Abstract
Background
More than 400,000 cases of oropharyngeal squamous cell cancer (OPSCC) are diagnosed every year worldwide and this is rising. Much of the increase has been attributed to human papillomavirus (HPV). HPV‐positive OPSCC patients are often younger and have significantly improved survival relative to HPV‐negative patients. Traditional management of OPSCC has been with radiotherapy with or without chemotherapy, as this was shown to have similar survival to open surgery but with significantly lower morbidity. Techniques have evolved, however, with the development of computerised planning and intensity‐modulated radiotherapy, and of minimally invasive surgical techniques. Acute and late toxicities associated with chemoradiotherapy are a significant burden for OPSCC patients and with an ever‐younger cohort, any strategies that could decrease treatment‐associated morbidity should be investigated.
Objectives
To assess the effects of de‐intensified adjuvant (chemo)radiotherapy in comparison to standard adjuvant (chemo)radiotherapy in patients treated with minimally invasive transoral surgery (transoral robotic surgery or transoral laser microsurgery) for resectable HPV‐positive oropharyngeal squamous cell carcinoma.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 26 April 2018.
Selection criteria
Randomised controlled trials (RCTs) in patients with carcinoma of the oropharynx (as defined by the World Health Organization classification C09, C10). Cancers included were primary HPV‐positive squamous cell tumours originating from the oropharyngeal mucosa. Tumours were classified as T1‐4a with or without nodal spread and with no evidence of distant metastatic spread. The intervention was minimally invasive transoral surgery followed by de‐intensified adjuvant therapy (either omission of chemotherapy or reduced‐dose radiotherapy). The comparator was minimally invasive transoral surgery followed by standard concurrent chemoradiotherapy or standard‐dose radiotherapy. The treatments received were of curative intent and patients had not undergone any prior intervention, other than diagnostic biopsy.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were overall survival (disease‐related survival was to be studied where possible) and disease‐free survival, measured at one, two, three and five years. Our secondary outcomes included assessment of swallowing ability and voice, measured at one, six, 12 and 24 months. We planned to use GRADE to assess the quality of evidence for each outcome.
Main results
We did not identify any completed RCTs that met our inclusion criteria. However, three eligible studies are in progress:
ADEPT is a phase III trial comparing postoperative radiotherapy with or without cisplatin in HPV‐positive T1‐4a OPSCC patients. Included patients must have received minimally invasive surgery and demonstrated extra‐capsular spread from disease in the neck.
ECOG‐E3311 is a phase II trial of treatment for HPV‐positive locally advanced OPSCC (stages III‐IVa + IVb without distant metastasis). Patients are stratified after minimally invasive surgery. Medium‐risk patients are randomised to either standard or reduced‐dose radiotherapy.
PATHOS is a phase III trial of treatment for HPV‐positive OPSCC (T1‐3, N0‐2b). Patients are stratified after minimally invasive surgery. Medium‐risk patients are randomised to either standard or reduced‐dose radiotherapy. High‐risk patients are randomised to radiotherapy with or without concurrent cisplatin.
Authors' conclusions
This review highlights the current lack of high‐quality randomised controlled trials studying treatment de‐escalation after minimally invasive surgery in patients with HPV‐positive OPSCC. However, trials that will meet the inclusion criteria for this review are in progress with results expected between 2021 and 2023.
Plain language summary
Reduced‐dose radiotherapy/chemotherapy compared to standard‐dose treatment after keyhole surgery for throat cancer caused by human papillomavirus
Review question
What are the effects of reduced‐dose radiotherapy/chemotherapy treatment compared to standard‐dose treatment after keyhole surgery for throat cancer caused by human papillomavirus (HPV)?
Background
More than 400,000 cases of cancer of the throat are diagnosed each year and this is increasing, with HPV being a significant factor. Throat cancer caused by this virus often affects younger patients but has a better prognosis than non‐viral throat cancer. Traditional treatment for throat cancer is with radiotherapy and chemotherapy as this had been shown to have similar survival outcomes to surgery but with fewer side effects. However, treatments have evolved, such as computerised planning and improvements in radiotherapy, and the development of keyhole surgery, which have the potential for fewer side effects. Chemotherapy and radiotherapy do have long‐term negative effects on quality of life. With younger patients being affected, any way of reducing these side effects should be investigated.
Study characteristics
In April 2018, we searched for randomised controlled trials (RCTs) that had compared reduced‐dose radiotherapy/chemotherapy treatment with standard‐dose treatment. We were interested in the outcomes of overall survival and disease‐free survival, as well as the effects on swallowing ability and voice. Our searches did not identify any completed RCTs, however three relevant studies are ongoing and the first results are expected between 2021 and 2023.
Key results
Currently there is no high‐quality evidence comparing these two treatments, however such trials are in progress.
Summary of findings
Background
Description of the condition
More than 400,000 cases of oropharyngeal squamous cell carcinoma (OPSCC) are diagnosed each year worldwide (Chaturvedi 2013). According to the US Centers for Disease Control and Prevention there were approximately 18,917 new cases of human papillomavirus‐associated OPSCC in the United States in 2015 (Van Dyne 2018). Worldwide, the incidence of OPSCC ranges from 7 to 17 cases per 100,000 persons and is steadily rising (Chaturvedi 2013), particularly in developed countries and young males (Chaturvedi 2011; Gillison 2015; Van Dyne 2018).
Human papillomavirus (HPV) is a major carcinogen, with an estimated 4.8% of total worldwide cancers in 2008 linked to the virus (de Martel 2012). HPV now meets the epidemiological criteria for OPSCC causality, especially in non‐smokers (Gillison 2015; Sudhoff 2011). Meta‐analysis of the world literature has demonstrated that the proportion of HPV‐associated oropharyngeal cancer has increased from 40.5% in studies recruiting before the year 2000 to 72.2% in studies reporting after 2005 (Mehanna 2013), although this is known to vary by individual population (Schache 2016). In contrast to this apparent overall trend, recent published work has shown that in the UK over the period 2002 to 2011, whilst the overall incidence of OPSCC doubled, the proportion that were HPV‐positive stayed the same, demonstrating a concomitant increase in non‐HPV associated OPSCC (Schache 2016). In the UK population, therefore, the increase cannot be attributed to HPV alone.
It is worth noting that HPV‐positive OPSCC patients have significantly improved rates of both overall and disease‐free survival compared to HPV‐negative tumour groups. HPV‐positive OPSCC is associated with a 58% reduction in the risk of death compared to HPV‐negative disease (Ang 2010; Fakhry 2008). Indeed the presence or absence of HPV with regard to the tumour may have a greater impact on five‐year survival than T stage or nodal status alone (Haughey 2011). This is illustrated by its inclusion as a significant factor in the progression from the TNM Classification of Malignant Tumours (TNM) 7th to 8th edition (TNM 2009; TNM 2017; O'Sullivan 2016).
