Abstract
Background
Adults in intensive care units (ICUs) often suffer from a lack of sleep or frequent sleep disruptions. Non‐pharmacological interventions can improve the duration and quality of sleep and decrease the risk of sleep disturbance, delirium, post‐traumatic stress disorder (PTSD), and the length of stay in the ICU. However, there is no clear evidence of the effectiveness and harms of different non‐pharmacological interventions for sleep promotion in adults admitted to the ICU.
Objectives
To assess the efficacy of non‐pharmacological interventions for sleep promotion in critically ill adults in the ICU.
To establish whether non‐pharmacological interventions are safe and clinically effective in improving sleep quality and reducing length of ICU stay in critically ill adults.
To establish whether non‐pharmacological interventions are cost effective.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 6), MEDLINE (OVID, 1950 to June 2014), EMBASE (1966 to June 2014), CINAHL (Cumulative Index to Nursing and Allied Health Literature, 1982 to June 2014), Institute for Scientific Information (ISI) Web of Science (1956 to June 2014), CAM on PubMed (1966 to June 2014), Alt HealthWatch (1997 to June 2014), PsycINFO (1967 to June 2014), the China Biological Medicine Database (CBM‐disc, 1979 to June 2014), and China National Knowledge Infrastructure (CNKI Database, 1999 to June 2014). We also searched the following repositories and registries to June 2014: ProQuest Dissertations & Theses Global, the US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov), the metaRegister of Controlled Trials (ISRCTN Register) (www.controlled‐trials.com), the Chinese Clinical Trial Registry (www.chictr.org.cn), the Clinical Trials Registry‐India (www.ctri.nic.in), the Grey Literature Report from the New York Academy of Medicine Library (www.greylit.org), OpenGrey (www.opengrey.eu), and the World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch). We handsearched critical care journals and reference lists and contacted relevant experts to identify relevant unpublished data.
Selection criteria
We included all randomized controlled trials (RCT) and quasi‐RCTs that evaluated the effects of non‐pharmacological interventions for sleep promotion in critically ill adults (aged 18 years and older) during admission to critical care units or ICUs.
Data collection and analysis
Two authors independently screened the search results and assessed the risk of bias in selected trials. One author extracted the data and a second checked the data for accuracy and completeness. Where possible, we combined results in meta‐analyses using mean differences and standardized mean differences for continuous outcomes and risk ratios for dichotomous outcomes. We used post‐test scores in this review.
Main results
We included 30 trials, with a total of 1569 participants, in this review. We included trials of ventilator mode or type, earplugs or eye masks or both, massage, relaxation interventions, foot baths, music interventions, nursing interventions, valerian acupressure, aromatherapy, and sound masking. Outcomes included objective sleep outcomes, subjective sleep quality and quantity, risk of delirium, participant satisfaction, length of ICU stay, and adverse events. Clinical heterogeneity (e.g., participant population, outcomes measured) and research design limited quantitative synthesis, and only a small number of studies were available for most interventions. The quality of the evidence for an effect of non‐pharmacological interventions on any of the outcomes examined was generally low or very low. Only three trials, all of earplugs or eye masks or both, provided data suitable for two separate meta‐analyses. These meta‐analyses, each of two studies, showed a lower incidence of delirium during ICU stay (risk ratio 0.55, 95% confidence interval (CI) 0.38 to 0.80, P value = 0.002, two studies, 177 participants) and a positive effect of earplugs or eye masks or both on total sleep time (mean difference 2.19 hours, 95% CI 0.41 to 3.96, P value = 0.02, two studies, 116 participants); we rated the quality of the evidence for both of these results as low.
There was also some low quality evidence that music (350 participants; four studies) may improve subjective sleep quality and quantity, but we could not pool the data. Similarly, there was some evidence that relaxation techniques, foot massage, acupressure, nursing or social intervention, and sound masking can provide small improvements in various subjective measures of sleep quality and quantity, but the quality of the evidence was low. The effects of non‐pharmacological interventions on objective sleep outcomes were inconsistent across 16 studies (we rated the quality of the evidence as very low): the majority of studies relating to the use of earplugs and eye masks found no benefit; results from six trials of ventilator modes suggested that certain ventilator settings might offer benefits over others, although the results of the individual trials did not always agree with each other. Only one study measured length of stay in the ICU and found no significant effect of earplugs plus eye masks. No studies examined the effect of any non‐pharmacological intervention on mortality, risk of post‐traumatic stress disorder, or cost‐effectiveness; the included studies did not clearly report adverse effects, although there was very low quality evidence that ventilator mode influenced the incidence of central apnoeas and patient‐ventilator asynchronies.
Authors' conclusions
The quality of existing evidence relating to the use of non‐pharmacological interventions for promoting sleep in adults in the ICU was low or very low. We found some evidence that the use of earplugs or eye masks or both may have beneficial effects on sleep and the incidence of delirium in this population, although the quality of the evidence was low. Further high‐quality research is needed to strengthen the evidence base.
Plain language summary
Non‐drug treatments for promoting sleep in adults in the intensive care unit
Review question
We reviewed the evidence on non‐pharmacological interventions (i.e. non‐drug treatments) for improving sleep in critically ill adults.
Background
Sleep is essential to enable adults in the intensive care unit (ICU) to recover from their illnesses. However, adults in the ICU often suffer from frequently disturbed sleep or a lack of sleep. The reasons for sleep disruption may include the underlying illness, uncomfortable therapy, psychological stress, or the ICU environment itself.
Interventions for sleep promotion include pharmacological treatments and non‐pharmacological interventions. Medications may produce side effects, such as a reduced ability to think clearly and negative effects on breathing, and they can also interfere with normal sleep patterns and lead to a risk of tolerance or drug dependency . Therefore, non‐pharmacological interventions, such as noise reduction, music therapy, alternative and complementary therapies, and social support, have been sought and are recommended for improving sleep in critically ill adults.
Search date
The evidence is current to June 2014.
Study characteristics
We found 30 trials, with a total of 1569 participants, and the interventions included changes to ventilator type and settings, earplugs and eye masks, relaxation therapy, sleep‐inducing music, massage, foot baths, aromatherapy, valerian acupressure, sound masking, and changing the visiting times of family members. We assessed the effects of these interventions on sleep outcomes (e.g., quality and amount of sleep), length of stay in the ICU, the occurrence of delirium, other adverse events, and death.
Key results and quality of evidence
Overall, the quality of the evidence for an effect of the interventions on any of the outcomes was low or very low. Normally, we would try to pool findings from similar trials of each intervention, but this was difficult because the design of the trials varied greatly. We were able to combine the results from three trials of earplugs and eye masks and found that their use increased the number of hours slept and prevented delirium in adults in the ICU. However, we cannot be certain about these findings because of problems with how the trials were carried out.
There was also some low quality evidence from four studies that music may improve subjective sleep quality and quantity, but we could not pool the data. Similarly, a low number of studies found that relaxation techniques, foot massage, acupressure, nursing or social intervention, and sound masking can provide small improvements in participant‐reported or nurse‐assessed sleep quality and quantity, but the quality of the evidence was low. The effects of the interventions on objective sleep outcomes (e.g., sleep measured by a machine) varied: the majority of studies that looked at the use of earplugs and eye masks found no benefit, and although the results from six trials of ventilator modes suggested that certain ventilator settings might offer benefits over others, the results of the individual trials did not always agree with each other. Only one study measured length of stay in the ICU and found no significant effect of earplugs plus eye masks. None of the included studies looked at economic outcomes, risk of post‐traumatic stress disorder, or deaths. The trials did not clearly report adverse effects, although there was very low quality evidence that ventilator mode might influence certain adverse effects that can happen when people are on a ventilator. In summary, further well‐designed and conducted research is needed to strengthen the evidence for the use of these interventions for improving sleep in critically ill adults.
Summary of findings
Summary of findings for the main comparison. Non‐pharmacological interventions for sleep promotion in ICU patients ‐ narrative summary.
| Non‐pharmacological interventions for sleep promotion versus usual care/no intervention | |||
| Patient or population: critically ill patients Settings: ICU Intervention: various non‐pharmacological interventions for sleep promotion Comparison: standard care or no intervention | |||
| Outcomes | Impact | Number of participants (studies)* | Quality of the evidence (GRADE) |
|
Changes in objective sleep variables (SEI, SFI, REM sleep) |
The evidence relating to effect of ventilator mode (89 participants; 6 studies) or type (40 participants; 2 studies) on objective sleep variables was inconsistent. The evidence relating to the use of earplugs or eye masks or both was also inconsistent (141 participants; 4 studies), with the majority of studies finding no benefit for this intervention type There was no evidence for an effect of relaxation via foot baths on objective sleep variables (6 participants; 1 study). There was no consistent effect of music intervention on objective sleep variables (58 participants; 2 studies). Only 1 study (69 participants) examined the effects of relaxation techniques on objective sleep variables, although a positive effect on SEI was noted |
403 (16 studies) |
⊕⊝⊝⊝ VERY LOW²,³ |
| Length of ICU stay | No effect of a combination of earplugs, eye mask, and sleep‐inducing music (45 participants; 1 study) was noted on length of ICU stay. No studies examined the effect of the other non‐pharmacological interventions on this outcome | 45 (1 study) |
⊕⊝⊝⊝ VERY LOW³,⁴,⁵ |
|
Subjective sleep quality or quantity |
No trials examined the effect of ventilator mode or type on subjectively measured sleep quality or quantity. Using various scales, 6 studies (395 participants) individually reported some benefit of earplugs or eye masks or both on subjective sleep quality; pooled analyses from 2 of these studies (116 participants) showed a benefit for the use of earplugs/eye masks compared with usual care. The mean difference in total sleep quantity versus usual care was 2.19 hours (95% CI 0.41 to 3.96) although evidence of heterogeneity was observed (I² statistic = 79%) There was some evidence that music (350 participants; 4 studies) may improve subjective sleep quality and quantity, but we could not pool the data. Similarly, there was some evidence that relaxation techniques (102 participants; 2 studies), foot massage (110 participants; 2 studies), acupressure (85 participants; 1 study), nursing or social intervention (158 participants; 2 studies), and sound masking (40 participants; 1 study) can provide small improvements in various subjective measures of sleep quality and quantity. Aromatherapy (25 participants; 1 study) was not found to influence subjective sleep quality |
1220 (18 studies)¹ |
⊕⊕⊝⊝ LOW³,⁶ |
| Risk of delirium | Data from 2 studies (177 participants) were pooled and showed a benefit of earplugs or eye masks or both versus usual care on the risk of delirium: the relative risk was 0.55 (95% CI 0.38 to 0.80). Assumed risk¶ was 489 per 1000 people, and the intervention reduced this risk to 269 per 1000 people (95% CI 186 to 391). No studies of other non‐pharmacological interventions assessed this outcome | 177 (2 studies) |
⊕⊕⊝⊝ LOW³,⁵ |
| Any adverse event | There was some evidence (72 participants; 5 studies) that ventilator mode influenced the incidence of adverse events, such as central apnoeas and patient‐ventilator asynchronies: more adverse events were noted with PSV compared with ACV and PAV. No studies examined the effect of non‐pharmacological interventions on other adverse events (including PTSD) | 72 (5 studies) |
⊕⊝⊝⊝ VERY LOW³,⁵,⁷ |
| Mortality | None of the included studies examined the effect of non‐pharmacological interventions for sleep promotion on the incidence of mortality | NA | NA |
| Economic outcomes | None of the included studies examined the cost effectiveness or health economic effects of non‐pharmacological interventions for sleep promotion in ICU patients | NA | NA |
|
*Number of participants refers to the number of participants analysed in each study. ¶The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACV: assist‐control ventilation;CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation; ICU: intensive care unit; NA: non applicable;PAV: proportional assist ventilation; PSV: pressure support ventilation; PTSD: post‐traumatic stress disorder; RR: risk ratio; SEI: Sleep Efficiency Index; REM: rapid eye movement sleep; SFI: sleep fragmentation index. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
General note: we assessed the effect of several interventions on each outcome; therefore, in some instances, the factors resulting in downgrading of the evidence varied by intervention type for a given outcome.
¹Hu 2010 contributed data on the use of eye masks/earplugs as well as music as non‐pharmacological interventions; the study and its 45 analysed participants were counted once towards the total number of studies and participants for this outcome. ²Evidence downgraded by 2 points (‐2) for inconsistency. Although we could not perform meta‐analysis because of clinical heterogeneity, reported treatment effects varied between individual studies. ³Evidence downgraded by 1 point (‐1) for risk of selection bias. We rated a number of the studies contributing evidence as at an unclear or high risk of selection bias. ⁴Evidence downgraded by 1 point (‐1) for indirectness as only a single study contributed data, and evidence was therefore based on a single patient population. ⁵Evidence downgraded by 1 point (‐1) for imprecision as the confidence intervals were wide. ⁶Evidence downgraded by 1 point (‐1) for either indirectness (data relevant to a single study population), inconsistency (findings across individual studies varied), or imprecision (confidence intervals were wide). ⁷Evidence downgraded by 1 point (‐1) for indirectness as only studies of ventilator mode or type were considered, so the evidence would be unlikely to be applicable to other intervention types (e.g., eye masks).
Background
Description of the condition
Sleep is a basic need for human survival and is essential for the recovery of critically ill adults. Normal human sleep is generally categorized as two states: non‐rapid eye movement (NREM) and rapid eye movement (REM), which alternate cyclically across a sleep episode. The American Academy of Sleep Medicine Scoring Manual (SiIber 2007) further subdivides NREM sleep into stages one to three. Sleep begins in NREM stage one (N1) and progresses through the deeper NREM stage two (N2) to NREM stage three (N3), which is also called delta sleep or slow‐wave sleep (SWS). A progressive increase in the threshold required for arousal (e.g., by noise) accompanies the progression of sleep from stage N1 through to stage N3. NREM sleep normally cycles with REM sleep approximately every 90 minutes. Normally, REM sleep accounts for about 25% of sleep time, and adults spend up to 50% of the night in stage N2 sleep.
Adults in intensive care units (ICUs) often suffer from a lack of sleep or frequent sleep disruptions (Gabor 2003; Meyer 1994). Both subjective and objective studies have demonstrated significant sleep disruption in critically ill patients (Freedman 1999; Freedman 2001; Friese 2007; Gabor 2001; Parthasarathy 2004; Simini 1999). In one study, as many as 38% of ICU patients experienced difficulty in falling asleep, and 61% reported shorter periods of sleep than usual (Orwelius 2008). Several studies using polysomnography (PSG) have consistently demonstrated that the sleep of ICU patients is characterized by sleep fragmentation, poor sleep efficiency, an increase in light sleep, and a decrease in both REM sleep and SWS (Cooper 2000; Freedman 2001; Friese 2007). Moreover, about 50% of sleep occurs during the day in ICU patients (Cooper 2000; Freedman 2001; Gabor 2003; Hardin 2006).
PSG represents the gold standard for techniques used to monitor sleep and is the only method to identify the individual sleep stages. However, many centres lack the facilities required for PSG (in terms of equipment and staff). Therefore, some studies (especially those performed in critical care units) have adopted other techniques for measuring sleep, such as actigraphy, Bispectral Index (BIS) monitoring, and nurse/patient assessment (Le Guen 2014; Jaber 2007). An ActiGraph is a small wristwatch device that can monitor whether a patient is asleep or awake based on the levels of patient wrist motor activity. ActiGraphs have been used in studies of sleep and circadian rhythms in ICU patients. However, actigraphy does not provide any information regarding either the stage or quality of sleep and tends to overestimate total sleep time compared with PSG and BIS. BIS is calculated from multiple analyses of the raw electroencephalography (EEG) waveform that is capable of detecting sleep, but the overlap of BIS values between given sleep stages currently prevents its use as a depth‐of‐sleep monitor (Nieuwenhuijs 2006). Furthermore, BIS values potentially provide an inaccurate indication of patients' sleep characteristics when patients have neurological abnormalities. ICU studies have often used subjective measurements of sleep: several visual analogue scales (VAS), such as the Verran/Snyder‐Halpern Sleep Scale (VSH) and the Richardson‐Campbell Sleep Questionnaire (RCSQ), have been developed and used to assess patients' sleep perception. The RCSQ score accounted for approximately 33% of the variance in the PSG indicator Sleep Efficiency Index (SEI) in one critical care group (Richards 2000). However, a problem with VAS scales is that patients may be incapable of completing the questionnaire; one study excluded half of the recruited participants because of unconsciousness or delirium (Frisk 2003).
