Abstract
Background
Knee arthroscopy is a common procedure and is associated with postoperative pain. Intra‐articular (IA) injection of morphine for pain control has been widely studied, but its analgesic effect after knee arthroscopy is uncertain.
Objectives
To evaluate the relative effects on pain relief and adverse events of IA morphine given for pain control after knee arthroscopy compared with placebo, other analgesics (local anaesthetics, non‐steroidal anti‐inflammatory drugs (NSAIDs), other opioids) and other routes of morphine administration.
Search methods
We searched CENTRAL (The Cochrane Library Issue 4, 2015), MEDLINE via Ovid (January 1966 to May 2015), EMBASE via Ovid (January 1988 to May 2015), and the reference lists of included articles. We also searched the metaRegister of controlled trials, clinicaltrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform for ongoing trials.
Selection criteria
We identified all the randomised, double‐blind controlled trials that compared single dose IA morphine with other interventions for the treatment of postoperative pain after knee arthroscopy. We excluded studies with fewer than 10 participants in each group, using spinal or epidural anaesthesia, or assessing the analgesic effect of IA morphine on chronic pain.
Data collection and analysis
Two authors independently assessed the quality of each trial and extracted information on pain intensity, supplementary analgesics consumption and adverse events. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created 'Summary of findings' tables.
Main results
We included 28 small, low quality studies (29 reports) involving 2564 participants. Of 20 studies (21 reports) comparing morphine with placebo, nine studies with adequate data were included in the meta‐analysis. Overall, the risk of bias was unclear. Overall, the quality of the evidence assessed using GRADE was low to very low, downgraded primarily due to risk of bias, small study size, and imprecision.
No statistical difference was found between 1 mg IA morphine and placebo in pain intensity (visual analogue scale (VAS)) at early phase (zero to two hours) (mean difference (MD) ‐0.50, 95% CI ‐1.15 to 0.14; participants = 297; studies = 7; low quality evidence), medium phase (two to six hours) (MD ‐0.47, 95% CI ‐1.09 to 0.14; participants = 297; studies = 7; low quality evidence) and late phase (six to 30 hours) (MD ‐0.88, 95% CI ‐1.81 to 0.04; participants = 297; studies = 7; low quality evidence). No significant difference was found between 1 mg and 2 mg morphine for pain intensity at early phase (MD ‐0.56, 95% CI ‐1.93 to 0.81; participants = 105; studies = 2; low quality evidence), while 4 mg/5 mg morphine provided better analgesia than 1 mg morphine at late phase (MD 0.67, 95% CI 0.08 to 1.25; participants = 97; studies = 3; low quality evidence). IA morphine was not better than local anaesthetic agents at early phase (MD 1.43, 95% CI 0.49 to 2.37; participants = 248; studies = 5; low quality evidence), NSAIDs at early phase (MD 0.95, 95% CI ‐0.95 to 2.85; participants = 80; studies = 2; very low quality evidence), sufentanil, fentanyl or pethidine for pain intensity. IA morphine was similar to intramuscular (IM) morphine for pain intensity at early phase (MD 0.21, 95% CI ‐0.48 to 0.90; participants = 72; studies = 2; very low quality evidence).
Meta‐analysis indicated that there was no difference between IA morphine and placebo or bupivacaine in time to first analgesic request. Eleven out of 20 studies comparing morphine with placebo reported adverse events and no statistical difference was obtained regarding the incidence of adverse events (risk ratio (RR) 1.09, 95% CI 0.51 to 2.36; participants = 314; studies = 8; low quality evidence). Seven of 28 studies reported participants' withdrawal. There were not enough data for withdrawals to be able to perform meta‐analysis.
Authors' conclusions
We have not found high quality evidence that 1 mg IA morphine is better than placebo at reducing pain intensity at early, medium or late phases. No statistical difference was reported between IA morphine and placebo regarding the incidence of adverse events. The relative effects of 1 mg morphine when compared with IA bupivacaine, NSAIDs, sufentanil, fentanyl and pethidine are uncertain. The quality of the evidence is limited by high risk of bias and small size of the included studies, which might bias the results. More high quality studies are needed to get more conclusive results.
Plain language summary
Morphine injections for pain relief after knee arthroscopy
Background
Knee arthroscopy is a surgical procedure on the knee. The surgery is minimally invasive, which means that only a small cut (incision) is needed. An examination, and sometimes treatment, of damage is performed using an arthroscope, which is inserted into the joint through the small incision. Knee arthroscopy is used to assess or treat many orthopaedic (musculoskeletal) conditions, and patients may have pain after surgery. Morphine injected directly into the knee (intra‐articular morphine) to relieve pain has been widely studied, but we do not know how well it works.
Key results and quality of the evidence
In May 2015, this review identified 28 small, low quality studies involving 2564 participants looking at intra‐articular morphine for pain relief after knee arthroscopy. From 9/20 studies we did not find evidence that intra‐articular morphine given at a dose of 1 mg was better than placebo for pain relief. From the limited evidence available we were unable to determine how intra‐articular morphine compared with morphine injected into the muscle (intra‐muscular morphine). There was also low quality evidence for the effects of 1 mg intra‐articular morphine compared with intra‐articular bupivacaine, non‐steroidal anti‐inflammatory drugs (NSAIDs), sufentanil, fentanyl and pethidine, so we were unsure which worked best. We were unable to determine how similar the rates of side effects such as nausea and vomiting were between intra‐articular morphine and placebo. Overall, the quality of the evidence was low.
Future research should focus on finding effective analgesics for knee arthroscopy.
Summary of findings
Background
Description of the condition
Knee arthroscopy is a very common procedure performed as day surgery and is associated with postoperative pain (Joshi 1992). The incidence of moderate or severe pain within an hour after knee arthroscopy is around 60%. Baseline scores are about 50 mm on the visual analogue scale (VAS) 10 minutes after test drug injection. However, pain scores quickly decreased to about 35 mm by 30 minutes and less than 5 mm by 12 hours (Solheim 2006). Women report more pain after knee arthroscopy than men (Rosseland 2004b). Adequate postoperative analgesia may accelerate the patient's return to normal activity.
Description of the intervention
Many trials have tried to find an ideal regimen which provides sufficient postoperative analgesia with fewer adverse events, including intra‐articular (IA) anaesthetics and analgesics for postoperative pain relief. The spinal or intravenous (IV) administration of morphine may cause side effects such as respiratory depression, sedation, dependence, pruritus and urinary retention. However, the adverse events of peripheral IA morphine administration were mild or absent. Morphine may therefore be a promising IA agent that is without obvious central side effects (Sawynok 2003). IA injection of morphine has been widely studied for its simplicity, safety and efficacy.
Opioid binding sites have been identified within synovial tissue, implying that the analgesic effect of morphine may be locally mediated (Khoury 1992). When given at the end of arthroscopic surgery, IA morphine could reduce postoperative pain through peripheral opioid receptors (Stein 1991). It has also been reported to reduce pain through other pathways (Kalso 1997) such as inflammatory reaction (Likar 1998; Stein 1995; Stein 1999). However, different opinions exist as to the postoperative effect of peripheral opioids. The reported dosages of IA morphine in studies vary from 0.5 mg to 5 mg (Joshi 1993). Stein 1991 reported that a dose of 1 mg morphine showed analgesic efficacy, whereas a dose of 0.5 mg did not. Allen 1993 reported that 2 mg morphine showed a better analgesic effect than 1 mg, but no dose response was detected in two other studies (Heine 1994; Laurent 1994). Other controlled trials, however, have failed to show any analgesic effect of morphine compared with placebo (Aasbø 1996; Drosos 2002; Gupta 1999; Ruwe 1995; Soderlund 1997).
In addition to opiates, many other interventions have been widely studied for the reduction of postoperative pain following knee arthroscopy. Local anaesthetics (such as bupivacaine, ropivacaine, carbocaine, lidocaine and prilocaine), non‐steroidal anti‐inflammatory drugs ((NSAIDs) such as ketorolac and tenoxicam) and other interventions (such as magnesium, clonidine, neostigmine and ketamine) have been intensely studied.
How the intervention might work
Opioid receptors exist on peripheral terminals of primary afferent neurons, demonstrated functionally and morphologically in Bartho 1990 and Stein 1990. As a μ‐opioid receptor agonist, morphine is effective in producing peripheral analgesia. Morphine activates peripheral opioid receptors, resulting in their interactions with G‐proteins and a decrease in cyclic adenosine monophosphate (cAMP) in sensory nerve terminals, an increase in K+ efflux and a decrease of Ca2+ entry. Thus, the excitability of the peripheral nerve terminal, the propagation of action potentials and release of neuropeptides are attenuated (Sawynok 2003).
Why it is important to do this review
Several systematic reviews have investigated IA morphine for the control of pain after knee surgery, however, consensus on the analgesic effect is still lacking. A previously published qualitative systematic review (Kalso 1997) showed that IA morphine was effective in reducing postoperative pain intensity and the consumption of rescue analgesics. All knee surgeries were included in the review, however, and it failed to focus on the effect of IA morphine on pain relief after knee arthroscopy. Also, no quantitative analysis of pooled data was performed here, nor in the authors' second systematic review on this topic (Kalso 2002). In another systematic review, variability was found not only between studies but also within one study (patient variability) (Gupta 2001). By calculating the weighted mean difference (WMD) of pain scores between treatment groups, Moiniche 1999 found that there was weak evidence for a reduction of postoperative pain after IA instillation of local anaesthetics. More trials comparing morphine and other interventions for knee arthroscopy were available recently and they added new knowledge to inform clinical practice (Rosseland 2003; Rosseland 2004a; Rosseland 2004b). Evidence is still lacking as to whether IA opioids offer clinically relevant pain relief. In light of the existing controversy, this systematic review aimed to investigate the analgesic effect of single dose IA morphine compared with other interventions in the management of pain control after knee arthroscopy.
Objectives
To evaluate the relative effects on pain relief and adverse events of IA morphine given for pain control after knee arthroscopy compared with placebo, other analgesics (local anaesthetics, non‐steroidal anti‐inflammatory drugs (NSAIDs), other opioids) and other routes of morphine administration.
Methods
Criteria for considering studies for this review
Types of studies
We included studies fulfilling the following selection criteria in this systematic review:
randomised, double‐blind controlled trials;
studies comparing single dose IA morphine with other interventions for the treatment of postoperative pain after knee arthroscopy; and
studies with more than 10 participants in each group.
We excluded the following studies:
studies in which spinal or epidural anaesthesia was used; and
studies whose primary aim was to assess the analgesic effect of IA morphine on chronic pain.
Types of participants
We included participants of either gender, aged 15 years or older, and undergoing knee arthroscopy.
Types of interventions
IA morphine versus any other interventions or administration methods:
IA morphine versus placebo;
IA morphine versus local anaesthetic agents (such as bupivacaine, ropivacaine, carbocaine, lidocaine and prilocaine);
IA morphine versus NSAIDs (such as ketorolac and tenoxicam);
different doses of IA morphine;
IA morphine versus intravenous (IV) or intra‐muscular (IM) morphine;
IA morphine versus other opioids.
Types of outcome measures
We included the following outcomes.
Primary outcomes
Patient‐reported postoperative pain intensity (a 0 ‐ 10 cm VAS score).
Use of supplementary analgesic (number of participants using rescue analgesics, time to first rescue analgesics, analgesic drug counts, patient‐controlled analgesia opioid consumption, etc.).
Secondary outcomes
Adverse events.
Withdrawals.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (The Cochrane Library, Issue 4, 2015), MEDLINE via Ovid (January 1966 to May 2015), EMBASE via Ovid (January 1988 to May 2015). Please see Appendix 1; Appendix 2; Appendix 3 for the database search strategies. Filters designed to limit the searches to RCTs were added to the MEDLINE and EMBASE strategies.
Searching other resources
Reference lists
We sought additional studies from the references of retrieved randomised trials, meta‐analyses and systematic reviews.
Unpublished studies
We searched trials registries for ongoing trials. We searched three web sites: the metaRegister of controlled trials (www.controlled-trials.com/mrct), clinicaltrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) up to May 2015.
Language
The search identified all relevant studies irrespective of language. We assessed non‐English language papers and translated as necessary.
Data collection and analysis
Selection of studies
Two authors independently assessed the eligibility of studies included in the review, and another author checked these results. For the studies that were reported several times, we used the first edition and added data from the secondary references to the first study to extract full data. We resolved any disagreement by discussion.
Data extraction and management
Two authors independently extracted data from each identified study and recorded data on a standardised data extraction form. We resolved any disagreement by discussion with a third author when necessary.
Assessment of risk of bias in included studies
Two authors assessed risk of bias for each study independently, based on the methods used to generate allocation sequence, allocation concealment, blinding, follow‐up, selective reporting and group size according to the 'Risk of bias' tool (Kjaergard 2001; Moher 1998; Schulz 1995; Higgins 2011a).
We assessed the methods used to deal with incomplete data as: low risk of bias (< 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); and high risk of bias (used 'completer' analysis).
"Size" was added to the 'Risk of bias' table. We assessed studies as low risk of bias (≧ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); and high risk of bias (< 50 participants per treatment arm).
We resolved any disagreement by discussion.
Dealing with missing data
We did not contact authors for missing data. We followed intention‐to‐treat principles. In some studies, the exact mean, standard deviation (SD) and standard error (SE) of pain scores were not reported or were difficult to decipher when results were presented in figures. In this situation, two review authors independently estimated the visual analogue scale (VAS) score presented in the figures in each study using Engauge Digitizer 4.1 (Lan 2010; Ma 2012) and achieved an agreement on the mean ± SE or SD (Gupta 2001). When the number of participants enrolled and the number of participants who reported outcomes were different, we noted this in the 'Characteristics of included studies' table. If the exclusions of participants was justifiable, we would use available data from the studies; if withdrawal of participants were not justifiable, we would carry out intention to treat (ITT) analysis.
Assessment of heterogeneity
We tested the heterogeneity between studies using the Chi2 test (with P < 0.1 indicating significant heterogeneity) and the I2 statistic, which described the proportion of variability due to heterogeneity (Higgins 2003). When P > 0.1, we carried out the meta‐analysis using a fixed‐effect model; otherwise we used a random‐effects model.
Data synthesis
Quantitative analysis
We performed a statistical analysis of outcomes. We merged both dichotomous and continuous data quantitatively in meta‐analysis.
Dichotomous data
For dichotomous data, such as the number of participants who suffered from adverse events, we calculated the risk ratio (RR) using Review Manager software (RevMan) 5.3 (RevMan 2014).
Continuous data
For continuous data, such as postoperative VAS score, analgesic duration of intervention drugs, we calculated mean differences (MDs). We applied a random‐effects or fixed‐effect model to assess outcomes, depending on the statistical heterogeneity among studies.
Qualitative analysis
Different analgesics were used to relieve postoperative pain and many studies did not report the exact doses consumed. Consequently, statistical analyses were not always possible. In situations where the data extracted from the original studies were insufficiently similar, we did not conduct a meta‐analysis but produced a qualitative description of the outcome study by study.
Quality of the evidence
Two review authors independently rated the quality of the outcomes pain intensity, analgesia duration and adverse events. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to rank the quality of the evidence using the GRADEprofiler Guideline Development Tool software (GRADEPro GDT 2015), and the guidelines provided in Chapter 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (Higgins 2011b).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence:
High = further research is very unlikely to change our confidence in the estimate of effect;
Moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
Low = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
Very low = any estimate of effect is very uncertain.
We decreased grade if:
Serious (‐1) or very serious (‐2) limitation to study quality;
Important inconsistency (‐1);
Some (‐1) or major (‐2) uncertainty about directness;
Imprecise or sparse data (‐1);
High probability of reporting bias (‐1).
'Summary of findings' table
We included 'Summary of findings' tables to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes listed above.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analysis to assess clinical heterogeneity rather than statistical heterogeneity. We carried out separate analyses for the most important clinical parameters as follows.
We identified three phases to assess postoperative pain: early phase (zero to two hours), medium phase (two to six hours) and late phase (six to 30 hours). We analysed the data from these different phases separately. Data of two hours and six hours were allocated to early phase and medium phase, respectively.
Comparisons: different agents were used as the comparator regimens in various studies. We only quantitatively merged the studies with the same drug categories in meta‐analysis.
Sensitivity analysis
We performed sensitivity analysis to evaluate the effect of methodological characteristics (quality assessment) of studies on the results of the meta‐analysis.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified 724 studies through the initial electronic search. We scanned these studies and found 35 studies which could not be excluded by scrutiny of the title and abstract only. Of these, seven were further excluded for reasons cited in the Characteristics of excluded studies table. Twenty eight studies (29 reports) satisfied the inclusion criteria comparing morphine with placebo or other analgesics (Figure 1). Studies that met the inclusion criteria contained 2482 enrolled participants, with trial size varying from 33 to 320 participants.
1.
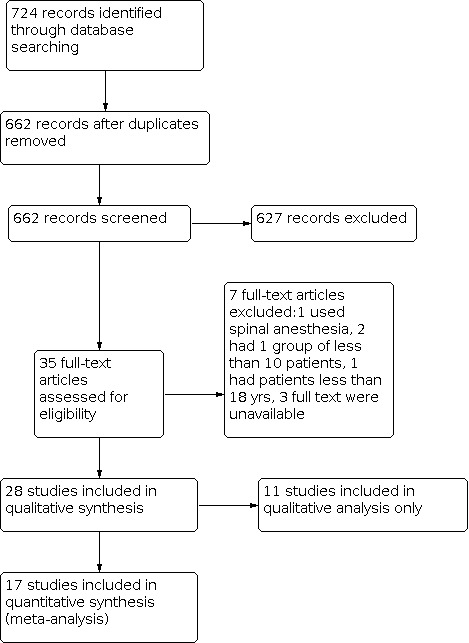
Flowchart showing the stepwise screening of search results
Included studies
Twenty studies (21 reports) enrolled participants undergoing elective arthroscopic knee surgery of multiple procedures such as diagnostic arthroscopy, meniscectomy, excision of plicae, full arthroscopic lateral retinacular release, synovectomy and chondral debridement (Aasbø 1996; Akinci 2003; Alagol 2005; Allen 1993; Bjornsson 1994; Christensen 1996; Follak 2001; Franceschi 2001; Kazemi 2004; Khoury 1992; Kizilkaya 2005; Likar 1995; Likar 1999; Muller 2001; Raj 2004; Richardson 1997; Rosseland 2003a; Ruwe 1995; Solheim 2006; Varkel 1999; Wrench 1996). Six studies (Calmet 2004; De Andres 1998; Dierking 1994; Elkousy 2013; Kanbak 1997; Lyons 1995) only enrolled participants undergoing arthroscopic meniscectomy and one study (Guler 2002) only enrolled participants undergoing arthroscopic anterior cruciate ligament (ACL) reconstruction with hamstring tendons.
