Abstract
Background
Mechanical ventilation is a critical component of paediatric intensive care therapy. It is indicated when the patient’s spontaneous ventilation is inadequate to sustain life. Weaning is the gradual reduction of ventilatory support and the transfer of respiratory control back to the patient. Weaning may represent a large proportion of the ventilatory period. Prolonged ventilation is associated with significant morbidity, hospital cost, psychosocial and physical risks to the child and even death. Timely and effective weaning may reduce the duration of mechanical ventilation and may reduce the morbidity and mortality associated with prolonged ventilation. However, no consensus has been reached on criteria that can be used to identify when patients are ready to wean or the best way to achieve it.
Objectives
To assess the effects of weaning by protocol on invasively ventilated critically ill children. To compare the total duration of invasive mechanical ventilation of critically ill children who are weaned using protocols versus those weaned through usual (non‐protocolized) practice. To ascertain any differences between protocolized weaning and usual care in terms of mortality, adverse events, intensive care unit length of stay and quality of life.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 10, 2012), MEDLINE (1966 to October 2012), EMBASE (1988 to October 2012), CINAHL (1982 to October 2012), ISI Web of Science and LILACS. We identified unpublished data in the Web of Science (1990 to October 2012), ISI Conference Proceedings (1990 to October 2012) and Cambridge Scientific Abstracts (earliest to October 2012). We contacted first authors of studies included in the review to obtain further information on unpublished studies or work in progress. We searched reference lists of all identified studies and review papers for further relevant studies. We applied no language or publication restrictions.
Selection criteria
We included randomized controlled trials comparing protocolized weaning (professional‐led or computer‐driven) versus non‐protocolized weaning practice conducted in children older than 28 days and younger than 18 years.
Data collection and analysis
Two review authors independently scanned titles and abstracts identified by electronic searching. Three review authors retrieved and evaluated full‐text versions of potentially relevant studies, independently extracted data and assessed risk of bias.
Main results
We included three trials at low risk of bias with 321 children in the analysis. Protocolized weaning significantly reduced total ventilation time in the largest trial (260 children) by a mean of 32 hours (95% confidence interval (CI) 8 to 56; P = 0.01). Two other trials (30 and 31 children, respectively) reported non‐significant reductions with a mean difference of ‐88 hours (95% CI ‐228 to 52; P = 0.2) and ‐24 hours (95% CI ‐10 to 58; P = 0.06). Protocolized weaning significantly reduced weaning time in these two smaller trials for a mean reduction of 106 hours (95% CI 28 to 184; P = 0.007) and 21 hours (95% CI 9 to 32; P < 0.001). These studies reported no significant effects for duration of mechanical ventilation before weaning, paediatric intensive care unit (PICU) and hospital length of stay, PICU mortality or adverse events.
Authors' conclusions
Limited evidence suggests that weaning protocols reduce the duration of mechanical ventilation, but evidence is inadequate to show whether the achievement of shorter ventilation by protocolized weaning causes children benefit or harm.
Plain language summary
The usefulness of protocols for reducing the time children spend mechanically ventilated in the intensive care unit
In a children’s intensive care unit, mechanical ventilation is used to help children to breathe when they are very ill and their spontaneous ventilation is inadequate to sustain life. Yet, if used for long periods of time, mechanical ventilation can cause problems. Ventilation is associated with complications such as ventilator‐induced lung injury, pneumonia, sedation complications and negative recollections of the experience. For this reason, it is important to recognize when the child has recovered enough to start breathing for himself and to reduce (or wean) the ventilator support. Unfortunately, no agreement has been reached on the best way to wean children off the ventilator.
In adults, researchers have studied the usefulness of standardized protocols to help guide doctors and nurses in intensive care to wean patients from the ventilator in a safe and timely manner. The purpose of this Cochrane review was to look at the weaning protocol studies in children to see whether a conclusion can be drawn regarding their usefulness in children.
We found three randomized controlled studies that analysed 321 children older than 28 days and younger than 18 years. The studies were of good quality and were carried out in Brazil, Canada and the United States. The largest study showed that weaning by protocol reduced the length of time on mechanical ventilation by an average of 32 hours; the other two studies did not show a significant effect. Two studies reported significant reductions in the time it took from start to end of weaning from the ventilator. Weaning protocols did not affect the child’s length of time in the intensive care unit or hospital, nor did they affect the number of complications associated with mechanical ventilation.
In two studies, participants represented a broad population of children in intensive care, although these studies did not include children undergoing heart surgery or with chronic neuromuscular, heart or lung disease. The third study included only those with pneumonia, bronchiolitis and acute respiratory distress syndrome. The included studies used a variety of criteria to establish readiness to wean, and their protocols took different approaches to the process of weaning. These studies were at low or unclear risk of bias.
Limited evidence suggests that weaning protocols reduce the duration of mechanical ventilation, but evidence is inadequate to show whether the achievement of shorter ventilation by protocolized weaning causes children benefit or harm.
Background
The most frequent cause of acute respiratory failure in infants and children leading to admission to a paediatric intensive care unit (PICU) is bronchiolitis with pneumonia (Randolph 2002). Mechanical ventilation is an important component of critical care (Byrd 2010). A pressurized volume of air is delivered via a tracheal tube or by tracheostomy, mask or nasopharyngeal tube. Evidence is insufficient to show the best ventilation modes in critically ill children (Duyndam 2011). Prolonged mechanical ventilation is associated with morbidity and mortality; as a result, clinical and research efforts have focused on early identification of weaning readiness to reduce unnecessary delays.
Description of the condition
Weaning consists of the gradual reduction of ventilatory support and the transfer of respiratory control and the work of breathing back to the patient, resulting in discontinuation of mechanical ventilation (Byrd 2010; Hess 2001; Intensive Care Society 2007). The most common ventilator weaning modes used in weaning children are pressure support ventilation, volume support ventilation, synchronized intermittent mandatory ventilation (Randolph 2002; Wolfler 2011) and a spontaneous breathing trial (Farias 2001). In pressure support mode, the level of pressure is adjusted to achieve acceptable respiratory parameters followed by gradual weaning to minimal pressure support. Volume support is an automated mode whereby the amount of pressure support required to maintain a pre‐set tidal volume is reduced automatically as respiratory mechanics improve. Synchronized intermittent mandatory ventilation is a combination mode by which patients receive mandatory (set) breaths synchronized with their breathing efforts and according to a pressure‐ or volume‐selected mode. Patients breathe spontaneously with pressure support between ventilator breaths; this results in patient‐ventilator synchrony. In this mode, weaning often involves combined reduction of all of the above. A spontaneous breathing trial involves allowing the child to breathe spontaneously on minimal pressure support or through a T‐piece attached to the ventilatory circuit. Each approach may be managed with or without written protocols, or with partial or fully automated ventilator loop algorithms. Investigators have tried to determine how to most effectively wean and extubate patients (Clement 1996; Ely 2001; Fortenberry 2009; Hubble 2000; Kahn 1996). Unfortunately no current, standard method is used to wean children (Newth 2009), and weaning practices vary even within the adult population (Blackwood 2010).
More than 50% of ventilated children are extubated within 48 hours of admission (Newth 2009), often without weaning; up to 50% of unplanned extubations are successful (Little 1990). Weaning refers to a gradual withdrawal of ventilatory support through a stepwise process, rather than extubation from full ventilatory support (Blackwood 2010; Cook 2000). For some children weaning may take weeks or months, and a few remain ventilator‐dependant.
Prolonged intubation and ventilation in children can compromise the child's comfort, feeding and mobility (Hoskote 2005). Furthermore, the requirement for continued sedation and risks of accidental extubation, vocal cord dysfunction, subglottic stenosis, ventilator‐induced lung injury and nosocomial pneumonia can present important morbidity (Hoskote 2005; Newth 2009). No less important are the well‐documented psychological sequelae, which include children's memories of pain and anxiety associated with inability to communicate and with the endotracheal tube (Noyes 2000; Playfor 2000); parental experiences of stress and emotional intensity (Latour 2011; Noyes 1999; Pooni 2013); and post‐traumatic stress disorder for both child and parents (Colville 2012). Ventilation is a life‐saving intervention; however, if unnecessarily prolonged, the child is needlessly exposed to these risks. Therefore, safely minimizing the duration of invasive mechanical support is an important goal of critical care medicine (MacIntyre 2001).
Extubation, which is defined as removal of the endotracheal tube, is a separate but closely related aspect of care (Alia 2000; Byrd 2010; Newth 2009). Concerns that must be addressed before extubation include level of consciousness, respiratory muscle strength, haemodynamic stability and airway oedema or trauma (Walters 2008). Once a patient has achieved a low level of ventilatory support and is capable of sustaining independent spontaneous breathing, his or her ability to safely maintain the airway should be assessed (Byrd 2010). Patients may require additional respiratory assistance after extubation, often in the form of non‐invasive positive‐pressure ventilation. Although non‐invasive ventilation is recognized as a form of mechanical ventilation, its place in weaning protocols has yet to be fully determined (Leclerc 2010).
Description of the intervention
Weaning protocols aim to safely and efficiently liberate patients from mechanical ventilation, reducing unnecessary or harmful variations in approach (Ely 2001). A protocol is defined by the United Kingdom National Health Service Institute as, "descriptions of the steps taken to care for and treat a patient..." enabling "...staff to put evidence into practice by addressing the key questions of what should be done, when, where and by whom at a local level" (NHS Institute 2010). A weaning protocol generally consists of an assessment of the patient's readiness to wean that is based on objective measurement of his or her clinical stability (cardiovascular and metabolic status) and adequate oxygenation, pulmonary function and mental status. This is followed by a method of removing or reducing support. One method involves undertaking a spontaneous breathing trial to identify patients ready for extubation (Farias 1998) that has been shown in children to be equally effective when performed with a T‐piece or with pressure support (Farias 2001). Another method involves following an algorithm outlining a step‐wise reduction in ventilatory support using pressure support ventilation or synchronized intermittent mandatory ventilation (Kollef 1997). Several automated, closed loop weaning systems have become commercially available that propose to wean in real time in accordance with patient ventilatory status (Rose 2008).
