Abstract
Background
Mortality rates among patients with sepsis, severe sepsis or septic shock are highly variable throughout different regions or services and can be upwards of 50%. Empirical broad‐spectrum antimicrobial treatment is aimed at achieving adequate antimicrobial therapy, thus reducing mortality; however, there is a risk that empirical broad‐spectrum antimicrobial treatment can expose patients to overuse of antimicrobials. De‐escalation has been proposed as a strategy to replace empirical broad‐spectrum antimicrobial treatment by using a narrower antimicrobial therapy. This is done by reviewing the patient’s microbial culture results and then making changes to the pharmacological agent or discontinuing a pharmacological combination.
Objectives
To evaluate the effectiveness and safety of de‐escalation antimicrobial treatment for adult patients diagnosed with sepsis, severe sepsis or septic shock caused by any micro‐organism.
Search methods
In this updated version, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 10); MEDLINE via PubMed (from inception to October 2012); EMBASE (from inception to October 2012); LILACS (from inception to October 2012); Current Controlled Trials; bibliographic references of relevant studies; and specialists in the area. We applied no language restriction. We had previously searched the databases to August 2010.
Selection criteria
We planned to include randomized controlled trials (RCTs) comparing de‐escalation (based on culture results) versus standard therapy for adults with sepsis, severe sepsis or septic shock. The primary outcome was mortality (at 28 days, hospital discharge or at the end of the follow‐up period). Studies including patients initially treated with an empirical but not adequate antimicrobial therapy were not considered for inclusion.
Data collection and analysis
Two authors planned to independently select and extract data and to evaluate methodological quality of all studies. We planned to use relative risk (risk ratio) for dichotomous data and mean difference (MD) for continuous data, with 95% confidence intervals. We planned to use the random‐effects statistical model when the estimate effects of two or more studies could be combined in a meta‐analysis.
Main results
Our search strategy retrieved 493 studies. No published RCTs testing de‐escalation of antimicrobial treatment for adult patients diagnosed with sepsis, severe sepsis or septic were included in this review. We found one ongoing RCT.
Authors' conclusions
There is no adequate, direct evidence as to whether de‐escalation of antimicrobial agents is effective and safe for adults with sepsis, severe sepsis or septic shock. This uncertainty warrants further research via RCTs and the authors are awaiting the results of an ongoing RCT testing the de‐escalation of empirical antimicrobial therapy for severe sepsis.
Plain language summary
Adjustment of antimicrobial agents for adults with sepsis, severe sepsis or septic shock
Broad‐spectrum antimicrobial treatment is defined as the use of an antibiotic or a combination of antibiotics which act against a wide range of disease‐causing bacteria. Broad‐spectrum antimicrobial treatment can reduce mortality rates in patients with sepsis, severe sepsis or septic shock. Sepsis is a serious medical condition which is characterized by an inflammatory response to an infection that can affect the whole body. The patient may develop this inflammatory response to microbes in their blood, urine, lungs, skin or other tissues. However, there is a risk that empirical broad‐spectrum antimicrobial treatment can expose patients to overuse of antimicrobials and increase the resistance of micro‐organisms to treatment. De‐escalation has been proposed as a means of adjusting initial, adequate broad‐spectrum treatment by changing the antimicrobial agent or discontinuing an antimicrobial combination according to the patient's culture results (a means of identifying the microbe causing the infection). In this updated Cochrane review we searched the databases until October 2012. We found no published randomized controlled trials (RCTs). We found one ongoing RCT. There is no adequate or direct evidence on whether de‐escalation of antimicrobial agents is effective and safe for adults with sepsis, severe sepsis or septic shock. Appropriate studies are needed to investigate the potential benefits proposed by de‐escalation treatment.
Background
Description of the condition
Sepsis is defined as a systemic inflammatory response to an infection (Bone 1992). Acute organ dysfunction caused by the infection is defined as severe sepsis, which when combined with persistent hypotension causes a condition defined as septic shock (Dellinger 2008). There are clinical and laboratory characteristics to be considered in the diagnosis of sepsis, severe sepsis or septic shock. These include fever, hypothermia, level of consciousness and inflammatory parameters (Levy 2003).
Irrespective of geographic and socio‐economic circumstances, sepsis, severe sepsis or septic shock have been associated with mortality. In a cohort study involving 3147 patients admitted to intensive care units (ICU) in 24 European countries, the rate of sepsis was 37% (mortality rate 27%); 30% had severe sepsis (mortality rate 32%) and 15% had septic shock (mortality rate 54%) (Vincent 2006). Similar findings could be seen in North America (from 1993 to 2003) (Dombrovskiy 2007). In the latter study an alarming prevalence of 2,857,476 cases of severe sepsis was found among more than eight million patients with sepsis. Higher mortality rates have been observed in other countries, for example in Brazil (Silva 2004; Teles 2008). Moreover, other studies from different countries have shown that the most prevalent infectious agents responsible for sepsis and severe sepsis are Staphylococcus sp.; Escherichia coli;Candida sp.;Pseudomonas sp.;Acinetobacter sp.; Streptococcus sp.;Klebsiella sp.; Enterococcus sp.;Enterobacter sp.;and Proteus sp. (Cheng 2007; Dougnac 2007; Garnacho‐Montero 2003; Iñigo 2006; Vincent 2006).
Before commencing antimicrobial therapy, it is necessary to obtain appropriate cultures in order to identify the pathogens responsible for the septic conditions. Factors that should be taken into account are that sampling should not delay the antimicrobial treatment in patients with severe sepsis; rapid sterilization of blood cultures can occur within a few hours after the first antibiotic dose (Dellinger 2008); and previous or concomitant antimicrobial administration can impair the culture results (Darby 1997).
A broad‐spectrum antimicrobial treatment is usually used to achieve adequate antibiotic therapy as soon as possible. This is because early and adequate antimicrobial therapy reduces mortality rates (ATS IDSA 2005; Harbarth 2003; Kumar 2006; Micek 2005; Proulx 2005). Unfortunately this approach can expose individuals to an overuse of antimicrobials. This is mainly because of emerging resistant pathogens, which increase the risk of inappropriate therapy (Leone 2007; Niederman 2006). Large pharmaceutical companies have recently decreased their antibiotic discovery efforts resulting in a dearth of resources being invested to target antibiotic resistance (IDSA 2004).
Strategies have been developed to solve the problems associated with the overuse of antimicrobials. For instance, a Cochrane systematic review offered favourable evidence for monotherapy (beta‐lactam alone) as compared to combination antibiotic therapy (beta‐lactam combined with aminoglycosides) (Paul 2006). According to Leone 2008, "restricting the use of antibiotics should remain the common rule" in order to minimize the chances for the emergence of multiresistant bacteria; and de‐escalation is one such strategy.
Description of the intervention
De‐escalation has been proposed by Kollef (Kollef 2006) and consists of the following.
1. Beginning treatment with an empirical broad‐spectrum antimicrobial therapy, aiming to cover the probable infectious agent(s).
2. Changing the empirical and appropriate broad‐spectrum antimicrobial to a narrower‐spectrum antimicrobial therapy by one of two ways:
changing the antimicrobial agent;
discontinuing an antimicrobial combination.
3. A further strategy is to shorten the course of the antimicrobial therapy.
Culture results are a prerequisite for the use of de‐escalation for patients with sepsis, severe sepsis or septic shock (Dellinger 2008; Höffken 2002) but the decision to de‐escalate has to also be based on the clinical evolution of the patient.
Some evidence on antibiotic de‐escalation is available for ventilator‐associated pneumonia (ATS IDSA 2005; Singh 2000). However antibiotic de‐escalation has been suggested by the 'Surviving Sepsis Campaign' for patients with sepsis, severe sepsis or septic shock based on poor quality evidence (Dellinger 2008).
How the intervention might work
Adequate antimicrobial therapy is associated with lower mortality rates in patients with sepsis, severe sepsis or shock septic (Harbarth 2003; McArthur 2004; Vallés 2003). The overuse of antimicrobials, usually characterized by broad spectrum antimicrobial therapies, may be related to adverse events, extra costs (Glowacki 2003) and the emergence of bacterial resistance (Leone 2008). Thus the use of an initial broad‐spectrum antimicrobial regimen with appropriate coverage would need to be balanced against the withdrawal of unnecessary drugs. Therefore, de‐escalation is essentially a proposed approach to minimize antimicrobial exposure, avoid the overuse of antibiotics, and to consequently minimize the adverse events and emergence of resistant micro‐organisms (Rello 2004).
Why it is important to do this review
The main guideline on sepsis, the 'Surviving Sepsis Campaign' (Dellinger 2008), has suggested de‐escalation as an option to avoid undesired manifestations associated with the overuse of antimicrobials. In view of the probable increase in de‐escalation of antimicrobial therapy, the authors of this current systematic review intended to combine all existing evidence in order to improve the directions for future trials involving patients with sepsis, severe sepsis or septic shock caused by any micro‐organism. The aim of this review was to assess the evidence from available randomized studies in order to improve practice in the area of sepsis.
Objectives
To evaluate the effectiveness and safety of antimicrobial de‐escalation when compared with the maintenance of broad‐spectrum therapy for adult patients diagnosed with sepsis, severe sepsis or septic shock caused by any micro‐organism.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized or quasi‐randomized controlled trials.
