Abstract
Background
Periodontal disease is a condition affecting tooth‐supporting tissues (gingiva, alveolar bone, periodontal ligament, and cementum), with the potential of introducing severe adverse effects on oral health. It has a complex pathogenesis which involves the combination of specific micro‐organisms and a predisposing host response. Infrabony defects are one of the morphological types of alveolar bone defects that can be observed during periodontitis. Recent approaches for the treatment of infrabony defects, combine advanced surgical techniques with platelet‐derived growth factors. These are naturally synthesized polypeptides, acting as mediators for various cellular activities during wound healing. It is believed that the adjunctive use of autologous platelet concentrates to periodontal surgical procedures produces a better and more predictable outcome for the treatment of infrabony defects.
Objectives
To assess the effects of autologous platelet concentrates (APC) used as an adjunct to periodontal surgical therapies (open flap debridement (OFD), OFD combined with bone grafting (BG), guided tissue regeneration (GTR), OFD combined with enamel matrix derivative (EMD)) for the treatment of infrabony defects.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 27 February 2018); the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library (searched 27 February 2018); MEDLINE Ovid (1946 to 27 February 2018); Embase Ovid (1980 to 27 February 2018); and LILACS BIREME Virtual Health Library (from 1982 to 27 February 2018). The US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials on 27 February 2018. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) of both parallel and split‐mouth design, involving patients with infrabony defects requiring surgical treatment. Studies had to compare treatment outcomes of a specific surgical technique combined with APC, with the same technique when used alone.
Data collection and analysis
Two review authors independently conducted data extraction and risk of bias assessment, and analysed data following Cochrane methods. The primary outcomes assessed were: change in probing pocket depth (PD), change in clinical attachment level (CAL), and change in radiographic bone defect filling (RBF). We organised all data in four groups, each comparing a specific surgical technique when applied with the adjunct of APC or alone: 1. APC + OFD versus OFD, 2. APC + OFD + BG versus OFD + BG, 3. APC + GTR versus GTR, and 4. APC + EMD versus EMD.
Main results
We included 38 RCTs. Twenty‐two had a split‐mouth design, and 16 had a parallel design. The overall evaluated data included 1402 defects. Two studies were at unclear overall risk of bias, while the remaining 36 studies had a high overall risk of bias.
1. APC + OFD versus OFD alone
Twelve studies were included in this comparison, with a total of 510 infrabony defects. There is evidence of an advantage in using APC globally from split‐mouth and parallel studies for all three primary outcomes: PD (mean difference (MD) 1.29 mm, 95% confidence interval (CI) 1.00 to 1.58 mm; P < 0.001; 12 studies; 510 defects; very low‐quality evidence); CAL (MD 1.47 mm, 95% CI 1.11 to 1.82 mm; P < 0.001; 12 studies; 510 defects; very low‐quality evidence); and RBF (MD 34.26%, 95% CI 30.07% to 38.46%; P < 0.001; 9 studies; 401 defects; very low‐quality evidence).
2. APC + OFD + BG versus OFD + BG
Seventeen studies were included in this comparison, with a total of 569 infrabony defects. Considering all follow‐ups, as well as 3 to 6 months and 9 to 12 months, there is evidence of an advantage in using APC from both split‐mouth and parallel studies for all three primary outcomes: PD (MD 0.54 mm, 95% CI 0.33 to 0.75 mm; P < 0.001; 17 studies; 569 defects; very low‐quality evidence); CAL (MD 0.72 mm, 95% CI 0.43 to 1.00 mm; P < 0.001; 17 studies; 569 defects; very low‐quality evidence); and RBF (MD 8.10%, 95% CI 5.26% to 10.94%; P < 0.001; 11 studies; 420 defects; very low‐quality evidence).
3. APC + GTR versus GTR alone
Seven studies were included in this comparison, with a total of 248 infrabony defects. Considering all follow‐ups, there is probably a benefit for APC for both PD (MD 0.92 mm, 95% CI ‐0.02 to 1.86 mm; P = 0.05; very low‐quality evidence) and CAL (MD 0.42 mm, 95% CI ‐0.02 to 0.86 mm; P = 0.06; very low‐quality evidence). However, given the wide confidence intervals, there might be a possibility of a slight benefit for the control. When considering a 3 to 6 months and a 9 to 12 months follow‐up there were no benefits evidenced, except for CAL at 3 to 6 months (MD 0.54 mm, 95% CI 0.18 to 0.89 mm; P = 0.003; 3 studies; 134 defects). No RBF data were available.
4. APC + EMD versus EMD
Two studies were included in this comparison, with a total of 75 infrabony defects. There is insufficient evidence of an overall advantage of using APC for all three primary outcomes: PD (MD 0.13 mm, 95% CI ‐0.05 to 0.30 mm; P = 0.16; 2 studies; 75 defects; very low‐quality evidence), CAL (MD 0.10 mm, 95% CI ‐0.13 to 0.32 mm; P = 0.40; 2 studies; 75 defects; very low‐quality evidence), and RBF (MD ‐0.60%, 95% CI ‐6.21% to 5.01%; P = 0.83; 1 study; 49 defects; very low‐quality evidence).
All studies in all groups reported a survival rate of 100% for the treated teeth. No complete pocket closure was reported. No quantitative analysis regarding patients' quality of life was possible.
Authors' conclusions
There is very low‐quality evidence that the adjunct of APC to OFD or OFD + BG when treating infrabony defects may improve probing pocket depth, clinical attachment level, and radiographic bone defect filling. For GTR or EMD, insufficient evidence of an advantage in using APC was observed.
Keywords: Adolescent; Adult; Aged; Female; Humans; Male; Middle Aged; Bone Transplantation; Guided Tissue Regeneration, Periodontal; Periodontal Debridement; Alveolar Bone Loss; Alveolar Bone Loss/diagnostic imaging; Alveolar Bone Loss/therapy; Autografts; Combined Modality Therapy; Combined Modality Therapy/methods; Dental Enamel Proteins; Dental Enamel Proteins/therapeutic use; Periodontal Diseases; Periodontal Diseases/diagnostic imaging; Periodontal Diseases/therapy; Periodontal Index; Platelet Transfusion; Platelet Transfusion/methods; Randomized Controlled Trials as Topic
Plain language summary
Autologous platelet concentrates for treating periodontal infrabony defects
Review question
Does the addition of autologous platelet concentrates (APC) improve surgical treatment outcomes of bone defects in gum disease?
Background
Teeth are maintained in their position by soft and hard tissues (gums and surrounding bone). Gum disease or periodontitis, is an inflammatory condition of all these tissues caused by the bacteria present in the dental plaque. If left untreated, gum disease can cause teeth to loosen and eventually lead to tooth loss. The destruction of jaw bone around teeth (called the alveolar bone) during gum disease, can be horizontal (where the whole level of bone around the root is reduced) or vertical, forming a bone defect within the bone (infrabony defect). There are several available surgical treatments for infrabony defects, including: 1. open flap debridement in which the gum is lifted back surgically in order to clean the deep tartar; 2. bone graft in which a portion of natural or synthetic bone is placed in the area of bone loss; 3. guided tissue regeneration in which a small piece of membrane‐like material is placed between the bone and gum tissue in order to keep the gum tissue from growing into the area where the bone should be; and 4. the use of enamel matrix derivative, a gel‐like material which is placed in the area where bone loss has occurred and promotes its regeneration. In order to accelerate the healing process, autologous platelet concentrates have been recently used. They are concentrates of the platelets of patient's own blood containing growth factors that are thought to promote tissue regeneration. The aim of this review was to assess if the addition of APC brings any benefits in the treatment of infrabony defects when combined with different surgical treatments.
Study characteristics
Authors from Cochrane Oral Health carried out this review and the evidence is up to date to 27 February 2018. We included 38 studies and a total of 1042 infrabony defects. We considered four different types of surgical treatments and compared each technique with the same one when APC was added. Overall we considered these comparisons: open flap debridement with APC versus without APC; open flap debridement and bone graft with APC versus without APC; guided tissue regeneration with APC versus without APC; and enamel matrix derivative with APC versus without APC.
Key results
There is very low‐quality evidence that the addition of APC to two types of treatment: open flap debridement and open flap debridement with bone graft, may bring some advantages in the treatment of infrabony defects. However, for the other two types of treatment, guided tissue regeneration and enamel matrix derivative, there is insufficient evidence of a benefit.
Quality of evidence
We judged the quality of the evidence to be very low due to problems with the design of the studies.
Summary of findings
Summary of findings for the main comparison. APC + OFD compared to OFD (9‐12 months follow‐up) for treating periodontal infrabony defects.
| APC + OFD compared to OFD (9‐12 months follow‐up) for treating periodontal infrabony defects | ||||||
| Patient or population: patients affected by infrabony defects requiring surgical treatment Settings: tertiary care Intervention: APC + OFD Comparison: OFD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants/defects (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| OFD | APC + OFD | |||||
|
Change in probing depth (PD) (mm) (9‐12 months follow‐up) |
Mean PD change (gain) across control groups ranged from 2.40 to 3.68 (2.36) mm Mean PD baseline value was 7.92 mm (95% CI 6.25 to 9.54) |
The mean PD change (gain) in the intervention groups was 1.29 mm higher (1.00 to 1.58 higher) | Mean difference 1.29 (1.00 to 1.58) mm | 510 (12 studies) | ⊕⊝⊝⊝ very low1, 2 | There is evidence of an advantage in using APC |
|
Change in clinical attachment level (CAL) (mm) (9‐12 months follow‐up) |
Mean CAL change (gain) across control groups ranged from 1.27 to 4.14 (2.03) mm Mean CAL baseline value was 6.78 mm (95% CI 5.56 to 7.54) |
The mean CAL change (gain) in the intervention groups was 1.47 mm higher (1.11 to 1.82 higher) | Mean difference 1.47 (1.11 to 1.82) mm | 510 (12 studies) | ⊕⊝⊝⊝ very low1, 2 | There is evidence of an advantage in using APC |
|
Change in radiographic bone defect filling (RBF) (%) (9‐12 months follow‐up) |
Mean RBF change (gain) across control groups ranged from ‐3.60% to 54.20% (16.90%) | The mean RBF change (gain) in the intervention groups was 34.26% higher (30.07 to 38.46 higher) | Mean difference 34.26% (30.07 to 38.46) | 401 (9 studies) | ⊕⊝⊝⊝ very low1, 2 | There is evidence of an advantage in using APC |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). APC: autologous platelet concentrates; CAL: clinical attachment level; CI: confidence interval; OFD: open flap debridement; PD: probing depth; RBF: radiographic bone defect filling. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded by 2 levels for high risk of performance bias. 2Downgraded by 2 levels for high heterogeneity.
Summary of findings 2. APC + OFD + BG compared to OFD + BG (all follow‐ups) for treating periodontal infrabony defects.
| APC + OFD + BG compared to OFD + BG (all follow‐ups) for treating periodontal infrabony defects | ||||||
| Patient or population: patients affected by infrabony defects requiring surgical treatment Settings: tertiary care Intervention: APC + OFD + BG Comparison: OFD + BG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants/defects (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| OFD + BG | APC + OFD + BG | |||||
|
Change in probing depth (PD) (mm) (All follow‐ups) |
Mean PD change (gain) across control groups ranged from 1.90 to 5.30 (3.54) mm Mean PD baseline value was 7.32 mm (95% CI 5.94 to 8.65) |
The mean PD change (gain) in the intervention groups was 0.54 mm higher (0.33 to 0.75 higher) | Mean difference 0.54 (0.33 to 0.75) mm | 569 (17 studies) | ⊕⊝⊝⊝ very low1, 2 | There is evidence of an advantage in using APC |
|
Change in clinical attachment level (CAL) (mm) (All follow‐ups) |
Mean CAL change (gain) across control groups ranged from 1.30 to 4.70 (3.20) mm Mean CAL baseline value was 7.34 mm (95% CI 5.21 to 9.82) |
The mean CAL change (gain) in the intervention groups was 0.72 mm higher (0.43 to 1.00 higher) | Mean difference 0.72 (0.43 to 1.00) mm | 569 (17 studies) | ⊕⊝⊝⊝ very low1, 2 | There is evidence of an advantage in using APC |
|
Change in radiographic bone defect filling (RBF) (%) (All follow‐ups) |
Mean RBF change (gain) across control groups ranged from 9.20% to 57.20% (40.54%) | The mean RBF change (gain) in the intervention groups was 8.10% higher (5.26 to 10.94 higher) | Mean difference 8.10% (5.26 to 10.94) | 420 (11 studies) | ⊕⊝⊝⊝ very low1, 2 | There is evidence of an advantage in using APC |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). APC: autologous platelet concentrates; BG: bone graft; CAL: clinical attachment level; CI: confidence interval; OFD: open flap debridement; PD: probing depth; RBF: radiographic bone defect filling. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded by 2 levels for high risk of performance bias. 2Downgraded by 2 levels for high heterogeneity.
Summary of findings 3. APC + GTR compared to GTR (all follow‐ups) for treating periodontal infrabony defects.
| APC + GTR compared to GTR (all follow‐ups) for treating periodontal infrabony defects | ||||||
| Patient or population: patients affected by infrabony defects requiring surgical treatment Settings: tertiary care Intervention: APC + GTR Comparison: GTR | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants/defects (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| GTR | APC + GTR | |||||
|
Change in probing depth (PD) (mm) (All follow‐ups) |
Mean PD change (gain) across control groups ranged from 3.19 to 6.00 mm (4.40 mm) Mean PD baseline value was 8.67 mm (95% CI 6.29 to 10.31) |
The mean PD change (gain) in the intervention groups was 0.92 mm higher (‐0.02 lower to 1.86 higher) | Mean difference 0.92 mm (‐0.02 to 1.86) | 248 (7 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | There is insufficient evidence of an advantage in using APC |
|
Change in clinical attachment level (CAL) (mm) (All follow‐ups) |
Mean CAL change (gain) across control groups ranged from 3.38 to 5.20 mm (4.38 mm) Mean CAL baseline value was 9.40 mm (95% CI 5.97 to 11.40) |
The mean CAL change (gain) in the intervention groups was 0.42 mm higher (‐0.02 lower to 0.86 higher) | Mean difference 0.42 mm (‐0.02 to 0.86) | 248 (7 studies) | ⊕⊝⊝⊝ very low1, 2, 3 | There is insufficient evidence of an advantage in using APC |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). APC: autologous platelet concentrates; CAL: clinical attachment level; CI: confidence interval; GTR: guided tissue regeneration; OFD: open flap debridement; PD: probing depth; RBF: radiographic bone defect filling. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded by 2 levels for high risk of performance bias. 2Downgraded by 2 levels for high heterogeneity. 3Downgraded by 2 levels for imprecision (wide confidence interval and small sample size).
Summary of findings 4. APC + EMD compared to EMD (all follow‐ups) for treating periodontal infrabony defects.
| APC + EMD compared to EMD (all follow‐ups) for treating periodontal infrabony defects | ||||||
| Patient or population: patients affected by infrabony defects requiring surgical treatment Settings: tertiary care Intervention: APC + EMD Comparison: EMD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants/defects (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| EMD | APC + EMD | |||||
|
Change in probing depth (PD) (mm) (All follow‐ups) |
Mean PD change (gain) across control groups ranged from 3.87 to 5.90 mm (4.89 mm) | The mean PD change (gain) in the intervention groups was 0.13 mm higher (‐0.05 lower to 0.30 higher) | Mean difference 0.13 mm (‐0.05 to 0.30) | 75 (2 studies) | ⊕⊝⊝⊝ very low1, 2 | There is insufficient evidence of an advantage in using APC |
|
Change in clinical attachment level (CAL) (mm) (All follow‐ups) |
Mean CAL change (gain) across control groups ranged from 3.30 to 5.00 mm (4.15 mm) | The mean CAL change (gain) in the intervention groups was 0.10 mm higher (‐0.13 lower to 0.32 higher) | Mean difference 0.10 mm (‐0.13 to 0.32) | 75 (2 studies) | ⊕⊝⊝⊝ very low1, 2 | There is insufficient evidence of an advantage in using APC |
|
Change in radiographic bone defect filling (RBF) (%) (All follow‐ups) |
Only 1 study reported RBF outcome with a mean change in control groups of 18.30% | The mean RBF change (gain) in the intervention group was 0.60% lower (‐6.21 lower to 5.01 higher) | Mean difference ‐0.60 (‐6.21 to 5.01) | 49 (1 study) | ⊕⊝⊝⊝ very low1, 2 | There is insufficient evidence of an advantage in using APC |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). APC: autologous platelet concentrates; CAL: clinical attachment level; CI: confidence interval; EMD: enamel matrix derivative; OFD: open flap debridement; PD: probing depth; RBF: radiographic bone defect filling. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded by 2 levels for high risk of performance bias. 2Downgraded by 2 levels for imprecision (wide confidence interval and small sample size).
Background
Description of the condition
Periodontitis is a disease of the periodontium characterized by the irreversible loss of connective tissue attachment and supporting alveolar bone (Pihlstrom 2005). For its onset, the presence of specific micro‐organisms together with an altered response of the host, are necessary. Despite its many variations, a typical course of periodontitis starts with pocket formation induced by bacterial plaque and a subsequent alveolar bone destruction typical of chronic periodontitis. Bone destruction during periodontitis can be of different morphological patterns including suprabony (horizontal) defects and infrabony (vertical) defects (Kinane 2001). An infrabony defect represents the anatomic sequelae resulting from the apical advancement of the dental plaque during the progression of the disease (Waerhaug 1979). Such defects, if left untreated, easily promote periodontitis progression and further loss of attachment (Papapanou 1991). Because infrabony defects are common in periodontitis (Vrotsos 1999), there is a considerable interest in approaches that will convert such defects, at risk for disease progression, to easily maintainable shallow probing sites (Crea 2014).
Description of the intervention
The ultimate goal of periodontal therapy is to preserve the natural dentition for as long as possible and enhance patient's comfort and aesthetic features by maintaining and improving the health and function of all tooth‐supporting tissues (gingiva, periodontal ligament, cementum, alveolar bone). Conventional treatment of periodontal disease may arrest bone destruction but usually does not restore the already lost alveolar bone or periodontal connective tissue. Various surgical techniques have been developed as an attempt to provide an efficient treatment to periodontitis. Open flap debridement (OFD) is among the earliest and most promising procedures to be used (Caffesse 1986; Cortellini 1996). Its main objective is to reduce the presence of micro‐organisms which develop and maintain the inflammatory process. By doing so, it consequently promotes the regeneration properties of the host, despite not being a regenerative procedure. Later, the combination of conventional OFD with various biomaterials such as bone grafts, enamel matrix derivative or membranes (guided tissue regeneration), resulted in the development of regenerative treatment protocols which introduced significant clinical benefits (Cochran 2003; Cortellini 1996; Esposito 2009; Hoidal 2008; Needleman 2006).
Despite advances in surgical procedures and materials, a complete and predictable regeneration, defined as the development of new bone, periodontal ligament and cementum on a root surface previously exposed to periodontal disease, remains a challenge (AAP 1992). Consequently, the concept of tissue engineering (Rose 2002) which requires the presence of cells, scaffold and signalling molecules, gained particular attention in terms of periodontal regeneration. Bone grafts and membranes used in guided tissue regeneration (GTR) can serve as scaffolds but there always exists a need of signalling molecules.
Recently, polypeptide growth factors have been investigated as possible signalling factors for enhancing periodontal regeneration. As preliminary evidence for their potential applications in periodontal wound healing, several polypeptide growth factors have been identified in the human periodontal tissues by immuno‐histochemistry and in‐situ hybridisation (Giannobile 1996). An abundant source of such growth factors are platelets, easily utilisable in the form of autologous platelet concentrates (APC). Therefore, the adjunctive use of APC in combination with periodontal surgery has emerged as a possible tool to enhance the predictability of infrabony defects treatment.
APCs based on their preparation protocol, can be of various types, including platelet‐rich plasma (PRP) (Marx 1998), platelet‐rich fibrin (PRF) (Choukroun 2001), and plasma‐rich growth factors (PRGF) (Anitua 2001). Several commercial techniques for obtaining platelet concentrates are available. However, their indication of use has been confusing because each method leads to a different product with different biological properties and possible applications. PRP represents the first generation of platelet concentrate, and shows a release of an array of growth factors for 7 days, with a peak release on its first day of application (Dohan Ehrenfest 2009). PRF represents the second generation APC, and its technique of preparation is simplified when compared to PRP. Moreover, PRF showed a sustained growth factors release for a period of 21 days with a peak release at 7 days (Carroll 2005). PRGF is also a second generation platelet concentrate, whose main difference when compared to PRP is the absence of leucocytes and the small blood volume required for its preparation (Anitua 2001). Following an upgrade in their classification (Dohan Ehrenfest 2009), platelet concentrates can be divided into four categories, based on the presence of leucocyte and fibrin: P‐PRP (pure PRP, without leucocytes, which includes PRGF), L‐PRP (leucocyte and platelet‐rich plasma), P‐PRF (pure PRF), and L‐PRF (leucocyte PRF).
How the intervention might work
The contribution of blood‐derived platelets to the bone healing process is thought to be based on the growth factors stored in their granules and released upon activation. The main growth factors released from platelet aggregates are the following: platelet derived growth factor (PDGF), transforming growth factor‐beta (TGF‐β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), insulin‐like growth factor‐1 (IGF‐1), and basic fibroblast growth factor (bFGF), as well as three blood proteins known to act as cell adhesion molecules for osteo‐conduction (fibrin, fibronectin and vitronectin). The set of these factors serve as biological mediators with the ability to regulate cell proliferation, chemotaxis, and differentiation.
Why it is important to do this review
The considerably increased interest in combining APC with surgical techniques for better outcomes in the treatment of infrabony defects, has made it necessary a thorough investigation of the actual benefits that can be obtained. The first systematic review that evaluated the effect of PRP on clinical applications in dentistry reported beneficial effects of PRP in the treatment of periodontal defects (Plachokova 2008). Another systematic review that evaluated the effect of a PRP adjunct in treatment of intraosseous defects, underlined the limits and the heterogeneity of available data and cautiously concluded that the specific selection of the graft type and the surgical procedures combined with PRP may be important (Kotsovilis 2010). A subsequent systematic review also evaluated the effect of platelet rich plasma in various regenerative procedures of periodontal defects, and concluded that PRP may be advantageously used as an adjunct to grafting procedures treatment for infrabony defects (Del Fabbro 2011). Such review also suggested that the use of PRP is ineffective when GTR procedure is used for treating infrabony defects.
Despite the numerous reports on the adjunctive use of autologous platelet concentrate to periodontal surgical procedures, its efficacy remains controversial. This is partly due to a large heterogeneity among different studies (Del Fabbro 2011; Del Fabbro 2013), concerning methods, study design, protocols for platelet concentrate preparation, participants selection criteria, outcome variables assessed, etc. Therefore, a review of the current state of the evidence is crucial in order to clarify if the adjunct of APCs eventually produces better outcomes in the treatment of infrabony defects, and if their effect is particularly enhanced when combined with a specific surgical technique. By doing so, clear and relevant guidelines can be addressed to clinicians.
Objectives
To assess the effects of autologous platelet concentrates used as an adjunct to periodontal surgical therapies (open flap debridement (OFD), OFD combined with bone grafting, guided tissue regeneration, OFD combined with enamel matrix derivative) for the treatment of infrabony defects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, of both parallel and split‐mouth design.
Types of participants
Patients affected by infrabony defects requiring surgical treatment, regardless of their age or gender.
Types of interventions
Experimental intervention: autologous platelet concentrates (APCs) (irrespective of the type: platelet‐rich plasma (PRP), plasma‐rich growth factors (PRGF), or platelet‐rich fibrin (PRF)) used in conjunction with a specific surgical technique (open flap debridement (OFD), OFD + bone grafts (BG), guided tissue regeneration (GTR), enamel matrix derivative (EMD)).
Comparison (control) intervention: the same surgical techniques when used alone (without the adjunct of APCs).
Types of outcome measures
Primary outcomes
Change in probing depth (PD), change in clinical attachment level (CAL), and change in radiographic bone defect filling (RBF).
Secondary outcomes
Tooth survival, pocket closure, and oral health‐related quality of life.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 27 February 2018) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library (searched 27 February 2018) (Appendix 2);
MEDLINE Ovid (1946 to 27 February 2018) (Appendix 3);
Embase Ovid (1980 to 27 February 2018) (Appendix 4);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 27 February 2018) (Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 27 February 2018) (Appendix 6);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 27 February 2018) (Appendix 7).
An adjunctive search was performed on the reference lists of the included articles and reviews retrieved.
Moreover, a handsearch was performed on the issues since January 2010 (including the 'early view' or equivalent section) of the following journals: International Journal of Periodontics and Restorative Dentistry, Journal of Clinical Periodontology, Journal of Periodontal Research, Journal of Periodontology, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology (last search was performed on 2 March 2018). Two review authors independently performed the searches (Saurav Panda (SP), Cristina Bucchi (CB)).
We also searched for grey literature, such as conference abstracts, proceedings and theses on the following databases: www.greylit.org; www.opengrey.eu (last search was performed on 2 March 2018, see Appendix 8).
Data collection and analysis
Selection of studies
Following the electronic search, two review authors (Jayakumar Nadathur Doraiswamy (JND), Malaiappan Sankari (MS)) independently screened the titles and abstracts (if available) to exclude all articles clearly not meeting the inclusion criteria. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. Of all the remaining articles, full texts were obtained and assessed independently by two review authors (JND, MS) and only articles fully meeting the inclusion criteria were considered. In cases of disagreement between the two review authors, a third review author (Massimo Del Fabbro (MDF)) was consulted. Detailed reasons were stated for all excluded studies. This process is summarised in Figure 1.
1.