Some studies had both placebo and active drug as control, and some had more than one active comparator. Twenty studies included a placebo control, whereas 23 used an active drug in the control group. The active drugs relevant to our studies included bupivacaine (Aasbø 1996; Alagol 2005; Allen 1993; Bjornsson 1994; Calmet 2004; De Andres 1998; Follak 2001; Khoury 1992; Richardson 1997; Ruwe 1995), ropivacaine (Franceschi 2001; Muller 2001), tenoxicam (Alagol 2005; Guler 2002), tramadol (Akinci 2003; Likar 1995), sufentanil (Kazemi 2004), fentanyl (Varkel 1999), pethidine (Lyons 1995), morphine injected IV/IM (Christensen 1996; Dierking 1994; Raj 2004; Richardson 1997; Rosseland 2003a) and different doses of morphine (Allen 1993; Kanbak 1997; Kizilkaya 2005; Likar 1999). Morphine was used from 1 mg to 10 mg. Two hundred and seventy three participants took a 1 mg dose (Allen 1993; Bjornsson 1994; Calmet 2004; De Andres 1998; Kanbak 1997; Khoury 1992; Kizilkaya 2005; Likar 1995; Likar 1999; Muller 2001; Richardson 1997; Wrench 1996), 178 took 2 mg (Alagol 2005; Allen 1993; Dierking 1994; Franceschi 2001; Guler 2002; Likar 1999; Rosseland 2003a; Ruwe 1995), 65 took 3 mg (Aasbø 1996; Kazemi 2004; Varkel 1999), 19 took 4 mg (Likar 1999), 325 took 5 mg (Akinci 2003; Bjornsson 1994; Christensen 1996; Follak 2001; Kanbak 1997; Kizilkaya 2005; Lyons 1995; Muller 2001; Richardson 1997; Solheim 2006), and 39 took 10 mg (Raj 2004).
Pain was rated using VAS in 26 trials, and a verbal rating scale (VRS) was used in six ( Akinci 2003; Christensen 1996; De Andres 1998; Rosseland 2003a; Solheim 2006; Wrench 1996). One study (Bjornsson 1994) assessed pain intensity using a modified VAS score, which was different from the conventional VAS score. In the modified score, "10" corresponded to "severe pain" instead of the conventional ''worst imaginable'' to increase the sensitivity of the scale. We excluded its results of VAS score from meta‐analysis. For those presenting both VAS at rest and VAS with movement, we only abstracted VAS at rest for meta‐analysis. However, the studies did not provide all the outcomes of interest. Some studies gave the central tendency of VAS as median, some presented mean values without SD, and some showed results in figures. We abstracted the data from figures using Engauge Digitizer 4.1. We did not include data presented as median and interquartile range in the meta‐analysis. Different analgesics and treatment regimens were employed for rescue medication, such as tylenol, paracetamol, tramadol, ketorolac, and metamizol, so a meta‐analysis of the rescue medication was not considered feasible.
Excluded studies
We excluded four studies (see Characteristics of excluded studies). One trial used spinal anaesthesia rather than general anaesthesia (Alvarez‐Cabo 1998). Two trials included fewer than 10 participants in the intervention group (Lehrberger 1994; Stein 1991). One trial included participants under 15 years old (De Andres 1993). Three studies were classified in Characteristics of studies awaiting classification because full texts were unavailable through database searching, handsearching or inter‐library loan (Altan 1994; Uzma 1997; VanNess 1994).
Risk of bias in included studies
All the included studies were prospective randomised double‐blind controlled trials (see Characteristics of included studies). We completed a 'Risk of bias' table and results were presented graphically in Figure 2 and summarised in Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the studies were described as 'randomised', and nine studies were of low risk (seven were randomised using a random table or a list of random numbers, two had randomisation stratified by a computerised allocation schedule). One study was assessed as high risk for its randomisation methods, which allocated patients according to the odd or even account number given on the day of surgery. Randomisation technique was not described in 18 studies, which were assessed as unclear risk. Allocation concealment was of low risk in 10 studies and of unclear risk in 18 studies. Although some studies failed to report the methods used to ensure randomisation and allocation concealment adequately, we included all of the studies in the analysis. We did sensitivity analysis to measure the effects of methodological quality.
Blinding
Twenty‐seven included trials were double‐blind and one was single‐blind. Thirteen trials stated the methods to maintain blindness of participants and personnel and were of low risk. Four studies stated the blinding of outcome assessment and were assessed as unclear risk.
Incomplete outcome data
Twenty‐six of the included studies were assessed as low risk and two studies as high risk. Seven studies (Aasbø 1996; Christensen 1996; Dierking 1994; Likar 1999; Lyons 1995; Raj 2004; Solheim 2006) reported the exact number of participants lost to follow‐up. Five studies (Aasbø 1996; Christensen 1996; Likar 1995; Lyons 1995; Raj 2004) had less than 10% of participants who did not complete the study and two studies (Dierking 1994; Likar 1999) had more than 10% withdrawals. Other studies did not mention dropouts. They included all the participants in the analysis as seen from the results section.
Selective reporting
Five studies (Akinci 2003; Alagol 2005; Calmet 2004; De Andres 1998; Raj 2004) were assessed as low risk and four studies (Guler 2002; Lyons 1995; Ruwe 1995; Wrench 1996) were assessed as high risk, while the other nineteen studies were assessed as unclear risk of selective reporting bias.
Other potential sources of bias
We considered study size as a potential source of bias because most of the included studies were small‐sized. Studies with fewer than 10 participants in each group were excluded. Twenty seven in 28 studies had fewer than 50 participants in each group, which were rated as high risk. These studies were all of small size and thus made the conclusions less robust. Only one study had a group size larger than 50 participants.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Morphine compared with placebo for pain control after knee arthroscopy.
| Morphine compared with placebo for pain control after knee arthroscopy | ||||||
|
Patient or population: patients undergoing knee arthroscopy
Settings: inpatients
Intervention: 1 mg morphine administered via the knee joint Comparison: placebo (saline) administered via the knee joint | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Morphine | |||||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 0‐2 hours | The mean pain score in placebo groups ranged from 2 cm to 5.4 cm | The mean pain score in the 1 mg morphine groups was 0.5 cm lower (1.15 lower to 0.14 higher) | 297 (7 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 2‐6 hours | The mean pain score in placebo groups ranged from 1.7 cm to 4.6 cm | The mean pain score in the 1 mg morphine groups was 0.47 cm lower (1.09 lower to 0.14 higher) | 297 (7 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 6‐30 hours | The mean pain score in placebo groups ranged from 0.8 cm to 4.6 cm | The mean pain score in the 1 mg morphine groups was 0.88 cm lower (1.81 lower to 0.04 higher) | 297 (7 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Analgesia duration Time duration from the end of surgery to the time of first analgesic consumption. Scale from: 0 to 100. Follow‐up: 30 hours | See comment | See comment | Not estimable | 124 (3 studies) | ⊕⊕⊝⊝ low3,4 | The data were not pooled. |
| Adverse events Percentage of participants with any adverse event Follow‐up: 30 hours | Study population | RR 1.09 (0.51 to 2.36) | 314 (8 studies) | ⊕⊕⊝⊝ low5,6 | ||
| 103 per 1000 | 97 per 1000 (51 to 179) | |||||
| Moderate | ||||||
| 80 per 1000 | 76 per 1000 (39 to 142) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: the included studies had small group size and were of low quality. Some studies did not describe randomisation and allocation concealment processes adequately.
2 Downgraded one level due to publication bias: we could not extract usable data for 11 out of 20 studies comparing morphine with placebo. Some studies presented data as figures and not enough data can be used.
3 Downgraded one level due to risk of bias: one of the included studies (Kanbak 1997) was of low quality.
4 Downgraded one level due to publication bias: only three out of 20 studies presented data of analgesic duration.
5 Downgraded one level due to risk of bias: two out of eight studies were of low quality.
6 Downgraded one level due to publication bias: the reported adverse events were not the same. Some only reported the overall incidence of adverse events and some reported a series of adverse events including nausea and vomiting.
Summary of findings 2. Morphine compared with bupivacaine for pain control after knee arthroscopy.
| Morphine compared with bupivacaine for pain control after knee arthroscopy | ||||||
|
Patient or population: patients undergoing knee arthroscopy
Settings: inpatients
Intervention: 1 mg morphine administered via the knee joint Comparison: bupivacaine administered via the knee joint | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bupivacaine | Morphine | |||||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 0‐2 hours | The mean pain score in bupivacaine groups ranged from 0.7 cm to 2.3 cm | The mean pain score in the morphine groups was 1.43 cm higher (0.49 to 2.37 higher) | 248 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 2‐6 hours | The mean pain score in bupivacaine groups ranged from 1.2 cm to 3.8 cm | The mean pain score in the morphine groups was 0.45 cm higher (0.47 lower to 1.36 higher) | 330 (6 studies) | ⊕⊕⊝⊝ low1,3 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 6‐30 hours | The mean pain score in bupivacaine groups ranged from 1.3 cm to 3.7 cm | The mean pain score in the morphine groups was 0.71 cm lower (1.23 to 0.19 lower) | 270 (5 studies) | ⊕⊕⊝⊝ low1,4 | ||
| Analgesia duration Time duration from the end of surgery to the time of first analgesic consumption. Scale from: 0 to 100. Follow‐up: 30 hours | See comment | See comment | Not estimable | 162 (3 studies) | ⊕⊕⊕⊝ moderate5 | The data were not pooled. |
| Adverse events Percentage of participants with any adverse event Follow‐up: 30 hours | Study population | RR 0.68 (0.09 to 5.17) | 210 (4 studies) | ⊕⊕⊕⊝ moderate6 | ||
| 76 per 1000 | 49 per 1000 (17 to 135) | |||||
| Moderate | ||||||
| 40 per 1000 | 26 per 1000 (9 to 73) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: the included studies have small group size and are of low quality. Some studies did not describe randomisation and allocation concealment processes adequately.
2 Downgraded one level due to publication bias: six out of 12 studies have exact data for meta‐analysis and others cannot be included in meta‐analysis due to incomplete reporting of outcomes.
3 Downgraded one level due to publication bias: seven out of 12 studies have exact data for meta‐analysis and others cannot be included in meta‐analysis due to incomplete reporting of outcomes.
4 Downgraded one level due to publication bias: five out of 12 studies have exact data for meta‐analysis and others cannot be included in meta‐analysis due to incomplete reporting of outcomes.
5 Downgraded one level due to publication bias: three out of ten studies reported analgesic duration.
6 Downgraded one level due to publication bias: four out of ten studies reported side effects.
Summary of findings 3. Morphine compared with NSAIDs for pain control after knee arthroscopy.
| Morphine compared with NSAIDs for pain control after knee arthroscopy | ||||||
|
Patient or population: patients undergoing knee arthroscopy
Settings: inpatients
Intervention: 1 mg morphine administered via the knee joint Comparison: NSAIDs administered via the knee joint | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| NSAIDs | Morphine | |||||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 0‐2 hours | The mean pain score in the NSAIDs groups ranged from 1 cm to 6.2 cm | The mean pain score in the morphine groups was 0.95 cm higher (0.95 lower to 2.85 higher) | 80 (2 studies) | ⊕⊝⊝⊝ verylow1,2,3 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 2‐6 hours | The mean pain score in the NSAIDs groups ranged from 2 cm to 2.7 cm | The mean pain score in the morphine groups was 1.00 cm higher (0.12 to 1.88 higher) | 80 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 6‐30 hours | The mean pain score in the NSAIDs groups ranged from 1.3 cm to 2 cm | The mean pain score in the morphine groups was 0.43 cm higher (0.54 lower to 1.39 higher) | 80 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Analgesia duration time duration from the end of surgery to the time of first analgesic consumption. Scale from: 0 to 100. Follow‐up: 30 hours | See comment | See comment | Not estimable | 50 (1 study) | ⊕⊕⊝⊝ low4,5 | The data were not pooled. |
| Adverse events Percentage of participants with any adverse event Follow‐up: 30 hours | Study population | RR 0.75 (0.19 to 3.04) | 120 (3 studies) | ⊕⊕⊕⊝ moderate6 | ||
| 67 per 1000 | 49 per 1000 (11 to 203) | |||||
| Moderate | ||||||
| 40 per 1000 | 29 per 1000 (6 to 129) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to risk of bias: one of the included studies (Guler 2002) was of low quality and has potential risk of bias.
2 Downgraded one level due to imprecision: the results showed wide confidence intervals.
3 Downgraded one level due to imprecision: wide confidence intervals.
4 Downgraded one level due to publication bias: three out of ten studies reported analgesic duration.
5 Downgraded one level due to publication bias: only one study reported analgesic duration.
6 Downgraded one level due to risk of bias: one study (Guler 2002) was of low quality.
Summary of findings 4. Different doses of morphine for pain control after knee arthroscopy.
| 1 mg morphine compared with 2 mg/4 mg/5 mg morphine for pain control after knee arthroscopy | ||||||
|
Patient or population: patients undergoing knee arthroscopy
Settings: inpatients
Intervention: 1 mg morphine administered via the knee joint Comparison: 2 mg/5 mg morphine administered via the knee joint | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 2 mg / 4 mg/5 mg morphine | 1 mg morphine | |||||
|
Pain intensity VAS score 1 mg morphine vs 2 mg morphine 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 0‐2 hours |
The mean pain score in the 2 mg morphine groups ranged from 1.8 cm to 3.9 cm | The mean pain score in the 1 mg morphine groups was 0.56 cm lower (1.93 lower to 0.81 higher) | 105 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
|
Pain intensity VAS score
1 mg morphine vs 2 mg morphine 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 2‐6 hours |
The mean pain score in the 2 mg morphine groups ranged from 1.6 cm to 3.8 cm | The mean pain score in the 1 mg morphine groups was 0.32 cm lower (1.69 lower to 1.05 higher) | 105 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
|
Pain intensity VAS score
1 mg morphine vs 2 mg morphine 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 6‐30 hours |
The mean pain score in the 2 mg morphine groups is 0.45 cm | The mean pain score in the 1 mg morphine groups was 0.55 cm higher (0.30 lower to 1.40 higher) | 45 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
|
Analgesia duration 1 mg morphine vs 2 mg morphine Time duration from the end of surgery to the time of first analgesic consumption Follow‐up: 30 hours |
See comment | See comment | Not estimable | 60 (1 study) | ⊕⊕⊕⊝ moderate3 | The data were not pooled. |
|
Adverse events 1 mg morphine vs 2 mg morphine Percentage of participants with any adverse event Follow‐up: 30 hours |
See comment | See comment | Not estimable | None | Not estimable | The were no data available. |
|
Pain intensity VAS score
1 mg morphine vs 4 mg/5 mg morphine 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 0‐2 hours |
The mean pain score in the 5 mg morphine groups ranged from 1.5 cm to 3.5 cm | The mean pain score in the1 mg morphine groups was 0.46 cm higher (0.24 lower to 1.16 higher) | 67 (2 studies) | ⊕⊕⊝⊝ low4,5 | ||
|
Pain intensity VAS score
1 mg morphine vs 4 mg/5 mg morphine 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 2‐6 hours |
The mean pain score in the 5 mg morphine groups ranged from 1.1 cm to 3.7 cm | The mean pain score in the 1 mg morphine groups was 0.44 cm higher (0.18 lower to 1.05 higher) | 67 (2 studies) | ⊕⊕⊝⊝ low4,5 | ||
|
Pain intensity VAS score
1 mg morphine vs 4 mg/5 mg morphine 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 6‐30 hours |
The mean pain score in the 5mg morphine groups ranged from 0.28 cm to 3.8 cm | The mean pain score in the1mg morphine groups was 0.67 cm higher (0.08 to 1.25 higher) | 67 (2 studies) | ⊕⊕⊝⊝ low4,5 | ||
|
Analgesia duration 1 mg morphine vs 4 mg/5 mg morphine time duration from the end of surgery to the first time of analgesic consumption Follow‐up: 30 hours |
See comment | See comment | Not estimable | 24 (1 study) | ⊕⊕⊕⊝ moderate6 | The data were not pooled. |
|
Adverse events 1 mg morphine vs 4 mg/5 mg morphine Percentage of participants with any adverse event Follow‐up: 30 hours |
See comment | See comment | Not estimable | None | Not estimable | The were no data available. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to publication bias: one out of three studies only presented data as figures and no usable data were available.
2 Downgraded one level due to imprecision: the results showed wide confidence intervals.
3 Downgraded one level due to publication bias: only one study reported analgesic duration.
4 Downgraded one level due to risk of bias: one of the included studies (Kanbak 1997) was of low quality.
5 Downgraded one level due to imprecision: imprecision arising from the small sample size.
6 Downgraded one level due to publication bias: only one study reported analgesic duration.
Summary of findings 5. IA morphine compared with IM morphine for pain control after knee arthroscopy.
| IA morphine compared with IM morphine for pain control after knee arthroscopy | ||||||
|
Patient or population: patients undergoing knee arthroscopy
Settings: inpatients
Intervention: 1 mg morphine administered via the knee joint Comparison: morphine administered intra‐muscularly | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IM morphine | IA morphine | |||||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 0‐2 hours | The mean pain score in the IM morphine groups ranged from 1.4 cm to 2.5 cm | The mean pain score in the IA morphine groups was 0.21 cm higher (0.48 lower to 0.9 higher) | 72 (2 studies) | ⊕⊝⊝⊝ verylow1,2,3 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 2‐6 hours | The mean pain score in the IM morphine groups ranged from 1cm to 1.7 cm | The mean pain score in the IA morphine groups was 0.14 cm lower (0.93 lower to 0.64 higher) | 72 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Pain intensity VAS score 0‐10 cm VAS score. Scale from: 0 to 10. Follow‐up: 6‐30 hours | The mean pain score in the IM morphine groups is 1.5 cm | The mean pain score in the IA morphine groups was 0.30 cm lower (1.39 lower to 0.79 higher) | 39 (1 studies) | ⊕⊕⊝⊝ low1,4 | ||
| Analgesia duration time duration from the end of surgery to the time of first analgesic consumption. Scale from: 0 to 100. Follow‐up: 30 hours | See comment | See comment | Not estimable | None | Not estimable | The were no data available. |
| Adverse events Percentage of participants with any adverse event Follow‐up: 30 hours | See comment | See comment | Not estimable | None | Not estimable | The were no data available. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; VAS: Visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to risk of bias: four out of five studies are of low quality.
2 Downgraded one level due to publication bias: only two out of five studies have usable data for meta‐analysis.
3 Downgraded one level due to imprecision: wide confidence intervals.
4 Downgraded one level due to publication bias: only one out of five studies have usable data for meta‐analysis.
We used pain intensity VAS score and use of supplementary analgesia (analgesia duration) as primary outcomes to assess analgesic effects of IA morphine, and adverse events and withdrawals as secondary outcomes to assess the safety of IA morphine.
Primary outcomes: patient‐reported postoperative pain intensity and use of supplementary analgesic
1. Morphine versus placebo
Twenty studies (20 reports) with 2066 participants compared morphine directly with placebo (Aasbø 1996; Akinci 2003; Alagol 2005; Bjornsson 1994; Calmet 2004; De Andres 1998; Follak 2001; Franceschi 2001; Guler 2002; Kanbak 1997; Kazemi 2004; Likar 1999; Lyons 1995; Muller 2001; Richardson 1997; Rosseland 2003a; Ruwe 1995; Solheim 2006; Varkel 1999; Wrench 1996). Thirteen of these studies found a beneficial effect of morphine (Akinci 2003; Alagol 2005; De Andres 1998; Follak 2001; Franceschi 2001; Guler 2002; Kanbak 1997; Kazemi 2004; Likar 1999; Lyons 1995; Muller 2001; Richardson 1997; Varkel 1999) whereas seven others did not find any beneficial effect (Aasbø 1996; Bjornsson 1994; Calmet 2004; Rosseland 2003a; Ruwe 1995; Solheim 2006; Wrench 1996). Among eight studies that compared 1 mg morphine with placebo (Bjornsson 1994; Calmet 2004; De Andres 1998; Kanbak 1997; Likar 1999; Muller 2001; Richardson 1997; Wrench 1996), four studies found a better analgesia effect of 1 mg morphine (De Andres 1998; Likar 1999; Muller 2001; Richardson 1997). Of the six studies that compared 2 mg morphine with placebo (Alagol 2005; Franceschi 2001; Guler 2002; Likar 1999; Rosseland 2003a; Ruwe 1995), four studies showed better analgesia of 2 mg morphine (Alagol 2005; Franceschi 2001; Guler 2002; Likar 1999).
Of studies comparing morphine with placebo, nine in 20 studies had suitable data for meta‐analysis (Calmet 2004; De Andres 1998; Kanbak 1997; Kazemi 2004; Likar 1999; Muller 2001; Richardson 1997; Varkel 1999; Wrench 1996), including seven studies comparing 1 mg morphine with placebo and two studies comparing 2 mg morphine with placebo. We included seven studies (297 participants) comparing 1 mg morphine with placebo in meta‐analysis. There was no difference between 1 mg IA morphine and placebo in pain intensity (VAS score) at early, medium or late phases (Figure 4). The MD and 95% confidence interval (CI) of resting pain was MD ‐0.50 (95% CI, ‐1.15 to 0.14), MD ‐0.47 (95% CI, ‐1.09 to 0.14), and MD ‐0.88 (95% CI, ‐1.81 to 0.04), respectively.
4.

