Abstract
Background
Midazolam is used for sedation before diagnostic and therapeutic medical procedures. It is an imidazole benzodiazepine that has depressant effects on the central nervous system (CNS) with rapid onset of action and few adverse effects. The drug can be administered by several routes including oral, intravenous, intranasal and intramuscular.
Objectives
To determine the evidence on the effectiveness of midazolam for sedation when administered before a procedure (diagnostic or therapeutic).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL to January 2016), MEDLINE in Ovid (1966 to January 2016) and Ovid EMBASE (1980 to January 2016). We imposed no language restrictions.
Selection criteria
Randomized controlled trials in which midazolam, administered to participants of any age, by any route, at any dose or any time before any procedure (apart from dental procedures), was compared with placebo or other medications including sedatives and analgesics.
Data collection and analysis
Two authors extracted data and assessed risk of bias for each included study. We performed a separate analysis for each different drug comparison.
Main results
We included 30 trials (2319 participants) of midazolam for gastrointestinal endoscopy (16 trials), bronchoscopy (3), diagnostic imaging (5), cardioversion (1), minor plastic surgery (1), lumbar puncture (1), suturing (2) and Kirschner wire removal (1). Comparisons were: intravenous diazepam (14), placebo (5) etomidate (1) fentanyl (1), flunitrazepam (1) and propofol (1); oral chloral hydrate (4), diazepam (2), diazepam and clonidine (1); ketamine (1) and placebo (3); and intranasal placebo (2). There was a high risk of bias due to inadequate reporting about randomization (75% of trials). Effect estimates were imprecise due to small sample sizes. None of the trials reported on allergic or anaphylactoid reactions.
Intravenous midazolam versus diazepam (14 trials; 1069 participants)
There was no difference in anxiety (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.39 to 1.62; 175 participants; 2 trials) or discomfort/pain (RR 0.60, 95% CI 0.24 to 1.49; 415 participants; 5 trials; I² = 67%). Midazolam produced greater anterograde amnesia (RR 0.45; 95% CI 0.30 to 0.66; 587 participants; 9 trials; low‐quality evidence).
Intravenous midazolam versus placebo (5 trials; 493 participants)
One trial reported that fewer participants who received midazolam were anxious (3/47 versus 15/35; low‐quality evidence). There was no difference in discomfort/pain identified in a further trial (3/85 in midazolam group; 4/82 in placebo group; P = 0.876; very low‐quality evidence).
Oral midazolam versus chloral hydrate (4 trials; 268 participants)
Midazolam increased the risk of incomplete procedures (RR 4.01; 95% CI 1.92 to 8.40; moderate‐quality evidence).
Oral midazolam versus placebo (3 trials; 176 participants)
Midazolam reduced pain (midazolam mean 2.56 (standard deviation (SD) 0.49); placebo mean 4.62 (SD 1.49); P < 0.005) and anxiety (midazolam mean 1.52 (SD 0.3); placebo mean 3.97 (SD 0.44); P < 0.0001) in one trial with 99 participants. Two other trials did not find a difference in numerical rating of anxiety (mean 1.7 (SD 2.4) for 20 participants randomized to midazolam; mean 2.6 (SD 2.9) for 22 participants randomized to placebo; P = 0.216; mean Spielberger's Trait Anxiety Inventory score 47.56 (SD 11.68) in the midazolam group; mean 52.78 (SD 9.61) in placebo group; P > 0.05).
Intranasal midazolam versus placebo (2 trials; 149 participants)
Midazolam induced sedation (midazolam mean 3.15 (SD 0.36); placebo mean 2.56 (SD 0.64); P < 0.001) and reduced the numerical rating of anxiety in one trial with 54 participants (midazolam mean 17.3 (SD 18.58); placebo mean 49.3 (SD 29.46); P < 0.001). There was no difference in meta‐analysis of results from both trials for risk of incomplete procedures (RR 0.14, 95% CI 0.02 to 1.12; downgraded to low‐quality evidence).
Authors' conclusions
We found no high‐quality evidence to determine if midazolam, when administered as the sole sedative agent prior to a procedure, produces more or less effective sedation than placebo or other medications. There is low‐quality evidence that intravenous midazolam reduced anxiety when compared with placebo. There is inconsistent evidence that oral midazolam decreased anxiety during procedures compared with placebo. Intranasal midazolam did not reduce the risk of incomplete procedures, although anxiolysis and sedation were observed. There is moderate‐quality evidence suggesting that oral midazolam produces less effective sedation than chloral hydrate for completion of procedures for children undergoing non‐invasive diagnostic procedures.
Plain language summary
Midazolam for sedation before procedures
Review question
We wanted to find out whether midazolam makes medical procedures more comfortable for children and adults, as well as whether it makes the procedure easier to perform.
Background
Children and adults can become anxious during medical procedures and the procedures can be painful. Pain and anxiety can sometimes make the procedure more difficult to perform for the medical staff, due to movement or a lack of co‐operation from the patient. Sedative medications, including midazolam, are used to reduce pain and anxiety. They can be injected directly into the bloodstream (with an almost immediate effect), injected into muscle tissue, given as a nasal spray, or swallowed as a tablet or solution.
Study characteristics
The evidence is up‐to‐date to January 2016. We included 30 trials involving 2319 participants. We looked at trials that compared midazolam with no active treatment ('dummy' treatment/placebo) or a different medication for sedation before a procedure. The trials involved children and adults having procedures to diagnose medical problems rather than procedures for treatment of a disease. We disregarded trials where people received a general anaesthetic or other medications for sedation or pain relief in addition to midazolam during their procedure.
Key results
Midazolam administered into the bloodstream compared with other medications did not seem to make the participants more drowsy, reduce anxiety or pain, or make the procedure easier to perform. This is based on the low‐quality evidence currently available. A potential benefit is that children and adults who received midazolam compared with no active treatment did not remember as much about the procedures. Midazolam made them drowsy, reduced anxiety and made it easier to perform a procedure. There is moderate‐quality evidence that a solution of midazolam given to children to drink before a procedure was not as effective as a different medication called chloral hydrate. A nasal spray of midazolam before a procedure made the participants drowsy and reduced their anxiety, but this did not make it easier to perform procedures on them. This review cannot be used to assess the harms of midazolam for sedation before a procedure.
Quality of the evidence
We rated the evidence, in the main, as being of low quality. Particularly concerning was that many trials did not explain how participants were randomized to either midazolam or to a different treatment, and that the results did not give us a very clearly defined answer.
Summary of findings
Background
Midazolam is used for sedation before diagnostic and therapeutic medical procedures. It is an imidazole benzodiazepine that has depressant effects on the central nervous system (CNS) with rapid onset of action, good effectiveness and few adverse effects. The drug can be administered by several routes including oral, intravenous, intranasal and intramuscular. We compare the effectiveness of midazolam versus placebo and other sedatives.
Description of the condition
The major goal of premedication is to provide sedation and anxiolysis in order to facilitate therapeutic and diagnostic interventions. The characteristics of midazolam are that it has a rapid onset of action, short duration of sedation and low toxicity.
Description of the intervention
Midazolam is one of the most commonly used sedative medications for surgical and non‐surgical procedures. It is currently indicated for sedation, anxiolysis and amnesia preoperatively and during procedures, including ventilation of critically‐ill patients; as a co‐induction agent; and as a supplement to nitrous oxide and oxygen.
Flumazenil is a benzodiazepine antagonist that can be used to rapidly reverse the sedative and other CNS effects of midazolam.
How the intervention might work
Midazolam has a fast recovery time and is used as premedication for many procedures including colonoscopy (Lazaraki 2007); gastrointestinal endoscopy (Fakheri 2010); magnetic resonance imaging (Hollenhorst 2001); and flexible bronchoscopy (Rolo 2012). The anterograde amnesic property of midazolam may be useful for premedication before a procedure, to reduce any associated unpleasant memories (Riss 2008). The disadvantages of midazolam include drug interactions, tolerance, and withdrawal syndrome, as well as adverse events including cognitive impairment (Riss 2008). There is also the possibility that midazolam‐induced anterograde amnesia may be viewed by some patients as undesirable.
Why it is important to do this review
Midazolam is used for sedation before procedures in a wide range of medical specialties. These include gastroenterology, respiratory medicine, gynaecology, cardiology and radiology. As midazolam is often not the only drug used for procedural sedation, it is important to determine if there are any relevant clinical differences between the effects of midazolam and other drugs. It may also be important to determine whether clinicians choose different medications according to patient characteristics (age, weight, gender), or if medication choice is influenced by the type of procedure or context. To our knowledge, no systematic review has been done on the use of midazolam for sedation prior to procedures. Our review aims to fill this gap.
Objectives
To determine the evidence on the effectiveness of midazolam for sedation when administered before a procedure (diagnostic or therapeutic).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in which midazolam was used for sedation before a procedure (diagnostic or therapeutic). We included studies irrespective of language and publication status. We excluded prospective cohort studies and quasi‐randomized studies.
Types of participants
We included participants of any age (adults and children) who were undergoing a procedure preceded by sedation. We excluded any participants undergoing dental procedures, because a Cochrane review has been published about sedation with midazolam specifically in that setting (Lourenço‐Matharu 2012).
Types of interventions
We included midazolam by any route, at any dose or time, administered before a procedure. Participants who received a placebo before a procedure constituted the control group. We also included studies that compared midazolam with another drug for sedation before a procedure. We performed a separate analysis for each different drug comparison (for example, midazolam versus sedative A; midazolam versus sedative B).
We excluded studies that simultaneously compared different drugs and different routes (for example, intranasal midazolam plus intravenous sedative A versus intranasal sedative A plus intravenous midazolam; intravenous midazolam versus intranasal sedative A). We excluded studies where dexmedetomidine was the comparator, as there is another Cochrane review about sedation for this medication (Shailaja 2013).
Types of outcome measures
Primary outcomes
Effective sedation corresponding to adequate sedation level, anxiolysis, ability to complete proposed procedure. This was evaluated by:
1.1. Level of sedation on a sedation assessment scale;
1.2. Numeric rating scale of anxiety or number of participants rated as anxious;
1.3. Vital signs (heart rate, blood pressure, respiratory rate, and oxygen saturation);
1.4. Tolerance of procedure or participant co‐operation (as defined/measured by the authors of the trial);
1.5. Participant or proceduralist satisfaction (as defined/measured by the authors of the trial);
1.6. Proportion of incomplete procedures or where there was difficulty performing the procedures.
Secondary outcomes
2.1. Duration of sedation;
2.2. Onset time of sedation;
2.3. Offset time of sedation;
2.4. Anterograde amnesia (defined by number of participants who recalled the procedure);
2.5. Oversedation (as defined/measured by the authors of the trial);
2.6. Disinhibition or excitation;
2.7. Quality of recovery (as defined/measured by the authors of the trial);
2.8. Discomfort/pain (as defined/measured by the authors of the trial);
2.9. Allergic or anaphylactoid reactions;
2.10. Sedation reversal.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) to January 2016, MEDLINE in Ovid (1966 to January 2016) and Ovid EMBASE (1980 to January 2016).
We combined the sensitive strategies described in Section 6.4 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search for RCTs in MEDLINE and EMBASE.
We searched CENTRAL using the terms given in Appendix 1. We adapted our MEDLINE search strategy (Appendix 2) to reflect the subject headings found in the thesauri used by EMBASE (Appendix 3). We used the free‐text terms in all databases and in combination with subject headings when thesauri are a component of a database.
We imposed no language restrictions.
Searching other resources
For ongoing trials, we searched the following databases on 13th July 2015: metaRegister of Controlled Trials (www.controlled‐trials.com/mrct) and Clinical Trials (clinicaltrials.gov). We also screened the reference lists of all eligible trials and reviews.
Data collection and analysis
Selection of studies
We screened all titles and abstracts for eligibility. Two authors (AC and JR) independently performed this screening (see Appendix 4 for a copy of the study selection form). We resolved disagreements by discussion to decide on trial inclusion. In the case of insufficient published information to make a decision about inclusion, we attempted to contact the first author of the relevant trial. We compiled a list of eligible trials, each with a unique identifier on a 'Form for eligible trials' (see Appendix 5).
Data extraction and management
Two authors (AC and JR) independently extracted data onto a paper form. A copy of this paper form is in Appendix 6. We resolved discrepancies by discussion. AC attempted to contact an author of the relevant trial if we required additional information.
Assessment of risk of bias in included studies
Two authors (AC and JR) independently assessed the methodological quality of the eligible trials. We resolved disagreements by discussion.
We performed 'Risk of bias' assessment using the 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and by Jüni 2001. A copy of the form we used for this is in Appendix 7.
We assessed each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and any other potential threats to validity.
We considered a trial as having a low risk of bias if we assessed all domains as adequate. We considered a trial as having a high risk of bias if we assessed one or more domains as inadequate or unclear. We planned to conduct sensitivity analyses to determine whether excluding studies at high risk of bias affected the results of the meta‐analysis.
We reported the 'Risk of bias' table as part of the Characteristics of included studies table, and present 'Risk of bias' summary figures that detail all of the judgements made for all included studies in the review.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR). For continuous variables, we calculated the mean difference (MD) when studies reported their results through the same variables measured with the same instruments (same units of measurement). When continuous data were related to the same aspect in the participants but were measured with different instruments (and did not have an interchangeable unit of measurement) we pooled them using the standardized mean difference (SMD). We calculated the 95% confidence interval (CI) as the measure of variance for all statistical methods.
Unit of analysis issues
To avoid unit of analysis issues, we planned to consider repeated observations as separate outcomes and group them accordingly for analysis (Morão 2011). However, the trials included in the review reported the change in time‐separated observations (such as the change in oxygen saturation from before to after the administration of sedation), so we were unable to do this (Differences between protocol and review). We sought pre‐cross‐over data for trials that used a cross‐over design.
Dealing with missing data
If trials did not report withdrawals, we assumed there were none. We used an available‐case analysis as the default for meta‐analysis and we also considered sensitivity analysis using best‐case (all participants who withdrew did not experience the event) and worst‐case (all participants who withdrew did experience the event) scenarios for any missing data. No outcomes measured with continuous variables had missing data that needed to be included in the meta‐analyses.
Assessment of heterogeneity
We assessed the clinical heterogeneity of included trials as:
clinical diversity (e.g. different types of procedures, different forms of midazolam administration, participants' ages, etc.);
methodological diversity ('Risk of bias' assessment);
statistical heterogeneity (a manifestation of clinical or methodological diversity, or both, among the trials).
We assessed statistical heterogeneity with the I² statistic, thereby estimating the percentage of total variance across studies due to heterogeneity rather than chance (Higgins 2002). We considered an I² statistic value greater than 50% as considerable heterogeneity or if the Chi² test was significant (see Data synthesis).
Assessment of reporting biases
As per the original protocol, we planned to assess publication bias and small‐study effects using a funnel plot if there were 10 or more studies included in the meta‐analysis (Morão 2011). However, we did not perform this analysis because fewer than 10 studies were included in each meta‐analysis.
Data synthesis
We generated meta‐analytic estimates for outcomes reported by two or more studies. We performed the analysis using Review Manager 5 software (Review Manager 2014). Because the population is varied, we included all types of procedures. Due to this variation, the intervention effect could have varied across the different studies. We therefore expected that a random‐effects model would be suitable for the meta‐analyses. However, a smaller value of the I² statistic (less than 50%) prompted consideration of the use of a fixed‐effect model. We performed all analyses according to the intention‐to‐treat (ITT) principle.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for age (children, adults (16 years of age or older)), type of procedure (diagnostic, therapeutic) and medical specialty (surgical, non‐surgical) (Morão 2011). However, there was not enough evidence to conduct any subgroup analysis.
Sensitivity analysis
We planned to perform sensitivity analyses by trials with a low risk of bias versus moderate or high risk of bias (Morão 2011). However, we rated most studies to be of either low or very low quality, and this was not appropriate.
'Summary of findings' table
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with the following specific outcomes: level of sedation on a sedation assessment scale; numerical rating of scale of anxiety or number of participants rated as anxious; incomplete procedures; anterograde amnesia (recalled procedures); disinhibition or excitation; discomfort/pain; allergic or anaphylactoid reactions; and we constructed 'Summary of findings' tables using the GRADE software.
The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence is based on within‐study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. The GRADE approach specifies four levels of quality (high, moderate, low, very low). The highest quality rating is for randomized trial evidence and the lowest is for triple‐downgraded randomized trials, downgraded observational studies or case series and case reports.
Results
Description of studies
Results of the search
Figure 1 summarizes the search results to January 2016. The searches identified 4033 hits. We retrieved 163 papers for consideration and included 30 trials in this review.
1.
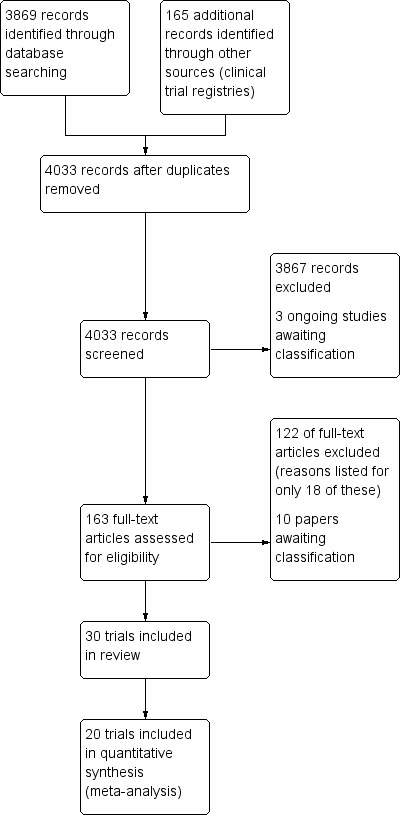
Study flow diagram.
Included studies
We include 30 trials with 2319 participants (Characteristics of included studies) that compared midazolam via the intravenous, oral and intranasal routes of administration, either to placebo or to another medication for sedation before a procedure. The included trials were conducted in both adult and paediatric populations. 16 trials enrolled participants having gastrointestinal endoscopy procedures (Bell 1988; Bhalla 2006; Bianchi Porro 1988; Cole 1983; Córdova 1992; Fakheri 2010; Gilvarry 1990; Kuganeswaran 1999; Lavies 1988; Lazaraki 2007; Lee 1989; Sainpy 1984; Takrouri 1988; Tolia 1990; Whitwam 1983; Yuno 1996) and there were three trials involving bronchoscopy (Aktogu 1994; Korttila 1985; Rolo 2012). Diagnostic imaging was performed in five trials (Akil 2005; D'Agostino 2000; Hollenhorst 2001; Stokland 2003; Wheeler 2001), one trial (Coll‐Vinent 2003) was conducted with participants undergoing cardioversion, and another (De Alencar 2010) for participants undergoing minor office‐based plastic surgery. Four trials were conducted with children undergoing minor procedures that required motion control, including lumbar puncture (Derakhshanfar 2013), suturing (Everitt 2002; Younge 2001) and Kirschner wire removal (Templeton 2010).
There was geographic variability across the included trials. Trials were performed in the United Kingdom (Gilvarry 1990; Templeton 2010; Whitwam 1983; Younge 2001), USA (Cole 1983; D'Agostino 2000; Kuganeswaran 1999; Lavies 1988; Tolia 1990; Wheeler 2001), Turkey (Akil 2005; Aktogu 1994), India (Bhalla 2006), Italy (Bianchi Porro 1988), Spain (Coll‐Vinent 2003), Mexico (Córdova 1992), Brazil (De Alencar 2010), Iran (Derakhshanfar 2013; Fakheri 2010), Australia (Everitt 2002), Germany (Hollenhorst 2001), Finland (Korttila 1985), Greece (Lazaraki 2007), Jamaica (Lee 1989), Portugal (Rolo 2012), France (Sainpy 1984), Sweden (Stokland 2003), Jordan (Takrouri 1988) and Japan (Yuno 1996).