Risk factors for oral HPV infection include a history of orogenital sexual practice, a large number of sexual partners and first intercourse at an early age. The same factors also reflect changes in modern society and combine to increase the cumulative effect of HPV infection in OPSCC (Chung 2009). HPV‐negative OPSCC tends to affect an older age group and is normally associated with smoking and alcohol. HPV‐positive OPSCC behaves differently, often presenting with a small primary in the oropharynx combined with a metastatic cystic deposit in the neck. In the TNM 7th edition this previously entailed a higher stage at presentation for the majority of patients (Ang 2010; Dwivedi 2013; Evans 2010), however this has been adjusted in the TNM 8th edition.
Description of the intervention
Over the last 20 years the management of oropharyngeal cancer has changed dramatically. In 2002, Parsons et al published a review of 51 studies of patients with OPSCC who were treated with surgery with or without radiotherapy or primary radiotherapy without neck dissection (Parsons 2002). The cumulative five‐year survival was 47% for patients undergoing primary surgical resection with or without neck dissection, and 43% for those undergoing primary radiotherapy with or without neck dissection. However, the severe complication rate was 23% in the primary surgical group and only 6% in the primary radiotherapy group. This led to the conclusion that non‐operative therapy was superior to operative therapy for OPSCC of all stages. More recently a large meta‐analysis comparing primary radiotherapy with chemoradiotherapy in 16,192 head and neck squamous cell carcinoma (HNSCC) patients provided updated results. The study concluded an absolute survival benefit of 8.1% after five years in OPSCC patients treated with concurrent chemoradiotherapy (Blanchard 2011).
Acute and late toxicities associated with chemoradiotherapy are, however, a significant burden for oropharyngeal cancer patients, with rates of acute and late grade 3 or higher toxicity at approximately 80% and 25% to 60% respectively (Kelly 2016). Recognised toxicities include: gastric tube dependence, pain, scarring, fibrosis, dysphagia, xerostomia, dental decay, osteoradionecrosis, hypothyroidism, carotid stenosis and stroke (Lee 2011). A relationship between the radiation dose to the constrictor muscles and long‐term swallowing difficulties has been well established: patients in whom more than 78% of their cricopharyngeus inlet receives over 60 Gy have a 50% risk of developing a stricture (Chen 2010). The addition of chemotherapy to radiotherapy worsens toxicity as demonstrated by the Intergroup trial, which found rates of grade 3 or higher toxicity of 89.5% in the chemoradiotherapy cohort compared to 52% in the radiotherapy alone cohort (Adelstein 2003). Furthermore, late toxicities may be under‐recognised as the 10‐year results of RTOG 91‐11 found increased non‐cancer mortality in the concurrent chemoradiotherapy arm (30.8%) compared to the induction chemotherapy arm (20.8%) or radiotherapy alone arm (16.9%) (Forastiere 2013). The incidence of dysphagia and feeding tube dependence post chemoradiotherapy cannot be entirely attributed to the treatment as there is evidence that part of the effect is due to disuse atrophy and resultant adverse remodelling of aerodigestive tract muscles (Hutcheson 2013).
Both open surgery and radiotherapy/concurrent chemoradiotherapy have drawbacks in terms of cost, overall survival and patient quality of life (Haigentz 2009; Machtay 2008). The benefit of a novel treatment regimen with lower toxicity is therefore clear, especially given the younger patient cohort who will have to live with treatment sequelae for longer.
As the biological differences in viral/non‐viral associated OPSCC are further elaborated (Masterson 2015; Pyeon 2007; Slebos 2006), radical change in most therapeutic interventions has taken place. In radiotherapy, intensity modulation and computerised planning have been introduced to external beam therapy. In surgery, the focus has shifted to the use of minimally invasive procedures such as transoral laser microsurgery or transoral robotic surgery, which demonstrate reduced immediate postoperative toxicity, reduced length of hospital stay and faster functional recovery compared with open surgery (Holsinger 2015). Furthermore, these techniques have the potential to improve organ preservation and function, and ameliorate the economic burden of treatment. Additionally, with regard to control of lymphatic spread, neck dissections have become more selective (resulting in the removal of fewer normal structures and therefore lower morbidity) (Adelstein 2012).
We have reviewed the evidence from studies comparing transoral minimally invasive surgery with (chemo)radiotherapy in the related Cochrane Review: 'Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma' (Howard 2016).
De‐intensified treatment strategies
The use of minimally invasive surgery for the primary site provides two principal areas of benefit. Firstly, it results in less morbidity for the patient compared to traditional open surgery. Secondly, in the context of HPV‐related OPSCC, it raises the potential option of 'de‐intensification therapy' with a concomitant reduction in radiation‐related morbidity (Masterson 2014a; Masterson 2014b; Moore 2009). So far this has been borne out by observational studies that suggest that transoral minimally invasive surgery may have an advantage by improving patient quality of life and functional outcome, and reducing the need for adjuvant concurrent chemoradiotherapy (Leonhardt 2012; Moore 2013).
De‐intensified treatment strategies fall into two groups. For primary concurrent chemoradiotherapy the options are to replace cisplatin with the epidermal growth factor receptor (EGFR) inhibitor cetuximab or to reduce the dose of radiation. These strategies have been covered by the Cochrane Review 'De‐escalation treatment protocols for human papillomavirus‐associated oropharyngeal squamous cell carcinoma' (Masterson 2014a). For primary surgery the options include:
administration of a lower dose of adjuvant radiotherapy; or
omission of chemotherapy from the adjuvant treatment regimen (radiotherapy alone).
It is these strategies that will be covered by this review.
The most appropriate option is decided based upon histopathological examination of the surgical specimen. This allows risk stratification of the individual patient, although it is worth noting that this stratification relies on traditional histopathological risk factors, which may not apply in HPV‐positive disease (Huang 2012; Masterson 2014b).
Patients are usually stratified into three cohorts:
Low‐risk: pathological findings associated with a low risk of locoregional relapse, which usually has no adjuvant treatment.
Medium‐risk: locoregional disease (or early disease with adverse histological features), which is usually treated with adjuvant radiotherapy.
High‐risk: presence of positive (< 1 mm) margins, extracapsular nodal spread or advanced disease, which is usually treated with adjuvant chemoradiotherapy.
How the intervention might work
Toxicities (both acute and late) as a result of chemotherapy and radiotherapy to the head and neck are well recognised. If survival outcomes can be maintained, de‐intensification of adjuvant therapy provides the opportunity for a reduction in these toxicities and an improved quality of life for patients. This is increasingly important, especially considering the younger age of some patients who will have to live with the consequences of treatment for many years.
Why it is important to do this review
The oropharynx plays an essential role in swallowing, speech and protecting the airway as it is situated at the bifurcation of the respiratory and digestive tract. The toxicities from standard‐dose chemotherapy and radiotherapy are well recognised and treatment modalities are therefore heavily influenced by the aim of reducing the risk of functional disability where possible. In the context of an expanding cohort of younger patients the potential effects of de‐intensification of adjuvant (chemo)radiotherapy facilitated by a minimally invasive surgical approach should be systematically reviewed.
Objectives
To assess the effects of de‐intensified adjuvant (chemo)radiotherapy in comparison to standard adjuvant (chemo)radiotherapy in patients treated with minimally invasive transoral surgery (transoral robotic surgery or transoral laser microsurgery) for resectable HPV‐positive oropharyngeal squamous cell carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We planned to exclude quasi‐randomised and cluster‐randomised trials.
