Abstract
Background
Supplementary feeding may help food insecure and vulnerable people by optimising the nutritional value and adequacy of the diet, improving quality of life and improving various health parameters of disadvantaged families. In low‐ and middle‐income countries (LMIC), the problems supplementary feeding aims to address are entangled with poverty and deprivation, the programmes are expensive and delivery is complicated.
Objectives
1. To summarise the evidence from systematic reviews of supplementary feeding for food insecure, vulnerable and malnourished populations, including children under five years of age, school‐aged children, pregnant and lactating women, people with HIV or tuberculosis (or both), and older populations.
2. To describe and explore the effects of supplementary feeding given to people in these groups, and to describe the range of outcomes between reviews and range of effects in the different groups.
Methods
In January 2017, we searched the Cochrane Database of Systematic Reviews, MEDLINE, Embase and nine other databases. We included systematic reviews evaluating community‐based supplementary feeding, and concerning food insecure, vulnerable and malnourished populations. Two review authors independently undertook selection of systematic reviews, data extraction and 'Risk of bias' assessment. We assessed review quality using the AMSTAR tool, and used GRADEpro 'Summary of findings' tables from each review to indicate the certainty of the evidence for the main comparisons. We summarised review findings in the text and reported the data for each outcome in additional tables. We also used forest plots to display results graphically.
Main results
This overview included eight systematic reviews (with last search dates between May 2006 and February 2016). Seven were Cochrane Reviews evaluating interventions in pregnant women; children (aged from birth to five years) from LMIC; disadvantaged infants and young children (aged three months to five years); children with moderate acute malnutrition (MAM); disadvantaged school children; adults and children who were HIV positive or with active tuberculosis (with or without HIV). One was a non‐Cochrane systematic review in older people with Alzheimer's disease. These reviews included 95 trials relevant to this overview, with the majority (74%) of participants from LMIC.
The number of included participants varied between 91 and 7940 adults, and 271 and more than 12,595 children. Trials included a wide array of nutritional interventions that varied in duration, frequency and format, with micronutrients often reported as cointerventions. Follow‐up ranged from six weeks to two years; three trials investigated outcomes at four to 17 years of age. All reviews were rated as high quality (AMSTAR score between eight and 11). The GRADE certainty ratings ranged from very low to moderate for individual comparisons, with the evidence often comprising only one or two small trials, thereby resulting in many underpowered analyses (too small to detect small but important differences). The main outcome categories reported across reviews were death, anthropometry (adults and children) and other markers of nutritional status, disease‐related outcomes, neurocognitive development and psychosocial outcomes, and adverse events.
Mortality data were limited and underpowered in meta‐analysis in all populations (children with MAM, in children with HIV, and in adults with tuberculosis) with the exception of balanced energy and protein supplementation in pregnancy, which may have reduced the risk of stillbirth (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.39 to 0.94; 5 trials, 3408 women). Supplementation in pregnancy also improved infant birth weight (mean difference (MD) 40.96 g, 95% CI 4.66 to 77.26; 11 trials, 5385 participants) and reduced risk of infants born small‐for‐gestational age (RR 0.79, 95% CI 0.69 to 0.90; 7 trials, 4408 participants). These effects did not translate into demonstrable long‐term benefits for children in terms of growth and neurocognitive development in the one to two trials reporting on longer‐term outcomes. In one study (505 participants), high‐protein supplementation was associated with increased risk of small‐for‐gestational age babies.
Effects on growth in children were mixed. In children under five years of age from LMIC, one review found that supplementary feeding had a little or no effect on child growth; however, a more recent review in a similar population found that those who received food supplementation gained an average of 0.12 kg more in weight (MD 0.12 kg, 95% CI 0.05 to 0.18; 9 trials, 1057 participants) and 0.27 cm more in height (MD 0.27 cm, 95% CI 0.07 to 0.48; 9 trials, 1463 participants) than those who were not supplemented. Supplementary food was generally more effective for younger children (younger than two years of age) and for those who were poorer or less well‐nourished. In children with MAM, the provision of specially formulated food improved their weight, weight‐for‐height z scores and other key outcomes such as recovery rate (by 29%), as well as reducing the number of participants dropping out (by 70%). In LMIC, school meals seemed to lead to small benefits for children, including improvements in weight z scores, especially in children from lower‐income countries, height z scores, cognition or intelligence quotient tests, and maths and spelling performance.
Supplementary feeding in adults who were HIV positive increased the daily energy and protein intake compared to nutritional counselling alone. Supplementation led to an initial improvement in weight gain or body mass index but did not seem to confer long‐term benefit.
In adults with tuberculosis, one small trial found a significant benefit on treatment completion and sputum conversion rate. There were also significant but modest benefits in terms of weight gain (up to 2.60 kg) during active tuberculosis.
The one study included in the Alzheimer's disease review found that three months of daily oral nutritional supplements improved nutritional outcomes in the intervention group.
There was little or no evidence regarding people's quality of life, adherence to treatment, attendance at clinic or the costs of supplementary feeding programmes.
Authors' conclusions
Considering the current evidence base included, supplementary food effects are modest at best, with inconsistent and limited mortality evidence. The trials reflected in the reviews mostly reported on short‐term outcomes and across the whole of the supplementation trial literature it appears important outcomes, such as quality of life and cost of programmes, are not systematically reported or summarised.
Plain language summary
Supplementary feeding for groups of people that are food insecure, vulnerable and malnourished
What was the aim of this review?
To summarise the effect of supplementary feeding on populations that were food insecure, vulnerable and malnourished. The overview authors found eight systematic reviews examining supplementary feeding in a variety of populations.
Key messages
Across a range of vulnerable populations, supplementary feeding programmes sometimes show modest benefit in nutritional outcomes. In a few studies examining mortality (death), effects were either small or absent, and research mostly looked at short‐term effects.
What was studied in the review?
Supplementary feeding means providing extra food to people or families over and above their home diet and has been used in populations that are food insecure (limited access to adequate and nutritious food) and vulnerable (including women and young children; school‐aged children; people living with diseases such as tuberculosis, HIV, and Alzheimer's disease; and older people) to improve their health and quality of life.
What are the main results of the review?
The evidence presented here was current to January 2017. We found eight systematic reviews to include in this summary. These reviews included 95 studies (including up to 7940 adults, and more than 12,595 children in a few studies). Most of the included studies lasted from six weeks to two years, with only three studies following people for longer periods of time (up to 17 years). In these reviews, there were a wide range of different types of supplementary feeding given to vulnerable groups over different periods of time, and often in combination with vitamins or minerals.
In pregnancy, we found that energy and protein supplements that were balanced (i.e. providing adequate amounts of energy and nutrients, in this case protein) may have decreased the rate of stillbirth (death or loss of a baby before or during delivery), improved infant birth weight and reduced the risk of infants born small‐for‐gestational age (infants that are smaller than expected). We observed no long‐term benefits for children in terms of growth and cognitive (intellectual) development (although very few studies reported long‐term effects). High‐protein supplements (containing protein in higher amounts) were associated with risk and harm (increased risk of small‐for‐gestational age babies).
We found that the effects of supplementary feeding on growth in children were varied. In children under five years of age from low‐ and middle‐income countries, supplementary feeding had a small impact on child growth. We observed some benefits in terms of weight and height gains, especially in younger children (those younger than two years of age) and in those who were poorer or less well‐nourished (or both). Some benefit could be seen in children with moderate acute malnutrition in terms of weight gain, other growth factors and recovery rate. School meals seemed to lead to a number of small benefits in school children (including improvements in weight, height, intelligence tests, and maths and spelling performance).
Supplementary feeding in adults who were HIV positive increased the daily intake of energy and protein and led to an early improvement in weight gain or body mass index (measure of whether someone is overweight or underweight), or both, but did not seem to lead to long‐term benefits (although few studies reported long‐term effects). In adults with tuberculosis (serious infectious lung disease), we observed small benefits in terms of weight gain during active tuberculosis.
In Alzheimer's disease (a type of dementia), providing a daily oral nutrition supplement for three months improved nutritional outcomes (such as weight and energy intake).
There was little or no evidence available regarding people's quality of life, adherence to treatment, attendance at clinic or the costs of supplementary feeding programmes.
Background
An adequate diet that includes the required macro‐ and micronutrients helps ensure human growth, physical and cognitive development, and a healthy immune system. What people need in their diet varies according to age, gender, physical activity and health status (Mahan 2011). Food security is defined as a situation in which "all people, at all times, have physical and economic access to sufficient, safe and nutritious food to meet their dietary needs and food preferences for an active and healthy life" (FAO 1996; FAO 2010). The definition, from the Food and Agriculture Organization (FAO), reinforces the multi‐dimensional nature and complexity of food security, which includes food availability, economic and physical access to food, food utilisation and stability of supplies over time (FAO 1996; FAO 2013). Food security is a prerequisite to adequate nutrition. Other factors, including child feeding and care practices, food choices, knowledge about and interest in food preparation, adequate water and a sanitary environment, as well as access to health care and the health status of a person, also play an important role in whether access to food translates into the consumption of an adequate diet and ultimately to adequate nutrition, health and well‐being.
Malnutrition, affecting one in three people worldwide, comes in a number of forms that not only affect a person's health and well‐being, but also place heavy burdens on families, communities and states (FAO 2017; FAO and WHO 2014; IFPRI 2016). The 'triple burden' of malnutrition include undernutrition, overweight/obesity and micronutrient deficiencies, and these forms can coexist within the same person, household and country (FAO 2017). According to FAO estimates, the prevalence of undernourishment remains high despite adequate food supplies and considerable progress in reducing hunger in some regions. More than 795 million people still presented with chronically inadequate levels of dietary energy intake between 2014 and 2016 (FAO 2015; Sundaram 2015), with women and children being particularly vulnerable. In 2016, stunting affected an estimated 22.9% or 154.8 million children and wasting continued to threaten the lives of an estimated 7.7% or nearly 52 million children under five years of age globally (UNICEF/WHO/World Bank Group 2017). Furthermore, 108 million people globally in 2016 were reported to be facing crisis‐level food insecurity or worse, representing a 35% increase compared to 2015 figures (FSIN 2017). Food security in the context of climate change has been highlighted as a significant risk in the 2016 Global Risk Report (WEF 2016). Weather patterns and climate change could jeopardise agricultural production and food security across geographies; the risk to food security is especially great as agriculture is already straining to meet a rapidly growing demand from a finite resource base (WEF 2016).
Ending hunger, achieving food security and improving nutrition have been prioritised as key steps towards sustainable development (UN 2016). The international community has fortunately recognised these challenges. The 2030 Agenda for Sustainable Development provides a vision on how multiple objectives can be combined to define new, sustainable development pathways. The second Sustainable Development Goal (SDG 2) aims at ending hunger, achieving food security and improved nutrition, and promoting sustainable agriculture simultaneously by 2030 (FAO 2017; UN 2015a). On 1 April 2016, the United Nations (UN) General Assembly adopted a resolution proclaiming a United Nations Decade of Action on Nutrition from 2016 to 2025 (UN 2015b). This Decade of Action aims to mobilise intensified action to end hunger and eradicate all forms of malnutrition worldwide, and ensure universal access to healthier and more sustainable diets for everyone (WHO 2016). In addition, and in the local context, strong political commitment (including placing food security and nutrition at the top of the political agenda and creating an enabling environment) is essential for hunger reduction, the latter which requires an integrated approach, including specific nutrition programmes.
Many factors influence vulnerability to malnutrition and food insecurity, with the well‐known United Nations Children's Fund (UNICEF) conceptual framework indicating that the causes of malnutrition are multi‐sectoral, taking into account food, health and caring practices. Causes are categorised as immediate (inadequate food intake and illness), underlying (poor household food security, inadequate maternal and child care, poor access to basic health services, and an unhealthy environment (with limited access to clean water and safe waste disposal)) and basic (poverty and lack of resources), whereby factors at one level influence other levels (UNICEF 1990). It is important that policymakers and community leaders take into account the causes of malnutrition when planning and prioritising health and nutrition interventions (Black 2013). As such, the multiple causes of malnutrition require a multi‐sectoral approach, including both nutrition‐specific and nutrition‐sensitive approaches (health; basic education; agriculture, forestry and fisheries; and social development at local, provincial and national levels) (Bhutta 2013; Garrett 2011; IFPRI 2014; Ruel 2013). Supplementary feeding programmes, targeting households and vulnerable people, are but one approach to address the complex issues surrounding food security and malnutrition. These programmes, operated by governments and agencies, can be expensive and complicated to deliver. This overview aims to summarise the evidence from existing systematic reviews of effects in the stated target groups.
Description of the condition
In 2014, 805 million people in the world experienced chronic hunger, not having enough food to ensure an optimal nutritional status and lead an active and healthy life (FAO 2014). A further one billion people were considered vulnerable to 'hidden hunger' as a result of micronutrient deficiencies (MI 2009). Many inter‐related factors influence vulnerability to food insecurity, including poverty, landlessness and conflict, as well as other factors such as gender, disease and age (FAO 2008).
We defined food security in this overview as a situation in which "all people, at all times, have physical and economic access to sufficient, safe and nutritious food to meet their dietary needs and food preferences for an active and healthy life" (FAO 1996; FAO 2010). Therefore, food insecurity exists when people do not have adequate physical, social or economic access to food.
Populations particularly at risk and vulnerable to food insecurity include:
women and young children;
school‐aged children;
people living with infectious diseases, notably tuberculosis and HIV; and
disabled and older populations.
Malnutrition is a broad term encompassing both under‐ and overnutrition. For the purpose of this overview, malnutrition refers to undernutrition. Undernutrition can result from a lack of macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), or both.
This overview is concerned with low‐income groups and populations in low‐ and middle‐income countries (LMIC) where food security for families and communities can be threatened, with consequent impacts on nutritional status, particularly those groups in the population that are vulnerable to malnutrition such as pregnant women, young children, older people or people with chronic diseases. Humanitarian aid (defined as "aid and action designed to save lives, alleviate suffering and maintain and protect human dignity during and in the aftermath of man‐made crises and natural disasters"), including emergency, short‐term food aid is of crucial importance (Global Humanitarian Assistance 2014), but this is not the topic of this overview.
Description of the interventions
In this overview, we defined supplementary feeding as providing extra food to people or families beyond the normal ration of their home diets (Beaton 1982). Supplementary feeding is used in both emergency and non‐emergency situations to address short‐term hunger, longer‐term food shortage, and to improve the nutritional status (or prevent the nutritional deterioration) of specific populations. Sometimes authorities provide food supplementation to increase use of health services, adherence to treatment regimens or attendance (and performance) at school. In humanitarian disasters, food aid aims to relieve absolute food shortage (although this overview is not concerned with food used in these circumstances). Many questions remain, however, about the cost‐effectiveness of supplementary feeding programmes, their design, and the appropriate mix of complementary activities to achieve the intended outcomes (Morris 2008).
Supplementary feeding can be undertaken in two ways (see Figure 1):
1.
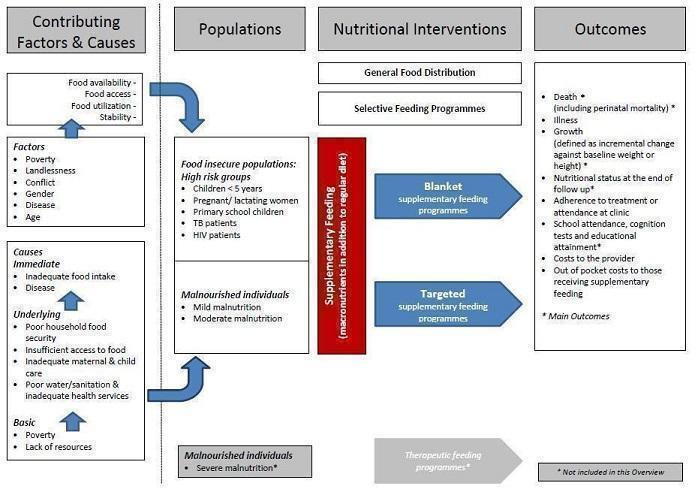
Conceptual framework of supplementary feeding to improve nutrition (adapted from UNICEF 1990).
blanket supplementary feeding, which aims to prevent malnutrition or its progression in food insecure populations, where the targeting is based on knowing that the population is high risk, and food is given to the whole at‐risk population without prior screening (UNHCR/WFP 1999); and
targeted supplementary feeding, which is directed at selected people who are at risk; treating mild or moderate malnutrition detected by screening at‐risk populations and providing supplementation only to people who fall below a prespecified nutritional status threshold.
We included both categories of supplementary feeding in this overview.
Food is sometimes used to induce people to behave in a particular way; for example, giving food to people with tuberculosis to ensure they attend clinics for treatment, or giving food to children at school to help improve school attendance (Devereux 2018). While this overview was not concerned with the use of food in these circumstances, where food may have been used to both improve nutrition and improve adherence to treatment (for example, in people with tuberculosis), we examined adherence outcomes as a secondary outcome.
We defined supplementary foods as additional foods to the normal diet. Supplementary foods also include specially formulated foods (for example, fortified blended foods) in ready‐to‐eat or in milled form, which are modified in their energy density, protein, fat or micronutrient composition to help meet the nutritional requirements of specific populations (WHO 2012). Supplementary foods are not intended to be the only source of nutrients in a given population (WHO 2012). They are different from food supplements that refer to vitamin and mineral supplements in unit‐dose forms (such as capsules, tablets, powders or solutions), which are not relevant to this overview (WHO 2012). Food fortification (with the aim of increasing the micronutrient content of the overall diet) as well as enteral and parenteral nutrition interventions are also not part of this overview.
How the intervention might work
Supplementary feeding can have direct nutrition and health benefits (Figure 1). It may also contribute to increased service utilisation, with secondary effects on improved health related to increased service uptake. In addition, it may contribute to social goals, such as food supplementation given at schools to improve school attendance.
Supplementary feeding may have negative effects by increasing dependency, creating expectations of food handouts at clinics and services, and impacting negatively on clinic attendance when it is discontinued. It is also expensive, and needs good management systems to ensure delivery and minimise leakage to people for whom it is not intended. In addition, food safety aspects are of crucial importance for safe delivery, especially if local production is encouraged.
Why it is important to do this overview
Although accurate figures are unavailable, a large proportion of development assistance funding allocated to food and nutrition is used for supplementary feeding programmes, including emergency assistance and food aid (Morris 2008). Thus, it is important to know if it is effective. Furthermore, it is important to try and identify the most successful (combination of) interventions for replication, as well as criteria to improve the cost‐effectiveness and efficiency of the interventions.
The target audience for this overview includes policymakers and programme implementers working in the fields of food security and public health nutrition. Development partners can also use this overview to inform the design of calls for research and programme proposals and to assist with the evaluation of current and proposed supplementary feeding programmes. Clinicians working in food insecure regions or where malnutrition is common will also find this overview useful in their efforts to advocate for cost‐effective preventive and promotional public health strategies.
Objectives
To summarise the evidence from systematic reviews of supplementary feeding for food insecure, vulnerable and malnourished populations, including children under five years of age, school‐aged children, pregnant and lactating women, people with HIV or tuberculosis (or both), and older populations.
To describe and explore the effects of supplementary feeding given to people in these groups, and to describe the range of outcomes between reviews and range of effects in the different groups. We examined possible influences on effects between reviews, including baseline nutritional status and comorbidities.
Methods
Criteria for considering reviews for inclusion
Types of studies
Published systematic reviews (with no restriction on date of last search) of supplementary feeding in vulnerable groups.
Inclusion criteria for non‐Cochrane Reviews were:
predetermined objectives;
predetermined eligibility criteria;
search conducted in at least two data sources, one of which must have been an electronic database; and
data extraction and 'Risk of bias' assessments performed independently and in duplicate by review authors.
Types of participants
Systematic reviews concerning vulnerable (food insecure and malnourished) populations, targeted food insecure populations or those identified as malnourished in these populations (children, school‐aged children, pregnant and lactating women, people with HIV or tuberculosis (or both), and older people). We included reviews provided some or all of these conditions (vulnerability, food insecurity and malnutrition) were met. We excluded groups for which specialised therapeutic care was necessary (such as preterm and low birth‐weight infants).
Types of interventions
Reviews evaluating community‐based, supplementary feeding in vulnerable groups (as defined under Description of the interventions).
Community‐based, supplementary feeding programmes were those that provided food to populations or ambulatory people in a non‐clinical setting. Supplementary feeding could thus take place at home, at a supervised feeding centre, or at other places adapted for this purpose (for example, healthcare centres and crèches).
Supplementary feeding programmes were where set criteria were applied to a population to determine eligibility for supplementary foods and the foods are provided to them. Supplementary foods were macronutrients (balanced diet or high protein, high carbohydrate, or high fat diets/foods) given as a supplement in addition to the usual diet (not a total dietary replacement). Supplementary foods could contain added micronutrients (vitamins and minerals), however, we excluded reviews of micronutrients only. Food supplements must have been taken orally. Supplementary feeding options included using additional foods, fortified foods, specially formulated foods (fortified blended foods such as corn‐soy blend, ready‐to‐use foods (RUFs) such as pastes, compressed bars or biscuits), or complementary food supplements (such as powdered complementary food supplements containing a combination of micronutrients, protein, amino acids and enzymes; or lipid‐based nutrient supplements (LNS) (120 kcal/day to 250 kcal/day), typically containing milk powder, high‐quality vegetable oil, peanut paste, sugar and micronutrients (De Pee 2009)). Ready‐to‐use therapeutic foods (RUTFs) are typically used for treating severe acute malnutrition (SAM), but in some instances these recipes are modified for use in moderate acute malnutrition (MAM), and thus could have been relevant to this overview (De Pee 2009).
We excluded:
reviews reporting on the effects of supplementary feeding in refugee settings or hospitals (after injury or surgery or other acute medical conditions);
vitamin and mineral supplements, enteral tube feeding or parenteral feeding products and
therapeutic feeds for the treatment of severe malnutrition.
The comparison groups were either those who did not receive the supplement or those who received a different supplement.
Types of outcomes
Death (including perinatal mortality).*
Illness (or disease‐related outcomes).
Growth in children (defined as an incremental change against baseline weight or height).*
Nutritional status of children (assessed by other anthropometry, biochemical markers and dietary intake) at the end of follow‐up.*
Nutritional status of adults (assessed by anthropometry, biochemical markers and dietary intake) at the end of follow‐up.*
Adherence to treatment or attendance at clinic.
School attendance, cognition tests and educational attainment.*
Costs to the provider.
Out‐of‐pocket costs to people receiving supplementary feeding.
*Main outcomes
Search methods for identification of reviews
We first searched the electronic sources listed below on 9 July 2013 and updated the searches on 29 January 2017.
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 1) in the Cochrane Library.
MEDLINE Ovid (searched from 1946).
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid.
MEDLINE Epub Ahead of Print Ovid.
Embase Ovid (searched from 1980).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2. Final issue), part of the Cochrane Library.
Health Technology Assessment Database (HTAD; 2016, Issue 4), part of the Cochrane Library.
Campbell Collaboration Online Library of Systematic Reviews (www.campbellcollaboration.org/library.html).
Virtual Health Library (bvsalud.org/en).
Database of Promoting Health Effectiveness Reviews (DoPHER; eppi.ioe.ac.uk/webdatabases4/Intro.aspx?ID=9).
3ie Database of Systematic Reviews (www.3ieimpact.org/en/evidence/systematic‐reviews).
PROSPERO (www.crd.york.ac.uk/prospero).
The core search strategy consisted of two concepts: supplementary feeding AND systematic reviews. Each concept was described using controlled vocabulary terms and free‐text terms. The systematic review filter for MEDLINE was adapted from the Scottish Intercollegiate Guidelines Network (SIGN; www.sign.ac.uk/search‐filters.html). We adapted the search terms for each database (Appendix 1). There were no date or language restrictions.
Data collection and analysis
Selection of reviews
For the July 2013 search, one overview author (JV) and one overview reviewer (LN; see Acknowledgements) independently screened titles and abstracts of records yielded by the search for relevance, and rated them as 'for exclusion', 'for inclusion' or 'potentially eligible'. Next, they obtained the full texts of those systematic reviews judged as 'for inclusion' or 'potentially eligible' and independently assessed them against the inclusion criteria (Criteria for considering reviews for inclusion). Two overview authors (JV; PG) and one overview reviewer (LN) resolved any differences of opinion as regards review selection by discussion until a consensus was reached.
For the January 2017 search, one overview author (NM) and two overview reviewers (NH; RW; see Acknowledgements) screened titles, abstracts and full texts using the same methods described above. Two overview authors (NM; JV) and two overview reviewers (NH; RW) resolved differences of opinion by discussion until a consensus was reached. For updated Cochrane Reviews, we included only the most recent publication.
The PRISMA flow diagram in Figure 2 illustrates the study selection process.
2.
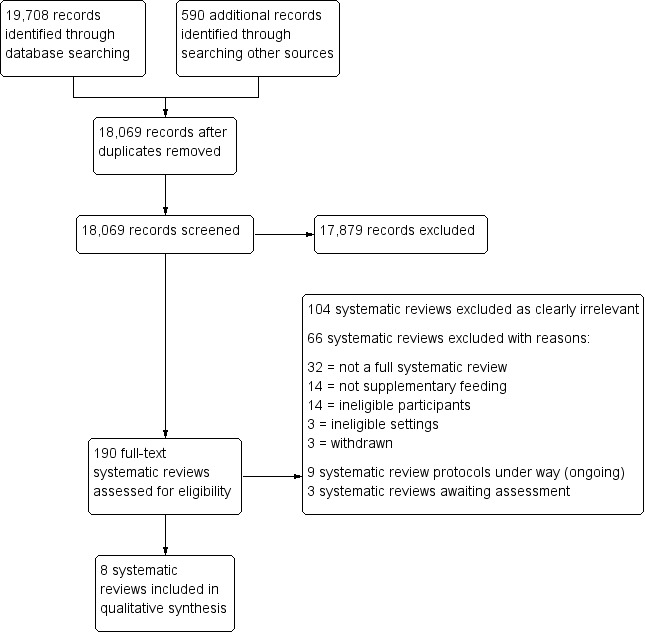
PRISMA flow diagram.
Data extraction and management
For the July 2013 search, one overview author (JV) extracted the data and one overview reviewer (LN) checked them for accuracy; one overview author (NM) and two overview reviewers (NH; RW) performed this for the January 2017 search. Reviewers resolved any disagreements by discussion, with assistance from the rest of the overview author team, as necessary.
We used a data collection form that was specifically designed by the overview author team to collect data on the key features of the systematic reviews such as objectives; inclusion and exclusion criteria; and information about participants, interventions and comparisons. We assessed whether each included review was up to date (and reported the date of the last search). We described the reviews in relation to background vulnerability, including equity‐related aspects and study characteristics, as available. In this regard, we referred to the relevant economies of the included trials (i.e. LMIC versus high‐income countries (HIC)), any summary of socioeconomic status of participants across trials, setting of the trials (community versus hospital or other) and if methods for targeting the interventions were used.
We present the key characteristics of each included systematic review in 'Characteristics of included systematic reviews' tables, the interventions used in the systematic reviews in a 'Summary of interventions' table, and details of the interventions used in separate 'Details of interventions' tables for each included systematic review. In the case of discordant results of included reviews, we presented all; where necessary, we contacted the authors of the primary reviews for clarification and consulted with a biostatistician experienced in meta‐analysis on statistical issues. We also summarised and presented the target groups of each systematic review by outcome in a 'Results matrix', and the results for each target group across included reviews in tables of comparisons by outcome for which data were available.
Assessment of methodological quality of included reviews
Quality of included reviews
For the July 2013 search, one overview author (JV) and one overview reviewer (LN) independently assessed the quality of the included systematic reviews using AMSTAR: A MeaSurement Tool to Assess Systematic Reviews (Shea 2007a; Shea 2007b; Shea 2009); one overview author (NM) and two overview reviewers (NH; RW) performed this for the January 2017 search. The overview author and reviewer(s) resolved any differences by discussion. We included other members of the author team in the discussion (if needed) until we reached a consensus.
AMSTAR assesses the degree to which review methods avoided bias by evaluating the methods against 11 distinct criteria (listed below).
Use of an a priori design.
Duplicate study selection and data extraction.
Comprehensive searching of the literature.
Use of publication status as an exclusion criterion.
Provision of (included and excluded) studies.
Provision of characteristics of included studies.
Assessment of methodological quality of included studies.
Appropriate use of quality of included studies in formulating conclusions.
Appropriate methods for combining results of studies.
Assessment of publication bias.
Conflict of interest (both review and included studies) stated.
Review authors rated each AMSTAR item as yes (clearly done), no (clearly not done), cannot answer or not applicable, based on the published and included systematic reviews.
We presented the review quality data in two ways. First, as a narrative report across all studies against the 11 criteria above. Second, using the standard scoring system provided by AMSTAR to classify reviews into three categories: high quality (those achieving scores between eight and 11); medium quality (those achieving scores between four and seven) and low quality (those achieving scores between zero and three). We identified and discussed differences in quality between reviews, and used the quality assessment to interpret the results of reviews when synthesised in this overview. We summarised the AMSTAR scores of each included systematic review in a table.