The reasons for sleep disruption are multifactorial and include underlying illness, uncomfortable therapy, psychological stress, age‐related changes in sleep patterns, pain, mechanical ventilation, and the ICU environment (Drouot 2008; Friese 2008; Weinhouse 2006; Weinhouse 2009). Environmental stimuli are thought to be important factors. Light, noise, patient‐care activities, and physician interventions all contribute to sleep deprivation; noise and patient‐care activities are thought to account for approximately 30% of observed sleep disruption (Gabor 2003). Continuous exposure to light can also disrupt the patient's naturally occurring circadian rhythms (Czeisler 1986).
There are several adverse consequences of sleep disruption, which may include an impaired immune function (Benca 1997), reduced inspiratory muscle endurance (Chen 1989), an altered weaning process (Pandharipande 2006), a degeneration in the quality of life (Dignani 2015), and prolonged neurocognitive dysfunction (O'Donoghue 2012). Importantly, these adverse consequences may be associated with ICU delirium and severe morbidity (Eddleston 2000; Novaes 1999; Pun 2007; Weinhouse 2006).
Interventions for sleep promotion involve both pharmacological treatment and non‐pharmacological interventions. Generally, pharmacological therapies are used for the treatment of sleep disturbances (Abad 2015). Pharmacological agents that induce sleep provide sedation and analgesia and are commonly used in the ICU setting. However, pharmacological interventions have potential side effects, including impaired cognitive function, risk of tolerance or dependency, depressed ventilation, and adversely affected normal sleep physiology (Mistraletti 2008). For example, benzodiazepines, opiates, or barbiturates disrupt normal sleep patterns and decrease REM activity and stage 3 sleep (Achermann 1987; Cronin 2001), whereas propofol leads to slow‐wave activity that mimics slow‐wave sleep and modifies circadian rhythms (Ozone 2000). Therefore, sedation in the ICU is both a cause and a potential treatment for sleep disruption in ICU patients (Weinhouse 2009). Additionally, induction of sleep by drugs is contraindicated in certain patient groups, such as non‐ventilated patients suffering from hypercapnic lung disease (Shilo 1999). Therefore, non‐pharmacological interventions have been sought, and a multifaceted approach is recommended to improve the sleep of critically ill patients (Jacobi 2002). In general, the efficacy of non‐pharmacological interventions for improving sleep has been considered to be less than pharmacological methods while having no risk of drug‐related tolerance or dependency (Hauri 1997; McClusky 1991).
Description of the intervention
A wide range of non‐pharmacological interventions have been used to improve sleep in ICU patients. These can be broadly categorized as follows: psychological (cognitive or behavioural) interventions, complementary therapies (e.g., music therapy, aromatherapy, massage, guided imagery, acupressure), environmental interventions (e.g., synchronization of ICU activities with daylight, noise reduction), social interventions (e.g., family support), and equipment modification (e.g., optimizing ventilator modes or ventilator types). Cognitive behavioural therapy (CBT) has been used to treat insomnia in the ambulant setting by changing poor sleep habits and prompting sleep hygiene practices (Gałuszko‐Węgielnik 2012). A meta‐analysis of 224 participants (aged > 60 years) who experienced insomnia in an ambulant setting indicated a mild effect of CBT for sleep problems and was best used for sleep maintenance insomnia (Montgomery 2003).
How the intervention might work
Complementary therapies, such as massage, music therapy, therapeutic touch, aromatherapy, relaxation, and mental imaginary, seem to comfort and reduce levels of stress and anxiety in critically ill patients, which in turn is likely to lead to improved sleep (Richards 2003). A combination of relaxation and imagery may be effective in improving the sleep of the critically ill adult (Richards 2003). Environmental interventions, such as reducing noise, controlling lighting, playing white noise, and adequate uninterrupted time for sleep, are safe and logical interventions to help patients sleep (Richards 2003). Several studies found that the use of earplugs and eye masks as methods of noise reduction and light control improved sleep quality (Koo 2008; Richardson 2007; Scotto 2009). Optimising modes of mechanical ventilation may also facilitate sleep, as some modes have been found to cause less arousals and awakenings per hour (Cabello 2008; Friese 2008; Parthasarathy 2004). However, the use of such non‐pharmacological interventions in critical care needs to take account of environmental and patient considerations. Interventions must be easy to implement (i.e., practical) and must not harm or diminish patient safety.
Why it is important to do this review
Several systematic reviews have highlighted benefits of non‐pharmacological interventions for improving sleep in different patient populations. Previous systematic reviews have assessed the efficacy of valerian and exogenous melatonin for improving sleep (Bent 2006; Buscemi 2005). Similarly, previous Cochrane reviews have examined the effects of bright light therapy, cognitive behavioural therapy, and acupuncture in improving sleep quality in patients with insomnia or elderly people (Cheuk 2012; Montgomery 2002; Montgomery 2003). However, there remains little clear evidence of the effectiveness of non‐pharmacological interventions for improving sleep quality in critically ill patients residing in critical care units. An earlier systematic review examined the effects of massage on relaxation, comfort, and sleep in acute and critical care settings and concluded that the existing clinical data at that time were insufficient and further studies were required (Richards 2000a). A subsequent review of complementary and alternative therapies to promote sleep in critically ill patients concluded that techniques to promote sleep through muscle relaxation might be difficult for critically ill patients because of the need for patients to be conscious to receive the therapy. The review also reported that interventions such as music therapy, environmental interventions, therapeutic touch, and relaxing massage appeared to be safe but that further randomized controlled trials were required to assess efficacy (Richards 2003). Therefore, it was important to perform this review, which examined recent studies, particularly as there remains little guidance on the potential efficacy and harms of these interventions for adult patients in the critical care unit.
Objectives
To assess the efficacy of non‐pharmacological interventions (Appendix 1) for sleep promotion in critically ill adult patients in the ICU.
To establish whether non‐pharmacological interventions are safe and clinically effective in improving sleep quality and reducing length of ICU stay in critically ill adults.
To establish whether non‐pharmacological interventions are cost effective.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs that evaluated the effects of non‐pharmacological interventions for sleep promotion in critical care units (CCU) or intensive care units (ICUs) for critically ill adult participants (aged 18 years and older).
We included all studies, published or unpublished, in any language.
Types of participants
Critically ill adult patients with stable haemodynamic status who were admitted to ICUs or critical care units and had a length of stay of more than 24 hours. We included studies of surgical or non‐surgical patients with or without mechanical ventilation. We imposed no restrictions on gender or ethnicity. We excluded studies enrolling participants who were diagnosed with obstructive sleep apnoea or dementia or those who were terminally ill or required palliative care.
Types of interventions
We included any non‐pharmacological intervention for improving sleep, such as those that examined one or a combination of interventions, and compared them with different non‐pharmacological interventions, pharmacological interventions (e.g., sedation), or standard care (e.g., routine nursing care).
We included the following types of non‐pharmacological interventions:
psychological (cognitive or behavioural) interventions, such as music therapy, back massage, muscle relaxation, imagery, and therapeutic touch;
environmental interventions, such as noise reduction, lighting control, and synchronization of ICU activities with daylight;
social support interventions;
equipment modification, including mechanical ventilation;
complementary and alternative therapies: aromatherapy, herbs, acupuncture; and
physical therapy modalities.
Types of outcome measures
Primary outcomes
Changes in objective sleep variables (as measured by polysomnography, ActiGraph, or Bispectral Index), including Sleep Efficiency Index (SEI), rapid eye movement (REM) sleep time, REM sleep latency, and sleep fragmentation index.
Length of ICU stay.
Mortality.
Secondary outcomes
Any adverse reactions or events.
Risk of delirium during ICU stay.
Changes in subjective sleep quality or quantity, measured by participant report or medical or nursing observation.
Risk of post‐traumatic stress disorder (PTSD) once discharged from hospital.
Participant satisfaction (as reported by the study authors).
Economic outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 6), 2014, Issue 6) (Appendix 2), MEDLINE (OVID, 1950 to June 2014) (Appendix 3), EMBASE (1966 to June 2014), CINAHL (Cumulative Index to Nursing and Allied Health Literature, 1982 to June 2014), Institute for Scientific Information (ISI) Web of Science (1956 to June 2014) (Appendix 4) , CAM on PubMed (1966 to June 2014), Alt HealthWatch (1997 to June 2014), PsycINFO (1967 to June 2014), the China Biological Medicine Database (CBM‐disc, 1979 to June 2014), and China National Knowledge Infrastructure (CNKI Database, 1999 to June 2014).
We searched for relevant ongoing trials up to June 2014 using the following websites.
The World Health Organization International Clinical Trials Registry platform (WHO ICTRP) (www.who.int/trialsearch) ‐ four WHO ICTRP Primary Registers.
Chinese Clinical Trial Registry (www.chictr.org.cn).
The metaRegister of Controlled Trials (ISRCTN Register) (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
Clinical Trials Registry‐India (www.ctri.nic.in).
We searched for grey literature using the following websites.
OpenGrey (www.opengrey.eu).
Grey Literature Report from the New York Academy of Medicine Library (www.greylit.org).
ProQuest Dissertations & Theses Global (www.search.proquest.com).
We modified the MEDLINE search strategy to search the other databases (Appendix 3).
Searching other resources
We handsearched appropriate journals and abstracts of relevant conference proceedings. We searched the reference lists of all retrieved articles. We did not limit the search by language or publication status.
We handsearched the following journals:
Critical Care Medicine (1995 to May 2014);
Critical Care (1997 to May 2014);
Journal of Critical Care (1995 to May 2014); and
American Journal of Respiratory and Critical Care Medicine (1995 to May 2014).
Data collection and analysis
Selection of studies
Two authors (HRF, CXY) independently examined the titles and abstracts identified from the search. We retrieved and evaluated the full text of potentially relevant studies. Two authors (HRF, ZZY) independently assessed their eligibility according to our inclusion and exclusion criteria, resolving any disagreements by discussion. A third author (CJM) settled any disagreements. Where appropriate, we corresponded with study authors by telephone or by email to clarify study eligibility. We recorded reasons for study exclusion in the 'Characteristics of excluded studies' tables.
Data extraction and management
Two authors (HRF, XHN) independently extracted data using a tool developed by the authors (Appendix 5). We resolved any disagreements by discussion with a third author (CJM). Two review authors entered the data into Review Manager software (RevMan 5.3), and a third author (JXY) checked the data.
Assessment of risk of bias in included studies
Two authors (HRF, LYP) independently assessed the quality of all included trials as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the methodological quality of all trials on the basis of the following six domains:
random sequence generation;
allocation concealment;
blinding of participants, personnel, and outcome assessors;
incomplete outcome data;
selective reporting; and
other sources of validity.
Measures of treatment effect
We calculated mean differences (MDs) with 95% confidence intervals (CI) for continuous data and standardized mean differences (SMDs) for outcome measures using results from different scales. Where possible, we obtained standard deviations from standard errors and confidence intervals. We analysed longer ordinal scales as continuous data. We combined adjacent categories together and made them into dichotomous data for trichotomous‐type outcomes. Where trichotomous‐type outcomes were summarized using methods for dichotomous data, we used risk ratios (RR) with 95% CIs to describe the intervention effect. We estimated heterogeneity using the I² statistic (Higgins 2011). In the case of significant clinical heterogeneity, we did not pool results.
Unit of analysis issues
We included both parallel and cross‐over randomized controlled trials. The participants in each intervention arm were the unit of analysis in a single parallel group design. According to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), the recommended method for including multiple groups from one study is to combine all relevant experimental intervention groups from the study into a single group and combine all relevant control intervention groups into a single control group. Although we found an orphan study with more than a two‐arm parallel intervention group and some cross‐over trials with more than two intervention groups in this review, we could not include them in a meta‐analysis. Considering the presence of carry‐over, we had planned to analyse the data from only the first period in cross‐over RCTs. However, only two cross‐over RCTs reported data from the first period and the cross‐over period, whereas the remaining studies only reported the whole period data. Thus, we took the decision to exclude cross‐over studies from the meta‐analyses.
Dealing with missing data
Whenever possible, we contacted the trial authors to request missing data. We calculated missing statistics (such as standard deviations or correlation coefficients) from other statistics, such as the standard error or confidence intervals.
Assessment of heterogeneity
We firstly explored clinical heterogeneity by assessing the clinical and methodological characteristics of the included studies (for example, trial design, participant characteristics, intervention, or outcome measurement). If we pooled data from multiple studies, we formally assessed heterogeneity using the I² statistic (Higgins 2011) and by visual inspection of the forest plots. We considered a Chi² statistic with a P value < 0.10 or an inconsistency between studies (I² statistic) greater than 50% as evidence of relevant heterogeneity.
Assessment of reporting biases
We assessed the scope for reporting bias by the absence of primary outcomes and by less detailed reporting of non‐significant outcomes. Due to the small number of studies included in each category, we did not perform funnel plots for publication bias.
Data synthesis
We anticipated that studies would use different scales to measure the same outcomes. We calculated standardized mean differences (SMDs) from different scales. We made the following intervention comparisons using meta‐analyses: use of earplugs or eye masks or both versus no use of earplugs or eye masks. We had planned to include the following additional treatment comparisons, but there were insufficient trials to do so, or the available trials had important clinical heterogeneity among them: acupressure versus other interventions or placebo, aromatherapy versus other interventions or placebo, back massage versus other interventions or placebo, foot baths versus other interventions or placebo, relaxation and imagery versus other interventions or placebo, foot massage versus other interventions or placebo, using sound masking versus other interventions or placebo, and social support intervention versus other interventions or placebo. Therefore, we included trials comparing these interventions with other therapies or placebo in the narrative but not the meta‐analysis of this review.
Subgroup analysis and investigation of heterogeneity
We had planned to explore the following subgroups:
age;
sex;
interventions (different methods, different duration, or difference frequency); and
trial quality (e.g., RCT and quasi‐RCT).
However, since we only pooled two studies for each meta‐analysis in this review, we did not perform subgroup analyses (see Differences between protocol and review).
Sensitivity analysis
We did not perform sensitivity analyses due to the small number of studies included in each group (see Differences between protocol and review).
'Summary of findings' tables
We used the principles of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes. Because of the number of interventions considered, the heterogeneity between studies, and the lack of meta‐analyses, we provided a narrative summary of findings: Table 1.
Results
Description of studies
Please see the 'Characteristics of included studies' tables; the 'Characteristics of excluded studies' tables; the 'Characteristics of studies awaiting classification' tables; and the 'Characteristics of ongoing studies' tables.
Results of the search
Please see Figure 1.
1.