Forest plot of comparison: 1 pain intensity VAS score, outcome: 1.1 1mg morphine vs placebo.
Eleven out of 20 studies reported time to first request of supplementary analgesics (Aasbø 1996; Akinci 2003; Alagol 2005; Calmet 2004; De Andres 1998; Follak 2001; Franceschi 2001; Kanbak 1997; Likar 1999; Kizilkaya 2005; Lyons 1995) and 17 out of 20 studies reported consumption of rescue medication (Aasbø 1996; Akinci 2003; Alagol 2005; Calmet 2004; Follak 2001; Guler 2002; Kanbak 1997; Kazemi 2004; Likar 1999; Lyons 1995; Muller 2001; Richardson 1997; Rosseland 2003a; Ruwe 1995; Solheim 2006; Varkel 1999; Wrench 1996). However, most studies did not present the exact dosages consumed and the number of different analgesic regimens was large. The data could not be subjected to any statistical analysis, so we summarised the results of each study. Three of 10 studies showed a significantly longer time to first analgesic request in the IA morphine group than the placebo group (Alagol 2005; Franceschi 2001; Kanbak 1997) while the remaining seven studies did not find a significant difference. Meta‐analysis of three studies (Alagol 2005; De Andres 1998; Kanbak 1997) indicated no difference between the IA morphine and placebo groups of time to first analgesic request. An I² statistic of 100% represented highly inconsistent findings across studies, and might indicate an error in the data. Seven studies showed a decrease in the postoperative consumption of analgesics in the IA morphine group (Alagol 2005; Kanbak 1997; Kazemi 2004; Likar 1999; Muller 2001; Richardson 1997; Varkel 1999), and four studies found that the number of participants who consumed supplementary analgesics in the IA morphine group was lower than the control group (Aasbø 1996; Calmet 2004; Follak 2001; Lyons 1995). Seven of 13 studies with significant differences favouring morphine also showed a decreased consumption of analgesics in the IA morphine group (Alagol 2005; Follak 2001; Kanbak 1997; Kazemi 2004; Likar 1999; Muller 2001; Varkel 1999), while in three studies analgesic consumption was equivalent between the IA morphine group and the placebo group (Akinci 2003; Lyons 1995; Richardson 1997).
We judged the quality of evidence for pain intensity VAS score, for morphine versus placebo, to be low. We downgraded the quality of evidence by two levels due to risk of bias and publication bias.
We judged the quality of evidence for analgesia duration, for morphine versus placebo, to be low. We downgraded the quality of evidence by two levels due to risk of bias and publication bias.
See Table 1.
2. Morphine versus local anaesthetics
Twelve studies (12 reports) compared IA morphine with IA local anaesthetics (Aasbø 1996; Alagol 2005; Allen 1993; Bjornsson 1994; Calmet 2004; De Andres 1998; Follak 2001; Franceschi 2001; Khoury 1992; Muller 2001; Richardson 1997; Ruwe 1995). Among the 10 studies that compared IA morphine with IA bupivacaine, five studies found a better analgesia effect of bupivacaine (Allen 1993; De Andres 1998; Follak 2001; Khoury 1992; Ruwe 1995) and the other five studies did not find significant differences. Of the six studies comparing 1 mg morphine with bupivacaine, three studies found IA bupivacaine provided better analgesia than IA morphine (Allen 1993; De Andres 1998; Khoury 1992), and the other three studies did not find a difference (Bjornsson 1994; Calmet 2004; Richardson 1997). Allen 1993 indicated that participants who received 1 mg IA morphine in combination with 0.25% bupivacaine provided superior postoperative analgesia for up to 24 hours after knee arthroscopy versus morphine or bupivacaine alone. Meta‐analysis of six studies comparing IA morphine with IA bupivacaine found no better analgesic effects of morphine (Figure 5). The MD and 95% CI of pain at rest was MD 1.43 (95% CI 0.49 to 2.37; participants = 248; studies = 5); at early phase, MD 0.45 (95% CI ‐0.47 to 1.36; participants = 330; studies = 6), and MD ‐0.71 (95% CI ‐1.23 to ‐0.19; participants = 270; studies = 5), respectively (Table 2). The results were highly heterogeneous because of the small size of the included studies. Three studies (Alagol 2005; Allen 1993; De Andres 1998) reported time to first analgesic request, and two studies (Allen 1993; De Andres 1998) found morphine had long analgesic duration. Two studies compared ropivacaine with morphine (Franceschi 2001; Muller 2001), where no significant difference of analgesic effect was found in one study (Franceschi 2001), and the other study (Muller 2001) favoured ropivacaine.
5.