Types of participants
We planned to include adult patients (aged 18 years and older) with sepsis, severe sepsis or septic shock caused by any micro‐organism.
Types of interventions
Our comparison groups of interest were as follows.
De‐escalation: defined as changing an initially appropriate antimicrobial therapy from an empirical broad‐spectrum characteristic to a narrower‐spectrum one (by either changing the antimicrobial agent or by discontinuing an eventual antimicrobial combination, or both) according to culture results (Kollef 2001; Leone 2007; Niederman 2006) or clinical conditions.
Standard therapy: defined as the maintenance of an initial empirical broad‐spectrum antimicrobial therapy (independent of whether the antimicrobial therapy was a combination or a single agent).
We also considered de‐escalation defined as the shortening of the time course of the antimicrobial therapy (for example short‐course versus long‐course antimicrobial therapy), trial by trial, to see whether it fulfilled the conditions for this review.
We planned to consider comparison arms for analysis irrespective of the types of antimicrobial agents and possible combinations.
Studies in which the patients were previously treated with an empirical but not adequate antimicrobial therapy were not considered for inclusion.
Types of outcome measures
Primary outcomes
Mortality at day 28
Mortality at hospital discharge or at the end of the follow‐up period
Secondary outcomes
Hospital length of stay
Intensive care unit (ICU) length of stay
Adverse events (e.g., hepatotoxicity, nephrotoxicity)
Individual antimicrobial resistance
Environmental antimicrobial resistance (de Jonge 2003)
Re‐infection
Search methods for identification of studies
Electronic searches
In our original review we searched the databases to August 2010. In this updated review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 9); MEDLINE via PubMed (from inception to October 2012); EMBASE (from inception to October 2012); and LILACS (from inception to October 2012).
We used the search terms and synonyms for "sepsis", "severe sepsis", "septic shock" (clinical conditions of interest), "antimicrobial therapy" and "de‐escalation" (intervention of interest) together with specialized filters for randomized controlled trials for MEDLINE and EMBASE (Appendix 1; Appendix 2; Appendix 3).
We searched for ongoing trials on the Current Controlled Trials website (www.controlled‐trials.com/).
We did not apply any language restriction.
Searching other resources
We searched the bibliographic references of relevant studies, irrespective of study design (narrative reviews, retrospective studies, etc) with the intention of finding cited randomized studies to be included in this review; as well as conference proceedings of relevant scientific societies, published in their official journals.
We contacted authors of relevant studies in the area for information on additional unpublished studies.
Data collection and analysis
Selection of studies
Two authors (BNGS and RBA) independently assessed the titles and abstracts of the identified articles to determine their potential relevance. We planned to resolve any disagreements by discussion with a third author (RS); this was not necessary for the first version of this systematic review. We planned to use the Kappa coefficient to formally test concordance between observers (Lattour 1997).
Data extraction and management
Two authors (BNGS and RBA) planned to independently extract data from each study using a data extraction form (see Appendix 4 ). We planned to resolve any disagreements by discussion with a third author (RS), but this was not necessary during preparation of the first version of this systematic review.
Assessment of risk of bias in included studies
Two authors (BNGS and RBA) planned to independently assess the methodological quality of included studies according to the study design, using the following items.
Selection bias
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Were there systematic differences between the baseline characteristics of the groups that were compared?
Performance bias
Were there systematic differences between groups in the care that was provided, or in exposure to factors other than the interventions of interest?
Detection bias
Were there systematic differences between groups in how outcomes were determined?
Attrition bias
Were incomplete outcome data adequately addressed?
Selective reporting bias
Were reports of the study free of suggestion of selective outcome reporting?
Other bias
Was the study apparently free of other problems that could put it at a high risk of bias?
For each one of the above items, we planned to classify studies according to their risk of systematic error
High risk: when the appropriate method to avoid systematic error (bias) was not met
Moderate risk: when the appropriate method to avoid systematic error (bias) was not described or the information was not acquired by contacting the authors of the primary studies
Low risk: when the appropriate method to avoid systematic error (bias) was met
Measures of treatment effect
For dichotomous variables, we planned to calculate the risk ratio (RR). For continuous variables, we planned to calculate the mean difference (MD) if the studies reported their results through the same variables measured with the same instruments (same units of measurement). On the other hand, when continuous data were relative to the same aspect in the patients but were measured with different instruments (and were not interchangeable units of measurement) it was intended to pool them by using standardized mean difference (SMD). The 95% confidence interval (95% CI) was to be determined for all statistical methods.
Unit of analysis issues
The unit of analysis was to be based on the individual patient. We had expected to find only parallel group study designs, not cross‐over studies. This is because of the natural history of sepsis, severe sepsis or septic shock. That is, the need to resolve the condition within a short time frame.
Dealing with missing data
For dichotomous data, we planned to use intention‐to‐treat analyses (ITT) by including all participants randomized to the intervention groups. For continuous data, we planned to try to contact the authors of the primary studies to supply missing information for participants who withdrew from the studies. We planned to analyse data based on the last individual data before the withdrawal. If we were unsuccessful in obtaining the missing data from the study authors, then we planned to perform available case analysis. If any studies did not report withdrawals, then we planned to assume that there were no withdrawals.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using the I2 statistic (Higgins 2002). We planned to assume a statistically significant heterogeneity between estimated effects of included studies when I2 > 50%. We planned to use the random‐effects model if significant heterogeneity was found.
Assessment of reporting biases
If there were a sufficient number of available studies, we had planned to assess publication bias by preparing a funnel plot. However, we were aware that asymmetry in the funnel plot can be associated with other reasons than publication bias (for example chance; real heterogeneity; clinical particularities inherent to each one of the included studies, such as patients at high risk of the outcome; etc).
Data synthesis
Qualitative data
We planned to synthesize and present qualitative information relative to methods, risk of bias, description of participants and outcomes measures and present them in the table 'Characteristics of included studies'.
Quantitative data
Irrespective of the nature of the data we planned to use the random‐effects model because substantial clinical and methodological heterogeneities were expected, which by themselves could generate substantial statistical heterogeneity. When data from primary studies were not parametric (for example effects reported as medians, quartiles, etc) or are without sufficient statistical information (for example standard deviations, number of patients, etc) we planned to insert them into an 'Additional table'.
Subgroup analysis and investigation of heterogeneity
We intended to carry out subgroup analyses by type of de‐escalation (guided by culture, stopping one drug of a combination, or guided by clinical signs). We planned to perform subgroup analysis according to: the type of infectious agent, fungi or bacteria; and site of infection (for example gastrointestinal, urinary, respiratory, abdominal, and surgical focus). We planned that heterogeneity in both the direction and length of estimate effect between subgroups would be assumed as a suspected causal relationship between them (the subgroup characteristic and the estimate effect).
Sensitivity analysis
We planned to use sensitivity analyses to examine the effects of study quality and any trials that were only reported as abstracts. This will be performed in updated versions of this systematic review.
Results
Description of studies
Results of the search
Our sensitive search strategy yielded 158 references in MEDLINE (PubMed), 52 in EMBASE, 302 in The Cochrane Library, 12 studies in Current Controlled Trials, and two in LILACS; and one ongoing trial by contacting the specialists in the area. We did not retrieve any studies in the reference lists of the main articles. After discarding duplicates we identified 493 publications.
Because of the lack of suitable studies in this area the two authors (BNGS and RBA), after screening the references by title and abstract, initially selected 71 studies. Although most were not RCTs they expressed the idea of a 'more restrictive' or 'rational' use of antimicrobial regimens or made suggestions about adjustment of an initial and empirical broad‐spectrum antimicrobial therapy to a narrowed‐spectrum antimicrobial therapy, irrespective of their inclusion criteria (participants) and study design. Of these 71 studies, 59 were not considered suitable because of their study design (Appendix 5). The 59 studies were comprised of 22 observational studies (cohort, case‐control, or prevalence studies), one an in vitro study, one a guideline, and 34 narrative or systematic reviews (including the previous version of this own systematic review). We did not calculate the Kappa coefficient because none of these studies met our inclusion criteria. For more details, see Figure 1.
1.
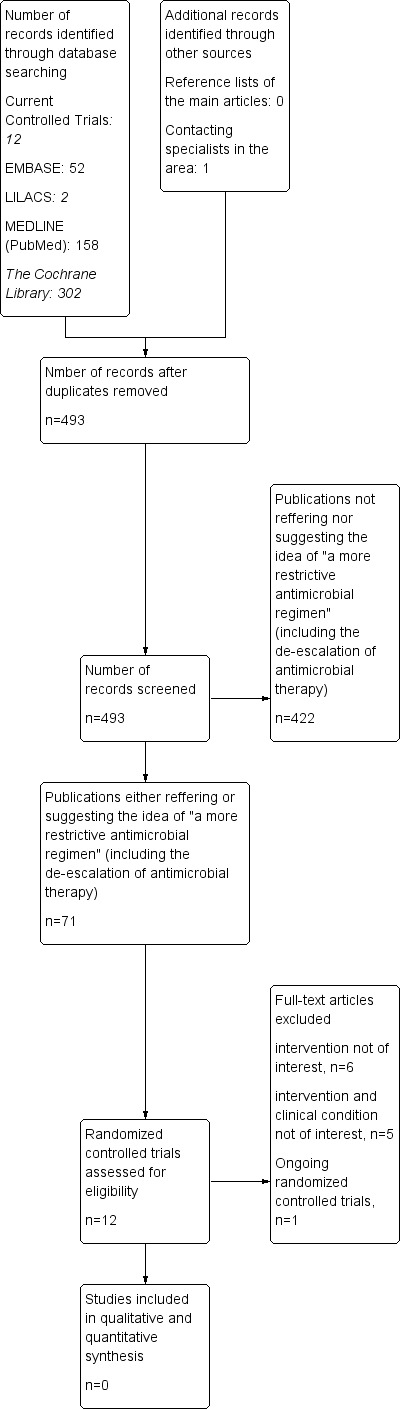
Study flow diagram.
