Abstract
Background
Peripheral arterial disease (PAD), caused by narrowing of the arteries in the limbs, is increasing in incidence and prevalence as our population is ageing and as diabetes is becoming more prevalent. PAD can cause pain in the limbs while walking, known as intermittent claudication, or can be more severe and cause pain while at rest, ulceration, and ultimately gangrene and limb loss. This more severe stage of PAD is known as 'critical limb ischaemia'. Treatments for PAD include medications that help to reduce the increased risk of cardiovascular events and help improve blood flow, as well as endovascular or surgical repair or bypass of the blocked arteries. However, many people are unresponsive to medications and are not suited to surgical or endovascular treatment, leaving amputation as the last option. Gene therapy is a novel approach in which genetic material encoding for proteins that may help increase revascularisation is injected into the affected limbs of patients. This type of treatment has been shown to be safe, but its efficacy, especially regarding ulcer healing, effects on quality of life, and other symptomatic outcomes remain unknown.
Objectives
To assess the effects of gene therapy for symptomatic peripheral arterial disease.
Search methods
The Cochrane Vascular Information Specialist searched Cochrane CENTRAL, the Cochrane Vascular Specialised Register, MEDLINE Ovid, Embase Ovid, CINAHL, and AMED, along with trials registries (all searched 27 November 2017). We also checked reference lists of included studies and systematic reviews for further studies.
Selection criteria
We included randomised and quasi‐randomised studies that evaluated gene therapy versus no gene therapy in people with PAD. We excluded studies that evaluated direct growth hormone treatment or cell‐based treatments.
Data collection and analysis
Two review authors independently selected studies, performed quality assessment, and extracted data from the included studies. We collected pertinent information on each study, as well as data for the outcomes of amputation‐free survival, ulcer healing, quality of life, amputation, all‐cause mortality, ankle brachial index, symptom scores, and claudication distance.
Main results
We included in this review a total of 17 studies with 1988 participants (evidence current until November 2017). Three studies limited their inclusion to people with intermittent claudication, 12 limited inclusion to people with varying levels of critical limb ischaemia, and two included people with either condition. Study investigators evaluated many different types of gene therapies, using different protocols. Most studies evaluated growth factor‐encoding gene therapy, with six studies using vascular endothelial growth factor (VEGF)‐encoding genes, four using hepatocyte growth factor (HGF)‐encoding genes, and three using fibroblast growth factor (FGF)‐encoded genes. Two studies evaluated hypoxia‐inducible factor 1‐alpha (HIF‐1α) gene therapy, one study used a developmental endothelial locus‐1 gene therapy, and the final study evaluated a stromal cell‐derived factor‐1 (SDF‐1) gene therapy. Most studies reported outcomes after 12 months of follow‐up, but follow‐up ranged from three months to two years.
Overall risk of bias varied between studies, with many studies not providing sufficient detail for adequate determination of low risk of bias for many domains. Two studies did not utilise a placebo control, leading to risk of performance bias. Several studies reported in previous protocols or in their Methods sections that they would report on certain outcomes for which no data were then reported, increasing risk of reporting bias. All included studies reported sponsorships from corporate entities that led to unclear risk of other bias. The overall quality of evidence ranged from moderate to very low, generally as the result of heterogeneity and imprecision, with few or no studies reporting on outcomes.
Evidence suggests no clear differences for the outcomes of amputation‐free survival, major amputation, and all‐cause mortality between those treated with gene therapy and those not receiving this treatment (all moderate‐quality evidence). Low‐quality evidence suggests improvement in complete ulcer healing with gene therapy (odds ratio (OR) 2.16, 95% confidence interval (CI) 1.02 to 4.59; P = 0.04). We could not combine data on quality of life and can draw no conclusions at this time regarding this outcome (very low‐quality evidence). We included one study in the meta‐analysis for ankle brachial index, which showed no clear differences between treatments, but we can draw no overall association (low‐quality evidence). We combined in a meta‐analysis pain symptom scores as assessed by visual analogue scales from two studies and found no clear differences between treatment groups (very low‐quality evidence). We carried out extensive subgroup analyses by PAD classification, dosage schedule, vector type, and gene used but identified no substantial differences.
Authors' conclusions
Moderate‐quality evidence shows no clear differences in amputation‐free survival, major amputation, and all‐cause mortality between those treated with gene therapy and those not receiving gene therapy. Some evidence suggests that gene therapy may lead to improved complete ulcer healing, but this outcome needs to be explored with improved reporting of the measure, such as decreased ulcer area in cm², and better description of ulcer types and healing. Further standardised data that are amenable to meta‐analysis are needed to evaluate other outcomes such as quality of life, ankle brachial index, symptom scores, and claudication distance.
Plain language summary
Gene therapy for peripheral arterial disease
Background
Peripheral arterial disease (PAD) occurs when the blood flow to the limbs is restricted because of narrowed arteries. This circulatory problem is increasing in the population because of increased levels of diabetes and because the population is ageing. Due to restricted blood flow, PAD can cause pain in the legs while walking, usually after some distance (known as 'intermittent claudication'). As the disease becomes more severe, a person can experience serious pain while at rest, as well as ulcers in the feet and legs (known as 'critical limb ischaemia'). PAD can be treated with medication or through interventions such as surgical or endovascular procedures (less invasive than surgery, endovascular intervention is carried out through a small incision to access the vessels). However, many people will not respond to medication, and surgical or endovascular procedures may not be appropriate because of medical risks. In these cases, for extreme PAD, the only option for treating the condition is amputation. Therapies are needed that can help repair the vessels in the limbs of people with PAD to restore adequate blood flow.
Gene therapy is a novel approach whereby genetic material, encoded for proteins that may help to improve blood flow by restoring blood vessels, is injected into a person's legs. Trials have shown that this treatment is safe, but whether it is effective in reducing the risk of amputation or improving quality of life remains unknown.
Review question
Is there a difference in outcomes of effectiveness (such as amputation, death, ulcer healing, and quality of life) between patients with symptomatic PAD who are given gene therapy and those who are not given gene therapy?
Study characteristics
We included 17 studies that had a total of 1988 participants (evidence current until November 2017). These studies used various types of gene therapy as well as different dosages, some providing single treatments and some repeated treatments. Most of the studies included people with critical limb ischaemia; three studies included people with intermittent claudication.
Key results
When combining the data, we found no clear differences between people who received gene therapy and those who did not in terms of amputation‐free survival (patients who did not have an amputation and did not die), major amputation (above the ankle), or death. We did see improvement in complete ulcer healing in the gene therapy treatment group compared to the control group. Studies show no clear differences in pain symptom scores, but we evaluated only two studies for this outcome. Not enough data are available to show if there was a difference between groups for the measure of blood flow known as the 'ankle brachial index'. We were not able to combine data on quality of life or pain‐free walking distances (distances one can walk without experiencing leg pain).
Quality of the evidence
Risk of bias of the included studies varied greatly, and this was a concern because studies did not clearly report on their methods nor on follow‐up of participants. Most studies used a placebo control, which increases the risk that outcomes may have been different if people knew they were given treatment or control. Corporations that produce the tested treatments sponsored all included trials.
The quality of evidence varied from moderate to very low. For amputation‐free survival, major amputation, and death, we considered the quality of evidence to be moderate because of differences between studies. For ulcer healing, risk of bias was a matter of concern, and study results were imprecise because few events were reported. The quality of evidence for quality of life was very low because of differences between studies and insufficient information to combine study findings. The quality of evidence for the ankle brachial index was low because only one study with few participants reported this outcome. For pain symptom scores, the quality of evidence was very low because of technical problems within one of the two studies, as well as differences between the two studies and few participants.
Summary of findings
Summary of findings for the main comparison. Gene therapy compared to no gene therapy control for peripheral arterial disease.
| Gene therapy compared to no gene therapy control for peripheral arterial disease | ||||||
| Patient or population: peripheral arterial disease Setting: inpatient treatment with outpatient follow‐up Intervention: gene therapy Comparison: no gene therapy control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no gene therapy control | Risk with gene therapy | |||||
| Amputation‐free survival Follow‐up: 12 months | Study population | OR 1.68 (0.75 to 3.76) | 756 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 622 per 1000 | 734 per 1000 (552 to 861) | |||||
| Ulcer healing Follow‐up: range 12 weeks to 12 months | Study population | OR 2.16 (1.02 to 4.59) | 238 (5 RCTs) | ⊕⊕⊝⊝ LOWb,c | ||
| 124 per 1000 | 233 per 1000 (126 to 393) | |||||
| Quality of life Follow‐up: range 3 months to 12 months | See comment | Not estimable | 699 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWd, e | Various QoL measures and reporting made meta‐analysis inappropriate for this outcome at this time. One of the 6 studies reporting on QoL found improvement in the treatment group, but in only 2 of 8 domains of the SF‐36. One study found improvement in the control group with regards to mental health using the SF‐36. Remaining studies found no differences between treatment groups, although most reported similar improvement in groups during the study | |
| Amputation (above‐ankle amputation of the index limb) | Study population | OR 1.06 (0.77 to 1.46) | 1336 (11 RCTs) | ⊕⊕⊕⊝ MODERATEf | ||
| 164 per 1000 | 172 per 1000 (131 to 223) | |||||
| All‐cause mortality | Study population | OR 0.93 (0.66 to 1.31) | 1685 (12 RCTs) | ⊕⊕⊕⊝ MODERATEf | ||
| 114 per 1000 | 107 per 1000 (78 to 144) | |||||
| ABI ‐ change from baseline | Mean ABI ‐ change from baseline was 0.01 | MD 0.04 higher (0.07 lower to 0.15 higher) | ‐ | 125 (1 RCT) | ⊕⊕⊝⊝ LOWg | |
| Pain symptom scores (VAS) ‐ change from baseline | Mean pain symptom scores (VAS) ‐ change from baseline was ‐0.02 | MD 0.22 cm lower (0.83 lower to 0.38 higher) | ‐ | 152 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWh,i,j | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABI: ankle brachial pressure index; CI: confidence interval; MD: mean difference; OR: odds ratio; QoL: quality of life; RCT: randomised controlled trial; SF‐36: Short Form‐36 quality of life tool; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence of substantial heterogeneity between studies (73%). bRisk of bias in most included studies due to study design or protocol execution. cImprecision in effect estimate due to few events, leading to wide confidence interval. dSubstantial heterogeneity between how studies reported on the outcome, making meta‐analysis inappropriate. eCannot estimate effect due to insufficient information provided by studies. fLittle overall heterogeneity detected, but the largest included study reported a very different rate of events compared with the other studies. gOnly one study in the meta‐analysis, with few participants, leading to imprecision. hRisk of bias; one of the two included studies incurred technical problems that study authors reported led to unreliable and uninterpretable data. iEvidence of moderate heterogeneity (46%). jOnly two studies included in the meta‐analysis, with few participants between them, leading to imprecision.
Background
Description of the condition
The global burden of peripheral arterial disease (PAD) is increasing because of the rising prevalence of diabetes mellitus and an ageing population (Fowkes 2013). However, not all individual countries are experiencing an increase in PAD, as was evidenced in a recent study in the UK (Cea‐Soriano 2018). Whilst PAD can be asymptomatic, it may also present with either intermittent claudication (IC) of varying severity or one or more manifestations of critical limb ischaemia (CLI), including rest pain, ischaemic ulcers, and gangrene (Norgren 2007). Treatment goals for claudicants versus patients with critical limb ischaemia are different, predominantly due to disease severity and concomitant comorbidity. Conventional management involves medical therapy for risk factor modification, pain relief, and treatment of infection, as well as interventions to relieve vascular obstruction through surgical procedures, endovascular approaches, or both (Mohler 2008). However, many cases are not amenable to these interventions because of patients' existing comorbidities and the complexity of their vascular anatomy due to multiple, diffuse, and distal disease. In CLI, this may result in amputation of the ischaemic limb. Hence, novel therapy is urgently needed to combat this unmet clinical need, and therapeutic revascularisation with gene therapy represents a promising new approach for the management of PAD.
Description of the intervention
Therapeutic revascularisation for management of PAD is possible via gene therapy. Recent randomised controlled trials (RCTs) utilising gene therapy for patients with PAD have involved the transfer of genetic material (DNA or RNA) into cells to modify their genetic expression. Gene therapy can be administered on one or more occasions via intra‐arterial or intramuscular routes. It can target a specific gene or multiple genes to either augment or attenuate specific gene expression, leading to therapeutic revascularisation.
A previous meta‐analysis has shown that gene therapy is safe and feasible, with some evidence of clinical improvement in patients with PAD (De Haro 2009), but it should be noted that systemic safety analysis has not been fully evaluated at this time. Gene therapy is a limb‐specific therapy that may not decrease mortality or risk of cardiac events.
How the intervention might work
Gene therapy can be performed by direct delivery of specific genetic materials (DNA or RNA) into cells via several viral‐ or non‐viral‐based methods (Kealy 2009; Liew 2013; Scougall 2003). This process can result in significant changes in specific gene expression leading to therapeutic revascularisation through stimulation of angiogenesis. Gene expression occurs when a cell's gene(s) are used to make a substance that changes the way the cell functions. In some cases, this change may improve or prevent a medical condition. The induction of therapeutic revascularisation can potentially lead to relief of symptoms associated with claudication in patients with PAD through formation of new blood vessels at ischaemic sites. It may also mobilise distant regenerative stem cell populations to ischaemic sites, thereby restoring the structure and function of surrounding ischaemic tissues (Asahara 1997; Kuliszewski 2011). Hence, improvement in blood flow in the affected limb(s) may potentially negate the need for amputation in critical ischaemia.
Why it is important to do this review
A previous meta‐analysis showed that gene therapy is safe and feasible, with some evidence of clinical improvement in patients with PAD (De Haro 2009). Since then, researchers have completed numerous RCTs using gene therapy to treat patients with PAD. However, these RCTs have reported inconsistent overall efficacy outcomes (Anghel 2011; Belch 2011; Creager 2011; Grossman 2007; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007; Shigematsu 2010). Meta‐analysis showed that gene therapy neither significantly increased nor decreased all‐cause mortality, amputation, or ulcer healing in patients with PAD (Hammer 2013). Furthermore, its effect on patients' quality of life is currently unclear.
In 2009, the Society for Vascular Surgery (SVS) published guidelines to improve the consistency and interpretability of all clinical trials conducted to evaluate potential treatment options for patients with CLI and suggested the following endpoints.
MALE (major adverse limb event: above‐ankle amputation of the index limb or major reintervention (new bypass graft, jump/interposition graft revision, or thrombectomy/ thrombolysis)).
MALE or POD (perioperative death (30 days), or any MALE).
MACE (major adverse cardiovascular event: myocardial infarction (MI), stroke, or death (any cause)).
Amputation (above‐ankle amputation of the index limb).
AFS (amputation‐free survival: above‐ankle amputation of the index limb or death (any cause)).
RAO (any reintervention or above‐ankle amputation of the index limb).
RAS (any reintervention, above‐ankle amputation of the index limb, or stenosis and all‐cause mortality).
Researchers presented these endpoints with suggested corresponding objective performance goals (OPGs) and designed them to meet US Federal Drug Administration (FDA) regulations by providing a framework for determining the appropriate entry of a novel therapy onto the market (Conte 2009).
Hence, the principal objective of this review is to provide the best estimate for the effects of gene therapy on two of these endpoints (amputation and amputation‐free survival) and on quality of life, as well as other commonly reported efficacy and safety outcome measures. This review will provide a better understanding of the efficacy of gene therapy in PAD, thereby helping to guide the future direction of gene therapy for this patient cohort.
Objectives
To assess the effects of gene therapy for symptomatic peripheral arterial disease.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) and quasi‐RCTs that compared gene therapy versus no gene therapy as treatment for patients with symptomatic PAD. We considered for inclusion cross‐over trials, cluster‐randomised trials, and multiple observations for the same outcome.
Types of participants
Our review included all patients (men and women with no age restriction) who had received a diagnosis of symptomatic PAD (intermittent claudication and critical limb ischaemia) of the lower extremities by an expert clinician after clinical and investigative assessment (by ankle brachial pressure index (ABI), exercise testing, duplex scanning, or angiography).
Types of interventions
We included only RCTs that compared gene therapy versus no gene therapy for patients with symptomatic PAD. We included all types of gene therapy, regardless of dosage or administration frequency or route of administration (systemic or local). We considered trials involving direct growth factor delivery (treatment with direct growth factor protein as opposed to a viral or plasmid vector containing genes encoding for a growth factor) or cell therapy to be not relevant. The minimum period of follow‐up allowed was three months.
Types of outcome measures
Primary outcomes
Amputation‐free survival (above‐ankle amputation of the index limb or death (any cause))
Ulcer healing
Quality of life (as assessed by formal questionnaires)
Secondary outcomes
Amputation (above‐ankle amputation of the index limb)
All‐cause mortality
Ankle brachial index (ABI)
Symptom scores (e.g. pain scores)
Claudication distance
Search methods for identification of studies
We applied no language, publication year or publication status restrictions.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases.
The Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched from inception to 27 November 2017).
The Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2017, Issue 10).
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 27 November 2017).
Embase Ovid (searched from 1 January 2017 to 27 November 2017).
CINAHL Ebsco (searched from 1 January 2017 to 27 November 2017).
AMED Ovid (searched from 1 January 2017 to 27 November 2017).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Cochrane Vascular Information Specialist also searched the following trials registries on 27 November 2017.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov)
Searching other resources
We handsearched references within identified RCTs and meta‐analyses for additional relevant studies.
Data collection and analysis
Selection of studies
Two review authors (AL and RF) independently selected RCTs for inclusion in the review, resolving discrepancies through joint discussion with the other review authors (VB, JS, and GS). Two review authors (AL and RF) independently reviewed the abstracts, titles, or both, of every record retrieved, to determine which studies needed further assessment. When we identified relevant articles, we obtained the full texts of these articles, and two review authors (AL and RF) independently applied review inclusion criteria.
Data extraction and management
Two review authors (AL and RF) independently examined all included RCTs and extracted all relevant data. We resolved disagreements by consensus with the other review authors (VB, JS, and GS). For primary RCTs with duplicate or multiple publications (e.g. interim analyses), we collated all available data and used the most complete data set aggregated across all known publications.
Assessment of risk of bias in included studies
Two review authors (AL and RF) independently assessed potential risks of bias for all included RCTs using the Cochrane tool for assessing risk of bias (Higgins 2011). This tool assesses bias in six different domains: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Each domain received a score of high, low, or unclear depending on each review author's judgement. The other review authors (VB, JS, and GS) were available to act as adjudicators in the event of disagreement.
We searched for protocols of included RCTs and compared outcomes in the protocol against those in the published report. If the protocol was not available, we compared outcomes listed in the Methods section of the RCT report versus actual reported results (Higgins 2011).
Measures of treatment effect
For dichotomous outcomes, we expressed results as odds ratios (ORs) with 95% confidence intervals (CIs). For continuous scales of measurement, we expressed results as mean differences (MDs). Furthermore, we planned to use standardised mean differences (SMDs) if RCTs used different scales. We planned to present time‐to‐event data as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
The unit of analysis within each trial was the individual participant. If necessary, two review authors (AL and RF) planned to consider the level at which randomisation occurred, such as in cross‐over and cluster‐randomised trials, and multiple observations for the same outcome. Again, we planned to resolve discrepancies through joint discussion with the other review authors (VB, JS, and GS).
Dealing with missing data
When necessary, we planned to request required further information from the original trial authors via written correspondence (e.g. emails to corresponding author(s)), and we planned to include in the review all relevant information obtained in this manner. We critically appraised issues related to missing data and imputation methods (e.g. last observation carried forward) and investigated attrition rates, including dropouts, losses to follow‐up, and withdrawals (Higgins 2011). For meta‐analysis, we included all participants randomised in each trial, when appropriate, to reduce the effects of attrition bias.
Assessment of heterogeneity
Clinical heterogeneity
Before performing data analysis, we assessed all included RCTs for potential clinical heterogeneity. We planned to conduct a subgroup analysis for any clinical outliers. However, we performed a meta‐analysis initially regardless of the presence of clinical heterogeneity.
Methodological heterogeneity
Before analysing data, we assessed all included RCTs for potential methodological heterogeneity. We planned to perform several subgroup analyses to detect methodological outliers. However, we performed a meta‐analysis initially regardless of methodological heterogeneity.
Statistical heterogeneity
Direct visual inspection
We assessed the possibility of statistical heterogeneity through direct visual inspection of the graphs.
I² statistic
We assessed heterogeneity between studies using the I² statistic with the associated Chi² test (Higgins 2003). We interpreted an I² estimate of 50% or above with a corresponding statistically significant Chi² test as evidence of substantial levels of heterogeneity. We performed subgroup analyses to explore reasons for the heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to use funnel plots to assess publication bias unless we identified 10 or fewer RCTs, or all RCTs were of similar size, because these circumstances would have limited power for detecting a small‐study effect (Higgins 2011).
Data synthesis
We used a fixed‐effect model to calculate pooled treatment of effect data and presented the estimates as ORs or MDs with their respective 95% CIs for binary and continuous outcome variables, as detailed above. We used the random‐effects model if we observed significant heterogeneity (defined as I² > 50%). We planned to report the absolute risk reduction/increase as a weighted estimate of the difference in event rates. We considered a two‐sided P value less than 0.05 to be the cutoff point for statistical significance. We created a forest plot for each outcome, as per Cochrane Vascular guidelines.
Subgroup analysis and investigation of heterogeneity
In the event of substantial clinical, methodological, or statistical heterogeneity, we attempted to determine possible reasons by examining individual study and subgroup characteristics. Nevertheless, we planned to perform the following subgroup analyses, regardless of the presence of any heterogeneity.
Intermittent claudication versus critical limb ischaemia.
Multiple‐gene therapy versus single‐gene therapy.
Repeated gene therapy versus single gene therapy.
Routes of administration: intramuscular versus intra‐arterial.
Vector type: virus versus plasmid.
Presence or absence of diabetes mellitus.