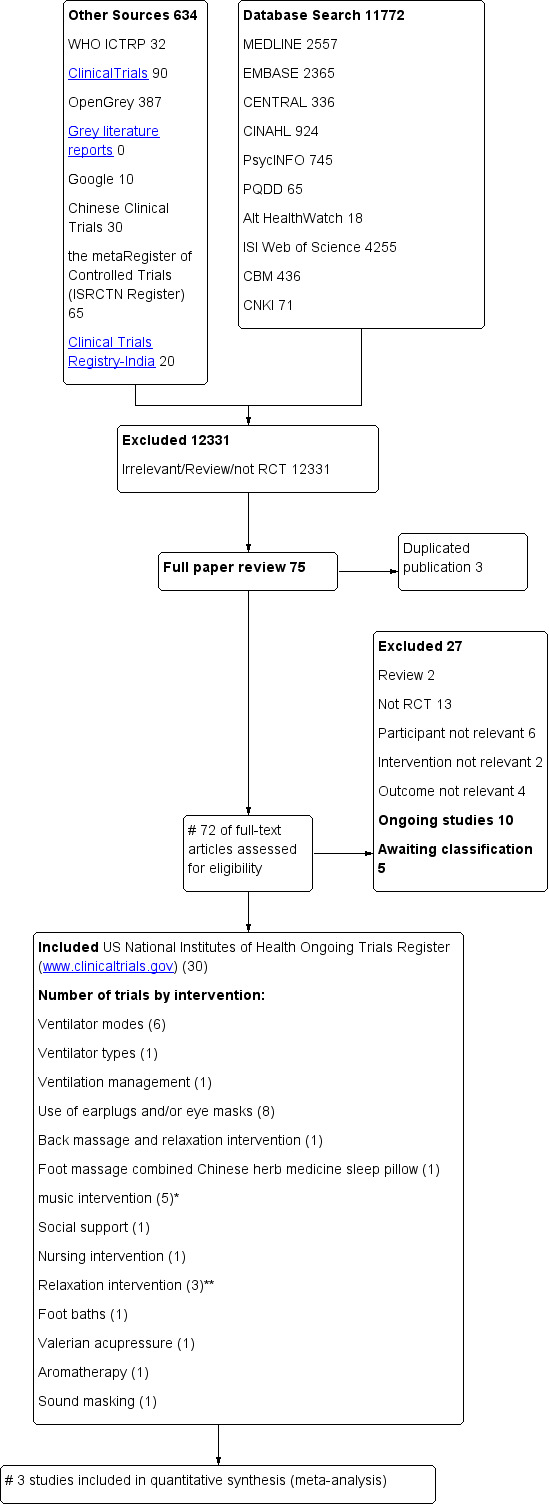
Study flow diagram. CBM = China Biological Medicine Database; CENTRAL = Cochrane Central Register of Controlled Trials; CINAHL = Cumulative Index to Nursing and Allied Health Literature; CNKI = China National Knowledge Infrastructure; ISI = Institute for Scientific Information; PQDD = ProQuest Dissertations & Theses Global; RCT = randomized controlled trial; WHO ICTRP = the World Health Organization International Clinical Trials Registry platform. *One trial examined music intervention and eye mask/earplugs and is counted under both categories. ** One trial examined relaxation interventions and back massage and is counted under both categories.
We identified 72 potentially relevant studies and retrieved them for further assessment. We included 30 studies (see the 'Characteristics of included studies' tables). We contacted the authors of five studies, Alexopoulou 2007; Andréjak 2013; Bosma 2007; Richards 1998; Wallace 1998, by email and retrieved details of study methods and data from them.
We excluded a total of 27 studies that did not meet the inclusion criteria (see the 'Characteristics of excluded studies' tables for detailed descriptions).
Ten trials registered on the US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) are ongoing (see the 'Characteristics of ongoing studies' tables for detailed descriptions), and five studies are awaiting classification (see the 'Characteristics of studies awaiting classification' tables).
Included studies
In this review, we included 30 randomized controlled trials, with 1569 participants; 12 trials using cross‐over design; and 18 trials using parallel group design. There were 29 randomized trials and one quasi‐randomized trial. Eight trials were conducted in China, one was conducted in Korea, one was conducted in Japan, 11 were conducted in Europe, and nine were conducted in the United States (see the 'Characteristics of included studies' tables for detailed descriptions).
Participants
The number of participants per study ranged from a minimum of six to a maximum of 136. Ten trials included ventilated participants (Alexopoulou 2007; Andréjak 2013; Bosma 2007; Cabello 2008; Córdoba‐Izquierdo 2013; Hu 2010; Jaber 2007; Parthasarathy 2002; Roche‐Campo 2013; Wallace 1998); most of these studies ventilated participants through an endotracheal tube or tracheostomy, and only one of these trials, Córdoba‐Izquierdo 2013, used non‐invasive ventilation. One study included both ventilated participants and non‐ventilated participants (Jaber 2007). Nine studies reported trials that were conducted in single‐bed rooms in the critical care unit (Alexopoulou 2007; Andréjak 2013; Borromeo 1998; Gragert 1990; Richards 1998; Richardson 2003; Su 2013; Toublanc 2007; Wallace 1998). Seven trials were conducted in coronary care units (Borromeo 1998; Gao 2008; Gragert 1990; Li 2011; Richards 1998; Ryu 2012; Wang 2012), one was performed in a cardiac surgical intensive care unit (Hu 2010), two were performed in a medicosurgical department of anaesthesia and resuscitation (Jaber 2007; Le Guen 2014), one was performed in a respiratory intensive care unit (ICU) (Toublanc 2007), one was performed in a pulmonary and critical care unit (Parthasarathy 2002), and the remaining studies were performed in medical ICUs.
Thirteen studies, Andréjak 2013; Bosma 2007; Córdoba‐Izquierdo 2013; Foreman 2013; Gao 2008; Hu 2010; Le Guen 2014; Parthasarathy 2002; Ruan 2006; Su 2013; Sha 2013; Toublanc 2007; Wang 2012, reported that baseline characteristics did not differ significantly between the groups.
Interventions
We included six trials of ventilator mode, eight trials using earplugs or eye masks or both, five trials of music interventions (which included one trial, Hu 2010, using earplugs and eye masks combined with music intervention), three trials of relaxation and imagery (which included one trial of back massage and relaxation intervention (Richards 1998)), one trial of back massage and relaxation intervention, one trial of foot massage combined with the use of a Chinese herb sleep pillow (Wang 2012), one trial of a foot bath intervention (Namba 2012), one trial of social support intervention through changing the ICU visit time for family members (Gao 2008), one trial of a nursing intervention (Li 2011), one trial of valerian acupressure (Chen 2012), one trial of ventilator type (Córdoba‐Izquierdo 2013), one trial of receiving mechanical versus spontaneous ventilation (Roche‐Campo 2013), one trial of aromatherapy (Borromeo 1998), and one trial of sound masking (using USASI noise, namely a continuous sound occurring at the same level over a long period) (Gragert 1990).
The interventions included in this review were heterogeneous with respect to components, methods, content, and intensity of use. The duration of the interventions ranged from 10 minutes, Chen 2012, to seven days (Wang 2012). Most cross‐over trials had no washout period between intervention periods (Alexopoulou 2007; Andréjak 2013; Bosma 2007; Cabello 2008; Jaber 2007; Martin 2008; Parthasarathy 2002; Roche‐Campo 2013; Toublanc 2007); only two trials used a washout period (Borromeo 1998; Namba 2012).
1. Optimizing ventilator mode, type, or management strategy
Six trials examined the effect of ventilator mode on sleep, namely three trials of assist‐control ventilation (ACV) versus pressure support ventilation (PSV) (Cabello 2008; Parthasarathy 2002; Toublanc 2007), two trials of proportional assist ventilation (PAV) versus PSV (Alexopoulou 2007; Bosma 2007), and one trial of pressure‐controlled ventilation (PCV) versus low PSV (Andréjak 2013).
One trial, Córdoba‐Izquierdo 2013, examined the effect of optimizing ventilator type on sleep.
One trial, Roche‐Campo 2013, examined the effect of mechanical versus spontaneous ventilation on sleep.
2. Earplugs or eye masks or both
We included eight trials using earplugs or eye masks or both. Four of these trials compared the use of earplugs versus no use of earplugs during regular night‐time sleeping hours (Martin 2008; Scotto 2009; Van Rompaey 2012; Wallace 1998). One trial compared the use of earplugs and eye masks combined with sleep‐inducing music versus no use of earplugs, no eye masks, and no music (Hu 2010). Two trials compared the use of earplugs and eye masks versus no use of earplugs and eye masks during night‐time (Le Guen 2014; Xie 2011). One trial compared oral melatonin, sound‐reducing headphones, and eye covers versus standard care (Foreman 2013). The duration of the interventions varied from one night, Le Guen 2014; Martin 2008; Scotto 2009; Wallace 1998, to four nights (Van Rompaey 2012).
3. Music intervention
Five studies included in this review used music intervention with sleep‐inducing or relaxing music, but the methods of the interventions, frequency and duration of music listening, and methods in the control group varied greatly between these trials. One trial compared earplug‐delivered sleep‐inducing music for 52 minutes versus control group (no music, but earplugs and eye shield worn) (Ryu 2012). One study compared a 45‐minute music‐listening intervention versus usual care without music (Su 2013). One trial combined the use of earplugs and eye masks with music listening versus no use of earplugs or eye masks and no music (Hu 2010). (We also counted this study under the eye mask/earplug category.) One trial compared a 20‐minute relaxing music therapy versus sitting and uninterrupted resting (Jaber 2007). One trial compared an individualized music intervention (12.30 p.m. to 1.30 p.m. and 8.30 p.m. to 9.30 p.m.) versus usual care during the period of ICU stay (Sha 2013).
4. Relaxation techniques
Three trials used relaxation techniques: Richardson 2003 used a combination of relaxation and imagery (13 to 18 minutes in length); Ruan 2006 used a combination of relaxation, imagery, and relaxing music; Richards 1998 used a combination of muscle relaxation, mental imagery, and music (a 7.5‐minute relaxation audiotape consisting of music; guided imagery; and muscle relaxation. We also included this trial under 'back massage' intervention below).
5. Massage
a) Back massage and relaxation intervention
Richards 1998 compared the effect of a back massage and relaxation intervention on sleep with two different groups: group one received a six‐minute back massage; group two received a teaching session on relaxation and a 7.5‐minute audiotape at bedtime consisting of muscle relaxation, mental imagery, and relaxing background music; group three received usual nursing care. The duration of the intervention was one night.
b) Foot massage or foot bath
Wang 2012 examined the effect of foot massage combined with use of a "sleep pillow" (ingredients: Chinese herbal medicine); the duration of the intervention was seven days.
Namba 2012 examined the efficacy of a foot bath intervention for sleep promotion.
6. Valerian acupressure
Chen 2012 compared valerian acupressure on the Shenmen, Neiguan, and Yongquan acupoints versus usual care; the duration of the intervention was one night.
7. Aromatherapy
Borromeo 1998 examined the effects of aromatherapy intervention on sleep.
8. Sound masking
Gragert 1990 compared sound masking (USASI noise) versus usual care.
9. Social support intervention and nursing intervention
Gao 2008 compared changing the ICU visit time for family members versus conventional care with standard visiting times.
Li 2011 compared a nursing intervention programme using the Roy Adaptation Model as a guide versus conventional care; the duration of the intervention was two weeks.
Outcomes
Not all trials measured all of the outcomes relevant for this review. Included studies examined objective sleep outcomes or subjective sleep outcomes or both.
Sleep was measured using polysomnography (Alexopoulou 2007; Andréjak 2013; Bosma 2007; Cabello 2008; Córdoba‐Izquierdo 2013; Namba 2012; Parthasarathy 2002; Richards 1998; Roche‐Campo 2013; Su 2013; Toublanc 2007Wallace 1998), ActiGraph (Chen 2012; Le Guen 2014), Bispectral Index (BIS) (Jaber 2007), electroencephalography (EEG) and methods of muscle tension (Foreman 2013; Xie 2011), nurse observation (Chen 2012; Gragert 1990; Gao 2008; Ruan 2006), and participant assessment (Borromeo 1998; Gragert 1990; Hu 2010; Le Guen 2014; Martin 2008; Richardson 2003; Ryu 2012; Scotto 2009; Toublanc 2007; Sha 2013; Van Rompaey 2012; Wang 2012; Xie 2011).
Sixteen trials used subjective sleep scales to measure sleep quality on the day following the intervention, but the sleep scales varied among these trials: five trials, Richardson 2003; Martin 2008; Scotto 2009; Su 2013; Ryu 2012, used the Verran/Synder‐Halpern (VSH (Snyder‐Halpern 1987)) Sleep Scale (although the versions of the VSH Scale used differed between these trials, and the rating methods were different); three studies, Borromeo 1998; Gragert 1990; Hu 2010, used the Richardson‐Campbell Sleep Questionnaire (RCSQ, a self‐reported visual analogy instrument (Richards 2000)); three trials, Li 2011; Sha 2013; Xie 2011, used a Chinese version of the Pittsburgh Sleep Quality Index (PSQI) scale (Liu 1996); one trial, Chen 2012, used the PSQI and Stanford Sleepiness Scale (SSS (Fichten 1995)); one trial, Wang 2012, used the Athens Insomnia Scale (AIS (Soldatos 2000)) to measure subjective sleep quality; one trial, Le Guen 2014, measured self‐assessment sleep quality by Spiegel score (Klimm 1987) and Medical Outcomes Study Sleep questionnaire (Hays 2005); and two trials, Toublanc 2007; Van Rompaey 2012, used participant‐perceived measures of sleep quality.
Two trials reported outcomes relating to the incidence of delirium (Le Guen 2014; Van Rompaey 2012). Van Rompaey 2012 assessed delirium using the validated Neelon/Champagne Confusion (NEECHAM) scale (Milisen 2005), which was based on the nurses' 24‐hour assessment of the level of processing information, the level of behaviour, and the physiological condition.
The majority of cross‐over trials included in this review only reported the whole‐period outcomes of the study. Two trials reported outcomes during the first period and the second period in addition to the whole period (Roche‐Campo 2013; Toublanc 2007).
Excluded studies
We excluded 27 studies (see the 'Characteristics of excluded studies' tables). We excluded these studies for the following reasons: four trials did not have relevant outcomes; 13 trials were not randomized or quasi‐randomized controlled trials; in six studies, the types of participants were not relevant; in two studies, the interventions were not relevant; and two articles were systematic reviews.
Studies awaiting classification
Five studies, NCT01607723; NCT01580956; NCT01343095; NCT01061242; Nerbass 2011, are awaiting classification. (Please refer to the 'Characteristics of studies awaiting classification' tables for more details.)
Ongoing studies
Ten studies, NCT02095496; NCT01082016; NCT01276652; NCT01284140; ChiCTR‐TRC‐14004405; IRCT2013030912749N1; NCT00638339; Qureshi 2014; NCT01523938; NCT01727375, are ongoing. (Please refer to the 'Characteristics of ongoing studies' tables for more details.)
Risk of bias in included studies
For details of the 'Risk of bias' rating for each study and the reasons for each rating, please see the 'Characteristics of included studies' tables. A summary of the 'Risk of bias' judgements by study and domain (sequence generation, allocation concealment, blinding, incomplete data, and selective reporting) can be found in Figure 2 and Figure 3.
2.