Study flow diagram.
Data extraction and management
Three review authors (SP, Lorena Karanxha (LK), CB) independently extracted and recorded data on ad hoc forms. Any disagreement was solved through discussion, or a third review author was consulted (MDF). In case of missing or unclear information, we contacted the authors of the included reports by email to provide clarification or missing information. In case of missing or incomplete data and absence of further clarification by study authors we excluded the report from the analysis.
We recorded the following data for each included report:
demographic characteristics of the population;
defect characteristics (PD, CAL, RBF);
type of platelet concentrate used (PRP, PRF, PRGF);
outcome characteristics (outcome variables assessed such as CAL and PD, follow‐up duration);
when possible, we also recorded the expertise of the clinician (years of experience with using platelet concentrates); and
source of funding.
Assessment of risk of bias in included studies
Three review authors (LK, SP, CB) independently assessed the risk of bias in the included studies. In case of disagreement a fourth review author (MDF) was consulted. Since some of the authors of one of the randomised controlled trials included (Panda 2016) are also authors of this review (SP, MDF, Silvio Taschieri (ST)), the risk of bias assessment for that study was carried out by other review authors not involved in the study (LK, CB).
The assessment was conducted following the instructions and the approach described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For each study, the following domains were considered: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data addressed), and reporting bias (selective reporting).
For each domain the risk was judged either low, unclear or high. If one study had low risk for all domains, the study was judged at low risk of bias. If it had an unclear risk for at least one domain, the study was judged at unclear risk of bias. If it had a high risk for at least one domain, the study was judged at high risk of bias. It was considered that blinding of patient and clinician might be difficult/impossible, as for many studies involving surgical procedures where interventions are quite different from each other.
We categorised the overall risk of bias of individual studies. Studies were categorised as being at low, high, or unclear risk of bias according to the following criteria:
low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were at low risk of bias;
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains were at high risk of bias; or
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains were at unclear risk of bias.
These assessments are reported in the Characteristics of included studies table and also graphically.
Measures of treatment effect
For continuous outcomes (e.g. PD, CAL, RBF), mean differences (change score) along with 95% confidence intervals (CIs) were used to summarise data for each treatment group. We expressed the data in mm for PD and CAL and in percentage for RBF, as they were reported in the studies.
Unit of analysis issues
The statistical unit of analysis in parallel studies was the patient, unless the study provided data only for defects. We considered one infrabony defect per patient in studies with parallel design. In the case of split‐mouth studies, the unit of analysis was the defect; a single defect per patient per group was considered.
Dealing with missing data
In case of missing data, we contacted the corresponding author of the article through e‐mail to obtain complete data. In case of no response, the same e‐mail was sent to co‐authors for a maximum of three times. If no answer was obtained, the study was excluded from the analysis. When feasible, missing standard deviations were estimated using the methods described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
Heterogeneity among studies was assessed with Cochran's test for heterogeneity, with a significance threshold of P < 0.1. The quantification of the heterogeneity was calculated with I2 statistic. For the interpretation of I2 the ranges suggested in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) were considered.
Assessment of reporting biases
We assessed publication bias by testing for funnel plot asymmetry, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry was evident, we investigated this and described possible causes.
Data synthesis
The meta‐analysis was performed only with studies with similar comparisons reporting the same outcome measures. We combined mean differences for continuous data, using random‐effects models if at least four studies were included in the meta‐analysis, while if there were less than four studies a fixed‐effect model was chosen. The software RevMan 5 (Review Manager 2014) was used for meta‐analysis computations. Data from split‐mouth and parallel‐group studies were combined (Elbourne 2002). The appropriate standard errors were estimated where they were not present in the trial reports (Follmann 1992). For the split‐mouth studies the standard error was calculated assuming an intraclass correlation coefficient of 0. The generic inverse variance procedure in RevMan 5 was used to combine these two subgroups in the analyses.
Subgroup analysis and investigation of heterogeneity
In addition to the different surgical protocols for different types of infrabony defects, duration of the follow‐up was investigated as a factor possibly affecting the outcome. The subgroups included data up to 6 months (3 to 6 months) and longer than 6 months (9 to 12 months).
Sensitivity analysis
Sensitivity analysis was performed in order to evaluate the effect of risk of bias and source of funding on the overall effects (e.g. omitting studies at unclear or high risk of bias or those sponsored by the manufacturer of the product under investigation). The effect of excluding specific studies that eventually appeared to be outliers was also investigated.
Summary of findings
We produced a 'Summary of findings' table for each comparison in which there was more than one study. We included the change in PD, CAL and RBF of the all follow‐up periods of each comparison group. We used GRADE methods, and GRADEpro software (GRADEpro GDT 2015) for developing 'Summary of findings' tables. We assessed the quality of the body of evidence for each comparison and outcome by considering the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias. We categorised the quality of each body of evidence as high, moderate, low, or very low.
Results
Description of studies
Results of the search
The electronic search retrieved 855 records, four trials were identified by handsearching and none by searching the grey literature. After discarding the duplicates, two review authors (Jayakumar Nadathur Doraiswamy (JND), Malaiappan Sankari (MS)) screened 480 titles and abstracts and rejected 402. The full text was obtained for 78 potentially eligible articles and of these, 40 were excluded with reasons (see Characteristics of excluded studies table). Finally, after agreement among the review authors 38 studies were included in this review (Figure 1).
Included studies
Design
Of the 38 included studies, 22 had a split‐mouth design, reporting for a total of 371 participants and 701 teeth (Agarwal 2014; Agarwal 2015; Agarwal 2016; Arabaci 2017; Aydemir 2016; Camargo 2009; Christgau 2006; Elgendy 2015; Gupta 2014; Hanna 2004; Hassan 2012; Kaushick 2011; Khosropanah 2015; Naqvi 2017; Ozdemir 2012; Panda 2016; Patel 2017; Ravi 2017; Rosamma Joseph 2012; Sezgin 2017; Shukla 2016; Thorat 2017); 16 studies had a parallel design with a total of 645 patients and 721 teeth (Chandradas 2016; Demir 2007; Döri 2007a; Döri 2007b; Döri 2008a; Döri 2008b; Döri 2009; Garg 2017; Kanoriya 2016; Martande 2016; Okuda 2005; Piemontese 2008; Pradeep 2015; Pradeep 2016; Sharma 2011; Thorat 2011). Of the 38 included studies only one was a multicentric study (Elgendy 2015). Finally, two studies declared that they were supported in part by companies whose products were used in the trials (Döri 2008a; Döri 2008b).
Sample size calculation was reported only by 15 studies (Döri 2007a; Döri 2007b; Döri 2008a; Döri 2008b; Döri 2009; Kanoriya 2016; Panda 2016; Patel 2017; Pradeep 2015; Pradeep 2016; Ravi 2017; Rosamma Joseph 2012; Sezgin 2017; Sharma 2011; Thorat 2011), meaning that in almost 60% of cases there was no rationale regarding the choice of the sample size.
Participants
The age range of the participants of included studies was between 17 and 74 years. However, four studies did not report the age of the participants (Agarwal 2016; Gupta 2014; Naqvi 2017; Shukla 2016) and 10 studies (Agarwal 2015; Aydemir 2016; Chandradas 2016; Demir 2007; Elgendy 2015; Hassan 2012; Khosropanah 2015; Okuda 2005; Ozdemir 2012; Sezgin 2017) reported only mean ages, ranging from 36.03 and 55.5 years.
35 studies included both men and women, but with different proportions, and three studies did not report this information (Gupta 2014; Elgendy 2015; Kaushick 2011). Finally, most of the studies did not include smokers (Agarwal 2014; Agarwal 2015; Agarwal 2016; Arabaci 2017; Aydemir 2016; Chandradas 2016; Döri 2007a; Döri 2007b; Döri 2008a; Döri 2008b; Döri 2009; Garg 2017; Gupta 2014; Hassan 2012; Kanoriya 2016; Kaushick 2011; Khosropanah 2015; Martande 2016; Naqvi 2017; Okuda 2005; Ozdemir 2012; Panda 2016; Patel 2017; Piemontese 2008; Pradeep 2015; Pradeep 2016; Ravi 2017; Rosamma Joseph 2012; Sezgin 2017; Sharma 2011; Shukla 2016; Thorat 2011; Thorat 2017).
Interventions
The general comparison was between a group that received autologous platelet concentrates (APC) as an adjunct to surgical treatment (experimental group), and a group that received surgical treatment alone (control group). Four different types of comparisons were assessed, based on the treatment type:
APC + open flap debridement (OFD) versus OFD alone (12 trials): Agarwal 2016; Arabaci 2017; Chandradas 2016; Kanoriya 2016; Martande 2016; Patel 2017; Pradeep 2015; Pradeep 2016; Rosamma Joseph 2012; Sharma 2011; Thorat 2011; Thorat 2017
APC + OFD + bone graft (BG) versus OFD + BG (17 trials): Agarwal 2014; Agarwal 2015; Demir 2007; Döri 2009; Elgendy 2015; Garg 2017; Gupta 2014; Hanna 2004; Hassan 2012; Kaushick 2011; Khosropanah 2015; Naqvi 2017; Okuda 2005; Ozdemir 2012; Piemontese 2008; Sezgin 2017; Shukla 2016
APC + guided tissue regeneration (GTR) versus GTR (7 trials): Camargo 2009; Christgau 2006; Döri 2007a; Döri 2007b; Döri 2008a; Panda 2016; Ravi 2017
APC + enamel matrix derivative (EMD) versus EMD (2 trials): Aydemir 2016; Döri 2008b.
Outcomes
Primary outcomes
Change in probing depth (PD), reported by all 38 included studies.
Change in clinical attachment level (CAL), defined relative attachment level (RAL) in some studies, reported by all 38 included studies.
Change in radiographic bone defect filling (RBF), reported by 31 studies.
Secondary outcomes
All articles in all groups reported a survival rate of 100% for the treated teeth. No complete pocket closure was reported. No quantitative analysis regarding patients' quality of life was possible.
Excluded studies
We excluded 40 studies from the review, for the following reasons (see Characteristics of excluded studies table):
no randomisation (Aleksić 2008; Jovicić 2013; Saini 2011)
no control group (Camargo 2002; Camargo 2005; Lekovic 2012)
gingival recession, not infrabony defects (Aroca 2009; Dogan 2015; Huang 2005; Jankovic 2010; Padma 2013; Shepherd 2009; Shivakumar 2016; Thamaraiselvan 2015)
same patients reported in a previous study (Cetinkaya 2014; Döri 2013; Moder 2012; Yajamanya 2017)
non‐independence of analysing unit (Gupta 2014b; Pradeep 2012a)
incomplete data (Cieplik 2018; Harnack 2009; Keceli 2008; Keles 2006; Menezes 2012; Shah 2015; Yassibag‐Berkman 2007; Yen 2007)
no APCs (fibrin glue) (Cortellini 1995; Trombelli 1995; Trombelli 1996)
APC not the only difference between groups (Cheung 2004; Eren 2014; Jankovic 2012)
studies with mixed (parallel/split‐mouth) design (Agarwal 2017; Bajaj 2017; Chatterjee 2017; Ouyang 2006; Pradeep 2017; Qiao 2016).
Risk of bias in included studies
The risk of bias in included studies is summarized in Figure 2 and Figure 3. Two studies were at unclear overall risk of bias (Ravi 2017; Rosamma Joseph 2012). The remaining 36 studies had a high overall risk of bias.
2.
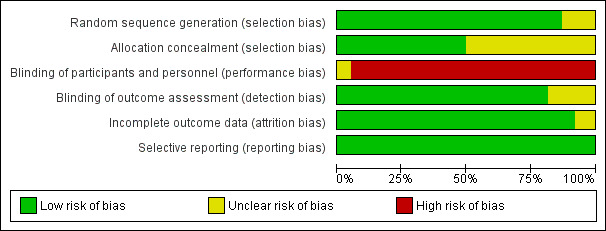
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
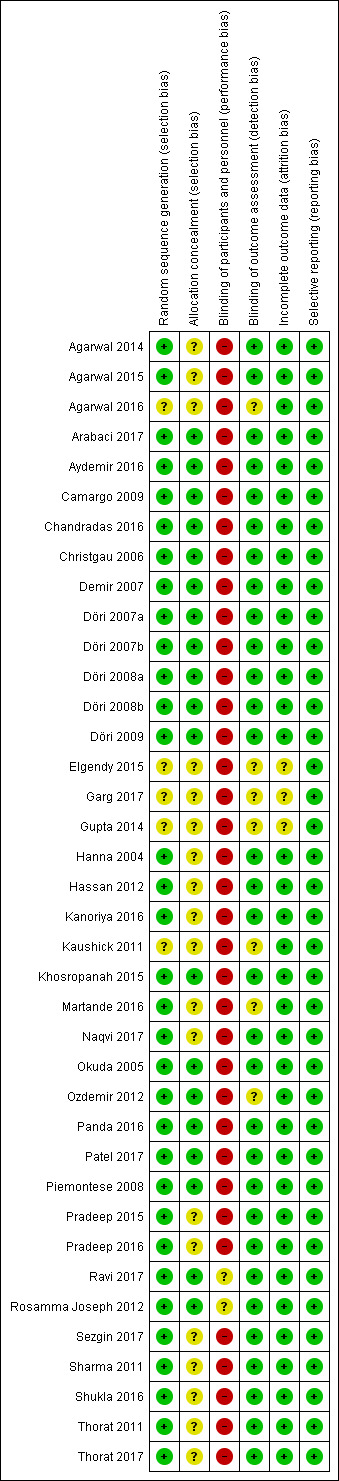
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The randomisation was performed correctly in most of the studies. The methods used were the tossing of a coin (Agarwal 2014; Agarwal 2015; Camargo 2009; Demir 2007; Gupta 2014; Hanna 2004; Khosropanah 2015; Okuda 2005; Ozdemir 2012; Panda 2016; Patel 2017; Piemontese 2008; Ravi 2017; Rosamma Joseph 2012; Sezgin 2017; Sharma 2011; Thorat 2011), the block approach (Döri 2007a; Döri 2007b; Döri 2008a; Döri 2008b; Döri 2009), the use of a freeware link (Chandradas 2016), computerized generated scheme (Aydemir 2016; Kanoriya 2016; Martande 2016; Pradeep 2016; Shukla 2016; Thorat 2011), biased coin randomisation (Hassan 2012), lottery method (Naqvi 2017), and a table of random numbers (Christgau 2006; Pradeep 2015). The randomisation method was not described in five articles, which were considered to be an unclear risk of bias (Agarwal 2016; Elgendy 2015; Garg 2017; Gupta 2014; Kaushick 2011).
Allocation concealment
The concealment of the allocation was correctly done in 19 studies (Arabaci 2017; Aydemir 2016; Camargo 2009; Chandradas 2016; Christgau 2006; Demir 2007; Döri 2007a; Döri 2007b; Döri 2008a; Döri 2008b; Döri 2009; Khosropanah 2015; Okuda 2005; Ozdemir 2012; Panda 2016; Patel 2017; Piemontese 2008; Ravi 2017; Rosamma Joseph 2012). In the remaining 19 studies, insufficient information was provided regarding the exact method used for allocation concealment (Agarwal 2014; Agarwal 2015; Agarwal 2016; Elgendy 2015; Garg 2017; Gupta 2014; Hanna 2004; Hassan 2012; Kanoriya 2016; Kaushick 2011; Martande 2016; Naqvi 2017; Pradeep 2015; Pradeep 2016; Sezgin 2017; Sharma 2011; Shukla 2016; Thorat 2011; Thorat 2017).
Blinding
Blinding of participants and personnel (performance bias)
Being the intervention surgical in nature, blinding of participants and treating clinicians is almost unfeasible either in a parallel or split‐mouth design: 36 out of 38 studies had a high risk of performance bias. For two studies an unclear risk of performance bias was assigned given that it was stated in the paper that blinding of the operator was performed but without specifying how (Ravi 2017; Rosamma Joseph 2012). The blinding of the personnel was also evaluated, which was reported in most of the studies except for eight studies (Agarwal 2016; Christgau 2006; Elgendy 2015; Garg 2017; Gupta 2014; Kaushick 2011; Okuda 2005; Ozdemir 2012). However, again for the fact that the intervention has a surgical nature, it is unlikely that blinding or not of the personnel could influence the outcome. Therefore such parameter did not influence the assignment of the risk of performance bias.
Blinding of outcome assessment (detection bias)
The blinding of the outcome assessor was done in most of the studies. However, it was not reported in seven studies, which were considered to be at unclear risk of detection bias (Agarwal 2016; Elgendy 2015; Garg 2017; Gupta 2014; Kaushick 2011; Martande 2016; Ozdemir 2012).
Incomplete outcome data
The completeness of outcome data was adequate in all but three studies in which the number of subjects that finished the study was not clear (Elgendy 2015; Garg 2017; Gupta 2014).
Selective reporting
All studies properly reported data for all patients.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
For the meta‐analyses of all follow‐ups, where the study presented multiple follow‐ups, we used the longest one.
1. Autologous platelet concentrates (APC) + open flap debridement (OFD) versus OFD
In this comparison we did not divide the data according to the follow‐up duration, because all studies had a follow‐up duration between 9 and 12 months.
Change in probing depth (PD) (mm)
Follow‐up between 9 and 12 months
There is evidence of an advantage in using APC from both split‐mouth studies (mean difference (MD) 1.86, 95% confidence interval (CI) 1.07 to 2.66; P < 0.001; 5 studies; 158 participants) and parallel studies (MD 0.99, 95% CI 0.90 to 1.07; P < 0.001; 7 studies, 352 participants). Overall, there is evidence of an advantage in using APC (MD 1.29, 95% CI 1.00 to 1.58; P < 0.001) (Figure 4; Analysis 1.1).
4.
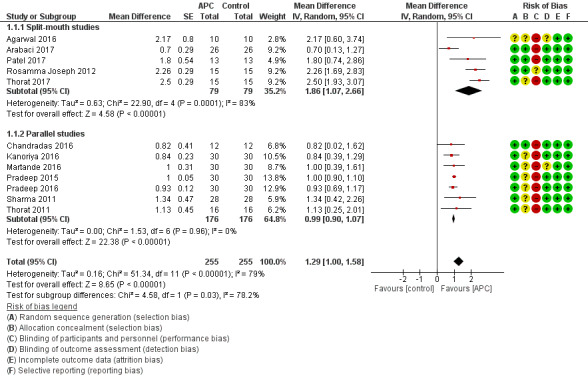
Forest plot of comparison: 1 APC + OFD versus OFD (9‐12 months follow‐up); outcome: 1.1 Probing depth (mm).
1.1. Analysis.

Comparison 1 APC + OFD versus OFD (9‐12 months), Outcome 1 Probing depth (mm).
Change in clinical attachment level (CAL) (mm)
Follow‐up between 9 and 12 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 2.36, 95% CI 1.19 to 3.54; P < 0.001; 5 studies; 158 participants) and parallel studies (MD 0.99, 95% CI 0.84 to 1.14; P < 0.001; 7 studies; 352 participants). Overall, there is evidence of an advantage in using APC (MD 1.47, 95% CI 1.11 to 1.82; P < 0.001) (Analysis 1.2).
1.2. Analysis.

Comparison 1 APC + OFD versus OFD (9‐12 months), Outcome 2 Clinical attachment level (mm).
Change in radiographic bone defect filling (RBF) (%)
Follow‐up between 9 and 12 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 27.32%, 95% CI 20.92% to 33.72%; P < 0.001; 2 studies; 49 participants) and parallel studies (MD 35.77%, 95% CI 31.20% to 40.35%; P < 0.001; 7 studies; 352 participants). Overall, there is evidence of an advantage in using APC (MD 34.26%, 95% CI 30.07% to 38.46%; P < 0.001) (Analysis 1.3).
1.3. Analysis.

Comparison 1 APC + OFD versus OFD (9‐12 months), Outcome 3 Radiographic bone defect filling (%).
2. APC + OFD + bone graft (BG) versus OFD + BG
Change in PD (mm)
All follow‐ups
There is evidence of an advantage in using APC from split‐mouth studies (MD 0.47, 95% CI 0.24 to 0.71; P < 0.001; 12 studies; 360 participants) and from parallel studies (MD 0.81, 95% CI 0.58 to 1.03; P < 0.001; 5 studies; 209 participants). Overall, there is evidence of an advantage in using APC (MD 0.54, 95% CI 0.33 to 0.75; P < 0.001) (Figure 5; Analysis 2.1).
5.
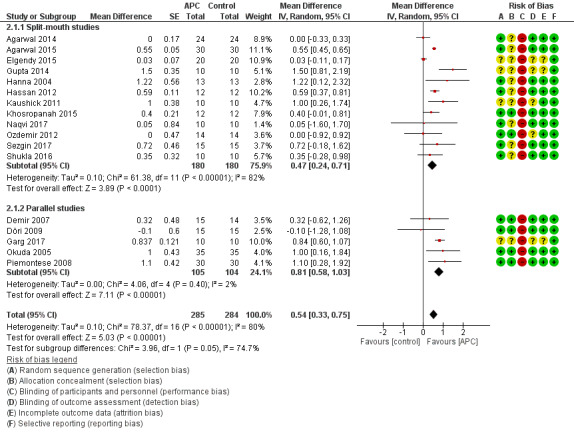
Forest plot of comparison: 2 APC + OFD + BG versus OFD + BG (all follow‐ups); outcome: 2.1 Probing depth (mm).
2.1. Analysis.

Comparison 2 APC + OFD + BG versus OFD + BG (all follow‐ups), Outcome 1 Probing depth (mm).
Follow‐up between 3 and 6 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 0.58, 95% CI 0.25 to 0.92; P = 0.0007; 10 studies; 252 participants). However, there is only one study to consider of parallel design (MD 0.84, 95% CI 0.60 to 1.07; P < 0.001; 20 participants). Overall, there is evidence of an advantage in using APC with a shorter follow‐up duration (MD 0.62, 95% CI 0.30 to 0.94; P = 0.0002) (Analysis 3.1).
3.1. Analysis.
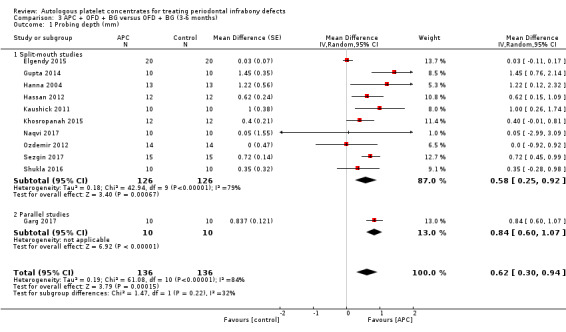
Comparison 3 APC + OFD + BG versus OFD + BG (3‐6 months), Outcome 1 Probing depth (mm).
Follow‐up between 9 and 12 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 0.49, 95% CI 0.26 to 0.72; P < 0.001; 6 studies; 192 participants), and from parallel studies (MD 0.58, 95% CI 0.09 to 1.06; P = 0.02; 4 studies; 189 participants). Overall, there is evidence of an advantage in using APC (MD 0.50, 95% CI 0.31 to 0.69; P < 0.0001) (Analysis 4.1).
4.1. Analysis.

