Abstract
Context
The lifetime prevalence of anabolic androgenic steroid (AAS) use is estimated at 1% to 5% worldwide. AAS use occurs primarily male elite athletes and men who want a muscular appearance. The evidence for effective, safe management of AAS cessation and withdrawal is weak.
Design
Key studies were extracted from PubMed (1990–2018) and Google Scholar with reference searches from relevant retrieved articles.
Results
The proven adverse effects of AASs include suppression of the gonadal axis and infertility, hirsutism and defeminization in women, and erythrocytosis. Alkylated AASs that are taken orally may cause hepatopathy. There is an association between high-dosage AAS use and increased risk of cardiovascular disease. Clues for AAS use include very low serum high-density cholesterol and sex hormone–binding globulin concentrations and unexplained erythrocytosis. For elite athletes, the biological passport (monitoring of blood or urinary androgen and androgen precursor concentrations after determining the athlete’s baseline) is useful for detecting AAS use. For nonelite athletes, the best method to confirm AAS use is to inquire in a nonjudgmental manner. Cessation of chronic AAS use is associated with a withdrawal syndrome of anxiety and depression.
Conclusions
Men who use AASs <1 year typically recover normal hypothalamic-pituitary-testicular axis function within 1 year after cessation. Men who have infertility due to high-dosage AAS use ≥1 year might benefit from short-term treatment with clomiphene or human chorionic gonadotropin.
AAS use suppresses gonadal function. Many AAS users return to normal gonadal function after discontinuation of AAS, but some might benefit from medical therapy.
There are few topics in endocrinology that generate more academic and public controversy than the use of anabolic androgenic steroids (AASs) or drugs that increase endogenous circulating AASs (e.g., testosterone) for the enhancement of athletic performance, physical function, sense of well-being, sexual function, or cosmetic appearance. The furor is fueled by the weakness of the data on the adverse effects of chronic use of AASs and the absence of evidence about effective management of AAS withdrawal.
There is an important distinction between androgen replacement therapy for male hypogonadism and the use of AASs (i.e., testosterone and its derivatives) at dosages to achieve effects above an individual’s baseline, eugonadal state. The former is an attempt to restore an individual to a “normal” state, whereas the latter use is pharmacotherapy for specific outcomes. Regulatory agencies and the scientific community historically have applied stricter safety standards and have required higher levels of evidence of benefit for pharmacotherapy. This review will focus on the pharmacological use of AASs and drugs that increase endogenous AASs for the purposes of enhancing athletic performance or a more muscular physical appearance.
History
The history of AAS use dates back to the 1870s when the physician-scientist Charles Edouard Brown self-administered an aqueous extract of canine and bovine testes. He reported that he had a remarkable improvement in energy and vigor. His report of this self-experiment led to widespread public use of the Brown-Sequard elixir of testicular extracts; however, Cussons et al. (1) demonstrated that Brown-Sequard’s elixir had homeopathic concentrations of testosterone. More than 60 years after Brown-Sequard’s famous demonstration of the placebo effect, Butenandt and Ruzicka (2) won the Nobel Prize for the synthesis of testosterone. Chemists made numerous derivatives of testosterone in the 1950s. Weightlifters and body builders were the first group to use AASs commonly for competitions, but athletes in other sports quickly adopted the practice (3). AASs were first banned for international competitions for the 1968 Olympics, but many athletes continued to use them and successfully evade detection. Curiously, the scientific community remained skeptical of the performance-enhancing effects of AASs. The athletes were right, but it was not until 1996 that the effects of AAS on strength were convincingly demonstrated in a rigorous study (4).
Epidemiology
The epidemiology of AAS use in athletes and in the general public has been difficult to establish. There are very few recent questionnaire-based studies of AAS use in elite athletes (5, 6). A 2013 questionnaire-based study of elite college athletes in the United States demonstrated a prevalent (lifetime) use of AASs of ∼20% (7). Data collected from standard questionnaires are unreliable because of a high likelihood of false answers (because of a tendency to give socially desirable or acceptable responses). Surveys with hypothetical questions have been used to increase the likelihood of veracity in elite athletes. A 2013 survey study of 212 elite track athletes found that >10% would take a hypothetical performance-enhancing drug that is illegal, but undetectable, if the drug would guarantee an Olympic gold medal (8).
Questionnaire-based studies of members of the US public have demonstrated lifetime prevalence rates of 1% to 15%; these data are sometimes erroneously reported in a manner that suggest current use or incidence (5, 9). These studies have been conducted primarily in adolescents. Data from questionnaires of US teenagers attending school indicate a decline in use over the past few years from a self-reported lifetime prevalence of 1.5% in 8th- to 12th-grade boys in 2013 to 1% in 2017 (10). A recent study using mathematical modeling suggests that 3 to 4 million boys and men between 13 and 50 years of age in the United States have used AASs and 30% to 35% of them have used AASs at high dosages for long enough to experience symptoms of AAS withdrawal (see “Adverse effects of AASs”).
The lifetime prevalence of any AAS use by adult members of the general public might be higher in the United States than many regions in the world, but the prevalence of AAS use is common worldwide (6, 9, 11, 12). A large 2012 Swedish national survey (using a standard questionnaire) study of a sample from the general population demonstrated an adjusted lifetime prevalence of AAS use of 0.7% in men and 0.002% in women (9). A 2014 meta-analysis of worldwide studies of AAS use indicated a lifetime prevalence of 6.4% in men and 1.6% in women, but this analysis was skewed by substantial overrepresentation of questionnaire studies of athletes, prisoners, athletic club and gym users, and other groups at higher risk for reporting AAS use (6).