Forest plot of comparison: 1 pain intensity VAS score, outcome: 1.2 1mg morphine vs bupivacaine.
We judged the quality of evidence for pain intensity VAS score, for morphine versus bupivacaine, to be low. We downgraded the quality of evidence by two levels due to risk of bias and publication bias.
We judged the quality of evidence for analgesia duration, for morphine versus bupivacaine, to be moderate. We downgraded the quality of evidence by one level due to publication bias.
See Table 2.
3. Morphine versus NSAIDs
Three studies (Alagol 2005; Calmet 2004; Guler 2002) compared morphine with NSAIDs. Two studies (Alagol 2005; Guler 2002) compared 20 mg tenoxicam with 2 mg morphine and no difference was found in VAS score in meta‐analysis. One study (Alagol 2005) suggested tenoxicam had better analgesic effects and a lower analgesic consumption compared with morphine. The other study (Alagol 2005) did not find a difference on VAS score between tenoxicam and morphine, while the tenoxicam group consumed fewer analgesics than the morphine group. The available data were few, and did not provide robust evidence. Another study (Calmet 2004) compared morphine with ketorolac and concluded that ketorolac was more effective than morphine in pain relief.
We judged the quality of evidence for pain intensity VAS score, for morphine versus NSAIDs, to be very low. We downgraded the quality of evidence by three levels due to risk of bias and imprecision.
We judged the quality of evidence for analgesia duration, for morphine versus NSAIDs, to be low. We downgraded the quality of evidence by two levels due to risk of bias and publication bias.
See Table 3.
4. Different doses of morphine
Four studies (four reports) compared different doses of IA morphine (Allen 1993; Kanbak 1997; Kizilkaya 2005; Likar 1999), of which three studies (Allen 1993; Kanbak 1997; Likar 1999) had usable data for meta‐analysis. Results of one study (Kizilkaya 2005) were presented as figures, which could not be used in meta‐analysis. No difference was found between 1 mg morphine and 2 mg morphine in meta‐analysis of two studies (Allen 1993; Likar 1999). Two studies (Kanbak 1997; Likar 1999) compared 1 mg morphine with 4 mg/5 mg morphine and meta‐analysis showed intensity of pain was lower in the 4 mg/5 mg morphine group than in the 1 mg morphine group at early, medium and late phases (Figure 6). Two studies (Kanbak 1997; Kizilkaya 2005) concluded that the analgesic effects of morphine were dose‐dependent and that 5 mg morphine might have systemic effects on pain relief in participants. Two studies reported analgesic duration, and no difference was found among different doses of morphine. The limited number of studies might bias the conclusion. The number of participants included was small and the differences between groups were not clinically significant. The limited number of studies on dose effects suggests there is a need for more clinical trials.
6.