Sensitivity analysis
We planned to perform sensitivity analyses following the exclusion of:
any substantially long or large RCTs, to establish how much their findings dominated the results;
cross‐over trials, cluster‐randomised trials, and multiple observations for the same outcome; and
any RCTs that we judged to be at high risk of bias across one or more domains of randomisation, allocation concealment, blinding, and outcome reporting for meta‐analysis of the primary outcome.
We planned to perform sensitivity analyses only if the outcome had at least three studies remaining after sensitivity analysis.
During study inclusion, we chose to include four studies that did not meet our robust inclusion criteria for diagnosis of PAD but did include various measures and descriptors of vascular disease that we deemed appropriate for inclusion, as investigators most likely were evaluating the same population as studies that fully met review criteria (Deev 2015; Kibbe 2014; Powell 2008; Powell 2010). We performed sensitivity analysis by excluding these studies from their respective meta‐analyses to make sure they did not have an overt effect on review results.
'Summary of findings' table
We have summarised the results of analyses on primary and secondary outcomes in a 'Summary of findings' table, which contains information regarding the quality of evidence for all relevant outcomes. We assessed the quality of the body of evidence by considering the overall risk of bias of included studies, directness of the evidence, inconsistency of the results, precision of the estimates, and risk of publication bias according to GRADE (Balshem 2011). We included in the 'Summary of findings' table seven outcomes (amputation‐free survival, ulcer healing, quality of life, amputation, all‐cause mortality, ABI, and symptom scores) that we considered essential for decision‐making.
Results
Description of studies
Results of the search
We retrieved a total of 3225 references, after de‐duplication, through comprehensive literature searches. After title and abstract review, we identified 117 references for full‐text assessment. Of these 117, we excluded 16 studies (18 records) with reasons, identified 11 as duplicate references, considered 40 to be not relevant, and identified four as ongoing studies. We included a total of 44 records from 17 studies (including one reference for two studies (Henry 2006 and Nikol 2008)). See Figure 1 for the search results flow diagram.
1.
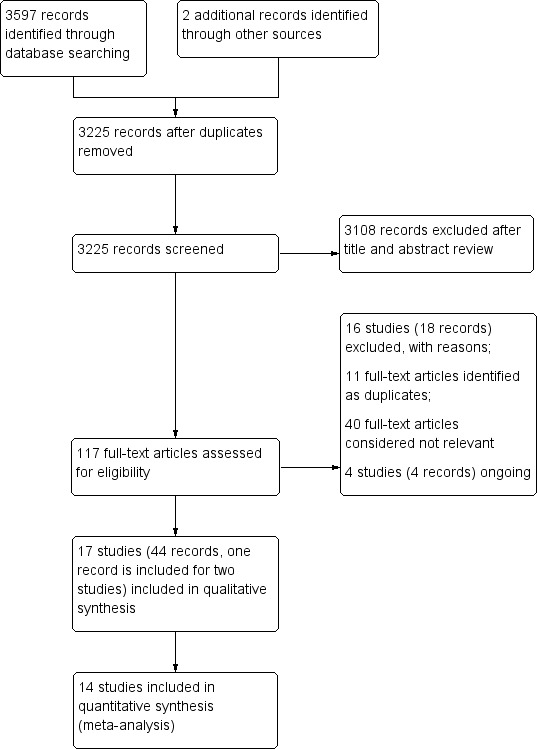
Study flow diagram.
Included studies
We included in this review a total of 17 randomised trials, with 1988 randomised participants (Belch 2011; Creager 2011; Deev 2015; Deev 2017; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Kusumanto 2006; Makinen 2002; Mohler 2003; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007; Shigematsu 2010). For detailed descriptions of these studies, please see Characteristics of included studies.
The types of gene therapy used varied between studies. A total of six studies evaluated various treatments encoding for vascular endothelial growth factor (VEGF) (Deev 2015; Deev 2017; Kusumanto 2006; Makinen 2002; Mohler 2003; Rajagopalan 2003). Four studies evaluated treatments that encoded for hepatocyte growth factor (HGF) (Kibbe 2016; Powell 2008; Powell 2010; Shigematsu 2010). Three studies utilised treatments encoding for fibroblast growth factor (FGF), all specifically using non‐viral 1 FGF (NV1FGF) (Belch 2011; Henry 2006; Nikol 2008). Two studies utilised a hypoxia‐inducible factor 1‐alpha (HIF‐1α)‐encoding treatment (Creager 2011; Rajagopalan 2007). One study evaluated a treatment encoding for the developmental endothelial locus‐1 (Del‐1) protein (Grossman 2007), and one study used a treatment that encoded for stromal cell‐derived factor‐1 (SDF‐1) (Kibbe 2014).
Three of the included trials evaluated participants with IC only (Creager 2011; Grossman 2007; Rajagopalan 2003), and 12 studies evaluated participants with varying levels of CLI (Belch 2011; Deev 2017; Henry 2006; Kibbe 2014; Kibbe 2016; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2007; Shigematsu 2010). Two studies evaluated participants with IC or CLI (Deev 2015; Mohler 2003).
Most included studies reported their primary analyses after 12 months (Belch 2011; Creager 2011; Kibbe 2014; Kibbe 2016; Mohler 2003; Nikol 2008; Powell 2008; Rajagopalan 2007). Four studies reported primary analysis after six months (Deev 2017; Grossman 2007; Powell 2010; Rajagopalan 2003), and two studies reported outcomes at or around three months (Kusumanto 2006; Makinen 2002). One study evaluated outcomes at 15 months (Deev 2015), and one at two years (Shigematsu 2010). The final study did not specify follow‐up time (Henry 2006), but from one reference it appears to be between one and three years. Several studies also reported longer follow‐up of safety outcomes.
Eight studies evaluated a range of dosages ‐ low, medium, high ‐ or used a dose‐escalation protocol (Creager 2011; Henry 2006; Kibbe 2014; Kibbe 2016; Mohler 2003; Powell 2008; Rajagopalan 2003; Rajagopalan 2007). One study evaluated the same growth factor in two treatments: one in a viral vector and one in a plasmid vector (Makinen 2002). The remaining eight studies evaluated one dose amount.
All studies administered treatment by intramuscular injection, aside from Makinen 2002, which used an intra‐articular route of administration. Twelve studies solely evaluated treatments using a plasmid vector (Belch 2011; Deev 2015; Deev 2017; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Kusumanto 2006; Nikol 2008; Powell 2008; Powell 2010; Shigematsu 2010), four using only a viral vector (Creager 2011; Mohler 2003; Rajagopalan 2003; Rajagopalan 2007), and, as stated above, one evaluating both a viral vector and a plasmid vector (Makinen 2002).
Eight studies utilised a repeat dosage schedule for treatment (Belch 2011; Deev 2015; Henry 2006; Kibbe 2016; Kusumanto 2006; Nikol 2008; Powell 2008; Powell 2010). Deev 2015, with the shortest duration, treated participants at baseline and then again on day 14. Kusumanto 2006 also undertook two dosages: at baseline and at day 28. Powell 2008 and Powell 2010 treated participants at baseline and at days 14 and 28. Belch 2011, Henry 2006, Kibbe 2016, and Nikol 2008 employed similar four‐times dosing schedules, around baseline and at days 14, 28, and 42, with some variation. The remaining nine studies provided a single treatment dose at baseline only.
Excluded studies
See Characteristics of excluded studies for the full list of excluded studies with reasons.
We excluded a total of 16 studies with reasons. Six of these excluded studies overall met the inclusion criteria, but their diagnosis of PAD was insufficient and generally just described their population as having PAD (CLI or IC) without presenting any specific diagnostic criteria, such as ankle/toe pressures, exercise testing, or angiography (Kalka 2000; Makinen 1999; NCT02544204; Powell 2003; Rauh 1999; Talitskiy 2012). We excluded five studies primarily because they were unlikely to be randomised, and secondarily, because they did not meet the diagnostic criteria for PAD (Gavrilenko 2015; Korpisalo 2015; Kusumanto 2001; Laitinen 1998; Morishita 2014). Two studies were non‐randomised (Anghel 2011; NCT02016755). Biggs 2009 did not describe the use of a comparison control group and provided insufficient evidence of PAD diagnosis. de Leeuw 2008 reported outcomes only after 28 days, and for Gavrilenko 2008, it is unclear if treatment fit within our inclusion criteria and if diagnosis of PAD was insufficient.
Ongoing studies
We identified four ongoing studies (Fujino 2013; NCT00080392; NCT00304837; NCT02144610). See Characteristics of ongoing studies for details of the ongoing studies.
Risk of bias in included studies
2.
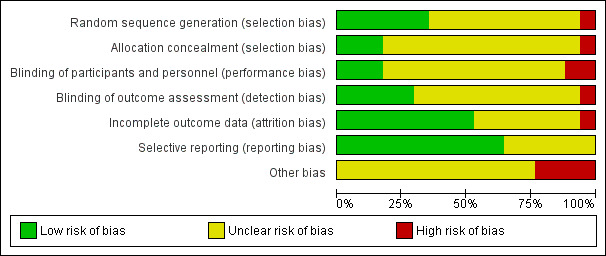
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
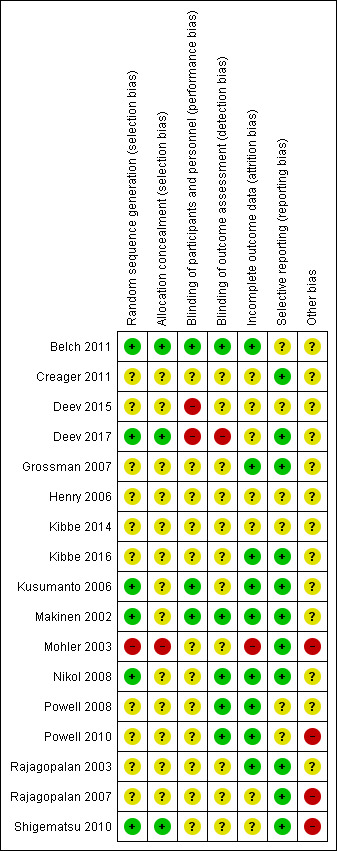
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 17 included studies, six provided sufficient information to indicate low risk of bias based on random sequence generation (Belch 2011; Deev 2017; Kusumanto 2006; Makinen 2002; Nikol 2008; Shigematsu 2010). We rated most studies (10) as having unclear risk because information on random sequence generation was insufficient (Creager 2011; Deev 2015; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Powell 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007). We rated Mohler 2003 as having high risk of selection bias because the protocol was altered during the study due to participant refusal to receive placebo over treatment, which could be evidence of improper random sequence generation and allocation concealment.
For allocation concealment, three studies used adequate methods (Belch 2011; Deev 2017; Shigematsu 2010). Thirteen studies provided insufficient detail to show whether researchers provided adequate allocation concealment, and we rated them as having unclear risk of bias (Creager 2011; Deev 2015; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007). We rated Mohler 2003 as having high risk of bias again for the reasons detailed above for random sequence generation.
Blinding
Three studies provided sufficient information to show that their blinding methods would ensure low risk of performance bias (Belch 2011; Kusumanto 2006; Makinen 2002). Study investigators described 12 studies as double‐blind and utilised a placebo control but gave no supporting information that described how blinding was maintained, for example, whether the placebo was exactly the same in appearance as the treatment, so those administering the treatment would not know the allocation (Creager 2011; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Mohler 2003; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007; Shigematsu 2010). Two studies did not use a placebo control, so we determined that they had high risk of performance bias (Deev 2015; Deev 2017).
In evaluating detection bias, we found that five studies provided sufficient information to show adequate blinding of outcome assessors (Belch 2011; Makinen 2002; Nikol 2008; Powell 2008; Powell 2010). Eleven studies did not provide sufficient information regarding outcome assessors, and we rated them as having unclear risk for detection bias (Creager 2011; Deev 2015; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Kusumanto 2006; Mohler 2003; Rajagopalan 2003; Rajagopalan 2007; Shigematsu 2010). We rated Deev 2017 as having high risk of detection bias because investigators did not implement blinding procedures.
Incomplete outcome data
We rated nine studies as having low risk of attrition bias because they clearly detailed the follow‐up of all participants, or they included sufficient intention‐to‐treat analysis methods (Belch 2011; Grossman 2007; Kibbe 2016; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2003). We rated seven studies as having unclear risk of attrition bias because they did not report on follow‐up of participants or they incurred withdrawals without clear explanation or description of which group they came from (Creager 2011; Deev 2015; Deev 2017; Henry 2006; Kibbe 2014; Rajagopalan 2007; Shigematsu 2010). We rated Mohler 2003 as having high risk of attrition bias because study authors reported a large number of withdrawals from the control group, leaving very few participants in this group.
Selective reporting
Eleven studies were at low risk of reporting bias, as they reported on all outcomes specified in the protocol or Methods section (Creager 2011; Deev 2017; Grossman 2007; Kibbe 2016; Kusumanto 2006; Makinen 2002; Mohler 2003; Nikol 2008; Rajagopalan 2003; Rajagopalan 2007; Shigematsu 2010). We rated six studies as having unclear risk of reporting bias, as they did not provide enough information in the report to show low risk of reporting bias, or they stated they would report on certain outcomes but provided no, or insufficient, data on those outcomes (Belch 2011; Deev 2015; Henry 2006; Kibbe 2014; Powell 2008; Powell 2010).
To assess publication bias, we generated funnel plots for outcomes reported by more than 10 studies. We generated funnel plots for the outcomes of amputation (above the ankle) and all‐cause mortality (Figure 4; Figure 5). We included 11 studies in the funnel plot for amputation and found no visual evidence of publication bias. We included 12 studies in the funnel plot for all‐cause mortality, and although the plot showed visual asymmetry, included studies were too few to determine of there is evidence of publication bias. Studies included in both funnel plots were too few for review authors to appropriately undertake hypothesis testing.
4.
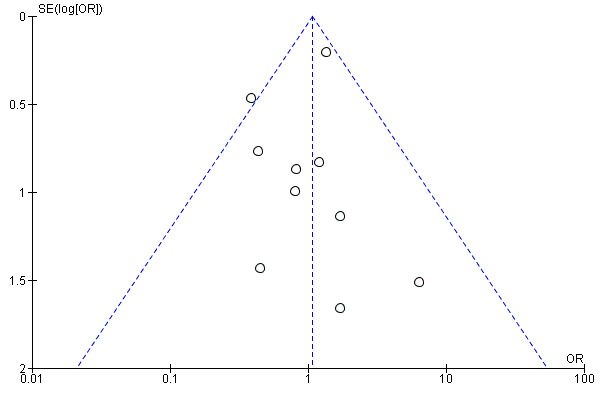
Funnel plot of comparison: 1 Gene therapy versus no gene therapy control, outcome: 1.3 Amputation (above‐ankle amputation of the index limb).
5.
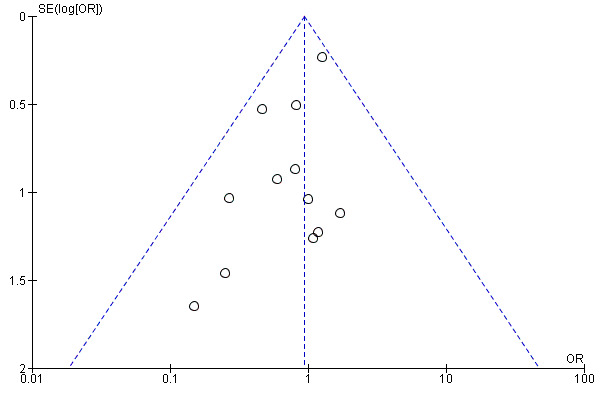
Funnel plot of comparison: 1 Gene therapy versus no gene therapy control, outcome: 1.4 All‐cause mortality.
Other potential sources of bias
We rated 13 studies as having unclear risk of other bias, mainly due to financial support form a commercial entity and/or unmet sample size requirements (Belch 2011; Creager 2011; Deev 2015; Deev 2017; Grossman 2007; Henry 2006; Kibbe 2014; Kibbe 2016; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2008; Rajagopalan 2003). We rated Mohler 2003 as having high risk of other bias in relation to points already made above regarding serious changes in the protocol during the study. Powell 2010 had concerns with enrolment and stated in the ClinicalTrials.gov report that there were "technical problems leading to unreliable or uninterpretable data". Therefore we rated this study as having high risk of other bias. We also rated Rajagopalan 2007 as having high risk of other bias because the investigators incorporated an open‐label phase after the initial blinded phase, during which several placebo participants were rolled over to treatment and therefore were counted twice in the analysis. We attempted to control for this in the data that we included in this review by considering participants as controls only if they had ever received placebo and were not rolled over to treatment. We rated Shigematsu 2010 as having high risk of other bias, as researchers encountered slow recruitment and ended up curtailing their enrolment numbers and conducting an interim analysis.
Effects of interventions
See: Table 1
For meta‐analysis, if an included study evaluated different dosages or types of gene therapy, we combined dosages or treatments into a single treatment group. For outcomes for which we conducted meta‐analyses, we attempted to include data as close to 12 months' follow‐up as possible, as most included studies reported data at this time point.
We did not include three studies in the meta‐analysis (Henry 2006; Kibbe 2014; Mohler 2003). Both Henry 2006 and Kibbe 2014 provided only published abstracts, which left us with insufficient information regarding the study and study results. For Mohler 2003, we had serious concerns regarding the study's high risk of bias due to protocol changes (see Risk of bias in included studies), so we chose not to include this study in the meta‐analysis.
Primary outcomes
Amputation‐free survival (above‐ankle amputation of the index limb or death (any cause))
We included a total of four studies in the meta‐analysis for amputation‐free survival (Belch 2011; Kibbe 2016; Kusumanto 2006; Nikol 2008). Due to high levels of heterogeneity, we utilised a random‐effects model and found no clear differences in outcomes between gene therapy groups and control groups (odds ratio (OR) 1.68, 95% confidence interval (CI) 0.75 to 3.76; 756 participants; I² = 73%; Analysis 1.1). We rated the evidence as moderate quality due to evidence of heterogeneity.
1.1. Analysis.
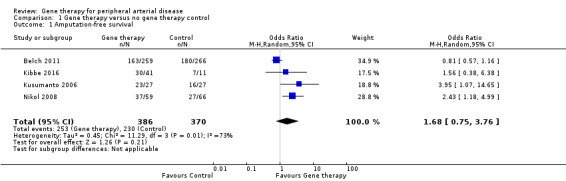
Comparison 1 Gene therapy versus no gene therapy control, Outcome 1 Amputation‐free survival.
Deev 2017 reported amputation‐free survival of 96% in the treatment group and 97% in the control group. We did not include these data in the meta‐analysis, as when we calculated participants using the percentages supplied, the numbers of those reported to have received an amputation and who had died were higher than this calculated figure, meaning that this was not likely a true amputation‐free survival outcome.
Ulcer healing
Seven studies reported on ulcer healing, but none of these studies met the criteria of reporting ulcer healing by change in area in cm². However, as several of these studies provided data on complete ulcer healing, we chose to include them in the meta‐analysis. We included five studies in the meta‐analysis of complete ulcer healing (Kibbe 2016; Nikol 2008; Powell 2010; Rajagopalan 2007; Shigematsu 2010), which showed an OR of 2.16 (95% CI 1.02 to 4.59; 238 participants; P = 0.04; Analysis 1.2). We rated the quality of the evidence as low because of risk of other bias in most of the included studies that was due to poor study design or poor execution of the protocol, and because the confidence interval was quite wide, with few events, leading to imprecision. It should be noted that only one of the five studies included in the meta‐analysis independently demonstrated significant improvement in ulcer healing in the gene therapy group (Kibbe 2016), and when we removed this study from the analysis, we noted no differences between treatment groups.
1.2. Analysis.
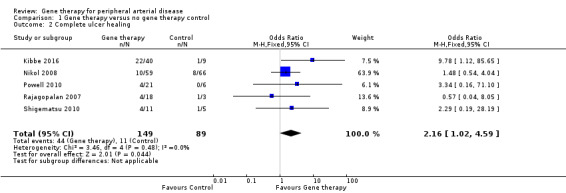
Comparison 1 Gene therapy versus no gene therapy control, Outcome 2 Complete ulcer healing.
Two additional studies reported on ulcer healing but did not meet the criteria of describing their outcomes as "complete ulcer healing". At 100 days, Kusumanto 2006 reported that seven of 21 ulcers in the treatment group showed a decrease in wound surface area greater than 60% but no ulcers in the placebo group met this criterion. Makinen 2002 reported ulcer healing in one of 18 participants in the VEGF‐adenovirus vector (VEGF‐AdV) group, in three of 17 in the VEGF‐plasmid/liposome (VEGF‐P/L) group, and in two of 19 in the control group. Investigators provided no further information on the definition of ulcer healing.
Quality of life (QoL)
Six studies reported on QoL as an outcome. One used solely the Walking Impairment Questionnaire (WIQ), two used only the Short Form‐36 (SF‐36) questionnaire, two evaluated QoL using both the WIQ and the SF‐36, and the sixth study evaluated QoL using the RAND‐36 questionnaire. Due to heterogeneity in collection and reporting of data, we did not undertake meta‐analysis for this outcome. Most studies found no differences between treatment groups in their measures of QoL; however, some studies found that all groups showed significant improvement during the follow‐up period. As with ulcer healing, we rated the findings from this outcome as very low quality due to heterogeneity and imprecision.
Using WIQ, Creager 2011 found no differences between groups at baseline and at 3, 6, and 12 months for any of the components measured: speed, claudication pain, or stair climbing. In Grossman 2007, both treatment and control groups showed significant improvement in WIQ speed and distance score from baseline to follow‐up and no between‐group differences. Findings also revealed no differences in SF‐36 between groups at 90 or 180 days. Kusumanto 2006 utilised the RAND‐36 questionnaire and found no improvement in QoL when comparing the 165‐amino‐acid isoform of VEGF (phVEGF165) versus control. Shigematsu 2010 reported at 12 weeks on the SF‐36 domains of physical functioning, role function (physical), bodily pain, general health perception, vitality, social functioning, role function (mental), and mental health. They found that the treatment group showed significant improvement in bodily pain and mental health domains over the placebo group. In Deev 2015, use of the SF‐36 questionnaire revealed similar increases in treatment and control arms for the physical health domain at six months. Researchers found higher QoL scores regarding mental health in the control group compared to the gene transfer treatment group. Last, Rajagopalan 2003, using both the SF‐36 questionnaire and the WIQ, reported improvements from baseline in both groups but no differences between groups at 12 or 26 weeks.