Certainty of evidence from primary studies in included reviews
We used the GRADEpro 'Summary of findings' tables from each review (if reported in the individual reviews) to indicate the certainty of the evidence for the main comparisons.
Data synthesis
We used a narrative approach to summarise the data, and included: the range of vulnerability assessment criteria; the range of types of food supplements given and the effects of supplementation. Because the different reviews were based on different population groups and the interventions were basically the same across reviews (considering variations in type, quantity and length of time that a food or supplement was provided), we did not explore indirect comparisons. For analyses with differing durations of supplementation and dietary compositions of supplements, we described this within and between reviews. We made reference to statistical heterogeneity indirectly via certainty of evidence ratings for outcomes in 'Summary of findings' tables, as reported for each outcome. We summarised the findings in additional tables ('Characteristics of included systematic reviews', 'Summary of interventions' and 'Details of interventions' per systematic review tables; 'Results matrix'; and tables of comparisons by outcome for which data were available). We also used forest plots to graphically display selected results.
Results
The searches identified 18,069 titles and abstracts. After initial screening of titles and abstracts, we retrieved 190 full texts and assessed them for eligibility against our inclusion criteria (Criteria for considering reviews for inclusion). Of these, we excluded 104 texts that clearly did not meet our inclusion criteria, and formally excluded a further 66 with reasons (see Table 1). We included eight systematic reviews. The reviews by Kramer 1996a, Kramer 1996b, and Kramer 1996c were all withdrawn and replaced by Ota 2015 and are listed in Table 1. We also identified nine potentially relevant protocols for reviews that are currently under way and have listed these in Appendix 2, and a further three reviews that are awaiting assessment (we were unable to locate the published reports for two of these reviews at the time of our last search, despite exhaustive efforts, and the third was published after the date of search of this review) (Appendix 3). See Figure 2.
1. Excluded reviews and reasons for exclusion.
| Review author and date | Reason for exclusion |
| Abdelhamid 2016 | Methods: stated that Cochrane methods were used but did not specify that 2 reviewers assessed risk of bias. Participants: majority of participants in care homes (1 in hospital); only 3/43 in community |
| Allen 2013 | Methods: no predetermined eligibility criteria Participants: mainly residing in long‐term care establishments (75%) |
| Arthur 2015 | Methods: no 'Risk of bias' assessment |
| Baldwin 2016 | Participants: 5/41 in community, including neurology outpatients and those enrolled at hospital discharge (Protocol published as Gibbs 2012) |
| Bally 2016 | Participants: hospital inpatients |
| Bandayrel 2011 | Methods: no mention that 'Risk of bias' assessment performed in duplicate. Intervention: only 2/15 studies on macronutrient supplementation |
| Beck 2011 | Methods: only 1 database used; single abstract screening; methods for 'Risk of bias' assessment and data extraction not reported. |
| Beck 2013 | Methods: only 1 reviewer assessed trials for inclusion, extracted data and assessed trial quality. |
| Beck 2016 | Methods: single screening of abstracts and single data extraction. |
| Bhutta 2008 | Methods: no 'Risk of bias' assessment |
| Bibas 2014 | Methods: only 1 data source; no 'Risk of bias' assessment |
| Campbell 2015 | Methods: only 1 data source; no 'Risk of bias' assessment |
| Cawood 2012 | Methods: no mention that abstract screening or data extraction performed in duplicate. Participants: hospital and community results reported together, very limited data reported separately for community. Quote: "The populations studied were mostly elderly including those with hip fractures, pressure ulcers, chronic obstructive pulmonary disease (COPD), cancer, gastro‐intestinal disease, and a range of critical and acute illnesses." |
| Choudhury 2014 | Methods: no details on screening; no 'Risk of bias' assessment |
| Collins 2015 | Methods: no mention that data extraction performed in duplicate. Participants: adult inpatients in rehabilitation, geriatric evaluation medicine wards or similar |
| Coyne‐Meyers 2004 | Methods: did not report methods; no 'Risk of bias' assessment |
| Daniels 2010 | Methods: no 'Risk of bias' assessment |
| de van der Schueren 2016 | Methods: no 'Risk of bias' assessment; based on 2 previous reviews |
| Dewey 2008 | Methods: search terms and databases not listed; no mention that screening or data extraction performed in duplicate. |
| Elia 2016 | Methods: AMSTAR score of 5; no mention that data extraction or 'Risk of bias' assessment performed in duplicate, though stated Cochrane methods were used to assess risk of bias. |
| Els 2013 | Methods: no mention that data extraction and 'Risk of bias' assessment performed in duplicate. |
| Fatima 2015 | Methods: methods not described |
| Ferreira 2010 | Methods: 2 databases searched; review methods not reported |
| Goudet 2017 | Methods: scoping review Methods: 1 reviewer screened; no 'Risk of bias' assessment |
| Grantham‐McGregor 2014 | Methods: no mention that screening or data extraction performed in duplicate; no 'Risk of bias' assessment |
| Gresham 2014 | Methods: not described Intervention: 25 studies on macronutrients reported, combined with 6 studies on micronutrients. |
| Gresham 2016 | Methods: second independent reviewer extracted data from half of the studies. Intervention: dietary intervention, macronutrients, micronutrients; data not reported separately; no information reported on intervention. |
| Gunaratna 2010 | Methods: no mention that screening or data extraction performed in duplicate. No 'Risk of bias' assessment. |
| Hubbard 2012 | Methods: no mention that data extraction performed in duplicate. Participants: included both well‐nourished and malnourished participants; mostly elderly (23 participants) with a range of acute and chronic conditions, including those with fractures (4 participants), renal disease (2 participants), cancer (5 participants) and respiratory disease (4 participants) Setting: community and hospital settings |
| Imdad 2011a | Methods: only 1 database searched; no mention that screening or 'Risk of bias' assessment performed in duplicate. |
| Imdad 2011b | Methods: no mention that screening or 'Risk of bias' assessment performed in duplicate but did mention that data extraction performed in duplicate. Quote: "Even though we included terms like 'supplementary food' and 'supplementary feed' in our literature search but only those studies were included where the term supplementary food was used for introduction of additional food to a breastfed child at the age of 6 months i.e. complementary feeding." |
| Imdad 2012 | Methods: only 1 database searched; no mention that screening, data extraction or 'Risk of bias' assessment performed in duplicate. |
| Kimber 2015 | Intervention: only 7/41 meal supplementation Participants: only 2/41 in community |
| Kramer 1996a | Status: withdrawn and replaced by Ota 2015 Intervention: nutrition advice (not food or supplements) |
| Kramer 1996b | Status: withdrawn and replaced by Ota 2015 |
| Kramer 1996c | Status: withdrawn and replaced by Ota 2015 |
| Larson 2017 | Intervention: majority of included studies had micronutrient interventions. |
| Lassi 2013a | Methods: method of data extraction and screening not described. |
| Lassi 2013b | Methods: screening and data extraction performed in duplicate but no mentioned that 'Risk of bias' assessment performed in duplicate. Intervention: education and complementary feeding |
| Lawson 2012 | Methods: did not list electronic sources; no mention that screening or data extraction performed in duplicate; no 'Risk of bias' assessment. |
| Lenters 2013 | Methods: AMSTAR score 7; did not describe that screening, data extraction or 'Risk of bias' assessment performed in duplicate. Participants: includes severe acute malnutrition and moderate acute malnutrition Interventions: ready‐to‐use supplementary food compared to corn soy blend. |
| Lerch 2007 | Intervention: not community‐based supplementary feeding; strategies/intervention to prevent rickets (4 included studies: 3 micronutrient interventions) Outcomes: few outcomes of interest |
| Liberato 2013 | Methods: single screening; no 'Risk of bias' assessment |
| Loveday 2012 | Methods: 'Risk of bias' assessment performed in duplicate but not reported that screening and data extraction performed in duplicate. Participants: acute and tertiary healthcare settings |
| Manders 2004 | Methods: 1 database searched |
| Marshall 2013 | Methods: single screening; second author checked included full texts. |
| Matsuyama 2017 | Methods: no mention that 'Risk of bias' assessment performed in duplicate. Intervention: micronutrient fortified milk |
| McGrath 2015 | Methods: no mention that screening and data extraction performed in duplicate; no 'Risk of bias' assessment. |
| McHenry 2015 | Methods: screening performed in duplicate but no mentioned that data extraction or 'Risk of bias' assessment performed in duplicate. Intervention: only 8/23 macronutrients |
| Milne 2009 | Setting: the majority of studies included were in a hospital, long‐care or nursing home setting (66%); only 1 subgroup analysis related to community versus hospital (mortality). |
| Milte 2013 | Methods: no mention that 'Risk of bias' assessment performed in duplicate. Participants: hospitalised, residential and aged, care and community dwelling populations (1/6 malnourished studies in community) |
| Morilla‐Herrera 2016 | Methods: 'Risk of bias' assessment performed in duplicate; no mention that screening or data extraction performed in duplicate. Setting: hospital and community; 2/7 in community but not reported separately |
| Potter 1998 | Methods: 1 database; no mention that double data extraction performed in duplicate; no 'Risk of bias' assessment. Intervention: oral or enteral protein energy supplementation |
| Ramakrishnan 2014 | Type of publication: abstract; no full‐text report available Intervention: mostly micronutrient, but also included balanced protein energy |
| Schultz 2015 | Methods: data extraction performed in duplicate but no mentioned that screening or 'Risk of bias' assessment performed in duplicate Intervention. Quote: "The focus of WIC [Women, Infants, and Children food packages] has transitioned from preventing malnourishment to concerns of childhood obesity and excessive energy consumption combined with a low intake of fruits, vegetables, and whole grains have become the primary dietary concern of WIC participants." |
| Stevens 2015 | Methods: single title and abstract screening (Protocol published as Stevens 2013) |
| Stratton 2000 | Methods: not described |
| Stratton 2013 | Methods: screening and 'Risk of bias' assessment performed in duplicate but no mention that data extraction performed in duplicate. Participants: community, care homes, rehabilitation/community hospitals Outcome: hospital admissions |
| Thorne 2014 | Methods: screening and 'Risk of bias' method not described; data extraction by 1 reviewer. |
| Trabal 2015 | Methods: screening, data extraction and 'Risk of bias' assessment performed by 1 reviewer and checked by a second. Participants: 1/9 studies in community |
| Tsiami 2013 | Type of publication: abstract; full‐text report published as Loveday 2012 and excluded. |
| Valle 2004 | Methods: not specified methodology for review; no report of number of reviewers Participants: not specified as vulnerable |
| Vandenplas 2014 | Methods: no systematic review methods described other than database search |
| Wang 2013 | Methods: no mention that screening, data extraction or 'Risk of bias' assessment performed in duplicate |
| Wright 2015 | Methods: screening and data extraction performed by 1 reviewer |
| Wrottesley 2016 | Methods: not described other than search |
AMSTAR: A Measurement Tool to Assess Systematic Reviews.
Description of included reviews
This overview of reviews included eight systematic reviews with 128 studies, of which 95 studies were relevant to this overview. All but two reviews included only randomised controlled trials (RCTs): Kristjansson 2007 also included controlled before‐and‐after (CBA) studies and interrupted time series (ITS) studies, and Kristjansson 2015a also included CBA studies. Five RCTs appeared in both Kristjansson 2015a and Sguassero 2012.
Studies included in the reviews were published between 1926 and 2015. The last date of search of these reviews varied between May 2006 (Kristjansson 2007) and February 2016 (Grobler 2016).
The reviews included:
pregnant women (Ota 2015);
children (aged birth to five years) from LMIC born at term (37 weeks or greater) (Sguassero 2012);
disadvantaged infants and young children (aged three months to five years) (Kristjansson 2015a, which was also published as Kristjansson 2015b and Kristjansson 2015c; however, for the purposes of this review we use Kristjansson 2015a);
children (aged six to 60 months) in LMIC with MAM (Lazzerini 2013);
disadvantaged children and adolescents (aged five to 19 years) attending primary or high school (Kristjansson 2007);
HIV‐positive adults and children (Grobler 2013);
adults and children with active tuberculosis (with or without HIV) (Grobler 2016); and
people with Alzheimer's disease (Droogsma 2014).
The number of participants (relevant to this overview) varied across reviews, ranging from 91 (Droogsma 2014) to 7940 (Ota 2015) adults, and 271 (Grobler 2013) to more than 12,595 (Kristjansson 2007) children. See Table 2 and Table 3 for a summary of the characteristics of included reviews.
2. Characteristics of included systematic reviews: part 1.
| Review | Vulnerability | Last search date | Population | Included studies (relevant to this overview) | Types of studies included | Participants (relevant to this overview) |
| Droogsma 2014 | Alzheimer's disease | April 2013 | Community‐dwelling people with Alzheimer's disease | 1 (1) | All RCTs | 91 adults (all relevant) |
| Grobler 2013 | HIV positive | August 2011 | Adults and children who were HIV positive | 14 (14) | All RCTs | 1725 adults (all relevant) |
| February 2012 | 271 children (all relevant) | |||||
| Grobler 2016 | TB | February 2016 | Adults and children with active TB (with/without HIV) | 35 (7)a | All RCTs | 7491 adults (986 relevant) |
| 792 children (none relevant) | ||||||
| Kristjansson 2007 | Disadvantaged school children | May 2006 | Children and adolescents (aged 5–19 years) attending primary or high school | 18 (18) | 7 RCTs | > 12,595 children (not accurately reported) (all relevant) |
| 9 CBAs | ||||||
| 2 ITSs | ||||||
| Kristjansson 2015a | Disadvantaged infants and young children | January 2014 | Infants and children aged 3 months to 5 years | 32 (32) | 21 RCTs (individual and cluster randomised) | 11,602 children (all relevant) |
| 11 CBAs (individual and cluster randomised) | ||||||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | October 2012 | Children with MAM (aged 6 to 60 months) in LMIC | 8 (8) | All RCTs (individual and cluster randomised) | 10,037 children (all relevant) |
| Ota 2015 | Pregnancy | January 2015 | Pregnant women | 17 (12)b | All RCTs (individual and cluster randomised) onlyc | 9030 adults (7940 relevant) |
| Sguassero 2012 | Children < 5 years of age in LMIC | January 2011 | Children (aged 0–5 years) in LMIC born at term (≥ 37 weeks) | 8 (8) | All RCTs (individual and cluster randomised) onlyc | 1243 children (all relevant) |
| CBA: controlled before‐and‐after study;ITS: interrupted time series; LMIC: low‐ and middle‐income countries; MAM: moderate acute malnutrition; RCTs: randomised controlled trial; TB: tuberculosis. | ||||||
aOnly seven trials (in adults) assessed macronutrient supplementation. bOnly 12 trials assessed macronutrient supplementation. cQuasi‐randomised designs were excluded.
3. Characteristics of included systematic reviews: part 2.
| Review | Vulnerability | Intervention categories (as per the original review) | Duration of intervention | Cointerventions | Associated interventions | Main outcome categories | Length of follow‐up |
| Droogsma 2014 | Alzheimer's disease | Oral nutritional supplements (1) | 3 months | — | — | Clinical | 3 months |
| Nutritional | |||||||
| Biochemical | |||||||
| Grobler 2013 | HIV positive | Supplementary food (2) | 6 weeks to 1 year | Micronutrients | Nutrition counselling | Mortality | 6 weeks to 1 year |
| Macronutrient formulas providing energy and protein (6) | Anthropometry | ||||||
| Dietary intake | |||||||
| Specific macronutrient supplements (6) | Disease parameters | ||||||
| Adverse events | |||||||
| Grobler 2016 | TB | Supplementary food (5) | 60 days to 6 months (for macronutrient interventions) |
Micronutrients | Nutrition counselling | Mortality | 8 weeks to 1 year |
| Macronutrient formulas providing energy and protein (2) | Anthropometry | ||||||
| Micronutrients (28)a | Disease‐related outcomes | ||||||
| Quality of life | |||||||
| Kristjansson 2007 | Disadvantaged school children | Supplementary food, snacks and drinks (18) | 20 days to 3 years | — | — | Anthropometry | Not consistently reported |
| Psychosocial outcomes | |||||||
| Kristjansson 2015a | Disadvantaged infants and young children | Supplementary food (12) | 3–32 months | Micronutrients | Additional rations for family | Growth | Not consistently reported (up to 8 years) |
| Cash transfers | Anthropometry | ||||||
| Stimulation | |||||||
| Macronutrient formulas providing energy and protein (20) | Health/nutritional education for mothers | Psychosocial outcomes | |||||
| Healthcare, deworming | Adverse events | ||||||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | Specially formulated foods, including LNS, blended foods, complementary LNS and blended foods (8) | 8–16 weeks (or upon recovery) |
Micronutrients | Nutrition education | Mortality | 8–16 weeks (outcomes in 2 trials: 6 months and 12 months) |
| Health education | Anthropometry | ||||||
| Medical care | Disease‐related outcomes | ||||||
| Psychosocial stimulation | |||||||
| Ota 2015 | Pregnancy | Balanced protein energy supplementation (12) | 2.5–9 months + during pregnancy (not consistently reported) |
Micronutrients | — | Mortality | Not consistently reported (up to 17 years) |
| High protein supplementation (1) | Anthropometry | ||||||
| Isocaloric protein supplementation (2) | Neurocognitive development | ||||||
| Nutritional advice (4)a | Adverse events | ||||||
| Sguassero 2012 | Children < 5 years of age in LMIC | Supplementary food, snacks and drinks (8) | 2–12 months | Micronutrients | — | Anthropometry | 2–12 months |
| Adverse events | |||||||
| LMIC: low and middle‐income country; LNS: lipid‐based nutrient supplement; MAM: moderate acute malnutrition; TB: tuberculosis. | |||||||
aExcluded from this overview.
The majority of studies (70 studies; 74%) relevant to this overview were conducted in LMIC. Socioeconomic and food security status of participants were poorly reported, and all but one study in one review were conducted in community settings. See Table 4. Studies in four reviews used a combination of blanket (Kristjansson 2007; Kristjansson 2015a) and targeted (Droogsma 2014; Lazzerini 2013) supplementary feeding approaches. See Table 5.
4. Characteristics of included systematic reviews: economies, socioeconomic status (SES) and setting.
| Review | Population | Included studies (relevant to this overview) | Economies (relevant to this overview) |
SES (relevant to this overview) |
Setting | |||
| LMIC | HIC | Economically disadvantaged, including undernourished, nutritionally‐at‐risk, rural | Economically advantaged, including well‐nourished | Community (or outpatient setting) | Hospital inpatients and other | |||
| Droogsma 2014 | Community‐dwelling people with Alzheimer's disease | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Grobler 2013 | Adults and children who were HIV positive | 14 | 7 | 7 | NR | NR | 14a | 0 |
| Grobler 2016 | Adults and children with TB | 35 (7) | 33 (6) | 2 (1) | NR | NR | 24 (6) | 11 (1)b |
| Kristjansson 2007 | Disadvantaged school children (aged 5–19 years) | 18 | 9 | 9 | 18 | 0 | 18 | 0 |
| Kristjansson 2015a* | Disadvantaged infants and young children (aged 3 months to 5 years) | 32 | 29 | 3c | 30 | 2 | 32 | 0 |
| Lazzerini 2013 | Children with MAM (< 5 years of age) | 8 | 8 | 0 | 8 | 0 | 8 | 0 |
| Ota 2015 | Pregnant women | 17 (12) | 10 (8) | 7 (4) | 7 (6) | 10 (6) | 17 (12) | 0 |
| Sguassero 2012e | Children < 5 years of age in LMIC | 8 | 8 | 0 | 6d | ? | 8 | 0 |
| ?: unknown; HIC: high‐income country; LMIC: low‐ and middle‐income country; MAM: moderate acute malnutrition; NR: not reported; SES: socioeconomic status. | ||||||||
aIn one study, Rollins 2007, children were included that were treated on an inpatient and outpatient basis. bOf the seven studies on macronutrients, one study recruited and treated people in a hospital setting (Pérez‐Guzmán 2005). cOne study included Aboriginal children. dSix studies included nutritionally‐at‐risk children, whereas in two studies there were no trial entry criteria based on child nutritional status. eFive studies appeared in both Kristjansson 2015a and Sguassero 2012.
5. Summary of interventions.
| Systematic review | Population | Supplementary feeding | Intervention categories (as per review) | Intervention summary (number of studies) | |||||
| Supplementary food/drinka | Non‐foodb | Other | |||||||
| No added micronutrients | Added micronutrients | No added micronutrients | Added micronutrients | ||||||
| Droogsma 2014 | Adults with Alzheimer's disease | — | T | Oral nutritional supplements (1) | 0 | 0 | 0 | 1 | 0 |
| Grobler 2013 | Adults who were HIV positive | Children who were HIV positive (3 studies) | B and T | Supplementary food (2) | 0 | 2 | 5 | 7 | 0 |
| Macronutrient supplements providing energy and protein (6) | |||||||||
| Specific macronutrient supplements (6) | |||||||||
| Grobler 2016 | Adults with TB | Children with TB (3 studies)c | B and T | Supplementary food (5) | 3 | 2 | 1 | 1 | 28 (micronutrients only) |
| Macronutrient formulas providing energy and protein (2) | |||||||||
| Micronutrients (28)d | |||||||||
| Kristjansson 2007 | — | Disadvantaged school children (aged 5 to 19 years) | B | Supplementary food, snacks and drinks (18) | 15 | 1 | 1 | 1 | 0 |
| Kristjansson 2015a | — | Disadvantaged infants and children (aged 3 months to 5 years) | B | Supplementary food, snacks and drinks (32) | 12 | 2 | 17 | 1 | 2 (nutritional counselling) |
| 1 (health‐sanitation programme) | |||||||||
| 1 (day‐care centre) | |||||||||
| 1 (day‐care centre + vitamin mineral supplement and sanitation) | |||||||||
| 1 (stimulation only) | |||||||||
| Lazzerini 2013 | — | Children with MAM (< 5 years of age) | T | Supplementary food, including LNS, blended foods, complementary LNS and blended foods (8) | 0 | 0 | 0 | 8e | 0 |
| Ota 2015 | Pregnant women | — | B and T | Balanced energy/protein supplementation (12) | 4 | 2 | 1 | 5 | 5 (nutrition advice only) |
| High protein supplementation (1) | |||||||||
| Isocaloric protein supplementation (2) | |||||||||
| Nutritional advice (4)d | |||||||||
| Sguassero 2012 | — | Children < 5 years of age in LMIC | B and T | Supplementary food, snacks and drinks (8) | 2 | 2 | 3 | 1 | 0 |
| B: blanket;LMIC: low and middle‐income country; LNS: lipid‐based nutrient supplement; MAM: moderate acute malnutrition; T: targeted; TB: tuberculosis. | |||||||||
aSupplementary food/drink: actual food to eat or fluid to drink as would be found in a household. bNon‐food: any powder, commercially prepared liquid supplement, mixtures. cNone relevant to this review. dExcluded from this overview. eThis review focused on foods developed for the treatment of MAM, including: LNS, blended food supplements, complementary food supplements.
The reviews evaluated a vast array of different nutritional interventions of varying duration, frequency and format, including solids versus liquids, meals, snacks or drinks, specially formulated foods, fortified foods, traditional foods, commercial macronutrient formulas, mixtures and powders, and specific supplements (e.g. L‐glutamine, spirulina). We included the latter macronutrient supplements for completeness, since they were compared to placebo, no supplements or usual diet in the relevant studies. Intervention categories (as per the relevant reviews) are reported in Table 3. Details of the interventions are summarised in Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, and Table 13. The duration of the intervention varied between 20 days (Kristjansson 2007) and three years (Kristjansson 2007), and follow‐up ranged between six weeks (Grobler 2013) and 17 years (Ota 2015). In all but two reviews (Droogsma 2014; Kristjansson 2007), micronutrients were reported as cointerventions in various studies, and associated interventions reported across reviews included nutrition education/counselling, health education, standard medical care, psychosocial stimulation and cash transfers (Table 3).
6. Droogsma 2014: details of interventions (as reported in systematic review).
| Review ID | Droogsma 2014 |
| Types of interventions considered | Nutritional intervention (i.e. any intervention that aimed to improve nutritional status (e.g. weight, upper‐arm anthropometry), such as oral nutritional supplements, dietary advice, food fortification, nutritional education programmes) |
| Details regarding interventions |
|
| Comments | Information provided as (and if) reported in systematic review. |
7. Grobler 2013: details of interventions (as reported in systematic review).
| Review ID | Grobler 2013 |
| Types of interventions considered |
|
| Details regarding the interventions |
|
| Comments | Information provided as (and if) reported in systematic review. |
AA: amino acid; AO: antioxidant; CHO: carbohydrate; FA: fatty acid; ID: identifier; kcal: kilocalories; kJ: kilojoules; RDA: recommended dietary allowances; USDA: US Department of Agriculture.
8. Grobler 2016: details of interventions (as reported in systematic review).
| Review ID | Grobler 2016 |
| Types of interventions considered |
|
| Details regarding the interventions |
|
| Comments | Only 7 trials of macronutrient supplementation were reported here (as relevant for this overview). We excluded 1 macronutrient trial, Pérez‐Guzmán 2005, as it was based in a hospital setting (inpatients). Information provided as (and if) reported in systematic review. |
BMI: body mass index; CHO: carbohydrate; ID: identifier; kcal: kilocalories; kJ: kilojoules; RNTCP: Revised National TB Control Program; TB: tuberculosis.
9. Kristjansson 2007: details of interventions (as reported in systematic review).
| Review ID | Kristjansson 2007 |
| Types of interventions considered | Meals (breakfast or lunch) or snacks (including milk) administered in a school setting |
| Details regarding the interventions |
|
| Comments | Information provided as (and if) reported in systematic review. |
DRI: daily recommended intake; ID: identifier; kcal: kilocalories; kJ: kilojoules; NR: not reported; RDA: recommended dietary allowances.
10. Kristjansson 2015a: details of interventions (as reported in systematic review).
| REVIEW ID | Kristjansson 2015a |
| Types of interventions considered | Provision of energy and macronutrients through:
|
| Details regarding interventions |
|
| Comments | Information provided as (and if) reported in systematic review. |
CSB: corn‐soy blend; DRA: daily recommended amount; DRI: daily recommended intake; ID: identifier; kcal: kilocalories; kJ: kilojoules; LNS: lipid‐based nutrient supplement; mJ: millijoules; NR: not reported; RDA: recommended dietary allowance; RUTF: ready‐to‐use therapeutic feeding; T: time point.
11. Lazzerini 2013: details of interventions (as reported in systematic review).
| Review ID | Lazzerini 2013 |
| Types of interventions considered | Any type of food used for children with moderate acute malnutrition, including:
|
| Details regarding the interventions |
|
| Comments | Information provided as (and if) reported in systematic review. |
CSB: corn‐soy blend; CSB++: corn‐soy blend enriched; ID: identifier; kcal: kilocalories; LNS: lipid‐based nutrient supplement; MN: micronutrient; RUTF: ready‐to‐use therapeutic feeding.
12. Ota 2015: details of interventions (as reported in systematic review).
| Review ID | Ota 2015 |
| Types of interventions considered |
|
| Details regarding the interventions |
|
| Comments | 4 trials provided nutrition advice only as intervention and are not reported here (see Ota 2015). Information provided as (and if) reported in systematic review. |
CHO: carbohydrate; ID: identifier; kcal: kilocalories; kJ: kilojoules; MMN: multiple micronutrient; N/A: not applicable; RDA: recommended dietary allowance.
13. Sguassero 2012: details of interventions (as reported in systematic review).
| Review ID | Sguassero 2012 |
| Types of interventions considered | Supplementary feeding was defined as the provision of extra food to children or families beyond the normal rations of their home diets. The intervention had to be community based in that young children could consume the supplementary food at home, at a supervised feeding centre or at other places adapted for this purpose such as healthcare centres and crèches. Supplementary feeding could comprise:
|
| Details regarding the interventions |
|
| Comments | Information provided as (and if) reported in systematic review. |
CHO: carbohydrate; ID: identifier; kcal: kilocalories.
The main outcome categories reported across reviews were: mortality; anthropometry (adults and children) and other markers of nutritional status assessment; disease‐related outcomes; neurocognitive development and psychosocial outcomes; and adverse events. A summary of the specific outcomes reported per review is presented in Table 14.
