Abstract
Background
The central venous catheter (CVC) is essential in managing acutely ill patients in hospitals. Bloodstream infection is a major complication in patients with a CVC. Several infection control measures have been developed to reduce bloodstream infections, one of which is impregnation of CVCs with various forms of antimicrobials (either with an antiseptic or with antibiotics). This review was originally published in June 2013 and updated in 2016.
Objectives
Our main objective was to assess the effectiveness of antimicrobial impregnation, coating or bonding on CVCs in reducing clinically‐diagnosed sepsis, catheter‐related blood stream infection (CRBSI), all‐cause mortality, catheter colonization and other catheter‐related infections in adult participants who required central venous catheterization, along with their safety and cost effectiveness where data were available. We undertook the following comparisons: 1) catheters with antimicrobial modifications in the form of antimicrobial impregnation, coating or bonding, against catheters without antimicrobial modifications and 2) catheters with one type of antimicrobial impregnation against catheters with another type of antimicrobial impregnation. We planned to analyse the comparison of catheters with any type of antimicrobial impregnation against catheters with other antimicrobial modifications, e.g. antiseptic dressings, hubs, tunnelling, needleless connectors or antiseptic lock solutions, but did not find any relevant studies. Additionally, we planned to conduct subgroup analyses based on the length of catheter use, settings or levels of care (e.g. intensive care unit, standard ward and oncology unit), baseline risks, definition of sepsis, presence or absence of co‐interventions and cost‐effectiveness in different currencies.
Search methods
We used the standard search strategy of the Cochrane Anaesthesia, Critical and Emergency Care Review Group (ACE). In the updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 3), MEDLINE (OVID SP; 1950 to March 2015), EMBASE (1980 to March 2015), CINAHL (1982 to March 2015), and other Internet resources using a combination of keywords and MeSH headings. The original search was run in March 2012.
Selection criteria
We included randomized controlled trials (RCTs) that assessed any type of impregnated catheter against either non‐impregnated catheters or catheters with another type of impregnation in adult patients cared for in the hospital setting who required CVCs. We planned to include quasi‐RCT and cluster‐RCTs, but we identified none. We excluded cross‐over studies.
Data collection and analysis
We extracted data using the standard methodological procedures expected by Cochrane. Two authors independently assessed the relevance and risk of bias of the retrieved records. We expressed our results using risk ratio (RR), absolute risk reduction (ARR) and number need to treat to benefit (NNTB) for categorical data and mean difference (MD) for continuous data, where appropriate, with their 95% confidence intervals (CIs).
Main results
We included one new study (338 participants/catheters) in this update, which brought the total included to 57 studies with 16,784 catheters and 11 types of impregnations. The total number of participants enrolled was unclear, as some studies did not provide this information. Most studies enrolled participants from the age of 18, including patients in intensive care units (ICU), oncology units and patients receiving long‐term total parenteral nutrition. There were low or unclear risks of bias in the included studies, except for blinding, which was impossible in most studies due to the catheters that were being assessed having different appearances. Overall, catheter impregnation significantly reduced catheter‐related blood stream infection (CRBSI), with an ARR of 2% (95% CI 3% to 1%), RR of 0.62 (95% CI 0.52 to 0.74) and NNTB of 50 (high‐quality evidence). Catheter impregnation also reduced catheter colonization, with an ARR of 9% (95% CI 12% to 7%), RR of 0.67 (95% CI 0.59 to 0.76) and NNTB of 11 (moderate‐quality evidence, downgraded due to substantial heterogeneity). However, catheter impregnation made no significant difference to the rates of clinically diagnosed sepsis (RR 1.0, 95% CI 0.88 to 1.13; moderate‐quality evidence, downgraded due to a suspicion of publication bias), all‐cause mortality (RR 0.92, 95% CI 0.80 to 1.07; high‐quality evidence) and catheter‐related local infections (RR 0.84, 95% CI 0.66 to 1.07; 2688 catheters, moderate quality evidence, downgraded due to wide 95% CI).
In our subgroup analyses, we found that the magnitudes of benefits for impregnated CVCs varied between studies that enrolled different types of participants. For the outcome of catheter colonization, catheter impregnation conferred significant benefit in studies conducted in ICUs (RR 0.70;95% CI 0.61 to 0.80) but not in studies conducted in haematological and oncological units (RR 0.75; 95% CI 0.51 to 1.11) or studies that assessed predominantly patients who required CVCs for long‐term total parenteral nutrition (RR 0.99; 95% CI 0.74 to 1.34). However, there was no such variation for the outcome of CRBSI. The magnitude of the effects was also not affected by the participants' baseline risks.
There were no significant differences between the impregnated and non‐impregnated groups in the rates of adverse effects, including thrombosis/thrombophlebitis, bleeding, erythema and/or tenderness at the insertion site.
Authors' conclusions
This review confirms the effectiveness of antimicrobial CVCs in reducing rates of CRBSI and catheter colonization. However, the magnitude of benefits regarding catheter colonization varied according to setting, with significant benefits only in studies conducted in ICUs. A comparatively smaller body of evidence suggests that antimicrobial CVCs do not appear to reduce clinically diagnosed sepsis or mortality significantly. Our findings call for caution in routinely recommending the use of antimicrobial‐impregnated CVCs across all settings. Further randomized controlled trials assessing antimicrobial CVCs should include important clinical outcomes like the overall rates of sepsis and mortality.
Keywords: Adult, Humans, Anti‐Infective Agents, Anti‐Infective Agents/administration & dosage, Catheter‐Related Infections, Catheter‐Related Infections/prevention & control, Central Venous Catheters, Central Venous Catheters/adverse effects, Randomized Controlled Trials as Topic
Plain language summary
Central venous catheter coating with antiseptics or antibiotics for reducing catheter‐related infections in adults
Background
CVCs are essential devices for giving fluids, medications, intravenous nutrition and cancer treatment to patients. Compared to peripheral catheters (i.e. tubes inserted via veins in the limbs that are designed for short‐term use), CVCs are longer and reach deeper into the major veins of the body, providing a more secure and durable intravenous access. However, infections, especially of the bloodstream, are common in patients with CVCs. Sometimes these infections are fatal. Several measures have been developed to reduce such infections, including coating or impregnation of CVCs with antiseptics or antibiotics. While these new technologies are promising, it is not clear whether they provide effective protection for a sufficiently long period against the wide variety of bacteria that might adapt to any strategy designed to overcome them. Furthermore, the benefits of these modified catheters in different settings, e.g. intensive care units (ICU), standard wards and cancer units, also require on‐going evaluation. Many clinical guidelines recommend the use of antimicrobial‐impregnated CVCs, although studies reveal conflicting results
Review question
We reviewed evidence about the effectiveness and safety of antimicrobial‐impregnated central venous catheters (CVCs) on bloodstream infections and death in adults who needed a CVC, and found 57 relevant studies.
Search date
In this update, we included evidence current to March 2015, updating the previous version of the review which was current to March 2012.
Study characteristics
We included 57 studies with 16,784 catheters and 11 types of antimicrobial impregnation. The total number of participants was not clear as some studies did not provide this information, and some participants may have had more than one CVC in the course of their treatment. The participants were mostly adults aged 18 and over in ICUs, cancer units or other healthcare settings in which CVCs were used for intravenous treatment or nutrition. All studies were completed when the participants left the unit or hospital, and no study followed up participants in the long‐term.
Source of funding
Twenty‐six out of 57 studies were funded fully or partially by the catheter manufacturers or distributors, two studies were government‐funded, and two received no funding. Funding sources were not stated in the remaining 27 studies.
Key results
Compared to those participants given non‐impregnated catheters, participants with impregnated catheters had 2% lower rates of bloodstream infections that were definitely catheter‐related (CRBSI) (average absolute reduction in CRBSI: 2%). There was also a 9% lower chance of finding bacteria on these impregnated catheters (catheter colonization) (average absolute reduction in catheter colonization: 9%). However, the benefits of these catheters in reducing catheter colonization varied according to study setting, with significant benefits observed only in studies conducted in the ICUs. There were no clinically significant differences in the overall rates of bloodstream infections (clinically‐diagnosed sepsis) or in death, although these outcomes were assessed in fewer studies than CRBSI and catheter colonization. Impregnated catheters appeared no more likely than non‐impregnated catheters to cause adverse effects such as bleeding, clots, pain or redness at the insertion site.
Quality of evidence
The amount of information in this review contributed to high‐quality evidence for the major outcomes of CRBSI, all‐cause mortality and adverse effects. However, for clinically‐diagnosed sepsis we considered the quality of the evidence to be moderate, as we suspected that there had been selective non‐publication of certain trials. We considered the quality of evidence to be moderate for catheter colonization too, due to major inconsistencies in the direction of the results amongst the included studies.
Authors' conclusion
While impregnated catheters are effective in reducing CRBSI and catheter colonization, particularly in ICUs, they may not be effective across all settings. Furthermore, our review shows that these impregnated catheters do not appear to reduce all bloodstream infections and numbers of deaths. The discrepancy between the findings for CRBSI, catheter colonization and overall bloodstream infections might be related to the limitations of the catheter and blood cultures that were used in most studies for detecting catheter‐related infections. Future research should include overall bloodstream infections and death as key outcomes, and include some advanced methods for detecting micro‐organisms on the catheters and in the bloodstream to evaluate the presence of catheter‐related infections more accurately.
Summary of findings
Summary of findings for the main comparison. Impregnated catheters versus non‐impregnated catheters for reducing the risk of central venous catheter‐related infections in adults.
| Impregnated catheters versus non‐impregnated catheters for reducing the risk of central venous catheter related infections in adults | ||||||
| Patient or population: adult patients who required central venous catheters Settings: hospital setting (medical and/or surgical intensive care unit, oncology, general wards or settings that catered for long‐term total parenteral nutrition) Intervention: antimicrobial‐impregnated central venous catheters | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Impregnated catheters | |||||
| Clinically‐diagnosed sepsis Identified by clinical, biochemical and/or microbiological methods | Study population1 | RR 1.00 (0.88 to 1.13) | 3686 (12 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 172 per 1000 | 172 per 1000 (152 to 195) | |||||
| Low1 | ||||||
| 7 per 1000 | 7 per 1000 (6 to 8) | |||||
| High1 | ||||||
| 443 per 1000 | 443 per 1000 (390 to 501) | |||||
| Catheter‐related blood stream infection (CRBSI) Identified by catheter culture and clinical features +/‐ haematological and biochemical parameters | Study population1 | RR 0.62 (0.52 to 0.74) | 10405 (42 studies) | ⊕⊕⊕⊕ high | ||
| 57 per 1000 | 35 per 1000 (29 to 41) | |||||
| Low1 | ||||||
| 13 per 1000 | 8 per 1000 (7 to 9) | |||||
| High1 | ||||||
| 286 per 1000 | 174 per 1000 (146 to 209) | |||||
| All‐cause mortality | Study population1 | RR 0.92 (0.80 to 1.07) | 2643 (10 studies) | ⊕⊕⊕⊕ high | ||
| 176 per 1000 | 155 per 1000 (132 to 185) | |||||
| Low1 | ||||||
| 77 per 1000 | 68 per 1000 (58 to 81) | |||||
| High1 | ||||||
| 420 per 1000 | 370 per 1000 (315 to 441) | |||||
| Catheter colonization Identified by catheter culture (microbiological methods) | Study population1 | RR 0.67 (0.59 to 0.76) | 9910 (43 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 270 per 1000 | 178 per 1000 (157 to 203) | |||||
| Low1 | ||||||
| 121 per 1000 | 80 per 1000 (70 to 91) | |||||
| High1 | ||||||
| 714 per 1000 | 471 per 1000 (414 to 536) | |||||
| Catheter‐related local infection Identified by catheter culture (microbiological methods) and clinical features | Study population1 | RR 0.84 (0.66 to 1.07) | 2688 (12 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 90 per 1000 | 76 per 1000 (60 to 97) | |||||
| Low1 | ||||||
| 20 per 1000 | 17 per 1000 (13 to 21) | |||||
| High1 | ||||||
| 171 per 1000 | 144 per 1000 (113 to 183) | |||||
| Adverse effects (combined) Identified by clinical assessment | Study population1 | RR 1.09 (0.94 to 1.27) | 3003 (10 studies) | ⊕⊕⊕⊕ high | ||
| 142 per 1000 | 155 per 1000 (134 to 180) | |||||
| Low1 | ||||||
| 46 per 1000 | 50 per 1000 (43 to 58) | |||||
| High1 | ||||||
| 224 per 1000 | 244 per 1000 (211 to 284) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 We provided three typical risk values for each outcome included in this table, namely, 'low risk', 'high risk' and average control risk. We chose the second lowest (non‐zero) control risk value from the included studies as 'low risk', and second highest control risk value as 'high risk', as recommended by the GRADE working group. 2 Publication bias is suspected as the funnel plot for this outcome shows asymmetry, with an apparent absence of smaller studies in which the outcome favours non‐impregnated catheters. We downgraded the quality of evidence by one level.
3 There was substantial heterogeneity in the results of the included studies for this outcome, as indicated by an I2 statistic of 64%. We downgraded the quality of evidence by one level. 4 The 95% confidence interval of the pooled estimate ranges from 0.66 to 1.07, which is not narrow enough for a confident judgment of the effect size. We downgraded the quality of evidence by one level.
Background
Description of the condition
Since its introduction over three decades ago, the central venous catheter (CVC) has been an essential device in managing patients with both acute, life‐threatening illnesses and chronic conditions such as cancer. Compared to a peripheral catheter, a CVC is inserted deeper into the larger veins of the body, providing a more secure and durable intravenous access. While a CVC can be used for administering various medications, large amounts of fluids, and total parenteral nutrition, there are significant risks to using this device. The major concern with CVCs is colonization by micro‐organisms which can lead to catheter‐related bloodstream infection (CRBSI), which is associated with increased morbidity, mortality, and healthcare costs (CDC 2011; Cicalini 2004; Olaechea 2013; Saint 2000; Tacconelli 2009). While the incidence of CRBSI varies depending upon the patient population evaluated, and adherence to recommendations based on up‐to‐date and high quality clinical evidence, CRBSI remains an important patient safety problem in high‐, middle‐ and low‐income countries (Norwood 1991; Peng 2013; Pronovost 2006; Rosenthal 2006; Saint 2000). For instance, the 2010 United States National Healthcare Safety Network (NHSN) report that covered 2473 hospitals reported nearly 11,000 cases of laboratory‐confirmed CRBSI, with estimated CRBSI rates of up to 3.5% (NHSN 2010). A study involving four European countries (France, Germany, Italy and the UK) estimated there were between 8400 to 14400 episodes of CRBSI per year in these counties, with associated annual costs of between EUR 35.9 and EUR 163.9 million (Tacconelli 2009).
Description of the intervention
Several methods have been evaluated to prevent CRBSI, including the maximal use of sterile barriers (namely cap, mask, sterile gown, gloves for staff and full‐sized sterile drapes for patients during catheter insertion; Hu 2004), chlorhexidine gluconate rather than povidone‐iodine for CVC site disinfection, and avoidance of the femoral site for catheter insertion (CDC 2011; Chaiyakunapruk 2002; Gnass 2004; Raad 1994). Additionally, modifications of the CVC itself, in the form of antimicrobial impregnation, coating, or bonding, have also been used to prevent CRBSI (Cicalini 2004). 'Antimicrobial' is a general term used to describe an agent that either kills or inhibits the growth of micro‐organisms, which include bacteria, fungi, viruses or parasites (CDC 2010). Currently, two major types of antimicrobial agents are used as CVC coatings: antiseptics and antibiotics. 'Antiseptic' refers to an agent that destroys or inhibits the growth of a range of micro‐organisms that are present in or on living tissues (e.g. hand washes or surgical scrubs), while 'antibiotic' refers to an agent that acts in similar fashion to an antiseptic, but targets selected micro‐organisms, especially bacteria, and works generally in low concentrations (McDonnell 1999). Various forms of antiseptic and antibiotic catheter impregnation have been introduced since the late 1980s, including chlorhexidine‐silver sulphadiazine (C‐SS) and minocycline‐rifampicin (MR) impregnation, which are the most commonly used and studied (Falagas 2007; Mermel 2001). Impregnation was only applied at the external surface of the first C‐SS‐impregnated catheters, but MR impregnation is applied to both external and luminal surfaces. More recently, second‐generation C‐SS‐impregnated catheters have been introduced, with both the external and luminal surfaces of the catheters impregnated (Ramritu 2008).
How the intervention might work
It is proposed that these compounds with well‐established antimicrobial properties inhibit the colonization of micro‐organisms ‐ like bacteria ‐ on the catheter surface, which in turn prevents the spread of these micro‐organisms into the bloodstream (Cicalini 2004). Several other compounds that have demonstrated antibacterial activities in vitro, like silver, platinum, carbon and heparin have also been evaluated as CVC‐impregnation materials in clinical studies (Abdelkefi 2007a; Hanna 2006; Khare 2007). Silver and platinum were found to inhibit bacterial cell growth and division (Jung 2008; Rosenberg 1967), while heparin was thought to reduce bacterial growth via a prevention of fibrin deposition and thrombus formation in the catheters (Abdelkefi 2007). Carbon nanotubes were seen to cause cell wall damage to bacteria that were in direct contact with them (Kang 2007), and combining these with platinum and silver enhanced their overall antibacterial properties (Narayan 2005). Initial in vitro and animal studies revealed the effectiveness of some of these impregnated catheters against certain common colonizing micro‐organisms (Raad 1995; Raad 1996).
Why it is important to do this review
While the effectiveness of these new catheter‐based technologies is promising, it has been challenged by the progressive discovery of different types of colonizing bacteria, factors that facilitate their adherence to the catheter and changes in their sensitivities to antibiotics over time (Raad 2002). Furthermore, the antibacterial activities of these modified catheters have been found to diminish after a period of use (Sampath 2001; Schmidt 1996; Yorganci 2002). Despite official recommendations regarding when these modified catheters should be used (CDC 2011), a number of systematic reviews have yielded discrepant findings (Gilbert 2008; Ramritu 2008; Veenstra 1999a; Walder 2002), reflecting a need to provide ongoing up‐to‐date collective evidence on the clinical impact of these modified catheters to inform current practice and direct future research. The benefits of these modified catheters in different hospital settings, e.g. intensive care units, standard wards and oncology units, also demands evaluation. Although people cared for at home with a CVC in place, like cancer patients, constitute an important population in terms of catheter care, it is unrealistic to expect them to be participants in such studies.
In this review, we aimed to assess the effectiveness of antimicrobial impregnation, coating and bonding on CVCs in reducing catheter‐related infections in adults. We also assessed their safety and cost effectiveness where possible.
Objectives
Our main objective was to assess the effectiveness of antimicrobial impregnation, coating or bonding on CVCs in reducing clinically‐diagnosed sepsis, catheter‐related blood stream infection (CRBSI), all‐cause mortality, catheter colonization and other catheter‐related infections in adult participants who required central venous catheterization, along with their safety and cost effectiveness where data were available. We undertook the following comparisons: 1) catheters with antimicrobial modifications in the form of antimicrobial impregnation, coating or bonding, against catheters without antimicrobial modifications and 2) catheters with one type of antimicrobial impregnation against catheters with another type of antimicrobial impregnation. We planned to analyse the comparison of catheters with any type of antimicrobial impregnation against catheters with other antimicrobial modifications, e.g. antiseptic dressings, hubs, tunnelling, needleless connectors or antiseptic lock solutions, but did not find any relevant studies. Additionally, we planned to conduct subgroup analyses based on the length of catheter use, settings or levels of care (e.g. intensive care unit, standard ward and oncology unit), baseline risks, definition of sepsis, presence or absence of co‐interventions and cost‐effectiveness in different currencies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), quasi‐randomized trials and cluster‐randomized trials comparing CVCs with antimicrobial impregnation, coating or bonding, against catheters without these modifications. We excluded cross‐over studies. We also excluded studies assessing CVCs for haemodialysis, as this is covered by another Cochrane review (McCann 2010).
Types of participants
We included studies with participants cared for in the adult inpatient unit in a hospital setting (intensive care unit (ICU) and non‐ICU) with a CVC in place. We accepted studies that enrolled a participant more than once. We addressed the issues arising from multiple enrolments using the approach detailed in the Unit of analysis issues section. We excluded studies on children because there is another Cochrane review that includes neonates and children as participants (Shah 2008).
Types of interventions
Intervention
The use of CVCs with antimicrobial impregnation, coating or bonding.
The main types of catheter impregnation were:
chlorhexidine‐silver sulphadiazine (C‐SS);
minocycline‐rifampin/rifampicin (MR);
others such as heparin and silver, platinum and carbon impregnation.
Comparison
The use of standard CVCs of matching material and design without antimicrobial modifications.
We also included studies comparing one type of impregnation to another (e.g. C‐SS versus MR). We also planned to include studies comparing catheters with antimicrobial impregnation against the use of catheters with the following modifications or procedures if such studies were available:
antimicrobial‐impregnated dressings;
silver iontophoretic device;
antiseptic‐filled catheter hubs (including iodinated alcohol or povidone iodine);
needleless connectors;
antimicrobial lock solutions;
tunnelling.
Each participant should only have one study catheter at any one time during the study. We attempted to identify whether any participant had multiple catheters concurrently from the descriptions of the participants in the methods and the results sections if such information was available. We did not place any limit on the minimum and maximum catheter indwelling time for each study.
Types of outcome measures
The following outcomes were measured during the indwelling time of the CVCs or at their removal, or, in the case of patient‐level outcomes such as all‐cause mortality and length of hospital stay, throughout the period in which the participants were being observed for the purpose of research, whether or not the CVCs were still in place.
Primary outcomes
Number of participants with clinically diagnosed sepsis. We used the diagnostic criteria developed from the 2001 Society of Critical Care Medicine/The European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society (SCCM/ESICM/ACCP/ATS/SIS) International Sepsis Definitions Conference (Levy 2003), as detailed in Appendix 1. This set of diagnostic criteria contains an extensive list of clinical features and investigation findings, with no clear statement regarding the minimum number or thresholds required to satisfy a diagnosis of sepsis. Therefore, we accepted various definitions adopted by the authors of each study, as long as the items included in their definitions were those contained in this set of diagnostic criteria. We would, however, also accept definitions that were not consistent with this set of diagnostic criteria, provided the authors justified their definitions with validated sources. We would then analyse those studies that followed such diagnostic criteria and those that adopted other definitions as subgroups .
Number of participants with laboratory‐proven catheter‐related bloodstream infection (CRBSI), defined as an isolate of the same organism from a semi‐quantitative or quantitative culture of a catheter segment and from separate percutaneous blood cultures, with no other identifiable source of infection (CDC 2011).
All‐cause mortality.
Secondary outcomes
Number of participants or catheters with catheter‐related local infections, including exit site and tunnel infection: defined as an isolate of an organism from a semi‐quantitative or quantitative culture of a catheter segment, with clinical signs of infection around the insertion site (CDC 2011).
Catheter colonization: number of participants or catheters with positive catheter cultures: defined as any positive semi‐quantitative or quantitative culture from a proximal or distal catheter segment (CDC 2011).
Number of participants or catheters with resistant organisms from catheter cultures.
Number of participants or catheters with skin or site colonization: defined as any positive semi‐quantitative or quantitative culture from the skin around the catheter site (CDC 2011).
Mortality from CRBSI, defined using diagnostic criteria as stated in the Primary outcomes (see number 2).
Number of participants or catheters with adverse effects: including skin irritation/contact dermatitis, thrombophlebitis, thrombo‐embolism and anaphylaxis.
Number of participants or catheters with catheter failure or premature catheter removal.
Use of systemic antibiotics: total courses of systemic antibiotics used during hospital stay or number of participants who required systemic antibiotics during the course of the study.
Length of hospital stay.
Cost of care, including the costs associated with the material and the number of catheters used or medication given (e.g. antibiotics).
Quality of life, measured using validated scales such as a disease‐specific adapted quality of life tool.
We also assessed whether the methods of outcome measurement, in particular the laboratory methods and survey tools such as the Quality of Life instrument had been previously validated by evaluating whether the authors cited relevant literature on the use of such tools. If there were a large number of studies that adopted non‐validated tools for measuring their major outcomes, we would explore the differences in the effect estimates between these studies and the studies that adopted previously validated tools further via a sensitivity analysis.
Search methods for identification of studies
Electronic searches
In this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 3), MEDLINE (OVID SP; 1950 to March 2015), EMBASE (OVID SP; 1980 to March 2015), and CINAHL (1982 to March 2015) databases. Our updated searches replaced the previous searches which were current to March 2012. The results of this 2015 search have been processed, with the exception of one study (Krikava 2011), which was awaiting classification in the previous version of our review (Lai 2013), and is still awaiting classification, as detailed in Figure 1, as we are awaiting further information from its authors (see Studies awaiting classification).
1.
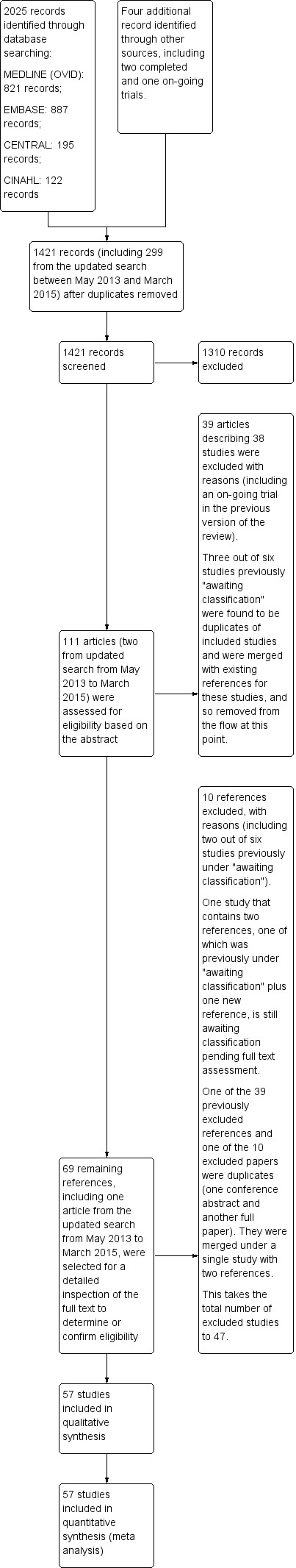
Study flow diagram.
We employed the search strategy as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Our detailed search strategies for MEDLINE (OVID SP), CENTRAL, EMBASE (OVID SP) and CINAHL are displayed in Appendix 2, Appendix 3, Appendix 4 and Appendix 5 respectively.
We also searched for ongoing clinical trials and unpublished studies via Internet searches on the following sites:
clinicaltrialresults.org
centrewatch.com
We did not apply language or publication restrictions.
Searching other resources
To identify further potential studies, we examined references cited in previous relevant Cochrane reviews, in other relevant studies, review articles and standard textbooks. We assessed handsearch results from ACE. We also sought relevant information from expert informants on additional published and unpublished studies. We accepted studies whether published or unpublished, in full article or abstract form, as long as assessment of study quality was possible and where the other inclusion criteria were fulfilled. If studies were published as abstracts, we contacted the study authors for further information if necessary. We contacted authors of all studies identified as relevant where possible, to clarify details of reported follow‐up studies where necessary, or to obtain any information about long‐term follow‐up where none had been reported, and to enquire about additional studies that might be suitable for inclusion.
Data collection and analysis
Selection of studies
We used Cochrane's standard methods as described in the Cochrane Handbook for Systematic Reviews of Interventions , and referred to the ACE's guidelines where appropriate (Higgins 2011; Chapter 7, section 2). Two authors (NML and NC) independently performed the first round of searching for studies that appeared to be relevant. Two authors (NML and WP) then screened these studies for inclusion in the review, using predefined inclusion and exclusion criteria to select eligible studies and determine their risks of bias, as detailed under Assessment of risk of bias in included studies. We resolved any disagreement by discussion leading to a consensus.
Data extraction and management
We extracted the following data from each included study: study characteristics, information relating to the risks of bias, outcomes assessed and data for each outcome that were relevant to this review. We used a standard data collection form from ACE for this purpose. One review author (NML) first entered all the data from the included studies. The data were then cross‐checked independently by other co‐authors (WP and EOR, NAL and SS) for accuracy. Any possible inaccuracy in the data was communicated with the first author (NML), which led to amendments of the data if necessary. Independently we also screened for duplicate entry of participants in each study by matching the initial number recruited against the total number at each step in the conduct of the study.
For studies with multiple comparisons, for example, antimicrobial‐impregnated CVCs versus non‐impregnated CVCs versus CVCs with non‐catheter‐related hygiene measures, we included only interventions that were relevant to this review (i.e. antimicrobial‐impregnated CVCs versus non‐impregnated CVCs). If there were more than two intervention groups that were relevant to this review, for example, antimicrobial A‐impregnated CVCs versus antimicrobial B‐impregnated CVCs versus non‐impregnated CVCs, we combined the intervention groups into a single pairwise comparison (combining the antimicrobial A‐ and antimicrobial B‐impregnated groups versus the non‐impregnated group), as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For studies in which data were given only in percentages, we converted the percentages back into the nearest round numbers by multiplying them by the total number of participants analysed in the assigned group and dividing by 100.
We assessed the definition of each outcome in the included studies. Some studies contained outcomes that were relevant to this review, but labelled them differently, for example, a study could assess an outcome that matched our definition for 'catheter colonization', but the study authors might label this outcome as 'catheter‐related infection'. In such cases, we allocated the data concerned to the prespecified review outcome that best matched the definition of the study authors' outcomes. We resolved any disagreements by discussion among the authors.
Assessment of risk of bias in included studies
Two authors (NML and EOR) independently assessed each included study for risk of bias against the following criteria, using the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
adequacy of sequence generation;
adequacy of allocation concealment;
completeness of follow‐up and handling of incomplete outcome data;
blinding of participants and care providers to intervention (medical and nursing staff who insert the catheters and were involved in the participants' day‐to‐day care) where possible. We sought statements in the study or clarification by the trialists on whether the catheters being compared were indistinguishable in appearance, and hence blinding was possible;
other issues (e.g. validity, reliability, objectivity, or blinding of outcome measurement, and whether there was extreme baseline imbalance).
The assessors assigned a judgment of low, high or unclear risk of bias for each item.
A detailed description of the 'Risk of bias' criteria is available in Appendix 6.
As an additional measure to assess the risk of performance bias, we looked for evidence in each study that a standard protocol was followed by all groups being studied for insertion, use, maintenance and removal of CVCs, and for concurrent use of catheter‐related antiseptic measures (including the use of prophylactic antibiotics) and sterile procedures. We made relevant comments in the corresponding tables for each study.
Measures of treatment effect
For categorical data, we pooled outcome estimates that were measured using the same scales with risk ratios (RR), absolute risk reduction (ARR) and number needed to treat for an additional beneficial outcome (NNTB) for each specific comparison, with their respective 95% confidence intervals (Higgins 2011). As the continuous data were provided by single studies, we expressed the results using mean difference (MD) with 95% confidence interval. Where pooled analyses were not possible, we reported the results of the individual studies separately.
Unit of analysis issues
We assessed unit of analysis issues in the included studies in two possible ways in which they might arise: firstly, multiple enrolments of the same participants either from individually randomized trials or cluster‐randomized trials; and secondly clustering at the level of the enrolled units in cluster‐randomized trials.
Unit of analysis issues might arise if there were multiple enrolments of the same individual following a need for repeated catheterization. We addressed this unit of analysis issue by assessing each included study for any evidence of multiple enrolments. If we found evidence of this, for example, the number of catheters exceeded the number of participants, and if there was sufficient information in the paper for us to do so, we assessed whether there were any participants with more than one event reported. We then excluded those with multiple enrolments by entering the data for those who were enrolled only once. However, if such information was not available, we performed our analysis based on whatever data the authors provided, and used the total number of catheters as the denominator.
In dealing with cluster‐randomized trials, we would have looked for evidence that the authors had made appropriate adjustments in their analyses in the Methods and Results. We would also have inspected the width of the standard error (SE) or 95% confidence interval (CI) of the estimated treatment effects. If we had found an inappropriately small SE or a narrow 95% CI, we would have asked the authors of the study to clarify the unit of analysis.
If we had found a unit of analysis error that was correctable with the information provided by the authors, for example when the included study analysed outcome data for individual participants without adjusting for the effects of clustering, we would have performed our own adjustments. We would have done this by adjusting the final estimates of the study, using the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions that is, by multiplying the SEs of the final effect estimates by the square root of the 'design effect': (1 + (M‐1) x ICC), where M is the average cluster size (number of participants in the units being studied) and ICC is the intracluster correlation coefficient among participants within each unit (Higgins 2011). We would have determined the average cluster size (M) from each trial by dividing the total number of participants by the number of units recruited. We would have sought the best estimate of ICC from reliable resources, such as landmark cluster‐randomized trials on central venous catheters, if such trials were available. We would then have combined the adjusted final effect estimates from each trial with their SEs in the meta‐analysis using the generic inverse variance methods available in Review Manager 5 (Revman 5.3). If we had failed to identify a reliable ICC for the relevant cluster‐randomized trials, we would have used the unadjusted estimates as reported by the study authors for our meta‐analysis, noting the absence of an appropriate adjustment. We would also have performed a sensitivity analysis to assess how the overall results were affected by the inclusion and exclusion of these studies.
We addressed the unit of analysis issues that might arise from multiple comparisons by combining all the intervention groups into a single combined intervention group to achieve a single pairwise comparison, as detailed under Data extraction and management.
Dealing with missing data
We obtained drop‐out rates from each study. We considered a drop‐out rate higher than the difference between the intervention and the control group event rates to be significant, namely, by using the worst case scenario model. If we found a significant drop‐out rate with no reasonable explanation, we contacted the authors of the individual studies where possible, to request further data. We also assessed whether an intention‐to‐treat analysis was performed.
We performed sensitivity analyses to assess how the overall results were affected by the inclusion and exclusion of those studies with a high risk of attrition bias and incomplete outcome data.
Assessment of heterogeneity
We assessed the treatment effects of individual trials and the heterogeneity between trial results by inspecting the forest plots.
We explored clinical heterogeneity by assessing clinical and methodological characteristics of the included studies (e.g. difference in study quality, participants, intervention or outcome assessment). We only attempted to pool data in a meta‐analysis if the clinical heterogeneity amongst the selected studies was negligible. If we found major discrepancies in clinical or methodological characteristics, we decided whether to exclude some studies altogether from the meta‐analysis, or to include them and perform a sensitivity analysis of the main outcome.
In addition, we used the I² statistic to measure inconsistency in the study results (Higgins 2002), and took values greater than 40% as indicative of substantial statistical heterogeneity. If significant statistical heterogeneity was found, but the studies were considered suitable for combining for a meta‐analysis based on the clinical and methodological characteristics as detailed above, we relied on the pooled effect estimates provided by a random‐effects model.
Assessment of reporting biases
For each study, we compared the outcomes reported in the results against the outcomes listed in the methods section. We also identified some key outcomes that might have been assessed but were not included. We contacted the study authors for clarification where necessary. In studies in which critical outcomes were missing, we sought the study protocol, either from PubMed, the relevant trial registry, the web link provided by the study, or directly from the study authors, to establish whether these outcomes had been prespecified. In addition to our description under 'reporting biases' in the risk of bias assessment tables (Characteristics of included studies), we present a matrix highlighting those studies in which there were discrepancies between the major outcomes listed in the methods versus those reported in the results, and also those studies in which there were critical outcomes that were not reported at all (Appendix 7).
Where possible, we also performed a sensitivity analysis taking an outcome that was reported by all studies, and comparing the overall results with and without inclusion of those studies in which key outcomes were missing.
Assessment of publication bias
If there were a sufficient number of studies (at least 10) included in the analysis, we screened for publication bias by constructing a funnel plot. If publication bias was suspected, that is, significant asymmetry was found on visual inspection of the funnel plot, we included a statement in our results and the 'Summary of findings' table with a corresponding note of caution in our discussion.
Data synthesis
We followed the procedures of ACE. We performed meta‐analysis of the included trials with Review Manager 5.3 (Revman 5.3), using a fixed‐effect model, unless significant statistical heterogeneity was found, as detailed under the previous heading, Assessment of heterogeneity. We used intention‐to‐treat data if possible in our analyses.
First, we presented the effects of antimicrobial‐impregnated CVCs versus non‐impregnated CVCs as a whole in our meta‐analysis. Since there might be differences in the effects of different types of antimicrobial impregnations, we also reported the effects of each specific type of impregnation in our subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
For rate data such as CRBSI per 1000 catheter days, we followed the methods outlined in Chapter section 9.4.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We obtained the rate ratio by dividing the rate in the intervention group by the rate in the control group. We then derived the natural log (ln) of the rate ratios and entered these into RevMan using the generic inverse variance method. We obtained the standard error (SE) of the ln(rate ratio) by the following formula: SE of ln(rate ratio) = square root of ((1/rate of the intervention group) + (1/rate of the control group)). For the study in which we combined two intervention groups (Arvaniti 2012), we obtained the adjusted rate data of the combined group using the following formula: adjusted event rate (per 1000 catheter days) = ((E₁ x CD₁/CD₁+₂) + (E₂ x CD₂/CD₁+₂)), where E₁ = event rate (group one), E₂ = event rate (group two), CD₁ = total catheter days (group one), CD₂ = total catheter days (group two), CD₁+₂ = total combined catheter days.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses if applicable for the following:
Participants with CVCs intended for use over the short term (less than 10 days) versus a long‐term period (10 days or more).
Studies using different types of catheter impregnation (e.g. C‐SS or MR) in the experimental arm against unimpregnated catheters.
Studies in different settings or with a certain type of patient as the predominant participants, e.g. those in intensive care units (ICUs), people receiving cancer treatments, those on long‐term parenteral nutrition, those requiring CVCs for other purposes, and studies with a mixture of different types of participant.
Studies in which the participants had higher or lower baseline risks, using the median event rates in the control group as cut‐offs.
Studies that adopted the definition of clinical sepsis developed from the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference versus studies with other definitions (Levy 2003).
Studies with and without co‐interventions (e.g. concurrent antiseptic device or procedures such as special dressing, hub, cutaneous antisepsis or the use of prophylactic antibiotics).
Studies that examined cost effectiveness ‐ these would have been analysed in different subgroups according to the currency used should there be data available.
Sensitivity analysis
We performed sensitivity analyses on four major outcomes, that is, our three primary outcomes of clinically diagnosed sepsis, CRBSI, and all‐cause mortality, and the most frequently reported secondary outcome, which was catheter colonization. We conducted our sensitivity analyses on the basis of two main criteria, namely, the risk of selection bias resulting from random sequence generation and allocation concealment, and the risk of attrition bias, as described under the headings of Assessment of risk of bias in included studies and Dealing with missing data, respectively.
Summary of findings table
We developed a 'Summary of findings' table highlighting the quality of evidence in six major outcomes, namely, clinically diagnosed sepsis, CRBSI, all‐cause mortality, catheter colonization, catheter‐related local infection and adverse effects (combined). We used the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence relating to the studies that contributed data to the meta‐analyses for each of these six outcomes. When we identified an issue that we considered to be serious in each of the five GRADE criteria, we downgraded the quality of evidence by one level, and when we considered the issue to be very serious, we downgraded the quality of evidence by two levels. Whenever we decided to downgrade the quality of evidence from the default high quality, we justified our decisions and described the level of downgrade in the footnotes of the table. We developed the 'Summary of findings' table using a web‐based version of the GRADEpro software (http://www.guidelinedevelopment.org/), according to the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Results
Description of studies
Results of the search
The initial search (up to May 2013) yielded 612 records from MEDLINE, 771 records from EMBASE, 179 records from CENTRAL and 108 records from CINAHL, giving a total of 1670 records. In our updated search from May 2013 to March 2015, we identified 355 further records, including 209 from MEDLINE, 116 from EMBASE, 16 from CENTRAL and 14 from CINAHL. We identified three further relevant studies from the initial searches in other Internet resources (as detailed in Appendix 8) in the previous version of the review (Lai 2013).
