Abstract
Background
Ventilator‐associated pneumonia (VAP) is one of the most common nosocomial infections in intubated and mechanically ventilated patients. Endotracheal tubes (ETTs) appear to be an independent risk factor for VAP. Silver‐coated ETTs slowly release silver cations. It is these silver ions that appear to have a strong antimicrobial effect. Because of this antimicrobial effect of silver, silver‐coated ETTs could be an effective intervention to prevent VAP in people who require mechanical ventilation for 24 hours or longer.
Objectives
Our primary objective was to investigate whether silver‐coated ETTs are effective in reducing the risk of VAP and hospital mortality in comparison with standard non‐coated ETTs in people who require mechanical ventilation for 24 hours or longer. Our secondary objective was to ascertain whether silver‐coated ETTs are effective in reducing the following clinical outcomes: device‐related adverse events, duration of intubation, length of hospital and intensive care unit (ICU) stay, costs, and time to VAP onset.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014 Issue 10, MEDLINE, EMBASE, EBSCO CINAHL, and reference lists of trials. We contacted corresponding authors for additional information and unpublished studies. We did not impose any restrictions on the basis of date of publication or language. The date of the last search was October 2014.
Selection criteria
We included all randomized controlled trials (RCTs) and quasi‐randomized trials that evaluated the effects of silver‐coated ETTs or a combination of silver with any antimicrobial‐coated ETTs with standard non‐coated ETTs or with other antimicrobial‐coated ETTs in critically ill people who required mechanical ventilation for 24 hours or longer. We also included studies that evaluated the cost‐effectiveness of silver‐coated ETTs or a combination of silver with any antimicrobial‐coated ETTs.
Data collection and analysis
Two review authors (GT, HV) independently extracted the data and summarized study details from all included studies using the specially designed data extraction form. We used standard methodological procedures expected by The Cochrane Collaboration. We performed meta‐analysis for outcomes when possible.
Main results
We found three eligible randomized controlled trials, with a total of 2081 participants. One of the three included studies did not mention the amount of participants and presented no outcome data. The 'Risk of bias' assessment indicated that there was a high risk of detection bias owing to lack of blinding of outcomes assessors, but we assessed all other domains to be at low risk of bias. Trial design and conduct were generally adequate, with the most common areas of weakness in blinding. The majority of participants were included in centres across North America. The mean age of participants ranged from 61 to 64 years, and the mean duration of intubation was between 3.2 and 7.7 days. One trial comparing silver‐coated ETTs versus non‐coated ETTs showed a statistically significant decrease in VAP in favour of the silver‐coated ETT (1 RCT, 1509 participants; 4.8% versus 7.5%, risk ratio (RR) 0.64, 95% confidence interval (CI) 0.43 to 0.96; number needed to treat for an additional beneficial outcome (NNTB) = 37; low‐quality evidence). The risk of VAP within 10 days of intubation was significantly lower with the silver‐coated ETTs compared with non‐coated ETTs (1 RCT, 1509 participants; 3.5% versus 6.7%, RR 0.51, 95% CI 0.31 to 0.82; NNTB = 32; low‐quality evidence). Silver‐coated ETT was associated with delayed time to VAP occurrence compared with non‐coated ETT (1 RCT, 1509 participants; hazard ratio 0.55, 95% CI 0.37 to 0.84). The confidence intervals for the results of the following outcomes did not exclude potentially important differences with either treatment. There were no statistically significant differences between groups in hospital mortality (1 RCT, 1509 participants; 30.4% versus 26.6%, RR 1.09, 95% CI 0.93 to 1.29; low‐quality evidence); device‐related adverse events (2 RCTs, 2081 participants; RR 0.65, 95% CI 0.37 to 1.16; low‐quality evidence); duration of intubation; and length of hospital and ICU stay. We found no clinical studies evaluating the cost‐effectiveness of silver‐coated ETTs.
Authors' conclusions
This review provides limited evidence that silver‐coated ETT reduces the risk of VAP, especially during the first 10 days of mechanical ventilation.
Plain language summary
Silver‐coated endotracheal tubes (ETTs) for prevention of ventilator‐associated pneumonia in critically ill people
Review question
We reviewed the evidence of whether silver‐coated endotracheal tubes (ETTs) are effective in reducing the risk of ventilator‐associated pneumonia (VAP) and hospital mortality in comparison with standard non‐coated ETTs in people who require mechanical ventilation for 24 hours or longer.
Background
Mechanical ventilation is used to help people breathe when they are very ill and find it difficult to breath on their own. An endotracheal tube is a disposable tube that is inserted into the person's windpipe to allow mechanical ventilation. In mechanical ventilation a machine is used to replace the natural spontaneous breathing and allows air in and out of the lungs. When used for a long period, mechanical ventilation can cause problems for the patient. Mechanical ventilation is associated with complications. Silver is an antimicrobial agent (an agent that kills bacteria) that has been used for many centuries to treat and prevent infections. Silver coating around the ETTs could prevent VAP because of its antimicrobial activity.
Study characteristics
We found three randomized controlled trials involving 2081 participants. No outcome data were available for one included study. The design of the other two RCTs was generally good, although there were some weaknesses. The majority of participants were included in centres around North America. The evidence is current to October 2014. Both RCTs were funded but stated that the sponsors contributed only to the study design and did not participate in the conduct of the study.
Key results
We found that silver‐coated ETT reduced the risk for developing VAP from 6.7% to 3.5% within 10 days of intubation compared with non‐coated ETT in people who required mechanical ventilation for 24 hours of longer, although the quality of evidence for this outcome was low. One study showed that an initial VAP occurrence appeared much later in time in people who had a silver‐coated ETT compared with a non‐coated ETT, although again the evidence was low quality. We could not draw conclusions about hospital mortality, device‐related complications, duration of intubation, and length of hospital and intensive care unit stay owing to uncertainty of the effects. We found no clinical studies evaluating the cost‐effectiveness of silver‐coated ETTs.
Quality of the evidence
The overall quality of evidence was low for all interested outcomes mentioned.
Conclusion
Limited evidence suggests that silver‐coated ETT seems to be an effective device in reducing the risk of VAP, although data for our other key outcome of hospital mortality were inconclusive. Larger studies with more participants who are at risk for VAP and who require mechanical ventilation for a longer period of time are needed.
Summary of findings
for the main comparison.
| Silver‐coated ETT compared with non‐coated ETT for VAP | ||||||
| Patient or population: People who are mechanically ventilated Settings: Intensive care unit Intervention: Silver‐coated ETT Comparison: Non‐coated ETT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐coated ETT | Silver‐coatedETT | |||||
| Risk of VAP at any time in participants intubated for ≥ 24 hours | 75 per 1000 | 48 per 1000 (32 to 72) |
RR 0.64 (0.43 to 0.96) |
1509 (1 study) | ⊕⊕⊝⊝ Low1,2,3,4,5,6 |
VAP rate was low for all mechanically ventilated participants in the analysis. VAP was diagnosed with new radiographic confirmed infiltrate with qualifying clinical signs followed by quantitative BAL. No standardization of other contemporary VAP prevention strategies. Wide confidence intervals around the estimate of the effect. |
| Risk of VAP within 10 days of intubation in participants intubated for ≥ 24 hours | 67 per 1000 | 35 per 1000 (22 to 56) | RR 0.51 (0.31 to 0.82) | 1509 (1 study) | ⊕⊕⊝⊝ Low1,2,3,4,5,6 |
VAP rate was low for all mechanically ventilated participants in the analysis. No standardization of other contemporary VAP prevention strategies. Wide confidence intervals around the estimate of the effect. |
| Hospital mortality | 266 per 1000 | 290 per 1000 (248 to 344) |
RR 1.09 (0.93 to 1.29) |
1509 (1 study) | ⊕⊕⊝⊝ Low1,2,3,4,5,6 |
The cause of hospital mortality was not clarified in the included studies. Wide confidence intervals around the estimate of the effect. Only included participants who were intubated for 24 hours or longer. |
| Device‐related adverse events | 28 per 1000 | 18 per 1000 (10 to 32) |
RR 0.65 (0.37 to 1.16) |
2081 (2 studies) | ⊕⊕⊝⊝ Low1,2,3,4,5,6 |
Participants who were intubated less than 24 hours were also included. Wide confidence intervals around the estimate of the effect. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BAL: bronchoalveolar lavage; CI: confidence interval; ETT: endotracheal tube; RR: risk ratio; VAP: ventilator‐associated pneumonia | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ||||||
1. Study design (‐1): Single‐blinded randomized controlled trial.
2. Low VAP rate.
3. No standardization of other contemporary VAP prevention strategies.
4. Inconsistency (‐0): I² = 0%.
5. Publication bias (‐0): Trial was registered.
6. Imprecision (‐1): Wide confidence intervals around the estimate of the effect.
Background
Description of the condition
Ventilator‐associated pneumonia (VAP) is one of the most common nosocomial infections in intubated and mechanically ventilated intensive care unit (ICU) patients (NNIS 2004; Vincent 1995). Nosocomial infections are hospital‐acquired infections. VAP occurs in 9% to 27% of all intubated ICU patients (Chastre 2002). The etiology of VAP seems to be related to colonization of the aerodigestive tract with pathogenic bacteria. Endotracheal tubes (ETTs) appear to be an independent risk factor for VAP (Girou 2000; Hubmayr 2002). ETTs are disposable catheters that are used for invasive mechanical ventilation and are inserted into the trachea primarily to maintain and establish a patent airway and to ensure sufficient exchange of oxygen and carbon dioxide. The median duration of mechanical ventilation with ETTs is estimated at less than 10 days, and up to 75% of the patients are extubated before 10 days (Esteban 2000; Esteban 2002; Freeman 2005). It has been suggested that the risk of developing VAP is at its highest in the first 10 days of mechanical intubation (Cook 1998a; Rello 2002a). Most ETTs used today are made of polyvinyl chloride. Polyvinyl chloride ETTs become colonized by bacteria after a few hours of mechanical ventilation. These bacteria often organize into a thick biofilm, representing a large reservoir of microorganisms that can spread to the lungs and cause pneumonia (Adair 1999; Costerton 2003; Inglis 1989; Sottile 1986). VAP is also linked with aspiration of contaminated secretions from the oropharynx or gastrointestinal tract (Kollef 2004; Kollef 2005a). VAP can be divided into early‐ and late‐onset disease. Early‐onset VAP occurs during the first four days that the person receives mechanical ventilation and is often caused by Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, or, uncommonly, by anaerobes. In comparison to early‐onset VAP, late‐onset VAP occurs over four days after intubation and is more commonly caused by Pseudomonas aeruginosa, Acinetobacter or Enterobacter species, or methicillin‐resistantStaphylococcus aureus (MRSA) (Bregeon 2001; Craven 1995; Kollef 1997; Kollef 1999; Mayhall 1997; Trouillet 1998). VAP leads to increased healthcare costs, longer stay in the ICU and hospital, and double the risk of hospital mortality (Safdar 2005).
