Abstract
Background
Polycystic ovarian syndrome (PCOS) occurs in 4% to 7% of all women of reproductive age and 50% of women presenting with subfertility. Subfertility affects 15% to 20% of couples trying to conceive. A significant proportion of these women ultimately need assisted reproductive technology (ART). In vitro fertilisation (IVF) is one of the assisted reproduction techniques employed to raise the chances of achieving a pregnancy. For the standard IVF technique, stimulating follicle development and growth before oocyte retrieval is essential, for which a large number of different methods combining gonadotrophins with a gonadotrophin‐releasing hormone (GnRH) agonist or antagonist are used. In women with PCOS, the supra‐physiological doses of gonadotrophins used for controlled ovarian hyperstimulation (COH) often result in an exaggerated ovarian response, characterised by the development of a large cohort of follicles of uneven quality, retrieval of immature oocytes, and increased risk of ovarian hyperstimulation syndrome (OHSS). A potentially effective intervention for women with PCOS‐related subfertility involves earlier retrieval of immature oocytes at the germinal‐vesicle stage followed by in vitro maturation (IVM). So far, the only data available have derived from observational studies and non‐randomised clinical trials.
Objectives
To assess the effectiveness and safety of IVM followed by IVF or ICSI versus conventional IVF or ICSI among women with PCOS undergoing assisted reproduction.
Search methods
This is the second update of this review. We performed the search on 17 April 2018.
The search was designed with the help of the Cochrane Gynaecology and Fertility Group Information Specialist, for all published and unpublished randomised controlled trials (RCTs).
We searched the the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, CENTRAL via the Cochrane Central Register of Studies Online, MEDLINE, Embase, CINAHL, and the trial registers for ongoing and registered trials and the Open Grey database for grey literature from Europe. We made further searches in the National Institute for Health and Care Excellence (NICE) fertility assessment and treatment guidelines. We handsearched reference lists of relevant systematic reviews and RCTs, together with PubMed and Google for any recent trials that have not yet been indexed in the major databases.
Selection criteria
All RCTs on the intention to perform IVM before IVF or ICSI compared with conventional IVF or ICSI for subfertile women with PCOS, irrespective of language and country of origin.
Data collection and analysis
Two review authors independently selected studies, assessed risk of bias, extracted data from studies, and attempted to contact the authors of studies for which data were missing. Our primary outcomes were live birth per woman randomised and miscarriage. We performed statistical analysis using Review Manager 5. We assessed evidence quality using GRADE methods.
Main results
We found two RCTs suitable for inclusion in the review and six ongoing trials that have not yet reported results. Both included studies were published as abstracts in international conferences.
Both studies were at unclear or high risk of bias for most of the seven domains assessed. Common problems were unclear reporting of study methods and lack of blinding. The main limitations in the overall quality of the evidence were high risk of bias and serious imprecision.
There were no data on the primary outcomes of this review, namely live birth per woman randomised and miscarriage.
Both studies reported clinical pregnancy rate: there was evidence of an effect between IVM and IVF, favouring the former (odds ratio 3.10, 95% confidence interval 1.06 to 9.00; 71 participants; 2 studies; I2 = 0%; very low‐quality evidence). The incidence of OHSS was zero in both studies in both groups.
There were no data for the other outcomes specified in this review.
Authors' conclusions
Though promising data on the in vitro maturation (IVM) technique have been published, unfortunately there is still no evidence from properly conducted randomised controlled trials upon which to base any practice recommendations regarding IVM before in vitro fertilisation (IVF) or intracytoplasmic sperm injection for women with polycystic ovarian syndrome. Regarding our secondary outcomes, very low‐quality evidence showed that clinical pregnancy was higher with IVM when compared to IVF, whereas the incidence of ovarian hyperstimulation syndrome was zero in both studies in both groups. We are awaiting the results of six ongoing trials and eagerly anticipate further evidence from good‐quality trials in the field.
Plain language summary
In vitro maturation in subfertile women with polycystic ovarian syndrome who are undergoing assisted reproduction
Review question
Is in vitro maturation efficient compared to standard assisted reproduction techniques (ART) in subfertile women with polycystic ovarian syndrome (PCOS)?
Background
Women with PCOS undergoing conventional ART are at an increased risk of ovarian hyperstimulation syndrome, a medical condition that can occur in women who take fertility medications to stimulate oocytes growth. The condition is associated with enlargement of both ovaries, fluid in the body of the woman, around her lungs and/or her heart, serious illness, and in rare cases, death. When women with PCOS take medications to stimulate their ovaries, the oocytes produced are often immature, which is the main reason that these oocytes are poorly fertilised and lead to low pregnancy rates. In addition, as previously noted, stimulation of the ovaries of women with PCOS with drugs often leads to ovarian hyperstimulation. These women may thus benefit from earlier retrieval of oocytes followed by their maturation in the laboratory, a technique known as in vitro maturation (IVM), as this would reduce the aforementioned risks. However, while successful fertilisation, embryo development, and term pregnancies resulting from IVM oocytes have been reported, some concern has been expressed regarding the safety of the method in terms of the health of the children born and the rate of genetic anomalies they carry. We reviewed the evidence up to April 2018, for the third time.
Study characteristics
We performed a comprehensive literature search of the standard medical databases (from database inception to 17 April 2018) in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist, for all randomised controlled trials (studies in which participants are assigned to a treatment group using a random method) investigating the efficiency of IVM compared to conventional ART in subfertile women with PCOS. We searched for and included studies irrespective of language and country of origin. Two review authors independently selected and evaluated studies, extracted data, and attempted to contact the authors of studies for which data were missing. We found two studies (71 women), published as abstracts in international conferences, and six ongoing trials that met our inclusion requirements.
Key results
Though promising data on the IVM technique have been published, unfortunately there is still no evidence concerning our primary outcomes of live‐birth and miscarriage rates from properly conducted randomised controlled trials upon which to base any practice recommendations regarding IVM before ART for women with PCOS. Of the secondary outcomes specified in this review, very low‐quality evidence showed that clinical pregnancy was higher when IVM was compared to conventional ART, whereas the incidence of ovarian hyperstimulation syndrome was zero in both studies in both groups. We are awaiting the results of six ongoing trials and eagerly anticipate further evidence from good‐quality trials in the field.
Quality of the evidence
The quality of the evidence was very low for all outcomes.
Summary of findings
Summary of findings for the main comparison. In vitro maturation compared to in vitro fertilisation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction.
| In vitro maturation compared to in vitro fertilisation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction | |||||
| Patient or population: subfertile women with polycystic ovarian syndrome undergoing assisted reproduction Setting: IVF Units Intervention: IVM Comparison: IVF | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with IVF | Risk with IVM | ||||
| Live birth | Not reported | ||||
| Miscarriage | Not reported | ||||
| Clinical pregnancy | Study population | OR 3.10 (1.06 to 9.00) | 71 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a, b, c, d | |
| 194 per 1000 | 428 per 1000 (204 to 685) | ||||
| Ovarian hyperstimulation syndrome incidence | Study population | Not estimable | 71 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a, b, c, d | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF: in vitro fertilisation; IVM: in vitro maturation; OR: odds ratio; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aInsufficient data about randomisation. bData obtained from abstract. cNo data available for heterogeneity. dIncluded women had anovulatory polycystic ovaries not polycystic ovarian syndrome.
Background
Description of the condition
Polycystic ovarian syndrome (PCOS) occurs in 4% to 7% of all women of reproductive age and 50% of women who will seek subfertility services at some point in their life (Archer 2004; Azziz 2005). Clear diagnostic criteria for this condition were drawn up at a consensus meeting of the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) (ESHRE/ASRM 2003), while more recently it has been proposed that definitions be based on anti‐Müllerian hormone levels of greater than 5 ng/mL (Dewailly 2011). In anovulatory women with PCOS, the first‐line approach to subfertility remains lifestyle changes (weight loss and exercise) along with clomiphene citrate. Other recognised options include surgical ovarian wedge resection, laparoscopic ovarian drilling, metformin, bromocryptine, or aromatase inhibitors (NICE 2004). A proportion of women with PCOS do not respond to these conventional treatments and will ultimately need assisted reproductive techniques (ART). In women with PCOS, supra‐physiological doses of gonadotrophins that are used for controlled ovarian hyperstimulation (COH) provoke the development of a large cohort of follicles of uneven quality. This can sometimes result in the retrieval of immature oocytes, which leads to poor fertilisation and lower cleavage, pregnancy, and live‐birth rates. The addition of melatonin into the in vitro maturation (IVM) media has been proposed to improve the cytoplasmic maturation of the immature oocytes and the subsequent clinical outcomes (Kim 2013). Controlled ovarian hyperstimulation in women with PCOS poses a particular challenge as many of these women exhibit an exaggerated response to exogenous gonadotrophins (MacDougall 1993), although there is evidence that COH in women with PCOS results in similar outcomes when compared to women without PCOS (Heijnen 2006). As a consequence, many women face an increased rate of cycle cancellations and potential life‐threatening complications due to ovarian hyperstimulation syndrome (OHSS). In addition, the retrieval of immature oocytes leads to reduced fertilisation and lower cleavage, pregnancy, and live‐birth rates compared to the conventional in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles (Son 2010; Qiao 2011; Zheng 2012).
Description of the intervention
In vitro maturation involves the in vitro culture of immature oocytes, from the germinal vesicle or the germinal vesicle breakdown stage, in special laboratory conditions until the metaphase II stage, along with the accompanying cytoplasmic maturation, when the oocyte is considered to be completely mature and ready to undergo fertilisation (Cha 1998; Yang 2017), before the largest follicle has surpassed 13 mm in mean diameter (Dahan 2016). This intervention is potentially useful for women with PCOS‐related subfertility since these oocytes can retain their maturational and developmental competence (Trounson 1994). It is believed that women with PCOS who are at risk of developing OHSS following a conventional COH regimen might benefit from earlier retrieval of oocytes followed by IVM, thus reducing the risk of OHSS. Furthermore, doses and cost of the drugs would be lower, although there will be a cost to laboratory. On the other hand, the process of IVM could affect the quality of oocytes, as any intervention in their growth phase could affect oocyte maturation, fertilisation, and subsequent embryo development (Trounson 2001), leading to poor pregnancy outcomes (Barnes 1996; Suikkari 2008; Son 2010; Qiao 2011; de Ziegler 2012; Zheng 2012). In addition, there are no data on long‐term outcomes.
How the intervention might work
An option for subfertile women with PCOS is the retrieval of oocytes that retain their functional competence with minimal or mild gonadotrophin stimulation, which stimulates the endometrium at a very low or zero level, mimicking natural environmental procedures. Of note, the ultrastructure of human oocytes matured in vitro is largely similar to those matured in vivo (Coticchio 2016). Successful fertilisation, embryo development, and term pregnancy resulting from IVM oocytes have been reported in stimulated cycles (Nagy 1996; Child 2001), natural cycles (Mikkelsen 2001; Yoon 2001; Benkhalifa 2009; Fadini 2009; Fadini 2013), and women with PCOS (Trounson 1994; Beckers 1999; Chian 1999; Cha 2000; Chian 2000; Child 2001; Child 2002; Lin 2003; Liu 2003; Gulekli 2004; Le Du 2005; Soderstrom‐Anttila 2005; Son 2005; Zhao 2006; Son 2007; Zhao 2009; Roesner 2012; Siristatidis 2015), the first report having been published in the mid‐1960s (Edwards 1965). While some concern has been expressed regarding the safety of the method with respect to the health of the children and the rate of congenital anomalies (Cha 2005), other studies have reported normal obstetric and neonatal outcomes (Mikkelsen 2005; Soderstrom‐Anttila 2005; Shu‐Chi 2006; Buckett 2007; Buckett 2008; Roesner 2017).