After removing duplicates of all records from our searches, a total of 1421 records remained that included 299 articles from our updated search from May 2013 to March 2015. We short listed 111 articles that appeared to be relevant after inspecting their titles. On further inspection of the abstracts, we excluded 39 articles, including all three articles identified through further Internet searches as described above. We found that three of the six studies we had previously put in the Studies awaiting classification section were duplicates of included studies and we merged them with the references of the corresponding included studies. We excluded a further 10 articles from the remaining 69, on the basis of the full text or cross‐inspection of related publications. We placed one article that we identified in this update as a poster publication of a previous trial record in 'Studies awaiting classification' pending further information from the author (Krikava 2011). The previous trial record is now a secondary reference for Krikava 2011 (see Characteristics of studies awaiting classification).
At the end of our screening and selection, we had included 57 eligible studies in our review. We checked the reference lists of all the full‐text articles we obtained, but did not identify any additional titles we considered relevant
A diagram of the flow of studies from the initial search to the meta‐analysis is shown in Figure 1. A description of all the included studies is provided in the Characteristics of included studies table, and details of the excluded studies with reasons for their exclusion are listed in the Characteristics of excluded studies table.
Included studies
The 57 included studies were randomized controlled trials (RCTs) conducted in 17 countries, including the USA (20 studies), Germany (nine studies), UK (six studies), Spain (three studies), Australia, Austria, France, Italy, Sweden, Turkey (two studies each), Belgium, Brazil, Greece, Netherland, South Africa, Taiwan and Tunisia (one study each). Thirty‐three trials were single‐centre RCTs and 24 were multicentre RCTs. We did not find any cluster‐randomized trials among our included studies. The initial sample sizes of the studies ranged from 20 to 960 participants (Bach 1996b; Walz 2010, respectively). Some studies only specified the number of catheters evaluated and not the number of participants (Darouiche 1999; Darouiche 2005; Fraenkel 2006; George 1997; Leon 2004; Maki 1988; Maki 1997; Mer 2009; Ostendorf 2005; Van Vliet 2001). In 25 studies, the minimum age for the participants was clearly stated. Among these studies, 22 included participants aged at least 18, and for the remaining three studies the minimum age for inclusion was 17 (Bennegard 1982), 12 (Collin 1999), and four years old (Abdelkefi 2007). Eleven studies did not provide the minimum age for inclusion but stated that their participants were 'adults'. For the remaining 21 studies, the minimum age for inclusion was not stated. In two studies (Bach 1996b; Bong 2003), participants were predominantly adult men, while all other studies included participants of both sexes in significant proportions.
Thirty‐five studies were conducted in medical/surgical ICU settings, 10 studies in haematology/oncology units, eight studies enrolled a mixture of participants including patients from ICU, general medical or surgical units and those receiving total parenteral nutrition (TPN), three studies enrolled only participants receiving TPN, and one study had no description of the study setting or participant type (Bennegard 1982).
There were three major categories of intervention:
two‐arm comparison between antimicrobial impregnation and no impregnation (48 studies);
two‐arm comparison between different catheter impregnations (five studies);
three‐arm comparison between different impregnations with or without a non‐impregnated group (four studies).
A total of 11 antimicrobial impregnations were assessed, including chlorhexidine‐silver sulphadiazine (C‐SS), minocycline‐rifampicin (MR), miconazole‐rifampicin, single antibiotics such as vancomycin, teicoplanin and cefazolin, silver‐platinum‐carbon, silver, silver‐impregnated cuff, heparin and benzalkonium. One study described the C‐SS impregnation used as 'second generation impregnation' (Rupp 2005). There was no evidence from any of the included studies that any participant had multiple study catheters concurrently, although there were participants who had multiple study catheters placed sequentially.
Catheter colonization was the most commonly evaluated outcome (50 studies), followed by catheter‐related bloodstream infection (CRBSI) (46 studies). The major clinical outcomes of clinically diagnosed sepsis, mortality attributed to catheter‐related infections, and all‐cause mortality were assessed in 13, 5 and 12 studies respectively. Adverse effects were evaluated in 13 studies. There were wide ranges of baseline risks in the included studies, from 0.4% to 58% for clinically diagnosed sepsis, 0% to 40% for CRBSI, 8% to 59% for all‐cause mortality and 12% to 80% for catheter colonization. Eight of the 13 studies that assessed our primary outcome of clinically diagnosed sepsis defined this outcome in accordance with the definition in this review. In another four studies, the authors did not provide sufficient information about the definition, and one study defined it in a way that was considered to be outside the scope of our definition for this review. Overall, the definition of CRBSI was consistent among the studies, which included suggestive clinical features, and a positive catheter culture with a positive blood culture growing the same organism. Most studies used previously validated laboratory methods to perform catheter and blood cultures, and adopted microbiological definitions for colonization and bloodstream infection that were consistent with the published literature in the evaluation of catheter‐related infections. All studies reported catheter‐related outcomes such as CRBSI and catheter colonization using the catheter as the unit, and none provided separate reports of these outcomes with participants as the unit. Thirty‐five studies provided the number of catheters as well as participants. The number of catheters matched the number of participants in 33 studies, and the number of catheters exceeded the number of participants by only one in two studies, suggesting that except for one participant who had two catheters evaluated, all participants had a single catheter. In terms of participant‐level outcomes, 12 studies reported clinically diagnosed sepsis, 10 reported all‐cause mortality, five reported mortality attributed to CRBSI and 10 studies reported adverse effects. None of the included studies assessed quality of life.
Source of funding
Twenty‐six of the 57 included studies were funded fully or partially by either the manufacturer or distributor of the catheters used in the studies. Two studies were government‐funded, and two studies received no funding, which was clearly stated by the authors. The remaining 27 studies provided no description of the sources of funding.
Excluded studies
We excluded 47 studies based on one or more of the following criteria.
Study design (28 studies): the studies were either retrospective or prospective cohort studies, before‐and‐after intervention studies, cross‐over studies, prospective non‐randomized intervention studies, meta‐analyses, economic analyses with no original trial data, in‐vitro experiments, or commentaries.
Population (17 studies): the participants in the studies were either children, people undergoing haemodialysis/extracorporeal detoxification or neurosurgical patients undergoing cerebral ventricular catheter placement.
Intervention (nine studies): the studies either assessed an athrombogenic‐coated CVC that was not designed to be antimicrobial, an antimicrobial‐impregnated dressing, a cerebral ventricular catheter, an impregnated CVC connector rather than a CVC itself or different methods of placing new CVCs.
A description of each study is available in the Characteristics of excluded studies table.
Studies awaiting classification
There is one study awaiting classification in this review update (Krikava 2011). This article, which was identified in this review update as the poster publication of a study previously available as a trial record and awaiting classification, describes the study methods and results without sufficient detail to allow us to determine its eligibility. We are awaiting a reply from the corresponding author.
Ongoing studies
There are no on‐going studies in this review update. In the previous version of the review (Lai 2013), there was an on‐going study, which has now been published in full and excluded (Jacob 2011).
Risk of bias in included studies
The majority of the studies had either low or unclear risks of bias for most criteria, except for blinding, which did not appear possible for the participants and carers in most studies, due to the different appearances of the catheters evaluated. The 'Risk of bias' graph, which shows the overall degree of risks of bias in the studies included in this review by depicting the proportions of studies with low, high and unclear risks of bias according to each criterion, and the 'Risk of bias' summary, which details the risk of bias of each included study, are illustrated in Figure 2 and Figure 3, respectively. A detailed description of the risk of bias in each study is provided in the Characteristics of included studies. Summaries of our 'Risk of bias' assessment for each major criterion are given below.
2.
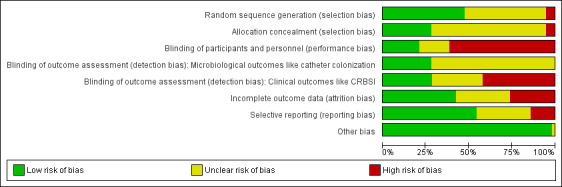
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For random sequence generation, 27 of the 57 included studies had a low risk of bias. For allocation concealment, 16 studies had a low risk of bias. Fourteen studies had low risks of bias for both random sequence generation and allocation concealment (Abdelkefi 2007; Antonelli 2012; Arvaniti 2012; Bong 2003; Darouiche 1999; Fraenkel 2006; Hanna 2004; Leon 2004; Maki 1997; Raad 1997; Raad 1998; Rupp 2005; Smith 1995; Yucel 2004).Three of the 57 included studies had high risks of bias in both random sequence generation and allocation concealment (Heard 1998; Kamal 1991; Van Vliet 2001). A large proportion of the studies had unclear risks of bias for these two criteria and for all other criteria, mainly due to a lack of information reported by the authors (Figure 2). In all studies with low risk of bias for allocation, the individual authors explicitly stated that some form of random number scheme was used, mostly computer‐based, to generate a random sequence. The study authors also made explicit statements about the independence of random sequence generation and allocation. All three studies with a high risk of bias in allocation used some form of alternation based on participants' identifying number or catheter type.
Blinding
The majority of the studies had unknown or high risks of bias for blinding. In 12 studies, the participants were described as 'blinded', and in 25 studies the participants were described as 'non‐blinded'. In the remaining studies, there was not enough information on blinding, although blinding appeared unlikely in most of them due to the different appearances of the catheters evaluated. For outcome assessment, 16 studies described the microbiological outcome assessors as 'blinded'. In the remaining studies, the blinding status of the microbiological outcome assessors was unknown. For clinical outcome assessment, 16 studies described the assessors as 'blinded', 24 described them as 'non‐blinded', and the blinding status of the clinical outcome assessors in the remaining studies was unknown. Overall, only six studies had low risks of bias in blinding of participants and personnel as well as blinding of microbiological and clinical outcome assessors (Bach 1996b; Darouiche 1999; Hanna 2004; Maki 1997; Mer 2009; Rupp 2005).
Incomplete outcome data
Judging from the completeness of the data across all the major outcomes including clinically diagnosed sepsis, CRBSI, mortality and catheter colonization, we considered 24 studies to have a low risk of attrition bias (Abdelkefi 2007; Arvaniti 2012; Bach 1996b; Bach 1999; Bennegard 1982; Bong 2003; Brun‐Buisson 2004; Carrasco 2004; Ciresi 1996; Fraenkel 2006; Goldschmidt 1995; Hanna 2004; Jaeger 2001; Jaeger 2005; Kahveci 2005; Kalfon 2007; Leon 2004; Maki 1997; Osma 2006; Raad 1998; Rupp 2005; Smith 1995; Theaker 2002; Walz 2010), and 15 studies to have a high risk (Antonelli 2012; Boswald 1999; Camargo 2009; Collin 1999; Corral 2003; Darouiche 1999; Harter 2002; Heard 1998; Maki 1988;Moretti 2005; Ostendorf 2005; Pemberton 1996; Stoiser 2002; Tennenberg 1997; Yucel 2004). We assessed a study as having a high risk of bias for one or both of the following reasons:
high attrition rates, either in absolute terms (≥ 20% attrition) or in relation to the event rates in the control group, or both;
marked imbalance in the attrition rates between the assigned groups.
Additionally, in five studies with a high risk of bias, the reasons stated for withdrawals appeared dubious, e.g. catheter removal prior to day three or four (two studies), catheter change (two studies), transfer to another unit or death (three studies each), as these did not preclude the participant or catheter, or both, from being assessed for at least some of the outcomes, and they might indeed have represented important and relevant outcomes, for example, excluding those who died might be inappropriate as the deaths might be related to the interventions assessed.
Selective reporting
Over half of the included studies (31) were at low risk of reporting bias(Abdelkefi 2007; Antonelli 2012; Arvaniti 2012; Babycos 1993; Bach 1999; Bennegard 1982; Bong 2003; Boswald 1999; Brun‐Buisson 2004; Camargo 2009; Darouiche 1999; Dunser 2005; Fraenkel 2006; George 1997; Goldschmidt 1995; Hanna 2004; Heard 1998; Kahveci 2005; Logghe 1997; Maki 1988; Maki 1997; Marik 1999; Mer 2009; Osma 2006; Pemberton 1996; Rupp 2005; Stoiser 2002; Tennenberg 1997; Theaker 2002; Van Vliet 2001; Walz 2010), and eight studies were at a high risk of reporting bias (Bach 1996a; Bach 1996b; Collin 1999; Raad 1998; Sherertz 1996; Smith 1995; Thornton 1996; Van Heerden 1996). In four of these eight studies, some outcomes were not reported in a format that would allow data extraction for meta‐analysis. For example, the authors presented the results in graphs without data labels, or reported continuous outcomes as means without standard deviations. In another four studies,the authors failed to include any important clinical outcome such as CRBSI, sepsis or mortality in their study report. We constructed a matrix that contains a more detailed description of these studies, along with other included studies in which there were discrepancies between the prespecified outcomes in the methods and reported outcomes in the results (Appendix 7).
Other potential sources of bias
Apart from a significant baseline imbalance in the major participant characteristics observed in one study (Smith 1995; for details see Characteristics of included studies), we observed no additional major sources of bias in the included studies.
Effects of interventions
See: Table 1
This review evaluated a total of 16,784 catheters in 57 studies. The total number of participants was unclear as some studies only specified the number of catheters and not the participants.
Comparison 1: Antimicrobial impregnation versus no impregnation
Primary outcomes
1. Clinically diagnosed sepsis
There was no difference between the impregnated group and the non‐impregnated group (risk ratio (RR) 1.0, 95% confidence interval (CI) 0.88 to 1.13; 12 studies, 3686 catheters; I² = 19%;Analysis 1.1; Figure 4). The funnel plot for this outcome (not shown) is asymmetrical, suggesting a possibility of publication bias, as smaller studies with outcomes that favour non‐impregnated catheters appear to be lacking. As a result, we downgraded the overall quality of evidence for this outcome from high to moderate.
1.1. Analysis.
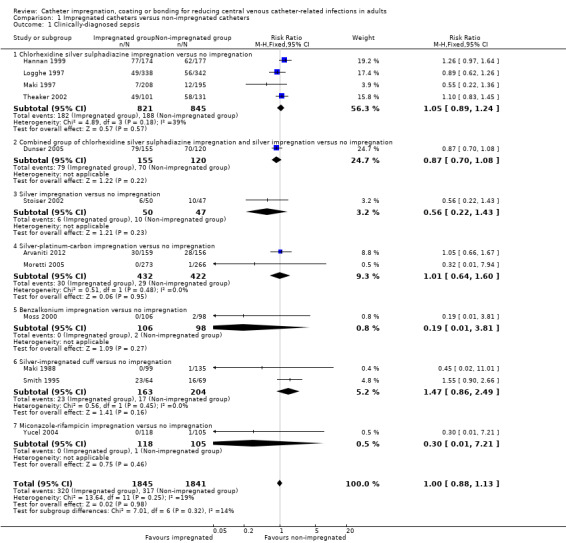
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 1 Clinically‐diagnosed sepsis.
4.
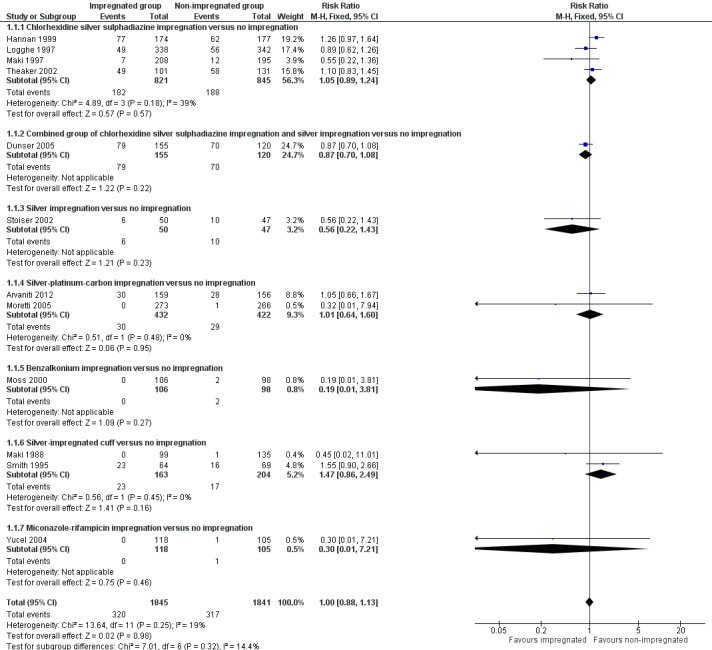
Forest plot of comparison: 1 Impregnated catheters versus non‐impregnated catheters, outcome: 1.1 Clinically‐diagnosed sepsis.
2. Catheter‐related bloodstream infection (CRBSI)
CRBSI: there was a significant reduction in CRBSI in the impregnated group (absolute risk reduction (ARR) 2%, 95% CI 3% to 1%, number needed to treat for an additional beneficial outcome (NNTB) 50; RR 0.62, 95% CI 0.52 to 0.74; 42 studies, 10,405 catheters; I² statistic = 20%; Analysis 1.2; Figure 5). There was no evidence of publication bias from the funnel plot and no other issues that affected the quality of evidence, so we rated this as high quality evidence in our 'Summary of findings' table.
CRBSI per 1000 catheter days: there was no difference between the impregnated group and the non‐impregnated group (RR 0.75, 95% CI 0.51 to 1.11; 15 studies; I² statistic = 19%; Analysis 1.3).
1.2. Analysis.
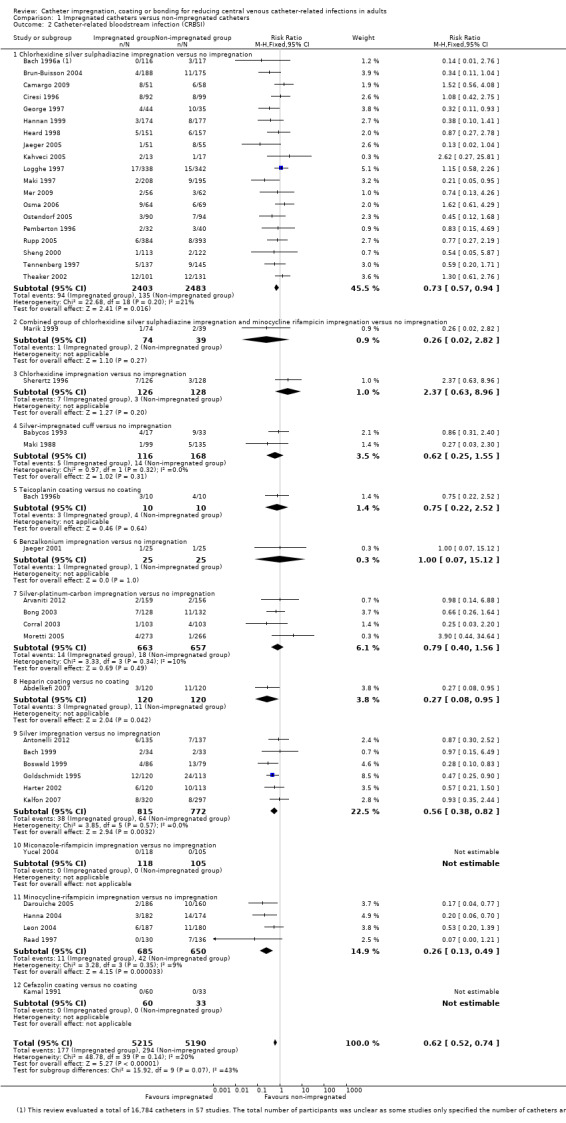
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 2 Catheter‐related bloodstream infection (CRBSI).
5.
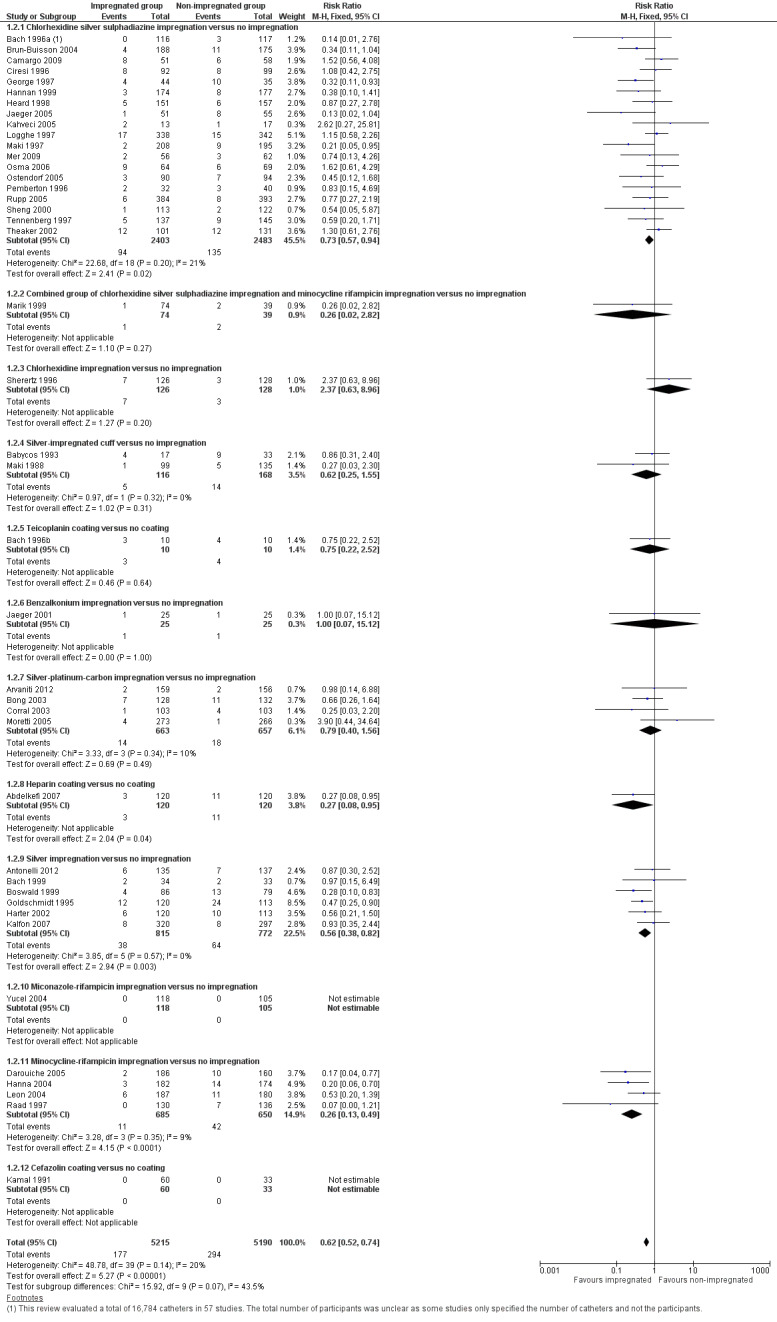
Forest plot of comparison: 1 Impregnated catheters versus non‐impregnated catheters, outcome: 1.2 Catheter related bloodstream infection (CRBSI).
1.3. Analysis.
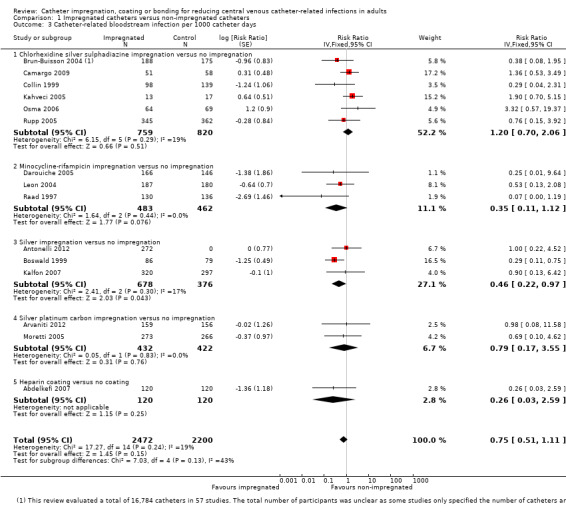
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 3 Catheter‐related bloodstream infection per 1000 catheter days.
3. All‐cause mortality
There was no difference between the impregnated group and the non‐impregnated group for all‐cause mortality (RR 0.92, 95% CI 0.80 to 1.07; 10 studies, 2643 catheters; I² statistic = 1%; Analysis 1.4). There was no evidence of publication bias from the funnel plot and no other issues that affected the quality of evidence, so we rated this as high quality evidence in our 'Summary of findings' table.
1.4. Analysis.
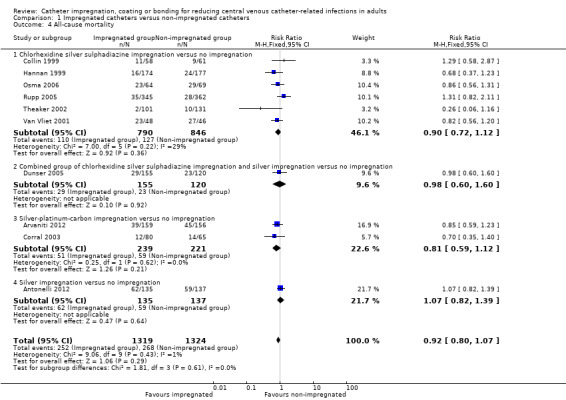
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 4 All‐cause mortality.
Secondary outcomes
1. Catheter‐related local infection
There was no difference between the impregnated group and the non‐impregnated group for catheter‐related local infection (RR 0.84, 95% CI 0.66 to 1.07;12 studies, 2688 catheters; I² statistic = 1%; Analysis 1.5). We downgraded the quality of evidence from high to moderate as the confidence interval was too wide, in our view, to enable a confident estimate of the effect size for consistent clinical decision making.
1.5. Analysis.
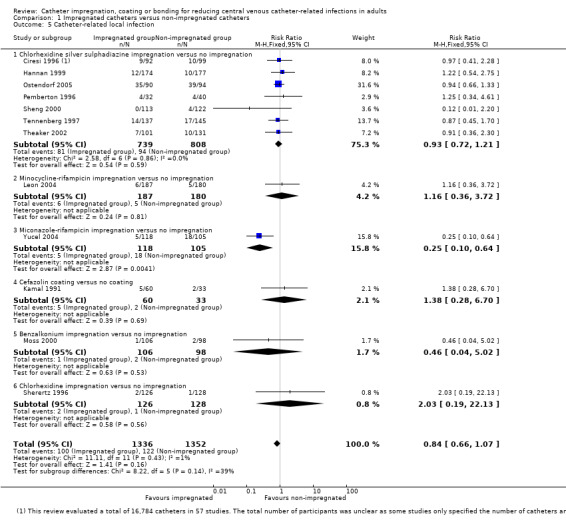
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 5 Catheter‐related local infection.
2. Catheter colonization
Catheter colonization: there was a significant reduction in catheter colonization in the impregnated group (ARR 9%, 95% CI 12% to 7%; NNTB 11; RR 0.67, 95% CI 0.59 to 0.76; 43 studies, 9910 catheters; I² statistic = 64%; Analysis 1.6; Figure 6). We downgraded the quality of evidence from high to moderate due to substantial heterogeneity among the included studies which led to our use of the random‐effects model in the analysis;
Catheter colonization per 1000 catheter days: there was a reduction of borderline statistical significance in the impregnated group (RR 0.74, 95% CI: 0.55 to 1.00; 12 studies; I² statistic = 51%; Analysis 1.7). There was no gross evidence of publication bias from the funnel plot. However, there was substantial heterogeneity among the included studies, indicated by the I² values of 64% and 51%, respectively, for outcomes 2.1 and 2.2. The degree of heterogeneity is not explained by the presence of many subgroups comprising different types of impregnation, as the I² statistic remains high within some of the subgroups, e.g. chlorhexidine‐silver sulphadiazine (C‐SS) impregnation versus no impregnation (20 studies, I² statistic = 58%), minocycline‐rifampicin (MR) impregnation versus no impregnation (four studies, I² statistic = 71%) and silver‐impregnated cuff versus no impregnation (two studies, I² statistic = 85%).
1.6. Analysis.
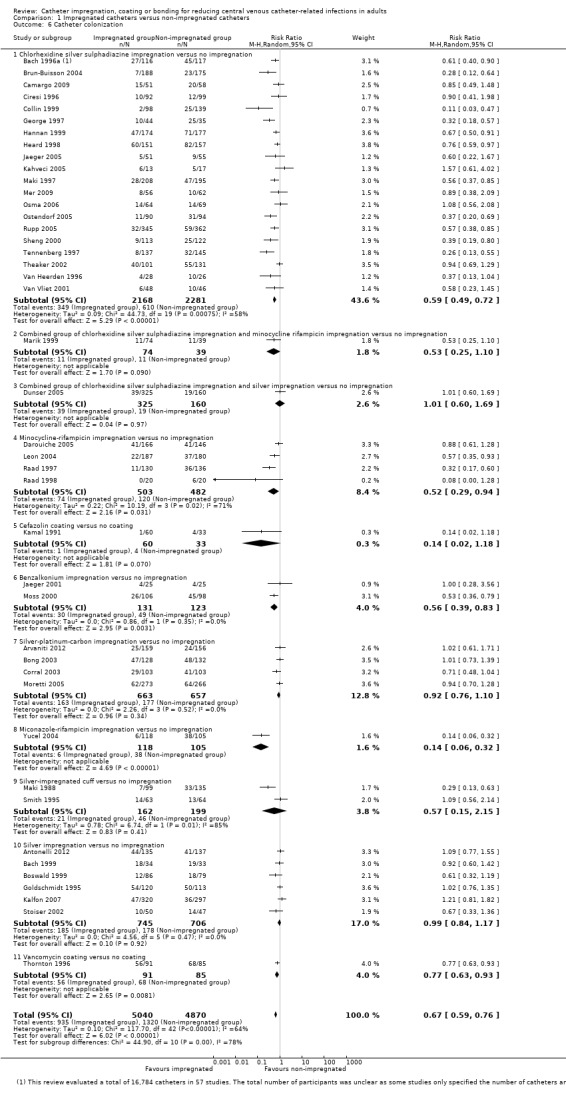
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 6 Catheter colonization.
6.
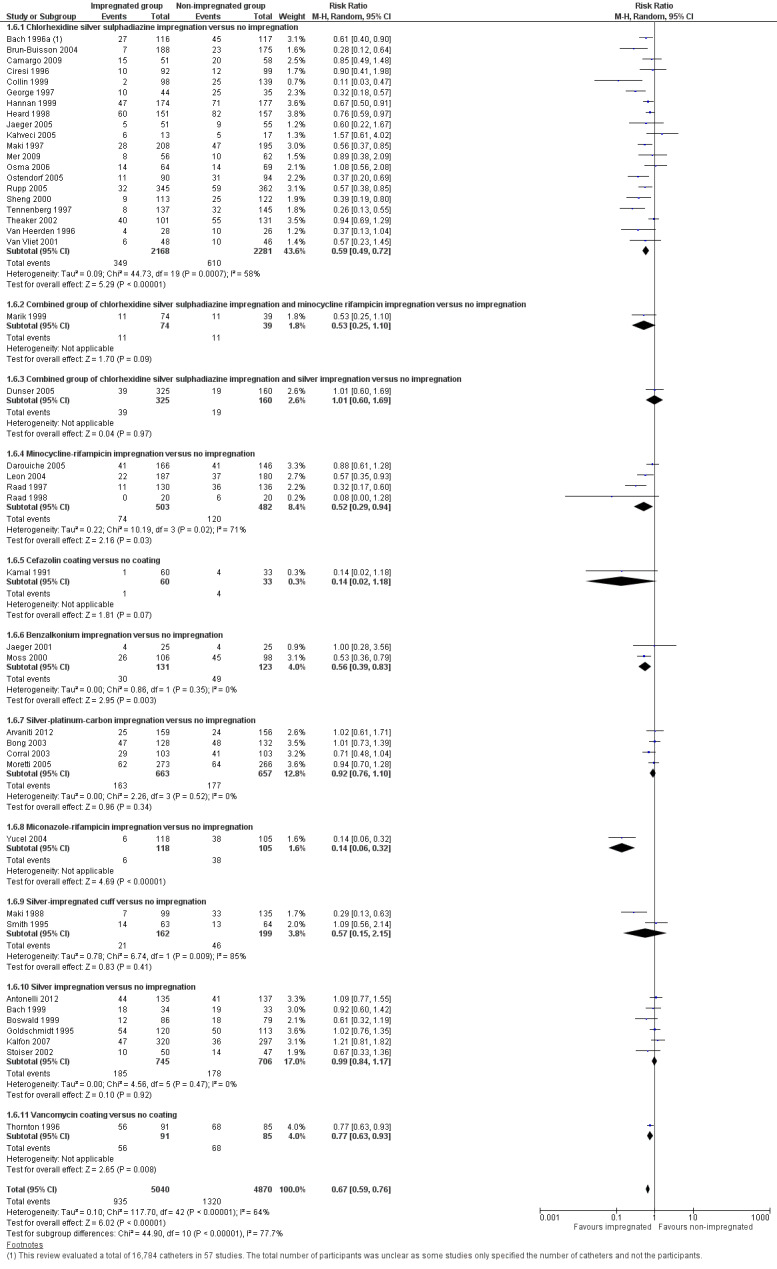
Forest plot of comparison: 1 Impregnated catheters versus non‐impregnated catheters, outcome: 1.6 Catheter colonization.
1.7. Analysis.
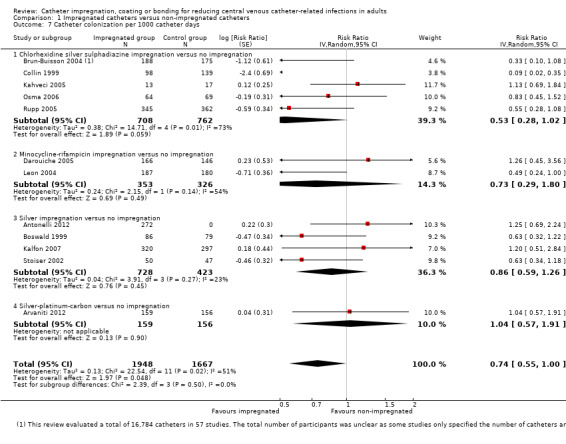
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 7 Catheter colonization per 1000 catheter days.
When we explored the possible reasons for the heterogeneity within each of the subgroups, it appeared that differences in study settings had probably contributed to it. We have provided a detailed description of the discordance in the pooled estimates between studies conducted in different settings under the heading of 'Subgroup analyses: 3. Participant type' below. After exploring the causes of heterogeneity, we decided to use the random‐effects model for the outcomes of catheter colonization and catheter colonization per 1000 catheter days.
3. Number of participants or catheters with resistant organisms from catheter cultures
No studies examined this outcome.
4. Skin or insertion site colonization
There was a significant reduction in skin or insertion site colonization in the impregnated group (RR 0.78, 95% CI 0.62 to 0.97; three studies, 366 catheters; I² statistic = 55%; Analysis 1.8).
1.8. Analysis.
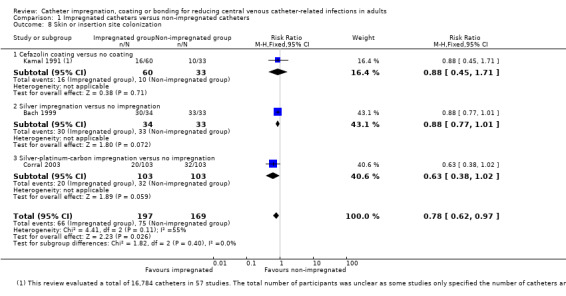
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 8 Skin or insertion site colonization.
5. Mortality attributed to CRBSI
There was no difference between the impregnated group and the non‐impregnated group for mortality attributed to CRBSI (RR 0.24, 95% CI 0.03 to 2.20; five studies, 1098 catheters; I² statistic = 0%; Analysis 1.9).
1.9. Analysis.

Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 9 Mortality attributed to catheter related blood stream infection (CRBSI).
6. Adverse effects
There were no differences between the impregnated group and the non‐impregnated group for any of the following adverse outcomes:
thrombosis/thrombophlebitis (RR 0.90, 95% CI 0.44 to 1.85; three studies, 829 catheters; I² statistic = 0%);
bleeding (RR 0.86, 95% CI 0.30 to 2.48; one study, 240 catheters);
combined adverse effects of bleeding, pain, erythema and/or tenderness at the insertion site (RR 1.09, 95% CI 0.94 to 1.27; 10 studies, 3003 catheters; I² statistic = 0%; Analysis 1.10). There was no evidence of publication bias from the funnel plot and no other issues that affected the quality of evidence, hence we rated this as high quality evidence in our 'Summary of findings' table.
1.10. Analysis.
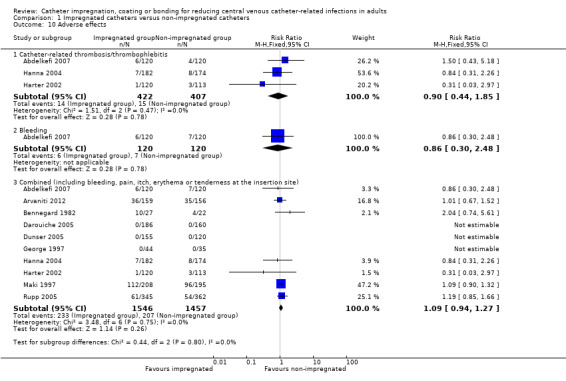
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 10 Adverse effects.
7. Number of catheters removed prematurely
There was no difference between the impregnated group and the non‐impregnated group for the number of catheters removed prematurely (RR 1.00, 95% CI 0.92 to 1.09; 15 studies, 3666 catheters; I² statistic = 28%; Analysis 1.11).
1.11. Analysis.
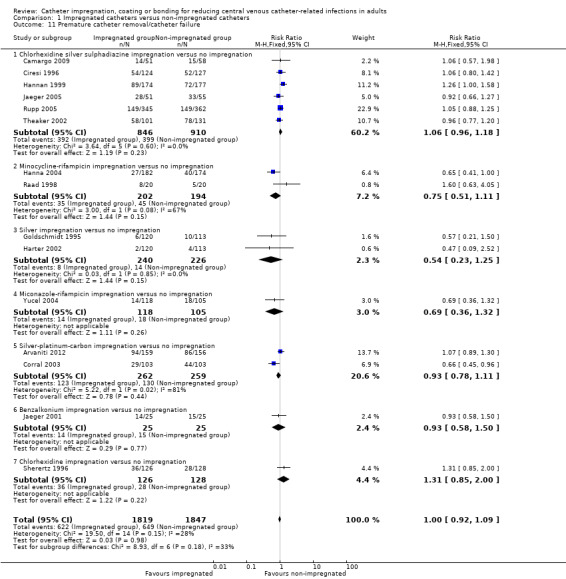
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 11 Premature catheter removal/catheter failure.
8. Number of participants who were on systemic antibiotics
There was no difference between the number of participants on systemic antibiotics in the impregnated group and the non‐impregnated group (RR 0.95, 95% CI 0.87 to 1.04; 2 studies, 541 participants; Analysis 1.12).