Comparison 4 APC + OFD + BG versus OFD + BG (9‐12 months), Outcome 1 Probing depth (mm).
Change in CAL (mm)
All follow‐ups
There is evidence of an advantage in using APC from split‐mouth studies (MD 0.67, 95% CI 0.35 to 0.99; P < 0.001; 12 studies; 360 participants) and from parallel design studies (MD 0.89, 95% CI 0.49 to 1.29; P < 0.001; 5 studies; 209 participants). Overall, there is evidence of an advantage in using APC (MD 0.72, 95% CI 0.43 to 1.00; P < 0.001) (Analysis 2.2).
2.2. Analysis.

Comparison 2 APC + OFD + BG versus OFD + BG (all follow‐ups), Outcome 2 Clinical attachment level (mm).
Follow‐up between 3 and 6 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 0.40, 95% CI 0.02 to 0.77; P = 0.04; 10 studies; 252 participants). However, there is only one study to consider of parallel design (MD 1.00, 95% CI 0.93 to 1.07; P < 0.001; 20 participants). Overall, there is evidence of an advantage in using APC (MD 0.47, 95% CI 0.11 to 0.84; P = 0.01) (Analysis 3.2).
3.2. Analysis.

Comparison 3 APC + OFD + BG versus OFD + BG (3‐6 months), Outcome 2 Clinical attachment level (mm).
Follow‐up between 9 and 12 months (only split‐mouth studies)
There is evidence of an advantage in using APC (MD 0.84, 95% CI 0.62 to 1.06; P < 0.001; 6 studies; 192 participants) (Analysis 4.2).
4.2. Analysis.

Comparison 4 APC + OFD + BG versus OFD + BG (9‐12 months), Outcome 2 Clinical attachment level (mm).
Change in RBF (%)
All follow‐ups
There is evidence of an advantage in using APC from both split‐mouth studies (MD 7.73%, 95% CI 4.50% to 10.97%; P < 0.001; 8 studies; 270 participants) and parallel studies (MD 9.66%, 95% CI 5.39% to 13.94%; P < 0.001; 3 studies; 150 participants). Overall, there is evidence of an advantage in using APC (MD 8.10%, 95% CI 5.26% to 10.94%; P < 0.001) (Analysis 2.3).
2.3. Analysis.

Comparison 2 APC + OFD + BG versus OFD + BG (all follow‐ups), Outcome 3 Radiographic bone defect filling (%).
Follow‐up between 3 and 6 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 3.59%, 95% CI 0.13% to 7.05%; P = 0.04; 5 studies; 142 participants) and from one parallel study (MD 10.00%, 95% CI 4.90% to 15.10%; P = 0.0001; 20 participants). Overall, there is evidence of an advantage in using APC (MD 4.76%, 95% CI 1.27% to 8.25%; P = 0.008) (Analysis 3.3).
3.3. Analysis.

Comparison 3 APC + OFD + BG versus OFD + BG (3‐6 months), Outcome 3 Radiographic bone defect filling (%).
Follow‐up between 9 and 12 months
There is evidence of an advantage in using APC from split‐mouth studies (MD 10.16%, 95% CI 6.18% to 14.14%; P < 0.001; 4 studies; 152 participants), and from parallel studies (MD 8.87%, 95% CI 1.03% to 16.71%; P = 0.03; 2 studies; 130 participants). Overall, there is evidence of an advantage in using APC (MD 9.99%, 95% CI 6.44% to 13.55%; P < 0.001) (Analysis 4.3).
4.3. Analysis.

Comparison 4 APC + OFD + BG versus OFD + BG (9‐12 months), Outcome 3 Radiographic bone defect filling (%).
3. APC + guided tissue regeneration (GTR) versus GTR
Change in PD (mm)
All follow‐ups
There is evidence of an advantage in using APC from split‐mouth studies (MD 1.52, 95% CI 0.54 to 2.51; P = 0.002; 4 studies; 166 participants) but not from parallel studies (MD 0.25, 95% CI ‐0.15 to 0.64; P = 0.22; 3 studies, 82 participants). Overall, there is evidence of an advantage in using APC (MD 0.92, 95% CI ‐0.02 to 1.86; P = 0.05). However, given the wide confidence intervals, there is a possibility of an advantage for the control group (Figure 6; Analysis 5.1).
6.
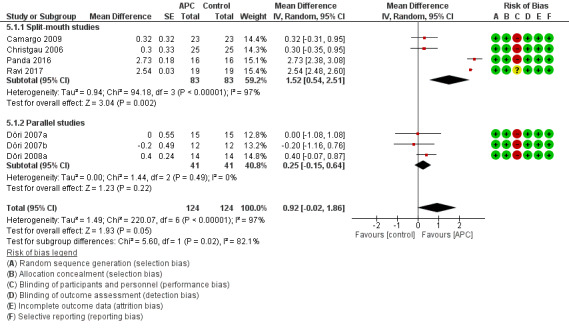
Forest plot of comparison: 5 APC + GTR versus GTR (all follow‐ups), outcome: 5.1 Probing depth (mm).
5.1. Analysis.

Comparison 5 APC + GTR versus GTR (all follow‐ups), Outcome 1 Probing depth (mm).
Follow‐up between 3 and 6 months (only split‐mouth studies)
There is insufficient evidence of an advantage in using APC (MD 1.07, 95% CI ‐0.71 to 2.86; P = 0.24; 3 studies; 134 participants) (Analysis 6.1).
6.1. Analysis.

Comparison 6 APC + GTR versus GTR (3‐6 months), Outcome 1 Probing depth (mm).
Follow‐up between 9 and 12 months
There is insufficient evidence of an advantage in using APC from both split‐mouth studies (MD 1.53, 95% CI ‐0.85 to 3.91; P = 0.21; 2 studies; 82 participants) and parallel studies (MD 0.25, 95% CI ‐0.15 to 0.64; P = 0.22; 3 studies; 82 participants). Overall, there is insufficient evidence of an advantage in using APC (MD 0.68, 95% CI ‐0.66 to 2.02; P = 0.32) (Analysis 7.1).
7.1. Analysis.

Comparison 7 APC + GTR versus GTR (9‐12 months), Outcome 1 Probing depth (mm).
Change in CAL (mm)
All follow‐ups
There is evidence of an advantage in using APC from split‐mouth studies (MD 0.67, 95% CI 0.20 to 1.14; P = 0.005; 4 studies; 166 participants) but not from parallel studies (MD 0.09, 95% CI ‐0.32 to 0.50; P = 0.66; 3 studies; 82 participants). Overall, there is insufficient evidence of an advantage in using APC (MD 0.42, 95% CI ‐0.02 to 0.86; P = 0.06) (Analysis 5.2).
5.2. Analysis.

Comparison 5 APC + GTR versus GTR (all follow‐ups), Outcome 2 Clinical attachment level (mm).
Follow‐up between 3 and 6 months (only split‐mouth studies)
There is evidence of an advantage in using APC (MD 0.54, 95% CI 0.18 to 0.89; P = 0.003; 3 studies; 134 participants) (Analysis 6.2).
6.2. Analysis.

Comparison 6 APC + GTR versus GTR (3‐6 months), Outcome 2 Clinical attachment level (mm).
Follow‐up between 9 and 12 months
There is insufficient evidence of an advantage in using APC from both split‐mouth studies (MD 0.51, 95% CI ‐0.72 to 1.73; P = 0.42; 2 studies; 82 participants) and parallel studies (MD 0.09, 95% CI ‐0.32 to 0.50; P = 0.66; 3 studies; 82 participants). Overall, there is no evidence of an advantage in using APC (MD 0.27, 95% CI ‐0.39 to 0.93; P = 0.42) (Analysis 7.2).
7.2. Analysis.

Comparison 7 APC + GTR versus GTR (9‐12 months), Outcome 2 Clinical attachment level (mm).
4. APC + enamel matrix derivative (EMD) versus EMD
Change in PD (mm)
All follow‐ups
Only one study had a split‐mouth design and showed insufficient evidence of an advantage in using APC (MD 0.13, 95% CI ‐0.05 to 0.31; P = 0.15; 49 participants). Equally only one study had a parallel design which showed insufficient evidence of an advantage in using APC (MD ‐0.10, 95% CI ‐1.32 to 1.12; P = 0.87; 26 participants). Overall, there is insufficient evidence of an advantage in using APC (MD 1.13, 95% CI ‐0.05 to 0.30; P = 0.16) (Analysis 8.1).
8.1. Analysis.

Comparison 8 APC + EMD versus EMD (all follow‐ups), Outcome 1 Probing depth (mm).
Change in CAL (mm)
All follow‐ups
Only one study had a split‐mouth design and showed insufficient evidence of an advantage in using APC (MD 0.12, 95% CI ‐0.12 to 0.36; P = 0.32; 49 participants). The only one study with a parallel design also showed insufficient evidence of an advantage in using APC (MD ‐0.20, 95% CI ‐1.06 to 0.66; P = 0.65; 26 participants). Overall, there is insufficient evidence of an advantage in using APC (MD 0.10, 95% CI ‐0.13 to 0.32; P = 0.40) (Analysis 8.2).
8.2. Analysis.

Comparison 8 APC + EMD versus EMD (all follow‐ups), Outcome 2 Clinical attachment level (mm).
Change in RBF (%)
All follow‐ups
Only one split‐mouth study provided data and showed insufficient evidence of an advantage in using APC (MD ‐0.60%, 95% CI ‐6.21% to 5.01%; P = 0.83; 49 participants) (Analysis 8.3).
8.3. Analysis.