A written protocol requires the vigilance and compliance of the clinician in the process, whereas an automated protocol changes the level of support provided by the ventilator in accordance with a computerised algorithm to decrease support and in some cases perform an automated spontaneous breathing trial (Lellouche 2006). An advantage of automated systems is that they may reduce adherence difficulties associated with paper‐based protocols (Rose 2008). Notwithstanding their potential benefits, weaning protocols have attracted criticism. In a review of the evidence for protocols for weaning and sedation management, Girard 2008 provided a sound rationale for the use of weaning protocols, but equally these investigators found that protocol applicability and efficacy remained a source of controversy. The clinical decision to wean or discontinue mechanical ventilation has traditionally been based on clinician judgement and experience (Sahn 1973); protocols may be perceived as removing clinical judgement and hindering consideration of all facets of the care of the participants involved, thereby creating resentment and frustration among healthcare professionals (Ely 2001). It may be argued that most protocols are not replicable because of their dependence on bedside clinician judgement for many decisions, which then become tacitly incorporated into the protocol. Because these judgements occur in a variable manner, it may not be possible to fully describe the protocol rules (Morris 2007).
How the intervention might work
Protocolized weaning, an intervention used by clinicians, may affect the duration of mechanical ventilation in a number of ways. First, it may reduce unwanted variability in weaning practice. In part because of their different experiences, skills and philosophies, clinicians may wean patients differently. Protocols are generally developed consensually by expert groups within the intensive care unit (ICU). They are intended to exemplify best practice, to provide guidelines and thus to reduce needless variation, thereby improving effectiveness and efficiency (Murtagh 2007). Second, weaning often is not considered early enough in the course of ventilation, and a protocol that incorporates assessment for readiness to wean will direct attention to patient readiness. Third, weaning protocols also have the potential to enable non‐medical healthcare professionals to lead or have responsibility in weaning from ventilation: this may reduce unnecessary delays in the weaning process due to limited availability of physicians (Blackwood 2010). Thus, using protocols to guide weaning may encourage best practice through timely recognition of readiness to wean, and adoption of effective weaning processes, so reducing risks and costs associated with unnecessary time on the ventilator.
Why it is important to do this review
A systematic review and meta‐analysis of 11 trials in adults indicated that protocolized weaning significantly reduced the duration of mechanical ventilation, weaning duration and ICU length of stay without adverse effects (Blackwood 2010). However, research evidence from studies of adult participants may not apply for children as children have a dynamic respiratory physiology, affected by growth demands and vulnerable to damage; they are not little adults (WHO 2008). With the growing interest amongst clinicians in developing weaning protocols, it is important to ensure that practice is evidence based and safe. Consequently, our review will rigorously and systematically examine the evidence concerning benefits and harms of protocols to wean children from mechanical ventilation. This is essential in guiding decisions on whether or not to adopt weaning protocols as a quality improvement measure.
Objectives
To assess the effects of weaning by protocol on invasively ventilated critically ill children.
To compare the total duration of invasive mechanical ventilation of critically ill children who are weaned using protocols versus those weaned through usual (non‐protocolized) practice.
To ascertain any differences between protocolized weaning and usual care in terms of mortality, adverse events, ICU length of stay and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We included in the review randomized controlled trials (RCTs) that compared protocolized with non‐protocolized weaning.
Types of participants
We included studies of children (younger than 18 years old) who were cared for in a PICU and were mechanically ventilated via a nasal or oral tracheal tube.
We excluded studies of neonates (from birth to 28 completed days after birth) (WHO 2010)) because of differences in their ventilation and weaning strategies (Alander 2013) and because a Cochrane systematic review of protocolized weaning in neonates is under way (Wielenga 2013).
We excluded studies in which children were ventilated exclusively via non‐invasive techniques or tracheostomy.
Types of interventions
We included studies that evaluated protocolized weaning compared with non‐protocolized weaning. For the purpose of this review, protocolized weaning was defined as the use of an algorithm (paper based or automated) intended to result in removal of children from invasive mechanical ventilation. Non‐protocolized weaning was defined as usual care, standard practice or clinician‐led care that incorporated any non‐protocolized practice.
Types of outcome measures
Primary outcomes
Duration of mechanical ventilation (MV), measured in hours.
Secondary outcomes
Weaning duration (from identification of weaning readiness to invasive MV discontinuation).
MV before weaning.
PICU and hospital length of stay.
PICU and hospital mortality.
Quality of life as defined by the authors.
Adverse events (such as re‐initiation of MV within 48 hours of removal, tracheostomy, self‐extubation or re‐admission within 48 hours).
Search methods for identification of studies
Electronic searches
We searched the literature using the standard strategy of the Cochrane Anaesthesia Review Group of the Cochrane Collaboration. We searched the Cochrane Central Register of Controlled trials (CENTRAL) (The Cochrane Library, Issue 10, 2012); MEDLINE In‐Process and other Non‐Indexed Citations and OVID MEDLINE (1946 to 22 October 2012); CINAHL Plus via EBSCO host (1982 to 22 October 2012); EMBASE via OVID (1980 to 22 October 2012); LILACS (1982 to 22 October 2012); unpublished data-Web of Science (1990 to 22 October 2012) and ISI Conference Proceedings (1990 to 22 October 2012). We did not restrict language of publication.
We used a specific search strategy for each database with descriptors that included synonyms for ventilator weaning, clinical protocols and randomized controlled trials; reflecting the clinical condition, intervention and research design, respectively. Search strategies for each database can be found in the appendices (Appendix 1: MEDLINE; Appendix 2: CINAHL; Appendix 3: EMBASE; Appendix 4: LILACS; Appendix 5: CENTRAL; Appendix 6: Web of Science).
Searching other resources
In our efforts to obtain grey literature, we searched reference lists of included studies; contacted authors of included studies by electronic mail for information; searched the major clinical trials registries (ProQuest; www.ClinicalTrials.gov) and searched for theses (www.theses.com).
Data collection and analysis
Selection of studies
Three review authors (MM, POH, BB) independently scanned identified titles and abstracts and excluded records that did not meet eligibility requirements. We obtained full‐text copies of potentially relevant studies.
Data extraction and management
Three review authors (AC, BB, MM) independently extracted data from the included studies using a piloted paper form (Appendix 7). We extracted information about study design, study setting and participants, inclusion and exclusion criteria, interventions and outcomes. We also collected information on sources of funding for the study and on ethical approval. Furthermore, we collected information, where available, regarding physician and nurse staffing numbers and sedation strategies as these can influence ventilator weaning (Hansen 2009; Playfor 2006). After independent data extraction, we met to resolve any disagreement through discussion and consultation. We did not require additional arbitration by a fourth review author (POH).
Assessment of risk of bias in included studies
Risk of bias in included studies was assessed independently by the same three review authors (AC, BB, MM) using the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8, version 5.1.0 (Higgins 2011). The 'Risk of bias' form (Appendix 7) extracted from Chapter 8.5.1 was used to evaluate each included study, and the review authors' judgements were directed by the criteria set out in Chapter 8.5.3 and Table 8.5c. Each study was judged as 'Yes' (low risk of bias), 'Unclear' (uncertain risk of bias) or 'No' (high risk of bias) for the following domains.
Random sequence generation.
Allocation concealment.
Blinding (of participants, personnel and outcome assessors).
Incomplete outcome data.
Selective reporting.
Free of other bias.
We categorized the risk of bias in all included studies according to the following:
Low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
These assessments are reported in the 'Risk of bias' tables in the review (Appendix 7). We also discuss in the review result section the impact of methodological quality on the results.
Measures of treatment effect
We planned to combine data using RevMan 5.2, when appropriate, by intervention, outcome and population.
Unit of analysis issues
The child was the unit of analysis in each trial. Children were randomly allocated to one of two parallel intervention groups, and a single measurement for each outcome from each participant was collected and analysed.
Dealing with missing data
Where necessary, we contacted the first author of included studies to obtain data.
Assessment of heterogeneity
Clinical heterogeneity was judged by the review authors (MM, AC), and these results are noted in the review. We planned to investigate heterogeneity by conducting subgroup analyses defined by type of PICU, protocol and approach to delivery.
Assessment of reporting biases
Studies were insufficient to allow the review authors to explore small study effects.
Data synthesis
Data were entered into RevMan (RevMan 5.2) by BB and were checked independently by MM. For the primary outcome (duration of mechanical ventilation), data were reported differently: median with 95% confidence interval (CI) using Kaplan‐Meier survival curves (Foronda 2011); mean and standard deviation (SD) (Jouvet 2013) or median and interquartile range (IQR) (Maloney 2007). Foronda 2011 supplied raw data to enable us to calculate the mean and SD. For the Maloney 2007 study, we approximated the mean using the median, and approximate SD estimates were calculated from the IQR, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Ventilation data from all three studies had skewed distributions. Whilst one study (Foronda 2011) provided raw data, the other two did not and would require approximations to calculate the mean and SD on the log scale before the meta‐analysis was performed. It was unclear how well these approximations would perform, particularly as two studies had small numbers (Jouvet 2013; Maloney 2007); therefore we did not conduct a meta‐analysis. Results from each study are presented in tables, along with mean differences and 95% CIs. If further trials are identified in the future, we will calculate pooled estimates of the difference in means and risk ratios (RRs) using the fixed‐effect model (FEM) or the random‐effects model (REM), depending on the degree of heterogeneity.