Included studies
We did not include any studies in this updated review.
Excluded studies
We excluded the remaining 12 references either because their interventions were not of interest (Bailey 1996; Bouadma 2010; Mabasa 2009; Masaoka 2000; Roberts 2009; Schroeder 2009) or because their interventions and inclusion criteria (clinical conditions) were not of interest to this review (Christ‐Crain 2004; Horisberger 2004; Jensen 2008; van den Anker 1995; Vuori‐Holopainen 2000) (see Characteristics of excluded studies). One study is an ongoing randomized controlled trial on the de‐escalation of empirical antimicrobial therapy for severe sepsis (Leone 2012) and the authors of this systematic review are awaiting its results.
Of the 12 studies we had paid special attention to, four studies (Bouadma 2010; Christ‐Crain 2004; Jensen 2008; Schroeder 2009) were excluded because they randomized the patients to either:
monitoring by procalcitonin (inflammatory marker) levels, or
a control group.
The patients' antibiotics were commenced or ceased based on procalcitonin concentrations. The patients were not randomized to have an initial empirical, broad‐spectrum antimicrobial therapy which was adjusted according to their culture results or clinical condition. Therefore, these four studies were not considered suitable for inclusion in this review.
Risk of bias in included studies
There was no eligible study.
Allocation
This category was not evaluated since no eligible study was found.
Blinding
This category was not evaluated since no eligible study was found.
Incomplete outcome data
This category was not evaluated since no eligible study was found.
Selective reporting
This category was not evaluated since no eligible study was found.
Other potential sources of bias
This category was not evaluated since no eligible study was found.
Effects of interventions
There was no eligible study.
Discussion
Summary of main results
We found no adequate, direct evidence as to whether de‐escalation of antimicrobial agents is effective and safe for adults with sepsis, severe sepsis or septic shock.
Overall completeness and applicability of evidence
We hope the information available in this systematic review will encourage researchers and specialists to test the de‐escalation of antimicrobial agents with the methodological rigour inherent in randomized controlled trials. Currently, there is no available evidence to recommend or not the de‐escalation of antimicrobial agents in clinical practice for septic patients. This lack of evidence justifies future randomized controlled trials or cohort studies. However, some clinical and methodological particularities should be considered (for example new infectious focus, recurrence, any intercurrent event needing changes in the antimicrobial therapy, or unavailability of microbiological culture) to avoid additional risks of harms (for example worsening of clinical condition, mortality). We offer a simplified model of patient flow for future randomized trials in this area, see Figure 2.
2.
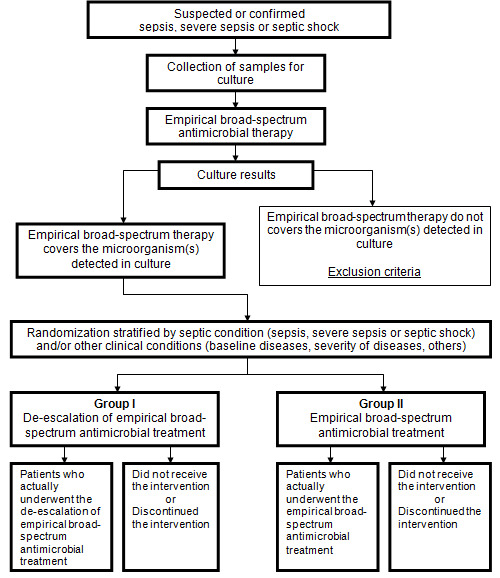
A simplified patients' flow for future randomized controlled trials testing the de‐escalation of antimicrobial therapy for septic patients. Adapted with kind permission of David Moher from the figure in Moher 2005.
We suggest sample sizes for two hypothesis.
Absence of difference in mortality between comparison groups (de‐escalation versus control)
Baseline risk of 27% for mortality among septic patients (Vincent 2006)
Assumed relative risk reduction of 10% for mortality in the de‐escalation group, corresponding to 24% of mortality (risk difference between comparison groups 3%)
4599 patients would be needed for each one of the comparison groups, according to the formula n = [2PC·(1‐PC)·(Zα+Zβ)2]·(PE‐PC)‐2 (Pocock 1983), where PC = 27%; PE = 24%; Zα = 1.96; Zβ = 1.28.
Reduction of mortality in the de‐escalation group (indirect evidence obtained from observational study in ventilator‐associated pneumonia)
Baseline risk of 27% for mortality among septic patients (Vincent 2006)
Indirect evidence of relative risk reduction of 28% for mortality in the de‐escalation group in patients with ventilator‐associated pneumonia, corresponding to a mortality rate of 19% in the de‐escalation group (risk difference between comparison groups of 8%) (Kollef 2006)
323 patients would be needed for each one of the comparison groups, according to the formula n = [PE·(1‐PE)·(Zα+Zβ)2]·(PE‐PC)‐2 (Pocock 1983), where PC = 27%; PE = 19%; Zα = 1.96; Zβ = 1.28.
Quality of the evidence
We found a complete absence of direct evidence regarding the de‐escalation of antimicrobial agents for adults with sepsis, severe sepsis or septic shock.
Potential biases in the review process
The high sensitivity of the search strategy we used in this systematic review should guarantee a low probability that we have missed any randomized controlled trials which would fulfil our inclusion criteria. Language bias was prevented by not imposing any language restriction. Other methodological issues of this review, such as data collection and analysis, cannot be judged since no adequate study could be found.
Agreements and disagreements with other studies or reviews
The World Health Organization and other health organizations have been encouraging the selection of interventions to minimize microorganisms that are resistant to antimicrobial agents, with important implications for world health and the economy (IDSA 2006; WHO 2002). Thus, several authors support the de‐escalation of antibiotics as a reasonable strategy to achieve this aim besides the minor adverse events and costs (Heenen 2012; Masterton 2011; Morel 2010; Shime 2011). In a narrative review, Deresinski 2007 suggests the de‐escalation of antimicrobial antibiotics in ICUs according to patients’ culture results and their clinical evolution. Available guidelines, specifically the 'Surviving Sepsis Campaign', have also suggested de‐escalation of antimicrobial agents for adults with sepsis, severe sepsis or septic shock based on specialists’ opinions or indirect evidence (Dellinger 2008).
Authors' conclusions
Implications for practice.
There is no adequate evidence as to whether de‐escalation of antimicrobial agents is, or is not, effective and safe for adults with sepsis, severe sepsis or septic shock.
Implications for research.
The information available in this systematic review should encourage researchers and specialists to test the de‐escalation of antimicrobial agents with the methodological rigour inherent in randomized controlled trials. This lack of information justifies future randomized controlled trials or cohort studies considering ethical, epidemiological and economical points of views. However, several clinical particularities as well as operational or methodological circumstances have to be better understood. Specific inclusion criteria and reasons for protocol deviations may be adopted to avoid additional risks of harms. Future trials can test for two hypothesis:
absence of difference in mortality between the de‐escalation and the control groups (maintained empirical broad‐spectrum antimicrobial therapy) (n ≃ 4600 patients for each of the comparison groups);
relative risk reduction of 28% for mortality in the de‐escalation group, considering the mortality baseline risk of 27% (n ≃ 323 for each of the comparison groups).
The authors of this review are awaiting the results of an ongoing randomized controlled trial by Leone 2012.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 18 February 2013 | New search has been performed | In the previous version (Silva 2010) we searched the databases until August 2010. In this updated version, we reran the searches until October 2012. |
| 18 February 2013 | New citation required but conclusions have not changed | We found no published randomized controlled trials (RCTs). We found one ongoing RCT (Leone 2012). |
Acknowledgements
We would like to thank the Cochrane Anaesthesia Review Group for their support throughout the entire editorial process.
We would like to thank Nicola Petrucci (content editor), Marissa M Alejandria, Marc Leone (peer reviewers) and Ann E Fonfa (consumer representative) for their help and editorial advice during the preparation of this review. In addition we also thank Nicola Petrucci (content editor), Marya Zilberberg and Mical Paul (peer reviewers) and Janet Wale and Tracey Lloyd (Cochrane Consumer Network) for their help and editorial advice during the preparation of the protocol for the review.