14. Results matrix.
| Systematic reviews | Vulnerability | Outcomes | ||||||||||
| Mortality | Disease‐related outcomes | Nutritional status assessment | Cognition tests, educational attainment and school attendance | Behavioural outcomesa | Quality of lifea | Adverse events | Costs | |||||
| Growth (weight and length/height) | Other anthropometry | Biochemistry | Dietary intake | |||||||||
| Droogsma 2014 | Alzheimer's disease | — | — | — | — | — | Adultsb | — | — | — | — | — |
| Grobler 2013 | HIV positive | Adults | Adults | Children | Adults | Adults | Adults | — | — | Adults | Adults | — |
| Children | Children | Children | ||||||||||
| Grobler 2016 | TB | Adults | Adults | — | Adults | — | — | — | — | Adults | — | — |
| Kristjansson 2007 | Disadvantaged school children | — | Children | Children | Children | Children | — | Children | Children | — | — | — |
| Kristjansson 2015a | Disadvantaged infants and children | Children | Children | Children | Children | Children | — | Children | Children | — | Children | — |
| Lazzerini 2013 | Children with MAM (< 5 years of age) | Children | — | Children | Children | — | — | — | — | — | Children | — |
| Ota 2015 | Pregnancy | Children | — | Children | Adults | — | — | Children | — | — | Adults | — |
| Children | Children | |||||||||||
| Sguassero 2012 | Children < 5 years of age in LMIC | — | — | Children | Children | — | — | — | — | — | Children | — |
| Total | 2 adults, 4 children | 2 adults, 2 children | 6 children | 3 adults, 6 children | 1 adult, 3 children | 2 adults | 3 children | 2 children | 2 adults | 2 adults, 4 children; | 0 children or adults | |
| LMIC: low‐ and middle‐income country; MAM: moderate acute malnutrition; TB: tuberculosis. | ||||||||||||
aOnly reported narratively.
Methodological quality of included reviews
Quality of systematic reviews
We rated the quality of the eight included systematic reviews using the AMSTAR tool, as described previously under Assessment of methodological quality of included reviews. We found that:
all reviews prespecified their clinical question and inclusion criteria;
all reviews conducted study selection and data extraction in duplicate;
all reviews conducted a comprehensive literature search;
seven of the reviews included defined searches of grey literature;
all reviews listed included and excluded studies;
all reviews described the characteristics of the included studies;
all reviews assessed study quality;
all reviews appropriately used the quality of included studies in formulating conclusions;
all reviews combined the studies using appropriate methods;
six reviews formally addressed the risk of publication bias, using a statistical test where appropriate; and
all reviews addressed the potential for conflict of interest.
Seven of the eight reviews had conducted a literature search between 2011 and 2016; the one remaining review had an older search date (Kristjansson 2007). We rated all reviews as high quality, as all had scores between eight and 11. See Table 15.
15. AMSTAR scores of included systematic reviews.
| Criteria | Droogsma 2014 | Grobler 2013 | Grobler 2016 | Kristjansson 2007 | Kristjansson 2015a | Lazzerini 2013 | Ota 2015 | Sguassero 2012 |
| Was an a priori design provided? | Y | Y | Y | Y | Y | Y | Y | Y |
| Was there duplicate study selection and data extraction? | Y | Y | Y | Y | Y | Y | Y | Y |
| Was a comprehensive literature search performed? | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the status of publication (i.e. grey literature) used as an exclusion criterion?a | N | Nb | N | N | Nb | N | N | Y |
| Was a list of studies (included and excluded) provided? | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the characteristics of the included studies provided? | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the scientific quality of the included studies assessed and documented? | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the scientific quality of the included studies used appropriately in formulating conclusions? | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the methods used to combine the findings of studies appropriate? | N/A | Y | Y | Y | Y | Y | Y | Y |
| Was the likelihood of publication bias assessed? (where relevant) | N/A | Nc | Y | Y | Y | Y | Y | Y |
| Was the conflict of interest stated? | Y | Y | Y | Y | Y | Y | Y | Y |
| AMSTAR scores | 8 | 10 | 11 | 11 | 11 | 11 | 11 | 10 |
| Y: yes; N: no; N/A: not applicable. | ||||||||
aFor all items except item 4, a rating of 'yes' was considered adequate. For item 4, a rating of 'no' was considered adequate. bExtensive handsearches not undertaken by authors, but trials not excluded if found. cAuthors discussed the risk involved; no formal assessment.
AMSTAR ratings (scores out of 11 criteria)
- High quality: 8–11.
- Medium quality: 4–7.
- Lower: low quality: ≤ 3.
Certainty of evidence from primary studies in included reviews
The included reviews used GRADE methods to rate the certainty of the evidence reported by the primary studies (as reported in 'Summary of findings' tables in the individual reviews). Ratings ranged from very low to moderate for individual comparisons (see Table 16; Table 17; Table 18; Table 19; Table 20; Table 21; Table 22; Table 23; Table 24; Table 25; Table 26; Table 27; Table 28 for details). We reported the certainty of the evidence in the individuals reviews in these tables and in the text, where available; however, ratings may not be directly comparable between reviews due to different approaches. The main reasons for the certainty of the evidence being downgraded across reviews were: inadequate reporting of allocation concealment and randomisation methods; lack of blinding; imprecision and indirectness. The evidence often comprised one or two small trials.
16. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: death.
| Review | Target group | Intervention | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Grobler 2013 | Children with HIV (aged 6–36 months) | Balancedb | Death (8 weeks) | 120 per 1000 | 163 per 1000 | RR 1.42 (0.59 to 3.40) | 169 (1) | NR |
| Death (26 weeks) | 217 per 1000 | 291 per 1000 | RR 1.48 (0.74 to 2.98) | 169 (1) | NR | |||
| Grobler 2016 | Adults with TB | Balancedc | Death (1 year follow‐up) | 3 per 100 | 1 per 100 (0 to 4) | RR 0.34 (0.10 to 1.20)d | 567 (4) | Very low |
| Lazzerini 2013 | Children with MAM (< 5 years of age) | High lipid and balancede | Death | 10 per 1000 | 4 per 1000 | RR 0.44 (0.14 to 1.36) | 1974 (1) | NR |
| Ota 2015 | Pregnant women | Balanced | Stillbirth | 30 per 1000 | 18 per 1000 (12 to 28) | RR 0.60 (0.39 to 0.94)f | 3408 (5) | Moderate |
| Neonatal death | 26 per 1000 | 18 per 1000 (11 to 28) | RR 0.68 (0.43 to 1.07) | 3381 (5) | Low | |||
| High protein | Stillbirth | 33 per 1000 | 27 per 1000 (10 to 72) | RR 0.81 (0.31 to 2.15) | 529 (1) | Low | ||
| Neonatal death | 11 per 1000 | 31 per 1000 (8 to 115) | RR 2.78 (0.75 to 10.36) | 529 (1) | Low | |||
| CI: confidence interval; MAM: moderate acute malnutrition; NR: not reported; RR: risk ratio; TB: tuberculosis. | ||||||||
aAs reported in 'Summary of findings' tables. bEnhanced diet: modified milk formula providing 150 kcal/kg/day and 15% of calories as protein. cProvided as a monthly ration. dNo subgroup differences between people who were HIV positive and people who were HIV negative. eComplementary lipid‐based nutrient supplement (Plumpy Doz) and blended foods (corn‐soy blended foods enriched) versus counselling (two subgroups, one study). fRisk of stillbirth significantly reduced in women given balanced energy and protein supplementation (biscuit (containing roasted groundnuts, rice flour, sugar, groundnut oil); supplement with sesame cake, jaggery, oil; fortified food supplement with peanut butter, soy flour, vegetable oil, sugar, micronutrients; supplement with dried skim milk, enriched bread, vegetable oil; oral supplement (beverage)).
Additional comments
- Stillbirth refers to death after 20 weeks' gestation and before birth.
- Neonatal death refers to death of a live infant within the first 28 days of life.
- 'Balanced' refer to additional energy or protein supplementation or both in 'balanced' proportions (balanced: carbohydrate: 45% to 65%; protein: 10% to 20%; fat: 25% to 35%).
- High protein refers to a protein content > 20% to 25% of total energy.
- Isocaloric balanced protein: a supplement in which the protein content is 'balanced', i.e. provides < 25% of total energy content, but the protein replaced an equal quantity of non‐protein energy in the control group.
- High lipid/fat refers to a lipid content > 35% of total energy.
- Adult mortality outcomes in the Grobler 2013 (HIV) review were reported narratively. Neither supplementary food (Sudarsanam 2011) nor daily supplement of spirulina (Yamani 2009) significantly altered the risk of death compared with no supplement or placebo in malnourished, antiretroviral therapy‐naive adults in these two studies.
- Child mortality outcomes in the Kristjansson 2015a review were reported narratively. One randomised controlled trial reported that there was no significant difference in mortality between children supplemented with ready‐to‐use therapeutic feeding (1671 children) and children who were not supplemented (1862 children; adjusted hazard ratio 0.76, 95% CI 0.51 to 1.13).
17. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: disease‐related treatment outcomes.
| Review | Target group | Intervention | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Grobler 2016 | Adults with TB | Balanced | Cured (at 6 months) | 48 per 100 | 44 per 100 (28 to 68) | RR 0.91 (0.59 to 1.41)b | 102 (1) | Very low |
| Balanced and high energy | Treatment completion (at 6 months) | 79 per 100 | 85 per 100 (70 to 100) | Not pooledc | 365 (2) | Very low | ||
| Balanced and high energy | Sputum negative (at 8 weeks) | 76 per 100 | 82 per 100 (65 to 100) | RR 1.08 (0.86 to 1.37) | 222 (3) | Very low | ||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | High lipid and balancedd | Recovered | 554 per 1000 | 715 per 1000 (664 to 765) | RR 1.29 (1.20 to 1.38)e | 2152 (2) | Moderate |
| High lipid and balancedf | Not recovered | 111 per 1000 | 107 per 1000 (82 to 141) | RR 0.97 (0.74 to 1.27) | 1974 (1) | Low | ||
| Progression to SAM | 116 per 1000 | 90 per 1000 | RR 0.78 (0.59 to 1.03) | 1974 (1) | NR | |||
| Defaulted | 185 per 1000 | 55 per 1000 (41 to 72) | RR 0.30 (0.22 to 0.30)g | 1974 (1) | Moderate | |||
| CI: confidence interval; MAM: moderate acute malnutrition; NR: not reported; RR: risk ratio; SAM: severe acute malnutrition; TB: tuberculosis. | ||||||||
aAs reported in 'Summary of findings' tables. bNo subgroup differences between people who were HIV positive and people who were HIV negative. cSubtotals were only given for people who were HIV negative (RR 1.20, 95% CI 1.04 to 1.37) and people with unknown HIV status (RR 0.98, 95% CI 0.86 to 1.12). dComplementary foods (Pusti Packet) and lipid‐based nutrient supplements (LNS) (i.e. Plumpy Doz and corn‐soy blend (CSB++)). eThe provision of complementary foods (Pusti Packet) and LNS (Plumpy Doz, CSB++) versus standard care significantly increased recovery rate by 29%. fComplementary foods (LNS: Plumpy Doz, CSB++). gThe provision of food (complementary foods (LNS: Plumpy Doz, CSB++) versus standard care significantly decreased the number dropping out by 70%.
Additional comments
- Kristjansson 2015a narratively reported morbidity outcomes in the review. Six studies (four randomised controlled trials (RCTs) and two controlled before‐and‐after (CBAs) studies) reported on morbidity. Three RCTs (Bhandari 2001; Iannotti 2014; Isanaka 2009) and two CBAS (Gopalan 1973; Tomedi 2012) found few differences between the supplemented group and the control group in the prevalence of morbidity. Roy 2005 (a CBA) reported mixed results; the prevalence of diarrhoea and fever was higher in the children who received supplementation (99 children), while the prevalence of respiratory infection was higher in the control group (90 children).
18. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: disease‐related biochemical parameters.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| CD4 (cells/mm3) | ||||||
| Grobler 2013 | Adults with HIV | Balanced | CD4 (12 weeks' follow‐up) | MD –114.48 (–233.20 to 4.23) | 81 (2) | Low |
| Specific (OKG)b | Mean CD4 count at study endpoint | MD –28.00 (–134.93 to 78.93) | 46 (1) | NR | ||
| Specific (GLN)c | MD 66.00 (–53.39 to 185.39) | 21 (1) | NR | |||
| Viral load (log10 copies/mL) | ||||||
| Grobler 2013 | Adults with HIV | Balanced | Viral load (12 weeks' follow‐up) | MD –3.71 (–12.16 to 4.74) | 66 (1) | Very low |
| Specific (OKG)b | Mean viral load at study endpoint | MD 0.20 (–0.58 to 0.98) | 46 (1) | NR | ||
| CI: confidence interval;GLN: L‐glutamine; MD: mean difference; NR: not reported; OKG: ornithine alpha‐ketoglutarate. | ||||||
aAs reported in 'Summary of findings' tables. bMonohydrated L‐ornithine alpha‐ketoglutarate versus placebo. c L‐glutamine versus placebo.
Additional comments
- Additional, disease‐related, biochemical parameter outcomes reported narratively in the Kristjansson 2015a review. One controlled before‐and‐after (CBA) study in a low‐ and middle‐income country reported a significant effect of supplementation on the risk of anaemia (P = 0.003; 110 participants at final survey); those who were supplemented had a lower risk of being anaemic (odds ratio (OR) 0.58, 95% CI 0.24 to 0.75) (Lutter 2008). Similarly, another CBA with 250 participants reported that while the prevalence of anaemia decreased by 27% in the intervention group, it decreased by only 13% in the control group (De Romaña 2000). In high‐income countries, one randomised controlled trial with 103 children found no significant difference between the intervention and the control groups in change in haemoglobin (Yeung 2000). One CBA with 116 children reported an increase in the number of Aboriginal children who had low haemoglobin levels in the intervention group and a decrease in the corresponding number in the control group (Coyne 1980).
19. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: growth in children, weight.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Weight gain (kg) | MD 0.39 (0.11 to 0.67)b,c | 1462 (3) | NR |
| MD 1.42 (1.19 to 1.65)d,e | 102 (1) | NR | ||||
| Change in weight (kg) | MD 0.13 (–0.23 to 0.49)f | 520 (1) | NR | |||
| Weight gain (adjusted ICC = 0.025) (kg) | MD 0.71 (0.48 to 0.95)g,h | 984 (3) | NR | |||
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplementary feedingi | Weight gain (kg) | MD 0.12 (0.05 to 0.18)j,k | 1057 (9) | Moderate |
| Supplementary feedingl | Weight gain (kg) | MD 0.24 (0.09 to 0.39)m | 1784 (7) | NR | ||
| Supplementary foodn | Weight gain (kg) | MD –0.10 (–0.52 to 0.32)o | 45 (1) | NR | ||
| Balanced | Weight gain (kg) | MD 0.95 (0.58 to 1.33)p | 116 (1) | NR | ||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | Balancedq | Weight gain total (kg) | MD 0.18 (0.04 to 0.33)r | 178 (1) | Low |
| Ota 2015 | Pregnant women | Balanced | Child's birth weight (g) | MD 40.96 (4.66 to 77.26)s,t | 5385 (11) | Moderate |
| Child's weight at 1 year (g) | MD 30.43 (–139.67 to 200.53) | 623 (2) | NR | |||
| Child's weight at 11 to 17 years (kg) | MD 0.46 (–0.77 to 1.69)u | 855 (2) | NR | |||
| High protein | Child's birth weight (g) | MD –73.0 (–171.26 to 25.26) | 504 (1) | Low | ||
| Child's weight at 1 year (g) | MD 61.0 (–184.60 to 306.60) | 409 (1) | NR | |||
| Isocaloric balanced protein | Child's birth weight (g) | MD 108.25 (–220.89 to 437.4) | 184 (2) | Very low | ||
| Sguassero 2012 | Children < 5 years of age (< 24 years) | High energy, protein and balancedv | Weight at end of intervention (kg) | MD –0.03 (–0.17 to 0.12)w | 587 (3) | NR |
| Balanced | Weight gain during the intervention (kg) | MD 0.04 (–0.03 to 0.11)j,x | 795 (2) | NR | ||
| CI: confidence interval;ICC: intracluster correlation; MAM: moderate acute malnutrition; MD: mean difference; NR: not reported. | ||||||
aAs reported in 'Summary of findings' tables. bDeveloping country/low‐ and middle‐income country (LMIC) randomised controlled trials (RCTs). cChildren who were fed (milk with calcium; githeri and meat; breakfast (patty with meat, vegetables, milk or banana cake)) at school gained significantly more weight (sensitivity analyses with ICCs at 0.01, 0.05 and 0.10 made little difference) (gain of 0.25 kg/year). In subgroup analyses, findings were significant for undernourished and adequately nourished children, as well as children aged 9 to 10 years specifically. dDeveloped country/high‐income country (HIC) controlled before‐and‐after study (CBA). eChildren who received milk at school gained significantly more weight. fDeveloped country/HIC RCT. gDeveloping country/LMIC CBAs. hChildren who were fed (school lunch; green gram and palm sugar; vegetable protein mixture) at school gained significantly more weight (sensitivity analyses with ICCs at 0.01, 0.05 and 0.10 made little difference) (gain of 0.75 kg/year). In subgroup analyses, findings were significant for boys and girls, and children aged 5 to 6, 6 to 8 and 9 to 10 years specifically. iBalanced (four studies); high energy (two studies); high lipid (one study); supplementary food (two studies). jAnalyses include the same RCT: Simondon 1996 (multi‐country study). kLow‐ and middle‐income country (LMIC) RCT. lBalanced (two studies); high energy (one study); high lipid (one study); high protein (one study); supplementary food (two studies). mLMIC CBA. n113 g wet ration fruit cereal, rice cereal with apple sauce, mixed cereal with apple sauce and bananas, and oatmeal with apple sauce and bananas (Gerber Products Company). oHigh‐income country (HIC) RCT. pAboriginal children, HIC CBA. qComplementary foods (Pusti Packet). rTotal weight gain significantly higher in group receiving complementary foods (Pusti Packet) than versus standard care. sBalanced energy and protein supplement associated with significant increases in mean birth weight (liquid, chocolate‐flavoured supplement; biscuit; milk; supplement with sesame cake, jaggery, oil; fortified food supplement with peanut butter, soy flour, vegetable oil, sugar, micronutrients; supplement as dry powder providing energy, protein, fat; supplement with dried skim milk, enriched bread, vegetable oil; mixture of beans, maize and micronutrients or porridge and micronutrients; oral supplement (beverage); glucose drink; glucose drink and skim milk powder). tNo subgroup differences between undernourished and adequately nourished groups (test for subgroup differences: Chi2 = 2.35, degrees of freedom (df) = 1 (P = 0.12), I2 = 57.5%). uNo subgroup differences between boys and girls (test for subgroup differences: Chi2 = 0.22, df = 1 (P = 0.64), I2 = 0%). vComparison group: no food or low‐protein, kcal supplementation. wNo subgroup differences based on age, nutritional status (stunted/wasted versus not) of the children and duration of feeding (< 12 months versus ≥ 12 months). xNo subgroup difference based on duration of feeding but subgroup difference based on age (test for subgroup differences: Chi2 = 7.24, df = 1 (P = 0.01), I2 = 86%): children > 24 months (MD 0.22, 95% CI 0.07 to 0.37).
Additional comments
- Grobler 2013 described weight outcomes narratively for one trial: children receiving enhanced nutrition support had significantly more weight gain in the first eight weeks than children receiving standard care (P < 0.0001) (Rollins 2007).
- Kristjansson 2015a narratively reported two additional RCTs in LMIC. One 14‐month RCT (60 children) found a large and significant effect of feeding on weight gain for boys (end‐of‐study difference 3.91 kg; statistically significant) and girls (end‐of‐study difference 2.55 kg; statistically significant) (Obatolu 2003). One study found that 48 children who received supplementary feeding gained a mean of 39 g more than the 43 children in the control group (six‐month intervention: not significant) (Fauveau 1992).
20. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: growth in children, length/height.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Height gain (cm) | MD 0.38 (–0.32 to 1.08)b | 1462 (3) | NR |
| Change in height (cm) | MD 0.28 (–0.01 to 0.57)c | 520 (1) | NR | |||
| Height gain (adjusted ICC = 0.0016) (cm) | MD 1.43 (0.46 to 2.41)d,e | 986 (6) | NR | |||
| MD 0.92 (0.16 to 1.67)f,g | 703 (4) | NR | ||||
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplementary feedingh | Height gain (cm) | MD 0.27 (0.07 to 0.48)i,j | 1463 (9) | Moderate |
| Supplementary feedingk | Height gain (cm) | MD 0.52 (–0.07 to 1.10)l | 1782 (7) | NR | ||
| Supplementary foodm | Height gain (cm) | MD –1.00 (–2.12 to 0.12)n | 45 (1) | NR | ||
| Balanced | Height gain (cm) | MD 0.61 (–0.31 to 1.54)o | 116 (1) | NR | ||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | Balancedp | Height gain (total) (mm) | MD 1.54 (–2.07 to 5.15) | 178 (1) | NR |
| Ota 2015 | Pregnant women | Balanced | Child's birth length (cm) | MD 0.16 (0.01 to 0.31)q | 3370 (5) | NR |
| Child's length at 1 year (cm) | MD 0.00 (–5.69 to 5.69) | 428 (1) | NR | |||
| High protein | Child's height at 11–17 years (cm) | MD –0.39 (–1.73 to 0.94)r | 855 (1) | NR | ||
| Child's length at 1 year (cm) | MD 0.20 (–5.59 to 5.99) | 412 (1) | NR | |||
| Sguassero 2012 | Children < 5 years of age | High energy, protein and balanceds | Length/height at the end of the intervention (cm) | MD 0.28 (–0.11 to 0.67)t | 587 (3) | NR |
| Balanced | Length/height gain during the intervention (cm) | MD 0.19 (0.07 to 0.31)i,u | 795 (2) | NR | ||
| CI: confidence interval; ICC: intracluster correlation coefficient; MAM: moderate acute malnutrition; MD: mean difference; NR: not reported. | ||||||
aAs reported in 'Summary of findings' tables. bLow‐ and middle‐income country (LMIC) randomised controlled trials (RCTs). cHigh‐income country (HIC) RCT. dLMIC controlled before‐and‐after studies (CBAs). eHeight gain significantly increased for children who received school meals (lunch; green gram and sugar; vegetable protein mixture). fHIC CBAs. gHeight gain significantly increased for children who received school meals (milk). hBalanced (five studies); high energy (two studies); high lipid (one study); supplementary food (one study). iAnalyses include the same RCT: Simondon 1996 (multi‐country study). jLMIC RCT. kBalanced (two studies); high energy (one study); high lipid (one study); high protein (one study); supplementary food (two studies). lLMIC CBA. m113 g wet ration fruit cereal, rice cereal with apple sauce, mixed cereal with apple sauce and bananas, and oatmeal with apple sauce and bananas (Gerber Products Company). nHIC RCT oAboriginal children, HIC CBA. pComplementary foods (Pusti Packet). qBirth length significantly increased in newborns of women given balanced energy, protein supplementation (liquid, chocolate‐flavoured supplement; biscuit; milk; supplement with sesame cake, jaggery, oil; fortified food supplement with peanut butter, soy flour, vegetable oil, sugar, micronutrients). rNo significant differences for boys and girls. sComparison group: no food or low‐protein, kcal supplement. tNo subgroup differences based on age, nutritional status (stunted/wasted versus not) of the children and duration of feeding (< 12 months versus > 12 months). uLength gain significantly increased in children given supplementary feeding (porridge and yogurt).
Additional comments:
- Kristjansson 2015a narratively reported two additional RCTs in LMIC. Pollitt 2000 studied effectiveness for two age cohorts, 12 and 18 months old. They found that supplementary feeding had a significant effect on height for the younger (12‐month‐old) cohort only. Obatolu 2003 (60 children) found a significant effect for feeding on length for boys (5.12 cm difference between intervention and control groups; end‐of‐study difference of 5.02; statistically significant) and girls (6.95 cm difference; end‐of‐study difference of 5.92 cm; statistically significant).
21. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: growth in children, z scores.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Weight‐for‐age z scores (WAZ) | ||||||
| Grobler 2013 | Children with HIV | Specific (spirulina)b | WAZ | MD 0.00 (–0.44 to 0.44) | 84 (1) | NR |
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | WAZ | MD 0.07 (0.04 to 0.10)c,d | 785 (1) | NR |
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplementary feedinge | WAZ | MD 0.15 (0.05 to 0.24)f | 1565 (8) | Moderate |
| Supplementary feedingg | WAZ | MD 0.27 (–0.13 to 0.68)h | 999 (4) | Very low | ||
| Supplementary foodi | Change in WAZ | MD 0.02 (0.01 to 0.03)j | 103 (1) | NR | ||
| Sguassero 2012 | Children < 5 years of age | Balanced | Change in WAZ during intervention | MD 0.12 (0.05 to 0.19)k | 348 (1) | Low |
| WAZ at end of intervention | MD –0.18 (–0.49 to 0.12) | 195 (2) | NR | |||
| Length/height‐for‐age z scores (HAZ) | ||||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | HAZ | MD 0.04 (0.02 to 0.06)l,m | 1021 (2) | NR |
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplementary feedingn | HAZ | MD 0.15 (0.06 to 0.24)f | 4544 (9) | Moderate |
| Supplementary feedingo | HAZ | MD 0.01 (–0.10 to 0.12)h | 999 (4) | Very low | ||
| Supplementary foodi | Change in HAZ | MD 0.04 (0.04 to 0.05)j | 103 (1) | NR | ||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | High lipid and balancedp | HAZ | MD 0.23 (–0.07 to 0.54) | 1546 (2) | Low |
| Sguassero 2012 | Children < 5 years of age | Balanced | HAZ at end of intervention | MD 0.02 (–0.29 to 0.32) | 195 (2) | NR |
| Weight‐for‐height/length z score (WHZ) | ||||||
| Grobler 2013 | Children with HIV | Specific (spirulina)b | WHZ | MD 0.35 (–0.21 to 0.91) | 84 (1) | NR |
| Kristjansson 2007 | School children (aged 5–19 years) | Balancedq | Change in WHZ | MD 0.20 (–0.24, 0.64)l | 236 (1) | NR |
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplementary feedingr | WHZ | MD 0.10 (–0.02 to 0.22)f | 4073 (7) | Moderate |
| Supplementary feedings | WHZ | MD 0.29 (–0.11 to 0.69)h | 999 (4) | Very low | ||
| Supplementary foodi | WHZ | MD –0.06 (–0.07 to –0.05)j | 103 (1) | NR | ||
| Lazzerini 2013 | Children with MAM (< 5 years of age) | High lipid and balancedl | WHZ (final) | MD 0.20 (0.03 to 0.37)t | 1546 (2) | Moderate |
| Balancedu | WHZ gain (total) | MD 0.28 (0.06 to 0.49)t | 178 (1) | NR | ||
| Sguassero 2012 | Children (< 5 years of age) | Balanced | WHZ at end of intervention | MD 0.10 (–0.33 to 0.13)v | 260 (3) | Moderate |
| BMI z score | ||||||
| Ota 2015 | Pregnant women | Balanced | Child's BMI z score at 11–17 years | MD 0.16 (0.01 to 0.31)w | 855 (1) | NR |
| BMI: body mass index; MAM: moderate acute malnutrition; MD: mean difference; NR: not reported. | ||||||
aAs reported in 'Summary of findings' tables. bSpirulina supplementation. cLow‐ and middle‐income country (LMIC) randomised controlled trial (RCT). dSignificant effect of school breakfast (cheese sandwich or spiced bun and cheese plus milk) versus control on weight‐for‐age z (WAZ) scores. eBalanced (two studies); high energy (two studies); high lipid (two studies); supplementary food (two studies). fLMIC RCT. gBalanced (one study); supplementary food (three studies). hLMIC controlled before‐and‐after study (CBA). iPuréed meat, iron‐fortified infant cereal and whole cows' milk. jHigh‐income country (HIC) RCT. kChange in WAZ significantly higher in the group supplemented with yoghurt. lA small, significant effect of school feeding on height‐for‐age z scores (HAZ) scores demonstrated; z score difference = 0.04 (school breakfast: cheese sandwich or spiced bun and cheese plus milk; githeri and meat). mLMIC RCTs. nBalanced (two studies); high energy (three studies); high lipid (two studies); supplementary food (two studies). oBalanced (one study); supplementary food (three studies). pComplementary foods (Pusti Packet) and lipid‐based nutrient supplement (LNS) (i.e. Plumpy Doz and corn‐soy blend (CSB++)). qLMIC RCT. rBalanced (three studies); high energy (two studies); high lipid (one study); supplementary food (one study). sBalanced (one study); supplementary food (three studies). tFinal weight‐for‐height/length z score (WHZ) score and WHZ gain significantly higher in the group receiving food than in standard care. uComplementary foods (Pusti Packet). vNo subgroup differences based on age, nutritional status of the children (stunted/wasted versus not stunted/wasted) and duration of feeding. wSmall increase in mean body mass index (BMI) z score for children receiving supplementary biscuits versus children in the control group (no subgroup differences between girls and boys).
Additional comments
- Kristjansson 2015a narratively reported one additional cluster‐RCT in an LMIC. In the cluster‐RCT with 282 children, Roy 2005 found significant effects of supplementation with maternal nutrition education. Those children in the intervention group gained 0.71 more in WAZ than the children who received no treatment (P < 0.001), and 0.26 more in WAZ than the children who received only maternal nutrition education (not significant). One additional CBA in an LMIC was also reported narratively; De Romaña 2000 (250 participants) found no significant difference between intervention and control groups in change in prevalence of stunting (i.e. HAZ scores).
22. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: nutritional status of children, other anthropometry indicators.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Head circumference | ||||||
| Ota 2015 | Pregnant women | Balanced | Child's birth head circumference (cm) | MD 0.04 (–0.08 to 0.17) | 3352 (5) | NR |
| Head circumference at 1 year (cm) | MD –0.13 (–0.35 to 0.10) | 627 (2) | NR | |||
| High protein | Head circumference at 1 year (cm) | MD 0.11 (–0.19 to 0.41) | 412 (1) | NR | ||
| Sguassero 2012 | Children < 5 years of age (stunted; after 12 months) | Balanced | Head circumference at end of the intervention (cm) | MD 0.40 (–0.21 to 1.01) | 65 (1) | NR |
| Children < 5 years of age (stunted/wasted; after 12 months) | High energy, proteinb | MD 0.19 (–0.41 to 0.79) | 75 (1) | NR | ||
| Mid‐upper arm circumference (MUAC) | ||||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | MUAC (mm) | MD 0.31 (0.16 to 0.46)c,d | 236 (1) | NR |
| Lazzerini 2013 | Children with MAM (< 5 years of age) | Balancedc,e | MUAC gain (total, mm) | MD 0.62 (–1.38 to 2.61) | 178 (1) | Very low |
| Sguassero 2012 | Children < 5 years of age (stunted; after 12 months) | Balanced | MUAC at end of intervention (cm) | MD 0.20 (–0.29 to 0.69) | 65 (1) | NR |
| Children < 5 years of age (stunted/wasted; after 12 months) | High energy, proteinb | MD 0.10 (–0.22 to 0.42) | 75 (1) | NR | ||
| Children < 5 years of age (nutritionally at risk; after 9 months) | Balanced | MD –0.08 (–0.31 to 0.15) | 348 (1) | NR | ||
| Triceps skinfold thickness | ||||||
| Sguassero 2012 | Children < 5 years of age (stunted; after 12 months) | Balanced | Triceps skinfold thickness at end of intervention (mm) | MD 0.20 (–0.51 to 0.91) | 65 (1) | NR |
| Subscapular skinfold thickness | ||||||
| Sguassero 2012 | Children < 5 years of age (stunted; after 12 months) | Balanced | Subscapular skinfold thickness at end of intervention (mm) | MD 0.20 (–0.34 to 0.74) | 65 (1) | NR |
| Mid‐upper arm muscle area | ||||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Mid‐upper arm muscle area (mm2) | MD 68.22 (39.57 to 96.87)d,f | 236 (1) | NR |
| Mid‐upper arm fat area | ||||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Mid‐upper arm fat area (mm2) | MD –0.31 (–26.12 to 25.50)d | 236 (1) | NR |
| Percentage body fat | ||||||
| Ota 2015 | Pregnant women | Balanced | Child's % body fat at 11–17 years | MD 0.06 (–0.41 to 0.52)g | 847 (1) | NR |
| CI: confidence interval; MAM: moderate acute malnutrition; MD: mean difference; NR: not reported. | ||||||
aAs reported in 'Summary of findings' tables. bComparison group: no food or low protein (kcal) supplementation. cSignificant increase in mid‐upper arm circumference (MUAC) in the meat group compared to the control group; school feeding (meat versus control) had a greater effect on MUAC for boys than for girls. dLow‐ and middle‐income country (LMIC) randomised controlled trial (RCT). eComplementary foods (Pusti Packet). fChildren in the intervention group, who were given meat, gained significantly more in the mid‐upper arm muscle area than the control group. gNo subgroup differences between boys and girls.
23. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: nutritional status of children, biochemical parameters.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Change in haemoglobin (g/L) | ||||||
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplementary feedingb | Change in haemoglobin (g/L) | SMD 0.49 (0.07 to 0.91)c | 300 (5) | NR |
| CI: confidence interval; NR: not reported; SMD: standardised mean difference. | ||||||
aAs reported in 'Summary of findings' tables. bBalanced (one study); high energy (two studies); high lipid (one study); supplementary food (one study). cLow‐ and middle‐income country (LMIC) randomised controlled trials (RCTs).
24. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome, nutritional status of adults, weight.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Grobler 2013 | Adults with HIV | Balancedb | Body weight (6–12 weeks' follow‐up) | MD –0.17 (–1.10 to 0.75) | 233 (4) | Moderate |
| Balancedc | Body weight at baseline (ART arm) | MD –0.58 (–1.47 to 0.31) | 617 (1) | NR | ||
| Body weight at baseline (pre‐ART arm) | MD 0.60 (–0.60 to 1.80) | 429 (1) | NR | |||
| Body weight at 1 month (ART arm) | MD 0.58 (–0.62 to 1.78) | 366 (1) | NR | |||
| Body weight at 1 month (pre‐ART arm) | MD 1.09 (–0.59 to 2.77) | 261 (1) | NR | |||
| Body weight at 3 months (ART arm) | MD 0.41 (–0.99 to 1.81) | 322 (1) | NR | |||
| Body weight at 3 months (pre‐ART arm) | MD 2.82 (1.02 to 4.62)d | 211 (1) | NR | |||
| Body weight at 6 months (ART arm) | MD 0.17 (–1.50 to 1.84) | 237 (1) | NR | |||
| Body weight at 6 months (pre‐ART arm) | MD 3.67 (1.50 to 5.84)d | 157 (1) | NR | |||
| Body weight at 12 months (ART arm) | MD –1.00 (–3.19 to 1.19) | 180 (1) | NR | |||
| Body weight at 12 months (pre‐ART arm) | MD 2.25 (–0.41 to 4.91) | 118 (1) | NR | |||
| Change in body weight at 1 month (ART arm) (kg) | MD 0.90 (0.40 to 1.41)e | 366 (1) | NR | |||
| Change in body weight at 1 month (pre‐ART arm) (kg) | MD 0.82 (0.28 to 1.36)f | 261 (1) | NR | |||
| Change in body weight at 3 months (ART arm) (kg) | MD 1.12 (0.29 to 1.95)e | 322 (1) | NR | |||
| Change in body weight at 3 months (pre‐ART arm) (kg) | MD 1.22 (0.31 to 2.12)f | 211 (1) | NR | |||
| Change in body weight at 6 months (ART arm) (kg) | MD 0.89 (–0.30 to 2.08) | 237 (1) | NR | |||
| Change in body weight at 6 months (pre‐ART arm) (kg) | MD 2.06 (0.82 to 3.30)f | 157 (1) | NR | |||
| Change in body weight at 12 months (ART arm) (kg) | MD –0.03 (–1.78 to 1.71) | 180 (1) | NR | |||
| Change in body weight at 12 months (pre‐ART arm) (kg) | MD 0.83 (–0.79 to 2.45) | 118 (1) | NR | |||
| Specific (AA mixture)g | Mean change in body weight (baseline to 8 weeks) (kg) | MD 2.63 (0.72 to 4.54)h | 43 (1) | NR | ||
| Specific (OKG)i | Mean weight at study endpoint (kg) | MD –5.00 (–11.68 to 1.68) | 46 (1) | NR | ||
| Specific (GLN)j | Mean weight at study endpoint (kg) | MD –1.30 (–10.18 to 7.58) | 21 (1) | NR | ||
| Grobler 2016 | Adults with TB | Balanced and high energy | Mean weight gain (after 6 weeks) (kg) | MD 1.73 (0.81 to 2.65)k | 34 (1) | NR |
| Mean weight gain (after 8 weeks) (kg) | MD 0.78 (–0.05 to 1.60) | 689 (3) | NR | |||
| Mean weight gain (after 12 weeks) (kg) | MD 2.60 (1.74 to 3.46)k | 100 (1) | NR | |||
| Mean weight gain (after 20 weeks) (kg) | MD –0.20 (–1.34 to 0.94) | 306 (1) | NR | |||
| Mean weight gain (after 24 weeks) (kg) | MD 1.78 (–0.25 to 3.81) | 26 (1) | NR | |||
| Mean weight gain (after 32 weeks) (kg) | MD 2.60 (0.52 to 4.68)k | 265 (1) | NR | |||
| Mean weight gain (at 8 weeks) (kg)l | Not pooled | 731 (4) | Moderate | |||
| Ota 2015 | Pregnant women | Balanced | Weekly gestational weight gain (g/week) | MD 18.63 (–1.81 to 39.07) | 2391 (9) | NR |
| Maternal weight 4 weeks' postpartum (kg) | MD –0.90 (–1.92 to 0.12) | 354 (1) | NR | |||
| High protein | Weekly gestational weight gain (g/week) | MD 4.50 (–33.55 to 42.55) | 486 (1) | NR | ||
| Isocaloric balanced protein | Weekly gestational weight gain (g/week) | MD 110.45 (–82.87 to 303.76) | 184 (2) | Very low | ||
| AA: amino acid; ART: antiretroviral therapy; CI: confidence intervals; GLN: L‐glutamine; MD: mean difference; NR: not reported; OKG: ornithine alpha‐ketoglutarate; TB: tuberculosis. | ||||||
aAs reported in 'Summary of findings' tables. bAll commercial balanced macronutrient formulas + nutrition counselling versus nutrition counselling in participants with weight loss. cFortified blended food + nutrition counselling versus nutrition counselling in malnourished adults on ART and pre‐ART. dAmong participants not receiving antiretroviral therapy (ART), the supplement group had a significantly greater mean body weight than the non‐supplement group at both three months (P = 0.0022) and at six months (P = 0.001). eAmong participants receiving ART, the supplement group appeared to gain weight more rapidly than the non‐supplement group in the first three months of the trial, as they had a significantly greater change in body weight gain compared to the non‐supplement group at these time points. After this time point, the change in body weight was not significantly different between the groups. fAmong participants not receiving ART, the supplement group gained significantly more body weight compared with the non‐supplement group in the first three months of the trial and at the six‐month time point. After this time point, the change in body weight was not significantly different between the groups. gAmino acid mixture (arginine, glutamine, β‐hydroxy‐β‐methylbutyrate versus placebo). hAfter eight weeks, the arginine‐rich group gained significantly more body weight than the control group. iOrnithine alpha‐ketoglutarate versus placebo. jL‐glutamine versus placebo. kSupplementation did seem to improve weight gain at specific time points during treatment, although one large trial exclusively in people coinfected with HIV found no difference at any time point (PrayGod 2011). lSupplementation probably increases weight gain during treatment. Four studies reported measures of weight gain but at different time points, which prevented meta‐analysis.
25. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: nutritional status of adults, anthropometry indicators.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Body mass index (BMI) | ||||||
| Grobler 2013 | Adults with HIV | Balancedb | BMI at baseline (ART arm) | MD 0.02 (–0.15 to 0.19) | 617 (1) | NR |
| BMI at baseline (pre‐ART arm) | MD 0.17 (–0.07 to 0.41) | 429 (1) | NR | |||
| BMI at 1 month (ART arm) | MD 0.36 (0.08 to 0.64)c | 366 (1) | NR | |||
| BMI at 1 month (pre‐ART arm) | MD 0.39 (0.05 to 0.74)d | 261 (1) | NR | |||
| BMI at 3 months (ART arm) | MD 0.43 (0.07 to 0.79)c | 322 (1) | NR | |||
| BMI at 3 months (pre‐ART arm) | MD 0.73 (0.31 to 1.15)d | 211 (1) | NR | |||
| BMI at 6 months (ART arm) | MD 0.42 (–0.07 to 0.91) | 237 (1) | NR | |||
| BMI at 6 months (pre‐ART arm) | MD 0.78 (0.22 to 1.34)c | 157 (1) | NR | |||
| BMI at 12 months (ART arm) | MD –0.08 (–0.72 to 0.56) | 180 (1) | NR | |||
| BMI at 12 months (pre‐ART arm) | MD 0.45 (–0.25 to 1.15) | 118 (1) | NR | |||
| Lean body mass (LBM) | ||||||
| Grobler 2013 | Adults with HIV | Balancedb | % LBM at baseline (ART arm) | MD 0.13 (–0.96 to 1.23) | 569 (1) | NR |
| % LBM at baseline (pre‐ART arm) | MD –0.30 (–1.51 to 0.92) | 394 (1) | NR | |||
| % LBM at 1 month (ART arm) | MD 0.47 (–1.20 to 2.13) | 253 (1) | NR | |||
| % LBM at 1 month (pre‐ART arm) | MD 0.41 (–1.40 to 2.22) | 185 (1) | NR | |||
| % LBM at 3 months (ART arm) | MD –0.53 (–2.13 to 1.07) | 283 (1) | NR | |||
| % LBM at 3 months (pre‐ART arm) | MD 1.14 (–0.70 to 2.98) | 179 (1) | NR | |||
| % LBM at 6 months (ART arm) | MD 0.32 (–1.48 to 2.12) | 202 (1) | NR | |||
| % LBM at 6 months (pre‐ART arm) | MD 1.65 (–0.79 to 4.09) | 129 (1) | NR | |||
| % LBM at 12 months (ART arm) | MD –1.53 (–3.55 to 0.49) | 169 (1) | NR | |||
| % LBM at 12 months (pre‐ART arm) | MD 0.67 (–1.82 to 3.16) | 107 (1) | NR | |||
| Fat mass | ||||||
| Grobler 2013 | Adults with HIV | Balancede | Fat mass measured in % of TBW | MD –1.14 (–2.58 to 0.29) | 233 (4) | Moderate |
| Specific (AA mixture)f | Change in fat mass (kg) | MD –0.64 (–2.69 to 1.41) | 43 (1) | NR | ||
| Specific (OKG)g | Mean fat mass (kg) at study endpoint | MD 0.00 (–2.00 to 2.00) | 46 (1) | NR | ||
| Specific (GLN)h | Mean fat mass (kg) at study endpoint | MD –1.00 (–32.40 to 30.40) | 21 (1) | NR | ||
| Fat‐free mass | ||||||
| Grobler 2013 | Adults with HIV | Balancede | Fat‐free mass | MD –0.37 (–2.77 to 2.03) | 218 (3) | Low |
| Specific (AA mixture)f | Change in fat‐free mass | MD 3.25 (1.25 to 5.25)i | 43 (1) | NR | ||
| Specific (OKG)g | Mean fat‐free mass (kg) at study endpoint | MD –5.10 (–11.11 to 0.91) | 46 (1) | NR | ||
| AA: amino acid; ART: antiretroviral therapy; BMI: body mass index; CI: confidence interval; GLN: L‐glutamine; LBW: lean body weight; MD: mean difference; NR: not reported; OKG: ornithine alpha‐ketoglutarate; TBW: total body weight. | ||||||
aAs reported in 'Summary of findings' tables. bFortified blended food + nutrition counselling versus nutrition counselling in malnourished adults on antiretroviral therapy (ART) and pre‐ART. cAmong participants receiving ART, mean body mass index (BMI) and change in BMI in the supplement group was significantly higher in the first three months compared to the no supplement group. After three months, there was no significant difference in BMI or BMI gain between the supplement and no supplement groups in the participants receiving ART. dAmong participants not receiving ART, mean BMI and change in BMI in the supplement group was significantly higher in the first six months compared to the no supplement group. After six months, there was no significant difference in BMI or BMI gain between the supplement and no supplement groups in the participants not receiving ART. eAll commercial balanced macronutrient formulas + nutrition counselling versus nutrition counselling in participants with weight loss. fAmino acid mixture (arginine, glutamine, β‐hydroxy‐β‐methylbutyrate versus placebo). gOrnithine alpha‐ketoglutarate versus placebo. hL‐glutamine versus placebo. iThe increase in fat‐free mass was significantly greater in the arginine group compared with the control group.
26. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: nutritional status of adults, dietary intake.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Energy intake | ||||||
| Grobler 2013 | Adults with HIV | Balancedb | Energy intake (6–12 weeks' follow‐up) (kcal/kg) | MD 393.57 (224.66 to 562.47)c | 131 (3) | Low |
| Specific (OKG)d | Mean daily energy intake at study endpoint (kcal/kg) | MD 0.66 (–564.63 to 432.63) | 46 (1) | NR | ||
| Protein intake | ||||||
| Grobler 2013 | Adults with HIV | Balancede | Protein intake (g/day) (6–12 weeks follow‐up) | MD 23.35 (12.68 to 34.01)c | 81 (2) | Low |
| Specific (OKG)d | Mean daily protein intake at study endpoint | MD –0.70 (–18.71 to 17.31) | 43 (1) | NR | ||
| CI: confidence interval;OKG: ornithine alpha‐ketoglutarate; MD: mean difference; NR: not reported. | ||||||
aAs reported in 'Summary of findings' tables. bMacronutrient formulas (Meritene, Ensure, range of fortified oral supplements). cSupplementation with balanced macronutrient formulas significantly improved energy intake and protein intake compared with no nutritional supplementation or nutrition counselling alone in adults with weight loss. dOrnithine alpha‐ketoglutarate versus placebo. eMacronutrient formulas (Meritene, Ensure).
Additional comments
- One systematic review described this outcome narratively (Droogsma 2014). In one study in the review, three months of daily oral nutritional supplements significantly improved nutritional outcomes in the intervention group (Lauque 2004). The nutritional status of the control group also improved after three months, although the intervention group improved significantly more than the control group. There were no significant changes on the clinical and biochemical outcomes.
27. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: school attendance, cognition tests and educational attainment.
| Review | Target group | Intervention | Outcome | Corresponding risk with intervention (95% CI) | Number of participants (studies) |
| Cognitive tests | |||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Full scale IQ (total) (adjusted ICC = 0.15) | MD 3.90 (–2.88 to 10.68)a,b | 231 (1) |
| Full scale IQ (separated) (cluster size as in analysis) (adjusted ICC = 0.15) | MD 3.80 (0.51 to 7.10)a,c,d | 231 (1) | |||
| Performance IQ (total) (adjusted ICC = 0.15) | MD 5.00 (–2.60 to 12.6)a,b | 231 (1) | |||
| Performance IQ (separated) (cluster size as in analysis) (adjusted ICC = 0.15) | MD 5.74 (1.73 to 9.74)a,c,e | 231 (1) | |||
| Verbal IQ (total) (adjusted ICC = 0.15) | MD 3.10 (–2.99 to 9.19)a,b | 231 (1) | |||
| Verbal IQ (separated) (cluster size as in analysis) (adjusted ICC = 0.15) | MD 3.35 (–0.21 to 6.92)a,c | 231 (1) | |||
| Kristjansson 2015a | Disadvantaged infants and young children (aged 3 months to 5 years) | Supplement food | Cognitive ability | SMD 0.58 (0.17 to 0.98)f,g | 99 (1) |
| High energy | Change on Bailey Scale of Mental Development (BSMD) | SMD –0.40 (–0.79 to –0.00)h | 113 (1) | ||
| Ota 2015 | Pregnant women | Balanced | Child's Bailey mental score (1 year) | MD –0.74 (–1.95 to 0.47) | 411 (1) |
| Child's IQ (5 years) | MD 0.00 (–4.98 to 4.98) | 153 (1) | |||
| High protein | Child's Bailey mental score (1 year) | MD 0.32 (–0.91 to 1.55) | 396 (1) | ||
| Educational attainment | |||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Maths change overall (ICC = 0.15) | SMD 0.31 (0.09 to 0.53)a,i | 337 (2) |
| Change in reading (ICC = 0.15) | MD 0.09 (–0.11 to 0.29)a | 106 (1) | |||
| Change in spelling (ICC = 0.15) | MD 0.24 (0.01 to 0.47)a,h | 106 (1) | |||
| School attendance | |||||
| Kristjansson 2007 | School children (aged 5–19 years) | Balanced | Change in attendance (ICC = 0.15) | MD 4.95 (–3.56 to 13.46)a | 108 (1) |
| End of study attendance (ICC = 0.15) | MD –0.23 (–17.93 to 17.47)a | 72 (1) | |||
| CI: confidence interval; ICC: intracluster correlations; IQ: intelligence quotient; MD: mean difference. | |||||
aAll comparisons: low‐ and middle‐income country (LMIC) controlled before‐and‐after studies (CBAs). bSensitivity analyses made very little difference to either the point estimate or the significance. cFour subgroups of one study (Agarwal 1989). dChildren who were given school lunches had an end‐of‐study full‐scale intelligence quotient (IQ) that was 3.8 points higher than children who were not given school lunch. Sensitivity analyses with intracluster correlation (ICCs) at 0.10 and 0.20 still significant. eChildren who were given school lunches had an end‐of‐study performance IQ that was 5.74 points higher than children who were not given school lunch. Sensitivity analyses with ICCs at 0.10 and 0.20 both significant. fTrial compared results for time point 4 children (supplemented with stimulation from 42 to 84 months of age) to those of time point 2 children (supplemented from 63 to 84 months of age) at 63 months. gLMIC randomised controlled trial (RCT). hChange in spelling achievement significantly greater for children who received school meals (breakfast). Sensitivity analysis with an ICC of 0.10 showed much the same results, however, the sensitivity analysis with an ICC of 0.20 was non‐significant. iChange in maths achievement significantly greater for children who received school meals (lunch and breakfast); results of an analysis with Agarwal 1989 broken down into four nutritional subgroups were similar (standardised mean difference 0.44, 95% confidence interval 0.22 to 0.67). Sensitivity analyses for ICCs of 0.10 and 0.20 made little difference.
Additional comments:
- An ICC of 0.15 was used for maths, reading, spelling, attendance and intelligence outcomes, with ICCs of 0.10 and 0.20 used for sensitivity analyses (Kristjansson 2007).
- Kristjansson 2015a narratively reported one additional cluster‐RCT in an LMIC (Pollitt 2000). The study found no main effects of supplementation on the Bailey Scales of Mental Development but reported positive effects in a contrast over time for the younger cohort but not for the older cohort (P < 0.05; 53 children).
- Kristjansson 2015a narratively reported long‐term follow‐up of cognitive development. Grantham‐McGregor 1997 followed up 97% (127 children) of the original cohort of stunted children (Grantham‐McGregor 1991; 129 children) after four years and tested them on a battery of cognitive and perceptual tests. A multiple regression found effects on perceptual motor tasks, but not on general cognition or memory. Interestingly, stimulation had a significant effect on later perceptual‐motor skills for all children (P < 0.05), but supplementation only had a significant effect for children whose mothers had higher scores on a test of verbal intelligence (P < 0.05). Grantham‐McGregor 2007 also found that supplemented children had higher mean scores than the control group on 14 out of 15 cognitive tests (P = 0.02). Pollitt 1997 performed a seven‐year follow‐up of Husaini 1991. They found no differences between the intervention (125 children) and control (106 children) groups on the Peabody Picture Vocabulary Test, emotionality, and maths. They found small (15‐second difference), positive effects of supplementation on working memory performance, although these are unlikely to be clinically significant.
28. Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice), outcome: adverse events.
| Review | Target group | Intervention | Outcome | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE)a |
| Grobler 2013 | Adults with HIV | Specific (OKG)b | GI adverse events | 542 per 1000 | 864 per 1000 | RR 1.59 (1.06 to 2.39)c | 46 (1) | NR |
| Ota 2015 | Pregnant women | Balanced | Small‐for‐gestational age | 173 per 1000 | 137 per 1000 (120 to 156) | RR 0.79 (0.69 to 0.90)d | 4408 (7) | Moderate |
| Preterm birth | 112 per 1000 | 108 per 1000 (90 to 130) | RR 0.96 (0.80 to 1.16) | 3384 (5) | Moderate | |||
| Pre‐eclampsia | 73 per 1000 | 108 per 1000 (60 to 195) | RR 1.48 (0.82 to 2.66) | 463 (2) | Very low | |||
| High protein | Small‐for‐gestational age | 117 per 1000 | 185 per 1000 (121 to 282) | RR 1.58 (1.03 to 2.41)e | 505 (1) | Moderate | ||
| Preterm birth | 219 per 1000 | 249 per 1000 (182 to 341) | RR 1.14 (0.83 to 1.56) | 505 (1) | Low | |||
| Sguassero 2012 | Children < 5 years of age | Balanced | Diarrhoea | — | — | OR 1.04 (0.67 to 1.62) | 108 (1) | NR |
| Vomiting | — | — | OR 0.89 (0.38 to 2.10) | 108 (1) | NR | |||
| CI: confidence interval; GI: gastrointestinal; NR: not reported; OKG: ornithine alpha‐ketoglutarate; OR: odds ratio; RR: risk ratio. | ||||||||
aAs reported in 'Summary of findings' tables. bMonohydrated L‐ornithine alpha‐ketoglutarate (OKG). cOKG associated with significantly more people reporting one or more GI adverse events. dIncidence of small‐for‐gestational age birth significantly reduced in women given balanced energy and protein supplementation (liquid, chocolate‐flavoured supplement; biscuit; milk; supplement with sesame cake, jaggery, oil; fortified food supplement with peanut butter, soy flour, vegetable oil, sugar, micronutrients; supplement with dried skim milk, enriched bread, vegetable oil; oral supplement (beverage)). eHigh‐protein supplementation associated with a significantly increased risk of small‐for‐gestational age babies (high protein oral supplement (beverage)).
Additional comments
- Lazzerini 2013 only reported adverse events in relation to lipid‐based nutrient supplements (LNS) versus all blended foods and LNS versus specific blended foods.
- Grobler 2013 poorly reported adverse effects in the included studies and, in general, they were related to tolerance rather than adverse effects. Keithley 2002 found no significant differences for acceptance and tolerance of the formulas (Ensure plus versus Advera). Rabeneck 1998 noted that one participant discontinued the supplement (lipisorb‐specialised medium chain triglycerides formula) due to nausea and epigastric pain, and one discontinued as he did not like the taste of the supplement.
- Kristjansson 2015a calculated the net benefit from supplementary feeding for seven studies that provided home‐delivered rations (randomised controlled trials (RCTs): Bhandari 2001; De Romaña 2000; Grantham‐McGregor 1991; Rivera 2004; controlled before‐and‐after studies (CBAs): Lutter 2008; Santos 2005; Tomedi 2012); and three of the day‐care/feeding centre studies (RCTs: Husaini 1991; Pollitt 2000; CBA: Devadas 1971). They found important differences in the number of calories provided by the supplementary food and the number of extra calories that the children actually consumed in addition to their regular food. In the take‐home studies, the net benefit to children was only 36% of the extra calories provide by the supplement. In the day‐care/feeding centres, the net benefit was 85% of the extra calories provided by the supplement.
Effect of interventions
The results reported here focused on the pooled analyses as undertaken in the various systematic reviews. We did not report on outcomes where no pooled analyses could be undertaken, but we did make reference to the individual studies, as reported in the reviews. For the Lazzerini 2013 review, we reported comparisons comparing specially formulated foods versus standard care only. We did not report comparisons of various types of specially formulated foods against each other.
Supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice)
Death
Four reviews reported mortality outcomes (Grobler 2013; Grobler 2016; Lazzerini 2013; Ota 2015). Mortality outcomes in the pregnancy review by Ota 2015 included stillbirth (death after 20 weeks' gestation and before birth) and neonatal death (death of a live infant within the first 28 days of life). Lazzerini 2013 reported death in children (aged less than five years) with MAM, and Grobler 2013 reported death (at eight and 26 weeks after study enrolment) for children who were HIV positive (aged six to 36 months) in one study. Grobler 2016 reported death at one‐year follow‐up in adults with tuberculosis. See Table 16 for details.
Figure 3 displays the meta‐analysis estimates for mortality across the four participant groups in the four reviews reporting data on this outcome. Estimates were all underpowered (too small to detect small but important differences), apart from balanced energy and protein supplementation in pregnancy, which suggested potential effects on reducing the risk of stillbirth (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.39 to 0.94; 5 studies, 3408 women; moderate‐certainty evidence).
3.
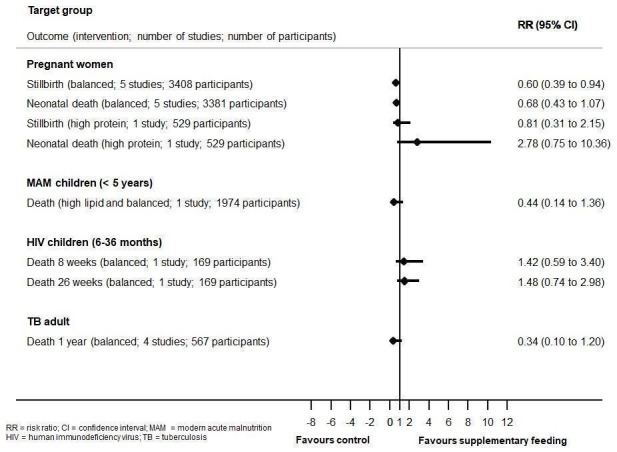
Outcome ‐ mortality: supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice).
In the HIV review, Grobler 2013 provided a narrative description of adult mortality outcomes, indicating that neither supplementary food (Sudarsanam 2011) nor daily supplementation of spirulina (Yamani 2009) significantly altered the risk of death compared with no supplement or placebo, in malnourished, antiretroviral therapy (ART)‐naive adults in the two studies that reported data on this outcome.