Why it is important to do this review
When choosing between different IVF protocols for subfertile women with PCOS, a balance needs to be achieved between benefits and harms. Successful fertilisation, embryo development, and term pregnancy resulting from IVM oocytes have been reported in stimulated cycles. However, despite the publication of a number of studies in this area showing that IVM is a low‐risk and patient‐friendly procedure (Sauerbrun‐Cutler 2015; Siristatidis 2015), it is unclear whether IVM offers any benefit to women with PCOS who are undergoing COH, as an alternative to conventional IVF. In addition, there are upcoming studies in the literature that are attempting to find the optimal protocol to be used in subfertile women with PCOS. We have therefore undertaken a second update of this systematic review to ascertain whether IVM is superior to conventional IVF treatment in women with PCOS (Siristatidis 2009; Siristatidis 2013).
Objectives
To assess the effectiveness and safety of IVM followed by IVF or ICSI versus conventional IVF or ICSI among women with PCOS undergoing assisted reproduction.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) that compared IVM with conventional ART (IVF or ICSI) in women with PCOS for inclusion in this review. If we identified a factorial design (e.g. trials with four groups: IVM alone, IVF alone, IVM + drug, IVF + drug), we would pool data where IVM followed by IVF or ICSI was compared with conventional IVF or ICSI.
We did not include quasi‐randomised trials. Cross‐over trials were not eligible for inclusion unless pre‐cross‐over data were available, in which case only these data would be used in this review.
Types of participants
Inclusion criteria
All studies that included subfertile women described as having PCOS were eligible. Polycystic ovarian syndrome was defined by the ESHRE/ASRM criteria (ESHRE/ASRM 2003). Considering the wide variation of diagnostic criteria used for PCOS studies, we included studies utilising different criteria if the broad definition matched that of the ESHRE/ASRM criteria.
We also included trials involving couples with various categories of subfertility alongside PCOS, irrespective of age, parity, and previously administered treatments for subfertility. If only pooled data were available, we considered a study to be eligible if at least 80% of the participants had PCOS.
Exclusion criteria
We excluded trials of ovum recipients and others in which assisted hatching was used, as an issue of embryo quality impairment might be introduced. Had there been insufficient information about the clinical diagnosis, we would have contacted the authors of primary trials.
Types of interventions
Conventional IVF or ICSI after COH versus IVM followed by IVF or ICSI. We included embryo transfers of both fresh and frozen, but not mixed embryos. We excluded trials evaluating alternative interventions in which the option of IVM or conventional ART was not randomised (e.g. ovarian drilling, use of the oral contraceptive pill, metformin, aromatase inhibitors, steroids, progestins, growth hormone, L‐arginine).
Studies using different protocols for IVM, for example human chorionic gonadotrophin (hCG) or gonadotrophin priming for the maturation of oocytes before IVM, were eligible as long as they compared women with PCOS undergoing COH programmes.
All ovarian stimulation protocols were eligible (use of gonadotrophin‐releasing hormone agonists (GnRHa), GnRH antagonists, gonadotrophins alone (recombinant or urinary preparations), or clomiphene with gonadotrophins). In all the above cases, pooling data was our intended purpose.
Types of outcome measures
Primary outcomes
Live birth per woman. Defined as the delivery of one or more living fetuses after 22 completed weeks of gestation. No cumulative results from more than one cycle were to be considered.
Miscarriage (defined as the loss of the pregnancy before 22 completed weeks of gestational age) rate per clinical pregnancy.
Secondary outcomes
-
Effectiveness
Clinical pregnancy per woman, where clinical pregnancy was defined as evidence of a foetal heart on ultrasound at seven plus two gestational weeks
Cycle cancellation
Oocyte fertilisation
-
Adverse effects
Ovarian hyperstimulation
Preterm birth
Congenital anomalies of the newborn
Search methods for identification of studies
We searched for all published and unpublished RCTs evaluating IVM versus IVF/ICSI in subfertile women with PCOS. We applied no language restrictions. The search was designed with the help of the Cochrane Gynaecology and Fertility Group Information Specialist.
Electronic searches
We searched:
the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials from inception to 17 April 2018 (Appendix 1) (ProCite platform);
CENTRAL via the Cochrane Central Register of Studies Online (CRSO) (searched on 17 April 2018) (Appendix 2) (web platform);
MEDLINE (from 1946 to 17 April 2018) (Appendix 3) (Ovid platform);
Embase (from 1980 to 17 April 2018) (Appendix 4) (Ovid platform);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (from 1961 to 17 April 2018) (Appendix 5) (EBSCO platform).
We also searched the following websites:
trial registers for ongoing and registered trials: US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov); and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) (searched 17 April 2018) (Appendix 6);
the Open Grey database for grey literature from Europe (www.opengrey.eu/) (searched 17 April 2018) (Appendix 7).
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials described in Chapter 6 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). The Embase and CINAHL searches are combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/search‐filters.html).
Searching other resources
We made further searches in the National Institute of Clinical Excellence (NICE) fertility assessment and treatment guidelines (NICE 2004). We handsearched reference lists of relevant systematic reviews and RCTs, together with PubMed and Google for any recent trials that have not yet been indexed in the major databases.
Data collection and analysis
Selection of studies
Two review authors (CS and AM) independently assessed the titles and abstracts retrieved by the search for compliance with the above criteria. We planned to seek additional information from the authors of trials that appeared to meet the eligibility criteria but for which aspects of methodology were unclear or data were in an unsuitable form for meta‐analysis. There was no disagreements related to study eligibility. We documented the selection process with a PRISMA flow chart.
Data extraction and management
We extracted the following data from the included studies.
-
Trial characteristics
Randomisation (truly randomised, pseudo‐randomised, or not stated)
Allocation concealment, scored according to the categories specified by Cochrane: adequate, unclear, inadequate, or not used
Trial design: multicentre or single centre, single‐phase or cross‐over design
-
Duration, timing, and location of the trial (single centre or multicentre), duration of follow‐up:
outcome data used for primary analysis complete (follow‐up to live birth); randomised women accounted for; intention‐to‐treat analysis;
completeness of data uncertain;
outcome data incomplete with 5% of cycles commenced missing some outcome data.
Source of funding
-
Co‐intervention:
where any intervention other than that being investigated in the study protocol was equivalent in the treatment and control groups;
issue of co‐intervention not considered;
co‐intervention definitely existed.
Definitions of PCOS were examined, e.g. if they matched the ESHRE/ASRM criteria, were too restrictive, or too vague
-
Baseline characteristics of the studied groups
Cause and duration of pre‐existing subfertility
Age of the women and parity
Investigative work‐up
Other causes of subfertility
Previously administered treatment(s)
-
Interventions
Type of intervention and control
Dose and type of regimen
-
Outcomes
Outcomes reported
How outcomes were defined
Timing of outcome measurements
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' assessment tool to assess the included studies for selection, performance, detection, attrition, reporting, and other bias. There were no disagreements. We described our judgements in detail the 'Risk of bias' table in the 'Characteristics of included studies' table.
Measures of treatment effect
For dichotomous data (e.g. live‐birth rates), our aim was to use the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CIs) for all outcomes. Where data to calculate ORs were not available, we would have utilised the most detailed numerical data available that might facilitate similar analyses of included studies.
Unit of analysis issues
We performed analysis per woman or couple randomised for live birth, clinical pregnancy, and complication rates. We counted multiple live births (twins, triplets) as a single live‐birth event. Data that prevented valid analysis (e.g. 'per cycle' data) would be briefly summarised in an Additional table but would not be meta‐analysed. We would include only first‐phase data from cross‐over trials. If studies reported only 'per cycle' data, we contacted the study authors to request 'per woman' data.
Dealing with missing data
We sent corresponding authors two emails in an attempt to obtain missing data from the original trials of the included and ongoing studies.
Our initial intention was to analyse data on an intention‐to‐treat basis. In the case of unobtainable data, we planned to undertake imputation of individual values for live‐birth rate only. We assumed that live births had not occurred in participants without a reported outcome. For other outcomes, we analysed only the available data.
Assessment of heterogeneity
We identified heterogeneity by visual inspection of forest plots and by using a standard Chi2 test with significance set at P < 0.1. We used the I2 statistic to estimate the total variation across RCTs that was due to heterogeneity, considering an I2 greater than 50% as indicative of substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In order to avoid, or at least minimise, reporting biases (e.g. publication bias, multiple publication bias, language bias, etc.), we performed a comprehensive search for eligible studies and were alert for duplication of data. If there were 10 or more studies in an analysis, we would use a funnel plot to explore the possibility of small‐study effects.
Data synthesis
We used Review Manager 5 for input of data and statistical analysis (RevMan 2012), in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We planned that if the studies were sufficiently similar, we would combine the data using a fixed‐effect model.
An increase in the odds of a particular outcome, which might be beneficial (e.g. live birth) or detrimental (e.g. adverse effects), would be displayed graphically in the meta‐analyses to the right of the centre‐line, and a decrease in the odds of an outcome to the left of the centre‐line. We would use a fixed‐effect analysis if the underlying effect size was the same for all trials in the analysis; if not, we would use a random‐effects analysis.
Subgroup analysis and investigation of heterogeneity
We planned that where data were available, we would conduct subgroup analyses to determine the separate evidence within the following subgroups:
average age for women of less than and over 35 years;
various types of subfertility;
different protocols for COH;
parity of women;
previous COH for IVF attempts.
Had we detected substantial heterogeneity, we would have explored possible explanations for it in sensitivity analyses. We would have taken any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses would consider whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias;
a random‐effects model had been adopted;
alternative imputation strategies had been implemented;
the summary effect measure was risk ratio rather than odds ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro GDT (GRADEpro GDT 2018). This table presents the overall quality of the body of evidence for the main review outcomes (clinical pregnancy and OHSS incidence) using the five GRADE criteria: study limitations, consistency of effect, imprecision, indirectness, and publication bias (Higgins 2011). Two review authors independently assessed the quality of the evidence for each outcome. We justified, documented, and incorporated judgements about the quality of the evidence (high, moderate, low, or very low) when reporting the results for each outcome. Data were not available for the outcomes of live birth per woman randomised and miscarriage, the primary outcomes specified in this review.
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies tables.
We initially identified 399 articles from the main databases and 25 articles from the databases of ongoing studies (424 in total) as providing data comparing conventional IVF with IVM in women with PCOS. Based on the abstract we excluded 354 records. We retrieved the full texts of the remaining 70 articles, and based on the full text excluded 62 articles. Two RCTs, published as abstracts, met the inclusion criteria for this review (Gidoni 2013; Shavit 2015), as well as six ongoing studies (ISRCTN61229302; NCT00867763; NCT01473459; NCT03134482; NCT03463772; NCT03405701). (See Figure 1)
1.