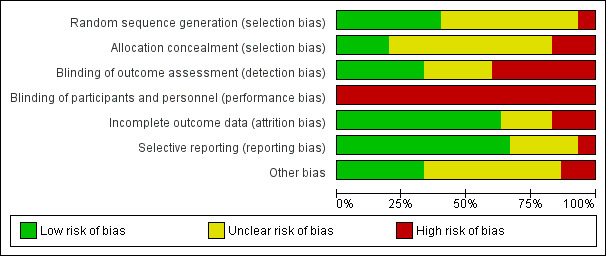
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
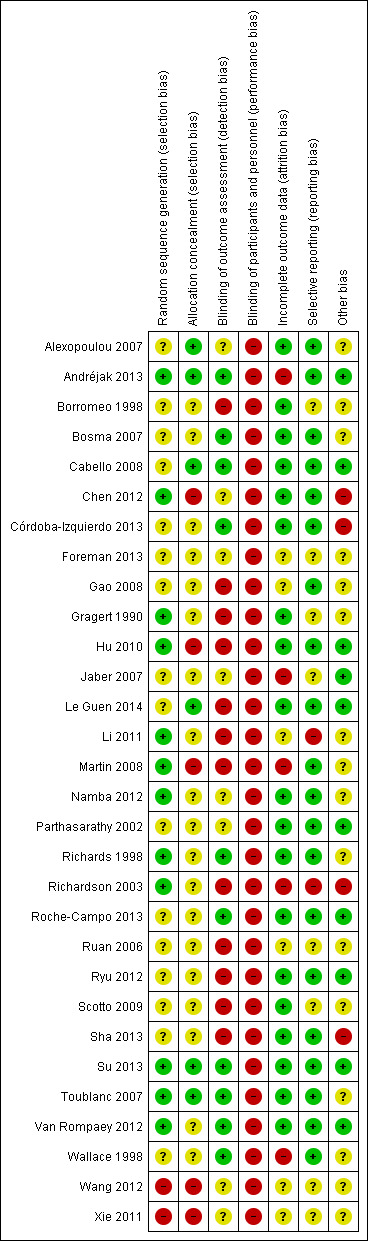
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
The method of random sequence generation may have introduced bias into the studies analysed in this review. Twelve studies provided details of adequate methods for random sequence generation: Richards 1998 used a random number generator; Richardson 2003 used coin toss; two trials used a computer randomization method (Toublanc 2007; Van Rompaey 2012); and three trials used a random number table (Hu 2010; Li 2011; Namba 2012). Five trials used a method involving drawing lots/random numbers (Andréjak 2013; Chen 2012; Gragert 1990; Martin 2008; Su 2013). Sixteen studies stated that participants were "randomly allocated" but lacked description about the method of sequence generation. (Therefore, the risk of bias was unclear.) Two studies used inadequate methods of sequence generation and the risk of bias was considered high (Wang 2012; Xie 2011).
We considered allocation concealment to be adequate in six studies (Alexopoulou 2007; Andréjak 2013; Cabello 2008; Le Guen 2014; Su 2013; Toublanc 2007): all of them used a sealed‐envelope technique. Five studies used inadequate methods of allocation concealment (Chen 2012; Hu 2010; Martin 2008; Wang 2012; Xie 2011), and it was unclear whether allocation concealment was adequate in the remaining 19 studies, so we considered that the risk of bias was unclear.
Blinding
Because of the nature of the interventions, it was not possible to blind personnel or participants or both to the intervention in any of the included studies. Therefore, we considered that all studies were at a high risk of performance and detection bias by participants and personnel, although we note that this was potentially less of a factor for the objective outcomes (e.g., mortality and objective sleep variables).
Seventeen studies considered objective sleep measures, and nine of these studies, Andréjak 2013; Bosma 2007; Cabello 2008; Córdoba‐Izquierdo 2013; Richards 1998; Roche‐Campo 2013; Su 2013; Toublanc 2007; Wallace 1998, were at a low risk of performance and detection bias by outcome assessors because polysomnography (PSG) sleep records (i.e., objective sleep measures) were scored by an expert who was blinded to the randomization assignment. The risk of bias for outcome assessors was unclear in six studies (Alexopoulou 2007; Chen 2012; Foreman 2013; Jaber 2007; Namba 2012; Parthasarathy 2002), and there was a high risk of bias for outcome assessors in one study (Le Guen 2014).
Incomplete outcome data
There was no risk of attrition bias in eight studies (Alexopoulou 2007; Cabello 2008; Chen 2012; Gragert 1990; Namba 2012; Parthasarathy 2002; Roche‐Campo 2013; Su 2013) as there were no dropouts or losses to follow up in these studies. We rated a further 11 studies as at a low risk of attrition bias as the reasons for dropout or loss to follow up were documented and acceptable (Borromeo 1998; Bosma 2007; Córdoba‐Izquierdo 2013; Hu 2010; Le Guen 2014; Richards 1998; Ryu 2012; Scotto 2009; Sha 2013; Toublanc 2007; Van Rompaey 2012). We considered five studies to be at a high risk of attrition bias (Andréjak 2013; Jaber 2007; Martin 2008; Richardson 2003; Wallace 1998). For six trials, it was unclear whether there were any participant withdrawals (Foreman 2013; Gao 2008; Li 2011; Ruan 2006; Wang 2012; Xie 2011).
Selective reporting
For two studies (Li 2011; Richardson 2003), it appeared that a degree of selective reporting had taken place, and we rated these studies as at a high risk of reporting bias. We considered 20 trials to be at a low risk of reporting bias, and it was unclear whether the remaining eight trials were at a risk of reporting bias.
Other potential sources of bias
Seven trials declared a conflict of interest; the other trials did not declare a conflict of interest, so we judged the potential bias to be "unclear" as we had insufficient information to permit a judgement. Most trials did not report a sample size calculation. Other potential sources of bias were evident in one trial (Richardson 2003); the author did not report the mean sleep scores on day one, day two, and day three in both groups, but reported the mean sleep scores on day one, day two, and day three by gender. We then combined the male group and the female group into a single group and calculated the mean sleep scores in both groups. The results showed that the mean sleep scores of the first night (namely baseline) were significantly different between the two groups. In Chen 2012, the baseline mean age and mean Acute Physiology Score (APS) scores of the experimental group were higher than those of the control group. In Córdoba‐Izquierdo 2013, the baseline Epworth Sleepiness Scale scores were higher in the NIVD group than in the NIVICU group. Sha 2013 did not assess the baseline of PSQI scores.
Effects of interventions
See: Table 1
Please see Table 1.
There was considerable clinical heterogeneity across the included studies due to the wide range of scales used to assess outcomes, the different participant populations, and study designs used (e.g., duration, time points). We could not pool the majority of results for meta‐analysis ‐ in which case, we have presented measures of treatment effect. If the published results did not provide sufficient detail to calculate between‐group differences and 95% confidence intervals, we present the data as reported in the study reports.
1 Primary outcome: objective sleep variables
In summary, the effects of non‐pharmacological interventions on objective measurements of sleep quality and quantity were inconsistent across studies. Overall, we rated the quality of the evidence as very low. The reasons for downgrading the quality of the evidence varied by intervention type and are summarized at the end of each subsection below.
a) Ventilator mode or type
Six cross‐over trials examined the effects of ventilator modes on objective sleep variables in ICU patients (Alexopoulou 2007; Andréjak 2013; Bosma 2007; Cabello 2008; Parthasarathy 2002; Toublanc 2007). All of these trials measured sleep using PSG, although there was inconsistency in the method of reporting outcomes between studies. Because of important clinical heterogeneity and missing data, we did not incorporate these studies into a meta‐analysis. We summarize below findings for these individual studies measuring PSG sleep variables (as reported by the authors) and present them in Table 2, Table 3, and Table 4.
1. Comparison of sleep quantity between ACV versus PSV.
| Toublanc 2007 | ACV group | PSV group | ||||
| Sleep outcomes |
Whole night (n = 20) |
1st period (n = 10) |
2nd period (n = 10) |
Whole night (n = 20) |
1st period (n = 10) |
2nd period (n = 10) |
| Stage 1, % | No reportb | 34.8 ± 18.6a | No reportb | No reportb | 17.1 ± 15.0a | No reportb |
| Stage 2, % | No reportb | 33.0 ± 24.6a | No reportb | No reportb | 11.4 ± 15.9a | No reportb |
| Stage 3, % | No reportb | No reportb | 6.3 ± 7.7a | No reportb | No reportb | 0.3 ± 1.0a |
| Stage 4, % | No reportb | No reportb | 5.4 ± 13.2a | No reportb | No reportb | 0.0 ± 0.0a |
| Wakefulness, per cent | 35.4 ± 25.6 | 30.8 ± 28.2a | No report | 50.7 ± 35.7 | 69.0 ± 26.2a | No reportb |
| REM, per cent | No reportb | No reportb | No reportb | No reportb | No reportb | No reportb |
| Awakening index | 7.1 ± 5.0 | No report | No report | 6.5 ± 4.9 | No reportb | No reportb |
| Parthasarathy 2002 | ||||||
| Sleep outcomes |
ACV (n = 11) |
PSV alone (n = 11) |
PSV with Dead space (n = 11) |
P value | ||
| Total sleep time, minutes | 90 ± 6 | 75 ± 6 | 82 ± 7 | ‐ | ||
| Sleep efficiency, per cent | 75 ± 5 | 63 ± 5 | 81 ± 7 | P value < 0.05 | ||
| Fragmentation index, n/hour | 54 ± 7 | 79 ± 7 | No report | P value < 0.05 | ||
| Arousals/hour | 35 ± 7 | 39 ± 6 | No report | P value = 0.8 | ||
| Awakenings/hour | 19 ± 3 | 39 ± 7 | No report | P value < 0.01 | ||
| REM, %c | ‐ | ‐ | ‐ | ‐ | ||
| Cabello 2008 | ||||||
| Sleep outcomes |
ACV (n = 15) |
cPSV (n = 15) |
aPSV (n = 15) |
P value | ||
| Sleep efficiency, per cent | 58 (44 to 82) | 44 (29 to 80) | 63 (29 to 80) | P value = 0.15 | ||
| Fragmentation index, n/hour | 30 (17 to 41) | 28 (17 to 53) | 23 (21 to 45) | P value = 0.62 | ||
| Stage 1, % | 8 (1 to 15) | 7 (1 to 23) | 5 (0 to 11) | P value = 0.62 | ||
| Stage 2, % | 54 (47 to 79) | 67 (54 to 84) | 39 (52 to 62) | P value = 0.02 | ||
| SWS, minutes | 37 (4 to 62) | 26 (0 to 68) | 24 (0 to 51) | P value = 0.79 | ||
| REM, per cent | 7 (0 to 13) | 4 (0 to 10) | 1 (0 to 7) | P value = 0.54 | ||
ACV = assist‐control ventilation. aPSV = automatically adjusted pressure support ventilation. cPSV = clinically adjusted pressure support ventilation. PSV = pressure support ventilation. REM = rapid eye movement. SWS = slow‐wave sleep.
Data sourced from Toublanc 2007 and Parthasarathy 2002 are expressed as mean ± standard deviation. Data sourced from Cabello 2008 are expressed as median (25th to 75th percentile). aP value < 0.05 compared ACV with PSV. bData were expressed in the source articles using figures; no numerical sleep data were provided: comparisons showed no significant difference for ACV versus PSV. c4 participants achieved REM sleep; only 1 participant achieved REM sleep with all 3 modes.
2. Comparison of sleep quantity between PAV versus PSV.
| Alexopoulou 2007 | PAV group | PSV group | ||
| Sleep outcomes | PAV+base | PAV+high | PSbase | PShigh |
| Sleep efficiency, per cent Protocol A Protocol B |
98.9 ± 2.3 75.6 ± 10.8 |
98.1 ± 4.7 70.7 ± 21.0 |
93.3 ± 10.8 68.1 ± 19.2 |
87.7 ± 16.4a 71.6 ± 14.9 |
| Stage 1, per cent Protocol A Protocol B |
40.5 ± 41.5 55.0 ± 38.1 |
39.4 ± 35.8 33.0 ± 30.4 |
50.6 ± 40.5 52.0 ± 39.9 |
55.2 ± 41.3 35.3 ± 34.7 |
| Stage 2, per cent Protocol A Protocol B |
50.5 ± 42.3 36.3 ± 32.1 |
48.1 ± 35.5 61.2 ± 27.6 |
39.4 ± 37.7 42.5 ± 34.9 |
35.0 ± 34.9 43.6 ± 31.6 |
| SWS, per cent Protocol A Protocol B |
9.9 ± 29.5 2.6 ± 7.4 |
12.9 ± 28.3 4.1 ± 9.4 |
11.01 ± 29.9 2.1 ± 3.9 |
10.6 ± 24.3 1.8 ± 4.9 |
| REM, per cent Protocol A Protocol B |
‐ 6.2 ± 13.9 |
0.88 ± 2.7b 1.7 ± 4.2 |
‐ 3.5 ± 6.2 |
‐ 19.3 ± 23.3 |
| Arousals/hour Protocol A Protocol B |
4.6 ± 4.9 12.2 ± 8.0 |
7.4 ± 10.7 11.4 ± 7.6 |
5.4 ± 3.6 8.4 ± 4.8 |
6.5 ± 6.7 10.5 ± 9.9 |
| Awakenings/hour Protocol A Protocol B |
0.6 ± 1.4 4.0 ± 3.0 |
0.8 ± 1.5 4.3 ± 3.2 |
1.3 ± 1.4 3.6 ± 3.1 |
2.7 ± 3.1 3.9 ± 3.4 |
| Fragmentation index, n/hour Protocol A Protocol B |
5.2 ± 5.1 17.5 ± 8.2 |
8.3 ± 11.1 16.8 ± 8.9 |
6.8 ± 4.5 13.0 ± 5.5 |
9.2 ± 8.5 15.3 ± 10.6 |
| Bosma 2007 | ||||
| Sleep outcomes | PAV (n = 13) | PSV (n = 13) | ||
| Total sleep time, minutes | 334 ± 124 | 314 ± 140 | ||
| Total sleep period, minutes | 451 ± 99 | 484 ± 63 | ||
| Sleep efficiency, per cent | 60 ± 23 | 58 ± 25 | ||
| Sleep maintenance efficiency, per cent | 69 ± 22 | 68 ± 21 | ||
| Arousals, n /hour | 12.8 ± 10.3 | 25.6 ± 23.2c | ||
| Awakenings, n/hour | 5.2 ± 6.1 | 8.3 ± 7.5 | ||
| Fragmentations index, n/hour | 18.0 ± 10.4 | 33.9 ± 28.9 | ||
| REM, per cent | 9 (0 to 3) | 4 (0 to 23) | ||
| SWS, per cent | 3 (0 to 16) | 19 [0 to 10) | ||
n = number. PAV = proportional assist ventilation. PAV+base = proportional assist ventilation with baseline level of assist. PAV+high = proportional assist ventilation with level of assist. PShigh = pressure support ventilation with high pressure support. PSbase = pressure support ventilation with baseline pressure support. PSV = pressure support ventilation. REM = rapid eye movement. SWS = slow‐wave sleep.
All data sourced from Alexopoulou 2007 are expressed as mean ± standard deviation. Data sourced from Bosma 2007 are expressed as mean ± standard deviation or median (range).
Protocol A: sedated participants. Protocol B: non‐sedated participants. aStatistically significantly different from PAV+mode. bREM was observed in 1 participant. cP value < 0.05 compared PAV with PSV.
3. Comparison of sleep quantity between PCV versus PSV.
|
Andréjak 2013 Sleep outcomes |
PCV (n = 26) | Low PSV (n = 26) | P value |
| Stages 1, % | 15 ± 14 | 15 ± 10 | P value > 0.05 |
| Stage 2, % | 35.3 ± 23.3 | 20 ± 21.9 | P value < 0.01 |
| Wakefulness, per cent | 37.7 ± 24.7 | 58.3 ± 28.8 | P value < 0.01 |
| REM, per cent | 3.4 ± 6.4 | 0.8 ± 2.1 | P value < 0.01 |
| Sleep efficiency, per cent | 61.5 ± 25.1 | 39.2 ± 29.1 | P value < 0.01 |
| SWS, per cent | 8.9 ± 10.1 | 3.5 ± 8.9 | P value < 0.01 |
PCV = pressure‐controlled ventilation. PSV = pressure support ventilation with 6 cm H₂O inspiratory pressure. REM = rapid eye movement. SWS = slow‐wave sleep.
All data are expressed as mean ± standard deviation (Andréjak 2013).