Description of the intervention
Silver has been used both as a prophylactic agent and as a treatment for infectious and other diseases for many centuries. Hippocrates, known as the 'father of medicine', described silver as having healing and anti‐disease properties (Magner 1992). In theory, silver is highly attractive because of its broad‐spectrum antibiotic activity (Petering 1976). Silver‐coated ETTs contain silver atoms that are slowly released as silver cations (Rello 2010; Thompson 1973). It is these silver ions that appear to have a strong antimicrobial effect. The silver ions bind to bacterial cell walls, causing disruption of the wall and death of the bacteria (Thompson 1973). Silver ions can also bind to bacterial enzymes and prevent them from performing their function (Thompson 1973). Ionic silver reacts strongly with the thiol groups of vital enzymes in the bacteria, thus inactivating the bacteria (Flemming 1990; Liau 1997; Russell 1994). Silver ions also bind to bacterial deoxyribonucleic acid (DNA), thus interfering with cell division and replication (Feng 2000). Moreover, silver has the ability to reduce bacterial adhesion to devices in vitro, in Ahearn 2000 and Gabriel 1995, and to block biofilm formation on medical devices in animal models (Berra 2004; Olson 2002). Recent studies have shown that the use of silver‐coated ETT to reduce lower lung colonization also reduces the risk of VAP (Balazs 2004; Berra 2004; Berra 2006; Chaiban 2005; Hartmann 1999; Jones 2003; Olson 2002; Pacheco‐Fowler 2004; Rello 2006; Rello 2010). Silver in combination with other antimicrobials, such as chlorhexidine, could be another option for coating ETTs. In an animal study, ETTs coated with a combination of silver and chlorhexidine showed no bacterial growth in comparison with non‐coated tubes and were associated with less bacterial colonization in bronchial samples and lung parenchyma (Berra 2004). Thus, silver‐coated ETT could be a valuable intervention to prevent VAP.
How the intervention might work
A number of factors could predispose a person to VAP. These factors are re‐intubation, nasogastric tubes, aspiration, supine positioning, pooling of subglottic secretions, coma, enteral nutrition, and pH‐altering agents (Collard 2003; Cook 1998b). Intubation and aspiration may bring the pathogenic bacteria into the respiratory tract and cause infection. Silver has a broad‐spectrum antimicrobial activity in vitro by binding to microbial DNA and thus preventing bacterial replication and by binding to the sulfhydryl groups of metabolic enzymes of the bacterial electron transport chain, causing their inactivation (Feng 2000; Flemming 1990; Illingworth 1998; Liau 1997; Petering 1976; Russell 1994; Thompson 1973). Due to the microbicidal effects of silver, silver‐coated ETTs could be an effective intervention against VAP.
Why it is important to do this review
The increase in length of ICU and hospital stay, healthcare costs, and infection with multidrug‐resistant pathogens due to VAP are major concerns (Cook 1998a; Kollef 2005a; Kollef 2005b; Rello 2002a). Prevention strategies aimed at reducing VAP often focus on modifiable risk factors for colonization and aspiration or use expensive prophylactic antimicrobial agents (Babcock 2004; de Jonge 2003), which may result in further antimicrobial resistance. As there is no single strategy that completely eliminates VAP, prevention strategies today consist of a combination of several effective interventions on multiple modifiable risk factors, which in total reduce infections. These combinations are known as care bundles. Unfortunately, compliance to such bundles may be hindered by costs and lack of education of personnel, resources, and leadership (Cook 2000; Craven 2006; Rello 2002b). The benefits may diminish if the initiatives for education of personnel are not continually reinforced or monitored (Zack 2002). Moreover, the claims of silver‐coated ETTs having antiseptic qualities that reduce the risk of VAP, bacterial airway colonization, and biofilm formation have in recent years been promoted to a high degree, which may have led, or may lead, to an increase in the use of such devices. However, we know little of the costs, effectiveness, and benefits of silver‐coated ETTs. A review of the literature on this topic is therefore needed to investigate the claims from an evidence‐based perspective.
Objectives
The primary objective of this review was to determine whether silver‐coated ETT is effective in reducing the risk of VAP and hospital mortality in comparison with standard non‐coated ETT in people who require mechanical ventilation for 24 hours or longer.
Our secondary objective was to ascertain whether silver‐coated ETTs are effective in reducing the following clinical outcomes:
Device‐related adverse events;
Duration of intubation;
Length of hospital and ICU stay;
Costs;
Time to VAP onset.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized trials that evaluated the effects of silver‐coated ETTs. We also included clinical studies that evaluated the cost‐effectiveness of silver‐coated ETTs.
Types of participants
We included studies that investigated intubated and mechanically ventilated critically ill participants. Due to the variety of criteria for admission to adult ICUs, we used authors' definitions of critically ill. We excluded studies investigating children under 16 years and participants who were re‐intubated. Where possible, we only included participants who were intubated for 24 hours or longer.
Types of interventions
We included trials that compared silver‐coated ETTs or a combination of silver and any antimicrobial‐coated ETTs with standard non‐coated ETTs or with other antimicrobial coated ETTs such as chlorhexidine‐coated ETTs. We excluded studies in which silver‐coated ETTs were not evaluated in the intervention or control groups.
Types of outcome measures
Primary outcomes
Risk rate of VAP;
hospital mortality.
Secondary outcomes
Device‐related events;
Duration of intubation;
Length of stay in ICU and hospital;
Cost‐effectiveness of silver‐coated ETTs (authors' definitions);
Time to VAP onset.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014 Issue 10), see Appendix 1; Ovid MEDLINE (1950 to October 2014), see Appendix 2; Ovid EMBASE (1980 to October 2014), see Appendix 3; and EBSCO CINAHL (1982 to October 2014), see Appendix 4. We imposed no restrictions on the basis of date of publication or language. We contacted experts in the field for information on unpublished studies.
We searched for unpublished studies and clinical trials using the following sites:
clinicaltrials.gov;
Searching other resources
We searched the reference lists of identified RCT reports and relevant systematic reviews for additional articles, contacted companies and expert informants for further details of unpublished and ongoing trials, and checked conference proceedings for any relevant trials. We also contacted experts in the field of VAP and silver technology to identify additional studies and information. Moreover, we contacted corresponding authors of relevant studies for detail clarification, where necessary.
Data collection and analysis
Selection of studies
Two review authors (GT, PK) independently assessed the titles and abstracts of studies identified in terms of their relevance and design. We obtained full versions of the articles if they appeared to meet the inclusion criteria in the initial assessment. A third review author (SZ) evaluated any discrepant judgements.
Data extraction and management
Two review authors (GT, HV) independently extracted the data and summarized study details from all included studies using the specially designed data extraction form shown in Appendix 5. In case of any disagreement on the details or quantitative data between GT and HV, a third review author (SZ) was consulted as a referee. One review author entered the data into Review Manager 5.3 (RevMan 5.3) for statistical analysis. We planned to extract data from any papers in languages not known to the review authors with help from appropriate translators. This was not necessary since all the included studies were in the English language. We also contacted study authors through an open‐ended request in order to obtain missing information or for clarification, if necessary.
Assessment of risk of bias in included studies
Two review authors (GT, SZ) independently assessed and rated the methodological quality of each trial using The Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). We judged the quality of the studies by evaluating them for the five domains found in Appendix 6. The five domains were as follows:
Random sequence generation;
Allocation concealment;
Blinding of participants and personnel;
Blinding of outcome assessment;
Incomplete outcome data.
We evaluated each study and assessed them separately for these domains. We judged each explicitly as follows:
Low risk of bias;
High risk of bias;
Unclear risk (lack of information or uncertainty over the potential for bias).
We entered data on what was reported to have happened in the study in the ’Risk of bias' table in Review Manager 5.3 (RevMan 5.3). We obtained further information from the study authors where we required clarification. We presented a summary figure of the risk of bias in the included studies in the review. This provided a context for discussing the reliability of the results. We resolved any disagreements by referring to a third review author (HV) to reach a consensus. Where there was still disagreement, we held a discussion amongst all the review authors to achieve consensus.
Measures of treatment effect
We calculated summary estimates of treatment effect (with 95% confidence intervals (CI)) for each comparison. We presented mean differences for continuous outcomes. For dichotomous outcomes we presented the absolute risk reduction, which is the risk difference. Risk difference is an absolute effect measure that expresses the difference between the experimental and the control event rates and allows calculation of the NNTB. We analysed time‐to‐event data and the risk rate of VAP as risk ratios. We reported measures from individual studies if we could not use them in a format suitable for meta‐analysis of the specified effect measures.