Subgroup analysis and investigation of heterogeneity
Studies were insufficient for review authors to conduct subgroup analyses.
Sensitivity analysis
Studies were insufficient for review authors to conduct sensitivity analyses.
Results
Description of studies
The studies were RCTs conducted with mechanically ventilated children older than 28 days.
Results of the search
The electronic searches identified a total of 10,983 citations: 9891 from electronic databases and 1092 from additional records. Three review authors (MM, POH, BB) reviewed these citations and listed eight studies for possible inclusion. Full papers for these citations were retrieved. Where necessary, the authors were contacted to clarify whether their study met inclusion criteria for our review. A flow diagram detailing the selection of studies is presented in Figure 1.
1.
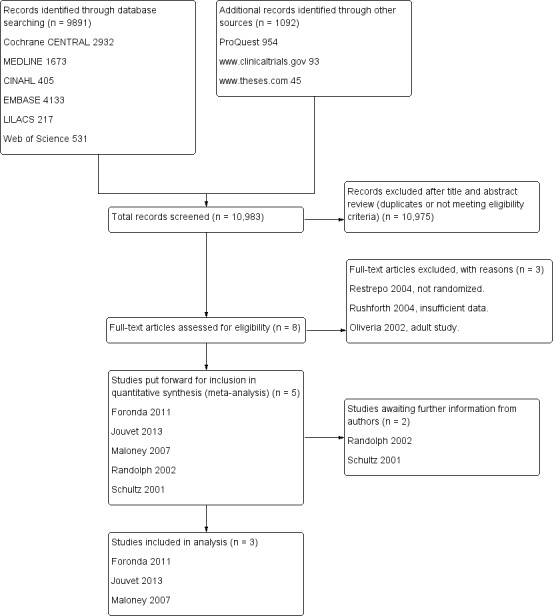
Review flow diagram.
Included studies
We included three RCTs conducted on mechanically ventilated children in PICUs. The intervention groups were weaned from mechanical ventilation in accordance with written or automated weaning protocols. The control groups were weaned by healthcare professionals without the use of written, formal guidelines.
Participants and settings
These studies analysed 321 children in two published papers (Foronda 2011; Jouvet 2013) and one thesis (Maloney 2007). Details are provided in the Characteristics of included studies table. The trials were conducted in Sao Paulo, Brazil (Foronda 2011), Montreal, Canada (Jouvet 2013), and Salt Lake City, Utah, United States (Maloney 2007). Trial sample sizes ranged from 30 to 260, and participants were recruited from mixed patient population paediatric (Foronda 2011; Jouvet 2013) or cardiothoracic (Maloney 2007) PICUs. The age range of participants was 28 days to 18 years. None of the studies provided information on physician and nurse staffing in the units, and none provided information on their sedation strategies or sedation protocols.
Interventions
Children were allocated to different interventions at different times in each study (see Appendix 8 for details). Weaning interventions included daily evaluation for readiness to wean and a spontaneous breathing trial (Foronda 2011); a computerized protocol using a commercially available closed‐loop system, SmartCare/PSTM (Jouvet 2013) and a non-commercially available computerized decision support tool and weaning protocol (Maloney 2007). Randomization to groups was conducted at different time points in the three trials: before meeting readiness to wean criteria (Foronda 2011); after passing a 30‐minute pressure support test (Jouvet 2013) and after two consecutive reductions in ventilator support (Maloney 2007). Only Foronda 2011 described usual care in which the most frequently used modes were pressure support, synchronized intermittent mandatory ventilation and pressure‐controlled ventilation; and weaning consisted of reductions in respiratory frequency, peak inspiratory pressure, fraction of inspired oxygen and positive end‐expiratory pressure as determined by the presence of ventilatory parameters.
Studies pending classification
Two studies (Randolph 2002; Schultz 2001) met the inclusion criteria but their study samples included neonates. All authors were contacted to ascertain whether the data for children and neonates could be separated for analysis. We are awaiting this information.
Excluded studies
Three studies were excluded. Two studies (Restrepo 2004, Oliveria 2002) did not meet the eligibility criteria, and one study (Rushforth 2004) included only three children. Details are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed the risk of bias using the domain‐based evaluation of risk of bias tool of the Cochrane Collaboration (Higgins 2011). Low or unclear risk was identified across all six domains. Our judgement on the classification of bias for individual studies is presented in the Characteristics of included studies table and is summarized in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies used adequate methods for random sequence generation and allocation concealment: Foronda 2011 and Jouvet 2013 used random selection of opaque sealed envelopes and Maloney 2007 used computer‐generated randomization.
Blinding
In the Foronda 2011 study, medical personnel were blinded to allocation up until the point at which the participant passed the spontaneous breathing trial, indicating low risk of performance bias. In the other two studies (Jouvet 2013; Maloney 2007), blinding was not possible, and it is unclear whether this produced performance bias. In all three studies, blinding of outcome assessors was not reported, and therefore risk of detection bias was unclear.
Incomplete outcome data
Data on recruitment and attrition were reported, and no evidence of attrition bias was found in the three studies.
Selective reporting
A trial protocol was registered by Foronda 2011 and Jouvet 2013, and no evidence of selective reporting was found. Maloney 2007 did not register a protocol but reported usual outcomes for trials in this area, so we assessed the risk of reporting bias as low.
Other potential sources of bias
We found no other potential sources of bias in included studies.
Effects of interventions
All study authors were contacted to confirm and supplement information related to methods and data, when needed. Results are reported for each outcome.
Primary analysis: Comparison of protocolized versus non‐protocolized weaning
Primary outcome: Total duration of mechanical ventilation (hours)
All three studies reported the review’s primary outcome, which was the total duration of mechanical ventilation (see Table 1). In all studies, this outcome was defined as initiation of mechanical ventilation to extubation. Jouvet 2013 further defined the endpoint of this outcome as including subsequent ventilation episodes if reintubation occurred within 48 hours of extubation; the endpoint in the other two studies was time to first extubation. All three studies reported results favouring protocolized weaning, but only the largest of the three trials (Foronda 2011) (with 260 participants) showed a statistically significant mean (95% CI) reduction of 32 (8 to 56) hours (P = 0.01). Jouvet 2013 reported a mean difference of ‐88 (‐228 to 52) hours (P = 0.2); and Maloney 2007 reported a difference of ‐24 (‐10 to 58) hours (P = 0.06).
1. Review Primary Outcome Results.
| Study |
Protocolized weaning N mean (SD) |
Non‐protocolized weaning N mean (SD) |
Difference in mean | 95% CI | P‐value | ||
| Foronda 2011 | 134 | 111 (85) | 126 | 143 (107) | ‐32 | ‐55.58 to ‐8.42 | 0.01± |
| Jouvet 2013 | 15 | 200 (186) | 15 | 288 (206) | ‐88 | ‐228.46 to 52.46 | 0.20§ |
| Maloney 2007 | 15 | 93.6 (27)* | 16 | 117.8 (64)* | ‐24.2† | ‐10.0 to 58.4 | 0.055|| |
* Standard deviation approximated from the interquartile range; † difference in median; ± from t‐test; § from Mann Whitney U‐test; || from Mann Whitney t‐test; NR not reported
Secondary outcomes
Two studies (Jouvet 2013; Maloney 2007) reported secondary outcomes relevant to the review. These are presented in Table 2. A statistically significant mean reduction in weaning duration was reported in the protocolized weaning group for Jouvet 2013 (106 hours, 95% CI 28 to 184, P = 0.007) and Maloney 2007 (21 hours, 95% CI 9 to 32, P < 0.001). Both studies defined weaning duration as initiation of weaning to extubation, but each study used different criteria for determining the start and endpoint of this outcome (see Appendix 8 for details). No significant differences in outcomes between protocolized and non‐protocolized weaning groups were reported for duration of MV before weaning or for PICU and hospital length of stay. No study reported quality of life.
2. Table of secondary outcomes.
| Study | Protocolized weaning | Non‐protocolized weaning | |||||
| N | Mean (SD) | N | Mean (SD) | Mean difference | 95% CI | P‐value | |
| Duration of mechanical ventilation before weaning (hours) | |||||||
| Jouvet 2013 | 15 | 157 (189) | 15 | 141 (104) | ‐16 | ‐125.17 to 93.17 | 0.89 |
| Maloney 2007 | 15 | 74.5 (39.3)* | 16 | 84 (53.3)* | 9.5 | ‐23.33 to 42.33 | 0.50 |
| Weaning duration (hours) | |||||||
| Jouvet 2013 | 15 | 36 (36) | 15 | 142 (150) | 106 | 27.94 to 184.06 | 0.007 |
| Maloney 2007 | 15 | 8 (9.3)* | 16 | 28.5 (22.2)* | 20.5 | 8.65 to 32.35 | <0.001 |
| PICU length of stay (hours) | |||||||
| Jouvet 2013 | 15 | 216 (120) | 15 | 696 (504) | 480 | 217.82 to 742.18 | 0.11 |
| Maloney 2007 | 15 | 176 (64) | 16 | 217 (114) | 41 | ‐23.57 to 105.57 | 0.23 |
| Hospital length of stay (hours) | |||||||
| Jouvet 2013 | 15 | 648 (432) | 15 | 696 (504) | 48 | ‐287.93 to 383.93 | 0.68 |
| Maloney 2007 | 15 | 312 (88) | 16 | 436 (338) | 124 | ‐47.50 to 295.50 | 0.18 |
* Standard deviation approximated from the interquartile range.
