Abstract
Background
Hypofibrinogenaemia is associated with increased morbidity and mortality, but the optimal treatment level, the use of preemptive treatment and the preferred source of fibrinogen remain disputed. Fibrinogen concentrate is increasingly used and recommended for bleeding with acquired haemostatic deficiencies in several countries, but evidence is lacking regarding indications, dosing, efficacy and safety.
Objectives
We assessed the benefits and harms of fibrinogen concentrate compared with placebo or usual treatment for bleeding patients.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 8); MEDLINE (1950 to 9 August 2013); EMBASE (1980 to 9 August 2013); International Web of Science (1964 to 9 August 2013); CINAHL (1980 to 9 August 2013); LILACS (1982 to 9 August 2013); and the Chinese Biomedical Literature Database (up to 10 November 2011), together with databases of ongoing trials. We contacted trial authors, authors of previous reviews and manufacturers in the field.
Selection criteria
We included all randomized controlled trials (RCTs), irrespective of blinding or language, that compared fibrinogen concentrate with placebo/other treatment or no treatment in bleeding patients, excluding neonates and patients with hereditary bleeding disorders.
Data collection and analysis
Three review authors independently abstracted data; we resolved any disagreements by discussion. Our primary outcome measure was all‐cause mortality. We performed subgroup and sensitivity analyses to assess the effects of fibrinogen concentrate in adults and children in terms of various clinical and physiological outcomes. We presented pooled estimates of the effects of intervention on dichotomous outcomes as risk ratios (RRs) and on continuous outcomes as mean differences, with 95% confidence intervals (CIs). We assessed the risk of bias through assessment of trial methodological components and the risk of random error through trial sequential analysis.
Main results
We included six RCTs with a total of 248 participants; none of the trials were determined to have overall low risk of bias. We found 12 ongoing trials, from which we were unable to retrieve any data. Only two trials provided data on mortality, and one was a zero event study; thus the meta‐analysis showed no statistically significant effect on overall mortality (2.6% vs 9.5%, RR 0.28, 95% CI 0.03 to 2.33). Our analyses on blood transfusion data suggest a beneficial effect of fibrinogen concentrate in reducing the incidence of allogenic transfusions (RR 0.47, 95% CI 0.31 to 0.72) but show no effect on other predefined outcomes, including adverse events such as thrombotic episodes.
Authors' conclusions
In the six available RCTs of elective surgery, fibrinogen concentrate appears to reduce transfusion requirements, but the included trials are of low quality with high risk of bias and are underpowered to detect mortality, benefit or harm. Furthermore, data on mortality are lacking, heterogeneity is high and acute or severe bleeding in a non‐elective surgical setting remains unexplored. Currently, weak evidence supports the use of fibrinogen concentrate in bleeding patients, as tested here in primarily elective cardiac surgery. More research is urgently needed.
Plain language summary
Use of fibrinogen concentrate in patients with bleeding
Fibrinogen is a natural blood protein involved in the coagulation process. Bleeding decreases the blood level, and low levels of this protein may increase bleeding even further, thereby increasing morbidity and mortality. Fibrinogen concentrate is widely used instead of traditional sources of fibrinogen, such as the blood products fresh frozen plasma and cryoprecipitate (a pooled concentrated plasma product), especially in some countries, despite the lack of adequate knowledge derived from previous research to support such an approach. In the present Cochrane systematic review, we set out to assess the benefits and harms of fibrinogen concentrate in patients with bleeding. We searched the databases to August 2013, we identified six randomized trials in cardiac and elective surgical settings that compared fibrinogen concentrate (248 participants) with placebo/other sources or no treatment. Additionally, we found 12 ongoing trials, but we were unable to retrieve any data from them. We could not identify beneficial effects of fibrinogen concentrate on patient survival. In our predefined outcomes, we identified a reduced proportion of patients requiring donor blood transfusion. We could not identify reduced blood loss or any harms or adverse events caused by treatment with fibrinogen concentrate. However, all trials were of low quality and were small, so evidence in support of fibrinogen concentrate in patients with bleeding remains weak.
Summary of findings
Summary of findings for the main comparison. Fibrinogen concentrate versus any comparator for patients with bleeding.
| Fibrinogen concentrate versus any comparator for patients with bleeding | ||||||
| Patient or population: bleeding patients or at risk of severe bleeding Settings: in‐hospital care, operative setting, intensive care or trauma Intervention: fibrinogen concentrate Comparison: any comparator | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Any comparator | Fibrinogen concentrate | |||||
| Mortality | Study population | RR 0.28 (0.03 to 2.33) | 81 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 95 per 1000 | 27 per 1000 (3 to 222) | |||||
| Moderate | ||||||
| 63 per 1000 | 18 per 1000 (2 to 147) | |||||
| Incidence of allogenic blood transfusion Proportion of participants transfused with RBC, FFP, PLT and/or cryo | Study population | RR 0.47 (0.31 to 0.72) | 207 (5 studies) | ⊕⊝⊝⊝ very low1,4,5,6 | ||
| 764 per 1000 | 359 per 1000 (237 to 550) | |||||
| Moderate | ||||||
| 800 per 1000 | 376 per 1000 (248 to 576) | |||||
| Thrombotic episodes (arterial and venous graft occlusion, pulmonary embolus, deep venous thrombosis) Proportion with event | Study population | RR 1.03 (0.27 to 3.97) | 124 (3 studies) | ⊕⊝⊝⊝ very low1,3,5,6 | ||
| 48 per 1000 | 49 per 1000 (13 to 189) | |||||
| Moderate | ||||||
| 48 per 1000 | 49 per 1000 (13 to 191) | |||||
| Intensive care unit (ICU) stay Duration of stay (hours) | The mean intensive care unit (ICU) stay ranged across control groups from 25 to 173 hours | The mean intensive care unit (ICU) stay in the intervention groups was 9.87 lower (20.67 lower to 0.93 higher) | 112 (3 studies) | ⊕⊝⊝⊝ very low1,3,4,6 | ||
| Re‐operation due to persistent bleeding Events during admission | Study population | RR 0.68 (0.01 to 36.71) | 124 (2 studies) | ⊕⊝⊝⊝ very low3,4,6 | ||
| 108 per 1000 | 73 per 1000 (1 to 1000) | |||||
| Moderate | ||||||
| 107 per 1000 | 73 per 1000 (1 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1One trial was a small pilot study without complete blinding of personnel and industry supported. Other trial was Industry sponsored. 2One trial was a zero event trial. 3Few participants and very few events. 4One trial with "high risk" or "unclear risk" in all aspects. 5Included trials biased by industry support, small sample sizes, selective reporting and insufficient blinding. 6Differing comparator, population (prophylactic against bleeding and with bleeding) and children/adults.
Background
Description of the condition
Fibrinogen (clotting factor I) plays a central role in normal haemostasis by acting as an endogenous substrate for fibrin formation and by inducing clot formation and platelet aggregation (Kreuz 2005; Mosesson 2001). A fibrin network formed by activated platelets and cross‐linked fibrin strings represents a climax of the coagulation process in vivo. The haemostatic clot formed at the site of injury involves a complex interaction among blood vessels, platelets, erythrocytes and plasma‐suspended coagulation factors (Monroe 2002). Fibrinogen is produced by the liver at a rate of 2 to 5 g per day, and the average plasma level is 2.0 to 4.5 g/L (5.88 to 13.23 μmol/L) (Kreuz 2005). In patients with congenital hypofibrinogenaemia or fibrinogen deficiency, the critical level of fibrinogen is estimated to be below 1 g/L (Bolton‐Maggs 2004; Bornikova 2011). The critical level in acquired hypofibrinogenaemia is somewhat more disputed, ranging from 1.0 to 1.5 g/L, as supported primarily by guidelines-not by evidence (Fries 2010; Rossaint 2010). Fibrinogen deficiency develops at an early stage of replacement therapy with red blood cells (RBCs) and fluids, depending on the transfusion strategy (Hiippala 1995), and is enhanced by dilution, consumption, acidosis and hypothermia (Martini 2009). In addition, infusion of colloids appears to impair coagulation even beyond the effect of dilution (Fenger‐Eriksen 2009a; Fenger‐Eriksen 2009c; Fries 2005; Fries 2006; Levi 2007; Mittermayr 2007). Bleeding and a low level of fibrinogen appear to be associated with increased morbidity and mortality in various clinical settings (Blome 2005; Brohi 2008; Charbit 2007; Fenger‐Eriksen 2008; Karlsson 2008; Lissalde‐Lavigne 2008; Parasnis 1992; Ucar 2007).
Description of the intervention
Fibrinogen substitution has been provided traditionally by sources such as fresh frozen plasma (FFP) and cryoprecipitate. FFP contains 2.0 to 4.5 g/L (5.88 to 13.23 μmol/L) of fibrinogen, and cryoprecipitate contains 15 to17 g/L (Caudill 2009). Substitution of fibrinogen is both historically and widely recommended at levels below 1 g/L (2.94 μmol/L) (ASA 2006; Jansen 2009), primarily on the basis of in vitro studies (Fenger‐Eriksen 2009b). However, the optimal goal for substitution or the relation to patient outcomes has not been established (Levy 2012; Sorensen 2010; Warmuth 2012). Fibrinogen concentrate is a pasteurized concentrate made from pooled human plasma. It is available in single‐use vials containing 900 to 1300 mg lyophilized fibrinogen concentrate powder for reconstitution (Fenger‐Eriksen 2009b; Kreuz 2005).
How the intervention might work
Fibrinogen substitution is believed to normalize and improve the environment for clot formation by providing sufficient amounts of substrate and by enhancing the strength and speed of clot generation (Nielsen 2005; Nielsen 2005a) in patients depleted of or with dysfunctional fibrinogen. Furthermore, the coagulopathic effects of colloids may cause a reduction in coagulation factor VIII and von Willebrand factor in combination with an acquired platelet dysfunction and impairment of the polymerization of fibrin monomers (de Jonge 1998). Several studies have suggested a role of fibrinogen concentrate in reversing this impairment of haemostasis (Fenger‐Eriksen 2005; Fenger‐Eriksen 2009a; Fries 2005; Fries 2006; Haas 2008a; Mittermayr 2007).
The level of fibrinogen is traditionally measured by the Clauss method (Fenger‐Eriksen 2009b) despite various limitations such as inability of the method to assess the functionality of fibrinogen (dysfibrinogenaemia) (Fenger‐Eriksen 2009b) and falsely high levels of measured fibrinogen when patients are transfused with certain colloids (Hiippala 1995a). Thrombelastography (TEG®) or thromboelastometry (ROTEM®) is established as a method that can be used to assess the coagulopathic function of fibrinogen (Huissoud 2009; Kalina 2008; Lang 2005). Fibrinogen concentrate improves clot strength (maximum amplitude, maximum clot firmness); clot formation (reaction time (R‐time), clotting time); and clot propagation (alpha angle) as measured by thrombelastography or thromboelastometry (Fenger‐Eriksen 2005; Lang 2005; Nielsen 2005; Nielsen 2005a).
Why it is important to do this review
Avoiding transfusions with allogeneic blood products represents a possible beneficial effect in terms of reduced morbidity and possibly reduced mortality (Carson 2011; Carson 2012; Hebert 1999). Fibrinogen substitution offers the possible effect of normalizing haemostasis and subsequently reducing bleeding and the need for blood transfusion. The use of fibrinogen concentrate is well established in the treatment of hereditary fibrinogen‐related bleeding disorders, for which the concentrate is preferred to both cryoprecipitate and FFP (Bornikova 2011; UKHCDO 2003). Fibrinogen concentrate has regained attention as a potential haemostatic agent in the context of acquired hypofibrinogenaemia and bleeding patients, probably as a result of practical issues, such as no requirement for thawing or blood group matching and low administration volume (Sorensen 2010), but also because of the increasing availability of fibrinogen and haemostatic monitoring. In addition, administering fibrinogen concentrate may be the most efficient way to correct a fibrinogen deficiency when compared with other sources of fibrinogen such as FFP and cryoprecipitate (Sorensen 2010). In addition to efficacy, the aspects of time needed for infusion and costs warrant further evaluation in future studies. Manufacturers recommend an infusion rate of a maximum of 1 gram over a period of 10 minutes, added to the 15 minutes for mixing, that is, longer than one hour of total time for mixing and infusing a recommended dose of 5 grams (70 mg/kg for a 70 kg person) (Riastap 2009). This should be compared with cryoprecipitate and FFP, which require only thawing (5 to 15 minutes) and carry no maximum infusion rate. Increasing use of fibrinogen concentrate should be accompanied by a systematic approach to gathering clinical evidence and identifying benefits as well as adverse effects, which will lead investigators to perform the randomized clinical trials that are needed. No previous systematic reviews have investigated this topic. The aim of this review was to assess the evidence suggesting that fibrinogen concentrate is beneficial or harmful for patients with bleeding when compared with placebo or usual treatment.
Objectives
We assessed the benefits and harms of fibrinogen concentrate compared with placebo or usual treatment for bleeding patients.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized clinical trials (RCTs) irrespective of publication status, date of publication, blinding status or language. We contacted the investigators and the authors to retrieve relevant data. We included unpublished trials only if trial data and methodological descriptions were provided in written form or could be retrieved from the study authors. We also included quasi‐randomized trials because of the expected low number of trials that could be included, but we excluded cross‐over trials and observational studies.
Types of participants
We included 'bleeding patients' as defined by the study authors.
We excluded trials that included neonates and patients with hereditary bleeding disorders resulting in fibrinogen deficiency or aberrant function.
Types of interventions
The primary analysis included trials on fibrinogen concentrate versus placebo. We included trials that used any dose of fibrinogen concentrate, any duration of administration and co‐interventions. We also included trials that compared fibrinogen concentrate with usual treatment (fresh frozen plasma, cryoprecipitate) or other haemostatic agents (e.g. recombinant factor VIIa, antifibrinolytic therapy, desmopressin and other plasma derivatives) or both.
We undertook separate subgroup analyses of trials in which fibrinogen concentrate was compared with other active interventions or was combined with co‐interventions.
Fibrinogen concentrate versus any comparator.
Fibrinogen concentrate versus placebo or no treatment.
Fibrinogen concentrate versus usual treatment (fresh frozen plasma or cryoprecipitate or both).
Fibrinogen concentrate versus other haemostatic agents (recombinant factor VIIa, antifibrinolytic therapy, desmopressin, other plasma derivatives or factor‐substitution products).
Fibrinogen concentrate in combination with other haemostatic agents versus placebo, no treatment or usual treatment.
Fibrinogen concentrate as part of a predefined transfusion algorithm versus placebo or no treatment.
Types of outcome measures
Primary outcomes
Overall mortality. We used the longest follow‐up data from each trial regardless of the period of follow‐up.*
Overall 28‐day mortality. We included data provided as 30‐day mortality in the same analysis.*
Secondary outcomes
Incidence of allogenic blood transfusion (e.g. avoidance of transfusion).*
Number of severe bleeding events and quantities of blood products transfused.*
Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, disseminated intravascular coagulation), major immunological and allergic reactions, infections and sepsis).*
Incidence of surgical interventions and re‐operation due to bleeding.*
Quality of life assessment, as defined by authors in included studies.
Complications during inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure).
Duration of mechanical ventilation.
Days free from ventilator (as defined by authors).
Number of days in hospital.
Mean length of stay in intensive care unit (ICU).*
* Indicates key outcomes included in the ‘Summary of findings’ tables.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2013, Issue 8); Ovid MEDLINE (1950 to 9 August 2013); EMBASE (Ovid SP,1980 to 9 August 2013); International Web of Science (1964 to 19 August 2013); Latin American Caribbean Health Sciences Literature (LILACS via BIREME, 1982 to 9 August 2013); the Chinese Biomedical Literature Database; advanced Google and the Cumulative Index to Nursing & Allied Health Literature (CINAHL via EBSCOhost, 1980 to 9 August 2013).
We used a systematic and sensitive search strategy to identify relevant RCTs with no language or date restrictions. For our detailed search strategies, please see Appendix 1.
Searching other resources
We handsearched the reference lists of reviews, randomized and non‐randomized studies and editorials to locate additional studies. We contacted the main authors of studies and experts in this field to ask for any missed, unreported or ongoing studies. We tried to contact the pharmaceutical companies CSL Behring (Switzerland/ Germany), LFB (France), Shanghai Raas Blood Products Company, Ltd (China), Benesis Corporation (Japan) and Pharming Group N.V. (Netherlands) to ask about any unpublished trials.
We searched for ongoing clinical trials and unpublished studies on the following Internet sites until 11 March 2012.
Data collection and analysis
Selection of studies
Identified reports retrieved from the search were assessed, and obviously irrelevant reports were excluded. Three review authors (JL, MJ, AW) independently examined them for eligibility. This process was performed without blinding of authors, institution, journal of publication or results. We resolved disagreements by discussion, and if no agreement was found, we consulted a fourth person (AA). We provide in this review a detailed description of the search and assessment (Figure 1).
1.

Study flow diagram.
Data extraction and management
Using a data extraction sheet (Appendix 2), we evaluated each study, entered the data in RevMan 5.1 and checked for accuracy. If data in the identified reports were somewhat unclear, we attempted to contact the authors of the original study to ask for further details.
Assessment of risk of bias in included studies
Three review authors (JL, MJ, AW) independently assessed the risk of bias without blinding using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion, and if no agreement was found, we consulted a third person. Each question of validity was answered systematically as described by the following.
1) Random sequence generation?
Assessment of randomization: the sufficiency of the method in producing two comparable groups before intervention.
Grading:
'Low risk' (a truly random process, e.g. random computer number generator, coin tossing or throwing dice); or
'High risk' (any non‐random process, e.g. date of birth, date of admission by hospital, clinic record number, or by availability of the intervention); or 'Unclear'.
2) Allocation concealment?
Allocation method prevented investigators or participants from foreseeing assignment.
Grading:
'Low risk' (central allocation or sealed envelopes); or
'High risk' (using open allocation schedule or other unconcealed procedure); or 'Unclear'.
3) Blinding of participants and personnel?
Assessment of appropriate blinding of investigation team and participants: person responsible for participant's care, participants and eventual others.
Grading:
'Low risk': We considered blinding as adequate if participants and personnel were kept unaware of intervention allocations after inclusion of participants into the study and if the method of blinding involved placebo or an intervention disguised in the same manner as a placebo, because mortality is an objective outcome;
'Unclear': blinding not described; or
'High risk': not double blinded, categorized as an open‐label study or without use of placebo or an intervention disguised in the same manner as placebo.
4) Blinding of outcome assessor?
Assessment of appropriate blinding of outcome assessor.
Grading:
'Low risk': We considered blinding as adequate if outcome assessors were kept unaware of intervention allocations after inclusion of participants into the study and if the method of blinding involved placebo or an intervention disguised in the same manner as a placebo, because mortality is an objective outcome;
'Unclear': blinding not described; or
'High risk': not double blinded, categorized as an open‐label study or without use of placebo or an intervention disguised in the same manner as placebo.
5) Incomplete outcome data?
Completeness of outcome data including attrition and exclusions.
Grading:
'Low risk' (if the numbers and the reasons for dropouts and withdrawals in the intervention groups were described, or if it was specified that no dropouts or withdrawals occurred);
'High risk' (if no description of dropouts and withdrawals was provided); or
'Unclear' (if the report gave the impression that no dropouts or withdrawals occurred, but this was not specifically stated).
6) Selective reporting?
The possibility of selective outcome reporting.
Grading:
'Low risk' (if the reported outcomes are those prespecified in an available study protocol or official trial registration, if this is not available, the published report includes all expected outcomes);
'High risk' (if not all prespecified outcomes have been reported, or if they have been reported using non‐prespecified subscales or have been reported incompletely or if report fails to include a key outcome that would have been expected to have been reported for such a study); or
'Unclear'.