Types of participants
We planned to include patients with HPV‐positive oropharyngeal carcinoma (subsites C09 and C10 as defined by the World Health Organization classification). Cancers included were T1‐4a with or without nodal disease with no evidence of distant metastatic spread. All patients had received minimally invasive transoral surgery.
We excluded carcinoma of the oral cavity (C01‐06), nasopharynx (C11), hypopharynx (C13) and larynx (C32) (WHO 2000).
We excluded patients receiving open surgery.
Types of interventions
Intervention
De‐intensified adjuvant (chemo)radiotherapy.
De‐intensified radiotherapy, total dose 50 Gy given in 25 fractions.
De‐intensified chemoradiotherapy (= standard dose radiotherapy with omission of concurrent chemotherapy): radiotherapy alone, total dose 60 Gy given in 30 fractions.
Control
Standard adjuvant (chemo)radiotherapy.
Radiotherapy, total dose 60 Gy given in 30 fractions.
+/‐
Chemotherapy: platinum‐based agent (usually cisplatin) administered concurrently with radiotherapy either weekly or three‐weekly.
The comparisons were:
Low‐dose adjuvant radiotherapy versus standard‐dose adjuvant radiotherapy.
Standard‐dose adjuvant radiotherapy alone versus adjuvant chemoradiotherapy.
Types of outcome measures
We planned to analyse the following outcomes in the review, but we did not use them as a basis for including or excluding studies. Primary outcomes focus on survival whilst the secondary outcomes focus on quality of life indices that may be affected by de‐intensification of treatment.
Primary outcomes
Overall survival/total mortality (disease‐related mortality was also to be studied if possible).
Disease‐free survival.
We planned to measure these outcomes at one, two, three and five years.
Secondary outcomes
-
Swallowing ability, as measured by:
the proportion of people with a gastrostomy tube (at one year);
the MD Anderson Dysphagia Inventory (MDADI);
modified barium swallow ratings.
return to normal diet, measured with the Performance Status Scale for Head and Neck cancer (PSS‐HN) normalcy of diet scale.
Voice, measured with the Voice Handicap Index (VHI).
Apart from the proportion of people with a gastrostomy tube we planned to measure these outcomes at one, six, 12 and 24 months.
Despite the increasing focus on quality of life the optimal patient‐reported outcome instrument that should be used to measure the impact of cancer therapy for the HPV‐associated OPSCC patient population is not clearly defined. Moreover, we feel that it is important to distinguish between patient‐reported outcomes and quality of life measures that may be more subjective. Finally, there is a danger that subtle differences in particular areas (e.g. dysphagia) may be lost within more global scoring systems, adding further complexity to the comparison (dilution effect).
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 26 April 2018.
Electronic searches
We identified published, unpublished and ongoing studies by searching the following databases from their inception:
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies to 26 April 2018);
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies to 26 April 2018);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 26 April 2018);
Ovid EMBASE (1974 to 26 April 2018);
LILACS, lilacs.bvsalud.org (searched 2 April 2018);
KoreaMed (searched via Google Scholar 27 April 2018);
Web of Knowledge, Web of Science (1945 to 26 April 2018);
ClinicalTrials.gov (searched via the Cochrane Register of Studies to 27 April 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched to 26 April 2018).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). The search strategy for CENTRAL is provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
Two authors (JH and LM) independently screened each abstract. We independently assessed full texts against the inclusion criteria. Any conflict was resolved through discussion with a senior author.
Data extraction and management
Two authors (JH and LM) planned to independently extract data using a specifically designed data extraction form. We planned to pilot the data extraction form on several studies and adjust it as necessary prior to use. Any disagreements were resolved through consultation with a senior author. Where required we contacted study authors for clarification or missing information.
For each study we planned to record the following:
Year of publication, country of origin and source of study funding.
Details of the participants, including demographic characteristics and criteria for inclusion and exclusion.
Details of the type of intervention, timing and duration (including type of surgery).
Details of survival outcomes reported, with time intervals.
Details of treatment‐related morbidity, categorised as acute (less than 90 days after treatment) or late (more than 90 days) and classified according to the Common Terminology Criteria for Adverse Events (CTCAE 4.03).
Details of all other outcomes reported, including method of assessment and time intervals.
Assessment of risk of bias in included studies
Had suitable studies been identified JH and LM would have assessed the risk of bias of the included studies independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We planned to use the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
Measures of treatment effect
We planned to express continuous outcomes as a mean endpoint (or change from baseline) for each group with standard deviation and number of people. For continuous outcomes measured using different (but compatible) scales we planned to express treatment effects as a standardised mean difference (SMD).
We planned to express dichotomous outcomes as a risk ratio (RR) with the number of people with the outcome and number of participants.
We planned to express time‐to‐event outcomes as a hazard ratio (HR) with standard deviation (SD).
We planned to preferentially report ordinal data as continuous, however if studies only reported data dichotomously then we would have expressed as dichotomous.
The analysis would have been on an intention‐to‐treat basis. Where useful we planned to calculate the number needed to treat to benefit/harm (NNTB/NNTH) to aid clinical interpretation of the findings.
Unit of analysis issues
We planned to use data only from individually randomised controlled trials to avoid unit of analysis issues. We excluded cluster‐randomised trials.
Dealing with missing data
Where standard deviations or hazard ratios were not reported we planned to impute these from other reported data.
We planned to contact study authors:
where a study protocol suggested that an outcome of interest had been measured but was not reported;
if not all data required for meta‐analysis were reported;
if standard deviation data or hazard ratios were not available or estimable from other reported data (using the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011)).
Assessment of heterogeneity
We intended to assess clinical heterogeneity by examining the types of participants, interventions and outcomes in each study. We planned to conduct formal assessment using the Chi² test (with a significance level of α = 0.1 in view of the low power of this test) and the I² statistic (with 75% or more indicating a considerable level of inconsistency), both available in RevMan 5.3 (RevMan 2014). If meta‐analysis was performed we planned to further assess heterogeneity by inspecting the overlap of confidence intervals for the results of individual studies within a forest plot.
Assessment of reporting biases
We planned to assess reporting bias as within‐study (outcome reporting) bias and between‐study (publication) bias.
Outcome reporting bias
Bias can occur if outcomes are not adequately reported to allow further analysis. We planned to assess outcome reporting bias by comparing the reported outcomes against the outcomes listed in the trial protocol or methods section. Where the protocol, methods or results indicated that an outcome had been measured but the results were not presented sufficiently we planned to contact the study authors. If no further information was found we planned to judge this a 'high' risk of bias. If insufficient information was found to allow adequate judgement we planned to judge this an 'unclear' risk of bias.
Publication bias
If sufficient studies were available we planned to create funnel plots for the outcomes overall survival and dysphagia (MDADI scores). If asymmetry was found we planned to investigate this further according to the methodology in the Cochrane Handbook of Systematic Reviews of Interventions (Handbook 2011).
Data synthesis
We planned to extract data from the included studies and enter the data into RevMan 5.3 for statistical analysis. In the event of incomplete data, we intended to contact the study authors to obtain further information and to seek statistical advice where necessary.