Secondary outcomes
Amputation (above‐ankle amputation of the index limb)
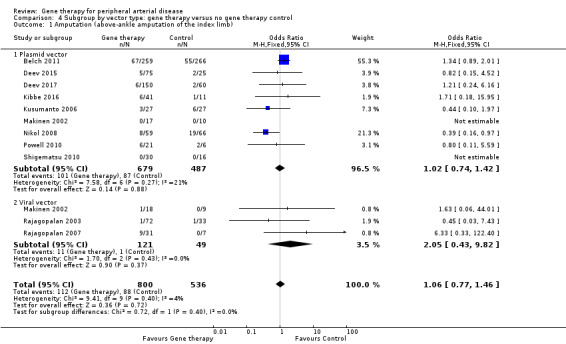
We included a total of 11 studies in the meta‐analysis for major amputation (Belch 2011; Deev 2015; Deev 2017; Kibbe 2016; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007; Shigematsu 2010). Five studies reported on this outcome after 12 months of follow‐up (Belch 2011; Deev 2015; Kibbe 2016; Nikol 2008; Rajagopalan 2007), three after six months (Deev 2017; Powell 2010; Rajagopalan 2003), two at three months (Makinen 2002; Shigematsu 2010), and one at 100 days (Kusumanto 2006). Results show no clear differences between treatment groups in the fixed‐effect model (OR 1.06, 95% CI 0.77 to 1.46; 1336 participants; Analysis 1.3). It should be noted that two studies did not clearly define their amputation outcomes (Deev 2015; Deev 2017). Results of the meta‐analysis did not differ when we removed these studies. We rated the quality of evidence on the outcome of amputation as moderate due to possible heterogeneity in outcomes between the largest study and the remaining studies. Also, study authors reported low numbers of amputation events, which could lead to imprecision, but we did not downgrade the quality of evidence based on this because the confidence interval was modestly narrow. We generated a funnel plot for this outcome but found no evidence of reporting bias (Figure 4). However, it should be noted that we included only 11 studies in the funnel plot, making interpretation difficult and subjective.
1.3. Analysis.
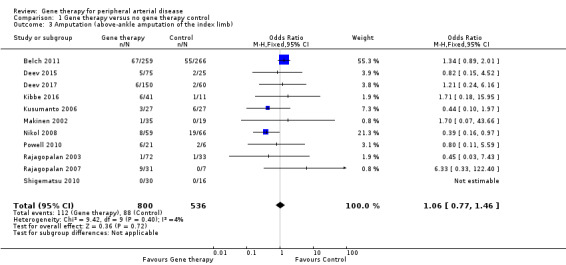
Comparison 1 Gene therapy versus no gene therapy control, Outcome 3 Amputation (above‐ankle amputation of the index limb).
Powell 2008 reported no differences in amputation at 12 months but did not report the number of participants, and Mohler 2003 reported amputation in 6/13 (46%) of those in the treatment group and in 1/2 (50%) participants in the control group at one year.
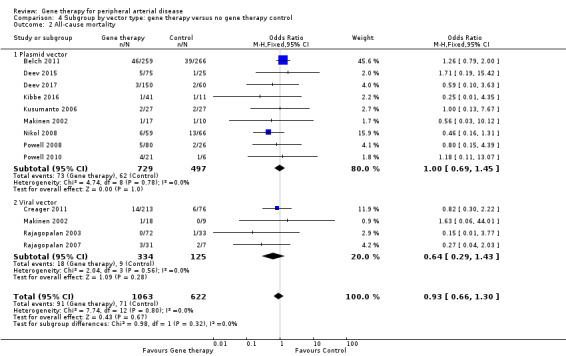
All‐cause mortality
We included 12 studies in the meta‐analysis that evaluated all‐cause mortality (Belch 2011; Creager 2011; Deev 2015; Deev 2017; Kibbe 2016; Kusumanto 2006; Makinen 2002; Nikol 2008; Powell 2008; Powell 2010; Rajagopalan 2003; Rajagopalan 2007). Seven studies reported on mortality at 12 months (Belch 2011; Creager 2011; Deev 2015; Kibbe 2016; Nikol 2008; Powell 2008; Rajagopalan 2007), three at six months (Deev 2017; Powell 2010; Rajagopalan 2003), and one at 100 days (Kusumanto 2006). Makinen 2002 reported outcome evaluation at one and three months after treatment but followed up with participants for safety outcomes, such as all‐cause mortality, for a median of 24 months (range, four to 36 months). Results show no clear differences in mortality between treatment groups in the fixed‐effect model (OR 0.93, 95% CI 0.66 to 1.31; 1685 participants; Analysis 1.4). We rated the quality of evidence for this outcome as moderate due to possible heterogeneity, as the largest included study reported a much higher rate of events compared with the other included studies. As with amputation, events were few, but we did not downgrade quality based on this, as the confidence interval was sufficiently narrow. We also generated a funnel plot for this outcome (Figure 5). Although visual analysis of the plot revealed some asymmetry, details were insufficient to determine if there was evidence of reporting bias. Studies included in the funnel plot were too few to allow adequate hypothesis testing.
1.4. Analysis.
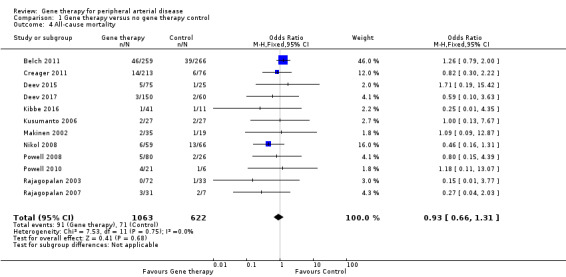
Comparison 1 Gene therapy versus no gene therapy control, Outcome 4 All‐cause mortality.
Shigematsu 2010 reported one death after 15 months but did not state from which group it came. Mohler 2003 reported one death among 13 (8%) participants in the treatment group and zero deaths among 2 (0%) participants in the control group at one year.
Ankle brachial index (ABI)
Only one study effectively reported change in ABI from baseline after 25 weeks of follow‐up (Nikol 2008). Results show no clear differences between groups in the single study, and no overall associations can be drawn (mean difference (MD) 0.04, 95% CI ‐0.07 to 0.15; 125 participants; Analysis 1.5). We rated the quality of evidence for change in ABI as low because we included only a single study, leading to serious imprecision.
1.5. Analysis.
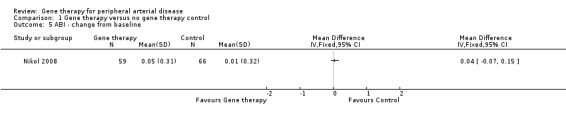
Comparison 1 Gene therapy versus no gene therapy control, Outcome 5 ABI ‐ change from baseline.
Eleven more studies did report on ABI but not in a way that was appropriate for adding data to the meta‐analysis. Most studies found no differences between treatment groups and control groups. Creager 2011 reported no differences between groups at baseline and at 3, 6, and 12 months. Deev 2015 reported an increase of 0.05 (P = 0.0009) in the treatment group at six months and no change in the control group. Powell 2010 provided no specific data on ABI in published references. However, data for this study are available on the ClinicalTrials.gov website, which reports ABI as the mean change in total ABI from baseline, but the data are difficult to interpret and appear to represent the absolute measurement, not the change score, so we have chosen not to report these figures. Powell 2008 reported on toe‐brachial index (TBI) but found no differences at 12 months. Kibbe 2016 reported that average ABI for each group was less than 0.5 at baseline and noted no significant differences within or between groups at any time point, nor with TBI. At 100 days, Kusumanto 2006 found an absolute increase greater than 15% in ABI or TBI for at least two time points among 7/21 (33%) in the treatment group and 1/17 (6%) in the control group. Makinen 2002 reported significant improvements in the two treatment groups at three months but noted similar improvements in control patients. Mohler 2003 found minimal or no improvements in ABI in four patients and "delayed improvement" in two others. The only study to report improvement in ABI in the treatment group, Deev 2017 reported an ABI at baseline of 0.49 ± 0.01 and at six months of 0.61 ± 0.02 in the treatment group, and 0.51 ± 0.01 at baseline and 0.50 ± 0.01 at six months in the control group, with a between‐treatment group P value less than 0.001. After 12 months, Rajagopalan 2007 observed no differences but noted that measurement was not possible in all participants due to arterial calcification, amputation, death, or early withdrawal. Shigematsu 2010 reported an initial increase in mean ABI in the placebo group after 10 weeks but at 12 weeks observed no statistically significant differences between groups.
Symptom scores
Two studies that we included in a meta‐analysis reported change in pain symptoms from baseline using a visual analogue scale (VAS). Powell 2010 reported on pain using a 100‐mm VAS scale at six months, and Nikol 2008 used a 10‐cm VAS scale at one year. We converted the scale used by Powell 2010 to a 10‐cm scale. Meta‐analysis showed no clear differences in pain scores between treatment groups (MD ‐0.22 cm, 95% CI ‐0.83 to 0.38; 152 participants; Analysis 1.6). We rated the quality of evidence as very low due to risk of bias, as one of the included studies incurred technical failures leading to poor data, moderate heterogeneity was evident, and inclusion of only two studies resulted in evidence of imprecision.
1.6. Analysis.
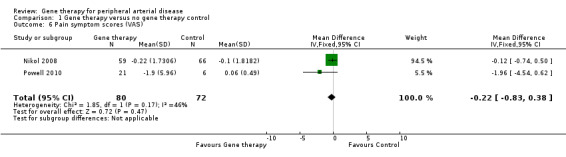
Comparison 1 Gene therapy versus no gene therapy control, Outcome 6 Pain symptom scores (VAS).
Shigematsu 2010 also evaluated pain symptom scores using VAS but found no differences between groups. Powell 2008 reported no difference in pain relief after 12 months. Makinen 2002, after three months, reported resolution of pain in 1/18 (6%) participants in the VEGF‐AdV group and in 1/19 (5%) in the control group, with none achieving resolution of rest pain in the VEGF‐P/L group (0%).
Claudication distance
Four studies reported claudication distances and/or times, but we could not include these studies in a meta‐analysis.
Creager 2011 observed no differences in peak walking time (PWT) and claudication onset time (COT) between groups after six months. Deev 2015 reported an increase in pain‐free walking distance (PWD) of 110%, or a change of 149.5 metres, in the treatment group, and a decrease of 1.5 metres from baseline in the control group after six months. Deev 2017 also reported improvement in PWD in the treatment group, with an increase of 176% in the treatment group and a P value less than 0.001 for differences between groups after six months. Rajagopalan 2003 reported increases in PWT and COT in all groups after 26 weeks but no differences between groups.
Subgoup and sensitivity analyses
At the outset of analysis, we found no substantial clinical or methodological heterogeneity between studies included in the meta‐analysis that was not already identified through planned subgroup or sensitivity analysis.
Subgroup analysis
To evaluate the effects of different subgroups on analyses, we included the results of four separate subgroup analyses, which consisted of (1) subgroup by PAD classification ‐ IC or CLI; (2) subgroup by dosage schedule ‐ single dosage or repeat dosages; (3) subgroup by vector type ‐ plasmid or viral; and (4) subgroup by gene type encoded in the treatment. For ease of incorporating these results, we created a separate comparison for each subgroup with the included outcomes. We evaluated an outcome in the subgroup analysis if it included at least three studies that were not all of the same subgroup category. We did not carry out subgroup analysis for single‐gene versus multi‐gene treatments, as all included studies evaluated only single‐gene treatments. We did not carry out subgroup analysis for treatment route, as all but one study utilised intramuscular injections, and we performed no subgroup analysis based on studies including participants with diabetes mellitus, as all studies included participants with diabetes and did not report their data based on diabetes status.
Comparison of participants with IC versus those with CLI revealed no differences between subgroups in major amputation (P = 0.79) or all‐cause mortality (P = 0.69) (Analysis 2.1; Analysis 2.2). Comparison of studies that evaluated single dosage schedules versus repeat dosages showed no differences between subgroups for both major amputation (P = 0.44) and all‐cause mortality (P = 0.23) (Analysis 3.1; Analysis 3.3). We noted evidence of superiority of a repeated dosage schedule over a single dosage schedule for complete ulcer healing, but this was most likely due to the fact that most of the studies that included this outcome utilised a repeat schedule (Analysis 3.2). Overall we noted no differences between subgroups (P = 0.51). Analysis revealed no differences between subgroups for comparisons of plasmid versus viral vectors in major amputation (P = 0.40) nor all‐cause mortality (P = 0.32) (Analysis 4.1; Analysis 4.2). In subgroup analysis of differences among genes encoded for in treatment groups, the single study that evaluated a VEGF‐encoding treatment showed evidence of improvement in the treatment group over the control group for the outcome of amputation‐free survival, but we cannot draw an overall conclusion based on the findings of a single study. The FGF‐ and HGF‐encoding treatments showed no differences from control (Analysis 5.1). Overall we found no differences between subgroups (P = 0.43). We also noted no differences for the outcomes of major amputation and all‐cause mortality between different gene treatment types (P = 0.55 and P = 0.79, respectively) (Analysis 5.3; Analysis 5.4). Studies that utilised HGF‐encoding vectors provided evidence of complete ulcer healing, and the single studies that evaluated FGF and HIF‐1α provided insufficient data to permit any conclusions at this time. Overall results show no differences between subgroups (P = 0.24) (Analysis 5.2).
2.1. Analysis.
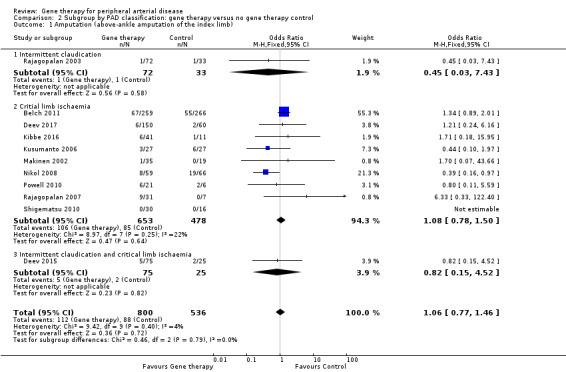
Comparison 2 Subgroup by PAD classification: gene therapy versus no gene therapy control, Outcome 1 Amputation (above‐ankle amputation of the index limb).
2.2. Analysis.
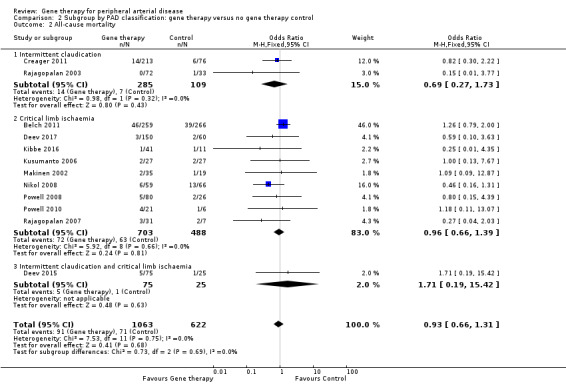
Comparison 2 Subgroup by PAD classification: gene therapy versus no gene therapy control, Outcome 2 All‐cause mortality.
3.1. Analysis.
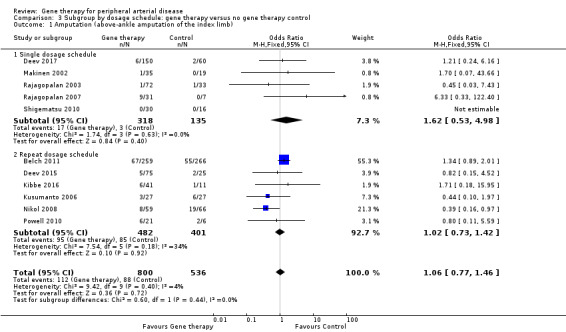
Comparison 3 Subgroup by dosage schedule: gene therapy versus no gene therapy control, Outcome 1 Amputation (above‐ankle amputation of the index limb).
3.3. Analysis.
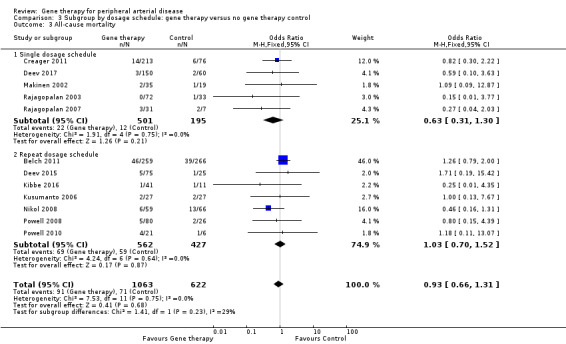
Comparison 3 Subgroup by dosage schedule: gene therapy versus no gene therapy control, Outcome 3 All‐cause mortality.
3.2. Analysis.
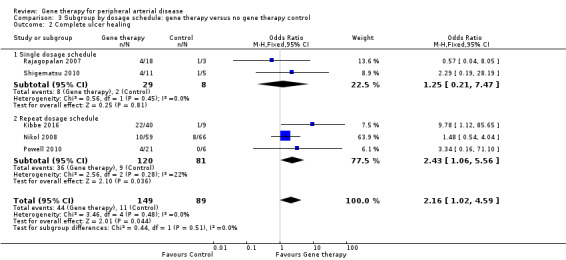
Comparison 3 Subgroup by dosage schedule: gene therapy versus no gene therapy control, Outcome 2 Complete ulcer healing.
4.1. Analysis.
Comparison 4 Subgroup by vector type: gene therapy versus no gene therapy control, Outcome 1 Amputation (above‐ankle amputation of the index limb).
4.2. Analysis.
Comparison 4 Subgroup by vector type: gene therapy versus no gene therapy control, Outcome 2 All‐cause mortality.
5.1. Analysis.
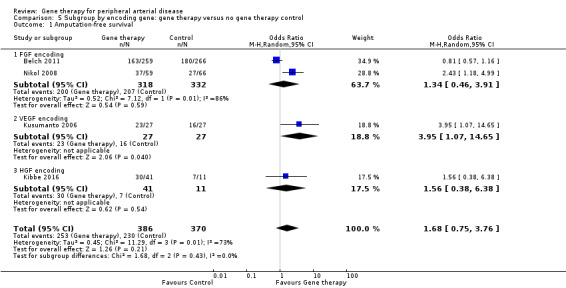
Comparison 5 Subgroup by encoding gene: gene therapy versus no gene therapy control, Outcome 1 Amputation‐free survival.
5.3. Analysis.
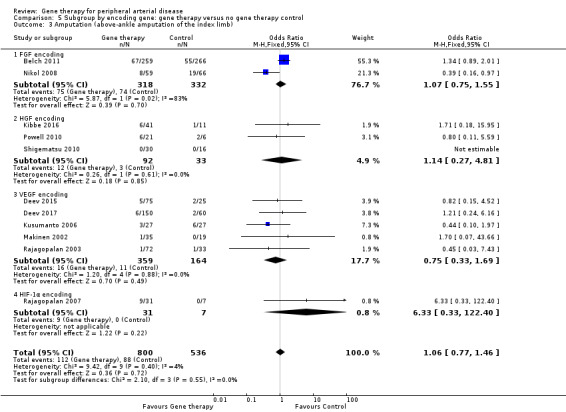
Comparison 5 Subgroup by encoding gene: gene therapy versus no gene therapy control, Outcome 3 Amputation (above‐ankle amputation of the index limb).
5.4. Analysis.
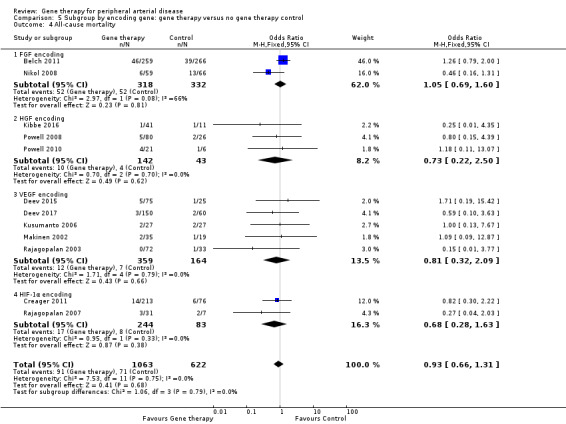
Comparison 5 Subgroup by encoding gene: gene therapy versus no gene therapy control, Outcome 4 All‐cause mortality.
5.2. Analysis.
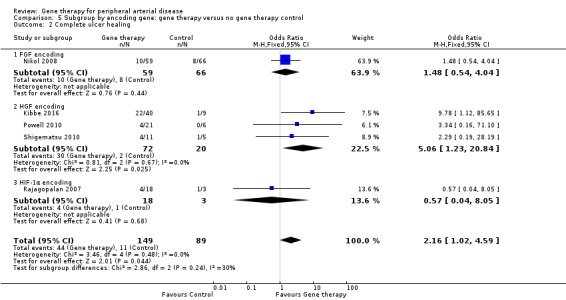
Comparison 5 Subgroup by encoding gene: gene therapy versus no gene therapy control, Outcome 2 Complete ulcer healing.
Sensitivity analysis
We carried out sensitivity analysis on outcomes from the primary comparison (non‐subgroup) if at least three studies reporting an outcome remained after sensitivity analysis, by excluding (1) studies that were particularly large or long; (2) cross‐over trials, cluster‐randomised trials, and multiple observations for the same outcome; and (3) any RCTs that we judged to be at high risk of bias across one or more of the domains evaluated. Upon conducting sensitivity analysis based on these criteria, we found no change in any of the results, which included outcomes of amputation‐free survival, major amputation, and all‐cause mortality.
