 10.1016/j.eclnm.2008.05.006] [DOI] [Google Scholar]
Palmese 2006 {published data only}
- Palmese S, Odierna I, Scarano D, Scibili AC, Natale A, Pezza M. Early enteral nutrition enriched with FOS and intravenous glutamine supplementation in intensive care unit patients. Nutritional Therapy Metabolism 2006;24(3):140‐6. [DOI: 10.5301/NTM.2012.9433] [DOI] [Google Scholar]
Schulman 2005 {published data only}
- Schulman AS, Willcutts KF, Claridge JA, Evans HL, Radigan AE, O'Donnell KB, et al. Does the addition of glutamine to enteral feeds affect patient mortality?. Critical Care Medicine 2005;33(11):2501‐6. [PUBMED: 16276173] [DOI] [PubMed] [Google Scholar]
Xiangdong 2012 {published data only}
- Xiangdong G, Xiaoyue L. The impact of glutamine on organ function and mortality of patients with severe sepsis. Intensive Care Medicine 2012;38:S31. [Google Scholar]
Xue 2008 {published data only}
- Xue P, Deng LH, Xia Q, Zhang ZD, Hu WM, Yang XN, et al. Impact of alanyl‐glutamine dipeptide on severe acute pancreatitis in early stage. World Journal of Gastroenterology 2008;14(3):474‐8. [PUBMED: 18200673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 2004 {published data only}
- Ziegler TR, Fernandez‐Estivariz C, Griffith DP, Szeszycki EE, Bazargan N, Luo M, et al. Parenteral nutrition supplemented with alanyl glutamine dipeptide decreases infectious morbidity and improves organ function in critically ill post‐operative patients: results of a double‐blind,randomized,controlled pilot study. Journal of Parenteral and Enteral Nutrition 2004;28:S11‐2. [Google Scholar]
Ziegler 2012 {published data only}
- Ziegler T, May A. Glutamine dipeptide‐supplemented parenteral nutrition in surgical ICU patients: Results of an American randomized, double‐blind, multicenter trial. Clinical Nutrition, Supplement 2012;7(1):265. [Google Scholar]
References to studies awaiting assessment
Hallay 2002 {published data only}
- Hallay J, Kovács G, Kiss Sz S, Farkas M, Lakos G, Sipka S, et al. Changes in the nutritional state and immune‐serological parameters of esophagectomized patients fed jejunaly with glutamine‐poor and glutamine‐rich nutriments. Hepatogastroenterology 2002;49(48):1555‐9. [PUBMED: 12397734] [PubMed] [Google Scholar]
References to ongoing studies
Al Balushi 2010 {published data only}
- Effect of Intravenous GLutamine Supplementation IN Trauma Patients Receiving Enteral Nutrition Study Protocol (GLINT Study): A Prospective, Blinded, Randomized, Placebo‐controlled Clinical Trial. Ongoing study March 2011. [DOI] [PMC free article] [PubMed]
Pérez‐Barcena 2010 {published data only}
- Effectiveness of Dipeptide N (2)‐L‐Alanyl‐L‐Glutamine in Trauma ICU Patients: Pilot, Prospective, Randomized and Double Blind Study. Ongoing study October 2010.
Rastmanesh 2010 {published data only}
- Iranian Intensive Care Unit (ICU) Glutamine Study. Ongoing study October 2010.
Talukdar 2012 {published data only}
- Efficacy of Enteral Glutamine Supplementation in Patients With Predicted Severe Acute Pancreatitis‐ A Double‐Blinded Randomized Controlled Trial. Ongoing study January 2012.
Wischmeyer 2010 {published data only}
- The RE‐ENERGIZE study: a RandomizEd trial of ENtERal Glutamine to minimIZE thermal injury. Ongoing study 01/06/2010. [DOI] [PMC free article] [PubMed]
Ziegler 2013 {published data only}
- A Randomized Controlled Trial of Glutamine Dipeptide in Severe Trauma (GLND Trauma). Ongoing study February 2013.