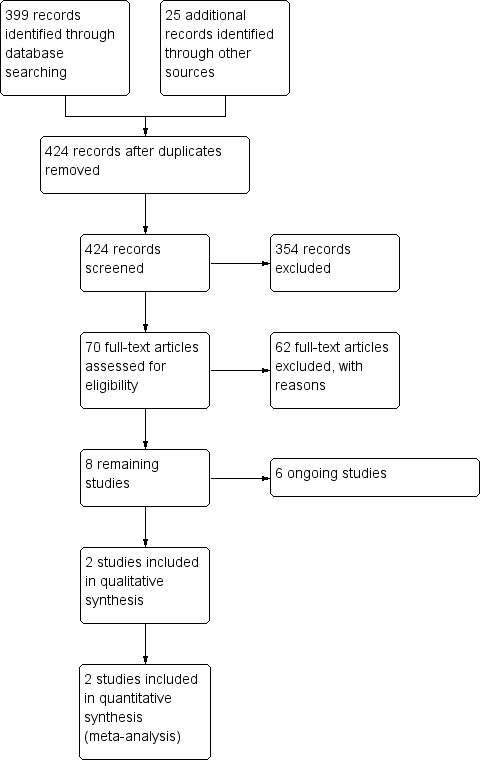
Study flow diagram.
We sent two emails to authors of the two included studies (published as abstracts), and four further emails (and the appropriate reminders) to the authors of three ongoing studies, ISRCTN61229302; NCT00867763; NCT01473459, and the study of Xu 2012 for unpublished data, or regarding data before inclusion, as well as for methodological details. We received responses from one of the ongoing studies (NCT01473459), and from the study of Xu 2012, but the data provided did not allow us to include these studies in the current review.
Included studies
Study design and setting
Two RCTs (Gidoni 2013; Shavit 2015), published as abstracts, met the inclusion criteria of this review. Both were single‐centre studies conducted in IVF units in Israel.
Participants
The included studies involved 71 subfertile women undergoing ART, one study of women with anovulatory polycystic ovaries (n = 50) and one study of women with PCOS (n = 21).
Interventions
Both studies compared IVM (primed with three days of gonadotrophins) with a GnRH antagonist protocol.
Outcomes
Neither of the studies reported live birth or miscarriage. Of the secondary outcomes specified in this review, both studies reported on clinical pregnancy and OHSS incidence.
Excluded studies
Of the 424 records identified after removal of duplicates, 354 studies were excluded initially on the basis of the abstract (Figure 1).
Of the remaining 70 papers, 62 were retrieved but were subsequently excluded as non‐RCTs or as not answering the precise review question (see Characteristics of excluded studies).
Risk of bias in included studies
As described in detail in the 'Risk of bias' table in Characteristics of included studies, we rated the risk of bias as unclear or high for most domains in both included studies (Figure 2, Figure 3). Both studies had a published conference abstract in international databases. After emailing the study authors we were unable to retrieve any further data. We assessed one study as at unclear risk of reporting bias (Gidoni 2013). However, we assessed both studies as at unclear risk of other sources of bias. The considerable impact of all types of bias on the reliability of the results contributed to our decision to reduce the quality of the evidence to very low for both outcomes.
2.
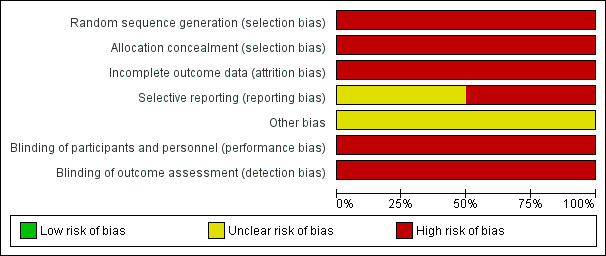
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
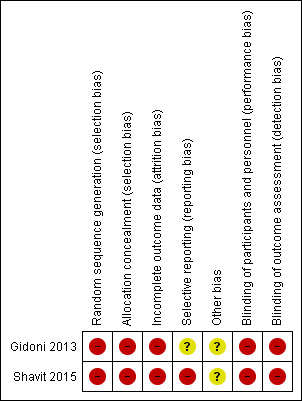
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
We found two studies assessing the role of IVM in subfertile women with PCOS undergoing ART in this second update of the review (Gidoni 2013; Shavit 2015; Table 1).
In vitro maturation versus in vitro fertilisation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction
Primary outcomes
Live‐birth rate per woman randomised
Data were not available.
Miscarriage
Data were not available.
Secondary outcomes
Effectiveness
Clinical pregnancy per woman
Both included studies reported this outcome. There was evidence of an effect between IVM and IVF, favoring IVM (odds ratio 3.10, 95% confidence interval 1.06 to 9.00; 71 participants; 2 studies; I2 = 0%; very low‐quality evidence; Analysis 1.1; Figure 4). This means that if the clinical pregnancy rate was 19.4% with conventional IVF, in women with PCOS undergoing IVM it would be between 20.4% and 68.5%.
1.1. Analysis.
Comparison 1 In vitro maturation versus in vitro fertilisation, Outcome 1 Clinical pregnancy.
4.
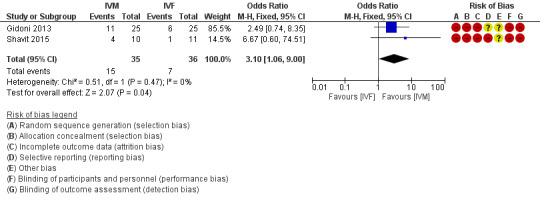
Forest plot of comparison: 1 IVM versus IVF, outcome: 1.1 Clinical pregnancy.
Cycle cancellation
Data were not available.
Oocyte fertilisation
Data were not available.
Adverse effects
Ovarian hyperstimulation
Both included studies reported this outcome. Zero cases of OHSS were reported in both groups (odds ratio not estimable; 71 participants; 2 studies; I2 not applicable; very low‐quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 In vitro maturation versus in vitro fertilisation, Outcome 2 OHSS incidence.
Preterm birth
Data were not available.
Congenital anomalies of the newborn
Data were not available.
Discussion
Summary of main results
In this 2018 update of the review, interpretation of results remains limited by the absence of adequately conducted RCTs. We found only two trials presented as abstracts in international conferences and published as supplements in journals. As we found no data on the primary outcomes set for this review, namely live birth and miscarriage, the data provided could not answer the question of this review. Of the secondary outcomes examined, very low‐quality evidence showed that clinical pregnancy was higher when IVM was compared to IVF, whereas the incidence of OHSS was zero in both groups in both studies that reported this outcome. However, we found six ongoing studies that are still recruiting participants. Consequently, any effect of IVM relative to traditional IVF/ICSI in terms of live‐birth rates for women with PCOS remains unknown.
Overall completeness and applicability of evidence
In theory, recovery of immature oocytes followed by IVM could be developed as a safe alternative method for the treatment of these women as their oocytes retain their maturational and developmental competence (Trounson 1994); this procedure was carried out successfully in a variety of different species during the previous century (Pincus 1935; Edwards 1965). During our initial search, we found some evidence that IVM could provide a promising alternative to conventional IVF/ICSI in women with PCOS, especially to prevent OHSS, but this evidence was not based on robust data reported in RCTs. Instead, observational studies showed a high maturation rate of the oocytes (up to 80.3%) (Child 2001); fertilisation rates from 10%, Beckers 1999, up to 76.5%, Child 2001; clinical pregnancy rates from 21.5%, Cha 2005, to 50% per cycle, Holzer 2006; implantation rates around 18% (Child 2002; Holzer 2006); live‐birth rates from 15.9% per retrieval, Child 2002, to 33% per cycle, Holzer 2006; and pregnancy complications at 13.2% (Cha 2005). Chromosomal abnormalities and outcomes were similar for IVM and conventional ART populations (Buckett 2005; Li 2005; Foix‐L'Hélias 2014). Case‐control retrospective studies reported similar results with oocyte maturation rates up to 84% (Chian 2000); fertilisation rates from 43%, Soderstrom‐Anttila 2005, to 70%, Le Du 2005; and pregnancy rates from 22%, Sandraa 2006, to 55.6%, Fukuda 2006; while rates of miscarriage, ectopic pregnancy, and late foetal loss were similar for IVM and IVF or ICSI groups of women with PCOS (Buckett 2008). The studies compared different protocols of IVM in women with PCOS, finding that human chorionic gonadotrophin priming, which was not dose‐dependent (Gulekli 2004), raised the maturation rate from 69% to 84% (Chian 2000); fertilisation rate from 45%, Chung 2000, up to 80%, Ge 2008; pregnancy rate from 31% up to 38.5%; and live‐birth rate up to 33% (Ge 2008). Follicle‐stimulating hormone priming increased the pregnancy rate from 0% to 29% (Mikkelsen 2001).
In the absence of robust results from high‐quality/appropriate trials, we are still unable to comment on the advantages or disadvantages of the technique. We must await the conclusions of the six ongoing studies identified in this review, and furthermore wish to highlight the need for properly designed RCTs in the field. There is still a requirement to offer women with PCOS a promising alternative to conventional ART, and IVM seems to be one (Cha 2005; Le Du 2005; Siristatidis 2015); the problem is that data showing this are from observational and non‐comparative studies. Moreover, adequate follow‐up of trial participants in terms of neonatal and postneonatal outcomes is necessary.
Quality of the evidence
There were no RCTs and no relevant data on live birth and miscarriage when IVM was compared to conventional IVF/ICSI in subfertile women with PCOS. Of the secondary outcomes examined, very low‐quality evidence showed that clinical pregnancy was higher when IVM was compared to IVF/ICSI, whereas the incidence of OHSS was zero in both groups in both studies that reported this outcome (as shown in Table 1). The limitations of this evidence include the absence of sufficient data on the randomisation process; the fact that data were obtained only from abstracts and no data were available for the assessment of heterogeneity; and finally the fact that women in one of the included studies were described as having anovulatory polycystic ovaries and not PCOS (Gidoni 2013).
Potential biases in the review process
We believe that we have made every effort to minimise biases in the review process. We conducted a systematic search of the literature for RCT evidence that was not restricted by language or date of publication. We attempted to make contact with authors of primary studies to obtain additional methodological or outcome data, or both. We followed Cochrane methods in the review process.
Agreements and disagreements with other studies or reviews
The conclusions of this review are in agreement with those of the previous versions of the review (Siristatidis 2009; Siristatidis 2013). In contrast, there are publications that support the standard use of IVM, including in subfertile women with PCOS, but the data are from non‐RCTs (Chian 2004; Reinblatt 2008; Grynberg 2013; Ellenbogen 2014), while others do not advise the standard administration of the technique, as new COH protocols are better in terms of pregnancy outcomes (de Ziegler 2012).
Authors' conclusions
Implications for practice.
Though promising data on the in vitro maturation (IVM) technique have been published, unfortunately there remains no evidence from properly conducted randomised controlled trials (RCTs) upon which to base any practice recommendations regarding IVM before in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) for women with polycystic ovarian syndrome (PCOS). Of the secondary outcomes specified in this review, very low‐quality evidence showed that clinical pregnancy was higher when IVM was compared to IVF, whereas the incidence of ovarian hyperstimulation syndrome was zero in both groups in both studies that reported this outcome. We are awaiting the results of six ongoing trials and eagerly anticipate further evidence from good‐quality trials in the field. Until more evidence is available, either from the six ongoing trials or from further well‐designed RCTs in the field, IVM cannot be the preferred first‐line treatment for subfertile women with PCOS, and it might be appropriate to continue to offer conventional IVF/ICSI.
Notably, there is growing evidence that for this category of patients the optimal method to be offered is a 'freeze‐all policy', after a conventional ovarian stimulation and IVF/ICSI, followed by transfers in frozen replacement cycles.
Implications for research.