Unit of analysis issues
We included no studies with a cross‐over design because such a study design was not appropriate for this research question.
Dealing with missing data
We planned to contact the trial authors to request additional missing data. We extracted data regarding intention to treat (ITT). If study authors did not perform ITT analysis and the number of participants lost to follow‐up was less than 20% of the total, but sufficient raw data were available, we planned to conduct an ITT analysis prior to entering data into RevMan 5.3. If more than 20% of the data were missing from the study, we planned to exclude the study from the meta‐analysis and perform an available case analysis. If possible, we calculated missing statistics (such as standard deviations or correlation coefficients) from other statistics (such as standard error or 95% CI). We performed sensitivity analysis to assess the impact of changing the assumptions made.
Assessment of heterogeneity
We planned to assess clinical diversity (for example different types endotracheal tubes, length of intubation, pulmonary comorbidities, age) and methodological diversity ('Risk of bias' assessment). We analysed the amount of heterogeneity using the I² statistic. If we detected statistical heterogeneity, we explored the sources by considering differences in study design or definitions of outcome measures in the trials. If there was sufficient homogeneity in populations, study design, and outcome measures (Higgins 2003), we planned to pool the results following assessment for statistical heterogeneity.
Assessment of reporting biases
We assessed reporting bias through careful attention to quality assessment, particularly methodology. We planned to use funnel plot analysis to assess publication bias when greater than 10 studies were included in the meta‐analysis. We also planned to use the Egger test to assess funnel plot asymmetry (Egger 1997). A thorough search for unpublished studies through grey literature searches and contact with known experts in the field also assisted in reducing the risk of publication bias.
Data synthesis
We carried out statistical analysis using Review Manager software. One review author (GT) entered data into Review Manager 5.3, and a second review author checked this for accuracy (RevMan 5.3). If the outcome of the heterogeneity was low, as indicated by an I² less than 30%, we planned to use the fixed‐effect model to synthesize the results. If heterogeneity was moderate or high, as indicated by an I² statiistic greater than 30% but below 60%, we planned to use the random‐effects model to synthesize the results. We planned to refrain from pooling and restricted the analysis to a qualitative overview when I² values were above 60%.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity by performing the following relevant subgroup analysis: length of intubation, that is participants intubated for 24 hours or more versus participants intubated for less than 24 hours. However, the number of studies was insufficient to perform any of these subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses to identify the effects of unpublished studies, small studies, allocation concealment, assessor blinding, and loss to follow‐up on the results. We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
Including or excluding unpublished studies;
Including or excluding studies judged as presenting a high risk of bias;
Sensitivity to ITT or per‐protocol data analysis assumptions;
Including or excluding studies with industry funding.
However, we could not carry out the planned analyses because of lack of relevant data in the included studies.
Summary of findings table
We used the principles of the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to assess the quality of the body of evidence associated with specific outcomes in our review and construct a Table 1 using the GRADEpro software (Guyatt 2008). The specific outcomes included in the table were as follows:
Risk of VAP at any time in participants intubated for ≥ 24 hours;
Risk of VAP within 10 days of intubation in participants intubated for ≥ 24 hours;
Hospital mortality;
Device‐related adverse events.
The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The assessment of the quality of a body of evidence considers within‐study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
See Characteristics of included studies for further details.
Results of the search
Figure 1 summarizes the results of the literature search. We identified 2303 publications from the initial search strategy. We excluded 433 publications as they were duplicates. We identified 1870 studies for further assessment. Of these 1870 studies, we selected eight based on title and abstract. After retrieving the full texts of these potentially relevant articles, we judged three trials as eligible for inclusion in this review after applying the inclusion and exclusion criteria (Kollef 2008; NCT00341354 2007; Rello 2006). No outcome data were available for one trial (NCT00341354 2007). All identified trials were published in English.
1.
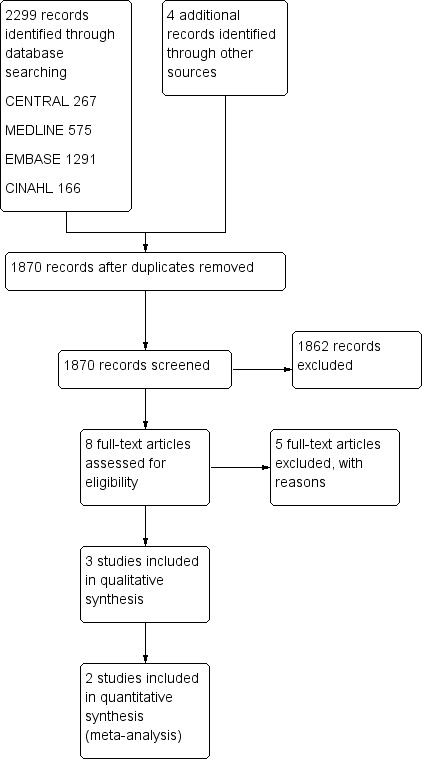
Study flow diagram.
Included studies
We included three RCTs in this review (Kollef 2008; NCT00341354 2007; Rello 2006).
Participants and settings
We analysed the two single‐blind multicentre RCTs and one single‐centre RCT with a total of 2081 participants. One of the three included studies did not mention the amount of participants and presented no outcome data (NCT00341354 2007). The two multicentre trials were conducted in the USA (Kollef 2008, Rello 2006), Canada (Kollef 2008), and Spain (Rello 2006). Study sample sizes ranged from 149, in Rello 2006, to 1932 participants, in Kollef 2008. The participants in the two studies were from different cohorts. The participant location at intubation varied, with the majority of participants intubated at the medical ICU, burn unit, and operating/recovery room. The mean age of the included participants ranged between 61.4 and 64.6 years. There were no differences observed between groups in Acute Physiology and Chronic Health Evaluation (APACHE) II scores, use of enteral nutrition, and presence of immunodeficiency. However, chronic obstructive pulmonary disease was more common in the group receiving the non‐coated tube (P value equals 0.007).
See Characteristics of included studies for additional details.
Interventions
The RCTs compared silver‐coated ETT with non‐coated ETT. One study used silver‐coated ETT with an internal diameter of 7 to 9 mm for 3.2 days (interquartile range, 1.2 to 7.1 days) in the intervention group (Kollef 2008), whereas the other study used silver‐coated ETT with an internal diameter of 7.5 to 8.5 mm for 7.7 days (range, 1.0 to 32.8 days) in the intervention group (Rello 2006). The study with no outcome data included ETT with an internal diameter of 7.5 mm or 8.0 mm (NCT00341354 2007). VAP was diagnosed by clinical and radiological criteria combined with positive culture of fluid obtained by bronchoalveolar lavage. Explicitly, new radiologically confirmed infiltrate with qualifying clinical signs were triggers for conducting quantitative bronchoalveolar lavage. Full analyses of the included studies are illustrated in Characteristics of included studies.
Excluded studies
We excluded five studies from this review. All five studies did not fulfil the required inclusion criteria. Two studies were retrospective studies (Afessa 2010; Morrow 2010); one study was a non‐randomized prospective study (Morrow 2009); one study was a RCT that included only participants who were intubated and mechanically ventilated for 12 to 24 hours (Berra 2008); and one study was a hypothetical 1000‐participant‐cohort cost‐effectiveness analysis. We have provided additional details in the table Characteristics of excluded studies.
Risk of bias in included studies
The 'Risk of bias' assessment for included studies is outlined in Figure 2. We have provided additional details in Characteristics of included studies.
2.
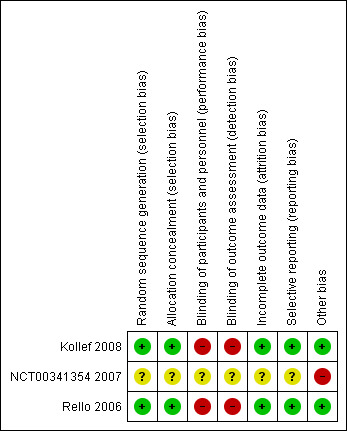
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both RCTs presented adequate details with regards to the methods used for random sequence generation (Kollef 2008; Rello 2006). Both trials used computer‐generated randomization sequence, with one study describing the utilization of a fixed block of four (Kollef 2008). Kollef 2008 described the use of allocation concealment with a series of sequentially numbered envelopes, each containing a randomization card for a study participant. No details were given regarding the allocation concealment in the study of Rello 2006. However, we contacted the study authors, and it seems that allocation concealment was conducted with central randomization by telephone, with different lists for each institution. We considered the overall risk of selection bias to be low.
Blinding
Both RCTs used a single‐blinded study design (Kollef 2008; Rello 2006). The investigators were blinded to block length, and the microbiology laboratory personnel were blinded to group assignments. However, the ICU investigators could not be blinded in the included studies, potentially introducing a bias in favour of silver‐coated ETTs. The authors stated that the experimental tube and the control tube were similar except for a silver coating on the experimental tube (Kollef 2008; Rello 2006). After investigating the products, we concluded that they look different from each other. Thus, both studies scored a high risk for detection and performance bias.
Incomplete outcome data
The included studies had 0% lost to follow‐up, thus making the risk for attrition bias low (Kollef 2008; Rello 2006).
Selective reporting
One study was registered as NCT00148642 (Kollef 2008). Both studies reported the outcomes in the methods section (Kollef 2008; Rello 2006). The risk for reporting bias for the included studies was therefore low.