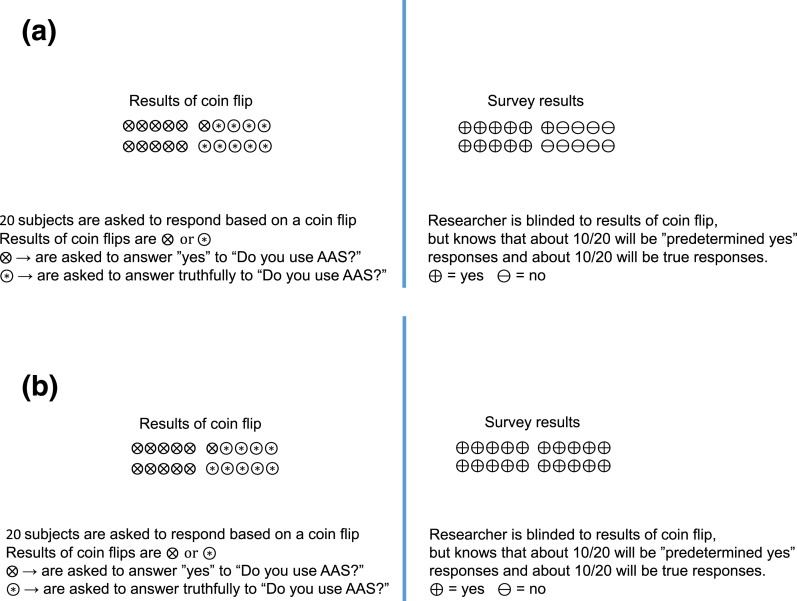
The most accurate data for estimating the prevalence of use of performance-enhancing drugs are generated from studies with questionnaires that use randomized response techniques (5, 13). This technique involves having respondents engage in an activity with a known random probability distribution and, based on the result of the activity, the respondent answers a question with a forced answer or with the truth. The researcher does not know the outcome of the stochastic activity and does not know whether the respondent has answered with a forced response or with the truth. For example, a subject is asked, “Have you used AASs for more than a month in the past 5 years?” The respondent is told to answer “yes” if the result of a coin flip is “heads” (forced response) and to answer truthfully if the result of a coin flip is “tails.” If nobody uses AASs, then the response rate will reflect the results of the coin flips (∼50%:50%). The researcher can estimate the prevalence (always expressed as a range) of AAS use in the truth tellers, but the researcher cannot know an individual’s response because the researcher does not know the outcome of the coin flip (Fig. 1). The use of randomized response techniques has consistently resulted in higher and more accurate estimations of socially undesirable behaviors (5, 13). The results of studies with randomized response questionnaires indicate that 20% to 60% of elite athletes report past-year use of some type of prohibited, performance-enhancing drug, but these studies did not characterize the type of performance-enhancing drugs and specifically did not determine the past-year usage of AAS (5, 14). Nonetheless, these studies suggest that AASs might be much more commonly used by elite and recreational athletes than standard questionnaire studies have suggested.
Figure 1.
Simplified description of randomized response surveys. (A) Scenario of randomized response survey in which no one in a group of 20 uses AASs. In this simplified diagram, each subject responds to a question with a predetermined answer or truthfully based on the results of a coin flip that the researcher cannot see. While protecting anonymity of individual respondents, the reviewer can determine that a very small percentage of the truth responders use AASs. This method provides a range of likely percentages based on statistical analysis of the probability of the results for a given number of coin flips. (B) Scenario of randomized response survey in which everyone in a group of 20 uses AASs. In this simplified diagram, each subject responds to a question with a predetermined answer or truthfully based on the results of a coin flip that the researcher cannot see. While protecting anonymity of individual respondents, the reviewer can determine that a very large percentage of the truth responders use AAS.
In summary, the lifetime prevalence of ever using AASs in men in the general population is probably 1% to 5% worldwide. It is much more common in males than females (>50:1), and long-term AAS users are also predominantly male (9, 12). There is a distinction between lifetime ever-use of AASs and chronic use. Lifetime prevalence use includes a high percentage of short-term (even a single episode of) experimental use in teenagers and young men. Most chronic AAS users have initiated use by their early 20s, and most AAS users are former elite (or near-elite) athletes, body builders, or weight lifters who have muscle dysmorphia or “bigorexia,” terms used to describe a chronic preoccupation about not looking sufficiently muscular (3).
Types of AASs
There are two general mechanisms for increasing circulating AAS: (i) administration of testosterone or synthetic derivatives or (ii) administration of drugs that increase endogenous testosterone production. Classes of drugs that increase endogenous testosterone production include drugs that have luteinizing hormone (LH) activity, drugs that raise LH concentrations, and precursors to testosterone (Table 1). Although drugs that increase endogenous testosterone production are not AASs, I will include them in this review because they are commonly used for the same outcome as AASs: increased muscle mass and strength by increased circulating AASs.
Table 1.