We aimed to provide a clear overview of the differences between IVM and conventional IVF/ICSI in order to facilitate a decision over which treatment is better for women with PCOS. Unfortunately, we included only two studies published in the form of abstracts, while another six studies are currently ongoing, hence we could arrive at no robust conclusions. Proper RCTs with sufficient power and appropriate endpoints (live‐birth and miscarriage rates must be the primary outcomes) that compare IVM with conventional assisted reproductive technology and that are performed according to CONSORT guidelines are urgently needed in women with PCOS and subfertility.
It may be that the only category of patients for whom IVM might be beneficial is fertility preservation cases, where there is no time to stimulate the ovaries. For these patients, IVM can be offered only by select IVF centres with adequate expertise, and RCT might not be the optimal type of study; instead, a prospective collection of data through cohort studies may be more appropriate.
What's new
| Date | Event | Description |
|---|---|---|
| 27 June 2018 | New search has been performed | The review has been updated. Updating of the Background section adding new references Update of search of studies: eight studies were excluded, six ongoing studies, two included studies in the form of abstracts |
| 27 June 2018 | New citation required but conclusions have not changed | Conclusions not changed |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 23 July 2013 | New search has been performed | Update of studies and Methods; one study reported as a randomised controlled trial was excluded; three ongoing studies were found reporting no results; final conclusion not changed |
| 23 July 2013 | New citation required but conclusions have not changed | Update of Methods; still no studies available for inclusion |
| 7 April 2011 | New search has been performed | To be made stable |
| 22 January 2008 | New citation required and major changes | Substantive amendment |
Notes
None.
Acknowledgements
We thank Helen Nagels (Managing Editor), Marian Showell (Information Specialist), and the editorial board of the Cochrane Gynaecology and Fertility Group for their invaluable assistance in developing this review. We thank Dr Maria Creatsa and Dr Nikos Vrachnis who contributed to the 2013 update of the review. We thank the following peer reviewers for their comments: Dr Selma Mourad, Rebecca Harmston, and Jack Wilkinson.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
Searched 17 April 2018
Procite Platform
Keywords CONTAINS "polycystic ovary syndrome" or "PCOS" or "anovulation" or "amenorrhea" or "amenorrhoea" or "ovarian dysfunction" or "ovarian failure" or "Oligo‐amenorrhea" or "oligo‐ovulation" or "oligoanovulatory "or "oligoamenorrhea" or "oligo‐ovulatory" or Title CONTAINS "polycystic ovary syndrome" or "PCOS" or "anovulation" or "amenorrhea" or "amenorrhoea" or "ovarian dysfunction" or "ovarian failure" or "Oligo‐amenorrhea" or "oligo‐ovulation" or "oligoanovulatory" or "oligoamenorrhea" or "oligo‐ovulatory"
AND
Keywords CONTAINS "IVM" or "in vitro maturation" or "in vivo maturation" or "oocyte immaturity" or "oocyte maturation" or "oocyte preparation techniques" or Title CONTAINS "IVM" or "in vitro maturation" or "in vivo maturation" or "oocyte immaturity" or "oocyte maturation" or "oocyte preparation techniques" (42 hits)
Appendix 2. CENTRAL via the Cochrane Central Register of Studies Online (CRSO) search strategy
Searched 17 April 2018
Web platform
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES 975 #2 (polycystic adj5 ovar*):TI,AB,KY 2107 #3 (PCOS or PCOD):TI,AB,KY 1686 #4 leventhal:TI,AB,KY 20 #5 #1 OR #2 OR #3 OR #4 2313 #6 MESH DESCRIPTOR In Vitro Oocyte Maturation Techniques EXPLODE ALL TREES 10 #7 (in vitro adj5 matur*):TI,AB,KY 138 #8 IVM:TI,AB,KY 106 #9 (oocyte* adj5 matur*):TI,AB,KY 457 #10 (immatur* adj5 oocyte*):TI,AB,KY 87 #11 #6 OR #7 OR #8 OR #9 OR #10 594 #12 #5 AND #11 81
Appendix 3. MEDLINE search strategy
Searched 1946 to 17 April 2018
Ovid platform
1 Polycystic Ovary Syndrome/ (12649) 2 (polycystic adj5 ovar$).ti,ab,sh. (14233) 3 PCOS.ti,ab,sh. (9117) 4 PCOD.ti,ab,sh. (280) 5 (stein‐leventhal or leventhal).tw. (715) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (85) 7 or/1‐6 (17100) 8 exp In Vitro Oocyte Maturation Techniques/ (902) 9 (in vitro adj5 matur$).tw. (10135) 10 IVM.tw. (2804) 11 (oocyte$ adj5 matur$).tw. (14095) 12 (immatur$ adj5 oocyte$).tw. (2268) 13 or/8‐12 (20891) 14 7 and 13 (472) 15 randomized controlled trial.pt. (458772) 16 controlled clinical trial.pt. (92329) 17 Randomized Controlled Trials/ (115529) 18 Random allocation/ (93877) 19 Double‐blind method/ (145297) 20 Single‐blind method/ (25008) 21 or/15‐20 (747335) 22 clinical trial.pt. (509690) 23 exp clinical trials/ (0) 24 (clin$ adj25 trial$).ti,ab,sh. (381378) 25 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. (162568) 26 Placebos/ (33868) 27 placebo$.ti,ab,sh. (208237) 28 random$.ti,ab,sh. (1185005) 29 Research design/ (95889) 30 or/22‐29 (1773035) 31 animal/ not (human/ and animal/) (4413998) 32 21 or 30 (1797409) 33 32 not 31 (1635933) 34 14 and 33 (87)
Appendix 4. Embase search strategy
Searched 1980 to 17 April 2018
Ovid platform
1 exp ovary polycystic disease/ (23246) 2 (polycystic adj5 ovar$).ti,ab,sh. (19704) 3 PCOS.ti,ab,sh. (13921) 4 PCOD.ti,ab,sh. (377) 5 (stein‐leventhal or leventhal).tw. (566) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (90) 7 or/1‐6 (27066) 8 exp in vitro oocyte maturation/ (1258) 9 (in vitro adj5 matur$).tw. (12100) 10 IVM.tw. (3841) 11 (oocyte$ adj5 matur$).tw. (17294) 12 (immatur$ adj5 oocyte$).tw. (2841) 13 or/8‐12 (25622) 14 7 and 13 (882) 15 Clinical Trial/ (964209) 16 Randomized Controlled Trial/ (495134) 17 exp randomization/ (77876) 18 Single Blind Procedure/ (31024) 19 Double Blind Procedure/ (145965) 20 Crossover Procedure/ (54970) 21 Placebo/ (309585) 22 Randomi?ed controlled trial$.tw. (179133) 23 Rct.tw. (28028) 24 random allocation.tw. (1767) 25 randomly.tw. (372435) 26 randomly allocated.tw. (29351) 27 allocated randomly.tw. (2307) 28 (allocated adj2 random).tw. (794) 29 Single blind$.tw. (20651) 30 Double blind$.tw. (181005) 31 ((treble or triple) adj blind$).tw. (765) 32 placebo$.tw. (266160) 33 prospective study/ (441083) 34 or/15‐33 (2098780) 35 case study/ (53662) 36 case report.tw. (350881) 37 abstract report/ or letter/ (1032536) 38 or/35‐37 (1428530) 39 34 not 38 (2050424) 40 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5940372) 41 39 not 40 (1911916) 42 14 and 41 (181)
Appendix 5. CINAHL search
Searched 1961 to 17 April 2018
EBSCO platform
| # | Query | Results |
| S22 | S5 AND S9 AND S21 | 8 |
| S21 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 1,224,729 |
| S20 | TX allocat* random* | 8,631 |
| S19 | (MH "Quantitative Studies") | 19,277 |
| S18 | (MH "Placebos") | 10,766 |
| S17 | TX placebo* | 50,582 |
| S16 | TX random* allocat* | 8,631 |
| S15 | (MH "Random Assignment") | 46,926 |
| S14 | TX randomi* control* trial* | 146,929 |
| S13 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 950,538 |
| S12 | TX clinic* n1 trial* | 223,249 |
| S11 | PT Clinical trial | 86,215 |
| S10 | (MH "Clinical Trials+") | 238,885 |
| S9 | S6 OR S7 OR S8 | 417 |
| S8 | TX (oocyte* N5 matur*) | 229 |
| S7 | TX IVM | 92 |
| S6 | TX (in vitro N5 matur*) | 210 |
| S5 | S1 OR S2 OR S3 OR S4 | 3,238 |
| S4 | TX ovar* N1 (scelerocystic or degeneration) | 6 |
| S3 | TX PCOS or TX PCOD | 1,688 |
| S2 | TX polycystic N3 ovar* | 2,737 |
| S1 | (MM "Polycystic Ovary Syndrome") | 1,708 |
Appendix 6. ClinicalTrials.gov and WHO ICTRP search
From inception to 17 April 2018
Web platform
1 Polycystic Ovary Syndrome
2 PCOS
3 stein‐leventhal or leventhal
4 In vitro adj matur$
5 IVM
6 In vitro maturation
Appendix 7. OpenGrey literature
From inception to 17 April 2018
Web platform
“IVM” and “PCOS”
Data and analyses
Comparison 1. In vitro maturation versus in vitro fertilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.06, 9.00] |
| 2 OHSS incidence | 2 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gidoni 2013.
| Methods | Authors only state that it was a "prospective randomized study". | |
| Participants | 50 women with anovulatory polycystic ovaries | |
| Interventions | IVM with 3 days of gonadotrophins vs GnRH antagonist flexible protocol | |
| Outcomes | Total number of mature oocytes, fertilisation, cleavage rates, "top quality" embryos, number of embryos transferred, clinical pregnancy, number of frozen embryos, and OHSS incidence | |
| Notes | Women described as having anovulatory polycystic ovaries but not PCOS. Data obtained from abstract presented in a conference. Assaf Harofeh Medical Center, IVF Unit, Zerifin, Israel |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No data available for the specific type of bias, even when sought |
| Allocation concealment (selection bias) | High risk | No data available for the specific type of bias, even when sought |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data available for the specific type of bias, even when sought |
| Selective reporting (reporting bias) | Unclear risk | Details not stated, outcomes relevant to this review were reported. |
| Other bias | Unclear risk | No data available for the specific type of bias, even when sought |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data available for the specific type of bias, even when sought |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No data available for the specific type of bias, even when sought |
Shavit 2015.