Regarding the characteristics of the interventions, for intravenous midazolam, four trials used weight‐based calculation with a dose of 0.1 mg/kg (Córdova 1992; Korttila 1985; Sainpy 1984; Tolia 1990). Other trials used smaller doses, including 0.07 mg/kg (Bianchi Porro 1988; Lee 1989; Whitwam 1983), 0.06 mg/kg (Aktogu 1994) and 0.05 mg/kg (Rolo 2012). One trial used a higher dose of 0.2 mg/kg (Coll‐Vinent 2003). Other trials did not use participants' weight to calculate doses. These trials used either 2.5 mg (Bell 1988), 5 mg (Bhalla 2006; Cole 1983;), 10 mg (Gilvarry 1990) or 15 mg (De Alencar 2010). Some trials used smaller doses for elderly participants (Bell 1988; Bhalla 2006; Cole 1983). Other trials reported only the mean or range of dose that was administered instead of the planned method of titration (Lavies 1988; Lazaraki 2007; Takrouri 1988). For oral midazolam, only one trial did not use a weight‐based dose calculation (Kuganeswaran 1999). Weight‐based doses of oral midazolam used were 0.5 mg/kg (D'Agostino 2000; Derakhshanfar 2013; Wheeler 2001), 0.6 mg/kg (Akil 2005), 0.7 mg/kg (Younge 2001) and 1 mg/kg (Everitt 2002; Templeton 2010). For intranasal midazolam, Hollenhorst 2001 used a standard dose of 4 mg whereas Stokland 2003 used a dose of 0.2 mg/kg up to 5 mg.
The comparator arms were: intravenous diazepam in 14 trials with 1069 participants (Aktogu 1994; Bhalla 2006; Bell 1988; Bianchi Porro 1988; Cole 1983; Córdova 1992; Gilvarry 1990; Korttila 1985; Lavies 1988; Lee 1989; Sainpy 1984; Takrouri 1988; Tolia 1990; Whitwam 1983); intravenous placebo in five trials with 493 participants (Bhalla 2006; Fakheri 2010; Lavies 1988; Rolo 2012; Yuno 1996); intravenous etomidate in one trial with 17 participants (Coll‐Vinent 2003); intravenous fentanyl in one trial with 126 participants(Lazaraki 2007); intravenous flunitrazepam in one trial with 86 participants (Takrouri 1988); intravenous propofol in one trial with 17 participants (Coll‐Vinent 2003); oral chloral hydrate in four trials with 268 participants (Akil 2005; D'Agostino 2000; Derakhshanfar 2013; Wheeler 2001); oral diazepam in two trials with 122 participants (De Alencar 2010; Everitt 2002); oral diazepam and clonidine in one trial with 34 participants (De Alencar 2010); oral ketamine in one trial with 59 participants (Younge 2001); oral placebo in three trials with 176 participants (Akil 2005; Kuganeswaran 1999; Templeton 2010); and intranasal placebo two trials with 149 participants (Hollenhorst 2001; Stokland 2003).
For three trials, we pooled two different groups, as the trials compared midazolam with: both a placebo and with chloral hydrate (Akil 2005); or both placebo and diazepam (Bhalla 2006; Lavies 1988). Three trials (Coll‐Vinent 2003; De Alencar 2010; Takrouri 1988) compared midazolam with two different medications. One trial compared two different doses of midazolam with another medication (Korttila 1985). For this review, we considered only the outcomes reported from the higher dose of midazolam used, as this dose was comparable with the doses used in the other included trials. Two included articles presented results from the same trial (Bhalla 2006).
Financial support was provided by industry for two trials (Cole 1983; Kuganeswaran 1999). Yuno 1996 and Templeton 2010 reported receiving funding for their trials from non‐industry sources. No other trials reported the source of funding in their publications.
Excluded studies
We needed to review a large number of papers in full text, as it was unclear from the title and abstract whether or not analgesia was administered concurrently with midazolam and whether or not the sedation was administered intraprocedurally or just before the procedure. We excluded 122 articles that we reviewed in full text. A selection of 18 of these excluded articles are included in the Characteristics of excluded studies table, to display the common reasons for exclusion. These include intraprocedural sedation used in addition to preprocedural sedation (Mui 2005; Muttu 2005), the concomitant use of analgesia or other sedative medication with midazolam (Brouillette 1989; Dere 2010; Nascimento 2007; Salmon 1992; Sajedi 2006; Sherry 1989; Tamayo 1993), routine use of flumazenil (Ristikankare 1999; Ristikankare 2000a; Ristikankare 2000b; Uygur‐Bayramiçli 2002), wrong research design (Sandler 1992; Tesoro 2007; Weinstein 2010), midazolam used in control group if initial sedation was ineffective (Bonta 2003), and placebo being administered by a different route to midazolam (Yildirim 2006).
Studies awaiting classification
We await more information in order to classify a further 10 studies (Characteristics of studies awaiting classification). We attempted to contact the authors of four of them (Bardhan 1984; Green 1984; Ogden 1993; Theroux 1993) to clarify details but we either could not locate their current contact details or we did not receive a response from the authors. The remaining six studies awaiting classification still require data extraction because they were not published in English (Frisancho 1996; Mendes 1986; Mignonsin 1994; Münte 2002; Thakur 2003; Wild 1988).
Ongoing studies
We identified three ongoing studies from our search of the clinical trials databases that might be eligible for inclusion in future updates of the review (NCT00563069; Puttapitakpong 2015; NCT01925898) (see Characteristics of ongoing studies).
Risk of bias in included studies
We present summaries of the judgements of the risk of bias of included trials in Figure 2 and Figure 3. Details of the included trials are in the Characteristics of included studies tables. The overall risk of performance bias and detection bias was low for 50% of included trials. For randomization sequence generation and allocation concealment the quality assessment yielded low risk of bias for approximately 25% or less of the included trials. The risk of attrition bias for the primary outcomes was low for more than 75% of trials.
2.
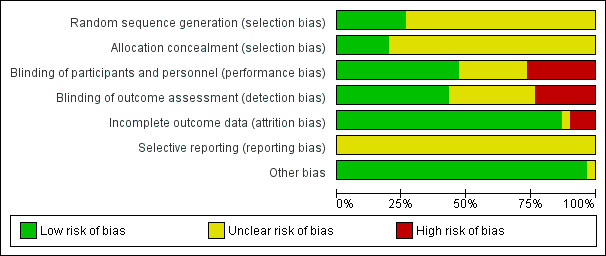
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
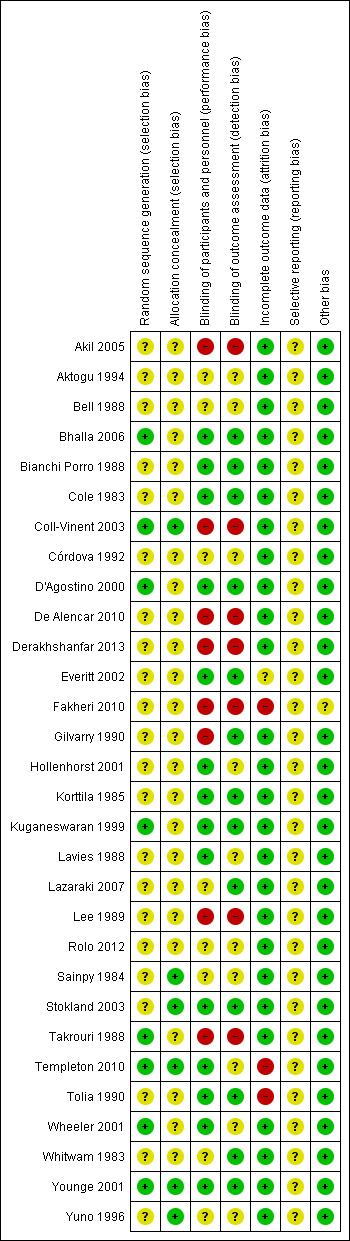
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Reporting of methods used for randomization sequence generation and allocation concealment was unclear in the majority of trials. As such, it is unclear as to the impact that potential selection bias might have on the estimates of the effects.
Blinding
Overall, there was a low risk of bias from blinding, due to the double‐blinded design used for most trials.
Incomplete outcome data
The trials were generally of short duration in an environment that was conducive to a low attrition rate for intra‐ and post‐procedural data that were collected before the participant was discharged. As such, there is low risk of attrition bias for the primary outcomes set for this review. However, one trial (Everitt 2002) reported high attrition rates for the 'quality of recovery' outcome, which was measured with a post‐discharge survey, meaning there is a high risk of attrition bias, but only for this secondary outcome.
Selective reporting
We found no definite evidence of selective reporting. However, we did not seek trial protocols because most included trials were published prior to the establishment of clinical trial registries. It is therefore unclear whether the outcomes infrequently reported or absent from the included trials, such as allergic or anaphylactoid reactions and sedation reversal, were collected but not reported.
Other potential sources of bias
We did not identify any other definite source of potential bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Intravenous midazolam compared to diazepam for sedation before procedures.
| Intravenous midazolam compared to diazepam for sedation before procedures | ||||||
| Patient or population: adults and children requiring sedation before gastrointestinal endoscopy and bronchoscopy Settings: hospitals in UK, USA, Mexico, India, Italy, Finland, Jamaica, France, Jordan and Turkey Intervention: intravenous midazolam Comparison: intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Midazolam | |||||
| Level of sedation on a sedation assessment scale | 75 (1 study) |
very low1 | The mean level of sedation in the midazolam group was 3.2 and the mean level of sedation in the diazepam group was 2.7 on a scale that ranged from 0 to 4 (higher scores indicating more sedation). Measured with a scale that ranged from 0 ‐ 4 (higher scores indicating the participant was more sedated). | |||
| Numeric rating of anxiety or rated as anxious | 167 per 1000 | 133 per 1000 (65 to 270) | RR 0.80 (0.39 to 1.62) | 175 (2 studies) | low2 | Effect estimate calculated for number of participants rated as anxious |
| Incomplete procedure | 170 (1 study) |
All procedures were completed in both groups | ||||
| Anterograde amnesia (defined by number of participants who recalled the procedure) | 481 per 1000 | 216 per 1000 (144 to 318) | RR 0.45 (0.3 to 0.66) | 587 (9 studies) | low3 | |
| Disinhibition or excitation | No studies reported on this outcome | |||||
| Discomfort/pain | 202 per 1000 | 121 per 1000 (48 to 301) | RR 0.60 (0.24 to 1.49) | 415 (5 studies) | low2 | |
| Allergic or anaphylactoid reaction | No studies reported on this outcome | |||||
| *The basis for the assumed risk is the control group risk across studies or the average risk for pooled data and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by three levels due to very serious concerns about study limitations (risk of bias) and very serious concerns about imprecision. 2Downgraded by two levels due to very serious concerns about study limitations (risk of bias) and imprecision. 3Downgraded by two level due to concerns about study limitations and inconsistency.
Summary of findings 2. Intravenous midazolam compared to placebo for sedation before procedures.
| Intravenous midazolam compared to placebo for sedation before procedures | ||||||
| Patient or population: adults requiring sedation before gastrointestinal endoscopy and bronchoscopy Settings: hospitals in India, Iran, UK, Portugal and Japan Intervention: intravenous midazolam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Intravenous midazolam | |||||
| Level of sedation on a sedation assessment scale | 100 (1 study) |
low1 | Participants who were randomized to midazolam were more sedated (the mean score on the Ramsay scale (1 to 6 with higher scores indicating the participant was more sedated) was 2.77 ± 1.19 in the midazolam group and 1.72 ± 0.50 in the placebo group. | |||
| Numeric rating of anxiety or rated as anxious | 100 (1 study) |
low1 | Authors of this trial reported that fewer participants who received midazolam were anxious (3/50 in midazolam group; 15/50 in placebo group) but results of statistical tests were not reported. | |||
| Incomplete procedures | No studies reported on this outcome | |||||
| Anterograde amnesia (defined by number of participants who recalled the procedure) | No studies reported on this outcome | |||||
| Disinhibition or excitation | No studies reported on this outcome | |||||
| Discomfort/pain | 167 (1 study) |
very low2 | There was no difference in the number of participants who had discomfort/pain during upper gastrointestinal endoscopy (3/85 in midazolam group; 4/82 in placebo group; P = 0.876). Measured in the trial as 'uncomfortable' | |||
| Allergic or anaphylactoid reaction | No studies reported on this outcome | |||||
| *The basis for the assumed risk is the control group risk across studies or the average risk for pooled data and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded two levels due to concerns about study limitations and imprecision. 2Downgraded three levels due to very serious concerns about study limitations and imprecision.
Summary of findings 3. Oral midazolam compared to chloral hydrate for sedation before procedures.
| Oral midazolam compared to chloral hydrate for sedation before procedures | ||||||
| Patient or population: children requiring sedation before procedures that require motion control, including echocardiography, lumbar puncture, micturating cystourethrograms and neuroimaging Settings: Paediatric ICU in USA, emergency departments in USA and Iran and Medical Imaging department in Turkey Intervention: oral midazolam Comparison: oral chloral hydrate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chloral hydrate | Oral midazolam | |||||
| Level of sedation on sedation assessment scale | 160 (1) |
moderate1 | 61 participants (76.25%) in the chloral hydrate group were rated at the highest level of sedation compared with 12 (15%) in the midazolam group (scores ranged from 1 = agitated to 4 = eyes closing spontaneously but response to minor stimuli) | |||
| Numeric rating of anxiety or rated as anxious | The mean rating of anxiety in D'Agostino 2000 was 2.5 The mean rating of anxiety in Akil 2005 was 49.4 |
The mean rating of anxiety in the D'Agostino 2000 trial (33 participants) was
1.1 lower
(on a scale of 1 ‐ 5 with higher scores indicating less anxiety) The mean rating of anxiety in the Akil 2005 trial (35 participants) was 1.83 lower (on the Spielberger's Trait Anxiety Inventory) |
88 (2) | very low2 | We did not conduct meta‐analysis because this outcome was measured differently in the trials (could have been answered by parents or children in Akil 2005). | |
| Incomplete procedures | 56 per 1000 | 226 per 1000 (108 to 474) | RR 4.01 (1.92 to 8.4) | 268 (4) | moderate1 | |
| Anterograde amnesia (defined by number of participants who recalled the procedure) | No studies reported on this outcome | |||||
| Disinhibition or excitation | No studies reported on this outcome | |||||
| Discomfort | No studies reported on this outcome | |||||
| Allergic or anaphylactoid reaction | No studies reported on this outcome | |||||
| *The basis for the assumed risk is the control group risk across studies or the average risk for pooled data and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to concerns about study limitations. 2Downgraded three levels due to concerns about study limitations, inconsistency and imprecision.
Summary of findings 4. Oral midazolam compared to placebo for sedation before procedures.
| Oral midazolam compared to placebo for sedation before procedures | ||||||
| Patient or population: children requiring sedation before micturating cystourethrograms and Kirschner wire removal and adults undergoing flexible sigmoidoscopy Settings: X‐ray department in Turkey, orthopaedic outpatient department in UK and endoscopy suite in USA Intervention: oral midazolam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Midazolam | |||||
| Level of sedation on a sedation assessment scale | 99 (1) |
moderate1 | Reported that level of sedation became statistically significantly different at 10 minutes after administration of medication but mean and standard deviations for midazolam and placebo group level of sedation were not reported in the article. Scores ranged from 0 = awake to 3 = asleep, responsive only to direct verbal or physical stimulus. | |||
| Numeric rating of anxiety or rated as anxious | The mean rating of anxiety in the Kuganeswaran 1999 trial was 4.2 The mean rating of anxiety in Templeton 2010 was 2.6 The mean rating of anxiety in Akil 2005 was 52.8 |
The mean anxiety score in Kuganeswaran 1999 (99 participants) was 2.52 lower (minimum score 0, maximum score 10; higher score indicates greater anxiety) The mean anxiety score in Templeton 2010 (42 participants) was 0.90 lower (minimum score 0, maximum score 8; higher score indicate greater anxiety) The mean anxiety score in Akil 2005 (35 participants) was 5.20 lower (on the Spielberger's Trait Anxiety Inventory) |
176 (3) | very low2 | We did not conduct meta‐analysis because of clinical heterogeneity (children and adults undergoing different procedures). | |
| Incomplete procedure | 179 (3 studies) |
very low3 | We did not conduct meta‐analysis because of clinical heterogeneity (children and adults undergoing different procedures). There was one incomplete procedure in the midazolam group in one of the three trials that reported on this outcome. | |||
| Anterograde amnesia (defined by number of participants who recalled the procedure) | No studies reported on this outcome | |||||
| Disinhibition or excitation | No studies reported on this outcome | |||||
| Discomfort/Pain | 99 (1 study) |
moderate1 | Statistically significant reduction in discomfort/pain (mean 2.56 (SD 0.49) in midazolam group; mean 4.62 (SD 1.49) in placebo group; P < 0.005; scores ranged from 0 to 10; higher score indicated more pain). | |||
| Allergic or anaphylactoid reaction | No studies reported on this outcome | |||||
| *The basis for the assumed risk is the control group risk across studies or the average risk for pooled data and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to concerns about study limitations. 2Downgraded three levels due to very serious concerns about study limitations, inconsistency and imprecision. 3Downgraded three levels due to concerns about study limitations and very serious concerns about imprecision.
Summary of findings 5. Intranasal midazolam compared to placebo for sedation before procedures.
| Intranasal midazolam compared to placebo for sedation before procedures | ||||||
| Patient or population: Children requiring sedation before voiding cystourethrograms and adults undergoing MRI Settings: Medical imaging departments in Germany and Sweden Intervention: intranasal midazolam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Intranasal midazolam | |||||
| Level of sedation on a sedation assessment scale | 54 (1 study) |
moderate1 | Deeper level of sedation was observed in the midazolam group (mean 3.15 (SD 0.36) in midazolam group; mean 2.56 (SD 0.64) in placebo group; P < 0.001). Level of sedation measured 15 minutes after medication by one of the authors using a five‐point sedation scale (1 = agitated, non‐co‐operative; 2 = alert, restless; 3 = calm, eyes spontaneously open; 4 = drowsy, responds to minor stimulation; 5 = asleep, rousable but does not respond to minor stimulation). | |||
| Numeric rating of anxiety or rated as anxious | 54 (1 study) |
moderate1 | Reduction in a numerical rating of anxiety among participants who received midazolam prior to magnetic resonance imaging procedure (mean 17.3 (SD 18.58) in midazolam group; mean 49.3 (SD 29.46) in placebo group; P < 0.001). Numerical rating of anxiety measured 15 minutes after medication on a Visual Analogue Scale of Anxiety comprised an undivided 100‐mm line, with 0 meaning “I am not anxious at all,” and 100 meaning “I am extremely anxious.” | |||
| Incomplete procedure | 81 per 1000 | 11 per 1000 (2 to 91) | RR 0.14 (0.02 to 1.12) | 149 (2 studies) | low2 | |
| Anterograde amnesia (defined by number of participants who recalled the procedure) | No studies reported on this outcome | |||||
| Disinhibition or excitation | No studies reported on this outcome | |||||
| Discomfort/pain | No studies reported on this outcome | |||||
| Allergic or anaphylactoid reaction | No studies reported on this outcome | |||||
| *The basis for the assumed risk is the control group risk across studies or the average risk for pooled data and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded two levels due to concerns about study limitations. 2Downgraded two levels due to concerns about study limitations and imprecision.
Intravenous midazolam versus diazepam (comparison 1, outcomes 1.1 to 1.6 and 2.1 to 2.10)
Intravenous midazolam was compared with diazepam in 14 trials in 1069 participants (Aktogu 1994; Bhalla 2006; Bell 1988; Bianchi Porro 1988; Cole 1983; Córdova 1992; Gilvarry 1990; Korttila 1985; Lavies 1988; Lee 1989; Sainpy 1984; Takrouri 1988; Tolia 1990; Whitwam 1983). We present the doses of midazolam and diazepam that were used in each of these trials in Table 6.