1.12. Analysis.
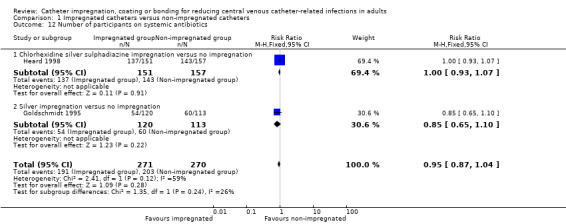
Comparison 1 Impregnated catheters versus non‐impregnated catheters, Outcome 12 Number of participants on systemic antibiotics.
9. Length of stay in ICU (days)
There was no difference between the impregnated group and the non‐impregnated group for length of stay in ICU (mean difference (MD): ‐1.0, 95% CI ‐4.81 to 2.81; 1 study, 275 participants; Additional Table 2; outcome 1.13).
1. Outcomes with only a single study included.
| Comparison | Outcome | Study ID | Data |
| 1. Impregnated catheters versus non‐impregnated catheters (1.12.1 Combined group of C‐SS and silver impregnation) |
1.13 Length of ICU stay (days) |
Dunser 2005 |
Impregnated group: mean 25.0, SD 15.95; total number of participants: 155 Non‐impregnated group: mean 26.0, SD 16; total number of participants: 120 Results: mean difference (days) ‐1.00 (95% CI ‐4.81 to 2.81) |
| 2. MR impregnation versus C‐SS impregnation (analysis GIV method) | 2.3 CRBSI per 1000 catheter days | Darouiche 1999 |
Analysis: GIV method Natural log (ln) of the rate ratio of the incidence density (per 1000 catheter days of MR group over C‐SS group): ‐2.61, SE: 0.9 Total number of catheters: MR group: 356, C‐SS group: 382 Results: RR 0.07 (95% CI 0.01 to 0.43) |
| 2.4 Mortality attributable to CRBSI |
Events/Total number of participants: MR group: 0/350, C‐SS group: 2/370 Results: RR 0.21 (95% CI 0.01 to 4.39) |
||
| 2.5 Adverse effects (combined) |
Events/Total number of participants: MR group: 0/350, C‐SS group: 0/370 Results: RR (95% CI): not estimable |
||
| 2.6 Premature catheter removal/catheter failure |
Events/Total number of catheters: MR group: 118/356, C‐SS group: 119/382 Results: RR 1.06 (95% CI 0.86 to 1.31) |
||
| 3. Silver impregnation versus C‐SS impregnation | 3.1 Clinically diagnosed sepsis | Dunser 2005 |
Events/Total number of participants: silver group: 40/85, C‐SS group: 39/70 Results: RR 0.84 (95% CI 0.62 to 1.15) |
| 3.2 All‐cause mortality |
Events/Total number of participants: silver group: 12/85, C‐SS group: 17/70 Results: RR 0.58 (95% CI 0.30 to 1.13) |
||
| 3.3 Catheter colonization |
Events/Total number of catheters: silver group: 27/160, C‐SS group: 12/165 Results: RR 2.32 (95% CI 1.22 to 4.42) |
||
| 3.4 Catheter colonization per 1000 catheter days |
Analysis: GIV method Natural log (ln) of the rate ratio of the incidence density (per 1000 catheter days of silver group over C‐SS group): 0.89, SE: 0.43 Total number of catheters: silver group: 160, C‐SS group: 165 Results: RR 2.44 (95% CI 1.05 to 5.66) |
||
| 3.5 Adverse effects (combined) |
Events/Total number of participants: silver group: 0/85, C‐SS group: 0/70 Results: RR (95% CI): not estimable |
||
| 3.6 Length of ICU stay (days) |
Silver group: mean 25.0, SD 16.0; total number of participants: 85 C‐SS group: mean 25.0, SD 16.0; total number of participants: 70 Results: mean difference (days) 0.00 (95% CI ‐5.06 to 5.06) |
||
| 4. Heparin coating versus C‐SS impregnation | 4.1 CRBSI | Carrasco 2004 |
Events/Total number of catheters: heparin group: 4/132, C‐SS group: 3/128 Results: RR 1.29 (95% CI 0.30 to 5.66) |
| 4.2 CRBSI per 1000 catheter days |
Analysis: GIV method Natural log (ln) of the rate ratio of the incidence density (per 1000 catheter days of heparin group over C‐SS group): 0.22, SE: 0.83 Total number of catheters: heparin group: 132, C‐SS group: 128 Results: RR1.25 (95% CI 0.24 to 6.34) |
||
| 4.3 Catheter colonization |
Events/Total number of catheters: heparin group: 29/132, C‐SS group: 13/128 Results: RR 2.16 (95% CI 1.18 to 3.97) |
||
| 4.4 Catheter colonization per 1000 catheter days |
Analysis: GIV method Natural log (ln) of the rate ratio of the incidence density (per 1000 catheter days of heparin group over C‐SS group): 0.73, SE: 0.36 Total number of catheters: heparin group: 132, C‐SS group: 128 Results: RR 2.08 (95% CI 1.02 to 4.20) |
||
| 5. MR versus SPC impregnation | 5.1 CRBSI | Fraenkel 2006 |
Events/Total number of catheters: MR group: 4/280, SPC group: 5/294 Results: RR 0.84 (95% CI 0.23 to 3.10) |
| 5.2 CRBSI per 1000 catheter days |
Analysis: GIV method Natural log (ln) of the rate ratio of the incidence density (per 1000 catheter days of MR group over SPC group): ‐0.16, SE: 0.9 Total number of catheters: MR group: 280, SPC group: 294 Results: RR 0.85 (95% CI 0.15 to 4.97) |
||
| 5.3 All‐cause mortality |
Events/Total number of participants: MR group: 45/319, SPC group: 45/327 Results: RR 1.03 (95% CI 0.70 to 1.50) |
||
| 5.4 Catheter colonization |
Events/Total number of catheters: MR group: 25/280, SPC group: 43/294 Results: RR 0.61 (95% CI 0.38 to 0.97) |
||
| 5.5 Catheter colonization per 1000 catheter days |
Analysis: GIV method Natural log (ln) of the rate ratio of the incidence density (per 1000 catheter days of MR group over SPC group): ‐0.02, SE: 0.37 Total number of catheters: MR group: 280, SPC group: 294 Results: RR 0.98 (95% CI 0.47 to 2.02) |
||
| 5.6 Adverse effects (combined) |
Events/Total number of participants: MR group: 29/319, SPC group: 20/327 Results: RR 1.49 (95% CI 0.86 to 2.57) |
||
| 6. Benzalkonium versus SPC impregnation | 6.1 CRBSI | Ranucci 2003 |
Events/Total number of catheters: benzalkonium group: 9/268, SPC group: 12/277 Results: RR 0.78 (95% CI 0.33 to 1.81) |
| 6.2 Catheter colonization |
Events/Total number of catheters: benzalkonium group: 50/268, SPC group: 82/277 Results: RR 0.63 (95% CI 0.46 to 0.86) |
||
| 7. 5‐FU versus C‐SS impregnation | 7.1 Clinically diagnosed sepsis |
Walz 2010 |
Events/Total number of participants: 5‐FU group: 65/480, C‐SS group: 71/480 Results: RR 0.92 (95% CI 0.67 to 1.25) |
| 7.2 CRBSI |
Events/Total number of catheters: 5‐FU group: 0/419, C‐SS group: 2/398 Results: RR 0.19 (95% CI 0.01 to 3.95) |
||
| 7.3 All‐cause mortality |
Events/Total number of participants: 5‐FU group: 38/480, C‐SS group: 39/480 Results: RR 0.97 (95% CI 0.63 to 1.50) |
||
| 7.4 Catheter colonization |
Events/Total number of catheters: 5‐FU group: 12/419, C‐SS group: 21/398 Results: RR 0.54 (95% CI 0.27 to 1.09) |
||
| 7.5 Catheter‐related local infection |
Events/Total number of participants: 5‐FU group: 5/480, C‐SS group: 3/480 Results: RR 1.67 (95% CI 0.40 to 6.93) |
||
| 7.6 Adverse effects (combined) |
Events/Total number of participants: 5‐FU group: 12/480, C‐SS group: 15/480 Results: RR 0.80 (95% CI 0.38 to 1.69) |
||
| 7.7 Duration of antibiotics use (days) |
5‐FU group: mean 6.7, SD 4.8; total number of participants: 480 C‐SS group: mean 6.8, SD 4.7; total number of participants: 480 Results: mean difference (days) ‐0.10 (95% CI ‐0.70 to 0.50) |
Abbreviations
5‐FU = 5‐fluorouracil
CI = confidence interval
CRBSI = catheter‐related blood stream infection
C‐SS = chlorhexidine‐silver sulphadiazine
GIV = generic inverse variance
ICU = intensive care unit
MR = minocycline‐rifampicin
RR = risk ratio or relative risk
SD = standard deviation
SE = standard error
SPC = silver‐platinum‐carbon
10. Cost
The authors of the two studies that reported this outcome based the costs on their data for CRBSI (Maki 1988; Maki 1997). Maki 1988 estimated that the use of a silver‐impregnated cuff could save at least USD 8600 for every 100 cuffs used, assuming that silver‐impregnated cuffs reduced CRBSI by at least two‐third. Maki 1997 estimated that the use of C‐SS‐impregnated catheters could save at least USD 55,000 in direct hospital costs for every 100 impregnated catheters used, again assuming that C‐SS‐impregnated catheters reduced CRBSI by at least two‐third.
11. Quality of life
No studies assessed quality of life.
Comparison 2: One antimicrobial impregnation versus another
1. Minocycline‐rifampicin (MR) versus chlorhexidine silver‐sulphadiazine (C‐SS) impregnation
MR impregnation was shown to significantly reduce CRBSI and catheter colonization compared to C‐SS impregnation, but there was no difference between the two groups in mortality attributed to CRBSI and the rate of premature catheter removal.
Primary outcomes
1. Catheter‐related bloodstream infection (CRBSI)
CRBSI: there were lower rates of CRBSI in the MR group (RR 0.11, 95% CI 0.02 to 0.58; NNTB 33; 2 studies, 812 catheters; I² statistic = 0%; Analysis 2.1).
CRBSI per 1000 catheter days: there were lower rates of CRBSI in the MR group (RR 0.07, 95% CI 0.01 to 0.43; 1 study; Additional Table 2; outcome 2.3).
2.1. Analysis.
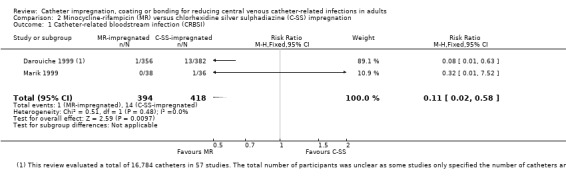
Comparison 2 Minocycline‐rifampicin (MR) versus chlorhexidine silver sulphadiazine (C‐SS) impregnation, Outcome 1 Catheter‐related bloodstream infection (CRBSI).
2. Catheter colonization
There were lower rates of catheter colonization in the MR group (RR 0.36, 95% CI 0.25 to 0.53, NNTB 7, 2 studies, 812 catheters, I² statistic = 0%; Analysis 2.2).
2.2. Analysis.
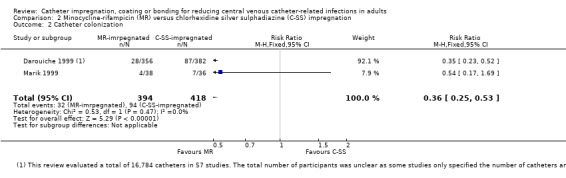
Comparison 2 Minocycline‐rifampicin (MR) versus chlorhexidine silver sulphadiazine (C‐SS) impregnation, Outcome 2 Catheter colonization.
3. Mortality attributed to CRBSI
There was no difference between catheters impregnated with MR or C‐SS for mortality attributed to CRBSI (RR 0.21, 95% CI 0.01 to 4.39; 1 study, 720 catheters; Additional Table 2; outcome 2.4).
Secondary outcome
Premature catheter removal/catheter failure
There was no difference between catheters impregnated with MR or C‐SS for premature catheter removal or catheter failure (RR 1.06, 95% CI 0.86 to 1.31; 1 study, 738 catheters; Additional Table 2; outcome 2.6).
2. Silver impregnation versus C‐SS impregnation
We analysed only one study (Dunser 2005), with 155 participants and 325 catheters, under this comparison. The results for all outcomes assessed in this study are tabulated in Additional Table 2. The results favoured C‐SS impregnation in two outcomes, namely catheter colonization (RR 2.32, 95% CI 1.22 to 4.42; NNTB 10; outcome 3.3), and catheter colonization per 1000 catheter days (RR 2.44, 95% CI 1.05 to 5.66; outcome 3.4). There were no differences between silver impregnation and C‐SS impregnation in clinically diagnosed sepsis (RR 0.84, 95% CI 0.62 to 1.15; outcome 3.1), all‐cause mortality (RR 0.58, 95% CI 0.30 to 1.13; outcome 3.2) or length of ICU stay (MD in days 0.00, 95% CI ‐5.06 to 5.06; outcome 3.6).
3. Heparin versus chlorhexidine silver‐sulphadiazine (C‐SS) impregnation
We analysed only one study with 260 catheters under this comparison (Carrasco 2004). The results for all outcomes assessed in this study are tabulated in Additional Table 2. The results favoured C‐SS impregnation for the outcomes of catheter colonization (RR 2.16, 95% CI 1.18 to 3.97; NNTB 8; outcome 4.3) and catheter colonization per 1000 catheter days (RR 2.08, 95% CI 1.02 to 4.20; outcome 4.4). There were no differences between the groups for the other outcomes assessed, namely, CRBSI (RR 1.29, 95% CI 0.30 to 5.66; outcome 4.1) and CRBSI per 1000 catheter days (RR 1.25, 95% CI 0.24 to 6.34; outcome 4.2).
4. Minocycline‐rifampicin (MR) versus silver‐platinum carbon (SPC) impregnation
We analysed only one study (Fraenkel 2006), with 646 participants and 574 evaluable catheters, under this comparison. The results for all outcomes assessed in this study are tabulated in Additional Table 2. In the only statistically significant result, MR impregnation was shown to reduce catheter colonization compared to SPC impregnation (RR 0.61, 95% CI 0.38 to 0.97; NNTB 17; outcome 5.4). There were no differences between the two groups for the five other outcomes assessed, namely, CRBSI (RR 0.84, 95% CI 0.23 to 3.10; outcome 5.1), CRBSI per 1000 catheter days (RR 0.85, 95% CI 0.15 to 4.97; outcome 5.2), all‐cause mortality (RR 1.03, 95% CI 0.70 to 1.50; outcome 5.3), catheter colonization per 1000 catheter days (RR 0.98, 95% CI 0.47 to 2.02; outcome 5.5) and combined adverse effects (RR 1.49, 95% CI 0.86 to 2.57; outcome 5.6).
5. Benzalkonium versus silver‐platinum carbon (SPC) impregnation
We analysed only one study with 545 catheters under this comparison (Ranucci 2003). The results for all outcomes assessed in this study are tabulated in Additional Table 2. Of the two outcomes assessed, one favoured benzalkonium impregnation (catheter colonization: RR 0.63, 95% CI 0.46 to 0.86; NNTB 9; outcome 6.2), and the other showed no difference between the two groups (CRBSI: RR 0.78, 95% CI 0.33 to 1.81; outcome 6.1).
6. 5‐Fluorouracil (5FU) versus C‐SS impregnation
We analysed only one study (Walz 2010), with 960 participants and 817 evaluable catheters, under this comparison. The results for all outcomes assessed in this study are tabulated in Additional Table 2. None of the seven outcomes assessed, including clinically diagnosed sepsis (outcome 7.1), CRBSI (outcome 7.2), all‐cause mortality (outcome 7.3), catheter colonization (outcome 7.4), catheter‐related local infection (outcome 7.5), any adverse effects (outcome 7.6) and duration of antibiotics used (outcome 7.7), showed any significant difference between the two groups. However, the effect estimates of certain outcomes were too imprecise to derive any clear conclusions about the relative effectiveness of the two interventions assessed, as the 95% CIs were wide for the outcomes of CRBSI (outcome 7.2), catheter colonization (outcome 7.4), catheter‐related local infection (outcome 7.5) and combined adverse effects (outcome 7.6).
Comparison 3: Antimicrobial impregnation versus other antimicrobial modifications
There was no eligible study that compared catheters with antimicrobial impregnation against catheters with other antimicrobial modifications, e.g. antiseptic dressings, hubs, tunnelling, needleless connectors or antiseptic lock solutions.
Subgroup analyses
We performed the following subgroup analyses, as specified in our Methods section, to test for substantial difference in the results based on the type of impregnation, duration of catheter use, predominant participant type, baseline risk, study definition of clinically diagnosed sepsis, catheter impregnation with and without co‐intervention, and cost effectiveness in different units of measurement. Specific data analyses and forest plots for these subgroup are not displayed separately.
1. Each specific type of impregnation versus no impregnation
We divided the studies from the overall comparison between impregnation and no impregnation (detailed under the heading of 'Comparison 1: Antimicrobial impregnation versus no impregnation' above) into subgroups comprising specific types of impregnation. Four of the 11 types of impregnation assessed showed significant reductions in the rates of CRBSI, and six types of impregnation showed significant reductions in catheter colonization rates. Details of the catheter impregnation type and magnitude of reduction in CRBSI and catheter colonization follow.
1. CRBSI
The greatest reduction was shown in studies that assessed MR impregnation (RR 0.26, 95% CI 0.13 to 0.49; NNTB 20; 4 studies, 1335 catheters; I² = 9%), followed by heparin coating (RR 0.27, 95% CI 0.08 to 0.95, NNTB 14, 1 study, 240 catheters), silver impregnation (RR 0.56, 95% CI 0.38 to 0.82, NNTB 25, 6 studies, 1587 catheters, I² = 0%), and C‐SS impregnation (RR 0.75, 95% CI 0.58 to 0.97, NNTB 100, 18 studies, 4653 catheters, I² = 21%), although there was no significant difference across the subgroups in the magnitudes of effect as indicated by a P value of 0.06 in the test of subgroup differences (Chi² test = 15.92, degrees of freedom (df) = 9, I² = 43.5%; Analysis 1.2; Figure 5).
2. Catheter colonization
The greatest reduction in catheter colonization was shown by studies that assessed miconazole‐rifampicin impregnation (RR 0.14, 95% CI 0.06 to 0.32; NNTB 3; 1 study; 223 catheters), followed by MR impregnation (RR 0.52, 95% CI 0.29 to 0.94; NNTB 8; 4 studies; 985 catheters; I² = 71%), benzalkonium impregnation (RR 0.56, 95% CI 0.39 to 0.83; NNTB 6; 2 studies; 254 catheters; I² = 0%), C‐SS impregnation (RR 0.59, 95% CI 0.49 to 0.72; NNTB 9; 20 studies; 4449 catheters; I² = 58%), and vancomycin coating (RR 0.77, 95% CI 0.63 to 0.93; NNTB 6; 1 study; 176 catheters). There was no difference between silver‐impregnated CVCs and non‐impregnated CVCs in catheter colonization (RR 0. 99, 95% CI 0.84 to 1.17; 6 studies; 1451 catheters; I² = 0%).The differences in the magnitude of the effect was highly significant across the subgroup (P < 0.0001 in the test for subgroup differences (Chi² test = 44.90; df = 10; I² statistic = 77.7%); Analysis 1.6; Figure 6). For the subgroup of silver‐impregnated cuff versus no impregnation, the results differed markedly between the two studies conducted in two different settings, as detailed under the earlier heading of 'Comparison 1: Antimicrobial impregnation versus no impregnation, Secondary outcomes 2. Catheter colonization'. We therefore considered it inappropriate to refer to the pooled estimate for this subgroup.
3. All other outcomes
The pooled estimates from each subgroup of a specific impregnation type revealed no significant difference between the impregnated and non‐impregnated groups for all other outcomes, with two exceptions. Firstly, for the outcome of CRBSI per 1000 catheter days, there was a significant reduction that favoured silver impregnation over no impregnation (RR 0.46, 95% CI 0.22 to 0.97; 3 studies; 1054 catheters; I² = 17%; Analysis 1.3.3); secondly, for the outcome of catheter‐related local infection, there was a significant reduction favouring miconazole‐rifampicin impregnation versus no impregnation (RR 0.25, 95% CI 0.10 to 0.64; 1 study; 223 catheters; Analysis 1.5.3). Notably, for the major outcomes of clinically diagnosed sepsis and mortality (all‐cause and attributable to CRBSI), there were no differences between impregnated and non‐impregnated groups for any types of impregnation assessed.
2. Short‐term and long‐term catheter use
We were unable to perform subgroup analysis based short‐term use of catheters (< 10 days) or long‐term use (10 days or more) because there were no separate data for these two subgroups in the included studies. However, Darouiche 1999, which compared MR impregnation versus C‐SS impregnation, provided separate data for catheters used for seven days or less against catheters used for more than seven days. The findings for catheter colonization were comparable between the two subgroups, in which MR impregnation was shown to reduce catheter colonization compared to C‐SS impregnation (seven days or less: RR 0.28, 95% CI 0.16 to 0.50; more than seven days: RR 0.44, 95% CI 0.26 to 0.76). However, for the outcome of CRBSI, the result was markedly in favour of MR impregnation for catheters used for more than seven days (RR 0.11, 95% CI 0.01 to 0.86), compared to the subgroup of catheters used for seven days or less, in which there was no significant difference between the two types of impregnation (RR 0.48, 95% CI 0.04 to 5.30).
3. Participant type
We evaluated two major outcomes of CRBSI and catheter colonization under the overall comparison of any antimicrobial impregnation versus no impregnation as there were a sufficient number of included studies. We grouped together studies that predominantly enrolled participants who were receiving intensive care, studies with a mixture of participants including ICU and non‐ICU, studies that predominantly enrolled people in haematological or oncological units, and studies that predominantly enrolled participants who required CVCs for long‐term total parenteral nutrition (TPN).
1. CRBSI
The overall result showed that antimicrobial impregnation significantly reduced CRBSI compared to no impregnation (RR 0.62, 95% CI 0.52 to 0.74; 42 studies; Analysis 1.2). Substantial reductions in CRBSI were shown in studies conducted in haematological and oncological units (RR 0.50, 95% CI 0.36 to 0.71; 8 studies), in studies with a mixture of ICU and non‐ICU patients (RR 0.47, 95% CI 0.27 to 0.81; 7 studies) and in studies conducted only in ICUs (RR 0.70, 95% CI 0.55 to 0.90; 24 studies). However, there was no difference in the rate of CRBSI between impregnated and non‐impregnated groups in studies conducted in participants in whom CVCs were inserted for TPN (RR 0.83, 95% CI 0.45 to 1.53; 3 studies). The overall difference in the magnitude of the results among the four subgroups was not significant, as indicated by a P value of 0.22 in the test for subgroup differences (Chi² test = 4.38; df = 3; I² = 31.5%; Analysis 3.1).
3.1. Analysis.
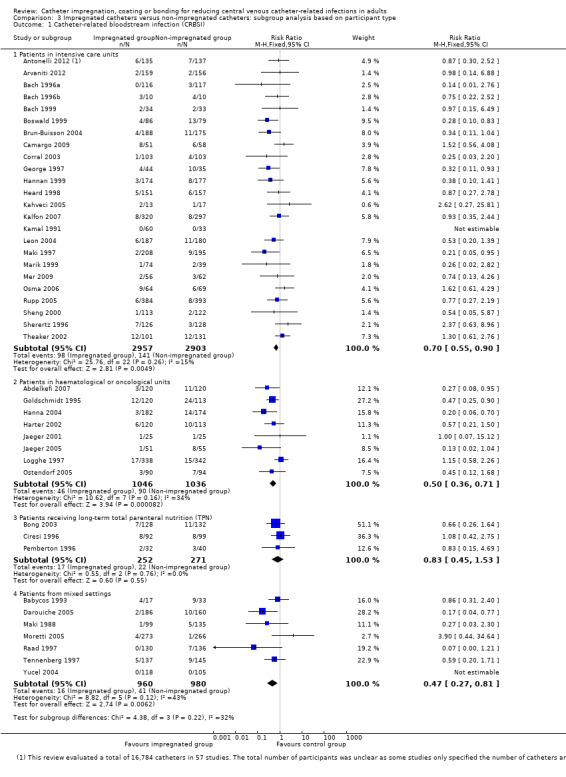
Comparison 3 Impregnated catheters versus non‐impregnated catheters: subgroup analysis based on participant type, Outcome 1 Catheter‐related bloodstream infection (CRBSI).
2. Catheter colonization
The overall result showed that antimicrobial impregnation significantly reduced catheter colonization compared to no impregnation (RR 0.67, 95% CI 0.59 to 0.76; 43 studies; Analysis 1.6). Studies conducted only in ICUs as well as studies with a mixture of ICU and non‐ICU participants demonstrated substantial reductions in catheter colonization favouring antimicrobial impregnation (ICU: RR 0.70, 95% CI 0.61 to 0.80; 29 studies; mixed participants: RR 0.40, 95% CI 0.22 to 0.74; 6 studies). However, there were no differences in the rates of catheter colonization between impregnated and non‐impregnated groups in studies conducted in haematological and oncological units, nor in studies conducted in participants in whom CVCs were inserted for TPN (haematological and oncological units: RR 0.75, 95% CI 0.51 to 1.11; 6 studies; TPN: RR 0.99, 95% CI 0.74 to 1.34; 2 studies).The overall difference in the magnitude of the results among the four subgroups was significant, as indicated by a P value of 0.04 in the test for subgroup differences (Chi² test = 8.28; df = 3; I² statistic = 63.8%; Analysis 3.2).
3.2. Analysis.
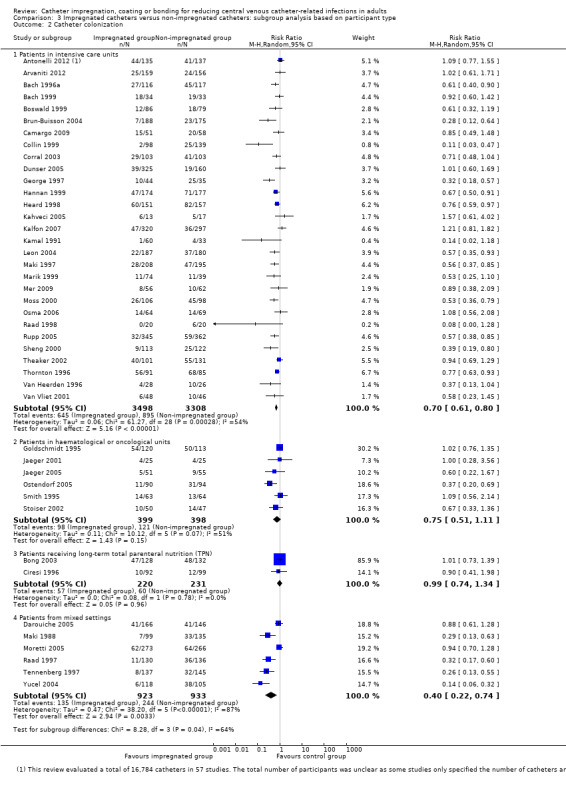
Comparison 3 Impregnated catheters versus non‐impregnated catheters: subgroup analysis based on participant type, Outcome 2 Catheter colonization.
4. Baseline risk
We screened for any major effect of baseline risks on the results by comparing the higher against the lower risk groups, using the median event rate of the control group across the included studies as the cut‐off. We assigned studies with control event rates equal to or higher than the median event rate as higher risk, and the remaining studies as lower risk. We evaluated the two most frequently‐assessed outcomes, namely, CRBSI and catheter colonization under the comparison of any antimicrobial impregnation versus no impregnation, as there were a sufficient number of included studies.
1. CRBSI
There was no difference in the magnitude of reduction in CRBSI between the higher and lower risk groups (higher risk group: RR 0.59, 95% CI 0.47 to 0.74; 21 studies; lower risk group: RR 0.67, 95% CI 0.50 to 0.90; 21 studies). The test for subgroup differences was non‐significant (P = 0.49; Chi² test = 0.47; df = 1; I² = 0%; Analysis 4.1).
4.1. Analysis.
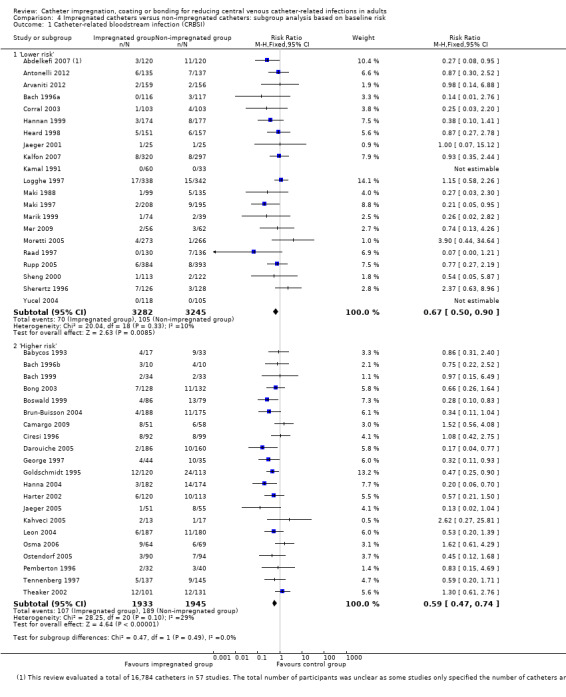
Comparison 4 Impregnated catheters versus non‐impregnated catheters: subgroup analysis based on baseline risk, Outcome 1 Catheter‐related bloodstream infection (CRBSI).
2. Catheter colonization
There was no difference in the magnitude of reduction in catheter colonization between the higher and lower risk groups (higher risk group: RR 0.66, 95% CI 0.56 to 0.78; 23 studies; lower risk group: RR 0.67, 95% CI 0.54 to 0.84; 20 studies). The test for subgroup differences was non‐significant (P value = 0.91; Chi² test = 0.01; df = 1; I² = 0%; Analysis 4.2).
4.2. Analysis.
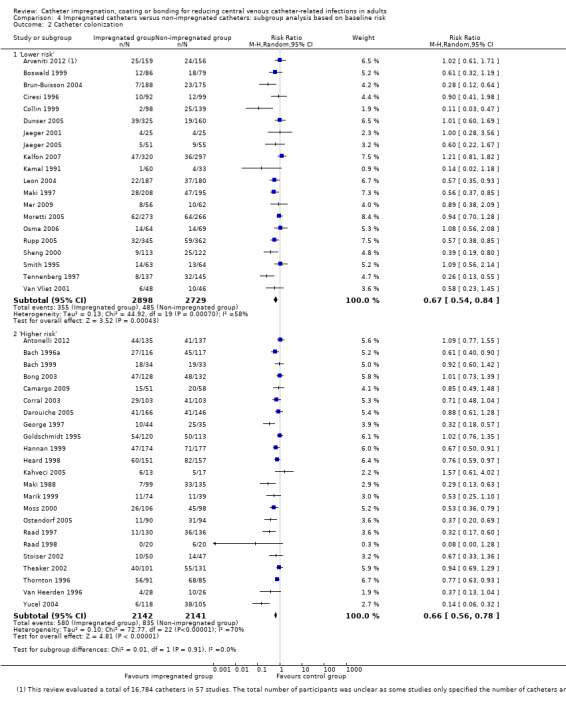
Comparison 4 Impregnated catheters versus non‐impregnated catheters: subgroup analysis based on baseline risk, Outcome 2 Catheter colonization.
5. Outcome of 'clinically diagnosed sepsis': definitions within and outside the scope of our definition
Eight of the 13 studies that evaluated clinically diagnosed sepsis defined this outcome in line with our prespecified definition in this review (see Appendix 1). Seven of these eight studies compared some form of antimicrobial impregnation against no impregnation, while the remaining study compared 5‐fluorouracil (5‐FU) impregnation against C‐SS impregnation. The result for these seven studies was almost identical to the overall result, showing no difference between the impregnated and non‐impregnated groups for this outcome (RR 0.97, 95% CI 0.84 to 1.13; analysis not displayed).
Only one study provided a definition for this outcome that the review authors considered to be outside the scope of our definition (Maki 1988). This study compared catheters with a silver‐impregnated cuff against non‐impregnated catheters, and showed no difference in the rate of sepsis between the two groups.
In the remaining four studies (Maki 1997;Moretti 2005; Theaker 2002; Smith 1995), the definition of this outcome was unclear.
6. Impregnation with and without co‐intervention
While most included studies had some form of hygiene protocol to follow, no study evaluated catheter impregnation with another specific intervention.
7. Cost effectiveness in different units of measurement
The two studies that included costs as an outcome reported their cost estimates in USD (Maki 1988; Maki 1997). Their findings are summarized under 'Comparison 1: Secondary outcomes: point 10' above.
Sensitivity analysis
We performed sensitivity analyses on four major outcomes, namely, clinically diagnosed sepsis, CRBSI, all‐cause mortality and catheter colonization, to evaluate the impact of excluding some studies based on the risks of selection and attrition bias. We only evaluated the comparison of catheters with any antimicrobial impregnation versus non‐impregnated catheters, as there were insufficient studies in the other comparisons to permit a meaningful analysis.
1. Clinically diagnosed sepsis
1. Selection bias
No study was excluded due to a high risk of selection bias.
Attrition bias
The exclusion of four studies with a high risk of attrition bias did not alter the pooled estimates substantively (RR 1.00, 95% CI 0.88 to 1.13, compared with RR 1.02, 95% CI 0.90 to 1.16) (Maki 1988; Moretti 2005; Stoiser 2002; Yucel 2004).
2. CRBSI
Selection bias
The pooled estimates were identical when the two studies with high risks of selection bias were included or excluded (RR 0.62, 95% CI 0.52 to 0.74) (Heard 1998; Kamal 1991).
Attrition bias
Exclusion of 12 studies with a high risk of attrition bias did not substantively alter the final estimates (RR 0.62, 95% CI 0.52 to 0.74, compared with RR 0.61, 95% CI 0.50 to 0.75 after exclusion) (Antonelli 2012; Boswald 1999; Camargo 2009; Corral 2003; Harter 2002; Heard 1998; Maki 1988; Moretti 2005; Ostendorf 2005; Pemberton 1996; Tennenberg 1997; Yucel 2004). However, in the subgroup of silver impregnation versus no impregnation, exclusion of three studies, Antonelli 2012, Boswald 1999, and Harter 2002, with high risks of attrition bias out of the six studies that made this comparison (Antonelli 2012; Bach 1999; Boswald 1999; Goldschmidt 1995; Harter 2002; Kalfon 2007), resulted in a substantial change from favouring impregnation, RR 0.56 (95% CI 0.38 to 0.82), to no significant difference, RR 0.61 (95% CI 0.37 to 1.01).
3. All‐cause mortality
Selection bias
Exclusion of one study, Van Vliet 2001, with a high risk of selection bias from 10 studies in total did not change the pooled estimates substantively (RR 0.92, 95% CI 0.80 to 1.07, compared with RR 0.94, 95% CI 0.80 to 1.09) (Antonelli 2012; Arvaniti 2012; Collin 1999; Corral 2003; Dunser 2005; Hannan 1999; Osma 2006; Rupp 2005; Theaker 2002; Van Vliet 2001).
Attrition bias
Exclusion of three studies with a high risk of attrition bias, Antonelli 2012, Collin 1999 and Corral 2003, from 10 studies in total did not change the pooled estimates substantively (RR 0.92, 95% CI 0.80 to 1.07, compared with RR 0.88, 95% CI 0.74 to 1.06) (Antonelli 2012; Arvaniti 2012; Collin 1999; Corral 2003; Dunser 2005; Hannan 1999; Osma 2006; Rupp 2005; Theaker 2002; Van Vliet 2001).
4. Catheter colonization
Selection bias
Exclusion of three studies with a high risk of selection bias, Heard 1998, Kamal 1991 and Van Vliet 2001, from a total of 43 studies did not change the pooled estimates substantively (RR 0.67, 95% CI 0.59 to 0.76, compared with RR 0.67, 95% CI 0.58 to 0.77).
Attrition bias
Exclusion of 12 studies with a high risk of attrition bias, Antonelli 2012, Boswald 1999, Camargo 2009, Collin 1999, Corral 2003, Heard 1998, Maki 1988, Moretti 2005, Ostendorf 2005, Stoiser 2002, Tennenberg 1997 and Yucel 2004, from a total of 43 studies did not substantively change the pooled estimates (RR 0.67, 95% CI 0.59 to 0.76, compared with RR 0.72, 95% CI 0.62 to 0.82).
Discussion
Summary of main results
Antimicrobial impregnations for central venous catheters (CVCs) did not reduce clinically diagnosed sepsis and all‐cause mortality, but did reduce the rate of catheter‐related bloodstream infections (CRBSIs) and catheter colonization, as shown by evidence from 57 studies with 16,784 catheters. There were no differences in the observed rates of adverse effects, such as thrombosis, bleeding, pain, itch, erythema or itch at the insertion site between participants with impregnated and non‐impregnated catheters. The discrepancy in the results between the outcomes of CRBSI (significant reduction favouring impregnated catheters) and CRBSI per 1000 catheter days (no significant difference) was due to the difference in the number of studies included in the analysis for each outcome. While a large number of studies evaluated CRBSI (42 studies, 10,405 catheters), only 15 of these studies, with 4672 catheters, also evaluated CRBSI per 1000 catheter days. Therefore, the findings for CRBSI in this review should be more representative than those for CRBSI per 1000 catheter days. Our findings for the seven major outcomes are displayed in the Table 1.
Most studies that assessed catheter impregnation included catheter‐specific outcomes, and fewer than a quarter assessed the non‐catheter‐specific ‐ but critical ‐ outcomes of clinical sepsis and mortality. The limited evidence offered by studies that compared different types of impregnation head‐to‐head suggests that in terms of microbiological outcomes, such as catheter colonization, minocycline‐rifampicin (MR) impregnation appeared to be superior to chlorhexidine‐silver sulphadiazine (C‐SS) impregnation, which was in turn superior to silver impregnation and to heparin coating.
For the outcome of catheter colonization, the magnitude of effects in the studies differed depending on the types of participants assessed, with significant benefits of antimicrobial impregnation observed only in studies conducted for patients in intensive care units (ICUs), and no significant benefits observed in studies conducted in haematological or oncological units or in studies for participants for whom CVCs were inserted for long‐term total parenteral nutrition (TPN).
There were wide ranges of baseline risks for major outcomes in the included studies, which were possibly explained by differences in factors such as study setting or participant type, study location and year of study (for instance, whether pre or post 2000). However, the degree of reduction in CRBSI and catheter colonization conferred by antimicrobial impregnation was similar between 'higher risk' and 'lower risk' participants, making baseline risk an unlikely effect modifier. It was unclear whether the variation observed in the reduction of CRBSI and catheter colonization among the studies could be accounted for by other factors such as the underlying conditions of the participants and existing infection control measures. In general, the exclusion of studies with high risks of selection or attrition biases (or both) from our sensitivity analyses for outcomes with a large number of included studies made no material difference to the results.
Overall completeness and applicability of evidence
We identified a large number of studies that contained the population, intervention, comparison and outcomes that matched our prespecified selection criteria. The studies were conducted in a range of settings in 17 different countries. The body of evidence that we have gathered is reflective of the current interest in the use of antimicrobial‐impregnated CVCs as one of the measures in reducing hospital‐associated infections. Statements supporting the use of antimicrobial‐impregnated CVCs are found in practice guidelines from authoritative sources such as the Centers for Disease Control and Prevention (CDC 2011).