We performed an additional sensitivity analysis to assess any overt impact that phase III trials may have had on the analysis. For this sensitivity analysis, we removed the two phase III RCTs ‐ Belch 2011 and Deev 2015 ‐ and found that with only three studies remaining, amputation‐free survival was increased in the gene therapy group as compared with the control group (OR 2.48, 95% CI 1.39 to 4.41; 231 participants; P = 0.002). For the same sensitivity analysis, the outcomes of amputation and all‐cause mortality showed no differences when phase III trials were removed.
We also conducted sensitivity analysis by removing studies that overall met our inclusion criteria and included diagnostic criteria for PAD, but for which the diagnostic criteria may not have matched precisely those laid out in the protocol of this review. We removed four studies from the analysis (Deev 2015; Kibbe 2014; Powell 2008; Powell 2010). When we excluded these studies from the analysis, we found no differences from the findings of original analyses.
Discussion
Summary of main results
We included in this review a total of 17 randomised controlled trials, totaling 1988 randomised participants. Included studies represent a varied range of gene therapy types, with most encoding for some kind of growth factor. Most studies compared gene therapy versus placebo, but two studies compared gene therapy versus conservative treatment.
Evidence from meta‐analysis showed no clear differences between gene therapy and control for amputation‐free survival, major amputation, and all‐cause mortality, although evidence revealed statistical heterogeneity in the amputation‐free survival outcome, for which we included only four studies. Limited evidence suggests improved complete ulcer healing in the gene therapy group. We could include in the analysis only one study that evaluated the ankle brachial pressure index (ABI), finding no evidence of a clear difference between groups. We included in the analysis two studies that reported pain symptom scores obtained on a visual analogue scale (VAS) and found no clear differences between treatment groups. We could not combine studies for meta‐analysis for the outcomes of quality of life (QoL) and claudication distance, although several studies did report on these outcomes. See Table 1 for further results for the main outcomes.
Overall completeness and applicability of evidence
We included 17 trials in this review. Even with this number of included studies, only a few or no studies assessed many of the outcomes of this review, including two of the three primary outcomes ‐ amputation‐free survival and quality of life ‐ and the secondary outcomes of ABI, symptom score, and claudication distance. However, a sufficient number of studies reported outcomes of major amputation and all‐cause mortality to permit a conclusion.
Although we found possible evidence of improved ulcer healing in the gene therapy group, this noted improvement should be accepted with awareness of the possibility that informative censoring bias may be affecting the other outcomes. This form of bias occurs when censoring time distribution is not independent of the time to event distribution, in this case, ulcer healing (i.e. participants who experience ulcer healing may be censored sooner), thereby missing out on subsequent deaths or amputations. Good study design and analysis would take this type of bias into account, but it is unclear to the review authors if we can fully accept these outcomes as free of informative censoring.
We applied stringent inclusion criteria surrounding the diagnosis of peripheral arterial disease (PAD) based on objective measures of disease. The included studies do evaluate a wide range of gene therapy types, mainly involving growth factor‐encoding genes. Most of the included studies evaluated participants with more severe PAD, with a diagnosis of critical limb ischaemia (CLI), but several studies also evaluated those with intermittent claudication (IC) without evidence of more severe disease. This review does not address use of cell‐based therapy nor use of direct angiogenic growth factors.
To fully explore the differences between study methods and participants, we conducted subgroup analyses by PAD severity (IC or CLI), by dosage schedule (single or repeat dosages), by vector type (plasmid or viral), and by the encoding genes involved in gene therapy. We found no evidence that any subgroups were more effective for the outcomes evaluated, which were limited mainly to amputation and all‐cause mortality. This type of subgroup analysis is critical for determining whether specific types of gene therapy or specific participant subgroups derive greater benefit from treatment than others; however, this review provides no evidence of such differences. Researchers are interested in evaluating differences in other haematological parameters between responders to treatment and non‐responders, which could yield greater detail on subgroups of interest, but this goal is outside the scope of the present review (Korpisalo 2015; Talitskiy 2012).
Quality of the evidence
Risk of bias was generally unclear due to lack of detailed reporting (Figure 2; Figure 3). This was especially true for selection bias, performance bias, and detection bias. Regarding performance bias, 15 of the 17 studies utilised a placebo and were described as double‐blind, but most of these studies did not clarify how blinding was maintained. We rated the two studies that were not blinded as having high risk of performance bias. Risk of attrition bias and reporting bias was mainly low, but several studies did not clarify findings on follow‐up of their participants, and some studies assessed outcomes that were not reported on, although evidence shows they were included in the protocol. Commercial groups that manufactured the treatment used in the trial supported all of the included studies, and not all reports clearly stated the role of the sponsor in data collection, interpretation, and reporting. We rated these studies as having unclear risk of other bias. Several studies also showed protocol differences that are indicative of bias, so we rated them as having high risk of other bias, including protocol deviations and counting participants twice for a single analysis.
Quality of the evidence, as evaluated by GRADE, ranged from moderate to very low. More included studies reported on outcomes rated as moderate (amputation‐free survival, amputation, all‐cause mortality), so we noted little imprecision but found evidence of heterogeneity. We evaluated outcomes rated as low and very low (ulcer healing, QoL, ABI, pain symptom scores) through meta‐analysis but found few or no data and identified issues related to risk of bias and heterogeneity. See Table 1.
Potential biases in the review process
To minimise potential bias, we undertook a comprehensive search of the literature, with two review authors reviewing all studies for inclusion. Two review authors extracted data, using a predefined data extraction format. However, the possibility remains that relevant literature and data, published or unpublished, were missed in the study selection and data extraction processes.
One study ‐ Rajagopalan 2007 ‐ incorporated a randomised, double‐blind, placebo‐controlled first phase of the study with an open‐label phase, after which several placebo participants were rolled over to treatment and were therefore counted twice in the analysis. In the first phase, researchers randomised 28 participants, with seven participants receiving placebo. For the open‐label phase, investigators added 10 participants for treatment and rolled over three placebo participants from the initial study to receive treatment. We initially planned to include only those enrolled in the first phase, but this was not possible because of reporting issues. Therefore, for our analysis, we included, where possible, control participants who only ever received placebo and were not rolled over, as well as those not initially treated with placebo. This is evident in the major amputation outcome, for which study authors reported 10 cases of major amputation in the treatment group; one of those cases was initially a placebo‐treated participant, so we did not include this case in our analyses.
We imputed several outcomes using data provided by included studies; both Nikol 2008 and Belch 2011 provided a combined death and/or amputation outcome that was then inverted to generate an amputation‐free survival outcome. Nikol 2008 and Powell 2010 provided a change in ABI and VAS with a standard error of the mean (SEM), which was converted to standard deviation (SD) using the formula: SD = SEM * √n. These methods should not lead to biased data but should be considered when data are interpreted.
Four included studies did not fully meet our criteria for PAD diagnosis. However, these four studies included various measures and descriptions of vascular disease that we deemed appropriate to include, as they most likely evaluated the same population as studies that fully met inclusion criteria (Deev 2015; Kibbe 2014; Powell 2008; Powell 2010). We performed sensitivity analysis by excluding these studies from their respective meta‐analyses, and we found no differences in results as compared with original analyses.
For analyses in this review, we utilised fixed‐effect models for meta‐analysis unless we found strong statistical evidence of heterogeneity, using the I² statistic as a reference. Analysis could also be undertaken solely with random‐effects models to account for clinical heterogeneity within studies. We will explore this method in future updates.
Agreements and disagreements with other studies or reviews
A systematic review and meta‐analysis published in 2013 utilised similar inclusion and exclusion criteria (Hammer 2013). The 12 included studies follow closely studies included in this review, and review authors also found no differences between treatment groups for the outcomes of amputation and all‐cause mortality. Hammer 2013 did include the outcome of ulcer healing in their meta‐analysis and found no differences between treatment groups in the number of healed ulcers. In our review, we used different criteria to evaluate ulcer healing and found evidence of possible improved complete ulcer healing in the gene therapy treatment group. Also, the single study in our review showing evidence of improved ulcer healing in the treatment group ‐ Kibbe 2016 ‐ was not yet published at the time of the Hammer 2013 meta‐analysis.
A recently published Cochrane Review evaluated the use of any growth factors for angiogenesis in PAD (Gorenoi 2017). Our review considered all types of gene therapy but excluded trials involving direct growth factor delivery (treatment with direct growth factor protein as opposed to a viral or plasmid vector containing genes encoding for a growth factor) or cell therapy. Many of the studies included in the Gorenoi 2017 review overlap with the studies included in our review. Gorenoi 2017 also found no differences in major amputation or mortality between treatment groups.
A review and meta‐analysis from 2008 included five studies, four of which we also included in this review and one that we considered not relevant, as it utilised a direct growth factor protein for treatment (Ghosh 2008). This meta‐analysis found no differences in peak walking time (PWT), claudication onset time (COT), ABI, or adverse events between treatment and control groups. This review evaluated low‐dose and high‐dose treatments against control separately.
De Haro 2009 produced a meta‐analysis of phase II randomised studies evaluating gene therapy, as well as cell therapy, in people with PAD. Of their six included studies with 543 participants, only three met the inclusion criteria for our review. Review authors found a statistically significant improvement in the treated group for the outcome "overall therapeutic angiogenesis efficacy" (odds ratio (OR) 1.437, 95% confidence interval (CI) 1.029 to 2.005), but it is unclear what was being measured. This meta‐analysis also showed no difference in mortality and, similar to Ghosh 2008, found no differences in overall treatment‐related adverse events.
Authors' conclusions
Implications for practice.
Moderate‐quality evidence shows no clear differences in effectiveness among people with PAD between gene therapy and no gene therapy for the outcomes of amputation‐free survival, major amputation, and all‐cause mortality. Low‐quality evidence suggests that gene therapy may improve complete ulcer healing when compared with control, but these findings have limitations. Very little or no evidence is available regarding other important outcomes such as quality of life and pain symptom scores.
Implications for research.
Further research must be undertaken to assess the effectiveness of gene therapy in people with PAD with focus on outcomes such as ulcer healing, quality of life, ABI, and claudication distance. Although several of the studies included in this review did report on these outcomes, researchers must give more thought to ways of disseminating these data, such as providing accurate change from baseline measures with measures of error, so data can be evaluated and compared appropriately through methods such as meta‐analysis. We found evidence of possible improvement in complete ulcer healing in the gene therapy group, which must be further investigated through the use of more robust measures of ulcer healing. Further evidence is needed around proper dose and timing protocols and regarding differences in treatment of patients with CLI versus IC.
Notes
Parts of the Methods section of the protocol for this review are based on a standard template established by Cochrane Vascular.
Acknowledgements
We would like to thank Tashtanbekova Cholpon Bolotbekovna, Liliya Eugenevna Ziganshina, and the remaining staff at Cochrane Russia for their incredible support and help in translation and data extraction for several studies reported solely in Russian.
We would like to thank the Cochrane Vascular Information Specialist and editorial board for their help and guidance and are grateful to the peer reviewers for their time and comments.
Appendices
Appendix 1. Search strategies
| Source | Search strategy | Hits retrieved |
| CENTRAL | #1 MESH DESCRIPTOR Arteriosclerosis 872 #2 MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 73 #4 MESH DESCRIPTOR Atherosclerosis 684 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 746 #6 MESH DESCRIPTOR Intermittent Claudication 738 #7 MESH DESCRIPTOR Ischemia 823 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2288 #9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY 10322 #10 (((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 9288 #11 (peripheral near3 dis*):TI,AB,KY 3862 #12 (claudic* or IC):TI,AB,KY 3571 #13 arteriopathic:TI,AB,KY 7 #14 dysvascular*:TI,AB,KY 12 #15 ((leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 110 #16 ((limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 185 #17 (((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*))):TI,AB,KY 91 #18 MESH DESCRIPTOR Iliac Artery EXPLODE ALL TREES 154 #19 MESH DESCRIPTOR Popliteal Artery EXPLODE ALL TREES 294 #20 MESH DESCRIPTOR Femoral Artery EXPLODE ALL TREES 873 #21 MESH DESCRIPTOR Tibial Arteries EXPLODE ALL TREES 35 #22 ((((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ))):TI,AB,KY 1362 #23 (isch* or CLI):TI,AB,KY 27272 #24 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 49656 #25 MESH DESCRIPTOR Genetic Therapy EXPLODE ALL TREES 148 #26 MESH DESCRIPTOR Gene Transfer Techniques EXPLODE ALL TREES 120 #27 MESH DESCRIPTOR Genes EXPLODE ALL TREES 1544 #28 MESH DESCRIPTOR Angiogenesis Inducing Agents EXPLODE ALL TREES 44 #29 MESH DESCRIPTOR DNA Viruses EXPLODE ALL TREES 2359 #30 MESH DESCRIPTOR RNA Viruses EXPLODE ALL TREES 6023 #31 angiogen* :TI,AB,KY 2935 #32 arteriogen* :TI,AB,KY 37 #33 vasculogen*:TI,AB,KY 137 #34 adenovirus:TI,AB,KY 455 #35 ((gene* near3 (therap* or treat* or transfer) )):TI,AB,KY 7837 #36 transgene*:TI,AB,KY 102 #37 MESH DESCRIPTOR Angiogenic Proteins EXPLODE ALL TREES 1032 #38 MESH DESCRIPTOR Fibroblast Growth Factors EXPLODE ALL TREES 304 #39 MESH DESCRIPTOR Endothelial Growth Factors EXPLODE ALL TREES 64 #40 MESH DESCRIPTOR Genetic Vectors EXPLODE ALL TREES 152 #41 MESH DESCRIPTOR Vascular Endothelial Growth Factors EXPLODE ALL TREES 968 #42 del‐1:TI,AB,KY 7 #43 VLTS:TI,AB,KY 3 #44 VEGF :TI,AB,KY 2229 #45 FGF:TI,AB,KY 315 #46 ((growth near3 factor)):TI,AB,KY 11682 #47 HGF*:TI,AB,KY 213 #48 HIF*:TI,AB,KY 337 #49 25# OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 30516 #50 #24 AND #49 1365 #51 * NOT SR‐PVD:CC 1088498 #52 #51 AND #50 1263 |
1263 |
| Clinicaltrials.gov | (peripheral OR arterial OR claudication OR ischemia OR ischaemia) AND (gene OR plasmid OR DNA) | 21 |
| ICTRP Search Portal | (peripheral OR arterial OR claudication OR ischemia OR ischaemia) AND (gene OR plasmid OR DNA) | 166 |
| MEDLINE (2017 only) | 1 *Arteriosclerosis/ 39872 2 exp Arteriolosclerosis/ 159 3 Arteriosclerosis Obliterans/ 4192 4 Atherosclerosis/ 32486 5 Arterial Occlusive Diseases/ 28247 6 Intermittent Claudication/ 8184 7 Ischemia/ 50319 8 exp Peripheral Vascular Diseases/ 53026 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 179650 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 151160 11 (peripheral adj3 dis*).ti,ab. 39826 12 (claudic* or IC).ti,ab. 64504 13 (isch* or CLI).ti,ab. 364490 14 arteriopathic.ti,ab. 181 15 dysvascular*.ti,ab. 222 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 745 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1860 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1539 19 Popliteal Artery/ 9465 20 Iliac Artery/ 14224 21 Femoral Artery/ 28635 22 Tibial Arteries/ 1574 23 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10135 24 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 815284 25 Genetic Therapy/ 48081 26 Gene Transfer Techniques/ 27752 27 Genes/ 60254 28 Angiogenesis Inducing Agents/ 3560 29 DNA Viruses/ 4246 30 RNA Viruses/ 7855 31 angiogen*.ti,ab. 103072 32 arteriogen*.ti,ab. 1388 33 vasculogen*.ti,ab. 4931 34 adenovirus.ti,ab. 40478 35 (gene* adj3 (therap* or treat* or transfer)).ti,ab. 138947 36 transgene*.ti,ab. 40856 37 Angiogenic Proteins/ 1373 38 Fibroblast Growth Factors/ 12333 39 Endothelial Growth Factors/ 8320 40 Genetic Vectors/ 77090 41 Vascular Endothelial Growth Factors/ 8783 42 del‐1.ti,ab. 332 43 VLTS.ti,ab. 33 44 VEGF.ti,ab. 58821 45 FGF.ti,ab. 17194 46 (growth adj3 factor).ti,ab. 308398 47 HGF*.ti,ab. 10542 48 HIF*.ti,ab. 23015 49 or/25‐48 735665 50 24 and 49 32344 51 randomized controlled trial.pt. 505458 52 controlled clinical trial.pt. 100426 53 randomized.ab. 442267 54 placebo.ab. 205474 55 drug therapy.fs. 2147127 56 randomly.ab. 305249 57 trial.ab. 465908 58 groups.ab. 1885345 59 or/51‐58 4448873 60 exp animals/ not humans.sh. 4743200 61 59 not 60 3847673 62 50 and 61 5415 63 2017*.ed. 953719 64 62 and 63 359 |
359 |
| Embase (2017 only) | 1 *Arteriosclerosis/ 8109 2 exp Arteriolosclerosis/ 453 3 Arteriosclerosis Obliterans/ 11088 4 Atherosclerosis/ 111646 5 Arterial Occlusive Diseases/ 5724 6 Intermittent Claudication/ 5963 7 Ischemia/ 58363 8 exp Peripheral Vascular Diseases/ 1248235 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 187583 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 140324 11 (peripheral adj3 dis*).ti,ab. 41933 12 (claudic* or IC).ti,ab. 50908 13 (isch* or CLI).ti,ab. 387776 14 arteriopathic.ti,ab. 81 15 dysvascular*.ti,ab. 168 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 671 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2084 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1417 19 Popliteal Artery/ 5109 20 Iliac Artery/ 9640 21 Femoral Artery/ 20298 22 Tibial Arteries/ 2033 23 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10508 24 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 1513904 25 Genetic Therapy/ 48294 26 Gene Transfer Techniques/ 35347 27 Genes/ 420137 28 Angiogenesis Inducing Agents/ 8523 29 DNA Viruses/ 3506 30 RNA Viruses/ 5745 31 angiogen*.ti,ab. 131283 32 arteriogen*.ti,ab. 1738 33 vasculogen*.ti,ab. 5978 34 adenovirus.ti,ab. 36199 35 (gene* adj3 (therap* or treat* or transfer)).ti,ab. 153680 36 transgene*.ti,ab. 43588 37 Angiogenic Proteins/ 721 38 Fibroblast Growth Factors/ 12569 39 Endothelial Growth Factors/ 1862 40 Genetic Vectors/ 15497 41 Vascular Endothelial Growth Factors/ 93342 42 del‐1.ti,ab. 302 43 VLTS.ti,ab. 32 44 VEGF.ti,ab. 81791 45 FGF.ti,ab. 18842 46 (growth adj3 factor).ti,ab. 306180 47 HGF*.ti,ab. 13163 48 HIF*.ti,ab. 31661 49 or/25‐48 1079608 50 24 and 49 79823 51 randomized controlled trial/ 435001 52 controlled clinical trial/ 407751 53 random$.ti,ab. 1126865 54 randomization/ 68057 55 intermethod comparison/ 222998 56 placebo.ti,ab. 214175 57 (compare or compared or comparison).ti. 325422 58 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1554249 59 (open adj label).ti,ab. 59761 60 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 152758 61 double blind procedure/ 118736 62 parallel group$1.ti,ab. 18876 63 (crossover or cross over).ti,ab. 69846 64 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 240046 65 (assigned or allocated).ti,ab. 281154 66 (controlled adj7 (study or design or trial)).ti,ab. 251707 67 (volunteer or volunteers).ti,ab. 167509 68 trial.ti. 205045 69 or/51‐68 3358247 70 50 and 69 15845 71 2017*.dc. 1625525 72 70 and 71 1280 |
1280 |
| CINAHL (2017 only) | S55 S53 AND S54 53 S54 EM 2017 177,369 S53 S45 AND S52 821 S52 S46 OR S47 OR S48 OR S49 OR S50 OR S51 951,352 S51 TX randomly 41,710 S50 TX "treatment as usual" 708 S49 TX "double‐blind*" 755,009 S48 TX "single‐blind*" 8,666 S47 TX trial 236,475 S46 MH "Clinical Trials" 90,793 S45 S24 AND S44 3,117 S44 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 51,639 S43 TX HIF* 794 S42 TX HGF* 268 S41 TX (growth N3 factor) 16,219 S40 TX FGF 578 S39 TX VEGF 2,696 S38 TX VLTS 58 S37 TX del‐1 445 S36 (MH "Vascular Endothelial Growth Factors+") 1235 S35 (MH "Endothelial Growth Factors") 1,003 S34 (MH "Angiogenic Proteins+") 1259 S33 TX transgene* 534 S32 TX (gene* N3 (therap* or treat* or transfer) ) 12,465 S31 TX adenovirus 927 S30 TX vasculogen* 148 S29 TX arteriogen* 70 S28 TX angiogen* 5,607 S27 (MH "RNA Viruses+") 4,533 S26 (MH "DNA Viruses+") 67 S25 (MH "Genes") 14,893 S24 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 87,781 S23 TX (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )) 1,035 S22 (MH "Tibial Arteries") 134 S21 (MH "Femoral Artery") 1,180 S20 (MH "Popliteal Artery") 352 S19 (MH "Iliac Artery") 449 S18 ((lower N3 extrem*) N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) 112 S17 (limb N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) 236 S16 TX (leg N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) 121 S15 TX dysvascular* 165 S14 TX arteriopathic 10 S13 TX (isch* or CLI) 37,892 S12 TX (claudic* or IC) 6,848 S11 (peripheral N3 dis*) 8,536 S10 TX (arter* or vascular or vein* or veno* or peripher*) N3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) 12,207 S9 TX (arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 0 S8 TX (atherosclero* or arteriosclero* or PVD or PAOD or PAD ) 25,447 S7 (MH "Peripheral Vascular Diseases+") 9,616 S6 (MH "Ischemia") 3,239 S5 (MH "Intermittent Claudication") 831 S4 (MH "Arterial Occlusive Diseases") 1,581 S3 (MH "Atherosclerosis") 3,138 S2 (MH "Atherosclerosis") 3,138 S1 (MH "Arteriosclerosis") 4,830 |
53 |
| AMED (2017 only) | 1 Atherosclerosis/ 209 2 Intermittent Claudication/ 72 3 Ischemia/ 253 4 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 783 5 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 451 6 (peripheral adj3 dis*).ti,ab. 431 7 (claudic* or IC).ti,ab. 1020 8 (isch* or CLI).ti,ab. 1615 9 arteriopathic.ti,ab. 1 10 dysvascular*.ti,ab. 56 11 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 21 12 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 31 13 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 14 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 109 15 Genes/ 112 16 angiogen*.ti,ab. 225 17 arteriogen*.ti,ab. 2 18 vasculogen*.ti,ab. 2 19 adenovirus.ti,ab. 15 20 (gene* adj3 (therap* or treat* or transfer)).ti,ab. 1302 21 transgene*.ti,ab. 15 22 VEGF.ti,ab. 117 23 FGF.ti,ab. 11 24 (growth adj3 factor).ti,ab. 414 25 HGF*.ti,ab. 19 26 HIF*.ti,ab. 51 27 or/1‐14 4166 28 or/15‐26 2075 29 27 and 28 88 30 2017*.up. 6951 31 29 and 30 4 |
3 |
| TOTAL before de‐duplication | 3597 | |
| TOTAL after de‐duplication | 3223 | |
Data and analyses
Comparison 1. Gene therapy versus no gene therapy control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation‐free survival | 4 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.75, 3.76] |
| 2 Complete ulcer healing | 5 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.02, 4.59] |
| 3 Amputation (above‐ankle amputation of the index limb) | 11 | 1336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 4 All‐cause mortality | 12 | 1685 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.31] |
| 5 ABI ‐ change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pain symptom scores (VAS) | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.83, 0.38] |
Comparison 2. Subgroup by PAD classification: gene therapy versus no gene therapy control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation (above‐ankle amputation of the index limb) | 11 | 1336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 1.1 Intermittent claudication | 1 | 105 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.03, 7.43] |
| 1.2 Critial limb ischaemia | 9 | 1131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.50] |
| 1.3 Intermittent claudication and critical limb ischaemia | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.15, 4.52] |
| 2 All‐cause mortality | 12 | 1685 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.31] |
| 2.1 Intermittent claudication | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.27, 1.73] |
| 2.2 Critical limb ischaemia | 9 | 1191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.39] |
| 2.3 Intermittent claudication and critical limb ischaemia | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.19, 15.42] |
Comparison 3. Subgroup by dosage schedule: gene therapy versus no gene therapy control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation (above‐ankle amputation of the index limb) | 11 | 1336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 1.1 Single dosage schedule | 5 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.53, 4.98] |
| 1.2 Repeat dosage schedule | 6 | 883 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.42] |
| 2 Complete ulcer healing | 5 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.02, 4.59] |
| 2.1 Single dosage schedule | 2 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.21, 7.47] |
| 2.2 Repeat dosage schedule | 3 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.06, 5.56] |
| 3 All‐cause mortality | 12 | 1685 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.31] |
| 3.1 Single dosage schedule | 5 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.31, 1.30] |
| 3.2 Repeat dosage schedule | 7 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
Comparison 4. Subgroup by vector type: gene therapy versus no gene therapy control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation (above‐ankle amputation of the index limb) | 11 | 1336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 1.1 Plasmid vector | 9 | 1166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.74, 1.42] |
| 1.2 Viral vector | 3 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.43, 9.82] |
| 2 All‐cause mortality | 12 | 1685 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 2.1 Plasmid vector | 9 | 1226 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 2.2 Viral vector | 4 | 459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.43] |
Comparison 5. Subgroup by encoding gene: gene therapy versus no gene therapy control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation‐free survival | 4 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.75, 3.76] |
| 1.1 FGF encoding | 2 | 650 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.46, 3.91] |
| 1.2 VEGF encoding | 1 | 54 | Odds Ratio (M‐H, Random, 95% CI) | 3.95 [1.07, 14.65] |
| 1.3 HGF encoding | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.38, 6.38] |
| 2 Complete ulcer healing | 5 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.02, 4.59] |
| 2.1 FGF encoding | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.54, 4.04] |
| 2.2 HGF encoding | 3 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.06 [1.23, 20.84] |
| 2.3 HIF‐1α encoding | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.04, 8.05] |
| 3 Amputation (above‐ankle amputation of the index limb) | 11 | 1336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 3.1 FGF encoding | 2 | 650 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.55] |
| 3.2 HGF encoding | 3 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.27, 4.81] |
| 3.3 VEGF encoding | 5 | 523 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.33, 1.69] |
| 3.4 HIF‐1α encoding | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.33 [0.33, 122.40] |
| 4 All‐cause mortality | 12 | 1685 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.31] |
| 4.1 FGF encoding | 2 | 650 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.60] |
| 4.2 HGF encoding | 3 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.22, 2.50] |
| 4.3 VEGF encoding | 5 | 523 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.09] |
| 4.4 HIF‐1α encoding | 2 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.28, 1.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belch 2011.