| Methods | Authors only state that it was a "prospective randomized controlled trial". | |
| Participants | 21 women with PCOS | |
| Interventions | IVM with 3 days of gonadotrophins and hCG vs GnRH antagonist "routine" protocol | |
| Outcomes | Total number of mature oocytes, fertilisation rates, "top quality" embryos, average dose of gonadotrophins, pregnancy rate, and OHSS incidence | |
| Notes | Data obtained from abstract presented in a conference. Hillel Yaffe Medical Center, IVF Unit, Hadera, Israel |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No data available for the specific type of bias, even when sought |
| Allocation concealment (selection bias) | High risk | No data available for the specific type of bias, even when sought |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data available for the specific type of bias, even when sought |
| Selective reporting (reporting bias) | High risk | No data available for the specific type of bias, even when sought |
| Other bias | Unclear risk | No data available for the specific type of bias, even when sought |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data available for the specific type of bias, even when sought |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No data available for the specific type of bias, even when sought |
GnRH: gonadotrophin‐releasing hormone hCG: human chorionic gonadotrophin IVF: in vitro fertilisation IVM: in vitro maturation OHSS: ovarian hyperstimulation syndrome PCOS: polycystic ovarian syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnes 1995 | Case report |
| Barnes 1996 | Prospective observational study |
| Beckers 1999 | Prospective observational study |
| Benkhalifa 2009 | Observational study |
| Bos‐Mikich 2011 | Observational study |
| Buckett 2005 | Retrospective observational study |
| Buckett 2007 | Retrospective analysis |
| Buckett 2008 | Retrospective study |
| Cha 1998 | Retrospective case‐control study |
| Cha 2000 | Prospective observational study |
| Cha 2005 | Prospective observational study |
| Chian 1999 | Retrospective observational study (as letter to the editor) |
| Chian 2000 | Prospective randomised study comparing priming versus non‐priming with hCG in women with PCOS undergoing IVM |
| Chian 2004 | Case series |
| Child 2001 | Retrospective case‐control study |
| Child 2002 | Prospective observational study |
| Choi 2012 | Not an RCT |
| Chung 2000 | Prospective randomised study comparing priming versus non‐priming with hCG in women with PCOS undergoing IVM |
| De Vos 2016 | Not an RCT |
| Filali 2008 | Retrospective observational study |
| Fukuda 2006 | Retrospective observational study |
| Ge 2008 | Prospective randomised study comparing different culture media in 62 women with PCOS undergoing IVM |
| Gremeau 2012 | Retrospective case–control study |
| Gulekli 2004 | Prospective controlled study of priming with 10,000 IU versus 20,000 IU of hCG in 57 women with PCOS undergoing IVM |
| Harris 2010 | Prospective observational study |
| Holzer 2006 | Prospective observational study |
| Hreinsson 2003 | Randomised prospective study comparing LH and hCG for oocyte maturation in an IVM programme |
| Junk 2012 | Prospective cohort study |
| Kedem 2013 | Not an RCT |
| Kim 2013 | RCT evaluating the effect of melatonin supplementation on the clinical outcomes of IVM IVF transfer in 111 women with PCOS |
| Le Du 2005 | Retrospective observational study |
| Li 2005 | Prospective observational study |
| Lim 2009 | Retrospective analysis |
| Lin 2003 | Prospective observational study |
| Liu 2010 | Prospective observational study |
| Lornage 2006 | Retrospective analysis |
| Mikkelsen 2001 | Randomised prospective study of 36 women with PCOS comparing priming versus non‐priming with FSH before IVM |
| Mikkelsen 2003 | Randomised prospective study on the interval between FSH administration and IVM in women with PCOS |
| NCT01237106 | Protocol of a non‐RCT |
| NCT01843569 | Protocol of a non‐RCT |
| Roesner 2012 | Retrospective analysis |
| Sandraa 2006 | Retrospective observational study |
| Shalom‐Paz 2012 | Retrospective evaluation |
| Shavit 2014 | Not an RCT |
| Shu‐Chi 2006 | Retrospective observational study |
| Soderstrom‐Anttila 2005 | Retrospective observational study |
| Son 2005 | Retrospective observational study |
| Son 2007 | Not an RCT |
| Sánchez 2017 | Does not answer the question of this review. Not an RCT |
| Trounson 1994 | Retrospective observational study |
| Vieira 2008 | Prospective controlled trial in 5 participants with PCOS and 8 participants with male or tubal causes of subfertility undergoing IVM comparing the meiotic spindle and chromosomal distribution of IVM oocytes and IVM rates |
| Vieira 2010 | Prospective controlled trial in 13 participants with PCOS and 27 participants with male or tubal causes of subfertility undergoing IVM comparing the meiotic spindle and chromosomal distribution of IVM oocytes and IVM rates |
| Walls 2012 | RCT comparing ICSI with IVF in 8 women with PCOS |
| Walls 2015a | Not an RCT |
| Walls 2015b | Not an RCT |
| Walls 2016 | Not an RCT |
| Xu 2012 | Not an RCT |
| Yu 2012 | Retrospective study |
| Zhang 2007 | Observational study |
| Zhao 2006 | Retrospective observational study |
| Zhao 2009 | Retrospective study |
| Zheng 2012 | RCT of 82 women with PCOS undergoing IVM cycles comparing priming versus non‐priming with hCG |
FSH: follicle‐stimulating hormone hCG: human chorionic gonadotrophin ICSI: intracytoplasmic sperm injection IU: international units IVF: in vitro fertilisation IVM: in vitro maturation LH: luteinising hormone PCOS: polycystic ovarian syndrome RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN61229302.
| Trial name or title | A multicentre randomised controlled trial comparing in vitro maturation of oocytes with in vitro fertilisation in women with an increased risk of ovarian hyperstimulation syndrome |
| Methods | Multicentre, randomised, active‐controlled, parallel‐group trial with non‐randomised pilot study |
| Participants | Target sample size 450 (400 couples, preceded by a pilot study of 50 non‐randomised IVM cycles) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Couples will be randomised to either: IVM/ICSI strategy
OR IVF/ICSI strategy
|
| Outcomes | Primary outcome: Cumulative live‐birth rate after IVM/ICSI or COH/IVF/ICSI strategy including pregnancies from cryoembryos transferred within 12 months after the end of IVM/ICSI or COH/IVF/ICSI treatment. Secondary outcomes: 1. Pregnancy/childbirth: detailed information on maternal complications will be obtained from the obstetrician treating the woman concerned. 6 weeks after the expected day of delivery all women will be contacted by telephone to ask for information on the delivery and on the health of the child, and for consent to contact the health centre where she gave birth. If a child has been hospitalised, the paediatrician treating the child will be contacted for further information. 2. Paediatric follow‐up: follow‐up will consist of evaluation on the following domains using internationally accredited and validated tests: motor development, cognitive development, and behaviour. Follow‐up visits will be scheduled at age 6 months, 1, 2, and 5 years. 3. Economic evaluation: a distinction will be made between costs of medical interventions (direct costs) and costs resulting from productivity losses (indirect or time costs). Standardised unit costs will be calculated for all centres based on actual expenses made during the study. Subsequently, unit costs will be applied to resource use as observed in the participating centres. Resource utilisation will be documented using individual patient data in the case record forms. In addition, each woman will receive a questionnaire for details on associated direct costs of professional care, and on indirect costs like transportation and productivity loss. These questionnaires will be sent at 4, 12, 24, and 48 weeks after treatment start. Resource unit prices will reflect the unit of staff, materials, equipment, housing, depreciation, and overhead. Endpoint for cost‐effectiveness will be euros/live birth for either strategy. 4. Patient quality of life study: before starting a treatment cycle, the day before oocyte retrieval and the day after oocyte retrieval and 3 weeks after a treatment cycle participants will be asked to fill out a validated questionnaire on quality of life (Fertility quality of life tool (FertiQoL), www.fertiqol.org). |
| Starting date | 1 January 2010 |
| Contact information | Register: Netherlands Trial Register Primary sponsor: Jeroen Bosch Hospital Name: JP Bruin, de Address: Postbus 90153 5200 Center for Reproductive Medicine P.O. Box 90153 ME Den Bosch the Netherlands Telephone: +31 (0)73 6998660 Email: j.d.bruin@jbz.nl |
| Notes | Trial website: www.studies‐obsgyn.nl/ivm Status of trial: recruiting |
NCT00867763.
| Trial name or title | In vitro maturation with in vitro fertilisation (IVF) compared to mild in vitro fertilisation (IVM vs IVF) |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: cross‐over assignment Masking: open‐label Primary purpose: health services research |
| Participants | Ages: 21 to 41 years Inclusion criteria: under 42 years of age, female, normal uterine cavity, BMI 18 to 34 kg/m2 Exclusion criteria: donor eggs, donor sperm, myoma over 5 cm, current hydrosalpinx, abnormal uterine cavity |
| Interventions | Arm 1: IVM and IVF. No stimulation with gonadotrophins, eggs harvested early Arm 2: mild IVF (low doses of gonadotrophin stimulation followed by conventional IVF) |
| Outcomes | Primary outcome measure: cost‐effectiveness Secondary outcome measure: ongoing pregnancy rates per cycle |
| Starting date | March 2009 |
| Contact information | Joe B Massey, MD, Batzofin Fertility Services |
| Notes | This study has suspended participant recruitment (investigator changed location, requires new training effort). ClinicalTrials.gov identifier: NCT00867763 |
NCT01473459.
| Trial name or title | Comparison between two fertility protocols in obese polycystic ovary syndrome patients |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | Estimated enrolment: 40 participants Age: 18 to 40 years Inclusion criteria: BMI > 30, PCOS, failure of COH treatment |
| Interventions | Arm 1: IVM treatment
No gonadotrophin stimulation. Ovum pick‐up will be from antral follicles of immature oocyte, which will be matured in the lab and fertilised by ICSI. Embryo transfer will take place on days 2 or 3. Arm 2: IVF antagonist protocol Stimulation with gonadotrophins and use of GnRH antagonists. Ovulation induction will be performed with GnRH agonist. After ovulation there will be ovum pick‐up and fertilisation in the lab. Embryo transfer will take place on days 2 or 3. |
| Outcomes | Reported as "fertility results" |
| Starting date | April 2012 |
| Contact information | Tal Shavit, MD; 972‐50‐6246712; email: tal.shavit@gmail.com |
| Notes | This study is not yet open for participant recruitment. Hillel Yaffe Medical Center, Hadera, Israel, 38100 ClinicalTrials.gov identifier: NCT01473459 |
NCT03134482.
| Trial name or title | Comparison of clinical outcomes between in vitro maturation and minimal stimulation in vitro fertilization in patients with polycystic ovarian syndrome; prospective, randomized controlled, parallel, open‐label, clinical trial |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | Ages eligible for study: 20 years to 39 years Estimated enrolment: 240 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: IVM
Active comparator: minimal‐stimulation IVF
|
| Outcomes | Primary outcome: clinical pregnancy rate Secondary outcome: OHSS incidence rate |
| Starting date | Actual start date of study: 1 May 2017 Estimated primary completion date: 23 September 2018 Estimated study completion date: 23 September 2019 |
| Contact information | Contact: You Shin Kim, MD, PhD; 82‐2‐2002‐0303; email: medikys@cha.ac.kr Principal Investigator: You Shin Kim, MD, PhD, Fertility Center of CHA Gangnam Medical Center, CHA University |
| Notes | CHA Fertility Center, Seoul station Recruiting Seoul, Korea, Republic of, 04367 Contact: You Shin Kim, MD, PhD; 2‐2‐2002‐0303; email: medikys@cha.ac.kr CHA Gangnam Medical Center Recruiting Seoul, Korea, Republic of, 06135 Contact: You Shin Kim, MD, PhD; 82‐2‐2002‐0303; email: medikys@cha.ac.kr ClinicalTrials.gov identifier: NCT03134482 Status: recruiting |
NCT03405701.
