Abstract
Background
This an update of the review first published in 2009.
Major abdominal and pelvic surgery carries a high risk of venous thromboembolism (VTE). The efficacy of thromboprophylaxis with low molecular weight heparin (LMWH) administered during the in‐hospital period is well‐documented, but the optimal duration of prophylaxis after surgery remains controversial. Some studies suggest that patients undergoing major abdominopelvic surgery benefit from prolongation of the prophylaxis up to 28 days after surgery.
Objectives
To evaluate the efficacy and safety of prolonged thromboprophylaxis with LMWH for at least 14 days after abdominal or pelvic surgery compared with thromboprophylaxis administered during the in‐hospital period only in preventing late onset VTE.
Search methods
We performed electronic searches on 28 October 2017 in the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, LILACS and registered trials (Clinicaltrials.gov October 28, 2017 and World Health Organization International Clinical Trials Registry Platform (ICTRP) 28 October 2017). Abstract books from major congresses addressing thromboembolism were handsearched from 1976 to 28 October 2017, as were reference lists from relevant studies.
Selection criteria
We assessed randomized controlled clinical trials (RCTs) comparing prolonged thromboprophylaxis (≥ fourteen days) with any LMWH agent with placebo, or other methods, or both to thromboprophylaxis during the admission period only. The population consisted of persons undergoing abdominal or pelvic surgery for both benign and malignant pathology. The outcome measures included VTE (deep venous thrombosis (DVT) or pulmonary embolism (PE)) as assessed by objective means (venography, ultrasonography, pulmonary ventilation/perfusion scintigraphy, spiral computed tomography (CT) scan or autopsy). We excluded studies exclusively reporting on clinical diagnosis of VTE without objective confirmation.
Data collection and analysis
Review authors identified studies and extracted data. Outcomes were VTE (DVT or PE) assessed by objective means. Safety outcomes were defined as bleeding complications within three months after surgery. Sensitivity analyses were also performed with unpublished studies excluded, and with study participants limited to those undergoing solely open and not laparoscopic surgery. We used a fixed‐effect model for analysis.
Main results
We identified seven RCTs (1728 participants) evaluating prolonged thromboprophylaxis with LMWH compared with control or placebo. The searches resulted in 1632 studies, of which we excluded 1528. One hundred and four abstracts, eligible for inclusion, were assessed of which seven studies met the inclusion criteria.
For the primary outcome, the incidence of overall VTE after major abdominal or pelvic surgery was 13.2% in the control group compared to 5.3% in the patients receiving out‐of‐hospital LMWH (Mantel Haentzel (M‐H) odds ratio (OR) 0.38, 95% confidence interval (CI) 0.26 to 0.54; I2 = 28%; seven studies, n = 1728; moderate‐quality evidence).
For the secondary outcome of all DVT, seven studies, n = 1728, showed prolonged thromboprophylaxis with LMWH to be associated with a statistically significant reduction in the incidence of all DVT (M‐H OR 0.39, 95% CI 0.27 to 0.55; I2 = 28%; moderate‐quality evidence). We found a similar reduction when analysis was limited to incidence in proximal DVT (M‐H OR 0.22, 95% CI 0.10 to 0.47; I2 = 0%; moderate‐quality evidence).
The incidence of symptomatic VTE was also reduced from 1.0% in the control group to 0.1% in patients receiving prolonged thromboprophylaxis (M‐H OR 0.30, 95% CI 0.08 to 1.11; I2 = 0%; moderate‐quality evidence).
No difference in the incidence of bleeding between the control and LMWH group was found, 2.8% and 3.4%, respectively (HM‐H OR 1.10, 95% CI 0.67 to 1.81; I2 = 0%; seven studies, n = 2239; moderate‐quality evidence).
Estimates of heterogeneity ranged between 0% and 28% depending on the analysis, suggesting low or unimportant heterogeneity.
Authors' conclusions
Prolonged thromboprophylaxis with LMWH significantly reduces the risk of VTE compared to thromboprophylaxis during hospital admittance only, without increasing bleeding complications after major abdominal or pelvic surgery. This finding also holds true for DVT alone, and for both proximal and symptomatic DVT. The quality of the evidence is moderate and provides moderate support for routine use of prolonged thromboprophylaxis. Given the low heterogeneity between studies and the consistent and moderate evidence of a decrease in risk for VTE, our findings suggest that additional studies may help refine the degree of risk reduction but would be unlikely to significantly influence these findings. This updated review provides additional evidence and supports the previous results reported in the 2009 review.
Keywords: Humans; Abdomen; Abdomen/surgery; Anticoagulants; Anticoagulants/administration & dosage; Drug Administration Schedule; Heparin, Low‐Molecular‐Weight; Heparin, Low‐Molecular‐Weight/administration & dosage; Pelvis; Pelvis/surgery; Postoperative Care; Postoperative Complications; Postoperative Complications/prevention & control; Pulmonary Embolism; Pulmonary Embolism/prevention & control; Venous Thromboembolism; Venous Thromboembolism/prevention & control
Do blood thinner injections given after abdominal surgery further reduce blood clots if continued after discharge from the hospital?
Review question
For persons having surgery on the abdomen and pelvis, does continuing blood thinner injections after discharge from a hospital stay decrease the likelihood of developing a blood clot in the lower limbs or lungs when compared to blood thinner injections given only while in the hospital?
Why is this important?
The complication of developing a blood clot can range from asymptomatic to potentially fatal, depending on the location and severity of the clot. After a postoperative patient is considered safe for discharge from the hospital, evidence suggests an ongoing risk for developing a blood clot in the weeks to months following the operation. Although recommended by some guidelines, not all physicians recommend discharging a postoperative patient home with a prolonged course of blood thinner injections.
What was found?
Seven studies were found that addressed this question, including a total of 1728 patients. Continuing blood thinning injections after hospital discharge decreased the risk of both blood clots in the limbs and in the lungs. This review determined that the overall incidence of having a blood clot is reduced from 13.2%, when no post‐discharge blood thinner injections are used, to 5.3% when a blood thinner injection is prescribed for at least 14 days following discharge in 30 days follow‐up. Both symptomatic and asymptomatic blood clots decreased with the use of prolonged duration blood thinner injections in postoperative patients. No increase in bleeding complications, a common concern when blood thinners are used, was observed in patients treated with prolonged duration blood thinner injections.
What does this mean?
Continuation of blood thinning injections for at least 14 days after abdominal or pelvic surgery reduces the risk of blood clots.
Summary of findings
Summary of findings for the main comparison.
| Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery | |||||
|
Patient or population: patients undergoing abdominal or pelvic surgery Settings: inpatient followed by outpatient, worldwide Intervention: thromboprophylaxis with low molecular weight heparin for ≥ 14 days Comparison: thromboprophylaxis during hospitalization only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Risk with only in‐hospital thrombopropylaxis | Risk with thromboprophylaxis ≥14 days | ||||
|
All VTE Follow‐up: 30 days postoperatively |
Study population | OR 0.38, (0.26 to 0.54) | 1728 (7 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 132 per 1000 | 50 per 1000 (34 to 71) | ||||
|
All DVT Follow‐up: 30 days postoperatively |
Study population | OR 0.39, (0.27 to 0.55) | 1728 (7 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 129 per 1000 | 50 per 1000 (35 to 71) | ||||
|
Proximal DVT Follow‐up: 30 days postoperatively |
Study population | OR 0.22, (0.10 to 0.47) | 1728 (7 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 39 per 1000 | 9 per 1000 (4 to 18) | ||||
|
Symptomatic VTE Follow‐up: 30 days postoperatively |
Study population | OR 0,30, (0.08 to 1.11) | 1728 (7 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 10 per 1000 | 3 per 1000 (1 to 11) | ||||
|
Bleeding complications Follow‐up: 3 months postoperatively |
Study population | OR 1.10, (0.67 to 1.81) | 2239 (7 RCTs) | ⊕⊕⊕⊝ moderate1 | |
| 28 per 1000 | 31 per 1000 (19 to 51) | ||||
| *The corresponding risk (and its 95% CI interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds Ratio; RCT: randomized Controlled Trial; VTE: venous Thromboembolism; DVT: deep venous thrombosis | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level for serious risk of bias (includes at least one study with overall high risk of bias)
Summary of findings 2.
Additional summary of findings
| Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery | |||||
|
Patient or population: patients undergoing abdominal or pelvic surgery Settings: inpatient followed by outpatient, worldwide Intervention: thromboprophylaxis with low molecular weight heparin for ≥ 14 days Comparison: thromboprophylaxis during hospitalization only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Risk with only in‐hospital thrombopropylaxis | Risk with thromboprophylaxis ≥14 days | ||||
|
All VTE (subgroup analysis open only) Follow‐up: 30 days postoperatively |
Study population | OR 0.42, (0.29 to 0.60) | 1503 (6 RCTs) |
⊕⊕⊕⊝ moderate1 | |
| 138 per 1000 | 58 per 1000 (40 to 83) | ||||
|
All DVT (subgroup analysis open only) Follow‐up: 30 days postoperatively |
Study population | OR 0.43, (0.30 to 0.62) | 1503 (6 RCTs) |
⊕⊕⊕⊝ moderate1 | |
| 134 per 1000 | 58 per 1000 (40 to 83) | ||||
|
All VTE (sensitivity analysis unpublished) Follow‐up: 30 days postoperatively |
Study population | OR 0.38, (0.27 to 0.56) | 1620 (6 RCTs) |
⊕⊕⊕⊝ moderate1 | |
| 128 per 1000 | 49 per 1000 (35 to 72) | ||||
|
All DVT (sensitivity analysis unpublished) Follow‐up: 30 days postoperatively |
Study population | OR 0.40, (0.27 to 0.58) | 1620 (6 RCTs) |
⊕⊕⊕⊝ moderate1 | |
| 124 per 1000 | 50 per 1000 (33 to 72) | ||||
| *The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds Ratio; RCT: randomized Controlled Trial; VTE: venous thromboembolism; DVT: deep venous thrombosis | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level for serious risk of bias (includes at least one study with overall high risk of bias)
Background
Description of the condition
Patients undergoing major abdominal or pelvic surgery are at increased risk of developing postoperative venous thromboembolic (VTE) complications (Najjar 2016; Rasmussen 2009). The incidence of VTE following abdominal surgery in the absence of thromboprophylaxis has been reported to be between 19% to 29% in high‐risk patients (Geerts 2001; Geerts 2004; Rasmussen 2009). For this reason, VTE thromboprophylaxis is routinely prescribed for postoperative patients, reducing the risk of VTE by up to 60% (Gross 2014). Prospective studies have demonstrated the risk for post‐discharge VTE remains elevated four to six weeks following surgery, with a cumulative incidence of VTE reaching up to 33.9% (Clarke‐Pearson 1984; Merkow 2011; Scurr 1988; Sørensen 1990). Though a large proportion of VTE‐related morbidity is attributable to postthrombotic syndrome, pulmonary hypertension, or recurrent thrombosis, VTE has been shown to be the most common cause of 30‐day mortality following cancer surgery and increase overall mortality by six‐fold (Agnelli 2006; Merkow 2011).
According to the Agency for Healthcare Research and Policy, VTE prevention for at‐risk patients presents the most significant opportunity to improve patient safety in hospitals among 79 patient safety practices due to its efficacy, cost‐effectiveness, and benefit‐risk ratio (Bahl 2010). Despite randomized trial data and clear guideline recommendations supporting post‐discharge extended‐VTE thromboprophylaxis following abdominal and pelvic cancer surgery (Gould 2012; NCCN 2016), a national sample of Medicare beneficiaries reported only 1.5% of patients receiving and then filling a prescription for its use (Merkow 2011).