Our analysis of survival and disease recurrence would have depended on the data available. We aimed to analyse the proportion surviving at one, two, three and five years as either:
proportion surviving; or
hazard ratios, for comparison in meta‐analysis if appropriate.
We planned to express the proportion of people with a gastrostomy tube as a risk ratio. The remaining secondary outcomes were to be restricted to assessment of validated assessment tools (where appropriate). If the data provided were in the form of means and standard deviations, we intended to display the effects on outcomes as a mean with standard deviation. If there was disparity in terms of scales we planned to express the data as a SMD with 95% confidence interval (CI). If hazard ratios were not quoted in studies, we planned to calculate them from available summary statistics such as observed events, expected events, variance, confidence intervals, P values or survival curves (Parmar 1998). If required, we planned to analyse the survival curves using the online tool: https://automeris.io/WebPlotDigitizer/.
We hoped to attempt a meta‐analysis if studies were available with similar comparisons and reporting the same outcome measures. If appropriate, we intended to calculate pooled estimates using a random‐effects model (Handbook 2011), as there is likely to be significant statistical or clinical heterogeneity (an I² value > 50%, as specified in the Cochrane Handbook for Systematic Reviews of Interventions).
Subgroup analysis and investigation of heterogeneity
We had no planned subgroup analyses.
We planned to assess heterogeneity using the methods advised in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Sensitivity analysis
If meta‐analysis had been performed we would have used sensitivity analysis. If we included studies with high risk of bias (e.g. poor follow‐up rate) we would have re‐calculated outcomes including these studies individually to see what influence this had on our presumed treatment effect.
Where thresholds had been set for inclusion or analysis we planned to re‐analyse the data using values either side of the set threshold to assess whether our decisions had influenced the outcomes.
GRADE and 'Summary of findings' table
We planned that three authors (JH, RD, LM) would ensure that each study was independently assessed twice by different authors using the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we planned to apply this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We planned to include 'Summary of findings' tables, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). The planned comparisons were:
low‐dose adjuvant radiotherapy versus standard‐dose adjuvant radiotherapy;
standard‐dose adjuvant radiotherapy alone versus adjuvant chemoradiotherapy.
We planned to include the following outcomes in the 'Summary of findings' tables:
Primary outcomes
Overall survival.
Disease‐free survival.
Secondary outcomes
-
Swallowing ability, as measured by:
the proportion of people with a gastrostomy tube (at one year);
the MD Anderson Dysphagia Inventory (MDADI);
modified barium swallow ratings;
return to normal diet, measured with the Performance Status Scale for Head and Neck cancer (PSS‐HN) normalcy of diet scale.
Voice, measured with the Voice Handicap Index (VHI).
Results
Description of studies
Results of the search
Our searches in April 2018 retrieved a total of 1959 records, which reduced to 1527 after the removal of duplicates. Following title and abstract screening we were able to discard 1502 irrelevant records. We screened 25 records in full text. We discarded 20 of these studies due to irrelevance (incorrect intervention, population, control, study design etc). We formally excluded two studies (see below).
We identified three ongoing RCTs that are eligible for inclusion in this review (see below).
The PRISMA diagram in Figure 1 shows our study search and selection process.
1.
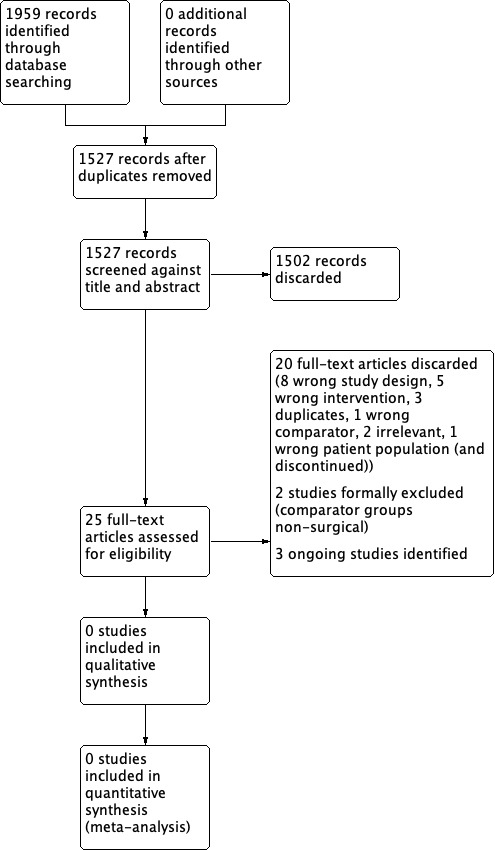
Study search and selection process (PRISMA diagram).
Included studies
We did not identify any completed studies that met the inclusion criteria for the review.
Excluded studies
We excluded two studies from the review. See Characteristics of excluded studies.
ORATOR ('Early‐stage squamous cell carcinoma of the oropharynx: radiotherapy versus trans‐oral robotic surgery') is a randomised controlled trial comparing primary radiotherapy with primary transoral robotic surgery for early‐stage (T1‐2, N0‐2) OPSCC. It is currently in progress with an estimated completion date of June 2021 for part 1 (phase 2, 68 patients) and 2028 for part 2 (phase 3, 120 patients). Patients will be randomised to receive either primary radiotherapy or primary transoral robotic surgery. The primary outcome for part 1 is quality of life (at one year) and the secondary outcomes include overall and progression‐free survival (at three and five years), toxicity and swallowing function (at five years). The primary outcome for part 2 (phase III trial) is two‐year progression‐free survival and the secondary outcomes are overall survival and quality of life outcomes. We excluded this study because the participants in the comparator group will not have undergone surgery.
EORTC‐1420 (European Organisation for Research and Treatment of Cancer 1420) is a phase III, randomised study assessing the "best of" radiotherapy compared to the "best of" minimally invasive head and neck surgery in patients with T1‐T2, N0 squamous cell carcinoma (EORTC‐1420). The study is recruiting, with an estimated enrollment of 170 patients and an estimated completion in May 2026. The primary outcome will be the assessment of swallowing function within the first year after the two treatment strategies. We excluded this study because again the participants in the comparator group will not have undergone surgery.
Ongoing studies
See Characteristics of ongoing studies.
ADEPT is a phase III prospective trial of de‐escalated adjuvant treatment after minimally invasive head and neck surgery (transoral robotic surgery or transoral laser microsurgery) for HPV‐positive OPSCC (T1‐4a) patients noted to have extracapsular extension detected in the nodal disease. This study is currently in progress and has finished recruiting (41 patients recruited), with an estimated completion date of August 2021. Patients are allocated to two arms after minimally invasive head and neck surgery through randomisation or patient choice, to receive either postoperative radiotherapy (60 Gy) alone or with concurrent systemic cisplatin therapy.
The primary outcomes are disease‐free survival and locoregional control at two years. Secondary outcomes include overall survival and distant metastasis rate up to five years, quality of life and functional outcomes to two years, and toxicities up to 4.5 months.