Illness (or disease‐related outcomes)
Three reviews reported illness‐related treatment outcomes (Grobler 2016; Kristjansson 2015a; Lazzerini 2013). Outcomes in the review of children with MAM included aspects of recovery, progression to SAM and defaulting (Lazzerini 2013). The tuberculosis review reported cure rate, treatment completion/failure and sputum conversion (Grobler 2016). Kristjansson 2015a narratively reported morbidity outcomes. All included comparisons included only one or two studies. See Table 17.
In the Lazzerini 2013 review on MAM, the provision of complementary foods (Pusti Packet) combined with LNS (Plumpy Doz, Corn‐Soy Blend (CSB++)), compared with standard care, increased 'recovery rate' by 29% (RR 1.29, 95% CI 1.20 to 1.38; 2 studies, 2152 children; moderate‐certainty evidence). The provision of complementary foods (LNS: Plumpy Doz, Corn‐Soy Blend (CSB++)) compared with standard care made little difference to the number recovering, or progression to SAM, but did reduce the number defaulting from the programme (RR 0.30, 95% CI 0.22 to 0.30; 1 study, 1974 children; moderate‐certainty evidence).
In the tuberculosis review, Grobler 2016, studies assessing the provision of free food or high energy supplements appeared to make little difference in terms of disease outcomes measured in various ways, although studies were all underpowered. One small study found a significant benefit in terms of treatment completion and sputum conversion, although these findings remain to be confirmed (very low‐certainty evidence). See Table 17.
In the review on disadvantaged infants and young children, Kristjansson 2015a provided a narrative description of the morbidity outcome data from six studies (four RCTs; two CBAs). Three RCTs (Bhandari 2001; Iannotti 2014; Isanaka 2009) and two CBAs (Gopalan 1973; Tomedi 2012) found few differences between the provision of food or high‐energy supplements and regular diet in the prevalence of morbidity. Roy 2005 (a CBA) reported mixed results; the prevalence of diarrhoea and fever was higher in the 99 children who received balanced protein supplementary food while the prevalence of respiratory infection was higher in the regular diet group (90 children).
Disease‐related biochemical parameters
One review reported disease‐related biochemical parameters (Grobler 2013). Outcomes in this HIV review included CD4 count and viral load. All comparisons included only one or two very small studies with fewer than 100 participants. None of the comparisons for this outcome were significant. See Table 18.
Growth in children
Weight
Five reviews reported on aspects of children's weight (Kristjansson 2007; Kristjansson 2015a; Lazzerini 2013; Ota 2015; Sguassero 2012). The pregnancy review, Ota 2015, reported birthweight and weight at two time points (aged one year and 11 to 17 years). The other reviews reported change in weight or weight at the end of the intervention. See Table 19 for details and Figure 4.
4.
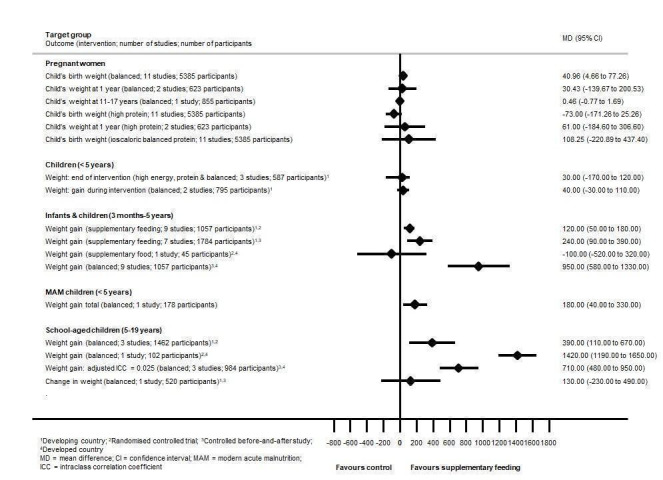
Outcome ‐ weight (g) in children: supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice).
Except for balanced energy and protein supplementation in the pregnancy review (Ota 2015), and food supplementation in the disadvantaged infants and young children review (Kristjansson 2015a), all comparisons included only one to three studies.
Balanced energy and protein supplementation in the pregnancy review was associated with increases in mean birth weight (mean difference (MD) 40.96 g, 95% CI 4.66 to 77.26; 11 studies, 5385 participants; moderate‐certainty evidence) (Ota 2015). Overall, the effects on weight in children as a result of pregnancy supplementation were limited, and not sustained.
Effects on growth in children were mixed. In children under five years of age from LMIC, one review found that supplementary feeding had a negligible impact on child growth (Sguassero 2012). However, a more recent review found that disadvantaged infants and young children in LMIC who received food supplementation gained a mean of 0.12 kg more than those who were not supplemented (MD 0.12 kg, 95% CI 0.05 to 0.18; 9 RCTs, 1057 participants; moderate‐certainty evidence) (Kristjansson 2015a). Sensitivity analyses with intracluster correlation coefficient (ICCs) at 0.10 made little difference, and findings from a subgroup analysis were significant for infants younger than 12 months of age and young children aged one to two years, but not in children older than two years of age. Supplementary feeding of undernourished children resulted in significant weight gain of 0.34 kg (95% CI 0.18 to 0.50) relative to controls, while the intervention was ineffective for well‐nourished children in a subgroup analysis of one trial at 0.08 kg (95% CI –0.09 to 0.25) (Thakwalakwa 2010). In further comparisons in CBAs in LMIC, infants and young children who received food supplementation gained a mean of 0.24 kg more than those who were not supplemented (MD 0.24 kg, 95% CI 0.09 to 0.39; 7 CBAs, 1784 participants). Sensitivity analyses with ICCs at 0.10 made little difference and, in subgroup analysis, findings were only significant for children aged two years. In HIC, one RCT assessed weight gain, and found that children who received supplementation in the form of an iron‐fortified cereal showed no additional gain in weight than children who received no supplementation (MD –0.10 kg, 95% CI –0.52 to 0.32; 45 participants) (Ziegler 2009). One CBA in young aboriginal children in Australia found that children receiving hot lunches in day care centres gained a mean of 0.95 kg more than those who did not (MD 0.95 kg, 95% CI 0.58 to 1.33; 116 participants) (Coyne 1980).
There seemed to be some gains in weight in children with MAM and school children, but the analyses were underpowered (Lazzerini 2013). In the MAM review, total weight gain was higher in children receiving complementary foods (Pusti Packet) when compared to those receiving standard care (MD 0.18 kg, 95% CI 0.04 to 0.33; 1 study, 178 participants; low‐certainty evidence) (Lazzerini 2013).
As reported in the Kristjansson 2007 review, disadvantaged school‐aged children who were fed at school gained an average of 0.39 kg more than those who were not supplemented (MD 0.39 kg, 95% CI 0.11 to 0.67; 3 RCTs in LMIC, 1462 participants). This translated to a gain of 0.25 kg per year for these children. Sensitivity analyses with ICCs at 0.01, 0.05 and 0.10 made little difference, and findings from a subgroup analysis were significant for undernourished and adequately nourished children, specifically children aged nine to 10 years. In further comparisons, children who received milk at school gained significantly more weight than those who did not (MD 1.42 kg, 95% CI 1.19 to 1.65; 1 CBA in a HIC, 102 participants). Children who were fed at school gained 0.71 kg more weight than controls (MD 0.71 kg, 95% CI 0.48 to 0.95; 3 CBA studies in LMIC, 984 participants). This translated to a gain of 0.75 kg per year for these children. Sensitivity analyses with ICCs at 0.01, 0.05 and 0.10 made little difference, and findings from a subgroup analysis were significant for boys and girls, and specifically for children aged five to six, six to eight and nine to 10 years of age.
Length/height
Five reviews reported on aspects of length/height in children (Kristjansson 2007; Kristjansson 2015a; Lazzerini 2013; Ota 2015; Sguassero 2012). The pregnancy review by Ota 2015 reported birth length and length/height at two time points: one year and 11 to 17 years. The other reviews reported change in length/height or length/height at the end of the intervention. See Table 20 for details and Figure 5.
5.
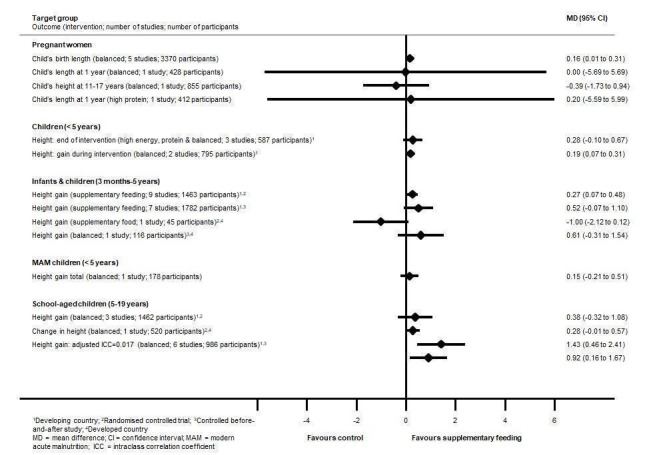
Outcome ‐ length/height (mm) in children: supplementary feeding versus no supplementary feeding (control, placebo, standard care, dietary advice).
Some gains in height were reported in children under five years of age (Sguassero 2012) and school children (Kristjansson 2007), but again analyses were underpowered (see Table 20 and Figure 5). For children with MAM, evidence was limited (as reported in one study) and no mean effect demonstrated (Lazzerini 2013).
In the pregnancy review by Ota 2015, birth length was significantly increased in newborns of women given balanced energy protein supplementation (MD 0.16 cm, 95% CI 0.01 to 0.31; 5 studies, 3370 participants). Overall, there was some increase in birth length with pregnancy supplementation but, as with the findings for weight, this was not sustained over time (as reported in one study; Rush 1980).
In the Sguassero 2012 review on children younger than five years of age in LMIC, length/height gain at the end of the intervention was significantly higher in children given supplementary feeding (porridge and yogurt) versus children in the control group (MD 0.19 cm, 95% CI 0.07 to 0.31; 2 studies, 795 participants). Kristjansson 2015a also found that disadvantaged infants and young children who received food supplementation gained a mean of 0.27 cm more in height than children who were not supplemented (MD 0.27 cm, 95% CI 0.07 to 0.48; 9 RCTs in LMIC, 1463 participants; moderate‐certainty evidence). A subgroup analysis revealed that supplementary feeding was effective for the youngest age groups (children younger than 12 months and children aged one to two years); there were no significant gains in height in the oldest age group (older than two years of age). In further comparisons of CBAs in LMIC, infants and young children who received food supplementation did not gain significantly more height than those who did not receive supplementation (MD 0.52 cm, 95% CI –0.07 to 1.10; 7 CBAs, 1782 participants). There were no significant effects of supplementation on height in HIC (MD 0.61, 95% CI ‐0.31 to 1.54; 1 study, 116 participants).
In the Kristjansson 2007 review on disadvantaged school children, compared with children in the control group, height gain was significantly increased in children who received school meals in LMIC (MD 1.43 cm, 95% CI 0.46 to 2.41; 6 CBAs, 986 participants) and HIC (MD 0.92 cm, 95% CI 0.16 to 1.67; 4 CBAs, 703 participants).
Growth z scores
Six of the eight reviews included growth z scores (Grobler 2013; Kristjansson 2007; Kristjansson 2015a; Lazzerini 2013; Ota 2015; Sguassero 2012). Four reviews reported data on weight‐for‐age z scores (WAZ) (Grobler 2013; Kristjansson 2007; Kristjansson 2015a; Sguassero 2012), four on length/height‐for‐age z scores (HAZ) (Kristjansson 2007; Kristjansson 2015a; Lazzerini 2013; Sguassero 2012), five on weight‐for‐height/length z (WHZ) scores (Grobler 2013; Kristjansson 2007; Kristjansson 2015a; Lazzerini 2013; Sguassero 2012), and one on body mass index (BMI) z score (Ota 2015). All comparisons included only one to three studies. See Table 21 for details.
Overall, the systematic reviews found modest improvements in z scores for children under five years of age and school‐aged children, but analyses were mostly underpowered.
Weight‐for‐age z scores
In the Sguassero 2012 review on children younger than five years of age in LMIC, the change in WAZ was higher in the group receiving yoghurt supplementation versus control (MD 0.12, 95% CI 0.05 to 0.19; 1 study, 348 participants; low‐certainty evidence). Similarly, RCTs in the Kristjansson 2015a review of disadvantaged infants and young children showed a significant effect on WAZ (MD 0.15, 95% CI 0.05 to 0.24; 8 RCTs in LMIC, 1565 participants; moderate‐certainty evidence) and change in WAZ (MD 0.02, 95% CI 0.01 to 0.03; 1 RCT in HIC, 103 participants). Further comparisons of CBAs in LMIC showed no significant improvement in WAZ (MD 0.27, 955 CI –0.13 to 0.68; 4 CBAs, 999 participants; very low‐certainty evidence). In the Kristjansson 2007 review on disadvantaged school children, a school breakfast (cheese sandwich or spiced bun and cheese plus milk) versus control demonstrated significant benefit in terms of WAZ (MD 0.07, 95% CI 0.04 to 0.10; 1 RCT in a LMIC, 785 participants). Supplementation with spirulina in children who were HIV positive did not lead to improvements in WAZ scores.
Length/height‐for‐age z scores
RCTs in the Kristjansson 2015a review on disadvantaged infants and young children showed a significant effect on HAZ (MD 0.15, 95% CI 0.06 to 0.24; 9 RCTs in LMIC, 4544 participants; moderate‐certainty evidence) and change in HAZ (MD 0.04, 95% CI 0.04 to 0.05; 1 RCT in HIC, 103 participants). Further comparisons of CBAs in LMIC showed no significant improvement in HAZ (MD 0.01, 95% CI –0.10 to 0.12; 4 CBAs, 999 participants; very low‐certainty evidence). In the Kristjansson 2007 review on disadvantaged school children, balanced school‐feeding interventions (school breakfast: cheese sandwich or spiced bun and cheese plus milk; githeri and meat versus control) demonstrated a small significant effect on HAZ with a z score difference of 0.04 (95% CI 0.02 to 0.06; 2 RCTs in LMIC, 1021 participants). Supplementation in children younger than five years of age and children with MAM did not lead to improvements in HAZ scores.
Weight‐for‐height/length z scores
Lazzerini 2013 found that final WHZ (MD 0.20, 95% CI 0.03 to 0.37; 2 studies, 1546 participants; moderate‐certainty evidence) and WHZ (MD 0.28, 95% CI 0.06 to 0.49; 1 trial, 178 participants) were significantly higher in the MAM group of children (younger than five years of age) receiving food versus those in the standard care group. Supplementary feeding in other groups (disadvantaged infants and young children, children younger than five years of age, school‐aged children and children who were HIV positive) did not lead to improvements in WHZ scores.
BMI z score
In the pregnancy review by Ota 2015, there was a small increase in children's mean BMI z score at 11 to 17 years of age in those receiving supplementary biscuits versus control (MD 0.16, 95% CI 0.01 to 0.31; 1 study, 855 participants). There were no subgroup differences between girls and boys.
Nutritional status of children
Other anthropometry indicators
Four of the included reviews reported on a variety of other anthropometrical indices in children. Two reviews reported on head circumference (Ota 2015; Sguassero 2012); three reviews on mid‐upper arm circumference (MUAC) (Kristjansson 2007; Lazzerini 2013; Sguassero 2012); and one review apiece on triceps and subscapular skinfold thickness (Sguassero 2012), mid‐upper arm muscle area and mid‐upper arm fat area (Kristjansson 2007), and percentage body fat (Ota 2015). Most comparisons included only one small study, except for balanced energy and protein supplementation in the pregnancy review (Ota 2015), which included seven studies in two comparison (five studies in one comparison and two studies in the other). See Table 22 for details.
Across reviews, supplementary feeding appeared to have little impact on the specified anthropometric indices, but estimates were all underpowered (apart from balanced energy and protein supplementation in pregnancy).
The only significant finding was in disadvantaged school children (aged five to 19 years) in the Kristjansson 2007 review, who were given meat and gained significantly more mid‐upper arm muscle area than the children in the control group (MD 68.22 mm2, 95% CI 39.57 to 96.87; 1 RCT in LMIC, 236 participants).
Biochemical parameters
Three systematic reviews provided a narrative description of biochemical parameters in children (Grobler 2013; Kristjansson 2007; Kristjansson 2015a).
A Kenyan study (Neumann 2003) (reported in Kristjansson 2007) assessed various micronutrient status indicators (including haemoglobin, plasma ferritin, serum iron, serum zinc, serum copper, plasma vitamin B12, folate and retinol, and erythrocyte riboflavin). School children receiving meat demonstrated significant increases in plasma vitamin B12 concentrations compared to children in the control group (P < 0.0001) after one year‐long intervention (Neumann 2003). All other findings were insignificant. Tisdall 1951 (reported in Kristjansson 2007) compared 'good attenders', 'poor attenders' and controls on serum ascorbic acid, serum carotene and serum vitamin A and reported "statistically significant differences" favouring children who received a school lunch.
The Kenyan study by Neumann 2003 found no differences in haemoglobin increase between the meat and control groups. In Devadas 1979 (reported in Kristjansson 2007), school children receiving a vegetable protein mixture reportedly had a greater increase in haemoglobin than the control group (significance level not reported). Tisdall 1951 found no significant difference in increase in haemoglobin between 'good attenders', 'poor attenders' and controls (statistics not given), while Paige 1976 found that school children receiving a high protein drink as a mid‐morning snack had a larger increase in percentage haematocrit than the control group (P < 0.001) (both studies reported in Kristjansson 2007).
One study in children with rapidly progressing HIV found no significant differences between groups (whey protein concentrate (WPC) versus maltodextrin placebo) for leukocytes, erythrocytes, haemoglobin and platelets (Moreno 2005).
The Kristjansson 2015a review on disadvantaged infants and young children found that children who were supplemented showed positive change in haemoglobin status compared to controls (standardised mean difference (SMD) 0.49 g/L, 95% CI 0.07 to 0.91; 5 RCTs in LMIC, 300 participants). See Table 23 for details. One CBA in an LMIC reported a significant effect of balanced protein supplementation on the risk of anaemia with those who were supplemented having a lower risk of being anaemic (odds ratio (OR) 0.58, 95% CI 0.24 to 0.75; 110 participants) (Lutter 2008). Similarly, another CBA with 250 participants reported that while the prevalence of anaemia decreased by 27% in the intervention group, it decreased by only 13% in the control group (De Romaña 2000). One RCT with 103 children in an HIC found no significant difference between the experimental and control group as regards change in haemoglobin (Yeung 2000); one CBA with 116 children reported an increase in the number of Aboriginal children who had low haemoglobin levels in the experimental group and a decrease in the corresponding number in the control group (Coyne 1980).
Dietary intake
None of the systematic reviews reported dietary intake in children (or individual trials included in these reviews).
Nutritional status of adults
Anthropometry indicators
The pregnancy, HIV and tuberculosis reviews reported weight outcomes in adults. The pregnancy review reported weekly gestational weight gain and maternal weight postpartum (Ota 2015), while the HIV (Grobler 2013) and tuberculosis (Grobler 2016) reviews reported body weight and weight gain at specific time points. See Table 24 for details.
Supplementary feeding had no effect on weight indices in pregnant women, but produced modest increases in weight gain at specific time points in adults with infectious diseases, although it did not seem to convey longer‐term benefits in people who were HIV positive. Estimates were mostly underpowered (apart from balanced energy and protein supplementation in pregnancy).
In one study among participants not receiving ART in the HIV review, the group receiving fortified blended foods had a significantly greater mean body weight than the no supplement group at three months (MD 2.82 kg, 95% CI 1.02 to 4.62; P = 0.0022, 211 participants) and six months (MD 3.67 kg, 95% CI 1.50 to 5.84; P = 0.001, 157 participants) (Grobler 2013). In the same study among participants receiving ART, the supplement group appeared to gain weight more rapidly than the no supplement group at month one (MD 0.90 kg, 95% CI 0.40 to 1.41; 366 participants) and month three (MD 1.12 kg, 95% CI 0.29 to 1.95; 322 participants), as they had a significantly greater change in body weight gain compared to the no supplement group at these time points. After this time point the change in body weight was not significantly different between the groups. Among participants not receiving ART, the supplement group had a significantly greater body weight gain compared with the no supplement group at month one (MD 0.82 kg, 95% CI 0.28 to 1.36; 261 participants), month three (MD 1.22 kg, 95% CI 0.31 to 2.12; 211 participants) and at six‐month time point (MD 2.06 kg, 95% CI 0.82 to 3.30; 157 participants). After this time point the change in body weight was not significantly different between the groups. Supplementation with ornithine alpha‐ketoglutarate (OKG) and L‐glutamine in adults who were HIV positive did not demonstrate any benefit in terms of weight gain.
In one small trial in the HIV review, after eight weeks of receiving an amino acid mixture (including arginine, glutamine and β‐hydroxy‐β‐methylbutyrate (HMB)), the arginine‐rich group gained significantly more body weight than the control group (MD 2.63 kg, 95% CI 0.72 to 4.54; 43 participants) (Grobler 2013).
In the tuberculosis review, balanced and high‐energy supplementation did seem to improve weight gain at specific time points during treatment (at six weeks: MD 1.73 kg, 95% CI 0.81 to 2.65; 1 study, 34 participants; 12 weeks: MD 2.60 kg, 95% CI 1.74 to 3.46; 1 study, 100 participants; 32 weeks: MD 2.60 kg, 95% CI 0.52 to 4.68; 1 study, 265 participants) (Grobler 2016), although one large study, exclusively in people coinfected with HIV, found no difference at any time point (PrayGod 2011). Review authors concluded that supplementation probably produced a modest increase in weight gain during treatment for active tuberculosis, although this was not seen consistently across all trials (data not pooled; 5 trials, 883 participants, moderate‐certainty evidence) (Grobler 2016).
The HIV review by Grobler 2013 reported a few other anthropometrical indices in adults, including BMI, lean body mass (LBM), fat mass and fat‐free mass. See Table 25 for details.
Studies assessing supplementary feeding appeared to have little impact on LBM, fat mass and fat‐free mass (again estimates were underpowered), with some benefit demonstrated in terms of improvements in BMI in the shorter term.
In one trial among participants receiving ART, mean BMI and change in BMI in the supplement group (receiving fortified blended foods) was significantly higher in the first three months compared to the no supplement group (month one: MD 0.36, 95% CI 0.08 to 0.64; 366 participants; month three: MD 0.43, 95% CI 0.07 to 0.79; 322 participants). After three months, there was no significant difference in BMI or BMI gain between the groups. In the same trial among participants not receiving ART, mean BMI and change in BMI in the supplement group was significantly higher in the first six months compared to the no supplement group (month one: MD 0.39, 95% CI 0.05 to 0.74; 261 participants; month three: MD 0.73, 95% CI 0.31 to 1.15; 211 participants; month six: MD 0.78, 95% CI 0.22 to 1.34; 157 participants). After six months, there was no significant difference in BMI or BMI gain between the groups.
In one small study, after eight weeks of receiving an amino acid mixture (including arginine, glutamine and HMB), the arginine‐rich group of participants had a significantly greater increase in fat‐free mass than the control group (MD 3.25 kg, 95% CI 1.25 to 5.25; 1 study, 43 participants).
Biochemical parameters
One systematic review provided a narrative description of biochemical parameters (Grobler 2013).
In one study, haemoglobin values decreased in both groups (spirulina versus green clay) between baseline and six months, with no difference between spirulina and placebo groups at the end of the study (Yamani 2009). In the Castleman 2011 study, ART participants receiving supplements (fortified blended food (Insta Foundation Plus), WPC, micronutrients, nutrition counselling) had a significantly higher increase in haemoglobin levels at month three compared to the no supplement (nutrition counselling only) group (P = 0.05). Supplemented pre‐ART participants had a significantly higher increase in haemoglobin levels at months three (P = 0.01) and six (P = 0.05) compared to the no supplement group.
There was no difference in changes in serum albumin at three months between the supplement and no supplement groups for either group (Castleman 2011).
Serum protein concentrations increased in both study groups (spirulina versus green clay) in the first three months and then decreased in the following three months. There was a significant increase in serum protein in the spirulina group between baseline and three months (P value not shown) and baseline and six months (P < 0.001). At three (P = 0.01) and six (P < 0.001) months, serum protein concentrations were significantly higher in the spirulina group compared to the placebo group (Yamani 2009).
Serum creatinine levels decreased in both study groups (spirulina versus green clay) at months three and six. At three months, serum creatinine levels were significantly higher in the spirulina group compared to the placebo group (P = 0.01) (Yamani 2009).
Dietary intake
The HIV review reported dietary intake (Grobler 2013), and the review on Alzheimer's disease narratively reported dietary intake (Droogsma 2014). See Table 26 for details.
In the HIV review, compared with no nutritional supplementation or nutrition counselling alone, supplementation with balanced macronutrient formulas significantly improved energy intake in adults with weight loss (MD 393.57 kcal/kg, 95% CI 224.66 to 562.47; 3 studies, 131 participants; low‐certainty evidence) and protein intake (MD 23.35 g/day, 95% CI 12.68 to 34.01; 2 studies, 81 participants; low‐certainty evidence) (Grobler 2013). OKG supplementation had no effect on energy and protein intake in one, very small study.
The one study included in the Lauque 2004 review on Alzheimer's disease found that three months of daily oral nutrition supplements (ONS) improved nutritional outcomes in the intervention group. The nutritional status of the control group also improved after three months (with standard care), although the intervention group improved more than the control group. There were no changes in clinical and biochemical outcomes.
School attendance, cognition tests and educational attainment
Three reviews reported outcomes related to school attendance, cognition and educational attainment (Kristjansson 2007; Kristjansson 2015a; Ota 2015). See Table 27 for details.
While supplementary feeding had no effect on cognition tests in the descendants of pregnant women (Ota 2015), there were some small benefits with regards to cognition (intelligence quotient (IQ)) and educational attainment (maths and spelling, but not reading) in disadvantaged school children (Kristjansson 2007), but again analyses were underpowered. Supplementary feeding did not affect school attendance in one small study (Powel 1983) (reported in Kristjansson 2007).
In the Kristjansson 2007 review, disadvantaged school children who were given school lunches had an end‐of‐study, full‐scale IQ (adjusted ICC = 0.15) that was 3.8 points higher than children who were not given school lunches (MD 3.80, 95% CI 0.51 to 7.10; 1 study, 231 participants). Sensitivity analyses with ICCs at 0.10 and 0.20 were still significant. Similarly, in the same study, the intervention group also had an end‐of‐study performance IQ (adjusted ICC = 0.15) that was 5.74 points higher than children who were not given a school lunch (MD 5.74, 95% CI 1.73 to 9.74; 1 study, 231 participants). Again, sensitivity analyses with ICCs at 0.10 and 0.20 were both significant.
Change in maths achievement (ICC = 0.15) was significantly greater for children who received school meals (lunch and breakfast) in two studies (SMD 0.31, 95% CI 0.09 to 0.53; 337 participants). Change in spelling achievement (ICC = 0.15) was greater for children who received a school breakfast compared to children in the control group (MD 0.24, 95% CI 0.01 to 0.47; 1 study, 106 participants). A sensitivity analysis with an ICC of 0.10 showed much the same results, but the sensitivity analysis with an ICC of 0.20 was not significant.
In the Kristjansson 2015a review on disadvantaged infants and young children in LMIC, cognitive ability improved more in children who were supplemented than in children who had not yet received supplementation (SMD 0.58, 95% CI 0.17 to 0.98; 1 RCT, 99 participants; McKay 1978). A further study in an LMIC measured change on the Bailey Scale of Mental Development (BSMD) and found a non‐significant difference (SMD –0.40, 95% CI –0.79 to –0.00; 1 RCT, 113 participants).
Behavioural outcomes
One systematic review provided a narrative description on behavioural outcomes (Kristjansson 2007).
The review presented the results from one study, which showed that playground activity levels, particularly prosocial activity, were higher for children who received school meals (githeri and meat versus no intervention) (P < 0.001; Neumann 2003). Using evidence from three studies, the review found that school feeding may have had positive effects on classroom behaviour in both HIC and LMIC (Bro 1994; Bro 1996; Chang 1996); however, the review authors concluded that effects may have depended on the quality of the educational attainment (Kristjansson 2007). Finally, the review reported one cluster‐RCT of breakfast clubs in the UK, which found no significant changes in hyperactivity levels after the intervention (Shemilt 2004).
Quality of life
Two systematic reviews provided a narrative description of quality of life (Grobler 2013; Grobler 2016). In the HIV review by Grobler 2013, only one study reported data on quality of life (Castleman 2011), where in the initial stages of the trial supplementary food had a significant beneficial effect on the quality of life of pre‐ART participants in particular. These benefits did not seem to persist over longer periods of follow‐up. In the tuberculosis review by Grobler 2016, two studies with 134 participants reported data on quality of life (Jahnavi 2010; Paton 2004). Review authors concluded that supplementation may have increased quality of life scores during the first two months of treatment (low‐certainty evidence).