Additional references
Avenell 2006
- Avenell A. Glutamine in critical care: current evidence from systematic reviews. Proceedings of the Nutrition Society 2006;65(3):236‐41. [PUBMED: 16923308] [DOI] [PubMed] [Google Scholar]
Avenell 2009
- Avenell A. Hot topics in parenteral nutrition. Current evidence and ongoing trials on the use of glutamine in critically‐ill patients and patients undergoing surgery. Proceedings of the Nutrition Society 2009;68(3):261‐8. [PUBMED: 19490739] [DOI] [PubMed] [Google Scholar]
Bakalar 2006
- Bakalar B, Duska F, Pachl J, Fric M, Otahal M, Pazout J, et al. Parenterally administered dipeptide alanyl‐glutamine prevents worsening of insulin sensitivity in multiple‐trauma patients. Critical Care Medicine 2006;34(2):381‐6. [PUBMED: 16424718] [DOI] [PubMed] [Google Scholar]
Bollhalder 2013
- Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta‐analysis of randomized clinical trials of parenteral glutamine supplementation. Clinical Nutrition 2013;32(2):213‐23. [PUBMED: 23196117] [DOI] [PubMed] [Google Scholar]
Bongers 2007
- Bongers T, Griffiths RD, McArdle A. Exogenous glutamine: the clinical evidence. Critical Care Medicine 2007;35(9 Suppl):S545‐52. [PUBMED: 17713407] [DOI] [PubMed] [Google Scholar]
Clinical Evaluation Research Unit 2013
- Clinical Evaluation Research Unit. Canadian Clinical Practice Guidelines: critical care nutrition. Available from www.criticalcarenutrition.com accessed on 1st July 2013.
Déchelotte 2006
- Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, et al. L‐alanyl‐L‐glutamine dipeptide‐supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double‐blind, multicenter study. Critical Care Medicine 2006;34(3):598‐604. [PUBMED: 16505644] [DOI] [PubMed] [Google Scholar]
Gamrin 1996
- Gamrin L, Essén P, Forsberg AM, Hultman E, Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy‐rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Critical Care Medicine 1996;24(4):575‐83. [PUBMED: 8612406 ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammarqvist 1989
- Hammarqvist F, Wernerman J, Ali R, Decken A, Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Annals of Surgery 1989;209(4):455‐61. [PUBMED: 2494960] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org accessed on 1st July 2013.
Heyland 2006
- Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, et al. REducing Deaths due to OXidative Stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically‐ill patients. Proceedings of the Nutrition Society 2006;65(3):250‐63. [PUBMED: 16923310] [DOI] [PubMed] [Google Scholar]
Heyland 2013 [pers comm]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PMID: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1993
- Jones C, Hussey R, Griffiths RD. A tool to measure the change in health status of selected adult patients before and after intensive care. Clinical Intensive Care 1993;4(4):160‐5. [PUBMED: 10146456] [PubMed] [Google Scholar]
Kim 2013
- Kim M, Wischmeyer PE. Glutamine. World Review of Nutrition and Dietetics 2013;105:90‐6. [PUBMED: 23075590] [DOI] [PubMed] [Google Scholar]
Koretz 2007a
- Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. American Journal of Gastroenterology 2007;102(2):412‐29. [PUBMED: 17311654] [DOI] [PubMed] [Google Scholar]
Koretz 2007b
- Koretz RL. Do data support nutrition support? Part I: intravenous nutrition. Journal of the American Dietetic Association 2007;107(6):988‐96. [PUBMED: 17524720] [DOI] [PubMed] [Google Scholar]
Koretz 2007c
- Koretz RL. Do data support nutrition support? Part II. enteral artificial nutrition. Journal of the American Dietetic Association 2007;107(8):1374‐80. [PUBMED: 17659905] [DOI] [PubMed] [Google Scholar]
Kreymann 2006
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clinical Nutrition 2006;25(2):210‐23. [PUBMED: 16697087 ] [DOI] [PubMed] [Google Scholar]