Description of the intervention
The increased risk of VTE has been definitively proven to extend beyond the inpatient stay, with up to one third of all VTE events occurring post‐discharge (Gross 2014; Merkow 2011). Abdominal surgery creates a hypercoagulable state (Dahl 1995; Galster 2000; Iversen 2002; Rahr 1994), which has been well‐measured by thromboelastography (TEG), a more sophisticated coagulation monitoring method that allows evaluation of all stages of the coagulation and fibrinolytic process (Akay 2009; Mahla 2001). A population‐based, prospective study from the UK reported the risk of VTE to remain 10 to 50 times higher in weeks seven to 12 following inpatient surgery (Sweetland 2009). Thus, it would seem reasonable to consider extending VTE prophylaxis beyond the time when the patient is usually hospitalized, since the risk persists after hospital discharge.
How the intervention might work
The efficacy of extended duration thromboprophylaxis in patients undergoing major abdominal or pelvic surgery has been studied and supported by data, despite the apparent slow adoption into clinical practice. The Cochrane Review published in 2009 included four eligible studies and demonstrated a statistically significant reduction in the incidence of overall VTE after major abdominal or pelvic surgery in the control group (14.3%) as compared to patients receiving extended duration low molecular weight heparin (LMWH) (6.1%) (P < 0.0005) (Rasmussen 2009). Prolonged thromboprophylaxis with LMWH was also associated with a statistically significant reduction in the incidence of symptomatic VTE from 1.7% in the control group to 0.2% in the extended thromboprophylaxis group (P = 0.02). In addition, bleeding complications were not increased with prolonged thromboprophylaxis with LMWH.
Why it is important to do this review
This update is necessary because adoption of extended VTE prophylaxis has been slow, but research has actively continued on this topic. A reassessment of the current evidence may help spur increased adoption of extended VTE prophylaxis.
This update includes three more trials (Kakkar 2010; Sakon 2010; Vedovati 2014), increasing the number of included persons analyzed from 1242 to 1728.
Objectives
To evaluate the efficacy and safety of prolonged thromboprophylaxis with low molecular weight heparin (LMWH) for at least 14 days after abdominal or pelvic surgery compared with thromboprophylaxis administered during the in‐hospital period only in preventing late onset venous thromboembolism (VTE).
Methods
Criteria for considering studies for this review
Types of studies
Randomized clinical trials (RCTs) comparing prolonged thromboprophylaxis interventions with solely in‐hospital prophylaxis followed by placebo or no treatment were included. Objective, systematic assessment of VTE via diagnostic methods (venography, ultrasonography/doppler, ventilation/perfusion lung scintigraphy, computed tomography (CT) and/or autopsy) were mandatory for studies to be eligible for inclusion. Studies using only symptomatic diagnosis of VTE rather than confirmatory objective methods were excluded. Cluster trials were not considered.
Types of participants
Trials that included patients undergoing open or minimally invasive abdominal or pelvic surgery were included. Specific restrictions were not placed on the disease process for which surgery was being performed, so patients with both benign and malignant disease were included.
Types of interventions
Trials reporting the use of LMWH were considered (including varying dosages). Patients allocated to the intervention of prolonged thromboprophylaxis required administration of a LMWH for ≥ 14 days postoperatively to be eligible for study inclusion.
Similar to the initial review, unfractionated heparin, mechanical methods (graded compression stockings, sequential compression devices), and Vitamin K antagonists were excluded since these differing methods of VTE prophylaxis would make comparison difficult. In addition, oral anticoagulants such direct thrombin inhibitors or Factor Xa inhibitors were not considered in this review for the same reason.
Types of outcome measures
Primary outcomes
The primary outcome for the review was:
incidence of VTE within 30 days after surgery, including both symptomatic and asymptomatic VTE and PE (verified by objective methods: radiographic diagnosis/confirmation (duplex ultrasonography, CT angiography)).
Secondary outcomes
Secondary outcomes included:
incidence of all deep venous thrombosis (DVT);
incidence of proximal DVT (defined as thrombi located in and above the popliteal vein);
incidence of symptomatic VTE;
bleeding complications within three months of surgery, defined as major or minor bleeding according to the definition provided in the individual studies;
Search methods for identification of studies
Electronic searches
Electronic searches were conducted with assistance from a medical librarian and Information Specialist trained in performing systematic reviews. Searches were conducted in the Cochrane Central Register of Controlled Trials (CENTRAL) 2017, issue 9, Embase from 1947, PubMed from 1967, and LILACS from 1967.
Details of the search strategy for each database are given in the Appendices:
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1);
Embase 1947 to October 2017 (Appendix 2);
PUBMED 1967 to October 2017 (Appendix 3);
LILACS 1967 to October 2017 (Appendix 4);
Registered Trials: Clinicaltrials.gov (Appendix 5);
Registered Trials: WHO ICTRP (Appendix 6).
Searching other resources
The bibliography of each trial report were checked for additional references. The abstract books of the congresses arranged by The International Society on Thrombosis and Haemostasis as well as The Mediterranean League against Thrombosis were handsearched back to 1976.
Data collection and analysis
Selection of studies
All identified reports from electronic and manual searches were reviewed independently by two review authors (SIF and RSK) for consideration for inclusion. Consensus was obtained in cases where the review authors disagreed on the inclusion of the study.
Studies reporting on different types of LMWH were entered into the same analysis if the dose of LMWH was found comparable in anti‐Xa units (20 mg enoxaparin equals 2500 anti‐Xa units).
Data extraction and management
Two review authors (SIF and RSK) independently extracted data and differences were resolved through discussion. The following data were extracted: type of prophylaxis, duration of thromboprophylaxis, type of VTE end point, total incidence of VTE including specific rates of DVT (total, proximal and distal) and PE (total and fatal) within 30 days after surgery, bleeding events, severity of bleeding and total number of transfusions. Data were entered into Review Manager 5 (RevMan 5.3) by one review author (SIF) and verified by another review author (CCJ) (Figure 1).
Figure 1.
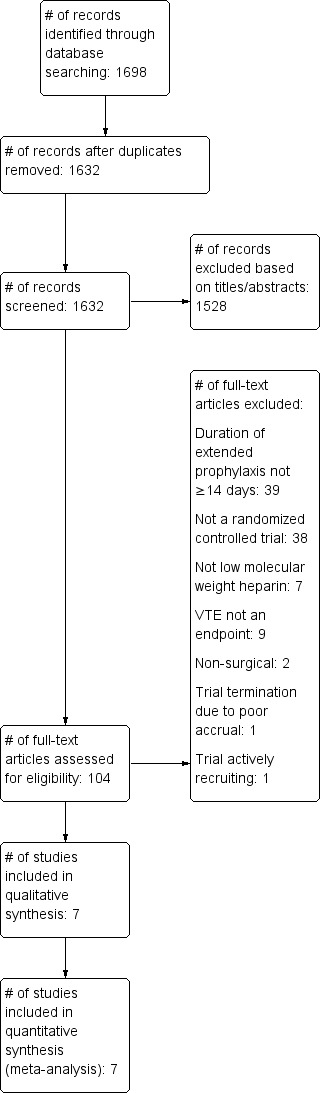
Study flow diagram
The primary search performed resulted 1698 studies, of which 1528 were excluded by reviewing the title and or removing duplicates, 104 were selected to be evaluated by the abstract, of these seven met the inclusion criteria. We excluded 97 studies by the primary selection because they lacked inclusion of patients undergoing abdominal or pelvic surgery, did not address thromboprophylaxis beyond day 14 after surgery, or were not clinical controlled trials. One trial were excluded as it was a double publication (Rasmussen 2003), and one because it was a review (Rasmussen 2003a). In addition, we found one trial by handsearching and only as an abstract presentation, and was reported as unpublished data (Jørgensen 2002). One study is actively recruiting patients (Zheng 2017), however, study methodology was not explicity outlined in the trial protocol.
Assessment of risk of bias in included studies
We used Cochrane's tool for assessing risk of bias, described in Table 8.5.d of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (SIF and CCJ) independently assessed risk of bias for each study and any differences were discussed and consensus reached.
We assessed the risks of bias of the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting bias.
Each domain was judged as low risk, high risk or unclear risk of bias according to predefined criteria described in Cochrane's 'Risk of bias' tool (Higgins 2011).
Measures of treatment effect
No comparisons across different trials between apparently equal groups were made because of the well‐documented substantial inter‐observer variation regarding the reported end points in various prophylactic trials, which substantially can influence the incidence of VTE (Wille‐Jørgensen 1992). Variations in diagnostic objective measures across trials were acceptable as long as the method was uniformly applied within the individual study to all patients.
Outcomes were measured as dichotomous, either present or absent. Treatment effect was estimated using Mantel Haentzel (M‐H) odds ratios (OR) with 95% confidence intervals (CIs). As the included studies were relatively clinically homogeneous, we applied a fixed‐effect model.
A P value < 0.05 was considered to represent statistical significance.
Unit of analysis issues
The unit of analysis was the individual patient. No trials with an alternate unit of analysis were identified in the search, and thus unit of analysis issues did not apply.
Dealing with missing data
This updated review was conducted with a modified intention‐to‐treat analysis of patients reaching an evaluable VTE end point. Because objective assessment of VTE/DVT can be invasive or inconvenient, or both, in each study there was a substantial minority of patients who did not reach an evaluable VTE end point.
Bergqvist 2002: 81 (32.7%) patients in placebo group and 88 (34.8%) patients in the experimental group excluded due to lack of adequate venography
Jørgensen 2002: Study terminated prematurely due to lack of funding and high attrition rate (rate not reported)
Kakkar 2010: 67 (21.3%) patients in experimental group, 70 (22.6%) patients excluded due to inadequate venography
Lausen 1998: Study terminated prematurely due to lack of funding. Of 176 eligible patients (87 in extended prophylaxis group and 89 in control group), 29 (33.3%) excluded in experimental group and 29 (32.6%) in the control group
Rasmussen 2006: 44 (19.8%) patients in control group and 40 (19.5%) patients in experimental group excluded
Sakon 2010: 26 patients (23.9%) in experimental group and seven patients (18.4%) in control group excluded from total treated population due to inadequate VTE assessment or measurement
Vedovati 2014: The study was interrupted after the results of the interim analysis showed a significant reduction in the rate of VTE in patients assigned to extended prophylaxis (P < 0.01)
Because the attrition rate was not reported in the Jørgensen study, we could not perform a best‐case/worst‐case scenario, however, a sensitivity analysis was performed excluding this study.
Assessment of heterogeneity
Clinical and methodological heterogeneity were assessed by evaluating the risk of bias as detailed in the 'Risk of bias' table (Figure 2) and Characteristics of included studies. We quantified statistical heterogeneity using the I2 statistic (I2=((Q‐df/Q) x100% where Q was the Chi2 statistic, and df represented the degrees of freedom). This illustrated the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error (Higgins 2011).
Figure 2.
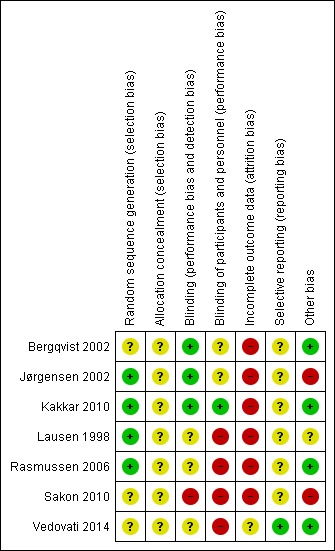
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Cut‐off values for the I2 statistic were:
0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We did not assess funnel plot asymmetry for outcomes reported, as there were fewer than 10 studies, which is considered unreliable as described in the Cochrane Handbook for Systematic Reviews of Intervention (Chapter 10, Sterne 2011).