Forest plot of comparison: 1 pain intensity VAS score, outcome: 1.6 1mg morphine vs 5mg morphine.
We judged the quality of evidence for pain intensity VAS score, for 1 mg morphine vs 2 mg morphine, to be low. We downgraded the quality of evidence by two levels due to publication bias and imprecision.
We judged the quality of evidence for analgesia duration, for 1 mg morphine vs 2 mg morphine, to be moderate. We downgraded the quality of evidence by one level due to publication bias.
See Table 4.
5. Different routes of administration
Five studies (five reports) compared different administration routes of morphine, including IA injection, IV injection and IM injection (Bjornsson 1994; Christensen 1996; Dierking 1994; Raj 2004; Richardson 1997). Of the four studies that compared IA morphine with IM morphine, three studies concluded that no significant difference in pain scores or in requirements for supplemental morphine was observed between participants receiving IA versus IM morphine (Bjornsson 1994; Christensen 1996; Dierking 1994). Only one study concluded that IA morphine provided better analgesia than the same dose of IM morphine and indicated a peripheral mechanism of IA morphine (Raj 2004). Two studies (72 participants) were included in meta‐analysis and no difference was found in pain intensity between IA and IM morphine (Analysis 1.4). The study comparing IA morphine with IV morphine indicated that IA morphine group had a lower VAS score and fewer additional analgesics (Richardson 1997).
1.4. Analysis.

Comparison 1: Pain intensity VAS score, Outcome 4: IA morphine vs IM morphine
We judged the quality of evidence for pain intensity VAS score at early phase (0 ‐ 2 hours), for IM morphine versus IA morphine, to be very low. We downgraded the quality of evidence by three levels due to risk of bias, publication bias and imprecision.
No data were available for analgesia duration.
See Table 5.
6. Morphine versus other opioids
IA morphine was compared with tramadol, sufentanil, fentanyl and pethidine (Akinci 2003; Kazemi 2004; Likar 1995; Lyons 1995; Varkel 1999). Sufentanil, fentanyl and pethidine showed improved analgesia compared to morphine (Kazemi 2004; Lyons 1995; Varkel 1999). IA injection of morphine and sufentanil both reduced the post‐arthroscopic‐knee procedural pain and the need for supplementary analgesics, but sufentanil 5 µg was more effective than morphine 3 mg (Kazemi 2004). Better postoperative analgesia was achieved with 50 µg IA fentanyl than with 3 mg IA morphine (Varkel 1999). The local anaesthetic effect of pethidine may have been responsible for the improved early analgesia, but its duration of action appeared to be less than that of morphine (Lyons 1995). Of the two studies that compared morphine with tramadol, one study found no difference between 5 mg morphine and 50 mg tramadol (Akinci 2003); while the other showed better analgesia of 1 mg morphine compared with 10 mg tramadol (Likar 1995). Because of the limited number of studies, no meta‐analysis was carried out.
Secondary outcomes: adverse events and withdrawals
In this review, twelve studies (13 reports) reported incidence of adverse events, and no difference was reported between groups. Eleven out of 20 studies comparing morphine with placebo reported side effects and nine studies (364 participants) were included in the meta‐analysis. No statistical difference was obtained regarding the incidence of side effects between IA morphine and placebo in meta‐analysis (Analysis 3.1). Two studies showed significant differences between IA morphine and IV morphine/ropivacaine. One study (Franceschi 2001) reported that three participants (10%) of the IA morphine group complained of nausea while no side effects were noted in ropivacaine group. One study (Richardson 1997) reported that the incidence of nausea was larger in the IV morphine group than in the IA morphine group.
3.1. Analysis.