Comparison 8 APC + EMD versus EMD (all follow‐ups), Outcome 3 Radiographic bone defect filling (%).
Secondary outcomes
All the studies in all groups reported a survival rate of 100% for the treated teeth. No complete pocket closure was reported. No quantitative analysis regarding patients' quality of life was possible.
Discussion
Summary of main results
We included 38 studies in this review. These studies assessed the effects of autologous platelet concentrates (APC) used as an adjunct to periodontal surgical therapies for the treatment of infrabony defects. We assessed the quality of the body of evidence using GRADE criteria, and our assessment is presented in Table 1 (for APC + open flap debridement (OFD) versus OFD alone); Table 2 (for APC + OFD + bone graft (BG) versus OFD + BG); Table 3 (for APC + guided tissue regeneration (GTR) versus GTR); and Table 4 (for APC + enamel matrix derivative (EMD) versus EMD).
All data were analysed separately by subgroups and for specific parameters. In an overall assessment of outcomes, there is evidence that the presence of APC brings advantages in the change of probing depth and clinical attachment level in two types of interventions (APC + OFD and APC + OFD + BG) but it did not show any benefit for probing depth for the APC + GTR and the APC + EMD groups. For the radiographic bone defect filling outcome, there is evidence that the adjunct of APC brings benefits in two types of treatment (APC + OFD and APC + OFD + BG) but it showed insufficient advantage when associated to the treatment with EMD, and no data were available for the GTR group. In the second comparison group (APC + OFD + BG versus OFD + BG) there was evidence of an advantage of APC in all follow‐ups and for all three parameters: probing depth, clinical attachment level, and radiographic bone defect filling. Conversely, when APC are used in combination with GTR or EMD insufficient benefits were observed at any follow‐up period except for clinical attachment level at the 3 to 6 months follow‐up. This would suggest that potential benefits of APC are masked by the well known advantages of gold standard treatments for infrabony defects such as GTR and EMD.
Regarding secondary outcomes, all the studies in all groups reported a survival rate of 100% for the treated teeth. No complete pocket closure was reported. No quantitative analysis regarding patients' quality of life was possible.
Overall completeness and applicability of evidence
Even though most of the studies were conducted by experienced professionals in university settings, we believe that with the adequate training the techniques are applicable in general everyday practice and therefore the generalisation of the results of this review is feasible.
Except for the radiographic bone filling, all other clinical parameters have some level of subjectivity in terms of measurements. However, the procedure for their assessment is generally well standardized and with basic training the result can be reproducible from one practitioner to another.
The follow‐up periods of the studies were, in general, adequate for each of the outcomes. All the included studies had a follow‐up period of at least 3 months for clinical outcomes (probing depth and clinical attachment level), which is adequate for this type of outcome. The radiographic bone defect filling, which requires a longer time in order to be detected, was measured in the majority of the studies between 9 and 12 months.
The vast majority of the patients completed the follow‐up periods in their respective studies and the dropouts never exceeded 20%. Furthermore, all 38 included studies reported the numerical data for the main clinical outcomes (probing depth and clinical attachment level), which made it possible to perform the meta‐analysis with a fair number of studies.
Quality of the evidence
Even though all studies included in this review were randomised controlled trials, 36 of them had a high risk of bias and 2 had an uncertain risk of bias. Consequently, to all of our study groups a high risk of bias was assigned because more than 50% of the studies included in each group had at least one domain rated at high risk of bias. This led to a downgrade of GRADE assessments for all groups.
The body of evidence for APC + OFD versus OFD was assessed as having a very low quality for all three parameters (probing depth, clinical attachment level, and radiographic bone filling). There was evidence of high heterogeneity, however, the study population was larger than 400.
The body of evidence for APC + OFD + BG versus OFD + BG was assessed as being of very low quality for all three parameters (probing depth, clinical attachment level, and radiographic bone filling). They had an adequate study population (larger than 400) but a high heterogeneity.
The body of evidence for APC + GTR versus GTR was assessed as being of very low quality for probing depth and clinical attachment level. There was evidence of imprecision for both parameters despite a good consistency.
The body of evidence for APC + EMD versus EMD was assessed as being of very low quality for probing depth, clinical attachment level, and radiographic bone filling. There was evidence of a high imprecision for all parameters.
Potential biases in the review process
A sensitive electronic search of multiple databases was conducted to identify suitable studies for this review. We did not apply restriction of language or date of publication. For the ongoing studies that met our inclusion criteria and for already published studies with missing data, we directly contacted the corresponding authors, but we were not always able to have a response from them. This led to an exclusion of all missing data from our review. One of the present review authors (Massimo Del Fabbro) is also among the authors of one of the reviews used as a comparative for the outcomes of the current review. We addressed this bias by not involving this author at the evaluation process of the 'Agreements and disagreements with other studies or reviews' session.
This review was aimed at analysing the effect of any type of autologous platelet concentrate for enhancing healing of infrabony defects, and no separate analysis was done for each type of APC. It is possible that the effect of different APCs is different in different subgroups, but since no study was found that compared two or more APCs among them and with a control group, we abandoned the idea of a comparison between APCs.
Agreements and disagreements with other studies or reviews
In general our results were concordant with those of previous systematic reviews.
A systematic review published in the Journal of Periodontology (Del Fabbro 2011) included 16 studies that evaluated treatment outcomes of infrabony defects and gingival recession with our without the adjunct of platelet‐rich plasma (PRP). They found a significant positive effect of the adjunct of PRP to OFD for the clinical attachment level parameter of infrabony defects. On the other hand, no significant difference was found between group with or without PRP in infrabony defects treated with GTR. These results are in agreement with the results of our current review.
Another review (Roselló‐Camps 2015) evaluated 21 studies about the use of PRP for periodontal regeneration compared to other regenerative procedures such as GTR. Similar to our results they found that APC significantly improved clinical attachment level and radiographic bone filling. However, they did not find additive benefits of APC for probing depth reduction.
Finally, a recent review (Castro 2017) analysed 21 articles about the use of leukocyte‐ and platelet‐rich fibrin (L‐PRF). Similar to our systematic review Castro et al found that APC was beneficial for probing depth reduction, clinical attachment level gain and radiographic bone filling, when comparing to OFD alone. However, they did not find differences on these outcomes when L‐PRF was compared to treatments consisting of a connective tissue graft utilisation.
Authors' conclusions
Implications for practice.
This review found very low‐quality evidence that the adjunct of autologous platelet concentrates (APC) to specific surgical techniques such as open flap debridement (OFD) and OFD + bone graft (BG) when treating infrabony defects, may improve probing pocket depth, clinical attachment level, and radiographic bone defect filling outcomes. For guided tissue regeneration (GTR) and enamel matrix derivative (EMD) interventions, insufficient evidence of an advantage in using APC was observed. The number of studies concerning these techniques was very limited (only two studies for EMD) and their quality was assessed as very low. Consequently, these assessments cannot be conclusive.
Implications for research.
The main problem we encountered while performing this review, was the high risk of bias for almost all included studies. Even though we very well understand the many difficulties in carrying out a randomised controlled trial, such a standard of evidence is mandatory in order to come to conclusive results and clinical guidelines. Furthermore, for some specific interventions such as GTR and EMD, there are few studies available that can be consulted in order to formulate conclusions. Therefore, we encourage investigators to further investigate this argument and to increase the quality of the evidence with attention paid to allocation concealment and blinding of the personnel which were not correctly performed in the majority of studies. Additionally, we advise authors of future studies to follow the CONSORT Statement, to clearly detail baseline and follow‐up data for the clinical outcomes and to always perform a sample size calculation.
Lastly, because of very few data available, we could not include in this review a comparison among different types of APC. Therefore, we encourage authors of future studies, to compare in the same study, different types of APC in combination with different surgical interventions in order to assess if one type of APC is more beneficial than another one when used as an adjunct to a specific surgical technique.
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2018 | Amended | Edits to authors' affiliations |
Acknowledgements
The review authors wish to thank Anne Littlewood, Information Specialist for Cochrane Oral Health, for developing and conducting the searches for this review; and peer reviewers for their comments.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
1. (periodont*:ti,ab) AND (INREGISTER) 2. ((infrabony or "infra bony" or intrabony or "intra bony" or infraosseous or "infra osseous" or endosseous or apicomarginal or "apico marginal" or interproximal or "inter proximal"):ti,ab) AND (INREGISTER) 3. (("vertical bone" and defect*):ti,ab) AND (INREGISTER) 4. ((bone and resorp*):ti,ab) AND (INREGISTER) 5. ((intraalveolar or "intra alveolar"):ti,ab) AND (INREGISTER) 6. (#1 or #2 or #3 or #4 or #5) AND (INREGISTER) 7. ((platelet* and (plasma* or fibrin* or concentrat*))) AND (INREGISTER) 8. ((PRP or L‐PRP or PRF or L‐PRF):ti,ab) AND (INREGISTER) 9. (#7 or #8) AND (INREGISTER) 10. (#6 and #9) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh "Platelet‐rich plasma"] #2 [mh Fibrin] #3 (platelet* near/5 (plasma* or fibrin* or concentrat*)) #4 (PRP or L‐PRP or PRF or L‐PRF):ti,ab #5 {or #1‐#4} #6 [mh "periodontal diseases"] #7 periodont* #8 (infrabony or "infra bony" or intrabony or "intra bony" or infraosseous or "infra osseous" or endosseous or apicomarginal or "apico marginal" or interproximal or "inter proximal") #9 ("vertical bone" and defect*) #10 (bone near/3 resorp*) #11 (intraalveolar or "intra alveolar") #12 {or #6‐#11} #13 #5 and #12
Appendix 3. MEDLINE Ovid search strategy
1. Platelet‐rich plasma/ 2. exp Fibrin/ 3. (platelet$ adj5 (plasma$ or fibrin$ or concentrat$)).mp. 4. (PRP or L‐PRP or PRF or L‐PRF).ti,ab. 5. or/1‐4 6. exp Periodontal diseases/ 7. periodont$.mp. 8. (infrabony or "infra bony" or intrabony or "intra bony" or infraosseous or "infra ossesous" or endosseous or apicomarginal or "apico marginal" or interproximal or "inter proximal").ti,ab. 9. ((vertical adj bone) and defect$).ti,ab. 10. (bone adj3 resorp$).ti,ab. 11. (intraalveolar or "intra alveolar").ti,ab. 12. or/6‐11 13. 5 and 12
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. Thrombocyte rish plasma/ 2. Fibrin/ 3. (platelet$ adj5 (plasma$ or fibrin$ or concentrat$)).mp. 4. (PRP or L‐PRP or PRF or L‐PRF).ti,ab. 5. or/1‐4 6. exp Periodontal disease/ 7. periodont$.mp. 8. (infrabony or "infra bony" or intrabony or "intra bony" or infraosseous or "infra ossesous" or endosseous or apicomarginal or "apico marginal" or interproximal or "inter proximal").ti,ab. 9. ((vertical adj bone) and defect$).ti,ab. 10. (bone adj3 resorp$).ti,ab. 11. (intraalveolar or "intra alveolar").ti,ab. 12. or/6‐11 13. 5 and 12
This subject search was linked to an adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see www.cochranelibrary.com/help/central‐creation‐details.html for information).
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database) search strategy
(Mh Platelet‐Rich Plasma or "platelet rich plasma" or "Plasma Rico en Plaquetas" or "Plasma Rico em Plaquetas" or Mh Fibrin or fibrin$)
AND
periodont$
Appendix 6. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
periodontal and platelet rich plasma
periodontal and fibrin
Appendix 7. World Health Organization International Clinical Trials Registry Platform search strategy
periodontal and platelet rich plasma
periodontal and fibrin
Appendix 8. Grey literature (www.greylit.org; www.opengrey.eu) search strategy
periodontal and platelet‐rich plasma
periodontal and fibrin
Data and analyses
Comparison 1. APC + OFD versus OFD (9‐12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 12 | 510 | Mean Difference (Random, 95% CI) | 1.29 [1.00, 1.58] |
| 1.1 Split‐mouth studies | 5 | 158 | Mean Difference (Random, 95% CI) | 1.86 [1.07, 2.66] |
| 1.2 Parallel studies | 7 | 352 | Mean Difference (Random, 95% CI) | 0.99 [0.90, 1.07] |
| 2 Clinical attachment level (mm) | 12 | 510 | Mean Difference (Random, 95% CI) | 1.47 [1.11, 1.82] |
| 2.1 Split‐mouth studies | 5 | 158 | Mean Difference (Random, 95% CI) | 2.36 [1.19, 3.54] |
| 2.2 Parallel studies | 7 | 352 | Mean Difference (Random, 95% CI) | 0.99 [0.84, 1.14] |
| 3 Radiographic bone defect filling (%) | 9 | 401 | Mean Difference (Random, 95% CI) | 34.26 [30.07, 38.46] |
| 3.1 Split‐mouth studies | 2 | 49 | Mean Difference (Random, 95% CI) | 27.32 [20.92, 33.72] |
| 3.2 Parallel studies | 7 | 352 | Mean Difference (Random, 95% CI) | 35.77 [31.20, 40.35] |
Comparison 2. APC + OFD + BG versus OFD + BG (all follow‐ups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 17 | 569 | Mean Difference (Random, 95% CI) | 0.54 [0.33, 0.75] |
| 1.1 Split‐mouth studies | 12 | 360 | Mean Difference (Random, 95% CI) | 0.47 [0.24, 0.71] |
| 1.2 Parallel studies | 5 | 209 | Mean Difference (Random, 95% CI) | 0.81 [0.58, 1.03] |
| 2 Clinical attachment level (mm) | 17 | 569 | Mean Difference (Random, 95% CI) | 0.72 [0.43, 1.00] |
| 2.1 Split‐mouth studies | 12 | 360 | Mean Difference (Random, 95% CI) | 0.67 [0.35, 0.99] |
| 2.2 Parallel studies | 5 | 209 | Mean Difference (Random, 95% CI) | 0.89 [0.49, 1.29] |
| 3 Radiographic bone defect filling (%) | 11 | 420 | Mean Difference (Random, 95% CI) | 8.10 [5.26, 10.94] |
| 3.1 Split‐mouth studies | 8 | 270 | Mean Difference (Random, 95% CI) | 7.73 [4.50, 10.97] |
| 3.2 Parallel studies | 3 | 150 | Mean Difference (Random, 95% CI) | 9.66 [5.39, 13.94] |
Comparison 3. APC + OFD + BG versus OFD + BG (3‐6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 11 | 272 | Mean Difference (Random, 95% CI) | 0.62 [0.30, 0.94] |
| 1.1 Split‐mouth studies | 10 | 252 | Mean Difference (Random, 95% CI) | 0.58 [0.25, 0.92] |
| 1.2 Parallel studies | 1 | 20 | Mean Difference (Random, 95% CI) | 0.84 [0.60, 1.07] |
| 2 Clinical attachment level (mm) | 11 | 272 | Mean Difference (Random, 95% CI) | 0.47 [0.11, 0.84] |
| 2.1 Split‐mouth studies | 10 | 252 | Mean Difference (Random, 95% CI) | 0.40 [0.02, 0.77] |
| 2.2 Parallel studies | 1 | 20 | Mean Difference (Random, 95% CI) | 1.0 [0.93, 1.07] |
| 3 Radiographic bone defect filling (%) | 6 | 162 | Mean Difference (Random, 95% CI) | 4.76 [1.27, 8.25] |
| 3.1 Split‐mouth studies | 5 | 142 | Mean Difference (Random, 95% CI) | 3.59 [0.13, 7.05] |
| 3.2 Parallel studies | 1 | 20 | Mean Difference (Random, 95% CI) | 10.0 [4.90, 15.10] |
Comparison 4. APC + OFD + BG versus OFD + BG (9‐12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 10 | 381 | Mean Difference (Random, 95% CI) | 0.50 [0.31, 0.69] |
| 1.1 Split‐mouth studies | 6 | 192 | Mean Difference (Random, 95% CI) | 0.49 [0.26, 0.72] |
| 1.2 Parallel studies | 4 | 189 | Mean Difference (Random, 95% CI) | 0.58 [0.09, 1.06] |
| 2 Clinical attachment level (mm) | 6 | 192 | Mean Difference (Random, 95% CI) | 0.84 [0.62, 1.06] |
| 2.1 Split‐mouth studies | 6 | 192 | Mean Difference (Random, 95% CI) | 0.84 [0.62, 1.06] |
| 3 Radiographic bone defect filling (%) | 6 | 282 | Mean Difference (Random, 95% CI) | 9.99 [6.44, 13.55] |
| 3.1 Split‐mouth studies | 4 | 152 | Mean Difference (Random, 95% CI) | 10.16 [6.18, 14.14] |
| 3.2 Parallel studies | 2 | 130 | Mean Difference (Random, 95% CI) | 8.87 [1.03, 16.71] |
Comparison 5. APC + GTR versus GTR (all follow‐ups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 7 | 248 | Mean Difference (Random, 95% CI) | 0.92 [‐0.02, 1.86] |
| 1.1 Split‐mouth studies | 4 | 166 | Mean Difference (Random, 95% CI) | 1.52 [0.54, 2.51] |
| 1.2 Parallel studies | 3 | 82 | Mean Difference (Random, 95% CI) | 0.25 [‐0.15, 0.64] |
| 2 Clinical attachment level (mm) | 7 | 248 | Mean Difference (Random, 95% CI) | 0.42 [‐0.02, 0.86] |
| 2.1 Split‐mouth studies | 4 | 166 | Mean Difference (Random, 95% CI) | 0.67 [0.20, 1.14] |
| 2.2 Parallel studies | 3 | 82 | Mean Difference (Random, 95% CI) | 0.09 [‐0.32, 0.50] |
Comparison 6. APC + GTR versus GTR (3‐6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 3 | 134 | Mean Difference (Random, 95% CI) | 1.07 [‐0.71, 2.86] |
| 1.1 Split‐mouth studies | 3 | 134 | Mean Difference (Random, 95% CI) | 1.07 [‐0.71, 2.86] |
| 2 Clinical attachment level (mm) | 3 | 134 | Mean Difference (Random, 95% CI) | 0.54 [0.18, 0.89] |
| 2.1 Split‐mouth studies | 3 | 134 | Mean Difference (Random, 95% CI) | 0.54 [0.18, 0.89] |
Comparison 7. APC + GTR versus GTR (9‐12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 5 | 164 | Mean Difference (Random, 95% CI) | 0.68 [‐0.66, 2.02] |
| 1.1 Split‐mouth studies | 2 | 82 | Mean Difference (Random, 95% CI) | 1.53 [‐0.85, 3.91] |
| 1.2 Parallel studies | 3 | 82 | Mean Difference (Random, 95% CI) | 0.25 [‐0.15, 0.64] |
| 2 Clinical attachment level (mm) | 5 | 164 | Mean Difference (Random, 95% CI) | 0.27 [‐0.39, 0.93] |
| 2.1 Split‐mouth studies | 2 | 82 | Mean Difference (Random, 95% CI) | 0.51 [‐0.72, 1.73] |
| 2.2 Parallel studies | 3 | 82 | Mean Difference (Random, 95% CI) | 0.09 [‐0.32, 0.50] |
Comparison 8. APC + EMD versus EMD (all follow‐ups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probing depth (mm) | 2 | 75 | Mean Difference (Random, 95% CI) | 0.13 [‐0.05, 0.30] |
| 1.1 Split‐mouth studies | 1 | 49 | Mean Difference (Random, 95% CI) | 0.13 [‐0.05, 0.31] |
| 1.2 Parallel studies | 1 | 26 | Mean Difference (Random, 95% CI) | ‐0.10 [‐1.32, 1.12] |
| 2 Clinical attachment level (mm) | 2 | 75 | Mean Difference (Random, 95% CI) | 0.10 [‐0.13, 0.32] |
| 2.1 Split‐mouth studies | 1 | 49 | Mean Difference (Random, 95% CI) | 0.12 [‐0.12, 0.36] |
| 2.2 Parallel studies | 1 | 26 | Mean Difference (Random, 95% CI) | ‐0.2 [‐1.06, 0.66] |
| 3 Radiographic bone defect filling (%) | 1 | 49 | Mean Difference (Random, 95% CI) | ‐0.6 [‐6.21, 5.01] |
| 3.1 Split‐mouth studies | 1 | 49 | Mean Difference (Random, 95% CI) | ‐0.6 [‐6.21, 5.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2014.
| Methods | Trial design: randomised, split‐mouth trial Location: Aligarh, India Number of centres: 1: Department of Periodontics, Dental College, Aligarh, India Recruitment period: not stated Source of funding: not stated Ethical approval: yes, ethical committee of Aligarh Muslim University Number of surgeons: 1 |
|
| Participants | Inclusion criteria: absence of any systemic disease, not taking any medication, no pregnancy or lactation, non‐smokers, no previous treatment for periodontal reasons, no furcation involved, matched pairs of intrabony defects with PD ≥ 6 mm following initial therapy Exclusion criteria: failure to satisfy inclusion criteria Age at baseline: mean not specified, range 30 to 65 years Gender: F 10/M 14 Smokers: excluded Teeth treated: maxillary/mandibular premolars and first/second molars Number randomised (participants/teeth): 24/48 Number evaluated (participants/teeth): 24/48 | |
| Interventions | Comparison: PRP + DFDBA versus DFDBA + saline solution Test group: PRP + DFDBA (n = 24 defects) Control group : DFDBA + saline (n = 24 defects) Surgical technique: OFD with the adjunct of a graft with DFDBA + PRP in test and saline in control Follow‐up duration: 12 months |
|
| Outcomes | Clinical: PD, CAL Radiographic: CEJ‐AC, AC‐BD, CEJ‐BD, defect width |
|
| Notes | Sample size calculation not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, but not specified if it was assessed Complications reported: yes (no complications) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "defects were randomly divided into 2 groups by the flip of a coin" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "defects were randomly divided into 2 groups by the flip of a coin" Comment: not sufficient information provided for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the clinician given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinical parameters were recorded preoperatively and at 12 months postoperatively by one trained examiner who was blind to the treatment assignments. Radiographs were assessed on a light box by a single experienced clinician who was blind to the treatment used" Comment: blinding likely to have been done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Agarwal 2015.
| Methods | Trial design: randomised, split‐mouth trial Location: Aligarh, India Number of centres: 1: Department of Periodontics, Dental College, Aligarh, India Recruitment period: not specified Source of funding: not reported Ethical approval: ethical committee of Dr ZA Dental College, Aligarh Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of a matched pair of interproximal, intrabony defects with PD ≥ 6 mm with defect depth ≥ 4 mm, in asymptomatic posterior teeth. Osseous defects needed to have 2 and/or 3 walls. The plaque and gingival indices, associated with interested tooth, achieved following re‐evaluation of initial therapy had to be ≤ 1. Radiographic evidence of intrabony defects Exclusion criteria: presence of any systemic disease, patients taking any medication, pregnancy or lactation, smokers, previously treated for periodontal reasons, 1‐wall defects and furcation involvement Age at baseline: mean age = 52 ± 7 years Gender: F 14/M 18 Smokers: excluded Teeth treated: 64 Number randomised (participants/teeth): 32/64 Number evaluated (participants/teeth): 30/60 | |
| Interventions | Comparison: PRF + DFDBA versus DFDBA + saline solution Test group: PRF + DFDBA Control group: DFDBA + saline Surgical technique: open flap debridement with the adjunct of a graft with DFDBA + PRP in test and saline in control Follow‐up duration: 12 months |
|
| Outcomes | Clinical: PD, CAL, measured from the CEJ Radiographic: CEJ‐AC, AC‐BD, CEJ‐BD. Differences between pre‐ and postoperative RBL measurements were considered as the radiographic bone loss/gain |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study used a split‐mouth design, in which 2 interproximal sites were randomly (toss of a coin, performed by the study therapists) assigned to the DFDBA with saline or DFDBA with the PRF group" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study used a split‐mouth design, in which 2 interproximal sites were randomly (toss of a coin, performed by the study therapists) assigned to the DFDBA with saline or DFDBA with the PRF group" Comment: insufficient information for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the clinician given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The research was designed as a randomized, double‐blinded, parallel, controlled clinical trial.." and "One operator (AA) performed all the surgeries, whereas another operator (NDG) performed all the clinical and radiographic measurements without knowledge of the groups" Comment: blinding likely to have been done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 patients (4 sites) did not return for follow‐up examinations, 1 of the test group and 1 of the control group |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Agarwal 2016.
| Methods | Trial design: randomised, split‐mouth trial Location: Institute of Dental Sciences, Bareilly, India Number of centres: 1 Recruitment period: not specified Source of funding: nil Ethical approval: Ethics Committee of MJP Rohilkhand University, Bareilly, India Number of surgeons: not reported |
|
| Participants | Inclusion criteria: adult patients in good general health and diagnosed with chronic advanced periodontitis, presence of 3 deep intrabony defects (3‐walled) with a PD > 5 mm located in the interproximal area in maxillary or mandibular posterior teeth in 3 different quadrants. Radiographic evidence of the defects should exist Exclusion criteria: smoking, antibiotic, or anti‐inflammatory treatment or the known use of any medication with the potential to affect periodontal tissues within the preceding 6 months, and pregnancy Age at baseline: not reported Gender: F 3/M 7 Smokers: excluded Teeth treated: not specified Number randomised (participants/teeth): 10/30 Number evaluated (participants/teeth): 8/28 | |
| Interventions | Comparison: the control group (C) consisted of sites treated with OFD alone. Whereas, test group A consisted of sites treated with PRP alone and test group B received PRP in combination with DFDBA Test group: OFD + PRP and PRP + DFDBA Control group: OFD Surgical technique: OFD Follow‐up duration: 12 months |
|
| Outcomes | Clinical: PI, GI, PD, and CAL Radiographic: defect depth reduction and defect resolution. Defect fill was assessed by measuring distance between CEJ and base of the defect. The distance between alveolar crest and base of the defect depicted defect resolution. Change in alveolar crest level was also seen as a measurement of distance between CEJ and alveolar crest |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The defects were assigned randomly to 3 groups" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The defects were assigned randomly to 3 groups" Comment: insufficient information to determine method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the clinician given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 patients (2 defects) did not return for follow‐up examination |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Arabaci 2017.
| Methods | Trial design: randomised, split‐mouth trial Location: Atatürk University, Department of Periodontics, Faculty of Dentistry, Erzurum, Turkey Number of centres: 1 Recruitment period: October 2013 to September 2015 Source of funding: the Scientific Research Fund of Atatürk University (AtaUni BAP‐2011/300) Ethical approval: ethics committee of Atatürk University Faculty of Dentistry, Turkey Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patients with moderate to severe chronic periodontitis with PD ≥ 5 mm and horizontal bone loss of at least 2 quadrants of the jaws after phase I therapy (SRP) Exclusion criteria: smoking or tobacco use in any form; medications known to affect periodontal treatment and blood coagulation; systemic conditions known to affect periodontal status; pregnancy/lactation; and disagreeable oral hygiene (PI > 1.5). Patients with teeth with 3‐wall deep intrabony defects, gingival recession, endodontic lesion, or furcation involvement were also excluded Age at baseline: 29 to 46 years (mean age = 36.49 ± 7.03 years) Gender: F 9/M 17 Smokers: excluded Teeth treated: tooth type was not specified Number randomised (participants/teeth): 26/52 Number evaluated (participants/teeth): 26/52 | |
| Interventions | Comparison: OFD + PRF versus OFD alone Test group: OFD + PRF (n = 26 defects) Control group: OFD alone (n = 26 defects) Surgical technique: full‐thickness mucoperiosteal flap with PRF in test site and full‐thickness mucoperiosteal flap alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, modified sulcus bleeding index, PD, relative attachment level, gingival margin level Radiographic: not reported Other: levels of growth factors (fibroblast growth factor‐2 (FGF‐2), platelet‐derived growth factor‐BB (PDGF‐BB), and transforming growth factor‐beta (TGF‐β)) in the gingival crevicular fluid |
|
| Notes | Sample size calculation: not reported Full‐mouth radiographs were taken only for diagnostic purpose Comparability at baseline: assessed for biochemical parameters, not reported for clinical parameters Complications: not reported Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The chosen sites were disunited fortuitously (using a coin toss method) into test groups and control" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "The chosen sites were disunited fortuitously (using a coin toss method) into test groups and control" comment: correct method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. The patients were blinded to their treatment group allocation. However blinding of the patients is unlikely to influence treatment outcome again because of the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The study, conducted from October 2013 to September 2015, was planned as a randomized, double‐blinded, controlled clinical trial that used a split‐mouth design to.." and "A single periodontal surgeon (TA) carried out all the surgical procedures and a second operator (AK) performed all clinical measurements without information of the groups" Comment: blinding of outcomes assessment done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts and outcomes were reported for all patients |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Aydemir 2016.
| Methods | Trial design: randomised, split‐mouth trial Location: Kirikkale University, Periodontology Department, Turkey Number of centres: 1 Recruitment period: February to August 2014 Source of funding: authors' institution Ethical approval: yes Number of surgeons: 1 |
|
| Participants | Inclusion criteria: existence of chronic periodontitis showing similar bilateral defects with minimum width of 2 mm and a maximum width of 4 mm in a radiographic evaluation at least 6 weeks after phase I therapy (consisted of SRP, oral hygiene instructions and occlusal adjustment, if necessary); existence of at least 2 mm keratinised gingiva; absence of caries and/or untreated endodontic problems; and full mouth plaque and bleeding scores ≤ 20 after phase I therapy Exclusion criteria: defects extending to the furcation area were not included. Systemic conditions, such as diabetes mellitus, rheumatoid arthritis, pregnancy, or lactation, that may affect the periodontal state or healing; antibiotic use in the last 6 months; and smoking (current, occasional or former) Age at baseline: mean = 38.5 ± 9.24 years Gender: F 14/M 14 Smokers: excluded Teeth treated: not specified Number randomised (participants/teeth): 28/56 Number evaluated (participants/teeth): 24/49 | |