ECOG‐E3311 is a phase II prospective randomised trial of reduced adjuvant treatment after transoral robotic surgery for HPV‐positive, locally advanced OPSCC (stages III‐IVa + IVb without distant metastases). The study is in progress but not recruiting (511 patients recruited), with an estimated completion date of February 2023. The primary foci for investigation are a feasibility study of risk‐adjusted adjuvant therapy and the oncologic outcomes for intermediate‐risk patients post transoral robotic surgery and standard or de‐escalated treatment.
Patients will be stratified into three groups depending on their surgical histology. The low‐risk group will have no adjuvant therapy as per standard treatment. The medium‐risk group will be randomised to receive either standard (60 Gy) or de‐escalated (50 Gy) postoperative radiotherapy. The high‐risk group will receive postoperative radiotherapy (60 Gy) with concurrent cisplatin.
The primary outcomes include progression‐free survival at two years, risk distribution and grade 3‐4 bleeding events during surgery. Secondary outcomes include overall survival, swallowing and voice function up to two years post treatment, and change in patient‐reported quality of life up to six months post radiation treatment.
PATHOS is a phase III prospective randomised controlled trial of reduced‐intensity adjuvant treatment for HPV‐positive OPSCC (T1‐3, N0‐2b) patients treated with transoral robotic or laser surgery, with an estimated recruitment of 242 patients and estimated completion in December 2019.
Patients will be stratified into three groups depending on their surgical histology. The low‐risk group will have no adjuvant therapy as per standard treatment. The medium‐risk group will be randomised to receive either standard (60 Gy) or de‐escalated (50 Gy) postoperative radiotherapy. The high‐risk group will be randomised to receive postoperative radiotherapy (60 Gy) with or without concurrent cisplatin.
The primary outcome measure is swallowing function (MDADI) at one year. Secondary outcomes include disease‐free and overall survival at six months, and swallowing function, quality of life and acute or late toxicities measured at up to 24 months.
Risk of bias in included studies
No studies are included in the review.
Effects of interventions
Summary of findings for the main comparison. Low‐dose adjuvant radiotherapy versus standard‐dose adjuvant radiotherapy.
| Low‐dose adjuvant radiotherapy compared with standard‐dose adjuvant radiotherapy for patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery | ||||||
|
Patient or population: patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery Settings: post minimally invasive transoral surgery Intervention: low‐dose adjuvant radiotherapy Comparison: standard‐dose adjuvant radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard‐dose adjuvant radiotherapy | Low‐dose adjuvant radiotherapy | |||||
| Overall survival Follow‐up: at 1, 2, 3 and 5 years |
No data available (no included studies) | |||||
| Disease‐free survival Follow‐up: at 1, 2, 3 and 5 years |
No data available (no included studies) | |||||
| Swallowing ability Follow‐up: at 1, 6, 12 and 24 months |
No data available (no included studies) | |||||
| Voice (Voice Handicap Index) Follow‐up: at 1, 6, 12 and 24 months |
No data available (no included studies) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Standard‐dose adjuvant radiotherapy alone versus adjuvant chemoradiotherapy.
| Standard‐dose adjuvant radiotherapy alone compared with adjuvant chemoradiotherapy for patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery | ||||||
|
Patient or population: patients with HPV‐positive oropharyngeal carcinoma who have received minimally invasive transoral surgery Settings: post minimally invasive transoral surgery Intervention: standard‐dose adjuvant radiotherapy alone Comparison: adjuvant chemoradiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Adjuvant chemoradiotherapy | Standard‐dose adjuvant radiotherapy alone | |||||
| Overall survival Follow‐up: at 1, 2, 3 and 5 years |
No data available (no included studies) | |||||
| Disease‐free survival Follow‐up: at 1, 2, 3 and 5 years |
No data available (no included studies) | |||||
| Swallowing ability Follow‐up: at 1, 6, 12 and 24 months |
No data available (no included studies) | |||||
| Voice (Voice Handicap Index) Follow‐up: at 1, 6, 12 and 24 months |
No data available (no included studies) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
No studies are included in the review.
Discussion
Summary of main results
We identified no completed randomised controlled trials (RCTs) that met the inclusion criteria for this review. We are aware of three RCTs meeting our inclusion criteria; however, all are currently in progress (ADEPT; ECOG‐E3311; PATHOS).
Overall completeness and applicability of evidence
We believe this is a thorough and unbiased review of the current literature available on this subject. Unfortunately, there were no eligible studies with results for inclusion in our analysis; however, applicable studies are ongoing.
Quality of the evidence
We did not identify any completed studies that could be included in the review.
Potential biases in the review process
We have striven to design a protocol that would include all the highest‐quality evidence in this area (Howard 2018). The search strategy was designed and run by a qualified Cochrane Information Specialist so any bias here should be minimal. The search was not limited to the English language. It is possible that suitable studies have been carried out and the results published elsewhere in another language; however, we feel that this is unlikely, as all applicable studies are likely to have been registered with one of the central trial registries.
All studies that we discarded during our search and selection process were rejected based on study design (e.g. they were not randomised) or because they were not on the topic of interest. We formally excluded two studies because they compared a surgical intervention with a non‐surgical comparator and thus did not meet our inclusion criteria.
Agreements and disagreements with other studies or reviews
The debate regarding the relevance of traditional risk factors in human papillomavirus (HPV)‐positive disease and the necessity of adjuvant therapy is ongoing, with conflicting evidence from retrospective and non‐randomised trials. In a combined analysis of the results from EORTC‐22931 and RTOG‐9501 Bernier et al demonstrated benefit from the addition of chemotherapy to radiotherapy with extracapsular extension status as one of the main risk factors warranting additional chemotherapy (Bernier 2005). Furthermore, in a meta‐analysis Blanchard et al showed improved survival with the addition of chemotherapy to radiotherapy (Blanchard 2011). More recent retrospective series, however, have questioned the relevance of extracapsular extension (Lewis 2011; Maxwell 2013; Sinha 2015a), with some suggesting that the total number of nodes is more prognostic than extracapsular extension or advanced N‐stage (Sinha 2015b).
Grant et al published a retrospective series showing no benefit in locoregional control from adjuvant treatment (HPV unknown population) (Grant 2009). By contrast, Pasalic et al published a retrospective series of 158 patients with intermediate or advanced disease showing a benefit from adjuvant therapy in patients with extracapsular extension compared to those without (hazard ratio (HR) 4.34, 95% confidence interval (CI) 1.540 to 12.213; P = 0.006) (Pasalic 2018). Of note, there was no difference in overall survival between the two groups; however, other series have again demonstrated benefit in disease‐free survival and overall survival from adjuvant therapy in advanced disease (T4 tumour burden) although the numbers were small (5 out of 62 treated surgically) (Zenga 2015). The overall picture from retrospective and non‐randomised controlled data is unclear at present and we therefore await the results of the prospective randomised controlled trials currently ongoing.
Authors' conclusions
Implications for practice.
This review highlights the current lack of high‐quality randomised controlled trials studying treatment de‐escalation after minimally invasive surgery in patients with human papillomavirus (HPV)‐positive oropharyngeal squamous cell cancer. As a result, we are unable to comment on current practice.
Implications for research.