7) Other bias?
Assessment of any possible sources of bias not addressed in domains 1 to 5.
Grading:
'Low risk' (if the report appears to be free of such bias); or
'High risk' (if at least one important bias related to study design is present, or early stopping due to some data‐dependent process, extreme baseline imbalance, claimed fraudulence or other problems); or 'Unclear' (insufficient information or evidence that an identified problem will introduce bias).
With reference to domains 1 to 6 above, we assessed the likely magnitude and direction of the bias and whether we considered it likely that it would have an impact on our findings. We planned to explore the impact of bias in the sensitivity analyses. Please see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
We calculated the risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (binary outcomes).
Continuous data
We used the mean difference (MD) if data were continuous and were measured in the same way between trials. The standardized mean difference (SMD) was used to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cross‐over trials
We excluded cross‐over trials because of the potential high risk of 'carry‐over' of treatment effect in the context of bleeding. However, if the authors provided relevant data for our analyses at a prespecified time point before cross‐over, these data were used in our analyses.
Studies with multiple intervention groups
In studies designed with multiple intervention groups, we combined groups to create a single pair‐wise comparison (Higgins 2011). In trials with two or more fibrinogen groups receiving different doses, we combined data, when possible, for the primary and secondary outcomes.
Dealing with missing data
We contacted the first authors and contact persons of the trials with missing data to try to retrieve the relevant data. For all included studies, we noted levels of attrition and any exclusions. We conducted a sensitivity analysis to explore the impact of included studies with high levels of missing data. In cases of missing data, we chose 'complete case analysis' for our primary outcome, which simply excludes from the analysis all participants with the outcome missing. Selective outcome reporting occurs when non‐significant results are selectively withheld from publication (Chan 2004) and is defined as the selection, on the basis of the results, of a subset of the original variables recorded for inclusion in publication of trials (Hutton 2000). The most important types of selective outcome reporting are:
selective omission of outcomes from reports;
selective selection of data for an outcome;
selective reporting of different analyses using the same data; and
selective reporting of subsets of the data and selective under‐reporting of data (Higgins 2011).
Statistical methods to detect within‐study selective reporting are still in their infant stages. We tried to check for selective outcome reporting by comparing publications with their protocols or official trial registrations when available.
Assessment of heterogeneity
We explored heterogeneity using the I2 statistic and the Chi2 test. A P value ≤ 0.1 is considered to identify significant heterogeneity. An I2 statistic above 50% represents substantial heterogeneity. In case of I2 > 0 (mortality outcome), we tried to determine the cause of heterogeneity by performing relevant subgroup analyses. We used the Chi2 test to obtain an indication of heterogeneity between studies, with P ≤ 0.1 considered significant.
Assessment of reporting biases
Publication bias: arises when the dissemination of research findings is influenced by the nature and direction of results (Higgins 2011). We planned to explore the level of publication bias related to the trials included in the review by providing a funnel plot. To quantify this asymmetry in meta‐analyses with binary outcomes, we also planned to apply the test proposed by Rücker (Rucker 2008). This test has the advantage of including trials with no events. However, because the number of included trials did not exceed 10, we chose not to carry out these tests as suggested by the Cochrane Handbook for Systematic Reviews of Interventions .
Funding bias: is related to possible publication delay or discouragement in relation to undesired results in trials sponsored by the industry (Higgins 2011). To explore the role of funding, we planned to conduct a sensitivity analysis based on our primary endpoint. However, because of lack of data, this approach was not applied.
Data synthesis
We used the Review Manager software (RevMan 5.1) to perform meta‐analyses on pre‐stated outcomes (Types of outcome measures) from included trials. If we performed the meta‐analyses and I2 = 0, we reported only the results from the fixed‐effect model; in cases of I2 > 0, we reported only the results from the random‐effects model unless one or two trials accounted for more than 60% of the total evidence provided, in which case the random‐effects model may be biased. The latter is provided only to make the review more readable. We believe there is little value in using a fixed‐effect model in cases of substantial heterogeneity, which we expect is due to the various reasons leading to massive bleeding. We chose to pool studies only in cases of low clinical heterogeneity.
When meta‐analysis is used in combining results from several studies with binary outcomes (i.e. event or no event), adverse effects may be rare but serious and hence important (Sutton 2002).
Most meta‐analytical software does not include trials with 'zero event' in both arms (intervention vs control) when calculating RR. Exempting these trials from the calculation of RR and 95% CI may lead to overestimation of treatment effect. The Cochrane Collaboration recommends application of the Peto odds ratio (OR), which is the best method of estimating odds ratio when many trials with no events in one or both arms are included (Higgins 2011). However, the Peto method is generally less useful when the trials are small, or when treatment effects are large. We planned to conduct a sensitivity analysis by applying the Peto OR if this sensitivity analysis was seen as a valid option. However, only two small trials provided data on mortality, and one included 'zero event' in both arms; therefore, because of lack of data, we chose not to proceed with these exploratory analyses.
In a single trial, interim analysis increases the risk of type I errors. To avoid type I errors, group sequential monitoring boundaries (Lan 1983) are applied to reveal whether a trial could be terminated early because of a sufficiently small P value, that is, the cumulative z‐curve crosses the monitoring boundaries. Sequential monitoring boundaries, called trial sequential monitoring boundaries, can be applied to meta‐analysis as well,. In trial sequential analysis (TSA), the addition of each trial into a cumulative meta‐analysis is regarded as an interim meta‐analysis and helps the investigator to decide whether additional trials are needed.
The idea behind TSA is that if the cumulative z‐curve crosses the boundary, a sufficient level of evidence is reached, and no further trials are needed. If the z‐curve does not cross the boundary, then evidence is insufficient to allow investigators to reach a conclusion. To construct the trial sequential monitoring boundaries, the information size is required and is calculated as the least number of participants needed in a well‐powered single trial (Brok 2008; Pogue 1997; Pogue 1998; Wetterslev 2008; Wetterslev 2009). We applied TSA (TSA 2010) because this prevents an increase in the risk of type I error (< 5%) due to potential multiple updating and sparse data in a cumulative meta‐analysis and provides us with important information to allow us to estimate the level of evidence for the experimental intervention. Additionally, TSA provides us with important information regarding the need for additional trials and the required information size. We wanted to perform TSA in anticipation of an intervention effect as indicated by the trials included in the traditional meta‐analysis, or even the intervention effect suggested by the upper confidence limit from the intervention effect estimate found in the traditional meta‐analysis, to cover any uncertainty displayed by the present data. We calculated the diversity‐adjusted required information size using the pooled variance from the traditional meta‐analysis (Turner 2013; Wetterslev 2009), as well as the control event proportion from the meta‐analysis of the included trials.
Subgroup analysis and investigation of heterogeneity
We aimed for the following subgroup analyses.
The benefits and harms of fibrinogen concentrate in trials investigating participants treated with plasma expanders (colloids) versus trials investigating participants not treated with plasma expanders. Treatment is defined as a methodical, prespecified and clinically evaluated level of haemodilution.
The benefits and harms of fibrinogen concentrate in trials investigating the cardiac surgery population versus trials investigating the non‐cardiac surgery population.
The benefits and harms of fibrinogen concentrate in trials investigating the emergency surgery population (defined as surgery that should be performed within 24 hours after the indication for surgery is identified) versus trials investigating the non‐emergency surgery population.
The benefits and harms of fibrinogen concentrate in trials investigating the trauma population versus trials investigating the non‐trauma population.
The benefits and harms of fibrinogen concentrate in trials investigating the obstetrical population versus trials investigating the non‐obstetrical population.
The benefits and harms of fibrinogen concentrate in trials investigating the paediatric population (age younger than 18 years, neonates not included) versus trials investigating the adult population.
The benefits and harms of fibrinogen concentrate in trials investigating critically ill participants (e.g. sepsis, septic shock, disseminated intravascular coagulation) versus trials investigating the population of participants not defined as critically ill.
The benefits and harms identified by comparing the pooled intervention effect in trials with a dose regimen that was higher than the median dose of administered fibrinogen concentrate with trials having a dose regimen equal to or smaller than the median dose. This is done to detect a possible dependency of the estimate of intervention effect with the dose regimen.
In case of considerable between‐trial heterogeneity, we planed to apply meta‐regression.
If analyses of various subgroups with binary data were significant, we planed to perform a test of interaction by applying the fixed inverse variance method incorporated in RevMan 5.1. Alternatively, we applied meta‐regression if a fixed‐effect model was not considered sensible because of considerable between‐study variability. We considered P < 0.05 as indicating significant interaction between the fibrinogen effect on mortality and the subgroup category (Higgins 2011, Chapters 9.6.1 and 9.7). Too few cases were available to conduct meta‐regression or Q‐partitioning.
Sensitivity analysis
We planned for the following sensitivity analyses.
Comparison of estimates of the pooled intervention effect in trials investigating fibrinogen concentrate as a part of a predefined transfusion algorithm with trials addressing only the isolated effect of fibrinogen concentrate (control of transfusion using an algorithm is considered a potential confounder).
Comparison of estimates of the pooled intervention effect in trials using quasi‐randomization with estimates from trials with proper randomization (i.e. adequate sequence generation and allocation concealment (Assessment of risk of bias in included studies)).
Comparison of estimates of the pooled intervention effect in trials with low risk of bias with estimates from trials with high risk of bias (i.e. trials having at least one inadequate risk of bias component).
Comparison of estimates of the pooled intervention effect in trials based on different components of risk of bias (random sequence generation, allocation concealment, blinding, completeness of outcome data, selective reporting and 'other' bias).
Comparison of estimates of the pooled intervention effect in trials with high levels of missing data. In cases of missing data, our strategy was to apply 'complete case analysis' for primary and secondary outcomes, thereby excluding from the analysis all participants for whom the outcome was missing.
Examination of the role of funding bias when trials that were exclusively sponsored by pharmaceutical and medical devices companies were excluded.
Comparison of estimates of the pooled intervention effect excluding data from studies published only as abstracts.
Assessment of the benefits and harms of fibrinogen by conducting a continuity correction of trials with zero events for the primary outcome.
We calculated RR with 95% CI and decided to apply complete case analysis, if possible, for our sensitivity and subgroup analyses based on our primary outcome measure (mortality).
Summary of findings tables
We used the principles of the GRADE system (GradePro; Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (overall mortality; incidence of allogenic blood transfusion; complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, disseminated intravascular coagulation (DIC)), incidence of surgical interventions and re‐operation due to bleeding; and mean length of stay in the ICU).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
Through electronic database search and handsearch of references, we identified 3280 publications. Identification of animal studies, duplicates and trials with irrelevant endpoints or populations excluded 3240 publications. Five studies are awaiting classification (Galas 2012a; Rahe‐Meyer 2011a; Rahe‐Meyer 2012; Rahe‐Meyer 2013a; Solomon 2012 see Characteristics of studies awaiting classification.On the basis of title and abstract, we found 33 studies relevant for full paper review. This excluded another 19 on the basis of design (not RCT), irrelevant endpoints and review publications (Characteristics of excluded studies). We included six randomized trials (Cui 2010; Fenger‐Eriksen 2009a; Galas 2012; Karlsson 2009; Lance 2011; Rahe‐Meyer 2013) comprising a total of 248 randomly assigned participants (Characteristics of included studies; Appendix 3). One of the included studies was published only as a posters/meeting abstract (Galas 2012). We found no quasi‐randomized studies.
We identified 11 ongoing studies but were unable to retrieve any data from the investigators at their current stage. For additional information on these studies (Fries 2011; Haas 2012; Innerhofer 2012; Jeppsson 2010; Kwapisz 2012; Nierich 2011; Nimmo 2009; ; Ranucci 2011; Sabate 2012; Tanaka 2011; Wikkelsoe 2011), see Characteristics of ongoing studies and Appendix 4. The three review authors (JL, AW and MJ) completely agreed on the selection of included studies. We obtained additional information from four study authors, as listed in the table (Characteristics of included studies).
Karlsson 2009 represents two publications of the same trial. Mortality was reported in two of the included studies (Rahe‐Meyer 2013; Karlsson 2009 (additional information obtained from the author)).
Included studies
See: Characteristics of included studies; Appendix 3.
We included six trials published or reported during the period from 2009 to 2013. The sample size varied between 20 and 63. Two trials (Cui 2010; Galas 2012) involved a paediatric population. All six trials included elective surgical participants but assessed different types of surgery: cardiac (Cui 2010; Galas 2012; Karlsson 2009; Rahe‐Meyer 2013); mixed population of cardiac surgery, major abdominal and spinal surgery (Lance 2011); and elective urological cancer operation (Fenger‐Eriksen 2009a). The objective of assessing the potential benefits of fibrinogen concentrate in the treatment of bleeding participants differed among the trials, ranging from one trial that explored the use of fibrinogen substitution for the reversal of colloid dilution and subsequent impairment of haemostasis (Fenger‐Eriksen 2009a), to another trial that assessed the role of preemptive treatment in participants at risk for postoperative bleeding (Karlsson 2009), to two trials that examined replacement of FFP or cryoprecipitate with fibrinogen concentrate in cases of major haemorrhage and need for haemostatic treatment (Galas 2012; Lance 2011) and finally to two trials that used a thrombelastography‐guided algorithm to treat acquired hypofibrinogenaemia (Cui 2010; Rahe‐Meyer 2013). Blood loss in the four studies involving adult participants ranged between mean 830 mL and 2933 mL. Four of six studies used synthetic colloids as part of the fluid resuscitation strategy before intervention was given (see Appendix 3), and the remaining two provided no information regarding this issue.
Three studies used single doses of fibrinogen concentrate (0.5 to 2 g) (Cui 2010; Karlsson 2009; Lance 2011), two derived the fibrinogen dose from the body weight (45 mg fibrinogen/kg: Fenger‐Eriksen 2009a and 60 mg fibrinogen/kg: Galas 2012) and one calculated the fibrinogen dose on the basis of ROTEM/FIBTEM measures (Rahe‐Meyer 2013, median fibrinogen dose administered was 8 g).
Cui 2010 combined fibrinogen concentrate substitution with routine transfusion therapy, and Lance 2011 used co‐application of two units of FFP. Two studies used placebo (isotonic saline) as a comparison (Fenger‐Eriksen 2009a; Rahe‐Meyer 2013), two compared fibrinogen with standard treatment (Cui 2010; Lance 2011), one used cryoprecipitate (Galas 2012) and another provided no additional treatment (Karlsson 2009). Follow‐up differed between studies and even between outcomes in each study (see Appendix 3), and no participants were followed beyond the end of hospitalisation.
Excluded studies
We excluded 14 potentially relevant publications. Most studies were designed as retrospective cohort studies involving a mixed group of participants. No studies evaluated mortality (see Characteristics of excluded studies; Bell 2010; Danes 2008; Glover 2010; Guasch 2008; Haas 2008; Mittermayr 2007; Morrison 2011; Rahe‐Meyer 2009; Rahe‐Meyer 2009a; Schöchl 2010a; Schöchl 2010b; Solomon 2010; Thorarinsdottir 2010; Weinkove 2007).
Risk of bias in included studies
The overall quality of trials was evaluated on the basis of major sources of bias (domains) as described above. No trials could be classified as overall 'Low risk of bias'. The various bias domains are presented in the 'risk of bias' graph (Figure 2) and in the 'risk of bias' summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies provided information on the method used to generate the allocation sequence (shuffling of cards or computer random sequence generation) (Fenger‐Eriksen 2009a; Karlsson 2009; Lance 2011; Rahe‐Meyer 2013), and the same four trials used adequate allocation concealment with closed opaque envelopes (Fenger‐Eriksen 2009a; Karlsson 2009; Lance 2011; Rahe‐Meyer 2013).
Blinding
One study was open‐label (Galas 2012). Two reports described adequate blinding provided to participants, caregivers (Fenger‐Eriksen 2009a; Rahe‐Meyer 2013) and outcome assessors (Fenger‐Eriksen 2009a; Karlsson 2009; Rahe‐Meyer 2013). Karlsson 2009 and Lance 2011 did not achieve complete blinding in that anaesthetic personnel at the operation theatre were not completely blinded. Cui 2010 gave no information on blinding, and attempts to contact the authors yielded no additional information.
Incomplete outcome data
One study reported on intention‐to‐treat (ITT) analysis (Rahe‐Meyer 2013). Three studies (Cui 2010; Fenger‐Eriksen 2009a; Lance 2011) excluded participants after randomization, and none performed ITT analysis or provided follow‐up data on excluded participants: Cui 2010 excluded 22.5% (9 of 40 randomly assigned participants) in accordance with exclusion criteria (history of blood disease, anticoagulant treatment before surgery, medication that affects haemostasis and difficult sternal closure). Fenger‐Eriksen 2009a excluded 5% (1 of 21) of randomly assigned participants because of cancelled operation, and Lance 2011 excluded 17% (9 of 52) of randomly assigned participants because FFP transfusion was less than required. We obtained no additional information on excluded participants through our attempts to contact study authors.
Selective reporting
We were able to compare three trials (Fenger‐Eriksen 2009a; Galas 2012; Rahe‐Meyer 2013) with the trial registration on clinicaltrials.gov and one with the protocol (Lance 2011). Galas 2012 appeared to have changed inclusion criteria (age of participants) and added one outcome parameter in the process. Lance 2011 seemed to have changed the primary outcome. None of the others were suspicious of selective reporting. We were unable to obtain protocol or trial registration material in the remaining studies to compare with the published material.
Other potential sources of bias
Funding bias
Three trials (Fenger‐Eriksen 2009a; Karlsson 2009; Lance 2011) registered the manufacturer of fibrinogen concentrate (Haemocomplettan®/RiaSTAP®), CSL Behring, Switzerland, as affiliated by means of unrestricted grants, and one (Rahe‐Meyer 2013) as receiving extensive support. Lance 2011 also acknowledges three persons from CSL Behring for "stimulating discussions". Cui 2010 and Galas 2012 provide no information on funding in their publications, and we were unable to obtain information regarding this issue.
A total of 45% (5/11) of the ongoing studies reported that they were independent of the industry (Haas 2012; Innerhofer 2012; Jeppsson 2010; Sabate 2012; Wikkelsoe 2011). Ten trials used (Haemocomplettan®/RiaSTAP®) CSL Behring and one (Fries 2011) used (FCTW/Clottafact®) Laboratoire Français du Fractionnement et des Biotechnologies (LFB) products (see Characteristics of ongoing studies; Appendix 4).
Pharming Group N.V. (Netherlands) replied that it had no ongoing clinical studies investigating fibrinogen concentrate. We received no reply from Shanghai Raas Blood Products Company, Ltd (China) or from Benesis Corporation (Japan) regarding this issue.
Study design and early stopping
Sample sizes were small in each trial (20 to 63 participants), immensely increasing the risk of random error. Only two studies provided sample size estimates used for calculation, and the two smallest trials (Fenger‐Eriksen 2009a; Karlsson 2009) described a primary surrogate outcome (coagulation parameters) or a pilot study that was clearly under‐dimensioned. No trials reported early stopping.
Baseline imbalance
One study showed operative recovery data with very large differences between groups, suggesting that the intervention group might have consisted of healthier individuals overall (Cui 2010), one provided no data to illustrate a reported balance in baseline parameters (Fenger‐Eriksen 2009a) and one provided no baseline parameters (Galas 2012). The rest were apparently free of baseline imbalance; however, no additional exploratory statistical analyses were performed, as recommended by The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice (ICH Harmonised Tripartite Guideline).