Adverse events
Adverse events are presented in Table 3. Foronda 2011; Jouvet 2013 and Maloney 2007 reported no significant differences in reintubation and self‐extubation rates. Jouvet 2013 reported one death in PICU in the automated group and none in the control group. Foronda 2011 reported no significant differences in PICU mortality between groups, with 23 (14.8%) and 15 (10.8%) deaths, respectively, reported in the protocol and control groups. Most deaths occurred before weaning; only two deaths per group occurred after weaning (personal communication). Foronda 2011 and Jouvet 2013 reported no significant differences in the use of non‐invasive ventilation post extubation. Foronda 2011 reported no significant differences in ventilator‐associated pneumonia, and Jouvet 2013 reported no significant differences in prolonged mechanical ventilation. No study reported hospital mortality.
3. Table of Adverse Events Results.
| Study |
Protocolized weaning n/N (%) |
Non‐protocolized weaning n/N (%) |
Risk Ratio | 95% CI | P‐value |
| PICU Mortality | |||||
| Foronda 2011 | 23/155 (14.8) | 15/139 (10.8) | 1.44 | 0.72 to 2.89 | 0.30 |
| Jouvet 2013 | 1/15 (6.7) | 0/15 (0.0) | 3.0 | 0.13 to 68.26 | NR |
| Reintubation | |||||
| Foronda 2011 | 15/134 (11.2) | 18/126 (14.3) | 0.78 | 0.41 to 1.49 | 0.45 |
| Jouvet 2013 | 2/15 (13.3) | 1/15 (6.7) | 2.0 | 0.2 to 19.78 | NR |
| Maloney 2007 | 2/15 (13.3) | 3/16 (12.5) | 0.71 | 0.14 to 3.68 | 1.0 |
| Self‐extubation | |||||
| Foronda 2011 | 3/134 (2.2) | 8/126 (6.3) | 0.35 | 0.1 to 1.3 | 0.10 |
| Jouvet 2013 | 1/15 (6.7) | 0/15 (0.0) | 3.0 | 0.13 to 68.26 | NR |
| Maloney 2007 | 0 | 0 | NE | NE | NE |
| Non‐invasive ventilation post extubation | |||||
| Foronda 2011 | 29/134 (21.6) | 39/126 (31.0) | 0.7 | 0.46 to 1.06 | 0.09 |
| Jouvet 2013 | 1/15 (6.7) | 2/15 (13.3) | 0.5 | 0.05 to 4.94 | NR |
| Ventilator‐associated pneumonia | |||||
| Foronda 2011 | 9/123 (6.7) | 12/126 (9.5) | 0.77 | 0.34 to 1.76 | 0.41 |
| Prolonged MV | |||||
| Jouvet 2013 | 0/15 (0.0) | 2/15 (13.3) | 0.20 | 0.01 to 3.85 | NR |
NE, not estimable; NR, not reported.
Discussion
A thorough search of the literature identified five studies that could potentially be included in our review. Two studies (Randolph 2002; Schultz 2001) included a proportion of neonates (17% and unknown proportion, respectively); the authors were unable to provide us with disaggregated data. Furthermore, Randolph 2002 included two weaning protocol groups (using pressure support and volume support ventilation) and one control group, and we were unable to obtain and combine intervention group data. Consequently, only three studies were included in the review. Ventilation outcome data in these studies were skewed and consequently require conversion to the log scale for meta‐analysis; these approximations are complicated by small numbers in two studies (Jouvet 2013; Maloney 2007), and this made meta‐analysis inadvisable. As a result, the review findings cannot provide sufficient strength of evidence to demonstrate benefit or harm.
Summary of main results
Only the largest study (Foronda 2011) showed a significant effect on the total duration of mechanical ventilation. In this study, the protocolized weaning group received a daily evaluation of readiness to wean and a two‐hour spontaneous breathing trial; duration of ventilation was reduced by a mean (95% CI) of 32 (8 to 56) hours. Using a SmartCare/PSTM automated system and a computerized weaning protocol, respectively, Jouvet 2013 and Maloney 2007 showed a statistically significant reduction in weaning duration in the protocolized groups by 106 and 21 hours, respectively, which is promising; however, this reduction did not significantly reduce total mechanical ventilation time or PICU or hospital length of stay. Foronda 2011 reported no significant differences in PICU mortality, reintubation, self‐extubation or use of non‐invasive ventilation after extubation. Adverse events and deaths were too few in the two smaller studies for the review authors to draw significant conclusions. Because of the small number of studies included in the review and inability to pool the data, we are not able to provide a meaningful summary of findings table.
Overall completeness and applicability of evidence
All three included studies included a population of children with respiratory conditions; therefore these studies are applicable to the general PICU, where respiratory disorders are the main cause of respiratory failure necessitating mechanical ventilation (Newth 2009). Jouvet 2013 was the only study that included postoperative surgical and trauma participants; additionally, this study group restricted participant age, as the SmartCare/PSTM automated system currently is not licensed for children younger than two years of age. The average age of children admitted to the PICU is seven months (Farias 2012), which explains the current lack of trials using this weaning method. All studies excluded children with complex conditions such as primary pulmonary hypertension, cyanotic heart disease and neuromuscular disease, which are associated with prolonged mechanical ventilation (Polito 2011); therefore the impact of protocolized weaning on prolonged mechanical ventilation in these groups is unknown.
Conclusions cannot be drawn on the effectiveness of specific weaning methods, as each study used a different approach to protocolized weaning. Methods included a professional‐led approach with a daily evaluation of readiness to wean, and with those meeting set criteria undergoing a two‐hour spontaneous breathing trial; an automated closed loop system with automatic adjustment of pressure support and physician‐led adjustment of positive end‐expiratory pressure and a computer‐driven protocol that automatically analysed data relevant to the participant's respiratory performance, formulated a recommended change in ventilator support and transmitted a paged message to a respiratory therapist to manually adjust settings in accordance with a protocol. Resources available to individual PICUs, including availability of computerized systems, may place restrictions on the choice of weaning method.
Context‐related information such as physician and nurse staffing, sedation strategies and sedation protocols are known to cause delays in the weaning process (Brattebø 2002; Marcin 2005). None of the studies provided contextual information; consequently, the influence of these factors on study outcomes cannot be assessed.
Quality of the evidence
The three studies included sample sizes ranging from 30 to 260 and involved 321 randomized children. Methodological quality of the studies was high. We assessed the largest study (Foronda 2011) as having low risk of bias in all domains of the domain‐based evaluation of risk of bias tool of the Cochrane Collaboration (Higgins 2011) and the two smaller studies as having low or unclear risk across all six domains. Blinding of the intervention is not feasible in studies comparing a weaning protocol with usual care; however, Foronda 2011 was able to conceal participant assignment up until the child passed the daily evaluation of weaning readiness and a spontaneous breathing trial was indicated, thus removing potential performance bias.
Potential biases in the review process
We adhered closely to our protocol, which outlined our procedures for minimising bias in the review: these included independent screening for trial inclusion, data extraction and assessment of risk of bias by three review authors. With the assistance of the Cochrane Anaesthesia Group's Search Trials Co‐ordinator and an experienced librarian, we conducted a thorough search strategy and believe we have identified all relevant studies.
Agreements and disagreements with other studies or reviews
This is the first published systematic review of trials comparing protocolized weaning with usual care in critically ill children in intensive care.
Authors' conclusions
Implications for practice.
Limited evidence suggests that weaning protocols reduce the duration of mechanical ventilation, and evidence is inadequate to determine whether achievement of shorter ventilation by protocolized weaning causes children benefit or harm.
Implications for research.
The small number and size of the trials limit our ability to provide evidence of protocolized weaning in children; therefore we believe that an adequately powered, multi‐centre, robustly randomized controlled trial is needed. Implementation of weaning protocols is a complex intervention that can be influenced by many contextual factors such as ICU organization, resources and staffing; inter‐professional working relationships; clinician willingness to adopt protocols; and skill mix, education and training of healthcare professionals (Blackwood 2006). Given the international variation in healthcare contexts, ventilator weaning requires careful evaluation not only of the intended clinical outcomes of the weaning protocol but also of the impact of associated contextual factors. Ideally, this should take place within a framework that incorporates a robustly randomized controlled trial and a process evaluation (such as that advocated by the Medical Research Council 2008) that will explain how context influences outcome and will provide insights to aid implementation in other settings. Additionally, such a trial should evaluate the cost‐effectiveness of implementing protocolized weaning against usual care.
Another important matter in the conduct of such a trial is the description of ‘usual care’ in the control group. A detailed description of usual care will enable a judgement to be made about the significance of the observed difference between groups and the likely impact of protocolized weaning in similar contexts.
Despite limited evidence of their benefits or harms in children, the prevalence of weaning protocols is increasing in PICUs (Blackwood 2013). The danger of rapid adoption without a robust evaluation of benefits and harms means that once the intervention has been adopted into practice, the control conditions essential for good effectiveness studies are no longer available (Girard 2008). This was observed with critical care outreach-a similar healthcare issue with rapid international implementation without robust evaluation (Priestley 2004). A randomized stepped wedge design (Brown 2006a), similar to that used by Priestley 2004, may be appropriate in a trial of weaning protocols, in that it will use the window of opportunity presented by the fact that during phased introduction of protocolized weaning, control conditions would prevail in PICUs that had not yet received the intervention. We provide a possible design for a future trial using the EPICOT+ framework as proposed by Brown and colleagues (Brown 2006b) (Appendix 9). This recommendation has been provided as an outline only and would require adaptation to the context in which any such study is undertaken.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Patricia Watt, Librarian, Medical Library, Queen's University, Belfast; Karen Hovhannisyan, Search Trials Co‐ordinator, Cochrane Anaesthesia Review Group (CARG); and Jane Cracknell, Managing Editor, CARG. Our thanks also go to John Carlisle (Content Editor, protocol and review), Nathan Pace (Statistical Editor, protocol), Cathal Walsh (Statistical Editor, review), Louise Rose and Neill Adhikari (Peer Reviewers, protocol and review), Anne Lyddiat (CARG Consumer Panel, protocol) and Janet Wale (Consumer Editor, review) for their help and editorial advice.