Data synthesis
Data analysis was performed using the RevMan 5 software (RevMan 2014). Summary ORs and 95% CIs were computed for:
all VTE;
all DVT;
proximal DVT;
symptomatic VTE;
bleeding complications.
A fixed‐effect model was used since the studies were relatively clinically homogeneous (e.g. comparing the same medication over a minimum time period).
'Summary of findings' Table
We assessed the overall quality of evidence for the main review outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach in Table 1). The 'Summary of findings' table highlighted the overall quality of the body of evidence for the main review outcomes, using the GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, indirectness, imprecision and publication bias). Judgements about the quality of the evidence (high, moderate, low or very low) were justified, documented and incorporated into the reporting of results for each outcome.
The GRADE system classifies the quality of evidence into one of four grades.
| Grade | Definition |
| High | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate | Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate |
| Very low | Any estimate of effect is very uncertain |
Subgroup analysis and investigation of heterogeneity
There was concern for clinical heterogeneity given that the Vedovati 2014 study was conducted in laparoscopic patients who may have a different baseline VTE risk. Therefore, a post‐hoc subgroup analysis was done for all VTE and all DVT to evaluate whether the results would differ if this study was excluded.
Sensitivity analysis
There was concern as the Jørgensen 2002 study was terminated prematurely due to lack of funding and attrition rate, but the attrition rate was not reported. An additional post‐hoc subgroup analysis for all VTE and all DVT was performed with this study excluded.
Results
Description of studies
Results of the search
The primary search revealed 1632 studies, of which 1528 were excluded by reviewing the title and/or removing duplicates. We selected 104 abstracts to be evaluated and of these seven met the inclusion criteria (Figure 1). The 97 studies excluded in the initial selection lacked inclusion of patients undergoing abdominal or pelvic surgery, did not address thromboprophylaxis beyond day 14 following surgery, or were not controlled trials. One trial was excluded as it was a double publication (Rasmussen 2003), and one because it was a review (Rasmussen 2003a) In addition, one trial was included by handsearch as an abstract presentation, and is thus reported as unpublished data (Jørgensen 2002). An ongoing study in China was identified (Zheng 2017), however, the trial protocol did not describe the duration of low molecular weight heparin (LMWH) prophylaxis.
Included studies
The initial version of this review was published in the Cochrane Library in 2009 and included four studies (Rasmussen 2009). This update adds three more studies (Kakkar 2010; Sakon 2010; Vedovati 2014) and increases the number of trial participants from 1242 to 1728. Studies were published between 1998 and 2014.
Six randomized controlled trials (RCTs) published as full‐text articles and one RCT published as an abstract met the inclusion criteria of this review and were included in the meta‐analysis (Bergqvist 2002; Jørgensen 2002; Kakkar 2010; Lausen 1998; Rasmussen 2006; Sakon 2010; Vedovati 2014). The trials by Bergqvist and Kakkar were double‐blinded RCT's, while the trials of Rasmussen, Lausen, and Vedovati were open‐label trials with blinded assessment of the venograms or compression ultrasounds. The Sakon trial did not explicity state whether the objective endpoint was assessor blinded. The unpublished double‐blinded Jorgensen RCT data were available within a meta‐analysis, which also included the data from Lausen 1998. Both of these trials were terminated prematurely due to lack of funding. The data from Lausen 1998 were entered into the present analysis once, thus the data obtained from the Jorgensen study for the present analysis are the unpublished data of the Jorgensen study from that meta‐analysis.
Five studies included only cancer patients (Bergqvist 2002; Jørgensen 2002; Kakkar 2010; Sakon 2010; Vedovati 2014). whereas two trials considered patients undergoing general surgery for both benign and malignant diseases (Lausen 1998; Rasmussen 2006). Only one study (Vedovati 2014) performed minimally invasive (laparoscopic) abdominal surgery, with the remaining studies strictly open surgery. Three trials (Kakkar 2010; Sakon 2010; Vedovati 2014) were added to the current update from the original review done in 2009, analyzing a total of 1728 participants in seven studies for the primary end point of venous thromboembolism.
Excluded studies
Relevant excluded studies and the reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
No trial had a low risk of bias across all the categories. All trials had at least one, and up to four, categories with high risk of bias. For the majority of categories, the risk of bias was unclear. Risk of bias is illustrated in Figure 2.
Allocation
All studies were RCTs, but there was variation in the detail provided as to how patients were randomized. In four studies, randomization was by computer‐generated allocation and therefore low risk (Jørgensen 2002; Kakkar 2010; Lausen 1998; Rasmussen 2006). In three studies, allocation was apparently random but the method was not described in the manuscript (Bergqvist 2002; Sakon 2010; Vedovati 2014), and therefore judged as an unclear risk of bias.
Blinding
Studies had a range of degree of blinding. In the majority, the outcomes assessors were blinded; however, in one study, neither patients, healthcare providers or outcomes assessors were blinded (Sakon 2010). In other studies, outcomes assessors (e.g. those reading the venograms) were blinded but the patients and healthcare providers were not (Lausen 1998; Rasmussen 2006; Vedovati 2014), and therefore there was some risk for bias. In three studies, patients, healthcare providers and outcomes assessors were all blinded (Bergqvist 2002; Jørgensen 2002; Kakkar 2010). Only four studies specifically reported whether data analysts were blinded, raising the possibility of bias in the other studies where this was not reported. In two of these studies, data analysts were blinded (Bergqvist 2002; Jørgensen 2002), and in two they were not (Lausen 1998; Rasmussen 2006). Thus, in only two studies were all participants, healthcare providers, outcomes assessors and data analysts specifically stated to have been blinded (Bergqvist 2002; Jørgensen 2002).
Incomplete outcome data
In all studies but one (Vedovati 2014), there was high risk of attrition bias due to significant attrition. This significant rate of attrition may be related to the invasiveness of the procedures used to determine DVT/VTE incidence (e.g. venogram) and also technical difficulties with the procedures. Attrition rates varied from 18.4% to 32.7% in the placebo groups and 19.5% to 34.8% in the treatment groups. In one study, the attrition rate was reported to be high and was a cause of the termination of the study, but the exact attrition rate was not reported (Jørgensen 2002).
Selective reporting
In all studies but one (Vedovati 2014), analysis was done on a modified intention‐to‐treat analysis of patients reaching an evaluable VTE end point (e.g. verification of symptomatic VTE, venogram or ultrasound), and were therefore of unclear risk of bias. Only one study performed an intention‐to‐treat analysis, and in that study all patients actually reached an evaluable VTE end point (Vedovati 2014).
Other potential sources of bias
In the Jørgensen 2002 study, the venograms were re‐evaluated by the same radiologists who assessed the venograms in the study by Lausen and colleagues (Lausen 1998), in order to perform a meta‐analysis with the results of the study of Lausen and colleagues, introducing the possibility of bias (Jørgensen 2002).
In Lausen 1998, a prespecified definition of bleeding complication was not used, and rather specific bleeding complications were listed, leaving which complications were considered bleeding complications open to interpretation.
Sakon 2010 was supported by a pharmaceutical manufacturer, which also provided editorial support. The principal investigator reported several relevant conflicts of interest.
Effects of interventions
The search provided the opportunity to compare extended prophylaxis with LMWH to placebo or no treatment with the following outcome parameters: Overall VTE, all deep venous thrombosis (DVT), proximal DVT, symptomatic VTE and bleeding complications.
The studies evaluated for VTE at o rnear the four‐week mark after surgery, as detailed in the Characteristics of included studies table.
Primary outcome
Incidence of venous thromboembolism (all VTE)
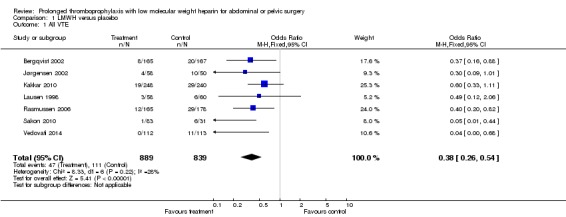
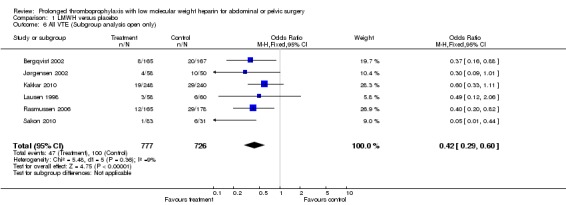
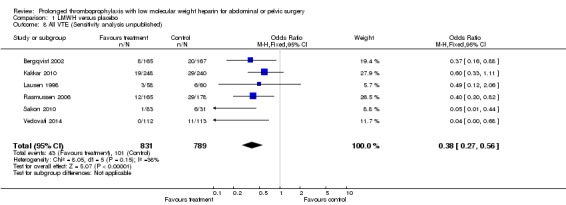
The incidence of VTE after major abdominal or pelvic surgery was 13.2% in the control group as compared to 5.3% in the patients receiving out‐of‐hospital low molecular weight heparin (LMWH) (Mantel Haentzel (M‐H) odds ratio (OR) 0.38, 95% confidence interval (CI) 0.26 to 0.54; I2 = 28%; 7 studies, n= 1728; moderate‐quality evidence) (Figure 3). Subgroup analysis of trials including only patients operated with an open technique (i.e. excluding patients operated upon laparoscopically (Vedovati 2014) showed similar findings with an incidence of VTE of 13.8% in the control group and 6.0% in the treatment group (M‐H 0.42, 95% CI 0.29 to 0.60; I2 = 9%; 6 studies, n= 1503; moderate‐quality evidence) (Figure 4). Similarly, sensitivity analysis with exclusion of the prematurely terminated trial (Jørgensen 2002) showed a benefit for extended prophylaxis with LMWH, with VTE occurring in 12.8% of the control group and 5.2% of the treatment group (M‐H OR 0.38, 95% CI 0.27 to 0.56; I2 = 38%;6 studies, n= 1620; moderate‐quality evidence) (Figure 5).
Figure 3.
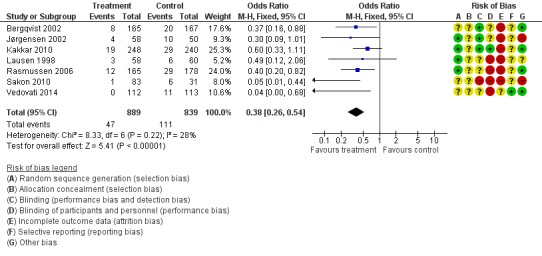
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.1 All VTE.
Figure 4.
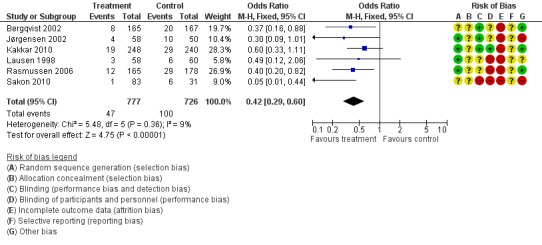
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.6 All VTE (Subgroup analysis: Open resections only, laparoscopic excluded (Vedovati 2014)).
Figure 5.
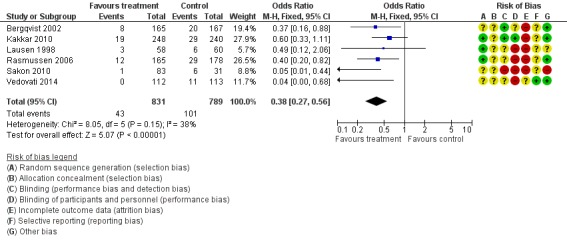
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.8 All VTE (Sensitivity analysis: Unpublished study excluded (Jørgensen 2002)).