Effects of interventions
See: Table 1
Primary outcome
Only two trials provided data on mortality, but onlyRahe‐Meyer 2013 contributed to the analysis because Karlsson 2009 was a zero event trial. No statistically beneficial effect was described for the longest follow‐up, and the outcome of 28 day mortality (2.6% vs 9.5%, RR 0.28, 95% CI 0.03 to 2.33) (Analysis 1.1; Appendix 5).
1.1. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 1 Mortality longest follow‐up.
Secondary outcomes
Comparison: Fibrinogen concentrate versus any comparator
Blood transfusion: Pooling data from five studies on the proportion of participants with allogenic blood transfusion (longest follow‐up) shows a statistically significant reduction favouring the use of fibrinogen concentrate (Analysis 1.5) (RR 0.47, 95% CI 0.31 to 0.72, I2 = 41%). This effect was not evident in the overall incidence of RBC transfusion (Analysis 1.10) (RR 0.81, 95% CI 0.32 to 2.02, I2 = 89%). The reporting of continuous transfusion requirement data was statistically skewed, and only median and interquartile range values were reported. Meta‐analyses based on data with median equal zero was not attempted. Thus, we are unable to report on the reduction in FFP, RBC or platelet (PLT) volume.
1.5. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 5 Incidence of allogenic blood transfusion (types of comparison).
1.10. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 10 Incidence of RBC transfusion longest follow‐up.
Adverse events: Three trials reported on thrombotic episodes with no statistically significant effect identified (Analysis 1.11) (RR 1.03, 95% CI 0.27 to 3.97, I2 = 0%). However, only six events were reported. No statistical difference was found related to complications unspecific to trial intervention (pleural effusion, abdominal ischaemias and other serious adverse events; Analysis 1.12) (RR 1.25, 95% CI 0.45 to 3.44, I2 = 0%), and only one study reported wound infection and sepsis as the outcome (Lance 2011), with no difference identified (Appendix 6).
1.11. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 11 Thrombotic episodes (arterial and venous graft occlusion, pulmonary embolus, deep venous thrombosis).
1.12. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 12 Complications not specific to trial intervention (pleural effusion, abdominal ischaemia and other serious adverse events).
Bleeding: No difference was found in any of the outcomes related to assessment of bleeding, including blood loss/drainage (longest follow‐up; Analysis 1.13) and 24 hour blood loss/drainage (Analysis 1.14) associated with re‐operation due to persistent bleeding (Analysis 1.9). Three additional bleeding outcomes were reported by only one trial (Appendix 8).
1.13. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 13 Blood loss/drainage, longest follow‐up.
1.14. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 14 Blood loss/Drainage (24 hours) mL/kg/h.
1.9. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 9 Re‐operation due to persistent bleeding.
Operative recovery: We assessed duration of ICU stay (Analysis 1.2), duration of mechanical ventilation (Analysis 1.3) and stay in hospital (Analysis 1.4) and obtained no statistically significant results. No studies provided data on "days free from ventilator".
1.2. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 2 ICU stay (hours).
1.3. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 3 Duration of mechanical ventilation (hours).
1.4. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 4 Stay in hospital (days).
Finally, none of the trials provided data on quality of life assessment or cost‐benefit analyses.
Comparison: Fibrinogen concentrate versus placebo or no treatment
Two trials compared fibrinogen with placebo or no treatment (Fenger‐Eriksen 2009a; Karlsson 2009). Fibrinogen concentrate appeared beneficial in reducing the proportion of participants in need of allogenic blood transfusion, but this was supported by only two trials with a total of 40 participants (Analysis 1.5) (RR 0.27, 95% CI 0.09 to 0.80, I2 = 0 %). Single‐study outcomes are reported in Appendix 9.
Comparison: Fibrinogen concentrate versus FFP or cryoprecipitate
Three trials compared fibrinogen with FFP or cryoprecipitate (Cui 2010; Galas 2012; Lance 2011). However, the reported data (single‐study) on relevant outcomes were sparse (Appendix 10).
Comparison: Fibrinogen concentrate in treatment algorithm versus placebo or no treatment
Only Rahe‐Meyer 2013 applied this treatment regimen (Appendix 11).
Subgroup analyses
We were unable to conduct our predefined subgroup and sensitivity analyses on the primary outcome because available data were insufficient.
Type of surgery: We were able to compare only trials with cardiac surgery versus non‐cardiac surgery. No trials investigating emergency surgery, trauma, critically ill or obstetrical participants were identified. Cardiac surgery participants were investigated in four trials (Cui 2010; Galas 2012; Karlsson 2009; Rahe‐Meyer 2013), but compared with the remaining two, no subgroup differences were found to explain the observed heterogeneity in the incidence of allogenic blood transfusion (Analysis 1.6).
1.6. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 6 Incidence of allogenic blood transfusion (cardiac vs non‐cardiac).
Age group: Two trials were conducted in paediatric populations (Cui 2010; Galas 2012). We found no significant difference in subgroups explaining heterogeneity in the incidence of allogenic blood transfusion (Analysis 1.7).
1.7. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 7 Incidence of allogenic blood transfusion (pediatric vs adult).
Use of plasma expanders: Only one trial (Fenger‐Eriksen 2009a) investigated participants specifically haemodiluted with synthetic plasma expanders before intervention. No subgroup comparison was conducted using this criterion.
Dose regimen: The median fibrinogen dose given was approximately 50 mg/kg body weight, with three trials giving higher doses (Cui 2010; Galas 2012; Rahe‐Meyer 2013) and three giving lower doses (Fenger‐Eriksen 2009a; Karlsson 2009; Lance 2011). The outcome of the incidence of allogenic blood transfusion revealed insignificant subgroup differences regarding dose regimen (Analysis 1.8).
1.8. Analysis.

Comparison 1 Fibrinogen concentrate versus any comparator, Outcome 8 Incidence of allogenic blood transfusion (high dose > 50 mg/kg vs low dose).
Sensitivity analyses
Bias assessment: No trials were evaluated as having overall "low risk of bias" (Characteristics of included studies); therefore, no sensitivity or subgroup analyses were carried out regarding this issue.
Types of comparison: We wished to assess whether differences in types of comparisons made a difference (e.g. if a predefined transfusion algorithm was different from the isolated effect of fibrinogen, or if comparison with placebo was different from that of FFP). The six included trials differ in terms of treatment regimens with regard to fibrinogen and type of comparison, and additional trials are needed to differentiate this aspect (Appendix 3).
Only published peer‐reviewed data: One trial was published only as an abstract (Galas 2012).
Funding bias: One trial (Rahe‐Meyer 2013) was industry initiated and sponsored, and three (Fenger‐Eriksen 2009a; Karlsson 2009; Lance 2011) were supported by unrestricted industry funding. Two trials (Cui 2010; Galas 2012) did not state their industry relations, and none of the included trials were clearly independent of the industry.
Trial sequential analysis (TSA)
Because data on mortality were very scarce (only two trials), we were unable to conduct a trial sequential analysis (TSA) on mortality. Using an anticipated clinically relevant intervention effect of 20% relative risk reduction and a control event proportion of 10% yields a required information size of at least 10,000 participants, which is more than 20 times the actual accrued information size.
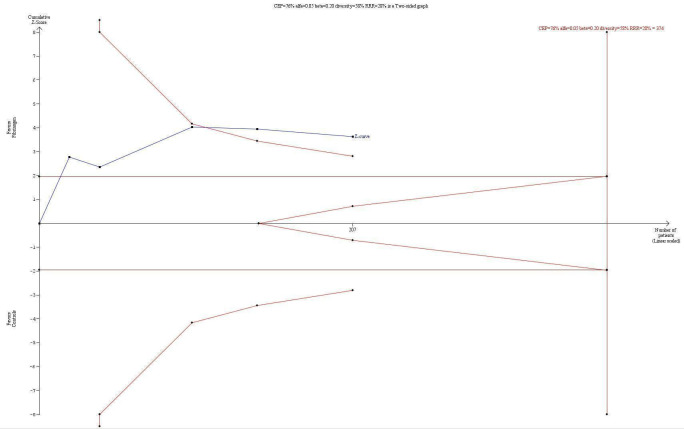
The TSA indicated statistical significance in favour of a reduction in the proportion of participants needing allogenic blood transfusion even with adjustment for repetitive testing of accumulating data in the cumulative meta‐analysis, because the z‐curve crossed the trial sequential monitoring boundary (Figure 4). However, bias is imminent, given that all trials are at high risk of bias.
4.
Trial sequential analysis of the effect of fibrinogen concentrate on the proportion of participants transfused using a control event proportion of 76% found in the included trials cumulated control groups. The diversity (58%) adjusted required information size for an anticipated intervention effect of 28% derived from the upper confidence limit of the RR (0.31 to 0.72) estimated in the traditional meta‐analysis is 374. The trial sequential monitoring boundary for benefit is crossed indicating lack of random error for the conclusion of an effect of 28% relative risk reduction even though the required information size has not been reached. However, risk of bias may have overestimated the intervention effect in the traditional meta‐analysis, so the results shall be interpreted with caution.
We did not conduct a TSA on complications specific to trial intervention (using an anticipated clinically relevant intervention effect of 20% RR and a control event proportion of approximately 10%) because the required information size was at least 10,000 participants, which is more than 20 times the actual accrued information size that would be needed. Thus, currently, firm evidence of fibrinogen reducing mortality and complications specific to trial intervention is lacking because very few data are available. Equally, bias is imminent for this outcome given that all trials were determined to have high risk of bias.
Discussion
In this systematic review of six randomized trials involving 248 participants with bleeding after elective surgery, we found that data on survival were lacking. Only two trials reported mortality as an endpoint (Karlsson 2009; Rahe‐Meyer 2013), and none were powered to detect a possible difference. Mortality may be contested by many as the choice of primary outcome, but it summarizes ultimate harms and benefits simultaneously. Even if participants in the trials included in this review represent patients with generally low mortality, the endpoint is of great clinical importance when data are extrapolated to the setting of severe, acute bleeding, as is often done in everyday practice.
We identified only six randomized trials that evaluated a treatment that has been used for decades in some countries and in some clinical cases is considered 'standard treatment', depending on tradition and region. Thus, this review summarizes an area in which the evidence differs widely from clinical practice, and the information provided here should indeed ultimately guide the direction of future research.
Eleven registered ongoing RCTs indicate that this is an area of ongoing development for which it is hoped that evidence will continue to be gathered during the coming years. The ongoing trials seem to address populations unexplored by the included trials in this review (such as trauma, liver surgery and obstetrical patients). Despite initial exploratory benefits of small RCTs with 20 to 60 participants in which surrogate outcomes such as changes in coagulation parameters/improvement in haemostasis were examined, findings remain inadequate to facilitate the goal of improving the overall quality of treatment. Thus, clinically relevant endpoints should be addressed, and trials must be powered to detect possible and relevant differences.
Our analyses on blood transfusion data indicate a beneficial effect of fibrinogen concentrate, which apparently reduces the proportion of patients in need of allogenic transfusions. However, the validity of our findings may be questioned by the heterogeneity of the included trials, which describe different clinical settings (participants receiving cardiopulmonary bypass(CPB) or haemodiluted with colloids, etc), statistical heterogeneity and different comparators (placebo/saline, FFP, cryoprecipitate or no treatment). Despite a general reduction in blood transfusions, no evidence of reduced blood loss or of reduction in re‐operations due to bleeding was identified. TSA on the proportion of participants in need of allogenic blood transfusion using a control event proportion of 76% and an anticipated intervention effect of 28% derived from the upper confidence limit of the intervention effect estimate in the traditional meta‐analysis showed firm evidence indicating benefit associated with the use of fibrinogen. However, given that all trials are determined to have high risk of bias, this finding should be interpreted with caution. Data on continuous outcomes such as quantity of FFP, RBC or PLT transfused were statistically skewed, often with the median equalling zero. Not only is meta‐analysis not recommended, but it questions the relevance of the trial population when apparently so few patients need blood transfusion.
Adverse events, especially thrombotic events, were reported as insignificant, with only very few cases reported in an overall small population. RCTs require large sample sizes if they are to show possible differences in adverse events. All six included trials were of overall 'high risk of bias', some with serious methodological flaws such as change in primary outcome (selective reporting) and exclusion of 22.5% of randomly assigned participants, with no explanation for the induced baseline imbalance. Blinding of participants, personnel and outcome assessors complicates the trial setup, especially in situations of ongoing severe haemorrhage, but it is crucial to reduce performance and detection bias. Furthermore, if mortality is not a primary endpoint of a study, then performing an open‐label study weakens other endpoints that are dependent on clinical practise.
Fibrinogen concentrate might be used with different objectives, including treatment of evident hypofibrinogenaemia in participants with bleeding, prophylactic treatment in participants with risk of bleeding due to a low fibrinogen level before surgery (Karlsson 2008) or treatment in categories of participants with bleeding known to be complicated by hypofibrinogenaemia (Charbit 2007), for whom early preemptive treatment might be provided. Too few trials were available to assess these different treatment objectives (Appendix 3), but when the findings of future trials are published, we may be able to explore this topic in detail (Appendix 4).
Colloids such as hydroxyethyl starch reduce haemostatic capacity, and fibrinogen concentrate may reverse this effect (Fenger‐Eriksen 2009a). Evidence of a beneficial effect related to the use of fibrinogen concentrate should therefore be sought in the light of the quantity of colloids transfused. Not all trials included in this review stated the volume of colloids transfused before intervention (Appendix 3), and we were not able to investigate this in a subgroup analysis, but future trials should address this matter. One thing that needs clarification in RCTs is the association between fluid resuscitation strategy (especially the use of synthetic plasma expanders) and the need for or effect of fibrinogen substitution.
Participants totaling 248 are too few for statistically meaningful subgroup analyses to be conducted. However, we have outlined our approaches for future updates of this review, when data from the many ongoing trials become available.
None of the included trials were clearly independent of the industry. Five ongoing trials report to be independent of industry, and it is hoped that the impact of funding bias may be addressed in greater detail when additional trials are published.
No studies addressed a cost‐benefit issue, but price remains a relevant issue: The price of 1 gram of fibrinogen concentrate in Denmark in April 2013 was 657 euro (4898 DKK, 1 DKK is 0.13 euro) (Pro.medicin.dk 2013), and for the recommended dose of 70 mg/mL (Riastap 2009) in an average person of 70 kg, the price was 3219 euro. Future studies should address cost benefit also in relation to treatment strategy (preemptive use or hypofibrinogenaemia replacement therapy).
Summary of main results
Our systematic review shows that overall few randomly assigned participants (n = 248) were included in only six overall 'high risk of bias' trials primarily performed in elective cardiac surgery. It should be kept in mind that the investigated intervention is being considered as a standard treatment in some countries and settings, and none of the presented trials is powered to detect harm or safety issues. Fibrinogen concentrate appears to reduce the proportion of patients in need of allogenic blood transfusions, but we were unable to evaluate our primary endpoint-mortality; clinical and statistical heterogeneity was substantial, and we were unable to explore settings with severe bleeding such as trauma or postpartum haemorrhage. Currently, evidence supporting the use of fibrinogen concentrate in bleeding patients is weak, as can be seen in this review of six trials of elective surgery.
Agreements and disagreements with other studies or reviews
Discussions related to the use of fibrinogen concentrate are generally complicated by the lack of a clear‐cut hypofibrinogenaemia level at which we should start treatment, including indications for preemptive treatment. In addition, discussions regarding the best source of fibrinogen (fibrinogen concentrate vs fresh frozen plasma vs cryoprecipitate) divide experts in often fierce discussions based on tradition, experience, preferences, lack of evidence and conflicts of interest (industry associations) (Ozier 2011; Rahe‐Meyer 2011c).
A recent review (excluding meta‐analysis) sought to address the optimal source of fibrinogen in trauma patients (Kozek‐Langenecker 2011). The review authors evaluated 70 publications on the use of FFP and 21 on the use of fibrinogen concentrate substitution, including all available study types ranging from case reports to RCTs. No consistent bias assessment was used, and thus RCTs were uncritically referred to as "high‐quality studies". Additionally, this review did not adhere to PRISMA (Moher 2009) guidelines and/or Cochrane methodology (Higgins 2011; Kozek‐Langenecker 2011a; Stanworth 2011).
In a recent publication with no meta‐analysis (Warmuth 2012), the efficacy and safety of fibrinogen concentrate substitution in adults were assessed. Only two RCTs were identified (Fenger‐Eriksen 2009a; Karlsson 2009) and judged to be of overall 'high risk of bias'/low quality. The authors' conclusions were consistent with our findings, indicating a possible benefit in terms of reduced transfusion requirements but with acute need of confirmation by large‐scale high‐quality trials.
Authors' conclusions
Implications for practice.
Given the low level of evidence in six trials of elective surgery with high risk of bias favouring the use of fibrinogen concentrate for patients with bleeding and acquired haemostatic deficiencies, caution is advised, and general widespread implementation is not warranted at the present time. Such general applications of the use of fibrinogen should be postponed until solid evidence is obtained, or usage should be confined to a controlled clinical setting or trial.
Implications for research.