Quality of the evidence
We included 57 studies with 16,784 catheters in our review. There was a sufficient number of studies to enable a meaningful meta‐analysis for most of our prespecified outcomes, including the primary outcomes of clinically diagnosed sepsis, CRBSI and all‐cause mortality, although fewer than a quarter of the included studies assessed the major outcomes of clinically diagnosed sepsis and all‐cause mortality. The majority of the included studies had low or unclear risks of bias for most criteria, except for blinding, which was not possible for the participants or healthcare providers in most studies due to the different appearances of the catheters. Furthermore, there were issues with: suspected publication bias for the outcomes of clinically diagnosed sepsis; heterogeneity for the outcome of catheter colonization that was not accounted for adequately by subgroup analyses; and imprecision due to wide 95% confidence intervals for the outcome of catheter‐related local infections, which led to downgrading of the quality of evidence from high to moderate for these outcomes. However, overall, the moderate‐to‐high quality of evidence for the major outcomes enabled us to draw general, robust conclusions (see Table 1).
Potential biases in the review process
The strengths of this review include a comprehensive search of multiple sources and extensive analyses incorporating non‐catheter‐specific ‐ but clinically important ‐ outcomes such as sepsis and mortality, as well as comprehensive subgroup analyses. A limitation of this review is that for catheter‐specific outcomes such as CRBSI and catheter colonization, we reported the results in terms of catheters rather than participants, as all the included studies reported their results this way and none provided the number of participants for each outcome. Our failure to address possible unit of analysis bias by adjusting the data for participants who had multiple catheters could have affected the results. Also, in the subgroup analysis of baseline risk, our decision to explore any possible effects of different baseline risks by simply comparing the 'higher risk' and 'lower risk' groups instead of performing a meta‐regression could have resulted in a loss of information.
Agreements and disagreements with other studies or reviews
There have been several systematic reviews published since 1999 that assessed the effectiveness of CVC impregnations. Many reviews assessed chiefly C‐SS and/or MR impregnation and found that impregnated CVCs significantly reduced CRBSI or catheter colonization, or both (Casey 2008; Falagas 2007; Hockenhull 2008; Hockenhull 2009; Niel‐Weise 2007; Ramritu 2008; Veenstra 1999a), and were estimated to be cost‐effective (Veenstra 1999b). However, other reviews found antimicrobial‐impregnated CVCs to have no significant benefits (Gilbert 2008; McConnell 2003; Niel‐Weise 2008). Notably, the authors in Niel‐Weise 2007 found substantial benefits of antimicrobial‐impregnated CVCs in a meta‐analysis of 21 trials conducted either in ICUs or other acute care settings, but found no benefits in a separate meta‐analysis of nine trials assessing CVCs for TPN and chemotherapy, which agrees with our results (Niel‐Weise 2008). The authors postulated that the difference in the duration of catheter placement between these reviews (mean of six to 12 days in the included studies in Niel‐Weise 2007 and 11 to 20 days in the included studies in Niel‐Weise 2008), the small sample sizes and the methodological limitations of the included studies in Niel‐Weise 2008, were possible factors that could have influenced the findings. In Niel‐Weise 2007, the authors included a study that assessed haemodialysis catheters and a study on children.
The systematic reviews cited above vary in scope, and most evaluated only catheter‐specific outcomes such as CRBSI and catheter colonization. There is no systematic review that incorporates non‐catheter‐specific critical outcomes such as clinical sepsis and mortality for a direct comparison with our findings.
Authors' conclusions
Implications for practice.
While there is high‐quality evidence for the benefits of antimicrobial‐impregnated central venous catheters (CVCs) in reducing catheter‐related blood stream infections (CRBSIs) and moderate quality evidence for reducing catheter colonization, there is also a high‐quality, but smaller body of evidence that shows no significant benefit of these catheters in reducing mortality, and moderate‐quality evidence shows no difference in clinically diagnosed sepsis. Therefore, there remains uncertainty about the value of these modified catheters in improving overall patient mortality and morbidity. Furthermore, this review shows that there were significant benefits with impregnated CVCs for catheter‐related outcomes, such as catheter colonization, in trials conducted in intensive care units (ICUs) only. Currently, while the overall body of evidence still allows recommendations in favour of their use in practice, there should be great caution in recommending the use of antimicrobial‐impregnated CVCs across all settings without incorporating the current uncertainties on their overall benefits.
Implications for research.
Despite strong evidence of the overall benefits of antimicrobial‐impregnated CVCs in reducing CRBSI, there is a need for ongoing research to evaluate their effects on major outcomes due to evolving patterns of hospital‐associated infections, infection control measures and microbiological diagnostic techniques. In this review, we found that a comparatively small number of studies reported clinical outcomes such as clinically diagnosed sepsis and mortality, and the quality of available evidence for major outcomes was adversely affected by either a suspicion of publication bias (clinically‐diagnosed sepsis), heterogeneity (catheter colonization) or wide confidence intervals (catheter‐related local infection). It appears that most studies evaluated catheter‐related outcomes with microbiological findings, as these might be more objective than clinical outcomes. While catheter‐related outcomes are important, they may not represent the true state of morbidities in a patient, as not all catheter‐related infections are proven by a positive catheter culture or blood culture, or both, despite the manifestation of clinical features suggestive of sepsis. Future research should include key clinical outcomes such as clinically diagnosed sepsis and mortality alongside catheter‐specific outcomes such as CRBSI and catheter colonization, and include other sensitive septic markers to detect the presence of micro‐organisms in case catheter and blood cultures fail. Any cost estimation should also base figures on the overall rates of sepsis rather than on CRBSI alone, as not all catheter related sepsis episodes are associated with a positive catheter and/or blood culture result, due to the limitations in currently available microbiological diagnostic techniques. The setting, type of participants and the existing infection control measures should be clearly described in future studies to enable an evaluation of the possible reasons for any difference in the effects of impregnated catheters in different settings.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 26 January 2016 | New citation required but conclusions have not changed | One new study included, with no change in the overall conclusion. There is no change to the team of authors. |
| 26 January 2016 | New search has been performed | Updated search current to March 2015, 299 new de‐duplicated studies identified (one included, one awaiting classification and the rest excluded). The review was rewritten in line with the MECIR recommendations with greater incorporation of quality of evidence. A section on grading the quality of evidence and the use of 'Summary of findings' table was included. |
| 14 June 2013 | Amended | Nathorn Chaiyakunapruk's contact details updated. |
Notes
May 2015: The updated review was rewritten in line with the MECIR recommendations to increase incorporation of quality of evidence judgements. A section on grading the quality of evidence and the use of 'Summary of findings' tables was included.
As part of the prepublication editorial process, the protocol for this systematic review was commented on by a content editor and three peer reviewers (who are external to the editorial team), members of the Cochrane Consumer Network’s international panel of consumers and the Cochrane Anaesthesia, Critical and Emergency Care Group’s Trials Search Co‐ordinator (Lai 2009).
Acknowledgements
We would like to thank Harald Herkner (Content Editor), Jane Cracknell (Managing Editor), Vibeke E Horstmann (Statistical Editor) and Sheila Page (Consumer Referee) for comments and help on the updated manuscript. Harald Herkner, Jane Cracknell, Karen Hovhanisyen, Cathal Walsh (Statistical Editor), Djillali Annane, Nasia Safdar, Donna Gillies (Peer Reviewers) and Anne Lyddiat (Consumer) commented on the published review (Lai 2013).
We thank the staff at the International‐Ann Arbor Safety Collaborative (I‐A²SC) for cross‐checking risk of bias assessment and data entry. Specifically, Andy Hickner and Todd Greene cross‐checked the risk of bias assessments; and Andy Hickner cross‐checked data entry and proof‐read the draft review (Lai 2013).
Appendices
Appendix 1. Diagnostic criteria for sepsis (2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference)
Infectiona (documented or suspected) and some of the following:b
General parameters:
fever (core temperature > 38.3°C)
hypothermia (core temperature < 36°C)
heart rate > 90 bpm or > 2 SD above the normal value for age
tachypnoea: > 30 bpm
altered mental status
significant oedema or positive fluid balance (> 20 ml/kg over 24 h)
hyperglycaemia (plasma glucose > 110 mg/dl or 7.7 mM/l) in the absence of diabetes
Inflammatory parameters:
leukocytosis (white blood cell count > 12,000/µl)
leukopenia (white blood cell count < 4,000/µl)
normal white blood cell count with > 10% immature forms
plasma C reactive protein > 2 SD above the normal value
plasma procalcitonin > 2 SD above the normal value
Haemodynamic parameters:
arterial hypotensionb (systolic blood pressure < 90 mmHg, mean arterial pressure < 70, or a systolic blood pressure decrease > 40 mmHg in adults or < 2 SD below normal for age)
mixed venous oxygen saturation > 70%b
cardiac index > 3.5 l min‐1m‐2c,d
Organ dysfunction parameters:
arterial hypoxaemia ( PaO2/FiO2 < 300)
acute oliguria (urine output < 0.5 ml kg‐1h‐1 or 45 mM/l for at least 2 h)
creatinine increase ≥ 0.5 mg/dl
coagulation abnormalities (international normalized ratio > 1.5 or activated partial thromboplastin time > 60 s)
ileus (absent bowel sounds)
thrombocytopenia (platelet count < 100,000/µl)
hyperbilirubinemia (plasma total bilirubin > 4 mg/dl or 70 mmol/l)
Tissue perfusion parameters:
hyperlactatemia ( > 3 mmol/l)
decreased capillary refill or mottling
a Defined as a pathological process induced by a micro‐organism.
b Values above 70% are normal in children (normally 75%–80%) and should therefore not be used as a sign of sepsis in newborns or children.
c Values of 3.5 ‐ 5.5 are normal in children and should therefore not be used as a sign of sepsis in newborns or children.
d Diagnostic criteria for sepsis in the paediatric population consist of signs and symptoms of inflammation plus infection with hyper‐ or hypothermia (rectal temperature > 38.5°C or < 35°C), tachycardia (may be absent in hypothermic patients) and at least one of the following indications of altered organ function: altered mental status, hypoxaemia, elevated serum lactate level, and bounding pulses.
Appendix 2. MEDLINE (OVID SP) search strategy
#1: exp Catheterization, Central Venous/ #2: (venous or vein) or catheter*.mp.
#3: #1 or #2 #4: (impregn* or bond* or coat*).mp. #5: (anti?microbial or antisep* or antibiotic*).mp. #6: (needleless and connector*).mp. #7: exp Chlorhexidine/ #8: exp Silver‐Sulfadiazine/ #9: exp Minocycline/ #10: exp rifampin/ #11: (Rifampi* or Minocyclin* or Silver?Sulfadiazine or Chlorhexidine or "Arrowgard" or "Cook Spectrum").mp. #12: #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13: #3 and #12 #14: Clinical trial.pt. #15: exp clinical trial/ #16: trial.ti. #17: random*.mp #18: #14 or #15 or #16 or #17 #19: #13 and #18
Appendix 3. CENTRAL search strategy
#1: MeSH descriptor Catheterization, Central Venous explode all trees #2: (catheter near impregnat* ):ti,ab,kw #3: (catheter* near coat*):ti,ab,kw #4: (catheter* near bond*):ti,ab,kw #5: #1 OR #2 OR #3 OR #4 (Restrict by product: Clinical Trials) #6: (antimicrobial OR antisep* OR antibiotics):ti,ab,kw #7: (needleless AND connector*):ti,ab,kw #8: (chlorhexidine):ti,ab,kw #9: (silver near sulphadiazine):ti,ab,kw #10: (minocyclin):ti,ab,kw #11: (rifampi*):ti,ab,kw #12: "Arrowgard" OR "Cook Spectrum":ti,ab,kw #13: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (Restrict by product: Clinical Trials) #14: #5 AND #13
Appendix 4. EMBASE search strategy
#1: Emtree: Explode: "Central Venous catheterization"/all subheadings #2: ((venous OR vein) AND catheter):ab,ti #3: #1 OR #2 #4: (impregn* OR bond* OR coat*):ab,ti #5: (anti?microbial OR antiseptic OR antibiotic*):ab,ti #6: (needleless AND connector*):ab,ti #7: Emtree: Explode: "Chlorhexidine"/ all subheadings #8: Emtree: Explode: "Sulfadiazine silver"/all subheadings #9: Emtree: Explode: "Minocycline"/all subheadings #10: Emtree: Explode: "Rifampicin"/all subheadings #11: (Rifampi* OR Minocyclin* OR Silver?Sulfadiazine OR Chlorhexidine OR "Arrowgard" OR "Cook Spectrum"):ab,ti #12: #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13: #3 AND #12 #14: Emtree: Explode: "RANDOMIZED‐CONTROLLED TRIAL"/ all subheadings #15: Emtree: Explode: "RANDOMIZATION"/ all subheadings #16: Emtree: Explode: "CONTROLLED STUDY"/ all subheadings #17: Emtree: Explode:"MULTICENTER STUDY"/ all subheadings #18: Emtree: Explode:"DOUBLE BLIND PROCEDURE"/ all subheadings #19: Emtree: Explode:"SINGLE BLIND PROCEDURE"/ all subheadings #20: #14 OR #15 OR #16 OR #17 OR #18 OR #19 #21: (RANDOM* OR CROSS?OVER* OR FACTORIAL* OR PLACEBO* OR VOLUNTEER*):ab,ti #22: (SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (BLIND* OR MASK*):ab,ti #23: #20 OR #21 OR #22 #24: #13 AND #23
Appendix 5. CINAHL search strategy (via EBSCOHost)
#1 MH "Catheterization, Central Venous"/explode
#2 TI venous or AB venous
#3 TI vein or AB vein
#4 TI catheter* or AB catheter*
#5 #2 or #3
#6 #4 and #5
#7 #1 or #6
#8 TI impregn* or AB impregn*
#9 TI bond* or AB bond*
#10 TI coat* or AB coat*
#11 #8 or #10 or #9
#12 TI antimicrobial or AB antimicrobial
#13 TI antisep* or AB antisep*
#14 TI antibiotic$ or AB antibiotic$
#15 #12 or #13 or #14
#16 TI needleless or AB needleless
#17 TI connector$ or AB connector$
#18 #16 and #17
#19 MH "Chlorhexidine"
#20 MH "Silver Sulfadiazine"
#21 MH "Minocycline"
#22 MH "Rifampin"
#23 TI Rifampi* or AB Rifampi*
#24 TI Minocyclin* or AB Minocyclin*
#25 TI Silver sulphadiazine or AB Silver sulphadiazine
#26 TI Chlorhexidine or AB Chlorhexidine
#27 TI Arrowgard or AB Arrowgard
#28 TI Cook Spectrum or AB Cook Spectrum
#29 #23 or #24 or #25 or #26 or #27 or #28
#30 #29 or #11 or #15 or #18 or #19 or #20 or #21 or #22
#31 #7 and #30
#32 PT Clinical trial
#33 AB randomized or AB randomized
#34 AB random*
#35 TI trial
#36 MH "Clinical Trials"/explode
#37 #32 or #33 or #34 or #35 or #36
#38 #31 and #37
Appendix 6. Criteria for a judgment on the sources of bias in the included studies
1. Was the allocation sequence randomly generated?
Yes, low risk of bias
A random (unpredictable) assignment sequence.
Examples of adequate methods of sequence generation are computer‐generated random sequence, coin toss (for studies with two groups), rolling a dice (for studies with two or more groups), drawing of balls of different colours, dealing previously shuffled cards.
No, high risk of bias
Quasi‐randomized approach: examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number.
Non‐random approaches: allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention.
Unclear
Insufficient information about the sequence generation process to permit judgement.
2. Was the treatment allocation adequately concealed?
Yes, low risk of bias
Assignment must be generated independently by a person not responsible for determining the eligibility of the participants. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about whether the person is eligible to enter the trial. Examples of adequate methods of allocation concealment are: central allocation, including telephone, web‐based, and pharmacy controlled, randomization; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
No, high risk of bias
Examples of inadequate methods of allocation concealment are: alternate medical record numbers, unsealed envelopes; date of birth; case record number; alternation or rotation; an open list of random numbers any information in the study that indicated that investigators or participants could influence the intervention group.
Unclear
Randomization stated but no information on method of allocation used is available.
3. Blinding was knowledge of the allocated interventions adequately prevented during the study?
Was the participant blinded to the intervention?
Yes, low risk of bias
The treatment and control groups are indistinguishable for the participants or if the participant was described as blinded and the method of blinding was described.
No, high risk of bias
Blinding of study participants attempted, but likely that the blinding could have been broken; participants were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Was the care provider blinded to the intervention?
Yes, low risk of bias
The treatment and control groups are indistinguishable for the care/treatment providers, or the care provider was described as blinded and the method of blinding was described.
No, high risk of bias
Blinding of care/treatment providers attempted, but likely that the blinding could have been broken; care/treatment providers were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Was the outcome assessor blinded to the intervention?
Yes, low risk of bias
Adequacy of blinding should be assessed for the primary outcomes. The outcome assessor was described as blinded and the method of blinding was described.
No, high risk of bias
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Unclear
4. Were incomplete outcome data adequately addressed?
Was the drop‐out rate described and acceptable?
The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given.
Yes, low risk of bias
If the percentage of withdrawals and drop‐outs does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias. (NB these percentages are arbitrary, not supported by literature).
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
Missing data have been imputed using appropriate methods.
No, high risk of bias
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
Unclear
Were all randomized participants analysed in the group to which they were allocated? (ITT analysis)
Yes, low risk of bias
Specifically reported by authors that ITT was undertaken and this was confirmed on study assessment, or not stated but evident from study assessment that all randomized participants are reported/analysed in the group they were allocated to for the most important time point of outcome measurement (minus missing values) irrespective of non‐compliance and co‐interventions.
No, high risk of bias
Lack of ITT confirmed on study assessment (patients who were randomized were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation) regardless of whether ITT reported or not.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; potentially inappropriate application of simple imputation.
Unclear
Described as ITT analysis, but unable to confirm on study assessment, or not reported and unable to confirm by study assessment.
5. Are reports of the study free of suggestion of selective outcome reporting?
Yes, low risk of bias
If all the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the final trial report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgment. Alternatively a judgement could be made if the trial report lists the outcomes of interest in the methods of the trial and then reports all these outcomes in the results section of the trial report.
No, high risk of bias
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
6. Other sources of potential bias:
PLEASE NOTE AUTHORS MUST DECIDE WHAT OTHER SOURCES OF POTENTIAL BIAS ARE APPROPRIATE TO THE REVIEW
THE DOMAINS BELOW ARE SUGGESTIONS:
Were the groups similar at baseline regarding the most important prognostic indicators?
Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, for example size and duration of ulcer. Alternatively if there were imbalances at baseline these have been accounted for in the analysis of the study.
Were co‐interventions avoided or similar?
There were no co‐interventions or there were co‐interventions but they were similar between the treatment and control groups.
Was the compliance acceptable in all groups?
The review author determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the treatment intervention and control intervention(s). For example, ultrasound treatment is usually administered over several sessions; therefore it is necessary to assess how many sessions each participant attended or if participants completed the course of an oral drug therapy. For single‐session interventions (for example: surgery), this item is irrelevant.
Were the trials or trialists in receipt of financial support from agencies or organisations with a financial interest in the outcome of the trial?
Appendix 7. Studies with discrepancies between the prespecified outcomes in the methods and the outcomes reported in the results
| Study ID | Major outcomes stated in the methods | Major outcomes reported in the results | Major relevant outcomes that were evaluable but not included |
| Abdelkefi 2007 |
|
|
|
| Babycos 1993 |
|
|
|
| Bach 1996a |
|
|
|
| Bach 1996b |
|
|
|
| Ciresi 1996 |
|
|
|
| Collin 1999 |
All the major outcomes prespecified in the Methods were reported in the Results |
The following 2 outcomes were incompletely reported:
|
None |
| Maki 1997 |
|
Although not specifically stated as the aim of this study, the authors put forward a cost‐effectiveness evaluation in the results based on various assumptions on the rate of bacteraemia in the control group. The data however were presented in a format that was unsuitable to be included in the meta‐analysis |
|
| Moss 2000 |
|
|
|
| Osma 2006 |
|
Additionally, the outcome of all‐cause mortality could be extracted from the study as it was reported as a part of the patient characteristics |
|
| Raad 1997 |
|
The authors provided a cost estimate of the two types of catheters but the figures were presented narratively and were not suitable to be included in our meta‐analysis. |
|
| Raad 1998 |
|
|
|
| Thornton 1996 |
|
|
|
| Van Heerden 1996 |
|
|
|
| Van Vliet 2001 |
|
Catheter‐related local infection, which was listed as an outcome in the Methods, was not reported in the Results |
None |
Appendix 8. Additional searches of Internet resources
| Resources | Terms searched | Hits | Relevant study (non‐duplicate) | Remarks |
| www.clinicaltrials.gov | Central venous catheter | 632 | 1. Antonelli 2011a 2. Pachl 2010a |
Both studies were completed but appear to be not yet published |
| catheter* AND impregnat* | 56 | 0 | ||
| catheter* AND coat* | 65 | Steinberg 2009 | Completed but appears to be not yet published | |
| catheter* AND bond* | 2 | 0 | ||
| www.control‐trials.com | central venous catheter | 238 | 0 | |
| impregnation | 9 | 0 | ||
| coating | 120 | 0 | ||
| www.update‐software.com | central venous catheter* OR impregnat* OR coat* OR bond* | 150 | 0 | |
| www.clinicaltrialsresults.org | catheter AND (impregnation OR coating OR bonding) | 0 | 0 | |
| http://centrewatch.com/ | central venous catheters OR catheter impregnation OR catheter coating OR catheter bonding | 0 | 0 |
Data and analyses
Comparison 1. Impregnated catheters versus non‐impregnated catheters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinically‐diagnosed sepsis | 12 | 3686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| 1.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 4 | 1666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 1.2 Combined group of chlorhexidine silver sulphadiazine impregnation and silver impregnation versus no impregnation | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.08] |
| 1.3 Silver impregnation versus no impregnation | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.43] |
| 1.4 Silver‐platinum‐carbon impregnation versus no impregnation | 2 | 854 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.60] |
| 1.5 Benzalkonium impregnation versus no impregnation | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.81] |
| 1.6 Silver‐impregnated cuff versus no impregnation | 2 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.86, 2.49] |
| 1.7 Miconazole‐rifampicin impregnation versus no impregnation | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.21] |
| 2 Catheter‐related bloodstream infection (CRBSI) | 42 | 10405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.52, 0.74] |
| 2.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 19 | 4886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.94] |
| 2.2 Combined group of chlorhexidine silver sulphadiazine impregnation and minocycline rifampicin impregnation versus no impregnation | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.02, 2.82] |
| 2.3 Chlorhexidine impregnation versus no impregnation | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.63, 8.96] |
| 2.4 Silver‐impregnated cuff versus no impregnation | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.55] |
| 2.5 Teicoplanin coating versus no coating | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.52] |
| 2.6 Benzalkonium impregnation versus no impregnation | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 2.7 Silver‐platinum‐carbon impregnation versus no impregnation | 4 | 1320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 2.8 Heparin coating versus no coating | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.95] |
| 2.9 Silver impregnation versus no impregnation | 6 | 1587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.38, 0.82] |
| 2.10 Miconazole‐rifampicin impregnation versus no impregnation | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Minocycline‐rifampicin impregnation versus no impregnation | 4 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.49] |
| 2.12 Cefazolin coating versus no coating | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Catheter‐related bloodstream infection per 1000 catheter days | 15 | 4672 | Risk Ratio (Fixed, 95% CI) | 0.75 [0.51, 1.11] |
| 3.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 6 | 1579 | Risk Ratio (Fixed, 95% CI) | 1.20 [0.70, 2.06] |
| 3.2 Minocycline‐rifampicin impregnation versus no impregnation | 3 | 945 | Risk Ratio (Fixed, 95% CI) | 0.35 [0.11, 1.12] |
| 3.3 Silver impregnation versus no impregnation | 3 | 1054 | Risk Ratio (Fixed, 95% CI) | 0.46 [0.22, 0.97] |
| 3.4 Silver platinum carbon impregnation versus no impregnation | 2 | 854 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.17, 3.55] |
| 3.5 Heparin coating versus no coating | 1 | 240 | Risk Ratio (Fixed, 95% CI) | 0.26 [0.03, 2.59] |
| 4 All‐cause mortality | 10 | 2643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.80, 1.07] |
| 4.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 6 | 1636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.12] |
| 4.2 Combined group of chlorhexidine silver sulphadiazine impregnation and silver impregnation versus no impregnation | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.60, 1.60] |
| 4.3 Silver‐platinum‐carbon impregnation versus no impregnation | 2 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.12] |
| 4.4 Silver impregnation versus no impregnation | 1 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.39] |
| 5 Catheter‐related local infection | 12 | 2688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 5.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 7 | 1547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 5.2 Minocycline‐rifampicin impregnation versus no impregnation | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.36, 3.72] |
| 5.3 Miconazole‐rifampicin impregnation versus no impregnation | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.64] |
| 5.4 Cefazolin coating versus no coating | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.28, 6.70] |
| 5.5 Benzalkonium impregnation versus no impregnation | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.02] |
| 5.6 Chlorhexidine impregnation versus no impregnation | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 6 Catheter colonization | 43 | 9910 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.59, 0.76] |
| 6.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 20 | 4449 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.49, 0.72] |
| 6.2 Combined group of chlorhexidine silver sulphadiazine impregnation and minocycline rifampicin impregnation versus no impregnation | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.25, 1.10] |
| 6.3 Combined group of chlorhexidine silver sulphadiazine impregnation and silver impregnation versus no impregnation | 1 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.60, 1.69] |
| 6.4 Minocycline‐rifampicin impregnation versus no impregnation | 4 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.94] |
| 6.5 Cefazolin coating versus no coating | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.18] |
| 6.6 Benzalkonium impregnation versus no impregnation | 2 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.39, 0.83] |
| 6.7 Silver‐platinum‐carbon impregnation versus no impregnation | 4 | 1320 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.10] |
| 6.8 Miconazole‐rifampicin impregnation versus no impregnation | 1 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.06, 0.32] |
| 6.9 Silver‐impregnated cuff versus no impregnation | 2 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.15, 2.15] |
| 6.10 Silver impregnation versus no impregnation | 6 | 1451 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.17] |
| 6.11 Vancomycin coating versus no coating | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.63, 0.93] |
| 7 Catheter colonization per 1000 catheter days | 12 | 3615 | Risk Ratio (Random, 95% CI) | 0.74 [0.55, 1.00] |
| 7.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 5 | 1470 | Risk Ratio (Random, 95% CI) | 0.53 [0.28, 1.02] |
| 7.2 Minocycline‐rifampicin impregnation versus no impregnation | 2 | 679 | Risk Ratio (Random, 95% CI) | 0.73 [0.29, 1.80] |
| 7.3 Silver impregnation versus no impregnation | 4 | 1151 | Risk Ratio (Random, 95% CI) | 0.86 [0.59, 1.26] |
| 7.4 Silver‐platinum‐carbon versus no impregnation | 1 | 315 | Risk Ratio (Random, 95% CI) | 1.04 [0.57, 1.91] |
| 8 Skin or insertion site colonization | 3 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.97] |
| 8.1 Cefazolin coating versus no coating | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.45, 1.71] |
| 8.2 Silver impregnation versus no impregnation | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 8.3 Silver‐platinum‐carbon impregnation versus no impregnation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.38, 1.02] |
| 9 Mortality attributed to catheter related blood stream infection (CRBSI) | 5 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.20] |
| 9.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 2 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.45] |
| 9.2 Minocycline‐rifampicin impregnation versus no impregnation | 1 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.00] |
| 9.3 Cefazolin coating versus no coating | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Heparin coating versus no coating | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse effects | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Catheter‐related thrombosis/thrombophlebitis | 3 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.44, 1.85] |
| 10.2 Bleeding | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.48] |
| 10.3 Combined (including bleeding, pain, itch, erythema or tenderness at the insertion site) | 10 | 3003 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.27] |
| 11 Premature catheter removal/catheter failure | 15 | 3666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 11.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 6 | 1756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.18] |
| 11.2 Minocycline‐rifampicin impregnation versus no impregnation | 2 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.51, 1.11] |
| 11.3 Silver impregnation versus no impregnation | 2 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.25] |
| 11.4 Miconazole‐rifampicin impregnation versus no impregnation | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.32] |
| 11.5 Silver‐platinum‐carbon impregnation versus no impregnation | 2 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.11] |
| 11.6 Benzalkonium impregnation versus no impregnation | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.50] |
| 11.7 Chlorhexidine impregnation versus no impregnation | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.85, 2.00] |
| 12 Number of participants on systemic antibiotics | 2 | 541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.04] |
| 12.1 Chlorhexidine silver sulphadiazine impregnation versus no impregnation | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 12.2 Silver impregnation versus no impregnation | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.10] |
Comparison 2. Minocycline‐rifampicin (MR) versus chlorhexidine silver sulphadiazine (C‐SS) impregnation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related bloodstream infection (CRBSI) | 2 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.58] |
| 2 Catheter colonization | 2 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.25, 0.53] |
Comparison 3. Impregnated catheters versus non‐impregnated catheters: subgroup analysis based on participant type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related bloodstream infection (CRBSI) | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Patients in intensive care units | 24 | 5860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.90] |
| 1.2 Patients in haematological or oncological units | 8 | 2082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.71] |
| 1.3 Patients receiving long‐term total parenteral nutrition (TPN) | 3 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.45, 1.53] |
| 1.4 Patients from mixed settings | 7 | 1940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.81] |
| 2 Catheter colonization | 43 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Patients in intensive care units | 29 | 6806 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.61, 0.80] |
| 2.2 Patients in haematological or oncological units | 6 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.11] |
| 2.3 Patients receiving long‐term total parenteral nutrition (TPN) | 2 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.34] |
| 2.4 Patients from mixed settings | 6 | 1856 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.74] |
Comparison 4. Impregnated catheters versus non‐impregnated catheters: subgroup analysis based on baseline risk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related bloodstream infection (CRBSI) | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 'Lower risk' | 21 | 6527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 1.2 'Higher risk' | 21 | 3878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.47, 0.74] |
| 2 Catheter colonization | 43 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 'Lower risk' | 20 | 5627 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.54, 0.84] |
| 2.2 'Higher risk' | 23 | 4283 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.56, 0.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelkefi 2007.
| Methods | Single‐centre RCT (Tunisia) | |
| Participants | Patients from 4 to 60 years of age with underlying haematological diseases including malignant and non‐malignant conditions requiring a CVC. Exclusion criteria were the presence of a CVC at admission, catheterization for less than 7 days, a contraindication to the use of subclavian catheterization due to major blood coagulation disorders (i.e. platelet count < 50 x 109/L, disseminated intravascular coagulation), and an absence of catheter‐tip culture at the time of catheter removal | |
| Interventions | Heparin coating versus heparin infusion: comparing heparin‐coated CVCs (with normal saline infusion) versus uncoated catheters with unfractionated heparin infusion | |
| Outcomes | CRBSI, mortality from CRBSI, catheter colonization and adverse events (bleeding and catheter thrombosis) | |
| Notes | This was a study that compared the use of heparin in CVCs in 2 forms: heparin coating versus heparin infusion. This study was included as it satisfied our selection criteria of catheter impregnation versus other form of catheter‐related intervention. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A simple randomization sequence was generated by a centralised computer” |
| Allocation concealment (selection bias) | Low risk | Quote: “A simple randomization sequence was generated by a centralised computer” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not stated whether the participants and the carers were blinded to the assigned status of the participants |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: “The principal investigator determined whether infections were catheter‐related and had no knowledge of 'the assigned arm' " Methods, data collection: “ ... the final analysis was performed by an independent statistical office” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: “The principal investigator determined whether infections were catheter‐related and had no knowledge of 'the assigned arm' ”. Methods, data collection: “ ... the final analysis was performed by an independent statistical office” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.4% of participants withdrew from the study with reasons for withdrawals stated, as follows: Quote: "... 6 patients (2.4%) were excluded (three in each group) because of catheter insertion failure." The missing outcome data were small, balanced across the two groups and the reason was unlikely to be related to the true outcomes assessed (CRBSI and catheter colonization) |
| Selective reporting (reporting bias) | Low risk | Two major outcomes that were prespecified in the Methods (CRBSI and catheter colonization) were reported |
| Other bias | Low risk | None identified |
Antonelli 2012.
| Methods | Multi‐centre RCT conducted in 5 ICUs (Italy) | |
| Participants | “Adult patients (> 18 years) scheduled to undergo central venous catheterization (via subclavian or internal jugular route) were enrolled with informed consent. Exclusion criteria were a history of unsuccessful attempts at catheterization or evidence of previous surgery, skeletal deformity and/or scarring involving the catheterization site” | |
| Interventions | Silver nanoparticle‐impregnated CVCs (AgTive) versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, infection‐free time (days from initial CVC insertion to initial blood culture positivity) and ICU mortality rates | |
| Notes | Sources of funding: Government research fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: patients were randomized using an “internet‐based scheme, stratified by centre, patient age and gender” |
| Allocation concealment (selection bias) | Low risk | The random sequence key appears to have been generated independently from allocation. Quote: “the key was held by the data manager” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel ‐ high risk: Quote: “… the physicians performing catheterization were aware of the type of catheter being used in each case…” Participants ‐ unclear risk: although the authors stated that the new catheters inserted were of the same type as the catheter being removed, it was not stated whether the catheters used in the 2 groups were similar in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: “For data collection purposes, patients were identified solely as members of Group A or B.” It was also stated that the statistician who performed the analysis was blinded to the patient’s group allocation |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: “For data collection purposes, patients were identified solely as members of Group A or B.” It was also stated that the statistician who performed the analysis was blinded to the patient’s group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 66/338 (19%) excluded from analysis due to “important clinical or microbiological data missing”. 34 of the excluded participants were in the AgTive group, and 32 in the control group. The rate of exclusion was high, and the exact reasons for exclusion, what data were missing, and whether the available data for the excluded participants could still be meaningfully analyzed were not clear. We judged the study to be at high risk for incomplete outcome data for the combination of the reasons mentioned above |
| Selective reporting (reporting bias) | Low risk | All the major outcomes specified in the Methods, namely, CRBSI, catheter colonization and ICU mortality, were reported in sufficient detail for meta‐analysis |
| Other bias | Low risk | None identified |
Arvaniti 2012.
| Methods | Multi‐centre RCT (Greece) | |
| Participants | Adult ICU patients who required a CVC for 3 days or more. Neutropenic patients, pregnant women and patients with allergy to silver or chlorhexidine were excluded | |
| Interventions | Three‐arm comparison: Oligon SPC impregnated CVCs versus CVCs with silver‐gluconate impregnated patch (placed over the skin underneath the CVC insertion site) versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, ICU death, local adverse effects, sepsis and the number of catheters removed due to suspected sepsis | |
| Notes | Only 2 of the 3 assigned groups (Oligon group and control group that received non‐impregnated CVCs) are included in this review, as the silver‐gluconate impregnated patch does not fit within the interventions prespecified. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated to one of the three groups, separately for each participating ICU, and according to computer‐generated randomization sequences.” |
| Allocation concealment (selection bias) | Low risk | Quote: “This procedure (randomization) was performed by the supervising ICU. The randomization number and the corresponding study group were sealed in envelopes in numeric order. Envelopes were posted to the ICUs with accompanying instructions to be opened by respecting their numerical order” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Double‐blind design was not possible as a result of apparent differences between the compared products" |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated clearly whether the assessors of the microbiological outcomes were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Although it was not stated clearly whether assessors of clinical outcomes were blinded to the status of the participants, the infectious disease physicians who verified the data were blinded Quote: : “Two ICU infectious diseases experts scrutinized all data blindly to the randomization group to identify concomitant infections and avoid erroneous attribution of the recorded events to the study catheters.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Catheter cultures were available in 376/465 participants randomized (80.9%). The proportions of uncultured catheters (not cultured for a variety of reasons) appeared balanced across the 3 groups. For person‐level outcomes such as CRBSI, all randomized participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All major outcomes specified in the Methods were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Babycos 1993.
| Methods | Two‐centre RCT (USA) | |
| Participants | Adult patients in surgical unit who required CVCs. Excluded people under the age of 18 years, acute trauma patients whose catheters were inserted in the emergency room, pregnant women, and people with sepsis of no known source | |
| Interventions | Three‐arm comparison: CVCs with silver‐impregnated cuff versus non‐impregnated tunnelled catheters with Opsite dressing versus non‐impregnated tunnelled catheters with collodion dressing | |
| Outcomes | Suspected and confirmed CRBSI and catheter colonization (labelled as insertion site infection) | |
| Notes | This study assessed the use of a silver‐impregnated cuff and not an impregnated catheter. However, the authors of this review were of the consensus that the cuff constitutes part of the catheter, and therefore this study should be included. Subgroup analysis would have been performed if there had been enough studies assessing cuff impregnation. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The only statement about sequence generation said: “The patients were randomly assigned to …” |
| Allocation concealment (selection bias) | Unclear risk | There was no statement about the methods and source of sequence generation and whether this was independent of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Whether participants and personnel were blinded was not clearly stated, although this was unlikely as the 2 types of catheters appear different and were inserted using different techniques, and there were no statements regarding any measures to standardize external appearance of the catheter insertion site |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no statement about whether the outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no statement about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no statement about the number of participants randomized versus the number that received the intervention, or about how many participants were analyzed and how many withdrew, or reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | The major outcomes specified in the Methods, included suspected and confirmed CRBSI (referred to as 'catheter related sepsis' in the paper) and catheter colonization (referred to as 'catheter infection' in the Methods and 'insertion site infection' in the Results, and defined as > 15 CFU/blood agar plate on catheter tip culture) were reported in the Results. However, catheter‐related local infection was not reported |
| Other bias | Low risk | None identified |
Bach 1996a.
| Methods | Single‐centre RCT (Germany) | |
| Participants | People scheduled for cardiac surgery, non‐pregnant, non‐lactating participants over the age of 18 years who were due to receive a CVC. Participants were excluded if there was a history of adverse reactions to silver, sulphonamides, or chlorhexidine, as were those with an immune deficiency. Participants who needed additional intravascular access (with the exception of an arterial line) were also excluded | |
| Interventions | Teicoplanin‐coated CVC versus standard uncoated catheters | |
| Outcomes | Catheter colonization and retention of antibiotic teicoplanin on the catheters | |
| Notes | The authors reported separately the incidence of colonization for subcutaneous and intravenous segments of the catheters. For this review, we took the higher incidences (subcutaneous segments) as our data for meta‐analysis. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The only statement regarding randomization said: “According to the randomization schedule … ” No specific method of sequence generation was mentioned |
| Allocation concealment (selection bias) | Unclear risk | The only statement regarding randomization said: “According to the randomization schedule… ” No details were given about how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the authors stated that "Both types of catheters were identical in appearance", there was no specific statement about blinding |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | Although the authors stated that "Both types of catheters were identical in appearance", there was no specific statement about blinding |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | Although the authors stated that "Both types of catheters were identical in appearance", there was no specific statement about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although there was a mention of the number of participants who entered the trial (116 in the intervention group and 117 in the control group), there was no mention of the number that were eventually analyzed and the number of withdrawals with their reasons |
| Selective reporting (reporting bias) | High risk | The outcomes specified in the methods, namely, catheter colonization and retention of antibiotics were reported in the Results. However, a major and clinically relevant outcome of bloodstream infection or sepsis (catheter‐related or otherwise) was not included |
| Other bias | Low risk | None identified |
Bach 1996b.