Commonly Used Exogenous AAS and Drugs That Increase Endogenous AAS
| AAS | Drugs That Increase Endogenous AAS |
|---|---|
| Designer AAS (generic/common name) | Gonadotropins |
| Bolandiol | Human chorionic gonadotropin |
| Clostebol (steranabol) | Recombinant human LH |
| Danazol | |
| Drostanolone (Masteron) | Aromatase inhibitors |
| Gestrinone | Anastrozole |
| Metandienone (Dianabol) | Letrozole |
| Metenolone (Primabolan) | Exemestane |
| Oxandrolone (Anavar) | |
| Oxymetholone (Anadrol) | |
| Stanozolol (Winstrol) | Selective estrogen receptor modulators |
| Tetrahydrogestrinone (The Clear) | Clomiphene |
| Trenbolone (Trenabol) | Raloxifene |
| Endogenous AASs used as drugs | Testosterone precursors |
| Testosterone | Androstenedione |
| Dihydrotestosterone | Androsterone |
| Boldenone (Equipoise) | Dehydroepiandrostenedione |
| Nandrolone (Durabolin) |
Human chorionic gonadotropin (hCG) has LH activity that stimulates endogenous testosterone production in men, but not women (15). Exogenous hCG is inexpensive (about the same price as intramuscular testosterone cypionate), widely available, and effective when administered twice weekly as a subcutaneous injection. It is commonly (much more commonly than injectable recombinant human LH) used by athletes and members of the general public who want increased AAS effects on muscle mass and strength. Aromatase inhibitors and selective estrogen receptor modulators such as clomiphene increase circulating LH that stimulates Leydig cells to synthesize testosterone (15). Finally, high dosages of testosterone precursors such dehydroepiandrostenedione increase testosterone production and circulating testosterone concentrations.
Effects of Supraphysiologic Dosages of AASs on Humans
Supraphysiologic dosages of AASs have been primarily used for performance enhancement in athletes and for a muscular appearance in body builders and nonelite athletes (3, 9). There was skepticism by the scientific community that AASs improved athletic performance in sports in which strength conferred an advantage. It was not until 1987 that the American College of Sports Medicine revised its conclusion that AASs were ineffective for muscle strength (16). Even in 1987, it concluded that effect is usually small and idiosyncratic. The athletes knew better. In 1987 and 1988, Ben Johnson shattered the world record for the 100-m sprint after taking AASs, but it was not until 1996 that Bhasin et al. demonstrated that 12 weeks of testosterone (at a dosage of 10 to 12 times the normal daily production rate) significantly increased strength in young trained men and that the effects of testosterone and exercise were additive (4). There is a dosage-dependent effect of AASs on muscle mass and strength (17, 18). The dose-response curve for AASs has not been clearly defined, but male athletes and body builders often use prodigious dosages. They also often use a variety of AASs simultaneously (“stacking”) or sequentially (“cyclically”) because of a belief that these regimens are safer.
With the exception of a few elite female athletes and body builders, women seldom use supraphysiologic dosages of AAS. There are few data on the effects of exogenous androgen therapy in women, but modestly supraphysiologic dosages of testosterone appear to increase muscle mass and strength (19, 20).
Adverse Effects of AASs
The majority of the data on the adverse effects of high dosages of AASs are derived from case reports and series, case-control studies, and cross-sectional studies of men. There are a number of important confounders in these studies. Users of high dosages of AASs are more likely to use tobacco, alcohol, marijuana, and illicit drugs, and they often take high dosages of dietary supplements and nutraceuticals that have not been carefully studied for safety (9, 21). In addition, AASs obtained on the open market or through the internet might be counterfeit or contaminated with other substances. Although the data are weak, chronic use of high dosages of AASs have been associated with adverse effects on several organ systems.
There are very few studies on the adverse effects of chronic use of supraphysiologic dosages of AASs on women. The comments that follow refer mostly to data from studies of men using AASs. I will cite data about adverse effects of AASs on women where studies have been done; there might be important sex-specific differences such as behavioral effects (22).
Reproductive system
Exogenous AASs use suppresses secretion of gonadotropins from the pituitary and thus suppresses endogenous testosterone production and spermatogenesis in men and estrogen and progestogen production and ovulation in women (21, 23–28). Many men who take AASs are infertile during the time of AAS use. Because chronic AAS use suppresses spermatogenesis, the testes shrink. Many men who use AASs will also take hCG to prevent testicular shrinkage. Many men who use AASs report decreased libido or erectile function. This sexual dysfunction might be due to lack of estrogen effect on the brain. In normal men, testosterone is aromatized to estradiol, and estradiol effects on the brain are important for normal libido and overall sexual function. Many AAS compounds are not aromatizable. In addition to amenorrhea or oligomenorrhea and anovulatory infertility, women often develop clitoromegaly with chronic high-dosage AAS use (27, 28).
Neuropsychiatric effects
AASs have been associated with a variety of adverse neuropsychiatric effects, including problems with impulse control, aggression, anxiety, and hypomania or mania (21, 22, 29). The relationship might not might causal, because many men take AASs to enhance their body image; these men often have underlying neuropsychiatric disorders including anxiety and depression. AASs might directly alter neurosignaling in the brain, including pathways using the neurotransmitter, gamma amino butyric acetate (21, 29). There is a dose-dependent effect of AASs on the brain, with very high dosages of AASs causing manic symptoms in normal men (22). Men who stop taking AASs often feel depressed, anxious, and a loss of self-esteem for weeks to months (21, 30). The AAS withdrawal syndrome is probably the result of persistent suppression of endogenous testosterone production during the recovery phase, direct effects of AAS withdrawal on brain neurosignaling, and loss of muscle mass that is disproportionately important for some men. AAS withdrawal syndrome provokes some men to reinitiate AAS use after discontinuation of chronic use.
Cardiovascular system
Exogenous AASs have been associated with a variety of adverse cardiovascular effects, including dyslipidemia, hypertension, increased atherosclerosis, left ventricular hypertrophy, abnormal cardiac remodeling and scarring, arrhythmia, and hypercoagulability (21, 31). Of these potential adverse effects, the strongest data relate to dyslipidemia. AASs have consistently been associated with low serum high-density lipoprotein (HDL) cholesterol concentrations in men and women; orally ingested alkylated AASs may cause profound suppression of serum HDL cholesterol.