| Trial name or title | The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with high antral follicle count (AFC): a randomised controlled trial |
| Methods | Study type: interventional (clinical trial) Allocation: randomised Intervention model: parallel assignment Masking: none (open‐label) Primary purpose: treatment |
| Participants | Estimated enrolment: 546 participants Ages eligible for study: 18 to 40 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | 1. Active comparator: IVM
Receiving FSH (menotrophin (Menopur), Ferring) for 2 days on day 2/3 of the menstrual cycle (spontaneous/OCP administration) and an ultrasound scan will be performed subsequently. Oocytes retrieval will be performed 42 hours after the last injection. Prematuration will last for 24 to 30 hours. Intracytoplasmic sperm injection will be used for insemination. Freeze‐only on day 3 and frozen embryo transfer will be performed on the subsequent cycle using HRT protocol with a maximum of 2 embryos transferred. 2. Active comparator: IVF Undergoing controlled ovarian hyperstimulation for IVF with rFSH (menotrophin (Menopur), Ferring) in GnRH antagonist protocol, treatment monitoring using ultrasound scans and blood tests. Gonadotrophin‐releasing hormone agonist triggering will be used for final oocytes maturation. Intracytoplasmic sperm injection will be used for insemination. Freeze‐only on day 3 and frozen embryo transfer will be performed on the subsequent cycle using HRT protocol with a maximum of 2 embryos transferred. |
| Outcomes | Primary outcome measure: ongoing pregnancy resulting in live birth resulting from the first embryo transfer of the started treatment cycle Secondary outcome measures: clinical pregnancy and foetal complications and characteristics |
| Starting date | Actual start date of study: 25 January 2018 Estimated primary completion date: 31 December 2019 Estimated study completion date: 30 June 2020 |
| Contact information | Contact: Vu NA Ho, MD; +84935843336; email: bsvu.hna@myduchospital.vn Contact: Lan N Vuong, MD, PhD; +84903008889; email: drlan@yahoo.com.vn |
| Notes | Mỹ Đức Hospital Recruitment status: recruiting ClinicalTrials.gov identifier: NCT03405701 |
NCT03463772.
| Trial name or title | In vitro maturation versus standard in vitro fertilization in infertile patients diagnosed with polycystic ovaries syndrome: a study protocol for a single‐center prospective, randomized controlled clinical trial |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | Ages eligible for study: 20 to 38 years Estimated enrolment: 350 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: IVM
Controlled ovarian stimulation protocol will not performed in this group of participants. Transvaginal ultrasound scanning on natural cycle will be used to monitor the follicle size. Oocyte retrieval scheduled to take place once the endometrium reached at least 6 mm thickness and there was no follicle larger than 10 mm. After oocyte retrieval, the COCs will be cultured for 28 h to 32 h in special IVM media to obtain the matured oocytes. All the metaphase II oocytes will be inseminated by means of ICSI. All embryos will be cultured to blastocyst stage and vitrified. Arm 2: Standard IVF Ovarian stimulation will be performed by a standard protocol using GnRH‐ant in association with rFSH and hCG. Oocyte retrieval is scheduled for 36 (±2) hours after hCG injection. The retrieved COC will be inseminated using ICSI or conventional IVF according to the semen analysis. All embryos will be cultured to blastocyst stage and vitrified. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | April 2018 |
| Contact information | Contact 1: Xiaoying Zheng; 86‐10‐82265033; email: zheng_xiaoying@126.com Contact 2: Rui Yang; 86‐10‐82265080; email: yrjeff@126.com |
| Notes | This study is not yet open for participant recruitment. Peking University Third Hospital Beijing, Beijing, China, 100191 ClinicalTrials.gov identifier: NCT03463772 |
AFC: antral follicle count ART: assisted reproductive technology BMI: body mass index COC: cumulus oocyte complexes COH: controlled ovarian hyperstimulation FSH: follicle‐stimulating hormone GnRH: gonadotrophin‐releasing hormone GnRH‐ant: gonadotrophin‐releasing hormone antagonist hCG: human chorionic gonadotrophin HRT: hormone replacement therapy ICSI: intracytoplasmic sperm injection IU: international units IVF: in vitro fertilisation IVM: in vitro maturation LEO: laparoscopic electrocoagulation of the ovaries OCP: oral contraceptive pills OHSS: ovarian hyperstimulation syndrome PCOS: polycystic ovarian syndrome PGD: pre‐implantation genetic diagnosis PGS: pre‐implantation genetic screening rFSH: recombinant follicle‐stimulating hormone
Differences between protocol and review
In this update, we moved the outcome of "miscarriage" from the secondary to the primary outcomes and defined it as "per clinical pregnancy".
Contributions of authors
Contact author
CS Siristatidis: wrote the protocol and updated the review twice.
Co‐review authors
Dr Abha Maheshwari: updated the protocol and made a major contribution to selection of studies.
Dr Dennis Vaidakis: made a major contribution in the last update in the selection of studies and writing of text.
Prof Siladitya Bhattacharya: initiated and corrected the protocol and the updates of the review.
All authors have agreed upon the final version of the review.
Sources of support
Internal sources
-
University of Aberdeen, UK.
Assisted Reproduction Unit, University of Aberdeen UK.
-
National and Kapodistrian University of Athens, Greece.
Assisted Reproduction Unit, Third Department of Obstetrics and Gynecology
External sources
No sources of support supplied
Declarations of interest
Charalampos S Siristatidis: no conflicts of interest to disclose.
Abha Maheshwari: no conflicts of interest to disclose.
Dennis Vaidakis: no conflicts of interest to disclose.
Siladitya Bhattacharya: no conflicts of interest to disclose.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gidoni 2013 {published data only}
- Gidoni YS, Raziel A, Friedler S, Strassburger D, Hadari D, Kasterstein E, et al. GnRH agonist for triggering final oocyte maturation versus IVM in patients at risk of OHSS. Human Reproduction 2013;28(Suppl 1):i311–56. [DOI: ] [Google Scholar]
Shavit 2015 {published data only}
- Shavit T, Shalom‐Paz E, Michaeli M, Ellenbogen A. Comparison between IVF antagonist protocol versus IVM protocol to treat infertile PCOS patients. A prospective randomized study. Preliminary results. Fertility and Sterility 2014;102(3 Suppl 1):e267. [DOI: ] [Google Scholar]
References to studies excluded from this review
Barnes 1995 {published data only}
- Barnes FL, Crombie A, Gardner DK, Kausche A. Blastocyst development and birth after in‐vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Human Reproduction 1995;10(12):3243‐7. [DOI] [PubMed] [Google Scholar]
Barnes 1996 {published data only}
- Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of embryos from in vitro‐matured primary human oocytes. Fertility and Sterility 1996;65(6):1151‐6. [DOI] [PubMed] [Google Scholar]
Beckers 1999 {published data only}
- Beckers N, Pieters M, Ramos L, Zeilmaker G, Fauser B, Braat D. Retrieval, maturation, and fertilization of immature oocytes obtained from unstimulated patients with polycystic ovary syndrome. Journal of Assisted Reproduction and Genetics 1999;16(2):81‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benkhalifa 2009 {published data only}
- Benkhalifa M, Demirol A, Ménézo Y, Balashova E, Abduljalil AK, Abbas S, et al. Natural cycle IVF and oocyte in‐vitro maturation in polycystic ovary syndrome: a collaborative prospective study. Reproductive Biomedicine Online 2009;18(1):29‐36. [DOI] [PubMed] [Google Scholar]
Bos‐Mikich 2011 {published data only}
- Bos‐Mikich A, Ferreira M, Höher M, Frantz G, Oliveira N, Dutra CG, et al. Fertilization outcome, embryo development and birth after unstimulated IVM. Journal of Assisted Reproduction and Genetics 2010;28(2):107‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckett 2005 {published data only}
- Buckett WM, Chian R, Holzer H, Usher R, Tan SL. Congenital abnormalities and perinatal outcome in pregnancies following IVM, IVF, and ICSI delivered in a single center. Fertility and Sterility 2005;84 Suppl 1:80‐1. [Google Scholar]
Buckett 2007 {published data only (unpublished sought but not used)}
- Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstetrics & Gynecology 2007;110(4):885‐91. [DOI] [PubMed] [Google Scholar]
Buckett 2008 {published data only}
- Buckett WM, Chian RC, Dean N, Sylvestre C, Holzer H, Tan SL. Pregnancy loss in pregnancies conceived after in vitro oocyte maturation, conventional in vitro fertilization, and intracytoplasmic sperm injection. Fertility and Sterility 2008;110(4):885‐91. [DOI] [PubMed] [Google Scholar]
Cha 1998 {published data only}
- Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Human Reproduction Update 1998;4(2):103‐20. [DOI] [PubMed] [Google Scholar]
Cha 2000 {published data only}
- Cha KY, Han SY, Chung HM, Choi DH. Pregnancies and deliveries after in vitro maturation culture followed by in vitro fertilization and embryo transfer without stimulation in women with polycystic ovary syndrome. Fertility and Sterility 2000;73(5):978‐83. [DOI] [PubMed] [Google Scholar]
Cha 2005 {published data only}
- Cha KY, Chung HM, Lee DR, Kwon H, Chung MK, Park LS, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization‐embryo transfer. Fertility and Sterility 2005;83:1461‐5. [DOI] [PubMed] [Google Scholar]
Chian 1999 {published data only}
- Chian RC, Gulekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. New England Journal of Medicine 1999;341(21):1624‐6. [DOI] [PubMed] [Google Scholar]
Chian 2000 {published data only}
- Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Human Reproduction 2000;15(1):165‐70. [DOI] [PubMed] [Google Scholar]
Chian 2004 {published data only}
- Chian RC, Buckett W, Jalil A, Son YU, Sylvestre C, Rao D, et al. Natural‐cycle in vitro fertilization combined with in vitro maturation of immature oocytes is a potential approach in infertility treatment. Fertility and Sterility 2004;82(6):1675‐8. [DOI] [PubMed] [Google Scholar]
Child 2001 {published data only}
- Child TJ, Abdul‐Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertility and Sterility 2001;76(5):936‐42. [DOI] [PubMed] [Google Scholar]
Child 2002 {published data only}
- Child TJ, Phillips SJ, Abdul‐Jalil AK, Gulakli B, Tan SL. A comparison of in vitro maturation and in vitro fertilization for women with polycystic ovaries. Obstetrics and Gynecology 2002;100(4):665‐70. [DOI] [PubMed] [Google Scholar]
Choi 2012 {published and unpublished data}
- Choi MH, Lee SH, Kim HO, Cha SH, Kim JY, Yang KM, et al. Comparison of assisted reproductive technology outcomes in infertile women with polycystic ovary syndrome: in vitro maturation, GnRH agonist, and GnRH antagonist cycles. Clinical and Experimental Reproductive Medicine 2012;39(4):166‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2000 {published data only}
- Chung MK, Chung HM, Lee WS, Han SY, Yoon TK, Cha KY. Applicability of a hCG priming of unstimulated PCOS patients for improving IVM/IVF‐ET outcome. Fertility and Sterility 2000;74 Suppl 3:32‐3. [Google Scholar]
De Vos 2016 {published data only}
- Vos M, Segers I, Santos‐Ribeiro S, Popovic‐Todorovic B, Verheyen G, Tournaye H, et al. Outcome after non‐hCG triggered serum‐free IVM in patients with PCOS: a freeze‐or‐fresh transfer strategy based on number of available embryos. Human Reproduction. Conference: 32nd Annual Meeting of the European Society of Human Reproduction and Embryology. Finland 2016;31:i443‐i444. [DOI: ] [Google Scholar]
Filali 2008 {published data only}
- Filali M, Hesters L, Fanchin R, Tachdjian G, Frydman R, Frydman N. Retrospective comparison of two media for invitro maturation of oocytes. Reproductive Biomedicine Online 2008;16(2):250‐6. [DOI] [PubMed] [Google Scholar]
Fukuda 2006 {published data only}