Secondary outcomes
Incidence of deep venous thromboembolism (all DVT)
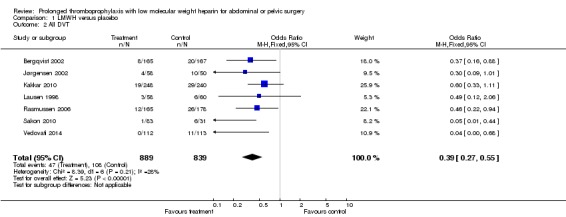
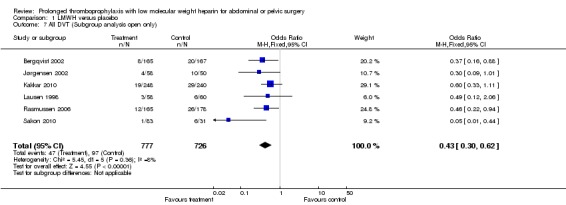
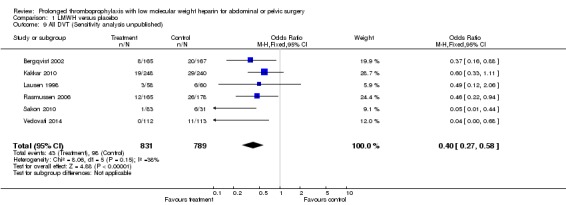
Prophylaxis with LMWH as compared to control also offered better protection against all DVT (M‐H OR 0.39, 95% CI 0.27 to 0.55; I2 = 28%; 7 studies, n= 1728; moderate‐quality evidence). DVT occurred in 12.9% of the control group versus 5.3% of the treatment group (Figure 6). In the subgroup analysis including only open technique, the rate of DVT was 13.3% in controls and 6.4% in the treatment group (M‐H OR 0.43, 95% CI 0.30 to 0.62; I2 = 8%; 6 studies, n= 1503; moderate‐quality evidence) (Figure 7). Exclusion of the prematurely terminated trial (Jørgensen 2002) did not affect the results, with DVT occurring in 12.4% of the control group versus 5.2% of the treatment group (M‐H OR 0.40, 95% CI 0.27 to 0.58; I2 = 38%; 6 studies, n= 1620; moderate‐quality evidence) (Figure 8).
Figure 6.
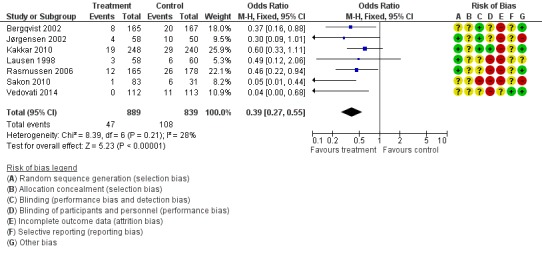
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.2 All DVT.
Figure 7.
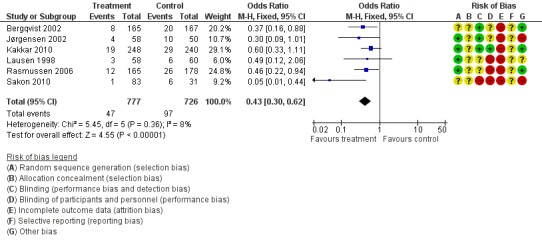
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.7 All DVT (Subgroup analysis: Open resections only, laparoscopic excluded (Vedovati 2014)).
Figure 8.
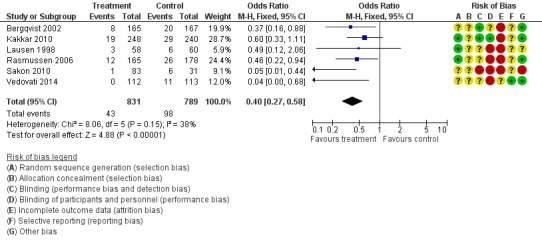
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.9 All DVT (Sensitivity analysis: Unpublished study excluded (Jørgensen 2002)).
Incidence of proximal deep venous thromboembolism (proximal DVT)
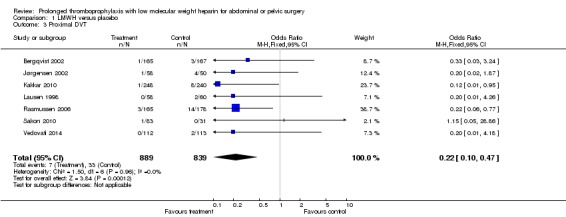
When the outcome was limited to proximal DVT (thus excluding more distal DVT which may not be clinically significant), there was still a reduction in DVT with extended LMWH. Rates of proximal DVT were 3.9% in the control group and 0.8% in the treatment group (M‐H OR 0.22, 95% CI 0.10 to 0.47; I2 = 0%; 7 studies, n= 1728; moderate‐quality evidence) (Figure 9).
Figure 9.
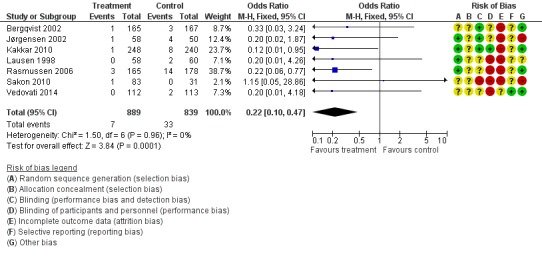
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.3 Proximal DVT.
Incidence of symptomatic venous thromboembolism (symptomatic VTE)
Prolonged thromboprophylaxis with LMWH was associated with a statistically significant reduction of symptomatic VTE, 1.3% in the control group versus 0.1% in the treatment group (M‐H OR 0.30, 95% CI 0.08 to 1.11; I2 = 0%; 7 studies, n= 1728; moderate‐quality evidence). (Figure 10).
Figure 10.
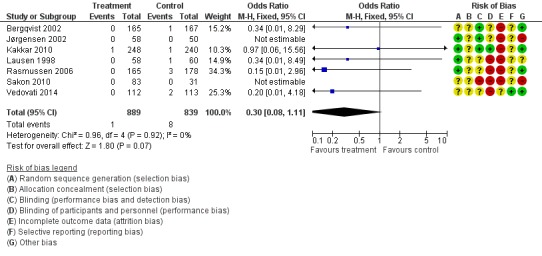
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.4 Symptomatic VTE.
Bleeding complications
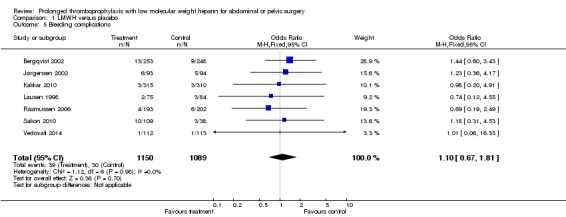
There was no significant difference regarding the incidence of overall (both major and minor) bleeding between the control group (2.8%) and the LMWH group (3.4%), (M‐H OR 1.10, 95% CI 0.67 to 1.81; I2 = 0%; 7 studies, n= 2239; moderate‐quality evidence). The number of patients in the safety population were higher than in the other outcome groups since all patients receiving at least one injection of the treatment and not necessarily reaching the evaluable end point were included for this analysis (Figure 11).
Figure 11.
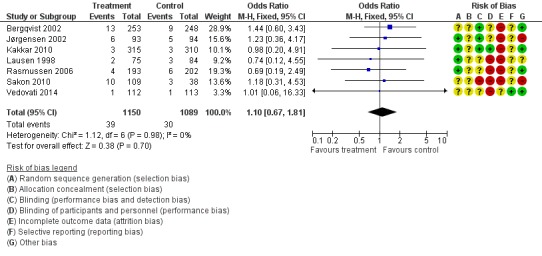
Forest plot of comparison: 1 LMWH versus placebo, outcome: 1.5 Bleeding complications.
Thus, extended LMWH demonstrated a reduction in all thrombotic outcomes (all VTE, all DVT, proximal DVT and symptomatic VTE) with no difference in bleeding complications.
Discussion
Summary of main results
In the first version of this review which included four studies, (Bergqvist 2002; Jørgensen 2002; Lausen 1998; Rasmussen 2006), prolonged thromboprophylaxis with low molecular weight heparin (LMWH) compared with prophylaxis limited to in‐hospital treatment only, significantly reduced the risk of major venous thromboembolism (VTE) in patients undergoing major abdominal or pelvic surgery. Three trials (Kakkar 2010; Sakon 2010; Vedovati 2014) were added to the current update, analyzing a total of 1728 participants in seven studies, confirming the initial review's findings.
The incidence of VTE after major abdominal or pelvic surgery was reduced from 13.2% in the control group to 5.3% in the patients receiving out‐of‐hospital LMWH (Mantel Haentzel (M‐H) odds ratio (OR) 0.38, 95% confidence interval (CI) 0.26 to 0.54; I2 = 28%). Similarly, all deep venous thrombosis (DVT) was also reduced: 12.9% of the controls versus 5.3% of the treatment group (M‐H OR 0.39, 95% CI 0.27 to 0.55; I2 = 28%).
When the analysis was limited to solely proximal DVT or symptomatic VTE, a benefit of extended prophylaxis was still apparent. Rates of proximal DVT were 3.9% in the control group and 0.8% in the treatment group (M‐H OR 0.22, 95% CI 0.10 to 0.47; I2 = 0%) and symptomatic VTE was 1.0% and 0.1%, respectively (M‐H OR 0.30, 95% CI 0.08 to 1.11; I2 = 0%).
Importantly, there was no significant difference regarding the incidence of overall (both major and minor) bleeding between the control group (2.8%) and the LMWH group (3.4%), (M‐H OR 1.10, 95% CI 0.67 to 1.81; I2 = 0%).
No study established mortality as a primary efficacy end point; however, several did report mortality as a secondary outcome. The incidence of objectively confirmed PE was extremely low across the studies, which would typically be considered the embolic event most likely to account for a mortality. However, no study identified a fatal PE with certainty. Causes of mortality among the studies, when described, were secondary to medical and/or surgical complications or progression of disease (for patients with malignancy). A small minority of patients died of a sudden cardiac arrest, however, among these patients, no patient was confirmed to have a PE nor was clinically suspected of experiencing a PE event.
No study correlated the VTE end point to the anatomic location of pathology (intra‐peritoneal versus extra‐peritoneal) or type of operation. For this reason, no analysis could be performed stratifying abdominal versus pelvic surgery.
In six of the seven included studies (Bergqvist 2002; Kakkar 2010; Lausen 1998; Rasmussen 2006; Sakon 2010; Vedovati 2014), objective confirmatory evaluation was used only for clinically suspected pulmonary embolism (PE). (Jørgensen 2002 is an unpublished abstract, such that the information of PE assessment was not available). Evaluation for PE was performed by ventilation/perfusion lung scintigraphy, pulmonary angiography, computed tomography (CT) scan, or autopsy. However, the event rate for PE among all trials was nominal, therefore a sensitivity analysis was not performed (Analysis 1.4) Bergqvist 2002 reported one PE occurring in the placebo group and confirmed by objective testing during the trial period, and one patient in the placebo group identified on autopsy during the follow‐up period (which was an unsuspected PE). Kakkar 2010 reported no objectively identified PE during the study trial period, nor in the follow‐up period. Nine deaths occurred during the double‐blind period, five associated with identifiable causal medical reasons, and the remaining four (one in the placebo group, three in the LMWH group) did not undergo confirmatory autopsy or have a positive objective test identifying PE. Lausen 1998 reported no objectively identified PE events. Eleven patients died during the trial, however, the authors reported these patients to have died under circumstances not clinically suggestive of a PE. Rasmussen 2006 reported three cases of symptomatic, non‐fatal cases of PE, with two of these events verified by ventilation/perfusion lung scintigraphy or CT scan, or both (all patients within the control group). Jørgensen 2002, Sakon 2010 and Vedovati 2014 reported no PE events in either group.