| Methods |
Study design: multi‐centre phase III double‐blind placebo‐controlled RCT Intention‐to‐treat: yes Countries: 30 countries |
|
| Participants |
Number randomised: N = 525 (NV1FGF n = 259; placebo n = 266) Losses to follow‐up and withdrawals: N = 0 Age (mean years (range)): 70 years (50 to 95) (NV1FGF 71 (50 to 95); placebo 69 (50 to 92)) Gender (M): 70% (NV1FGF 69%; placebo 70%) Inclusion criteria: age > 50 years; CLI with ischaemic lesions (Fontaine stage IV) with diagnosis confirmed by at least 1 haemodynamic measurement (ankle pressure < 70 mmHg, toe pressure < 50 mmHg, or TcPO₂ < 30 mmHg) and by 1 imaging technique (angiography or doppler examination), and confirmed by vascular surgeons that participant was unsuitable for revascularisation; and to justify this decision to the independent adjudication panel, patent femoral artery inflow assessed by digital angiography, magnetic resonance, or CT angiography (doppler if previous angiography is available) < 6 months before first administration of study treatment; negative screening for cancer (including family history, complete physical examination of every system organ including the skin, haematological blood testing, chest radiography, stool haemoccult test, measurement of prostate‐specific antigen for men, and mammography and Papanicolaou test for women, and any investigation required by national guidelines for cancer screening) Exclusion criteria: previous major amputation of the leg to be treated; planned major amputation within the first month after randomisation; infected gangrene affecting the forefoot evidenced by imaging (radiography); CLI caused by Buerger’s disease; ulcers from venous or neuropathic origin if not associated with at least 1 ulcer of arterial origin; successful revascularisation procedure of the lower leg or any other successful treatment of the leg to be treated < 3 months before randomisation; uncontrolled blood pressure defined as systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg; severe comorbid disorder, not expected to survive longer than 12 months; acute cardiovascular events within 3 months before randomisation; active or proliferative retinopathy and severe macular oedema; previous or present history of malignant disease, other than basal cell carcinoma and cervical carcinoma in situ, within the past 5 years; previous malignant disease with relapse or therapy within the past 5 years; previous treatment with systemic growth angiogenic factors or with stem cell therapy; women pregnant or breastfeeding, or of childbearing potential not protected by an effective contraceptive method of birth control; men not following effective contraceptive method with their partner of childbearing potential during the study |
|
| Interventions |
Treatment: NV1FGF, 0.2 mg/mL, eight 0.5‐mg intramuscular injections in the index leg (affected leg; if disease affected both legs and both were unsuitable for revascularisation, the leg with the lowest ABI or TBI), 4 injections into the calf (anterior and posterior regions) and 4 into the thigh on days 1, 15, 29, and 43; injection sites selected according to an accessible good striated muscle mass and as close as possible to areas of known collateral blood flow development Control: placebo, given in the same manner as treatment |
|
| Outcomes |
Follow‐up times: 2, 4, and 6 weeks and 2, 6, 9, and 12 months (exploratory extended safety assessment at 18, 24, 30, and 36 months) Outcomes: Amputation, Death, Skin lesion status, Pain intensity at rest (VAS), Functionality and general health assessment ‐ ambulatory function and residential status for patients (Deneuville questionnaire) and overall QoL (using EuroQoL), Admittance to hospital for amputation and other CLI‐related issues; ABI; TBI; safety assessment (adverse events, subjective symptoms, vital signs, ECG, ophthalmic exam, blood tests) |
|
| Notes |
Study period: recruitment from 1 December 2007 to 31 July 2009 NCT00566657 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a central interactive voice response system by block size of 4 and stratified by diabetes status and country; "generated by an electronic technique"; randomised 1:1 |
| Allocation concealment (selection bias) | Low risk | Used a central interactive voice response system for randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Visually identical matching placebo; "Investigators, patients and study teams were masked to treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators, patients and study teams were masked to treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up and all discontinued clearly reported; ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Did not report ABI/TBI; pain severity by VAS; QoL outcomes, which were indicated in the Methods; the additional publication ‐ Van Belle 2011 ‐ does mention baseline geographical and diabetes status but does not provide the data in a meaningful way |
| Other bias | Unclear risk | Funded by Sanofi‐Aventis; the sponsor was responsible for data monitoring, data collection, and data analysis but had no role in data interpretation or writing of the report |
Creager 2011.
| Methods |
Study design: multi‐centre prospective double‐blind placebo‐controlled parallel‐group RCT Intention‐to‐treat: no; utilised "efficacy set", which includes all patients who were randomised and had at least 1 post‐randomisation treadmill exercise test; utilised last observation carried forward methods Countries: USA (27 sites), UK (4 sites), Germany (4 sites) |
|
| Participants |
Number randomised: total N = 289 (low‐dose HIF‐1α n = 74; mid‐dose HIF‐1α n = 74; high‐dose HIF‐1α n = 65; placebo n = 76); "efficacy set": N = 273 (low‐dose HIF‐1α n = 69; mid‐dose HIF‐1α n = 71; high‐dose HIF‐1α n = 62; placebo n = 71) Losses to follow‐up and withdrawals: not reported; only report n = 16 with no follow‐up treadmill tests Age (mean years ± SD): 68.4 ± 8.4 (low‐dose 65.7; mid‐dose 68.8; high‐dose 66.7; placebo 66.2) Gender (M): low‐dose 78.4%; mid‐dose 78.4%; high‐dose 72.3%; placebo 72.4% Inclusion criteria: men and women 40 to 80 years of age; bilateral atherosclerotic PAD and IC ascertained by resting ABI ≤ 0.90 in the index leg (if arteries non‐compressible, TBI ≤ 0.70); PAD in non‐index leg confirmed by resting ABI ≤ 0.90, reduction in ABI by ≥ 20% after exercise if ABI at rest was > 0.90, or stenosis ≥ 50% as evidenced by duplex ultrasonography, magnetic resonance angiography, or computed tomographic angiography; catheter‐based angiography for diagnosis if necessary; PWT between 1 and 12 minutes on a graded exercise treadmill test and confirmation of PAD as a reason for claudication by a decrease in ABI in the index leg or ≥ 20% immediately after exercise; stable claudication symptom for at least 6 months; smoking status; exercise habits; other medical therapy for claudication; stable for 3 months before enrolment Exclusion criteria: aortoiliac disease limiting the inflow of blood to areas of the limb that were to receive study treatment injections (thighs and calves); type 1 diabetes mellitus; CLI defined as the presence of rest pain, non‐healing ulcers, or tissue loss; PAD‐specific surgical revascularisation within 6 months or an endovascular procedure within 3 months of enrolment; conditions other than PAD that could confound assessment of walking time such as angina, congestive heart failure, or chronic lung disease; cancer within the previous 5 years and not current with American Cancer Society‐recommended cancer screening tests; proliferative diabetic retinopathy and clinically significant abnormal haematological, renal, and hepatic laboratory values |
|
| Interventions |
Treatment: Low‐dose HIF‐1α ‐ 2 × 10⁹ viral particles Ad2/HIF‐1α/VP16, 20 intramuscular injections to each leg (100 µL per injection for a total of 2.0 mL per limb) at predefined sites in the thigh (11 injections) and calf (9 injections) Mid‐dose HIF‐1α ‐ 2 × 10¹⁰ viral particles Ad2/HIF‐1‐α/VP16, as in manner of treatment above High‐dose HIF‐1α ‐ 2 × 10¹¹ viral particles Ad2/HIF‐1‐α/VP16, as in manner of treatment above Control: placebo, phosphate‐buffered saline, 10% sucrose, and 0.02% polysorbate 80, given in the same manner as treatment above |
|
| Outcomes |
Follow‐up times: 3, 6, and 12 months Outcomes: PWT and COT (graded treadmill test, modified Gardner protocol), ABI, QoL (WIQ) |
|
| Notes |
Study period: not reported NCT00117650 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine random sequence generation; randomisation at a ratio of 1:1:1:1 per treatment group |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how saline placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16/289 participants not included in efficacy set due to not having a treadmill test after baseline, but no discussion of other losses or dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the ClinicalTrials.gov protocol reported on appropriately |
| Other bias | Unclear risk | Power calculation required 75 participants in each arm for 80% power to detect a treatment effect of 1.5 minutes in the 26‐week change from baseline in PWT Data were collected and analysed by the sponsor, Genzyme Corp., manufacturer of Ad2/HIF‐1α/VP16 |
Deev 2015.
| Methods |
Study design: multi‐centre phase IIb/III open‐label RCT Intention‐to‐treat: not specified; for PWD, n = 5 in treatment group and n = 1 in control group had amputation before enrolment and therefore could not perform treadmill test; reported "analyzed population in the study included 94 patients: 70‐in the test group and 24‐in the control group" Country: Russia |
|
| Participants |
Number randomised: N = 100 (pCMV‐vegf165 n = 75; control n = 25) Losses to follow‐up and withdrawals: not specified Age (mean years ± SD): pCMV‐vegf165 67.8 ± 9.0; control 70.9 ± 7.8 Gender (M): pCMV‐vegf165 80%; control 80% Inclusion criteria: inclusion decision made by a team of vascular surgeons and radiologists based on angiographic and echographic findings, history of the disease, previous procedures, and concomitant pathology; age > 40 years; history of stable claudication for at least 3 months; stage II to III chronic ischaemia according to Fontaine classification; absence of haemodynamically significant (> 70%) stenosis of the aortoiliofemoral arterial segment or (if present) a patent proximal bypass graft if revascularisation surgery was performed no earlier than 3 months before inclusion in the study; satisfactory patency of the deep femoral artery in the presence of haemodynamically significant femoropopliteal arterial lesions; presence of haemodynamically significant stenosis (stenosis > 70% and/or occlusion); diffuse lesions of the anterior and/or posterior tibial arteries; voluntary informed consent signed Exclusion criteria: CLI of non‐atherosclerotic genesis (autoimmune disorders, Buerger's disease, congenital abnormalities, vascular injuries, etc.); stage IV chronic ischaemia according to Fontaine classification; severe concomitant pathology with life expectancy < 1 year; infectious disease; history of cancer or suspected malignancy; decompensated diabetes mellitus |
|
| Interventions |
Treatment: pCMV‐vegf165 (Neovasculgen) ‐ intramuscular injection of 1.2 mg of pCMV‐vegf165, administered at 4 to 5 injection sites in the lower and middle thirds of the posterior part of the calf; a second 1.2‐mg injection administered 14 days after first treatment, in conjunction with standard treatment Control: standard treatment only |
|
| Outcomes |
Follow‐up times: 6 months, 1 and 2 years Outcomes: PWD, ABI, Blood flow velocity; Additionally QoL (SF‐36) at 6 months only; Safety (adverse events, blood and urine lab tests, chest X‐rays, and abdominal echography) |
|
| Notes |
Study period: protocol approved April 2010 NCT03068585 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine adequate random sequence generation; randomised to 2 groups at a ratio of 3:1 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not undertaken and not feasible due to the nature of treatment and control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses and withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | ClinicalTrials.gov protocol states researchers will evaluate transcutaneous oximetry, but this is not reported in the results |
| Other bias | Unclear risk | With 3:1 randomisation, there were only 25 participants in the control group, which limited the subgroup analysis; as stated in the report, no participants in the control group had stage IIa disease Sample size calculation estimated 28 participants in each group to detect a 0.75 standardised difference (80% power) Several study authors are employees of the OJSC Human Stem Cell Institute, which funded the study |
Deev 2017.
| Methods |
Study design: multi‐centre open controlled prospective comparative RCT Intention‐to‐treat: not reported Countries: Russia, Ukraine |
|
| Participants |
Number randomised: N = 210 (pl‐VEGF165 n = 150; control n = 60) Losses to follow‐up and withdrawals: not reported Age (mean years ± SD): pl‐VEGF165 62.7 ± 9.4; control 68.9 ± 7.1 Gender (M): pl‐VEGF165 85%; control 74% Inclusion criteria: people age ≥ 40 years with diagnosis of IC and CLI of atherosclerotic genesis that correlated with stage II to III according to Fontaine‐Pokrovsky classification (pain‐free walking distance < 200 m and resting pain); unsuitable for surgical and endovascular vessel reconstruction; signed consent form Exclusion criteria: any disease that can, in the opinion of the treating physician, affect the outcome of the study; ulcerous‐necrotic changes in limb tissues; addictive disorders or substance abuse; pregnancy or nursing; all other exclusion criteria listed in the summary of product characteristics |
|
| Interventions |
Treatment: pl‐VEGF165 (Neovasculgen) ‐ 2 × 1.2 mg intramuscular injections for a total dose of 2.4 mg, administered at 4 to 5 injection sites in the lower and middle thirds of the posterior part of the calf muscle Control: conservative therapy without prostaglandins and prostacyclins |
|
| Outcomes |
Follow‐up times: 3 months and 6 months Outcomes: PWD, Adverse drug reactions (using ECG, blood haematology and biochemistry, a coagulation panel, and urinalysis), ABI, blood flow linear velocity, TcPO₂, Mortality, Amputation |
|
| Notes | Study period: completed January 2017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a computer‐generated block randomisation list (block size 5) with consecutively numbered and sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes prepared in advance of the study by a researcher; local trial co‐ordinator who enrolled patients and assigned them to groups was unable to access the randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, no blinding; high chance of leading to bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals reported |
| Selective reporting (reporting bias) | Low risk | All outcomes from ClinicalTrials.gov protocol reported |
| Other bias | Unclear risk | Funding from Human Stem Cells Institute OJSC, Moscow, Russia No reporting of results from the post‐marketing phase |
Grossman 2007.
| Methods |
Study design: multi‐centre phase II double‐blind placebo‐controlled trial Intention‐to‐treat: not specified Country: USA |
|
| Participants |
Number randomised: N = 105 (VLTS‐589 n = 52; control n = 53) Losses to follow‐up and withdrawals: N = 7 withdrew (VLTS‐589 n = 4; control n = 3); reasons: death n = 3, withdrawal of consent n = 1, loss to follow‐up n = 2, pre‐existing condition n = 1 Age (mean years ± SD): 67.7 ± 8.95 (VLTS‐589 67.3 ± 8.16; control 68.1 ± 9.73) Gender (M): 84.8% (VLTS‐589 88.5%; control 81.1%) Inclusion criteria: between the ages of 40 and 81; significant bilateral infrainguinal PAD as assessed by duplex ultrasound, magnetic resonance angiography, computed tomography angiography, or cineangiography within 6 months before screening; stable exercise limiting IC of the lower extremities of > 2 months' duration with a diagnosis of PAD confirmed with ABI ≤ 0.80 in both lower extremities or TBI < 0.70 Exclusion criteria: significant in‐flow disease defined as > 50% stenosis in the distal aorta, common iliac, external iliac, or common femoral arteries; CLI, change in claudication symptoms within 2 months; terminated the treadmill for reasons other than claudication; lower extremity percutaneous intervention within 2 months; lower limb surgical revascularisation within 6 months before study entry or participation in a structured exercise treatment protocol within 30 days of the study; unstable angina; recent MI; recent coronary artery bypass grafting or coronary percutaneous intervention; stroke; congestive heart failure or deep venous thrombosis; history of malignant neoplasm within the past 5 years or presence of proliferative retinopathy; women of reproductive potential required to have a negative pregnancy test at the time of study drug administration |
|
| Interventions |
Treatment: 42 mg VLTS‐589 (Del‐1) ‐ an investigational, non‐viral, plasmid‐based therapeutic comprising a plasmid (pDL1680) expression system formulated with poloxamer 188 ‐ delivered via 21 percutaneous intramuscular injections of 2 mL each Control: placebo, poloxamer 188 alone, delivered in the same manner as treatment |
|
| Outcomes |
Follow‐up times: 30, 90, and 180 days Outcomes: PWT (Gardner exercise treadmill test protocol), ABI, COT, QoL (WIQ and SF‐36 v2) |
|
| Notes |
Study period: June 2003 to June 2005 ‐ estimated dates NCT00068133 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how saline placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven dropouts clearly described and similar between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sponsored by Valentis, Inc. |
Henry 2006.