Appendices
Appendix 1. Search for MEDLINE (via PubMed)
#1 (Sepsis [Mesh]) OR (Septicemia) OR (Blood stream infection) OR (Septic shock) OR (Endotoxic Shock) OR (Toxic Shock) OR (Severe sepsis)
#2 (Anti‐Bacterial agents [Mesh]) OR (antibiotic therapy) OR (Anti Bacterial) OR (Antibacterial) OR (Anti‐Mycobacterial) OR (Bactericidal) OR (Anti‐Mycobacterial) OR (Anti Mycobacterial) OR (Antimycobacterial) OR (Antibiotics) OR (Antibiotic) OR (Bacteriocidal) OR (Bacteriocides) OR (Antifungal agents [Mesh]) OR (Anti‐fungal) OR (Antifungic) OR (Anti‐fungic) OR (Fungicides) OR (Chemotherapies) OR (Chemotherapy) OR (Drug Therapies) OR (Drug Therapy [Mesh]) OR (Pharmacotherapies) OR (Pharmacotherapy)
#3 (Adequacy) OR (Adequate) OR (Extended‐spectrum) OR (Appropriate) OR (Empiric) OR (Empirical) OR (Broad‐spectrum) OR (Broad spectrum)
#4 (De‐escalation) OR (De escalation) OR (Deescalate) OR (Narrow spectrum) OR (Narrow‐spectrum) OR (Narrower spectrum) OR (Narrower‐spectrum) OR (Narrowered‐spectrum) OR (Narrowered spectrum) OR (Narrowing) OR (Adjustment) OR (Adjust) OR (Tailoring) OR (Tailored) OR (Tailor) OR (Downgrading) OR (Discontinue) OR (discontinuing)
#5 ((randomized controlled trial [pt]) OR (controlled clinical trial [pt]) OR (randomized [tiab]) OR (placebo [tiab]) OR (drug therapy [sh]) OR (randomly [tiab]) OR (trial [tiab]) OR (groups [tiab])) AND (humans[mh])
#6 #1 AND #2 AND #3 AND #4 AND #5
Appendix 2. EMBASE via Ovid
1 'sepsis[emtree]'/exp OR sepsis OR 'septicemia'/exp OR septicemia OR ('blood'/exp OR blood AND stream AND ('infection'/exp OR infection)) OR (septic AND ('shock'/exp OR shock)) OR (endotoxic AND ('shock'/exp OR shock)) OR (toxic AND ('shock'/exp OR shock)) OR (severe AND ('sepsis'/exp OR sepsis))
2 'antiinfective agent[emtree]' OR 'anti bacterial' OR ('antibiotic' OR 'antibiotic'/exp OR antibiotic AND ('therapy' OR 'therapy'/exp OR therapy)) OR (anti AND bacterial) OR antibacterial OR bactericidal OR 'anti mycobacterial'OR (anti AND mycobacterial) OR antimycobacterial OR 'antibiotics' OR 'antibiotics'/exp OR antibioticsOR 'antibiotic' OR 'antibiotic'/exp OR antibiotic OR bacteriocidalOR bacteriocides OR 'antifungal'OR 'antifungal'/exp OR antifungalOR 'anti fungal' OR antifungicOR 'anti fungic' OR fungicidesOR chemotherapies OR 'chemotherapy'OR 'chemotherapy'/exp OR chemotherapyOR ('drug' OR 'drug'/exp OR drug AND therapies) OR ('drug' OR 'drug'/exp OR drug AND ('therapy' OR 'therapy'/exp OR therapy)) OR pharmacotherapies OR 'pharmacotherapy'OR 'pharmacotherapy'/exp OR pharmacotherapy
3 adequacy OR adequate OR 'extended spectrum' OR appropriate OR empiric OR empirical OR 'broad spectrum' OR (broad AND ('spectrum'/exp OR spectrum))
4 narrow AND ('spectrum'/exp OR spectrum) OR 'narrow spectrum' OR (narrower AND ('spectrum'/exp OR spectrum)) OR 'narrower spectrum' OR 'narrowered spectrum' OR (narrowered AND ('spectrum'/exp OR spectrum)) OR (de AND escalation) OR narrowing ORdeescalate OR 'de escalation' OR 'adjustment'/exp OR adjustment OR adjust OR tailoring OR tailored OR tailor OR downgrading OR discontinue OR discontinuing OR switch$
5 (random$) OR (factorial$) OR (crossover$) OR (cross over$) OR (cross‐over$) OR (placebo$) OR (doubl$ adj blind$) OR (singl$ adj blind$) OR (assign$) OR (allocat$) OR (volunteer$) OR (crossover‐procedure) OR (double‐blind procedure) OR (randomized controlled trial) OR (single‐blind procedure)
6 1 and 2 and 3 and 4 and 5
Appendix 3. Search strategy for LILACS (via Bireme)
#1 (Sepsis) OR (Septicemia) OR (Blood stream infection) OR (Septic shock) OR (Endotoxic Shock) OR (Toxic Shock) OR (Severe sepsis)
#2 ((Anti‐Bacterial agents) OR (antibiotic therapy) OR (Anti Bacterial) OR (Antibacterial) OR (Anti‐Mycobacterial) OR (Bactericidal) OR (Anti‐Mycobacterial) OR (Anti Mycobacterial) OR (Antimycobacterial) OR (Antibiotics) OR (Antibiotic) OR (Bacteriocidal) OR (Bacteriocides) OR (Antifungal agents) OR (Anti‐fungal) OR (Antifungic) OR (Anti‐fungic) OR (Fungicides) OR (Chemotherapies) OR (Chemotherapy) OR (Drug Therapies) OR (Drug Therapy) OR (Pharmacotherapies) OR (Pharmacotherapy)) AND ((Adequacy) OR (Adequate) OR (Extended‐spectrum) OR (Appropriate) OR (Empiric) OR (Empirical) OR (Broad‐spectrum) OR (Broad spectrum))
#3 (De‐escalation) OR (De escalation) OR (Deescalate) OR (Narrow spectrum) OR (Narrow‐spectrum) OR (Narrower spectrum) OR (Narrower‐spectrum) OR (Narrowered‐spectrum) OR (Narrowered spectrum) OR (Narrowing) OR (Adjustment) OR (Adjust) OR (Tailoring) OR (Tailored) OR (Tailor) OR (Downgrading) OR (Discontinue) OR (discontinuing)
#4 #1 and #2 and #3
Appendix 4. Data extraction form
Study Selection, Quality Assessment & Data Extraction Form
| First author | Journal/Conference Proceedings etc | Year |
Study eligibility
| RCT/Quasi‐randomized | Participants with sepsis, severe sepsis or septic shock | De‐escalation* | Relevant outcomes |
| Yes / No / Unclear | Yes / No / Unclear | Yes / No / Unclear | Yes / No* / Unclear |
* De‐escalation, as defined by the changing the empirical and adequate broad spectrum to a narrower spectrum antimicrobial therapy by changing the antimicrobial agent or discontinuing an antimicrobial combination
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| Freehand space for comments on study design and treatment: |
References to trial (Secondary references)
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers |
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Disease status / type, etc (if applicable) | |
| Undelying disease | |
| % of appropriate empirical antibiotic treatment | |
| Setting | |
| Other | |
Trial characteristics
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Adequate (Random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate | |
| Inadequate | |
| Unclear | |
| Blinding | |
| Person responsible for participants care | Yes / No |
| Participant | Yes / No |
| Outcome assessor | Yes / No |
| Other (please specify) | Yes / No |
|
Intention‐to‐treat (consider each one of the outcomes) An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes ? No ? not clear ?