Hepatic system
It is a common myth that testosterone is associated with hepatotoxic effects. It is not. However, alkylated testosterone derivatives are hepatoxic, particularly when taken orally (21). These drugs also suppress the hepatic microsomal metabolizing system, leading to prolonged half-lives of many drugs and substances. Hepatic peliosis, cholestasis, and hepatic tumors have also been associated with oral ingestion of alkylated testosterone derivatives.
Other organ systems
There is a known association between the use of exogenous testosterone and other AASs and increased erythropoiesis with a potential risk of erythrocytosis (26). There are a number of case reports of AAS-induced erythrocytosis, but the prevalence of this adverse effect is unknown. Some men report gynecomastia with AASs, particularly with high dosages of testosterone or hCG. This effect is presumably the result of higher estrogen to androgen ratios that occur with the initiation or a dosage increase of these drugs. Many men will use an aromatase inhibitor (to decrease conversion of testosterone to estradiol) in combination with AAS to prevent gynecomastia, but this approach has not been shown to be proven effective. For men who use an aromatase inhibitor to prevent gynecomastia resulting from AAS use, the decreased estradiol production is likely to cause loss of bone mineral density, increased total body fat, and may result in decreased libido and sexual function (32). Women using high dosages of AASs will chronically experience breast atrophy. Long-term AAS use is associated with increased risk of tendinous rupture (33). Upper extremity tendinous ruptures are much more common among AAS users than the general population. AAS use might be associated with chronic kidney disease, but this association is weak and might be due to contaminants or adulterants in AASs (21). Chronic AAS use may cause acne and male pattern alopecia in men and women and hirsutism in women.
In summary, there is strong evidence that AAS causes reversible suppression of endogenous sex steroid hormone production, marked decreases in serum HDL cholesterol, and decreased fertility in men and women. In addition, AASs are known to increase erythropoiesis and may induce erythrocytosis. There is emerging evidence of an AAS withdrawal syndrome, and there is weak, inferential evidence for an association with adverse cardiovascular effects. Alkylated AASs taken orally cause hepatopathy. There is some evidence that that AAS use is associated with increased risk of tendinous ruptures, particularly of the upper extremity.
Detection of AAS Use
Detection in the elite athlete
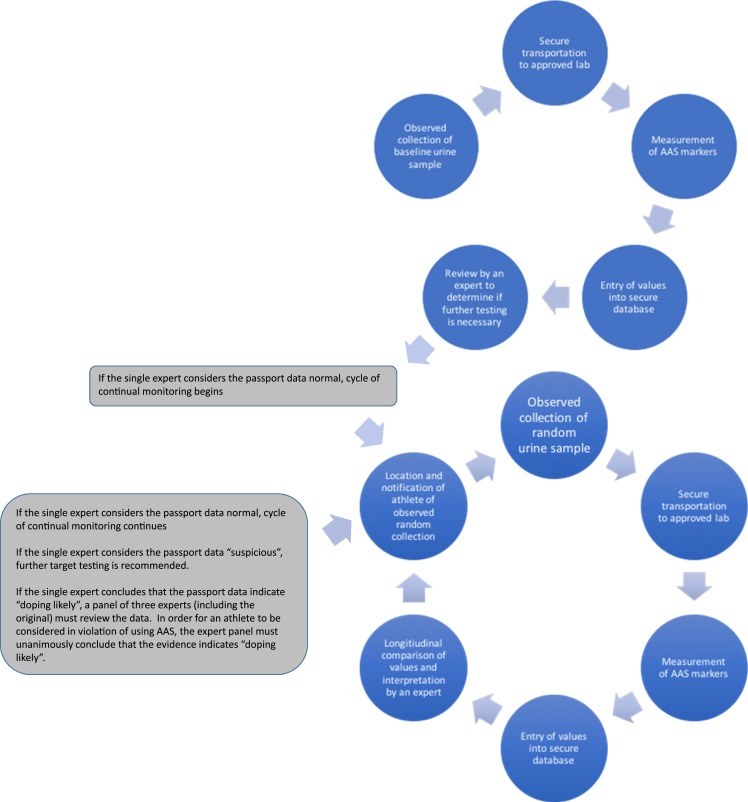
Detection of AAS use in the elite athlete has become a small industry. Sophisticated laboratory techniques have developed with funding from governments and sports industries. The latest development in the detection of use of AASs (and other performance-enhancing drugs) has been the biological passport. The concept of a biological passport assumes that an individual’s serum and urine hormone and prohormones do not change significantly over time (34). An athlete’s urinary and serum testosterone, dihydrotestosterone, epitestosterone (another endogenous testicular steroid), and their precursors are measured at baseline (before the first competition that has antidoping regulations) and are followed longitudinally. Any deviation from baseline that is considered important is followed with additional testing (Fig. 2). The biological passport is intended to detect attempts to use exogenous AASs or attempts to increase endogenous AASs by drugs that stimulate endogenous production of AASs.
Figure 2.
The biological passport. The circles at top show the steps for creating baseline measurements for an individual athlete’s biological passport. The circles at bottom show the monitoring cycle after establishment of the baseline. [Reproduced with permission from Anawalt BD. Detection of anabolic androgenic steroid use by elite athletes and by members of the general public. Mol Cell Endocrinol. 2018;464:21–27.]