| Methods |
Study design: multi‐centre phase II double‐blind placebo‐controlled randomised study Intention‐to‐treat: not reported Country: USA |
|
| Participants |
Number randomised: N = 71 Losses to follow‐up and withdrawals: not reported Age (mean years ± SD): not reported Gender (M): not reported Inclusion criteria: 45 years old; informed consent signed before proceeding with any study procedure; severe PAD; trophic lesions with no signs of healing for at least 2 weeks before first study treatment administration; objective evidence of peripheral vascular disease in the diseased limb on 2 consecutive examinations performed at least 1 week apart; demonstration or documentation of total occlusion of the affected limb of 1 or more iliac, superficial femoral, popliteal, and/or 1 or more infrapopliteal arteries as assessed by angiography or magnetic resonance angiography; mean resting supine TcPO₂ of the foot ≤ 40 mmHg based on 2 separate measures performed at least 1 week apart; poor/not candidates for revascularisation Exclusion criteria: previous or current history of malignant disease; positive cancer screening; successful lower extremity surgery; planning to undergo amputation of target limb within 1 month following first administration of study treatment; history of severe renal failure; creatinine > 2.0 mg/dL or estimated creatinine clearance < 30 mL; serious concomitant medical conditions not adequately controlled; Buerger's disease; on dialysis; active proliferative retinopathy with stroke or neurological deficit presumed to be due to stroke within 3 months before first administration of study treatment; previous treatment with any angiogenic growth factor; positive serology for HIV 1 or 2; participation in clinical trials of non‐approved experimental agents within 4 weeks before study entry |
|
| Interventions |
Treatment: NV1FGF, 1 of 5 treatment regimens of 2 to 16 mg, delivered by 8 intramuscular injections in the affected leg every 2 weeks for 4 sessions Control: placebo |
|
| Outcomes |
Follow‐up times: not reported, but 1 reference suggests between 1 and 3 years Outcomes: TcPO₂, Ulcer healing |
|
| Notes |
Study period: June 2002 to July 2005 NCT00798005 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported on study population during follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine selective reporting bias |
| Other bias | Unclear risk | Sponsored by Sanofi |
Kibbe 2014.
| Methods |
Study design: multi‐centre phase IIa double‐blind placebo‐controlled randomised trial Intention‐to‐treat: not reported Country: not reported |
|
| Participants |
Number randomised: N = 48 Losses to follow‐up and withdrawals: not reported Age (mean years ± SD): 58.6 ± 13.7 Gender (M): 88% Inclusion criteria: CLI (Rutherford 4 or 5); poor candidates for surgical revascularisation; receiving stable medical therapy; ankle systolic pressure ≤ 70 mmHg or toe systolic pressure ≤ 50 mmHg Exclusion criteria: not reported |
|
| Interventions |
Treatment: plasmid stromal cell‐derived factor‐1 (pSDF‐1), 4 cohorts, single set of direct intramuscular injections (8 or 16) to the ischaemic limb at escalating doses of 1 mg/mL pSDF‐1 (4, 8, 8, or 16 mg) Control: placebo |
|
| Outcomes |
Follow‐up times: 12 months Outcomes: QoL (SF‐36), VAS, Rutherford class, Time to first/Number of amputations, Wound healing, Survival |
|
| Notes |
Study period: enrolment completed July 2013 Only conference proceedings available from interim report; stated 12‐month data would be available September 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported on study population during follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine selective reporting bias |
| Other bias | Unclear risk | Support from Juventas Therapeutics |
Kibbe 2016.
| Methods |
Study design: multi‐centre phase II double‐blind RCT Intention‐to‐treat: yes, used LOCF (last observation carried forward) Countries: USA, Korea |
|
| Participants |
Number randomised: N = 52 (VM202 low‐dose n = 21; VM202 high‐dose n = 20; placebo n = 11) Losses to follow‐up and withdrawals: VM202 low‐dose n = 1, VM202 high‐dose n = 2, placebo n = 1; 3/21 (14.3%) did not complete study in VM202 low‐dose group, 3/20 (15.0%) did not complete study in VM202 high‐dose group, 3/11 (27.3%) did not complete study in placebo group; 1 person from each group withdrew; 1 person in the placebo group died, as did 1 person in the low‐dose group Age (mean years ± SD): VM202 low‐dose 65.9 ± 10.7; VM202 high‐dose 67.2 ± 10.9; placebo 64.3 ± 14.5 Gender (M): VM202 low‐dose 66.7%; VM202 high‐dose 65.0%; placebo 54.5% Inclusion criteria: 18 to 90 years old; CLI (Rutherford Class 4 to 5); deemed to be poor or suboptimal candidates for bypass graft surgery or endovascular revascularisation; ≥ 1 hallmark symptom of CLI (ischaemic rest pain, focal gangrene (< 3 cm)) Exclusion criteria: pregnant women; successful revascularisation procedure or sympathectomy within 12 weeks before study initiation; major amputation anticipated in the target leg within 4 weeks of the start of treatment; estimated life expectancy < 6 months; thromboangiitis obliterans; deep tissue ulcerations with bone or tendon exposure or clinical evidence of invasive infection uncontrollable by antibiotics; required > 81 mg per day aspirin; currently receiving immunosuppressive medications, COX‐1/COX‐2 inhibitor drugs, high‐dose steroids, chemotherapy, or radiation; history within 5 years or new finding of malignant neoplasm; New York Heart Association Class III or IV heart failure; history of stroke or myocardial infarction within the last 6 months; unstable angina or proliferative retinopathy; any of the following laboratory findings: positive HIV, human T‐lymphotrophic virus, hepatitis B or C |
|
| Interventions |
Treatment VM202 (plasmid DNA expressing 2 isoforms of HGF) low‐dose ‐ 1 × 4 mg VM202 intramuscular injections, 16 total injections into the affected leg according to a schedule that targeted the vascular compartments corresponding to occluded segments, given on day 0 and again on day 14 (8 mg total), followed by saline on days 28 and 42 VM202 high‐dose ‐ 1 × 4 mg VM202, in the same manner as above, on day 0, and again on days 14, 28, and 42 (16 mg total) Control: placebo, saline, in the same manner as above, on days 0, 14, 28, and 42 |
|
| Outcomes |
Follow‐up times: days 14, 28, and 42, and 3, 6, 9, and 12 months Outcomes: Adverse events, Difference in pain severity measured by VAS between baseline and 9 months, Change in VAS, Ulcer healing, Skin perfusion by TcPO₂, ABI and TBI, Rutherford Classification, Quality of life score using VascuQoL, Amputation, Mortality during 12 months |
|
| Notes |
Study period: July 2010 to July 2012 NCT01064440 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine random sequence generation; described only as a "1:2:2 scheme to placebo, low‐dose or high‐dose" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how saline placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk of attrition bias, as all participants accounted for; ITT analysis and LOCF performed |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in Methods reported on |
| Other bias | Unclear risk | Funding by ViroMed; 1 study author receives consulting fees from ViroMed but specified that sole responsibility for data, statistical analysis, and manuscript content lies with the study authors ‐ not the funders |
Kusumanto 2006.
| Methods |
Study design: multi‐centre double‐blind placebo‐controlled RCT Intention‐to‐treat: not specified but all participants evaluated for all endpoints Country: The Netherlands |
|
| Participants |
Number randomised: N = 54 (phVEGF165 n = 27; placebo n = 27) Losses to follow‐up and withdrawals: N = 0 Age (mean years (range)): phVEGF165 68.7 (45 to 85); control 68.4 (40 to 84) Gender (M): phVEGF165 59.2%; control 55.6% Inclusion criteria: type 1 or type 2 diabetes mellitus established according to current American Diabetes Association criteria; evidence of CLI including rest pain and/or ulcers that had not healed for a minimum of 2 weeks despite conventional therapy; compressible vessels with resting ankle systolic blood pressure < 50 mmHg or toe systolic blood pressure < 30 mmHg; unsuitable candidates for surgical or percutaneous revascularisation judged after contrast angiography by vascular surgeon and intervention radiologist Exclusion criteria: active proliferative diabetic retinopathy; history of malignancy; severe comorbidity, compromising comedications |
|
| Interventions |
Treatment: phVEGF165, 2000 µg, on days 0 and 28, 4 aliquots, 500 µg each, diluted in 1.0 mL NaCl, injected intramuscularly into the thigh and calf of the most ischaemic limb; injection sites chosen arbitrarily according to available muscle mass Control: placebo, on days 0 and 28 |
|
| Outcomes |
Follow‐up times: days 7, 14, 35, 42, 72, and 100 Outcomes: Amputation, ABI, TBI, Skin improvements, Pain, QoL using RAND‐36 questionnaire, Safety outcomes |
|
| Notes | Study period: February 2000 to January 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation without stratification or matching, performed by the pharmacy of the University Medical Center Groningen |
| Allocation concealment (selection bias) | Unclear risk | Not specified how allocation concealment was carried out |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double‐blind; "no difference between the phVEGF165 and placebo could be seen or felt by the physician who performed the injection" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Supported by a grant from Fornix BioSciences |
Makinen 2002.
| Methods |
Study design: phase II placebo‐controlled double‐blind RCT Intention‐to‐treat: yes Country: Finland |
|
| Participants |
Number randomised: N = 54 (VEGF‐AdV n = 18; VEGF‐P/L n = 17; control n = 19) Losses to follow‐up and withdrawals: at 3 months: VEGF‐Ad n = 3; VEGF‐P/L n = 1; control n = 2) Age (mean years (range)): VEGF‐AdV 70 (53 to 86); VEGF‐P/L 74 (55 to 84); control 73 (61 to 86) Gender (M): VEGF‐AdV 50.0%; VEGF‐P/L 35.3%; control 42.1% Inclusion criteria: angiographically proven atherosclerotic infrainguinal stenosis or occlusion suitable for PTA Exclusion criteria: type 1 diabetes; malignancy; osteomyelitis; fertile women age < 50 years; signs of active inflammation; abnormal prostate‐specific antigen or carcinoembryonic antigen values; poor cooperation |
|
| Interventions |
Treatment: 2 × 10¹⁰ pfu VEGF‐AdV, intra‐articular catheter administration following PTA VEGF‐P/L (2000 µg VEGF plasmid plus 2000 µL DOTMA:DOPE) intra‐articular catheter administration following PTA Control: placebo, Ringer's lactate, intra‐articular catheter administration following PTA |
|
| Outcomes |
Follow‐up times: 1 and 3 months, median follow‐up 24 months for safety outcomes Outcomes: Ischaemic status using Rutherford Classification, ABI, Vascular assessment, Restenosis rate |
|
| Notes | Study period: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done before beginning of study; block of 9 people; used a procedure based on random digits |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used control placebo treatment; "treatment and follow up were made in double‐blinded manner" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Treatment and follow up were made in double‐blinded manner"; image analysis was carried out by blinded assessors who did not have access to follow‐up laboratory or clinical information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for and dropouts clearly explained |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Supported by a grant from Kuopio University Hospital, Ark Therapeutics Ltd., Boston Scientific Inc., and Valentis |
Mohler 2003.
| Methods |
Study design: phase I double‐blind placebo‐controlled dose‐escalating RCT Intention‐to‐treat: not specified Country: USA |
|
| Participants |
Number randomised with IC: n = 18 (CI‐1023 n = 15 (4 × 10⁸ n = 3, 4 × 10⁸.⁵ n = 3, 4 × 10⁹ n = 3, 4 × 10⁹.⁵ n = 3, 4 × 10¹⁰ n = 3); placebo n = 3) Number randomised with CLI: n = 15 (CI‐1023 n = 13 (4 × 10⁸ n = 3, 4 × 10⁸.⁵ n = 3, 4 × 10⁹ n = 3, 4 × 10⁹.⁵ n = 3, 4 × 10¹⁰ n = 1); placebo n = 2) Losses to follow‐up and withdrawals with IC: n = 1 in CI‐1023 lost to follow‐up; n = 5 withdrew (n = 3 in CI‐1023; n = 2 in placebo) Losses to follow‐up and withdrawals with CLI: n = 5 (CI‐1023 n = 5; placebo n = 1) Age (mean years ± SD) with IC: not specified Age (mean years ± SD) with CLI: 73 ± 8 Gender (M) with IC: 78% Gender (M) with CLI: 67% Inclusion criteria with IC: men or women > 40 years of age; patent inflow (aorto‐iliac segments); angiographic evidence of > 35% stenosis involving infrageniculate vessels and disabling claudication; demonstrable ABI at rest < 0.90 and/or exercise ABI < 0.75 confirmed on 2 different occasions 2 days apart Exclusion criteria with IC: advanced or unstable medical disease; renal insufficiency; proliferative retinopathy; history of malignancy other than non‐melanoma skin cancers Inclusion criteria with CLI: atherosclerotic peripheral arterial disease (PAD); > 35 years of age; patent inflow and angiographic evidence of infra‐inguinal disease (> 50% stenosis) involving the common femoral, superficial femoral, popliteal artery or infrapopliteal vessels and ongoing rest pain or tissue loss (grades II and II of the Joint Council of the Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular Surgery Classification comprising categories 4 and 5 with demonstrable resting ABI < 0.70 and exercise ABI < 0.60 confirmed on 2 different occasions 2 days apart) Exclusion criteria with CLI: advanced renal or liver disease; evidence of infection of any type, including adenovirus, hepatitis virus (A, B, or C), or HIV; ophthalmological exam indicative of retinopathy; history of malignancy other than non‐melanoma skin cancers; successful surgical or endoluminal revascularisation of lower extremity to be treated; unstable angina; coronary artery disease requiring immediate surgical or angioplasty intervention, or recent transmural MI or CVA; serious CNS, psychiatric, musculoskeletal, or immune disease |
|
| Interventions |
Treatment: CI‐1023 (AdGVVEGF121.10), dose escalation from 4 × 10⁸ to 4 × 10¹⁰ particle units in half‐log increments with 1 week between each dosage group for safety; 1‐mL intramuscular injections into 20 sites of the ischaemic lower limb; anatomical region of administration varied dependent on location of disease and vascular anatomy Control: placebo diluent, in the same manner as treatment |
|
| Outcomes |
Follow‐up times with IC: days 1, 7, 15, 30, 90, and 180 and 12 months Outcomes with IC: Safety parameters, Walking ability using Gardner protocol, ABI, Anti‐adenovirus neutralising antibodies, Adenoviral cultures, VEGF levels Follow‐up times with CLI: 1 year Outcomes with CLI: Safety parameters including gangrene and amputation, ABI |
|
| Notes | Study period: reported all 13 participants receiving CI‐1023 as 1 group, although different doses were received based on dose‐escalation schedule | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insufficient information provided to determine randomisation sequence; protocol was altered due to participants' refusal of placebo over treatment, which could be evidence of improper random sequence generation and allocation concealment; at each week or dose, 3 participants were meant to receive treatment and 1 placebo; this was altered after 3 doses for above reasons |
| Allocation concealment (selection bias) | High risk | Insufficient information provided to determine randomisation sequence; protocol altered due to participants' refusal of placebo over treatment, which could be evidence of improper random sequence generation and allocation concealment; at each week or dose, 3 participants were meant to receive treatment and 1 placebo; this was altered after 3 doses for above reasons |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how saline placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts of all participants reported, but in CLI group, 5 of 15 participants withdrew or were lost to follow‐up (this left only 1 participant in the placebo arm), and 6 of 18 participants withdrew or were lost to follow‐up in the IC group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported but no protocol identified |
| Other bias | High risk | Very few numbers in this study and only 2 participants in the control group for the CLI study; 3 for IC Major change in protocol: after first 3 dosing cohorts, protocol was modified to an open‐label format with no placebo arm because of refusal to participate due to placebo arm ‐ same for both IC and CLI studies |
Nikol 2008.
| Methods |
Study design: multi‐centre phase II double‐blind placebo‐controlled RCT Intention‐to‐treat: yes, modified intention‐to‐treat (MITT): those who (1) received at least 2 treatment injections of a study drug, (2) had undergone an evaluation for aggregate ulcer size at baseline and had at least 1 non‐healing ulcer, and (3) had undergone an evaluation for aggregate ulcer size at or after week 5; safety population included all those who received at least 1 treatment injection Countries: Belgium, France, Germany, Italy, Switzerland, UK |
|
| Participants |
Number randomised: N = 125 (NV1FGF n = 59; placebo n = 66); MITT N = 107 (NV1FGF n = 51; placebo n = 56) Losses to follow‐up and withdrawals: N = 18; rate of discontinuation NV1FGF 45.5%; placebo 30.5%; discontinuation from adverse events NV1FGF n = 4; placebo n = 10 Age (mean years ± SD): NV1FGF 71.1 ± 10.4; placebo 73.3 ± 9.8 Gender (M): NV1FGF 64.7%; placebo 75% Inclusion criteria: men and women aged ≥ 45 years, with CLI (defined according to TASC, both arterial occlusion (angiography or doppler) and pressure (resting ankle pressure ≤ 70 mmHg and/or toe pressure ≤ 50 mmHg, and or TcPO₂ ≤ 20 mmHg and/or metatarsal pulse volume recording barely pulsatile) who presented with non‐healing ulcers and for whom revascularisation was not considered a suitable option, with signs of healing of trophic lesions absent for ≥ 2 weeks before first administration of study drug; unsuitable for revascularisation for 1 or more of the following reasons: (1) poor or no autologous graft material, (2) revascularisation would result in incomplete perfusion of the foot, (3) high risk of failure for technical reasons, (4) safety risk associated with the procedure, and (5) high risk of amputation on account of conditions such as gangrene Exclusion criteria: previous or current history of malignant disease (patients who had successful tumour resection or radiochemotherapy more than 5 years before inclusion in the study and no recurrence allowed for inclusion); suspicion of malignant disease (abnormal X‐ray, positive stool haemoccult, positive prostate‐specific antigen, abnormal mammography, Papanicolaou smear of Class IV or Class V characterisation); lower extremity surgery: bypass/angioplasty of the leg to be treated within 2 months before first administration of study treatment (day 1); active PDR; Buerger's disease |
|
| Interventions |
Treatment: 2.5 mL NV1FGF at 0.2 mg/mL, 8 intramuscular injections in a single leg (if bilateral CLI, leg estimated to benefit the most based on lower haemodynamic parameters), with 4 into the calf and 4 into the thigh, with sites selected based on muscle mass, ulcer location, and distance from an artery or main nerve; injections given on days 1, 15, 30, and 45 for a total of 16 mg Control: placebo, saline, given in the same manner as treatment |
|
| Outcomes |
Follow‐up times: weeks 13, 25, 38, and 52 Outcomes: Complete healing of at least 1 ulcer, ABI, Amputation, Death, Ischaemic rest pain during previous 7 days (VAS), Safety (adverse events, physical exam, vital signs, lab tests, ophthalmological exams, chest X‐ray, mammography) |
|
| Notes |
Study period: enrolment April 2002 to April 2004 NCT00368797 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in order enrolled via permuted‐block randomisation in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Utilised a blinded review panel to reconcile discordance between investigators' assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses and discontinuations reported |
| Selective reporting (reporting bias) | Low risk | All outcomes from ClinicalTrials.gov protocol reported |
| Other bias | Unclear risk | Sponsored by Centelion SAS, a subsidiary of Sanofi; sponsor collected data, monitored the conduct of the study, and co‐ordinated writing of the manuscript |
Powell 2008.
| Methods |
Study design: multi‐centre phase II double‐blind placebo‐controlled RCT Intention‐to‐treat: not specified but last observation carried forward method used for missing data; evaluated participants who received at least 1 dose n = 104 (low‐dose n = 26; mid‐dose n = 25; high‐dose n = 27; placebo n = 26) Country: USA |
|
| Participants |
Number randomised: N = 106 (low‐dose AMG0001 n = 27; mid‐dose AMG0001 n = 26; high‐dose AMG0001 n = 27; placebo n = 26) Losses to follow‐up and withdrawals: reported N = 93 evaluated for safety Age (mean years): low‐dose 70.1; mid‐dose 73.0; high‐dose 68.1; placebo 70.2 Gender (M): low‐dose 76%; mid‐dose 57%; high‐dose 57%; placebo 63% Inclusion criteria: age ≥ 40 years; 1 or more clinical indications diagnostic of CLI: distal extremity pain at rest that requires the patient to use analgesics for > 2 weeks, or peripheral ischaemic ulcers or areas of gangrene; TcPO₂ < 40 mmHg; ankle systolic pressure < 70 mmHg or toe pressure < 50 mmHg; poor candidates for standard revascularisation treatment on the basis of inadequate bypass conduit, unfavourable anatomy, or poor operative risk Exclusion criteria: people who, in the opinion of the investigator, had a vascular disease prognosis that indicated they would require a major amputation within 4 weeks of the start of treatment; diagnosis of Buerger's disease (thromboangiitis obliterans); haemodynamically significant aorto‐iliac occlusive disease; deep ulcerations with bone or tendon exposure or clinical evidence of invasive infection uncontrollable by antibiotics; receiving immunosuppressive medication, chemotherapy, or radiation; end‐stage renal disease and receiving long‐term haemodialysis, with evidence of malignant neoplasm (except for fully resolved basal cell carcinoma); PDR; severe non‐proliferative retinopathy; recent retinal vein occlusion; macular degeneration with choroidal neovascularisation; macular oedema on fundus evaluation by ophthalmologist; intraocular surgery within 3 months |
|
| Interventions |
Treatment: Low‐dose AMG0001 ‐ 0.4 mg at days 0, 14, and 28; intramuscular injections, 8 locations: 4 injections at lateral and medial locations in the anterior and posterior distal limb and 4 locations in the posterior calf of the affected limb Mid‐dose AMG0001 ‐ 4.9 mg at days 0 and 28; saline placebo given on day 14, in the same manner as above High‐dose AMG0001 ‐ 4.0 mg at days 0, 14, and 28, in the same manner as above Control: placebo, saline, at days 0, 14, and 28, in the same manner as above |
|
| Outcomes |
Follow‐up times: weeks 1 to 5 and week 7, as well as months 3, 6, and 12 Outcomes: Safety (adverse events, concomitant medication use, ECG, blood chemistry, haematology, coagulation, urinalysis, vital signs, physical exam, cancer and retinopathy screening, assays for HGF plasmid, protein and antibodies), TcPO₂, ABI/TBI, Amputation and ulcer healing, Mortality, Pain (VAS), Rutherford Classification, QoL (SF‐36) |
|
| Notes |
Study period: April 2003 to January 2007 NCT00060892 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine adequate random sequence generation; randomised to 4 groups at ratio 1:1:1:1 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ClinicalTrials.gov protocol describes quadruple blinding that included the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported outcomes for all participants receiving at least 1 dose and provided data for withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No data provided on pain (VAS) or QoL |
| Other bias | Unclear risk | Funded by AnGes Inc. |
Powell 2010.