Appendices
Appendix 1. MEDLINE search strategy.
#1 exp Ventilator Weaning/
#2 mechanical ventilat$ weaning.mp.
#3 mechanical ventilation.mp.
#4 (protocol$ adj5 weaning).mp.
#5 (ventilat$ adj5 weaning).mp.
#6 exp Ventilators, Mechanical/
#7 exp Ventilators, Negative‐Pressure/
#8 (mechanical adj5 ventilat$).mp.
#9 (mechanical adj5 weaning).mp.
#10 ventilat$.ab,ti.
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 protocol$.mp.
#13 exp Clinical Protocols/
#14 exp Patient Care Management/
#15 Practice Guidelines/
#16 #12 or #13 or #14 or #15
#17 #11 and #16
#18 randomized controlled trial.pt
#19 controlled clinical trial.pt.
#20 randomized.ab.
#21 placebo.ab.
#22 randomly.ab
#23 trial.ab
#24 groups.ab
#25 #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #17 and #25
#27 animals.sh. not (humans.sh. and animals.sh.)
#28 #26 not #27
[mp = title, original title, abstract, name of substance word, subject heading word]
Appendix 2. CINAHL search strategy
S1 TX Ventilator Weaning
S2 TX mechanical ventilat* weaning
S3 "mechanical ventilat* weaning"
S4 TX mechanical ventilation
S5 TX protocol* N5 weaning
S6 TX ventilat* N5 weaning
S7 (MH "Ventilator Weaning") OR (MH "Respiration, Artificial") OR "exp Ventilators, Mechanical" OR (MH "Mechanical Ventilation (Iowa NIC)") OR (MH "Ventilation, Mechanical, Differentiated") OR (MH "Ventilators, Mechanical")
S8 (MH "Ventilation, Negative Pressure") OR (MH "Negative End‐Expiratory Pressure") OR "Ventilators, Negative‐Pressure" OR (MH "Positive Pressure Ventilation") OR (MH "Pressure Support Ventilation")
S9 TX mechanical N5 ventilat*
S10 TX mechanical N5 weaning
S11 TI ventilat*
S12 AB ventilat*
S13 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12
S14 TX protocol*
S15 TX Patient Care Management
S16 TX Clinical Protocols
S17 (MH "Practice Guidelines") OR "Practice Guidelines"
S18 TX Practice Guidelines
S19 S14 or S15 or S16 or S17 or S18
S20 S13 and S19
S21 TX randomized controlled trial
S22 TX controlled clinical trial
S23 AB randomized
S24 AB placebo
S25 AB randomly
S26 AB trial
S27 AB groups
S28 (MH "Clinical Trials") OR (MH "Randomized Controlled Trials") OR (MH "Clinical Trial Registry") OR (MH "Multicenter Studies") OR (MH "Cochrane Library")
S29 S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28
S30 not AB animal*
Appendix 3. EMBASE search strategy
#1 exp Ventilator Weaning/
#2 mechanical ventilat$ weaning.mp.
#3 mechanical ventilation.mp.
#4 (protocol$ adj5 weaning).mp.
#5 (ventilat$ adj5 weaning).mp.
#6 exp Ventilators, Mechanical/
#7 exp Ventilators, Negative‐Pressure/
#8 (mechanical adj5 ventilat$).mp.
#9 (mechanical adj5 weaning).mp.
#10 ventilat$.ab,ti.
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 protocol$.mp.
#13 exp Clinical Protocols/
#14 exp Patient Care Management/
#15 Practice Guidelines/
#16 #12 or #13 or #14 or #15
#17 #11 and #16
#18 randomized controlled trial/
#19 controlled clinical trial/
#20 randomized.ab.
#21 placebo.ab.
#22 randomly.ab
#23 trial.ab
#24 groups.ab
#25 #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #17 and #25
#27 animals/
#28 humans/
#29 #27 not (#27 and #28)
#30 #26 not 29
Appendix 4. LILACS search strategy
Search string:
(WEAN$ or "MECHANICAL VENTILATION" or VENTILAT$ or "NEGATIVE PRESSURE") protocol$
Appendix 5. CENTRAL Cochrane database search strategy
(search all text)
"Ventilator Wean*" or mechanical ventilat* wean* or "mechanical ventilation" or protocol* wean* or "negative pressure"
Appendix 6. Web of Science search strategy
| #9 | #5 NOT #8 DocType=All document types; Language=All languages; |
| #8 | #7 AND #6 DocType=All document types; Language=All languages; |
| #7 | TI=(animal*) DocType=All document types; Language=All languages; |
| #6 | Topic=(animal*) DocType=All document types; Language=All languages; |
| #5 | #4 AND #3 DocType=All document types; Language=All languages; |
| #4 | Title=(randomi$ed controlled trial) OR Title=(controlled clinical trial) OR Title=(random*) OR Title=(placebo) OR Title=(trial*) OR Title=(group*) OR Topic=(randomi$ed controlled trial) OR Topic=(controlled clinical trial) OR Topic=(random*) OR Topic=(placebo) OR Topic=(trial*) OR Topic=(group*) DocType=All document types; Language=All languages; |
| #3 | #2 AND #1 DocType=All document types; Language=All languages; |
| #2 | Title=(protocol*) OR Title=(Clinical Protocol*) OR Title=(Patient Care Management) OR Title=(Practice Guideline*) OR Topic=(protocol*) OR Topic=(Clinical Protocol*) OR Topic=(Patient Care Management) AND Topic=(Practice Guideline*) DocType=All document types; Language=All languages; |
| #1 | Title=(Ventilator Weaning) OR Title=(mechanical ventilat* weaning) OR Title=(mechanical ventilat*) OR Title=(protocol* adj5 weaning) OR Title=(ventilat* adj5 weaning) OR Title=(Ventilator* Mechanical) OR Title=(Ventilator* Negative Pressure) OR Title=(mechanical adj5 ventilat*) OR Title=(mechanical adj5 weaning) OR Title=(ventilat*) DocType=All document types; Language=All languages; |
Appendix 7. Study eligibility and data extraction form
Name of author extracting data:
Date form completed:
Study ID:
| Title: | |
|
Study ID for RevMan: (Family name of first author and year of publication + letter if more than one per year, e.g. Smith 2001b) |
|
|
Are there other articles of the same study? (If yes, write Study ID’s) |
Yes Unclear No |
Study eligibility
| |
Judgement (please circle) |
Source (page no in report) |
|
A. Types of study Can the study be described as randomized or quasi‐randomized? |
Yes Unclear No |
|
|
B. Participants Were any participants children (18 years or younger)? |
Yes Unclear No |
|
|
C. Interventions 1. Was one group weaned using a formal protocol1? 2. Was the other group weaned without reference to a formal protocol? |
Yes Unclear No Yes Unclear No |
|
|
D. Outcomes Did the study report: 1. Total duration of MV (time from initiation of MV to invasive MV discontinuation as stated by the authors)? 2. Weaning duration (time from identification of weaning readiness to MV discontinuation as stated by the authors)? 3. MV time before weaning (time from initiation of MV to identification of weaning readiness as stated by the authors)? 4. PICU length of stay? 5. Hospital length of stay? 6. Qualtiy of life for participants? 7. Adverse events? |
Yes Unclear No Yes Unclear No Yes Unclear No Yes Unclear No Yes Unclear No Yes Unclear No Yes Unclear No |
|
|
Conclusion: Do not proceed if any answers to A, B or C are ‘No’. Do not proceed if all answers to D are ‘No’. Excluded and listed in excluded studies table: Included: (continue to page 2) More information needed before inclusion decision (specify): Record for tables: If study to be ‘included' or ‘listed in excluded table’, record below the information to be inserted into tables: | ||
1Protocol = a written algorithm for identifying readiness to wean and/or for reducing ventilator support.