The biological passport is a simple concept, but is fraught with potential problems. The concept assumes that athletes have not used AAS before initial, baseline collection for the passport process. Many athletes begin using AASs at ages 16 through 22, before they might become accomplished enough to compete in events that require a biological passport; their “baseline” blood and urine samples at the time of enrollment in the biological passport program might not reflect their true baseline status (12). The biological passport also depends on the premise that all compounds with androgenic anabolic properties will significantly affect endogenous production and secretion of pituitary gonadotropins and/or sex steroid hormone precursors. That supposition might prove to be false as chemists continue to develop selective androgen receptor modulators with differential effects on tissues and organs. The biological passport also assumes that urinary samples are securely transported and stored, an assumption that has not always been true. There is also an assumption that data are entered correctly and stored in a secure database. Breaches of “secure databases” have been common in recent years (35).
Detection in the general public
Men (and a very small number of women) may use AASs to enhance their appearance or improve performance in nonelite athletic competition. There is no practical diagnostic test to detect their AAS use. The tests to detect AASs that are used by the World Anti-Doping Agency and other antidoping agencies are not clinically available. Also, if a patient wishes to avoid detection, he or she can easily do so by avoiding use of AASs at the time of testing or by submitting someone else’s urine sample.
Chronic AAS use should be considered when a muscular man presents with infertility or onset of gynecomastia, or a hirsute, muscular woman reports amenorrhea. Serum high-density cholesterol and sex hormone–binding globulin concentrations are often very low. The most useful of the commonly available tests are measurement of serum testosterone, follicle stimulating hormone (FSH), and LH concentrations. Because exogenous testosterone, nontestosterone AASs, or hCG suppress circulating gonadotropin concentrations, measurement of serum testosterone, FSH, and LH concentrations are useful for determining the likelihood of AAS use (Table 2). In general, the most useful “diagnostic test” is to ask about the use of AASs.
Table 2.
Serum Hormone Concentrations During Chronic Use of AASs or Drugs That Increase Endogenous AASs
| AAS Used | Testosterone | FSH | LH |
|---|---|---|---|
| Testosterone | ↑ | ↓ | ↓ |
| Testosterone precursorsa,b | ↑ | ↓ | ↓ |
| Nontestosterone AAS | ↓ | ↓ | ↓ |
| hCGb | ↑ | ↓ | ↓ |
| Aromatase inhibitor aloneb | ↑ | ↑ | ↑ |
| Clomipheneb | ↑ | ↑ | ↑ |
High dosages of precursors required.
Not technically AAS; see text.
Management of AAS Use
Management in the elite athlete
For elite athletes participating in sports in which AASs are prohibited, there is a process for obtaining permission (therapeutic use exemption) from the relevant antidoping agency for the use of a banned substance (e.g., testosterone replacement therapy for treatment of hypogonadism resulting from Klinefelter syndrome). Endocrinologists may be asked to provide documentation (e.g., chart notes, results of diagnostic studies) of the medical indication for testosterone replacement therapy. The granting of a therapeutic use exemption for testosterone has strict guidelines, including documentation of symptoms or signs of androgen deficiency, unequivocally low early morning serum testosterone concentrations on more than one occasion, and a specific cause for the diagnosis of male hypogonadism. Therapeutic use exemptions for the treatment of male hypogonadism are usually granted only for testosterone or gonadotropin therapy. Therapeutic use exemptions are not usually approved retrospectively or for nontestosterone AASs.
For the elite athlete who has been punished for the unapproved use of AASs, the management is generally straightforward and not under the purview of the endocrinologist. The athlete must discontinue using the banned drug, generally without the use of any hormone therapy to mitigate against AAS withdrawal syndrome.
The endocrinologist may have an important role in the treatment of retired elite male athletes who may have used AASs while active in sports. Some of these retired athletes have persistently low serum testosterone concentrations and normal serum gonadotropin concentrations without an identifiable cause of secondary hypogonadism. These retired athletes might not be willing to divulge that they used AASs because of the fear of consequences of leaked information to the public, such as a damaged reputation and loss of lucrative endorsements and employment opportunities in their sport. The medical management of AASs is the same as the management for nonathletes (see the following section).
Management in the general public
Because there are no clinical trials of the management of long-term AAS use by members of the general public, care is based on the likelihood of severe AAS withdrawal symptoms, the near-term goals of the patient, and a risk-benefit analysis of the treatment options compared with continued AAS use. All AAS users should be queried about anxiety and depressive disorders and alcohol or illicit drug abuse and offered a referral for treatment if appropriate. The key questions when caring for a patient who has been using AASs for more than a year include the following: Does the patient want to quit using AASs? Why does the patient want to quit using AASs? How long has the patient been using AASs and what regimen(s)?
Some men do not want to stop using AASs. In this setting, the endocrinologist should inform the man about the known side effects (including reduced fertility that might take 1 to 2 years to normalize after discontinuation of AASs, erythrocytosis, and dyslipidemia) and potential adverse effects with a focus on the cardiovascular system. Some of these men may be willing to “convert” to prescription testosterone. For these patients, the author has prescribed intramuscular testosterone at dosages of up to twice the typical replacement dosage with a taper to a physiological dosage over several months. With this approach, the patient avoids severe AAS withdrawal symptoms and the uncertain safety of AASs purchased over the internet and other unregulated sources. In addition, the clinician builds a relationship with the patient that facilitates safety monitoring and possible eventual discontinuation of AASs.
For men who want to quit using AASs, there are several management options: (i) immediate discontinuation with no medical therapy; (ii) discontinuation and initiation of a limited course of clomiphene therapy; (iii) discontinuation and initiation of a limited course of hCG therapy; or (iv) conversion of nonprescription AASs to prescription testosterone. Note that the US Food and Drug Administration has not approved the use of clomiphene, hCG, or testosterone for the treatment of AAS withdrawal, and there are no clinical trials of medical therapy for AAS withdrawal.