Analysis 1.4.
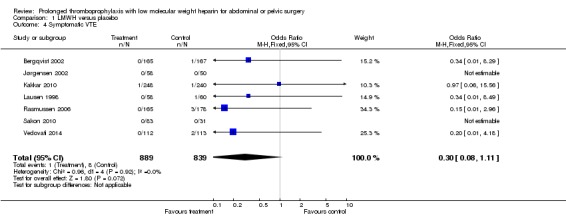
Comparison 1 LMWH versus placebo, Outcome 4 Symptomatic VTE.
Overall completeness and applicability of evidence
The studies included in this analysis were all designed on 'surrogate' end points based on objective diagnosis (venography, ultrasonography, ventilation/perfusion scintigraphy, CT or autopsy) performed at extended time intervals following surgery. The clinical relevance of asymptomatic cases of VTE detected objectively has been questioned, as the majority of these are confined to the lower extremity veins with limited potential for propagation or embolization. However, even asymptomatic postoperative VTE is associated with a 59% relative risk increment of developing late post‐thrombotic syndrome (Wille‐Jørgensen 2005). Furthermore, a highly significant association between asymptomatic proximal VTE and 90‐day mortality has been described in medical patients (Vaitkus 2007). This increased mortality risk supports prior autopsy series reporting symptomatic DVT seldom precede a fatal PE (Huber 1992; Lindblad 1991). Additionally, orthopedic surgery patients receiving extended thromboprophylaxis with LMWH have shown a relative reduction of asymptomatic DVT translating into a corresponding reduction of symptomatic DVT (Arnesen 2003; Eilkelboom 2001; Hull 2001). Based on these observations, asymptomatic VTE does have clinical importance, thus representing more than a 'surrogate' end point.
Although inpatient thromboprophylaxis is considered standard hospital practice and prolonged thromboprophylaxis is both effective and safe under most circumstances, outpatient VTE thromboprophylaxis is infrequently utilized for at‐risk patients (Kalka 2009; Merkow 2011). Two of the included studies described a high rate (more than 97% of the patients) of compliance during the study trial, (Lausen 1998; Rasmussen 2006), while compliance was not reported in the remaining five studies (Bergqvist 2002; Jørgensen 2002; Kakkar 2010; Sakon 2010; Vedovati 2014). Despite the strong evidence reported in the initial version of the review (Rasmussen 2009), along with the American Society of Clinical Oncology, (Lyman 2015) National Cancer Center Network (Version 1.2016) ,and the American College of Chest Physician guidelines (Gould 2012), the proportion of patients receiving prolonged thromboprophylaxis is surprisingly low. It remains unclear why these data‐driven recommendations have not translated into routine clinical practice, however, a number of financial, social, and provider‐level barriers may discourage prolonged VTE thromboprophylaxis (Merkow 2011). In a national study using administrative data, Amin and colleagues found that among patients undergoing major abdominal surgery, approximately 60% received inpatient thromboprophylaxis, yet only 1.6% of patients meeting the American College of Chest Physician guidelines for extended prophylaxis filled an outpatient prescription within 30 days of discharge (Amin 2010). Interestingly, the authors also found that among orthopedic surgery patients analyzed separately within the study, 54.4% filled a prescription for outpatient VTE prophylaxis. Other reports have documented post‐discharge VTE thromboprophylaxis in the orthopedic population approaching 90% (Bergqvist 2012). In another study focusing on colorectal surgical patients, only 1.2% of Medicare beneficiaries received guideline‐recommended post‐discharge thromboprophylaxis (Merkow 2011).
Accurate assessment of a patient’s VTE risk is critical to improving compliance with prophylaxis guidelines. Practical methods for VTE risk stratifying surgical inpatients have been developed (Caprini 2001; Rogers 2007), however, these models have been criticized for being cumbersome as well lacking strong external validation (Gould 2012). The weight each risk factor confers to a patient’s postoperative VTE relative risk is cumulative. Notably, malignancy is recognized as a moderate risk factor in the Caprini model (but not in the Rogers Risk Score), but inflammatory bowel disease is a minor risk factor, (Bahl 2010, Gross 2014; Merkow 2014). In a NSQIP analysis, the postoperative rate of VTE in IBD patients was found to be significantly greater than the rate of VTE in patients who have colorectal cancer undergoing similar operations, particularly in the post‐discharge timeframe (40% occurred post‐discharge), emphasizing the deficiencies in currently utilized VTE risk‐assessment tools (Gross 2014).
Quality of the evidence
The robustness of the outcome in the primary analysis of overall VTE and of symptomatic VTE was demonstrated by the fact that exclusion of the unpublished data (Jørgensen 2002) and exclusion of the exclusively laparoscopic study (Vedovati 2014) did not alter the conclusions of this analysis. These findings also occur despite a relatively high attrition rate in all trials included. In the Bergqvist study, almost one third of the patients did not undergo venography or had un‐interpretable venogram. In the study by Kakkar and colleagues, the proportion of patients in whom inadequate or no venography was obtained (21.5% LMWH group and 22.5% control) was also relatively high. The inability to objectively measure the primary VTE end point for a significant number of trial participants (attrition rates varied from 18.4% to 32.7% in the placebo groups and 19.5% to 34.8% in the treatment groups, exclusive of the Vedovati 2014), may have affected the study end point.
Some differences were identified regarding trial design. Three studies were double‐blinded and placebo‐controlled (Bergqvist 2002; Jørgensen 2002; Kakkar 2010), whereas three reported assessor‐blinded evaluations of the venous system (Lausen 1998; Rasmussen 2006; Vedovati 2014). The trial from Sakon et al did not explicitly describe if the objective VTE endpoint assessment was assessor‐blinded (Sakon 2010). Differences regarding patient characteristics were also present. Five studies included only cancer patients (Bergqvist 2002; Jørgensen 2002; Kakkar 2010; Sakon 2010; Vedovati 2014), whereas the trials by Lausen and Rasmussen also considered patients undergoing surgery for both benign and malignant diseases (Lausen 1998; Rasmussen 2006). These two studies included a substantial proportion of patients with benign disease ( Rasmussen 2006: 42%) and (Lausen 1998: 31%). It was not possible to make a comparison between these two patients groups, because no separate data were provided. Additionally, only one study (Vedovati 2014) performed laparoscopic surgery. The number of patients included in this trial was not large enough to make a reliable comparison between the different open and minimally invasive techniques. Multiple studies have reported that laparoscopic surgery induces a similar postoperative hypercoaguable state when compared to open surgery, however, the degree and duration of the hypercoagulability is not yet well understood (Diamantis 2007; Nguyen 2001; Tsiminikakis 2009). It is possible that the increased number of minimally invasive surgical procedures (Rahr 1999; SAGES 2007) and the current trend for fast‐track recovery (Kehlet 2005) following gastrointestinal surgery may lower the risk of postoperative VTE. Because patients undergoing minimally invasive abdominal or pelvic surgeries may have shorter lengths of hospitalization, these patients might presumably receive a shorter time of sufficient thromboprophylaxis. Future clinical trials will need to evaluate this additional variable when assessing patient VTE risk and the appropriate duration of thromboprophylaxis.
Potential biases in the review process
Trial author involvement in review (review author MSR).
Agreements and disagreements with other studies or reviews
This version agrees with the findings of the prior version of this review, demonstrating a benefit to extended prophylaxis with LMWH after abdominal or pelvic surgery.
Authors' conclusions
Administration of low molecular weight heparin (LMWH for) ≥14 days compared to inpatient only prophylaxis after major abdominal or pelvic surgery significantly reduces the incidence of venous thromboembolism (VTE) without jeopardizing safety, and should be utilized.
Unfractionated heparin and LMWH in general surgery patients has been shown to likely be comparable in efficacy for thromboprophylaxis (Geerts 2001; Geerts 2004; Gould 2012; Mismetti 2001). However, LMWH allows once‐daily injection and carries a lesser risk of heparin‐induced thrombocytopenia (Jørgensen 1993). Although LMWHs belong to the same drug category, the use of single drug variations in terms of anticoagulant profiles may have added some bias in this analysis. However, the number of patients included in each trial was not large enough to make a reliable comparison between the different types of LMWHs. This review was limited to exclusively LMWH and did not assess unfractionated heparin, vitamin K antagonists, oral direct thrombin inhibitors or oral factor Xa inhibitors for prolonged surgical thromboprophylaxis. The emerging use of oral anticoagulants may help improve the barriers related to compliance, however, clinical studies evaluating abdominal and pelvic surgical patient populations are limited at this time (Sakon 2012).
Future research of optimizing VTE thromboprophylaxis should include patients undergoing laparoscopic surgery under a fast track, enhanced recovery protocol. New antithrombotic drugs with oral administration might improve the ease and compliance of prolonged thromboprophylaxis and should be studied to determine if they are of equal efficacy as LMWH.
Acknowledgements
Drs. L.N. Jørgensen and P. Wille‐Jørgensen were authors on the original version of the review. Susan Sharpe, Library Supervisor, Moffitt Cancer Center, Tampa, Florida assisted with database searches. Caitlin Bakker, Information specialist, University of Minnesota Bio‐Medical Library.
The authors acknowledge the Cochrane Colorectal Cancer editorial office, Josephine Lyngh Steenberg and Henning Keinke Andersen, and the peer reviewers for their valuable comments and assistance.