Discuss if appropriate
Data extraction
|
Outcomes relevant to your review Copy and paste from ‘Types of outcome measures’ | |
| Reported in paper (circle) | |
| Primary outcomes | |
| 1) mortality. | Yes / No |
| 2) hospital length stay; | Yes / No |
| 3) intensive care unit (ICU) length stay | Yes / No |
| Secondary outcomes | |
| 1) adverse events (e.g., hepatotoxicity, nephrotoxicity); | Yes / No |
| 2) individual antimicrobial resistance; | Yes / No |
| 3) environmental antimicrobial resistance | Yes / No |
| 4) re‐infection | Yes / No |
| For Continuous data | |||||||
| Code of paper |
Outcomes (rename) |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A etc | 1) Mean time to mortality. | ||||||
| 2) Mean hospital length stay; | |||||||
| 3) Mean intensive care unit (ICU) length stay | |||||||
| For Dichotomous data | |||
| Code of paper | Outcomes (rename) | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| Primary outcomes | |||
| 1) mortality. | |||
| 2) hospital length stay | |||
| 3) intensive care unit (ICU) length stay | |||
| Secondary outcomes | |||
| 1) adverse events (e.g., hepatotoxicity, nephrotoxicity) | |||
| 2) individual antimicrobial resistance | |||
| 3) environmental antimicrobial resistance | |||
| 4) re‐infection | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
| Trial characteristics | |
| Further details | |
| Single centre / multicentre | |
| Country / Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose / frequency of administration | |
| Duration of treatment (State weeks / months, etc, if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design (e.g. parallel / cross‐over*) | |
| Other | |
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data
Appendix 5. Studies referring to the idea of 'de‐escalation' of antimicrobial agents for diverse clinical conditions
| Study | Study design | |
| 1 | Adukauskiene 2006 | Narrative review |
| 2 | Alexandraki 2008 | Observational study |
| 3 | Apisarnthanarak 2004 | Observational study |
| 4 | Bagshaw 2009 | Observational study |
| 5 | Balk 2004 | Narrative review |
| 6 | Berild 2006 | Observational study |
| 7 | Brunkhorst 2009 | Narrative review |
| 8 | Carcelero 2012 | Narrative review |
| 9 | Cattoir 2010 | Narrative review |
| 10 | Cheadle 1992 | Narrative review |
| 11 | Colardyn 2005 | Narrative review |
| 12 | Cordero 2006 | Observational study |
| 13 | Cunha 2008 | Narrative review |
| 14 | De Angelis 2011 | systematic review |
| 15 | Dellinger 2008 | Guideline |
| 16 | Deresinski 2007 | Narrative review |
| 17 | Drekonja 2008 | Narrative review |
| 18 | Erlandsson 2007 | Observational study |
| 19 | Filius 2002 | Narrative review |
| 20 | Fluckiger 2000 | Observational study |
| 21 | Galal 2010 | Narrative review |
| 22 | Garnacho‐Montero 2003 | Observational study |
| 23 | Gomes Silva 2010 | Systematic review |
| 24 | Guillon 2010 | in vitro study |
| 25 | Harbarth 2003 | Observational study |
| 26 | Heenen 2012 | Observational study |
| 27 | Hitt 1997 | Narrative review |
| 28 | Kielstein 2011 | Narrative review |
| 29 | Kumar 2009 | Observational study |
| 30 | Kumar 2011 | Narrative review |
| 31 | Lane 2011 | Narrative review |
| 32 | Leone 2007 | Observational study |
| 33 | Liew 2011 | Observational study |
| 34 | Lipman 2009 | Observational study |
| 35 | Malacarne 2004 | Observational study |
| 36 | Masterton 2011 | Narrative review |
| 37 | McCabe 2010 | Observational study |
| 38 | McNulty 1997 | Observational study |
| 39 | Miano 2012 | Observational study |
| 40 | Mol 2006 | Observational study |
| 41 | Morel 2010 | Observational study |
| 42 | Mutlu 2006 | Narrative review |
| 43 | Napolitano 2009 | Narrative review |
| 44 | Niederman 2006 | Narrative review |
| 45 | Pea 2009 | Narrative review |
| 46 | Raisch 1988 | Observational study |
| 47 | Richards 2005 | Narrative review |
| 48 | Rodloff 2006 | Narrative review |
| 49 | Sanchez 1997 | Observational study |
| 50 | Schierbeck 207 | Narrative review |
| 51 | Schuler 1994 | Narrative review |
| 52 | Shani 2009 | Systematic review |
| 53 | Shime 2011 | Observational study |
| 54 | Spies 2009 | Observational study |
| 55 | Textoris 2011 | Narrative review |
| 56 | Tripathi 2012 | Narrative review |
| 57 | Welte 2004 | Narrative review |
| 58 | West 2008 | Narrative review |
| 59 | Zaragoza 2008 | Narrative review |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bailey 1996 | Intervention not of interest: single large iv dose (10 mg/kg) of gentamicin with a standard multiple dose regimen of gentamicin. |
| Bouadma 2010 | Intervention not of interest, patients were randomized to:
The patients were not randomized to have an initial empirical and broad‐spectrum antimicrobial therapy, adjusted according to the culture results or clinical condition. |
| Christ‐Crain 2004 | Intervention not of interest: patients randomized to be monitored by inflammatory marker (procalcitonin) or control group. The patients were not randomized to have their antimicrobial therapy adjusted according to the culture results or clinical condition. Clinical condition out of area of interest: ICU patients with no obvious site of Infection. |
| Horisberger 2004 | Interventions not of interest: routine sepsis work up versus intervention strategy with additional cytokine measurements. Clinical condition not of interest: paediatric patients. |
| Jensen 2008 | Interventions not of interest: procalcitonin measurements. Clinical condition out of area of interest: ICU patients. |
| Mabasa 2009 | Intervention out of area of interest: participants with septic shock were randomized to have renally adjusted dosage of antibiotics. |
| Masaoka 2000 | Interventions out of area of interest: intravenous immunoglobulin in combination therapy with antibiotics versus antibiotics monotherapy. |
| Roberts 2009 | Intervention out of area of interest: different daily doses of piperacillin‐tazobactam by bolus dosing or continuous infusion. |
| Schroeder 2009 | Intervention out of area of interest, patients were randomized to:
|
| van den Anker 1995 | Intervention not of interest (once‐daily versus twice‐daily administration of ceftazidime), clinical condition not of interest (preterm infants). |
| Vuori‐Holopainen 2000 | Interventions out of area of interest: procaine penicillin intramuscularly (narrow‐spectrum antimicrobial) versus cefuroxime intravenously (broad‐spectrum antimicrobial) for 4 to 7 days. Clinical condition out of area of interest: common infections of childhood. |
ICU ‐ intensive care unit
iv ‐ intravenous
Characteristics of ongoing studies [ordered by study ID]
Leone 2012.
| Trial name or title | De‐escalation of Empirical Antimicrobial Therapy Study in Severe Sepsis |
| Methods | Open label randomized controlled trial |
| Participants |
|
| Interventions | 1. Experimental: a strategy based on de‐escalation intervention. Procedure: streamlining of the empirical antimicrobial therapy 2. Active comparator: a conservative strategy intervention. Procedure: continuation of the empirical antimicrobial therapy |
| Outcomes | |
| Starting date | October 2011 |
| Contact information | Marc Leone marc.leone@ap‐hm.fr |
| Notes |
Differences between protocol and review
We inserted two new items in the Assessment of risk of bias in included studies according to the updated Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): selective reporting bias and other bias.
The comparison group was inserted into the objective. Thus, the objective was changed from 'To evaluate the effectiveness and safety of antimicrobial de‐escalation for adult patients diagnosed with sepsis, severe sepsis or septic shock caused by any micro‐organism' to 'To evaluate the effectiveness and safety of antimicrobial de‐escalation when compared with the maintenance of broad‐spectrum therapy for adult patients diagnosed with sepsis, severe sepsis or septic shock caused by any micro‐organism'.
The filter for randomized controlled trials previously planned to be used in the LILACS database was removed from the search strategy.
Contributions of authors
Conceiving the review: Brenda NG Silva (BGNS), Reinaldo Salomão (RS)
Co‐ordinating the review: BNGS
Screening search results: BNGS, Régis B Andriolo (RBA)
Organizing retrieval of papers: BNGS
Screening retrieved papers against inclusion criteria: BNGS, RBA, RS
Appraising quality of papers: BNGS, RBA, Álvaro N Atallah (ANA)
Abstracting data from papers: BNGS, RBA
Writing to authors of papers for additional information: BNGS
Providing additional data about papers: BNGS
Obtaining and screening data on unpublished studies: BNGS, RS
Data management for the review: BNGS
Entering data into Review Manager (RevMan 5.1): BNGS, RBA
RevMan statistical data: BNGS, RA, ANA
Other statistical analysis not using RevMan: RBA
Interpretation of data: BNGS, RBA, ANA
Statistical inferences: BNGS, RBA
Writing the review: BNGS, RS, ANA
Guarantor for the review (one author): BNGS
Person responsible for reading and checking review before submission: BNGS, RS, ANA
Sources of support
Internal sources
CAPES ‐ Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
External sources
No sources of support supplied
Declarations of interest
Brenda NG Silva: none known
Régis B Andriolo: none known
Álvaro N Atallah: none known
Reinaldo Salomão: none known
Edited (no change to conclusions)
References
References to studies excluded from this review
Bailey 1996 {published data only}
- Bailey RR, Begg EJ, Smith AH, Robson RA, Lynn KL, Chambers ST, et al. Prospective, randomized, controlled study comparing two dosing regimens of gentamicin/oral ciprofloxacin switch therapy for acute pyelonephritis. Clinical Nephrology 1996;46(3):183‐6. [PUBMED: 8879853] [PubMed] [Google Scholar]
Bouadma 2010 {published data only}
- Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463‐74. [NCT00472667; PUBMED: 20097417] [DOI] [PubMed] [Google Scholar]
Christ‐Crain 2004 {published data only}
- Bergmans DC, Bonten MJ, Gaillard CA, Tiel FH, Geest S, Leeuw PW, et al. Indications for antibiotic use in ICU patients: a one‐year prospective surveillance. Journal of Antimicrobial Chemotherapy 1997;39(4):527‐35. [PUBMED: 9145828] [DOI] [PubMed] [Google Scholar]
- Christ‐Crain M, Jaccard‐Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet 2004;363(9409):600‐7. [NCT00407147; PUBMED: 14987884] [DOI] [PubMed] [Google Scholar]
- Garnacho‐Montero J, Garcia‐Garmendia JL, Barrero‐Almodovar A, Jimenez‐Jimenez FJ, Perez‐Paredes C, Ortiz‐Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Critical Care Medicine 2003;31(12):2742‐51. [PUBMED: 14668610] [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine 1985;13(10):818‐29. [PUBMED: 3928249] [PubMed] [Google Scholar]
- Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C‐reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Critical Care 1999;3(1):45‐50. [PUBMED: 11056723] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau B, Steinbach G, Gansauge F, Mayer JM, Grunert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 1997;41(6):832‐40. [PUBMED: 9462219 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder BL, Nielsen SL, Magnussen P, Engquist A, Frimodt‐Moller N. Antibiotic usage in an intensive care unit in a Danish university hospital. Journal of Antimicrobial Chemotherapy 1993;32(4):633‐42. [PUBMED: 8288506] [DOI] [PubMed] [Google Scholar]
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas‐Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995;274(8):639‐44. [PUBMED: 7637145] [PubMed] [Google Scholar]
Horisberger 2004 {published data only}
- Horisberger T, Harbarth S, Nadal D, Baenziger O, Fischer JE. G‐CSF and IL‐8 for early diagnosis of sepsis in neonates and critically ill children ‐ safety and cost effectiveness of a new laboratory prediction model: study protocol of a randomized controlled trial [ISRCTN91123847]. Critical Care 2004;8(6):R443‐50. [PUBMED: 15566590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2008 {published data only}
- Jensen JU, Hein L, Thornberg K, Loeken J, Tousi H, Larsen KM, et al. Dynamic use of procalcitonin in the intensive care unit. International Journal of Intensive Care 2007;14(2):52‐7. [Google Scholar]
- Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Critical Care Medicine 2006;34(10):2596‐602. [PUBMED: 16915118] [DOI] [PubMed] [Google Scholar]
- Jensen JU, Lundgren B, Hein L, Mohr T, Petersen PL, Andersen LH, et al. The Procalcitonin And Survival Study (PASS) ‐ a randomised multi‐center investigator‐initiated trial to investigate whether daily measurements biomarker Procalcitonin and pro‐active diagnostic and therapeutic responses to abnormal Procalcitonin levels, can improve survival in intensive care unit patients. Calculated sample size (target population): 1000 patients. BMC Infectious Disease 2008;8:91. [NCT00271752; PUBMED: 18620598] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JU, Lundgren B, Lundgren JD. Meta‐analysis of procalcitonin for sepsis detection. Lancet Infectious Diseases 2007;7(8):499‐500. [PUBMED: 17646020] [DOI] [PubMed] [Google Scholar]
- Jensen JU, Lundgren JD. Procalcitonin in liver transplant patients ‐ yet another stone turned. Critical Care 2008;12(1):108. [PUBMED: 18254924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mabasa 2009 {published data only}
- Mabasa V, Keenan S, Wiens M, Kangura S. Standard vs adjusted dosing of piperacillin/tazobactam in acute renal failure and septic shock. http://www.controlled‐trials.com/mrct/trial/477949/NCT00816790 2009.