Other potential sources of bias
Both RCTs were funded by sponsors but stated that the sponsors only contributed to the study design and did not participate in the conduct of the study (the collection, analysis, interpretation of the data or the review or approval of the manuscript). We found no other potential sources of bias in the included studies. There is possible publication bias for one included study that did not present any outcome data (NCT00341354 2007).
Effects of interventions
See: Table 1
Primary outcomes
Risk rate of VAP
One RCT involving 1509 participants who were intubated for at least 24 hours reported the risk of VAP (Kollef 2008). The risk for developing VAP was significantly lower in the silver‐coated ETT group compared with the non‐coated ETT group (4.8% versus 7.5%, risk ratio (RR) 0.64, 95% confidence interval (CI) 0.43 to 0.96; P value = 0.03) (Analysis 1.1). The absolute risk reduction was 2.7% (95% CI 0.3% to 2.7%) with a number needed to treat for an additional beneficial outcome (NNTB) of 37 (95% CI 19 to 369). The risk for VAP was at its highest in the first 10 days of intubation. The risk of VAP within 10 days of intubation was significantly lower in participants intubated with silver‐coated ETTs for 24 hours or longer than in participants with non‐coated ETTs (3.5% versus 6.7%, RR 0.51, 95% CI 0.31 to 0.82; P value = 0.05) (Analysis 1.2). The absolute risk reduction was 3.2% with a NNTB of 32 (95% CI 18 to 102). Of note, the VAP rate was low overall for all mechanically ventilated participants in the analysis. The ICU investigators were not blinded in this study, thus potentially introducing detection bias in favour of silver‐coated ETTs. The study did not standardize other contemporary VAP prevention strategies, thus potentially influencing between‐group VAP rates. The observed risk of VAP in the included study was 7.5% in the control group, whereas we predicted up to 15% using target sample size for between‐group comparison (Kollef 2008).
1.1. Analysis.
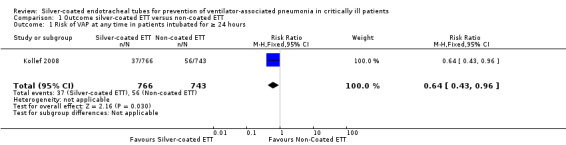
Comparison 1 Outcome silver‐coated ETT versus non‐coated ETT, Outcome 1 Risk of VAP at any time in patients intubated for ≥ 24 hours.
1.2. Analysis.
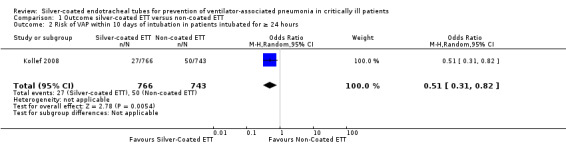
Comparison 1 Outcome silver‐coated ETT versus non‐coated ETT, Outcome 2 Risk of VAP within 10 days of intubation in patients intubated for ≥ 24 hours.
Mortality
Two RCTs reported hospital mortality as an endpoint (Kollef 2008; Rello 2006). Only one study presented mortality data for participants who were intubated for 24 hours or longer (Kollef 2008). mortality was defined as hospital mortality according to the study authors of the included trials. The study involving 1509 participants demonstrated no significant difference in hospital mortality between participants receiving silver‐coated ETT versus non‐coated ETT in participants who were intubated for 24 hours or longer (30.4% versus 26.6%, RR 1.09, 95% CI 0.93 to 1.29; P value = 0.29) (Analysis 1.3). The included studies did not provide any details with regards to the cause of hospital mortality. The study authors did not have the data regarding the cause of hospital mortality. We excluded participants who were intubated for less than 24 hours.
1.3. Analysis.
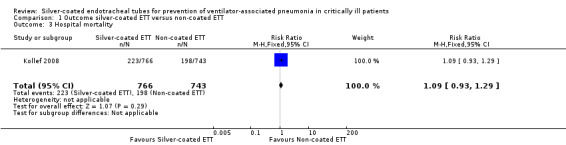
Comparison 1 Outcome silver‐coated ETT versus non‐coated ETT, Outcome 3 Hospital mortality.
Secondary outcomes
Device‐related adverse events
Two studies with a total of 2081 participants reported device‐related adverse events (Kollef 2008; Rello 2006). As Kollef 2008 reported all intubated participants for this outcome, it was not possible to include participants who were only intubated for 24 hours or longer for this outcome. Device‐related adverse events that were observed were cough, sore throat, dysphagia, laryngitis, discoloured tongue, glottis oedema, device malfunction, and abnormal breathing sounds. According to our meta‐analysis, there was no statistically significant difference observed between silver‐coated ETT and non‐coated ETT regarding device‐related adverse events (1.8% versus 2.8%, RR 0.65, 95% CI 0.37 to 1.16; P value = 0.14; I² statistic = 0) (Analysis 1.4).
1.4. Analysis.
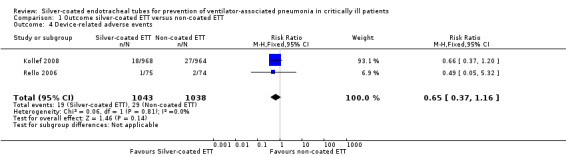
Comparison 1 Outcome silver‐coated ETT versus non‐coated ETT, Outcome 4 Device‐related adverse events.
Duration of intubation
Only one study reported duration of intubation in 1509 participants who received mechanical ventilation for 24 hours or longer (Kollef 2008). We observed no between‐group differences with regards to duration of intubation (4.0 days, interquartile range (IQR) 1.9 to 7.9 days; P value = 0.59). The overall duration of intubation in this study was less than 10 days.
Length of ICU and hospital stay
One study reported length of hospital stay involving 1509 participants who were intubated for 24 hours or longer (Kollef 2008). Silver‐coated ETT was not associated with a significant reduction with regards to length of hospital stay (silver‐coated ETT of 16 days (IQR 10.0 to 26.0 days) versus non‐coated ETT of 16 days (IQR 10.0 to 27.0 days); P value = 0.57) and ICU stay (both groups: 8.0 days IQR 4.0 to 14.0 days; P value = 0.92) when compared with non‐coated ETT.
Time to VAP onset
One study involving 1509 participants who were intubated for 24 hours or longer reported time to VAP onset using a risk factor‐adjusted Cox regression analysis (Kollef 2008). The study showed that silver‐coated ETT was associated with a delayed time to VAP occurrence compared with non‐coated ETT (hazard ratio 0.55, 95% CI 0.37 to 0.84; P value = 0.006).
Cost‐effectiveness of silver‐coated ETTs
We included no studies with regards to cost‐effectiveness of silver‐coated ETTs.
Discussion
Summary of main results
This systematic review examined the clinical effectiveness of silver‐coated ETTs with regards to risk reduction of VAP and hospital mortality in comparison to standard non‐coated ETTs in participants who required mechanical ventilation for 24 hours or longer. Secondary outcome measures were device‐related adverse events, duration of intubation, cost‐effectiveness of silver‐coated ETTs, length of hospital and ICU stay, and time to VAP onset. We identified two eligible studies with a total of 2081 participants (Kollef 2008; Rello 2006). Both RCTs had the same participant population, namely ICU patients who required ventilation for 24 hours or longer. Despite the low VAP rate in the included study, our systematic review showed that silver‐coated ETT was associated with risk reduction of VAP and time to VAP onset compared with non‐coated ETT (Kollef 2008). Silver‐coated ETT was not associated with reducing the risk of hospital mortality, device‐related adverse events, and length of hospital and ICU stay compared with non‐coated ETT.
Overall completeness and applicability of evidence
One of the key issues in trials that investigate a novel preventive strategy in the ICU is how the care of the participants in the control group were managed and which standard safety measures were utilized for preventing VAP. Both RCTs included participants who were mechanically ventilated for 24 hours or longer with either silver‐coated ETTs or non‐coated ETTs. The vast majority of participants who were intubated for 24 hours or longer were either intubated at the ICU, operating or recovering rooms. Most of the data we obtained were from one study (Kollef 2008). Kollef 2008 used clinical criteria for diagnosing VAP, including assessing quantitative culture results of distal respiratory secretion obtained by bronchoalveolar lavage. New radiographically confirmed infiltrate with qualifying clinical signs were triggers for conducting bronchoalveolar lavage. Kollef 2008 did not standardize prevention strategies at the clinical sites that participated in the study. This could potentially have introduced bias in favour of the clinical sites that had a high standard of VAP prevention strategies in combination with silver‐coated ETT utilization. The observed risk ratio of VAP in the included study was 7.5% in the control group, whereas up to 15% was predicted from the target sample size for detecting between‐group differences for VAP (Klompas 2008; Kollef 2008). This could potentially downgrade the estimate of treatment effect for all interested outcomes. This also could potentially have led to inadequate sample size for the interested outcomes. The results for these outcomes should therefore be taken with caution. Furthermore, the study investigators did not examine which antibiotics were given to treat VAP, thus making it unclear whether the participants who were randomized to receive silver‐coated ETTs indeed received fewer antibiotics during the trial period compared with the control group. This could potentially have been the situation if the silver‐coated ETT was effectively to reduce the risk of VAP. Due to the proper design of the RCTs with regards to our interested participants, interventions, and outcomes, the results are definitely applicable to general ICU patients who are mechanically ventilated for 24 hours or longer. We excluded one cost‐effectiveness analysis study from this review because of the study design (Shorr 2009). However, it is interesting to note that this simulation study, which used a simple decision model to investigate the economical endpoints regarding VAP prevention, showed a cost savings of USD12,840 per case of VAP in favour of silver‐coated ETT. Although we included a study with a relative large patient cohort (Kollef 2008), the generalizability of our results is potentially limited due to the fact that we obtained data from only two studies for the outcomes of interest (Kollef 2008; Rello 2006).