Further trials are urgently needed, and great emphasis must be placed on attempts to reduce bias and increase power to show differences in patient‐relevant clinical outcomes (i.e. mortality). Additionally, further assessment of potential benefits or harms and of the cost benefit of fibrinogen in acute non‐elective settings and with major haemorrhage remains essential and of great importance. Last, fibrinogen dosing protocols, fluid resuscitation protocols and pre‐intervention monitoring will be improved by further elucidation.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We thank Dr Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia Review Group (CARG)) for his assistance in providing our different search strategies and Prof Nathan Leon Pace, MD, MSTAT (University of Utah, USA), for providing statistical guidance. We thank Jane Cracknell (Managing Editor, CARG) for her valuable assistance during the entire process. We would also like to thank Harald Herkner (Content Editor); Christian Fenger‐Eriksen, Benny Sørensen and Jørgen Ingerslev (peer reviewers) for their help and editorial advice during the preparation of the protocol; and Harald Herkner (Content Editor), Nathan Pace (Statistical Editor) and David Keeling and Ole Halfdan Larsen (peer reviewers) for their help and editorial advice during the preparation of this systematic review. We also thank CSL Behring and Stephan Grass, MD, for providing additional information on ongoing studies, and trial authors for providing requested information on published and ongoing studies.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL, the Cochrane Library #1 MeSH descriptor: [Fibrin Tissue Adhesive] explode all trees #2 MeSH descriptor: [Fibrinogen] this term only #3 (fibrinogen?concentrate or fibrin*):ti,ab #4 #1 or #2 or #3 #5 MeSH descriptor: [Hemorrhage] this term only #6 MeSH descriptor: [Afibrinogenemia] explode all trees #7 (hypofibrinogen?em* or bleeding):ti,ab #8 #5 or #6 or #7 #9 #4 and #8
Ovid MEDLINE search strategy
1. Fibrin Tissue Adhesive/ or exp Fibrinogen/ 2. fibrinogen‐concentrate.mp. or fibrin*.ti,ab. 3. 1 or 2 4. exp Hemorrhage/ or Afibrinogenemia/ 5. (hypofibrinogen?em* or bleeding).mp. 6. 4 or 5 7. 3 and 6 8. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 9. 7 and 8
Ovid EMBASE search strategy
1. fibrin glue/ or fibrinogen/ or fibrinogen‐concentrate.mp. or fibrin*.ti,ab. 2. bleeding/ or afibrinogenemia/ or (hypofibrinogen?em* or bleeding).ti,ab. 3. 1 and 2 4. ((controlled adj2 (study or trial*)) or random* or multicentre or prospective).ti,ab. 5. 3 and 4
CINAHL (EBSCO host) search strategy
S1 ( (MH "Fibrin Tissue Adhesive") OR (MH "Fibrinogen") ) OR fibrinogen‐concentrate OR TI fibrin* OR AB fibrin* S2 ( (MH "Hemorrhage") OR (MM "Afibrinogenemia") ) OR AB ( hypofibrinogen?em* or bleeding ) S3 S1 and S2
ISI Web of Science search strategy
#1 TS=(fibrin tissue adhesive) or TS=(fibrinogen SAME concentrate) or TI= fibrin* #2 TS=(hemorrhage or afibrinogen?em* or hypofibrinogen?em* or bleeding) #3 #1 and #2 #4 TS=(random* or placebo*) or TS=(controled SAME (stud* or trial*)) or TS=((blind* or mask*) SAME (single or double or triple)) or TS=(multicenter* or prospective) #5 #4 and #3
Search strategy for LILACS (BIREME interface)
("fibrin$" and ("adhesiv$" or "concentrate")) and ("hemorragia" or "hemorrhage" or "afibrinogenem$" or "afibrinogenaem$" or "hypofibrinogenem$" or "hypofibrinogenaem$" or "bleeding" or "sangradura")
Appendix 2. Data extraction form
Study Selection, Quality Assessment & Data Extraction Form
| First author | Journal/Conference proceedings, etc | Year |
| |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/No*/Unclear |
*Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Review authors should contact trialists for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If further references to this trial are included, link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference proceedings, etc | Year |
| A | The paper listed above | ||
| B | Further papers | ||
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers/%, etc) | |
| Disease status/type, etc (if applicable) | |
| Patients treated with plasma expanders (colloids) before intervention (Treatment is defined as a methodical prespecified and clinically evaluated level of haemodilution) | |
| Clinical setting: (mark with an X) | Cardiac Non‐cardiac Emergency (surgery that should be performed within 24 hours after meeting the indication for surgery) Trauma Obstetrics Paediatrics (age younger than 18 years, neonates not included) Neonates (born preterm) Critically ill (sepsis, septic shock, DIC) Other |
| Other | |
Trial characteristics
SeeAppendix 1, usually just completed by one review author
Methodological quality
We recommend you refer to and use the method described by Juni (Juni 2001)
| Allocation of intervention (adequate sequence generation?) | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Adequate (random) (yes) |
| Inadequate (e.g. alternate) (no) | |
| Unclear | |
|
Concealment of allocation (allocation concealment?) Process used to prevent foreknowledge of group assignment in an RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate (yes) | |
| Inadequate (no) | |
| Unclear | |
| Blinding (blinding?) | |
| Person responsible for participants' care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes ? No ? Not clear ?
| Incomplete outcome data addressed? | |
| Completeness of outcome data, including attritions and exclusions | Grade (circle) |
| Adequate (yes) | |
| Inadequate (no) | |
| Unclear | |
| Free of selective reporting? | |
| The possibility of selective outcome reporting | Grade (circle) |
| Adequate (yes) | |
| Inadequate (no) | |
| Unclear | |
|
Free of other bias? (bias not addressed in the other domains) | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate (yes) | |
| Inadequate (no) | |
| Unclear | |
Data extraction
|
Outcomes relevant to your review Copy and paste from ‘Types of outcome measures’ | |
| Reported in paper (circle) | |
| Overall mortality | Yes/No |
| Overall 28 days' mortality (30 days M. included) | Yes/No |
| Incidence of allogenic transfusion (e.g. avoidance of transfusion) | Yes/No |
| Bleeding events | Yes/No |
| Quantity of blood products transfused | Yes/No |
| Incidence of surgical interventions | Yes/No |
| Incidence of re‐operation due to bleeding | Yes/No |
| Quality of life assessment, as defined by authors in included studies. | Yes/No |
| Complications during the inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure). | Yes/No |
| Duration of mechanical ventilation | Yes/No |
| Days free from ventilator (as defined by authors) | Yes/No |
| Number of days in hospital | Yes/No |
| Mean length of stay in intensive care unit (ICU) | Yes/No |
| Yes/No | |
| For continuous data | |||||||
| Code of paper |
Outcomes (rename) |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A etc | Bleeding events and amount of blood transfused | ||||||
| Quality of life assessment | |||||||
| Duration of mechanical ventilation and/or improvement in respiratory failure (ventilator‐free days) | |||||||
| Days free from ventilator (as defined by authors) | |||||||
| Number of days in hospital | |||||||
| Mean length of stay in intensive care unit (ICU) | |||||||
| For dichotomous data | |||
| Code of paper | Outcomes (rename) | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| A | Overall mortality | ||
| Overall mortality (28 days) | |||
| Incidence of re‐operation due to bleeding | |||
| Incidence of surgical interventions | |||
| Complications probably related to the intervention (e.g. thrombotic episodes (pulmonary embolism, myocardial infarction, DIC), major immunological and allergic reactions, infections and sepsis) | |||
| Complications during the inpatient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure) | |||
| Incidence of allogenic blood transfusions (e.g. avoidance of transfusion) | |||
|
Other information that you feel is relevant to the results Indicate if any data were obtained from the primary author; if results were estimated from graphs, etc or calculated by you using a formula (this should be stated and the formula given). In general, if results not reported in paper(s) are obtained, this should be made clear here to be cited in review. |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| Trial characteristics | |
| Further details | |
| Single centre/multi‐centre | |
| Country/countries | |
| How was participant eligibility defined? | |
| How many people were randomly assigned? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose/frequency of administration | |
| Co‐interventions (including if fibrinogen concentrate was given as part of a predefined transfusion algorithm) |
|
| Duration of treatment (state weeks/months, etc, if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Appendix 3. Detailes of included studies
| Study | Population | Intervention | Control | Outcome | Follow‐up | Objective | Blood loss + amount colloid transfused in control group (mean if otherwise not stated) |
| Cui 2010 | N = 40 (14/17; 9 excluded) Elective cardiac surgery, children |
Single dose, 0.5 to 1 g fibrinogen concentrate (manufacturer not stated) administration combined with traditional transfusion therapy guided by thrombelastography Dose approx = 39 to 80 mg/kg |
Traditional transfusion therapy guided by clinical experience |
Closure time, transfusion at closure (FFP/PLT), transfusion requirements at ICU (FFP/PLT/RBC), chest tube drainage (1, 6, 24 hours) and total transfusion requirements |
Until end of hospitalization, but blood loss was only recorded the first 24 hours postsurgery |
Treatment of hypofibrinogenaemia in thrombelastography‐guided algorithm |
Blood loss:
At 1 hour: 3.5 mL/kg/h At 6 hours: 1.5 mL/kg/h At 24 hours: 0.7 mL/kg/h Colloid transfused: 82.5 mL/kg |
| Lance 2011 | N = 43 (21/22) Elective cardiac, major abdominal and spinal column surgery, adults |
Hemostatic transfusion with 2 units of FFP combined with single‐dose 2 g fibrinogen concentrate (Haemocomplettan®) Dose approx = 25 mg/kg |
Hemostatic transfusion with 4 units of FFP | Arrest of bleeding and fibrin clot formation (measured by whole‐blood thromboelastometry and thrombin generation) | No follow‐up after successful haemostasis (possible adverse events were recorded postsurgery) | Replacement of FFP |
Blood loss: not stated Colloid transfused: not stated |
| Fenger‐Eriksen 2009a | N = 21 (10/10; 1 excluded) Elective urological cancer operation, adults |
Fibrinogen concentrate (Haemocomplettan®) Dose 45 mg/kg |
Placebo, isotonic saline 2.25 mL/kg |
Primary: whole‐blood maximum clot firmness as determined by thromboelastometry (ROTEM) Secondary: other thromboelastometric variables; platelet function; thrombin generation; bleeding and perioperative and postoperative blood product requirements |
48 hours postsurgery | Reversal of colloid dilution |
Blood loss: 2933 mL (at time of intervention) Colloid transfused: 2795 mL (at time of intervention) |
| Karlsson 2009 | N = 20 (10/10), elective cardiac surgery, adults | Preoperative infusion of 2 g fibrinogen concentrate (Haemocomplettan®) Dose 18 to 34 mg/kg |
No treatment |
Primary: clinical adverse events and graft occlusion assessed by multi‐slice computed tomography 3 to 4 days after surgery Secondary: postoperative blood loss, blood transfusions, haemoglobin levels 24 hours after surgery and global haemostasis assessed with thromboelastometry 2 and 24 hours after surgery |
3 to 4 days' follow‐up on clinical adverse events (blood loss was recorded only 12 hours postsurgery) |
Preemptive treatment |
Blood loss: 830 mL (postsurgery) Colloid transfused: 1000 mL |
| Rahe‐Meyer 2013 | N = 61 (29/32), elective thoracic or thoracoabdominal aortic replacement surgery, adults |
Fibrinogen concentrate (Haemocomplettan P®) Individualized dose using maximum amplitude of ROTEM/FIBTEM measurements before the end of CPB. (Median dose 8 g.) Dose approx = 90 mg/kg | Placebo (saline 0.9%) same volume |
Primary outcome: total allogenic transfusion requirements in the 24 hours post administration phase (Transfusion therapy guided according to algorithm) Secondary outcomes: intensive care length of stay, number of allogenic units transfused, mortality and adverse events and re‐operation due to persistent bleeding |
24 hours post intervention | Treatment of hypofibrinogenaemia in thrombelastography‐guided algorithm |
Blood loss: postoperative blood loss 850 mL (655 to 1565 mL) (median, IQ) Intraoperative not recorded Colloid transfused: only gelatins were used, but amount not recorded |
| Galas 2012 | N = 63 (30/33) Children (< 15 years) receiving cardiac surgery with cardiopulmonary bypass and clinically important intraoperative bleeding, and hypofibrinogenaemia |
Fibrinogen concentrate dose 60 mg/kg body weight (RiaSTAP®) |
Cryoprecipitate (10 mL/kg body weight) |
Primary outcome measures: number of participants not receiving any allogeneic blood products (intraoperative until hospital discharge). Transfusional requirements were based on clinical judgement Secondary outcome measures: haemostatic tests, length of ICU stay, clinical complications (renal failure, respiratory failure, sepsis, myocardial ischaemias, stroke), transfusion requirements, mechanical ventilation‐free days, length of hospital stay, vasopressor‐free days, perioperative bleeding and re‐operation due to persistent bleeding |
At least 48 hours |
Treatment of hypofibrinogenaemia and replacement of cryoprecipitate |
Blood loss: not stated Colloid transfused: not stated |
Appendix 4. Details of ongoing studies
| Study | Planned size | Population | Intervention | Control | Outcome | Funding | Objective |
| Fries 2011 | 60 | Trauma patients with hemorrhagic shock | 50 mg/kg fibrinogen concentrate (Clottafact/LFB) | Placebo (buffer substance) | ROTEM/FIBTEM change at 60 minutes post infusion | LFB provides medicine | Preemptive treatment |
| Haas 2012 | 60 | Elective paediatric patients: scoliosis or craniofacial surgery with hypofibrinogenaemia | 30 mg/kg fibrinogen concentrate (Haemocomplettan/CSL Behring) and hourly repeated administration guided by ROTEM FIBTEM MCF < 13 mm | 30 mg/kg fibrinogen concentrate (Haemocomplettan/CSL Behring) and hourly repeated administration guided by ROTEM FIBTEM MCF < 8 mm | Total quantity of transfused red cells | Independent | Treatment of hypofibrinogenaemia in a thrombelastography‐guided algorithm |
| Innerhofer 2012 | 200 | Severe trauma patients with clinical bleeding or at risk of bleeding and coagulopathy (measured by ROTEM) | Fibrinogen concentrate (if FIBTEM MA < 7 mm) and/or prothrombin complex concentrate and/or factor XIII concentrate | 15 mL/kg fresh frozen plasma (if FIBTEM MA < 7 mm or EXTEM CT > 90 s) | Multiple organ failure within 24 hours on intensive care unit | Independent | Treatment of hypofibrinogenaemia/ coagulopathy and replacement of FFP |
| Jeppsson 2010 | 60 | Elective coronary artery bypass with preoperative fibrinogen level below 3.8 g/L | 2 g fibrinogen concentrate (RiaSTAP/ CSL Behring) | Placebo (saline) | 2 years' follow‐up on safety and 12 hours' postoperative blood loss | Independent | Preemptive treatment |
| Nierich 2011 | 120 | Complex elective cardiac surgery and microvascular bleeding | Fibrinogen concentrate (Haemocomplettan/CSL Behring) guided by plasma concentration (Clauss method) | Placebo (human albumin) | Perioperative blood loss 12 hours post intervention | Receives study medication from CSL Behring | Treatment of hypofibrinogenaemia |
| Nimmo 2009 | 20 | Elective thoracoabdominal aneurysm repair | 2 g fibrinogen concentrate (Haemocomplettan/CSL Behring) per hour guided by clinical picture and point‐of‐care testing | Fresh frozen plasma (dose not stated) | Intraoperative coagulation pattern | Study collaborates with CSL Behring | Treatment of hypofibrinogenaemia in a guided algorithm |
| Rahe‐Meyer 2011c | 200 | Open elective cardiac surgery and 5 min bleeding mass of 60 to 250 g | Fibrinogen concentrate (Haemocomplettan/CSL Behring) guided by body weight and ROTEM measures | Placebo (saline) | Number of allogenic blood products at 24 hours post intervention | Industry initiated, collaborates with CSL Behring | Treatment of hypofibrinogenaemia in thrombelastography‐guided algorithm |
| Ranucci 2011 | 120 | Complex elective cardiac surgery with CPB > 90 minutes | Fibrinogen concentrate (Haemocomplettan/CSL Behring) and prothrombin complex concentrate guided by ROTEM measures | Placebo (saline) | Avoidance of allogenic blood products at 30 days' post surgery | Study collaborates with CSL Behring | Treatment of hypofibrinogenaemia/ coagulopathy in thrombelastography‐guided algorithm |
| Sabate 2012 | 132 | Liver transplantation with pretransplant plasma fibrinogen level < 2.9 g/L | Fibrinogen concentrate (RiaSTAP/CSL Behring) until expected level of 2.9 g/L is reached | Placebo (saline) | Incidence of RBC transfusion during operation | Independent | Preemptive treatment |
| Tanaka 2011 | 60 | Elective cardiac surgery with CPB and microvascular bleeding | 4 g fibrinogen concentrate (RiaSTAP/CSL Behring) | A single apheresis platelet unit | Coagulation disturbances intraoperatively and up to 24 hours postoperatively | Study collaborates with CSL Behring | Treatment of hypofibrinogenaemia/ coagulopathy |
| Wikkelsoe 2011 | 245 | Severe postpartum haemorrhage (post vaginal delivery and during caesarean section) | 2 g fibrinogen concentrate (RiaSTAP/CSL Behring) | Placebo (saline) | Incidence of allogenic transfusions within 6 weeks postpartum | Independent | Preemptive treatment |
| Kwapisz 2012 | 40 | Adult elective complex cardiac surgical procedures | Fibrinogen concentrate (Haemocomplettan/CSL Behring) guided by body weight | Placebo (saline) | Number of allogenic blood products at 48 hours post intervention | Study collaborates with CSL Behring | Treatment of hypofibrinogenaemia in a thrombelastography‐guided algorithm |
Appendix 5. Mortality: Fibrinogen versus any comparator, single‐study analysis
| Analysis | Study | Participants | Statistical method | Effect estimate |
| Mortality, 28 days |
Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) |
0.28 [0.03 to 2.33] |
Appendix 6. Adverse events: Fibrinogen versus any comparator, single study analysis
| Analysis | Study | Participants | Statistical method | Effect estimate |
| Infections (wound) |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) |
0.32 [0.04 to 2.82] |
| Sepsis |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.78 [0.24 to 94.12] |
Appendix 7. Blood transfusion: Fibrinogen versus any comparator, single‐study analysis
| Analysis | Study | Participants | Statistical method | Effect estimate |
| Incidence of transfusion with PLT in ICU |
Cui 2010 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) |
1.10 [0.67 to 1.80] |
| Incidence of RBC transfusion in ICU |
Cui 2010 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) |
1.28 [0.82 to 2.01] |
| ICU transfusion FFP (ml/kg) |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) |
‐11.90 [‐19.43 to ‐4.37] |
| ICU transfusion PLT (units) |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) |
Not estimable |
| Total FFP usage (mL/kg) |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) |
25.90 [8.56 to 43.24] |
| RBC transfusion during operation (units) |
Fenger‐Eriksen 2009a | 20 |
Mean Difference (IV, Fixed, 95% CI) |
‐0.50 [‐4.08 to 3.08] |
| Total number of allogenic transfusions |
Rahe‐Meyer 2013 | 61 | Mean Difference (IV, Fixed, 95% CI) |
‐11.00 [‐14.55 to ‐7.45] |
| Incidence of FFP transfusion, longest follow‐up | Rahe‐Meyer 2013 | 61 |
Risk Ratio (M‐H, Fixed, 95% CI) |
0.36 [0.22 to 0.58] |
| Incidence of cell saver blood use |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.66 to 2.61] |
| Transfused cell saver blood (mL) |
Lance 2011 | 43 | Mean Difference (IV, Fixed, 95% CI) |
‐286.29 [‐1326.93 to754.35] |
Appendix 8. Bleeding: Fibrinogen versus any comparator, single‐study analysis
| Analysis | Study | Participants | Statistical method | Effect estimate |
| Bleeding arrest with sufficient haemostasis |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73 to 1.41] |
| Blood loss/Drainage (1 hour) mL/kg/h |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.87 to 0.67] |
| Blood loss/Drainage (6 hour) mL/kg/h |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI | ‐0.20 [‐0.52 to 0.12] |
Appendix 9. Fibrinogen versus placebo or no treatment, single‐study analysis
| Analysis | Study | Participants | Statistical method | Effect estimate |
| Mortality longest follow‐up | Karlsson 2009 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| ICU stay (hours) | Karlsson 2009 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐11.20 [‐22.84 to 0.44] |
| Duration of mechanical ventilation (hours) | Karlsson 2009 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐3.03 to 0.23] |
| Stay in hospital (days) | Karlsson 2009 | 20 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.24 to 2.24] |
| ICU transfusion RBC (units) | Fenger‐Eriksen 2009a | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.80 to ‐0.20] |
| Thrombotic episodes (arterial and venous graft occlusion, pulmonary embolus, deep venous thrombosis) | Karlsson 2009 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.27 to 92.62] |
| Blood loss/drainage, longest follow‐up | Karlsson 2009 | 20 | Mean Difference (IV, Random, 95% CI) | ‐265.00 [‐455.35 to ‐74.65] |
| RBC transfusion during operation (units) |
Fenger‐Eriksen 2009a | 20 |
Mean Difference (IV, Fixed, 95% CI) | RBC transfusion during operation (units) |
Appendix 10. Fibrinogen versus FFP or cryo, single‐study analysis
| Analysis | Study | Participants | Statistical method | Effect estimate |
| ICU stay (hours) | Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐36.10 [‐169.22 to 97.02] |
| Duration of mechanical ventilation (hours) | Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐66.50 [‐124.25 to ‐8.75] |
| Stay in hospital (days) | Cui 2010 | 31 | Mean Difference (IV, Random, 95% CI) | ‐11.00 [‐19.04 to ‐2.96] |
| ICU transfusion RBCs (units) | Cui 2010 | 17 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| PLT Transfusion (mL)-longest follow‐up | Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| Incidence of RBC transfusion-longest follow‐up | Cui 2010 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.82 to 2.01] |
| Thrombotic episodes (arterial and venous graft occlusion, pulmonary embolus, deep venous thrombosis) | Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01 to 7.42] |
| Complications not specific to trial intervention (pleural effusion, abdominal ischaemia and other serious adverse events) | Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.19 to 19.52] |
| Blood loss/drainage, longest follow‐up | Cui 2010 | 31 | Mean Difference (IV, Random, 95% CI) | 59.28 [8.09 to 110.47] |
| Blood loss/Drainage (24 hours) mL/kg/h | Cui 2010 | 31 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.28 to 0.08] |
| Re‐operation due to persistent bleeding | Galas 2012 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00 to 1.44] |
| Infections (wound) |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04 to 2.82] |
| Sepsis |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.78 [0.24 to 94.12] |
| Incidence of transfusion with PLT in ICU |
Cui 2010 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.67 to 1.80] |
| Incidence of RBC transfusion in ICU |
Cui 2010 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.82 to 2.01] |
| ICU transfusion FFP (mL/kg) |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐11.90 [‐19.43 to ‐4.37] |
| ICU transfusion PLT (units) |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| Total FFP usage (mL/kg) |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | 25.90 [8.56 to 43.24] |
| Incidence of cell saver blood use |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | Incidence of cell saver blood use |
| Transfused cell saver blood (mL) |
Lance 2011 | 43 | Mean Difference (IV, Fixed, 95% CI) | Transfused cell saver blood (mL) |
| Bleeding arrest with sufficient haemostasis |
Lance 2011 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | Bleeding arrest with sufficient haemostasis |
| Blood loss/Drainage (1 hour) mL/kg/h |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | Blood loss/Drainage (1 hour) mL/kg/h |
| Blood loss/Drainage (6 hour) mL/kg/h |
Cui 2010 | 31 | Mean Difference (IV, Fixed, 95% CI) | Blood loss/Drainage (6 hour) mL/kg/h |
Appendix 11. Fibrinogen in algorithm versus placebo or no treatment
| Analysis | Study | Participants | Statistical method | Effect estimate |
| Mortality longest follow‐up | Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03 to 2.33] |
| ICU stay (hours) | Rahe‐Meyer 2013 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐29.56 to 29.56] |
| Stay in hospital (days) | Rahe‐Meyer 2013 | 56 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐5.29 to 2.29] |
| Incidence of RBC transfusion longest follow‐up | Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34 to 0.78] |
| Thrombotic episodes (arterial and venous graft occlusion, pulmonary embolus, deep venous thrombosis) | Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05 to 5.77] |
| Complications not specific to trial intervention (pleural effusion, abdominal ischaemia and other serious adverse events) | Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.36 to 3.43] |
| Blood loss/Drainage (24 hours) mL/kg/h | Rahe‐Meyer 2013 | 61 | Mean Difference (IV, Random, 95% CI) | ‐200.00 [‐522.47 to 122.47] |
| Re‐operation due to persistent bleeding | Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 4.41 [0.52 to 37.25] |
| Mortality, 28 days |
Rahe‐Meyer 2013 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) |
0.28 [0.03 to 2.33] |
| Total number of allogenic transfusions |
Rahe‐Meyer 2013 | 61 | Mean Difference (IV, Fixed, 95% CI) |
‐11.00 [‐14.55 to ‐7.45] |
| Incidence of FFP transfusion, longest follow‐up | Rahe‐Meyer 2013 | 61 |
Risk Ratio (M‐H, Fixed, 95% CI) |
0.36 [0.22 to 0.58] |
Data and analyses
Comparison 1. Fibrinogen concentrate versus any comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality longest follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 ICU stay (hours) | 3 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐9.87 [‐20.67, 0.93] |
| 3 Duration of mechanical ventilation (hours) | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐27.29 [‐89.73, 35.16] |
| 4 Stay in hospital (days) | 3 | 107 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐7.04, 2.48] |
| 5 Incidence of allogenic blood transfusion (types of comparison) | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 5.1 Fibrinogen versus placebo/no treatment | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.09, 0.80] |
| 5.2 Fibrinogen versus FFP/cryoprecipitate | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.15, 0.56] |
| 5.3 Fibrinogen in combination with FFP versus placebo/no treatment/usual treatment | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.41, 1.49] |
| 5.4 Fibrinogen as part of transfusion algorithm versus placebo or no treatment | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.77] |
| 6 Incidence of allogenic blood transfusion (cardiac vs non‐cardiac) | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 6.1 Cardiac surgery | 3 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.76] |
| 6.2 Non‐cardiac surgery | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.17, 1.53] |
| 7 Incidence of allogenic blood transfusion (pediatric vs adult) | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 7.1 Paediatric studies | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.15, 0.56] |
| 7.2 Adult studies | 4 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.75] |
| 8 Incidence of allogenic blood transfusion (high dose > 50 mg/kg vs low dose) | 5 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 8.1 High‐dose studies (> 50 mg/kg) | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.86] |
| 8.2 Low‐dose studies (≤ 50 mg/kg) | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.14] |
| 9 Re‐operation due to persistent bleeding | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.01, 36.71] |
| 10 Incidence of RBC transfusion longest follow‐up | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.32, 2.02] |
| 11 Thrombotic episodes (arterial and venous graft occlusion, pulmonary embolus, deep venous thrombosis) | 3 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.27, 3.97] |
| 12 Complications not specific to trial intervention (pleural effusion, abdominal ischaemia and other serious adverse events) | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.45, 3.44] |
| 13 Blood loss/drainage, longest follow‐up | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐89.37 [‐406.05, 227.32] |
| 14 Blood loss/Drainage (24 hours) mL/kg/h | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐32.34 [‐176.45, 111.76] |
Comparison 2. Fibrinogen versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of allogenic blood transfusion (cardiac vs non‐cardiac) | 2 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.81] |
| 1.1 Cardiac surgery | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.69] |
| 1.2 Non‐cardiac surgery | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.90] |