1. Intravenous midazolam versus diazepam doses.
| Study | Dose of midazolam | Dose of diazepam |
| Aktogu 1994 | 0.06 mg/kg | 0.15 mg/kg |
| Bhalla 2006 | 5 mg (3 mg if older than 65) | 5 mg (3 mg if older than 65) |
| Bell 1988 | 2.5 mg or 1 mg for elderly (mean 6.0 ± 2.8) | 5 mg or 2 mg for elderly (mean 11.5 ± 6.7) |
| Bianchi Porro 1988 | 0.07 mg/kg | 0.15 mg/kg |
| Cole 1983 | 5 mg bolus with 2.5 ‐ 3.75 mg increments at 30‐ ‐ 60‐second intervals as required. Half doses for elderly | 5 mg bolus with 2.5 ‐ 3.75 mg increments at 30‐ ‐ 60‐second intervals as required. Half doses for elderly |
| Córdova 1992 | 0.10 mg/kg | 0.15 mg/kg |
| Gilvarry 1990 | 10 mg | 20 mg |
| Korttila 1985 | 0.1 mg/kg | 0.2 mg/kg |
| Lavies 1988 | 2.5 ‐ 7.5 mg | 2.5 ‐ 10 mg |
| Lee 1989 | 0.07 mg/kg | 0.15 mg/kg |
| Sainpy 1984 | 0.1 mg under 65 yrs and 0.085 mg over 65 yrs infused in 30 seconds | 0.2 mg under 65 yrs and 0.15 mg over 65 yrs infused in 30 seconds |
| Takrouri 1988 | mean 5.8 mg | mean 5 mg |
| Tolia 1990 | 0.1 ‐ 0.15 mg/kg | 0.2 ‐ 0.4 mg/kg |
| Whitwam 1983 | 0.07 mg/kg | 0.15 mg/kg |
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
One trial with 75 participants (Takrouri 1988) reported on the difference in level of sedation on a sedation assessment scale. The midazolam group were given a mean dose of 5.8 mg and the diazepam group a mean dose of 5 mg. The mean level of sedation in the midazolam group was 3.2 and the mean level of sedation in the diazepam group was 2.7 on a scale that ranged from 0 to 4 (higher scores indicating more sedation). No effect estimate was reported in the trial.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
Two trials (175 participants) reported on this outcome, with 91 (52%) receiving midazolam and 84 (48%) diazepam (Takrouri 1988; Whitwam 1983). Twelve participants (13.2%) receiving midazolam were rated as anxious compared to 14 (16.7%) receiving diazepam (RR 0.80, 95% CI 0.39 to 1.62, I² = 0%; Analysis 1.1). In Takrouri 1988, both groups were given a similar mean dose of sedative medication (midazolam 5.8 mg; diazepam 5 mg). In Whitwam 1983, the midazolam group was given a dose of 0.07 mg/kg and the diazepam group was given 0.15 mg/kg.
1.1. Analysis.
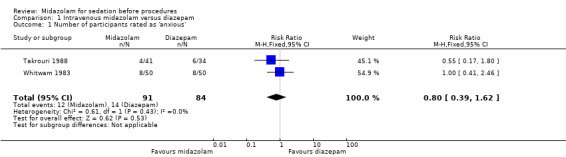
Comparison 1 Intravenous midazolam versus diazepam, Outcome 1 Number of participants rated as 'anxious'.
Outcome 1.3. Vital signs
The difference in vitals signs between the midazolam and diazepam groups could not be combined in a meta‐analysis due to disparities in how they were reported. In a trial of 170 participants, a 5 mg (or 3 mg if older than 65) dose of sedative medication was given to both the midazolam and diazepam groups (Bhalla 2006). They found no difference between groups in oxygen desaturation (35/85 in midazolam group; 29/85 in diazepam group), minimum oxygen saturation (mean 90.7 (SD 3.9) in midazolam group; mean 90.8 (SD 3.15) in diazepam group), tachycardia (15/85 in midazolam group; 10/85 in diazepam group), bradycardia (no events in either group) or hypertension (2/85 in midazolam group; 2/85 in diazepam group). Bell 1988 reported a difference between groups in the change in oxygen saturation post‐sedation (mean 3.5 (SD 2.07) in midazolam group; mean 2.8 (SD 3.7) in diazepam group; P < 0.001) in their trial of 102 participants (doses used were: midazolam 2.5 mg or 1 mg for elderly, diazepam 5 mg or 2.5 mg for elderly). In a smaller trial with 40 participants, Cole 1983 identified that the change in respiration rate was greater after administration of midazolam compared with diazepam (mean 1.7 (SD 0.7) in the 19 participants randomized to midazolam compared with mean 3.9 (SD 0.8) in the 21 participants randomized to midazolam) but there was no difference between groups for the change in heart rate (mean 3.3 (SD 1.6) in midazolam group; mean 4.6 (SD 2) in diazepam group) or diastolic blood pressure (mean ‐3.7 (SD 1.9) in midazolam group; mean 1.5 (SD 1.9) in diazepam group). The doses used in both groups were a 5 mg bolus with 2.5 to 3.75 mg increments at 30‐ to 60‐second intervals as required with half doses for elderly (Cole 1983). In a similar‐sized trial (46 participants), Bianchi Porro 1988 reported that systolic blood pressure (mean 116.1 (SD 26.5) in midazolam group; mean 129.1 (SD 14.8) in diazepam group) and diastolic blood pressure (mean 83.7 (SD 10.7) in midazolam group; mean 85.4 (SD 9.3) in diazepam group) was similar between groups. The midazolam group was given a dose of 0.07 mg/kg and the diazepam group was given 0.15 mg/kg in this trial.
Outcome 1.4. Tolerance of procedure or participant co‐operation
Five trials (Lee 1989; Bhalla 2006; Takrouri 1988; Tolia 1990; Whitwam 1983) including 486 participants reported on this outcome, with 247 (51%) receiving midazolam and 239 (49%) diazepam. Forty‐eight participants (19%) who received midazolam were deemed 'not co‐operative' in comparison with 55 (23%) in the diazepam group (RR 0.96, 95% CI 0.53 to 1.72; I² = 63%; Analysis 1.2). The dosing strategy differed between these trials, which may account for the considerable degree of inconsistency observed for this result (Table 6).
1.2. Analysis.
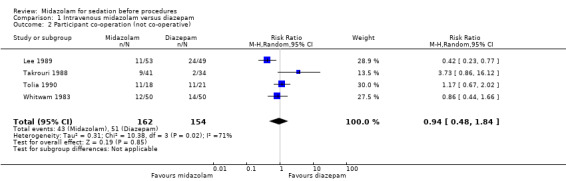
Comparison 1 Intravenous midazolam versus diazepam, Outcome 2 Participant co‐operation (not co‐operative).
Outcome 1.5. Participant or proceduralist satisfaction
Two trials (Cole 1983; Korttila 1985) with 91 participants reported proceduralist satisfaction and participant satisfaction using a scale from 0 to 100, with higher scores equating to better satisfaction. Higher doses of midazolam than diazepam were used in both of these trials (Table 6). Meta‐analysis of participant satisfaction (MD 2.17, 95% CI ‐0.51 to 4.85; I² = 12%; Analysis 1.3) and proceduralist satisfaction (MD 1.09, 95% CI ‐10.43 to 12.60; Analysis 1.4) was not statistically significantly different between the midazolam group (43 participants; 47%) and the diazepam group (48 participants; 53%).
1.3. Analysis.
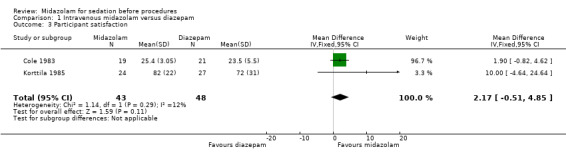
Comparison 1 Intravenous midazolam versus diazepam, Outcome 3 Participant satisfaction.
1.4. Analysis.
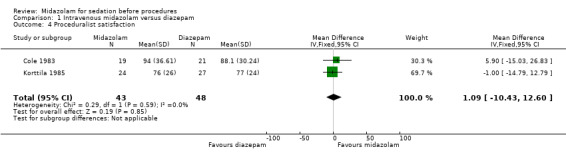
Comparison 1 Intravenous midazolam versus diazepam, Outcome 4 Proceduralist satisfaction.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
Meta‐analysisof results from three trials (Lee 1989; Takrouri 1988; Whitwam 1983) for the number of procedures rated as 'difficult to perform' revealed no important difference between the midazolam group (procedures for 23 of 144 participants were difficult to perform) and the diazepam group (procedures for 32 of 133 participants were difficult to perform) (RR 0.66, 95% CI 0.41 to 1.07; I² = 0%; Analysis 1.5). The dosing strategies differed between these trials (Table 6).One trial with 170 participants, where both groups received the same dose of midazolam or diazepam (5 mg, or 3 mg for elderly participants), reported that there were no incomplete procedures in either group (Bhalla 2006).
1.5. Analysis.
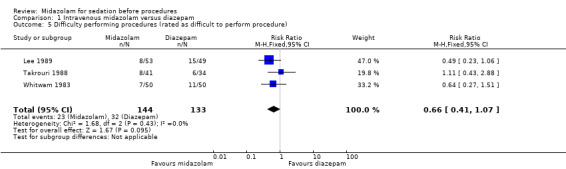
Comparison 1 Intravenous midazolam versus diazepam, Outcome 5 Difficulty performing procedures (rated as difficult to perform procedure).
Secondary outcomes
Outcome 2.1. Duration of sedation
Three trials with 224 participants reported on the duration of sedation using different definitions (Córdova 1992; Sainpy 1984; Whitwam 1983). For this reason, we did not conduct meta‐analysis. The dose of midazolam was lower than the diazepam dose in all three trials (Table 6). Córdova 1992, with 60 participants, reported that there was a reduction in minutes until recovery (mean 16 minutes (SD 8) in midazolam group; mean 35 minutes (SD 19) in diazepam group; P < 0.01). The other two trials did not identify a difference in duration of sedation between groups. The mean duration of sedation recovery (measured in minutes until sense of direction and temporospatial recovery) in the 32 participants randomized to midazolam in Sainpy 1984 trial was 24.9 minutes (SD 14.4) compared with 25.2 minutes (SD 14.9) for the 32 participants randomized to diazepam. In Whitwam 1983, the mean duration of sedation recovery (time in minutes until ready for discharge) was 75.3 minutes (SD 23.2) for the 50 participants randomized to midazolam compared with 76.4 minutes (SD 30.9) for the 50 participants randomized to diazepam.
Outcome 2.2. Onset time of sedation
Meta‐analysis of two trials (Cole 1983; Whitwam 1983) with 140 participants (69 participants received midazolam; 50%) demonstrated that the onset of sedation was similar for participants who received midazolam0 (MD ‐1.80 minutes, 95% CI ‐3.76 to 0.16; I2 = 99%; Analysis 1.6). The high heterogeneity could be explained by the doses of sedation used, with Cole 1983 using the same dosing regimen for both midazolam and diazepam (5 mg) whereas Whitwam 1983 administered 0.07 mg/kg midazolam or 0.15 mg/kg diazepam.
1.6. Analysis.
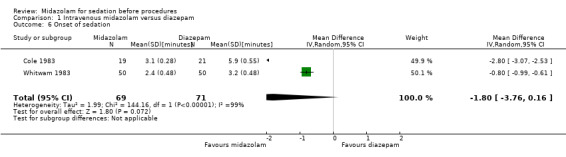
Comparison 1 Intravenous midazolam versus diazepam, Outcome 6 Onset of sedation.
Outcome 2.3. Offset time of sedation
Whitwam 1983, with 100 participants, reported this outcome. There was no clear difference between the midazolam and diazepam groups (14.6 minutes (SD 5.7) in the midazolam group; 12.9 minutes (SD 5.2) in the diazepam group).
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
A meta‐analysis of results from nine trials (Aktogu 1994; Córdova 1992; Gilvarry 1990; Korttila 1985; Lee 1989; Sainpy 1984; Takrouri 1988; Tolia 1990; Whitwam 1983) with 587 participants revealed that those who received midazolam (58 of 296 participants recalled the procedure) had greater anterograde amnesia than those who received diazepam (140 of 291 participants recalled the procedure) (RR 0.45, 95% CI 0.30 to 0.66; downgraded to low‐quality evidence due to concerns about study limitations and inconsistency; I² = 65%; Analysis 1.7). It should be noted that two participants randomized to the midazolam group in Tolia 1990 withdrew, which meant that there were missing data for this outcome. Sensitivity analysis using best‐case and worst‐case scenarios did not significantly change the result.
1.7. Analysis.
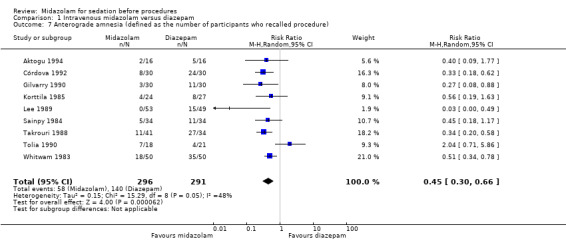
Comparison 1 Intravenous midazolam versus diazepam, Outcome 7 Anterograde amnesia (defined as the number of participants who recalled procedure).
Outcome 2.5. Oversedation
One participant in the midazolam group of Whitwam 1983, with 100 participants, was rated as being oversedated compared with no participants in the diazepam group.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the intravenous midazolam versus diazepam comparison.
Outcome 2.7. Quality of recovery
Three trials (Cole 1983; Korttila 1985; Takrouri 1988) with 166 participants reported on quality of recovery (measured in the trials as delayed recovery). There was no difference between those who received midazolam (8 of 84 participants; 10%) or diazepam (13 of 82 participants; 16%) (RR 0.72, 95% CI 0.08 to 6.63; I² = 67%; Analysis 1.8). This result was inconsistent and imprecise, so we downgraded the evidence that informed this outcome to low quality.
1.8. Analysis.
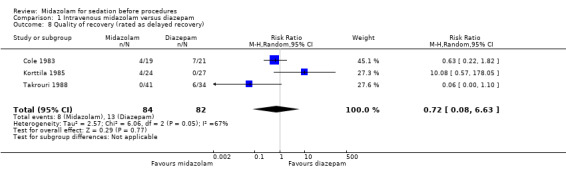
Comparison 1 Intravenous midazolam versus diazepam, Outcome 8 Quality of recovery (rated as delayed recovery).
Outcome 2.8. Discomfort/pain
There was no difference in the occurrence of discomfort/pain during the procedure between midazolam (24 of 207 participants; 12%) and diazepam (42 of 208 participants; 20%) in a meta‐analysis of five trials (Cole 1983; Lee 1989; Bhalla 2006; Sainpy 1984; Tolia 1990) with 415 participants (RR 0.60, 95% CI 0.24 to 1.49; Analysis 1.9). However, there was substantial statistical heterogeneity (I² = 76%) and the result was imprecise.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the intravenous midazolam versus diazepam comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the intravenous midazolam versus diazepam comparison.
Intravenous midazolam versus etomidate (comparison 2, outcomes 1.1 to 1.6 and 2.1 to 2.10)
We identified one trial with 17 participants that investigated the use of midazolam versus etomidate before electrical cardioversion (Coll‐Vinent 2003). Eight participants were randomized to midazolam and nine to receive etomidate. The doses of both midazolam and etomidate were 0.2 mg/kg.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
Level of sedation (measured using the Ramsay scale) was similar between groups: median score of 6 with interquartile range 5 and 6 in both groups.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 1.3. Vital signs
There were no statistically significant differences in systolic blood pressure, measured by the Kruskall‐Wallis test. Median systolic blood pressure in the midazolam group was 141 mmHg (range 99 ‐ 165) compared with 139 mmHg in the etomidate group (range 118 ‐ 150). One participant randomized to midazolam experienced an oxygen desaturation event (SpO₂ < 90%) in comparison to four events in the etomidate group.
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Secondary outcomes
Outcome 2.1. Duration of sedation
The median duration of sedation was lower in the etomidate group compared with midazolam by 11.5 minutes, and this difference was noted to be significantly different (P = 0.05).
Outcome 2.2 Onset time of sedation
The median onset time of sedation was 30 seconds lower in the etomidate group compared with the midazolam group. This difference was noted to be statistically non‐significant (P value for this comparison was not reported in publication Coll‐Vinent 2003).
Outcome 2.3. Offset time of sedation
The median offset time of sedation was 31 minutes lower in the etomidate group compared with the midazolam group, and this difference was noted to be statistically significantly (P = 0.015).
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 2.5. Oversedation
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 2.7. Quality of recovery
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the intravenous midazolam versus etomidate comparison.
Intravenous midazolam versus fentanyl (comparison 3, outcomes 1.1 to 1.6 and 2.1 to 2.10)
Intravenous midazolam was compared with fentanyl for sedation before colonoscopy in one trial with 126 participants (Lazaraki 2007). Mean dosage for midazolam was 4.6 mg and for fentanyl was 36 mcg.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 1.3. Vital signs
Midazolam was associated with more oxygen desaturation, defined as SpO₂ below 90% (23/60 in midazolam group; 0/66 in fentanyl group; P = 0.001).
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 1.5. Participant or proceduralist satisfaction
There were no differences between the groups in the effectiveness of the sedation in terms of participant satisfaction, which was measured in this trial as the acceptability of undergoing another procedure with the same sedative medication (5/60 in midazolam group; 4/66 in fentanyl group).
Outcome 1.6. Incomplete procedures/difficulty performing procedures
There were no differences between the groups in the effectiveness of the sedation in terms of incomplete procedures (3/60 in midazolam group; 1/66 in fentanyl group).
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.2. Onset time of sedation
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.3. Offset time of sedation
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
Midazolam produced more anterograde amnesia (32/60 in midazolam group; 66/66 in fentanyl group; P = 0.001).
Outcome 2.5. Oversedation
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.7. Quality of recovery
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the intravenous midazolam versus fentanyl comparison.
Outcome 2.10. Sedation reversal
There were no differences between groups in the number of participants who required sedation reversal (no events in either group).
Intravenous midazolam versus flunitrazepam (comparison 4, outcomes 1.1 to 1.6 and 2.1 to 2.10)
We identified one trial with 86 participants that compared intravenous midazolam with flunitrazepam (Takrouri 1988) before gastrointestinal endoscopy. The mean dose of midazolam was 5.8 mg and 0.65 mg for flunitrazepam. In this trial 41 participants were randomized to midazolam and 45 participants to flunitrazepam.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
The mean level of sedation was 0.5 higher (on a scale that ranged from 0 to 4) in the midazolam group.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 1.3. Vital signs
Post‐sedation heart rate (mean 88 (SD 7.2) in midazolam group; mean 92 (SD 6.3) in flunitrazepam group; P = 0.001) was lower in participants randomized to midazolam. There was no difference between groups for systolic blood pressure (mean 91 mmHg (SD 6.3) in the midazolam group; mean 98 mmHg (SD 10) in the flunitrazepam group; P = 0.5) or for diastolic blood pressure (mean 58 mmHg (SD 7) in midazolam group; mean 61 mmHg (SD 6.7) in flunitrazepam group; P = 0.5). Mean respiration rate was similar between groups, with 21 in the flunitrazepam group and 20 in the midazolam group.
Outcome 1.4. Tolerance of procedure or participant co‐operation
There were no differences between groups for participant co‐operation, which was measured in this trial as the number of participants who were rated as 'not co‐operative' (9/41 in the midazolam group; 12/45 in the flunitrazepam group).
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
There were no differences between groups for difficulty performing procedures (8/41 in the midazolam group; 7/45 in the flunitrazepam group).
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.2. Onset time of sedation
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.3. Offset time of sedation
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
The risk of recalling a procedure was reduced in the midazolam group (11/41 in the midazolam group; 36/45 in the flunitrazepam group).
Outcome 2.5. Oversedation
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.7. Quality of recovery
Recovery was delayed in no participants randomized to midazolam, compared with 17/45 in the flunitrazepam group.
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the intravenous midazolam versus flunitrazepam comparison.
Intravenous midazolam versus placebo (comparison 5, outcome 1.1 to 1.6 and 2.1 to 2.10)
Intravenous midazolam was compared with placebo in five trials with 493 participants (Bhalla 2006; Fakheri 2010; Lavies 1988; Rolo 2012; Yuno 1996). The doses of midazolam used are presented in Table 7. We downgraded the evidence identified to inform the intravenous midazolam versus placebo comparison to low quality, due to concerns about study limitations and imprecision (Table 2).
2. Intravenous midazolam versus placebo comparison ‐ doses used.
| Study | Dose of midazolam |
| Bhalla 2006 | 5 mg (3 mg if over 65 years old) |
| Fakheri 2010 | mean 3.2 mg (SD 1.6) |
| Lavies 1988 | 2.5 ‐ 7.5 mg |
| Rolo 2012 | 0.05 mg/kg |
| Yuno 1996 | 0.05 mg/kg |
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
Participants who were randomized to midazolam were more sedated: the mean score on the Ramsay scale (1 to 6, with higher scores indicating the participant was more sedated) was 2.77 ± 1.19 in the midazolam group and 1.72 ± 0.50 in the placebo group) (Rolo 2012; 100 participants).