| Methods | Single‐centre RCT (Germany) | |
| Participants | Adult male participants scheduled to be admitted to the ICU after major abdominal surgery were included. No exclusion criteria were stated in the paper | |
| Interventions | Teicoplanin‐coated CVCs versus uncoated polyurethane single lumen catheters | |
| Outcomes | Catheter colonization | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was generated by a “computer‐generated random list ... ” |
| Allocation concealment (selection bias) | Unclear risk | No details were given about how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The inserter in the OR [operating room] was not blinded for randomization, but the patients were; after catheterization, no note was made on the patients' files and the physicians and nursing staff in the ICU were blind to the randomization. The laboratory personnel were also blinded.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: “The inserter in the OR [operating room] was not blinded for randomization, but the patients were; after catheterization, no note was made on the patients' files and the physicians and nursing staff in the ICU were blind to the randomization. The laboratory personnel were also blinded.” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: “The inserter in the OR [operating room] was not blinded for randomization, but the patients were; after catheterization, no note was made on the patients' files and the physicians and nursing staff in the ICU were blind to the randomization. The laboratory personnel were also blinded.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants (20 in total, 10 each group) were analyzed |
| Selective reporting (reporting bias) | High risk | The study reported only the microbiological outcome of catheter colonization, the major clinical outcome of CRBSI was not reported |
| Other bias | Low risk | None identified |
Bach 1999.
| Methods | Single‐centre RCT (Germany) | |
| Participants | People admitted to a 16‐bed ICU after cardiac surgery who required CVCs. Age was not stated as an inclusion criterion, and no details were given about the ages of the participants. Exclusion criteria were not stated | |
| Interventions | Silver‐impregnated CVCs versus standard uncoated CVCs | |
| Outcomes | Catheter colonization and CRBSI (referred to as 'catheter related bacteraemia') | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The only statement concerning randomization said: “Assignment to one of the two catheter groups was randomized." No details were given about how randomization was achieved. |
| Allocation concealment (selection bias) | Unclear risk | The only statement concerning randomization said: “Assignment to one of the two catheter groups was randomized.” No details were given about how allocation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no direct statement about blinding nor any statement concerning the appearance of the catheters used in the experimental and the control groups |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no statement about blinding of the outcome assessors |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no statement about blinding of the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/74 patients (4 in the experimental group and 3 in the control group: 9.5%) were excluded “because they were transferred to other hospitals, and the catheters were not available for detailed microbiological analysis.” The attrition rates were balanced between the 2 groups |
| Selective reporting (reporting bias) | Low risk | All the outcomes prespecified in the Methods were reported in the Results, including the major clinically relevant outcome of CRBSI (referred to as 'catheter related bacteraemia') |
| Other bias | Low risk | None identified |
Bennegard 1982.
| Methods | Single‐centre prospective controlled clinical study (Sweden) | |
| Participants | Participants between 17 and 84 years old requiring CVCs. No further details were given regarding the eligibility and exclusion criteria of the participants | |
| Interventions | Heparin‐coated CVCs versus uncoated CVCs inserted through the cubital fossa (antebrachial veins) | |
| Outcomes | The primary aims of the study were to assess thrombogenicity and thrombophlebitis | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was described by the authors as "double‐blind, prospective, controlled clinical study" without further description of the methods used for sequence generation and allocation, and so it was unclear whether this was truly a randomized study |
| Allocation concealment (selection bias) | Unclear risk | The study was described by the authors as "double‐blind, prospective, controlled clinical study" without further description of the methods used for sequence generation and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors stated that the study was 'double‐blind' without specifically mentioning who was blinded. It was very likely that the participants were blinded, but it was unclear whether the second blinded element was the investigator, the carer or the outcome assessor |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The authors stated that the study was 'double‐blind' without specifically mentioning who was blinded. It was very likely that the participants were blinded, but it was unclear whether the second blinded element was the investigator, the carer or the outcome assessor |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The authors stated that the study was 'double‐blind' without specifically mentioning who was blinded. It was very likely that the participants were blinded, but it was unclear whether the second blinded element was the investigator, the carer or the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 49 participants recruited were included in the analysis |
| Selective reporting (reporting bias) | Low risk | The major outcomes specified in the Methods, namely thrombosis and thrombophlebitis, were reported in the Results. The direct anti‐infective properties of heparin were probably not of major interest at the time of the study, so it appeared acceptable that major infective outcomes like CRBSI were not included in this study |
| Other bias | Low risk | None identified |
Bong 2003.
| Methods | Single‐centre RCT (UK) | |
| Participants | Participants comprised "all patients who required central venous access over a period greater than seven days." The setting of the study was not described specifically but the authors stated that "Most patients in our study required central venous access for total parenteral nutrition." "Patients were excluded if they were younger than 18 years of age, had a history of allergy to silver, needed multilumen central venous access, or were pregnant.” | |
| Interventions | Silver‐platinum‐carbon (Oligon) CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was generated by computer in blocks of 10.” |
| Allocation concealment (selection bias) | Low risk | The authors stratified the participants into those with high or low risk of sepsis before randomization Quote: “Patients were then assigned the study catheters sequentially according to randomization codes, which were concealed in separate envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no specific description of blinding in the paper, but it was highly unlikely that any blinding occurred as the experimental and control catheters differed in appearance, and there was no description of any measure taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no specific statement about whether the investigator/data collector or analyst were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no specific statement about whether the investigator/data collector or analyst were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Complete data could be evaluated in 270 catheters (89%): 128 silver iontophoretic catheters and 140 untreated catheters. The remaining 34 catheters (15 silver iontophoretic catheters and 19 untreated catheters) were not cultured (either being removed without notification or lost to follow up) and hence were excluded from our study.” The number excluded was acceptable and appeared to be balanced between the 2 groups |
| Selective reporting (reporting bias) | Low risk | Both the major outcomes specified in the Methods, namely, catheter colonization and CRBSI were reported in the Results |
| Other bias | Low risk | None identified |
Boswald 1999.
| Methods | Single‐centre RCT (Germany) | |
| Participants | Participants included those recruited from acute emergencies, postsurgical ICU patients and those requiring long‐term parenteral nutrition. Pregnant women and people whose body weight was below 30 kg were excluded from the study. Median age of the participants: 55 years in the intervention group, 53 years in the control group | |
| Interventions | Silver‐impregnated CVCs versus uncoated CVCs | |
| Outcomes | Catheter colonization and CRBSI (referred to as 'catheter associated infection') | |
| Notes | Sources of funding: public cross‐institutional research fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Catheters were allocated in the study by block randomization" |
| Allocation concealment (selection bias) | Unclear risk | The authors did not explain whether random sequence was generated independently of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no clear statement about blinding, or whetherthe experimental and control catheters were identical in appearance, or there was any measure taken to blind those involved in the study. The only statements about the catheters said: ”control catheters were commercially available catheters without silver or antibiotic impregnation with the same dimensions.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no clear statement about blinding, or whether the experimental and control catheters were identical in appearance, or there was any measure taken to blind those involved in the study. The only statements about the catheters said: ”control catheters were commercially available catheters without silver or antibiotic impregnation with the same dimensions.” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no clear statement about blinding, or whether the experimental and control catheters were identical in appearance, or there was any measure taken to mask those involved in the study. The only statements about the catheters said: ”control catheters were commercially available catheters without silver or antibiotic impregnation with the same dimensions.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 165/263 participants (62.7%) with evaluable microbiological results were analyzed for the outcome of catheter colonization and catheter associated infections for which microbiological results were required as a part of the diagnosis. However, the authors did not explain why the microbiological results were unavailable for the 37.3% excluded |
| Selective reporting (reporting bias) | Low risk | Both the major outcomes specified in the Methods, namely, catheter colonization and CRBSI, were reported in the Results |
| Other bias | Low risk | None identified |
Brun‐Buisson 2004.
| Methods | Multicentre RCT (France) | |
| Participants | Adult patients requiring a CVC at a new site for at least 3 days. Detailed exclusion criteria were not stated but the author stated that they were available upon request | |
| Interventions | C‐SS‐impregnated CVCs versus uncoated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | The authors stated in the Results that "the trial was stopped after 42 months because of the slow enrolment rate and lower than expected infection rate, which did not allow reaching the prespecified objectives within a reasonable time frame." Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | While the authors stated that the study was randomized, there was no statement about how, and by whom, the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | While the authors stated that the study was randomized, there was no statement about how the allocation was carried out |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Catheters, whether or not antiseptic‐coated, were provided with identical appearance” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no specific statement about whether the investigator/data collector or analyst were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no specific statement about whether the investigator/data collector or analyst were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 363/397 participants initially randomized were analyzed (91.4%). 17 participants in each group were not included in the analysis due to failure of catheter insertion, consent withdrawal (1 in the antiseptic impregnated group) and catheters not cultured (3 all in the antiseptic‐impregnated group). With regard to the latter 2 reasons, although they occurred only in 1 group, the number was small and was unlikely to influence the results of this study materially |
| Selective reporting (reporting bias) | Low risk | The 2 major outcomes prespecified in the Methods, catheter colonization and CRBSI, were reported in the Results |
| Other bias | Low risk | None identified |
Camargo 2009.
| Methods | Single‐centre RCT (Brazil) | |
| Participants | Adult participants aged over 18 years in a medical‐surgical ICU in Brazil who required a new double‐lumen CVC. Pregnant women and people with sulphonamide allergy were excluded | |
| Interventions | C‐SS‐impregnated CVCs versus uncoated CVCs | |
| Outcomes | Catheter colonization, CRBSI and premature catheter removal | |
| Notes | Sources of funding: stated as none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Folders were randomly assigned in a blinded fashion as ‘impregnated’ or ‘standard’ for the same number of available catheters." No further details were given about how the randomization sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: “A randomization scheme with concealed allocation was used to help match the two study groups closely. Folders were randomly assigned in a blinded fashion as ‘impregnated’ or ‘standard’ for the same number of available catheters. After the need for a CVC had been determined, and participants or their legal guardians had given informed consent, a nurse not otherwise involved the study chose one folder.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors did not provide any clear statement on blinding, although blinding seemed unlikely in this study as folders containing the code for the group assigned had either 'impregnated' or 'standard' clearly written on them. It was also not stated whether the 2 types of catheter were identical in their external appearances and whether any measures had been taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The authors did not describe whether the outcome assessors were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The authors did not describe whether the outcome assessors were blinded to the status of the participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32 catheters from 141 randomized participants (22.7%) were excluded because they were lost after removal. 20 of these were from the antiseptic impregnated group and 12 from the control group. The high attrition rate and the imbalance in loss of data between the 2 groups put the study at high risk of bias due to incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | The major outcomes of catheter colonization, CRBSI and premature catheter removal were reported in the Results |
| Other bias | Low risk | None identified |
Carrasco 2004.
| Methods | Single‐centre RCT (Spain) | |
| Participants | Participants admitted to a medical‐surgical ICU in a university hospital of 600 beds who required triple‐lumen CVCs | |
| Interventions | Heparin‐coated CVCs versus C‐SS‐coated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… 196 consecutive patients admitted to the M/SICU [Medical/Surgical Intensive Care Unit] and who needed a triple‐lumen CVC were first randomized to receive either a triple‐lumen polyurethane HC [healthcare] (Abbott) catheter or one coated with C‐SS on the outer surface (Arrow)." No further details were given about the methods of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details were given about the method of allocation and whether this was independent of sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not clearly stated whether the participants and personnel were blinded, but blinding appeared very unlikely as both types of catheters were produced by different manufacturers and therefore would appear different (Quote: "... 196 consecutive patients admitted to the M/SICU [Medical/Surgical Intensive Care Unit] and who needed a triple‐lumen CVC were first randomized to receive either a triple‐lumen polyurethane HC [healthcare] (Abbott) catheter or one coated with C‐SS on the outer surface (Arrow).") |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "The microbiologist who processed all cultures was blinded to each catheter group" |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors did not explain whether the clinicians responsible for diagnosing CRBSI were blinded, but blinding appeared very unlikely as both types of catheters were produced by different manufacturers and therefore would look different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 260/276 catheters (94.2%) were available for analysis. 16 catheters (7 from the heparin group and 9 from the C‐SS group) were excluded from analysis because these catheters were not cultured. |
| Selective reporting (reporting bias) | Unclear risk | The major outcomes of catheter colonization and CRBSI, as specified in the Methods, were also reported in the Results |
| Other bias | Low risk | None identified |
Ciresi 1996.
| Methods | Single‐centre RCT (USA) | |
| Participants | Adults 18 years or over who needed a triple‐lumen CVC for TPN. Exclusion criteria: < 18 years of age; unable to give consent; pregnant women; people for whom cultures were not obtained for whatever reason; allergy to sulfa for the Arrowgard group. It appeared that the last 2 criteria referred to postrandomization exclusions | |
| Interventions | C‐SS‐impregnated CVCs versus uncoated CVCs | |
| Outcomes | Catheter colonization, CRBSI, catheter‐related local infection, premature catheter removal and length of hospital stay | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients meeting the enrolment criteria were randomized on the basis of the last digit of the patient’s record number to receive either the antiseptic‐coated Arrowgard or standard triple lumen catheter." The author did not elaborate on how the last digit of the record number was used in randomization, i.e. whether some form of alternate allocation was used or whether a truly randomized sequence was deployed |
| Allocation concealment (selection bias) | Unclear risk | See the comments above for Random sequence generation (selection bias). Furthermore, the authors did not provide the source of sequence generation and whether this was independent of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no description of blinding in the paper, and it was highly unlikely that any blinding occurred as the experimental catheter and control catheter differed in appearance, and there was no description of any measures taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no description of blinding in the paper, and it was highly unlikely that any blinding occurred as the experimental catheter and control catheter differed in appearance, and there was no description of any measures taken to blind those involved in the study. It was unclear whether the assessors of microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was description of blinding in the paper, and it was highly unlikely that any blinding occurred as the experimental catheter and control catheter differed in appearance, and there was no description of any measures taken to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/202 (5.5%) participants were excluded postrandomization (7 in the experimental group and 4 in the control group). The reason for exclusion was failure to obtain a culture. The number excluded was small and unlikely to change the results of this study significantly. |
| Selective reporting (reporting bias) | Unclear risk | The major outcomes specified and defined in the Methods, including catheter colonization (referred to as 'catheter related infection') and CRBSI (referred to as 'catheter related sepsis') and premature catheter removal were reported in the Results |
| Other bias | Low risk | None identified |
Collin 1999.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: "All patients > 12 years of age who were to receive a central venous catheter in the emergency room, neurotrauma ICU or medical/surgical ICU were eligible for inclusion." The author did not provide exclusion criteria | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, all‐cause mortality, premature catheter removal, length of hospital stay | |
| Notes | Two‐part study: part I was an RCT that compared an antiseptic‐impregnated catheter with a non‐impregnated catheter. Part II was a phase II study: a single group of participants, all received antiseptic‐impregnated catheters. Only data from phase I were extracted for meta‐analysis in this systematic review. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients .… were allocated by a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | It was not stated whether the random number sequence was generated independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The author did not provide any information about blinding, or the similarity or differences in the appearances of the 2 types of catheter. However, it was unlikely that participants or investigators in contact with them would have been blinded, as the antiseptic‐impregnated catheter had an appearance that differed from most standard non‐impregnated catheters in use at the time of the study, and there was no mention of any measures taken to blind the participants and personnel involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The author did not provide any statement about whether the outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | It was unlikely that the clinicians responsible for the diagnosis of CRBSI were blinded. See comments above under blinding of participants and personnel (performance bias) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 242 catheters were placed in 123 patients. 5 participants were excluded (4 died prior to the removal/exchange of the first catheter, "all as a result of their original injury or illness"), and 1 participant pulled out his own catheter. It was unclear what basis the author had for making the statement that the 4 participants who died died as a result of their own prior illness, and it seemed inappropriate to exclude them for this reason when one of the outcomes examined was all‐cause mortality. Furthermore, it was not stated from which group these 4 participants came. The results for mortality could have been altered significantly if at least 3 of the 4 had been allocated to 1 group |
| Selective reporting (reporting bias) | High risk | All the major outcomes prespecified in the Methods were reported in the Results. However, for the outcome of premature catheter removal, the author presented the results in charts without providing the data as numbers. For the length of hospital stay, only means were reported without standard deviations or standard errors. It was therefore impossible to extract data for these 2 outcomes for meta‐analysis |
| Other bias | Low risk | None identified |
Corral 2003.
| Methods | Single‐centre RCT (Spain) | |
| Participants | Quote: “Patients requiring non‐tunnelled insertion of a triple‐lumen central venous catheter (CVC), which remained in place for four or more days, were included in the study.” No exclusion criteria were given | |
| Interventions | SPC‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, skin colonization, all‐cause mortality in ICU and number of catheters removed prematurely | |
| Notes | Sources of funding: stated as none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “First, ICU beds were divided randomly in two groups: the control group (CG) received a standard polyurethane catheter and the silver group (SG) an OVSC [Oligon Vantex silver catheter]. Second, nursing staff were rotated between the two groups according to a pre‐designed timetable. To avoid the possible influence of location, the two groups of beds were switched halfway through the study. At ICU admission, patients were allocated to beds by nursing staff, who were unaware of the catheter group assigned to the beds.” It was unclear how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: “First, ICU beds were divided randomly in two groups: the control group (CG) received a standard polyurethane catheter and the silver group (SG) an OVSC [Oligon Vantex silver catheter]. Second, nursing staff were rotated between the two groups according to a pre‐designed timetable. To avoid the possible influence of location, the two groups of beds were switched halfway through the study. At ICU admission, patients were allocated to beds by nursing staff, who were unaware of the catheter group assigned to the beds.” It was unclear how effective this method of randomization was in achieving concealment of allocation. There was a risk that the nurses who allocated the participants would gain knowledge of the pattern of allocation based on the "catheter type and bed" association after a period of observation, especially since the catheters evaluated were of different appearances |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no specific statement about blinding, although blinding appeared highly unlikely as the catheters evaluated differed in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The author did not provide any statement about whether the outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The author did not provide any statement about whether the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Complete data were evaluated for 145 patients (83%) and 206 catheters (80%): 103 catheters in each group. The remaining 51 catheters (28 CG [control group] and 23 SG [SPC group]) were not cultured (23 were not cultured after removal as the protocol could not be carried out and 28 remained in place for less than four days)." The high proportion of excluded catheters and the seemingly dubious reasons for their exclusion (for example, violation of protocol and shorter than required indwelling time did not preclude the catheters from being cultured) placed the study at high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | All the major outcomes specified in the Methods were reported in sufficient details in the Results |
| Other bias | Low risk | None identified |
Darouiche 1999.
| Methods | Multicentre RCT (USA) | |
| Participants | Hospitalized adults who were at high risk for catheter‐related infection (such as people in ICUs or those who were immunocompromised) and were likely to require a CVC for 3 or more days were eligible for the study. Pregnant women and people with a history of allergy to any of the antimicrobial agents used for impregnating the catheters were excluded | |
| Interventions | MR‐impregnated CVCs versus C‐SS‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI and mortality attributed to CRBSI, premature catheter removal and adverse effects | |
| Notes | The authors presented the outcomes separately for catheters in place for ≤ 7 days and > 7 days along with the overall outcomes. Sources of funding: partly from the industry (catheter manufacturer or distributor) and partly from institutional research fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was described in detail and appeared truly random: Quote: "A special randomization scheme was used to help match the two study groups closely. Catheter trays wrapped in identical folders were randomly assigned in blinded fashion according to computer‐generated identification numbers, in blocks of six (three from each group), so that the catheter trays would be removed from the box one at a time in the prescribed, random order from the top to the bottom. Blocks of six catheters were then shipped to the participating hospitals for assignment to specified patient care units.” |
| Allocation concealment (selection bias) | Low risk | From the statements above, the random sequence appeared to be centrally generated, i.e. away from the recruitment sites |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In each case, the patients, nurses, physicians, and principal investigators who assessed the outcomes in each hospital were unaware of the type of catheter inserted.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "In each case, the patients, nurses, physicians, and principal investigators who assessed the outcomes in each hospital were unaware of the type of catheter inserted.” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "In each case, the patients, nurses, physicians, and principal investigators who assessed the outcomes in each hospital were unaware of the type of catheter inserted.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 127/856 catheters (14.7%) were excluded from analysis (58 MR‐impregnated and 69 impregnated with C‐SS). The authors stated that the excluded samples had "similar patient and catheter characteristics". The catheters were excluded because they were not cultured, due to the following: " ... 84 were removed without notification of study coordinators, 19 were grossly contaminated during removal, and 24 were not available for other reasons" Although the excluded samples had similar characteristics across the 2 groups according to the authors, in view of the low event rates ‐ especially for the outcomes of CRBSI and mortality from CRBSI ‐ inclusion of these catheters might have altered the results significantly with or without assuming the worst‐case scenarios for either group |
| Selective reporting (reporting bias) | Low risk | All the major outcomes specified in the Methods, including catheter colonization and CRBSI, were reported in the Results |
| Other bias | Low risk | None identified |
Darouiche 2005.
| Methods | Multicentre RCT (USA) | |
| Participants | Quote: "Men and women 18 years of age or older who required a central venous catheter for ≥ 2 weeks were eligible. Pregnant women and patients with a history of allergy to any of the antimicrobial agents used for impregnating the catheters were excluded." | |
| Interventions | MR‐impregnated, non‐tunnelled CVCs versus non‐impregnated, tunnelled CVCs | |
| Outcomes | Catheter colonization, CRBSI and mortality attributed to CRBSI, premature catheter removal, antimicrobial activities of the antiseptic‐impregnated catheters and adverse effects | |
| Notes | The major comparison was antimicrobial impregnated CVCs versus non‐impregnated CVCs, although the comparison was confounded by the presence of tunneling in the non‐impregnated group. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no explicit statement about how or where the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | There was no information that would enable an assessment of whether the random sequence generation was achieved independently of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors did not provide any specific statement on blinding. However blinding appeared unlikely as the 2 types of catheters used in the study differed in appearance: the tunnelled catheter had a cuff, while the MR‐impregnated catheter had not. Furthermore, there was no statement about any measures taken to blind the participants and personnel involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "The investigators who assessed the outcomes of catheter colonization and bloodstream infection in each hospital relied on information contained in the completed case forms with regard to clinical findings and results of cultures, and were unaware of the type of inserted catheter." |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "The investigators who assessed the outcomes of catheter colonization and bloodstream infection in each hospital relied on information contained in the completed case forms with regard to clinical findings and results of cultures, and were unaware of the type of inserted catheter." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 351 study catheters were inserted. Because 39 catheters were not cultured (15 were removed without notification of study co‐ordinators, 10 were grossly contaminated at the time of removal, 10 were inserted in participants who subsequently died, and 4 were inserted in participants who were lost to follow‐up), a total of 312 catheters (166 MR‐impregnated and 146 tunnelled) were evaluable for analysis of catheter colonization. 5 catheters (4 inserted in participants lost to follow‐up and one inserted in a participant whose cause of death was not investigated) were excluded from the analysis of CRBSI. Of the total of 346 catheters that were included in the analysis of CRBSI, 186 were MR‐impregnated and 160 were tunnelled. These two groups of evaluable participants were well matched with respect to most characteristics, including gender, age, type of underlying disease, risk factors for infection, receipt of systemic antibiotics, site of catheter insertion, and number of catheter lumens |
| Selective reporting (reporting bias) | Unclear risk | All the major outcomes, including catheter colonization, CRBSI, premature catheter removal, adverse effects and antimicrobial activities of the impregnated catheters were reported in the Results |
| Other bias | Low risk | None identified |
Dunser 2005.
| Methods | Single‐centre RCT (Austria) with three‐arm comparison. | |
| Participants | Quote: “All patients older than 18 yr who required a new CVC during their intensive care unit stay were eligible for study entry. Pregnant women and patients with a history of allergy to any of the components of the study catheter were excluded from study enrolment.” | |
| Interventions | Three‐arm comparison: silver‐impregnated CVCs versus C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Clinical sepsis (referred to as "systemic inflammatory response syndrome (SIRS)"), catheter colonization, adverse effects and length of ICU stay | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization of patients was performed using a random number generating scheme.” |
| Allocation concealment (selection bias) | Unclear risk | The authors did not explain who performed the random sequence generation or provide information to enable an assessment of whether sequence generation was performed independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Because of the different colours and packages of the individual catheters, doctors and nurses could not be blinded to the type of study catheter." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The authors did not provide any explanation about whether the assessors of microbiological outcomes were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | Quote: "Because of the different colours and packages of the individual catheters, doctors and nurses could not be blinded to the type of study catheter." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The numbers of participants analyzed in each group were identical to the numbers randomized, and it appears that all participants recruited were analyzed. However there were no confirmatory statements that this was the case and no statements about withdrawals |
| Selective reporting (reporting bias) | Low risk | The major outcomes specified in the Methods, namely catheter colonization and CRBSI (labelled as SIRS by the authors) were reported in the Results along with some other outcomes that were listed alongside the participants' other characteristics, like ICU mortality and length of ICU stay |
| Other bias | Low risk | None identified |
Fraenkel 2006.
| Methods | Single‐centre RCT (Australia) | |
| Participants | Quote: "All patients requiring insertion of a CVC while in the ICU were eligible. Exclusion criteria were allergies to the constituent antimicrobials." | |
| Interventions | MR‐impregnated CVCs versus SPC impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, all‐cause mortality and adverse effects | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocks of ten computer‐generated random numbers were used. Burn patients were randomized in blocks of six, given their higher risk and smaller numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomly sequenced CVC sets in identical individual opaque covers were placed in a sealed dispenser box, which allowed removal of only the lowest CVC in the stack. The set was drawn from the box after the decision to insert a CVC for an individual patient and was brought to the bedside still in its opaque cover. No other triple‐lumen catheters were available for the duration of the trial.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Staff inserting and caring for the CVCs could not be blinded due to the different colours of the two catheter types; however, all other aspects of the study were blinded and concealed. Culture results were reported by laboratory staff unaware of the catheter type. Clinical and microbiological data were collected, assessed, and analyzed by research staff without knowledge of the catheter type or any role in catheter insertion or care.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: “Staff inserting and caring for the CVCs could not be blinded due to the different colours of the two catheter types; however, all other aspects of the study were blinded and concealed. Culture results were reported by laboratory staff unaware of the catheter type. Clinical and microbiological data were collected, assessed, and analyzed by research staff without knowledge of the catheter type or any role in catheter insertion or care.” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: “Staff inserting and caring for the CVCs could not be blinded due to the different colours of the two catheter types; however, all other aspects of the study were blinded and concealed. Culture results were reported by laboratory staff unaware of the catheter type. Clinical and microbiological data were collected, assessed, and analyzed by research staff without knowledge of the catheter type or any role in catheter insertion or care.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72/646 catheters (11.1%) could not be cultured and were excluded from analysis. 39 of these were from the MR‐impregnated group and 33 from the SPC‐impregnated group. The reasons for failure to obtain cultures from these catheters included death (total 43: 24 in MR group and 19 in SPC group), dislodgement (total 14: 8 in MR group and 6 in SPC group) and missed culture (total 15: 7 in MR group and 8 in SPC group). The authors included all 646 participants for the outcome of all‐cause mortality The authors stated that there were no significant differences between the baseline characteristics of the initial participants in the 2 groups. The numbers of withdrawals appeared to be balanced between the 2 groups |
| Selective reporting (reporting bias) | Low risk | The major outcomes specified in the Methods, namely catheter colonization and CRBSI, were reported in the Results alongside some other clinically important outcomes like all‐cause mortality and adverse effects |
| Other bias | Low risk | None identified |
George 1997.
| Methods | Single‐centre RCT (UK) | |
| Participants | Quote: "Patients undergoing or having undergone transplant of the heart, heart‐lung, or lung(s) with concomitant immunosuppression, requiring central venous access, were recruited." There were no exclusion criteria provided | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, 'associated infections' which included CRBSI or positive cultures from other sites (like urine or other lines) and adverse effects | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated by computer‐generated random number" |
| Allocation concealment (selection bias) | Unclear risk | The authors did not explain who performed the random sequence generation or provide information to enable an assessment of whether sequence generation was performed independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors stated specifically that blinding was not possible since the catheters in the 2 arms differed in colour |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The authors did not describe whether the assessors of microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors stated specifically that blinding was not possible since the catheters in the 2 arms differed in colour |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/86 catheters (8.1%) were excluded from analysis. The authors did not state the assigned groups of these 7 catheters, but stated that they were excluded because they "were lost owing to deviation from the protocol, contamination of the catheter tips and death.”Due to the lack of information regarding the assigned groups of the excluded catheters, the risk of attrition bias in this study is unclear |
| Selective reporting (reporting bias) | Low risk | The important outcomes specified in the Methods including catheter colonization and CRBSI were reported in the Results |
| Other bias | Low risk | None identified |
Goldschmidt 1995.
| Methods | Single‐centre RCT (phase II study) (Germany) | |
| Participants | Quote: "Adult patients with hematological‐oncological diseases admitted to the hospital who required CVC for treatment purposes were offered the opportunity to participate in the study. Exclusion criteria were: catheterization for less than 48 hours, second central venous access, open infection, catheter‐related infection, pregnancy and age < 18 years." | |
| Interventions | Silver‐coated CVCs versus uncoated CVCs. | |
| Outcomes | Catheter colonization, CRBSI, catheter‐related local infection, number of catheters removed prematurely and number of participants on systemic antibiotics. | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to receive silver‐coated or uncoated control catheters.” No further details were given about how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to receive silver‐coated or uncoated control catheters.” The authors did not provide enough information to enable an assessment of whether sequence generation was performed independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There were no specific statements on blinding, but blinding appeared unlikely as the catheters evaluated differed in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The authors did not describe whether the assessors of microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The authors did not describe whether the assessors of clinical outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "33 inserted catheters (12.4%) were excluded because of catheterization < 48 hrs (5 patients), failure to notify the study team when a catheter was removed (15 patients) and violations of microbiological test requirements (> 24 hrs between removal and microbiological examination, 13 patients). The number of excluded catheters was 20 in the standard and 13 in the silver catheter group." The proportion of excluded catheters as well as the reasons for their exclusion appeared to be acceptable |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the Methods were reported in sufficient detail in the Results, except for the outcome of catheter‐related local infection, for which the results were presented in a graph without a data label, and so these data were not extractable for meta‐analysis |
| Other bias | Low risk | None identified |
Hanna 2004.
| Methods | Single‐centre RCT (USA) | |
| Participants | Adults with cancer. Those who were allergic to rifampin or tetracyclines and pregnant women were excluded | |
| Interventions | MR‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | CRBSI, catheter‐related local adverse reactions (referred to as 'aseptic thrombophlebitis') and premature catheter removal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization numbers were used." |
| Allocation concealment (selection bias) | Low risk | Comprehensive description on allocation, indicating that allocation concealment was achieved in this study. Quote: "Each catheter was sterilized and placed in an assembled sterile catheter tray which in turn was individually wrapped in identical wrappings and shipped to the hospital in large cartons. Each carton contained six catheters, each in its own tray; three were impregnated with M‐R and three were uncoated (control). When an eligible patient was identified, a wrapped catheter tray was removed from the carton in the order in which trays were placed according to the randomization scheme." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, microbiologists, and other research personnel involved in the evaluation of outcome were blinded to the type of catheter used.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "Patients, microbiologists, and other research personnel involved in the evaluation of outcome were blinded to the type of catheter used.” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "Patients, microbiologists, and other research personnel involved in the evaluation of outcome were blinded to the type of catheter used.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15/371 catheters (4%) and 15/370 participants (4.1%) randomized were excluded from analysis. The reason for exclusion was failure to insert the assigned catheters. 10/15 excluded catheters were from the MR‐impregnated group and 5 from the control group. The number of excluded catheters, although not balanced between the groups, was small and the reason for exclusion appeared not to be related to the true outcomes |
| Selective reporting (reporting bias) | Low risk | All the important outcomes specified in the Methods, including CRBSI and septic thrombophlebitis, were reported in the Results |
| Other bias | Low risk | None identified |
Hannan 1999.
| Methods | Single‐centre RCT (UK) | |
| Participants | Quote: "All ICU patients requiring elective central venous access were considered eligible for inclusion in this study." The author did not provide any exclusion criteria | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Clincially diagnosed sepsis (referred to as 'SIRS'), CRBSI, all‐cause mortality, catheter colonization, catheter‐related local infections, premature catheter removal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… the patient was randomly allocated to receive either a standard triple lumen CVC (Arrow International) or a C‐SS bonded triple‐lumen type (Arrowguard TM).” No further details were given about how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | The authors did not provide enough information to enable an assessment of whether the random sequence was generated independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no clear statement about blinding, although it was unlikely as the study catheter (Arrowgard) and control catheter (Arrow International) differed in appearance, and the authors did not report any measures taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the microbiological outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was no clear statement about blinding, although it was unlikely as the study catheter (Arrowgard) and control catheter (Arrow International) differed in appearance, and the authors did not report any measures taken to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number analyzed in each arm was the same as the number of participants initially randomized. It was unclear whether this represented 100% follow‐up or whether the authors had included the drop‐outs. The authors did not provide any explanation of the number of withdrawals |
| Selective reporting (reporting bias) | Unclear risk | All the important outcomes as specified in the Methods, including sepsis, proven CRBSI, catheter colonization and all‐cause mortality were reported in the Results |
| Other bias | Low risk | None identified |
Harter 2002.
| Methods | Single‐centre RCT (Sweden) | |
| Participants | Quote: "Adult patients with hematologic‐oncologic disease admitted to the hospital who required CVCs for treatment purposes were offered the opportunity to participate in the study. Exclusion criteria were catheterization for less than 48 hours, second central venous access, existing severe infection, current catheter‐related infection, pregnancy and age younger than 18 years." | |
| Interventions | Silver‐coated CVCs versus uncoated CVCs | |
| Outcomes | CRBSI, premature catheter removal, adverse effects (specifically catheter‐related thrombosis) | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not provide any information about the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The authors did not provide any information about the method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not specifically mentioned, although it was unlikely as the intervention and control catheters differed in appearance, and there was no mention of any effort to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the outcome assessors in the microbiological laboratory and the sonographer (for catheter‐related thrombosis) were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | Blinding was not specifically mentioned, although it was unlikely as the intervention and control catheters differed in appearance, and there was no mention of any effort to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33/266 participants (12.4%) were excluded from the analysis. 13 excluded participants were from the experimental group (silver‐coated CVCs) and 20 were from the control group (uncoated CVCs). The reasons for exclusion included "catheterization within 48 hours before randomization (five patients), failure to notify the study team when a catheter was removed (15 patients), and violations of microbiologic test requirements (more than 24 hours between removal and microbiologic examination)." It was unclear whether the 2 groups remained prognostically balanced after the exclusion of the 33 participants. In view of the relatively low event rates for all the major outcomes examined, the number of excluded participants could have altered the results substantially, had they be included, assuming the worst‐case scenario for each outcome |
| Selective reporting (reporting bias) | Unclear risk | All the major outcomes specified in the Methods, including CRBSI and catheter‐related thrombosis were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Heard 1998.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: "All patients who were admitted to the surgical intensive care units at the University of Massachusetts Medical Center from March 1994 through June 1995 and who needed a CVC were eligible for the study." The authors did not provide any exclusion criteria | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, use of antibiotics | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study used an alternate form of allocation. Quote: "Randomization was based on the last digit of the medical record number: patients with an odd number received a standard uncoated CVC." |
| Allocation concealment (selection bias) | High risk | The study used an alternate form of allocation. Quote: "Randomization was based on the last digit of the medical record number: patients with an odd number received a standard uncoated CVC." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no clear statement about blinding, although blinding appeared unlikely as the study and control catheters differed in appearance, and the authors did not report any measures taken to blind the personnel involved (for example: a transparent dressing was used to dress the catheters) |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the microbiological outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was no clear statement about blinding, although blinding appeared unlikely as the study and control catheters differed in appearance, and the authors did not report any measures taken to blind the personnel involved (for example: a transparent dressing was used to dress the catheters) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 308/351 catheters (87.7%) were analyzed. However some of the reasons for excluding the participants (detailed below) appeared dubious (for example, exchanged with other catheters and transferred to other unit), as some or all of the outcomes could still have been assessed. Quote: "Fifty seven catheters were removed or exchanged with other catheters and not cultured because the patient was transferred to a rehabilitation facility with the catheter in place or the patient had been transferred to a different area of the hospital (e.g., operating room or ward) and the intensive care unit team was not notified that the catheter was being removed." |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the Methods, including CRBSI, catheter colonization and the use of antibiotics, were reported in the Results |
| Other bias | Low risk | None identified |
Jaeger 2001.
| Methods | Single‐centre RCT (Germany) | |
| Participants | Quote: “Cancer patients requiring CVCs for chemotherapy application were entered into the study …” There were no exclusion criteria stated in the paper | |
| Interventions | Benzalkonium‐chloride‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, premature catheter removal | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “… they were randomly allocated …” ‐ no further statements given about how the random sequence was generated and whether this was independent of allocation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “… they were randomly allocated …” ‐ no further statements given about how the random sequence was generated and whether this was independent of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors clearly stated that the study was not blinded to the participants and personnel, as the physicians were "... aware of the CVC type required ..." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "Microbiological analysis was performed by a physician unaware of the difference between the two CVCs" |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors clearly stated that the study was not blinded to the participants and personnel, as the physicians were "... aware of the CVC type required ..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 50 participants recruited initially (25 each group) were analyzed |
| Selective reporting (reporting bias) | Unclear risk | All major outcomes specified in the Methods, including CRBSI, catheter colonization and premature catheter removal, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Jaeger 2005.
| Methods | Single‐centre RCT (Germany) | |
| Participants | Quote: “... leukaemic patients requiring CVCs for chemotherapy application were entered into the study.” There were no exclusion criteria stated | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVC | |
| Outcomes | Catheter colonization, CRBSI, premature catheter removal | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly allocated, either to a trial group (group I) with insertion of a CH‐SS‐impregnated CVC (ARROWgard Blue, Arrow International, Inc., Reading, Pa., USA) or to a control group (group II) with insertion of a standard triple‐lumen polyurethane CVC (Arrow‐Howes, Arrow International, Inc., Reading, Pa., USA).” No further details were given about how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information was provided to enable an assessment of whether the sequence generation was independent from allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors clearly stated that the study was not blinded to the participants and personnel, as the physicians were "... aware of the CVC type required ...." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "Microbiological analysis was performed by a physician unaware of the difference between the two CVCs" |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors clearly stated that the study was not blinded to the participants and personnel, as the physicians were "... aware of the CVC type required ...." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 106 patients randomized were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All major outcomes specified in the Methods, including CRBSI, catheter colonization and premature catheter removal, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Kahveci 2005.