| Interventions | Comparison: EMD + PRF and EMD Test group: EMD + PRF (25 defects) Control group : EMD (24 defects) Surgical technique: OFD Follow‐up duration: 6 months |
|
| Outcomes | Clinical: GI, PI, PD, CAL, GR Radiographic: total defect depth from the CEJ to the base of the defect at a line tangent to the adjacent root surface; suprabony defect depth from the CEJ to the alveolar crest; defect width: the horizontal distance from the alveolar crest to the root surface; defect angle: the angle between the line connecting the CEJ to the base of the defect and the lateral border of the defect; linear bone growth and bone fill percentage (BF%) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To assign the defects into 2 groups, EMD + PRF (28 defects ‐ test) and EMD (28 defects ‐ control), a computer‐generated randomization scheme (without blocking) was utilized by 1 author (AD)" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "The use of opaque, numbered envelopes that contained the assigned intervention concealed the allocation" Comment: correct method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The author who performed the measurements on the participants (HGK) and the statistician (AD) were blinded to the surgical procedures and measurements" Comment: blinding done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 patients (6 defects) did not return for follow‐up examination and the reason was provided. (Data of 1 patient from the EMD group was removed from the study due to an acute mechanical trauma 7 days after surgery) |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Camargo 2009.
| Methods | Trial design: randomised, split‐mouth trial Location: School of Dentistry, University of Belgrade, Belgrade, Republic of Serbia Number of centres: 1 Recruitment period: 15 May 1999 to 20 March 2000 Source of funding: not stated Ethical approval: yes, University Institutional Review Board Number of surgeons: 2 |
|
| Participants | Inclusion criteria: patients having 2 similar interproximal defects with PD > 6 mm after initial therapy. Radiographic evidence of intrabony defects had to exist. Upon surgical exposure, defects needed to have a minimum depth of 3 mm and present with 2 or 3 walled defects
Exclusion criteria: systemic illnesses, compromised immune system, pregnant and/or lactating women, and patients taking any drug known to cause gingival enlargement. Patients allergic or sensitive to any of the medications to be used, teeth non‐responsive to cold or endodontically treated
Age at baseline: 34 to 67 years (mean age = 47 ± 10 years) Gender: F 14/M 9 Smokers: 11 smokers/12 non‐smokers Teeth treated: maxillary and mandibular posteriors Number randomised (participants/teeth): 23/46 Number evaluated (participants/teeth): 23/46 |
|
| Interventions | Comparison: PRP/BPBM/GTR versus BPBM/GTR Test group: PRP/BPBM/GTR (n = 23) Control group: BPBM/GTR (n = 23) Surgical technique: intrabony defects treated with PRP/BPBM/GTR for test group and BPBM/GTR for control group Follow‐up duration: 6 months |
|
| Outcomes | Clinical: PD, CAL, defect fill (re‐entry surgery) Radiographic: none Other: alveolar crest resorption |
|
| Notes | Sample size calculation: not reported Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study used a split‐mouth design, and 2 interproximal sites were randomly (toss of a coin) assigned to the control and experimental groups" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "The study used a split‐mouth design, and 2 interproximal sites were randomly (toss of a coin) assigned to the control and experimental groups" Comment: correct method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An examiner other than the surgeons performed all clinical measurements without knowledge of the treatment groups" Comment: blinding of outcome assessment done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Chandradas 2016.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontics, Sree Mookambika Institute of Dental Sciences, India Number of centres: 1 Recruitment period: not specified Source of funding: nil Ethical approval: institutional ethics committee Number of surgeons: 1 |
|
| Participants | Inclusion criteria: systemically healthy patients diagnosed with chronic periodontitis based on the international workshop for the classification of periodontal disease, having ≥ 20 teeth and ≥ 30% of sites with > 4 mm clinical attachment loss, PD ≥ 5 mm, and presence of intrabony defect ≥ 3 mm (measured from alveolar crest to the base of the defect on intraoral periapical radiograph) Exclusion criteria: patients with use of tobacco or tobacco‐related products; systemic or local application of antibiotics within the previous 6 months; patients with poor oral hygiene (PI ≥ 3) after the revaluation of cause‐related therapy Age at baseline: 44.4 years Gender: F 18/M 18 Smokers: excluded Teeth treated: maxilla and mandible Number randomised (participants/teeth): 36/36 Number evaluated (participants/teeth): 36/36 |
|
| Interventions | Comparison: group A, PRF + DBM; group B, PRF alone; and group C, control (OFD) Test groups: PRF + DBM (n = 12), PRF alone (n = 12) Control group: OFD (n = 12) Surgical technique: OFD Follow‐up duration: 9 months |
|
| Outcomes | Clinical: GI, GR, PD, relative attachment level was measured from apical border of the stent to the base of the pocket Radiographic: linear bone growth and percentage in bone fill |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allotment of participants within the groups was performed randomly by creating a randomization list by means of a freeware link (http://www.graphad.com/quickcalcs/randomize1.cfm)" Comment: likely to have been done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment allocation of the patients was prepared and sealed in the numbered opaque envelopes and were opened during surgery immediately after completing the defect debridement. Allocation protocol was unavailable to the periodontal examiner (RS) throughout the study" Comment: correct method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. The patients were blinded to their treatment group allocation. However blinding of the patients is unlikely to influence treatment outcome again because of the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The pre‐ and postoperative assessments were performed by another examiner (RS) without knowledge of the nature of intervention" Comment: blinding done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
Christgau 2006.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Operative Dentistry and Periodontology, University of Regensburg, Germany Number of centres: 1 Recruitment period: not stated Source of funding: reported, Robert Mathys Foundation, Bettlach, Schweiz Ethical approval: ethical committee of the medical facility of University of Regensburg Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient having 1 pair of contralateral deep, intrabony, inter‐proximal periodontal defects with a PPD of at least 6 mm, radiographic evidence of angular bone loss of at least 4 mm at baseline, none of the defects to show furcation involvement Exclusion criteria: not meeting the inclusion criteria Age at baseline: 26 to 62 years (median 42 years) Gender: F 15/M 10 Smokers: 5 patients (smoking 8 cigarettes per day) Teeth treated: not specified Number randomised (participants/teeth): 25/50 Number evaluated (participants/teeth): 25/50 | |
| Interventions | Comparison: β‐TCP/GTR + APC versus β‐TCP/GTR Test group: β‐TCP/GTR + APC (n = 25) Control group : β‐TCP/GTR (n = 25) Surgical technique: intrabony defects were treated with β‐TCP/GTR bioresorbable barrier membrane at control site and APC was additionally applied on test group Follow‐up duration: 12 months |
|
| Outcomes | Clinical: papillary bleeding index, approximal plaque index, CAL, gingival recession, PPD, depth of osseous defect Radiographic: digital subtraction radiography ‐ bone density Other: vertical relative attachment gain |
|
| Notes | Sample size calculation: not reported Radiographs were taken at baseline and at end of follow‐up to analyse by digital subtraction radiography Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For randomized treatment allocation, a randomizing table was created by our mathematician (K‐AH) using the SPSS software (Version 13.0, SPSS Inc., Chicago, IL, USA)" Comment: likely to have been done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization table comprised the patient numbers (1–25) and the corresponding defect numbers (1 and 2) per patient. The therapy methods (test or control) were randomly allocated to the defect numbers. By entering the study, the patient numbers were consecutively allocated to the patients and the defect numbers were allocated to the 2 teeth to be treated. Treatment allocation was concealed to the surgeon until the beginning of the surgery" Comment: likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinical examination was performed by 2 masked examiners .." Comment: blinding of outcome assessment done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Demir 2007.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontology, Faculty of Dentistry, Hacettepe University, Ankara, Turkey Number of centres: 1 Recruitment period: not stated Source of funding: mentioned, The Research Foundation of Hacettepe University Ethical approval: yes, Faculty of Medicine, Ethical Committee of Medical, Surgical and Drug Research, Hacettepe University Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient with no systemic diseases, having a good level of oral hygiene, mobility < 1 mm in total, radiographic evidence of vertical alveolar bone loss at the mesial aspect of the tooth, presence of a mesial inter‐proximal probing pocket depth > 6 mm following initial therapy, no prosthetic restoration or endodontic treatment on the related tooth, any medications affecting the coagulation mechanism Exclusion criteria: failing to meet the inclusion criteria Age at baseline: mean = 36.03 + 12.02 years Gender: F 16/M 13 Smokers: yes (9 smokers ‐ 6 to 10 cigarettes per day) Teeth treated: maxillary and mandibular anterior and posterior teeth Number randomised (participants/teeth): 29/29 Number evaluated (participants/teeth): 29/29 | |
| Interventions | Comparison: PRP/BG versus BG alone Test group: PRP/BG (n = 15) Control group: BG alone (n = 14) Surgical technique: OFD + intrabony defects treated with BG in control group and addition of PRP with BG in test group Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, GI, BOP, PD, GR, CAL Radiographic: none reported Other: surgical re‐entry (CEJ‐BD, CEJ‐CD, intrabony defect depth) |
|
| Notes | Sample size calculation: not reported Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients included in the study were divided into 2 groups randomly by the flip of a coin" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients included in the study were divided into 2 groups randomly by the flip of a coin" Comment: correct method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All clinical and intrasurgical measurements were performed by a single examiner (author AB) at baseline and 9 months after the surgical procedure without knowledge of the treatment groups" Comment: blinding done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | Data were properly reported |
Döri 2007a.
| Methods | Trial design: randomised, parallel trial Location: Semmelweis University, Budapest, Hungary Number of centres: 1 Recruitment period: not stated Source of funding: Semmelweis University Ethical approval: yes, University Ethical Board Number of surgeons: 1 |
|
| Participants | Inclusion criteria: no systemic diseases that could influence the outcome of the therapy, good level of oral hygiene ‐ plaque index, compliance with the maintenance program and presence of 1 intrabony defect with a PD of at least 6 mm and an intrabony component of at least 3 mm as detected on the radiographs, non‐smoker Exclusion criteria: failing to meet inclusion criteria Age at baseline: 28 to 56 years Gender: F 16/M 14 Smokers: excluded Teeth treated: maxillary and mandibular anterior, premolars and molars Number randomised (participants/teeth): 30/30 Number evaluated (participants/teeth): 30/30 | |
| Interventions | Comparison: PRP + NBM/GTR versus NBM/GTR Test group: PRP + NBM/GTR (n = 15/15) Control group: NBM/GTR (n = 15/15) Surgical technique: intrabony defects treated with NBM/GTR in control group and with addition of PRP in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, BOP, PD, GR, CAL Radiographic: not reported Other: INTRA (defined as the distance from the alveolar bone crest to the bottom of the defect) (before surgery) |
|
| Notes | Sample size calculation: reported Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The defects were randomly assigned before surgery to the 2 treatment groups with the randomized block approach. Blocking was performed to control for the effects of the prognostic variables INTRA and CAL to decrease outcome variability (Fleiss 1986). For allowing randomization, INTRA (defined as the distance from the alveolar bone crest to the bottom of the defect) was estimated before surgery on pre‐operative radiographs and by performing trans‐gingival bone sounding" Comment: random sequence generation likely to have been done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "The defects were randomly assigned before surgery to the 2 treatment groups with the randomized block approach. Blocking was performed to control for the effects of the prognostic variables INTRA and CAL to decrease outcome variability (Fleiss 1986). For allowing randomization, INTRA (defined as the distance from the alveolar bone crest to the bottom of the defect) was estimated before surgery on pre‐operative radiographs and by performing transgingival bone sounding. In each case, the surgeon was informed of the assigned treatment option after completion of flap elevation and defect debridement. Also, blood samples were collected from all patients regardless of the subsequent PRP application" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner was not aware, in any of the cases, of the type of treatment rendered" Comment: blinding done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Döri 2007b.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontology, Semmelweis University, Budapest, Hungary Number of centres: 1 Recruitment period: July 2002 to September 2003 Source of funding: not stated Ethical approval: yes, Semmelweis University Ethical Board Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient having no systemic diseases that could influence the outcome of the therapy; having good level of oral hygiene (PI < 1); having compliance with the maintenance program; with presence of 1 intrabony defect with PD > 6 mm and an intrabony component (INTRA) > 3 mm as detected on the radiographs and measured at bone sounding; no intrabony defects extending into a furcation area; and no teeth presenting furcation involvements Exclusion criteria: patients failing to meet the inclusion criteria Age at baseline: 26 to 55 years Gender: F 14/M 10 Smokers: none of the patients were smokers Teeth treated: maxillary and mandibular anterior, premolars and molars Number randomised (participants/teeth): 24/24 Number evaluated (participants/teeth): 24/24 | |
| Interventions | Comparison: PRP + ABBM + GTR versus ABBM + GTR Test group: PRP + ABBM + GTR (n = 12/12) Control group: ABBM + GTR (n = 12/12) Surgical technique: intrabony defects were treated with ABBM + GTR in control group and PRP was additionally applied in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, BOP, PD, GR, and CAL Radiographic: preoperative non‐standardized radiographs were taken with the long cone parallel technique for the purpose of baseline defect characteristics for inclusion Other: none reported |
|
| Notes | Sample size calculation: reported Radiographs were taken without a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a randomized block approach, the defects were randomly assigned before surgery to the 2 treatment groups. Blocking to control for the effects of the prognostic variables, the distance from the alveolar bone crest to the bottom of the defect (INTRA) and CAL was used to decrease outcome variability. 34 INTRA was estimated before surgery based on radiographs and transgingival bone sounding recordings" Comment: random sequence generation likely to have been done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a randomized block approach, the defects were randomly assigned before surgery to the 2 treatment groups. Blocking to control for the effects of the prognostic variables, the distance from the alveolar bone crest to the bottom of the defect (INTRA) and CAL was used to decrease outcome variability. 34 INTRA was estimated before surgery based on radiographs and transgingival bone sounding recordings" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner was not aware, in any of the cases, of the type of treatment rendered" Comment: blinding done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Döri 2008a.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontology, Semmelweis University, Budapest, Hungary Number of centres: 1 Recruitment period: June 2002 and November 2003 Source of funding: Department of Periodontology, Semmelweis University. Part of the grafting material was provided by Curasan, Kleinostheim, Germany Ethical approval: yes, Semmelweis University Ethical Board Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient having no systemic diseases that could influence the outcome of the therapy; having good level of oral hygiene (PI < 1); having compliance with the maintenance program; with presence of 1 intrabony defect with PD > 6 mm and an intrabony component > 3 mm as detected on the radiographs and measured at bone sounding; no intrabony defects extending into a furcation area; and no teeth presenting furcation involvements Exclusion criteria: patients failing to meet the inclusion criteria Age at baseline: 28 to 58 years Gender: F16/M 12 Smokers: none of the patients were smokers Teeth treated: maxillary and mandibular anterior, premolars and molars Number randomised (participants/teeth): 28/28 Number evaluated (participants/teeth): 28/28 | |
| Interventions | Comparison: PRP + β‐TCP + GTR versus β‐TCP + GTR Test group: PRP + β‐TCP + GTR (n = 14/14) Control group: β‐TCP + GTR (n = 14/14) Surgical technique: intrabony defects were treated with β‐TCP + GTR in control group and PRP was additionally applied in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, BOP, PD, GR, and CAL Radiographic: preoperative non‐standardized radiographs were taken with the long cone parallel technique for the purpose of baseline defect characteristics for inclusion Other: none reported |
|
| Notes | Sample size calculation: reported Radiographs were taken without a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a randomized block approach, the defects were assigned to the 2 treatment groups before surgery. Blocking to control for the effects of the prognostic variables, INTRA (the distance from the alveolar bone crest to the bottom of the defect) and CAL were used to decrease outcome variability. 42 INTRA was estimated before surgery based on radiographs and transgingival bone sounding recordings" Comment: random sequence generation likely to have been done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a randomized block approach, the defects were assigned to the 2 treatment groups before surgery. Blocking to control for the effects of the prognostic variables, INTRA (the distance from the alveolar bone crest to the bottom of the defect) and CAL were used to decrease outcome variability. 42 INTRA was estimated before surgery based on radiographs and transgingival bone sounding recordings" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner was not aware of the type of treatment rendered" Comment: blinding done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Döri 2008b.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontology, Semmelweis University, Budapest, Hungary Number of centres: 1 Recruitment period: September 2004 and September 2005 Source of funding: the study was funded by the author's own institution. Part of the graft material was kindly provided by Geistlich, Wolhusen, Switzerland Ethical approval: yes, Semmelweis University Ethical Board Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient having no systemic diseases that could influence the outcome of the therapy; having good level of oral hygiene (PI < 1); having compliance with the maintenance program; with presence of 1 intrabony defect with PD at least 6 mm and an intrabony component > 4 mm as detected on the radiographs Exclusion criteria: patients failing to meet the inclusion criteria Age at baseline: 32 to 56 years Gender: F 14/M 12 Smokers: none of the patients were smokers Teeth treated: maxillary and mandibular anterior, premolars and molars Number randomised (participants/teeth): 26/26 Number evaluated (participants/teeth): 26/26 | |
| Interventions | Comparison: EMD + NBM + PRP versus EMD + NBM Test group: EMD + NBM + PRP (n = 13/13) Control group: EMD + NBM (n = 13/13) Surgical technique: intrabony defects were treated with EMD + NBM in control group and PRP was additionally applied in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, BOP, PD, GR, and CAL Radiographic: preoperative non‐standardized radiographs were taken with the long cone parallel technique for the purpose of baseline defect characteristics for inclusion Other: none reported |
|
| Notes | Sample size calculation: reported Radiographs were taken without a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The defects were randomly assigned before surgery to the 2 treatment groups with the randomized block approach. Blocking to control for the effects of the prognostic variables INTRA and CAL was used to decrease outcome variability (Fleiss 1986). To allow randomization, INTRA (defined as the distance from the alveolar bone crest to the bottom of the defect) was estimated before surgery on pre‐operative radiographs and by performing transgingival bone sounding" Comment: random sequence generation done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "The defects were randomly assigned before surgery to the 2 treatment groups with the randomized block approach. Blocking to control for the effects of the prognostic variables INTRA and CAL was used to decrease outcome variability (Fleiss 1986). To allow randomization, INTRA (defined as the distance from the alveolar bone crest to the bottom of the defect) was estimated before surgery on pre‐operative radiographs and by performing transgingival bone sounding" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner was not aware, in any of the cases, of the type of treatment administered" Comment: blinding done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Döri 2009.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontology, Semmelweis University, Budapest, Hungary Number of centres: 1 Recruitment period: June 2006 and May 2007 Source of funding: stated, Department of Periodontology and Oral and Maxillofacial Surgery, Semmelweis University Ethical approval: yes, Semmelweis University Ethical Board Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient having no systemic diseases that could influence the outcome of the therapy; having good level of oral hygiene (PI < 1); having compliance with the maintenance program; with presence of 1 intrabony defect with PD > 6 mm and an intrabony component (INTRA) > 3 mm as detected on the radiographs and measured at bone sounding; no intrabony defects extending into a furcation area; and no teeth presenting furcation involvements Exclusion criteria: patients failing to meet the inclusion criteria Age at baseline: 28 to 65 years Gender: F 21/M 9 Smokers: none of the patients were smokers Teeth treated: maxillary and mandibular anterior, premolars and molars Number randomised (participants/teeth): 30/30 Number evaluated (participants/teeth): 30/30 | |
| Interventions | Comparison: PRP + ABBM versus ABBM alone Test group: PRP + ABBM (n = 15/15) Control group: ABBM alone (n = 15/15) Surgical technique: CAF + intrabony defects were treated with ABBM alone in control group and PRP was additionally applied in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, BOP, PD, GR, and CAL Radiographic: preoperative non‐standardized radiographs were taken with the long cone parallel technique for the purpose of baseline defect characteristics for inclusion Other: none reported |
|
| Notes | Sample size calculation: reported Radiographs were taken without a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a randomized block approach, the defects were randomly assigned before surgery to the 2 treatment groups..." Comment: random sequence generation likely to have been done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a randomized block approach, the defects were randomly assigned before surgery to the 2 treatment groups..." Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner was not aware of the type of treatment rendered" Commet: blinding of outcomes assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Elgendy 2015.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontology, Faculty of Dentistry, October 6 University and Tanta University, Egypt Number of centres: 2 Recruitment period: February to December 2013 Source of funding: not stated Ethical approval: Research Ethical Committee of Tanta University, Egypt Number of surgeons: not stated |
|
| Participants | Inclusion criteria: presence of 2 almost identical interproximal intrabony defects, 1 on either side of the arch based on radiographic observations with clinical probing depth ≥ 6 mm in teeth Exclusion criteria: any systemic disease that affect the periodontium and contraindicate for periodontal surgery; patients having insufficient platelet count for PRF preparation; patients with coagulation defect or anticoagulation treatment; pregnant or lactating mothers; postmenopausal women; people who take anti‐inflammatory drugs, antibiotics or vitamins within the previous 3 months; people who use mouthwashes regularly; heavy smoking (> 10 cigarettes/day); history of alcohol abuse; unacceptable oral hygiene after the re‑evaluation of phase I therapy Age at baseline: group I 44.25 ± 8.45 years, group II 39.70 ± 6.36 years Gender: not stated Smokers: heavy smokers (> 10 cigarettes/day) were excluded Teeth treated: not reported Number randomised (participants/teeth): 20/40 Number evaluated (participants/teeth): 20/40 | |
| Interventions | Comparison: PRF + NcHA bone graft versus NcHA bone graft alone Test group: PRF + NcHA bone graft (n =20) Control group: NcHA bone graft alone (n = 20) Surgical technique: OFD + intrabony defects were treated with NcHA bone graft alone in control group and PRF was additionally applied in test group Follow‐up duration: 6 months |
|
| Outcomes | Clinical: PI, GI, PPD, CAL Radiographic: bone density Other: none |
|
| Notes | Sample size calculation: reported Comparability at baseline: yes, assessed Complications reported: no Dropouts: not reported, reasons not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Selected sites were randomly divided into 2 groups" Comment: insufficient information regarding the random sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Selected sites were randomly divided into 2 groups" Comment: insufficient information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear wether or not all patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Garg 2017.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontics, Azamgarh Dental College, Azamgarh, India Number of centres: 1 Recruitment period: March 2013 to February 2014 Source of funding: not stated Ethical approval: Institutional Ethical Committee and Review Board of the Government Dental College and Research Institute, Bangalore, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: good general health with no history of allergy, presence of moderate to severe periodontitis, presence of a 3‐wall intrabony defect with PD > 5 mm and CAL > 5 mm with radiographic angular defect depth > 3 mm, located in the interproximal area Exclusion criteria: medically compromised patients, smokers, generalized aggressive periodontitis, pregnant and lactating women, and teeth with grade III mobility Age at baseline: 28 to 47 years Gender: F 15/M 9 Smokers: excluded Teeth treated: not stated Number randomised (participants/teeth): 24/24 Number evaluated (participants/teeth): 24/24 | |
| Interventions | Comparison: OFD + HA/β‐TCP + PRF versus OFD + HA/β‐TCP alone Test group: OFD + HA/β‐TCP + PRF (n = 12/12) Control group: OFD + HA/β‐TCP alone (n = 12/12) Surgical technique: a mucoperiosteal flap was elevated with sulcular incisions. After complete debridement of the defect, scaling and root planing, the defect were filled with PRF and HA/β‐TCP in test site and HA/β‐TCP alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: BOP, PD, CAL Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: not stated Standardized parallel cone technique with grid mount was used to take radiographs Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who met all criteria for entry into surgical phase of the study were then randomized to 'test Group‐I' (PRP + HA and β‐TCP) and 'control Group‐II' (saline + HA and β‐TCP)" Comment: insufficient information provided on the method used for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided on the method used for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Gupta 2014.
| Methods | Trial design: randomised, split‐mouth trial Location: not reported Number of centres: not reported Recruitment period: not stated Source of funding: reported, no funding Ethical approval: yes, Institutional Review Board Number of surgeons: not stated |
|
| Participants | Inclusion criteria: patients selected were in good general health, having an intrabony defect ≥ 2 mm with PD ≥ 6 mm Exclusion criteria: patients with abnormal platelet count, smokers, and pregnant women Age at baseline: not stated Gender: not stated Smokers: excluded Teeth treated: maxillary and mandibular arch Number randomised (participants/teeth): 10/20 Number evaluated (participants/teeth): 10/20 | |
| Interventions | Comparison: PRP/HA versus HA alone Test group: PRP/HA (n = 10) Control group: HA alone (n = 10) Surgical technique: OFD + intrabony defects were treated with HA bone graft in control group and PRP was additionally applied in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: plaque control record, BOP, PD, and relative attachment level Radiographic: INFRA (size of the defect) Other: none |
|