2.1. Analysis.

Comparison 2 Fibrinogen versus placebo or no treatment, Outcome 1 Incidence of allogenic blood transfusion (cardiac vs non‐cardiac).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cui 2010.
| Methods | Two‐group parallel RCT, single centre Overall study quality: high risk of bias Sample size calculation: none reported Funding: not stated |
|
| Participants | 40 participants randomly assigned, of which 31 received intervention (17 in fibrinogen group) Inclusion criteria: cyanotic paediatric patients diagnosed with transposition of the great arteries (TGA) or double‐outlet right ventricle (DORV); the operation that the patients underwent was arterial switch operation (ASO) or double roots transplantation (DRT). Hematocrit higher than 54% before operation Exclusion criteria: history of blood disease; anticoagulation treatment before surgery; medication that affects haemostasis (such as prostaglandin E1); difficult sternal closure caused by anatomical or surgical reasons |
|
| Interventions |
Intervention: fibrinogen (0.5 to 1 g) administration combined with traditional transfusion guided by thrombelastography (TEG, Haemoscope Corp) The type of fibrinogen substitution is not specifically described Control: traditional transfusion guided by clinical experience |
|
| Outcomes | No primary outcome is stated Closure time, transfusion at closure (FFP/PLT), transfusion requirements at ICU (FFP/PLT/RBC), chest tube drainage (1,6,24 hours) and total transfusion requirements (FFP/PLT/RBC*) *RBC at closure time was not assessed because residuals of blood were present in the CPB machine |
|
| Notes |
Country: China We have converted the missing standard deviation in accordance with recommendations stated in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 7.7.3.5 We used mean body weight for each group to calculate transfused amount, when outcome was reported in mL/kg Letter to author 2 March 2012, repeated 23 May 2012. No reply received Authors' conclusion: "The present study suggests that fibrinogen might be a better haemostatic agent for paediatric patients with severely cyanotic CCHD than FFP. This new therapy method could reduce the use of allogeneic blood products and shorten the operative recovery period. In addition, TEG is effective for blood protection" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22.5% excluded (9/40), not accounted for |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | High risk | Sample size not stated, funding not stated, baseline parameters are provided, but it is unclear if they include the excluded patients, and if the exclusions influence baseline balance. A small study and operative recovery data show very large differences between small trial groups, suggesting that the intervention group might have consisted of healthier individuals overall |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described. The intervention would be difficult to blind, so we expect this to be provided without blinding of personnel. It may be blinded to participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described (see above) |
Fenger‐Eriksen 2009a.
| Methods | Two‐group parallel RCT Funding: The study was supported by an unrestricted research grant from CSL Behring, the University of Aarhus Research Foundation and the A. P. Møller and Hustru Chastine McKinney Møllers Foundation Overall study quality: high risk of bias Sample size calculation: yes, based on 10% change in maximum clot strength |
|
| Participants | 21 participants randomly assigned; 20 participants received intervention (10 in fibrinogen group) Inclusion criteria: Patients older than 17 years of age suffering from localized bladder cancer and admitted for radical cystectomy. Furthermore, perioperative dilution with hydroxyethyl starch (HES) to a 30% reduction in hematocrit level Exclusion criteria: 1: Presence of coagulation disorders defined as abnormal values of platelet count, PT, APTT, fibrinogen, antithrombin or D‐dimer; 2: Treatment with oral vitamin K antagonists; 3: Intake of non‐steroid anti‐inflammatory drugs within 2 days before surgery; 4: Renal or hepatic dysfunction; 5: Ischaemic heart disease; 6: Pregnancy; 7: Known hypersensitivity to HES |
|
| Interventions |
Intervention: fibrinogen concentrate, Haemocomplettan, CSL Behring (dosage 45 mg/kg) Control: placebo in equivalent volume (isotonic saline 2.25 mL/kg) |
|
| Outcomes |
Primary: whole‐blood maximum clot firmness as determined by thromboelastometry (ROTEM, thromboelastometry, Pentafarm, Munich, Germany) Secondary: other thromboelastometric variables; platelet function; thrombin generation; bleeding and perioperative and postoperative blood product requirements |
|
| Notes | Letter to author sent 2 March 2012. Reply received 13 March 2012, additional data were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of cards (additional information) |
| Allocation concealment (selection bias) | Low risk | Randomly assigned using the closed envelope principle |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | According to published article, one participant was excluded after randomization because of an interrupted operation. Participant did not receive intervention. Data from this participant were not included in the analysis (no intention‐to‐treat analysis). (additional information) Exclusion was due to "Spread of cancer, so it was not possible to perform planned cystectomy hence the operation was interrupted". No available data on this participant regarding outcomes of this review |
| Selective reporting (reporting bias) | Low risk | Compared with the official description on clinicaltrials.gov. NCT00493272 |
| Other bias | High risk | Unrestricted funding from CSL Behring. Very small sample size aimed to assess a surrogate outcome. Baseline imbalance described but analysis not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study medicine was prepared and administered by a second person; thus, the study staff was blinded to infusion of the drug vehicle and to patients participating in the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Allocation concealment was sustained until all recruitment, data collection, transfusion requirement data and laboratory analyses were complete". Statistician was not blinded (additional information) |
Galas 2012.
| Methods | Randomized double‐blinded parallel‐assigned single‐centre clinical trial Data from abstract. No additional information available Funding: not stated Overall study quality: high risk of bias Sample size calculation: not stated |
|
| Participants | 63 participants completed the study (30 in fibrinogen group) Mean age, 3 years and 5 months; mean weight, 6.7 kg Inclusion criteria: children (< 15 years) receiving cardiac surgery with cardiopulmonary bypass, clinically important intraoperative bleeding and hypofibrinogenaemia (fibrinogen level < 1 g/L or TEG < 7 mm) Exclusion criteria: previous coagulopathy (clinical history or INR > 1.5); low platelet count (lower than 100.000); product allergy; urgent procedures; active infection |
|
| Interventions |
Intervention group: fibrinogen concentrate (60 mg/kg body weight) (RiaSTAP®/CSL Behring) Median doses were 504 mg fibrinogen concentrate Control group: cryoprecipiate (10 mL/kg body weight) Median doses were 402 mg cryoprecipitate |
|
| Outcomes |
Primary outcome measures: number of patients not receiving any allogeneic blood products (intraoperative until hospital discharge). Transfusional requirements were based on clinical judgement Secondary outcome measures: haemostatic tests, length of ICU stay, clinical complications (renal failure, respiratory failure, sepsis, myocardial ischemias, stroke), transfusion requirements, mechanical ventilation free‐days, length of hospital stay, vasopressors free‐days, perioperative bleeding |
|
| Notes |
Estimated enrolment: 80 participants. Finished. Data submitted for publication. Abstract available International Symposium on Intensive Care and Emergency Medicine 2012 Collaborator: CSL Behring Contacted 4 April 2012. Reply received 8 April 2012. No additional information provided Participating hospitals: (Brazil) Incor ‐ Heart Institute ‐ University of Sao Paulo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | Inclusion criteria changed from < 18 years to < 15 years compared with trial registration (NCT01187225) "Reoperation due to bleeding" not stated as secondary outcome in trial registration (NCT01187225) |
| Other bias | Unclear risk | Sample size not stated, funding not stated, baseline imbalance not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
Karlsson 2009.
| Methods | Single‐center parallel‐group RCT, pilot study Funding: The study was supported by the Swedish Heart & Lung Foundation, CSL Behring, Marburg, Germany, and Sahlgrenska University Hospital Overall study quality: high risk of bias Sample size calculation: pilot study: sample size was determined in agreement with the Regional Research Ethics Committee and the Swedish Medical Products Agency |
|
| Participants | 20 participants randomly assigned (10 in fibrinogen group) Inclusion criteria: elective CABG patients with plasma fibrinogen < 3.8 g/L Exclusion criteria: known kidney/liver disease, bleeding disorder and a surgical source of bleeding at acute re‐exploration |
|
| Interventions | Intervention: preoperative infusion of 2 g fibrinogen concentrate (Haemocomplettan, CSL Behring) Control: no treatment | |
| Outcomes |
Primary: clinical adverse events and graft occlusion assessed by multi‐slice computed tomography 3 to 4 days after surgery Secondary: postoperative blood loss, blood transfusions, haemoglobin levels 24 hours after surgery, and global haemostasis assessed with thromboelastometry, 2 and 24 hours after surgery and clinical adverse events |
|
| Notes |
Country: Sweden Letter to author 14 March 2012. Reply by phone 29 May 2012 Provided additional data on mortality (6 year follow‐up), ICU stay, mechanical ventilation and length of stay Authors' conclusion: "In summary, the results of this pilot study indicate that prophylactic treatment with fibrinogen concentrate in cardiac surgery patients is feasible and reduces postoperative bleeding. Further prospective, randomized, placebo‐controlled studies with sufficient statistical power are necessary to ensure safety, confirm efficacy, and to determine cost‐effectiveness of fibrinogen prophylaxis in cardiac surgery" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of cards (additional information) |
| Allocation concealment (selection bias) | Low risk | Random assignment was conducted using unmarked envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. All 20 randomly assigned participants were described |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Apparently free of selective reporting |
| Other bias | High risk | Unrestricted funding from CSL Behring. Apparently without baseline imbalance but with a very small sample size because it was a pilot study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "Only the study coordinator was informed about group assignment, that is neither the operating surgeon, the operating room staff, anaesthesiologists nor the staff at the intensive care unit, was informed." Fibrinogen was administered after induction of anaesthesia as an infusion during 5 minutes in a central venous catheter line, meaning that anaesthetic personnel at the operation theatre were not completely blinded (additional information). Pharmacist randomly assigned and dispensed the medicine |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of postoperative bleeding: This was assessed by ICU nurses, who were not informed of group assignment |
Lance 2011.
| Methods | Single‐centre, parallel‐group RCT Funding: The study group received unrestricted grants from CSL Behring Overall study quality: high risk of bias Sample size calculation: yes, based on 10% reduction in blood loss |
|
| Participants | 52 participants randomly assigned, of which 43 received intervention (22 in fibrinogen group) Patients with massive bleeding requiring less than 4 FFP were excluded after randomization Inclusion criteria: Major surgery (cardiovascular, abdominal, and orthopaedic spine surgery) with expected duration of operation exceeding 120 minutes. Patients requiring massive transfusion (defined as "prolonged blood loss of > 150 ml/hour or > 1.5 ml/kg/20 min, or acute blood loss of > 700 ml at once") during or after surgery Exclusion criteria: age younger than 18, active HIV infection, known coagulation abnormalities, deep hypothermia with circulatory arrest or preoperative need of transfusion |
|
| Interventions |
Intervention: haemostatic transfusion with 2 units FFP plus 2 g fibrinogen concentrate (Haemocomplettan®) Control: haemostatic transfusion with 4 units FFP Haemostatic therapy to stop bleeding was started on the basis of clinical decision Comment: 25 participants (13 in fibrinogen group and 12 in control group) received heparin (150 to 300 mg/kg) as the result of extracorporal circulation |
|
| Outcomes | According to protocol, primary outcome was blood loss: "Primary endpoint is the amount of blood loss (expressed in ml per time unit) and the further use of blood products. As secondary endpoints, the levels of thrombin generation and thromboelastografische provisions compared with conventional coagulation tests" In article: bleeding arrest after intervention, fibrin clot formation (measured by whole‐blood thromboelastometry and thrombin generation), adverse events Participants were followed up to 30 days or at least until discharge |
|
| Notes |
Country: the Netherlands Letter to author 6 March 2012. Reply received 3 April 2012 and 22 June Authors' conclusion: "In conclusion, this trial showed a similar effect of 2 g fibrinogen additive to 2 units FFP compared with transfusion of 4 units FFP on haemostasis. The transfused fibrinogen had an important contribution to fibrin clot formation in thromboelastometry analysis, while the transfused 4 units FFP more increased thrombin generation and fibrinogen levels remained lower. We hence postulate that transfused FFP and fibrinogen are both relevant for early haemostasis and that both should be combined in massive haemorrhage protocols. These results argue for a larger‐scale follow‐up study, where the effects are determined of supplementing fibrinogen concentrate together with a standard dose of FFP. Herein, a pro‐haemostatic effect of fibrinogen is expected on top of the effect of normalization of other coagulation factors." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of cards (additional information) |
| Allocation concealment (selection bias) | Low risk | Closed envelope method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No intention‐to‐treat analysis, 9/52 (17%) patients were excluded after randomization because an inadequate amount of FFP was transfused; no data available on these |
| Selective reporting (reporting bias) | High risk | In protocol, authors state blood loss and transfusions as primary outcome (with sample size calculated on reduction in blood loss). In publication, authors describe arrest of bleeding instead |
| Other bias | High risk | Two of authors (not primary author) hold an unrestricted grant from CSL Behring. Three persons from CSL Behring are acknowledged for "stimulating discussions". Sample size provided. Apparently without baseline imbalance but with no data on exclusions. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Inclusion was based on clinical decision, with the attending anaesthesiologist not blinded to the study intervention (additional information): "Patients and surgeons were not aware of group. Only the attending anaesthesiologist received the products and transfused them‐ so she/he was aware of the treatment group, but lab‐parameters were taken from researchers and worked up by analysts who did not know the groups. Registration of bleeding and further treatment was done by researchers" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above |
Rahe‐Meyer 2013.