Three studies examined objective sleep variables in participants receiving ACV versus PSV (Cabello 2008; Parthasarathy 2002; Toublanc 2007).
i) One trial, Parthasarathy 2002, demonstrated a significant increase in Sleep Efficiency Index (SEI) in the ACV group (mean = 75, standard deviation (SD) = 5) compared with the PSV group (mean = 63, SD = 5) (P value < 0.05). However, no significant improvement in SEI was found by Cabello and colleagues (P value > 0.05; Cabello 2008).
ii) Two trials, Cabello 2008; Parthasarathy 2002, reported sleep fragmentation index, but only one, Parthasarathy 2002, indicated a significant reduction in sleep fragmentation index in the ACV group (mean = 54, SD =7) compared with the PSV group (mean = 79, SD = 7) (P value < 0.05). Cabello 2008 found no significant reduction in sleep fragmentation index (P value > 0.05).
iii) Toublanc 2007 reported no significant reduction in awakening index between ACV and PSV groups (P value > 0.05).
iv) Two trials, Cabello 2008; Parthasarathy 2002, measured the percentage of stage three and four sleep, but no significant difference was found between the PSV and ACV groups in either trial (P value > 0.05). However, during the second period of the cross‐over study by Toublanc et al (Toublanc 2007), higher percentages of stage three sleep (mean = 6.3, SD = 7.7 versus mean = 0.3, SD = 1.0) (P value < 0.01) and stage four sleep (mean = 5.4, SD = 13.2 versus mean = 0, SD = 0) (P value < 0.05) were observed in the ACV group compared with those in the low PSV group.
Two studies compared PAV versus PSV (Alexopoulou 2007; Bosma 2007).
i) In Alexopoulou 2007, SEI was significantly higher in the PAV group (mean = 98.9, SD = 2.3) compared with the PSV group (mean =87.7, SD = 16.4) (P value < 0.05). Bosma 2007 found no significant difference in SEI (P value > 0.05).
ii) No significant reductions in sleep fragmentation index and slow‐wave sleep (SWS) per cent were found in either trial (P > 0.05).
Only one study compared PCV versus PSV (Andréjak 2013).
i) SEI was significantly higher in the PCV group (mean = 61.5, SD = 25.1) compared with the PSV group (mean = 39.2, SD = 29.1) (P value < 0.01).
ii) A significant increase in the number of hours of REM sleep time was reported in the PCV group (mean = 3.4, SD=6.4) compared with the PSV group (mean = 0.8, SD = 2.1) (P value < 0.01).
iii) No significant difference in the percentage of stage three and four sleep was observed between groups (P value > 0.05).
Two studies examined the effect of ventilator type on objective sleep variables (Córdoba‐Izquierdo 2013; Roche‐Campo 2013). One study of 24 participants with acute hypercapnic respiratory failure requiring non‐invasive ventilation, Córdoba‐Izquierdo 2013, compared the use of conventional ICU ventilators versus dedicated non‐invasive ventilators and found no significant difference between the groups in sleep fragmentation index, total sleep time (TST), stage one per cent, stage two per cent, SWS per cent, and REM per cent (P value > 0.05).
One cross‐over study examined spontaneous ventilation versus mechanical ventilation at low levels of pressure support in 16 tracheostomized participants during weaning (Roche‐Campo 2013). Total sleep time was greater during mechanical ventilation than during spontaneous ventilation (183 minutes versus 132 minutes, P value = 0.04). This study found no significant difference between the groups in SWS time, REM time, and sleep fragmentation index (P value > 0.05).
We rated the quality of the evidence as low for the effect of ventilator mode or type on objective sleep variables, having downgraded once for inconsistency (findings differed between studies) and once for risk of selection bias.
b) Earplugs or eye masks or both
Two studies assessed the effect of eye masks or earplugs or both on objective sleep variables as measured using PSG (Foreman 2013; Wallace 1998). Due to clinical heterogeneity in study design, the results from these studies could not be combined statistically. Wallace 1998 reported significantly higher percentages of REM sleep during the night in the group assigned to earplugs compared with the control group (mean = 5.60, SD = 8.00 versus mean = 2.40, SD = 5.60) (P value = 0.04). No significant difference in other objective sleep variables (sleep period time, SEI, sleep maintenance efficiency index, number of awakenings) was found between the groups in this study (each P value > 0.05). Foreman 2013 examined objective sleep variables in 12 neurological ICU patients who received oral melatonin, sound‐reducing headphones, and eye covers versus standard care, finding no significant difference between the groups in terms of sleep architecture (no P value or 95% CI reported).
One quasi‐RCT of 75 ICU patients, Xie 2011, compared the use of earplugs and eye masks versus usual care on objective sleep variables, as measured by EEG. There was a greater improvement in the mean number of hours of SWS in the intervention group compared with the control group (SWS: post‐test mean = 2.18, SD = 0.34 versus post‐test mean = 1.43, SD = 0.28) (P value < 0.01) (REM: post‐test mean = 2.09, SD = 0.28 versus post‐test mean = 0.71, SD = 0.36) (P value < 0.01). A greater reduction in the mean number of hours of waking time was also reported in the intervention group compared with the control group (post‐test mean = 1.79, SD = 0.75 versus post‐test mean = 3.8, SD = 0.79) (P value < 0.01); no significant difference in NREM time was observed between groups (P value > 0.05).
One study of 41 postoperative patients compared the use of earplugs and eye masks versus usual care on objective sleep variables, as measured by ActiGraph (Le Guen 2014). This study found no significant between‐group difference (P value > 0.05) in sleep variables, including sleep efficiency, sleep fragmentation, sleep disruptions, movement numbers, or activity scores.
We rated the quality of the evidence as very low for the effect of earplugs or eye masks or both on objective sleep variables, having downgraded twice for inconsistency (findings differed between studies) and once for risk of selection bias.
c) Music intervention
One study examined the effects of listening to music (versus usual care) on PSG sleep variables in 28 ICU patients (Su 2013). The authors reported that participants in the music group had shorter stage two sleep time (P value = 0.014) and longer stage three sleep time (P value = 0.008) in the first two hours of the nocturnal sleep as calculated by generalized estimating equation analysis. No statistically significant differences in the mean total sleep time, sleep efficiency, and stage one sleep times were reported between groups (P value > 0.05).
One study measured objective sleep variables as measured by BIS (Jaber 2007). The author reported a significantly greater reduction in BIS in the music intervention group (post‐test mean = 81, SD = 10) compared with the control group (post‐test mean = 94, SD = 5) (P value < 0.01).
We rated the quality of the evidence as very low for the effect of music on objective sleep variables, having downgraded once for inconsistency (findings differed between studies), once for indirectness (only two small studies included), and once for risk of selection bias in Jaber 2007.
d) Relaxation techniques
Richards 1998 compared a six‐minute back massage versus relaxation intervention plus relaxing music (combined muscle relaxation, mental imagery, and audiotape) versus usual care (control). The study measured objective sleep variables using PSG in 69 older men with cardiovascular illness. Participants in the back‐massage group slept more than one hour longer than those in the control group (mean = 319.82, SD = 48.45 versus mean = 257.33, SD = 108.22; no significance value reported). This study found a significant difference among the three groups in SEI (control group: mean = 62.84, SD = 24.46; back‐massage group: mean = 77.32, SD = 10.53; relaxation group: mean = 73.13, SD = 15.66, F = 3.73) (P value = 0.03). No significant differences in other PSG sleep variables were found in this study.
We rated the quality of the evidence as very low for the effect of relaxation techniques on objective sleep variables, having downgraded once for risk of selection bias, once for indirectness (only one study population), and once for imprecision (large standard deviations).
e) Foot massage or foot bath
One study of six participants compared using foot baths at 40℃ for 10 minutes before sleep onset with usual care and measured PSG sleep (Namba 2012). There was no significant difference in total sleep time, sleep efficiency, time spent in REM or sleep stages, and sleep fragmentation (all P values > 0.05).
We rated the quality of the evidence as very low for the effect of foot massage/bath on objective sleep variables, having downgraded once for risk of selection bias, once for indirectness (only one study population), and once for imprecision.
f) Other interventions
None of the studies examined the effect of valerian acupressure, aromatherapy, sound masking, or nursing/social interventions on objective sleep variables.
2) Primary outcome: length of ICU stay
We rated the quality of the evidence as very low for this outcome, having downgraded once for risk of selection bias, once for indirectness (only one population considered), and once for imprecision (wide confidence intervals).
a) Earplugs or eye masks or both
Hu 2010 examined the effect of earplugs plus eye masks plus sleep‐inducing music versus usual care on the length of ICU stay. No significant difference in the length of ICU stay was found between groups (MD = ‐5.90, 95% CI ‐16.42 to 4.62) (P value > 0.05).
b) Other interventions
No other trials examined the effect of the other non‐pharmacological intervention types on the length of ICU stay.
3) Primary outcome: mortality
None of the included studies examined mortality.
4) Secondary outcome: adverse events
We rated the quality of the evidence as very low for this outcome, having downgraded once for risk of selection bias, once for indirectness (the evidence was based only on studies of ventilator mode or type), and once for imprecision (large standard deviations reported in individual studies).
a) Ventilator mode or type
Five trials assessed the effect of ventilator mode on adverse events, such as central apnoeas, patient‐ventilator asynchronies, and ineffective efforts. In Bosma 2007, total patient‐ventilator asynchronies per hour were more frequent during PSV than during PAV (53 ± 59 versus 24 ±15) (P value = 0.02); episodes of central apnoeas were observed during the night with PSV, whereas no participants showed central apnoeas during the night on PAV. In Cabello 2008, no apnoeas occurred during ACV, whereas nine of 15 participants presented sleep apnoeas during PSV, and the mean number of ineffective efforts per hour of sleep were similar with ACV, automatically adjusted pressure support ventilation (aPSV), and clinically adjusted pressure support ventilation (cPSV) (P value > 0.05). In Parthasarathy 2002, apnoeas occurred in six of 11 participants during PSV alone, but not during ACV; the use of PSV with dead space decreased the frequency of apnoeas significantly (P value < 0.05). In Alexopoulou 2007, the two modes (PAV and PSV) had comparable effects on respiratory variables, particularly at high assist, and a significant proportion of participants in both groups developed periodic breathing during sleep. In Roche‐Campo 2013, one participant experienced periodic breathing and one participant experienced central apnoeas regardless of the ventilatory mode used; nobody exhibited ineffective efforts.
b) Other interventions
No trials of the other non‐pharmacological interventions examined adverse events.
5) Secondary outcome: delirium
We rated the quality of the evidence as low for this outcome, having downgraded once for risk of selection bias and once for imprecision (wide confidence intervals ‐ see Table 1).
a) Earplugs or eye masks or both
Two studies examined the effect of earplugs or eye masks or both on the risk of delirium (Le Guen 2014; Van Rompaey 2012). Van Rompaey and colleagues used the validated Neelon and Champagne Confusion Scale (NEECHAM) (Van Rompaey 2008). In Le Guen 2014, the author did not report the method of assessment of delirium. A meta‐analysis of these two studies showed that use of earplugs or eye masks or both significantly decreased the risk of delirium or confusion (risk ratio (RR) 0.55, 95% CI 0.38 to 0.80) (P value = 0.002) (Analysis 1.1; Figure 4).
1.1. Analysis.
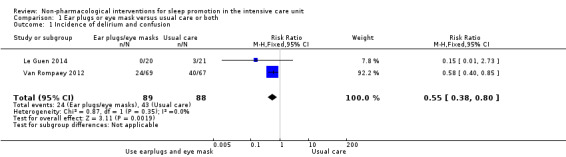
Comparison 1 Ear plugs or eye mask versus usual care or both, Outcome 1 Incidence of delirium and confusion.
4.
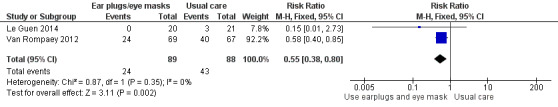
Forest plot of comparison: earplugs or eye mask or both versus usual care, outcome: 2.2 incidence of delirium and confusion.
b) Other interventions
No trials of the other non‐pharmacological interventions examined delirium.
6) Secondary outcome: subjective sleep quantity or quality
Overall, we rated the quality of the evidence for this outcome as low. The reasons for downgrading the quality of the evidence varied by intervention type and are summarized at the end of each subsection below.
a) Ventilator mode or type
Toublanc 2007 reported that self‐perceived quality of sleep in ICU patients was poor, but did not compare subjective sleep quality between the different ventilator modes.
b) Earplugs or eye masks or both
Six studies of earplugs or eye masks or both assessed sleep quality or quantity using subjective sleep scales (Hu 2010; Le Guen 2014; Martin 2008; Scotto 2009; Van Rompaey 2012; Xie 2011); the scales used varied among these trials.
Two studies, involving 120 participants, compared earplugs and eye masks versus usual care and assessed nurse‐measured (subjective) sleep quantity (Le Guen 2014; Xie 2011). Meta‐analysis of these two studies showed that total sleep time was significantly greater in the intervention group compared with the control group (MD 2.19 hours, 95% CI 0.41 to 3.96, two studies, 116 participants). However, there was evidence of heterogeneity between studies for this outcome (I² statistic = 79%; P value = 0.03) (Analysis 1.2; Figure 5).
1.2. Analysis.
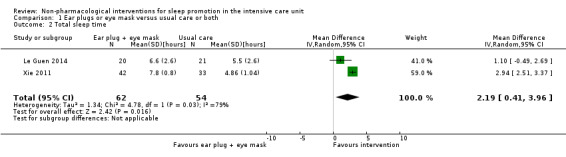
Comparison 1 Ear plugs or eye mask versus usual care or both, Outcome 2 Total sleep time.
5.
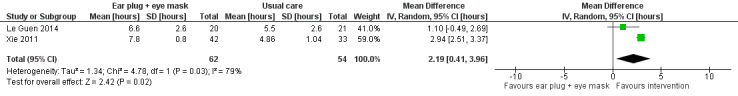
Forest plot of comparison: Use of ear plugs and eye mask versus usual care, outcome: 2.3 subjective sleep quantity; total sleep time (hours).
Two studies, Hu 2010 and Xie 2011, compared the use of earplugs and eye masks versus usual care and assessed subjective sleep quality using the RCSQ and PSQI scales, respectively. As Hu 2010 combined music and the use of earplugs/eye masks in the intervention group whereas Xie 2011 examined earplugs plus eye masks only, we could not pool data from the two studies. Subjective sleep quality in the intervention group was greater in the intervention versus control groups of both studies. In Hu 2010, the mean difference in the Chinese version of RCSQ scores of perceived quality (0 = better sleep, 100 = poor sleep) for intervention versus control was ‐27.00 (95% CI ‐40.15 to ‐13.85). In Xie 2011, the mean difference in PSQI score (0 = best sleep, 21 = worst sleep) for intervention versus control was ‐7.25 (95% CI ‐8.46 to ‐6.04).
Le Guen 2014 evaluated subjective sleep quality using the Spiegel score for which higher scores indicate a better sleep quality; a total score below 15 signifies a pathological sleep, and a score above 20, good sleep. Postoperatively, the mean Spiegel score in the earplug and eye mask group was 20 (SD = 4) compared with 15 (SD = 5) in the control group (comparison P value = 0.006). Additionally, only 50% of the participants in the intervention group reported the need for a nap compared with 95% of those in the control group (P value = 0.001).
Martin 2008 reported no significant difference in VSH sleep scores between earplug and usual care groups (post‐test mean = 56.7, SD = 25.6 versus post‐test mean = 59.2, SD = 27.0; significance value not reported). Scotto 2009 also assessed sleep quality using the VSH sleep score. The author reported that use of earplugs improved the subjective sleep quality (P value < 0.05), but no mean scores were reported.
One study, Van Rompaey 2012, of 136 participants compared sleep perception using five dichotomous questions on the self‐reported sleep quality of the participant, which they categorized as: bad sleep (sum < 2), moderate sleep (sum 2 < 4), and good sleep (sum ≥ 4). More participants perceived good sleep in the intervention group than those in the control group after the first night (P value = 0.042, no Chi² test value reported).