| Notes | Sample size calculation: not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: no Dropouts: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "10 L‐AgP [localized aggressive periodontitis] patients having bilateral intrabony defect ≥ 2 mm and probing depth (PD) ≥ 6 mm were randomly treated either with the PRP/HA graft or HA graft alone" Comment: not sufficient information provided regarding the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "10 L‐AgP patients having bilateral intrabony defect ≥ 2 mm and probing depth (PD) ≥ 6 mm were randomly treated either with the PRP/HA graft or HA graft alone" Comment: not sufficient information provided regarding the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not reported wether all patients concluded the study or not |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Hanna 2004.
| Methods | Trial design: randomised, split‐mouth, double‐blinded trial Location: The University of Texas Health Science Center at Houston, Texas, USA Number of centres: 1 Recruitment period: not reported Source of funding: not stated Ethical approval: yes, Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston, Texas, USA Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patients between 35 to 75 years of age; exhibited plaque score of 20% or less prior to the surgical phase; teeth with mobility less than Miller's Class III or mobile teeth requiring splinting; and teeth responding normally to vitality testing or with stable endodontic therapy Exclusion criteria: known systemic diseases and/or drug therapy known to interfere with wound healing; known drug allergies to any of the medications used in the study; using systemic antibiotics or having received antibiotic therapy in the last 3 months; abnormal platelet counts disclosed by a complete blood count (CBC) test performed within 1 month prior to surgery; and participation in other dental clinical trials Age at baseline: 37 to 74 years Gender: F 8/M 5 Smokers: yes, 1 heavy smoker (> 20 cigarettes/day) Number randomised (participants/teeth): 13/26 Number evaluated (participants/teeth): 13/26 | |
| Interventions | Comparison: BDX + PRP versus BDX alone Test group: BDX + PRP (n = 13 defects) Control group: BDX alone (n = 13 defects) Surgical technique: OFD + intrabony defects treated with BDX alone in control group and additionally PRP was applied in test group Follow‐up duration: 6 months |
|
| Outcomes | Clinical: GI, PI, PD, CAL, recession as the position of the gingival margin from the CEJ, and BOP Radiographic: none reported Other: none |
|
| Notes | Sample size calculation: not reported Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed immediately following defect debridement by the flip of a coin" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed immediately following defect debridement by the flip of a coin" Comment: not sufficient information provided regarding the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "13 patients were enrolled in a randomized, split‐mouth, double‐masked clinical trial" Comment: blinding of outcomes likely to have been done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Hassan 2012.
| Methods | Trial design: randomised, split‐mouth trial Location: University of Dammam, College of Dentistry, Kingdom of Saudi Arabia Number of centres: 1 Recruitment period: not stated Source of funding: self‐funded Ethical approval: yes, Ethical Committee of the College of Dentistry, Dammam University, Kingdom of Saudi Arabia Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patient free from any systemic diseases, non‐smokers, not pregnant (female cases), had a good level of oral hygiene, and had infrabony 2 osseous walls defect with PPD 6 mm and CAL = 5 mm Exclusion criteria: failing to meet the inclusion criteria Age at baseline: mean age = 41.4 + 2.61 years Gender: F 5/M 7 Smokers: excluded Teeth treated: not stated Number randomised (participants/teeth): 12/24 Number evaluated (participants/teeth): 12/24 | |
| Interventions | Comparison: Torus mandibularis bone chips with PRP versus Torus mandibularis bone chips alone Test group: Torus mandibularis bone chips with PRP (n = 12 defects) Control group: Torus mandibularis bone chips alone (n = 12 defects) Surgical technique: OFD + intrabony defects were surgically treated using Torus mandibularis bone chips alone in control group and Torus mandibularis bone chips with PRP in test group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, PPD, CAL Radiographic: bone density, marginal bone loss Other: none |
|
| Notes | Sample size calculation: not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: no Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "24 sites were selected by using a split‐mouth design for each patient determined randomly through a biased coin randomization" Comment: random sequence generation done correctly |
| Allocation concealment (selection bias) | Unclear risk | Quote: "24 sites were selected by using a split‐mouth design for each patient determined randomly through a biased coin randomization" Comment: not enough information to understand if allocation concealment was done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded clinical and radiological assessments were performed at baseline and after 3, 6, 9 and 12 months" Comment: blinding of outcomes likely to have been done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Kanoriya 2016.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontology, Government Dental College and Research Institute (GDCRI), Bengaluru, Karnataka, India Number of centres: 1 Recruitment period: October 2014 to June 2015 Source of funding: not stated Ethical approval: Institutional Ethical Committee, GDCRI, Bengaluru, Karnataka, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of 3‐walled intrabony defects ≥ 3 mm deep (distance measured on intraoral periapical radiographs between alveolar crest and defect base) and interproximal probing depth ≥ 5 mm after etiotropic phase in asymptomatic teeth Exclusion criteria: participants with aggressive periodontitis; known systemic conditions that affect periodontal status; blood disorders and inadequate platelet count (< 200,000/mm3); known medications that affect periodontal therapy outcomes; pregnancy or lactation; smokers and tobacco users; immunodeficient patients; allergies to bisphosphonates; and under systemic bisphosphonate therapy. Patients with poor oral hygiene (PI > 1.5) after etiotropic phase re‐evaluation were also excluded. Apart from this, furcation involved non‐vital teeth, carious teeth indicated for restorative therapy, and grade II mobile teeth were also eliminated Age at baseline: mean age = 39 years Gender: F 55/M 53 (for all 3 groups) Smokers: excluded Teeth treated: 36 were maxillary and mandibular single‐rooted teeth and 54 were maxillary and mandibular multirooted teeth sites Number randomised (participants/teeth): 64/64 Number evaluated (participants/teeth): 60/60 | |
| Interventions | Comparison: OFD + PRF versus OFD alone Test group: OFD + PRF (n = 30/30) Control group: OFD alone (n = 30/30) Surgical technique: full thickness mucoperiosteal flap with PRF in test site and full thickness mucoperiosteal flap alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, modified sulcus bleeding index, PD, CAL Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes For radiographs, single customized bite blocks and paralleling technique were used. Radiographs were taken with a scanner of 6400 dots per inch Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: 4 dropouts 3rd group data (PRF + 1% alendronate gel) not included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "These patients were divided into 3 groups randomly using a computer" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not enough information is provided regarding the allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. For the same reason, even though the patient was blinded, it does not influence the outcome of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One operator (DK) performed all surgeries and a different operator (ARP) performed all parameter measurements without information about the groups" Comment: blinding of outcome assessment done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 patients did not complete the study (4 patients for the 2 groups considered in this review) |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Kaushick 2011.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontics, Saveetha Dental College and Hospitals Chennai, India Number of centres: 1 Recruitment period: not stated Source of funding: nil Ethical approval: Instutional Review Board Number of surgeons: 1 |
|
| Participants | Inclusion criteria: males and females aged between 20 and 50 years; attachment loss > 3 mm as assessed by periodontal probe with a diagnosis of chronic periodontitis; presence of infrabony defects (2/3 wall confirmed upon surgical exposure); patients with a minimum of 2 intrabony defects in different quadrants; vital teeth; teeth with mobility less than grade I; patients willing to comply with multiple recall schedules Exclusion criteria: patients with systemic illness such as diabetes, hypertension, bleeding disorders, epilepsy, or abnormal blood picture; pregnant/lactating women; patients on medications known to cause gingival overgrowth or interfere with wound healing; patients allergic to routine medications prescribed following surgery; mucogingival problems; aggressive periodontitis; smokers; trauma from occlusion Age at baseline: 20 to 50 years Gender: not stated Smokers: excluded Teeth treated: not stated Number randomised (participants/teeth): 10/20 Number evaluated (participants/teeth): 10/20 | |
| Interventions | Comparison: PRP + bone graft (HA + ß‐TCP) versus saline + bone graft (HA + ß‐TCP) Test group: PRP + bone graft (HA + ß‐TCP) (n = 10) Control group: saline + bone graft (HA + ß‐TCP) (n = 10) Surgical technique: OFD+ intrabony defects were treated with PRP + bone graft (HA + ß‐TCP) on test group sites and saline + bone graft (HA + ß‐TCP) on control group Follow‐up duration: 6 months |
|
| Outcomes | Clinical: PI (Silness and Löe), GI (Löe and Silness), PD; relative attachment levels (distance between the most apical portion of the stent and the base of the pocket), relative gingival margin levels (distance between the apical most part of the stent and the coronal limit of the gingival margin) Radiographic: radiographic measurements. Radio density Other: none |
|
| Notes | Sample size calculation: not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then randomized into the designated study groups" Comment: insufficient information provided regarding the method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided regarding the method for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided regarding the blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Khosropanah 2015.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontology, Shiraz Dental School, Iran Number of centres: 1 Recruitment period: not specified Source of funding: Vice‐Chancellery of Research of Shiraz University, Iran Ethical approval: ethical approval (CT‐90‐5834) Number of surgeons: not reported |
|
| Participants | Inclusion criteria: moderate to advanced periodontitis, at least 2 intrabony defects with > 4 mm depth based on clinical examination, and at least 3 mm of keratinized tissue Exclusion criteria: systemic diseases or pregnancy, tobacco use, antibiotic intake in the past 3 months, taking anticoagulants for any reason, and history of periodontal therapy Age at baseline: 45 ± 10.7 years Gender: F 7/M 5 Smokers: excluded Number randomised (participants/teeth): 12/24 Number evaluated (participants/teeth):12/24 | |
| Interventions | Comparison: DFDBA + PRP versus DFDBA Test group: DFDBA + PRP (n = 12) Control group: DFDBA (n = 12) Surgical technique: OFD Follow‐up duration: 6 months |
|
| Outcomes | Clinical: PI, BOP, PD, CAL, recession Radiographic: defect height, defect width and angle and hard tissue fills |
|
| Notes | Radiographs were taken with cone beam computed tomography (CBCT) Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In this study, randomization was done using a 2‐step coin tossing method. The first step of coin tossing was performed to choose the right side (tails) versus the left side (heads) and in the second step of coin tossing, the tails indicated controls and the heads indicated the test group. This way, location and type of intervention were both randomized" Comment: random sequence generation properly done |
| Allocation concealment (selection bias) | Low risk | Quote: "In this study, randomization was done using a 2‐step coin tossing method. The first step of coin tossing was performed to choose the right side (tails) versus the left side (heads) and in the second step of coin tossing, the tails indicated controls and the heads indicated the test group. This way, location and type of intervention were both randomized" Comment: allocation concealment done correctly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All measurements, including defect height, defect width and angle at baseline and 6 months later were recorded by an expert radiologist who was blinded to the type of surgical procedure" Comment: correct method for blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients concluded the study |
| Selective reporting (reporting bias) | Low risk | Outcomes properly reported |
Martande 2016.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontics, Government Dental College and Research Institute, Bangalore, India Number of centres: 1 Recruitment period: March 2013 to February 2014 Source of funding: not stated Ethical approval: Institutional Ethical Committee and Review Board of the Government Dental College and Research Institute, Bangalore, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patients with moderate‐to‐severe chronic periodontitis, based on the 1999 consensus classification of periodontal diseases; and presence of a 3‐walled infrabony defect ≥ 3 mm deep in which depth was measured radiographically from the alveolar crest to the base of defect on an intraoral periapical radiograph (IOPA) and the architecture of the 3‐walled infrabony defect confirmed upon surgical exposure of the defect Exclusion criteria: patients with aggressive periodontitis; patients with systemic diseases affecting periodontal condition; those who had received periodontal therapy during the previous 6 months and/or are taking antibiotics for any chronic inflammatory conditions; smokers; pregnant and/or lactating females. Individuals with unacceptable oral hygiene (PI > 1.5) after re‐evaluation of phase I therapy, and teeth with questionable to poor prognosis including: furcation defects; gingival recessions; carious teeth requiring extensive restorations; and non‐vital teeth were also excluded. In addition, 1‐walled and combined 1‐ and 2‐walled defects confirmed upon surgical exposure were also excluded from the study Age at baseline: 30 to 50 years; mean age = 37.6 years Gender: F 48/M 48 (for all 3 groups) Smokers: excluded Teeth treated: 42 sites were from maxillary and mandibular single‐rooted teeth, and the remaining 48 sites were from maxillary and mandibular multirooted teeth Number randomised (participants/teeth): 64/64 (96/96 for all 3 groups) Number evaluated (participants/teeth): 60/60 (90/90 for all 3 groups) | |
| Interventions | Comparison: OFD + PRF versus OFD alone Test group: OFD + PRF (n = 30) Control group: OFD alone (n = 30) Surgical technique: full thickness mucoperiosteal flap with PRF in test site and full thickness mucoperiosteal flap alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, modified sulcus bleeding index, PD, relative attachment level, gingival margin level Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes Radiographs were standardized using customized bite blocks and parallel angle technique and scanned with a scanner of 6400 dots per inch Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: reported, 4 dropouts (6 for all 3 groups, 2 for each group) 3rd group data (OFD + PRF + 1.2% atorvastatin gel) not included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Selected sites were divided randomly (computer‐generated tables) into control and test groups (PRF or PRF + 1.2% ATV [atorvastatin])" Comment: random sequence generation likely to have been done properly |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were masked regarding their allocation to specific group and treatment" Comment: insufficient information is provided for the allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. For the same reason, blinding of the patient, even though it was done, does not influence the outcome of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "To avoid interoperative and inter‐examiner bias, all surgical procedures were performed by a single operator (SSM) and all clinical and radiographic measurements were performed by a single examiner (ARP)" Comment: insufficient information is provided regarding blinding of the outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6 patients did not complete the study (4 patients for the 2 groups considered in this review) |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Naqvi 2017.
| Methods | Trial design: randomised, split‐mouth, double‐blinded clinical trial Location: Department of Periodontics and Oral Implantology, Santosh Dental College and Hospital, Santosh University, Ghaziabad, Uttar Pradesh, India Recruitment period: not reported Source of funding: not stated Ethical approval: the institutional ethical committee Number of surgeons: not stated |
|
| Participants | Inclusion criteria: presence of moderate to severe localized chronic periodontitis, having radiographic evidence of 1 or more vertical defects (2‐ or 3‐walled) and probing pocket depth of 5 mm or more at the experimental site Exclusion criteria: patients with systemic diseases, on anticoagulants, those with habit of smoking and alcohol, with known history of allergy to graft material and who have undergone periodontal surgical treatment for chronic periodontitis within 12 months for the same defects. Pregnant and lactating females as well as patients on antibiotic therapy Age at baseline: 20 to 50 years Gender: F 3/M 7 Smokers: excluded Teeth treated: not stated Number randomised (participants/teeth): 10/20 Number evaluated (participants/teeth): 10/20 | |
| Interventions | Comparison: OFD + bioactive glass putty + PRF versus OFD + bioactive glass putty alone Test group: OFD + bioactive glass putty + PRF (n = 10) Control group: OFD + bioactive glass putty alone (n = 10) Surgical technique: full thickness mucoperiosteal flap with OFD + intrabony defects treated with bioactive glass putty alone in control group and additionally PRF was applied in test group Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PD, CAL Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: not reported Standardized intraoral periapical radiographs of the defects were taken using a paralleling technique Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The intrabony defects were randomly assigned to either control group (bioactive glass putty alone) and test group (bioactive glass putty and PRF) by draw of chits" Comment: random sequence generation properly done |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The intrabony defects were randomly assigned to either control group (bioactive glass putty alone) and test group (bioactive glass putty and PRF) by draw of chits" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. For the same reason, blinding of the patient, even though it was done, does not influence the outcome of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the patients nor the investigator was aware of the group assignment, thereby assuring double blindness" Comment: blinding of outcome assessment done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients concluded the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Okuda 2005.
| Methods | Trial design: randomised, parallel trial Location: Niigata University Medical and Dental Hospital, Japan Number of centres: 1 Recruitment period: not stated Ethical approval: ethical committee for human subject use at Niigata University Medical and Dental Hospital in accordance with the Helsinki Declaration of 1975 as revised in 1983 Number of surgeons: 3 |
|
| Participants | Inclusion criteria: individuals who were non‐smoking, free of systemic complications, and without a history of allergies; had not used antibiotics within the previous 6 months prior to treatment; had not been treated for periodontitis during the previous 2 years; had 1 intrabony defect with PD) ≥ 6 mm, CAL loss ≥ 6 mm, and an osseous defect depth estimated from radiographic evaluation as ≥ 3 mm; and had at least 2 mm of keratinized gingiva on the facial aspect of the selected tooth Exclusion criteria: failing to meet inclusion criteria Age at baseline: mean age = 55.5 ± 8.2 years Gender: F 49/M 21 Smokers: excluded Number randomised (participants/teeth): 70/70 Number evaluated (participants/teeth): 70/70 | |
| Interventions | Comparison: PRP + HA versus saline + HA Test group: PRP + HA (n = 35/35) Control group: saline + HA (n = 35/35) Surgical technique: OFD + intrabony defects were treated with PRP + HA on test group sites and saline + HA on control group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PD, CAL, GR, vertical relative attachment gain Radiographic: intrabony defect depth fill Other: none |
|
| Notes | Sample size calculation: not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients who met all criteria for entry into the surgical phase of the study were then randomized by a coin toss to the test (PRP + HA) or control (saline + HA) study groups" Comment: random sequence generation done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients who met all criteria for entry into the surgical phase of the study were then randomized by a coin toss to the test (PRP + HA) or control (saline + HA) study groups" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All radiographs were evaluated by a single examiner (author KT) who was masked to the treatment group to which a patient was assigned" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Ozdemir 2012.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontology, Faculty of Dentistry, Gazi University, Turkey Number of centres: 1 Recruitment period: not reported Source of funding: not reported Ethical approval: Ethical Board of Gazi University School of Medicine, Turkey Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patients with no periodontal treatment and consumption of medicine 6 months before the study; a good level of oral hygiene (PI < 1) at re‐evaluation sessions; no orthodontic treatment; compliance with the maintenance program; the involved teeth were vital and had no mobility, occlusal trauma, endodontic treatment, or prosthetic restoration; at least 2 similar 3‐walled intrabony defects with 6 mm PD at interproximal region, which was supported by periapical radiographs; intrabony defects that were not on the same tooth or at the same interproximal region and were localized to the interproximal region of mandibular and maxillary anterior and premolar teeth and mesial root of the first mandibular molars; and keratinized gingival width of at least 2 to 3 mm in the defect region Exclusion criteria: pregnant and/or lactating women; smokers; abnormal platelet counts disclosed by a complete blood count test performed within 2 weeks before surgery; and participation in other dental clinical trials at the time of this trial Age at baseline: mean = 48.9 + 6.6 years Gender: F 5/M 9 Smokers: excluded Teeth treated: not reported Number randomised (participants/teeth): 14/28 Number evaluated (participants/teeth): 14/28 | |
| Interventions | Comparison: PRP/ß‐TCP versus ß‐TCP alone Test group: PRP/ß‐TCP (n = 14) Control group: ß‐TCP alone (n = 14) Surgical technique: OFD intrabony defects were treated with ß‐TCP alone in control group and additionally PRP was applied to the test group Follow‐up duration: 6 months |
|
| Outcomes | Clinical: PI, GI, PPD, CAL, BOP, and GR measured between CEJ and gingival margin Radiographic: radiographic intrabony defect depth Other: none |
|
| Notes | Sample size calculation: not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "ß‐TCP (n = 14) and PRP/ß‐TCP groups (n = 14) were selected randomly by the toss of a coin and each patient had 1 pair of both ß‐TCP and PRP/ß‐TCP group defects" Comment: random sequence generation done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "ß‐TCP (n = 14) and PRP/ß‐TCP groups (n = 14) were selected randomly by the toss of a coin and each patient had 1 pair of both ß‐TCP and PRP/ß‐TCP group defects" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information is provided regarding the blinding of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients concluded the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Panda 2016.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontics, Saveetha Dental College and Hospitals Saveetha University, Tamil Nadu, India Number of centres: 1 Recruitment period: March to December 2012 Source of funding: no funding Ethical approval: Institutional Human Ethical Committee of Saveetha Dental College and Hospitals, Chennai, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of interproximal intrabony defects ≥ 2 mm (distance between alveolar crest and base of the defect evaluated on intraoral periapical radiographs) along with an interproximal PD ≥ 5 mm following phase I therapy (SRP) Exclusion criteria: present or past systemic illnesses known to affect the outcomes of periodontal therapy; immunocompromised status; tobacco use in any form; current medications that may interfere with periodontal therapy; haematologic disorders, or insufficient platelet count (< 200,000/mm3) and poor oral hygiene after the re‐evaluation of phase I therapy (PI ≥ 1.5); pregnancy and lactation; teeth with furcation defects; mobility of at least Grade II, and carious lesions needing restorations; 2‐ and 1‐wall defects and interdental craters Age at baseline: mean = 38.12 ± 2.06 years Gender: F 8/M 10 Smokers: excluded Teeth treated: not stated Number randomised (participants/teeth): 18/36 Number evaluated (participants/teeth): 16/32 | |
| Interventions | Comparison: GTR + PRF versus GTR alone Test group: GTR + PRF (n = 16) Control group: GTR alone (n = 16) Surgical technique: GTR and PRF in test site and GTR alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, modified sulcus bleeding index, PPD, CAL, and gingival marginal level Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes PD and the CAL were measured using customized acrylic stents with grooves to ensure a reproducible placement of the probe Radiographs were made using customized bite blocks and long cone paralleling angle technique Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: 2 dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A simple randomization (coin toss) scheme was used by 1 of the authors (MDF) to assign the patients with an allocation ratio of 1:1 into 2 study groups: PRF + GTR (18 patients, test) and GTR alone (18 patients, control)" Comment: random sequence generation properly done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocations were concealed by using number‐labelled opaque envelopes containing the name of the assigned intervention" Comment: allocation concealment properly done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. For the same reason, blinding of the patient, even though it was done, does not influence the outcome of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Since only 1 examiner (SM) measured the clinical and radiographic parameters in the study, intra‐examiner reliability assessment was done to validate the ability of the examiner to constantly replicate the quantitative outcome measurements of the parameters used." "The examiner was blinded to treatment" (information provided by the author) Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 patients did not conclude the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Patel 2017.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontology, Jagadguru Sri Shivarathreshwara (JSS) Dental College and Hospital, Mysore, India Number of centres: 1 Recruitment period: from October 2010 to (not stated) Source of funding: not stated Ethical approval: Institutional Review Board of the JSS University governing the use of human patients in clinical experimentation Number of surgeons: 1 |
|
| Participants | Inclusion criteria: the presence of 2 similar mandibular interproximal, 3‐walled intrabony defects with PD ≥ 6 mm and radiographic evidence of ≥ 3 mm distance between alveolar crest and base of the defect. PI and GI achieved after initial therapy had to be < 1. Only vital teeth were included in the study Exclusion criteria: individuals with underlying systemic illnesses and those taking any drug known to affect the outcome of periodontal therapy and/or drugs effecting platelets; smokers, immunocompromised individuals; and pregnant or lactating individuals. Defect sites which were found to be 1‐walled on flap reflection were also excluded Age at baseline: mean = 44 ± 9 years Gender: F 9/M 4 Smokers: excluded Teeth treated: lower single‐rooted and multirooted teeth Number randomised (participants/teeth): 13/26 Number evaluated (participants/teeth): 13/26 | |
| Interventions | Comparison: OFD + PRF versus OFD alone Test group: OFD + PRF (n = 13) Control group: OFD alone (n = 13) Surgical technique: full thickness mucoperiosteal flap and debridement + PRF in test site and full thickness mucoperiosteal flap and debridement alone in control site Follow‐up duration: 12 months |
|
| Outcomes | Clinical: PI, GI, reduction in PD, gain in CAL Radiographic: radiographic bone filling Other: wound healing index |
|
| Notes | Sample size calculation: yes PD and CAL were measured by a manual periodontal probe using customized acrylic stents Radiographic evaluation was done using digital radiography/radiovisiography with the long cone parallel technique Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of the selected sites (i.e. 2 similar interproximal sites in each individual) was done by toss of a coin by the study therapist (GP)" Comment: random sequence generation done properly |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of the selected sites (i.e. 2 similar interproximal sites in each individual) was done by toss of a coin by the study therapist (GP)" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "It was a double‐masked, single‐center, prospective study of 12 months duration" Comment: blinding of the outcome assessment likely to have been done properly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients concluded the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Piemontese 2008.
| Methods | Trial design: randomised, parallel trial Location: Polytechnic University of Marche, Ancona, Italy Number of centres: 1 Recruitment period: 2002 to 2003 Source of funding: study supported by the Polytechnic University of Marche, Ancona, Italy Ethical approval: Committee for the Protection of Human Subjects at the Polytechnic University of Marche Number of surgeons: 1 |
|