This is clearly an area of interest to both patients and clinicians. Future trials should include patients with HPV‐positive oropharyngeal squamous cell cancer, who have been treated with minimally invasive surgery and are then randomised to either standard adjuvant treatment or de‐intensified adjuvant treatment. Outcomes of interest include both survival and quality of life indices, particularly assessment of swallowing. Trials that will meet the inclusion criteria for this review are in progress, which should hopefully shed more light on this topic.
History
Protocol first published: Issue 2, 2018 Review first published: Issue 12, 2018
| Date | Event | Description |
|---|---|---|
| 24 May 2018 | Amended | Minor edit to Types of participants to correct inclusion criteria for T stage. |
Acknowledgements
We would like to thank Mr David Hamilton for peer reviewing the draft review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Oropharyngeal Neoplasms] explode all trees
#2 MeSH descriptor: [Head and Neck Neoplasms] this term only
#3 MeSH descriptor: [Otorhinolaryngologic Neoplasms] explode all trees
#4 MeSH descriptor: [Neoplasms] explode all trees
#5 cancer* or carcinoma* or neoplas* or tumour* or tumour* or malignan* or SCC*
#6 #4 or #5
#7 MeSH descriptor: [Oropharynx] explode all trees
#8 oropharyn* or mesopharyn* or tonsil* or "head and neck" or "head neck" or "head‐neck" or "head‐and‐neck" or tongue
#9 #7 or #8
#10 #6 and #9
#11 HNSCC or SCCHN or OP‐SCC or OPSCC or OPC or SCCOP
#12 #1 or #2 or #3 or #10 or #11
#13 MeSH descriptor: [Surgical Procedures, Operative] explode all trees 93705
#14 surg* or operat* or microsurg* or resect* or dissect* or microdissect* or excis or microresect*
#15 #13 or #14
#16 #12 and #15
#17 MeSH descriptor: [Head and Neck Neoplasms] this term only and with qualifier(s): [Surgery ‐ SU]
#18 MeSH descriptor: [Otorhinolaryngologic Neoplasms] explode all trees and with qualifier(s): [Surgery ‐ SU]
#19 MeSH descriptor: [Oropharyngeal Neoplasms] explode all trees and with qualifier(s): [Surgery ‐ SU]
#20 #16 or #17 or #18 or #19
#21 (minimal* and invasive) or transoral* or trans‐oral* or "trans oral*" or TORS or TOLS or (minimal* and access) or TLM or ORATOR or TOVANS or "video assist*" or video‐assist* or "computer guid*" or computer‐guid* or laser or robot* or TOL
#22 MeSH descriptor: [Robotics] explode all trees
#23 MeSH descriptor: [Laser Therapy] this term only
#24 MeSH descriptor: [Microsurgery] explode all trees
#25 MeSH descriptor: [Surgical Procedures, Minimally Invasive] this term only 813
#26 MeSH descriptor: [Lasers] explode all trees
#27 #21 or #22 or #23 or #24 or #25 or #26
#28 #20 and #27
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| EORTC‐1420 | ALLOCATION: prospective randomised controlled trial PARTICIPANTS: HPV‐positive OPSCC INTERVENTION: minimally invasive (endoscopic) head and neck surgery versus standard radiotherapy ± adjuvant chemotherapy (non‐surgical, therefore excluded) |
| ORATOR | ALLOCATION: prospective randomised controlled trial PARTICIPANTS: HPV‐positive OPSCC INTERVENTION: minimally invasive (endoscopic) head and neck surgery versus standard radiotherapy ± adjuvant chemotherapy (non‐surgical, therefore excluded) |
HPV: human papillomavirus OPSCC: oropharyngeal squamous cell cancer
Characteristics of ongoing studies [ordered by study ID]
ADEPT.
| Trial name or title | 'Adjuvant De‐escalation, Extracapsular Spread, P16+, Transoral (ADEPT) Trial for Oropharynx Malignancy (ADEPT)' |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Surgery + neck dissection followed by: Intervention group: radiotherapy (IMRT: 60 Gy in 30 fractions over 6 weeks) Comparator group: radiotherapy + chemotherapy (IMRT: 60 Gy in 30 fractions over 6 weeks + cisplatin 40 mg/m2 on days 1, 8, 15, 22, 29 and 36 of radiation therapy (6 doses, total 240 mg/m2)) |
| Outcomes | Primary outcomes:
Secondary outcomes:
Function and quality of life (time frame 2 years) |
| Starting date | 10 January 2013 |
| Contact information | Primary investigator: Jason Rich MD Washington University School of Medicine |
| Notes | https://clinicaltrials.gov/ct2/show/NCT01687413 |
ECOG‐E3311.
| Trial name or title | 'Phase II randomized trial of transoral surgical resection followed by low‐dose or standard‐dose IMRT in resectable p16+ locally advanced oropharynx cancer' |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group A (TOS): patients undergo transoral surgical resection of the oropharyngeal tumour Intervention group B (TOS, low‐dose IMRT): patients undergo transoral surgical resection of the oropharyngeal tumour. Patients then undergo low‐dose IMRT 5 days a week for 5 weeks. Intervention group C (TOS, standard‐dose IMRT): patients undergo transoral surgical resection of the oropharyngeal tumour. Patients then undergo standard‐dose IMRT 5 days a week for 6 weeks. Intervention group D (TOS, standard‐dose IMRT, chemotherapy): patients undergo transoral surgical resection of the oropharyngeal tumour. Patients then undergo standard‐dose IMRT 5 days a week for 6 to 7 weeks. Patients also receive cisplatin IV over 60 minutes or carboplatin IV over 30 minutes on days 1, 8, 15, 22, 29, 36 and 43 during radiation therapy. |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 10 July 2013 |
| Contact information | Primary investigator: Robert Ferris MD Eastern Cooperative Oncology Group |
| Notes | https://clinicaltrials.gov/ct2/show/study/NCT01898494 |
PATHOS.
| Trial name or title | 'Post‐operative Adjuvant Treatment for HPV‐positive Tumours (PATHOS)' |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Surgery (TLM or TORS) + neck dissection then stratification by pathology: Low‐risk (Intervention group A): no adjuvant therapy Intermediate‐risk (Intervention group B) then randomised to: (B1) Postoperative RT 60 Gy in 30 fractions (Control arm) (B2) Postoperative RT 50 Gy in 25 fractions (Test arm) High‐risk (Intervention group C) then randomised to: (C1) Postoperative RT 60 Gy in 30 fractions with concurrent cisplatin (Test arm) (C2) Postoperative RT 60 Gy in 30 fractions (Test arm) |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | December 2014 |
| Contact information | Principal Investigator: Mererid Evans PhD, Velindre NHS Trust Trial Manager: Lisette Nixon, Velindre NHS Trust |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02215265 |
CT: computerised tomography CTCAE: Common Terminology Criteria for Adverse Events ECOG: Eastern Cooperative Oncology Group EORTC: European Organisation for Research and Treatment of Cancer IMRT: intensity‐modulated radiotherapy MDT: multidisciplinary team NCI: National Cancer Institute OPSCC: oropharyngeal squamous cell cancer PET‐CT: positron emission tomography–computed tomography SCC: squamous cell cancer TLM: transoral laser microsurgery TORS: transoral robotic surgery TOS: transoral surgery UICC: Union for International Cancer Control
Contributions of authors
James Howard (JH): protocol drafting, search strategy development, acquiring study report copies, study selection, data extraction, data analysis, data interpretation, review drafting and future review updating.