Most men who want to quit using AASs come to the endocrinologist because of infertility. These men often feel some urgency to have improved spermatogenesis and fertility. The endocrinologist should assess for and treat other causes of impaired male fertility (36) and confirm that the man has secondary hypogonadism with an early morning serum testosterone and gonadotropins. At least two baseline sperm samples obtained (each after 3-7 days of ejaculatory abstinence) should be submitted for seminal fluid analysis for assessment of sperm concentration, motility, and morphology. In addition, the endocrinologist should inquire about the likely fertility of the patient’s female partner; causes of decreased female fertility should be addressed (37). The key questions are the female’s age, the regularity of her menses, and her history of pregnancy. If she is younger than age 30 years and has had regular periods or a history of pregnancy in the past 5 years, then the “urgency” to treat to improve spermatogenesis and fertility in the male patient is based on the couples’ preference for timing of having children. In this case, a reasonable option is discontinuation of AASs with monitoring of serum testosterone and gonadotropins every 1 to 3 months and seminal fluid analysis 3 to 6 months after serum testosterone has normalized. Because female fertility is significantly lower by age 35, it is clinically urgent to improve spermatogenesis and fertility in the male partner of women who are older than 30 years. For men who have been using AASs for ≤1 year, cessation of AAS use might be sufficient intervention. For men who are infertile and have a recent history of using high dosages of AASs for >1 year, the time to recovery of normal serum gonadotropins and testosterone and the even longer lag time for recovery of normal spermatogenesis might be excessive. Initiation of clomiphene or gonadotropin therapy after discontinuation of chronic AAS use (>1 year of use) might increase spermatogenesis and fertility months earlier than discontinuation of AASs without additional therapy. Initiation of a testosterone taper is not recommended in men who are seeking improved fertility because exogenous testosterone therapy suppresses the hypothalamic-pituitary-gonadal axis.
Some experts recommend reassurance only for men who are discontinuing chronic AAS use. This approach is based on the observation that spontaneous recovery of the hypothalamic-pituitary-testicular axis occurs in many men who use AASs. In clinical trials of testosterone-based male hormone regimens, serum gonadotropin and testosterone concentrations typically normalize within 1 to 3 months and sperm concentrations typically normalize within 12 to 16 months after cessation of exogenous testosterone (38–41). These male hormone contraceptive regimens have used physiologic to modestly supraphysiologic dosages of testosterone for <2 years. A 2017 meta-analysis of the effects of much higher dosages of potent AASs on the reproductive system of users found only 33 studies (1766 users and 2113 nonuser controls) that met criteria for inclusion; one-half of the subjects were in one cohort study) (25). Three studies were randomized, controlled trials, and six studies (no randomized, controlled trials) had data on women. There was substantial heterogeneity of the studies and the risk of bias in the studies was unclear. There were seven studies with hormone data (testosterone and/or gonadotropin concentrations) after immediate cessation of AASs and during recovery, and there were three studies (n = 17 AAS users) with hormone data before initiation of AAS use and during recovery from cessation of AAS use. The studies included in this meta-analysis generally had an AAS exposure ≤1 year. Based on limited data, it appears that serum gonadotropins recover to normal concentrations within 3 to 6 months, followed by recovery of serum testosterone to baseline within 6 months in most but not all men who use AASs ≤1 year. Case series have shown that many men who use high-dosage AASs for >1 year will have symptoms suggestive of hypogonadism and serum testosterone concentrations below normal for years after cessation of AAS use (23, 24).
Clomiphene is a selective estrogen receptor modulator that increases serum gonadotropins and testosterone concentrations within 2 to 4 weeks in normal men and in men with intact pituitary function and low or normal serum gonadotropin but low serum testosterone concentrations (15, 42–44). One 3-month randomized prospective study and three longer-term cohort studies (mean time of therapy, 1.5 to 2 years) reported no substantial adverse events at dosages of up 100 mg every other day in younger men (<50 years old) with baseline low testosterone concentrations (42–45). There were no important adverse events in these studies, but safety data did not appear to be systematically reported. In women, selective estrogen receptor modulators (e.g., clomiphene) increase the risk of venous thrombosis (46). There are case reports of clomiphene-induced venous thrombosis in men (47, 48). Anecdotal reports suggest that some male chronic AAS users may have recovery of their hypothalamic-pituitary-gonadal axis after clomiphene therapy for AAS withdrawal (49, 50).
The benefits of normalization of serum gonadotropins and testosterone followed by potentially improved spermatogenesis 3 to 6 months later might be worth the risk of uncertain safety for some men with AAS use >1 year who are willing to quit using AASs and who are attempting to conceive a child with a female partner as soon as possible. A 6- to 12-month trial of clomiphene at a dosage sufficient to increase serum testosterone to the upper half of the normal range is reasonable in these men. Initiate oral clomiphene 25 mg every other day and increase the dosage by 12.5 to 25 mg every other day (up to 100 mg every other day) based on monthly serum testosterone concentrations. After 3 to 4 months of maintenance of serum testosterone concentrations within the normal range, the dosage of clomiphene is reduced by 12.5 mg every other day at monthly intervals. The clinician should inform the patient that the safety and effectiveness of the approach has not been proven and that clomiphene might increase the risk of venous thrombosis. The clinician must document the conversation in the medical record before prescribing clomiphene.