Adverse events
Four reviews reported on adverse events (Grobler 2013; Kristjansson 2015a; Ota 2015; Sguassero 2012), and included serious adverse events (small‐for‐gestational age (SGA), preterm birth and pre‐eclampsia) in the pregnancy review (Ota 2015), and milder adverse events and discomfort (including diarrhoea, vomiting and general gastrointestinal adverse events) in the three remaining reviews (Grobler 2013; Kristjansson 2015a; Sguassero 2012). See Table 28 for details. Additionally, one review described substitution or leakage (where the family cut home rations for the child who has been fed in order to spread food to other family members, or shared the child's supplementary rations with other family members) as an adverse event (Kristjansson 2015a).
Data on adverse events were generally limited or lacking. One exception was the pregnancy review (Ota 2015). It reported the incidence of SGA birth as significantly reduced in women given balanced energy and protein supplementation (RR 0.79, 95% CI 0.69 to 0.90; 7 studies, 4408 participants; moderate‐certainty evidence). In contrast, high protein supplementation was associated with a significantly increased risk of SGA babies (RR 1.58, 95% CI 1.03 to 2.41; 1 study, 505 participants; moderate‐certainty evidence). Balanced energy and protein supplementation and high protein supplementation had no effect on risk of preterm birth (balanced: moderate‐certainty evidence; high protein: low‐certainty evidence) and pre‐eclampsia (very low‐certainty evidence).
The provision of a multi‐mixture to children younger than five years of age in one small study had no effect on incidence of diarrhoea and vomiting (Sguassero 2012). In one small study, participants who were HIV positive and receiving OKG reported significantly more gastrointestinal adverse events than participants in the placebo group (RR 1.59, 95% CI 1.06 to 2.39; 46 participants) (Grobler 2013).
One review described substitution or leakage by calculating the net benefit from supplementary feeding (Kristjansson 2015a). This review included seven studies that provided home‐delivered rations (RCTs: Bhandari 2001; De Romaña 2000; Grantham‐McGregor 1991; Rivera 2004; CBAs: Lutter 2008; Santos 2005; Tomedi 2012), and three day‐care and feeding centre studies (RCTs: Husaini 1991; Pollitt 2000; CBA: Devadas 1971). It found differences in the number of calories provided by the supplementary food and the number of extra calories that the children had actually consumed in addition to their regular food. In the take‐home studies, the net benefit to children was only 36% of the extra calories provided by the supplement, while in the day‐care and feeding centres the net benefit was 85% of the extra calories provided by the supplement.
None of the included reviews reported on the following outcomes: adherence to treatment or attendance at clinic; costs to the provider and out‐of‐pocket costs to people receiving supplementary feeding.
Discussion
According to FAO estimates, the prevalence of undernourishment remains high, despite adequate food supplies and considerable progress in reducing hunger in some regions. More than 795 million people still present with chronically inadequate levels of dietary energy intake (FAO 2015; Sundaram 2015), with women and children being particularly vulnerable. In 2016, the prevalence of stunting (22.9%) and wasting (7.7%) remained high in children under five years of age globally (UNICEF/WHO/World Bank Group 2017), and 108 million people were reported to be facing crisis‐level food insecurity or worse (FSIN 2017). Supplementary feeding is thought to be beneficial in food insecure and vulnerable groups by optimising the nutritional value and adequacy of the diet, improving quality of life and improving various health parameters of disadvantaged families. In LMIC, the problems that supplementary feeding aims to address are entangled with poverty and deprivation, necessitating a multi‐dimensional and integrated approach. In addition, appropriate sanitation facilities and safe drinking water are essential components to ensure the effective impact of supplementary feeding. Other relevant contextual factors include availability of basic health services and medical care, nutrition education, and parenteral knowledge and care (WHO 1999). This overview undertook to describe and explore the effects of supplementary feeding, specifically across an array of vulnerable groups.
Summary of main results
The eight systematic reviews (search dates between May 2006 and February 2016) evaluated interventions in pregnant women, children under five years from LMIC, disadvantaged infants and young children (three months to five years), children with MAM, disadvantaged school children, HIV‐positive adults and children, adults and children with active tuberculosis (with or without HIV), and older people with Alzheimer's disease. These reviews included 95 trials relevant to this overview, with the majority of participants from LMIC. Trials included a vast array of different nutritional interventions of varying duration, frequency and format, with micronutrients often reported as cointerventions in various trials. Follow‐up ranged from six weeks to two years in the reviews, with three trials investigating outcomes at four to 17 years of age.
Pregnant women: there is a suggestion that supplementing pregnant women may have an effect on stillbirth, infant birth weight and risk of infants born SGA (all moderate‐certainty evidence). Although clinically small, supplementation significantly increased birth length. These demonstrated effects did not translate into long‐term benefits for the child in terms of growth and neurocognitive development in the one or two trials that reported on longer term outcomes. High‐protein supplementation was associated with harm (increased risk of SGA babies) (moderate‐certainty evidence), indicating that there is currently no justification to prescribe these supplements to pregnant women (considering that this finding is based on results reported in one trial).
Children: in children under five years of age from LMIC, supplementary feeding had a negligible impact on child growth, with authors warning of interpreting pooled results with caution due to the presence of clinical heterogeneity. However, a review published on 2015 found that children who received food supplementation gained a mean of 0.12 kg more in weight and 0.27 cm more in height than children who were not supplemented (moderate‐certainty evidence). In children under five years of age with MAM, the provision of specially formulated food significantly improved weight (low‐certainty evidence), WHZ scores (moderate‐certainty evidence) and other key outcomes such as recovery rate (by 29%) (moderate‐certainty evidence) and a decrease in the number of participants dropping out (by 70%) (moderate‐certainty evidence). Other comparisons in this review compared types of specially formulated foods with each other, with authors concluding that both LNS and blended foods (such as CSB++ (corn‐soy blended foods)) are effective in treating children with MAM (moderate‐ to high‐certainty evidence). In children school meals seemed to lead to a number of small benefits, including improvements in weight (especially lower‐income countries), height, WAZ scores, HAZ scores, mid‐upper arm muscle area (reported in one small study), cognition tests (in LMIC), maths and spelling performance (in LMIC), and behaviour (described narratively).
Infectious diseases: supplementary feeding in HIV‐positive adults increased daily energy and protein intake when compared to nutritional counselling alone (low‐certainty evidence). Supplementation led to an initial improvement in weight gain/BMI, but did not seem to confer long‐term benefit. No firm conclusions could be drawn regarding supplementation in children diagnosed with HIV. In adults with tuberculosis, one small trial found a significant benefit on treatment completion and sputum conversion rate (very low‐certainty evidence). There were significant but modest benefits in terms of weight gain during active tuberculosis (moderate‐certainty evidence). Supplementation may have increased quality of life scores during the first two months of tuberculosis treatment (low‐certainty evidence; narratively described).
Alzheimer's disease: the one study included in the Alzheimer's disease review found that three months of daily ONS significantly improved nutritional outcomes in the intervention group. There were no significant changes on the clinical and biochemical outcomes.
None of the systematic reviews reported outcomes related to costs.
Overall: mortality data indicated an effect of supplementation on stillbirth when given to pregnant women; there were data from trials in supplementation for children with MAM, HIV‐infected children and adults with tuberculosis, but these studies were few and small. There were mixed effects on weight and weight gain in children. In children under five years of age from LMIC, one review found that supplementary feeding had a negligible impact on child growth, however a more recent review found that children who received food supplementation gained a mean of 0.12 kg more in weight and 0.27 cm more in height than children who were not supplemented. Supplementary food was generally more effective for younger children (less than two years of age) and for those who were poorer/less well‐nourished. In children with MAM, there were modest benefits. Pregnancy supplementation did not provide evidence of long‐term effects; and in school children, there were bigger apparent effects, but follow‐up was 24 months in the study with the longest duration. Length/height gain in children showed a similar pattern. There were modest improvements in z scores for children under five years of age and school children, but mostly analyses were underpowered. There were some small benefits regarding cognition (IQ) and educational attainment (maths and spelling) in children, but again analyses were underpowered. In adults with infectious diseases supplementary feeding did not seem to convey longer‐term benefits in terms of weight gain and other anthropometric indices. In disadvantaged school children under five years of age, substitution or leakage was a substantial problem when feeding was given at home (as opposed to when food was given in day‐care/feeding centres).
We found good coverage of vulnerable groups by systematic reviews and fewer than expected trials per review (the latter should be considered in the context of probable under‐reporting, as many international agencies and NGOs (non‐profit organisations) probably have unpublished data that might have been relevant to the individual systematic reviews, and thus also this overview). Furthermore, we found limited evidence overall of an effect on mortality (also considering that we excluded children with SAM from this overview) or nutritional status or school performance.
Overall completeness and applicability of evidence
This overview summarised published systematic reviews of supplementary feeding in vulnerable groups. Although we intended to include older people as a vulnerable group a priori (Visser 2013), we included just one review (with one trial) investigating community‐dwelling older participants with Alzheimer's disease. We consider the overview to be complete, although we also acknowledge that not all systematic reviews included in this overview were up to date. While we found evidence within each of our outcome categories (except for costs), there was often only a small number of reviews and studies within any one category. Some of the reviews did not consider socioeconomic status or nutritional status at baseline in their inclusion criteria or analyses, or did not report this clearly, which could have had an impact on some findings. Many individual studies did not report clinical and other important endpoints (e.g. mortality, quality‐of‐life aspects and cost‐effectiveness), and for many interventions in the various reviews there were too few data to reach a firm conclusion.
Policymakers and programme implementers can use the information summarised in this overview to obtain a 'bird's eye view' of effects demonstrated (and the certainty of the evidence supporting these effects) of supplementary feeding across reviews and groups (including pregnant women, infants, children, adolescents, people living with HIV/AIDS or tuberculosis or both, and older people with Alzheimer's disease), with a view to inform future programme designs and assist with evaluations of supplementary feeding programmes. We also believe this overview highlights areas that need further investigation.
Certainty of the evidence
We included eight systematic reviews (with 95 relevant trials) of supplementary feeding in vulnerable groups in this overview, with the number of relevant included participants across reviews varying between 91 and 7940 adults, and 271 to more than 12,595 children. All systematic reviews included were rated as high quality (with AMSTAR scores between eight and 11). In one review, authors did not formally assess publication bias (Grobler 2013).
The certainty of the evidence reported by the primary studies in the included reviews ranged from very low to moderate for individual comparisons, with the evidence often comprising one or two small trials. We used the GRADEpro 'Summary of findings' tables from each review (if reported in the individual reviews for the main comparisons). Six of the eight reviews included such tables.
There was moderate‐certainty evidence was reported for balanced protein energy supplementation in relation to reduction in the stillbirth, increase in infant birth weight and reduction in the risk of infants born SGA in the pregnancy review. In addition, the evidence linking high protein supplementation to increased risk of SGA babies was of moderate certainty. There was moderate‐certainty evidence for growth outcomes in disadvantaged infants and young children in LMIC. Furthermore, there was moderate‐certainty evidence for the provision of specially formulated food in relation to WHZ scores, recovery rate and defaulting in the MAM review. The benefits demonstrated in terms of weight gain in the tuberculosis review were also considered to be of moderate certainty. All other significant findings were considered to be of low‐ or very low‐certainty evidence as assessed in the individual reviews.
Potential biases in the overview process
There were potential biases in the overview process. Since our data extraction was limited to systematic reviews and not the original studies, it is possible that we may not have captured all minor outcomes. One overview author and one overview reviewer independently applied eligibility criteria, assessed the studies for inclusion, extracted data and assessed the scientific quality of reviews (using the AMSTAR tool), which should have reduced the risk of bias in the overview process. An overlap in included studies between systematic reviews should be noted and considered, since five RCTs appeared in both Kristjansson 2015a and Sguassero 2012, which could potentially influence the overall interpretation of outcomes related to weight and length/height in children.
Agreements and disagreements with other studies or reviews
We are not aware of any other published overviews of reviews of supplementary feeding in vulnerable groups.
Authors' conclusions
We have observed modest benefits (mostly in terms of anthropometric parameters) for some outcomes across studies, with a variety of supplementary feeding interventions. Mortality evidence is limited, with some evidence in newborns that supplementing the mother reduces the risk of stillbirth. The certainty of the evidence overall was moderate to very low (often including one or two small trials). Reviews mostly reported on short‐term outcomes, with very few trials investigating crucial long‐term outcomes. Some important outcomes were scarcely (e.g. quality of life) or not reported (e.g. cost aspects). The findings reinforce the multi‐dimensional nature of food insecurity and malnutrition (Figure 1), with supplementary feeding programmes again clear to be but one approach to address these complex issues. An integrated approach seems crucial, with investigations on how best to combine supplementary feeding programmes with other interventions to achieve the desired nutrition and health outcomes. Ultimately it remains unrealistic to expect that a single intervention will be the ultimate solution.
The vast array of nutritional interventions of varying duration, frequency and format in the various systematic reviews and trials make conclusions regarding specific supplementary foods or practices almost impossible. It is clear that aspects such as non‐compliance and dietary substitution/leakage, the amount of energy and specific nutrients provided by the supplement/meal/snack as well as the timing thereof, and the provision of additional micronutrients that in itself could impact on the outcomes, are key factors related to outcome. Place of administration (e.g. 'on‐site'/feeding centres versus at home) also has an impact on supplementation reaching those it is intended for.
Critical aspects related to the outcomes or benefits (or lack thereof) demonstrated across studies, probably include a combination of factors, including programme design, aspects related to the intervention (see above), the participant inclusion criteria (such as baseline nutritional status or vulnerability), the social environment at home, aspects of sanitation and access to clean water. Given the political economy of supplementary feeding there will continue to be investment in these programmes in the foreseeable future. Paying more attention to supplementary feeding programme design, being specific about the 'conditions' that they address, and experimenting with different combinations of interventions seems crucially important.
Implications for practice.
This overview has demonstrated that supplementary feeding programmes across vulnerable groups are probably not performing as expected. Aspects to consider when designing, planning and prioritising health and nutrition interventions include investigating the causes of malnutrition, identifying the relevant target populations (those that could potentially benefit most), as well as the best combinations of interventions to address the complex and multi‐dimensional nature of food insecurity and malnutrition. In addition, strong political commitment (including placing food security and nutrition at the top of the political agenda and creating an enabling environment) is essential for hunger reduction and addressing malnutrition, the latter which requires an integrated approach, including specific nutrition programmes. Proper programme implementation, evaluation and monitoring are key components for success.
Considerations for programme development include the following.
Target people who are undernourished and vulnerable.
Minimise leakage or substitution by considering place of administration (onsite versus at home) and supervision.
Provide sufficient energy and nutrients (some studies suggest at least 30% of the dietary reference intakes).
Start early (in infants and children; supplementation earlier in life may optimise benefit).
Start with the end in mind (pilot a combination of potentially relevant interventions, assess outcomes in specific contexts, scale the intervention/principles/models that work, rather than specific interventions).
Involve stakeholders in the design and piloting of interventions and build capacity for programme implementation, including education and nutritional counselling.
Ongoing monitoring and evaluation (including relevant and important outcomes and factors impacting programme success).
Implications for research.
It is clear from our findings that many of the key outcomes are rarely reported, and high‐quality randomised trials addressing relevant outcomes (rather than specific, narrowly defined 'interventions') may be needed, focusing on those that are nutritionally deprived or malnourished in order to possibly convey the most benefit. This overview does not call for more of the same research, but rather research with a focus on relevant and understudied outcomes, with follow‐up over longer periods of time, and investigating a combination of interventions that are needed to address such complex issues as malnutrition and food insecurity.
Better project conceptualisation, design and combinations of interventions (and being much more alert to specific contexts) seem critically important. Well‐conducted research, including multi‐sectoral efforts to address nutrition challenges, is essential for the optimal allocation of resources and scaling‐up of public healthcare interventions. In the meantime, disadvantaged and vulnerable families and children cannot wait indefinitely for future trials and should have access both to appropriate healthcare and sanitation, as well as adequate amounts of nutritious food. Researchers should work with programme implementers (governments, UN and other international agencies, etc.), to build robust evaluation and learning components into existing and planned programs (including RCTs, where appropriate). Considering the complexity of the interventions, component network meta‐analysis could be considered in future systematic reviews.
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2018 | Amended | Amendments to Acknowledgements, Declarations of interests, and Sources of support sections. |
History
Protocol first published: Issue 6, 2013 Review first published: Issue 11, 2018
| Date | Event | Description |
|---|---|---|
| 2 November 2018 | Amended | Remarked for publication for immediate open access. |
Acknowledgements
JV was supported by the Effective Health Care Research Consortium; this was in the form of grants from the Consortium. PG was also supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
We thank Liesl Nicol (LN), Nicholas Henschke (NH), and Rachel Wheeler (RW) for their assistance with review selection, assessment of methodological quality of included reviews, and data extraction.
We also thank Alfred Musekiwa and Nicholas Henschke for statistical assistance (graphical display of selected results).
This overview of reviews has been produced within Cochrane Developmental, Psychosocial and Learning Problems.
Appendices
Appendix 1. Search strategies
Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library
#1 MeSH descriptor: [Dietary Supplements] this term only #2 MeSH descriptor: [Dietary Proteins] this term only #3 MeSH descriptor: [Dietary Fats] 1 tree(s) exploded #4 MeSH descriptor: [Energy Intake] this term only #5 MeSH descriptor: [Nutrition Therapy] explode all trees #6 Any MeSH descriptor with qualifier(s): [Diet therapy ‐ DH] #7 ((diet* or food* or feed or feeding or meal* or nutrition* or nutrient*) near/3 (additional or extra or supplement*)):ti,ab #8 MeSH descriptor: [Food, Fortified] this term only #9 MeSH descriptor: [Foods, Specialized] this term only #10 MeSH descriptor: [Functional Food] this term only #11 ((fortif* or special* or functional* or formulat*) near/3 food*):ti,ab #12 (therapeutic* near/3 (diet* or food* or feeding)):ti,ab #13 ((ready next to next use near/3 food*) or RUTF* or RTUF* or RUF*):ti,ab #14 MeSH descriptor: [Food, Formulated] this term only #15 ((food or feeding) near/3 (intervention* or program*)) #16 (food next secur* or food next insecur* or food next in‐secur*):ti,ab #17 ((nutrient* or nutrition*) near/3 (intervention* or program*)):ti,ab #18 MeSH descriptor: [Dietary Carbohydrates] this term only #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
MEDLINE Ovid
1 Dietary Supplements/ 2 dietary proteins/ 3 dietary carbohydrates/ 4 exp dietary fats/ 5 energy intake/ 6 nutrition therapy/ 7 Diet Therapy/ 8 diet therapy.fs. 9 ((diet$ or food$ or feed or feeding or meal$ or nutrition$ or nutrient$) adj3 (additional or extra or supplement$)).tw. 10 exp Food, Fortified/ 11 (therapeutic$ adj3 (food$ or feeding)).tw. 12 (therapeutic$ adj3 diet$).tw. 13 ((ready‐ to‐use adj3 food$) or RUTF$1 or RTUF$1 or RUF$1).tw. 14 ((food or feeding) adj3 (intervention$ or program$)).tw. 15 (food secur$ or food insecur$ or food in‐secur$).tw. 16 ((nutrient$ or nutrition$) adj3 (intervention$ or program$)).tw. 17 or/1‐16 18 meta‐analysis/ 19 meta‐analysis as topic/ 20 meta analy$.tw. 21 metaanaly$.tw. 22 (systematic adj (review$1 or overview$1)).tw. 23 exp Review Literature as Topic/ 24 or/18‐23 25 cochrane.ab. 26 embase.ab. 27 (psychlit or psyclit).ab. 28 (psychinfo or psycinfo).ab. 29 (cinahl or cinhal).ab. 30 science citation index.ab. 31 or/25‐30 32 hand‐search.ab. 33 manual search.ab. 34 relevant journals.ab. 35 reference list$.ab. 36 bibliograph$.ab. 37 or/32‐36 38 (selection criteria or data extraction).ab. 39 "Review"/ 40 38 and 39 41 randomized controlled trials as topic/ 42 24 or 31 or 37 or 40 or 41 43 comment/ 44 letter/ 45 editorial/ 46 animal/ 47 human/ 48 46 not (46 and 47) 49 or/43‐45,48 50 42 not 49 51 17 and 50
MEDLINE In‐Process and Other In‐Process Citations Ovid
1. (nutriti* adj (advice or assisted or enrich* or intervention* or program* or support)).ti,ab,kf,hw. 2. ((fortif* or supplement*) adj3 (protein or carbohydrate or fat or energy or calorie*)).ti,ab,kf. 3. ((fortif* or formula or formulated or supplement* or therapeutic*) adj (diet* or feed* or fed or food* or meal? or nutriti* or milk)).ti,ab,kf. 4. ((fortif* or supplement*) adj (calcium or phosph* or iron or iodine or magnesium or zinc or vitamin* or mineral*)).ti,ab,kf,hw. 5. or/1‐4
MEDLINE EPub Ahead of Print Ovid
1. (nutriti* adj (advice or assisted or enrich* or intervention* or program* or support)).ti,ab,kf,hw. 2. ((fortif* or supplement*) adj3 (protein or carbohydrate or fat or energy or calorie*)).ti,ab,kf. 3. ((fortif* or formula or formulated or supplement* or therapeutic*) adj (diet* or feed* or fed or food* or meal? or nutriti* or milk)).ti,ab,kf. 4. ((fortif* or supplement*) adj (calcium or phosph* or iron or iodine or magnesium or zinc or vitamin* or mineral*)).ti,ab,kf,hw. 5. or/1‐4
Embase Ovid
1 diet supplementation/ 2 diet therapy/ 3 carbohydrate diet/ 4 fat intake/ 5 protein intake/ 6 caloric intake/ 7 dietary intake/ 8 food availability/ 9 nutrition/ 10 ((diet$ or food$ or feed or feeding or meal$ or nutrition$ or nutrient$) adj3 (additional or extra or supplement$)).tw. 11 (therapeutic$ adj3 (food$ or feeding)).tw. 12 (therapeutic$ adj3 diet$).tw. 13 ((ready‐ to‐use adj3 food$) or RUTF$1 or RTUF$1 or RUF$1).tw. 14 ((food or feeding) adj3 (intervention$ or program$)).tw. 15 (food secur$ or food insecur$ or food in‐secur$).tw. 16 ((nutrient$ or nutrition$) adj3 (intervention$ or program$)).tw. 17 ((fortif* or special$ or functional$ or formulat$) adj3 food$).tw. 18 or/1‐17 19 "systematic review"/ 20 "systematic review (topic)"/ 21 (systematic adj (review$1 or overview$1)).tw. 22 meta analysis/ 23 meta analy$.tw. 24 metaanaly$.tw. 25 cochrane.ab. 26 embase.ab. 27 (psychlit or psyclit).ab. 28 (psychinfo or psycinfo).ab. 29 (cinahl or cinhal).ab. 30 science citation index.ab. 31 web of science.ab. 32 manual search$.ab. 33 (hand‐search$ or handsearch$).ab. 34 relevant journals.ab. 35 reference list$.ab. 36 bibliograph$.ab. 37 or/19‐36 38 "randomized controlled trial (topic)"/ 39 review/ 40 (selection criteria or data extraction).ab. 41 39 and 40 42 37 or 38 or 41 43 letter.pt. 44 editorial.pt. (431095) 45 note.pt. (562132) 46 or/43‐45 (1814268) 47 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 48 human/ or human cell/ 49 47 and 48 50 47 not 49 51 46 or 50 52 18 and 42 53 52 not 51
Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library
#1 MeSH descriptor: [Dietary Supplements] this term only #2 MeSH descriptor: [Dietary Proteins] this term only #3 MeSH descriptor: [Dietary Fats] 1 tree(s) exploded #4 MeSH descriptor: [Energy Intake] this term only #5 MeSH descriptor: [Nutrition Therapy] explode all trees #6 Any MeSH descriptor with qualifier(s): [Diet therapy ‐ DH] #7 ((diet* or food* or feed or feeding or meal* or nutrition* or nutrient*) near/3 (additional or extra or supplement*)):ti,ab #8 MeSH descriptor: [Food, Fortified] this term only #9 MeSH descriptor: [Foods, Specialized] this term only #10 MeSH descriptor: [Functional Food] this term only #11 ((fortif* or special* or functional* or formulat*) near/3 food*):ti,ab #12 (therapeutic* near/3 (diet* or food* or feeding)):ti,ab #13 ((ready next to next use near/3 food*) or RUTF* or RTUF* or RUF*):ti,ab #14 MeSH descriptor: [Food, Formulated] this term only #15 ((food or feeding) near/3 (intervention* or program*)) #16 (food next secur* or food next insecur* or food next in‐secur*):ti,ab #17 ((nutrient* or nutrition*) near/3 (intervention* or program*)):ti,ab #18 MeSH descriptor: [Dietary Carbohydrates] this term only #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
Health Technology Assessment Database (HTA), part of the Cochrane Library
#1 MeSH descriptor: [Dietary Supplements] this term only #2 MeSH descriptor: [Dietary Proteins] this term only #3 MeSH descriptor: [Dietary Fats] 1 tree(s) exploded #4 MeSH descriptor: [Energy Intake] this term only #5 MeSH descriptor: [Nutrition Therapy] explode all trees #6 Any MeSH descriptor with qualifier(s): [Diet therapy ‐ DH] #7 ((diet* or food* or feed or feeding or meal* or nutrition* or nutrient*) near/3 (additional or extra or supplement*)):ti,ab #8 MeSH descriptor: [Food, Fortified] this term only #9 MeSH descriptor: [Foods, Specialized] this term only #10 MeSH descriptor: [Functional Food] this term only #11 ((fortif* or special* or functional* or formulat*) near/3 food*):ti,ab #12 (therapeutic* near/3 (diet* or food* or feeding)):ti,ab #13 ((ready next to next use near/3 food*) or RUTF* or RTUF* or RUF*):ti,ab #14 MeSH descriptor: [Food, Formulated] this term only #15 ((food or feeding) near/3 (intervention* or program*)) #16 (food next secur* or food next insecur* or food next in‐secur*):ti,ab #17 ((nutrient* or nutrition*) near/3 (intervention* or program*)):ti,ab #18 MeSH descriptor: [Dietary Carbohydrates] this term only #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
Campbell Collaboration Online Library of Systematic Reviews (www.campbellcollaboration.org/library.html)
Individual searches were run for each of the following search terms:
TITLE: (food* or feed* or nutrition* or malnutrition or malnourish* or undernourish* or diet* or nutrient* or meal*)
OR
KEYWORD: (food or foods or feeding or nutrition or nutritional or nutrients
Virtual Health Library (bvsalud.org/en/)
tw:((((diet* OR food* OR feed OR feeding OR meal* OR nutrient* OR nutrition* ) AND (additional OR extra OR supplement* OR fortif* OR special* OR functional* OR formulat* OR intervention* OR program* OR therap*)) OR (food secur* OR food insecur* OR rutf* OR rtuf* OR ruf* ))) AND (instance:"regional") AND ( db:("LILACS" OR "WHOLIS" OR "IBECS" OR "REPIDISCA" OR "PAHO" OR "MedCarib" OR "LIS" OR "CUMED" OR "SES‐SP" OR "BDENF") AND type_of_study:("systematic_reviews" OR "health_economic_evaluation" OR "health_technology_assessment" OR "evidence_synthesis"))
Database of Promoting Health Effectiveness Reviews (DoPHER; eppi.ioe.ac.uk/webdatabases/Search.aspx)
1. Freetext: “supplementary food*” 2. TI:”supplement*” 3. TI: feeding 4. TI: “nutrition*” 5. TI: “nutrient*” ) 6. Freetext: malnourished 7. Freetext: undernourished or “under nourished” 8. TI: “meal*” 9. TI: (”diet*” and “intervention*”) 10. Freetext: “fortifi*” 11. Freetext: RUTF 12. Freetext: RTUF 13. Freetext: RUF 14. or/1‐13,
3ie Database of Systematic Reviews (www.3ieimpact.org/en/evidence/systematic‐reviews/)
The search was limited to systematic reviews. The following search terms were input individually. Relevant records were selected before being added to the EndNote library.
Food ; Feeding ; Nutrition ; Meals ; Malnourished ; Undernourished ; RUTF; RTUF; RUF
PROSPERO (www.crd.york.ac.uk/prospero/search.asp)
The following search strings were combined using OR.