Appendices
Appendix 1. Appendix: Cochrane Central Register of Controlled Trials (CENTRAL)
general near/3 surgery (MESH) OR abdom* near/3 (MESH) OR pelvi* near/3 (MESH) OR gynecolo* near/3 (MESH)
AND thrombos* OR thromboemb* OR embol* or "Embolism and Thrombosis" (MESH)
Appendix 2. Appendix: Embase (Ovid) 1947 to October 2017
| 1 | 'general surgery' OR 'abdominal surgery' OR 'pelvic surgery' OR 'gynecologic surgery' |
| 2 | 'abdominal surgery'/exp |
| 3 | 'general surgery'/exp |
| 4 | 'pelvis surgery'/exp |
| 5 | 'gynecologic surgery'/exp |
| 6 | #1 OR #2 OR #3 OR #4 OR #5 |
| 7 | thrombo* OR thromboem* OR embol* |
| 8 | 'thrombosis'/exp OR 'thrombosis prevention'/exp |
| 9 | 'thromboembolism'/exp |
| 10 | 'embolism'/exp |
| 11 | #7 OR #8 OR #9 OR #10 |
| 12 | 'prophylaxis' OR 'prophylactic' OR 'prevention' OR 'preventative' OR 'chemoprophylaxis' OR 'antithrombotic prophylaxis' OR 'reduction' OR anticoag* OR 'heparin' OR 'thromboprophylaxis' |
| 13 | 'prevention'/exp OR 'thrombosis prevention'/exp |
| 14 | 'heparin'/exp |
| 15 | 'anticoagulant agent'/exp |
| 16 | #12 OR #13 OR #14 OR #15 |
| 17 | 'prolonged' OR 'long term' OR 'duration' OR 'late' OR 'extended' OR 'discharge' |
| 18 | #6 AND #11 AND #16 AND #17 |
| 19 | 'crossover procedure':de OR 'double blind procedure':de OR 'randomized controlled trial':de OR 'single blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti |
| 20 | #18 AND #19 |
Appendix 3. Appendix: PUBMED 1967 to October 2017
((((((((((((("general surgery" OR "General Surgery" [MESH] OR "abdominal surgery" OR "Abdomen/surgery" [MESH] OR "pelvic surgery" OR "Pelvis/surgery" [MESH] OR "gynecologic surgery" OR "Gynecologic Surgical Procedures" [MESH]))) AND ((({("general surgery" OR "General Surgery" [MESH] OR "abdominal surgery" OR "Abdomen/surgery" [MESH] OR "pelvic surgery" OR "Pelvis/surgery" [MESH] OR "gynecologic surgery" OR "Gynecologic Surgical Procedures" [MESH]))) AND ((thrombos* OR thromboesm* OR embol* OR "Embolism and Thrombosis" [MESH])))) AND (((((((("general surgery" OR "General Surgery" [MESH] OR "abdominal surgery" OR "Abdomen/surgery" [MESH] OR "pelvic surgery" OR "Pelvis/surgery" [MESH] OR "gynecologic surgery" OR "Gynecologic Surgical Procedures" [MESH]))) AND ((thrombos* OR thromboesm* OR embol* OR "Embolism and Thrombosis" [MESH])}}) AND ((("general surgery" OR "General Surgery" [MESH] OR "abdominal surgery" OR "Abdomen/surgery" [MESH] OR "pelvic surgery" OR "Pelvis/surgery" [MESH] OR "gynecologic surgery" OR "Gynecologic Surgical Procedures" [MESH]))) AND ("antithrombotic prophylaxis" OR reduction OR anticoag* OR "prevention and control" [MESH Subheading] OR heparin OR "Heparin, Low‐Molecular‐Weight" [MESH Terms] OR Anticoagulants [MESH Terms] OR thromboprophylaxis)))) AND ((prolonged OR "long term" OR duration OR late OR extended OR discharge [MESH Terms]) OR "administration and dosage" [MESH Subheading])))))) AND ((((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab] OR randomised[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh])))
Appendix 4. AppendixL LILACS 1967 to October 2017
(MH:"General Surgery" OR MH:"Cirugfa General" OR MH:"Cirurgia Geral" OR TW:"General Surgery" OR TW:"Cirugia General" OR TW:"Cirurgia Geral" OR MH:H02.403.810.300) AND (TW:Embolism OR TW:Thrombosis OR TW:Embolia OR TW:Trombosis OR MH:"Embolia y Trombosis" OR TW:Embolia OR TW:Trombose OR MH:"Embolia e Trombose" OR MH:C14.907.355)
Appendix 5. Appendix: Clinicaltrials.gov
Condition/ Disease: embolism OR thrombosis
Other Terms: general surgery OR abdominal surgery OR pelvic surgery OR pelvis surgery OR gynecologic surgery
| Terms | Search Results* | Entire Database** |
| Synonyms | ||
| general surgery | 401 studies | 31,181 studies |
| surgery | 401 studies | 31,181 studies |
| surgery | 403 studies | 39,899 studies |
| Surgical | 98 studies | 15,174 studies |
| operations | 25 studies | 4,764 studies |
| invasive procedures | 8 studies | 413 studies |
| operative procedures | 3 studies | 133 studies |
| operative therapy | ‐‐ | 41 studies |
| Surgically | ‐‐ | 975 studies |
| general | 72 studies | 39,430 studies |
| Global | 8 studies | 12,389 studies |
| Generalised | ‐‐ | 98 studies |
| Generalized | ‐‐ | 1,089 studies |
| Generalizes | ‐‐ | 4 studies |
| abdominal surgery | 11 studies | 695 studies |
| abdomen surgeries | ‐‐ | 2 studies |
| abdominal operations | ‐‐ | 39 studies |
| abdominal | 25 studies | 6,805 studies |
| Abdomen | 2 studies | 1,444 studies |
| abd | 1 studies | 273 studies |
| Abdominopelvis | ‐‐ | 1 studies |
| pelvic surgery | 1 studies | 103 studies |
| pelvic | 8 studies | 2,907 studies |
| Pelvis | 1 studies | 711 studies |
| pelvis surgery | ‐‐ | 0 studies |
| pelvis | 8 studies | 2,908 studies |
| pelvic | 7 studies | 2,423 studies |
| intrapelvic | ‐‐ | 4 studies |
| gynecologic surgery | 2 studies | 203 studies |
| Gynecological Surgeries | 1 studies | 90 studies |
| Gynecologic Surgical Procedure | ‐‐ | 12 studies |
| Gynecological Surgical Procedure | ‐‐ | 3 studies |
| gynecologic | 6 studies | 1,155 studies |
| Gynaecologic | ‐‐ | 64 studies |
| embolism | 367 studies | 1,764 studies |
| Embolus | 20 studies | 49 studies |
| thrombosis | 385 studies | 2,004 studies |
| Blood Clots | 21 studies | 78 studies |
| Thrombi | 20 studies | 141 studies |
| Blood Clotting | 8 studies | 32 studies |
| thrombotic disorder | ‐‐ | 3 studies |
| ‐‐ | No studies found |
| * | Number of studies in the search results containing the term or synonym |
| ** | Number of studies in the entire database containing the term or synonym |
Appendix 6. Appendix 5: WHO ICTRP
Condition: Embolism OR Thrombosis
Intervention: heparin OR antithrombotic prophylaxis OR prevention
Appendix 7. Data Extraction Form
Data collection form
| Study ID | |
| Notes | |
General Information
| Date form completed (dd/mm/yyyy) | |
| Name/ID of person extracting data | |
| Reference citation (e.g. Medline) | |
| Study author contact details | |
| Publication type (e.g. full report, abstract, letter) |
|
| Notes: | |
Study eligibility
| Study Characteristics | Eligibility criteria | Eligibility criteria met? | Location in text or source | |||
| Yes | No | Unclear | ||||
| Type of study | Randomized controlled trials (RCT) | |||||
| Quasi‐experimental studies including quasi‐randomized trials | ||||||
| Observational studies including cohort, case‐control and cross‐sectional studies. | ||||||
| Participants | Abdominal or pelvic operations (benign or malignant pathology) | |||||
| Type of intervention | LMWH for extended duration (>= 14 days) | |||||
| Types of comparison | LMWH <14 days or other method of thromboprophylaxis | |||||
| Types of outcome measures | Primary outcome: VTE, or pulmonary embolism within 30 days of surgery Secondary outcome: Bleeding complications |
|||||
| Types of determinants | Determinants of concern are: 1) Socioeconomic status ‐ assessed by income, expenditure, household characteristics and/or assets, occupational or contractual status – and education (highest level of education completed, years of schooling, literacy); 2) Geographic (euclidian distance ‐ km ‐ to a health center, travel time, location ‐ rural vs. urban residence); 3) Demographic (ethnicity, marital status, immigration status). |
|||||
| Results | Quantitative results of the association between potential determinants and postnatal care services utilization | |||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
Characteristics of included studies
Methods
| Descriptions as stated in report/paper | |
| Aim of study | |
| Design | |
| Study Setting (single/multi‐instituional) | |
| Start date | |
| End date | |
| Duration of participation | |
| Notes: | |
Participants
| Description | Location in text or source (pg & ¶/fig/table) | ||
| Population description | |||
| Setting and context | |||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method of recruitment of participants | |||
| Informed consent obtained | Yes No Unclear | ||
| Total no. of participants | |||
| Withdrawals and exclusions | |||
| Missing data | |||
| Outcome(s) Definition, measure & classification |
Primary outcome – VTE or PE | ||
| Secondary outcomes 1) Bleeding complication |
|||
| Confounding factors/ effect modifiers accounted for | |||
| Results (specify, e.g. OR, RR, IRR) (specify the reference group) |
Crude | ||
| Adjusted | |||
| Authors’ reported limitations of study’s methods/results | |||
| Scientific quality (specify tool, e.g. modified EPHPP tool) | |||
| Notes: | |||
Other information
|
Study funding sources (including role of funders) |
||
|
Possible conflicts of interest (for study authors) |
||
| Description as stated in report/paper | Location in text or source | |
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Notes: | ||
Data and analyses
Comparison 1.
LMWH versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All VTE | 7 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.26, 0.54] |
| 2 All DVT | 7 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.55] |
| 3 Proximal DVT | 7 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.10, 0.47] |
| 4 Symptomatic VTE | 7 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.08, 1.11] |
| 5 Bleeding complications | 7 | 2239 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.67, 1.81] |
| 6 All VTE (Subgroup analysis open only) | 6 | 1503 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.29, 0.60] |
| 7 All DVT (Subgroup analysis open only) | 6 | 1503 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.30, 0.62] |
| 8 All VTE (Sensitivity analysis unpublished) | 6 | 1620 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.27, 0.56] |
| 9 All DVT (Sensitivity analysis unpublished) | 6 | 1620 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.27, 0.58] |
Analysis 1.1.
Comparison 1 LMWH versus placebo, Outcome 1 All VTE.
Analysis 1.2.
Comparison 1 LMWH versus placebo, Outcome 2 All DVT.
Analysis 1.3.
Comparison 1 LMWH versus placebo, Outcome 3 Proximal DVT.
Analysis 1.5.
Comparison 1 LMWH versus placebo, Outcome 5 Bleeding complications.
Analysis 1.6.
Comparison 1 LMWH versus placebo, Outcome 6 All VTE (Subgroup analysis open only).
Analysis 1.7.
Comparison 1 LMWH versus placebo, Outcome 7 All DVT (Subgroup analysis open only).
Analysis 1.8.
Comparison 1 LMWH versus placebo, Outcome 8 All VTE (Sensitivity analysis unpublished).
Analysis 1.9.
Comparison 1 LMWH versus placebo, Outcome 9 All DVT (Sensitivity analysis unpublished).