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
One trial (Rolo 2012; 100 participants) reported that fewer participants who received midazolam were anxious (3/50 in midazolam group; 15/50 in placebo group).
Outcome 1.3. Vital signs
Meta‐analysis of two trials (Bhalla 2006; Yuno 1996), with 207 participants (105 randomized to midazolam; 51%) revealed that midazolam was associated with a statistically significant reduction in the lowest recorded oxygen saturation (MD ‐1.50 %, 95% CI ‐1.77 to ‐1.23; I2 = 0%; Analysis 2.1). The rate of oxygen desaturation was reported by three trials (Bhalla 2006; Fakheri 2010; Rolo 2012), with 535 participants. Forty‐seven of 225 participants (21%) randomized to midazolam experienced oxygen desaturation in comparison to 41 of the 222 participants (18%) randomized to placebo (RR 1.12, 95% CI 0.79 to 1.58; I² = 0%; Analysis 2.2). Three trials (307 participants) reported on the rate of hypotension (Rolo 2012; Yuno 1996). No events occurred in either group in the Bhalla 2006 trial. One of 70 participants randomized to midazolam became hypotensive in comparison to three of 70 participants randomized to placebo (RR 0.43, 95% CI 0.07 to 2.78; participants = 140; studies = 2; I2 = 0%; Analysis 2.3). Two trials (207 participants) reported on the rate of tachycardia (Bhalla 2006; Yuno 1996). Eighteen of the 105 participants (17%) randomized to midazolam became tachycardic in comparison to 12 of the 152 participants (8%) randomized to placebo (RR 1.46, 95% CI 0.74 to 2.87; I² = 0%; Analysis 2.4). Two trials (207 participants) reported on the rate of hypertension (Bhalla 2006; Yuno 1996). Seven of the 105 participants (7%) randomized to midazolam became hypertensive in comparison to six of the 152 participants (4%) randomized to placebo (RR 1.15, 95% CI 0.43 to 3.13; I² = 0%; Analysis 2.5).
2.1. Analysis.
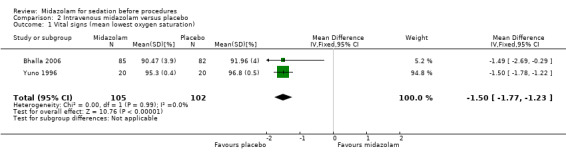
Comparison 2 Intravenous midazolam versus placebo, Outcome 1 Vital signs (mean lowest oxygen saturation).
2.2. Analysis.
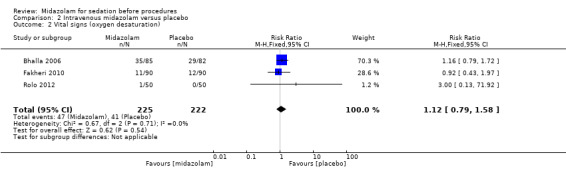
Comparison 2 Intravenous midazolam versus placebo, Outcome 2 Vital signs (oxygen desaturation).
2.3. Analysis.
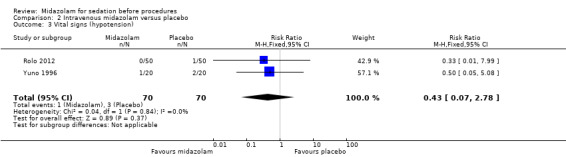
Comparison 2 Intravenous midazolam versus placebo, Outcome 3 Vital signs (hypotension).
2.4. Analysis.
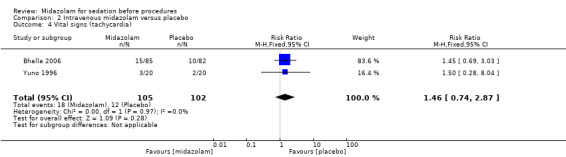
Comparison 2 Intravenous midazolam versus placebo, Outcome 4 Vital signs (tachycardia).
2.5. Analysis.
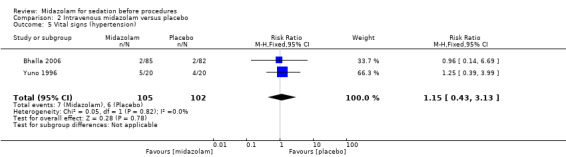
Comparison 2 Intravenous midazolam versus placebo, Outcome 5 Vital signs (hypertension).
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 1.5. Participant or proceduralist satisfaction
Proceduralist satisfaction was greater for participants randomized to midazolam in a trial of 40 participants (Yuno 1996): mean 0.9 (SD 0.22) for 20 participants randomized to midazolam; mean 2.7 (SD 0.22) for 20 participants randomized to placebo; P < 0.001; measured on a four‐point scale with lower scores indicating greater satisfaction. Participant satisfaction (measured as the number of participants reporting that they would not be willing to undergo another procedure with the same medication) was greater in the midazolam group (50/50 in midazolam group versus 41/50 in placebo group; P = 0.003; Rolo 2012). Participant satisfaction was also greater in the midazolam group of the trial of 40 participants (Yuno 1996); mean 1.45 (SD 0.15) for 20 participants randomized to midazolam; mean 3.1 (SD 0.16) for 20 participants randomized to placebo; P < 0.001; measured on a four‐point scale with lower scores indicating greater satisfaction.
Outcome 1.6. Incomplete procedures/difficulty performing procedure
Midazolam reduced the risk of difficulty performing the procedure in Bhalla 2006, with 167 participants (3/85 in midazolam group; 8/82 in placebo group; P = 0.129).
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.2. Onset time of sedation
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.3. Offset time of sedation
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.5. Oversedation
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.7. Quality of recovery
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.8. Discomfort/pain
There was no difference in the number of participants who had discomfort/pain in Bhalla 2006 (167 participants) who had upper gastrointestinal endoscopy (3/85 in the midazolam group; 4/82 in the placebo group; P = 0.876).
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the intravenous midazolam versus placebo comparison.
Outcome 2.10. Sedation reversal
Rolo 2012 (100 participants) reported on the requirement for sedation reversal; however, no events were reported in either group.
Intravenous midazolam versus propofol (comparison 6, outcomes 1.1 to 1.6 and 2.1 to 2.10)
We identified one trial with 17 participants that investigated the use of midazolam versus propofol before electrical cardioversion (Coll‐Vinent 2003). The midazolam group (eight participants) were given a dose of 0.2 mg/kg and the propofol group (nine participants) were given a dose of 1.5 mg/kg.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
Level of sedation (measured using the Ramsay scale) was similar between groups: median score of 6 with interquartile range 5 and 6 in both groups.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 1.3. Vital signs
There were no statistically significant differences in the vital signs measured between groups. Median systolic blood pressure after sedation in the midazolam group was 141 mmHg (range 99 ‐ 165) and 120 mmHg (range 100 ‐ 172) in the propofol group. One participant in the midazolam group experienced oxygen desaturation, with SpO₂ below 90%, and no events occurring in the propofol group.
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Secondary outcomes
Outcome 2.1. Duration of sedation
The median duration of sedation was lower in the propofol group compared with midazolam by 13 minutes (median 21 minutes with range from 1 ‐ 42 minutes in midazolam group; median 8 minutes with range from 3 ‐ 15 minutes in propofol group; P = 0.021).
Outcome 2.2. Onset time of sedation
The median onset time of sedation was 70 seconds lower in the propofol group compared with midazolam (median 120 seconds in the midazolam group; median 50 seconds in the propofol group, with range from 30 ‐ 100 seconds; P = 0.28).
Outcome 2.3. Offset time of sedation
The median offset time of sedation was 35 minutes lower in the propofol group compared with midazolam (median offset time was 45 minutes with range from 20 ‐ 60 minutes in the midazolam group; median offset time was 10 minutes with range from 5 ‐ 15 minutes in the propofol group; P = 0.002).
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 2.5. Oversedation
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 2.7. Quality of recovery
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the intravenous midazolam versus propofol comparison.
Oral midazolam versus chloral hydrate (comparison 7, outcomes 1.1 to 1.6 to 2.1 to 2.10)
Four trials (Akil 2005; D'Agostino 2000; Derakhshanfar 2013; Wheeler 2001) with 268 participants compared oral midazolam with chloral hydrate for sedation of children (Table 3). Doses for midazolam and chloral hydrate differed between the trials (Table 8).
3. Oral midazolam versus chloral hydrate doses.
| Study | Oral midazolam | Oral chloral hydrate |
| Akil 2005 | 0.6 mg/kg (max 15 mg) | 25 mg/kg (max 0.5 g) |
| D'Agostino 2000 | 0.5 mg/kg (max 10 mg) | 75 mg/kg (max 2 g) |
| Derakhshanfar 2013 | 0.5 mg/kg midazolam. Additional dose up to 8 mg for inadequate sedation | 80 mg/kg chloral hydrate followed by further dose 20 mg/kg if required 20 minutes later |
| Wheeler 2001 | 0.5 mg/kg midazolam; second dose 30 minutes after the initial dose, either 0.25 mg/kg | 75 mg/kg oral chloral hydrate; 30 minutes after the initial dose 25 mg/kg |
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
In one trial (Derakhshanfar 2013) with 160 participants, more children (n = 61; 76.25%) in the chloral hydrate group were rated at the highest level of sedation compared with the midazolam group (n = 12; 15%), measured using Wheeler's sedation scale (Wheeler 2001) with scores ranging from 1 = agitated to 4 = eyes closing spontaneously but response to minor stimuli. We downgraded the evidence from this trial to moderate quality, due to concerns about study limitations.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
Although a numerical rating of anxiety was reported in two trials with 88 participants, we did not conduct meta‐analysis because of differences in how this outcome was measured (by children using a numerical rating scale in D'Agostino 2000, and by parents using the Speilberger's Trait Anxiety Inventory in Akil 2005). There was no difference between groups in either D'Agostino 2000 (mean 1.4 (SD 2.26) in midazolam group; mean 2.5 (SD 0.97) in the chloral hydrate group; P = 0.07) or in Akil 2005 (mean 47.56 (SD 11.68) in midazolam group; 49.39 (SD 16) in chloral hydrate group; not significant at P < 0.05 level). We downgraded the evidence for this outcome to very low, due to concerns about study limitation, inconsistency and imprecision.
Outcome 1.3. Vital signs
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 1.4. Tolerance of procedure or participant co‐operation
Tolerance of the procedure was measured using the Frankl behaviour rating scale (range 1 to 4, with higher scores indicating better tolerance) in Akil 2005 with 35 participants. The difference between groups in tolerance was not statistically significant (mean 2.25 (SD 0.86) in the midazolam group; mean 2.5 (SD 1.1) in the chloral hydrate group; not significant at P < 0.05 level). Participant co‐operation was measured using the Houpt behavioural scale (range 1 to 6, with higher scores indicating better co‐operation) in Akil 2005. Participant co‐operation was rated better in the midazolam group (mean 4.94 (SD 1.12) in the midazolam group; mean 4.78 (SD 1) in the chloral hydrate group; P = 0.018).
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
All four trials (268 participants) included in this comparison reported on this outcome (Akil 2005; D'Agostino 2000; Derakhshanfar 2013; Wheeler 2001). There were 37 incomplete procedures in the midazolam group (from 144 participants; 26%) in comparison to seven incomplete procedures in the chloral hydrate group (124 participants; 6%) (RR 4.01, 95% CI 1.92 to 8.40; I² = 0%; Analysis 3.1). We downgraded the quality of evidence to moderate, due to concerns about study limitations.
3.1. Analysis.
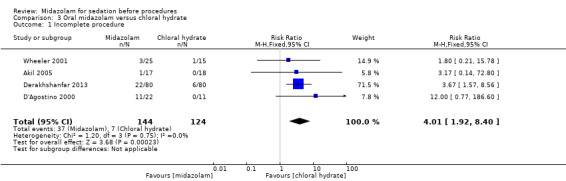
Comparison 3 Oral midazolam versus chloral hydrate, Outcome 1 Incomplete procedure.
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 2.2. Onset time of sedation
Meta‐analysis of the two trials (Derakhshanfar 2013; Wheeler 2001) with 200 participants (105 in midazolam group; 53%) revealed that there was no difference in the onset of sedation between midazolam and chloral hydrate (MD 8.37 minutes, 95% CI ‐3.49 to 20.23; Analysis 3.2). The results were inconsistent (I² = 98%) even though similar dosing regimens were used (Table 8).
3.2. Analysis.
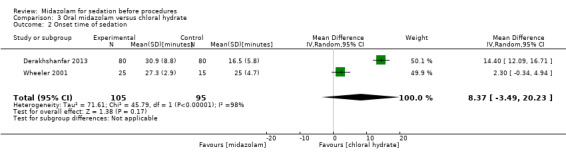
Comparison 3 Oral midazolam versus chloral hydrate, Outcome 2 Onset time of sedation.
Outcome 2.3. Offset time of sedation
There was no difference in the offset of sedation (MD ‐12.87 minutes, 95% CI ‐63.24 to 37.50; Analysis 3.3) between midazolam (127 participants; 55%) and chloral hydrate (106 participants; 45%) in meta‐analysis of three trials (D'Agostino 2000; Derakhshanfar 2013; Wheeler 2001) with 233 participants. There was unexplained heterogeneity (I² = 99%).
3.3. Analysis.
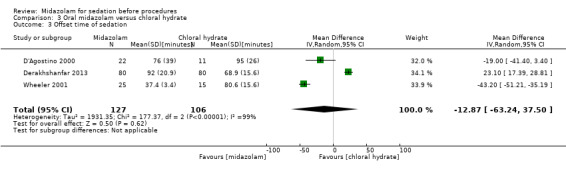
Comparison 3 Oral midazolam versus chloral hydrate, Outcome 3 Offset time of sedation.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 2.5. Oversedation
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 2.6. Disinhibition or excitation
There was no difference in disinhibition or excitation between midazolam or chloral hydrate groups in Derakhshanfar 2013 (13/67 in midazolam group; 10/70 in chloral hydrate group; P = 0.208). No events were observed in either group in Wheeler 2001 (40 participants).
Outcome 2.7. Quality of recovery
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the oral midazolam versus chloral hydrate comparison.
Oral midazolam versus diazepam (comparison 8, outcomes 1.1 to 1.6 and 2.1 to 2.10)
Oral midazolam was compared with diazepam in two trials with 122 participants (De Alencar 2010; Everitt 2002). In De Alencar 2010 the midazolam group was given a 15 mg dose and the diazepam group was given a 10 mg dose. The midazolam dose was 1.0 mg/kg (maximum 15 mg) and the diazepam dose was 0.5 mg/kg (maximum 10 mg) in Everitt 2002.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
More participants who received midazolam (14/17) in De Alencar 2010 were sedated to the level of 'somnolence' than the diazepam group (3/18). In a trial with 87 participants that compared oral midazolam with diazepam in children undergoing laceration repair, midazolam produced higher levels of sedation, regardless of whether sedation was rated by the investigator: mean 16 (SD 19) for 45 participants randomized to midazolam; mean 33 (SD 31) for 42 participants randomized to diazepam (Everitt 2002).
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 1.3. Vital signs
Changes in vital signs one hour after the administration of sedation were reported in one trial with 35 participants that compared oral midazolam with diazepam administered before undergoing blepharoplasty (De Alencar 2010). The mean change in systolic blood pressure after administration of midazolam was ‐1.9 mmHg (SD 3.06) compared with an increase of 3.8 mmHg (SD 6.77) after administration of diazepam. The mean change in diastolic blood pressure after administration of midazolam was ‐9.7 mmHg (SD 3.43) compared with an increase of 4.4 mmHg (SD 3.78) after administration of diazepam. The mean change in heart rate after administration of midazolam was ‐4.5 (SD 2.87) compared with ‐6.3 (SD 2.39) after administration of diazepam. The mean change in oxygen saturation after administration of midazolam was ‐0.31% (SD 0.38) compared with ‐1.35% (SD 1.06) after administration of diazepam.
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.2. Onset time of sedation
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.3. Offset time of sedation
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.5. Oversedation
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.7. Quality of recovery
Quality of recovery was reported in one trial (Everitt 2002). More children were reported to be drowsy after discharge in the midazolam group (18/35 compared with 10/31; P = 0.032).
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the oral midazolam versus diazepam comparison.
Oral midazolam versus diazepam and clonidine (comparison 9, outcomes 1.1 to 1.6)
Oral midazolam was compared with a combination of diazepam and clonidine in one trial with 34 participants (De Alencar 2010). Seventeen participants were randomized to receive 15 mg midazolam and 17 participants to receive 10 mg diazepam with 0.15 mg clonidine.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
Level of sedation was measured using the Michigan University Scale, with a range in scores from 0 = awake to 4 = unrousable to stimuli. Measures of central tendency were not reported in the article.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the oral midazolam versus diazepam and clonidine comparison.
Outcome 1.3. Vital signs
The mean change in systolic blood pressure one hour after administration of midazolam was ‐1.9 mmHg (SD 3.06) compared with an increase of 5.8 mmHg (SD 4.65) after administration of diazepam. The mean change in diastolic blood pressure after administration of midazolam was ‐9.7 mmHg (SD 3.43) compared with an increase of 6.1 mmHg (SD 2.54) after administration of diazepam. The mean change in heart rate after administration of midazolam was ‐4.5 (SD 2.87) compared with ‐9.2 (SD 1.98) after administration of diazepam. The mean change in oxygen saturation after administration of midazolam was ‐0.31% (SD 0.38) compared with ‐0.11% (SD 0.48) after administration of diazepam.
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the oral midazolam versus diazepam and clonidine comparison.
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the oral midazolam versus diazepam and clonidine comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
No trials reported this outcome for the oral midazolam versus diazepam and clonidine comparison.
Secondary outcomes
None of the secondary outcomes were reported by the included trials.
Oral midazolam versus ketamine (comparison 10, outcomes 1.1 to 1.6 and 2.1. to 2.10)
Younge 2001 compared 0.7 mg/kg oral midazolam with 10 mg/kg oral ketamine in a RCT for sedation before laceration repair in 59 children.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
Children who received ketamine were more deeply sedated (median score 2 versus 3 (lower score = deeper sedation)).
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 1.3. Vital signs
There was no significant difference between the medications for oxygen desaturation (2/29 in midazolam group; 1/30 in ketamine group).
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.2. Onset time of sedation
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.3. Offset time of sedation
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.5. Oversedation
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.6. Disinhibition or excitation
Six of the 29 participants randomized to midazolam experienced disinhibition/excitation in comparison to none of the 30 participants randomized to ketamine (P = 0.01).
Outcome 2.7. Quality of recovery
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.8. Discomfort/pain
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the oral midazolam versus ketamine comparison.
Oral midazolam versus placebo (comparison 11, outcomes 1.1 to 1.6 and 2.1 to 2.10)
Three trials (Akil 2005; Kuganeswaran 1999; Templeton 2010) with 176 participants compared midazolam administered via the oral route with a placebo (Table 4). Kuganeswaran 1999 was conducted in adults undergoing outpatient sigmoidoscopy (99 participants) and used a 7.5 mg dose of midazolam. Templeton 2010 was conducted in children undergoing removal of Kirschner wires (42 participants) and the 20 participants randomized to midazolam received 0.2 mL/kg of 1 mg/mL midazolam oral solution. Akil 2005 enrolled children undergoing micturating cystourethrography (35 participants). The 16 participants randomized to midazolam received a 0.6 mg/kg maximum 15 mg) dose.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
Kuganeswaran 1999 reported that levels of sedation became statistically significantly different between the midazolam and placebo groups 10 minutes after administration. However, the actual summary statistics were not reported in the article.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
There was no difference in anxiety between groups in Templeton 2010 (mean 1.7 (SD 2.4) for 20 participants randomized to midazolam; mean 2.6 (SD 2.9) for 22 participants randomized to placebo; P = 0.216), nor in Akil 2005 (mean Spielberger's Trait Anxiety Inventory score was 47.56 (SD 11.68) in the midazolam group compared with mean 52.78 (SD 9.61) in placebo group; P > 0.05). In contrast, there was a statistically significant reduction in numerical rating of anxiety in Kuganeswaran 1999 (mean 1.52 (SD 0.3) in midazolam group; mean 3.97 (SD 0.44) in placebo group; P < 0.0001).
Outcome 1.3. Vital signs
The rate of hypotension was reported in Kuganeswaran 1999. There was no difference between groups (2/51 midazolam; 0/48 placebo).