1. ( (diet* OR food* OR feed OR feeding OR meal* OR nutrient* OR nutrition*) AND (additional OR advice OR enrich* OR extra OR supplement* OR fortif* OR special* OR functional* OR formulat* OR intervention* OR program* OR support* OR therap*)):TI 2. ((fortif* or formula or formulated or supplement* or therapeutic*) and (diet* or feed* or fed or food* or meal? or nutriti* or milk)):TI 3. ((fortif* or supplement*) adj (calcium or phosph* or iron or iodine or magnesium or zinc or vitamin* or mineral*)):TI ((supplement* or fortifi*) and (diet* or food* or meal*)):TI 4. (food secur* or food insecure* or food in‐secur*):TI 5. (malnourish* or under nourish* or undernourish*):TI, 6. INTERVENTION = ((supplement* or fortifi*) and (diet* or food* or meal*)) 7. MeSH DESCRIPTOR NUTRITIONAL SUPPORT 8. MeSH DESCRIPTOR DIETARY SUPPLEMENTS 9. MeSH DESCRIPTOR NUTRITION THERAPY 10. MeSH DESCRIPTOR DIET THERAPY 11. MeSH DESCRIPTOR FOOD, FORTIFIED 12. OR/1‐11
Appendix 2. Protocols for future assessment and possible inclusion in this review
| Reference | Protocol title |
| Ashman 2014 | The effectiveness of nutrition interventions for pregnant indigenous women: a systematic review |
| Burns 2010 | Community level interventions to improve food security in developed countries |
| Durao 2015 | Community‐level interventions for improving access to food in low‐ and middle‐income countries |
| Goudet 2015 | Nutritional interventions for preventing stunting in children (0 to 5 years) living in urban slums in low and middle‐income countries (LMIC) |
| Grobler 2014 | Effects of oral supplementary foods or nutrition counselling or both on infants and children (6 –59m) with severe or moderate under nutrition on linear growth, becoming overweight or obese, developing risk factors for cardiovascular disease or diabetes mellitus, and developing cardiovascular disease or diabetes mellitus later in life |
| Gwynn 2015 | Dietary interventions to improve nutritional status and health outcomes in Aboriginal and Torres Strait Islander peoples in Australia: a systematic review |
| Oluwaniyi 2015 | Impact of lipid based nutrient supplements for prevention of childhood malnutrition: a systematic review |
| Soboka 2015 | The effectiveness of counselling, material support and/or nutritional supplementation on improving adherence to anti‐retroviral therapy and clinical outcomes among HIV patients: a systematic review of quantitative evidence protocol |
| Thorley 2015 | Interventions for preventing or treating malnutrition in problem drinkers who are homeless or vulnerably housed: protocol for a systematic review |
Appendix 3. Reviews awaiting assessment
| Reference | Review title |
| D'Souza 2005 | The effectiveness of food support programmes for low‐income women in developed country settings: a systematic review of the evidence |
| Gera 2016 | Lipid‐based nutrient supplements for the treatment of 6‐ to 59‐month‐old children with moderate acute malnutrition (Only protocol available at the time of the last search; subsequently published as Gera 2017) |
| Milne 2005 | Oral protein and energy supplementation in older people: a systematic review of randomised trials |
Contributions of authors
JV, MHMcL, PG: conception and design of the protocol, writing of the overview.
NM: screening, data extraction and writing of the overview.
PG: is the guarantor of the overview.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
JV was supported by the Effective Health Care Research Consortium; this support was in the form of grants from the Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of LMIC (Grant: 5242).
MHMcL was previously hired as a Consultant by Synergos Institute to provide technical advice and facilitation services to a private sector/government partnership working on extending a CMAM (Community‐based Management of Acute Malnutrition) programme in Nigeria.
NM previously worked for Enhanced Reviews Ltd., a company that conducts systematic reviews, mostly for the public sector. NM was employed by Cochrane Response, an evidence services unit operated by Cochrane. Cochrane Response was contracted by the Effective Health Care Research Consortium (Grant: 5242) to work on this review.
PG is the Director of the Effective Health Care Research Consortium, a Department for International Development (DFID) UK funded research programme to support people carrying out Cochrane Reviews for the benefit of the poor in LMIC (Grant: 5242). DFID had no part in writing this review. PG is the Co‐ordinating Editor of the Cochrane Infectious Diseases Group.
Disclaimer: the views expressed in this publication are those of the authors and do not necessarily reflect UK government policy.
New
References
References to included reviews
Droogsma 2014
- Droogsma E, Asselt D, Steijn J, Veeger N, Dusseldorp I, Deyn PP. Nutritional interventions in community‐dwelling Alzheimer patients with (risk of) undernutrition: a systematic review. International Psychogeriatrics 2014;26(9):1445‐53. [DOI: 10.1017/S1041610214000817; PUBMED: 24846712] [DOI] [PubMed] [Google Scholar]
Grobler 2013
- Grobler L, Siegfried N, Visser ME, Mahlungulu SS, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004536.pub3; PUBMED: 23450554] [DOI] [PubMed] [Google Scholar]
Grobler 2016
- Grobler L, Nagpal S, Sudarsanam TD, Sinclair D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD006086.pub4; PMC4981643; PUBMED: 27355911] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristjansson 2007
- Kristjansson B, Petticrew M, MacDonald B, Krasevec J, Janzen L, Greenhalgh T, et al. School feeding for improving the physical and psychosocial health of disadvantaged students. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004676.pub2] [DOI] [PubMed] [Google Scholar]
Kristjansson 2015a
- Kristjansson E, Francis DK, Liberato S, Benkhalti JM, Welch V, Batal M, et al. Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009924.pub2; PUBMED: 25739460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazzerini 2013
- Lazzerini M, Rubert L, Pani P. Specially formulated foods for treating children with moderate acute malnutrition in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009584.pub2; PUBMED: 23794237] [DOI] [PubMed] [Google Scholar]
Ota 2015
- Ota E, Hori H, Mori R, Tobe‐Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000032.pub3; PUBMED: 26031211 ] [DOI] [PubMed] [Google Scholar]
Sguassero 2012
- Sguassero Y, Onis M, Bonotti AM, Carroli G. Community‐based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005039.pub3; PUBMED: 22696347] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Abdelhamid 2016
- Abdelhamid A, Bunn DK, Copley M, Cowap V, Dickinson A, Gray L, et al. Effectiveness of interventions to directly support food and drink intake in people with dementia: systematic review and meta‐analysis. BMC Geriatrics 2016;16:26. [DOI: 10.1186/s12877-016-0196-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2013
- Allen VJ, Methven L, Gosney MA. Use of nutritional complete supplements in older adults with dementia: systematic review and meta‐analysis of clinical outcomes. Clinical Nutrition 2013;32(6):950‐7. [DOI: 10.1016/j.clnu.2013.03.015; PUBMED: 23591150] [DOI] [PubMed] [Google Scholar]
Arthur 2015
- Arthur SS, Nyide B, Soura AB, Kahn K, Weston M, Sankoh O. Tackling malnutrition: a systematic review of 15‐year research evidence from INDEPTH health and demographic surveillance systems. Global Health Action 2015;8:28298. [DOI: 10.3402/gha.v8.28298; PMC4627942; PUBMED: 26519130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 2016
- Baldwin C, Kimber KL, Gibbs M, Weekes CE. Supportive interventions for enhancing dietary intake in malnourished or nutritionally at‐risk adults. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD009840.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bally 2016
- Bally MR, Blaser Yildirim PZ, Bounoure L, Gloy VL, Mueller B, Briel M, et al. Nutritional support and outcomes in malnourished medical inpatients: a systematic review and meta‐analysis. JAMA Internal Medicine 2016;176(1):43‐53. [DOI: 10.1001/jamainternmed.2015.6587; PUBMED: 26720894] [DOI] [PubMed] [Google Scholar]
Bandayrel 2011
- Bandayrel K, Wong S. Systematic literature review of randomized control trials assessing the effectiveness of nutrition interventions in community‐dwelling older adults. Journal of Nutrition Education and Behavior 2011;43(4):251‐62. [DOI: 10.1016/j.jneb.2010.01.004; PUBMED: 21371944] [DOI] [PubMed] [Google Scholar]
Beck 2011
- Beck AM, Wijnhoven HA, Lassen KO. A review of the effect of oral nutritional interventions on both weight change and functional outcomes in older nursing home residents. E‐SPEN, the European E‐journal of Clinical Nutrition and Metabolism 2011; Vol. 6, issue 3:e101‐5. [DOI: 10.1016/j.eclnm.2011.03.003] [DOI]
Beck 2013
- Beck AM, Holst M, Rasmussen HH. Oral nutritional support of older (65 years+) medical and surgical patients after discharge from hospital: systematic review and meta‐analysis of randomized controlled trials. Clinical Rehabilitation 2013;27(1):19‐27. [DOI: 10.1177/0269215512445396; PUBMED: 22643726] [DOI] [PubMed] [Google Scholar]
Beck 2016
- Beck AM, Dent E, Baldwin C. Nutritional intervention as part of functional rehabilitation in older people with reduced functional ability: a systematic review and meta‐analysis of randomised controlled studies. Journal of Human Nutrition and Dietetics 2016;29(6):733‐45. [DOI: 10.1111/jhn.12382; PUBMED: 27231148] [DOI] [PubMed] [Google Scholar]
Bhutta 2008
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417‐40. [DOI: 10.1016/S0140-6736(07)61693-6] [DOI] [PubMed] [Google Scholar]
Bibas 2014
- Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Progress in Cardiovascular Diseases 2014;57(2):134‐43. [DOI: 10.1016/j.pcad.2014.07.004; PUBMED: 25216612] [DOI] [PubMed] [Google Scholar]
Campbell 2015
- Campbell AD, Godfryd A, Buys DR, Locher JL. Does participation in home‐delivered meals programs improve outcomes for older adults? Results of a systematic review. Journal of Nutrition in Gerontology and Geriatrics 2015;34(2):124‐67. [DOI: 10.1080/21551197.2015.1038463; NIHMS692744; PMC4480596; PUBMED: 26106985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cawood 2012
- Cawood AL, Elia M, Stratton RJ. Systematic review and meta‐analysis of the effects of high protein oral nutritional supplements. Ageing Research Reviews 2012;11(2):278‐96. [DOI: 10.1016/j.arr.2011.12.008; PUBMED: 22212388] [DOI] [PubMed] [Google Scholar]
Choudhury 2014
- Choudhury N, Ahmed T, Hossain MI, Mandal BN, Mothabbir G, Rahman M, et al. Community‐based management of acute malnutrition in Bangladesh: feasibility and constraints. Food and Nutrition Bulletin 2014;35(2):277‐85. [DOI: 10.1177/156482651403500214; PUBMED: 25076775] [DOI] [PubMed] [Google Scholar]
Collins 2015
- Collins J, Porter J. The effect of interventions to prevent and treat malnutrition in patients admitted for rehabilitation: a systematic review with meta‐analysis. Journal of Human Nutrition and Dietetics 2015;28(1):1‐15. [DOI: 10.1111/jhn.12230; PUBMED: 24811842] [DOI] [PubMed] [Google Scholar]
Coyne‐Meyers 2004
- Coyne‐Meyers K, Trombley LE. A review of nutrition in human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Nutrition in Clinical Practice 2004;19(4):340‐55. [DOI: 10.1177/0115426504019004340; PUBMED: 16215125] [DOI] [PubMed] [Google Scholar]
Daniels 2010
- Daniels R, Metzelthin S, Rossum E, Witte L, Heuvel W. Interventions to prevent disability in frail community‐dwelling older persons: an overview. European Journal of Ageing 2010; Vol. 7, issue 1:37‐55. [DOI: 10.1007/s10433-010-0141-9; PMC5547279; PUBMED: 28798616] [DOI] [PMC free article] [PubMed]
de van der Schueren 2016
- van der Schueren MA, Wijnhoven HA, Kruizenga HM, Visser M. A critical appraisal of nutritional intervention studies in malnourished, community dwelling older persons. Clinical Nutrition 2016;35(5):1008‐14. [DOI: 10.1016/j.clnu.2015.12.013; PUBMED: 26774525] [DOI] [PubMed] [Google Scholar]
Dewey 2008
- Dewey KG, Adu‐Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition 2008; Vol. 4, issue Suppl 1:24‐85. [DOI: 10.1111/j.1740-8709.2007.00124.x; PUBMED: 18289157] [DOI] [PMC free article] [PubMed]
Elia 2016
- Elia M, Normand C, Laviano A, Norman K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in community and care home settings. Clinical Nutrition 2016;35(1):125‐37. [DOI: 10.1016/j.clnu.2015.07.012; PUBMED: 26309240] [DOI] [PubMed] [Google Scholar]
Els 2013
- Els A, Walsh C. The impact of preschool feeding programmes on the growth of disadvantaged young children in developing countries: a systematic review of randomised trials. South African Journal of Clinical Nutrition 2013;26(2):33‐40. [DOI: 10.1080/16070658.2013.11734448] [DOI] [Google Scholar]
Fatima 2015
- Fatima S, Ali Khan S, Fatima F. Nutritional supplements and their use in the treatment of malnutrition in developing countries. Journal of Ayub Medical College Abbottabad 2015;27(4):911‐22. [PUBMED: 27004351] [PubMed] [Google Scholar]
Ferreira 2010
- Ferreira HS, Cavalcante SA, Assunção ML. Chemical composition and efficacy of the multimixture as a dietary supplement: a literature review [Composição química e eficácia da multimistura como suplemento dietético: revisão da literatura]. Ciência & Saúde Coletiva 2010;15(Suppl 2):3207‐20. [PUBMED: 21049162 ] [DOI] [PubMed] [Google Scholar]
Goudet 2017
- Goudet S, Griffiths P, Bogin B, Madise N. Interventions to tackle malnutrition and its risk factors in children living in slums: a scoping review. Annals of Human Biology 2017;44(1):1‐10. [DOI: 10.1080/03014460.2016.1205660; PUBMED: 27356853] [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 2014
- Grantham‐McGregor SM, Fernald LC, Kagawa RMC, Walker S. Effects of integrated child development and nutrition interventions on child development and nutritional status. Annals of the New York Academy of Sciences 2014;1308(1):11‐32. [DOI: 10.1111/nyas.12284] [DOI] [PubMed] [Google Scholar]
Gresham 2014
- Gresham E, Byles JE, Bisquera A, Hure AJ. Effects of dietary interventions on neonatal and infant outcomes: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2014;100(5):1298‐321. [DOI: 10.3945/ajcn.113.080655; PUBMED: 25332328] [DOI] [PubMed] [Google Scholar]
Gresham 2016
- Gresham E, Bisquera A, Byles JE, Hure AJ. Effects of dietary interventions on pregnancy outcomes: a systematic review and meta‐analysis. Maternal & Child Nutrition 2016;12(1):5‐23. [DOI: 10.1111/mcn.12142; PUBMED: 25048387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunaratna 2010
- Gunaratna NS, Groote H, Nestel P, Pixley KV, McCabe GP. A meta‐analysis of community‐based studies on quality protein maize. Food Policy 2010;35(3):202‐10. [DOI: 10.1080/16070658.2013.11734448] [DOI] [Google Scholar]
Hubbard 2012
- Hubbard GP, Elia M, Holdoway A, Stratton RJ. A systematic review of compliance to oral nutritional supplements. Clinical Nutrition 2012;31(3):293‐312. [DOI: 10.1016/j.clnu.2011.11.020; PUBMED: 22257636] [DOI] [PubMed] [Google Scholar]
Imdad 2011a
- Imdad A, Bhutta ZA. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011;11(Suppl 3):S17. [DOI: 10.1186/1471-2458-11-S3-S17; PMC3231890; PUBMED: 21501434] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2011b
- Imdad A, Yakoob MY, Bhutta ZA. The effect of folic acid, protein energy and multiple micronutrient supplements in pregnancy on stillbirths. BMC Public Health 2011;11(Suppl 3):S4. [DOI: 10.1186/1471-2458-11-S3-S4; PMC3231910; PUBMED: 21501455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2012
- Imdad A, Bhutta ZA. Maternal nutrition and birth outcomes: effect of balanced protein‐energy supplementation. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):178‐90. [DOI: 10.1111/j.1365-3016.2012.01308.x; PUBMED: 22742610] [DOI] [PubMed] [Google Scholar]
Kimber 2015
- Kimber K, Gibbs M, Weekes CE, Baldwin C. Supportive interventions for enhancing dietary intake in malnourished or nutritionally at‐risk adults: a systematic review of non‐randomised studies. Journal of Human Nutrition and Dietetics 2015;28(6):517‐45. [DOI: 10.1111/jhn.12329; PUBMED: 26300378] [DOI] [PubMed] [Google Scholar]
Kramer 1996a
- Kramer MS. Nutritional advice in pregnancy. Cochrane Database of Systematic Reviews 1996, Issue 4. [DOI: 10.1002/14651858.CD000149] [DOI] [PubMed] [Google Scholar]
Kramer 1996b
- Kramer MS. Isocaloric balanced protein supplementation in pregnancy. Cochrane Database of Systematic Reviews 1996, Issue 4. [DOI: 10.1002/14651858.CD000118] [DOI] [PubMed] [Google Scholar]
Kramer 1996c
- Kramer MS. High protein supplementation in pregnancy. Cochrane Database of Systematic Reviews 1996, Issue 4. [DOI: 10.1002/14651858.CD000105] [DOI] [PubMed] [Google Scholar]
Larson 2017
- Larson LM, Yousafzai AK. A meta‐analysis of nutrition interventions on mental development of children under‐two in low‐ and middle‐income countries. Maternal & Child Nutrition 2017;13(1):e12229. [DOI: 10.1111/mcn.12229; PUBMED: 26607403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2013a
- Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health 2013;13(Suppl 3):S13. [DOI: 10.1186/1471-2458-13-S3-S13; PMC3847349; PUBMED: 24564534] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2013b
- Lassi ZS, Zahid G, Das JK, Bhutta ZA. Systematic review of complementary feeding strategies amongst children less than two years of age. assets.publishing.service.gov.uk/media/57a08a38ed915d3cfd000654/Revised‐Complementary‐Feeding‐desk‐study_5‐13‐2013.pdf (accessed prior to 25 September 2018).
Lawson 2012
- Lawson TM. Impact of School Feeding Programs on Educational, Nutritional, and Agricultural Development Goals: A Systematic Review of Literature [Masters Thesis]. East Lansing (MI): Michigan State University; Department of Agricultural, Food, and Resource Economics, 2012. [ageconsearch.umn.edu/bitstream/142466/2/2012LawsonPlanB.pdf] [Google Scholar]
Lenters 2013
- Lenters LM, Wazny K, Webb P, Ahmed T, Bhutta ZA. Treatment of severe and moderate acute malnutrition in low‐ and middle‐income settings: a systematic review, meta‐analysis and Delphi process. BMC Public Health 2013;13(Suppl 3):S23. [DOI: 10.1186/1471-2458-13-S3-S23; PMC3847503; PUBMED: 24564235] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerch 2007
- Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006164.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberato 2013
- Liberato SC, Singh G, Mulholland K. Effects of protein energy supplementation during pregnancy on fetal growth: a review of the literature focusing on contextual factors. Food & Nutrition Research 2013;57:20499. [DOI: 10.3402/fnr.v57i0.20499; PMC3827488; PUBMED: 24235913] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loveday 2012
- Loveday H, Smales C, Tsiami A, Browne J. The effectiveness of nutritional interventions to reduce the risk of healthcare‐associated infection in the undernourished elderly: a systematic review. JBI Library of Systematic Reviews 2012;10(Suppl 14):1‐18. [DOI: 10.11124/jbisrir-2012-276; PUBMED: 27820446] [DOI] [PubMed] [Google Scholar]
Manders 2004
- Manders M, Groot LC, Staveren WA, Wouters‐Wesseling W, Mulders AJ, Schols JM, et al. Effectiveness of nutritional supplements on cognitive functioning in elderly persons: a systematic review. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2004; Vol. 59, issue 10:M1041‐9. [DOI: 10.1093/gerona/59.10.M1041; PUBMED: 15528776] [DOI] [PubMed]
Marshall 2013
- Marshall S, Bauer J, Capra S, Isenring E. Are informal carers and community care workers effective in managing malnutrition in the older adult community? A systematic review of current evidence. Journal of Nutrition, Health & Aging 2013;17(8):645‐51. [DOI: 10.1007/s12603-013-0341-z; PUBMED: 24097017] [DOI] [PubMed] [Google Scholar]
Matsuyama 2017
- Matsuyama M, Harb T, David M, Davies PS, Hill RJ. Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta‐analysis. Public Health Nutrition 2017;20(7):1214‐25. [DOI: 10.1017/S1368980016003189; PUBMED: 27938461] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGrath 2015
- McGrath CJ, Diener L, Richardson BA, Peacock‐Chambers E, John‐Stewart GC. Growth reconstitution following antiretroviral therapy and nutritional supplementation: systematic review and meta‐analysis. AIDS 2015;29(15):2009‐23. [DOI: 10.1097/QAD.0000000000000783; PMC4579534; PUBMED: 26355573] [DOI] [PMC free article] [PubMed] [Google Scholar]
McHenry 2015
- McHenry MS, Dixit A, Vreeman RC. A systematic review of nutritional supplementation in HIV‐Infected children in resource‐limited settings. Journal of the International Association of Providers of AIDS Care 2015;14(4):313‐23. [DOI: 10.1177/2325957414539044; PUBMED: 24943654] [DOI] [PubMed] [Google Scholar]
Milne 2009
- Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003288.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milte 2013
- Milte RK, Ratcliffe J, Miller MD, Crotty M. Economic evaluation for protein and energy supplementation in adults: opportunities to strengthen the evidence. European Journal of Clinical Nutrition 2013;67(12):1243‐50. [DOI: 10.1038/ejcn.2013.206; PUBMED: 24169464] [DOI] [PubMed] [Google Scholar]
Morilla‐Herrera 2016
- Morilla‐Herrera JC, Martin‐Santos FJ, Caro‐Bautista J, Saucedo‐Figueredo C, Garcia‐Mayor S, Morales‐Asencio JM. Effectiveness of food‐based fortification in older people. A systematic review and meta‐analysis. Journal of Nutrition, Health & Aging 2016;20(2):178‐84. [DOI: 10.1007/s12603-015-0591-z; PUBMED: 26812514] [DOI] [PubMed] [Google Scholar]
Potter 1998
- Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review. BMJ 1998;317(7157):495‐501. [DOI: 10.1136/bmj.317.7157.495; PUBMED: 9712593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramakrishnan 2014
- Ramakrishnan U, Imhoff‐Kunsch B, Martorell R. Maternal nutrition interventions to improve maternal, newborn, and child health outcomes. Nestle Nutrition Institute Workshop Series 2014;78:71‐80. [DOI: 10.1159/000354942; PUBMED: 24504208] [DOI] [PubMed] [Google Scholar]
Schultz 2015
- Schultz DJ, Byker Shanks C, Houghtaling B. The impact of the 2009 Special Supplemental Nutrition Program for Women, Infants, and Children Food Package revisions on participants: a systematic review. Journal of the Academy of Nutrition and Dietetics 2015;115(11):1832‐46. [DOI: 10.1016/j.jand.2015.06.381; PUBMED: 26276067 ] [DOI] [PubMed] [Google Scholar]
Stevens 2015
- Stevens B, Buettner P, Watt K, Clough A, Brimblecombe J, Judd J. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low‐ and middle‐income countries: a systematic review and meta‐analysis. Maternal & Child Nutrition 2015;11(4):415‐32. [DOI: 10.1111/mcn.12183; PUBMED: 25857334] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratton 2000
- Stratton RJ, Elia M. Are oral nutritional supplements of benefit to patients in the community? Findings from a systematic review. Current Opinion in Clinical Nutrition and Metabolic Care 2000;3(4):311‐5. [PUBMED: 10929679] [DOI] [PubMed] [Google Scholar]
Stratton 2013
- Stratton RJ, Hébuterne X, Elia M. A systematic review and meta‐analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Research Reviews 2013;12(4):884‐97. [DOI: 10.1016/j.arr.2013.07.002; PUBMED: 23891685] [DOI] [PubMed] [Google Scholar]
Thorne 2014
- Thorne F, Baldwin C. Multimodal interventions including nutrition in the prevention and management of disease‐related malnutrition in adults: a systematic review of randomised control trials. Clinical Nutrition 2014;33(3):375‐84. [DOI: 10.1016/j.clnu.2013.12.018; PUBMED: 24485001] [DOI] [PubMed] [Google Scholar]
Trabal 2015
- Trabal J, Farran‐Codina A. Effects of dietary enrichment with conventional foods on energy and protein intake in older adults: a systematic review. Nutrition Reviews 2015;73(9):624‐33. [DOI: 10.1093/nutrit/nuv023; PUBMED: 26180256] [DOI] [PubMed] [Google Scholar]
Tsiami 2013
- Tsiami A, Bak, A, Browne J, Loveday HP. The effectiveness of nutritional interventions to reduce the risk of healthcare‐associated infection in the undernourished elderly: a systematic review. International Journal of Evidence‐Based Healthcare 2013;11(3):255‐6. [DOI] [PubMed] [Google Scholar]
Valle 2004
- Valle NJ, Santos IS, Gigante DP. Nutritional interventions and child growth among under‐two‐year‐olds: a systematic review [Intervenções nutricionais e crescimento infantil em crianças de até dois anos de idade: uma revisão sistemática]. Cadernos de Saúde Pública 2004;20(6):1458‐67. [DOI: 10.1590/S0102-311X2004000600003] [DOI] [PubMed] [Google Scholar]
Vandenplas 2014
- Vandenplas Y, Ronne N, Sompel A, Huysentruyt K, Robert M, Rigo J, et al. A Belgian consensus‐statement on growing‐up milks for children 12‐36 months old. European Journal of Pediatrics 2014;173(10):1365‐71. [DOI: 10.1007/s00431-014-2321-7; PUBMED: 24764116] [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang D, Stewart D. The implementation and effectiveness of school‐based nutrition promotion programmes using a health‐promoting schools approach: a systematic review. Public Health Nutrition 2013;16(6):1082‐100. [DOI: 10.1017/S1368980012003497; PUBMED: 22850118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2015
- Wright J, Baldwin C. A systematic review of oral nutritional support with or without exercise in the management of malnutrition in nutritionally vulnerable older people. www.crd.york.ac.uk/prospero/display_record.php?RecordID=27323 (accessed prior to 25 September 2018). [CRD42015027323] [DOI] [PubMed]
Wrottesley 2016
- Wrottesley SV, Lamper C, Pisa PT. Review of the importance of nutrition during the first 1000 days: maternal nutritional status and its associations with fetal growth and birth, neonatal and infant outcomes among African women. Journal of Developmental Origins of Health and Disease 2016;7(2):144‐62. [DOI: 10.1017/S2040174415001439; PUBMED: 26279311] [DOI] [PubMed] [Google Scholar]
Additional references
Agarwal 1989
- Agarwal DK, Agarwal KN, Upadhyay SK. Effect of mid‐day meal programme on physical growth & mental function. Indian Journal of Medical Research 1989;90:163‐74. [PUBMED: 2767740] [PubMed] [Google Scholar]
Ashman 2014
- Ashman A, Collins C, Rae K, Rollo M, Brown L. The effectiveness of nutrition interventions for pregnant indigenous women: a systematic review. www.crd.york.ac.uk/prospero/display_record.php?RecordID=12984 (accessed prior to 25 September 2018). [CRD42014012984]
Beaton 1982
- Beaton GH, Ghassemi H. Supplementary feeding programs for young children in developing countries. American Journal of Clinical Nutrition 1982;35(Suppl 4):863‐916. [DOI: 10.1093/ajcn/35.4.864; PUBMED: 7039297] [DOI] [PubMed] [Google Scholar]
Bhandari 2001
- Bhandari N, Bahl R, Nayyar B, Khokhar P, Rohde JE, Bhan MK. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. Journal of Nutrition 2001;131(7):1946‐51. [DOI: 10.1093/jn/131.7.1946; PUBMED: 11435512] [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet 2013;382(9890):452‐77. [DOI: 10.1016/S0140-6736(13)60996-4] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. [DOI: 10.1016/S0140-6736(13)60937-X; PUBMED: 23746772] [DOI] [PubMed] [Google Scholar]
Bro 1994
- Bro RT, Shank L, Williams R, McLaughlin TF. The effects of an in‐class breakfast program on attendance and on‐task behaviour of high school students. Child & Family Behavior Therapy 1994;16(3):1‐8. [DOI: 10.1300/J019v16n03_01] [DOI] [Google Scholar]
Bro 1996
- Bro RT, Shank LL, McLaughlin TF, Williams RL. Effects of a breakfast program on on‐task behaviours of vocational high school students. Journal of Educational Research 1996;90(2):111–5. [DOI: 10.1080/00220671.1996.9944452] [DOI] [Google Scholar]
Burns 2010
- Burns C, Kristjansson B, Harris G, Armstrong R, Cummins S, Black A, et al. Community level interventions to improve food security in developed countries. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008913] [DOI] [Google Scholar]
Castleman 2011
- Castleman T, Mwadime R. Randomised Controlled Trial of the Impact of Supplementary Food on Malnourished Adult ART Clients and Adult Pre‐ART Clients in Kenya. Nairobi: FANTA‐KEMRI, 2011. [Google Scholar]
Chang 1996
- Chang SM, Walker SP, Himes J, Grantham‐McGregor SM. Effects of breakfast on classroom behavior in rural Jamaican schoolchildren. Food and Nutrition Bulletin 1996;17(3):248‐57. [archive.unu.edu/unupress/food/8F173e/8F173E0k.htm] [Google Scholar]
Coyne 1980
- Coyne T, Dowling M, Condon‐Paoloni D. Evaluation of the preschool meals program on the nutritional health of Aboriginal children. Medical Journal of Australia 1980;2(7):369‐75. [PUBMED: 7453609 ] [DOI] [PubMed] [Google Scholar]
D'Souza 2005
- D'Souza L, Renfrew M, McCormick F, Dyson L, Wright K, Henderson J, et al. The effectiveness of food support programmes for low‐income women in developed country settings: a systematic review of the evidence. www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?ID=12006008123 (accessed prior to 25 September 2018).