Source of key information
|
Electronic database Which one? |
................................................................................... |
|
Unpublished source Where? |
................................................................................... |
|
Personal communication From whom? |
................................................................................... |
The Cochrane Collaboration risk of bias tool (Table 8.5.a Cochrane Handbook for Systematic Reviews of Interventions)
| Domain | Description | Review author’s judgement |
|
Sequence generation |
Was the sequence adequately generated? Yes Unclear No |
|
|
Allocation concealment |
Was allocation adequately concealed? Yes Unclear No |
|
|
Blinding of participants, personnel and outcome assessors Assessments should be made for each main outcome (or class of outcome) |
Was knowledge of the allocated intervention adequately prevented during the study? Yes Unclear No |
|
|
Incomplete outcome data Assessments should be made for each main outcome (or class of outcome) |
Were incomplete outcome data adequately addressed? Yes Unclear No |
|
|
Selective outcome reporting |
Are reports of the study free of suggestion of selective outcome reporting? Yes Unclear No |
|
|
Other sources of bias |
Was the study apparently free of other problems that could put it at high risk? Yes Unclear No |
Study setting (circle as appropriate)
|
Country |
|
|
Hospital setting Type Location(s) Bed numbers |
Single hospital > 1 (specify no): |
|
PICU setting Type Number of paediatric beds |
Single PICU > 1 PICU (specify no) Paediatric only patients Mixed adult and paediatric patients Medical Surgical Cardiac Mixed medical and surgical Other (specify): |
|
Organization of care PICU staffing levels (specify numbers or staff/patient ratio) |
Nurses: Medical personnel: Respiratory therapists: Other (specify): |
|
Sedation characteristics Sedation used? (specify) Sedation protocol used Daily sedation break |
Yes Unclear No Yes Unclear No |
Participants
| Source of funding for study | |
|
No of participants who were randomly assigned |
Intervention group n = Control group n = |
|
No of participants who were analysed |
Intervention group n = Control group n = |
|
Age of participants (mean/SD) |
Intervention group Control group |
|
Sex of participants (M/F numbers or %) |
Intervention group Control group |
| Inclusion criteria | |
| Exclusion criteria | |
|
Types of medical conditions treated (specify) |
Intervention delivery
|
Protocolized weaning (characteristics defined by the authors) |
Standard practice (characteristics defined by the authors) |
|
| Delivered by | Nurse Respiratory therapist Nurse and respiratory therapist Doctors All Other (specify) Not specified |
Nurse Respiratory therapist Nurse and respiratory therapist Doctors All Other (specify) Not specified |
|
Training required (specify) (e.g. degree, respiratory module, PICU course) |
Nurse Respiratory therapist Doctors Other |
Nurse Respiratory therapist Doctors Other |
| Compliance with treatment (% or nos) |
Type of intervention
| Readiness to wean criteria |
Yes Unclear No If yes, please comment on the following or state not reported (NR): Assessment frequency: Oxygenation: Other respiratory factors: Cardiovascular: Neurological: Inflammatory response: Medication: Other indicators (specify): |
| Spontaneous breathing trial (SBT) |
Yes Unclear No Techniques used for SBT: (e.g. PS, T‐piece, CPAP, not specified) Length of SBT: (e.g. 2 hours) |
| Step‐wise reduction in support (circle type) |
Yes Unclear No If yes, select one of the following: SIMV PS Daily T‐piece Intermittent T‐piece Mixed (specify): Other (specify): |
| Extubation criteria |
Yes Unclear No If yes please specify: |
Outcomes: continuous data
| Outcomes | Unit of measurement | Intervention group | Control group | 95% CI or any further details if outcome only described in text | |||||
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | P value | |||
| Total duration of mechanical ventilation (initiation to mechanical ventilation to discontinuation) | |||||||||
| Weaning duration (identification of weaning to mechanical ventilation discontinuation) | |||||||||
| Mechanical ventilation time before weaning (initiation of mechanical ventilation to identification of weaning) | |||||||||
| PICU length of stay | |||||||||
| Hospital length of stay | |||||||||
| Quality of life for patient | |||||||||
Outcomes: dichotomous data
| Outcomes |
Intervention group (n = ) |
Control group (n = ) |
P‐value | Any further information |
| Reintubation | ||||
| Self‐extubation | ||||
| Tracheostomy | ||||
| PICU mortality | ||||
| Hospital mortality | ||||
| Other |
Other information relevant to the results
| Indicate if any data were obtained from the primary author; if results were estimated from graphs etc. or were calculated by you, using a formula (this should be stated and the formula given). In general, if results not reported in paper(s) are obtained, this should be made clear here, to be cited in review. |
| Freehand space for writing actions such as contact with the authors and changes: |
Appendix 8. Study inclusion criteria and characteristics of the interventions
| Study | Study inclusion | Protocol intervention | Usual care |
| Foronda 2011 | Participants randomly assigned to groups when they met the study inclusion criteria: · 28 days to 15 years · Mechanically ventilated > 24 hours |
1. Daily evaluation of readiness to wean with following criteria: · FiO2 ≤ 0.5 · PEEP ≤ 8 cm H2O · PIP ≤ 25 cm H2O · CXR nothing new · Respiratory drive · No IV sedatives · No neuromuscular blockade · Electrolytes normal · Hemodynamically stable 2. Two‐hour SBT |
Weaning according to discretion of medical team with no influence from protocols, usually when following criteria met: · Wheezing controlled/ respiratory drive · pH ≥ 7.3 · FiO2 ≤ 0.4 · PEEP ≤ 5 cm H2O · PIP ≤ 20 cm H2O · CXR nothing new · No IV sedatives/neuromuscular blockade · Electrolytes normal · Haemodynamically stable |
| Jouvet 2013 | Participants randomly assigned to groups when they met the study inclusion criteria AND passed a PS test: · 2 to 18 years · Weight > 15 kg · Able to breathe spontaneously · No vasopressors/inotropes · FiO2 ≤ 0.60 · PEEP ≤ 8 cm H2O · Plateau pressure ≤ 25 cm H2O · PaCO2 < 70 · ET tube leak ≤ 20% · Ventilator available PS test: PS ± 5 cm H2O of pre‐inclusion plateau pressure for 30 min. Repeated daily until passed (fail if RR > 40 breaths/min; FiO2 > 0.6 on 95% SaO2) |
Weaned using the SmartCare/PS™ computer‐driven explicit computerized protocol. In addition, PEEP was adjusted using a written protocol including the following two guidelines: (1) decrease of PEEP level by 1 cm H2O per 8 hours as far as 5 cm H2O, if FiO2 ≤ 50% with SpO2 ≥ 95%; (2) if FiO2 ≥ 60% to maintain SpO2 ≥ 95% during 1 hour, the attending physician could decide if an increase in PEEP was necessary | Weaned according to individual discretion of medical team without a protocol |
| Maloney 2007 | Participants randomly assigned to groups when they met the study inclusion criteria: Intubated (oral/nasal) for acute respiratory failure AND the following weaning actions had begun: MV > 48 hours and two consecutive reductions in vT, PEEP, mRR or PS |
Computerized decision support tool based on a paper‐based paediatric ventilator weaning protocol Computer system collected real‐time data from other sources. Then using rules (based on the paper protocol), it determined when changes to ventilation were necessary. The computer alerted the RT by using a paging system to log on to view the changes required to the ventilator. Changes were made manually by RTs |
Weaned according to personal clinical judgment of the physician |
Appendix 9. Research recommendation
| Existing evidence | One systematic review (this review) dominated by a large randomized controlled study conducted in a paediatric intensive care unit |
| Population | Children > 28 days and < 18 years old cared for in an intensive care unit; mechanically ventilated via a nasal or oral endotracheal tube |
| Intervention | Protocolized weaning (i.e. the use of an algorithm or written protocol intended to result in early identification of readiness to wean and liberation of patients from invasive mechanical ventilation). This should include frequent assessment of readiness to wean using a set of agreed criteria, followed by a spontaneous breathing trial or step‐wise reduction in ventilator support |
| Comparison | Usual care, which incorporates any non‐protocolized practice. A clear description of usual care should be documented |
| Outcomes | Duration of mechanical ventilation (MV), measured in hours, from initiation of invasive MV to removal of invasive MV Mortality. Reintubation Implementation success (initial acceptance, continued adherence and sustainability) |
| Time stamp | April 2013 |
| Study type | Cluster randomized controlled trial or randomized stepped wedge design with process evaluation of implementation success Blindness: participants and therapists not blind, assessors blind |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Foronda 2011.
| Methods | Multicentre, randomized controlled trial | |
| Participants |
Setting: two hospitals in Sao Paulo, Brazil. Two paediatric ICUs Inclusion criteria: age between 28 days and 15 years; receiving mechanical ventilation for > 24 hours Exclusion criteria: intubation due to upper airway obstruction; diaphragmatic hernia or paralysis; long‐term ventilator use (dependent on invasive or non‐invasive ventilation before ICU admission); cyanotic congenital heart disease; primary pulmonary hypertension; neuromuscular disease; tracheostomy Participant numbers: 312 randomly assigned; 52 withdrawn (29 from protocol group, 23 from standard care group); 260 analysed |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The research fellow conducting the daily evaluation randomly assigned eligible patients by randomly selecting a sealed envelope from an opaque plastic bag containing a 1:1 ratio of test/control group numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medical staff were unaware of participant assignment until the participant passed the daily evaluation and a spontaneous breathing trial was indicated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research fellow was responsible for screening, randomisation and daily evaluations but was not involved in the decision to extubate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Recruitment and attrition were reported |
| Selective reporting (reporting bias) | Low risk | Protocol was registered as ISRCTN37806223 and outcomes were reported |
| Other bias | Low risk | None was apparent |
Jouvet 2013.
| Methods | Single‐centre, randomized controlled trial | |
| Participants |
Setting: pediatric ICU, Montreal, Canada Inclusion criteria: age between 2 and 18 years; body weight ≥ 15 kg; mechanically ventilated > 12 hours; availability of Evita XL respirator with SmartCare/PSTM ; fulfilling weaning criteria (able to breathe spontaneously; no vasopressor or inotropic medication; FiO2 ≤ 60% with pulse oximetry ≥ 95%; PEEP ≤ 8 cm H2O; PaCO2 < 70 mmHg; endotracheal tube leakage ≤ 20%) Exclusion criteria: chronic respiratory insufficiency due to neurological, neuromuscular or lung disease before ICU admission; primary pulmonary hypertension; cyanotic congenital heart disease with unrepaired/palliated right to left intracardiac shunt; not expected to survive; decision to withdraw care; no parental consent Participant numbers: 30 randomly assigned; 30 analysed |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 30 sealed envelopes contained control or SmartCare group sheet of paper (15 each) in a random manner and numbered from 1 to 30. After inclusion of a participant, the research assistant took the envelope n°1, 2, etc (author communication) |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel were unblinded and extubation decision was made by attending clinicians in both groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Recruitment and attrition were reported |
| Selective reporting (reporting bias) | Low risk | Protocol was registered as NCT00678912 and outcomes were reported |
| Other bias | Low risk | None was apparent |
Maloney 2007.