Outcome 1.4. Tolerance of procedure or participant co‐operation
Tolerance of the procedure was measured using the Frankl behaviour rating scale (range 1 to 4, with higher scores indicating better tolerance) in Akil 2005 with 35 participants. The difference between groups in tolerance was not statistically significant (mean 2.25 (SD 0.86) in midazolam group; mean 2.12 (SD 1.05) in placebo group). Participant co‐operation was measured using the Houpt behavioural scale (range 1 to 6, with higher scores indicating better co‐operation) in Akil 2005 with 35 participants. The mean participant co‐operation rating was 4.94 (SD 1.12) in the midazolam group compared with 4.12 (SD 1.05) in the placebo group.
Outcome 1.5. Participant or proceduralist satisfaction
Participant satisfaction (measured by participants' perception that they received inadequate sedation for their procedure) in Kuganeswaran 1999 was superior in the midazolam group (14/51 in midazolam group; 31/48 in placebo group; P < 0.05).
Outcome 1.6. Incomplete procedures/difficulty performing procedures
There were no incomplete procedures in either the midazolam or placebo groups in either Kuganeswaran 1999 or Templeton 2010, and only one procedure could not be completed in the midazolam group in Akil 2005.
Secondary outcomes
Outcome 2.1. Duration of sedation
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.2. Onset time of sedation
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.3. Offset time of sedation
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.4. Anterograde amnesia (defined by number of participants who recalled the procedure)
There was no difference in anterograde amnesia between midazolam (41/51 participants) and placebo (44/48 participants) in Kuganeswaran 1999.
Outcome 2.5. Oversedation
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.6. Disinhibition or excitation
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.7. Quality of recovery
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.8. Discomfort/pain
Kuganeswaran 1999, conducted with 99 participants undergoing sigmoidoscopy, reported that the administration of oral midazolam resulted in a statistically significant reduction in discomfort/pain compared with placebo (mean 2.56 (SD 0.49) in midazolam group; mean 4.62 (SD 1.49) in placebo group; P < 0.005).
Outcome 2.9. Allergic or anaphylactoid reactions
No trials reported this outcome for the oral midazolam versus placebo comparison.
Outcome 2.10. Sedation reversal
No trials reported this outcome for the oral midazolam versus placebo comparison.
Intranasal midazolam versus placebo (comparison 12, outcomes 1.1 to 1.6)
Two trials (149 participants) compared midazolam administered via the intranasal route for sedation before a procedure with placebo (Hollenhorst 2001; Stokland 2003). Hollenhorst 2001 compared intranasal midazolam with placebo in adults undergoing magnetic resonance imaging, while Stokland 2003 was conducted in children requiring cystourethrography.
Primary outcomes
Outcome 1.1. Level of sedation on a sedation assessment scale
A deeper level of sedation was observed in the midazolam group (mean 3.15 (SD 0.36) in midazolam group; mean 2.56 (SD 0.64) in placebo group; P < 0.001) in Hollenhorst 2001. We downgraded this evidence to moderate quality, due to concerns about study limitations arising from an unclear risk of bias from randomization sequence generation and allocation concealment.
Outcome 1.2. Numeric rating scale of anxiety or number of participants rated as anxious
Hollenhorst 2001 reported a marked reduction in a numerical rating of anxiety among participants who received midazolam prior to their magnetic resonance imaging procedure (mean 17.3 (SD 18.58) in midazolam group; mean 49.3 (SD 29.46) in placebo group; P < 0.001). We downgraded this evidence to moderate quality, due to concerns about study limitations arising from an unclear risk of bias from randomization sequence generation and allocation concealment.
Outcome 1.3. Vital signs
No trials reported this outcome for the intranasal midazolam versus placebo comparison.
Outcome 1.4. Tolerance of procedure or participant co‐operation
No trials reported this outcome for the intranasal midazolam versus placebo comparison.
Outcome 1.5. Participant or proceduralist satisfaction
No trials reported this outcome for the intranasal midazolam versus placebo comparison.
Outcome 1.6. Incomplete procedures/difficulty performing procedures
One of the primary outcomes, incomplete procedures, was reported in both of these trials (Hollenhorst 2001; Stokland 2003). Meta‐analysis of results from the 149 participants showed that the administration of midazolam compared with placebo had no impact on incomplete procedures (no incomplete procedures from 75 participants in midazolam group compared with six incomplete procedures from 74 participants in placebo group; RR 0.14, 95% CI 0.02 to 1.12; I² = 0%; Analysis 4.1). It should be noted that results from a further study that is awaiting classification, which is published in German and requires translation, is likely to have an impact on the effect estimates, so we have downgraded the quality of this evidence to 'low'.
4.1. Analysis.
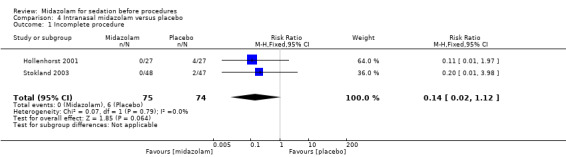
Comparison 4 Intranasal midazolam versus placebo, Outcome 1 Incomplete procedure.
Secondary outcomes
None of the secondary outcomes for this comparison were reported by the included trials.
Discussion
Summary of main results
The largest amount of evidence involved the comparison of intravenous midazolam with diazepam for endoscopic procedures (Table 1). The administration of intravenous midazolam produced greater anterograde amnesia, as the risk of participants recalling the procedures was reduced by 55% (RR 0.45, 95% CI 0.30 to 0.66; 587 participants; 9 trials; Analysis 1.7). However, it is unclear how important anterograde amnesia is in regard to the effectiveness of sedation, as there was no difference in participant satisfaction between midazolam and diazepam (MD 2.17, 95% CI ‐0.51 to 4.85; scale ranged from 0 to 100 with higher scores equating to better satisfaction; 91 participants; 2 trials; Analysis 1.3). We judged the quality of this evidence to be very low or low.
Intravenous midazolam may be more effective than placebo for procedural sedation because fewer participants were rated as anxious (one trial; 100 participants; RR 0.20, 95% CI 0.06 to 0.65; Rolo 2012). However, we judged the quality of the evidence to be low (Table 2).
Based on meta‐analysis of four trials (Akil 2005; D'Agostino 2000; Derakhshanfar 2013; Wheeler 2001) with 268 participants, midazolam was found to be inferior to chloral hydrate for sedation before procedures in children in regard to the number of incomplete procedures (RR 4.01, 95% CI 1.92 to 8.40; Analysis 3.1) However, we rated the quality of the evidence as moderate (Table 3).
In two trials (De Alencar 2010; Everitt 2002) conducted with 122 participants (results could not be combined in meta‐analysis because of differences in measurement), midazolam produced deeper levels of sedation than diazepam when administered orally. There were no other data reported in the trials to make a definitive determination as to the superiority of midazolam over diazepam in terms of pain, anxiety or the participants' satisfaction with or tolerance of the procedures.
The evidence for the effect of oral midazolam compared with placebo on anxiety was inconsistent (Table 4) and we judged it to be of very low quality, meaning that we are very uncertain about the effect estimates.
The administration of intranasal midazolam compared with placebo had no impact on incomplete procedures (RR 0.14, 95% CI 0.02 to 1.12; 149 participants; 2 trials; I² = 0%; Analysis 4.1). It should be noted that Hollenhorst 2001 reported a reduction in a numerical rating of anxiety (100 mm line, with 0 meaning “I am not anxious at all,” and 100 meaning “I am extremely anxious”) among participants who received midazolam prior to their magnetic resonance imaging procedure (MD ‐32.00, 95% CI ‐45.14 to ‐18.86). This result is likely due to the higher level of sedation observed in the midazolam group (MD 0.59, 95% CI 0.31 to 0.87; measured on a five‐point scale with a higher score indicating higher level of sedation). We downgraded this evidence to moderate quality, due to concerns about study limitations arising from an unclear risk of bias from randomization sequence generation and allocation concealment (Table 5).
Overall completeness and applicability of evidence
The evidence from trials included in this review and conducted in the 1980s and 1990s that compared midazolam with diazepam have been superceded by more contemporary sedative regimens that include continuous infusions of propofol or bolus doses of benzodiazepines and opioids, which are titrated to effect throughout the procedure (Qadeer 2005; Thomson 2010; Wang 2013).
We were not able to collect data on the primary outcome from all the included trials. It is not clear that selective reporting of outcomes, if present, would favour midazolam over placebo or another medication for sedation before a procedure.
It should be noted that the participant populations were from a range of elective, mostly adult outpatients with low risk of anaesthesia‐related adverse events (American Society of Anesthesiology Class < III). The results should therefore not be considered generalizable to people undergoing urgent procedures or people at higher risk of adverse events.
This review cannot be used to draw conclusions about the harms of midazolam administered for sedation before procedures. Only allergic or anaphylactoid reactions were a prespecified outcome and no trials reported on them. Further, as only randomized controlled trials were included in this review, we were unable to detect rare adverse events that are known to be associated with the administration of sedation in large observational studies, such as death arising from undetected respiratory depression and hypoxia.
Quality of the evidence
Incomplete reporting of trial designs led to challenges in interpreting the risk of bias. Attrition was low, as is expected with short‐term trials of sedative medications. There were few data for most outcomes. As such, there was considerable uncertainty in the estimates of the effects of the interventions due to inconsistency and imprecision. For these reasons, we downgraded the evidence to moderate, low and very low quality for all outcomes included in the 'Summary of findings' tables.
Potential biases in the review process
Due to relative ambiguity in the primary outcome set in the original protocol for this review (Morão 2011), we needed to make decisions about the handling of the data after seeing it, which may have introduced bias to the review process. Another potential source of bias is that we did not seek trial protocols because most included trials were published prior to the establishment of clinical trial registries. It is also possible that if future trials use alternative (either higher or lower) doses of midazolam, results may be different from those found in the trials included in this review. For these reasons, we have been cautious about the interpretations of results of the evidence syntheses.
We were unable to classify 10 potentially eligible papers because they were not published in English and we were not able to have them translated in time (Frisancho 1996; Mendes 1986; Mignonsin 1994; Münte 2002; Thakur 2003; Wild 1988), we could not locate current contact details of authors (Bardhan 1984; Green 1984; Theroux 1993) or results were published only in abstract form and we were unable to retrieve further information from the study authors (Ogden 1993) (Characteristics of studies awaiting classification). Four of the unclassified studies could have been included in the comparison of intravenous midazolam versus diazepam (Bardhan 1984; Frisancho 1996; Green 1984; Mignonsin 1994). One of the studies could contribute further evidence about the effectiveness of intranasal midazolam versus placebo (Münte 2002). Two unclassified studies compared intramuscular midazolam with hydrocodonum for sedation before bronchoscopy (Mendes 1986; Wild 1988). One unclassified study compared oral midazolam with tricolofos sodium for sedation of children undergoing echocardiography (Thakur 2003).
Agreements and disagreements with other studies or reviews
Some results of our review are consistent with a previous meta‐analysis (McQuaid 2008), which focused only on endoscopic procedures. McQuaid 2008 also found that midazolam has a greater amnesic effect than diazepam undergoing endoscopic procedures and that participants preferred to undergo procedures with sedation rather than without. Guidelines for sedation during endoscopy recommend that a combination of a benzodiazepine and an opioid can be used to adequately sedate most patients (Cohen 2006). Results of our review do not provide any evidence to suggest otherwise.
Single‐agent sedation without additional analgesia is more often used in contemporary clinical practice for 'motion control', mainly in children for diagnostic procedures that are not painful. It is important to note that our results contrast with recommendations from the National Institute of Health and Clinical Excellence in the United Kingdom regarding the use of midazolam and chloral hydrate for paediatric sedation during diagnostic procedures (NICE 2010). Both of these medications are recommended in the guidelines if sedation is required for painless diagnostic procedures, whereas results from our meta‐analysis suggest that chloral hydrate is superior to midazolam in terms of procedural completion. A potential reason for the disagreement is that the trials included in our systematic review were not included in the guidelines report (NICE 2010) even though three of the four studies were published prior to publication of the guidelines (Akil 2005; D'Agostino 2000; Wheeler 2001). We are unsure whether they were excluded from the review or were not uncovered in the literature search strategy used by the guideline developers.
Authors' conclusions
Implications for practice.
The evidence from comparison 1 (intravenous midazolam versus diazepam) is not relevant to current clinical practice because it has been superceded by more contemporary sedative regimens that include continuous infusions of propofol or bolus doses of benzodiazepines and opioids, which are titrated to effect throughout the procedure. We do not have sufficient high‐quality evidence to determine whether midazolam produces more effective sedation than other medications in any specific population included in this review. Moderate‐quality evidence demonstrated that midazolam administered orally to children who require sedation for motion control during diagnostic procedures produced less effective sedation compared with chloral hydrate in terms of the ability to complete procedures. For this reason, chloral hydrate could be considered a preferred option. Patients appear to prefer to be sedated with midazolam when undergoing a procedure than receive no sedation at all. For this reason, sedation with midazolam could be offered if it is clinically appropriate to do so.
Implications for research.
A focus on more contemporary anaesthetic approaches in future systematic reviews focused on the use of midazolam for sedation would be important in order to account for the outdated single‐agent approaches to sedation for endoscopic procedures included in this review. Standardizing measurement and definitions for outcomes important to the practice of sedation, such as anxiety, would also help to strengthen the evidence base.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to acknowledge the authors of the protocol of this review (Morão 2011).
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care Review Group) for her help and availability during the preparation of this review.
We would like to thank Christian Byhahn, Jesus Barea Mendoza, Marc Gentili, Eva Madrid, Mukadder Orhan Sungur, Javier Zamora, Patrick Brass, Djillali Annane and Ozlem Serpil Cakmakkaya for assistance extracting data and risk of bias assessment from papers not published in English.
We would like to thank Mike Bennett (content editor), Vibeke Horstmann (statistical editor), Brett Doleman, Robert Seal (peer reviewers), Rosanna Fennessy (consumer editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. CENTRAL search
#1 MeSH descriptor Midazolam explode all trees #2 (midazolam near (intranasal or endonasal or intravenous or oral or intramuscular or rectal or sub?lingual)):ti,ab #3 (#1 OR #2) #4 MeSH descriptor Conscious Sedation explode all trees #5 MeSH descriptor Anesthesia Recovery Period explode all trees #6 MeSH descriptor Anesthesia, Intravenous explode all trees #7 MeSH descriptor Preanesthetic Medication explode all trees #8 (anxiolysis or sedat* or pre?medicat* or analges* or surgery or endoscop* or fibroscopy or biopsy or tomography or magnetic resonance or lumbar puncture):ti,ab #9 (#4 OR #5 OR #6 OR #7 OR #8) #10 (#3 AND #9)
Appendix 2. Ovid MEDLINE search
1. Midazolam/ or (midazolam adj5 (intranasal or endonasal or intravenous or oral or intramuscular or rectal or sub?lingual)).ti,ab. 2. Conscious Sedation/ or Anesthesia Recovery Period/ or Anesthesia, Intravenous/ or exp Preanesthetic Medication/ or (anxiolysis or sedat* or pre?medicat* or analges* or surgery or endoscop* or fibroscopy or biopsy or tomography or magnetic resonance or lumbar puncture).ti,ab. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Ovid EMBASE search
1. midazolam.ti,ab. 2. sedation/ or anesthetic recovery/ or intravenous anesthesia/ or premedication/ or (anxiolysis or sedat* or pre?medicat* or analges* or surgery or endoscop* or fibroscopy or biopsy or tomography or magnetic resonance or lumbar puncture).ti,ab. 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Study Selection Form
| First author | Journal/Conference Proceedings etc | Year |
Study eligibility
| RCT | Relevant participants [age >16, type of procedures (diagnostic, therapeutic), medical specialty (surgical, non‐surgical)] |
Relevant interventions [comparisons between midazolam with placebo, midazolam with other drugs, timings and doses of midazolam administration] |
Relevant outcomes (Midazolam's efficacy and level of sedation) (duration of sedation, onset time of sedation, offset time of sedation, retrograde and anterograde amnesia duration, oversedation, disinhibition/excitation, quality of recovery, discomfort, pain, allergic or anaphylactoid reactions, use of flumazenil) |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
Appendix 5. Eligible trial forms
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| A | |||
| B | |||
| C |
Appendix 6. Data Extraction Form
|
Outcomes |
Reported in paper (circle) |
Subgroups | Information available in paper (circle) |
| Primary outcome effective sedation | Yes/No | Age (<16) | Yes/No |
| Type of procedure ‐ diagnostic | Yes/No | ||
| Type of procedure ‐ therapeutic | Yes/No | ||
| Surgical specialty | Yes/No | ||
| Secondary outcomes | Yes/No | ||
| Outcome 1 duration of sedation | Yes/No | ||
| Outcome 2 onset time of sedation | Yes/No | ||
| Outcome 3 offset time of sedation | Yes/No | ||
| Outcome 4 retrograde and anterograde amnesia | Yes/No | ||
| Outcome 5 oversedation | Yes/No | ||
| Outcome 6 discomfort, pain | Yes/No | ||
| Outcome 7 disinhibition/excitation | Yes/No | ||
| Outcome 8 quality of recovery | Yes/No | ||
| Outcome 9 allergic or anaphylactoid reactions | Yes/No | ||
| Outcome 10 need for sedation reversal | Yes/No |
| For continuous data (with a separate copy for each relevant subgroup) | |||||||
| Code of paper | Outcomes | Unit of measurement | Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Primary Outcome effective sedation | |||||||
| Outcome 1 duration of sedation | |||||||
| Outcome 2 onset time of sedation | |||||||
| Outcome 3 offset time of sedation | |||||||
| Outcome 4 retrograde and anterograde amnesia | |||||||
| Outcome 5 oversedation | |||||||
| Outcome 6 discomfort, pain | |||||||
| Outcome 7 disinhibition/excitation | |||||||
| Outcome 8 quality of recovery | |||||||
| Outcome 9 allergic or anaphylactoid reactions | |||||||
| Outcome 10 need for sedation reversal | |||||||
| For dichotomous data (with a separate copy for each relevant subgroup) | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| Primary outcome effective sedation | |||
| Outcome 1 duration of sedation | |||
| Outcome 2 onset time of sedation | |||
| Outcome 3 offset time of sedation | |||
| Outcome 4 retrograde and anterograde amnesia | |||
| Outcome 5 oversedation | |||
| Outcome 6 discomfort, pain | |||
| Outcome 7 disinhibition/excitation | |||
| Outcome 8 quality of recovery | |||
| Outcome 9 allergic or anaphylactoid reactions | |||
| Outcome 10 need for sedation reversal | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
|
Freehand space for writing actions such as contact with study authors and changes Were original authors contacted? Yes/No What questions were addressed? |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| Trial characteristics | |
| Further details | |
| Single centre/multicenter | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose/frequency of administration | |
| Duration of treatment (state weeks/months, etc, if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow up reported in this paper (state weeks, months or years or if not | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan 5.0 | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Appendix 7. Quality Assessment of Eligible Trial Form
Methodological quality
Trial:__________
| Random sequence generation | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Comment on allocation by review authors or included study quote concerning allocation: | Low risk of bias (Random) |
| High risk of bias (e.g. alternate) | |
| Unclear | |
|
Allocation concealment Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Comment on allocation concealment by review authors or included study quote concerning allocation: | Low risk of bias |
| High risk of bias | |
| Unclear | |
| Blinding | |
| Participant | High/ Low/ Unclear Risk |
| Outcome assessor | High/ Low/ Unclear Risk |
| Other (please specify) | High/ Low/ Unclear Risk |
| Comment on blinding by review authors or included study quote concerning allocation: | |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals/dropouts described? Yes ? No ? Not clear ?