| Methods | Single‐centre RCT (Turkey) | |
| Participants | Adult participants over 18 years old needing a CVC for TPN. Patients who were estimated to need a CVC for less than 3 days, allergic to silver sulphadiazine, burn patients or those with skin lesions at the catheterization area and pregnant women were excluded | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Article was in Turkish and the information was obtained through a translation. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors stated that the 2 groups were randomly assigned to receive the study and control catheters, but no further details were given about the methods of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | There was no information available that would enable an assessment of whether random sequence was generated independently from allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There were no clear statements about blinding, although blinding appeared unlikely as the 2 types of CVCs differed in appearance and there was no mention of any measures taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the microbiological outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There were no clear statements about blinding, although blinding appeared unlikely as the 2 types of CVCs used differed in appearance and there was no mention of any measures taken to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors stated in the Methods that they would enrol 30 participants in this study. In the Results, a total of 30 participants (13 in the study group and 17 in the control group) were analyzed, suggesting that there was no loss of follow‐up in this study |
| Selective reporting (reporting bias) | Low risk | The 2 major outcomes specified in the Methods, namely, catheter colonization and CRBSI, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Kalfon 2007.
| Methods | Multicentre RCT (France) | |
| Participants | Quote: "Patients were eligible for entry to the study if they were hospitalized in the ICU for either medical or surgical pathology and required a multi‐lumen CVC for three days. We excluded patients under 18 yrs of age, pregnant women, patients with a burn over the insertion site, neutropenic patients (500/mm3), patients for whom the anticipated duration of placement of the catheter was less than 3 days, and patients who had been enrolled in any clinical trial during the previous 3 months." | |
| Interventions | Silver‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Catheter trays were randomly assigned in a blinded fashion according to computer‐generated identification numbers, in blocks of ten for each centre." |
| Allocation concealment (selection bias) | Unclear risk | Quote: “To avoid the potential bias related to the impossible blinding of the investigators, the randomization occurred after the preparation by the nurse of the skin at the insertion site selected by the physician." It was unclear whether the sequence was centrally generated or generated by each participating centre, and if so whether the sequence was generated independently from participant recruitment, allocation and consent Although the quoted statements provided by the authors appeared to be sufficient to protect against bias introduced by differential selection of catheter insertion site as a result of allocation, they did not seem sufficient to convince the readers that allocation concealment was achieved, as there remained a risk of selection bias introduced by differential influences of the investigators on participant consent, and, judging from the study flow chart, consent was refused in 14.1% of the sample (108 catheters) after randomization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors clearly stated that blinding was impossible to those involved in caring and assessing the catheters clinically |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "... the diagnoses of CRBSI and nonbacteraemic catheter‐related infection were established by an independent and blinded clinical evaluation committee composed of three experts on infectious diseases. The clinical evaluation committee used a four‐level scale (with One being a very high probability and Four being a very low probability) blindly to the randomization group for the assessment of catheters according to the above definitions." |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors clearly stated that blinding was impossible to those involved in caring and clinically assessing the catheters clinically |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From those who had consented to participate, 617/656 catheters (94.1%) were analyzed. 39 catheters were not analyzed because they were not cultured (17 from the study group and 22 from the control group). The number of catheters excluded was low compared to the total number of catheters, and the 2 groups appeared well‐balanced in terms of baseline characteristics after the exclusion of these 39 catheters |
| Selective reporting (reporting bias) | Unclear risk | All the major outcomes specified in the Methods, including catheter colonization and CRBSI, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Kamal 1991.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: “Patients who required the insertion of a fresh central venous or arterial catheter were invited to participate in the project. Patients allergic to cephalosporins and patients who were anticipated to need catheters for less than 2 days were excluded." Patients with a catheter in place for longer than 7 days were excluded as well, as stated in the Results, although this criterion was not stated in the Methods | |
| Interventions | CVCs with the antibiotic cefazolin bonded to TDMAC (cationic surfactant trododecylmethylammonium chloride) material compared with uncoated CVCs | |
| Outcomes | Catheter colonization (referred to as 'catheter infection' in this study), CRBSI and attributed mortality, skin or insertion site colonization, catheter‐related local infection or inflammation | |
| Notes | This study included both venous and arterial catheters, although data were available specifically for venous catheters. Only data for venous catheters were included in this systematic review. Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Catheters were randomized by the patient’s hospital number." This suggests an alternate rather than a truly randomized form of allocation |
| Allocation concealment (selection bias) | High risk | Quote: “Catheters were randomized by the patient’s hospital number." This suggests an alternate form of allocation, which carries a high risk of bias due to inadequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors stated that the 2 catheters were identical in appearance, except that the study catheters were coated with extra materials. They stated that all catheters were inspected daily by a member of the study team who was blinded to the participants' catheter group. The statements suggest that the participants and clinical outcome assessors were likely to be blinded |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not mentioned whether the microbiological outcome assessors were blinded to the status of the catheter samples |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | The authors stated that the 2 catheters were identical in appearance except that the study catheters were coated with extra materials. They stated that all catheters were inspected daily by a member of the study team who was blinded to the participants in the catheter group. The statements suggest that the participants and clinical outcome assessors were likely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 147/178 catheters (82.6%) and 124/141 participants (87.9%) in total (venous and arterial combined) were analyzed. However there was no separate mention of drop‐outs for CVCs only. Reasons for exclusion included a catheter indwelling time of over seven days and non‐removal of the catheters when the participants left the ICU |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes defined in the Methods, including catheter colonization, CRBSI and catheter‐related local infection or inflammation were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Leon 2004.
| Methods | Multicentre RCT (Spain) | |
| Participants | Methods, study population: " All consecutive patients aged 18 years or older admitted to the ICUs of seven teaching hospitals in Spain from November, 1999, to April, 2002, who were likely to require a CVC at a new insertion site for 3 days or more were eligible. Only one catheter per patient was studied. Allergy to minocycline or rifampin was an exclusion criterion." | |
| Interventions | MR‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, catheter‐related local infection | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Catheter trays wrapped in identical folders were randomly assigned in blinded fashion according to computer‐generated identification numbers, in blocks of six (three from each group), so that the catheter trays would be removed from the box one at a time in the prescribed, random order from the top to the bottom." |
| Allocation concealment (selection bias) | Low risk | Quote: "Catheter trays wrapped in identical folders were randomly assigned in blinded fashion according to computer‐generated identification numbers, in blocks of six (three from each group), so that the catheter trays would be removed from the box one at a time in the prescribed, random order from the top to the bottom." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The author stated that this was a 'double‐blind' trial, but did not specify how blinding was achieved for the participants and carer, as MR catheters and control catheters differed in appearance: although produced by the same manufacturer, MR catheters and non‐impregnated catheters were likely to have different external labelling |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The author stated that this was a 'double‐blind' trial, but did not specify how blinding was achieved for the participants and carer, as MR catheters and control catheters differed in appearance: although produced by the same manufacturer, MR catheters and non‐impregnated catheters were likely to have different external labelling |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41/228 MR study catheters (18%) and 57/237 control catheters (24%) were excluded from analysis for microbiological outcomes. The reasons for exclusion were as follows: removal without notification (26 in the MR group and 37 in the control group), death (14 in the MR group and 17 in the control group), administrative reasons (1 in the MR group and 3 in the control group). The authors reported that the baseline characteristics of the analyzed participants (187 in the MR group and 180 in the control group) were well‐balanced between the 2 groups, and all the major outcomes that could be assessed, including 'septic episodes' and CRBSI were also measured in the 98 participants excluded from microbiological analysis |
| Selective reporting (reporting bias) | Unclear risk | All the major outcomes specified in the Methods, including clinical septic episodes, CRBSI and catheter colonization were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Logghe 1997.
| Methods | Single‐centre RCT (Belgium) | |
| Participants | Adults with haematological malignancies: leukaemia, lymphoma, myeloma undergoing chemotherapy via a CVC | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Clinically diagnosed sepsis/bacteraemia and CRBSI | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At random, either an non‐impregnated control catheter or the study catheter was inserted." No further descriptions were provided about the methods of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "At random, either an unimpregnated control catheter or the study catheter was inserted." No information was provided that would enable an assessment of whether the random sequence was generated independently from allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no information about whether any blinding was achieved, although it appeared very unlikely that blinding was achieved for those involved in the study who had direct contact with the participants, as the catheters in the 2 arms differed in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no statement about whether the assessors of microbiological outcomes were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was no information about whether any blinding was achieved, although it appeared very unlikely that blinding was achieved for those involved in the study who had direct contact with the participants, as the catheters in the 2 arms differed in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no information in the paper regarding the number of catheters initially randomized, or withdrawals leading to the number that were eventually analyzed |
| Selective reporting (reporting bias) | Low risk | The 2 major clinically relevant outcomes, clinical sepsis/bacteraemia and CRBSI, as defined in the Methods, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Maki 1988.
| Methods | Multicentre RCT (USA) | |
| Participants | Adults over 18 years of age without granulocytopaenia who were scheduled to receive CVCs | |
| Interventions | Silver‐impregnated cuff versus non‐impregnated CVC | |
| Outcomes | Catheter colonization, "septicaemia from contaminated infusate", CRBSI, all‐cause mortality, adverse effects and cost effectiveness | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " ... randomization schedule prepared using a random number table" |
| Allocation concealment (selection bias) | Unclear risk | It was not stated whether random sequence was generated independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not stated whether blinding occurred, but it appeared unlikely that it would have for those involved in the insertion, care and inspection of the catheters, as the catheter appearance and insertion techniques were different for the 2 catheters, and there was no mention of any specific measures that were taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the interpreter of the microbiological results was blinded to the status of the catheter samples |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | It was not stated whether blinding occurred, but it appeared unlikely that it would have for those involved in the insertion, care and inspection of the catheters, as the catheter appearance and insertion techniques were different for the 2 catheters, and there was no mention of any specific measures that were taken to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors stated that > 90% of the patients invited consented to participate, and only presented the number of participants that were analyzed eventually (234 catheters in total, 99 silver cuff and 135 control). However, 55 catheters were not analyzed for local adverse effects (23 in cuff group, 22 in control group), which represented a 23.5% exclusion. The reasons for exclusion were not stated |
| Selective reporting (reporting bias) | Low risk | The major outcomes specified in the Methods, namely, septicaemia, CRBSI, all‐cause mortality and catheter colonization, were all reported in sufficient detail in the Results. Although not specifically stated as the aim of this study, the authors put forward a cost effectiveness evaluation in the results based on various assumptions on the rate of bacteraemia in the control group. The data were unsuitable for meta‐analysis, so we have reported them narratively in our Results |
| Other bias | Low risk | None identified |
Maki 1997.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: "All adult patients who were not known to be allergic to chlorhexidine, silver, or sulphonamides and were scheduled to receive a central venous catheter for short‐term use were eligible to participate. Catheters should be in place for at least 8 hours and culturable." | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Clinical sepsis ("bloodstream infection: catheter and non‐catheter related"), CRBSI, catheter colonization and adverse effects | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each catheter included was assigned via a blinded preset randomization schedule to be a control catheter or an antiseptic catheter." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each catheter included was assigned via a blinded preset randomization schedule to be a control catheter or an antiseptic catheter." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients' physicians and nurses, the principal investigator, and the research microbiologist who processed all cultures were blinded to each study catheter's group." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "The patients' physicians and nurses, the principal investigator, and the research microbiologist who processed all cultures were blinded to each study catheter's group." |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "The patients' physicians and nurses, the principal investigator, and the research microbiologist who processed all cultures were blinded to each study catheter's group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors stated that the criteria for inclusion in analysis were that the catheter should be in place for at least 8 hours and be culturable. It appeared that all catheters that met these criteria were included in the analysis |
| Selective reporting (reporting bias) | Low risk | The major outcomes specified in the Methods, namely, clinical sepsis ("bloodstream infection: catheter and non‐catheter related"), CRBSI, catheter colonization and adverse effects were reported in sufficient detail in the Results. Although not specifically stated as the aim of this study, the authors put forward a cost effectiveness evaluation in the results based on various assumptions about the rate of bacteraemia in the control group. The data were unsuitable for meta‐analysis, so we have reported them narratively in our Results |
| Other bias | Low risk | None identified |
Marik 1999.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: “…. consecutive medical intensive care patients requiring new central venous catheters." No exclusion criteria were stated | |
| Interventions | Three‐arm comparison: C‐SS‐impregnated CVCs versus MR‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI and 'ex‐vivo' antimicrobial effects of the study catheters against 5 tested organisms | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated ”Using random number generator…” |
| Allocation concealment (selection bias) | Unclear risk | There was no information provided that would enable an assessment of whether random sequence generation was performed independently of recruitment and allocation, and what the methods of allocation were, e.g. whether sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors stated that this was a 'non‐blinded' study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the microbiological outcomes assessors were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors stated that this was a 'non‐blinded' study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 participants (5.8%) were excluded from the analysis (3 catheters were removed before 24 hours and 4 were discarded in error). It was unlikely that the reasons stated for exclusion were related to true outcomes. However the groups to which the excluded participants had been assigned were not reported, so it was unclear whether the drop‐outs were balanced across the 3 groups. In view of the relatively low event rates across the 3 groups for the outcomes of CRBSI, we felt that it was best to assign the risk of attrition bias as 'unclear' |
| Selective reporting (reporting bias) | Low risk | All 3 outcomes specified in the Methods were reported in the Results. However, the data on the 'ex‐vivo' antimicrobial effects of the catheters for each organism were not incorporated into our meta‐analysis as they were not part of our prespecified outcomes |
| Other bias | Low risk | None identified |
Mer 2009.
| Methods | Single‐centre RCT (South Africa) | |
| Participants | Quote: "The study included 118 critically ill medical, surgical, trauma, and obstetric/gynaecological patients ...” in the setting of a multidisciplinary ICU | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization protocol involved equal numbers of the 2 types of non distinguishable catheters being mixed in consignments and then selected in a consecutive fashion for placement in study candidates." It was unclear how the catheters were mixed: whether a random sequence was generated and the catheters placed according to the sequence or whether they were hand‐shuffled |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization protocol involved equal numbers of the 2 types of non distinguishable catheters being mixed in consignments and then selected in a consecutive fashion for placement in study candidates." This statement suggests that there was a low risk of any foreknowledge of the investigators who were recruiting concerning the allocation of the potential participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The 2 types of catheters were of identical appearance and were subsequently differentiated by a numerical code, which was broken only on completion of the study." This suggest that all those involved in the study were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "The 2 types of catheters were of identical appearance and were subsequently differentiated by a numerical code, which was broken only on completion of the study." This suggests that all those involved in the study were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "The 2 types of catheters were of identical appearance and were subsequently differentiated by a numerical code, which was broken only on completion of the study." This suggests that all those involved in the study were blinded to the status of the participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors stated that 56 participants received C‐SS‐impregnated catheters and 62 received non‐impregnated catheters, and these were the figures reported in the analysis. It was unclear whether there was any non‐evaluable catheter that was excluded from the final analysis |
| Selective reporting (reporting bias) | Low risk | The outcomes specified in the Methods, including catheter colonization and CRBSI, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Moretti 2005.
| Methods | Multicentre RCT (USA) | |
| Participants | Quote: "Adults expected to require a CVC for more than 60 hours were eligible. There were no eligibility constraints related to diagnoses, hospital unit, or anticipated treatments or procedures. Patients were excluded if: they had a history of allergic reactions to silver, platinum or carbon black; were expected to live for seven days or less; had evidence of a burn or dermatitis at the catheter insertion site; were pregnant or lactating; or had a catheter placed in the same proposed site previously." | |
| Interventions | SPC‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Bacteraemia, CRBSI and catheter colonization | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization schedule was used to avoid potential bias in catheter selection." |
| Allocation concealment (selection bias) | Unclear risk | There was no information about how allocation was implemented to enable an assessment of whether random sequence was generated independently of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "... due to the difference in appearance of the CVCs, blinding could not be achieved.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no specific statement about whether the microbiological outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | Quote: "... due to the difference in appearance of the CVCs, blinding could not be achieved.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 160 catheters (23%) were excluded from the analysis. The reasons for exclusion were inadvertent contamination or disposal of the catheters, or a transit time of more than 24 hours before culture. The authors stated that "There were no significant differences in patient characteristics between the evaluable (266 control, 273 test) and non‐evaluable (83 control, 77 test) groups." However, the high exclusion rate in this study posed a high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes specified in the Methods, including bacteraemia, CRBSI and catheter colonization, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Moss 2000.
| Methods | Single‐centre RCT (UK) | |
| Participants | Participants over 18 years of age who were admitted for routine surgical procedures including coronary bypass grafting who required a CVC, with no known allergy to benzalkonium chloride | |
| Interventions | Benzalkonium‐coated CVCs versus uncoated CVCs | |
| Outcomes | Catheter colonization at subcutaneous and distal catheter segments, clinically diagnosed sepsis, catheter‐related local infection and in‐vitro antibacterial activity | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: participant recruitment: "Following informed consent each patient was randomly assigned to either the benzalkonium chloride catheter group or the control group." The method of random sequence generation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote “The randomization system consisted of sequentially sealed envelopes each of which contained information stating the specified study catheter the patient should receive.” It was not stated whether the envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no clear statement about blinding. The study and control catheters were produced by the same manufacturer, but it was unclear whether the 2 types of catheter were identical in their appearance, and whether any measures were taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no clear statement about blinding. The study and control catheters were produced by the same manufacturer, but it was unclear whether the 2 types of catheter were identical in their appearance, and whether any measures were taken to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 31/235 catheters (13.2%) were excluded from the analysis (11 from the study group and 20 from the control group). The authors stated that the catheters were "not available for microbiological analysis", and gave no further reasons. The authors stated that the characteristics of the 2 study groups (after postrandomization exclusions) were similar. However, in view of the low event rates for the outcomes of clinically diagnosed sepsis and catheter‐related local infection, it was unclear whether data from the excluded catheters, had they be available, would have changed the results substantially |
| Selective reporting (reporting bias) | Unclear risk | All the clinically relevant outcomes specified in the Methods, including clinically diagnosed sepsis, catheter‐related local infection, catheter colonization and in‐vitro antimicrobial activity were reported in sufficient detail in the Results. However, the latter outcome was not included in our meta‐analysis as it was not a prespecified outcome for our review |
| Other bias | Low risk | None identified |
Osma 2006.
| Methods | Single‐centre RCT (Turkey) | |
| Participants | Quote: "All patients were more than 18 years of age and all were mechanically ventilated. Exclusion criteria were: anticipated duration of catheterization of less than three days; allergy to chlorhexidine, silver or sulphonamides; pregnancy; dermatitis or a burn over the insertion site; and signs of systemic inflammatory response syndrome and sepsis.” | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed using a computer‐generated randomization list.” |
| Allocation concealment (selection bias) | Unclear risk | It was not stated how the allocation was performed, and so it was not possible to assess whether random sequence was generated independently of recruitment and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no specific statement about blinding. However blinding appeared unlikely for the participants and the personnel as the 2 types of catheters differed in appearance, and there was no mention of any measures taken to blind those involved in the study |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of the microbiological outcomes were blinded. |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was no specific statement about blinding. However blinding appeared unlikely for the participants and the personnel as the 2 types of catheters differed in appearance, and there was no mention of any measures taken to blind those involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors stated in the Results that no participants were excluded from the study |
| Selective reporting (reporting bias) | Low risk | The outcomes specified in the Methods, including catheter colonization and CRBSI, were reported in sufficient detail in the Results. Additionally, the outcome of all‐cause mortality could be extracted from the study, as it was reported as a part of the participant characteristics |
| Other bias | Low risk | None identified |
Ostendorf 2005.
| Methods | Single‐centre RCT (Germany) | |
| Participants | Participants with haematological malignancy and needing a CVC for at least 7 days. No exclusion criteria were mentioned | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI (referred to as 'catheter related bacteraemia') and attributed mortality and catheter‐related local infection | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were allocated "through randomization ...." No further statements were made about how random sequence was generated and how allocation was implemented |
| Allocation concealment (selection bias) | Unclear risk | Participants were allocated "through randomization ...." No further statements were made about how random sequence was generated and how allocation was implemented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The two catheter types were indistinguishable to users and patients (double‐blinded study design)." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the assessors of the microbiological outcomes were blinded to the status of the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "The two catheter types were indistinguishable to users and patients (double‐blinded study design)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Sixty‐one catheters (24.9%) were excluded because of patient’s failure to notify the study team when the catheter was removed, or catheterization <24 h.” The authors did not state the number of catheters excluded from each group. They stated that the baseline characteristics of the 2 groups after excluding the 61 catheters were similar In view of the high exclusion rate and the relatively low event rates, particularly for CRBSI and attributed mortality, we judged the risk of attrition bias in this study as high |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes specified in the Methods, including catheter colonization, CRBSI and attributed mortality and catheter‐related local infection, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Pemberton 1996.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: "The study group was all inpatients, both men and women, who received a CVC for the infusion of TPN. The exclusion criteria were any patient with a high risk for contamination during insertion, such as a catheter placed through an introducer, changed over a guidewire into the same infected site or inserted in the emergency department" | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | CRBSI and catheter‐related local infection | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random number table was used prospectively to divide patients into two groups" |
| Allocation concealment (selection bias) | Unclear risk | There was no information provided about how allocation was performed that might enable an assessment of whether the random sequence was generated independently of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The Arrow Company (Reading, Pa) made both catheters, which were yellow and identical except for the manufacturer's numbers on the hub of the antiseptic catheter and the antiseptic catheter label, which was on top when the catheter kit was opened." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of the microbiological outcomes were blinded to the status of the catheter sample |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | Quote: “The Arrow Company (Reading, Pa) made both catheters, which were yellow and identical except for the manufacturer's numbers on the hub of the antiseptic catheter and the antiseptic catheter label, which was on top when the catheter kit was opened." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/88 catheters (18%) were excluded. The reasons for exclusion included death (4 participants), misplaced catheters (4 catheters), urgent catheter change (3 catheters), hospital transfer with catheter in place (1 catheter) and no reason noted (3 catheters). The authors did not provide the number excluded from each group, but they noted that "no significant difference between groups owing to these reasons for exclusion was found." We feel that some of the reasons for exclusion, including death and urgent catheter change, were not further clarified and they could be related to the true outcome of CRBSI or catheter‐related local infection. Also due to the low event rates for CRBSI and catheter‐related local infection, we feel that this study was at high risk for attrition bias |
| Selective reporting (reporting bias) | Low risk | The outcomes of CRBSI and catheter‐related local infection were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Raad 1997.
| Methods | Multicentre RCT (USA) | |
| Participants | Quote: "Hospitalized patients 18 years of age or older who required a triple‐lumen polyurethane central venous catheter at a new insertion site were asked to participate. We excluded pregnant women, patients who were allergic to rifampin or tetracycline, patients with dermatitis or a burn over the insertion site, and patients for whom the anticipated duration of catheterization was less than 3 days.” | |
| Interventions | MR‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: partly from the industry (catheter manufacturer or distributor) and partly from institutional research fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation and allocation were described in detail, as follows: Quote: ”All catheters were gas sterilized and placed in identical trays, and each tray was assigned an identification number. The trays were then randomly assigned into blocks of six: three with coated catheters and three with control catheters. Each block of trays was placed in boxes by Cook Critical Care, and the boxes were shipped to the five hospitals. When a patient was determined to be eligible, a tray was removed from the box (trays were removed one at a time, in sequential order from top to bottom), and that catheter was used for the patient. The catheter identification number was recorded on a data entry form and on the patient's medical chart.” |
| Allocation concealment (selection bias) | Low risk | Random sequence generation and allocation were described in detail, as follows: Quote: ”All catheters were gas sterilized and placed in identical trays, and each tray was assigned an identification number. The trays were then randomly assigned into blocks of six: three with coated catheters and three with control catheters. Each block of trays was placed in boxes by Cook Critical Care, and the boxes were shipped to the five hospitals. When a patient was determined to be eligible, a tray was removed from the box (trays were removed one at a time, in sequential order from top to bottom), and that catheter was used for the patient. The catheter identification number was recorded on a data entry form and on the patient's medical chart.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the patient nor the clinician who inserted the device knew which catheter (coated or uncoated) had been used." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no specific statement on whether the assessors of the microbiological outcomes were blinded to the status of the catheter sample |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "Neither the patient nor the clinician who inserted the device knew which catheter (coated or uncoated) had been used." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32/298 catheters (10.4%) were excluded from the analysis due to a failure to obtain cultures (17 from the study group and 15 from the control group). Although the number excluded appeared balanced between the 2 groups, in view of the relatively low event rates for CRBSI and catheter colonization, it was unclear whether the results would be altered substantially should data from the excluded samples be available |
| Selective reporting (reporting bias) | Unclear risk | The major clinical outcomes of CRBSI and catheter colonization were reported in sufficient detail in the Results. The authors provided a cost estimate of the 2 types of catheters, but the figures were presented narratively and were not suitable to be included in our meta‐analysis |
| Other bias | Low risk | None identified |
Raad 1998.
| Methods | Multicentre RCT (USA) | |
| Participants | The only information provided by the authors of this paper on the participants was that they were ".... critically ill patients ..." | |
| Interventions | MR‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, premature catheter removal, duration of systemic antibiotics use and durability of the antimicrobial activity | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation and allocation were described in detail, as follows: Quote: "Coated and uncoated catheters were packaged in identical trays, assigned a random number, and shipped to our study sites ... The randomization scheme consisted of blocks of six identical catheter trays: three for coated central venous catheters and three for control central venous catheters. Every block of six catheter trays was packed in a box. Central venous catheters were allotted to each eligible patient sequentially from the catheter trays which were removed from the box, one at a time, from top to bottom." |
| Allocation concealment (selection bias) | Low risk | Random sequence generation and allocation were described in detail, as follows: Quote: "Coated and uncoated catheters were packaged in identical trays, assigned a random number, and shipped to our study sites … The randomization scheme consisted of blocks of six identical catheter trays: three for coated central venous catheters and three for control central venous catheters. Every block of six catheter trays was packed in a box. Central venous catheters were allotted to each eligible patient sequentially from the catheter trays which were removed from the box, one at a time, from top to bottom." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The catheters were prepared in‐house from identical materials but the authors did not mention whether the final appearance of the prepared and unprepared catheters remained identical in appearance, and whether those involved in the study knew the difference |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | The authors did not mention whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The catheters were prepared in‐house from identical materials but the authors did not mention whether the final appearance of the prepared and unprepared catheters remained identical in appearance, and whether those involved in the study knew the difference |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appeared that all 40 participants initially randomized were analyzed |
| Selective reporting (reporting bias) | High risk | The outcomes of catheter colonization and premature catheter removal were reported in sufficient detail in the Results. However the usage of systemic antibiotics was reported in the form of median and range and not mean and standard deviation, and so the data were unsuitable to be included in our meta‐analysis |
| Other bias | Low risk | None identified |
Ranucci 2003.
| Methods | Multicentre RCT (Italy) | |
| Participants | Quote: “All the patients enrolled were undergoing a CVC insertion likely to require an in‐dwelling period of more than three days, for either medical or surgical pathologies. We excluded anyone less than 18 yrs old, pregnant women, patients with a diagnosis of systemic infection at the moment of insertion, and patients with a history of allergy to any of the components of the study or control catheters.” | |
| Interventions | SPC (Oligon)‐impregnated CVCs versus benzalkonium‐impregnated CVCs | |
| Outcomes | Catheter colonization and CRBSI | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomly allocated to the oligon or the control group, according to a computer‐generated randomization code. Randomization was in blocks of ten; each participating centre was required to enrol at least ten and no more than 100 patients.” |
| Allocation concealment (selection bias) | Unclear risk | Although the methods of random sequence generation were adequately described as detailed above, there was no information about how the allocation was carried out in each centre to enable an assessment of whether sequence generation was independent of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding of the participants and personnel appeared unlikely as catheters from different manufacturers were used and they differed in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the microbiological outcome assessors were blinded to the status of the catheter samples |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | Although not clearly stated, blinding of the participants and personnel appeared unlikely as catheters from different manufacturers were used and they differed in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 62/607 catheters (10.2%) were excluded from the analysis (33 from the SPC group and 29 from the benzalkonium group). The reasons for exclusion were stated as follows: Quote: "Twenty‐nine cases were missed in the control group (ten due to catheter contamination during the removal, 19 due to noncultured catheters) and 33 in the oligon group (12 due to catheter contamination during the removal, 20 due to noncultured catheters, one due to intraoperative death). The noncultured catheters were wrongly removed without notification of study co‐ordinators." The authors reported that the characteristics of the 2 groups of participants after postrandomization exclusion were similar. However, in view of the low event rates in CRBSI and the event rates and number of excluded sample in the outcome of catheter colonization, it was unclear whether the results would be substantially different if data from the excluded sample were available |
| Selective reporting (reporting bias) | Unclear risk | The 2 major outcomes specified in the Methods, namely catheter colonization and CRBSI, were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Rupp 2005.
| Methods | Multicentre RCT (USA) | |
| Participants | Quote: “Adult patients who were cared for in critical care units and who required a triple lumen central venous catheter were eligible for participation. Patients who were pregnant, were allergic to chlorhexidine or sulfa drugs, were hospitalized for burn injuries, had a chronic inflammatory skin disorder at the catheter insertion site, were suspected of having a catheter‐associated infection, or were enrolled in another investigational trial were not eligible for participation.” | |
| Interventions | Second‐generation C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, all‐cause mortality, catheter‐related local infection, adverse effects and premature catheter removal | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization code was developed by using a computerized random‐number generator to select permuted blocks. The block length was 4.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Catheter allocation was concealed, and patients, study personnel, and all health care workers were unaware of whether the catheters were coated or uncoated.” There was however no statement about how allocation was carried out and whether the randomization code was generated independently of allocation. In this review, we assigned the risk as low for allocation concealment, based on the authors’ statement that “Catheter allocation was concealed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors stated that all catheters were indistinguishable in appearance and packaging, and all participants, personnel and adjudicators involved in the study were blinded to the status of the participants and the catheter samples |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | The authors stated that all catheters were indistinguishable in appearance and packaging, and all participants, personnel and adjudicators involved in the study were blinded to the status of the participants and the catheter samples |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | The authors stated that all catheters were indistinguishable in appearance and packaging, and all participants, personnel and adjudicators involved in the study were blinded to the status of the participants and the catheter samples |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/780 participants (0.3%) were excluded after randomization because they did not received a catheter. 70 additional patients (9%) were excluded because cultures were not taken at the time of catheter removal and study end points could not be assessed. The authors stated that the characteristics of the 2 groups were similar without the excluded samples, and "all catheters were included in the safety analysis." The authors also stated that "A modified intention‐to‐treat analysis was conducted on all patients who received a study catheter and had a catheter culture." On the basis of the above statements we felt that overall the risk of attrition bias in this study was low |
| Selective reporting (reporting bias) | Low risk | All the outcomes defined in the Methods were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Sheng 2000.
| Methods | Single‐centre RCT (Taiwan) | |
| Participants | Quote: “All adult patients who were not allergic to chlorhexidine, silver, or sulphonamides and were scheduled to receive a central venous catheter were eligible to participate. Those who had known bacteraemia or fungaemia episodes within two weeks before the central venous catheter insertion were excluded. Febrile patients (oral temperature more than 38°C) and patients with sepsis syndrome within one week or at the time of catheter insertion were also excluded.” | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI and attributed mortality, catheter‐related local infection | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each time a catheter was scheduled to be inserted in a study patient, it was randomly assigned to be a control or an antiseptic catheter.” There was no further statement about how sequence generation was performed and how allocation was implemented |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Each time a catheter was scheduled to be inserted in a study patient, it was randomly assigned to be a control or an antiseptic catheter.” There was no further statement about how sequence generation was performed and how allocation was implemented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The antiseptic catheter and the standard catheter were totally identical in the outlook and during the study, the users did not know which type of catheter was used" and “The patients’ physicians and nurses, the investigators, and the laboratory technologists who processed the cultures were blinded to know which kind of catheter was used” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "The antiseptic catheter and the standard catheter were totally identical in the outlook and during the study, the users did not know which type of catheter was used" and “The patients’ physicians and nurses, the investigators, and the laboratory technologists who processed the cultures were blinded to know which kind of catheter was used” |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | Quote: "The antiseptic catheter and the standard catheter were totally identical in the outlook and during the study, the users did not know which type of catheter was used" and “The patients’ physicians and nurses, the investigators, and the laboratory technologists who processed the cultures were blinded to know which kind of catheter was used” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The only statement about the number of participants said: "A total of 235 cases (122 cases of control group and 113 cases of antiseptic group) were included in this study". It was unclear how many participants were recruited, how many received intervention and how many were analyzed |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes specified in the Methods were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Sherertz 1996.
| Methods | Multicentre RCT (USA) | |
| Participants | Quote: "Patients in intensive care unit". Exclusion criteria: patients < 18 years of age, with dermatitis over the proposed insertion site, neutropenia, pregnant, or allergic to topical disinfectants including chlorhexidine. No patient was enrolled in the study twice | |
| Interventions | Chlorhexidine‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, catheter‐related local infection, premature catheter removal | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This was a two‐armed, randomized, double‐blind, multicenter trial. It was carried out in the intensive care units of four tertiary‐care university hospitals.” There was no statement on how random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | There was no information about how allocation was performed that would enable an assessment of whether the random sequence was generated independently of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors described this as a 'double‐blind' study and stated that "The catheters coated with chlorhexidine were identical in appearance to uncoated catheters". It was likely that the participants and personnel were blinded to the catheter type |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There were no specific statements on whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Low risk | The authors described this as a 'double‐blind' study and stated that "The catheters coated with chlorhexidine were identical in appearance to uncoated catheters". It was likely that the participants and personnel were blinded to the catheter type |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/263 catheters (3.4%) were excluded from the analysis because "the catheters were removed from eight patients without culturing and incomplete data entry occurred for one patient." It was unclear how many of these excluded catheters were assigned to the study group and how many were assigned to the control group. Furthermore, as the event rates were low for the outcomes of CRBSI and catheter‐related local site infection, it was unclear whether the inclusion of these 9 catheters, had their outcome data been available, would have altered the results |
| Selective reporting (reporting bias) | High risk | All the outcomes specified in the Methods were reported in sufficient detail in the Results. However, all the outcomes were presented as percentages, and we could not translate most of these into round figures for meta‐analysis, taking the possible total sample as denominators |
| Other bias | Low risk | None identified |
Smith 1995.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: "in the absence of fever or suspected infection, and after recovery of all hematologic side effects of chemotherapy, all patients admitted to the gynaecologic oncology service requiring central venous access for medical or surgical conditions were eligible.” | |
| Interventions | CVCs with silver‐impregnated cuff versus standard uncoated CVCs | |
| Outcomes | Clinical sepsis, catheter colonization and adverse effects | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed “based on a table of random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote: “… randomization did not occur until after informed consent was obtained." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not stated whether the catheters were identical in appearance or any blinding occurred, although blinding appeared unlikely as the study catheter had a different cuff compared to the control catheter, and an additional incision had to be made when inserting the study catheter |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the assessors of the microbiological outcomes were blinded to the status of the catheter samples |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | It was not stated whether the catheters were identical in appearance or any blinding occurred, although blinding appeared unlikely as the study catheter had a different cuff compared to the control catheter, and an additional incision had to be made when inserting the study catheter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/142 (6.3%) catheters were excluded from the analysis. The reasons for exclusion were inability to access the subclavian vein (5 participants), operative findings of peritonitis or intra‐abdominal abscess (3 participants) and inadvertent catheter removal during transport (1 participant). The authors did not provide the number excluded from each groups. In view of the relatively high event rates for the outcomes in relation to the number excluded, we felt that the study had a low risk of attrition bias |
| Selective reporting (reporting bias) | High risk | All the outcomes specified in the Methods were reported in the Results. However, the adverse effects ‐ including pneumothorax and thrombosis ‐ were only reported as the overall rate and not the numbers in each group, and therefore the data could not be included in the meta‐analysis |
| Other bias | Unclear risk | It was unclear whether randomization was effective in this study, as the assigned groups did not appear to be prognostically comparable: the Vitacuff group had more ICU cases, more cases needing assisted ventilation, venous blood sampling, hyperalimentation and broad spectrum antibiotics use |
Stoiser 2002.
| Methods | Multicentre RCT (Austria) | |
| Participants | Patients who were immunocompromised from various causes including cancer, haematological disorders, bone marrow and other organ transplant requiring implantation of a CVC were included in this study. No exclusion criteria were stated | |
| Interventions | Silver‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization (referred to as 'catheter contamination'), clinically diagnosed sepsis (referred to as 'clinical infection') and the use of antibiotics (referred to as the 'antibiotic index') | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After giving informed consent, the patients were randomized to receive either a conventional polyurethane catheter (Arrow1, Reading, PA, USA), or a silver‐impregnated catheter (ArgenTec1, Fa, City, Country).” It was not stated how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | There was no information about the method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There were no statements about blinding in the paper. Blinding appeared unlikely as the 2 types of catheters were from 2 different manufacturers (Arrow and Argen Tec) and they differed in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There were no statements about blinding in the paper. Blinding appeared unlikely as the 2 types of catheters were from 2 different manufacturers (Arrow and Argen Tec) and they differed in appearance |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 97/154 (63%) of the catheters were analyzed. The reasons for excluding 54 participants were given as follows: "Fifty‐seven patients had to be excluded from evaluation due to removal of the catheter prior to day 3, accidental removal of the catheter, transfer of the patient to another hospital, or death prior to the end of the study." We felt that the rate of exclusion was high, and the reasons given for exclusion could be related to the true outcomes, for example, catheter removal prior to day 3 or death might be related to infection; accidental removal of the catheter would not preclude a catheter culture unless it occurred without knowledge of the personnel involved in the study |
| Selective reporting (reporting bias) | Low risk | The outcomes specified in the Methods including clinically diagnosed sepsis, catheter colonization and the use of antibiotics were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Tennenberg 1997.
| Methods | Single‐centre RCT (USA) | |
| Participants | Quote: "Patients eligible for the study were inpatients on the surgical, medical, and intensive care unit wards who were deemed by their physicians to require CVC placement for their care. To eliminate the potential contamination associated with CVCs placed over guidewires, only patients who required fresh‐stick CVC insertion in the subclavian, jugular, or femoral sites were eligible for study." | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI and catheter‐related local infection | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “….disparate allocations from a large master computer‐generated randomization list in blocks of 8 were used for these different patient locations and for the number of CVC lumens.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was carried out using sealed envelope during randomization which occurred after informed consent." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The study was not blinded because the physician became aware (by opening a sealed envelope) of the CVC type required after randomization.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | There was no mention of whether the assessors of the microbiological outcomes were blinded to the status of the sample |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | Quote:“The study was not blinded because the physician became aware (by opening a sealed envelope) of the CVC type required after randomization.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 70/350 catheters (19.9%) were excluded from the analysis. It was unclear how many of the excluded catheters belonged to each group. The reasons for exclusion were as follows: "less than 48 hours of catheterization (34 patients), incomplete cultures (26 patients), ongoing sepsis from another source (3 patients), and other reasons (CVC not inserted, wrong CVC inserted, CVC misplaced, and CVC accidentally removed) (7 patients)." In view of the high rate of exclusion and relatively low event rates for the outcomes of CRBSI and catheter colonization, we felt that there was a high risk of attrition bias in this study |
| Selective reporting (reporting bias) | Low risk | All the outcomes specified in the Methods, including catheter colonization, CRBSI and catheter‐related local infections were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Theaker 2002.