What's new
| Date | Event | Description |
|---|---|---|
| 28 October 2017 | New search has been performed | New version submitted. |
| 28 October 2017 | New citation required and conclusions have changed | Update of the version published in the Cochrane Library 2009, Issue 1. Three new studies identified and included. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 30 June 2008 | Amended | Converted to new review format. |
| 8 January 2008 | New citation required and conclusions have changed | Substantive amendment. |
Differences between protocol and review
After recommendation from the editorial team, 'pelvic surgery' has been added to the title.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, double‐blinded, venography | |
| Participants | Patients undergoing elective, open, curative surgery for malignant disease of the gastrointestinal (excluding esophagus) tract, genitourinary tract or female reproductive organs. | |
| Interventions | LMWH (enoxaparin 40 mg, total treatment period of 25 to 31 days) or placebo. | |
| Outcomes | LMWH 165 Placebo167 | |
| Notes | ENOXACAN II trial. Follow‐up period 3 months. Complete follow‐up. All patients were scheduled for bilateral venography. Adequate definitions of VTE and bleeding complications were described in the paper. Venography performed between 25 and 31 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the manuscript. Randomization stratified according to the country where institution was located. |
| Allocation concealment (selection bias) | Unclear risk | Not described in manuscript. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled double‐blind study. Patients, healthcare providers, data collectors, outcome assessors, and data analysts were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eighty‐one (32.7%) patients in placebo group and 88 (34.8%) patients in experimental group excluded due to lack of adequate venography. |
| Selective reporting (reporting bias) | Unclear risk | Modified intention‐to‐treat analysis of patients reaching an evaluable VTE end point (venogram or objection verification of symptomatic VTE). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | RCT, double‐blinded, venography. | |
| Participants | Patients undergoing curative surgery for abdominal or pelvic cancer. | |
| Interventions | LMWH (tinzaparin 3500 IU, total treatment period of 28 days) or placebo. | |
| Outcomes | LMWH 58 Placebo 50 | |
| Notes | Unpublished data. Presented as an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation. |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled double‐blind study. Patients, healthcare providers, data collectors, outcome assessors and data analysts were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in abstract. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated prematurely due to lack of funding and high attrition rate (rate not reported). |
| Selective reporting (reporting bias) | Unclear risk | Intention‐to‐treat analysis of patients reaching an evaluable VTE end point (venogram or objection verification of symptomatic VTE). Adequate definitions of VTE and bleeding complications were described. |
| Other bias | High risk | Premature termination of trial. Full details of study unclear because Abstract only. Bilateral venography performed on day 28 to 35. |
| Methods | RCT, double‐blinded, placebo‐controlled, venography. | |
| Participants | Patients undergoing elective, open, curative or palliative surgery for malignant disease of the gastrointestinal (excluding esophagus) tract, genitourinary tract or female reproductive organs. | |
| Interventions | LMWH (bemiparin 3500 IU, total treatment period of 24 to 32 days), or placebo. | |
| Outcomes | LMWH 248 Placebo 240 |
|
| Notes | CANBESURE trial. Follow‐up 74 to 90 days after randomization. Follow‐up complete. All patients were scheduled for bilateral venography. Adequate definitions of VTE and bleeding complications were described in the paper. Venography performed on day 18 to 22, two days before the last LMWH injection. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the manuscript. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled. All deaths and symptomatic VTE events centrally evaluated by independent blinded committee. Venograms blinded and centrally evaluated by independent committee. Bleeding events adjudicated by an independent data safety monitoring board. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo injection 0.9% sodium chloride, 0.2 mL. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sixty‐seven (21.3%) patients in experimental group, 70 (22.6%) patients excluded due to inadequate venography. |
| Selective reporting (reporting bias) | Unclear risk | Modified intention‐to‐treat population, included all randomized patients who received at least one dose of randomized treatment and had an assessable venogram, or documented symptomatic VTE, or died during the double‐blind period. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | RCT, assessor‐blinded, venography. | |
| Participants | Patients undergoing major abdominal or non‐cardiac thoracic surgery for either malignant or benign diseases. | |
| Interventions | LMWH (tinzaparin 3500 IU, total treatment period 28 days) or no treatment. | |
| Outcomes | LMWH 58 Control 60 | |
| Notes | There was no defined follow‐up period. All patients were scheduled for bilateral venography. An adequate definition of VTE was described in the paper. No definition of bleeding complications was given in the paper, but bleeding episodes were described. Venography performed on day 28. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation list. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the manuscript. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Assessor‐blinded evaluation of the venograms by two radiologists. Patients, healthcare providers and data‐analysts were not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, patients not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated prematurely due to lack of funding. Of 176 eligible patients (87 in extended prophylaxis group and 89 in control group), 29 (33.3%) excluded in experimental group and 29 (32.6%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Modified intention‐to‐treat analysis of patients reaching an evaluable VTE end point (venogram or objection verification of symptomatic VTE). |
| Other bias | Unclear risk | No definition for bleeding complications was specified. |
| Methods | RCT, assessor blinded, venography. | |
| Participants | Patients undergoing major abdominal surgery for either malignant or benign diseases. | |
| Interventions | LMWH (dalteparin 5000 IU, total treatment period 28 days) or no treatment. | |
| Outcomes | LMWH 165 Control 178 | |
| Notes | Follow‐up period 3 months. Complete follow‐up. All patients were scheduled for bilateral venography. Adequate definitions of VTE and bleeding complications were described in the paper. Venography performed on day 28. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation list. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open‐label study with assessor‐blinded evaluation of the venograms. Patients, healthcare providers and data‐analysts were not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Forty‐four (19.8%) patients in control group and 40 (19.5%) patients in experimental group excluded. |
| Selective reporting (reporting bias) | Unclear risk | Modified intention‐to‐treat analysis of patients reaching an evaluable VTE end point (venogram or objection verification of symptomatic VTE). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | RCT, assessor interpretation of venography or ultrasonography not described. | |
| Participants | Patients undergoing elective, curative laparotomy for cancer of > 45 minutes duration with a life expectancy of > 6 months after surgery. | |
| Interventions | LMWH (enoxaparin 20 mg twice daily for 14 days) or no treatment. | |
| Outcomes | LMWH/IPC = 113 IPC = 38 |
|
| Notes | The study used patients receiving mechanical prophylaxis (IPC) as a reference group for VTE incidence during the study period, but was not intended to be compared statistically with the enoxaparin group. In the total treated population, (n = 109) LMWH mean treatment duration was 10.5 +/‐ 3.3 days, and in the modified‐intention‐to‐treat population (n = 83), LMWH mean treatment duration was 11 +/‐ 2.8 days. The defined follow‐up period was 14 days following venography (day 28 +/‐ 5). All patients were scheduled for bilateral venography on day 14 after surgery. Adequate definitions of VTE and bleeding complications were described in the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized in a 3:1 ratio (LMWH to IPC), method not stated in the manuscript. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the manuscript. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study, no stated blinding of VTE objective end points or primary safety end point (bleeding). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26 patients (23.9%) in LMWH group and 7 patients (18.4%) in IPC group excluded from total treated population due to inadequate VTE assessment or measurement. |
| Selective reporting (reporting bias) | Unclear risk | Modified intention‐to‐treat analysis of patients reaching an evaluable VTE end point (venogram or objection verification of symptomatic VTE). |
| Other bias | High risk | Study and editorial support financially supported by Sanofi‐Aventis K.K., Japan. The principle investigator (MS) reported conflict of interest with Sanofi‐Aventis (Japan), GlaxoSmithKline (Japan), Astellas, and Bayer pharmaceutical companies. |
| Methods | RCT, assessor‐blinded, compression ultrasonography. | |
| Participants | Patients undergoing elective laparoscopic surgery for colorectal cancer. | |
| Interventions | LMWH (enoxaparin 4000 IU in 79% allocated to extended prophylaxis, dalteparin 5000 IU in 21% allocated to extended prophylaxis, total treatment period 27 to 31 days). | |
| Outcomes | LMWH 112 Control 113 |
|
| Notes | LMWH prophylaxis medication varied prior to randomization to extended duration LMWH prophylaxis (enoxaparin 4000 UI or dalterparin 5000 UI or nadroparin 2850 IU). The study was interrupted after the results of the interim analysis showed a reduction in the rate of VTE in patients assigned to extended prophylaxis (P < 0.01). Compression ultrasonography day 26 to 30. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Centralized randomization, method not stated in manuscript. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Compression ultrasonography evaluated by blinded investigator. Unclear if data‐analysts blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and presumably caregivers not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Terminated prematurely after interim analysis showed a reduction in the rate of VTE in patients assigned to extended prophylaxis. |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis on all patients with at least 26 days of follow up and at least one dose of medication; it appears all randomized patients met these parameters. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
IPC: intermittent pneumatic compression; IU: international units: LMWH: low molecular weight heparin; RCT: randomized controlled trial; VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Corr 2015 | Cohort study using historical control patients. |
| Downs 2012 | Phase 2 open‐label, non‐randomized (single‐group assignment) trial using fondaparinux days 1‐28 with duplex ultrasonography of the lower extremities between days 28‐35. Results are not published. Recruitment was completed January 2009 (n = 44), of which 17 patients did not complete the study. Of the 27 remaining patients, 0 patients were diagnosed with a VTE at week 4, although 1 patient of the 33 patients who received at least one dose of fondaparinux (and could be assessed for adverse events) experienced a pulmonary embolism and 1 developed a retroperitoneal hematoma. |
| GlaxoSmithKline 2009 | Phase 3 trial enrollment reported as complete (n = 127) February 2007. No results reported. No duration of LMWH or objective VTE measurement defined in protocol. |
| Huh, 2017 | Terminated due to poor enrollment (total patients, n = 7; 4 in control arm, 3 in experimental). No statistical analysis performed. |
| Krasinski 2014 | Prospective, non‐randomized trial. |
| Rasmussen 2003 | Double publication. Data included in the Rasmussen 2006 trial. |
| Rasmussen 2003a | Publication is a review and not a primary trial. |
| Sakon 2012 | Extended prophylaxis using an oral direct factor Xa inhibitor (Darexaban, YM150). |
| Schmeler 2013 | Cohort study using historical control patients. |
LMWH: low molecular weight heparin; VTE: venous thromboembolism.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized parallel controlled trial. |
| Participants | Patients with gynecologic malignancy diagnosis undergoing surgery. |
| Interventions | Graduated compression stocking and/or intermittent pneumatic compression and/or LMWH. |
| Outcomes | Ultrasound diagnosis of VTE. |
| Notes | Duration of LMWH duration not specified in protocol. Date of trial registration 30 October 2015. Each of 4 arms of trial anticipate 250 patients enrolled. Study contact zhangzhenyu@coga.org.cn, telephone +86 13801237287 |
LMWH: low molecular weight heparin; VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Rivaroxaban or placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer: a randomized, double blind, placebo‐controlled study |
| Methods | Randomized, parallel assignment, triple‐blinded. |
| Participants | Diagnosis of colorectal cancer (any stage), elective laparoscopic surgery planned. |
| Interventions | Postoperative LMWH then oral rivaroxaban for 3 weeks versus placebo for 3 weeks. |
| Outcomes | Composite of symptomatic objectively confirmed VTE, asymptomatic ultrasonography‐confirmed DVT or VTE‐related death. |
| Starting date | May 3, 2017 |
| Contact information | cecilia.becattini@unipg.it |
| Notes | Rivaroxaban is a direct Factor Xa inhibitor. |
DVT: deep venous thrombosis; LMWH: low molecular weight heparin; VTE: venous thromboembolism.
Contributions of authors
SIF structured the work, extracted the data, completed data entry and analysis and wrote the final manuscript. RSK and CCJ extracted data and proofread the manuscript. MSR, BS, MRK and RDM proofread the manuscript.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
MSR is a member the advisory board of Pfizer, Denmark.
One author has been investigators on three of the randomized trials included in this review update (Rasmussen 2006).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Moigne‐Amrani AL, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. New England Journal of Medicine 2002;346(13):975‐90. [DOI] [PubMed] [Google Scholar]
- Jørgensen LN, Lausen I, Rasmussen MS, Wille‐Jørgensen P, Bergqvist D. Prolonged thromboprophylaxis with low‐molecular weight heparin following major general surgery: an individual patient data meta‐analysis. Blood. 2002; Vol. 100:abstract 1952 (poster). [Google Scholar]
- Kakkar VV, Balibrea JL, Martinez‐Gonzalez J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. Journal of Thrombosis and Haemostasis 2010;8(6):1223‐39. [DOI] [PubMed] [Google Scholar]
- Lausen I, Jensen R, Jorgensen LN, Rasmussen MS, Lyng KM, Andersen M, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: Randomised controlled study of prolonged thromboprophylaxis. European Journal of Surgery 1998;164:657‐63. [DOI] [PubMed] [Google Scholar]
- Rasmussen MS, Jorgensen LN, Wille‐Jørgensen P, Nielsen JD, Horn A, Mohn AC, et al. Prolonged prophylaxis with dalteparin to prevent late thromboeembolic complications in patients undergoing major abdominal surgery: a multicenter randomised open‐label study. Thrombosis and Haemostasis 2006;4:2384‐90. [DOI] [PubMed] [Google Scholar]
- Sakon M, Kobayashi T, Shimazui T. Efficacy and safety of enoxaparin in Japanese patients undergoing curative abdominal or pelvic cancer surgery: Results from a multicenter, randomized, open‐label study. Thrombosis Research 2010;125:65‐70. [DOI] [PubMed] [Google Scholar]
- Vedovati MC, Becattini C, Rondelli F, Boncompagni M, Camporese G, Balzarotti R, et al. Randomized study on 1‐week versus 4‐week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Annals of Surgery 2014;259(4):665‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Corr BR, Winter AM, Sammel MD, Chu CS, Gage BF, Hagemann AR. Effectiveness and safety of expanded perioperative thromboprophlaxis in complex gynecologic surgery. Gynecologic Oncology 2015;138(3):501‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs LS. Women's Cancer Center Protocol #45: Prolonged venous thromboembolism prophylaxis with fondaparinux in gynecologic oncology patients: an open label phase ii trial. Clinicaltrials.gov2012.