| Methods |
Study design: multi‐centre double‐blind placebo‐controlled RCT Intention‐to‐treat: safety outcomes analysed by ITT, defined as all randomised participants who received at least 1 dose of treatment; efficacy evaluable (EE) population included all participants who received all 3 doses and had at least 1 follow‐up visit after receiving all 3 doses but before having a peripheral vascular intervention or major amputation Country: USA |
|
| Participants |
Number randomised: N = 27 (AMG0001 n = 21; placebo n = 6) Losses to follow‐up and withdrawals: withdrawal by participant AMG0001 n = 3; placebo n = 0 Age (mean years ± SD): 76.2 ± 1.97 (AMG0001 75.7 ± 2.49; placebo 78.0 ± 1.86) Gender (M): 55.6% (AMG0001 61.9%; placebo 33.3%) Inclusion criteria: appropriately sized ischaemic peripheral ulcer(s) or tissue loss (photographs of wounds reviewed by a vascular specialist before enrolment); 1 or both of the following haemodynamic indicators of severe peripheral arterial occlusive disease: ankle systolic pressure ≤ 70 mmHg or toe systolic pressure ≤ 50 mmHg, poor candidate for standard revascularisation treatment options for peripheral arterial disease based on inadequate bypass conduit, unfavourable anatomy, or poor operative risk Exclusion criteria: patients who, in the opinion of the investigator, had a vascular disease prognosis that indicated they may require a major amputation (at or above the ankle) within 4 weeks of the start of treatment; diagnosis of Buerger’s disease (thromboangitis obliterans); haemodynamically significant aorto‐iliac occlusive disease; revascularisation procedure within 12 weeks before treatment initiation that remained patent (revascularisation procedures evidenced to have failed (completely occluded) for > 2 weeks before treatment initiation were acceptable); deep ulcerations with bone or tendon exposure, or clinical evidence of invasive infection uncontrollable by antibiotics; evidence or history of malignant neoplasm (clinical, laboratory, or imaging), except for fully resolved basal cell carcinoma of the skin (people who underwent successful tumour resection or radiochemotherapy of breast cancer more than 10 years before inclusion in the study, and with no recurrence, could be enrolled, and who had successful tumour resection or radiochemotherapy of all other tumour types more than 5 years before inclusion in the study, and with no recurrence, could be enrolled in the study); proliferative diabetic retinopathy; severe nonproliferative retinopathy; recent (within 6 months) retinal vein occlusion; macular degeneration with choroidal neovascularisation; macular oedema on fundus evaluation by ophthalmologist; intraocular surgery within 3 months; history of ESRD defined as significant by creatinine of 2.5 mg/dL, or receiving long‐term haemodialysis |
|
| Interventions |
Treatment: HGF plasmid AMG0001, 4.0 mg in 8 intramuscular injections, performed under duplex ultrasound guidance in arteriographically chosen (by a central committee of vascular specialists) locations for each participant based on regions of most severe vascular disease; injection given at 3 time points 2 weeks apart (days 0, 14, and 28) Control: placebo, given in the same manner as treatment |
|
| Outcomes |
Follow‐up times: 3 months and 6 months Outcomes: Adverse events, ABI and TBI, Rest pain (VAS), Wound healing (change in size of ulcer), Amputation, Survival, QoL |
|
| Notes |
Study period: August 2005 to August 2008 NCT00189540 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine adequate random sequence generation; randomisation ratio was 4:1 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ClinicalTrials.gov protocol describes quadruple blinding that included the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Utilised ITT efficacy analysis; ClinicalTrials.gov report includes withdrawals |
| Selective reporting (reporting bias) | Unclear risk | QoL not reported |
| Other bias | High risk | Supported by AnGes Inc., for whom 2 of the study authors are consultants Sample size calculation estimated the need for N = 39 evaluable participants (AMG0001 n = 26; placebo n = 13); actual evaluated numbers are far lower due to early termination of the study Reasons given for early termination: (1) sufficient numbers to assess safety, (2) demonstrated a signal of efficacy, and (3) difficulty and slowness of recruitment ClinicalTrials.gov report states that there were "technical problems leading to unreliable or uninterpretable data" |
Rajagopalan 2003.
| Methods |
Study design: multi‐centre phase II double‐blind placebo‐controlled RCT Intention‐to‐treat: yes, missing data analysed via last observation carried forward procedure Country: USA |
|
| Participants |
Number randomised: N = 105 (low‐dose AdVEGF121 n = 32; high‐dose AdVEGF121 n = 40; placebo n = 33) Losses to follow‐up and withdrawals: N = 18 (low dose AdVEGF121 n = 9; high‐dose AdVEGF121 n = 2; placebo n = 8) Age (mean years ± SD): low‐dose AdVEGF121 66 ± 9; high‐dose AdVEGF121 64 ± 9; placebo 68 ± 10 Gender (M): low‐dose AdVEGF121 81%; high‐dose AdVEGF121 68%; placebo 91% Inclusion criteria: male and female; 40 to 80 years of age, with PAD (resting ABI < 0.80 in affected limb) and chronic, stable, predominantly unilateral intermittent claudication ≥ 6 months on a stable medication regimen, with exercise‐associated flow limitation (> 20% fall in ABI with exercise) and unilateral exercise‐limiting claudication, with exercise duration between 1 and 10 minutes (and variability within 20%) on 2 consecutive graded Gardner‐Skinner protocols Exclusion criteria: significant contralateral lower extremity symptoms and signs |
|
| Interventions |
Treatment: Low‐dose AdVEGF121 4 × 10⁹ particle units ‐ 20 1.0‐mL intramuscular injections into the index leg in a single session both anterior and posteriorly into the lower thigh or into the lower thigh and the upper calf High‐dose AdVEGF121 4 × 10¹⁰ particle units, given in the same manner as above Control: vehicle alone, given in the same manner as above |
|
| Outcomes |
Follow‐up times: 12 weeks and 26 weeks Outcomes: PWT (graded Gardner‐Skinner protocol), ABPI, COT, QoL (using SF‐36 and WIQ), Safety (adverse event monitoring, physical exam, lab tests, resting ECGs, ophthalmological exams, and cancer screens) |
|
| Notes | Study period: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine adequate random sequence generation; stratified on the basis of diabetic status |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and used placebo but did not describe how placebo was disguised for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Utilised ITT analysis and clearly stated numbers and reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes from trial design paper reported |
| Other bias | Unclear risk | Funded by GenVec; several study authors are employees of or own stock in GenVec Sample size calculation estimated for 35 people in each treatment group to provide 80% power to detect a mean difference of 1.5 minutes in change in PWT |
Rajagopalan 2007.
| Methods |
Study design: multi‐centre double‐blind placebo‐controlled; 2 trial phases: first phase conducted as an RCT with n = 28 participants; second open‐label phase with n = 10 participants added in the treatment group and n = 3 original placebo‐treated participants rolled over to receive gene therapy Intention‐to‐treat: no, all participants receiving ≥ 1 HIF‐1α or placebo injection were included in the safety analysis Country: USA |
|
| Participants |
Number randomised: N = 38 (HIF‐1α n = 31; placebo n = 7) Losses to follow‐up and withdrawals: not reported Age (mean years (range)): 66 (39 to 87) (HIF‐1α 66 (39 to 87); placebo 67 (46 to 80)) Gender (M): 66% (HIF‐1α 62%; placebo 100%) Inclusion criteria: between 21 and 45 years of age; no options for surgical or endovascular revascularisation and total or subtotal occlusion of at least 1 main artery in a limb confirmed by angiography; CLI (defined as Rutherford Category 4 or 5 present for a minimum of 4 weeks without response to conventional therapies with lack of further revascularisation options confirmed by both the investigator and an independent reviewer) Exclusion criteria: contraindications to growth factor therapy that have been published previously; inflammatory arthritis; Rutherford Category 6 status; prior successful lower extremity arterial surgery, angioplasty, or lumbar sympathectomy during the 2 months before screening; participated in other experimental protocols within 30 days of enrolment or had ever been enrolled in a similar vascular endothelial growth factor or fibroblast growth factor adenoviral or plasmid gene therapy protocol |
|
| Interventions |
Treatment: Ad2/HIF‐1α /VP16 ‐ 1 × 10⁸ to 1 × 10¹⁰ viral particles (5 different treatment groups), 10 × 100 µL intramuscular injections for a total volume of 1.0 mL, into a single limb, placement of injections at discretion of investigator based on patient anatomy and location of occluded artery or arteries Control: placebo, phosphate‐buffered saline with 10% sucrose, given in the same manner as treatment |
|
| Outcomes |
Follow‐up times: days 3, 7, 14, 21, 30, 45, 60, and 90, 6 months, and 1 year Outcomes: Adverse events, Changes in baseline physical examinations, Clinical laboratory evaluations, Adenoviral antibody titre measurement, Retinal eye examinations and examinations to assess rest pain, Healing of ischaemic ulcers, Rutherford Category, ABI, MRA to detect vascular changes |
|
| Notes |
Study period: October 1999 to June 2004 Study reported pooled HIF‐1α results and not per dosage; we are reporting HIF‐1α as a single treatment group Treatment numbers reported in this review (HIF‐1α n = 31) differ from the report, as their n = 34 treated includes 3 participants originally randomised to placebo who were rolled over, so are counted twice (in the control group as well) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to determine allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | First part of study was double‐blind, but second phase was open‐label, where several participants originally assigned to placebo were rolled over to treatment; blinding methods not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information provided only as total trial, not separately by trial phases; withdrawals reported in Figures 2 and 3 but only for those with rest pain or ulcers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported but ABI reportedly not available for all study participants |
| Other bias | High risk | Study sponsored by Genzyme Corp., manufacturer of Ad2/HIF‐1α/VP16 Study incorporated a randomised, double‐blind, placebo‐controlled first phase of the study with an open‐label phase after, where several placebo participants were rolled over to treatment and therefore were counted twice in the analysis |
Shigematsu 2010.
| Methods |
Study design: multi‐centre double‐blind placebo‐controlled RCT Intention‐to‐treat: no, interim analysis carried out when participants reached N = 40 (HGF n = 27; placebo n = 13), and safety analysis N = 41 (HGF n = 28; placebo n = 13) Country: Japan |
|
| Participants |
Number randomised: N = 46 (HGF n = 30; placebo n = 16) Losses to follow‐up and withdrawals: N = 6 (HGF n = 3; placebo n = 3) Age (mean years ± SD): HGF 71.9 ± 7.6; placebo 72.8 ± 7.3 Gender (M): HGF 77.8%; placebo 53.8% Inclusion criteria: all eligible participants screened by an eligibility committee composed of vascular surgeons: aged 40 to 84 years with chronic CLI and rest pain or non‐healing ischaemic ulcers (Rutherford 4/Fontaine III or Rutherfor 5/Fontaine IV) persisting for a minimum of 4 weeks; resting ABI < 0.6 and mean ankle blood pressure < 70 mmHg in the affected limb according to 3 consecutive measurements performed at weeks ‐4, ‐2, and 0, or TBI < 0.5 if ABI not measurable; ineligible for standard surgical or percutaneous revascularisation and showed no response to conventional drug therapy for at least 4 weeks Exclusion criteria: deep ulcers that exposed bone or tendon; clinical evidence of invasive infection uncontrolled by antibiotics; serious cardiac, hepatic, renal, or haematological disease; current evidence or history of malignancy; PDR; neovascular age‐related macular degeneration; sympathectomy or sympathetic block within 6 months; revascularisation or major amputation within 3 months |
|
| Interventions |
Treatment: naked plasmid encoding human HGF gene (beperminogene perplasmid, Collategene) ‐ 0.5 mg of HGF plasmid in 3 mL saline given by 8 intramuscular injections into the calf muscles and/or the distal thigh of the ischaemic limb under ultrasound guidance; injection schedule repeated after 28 days Control: placebo, saline, given in the same method as HGF plasmid |
|
| Outcomes |
Follow‐up times: 12 and 24 weeks and 9 and 15 months Outcomes: Improvement in rest pain (reduction in VAS scale > 20 mm compared with baseline) in patients without ulcers or reduction in ulcer size (> 25% (approximately 50% change in area)) in patients with ulcers, ABI, Amputation, QoL using SF‐36, Safety (adverse events, concomitant medications, ECG, lab blood and urine tests, vital signs, physical findings, cancer and retinopathy screenings, assays for HGF protein and antibodies, Escherichia coli protein antibodies and DNA antibodies) |
|
| Notes |
Study period: pre‐screened February 2004 to June 2007 After 12 weeks, participants were unblinded; those who received placebo could choose to enter the next stage and receive active drug |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a modified minimisation method, allocated by the central registration centre; randomisation ratio for plasmid‐to‐placebo was 2:1 |
| Allocation concealment (selection bias) | Low risk | Allocated by the central registration centre |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind, used placebo, and described administration as given in a blinded manner; at the time the study reached N = 40, trial was terminated and information about allocation of treatment was opened to investigators; 3 patients had not been evaluated and were excluded from the analysis; 8 weeks after second administration (12 weeks from first treatment), the study treatment code was opened for each participant, who could then receive HGF if previously receiving placebo, if they wished |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to determine blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals and losses clearly presented in the figure; due to trial terminated early and unblinded, n = 3 were not yet evaluated and were excluded from the final analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Funded and designed by AnGes MG, Inc. (Osaka, Japan); formal data analysis performed by a contract research organisation Study power calculation required for n = 80 in the HGF plasmid group and n = 40 in the placebo group, but with slow recruitment, the analysis was changed to an interim analysis with total N = 40, with only n = 13 in the placebo group |
ABI: ankle brachial pressure index. CLI: critical limb ischaemia. CNS: central nervous system. COT: claudication onset time. CT: computerised tomography. CVA: cerebrovascular accident. Del‐1: developmental endothelial locus‐1. DNA: deoxyribonucleic acid. dL: decilitre. ECG: electrocardiogram. EE: efficacy evaluable. ESRD: end‐stage renal disease. EuroQol: quality of life tool. FGF: fibroblast growth factor. HGF: hepatocyte growth factor. HIF‐1α: hypoxia‐inducible factor 1‐alpha. HIV: human immunodeficiency virus. IC: intermittent claudication. ITT: intention‐to‐treat. LOCF: last observation carried forward. mg: milligram. MI: myocardial infarction. MITT: modified intention‐to‐treat. mL: millilitre. mmHg: millimetre of mercury. MRA: magnetic resonance angiography. NaCl: sodium chloride. NV1FGF: non‐viral 1 FGF. PAD: peripheral arterial disease. PDR: proliferative diabetic retinopathy. Pfu: plaque forming unit. PTA: percutaneous transluminal angioplasty. PWD: pain‐free walking distance. PWT: peak walking time. QoL: quality of life. RAND‐36: quality of life tool. RCT: randomised controlled trial. SD: standard deviation. SDF‐1: stromal cell‐derived factor‐1. SF‐36: Short Form‐36; quality of life tool. TASC: Trans‐Atlantic Inter‐Society Consensus. TBI: toe brachial pressure index. TcPO₂: transcutaneous oximetry. VascuQoL: vascular quality of life questionnaire. VAS: visual analogue scale. VEGF: vascular endothelial growth factor. VEGF‐AdV: VEGF‐adenovirus. VEGF‐P/L: VEGF‐plasmid/liposome. WIQ: Walking Impairment Questionnaire; quality of life tool. µg: microgram. µL: microlitre. TcPO₂: transcutaneous oximetry.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anghel 2011 | Not a randomised study |
| Biggs 2009 | No use of a control comparator group and insufficient diagnostic criteria for PAD |
| de Leeuw 2008 | Insufficient follow‐up period (28 days) |
| Gavrilenko 2008 | Insufficient diagnostic criteria for PAD; unclear if treatment fits inclusion criteria |
| Gavrilenko 2015 | Only partially randomised and insufficient diagnostic criteria for PAD |
| Kalka 2000 | Insufficient diagnostic criteria for PAD |
| Korpisalo 2015 | Insufficient diagnostic criteria for PAD; unclear if this is a randomised study |
| Kusumanto 2001 | Insufficient diagnostic criteria for PAD; unclear if this is a randomised study |
| Laitinen 1998 | Insufficient diagnostic criteria for PAD; unclear if this is a randomised study |
| Makinen 1999 | Insufficient diagnostic criteria for PAD |
| Morishita 2014 | Insufficient diagnostic criteria for PAD; unclear if this is a randomised study |
| NCT02016755 | Not a randomised study |
| NCT02544204 | Insufficient diagnostic criteria for PAD |
| Powell 2003 | Insufficient diagnostic criteria for PAD |
| Rauh 1999 | Insufficient diagnostic criteria for PAD |
| Talitskiy 2012 | Insufficient diagnostic criteria for PAD |
PAD: peripheral arterial disease.
Characteristics of ongoing studies [ordered by study ID]
Fujino 2013.
| Trial name or title | The efficacy and safety of DVC1‐0101 for intermittent claudication secondary to peripheral artery disease: study protocol of a randomised phase IIb trial |
| Methods | Phase IIb randomised placebo‐controlled parallel‐design single‐dose blinded single‐centre clinical trial in Japan |
| Participants | Plan to enrol 60 participants with diagnosis of PAD with intermittent claudication |
| Interventions | DVC1‐0101 (low dose or high dose) or placebo administered by direct intramuscular injection |
| Outcomes | Peak walking time, Safety and tolerability, Claudication onset time, Quality of life measured by the Walking Impairment Questionnaire, Qualifying limb haemodynamics, Pharmacodynamics of DVC1‐0101 by evaluating biomarkers |
| Starting date | March 2014 |
| Contact information | Michiko Tanaka; tmiciko@med.kyushu‐u.ac.jp |
| Notes |
NCT00080392.
| Trial name or title | EW‐A‐401 to treat intermittent claudication |
| Methods | Randomised double‐blind dose‐escalation placebo‐controlled study |
| Participants | Participants with intermittent claudication |
| Interventions | EW‐A‐401 or placebo |
| Outcomes | Safety and toxicity, Blood flow, Walking capacity, Quality of life, Inspection of blood vessels |
| Starting date | March 2004 |
| Contact information | National Institutes of Health Clinical Center (CC) |
| Notes |
NCT00304837.
| Trial name or title | VEGF gene transfer for critical limb ischemia |
| Methods | Randomised cross‐over double‐blind clinical trial |
| Participants | Moderate‐ to high‐risk critical limb ischaemia |
| Interventions | pVGI.1 (VEGF‐2) or placebo |
| Outcomes | Rest pain, Ulcer healing |
| Starting date | March 2006 |
| Contact information | Douglas Losordo |
| Notes | Completed in April 2008 |
NCT02144610.
| Trial name or title | Efficacy and safety of AMG0001 in subjects with critical limb ischemia (AGILITY) |
| Methods | Phase III randomised parallel‐assignment quadruple‐blinded placebo‐controlled study |
| Participants | Participants with critical limb ischaemia |
| Interventions | HGF plasmid (AMG0001) or placebo |
| Outcomes | Time to major amputation, Major amputation and revascularisation, Complete ulcer healing, Ischaemic rest pain, Quality of life, Incident stroke and myocardial infarction, Primary bypass graft patency |
| Starting date | May 2014 |
| Contact information | Richard J Powell; Dartmouth‐Hitchcock Medical Center |
| Notes |
HGF: hepatocyte growth factor. PAD: peripheral arterial disease.
Differences between protocol and review
Several of the outcomes from the protocol were written as "improvement in...", which indicates that we are looking only for improvements and would not report a worsening of the outcome if we found this information. We have amended this and removed "improvement".
For the outcome "Ulcer healing", we removed the description "as measured by surface area of ulceration in cm²", as none of the included studies reported ulcer healing in this manner. However, several of the included studies reported the number of ulcers that healed completely, which we deemed as sufficiently objective; we chose to include these studies in the meta‐analysis.
For clarification of our methods, we changed the way we dealt with studies involving direct growth factor treatment or cell therapy from "excluded" to "not relevant"; therefore we have not included them in the list of excluded studies.
Contributions of authors
RF: acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, writing and reviewing of the manuscript draft. AL: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates. VB: protocol drafting, review drafting, and future review updates. JS: protocol drafting, review drafting, and future review updates. GS: protocol drafting, review drafting, and future review updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Incentive Scheme Award funding to Cochrane Vascular (17/62/09). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health.
Declarations of interest
RF: none known. AL: has received travel, accommodation, and meeting expenses from Pfizer, Bristol‐Myers Squibb, and Novartis; travel expenses from Novo Nordisk, Lilly, and Sanofi; and educational funding from Novartis for completion of a Post Graduate Diploma in Clinical Education. AL is a member of the Scientific Advisory Board for pharmacological treatment of diabetes mellitus and dyslipidaemia (Novo Nordisk, AstraZeneca, Sanofi, Lilly, Janssen, and Amgen). AL has received payment from Novo Nordisk and Lilly for lectures related to diabetes mellitus. AL is an author on the patent "Osteopontin for the prediction and treatment of cardiovascular diseases" (US Patent Number: US8323968B2). This invention relates to the use of endothelial progenitor cells (EPCs) and osteopontin for treatment of cardiovascular disease or complications. The invention also relates to the use of EPC osteopontin levels as a marker of the risk of development of these cardiovascular complications. In particular, the invention provides compositions and methods based on osteopontin and the genes encoding osteopontin. However, this patent is not directly related to the use of osteopontin or any other aspect of this review. In our Cochrane review, we focus specifically on genetic modification of muscle and surrounding tissues (by direct intramuscular or intra‐arterial injections) of the lower limbs of patients with peripheral arterial disease. Whilst both the patent and the Cochrane Review involved gene transfer, they are two completely distinct entities. VB: none known. JS: has received travel support from Novo Nordisk to attend American Diabetes Association meetings and has received grant funding from Dompe for participation in an RCT of reparixin vs placebo in pancreatic islet transplant recipients. GS: none known.