Masaoka 2000 {published data only}
- Masaoka T, Hasegawa H, Takaku F, Mizoguchi H, Asano S, Ikeda Y, et al. The efficacy of intravenous immunoglobulin in combination therapy with antibiotics for severe infections. Japanese Journal of Chemotherapy 2000;48(3):199‐217. [CENTRAL: CN‐00418781] [Google Scholar]
Roberts 2009 {published data only}
- Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Piperacillin penetration into tissue of critically ill patients with sepsis‐‐bolus versus continuous administration?. Critical Care Medicine 2009;37(3):926‐33. [CENTRAL: CN‐00684456] [DOI] [PubMed] [Google Scholar]
Schroeder 2009 {published data only}
- Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Critical Care 2009;13(3):R83. [PUBMED: 19493352] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbeck's Archives of Surgery / Deutsche Gesellschaft für Chirurgie 2009;394(2):221‐6. [CENTRAL: CN‐00703948] [DOI] [PubMed] [Google Scholar]
van den Anker 1995 {published data only}
- Anker JN, Schoemaker RC, Heijden BJ, Broerse HM, Neijens HJ, Groot R. Once‐daily versus twice‐daily administration of ceftazidime in the preterm infant. Antimicrobial Agents and Chemotherapy 1995;39(9):2048‐50. [CENTRAL: CN‐00119763; PUBMED: 8540714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuori‐Holopainen 2000 {published data only}
- Vuori‐Holopainen E, Peltola H, Kallio MJ. Narrow‐ versus broad‐spectrum parenteral anatimicrobials against common infections of childhood: a prospective and randomised comparison between penicillin and cefuroxime. European Journal of Pediatrics 2000;159(12):878‐84. [PUBMED: 11131342] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Leone 2012 {published data only}
- De‐escalation of Empirical Antimicrobial Therapy Study in Severe Sepsis. Ongoing study October 2011.
Additional references
Adukauskiene 2006
- Adukauskiene D, Vitkauskiene A. Empiric de‐escalation strategy of antibiotic treatment [Empirinis plataus antimikrobinio veikimo gydymas]. Medicina (Kaunas) 2006;42(9):703‐8. [PUBMED: 17028467] [PubMed] [Google Scholar]
Alexandraki 2008
- Alexandraki I, Sullivan R, Zaiden R, Bailey C, McCarter Y, Khan A, et al. Blood culture isolates in hemodialysis vascular catheter‐related bacteremia. American Journal of the Medical Sciences 2008;336(4):297‐302. [PUBMED: 18854670] [DOI] [PubMed] [Google Scholar]
Apisarnthanarak 2004
- Apisarnthanarak A, Holzmann‐Pazgal G, Hamvas A, Olsen MA, Fraser VJ. Antimicrobial use and the influence of inadequate empiric antimicrobial therapy on the outcomes of nosocomial bloodstream infections in a neonatal intensive care unit. Infection Control and Hospital Epidemiology 2004;25(9):735‐41. [PUBMED: 15484797] [DOI] [PubMed] [Google Scholar]
ATS IDSA 2005
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired,ventilator‐associated, and healthcare‐associated pneumonia. American Journal Respiratory and Critical Care Medicine 2005;171(4):388‐416. [PUBMED: 15699079] [DOI] [PubMed] [Google Scholar]
Bagshaw 2009
- Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Medicine 2009;35(5):871‐81. [PUBMED: 19066848] [DOI] [PubMed] [Google Scholar]
Balk 2004
- Balk RA. Optimum treatment of severe sepsis and septic shock: Evidence in support of the recommendations. Disease‐a‐Month 2004;50(4):168‐213. [PUBMED: 15133467] [DOI] [PubMed] [Google Scholar]
Berild 2006
- Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. Journal of Antimicrobial Chemotherapy 2006;52(2):326‐30. [PUBMED: 16387751] [DOI] [PubMed] [Google Scholar]
Bone 1992
- Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Critical Care Medicine 1992;20(6):724‐6. [PUBMED: 1600757] [DOI] [PubMed] [Google Scholar]
Brunkhorst 2009
- Brunkhorst FM, Reinhart K. Diagnosis and causal treatment of sepsis [Diagnose und kausale Therapie der Sepsis]. Der Internist 2009;50(7):810‐6. [PUBMED: 19506808] [DOI] [PubMed] [Google Scholar]
Carcelero 2012
- Carcelero E, Soy D. Antibiotic dose adjustment in the treatment of MRSA infections in patients with acute renal failure undergoing continuous renal replacement therapies. Enfermedades Infecciosas y Microbiología Clínica 2012;30(5):249‐56. [PUBMED: 22130573] [DOI] [PubMed] [Google Scholar]
Cattoir 2010
- Cattoir V, Daurel C. Update on antimicrobial chemotherapy [Quelles nouveautés en antibiothérapie ?]. Medecine et Maladies Infectieuses 2010;40(3):135‐54. [PUBMED: 19959306] [DOI] [PubMed] [Google Scholar]
Cheadle 1992
- Cheadle WG. Current perspectives on antibiotic use in the treatment of surgical infections. American Journal of Surgery 1992;164(4A Suppl):44S‐7S. [PUBMED: 1443360] [DOI] [PubMed] [Google Scholar]
Cheng 2007
- Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Critical Care Medicine 2007;35(11):2538‐46. [PUBMED: 17828034] [DOI] [PubMed] [Google Scholar]
Colardyn 2005
- Colardyn F. Appropriate and timely empirical antimicrobial treatment of icu infections‐‐a role for carbapenems. Acta Clinica Belgica 2005;60(2):51‐62. [PUBMED: 16082989] [DOI] [PubMed] [Google Scholar]
Cordero 2006
- Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early‐onset sepsis in extremely low birth weight infants. Infection Control and Hospital Epidemiology 2003;24(9):662‐6. [PUBMED: 14510248] [DOI] [PubMed] [Google Scholar]
Cunha 2008
- Cunha BA. Sepsis and septic shock: selection of empiric antimicrobial therapy. Critical Care Clinics 2008;24(2):313‐34, ix. [PUBMED: 18361948] [DOI] [PubMed] [Google Scholar]
Darby 1997
- Darby JM, Linden P, Pasculle W, Saul M. Utilization and diagnostic yield of blood cultures in a surgical intensive care unit. Critical Care Medicine 1997;25(6):989‐94. [PUBMED: 9201052] [DOI] [PubMed] [Google Scholar]
De Angelis 2011
- Angelis G, Restuccia G, Muzio F, Cipriani M, Milozzi E, Cauda R, et al. Evidence‐based recommendations for antibiotic usage in the intensive care unit: A systematic review. Clinical Microbiology and Infection 2011;17:S358. [Google Scholar]
de Jonge 2003
- Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362(9389):1011‐6. [PUBMED: 14522530] [DOI] [PubMed] [Google Scholar]
Dellinger 2008
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Medicine 2008;34(1):17‐60. [PUBMED: 18058085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deresinski 2007
- Deresinski S. Principles of antibiotic therapy in severe infections: optimizing the therapeutic approach by use of laboratory and clinical data. Clinical Infectious Diseases 2007;45 Suppl 3:177‐83. [PUBMED: 17712744] [DOI] [PubMed] [Google Scholar]
Dombrovskiy 2007
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Critical Care Medicine 2007;35(5):1244‐50. [PUBMED: 17414736] [DOI] [PubMed] [Google Scholar]
Dougnac 2007
- Dougnac AL, Mercado MF, Cornejo RR, Cariaga MV, Hernández GP, Andresen MH, et al. Prevalence of severe sepsis in intensive care units. A national multicentric study [Prevalencia de sepsis grave en las Unidades de Cuidado Intensivo. Primer estudio nacional multicéntrico]. Revista Médica de Chile 2007;135(5):620‐30. [PUBMED: 17657331] [DOI] [PubMed] [Google Scholar]
Drekonja 2008
- Drekonja DM, Johnson JR. Urinary Tract Infections. Primary Care ‐ Clinics in Office Practice 2008;35(2):345‐67. [PUBMED: 18486719] [DOI] [PubMed] [Google Scholar]
Erlandsson 2007
- Erlandsson M, Burman LG, Cars O, Gill H, Nilsson LE, Walther SM, et al. Prescription of antibiotic agents in Swedish intensive care units is empiric and precise. Scandinavian Journal of Infectious Diseases 2007;39(1):63‐9. [PUBMED: 17366015] [DOI] [PubMed] [Google Scholar]
Filius 2002
- Filius PM, Gyssens IC. Impact of increasing antimicrobial resistance on wound management. American Journal of Clinical Dermatology 2002;3(1):1‐7. [PUBMED: 11817964] [DOI] [PubMed] [Google Scholar]