Raghav C Dwivedi (RD): protocol drafting, search strategy development, study selection, data interpretation and review drafting.
Liam Masterson (LM): protocol drafting, search strategy development, study selection, data interpretation and review drafting.
Prasad Kothari (PK): protocol drafting, data interpretation and review drafting.
Harry Quon (HQ): protocol drafting, data interpretation and review drafting.
Chris Holsinger (CH): protocol drafting, data interpretation and review drafting.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
James Howard: none known.
Raghav C Dwivedi: none known.
Liam Masterson: none known.
Prasad Kothari: none known.
Harry Quon: none known.
Chris Holsinger: none known.
New
References
References to studies excluded from this review
EORTC‐1420 {published data only}
- Phase III study assessing the "best of" radiotherapy compared to the "best of" surgery (trans‐oral surgery (TOS)) in patients with T1‐T2, N0 oropharyngeal carcinoma. https://clinicaltrials.gov/ct2/show/NCT02984410 (first received 6 December 2016). [NCT02984410]
ORATOR {published data only}
- Nichols AC, Yoo J, Hammond JA, Fung K, Winquist E, Read N, et al. Early‐stage squamous cell carcinoma of the oropharynx: Radiotherapy vs. Trans‐Oral Robotic Surgery (ORATOR) ‐ study protocol for a randomized phase II trial. BMC Cancer 2013;13:133. [NCT01590355] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ADEPT {published data only}
- Haughey B. Adjuvant De‐escalation, Extracapsular Spread, P16+, Transoral (ADEPT) trial for oropharynx malignancy. https://clinicaltrials.gov/ct2/show/NCT01687413 (first received 18 September 2012). [NCT01687413]
ECOG‐E3311 {published data only}
- Ferris R. Transoral surgery followed by low‐dose or standard‐dose radiation therapy with or without chemotherapy in treating patients with HPV positive stage III‐IVA oropharyngeal cancer. https://clinicaltrials.gov/ct2/show/NCT01898494 (first received 12 July 2013). [NCT01898494]
PATHOS {published data only}
- Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, et al. PATHOS: a phase II/III trial of risk‐stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer 2015;15:602. [NCT02215265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adelstein 2003
- Adelstein DJ, Li Y, Adams GL, Wagner Jr H, Kish JA, Ensley JF, et al. An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. Journal of Clinical Oncology 2003;21(1):92‐8. [DOI] [PubMed] [Google Scholar]
Adelstein 2012
- Adelstein DJ, Ridge JA, Brizel DM, Holsinger FC, Haughey BH, O'Sullivan B, et al. Transoral resection of pharyngeal cancer: summary of a National Cancer Institute Head and Neck Cancer Steering Committee Clinical Trials Planning Meeting, November 6‐7, 2011, Arlington, Virginia. Head and Neck 2012;34(12):1681‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ang 2010
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen‐Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine 2010;363(1):24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernier 2005
- Bernier J, Cooper JS, Pajak TF, Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head & Neck 2005;27(10):843‐50. [DOI] [PubMed] [Google Scholar]
Blanchard 2011
- Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J, et al. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): a comprehensive analysis by tumour site. Radiotherapy Oncology 2011;100(1):33‐40. [DOI] [PubMed] [Google Scholar]
Chaturvedi 2011
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of Clinical Oncology 2011;29(32):4294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaturvedi 2013
- Chaturvedi AK, Anderson WF, Lortet‐Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. Journal of Clinical Oncology 2013;31(36):4550‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2010
- Chen AM, Li BQ, Jennelle RL, Lau DH, Yang CC, Courquin J, et al. Late esophageal toxicity after radiation therapy for head and neck cancer. Head and Neck 2010;32(2):178‐83. [DOI] [PubMed] [Google Scholar]
Chung 2009
- Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clinical Cancer Research 2009;15:6758‐62. [DOI] [PubMed] [Google Scholar]
de Martel 2012
- Martel C, Ferlay F, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncology 2012;13(6):607‐15. [DOI] [PubMed] [Google Scholar]
Dwivedi 2013
- Masterson L, Dwivedi RC, Allam M, Jani P. An adult with a neck lump. BMJ 2013;347:f5473. [DOI] [PubMed] [Google Scholar]
Evans 2010
- Evans M, Powell NG. The changing aetiology of head and neck cancer: the role of human papillomavirus. Clinical Oncology 2010;22:538‐46. [DOI] [PubMed] [Google Scholar]
Fakhry 2008
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus‐positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute 2008;100(4):261‐9. [DOI] [PubMed] [Google Scholar]
Forastiere 2013
- Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long‐term results of RTOG 91‐11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. Journal of Clinical Oncology 2013;31(7):845‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillison 2015
- Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus‐positive head and neck squamous cell carcinoma. Journal of Clinical Oncology 2015;33(29):3235‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2009
- Grant DG, Hinni ML, Salassa JR, Perry WC, Hayden RE, Casler JD. A case for single modality treatment with transoral laser microsurgery. JAMA Otolaryngology‐Head & Neck Surgery 2009;135(12):1225‐30. [DOI] [PubMed] [Google Scholar]
Haigentz 2009
- Haigentz M Jr, Silver CE, Corry J, Genden EM, Takes RP, Rinaldo A, et al. Current trends in initial management of oropharyngeal cancer: the declining use of open surgery. European Archives of Oto‐rhino‐laryngology 2009;266(12):1845‐55. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Haughey 2011
- Haughey BH, Hinni ML, Salassa JR, Hayden RE, Grant DG, Rich JT, et al. Transoral laser microsurgery as primary treatment for advanced stage oropharyngeal cancer: a United States multicentre study. Head and Neck 2011;33(12):1683‐94. [DOI: 10.1002/hed.21669] [DOI] [PubMed] [Google Scholar]
Holsinger 2015
- Holsinger FC, Ferris RL. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: robotics, lasers and clinical trials. Journal of Clinical Oncology 2015;33(29):3285‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2016
- Howard J, Masterson L, Dwivedi RC, Riffat F, Benson R, Jefferies S, et al. Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD010963.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2012
- Huang S, O'Sullivan B. Temporal regression of cervical lymph node in N2‐N3 head‐and‐neck cancer treated with primary radiation therapy +/‐ chemotherapy: stratified by HPV status. International Journal of Radiation Oncology, Biology, Physics 2012;1:S205. [DOI] [PubMed] [Google Scholar]
Hutcheson 2013
- Hutcheson KA, Bhayani MK, Beadle BM, Gold KA, Shinn EH, Lai SY, et al. Use it or lose it: eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers. JAMA Otolaryngology, Head and Neck Surgery 2013;139(11):1127‐34. [10.1001/jamaoto. 2013.4715.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2016