| Participants | Inclusion criteria: individuals who were non‐smoking, free of systemic complications, and without a history of allergies; had not used antibiotics within the previous 6 months prior to treatment; had not had abnormal platelet counts disclosed by a complete blood cell count performed within 1 month prior to surgery; had not been treated for periodontitis during the previous 2 years; had radiographic and clinical evidence of 1 defect with PD > 6 mm, CAL > 6 mm, osseous defect depth estimated from radiographic evaluation as > 3 mm, and 2 or 3 osseous walls; had no intrabony defects extending into a furcation area; and had no teeth presenting furcation involvement Exclusion criteria: patients failing to meet inclusion criteria Age at baseline: 47 to 72 years Gender: F 29/M 31 Smokers: excluded Teeth treated: maxillary and mandibular incisor and premolar and maxillary molar Number randomised (participants/teeth): 60/60 Number evaluated (participants/teeth): 60/60 | |
| Interventions | Comparison: PRP + DFDBA versus DFDBA + saline Test group: PRP + DFDBA (n = 30/30) Control group: DFDBA + saline (n = 30/30) Surgical technique: OFD intrabony defects were treated with PRP/DFDBA in test group and saline/DFDBA in control group Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PI, GI, BOP, PD, CAL, REC Radiographic: CEJ‐BD , AC‐BD, CEJ‐AC Other: none |
|
| Notes | Sample size calculation: not reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by the toss of a coin immediately following defect debridement" Comment: random sequence generation done correctly |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by the toss of a coin immediately following defect debridement" Comment: allocation concealment likely to have been done correctly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The study was designed as a randomized, double‐masked, clinical trial comparing the periodontal outcomes ..." and "On the day of the surgical procedure, baseline clinical measurements were recorded by the same calibrated examiner (SDA) masked to the treatment" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients concluded the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Pradeep 2015.
| Methods | Trial design: randomised, longitudinal, triple‐masked, parallel trial Location: Department of Periodontics, Government Dental College and Research Institute, Bangalore, India Number of centres: 1 Recruitment period: November 2013 to July 2014 Source of funding: not stated Ethical approval: Institutional Ethical Committee and Review Board of the Government Dental College and Research Institute, Bangalore, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of intrabony defect ≥ 3 mm deep (distance between alveolar crest and base of the defect on an intraoral periapical radiograph (IOPA)) along with an interproximal PD ≥ 5 mm after phase I therapy (scaling and root planing) in asymptomatic maxillary/mandibular molar teeth Exclusion criteria: aggressive periodontitis patients; patients with systemic conditions known to affect the periodontal status; medications known to affect the outcomes of periodontal therapy; haematological disorders and insufficient platelet count (< 200,000/mm3); pregnancy/lactation; smoking and tobacco use in any form; and immunocompromised individuals. Those having unacceptable oral hygiene (PI > 1.5) after re‐evaluation of phase I therapy were also excluded. In addition, teeth with furcation defects, non‐vital teeth, carious teeth warranting restorations and mobility of at least grade II were also excluded Age at baseline: mean = 41 years Gender: F 68/M 68 (for all 4 groups) Smokers: excluded Teeth treated: maxillary and mandibular molar Number randomised (participants/teeth): 64/64 (126/126 for all 4 groups; 136 eligible but 10 excluded at time of surgery) Number evaluated (participants/teeth): 60/60 (120/120 for all 4 groups) | |
| Interventions | Comparison: OFD alone versus OFD + PRF Group 1: OFD alone (n = 30) Group 2: OFD + PRF (n = 30) Surgical technique: in group 1, only OFD was done, without addition of any regenerative material into the bone defect; in group 2, PRF of the required size was filled into the intrabony defect after OFD Follow‐up duration: 9 months |
|
| Outcomes | Clinical: site specific PI, modified sulcus bleeding index, relative attachment level, gingival marginal level Radiographic: radiographic intrabony defect depth Other: none |
|
| Notes | Sample size calculation: reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, 4 dropouts (6 for all 4 groups) 3rd and 4th group data (OFD + 1% metformin and OFD + PRF + 1% metformin) not included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "These patients were divided randomly (computer generated tables) into 4 groups" Comment: random sequence generation properly done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided for allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment. For the same reason, blinding of the patient, even though it was done, does not influence the outcome of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "This was a randomized, single‐centre, longitudinal, triple‐masked (investigators, individuals and statistician), parallel arm design study" and "One operator (KN) performed all the surgeries, whereas another operator (ARP) performed all the clinical and radiographic measurements without knowledge of the groups" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 dropouts (6 for all 4 groups) |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Pradeep 2016.
| Methods | Trial design: randomised, parallel trial Location: Periodontics Clinic, GDCRI, Bengaluru, Karnataka, India Number of centres: 1 Recruitment period: January to October 2015 Source of funding: not stated Ethical approval: ethical approval from the Institutional Ethical Committee and Review Board of Government Dental College and Research Institute (GDCRI), Bengaluru, Karnataka, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: systemically healthy with diagnosis of chronic periodontitis; PD ≥ 5 mm; CAL ≥ 3 mm; and 2‐ or 3‐walled intrabony defect on at least 1 mandibular molar; vertical bone loss ≥ 3 mm on intraoral periapical radiographs and no antibiotic or periodontal therapy in 6 months before study Exclusion criteria: statin allergy; statin therapy; any systemic condition or medication altering periodontal condition; an immunocompromised state; haematologic disorders; insufficient platelet count (< 200,000/mm3); aggressive periodontitis; substance/tobacco abuse; lactating or pregnant females. Age at baseline: mean age = 35 years Gender: F 45/M 45 (for all 3 groups) Smokers: excluded Teeth treated: 42 sites were from maxillary and mandibular single‐rooted teeth, and the remaining 48 sites were from maxillary and mandibular multirooted teeth Number randomised (participants/teeth): 60/60 (90/90 for all 3 groups) Number evaluated (participants/teeth): 60/60 (90/90 for all 3 groups) | |
| Interventions | Comparison: OFD + PRF versus OFD alone Test group: OFD + PRF (n = 30/30) Control group: OFD alone (n = 30/30) Surgical technique: full thickness mucoperiosteal flap with PRF in test site and full thickness mucoperiosteal flap alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, modified sulcus bleeding index, PD, CAL Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes Reproducible parallel‐angle radiographs of concerned sites using customized bite blocks Comparability at baseline: yes, assessed Complications reported: yes, no complications Dropouts: no dropouts 3rd test group data (OFD + PRF + 1.2% rosuvastatin gel) not included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After power calculations‐based (90% confidence at P ≤ 0.05) enrolment, computer‐assisted random allocation of the 90 patients was done into 3 treatment groups" Comment: random sequence generation done correctly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided for allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator given the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "90 patients ...... were enrolled for this placebo‐controlled, triple‐masked, single‐center randomized controlled clinical trial from January 2015 to October 2015 (9‐month study)" Comment: blinding of outcome assessment done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Ravi 2017.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontology, Saveetha University, India Number of centres: 1 Recruitment period: September 2015 to September 2016 Source of funding: no funding Ethical approval: Institution Human Ethics Committee Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of generalized chronic periodontitis (on the basis of the 1999 consensus classification of periodontal diseases); presence of bilateral intrabony defect ≥ 3 mm deep (distance between alveolar bone crest and base of defect on intraoral periapical radiograph); presence of interproximal PD ≥ 5 mm after phase I periodontal therapy (scaling and root planing); systemically healthy condition Exclusion criteria: history of periodontal surgical treatment within the last 6 months, smokers, pregnant or lactating women Age at baseline: mean age = 43.26 ± 9.45 years Gender: F 9/M 5 Smokers: excluded Teeth treated: premolars and molars Number randomised (participants/teeth): 14/42 Number evaluated (participants/teeth): 12/38 | |
| Interventions | Comparison: GTR + PRGF versus GTR alone Test group: GTR + PRGF (n = 19 sites) Control group : GTR alone (n= 19 sites) Surgical technique: GTR and PRGF in test site and GTR alone in control site Follow‐up duration: 6 months |
|
| Outcomes | Clinical: GI, PD, CAL Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes Customized putty bite blocks were made for each patient to standardize positioning of the sensor and angle with which radiographs were taken Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: 2 patients, 4 sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Selected sites were randomly assigned to 1 of the following groups: 1) PRGF plus GTR or 2) GTR alone by using the coin toss method for each patient (NJ)" Comment: random sequence generation properly done |
| Allocation concealment (selection bias) | Low risk | Quote: "Selected sites were randomly assigned to 1 of the following groups: 1) PRGF plus GTR or 2) GTR alone by using the coin toss method for each patient (NJ)" Comment: allocation concealment likely to have been done properly |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The present study was a split‐mouth randomized control trial in which the operator and assessor were masked" It is stated that the operator was blinded but no further information is provided on the exact method in which it was done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The present study was a split‐mouth randomized control trial in which the operator and assessor were masked" and "The examiner, however, was not aware, in any of the cases, of the type of treatment rendered (SV and SM)" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 patients failed to complete the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Rosamma Joseph 2012.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontics, Government Dental College, Kozhikode, Kerala, India Number of centres: 1 Recruitment period: September 2009 to October 2010 Source of funding: not stated Ethical approval: Institutional Ethics Committee, Government Dental College, Kozhikode, in accordance with the Helsinki Declaration of 1975 as revised in 2000 Number of surgeons: 1 |
|
| Participants | Inclusion criteria: patients had paired, contralateral interproximal infrabony defect with a probing PD > 6 mm, CAL loss > 5 mm, and an osseous defect depth estimated from radiographic evaluation as > 4 mm; were systemically healthy without a history of allergies; and had at least 2 mm of keratinized gingiva on the facial aspect of the selected tooth Exclusion criteria: haematological or immunological disorders; pregnancy or lactation; smoking or the use of other tobacco products; those taking drugs known to interfere with wound healing; had used antibiotics within the previous 1 year; had been treated for periodontitis during the previous 2 years; those with unacceptable oral hygiene (PI) after the re‐evaluation of phase I therapy; were not willing to sign an informed consent Age at baseline: mean = 29.47 + 7.65 years (range 17 to 44 years) Gender: F 9/M 6 Smokers: excluded Teeth treated: not reported Number randomised (participants/teeth): 15/30 Number evaluated (participants/teeth): 15/30 | |
| Interventions | Comparison: OFD + PRFm versus OFD alone Test group: OFD + PRFm (n = 15/15) Control group: OFD alone (n = 15/15) Surgical technique: test group was treated by placement of platelet‐rich fibrin matrix following OFD and control group was treated by OFD alone Follow‐up duration: 1 year |
|
| Outcomes | Clinical: PD, recession/enlargement, CAL, PI, modified GI Radiographic: the vertical dimension between the projection of the bone crest on the root surface (BCP) and the most coronal level along the root surface where the periodontal ligament space was considered to have a normal width (BoBD‐base of bone defect) was measured and designated as infrabony defect depth (IBD = BCP ‐ BoBD). The distance from the crest of remaining alveolar bone to CEJ was also recorded (CEJ‐BC) Other: a visual analogue scale (VAS1) was used to assess the patient experience with the 2 treatment modalities. Another visual analogue scale (VAS2) was designed and used to assess the initial soft tissue healing |
|
| Notes | Sample size calculation: reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Either right sided or maxillary defects were operated first and whether the site belonged to experimental or control group was determined by a simple lottery method by the toss of a coin" Comment: random sequence generation done correctly |
| Allocation concealment (selection bias) | Low risk | Quote: "The sites were divided into experimental and control groups at the time of periodontal surgery. Either right sided or maxillary defects were operated first and whether the site belonged to experimental or control group was determined by a simple lottery method by the toss of a coin" Comment: allocation concealment likely to have been done correctly |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is stated that the operator was blinded but no further information is provided on the exact method in which it was done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All radiographs were evaluated by a single examiner (RJ) who was masked to the treatment group to which a patient was assigned and also to whether the radiograph was taken at baseline or re‐evaluation" Comment: blinding of outcome assessment done correctly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Sezgin 2017.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontology, Gazi University, Turkey Number of centres: 1 Recruitment period: not reported Source of funding: Gazi University Research Grant, Turkey Ethical approval: approved by the ethics board at the Faculty of Dentistry, Gazi University, Turkey Number of surgeons: 2 |
|
| Participants | Inclusion criteria: no systemic diseases; a good level of oral hygiene (PI < 0.15); presence of 2 paired, 2‐ or 3‐walled intrabony defects with PD ≥ 6 mm and an intrabony component of ≥ 3 mm, as detected on radiographs; no intrabony defects extending into the furcation area; tooth mobility ≤ 1; tooth and adjoining teeth testing vital and without symptoms or signs of endodontic involvement; and tooth and adjoining teeth free of caries or inadequate restorations Exclusion criteria: patients with compromised immune systems; pregnant and/or lactating women; patients taking any drug known to affect the periodontal status or the coagulation system; and smokers Age at baseline: 38 to 61 years Gender: F 7/M 8 Smokers: excluded Teeth treated: all Number randomised (participants/teeth): 21/42 Number evaluated (participants/teeth): 15/30 | |
| Interventions | Comparison: ABBM + PRF versus ABBM alone Test group: ABBM + PRF (n = 15) Control group: ABBM alone (n = 15) Surgical technique: OFD Follow‐up duration: 6 months |
|
| Outcomes | Clinical: PI, GI, PD, CAL and GR Radiographic: vertical bone loss, depth of intrabony defect, radiographic defect angle |
|
| Notes | Sample size calculation: reported Radiographs were taken using long cone parallel and direct digital radiography Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: reported, 1 dropout (5 patients excluded because the defects did not meet the study criteria at surgery) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The selected sites were randomly (coin toss) divided into control (ABBM alone) and test (ABBM‐PRF) groups" Comment: correct method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided for the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One examiner other than the surgeons performed all clinical measurements, and another examiner performed all radiographical measurements. Both examiners were blinded to the study groups" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient failed to complete the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Sharma 2011.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontics, Government Dental College and Research Institute, Bangalore, India Number of centres: 1 Recruitment period: June 2009 to March 2010 Source of funding: nil Ethical approval: Institutional Ethical Committee and Review Board, Government Dental College and Research Institute, Bangalore, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of 3‐walled intrabony defects > 3 mm deep (the distance between the alveolar crest and base of the defect on an intraoral periapical radiograph (IOPA)) along with an interproximal PD > 5 mm after phase 1 therapy (scaling and root planing) in an asymptomatic tooth
Exclusion criteria: patients with aggressive periodontitis with known systemic illness and taking any medications known to affect the outcomes of periodontal therapy; an insufficient platelet count (< 200,000/mm3); pregnancy or lactation; use of any form of tobacco; patients who had unacceptable oral hygiene (PI > 1.5) after the re‐evaluation of phase 1 therapy; teeth with furcation defects, non‐vital teeth or teeth with mobility > grade II
Age at baseline: 30 to 50 years; mean = 35.34 + 6.45 years Gender: F 18/M 24 Smokers: excluded Teeth treated: 17 of the 56 sites were from upper and lower single‐rooted teeth, and the remaining 39 sites were from upper and lower multirooted teeth Number randomised (participants/teeth): 42/69 Number evaluated (participants/teeth): 35/56 |
|
| Interventions | Comparison: PRF/OFD versus OFD alone Test group: PRF/OFD (n = 18/28) Control group: OFD alone (n = 17/28) Surgical technique: intrabony defects treated with OFD alone in control group and additionally PRF was added in test group Follow‐up duration: 9 months |
|
| Outcomes | Clinical: site specific PI, modified sulcus bleeding index, PD, periodontal attachment level, gingival margin level Radiographic: radiographic intrabony defect depth Other: none |
|
| Notes | Sample size calculation: reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, reasons given, 7 patients, 13 sites did not return for follow‐up examinations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The selected sites were divided randomly (by using a coin‐toss method) into control and test groups" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided for allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One operator (AS) performed all surgeries whereas another operator (ARP) performed all clinical and radiographic measurements without knowledge of the groups" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 patients out of 42 failed to complete the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Shukla 2016.
| Methods | Trial design: randomised, split‐mouth trial Location: outpatient service on a teaching dental institute in North India (no further details given) Number of centres: 1 Recruitment period: not specified Source of funding: not stated Ethical approval: Institutional Ethical Committee and Review Board of the Government Dental College and Research Institute, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of intrabony defects > 3 mm deep (distance between alveolar crest and base of the defect on intraoral periapical radiograph (IOPA)) and an interproximal PD > 5 mm Exclusion criteria: known systemic illness; taking any medications known to affect the outcomes of periodontal therapy; pregnancy/lactation; use of any form of tobacco; allergy to calcium phosphosilicate putty Age at baseline: mean = 40 + 10.5 years Gender: F 7/M 13 Smokers: excluded Teeth treated: not stated Number randomised (participants/teeth): 20/40 Number evaluated (participants/teeth): 20/40 | |
| Interventions | Comparison: OFD + calcium phosphosilicate (CPS) + PRF versus OFD + CPS alone Test group: OFD + CPS + PRF (n = 20) Control group: OFD + CPS alone (n = 20) Surgical technique: full thickness mucoperiosteal flap with PRF and CPS in test site and full thickness mucoperiosteal flap with CPS alone in control site Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, PD, CAL, GI Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using a computer‐generated randomization list" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided on the allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the evaluations were performed by an independent trained observer not involved in the study" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
Thorat 2011.
| Methods | Trial design: randomised, parallel trial Location: Department of Periodontics, Government Dental College and Research Institute, Bangalore, India Number of centres: 1 Recruitment period: April 2009 to January 2010 Source of funding: self‐funded Ethical approval: Institutional Review Board, India Number of surgeons: 1 |
|
| Participants | Inclusion criteria: presence of interproximal intrabony defects > 3 mm deep (distance between alveolar crest and base of the defect on intraoral periapical radiograph (IOPA)) along with an interproximal PD > 5 mm following phase I therapy (scaling and root planing in vital, asymptomatic first and second mandibular molars without furcation involvement Exclusion criteria: patients with present or past systemic illness that were known to affect the outcomes of periodontal therapy; insufficient platelet count (< 200,000/mm3); immunocompromised patients; pregnancy/lactation; smoking (any other tobacco products); patients taking medications that may interfere with wound healing; those allergic to other medication and having unacceptable oral hygiene (PI > 3) after the re‐evaluation of phase I therapy Age at baseline: 25 to 45 years; mean = 31.1 + 2.06 years Gender: F 18/M 22 Smokers: excluded Teeth treated: first and second mandibular molars Number randomised (participants/teeth): 40/40 Number evaluated (participants/teeth): 32/32 | |
| Interventions | Comparison: PRF + OFD versus OFD alone Test group: PRF + OFD (n = 16/16) Control group: OFD alone (n = 16/16) Surgical technique: intrabony defects treated with OFD alone in control group and additionally PRF was added in test group Follow‐up duration: 9 months |
|
| Outcomes | Clinical: PI, sulcus bleeding index, PD, CAL, and gingival marginal level Radiographic: bone defect fill Other: none |
|
| Notes | Sample size calculation: reported Radiographs were taken with a bite block for ensuring reproducibility Comparability at baseline: yes, assessed Complications reported: yes Dropouts: reported, reasons given, 8 dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The selected sites were divided randomly (coin toss) into the control and test groups" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided regarding the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossibe to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "A review of all the radiographs was performed in a single reference center by a blind evaluator" and "An examiner (ARP) other than the operator performed all clinical measurements without knowledge of the treatment groups" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 out of 40 patients failed to complete the study |
| Selective reporting (reporting bias) | Low risk | All outcomes properly reported |
Thorat 2017.
| Methods | Trial design: randomised, split‐mouth trial Location: Department of Periodontics, Government Dental College and Research Institute, Bangalore, India Number of centres: 1 Recruitment period: not stated Source of funding: not stated Ethical approval: Institutional Ethical Committee and registered with Clinical Trials Registry India REF/12/006069) Number of surgeons: 1 |
|
| Participants | Inclusion criteria: localized aggressive periodontitis; presence of at least 2 contralateral interproximal intrabony defects; intrabony defect ≥ 3 mm (vertical distance between alveolar crest and base of the defect on standardized intraoral periapical radiographs) with corresponding PD ≥ 5 mm following phase I therapy; individual PI score ≤ 2; asymptomatic first/second molars without furcation involvement Exclusion criteria: present or past systemic illness known to affect the outcomes of periodontal therapy; insufficient platelet counts (< 200,000/mm3); immunocompromised status; pregnancy/lactation; taking medications that might interfere with wound healing; and tobacco habits Age at baseline: mean = 25 ± 1.5 years Gender: F 10/M 8 (3 did not receive surgery, their gender not specified) Smokers: excluded Teeth treated: first/second molars Number randomised (participants/teeth): 15/30 Number evaluated (participants/teeth): 15/30 | |
| Interventions | Comparison: OFD + PRF versus OFD alone Test group: OFD + PRF (n = 15) Control group: OFD alone (n = 15) Surgical technique: Kirkland modified flap operation Follow‐up duration: 12 months |
|
| Outcomes | Clinical: gain in CAL, reduction in PD, change in gingival margin level Radiographic: radiographic bone filling Other: none |
|
| Notes | Sample size calculation: yes PD and the CAL were measured by a manual periodontal probe using customized acrylic stents Radiographic evaluation was done on intraoral periapical radiographs using long cone paralleling angle technique and individualized bite blocks with a positioning device Comparability at baseline: yes, assessed Complications reported: no complications Dropouts: no dropouts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sites were assigned using a computer‐generated randomization process" Comment: correct method for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information regarding allocation concealment method was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind the operator due to the surgical nature of the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The preoperative and postoperative clinical parameters were checked by a single blinded examiner. Another blinded and calibrated examiner (radiologist) recorded the radiographic parameters" Comment: blinding of outcome assessment properly done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All results properly reported |
ABBM = anorganic bovine bone mineral; AC = alveolar crest; APC = autologous platelet concentrate; BD = base of the defect; BDX = bovine derived xenograft; BG = bone graft; BOP = bleeding on probing; BPBM = bovine porous bone mineral; ß‐TCP = beta‐tricalcium phosphate; CAF = coronally advanced flap; CAL = clinical attachment level; CD = crest of the defect; CEJ = cemento‐enamel junction; DBM = demineralized bone matrix; DFDBA = demineralized freeze‐dried bone allograft; EMD = enamel matrix derivative; F = female; GI = gingival index; GR = gingival recession; GTR = guided tissue regeneration; HA = hydroxyapatite; M = male; n = number; NBM = natural bone mineral; NcHA = nanocrystalline hydroxyapatite; OFD = open flap debridement; PD = probing depth; PI = plaque index; PPD = probing pocket depth; PRF = platelet‐rich fibrin; PRFm = platelet‐rich fibrin matrix; PRGF = plasma‐rich growth factors; PRP = platelet‐rich plasma; RBL = radiographic bone loss; REC = gingival recession; SRP = scaling and root planing; TDD = total defect depth.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2017 | Mixed design ‐ randomised controlled trial |
| Aleksić 2008 | No randomisation |
| Aroca 2009 | Gingival recession (not infrabony defects) |
| Bajaj 2017 | Mixed design ‐ randomised controlled trial |
| Camargo 2002 | No control group |
| Camargo 2005 | No control group |
| Cetinkaya 2014 | Same participants of Keles 2006 |
| Chatterjee 2017 | Mixed design ‐ randomised controlled trial |
| Cheung 2004 | Autologous platelet concentrates not the only difference between groups |
| Cieplik 2018 | Incomplete data |
| Cortellini 1995 | No platelet concentrate (fibrin glue) |
| Dogan 2015 | Gingival recession (not infrabony defects) |
| Döri 2013 | Same participants of Döri 2008b |
| Eren 2014 | Autologous platelet concentrates not the only difference between groups |
| Gupta 2014b | Non‐independence of analysis unit |
| Harnack 2009 | Incomplete data |
| Huang 2005 | Gingival recession (not infrabony defects) |
| Jankovic 2010 | Gingival recession (not infrabony defects) |
| Jankovic 2012 | Autologous platelet concentrates not the only difference between groups |
| Jovicić 2013 | No randomisation |
| Keceli 2008 | Incomplete data |
| Keles 2006 | Incomplete data |
| Lekovic 2012 | No control group |
| Menezes 2012 | Incomplete data |
| Moder 2012 | Same participants of Christgau 2006 |
| Ouyang 2006 | Mixed design ‐ randomised controlled trial |
| Padma 2013 | Gingival recession (not infrabony defects) |
| Pradeep 2012a | Non‐independence of analysis unit |
| Pradeep 2017 | Mixed design ‐ randomised controlled trial |
| Qiao 2016 | Mixed design ‐ randomised controlled trial |
| Saini 2011 | No randomisation |
| Shah 2015 | Incomplete data |
| Shepherd 2009 | Gingival recession (not infrabony defects) |
| Shivakumar 2016 | Gingival recession (not infrabony defects) |
| Thamaraiselvan 2015 | Gingival recession (not infrabony defects) |
| Trombelli 1995 | No platelet concentrate (fibrin glue) |
| Trombelli 1996 | No platelet concentrate (fibrin glue) |
| Yajamanya 2017 | Same participants of Chatterjee 2017 |
| Yassibag‐Berkman 2007 | Incomplete data |
| Yen 2007 | Incomplete data |
Differences between protocol and review
We did not perform a global comparison between the groups using autologous platelet concentrates and the control groups, because the differences in the surgical protocols among subgroups were consistent, and preferred to directly perform subgroup analyses.
The primary outcomes of the protocol are the secondary outcomes in the review and vice versa. We did not consider other participant‐reported outcomes (including preference, pain and cost‐effectiveness).
Contributions of authors
| Draft the protocol | Massimo Del Fabbro, Saurav Panda |
| Develop a search strategy | Saurav Panda, Surendar Ramamoorthi |
| Search for trials | Saurav Panda, Cristina Bucchi |
| Obtain copies of trials | Massimo Del Fabbro |
| Select which trials to include (two + one arbiter) | Jayakumar Nadathur Doraiswamy, Malaiappan Sankari, Massimo Del Fabbro |
| Extract data from trials (three people) | Lorena Karanxha, Saurav Panda, Cristina Bucchi |
| Enter data into Review Manager | Massimo Del Fabbro, Lorena Karanxha, Cristina Bucchi |