Quality of the evidence
The methodological quality with regards to the published studies was moderate. However, we did have some concerns about detection and performance bias in both studies. It was not possible to blind the ICU investigators, which could potentially have initiated bias in favour of silver‐coated ETT. One included study that did not present any outcome data may potentially have publication bias. Both Kollef 2008 and Rello 2006 were funded but stated that the sponsors only contributed to the study design and did not participate in the conduct of the study. A statistically significant imbalance (P value = 0.007) was observed in Kollef 2008 with regards to the number of participants with pre‐existing chronic obstructive pulmonary disease in the control group, thus favouring the intervention group, which received the silver‐coated ETT (Kollef 2008). We deducted one point for all interested outcomes in the 'Summary of findings' table for risk of bias for these reasons. It should also be noted that the primary outcomes (the overall risk of VAP and the risk of VAP within 10 days of intubation) have wide NNTB confidence intervals, thus downgrading the estimate of treatment effect for these outcomes. Moreover, it should be noted that the current evidence regarding hospital mortality and device‐related adverse events is limited due to the relatively large confidence interval, thus lacking precision as to the direction of effect. For this reason, we deducted one point for imprecision in the 'Summary of findings' table for all the interested outcomes. Due to limited study data, it was not possible to include only participants who were intubated for 24 hours or longer with regard to device‐related adverse events. The included studies reported all intubated participants for this outcome, thus increasing the risk for bias (Kollef 2008; Rello 2006). Experts in the field did not know of any unpublished studies on this topic. However, we detected one unpublished study, which we included in our review. In general, we considered the findings of our review to be valid.
Potential biases in the review process
With the assistance of the Cochrane Anaesthesia, Critical and Emergency Care Group’s Trials Search Co‐ordinator, we conducted a comprehensive search strategy. We are confident that we have identified all relevant studies that compared the clinical outcome assessment of silver‐coated ETT versus non‐coated ETT. We were unable to conduct a funnel plot analysis or assess publication bias because we identified fewer than 10 studies for any interested outcome. However, there may be bias in this review from the author side due to foreknowledge we had concerning the included studies when we changed the primary and secondary outcomes. We have provided additional details in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
Li 2012 systematically reviewed the effects of silver‐coated ETT versus non‐coated ETT with regards to the risk of VAP and hospital mortality in adult participants. The objectives and methods were generally the same as in our review. They included the risk of VAP, hospital mortality, microbiologic burden, modified clinical pulmonary infection score, and device‐related adverse events as interested outcome measurements, while we included several other clinically important outcomes including duration of intubation, length of hospital and ICU stay, costs, and time to VAP onset. They searched the Chinese Biomedical Literature Database, while we searched EBSCO CINAHL and other additional websites mentioned in Search methods for identification of studies. Our search date and search strategy were more recently updated, and we also contacted experts in the field for additional information. Their review identified and included the same RCTs that we included in our systematic review; however, our review went into more depth concerning potential biases in the included studies. Their conclusion was generally consistent with our conclusion regarding the effect of silver‐coated ETT on the risk of VAP and hospital mortality. However, we found no statistically significant difference between silver‐coated ETT and non‐coated ETT with regards to device‐related adverse events, contradicting their results. The reason for this difference is that we did not treat count data as dichotomous data. We divided the number of participants in the intervention or control group over the total number of participants for an interested outcome, while Li 2012 divided the events that occurred for an interested outcome over the total number of participants, thus making the result and conclusion for this interested outcome in the review of Li 2012 inconclusive. Moreover, we went into more depth regarding recommendations for future trials.
Authors' conclusions
Implications for practice.
Silver‐coated ETT seems to show a significant reduction with regards to the risk of VAP, especially during the first 10 days of mechanical ventilation in participants who require mechanical ventilation for 24 hours or longer. This finding is of clinical importance because the peak time of a VAP event in mechanically ventilated patients is usually within 10 days after intubation. In addition, silver‐coated ETT reduces time to VAP onset. However, due to the low quality of evidence, it is possible that the findings of this review could merely be a reflection of random error. Also, due to the fact that confidence intervals were insufficiently narrow to rule out an important magnitude of effect, there is a lack of evidence of effect regarding other important clinical outcomes, including hospital mortality, duration of intubation, and length of ICU and hospital stay. We did not have enough evidence in this review to draw conclusions about these important clinical outcomes.
Implications for research.
Further research should focus on the clinical effectiveness of silver‐coated ETT in high‐risk patients. Also, a thorough investigation should be conducted on whether silver‐coated ETTs are effective in people who require prolonged mechanical ventilation (longer than seven days). These people may develop late‐onset infections that are particularly challenging with regards to treatment and prevention. Silver‐coated ETTs should also be compared with other anti‐VAP strategies such as chlorhexidine/selective decontamination or chlorhexidine‐coated ETTs. A prospective cost‐effectiveness trial of silver‐coated ETTs is advisable to present the true cost‐effectiveness of these novel devices. In future trials, investigators should rely on the same care bundles in clinical sites where patients will be included in order increase the precision of the estimate of treatment effect. Larger randomized trials should also increase the estimate of the effect of silver‐coated ETTs on patient‐important outcomes and should therefore be conducted.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would also like to thank Arash Afshari (content editor), Nathan Pace (statistical editor), Sujanthy S Rajaram, Steven Deem, Andrew MacDuff (peer reviewers), and Ann E Fonfa (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank the following people who provided assistance with the protocol: Jane Cracknell, Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care Group (ACE), Karen Hovhanisyen Trials Search Co‐ordinator, Mathew Zacharias (content editor), Marialena Trivella (statistical editor), Karen Burns, Steven Deem, Sujanthy Rajaram (peer reviewers), and Janet Wale (CARG consumer panel) for their help and editorial advice during the preparation of the protocol for the review (Tokmaji 2011).
Appendices
Appendix 1. Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Silver explode all trees #2 silver* or (silver near (endotracheal or impregnate* or coat* or tube* or ETT or antimicrob* or reduc*)) #3 (#1 OR #2) #4 (cross infection near (respiration or intubation)) or (ventilator near (pneumonia or infect*)) or VAP or (Infect* near (intubate* or mechanical ventilat* or MV)) or pneumonia or (biofilm near format*) or (bacteria* near (airway and coloni?at*)) #5 MeSH descriptor Pneumonia, Ventilator‐Associated explode all trees #6 MeSH descriptor Costs and Cost Analysis explode all trees #7 MeSH descriptor Cost‐Benefit Analysis explode all trees #8 (#4 OR #5 OR #6 OR #7) #9 (#3 AND #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1 exp Silver/ or silver*.mp. or (silver adj5 (endotracheal or impregnate* or coat* or tube* or ETT or antimicrob* or reduc*)).mp.
2 exp Pneumonia, Ventilator‐Associated/ or (Cross Infection/ and (Respiration, Artificial/ or Intubation, Intratracheal/)) or (ventilator adj3 (pneumonia or infect*)).mp. or VAP.mp. or (Infect* adj3 (intubate* or mechanical ventilat* or MV)).mp. or pneumonia.mp. or (biofilm adj3 format*).mp. or (bacteria* adj3 (airway and coloni?at*)).mp. or exp "Costs and Cost Analysis"/ or exp Cost‐Benefit Analysis/
3 1 and 2
Appendix 3. Search strategy for EMBASE (Ovid SP)
1 exp SILVER/ or silver*.mp. or (silver adj5 (endotracheal or impregnate* or coat* or tube* or ETT or antimicrob* or reduc*)).mp. 2 exp ventilator associated pneumonia/ or (cross infection/ and (artificial ventilation/ or endotracheal intubation/)) or (ventilator adj3 (pneumonia or infect*)).mp. or VAP.mp. or (Infect* adj3 (intubate* or mechanical ventilat* or MV)).mp. or pneumonia.mp. or (biofilm adj3 format*).mp. or (bacteria* adj3 (airway and coloni?at*)).mp. or exp "COST"/ or exp "COST BENEFIT ANALYSIS"/ 3 1 and 2
Appendix 4. Search strategy for CINAHL (EBSCO host)
S1 (MM "Silver") S2 TX silver and TX ( endotracheal or impregnate* or coat* or tube* or ETT or antimicrob* or reduc* ) S3 S1 or S2 S4( MM "Pneumonia, Ventilator‐Associated") OR (MM "Costs and Cost Analysis") OR (MM "Cost Benefit Analysis") S5 cross infection and ( respiration or intubation ) S6 ventilator and ( pneumonia or infect* ) S7 AB VAP or AB pneumonia S8 TX Infect* and TX ( intubate* or mechanical ventilat* or MV ) S9 TX biofilm and TX format* S10 TX bacteria* and TX ( airway and coloni?at* ) S11 S4 or S5 or S6 or S7 or S8 or S9 or S10 S12 S3 and S11
Appendix 5. Data extraction form
| Part 1: Review, Review Author and Study Information | ||||||||
| Review title: | ||||||||
| Review author: | ||||||||
| Date of completion of this form: | Year / Month / Day | |||||||