These authors contributed equally to this work
These authors contributed equally to this work
New
References
References to studies included in this review
Belch 2011 {published data only}
- Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo‐controlled trial of gene therapy in critical limb ischaemia. Lancet 2011;377(9781):1929‐37. [DOI] [PubMed] [Google Scholar]
- Hiatt WR. Result of phase III TAMARIS trial. Journal of Gene Medicine 2014;16:209‐10. [Google Scholar]
- NCT00566657. Efficacy and safety of XRP0038/NV1FGF in critical limb ischemia patients with skin lesions (TAMARIS). clinicaltrials.gov/ct2/show/NCT00566657 (first received 3 December 2007).
- Belle E, Nikol S, Norgren L, Baumgartner I, Driver V, Hiatt W, et al. A randomized, double‐blind placebo‐controlled study of NV1FGF gene therapy in critical limb ischemia patients (TAMARIS Study). Rationale, design and baseline patient characteristics. Archives of Cardiovascular Diseases Supplements 2011; Vol. 3, issue 1:77‐8.
- Belle E, Nikol S, Norgren L, Baumgartner I, Driver V, Hiatt WR, et al. Insights on the role of diabetes and geographic variation in patients with critical limb ischaemia. European Journal of Vascular & Endovascular Surgery 2011;42(3):365‐73. [DOI] [PubMed] [Google Scholar]
Creager 2011 {published data only}
- Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S, et al. Effect of hypoxia‐inducible factor‐1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation 2011;124(16):1765‐73. [DOI] [PubMed] [Google Scholar]
- NCT00117650. Safety and efficacy study of Ad2/Hypoxia inducible factor (HIF)‐1a/VP16 gene transfer in patients with intermittent claudication (WALK). clinicaltrials.gov/ct2/show/NCT00117650 (first received 8 July 2005).
Deev 2015 {published data only}
- Bozo IY, Deev RV, Plaksa IL, Mzhavanadze ND, Chervyakov YV, Staroverov IN, et al. Long‐term results of PCMV‐VEGF165 intramuscular gene transfer in patients with chronic lower limb ischemia. Molecular Therapy 2015;23(Suppl 1):S74‐5. [DOI] [PubMed] [Google Scholar]
- Deev RV, Bozo IY, Mzhavanadze ND, Voronov DA, Gavrilenko AV, Chervyakov YV, et al. PCMV‐vegf165 intramuscular gene transfer is an effective method of treatment for patients with chronic lower limb ischemia. Journal of Cardiovascular Pharmacology and Therapeutics 2015;20(5):473‐82. [DOI] [PubMed] [Google Scholar]
- Deev RV, Bozo Ila, Mzhavanadze ND, Nersesian EG, Chukhralia OV, Shval'b PG, et al. Efficacy of using VEGF165 gene in comprehensive treatment of patients with stage 2A‐3 lower limb chronic ischaemia. Angiologiia i Sosudistaia Khirurgiia 2014;20(2):38‐48. [PubMed] [Google Scholar]
- NCT03068585. Efficiency, safety and portability of neovasculgen. clinicaltrials.gov/ct2/show/NCT03068585 (first received 3 March 2017).
Deev 2017 {published data only}
- Deev R, Plaksa I, Bozo I, Isaev A. Results of an international postmarketing surveillance study of pl‐VEGF165 safety and efficacy in 210 patients with peripheral arterial disease. American Journal of Cardiovascular Drugs 2017;17(3):235‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaksa I, Deev R, Bozo I, Livanova A, Isaev A. Four‐year results of an international, multicenter, randomized clinical trial of a pCMV‐vegf165 in progressive ischemia caused by atherosclerotic peripheral arterial disease: results from 332 participants. Journal of Vascular Surgery 2016;63(6 Suppl):37S‐38S. [Google Scholar]
Grossman 2007 {published data only}
- Grossman PM, Mendelsohn F, Henry TD, Hermiller JB, Litt M, Saucedo JF, et al. Results from a phase II multicenter, double‐blind placebo‐controlled study of Del‐1 (VLTS‐589) for intermittent claudication in subjects with peripheral arterial disease. American Heart Journal 2007;153(5):874‐80. [DOI] [PubMed] [Google Scholar]
- NCT00068133. A phase II multicenter, double‐blind, placebo‐controlled, trial of VLTS‐589 in subjects with intermittent claudication secondary to peripheral arterial disease. clinicaltrials.gov/show/NCT00068133 (first received 10 September 2003).
- Rajagopalan S, Olin JW, Young S, Erikson M, Grossman PM, Mendelsohn FO, et al. Design of the Del‐1 for therapeutic angiogenesis trial (DELTA‐1), a phase II multicenter, double‐blind, placebo‐controlled trial of VLTS‐589 in subjects with intermittent claudication secondary to peripheral arterial disease. Human Gene Therapy 2004;15(6):619‐24. [DOI] [PubMed] [Google Scholar]
Henry 2006 {published data only}
- Henry TD, Mendelsohn F, Comerota A, Pham E, Grek V, Coleman M, et al. Dose and regimen effects of intramuscular NV1FGF in patients with critical limb ischemia: a randomized, double‐blind, placebo controlled study. European Heart Journal 2006;27(Suppl 1):P1497. [Google Scholar]
- NCT00798005. Efficacy and safety study of NV1FGF in patients with severe peripheral artery occlusive disease. (TALISMAN202). clinicaltrials.gov/ct2/show/NCT00798005 (first received 25 November 2008).
- Niebuhr A, Henry T, Goldman J, Baumgartner I, Belle E, Gerss J, et al. Long‐term safety of intramuscular gene transfer of non‐viral FGF1 for peripheral artery disease. Gene Therapy 2012;19(3):264‐70. [DOI] [PubMed] [Google Scholar]
Kibbe 2014 {published data only}
- Kibbe MR, Yadav A, Parakh R, Mendelsohn FO, Alexander JQ, McShannic JR, et al. A phase IIA randomized double‐blind, placebo controlled study to evaluate plasmid stromal cell‐derived factor‐1 for treatment of critical limb ischemia ‐ the STOP‐CLI trial. Circulation 2014;130:A19419. [Google Scholar]
Kibbe 2016 {published data only}
- Kibbe MR, Hirsch AT, Medelsohn FO, Davies MG, Pham H, Saucedo J, et al. Safety and efficacy of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with critical limb ischemia. Gene Therapy 2016;23(3):306‐12. [DOI] [PubMed] [Google Scholar]
- NCT01064440. Safety and efficacy study using gene therapy for critical limb ischemia. clinicaltrials.gov/ct2/show/NCT01064440 (first received 8 February 2010).
- Perin ECM. A phase 2, double‐blind, randomized, placebo‐controlled, multicenter trial of the safety and efficacy of plasmid DNA expressing 2 isoforms of hepatocyte growth factor in patients with critical limb ischemia. Journal of the American College of Cardiology 2014; Vol. 63, issue 12 Suppl 1:A2092.
Kusumanto 2006 {published data only}
- Kusumanto YH, Weel V, Mulder NH, Smit AJ, Dungen JJ, Hooymans JM, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double‐blind randomized trial. Human Gene Therapy 2006;17(6):683‐91. [DOI] [PubMed] [Google Scholar]
Makinen 2002 {published data only}
- Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo‐controlled, double‐blinded phase II study. Molecular Therapy 2002;6(1):127‐33. [DOI] [PubMed] [Google Scholar]
- Muona K, Makinen K, Hedman M, Manninen H, Yla‐Herttuala S. 10‐year safety follow‐up in patients with local VEGF gene transfer to ischemic lower limb. Gene Therapy 2012;19(4):392‐5. [DOI] [PubMed] [Google Scholar]
Mohler 2003 {published data only}
- Mohler ER III, Rajagopalan S, Olin JW, Trachtenberg JD, Rasmussen H, Pak R, et al. Adenoviral‐mediated gene transfer of vascular endothelial growth factor in critical limb ischemia: safety results from a phase I trial. Vascular Medicine 2003;8(1):9‐13. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Trachtenberg J, Mohler E, Olin J, McBride S, Pak R, et al. Phase I study of direct administration of a replication deficient adenovirus vector containing the vascular endothelial growth factor cDNA (CI‐1023) to patients with claudication. American Journal of Cardiology 2002;90(5):512‐6. [DOI] [PubMed] [Google Scholar]
Nikol 2008 {published data only}
- NCT00368797. Efficacy and safety study of NV1FGF in patients with severe peripheral artery occlusive disease. clinicaltrials.gov/ct2/show/NCT00368797 (first received 25 August 2006).
- Niebuhr A, Henry T, Goldman J, Baumgartner I, Belle E, Gerss J, et al. Long‐term safety of intramuscular gene transfer of non‐viral FGF1 for peripheral artery disease. Gene Therapy 2012;19(3):264‐70. [DOI] [PubMed] [Google Scholar]
- Nikol S, Baumgartner I, Belle E, Diehm C, Visona A, Capogrossi MC, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation‐free survival in patients with critical limb ischemia. Molecular Therapy 2008;16(5):972‐8. [DOI] [PubMed] [Google Scholar]
- Prokosch V, Stupp T, Spaniol K, Pham E, Nikol S. Angiogenic gene therapy does not cause retinal pathology. Journal of Gene Medicine 2014;16(9‐10):309‐16. [DOI] [PubMed] [Google Scholar]
Powell 2008 {published data only}
- NCT00060892. Study of HGF via plasmid vector to improve perfusion in critical limb ischemia. clinicaltrials.gov/ct2/show/NCT00060892 (first received 16 May 2003).
- Powell RJ, Dormandy J, Simons M, Morishita R, Annex BH. Therapeutic angiogenesis for critical limb ischemia: design of the hepatocyte growth factor therapeutic angiogenesis clinical trial. Vascular Medicine 2004;9(3):193‐8. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, et al. Results of a double‐blind, placebo‐controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 2008;118(1):58‐65. [DOI] [PubMed] [Google Scholar]
Powell 2010 {published data only}
- NCT00189540. Study of hepatocyte growth factor (HGF) via plasmid vector to improve perfusion in critical limb ischemia patients with peripheral ischemic ulcers. clinicaltrials.gov/ct2/show/NCT00189540 (first received 19 September 2005).
- Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH, HGF‐0205 Trial Investigators. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF‐0205 trial. Journal of Vascular Surgery 2010;52(6):1525‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RJ, Marrot P, Annex BH. Safety and efficacy of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF‐0205 trial. Journal of Vascular Surgery 2009; Vol. 50, issue 2:451. [DOI] [PMC free article] [PubMed]
Rajagopalan 2003 {published data only}
- Rajagopalan S, Mohler E III, Lederman RJ, Saucedo J, Mendelsohn FO, Olin J, et al. Regional angiogenesis with vascular endothelial growth factor (VEGF) in peripheral arterial disease: design of the RAVE trial. American Heart Journal 2003;145(6):1114‐8. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Mohler ER III, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double‐blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 2003;108(16):1933‐8. [DOI] [PubMed] [Google Scholar]
Rajagopalan 2007 {published data only}
- Rajagopalan S, Olin J, Deitcher S, Pieczek A, Laird J, Grossman PM, et al. Use of a constitutively active hypoxia‐inducible factor‐1alpha transgene as a therapeutic strategy in no‐option critical limb ischemia patients: phase I dose‐escalation experience. Circulation 2007;115(10):1234‐43. [DOI] [PubMed] [Google Scholar]
Shigematsu 2010 {published data only}
- AnGes MG Inc. Report on long‐term data of Collategene (HGF plasmid). Japanese Phase III study for CLI. AnGes MG Inc 2010.
- Anon. Academic release of data from phase III clinical trial of HGF gene therapy conducted in Japan. https://www.anges.co.jp/en/index.php (date accessed 11 Dec 2008).
- Anon. Announcement of results of phase III clinical trials of HGF gene therapy in Japan. AnGes MG, Inc.. AnGes MG, Inc., 2007.
- Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, et al. Randomized, double‐blind, placebo‐controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Therapy 2010;17(9):1152‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anghel 2011 {published data only}
- Anghel A, Taranu G, Seclaman E, Rata A, Tamas L, Moldovan H, et al. Safety of vascular endothelial and hepatocyte growth factor gene therapy in patients with critical limb ischemia. Current Neurovascular Research 2011;8:183‐9. [DOI] [PubMed] [Google Scholar]
Biggs 2009 {published data only}
- Biggs T, Dulas D, Duval S, Goldman J, Henry T, Hirsch A, et al. Hepatocyte growth factor gene therapy for patients with critical limb ischemia: results of a Phase I dose‐escalation trial. Catheterization and Cardiovascular Interventions 2009;73:S20. [Google Scholar]
de Leeuw 2008 {published data only}
- Leeuw K, Kusumanto Y, Smit AJ, Oomen P, Hoeven JH, Mulder NH, et al. Skin permeability in the diabetic foot with critical limb ischaemia: the effects of a phVEGF165 gene product. Diabetic Medicine 2008;25(10):1241‐4. [DOI] [PubMed] [Google Scholar]
Gavrilenko 2008 {published data only}
- Gavrilenko AV, Voronov DA, Konstantinov BA, Bochkov NP. Combination of reconstructive vascular operations with gene‐engineering technologies of angiogenesis stimulation: a present‐day policy aimed at improving the remote results of treating patients with lower limb chronic ischaemia. Angiologiia i Sosudistaia Khirurgiia/Angiology & Vascular Surgery 2008;14(4):49‐53. [PubMed] [Google Scholar]
Gavrilenko 2015 {published data only}
- Gavrilenko AV, Voronov DA. Results of comprehensive treatment of patients with chronic lower limb ischaemia using gene‐engineering technologies of angiogenesis stimulation. Angiology and Vascular Surgery 2015;21(4):29‐35. [PubMed] [Google Scholar]
Kalka 2000 {published data only}
- Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circulation Research 2000;86(12):1198‐202. [DOI] [PubMed] [Google Scholar]
Korpisalo 2015 {published data only}
- Korpisalo P, Tarvainen S, Auvinen T, Makinen K, Hytonen J, Mussalo H, et al. Differences in collateral‐dependent muscle perfusion may explain efficacy variation in clinical angiogenic gene therapy. Human Gene Therapy 2015;26(10):OR067. [Google Scholar]
Kusumanto 2001 {published data only}
- Kusumanto YH, Mulder NH, Dullaart RPF, Dungen JJAM, Gans ROB, Hoeven HH, et al. Phase III comparison of intramuscular delivery of ANG1 (a vascular endothelial growth factor containing plasmid) with placebo in diabetic patients with critical limb ischaemia. Molecular Therapy 2001;3(5):S73. [Google Scholar]
Laitinen 1998 {published data only}
- Laitinen M, Makinen K, Manninen H, Matsi P, Kossila M, Agrawal RS, et al. Adenovirus‐mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Human Gene Therapy 1998;9(10):1481‐6. [DOI] [PubMed] [Google Scholar]
Makinen 1999 {published data only}
- Makinen K, Laitinen M, Manninen H, Matsi P, Alhava E. Catheter‐mediated VEGF gene transfer to human lower limb arteries after PTA. Circulation 1999;100(18):4063. [Google Scholar]
Morishita 2014 {published data only}
- Morishita R. Development of therapeutic angiogenesis gene therapy using HGF. Journal of Gene Medicine 2014;16(7‐8):210. [Google Scholar]
NCT02016755 {published data only}
- NCT02016755. A phase IIB pilot study of a modified dosage regimen of AMG0001 in subjects with critical limb ischemia. clinicaltrials.gov/ct2/show/NCT02016755 (first received 20 December 2013).
NCT02544204 {published data only}
- NCT02544204. SDF1 plasmid treatment for patients with peripheral artery disease (STOP‐PAD). clinicaltrials.gov/ct2/show/NCT02544204 (first received 9 September 2015).
Powell 2003 {published data only}
- Powell RJ. Protocol#0207‐546: a phase I/II, double‐blind, randomized, placebo‐controlled study to assess the safety and efficacy of AMG0001 to improve perfusion in critical leg ischemia. Human Gene Therapy 2003;14(3):302‐6. [PubMed] [Google Scholar]
Rauh 1999 {published data only}
- Rauh G, Gravereaux E, Pieczed A, Curry C, Schainfeld R, Isner JM. Assessment of safety and efficiency of intramuscular gene therapy with VEGF‐2 in patients with critical limb ischaemia. Circulation 1999;100:770. [Google Scholar]
Talitskiy 2012 {published data only}
- Talitskiy K, Bulkina O, Arefieva T, Vorobieva O, Balkhonova T, Samko A, et al. Blood pressure response and higher count of circulating endothelial progenitors predict angiogenic gene therapy effectiveness in hypertensive patients with chronic limb ischemia. Journal of Hypertension 2010;28(e‐Suppl A):e539. [Google Scholar]
- Talitskiy K, Stukalova O, Bulkina O, Parfyonova Y, Karpov Y. Magnetic resonance imaging is useful for the estimation of therapeutic angiogenesis effect in patients with chronic limb ischemia. Journal of the American College of Cardiology 2012;59(13 Suppl 1):E2069. [Google Scholar]
- Talitskiy K, Stukalova O, Fedorovich A, Bulkina O, Parfyonova Y, Karpov Y. Evaluation of angiogenic gene therapy effect with laser doppler flowmetry and magnetic resonance imaging in patients with chronic limb ischemia. European Heart Journal 2012;33:1011. [Google Scholar]
References to ongoing studies
Fujino 2013 {published data only}
- Fujino Y, Tanaka M, Yonemitsu Y. The efficacy and safety of DVC1‐0101 for intermittent claudication secondary to peripheral artery disease: study protocol of a randomized phase IIb trial. Human Gene Therapy 2013;24(12):A73. [Google Scholar]
NCT00080392 {published data only}
- NCT00080392. EW‐A‐401 to treat intermittent claudication. clinicaltrials.gov/ct2/show/NCT00080392 (first received 30 March 2004).
NCT00304837 {published data only}
- NCT00304837. VEGF gene transfer for critical limb ischemia. clinicaltrials.gov/ct2/show/NCT00304837 (first received 20 March 2006).
NCT02144610 {published data only}
- NCT02144610. Efficacy and safety of AMG0001 in subjects with critical limb ischemia (AGILITY). clinicaltrials.gov/show/NCT02144610 (first received 22 May 2014).
Additional references
Asahara 1997
- Asahara T, Murohara T, Sullivan A, Silver M, Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275(5302):964‐7. [PUBMED: 9020076] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Cea‐Soriano 2018
- Cea‐Soriano L, Fowkes FGR, Johansson S, Allum AM, Rodriguez LAG. Time trends in peripheral artery disease incidence, prevalence and secondary preventive therapy: a cohort study in The Health Improvement Network in the UK. BMJ Open 2018;8(2):e018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conte 2009
- Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, et al. Suggested objective performance goals and clinical trial design for evaluating catheter‐based treatment of critical limb ischemia. Journal of Vascular Surgery 2009;50(6):1462‐73.e1‐3. [PUBMED: 19897335] [DOI] [PubMed] [Google Scholar]
De Haro 2009
Fowkes 2013
- Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382(9901):1329‐40. [DOI] [PubMed] [Google Scholar]
Ghosh 2008
- Ghosh R, Walsh SR, Tang TY, Noorani A, Hayes PD. Gene therapy as a novel therapeutic option in the treatment of peripheral vascular disease: systematic review and meta‐analysis. International Journal of Clinical Practice 2008;62(9):1383‐90. [DOI] [PubMed] [Google Scholar]
Gorenoi 2017
- Gorenoi V, Brehm MU, Kock A, Hagen A. Growth factors for angiogenesis in peripheral arterial disease. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD011741.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammer 2013
- Hammer A, Steiner S. Gene therapy for therapeutic angiogenesis in peripheral arterial disease ‐ a systematic review and meta‐analysis of randomized, controlled trials [Gentherapie zur therapeutischen Angiogenese bei peripherer arterieller Verschlusskrankheit‐Ein systematischer review und Metaanalyse von randomisierten, kontrollierten Studien]. VASA. Zeitschrift fur Gefasskrankheiten 2013;42(5):331‐9. [PUBMED: 23989068] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Kealy 2009
- Kealy B, Liew A, McMahon JM, Ritter T, O'Doherty A, Hoare M, et al. Comparison of viral and nonviral vectors for gene transfer to human endothelial progenitor cells. Tissue Engineering. Part C, Methods 2009;15(2):223‐31. [PUBMED: 19196124] [DOI] [PubMed] [Google Scholar]
Kuliszewski 2011
- Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong‐Poi H. Vascular gene transfer of SDF‐1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Molecular Therapy 2011;19(5):895‐902. [PUBMED: 21364544] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liew 2013
- Liew A, André FM, Lesueur LL, Menorval MA, O'Brien T, Mir LM. Robust, efficient, and practical electrogene transfer method for human mesenchymal stem cells using square electric pulses. Human Gene Therapy Methods 2013;24(5):289‐97. [PUBMED: 23931158] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohler 2008
- Mohler E, Giri J. Management of peripheral arterial disease patients: comparing the ACC/AHA and TASC‐II guidelines. Current Medical Research and Opinion 2008;24(9):2509‐22. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(Suppl S):S5‐67. [PUBMED: 17223489] [DOI] [PubMed] [Google Scholar]
Scougall 2003
- Scougall KT, Maltin CA, Shaw JA. Tetracycline‐regulated secretion of human insulin in a transfected non‐endocrine cell line. Journal of Molecular Endocrinology 2003;30(3):331‐46. [PUBMED: 12790803] [DOI] [PubMed] [Google Scholar]
Van Belle 2011
- Belle E, Nikol S, Norgren L, Baumgartner I, Driver V, Hiatt WR, et al. Insights on the role of diabetes and geographic variation in patients with critical limb ischaemia. European Journal of Vascular & Endovascular Surgery 2011;42(3):365‐73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liew 2016
- Liew A, Bhattacharya V, Shaw J, Stansby G. Gene therapy for peripheral arterial disease. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012058] [DOI] [PMC free article] [PubMed] [Google Scholar]