Number of withdrawals/dropouts
Reasons for withdrawals/dropouts
Description of withdrawals/dropouts
Discuss if appropriate
Data and analyses
Comparison 1. Intravenous midazolam versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants rated as 'anxious' | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.39, 1.62] |
| 2 Participant co‐operation (not co‐operative) | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.48, 1.84] |
| 3 Participant satisfaction | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [‐0.51, 4.85] |
| 4 Proceduralist satisfaction | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 1.09 [‐10.43, 12.60] |
| 5 Difficulty performing procedures (rated as difficult to perform procedure) | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.41, 1.07] |
| 6 Onset of sedation | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.76, 0.16] |
| 7 Anterograde amnesia (defined as the number of participants who recalled procedure) | 9 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.30, 0.66] |
| 8 Quality of recovery (rated as delayed recovery) | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.63] |
| 9 Discomfort/Pain | 5 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.24, 1.49] |
1.9. Analysis.
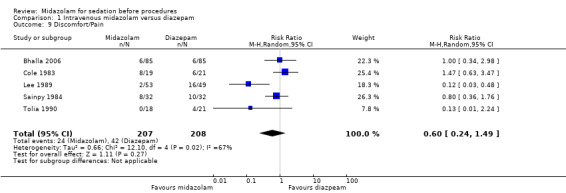
Comparison 1 Intravenous midazolam versus diazepam, Outcome 9 Discomfort/Pain.
Comparison 2. Intravenous midazolam versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vital signs (mean lowest oxygen saturation) | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐1.77, ‐1.23] |
| 2 Vital signs (oxygen desaturation) | 3 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.79, 1.58] |
| 3 Vital signs (hypotension) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.07, 2.78] |
| 4 Vital signs (tachycardia) | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.74, 2.87] |
| 5 Vital signs (hypertension) | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.43, 3.13] |
Comparison 3. Oral midazolam versus chloral hydrate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incomplete procedure | 4 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.01 [1.92, 8.40] |
| 2 Onset time of sedation | 2 | 200 | Mean Difference (IV, Random, 95% CI) | 8.37 [‐3.49, 20.23] |
| 3 Offset time of sedation | 3 | 233 | Mean Difference (IV, Random, 95% CI) | ‐12.87 [‐63.24, 37.50] |
Comparison 4. Intranasal midazolam versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incomplete procedure | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akil 2005.
| Methods | Parallel‐group single‐centre randomized controlled trial conducted in Turkey | |
| Participants | 53 children requiring micturating cystourethrogram with sedation (39 girls, 14 boys; mean age of 5.8 ± 3.5 years) | |
| Interventions | Oral midazolam 0.6 mg/kg (max 15 mg) versus chloral hydrate 25 mg/kg (max 0.5 g) and placebo (saline) 15 ‐ 30 minutes before procedure | |
| Outcomes |
Measured during procedures: Incomplete procedures Anxiety (measured during the procedure using Spielberger's Trait Anxiety Inventory) Participant co‐operation (measured during the procedure using Houpt behavioural scale; range 1 ‐ 6 with higher scores indicating better co‐operation) Tolerance of procedure (measured during the procedure using Frankl behaviour rating score; range 1 ‐ 4 with higher scores indicating better tolerance) Duration of sedation Onset of sedation Oversedation |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes included in this review were not blinded ‐ only assessment of image quality was performed by a blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Aktogu 1994.
| Methods | Parallel‐group single‐centre randomized controlled trial conducted in Turkey | |
| Participants | 32 adults undergoing bronchoscopy (mean age midazolam group 49.4 ± 13.3 and diazepam group 50.9 ± 12.1; 50% men in both groups) | |
| Interventions | Midazolam 0.06 mg/kg administered intravenously Diazepam 0.15 mg/kg administered intravenously |
|
| Outcomes |
Measured 5 and 10 minutes after start of procedure: Level of sedation on a sedation assessment scale (only the percentage of participants who scored in the 'awake' rank of a sedation scale that ranged from 0 to 4 (0 = awake and 4 = reactive to pain but no verbal communication) that was measured 5 minutes after sedation was administered) Measured 24 hours after procedure: Anterograde amnesia (defined by number of participants who recalled the procedure) |
|
| Notes | Conflicts of interest or funding sources were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (no mention of randomization method, just a statement that participants were randomized) |
| Allocation concealment (selection bias) | Unclear risk | Unclear (no mention of allocation method, just a statement that participants were randomized) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear (no mention) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only statistical analysis of the questionnaires was reported to have been performed by a blinded statistician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Bell 1988.
| Methods | Parallel‐group single‐centre randomized controlled trial conducted in the UK | |
| Participants | 102 adults undergoing upper gastrointestinal endoscopy (mean age midazolam group was 62.8 ± 16.1 years and 65.8 ± 11.5 in the diazepam group) | |
| Interventions | Intravenous midazolam 2.5 mg or 1 mg for elderly (mean 6.0 ± 2.8) vs intravenous diazepam 5 mg or 2 mg for elderly (mean 11.5 ± 6.7) | |
| Outcomes | Vital signs (change in oxygen saturation from baseline to post‐sedation) | |
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Bhalla 2006.
| Methods | Parallel‐group single‐centre randomized controlled trial conducted in India in 2000 | |
| Participants | 252 adults undergoing diagnostic or therapeutic upper gastrointestinal endoscopy. Authors stated that there were no difference in baseline characteristics of the 3 groups | |
| Interventions | 1) Intravenous midazolam 5 mg (3 mg if older than 65) 2) Intravenous diazepam 5 mg (3 mg if older than 65) 3) Intravenous saline (placebo) |
|
| Outcomes |
Measured during the procedure: Vital signs (minimum oxygen saturation) Vital signs (oxygen desaturation < 90%) Vital signs (tachycardia defined as heart rate > 140 bpm) Vital signs (bradycardia) Vital signs (hypertension defined as systolic blood pressure > 200 mmHg) Incomplete procedures Difficulty performing procedure Discomfort/pain |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Endoscopist and investigator recording haemodynamic data were not aware of the nature of the medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Endoscopist and investigator recording haemodynamic data were not aware of the nature of the medications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Bianchi Porro 1988.
| Methods | Single‐centre cross‐over randomized controlled trial conducted in Italy | |
| Participants | 23 adults undergoing upper gastrointestinal endoscopy (14 men and 9 women; mean weight 60.7 kilograms; age range 20 to 48 years; mean age 32.5) | |
| Interventions | 1) Intravenous midazolam 0.07 mg/kg 2) Intravenous Diazepam 0.15 mg/kg |
|
| Outcomes |
Measured during the procedure: Vital signs (blood pressure 5 minutes after sedation) Vital signs (heart rate 5 minutes after sedation Level of sedation using a sedation assessment scale 4 Participant co‐operation Measured 2 hours after the procedure: Quality of recovery Measured 24 hours after the procedure: Anterograde amnesia (defined by number of participants who recalled the procedure) |
|
| Notes | At least 30 days between procedures We were unable to locate contact details of the authors to access pre‐cross‐over data Conflicts of interest or funding sources were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drugs prepared and administered by physician not performing endoscopy or assessments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Drugs prepared and administered by physician not performing endoscopy or assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Cole 1983.
| Methods | Parallel‐group randomized controlled trial conducted in 2 sites in the USA | |
| Participants | 40 adult participants (American Society of Anesthesiology physical classification status I ‐ II) undergoing upper gastrointestinal endoscopy for the first time (aged 18 ‐ 70 years; authors reported that the groups were comparable in all parameters evaluated: age, sex, weight, race (white/non‐white), psychoactive drug history, duration of procedure, injection speed, data collection site) | |
| Interventions | 1) Intravenous midazolam 5 mg bolus with 2.5 ‐ 3.75 mg increments at 30‐ ‐ 60‐second intervals as required. Half doses for elderly 2) Same dose for diazepam |
|
| Outcomes |
Measured during the procedure: Onset of sedation Vital signs (change in heart rate) Vital signs (change in respiration rate) Vital signs (change in diastolic blood pressure) Discomfort/pain (measured in the study as absolute number with pain) Measured after the procedure: Proceduralist satisfaction Measured the day after the procedure: Participant satisfaction Quality of recovery (number of participants reporting unusual sensations the day after the procedure) Anterograde amnesia (numerical rating from 0 to 100 with a lower number indicating greater amnesia) |
|
| Notes | Authors declared that Hoffman‐La Riche Inc. provided financial assistance for the study, but did not disclose the role of the funder in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participants and the endoscopist were masked to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both the participant and the endoscopist were masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Coll‐Vinent 2003.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Spain | |
| Participants | 32 consecutive adults undergoing cardioversion for supraventricular arrhythmia in an emergency department | |
| Interventions | Randomized to 1 of 4 treatment arms (all intravenous administration): 1) 0.2 mg/kg midazolam 2) 0.2 mg/kg etomidate 3) 1.5 mg/kg propofol 4) 0.2 mg/kg midazolam and flumazenil 0.5 mg bolus followed by 0.5 mg infusion for 1 hour If induction was not achieved within 3 ‐ 5 minutes, supplementary doses of etomidate (0.05 mg/kg), midazolam (0.05 mg/kg) or propofol (0.5 mg/kg) were injected at 1‐minute intervals until the desired effect was obtained |
|
| Outcomes |
Measured during the procedure: Level of sedation using a sedation assessment scale (Ramsay scale) Vital signs (systolic blood pressure after sedation) Vital signs (desaturation < 90%) Duration of sedation Onset of sedation Measured after the procedure: Offset time of sedation |
|
| Notes | Only median values reported for continuous outcomes (skewed distributions because of small sample size). We did not include the midazolam and flumazenil group in our review because combinations of medications with midazolam were excluded. The authors declared that no outside funding or support was received for the study. No other conflicts of interest were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Córdova 1992.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Mexico | |
| Participants | 60 adults aged 18 ‐ 65 years who underwent upper digestive endoscopy. Participants with allergy to midazolam or diazepam were excluded | |
| Interventions | Intravenous diazepam (0.15 mg/kg) Intravenous midazolam (0.10 mg/kg) |
|
| Outcomes |
Measured during the procedure: Tolerance of procedure Measured after the procedure: Duration of sedation Anterograde amnesia (defined by number of participants who recalled the procedure) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explanation about method to generate allocation. Just stated “randomly allocated to two groups” |
| Allocation concealment (selection bias) | Unclear risk | Authors describe no method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Methods include a mention of “double blinding”, but no further details about who and how |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method include a mention of “double blinding”, but no further details about who and how |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
D'Agostino 2000.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in USA | |
| Participants | 40 2‐months – 8‐year‐old children requiring neuroimaging with sedation. Average age 31 ± 23 months and 45% were boys. | |
| Interventions | Oral midazolam 0.5 mg/kg (max 10 mg) vs oral chloral hydrate 75 mg/kg (max 2 g) Could received additional dose (50% of original dose) if required |
|
| Outcomes |
Measured during the procedure:
1) Numerical rating of anxiety 2) Duration of sedation 3) Incomplete procedures |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Children were administered freshly‐prepared, identically‐appearing, cherry‐flavoured liquids in body weight equivalent volumes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the participant nor any of the investigators were aware of the active component given to individual participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals were reported. Randomized children who did not complete the protocol included 1 with respiratory distress, 1 who ate a full meal prior to intended drug administration, 1 who fell asleep after intravenous line placement and 4 who cancelled their appointments after randomization |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
De Alencar 2010.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Brazil | |
| Participants | 70 adult participants (American Society of Anesthesiology physical classification status I ‐ II)undergoing lower eyelid blepharoplasty under local anaesthetic. Mean age in the groups ranged from 57.9 ± 10.2 years to 64 ± 14.4 years. There was more women than men in each group. | |
| Interventions | Intravenous administration of: 1) diazepam 10 mg and clonidine 0.15 mg 2) diazepam 10 mg 3) Midazolam 15 mg 4) Midazolam 15 mg and clonidine 0.15 mg (not used in this review) |
|
| Outcomes |
Measured before the procedure: Level of sedation using a sedation assessment scale (Michigan University Scale range from 0 = awake to 4 = unarousable to stimuli) Change in vital signs at 1 hour after administration of medication |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Derakhshanfar 2013.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Iran | |
| Participants | 160 children aged 2 to 7 years requiring lumbar puncture (mean age: 3.4 ± 1.9 years old in chloral hydrate, 3.6 ± 2.6 years old in midazolam group; 42 girls and 38 boys in chloral hydrate group and 48 girls and 32 boys in the midazolam group) | |
| Interventions | Oral administration of: 1) 80 mg/kg chloral hydrate followed by further dose 20 mg/kg if required 20 minutes later 2) 0.5 mg/kg midazolam. Additional dose up to 8 mg for inadequate sedation |
|
| Outcomes |
Measured before the procedure: Level of sedation using a sedation assessment scale (Wheeler's sedation level with scores ranging from 1 = agitated to 4 = eyes closing spontaneously but response to minor stimuli) Onset of sedation Offset of sedation Incomplete procedures Disinhibition/excitation Measured after the procedure: Quality of recovery (prolonged sedation) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the investigator, proceduralist and nurse were 'unaware' of the drug used, the parents (who also rated sedation) did know which |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although the investigator, proceduralist and nurse were 'unaware' of the drug used, the parents (who also rated sedation) did know which medication was administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Everitt 2002.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Australia | |
| Participants | 129 children aged 1 ‐ 4 years with uncomplicated lacerations that required 2 or more sutures (42 excluded from the review due to comparison between different routes of administration). Similar at baseline for age, heart rate, respiratory rate, blood pressure, oxygen saturation, anxiety score and laceration characteristics (summary statistics were not reported) | |
| Interventions | Oral administration of: 1) 0.5 mg/kg diazepam 2) 1 mg/kg midazolam 3) 0.4 mg/kg intranasal midazolam (not included) |
|
| Outcomes |
Measured during the procedure immediately after suturing: Level of sedation on a sedation assessment scale (rated on a scale of 0 to 100 (lower score = better sedation) by proceduralist, nurse, parent and investigator) Measured 24 to 48 hours after the procedure: Quality of recovery (proportion of participants who were 'drowsy' at home) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A nurse not involved in the participants' care performed the drug administration. The investigator (I.J.E.), suturing doctor, and nurse assisting with suturing were unaware which sedative had been given. Parents were asked not to reveal which drug or route of delivery their child had received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A nurse not involved in the participants' care performed the drug administration. The investigator (I.J.E.), suturing doctor, and nurse assisting with suturing were unaware which sedative had been given. Parents were asked not to reveal which drug or route of delivery their child had received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how much data were missing for parents' assessments |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Fakheri 2010.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Iran in 2008 | |
| Participants | 180 adults over 18 years of age without serious comorbidities undergoing upper gastrointestinal endoscopy (mean age: 46.9 ± 17.5 years in midazolam group and 47 ± 17.5 years in placebo group; 49% men in midazolam group and 43% men in placebo group) | |
| Interventions | 1) Intravenous midazolam ‐ mean dose 3.2 (1.6) mg 2) Saline placebo |
|
| Outcomes |
Measured during the procedure: Vital signs (oxygen desaturation) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded participants who required more than 10 mg midazolam for sedation |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Unclear risk | None expected |
Gilvarry 1990.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in the United Kingdom | |
| Participants | 60 adults undergoing upper gastrointestinal endoscopy (mean age: 41.4 in diazepam group and 42.2 in midazolam group; 46% men in diazepam group and 43% men in diazepam group) | |
| Interventions | 10 mg intravenous midazolam 20 mg intravenous diazepam |
|
| Outcomes |
Measured 24 hours after the procedure: Participant satisfaction (measured in the trial as the participant considered sedation was inadequate) Anterograde amnesia (recalled procedures) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Stratified randomised order" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An author who did not know which medication had been administered assessed outcomes 24 hours after the procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Hollenhorst 2001.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Germany | |
| Participants | 54 participants aged 18 to 65 years old scheduled for magnetic resonance imaging (MRI) for the first time (mean age: 43 ± 14.6 years in the midazolam group and 49 ± 11.7 years in the placebo group; 48% men in midazolam group and 37% men in placebo group) | |
| Interventions | 1) Intranasal midazolam 4 mg 2) placebo |
|
| Outcomes | 1) Level of sedation measured 15 minutes after medication and after MRI. Participant sedation was evaluated by 1of the authors using a 5‐point sedation scale (1 = agitated, non co‐operative; 2 = alert, restless; 3 = calm, eyes spontaneously open; 4 = drowsy, responds to minor stimulation; 5 = asleep, rousable but does not respond to minor stimulation) 2) Numerical rating of anxiety measured 15 minutes after medication and after MRI. Visual Analogue Scale of Anxiety comprised an undivided 100‐mm line, with 0 meaning “I am not anxious at all,” and 100 meaning “I am extremely anxious.” Participants were instructed to mark 1 point on the line that corresponded to the intensity of their anxiety at that moment 3) Incomplete procedures |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding of assessor of sedation level. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Korttila 1985.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Finland | |
| Participants | 76 adults undergoing rigid diagnostic bronchoscopy (25 participants randomized to 'low dose' midazolam were excluded from the review). Mean age: 55 ± 11 in the diazepam group and 55 ± 11 in the midazolam group. 70% men in the diazepam group and 58% men in the midazolam group | |
| Interventions | Intravenous administration of: Midazolam 0.05 mg/kg (not used in the review) Midazolam 0.1 mg/kg Diazepam 0.2 mg/kg |
|
| Outcomes |
Measured during the procedure: Participant and proceduralist satisfaction (0 = poor, 100 = good) Measured 2 hours after procedure: Anterograde amnesia (recalled procedures) Quality of recovery (could not walk in straight line at 2 hours after procedure) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participant and the bronchoscopist were unaware of the identity of the drug being administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigator was unaware of the identity of the drug being administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Kuganeswaran 1999.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in USA | |
| Participants | 99 adults undergoing sigmoidoscopy (age 51 ± 2 years; 13 men and 35 women) | |
| Interventions | 1) Oral midazolam 7.5 mg (participants were asked to swish medication in mouth 15 times before swallowing to allow improved absorption by the oral mucosa) 2) Placebo |
|
| Outcomes |
Measured during the procedure: Level of sedation using a sedation assessment scale (scores ranged from 0 = awake to 3 = asleep, responsive only to direct verbal or physical stimulus) Numerical rating of anxiety reported by proceduralist and participant (0 = no anxiety; 10 = severe anxiety) Discomfort/pain reported by proceduralist and participant (0 = no pain; 10 = severe pain) Vital signs (hypotension) Incomplete procedures Measured after the procedure: Participant satisfaction (refuse repeat procedure with same sedation) Participant satisfaction (participant considered sedation was not adequate) Anterograde amnesia (defined by number of participants who recalled the procedure) |
|
| Notes | Conflicts of interest were not reported but it was noted that Roche Pharmaceuticals provided the study medications and "funded in part" the study. The role of the funder in design, analysis or reporting was not disclosed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind ‐ "the study medication was prepared by the inpatient pharmacy and physicians, nurses and patients were blinded to its identity" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind ‐ "the study medication was prepared by the inpatient pharmacy and physicians, nurses and patients were blinded to its identity" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew before receiving medication |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Lavies 1988.
| Methods | Single‐centre parallel group randomized controlled trial conducted in USA | |
| Participants | 120 adults undergoing upper gastrointestinal endoscopy (mean age: 58 years in placebo group, 55 in diazepam group, 50 in midazolam group; 55% men in the placebo group, 70% men in the diazepam group, 48% men in the midazolam group) | |
| Interventions | Intravenous administration of: 1) Midazolam 2.5 ‐ 7.5 mg 2) Diazepam 2.5 ‐ 10 mg 3) Placebo |
|
| Outcomes |
Measured during the procedure: Vital signs Tolerance of procedure |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random‐number sequence used but "The study was continued until 40 patients were included in each group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant was unaware of medication |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Lazaraki 2007.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Greece in 2004 | |
| Participants | 126 adults who were 23 to 84 years of age undergoing colonoscopy for the first time | |
| Interventions | Intravenous administration of: 1) 25 ‐ 50 mcg fentanyl (mean 36 mcg) 2) 2 ‐ 5 mg midazolam (mean 4.6 mg) |
|
| Outcomes |
Measured during the procedure: Discomfort, pain (0 = very well/no discomfort, 4 = unbearable) Incomplete procedures Vital signs Sedation reversal Measured after the procedure: Offset of sedation Participant satisfaction (measured in the trial as willingness to undergo another procedure with same medication) Anterograde amnesia (recalled procedures) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Monitoring was performed by a single specialist nurse blinded to the randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Lee 1989.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Jamaica | |
| Participants | 149 adults undergoing upper GI endoscopy for the first time. 79 men with a mean age of 52.5 years (range 18 to 81) and 70 women with a mean age of 46 years (17 to 82 years) | |
| Interventions | Intravenous administration of: Diazepam to a maximum of 0.15 mg/kg Midazolam to a maximum of 0.07 mg/kg No sedation (not used in this review) (47 participants) |
|
| Outcomes |
Measured during the procedure: Participant co‐operation Difficulty performing procedure Measured 24 to 48 hours after the procedure: Anterograde amnesia (defined by number of participants who recalled the procedure) Discomfort/pain (measured in the trial as 'uncomfortable') |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The endoscopist who was unaware of the drug used completed a questionnaire to assess the participant co‐operation and difficulty performing procedure outcomes, but anterograde amnesia and discomfort/pain were not assessed in a blinded fashion (no information about participant blinding) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Rolo 2012.