| Study ID: | ||||||||
| Author(s): | ||||||||
| Title of Report: | ||||||||
| Year of publication: | ||||||||
| Source: | ||||||||
| Language of publication: | English / Dutch / French / German / Other (specify) | |||||||
| Type of report: | Full paper / Abstract / Unpublished | |||||||
| Number of centres: | Single centre / Multicentre (if multicentre, number of centres) | |||||||
| Date trial was conducted: | ||||||||
| Country where study performed: | ||||||||
| Funders of the trial: | ||||||||
| Study found in: | Electronic databases / Trials registries / Manual searches of conference proceedings | |||||||
| Unpublished data: | Yes / No | |||||||
| Part 2: Eligibility of Study for Review | ||||||||
| Study Type | ||||||||
| A randomized controlled trial: | Yes / No / Unclear | |||||||
| Type of randomization: | ||||||||
| Study Participants | ||||||||
| (critically ill) adults: | Yes / No / Unclear | |||||||
| Invasively ventilated: | Yes / No / Unclear | |||||||
| Study Intervention | ||||||||
| Comparison between silver‐coated ETTs or a combination of silver with any antimicrobial‐coated ETTs with standard non‐coated ETTs or with other antimicrobial‐coated ETTs such as chlorhexidine‐coated ETTs. | Yes / No / Unclear | |||||||
| Outcome | ||||||||
| Primary Outcome: | ||||||||
| The study reports the following outcomes: | ||||||||
| Number of participants with VAP: | Yes / No / Unclear | |||||||
| Hospital mortality: | Yes / No / Unclear | |||||||
| Secondary Outcome: | ||||||||
| Device‐related adverse events: | Yes / No / Unclear | |||||||
| Cost‐effectiveness of the silver‐coated ETTs (author's definitions): | Yes / No / Unclear | |||||||
| Length of hospital and ICU stay: | Yes / No / Unclear | |||||||
| Time to VAP onset: | Yes / No / Unclear | |||||||
| Review Exclusion Criteria | ||||||||
| The study includes: | ||||||||
| Children under 16 years: | Yes / No / Unclear | |||||||
| Participants who are re‐intubated: | Yes / No / Unclear | |||||||
| Participants requiring planned short‐term ventilation (< 24 hours): | Yes / No / Unclear | |||||||
| Part 3: Description of the Study | ||||||||
| Characteristics of the Participants | ||||||||
| Study inclusion criteria: | ||||||||
| Study exclusion criteria: | ||||||||
| Intervention group | Control group | TOTAL | ||||||
| Number of participants: | ||||||||
| Mean age (SD) (range): | ||||||||
| Sex (F/M): | ||||||||
| Participants excluded from the study before randomization: | Number: | Number: | Number: | |||||
| Reason: | Reason: | |||||||
| Participants excluded from the study after randomization: | Number: | Number: | Number: | |||||
| Reason: | Reason: | |||||||
| Withdrawals: | Number: | Number: | Number: | |||||
| Reason: | Reason: | |||||||
| Length of follow‐up: | Number: | Number: | Number: | |||||
| Unit of randomization: | Participant / endotracheal tube / other | |||||||
| Types of Interventions | ||||||||
| The intervention group: | ||||||||
| The control group: | ||||||||
| Setting of intervention: | Emergency department / Operating theatre / ICU / Other (specify) | |||||||
| Intervention group | Control group | |||||||
| Antibiotics given: | Yes / No / Unclear | Yes / No / Unclear | ||||||
| If yes, mention the duration of antibiotics given in hours: | ||||||||
| Continuous Outcomes | ||||||||
| Intervention group | Control group | 95% CI or additional information | ||||||
| Outcomes | n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | P value | |
| Primary Outcome: | ||||||||
| Participants with VAP: | ||||||||
| Hospital mortality: | ||||||||
| Other (specify): | ||||||||
| Secondary Outcome: | ||||||||
| Device‐related adverse events: | ||||||||
| Length of hospital and ICU stay: | ||||||||
| Cost‐effectiveness of the silver‐coated ETTs: | ||||||||
| Time to VAP onset: | ||||||||
| Other (specify): | ||||||||
| Dichotomous Outcomes | ||||||||
| Intervention group | Control group | |||||||
| Outcomes | n | n | P value | Risk difference (RD) | Number needed to treat for an additional beneficial outcome (NNTB) | Risk Ratio | Additional information | |
| Primary Outcome: | ||||||||
| Participants with VAP: | ||||||||
| Hospital mortality: | ||||||||
| Other (specify): | ||||||||
| Secondary Outcome: | ||||||||
| Device‐related adverse events: | ||||||||
| Length of hospital and ICU stay: | ||||||||
| Cost‐effectiveness of the silver‐coated ETTs: | ||||||||
| Time to VAP onset: | ||||||||
| Other (specify): | ||||||||
| Scale/Measure (Definition/Description of How It Was Measured) | ||||||||
| Hospital mortality: | ||||||||
| Device‐related adverse events: | ||||||||
| Cost‐effectiveness of the silver‐coated ETTs: | ||||||||
| Other (specify): | ||||||||
| Date:___/___/___ | Review author‘s signature | |||||||
Appendix 6. Risk of bias assessment
| Risk of bias assessment (RoB assessment) | ||
| Entry | Judgement | Support for judgement |
| 1. Random sequence generation (selection bias) | Low risk / High risk / Unclear risk | Quote: |
| 2. Allocation concealment (selection bias) | Low risk / High risk / Unclear risk | Quote: |
| 3. Blinding of participants and personnel (performance bias) | Low risk / High risk / Unclear risk | Quote: |
| 4. Blinding of outcome assessment (detection bias) (patient‐reported outcomes; hospital mortality) | Low risk / High risk / Unclear risk | Quote: |
| 5. Incomplete outcome data | Low risk / High risk / Unclear risk | Quote: |
| Date:___/___/___ | Review author‘s signature: | |
Data and analyses
Comparison 1. Outcome silver‐coated ETT versus non‐coated ETT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of VAP at any time in patients intubated for ≥ 24 hours | 1 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.96] |
| 2 Risk of VAP within 10 days of intubation in patients intubated for ≥ 24 hours | 1 | 1509 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.31, 0.82] |
| 3 Hospital mortality | 1 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.29] |
| 4 Device‐related adverse events | 2 | 2081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kollef 2008.
| Methods | Mulitcentre, prospective, randomized, single‐blind, controlled study | |
| Participants | Setting: 54 centres throughout North America (Canada & USA). Inclusion criteria: Adults at least 18 years old eligible for enrolment if they were expected to require mechanical ventilation with an endotracheal tube for 24 hours or longer Exclusion criteria: Participation in another study that conflicted with the current study, bronchiectasis, severe or massive haemoptysis, cystic fibrosis, pregnancy, silver sensitivity, and endotracheal intubation for longer than 12 hours within the preceding 30 days Participant numbers: 2003 randomly assigned; 494 excluded (71 not intubated, 423 intubated < 24 h); 1932 all intubated; 1509 intubated ≥ 24 h analysed. 766 silver‐coated ETT versus 743 non‐coated ETT who were intubated for ≥ 24 h | |
| Interventions | Silver‐coated ETT (7 mm to 9 mm internal diameter, high‐volume/low‐pressure) for 3.2 days versus non‐coated ETT (7 mm to 9 mm internal diameter, high‐volume/low‐pressure) for 3.2 days | |
| Outcomes | Risk of VAP; hospital mortality; device‐related adverse events; duration of intubation; time to VAP onset; length of hospital and ICU stay | |
| Notes | No differences were noted between groups in APACHE II scores, use of enteral nutrition, presence of immunodeficiency, or other risk factors for VAP Chronic obstructive pulmonary disease was more common in the group receiving the non‐coated tube (P value = 0.007) Cause of hospital mortality was not specified in this study VAP was diagnosed by clinical and radiographic parameters combined with culture‐positive fluid obtained by BAL. Explicitly, new radiographic confirmed infiltrate with qualifying clinical signs were triggers for conducting quantitative BAL No standardization of prevention strategies at the clinical sites that participated in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization was provided in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment with a series of sequentially numbered envelopes, each containing a randomization card for a study participant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were blinded to block length; microbiology laboratory personnel were blinded to group assignments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blinded study design. The ICU investigators could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Protocol was registered as NCT00148642, and outcomes were reported |
| Other bias | Low risk | None was apparent |
NCT00341354 2007.
| Methods | Unicentre, randomized, controlled study | |
| Participants | Setting: San Gerardo Hospital in Milan, Italy Inclusion criteria: Adults at least 18 years old eligible for enrolment if they were expected to require mechanical ventilation with an endotracheal tube with an internal diameter of 7.5 mm or 8.0 mm for 48 hours or longer Exclusion criteria: Males and females less than 18 years old; patients who are expected to be intubated for less than 48 hours; patients who are allergic to silver‐sulfadiazine; patients who require an endotracheal tube with an internal diameter less than 7.5 mm or greater than 8.0 mm; patients who do not tolerate disconnection from the ventilator; haemodynamically unstable; severe ARDS: PaO2/FiO2 ≤ 200 at PEEP ≤ 5 cmH20 Participant numbers: not mentioned | |
| Interventions | Silver‐sulfadiazine tracheal tubes/mucus shaver in intubated patients expected to have a prolonged mechanical ventilation | |
| Outcomes | No outcome data were available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No outcome data were available to judge this bias |
| Allocation concealment (selection bias) | Unclear risk | No outcome data were available to judge this bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No outcome data were available to judge this bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No outcome data were available to judge this bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No outcome data were available to judge this bias |
| Selective reporting (reporting bias) | Unclear risk | No outcome data were available to judge this bias |
| Other bias | High risk | Possible publication bias |
Rello 2006.