| Methods | Single‐centre parallel‐group RCT Data from publication and additional information provided by authors Funding: CSL Behring supported the trial Overall study quality: high risk of bias Sample size calculation: not stated |
|
| Participants | 61 randomly assigned, of which 29 received fibrinogen concentrate Inclusion criteria: Age > 18 years and elective thoracic or thoracoabdominal aortic replacement surgery involving cardiopulmonary bypass (CPB) (aortic valve operations with root/ascending aorta replacement (thoracic aortic aneurysm) with or without aortic bow replacements and thoracoabdominal replacements (thoracoabdominal aortic aneurysm)) and clinically relevant bleeding (defined as bleeding of 60 to 250 g into the surgical site within 5 minutes after CPB and completion of surgical haemostasis) Exclusion criteria:
|
|
| Interventions |
Intervention: Fibrinogen concentrate (Haemocomplettan P®/CSL Behring). Individualized dose using maximum amplitude of ROTEM/FIBTEM measurements before the end of CPB. Median dose given was 8 g (range, 3 to 12 g). Control: placebo (saline 0.9%) same volume |
|
| Outcomes |
Primary outcome: total allogenic transfusion requirements in the 24 hours post administration phase Transfusion therapy in both groups was guided by algorithm based on postoperative blood drainage and platelet count Secondary outcomes: Intensive care length of stay, number of allogenic units transfused, mortality and adverse events and re‐operation due to persistent bleeding |
|
| Notes |
Country: Germany Letter to author: sent 8 April 2012: reply with additional data received 18 April 2012 Authors' conclusion: "Hemostatic therapy with fibrinogen concentrate in patients undergoing aortic surgery significantly reduced the transfusion of allogeneic blood products. Larger multicenter studies are necessary to confirm the role of fibrinogen concentrate in the management of perioperative bleeding in patients with life‐threatening coagulopathy" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. 1:1 with blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Closed opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in placebo/control group died before 24 hours and is not included in primary outcome analysis. One participant in fibrinogen group and 4 participants in control group did not have data on length of stay. Intention‐to‐treat analyses were used to assess adverse events |
| Selective reporting (reporting bias) | Low risk | Compared with clinical registration NCT00701142, apparently free of bias |
| Other bias | High risk | (Additional information provided by author): "CSL Behring funded the trial. Sponsor participated in study design but was not involved with data collection. Data analysis was performed independently by a contract research organization funded by the study sponsor. The sponsor paid for the services of biometrics service providers and medical writing support. The clinical study report was written by medical writing vendor funded by the sponsor." Apperently free of baseline imbalance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomization and dispensation of trial medication were performed by the local hospital pharmacy, and medication was delivered to the operation theatre in syringes blinded to personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only pharmacists dispensing the medicine and randomly assigning participants were unblinded and had no participant contact |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bell 2010 |
Case report, obstetrical setting Six cases characterized by significant (> 1500 mL) peripartum blood loss and severe acquired hypofibrinogenaemia. Patients were treated with fibrinogen concentrate (mean dose, 3.16 g) along with allogeneic blood products (PLT, FFP, RBC) Findings: rapid normalization of coagulation parameters and improved haemorrhage Reason for exclusion: non‐RCT |
| Danes 2008 |
Retrospective cohort study, non‐controlled, single centre, mixed patient population Patients given fibrinogen concentrate according to pharmacy‐dispensing records were included, receiving a median dose of 4 g The cohort consisted of 69 participants suffering from various forms of acquired severe hypofibrinogenaemia with life‐threatening consumptive thrombo‐haemorrhagic disorders (surgery, trauma and digestive haemorrhage) (43 participants), or with underlying disease states that limit fibrinogen synthesis (hepatic dysfunction, haematological malignancies) (26 participants) Findings: improved coagulation after treatment. Acute fibrinogen deficiency probably related to mortality Reason for exclusion: non‐RCT |
| Glover 2010 |
Case report, obstetrical setting One patient suffering major antenatal obstetrical haemorrhage with DIC and severe hypofibrinogenaemia. Treated with fibrinogen concentrate (4 g) in combination with RBC and FFP transfusion Findings: Emphasis on early and timely monitoring of coagulation and rapid administration and availability of fibrinogen substitution Reason for exclusion: non‐RCT |
| Guasch 2008 |
Prospective observational cohort, non‐controlled (descriptive), single centre, obstetrical setting 124 women admitted for postpartum haemorrhage. Treatment was provided with RBCs (96.8%), prothrombin complex concentrate (7.25%), recombinant factor VIIa (3.2%) and fibrinogen concentrate (49.2%) Findings: Use of fibrinogen concentrate is common and provides good results Reason for exclusion: non‐RCT |
| Haas 2008 |
Retrospective non‐controlled study, single centre, craniofacial surgery, children Nine children undergoing major craniofacial surgery with massive blood loss. Thromboelastometry‐guided assessment of coagulopathy and guided administration of fibrinogen concentrate (30 mg/kg) Findings: no need for PLT and FFP. Fibrinogen concentrate effectively improved fibrinogen polymerisation and total cloth strength Reason for exclusion: non‐RCT |
| Mittermayr 2007 |
Randomized clinical trial, placebo‐controlled, single centre, orthopaedic spine surgery Intervention: three groups receiving modified gelatin solution, hydroxyethyl starch 130/0.4 or Ringer lactate Fibrinogen concentrate (Hemocomplettan®) was administered (30 mg/kg) to all three groups when fibrin polymerisation was critically decreased as evaluated by thromboelastometry (ROTEM) Population: 61 patients undergoing spine surgery of more than three segments with an expected duration of surgery of longer than 3 hours Outcomes: allogeneic blood products transfused; estimated intraoperative blood loss; maximum clot firmness measured by FIBTEM Findings: fibrin/fibrinogen polymerisation is disturbed because of dilution, especially in participants receiving hydroxyethyl starch 130/0.4. This effect may be reversed by fibrinogen concentrate Reason for exclusion: Fibrinogen concentrate was not given in accordance with randomization. In this trial, it served as a rescue treatment to correct dilutional coagulopathy and was given to all three groups |
| Morrison 2011 |
Case report, elective vascular surgery Three patients undergoing elective type IV thoracoabdominal aortic aneurysm treatment. Patients were treated with continuous infusion of fibrinogen concentrate (mean dose, 13.3 g) guided by FIBTEM maximum clot firmness Findings: despite blood loss of up to 11 L, no need for FFP and cryoprecipitate transfusion Reason for exclusion: non‐RCT |
| Rahe‐Meyer 2009 |
Prospective interventional cohort study non‐randomized and non‐blinded, single centre, elective aortic valve operation and ascending aorta replacement surgery Fifteen participants included: standard treatment algorithm based on ROTEM (group with five participants) compared with a group receiving fibrinogen concentrate before standard treatment targeting ROTEM/FIBTEM CF > 22 mm (group with ten participants). Increased risk of all types of bias Findings: FIBTEM‐guided fibrinogen concentrate administration was associated with reduced transfusion requirements and 24 hour postoperative bleeding Reason for exclusion: non‐RCT |
| Rahe‐Meyer 2009a |
Prospective interventional cohort study with a retrospective control group, single centre, elective thoracoabdominal aortic aneurysm surgery Retrospective control group (12 participants) with perioperative clinically relevant diffuse bleeding after weaning from cardiopulmonary bypass treated with allogeneic blood products according to predetermined algorithm Prospective intervention group (6 participants) treated with fibrinogen concentrate guided by thromboelastometry (ROTEM), in addition to the algorithm Findings: administration of 7.8 ± 2.7 g of fibrinogen concentrate established haemostasis, completely avoiding intraoperative transfusion of FFP and PLT. Lower 24 hour drainage volume in fibrinogen group Reason for exclusion: non‐RCT |
| Schöchl 2010a |
Case report, trauma setting A severely injured patient with multiple trauma with massive blood loss. Patient receiving haemostatic therapy with coagulation factor concentrates, guided by thromboelastometry (ROTEM) Initial therapy consisted of fibrinogen concentrate Findings: suggest successful haemostatic treatment with fibrinogen concentrate and prothrombin complex concentrate, minimizing the need for allogeneic blood products Reason for exclusion: non‐RCT |
| Schöchl 2010b |
Retrospective noncontrolled cohort study, single centre, trauma setting The cohort consists of 128 trauma patients receiving at least five units of RBC within the first 24 hours after arrival at trauma centre. Patients who died in the first hour after admission and patients who received no haemostatic therapy within the first 24 hours were excluded. Evaluation of goal‐directed coagulation management using thromboelastometry (ROTEM) with guided administration of fibrinogen concentrate and prothrombin complex concentrate Findings: Thromboelastometry was rapid and reliable for diagnosing coagulopathy and guiding transfusion of concentrates. First‐line fibrinogen concentrate and prothrombin complex concentrate seemed efficacious and quick to administer Reason for exclusion: non‐RCT |
| Solomon 2010 |
Retrospective noncontrolled study, single centre, cardiovascular surgery Aimed to assess fibrinogen recovery parameters after administration of fibrinogen concentrate. 39 patients with diffuse bleeding in relation to cardiovascular surgery and treated with fibrinogen concentrate. Patients with concomitant administration of FFP or with non‐elective or emergency intervention, age younger than 18 and terminal illness were excluded. Dosing of fibrinogen concentrate was guided by FIBTEM thromboelastometry Findings: Fibrinogen concentrate effectively increased plasma fibrinogen level. Suggests a favourable survival Reason for exclusion: non‐RCT |
| Thorarinsdottir 2010 |
Retrospective non‐controlled study, single centre, mixed patient population 37 patients receiving fibrinogen concentrate were identified using hospital database. If repeated dosage was necessary as well as recombinant factor VIIa, patients were excluded. Fibrinogen was administered when standard treatment was deemed to have failed to control the bleeding, aiming to maintain fibrinogen level above 1.5 g/L Findings: Administration of fibrinogen in the context of severe haemorrhage was associated with increased fibrinogen concentration. A significant decrease in RBC transfusions was identified after fibrinogen administration (but no control group) Reason for exclusion: non‐RCT |
| Weinkove 2007 |
Retrospective non‐controlled cohort study, single centre, mixed patient population 30 patients receiving fibrinogen concentrate in treatment of acquired hypofibrinogenaemia Findings: Fibrinogen concentrate is well suited for fibrinogen substitution Reason for exclusion: non‐RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Galas 2012a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Need full paper review, but apparently secondary publication of Galas 2012 |
Rahe‐Meyer 2011a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Need full paper review, but apparently not RCT |
Rahe‐Meyer 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Need full paper review, but apparently publication of Rahe‐Meyer 2013 |
Rahe‐Meyer 2013a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Need full paper review, but apparently secondary publication of Rahe‐Meyer 2013 |
Solomon 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Need full paper review, but apparently secondary publication of Rahe‐Meyer 2013 |
Characteristics of ongoing studies [ordered by study ID]
Fries 2011.
| Trial name or title | Fibrinogen Concentrate (FGTW) in Trauma Patients, Presumed to Bleed (FI in TIC) |
| Methods | Randomized double‐blind parallel‐assigned multicentre clinical trial |
| Participants |
Inclusion criteria: adult trauma patients > 18 years, with major or occult bleeding, indicating shock and need of volume replacement therapy Exclusion criteria: penetrating trauma. Solely head injury. Hemodynamic instability (patient has to be excluded if haemodynamic stabilisation (SBP below 90 mmHg and HR more than 100 per min) is not achieved after 15 minutes of resuscitation management in spite of volume therapy and administration of catecholamines). Patient with inevitable lethal course as evaluated by emergency physician. Need for CPR on the scene. Deep hypothermia (below 30°C). Obviously pregnant women. Known recent history of thromboembolic events within the last 6 months. Patients receiving anticoagulant therapy |
| Interventions |
Intervention group: infusion of 50 mg/kg body weight fibrinogen concentrate (FGTW/Clottafact®-LFB)-one vial for each 30 kg body weight, estimated by the emergency physician: Body weight: < 30 kg/30 to 60 kg/60 to 90 kg/> 90 kg No. of vials: 1 vial (100 mL)/2 vials (200 mL)/3 vials (300 mL)/4 vials (400 mL) Fibrinogen (if applicable): 1.5 g/3 g/4.5 g/6 g Control group: placebo (a buffer substance consisting of mannitol/sucrose/sodium chloride/polysorbate 80) Infusion of one vial including 100 mL for each 30 kg body weight The flow rate should not exceed 100 mL within 5 minutes |
| Outcomes |
Primary outcome measure: change in the fibrinogen polymerisation measured with FIBTEM® MCF at average 60 min (arrival at hospital) post infusion Further measurements and investigations will be done until 7 days after the hospital admission and final at 30 days |
| Starting date | October 2011 |
| Contact information | Pamela Schech, pamela.schech@i‐med.ac.at |
| Notes | Estimated enrolment: 60 participants, by 22 May, 6 participants included. The study is currently recruiting participants Participating hospitals: (Austria) Medical University Innsbruck, Emergency Hospital Salzburg, PMU Salzburg, Regional Hospital Vöcklabruck, Medical University Graz Contacted 3 April 2012 and again 22 May 2012; reply 22 May 2012 Funding: US Department of Defence. LFB provides medicine and placebo‐drug together with unrestricted grant |
Haas 2012.
| Trial name or title | Fibrinogen for Treatment of Pediatric Dilutional Coagulopathy: FibPaed Study |
| Methods | Randomized single‐blinded parallel‐assigned single‐centre clinical trial |
| Participants |
Inclusion criteria: children aged 6 months to 17 years scheduled for elective scoliosis surgery or major craniofacial surgery presenting with intraoperative hypofibrinogenaemia according to definition of treatment groups Exclusion criteria: preexisting congenital or acquired coagulation disorder. Medical history of estimated increased bleeding tendency. Ongoing coagulation therapy. Clinical signs or diagnosis of acute thromboembolism. Intolerance of study drug. Pregnant or lactating women. Participation in another clinical trial |
| Interventions |
Intervention group: fibrinogen concentrate (Haemocomplettan P, CSL Behring, 30 mg/kg body weight) over 15 min Repetition if hourly intraoperative ROTEM measurements revealed hypofibrinogenaemia according to treatment group definition (administration of fibrinogen concentrate if ROTEM FIBTEM revealed MCF < 13 mm) Control group: fibrinogen concentrate (Haemocomplettan P, CSL Behring, 30 mg/kg body weight) over 15 min Repetition if hourly intraoperative ROTEM measurements revealed hypofibrinogenaemia according to treatment group definition (administration of fibrinogen concentrate if ROTEM FIBTEM revealed MCF < 8 mm) |
| Outcomes |
Primary outcome measures: total amount of transfused red cell concentrate at 24 hours after start of surgery Secondary outcome measures: coagulation measurements, length of stay on paediatric intensive care unit, additional transfusion/blood product requirements, occurrence of rebleeding, surgical revision, occurrence of (severe) adverse events |
| Starting date | January 2012 |
| Contact information | Thorsten Haas, MD, thorsten.haas@kispi.uzh.ch |
| Notes | Estimated enrolments: 60 participants. The study is currently recruiting participants Collaborator/funding: independent Contacted 4 April; reply 4 April 2012 Participating hospitals: (Switzerland) University Children's Hospital, Zurich |
Innerhofer 2012.
| Trial name or title | RETIC Trial: Reversal of Trauma Induced Coagulopathy Using Coagulation Factor Concentrates or Fresh Frozen Plasma |
| Methods | Single‐centre parallel open‐label randomized trial |
| Participants | Severely traumatized patients (ISS > 15) admitted to emergency department with obvious bleeding and/or at risk for significant haemorrhage will be screened by rotational thromboelastometry (ROTEM) assays during ED treatment and subsequent surgical/radiological interventions for having coagulopathy Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: receives fibrinogen concentrate and/or prothrombin complex concentrate (PCC) and/or FXIII concentrate If FIBTEM A10 < 7 mm: fibrinogen concentrate (RiaSTAP®/CLS Behring) dose at 50 mg/kg body weight intravenously as single dose or repeated, each single vial (1 g) over 5 min If EXTEM CT > 90 s and FIBTEM A10 > 7 mm: prothrombin complex concentrate dose at 20 IE/kg body weight PCC intravenously as single dose or repeated, each single dose over 10 min If FXIII decreases below 60% as detected by laboratory measurements: FXIII concentrate dose at 20 IU/kg body weight Fibrogammin® P administered with the second dose of fibrinogen concentrate (100 mg/kg) intravenously as single dose or repeated, each single dose over 10 min Control group: fresh frozen plasma (FFP) blood type 0, A, B and AB If FIBTEM A10 < 7 mm and/or EXTEM CT > 90 s: fresh frozen plasma dose of 15 mL/kg body weight intravenously as single dose or repeated, each single U (200 mL) over 5 min Additional treatment/rescue treatment: Treatment failure will be registered if bleeding persists and ROTEM parameters do not improve after two times dosages of study drug. In these cases, haemostatic rescue therapy will be administered. CFC (fibrinogen concentrate and/or PCC, and/or FXIII concentrate) will be administered to participants randomly assigned to receive FFP, and FFP will be administered to participants in the CFC group. In cases unresponsive to comprehensive treatment or normal ROTEM combined with diffuse bleeding, other haemostatic medications can be administered (e.g. rFVIIa, DDAVP, VWF/FVIII concentrate) as judged by the anaesthetist in charge. The need for and type of any rescue therapy will be documented, and ROTEM will be performed thereafter |
| Outcomes | Multiple organ failure (MOF) until 24 h on ICU Difference between treatment groups in MOF as assessed by the Sequential Organ Failure Assessment score (SOFA) |
| Starting date | March 2012 |
| Contact information | Petra Innerhofer, MD, petra.innerhofer@uki.at |
| Notes | Estimated enrolment: 200 participants. The study is currently recruiting participants Participating hospital: University Hospital Innsbruck Contacted 3 April 2012; reply 3 April 2012 Relation to the industry: independent |
Jeppsson 2010.
| Trial name or title | Fibrinogen and Bleeding After Cardiac Surgery (Fibro‐3) |
| Methods | Randomized double‐blind placebo‐controlled parallel‐assigned single‐centre study |
| Participants |
Inclusion criteria: adults 18 to 85 years eligible for first‐time coronary artery bypass (CABG) surgery with a preoperative fibrinogen plasma concentration less than 3.8 g/L Exclusion criteria: patients undergoing re‐do surgery. Clinical or laboratory signs of bleeding disorder, liver disease or other significant disease/condition, which in the investigators' judgment interferes with haemostasis. Any medications with agents that may interfere with haemostasis within 14 days before study start. Clopidogrel and warfarin are withdrawn at least 24 hours before surgery. Oral aspirin is allowed as co‐medication. Administration of other investigational drugs within eight weeks before the pre‐entry examination. Pregnant or lactating women |
| Interventions |
Intervention group: fibrinogen concentrate 2 g (RiaSTAP®, CSL Behring) in 100 mL sterile water Control group: (placebo) saline 100 mL Administration during a period of 15 minutes after induction of anaesthesia and before start of surgery. Pharmacist randomly assigns and dispenses the medicine |
| Outcomes |
Primary outcome measures: to evaluate safety of prophylactic fibrinogen infusion in patients with fibrinogen levels in the lower normal range undergoing cardiac surgery (2 years' follow‐up) Blood loss at first 12 postoperative hours Secondary outcome measures: transfusion requirements at 7 days. Biomarkers of coagulation, fibrinolysis and platelet function and pharmacoeconomic analysis |
| Starting date | April 2009 |
| Contact information | Contact: Anders Jeppsson, MD, PhD, anders.jeppsson@vgregion.se |
| Notes | Same group behind Karlsson 2009 Estimated enrolment: 60 participants. The study is currently recruiting participants Contacted 14 March 2012 and again 22 May 2012; reply by phone 29 May 2012 Participating hospitals: Cardiothoracic Surgery Unit, Sahlgrenska University Hospital Funding/relation to the industry: independent |
Kwapisz 2012.