| Methods | Multicentre (2 sites) parallel‐group randomized controlled trial conducted in Portugal | |
| Participants | 100 adults undergoing fibreoptic bronchoscopy (Mean age was 56 ± 14 years (range 18 ‐ 79 years); 66% were men | |
| Interventions | 0.05 mg/kg intravenous midazolam Placebo |
|
| Outcomes |
Measured during the procedure: Level of sedation on a sedation assessment scale Vitals signs Disinhibition/excitation (measured in the trial as 'agitation') Sedation reversal Measured 1 hour after the procedure: Participant satisfaction (willingness to undergo another procedure with the same medication) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did state it was double‐blind but no specific information provided about how this was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Sainpy 1984.
| Methods | Parallel‐group single‐centre randomized controlled trial conducted in France | |
| Participants | 64 adults who underwent a gastroduodenal endoscopy. Exclusion of myasthenia gravis, pregnancy, past history of adverse reaction to benzodiazepines, long‐term treatment with psychotropes | |
| Interventions | Intravenous midazolam: 0.1 mg under 65 yrs and 0.085 mg over 65 yrs infused in 30 seconds Intravenous diazepam: 0.2 mg under 65 yrs and 0.15 mg over 65 yrs infused in 30 seconds |
|
| Outcomes |
Measured during the procedure: Discomfort/pain Measured after the procedure Duration of sedation Anterograde amnesia (defined by number of participants who recalled the procedure) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The gastroenterologist was the only person blinded to sedative drug |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The gastroenterologist was the only person blinded to sedative drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Stokland 2003.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Sweden | |
| Participants | 95 children referred for voiding cystourethrography (median age 2.2 years in midazolam group and 3.2 years in placebo group) | |
| Interventions | Intranasal midazolam given in a dose of 0.2 mg/kg body weight with a maximum dose of 5 mg Placebo group was given saline 0.9 mg/ml sterile solution |
|
| Outcomes | Incomplete procedures | |
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | The children were stratified by gender and randomly allocated to 1 of 2 groups, midazolam or placebo, by opening a sealed envelope prepared in blocks of 4 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The substances were available in bottles with serial numbers, but were otherwise of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Takrouri 1988.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Jordan | |
| Participants | 120 adults undergoing endoscopy (mean age 39 ± 0.6 in diazepam group, 41 ± 0.9 in flunitrazepam group and 34 ± 0.6 in midazolam group) | |
| Interventions | Mean doses: diazepam 5 mg; flunitrazepam 0.65 mg; midazolam 5.8 mg (all intravenous) | |
| Outcomes |
Measured during the procedure: Level of sedation on a sedation assessment scale Number of participants rated as 'anxious' Difficulty performing procedure (rated as difficult to perform procedure) Participant co‐operation (not co‐operative) Measured after the procedure (before discharge): Anterograde amnesia (defined by number of participants who recalled the procedure) Quality of recovery (rated as delayed recovery) |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Templeton 2010.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in UK | |
| Participants | 42 children requiring removal of Kirschner wires in an orthopaedic outpatient department (average age 7.1 years; range 3.6 to 12.3 years) | |
| Interventions | 1) 0.2 mL/kg of 1 mg/mL oral midazolam 2) Placebo |
|
| Outcomes | Numerical rating of anxiety | |
| Notes | Funding source was reported (research grant from Beatrice Jennings Trust Fund at The General Infirmary at Leeds) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by the pharmacy department using random‐number tables |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes each containing a code number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Corresponding coded bottles contained either midazolam 1.0 mg/mL or the placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis not used |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Tolia 1990.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in USA | |
| Participants | 41 children undergoing upper GI endoscopy | |
| Interventions | Intravenous administration of: 1) 0.1 ‐ 0.15 mg/kg midazolam 2) 0.2 ‐ 0.4 mg/kg diazepam |
|
| Outcomes |
Measured during procedures: Participant co‐operation Measured 24 hours after procedures: Anterograde amnesia (recalled procedures) Discomfort, pain |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both endoscopist and participant were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both endoscopist and participant were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not analysed as intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Wheeler 2001.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in USA | |
| Participants | 40 children < 5 years of age undergoing echocardiography (13 boys (52%) in midazolam group and 10 boys (66%) in chloral hydrate group) | |
| Interventions | 1) 75 mg/kg oral chloral hydrate 2) 0.5 mg/kg oral midazolam Children requiring further sedation (as determined by the assigned nurse) received a second dose of the same medication 30 minutes after the initial dose, either 25 mg/kg chloral hydrate or 0.25 mg/kg midazolam |
|
| Outcomes |
Measured during the procedure: Level of sedation on a sedation assessment scale Incomplete procedures Onset time of sedation Disinhibition/excitation Measured after the procedure: Offset time of sedation |
|
| Notes | Conflicts of interest or funding sources were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Echocardiographer blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Whitwam 1983.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in the United Kingdom | |
| Participants | 100 adult ASA I ‐ II participants undergoing upper GI endoscopy (29 men (58%) in midazolam group and 30 men (60%) in diazepam group; mean age 42 ± 19.9 in midazolam group and 44 ± 18.5 in diazepam group) | |
| Interventions | Intravenous administration of: 1) midazolam 0.07 mg/kg 2) diazepam 0.15 mg/kg |
|
| Outcomes |
Measured during the procedure: Number of participants rated as 'anxious' Difficulty performing procedure Participant co‐operation Onset of sedation Offset of sedation Oversedation Measured on discharge from recovery area: Anterograde amnesia (recalled procedures) Measured after the procedure (questionnaire sent to participants ‐ no timeframe reported) Quality of recovery |
|
| Notes | Reported that 2 of the authors were supported by Roche Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind to investigators assessing participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Younge 2001.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in the United Kingdom | |
| Participants | 59 children ages 1 to 7 needing laceration repair (mean age 4.1 years in both groups; 54% boys in midazolam group and 53% boys in ketamine group) | |
| Interventions | Oral midazolam 0.7 mg/kg Oral ketamine 10 mg/kg |
|
| Outcomes |
Measured during the procedure: Level of sedation on a sedation assessment scale Offset time of sedation Vital signs Disinhibition/excitation |
|
| Notes | Reported that there were no conflicts of interest or funding received for the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug solutions were pre‐prepared by the hospital pharmacy and numbered sequentially, randomly containing 1 or other drug |
| Allocation concealment (selection bias) | Low risk | Drug solutions were pre‐prepared by the hospital pharmacy and numbered sequentially, randomly containing 1 or other drug |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | None expected |
Yuno 1996.
| Methods | Single‐centre parallel‐group randomized controlled trial conducted in Japan | |
| Participants | 40 adults undergoing colonoscopy for polyp removal | |
| Interventions | 0.05 mg/kg intravenous midazolam Placebo |
|
| Outcomes |
Measured during the procedure: Level of sedation on a sedation assessment scale Participant satisfaction (measured in the trial as participant‐assessed adequacy of sedation) Proceduralist satisfaction (measured in the trial as proceduralist‐assessed adequacy of sedation) Vital signs |
|
| Notes | Reported that a grant from the Osaka Association for Prevention of Adult Diseases funded the study. No conflicts of interest were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Noted it was double‐blind but no specific information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Noted it was double‐blind but no specific information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence that measured outcomes were not reported (trial protocols were not sought for confirmation) |
| Other bias | Low risk | No withdrawals reported |
ASA: American Society of Anesthesiology BPM: beats per minute GI; gastrointestinal HR: heart rate MRI: magnetic resonance Imaging RCT: randomized controlled trial SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonta 2003 | Both groups could receive midazolam if initial sedation not effective |
| Brouillette 1989 | Meperidine administered in both groups |
| Dere 2010 | Fentanyl used in both groups |
| Mui 2005 | Additional IV midazolam used in placebo group |
| Muttu 2005 | Intra‐procedural sedation used |
| Nascimento 2007 | Meperidine used in all groups if sedation inadequate |
| Ristikankare 1999 | Flumazenil for all participants |
| Ristikankare 2000a | Flumazenil for all participants |
| Ristikankare 2000b | Flumazenil for all participants |
| Sajedi 2006 | Fentanyl used in both groups |
| Salmon 1992 | Temazepam for all groups |
| Sandler 1992 | Quasi‐RCT |
| Sherry 1989 | Nitrous oxide used on both groups |
| Tamayo 1993 | Fentanyl used in both groups |
| Tesoro 2007 | Quasi‐RCT |
| Uygur‐Bayramiçli 2002 | Flumazenil at end of procedure |
| Weinstein 2010 | Post hoc analysis |
| Yildirim 2006 | Placebo administered in different route of administration |
Only a selection of the studies the authors excluded after review of full text are listed, as we identified a large number of potentially relevant articles in initial screening.
IV: intravenous
RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Bardhan 1984.
| Methods | RCT |
| Participants | Endoscopy |
| Interventions | Intravenous midazolam versus diazepam; Unclear whether intra‐procedural sedation was used |
| Outcomes | Full details not known |
| Notes | Could not locate current contact details for authors |
Frisancho 1996.
| Methods | RCT |
| Participants | GI endoscopy |
| Interventions | Intravenous midazolam versus diazepam |
| Outcomes | Full details not known |
| Notes | Article published in Spanish. Awaiting data extraction from a second person |
Green 1984.
| Methods | RCT |
| Participants | GI endoscopy |
| Interventions | Intravenous midazolam versus diazepam; Time point at which medications was administered is not clear |
| Outcomes | Full details not known |
| Notes | Could not locate current contact details for authors |
Mendes 1986.
| Methods | RCT |
| Participants | Bronchoscopy |
| Interventions | Intramuscular midazolam versus hydrocodonum |
| Outcomes | Full details not known |
| Notes | Article published in German. Awaiting data extraction from a second person |
Mignonsin 1994.
| Methods | RCT |
| Participants | GI endoscopy |
| Interventions | Intravenous midazolam versus diazepam |
| Outcomes | Full details not known |
| Notes | Article published in German. Awaiting data extraction from a second person |
Münte 2002.
| Methods | RCT |
| Participants | MRI scan |
| Interventions | Intranasal midazolam versus placebo |
| Outcomes | Full details not known. 4/27 randomized to placebo did not complete procedure, whereas all participants randomized to midazolam completed the procedure. |
| Notes | Article published in German. Awaiting data extraction from a second person |
Ogden 1993.
| Methods | RCT |
| Participants | Children undergoing bone marrow aspiration/biopsy |
| Interventions | Oral midazolam 1 mg/kg Oral ketamine 10 mg/kg |
| Outcomes | Full details not known |
| Notes | The design of this trial is unclear because the abstract reports that only 29 participants were enrolled but 89 procedures are included. It also states it is a non‐cross‐over study. We could not locate current contact details for authors |
Thakur 2003.
| Methods | RCT |
| Participants | Children undergoing echocardiography |
| Interventions | Oral midazolam versus triclofos sodium |
| Outcomes | Full details not known. Children who received midazolam had a quicker onset of sedation and recovery from sedation |
| Notes | Article published in French. Awaiting data extraction by 2 people |
Theroux 1993.
| Methods | RCT |
| Participants | Children requiring laceration repair |
| Interventions | Intranasal midazolam versus placebo versus no sedation |
| Outcomes | Placebo group results were reported with control |
| Notes | Contacted authors by email on 12th December 2014 for clarification about just the results of the placebo group, but did not receive a response |
Wild 1988.
| Methods | RCT |
| Participants | Bronchoscopy |
| Interventions | Intramuscular midazolam versus hydrocodonum |
| Outcomes | Full details not known |
| Notes | Article published in German. Awaiting data extraction from a second person |
GI: gastrointestinal MRI: magnetic resonance imaging RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00563069.
| Trial name or title | Premedication with oral midazolam in patients undergoing rigid cystoscopy: a randomized, double‐blind, placebo‐controlled trial |
| Methods | RCT |
| Participants | Elective rigid cystoscopy Either diagnostic or therapeutic Age between 18 and 80 |
| Interventions | Oral midazolam |
| Outcomes | Pain score during the procedure as assessed by visual analogue scale |
| Starting date | September 2005 |
| Contact information | Shirley YW Liu |
| Notes | NCT00563069 |
NCT01925898.
| Trial name or title | A randomized, clinical trial of oral midazolam versus oral ketamine for sedation during laceration repair |
| Methods | RCT |
| Participants | Any child with laceration requiring sedation |
| Interventions | Oral midazolam versus oral ketamine |
| Outcomes | Number of participants requiring IV sedation (Time frame: Ddring the procedure ‐ up to 1 hour) Pain score: visual analogue score (VAS) by a parent (Time frame: during the procedure ‐ up to 1 hour) |
| Starting date | August 2013 |
| Contact information | Orit Rubinstein |
| Notes | NCT01925898 |
Puttapitakpong 2015.
| Trial name or title | Oral midazolam for sedation in esophagogastroduodenoscopy(EGD) |
| Methods | RCT |
| Participants | Scheduled to undergo elective diagnostic EGD American Society of Anesthesia (ASA) criteria to be class 1 to 2 |
| Interventions | Oral midazolam |
| Outcomes | Difference of anxiety score (Time frame: asked the participant 5 minutes before EGD and then after fully recovery from sedation) |
| Starting date | October 2013 |
| Contact information | Chaipichit Puttapitakpong, Doctor |
| Notes | NCT01990937 |
ASA: American Society of Anesthesiology Physical Classification Status EGD: oesophagogastroduodenoscopy IV: intravenous RCT: randomized controlled trial VAS: visual analogue scale
Differences between protocol and review
The authors of this review carried out the protocol as originally designed by a different set of authors (Morão 2011).
We did not search the LILACS database, as we decided that it would probably not result in the identification of relevant studies not already captured through searches of CENTRAL, MEDLINE, and EMBASE.
We removed 'time to sedation' from the primary outcome section because we could not determine how this was different to onset of sedation, which was one of the secondary outcomes.
We removed 'correct dosing' from the primary outcome section because there was no definition for this term provided in the protocol.
We changed 'vital signs' in the primary outcome section from vital signs '(heart rate, electrocardiogram, blood pressure, respiratory rate and oxygen saturation)' to 'vital signs (heart rate, blood pressure, respiratory rate, and oxygen saturation)'.
We removed Ramsay scale, motor activity assessment scale and sedation scale from the primary outcomes section and replaced these with the collective term 'sedation assessment scale'.
We changed 'numeric rating scale of pain' in the primary outcomes section to 'numerical rating scale of anxiety or number of participants rated as anxious' because discomfort/pain was already also listed in the secondary outcomes section.
We changed 'use of flumazenil' in the secondary outcomes section to 'sedation reversal'.
We added participant and proceduralist satisfaction as one of the ways that 'effective sedation' could be evaluated.
We added 'tolerance of the procedure or participant co‐operation' as one of the ways that 'effective sedation' could be evaluated.
'Adequate sedation level' was not displayed in the 'Summary of findings' tables because no clear definition for this outcome was provided in the protocol. Instead we used 'level of sedation on a sedation assessment scale'.
Anxiolysis was not defined in the original protocol so we collected data from included trials in both the continuous (numerical rating of anxiety) and dichotomous (rated as anxious or not) outcomes sections of the data extraction form. We decided to report either a numerical rating of anxiety or ratings of being anxious in the primary 'Summary of findings' tables, as 'low levels of anxiolysis' was not defined clearly in the original protocol.
As retrograde or anterograde amnesia was listed in both the continuous and dichotomous outcome sections of the data extraction forms, we attempted to collect both the duration of amnesia and its occurrence. However, only the occurrence of anterograde amnesia was reported. For this reason, we changed the outcome, 'Retrograde and anterograde amnesia duration: defined by loss of the ability of recalling events that occurred before being sedated or creating new memories whilst sedated, respectively' to 'Anterograde amnesia (defined by number of participants who recalled the procedure)'. We used the number of participants who recalled the procedure as opposed to the number who did not recall the procedure, in order to reduce the risk of calculation errors because this is how the data were presented in the articles.
In order to reduce the risk of calculation errors, we reported the number of incomplete procedures instead of 'ability to complete procedures' as planned in the protocol, because this is how the data were presented in the articles.
We included 'Discomfort/pain' in the 'Summary of findings' tables instead of 'Discomfort' as planned in the protocol, to make the tables consistent with the outcomes section of the review.
We had planned in the protocol to consider repeated observations as separate outcomes and group them accordingly for analysis. However, the studies included in the review reported the change in time‐separated observations (such as the change in oxygen saturation from before to after the administration of sedation), so we did not conduct this.
We changed the section 'Dealing with missing data' in the protocol from 'For dichotomous data, we will perform intention‐to‐treat (ITT) analyses to include all participants randomized to the intervention groups. For continuous data, we will contact the authors of the primary studies to supply missing information from participants who withdrew from the studies so that we can include in the analysis the last individual data before the withdrawal of the participant. If studies do not report withdrawals, or no further data are available, we will assume there were none.' to 'We analysed the available data on an intention‐to‐treat basis. If studies did not report withdrawals, we assumed there were none.' The reason we changed this is because data collected over multiple time points were all intra‐procedural and there were no missing data, so we did not need to collect 'the last individual data before the withdrawal of the participant.'
We now state that we excluded studies where dexmedetomidine was the comparator, as there is another Cochrane review about sedation for this medication (Shailaja 2013).
We now state that we used an available‐case analysis as the default for meta‐analysis and we also considered sensitivity analysis using best‐case (all participants who withdrew did not experience the event) and worst‐case (all participants who withdrew did experience the event) scenarios for any missing data. No outcomes measured with continuous variables had missing data that needed to be included in the meta‐analyses.
Contributions of authors
Co‐ordinating the review: Aaron Conway (AC) Undertaking manual searches: AC Screening search results: AC, John Rolley (JR) Organizing retrieval of papers: AC Screening retrieved papers against inclusion criteria: AC, JR Appraising quality of papers: AC, JR Abstracting data from papers: AC, JR Writing to authors of papers for additional information: AC Providing additional data about papers: Obtaining and screening data on unpublished studies: AC, JR Data management for the review: AC, JR Entering data into Review Manager 5 (Review Manager 2014): AC, JR Data entry checking: AC, JR RevMan statistical data: AC, JR Other statistical analysis not using RevMan: AC, JR Interpretation of data: AC, JR, Joanna Sutherland (JS) Statistical inferences: AC, JR Writing the review: AC, JR, JS Securing funding for the review: n/a Performing previous work that was the foundation of the present study: n/a Guarantor for the review (one author): AC Person responsible for reading and checking review before submission: AC
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Aaron Conway: none known
John Rolley is currently researching aspects of pre‐procedural sedation and fasting for people undergoing diagnostic and interventional cardiac catheterization laboratory procedures. He has no relationships with any company providing drugs associated with this systematic review.
Joanna Sutherland was a member of the working party reviewing ANZCA PSO9 prior to 2014 and was the Clinical Lead for the NSW Agency for Clinical Innovation working party which developed the Minimum Standards for Safe Procedural Sedation.