| Methods | Mulitcentre, prospective, randomized, single‐blind, controlled study | |
| Participants | Setting: 4 centres (3 Spain & 1 USA) Inclusion criteria: Mechanical ventilation for 24 hours and 18 years old Exclusion criteria: Respiratory infection, bronchiectasis, haematemesis, haemoptysis, or cystic fibrosis; sensitivity to silver or silver compounds; immunosuppression; and, in the substudy only, intubation within 30 days and pregnancy Participant numbers: 155 randomly assigned, 34 excluded (6 not intubated, 28 intubated < 24 h); 149 all intubated; 121 intubated ≥ 24 h analysed; 61 silver‐coated ETT versus 60 non‐coated ETT who were intubated for ≥ 24 h | |
| Interventions | Silver‐coated ETT (7.5 mm to 8.5 mm internal diameter, high‐volume/low‐pressure) for 7.7 days versus non‐coated ETT (7.5 mm to 8.5 mm internal diameter, high‐volume/low‐pressure) for 5.9 days | |
| Outcomes | Hospital mortality; device‐related adverse events | |
| Notes | Risk of VAP was not reported Cause of hospital mortality was not specified in this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | No details given in manuscript. After contacting study authors: Adequate allocation concealment with central randomization by telephone, with different lists for each institution |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were blinded to block length; microbiology laboratory personnel were blinded to group assignments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blinded study design. The ICU investigators could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0% of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported |
| Other bias | Low risk | None was apparent |
APACHE = Acute Physiology and Chronic Health Evaluation;
ARDS = acute respiratory distress syndrome;
BAL = bronchoalveolar lavage;
ETT = endotracheal tube;
ICU = intensive care unit;
PEEP = positive end‐expiratory pressure;
VAP = ventilator‐associated pneumonia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afessa 2010 | Retrospective cohort analysis |
| Berra 2008 | Randomized controlled trial comparing participants intubated and mechanically ventilated for 12 to 24 hours with silver sulfadiazine NASCENT versus non‐coated ETT. Excluded due to absence of participants who were intubated for 24 hours or longer |
| Morrow 2009 | Prospective non‐randomized study |
| Morrow 2010 | Retrospective study |
| Shorr 2009 | A simple decision model with a hypothetical 1000‐patient cohort cost‐effectiveness analysis based on the NASCENT trial. Excluded due to the study design (hypothetical 1000‐patient cohort) |
ETT = endotracheal tube
NASCENT = North American Silver‐Coated Endotracheal Tube.
Differences between protocol and review
Differences between published protocol (Tokmaji 2011) and review
1. We changed the title from: "Silver coated endotracheal tubes for prevention of ventilator‐associated pneumonia in critically ill patients" to "Silver‐coated endotracheal tubes for prevention of ventilator‐associated pneumonia in critically ill patients". The correct phrase is "silver‐coated" and not "silver coated".
2. Types of outcome measures: We included length of intensive care unit (ICU) stay because it is a clinically relevant outcome and it was mentioned in the included study (Kollef 2008).
3. Types of outcome measures: We changed one of the primary endpoints from "mortality (defined as early (less than 30 days) or late (more than 30 days))" to "hospital mortality", as we did not want to limit the definition of hospital mortality to early and late.
4. Types of outcome measures: Upon reviewing the studies, it became apparent that "duration of intubation" was a more correct term than "duration of mechanical ventilation". Corresponding authors explained that they measured time of exposure to the endotracheal tubes, and therefore coded time of intubation and extubation. They explained that this is essentially the time on mechanical ventilation. However, it is possible that a participant could have been intubated for a spontaneous breathing trial for six to eight hours prior to extubation. This could have resulted in a minor discrepancy between these terms, but was unlikely.
5. Types of outcome measures: We included clinical cost‐effectiveness analysis studies based on real patient cohort in this review because we believe that this study type is a legitimate method to answer the question regarding the cost‐effectiveness of silver‐coated ETT compared with non‐coated ETT in VAP prevention.
6. Table 1: Upon reviewing the studies, we changed the outcomes in the 'Summary of findings' table. The new outcomes are generally the same as the previous outcomes but are more detailed. We excluded "time to VAP onset" because the studies provided no additional details to include this outcome in the 'Summary of findings' table.
We changed Types of outcome measures from:
Risk rate of VAP;
Mortality (defined as early (< 30 days) or late (> 30 days));
Adverse events (authors’ definitions);
Time to VAP onset.
to:
Risk of VAP at any time in participants intubated for ≥ 24 hours;
Risk of VAP within 10 days of intubation in participants intubated for ≥ 24 hours;
Hospital mortality;
Device‐related adverse events.
7. The small number of studies included in this review limited our ability to:
Perform subgroup and sensitivity analyses;
Construct funnel plots to assess reporting biases.
Contributions of authors
George Tokmaji (GT), Hester Vermeulen (HV), Marcella CA Müller (MM), Paulus HS Kwakman (PK), Marcus J Schultz (MS), Sebastian AJ Zaat (SZ)
Conceiving the review: GT
Co‐ordinating the review: GT
Undertaking manual searches: GT
Screening search results: GT, PK; Arbiter: SZ
Organizing retrieval of papers: GT
Screening retrieved papers against inclusion criteria: GT, SZ; Arbiter: PK
Appraising the quality of papers: GT, SZ; Arbiter: PK
Abstracting data from papers: GT, SZ; Arbiter: PK
Writing to authors of papers for additional information: GT
Providing additional data about papers: GT, HV, SZ
Obtaining and screening data on unpublished studies: GT
Data management for the review: GT, SZ, HV
Entering data into Review Manager (RevMan 5.3): GT, SZ
RevMan statistical data: GT, HV
Other statistical analysis not using RevMan: GT, HV, MS
Double entry of data: GT, HV
Interpretation of data: GT, SZ, PK, HV, MS, MM
Statistical inferences: GT, SZ, MS, MM
Writing the review: GT, SZ, HV
Securing funding for the review: GT
Performing previous work that was the foundation of the present study: GT
Guarantor for the review (one review author): HV
Person responsible for reading and checking review before submission: GT, HV, SZ
Sources of support
Internal sources
Infrastructure supplied by the hospital, Academic Medical Center at the University of Amsterdam, Netherlands.
External sources
No sources of support supplied, Other.
Declarations of interest
George Tokmaji: None known.
Hester Vermeulen: None known.
Marcella CA Müller: None known.
Paulus HS Kwakman: 'Zon MW Vemi' grant from the Netherlands Organisation for Scientific Research for a clinical trial on the prevention of catheter insertion risk colonization using medical‐grade honey.
Marcus J Schultz: None known.
Sebastian AJ Zaat: Monies paid to institution; grants: Technologisch Topinstituut BioMedical Materials (BMM), funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation: Projects: (i) BMM grant P4.01 NANTICO; Non‐adherent ANTIbacterial COatings: http://www.bmm‐program.nl/SITE/PUBLIC/GO/article.aspx?id=89&title=Anti‐bacterial+coatings
(ii) BMM grant P5.03 IBIZA: Imaging of Biomaterial‐associated Inflammation and Infection using Zebrafish Analysis
http://www.bmm‐program.nl/SITE/PUBLIC/GO/article.aspx?id=91&title=Imaging+biocompatibility
(iii) European Commission seventh framework programme grant 278890 BALI: Biofilm alliance: http://www.bali‐consortium.eu.
Edited (no change to conclusions)
References
References to studies included in this review
Kollef 2008 {published data only}
- Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, et al. Silver‐coated endotracheal tubes and incidence of ventilator‐associated pneumonia. The NASCENT Randomized Trial. JAMA 2008;300(7):805‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00341354 2007 {unpublished data only}
- National Heart, Lung, and Blood Institute (NHLBI). Evaluation of silver‐sulfadiazine tracheal tubes/mucus shaver in intubated patients expected to have a prolonged mechanical ventilation. Clinicaltrials.gov; NCT00341354 June 19, 2006.
Rello 2006 {published data only}
- Rello J, Kollef M, Diaz E, Sandiumenge A, Castillo Y, Corbella X, et al. Reduced burden of bacterial airway colonization with a novel silver‐coated endotracheal tube in a randomized multiple‐center feasibility study. Critical Care Medicine 2006;34(11):2766‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Afessa 2010 {published data only}
- Afessa B, Shorr AF, Anzueto AR, Craven DE, Schinner R, Kollef MH. Association between a silver‐coated endotracheal tube and reduced mortality in patients with ventilator‐associated pneumonia. Chest 2010;2010(5):1015‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berra 2008 {published data only}
- Berra L, Kolobow T, Laquerriere P, Pitts B, Bramati S, Pohlmann J, et al. Internally coated endotracheal tubes with silver sulfadiazine in polyurethane to prevent bacterial colonization: a clinical trial. Intensive Care Medicine 2008;34(6):1030‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morrow 2009 {published data only}
- Morrow LE, Jarrett JE, Malesker MA, Kollef MH. Systematic use of a silver‐coated endotracheal tube reduces rates of ventilator‐associated pneumonia .. American Journal of Respiratory and Critical Care Medicine. San Diego Convention Center: American Thoracic Society International Conference, May 15‐20; 2009.
Morrow 2010 {published data only}
- Morrow LE, Mintz EP, Malesker MA. Effect of a silver‐coated endotracheal tube on ventilator‐associated pneumonia and medical resource utilization in clinical practice. Fifth Decennial International Conference on Healthcare‐Associated Infections. Critical Care Medicine. Miami Beach, FL: Society of Critical Care Medicine, March 18‐22; 2010:388.