Fluckiger 2000
- Fluckiger U, Zimmerli W, Sax H, Frei R, Widmer AF. Clinical impact of an infectious disease service on the management of bloodstream infection. European Journal of Clinical Microbiology and Infectious Diseases 2000;19(7):493‐500. [PUBMED: 10968319] [DOI] [PubMed] [Google Scholar]
Galal 2010
- Galal AM, Gul W, Noreddin AM, Slade D. An update on the synthesis and antibacterial effects of carbapenems. Recent Patents on Anti‐infective Drug Discovery 2010;5(1):23‐43. [PUBMED: 19929840] [DOI] [PubMed] [Google Scholar]
Garnacho‐Montero 2003
- Garnacho‐Montero J, Garcia‐Garmendia JL, Barrero‐Almodovar A, Jimenez‐Jimenez FJ, Perez‐Paredes C, Ortiz‐Leyba C. Impact of adequate empiric antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Critical Care Medicine 2003;31(12):2742‐51. [PUBMED: 14668610] [DOI] [PubMed] [Google Scholar]
Glowacki 2003
- Glowacki RC, Schwartz DN, Itokazu GS, Wisniewski MF, Kieszkowski P, Weinstein RA. Antibiotic combinations with redundant antimicrobial spectra: clinical epidemiology and pilot intervention of computer‐assisted surveillance. Clinical Infectious Diseases 2003;37(1):59‐64. [PUBMED: 12830409] [DOI] [PubMed] [Google Scholar]
Gomes Silva 2010
- Gomes Silva BN, Andriolo RB, Atallah AN, Salomão R. De‐escalation of antimicrobial treatment for adults with sepsis, severe sepsis or septic shock. Cochrane Database of Systematic Reviews 2010;8(12):CD007934. [PUBMED: 21154391] [DOI] [PubMed] [Google Scholar]
Guillon 2010
- Guillon H, Eb F, Mammeri H. Characterization of CSP‐1, a novel extended‐spectrum beta‐lactamase produced by a clinical isolate of Capnocytophaga sputigena. Antimicrobial Agents and Chemotherapy 2010;54(5):2231‐34. [PUBMED: 20308380] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbarth 2003
- Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. American Journal of Medicine 2003;115(7):529‐35. [PUBMED: 14599631] [DOI] [PubMed] [Google Scholar]
Heenen 2012
- Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de‐escalate more often?. Critical Care Medicine 2012;40(5):1404‐9. [PUBMED: 22430235] [DOI] [PubMed] [Google Scholar]
Higgins 2002
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March, 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hitt 1997
- Hitt CM, Nightingale CH, Quintiliani R, Nicolau DP. Streamlining antimicrobial therapy for lower respiratory tract infections. Clinical Infectious Diseases 1997;24 Suppl 2:231‐7. [PUBMED: 9126698] [DOI] [PubMed] [Google Scholar]
Höffken 2002
- Höffken G, Niederman MS. Nosocomial pneumonia: The importance of a de‐escalating strategy for antibiotic treatment of pneumonia in the ICU. Chest 2002;122:2183‐96. [PUBMED: 12475862 ] [DOI] [PubMed] [Google Scholar]
IDSA 2004
- Infectious Diseases Society of America. Bad bugs, no drugs. As antibiotic discovery stagnates . . . a public health crisis brews. http://www.idsociety.org/badbugsnodrugs.html. Alexandria: Infectious Diseases Society of America, 2004.
IDSA 2006
- Infectious Diseases Society of America. Principles and strategies intended to limit the impact of antimicrobial resistance. http://www.idsociety.org/Content.aspx?id=6252 2006.
Iñigo 2006
- Iñigo J, Sendra JM, Díaz R, Bouza C, Sarría‐Santamera A. Epidemiology and costs of severe sepsis in Madrid. A hospital discharge study [Epidemiología y costes de la sepsis grave en Madrid. Estudio de altas hospitalarias]. Medicina Intensiva 2006;30(5):197‐203. [PUBMED: 16938192] [DOI] [PubMed] [Google Scholar]
Kielstein 2011
- Kielstein JT, Burkhardt O. Dosing of antibiotics in critically ill patients undergoing renal replacement therapy. Current Pharmaceutical Biotechnology 2011;12(12):2015‐9. [PUBMED: 21554216] [DOI] [PubMed] [Google Scholar]
Kollef 2001
- Kollef MH. Hospital‐acquired pneumonia and de‐escalation of antimicrobial treatment. Critical Care Medicine 2001;29(7):1473‐5. [PUBMED: 11445712] [DOI] [PubMed] [Google Scholar]
Kollef 2006
- Kollef MH. Providing appropriate antimicrobial therapy in the intensive care unit: surveillance vs. de‐escalation. Critical Care Medicine 2006;34(3):903‐5. [PUBMED: 16505677] [DOI] [PubMed] [Google Scholar]
Kumar 2006
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine 2006;34(6):1589‐96. [PUBMED: 16625125] [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136(5):1237‐48. [PUBMED: 19696123] [DOI] [PubMed] [Google Scholar]
Kumar 2011
- Kumar A. Optimizing antimicrobial therapy in sepsis and septic shock. Critical Care Nursing Clinics of North America 2011;23(1):79‐97. [PUBMED: 21316569] [DOI] [PubMed] [Google Scholar]
Lane 2011
- Lane DR, Takhar SS. Diagnosis and management of urinary tract infection and pyelonephritis. Emergency Medicine Clinics of North America 2011;29(3):539‐52. [PUBMED: 21782073] [DOI] [PubMed] [Google Scholar]
Lattour 1997
- Latour J, Abraira V, Cabello JB, López Sánchez J. Investigation methods in clinical cardiology. IV. Clinical measurements incardiology: validity and errors of measurements [Las mediciones clínicas en cardiología: validez y errores de medición]. Revista Española de Cardiología 1997;50(2):117‐28. [PUBMED: 9091999 ] [DOI] [PubMed] [Google Scholar]
Leone 2007
- Leone M, Garcin F, Bouvenot J, Boyadjev I, Visintini, Albanèse J, et al. Ventilator‐associated pneumonia: Breaking the vicious circle of antibiotic overuse. Critical Care Medicine 2007;35(2):379‐85. [PUBMED: 17205011] [DOI] [PubMed] [Google Scholar]
Leone 2008
- Leone M, Martin C. How to break the vicious circle of antibiotic resistances?. Current Opinion in Critical Care 2008;14(5):587‐92. [PUBMED: 18787454] [DOI] [PubMed] [Google Scholar]
Levy 2003
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Medicine 2003;29(4):530‐8. [PUBMED: 12664219] [DOI] [PubMed] [Google Scholar]
Liew 2011
- Liew YX, Chlebicki MP, Lee W, Hsu LY, Kwa AL. Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). European Journal of Clinical Microbiology & Infectious Diseases 2011;30(7):853‐5. [PUBMED: 21279532] [DOI] [PubMed] [Google Scholar]
Lipman 2009
- Lipman J, Boots R. A new paradigm for treating infections: "go hard and go home" initiation of antimicrobial therapy. Critical Care and Resuscitation 2009;35(5):871‐81. [PUBMED: 20001878] [PubMed] [Google Scholar]
Malacarne 2004
- Malacarne P, Rossi C, Bertolini G. Antibiotic usage in intensive care units: a pharmaco‐epidemiological multicentre study. Journal of Antimicrobial Chemotherapy 2004;54(1):221‐4. [PUBMED: 15190030] [DOI] [PubMed] [Google Scholar]
Masterton 2011
- Masterton RG. Antibiotic de‐escalation. Critical Care Clinics 2011;27(1):149‐62. [21144991] [DOI] [PubMed] [Google Scholar]
McArthur 2004
- MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clinical Infectious Diseases 2004;38(2):284‐8. [PUBMED: 14699463] [DOI] [PubMed] [Google Scholar]
McCabe 2010
- McCabe C, Kirchner C, Zhang H, Daley J, Fisman DN. Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Archives of Internal Medicine 2009;169(16):1525‐31. [PUBMED: 19752411] [DOI] [PubMed] [Google Scholar]
McNulty 1997
- McNulty C, Logan M, Donald IP, Ennis D, Taylor D, Baldwin RN, et al. Successful control of Clostridium difficile infection in an elderly care unit through use of a restrictive antibiotic policy. Journal of Antimicrobial Chemotherapy 1997;40(5):707‐11. [PUBMED: 9421320] [DOI] [PubMed] [Google Scholar]
Miano 2012
- Miano TA, Powell E, Schweickert WD, Morgan S, Binkley S, Sarani B. Effect of an antibiotic algorithm on the adequacy of empiric antibiotic therapy given by a medical emergency team. Journal of Critical Care 2012;27(1):45‐50. [PUBMED: 21798704] [DOI] [PubMed] [Google Scholar]
Micek 2005
- Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrobial Agents Chemotherapy 2005;49(4):1306‐11. [PUBMED: 15793102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2005
- Moher D, Schulz KF, Altman D, CONSORT Group. The CONSORT Statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials 2001. Explore (NY) 2005;1(1):40‐5. [PUBMED: 16791967] [DOI] [PubMed] [Google Scholar]
Mol 2006
- Mol PG, Denig P, Gans RO, Nannanpanday PV, Degener JE, Laseur M, Haaijer‐Ruskamp FM. Limited effect of patient and disease characteristics on compliance with hospital antimicrobial guidelines. European Journal of Clinical Pharmacology 2006;62(4):297‐305. [PUBMED: 16432716] [DOI] [PubMed] [Google Scholar]
Morel 2010
- Morel J, Casoetto J, Jospe R, Aubert G, Terrana R, Dumont A, et al. De‐escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico‐surgical intensive care unit. Critical Care 2010;14(6):R225. [PUBMED: 21167047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutlu 2006
- Mutlu GM, Wunderink RG. Severe pseudomonal infections. Current Opinion in Critical Care 2006;12(5):458‐63. [PUBMED: 16943726] [DOI] [PubMed] [Google Scholar]
Napolitano 2009
- Napolitano. Severe soft tissue infections. Infectious Disease Clinics of North America 2009;23(3):571‐91. [PUBMED: 19665084] [DOI] [PubMed] [Google Scholar]
Niederman 2006
- Niederman MS. De‐escalation therapy in ventilator‐associated pneumonia. Current Opinion in Critical Care 2006;12(5):425‐7. [PUBMED: 16943725] [DOI] [PubMed] [Google Scholar]
Paul 2006
- Paul M, Silbiger I, Grozinsky S, Soares‐Weiser K, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam‐aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003344] [DOI] [PubMed] [Google Scholar]
Pea 2009
- Pea F, Viale P. Bench‐to‐bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock‐‐does the dose matter?. Critical Care 2009;13(3):214. [PUBMED: 19519961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pocock 1983
- Pocock SJ. The size of a clinical trial. In: Pocock SJ editor(s). Clinical Trials, a practical approach. 1st Edition. Chichester: John Wiley & Sons, 1983:123‐41. [Google Scholar]
Proulx 2005
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. Quarterly Journal of Medicine 2005;98(4):291‐8. [PUBMED: 15760921] [DOI] [PubMed] [Google Scholar]
Raisch 1988
- Raisch DW, Bootman JL, McGhan WF. Association of length of stay and total hospital charges with antimicrobial regimen changes. American Journal of Hospital Pharmacy 1988;45(4):819‐23. [PUBMED: 3132038] [PubMed] [Google Scholar]
Rello 2004
- Rello J, Vidaur L, Sandiumenge A, Rodríguez A, Gualis B, Boque C, et al. De‐escalation therapy in ventilator‐associated pneumonia. Critical Care Medicine 2004;32(11):2183‐90. [PUBMED: 15640629] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richards 2005
- Richards GA. The therapeutic challenge of gram‐negative sepsis: Prolonging the lifespan of a scarce resource. Clinical Microbiology and Infection 2005;11 Suppl 6:18‐22. [DOI] [PubMed] [Google Scholar]
Rodloff 2006
- Rodloff AC, Goldstein EJ, Torres A. Two decades of imipenem therapy. Journal of Antimicrobial Chemotherapy 2006;58(5):916‐29. [PUBMED: 16997845] [DOI] [PubMed] [Google Scholar]
Sanchez 1997
- Sanchez C, Matamala A, Salavert M, Cuchi E, Pons M, Angles F, et al. Cotrimoxazole plus rifampicin in the treatment of staphylococcal osteoarticular infection [Cotrimoxazol más rifampicina en el tratamiento de la infección osteoarticular estafilocócica]. Enfermedades Infecciosas y Microbiologia Clinica 1997;15(1):10‐3. [PUBMED: 9147500] [PubMed] [Google Scholar]
Schierbeck 207
- Schierbeck J, Kolmos HJ. Antibiotic strategies in the treatment of infection in critically ill patients. Ugeskrift for Laeger 207;169(8):699‐702. [PUBMED: 17313920] [PubMed] [Google Scholar]
Schuler 1994
- Schuler G. Antibiotic therapy of infectious endocarditis (when, with what drug, how long?) [Antibiotische Therapie der infektiösen Endokarditis (wann, womit, wie lange?)]. Zeitschrift für Kardiologie 1994;83(1):2‐8. [PubMed] [Google Scholar]
Shani 2009
- Shani V, Kariv G, Muchtar E, Leibovici L, Paul M. Appropriate vs. inappropriate empirical antibiotic treatment: Systematic review and meta‐analysis of effects and modifiers. Clinical Microbiology and Infection 2009;15:S46‐7. [DOI] [PubMed] [Google Scholar]
Shime 2011
- Shime N, Satake S, Fujita N. De‐escalation of antimicrobials in the treatment of bacteraemia due to antibiotic‐sensitive pathogens in immunocompetent patients. Infection 2011;39(4):319‐25. [21509424] [DOI] [PubMed] [Google Scholar]
Silva 2004
- Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian Sepsis Epidemiological Study (BASES study). Critical Care 2004;8(4):R251‐60. [PUBMED: 15312226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2000
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short‐course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. American Journal of Respiratory and Critical Care Medicine 2000;162(2 Pt1):505‐11. [PUBMED: 10934078] [DOI] [PubMed] [Google Scholar]
Spies 2009
- Spies C. Implementation of de‐escalation algorithm in patients with ventilator‐associated pneumonia at two anaesthesiological intensive care units (ICUs) of Charité. http://www.controlled‐trials.com/. Berlin, 2009. [ISRCTN80881420]
Teles 2008
- Teles JMM, Silva E, Westphal G, Filho RC, Machado FR. Surviving sepsis campaign in Brazil. Shock 2008;30(7):1‐6. [PUBMED: 18704009] [DOI] [PubMed] [Google Scholar]
Textoris 2011
- Textoris J, Wiramus S, Martin C, Leone M. Antibiotic therapy in patients with septic shock. European Journal of Anaesthesiology 2011;28(5):318‐24. [PUBMED: 21464717] [DOI] [PubMed] [Google Scholar]
Tripathi 2012
- Tripathi N, Cotten CM, Smith PB. Antibiotic use and misuse in the neonatal intensive care unit. Clinics in Perinatology 2012;39(1):61‐8. [PUBMED: 22341537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vallés 2003
- Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community‐acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest 2003;125(5):1615‐24. [PUBMED: 12740282] [DOI] [PubMed] [Google Scholar]
Vincent 2006
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Critical Care Medicine 2006;34(2):344‐53. [PUBMED: 16424713] [DOI] [PubMed] [Google Scholar]
Welte 2004
- Welte T. Sepsis management ‐‐ antibiotic therapy [Antibiotikatherapie der Sepsis]. Deutsche Medizinische Wochenschrift 2004;129(48):2609‐13. [PUBMED: 15558411] [DOI] [PubMed] [Google Scholar]
West 2008
- West MA, Moore EE, Shapiro MB, Nathens AB, Cuschieri J, Johnson JL, et al. Inflammation and the host response to injury, a large‐scale collaborative project: patient‐oriented research core‐‐standard operating procedures for clinical care VII‐‐Guidelines for antibiotic administration in severely injured patients. The Journal of Trauma 2008;65(6):1511‐9. [PUBMED: 19077651] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Antimicrobial resistance. http://www.who.int/mediacentre/factsheets/fs194/en/index.html.. Geneva, 2002; Vol. Fact sheet:194.
Zaragoza 2008
- Zaragoza R, Peman J, Salavert M, Viudes A, Sole A, Jarque I, et al. Multidisciplinary approach to the treatment of invasive fungal infections in adult patients. Prophylaxis, empirical, preemptive or targeted therapy, which is the best in the different hosts?. Therapeutics and Clinical Risk Management 2008;4(6):1261‐80. [PUBMED: 19337433] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Silva 2010
- Silva BNG, Andriolo RB, Atallah ÁN, Salomão R. De‐escalation of antimicrobial treatment for adults with sepsis, severe sepsis or septic shock. Cochrane Database of Systematic Reviews 2010;12:CD007934. [DOI: 10.1002/14651858.CD007934.pub2] [DOI] [PubMed] [Google Scholar]