| Methods | Single‐centre, randomized controlled trial | |
| Participants |
Setting: US, paediatric ICU Inclusion criteria: intubated patients with intrinsic lung injury Exclusion criteria: mechanical ventilation through tracheostomy; after surgery/trauma; neuromuscular disease; upper airway disease; cyanotic heart failure Participant numbers: 34 randomly assigned; 3 withdrawn (2 from automated weaning group, 1 from standard care group); 31 analysed |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization was provided in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomization was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Recruitment and attrition were reported |
| Selective reporting (reporting bias) | Low risk | No protocol was provided, but outcomes relevant to trials in this area are reported |
| Other bias | Low risk | None was apparent |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Oliveria 2002 | Study of adult population |
| Restrepo 2004 | Not randomized. Before and after study design |
| Rushforth 2004 | Only three children included in the study who could have contributed data to this review |
Characteristics of studies awaiting assessment [ordered by study ID]
Randolph 2002.
| Methods | Multi‐centre, randomized controlled trial |
| Participants |
Setting: paediatric intensive care units of 10 children's hospitals in North America Inclusion criteria1: children admitted to paediatric ICUs requiring > 24 hours of ventilator support Exclusion criteria: 18 years or older, corrected gestational age < 38 weeks; diaphragmatic hernia or paralysis; ventilator use before admission; cyanotic congenital heart disease with unrepaired/palliated right‐to‐left intracardiac shunt; history of single ventricular defect; significantly diminished lung capacity (resting tidal volume < 6 ml/kg); decreased lung vascularity; anatomical obstruction lower airways; primary pulmonary hypertension or anticipated need for nitric oxide after extubation; previous bone marrow or lung transplant; spinal cord injury above lumbar region; tracheal/upper airway obstructive conditions; status asthmaticus in children 2 years or older; currently enrolled in another trial; decision to withdraw or limit life support Participant numbers: 182 randomly assigned; 3 excluded from analysis (one in each of the three groups); 179 analysed |
| Interventions |
Primary hypothesis: Time to successful extubation for children receiving protocol‐directed weaning (PSV and VSV combined) was equivalent to or less than that seen in children receiving traditional physician‐directed weaning (no protocol) |
| Outcomes |
|
| User defined 1 | |
| Notes | 1The sample included neonates. We are awaiting further communication regarding ability to separate neonatal data from children's data. |
Schultz 2001.
| Methods | Multi‐centre, randomized controlled trial |
| Participants |
Setting: one children's hospital in Philadelphia, Pennsylvania, US. Two ICUs: (a) 38‐bed paediatric ICU and (b) 20‐bed cardiac ICU Inclusion criteria1: all patients requiring intubation and mechanical ventilation Exclusion criteria: central apnoea; requirement for chronic mechanical ventilation; meeting criteria for brain death Participant numbers: 223 enrolled; 4 did not reach study endpoint; 219 analysed |
| Interventions |
|
| Outcomes |
|
| User defined 1 | |
| Notes | 1The sample included neonates. We are awaiting further communication regarding ability to separate neonatal data from children's data |
Differences between protocol and review
The small number of studies included in this review limited our ability to:
synthesize data and conduct a meta‐analysis;
assess statistical heterogeneity;
perform subgroup and sensitivity analyses; and
construct funnel plots to assess reporting biases.
The order of review author names has changed to reflect their relative contributions to the review.
Contributions of authors
Conceiving of the review: Bronagh Blackwood (BB), Peter O'Halloran (POH)
Co‐ordinating the review: Maeve Murray (MM)
Undertaking manual searches: MM, POH
Screening search results: MM, POH, BB
Organizing retrieval of papers: MM, BB
Screening retrieved papers against inclusion criteria: MM, BB, Anthony Chisakuta (AC)
Appraising quality of papers: MM, AC, BB
Abstracting data from papers: MM, AC, BB
Writing to authors of papers for additional information: MM, BB
Providing additional data about papers: MM, BB
Obtaining and screening data on unpublished studies: MM, BB
Providing data management for the review: MM, BB
Entering data into Review Manager (RevMan 5.2): BB
Managing RevMan statistical data: Not applicable
Performing other statistical analysis not using RevMan: BB, Chris R Cardwell (CRC)
Providing double entry of data: Not applicable
Interpreting data: CRC, MM, BB, POH, AC
Making statistical inferences: CRC, BB, POH,
Writing the review: MM, BB, POH, AC
Securing funding for the review: MM, POH
Performing previous work that served as the foundation of the present study: BB, POH
Serving as guarantor for the review (one author): BB
Taking responsibility for reading and checking the review before submission: BB
Sources of support
Internal sources
School of Nursing and Midwifery, and School of Medicine, Dentistry and Biomedical Science, Queen's University Belfast, Northern Ireland, UK.
Belfast Health and Social Care Trust, Research and Development Office, Belfast, Northern Ireland, UK.
External sources
Health and Social Care Public Health Agency, Research and Development Office, Belfast, Northern Ireland. Cochrane Fellowship (Maeve Murray), UK.
Declarations of interest
No financial conflicts of interest have been reported, and the review authors declare that they do not have any associations with any parties who may have vested interests in the results of this review.
Edited (no change to conclusions)
References
References to studies included in this review
Foronda 2011 {published data only}
- Foronda FA, Troster EJ, Farias JA, Barbas CS, Ferraro AA, Faria LS, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Critical Care Medicine 2011;39(11):1‐11. [DOI: 10.1097/CCM.0b013e3182257520] [DOI] [PubMed] [Google Scholar]
Jouvet 2013 {published and unpublished data}
- Jouvet PA, Payen V, Gauvin F, Ereriaud G, Lacroix J. Weaning children from mechanical ventilation with an explicit computerized protocol: a pilot randomized controlled trial. Intensive Care Medicine 2013;39(5):919‐25. [DOI: 10.1007/s00134-013-2837-8] [DOI] [PubMed] [Google Scholar]
Maloney 2007 {unpublished data only}
- Maloney C. Computerized weaning of childhood respiratory failure. PhD Thesis, University of Utah, 2007.
References to studies excluded from this review
Oliveria 2002 {published data only}
- Oliveria LR, Jose A, Dias EC, Santos VLA, Chiavone PA. Weaning protocol for mechanical ventilation: effects of its use in an intensive care unit. A controlled, prospective and randomized trial. Revista Brasileira de Terapia Intensiva 2002;14(1):22‐32. [LILACS: 320673] [Google Scholar]
Restrepo 2004 {published data only}
- Restrepo RD, Fortenberry JD, Spainhour C. Protocol‐driven ventilation management in children: comparison to nonprotocol care. Journal of Intensive Care Medicine 2004;19(5):274‐84. [PUBMED: 15358946] [DOI] [PubMed] [Google Scholar]
Rushforth 2004 {published data only}
- Rushforth K. A randomised controlled trial of weaning from mechanical ventilation in Paediatric Intensive Care (PIC): methodological and practical issues. Intensive and Critical Care Nursing 2005;21:76‐86. [PUBMED: 15778071] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Randolph 2002 {published data only}
- Randolph AG, Wypij DW, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children. A randomized controlled trial. JAMA 2002;288(20):2561‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schultz 2001 {published data only}
- Schultz TR, Lin RJ, Watzman HM, Durning SM, Hales R, Woodson A, et al. Weaning children from mechanical ventilation: a prospective randomized trial of protocol‐directed versus physician‐directed weaning. Respiratory Care 2001;46(8):772‐82. [PubMed] [Google Scholar]
Additional references
Alander 2013
- Ålander M, Peltoniemi O, Saarela T, Anttila E, Pokka T, Kontiokari T. Current trends in paediatric and neonatal ventilatory care – a nationwide survey. Acta Pædiatrica 2013;102:123‐8. [DOI] [PubMed] [Google Scholar]
Alia 2000
- Alía I, Esteban A. Weaning from mechanical ventilation. Critical Care 2000;4(2):72‐80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackwood 2006
- Blackwood B. Methodological issues in evaluating complex health care interventions. Journal of Advanced Nursing 2006;54(5):612‐22. [PUBMED: 16722959] [DOI] [PubMed] [Google Scholar]
Blackwood 2010
- Blackwood B, Alderdice F, Burns KEA, Cardwell CR, Lavery G, O'Halloran P. Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD006904.pub2; PUBMED: 20464747] [DOI] [PubMed] [Google Scholar]
Blackwood 2013
- Blackwood B, Junk C, Lyons J, McAuley DF, Rose L. Roles responsibilities in mechanical ventilation and weaning in pediatric intensive care units: a national survey. American Journal of Critical Care 2013;22(3):189‐97. [doi: http://dx.doi.org/10.4037/ajcc2013xxx] [DOI] [PubMed] [Google Scholar]
Brattebø 2002
- Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients' need for ventilator support in a surgical intensive care unit. BMJ 2002;324:1386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2006a
- Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Medical Research Methodology 2006;6:54. [DOI: 10.1186/1471-2288-6-54] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2006b
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ 2006;333:804‐6. [DOI: 10.1136/bmj.38987.492014.94] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byrd 2010
- Byrd RP Jr. Ventilation, mechanical. eMedicine; http://emedicine.medscape.com/article/304068‐overview updated 13 April 2010 (accessed 13 May 2010).