Overall, we deemed the quality of the evidence for the effect of earplugs or eye masks or both on objective sleep variables as low, having downgraded once for inconsistency (findings differed between studies) and once for risk of selection bias.
c) Music interventions
Four studies of music intervention reported subjective sleep quality. One study, Sha 2013, used the PSQI sleep scale, and one study, Hu 2010, used a Chinese version of RSCQ. We could not pool data from these studies as they reported no post‐test PSQI total scores (Sha 2013), and the sleep quality scales were incompatible (Hu 2010). In Sha 2013, the subjective sleep quality, sleep time, sleep efficiency, and total PSQI scores were significantly improved in the intervention group compared with the control group (P value < 0.05). Additionally, the incidence of sleep disorder in the music intervention group was significantly lower than that in the control group (P value = 0.036).
Two studies examined subjective sleep quality using different versions of the VSH sleep scale (Ryu 2012; Su 2013). However, Ryu 2012 combined music with the use of earplugs and eye masks, whereas Su 2013 did not; for this reason, we could not combine the results in a meta‐analysis. Ryu 2012 reported that participants receiving a music intervention had improved sleep quality versus those receiving usual care (standardized mean difference (SMD) 0.93, 95% CI 0.15 to 1.72; N = 28). Similarly, sleep quality was improved in participants receiving music intervention plus eye masks and earplugs versus usual care (SMD 1.37, 95% CI 0.79 to 1.94; N = 58; Su 2013).
Overall, we deemed the quality of the evidence for the effect of music interventions on objective sleep variables as very low, having downgraded once for inconsistency (findings differed between studies) and twice for a high risk of selection bias.
d) Relaxation techniques
One study measured perception of sleep quality using the VSH sleep scale (Richardson 2003). No differences were observed between control and experimental sleep scores on day one, two, and three (each P value > 0.05; no mean sleep score values were reported by group). We calculated mean sleep scores and used intention‐to‐treat (ITT) analysis; the results showed the intervention group (namely a combination of relaxation and imagery) exhibited significantly less change in sleep scores from the first night to the third night (MD ‐13.52, 95% CI ‐34.24 to 7.20), indicating better sleep in the intervention group (higher sleep scores indicated a perception of improved sleep in this trial). However, we also found the baseline of sleep scores in the intervention group was significantly higher than those in the control group, which resulted in a high risk of selection bias in the study, so it was difficult to ascertain if there was a real effect.
One study measured sleep quality and quantity by nursing observation (Ruan 2006). The main outcomes were trichotomous types; the study classified the outcome of the time taken to fall asleep into "less than 30 minutes", "0 to 60 minutes", or "greater than 60 minutes", and it classified the outcome of total nocturnal sleep time into "less than three hours", "three to five hours", or "greater than five hours". Using methodology recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we transformed the published data into a dichotomous format by combining adjacent categories together, using "greater than 60 minutes" and "greater than five hours" as cut‐off points. The results showed that it took significantly less time to fall asleep in the intervention group than in the control group (RR 0.34, 95% CI 0.11 to 1.06), but there was no significant difference between the groups for total nocturnal sleep time (RR 0.26, 95% CI 0.09 to 0.74).
We deemed the quality of the evidence for the effect of foot massage/bath on objective sleep variables as very low, having downgraded once for indirectness (evidence based on two small populations), once for risk of selection bias, and once for precision (wide confidence intervals).
e) Foot massage or foot bath
In Namba 2012, the participants claimed that they slept well the night after receiving a foot bath. One study, Wang 2012, of 104 participants with sleep problems in a coronary critical care unit compared foot massage plus 'sleep pillow' (ingredients: Chinese herbal medicine) and measured perceived sleep quality using the Athens Insomnia Scale (AIS). This study found that the mean change scores of AIS in the intervention group were higher than those in the control group (mean = 1.06, SD = 0.72 versus mean = 0.74, SD = 0.61) (P value < 0.05). We deemed the quality of the evidence for the effect of foot massage/bath on objective sleep variables as low, having downgraded once for indirectness (evidence based on two small populations) and once for risk of selection bias.
f) Valerian acupressure
One study of 85 ICU patients, Chen 2012, compared valerian acupressure on the Shenmen, Neiguan, and Yongquan acupoints versus usual care and measured subjective sleep quality using the Stanford Sleepiness Scale (SSS). This study found that, compared with the control group, the acupressure group had lower SSS ratings (i.e., better sleep; mean = 2.5, SD = 0.5 versus mean = 3.4, SD =1.1) (P value < 0 .001) and a greater number of hours sleep as observed by nursing staff (mean = 3.4, SD = 1.7 versus mean = 2.6, SD = 1.5) (P value < 0.05 ). We calculated the mean changes and the standard deviations in each group from baseline and calculated the mean difference. We found evidence of a difference between the two groups for number of hours of sleep (MD 0.7, 95% CI 0.29 to 1.11) (P value = 0.0008) and waking frequency (MD ‐4.30, 95% CI ‐6.36 to ‐2.24) (P value < 0.0001), but not for SSS ratings (MD ‐0.10, 95% CI ‐0.35 to 0.15) (P value = 0.44). We deemed the quality of the evidence for the effect of valerian acupressure on objective sleep variables as low, having downgraded once for indirectness (evidence based on one small population) and once for risk of selection bias.
g) Aromatherapy
One study compared aromatherapy intervention versus usual care and measured perceived sleep quality by RCSQ (Borromeo 1998). The study indicated no significant between‐group differences in sleep scores (intervention group: mean = 59.84, SD = 2.91; control group: mean = 63.28, SD = 2.48) (P value > 0.05). We deemed the quality of the evidence for the effect of aromatherapy on objective sleep variables as low, having downgraded once for indirectness (evidence based on one small population) and once for risk of selection bias.
h) Sound masking
One study of 40 older patients in a critical care unit assessed the effect of sound masking on subjective sleep quality measured by RCSG and nursing observation (Gragert 1990). The results indicated a significant difference in mean SEI between the intervention group and the control group (75% versus 61%; P value = 0.016), a greater total sleep time (308.70 minutes versus 249.5 minutes, P value = 0.012), and a reduced sleep latency time (35.12 minutes versus 102.60 minutes, P value = 0.000). No standard deviations were provided. No significant difference was seen in the number of awakenings (P value = 0.60). The following six variables were scored from 0 to 100 mm using the RCSQ: sleep depth, falling asleep, awakenings, returning to sleep, quality of sleep, and noise level (0 represented the best possible score, and 100 represented the worst possible score). The results showed that there was greater sleep depth (81.55 versus 54., P value = 0.001), less sleep latency (79.80 versus 56.15, P value = 0.002), and fewer awakenings (79.40 versus 56.20, P value = 0.002) in the intervention group compared with the control group. Subjective sleep quality was greater (81.20 versus 54.60, P value = 0.002); participants had less difficulty returning to sleep (79.90 versus 58.35, P value = 0.005) and lower subjective impressions of the noise level during the night‐time (90.85 versus 38.40, P value = 0.000) in the intervention group compared with the control group. We deemed the quality of the evidence for the effect of sound masking on objective sleep variables as low, having downgraded once for indirectness (evidence based on one small population) and once for risk of selection bias.
i) Nursing intervention or social intervention
One study, Gao 2008, compared changing the ICU visiting time for family members versus conventional care and demonstrated a significant increase in hours of total sleep time in the intervention group (post‐test mean = 6.7, SD = 1.1 versus post‐test mean = 3.6, SD = 2.4) (P value < 0.05).
One study, Li 2011, compared a nursing intervention programme with the Roy Adaptation Model as a guide versus conventional care and measured subjective sleep quality by PSQI (0 = better sleep, 21 = worse sleep). The author reported a significantly higher subjective sleep quality in the intervention group than in the control group (post‐test mean = 5.57, SD = 2.62 versus post‐test mean = 10.03, SD = 2.62) (P value < 0.05).
7. Secondary outcome: PTSD
None of the included studies examined PTSD.
8. Secondary outcome: participant satisfaction
a) Music interventions
One trial reported that five participants did not complete the study because they refused or resented the music therapy (Jaber 2007).
b) Other interventions
No trials examined the effect of the other non‐pharmacological intervention types on participant satisfaction.
9. Secondary outcome: economic outcomes
None of the included studies examined economic outcomes.
Discussion
Summary of main results
We included non‐pharmacological interventions, such as ventilator modes and type, earplugs or eye masks or both, massage, relaxation techniques, foot baths, music interventions, nursing interventions, valerian acupressure, aromatherapy, and the use of sound masking, in this review. Thirty studies, with a total of 1569 adult participants, were eligible for inclusion, three of which provided data suitable for meta‐analysis (all three studies assessed the use of earplugs or eye masks or both). Outcomes included objective sleep outcomes (as measured by polysomnography (PSG), Bispectral Index (BIS), or ActiGraph), subjective sleep quality and quantity by participant assessment or nursing observation, risk of delirium during intensive care unit (ICU) stay, participant satisfaction, length of ICU stay, and adverse events.
We considered the overall quality of the evidence for an effect of non‐pharmacological interventions on objective sleep variables in ICU patients as very low. Clinical heterogeneity prevented meaningful meta‐analysis of data from individual studies that examined the same intervention, and findings across studies of the same intervention were often inconsistent; the following text discusses our findings for this outcome by intervention type.
Four included studies examined the effect of earplugs or eye masks or both on objective sleep variables, all versus usual care (i.e., without using earplugs or eye masks). Individual studies provided some evidence that the use of earplugs or eye masks or both may increase rapid eye movement (REM) sleep time (Wallace 1998; Xie 2011) and non‐REM (NREM) 3˜4 time (Xie 2011). However, the trials contributing evidence for this outcome were potentially at a risk of selection bias, and there were inconsistent findings between studies (Le Guen 2014). Therefore, our overall rating of the evidence for an effect of earplugs or eye masks or both on objective sleep variables was very low. Mechanical ventilation has been cited as an important contributing factor to sleep disruption, and the optimization of ventilator mode is recommended for sleep promotion in ICU patients (Friese 2008; Parthasarathy 2004). Six randomized cross‐over studies also examined the effect of ventilator mode or type on objectively measured sleep variables (Alexopoulou 2007; Andréjak 2013; Bosma 2007; Cabello 2008; Parthasarathy 2002; Toublanc 2007). Clinical heterogeneity in the types and methods of interventions assessed and the specific outcomes measured meant that it was not possible to pool data from these studies.
Results from individual studies suggested that optimizing ventilator modes may improve sleep quality and reduce patient–ventilator asynchrony. In particular, pressure‐controlled ventilation mode (PCV), assist‐control ventilation mode (ACV), and proportional assist ventilation (PAV) mode appeared to offer some benefit in terms of sleep quantity or quality or both compared with pressure support ventilation mode (PSV). For example, in one study, Toublanc 2007, participants on ACV had lower wakefulness and longer stage one and two NREM sleep than participants on PSV. In a separate study (Parthasarathy 2002), differences in respiratory rate, mechanical expiratory time, mechanical inspiratory time, and end‐tidal CO₂ between sleep and wakefulness were greater during PSV than during ACV. However, we considered many of the included studies to be at a risk of selection bias, and findings were inconsistent between studies. For example, Parthasarathy 2002 reported that participants with ACV had a higher Sleep Efficiency Index (SEI) and lower sleep fragmentation than those during PSV, whereas Cabello 2008 reported no significant difference in SEI and sleep fragmentation. In addition to ventilator mode, the effect of ventilator type was also examined. One included study, Córdoba‐Izquierdo 2013, examined the effects of dedicated non‐invasive ventilators versus conventional ICU ventilators on sleep and reported no significant difference between groups. Similarly, another included study, Roche‐Campo 2013, reported that sleep quality was similar during mechanical ventilation (MV) and spontaneous ventilation (SV), but noted a greater quantity of sleep during MV than during SV in tracheostomized participants with prolonged weaning. Both studies were of an unclear risk of selection bias and represented only small populations of participants. Overall, we rated the quality of the evidence for the effect of ventilator mode or type on objective sleep variables as low. Similarly, the quality of evidence for an effect of music interventions on objective sleep variables was very low, with only two studies contributing relevant data, which we could not pool because of clinical heterogeneity. The two included studies reported contrasting findings: in one study, Jaber 2007, music interventions appeared effective in reducing the BIS with a difference of 13 points between groups. However, Su 2013 reported no effect of music interventions on PSG sleep outcomes.
Only one included study, Hu 2010, incorporated length of ICU stay as an outcome (a secondary outcome for this review). No significant effect of earplugs plus eye masks was found on length of ICU stay. We rated the overall quality of the evidence for this outcome as very low. None of the interventions examined in this review were assessed in relation to effect on mortality.
In terms of the review's secondary outcomes, few included studies assessed the effect of the interventions on adverse events in ICU patients. There was some evidence that ventilator mode influenced the incidence of adverse events, such as central apnoeas and patient‐ventilator asynchronies. Generally, more adverse events were noted with PSV compared with ACV or PAV. For example, two included studies reported that no central apnoeas occurred during ACV whereas more than 50% of participants had apnoeas during PSV (Cabello 2008; Parthasarathy 2002). However, clinical heterogeneity between studies prevented meta‐analysis, and we rated the quality of the evidence for this outcome (and thus the effect of ventilator mode on adverse events) as low.
Two included studies examined the incidence of delirium in ICU patients (Le Guen 2014; Van Rompaey 2012). Both of these studies examined the effect of earplugs or eye masks or both, and we pooled data from these studies for meta‐analysis. In participants using earplugs or eye masks or both, the risk of delirium was lower than for participants in the control group (risk ratio (RR) 0.55, 95% confidence interval (CI) 0.38 to 0.80). Assuming an incidence of delirium of 489 per 1000 people in the ICU with usual care, we estimated that 220 fewer people per thousand would experience delirium if using earplugs or eye masks or both (CI 98 to 303 fewer people per thousand). However, we rated the quality of the evidence for this finding as low.
Several studies assessed subjective sleep quantity or quality with the various non‐pharmacological interventions in ICU patients (Borromeo 1998; Chen 2012; Gao 2008; Gragert 1990; Hu 2010; Le Guen 2014; Li 2011; Martin 2008; Namba 2012; Richardson 2003; Ruan 2006; Ryu 2012; Scotto 2009; Sha 2013; Su 2013; Toublanc 2007; Van Rompaey 2012; Wang 2012; Xie 2011). Overall, we rated the quality of the evidence for objective sleep quality/quantity as low. Using various subjective scales, six studies individually reported some benefit of earplugs or eye masks or both on subjective sleep quality (Hu 2010; Le Guen 2014; Martin 2008; Scotto 2009; Van Rompaey 2012; Xie 2011). Pooled data from two of these studies showed a benefit for the use of earplugs/eye masks compared with usual care (Le Guen 2014; Xie 2011; 116 participants). The mean difference in total sleep quantity versus usual care was 2.19 hours (95% CI 0.41 to 3.96) although we observed evidence of heterogeneity (I² statistic = 79%). The quality of the evidence for the effect of this intervention on sleep quantity (assessed subjectively) was low due to heterogeneity and an unclear or high risk of selection and detection bias in these studies. Individual studies also provided some evidence that music interventions may improve subjective sleep quantity or quality (Ryu 2012; Sha 2013; Su 2013). However, findings were inconsistent across studies, and the studies had a high risk of selection bias. Therefore, we considered the quality of the evidence for an effect of music intervention on subjective sleep quantity/quality as very low. Several included studies examined alternative and complementary therapies; relaxation techniques (Richardson 2003; Ruan 2006), foot massage or foot bath (Namba 2012; Wang 2012), acupressure (Chen 2012), nurse or social intervention (Gao 2008; Li 2011), and sound masking (Gragert 1990) may offer some benefit in terms of subjectively measured sleep quantity or quality. However, the number of studies per intervention type was minimal (i.e., one or two studies), and the studies had an unclear or high risk of selection bias. Therefore, we rated the quality of the evidence for an effect of these interventions on subjectively measured sleep quantity/quality as low.
None of the interventions examined in this review were assessed in relation to mortality, risk of post‐traumatic stress disorder, or economic cost.