| Trial name or title | Prospective Double Blinded Randomized Control Study of the Use of Fibrinogen in High‐Risk Cardiac Surgery |
| Methods | Randomized double‐blind placebo‐controlled parallel‐assigned single‐centre study |
| Participants |
Inclusion criteria: adults, elective complex cardiac surgical procedures (double procedures re‐do sternotomies, thoraco‐abdominal aortic aneurysm, aortic root) Participants will be randomly assigned after protamine administration. Surgeon and anaesthetist have to agree on diffuse, non‐surgical bleeding Exclusion criteria: any known congenital or preexisting bleeding disorder, preexisting abnormal fibrinogen level (normal: 1.8 to 4.7 g/L), severe liver disease (alanine aminotransferase or aspartate aminotransferase > 150 U/L), inability to provide informed consent, emergency surgery, pregnancy or nursing, younger than 18 years, intake of anti‐platelet drugs within three days preoperatively (low‐dose ASA is allowed), allergy to concentrated fibrinogen or other components in the product, anaemia (Hgb < 110), diagnosed deep vein thrombosis (DVT), pulmonary embolism, acute stroke |
| Interventions |
Intervention group: fibrinogen concentrate (Haemocomplettan P®/CSL Behring) infused according to a haemostatic algorithm based on FIBTEM (MCF, maximum clot firmness) Control group: intravenous saline All syringes and tubing will be covered. A study nurse who is not involved in treatment of the participant will prepare the syringes |
| Outcomes |
Primary outcome measure: the number of used blood products, including packed red cells, fresh frozen plasma, platelets and cryoprecipitate within 48 hours after surgery Secondary outcome measures: (one‐year follow‐up) fibrinogen levels, TEG (R‐value, K‐value, angle), CVICU stay, hospital stay, in‐hospital mortality, haemoglobin, adverse events (anaphylaxis, stroke, myocardial infarction, pulmonary embolism, and deep vein thromboembolism) and usage of factor VII concentrate and prothrombin complex concentrate |
| Starting date | Not yet recruiting |
| Contact information | Myron M Kwapisz, MD, myron.kwapisz@gmail.com, Heather E Mingo, RN, PhDc, heather.mingo@cdha.nshealth.ca |
| Notes | Information is based on clinical trials registration and contact with authors Estimated enrolment: 40 participants. Currently awaiting IRB approval Participating hospital: Halifax, Nova Scotia, Canada, B3H3A7 Funding/relation to the industry: collaborates with CSL Behring Contacted 20 March 2013; reply 22 March 2013 |
Nierich 2011.
| Trial name or title | Haemocomplettan® P During Elective Complex Cardiac Surgery |
| Methods | Randomized double‐blind parallel‐assigned single‐centre clinical trial |
| Participants |
Inclusion criteria: adults (> 18 years) undergoing elective complex cardiac surgery with clinically relevant non‐surgical microvascular bleeding after removal of cardiopulmonary bypass. Exclusion criteria: positive pregnancy test, pregnancy or lactation. Proof or suspicion of a congenital or acquired coagulation disorder. Emergency operation. Clopidogrel use in the 5 days preceding surgery. INR > 1.4 if on Coumadin |
| Interventions |
Intervention group: fibrinogen concentrate (Haemocomplettan P®/CSL Behring) Dose: study medication will be individually determined on the basis of plasma fibrinogen concentrations (measured with Clauss method during the reperfusion period on CPB) and body weight. Intravenous infusion within 10 minutes Control group: human albumin with concentration 200 g/L Study bottles of 50 mL will be diluted with saline and will contain 2 g in total. This concentration resembles the total protein load in the bottles with Haemocomplettan® P |
| Outcomes |
Primary outcome measure: perioperative blood loss within 12 hours (measured as blood loss in mL between infusion of study medication and closure of chest) Secondary outcome measures: postoperative blood loss (measured as blood loss at the ICU between closure of chest and 1st hour and 6th hour and after 24 hours). Postoperative transfusion requirements within 24 hours after closure of chest. Major clinical events: mortality at 30 days post‐surgery, MACE (major adverse cardiac event), cerebrovascular accident/ transient ischaemic attack, renal insufficiency or failure, venous thromboembolism/pulmonary embolism, allergic or other systemic reaction to study medication |
| Starting date | February 2011 |
| Contact information | Marga Hoogendoorn, MSc, m.e.hoogendoorn@isala.nl |
| Notes | Estimated enrolment: 120 participants. The study is currently recruiting participants Participating hospitals: (Netherlands) Isala Klinieken, Zwolle, Overijssel Funding/relation to the industry: investigator initiated but receives study medication from industry Contacted 4 April 2012 and again 22 May 2012; reply 22 May 2012 |
Nimmo 2009.
| Trial name or title | Fibrinogen as an Alternative to Fresh Frozen Plasma in Aortic Surgery |
| Methods | Randomized single‐blinded parallel‐assigned single‐centre clinical trial |
| Participants |
Inclusion criteria: patients > 18 years undergoing elective thoracoabdominal aneurysm repair Exclusion criteria: previous aortic surgery (re‐do surgery). Emergency surgery. Pregnancy. Females of child‐bearing age (younger than 45 years) not using medically approved method of contraception. Congenital or acquired coagulopathy. Known allergy to study drug |
| Interventions |
Intervention group: fibrinogen concentrate (Haemocomplettan P®/CSL Behring) administered initially at 2 g per hour by continuous infusion. This rate will be adjusted in response to the clinical picture and results of point‐of‐care coagulation tests. The infusion will be used intraoperatively Control group: fresh frozen plasma (dose not described) |
| Outcomes |
Primary outcome measures: pattern of coagulation disturbance intraoperatively and up to 24 hours postoperatively Secondary outcome measures: proportion of participants in the fibrinogen group requiring FFP during operation. Number of FFP units transfused-during surgery and up to 24 hours after surgery. Numbers of platelet and red blood cell units transfused-during surgery and up to 24 hours after surgery |
| Starting date | October 2009 |
| Contact information | Alastair Nimmo, MBChB, a.nimmo@ed.ac.uk, University of Edinburgh |
| Notes | Estimated enrolment: 20 participants. Apparently not yet recruiting but registered in 2009 Collaborators: NHS Lothian and CSL Behring Participating hospitals: (United Kingdom) The Royal Infirmary of Edinburgh Funding/relation to the industry: yes, collaborates with CSL Behring Contacted 4 April 2012 and again 22 May 2012; no reply received |
Ranucci 2011.
| Trial name or title | The ZEro PLASma Trial (ZEPLAST): Avoidance of Fresh Frozen Plasma in Cardiac Surgery |
| Methods | Prospective randomized double‐blind comparative parallel‐assigned single‐centre clinical trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
Withdrawal/additional exclusion criterion (exclusion following randomization):
Participants randomly assigned and not withdrawn will be given the investigational/place drugs in accordance with allocation All participants randomly assigned and not withdrawn will be tested 20 minutes before removal of aortic cross‐clamping with a thromboelastometric fibrinogen test ROTEM®/FIBTEM |
| Interventions |
Intervention group: fibrinogen concentrate (Haempcpmplettan P®/CSL Behring) and prothrombin complex concentrate (PCC) (Confidex®/CSL Behring) Dose fibrinogen: According to the formula based on ROTEM®/FIBTEM test: (22 [mm] − MCF [mm]) * body weight [kg]/140 [m] = whole g fibrinogen Co‐intervention: After 15 min from study drug administration and in the presence of ongoing microvascular bleeding: ROTEM®/EXTEM ‐ prolonged CT time (> 80 seconds): PCC at a weight‐based dose of 7 U/kg body weight Control group: (placebo) saline Study drugs or placebo has to be administered after protamine |
| Outcomes | Primary outcome measures: avoidance of allogeneic blood product transfusion within 30 days (packed red cells, FFP, platelet concentrates, cryoprecipitates) Secondary outcome measures: reduction in allogeneic blood product transfusions within 30 days |
| Starting date | November 2011 |
| Contact information | Marco Ranucci, MD, cardioanestesia@virgilio.it, |
| Notes | Estimated enrolment: 120 participants. 16 included by 4 April 2012 Participating hospitals: (Italy) IRCCS Policlinico San Donato Funding/relation to the industry: collaborates with CSL Behring Contacted 4 April 2012; reply 4 April 2012 |
Sabate 2012.
| Trial name or title | The Efficacy of the Administration of Fibrinogen in Liver Transplantation (FibstudLT) |
| Methods | Randomized placebo‐controlled double‐blind parallel‐assigned multi‐centre clinical trial |
| Participants |
Inclusion criteria: patients who are candidates for liver transplantation with a value of pretransplant plasma fibrinogen less than 2.9 g/L Exclusion criteria: Patients with a value of fibrinogen in the 24 hours before the intervention greater than 2.9 g/L, known history of thromboembolic events in 30 days, known or suspected pregnancy, previous randomization in this trial, known or suspected allergy to trial products or related products, known presence of congenital bleeding disorder, patients treated with aspirin or warfarin. The following indications for transplantation: familial polyneuropathy, acute liver failure, biliary cirrhosis and sclerosing cholangitis, Budd‐Chiari syndrome. Heart beating donors and living donors |
| Interventions |
Intervention group: fibrinogen concentrate (RiaSTAP®/CSL Behring) will be administered until an expected plasmatic value of 2.9 g/L is achieved: The dose calculated as 1 g of fibrinogen to obtain an increase in plasma fibrinogen value of 0.29 g/L to reach a final value of 2.9 g/L Control group: (placebo) saline in same volume Administration before surgery starts, intravenous infusion for 10 minutes |
| Outcomes | Primary outcome measures: percentage of participants requiring transfusion of packed red blood cells during the procedure Secondary outcome measures: Percentage of participants requiring intraoperative blood products other than red cell concentrates, number of units of fresh frozen plasma transfused during surgery, number of platelet units transfused during surgery, grams of fibrinogen administered during surgery, operative outcome until 4 weeks' follow‐up, operative mortality, liver graft survival. Thrombotic complications of all types and causes. Liver transplantation outcome at follow‐up 1 year |
| Starting date | March 2012 |
| Contact information | Antoni Sabate, MD, asabatep@bellvitgehospital.cat |
| Notes | Estimated enrolment: 132 participants. The study is currently recruiting participants Participating hospitals: (Spain) Hospital de Cruces, Bilbao, Vizcaya, Hospital Universitari de Bellvitge, Barcelona, Hospital Clinic, Barcelona, Hospital Virgen de la Arrixaca, Murcia, Hospital Virgen del Rocio, Sevilla Funding/relation to the industry: independent of industry with institutional and governmental support Contacted 4 April 2012; reply 20 April 2012 |
Tanaka 2011.
| Trial name or title | RiaSTAP vs. Conventional Transfusion in Patients Having Heart Valve Surgery (RiaCT) |
| Methods | Randomized parallel‐assigned single‐centre clinical trial |
| Participants |
Inclusion criteria: adults 18 to 85 years undergoing planned cardiopulmonary bypass (CPB) for combined coronary artery bypass grafting and valve replacement/repair surgery, single valve replacement surgery, mitral valve repair surgery or double valve surgery (aortic and mitral). Presence of clinically relevant microvascular bleeding after protamine administration Patients should fulfil the following parameters before the study intervention: body temperature > 35.0°C, blood pH > 7.2, Hb > 7.0 mg/dL, activated clotting time (ACT) < 155 seconds, CPB time > 60 minutes and evidence of significant microvascular bleeding Exclusion criteria: replacement of aorta, planned valve replacement without median sternotomy, previous valve replacement surgery (previous CABG acceptable), history or suspicion of a congenital or acquired coagulation disorder such as haemophilia, von Willebrand disease, and liver disease, haemodialysis dependent renal failure, liver dysfunction (aspartate aminotransferase (AST) or alanine aminotransferase (ALT) increased ≥ 2‐fold above the upper limit of local laboratory normal ranges), known allergy/anaphylaxis to fibrinogen concentrate or apheresis platelet units, clopidogrel administration within 5 days of surgery, Coumadin (warfarin) administration within 5 days of surgery, participation in another clinical study in the 4 weeks preceding surgery, any indication that a potential participant did not comprehend the study restrictions, procedures, or consequences (therein an informed consent cannot be convincingly given), life expectancy less than 48 hours |
| Interventions |
Intervention group: fibrinogen concentrate (RiaSTAP®/CSL Behring) 4 g IV Control group: a single apheresis platelet unit Administration within 30 minutes of ACT < 155 seconds and post CPB |
| Outcomes | Primary outcome measures: pattern of coagulation disturbance intraoperatively and up to 24 hours postoperatively Secondary outcome measures: proportion requiring FFP or platelets during or after surgery. Number of FFP, RBC and platelet units transfused during surgery and up to 24 hours after surgery and intraoperative blood loss |
| Starting date | January 2011 |
| Contact information | Kathy F Egan, BSN, RN, kfegan@emory.edu |
| Notes | Estimated enrolment: 60 participants. The study is currently recruiting participants, 14 included by 4 April Participating hospitals: (United States) Emory University Hospital, Atlanta, Georgia Funding/relation to industry: collaborates with CSL Behring Contacted 4 April 2012; reply received 4 April 2012 |
Wikkelsoe 2011.
| Trial name or title | Fibrinogen Concentrate as Initial Treatment for Postpartum Haemorrhage: A Randomized Clinically Controlled Trial (FIB‐PPH) |
| Methods | Randomized placebo‐controlled double‐blind parallel‐assigned multi‐centre clinical trial |
| Participants |
Inclusion criteria: parturients > 18 years developing postpartum haemorrhage (PPH), defined as bleeding from uterus and/or the birth canal within 24 hours postpartum. If vaginal birth: indication of one of the following procedures at the operation theatre with anaesthetic assistance: (a) estimated blood loss ≥ 500 mL and indication of manual removal of placenta or (b) indication of manual exploration of the uterus due to continuous bleeding after the birth of placenta. If birth by caesarean section: perioperative blood loss ≥ 1000 mL Exclusion criteria: known inherited deficiencies of coagulation, anti‐thrombotic treatment prepartum due to increased risk of thrombosis, patients with a prepregnancy body weight < 45 kg. Refuse to receive blood transfusion |
| Interventions |
Intervention group: intravenous administration of 2 g fibrinogen concentrate (Haemocomplettan, CSL Behring) Control group: intravenous administration of isotonic saline in equivalent volume: 100 mL |
| Outcomes |
Primary outcome measures: incidence of transfusion with allogenic blood products during hospital stay or until 6 weeks postintervention Secondary outcome measures: severe PPH, defined as: "Decrease of haemoglobin (Hb) of > 2.5 mmol/L, transfusion of at least 4 red blood cell (RBC) units, haemostatic intervention (angiographic embolization, surgical arterial ligation or hysterectomy) or death, Estimated blood loss, total amount of blood transfused, the development of re‐bleeding defined as bleeding re‐occurring after primary haemostasis and requiring surgical procedures or intervention, haemoglobin level below 3,6 mmol/L." Side effects including thromboembolic complications (Safety measures/Potential known side effects): fever, headache, nausea, vomiting, allergic reactions, anaphylaxis and thromboembolic complications (deep venous thrombosis, acute myocardial infarct and lung embolus). All suspected unexpected serious adverse reactions will also be reported in accordance with the Good Clinical Practice (GCP) and the Danish Medicines Agency guidelines on follow‐up until 6 weeks postintervention |
| Starting date | May 2011 |
| Contact information | Anne Juul Wikkelsoe, MD, wikkelso@gmail.com |
| Notes | Estimated enrolment: 245 participants. Completed. No data available yet (August 2013). Participating hospitals: (Denmark) Rigshospitalet, Copenhagen University Hospital Denmark, Herlev Hospital, Copenhagen University Hospital, Hvidovre Hospital, Copenhagen University Hospital, Hillerød Hospital, Copenhagen University Hospital Funding/relation to industry: independent Collaborators: Haemonetics Corporation (provides TEG® assays) |
Differences between protocol and review
We have made the following changes to the published protocol (Wikkelsø 2010).
Change of title
Only one study (Lance 2011) involved participants with massive or severe bleeding. To evaluate the benefits and harms of fibrinogen concentrate in bleeding patients in general, we changed the title and population, thus skipping the restriction of a population defined by "severely bleeding" by the authors' definition. The background section was modified accordingly.
The title previously stated "..for acquired hypofibrinogenaemia", but to be able to include bleeding patients in risk of acquired hypofibrinogenaemia and not only those diagnosed with it, we removed this from the title.
Addition of comparator
We have added an overall comparison, "fibrinogen concentrate versus any comparator".
Contributions of authors
Conceiving the review: Anne Wikkelsø (AW), Jens Lunde (JL), Mathias Johansen (MJ), Arash Afshari (AA), Ann Merete Møller (AM), Jakob Stensballe (JS), Jørn Wetterslev (JW)
Co‐ordinating the review: AW
Undertaking manual searches: AW, JL
Screening search results: AW, MJ, JL
Organizing retrieval of papers: JL
Screening retrieved papers against inclusion criteria: AW, JL, MJ, AA
Appraising quality of papers: AW, JL, MJ, AA
Abstracting data from papers: AW, JL, MJ
Writing to authors of papers for additional information: AW, MJ, JL, AA, JS
Providing additional data about papers: AW, JS
Obtaining and screening data on unpublished studies: AW, MJ
Providing data management for the review: AW
Entering data into Review Manager (RevMan 5.1): AW, JL, MJ
Interpreting RevMan statistical data: AW, AA
Performing other statistical analysis while not using RevMan: AA, JW
Performing double entry of data: (data entered by person one: MJ; data entered by person two: JL)
Interpreting data: AW, AA, AM, JL, MJ, JS, JW
Writing the review: AW
Securing funding for the review: AW, AM
Performing previous work that served as the foundation of the present study: AW, AA, AM, JS, JW
Serving as guarantor for the review (one author): AW
Taking responsibility for reading and checking the review before submission: AW
Sources of support
Internal sources
Cochrane Anaesthesia Review Group (CARG), Denmark.
-
Karen Hovhannisyan (CARG), Denmark.
Technical support and search strategy design
-
University of Copenhagen Herlev Hospital, Department of Anaesthesia, Denmark.
Salary for primary author.
External sources
No sources of support supplied
Declarations of interest
The authors of this protocol are involved in the preparation of a potentially eligible study: Fibrinogen concentrate for postpartum haemorrhage (Wikkelsoe 2011). This study is unrelated and is not sponsored by any groups or companies with economic interests.
No other conflicts of interests.