| Methods | Single‐centre RCT (UK) | |
| Participants | Quote: "Longer‐stay critically ill patients electively having four lumen catheters placed to facilitate management were included in this study." There were no exclusion criteria stated | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Clinical sepsis (referred to as 'SIRS/sepsis'), CRBSI, all‐cause mortality, catheter colonization, catheter‐related local infection (referred to as 'local sepsis'), premature catheter removal | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to either a pure polymer (Tecoflex) catheter (InfectguardTM MedexMedical Ltd) or an antiseptic bonded (C‐SS) catheter (Arrowguard TM Arrow International).” No further details were given about how randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided about how allocation was performed and whether any measure was in place to prevent foreknowledge of group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no mention of blinding, although blinding to the participants and personnel was unlikely as the 2 types of catheters differed in appearance |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was no mention of blinding, although blinding to the participants and personnel was unlikely as the 2 types of catheters differed in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors stated that "232 catheters were inserted into 181 participants" and "analysis was conducted on 131 pure‐polymer catheters and 101 antiseptic‐impregnated catheters," suggesting that there were no postrandomization exclusions |
| Selective reporting (reporting bias) | Low risk | The major outcomes defined in the Methods, namely catheter colonization and CRBSI were reported in sufficient detail in the Results, alongside other clinically important outcomes such as clinical sepsis, death, catheter‐related local infection and premature catheter removal |
| Other bias | Low risk | None identified |
Thornton 1996.
| Methods | Single‐centre RCT (UK) | |
| Participants | Quote: “… all patients expected to require intensive care unit (ICU) treatment for 48 h or more." No exclusion criteria were given | |
| Interventions | Vancomycin‐bonded CVCs versus unbonded CVCs | |
| Outcomes | Catheter colonization and adverse effects | |
| Notes | Despite the suggestion to the contrary in the title of the trial report, the study assessed only microbiological outcomes in terms of catheter colonization, and not central line sepsis. Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no statement about the methods of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | There was no information about the methods of allocation and whether sequence generation and allocation were independent |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors stated that “all catheters were the same colour” but it was not clear whether they appeared identical. There were no specific statements about blinding |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | The authors stated that “all catheters were the same colour” but it was not clear whether they appeared identical. There were no specific statements about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of postrandomization exclusions ‐ the data were presented as if all participants recruited completed the study and were analyzed |
| Selective reporting (reporting bias) | High risk | Although the major outcome specified in the Methods, catheter colonization, was reported in the Results, clinically important outcomes such as CRBSI and death were not reported |
| Other bias | Low risk | None identified |
Van Heerden 1996.
| Methods | Single‐centre RCT (Australia) | |
| Participants | Quote: "ICU patients who required a CVC for drug administration, parenteral nutrition or monitoring purposes or who were expected to have the device in‐situ for at least five days were eligible for the study." | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization | |
| Notes | The study consisted of 2 parts: first was a comparison between C‐SS‐impregnated CVCs and non‐impregnated CVCs for reducing catheter colonization and second was an evaluation of the Fibrin Analysing System (FAS) brush in detecting early catheter colonization. The first part of the study was included in this review. Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The word 'randomized' was used in the abstract, but there was no mention of randomization in the full text |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of randomization or the methods of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no mention of blinding in the paper, but blinding appeared unlikely as the 2 types of catheter used differed in appearance, and it was mentioned in the Methods that “Once inserted, the CVC insertion site was covered with a transparent dressing." |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | There was no mention of blinding in the paper, but blinding appeared unlikely as the 2 types of catheter used differed in appearance, and it was mentioned in the Methods that “Once inserted, the CVC insertion site was covered with a transparent dressing." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/61 catheters were excluded from the analysis because the participants "did not complete the five‐day study period." It was unclear how many of the 7 excluded catheters were in each assigned group, and the authors did not provide a description of the participants characteristics of the 2 groups without the 7 excluded participants. The event rates of the outcomes of catheter colonization were comparable to the number of catheters excluded (4 in the study group and 10 in the control group), it was unclear whether the results would have been altered substantially if the data of these excluded catheters had been available |
| Selective reporting (reporting bias) | High risk | Catheter colonization was the only outcome reported in this part of the study. Other clinically relevant outcomes like CRBSI and death were not reported |
| Other bias | Low risk | None identified |
Van Vliet 2001.
| Methods | Single‐centre RCT (Netherlands) | |
| Participants | All adult patients who received a double‐lumen CVC in the study period. Participants who were allergic to chlorhexidine and sulphur were excluded | |
| Interventions | C‐SS‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Catheter colonization, CRBSI, all‐cause mortality, catheter‐related local infection | |
| Notes | Sources of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A standard catheter was placed on even days, while an antiseptic‐bonded catheter was placed on odd days." This suggested an alternate form of allocation |
| Allocation concealment (selection bias) | High risk | Quote: "A standard catheter was placed on even days, while an antiseptic‐bonded catheter was placed on odd days." This suggested an alternate form of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no statement about blinding, and it was unclear whether the catheters used were identical in appearance. The authors stated that the standard catheters were made of the same materials as the study catheters, although it was not stated whether the final appearance was identical between the types of catheters |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was not stated whether the assessors of the microbiological outcomes were blinded |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | Unclear risk | There was no statement about blinding, and it was unclear whether the catheters used were identical in appearance. The authors stated that the standard catheters were made of the same materials as the study catheters, although it was not stated whether the final appearance was identical between the types of catheters |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of postrandomization withdrawals |
| Selective reporting (reporting bias) | Low risk | Major outcomes such as catheter colonization and all‐cause mortality were listed in the Methods and reported in the Results. However, catheter‐related local infection, which was listed as an outcome in the Methods, was not reported in the Results. For another major outcome of CRBSI, the authors stated that there were cases where CRBSI was suspected, but in all cases, they were unable to prove that the infections were catheter‐related, implying that there was no case of CRBSI that was established with confidence in either group. Overall, due to the acceptable reporting of the major outcomes, as detailed above, we judged the study to have a low risk for selective reporting, despite the non‐reporting of a relatively minor outcome of catheter‐related local infection |
| Other bias | Low risk | None identified |
Walz 2010.
| Methods | Multicentre non‐inferiority RCT (USA) | |
| Participants | Quote: "Adult patients were enrolled who were initially hospitalized in an intensive care setting and required insertion of a triple‐lumen CVC for an anticipated period of up to 28 days. Subjects were excluded if they were pregnant, had participated in another research study within the last 30 days, had a life expectancy of less than one month, or had an allergy to 5‐FU chlorhexidine or sulfa." | |
| Interventions | 5‐fluorouracil‐coated (5‐FU) CVCs versus C‐SS coated CVCs | |
| Outcomes | Clinically‐diagnosed sepsis, CRBSI, all‐cause mortality, catheter‐related local infection, catheter colonization, adverse effects and the use of systematic antibiotics | |
| Notes | In this study, all CVCs used were externally coated. Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects meeting the inclusion criteria were randomized in a 1:1 ratio to receive …” No further details were given about how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No details were given about the methods of allocation that would enable an assessment of whether this was done independently of sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors stated that the trial was a 'single‐blind' trial. Quote: “Subjects and individuals involved with data analysis and management were blinded to treatment, but not the hospital staff as a result of visible differences in CVC colouring.” |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Low risk | Quote: "Subjects and individuals involved with data analysis and management were blinded to treatment ..." |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors stated that the trial was a 'single‐blind' trial. Quote: “Subjects and individuals involved with data analysis and management were blinded to treatment, but not the hospital staff as a result of visible differences in CVC colouring.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 817/960 catheters (85.1%) completed the study (62 from the 5‐FU group and 81 from the C‐SS group). The authors clearly described the postrandomization drop‐outs at various stages of the study in the form of a flow diagram. The reasons for exclusion included failure of catheter insertion, death with catheters in place, site errors and contamination of tips during removal. The number excluded appeared sufficiently balanced between the 2 groups and the reasons for exclusion seemed unrelated to the true outcomes. The authors also performed an intention‐to‐treat analysis by including all participants in all evaluable outcomes, such as death. Overall, we feel that the risk of attrition bias was low in this study |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the Methods were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Yucel 2004.
| Methods | Multicentre RCT (Germany) | |
| Participants | Hospitalized adult (18‐80 years) surgical patients requiring CVC for at least 3 days and undergoing their first central venous catheterization. Exclusion criteria were pregnancy, known allergy to miconazole and/or rifampicin, anatomic defect or skin lesion at the potential site of insertion, and previous inclusion in the trial | |
| Interventions | MR‐impregnated CVCs versus non‐impregnated CVCs | |
| Outcomes | Clinically diagnosed sepsis, CRBSI, catheter colonization, catheter‐related local infection and premature catheter removal | |
| Notes | Sources of funding: industry (catheter manufacturer or distributor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated random list with varying block size was generated separately for each of the two participating study centres." |
| Allocation concealment (selection bias) | Low risk | Quote: "The information whether to use a modified or non‐modified catheter was put in opaque envelopes with a serial number. After insertion of the catheter the individual identification number was recorded in the patient’s medical chart and on a separate documentation sheet (case report form)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors stated that the trial was 'non‐blinded' |
| Blinding of outcome assessment (detection bias) Microbiological outcomes like catheter colonization | Unclear risk | It was unclear whether the assessors of the microbiological outcomes were blinded to the status of the samples |
| Blinding of outcome assessment (detection bias) Clinical outcomes like CRBSI | High risk | The authors stated that the trial was 'non‐blinded' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluding primary drop‐outs (56 that did not receive CVCs post‐randomization), 37/260 of the participants who received CVCs were excluded (14.2%: 29 in the control group and 8 in the study group). The reasons for exclusion included accidental removal of the catheter by the medical staff (22 in the control group and 1 in the study group) or by the participants (4 in the control group and 1 in the study group), death (2 in the control group and 1 in the study group), or missing microbiological analysis (5 in the control group and 1 in the study group). There were many more drop‐outs from the control group than the study group. The event rates were low, especially for the outcomes of clinically diagnosed sepsis and CRBSI. Overall we felt that this study had a high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes specified in the Methods were reported in sufficient detail in the Results |
| Other bias | Low risk | None identified |
Abbreviations
CFU = colony‐forming unit
CRBSI = catheter‐related bloodstream infection
C‐SS = chlorhexidine‐silver sulphadiazine
CVC= central venous catheter
ICU = intensive care unit
MR = minocycline‐rifampicin
RCT = randomized controlled trial
SIRS = Systemic Inflammatory Response Syndrome
SPC = silver‐platinum‐carbon
TPN = total parenteral nutrition
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Hwiesh 2007 | RCT assessing the tunnelled cuff catheter with antibiotic lock compared with a standard untreated tunnelled cuff catheter for haemodialysis; outcomes included CRBSI and access site infections. Excluded on the basis of the population group and study design |
| Alderman 2005 | A non‐randomized study comparing a central venous catheter with a silver‐impregnated cuff (Hohn catheter) with a polypropylene catheter in reducing central line infections and pulmonary embolism. Excluded on the basis of study design |
| Alonso‐Echanove 2003 | A prospective observational study assessing risk and protective factors for CRBSI in 8 adult ICUs, including the type of CVC used, the main purpose of CVC use, nurse staffing and participant‐related variables. Excluded on the basis of study design |
| Anton 2009 | A blinded single‐centre RCT assessing heparin‐bonded CVCs for infants with congenital heart disease, with risk of thrombosis as the main outcome. Excluded on the basis of the population group |
| Bambauer 1998 | RCT assessing silver‐coated large‐bore catheters against untreated catheters for patients undergoing extracorporeal detoxification (haemodialysis), with major outcomes being bacterial catheter colonization and thrombogenicity. Excluded on the basis of the population group |
| Bambauer 2000 | A similar study to Bambauer 1998, assessing silver‐coated large‐bore catheters against untreated catheters for patients undergoing extracorporeal detoxification (haemodialysis), with major outcomes of bacterial catheter colonization and thombogenicity. This differed from Bambauer 1998 only in the total number of participants. Excluded on the basis of the population group |
| Betjes 2004 | RCT assessing catheters locked with either heparin or a citrate‐containing solution in reducing catheter‐related infections for patients undergoing haemodialysis. Excluded on the basis of the population group. |
| Borschel 2006 | A pre‐test post‐test cohort study comparing CRBSI rates 2 years after the universal introduction of C‐SS‐impregnated CVCs in an adult ICU 2 years before the introduction of the impregnated catheters. Costs were also included as an outcome. Excluded on the basis of study design |
| Carbon 1999 | A two‐part study assessing catheter‐related infections before and after the introduction silver‐impregnated CVC for long‐term therapy in children. The first part was a retrospective analysis of catheter‐related infections during a six‐year period (1990‐1995) when conventional, untreated catheters were used, and the second part comprised an RCT comparing catheter‐related infections in silver‐impregnated catheters and untreated catheters. Excluded on the basis of the population group, as the study only included children |
| Casey 2012 | RCT comparing the effectiveness of silver‐coated versus non‐coated needleless intravascular connector of CVCs. Excluded on the basis of intervention |
| Chaftari 2011 | A retrospective cohort study involving cancer patients with a CVC in place who had developed CRBSI. The study compared the outcomes of patients who had their catheters exchanged with MR‐coated catheters versus those who had their catheter removed upon the diagnosis of CRBSI. Excluded on the basis of study design |
| Chatzinikolaou 2003 | RCT comparing MR‐coated catheters with uncoated catheters in reducing catheter‐related infections in patients with acute renal failure requiring haemodialysis. Excluded on the basis of the population group |
| Chelliah 2007 | A prospective observational study on the catheter type (antimicrobial‐coated and uncoated) and catheter‐related infections in children. Excluded on the basis of the population group |
| Cherry‐Bukowiec 2009 | A before‐and‐after trial comparing CRBSI between 2 periods, from November 2006 to October 2007, during which time C‐SS‐impregnated CVCs were used universally, and from November 2007 to October 2008, during which time uncoated CVCs were used universally in the ICU. Excluded on the basis of study design. |
| Dahlberg 1995 | RCT comparing the use of a haemodialysis catheter with a silver‐impregnated cuff with a standard non‐impregnated catheter in reducing catheter‐related infections. Excluded on the basis of the population group |
| Frank 2003 | A study on the development of a cost‐effectiveness model of the antiseptic‐impregnated CVCs in a single hospital setting using a two‐step approach: firstly, a prospectively planned comparison between the overall cost of care of a group of 30 patients who had developed catheter‐related infections over a 15‐month period from 1998 to 1999 and 20 matching controls without catheter‐related infections, and secondly, an estimate of the difference in catheter‐related infections through the use of antiseptic‐impregnated CVCs via a meta‐analysis of studies comparing antiseptic‐impregnated versus non‐impregnated CVCs. Excluded on the basis of study design |
| Garland 2001 | RCT comparing 2 antiseptic‐impregnated catheter dressings for preventing CVC‐related infections in neonates. Excluded on the basis of the population group, as the study only included neonates |
| Geyik 2010 | An in‐vitro study comparing 3 types of CVCs (C‐SS, MR and rifampicin‐miconazole‐coated catheters) in their antifungal activities against Candida albicans. Excluded on the basis of study type and lack of a population group. |
| Guggenbichler 2003 | A prospective observational study involving a single group of participants who were patients in a critical care unit. All participants received silver‐coated CVCs, and there was no control group. Excluded on the basis of study design |
| Halton 2009 | A cost‐effectiveness analysis of 4 types of antimicrobial‐impregnated CVCs (MR, SPC and 2 types of C‐SS‐impregnated catheters, 1 with external coating only and the other with both internal and external coatings). The analysis was not based on any single RCT. Excluded on the basis of study design |
| Hanley 2000 | A retrospective review of the factors that influenced CRBSI in an ICU, including the use of antiseptic‐impregnated triple‐lumen CVCs. Excluded on the basis of study design |
| Hanna 2003 | A non‐randomized, pre‐and‐post study comparing 2 periods in which different types of CVCs were used predominantly in the setting of an ICU in a university hospital. The 2 periods assessed were: September 1997 to August 1998, during which most of the CVCs used were uncoated, and September 1998 to August 1999, during which most of the CVCs used were MR‐impregnated. Excluded on the basis of study design |
| Hitz 2012 | An RCT that compared an athrombogenic CVC coating against conventional, uncoated CVC. The coating was not designed to be antimicrobial in nature, and although infection was included as a secondary outcome, it was measured as late complication, of up to 6 months post‐CVC insertion, and there was no definition of infection provided. Excluded on the basis of intervention and outcome measurement |
| Jacob 2011 | A prospective cross‐over study comparing a silver‐coated CVC against a standard non‐coated CVC. Excluded on the basis of study design |
| Jansen 1992 | A paper describing an in‐vitro experiment in which a CVC was coated with iodine and challenged with Staphylococcus epidermidis. The degree of bacterial inhibition was then assessed. Excluded on the basis of study design. |
| Jung 2005 | A retrospective analysis of participant characteristics, catheter insertion site and catheter‐related infections with respect to the type of CVC (C‐SS‐impregnated or non‐impregnated) inserted. Excluded on the basis on study design |
| Khare 2007 | A prospective sequential study in an adult critical care unit comparing the universal use of silver zeolite‐impregnated polyurethane catheters for 7 months with non‐impregnated polyurethane catheters for the next 7 months, with the main outcome being catheter‐related colonization. Excluded on the basis of study design |
| Krafte‐Jacobs 1995 | A prospective controlled trial in children, comparing heparin‐bonded femoral venous catheters versus standard unbonded catheters in reducing catheter‐related infection and thrombosis. Excluded on the basis of population group |
| Lenz 2010 | RCT comparing C‐SS‐impregnated CVCs against uncoated catheters in children admitted to the cardiac ICU. Minor outcomes included catheter‐related infection and costs. Excluded on the basis of population group |
| Levy 2005 | RCT comparing chlorhexidine‐impregnated CVC dressing against standard catheters without impregnated dressing in reducing catheter colonization for infants and children. Excluded on the basis of population group and intervention |
| Marin 2000 | A meta‐analysis of 11 studies comparing antimicrobial‐impregnated and heparin‐bonded central venous catheters in reducing CRBSI, Excluded on the basis of study design |
| Misra 2014 | RCT comparing the effects of 2 methods of placing new CVCs: guide wire exchange method versus new insertion. All CVCs used in the study were treated with antiseptics. Excluded on the basis on intervention |
| Pierce 2000 | RCT comparing heparin‐bonded CVC and standard non‐bonded catheters in reducing catheter‐related thrombosis and infection in children. Excluded on the basis of the population group |
| Richards 2003 | A non‐randomized, block clinical trial in which all participants in the first 2 months of study received the C‐SS‐coated catheters and all in the next 2 months received non‐coated catheters. The allocation would alternate on a two‐monthly basis. Excluded on the basis of study design |
| Roberts 1998 | RCT comparing CVCs modified with Biopatch dressing with standard unmodified catheters in reducing catheter‐related infection. Excluded on the basis of the intervention |
| Ruschulte 2009 | RCT using C‐SS‐impregnated CVCs throughout, and comparing catheters with a chlorhexidine gluconate‐impregnated wound dressing versus those with a standard unimpregnated dressing for reducing catheter‐related infections in people undergoing cancer chemotherapy. Excluded on the basis of the intervention |
| Schmitt 1996 | A series of in‐vitro experiments assessing the duration of antiseptic effects of C‐SS‐impregnated CVCs and unimpregnated catheters when challenged by Staphyloccocus epidermidis. Excluded on the basis of study design |
| Schuerer 2007 | A before‐and‐after trial comparing CRBSI before and after the universal use of C‐SS‐impregnated CVCs in an ICU with a low baseline CRBSI rate. Excluded on the basis of study design |
| Schutze 2002 | A review article on 2 major types of antimicrobial‐impregnated CVCs (C‐SS and MR) in general and for children. Excluded on the basis of study design |
| Sherertz 1997 | RCT comparing C‐SS‐impregnated peripheral venous catheters against non‐impregnated catheters for reducing phlebitis. Excluded on the basis of the intervention |
| Timsit 2010a | A cost‐effectiveness analysis of a previously published study (Timsit 2009), comparing chlorhexidine‐impregnated sponges against less frequent dressing changes in critically ill patients with a CVC in place. Excluded on the basis of study design and intervention |
| Timsit 2010b | A conference abstract that described a prospective, non‐randomized study in the form of a multicentre questionnaire survey assessing various possible risk factors for catheter‐related infections. Excluded on the basis of study design |
| Trerotola 1998 | RCT comparing silver‐coated haemodialysis catheters against standard uncoated catheters in reducing catheter‐related infection, venous thrombosis and stenosis. Excluded on the basis of the population |
| Vokurka 2009 | A non‐randomized study in which C‐SS‐impregnated CVCs were used in a group of participants, and their outcomes compared with a group of historical controls. Excluded on the basis of study design |
| Wong 2010 | RCT comparing antibiotic‐impregnated ventricular catheters against conventional non‐impregnated catheters coupled with systemic antibiotics in people undergoing emergency neurosurgical procedures for reducing cerebrospinal fluid infections. Excluded on the basis of population group |
| Ye 2011 | An economic analysis of the use of a chlorhexidine‐impregnated sponge dressing for preventing CVC‐related infections. The data from this study were taken from several published studies and not from a single RCT. Excluded on the basis of study design and intervention type |
| Yorganci 2002 | An in‐vitro study in which a sample of 150 catheter segments from 4 catheter types (3 antiseptic bonded and 1 uncoated) were tested for bactericidal and bacteriostatic activities against Klebsiella pneumonia. Excluded on the basis of study design |
Abbreviations
CRBSI = catheter‐related bloodstream infection CVC = central venous catheter C‐SS = chlorhexidine‐silver sulphadiazine ICU = intensive care unit MR = minocycline‐rifampicin RCT = randomized controlled trial SPC = silver‐platinum‐carbon
Characteristics of studies awaiting assessment [ordered by study ID]
Krikava 2011.
| Methods | Multi‐centre RCT involving 2 tertiary ICUs (Czec Republic) |
| Participants | ICU patients. Further information not available |
| Interventions | CVCs with polyhexanide anti‐infective coating (Cetofix Protect, internal and external coating) versus uncoated CVCs |
| Outcomes | Catheter colonization, CRBSI |
| Notes | The article was published as a conference proceeding. The data were reported as percentages without the accompanying number of participants or catheters in each group. Awaiting further information from the authors |
Abbreviations
CVC= central venous catheter
CRBSI = catheter related blood stream infection
ICU = intensive care unit
RCT = randomized controlled trial
Differences between protocol and review
We made the following changes to the published protocol (Lai 2009).
We changed the search platform from PubMed (National Library of Medicine) to MEDLINE (OVID SP), following a change of the contact author's affiliation where access to OVID database was possible. We translated the PubMed search strategy to MEDLINE (OVID SP) equivalent without any alteration of the content. We made corresponding revisions in our texts in the 'electronic searches' section.
Search strategies for EMBASE and CINAHL were reformatted, without changing the search terms, to be in line with the format required by the host database of the contact author (see Appendix 4; Appendix 5).
Types of studies: we included cluster‐randomized trials.
Types of participants: amended with the addition of a statement accepting studies with multiple enrolments of the same participant.
Types of outcomes: we did not include the duration of catheterization as an outcome, as specified in our protocol, because this was listed as one of the characteristics of the population rather than as an outcome in all the studies. Instead, we assessed catheter durability via the outcome of premature catheter removal, which was also listed in our protocol and reported as an outcome in the included studies.
Types of outcomes: under Secondary outcomes, the unit of analysis has been amended from 'the number of participants' to 'the number of participants or catheters', to account for the effect of multiple enrolments.
Types of outcomes: we removed the secondary outcome of 'duration of catheter use' as prespecified in our protocol, and chose to assess catheter durability via a single outcome of 'number of participants or catheters with catheter failure or premature catheter removal'. This was because duration of catheter use was reported as a study characteristic rather than an outcome in all the included studies, in which there was a wide range of catheter indwelling time depending on the underlying conditions and needs of the participants.
Types of outcomes: in the eighth secondary outcome, we added the number of participants who required systemic antibiotics during the course of the study alongside the total duration of antibiotic use.
Assessment of risk of bias in included studies: rewritten in accordance with the new 'Risk of bias' assessment criteria. A detailed description of the assessment criteria is included in the new Appendix 6.
We added a paragraph under 'Data synthesis' describing how we handled the rate data (such as CRBSI per 1000 catheter days) in individual groups as well as combined groups.
Unit of analysis issues: an extensive section was added to describe our approach to handling unit of analysis issues that might arise from multiple enrolments of the same participants and from analysing cluster‐randomized trials.
We added a paragraph under the previously empty heading of 'Sensitivity analysis' detailing how we conducted our sensitivity analyses and later reported our findings.
In subgroup analysis point number three, we included the term 'predominant participant type' alongside 'study setting' as the criterion for separating studies into subgroups. We have also added baseline risk as a criterion for our subgroup analysis, and placed this as subgroup analysis number four. Additionally, we have revised our wording for subgroup analysis point number seven to indicate that we would separate studies conducted in different countries with different currencies used to measure costs in the outcome of cost effectiveness.
References: we have replaced the CDC guideline for the prevention of intravascular‐related infections to an updated document that was published in 2011 (O'Grady 2002 has been replaced by CDC 2011). We have also replaced cited references for many of our outcomes from another Cochrane review on CVCs, Webster 2011, to the primary source of reference, which is the CDC guideline (CDC 2011).
We have included a section on the assessment of quality of the evidence and the development of the 'Summary of findings' table in the Methods to reflect what was done post‐protocol.
Contributions of authors
Conceiving the review: Nai Ming Lai (NML) Co‐ordinating the review: NML Undertaking manual searches: NML, Wilson Shu Cheng Pau (WP) Screening search results: NML, Nathorn Chaiyakunapruk (NC) Organizing retrieval of papers: Nai An Lai (NAL) , Elizabeth O'Riordan (EOR) Screening retrieved papers against inclusion criteria: NAL, EOR Appraising quality of papers: NML, NC Abstracting data from papers: NML, NAL, NC, EOR, WP Writing to authors of papers for additional information: NML, NAL, EOR, WP, NC Providing additional data about papers: NML, NAL, NC, EOR Obtaining and screening data on unpublished studies: NML, NAL, NC, EOR Data management for the review: NML, NC, WP, EOR Entering data into Review Manager 5 (Revman 5.3): NML RevMan statistical data: NML, NC, EOR, NAL Other statistical analysis not using RevMan: NML Double entry of data: data entered by NML and cross‐checked by a member of Sanjay Saint (SS)’s team (Andy Hickner), as acknowledged above. Interpretation of data: NML, NC, NAL, EOR, SS Statistical inferences: NML Writing the review: NML, NC, NAL, WP, EOR, SS Securing funding for the review: NML, SS Performing previous work that was the foundation of the present study: N/A Guarantor for the review: NML Person responsible for reading and checking review before submission: NAL, EOR, NC, WP Updating the review: NML, NC
Sources of support
Internal sources
No sources of support supplied
External sources
-
SEA‐ORCHID (South East Asian Optimising Reproductive and Child Health Outcomes in developing countries) Project, Other.
Five‐year project (2003 to 2008) aiming to promote synthesis and application of high level evidence in clinical practice especially on issues relevant to this region.
International‐Ann Arbor Safety Collaborative (I‐A2SC), USA.
Declarations of interest
Nai Ming Lai: none known
Nathorn Chaiyakunapruk. none known
Nai An Lai: none known
Elizabeth O'Riordan: none known
Wilson Shu Cheng Pau: none known
Sanjay Saint has received numerous honoraria and speaking fees from academic medical centres, hospitals, group‐purchasing organizations (e.g., Premier, VHA), specialty societies, state‐based hospital associations, and non‐profit foundations (e.g., Michigan Health and Hospital Association, Institute for Healthcare Improvement) for lectures about preventing healthcare‐associated infection, implementing change, clinical problem‐solving, and leadership. Dr Saint has provided expert testimony for legal cases focusing on medical malpractice. He has received grants/has grants pending from National Institute of Health (NIH); Veterans Affairs (VA); Agency for Healthcare Research and Quality (AHRQ); Blue Cross Blue Shield of Michigan Foundation. Dr Saint has stock options with Doximity and is on the medical advisory board of Doximity, a new social networking site for physicians. However, Dr Saint has stated that these activities are not related to the topic of the review.
Edited (no change to conclusions)
References
References to studies included in this review
Abdelkefi 2007 {published data only}
- Abdelkefi A, Achour W, Ben Othman T, Ladeb S, Torjman L, Lakhal A, et al. Use of heparin‐coated central venous lines to prevent catheter‐related bloodstream infection. Journal of Supportive Oncology 2007;5(6):273‐8. [PUBMED: 17624052] [PubMed] [Google Scholar]
Antonelli 2012 {published data only}
- Antonelli M. Comparison of central venous catheters with silver nanoparticles versus conventional catheters (NanoAgCVC). www.clinicaltrials.gov 13 May 2011.