Shorr 2009 {published data only}
- Shorr AF, Zilberberg MD, Kollef M. Cost‐effectiveness analysis of a silver‐coated endotracheal tube to reduce the incidence of ventilator‐associated pneumonia. Infection Control and Hospital Epidemiology 2009;30(8):759‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Adair 1999
- Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, et al. Implications of endotracheal tube biofilm for ventilator‐associated pneumonia. Intensive Care Medicine 1999;25(10):1072‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahearn 2000
- Ahearn DG, Grace DT, Jennings MJ. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infections. Current Microbiology 2000;41(2):120‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Babcock 2004
- Babcock HM, Zack JE, Garrison T. An educational intervention to reduce ventilator‐associated pneumonia in an integrated health system: a comparison of effects. Chest 2004;125(6):2224‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balazs 2004
- Balazs DJ, Triandafillu K, Wood P, Chevolot Y, Delden C, Harms H, et al. Inhibition of bacterial adhesion on PVC endotracheal tubes by RF‐oxygenglow discharge, sodium hydroxide and silver nitrate treatments. Biomaterials 2004;25(11):2139–51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berra 2004
- Berra L, Marchi L, Yu ZX, Laquerriere P. Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs, and endotracheal tube. Anesthesiology 2004;100(6):1446‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berra 2006
- Berra L, Curto F, Li Bassi G, Laquerriere P, Baccarelli A, Kolobow T. Antibacterial‐coated tracheal tubes cleaned with the Mucus Shaver: a novel method to retain long‐term bactericidal activity of coated tracheal tubes. Intensive Care Medicine 2006;32(6):888–93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bregeon 2001
- Bregeon F, Ciais V, Carret V. Is ventilator‐associated pneumonia an independent risk factor for death?. Anesthesiology 2001;94(4):554‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chaiban 2005
- Chaiban G, Hanna H, Dvorak T, Raad I. A rapid method of impregnating endotracheal tubes and urinary catheters with gendine: a novel antiseptic agent. Journal of Antimicrobial Chemotherapy 2005;55(1):51–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chastre 2002
- Chastre J, Fagon JY. Ventilator‐associated pneumonia. American Journal of Respiratory and Critical Care Medicine 2002;165(7):867–903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collard 2003
- Collard HR, Saint S, Matthay MA. Prevention of ventilator‐associated pneumonia: an evidence‐based systematic review. Annals of Internal Medicine 2003;138(6):494‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cook 1998a
- Cook DJ, Walter SD, Cook RJ. Incidence of and risk factors for ventilator‐associated pneumonia in critically ill patients. Annals of Internal Medicine 1998;129(6):433‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cook 1998b
- Cook DJ, Kollef MH. Risk factors for ICU‐acquired pneumonia. JAMA 1998;279(20):1605‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cook 2000
- Cook D, Ricard JD, Reeve B. Ventilator circuit and secretion management strategies: a Franco‐Canadian survey. Critical Care Medicine 2000;28(10):3547‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Costerton 2003
- Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. Journal of Clinical Investigation 2003;112(10):1466‐77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craven 1995
- Craven DE, Steger KA. Epidemiology of nosocomial pneumonia: new perspectives on an old disease. Chest 1995;108 Suppl(2):1‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Craven 2006
- Craven DE. Preventing ventilator‐associated pneumonia in adults: sowing seeds of change. Chest 2006;130(1):251‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jonge 2003
- Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. The Lancet 2003;362(9389):1011‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esteban 2000
- Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1450‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteban 2002
- Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA 2002;287(3):345‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feng 2000
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Materials Research 2000;52(4):662‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flemming 1990
- Flemming CA, Ferris FG, Beveridge TJ, Bailey GW. Remobilization of toxic heavy metals adsorbed to bacterial wall‐clay composites. Applied and Environmental Microbiology 1990;56(10):3191‐203. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 2005
- Freeman BD, Borecki IB, Coopersmith CM, Buchman TG. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Critical Care Medicine 2005;33(11):2513‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gabriel 1995
- Gabriel MM, Sawant AD, Simmons RB, Ahearn. Effects of silver on adherence of bacteria to urinary catheters: in vitro studies. Current Microbiology 1995;30(1):17‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girou 2000
- Girou E, Schortgen F, Delclaux C, Brun‐Buisson C, Blot F, Lefort Y, et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA 2000;284(18):2361‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336(7651):995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 1999
- Hartmann M, Guttmann J, Muller B, Hallmann T, Geiger K. Reduction of the bacterial load by the silver‐coated endotracheal tube (SCET), a laboratory investigation. Technology and Health Care 1999;7(5):359–70. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557–60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hubmayr 2002
- Hubmayr RD, Burchardi H, Elliot M, Fessler H, Georgopoulos D, Jubran A, et al. Statement of the 4th International Consensus Conference in Critical Care on ICU‐Acquired Pneumonia. Intensive Care Medicine 2002;28(11):1521‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Illingworth 1998
- Illingworth B, Tweden K, Schroeder R, Cameron JD. In vivo efficacy of silver‐coated (Silzone) infection‐resistant polyester fabric against a biofilm‐producing bacteria, Staphylococcus epidermidis. Journal of Heart Valve Disease 1998;7(5):524–30. [MEDLINE: ] [PubMed] [Google Scholar]
Inglis 1989
- Inglis TJ, Millar MR, Jones JG, Robinson DA. Tracheal tube biofilm as a source of bacterial colonization of the lung. Journal of Clinical Microbiology 1989;27(9):2014‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2003
- Jones DS, McMeel S, Adair CG, Gorman SP. Characterisation and evaluation of novel surfactant bacterial anti‐adherent coatings for endotracheal tubes designed for the prevention of ventilator‐associated pneumonia. Journal of Pharmacy and Pharmacology 2003;55(1):43–52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klompas 2008
- Klompas M. Silver‐coated endotracheal tubes and patient outcomes in ventilator‐associated pneumonia. JAMA 2008;300(22):2605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kollef 1997
- Kollef MH, Sharpless L, Vlasnik J, Plasque C, Murphy D, Fraser VJ. The impact of nosocomial infections on patient outcomes following cardiac surgery. Chest 1997;112(3):666‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kollef 1999
- Kollef MH. Epidemiology and risk factors for nosocomial pneumonia. Clinics in Chest Medicine 1999;20(3):653‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kollef 2004
- Kollef MH. Prevention of hospital‐associated pneumonia and ventilator‐associated pneumonia. Critical Care Medicine 2004;32(6):1396‐405. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kollef 2005a
- Kollef MH. What is ventilator‐associated pneumonia and why is it important?. Respiratory Care 2005;50(6):714‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Kollef 2005b
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ. Epidemiology and outcomes of health‐care‐associated pneumonia: results from a large US database of culture‐positive pneumonia. Chest 2005;128(6):3854‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2012
- Li X, Yuan Q, Wang L, Du L, Deng L. Silver‐coated endotracheal tube versus non‐coated endotracheal tube for preventing ventilator‐associated pneumonia among adults: a systematic review of randomized controlled trials. Journal of Evidence‐Based Medicine 2012;5(1):25‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liau 1997
- Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Letters in Applied Microbiology 1997;25(4):279‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Magner 1992
- Magner LN. Hippocrates and the Hippocratic tradition. A History of Medicine. 1st Edition. Marcel Dekker, 1992:66‐8. [9780824786731] [Google Scholar]
Mayhall 1997
- Mayhall CG. Nosocomial pneumonia, diagnosis and prevention. Infectious Disease Clinics of North America 1997;11(2):427‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NNIS 2004
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report. American Journal of Infection Control 2004;32(8):470‐85. [DOI] [PubMed] [Google Scholar]
Olson 2002
- Olson ME, Harmon BG, Kollef MH. Silver‐coated endotracheal tubes associated with reduced bacterial burden in the lungs of mechanically ventilated dogs. Chest 2002;121(3):863–70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pacheco‐Fowler 2004
- Pacheco‐Fowler V, Gaonkar T, Wyer PC, Modak S. Antiseptic impregnated endotracheal tubes for the prevention of bacterial colonization. Journal of Hospital Infection 2004;57(2):170–4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Petering 1976
- Petering HG. Pharmacology and toxicology of heavy metals: silver. Pharmacology & Therapeutics 1976;1(2):127‐30. [Google Scholar]
Rello 2002a
- Rello J, Ollendorf DA, Oster G. Epidemiology and outcomes of ventilator‐associated pneumonia in a large US database. Chest 2002;122(6):2115‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rello 2002b
- Rello J, Lorente C, Bodi M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evidence‐based guidelines for preventing ventilator‐associated pneumonia? A survey based on the opinions of an international panel of intensivists. Chest 2002;122(2):656‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rello 2010
- Rello J, Afessa B, Anzueto A, Arroliga AC, Olson ME, Restrepo MI, , et al. Activity of a silver‐coated endotracheal tube in preclinical models of ventilator‐associated pneumonia and a study after extubation. Critical Care Medicine 2010;38(4):1135‐40. [PUBMED: 20081533] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 1994
- Russell AD, Hugo WB. Antimicrobial activity and action of silver. Progress in Medicinal Chemistry 1994;31:351‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Safdar 2005
- Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator‐associated pneumonia: a systematic review. Critical Care Medicine 2005;33(10):2184‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sottile 1986
- Sottile FD, Marrie TJ, Prough DS, Hobgood CD, Gower DJ, Webb LX, et al. Nosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubes. Critical Care Medicine 1986;14(4):265‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Thompson 1973
- Thompson S, Bailey JC. Comprehensive Inorganic Chemistry. Oxford, UK: Pergamon Press, 1973. [Google Scholar]
Trouillet 1998
- Trouillet JL, Chastre J, Vuagnat A. Ventilator associated pneumonia caused by potentially drug‐resistant bacteria. American Journal of Respiratory and Critical Care Medicine 1998;157(2):531‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vincent 1995
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas‐Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA 1995;274(8):639–44. [MEDLINE: ] [PubMed] [Google Scholar]
Zack 2002
- Zack JE, Garrison T, Trovillion E. Effect of an education program aimed at reducing the occurrence of ventilator‐associated pneumonia. Critical Care Medicine 2002;30(11):2407‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tokmaji 2011
- Tokmaji G, Vermeulen H, Müller MCA, Kwakman PHS, Schultz MJ, Zaat SAJ. Silver coated endotracheal tubes for prevention of ventilator‐associated pneumonia in critically ill patients. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009201] [DOI] [PMC free article] [PubMed] [Google Scholar]