Overall completeness and applicability of evidence
The review included 29 randomized controlled trials (RCTs) and one quasi‐RCT. Because of the small number of studies per intervention and the different outcomes used across studies, we could not incorporate many studies into meta‐analyses in this review.
We found very limited evidence supporting non‐pharmacological interventions, such as massage, acupressure, imagery relaxation, nursing intervention, and social support. Most of these trials had small sample sizes, and none of the trials measured longer‐term clinical outcomes.
Interestingly, we found that ongoing studies are assessing several other non‐pharmacological interventions, including environmental modification, behavioural interventions, massage therapy, and 'device modifications' (see Ongoing studies). The excluded studies also examined several other non‐pharmacological interventions; these included aromatherapy (Cho 2013), use of earplugs and eye protective devices (House 2003; Koo 2008), an ICU‐wide quality improvement intervention (Kamdar 2013), therapeutic touch (Cox 1999), a postoperative pain treatment programme (Diby 2008), a sedation wake‐up trial and spontaneous breathing trial (Figueroa‐Ramos 2010), and implementing a "quiet time" protocol to reduce ICU environmental stimuli (Olson 2001). We excluded the majority of these trials as they were not RCTs, and most used non‐equivalent group designs.
The frequency and duration of the interventions varied widely across the trials. There were relatively small numbers of participants in all of the included studies, and few studies used power analysis, thereby, limiting study power. It was often difficult to collate and interpret information from the included studies due to inconsistency in the outcomes studied between the included trials. For example, few studies reported the same sleep outcomes or type of data with respect to PSG sleep variables. Similarly, few studies that assessed subjective sleep outcomes used the same sleep scales to measure subjective sleep quality. All of these factors contributed to our overall rating of the quality of the evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE). None of the included trials provided data on the effects of the non‐pharmacological interventions on mortality, risk of post‐traumatic stress disorder (PTSD), and cost effectiveness in ICU patients.
Quality of the evidence
A large number of the included studies had an unclear or high risk of allocation bias as methods of random sequence generation or allocation concealment or both were often inadequately reported or inappropriate. Furthermore, blinding of participants and personnel was often not possible for non‐pharmacological treatments, such as massage, use of earplugs and eye masks, imagery, relaxation, music therapy, or social support. As many of the trials in this review included subjective outcomes, such as subjective sleep scores, there was a high risk of performance bias associated with many of the studies. For many of the included studies, there was a need for additional methodological and statistical information, which if available, could have improved the quality of the evidence in the review. Additionally, many of the included studies provided insufficient information about general characteristics before randomization, and the majority of included studies had relatively small numbers of participants (most trials did not use power analysis), thus, limiting the power of the trials. Finally, due to substantial clinical heterogeneity, it was generally not possible to pool data across studies of the same intervention type, and findings from individual studies of the same intervention type were often inconsistent. In summary, all of these factors provided rationale for rating the quality of the evidence as low or very low (Table 1).
Potential biases in the review process
Our goal was to determine whether a range of non‐pharmacological interventions were effective for sleep promotion in ICU patients. We developed our search strategy to cover as many terms as possible. We searched all available databases, checked the reference lists of all relevant trials, and included trials without restricting language for both published and unpublished studies. Where necessary, we contacted the authors for additional unpublished information. However, it remains possible that we missed some published and unpublished trials. In the several instances where we contacted lead authors to request additional data and detailed information regarding research practice, we often failed to receive a reply from the authors (See Characteristics of included studies).
Agreements and disagreements with other studies or reviews
Music interventions have been cited as helpful measures to improve mood and reduce anxiety in coronary heart disease patients (Bradt 2009) and mechanically ventilated patients (Bradt 2010) and to reduce pain in cancer patients (Bradt 2011). In a systematic review (de Niet 2009), music‐assisted relaxation had a moderate effect on the sleep quality of participants with sleep complaints, possibly via effect on psychological outcomes (e.g., by assisting the relaxation for ICU patients). These findings are supported by those of a Cochrane systematic review, which suggested that music listening may have a large anxiety‐reducing effect on mechanically ventilated patients (Bradt 2014). These reviews reported no adverse reactions or outcomes relating to participant satisfaction.
An earlier systematic review by Richards and colleagues, Richards 2000a, examined the effects of massage on relaxation, comfort, and sleep in acute and critical care settings. In agreement with our findings, the review concluded that the clinical data were insufficient and further studies were required. Another systematic review, Richards 2003, presented the complementary and alternative therapies for promoting sleep in critically ill patients. The review searched the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and MEDLINE databases and limited to papers in the English language from 1982 to 2002. Therapies included massage, relaxation technique, aromatherapy, therapeutic touch, environmental interventions, music therapy, and alternative sedatives. Although this review focused on the interventions and did not assess quality of the evidence, the authors conclusions were similar to those that we obtained: that there is currently insufficient evidence relating to the efficacy of non‐pharmacological interventions for sleep promotion in critically ill patients. A more recent systematic review, Tamrat 2014, identified non‐pharmacologic interventions for improving sleep quality and quantity of non‐intensive care unit inpatients. Again, this review found insufficient evidence to support the use of any non‐pharmacologic intervention for improving sleep quality or quantity in general inpatients. Finally, it will be interesting to examine our findings alongside those of a future Cochrane systematic review, which plans to evaluate the use of pharmacological agents for the promotion of sleep in the intensive care unit (Evans 2016).
Authors' conclusions
Implications for practice.
Mechanical ventilation is an important contributing factor to sleep deprivation. In this review, several studies investigated the effects of ventilator modes on sleep outcomes, although we were unable to perform meta‐analysis of these studies. There was some evidence from individual studies to suggest that pressure‐controlled ventilation mode, assist‐control ventilation mode, and proportional assist ventilation mode may all improve sleep quantity or quality or both compared with pressure support ventilation mode. However, we noted some inconsistent findings between studies, and we rated the overall quality of the evidence as very low.
Our findings suggest that non‐pharmacological interventions, such as the use of earplugs or eye masks or both, may have some beneficial effects on sleep promotion and potentially decrease the risk of delirium in intensive care unit (ICU) adult patients. However, again, the quality of the evidence was generally low due to inconsistency in the findings of the contributing studies and the risk of bias associated with these studies. If using earplugs and eye masks, careful consideration should be given to implementation. For example, Scotto 2009 reported that some ICU patients were unwilling to use earplugs or eye masks or both because they found them uncomfortable or they fell out during sleep. Therefore, it may be important to provide alternative designs of earplugs or eye masks, or for clinical staff to help with the correct insertion of the earplugs.
Implications for research.
The quality of existing evidence relating to the use of non‐pharmacological interventions for sleep promotion in ICU patients is low or very low. Whilst these interventions are often difficult to assess in the ICU setting and some of the methodological difficulties (e.g., blinding) relate to the nature of the interventions, we have several recommendations for future research in this area.
Generally, future studies should ensure the following:
Provide power calculations so that adequate sample sizes are used and where possible use as large a sample size of participants as is feasible.
Ensure low risk of bias through rigorous methodological development and reporting. For example, trials need to use reliable methods of allocation concealment, and methods of blinding should be as robust as possible. It is essential that trial design methods and outcomes are better reported, including randomization methods, loss to follow up, and details of prespecified outcomes measures.
Include an assessment of sleep‐related outcomes using polysomnography, which represents the gold standard of sleep measurement; relatively few published studies use this technique.
Outcomes should focus not only on sleep outcomes but also on the clinical outcomes, such as mortality, incidence of adverse events, or the risk of delirium or PTSD. Greater inclusion of outcomes relating to participant satisfaction, length of ICU stay, or health economics would also be desirable.
Include a validated sleep scale to measure subjective sleep quality. (A validated consensus instrument is required for comparison of studies in different countries.)
Specifically, we would recommend that more research is needed to test the effects of music intervention on objective sleep outcomes, ideally using polysomnography (PSG). A greater volume of research is needed for interventions, such as massage, acupressure, music therapy, environmental intervention, behaviour therapy, and psychological support, all in the ICU setting. (These interventions are used widely for sleep promotion in other clinical settings.)
Finally, we note that the analysis of data from cross‐over trials is critical for systematic reviews in this area. Therefore, a consensus in the method of reporting outcomes from cross‐over trials is required (e.g., reporting first period data and full period data separately). We also recommend that cross‐over trials include an adequate washout period between interventions; an inadequate washout period could potentially confound the findings of studies where the intervention serves to improve sleep via anxiolytic effects.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Professor Wu Taixiang (Chinese Cochrane Centre); Professor Chen Junmin (Department of Haematology and Rheumatology, The First Affiliated Hospital of Fujian Medical University); Dr Karen Hovhannisyan (Cochrane Anaesthesia Review Group (CARG) Trials Search Co‐ordinator); and Jane Cracknell (Cochrane Anaesthesia, Critical and Emergency Care (ACE) Managing Editor).
We would like to thank Mathew Zacharias (content editor), Georgopoulos Dimitris, G Iapichino, G Mistraletti, Matthew Bailey (peer reviewers), NL Pace (statistical editor), and Janet Wale (consumer editor) for their help and editorial advice during the preparation of the protocol (Hu 2010) for the systematic review.
We would like to thank Bronagh Blackwood (content editor); Janet Wale (consumer editor); Matthew Bailey, Jaap Lancee, Paul Montgomery, and Paula L Watson (peer reviewers) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Interventions
01 Psychological interventions, cognitive or behavioural therapy such as music therapy, back massage, muscle relaxation, imagery, therapeutic touch.
02 Environmental interventions, such as noise reduction, lighting control, synchronization of ICU activities with daylight.
03 Social support interventions
04 Physical therapy modalities
05 Equipment modification, including mechanical ventilation.
06 Comentary therapy such as aromatherapy, herbs, acupuncture, acupressure.
Appendix 2. Search strategy for CENTRAL
| #1 | MeSH descriptor Complementary Therapies explode all trees |
| #2 | MeSH descriptor Music Therapy explode all trees |
| #3 | MeSH descriptor Massage |
| #4 | MeSH descriptor Muscle Relaxation explode all trees |
| #5 | MeSH descriptor Imagery (Psychotherapy) explode all trees |
| #6 | MeSH descriptor Cognitive Therapy explode all trees |
| #7 | MeSH descriptor Behavior Therapy explode all trees |
| #8 | MeSH descriptor Social Support explode all trees |
| #9 | MeSH descriptor Physical Therapy Modalities explode all trees |
| #10 | MeSH descriptor Aromatherapy explode all trees |
| #11 | MeSH descriptor Sleep explode all trees |
| #12 | ((Music or complementary or alternative or cognitive or behavioural) near therap*):ti,ab |
| #13 | (imagery or massage or muscle relaxation or therapeutic touch or aromatherapy):ti,ab |
| #14 | ((environmental or cognitive or behavioural or interventions or social support) near intervention*):ti |
| #15 | (nighttime light or noise level* ):ti,ab |
| #16 | (sleep near (promot* or help* or support* or Initiat*)) |
| #17 | sleep:yi,ab |
| #18 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) |
| #19 | MeSH descriptor Critical Illness explode all trees |
| #20 | MeSH descriptor Critical Care explode all trees |
| #21 | MeSH descriptor Intensive Care explode all trees |
| #22 | MeSH descriptor Intensive Care Units |
| #23 | ((intensive or critical) near unit*) |
| #24 | (critical* near ill*) |
| #25 | (#19 OR #20 OR #21 OR #22 OR #23 OR #24) |
| #26 | (#18 AND #25) |
Appendix 3. Search strategies
01 Search Strategy for MEDLINE (via OVID 1950 to May 2014)
1. Complementary Therapies/ or Music Therapy/ or Massage/ or Muscle Relaxation/ or "Imagery (Psychotherapy)"/ or Cognitive Therapy/ or Behavior Therapy/ or Social Support/ or Physical Therapy Modalities/ or Aromatherapy/ or ((Music or complementary or alternative or cognitive or behavioural) adj3 therapy*).ti,ab. or (imagery or massage or muscle relaxation or therapeutic touch or aromatherapy).ti,ab. or ((environmental or cognitive or behavioural or interventions or social support) adj3 intervention*).ti,ab. or (nighttime light or noise level* ).ti,ab. 2. exp Sleep/ or (sleep adj3 (promot* or help* or support* or Initiat*)).mp. or sleep.ti,ab. 3. 1 or 2 4. exp Critical Illness/ or exp Critical Care/ or exp Intensive Care/ or exp Intensive Care Units/ or (((intensive or critical) adj3 unit*) or (critical* adj3 ill*)).mp. 5. 4 and 3 6. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trial.af. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 7. 6 and 5
02 Search Strategy for EMBASE <1980 to April 2014> #1 (((Music or complementary or alternative or cognitive or behavioural) adj3 therap*) or (imagery or massage or muscle relaxation or therapeutic touch or aromatherapy) or ((environmental or cognitive or behavioural or interventions or social support) adj3 intervention*) or (nighttime light or noise level* or melatonin)).ti,ab. or (sleep adj3 (promot* or help* or support* or Initiat*)).mp. or sleep.ti,ab. #2 alternative medicine/ or music therapy/ or massage/ or muscle relaxation/ or imagery/ or cognitive therapy/ or behavior therapy/ or social support/ or physiotherapy/ or aromatherapy/ or exp sleep/ #3 #1 or #2 #4 intensive care unit/ or ((intensive or critical) adj3 unit*).ti,ab. #5 #3 and #4 #6 (controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. #7 #5 and #6 #8 from #7 keep #1
03 Search Strategy for CINAHL via EBSCO host <1982 to July 2013> #S1 (MH "Alternative Therapies") #S2 (MM "Music Therapy") #S3 (MH "Massage") #S4 (MH "Muscle Relaxation") #S5 (MH "Cognitive Therapy") #S6 (MH "Behavior Therapy") #S7 (MH "Social Support (Iowa NOC)") or (MH "Social Support Index") #S8 (MH "Physical Therapy") #S9 (MH "Aromatherapy") #S10 (MH "Sleep") #S11 AB ((Music or complementary or alternative or cognitive or behavioural) and therap*) #S12 AB imagery or massage or muscle relaxation or therapeutic touch or aromatherapy #S13 AB ((environmental or cognitive or behavioural or interventions or social support) and intervention*) #S14 AB nighttime light or noise level* #S15 AB ( (sleep and (promot* or help* or support* or Initiat*)) ) or TI sleep #S16 #S1 or #S2 or #S3 or #S4 or #S5 or #S6 or #S7 or #S8 or #S9 or #S10 or #S11 or #S12 or #S13 or #S14 or #S15 #S17 (MM "Critical Illness") or (MM "Critically Ill Patients") #S18 (MM "Critical Care") #S19 (MH "Intensive Care Units") #S20 AB ( (intensive or critical) and unit* ) or AB ( critical* and ill* ) #S21 #S17 or #S18 or #S19 or #S20 #S22 #S16 and #S21 #S23 (MM "Random Assignment") #S24 AB random* or AB controlled trial* #S25 #S23 or #S24 #S26 #S22 and #S25
Appendix 4. Search strategy for ISI Web of Science
#1 Topic=(Critical Illness or Critical Care or Intensive Care or Intensive Care Units or critical* ill*) #2 Topic=(Sleep or sleep promot* or help* or support* or Initiat*) #3 Topic=(randomized controlled trial or controlled clinical trial or placebo or clinical trial or randomly or trial) #4 Topic=(Music or complementary or alternative or cognitive or behavioural therap* or imagery or massage or muscle relaxation or therapeutic touch or ventilation or aromatherapy or environmental or cognitive or behavioural or interventions or social support intervention* or nighttime light or noise level*) $5 #4 AND #3 AND #2 AND #1
Appendix 5. Data Extraction Form
CARG 200 Non‐pharmacological interventions for sleep promotion in intensive care unit
Data Extraction Form
Reviewer ________________________
Reference number ________________
Study ID ________________________
Date of review ___________________
| First author | Journal/Conference Proceedings, etc. | Year/language |
| title | ||
Study eligibility form
| RCT/Quasi/CCT? (delete as appropriate) |
Relevant participants Adult critically ill patients admitted to the intensive or critical care unit |
Relevant interventions Complementary and alternative therapies (music, therapy, back massage, muscle relaxation, imagery, therapeutic touch, aromatherapy, herbs, etc.), physical therapy modalities, environmental interventions,social support interventions, equipment modification. |
Relevant outcomes Changes in objective and subjective sleep variables, length of stay in ICU, patient satisfaction, any adverse reactions or events, mortality, risk of delirium during ICU stay, risk of PTSD, cost |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/No*/Unclear |
I f issue relates to selective reporting (i.e. when authors may have taken measurements for particular outcomes, but not reported these), reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in 'Studies awaiting assessment' until clarified. If no clarification is received after three attempts, study should then be excluded.