Melis 2004
- Melis GC, ter Wengel N, Boelens PG, Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Current Opinion in Clinical Nutrition and Metabolic Care 2004;7(1):59‐70. [PUBMED: 15090905] [DOI] [PubMed] [Google Scholar]
Mizock 2010
- Mizock BA. Immunonutrition and critical illness: an update. Nutrition 2010;26(7‐8):701‐7. [PUBMED: 20381315] [DOI] [PubMed] [Google Scholar]
Novak 2002
- Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Critical Care Medicine 2002;30(9):2022‐9. [PUBMED: 12352035] [DOI] [PubMed] [Google Scholar]
O'Leary 1996
- O'Leary MJ, Coakley JH. Nutrition and immunonutrition. British Journal of Anaesthesia 1996;77(1):118‐27. [PUBMED: 8703621] [DOI] [PubMed] [Google Scholar]
O'Riordain 1996
- O'Riordain MG, Beaux A, Fearon KC. Effect of glutamine on immune function in the surgical patient. Nutrition 1996;12(11‐12 Suppl):S82‐4. [PUBMED: 8974126] [DOI] [PubMed] [Google Scholar]
Ogle 1994
- Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, et al. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. Journal of Parenteral and Enteral Nutrition 1994;18(2):128‐33. [PUBMED: 8201747] [DOI] [PubMed] [Google Scholar]
Parry‐Billings 1992
- Parry‐Billings M, Baigrie RJ, Lamont PM, Morris PJ, Newsholme EA. Effects of major and minor surgery on plasma glutamine and cytokine levels. Archives of Surgery 1992;127(10):1237‐40. [PUBMED: 1358047] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roth 2008
- Roth E. Nonnutritive effects of glutamine. The Journal of Nutrition 2008;138(10):2025S‐31S. [PUBMED: 18806119] [DOI] [PubMed] [Google Scholar]
Salloum 1991
- Salloum RM, Copeland EM, Souba WW. Brush border transport of glutamine and other substrates during sepsis and endotoxemia. Annals of Surgery 1991;213(5):401‐9. [PUBMED: 2025060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singer 2009
- Singer P, Berger MM, Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clinical Nutrition 2009;28(4):387‐400. [PUBMED: 19505748] [DOI] [PubMed] [Google Scholar]
Tjader 2007
- Tjader I, Berg A, Wernerman J. Exogenous glutamine‐compensating a shortage?. Critical Care Medicine 2007;35(9 Suppl):S553‐6. [PUBMED: 17713408] [DOI] [PubMed] [Google Scholar]
Tschoeke 2007
- Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury 2007;38(12):1346‐57. [PUBMED: 18048039] [DOI] [PubMed] [Google Scholar]
Vinnars 1975
- Vinnars E, Bergstom J, Furst P. Influence of the postoperative state on the intracellular free amino acids in human muscle tissue. Annals of Surgery 1975;182(6):665‐71. [PUBMED: 1190870] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang Y, Jiang ZM, Nolan MT, Jiang H, Han HR, Yu K, et al. The impact of glutamine dipeptide‐supplemented parenteral nutrition on outcomes of surgical patients: a meta‐analysis of randomized clinical trials. Journal of Parenteral and Enteral Nutrition 2010;34(5):521‐9. [PUBMED: 20852180] [DOI] [PubMed] [Google Scholar]
Yue 2013
- Yue C, Tian W, Wang W, Huang Q, Zhao R, Zhao Y, et al. The impact of perioperative glutamine‐supplemented parenteral nutrition on outcomes of patients undergoing abdominal surgery: a meta‐analysis of randomized clinical trials. The American Surgeon 2013;79(5):506‐13. [PUBMED: 23635587] [PubMed] [Google Scholar]
Zheng 2006
- Zheng YM, Li F, Zhang MM, Wu XT. Glutamine dipeptide for parenteral nutrition in abdominal surgery: a meta‐analysis of randomized controlled trials. World Journal of Gastroenterology 2006;12(46):7537‐41. [PUBMED: 17167847] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 2005
- Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez‐Estivariz C, Griffith DP, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Medicine 2005;31(8):1079‐86. [PUBMED: 15973519] [DOI] [PubMed] [Google Scholar]