Edited (no change to conclusions)
References
References to studies included in this review
Cui 2010 {published data only (unpublished sought but not used)}
- Cui Y, Hei F, Long C, Feng Z, Zhao J, Yan F, et al. Perioperative monitoring of thromboelastograph on blood protection and recovery for severely cyanotic patients undergoing complex cardiac surgery. Artificial Organs 2010;34(11):955–60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fenger‐Eriksen 2009a {published and unpublished data}
- Fenger‐Eriksen C, Jensen TM, Kristensen BS, Jensen KM, Tonnesen E, Ingerslev J, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo‐controlled clinical trial. Journal of Thrombosis and Haemostasis 2009;7:795‐802. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Galas 2012 {published data only (unpublished sought but not used)}
- Galas F, Hajjar L, Sorensen B, Almeida J, Sundin M, Guimaraes V, et al. Randomized comparison of fibrinogen concentrate versus cryoprecipitate for bleeding control in pediatric cardiac surgery (FICCS study). Critical Care 2012;16(Suppl 1):P438 . [DOI: ] [Google Scholar]
Karlsson 2009 {published and unpublished data}
- Karlsson M, Ternström L, Hyllner M, Baghaei F, Flinck A, Skrtic S, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thrombosis and Haemostasis 2009;102:137‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karlsson M, Ternström L, Hyllner M, Baghaei F, Skrtic S, Jeppson A. Prophylactic fibrinogen Infusion in cardiac surgery patients: effects on biomarkers of coagulation, fibrinolysis, and platelet function. Clinical and Applied Thrombosis/Hemostasis 2011;17(4):396‐404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lance 2011 {published and unpublished data}
- Lance MD, Ninivaggi M, Schols SE, Feijge MA, Oehrl SK, Kuiper GJ, et al. Perioperative dilutional coagulopathy treated with fresh frozen plasma and fibrinogen concentrate: a prospective randomized intervention trial. Vox Sanguinis 2012;103(1):25‐34. [PUBMED: 22211833] [DOI] [PubMed] [Google Scholar]
Rahe‐Meyer 2013 {published and unpublished data}
- Rahe‐Meyer N, Solomon C, Hanke A, Schmidt DS, Knoerzer D, Hochleitner G, et al. Effects of fibrinogen concentrate as first‐line therapy during major aortic replacement surgery: a randomized, placebo‐controlled trial. Anesthesiology 2013;118(1):40‐50. [PUBMED: 23249928] [DOI] [PubMed] [Google Scholar]
- Rahe‐Meyer N, Solomon C, Hanke A, Schmidt DS, Pichlmaier M. Efficacy and safety of fibrinogen concentrate in the treatment of major bleeding during cardiac surgery: results of a randomized, placebo‐controlled trial. No. LBC07. American Society of Anesthesiologists Annual Meeting, october 15‐19, 2011; Chicago Illinois, USA. [http://www.asaabstracts.com/strands/asaabstracts/abstract.htm;jsessionid=33D06092FED7196AF490D3E3FBAB06B3?year=2011&index=6&absnum=7668]
References to studies excluded from this review
Bell 2010 {published data only}
- Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct hypofibrinogenaemia rapidly during obstetric haemorrhage. International Journal of Obstetrical Anesthesiology 2010;19(2):218‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Danes 2008 {published data only}
- Danés AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high‐risk severe bleeding. Vox Sanguinis 2008;94:221‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glover 2010 {published data only}
- Glover NJ, Collis RE, Collins P. Fibrinogen concentrate use during major obstetric haemorrhage. Anaesthesia 2010;65:1229‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guasch 2008 {published data only}
- Guasch E, Alsina E, Diez J, Ruiz R, Gilsanz F. [Postpartum hemorrhage: an observational study of 21,726 deliveries in 28 months] [Hemorragia obstetrica: estudio observacional sobre 21.726 partos en 28 meses.]. Revista Espanola de Anestesiologia y Reanimacion 2009;56(3):139‐46. [PUBMED: 19408780] [DOI] [PubMed] [Google Scholar]
Haas 2008 {published data only}
- Haas T, Fries D, Velik‐Salchner C, Oswald E, Innerhofer P. Fibrinogen in craniosynostosis surgery. Anesthesia and Analgesia 2008;106(3):725‐31, table of contents. [PUBMED: 18292409] [DOI] [PubMed] [Google Scholar]
Mittermayr 2007 {published data only}
- Mittermayr M, Streif W, Haas T, Fries D, Velik‐Salchner C, Klingler A, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesthesia and Analgesia 2007;105(4):905‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morrison 2011 {published data only}
- Morrison GA, Chalmers RT, Solomon C, Nimmo AF. Fibrinogen concentrate therapy guided by thromboelastometry as an alternative to fresh frozen plasma in major vascular surgery. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(4):654‐9. [PUBMED: 21955829] [DOI] [PubMed] [Google Scholar]
Rahe‐Meyer 2009 {published data only}
- Rahe‐Meyer N, Pichlmaier M, Haverich A, Solomon C, Winterhalter M, Piepenbrock S, et al. Bleeding management with fibrinogen concentrate targeting a high‐normal plasma fibrinogen level: a pilot study. British Journal of Anaesthesia 2009;102(6):785‐92. [PUBMED: 19411671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahe‐Meyer 2009a {published data only}
- Rahe‐Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, et al. Thromboelastometry‐guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. The Journal of Thoracic and Cardiovascular Surgery 2009;138(3):694‐702. [PUBMED: 19698858] [DOI] [PubMed] [Google Scholar]
Schöchl 2010a {published data only}
- Schochl H, Forster L, Woidke R, Solomon C, Voelckel W. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia 2010;65(2):199‐203. [PUBMED: 19995349] [DOI] [PubMed] [Google Scholar]
Schöchl 2010b {published data only}
- Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, et al. Goal‐directed coagulation management of major trauma patients using thromboelastometry (ROTEM)‐guided administration of fibrinogen concentrate and prothrombin complex concentrate. Critical Care (London, England) 2010;14(2):R55. [PUBMED: 20374650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2010 {published data only}
- Solomon C, Pichlmaier U, Schoechl H, Hagl C, Raymondos K, Scheinichen D, et al. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. British Journal of Anaesthesia 2010;104(5):555‐62. [PUBMED: 20348140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorarinsdottir 2010 {published data only}
- Thorarinsdottir HR, Sigurbjornsson FT, Hreinsson K, Onundarson PT, Gudbjartsson T, Sigurdsson GH. Effects of fibrinogen concentrate administration during severe hemorrhage. Acta Anaesthesiologica Scandinavica 2010;54(9):1077‐82. [PUBMED: 20887409] [DOI] [PubMed] [Google Scholar]
Weinkove 2007 {published data only}
- Weinkove R, Rangarajan S. Fibrinogen concentrate for acquired hypofibrinogenaemic states. Transfusion Medicine (Oxford, England) 2008;18(3):151‐7. [PUBMED: 18598277] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Galas 2012a {published data only}
- Galas F, Hajjar L, Sorensen B, Almeida J, Sundin M, Guimaraes V, et al. Randomized comparison of fibrinogen concentrate versus cryoprecipitate for bleeding control in pediatric cardiac surgery (FICCS study). Critical Care 2012;16:S156‐7. [Google Scholar]
Rahe‐Meyer 2011a {published data only}
- Rahe‐Meyer N. Fibrinogen Concentrate in the Treatment of Severe Bleeding After Aortic Aneurysm Graft Surgery. Thrombosis Research 2011;128:S17‐9. [DOI] [PubMed] [Google Scholar]
Rahe‐Meyer 2012 {published data only}
- Rahe‐Meyer N, Solomon C, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Haemostatic therapy with fibrinogen concentrate for controlling major bleeding during complex cardiovascular surgery: Results of a randomised, placebo‐controlled trial. British Journal of Anaesthesia 2012;108:ii86. [Google Scholar]
Rahe‐Meyer 2013a {published data only}
- Rahe‐Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first‐line hemostatic therapy during major aortic replacement surgery: Results from a randomized, placebo‐controlled trial. Journal of Thoracic and Cardiovascular Surgery 2013;145:S178‐85. [DOI] [PubMed] [Google Scholar]
Solomon 2012 {published data only}
- Solomon C, Schoechl H, Hagl C, Rahe‐Meyer N. Fibrinogen concentrate as first‐line haemostatic therapy during aortic replacement surgery. A randomised trial. Journal of Thrombosis and Haemostasis 2012;10:e7. [Google Scholar]
References to ongoing studies
Fries 2011 {published data only}
- Fibrinogen Concentrate (FGTW) in Trauma Patients, Presumed to Bleed (FI in TIC). Ongoing study October 2011.
Haas 2012 {published data only}
- Fibrinogen for Treatment of Pediatric Dilutional Coagulopathy: FibPaed Study. Ongoing study January 2012.
Innerhofer 2012 {published data only}
Jeppsson 2010 {published data only}
- Fibrinogen and Bleeding After Cardiac Surgery (Fibro‐3). Ongoing study April 2009.
Kwapisz 2012 {published data only}
- Prospective Double Blinded Randomized Control Study of the Use of Fibrinogen in High‐Risk Cardiac Surgery. Ongoing study Not yet recruiting.
Nierich 2011 {published data only}
- Haemocomplettan® P During Elective Complex Cardiac Surgery. Ongoing study February 2011.
Nimmo 2009 {published data only}
- Fibrinogen as an Alternative to Fresh Frozen Plasma in Aortic Surgery. Ongoing study October 2009.
Ranucci 2011 {published data only}
- The ZEro PLASma Trial (ZEPLAST): Avoidance of Fresh Frozen Plasma in Cardiac Surgery. Ongoing study November 2011.
Sabate 2012 {published data only}
- The Efficacy of the Administration of Fibrinogen in Liver Transplantation (FibstudLT). Ongoing study March 2012.
Tanaka 2011 {published data only}
- RiaSTAP vs. Conventional Transfusion in Patients Having Heart Valve Surgery (RiaCT). Ongoing study January 2011.
Wikkelsoe 2011 {published data only}
- Wikkelsoe AJ, Afshari A, Stensballe J, Langhoff‐Roos J, Albrechtsen C, Ekelund K, et al. The FIB‐PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial. Trials 2012;13(1):110. [PUBMED: 22805300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ASA 2006
- The American Society of Anesthesiologists. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006;105:198‐208. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blome 2005
- Blome M, Isgro F, Kiessling AH, Skuras J, Haubelt H, Hellstern P, et al. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thrombosis and Haemostasis 2005;93:1101‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bolton‐Maggs 2004
- Bolton‐Maggs PH, Perry DJ, Chalmers EA, Parapia LA, Wilde JT, Williams MD, et al. The rare coagulation disorders-review with guidelines for management from the United Kingdom Haemophilia Centre Doctors' Organisation. Haemophilia 2004;10(5):593‐628. [PUBMED: 15357789] [DOI] [PubMed] [Google Scholar]
Bornikova 2011
- Bornikova L, Peyvandi F, Allen G, Bernstein J, Manco‐Johnson MJ. Fibrinogen replacement therapy for congenital fibrinogen deficiency. Journal of Thrombosis and Haemostasis 2011;9(9):1687‐704. [PUBMED: 21711446] [DOI] [PubMed] [Google Scholar]
Brohi 2008
- Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. The Journal of Trauma 2008;64(1529‐8809 (Electronic), 5):1211‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carson 2011
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. The New England Journal of Medicine 2011;365(26):2453‐62. [PUBMED: 22168590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002042.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caudill 2009
- Caudill JS, Nichols WL, Plumhoff EA, Schulte SL, Winters JL, Gastineau DA, et al. Comparison of coagulation factor XIII content and concentration in cryoprecipitate and fresh‐frozen plasma. Transfusion 2009;49(4):765‐70. [PUBMED: 19192257] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Charbit 2007
- Charbit B, Mandelbrot L, Samain E, Baron G, Haddaoui B, Keita H, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. Journal of Thrombosis and Haemostasis 2007;5:266‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jonge 1998
- Jonge E, Levi M, Berends F, Ende AE, Cate JW, Stoutenbeek CP. Impaired haemostasis by intravenous administration of a gelatin‐based plasma expander in human subjects. Thrombosis and Haemostasis 1998;79(0340‐6245 (Print), 0340‐6245 (Linking), 2):286‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Fenger‐Eriksen 2005
- Fenger‐Eriksen C, Anker‐Moller E, Heslop J, Ingerslev J, Sorensen B. Thrombelastographic whole blood clot formation after ex vivo addition of plasma substitutes: improvements of the induced coagulopathy with fibrinogen concentrate. British Journal of Anaesthesia 2005;94:324‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fenger‐Eriksen 2008
- Fenger‐Eriksen C, Lindberg‐Larsen M, Christensen AQ, Ingerslev J, Sorensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. British Journal of Anaesthesia 2008;101:769‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fenger‐Eriksen 2009b
- Fenger‐Eriksen C, Ingerslev J, Sorensen B. Fibrinogen concentrate-a potential universal hemostatic agent. Expert Opinion on Biological Therapy 2009;9(10):1325‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fenger‐Eriksen 2009c
- Fenger‐Eriksen C, Tonnesen E, Ingerslev J, Sorensen B. Mechanisms of hydroxyethyl starch induced dilutional coagulopathy. Journal of Thrombosis and Haemostasis 2009;7(7):1099‐105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fries 2005
- Fries D, Krismer A, Klingler A, Streif W, Klima G, Wenzel V, et al. Effect of fibrinogen on reversal of dilutional coagulopathy: a porcine model. British Joural of Anaesthesia 2005;95:172‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fries 2006
- Fries D, Haas T, Klingler A, Streif W, Klima G, Martini J, et al. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy-a porcine model. British Journal of Anaesthesia 2006;97:460‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fries 2010
- Fries D, Martini WZ. Role of fibrinogen in trauma‐induced coagulopathy. British Journal of Anaesthesia 2010;105(2):116‐21. [PUBMED: 20627882] [DOI] [PubMed] [Google Scholar]
GradePro [Computer program]
- GradePro Working Team. GRADEPro. Version 3.2 for Windows. GradePro Working Team, 2008, http://ims.cochrane.org/revman/other‐resources/gradepro/download.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(1468‐5833 (Electronic), 0959‐535X (Linking), 7651):995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2008a
- Haas T, Fries D, Velik‐Salchner C, Reif C, Klingler A, Innerhofer P. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesthesia and Analgesia 2008;106:1360‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hebert 1999
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. The New England Journal of Medicine 1999;340(6):409‐17. [PUBMED: 9971864] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hiippala 1995
- Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma‐poor red cell concentrates. Anesthesia and Analgesia 1995;81:360‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hiippala 1995a
- Hiippala ST. Dextran and hydroxyethyl starch interfere with fibrinogen assays. Blood Coagulation & Fibrinolysis 1995;6:743‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Huissoud 2009
- Huissoud C, Carrabin N, Audibert F, Levrat A, Massignon D, Berland M, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. British Journal of Obstetrics and Gynecology 2009;116:1097‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series C 2000;49:359‐70. [MEDLINE: ] [Google Scholar]
ICH Harmonised Tripartite Guideline
- ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Statistics in Medicine 1999;18(15):1905‐42. [PUBMED: 10532877] [PubMed] [Google Scholar]
Jansen 2009
- Jansen JO, Thomas R, Loudon MA, Brooks A. Damage control resuscitation for patients with major trauma. British Medical Journal 2009;338:b1778. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kalina 2008
- Kalina U, Stohr HA, Bickhard H, Knaub S, Siboni SM, Mannucci PM, et al. Rotational thromboelastography for monitoring of fibrinogen concentrate therapy in fibrinogen deficiency. Blood Coagulation & Fibrinolysis 2008;19:777‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karlsson 2008
- Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on‐pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion 2008;48:2152‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kozek‐Langenecker 2011
- Kozek‐Langenecker S, Sorensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Critical Care (London, England) 2011;15(5):R239. [PUBMED: 21999308] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kozek‐Langenecker 2011a
- Kozek‐Langenecker S, Sorensen B, Hess J, Spahn DR. Emotional or evidence‐based medicine-is there a moral tragedy in haemostatic therapy?. Critical Care (London, England) 2011; Vol. 15, issue 6:462. [PUBMED: 22236360] [DOI] [PMC free article] [PubMed]
Kreuz 2005
- Kreuz W, Meili E, Peter‐Salonen K, Dobrkovska A, Devay J, Haertel S, et al. Pharmacokinetic properties of a pasteurised fibrinogen concentrate. Transfusion and Apheresis Science 2005;32:239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63. [Google Scholar]
Lang 2005
- Lang T, Bauters A, Braun SL, Potzsch B, Pape KW, Kolde HJ, et al. Multi‐centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagulation & Fibrinolysis 2005;16:301‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levi 2007
- Levi M, Jonge E. Clinical relevance of the effects of plasma expanders on coagulation. Seminars in Thrombosis and Hemostasis 2007;33:810‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levy 2012
- Levy JH, Szlam F, Tanaka KA, Sniecienski RM. Fibrinogen and hemostasis: a primary hemostatic target for the management of acquired bleeding. Anesthesia and Analgesia 2012;114(2):261‐74. [PUBMED: 21965371] [DOI] [PubMed] [Google Scholar]
Lissalde‐Lavigne 2008
- Lissalde‐Lavigne G, Combescure C, Muller L, Bengler C, Raillard A, Lefrant JY, et al. Simple coagulation tests improve survival prediction in patients with septic shock. Journal of Thrombosis and Haemostasis 2008;6:645‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martini 2009
- Martini WZ. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. The Journal of Trauma 2009;67:202‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
Monroe 2002
- Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arteriosclerosis, Thrombosis, and Vascular Biology 2002;22:1381‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mosesson 2001
- Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Annals of the New York Academy of Sciences 2001;936:11‐30. [PUBMED: 11460466] [DOI] [PubMed] [Google Scholar]
Nielsen 2005
- Nielsen VG, Cohen BM, Cohen E. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiologica Scandinavica 2005;49:222‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nielsen 2005a
- Nielsen VG. Colloids decrease clot propagation and strength: role of factor XIII‐fibrin polymer and thrombin‐fibrinogen interactions. Acta Anaesthesiologica Scandinavica 2005;49:1163‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozier 2011
- Ozier Y, Hunt BJ. Against: fibrinogen concentrate for management of bleeding: against indiscriminate use. Journal of Thrombosis and Haemostasis 2011;9(1):6‐8. [PUBMED: 21210948] [DOI] [PubMed] [Google Scholar]
Parasnis 1992
- Parasnis H, Raje B, Hinduja IN. Relevance of plasma fibrinogen estimation in obstetric complications. Jourmal of Postgraduate Medicine 1992;38:183‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):45‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pro.medicin.dk 2013
- Dansk Lægemiddel Information A/S. Information on drugs for professionals, fibrinogen concentrate. http://pro.medicin.dk/Medicin/Praeparater/6505 (accessed 3 April 2013).
Rahe‐Meyer 2011c
- Rahe‐Meyer N, Sorensen B. Fibrinogen concentrate for management of bleeding. Journal of Thrombosis and Haemostasis 2011;9(1):1‐5. [PUBMED: 20946151] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Riastap 2009
- CSL Behring. RiSTAP-Full prescribing information. http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm094006.pdf (accessed 6 February 2013).
Rossaint 2010
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez‐Mondejar E, et al. Management of bleeding following major trauma: an updated European guideline. Critical Care (London, England) 2010;14(2):R52. [PUBMED: 20370902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rucker 2008
- Rucker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sorensen 2010
- Sorensen B, Bevan D. A critical evaluation of cryoprecipitate for replacement of fibrinogen. British Journal of Haematology 2010;149(6):834‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stanworth 2011
- Stanworth SJ, Hunt BJ. The desperate need for good‐quality clinical trials to evaluate the optimal source and dose of fibrinogen in managing bleeding. Critical Care (London, England) 2011; Vol. 15, issue 6:1006. [PUBMED: 22082120] [DOI] [PMC free article] [PubMed]
Sutton 2002
- Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta‐analysis of rare and adverse event data. Expert Review in Pharmacoeconomics Outcomes Research 2002;2:367‐79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TSA 2010 [Computer program]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis. Version version 0.8. Copenhagen: Copenhagen Trial Unit, 2010.
Turner 2013
- Turner RM, Bird SM, Higgins JP. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PloS One 2013;8(3):e59202. [PUBMED: 23544056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ucar 2007
- Ucar HI, Oc M, Tok M, Dogan OF, Oc B, Aydin A, et al. Preoperative fibrinogen levels as a predictor of postoperative bleeding after open heart surgery. The Heart Surgery Forum 2007;10:E392‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UKHCDO 2003
- United Kingdom Haemophelia Centre Doctors' Organisation. Guidelines on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. Haemophilia 2003;9(1):1‐23. [PUBMED: 12558775] [DOI] [PubMed] [Google Scholar]
Warmuth 2012
- Warmuth M, Mad P, Wild C. Systematic review of the efficacy and safety of fibrinogen concentrate substitution in adults. Acta Anaesthesiologica Scandinavica 2012;56(5):539‐48. [PUBMED: 22150561] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wikkelsø 2010
- Wikkelsø A, Lunde J, Johansen M, Stensballe J, Wetterslev J, Møller AM, et al. Fibrinogen concentrate in severely bleeding patients for acquired hypofibrinogenaemia. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008864] [DOI] [Google Scholar]