Edited (no change to conclusions)
References
References to studies included in this review
Akil 2005 {published data only}
- Akil I, Ozkol M, Ikizoglu O, Polat M, Tuncyurek O, Taskin O, et al. Premedication during micturating cystourethrogram to achieve sedation and anxiolysis. Pediatric Nephrology 2005;20(8):1106‐10. [DOI] [PubMed] [Google Scholar]
Aktogu 1994 {published data only}
- Aktogu S, Hasegeli L, Dereli M. Comparison of the sedative effects of midazolam and diazepam during fibreoptic bronchoscopy. Turk Anesteziyoloji ve Reanimasyon 1994;22:157‐60. [Google Scholar]
Bell 1988 {published data only}
- Bell GD, Morden A, Coady T, Lee J, Logan RF. A comparison of diazepam and midazolam as endoscopy premedication assessing changes in ventilation and oxygen saturation. British Journal of Clinical Pharmacology 1988;26(5):595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhalla 2006 {published data only}
- Bhalla A, Sood A, Gupta V, Duseja A. Cardiorespiratory compromise under conscious sedation during upper gastrointestinal endoscopy. Journal of the College of Physicians and Surgeons 2006;16(9):585‐9. [PubMed] [Google Scholar]
- Sachdeva A, Bhalla A, Sood A, Duseja A, Gupta V. The effect of sedation during upper gastrointestinal endoscopy. Saudi Journal of Gastroenterology 2010;16(4):280‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bianchi Porro 1988 {published data only (unpublished sought but not used)}
- Bianchi Porro G, Baroni S, Parente F, Lazzaroni M. Midazolam versus diazepam as premedication for upper gastrointestinal endoscopy: a randomized, double‐blind, crossover study. Gastrointestinal Endoscopy 1988;34(3):252‐4. [DOI] [PubMed] [Google Scholar]
Cole 1983 {published data only}
- Cole SG, Brozinsky S, Isenberg JI. Midazolam, a new more potent benzodiazepine, compared with diazepam: a randomized, double‐blind study of preendoscopic sedatives. Gastrointestinal Endoscopy 1983;29(3):219‐22. [DOI] [PubMed] [Google Scholar]
Coll‐Vinent 2003 {published data only}
- Coll‐Vinent B, Sala X, Fernández C, Bragulat E, Espinosa G, Miró O, et al. Sedation for cardioversion in the emergency department: analysis of effectiveness in four protocols. Annals of Emergency Medicine 2003;42(6):767‐72. [DOI] [PubMed] [Google Scholar]
Córdova 1992 {published data only}
- Córdova Villalobos JA, Rojas Hernández R, Hernández Ortiz TM, Ramírez Barba EJ. Diazepam versus midazolam as pre‐endoscopic medication. Revista de Gastroenterología de México 1992;57(4):238‐41. [PubMed] [Google Scholar]
D'Agostino 2000 {published data only}
- D'Agostino J, Terndrup T. Chloral hydrate versus midazolam for sedation of children for neuroimaging: A randomized clinical trial. Pediatric Emergency Care 2000;16(1):1‐4. [DOI] [PubMed] [Google Scholar]
De Alencar 2010 {published data only}
- Alencar VM, Gonçalves RD, Cruz AA. Oral medication with diazepam or midazolam associated or not with clonidine for oculoplastic office surgery under local anesthesia. Ophthalmic Plastic and Reconstructive Surgery 2010;26:269‐72. [DOI] [PubMed] [Google Scholar]
Derakhshanfar 2013 {published data only}
- Derakhshanfar H, Kordi MM, Amini A, Shojahee M. A comparative study on the sedative effect of oral midazolam and oral chloral hydrate medication in lumbar puncture. Acta Medica Croatica 2013;67(5):401‐5. [PubMed] [Google Scholar]
Everitt 2002 {published data only}
- Everitt IJ, Barnett P. Comparison of two benzodiazepines used for sedation of children undergoing suturing of a laceration in an emergency department. Pediatric Emergency Care 2002;18(2):72‐4. [DOI] [PubMed] [Google Scholar]
Fakheri 2010 {published data only}
- Fakheri H, Kiasari A, Taghvaii T, Hosseini V, Mohammadpour R, Nasrollah A, et al. Assessment the effect of midazolam sedation on hypoxia during upper gastrointestinal endoscopy. Pakistan Journal of Biological Sciences 2010;13(4):152‐7. [DOI] [PubMed] [Google Scholar]
Gilvarry 1990 {published data only}
- Gilvarry JM, Craig M, Fielding JF. Short report: sedation for upper gastrointestinal endoscopy‐‐diazepam versus midazolam. Alimentary Pharmacology & Therapeutics 1990;4(4):423‐5. [DOI] [PubMed] [Google Scholar]
Hollenhorst 2001 {published data only}
- Hollenhorst J, Münte S, Friedrich L, Heine J, Leuwer M, Becker H, et al. Using intranasal midazolam spray to prevent claustrophobia induced by MR imaging. American Journal of Roentgenology 2001;176(4):865‐8. [DOI] [PubMed] [Google Scholar]
Korttila 1985 {published data only}
- Korttila K, Tarkkanen J. Comparison of diazepam and midazolam for sedation during local anaesthesia for bronchoscopy. British Journal of Anaesthesia 1985;57(6):581‐6. [DOI] [PubMed] [Google Scholar]
Kuganeswaran 1999 {published data only}
- Kuganeswaran E, Clarkston WK, Cuddy PG, Quiason SG, Pandya PK, Dierenfeldt WT, et al. A double‐blind placebo controlled trial of oral midazolam as premedication before flexible sigmoidoscopy. The American Journal of Gastroenterology 1999;94(11):3215‐9. [DOI] [PubMed] [Google Scholar]
Lavies 1988 {published data only}
- Lavies NG, Creasy T, Harris K, Hanning CD. Arterial oxygen saturation during upper gastrointestinal endoscopy: influence of sedation and operator experience. The American Journal of Gastroenterology 1988;83(6):618‐22. [PubMed] [Google Scholar]
Lazaraki 2007 {published data only}
- Lazaraki G, Kountouras J, Metallidis S, Dokas S, Bakaloudis T, Chatzopoulos D, et al. Single use of fentanyl in colonoscopy is safe and effective and significantly shortens recovery time. Surgical Endoscopy 2007;21(9):1631‐6. [DOI] [PubMed] [Google Scholar]
Lee 1989 {published data only}
- Lee MG, Hanna W, Harding H. Sedation for upper gastrointestinal endoscopy: a comparative study of midazolam and diazepam. Gastrointestinal Endoscopy 1989;35(2):82‐4. [DOI] [PubMed] [Google Scholar]
Rolo 2012 {published data only}
- Rolo R, Mota PC, Coelho F, Alves D, Fernandes G, Cunha J, et al. Sedation with midazolam in flexible bronchoscopy ‐ a prospective study. Revista Portugesa de Pneumologia 2012;18(5):226‐32. [DOI] [PubMed] [Google Scholar]
Sainpy 1984 {published data only}
- Sainpy D, Boileau S, Vicari F. Comparative study of intravenous midazolam and diazepam used as sedative agents during gastroscopy. Annales Françaises d Anesthèsie et de Rèanimation 1984;3(3):177‐80. [DOI] [PubMed] [Google Scholar]
Stokland 2003 {published data only}
- Stokland E, Andréasson S, Jacobsson B, Jodal U, Ljung B. Sedation with midazolam for voiding cystourethrography in children: a randomised double‐blind study. Pediatric Radiology 2003;33(4):247‐9. [DOI] [PubMed] [Google Scholar]
Takrouri 1988 {published data only}
- Takrouri MS, Toukan A, Badran I, Sbeih Z, Hunjul N. Diazepam, flunitrazepam and midazolam for elective endoscopy‐‐a comparative study. Middle East Journal of Anaesthesiology 1988;9(6):529‐36. [PubMed] [Google Scholar]
Templeton 2010 {published data only}
- Templeton P, Burton D, Cullen E, Lewis H, Allgar V, Wilson R. Oral midazolam for removal of Kirschner wires in the children's orthopaedic outpatient department: A randomized controlled trial. Journal of Pediatric Orthopedics 2010;30(2):130‐4. [DOI] [PubMed] [Google Scholar]
Tolia 1990 {published data only}
- Tolia V, Fleming SL, Kauffman RE. Randomized, double‐blind trial of midazolam and diazepam for endoscopic sedation in children. Developmental Pharmacology and Therapeutics 1990;14(3):141‐7. [PubMed] [Google Scholar]
Wheeler 2001 {published data only}
- Wheeler DS, Jensen RA, Poss WB. A randomized, blinded comparison of chloral hydrate and midazolam sedation in children undergoing echocardiography. Clinical Pediatrics 2001;40(7):381‐7. [DOI] [PubMed] [Google Scholar]
Whitwam 1983 {published data only}
- Whitwam JG, Al‐Khudhairi D, McCloy RF. Comparison of midazolam and diazepam in doses of comparable potency during gastroscopy. British Journal of Anaesthesia 1983;55(8):773‐7. [DOI] [PubMed] [Google Scholar]
Younge 2001 {published data only}
- Younge PA, Kendall JM. Sedation for children requiring wound repair: a randomised controlled double blind comparison of oral midazolam and oral ketamine. Emergency Medicine Journal 2001;18(1):30‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuno 1996 {published data only}
- Yuno K, Iishi H, Tatsuta M, Hifumi K, Omori M. Intravenous midazolam as a sedative for colonoscopy: a randomized, double‐blind clinical trial. Alimentary Pharmacology & Therapeutics 1996;10(6):981‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bonta 2003 {published data only}
- Bonta PI, Kok MF, Bergman JJ, Brink GR, Lemkes JS, Tytgat GN, et al. Conscious sedation for EUS of the esophagus and stomach: a double‐blind, randomized, controlled trial comparing midazolam with placebo. Gastrointestinal Endoscopy 2003;57(7):842‐7. [DOI] [PubMed] [Google Scholar]
Brouillette 1989 {published data only}
- Brouillette D, Leventhal R, Kumar S, Berman D, Kajani M, Yoo Y, et al. Midazolam versus diazepam for combined esophogastroduodenoscopy and colonoscopy. Digestive Diseases and Sciences 1989;34(8):1265‐71. [DOI] [PubMed] [Google Scholar]
Dere 2010 {published data only}
- Dere K, Sucullu I, Budak ET, Yeyen S, Filiz AI, Ozkan S, et al. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. European Journal of Anaesthesiology 2010;27(7):648‐52. [DOI] [PubMed] [Google Scholar]
Mui 2005 {published data only}
- Mui LM, Teoh AY, Ng EK, Lee YT, Au Yeung AC, Chan YL, et al. Premedication with orally administered midazolam in adults undergoing diagnostic upper endoscopy: a double‐blind placebo‐controlled randomized trial. Gastrointestinal Endoscopy 2005;61(2):195‐200. [DOI] [PubMed] [Google Scholar]
Muttu 2005 {published data only}
- Muttu S, Liu EH, Ang SB, Chew PT, Lee TL, Ti LK. Comparison of dexmedetomidine and midazolam sedation for cataract surgery under topical anesthesia. Journal of Cataract and Refractive Surgery 2005;31(9):1845‐6. [DOI] [PubMed] [Google Scholar]
Nascimento 2007 {published data only}
- Nascimento Jdos S, Modolo NS, Silva RC, Santos KP, Carvalho HG. Sedative and cardiovascular effects of midazolam and diazepam alone or combined with clonidine in patients undergoing hemodynamic studies for suspected coronary artery disease. Arquivos Brasileiros de Cardiologia 2007;89(6):403‐8. [DOI] [PubMed] [Google Scholar]
Ristikankare 1999 {published data only}
- Ristikankare M, Hartikainen J, Heikkinen M, Janatuinen E, Julkunen R. Is routinely given conscious sedation of benefit during colonoscopy?. Gastrointestinal Endoscopy 1999;49(5):566‐72. [DOI] [PubMed] [Google Scholar]
Ristikankare 2000a {published data only}
- Ristikankare M, Julkunen R, Laitinen T, Wang SX, Heikkinen M, Janatuinen E, et al. Effect of conscious sedation on cardiac autonomic regulation during colonoscopy. Scandinavian Journal of Gastroenterology 2000;35(9):990‐6. [DOI] [PubMed] [Google Scholar]
Ristikankare 2000b {published data only}
- Ristikankare M, Julkunen R, Mattila M, Laitinen T, Wang SX, Heikkinen M, et al. Conscious sedation and cardiorespiratory safety during colonoscopy. Gastrointestinal Endoscopy 2000;52:48‐54. [DOI] [PubMed] [Google Scholar]
Sajedi 2006 {published data only}
- Sajedi P, Yaraghi A, Niareisy L. A single dose of propofol can produce excellent sedation and comparable amnesia with midazolam in cystoscopic examination. Journal of Research in Medical Sciences 2006;11:160‐3. [Google Scholar]
Salmon 1992 {published data only}
- Salmon JF, Mets B, James MF, Murray AD. Intravenous sedation for ocular surgery under local anaesthesia. The British Journal of Ophthalmology 1992;76(10):598‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandler 1992 {published data only}
- Sandler ES, Weyman C, Conner K, Reilly K, Dickson N, Luzins J, et al. Midazolam versus fentanyl as premedication for painful procedures in children with cancer. Pediatrics 1992;89(4 Pt 1):631‐4. [PubMed] [Google Scholar]
Sherry 1989 {published data only}
- Sherry E, Henderson A, Cotton J. Comparison of midazolam and diazepam for the reduction of shoulder dislocations and Colles' fractures in skiers on an outpatient basis. Australian Journal of Science and Medicine in Sport 1989;21:23‐7. [Google Scholar]
Tamayo 1993 {published data only}
- Tamayo E, Llorente A, Munoz R, Alvarez F J. Cardiorespiratory evaluation of midazolam and thiopental during their use in patients undergoing translumbar aortography in decubitus prone position. Revista Española de Anestesiología y Reanimación 1993;40(3):119‐24. [PubMed] [Google Scholar]
Tesoro 2007 {published data only}
- Tesoro S, Vicchio N, Marchesini L, Aldo Peduto V. Sedation of children with ADHD: trazodone or midazolam?. Paediatric anaesthesia 2007;17(10):1008‐9. [DOI] [PubMed] [Google Scholar]
Uygur‐Bayramiçli 2002 {published data only}
- Bayramicli OU, Dabak R, Kuzucuoglu T, Kavakli B, Yayla A. Endoscopy 2000, 32:E32. Premedication with intranasal midazolam in adults undergoing upper gastrointestinal endoscopy. [abstract].. Endoscopy 2000;32(e32). [DOI] [PubMed] [Google Scholar]
- Uygur‐Bayramiçli O, Dabak R, Kuzucuoglu T, Kavakli B. Sedation with intranasal midazolam in adults undergoing upper gastrointestinal endoscopy. Journal of Clinical Gastroenterology 2002;35(2):133‐7. [DOI] [PubMed] [Google Scholar]
Weinstein 2010 {published data only}
- Weinstein M, Russell L, Kesler J, Niklewski P. Level of sedation and patient / clinician satisfaction. Gastrointestinal Endoscopy 2010;71:AB211. [Google Scholar]
Yildirim 2006 {published data only}
- Yildirim S, Guc B, Bozdogan N, Tokel K. Oral versus intranasal midazolam premedication for infants during echocardiographic study. Advances in Therapy 2006;23(5):719‐24. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bardhan 1984 {published data only}
- Bardhan KD, Morris P, Taylor PC, Hinchliffe RF, Harris PA. Intravenous sedation for upper gastrointestinal endoscopy: diazepam versus midazolam. BMJ (Clinical Research Ed) 1984;288(6423):1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frisancho 1996 {published data only}
- Frisancho Velarde O, Contardo Zambrano C. Comparative study between midazolam and diazepam in upper gastrointestinal endoscopy premedication. Revista de Gastroenterología del Perú 1996;16(3):222‐7. [PubMed] [Google Scholar]
Green 1984 {published data only}
- Green JR, Ravenscroft MM, Swan CH. Diazepam or midazolam for endoscopy?. BMJ (Clinical Research Ed) 1984;288(6427):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendes 1986 {published data only}
- Mendes de Leon C, Bezel R, Karrer W, Brändli O. Premedication in fiber optic bronchoscopy from the patient's and the physician's viewpoint‐‐a randomized study for the comparison of midazolam and hydrocodone. Schweizerische Medizinsche Wochenschrifte 1986;116(37):1267‐72. [PubMed] [Google Scholar]
Mignonsin 1994 {published data only}
- Mignonsin D, Kouame K, Camara B, Bondurand M. Comparative study of intravenous midazolam and diazepam used as sedative agents in upper digestive endoscopy. Cahiers d'Anesthésiologie 1994;42(1):25‐30. [PubMed] [Google Scholar]
Münte 2002 {published data only}
- Münte S, Hollenhorst J, Friedrich L, Heine J, Leuwer M, Piepenbrock S, et al. Intranasal midazolam‐application for anxiolysis during MR imaging. Klinische Neuroradiologie 2002;12:82‐7. [Google Scholar]
Ogden 1993 {published data only}
- Ogden A, Kennedy L, Glass N. Oral midazolam versus oral ketamine for sedation of children with cancer for painful procedures [abstract]. Proceedings of the American Society of Clinical Oncology 1993;12:436, Abstract 1503. [Google Scholar]
Thakur 2003 {published data only}
- Thakur M, Salgaonkar S, Nyayadhish P Y, Sinhal A, Thakkar BM, Choudhari L, et al. Comparison of triclofos sodium and midazolam as oral sedative agents for echocardiography in children [abstract]. Indian Heart Journal 2003;55:Abstract no:264. [Google Scholar]
Theroux 1993 {published data only}
- Theroux M, West D, Corddry D, Hyde P, Bachrach S, Cronan K, et al. Efficacy of intranasal midazolam in facilitating suturing of lacerations in preschool children in the emergency department. Pediatrics 1993;91(3):624‐7. [PubMed] [Google Scholar]
Wild 1988 {published data only}
- Wild A, Bertolaso E. Is there a risk of global respiratory insufficiency with the use of intramuscular midazolam as premedication for bronchoscopy?. Schweizerische Medizinische Wochenschrift 1988;118(38):1375‐7. [PubMed] [Google Scholar]
References to ongoing studies
NCT00563069 {unpublished data only}
- Premedication with oral midazolam in patients undergoing rigid cystoscopy: a randomized, double‐blind, placebo‐controlled trial. Ongoing study September 2005.
NCT01925898 {unpublished data only}
- A randomized, clinical trial of oral midazolam versus oral ketamine for sedation during laceration repair. Ongoing study August 2013.
Puttapitakpong 2015 {unpublished data only}
- Oral midazolam for sedation in esophagogastroduodenoscopy(EGD). Ongoing study October 2013.
Additional references
ANZCA PSO9
- ANZCA Guidelines on Sedation and/or Analgesia for Diagnostic and Interventional Medical, Dental or Surgical Procedures. Users/JCRA0002/Downloads/ps09‐2014‐guidelines‐on‐sedation‐and‐or‐analgesia‐for‐diagnostic‐and‐interventional‐medical‐dental‐or‐surgical‐procedures.pdf (accessed September 11 2015).
Cohen 2006
- Cohen L, Wecsler J, Gaetano J, Benson A, Miller K, Durkalski V, et al. Endoscopic sedation in the United States: results from a nationwide survey. The American Journal of Gastroenterology 2006;101(5):967‐74. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336(7651):995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thomson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org.
Jüni 2001
- Jüni P, Altman D, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lourenço‐Matharu 2012
- Lourenço‐Matharu L, Ashley PF, Furness S. Sedation of children undergoing dental treatment. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD003877.pub4] [DOI] [PubMed] [Google Scholar]
McQuaid 2008
- McQuaid KR, Laine L. A systematic review and meta‐analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointestinal Endoscopy 2008;67(6):910‐23. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institue of Health and Clinical Excellence. Sedation in children and young people. www.rcpch.ac.uk/sites/default/files/asset_library/Research/Clinical%20Effectiveness/Endorsed%20guidelines/Sedation%20in%20Children%20and%20Young%20People/Quick%20Reference%20GuideA.pdf. London, UK: National Clinical Guideline Centre, 2010 (accessed May 2016).
Qadeer 2005
- Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta‐analysis. Clinical Gastroenterology and Hepatology 2005;3(11):1049‐56. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014; Vol. .:..
Riss 2008
- Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurologica Scandinavica 2008;118(2):69‐86. [DOI] [PubMed] [Google Scholar]
Shailaja 2013
- Shailaja S, Ray A, Ray S, Kirubakaran R. Dexmedetomidine for procedural sedation in children. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010886] [DOI] [Google Scholar]
Thomson 2010
- Thomson A, Andrew G, Jones B. Optimal sedation for gastrointestinal endoscopy: review and recommendations. Journal of Gastroenterology and Hepatology 2010;25(3):469‐78. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang D, Chen C, Chen J, Xu Y, Wang L, Zhu Z, et al. The use of propofol as a sedative agent in gastrointestinal endoscopy: a meta‐analysis. PLoS One 2013;8(1):e53311. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Morão 2011
- Morão S, Ratilal BO, Santos H, Sampaio C. Midazolam for sedation before procedures. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009491] [DOI] [Google Scholar]