- Kelly JR, Husain ZA, Burtness B. Treatment de‐intensification strategies for head and neck cancer. European Journal of Cancer 2016;68:125‐33. [DOI: 10.1016/j.ejca.2016.09.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011
- Lee MK, Nalliah RP, Kim MK, Elangovan S, Allareddy V, Kumar‐Gajendrareddy P, et al. Prevalence and impact of complications on outcomes in patients hospitalized for oral and oropharyngeal cancer treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2011;112(5):581‐91. [DOI] [PubMed] [Google Scholar]
Leonhardt 2012
- Leonhardt FD, Quon H, Abrahao M, O'Malley Jr BW, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient‐reported quality of life and function. Head and Neck 2012;34(2):146‐54. [DOI] [PubMed] [Google Scholar]
Lewis 2011
- Lewis JS, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Modern Pathology 2011;24(11):1413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Machtay 2008
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. Journal of Clinical Oncology 2008;26(21):3582‐9. [DOI: 10.1200/JCO.2007.14.8841; PUBMED: PMC4911537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masterson 2014a
- Masterson L, Moualed D, Masood A, Dwivedi RC, Benson R, Sterling JC, et al. De‐escalation treatment protocols for human papillomavirus‐associated oropharyngeal squamous cell carcinoma. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010271.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masterson 2014b
- Masterson L, Moualed D, Liu ZW, Howard JE, Dwivedi RC, Tysome JR, et al. De‐escalation treatment protocols for human papillomavirus‐associated oropharyngeal squamous cell carcinoma: a systematic review and meta‐analysis of current clinical trials. European Journal of Cancer 2014;50(15):2636‐48. [DOI: 10.1016/j.ejca.2014.07.001] [DOI] [PubMed] [Google Scholar]
Masterson 2015
- Masterson L, Sorgeloos F, Winder D, Lechner M, Marker A, Malhotra S, et al. Deregulation of SYCP2 predicts early stage HPV+ oropharyngeal carcinoma – a prospective whole transcriptome analysis. Cancer Science 2015;106(11):1568‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxwell 2013
- Maxwell JH, Ferris RL, Gooding W, Cunningham D, Mehta V, Kim S, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer 2013;119(18):3302‐8. [DOI] [PubMed] [Google Scholar]
Mehanna 2013
- Mehanna H, Beech T, Nicholson T, El‐Hariry I, McConkey C, Paleri V, et al. The prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer: a systematic review and meta‐analysis of trends by time and region. Head and Neck 2013;35(5):747‐55. [DOI: 10.1002/hed.22015] [DOI] [PubMed] [Google Scholar]
Moore 2009
- Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope 2009;119(11):2156–64. [DOI: 10.1002/lary.20647] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore EJ, Hinni ML. Critical review: transoral laser microsurgery and robotic‐assisted surgery for oropharynx cancer including human papillomavirus‐related cancer. International Journal of Radiation Oncology, Biology, Physics 2013;85(5):1163‐7. [PUBMED: 23182390] [DOI] [PubMed] [Google Scholar]
O'Sullivan 2016
- O'Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV‐related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON‐S): a multicentre cohort study. Lancet Oncology 2016;17(4):440‐51. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Parsons 2002
- Parsons JT, Mendenhall WM, Stringer SP, Amdur RJ, Hinerman RW, Villaret DB, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer 2002;94(11):2967‐80. [PUBMED: 12115386] [DOI] [PubMed] [Google Scholar]
Pasalic 2018
- Pasalic D, Funk RK, Garcia JJ, Price DL, Price KA, Harmsen WS, et al. Magnitude of benefit for adjuvant radiotherapy following minimally invasive surgery in intermediate to high risk HPV‐positive oropharyngeal squamous cell carcinoma. Oral Oncology 2018;82:181‐6. [DOI] [PubMed] [Google Scholar]
Pyeon 2007
- Pyeon D, Newton MA, Lambert PF, Boon JA, Sengupta S, Marsit CJ, et al. Fundamental differences in cell cycle deregulation in human papillomavirus‐positive and human papillomavirus‐negative head/neck and cervical cancers. Cancer Research 2007;67:4605‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schache 2016
- Schache AG, Powell N, Cuschieri K, Robinson M, Leary S, Mehanna H, et al. HPV‐related oropharynx cancer in the United Kingdom ‐ an evolution in the understanding of disease aetiology. Cancer Research 2016;76:1‐9. [DOI: 10.1158/0008-5472.CAN-16-0633; PUBMED: 27569214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2015a
- Sinha P, Piccirillo JF, Kallogjeri D, Spitznagel EL, Haughey BH. The role of postoperative chemoradiation for oropharynx carcinoma: a critical appraisal of the published literature and National Comprehensive Cancer Network guidelines. Cancer 2015;121(11):1747‐54. [DOI] [PubMed] [Google Scholar]
Sinha 2015b
- Sinha P, Kallogjeri D, Gay H, Thorstad WL, Lewis JS Jr, Chernock R, et al. High metastatic node number, not extracapsular spread or N‐classification is a node‐related prognosticator in transorally‐resected, neck‐dissected p16‐positive oropharynx cancer. Oral Oncology 2015;51(5):514‐20. [DOI] [PubMed] [Google Scholar]
Slebos 2006
- Slebos RJ, Yi Y, Ely K, Carter J, Evjen A, Zhang X, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clinical Cancer Research 2006;12(3):701‐9. [DOI] [PubMed] [Google Scholar]
Sudhoff 2011
- Sudhoff HH, Schwarze HP, Winder D, Steinstraesser L, Gormer M, Stanley M, et al. Evidence for a causal association for HPV in head and neck cancers. European Archives of Oto‐Rhino‐Laryngology 2011;268:1541–7. [DOI] [PubMed] [Google Scholar]
TNM 2009
- Sobin LH, Gospodarowicz MK, Wittekind C (editors). TNM Classification of Malignant Tumours. 7th Edition. Chichester, UK: Wiley‐Blackwell, 2009. [Google Scholar]
TNM 2017
- Brierley JD, Gospodarowicz MK, Wittekind C (editors). TNM classification of malignant tumours. 8th Edition. Chichester, UK: Wiley‐Blackwell, 2017. [Google Scholar]
Van Dyne 2018
- Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Bernard VB. Trends in human papillomavirus‐associated cancers ‐ United States, 1999‐2015. Morbidity and Mortality Weekly Report: MMWR 2018;67:918‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- WHO. International Classification of Diseases for Oncology (ICD‐O). 3rd Edition. Geneva: World Health Organization, 2000. [Google Scholar]
Zenga 2015
- Zenga J, Wilson M, Adkins DR, Gay HA, Haughey BH, Kallogjeri D, et al. Treatment outcomes for T4 oropharyngeal squamous cell carcinoma. JAMA Otolaryngology Head & Neck Surgery 2015;141(12):1118‐27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Howard 2018
- Howard J, Dwivedi RC, Masterson L, Kothari P, Quon H, Holsinger FC. De‐intensified adjuvant (chemo)radiotherapy versus standard adjuvant chemoradiotherapy post transoral minimally invasive surgery for resectable HPV‐positive oropharyngeal carcinoma. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD012939] [DOI] [PMC free article] [PubMed] [Google Scholar]