Clement 1996
- Clement JM, Buck EA. Weaning from mechanical ventilatory support. Dimensions of Critical Care Nursing 1996;15(2):114‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Colville 2012
- Colville G, Pierce C. Patterns of post‐traumatic stress symptoms in families after paediatric intensive care. Intensive Care Medicine 2012;38:1523‐31. [DOI: 10.1007/s00134-012-2612-2] [DOI] [PubMed] [Google Scholar]
Cook 2000
- Cook DJ, Walter SD, Cook RJ, Griffith GH, Leasa D. Incidence and risk factors for ventilator‐associated pneumonia in critically ill patients. Annals of Internal Medicine. Evidence Report /Technology Assessment No. 23 AHRQ Publication No. 01‐E005 2000;23:1‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duyndam 2011
- Duyndam A, Ista E, Houmes RJ, Driel B, Reiss I, Tibboel D. Invasive ventilation modes in children: a systematic review and meta‐analysis. Critical Care 2011;15:R24. [DOI: 10.1186/cc9969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2001
- Ely EW, Meade MO, Haponik EF, Kollef MH, Cook DJ, Guyatt GH, et al. Mechanical ventilator weaning protocols driven by non‐physician health‐care professionals: evidence‐based clinical practice guidelines. Chest 2001;120 Suppl(6):454‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farias 1998
- Farias JA, Alía I, Esteban A, Golubicki AN, Olazarri FA. Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Medicine 1998;24:1070‐5. [DOI] [PubMed] [Google Scholar]
Farias 2001
- Farias JA, Retta A, Alía I, Olazarri F, Esteban A, Golubicki A, et al. Comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Medicine 2001;27:1649‐54. [DOI] [PubMed] [Google Scholar]
Farias 2012
- Farias JA, Fernández A, Monteverde E, Flores JC, Baltodano A, Menchaca A, et al. Latin‐American Group for Mechanical Ventilation in Children. Mechanical ventilation in pediatric intensive care units during the season for acute lower respiratory infection: a multicenter study. Pediatric Critical Care Medicine 2012;13(2):158‐64. [DOI: 10.1097/PCC.0b013e3182257b82] [DOI] [PubMed] [Google Scholar]
Fortenberry 2009
- Fortenberry JD. Do we know when to say "wean"?. Pediatric Critical Care Medicine. 2009;10(1):126‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girard 2008
- Girard TD, Ely EW. Protocol driven ventilator weaning: reviewing the evidence. Clinics in Chest Medicine 2008;29:241‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hansen 2009
- Hansen BS, Severinsson E. Dissemination of research‐based knowledge in an intensive care unit: a qualitative study. Intensive and Critical Care Nursing 2009;25:147‐54. [DOI: 10.1016/j.iccn.2009.02.005] [DOI] [PubMed] [Google Scholar]
Hess 2001
- Hess D. Ventilator modes used in weaning. Chest 2001;120 Suppl:474‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hoskote 2005
- Hoskote A, Cohen G, Goldman A, Shekerdemian L. Tracheostomy in infants and children after cardiothoracic surgery: Indications, associated risk factors, and timing. The Journal of Thoracic and Cardiovascular Surgery 2005;130(4):1086–93. [PMID: 16214524 ] [DOI] [PubMed] [Google Scholar]
Hubble 2000
- Hubble CL, Gentile MA, Tripp DS, Craig DM, Meliones JN, Cheifetz IM. Deadspace to tidal volume ratio predicts successful extubation in infants and children. Critical Care Medicine 2000;28(6):2034‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Intensive Care Society 2007
- Intensive Care Society. National guidelines ‐ when and how to wean. http://www.ics.ac.uk/intensive_care_professional/standards_and_guidelines/weaning_guidelines_2007_ (accessed 3 November 2010).
Kahn 1996
- Khan, N, Brown A, Venkataraman ST. Predictors of extubation success and failure in mechanically ventilated infants and children. Critical Care Medicine 1996;24(9):1568‐79. [Accession Number: 00003246‐199609000‐00023] [DOI] [PubMed] [Google Scholar]
Kollef 1997
- Kollef MH, Shapiro SD, Silver P, John RE, Prentice D, Sauer S, et al. A randomised controlled trial of protocol‐directed weaning versus physician‐directed weaning from mechanical ventilation. Critical Care Medicine 1997 Apr;25(4):567‐74. [PUBMED: 9142019] [DOI] [PubMed] [Google Scholar]
Latour 2011
- Latour JM, Goudoever JB, Schuurman BE, Albers MJIJ, Dam NAM, Dullaart E, et al. A qualitative study exploring the experiences of parents of children admitted to seven Dutch pediatric intensive care units. Intensive Care Medicine 2011;37:319‐25. [DOI: 10.1007/s00134-010-2074-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leclerc 2010
- Leclerc F, Noizet O, Botte A, Binoche A, Chaari W, Sadik A, et al. Weaning from invasive mechanical ventilation in pediatric patients (excluding premature neonates). Archives de Pediatrie 2010;17(4):399‐406. [DOI] [PubMed] [Google Scholar]
Lellouche 2006
- Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, Dojat M. A multicenter randomized trial of computer‐driven protocolized weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 2006 Oct 15;74(8):894‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 1990
- Little LA, Koenig JC Jr, Newth CJ. Factors affecting accidental extubations in neonatal and pediatric intensive care patients. Critical Care Medicine 1990;18(2):163‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacIntyre 2001
- MacIntyre NR, Cook DJ, Ely EW, Epstein SK, Fink JB, Heffner JE, et al. Evidence‐based guidelines for weaning and discontinuing ventilatory support. A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001;6 Suppl:375‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marcin 2005
- Marcin JP, Rutan E, Rapetti PM, Brown JP, Rahnamayi R, Pretzlaff RK. Nurse staffing and unplanned extubation in the PICU.. Pediatric Critical Care Medicine 2005;6(3):254‐7. [PUBMED: 15857520 ] [DOI] [PubMed] [Google Scholar]
Medical Research Council 2008
- Medical Research Council. Developing and evaluating complex interventions: new guidance. www.mrc.ac.uk/complexinterventionsguidance (accessed 22 October 2012).
Morris 2007
- Morris A. Using a computer‐driven system to wean children from mechanical ventilation. Pediatric Critical Care 2007;8(5):494‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murtagh 2007
- Murtagh D, Baum N. Protocols put more pennies in your practice pockets or protocols lead to practice improvement. http://www.neilbaum.com/protocols‐put‐more‐than‐pennies‐in‐your‐practice‐pockets.html (accessed 19 May 2010).
Newth 2009
- Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, et al. Weaning and extubation readiness in pediatric patients. Pediatric Critical Care Medicine 2009;10(1):1‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS Institute 2010
- NHS Institute for Innovation and Improvement. Protocol Based Care. http://www.institute.nhs.uk/quality_and_service_improvement_tools/quality_and_service_improvement_tools/protocol_based_care.html (accessed 13 May 2010).
Noyes 1999
- Noyes J. The impact of knowing your child is critically ill: a qualitative study of mothers' experiences. Journal of Advanced Nursing 1999;29(2):427‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noyes 2000
- Noyes J. 'Ventilator‐dependent' children who spend prolonged periods of time in intensive care units when they no longer have a medical need or want to be there. Journal of Clinical Nursing 2000;9:774‐83. [Google Scholar]
Playfor 2000
- Playfor S. Thomas D. Choonara I. Recollections of children following intensive care. Archives of Disease in Childhood 2000;83:445‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Playfor 2006
- Playfor S, Jenkins I, Boyles C, Choonara I, Davies G, Haywood T, et al. for the United Kingdom Paediatric Intensive Care Society Sedation, Analgesia and Neuromuscular Blockade Working Group. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Medicine 2006;32(8):1125‐36. [PUBMED: 16699772] [DOI] [PubMed] [Google Scholar]
Polito 2011
- Polito A, Patorno E, Costello JM, Salvin JW, Emani SM, Rajagopal S, et al. Perioperative factors associated with prolonged mechanical ventilation after complex congenital heart surgery. Pediatric Critical Care Medicine 2011;12(3):e122‐6. [DOI: 10.1097/PCC.0b013e3181e912bd.] [DOI] [PubMed] [Google Scholar]
Pooni 2013
- Pooni PA, Singh D, Bains HS, Misra BP, Soni RK. Parental stress in a paediatric intensive care unit in Punjab, India. Journal of Paediatrics and Child Health 2013;49(3):204‐9. [DOI: 10.1111/jpc.12127] [DOI] [PubMed] [Google Scholar]
Priestley 2004
- Priestley G, Watson W, Rashidian A, Mozley C, Russell D, Wilson J, et al. Introducing critical care outreach: a ward‐randomized trial of phased introduction in a general hospital. Intensive Care Medicine 2004;30:1398‐404. [PUBMED: 15112033] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rose 2008
- Rose L, Presneill JJ, Johnston L, Cade JF. A randomised,controlled trial of conventional weaning versus an automated system (SmartCareTM/PS) in mechanically ventilated critically‐ill patients. Intensive Care Medicine 2008;34:1788–95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sahn 1973
- Sahn SA, Lakshminarayan S. Bedside criteria for discontinuation of mechanical ventilation. Chest 1973;63(6):1002‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walters 2008
- Walters N, Polkey MI. Weaning strategies in problem patients. Journal of the Intensive Care Society 2008;9(2):173‐5. [Google Scholar]
WHO 2008
- World Health Organisation. Children are not little adults: Children's Health and the Environment WHO Training Package for the Health Sector. www.who.int/entity/ceh/capacity/Children_are_not_little_adults.pdf (accessed 3 May 2013).
WHO 2010
- World Health Organization. Health Status Statistics: Mortality. http://www.who.int/healthinfo/statistics/indneonatalmortality/en/ (accessed 20 October 2010).
Wielenga 2013
- Wielenga JM, Hoogen A, Helder OK, Zanten HA, Bol SP, Blackwood B. Protocolized versus non‐protocolized weaning for reducing the duration of invasive mechanical ventilation in newborn infants (Intervention Review Title). Cochrane Neonatal Group. Title registered 30 January 2013.
Wolfler 2011
- Wolfler A, Calderoni E, Ottonello G, Conti G, Baroncini S, Santuz P, et al. on behalf of the SISPE Study Group. Daily practice of mechanical ventilation in Italian pediatric intensive care units: a prospective survey. Pediatric Critical Care Medicine 2011;12(2):141‐6. [DOI] [PubMed] [Google Scholar]