Comparison 3: Adverse events, Outcome 1: morphine vs placebo
We judged the quality of evidence for adverse events, for morphine versus placebo, to be low. We downgraded the quality of evidence by two levels due to risk of bias and publication bias.
We judged the quality of evidence for adverse events, for morphine versus bupivacaine, to be moderate. We downgraded the quality of evidence by one level due to publication bias.
We judged the quality of evidence for adverse events, for morphine versus NSAIDs, to be moderate. We downgraded the quality of evidence by one level due to risk of bias.
Seven of 28 studies reported participants' withdrawal. Five studies (Aasbø 1996; Christensen 1996; Likar 1995; Lyons 1995; Raj 2004) had less than 10% of participants who did not complete the study and two studies (Dierking 1994; Likar 1999) had more than 10% withdrawals. Only one study (Raj 2004) reported the allocated group of the participants lost to follow‐up. One participant withdrew from the IM morphine group while no participants withdrew from the IA morphine group. There were not enough data for meta‐analysis.
Sensitivity analysis
We excluded nine low‐quality studies (743 participants) from meta‐analysis (Aasbø 1996; Christensen 1996; Dierking 1994; Elkousy 2013; Follak 2001; Guler 2002; Kanbak 1997; Khoury 1992; Likar 1995) to measure the effects of methodological quality. The pain intensity VAS score comparisons of 1 mg IA morphine with placebo and 1 mg morphine with bupivacaine did not change.
Discussion
Summary of main results
This review evaluated the relative effects on pain intensity and adverse events of IA morphine after knee arthroscopy. We compared IA morphine with placebo, active analgesics (local anaesthetics, NSAIDs, other opioids) and other routes of morphine administration. We found that IA morphine did not show beneficial effects in pain intensity compared with placebo. IA morphine was not better than IA bupivacaine, NSAIDs, sufentanil, fentanyl and pethidine in pain control. The comparison of effects between morphine and bupivacaine at different time points presented for the comparison of morphine and bupivacaine varied (zero to two hours favoured bupivacaine but this effect had reversed at 24 to 30 hours). The evidence was low quality and the relative effects of the interventions listed were uncertain. The conclusion is not robust because some of the included studies were of low quality and were poorly reported.
Of the 20 studies that compared IA morphine with placebo, we included nine studies with suitable data in the meta‐analysis, which did not show a difference between 1 mg IA morphine and placebo in pain intensity at early, medium or late phases. There was low quality evidence for IA morphine compared with IA bupivacaine, NSAIDs, sufentanil, fentanyl and pethidine, and the relative effects are uncertain. Meta‐analysis indicated no difference between IA morphine and placebo/bupivacaine in time to first analgesic request. None of the included studies showed any statistical difference regarding the incidence of adverse events between IA morphine and placebo.
Overall completeness and applicability of evidence
We searched for published and ongoing trials on IA morphine to identify all the relevant studies. We could only extract useful data from 17 of the 28 included studies. Our evidence showed that IA morphine given after knee arthroscopy did not have beneficial effects in pain relief compared with placebo, and IA morphine was not better than IA bupivacaine, NSAIDs, sufentanil, fentanyl or pethidine. We could not find any robust evidence to support the analgesic effects of IA morphine.
Included studies enrolled participants undergoing elective arthroscopic knee surgery of multiple procedures, such as diagnostic arthroscopy, meniscectomy, excision of plicae, full arthroscopic lateral retinacular release, synovectomy, and chondral debridement, covering most of the knee arthroscopic surgeries. The number of studies favouring morphine exceeded the number of studies without significant difference.
More than half of the included studies did not report adverse events. Of the studies reporting adverse events, we found no statistical difference regarding the incidence of side effects between IA morphine and placebo. No severe side effects of IA morphine were reported.
Quality of the evidence
This systematic review was limited by the quality of existing data. All the included studies were described as randomised and double‐blind, however, some studies failed to report the methods used to ensure randomisation and blinding adequately. In addition, we could not pool some data due to divergent outcome measurements and different types of rescue agents used in the studies. The group sizes of the included studies were small, which indicated low quality of the included studies. Some of the included RCTs with usable information were of low methodological quality. Lack of allocation concealment and blinding might be a source of bias which threatened the validity of the reported results. There was clinical heterogeneity among some trials, which resulted in high levels of statistical heterogeneity in some analyses. Most outcomes were assessed as low quality according to GRADE. Three full texts were unavailable and some outcomes were not fully reported, which might have had an impact on the quality of this review.
Potential biases in the review process
Thirty five studies appeared to satisfy the inclusion criteria through searching, but seven studies were not included in this review (see Figure 1). For example, despite our great efforts, three full texts were unavailable through database searching, handsearching or inter‐library loan (Altan 1994; Uzma 1997; VanNess 1994) (Characteristics of studies awaiting classification). The previously related published systematic reviews also did not include these three studies. Several studies were excluded from meta‐analysis because not enough data were presented in the text. Moreover, not all the included studies reported all the outcomes of interest. Two high‐quality studies only presented results as median and interquartile. The retrieved studies were heterogenous in data and the results of the meta‐analyses might then be less robust.
Agreements and disagreements with other studies or reviews
Four systematic reviews focusing on the same topic have been published previously. Three of them (Gupta 2001; Kalso 1997; Kalso 2002) came up with a positive conclusion that IA morphine might alleviate postoperative pain after knee arthroscopy, while one study found no analgesic effects of morphine compared with placebo (Rosseland 2004b). In the first review by Kalso in 1997, 33 RCTs published before 1996 were included, with no quantitative analysis of pooled data made. Seven RCTs from the included articles showed a significant analgesic effect of IA morphine compared with placebo. The study authors felt that sensitivity of analgesic measurement was important, based on the judgment that low pain intensity in the immediate postoperative period could render the studies insensitive (i.e. no significant difference). In the review, the effectiveness of internal sensitivity was defined as a significant difference between the active and placebo in pain intensity or total consumption of rescue analgesics, as reported by original studies. For example, bupivacaine as an active control in four studies showed a significantly lower VAS compared with placebo, and these four studies were considered sensitive (i.e. significant differences between groups), and the comparison between IA morphine and placebo can be made.
Later, bupivacaine was found to have no better analgesic effect than placebo, thus it was not suitable for testing study sensitivity. Therefore, in 2002, Kalso et al suggested a study was sensitive if VAS was above 3/10 in the control group. Fifteen of 25 trials were considered sensitive. The article concluded that IA morphine was superior to placebo, especially when a dose of 5 mg was used. However, the author also thought that a systemic effect still had to be considered with 5 mg of IA morphine.
In another systematic review (Gupta 2001), the authors did a quantitative analysis of data from 19 (of 45) studies and found an improvement in analgesia in the morphine group compared with placebo. However, in this review, the original articles included participants who underwent arthroscopic knee procedures under local, regional or general anaesthesia. Minor surgeries often cause less tissue damage and less postoperative pain, and spinal anaesthesia can provide longer lasting analgesia compared with general anaesthesia. Therefore, in our present systematic review, only patients under general anaesthesia were included.
Different from the above three reviews, the negative result from Rosseland 2004b suggested that, in properly controlled trials, there was no added analgesic effect of IA morphine compared with placebo alone. The authors thought that most trials with positive results were of low quality or had a small sample size, which must be interpreted cautiously because of the high likelihood of having a randomisation failure. The authors also considered that the definition of sensitive studies in previous systematic reviews (Kalso 1997; Kalso 2002) possibly introduced a bias, because it was difficult to document a baseline pain, making high pain intensity in the placebo group and the positive results simply an outcome of imbalanced allocation of participants.
Two studies compared IA morphine with placebo given to participants who experienced baseline pain of moderate to severe intensity after knee arthroscopy (Rosseland 2003a; Solheim 2006). They found no difference between groups. They had an IA catheter inserted at the end of arthroscopy and only included participants who reported moderate or severe postoperative pain during the first hour after surgery. One of the studies (Solheim 2006) reported 40 of 60 participants (67%) developed moderate to severe pain within one hour. They found that a significantly higher proportion of women (24/26) than men (26/39) reported at least moderate pain (P = 0.018) during the first hour after surgery. The study authors found no difference between IA 5 mg morphine and placebo in pain intensity or pain relief at any time during the 48‐hour observation period and concluded that IA 5 mg morphine did not produce clinically significant pain relief in participants with moderate or severe pain after knee arthroscopy. These controversies originated from the preemptive design of the included articles. Besides, according to Rosseland et al, the incidence of moderate to severe pain after arthroscopy was only 60%. We had to take the participants with mild postoperative pain into account in clinical practice, therefore, we did no test of internal sensitivity, especially when the test may have actually turned out to be biased itself, and just combined data as Gupta et al did.
Of the four dose‐response comparisons, only one was defined as sensitive by Kalso 2002. The result showed that 5 mg of IA morphine provided statistically significantly better analgesia compared with 1 mg of IA morphine. This was confirmed by our meta‐analysis, although the data were quite limited even after all these years of researching.
We believed that a systemic effect of 5 mg IA morphine still had to be considered, particularly in the early period, because no difference could be detected in the efficacy of 5 mg of morphine whether it was injected through IA or IM (Kalso 1997). Additionally, Kalso et al found that 1 mg IA morphine in a 20 ml injection was equivalent to a concentration of about 50 μg/ml. This high concentration would be expected to saturate all the opioid receptors in the knee joint (Kalso 1997). From this point of view, any dose response may be a consequence of systemic effect and residual morphine concentration. The proper dose of IA morphine, and whether this specific dose of morphine is superior to placebo, are still uncertain with the evidence currently available. More trials are needed to make dose‐response comparisons.
Authors' conclusions
Implications for practice.
This review did not find high quality evidence that 1 mg IA morphine is better than placebo at reducing pain intensity at early, medium or late phases. No statistical difference was reported between IA morphine and placebo regarding the incidence of adverse events. The relative effects of 1 mg morphine when compared with IA bupivacaine, NSAIDs, sufentanil, fentanyl and pethidine are uncertain. The quality of the evidence is limited by high risk of bias and small size of the included studies, which might bias the results. More high quality studies are needed to get more conclusive results.
Implications for research.
Though many trials in this area have been conducted, the quality of reporting in these trials is disappointing. Most of the included studies were of small size, so likely to introduce bias and make the conclusions less robust. Some of the studies did not set a primary end point or calculate sample size. Most studies were published at least ten years before we ran our search. Data from the studies were insufficient to show whether IA morphine is beneficial or not.
New standards for clinical trials are now in place, which aim to make clinical trials more strict and precise. Several previous systematic reviews reached different conclusions to this review, but high quality trials with at least 200 participants in each arm are needed to get a more conclusive result of the efficacy and safety of single dose intra‐articular morphine for post‐operative pain after knee arthroscopy. Future trials should focus on quality of reporting, for example by specifying the randomisation process and attempting to conceal allocation of participants to study groups. Better reporting of adverse events and withdrawal is required to fully evaluate the safety of morphine. The incidence of adverse events and their severity should be clearly reported in the trials.
What's new
| Date | Event | Description |
|---|---|---|
| 25 January 2021 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 5, 2016
| Date | Event | Description |
|---|---|---|
| 3 December 2018 | Review declared as stable | See Published notes. |
Notes
Assessed for updating in 2018
A restricted search in December 2018 identified one potentially relevant study which is unlikely to change the conclusions (J Clin Diagn Res. 2017 Apr;11(4):UC13‐15). Therefore, this review has now been stabilised for two years following discussion with the authors and editors. If appropriate, we will update the review sooner if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2021
At January 2021 we are not aware of any potentially relevant studies likely to change the conclusions. Therefore, the PaPaS editorial team has stabilised this review which will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The review was supported by the fund of National Nature Science Foundation of China (81000525), Shanghai Chen‐guang programme (10CG40) and Shanghai Health Bureau (XYQ2011022), and the authors thank Cochrane editors and reviewers for valuable advice.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 MESH DESCRIPTOR Analgesics, Opioid EXPLODE ALL TREES
#2 MESH DESCRIPTOR Opioid Peptides EXPLODE ALL TREES
#3 MESH DESCRIPTOR Morphine EXPLODE ALL TREES
#4 ( (opiate* or opioid* or endorphin* or morphin*)):TI,AB,KY
#5 MESH DESCRIPTOR Knee Joint EXPLODE ALL TREES
#6 MESH DESCRIPTOR Menisci, Tibial EXPLODE ALL TREES
#7 MESH DESCRIPTOR Arthroscopy EXPLODE ALL TREES
#8 MESH DESCRIPTOR Injections, Intra‐Articular EXPLODE ALL TREES
#9 (((inject* or needl*) near10 (joint* or articul* or intra‐articular or intraarticular or "intra articular"))):TI,AB,KY
#10 ( (arthroscop* or menisect* or (knee* near6 surg*))):TI,AB,KY
#11 #1 OR #2 OR #3 OR #4
#12 #5 OR #6 OR #7 OR #10
#13 #8 OR #9
#14 #11 AND #12 AND #13
Appendix 2. Search strategy for MEDLINE (via Ovid)
[mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
1 exp Analgesics, Opioid/
2 exp Opioid Peptides/
3 exp Morphine/
4 (opiate* or opioid* or endorphin* or morphin*).mp.
5 exp Knee Joint/
6 exp Menisci, Tibial/
7 exp Arthroscopy/
8 (arthroscop* or menisect* or (knee* adj6 surg*)).mp.
9 exp Injections, Intra‐Articular/
10 ((inject* or needl*) adj10 (joint* or articul* or intra‐articular or intraarticular or "intra articular")).mp.
11 1 or 2 or 3 or 4
12 5 or 6 or 7 or 8
13 9 or 10
14 11 and 12 and 13
15 randomized controlled trial.pt.
16 controlled clinical trial.pt.
17 randomized.ab.
18 placebo.ab.
19 drug therapy.fs.
20 randomly.ab.
21 trial.ab.
22 or/15‐21
23 exp animals/ not humans.sh.
24 22 not 23
25 14 and 24
Appendix 3. Search strategy for EMBASE
1 exp Narcotic analgesic agent/
2 exp Opiate peptide/
3 exp Morphine/
4 (opiate* or opioid* or endorphin* or morphin*).mp.
5 exp Knee/
6 exp Knee meniscus/
7 exp Arthroscopy/
8 (arthroscop* or menisect* or (knee* adj6 surg*)).mp.
9 exp Intraarticular drug administration/
10 ((inject* or needl*) adj10 (joint* or articul* or intra‐articular or intraarticular or "intra articular")).mp.
11 1 or 2 or 3 or 4
12 5 or 6 or 7 or 8
13 9 or 10
14 11 and 12 and 13
15 random$.tw.
16 factorial$.tw.
17 crossover$.tw.
18 cross over$.tw.
19 cross‐over$.tw.
20 placebo$.tw.
21 (doubl$ adj blind$).tw.
22 (singl$ adj blind$).tw.
23 assign$.tw.
24 allocat$.tw.
25 volunteer$.tw.
26 Crossover Procedure/
27 double‐blind procedure.tw.
28 Randomized Controlled Trial/
29 Single Blind Procedure/
30 or/15‐29
31 (animal/ or nonhuman/) not human/
32 30 not 31
33 14 and 32
Data and analyses
Comparison 1. Pain intensity VAS score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 1mg morphine vs placebo | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 0‐2h | 7 | 297 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.15, 0.14] |
| 1.1.2 2‐6h | 7 | 297 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.09, 0.14] |
| 1.1.3 6‐30h | 7 | 297 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.81, 0.04] |
| 1.2 1mg morphine vs bupivacaine | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 0‐2h | 5 | 248 | Mean Difference (IV, Random, 95% CI) | 1.43 [0.49, 2.37] |
| 1.2.2 2‐6h | 6 | 330 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.47, 1.36] |
| 1.2.3 6‐30h | 5 | 270 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.23, ‐0.19] |
| 1.3 morphine vs NSAIDs | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 0‐2h | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.95, 2.85] |
| 1.3.2 2‐6h | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 1.00 [0.12, 1.88] |
| 1.3.3 6‐30h | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.54, 1.39] |
| 1.4 IA morphine vs IM morphine | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 0‐2h | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.48, 0.90] |
| 1.4.2 2‐6h | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.93, 0.64] |
| 1.4.3 6‐30h | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.39, 0.79] |
| 1.5 1mg morphine vs 2mg morphine | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 0‐2h | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.93, 0.81] |
| 1.5.2 2‐6h | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.69, 1.05] |
| 1.5.3 6‐30h | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.30, 1.40] |
| 1.6 1mg morphine vs 4mg/5mg morphine | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 0‐2h | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐0.24, 1.16] |
| 1.6.2 2‐6h | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐0.18, 1.05] |
| 1.6.3 6‐30h | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.08, 1.25] |
1.1. Analysis.

Comparison 1: Pain intensity VAS score, Outcome 1: 1mg morphine vs placebo
1.2. Analysis.

Comparison 1: Pain intensity VAS score, Outcome 2: 1mg morphine vs bupivacaine
1.3. Analysis.

Comparison 1: Pain intensity VAS score, Outcome 3: morphine vs NSAIDs
1.5. Analysis.

Comparison 1: Pain intensity VAS score, Outcome 5: 1mg morphine vs 2mg morphine
1.6. Analysis.

Comparison 1: Pain intensity VAS score, Outcome 6: 1mg morphine vs 4mg/5mg morphine
Comparison 2. Analgesia duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 morphine vs placebo | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 morphine vs bupivacaine | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 morphine vs NSAIDS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 1mg morphine vs 2mg morphine | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 1mg morphine vs 5mg morphine | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2: Analgesia duration, Outcome 1: morphine vs placebo
2.2. Analysis.

Comparison 2: Analgesia duration, Outcome 2: morphine vs bupivacaine
2.3. Analysis.

Comparison 2: Analgesia duration, Outcome 3: morphine vs NSAIDS
2.4. Analysis.

Comparison 2: Analgesia duration, Outcome 4: 1mg morphine vs 2mg morphine
2.5. Analysis.

Comparison 2: Analgesia duration, Outcome 5: 1mg morphine vs 5mg morphine
Comparison 3. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 morphine vs placebo | 8 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.51, 2.36] |
| 3.2 morphine vs bupivacaine | 4 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.09, 5.17] |
| 3.3 morphine vs NSAIDs | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 3.04] |
3.2. Analysis.