De Pee 2009
- Pee S, Bloem MW. Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6‐ to 23‐month‐old children and for treating moderate malnutrition among 6‐ to 59‐month‐old children. Food and Nutrition Bulletin 2009;30(Suppl 3):S434‐63. [DOI: 10.1177/15648265090303S305; PUBMED: 19998866] [DOI] [PubMed] [Google Scholar]
De Romaña 2000
- Romaño GL. Experience with complementary feeding in the FONCODES Project. Food and Nutrition Bulletin 2000;21(1):43‐8. [DOI: 10.1177/156482650002100107] [DOI] [Google Scholar]
Devadas 1971
- Devadas RP, Balambal M, Kumari NU. Impact of an applied nutrition programme on the nutritional status of pre‐school children in a village. Indian Journal of Nutrition and Dietetics 1971;8(5):260‐3. [19721406619] [Google Scholar]
Devadas 1979
- Devadas RP, Jamala S, Surabhi VA, Murthy NK. Evaluation of a food supplement to school children. Indian Journal of Nutrition and Dietetics 1979;16:335‐41. [agris.fao.org/agris‐search/search.do?recordID=IN8000386] [Google Scholar]
Devereux 2018
- Devereux S, Hochfeld T, Karriem A, Mensah C, Morahanye M, Msimango T, et al. School feeding in South Africa: what we know, what we don't know, what we need to know, what we need to do. foodsecurity.ac.za/wp‐content/uploads/2018/06/CoE‐FS‐WP4‐School‐Feeding‐in‐South‐Africa‐11‐jun‐18.pdf (accessed prior to 25 September 2018). [foodsecurity.ac.za/wp‐content/uploads/2018/06/CoE‐FS‐WP4‐School‐Feeding‐in‐South‐Africa‐11‐jun‐18.pdf]
Durao 2015
- Durao S, Schoonees A, Ramokolo V, Oliveira JM, Kristjansson E. Community‐level interventions for improving access to food in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011504] [DOI] [PMC free article] [PubMed] [Google Scholar]
FAO 1996
- Food, Agriculture Organization of the United Nations (FAO). Rome declaration on world food security. www.fao.org/docrep/003/w3613e/w3613e00.HTM (accessed 10 July 2017).
FAO 2008
- Food, Agriculture Organization of the United Nations (FAO). The State of Food Insecurity in the World 2008: High food Prices and Food Security ‐ Threats and Opportunities. Rome: FAO, 2008. [ISBN 978‐92‐5‐106049‐0; www.fao.org/docrep/011/i0291e/i0291e00.htm] [Google Scholar]
FAO 2010
- Food, Agriculture Organization of the United Nations (FAO). The State of Food Insecurity in the World: Addressing Food Insecurity in Protracted Crises. Rome: FAO, 2010. [ISBN 978‐92‐5‐106610‐2; www.fao.org/docrep/013/i1683e/i1683e.pdf] [Google Scholar]
FAO 2013
- Food, Agriculture Organization of the United Nations (FAO). International Fund for Agricultural Development (IFAD), World Food Programme (WFP). The State of Food Insecurity in the World: The Multiple Dimensions of Food Security. Rome: FAO, 2013. [ISBN 978‐92‐5‐107916‐4 ; www.fao.org/docrep/018/i3434e/i3434e.pdf] [Google Scholar]
FAO 2014
- Food, Agriculture Organization of the United Nations (FAO). International Fund for Agricultural Development (IFAD), World Food Programme (WFP). The State of Food Insecurity in the World: Strengthening the Enabling Environment for Food Security and Nutrition. Rome: FAO, 2014. [ISBN 978‐92‐5‐108542‐4; reliefweb.int/sites/reliefweb.int/files/resources/a‐i4030e.pdf] [Google Scholar]
FAO 2015
- Food, Agriculture Organization of the United Nations (FAO). International Fund for Agricultural Development (IFAD), World Food Programme (WFP). The State of Food Insecurity in the World, 2015: Meeting the 2015 International Hunger targets: Taking Stock of Uneven Progress. Rome: FAO, 2015. [ISBN 978‐92‐5‐108785‐5; www.fao.org/3/a‐i4646e.pdf] [Google Scholar]
FAO 2017
- Food, Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture: Trends and Challenges. Rome: FAO, 2017. [ISBN 978‐92‐5‐109551‐5] [Google Scholar]
FAO and WHO 2014
- Food, Agriculture Organization of the United Nations (FAO). World Health Organization (WHO). Conference outcome document: Rome declaration on nutrition. www.fao.org/3/a‐ml542e.pdf (accessed prior to 25 September 2018).
Fauveau 1992
- Fauveau C, Siddiqui M, Briend A, Silimperi DR, Begum N, Fauveau V. Limited impact of a targeted food supplementation programme in Bangladeshi urban slum children. Annals of Tropical Paediatrics: International Child Health 1992;12(1):41‐6. [DOI: 10.1080/02724936.1992.11747545] [DOI] [PubMed] [Google Scholar]
FSIN 2017
- Food Security Information Network (FSIN). Global report on food crises 2017. www.wfp.org/content/global‐report‐food‐crisis‐2017 (accessed 10 July 2017).
Garrett 2011
- Garrett J, Natalicchio M, editor(s). Working Multisectorally in Nutrition: Principles, Practices and Case Studies. Washington (DC): International Food Policy Research Institute, 2011. [ISBN 978‐0‐89629‐181‐2] [Google Scholar]
Gera 2016
- Gera T, Sachdev HS. Lipid‐based nutrient supplements for the treatment of 6‐ to 59‐month‐old children with moderate acute malnutrition. www.crd.york.ac.uk/prospero/display_record.php?RecordID=36458 (accessed prior to 25 September 2018). [CRD42016036458]
Gera 2017
- Gera T, Pena‐Rosas JP, Boy‐Mena E, Sachdev HS. Lipid based nutrient supplements (LNS) for treatment of children (6 months to 59 months) with moderate acute malnutrition (MAM): a systematic review. PloS One 2017;12(9):e0182096. [DOI: 10.1371/journal.pone.0182096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbs 2012
- Gibbs M, Baldwin C, Weekes CE. Supportive interventions for enhancing dietary intake in malnourished or nutritionally at‐risk adults. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009840] [DOI] [PMC free article] [PubMed] [Google Scholar]
Global Humanitarian Assistance 2014
- Global Humanitarian Assistance. Defining humanitarian assistance. www.globalhumanitarianassistance.org/data‐guides/defining‐humanitarian‐aid (accessed 10 July 2017).
Gopalan 1973
- Gopalan C, Swaninathan MC, Kumari VK, Rao DH, Vijayaraghavan K. Effect of calorie supplementation on growth of undernourished children. American Journal of Clinical Nutrition 1973;26(5):563‐6. [DOI: 10.1093/ajcn/26.5.563; PUBMED: 4633703] [DOI] [PubMed] [Google Scholar]
Goudet 2015
- Goudet SM, Griffiths PL, Bogin BA, Madise NJ. Nutritional interventions for preventing stunting in children (0 to 5 years) living in urban slums in low and middle‐income countries (LMIC). Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grantham‐McGregor 1991
- Grantham‐McGregor SM, Powell CA, Walker SP, Himes JH. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican Study. Lancet 1991;338(8758):1‐5. [PUBMED: 1676083] [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 1997
- Grantham‐McGregor SM, Walker SP, Chang SM, Powell CA. Effects of early childhood supplementation with and without stimulation on later development in stunted Jamaican children. American Journal of Clinical Nutrition 1997;66(2):247‐53. [DOI: 10.1093/ajcn/66.2.247; PUBMED: 9250101] [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 2007
- Grantham‐McGregor S, International Child Development Committee. Early child development in developing countries. Lancet 2007;369(9564):824. [DOI: 10.1016/S0140-6736(07)60404-8; PUBMED: 17350449] [DOI] [PubMed] [Google Scholar]
Grobler 2014
- Grobler L, Visser M, Siegfried N, Young T, Schoonees A. Effects of oral supplementary foods or nutrition counselling or both on infants and children (6–59 m) with severe or moderate under nutrition on linear growth, becoming overweight or obese, developing risk factors for cardiovascular disease or diabetes mellitus, and developing cardiovascular disease or diabetes mellitus later in life. www.crd.york.ac.uk/prospero/display_record.php?RecordID=13863 (accessed prior to 25 September 2018). [CRD42014013863]
Gwynn 2015
- Gwynn J, Sim K, Brimblecombe J, Lee A, O'Dea K, Turner N, et al. Dietary interventions to improve nutritional status and health outcomes in Aboriginal and Torres Strait Islander peoples in Australia: a systematic review. www.crd.york.ac.uk/prospero/display_record.php?RecordID=29551 (accessed prior to 25 September 2018). [CRD42015029551]
Husaini 1991
- Husaini MA, Karyadi L, Husaini YK, Sandjaja, Karyadi D, Pollitt E. Developmental effects of short‐term supplementary feeding in nutritionally‐at‐risk Indonesian infants. American Journal of Clinical Nutrition 1991;54(5):799‐804. [DOI: 10.1093/ajcn/54.5.799; PUBMED: 1951149] [DOI] [PubMed] [Google Scholar]
Iannotti 2014
- Iannotti LL, Dulience SJ, Green J, Joseph S, Francois J, Anténor ML, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of lipid‐based nutrient supplement. American Journal of Clinical Nutrition 2014;99(1):198‐208. [DOI: 10.3945/ajcn.113.063883; NCT01552512; PMC3862455; PUBMED: 24225356] [DOI] [PMC free article] [PubMed] [Google Scholar]
IFPRI 2014
- International Food Policy Research Institute (IFPRI). Global Nutrition Report 2014: Actions and Accountability to Accelerate the World's Progress on Nutrition. Washington (DC): IFRPI, 2014. [DOI: 10.2499/9780896295643; ISBN 978‐0‐89629‐564‐3; www.ifpri.org/publication/global‐nutrition‐report‐2014‐actions‐and‐accountability‐accelerate‐worlds‐progress] [DOI] [PMC free article] [PubMed] [Google Scholar]
IFPRI 2016
- International Food Policy Research Institute (IFPRI). Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2030. Washington (DC): IFPRI, 2016. [DOI: 10.2499/9780896295841; ISBN 2380‐6443; www.ifpri.org/publication/global‐nutrition‐report‐2016‐promise‐impact‐ending‐malnutrition‐2030] [DOI] [Google Scholar]
Isanaka 2009
- Isanaka S, Nombela N, Djibo A, Poupard M, Beckhoven D, Gaboulaud V, et al. Effect of preventive supplementation with ready‐to‐use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 2009;301(3):277‐85. [DOI: 10.1001/jama.2008.1018; NCT00682708; PMC3144630; PUBMED: 19155454] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahnavi 2010
- Jahnavi G, Sudha CH. Randomised controlled trial of food supplements in patients with newly diagnosed tuberculosis and wasting. Singapore Medical Journal 2010;51(12):957‐62. [PUBMED: 21221502] [PubMed] [Google Scholar]
Jeremiah 2014
- Jeremiah K, Denti P, Chigutsa E, Faurholt‐Jepsen D, PrayGod G, Range N, et al. Nutritional supplementation increases rifampin exposure among tuberculosis patients coinfected with HIV. Antimicrobial Agents and Chemotherapy 2014;58(6):3468‐74. [DOI: 10.1128/AAC.02307-13; PMC4068463; PUBMED: 24709267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keithley 2002
- Keithley JK, Swanson B, Zeller JM, Sha BE, Cohen M, Hershow R, et al. Comparison of standard and immune‐enhancing oral formulas in asymptomatic HIV‐infected persons: a multicenter randomized controlled clinical trial. Journal of Parenteral and Enteral Nutrition 2002;26(1):6‐14. [DOI: 10.1177/014860710202600106; PUBMED: 11833753] [DOI] [PubMed] [Google Scholar]
Kristjansson 2015b
- Kristjansson E, Francis DK, Liberato S, Jandu MB, Welch V, Batal M, et al. Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years: a systematic review. Campbell Systematic Reviews 2015;11:1‐226. [DOI: 10.4073/csr.2015:11] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristjansson 2015c
- Kristjansson E, Francis D, Liberato S, Greenhalgh T, Welch V, Jandu MB, et al. Supplementary Feeding for Improving the Health of Disadvantaged Infants and Young Children: A Systematic and Realist Review, 3ie Systematic Review 15. London: 3ie International Initiative for Impact Evaluation, 2016. [Google Scholar]
Lauque 2004
- Lauque S, Arnaud‐Battandier F, Gillette S, Plaze JM, Andrieu S, Cantet C, et al. Improvement of weight and fat‐free mass with oral nutritional supplementation in patients with Alzheimer's disease at risk of malnutrition: a prospective randomized study. Journal of the American Geriatrics Society 2004;52(10):1702‐7. [DOI: 10.1111/j.1532-5415.2004.52464.x; PUBMED: 15450048] [DOI] [PubMed] [Google Scholar]
Lutter 2008
- Lutter CK, Rodríguez A, Fuenmayor G, Avila L, Sempertegui F, Escobar J. Growth and micronutrient status in children receiving a fortified complementary food. Journal of Nutrition 2008;138(2):379‐88. [DOI: 10.1093/jn/138.2.379] [DOI] [PubMed] [Google Scholar]
Mahan 2011
- Mahan LK, Escott‐Stump S, Raymond JL. Krause's Food and the Nutrition Care Process. 13th Edition. Missouri (MO): Elsevier Saunders, 2011. [ISBN 978‐1‐4377‐2233‐8] [Google Scholar]
McKay 1978
- McKay H, Sinisterra L, McKay A, Gomez H, Lloreda P. Improving cognitive ability in chronically deprived children. Science 1978;200(4339):270‐8. [DOI: 10.1126/science.635585; PUBMED: 635585] [DOI] [PubMed] [Google Scholar]
MI 2009
- Micronutrient Initiative (MI). Investing in the future: a united call to action on vitamin and mineral deficiencies. Global report 2009. www.unitedcalltoaction.org/documents/Investing_in_the_future.pdf (accessed 13 February 2013).
Milne 2005
- Milne AC, Avenell A, Potter J. Oral protein and energy supplementation in older people: a systematic review of randomised trials. Nestle Nutrition Workshop Series. Clinical and Performance Program 2005;10:103‐25. [DOI: 10.1159/000083301; PUBMED: 15818025] [DOI] [PubMed] [Google Scholar]
Moreno 2005
- Moreno YF, Sgarbieri VC, Silva MN, Toro AA, Vilela MM. Features of whey protein concentrate supplementation in children with rapidly progressive HIV infection. Journal of Tropical Pediatrics 2005;52(1):34‐8. [DOI: 10.1093/tropej/fmi074; PUBMED: 16014759] [DOI] [PubMed] [Google Scholar]
Morris 2008
- Morris S, Cogill B, Uauy R, Maternal and Child Undernutrition Study Group. Effective international action against malnutrition: why has it proven so difficult and what can be done to accelerate progress?. Lancet 2008;371(9612):608‐21. [DOI: 10.1016/S0140-6736(07)61695-X; PUBMED: 18206225] [DOI] [PubMed] [Google Scholar]
Neumann 2003
- Neumann CG, Bwibo NO, Murphy SP, Sigman M, Whaley S, Allen LH, et al. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design and baseline findings. Journal of Nutrition 2003;133(11 Suppl 2):3941S‐9S. [DOI: 10.1093/jn/133.11.3941S; PUBMED: 14672294] [DOI] [PubMed] [Google Scholar]
Obatolu 2003
- Obatolu VA. Growth pattern of infants fed with a mixture of extruded malted maize and cowpea. Nutrition 2003;19(2):174‐8. [DOI: 10.1016/S0899-9007(02)01102-4; PUBMED: 12591556] [DOI] [PubMed] [Google Scholar]
Oluwaniyi 2015
- Oluwaniyi T, Adegboye A. Impact of lipid based nutrient supplements for prevention of childhood malnutrition: a systematic review. www.crd.york.ac.uk/prospero/display_record.php?RecordID=25019 (accessed prior to 25 September 2018). [CRD42015025019]
Paige 1976
- Paige DM, Cordano AP, Huang SS. Nutritional supplementation of disadvantaged elementary‐school children. Pediatrics 1976;58(5):697‐703. [PUBMED: 980602] [PubMed] [Google Scholar]
Paton 2004
- Paton NI, Chua YK, Earnest A, Chee CB. Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. American Journal of Clinical Nutrition 2004;80(2):460‐5. [DOI: 10.1093/ajcn/80.2.460; PUBMED: 15277171] [DOI] [PubMed] [Google Scholar]
Pollitt 1997
- Pollitt E, Watkins WE, Husaini MA. Three‐month nutritional supplementation in Indonesian infants and toddlers benefits memory function 8 y later. American Journal of Clinical Nutrition 1997;66(6):1357‐63. [DOI: 10.1093/ajcn/66.6.1357; PUBMED: 9394687] [DOI] [PubMed] [Google Scholar]
Pollitt 2000
- Pollitt E, Durnin JV, Husani M, Jahari A. Effect of an energy and micro‐nutrient supplement on growth and development in undernourished children in Indonesia: methods. European Journal of Clinical Nutrition 2000;54(Suppl 2):S16‐20. [PUBMED: 10902983] [DOI] [PubMed] [Google Scholar]
Powel 1983
- Powell C, Grantham‐McGregor S, Elston M. An evaluation of giving the Jamaican government school meal to a class of children. Human Nutrition. Clinical Nutrition 1983;37(5):381–8. [PUBMED: 6654697] [PubMed] [Google Scholar]
PrayGod 2011
- PrayGod G, Range N, Faurholt‐Jepsen D, Jeremiah K, Faurholt‐Jepsen M, Aabye MG, et al. Daily multi‐micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV‐uninfected but not HIV‐infected patients in Mwanza, Tanzania. Journal of Nutrition 2011;141(4):685‐91. [DOI: 10.3945/jn.110.131672; PUBMED: 21346105] [DOI] [PubMed] [Google Scholar]
Pérez‐Guzmán 2005
- Pérez‐Guzmán C, Vargas MH, Quiñonez F, Bazavilvazo N, Aguilar A. A cholesterol‐rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005;127(2):643‐51. [DOI: 10.1378/chest.127.2.643; PUBMED: 15706008] [DOI] [PubMed] [Google Scholar]
Rabeneck 1998
- Rabeneck L, Palmer A, Knowles JB, Seidehamel RJ, Harris CL, Merkel KL, et al. A randomized controlled trial evaluating nutrition counselling with or without supplementation in malnourished HIV‐infected patients. Journal of American Dietary Association 1998;98(4):434‐8. [DOI: 10.1016/S0002-8223(98)00099-6; PUBMED: 9550167] [DOI] [PubMed] [Google Scholar]
Rivera 2004
- Rivera JA, Sotres‐Alvarez D, Habicht JP, Shamah T, Villalpando S. Impact of the Mexican program for education, health, and nutrition (Progresa) on rates of growth and anaemia in infants and young children: a randomized effectiveness study. JAMA 2004;291(21):2563‐70. [DOI: 10.1001/jama.291.21.2563; PUBMED: 15173147] [DOI] [PubMed] [Google Scholar]
Rollins 2007
- Rollins NC, Broeck J, Kindra G, Pent M, Kasambira T, Bennish ML. The effect of nutritional support on weight gain of HIV‐infected children with prolonged diarrhoea. Acta Paediatrica 2007;96(1):62‐8. [DOI: 10.1111/j.1651-2227.2006.00013.x; PUBMED: 17187606] [DOI] [PubMed] [Google Scholar]
Roy 2005
- Roy SK, Fuchs GJ, Mahmud Z, Ara G, Islam S, Shafique S, et al. Intensive nutrition education with or without supplementary feeding improves the nutritional status of moderately‐malnourished children in Bangladesh. Journal of Health, Population, and Nutrition 2005;23(4):320‐3. [PUBMED: 16599102] [PubMed] [Google Scholar]
Ruel 2013
- Ruel MT, Alderman H, Maternal and Child Nutrition Study Group. Nutrition‐sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? . Lancet 2013;382(9891):536‐51. [DOI: 10.1016/S0140-6736(13)60843-0; PUBMED: 23746780] [DOI] [PubMed] [Google Scholar]
Rush 1980
- Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 1980;66(4):683‐97. [PUBMED: 6988785] [PubMed] [Google Scholar]
Santos 2005
- Santos IS, Gigante DP, Coitinho DC, Haisma H, Valle NC, Valente G. Evaluation of the impact of a nutritional program for undernourished children in Brazil. Cadernos de Saúde Pública 2005;21(3):776‐85. [DOI: 10.1590/S0102-311X2005000300011] [DOI] [PubMed] [Google Scholar]
Shea 2007a
- Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PloS One 2007;2(12):e1350. [DOI: 10.1371/journal.pone.0001350; PMC2131785; PUBMED: 18159233] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shea 2007b
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI: 10.1186/1471-2288-7-10; PMC1810543; PUBMED: 17302989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shea 2009
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw JM, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology 2009;62(10):1013‐20. [DOI: 10.1016/j.jclinepi.2008.10.009; PUBMED: 19230606] [DOI] [PubMed] [Google Scholar]
Shemilt 2004
- Shemilt I, Harvey I, Shepstone L, Swift L, Reading R, Mugford M, et al. A national evaluation of school breakfast clubs: evidence from a cluster randomized controlled trial and an observational analysis. Child: Care, Health and Development 2004;30(5):413‐27. [DOI: 10.1111/j.1365-2214.2004.00453.x; PUBMED: 15320919] [DOI] [PubMed] [Google Scholar]
Simondon 1996
- Simondon KB, Gartner A, Berger J, Cornu A, Massamba JP, San Miguel JL, et al. Effect of early, short‐term supplementation on weight and linear growth of 4‐7‐mo‐old infants in developing countries: a four‐country randomized trial. American Journal of Clinical Nutrition 1996;64(4):537‐45. [DOI: 10.1093/ajcn/64.4.537; PUBMED: 8839497] [DOI] [PubMed] [Google Scholar]
Soboka 2015
- Soboka M, Feyissa GT. The effectiveness of counselling, material support and/or nutritional supplementation on improving adherence to anti‐retroviral therapy and clinical outcomes among HIV patients: a systematic review of quantitative evidence protocol. JBI Database of Systematic Reviews and Implementation Reports 2015; Vol. 13, issue 7:142‐52. [DOI: 10.11124/jbisrir-2015-2243; PUBMED: 26455853] [DOI] [PubMed]
Stevens 2013
- Stevens B, Judd J, Buettner P, Brimblecombe J, Watt K, Clough A. Balanced protein energy supplementation for undernourished pregnant women in low income countries and child physical growth: a systematic review. www.crd.york.ac.uk/prospero/display_record.php?RecordID=5115 (accessed prior to 25 September 2018). [CRD42013005115] [DOI] [PMC free article] [PubMed]
Sudarsanam 2011
- Sudarsanam TD, John J, Kang G, Mahendri V, Gerrior J, Franciosa M, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV‐tuberculosis coinfection receiving directly observed short course chemotherapy for tuberculosis. Tropical Medicine & International Health 2011;16(6):699‐706. [DOI: 10.1111/j.1365-3156.2011.02761.x; PMC3918515; PUBMED: 21418447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundaram 2015
- Sundaram JK, Rawal V, Clark MT. Ending Malnutrition: from Commitment to Action. Rome: Food and Agriculture Organisation of the United Nations (FAO), 2015. [978‐92‐5‐108872‐2] [Google Scholar]
Thakwalakwa 2010
- Thakwalakwa C, Ashorn P, Phuka J, Cheung YB, Briend A, Puumalainen T, et al. A lipid‐based nutrient supplement but not corn‐soy blend modestly increases weight gain among 6‐ to18‐month‐old moderately underweight children in rural Malawi. Journal of Nutrition 2010;140(11):2008‐13. [DOI: 10.3945/jn.110.122499; NCT00420368; PUBMED: 20861218] [DOI] [PubMed] [Google Scholar]
Thorley 2015
- Thorley H, Porter K, Fleming C, Jones T, Kesten J, Marques E, et al. Interventions for preventing or treating malnutrition in problem drinkers who are homeless or vulnerably housed: protocol for a systematic review. Systematic Reviews 2015; Vol. 4:131. [DOI: 10.1186/s13643-015-0114-3; CRD42015024247; PMC4589081; PUBMED: 26424296] [DOI] [PMC free article] [PubMed]
Tisdall 1951
- Tisdall FF, Robertson EC, Drake TG, Jackson SH, Fowler HM, Long JA, et al. The Canadian Red Cross School Meals Study. Canadian Medical Association Journal 1951;64(6):477‐89. [PMC1821925; PUBMED: 14839578] [PMC free article] [PubMed] [Google Scholar]
Tomedi 2012
- Tomedi A, Rohan‐Minjares F, McCalmont K, Ashton R, Opiyo R, Mwanthi M. Feasibility and effectiveness of supplementation with locally available foods in prevention of child malnutrition in Kenya. Public Health Nutrition 2012;15(4):749‐56. [DOI: 10.1017/S1368980011002217; PUBMED: 21896234] [DOI] [PubMed] [Google Scholar]
UN 2015a
- United Nations (UN). Transforming our world: the 2030 agenda for sustainable development. sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed 10 July 2017).
UN 2015b
- United Nations (UN). United Nations decade of action on nutrition (2016‐2025). www.un.org/ga/search/view_doc.asp?symbol=A/70/L.42 (accessed 14 August 2017).
UN 2016
- United Nations (UN). Sustainable development knowledge platform. sustainabledevelopment.un.org (accessed 14 August 2017).
UNHCR/WFP 1999
- United Nations High Commissioner for Refugees (UNHCR), World Food Programme (WFP). UNHCR/WFP guidelines for selective feeding programmes in emergency situations. conflict.lshtm.ac.uk/media/UNHCR_WFP_Guidelines_for_SFP_In_Emergencies.pdf (accessed 10 July 2017).
UNICEF 1990
UNICEF/WHO/World Bank Group 2017
- United Nations Children's Fund (UNICEF), World Health Organization (WHO), World Bank Group. Levels and trends in child malnutrition. UNICEF/WHO/World Bank Group joint child malnutrition estimates. Key findings of the 2017 edition. www.who.int/nutgrowthdb/jme_brochoure2017.pdf?ua=1 (accessed 14 August 2017).
WEF 2016
- World Economic Forum. The global risks report 2016, 11th edition. www3.weforum.org/docs/GRR/WEF_GRR16.pdf (accessed 10 July 2017).
WHO 1999
- World Health Organization (WHO). A critical link: interventions for physical growth and psychological development. apps.who.int/iris/bitstream/handle/10665/66677/WHO_CHS_CAH_99.3.pdf?sequence=1 (accessed 10 July 2017).
WHO 2012
- World Health Organization (WHO). Technical note: supplementary foods for the management of moderate acute malnutrition in infants and children 6–59 months of age. apps.who.int/iris/bitstream/10665/75836/1/9789241504423_eng.pdf (accessed 13 February 2013).
WHO 2016
- World Health Organization (WHO). The double burden of malnutrition: policy brief. apps.who.int/iris/bitstream/handle/10665/255413/WHO‐NMH‐NHD‐17.3‐eng.pdf;jsessionid=6689B87F0A898787C35A4699A11A16B5?sequence=1 (accessed 10 July 2017).
Yamani 2009
- Yamani E, Kaba‐Mebri J, Mouala C, Gresenguet G, Rey JL. Use of spirulina supplement for nutritional management of HIV‐infected patients: study in Bangui, Central African Republic [Intérêt de la spiruline chez les personnes vivant avec le VIH a Bangui (RCA)]. Medecine Tropicale 2009;69(1):66‐70. [PUBMED: 19499738] [PubMed] [Google Scholar]
Yeung 2000
- Yeung GS, Zlotkin SH. Efficacy of meat and iron‐fortified commercial cereal to prevent iron depletion in cow milk‐fed infants 6 to 12 months of age: a randomized controlled trial. Canadian Journal of Public Health 2000;91(4):263‐7. [DOI: 10.17269/cjph.91.262] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 2009
- Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. American Journal of Clinical Nutrition 2009;89(2):525‐32. [DOI: 10.3945/ajcn.2008.26591; PMC2629145; PUBMED: 19073791] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Visser 2013
- Visser J, McLachlan MH, Fergusson P, Volmink J, Garner P. Supplementary feeding for food insecure, vulnerable and malnourished populations ‐ an overview of systematic reviews. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010578] [DOI] [PMC free article] [PubMed] [Google Scholar]