| Carry out the analysis | Massimo Del Fabbro, Lorena Karanxha, Saurav Panda, Sheeja Varghese, Cristina Bucchi |
| Interpret the analysis | Sheeja Varghese, Jayakumar Nadathur Doraiswamy, Silvio Taschieri |
| Draft the final review | Massimo Del Fabbro, Lorena Karanxha, Malaiappan Sankari, Cristina Bucchi |
| Update the review | Massimo Del Fabbro, Lorena Karanxha, Saurav Panda |
Sources of support
Internal sources
This study received no support, Other.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been AS‐Akademie, Germany; American Association of Public Health Dentistry, USA; British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; Canadian Dental Hygienists Association, Canada; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Swiss Society for Endodontology, Switzerland.
Declarations of interest
The review authors declare they have no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Agarwal 2014 {published data only}
- Agarwal A, Gupta ND. Platelet‐rich plasma combined with decalcified freeze‐dried bone allograft for the treatment of noncontained human intrabony periodontal defects: a randomized controlled split‐mouth study. International Journal of Periodontics and Restorative Dentistry 2014;34(5):705‐11. [DOI] [PubMed] [Google Scholar]
Agarwal 2015 {published data only}
- Agarwal A, Gupta ND, Jain A. Platelet rich fibrin combined with decalcified freeze‐dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split‐mouth clinical trail. Acta Odontologica Scandinavica 2016;74(1):36‐43. [DOI] [PubMed] [Google Scholar]
Agarwal 2016 {published data only}
- Agarwal P, Chatterjee A, Gokhale S, Singh HP, Kandwal A. Evaluation of platelet‐rich plasma alone or in combination with demineralized freeze dried bone allograft in treatment of periodontal infrabony defects: a comparative clinical trial. Journal of Indian Society of Periodontology 2016;20(1):42‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arabaci 2017 {published data only}
- Arabaci T, Kose O, Albayrak M, Cicek Y, Kizildag A. Advantages of autologous platelet‐rich fibrin membrane on gingival crevicular fluid growth factor levels and periodontal healing: a randomized split‐mouth clinical study. Journal of Periodontology 2017;88(8):771‐7. [DOI: 10.1902/jop.2017.160485] [DOI] [PubMed] [Google Scholar]
Aydemir 2016 {published data only}
- Aydemir Turkal H, Demirer S, Dolgun A, Keceli HG. Evaluation of the adjunctive effect of platelet‐rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six‐month results of a randomized, split‐mouth, controlled clinical study. Journal of Clinical Periodontology 2016;43(11):955‐64. [DOI: 10.1111/jcpe.12598] [DOI] [PubMed] [Google Scholar]
Camargo 2009 {published data only}
- Camargo PM, Lekovic V, Weinlaender M, Divnic‐Resnik T, Pavlovic M, Kenney EB. A surgical reentry study on the influence of platelet‐rich plasma in enhancing the regenerative effects of bovine porous bone mineral and guided tissue regeneration in the treatment of intrabony defects in humans. Journal of Periodontology 2009;80(6):915‐23. [DOI] [PubMed] [Google Scholar]
Chandradas 2016 {published data only}
- Chandradas ND, Ravindra S, Rangaraju VM, Jain S, Dasappa S. Efficacy of platelet rich fibrin in the treatment of human intrabony defects with or without bone graft: a randomized controlled trial. Journal of International Society of Preventive & Community Dentistry 2016;6(Suppl 2):S153‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christgau 2006 {published data only}
- Christgau M, Moder D, Wagner J, Glässl M, Hiller KA, Wenzel A, et al. Influence of autologous platelet concentrate on healing in intrabony defects following guided tissue regeneration therapy: a randomized prospective clinical split‐mouth study. Journal of Clinical Periodontology 2006;33(12):908‐21. [DOI] [PubMed] [Google Scholar]
Demir 2007 {published data only}
- Demir B, Sengün D, Berberoğlu A. Clinical evaluation of platelet‐rich plasma and bioactive glass in the treatment of intrabony defects. Journal of Clinical Periodontology 2007;34(8):709‐15. [DOI] [PubMed] [Google Scholar]
Döri 2007a {published data only}
- Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet‐rich plasma on the healing of intrabony defects treated with a natural bone mineral and a collagen membrane. Journal of Clinical Periodontology 2007;34(3):254‐61. [DOI] [PubMed] [Google Scholar]
Döri 2007b {published data only}
- Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet‐rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral and expanded polytetrafluoroethylene membranes. Journal of Periodontology 2007;78(6):983‐90. [DOI] [PubMed] [Google Scholar]
Döri 2008a {published data only}
- Döri F, Huszár T, Nikolidakis D, Tihanyi D, Horváth A, Arweiler NB, et al. Effect of platelet‐rich plasma on the healing of intrabony defects treated with Beta tricalcium phosphate and expanded polytetrafluoroethylene membranes. Journal of Periodontology 2008;79(4):660‐9. [DOI] [PubMed] [Google Scholar]
Döri 2008b {published data only}
- Döri F, Nikolidakis D, Húszár T, Arweiler NB, Gera I, Sculean A. Effect of platelet‐rich plasma on the healing of intrabony defects treated with an enamel matrix protein derivative and a natural bone mineral. Journal of Clinical Periodontology 2008;35(1):44‐50. [DOI] [PubMed] [Google Scholar]
Döri 2009 {published data only}
- Döri F, Kovács V, Arweiler NB, Huszár T, Gera I, Nikolidakis D, et al. Effect of platelet‐rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral: a pilot study. Journal of Periodontology 2009;80(10):1599‐605. [DOI] [PubMed] [Google Scholar]
Elgendy 2015 {published data only}
- Elgendy EA, Abo Shady TE. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet‐rich fibrin membrane in the treatment of periodontal intrabony defects. Journal of Indian Society of Periodontology 2015;19(1):61‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 2017 {published data only}
- Garg K, Srivastava R, Verma PK, Gautam A, Tripathi V, Agarwal S. Clinical evaluation of platelet rich plasma when combined with an alloplastic bone graft material in the treatment of intrabony periodontal defects. Saudi Journal of Oral Sciences 2017;4(1):33‐40. [Google Scholar]
Gupta 2014 {published data only}
- Gupta G. Clinical and radiographic evaluation of intrabony defects in localized aggressive periodontitis patients with platelet rich plasma/hydroxyapatite graft: a comparative controlled clinical trial. Contemporary Clinical Dentistry 2014;5(4):445‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanna 2004 {published data only}
- Hanna R, Trejo PM, Weltman RL. Treatment of intrabony defects with bovine‐derived xenograft alone and in combination with platelet‐rich plasma: a randomized clinical trial. Journal of Periodontology 2004;75(12):1668‐77. [DOI] [PubMed] [Google Scholar]
Hassan 2012 {published data only}
- Hassan KS, Alagl AS, Abdel‐Hady A. Torus mandibularis bone chips combined with platelet rich plasma gel for treatment of intrabony osseous defects: clinical and radiographic evaluation. International Journal of Oral and Maxillofacial Surgery 2012;41(12):1519‐26. [DOI] [PubMed] [Google Scholar]
Kanoriya 2016 {published data only}
- Kanoriya D, Pradeep AR, Singhal S, Garg V, Guruprasad CN. Synergistic approach using platelet‐rich fibrin and 1% alendronate for intrabony defect treatment in chronic periodontitis: a randomized clinical trial. Journal of Periodontology 2016;87(12):1427‐35. [DOI] [PubMed] [Google Scholar]
Kaushick 2011 {published data only}
- Kaushick BT, Jayakumar ND, Padmalatha O, Varghese S. Treatment of human periodontal infrabony defects with hydroxyapatite + β tricalcium phosphate bone graft alone and in combination with platelet rich plasma: a randomized clinical trial. Indian Journal of Dental Research 2011;22(4):505‐10. [DOI] [PubMed] [Google Scholar]
Khosropanah 2015 {published data only}
- Khosropanah H, Shahidi S, Basri A, Houshyar M. Treatment of intrabony defects by DFDBA alone or in combination with PRP: a split‐mouth randomized clinical and three‐dimensional radiographic trial. Journal of Dentistry (Tehran, Iran) 2015;12(10):764‐73. [PMC free article] [PubMed] [Google Scholar]
Martande 2016 {published data only}
- Martande SS, Kumari M, Pradeep AR, Singh SP, Suke DK, Guruprasad CN. Platelet‐rich fibrin combined with 1.2% atorvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2016;87(9):1039‐46. [DOI] [PubMed] [Google Scholar]
Naqvi 2017 {published data only}
- Naqvi A, Gopalakrishnan D, Bhasin MT, Sharma N, Haider K, Martande S. Comparative evaluation of bioactive glass putty and platelet rich fibrin in the treatment of human periodontal intrabony defects: a randomized control trial. Journal of Clinical and Diagnostic Research 2017;11(7):ZC09‐ZC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuda 2005 {published data only}
- Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, et al. Platelet‐rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: a comparative controlled clinical study. Journal of Periodontology 2005;76(6):890‐8. [DOI] [PubMed] [Google Scholar]
Ozdemir 2012 {published data only}
- Ozdemir B, Okte E. Treatment of intrabony defects with beta‐tricalciumphosphate alone and in combination with platelet‐rich plasma. Journal of Biomedical Materials Research. Part B, Applied Biomaterials 2012;100(4):976‐83. [DOI] [PubMed] [Google Scholar]
Panda 2016 {published data only}
- Panda S, Sankari M, Satpathy A, Jayakumar D, Mozzati M, Mortellaro C, et al. Adjunctive effect of autologus platelet‐rich fibrin to barrier membrane in the treatment of periodontal intrabony defects. Journal of Craniofacial Surgery 2016;27(3):691‐6. [DOI] [PubMed] [Google Scholar]
Patel 2017 {published data only}
- Patel GK, Gaekwad SS, Gujjari SK, Kumar V. Platelet‐rich fibrin in regeneration of intrabony defects: a randomized controlled trial. Journal of Periodontology 2017;88(11):1192‐9. [DOI] [PubMed] [Google Scholar]
Piemontese 2008 {published data only}
- Piemontese M, Aspriello SD, Rubini C, Ferrante L, Procaccini M. Treatment of periodontal intrabony defects with demineralized freeze‐dried bone allograft in combination with platelet‐rich plasma: a comparative clinical trial. Journal of Periodontology 2008;79(5):802‐10. [DOI] [PubMed] [Google Scholar]
Pradeep 2015 {published data only}
- Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN. Platelet rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2015;86(6):729‐37. [DOI] [PubMed] [Google Scholar]
Pradeep 2016 {published data only}
- Pradeep AR, Garg V, Kanoriya D, Singhal S. Platelet‐rich fibrin with 1.2% rosuvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2016;87(12):1468‐73. [DOI] [PubMed] [Google Scholar]
Ravi 2017 {published data only}
- Ravi S, Malaiappan S, Varghese S, Jayakumar ND, Prakasam G. Additive effect of plasma rich in growth factors with guided tissue regeneration in treatment of intrabony defects in patients with chronic periodontitis: a split‐mouth randomized controlled clinical trial. Journal of Periodontology 2017;88(9):839‐45. [DOI] [PubMed] [Google Scholar]
Rosamma Joseph 2012 {published data only}
- Rosamma Joseph V, Raghunath A, Sharma N. Clinical effectiveness of autologous platelet rich fibrin in the management of infrabony periodontal defects. Singapore Dental Journal 2012;33(1):5‐12. [DOI] [PubMed] [Google Scholar]
Sezgin 2017 {published data only}
- Sezgin Y, Uraz A, Taner IL, Çulhaoğlu R. Effects of platelet‐rich fibrin on healing of intra‐bony defects treated with anorganic bovine bone mineral. Brazilian Oral Research 2017;31:e15. [DOI] [PubMed] [Google Scholar]
Sharma 2011 {published data only}
- Sharma A, Pradeep AR. Treatment of 3‐wall intrabony defects in patients with chronic periodontitis with autologous platelet‐rich fibrin: a randomized controlled clinical trial. Journal of Periodontology 2011;82(12):1705‐12. [DOI] [PubMed] [Google Scholar]
Shukla 2016 {published data only}
- Shukla S, Chug A, Mahesh L, Grover HS. Effect of addition of platelet‐rich plasma to calcium phosphosilicate on healing at 9 months in periodontal intrabony defects. Journal of Contemporary Dental Practice 2016;17(3):230‐4. [DOI] [PubMed] [Google Scholar]
Thorat 2011 {published data only}
- Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet‐rich fibrin in the treatment of intrabony defects: a controlled clinical trial. Journal of Clinical Periodontology 2011;38(10):925‐32. [DOI] [PubMed] [Google Scholar]
Thorat 2017 {published data only}
- Thorat M, Baghele ON, S RP. Adjunctive effect of autologous platelet‐rich fibrin in the treatment of intrabony defects in localized aggressive periodontitis patients: a randomized controlled split‐mouth clinical trial. International Journal of Periodontics & Restorative Dentistry 2017;37(6):e302‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2017 {published data only}
- Agarwal I, Chandran S, Nadig P. Comparative evaluation of the efficacy of platelet‐rich fibrin and calcium phosphosilicate putty alone and in combination in the treatment of intrabony defects: a randomized clinical and radiographic study. Contemporary Clinical Dentistry 2017;8(2):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aleksić 2008 {published data only}
- Aleksić Z, Janković S, Dimitrijević B, Pucar A, Lazić V, Leković V. Clinical impact of platelet rich plasma in treatment of gingival recessions [Primena plazme bogate trombocitima urekonstruktivnoj mukogingivnoj hirurgiji]. Srpski Arhiv za Celokupno Lekarstvo 2008;136(3‐4):95‐103. [DOI] [PubMed] [Google Scholar]
Aroca 2009 {published data only}
- Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet‐rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6‐month study. Journal of Periodontology 2009;80(2):244‐52. [DOI] [PubMed] [Google Scholar]
Bajaj 2017 {published data only}
- Bajaj P, Agarwal E, Rao NS, Naik SB, Pradeep AR, Kalra N, et al. Autologous platelet‐rich fibrin in the treatment of 3‐wall intrabony defects in aggressive periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2017;88(11):1186‐91. [DOI] [PubMed] [Google Scholar]
Camargo 2002 {published data only}
- Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet‐rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. Journal of Periodontal Research 2002;37(4):300‐6. [DOI] [PubMed] [Google Scholar]
Camargo 2005 {published data only}
- Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. A reentry study on the use of bovine porous bone mineral, GTR, and platelet‐rich plasma in the regenerative treatment of intrabony defects in humans. International Journal of Periodontics and Restorative Dentistry 2005;25(1):49‐59. [PubMed] [Google Scholar]
Cetinkaya 2014 {published data only}
- Cetinkaya BO, Keles GC, Pamuk F, Balli U, Keles ZP. Long‐term clinical results on the use of platelet concentrate in the treatment of intrabony periodontal defects. Acta Odontologica Scandinavica 2014;72(2):92‐8. [DOI] [PubMed] [Google Scholar]
Chatterjee 2017 {published data only}
- Chatterjee A, Pradeep AR, Garg V, Yajamanya S, Ali MM, Priya VS. Treatment of periodontal intrabony defects using autologous platelet‐rich fibrin and titanium platelet‐rich fibrin: a randomized, clinical, comparative study. Journal of Investigative and Clinical Dentistry 2017;8(3):e12231. [DOI] [PubMed] [Google Scholar]
Cheung 2004 {published data only}
- Cheung WS, Griffin TJ. A comparative study of root coverage with connective tissue and platelet concentrate grafts: 8‐month results. Journal of Periodontology 2004;75(12):1678‐87. [DOI] [PubMed] [Google Scholar]
Cieplik 2018 {published data only}
- Cieplik F, Tabenski L, Hiller KA, Schmalz G, Buchalla W, Christgau M. Influence of autogenous platelet concentrate on combined GTR/graft therapy in intrabony defects: a 13‐year follow‐up of a randomized controlled clinical split‐mouth study. Journal of Clinical Periodontology 2018;45(1):382‐91. [DOI] [PubMed] [Google Scholar]
Cortellini 1995 {published data only}
- Cortellini P, Pini Prato GP, Tonetti MS. No detrimental effect of fibrin glue on the regeneration of intrabony defects. A controlled clinical trial. Journal of Clinical Periodontology 1995;22(9):697‐702. [DOI] [PubMed] [Google Scholar]
Dogan 2015 {published data only}
- Bozkurt Dogan S, Ongoz Dede F, Balli U, Atalay EN, Durmuslar MC. Concentrated growth factor in the treatment of adjacent multiple gingival recessions: a split‐mouth randomized clinical trial. Journal of Clinical Periodontology 2015;42(9):868‐75. [DOI] [PubMed] [Google Scholar]
Döri 2013 {published data only}
- Döri F, Arweiler N, Húszár T, Gera I, Miron RJ, Sculean A. Five‐year results evaluating the effects of platelet‐rich plasma on the healing of intrabony defects treated with enamel matrix derivative and natural bone mineral. Journal of Periodontology 2013;84(11):1546‐55. [DOI] [PubMed] [Google Scholar]
Eren 2014 {published data only}
- Eren G, Atilla G. Platelet‐rich fibrin in the treatment of localized gingival recessions: a split‐mouth randomized clinical trial. Clinical Oral Investigations 2014;18(8):1941‐8. [DOI] [PubMed] [Google Scholar]
Gupta 2014b {published data only}
- Gupta SJ, Jhingran R, Gupta V, Bains VK, Madan R, Rizvi I. Efficacy of platelet‐rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: a clinical and cone beam computed tomography study. Journal of the International Academy of Periodontology 2014;16(3):86‐96. [PubMed] [Google Scholar]
Harnack 2009 {published data only}
- Harnack L, Boedeker RH, Kurtulus I, Boehm S, Gonzales J, Meyle J. Use of platelet‐rich plasma in periodontal surgery ‐ a prospective randomised double blind clinical trial. Clinical Oral Investigations 2009;13(2):179‐87. [DOI] [PubMed] [Google Scholar]
Huang 2005 {published data only}
- Huang LH, Neiva RE, Soehren SE, Giannobile WV, Wang HL. The effect of platelet‐rich plasma on the coronally advanced flap root coverage procedure: a pilot human trial. Journal of Periodontology 2005;76(10):1768‐77. [DOI] [PubMed] [Google Scholar]
Jankovic 2010 {published data only}
- Jankovic S, Aleksic Z, Milinkovic I, Dimitrijevic B. The coronally advanced flap in combination with platelet‐rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. European Journal of Esthetic Dentistry 2010;5(3):260‐73. [PubMed] [Google Scholar]
Jankovic 2012 {published data only}
- Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, et al. Use of platelet‐rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. International Journal of Periodontics & Restorative Dentistry 2012;32(2):e41‐50. [PubMed] [Google Scholar]
Jovicić 2013 {published data only}
- Jovicić B, Lazić Z, Nedić M, Matijević S, Gostović‐Spadijer A. Therapeutic efficacy of connective tissue autotransplants with periosteum and platelet rich plasma in the menagement of gingival recession. Vojnosanitetski Pregled 2013;70(7):664‐9. [DOI] [PubMed] [Google Scholar]
Keceli 2008 {published data only}
- Keceli HG, Sengun D, Berberoğlu A, Karabulut E. Use of platelet gel with connective tissue grafts for root coverage: a randomized controlled trial. Journal of Clinical Periodontology 2008;35(3):255‐62. [DOI] [PubMed] [Google Scholar]
Keles 2006 {published data only}
- Keles GC, Cetinkaya BO, Albayrak D, Koprulu H, Acikgoz G. Comparison of platelet pellet and bioactive glass in periodontal regenerative therapy. Acta Odontologica Scandinavica 2006;64(6):327‐33. [DOI] [PubMed] [Google Scholar]
Lekovic 2012 {published data only}
- Lekovic V, Milinkovic I, Aleksic Z, Jankovic S, Stankovic P, Kenney EB, et al. Platelet‐rich fibrin and bovine porous bone mineral vs platelet‐rich fibrin in the treatment of intrabony periodontal defects. Journal of Periodontal Research 2012;47(4):409‐17. [DOI] [PubMed] [Google Scholar]
Menezes 2012 {published data only}
- Menezes LM, Rao J. Long‐term clinical evaluation of platelet‐rich plasma in the treatment of human periodontal intraosseous defects: a comparative clinical trial. Quintessence International 2012;43(7):571‐82. [PubMed] [Google Scholar]
Moder 2012 {published data only}
- Moder D, Taubenhansl F, Hiller KA, Schmalz G, Christgau M. Influence of autogenous platelet concentrate on combined GTR/graft therapy in intrabony defects: a 7‐year follow‐up of a randomized prospective clinical split‐mouth study. Journal of Clinical Periodontology 2012;39(5):457‐65. [DOI] [PubMed] [Google Scholar]
Ouyang 2006 {published data only}
- Ouyang XY, Qiao J. Effect of platelet‐rich plasma in the treatment of periodontal intrabony defects in humans. Chinese Medical Journal 2006;119(18):1511‐21. [PubMed] [Google Scholar]
Padma 2013 {published data only}
- Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, Sreedhar A. A split‐mouth randomized controlled study to evaluate the adjunctive effect of platelet‐rich fibrin to coronally advanced flap in Miller's class‐I and II recession defects. Journal of Indian Society of Periodontology 2013;17(5):631‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pradeep 2012a {published data only}
- Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet‐rich fibrin and platelet‐rich plasma in the treatment of 3‐wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2012;83(12):1499‐507. [DOI] [PubMed] [Google Scholar]
Pradeep 2017 {published data only}
- Pradeep AR, Bajaj P, Rao NS, Agarwal E, Naik SB. Platelet‐rich fibrin combined with a porous hydroxyapatite graft for the treatment of 3‐wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. Journal of Periodontology 2017;88(12):1288‐96. [DOI] [PubMed] [Google Scholar]
Qiao 2016 {published data only}
- Qiao J, Duan J, Zhang Y, Chu Y, Sun C. The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Future Science OA 2016;2(4):FS136. [DOI: 10.4155/fsoa-2016-0019.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saini 2011 {published data only}
- Saini N, Sikri P, Gupta H. Evaluation of the relative efficacy of autologous platelet‐rich plasma in combination with β‐tricalcium phosphate alloplast versus an alloplast alone in the treatment of human periodontal infrabony defects: a clinical and radiological study. Indian Journal of Dental Research 2011;22(1):107‐15. [DOI] [PubMed] [Google Scholar]
Shah 2015 {published data only}
- Shah M, Patel J, Dave D, Shah S. Comparative evaluation of platelet‐rich fibrin with demineralized freeze‐dried bone allograft in periodontal infrabony defects: a randomized controlled clinical study. Journal of Indian Society of Periodontology 2015;19(1):56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 2009 {published data only}
- Shepherd N, Greenwell H, Hill M, Vidal R, Scheetz JP. Root coverage using acellular dermal matrix and comparing a coronally positioned tunnel with and without platelet‐rich plasma: a pilot study in humans. Journal of Periodontology 2009;80(3):397‐404. [DOI] [PubMed] [Google Scholar]
Shivakumar 2016 {published data only}
- Shivakumar MA, Gopal SV, Govindaraju P, Ramayya S, Bennadi D, Mruthyuenjaya RK. Evaluation of treatment for isolated bilateral Miller's class I or II gingival recession with platelet rich fibrin membrane ‐ a comparative study. Journal of Young Pharmacists 2016;8(3):206‐13. [Google Scholar]
Thamaraiselvan 2015 {published data only}
- Thamaraiselvan M, Elavarasu S, Thangakumaran S, Gadagi JS, Arthie T. Comparative clinical evaluation of coronally advanced flap with or without platelet rich fibrin membrane in the treatment of isolated gingival recession. Journal of Indian Society of Periodontology 2015;19(1):66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trombelli 1995 {published data only}
- Trombelli L, Schincaglia GP, Zangari F, Griselli A, Scabbia A, Calura G. Effects of tetracycline HCl conditioning and fibrin‐fibronectin system application in the treatment of buccal gingival recession with guided tissue regeneration. Journal of Periodontology 1995;66(5):313‐20. [DOI] [PubMed] [Google Scholar]
Trombelli 1996 {published data only}
- Trombelli L, Scabbia A, Wikesjö UM, Calura G. Fibrin glue application in conjunction with tetracycline root conditioning and coronally positioned flap procedure in the treatment of human gingival recession defects. Journal of Clinical Periodontology 1996;23(9):861‐7. [DOI] [PubMed] [Google Scholar]
Yajamanya 2017 {published data only}
- Yajamanya SR, Chatterjee A, Hussain A, Coutinho A, Das S, Subbaiah S. Bioactive glass versus autologous platelet‐rich fibrin for treating periodontal intrabony defects: Aa comparative clinical study. Journal of Indian Society of Periodontology 2017;21(1):32‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yassibag‐Berkman 2007 {published data only}
- Yassibag‐Berkman Z, Tuncer O, Subasioglu T, Kantarci A. Combined use of platelet‐rich plasma and bone grafting with or without guided tissue regeneration in the treatment of anterior interproximal defects. Journal of Periodontology 2007;78(5):801‐9. [DOI] [PubMed] [Google Scholar]
Yen 2007 {published data only}
- Yen CA, Griffin TJ, Cheung WS, Chen J. Effects of platelet concentrate on palatal wound healing after connective tissue graft harvesting. Journal of Periodontology 2007;78(4):601‐10. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 1992
- AAP. Glossary of Periodontal Terms. 3rd Edition. Chicago (IL): American Academy of Periodontology, 1992. [Google Scholar]
Anitua 2001
- Anitua E. The use of plasma‐rich growth factors (PRGF) in oral surgery. Practical Procedures and Aesthetic Dentistry 2001;13(6):487‐93. [PubMed] [Google Scholar]
Caffesse 1986
- Caffesse RG, Sweeney PL, Smith BA. Scaling and root planing with and without periodontal flap surgery. Journal of Clinical Periodontology 1986;13(3):205‐10. [PUBMED: 3517073] [DOI] [PubMed] [Google Scholar]
Carroll 2005
- Carroll RJ, Amoczky SP, Graham S, O'Connell SM. Characterization of Autologous Growth Factors in Cascade Platelet Rich Fibrin Matrix (PRFM). Edison (NJ): Musculoskeletal Transplant Foundation, 2005. [Google Scholar]
Castro 2017
- Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, et al. Regenerative potential of leucocyte‐ and platelet‐rich fibrin. Part A: intrabony defects, furcation defects and periodontal plastic surgery. A systematic review and meta‐analysis. Journal of Clinical Periodontology 2017;44(1):67‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choukroun 2001
- Choukroun J, Adda F, Schoeffler C, Vervelle A. An opportunity in perio‐implantology: the PRF [Une opportunité en paro‐implantologie: le PRF]. Implantodontie 2001;42:55‐62. [Google Scholar]
Cochran 2003
- Cochran DL, Jones A, Heijl L, Mellonig JT, Schoolfield J, King GN. Periodontal regeneration with a combination of enamel matrix proteins and autogenous bone grafting. Journal of Periodontology 2003;74(9):1269‐81. [PUBMED: 14584859] [DOI] [PubMed] [Google Scholar]
Cortellini 1996
- Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human intrabony defects with bioresorbable membranes. A controlled clinical trial. Journal of Periodontology 1996;67(3):217‐23. [PUBMED: 8708952] [DOI] [PubMed] [Google Scholar]
Crea 2014
- Crea A, Deli G, Littarru C, Lajolo C, Orgeas GV, Tatakis DN. Intrabony defects, open‐flap debridement, and decortication: a randomized clinical trial. Journal of Periodontology 2014;85(1):34‐42. [PUBMED: 23537123] [DOI] [PubMed] [Google Scholar]
Del Fabbro 2011
- Fabbro M, Bortolin M, Taschieri S, Weinstein R. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta‐analysis. Journal of Periodontology 2011;82(8):1100‐11. [DOI] [PubMed] [Google Scholar]
Del Fabbro 2013
- Fabbro M, Ceci C, Taschieri S. Systematic review on the effect of platelet concentrates for the surgical treatment of periodontal defects [Revisione sistematica della letteratura sull'effetto dei concentrati piastrinici nel trattamento chirurgico dei difetti parodontali]. Dental Cadmos 2013;81(3):138‐45. [Google Scholar]
Dohan Ehrenfest 2009
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet‐rich plasma (P‐PRP) to leucocyte‐ and platelet‐rich fibrin (L‐PRF). Trends in Biotechnology 2009;27(3):158‐67. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Esposito 2009
- Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003875.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Giannobile 1996
- Giannobile WV. The potential role of growth and differentiation factors in periodontal regeneration. Journal of Periodontology 1996;67(5):545‐53. [PUBMED: 8724716] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 27 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoidal 2008
- Hoidal MJ, Grimard BA, Mills MP, Schoolfield JD, Mellonig JT, Mealey BL. Clinical evaluation of demineralized freeze‐dried bone allograft with and without enamel matrix derivative for the treatment of periodontal osseous defects in humans. Journal of Periodontology 2008;79(12):2273‐80. [PUBMED: 19053917] [DOI] [PubMed] [Google Scholar]
Kinane 2001
- Kinane DF. Causation and pathogenesis of periodontal disease. Periodontology 2000 2001;25:8‐20. [DOI] [PubMed] [Google Scholar]
Kotsovilis 2010
- Kotsovilis S, Markou N, Pepelassi E, Nikolidakis D. The adjunctive use of platelet‐rich plasma in the therapy of periodontal intraosseous defects: a systematic review. Journal of Periodontal Research 2010;45(3):428‐43. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Marx 1998
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet‐rich plasma: growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1998;85(6):638‐46. [DOI] [PubMed] [Google Scholar]
Needleman 2006
- Needleman I, Worthington HV, Giedrys‐Leeper E, Tucker R. Guided tissue regeneration for periodontal infra‐bony defects. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001724.pub2] [DOI] [PubMed] [Google Scholar]
Papapanou 1991
- Papapanou PN, Wennström JL. The angular bony defect as indicator of further alveolar bone loss. Journal of Clinical Periodontology 1991;18(5):317‐22. [PUBMED: 2066446 ] [DOI] [PubMed] [Google Scholar]
Pihlstrom 2005
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366(9499):1809‐20. [PUBMED: 16298220] [DOI] [PubMed] [Google Scholar]
Plachokova 2008
- Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH. Effect of platelet‐rich plasma on bone regeneration in dentistry: a systematic review. Clinical Oral Implants Research 2008;19(6):539‐45. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2002
- Rose FR, Oreffo RO. Bone tissue engineering: hope vs hype. Biochemical and Biophysical Research Communications 2002;292(1):1‐7. [PUBMED: 11890663] [DOI] [PubMed] [Google Scholar]
Roselló‐Camps 2015
- Roselló‐Camps À, Monje A, Lin GH, Khoshkam V, Chávez‐Gatty M, Wang HL, et al. Platelet‐rich plasma for periodontal regeneration in the treatment of intrabony defects: a meta‐analysis on prospective clinical trials. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2015;120(5):562‐74. [DOI] [PubMed] [Google Scholar]
Vrotsos 1999
- Vrotsos JA, Parashis AO, Theofanatos GD, Smulow JB. Prevalence and distribution of bone defects in moderate and advanced adult periodontitis. Journal of Clinical Periodontology 1999;26(1):44‐8. [PUBMED: 9923510] [DOI] [PubMed] [Google Scholar]
Waerhaug 1979
- Waerhaug J. The infrabony pocket and its relationship to trauma from occlusion and subgingival plaque. Journal of Periodontology 1979;50(7):355‐65. [PUBMED: 381633 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Del Fabbro 2014
- Fabbro M, Panda S, Jayakumar ND, Sankari M, Varghese S, Ramamoorthi S, et al. Autologous platelet concentrates for treatment of periodontal defects. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011423] [DOI] [Google Scholar]