Comparison 3: Adverse events, Outcome 2: morphine vs bupivacaine
3.3. Analysis.

Comparison 3: Adverse events, Outcome 3: morphine vs NSAIDs
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aasbø 1996.
| Study characteristics | ||
| Methods | Prospective, randomised double‐blind trial | |
| Participants | 107 patients scheduled for elective, diagnostic knee arthroscopy | |
| Interventions | Group 1: 20 ml of bupivacaine 2.5 mg/ml with 3 mg of morphine; Group 2: 20 ml of bupivacaine 2.5 mg/ml; Group 3: 20 ml isotonic saline with 3 mg morphine; Group 4: 20 ml of isotonic saline |
|
| Outcomes | VAS, time to first analgesic administered, analgesic consumption | |
| Notes | No additional analgesic effect of IA morphine or bupivacaine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: The participants were randomly assigned to double‐blind administration of 20 ml of the test drug |
| Allocation concealment (selection bias) | Unclear risk | Quote: The test drugs were drawn into a syringe by an independent nurse |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no patient was excluded, but 3 in 107 (less than 10%) participants did not complete the questionnaire. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: was given to the surgeon who administrated the injection at the end of the procedure without knowing the contents |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm. |
Akinci 2003.
| Study characteristics | ||
| Methods | Prospective double‐blind randomised trial | |
| Participants | 75 patients having elective arthroscopic surgery of the knee, included diagnostic arthroscopy, meniscectomy, and minimal debridement or loose body removal, or both | |
| Interventions | 3 groups ‐ IA tramadol 50 mg (tramadol group), IA morphine 5 mg (morphine group), or IA normal saline (control group), injected through the arthroscope with the study drug supplied in a coded syringe | |
| Outcomes | VRS, supplemental analgesic requirements, incidence of side effects | |
| Notes | There was no significance with respect to the pain scores postoperatively except the first VRS pain score when the participants arrived at the PACU. Nausea and vomiting were reported in 32% of participants in the control group and 24% of participants in both the morphine and the tramadol groups (P>0.05). Somnolence was noted in 24% of the control group, 16% of the morphine group, and 8% of the tramadol group (P=0.10) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: a computerized allocation schedule |
| Allocation concealment (selection bias) | Low risk | Quote: coded syringes containing the study drugs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Low risk | Comment: all the outcomes in methods section were reported. And VRS score of continuous time course were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: the coded syringes were prepared by an anaesthesiologist not involved in the administration of the drug, patient care, or data collection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: A blinded observer recorded pain, supplemental analgesic requirements, and incidence of side effects |
| Size | High risk | Comment: < 50 participants per treatment arm |
Alagol 2005.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blinded study | |
| Participants | 150 patients undergoing arthroscopic knee surgery, multiple arthroscopic procedures | |
| Interventions | Group N received 500 μg neostigmine, Group M received 2 mg morphine, Group T received 20 mg tenoxicam, Group C received clonidine, Group B received 100 mg bupivacaine and Group S received saline 20 ml. | |
| Outcomes | VAS, duration of analgesia | |
| Notes | Neostigmine, clonidine, tenoxicam, morphine and bupivacaine decreased postoperative pain intensity and reduced analgesic consumption when compared with placebo. The most effective drugs that are administered intra articularly are neostigmine and clonidine among the five drugs we studied. Tenoxicam provided longer analgesia when compared with morphine and bupivacaine, postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Low risk | Comment: all the outcomes in methods section were reported and VAS score of continuous time course were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: The solutions were prepared in 20‐ml volume by an anaesthesiologist and administered by a surgeon who was blinded to the contents of the syringe |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: After extubation, participants were observed in the postanaesthesia care unit for 1 h and in the clinic of orthopaedic surgery by an anaesthesiologist who was blinded to the groups |
| Size | High risk | Comment: < 50 participants per treatment arm |
Allen 1993.
| Study characteristics | ||
| Methods | Randomised, double‐blind trial | |
| Participants | 120 patients ASA 1‐2, need arthroscopy surgery | |
| Interventions | Group 1: bupivacaine Group 2: 1 mg morphine Group 3: 2 mg morphine Group 4: morphine & bupivacaine |
|
| Outcomes | VAS, duration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: use a random table to ensure randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Bjornsson 1994.
| Study characteristics | ||
| Methods | Two‐stage, randomised, double‐blind, controlled trial | |
| Participants | 149 patients ASA 1‐2, part 1: 78 part 2: 71 | |
| Interventions | Morphine 1 mg, saline, bupivacaine, morphine & bupivacaine 5 mg morphine IA, saline, 5 mg morphine IM |
|
| Outcomes | VAS (severe, sensitivity) | |
| Notes | VAS scores similar | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: After the operation, a nurse who was blind to the method asked the patient to assess the severity of pain |
| Size | High risk | Comment: < 50 participants per treatment arm |
Calmet 2004.
| Study characteristics | ||
| Methods | Prospective, double‐blind, randomised study | |
| Participants | 80 consecutive patients were studied who had been diagnosed with torn meniscus and recommended to have arthroscopic surgery | |
| Interventions | Group 1 participants (n = 20) received postoperative injection of 60 mg IA ketorolac, Group 2 participants (n = 20) 10 cc IA bupivacaine 0.25% Group 3 participants (n = 20) 1 mg IA morphine diluted in 10 cc saline, and Group 4 participants (n = 20, controls) only 10 cc saline |
|
| Outcomes | VAS, analgesic duration | |
| Notes | The best analgesic effect was IA ketorolac | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | Comment: all the outcomes in methods section were reported and VAS score of continuous time course were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Christensen 1996.
| Study characteristics | ||
| Methods | A controlled, randomised and double‐blind study | |
| Participants | 61 ASA 1‐2, having elective arthroscopic surgery of the knee (61 participants recruited, 58 participants completed trial) | |
| Interventions | Group 1: (n = 29) 5 mg morphine IA, Group 2: (n = 29) 5 mg morphine IM |
|
| Outcomes | VRS and VAS, analgesic consumption | |
| Notes | The clinical analgesic effect of 5 mg morphine given intra‐articularly is equal to 5 mg morphine given intra‐muscularly. The occurrence of villous synovitis seems to be of no clinical importance concerning the local effect of morphine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3/61 participants excluded, < 10% of participants did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: all participants were assessed by an observer blinded to the randomisation of the patient |
| Size | High risk | Comment: < 50 participants per treatment arm |
De Andres 1998.
| Study characteristics | ||
| Methods | Randomised double‐blind, placebo‐controlled trial | |
| Participants | 78 patients having elective arthroscopic meniscectomy | |
| Interventions | Group 1 (n = 25): 0.25% bupivacaine (50 mg) (IA), Group 2 (n = 27): 1 mg morphine, Group 3 (n = 26): morphine bupivacaine mixture, Group 4 (n = 25): normal saline |
|
| Outcomes | VAS, VRS, side effect, time to first request of analgesics | |
| Notes | Side effects occurred in 13.7% of the participants, with urinary retention being the most common (n = 8, 10%). In this regard there were no significant differences. These results show no significant differences between the groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: a random number table |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Low risk | Comment: all the outcomes in methods section were reported and VRS score of continuous time course were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: The surgeon injected 20 ml solution into the knee joint. The patient, the anaesthesiologist in charge and the surgeon were blind to the solution injected. Participants were informed pre‐operatively by a blinded observer about all pain scores |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Dierking 1994.
| Study characteristics | ||
| Methods | Double‐blind, randomised study | |
| Participants | 40 healthy patients undergoing elective arthroscopic meniscectomy | |
| Interventions | 18 participants received IA morphine and 15 participants IM morphine 2 mg | |
| Outcomes | VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 7 in 40 of participants did not complete the study, used 'completer analysis' |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: Study medication was drawn from coded ampoules to ensure the double‐blind nature of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Elkousy 2013.
| Study characteristics | ||
| Methods | Randomised | |
| Participants | 82 patients underwent partial meniscectomy, chondral debridement, or both | |
| Interventions | 10 mg morphine versus 10 cc bupivacaine | |
| Outcomes | VAS score, side effects | |
| Notes | Quasi‐randomised trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: group assignment was determined by an odd or even account number given on the day of surgery |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient data were available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: eighteen participants (11 in the morphine group and 7 in the bupivacaine group) had data available through hospital discharge but no data in the subsequent 24 hours. The remaining 64 participants (36 in the morphine group and 28 in the bupivacaine group) had post‐anaesthesia care unit data, as well as follow‐up VAS and medication use data through 24 hours postoperatively. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available. The time point of VAS recording was not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: single‐blind. The participants were blinded to group assignment, but the investigators were not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Follak 2001.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind study | |
| Participants | 320 patients, subdivided into 4 groups of 80, who underwent arthroscopic knee surgery | |
| Interventions | Each of the 4 groups received a different solution: 15 ml of bupivacaine 0.5%, 5 mg of morphine in 15 ml of isotonic saline solution, 15 ml of bupivacaine 0.5% with epinephrine 0.0005% 15 ml of isotonic saline solution (control group) |
|
| Outcomes | VAS, consumption of analgesics and time point of first analgesic application | |
| Notes | Bupivacaine 0.5% with epinephrine 0.0005% was found to be the most effective | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | Unclear risk | Comment: 50 to 199 participants per treatment arm |
Franceschi 2001.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 90 patients scheduled to undergo elective knee arthroscopy | |
| Interventions | Group 1 received ropivacaine 75 mg, Group 2 received 2 mg morphine, Group 3 received 20 ml of saline solution. |
|
| Outcomes | VAS, duration, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Low risk | Quote: the 3 different analgesics were administered in a double‐blinded randomised fashion from a coded syringe into the joint space |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: the 3 different analgesics were administered in a double‐blinded randomised fashion from a coded syringe |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Guler 2002.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 42 patients only arthroscopic ACL reconstruction using hamstring tendons | |
| Interventions | Group 1: 20 mg tenoxicam, Group 2: 2 mg morphine, Group 3: control NS |
|
| Outcomes | VAS, analgesic requirements, side effects | |
| Notes | VAS data without SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | High risk | Comment: insufficient data were available. Data of VAS score were presented as figures only |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Kanbak 1997.
| Study characteristics | ||
| Methods | Prospective, randomised, controlled, double‐blinded | |
| Participants | 35 patients, included partial or total meniscectomy and repair of ruptured ligaments | |
| Interventions | Group 1: n = 11, NS intra‐articularly Group 2: n = 11, 1 mg morphine intra‐articularly Group 3: n = 13, 5 mg morphine intra‐articularly |
|
| Outcomes | VAS, analgesic consumption, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: VAS score recorded by a blinded observer |
| Size | High risk | Comment: < 50 participants per treatment arm |
Kazemi 2004.
| Study characteristics | ||
| Methods | Prospective, double‐blind study | |
| Participants | 45 patients who were ASA physical status I and II and scheduled for arthroscopic knee surgery | |
| Interventions | Sufentanil 5 µg (group S), morphine 3 mg (group M) or normal saline 20 cc as placebo (group p), intra‐articularly at the end of arthroscopic knee surgery | |
| Outcomes | VAS, consumption of rescue medication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Low risk | Quote: The contents of these syringes were unknown to anesthesiologist and surgeon who performed the study. The codes were not revealed until completion of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: the content of syringe in unknown to surgeon and anaesthesiologists |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Khoury 1992.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 33 patients of various kinds of knee surgeries | |
| Interventions | Group 1:1 mg morphine Group 2: 0.25% bupivacaine Group 3: morphine + bupivacaine |
|
| Outcomes | VAS, analgesic consumption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Kizilkaya 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomised | |
| Participants | 72 patients scheduled for arthroscopic knee surgery either for diagnostic purposes or for partial meniscectomy | |
| Interventions | Group C (n = 18), 20 ml of IA isotonic saline and 5 mg morphine in 5 ml of isotonic saline IV;
Group M5 (n = 17) received 5 mg morphine in 20 ml of isotonic saline intra‐articularly and 5 ml isotonic saline IV; Group M1 (n = 18) received 1 mg morphine in 20 ml of isotonic saline intra‐articularly and 5 ml of isotonic saline IV; Group M1M (n = 19) received 1 mg morphine plus 40 mg methylprednisolone in 20 ml of isotonic saline intra‐articularly and 5 ml of isotonic saline IV. Injected the solution through the arthro‐scope |
|
| Outcomes | VAS, analgesic consumption | |
| Notes | The analgesic effect of morphine given intra‐articularly is dose dependent and that combination of methylprednisolone with morphine has an additive effect on analgesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By a computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: iInsufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: without knowing the contents measured and recorded by a anaesthesiologist who is blind to the group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Likar 1995.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 93 patients (93 recruited, 86 completed trial), multiple kinds of knee surgeries | |
| Interventions | Group 1: n = 41, 1 mg morphine Group 2: n = 45, 10 mg tramadol |
|
| Outcomes | VAS, analgesic consumption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 7 participants were excluded from centre 2, < 10% of participants did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Likar 1999.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 108 patients (108 recruited, 86 completed trial), various kinds of knee surgeries | |
| Interventions | Group 1: NS Group 2: morphine 1 mg Group 3: morphine 2 mg Group 4: morphine 4 mg |
|
| Outcomes | VAS, analgesic consumption | |
| Notes | Increasing doses of IA morphine were associated with better analgesic effect and less analgesic consumption | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: using a random table |
| Allocation concealment (selection bias) | Low risk | Quote: each bottle of test solution was coded by the hospital pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 22 in 108 of participants did not complete the study, used 'completer analysis'. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: VAS scores were recorded by a blinded observer |
| Size | High risk | Comment: < 50 participants per treatment arm |
Lyons 1995.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 66 patients (66 recruited, 60 completed trial, without ITT analysis), only enrolled participants undergoing arthroscopic meniscectomy |
|
| Interventions | Group 1: pethidine 50 mg Group 2: morphine 5 mg Group 3: NS |
|
| Outcomes | VAS, analgesic consumption | |
| Notes | Morphine & pethidine effective | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Quote: each injection was drawn up by an anaesthesiologist not involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: replies were received from 90% (60/66) of participants studied. < 10% of participants did not complete the study |
| Selective reporting (reporting bias) | High risk | Comment: no exact data were provided. Only one figure showed the results. No exact P value was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: anaesthesiologists and operating surgeon involved were not aware of the contents of the Injectate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Muller 2001.
| Study characteristics | ||
| Methods | Prospective randomised, double‐blind controlled trial | |
| Participants | 135 patients > 18 yrs, knee arthroscopy | |
| Interventions | 9 groups, each has 15 participants 1A NS 2A morphine 5 mg 3A morphine 1 mg 4A mor + ropivacaine 5A ropivacaine 20 mg 1B NS 2B morphine 5 mg 3B morphine 1 mg 4B mor + ropivacaine 5B ropivacaine 20 mg |
|
| Outcomes | VAS, tramadol consumption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessor was blind |
| Size | High risk | Comment: < 50 participants per treatment arm |
Raj 2004.
| Study characteristics | ||
| Methods | Randomised, double‐blind | |
| Participants | 40 patients, various kinds of knee surgery | |
| Interventions | Group 1: n = 20, 10 mg morphine IA Group 2: n = 19, 10 mg morphine IM |
|
| Outcomes | VAS, plasma concentration | |
| Notes | IA morphine is better than IM morphine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: allocated randomly using Lab5.1 |
| Allocation concealment (selection bias) | Low risk | Quote: using a sealed envelope method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: one out of 40 participants withdrew from the study |
| Selective reporting (reporting bias) | Low risk | Comment: all the outcomes in methods section were reported and VAS score of continuous time course were reported. Exact P value was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: all participants received a small dressing where the IM injection would have been or actually placed. The anaesthetist involved was not aware of which treat the participants received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Richardson 1997.
| Study characteristics | ||
| Methods | Two prospective, randomised, double‐blind clinical trials | |
| Participants | Trial 1: n = 106 Trial 2: n = 48, various kinds of knee surgery | |
| Interventions | Trial 1: 1A 1 mg morphine in 20 ml normal saline IA, 1B 20 ml normal saline IA, 1C 20 ml 0.5% bupivacaine IA Trial 2: 20 ml normal saline IA plus 5 mg morphine in 10 ml normal saline IV, 2B 1 mg morphine in 20 ml normal saline IA plus 10 ml normal saline IV 2C 5 mg morphine in 20 ml normal saline IA plus 10 ml normal saline IV |
|
| Outcomes | VAS, analgesia consumption | |
| Notes | 5 mg IA was the most effective analgesic following knee arthroscopy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: solution was injected into the knee by the surgeon, who was blinded to its contents |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Rosseland 2003a.
| Study characteristics | ||
| Methods | A randomised, double‐blind controlled clinical study | |
| Participants | 40 patients (59 recruited, 40 included in analysis), various kinds of knee surgery | |
| Interventions | Saline 10 ml with morphine 2 mg (n = 19), without morphine (n = 21) | |
| Outcomes | VAS, VRS, analgesic time and consumption | |
| Notes | Only 70% of 57 participants had pain of moderate to severe intensity within 1 h after an arthroscopic procedure of the knee joint under general anaesthesia. IA injection of saline 10 ml and saline 10 ml with morphine 2 mg were both associated with pain relief | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: list of random numbers |
| Allocation concealment (selection bias) | Low risk | Quote: by a person not involved in the study. Block size and randomisation codes were not revealed to the investigators until all measurements and calculations had been entered into the database. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: maintain blinding of both participants and examiner throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Ruwe 1995.
| Study characteristics | ||
| Methods | A double‐blind, randomised trial | |
| Participants | 124 patients elective outpatient arthroscopic knee surgery | |
| Interventions | Group 1 (n = 23) received 15 ml of 0.5% bupivacaine and 5 ml of normal saline Group 2 (n = 26) received 15 ml of normal saline and 2 mg of morphine in 5 ml of normal saline Group 3 (n = 25) received 15 ml of 0.5% bupivacaine with 1 mg morphine in 5 ml of normal saline Group 4 (n = 22) received 15 ml of 0.5% bupivacaine with 2 mg morphine in 5 ml of normal saline Group 5 (n = 28) received 20 ml of normal saline |
|
| Outcomes | VAS, supplemental analgesic, weightbearing status | |
| Notes | Our results failed to show any beneficial effect of morphine used for postoperative analgesia, either alone or in combination with bupivacaine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Low risk | Quote: Syringes were coded by the pharmacy with their contents unknown to the investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | High risk | Comment: no exact data were provided. Only one figure showed the results. No exact P value was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Solheim 2006.