- Antonelli M, Pascale G, Ranieri V M, Pelaia P, Tufano R, Piazza O, et al. Comparison of triple‐lumen central venous catheters impregnated with silver nanoparticles (AgTive®) vs conventional catheters in intensive care unit patients. Journal of Hospital Infection 2012;82:101‐7. [DOI] [PubMed] [Google Scholar]
Arvaniti 2012 {published data only}
- Arvaniti K, Lathyris D, Clouva‐Molyvdas P, Haidich AB, Mouloudi E, Synnefaki E, et al. Comparison of Oligon catheters and chlorhexidine‐impregnated sponges with standard multilumen central venous catheters for prevention of associated colonization and infections in intensive care unit patients: a multicenter, randomized, controlled study. Critical Care Medicine 2012;40(2):420‐9. [PUBMED: 21926583] [DOI] [PubMed] [Google Scholar]
Babycos 1993 {published data only}
- Babycos CR, Barrocas A, Webb WR. A prospective randomized trial comparing the silver‐impregnated collagen cuff with the bedside tunneled subclavian catheter. Journal of Parenteral and Enteral Nutrition 1993;17(1):61‐3. [PUBMED: 8437326] [DOI] [PubMed] [Google Scholar]
Bach 1996a {published data only}
- Bach A, Darby D, Bottiger B, Bohrer H, Motsch J, Martin E. Retention of the antibiotic teicoplanin on a hydromer‐coated central venous catheter to prevent bacterial colonization in postoperative surgical patients. Intensive Care Medicine 1996;22(10):1066‐9. [PUBMED: 8923071] [DOI] [PubMed] [Google Scholar]
Bach 1996b {published data only}
- Bach A, Schmidt H, Bottiger B, Schreiber B, Bohrer H, Motsch J, et al. Retention of antibacterial activity and bacterial colonization of antiseptic‐bonded central venous catheters. Journal of Antimicrobial Chemotherapy 1996;37(2):315‐22. [PUBMED: 8707741] [DOI] [PubMed] [Google Scholar]
Bach 1999 {published data only}
- Bach A, Eberhardt H, Frick A, Schmidt H, Bottiger BW, Martin E. Efficacy of silver‐coating central venous catheters in reducing bacterial colonization. Criticical Care Medicine 1999;27(3):515‐21. [PUBMED: 10199530] [DOI] [PubMed] [Google Scholar]
Bennegard 1982 {published data only}
- Bennegard K, Curelaru I, Gustavsson B, Linder LE, Zachrisson BF. Material thrombogenicity in central venous catheterization. I. A comparison between uncoated and heparin‐coated, long antebrachial, polyethylene catheters. Acta Anaesthesiology Scandinavia 1982;26(2):112‐20. [PUBMED: 7048839] [DOI] [PubMed] [Google Scholar]
Bong 2003 {published data only}
- Bong JJ, Kite P, Wilco MH, McMahon MJ. Prevention of catheter related bloodstream infection by silver iontophoretic central venous catheters: a randomised controlled trial. Journal of Clinical Pathology 2003;56:731‐5. [PUBMED: 14514774] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boswald 1999 {published data only}
- Boswald M, Lugauer S, Regenfus A, Braun GG, Martus P, Geis C, et al. Reduced rates of catheter‐associated infection by use of a new silver‐impregnated central venous catheter. Infection 1999;27 Suppl 1:S56‐60. [PUBMED: 10379447] [DOI] [PubMed] [Google Scholar]
- Guggenbichler JP, Boswald M, Lugauer S, Bechert T, Krall T. Silver‐impregnation reduces the risk of catheter related blood stream infections. Klinikarzt 1998;27(7‐8):197‐202. [Google Scholar]
Brun‐Buisson 2004 {published data only}
- Brun‐Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter‐related infection with newer chlorhexidine‐silver sulfadiazine‐coated catheters: a randomized controlled trial. Intensive Care Medicine 2004;30(5):837‐43. [PUBMED: 15060765] [DOI] [PubMed] [Google Scholar]
Camargo 2009 {published data only}
- Camargo LF, Marra AR, Buchele GL, Sogayar AM, Cal RG, Sousa JM, et al. Double‐lumen central venous catheters impregnated with chlorhexidine and silver sulfadiazine to prevent catheter colonization in the intensive care unit setting: a prospective randomized study. Journal of Hospital Infections 2009;72(3):227‐33. [PUBMED: 19443078] [DOI] [PubMed] [Google Scholar]
Carrasco 2004 {published data only}
- Carrasco MN, Bueno A, las Cuevas C, Jimenez S, Salinas I, Sartorius A, et al. Evaluation of a triple‐lumen central venous heparin‐coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Medicine 2004;30(4):633‐8. [PUBMED: 14722639] [DOI] [PubMed] [Google Scholar]
Ciresi 1996 {published data only}
- Ciresi DL, Albrecht RM, Volkers PA, Scholten DJ. Failure of antiseptic bonding to prevent central venous catheter‐related infection and sepsis. American Surgeon 1996;62(8):641‐6. [PUBMED: 8712561] [PubMed] [Google Scholar]
Collin 1999 {published data only}
- Collin GR. Decreasing catheter colonization through the use of an antiseptic‐impregnated catheter: a continuous quality improvement project. Chest 1999;115(6):1632‐40. [PUBMED: 10378561] [DOI] [PubMed] [Google Scholar]
Corral 2003 {published data only}
- Corral L, Nolla‐Salas M, Ibanez‐Nolla J, Leon MA, Diaz RM, Cruz Martin M, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. Journal of Hospital Infection 2003;55:212‐9. [PUBMED: 14572489] [DOI] [PubMed] [Google Scholar]
Darouiche 1999 {published data only}
- Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, et al. Catheter Study Group. A comparison of two antimicrobial‐impregnated central venous catheters. New England Journal of Medicine 1999;340(1):1‐8. [PUBMED: 9878638] [DOI] [PubMed] [Google Scholar]
- Marciante KD, Veenstra DL, Lipsky BA, Saint S. Which antimicrobial impregnated central venous catheter should we use? Modeling the costs and outcomes of antimicrobial catheter use. American Journal of Infection Control 2003;31(1):1‐8. [PUBMED: 12548250] [DOI] [PubMed] [Google Scholar]
Darouiche 2005 {published data only}
- Darouiche RO, Berger DH, Khardori N, Robertson CS, Wall MJ Jr, Metzler MH, et al. Comparison of antimicrobial impregnation with tunneling of long‐term central venous catheters: a randomized controlled trial. Annals of Surgery 2005;242(2):193‐200. [PUBMED: 16041209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunser 2005 {published data only}
- Dunser MW, Mayr AJ, Hinterberger G, Florl CL, Ulmer H, Schmid S, et al. Central venous catheter colonization in critically ill patients: a prospective, randomized, controlled study comparing standard with two antiseptic‐impregnated catheters. Anesthesia Analgesia 2005;101(6):1778‐84. [PUBMED: 16301258] [DOI] [PubMed] [Google Scholar]
Fraenkel 2006 {published data only}
- Fraenkel D, Rickard C, Thomas P, Faoagali J, George N, Ware R. A prospective, randomized trial of rifampicin‐minocycline‐coated and silver‐platinum‐carbon‐impregnated central venous catheters. Critical Care Medicine 2006;34(3):668‐75. [PUBMED: 16505651] [DOI] [PubMed] [Google Scholar]
George 1997 {published data only}
- George SJ, Vuddamalay P, Boscoe MJ. Antiseptic‐impregnated central venous catheters reduce the incidence of bacterial colonization and associated infection in immunocompromised transplant patients. European Journal of Anaesthesiology 1997;14(4):428‐31. [PUBMED: 9253572] [DOI] [PubMed] [Google Scholar]
Goldschmidt 1995 {published data only}
- Goldschmidt H, Hahn U, Salwender HJ, Haas R, Jansen B, Wolbring P, et al. Prevention of catheter‐related infections by silver coated central venous catheters in oncological patients. Zentralblatt fur Bakteriologie 1995;283(2):215‐23. [PUBMED: 8825113] [DOI] [PubMed] [Google Scholar]
Hanna 2004 {published data only}
- Hanna H, Benjamin R, Chatzinikolaou I, Alakech B, Richardson D, Mansfield P, et al. Long‐term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter‐related bloodstream infection in cancer patients: a prospective randomized clinical trial. Journal of Clinical Oncology 2004;22(15):3163‐71. Erratum in: Journal of Clinical Oncology 2005; 23 (15): 3652. [PUBMED: 15284269] [DOI] [PubMed] [Google Scholar]
Hannan 1999 {published data only}
- Hannan M, Juste RN, Umasanker S, Glendenning A, Nightingale C, Azadian B, et al. Antiseptic‐bonded central venous catheters and bacterial colonization. Anaesthesia 1999;54(9):868‐72. [PUBMED: 10460558] [DOI] [PubMed] [Google Scholar]
Harter 2002 {published data only}
- Harter C, Salwender HJ, Bach A, Egerer G, Goldschmidt H, Ho AD. Catheter‐related infection and thrombosis of the internal jugular vein in hematologic‐oncologic patients undergoing chemotherapy: a prospective comparison of silver‐coated and uncoated catheters. Cancer 2002;94(1):245‐51. [PUBMED: 11815983] [DOI] [PubMed] [Google Scholar]
Heard 1998 {published data only}
- Heard SO, Wagle M, Vijayakumar E, McLean S, Brueggemann A, Napolitano LM, et al. Influence of triple‐lumen central venous catheters coated with chlorhexidine and silver sulfadiazine on the incidence of catheter‐related bacteremia. Archives of Internal Medicine 1998;158(1):81‐7. [PUBMED: 9437382] [DOI] [PubMed] [Google Scholar]
Jaeger 2001 {published data only}
- Jaeger K, Osthaus A, Heine J, Ruschulte H, Kuhlmann C, Weissbrodt H, et al. Efficacy of a benzalkonium chloride‐impregnated central venous catheter to prevent catheter‐associated infection in cancer patients. Chemotherapy 2001;47(1):50‐5. [PUBMED: 11125233] [DOI] [PubMed] [Google Scholar]
Jaeger 2005 {published data only}
- Jaeger K, Zenz S, Juttner B, Ruschulte H, Kuse E, Heine J, et al. Reduction of catheter‐related infections in neutropenic patients: a prospective controlled randomized trial using a chlorhexidine and silver sulfadiazine‐impregnated central venous catheter. Annals of Hematology 2005;84(4):258‐62. [PUBMED: 15549302] [DOI] [PubMed] [Google Scholar]
Kahveci 2005 {published data only}
- Kahveci F, Kaya FN, Osma S, Kutlay O. Influence of antiseptic‐impregnated central venous catheters on catheter colonization and catheter‐related bacteremia incidences in patients receiving TPN. Turk Anesteziyoloji ve Reanimasyon Dernegi Dergisi (Journal of Turkish Society of Anaesthesiology) 2005;33(4):321‐7. [Google Scholar]
Kalfon 2007 {published data only}
- Kalfon P, Vaumas C, Samba D, Boulet E, Lefrant JY, Eyraud D, et al. Comparison of silver‐impregnated with standard multi‐lumen central venous catheters in critically ill patients. Critical Care Medicine 2007;35(4):1032‐9. [PUBMED: 17334256] [DOI] [PubMed] [Google Scholar]
Kamal 1991 {published data only}
- Kamal GD, Pfaller MA, Rempe LE, Jebson PJ. Reduced intravascular catheter infection by antibiotic bonding. A prospective, randomized, controlled trial. JAMA 1991;265(18):2364‐8. [PUBMED: 2016833] [PubMed] [Google Scholar]
Leon 2004 {published data only}
- Leon C, Ruiz‐Santana S, Rello J, Torre MV, Valles J, Alvarez‐Lerma F, et al. Benefits of minocycline and rifampin‐impregnated central venous catheters. A prospective, randomized, double‐blind, controlled, multicenter trial. Intensive Care Medicine 2004;30(10):1891‐9. [PUBMED: 15278273] [DOI] [PubMed] [Google Scholar]
Logghe 1997 {published data only}
- Logghe C, Ossel C, D'Hoore W, Ezzedine H, Wauters G, Haxhe JJ. Evaluation of chlorhexidine and silver‐sulfadiazine impregnated central venous catheters for the prevention of bloodstream infection in leukaemic patients: a randomized controlled trial. Journal of Hospital Infections 1997;37(2):145‐56. [PUBMED: 9364263] [DOI] [PubMed] [Google Scholar]
Maki 1988 {published data only}
- Maki DG, Cobb L, Garman JK, Shapiro JM, Ringer M, Helgerson RB. An attachable silver‐impregnated cuff for prevention of infection with central venous catheters: a prospective randomized multicenter trial. American Journal of Medicine 1988;85(3):307‐14. [PUBMED: 3046351] [DOI] [PubMed] [Google Scholar]
Maki 1997 {published data only}
- Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter‐related bloodstream infection by use of an antiseptic‐impregnated catheter. A randomized, controlled trial. Annals of Internal Medicine 1997;127(4):257‐66. [PUBMED: 9265424] [DOI] [PubMed] [Google Scholar]
Marik 1999 {published data only}
- Marik PE, Abraham G, Careau P, Varon J, Fromm RE Jr. The ex vivo antimicrobial activity and colonization rate of two antimicrobial‐bonded central venous catheters. Critical Care Medicine 1999;27(6):1128‐31. [PUBMED: 10397217] [DOI] [PubMed] [Google Scholar]
Mer 2009 {published data only}
- Mer M, Duse AG, Galpin JS, Richards GA. Central venous catheterization: a prospective, randomized, double‐blind study. Clinical and Applied Thrombosis/Hemostasis 2009;15(1):19‐26. [PUBMED: 18593746] [DOI] [PubMed] [Google Scholar]
Moretti 2005 {published data only}
- Moretti EW, Ofstead CL, Kristy RM, Wetzler HP. Impact of central venous catheter type and methods on catheter‐related colonization and bacteraemia. Journal of Hospital Infection 2005;61(2):139‐45. [PUBMED: 16026898] [DOI] [PubMed] [Google Scholar]
Moss 2000 {published data only}
- Moss HA, Tebbs SE, Faroqui MH, Herbst T, Isaac JL, Brown J, et al. A central venous catheter coated with benzalkonium chloride for the prevention of catheter‐related microbial colonization. European Journal of Anaesthesiology 2000;17(11):680‐7. [PUBMED: 11029566] [DOI] [PubMed] [Google Scholar]
Osma 2006 {published data only}
- Osma S, Kahveci SF, Kaya FN, Akalin H, Ozakin C, Yilmaz E, et al. Efficacy of antiseptic‐impregnated catheters on catheter colonization and catheter‐related bloodstream infections in patients in an intensive care unit. Journal of Hospital Infection 2006;62(2):156‐62. [PUBMED: 16307824] [DOI] [PubMed] [Google Scholar]
Ostendorf 2005 {published data only}
- Ostendorf T, Meinhold A, Harter C, Salwender H, Egerer G, Geiss HK, et al. Chlorhexidine and silver‐sulfadiazine coated central venous catheters in haematological patients‐‐a double‐blind, randomized, prospective, controlled trial. Supportive Care in Cancer 2005;13(12):993‐1000. [PUBMED: 15834740] [DOI] [PubMed] [Google Scholar]
Pemberton 1996 {published data only}
- Pemberton LB, Ross V, Cuddy P, Kremer H, Fessler T, McGurk E. No difference in catheter sepsis between standard and antiseptic central venous catheters. A prospective randomized trial. Archives of Surgery 1996;131(9):986‐9. [PUBMED: 8790170] [DOI] [PubMed] [Google Scholar]
Raad 1997 {published data only}
- Raad I, Darouiche R, Dupuis J, Abi‐Said D, Gabrielli A, Hachem R, et al. The Texas Medical Center Catheter Study Group. Central venous catheters coated with minocycline and rifampin for the prevention of catheter‐related colonization and bloodstream infections. A randomized, double‐blind trial. Annals of Internal Medicine 1997;127(4):267‐74. [PUBMED: 9265425] [DOI] [PubMed] [Google Scholar]
Raad 1998 {published data only}
- Raad II, Darouiche RO, Hachem R, Abi‐Said D, Safar H, Darnule T, et al. Antimicrobial durability and rare ultrastructural colonization of indwelling central catheters coated with minocycline and rifampin. Critical Care Medicine 1998;26(2):219‐24. [PUBMED: 9468157] [DOI] [PubMed] [Google Scholar]
Ranucci 2003 {published data only}
- Ranucci M, Isgrò G, Giomarelli PP, Pavesi M, Luzzani A, Cattabriga I, et al. Impact of oligon central venous catheters on catheter colonization and catheter‐related bloodstream infection. Critical Care Medicine 2003;31(1):52‐9. [PUBMED: 12544993] [DOI] [PubMed] [Google Scholar]
Rupp 2005 {published data only}
- Rupp ME, Lisco SJ, Lipsett PA, Perl TM, Keating K, Civetta JM, et al. Effect of a second‐generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter‐related infections: a randomized, controlled trial. Annals of Internal Medicine 2005;143(8):570‐80. [PUBMED: 16230723] [DOI] [PubMed] [Google Scholar]
Sheng 2000 {published data only}
- Sheng WH, Ko WJ, Wang JT, Chang SC, Hsueh PR, Luh KT. Evaluation of antiseptic‐impregnated central venous catheters for prevention of catheter‐related infection in intensive care unit patients. Diagnostic Microbiology and Infectious Disease 2000;38(1):1‐5. [PUBMED: 11025176.] [DOI] [PubMed] [Google Scholar]
Sherertz 1996 {published data only}
- Sherertz RJ, Heard SO, Raad II, Gentry L, Bowton D, Scuderi P, et al. Gamma radiation‐sterilized, triple‐lumen catheters coated with a low concentration of chlorhexidine were not efficacious at preventing catheter infections in intensive care unit patients. Antimicrobial Agents and Chemotherapy 1996;40(9):1995‐7. [CENTRAL: CN‐00132224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1995 {published data only}
- Smith HO, DeVictoria CL, Garfinkel D, Anderson P, Goldberg GL, Soeiro R, et al. A prospective randomized comparison of an attached silver‐impregnated cuff to prevent central venous catheter‐associated infection. Gynaecologic Oncology 1995;58(1):92‐100. [PUBMED: 7789897] [DOI] [PubMed] [Google Scholar]
Stoiser 2002 {published data only}
- Stoiser B, Kofler J, Staudinger T, Georgopoulos A, Lugauer S, Guggenbichler JP, et al. Contamination of central venous catheters in immunocompromised patients: a comparison between two different types of central venous catheters. Journal of Hospital Infection 2002;50(3):202‐6. [PUBMED: 11886196] [DOI] [PubMed] [Google Scholar]
Tennenberg 1997 {published data only}
- Bach A. A randomized trial of an antibiotic‐ and antiseptic‐coated central venous catheter in the prevention of catheter‐related infections (comment). Archives of Surgery 1998;133(9):1022. [PUBMED: 9749860] [DOI] [PubMed] [Google Scholar]
- Tennenberg S, Lieser M, McCurdy B, Boomer G, Howington E, Newman C, et al. A prospective randomized trial of an antibiotic‐ and antiseptic‐coated central venous catheter in the prevention of catheter‐related infections. Archives of Surgery 1997;132(12):1348‐51. [PUBMED: 9403542] [DOI] [PubMed] [Google Scholar]
Theaker 2002 {published data only}
- Theaker C, Juste R, Lucas N, Tallboys C, Azadian B, Soni N. Comparison of bacterial colonization rates of antiseptic impregnated and pure polymer central venous catheters in the critically ill. Journal of Hospital Infection 2002;52(4):310‐2. [PUBMED: 12473479] [DOI] [PubMed] [Google Scholar]
Thornton 1996 {published data only}
- Thornton J, Todd NJ, Webster NR. Central venous line sepsis in the intensive care unit. A study comparing antibiotic coated catheters with plain catheters. Anaesthesia 1996;51(11):1018‐20. [PUBMED: 8967579] [DOI] [PubMed] [Google Scholar]
Van Heerden 1996 {published data only}
- Heerden PV, Webb SA, Fong S, Golledge CL, Roberts BL, Thompson WR. Central venous catheters revisited‐‐infection rates and an assessment of the new Fibrin Analysing System brush. Anaesthesia and Intensive Care 1996;24(3):330‐3. [PUBMED: 8805887] [DOI] [PubMed] [Google Scholar]
Van Vliet 2001 {published data only}
- Vliet J, Leusink JA, Jongh BM, Boer A. A comparison between two types of central venous catheters in the prevention of catheter‐related infections: the importance of performing all the relevant cultures. Clinical Intensive Care 2001;12(3):135‐40. [EMBASE: 2001264184] [Google Scholar]
Walz 2010 {published data only}
- Walz JM, Avelar RL, Longtine KJ, Carter KL, Mermel LA, Heard SO. Anti‐infective external coating of central venous catheters: a randomized, noninferiority trial comparing 5‐fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization. Critical Care Medicine 2010;38(11):2095‐102. [PUBMED: 20711070] [DOI] [PubMed] [Google Scholar]
Yucel 2004 {published data only}
- Schierholz JM, Nagelschmidt K, Nagelschmidt M, Lefering R, Yucel N, Beuth J. Antimicrobial central venous catheters in oncology: efficacy of a rifampicin‐miconazole‐releasing catheter. Anticancer Research 2010;30(4):1353‐8. [PUBMED: 20530452] [PubMed] [Google Scholar]
- Yucel N, Lefering R, Maegele M, Max M, Rossaint R, Koch A, et al. Reduced colonization and infection with miconazole‐rifampicin modified central venous catheters: a randomized controlled clinical trial. Journal of Antimicrobial Chemotherapy 2004;54(6):1109‐15. [PUBMED: 15537696] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alderman 2005 {published data only}
- Alderman RL, Sugarbaker PH. Prospective non randomized trial of silver impregnated cuff central lines. International Surgery 2005;90(4):219‐22. [PUBMED: 16548318] [PubMed] [Google Scholar]
Al‐Hwiesh 2007 {published data only}
- Al‐Hwiesh AK. Tunneled catheter‐antibiotic lock therapy for prevention of dialysis catheter‐related infections: a single center experience. Saudi Journal of Kidney Diseases and Transplantation 2008;19(4):593‐602. [PUBMED: 18580019] [PubMed] [Google Scholar]
- Al‐Hwiesh AK, Abdul‐Rahman IS. Successful prevention of tunneled, central catheter infection by antibiotic lock therapy using vancomycin and gentamycin. Saudi Journal of Kidney Diseases and Transplantation 2007;18(2):239‐47. [PUBMED: 17496402] [PubMed] [Google Scholar]
Alonso‐Echanove 2003 {published data only}
- Alonso‐Echanove J, Edwards JR, Richards MJ, Brennan P, Venezia RA, Keen J, et al. Effect of nurse staffing and antimicrobial‐impregnated central venous catheters on the risk for bloodstream infections in intensive care units. Infection Control and Hospital Epidemiology 2003;24(12):916‐25. [PUBMED: 14700407] [DOI] [PubMed] [Google Scholar]
Anton 2009 {published data only}
- Anton N, Cox PN, Massicotte MP, Chait P, Yasui Y, Dinyari PM, et al. Heparin‐bonded central venous catheters do not reduce thrombosis in infants with congenital heart disease: a blinded randomized, controlled trial. Pediatrics 2009;123(3):e453‐8. [PUBMED: 19237438] [DOI] [PubMed] [Google Scholar]
Bambauer 1998 {published data only}
- Bambauer R, Mestres P, Schiel R, Schneidewind JM, Goudjinou R, Latza R, et al. Surface treated large bore catheters with silver based coatings versus untreated catheters for extracorporeal detoxification methods. American Society for Artificial Internal Organs (ASAIO) Journal 1998;44(4):303‐8. [PUBMED: 9682957] [DOI] [PubMed] [Google Scholar]
Bambauer 2000 {published data only}
- Bambauer R, Mestres P, Schiel R, Schneidewind JM, Latza R, Bambauer S, et al. Surface treated catheters with ion beam based process for blood access. Therapeutic Apheresis and Dialysis 2000;4(5):342‐7. [PUBMED: 11111815] [DOI] [PubMed] [Google Scholar]
Betjes 2004 {published data only}
- Betjes MG, Agteren M. Prevention of dialysis catheter‐related sepsis with a citrate‐taurolidine‐containing lock solution. Nephrology Dialysis Transplantation 2004;19(6):1546‐51. [PUBMED: 14993498] [DOI] [PubMed] [Google Scholar]
Borschel 2006 {published data only}
- Borschel DM, Chenoweth CE, Kaufman SR, Hyde KV, Elzen KA, Raghunathan TE, et al. Are antiseptic‐coated central venous catheters effective in a real‐world setting?. American Journal of Infection Control 2006;34(6):388‐93. [PUBMED: 16877109] [DOI] [PubMed] [Google Scholar]
Carbon 1999 {published data only}
- Carbon RT, Lugauer S, Geitner U, Regenfus A, Boswald M, Greil J, et al. Reducing catheter‐associated infections with silver‐impregnated catheters in long‐term therapy of children. Infection 1999;27 Suppl 1:S69‐73. [PUBMED: 10379449] [DOI] [PubMed] [Google Scholar]
Casey 2012 {published data only}
- Casey AL, Karpanen TJ, Nightingale P, Cook M, Elliott TSJ. Microbiological comparison of a silver‐coated and a non‐coated needleless intravascular connector in clinical use. Journal of Hospital Infection 2012;80:299‐303. [DOI] [PubMed] [Google Scholar]
Chaftari 2011 {published data only}
- Chaftari AM, Kassis C, Issa H, Al Wohoush I, Jiang Y, Rangaraj G, et al. Novel approach using antimicrobial catheters to improve the management of central line‐associated bloodstream infections in cancer patients. Cancer 2011;117(11):2551‐8. [EMBASE: 1097‐0142] [DOI] [PubMed] [Google Scholar]
Chatzinikolaou 2003 {published data only}
- Chatzinikolaou I, Finkel K, Hanna H, Boktour M, Foringer J, Ho T, et al. Antibiotic‐coated hemodialysis catheters for the prevention of vascular catheter‐related infections: a prospective, randomized study. American Journal of Medicine 2003;115(5):352‐7. [PUBMED: 14553869] [DOI] [PubMed] [Google Scholar]
Chelliah 2007 {published data only}
- Chelliah A, Heydon KH, Zaoutis TE, Rettig SL, Dominguez TE, Lin R, et al. Observational trial of antibiotic‐coated central venous catheters in critically ill paediatric patients. Pediatric Infectious Disease Journal 2007;26(9):816‐20. [PUBMED: 17721377] [DOI] [PubMed] [Google Scholar]
Cherry‐Bukowiec 2009 {published data only}
- Cherry‐Bukowiec JR, Denchev K, Dickinson S, Chenoweth CE, Zalewski C, Meldrum, C, et al. Prevention of catheter‐related blood stream infection: back to basics?. Surgical Infections 2011;12(1):27‐32. [PUBMED: 21171811] [DOI] [PubMed] [Google Scholar]
- Cherry‐Bukowiec JR, Denchev KL, Dickinson S, Chenoweth C, Zalewski C, Meldrum C, et al. Prevention of catheter‐related sepsis: back to basics?. Surgical Infections 2009;10(2):207. [DOI] [PubMed] [Google Scholar]
Dahlberg 1995 {published data only}
- Dahlberg PJ, Agger WA, Singer JR, Yutuc WR, Newcomer KL, Schaper A, et al. Subclavian hemodialysis catheter infections: a prospective, randomized trial of an attachable silver‐impregnated cuff for prevention of catheter‐related infections. Infection Control and Hospital Epidemiology 1995;16(9):506‐11. [PUBMED: 8537627] [DOI] [PubMed] [Google Scholar]
Frank 2003 {published data only}
- Frank U, Chojnacki T, Dettenkofer M, Daschner FD. Cost‐effectiveness of an antiseptic‐impregnated central venous catheter in the ICU. Intensive Care Medicine 2003;29(1):139. [PUBMED: 12528036] [DOI] [PubMed] [Google Scholar]
Garland 2001 {published data only}
- Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, et al. A randomized trial comparing povidone‐iodine to a chlorhexidine gluconate‐impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001;107(6):1431‐6. [PUBMED: 11389271] [DOI] [PubMed] [Google Scholar]
Geyik 2010 {published data only}
- Geyik SG, Gursoy B, Sancak B, Arikan S, Yorganci K. In vitro antifungal activities of antimicrobial coated/impregnated central venous catheters against Candida albicans. Journal of Medical and Surgical Intensive Care Medicine 2010;1(1):10‐3. [EMBASE: 2011123033] [Google Scholar]
Guggenbichler 2003 {published data only}
- Guggenbichler JP, Juhl G, Braun GG, Frass M, Kunstle OA, Plotz J, et al. Clinical investigation of a new central venous catheter impregnated with silver nanoparticles. Hygiene & Medizin (Hygiene and Medicine) 2003;28(6):228‐42. [EMBASE: 2003275496] [Google Scholar]
Halton 2009 {published data only}
- Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Critical Care 2009;13(2):R35. [PUBMED: 19284570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanley 2000 {published data only}
- Hanley EM, Veeder A, Smith T, Drusano G, Currie E, Venezia RA. Evaluation of an antiseptic triple‐lumen catheter in an intensive care unit. Critical Care Medicine 2000;28(2):366‐70. [PUBMED: 10708168] [DOI] [PubMed] [Google Scholar]
Hanna 2003 {published data only}
- Hanna HA, Raad II, Hackett B, Wallace SK, Price KJ, Coyle DE, et al. Antibiotic‐impregnated catheters associated with significant decrease in nosocomial and multidrug‐resistant bacteremias in critically ill patients. Chest 2003;124(3):1030‐8. [PUBMED: 12970034 ] [DOI] [PubMed] [Google Scholar]
Hitz 2012 {published data only}
- Hitz F, Klingbiel D, Omlin A, Riniker S, Zerz A, Cerny T. Athrombogenic coating of long‐term venous catheter for cancer patients: a prospective, randomised, double‐blind trial. Annals of Hematology 2012;91:613‐20. [DOI] [PubMed] [Google Scholar]
Jacob 2011 {published data only}
- Jacob J, Tejedor SC, Reyes MD, Switchenko J, Easley K, Garrett G, et al. Comparison of a novel silver‐coated catheter access device and a standard catheter access device: a dual center cross‐over study. 49th Annual Conference of the Infectous Disease Society of America (IDSA); 2011 October 20‐23; Boston. Boston: IDSA, 2011:Abstract no 676.
Jansen 1992 {published data only}
- Jansen B, Kristinsson KG, Jansen S, Peters G, Pulverer G. In‐vitro efficacy of a central venous catheter complexed with iodine to prevent bacterial colonization. Journal of Antimicrobial Chemotherapy 1992;30(2):135‐9. [PUBMED: 1399922] [DOI] [PubMed] [Google Scholar]
Jung 2005 {published data only}
- Jung YJ, Koh Y, Lim CM, Lee JS, Yu H, Oh YM, et al. The central venous catheter‐related infection of chlorhexidine‐silver sulfadiazine coated catheters in medical ICU. Tuberculosis and Respiratory Diseases 2005;59(4):389‐96. [Google Scholar]
Khare 2007 {published data only}
- Khare MD, Bukhari SS, Swann A, Spiers P, McLaren I, Myers J. Reduction of catheter‐related colonization by the use of a silver zeolite‐impregnated central vascular catheter in adult critical care. Journal of Infection 2007;54(2):146‐50. [PUBMED: 16678904] [DOI] [PubMed] [Google Scholar]
Krafte‐Jacobs 1995 {published data only}
- Krafte‐Jacobs B, Sivit CJ, Mejia R, Pollack MM. Catheter‐related thrombosis in critically ill children: comparison of catheters with and without heparin bonding. Journal of Pediatrics 1995;126(1):50‐4. [PUBMED: 7815223] [DOI] [PubMed] [Google Scholar]
Lenz 2010 {published data only}
- Lenz AM, Vassallo JC, Moreno GE, Althabe M, Gomez S, Magliola R, et al. Prevention of catheter‐related infection; usefulness and cost‐effectiveness of antiseptic catheters in children. Archivos Argentinos de Pediatria 2010;108(3):209‐15. [PUBMED: 20544135] [DOI] [PubMed] [Google Scholar]
Levy 2005 {published data only}
- Levy I, Katz J, Solter E, Samra Z, Vidne B, Birk E, et al. Chlorhexidine‐impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. Pediatric Infectious Disease Journal 2005;24(8):676‐9. [PUBMED: 16094219] [DOI] [PubMed] [Google Scholar]
Marin 2000 {published data only}
- Marin MG, Lee JC, Skurnick JH. Prevention of nosocomial bloodstream infections: effectiveness of antimicrobial‐impregnated and heparin‐bonded central venous catheters. Critical Care Medicine 2000;28(9):3332‐8. [PUBMED: 11008998 ] [DOI] [PubMed] [Google Scholar]
Misra 2014 {published data only}
- Misra A, Thatte W S, Ambike D. A comparative study of the microbial and clinical outcome of antimicrobial surface treated central venous catheters exchanged over the guide wire versus newly inserted catheters. Indian Journal of Critical Care Medicine 2014;18:S2. [Google Scholar]
Pierce 2000 {published data only}
- Pierce CM, Wade A, Mok Q. Heparin‐bonded central venous lines reduce thrombotic and infective complications in critically ill children. Intensive Care Medicine 2000;26(7):967‐72. [PUBMED: 10990114] [DOI] [PubMed] [Google Scholar]
Richards 2003 {published data only}
- Richards B, Chaboyer W, Bladen T, Schluter PJ. Effect of central venous catheter type on infections: a prospective clinical trial. Journal of Hospital Infection 2003;54(1):10‐7. [PUBMED: 12767841] [DOI] [PubMed] [Google Scholar]
Roberts 1998 {published data only}
- Roberts B, Cheung D. Biopatch‐‐a new concept in antimicrobial dressings for invasive devices. Australian Critical Care 1998;11(1):16‐9. [PUBMED: 9708081] [DOI] [PubMed] [Google Scholar]
Ruschulte 2009 {published data only}
- Ruschulte H, Franke M, Gastmeier P, Zenz S, Mahr KH, Buchholz S, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Annals of Hematology 2009;88(3):267‐72. [PUBMED: 18679683] [DOI] [PubMed] [Google Scholar]
Schmitt 1996 {published data only}
- Schmitt SK, Knapp C, Hall GS, Longworth DL, McMahon JT, Washington JA. Impact of chlorhexidine‐silver sulfadiazine‐impregnated central venous catheters on in vitro quantitation of catheter‐associated bacteria. Journal of Clinical Microbiology 1996;34(3):508‐11. [PUBMED: 8904403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuerer 2007 {published data only}
- Schuerer DJ, Zack JE, Thomas J, Borecki IB, Sona CS, Schallom ME, et al. Effect of chlorhexidine/silver sulfadiazine‐impregnated central venous catheters in an intensive care unit with a low blood stream infection rate after implementation of an educational program: a before‐after trial. Surgical Infection 2007;8(4):445‐54. [PUBMED: 17883361] [DOI] [PubMed] [Google Scholar]
Schutze 2002 {published data only}
- Schutze GE. Antimicrobial‐impregnated central venous catheters. Pediatric Infectious Disease Journal 2002;21(1):63‐4. [PUBMED: 11791103] [DOI] [PubMed] [Google Scholar]
Sherertz 1997 {published data only}
- Sherertz RJ, Stephens JL, Marosok RD, Carruth WA, Rich HA, Hampton KD, et al. The risk of peripheral vein phlebitis associated with chlorhexidine‐coated catheters: a randomized, double‐blind trial. Infection Control and Hospital Epidemiology 1997;18(4):230‐6. [PUBMED: 9131364] [DOI] [PubMed] [Google Scholar]
Timsit 2010a {published data only}
- Timsit JF, Schwebel C, Vesin A, Bouadma L, Geffroy A, Garrouste‐Orgeas M, et al. Cost‐benefit of chlorhexidine impregnated sponges for prevention of catheter‐related infections in adult ICU patients. Intensive Care Medicine 2010;36:S207. [Google Scholar]
Timsit 2010b {published data only}
- Timsit JF, Francais A, L'Heriteau F, Coignard B, Venier AG, Jarno P, et al. Center, patient and catheter‐related determinants of catheter‐related infection in the ICU: a multicenter analysis based on a hierarchical model. Intensive Care Medicine 2010;36:S208. [DOI] [PubMed] [Google Scholar]
Trerotola 1998 {published data only}
- Trerotola SO, Johnson MS, Shah H, Kraus MA, McKusky MA, Ambrosius WT, et al. Tunneled hemodialysis catheters: use of a silver‐coated catheter for prevention of infection‐‐a randomized study. Radiology 1998;207(2):491‐6. [PUBMED: 9577500] [DOI] [PubMed] [Google Scholar]
Vokurka 2009 {published data only}
- Vokurka S, Kabatova‐Maxova K, Skardova J, Bystricka E. Antimicrobial chlorhexidine/silver sulfadiazine‐coated central venous catheters versus those uncoated in patients undergoing allogeneic stem cell transplantation. Supportive Care in Cancer 2009;17(2):145‐51. [PUBMED: 18449570] [DOI] [PubMed] [Google Scholar]
Wong 2010 {published data only}
- Wong GK, Ip M, Poon WS, Mak CW, Ng RY. Antibiotics‐impregnated ventricular catheter versus systemic antibiotics for prevention of nosocomial CSF and non‐CSF infections: a prospective randomized clinical trial. Journal of Neurology, Neurosurgery, and Psychiatry 2010;81(10):1064‐7. [PUBMED: 20466698] [DOI] [PubMed] [Google Scholar]
Ye 2011 {published data only}
- Ye X, Rupnow M, Bastide P, Lafuma A, Ovington L, Jarvis WR. Economic impact of use of chlorhexidine‐impregnated sponge dressing for prevention of central line‐associated infections in the United States. American Journal of Infection Control 2011;39(8):647‐54. [PUBMED: 21641681] [DOI] [PubMed] [Google Scholar]
Yorganci 2002 {published data only}
- Yorganci K, Krepel C, Weigelt JA, Edmiston CE. Activity of antibacterial impregnated central venous catheters against Klebsiella pneumoniae. Intensive Care Medicine 2002;28(4):438‐42. [PUBMED: 11967598 ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Krikava 2011 {published data only}
- Krikava I, Kolar M, Garajova B, Balik T, Sevcikova A, Pachl J, et al. Polyhexanide anti‐infective coating of central venous catheters in prevention of catheter colonization and bloodstream infection: Study HC‐G‐H‐0507. Critical Care 2011;15:P229. [Google Scholar]
- Pachl J, Sevcik P, Brauer B. Rate of catheter colonization and risk of bloodstream infection during use of two different central venous catheters (CVC). www.clinicaltrials.gov 4 February 2010.
Additional references
Abdelkefi 2007a
- Abdelkefi A, Achour W, Ben Othman T, Ladeb S, Torjman L, Lakhal A, et al. Use of heparin‐coated central venous lines to prevent catheter‐related bloodstream infection. The Journal of Supportive Oncology 2007;5(6):273‐8. [PUBMED: 17624052] [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial resistance: glossary. Centers for Disease Control and Prevention health topics 22 July 2010.
CDC 2011
- O'Grady N, Alexander M, Burns LA, Dellinger P, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter‐related infections, 2011. Centers for Disease Control and Prevention (CDC), Healthcare Infection Control Practices Advisory Committee (HICPAC) publications 1 April 2011.
Chaiyakunapruk 2002
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone‐iodine solution for vascular catheter‐site care: a meta‐analysis. Annals of Internal Medicine 2002;136(11):792‐801. [PUBMED: 12044127] [DOI] [PubMed] [Google Scholar]
Cicalini 2004
- Cicalini S, Palmieri F, Petrosillo N. Clinical review: new technologies for prevention of intravascular catheter‐related infections. Critical Care 2004;8:157‐62. [PUBMED: 15153233] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falagas 2007
- Falagas ME, Fragoulis K, Bliziotis IA, Chatzinikolaou I. Rifampicin‐impregnated central venous catheters: a meta‐analysis of randomized controlled trials. The Journal of Antimicrobial Chemotherapy 2007;59:359‐69. [PUBMED: 17255143] [DOI] [PubMed] [Google Scholar]
Gilbert 2008
- Gilbert RE, Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: a systematic review. Current Opinion in Infectious Diseases 2008;21(3):235‐45. [PUBMED: 18448967] [DOI] [PubMed] [Google Scholar]
Gnass 2004
- Gnass SA, Barboza L, Bilicich D, Angeloro P, Treiyer W, Grenovero S, et al. Prevention of central venous catheter‐related bloodstream infections using non‐technologic strategies. Infection Control and Hospital Epidemiology 2004;25:675‐7. [PUBMED: 15357160] [DOI] [PubMed] [Google Scholar]
Hanna 2006
- Hanna H, Bahna P, Reitzel R, Dvorak T, Chaiban G, Hachem R, et al. Comparative in vitro efficacies and antimicrobial durabilities of novel antimicrobial central venous catheters. Antimicrobial Agents and Chemotherapy 2006;50(10):3283‐8. [PUBMED: 17005806] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. The Cochrane Collaboration.
Hockenhull 2008
- Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al. The clinical effectiveness and cost‐effectiveness of central venous catheters treated with anti‐infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technology Assessment 2008;12:iii‐92. [PUBMED: 18405471] [DOI] [PubMed] [Google Scholar]
Hockenhull 2009
- Hockenhull JC, Dwan KM, Smith GW, Gamble CL, Boland A, Walley TJ, et al. The clinical effectiveness of central venous catheters treated with anti‐infective agents in preventing catheter‐related bloodstream infections: a systematic review. Critical Care Medicine 2009;37:702‐12. [PUBMED: 19114884] [DOI] [PubMed] [Google Scholar]
Hu 2004
- Hu KK, Lipsky BA, Veenstra DL, Saint S. Using maximal sterile barriers to prevent central venous catheter‐related infection: a systematic evidence‐based review. American Journal of Infection Control 2004;32(3):142‐6. [PUBMED: 15153925] [DOI] [PubMed] [Google Scholar]
Jung 2008
- Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Applied and Environmental Microbiology 2008;74(7):2171‐8. [PUBMED: 18245232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2007
- Kang S, Pinault M, Pfefferle LD, Elimelech M. Single‐walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007;23(17):8670‐3. [PUBMED: 17658863] [DOI] [PubMed] [Google Scholar]
Levy 2003
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Medicine 2003;29(4):530‐8. [PUBMED: 12664219] [DOI] [PubMed] [Google Scholar]
McCann 2010
- McCann M, Moore ZEH. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006894.pub2] [DOI] [PubMed] [Google Scholar]
McConnell 2003
- McConnell SA, Gubbins PO, Anaissie EJ. Do antimicrobial‐impregnated central venous catheters prevent catheter‐related bloodstream infection?. Clinical Infectious Diseases 2003;37:65‐72. [PUBMED: 12830410] [DOI] [PubMed] [Google Scholar]
McDonnell 1999
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews 1999;12(1):147‐79. [PUBMED: 9880479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mermel 2001
- Mermel LA. New technologies to prevent intravascular catheter‐related bloodstream infections. Emerging Infectious Diseases 2001;7:197‐9. [PUBMED: 11294705] [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayan 2005
- Narayan R, Abernathy H, Riester L, Berry C, Brigmon R. Antimicrobial properties of diamond‐like carbon‐silver‐platinum nanocomposite thin films. Journal of Materials Engineering and Performance 2005;14:435‐40. [DOI: 10.1361/105994905X56197] [DOI] [Google Scholar]
NHSN 2010
Niel‐Weise 2007
- Niel‐Weise BS, Stijnen T, Broek PJ. Anti‐infective‐treated central venous catheters: a systematic review of randomized controlled trials. Intensive Care Medicine 2007;33:2058‐68. [PUBMED: 17940746] [DOI] [PubMed] [Google Scholar]
Niel‐Weise 2008
- Niel‐Weise BS, Stijnen T, Broek PJ. Anti‐infective‐treated central venous catheters for total parenteral nutrition or chemotherapy: a systematic review. Journal of Hospital Infection 2008;69:114‐23. [PUBMED: 18439717] [DOI] [PubMed] [Google Scholar]
Norwood 1991
- Norwood S, Ruby A, Civetta J, Cortes V. Catheter‐related infections and associated septicemia. Chest 1991;99:968‐75. [PUBMED: 2009804] [DOI] [PubMed] [Google Scholar]
Olaechea 2013
- Olaechea PM, Palomar M, Alvarez‐Lerma F, Otal JJ, Insausti J, Lopez‐Pueyo MJ. Morbidity and mortality associated with primary and catheter‐related bloodstream infections in critically ill patients. Revista espanola de quimioterapia : publicacion oficial de la Sociedad Espanola de Quimioterapia 2013;26(1):21‐9. [PUBMED: 23546458] [PubMed] [Google Scholar]
Peng 2013
- Peng S, Lu Y. Clinical epidemiology of central venous catheter‐related bloodstream infections in an intensive care unit in China. Journal of Critical Care 2013;28(3):277‐83. [PUBMED: 23265289] [DOI] [PubMed] [Google Scholar]
Pronovost 2006
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. The New England Journal of Medicine 2006;355:2725‐32. [PUBMED: 17192537] [DOI] [PubMed] [Google Scholar]
Raad 1994
- Raad II, Hohn DC, Gilbreath BJ, Suleiman N, Hill LA, Bruso PA, et al. Prevention of central venous catheter‐related infections by using maximal sterile barrier precautions during insertion. Infection Control and Hospital Epidemiology 1994;15:231‐8. [PUBMED: 8207189] [PubMed] [Google Scholar]
Raad 1995
- Raad I, Darouiche R, Hachem R, Sacilowski M, Bodey GP. Antibiotics and prevention of microbial colonization of catheters. Antimicrobial Agents and Chemotherapy 1995;39:2397‐400. [PUBMED: 8585715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raad 1996
- Raad I, Darouiche R, Hachem R, Mansouri M, Bodey GP. The broad‐spectrum activity and efficacy of catheters coated with minocycline and rifampicin. The Journal of Infectious Diseases 1996;173:418‐24. [PUBMED: 8568304] [DOI] [PubMed] [Google Scholar]
Raad 2002
- Raad II, Hanna HA. Intravascular catheter‐related infections: new horizons and recent advances. Archives of Internal Medicine 2002;162(8):871‐8. [PUBMED: 11966337] [DOI] [PubMed] [Google Scholar]
Ramritu 2008
- Ramritu P, Halton K, Collignon P, Cook D, Fraenkel D, Battistutta D, et al. A systematic review comparing the relative effectiveness of antimicrobial‐coated catheters in intensive care units. American Journal of Infection Control 2008;36:104‐17. [PUBMED: 18313512 ] [DOI] [PubMed] [Google Scholar]
Revman 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenberg 1967
- Rosenberg B, Camp L, Grimley EB, Thomson AJ. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum (IV) complexes. The Journal of Biological Chemistry 1967;242(6):1347‐52. [PUBMED: 5337590] [PubMed] [Google Scholar]
Rosenthal 2006
- Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, et al. Device‐associated nosocomial infections in 55 intensive care units of 8 developing countries. Annals of Internal Medicine 2006;145:582‐91. [PUBMED: 17043340] [DOI] [PubMed] [Google Scholar]
Saint 2000
- Saint S, Veenstra DL, Lipsky BA. The clinical and economic consequences of nosocomial central venous catheter‐related infection: are antimicrobial catheters useful?. Infection Control and Hospital Epidemiology 2000;21:375‐80. [PUBMED: 10879567] [DOI] [PubMed] [Google Scholar]
Sampath 2001
- Sampath LA, Tambe SM, Modak SM. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infection Control and Hospital Epidemiology 2001;22:640‐6. [PUBMED: 11776351] [DOI] [PubMed] [Google Scholar]
Schmidt 1996
- Schmidt SK, Knapp C, Hall G, Longworth D, McMahon JT, Washington JA. Impact of chlorhexidine‐silver sulfadiazine‐Impregnated central venous catheters on in vitro quantitation of catheter‐associated bacteria. Journal of Clinical Microbiology 1996;34:508‐11. [PUBMED: 8904403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2008
- Shah PS, Shah VS. Continuous heparin infusion to prevent thrombosis and catheter occlusion in neonates with peripherally placed percutaneous central venous catheters. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002772.pub3] [DOI] [PubMed] [Google Scholar]
Tacconelli 2009
- Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. Epidemiology, medical outcomes and costs of catheter‐related bloodstream infections in intensive care units of four European countries: literature‐ and registry‐based estimates. The Journal of Hospital Infection 2009;72(2):97‐103. [PUBMED: 19246122] [DOI] [PubMed] [Google Scholar]
Timsit 2009
- Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste‐Orgeas M, Pease S, et al. Chlorhexidine‐impregnated sponges and less frequent dressing changes for prevention of catheter‐related infections in critically ill adults: a randomized controlled trial. JAMA 2009;301(12):1231‐41. [PUBMED: 19318651 ] [DOI] [PubMed] [Google Scholar]
Veenstra 1999a
- Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. Efficacy of antiseptic‐impregnated central venous catheters in preventing catheter‐related bloodstream infection: a meta‐analysis. JAMA 1999;281:261‐7. [PUBMED: 9918482] [DOI] [PubMed] [Google Scholar]
Veenstra 1999b
- Veenstra DL, Saint S, Sullivan SD. Cost‐effectiveness of antiseptic‐impregnated central venous catheters for the prevention of catheter‐related bloodstream infection. JAMA 1999;282:554‐60. [PUBMED: 10450717] [DOI] [PubMed] [Google Scholar]
Walder 2002
- Walder B, Pittet D, Tramer MR. Prevention of bloodstream infections with central venous catheters treated with anti‐infective agents depends on catheter type and insertion time: evidence from a meta‐analysis. Infection Control and Hospital Epidemiology 2002;23:748‐56. [PUBMED: 12517018] [DOI] [PubMed] [Google Scholar]
Webster 2011
- Webster J, Gillies D, O'Riordan E, Sherriff KL, Rickard CM. Gauze and tape and transparent polyurethane dressings for central venous catheters. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD003827.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lai 2009
- Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WS, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter related infections in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007878] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2013
- Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WSC, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter‐related infections in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD007878.pub2] [DOI] [PubMed] [Google Scholar]