Similarly, hCG therapy might be useful in the same group of men. Subcutaneous hCG therapy stimulates testosterone production and spermatogenesis in most men with hypogonadotropic hypogonadism with testes that are ≥6 mL (36). Administration of hCG suppresses endogenous production of LH and FSH; therefore, the gonadal axis will remain suppressed after discontinuation of hCG. The usual dosage of hCG is 1000 to 2000 IU subcutaneously 2 to 3 times weekly. The dosage is adjusted until serum total testosterone is in the normal range and continued at least until conception (36). There is more clinical experience and published data on the safety and effectiveness of hCG therapy than clomiphene therapy in men, but there are no data on the comparative effectiveness of these drugs. If a man with a history of long-term AAS use is treated with either hCG or clomiphene fails to have increased serum testosterone concentrations after 3 months of treatment, the clinician should consider the possibility of underlying classic causes of hypogonadism such as Klinefelter syndrome or pituitary tumor.
For the chronic, high-dosage AAS male user who is not interested fertility in the near future, the author sometimes prescribes relatively high dosages of injectable testosterone with a tapering of testosterone over several months. This approach may help facilitate convincing a man to quit AAS use and mitigates AAS withdrawal syndrome.
Conclusion
There are many gaps in our knowledge about the management of chronic AAS use. The most important gaps relate to the long-term outcomes on health and the safest and most effective treatments of AAS withdrawal syndrome and AAS-induced infertility. Most men who use AASs for ≤1 year will recover normal hypothalamic-pituitary-testicular axis function within 3 to 6 months after discontinuation of AAS use. Short treatment (≤1 year) with clomiphene or hCG therapy is likely to be useful for men with suppressed gonadotropins and spermatogenesis resulting from high-dosage AAS usage for >1 year. Men who are treated with clomiphene or hCG after cessation of AASs tend to have minimal or less AAS withdrawal symptoms than those men who are simply stop taking AASs. The diagnosis and management of chronic AAS use requires a good rapport with the patient, clinical judgment, and shared decision-making.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants HSN27500006, HHSN27500003, and U54HD042454-06.
Disclosure Summary: B.D.A. is a consultant for the US Anti-Doping Agency and is an UpToDate author.
Glossary
Abbreviations:
- AAS
anabolic androgenic steroid
- FSH
follicle stimulating hormone
- hCG
human chorionic gonadotropin
- HDL
high-density lipoprotein
- LH
luteinizing hormone
References
- 1. Cussons AJ, Bhagat CI, Fletcher SJ, Walsh JP. Brown-Séquard revisited: a lesson from history on the placebo effect of androgen treatment. Med J Aust. 2002;177(11-12):678–679. [DOI] [PubMed] [Google Scholar]
- 2. Nieschlag E, Nieschlag S. Testosterone deficiency: a historical perspective. Asian J Androl. 2014;16(2):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanayama G, Pope HG Jr. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol Cell Endocrinol. 2018;464:4–13. [DOI] [PubMed] [Google Scholar]
- 4. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7. [DOI] [PubMed] [Google Scholar]
- 5. de Hon O, Kuipers H, van Bottenburg M. Prevalence of doping use in elite sports: a review of numbers and methods. Sports Med. 2015;45(1):57–69. [DOI] [PubMed] [Google Scholar]
- 6. Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24(5):383–398. [DOI] [PubMed] [Google Scholar]
- 7. Buckman JF, Farris SG, Yusko DA. A national study of substance use behaviors among NCAA male athletes who use banner performance enhancing substances. Drug Alcohol Depend. 2013;131(1–2):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connor J, Woolf J, Mazanov J. Would they dope? Revisiting the Goldman dilemma. Br J Sports Med. 2013;47(11):697–700. [DOI] [PubMed] [Google Scholar]
- 9. Hakansson A, Mickelsson K, Wallin C, Berglund M. Anabolic androgenic steroids in the general population: user characteristics and associations with substance use. Eur Addict Res. 2012;18(2):83–90. [DOI] [PubMed] [Google Scholar]
- 10.The University of Michigan Institute for Social Research. Monitoring the future. National survey results on drug use. Available at: http://monitoringthefuture.org/pubs/monographs/mtf-overview2017.pdf. Accessed 1 August 2018.
- 11. Abrahin OS, Sousa EC, Santos AM. Prevalence of the use of anabolic-androgenic steroids in Brazil: a systematic review. Subst Use Misuse. 2014;49(9):1156–1162. [DOI] [PubMed] [Google Scholar]
- 12. Pope HG Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am J Addict. 2014;23(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lensvelt-Mulders GJLM, Hox JJ, Van der Heijden PGM, Maas CJM. Meta-analysis of randomized response research: thirty-five years of validation. Sociol Methods Res. 2005;33(3):319–348. [Google Scholar]
- 14. Ulrich R, Pope HG Jr, Cléret L, Petróczi A, Nepusz T, Schaffer J, Kanayama G, Comstock RD, Simon P. Doping in two elite athletics competitions assessed by randomized-response surveys. Sports Med. 2018;48(1):211–219. [DOI] [PubMed] [Google Scholar]
- 15. Handelsman DJ. Clinical review: the rationale for banning human chorionic gonadotropin and estrogen blockers in sport. J Clin Endocrinol Metab. 2006;91(5):1646–1653. [DOI] [PubMed] [Google Scholar]
- 16.American College of Sports Medicine position stand on the use and abuse of anabolic-androgenic steroids in sports. Med Sci Sports Exerc. 1987;19:534–539. [PubMed] [Google Scholar]
- 17. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–E1181. [DOI] [PubMed] [Google Scholar]
- 18. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688. [DOI] [PubMed] [Google Scholar]