| Study characteristics | ||
| Methods | Randomised, double blind | |
| Participants | 40 patients (60 recruited, 40 included in analysis), scheduled for day‐case knee arthroscopy, such as meniscectomies, removal of loose body, or shaving and lavage of degenerated cartilage | |
| Interventions | IA injection was given through a 20‐gauge catheter IA saline 1 ml (placebo) or IA morphine 5 mg and to immediate or delayed removal of IA catheter |
|
| Outcomes | VAS, VRS, time to and consumption of rescue analgesic drugs, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: by using a list of random numbers |
| Allocation concealment (selection bias) | Low risk | Quote: by the senior author who did not deal with the participants. Block size and randomisation codes were not revealed to the investigators until all measurements and calculations had been entered into the database |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: None lost to follow‐up during hospital day and 9 participants lost to follow‐up during 1 and 2 day. Used all the included participants' data in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: To maintain blinding of both participants and examiner throughout the study, syringes for each patient were prepared in the morning of surgery by a nurse not involved in the treatment or assessment of the participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
Varkel 1999.
| Study characteristics | ||
| Methods | Double blind, randomised | |
| Participants | 69 patients, arthroscopic surgery | |
| Interventions | Group I (n = 23) fentanyl in 20 ml saline; Group II (n = 24) 3 mg morphine in 20 ml saline; Group III (n = 22) 20 ml saline |
|
| Outcomes | VAS, consumption of rescue medication | |
| Notes | Better postoperative analgesia was achieved with 50 Hg IA fentanyl than with 3 mg IA morphine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Low risk | Quote: using a sealed envelope technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdraw from the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient data were available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: by an observer blinded to patient group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified, double blind, probably done |
| Size | High risk | Comment: < 50 participants per treatment arm |
Wrench 1996.
| Study characteristics | ||
| Methods | A randomised double‐blind placebo‐controlled trial | |
| Participants | 60 patients (ASA 1 or 2) scheduled to undergo day‐case arthroscopic knee surgery | |
| Interventions | (i) 0.9% saline 20 ml, n=20 (ii) morphine 1 mg in 20 ml of 0.9% saline 20 ml, n=19 (iii) buprenorphine 30 jig in 20 ml of 0.9% saline 20 ml, n=19 |
|
| Outcomes | VRS, consumption of rescue medication | |
| Notes | There were no differences in pain scores among groups, first 24 h postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not specified |
| Allocation concealment (selection bias) | Low risk | Quote: these solutions were prepared by an anaesthetist who was not one of the investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew from the study |
| Selective reporting (reporting bias) | High risk | Comment: no exact data were provided. Only one figure showed the results. No exact P value was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: These solutions were prepared by an anaesthetist who was not one of the investigators; the patient and the investigator were blinded to the identity of the test solution |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Size | High risk | Comment: < 50 participants per treatment arm |
IA: Intra‐articular
IM: Intramuscular
IV: Intravenous
NS: Normal saline
VAS: Visual analogue scale
VRS: Verbal rating scales
PACU: Postanesthesia care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez‐Cabo 1998 | Participants received spinal anaesthesia |
| De Andres 1993 | Some participants were younger than 15 years old |
| Lehrberger 1994 | Group size. Number of participants in group who completed the study less than ten |
| Stein 1991 | Group size. Number of participants in group who completed the study less than ten |
Characteristics of studies awaiting classification [ordered by study ID]
Altan 1994.
| Methods | Randomised clinical trial |
| Participants | ASA I, II 60 participants |
| Interventions | 3 groups: 1. 0.5 % bupivacaine, 2. 0.025% morphine, 3. normal saline IA |
| Outcomes | VAS score |
| Notes | Only abstract |
Uzma 1997.
| Methods | A randomised, double‐blind trial |
| Participants | Sixty participants who underwent arthroscopic knee surgery |
| Interventions | 4 groups, Group M: morphine, Group B: bupivacaine Group M+B: morphine+bupivacaine, Group SF: normal saline |
| Outcomes | VAS |
| Notes | Only abstract. |
VanNess 1994.
| Methods | A prospective study |
| Participants | Participants undergoing elective knee arthroscopy performed under general anaesthesia |
| Interventions | Group 1 (n = 41) received 30 cc of 0.25% bupivacaine with 1:200,000 epinephrine; Group 2 (n = 40) received 2 mg morphine (1 mg/cc) in 28 cc normal saline (total volume 30 cc) |
| Outcomes | Postoperative pain scores and the amount of supplemental analgesic agents used in a 24‐hour period |
| Notes | Only abstract |
Differences between protocol and review
The subgroup analyses of additional analgesics and different types of operation proposed in our protocol were not performed due to the lack of data available to perform subgroup analysis.
We separated the outcomes into primary and secondary. Patient‐reported pain intensity and use of supplementary analgesic are listed as primary outcomes. These outcomes can reflect the efficacy of the drug.
Subgroup analysis about different methods of application of morphine (wound/synovial infiltration/intra‐articular instillation, etc.) is called IA morphine versus IM morphine. In all the included studies, neither wound nor synovial infiltration was used, and IM morphine was used. So we compared IA morphine with IM morphine in the subgroup analysis.
We considered 'size' and 'selective outcome reporting' as potential sources of bias so we added these two domains in the characteristics of studies table.
Ten out of 20 studies reported time to first request of supplementary analgesics and 17 out of 20 studies reported consumption of rescue medication. However, many studies did not present the exact dosage consumed and the number of different analgesic regimens was large. The data could not be subjected to any statistical analysis as no usable data could be extracted from studies, so we summarised the results of each study.
We added IA morphine versus other opioids to Types of interventions.
Contributions of authors
| Draft the protocol | Zui Zou, Xue Yin Shi |
| Develop a search strategy | Mao Mao An, Zui Zou |
| Search for trials | Zui Zou, Xiao‐Yan Chen |
| Obtain copies of trials | Xiao‐Yan Chen |
| Select which trials to include | Xue‐Yin Shi, Qun Xie, Xue Yin Shi (arbiter) |
| Extract data from trials | Zui Zou, Qun Xie |
| Enter data into RevMan | Qun Xie |
| Carry out the analysis | Qun Xie |
| Interpret the analysis | Qun Xie |
| Draft the final review | Xue‐Yin Shi, Zui Zou |
| Update the review | Xue‐Yin Shi, Zui Zou |
| Content expert | Xue‐Yin Shi |
| Methodologist | Mao Mao An |
| Statistician | Guan‐Jian Liu |
Sources of support
Internal sources
-
National Nature Science Foundation of China, China
81000525
-
Shanghai Chen‐guang program, China
10CG40
-
Shanghai Health Bureau, China
XYQ2011022
External sources
No sources of support supplied
Declarations of interest
Zui Zou: none known.
Mao Mao An: none known.
Qun Xie: none known.
MMA: none known.
Xiao Y Chen: none known.
Hao Zhang: none known.
Guan J Liu: none known.
Xue Y Shi: none known.
ZZ's and XYS's institution received funding support from the National Nature Science Foundation of China (81000525), Shanghai Chen‐guang program (10CG40) and Shanghai Health Bureau (XYQ2011022), to complete this review.
Joint first author
Joint first author
Joint first author
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aasbø 1996 {published data only}
Akinci 2003 {published data only}
- Akinci SB, Saricaoglu F, Atay A, Doral MN, Kanbak M. Analgesic effect of intra-articular tramadol compared to morphine after arthroscopic knee surgery. Canadian Journal of Anesthesia 2003;50:423-4. [DOI] [PubMed] [Google Scholar]
- Akinci SB, Saricaoglu F, Atay OA, Doral MN, Kanbak M. Analgesic effect of intra-articular tramadol compared with morphine after arthroscopic knee surgery. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2005;21:1060-5. [DOI] [PubMed] [Google Scholar]
Alagol 2005 {published data only}
- Alagol A, Calpur OU, Usar PS, Turan N, Pamukcu Z. Intraarticular analgesia after arthroscopic knee surgery: comparison of neostigmine, clonidine, tenoxicam, morphine and bupivacaine. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal of the ESSKA 2005;13:658-63. [DOI] [PubMed] [Google Scholar]
Allen 1993 {published data only}
- Allen GC, St Amand MA, Lui AC, Johnson DH, Lindsay MP. Postarthroscopy analgesia with intraarticular bupivacaine/morphine. A randomized clinical trial. Anesthesiology 1993;79:475-80. [DOI] [PubMed] [Google Scholar]
Bjornsson 1994 {published data only}
- Bjornsson A, Gupta A, Vegfors M, Lennmarken C, Sjoberg F. Intraarticular morphine for postoperative analgesia following knee arthroscopy. Regional Anesthesia 1994;19:104-8. [Google Scholar]
Calmet 2004 {published data only}
- Calmet J, Esteve C, Boada S, Gine J. Analgesic effect of intra-articular ketorolac in knee arthroscopy: comparison of morphine and bupivacaine. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 2004;12:552-5. [DOI] [PubMed] [Google Scholar]
Christensen 1996 {published data only}
- Christensen O, Christensen P, Sonnenschein C, Nielsen PR, Jacobsen S. Analgesic effect of intraarticular morphine. A controlled, randomised and double-blind study. Acta Anaesthesiologica Scandinavica 1996;40:842-6. [DOI] [PubMed] [Google Scholar]
De Andres 1998 {published data only}
- De Andres J, Valia JC, Barrera L, Colomina R. Intra-articular analgesia after arthroscopic knee surgery: comparison of three different regimens. European Journal of Anaesthesiology 1998;15:10-5. [DOI] [PubMed] [Google Scholar]
Dierking 1994 {published data only}
- Dierking GW, Ostergaard HT, Dissing CK, Kristensen JE, Dahl JB. Analgesic effect of intra-articular morphine after arthroscopic meniscectomy. Anaesthesia 1994;49:627-9. [DOI] [PubMed] [Google Scholar]
Elkousy 2013 {published data only}
- Elkousy H, Kannan V, Calder CT, Zumwalt J, O'Connor DP, Woods GW. Intra-articular morphine versus bupivacaine for postoperative pain management. Orthopedics 2013;36(9):e1121-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Follak 2001 {published data only}
- Follak N, Ganzer D. Postoperative analgesic value of the intra-articular instillation of bupivacaine and morphine after arthroscopic knee surgery. Archives of Orthopaedic and Trauma Surgery 2001;121:278-81. [DOI] [PubMed] [Google Scholar]
Franceschi 2001 {published data only}
- Franceschi F, Rizzello G, Cataldo R, Denaro V. Comparison of morphine and ropivacaine following knee arthroscopy. Arthroscopy 2001;17:477-80. [DOI] [PubMed] [Google Scholar]
Guler 2002 {published data only}
- Guler G, Karaoglu S, Velibasoglu H, Ramazanogullari N, Boyaci A. Comparison of analgesic effects of intra-articular tenoxicam and morphine in anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal of the ESSKA 2002;10:229-32. [DOI] [PubMed] [Google Scholar]
Kanbak 1997 {published data only}
- Kanbak M, Akpolat N, Ocal T, Doral MN, Ercan M, Erdem K. Intraarticular morphine administration provides pain relief after knee arthroscopy. European Journal of Anaesthesiology 1997;14:153-6. [DOI] [PubMed] [Google Scholar]
Kazemi 2004 {published data only}
- Kazemi AP, Rezazadeh S, Gharacheh HR. Pain relief after arthroscopic knee surgery--intraarticular sufentanil vs morphine. Middle East Journal of Anesthesiology 2004;17:1099-112. [PubMed] [Google Scholar]
Khoury 1992 {published data only}
- Khoury GF, Chen ACN, Garland DE, Stein C. Intraarticular morphine, bupivacaine, and morphine/bupivacaine for pain control after knee videoarthroscopy. Anesthesiology 1992;77:263-6. [DOI] [PubMed] [Google Scholar]
Kizilkaya 2005 {published data only}
- Kizilkaya M, Yildirim OS, Ezirmik N, Kursad H, Karsan O. Comparisons of analgesic effects of different doses of morphine and morphine plus methylprednisolone after knee surgery. European Journal of Anaesthesiology 2005;22:603-8. [DOI] [PubMed] [Google Scholar]
Likar 1995 {published data only}
- Likar R, Mathiaschitz K, Burtscher M, Stettner H. Randomised, double-blind, comparative study of morphine and tramadol administered intra-articularly for postoperative analgesia following arthroscopic surgery. Clinical Drug Investigation 1995;10:17-21. [Google Scholar]
Likar 1999 {published data only}
- Likar R, Kapral S, Steinkellner H, Stein C, Schafer M. Dose-dependency of intra-articular morphine analgesia. British Journal of Anaesthesia 1999;83:241-4. [DOI] [PubMed] [Google Scholar]
Lyons 1995 {published data only}
- Lyons B, Lohan D, Flynn CG, Joshi GP, O'Brien TM, McCarroll M. Intra-articular analgesia for arthroscopic meniscectomy. British Journal of Anaesthesia 1995;75:552-5. [DOI] [PubMed] [Google Scholar]
Muller 2001 {published data only}
- Muller M, Burkhardt J, Borchardt E, Buttner-Janz K. Postoperative analgesic effect after intra-articular morphine or ropivacaine following knee arthroscopy - a prospective randomized, doubleblinded study [Postoperative analgetische Wirksamkeit intraartikulärer Morphin- oder Ropivacaingabe nach Kniegelenkarthroskopie]. Der Schmerz 2001;15:3-9. [DOI] [PubMed] [Google Scholar]
Raj 2004 {published data only}
- Raj N, Sehgal A, Hall JE, Sharma A, Murrin KR, Groves ND. Comparison of the analgesic efficacy and plasma concentrations of high-dose intra-articular and intramuscular morphine for knee arthroscopy. European Journal of Anaesthesiology 2004;21:932-7. [DOI] [PubMed] [Google Scholar]
Richardson 1997 {published data only}
- Richardson MD, Bjorksten AR, Hart JA, McCullough K. The efficacy of intra-articular morphine for postoperative knee arthroscopy analgesia. Arthroscopy 1997;13:584-9. [DOI] [PubMed] [Google Scholar]
Rosseland 2003a {published data only}
- Rosseland LA, Stubhaug A, Grevbo F, Reikeras O, Breivik H. Effective pain relief from intra-articular saline with or without morphine 2 mg in patients with moderate-to-severe pain after knee arthroscopy: a randomized, double-blind controlled clinical study. Acta Anaesthesiologica Scandinavica 2003;47:732-8. [DOI] [PubMed] [Google Scholar]
Ruwe 1995 {published data only}
- Ruwe PA, Klein I, Shields CL. The effect of intraarticular injection of morphine and bupivacaine on postarthroscopic pain control. American Journal of Sports Medicine 1995;23:59-64. [DOI] [PubMed] [Google Scholar]
Solheim 2006 {published data only}
- Solheim N, Rosseland LA, Stubhaug A. Intra-articular morphine 5 mg after knee arthroscopy does not produce significant pain relief when administered to patients with moderate to severe pain via an intra-articular catheter. Regional Anesthesia and Pain Medicine 2006;31:506-13. [DOI] [PubMed] [Google Scholar]
Varkel 1999 {published data only}
- Varkel V, Volpin G, Ben-David B, Said R, Grimberg B, Simon K, et al. Intraarticular fentanyl compared with morphine for pain relief following arthroscopic knee surgery. Canadian Journal of Anaesthesia 1999;46:867-71. [DOI] [PubMed] [Google Scholar]
Wrench 1996 {published data only}
- Wrench IJ, Taylor P, Hobbs GJ. Lack of efficacy of intra-articular opioids for analgesia after day-case arthroscopy. Anaesthesia 1996;51:920-2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez‐Cabo 1998 {published data only}
- Alvarez-Cabo JM, Lopez-Rouco M, Gonzalez-Paleo JR. Analgesic effect of intra-articular morphine after arthroscopic knee surgery. Ambulatory Surgery 1998;6:179-82. [Google Scholar]
De Andres 1993 {published data only}
- De Andres J, Bellver J, Barrera L, Febre E, Bolinches R. A comparative study of analgesia after knee surgery with intraarticular bupivacaine, intraarticular morphine, and lumbar plexus block. Anesthesia and Analgesia 1993:727-30. [PubMed]
Lehrberger 1994 {published data only}
- Lehrberger K, Stein C, Hassan A, Yassouridis A. Opioids as novel intra-articular agents for analgesia following arthroscopic knee surgery. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal of the ESSKA 1994;2:174-5. [DOI] [PubMed] [Google Scholar]
Stein 1991 {published data only}
- Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, et al. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. New England Journal of Medicine 1991;325:1123-6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Altan 1994 {published data only}
- Altan A, Kutlu F, Ozkan C, Bahat H, Altun M, Sungar D, et al. Postoperative analgesia with bupivacaine or morphine after arthroscopic knee surgery. Agri Dergisi 1994;6:35-7. [Google Scholar]
Uzma 1997 {published data only}
- Uzma O, Aydingoz O, Erdogan F, Kose Y, Akgun I. Comparison of analgesic effects of intraarticular morphine, bupivacaine and morphine-bupivacaine combination following arthroscopic knee surgery. Turk Anesteziyoloji ve Reanimasyon 1997;25:373-9. [Google Scholar]
VanNess 1994 {published data only}
- VanNess SA, Gittins ME. Comparison of intra-articular morphine and bupivacaine following knee arthroscopy. Orthopaedic Review 1994;23:743-7. [PubMed] [Google Scholar]
Additional references
Bartho 1990
- Bartho L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn-Schmiedebergs Archives of Pharmacology 1990;342(6):666-70. [DOI] [PubMed] [Google Scholar]
Drosos 2002
- Drosos GI, Vlachonikolis IG, Papoutsidakis AN, Gavalas NS, Anthopoulos G. Intra-articular morphine and postoperative analgesia after knee arthroscopy. The Knee 2002;9:335-40. [DOI] [PubMed] [Google Scholar]
GRADEPro GDT 2015 [Computer program]
- McMaster University, 2015 (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.), 2015. Available from www.gradepro.org.
Gupta 1999
- Gupta A, Axelsson K, Allvin R, Liszka-Hackzell J, Rawal N, Althoff B, et al. Postoperative pain following knee arthroscopy: the effects of intra-articular ketorolac and/or morphine. Regional Anesthesia and Pain Medicine 1999;24:225-30. [DOI] [PubMed] [Google Scholar]
Gupta 2001
- Gupta A, Bodin L, Holmstrom B, Berggren L. A systematic review of the peripheral analgesic effects of intraarticular morphine. Anesthesia and Analgesia 2001;93:761-70. [DOI] [PubMed] [Google Scholar]
Heine 1994
- Heine MF, Tillet ED, Tsueda K, Loyd GE, Schroeder JA, Vogel RL, et al. Intra-articular morphine after arthroscopic knee operation. British Journal of Anaesthesia 1994;73:413-5. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing Risk of Bias in Included Studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Available from www.cochrane-handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Joshi 1992
- Joshi GP, McCarroll SM, Cooney CM, Blunnie WP, O'Brien TM, Lawrence AJ. Intra-articular morphine for pain relief after knee arthroscopy. Journal of Bone and Joint Surgery 1992;74:749-51. [DOI] [PubMed] [Google Scholar]
Joshi 1993
- Joshi GP, McCarroll SM, O'Brien TM, Lenane P. Intraarticular analgesia following knee arthroscopy. Anesthesia and Analgesia 1993;76:333-6. [PubMed] [Google Scholar]
Kalso 1997
- Kalso E, Tramer MR, Carroll D, McQuay HJ, Moore RA. Pain relief from intra-articular morphine after knee surgery: a qualitative systematic review. Pain 1997;71:127-34. [DOI] [PubMed] [Google Scholar]
Kalso 2002
- Kalso E, Smith L, McQuay HJ, Moore AR. No pain, no gain: clinical excellence and scientific rigour - lessons learned from IA morphine. Pain 2002;98:269-75. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135:982-9. [DOI] [PubMed] [Google Scholar]
Lan 2010
- Lan X, Zhang MM, Pu CL, Guo CB, Kang Q, Li YC, et al. Impact of human leukocyte antigen mismatching on outcomes of liver transplantation: a meta-analysis. World Journal of Gastroenterology: WJG 2010;16(27):3457-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laurent 1994
- Laurent SC, Nolan JP, Pozo JL, Jones CJ. Addition of morphine to intra-articular bupivacaine does not improve analgesia after day-case arthroscopy. British Journal of Anaesthesia 1994;72:170-3. [DOI] [PubMed] [Google Scholar]
Likar 1998
- Likar R, Sittl R, Gragger K, Pipam W, Blatnig H, Breschan C, et al. Peripheral morphine analgesia in dental surgery. Pain 1998;76:145-50. [DOI] [PubMed] [Google Scholar]
Ma 2012
- Ma J, Liu Y, Huang XL, Zhang ZY, Myers JN, Neskey DM, et al. Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: a meta-analysis. Oral oncology 2012;48(11):1076-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
Moiniche 1999
- Moiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A systematic review of intra-articular local anesthesia for postoperative pain relief after arthroscopic knee surgery. Regional Anesthesia and Pain Medicine 1999;24:430-7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosseland 2003
- Rosseland LA, Stubhaug A, Sandberg L, Breivik H. Intra-articular (IA) catheter administration of postoperative analgesics. A new trial design allows evaluation of baseline pain, demonstrates large variation in need of analgesics, and finds no analgesic effect of IA ketamine compared with IA saline. Pain 2003;104:25-34. [DOI] [PubMed] [Google Scholar]
Rosseland 2004a
- Rosseland LA, Helgesen KG, Breivik H, Stubhaug A. Moderate-to-severe pain after knee arthroscopy is relieved by intraarticular saline: a randomized controlled trial. Anesthesia and Analgesia 2004;98:1546-51. [DOI] [PubMed] [Google Scholar]
Rosseland 2004b
- Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain 2004;112(3):248-53. [DOI] [PubMed] [Google Scholar]
Sawynok 2003
- Sawynok J. Topical and peripherally acting analgesics. Pharmacological Reviews 2003;55(1):1-20. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
Soderlund 1997
- Soderlund A, Westman L, Ersmark H, Eriksson E, Valentin A, Ekblom A. Analgesia following arthroscopy - a comparison of intra-articular morphine, pethidine and fentanyl. Acta Anaesthesiologica Scandinavica 1997;41:6-11. [DOI] [PubMed] [Google Scholar]
Stein 1990
- Stein C, Hassan AH, Przewlocki R, Gramsch C, Peter K, Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proceedings of the National Academy of Sciences of the United States of America 1990;87(15):5935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 1995
- Stein C. The control of pain in peripheral tissue by opioids. New England Journal of Medicine 1995;332:1685-90. [DOI] [PubMed] [Google Scholar]
Stein 1999
- Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain 1999;83:525-32. [DOI] [PubMed] [Google Scholar]