- Fukuda AI, Sato M, Sugihara K, Nagata F, Nakaoka Y, Morimoto Y. In vitro maturation, in vitro fertilisation and embryo transfer (IVM‐IVF) combined with low dose FSH over metformin pretreatment and frozen‐thawed cycles should be a routine ART option for polycystic ovarian syndrome (PCOS) patients. Fertility and Sterility 2006;86 Suppl 2:129. [Google Scholar]
Ge 2008 {published data only}
- Ge HS, Huang XF, Zhang W, Zhao JZ, Lin JJ, Zhou WZ. Exposure to human chorionic gonadotropin during in vitro maturation does not improve the maturation rate and developmental potential of immature oocytes from patients with polycystic ovary syndrome. Fertility and Sterility 2008;89(1):98‐103. [DOI] [PubMed] [Google Scholar]
Gremeau 2012 {published data only}
- Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, McVeigh E, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case‐control study of 194 treatment cycles. Fertility and Sterility 2012;98(2):355‐60. [DOI] [PubMed] [Google Scholar]
Gulekli 2004 {published data only}
- Gulekli B, Buckett WM, Chian RC, Child TJ, Abdul‐Jalil AK, Tan SL. Randomized, controlled trial of priming with 10,000 IU versus 20,000 IU of human chorionic gonadotropin in women with polycystic ovary syndrome who are undergoing in vitro maturation. Fertility and Sterility 2004;82(5):1458‐9. [DOI] [PubMed] [Google Scholar]
Harris 2010 {published data only}
- Harris SE, Maruthini D, Tang T, Balen AH, Picton HM. Metabolism and karyotype analysis of oocytes from patients with polycystic ovary syndrome. Human Reproduction 2010;25(9):2305‐15. [DOI] [PubMed] [Google Scholar]
Holzer 2006 {published data only}
- Holzer H, Scharf E, Chian RC, Demirtas E, Buckett W, Tan SL. In vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertility and Sterility 2007;88(1):62‐7. [DOI] [PubMed] [Google Scholar]
Hreinsson 2003 {published data only}
- Hreinsson J, Rosenland B, Fridén B, Levkov L, Ek I, Suikkari AM, et al. Recombinant LH is equally effective as recombinant HCG in promoting oocyte maturation in a clinical in vitro maturation program: a randomised study. Human Reproduction 2003;18(10):2131‐6. [DOI] [PubMed] [Google Scholar]
Junk 2012 {published data only}
- Junk SM, Yeap D. Improved implantation and ongoing pregnancy rates after single‐embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertility and Sterility 2012;98(4):888‐92. [DOI] [PubMed] [Google Scholar]
Kedem 2013 {published data only}
- Kedem A, Hourvitz A, Yung Y, Shalev L, Yerushalmi GM, Kanety H, et al. Anti‐Müllerian hormone (AMH) downregulation in late antral stages is impaired in PCOS patients. A study in normo‐ovulatory and PCOS patients undergoing in vitro maturation (IVM) treatments. Gynecological Endocrinology 2013;29(7):651‐6. [DOI: 10.3109/09513590.2013.798279] [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, et al. Does supplementation of in‐vitro culture medium with melatonin improve IVF outcome in PCOS?. Reproductive Biomedicine Online 2013;26(1):22‐9. [DOI] [PubMed] [Google Scholar]
Le Du 2005 {published data only}
- Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier MC, Chevalier N, et al. In vitro maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Human Reproduction 2005;20(2):420‐4. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, et al. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertility and Sterility 2006;85(4):827‐32. [DOI] [PubMed] [Google Scholar]
Lim 2009 {published data only}
- Lim JH, Yang SH, Xu Y, Yoon SH, Chian RC. Selection of patients for natural cycle in vitro fertilization combined with in vitro maturation of immature oocytes. Fertility and Sterility 2009;91(4):1050‐5. [DOI] [PubMed] [Google Scholar]
Lin 2003 {published data only}
- Lin YH, Hwang JL, Huang LW, Mu SC, Seow KM, Chung J, et al. Combination of FSH priming and hCG priming for in‐vitro maturation of human oocytes. Human Reproduction 2003;18(8):1632‐6. [DOI] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu S, Jiang JJ, Feng HL, Ma SY, Li M, Li Y. Evaluation of the immature human oocytes from unstimulated cycles in polycystic ovary syndrome patients using a novel scoring system. Fertility and Sterility 2010;93(7):2202‐9. [DOI] [PubMed] [Google Scholar]
Lornage 2006 {published data only}
- Lornage J. In vitro maturation: the French experience. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction (Paris) 2006;35(5 Pt 2):2S3‐7. [PubMed] [Google Scholar]
Mikkelsen 2001 {published data only}
- Mikkelsen AL, Lindenberg S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction 2001;122(4):587‐92. [DOI] [PubMed] [Google Scholar]
Mikkelsen 2003 {published data only}
- Mikkelsen AL, Host E, Blaabjerg J, Lindenberg S. Time interval between FSH priming and aspiration of immature human oocytes for in vitro maturation: a prospective randomised study. Reproductive Biomedicine Online 2003;6(4):416‐20. [DOI] [PubMed] [Google Scholar]
NCT01237106 {published data only}
- NCT01237106. In vitro maturation (IVM) for polycystic ovary syndrome (PCOS) [In Vitro Maturation for Polycystic Ovary Syndrome (PCOS)‐ A Pilot Study]. Clinicaltrials.gov/show/NCT01237106 (first received December 3, 2015). [NCT01237106]
NCT01843569 {published data only}
- NCT01843569. In Vitro Maturation (IVM) of Human Oocytes (IVM). clinicaltrials.gov/show/NCT01843569 2015. [NCT01843569]
Roesner 2012 {published data only}
- Roesner S, Wolff M, Eberhardt I, Beuter‐Winkler P, Toth B, Strowitzki T. In vitro maturation: a five‐year experience. Acta Obstetricia et Gynecologica Scandinavica 2012;91(1):22‐7. [DOI] [PubMed] [Google Scholar]
Sánchez 2017 {published data only}
- Sánchez F, Lolicato F, Romero S, Vos M, Ranst H, Verheyen G, et al. An improved IVM method for cumulus‐oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Human Reproduction 2017;32(10):2056–68. [DOI: 10.1093/humrep/dex262] [DOI] [PubMed] [Google Scholar]
Sandraa 2006 {published data only}
- Sandraa P, Cheloa E, Cuomoa S, Livia C. IVM is a promising alternative to conventional IVF treatment for women with polycystic ovarian syndrome (PCOS). Fertility and Sterility 2006;86 Suppl 3:128. [Google Scholar]
Shalom‐Paz 2012 {published data only}
- Shalom‐Paz E, Holzer H, Son W, Levin I, Tan SL, Almog B. PCOS patients can benefit from in vitro maturation (IVM) of oocytes. European Journal of Obstetrics, Gynecology and Reproductive Biology 2012;165(1):53‐6. [DOI] [PubMed] [Google Scholar]
Shavit 2014 {published data only}
- Shavit T, Ellenbogen A, Michaeli M, Kartchovsky E, Ruzov O, Shalom‐Paz E. In‐vitro maturation of oocytes vs in‐vitro fertilization with a gonadotropin‐releasing hormone antagonist for women with polycystic ovarian syndrome: can superiority be defined?. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;179:46‐50. [DOI: 10.1016/j.ejogrb.2014.05.013] [DOI] [PubMed] [Google Scholar]
Shu‐Chi 2006 {published data only}
- Shu‐Chi M, Jiann‐Loung H, Yu‐Hung L, Tseng‐Chen S, Ming‐I L, Tsu‐Fuh Y. Growth and development of children conceived by in‐vitro maturation of human oocytes. Early Human Development 2006;82(10):677‐82. [DOI] [PubMed] [Google Scholar]
Soderstrom‐Anttila 2005 {published data only}
- Soderstrom‐Anttila V, Makinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Human Reproduction 2005;20(6):1534‐40. [DOI] [PubMed] [Google Scholar]
Son 2005 {published data only}
- Son WY, Lee SY, Lim JH. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Human Reproduction 2005;20(11):3204‐7. [DOI] [PubMed] [Google Scholar]
Son 2007 {published data only}
- Son WY, Lee SY, Yoon SH, Lim JH. Pregnancies and deliveries after transfer of human blastocysts derived from in vitro matured oocytes in in vitro maturation cycles. Fertility and Sterility 2007;87(6):1491‐3. [DOI] [PubMed] [Google Scholar]
Trounson 1994 {published data only}
- Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertility and Sterility 1994;62(2):353‐62. [DOI] [PubMed] [Google Scholar]
Vieira 2008 {published data only}
- Vieira RC, Barcelos ID, Ferreira EM, Araújo MC, dos Reis RM, Ferriani RA, et al. Evaluation of meiotic abnormalities of oocytes from polycystic ovary syndrome patients submitted to ovarian stimulation. Revista Brasileira de Ginecologia e Obstetrícia 2008;30(5):241‐7. [DOI] [PubMed] [Google Scholar]
Vieira 2010 {published data only}
- Vieira RC, Barcelos ID, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Spindle and chromosome configurations of in vitro‐matured oocytes from polycystic ovary syndrome and ovulatory infertile women: a pilot study. Journal of Assisted Reproduction and Genetics 2011; Vol. 28, issue 1:15‐21. [DOI: 10.1007/s10815-010-9475-7] [DOI] [PMC free article] [PubMed]
Walls 2012 {published data only}
- Walls M, Junk S, Ryan JP, Hart R. IVF versus ICSI for the fertilization of in‐vitro matured human oocytes. Reproductive Biomedicine Online 2012;25(6):603‐7. [DOI] [PubMed] [Google Scholar]
Walls 2015a {published data only}
- Walls ML, Ryan JP, Keelan JA, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Human Reproduction 2015;30(8):1842–9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Walls 2015b {published data only}
- Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Human Reproduction 2015;30(1):88–96. [DOI: 10.1093/humrep/deu248] [DOI] [PubMed] [Google Scholar]
Walls 2016 {published data only}
- Walls M, Hart R, Keelan JA, Ryan JP. Structural and morphologic differences in human oocytes after in vitro maturation compared with standard in vitro fertilization. Fertility and Sterility 2016;106(6):1392‐8.e5. [DOI: 10.1016/j.fertnstert.2016.08.014] [DOI] [PubMed] [Google Scholar]
Xu 2012 {published data only}
- Xu YP, Xiang HF, Zou WW, Li ZL, Zhang ZG, Zhou P, et al. Clinical application of in vitro maturation of human immature oocytes for infertile women with polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi 2012;47(1):14‐8. [PubMed] [Google Scholar]
Yu 2012 {published data only}
- Yu R, Lin J, Zhao JZ, Wang PY, Xiao SQ, Zhang W. Study on clinical effect on infertility women with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization‐embryo transfer. Zhonghua Fu Chan Ke Za Zhi 2012;47(4):250‐4. [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang ZG, Zhao JH, Wei ZL, Cong L, Zhou P, Cao YX. Human umbilical cord blood serum in culture medium on oocyte maturation in vitro. Archives of Andrology 2007;53(6):303‐7. [DOI] [PubMed] [Google Scholar]
Zhao 2006 {published data only}
- Zhao JZ, Ge HS, Ye BL, Huang XF, Zhou HW, Chi HH, et al. In vitro maturation and fertilization of unstimulated immature oocyte for the treatment of infertile women. Zhonghua Fu Chan Ke Za Zhi 2006;41(3):173‐6. [PubMed] [Google Scholar]
Zhao 2009 {published data only}
- Zhao JZ, Chen X, Wang PY, Zhou W, Lin JJ, Zhang W, et al. Outcome of pregnancy in women with polycystic ovary syndrome treated by in vitro maturation of immature oocytes. Zhonghua Fu Chan Ke Za Zhi 2009;44(6):409‐12. [PubMed] [Google Scholar]
Zheng 2012 {published data only}
- Zheng X, Wang L, Zhen X, Lian Y, Liu P, Qiao J. Effect of hCG priming on embryonic development of immature oocytes collected from unstimulated women with polycystic ovarian syndrome. Reproductive Biology and Endocrinology 2012;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN61229302 {published data only}
- ISRCTN61229302. A multicentre randomised controlled trial comparing in vitro maturation of oocytes with in vitro fertilisation in women with an increased risk of ovarian hyperstimulation syndrome [Comparing in vitro maturation of oocytes with in vitro fertilisation in women with an increased risk of ovarian hyperstimulation syndrome: A multicentre randomised controlled trial with non‐randomised pilot study]. http://www.isrctn.com/ISRCTN61229302 (first received 27 May 2010). [DOI: 10.1186/ISRCTN61229302] [DOI]
NCT00867763 {published data only}
- NCT00867763. In vitro maturation with in vitro fertilization (IVF) compared to mild in vitro fertilization (IVM vs IVF) [Clinical Research Study on in Vitro Maturation With IVF Compared to Mild Stimulation IVF]. http://clinicaltrials.gov/show/NCT00867763 (first received March 24, 2009).