- GlaxoSmithKline. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism after abdominal surgery. Clinicaltrials.gov July 10, 2009.
- Huh W. Extended deep venous thrombosis prophylaxis in gynecologic oncology surgery with intermittent compression devices (ICD) with or without postoperative arixtra (fondaparinux sodium): a randomized controlled trial. Clinicaltrials.gov March 27, 2017.
- Krasiński Z, Szpurek D, Staniszewski R, Dzieciuchowicz Ł, Pawlaczyk K, Krasińska B, et al. The value of extended preoperative thromboprophylaxis with dalteparin in patients with ovarian cancer qualified to surgical treatment. International Angiology 2014;33(4):365‐71. [PubMed] [Google Scholar]
- Rasmussen MS, Wille‐Jørgensen P, Jørgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery prevents late VTE: the FAME study. Pathophysiology of Haemostasis and Thrombosis 2003;33(1):77‐104. [Google Scholar]
- Rasmussen MS. Does prolonged thromboprophylaxis improve outcome in patients undergoing surgery?. Cancer Treatment Reviews 2003;Suppl 2:15‐7. [DOI] [PubMed] [Google Scholar]
- Sakon M, Nakamura, M. Darexaban (YM150) prevents venous thromboembolism in Japanese patients undergoing major abdominal surgery: Phase III randomized, mechanical prophylaxis‐controlled, open‐label study. Thrombosis Research 2012;130(3):52‐9. [DOI] [PubMed] [Google Scholar]
- Schmeler KM, Langley G, Cain K, Munsell MF, Ramirez PT, Soliman PT, et al. Venous thromboembolism (VTE) rates following the implementation of extended duration prophylaxis for patients undergoing surgery for gynecologic malignancies. Gynecologic Oncology 2013;128(2):204‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Zhang, Z. The mechanical and medical prevention of lower extremity deep venous thrombosis formation post gynecologic pelvic surgery, a multiple center randomized case control study. ChiCTR‐IPR‐15007324 Recruiting.
References to ongoing studies
- Campanini M (FADOI Foundation, Italy). Rivaroxaban or placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer: a randomized, double blind, placebo‐controlled study. Clinicaltrials.gov2017.
Additional references
- Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Annals of Surgery 2006;243(1):89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Medical Oncology 2009;26(3):358‐64. [DOI] [PubMed] [Google Scholar]
- Amin AN, Lin J, Ryan A. Need to improve thromboprophylaxis across the continuum of care for surgical patients. Advances in Therapy 2010;27(2):81‐93. [DOI] [PubMed] [Google Scholar]
- Arnesen H, Dahl OE, Aspelin T, Seljeflot I, Kierulf P, Lyberg T. Sustained prothrombotic profile after hip replacement surgery: the influence of prolonged prophylaxis with dalteparin. Journal of Thrombosis and Haemostasis 2003;1:971‐5. [DOI] [PubMed] [Google Scholar]
- Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Annals of Surgery 2010;251(2):344‐50. [DOI] [PubMed] [Google Scholar]
- Bergqvist D, Arcelus JI, Felicissimo P, ETHOS investigators. Post‐discharge compliance to venous thromboembolism prophylaxis in high‐risk orthopaedic surgery:results from the ETHOS registry. Thrombosis and Haemostasis 2012;107(2):280‐7. [DOI] [PubMed] [Google Scholar]
- Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Seminars in Hamatology 2001;38(2 Suppl 5):12‐9. [DOI] [PubMed] [Google Scholar]
- Clarke‐Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstetrics and Gynecology 1984;63:92‐8. [PubMed] [Google Scholar]
- Dahl OE, Aspelin T, Arnesen H, Sejlefjot I, Kierulf P, Ruyter R, et al. Increased activation of coagulation and formation of late deep venous thrombosis following discontinuation of thromboprophylaxis after hip replacement surgery. Thrombosis Research 1995;80:299‐306. [DOI] [PubMed] [Google Scholar]
- Diamantis T, Tsiminikakis N, Skordylaki A, Samiotaki F, Vernadakis S, Bongiorni C, et al. Alterations of hemostasis after laparoscopic and open surgery. Hematology 2007;12(6):561‐70. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Quinlan DJ, Douketis JD. Extended‐duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta‐analysis of the randomised trials. Lancet 2001;358:9‐15. [DOI] [PubMed] [Google Scholar]
- Galster H, Kolb G, Kohsytorz A, Seidlmayer C, Paal V. The pre‐, peri‐, and postsurgical activation of coagulation and the thromboembolic risk for different risk groups. Thrombosis Research 2000;100(5):381‐8. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, et al. Prevention of venous thromboembolism. Chest 2001;119(suppl 1):132S‐175S. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:338S‐400S. [DOI] [PubMed] [Google Scholar]
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(Suppl 2):e227S‐77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ME, Vogler SA, Mone MC, Sheng X, Sklow B. The importance of extended postoperative venous thromboembolism prophylaxis in IBD: a National Surgical Quality Improvement Program analysis. Diseases of the Colon and Rectum 2014;57(4):482‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge: an underestimated risk. Archives of Surgery 1992;127:310‐3. [DOI] [PubMed] [Google Scholar]
- Hull RD, Pineo GF, Stein PD, Mah AF, MacIsaac SM, Dahl OE, et al. Extended out‐of‐hospital low‐molecular‐weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Annals of Internal Medicine 2001;135:858‐69. [DOI] [PubMed] [Google Scholar]
- Iversen LH, Thorlasius‐Ussing O. Relations of coagulation test abnormalities to tumour burden and postoperative DVT in resected colorectal cancer. Thrombosis Haemostasis 2002;87:402‐8.. [PubMed] [Google Scholar]
- Jørgensen LN, Wille‐Jørgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. British Journal of Surgery 1993;80:689‐704. [DOI] [PubMed] [Google Scholar]
- Kalka C, Spirk D, Siebenrock KA, Metzger U, Tuor P, Sterzing D, et al. Lack of extended venous thromboembolism prophylaxis in high‐risk patients undergoing major orthopaedic or major cancer surgery. Electronic Assessment of VTE Prophylaxis in High‐Risk Surgical Patients at Discharge from Swiss Hospitals (ESSENTIAL). Thrombosis and Haemostasis 2009;102(1):56‐61. [DOI] [PubMed] [Google Scholar]
- Kehlet H. Fast‐track colonic surgery: status and perspectives. Recent Results Cancer Research 2005;165:8‐13. [DOI] [PubMed] [Google Scholar]
- Lindblad B, Sternby NH, Bergqvist D. Incidence of venous thromboembolism verified by necropsy over 30 years. BMJ 1991;302:709‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman GH, Bohlke K, Falanga A, American Society of Clinical Oncology. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Oncology Practice 2015;11(3):e442‐4. [DOI] [PubMed] [Google Scholar]
- Mahla E, Lang T, Vicenzi MN, Werkgartner G, Maier R, Probst C, et al. Thromboelastography for monitoring prolonged hypercoagulability after major abdominalsurgery. Anesthesia and Analgesia 2001 March;92(3):572‐7. [DOI] [PubMed] [Google Scholar]
- Merkow RP, Bilimoria KY, McCarter MD, Cohen ME, Barnett CC, Raval MV, et al. Post‐discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Annals of Surgery 2011 Jul;254(1):131‐7. [DOI] [PubMed] [Google Scholar]
- Merkow RP. Is there a role for extended venous thromboembolism chemoprophylaxis in IBD?. Diseases of the Colon and Rectum 2014;57(4):413‐4. [DOI] [PubMed] [Google Scholar]
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta‐analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001;88(7):913‐30. [DOI] [PubMed] [Google Scholar]
- Najjar PA, Madenci AL, Zogg CK, Schneider EB, Dankers CA, Pimentel MT, et al. Implementation of a comprehensive post‐discharge venous thromboembolism prophylaxis program for abdominal and pelvic surgery patients. Joural of the American College of Surgeons 2016;223(16):804‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. Cancer‐Associated Venous Thromboembolic Disease. https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf (Version 1.2016); Vol. Accessed August 1, 2016.. [DOI] [PubMed]
- Nguyen NT, Owings JT, Gosselin R, Pevec WC, Lee SJ, Goldman C, et al. Systemic coagulation and fibrinolysis after laparoscopic and open gastric bypass. Archives of Surgery 2001;136(8):909‐16. [DOI] [PubMed] [Google Scholar]
- Rahr HB, Sorensen JV, Larsen JF, Jensen FS, Bredahl C, Nieuwenhuizen W. Haemostatic activation before and after surgery in patients with and without gastric malignancy. Thrombosis and Haemostasis 1994;71:713‐8. [PubMed] [Google Scholar]
- Rahr HB, Fabrin K, Larsen JF, Thorlacius‐Ussing O. Coagulation and fibrinolysis during laparoscopic Cholecystectomy. Thrombosis Research 1999;93:121‐7. [DOI] [PubMed] [Google Scholar]
- Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3.. Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rogers SO Jr, Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, Khuri SF. Multivariable predictors of postoperative venous thromboembolic events after general and vascularsurgery: results from the patient safety in surgery study. Journal of the American College of Surgeons 2007;204(6):1211‐21. [DOI] [PubMed] [Google Scholar]
- Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Guidelines Committee. Guidelines for deep venous thrombosis prophylaxis during laparoscopic surgery. Surgical Endoscopy 2007;April 5:1. [Google Scholar]
- Scurr JH, Colerigde‐Smith PD, Hasty JH. Deep Venous thrombosis: a continuing problem. British Medical Journal 1988;297:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration,. Available from www.cochrane‐handbook.org.
- Sweetland S, Green J, Liu B, Berrington de Gonzalez A, Canonico M, Reeves G, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009;339:b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen C, Andersen M, Kristiansen VB, Jensen R, Wille‐Jørgensen P. The occurrence of late thromboembolic complications after elective abdominal surgery. Ugskrift for Laeger 1990;152:1586‐7. [PubMed] [Google Scholar]
- Tsiminikakis N, Chouillard E, Tsigris C, Diamantis T, Bongiorni C, Ekonomou C, et al. Fibrinolytic and coagulation pathways after laparoscopic and open surgery: a prospective randomized trial. Surgical Endoscopy 2009;23(12):2762‐9. [DOI] [PubMed] [Google Scholar]
- Vaitkus PT, Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Goldhaber SZ, PREVENT Medical Thromboprophylaxis Study Group. Mortality rates and risk factors for asymptomatic deep vein thrombosis in medical patients. Thrombosis and Haemostasis 2005;93:76‐9. [DOI] [PubMed] [Google Scholar]
- Wille‐Jørgensen P, Jørgensen LN, Hauch O, Borris LC, Lassen MR, Nehen AM, et al. Potential influence of observer variation on thromboprophylactic studies. Hæmostasis 1992;22:211‐5. [DOI] [PubMed] [Google Scholar]
- Wille‐Jørgensen P, Jorgensen LN, Crawford M. Asymptomatic postoperative deep vein thrombosis and the developement of postthrombotic syndrome. A systematic review and meta‐analysis. Thrombosis and Haemostasis 2005;93:236‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Rasmussen MS, Wille‐Jørgensen P. Prolonged thromboprophylaxis for abdominal surgery. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004318] [DOI] [Google Scholar]
- Rasmussen MS, Jørgensen LN, Wille‐Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004318.pub2] [DOI] [PubMed] [Google Scholar]