- 19. Huang G, Basaria S, Travison TG, Ho MH, Davda M, Mazer NA, Miciek R, Knapp PE, Zhang A, Collins L, Ursino M, Appleman E, Dzekov C, Stroh H, Ouellette M, Rundell T, Baby M, Bhatia NN, Khorram O, Friedman T, Storer TW, Bhasin S. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21(6):612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang G, Basaria S. Do anabolic-androgenic steroids have performance-enhancing effects in female athletes? Mol Cell Endocrinol. 2018;464:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pope HG Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014;35(3):341–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onakomaiya MM, Henderson LP. Mad men, women and steroid cocktails: a review of the impact of sex and other factors on anabolic androgenic steroids effects on affective behaviors. Psychopharmacology (Berl). 2016;233(4):549–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanayama G, Hudson JI, DeLuca J, Isaacs S, Baggish A, Weiner R, Bhasin S, Pope HG Jr. Prolonged hypogonadism in males following withdrawal from anabolic-androgenic steroids: an under-recognized problem. Addiction. 2015;110(5):823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasmussen JJ, Selmer C, Østergren PB, Pedersen KB, Schou M, Gustafsson F, Faber J, Juul A, Kistorp C. Former abusers of anabolic androgenic steroids exhibit decreased testosterone levels and hypogonadal symptoms years after cessation: a case-control study. PLoS One. 2016;11(8):e0161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Christou MA, Christou PA, Markozannes G, Tsatsoulis A, Mastorakos G, Tigas S. Effects of anabolic androgenic steroids on the reproductive system of athletes and recreational users: a systematic review and meta-analysis. Sports Med. 2017;47(9):1869–1883. [DOI] [PubMed] [Google Scholar]
- 26. Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101(5):1271–1279. [DOI] [PubMed] [Google Scholar]
- 27. Ip EJ, Barnett MJ, Tenerowicz MJ, Kim JA, Wei H, Perry PJ. Women and anabolic steroids: an analysis of a dozen users. Clin J Sport Med. 2010;20(6):475–481. [DOI] [PubMed] [Google Scholar]
- 28. Gruber AJ, Pope HG Jr. Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69(1):19–26. [DOI] [PubMed] [Google Scholar]
- 29. Piacentino D, Kotzalidis GD, Del Casale A, Aromatario MR, Pomara C, Girardi P, Sani G. Anabolic-androgenic steroid use and psychopathology in athletes. A systematic review. Curr Neuropharmacol. 2015;13(1):101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grönbladh A, Nylander E, Hallberg M. The neurobiology and addiction potential of anabolic androgenic steroids and the effects of growth hormone. Brain Res Bull. 2016;126(Pt 1):127–137. [DOI] [PubMed] [Google Scholar]
- 31. Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, Pope HG Jr. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation. 2017;135(21):1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanayama G, DeLuca J, Meehan WP III, Hudson JI, Isaacs S, Baggish A, Weiner R, Micheli L, Pope HG Jr. Ruptured tendons in anabolic-androgenic steroid users: a cross-sectional cohort study. Am J Sports Med. 2015;43(11):2638–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anawalt BD. Detection of anabolic androgenic steroid use by elite athletes and by members of the general public. Mol Cell Endocrinol. 2018;464:21–27. [DOI] [PubMed] [Google Scholar]
- 35. Mele C. Data breaches keep happening. So why don't you do something? New York Times. August 1, 2018. https://www.nytimes.com/2018/08/01/technology/data-breaches.html. Accessed 6 May 2019. [Google Scholar]
- 36. Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab. 2013;98(9):3532–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McLaren JF. Infertility evaluation. Obstet Gynecol Clin North Am. 2012;39(4):453–463. [DOI] [PubMed] [Google Scholar]
- 38. Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C; Hormonal Male Contraception Summit Group . Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367(9520):1412–1420. [DOI] [PubMed] [Google Scholar]
- 39. Gu YQ, Tong JS, Ma DZ, Wang XH, Yuan D, Tang WH, Bremner WJ. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in chinese men. J Clin Endocrinol Metab. 2004;89(5):2254–2262. [DOI] [PubMed] [Google Scholar]
- 40. Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81(2):757–762. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization Task Force on Methods for the Regulation of Male Fertility Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65(4):821–829. [PubMed] [Google Scholar]
- 42. Habous M, Giona S, Tealab A, Aziz M, Williamson B, Nassar M, Abdelrahman Z, Remeah A, Abdelkader M, Binsaleh S, Muir G. Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study. BJU Int. 2018;122(5):889–897. [DOI] [PubMed] [Google Scholar]
- 43. Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110(10):1524–1528. [DOI] [PubMed] [Google Scholar]
- 44. Katz DJ, Nabulsi O, Tal R, Mulhall JP. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110(4):573–578. [DOI] [PubMed] [Google Scholar]
- 45. Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med. 2010;7(1 Pt 1):269–276. [DOI] [PubMed] [Google Scholar]
- 46. Artero A, Tarín JJ, Cano A. The adverse effects of estrogen and selective estrogen receptor modulators on hemostasis and thrombosis. Semin Thromb Hemost. 2012;38(8):797–807. [DOI] [PubMed] [Google Scholar]
- 47. Politou M, Gialeraki A, Merkouri E, Travlou A, Baltatzis S. Central retinal vein occlusion secondary to clomiphene treatment in a male carrier of factor V Leiden. Genet Test Mol Biomarkers. 2009;13(2):155–157. [DOI] [PubMed] [Google Scholar]
- 48. Zahid M, Arshad A, Zafar A, Al-Mohannadi D. Intracranial venous thrombosis in a man taking clomiphene citrate. BMJ Case Rep. 2016;2016:bcr2016217403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan RS, Vasudevan D. Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertil Steril. 2003;79(1):203–205. [DOI] [PubMed] [Google Scholar]
- 50. Guay AT, Jacobson J, Perez JB, Hodge MB, Velasquez E. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res. 2003;15(3):156–165. [DOI] [PubMed] [Google Scholar]