NCT01473459 {published data only}
- NCT01473459. Comparison between two fertility protocols in obese polycystic ovary syndrome patients [Comparison Between Two Fertility Protocols in Obese Polycystic Ovary Syndrome]. clinicaltrials.gov/show/NCT01473459 (first received November 17, 2011). [ClinicalTrials.gov Identifier:NCT01473459]
NCT03134482 {published data only}
- NCT03134482. Comparison of Clinical Outcomes Between IVM and Minimal Stimulation IVF in Patients With PCOS [Comparison of Clinical Outcomes Between in Vitro Maturation and Minimal Stimulation in Vitro Fertilization in Patients With Polycystic Ovarian Syndrome; Prospective, Randomized Controlled, Parallel, Open‐label, Clinical Trial]. ClinicalTrials.gov/show/NCT03134482 (first received May 1, 2017). [https://www.clinicaltrials.gov/ct2https://clinicaltrials.gov/ct2/show/record/NCT03134482]
NCT03405701 {published data only}
- NCT03405701. IVM Versus IVF in High Antral Follicle Count Patients [The Effectiveness and Safety of in Vitro Maturation of Oocytes Versus in Vitro Fertilization in Women With High Antral Follicle Count (AFC): a Randomised Controlled Trial]. clinicaltrials.gov/show/NCT03405701 (first received January 23, 2018). [https://www.clinicaltriClinicalTrials.gov Identifier: NCT03405701]
NCT03463772 {published data only}
- NCT03463772. IVM versus standard in vitro fertilization in infertile patients diagnosed with PCOS [In Vitro Maturation Versus Standard in Vitro Fertilization in Infertile Patients Diagnosed With Polycystic Ovaries Syndrome: a Study Protocol for a Single‐center Prospective, Randomized Controlled Clinical Trial]. ClinicalTrials.gov/show/NCT03463772 (first reveived March 13, 2018). [ClinicalTrials.gov Identifier: NCT03463772]
Additional references
Archer 2004
- Archer J, Chang J. Hirsutism and acne in polycystic ovary syndrome. Best Practice & Research Clinical Obstetrics & Gynaecology 2004;18(5):737‐754. [DOI: ] [DOI] [PubMed] [Google Scholar]
Azziz 2005
- Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care‐related economic burden of the polycystic ovary syndrome during the reproductive life span. The Journal of Clinical Endocrinology & Metabolism 2005;90(8):4650‐4658. [DOI: ] [DOI] [PubMed] [Google Scholar]
Coticchio 2016
- Coticchio G, Dal Canto M, Fadini R, Mignini Renzini M, Guglielmo MC, Miglietta S, et al. Ultrastructure of human oocytes after in vitro maturation. Molecular Human Reproduction 2016;22(2):110‐8. [DOI: 10.1093/molehr/gav071] [DOI] [PubMed] [Google Scholar]
Dahan 2016
- Dahan MH, Tan SL, Chung J, Son WY. Clinical definition paper on in vitro maturation of human oocytes. Human Reproduction 2016;31(7):1383‐6. [DOI: 10.1093/humrep/dew109] [DOI] [PubMed] [Google Scholar]
de Ziegler 2012
- Ziegler D, Streuli I, Gayet V, Frydman N, Bajouh O, Chapron C. Retrieving oocytes from small non‐stimulated follicles in polycystic ovary syndrome (PCOS): in vitro maturation (IVM) is not indicated in the new GnRH antagonist era. Fertility and Sterility 2012;98(2):290‐293. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dewailly 2011
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau‐Jonard S. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Human Reproduction 2011;26(11):3123‐3129. [DOI: ] [DOI] [PubMed] [Google Scholar]
Edwards 1965
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 1965;208:349‐351. [PUBMED: 4957259] [DOI] [PubMed] [Google Scholar]
Ellenbogen 2014
- Ellenbogen A, Shavit T, Shalom‐Paz E. IVM results are comparable and may have advantages over standard IVF. Facts, Views and Vision in Obstetrics and Gynaecology 2014;6(2):77‐80. [PUBMED: 25009730] [PMC free article] [PubMed] [Google Scholar]
ESHRE/ASRM 2003
- The Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility 2003;81:19‐25. [PUBMED: 14711538] [DOI] [PubMed] [Google Scholar]
Fadini 2009
- Fadini R, Dal Canto MB, Renzini MM, Brambillasca F, Comi R, Fumagalli D, et al. Predictive factors in in‐vitro maturation in unstimulated women with normal ovaries. Reproductive Biomedicine Online 2009;18(2):251‐261. [PUBMED: 19192347] [DOI] [PubMed] [Google Scholar]
Fadini 2013
- Fadini R, Mignini Renzini M, Dal Canto M, Epis A, Crippa M, Caliari I, Brigante C, Coticchio G. Oocyte in vitro maturation in normo‐ovulatory women. Fertility and Sterility 2013;99(5):1162‐1169. [DOI: ] [DOI] [PubMed] [Google Scholar]
Foix‐L'Hélias 2014
- Foix‐L'Hélias L, Grynberg M, Ducot B, Frydman N, Kerbrat V, Bouyer J, et al. Growth development of French children born after in vitro maturation. PLoS ONE 2014;9(2):e89713. [DOI: 10.1371/journal.pone.0089713] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2018 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 30 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grynberg 2013
- Grynberg M, Hachem H, Bantel A, Benard J, Parco S, Fanchin R. In vitro maturation of oocytes: uncommon indications. Fertility and Sterility 2013;99(5):1182‐1188. [DOI: ] [DOI] [PubMed] [Google Scholar]
Heijnen 2006
- Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta‐analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Human Reproduction Update 2006;12(1):13–21. [DOI: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2003
- Liu JY, Qian Y, Mao YD, Ding W, Yang NM. In vitro maturation, fertilization and embryo transfer of human immature oocyte. Zhonghua Fu Chan Ke Za Zhi 2003;38(4):230‐2. [PUBMED: 12885372] [PubMed] [Google Scholar]
MacDougall 1993
- MacDougall MJ, Tan SL, Balen A, Jacobs HS. A controlled study comparing patients with and without polycystic ovaries undergoing in‐vitro fertilization. Human Reproduction 1993;8(2):233‐237. [PUBMED: 8473426] [DOI] [PubMed] [Google Scholar]
Mikkelsen 2005
- Mikkelsen AL. Strategies in human in‐vitro maturation and their clinical outcome. Reproductive Biomedicine Online 2005;10(5):593‐599. [PUBMED: 15949216] [DOI] [PubMed] [Google Scholar]
Nagy 1996
- Nagy ZP, Cecile J, Liu J, Loccufier A, Devroey P, Steirteghem A. Pregnancy and birth after intracytoplasmic sperm injection of in vitro matured germinal‐vesicle stage oocytes: case report. Fertility and Sterility 1996;65(5):1047‐50. [PUBMED: 8612833] [DOI] [PubMed] [Google Scholar]
NICE 2004
- National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK), 2003‐. Fertility: Assessment and Treatment for People with Fertility Problems [Available from: https://www.ncbi.nlm.nih.gov/books/NBK11822/]. (accessed 2 June 2018).
Pincus 1935
- Pincus G, Enzmann EV. The comparative behaviour of mammalian eggs in vivo and in vitro. Journal Of Experimental Medicine 1935;62:665‐675. [PUBMED: 19870440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiao 2011
- Qiao J, Feng HL. Extra‐ and intra‐ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Human Reproduction Update 2011;17(1):17‐33. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinblatt 2008
- Reinblatt SL, Buckett W. In vitro maturation for patients with polycystic ovary syndrome. Seminars in Reproductive Medicine 2008;26(1):121‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roesner 2017
- Roesner S, Wolff M, Elsaesser M, Roesner K, Reuner G, Pietz J, et al. Two‐year development of children conceived by IVM: a prospective controlled single‐blinded study. Human Reproduction 2017;32(6):1341–50. [DOI: 10.1093/humrep/dex068] [DOI] [PubMed] [Google Scholar]
Sauerbrun‐Cutler 2015
- Sauerbrun‐Cutler MT, Vega M, Keltz M, McGovern PG. In vitro maturation and its role in clinical assisted reproductive technology. Obstetrical and Gynecological Survey 2015;70(1):45‐57. [DOI: 10.1097/OGX.0000000000000150] [DOI] [PubMed] [Google Scholar]
Siristatidis 2015
- Siristatidis C, Sergentanis TN, Vogiatzi P, Kanavidis P, Chrelias C, Papantoniou N, et al. In vitro maturation in women with vs. without polycystic ovarian syndrome: a systematic review and meta‐analysis. PLoS ONE 2015;10(8):e0134696. [DOI: 10.1371/journal.pone.0134696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Son 2010
- Son WY, Tan SL. Laboratory and embryological aspects of hCG‐primed in vitro maturation cycles for patients with polycystic ovaries. Human Reproduction Update 2010;16(6):675‐689. [DOI: ] [DOI] [PubMed] [Google Scholar]
Suikkari 2008
- Suikkari AM. In‐vitro maturation: its role in fertility treatment. Current Opinion in Gynecology and Obstetrics 2008;20(3):242‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Trounson 2001
- Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction 2001;121(1):51‐75. [PUBMED: 11226029] [DOI] [PubMed] [Google Scholar]
Yang 2017
- Yang ZY, Chian RC. Development of in vitro maturation techniques for clinical applications. Fertility and Sterility 2017;108(4):577‐84. [DOI: 10.1016/j.fertnstert.2017.08.020] [DOI] [PubMed] [Google Scholar]
Yoon 2001
- Yoon HG, Yoon SH, Son WY, Lee SW, Park SP, Im KS, Lim jH. Pregnancies resulting from in vitro matured oocytes collected from women with regular menstrual cycle. Journal of Assisted Reproduction and Genetics 2001;18(6):325‐9. [PUBMED: 11495408] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Siristatidis 2009
- Siristatidis CS, Maheshwari A, Bhattacharya S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006606.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siristatidis 2013
- Siristatidis CS, Vrachnis N, Creatsa M, Maheshwari A, Bhattacharya S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD006606.pub3] [DOI] [PubMed] [Google Scholar]