Final decision: Include □ Unclear □ Exclude □
| Do not proceed if any of the above answers are 'No'. If study to be included in 'Excluded studies' section of the review, record below the information to be inserted into 'Table of excluded studies' |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings, etc. | Year |
| A | The paper listed above | ||
| B | Further papers |
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc.) | |
| Sex of participants (numbers/%, etc.) | |
| Disease status/type, etc. (if applicable) | |
| Settings | |
| Other | |
| Trial characteristics | |
| Further details | |
| Single centre/multicentre | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Treatment(s) used | |
| Dose/frequency of administration | |
| Duration of treatment (State weeks/months, etc., if cross‐over trial, give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months, or years or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan | |
| Trial design (e.g., parallel/cross‐over*) | |
| Other | |
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (delete as appropriate) |
| Note reason for allocation: |
Adequate (Random) |
| Inadequate (e.g., alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (delete as appropriate) |
| Note reason for allocation: | Adequate |
| Inadequate | |
| Unclear | |
| Blinding | |
| Person responsible for participant's care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
| Note reason for blinding: | |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as 'intention‐to‐treat' | |
| Unclear | |
Were withdrawals described? Yes □ No □ not clear □
Discuss if appropriate
Data extraction
| Outcomes relevant to your review | |
| Reported in paper | |
| Outcome 1 Changes in objective sleep variables including sleep efficiency index and/or REM sleep latency and/or REM sleep time and/or arousals index and/or latency to sleep onset and/or total sleep time and/or percentage of stage 1, 2, 3, 4 | Yes/No Specify: |
| Outcome 2 Changes in subjective sleep quality and quantity | Yes/No Specify: |
| Outcome 3 Length of stay in ICU | Yes/No Specify: |
| Outcome 4 Cost | Yes/No Specify: |
| Outcome 5 Patient satisfaction | Yes/No |
| Outcome 6 Adverse reactions or events | Yes/No Specify: |
| Outcome 7 Mortality | Yes/No Specify |
| Outcome 8 Risk of delirium during ICU stay | Yes/No Specify |
| Outcome 9 Risk of PTSD once discharged from hospital | Yes/No Specify |
| For continuous data | |||||||
| Code of paper | Outcomes (rename) |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A etc | Objective sleep variables, such as REM sleep latency and/or REM sleep time and/or total sleep time and/or latency to sleep onset and/or sleep period time | Minute | |||||
| Objective sleep variables, such as sleep efficiency index and/or arousals index and/or percentage of stage 1, 2, 3, 4 and/or numbers of awakenings | |||||||
| Subjective sleep quality | |||||||
| Subjective sleep quantity | Minute | ||||||
| Length of stay in ICU | hour | ||||||
| Patient satisfaction | |||||||
| Cost | dollar | ||||||
| For dichotomous data | |||
| Code of paper | Outcomes (rename) | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
|
A B C D E |
Adverse reactions or events Mortality Risk of delirium during ICU stay Risk of PTSD once discharged from hospital |
||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
Data and analyses
Comparison 1. Ear plugs or eye mask versus usual care or both.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of delirium and confusion | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.80] |
| 2 Total sleep time | 2 | 116 | Mean Difference (IV, Random, 95% CI) | 2.19 [0.41, 3.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alexopoulou 2007.
| Methods | Design: cross‐over RCT Setting: single‐bed rooms in an intensive care unit, Greece |
|
| Participants |
Inclusion criteria
Exclusion criteria
17 participants (received mechanical ventilation for at least 48 hours) were studied Age: 63.9 ± 16.7 years Sex: 6 women, 11 men Number included: 17 Number analysed in protocol A (sedated): 11 Number analysed in protocol B (without sedation): 9 3 participants were studied in both protocols |
|
| Interventions |
"PShigh was obtained by increasing the pressure assist level by 40‐50% or until Paw reached 30 cm H₂O. PAV+high was obtained by increasing the percentage of unloading by 40‐50% or until the assist reached a value of 85%" Intervention duration: 2.5 hours for each period in protocol A (from 9:00 p.m. to 7 a.m. over 1 night) and at least 3 hours in protocol B (from 11.00 p.m. to 6.00 a.m. over 2 consecutive nights) There was no washout between cross‐over periods |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Protocol A: sedated participants Protocol B: non‐sedated participants |
|
| Notes | Author Georgopoulos D provided additional data and the study protocol via email Only the whole period of the cross‐over study was analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was randomized (concealed envelopes) to receive each mode Comment: insufficient details were provided |
| Allocation concealment (selection bias) | Low risk | The trial used concealed envelopes (emailed author response) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants were ventilated randomly either with pressure support or with PAV+ mode, so it was not possible to blind personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Unclear risk | In 2006, Dimitris Georgopoulos received 4500 euros as a lecture fee (honoraria) from the company TYCO |
Andréjak 2013.
| Methods | Design: randomized, cross‐over study to compare the impact of pressure‐controlled ventilation (PCV) with spontaneous ventilation with 6 cm H₂O inspiratory pressure (low PSV) on sleep Setting: isolated single rooms, respiratory ICU, France |
|
| Participants | ICU patients with acute‐on‐chronic respiratory failure and near to being weaned off mechanical ventilation Inclusion criteria
Exclusion criteria
Age: 67 ± 11 years Sex: 23 men, 3 women Number included: 35 Number analysed: 26 Number of assessable participants in PCV first/low PSV first arms: 13/13 |
|
| Interventions |
PCV: inspiratory pressure support was set at 20 cm H₂O with the respirator‐frequency set to provide complete disappearance of spontaneous inspiratory efforts. Inspiratory time was set to provide an I/E ratio of between 1/1.2 and 1/1.5 Low PSV: participants breathed spontaneously via the respirator's circuitry, with a pressure‐support level of 6 cm H₂O and a trigger sensitivity of 0.5 cm H₂O Intervention duration: 13 participants received PCV first (10 p.m. to 2 a.m.) and then low PSV (2 a.m. to 6 a.m.); 13 participants received low PSV first and then PCV There was no washout between cross‐over periods |
|
| Outcomes |
Primary outcomes
|
|
| Notes | The 2 groups were similar in terms of anthropometric data, pulmonary function tests, and arterial blood gas data sampled before randomization Only the whole period of the cross‐over study was analysed Sample size calculation: a power of 80% and an alpha risk of 5% We contacted the author via email and acquired additional information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated through lots |
| Allocation concealment (selection bias) | Low risk | The trial used the closed‐envelope method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The physician who scored the polysomnographic recordings was not aware of the randomization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The personnel were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35 participants were included; 9 were discarded |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | No other bias was described |
Borromeo 1998.
| Methods | Design: randomized cross‐over trial Settings: coronary care unit with 20 beds, single room for each bed, USA |
|
| Participants | All participants were diagnosed with 1 of the following diseases: chest pain, R/O MI, unstable angina Inclusion criteria
Exclusion criteria
Total number randomized: 25 Total number analysed: 25 Age: from 38 to 82 years old, 62 ± 3 years Sex: 18 men, 7 women |
|
| Interventions |
Study duration: 1 night for the aromatherapy treatment, 1 night for the control treatment, 15 hours for washout Washout period = 15 hours |
|
| Outcomes |
Primary outcomes
|
|
| Notes | We were unable to contact the author by email A power calculation was used for the sample size Only the whole period of the cross‐over study was analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The treatment order was randomized. There was insufficient detail of the sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measures were used for subjective sleep quality |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was reported |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgement |
| Other bias | Unclear risk | There may have been some confounding variables, such as medication and ICU environment, which may have disturbed the effects |
Bosma 2007.
| Methods | Design: randomized, cross‐over clinical trial to assess quality and quantity of sleep during PSV and PAV Setting: a 12‐bed ICU, arranged as a row of 3 rooms with 4 participants per room, Italy |
|
| Participants | "Patients during weaning from mechanical ventilation, between 18 and 75 yrs of age, mechanically ventilated for ≥ 3 days and sedated with midazolam, lorazepam, or propofol according to the daily interruption protocol at doses not higher than 0.05, 0.01, and 2 mg/kg/hr, respectively, were eligible to participate in the study" Inclusion criteria
Exclusion criteria
Participants were withdrawn from the study at any time for the following a priori defined conditions: "a) need for inotropic support, sedation, or analgesia with morphine at a dosage > 0.01 mg/kg/hr; b) readiness for extubation; c) haemodynamic instability, arrhythmia, PaO₂/FIO₂ ratio less than 200, PH less than 7.35 or less than 7.45, or temperature > 37.5 ∘C" Total number randomized: 16 Total number analysed: 13 Numbers of assessable participants in PAV first/PSV first arms: 7/6 Mean age: 63 ± 13 years Sex: 3 women, 10 men |
|
| Interventions |
"Patients were randomized to receive PSV or PAV on the first night and then crossed over to the alternative mode for the second night" Study duration: 1 day for each period. There was no washout between cross‐over periods |
|
| Outcomes |
Primary outcomes
Secondary outcomes
"All data were recorded from 10:00 PM to 8:00 AM for the two consecutive study nights" |
|
| Notes | On the first study nights, baseline values of PaO₂, PaCO₂, and arterial pH did not differ between PAV and PSV. Maximum and mean environmental noise and light did not differ between PSV and PAV Author Bosma K provided additional data via email Only the whole period of the cross‐over study was analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of the randomization method were not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An expert blinded to respiratory signals manually scored all polysomnography records |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data were described in detail. 16 participants met enrolment criteria; 3 participants were withdrawn because of sepsis (2 participants) and severe hypoxaemia (1 participant) |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Unclear risk | Università di Torino (grant PR60ANRA02) and Regione Piemonte (grant CEPANMAS03) supported, in part, the trial "Dr. Ranieri is on the advisory board for Maquet and received unopposed research grants from Tyco, Draeger, and Hamilton. The remaining authors have not disclosed any potential conflicts of interest" |
Cabello 2008.
| Methods | Design: randomized, cross‐over clinical trial to compare the influence of 3 ventilatory modes on sleep Setting: a 24‐bed medical ICU, France |
|
| Participants |
Inclusion criteria
"All were ventilated through an endotracheal tube or a tracheostomy" Exclusion criteria
Number randomized: 15 Number analysed: 15 Ages: range from 47 to 84 years, mean ages = 70 ± 13 years old Sex: 11 men and 4 women During the first study stage, there were 4 participants in ACV, 5 in aPSV, and 6 in cPSV During the second study stage, there were 5 participants in each ventilatory mode During the third study stage, there were 6 participants in ACV, 5 in aPSV, and 4 in cPSV |
|
| Interventions |
Participants were successively ventilated with ACV, cPSV, and aPSV in a randomized order during 3 successive periods of 6 hours: a daytime period from 2 p.m. to 8 p.m., a first nocturnal period from 8 p.m. to 2 a.m., and a second nocturnal period from 2 p.m. to 8 a.m. Study duration: 6 hours for each period There was no washout between cross‐over periods |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Sleep variables were expressed as a median (25th–75th percentile) Only the whole period of the cross‐over study was analysed We attempted to contact author Dr Cabello via email; however, we received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized order" was mentioned, but there was a lack of description about the randomization procedure or method |
| Allocation concealment (selection bias) | Low risk | The trial used a closed‐envelope technique |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor was blinded. A neurologist blinded to the study manually scored sleep recordings |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were successively ventilated with ACV, cPSV, and aPSV; it was not possible to blind the personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported. All participants completed the study and were included in analyses |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | Grants from the Instituto de Salud Carlos III (expedient CM04/00096, Ministerio deSanidad) and the Instituto de Recerca Hospital de la Santa Creu Sant Pau (BC) in part supported the study |
Chen 2012.
| Methods | Design: randomized clinical trial to test the effectiveness of valerian acupressure on the sleep of participants in the ICU Settings: a 42‐bed adult intensive care unit, 28 single‐bed rooms and a 24‐bed ward, Taiwan, China |
|
| Participants |
Inclusion criteria
Exclusion criteria
Number randomized: 85 (41 in the experimental group and 44 in the control group) Number analysed: 85 Mean ages: 72.1 years old in the experimental group, 69.1 years old in the control group Sex: 30 men/41 women in the experimental group, 35 men/9 women in the control group |
|
| Interventions |
|
|
| Outcomes |
Primary outcomes
Other outcome
|
|
| Notes | As for baseline, the mean age and mean APS scores of the experimental group were higher than those of the control group; the 2 groups did not show any statistically significant differences in their baseline observed sleep and sleep measurements (the first night) Sample size calculation was used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly selected a numbered (1 to 10) stick from a bin. Participants who had selected odd numbers were assigned to the control group, and the participants who had drawn even numbers were assigned to the experimental group |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed as the investigator knew the relevance of an odd or even draw |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was unable to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and were included in analyses |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | High risk | The mean age and mean APS scores of the experimental group were higher than those of the control group. SSS ratings in the baseline (namely first night) were significantly lower in the intervention group than the control group (P value < 0.01) |
Córdoba‐Izquierdo 2013.
| Methods | Design: RCT, 2‐arm, parallel group design Setting: medical ICU, France |
|
| Participants | 24 participants admitted for acute hypercapnic respiratory failure requiring non‐invasive ventilation Inclusion criteria
Exclusion criteria
Total number randomized: 25 (12 in the NIVICU group and 13 in the NIVD group) Total number analysed: 24 (12 in both groups) Age: mean age: 69 years, range from 65 to 77 years Sex: 14 men, 10 women |
|
| Interventions |
Study duration: 1 night |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | There were no differences between groups, including the time under NIV previous to the study inclusion. The only difference was a higher Epworth Sleepiness Scale score in the NIVD group than in the NIVICU group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomized, but sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The sleep scorer was blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was excluded from the analysis as a result of technical problems during the recordings |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | High risk | The baseline Epworth Sleepiness Scale scores significantly differed between the 2 groups |
Foreman 2013.
| Methods | Design: 2‐arm, parallel group design RCT Setting: neurological ICU |
|
| Participants | Adult neurological ICU patients undergoing continuous electroencephalography Total number randomized: 12 participants (6 in each arm) Mean age: 57.9 years old |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There were no significant differences between those who received the intervention and those who did not regarding illness severity, intubation, or neurological exam As this was a conference abstract, we could not access the original full paper and contact author The sample size was small |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomized, but there was no description of the randomization |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This was a conference abstract, so there was insufficient information to permit judgement of risk of bias |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgement of risk of bias |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Gao 2008.
| Methods | Design: 2‐arm, parallel RCT Settings: coronary care unit, China |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number randomized: 106 (53 in each group) Total number analysed: 106 Mean age: 54.94 ± 10.51 years Sex: 63 men, 43 women |
|
| Interventions |
Study duration: 7 days |
|
| Outcomes |
Primary outcomes
Other outcome
|
|
| Notes | The general characteristic of the 2 groups before randomization did not differ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The daily cumulative sleeping time was measured by unblinded nurses' observation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was unable to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant flow was not described |
| Selective reporting (reporting bias) | Low risk | The stated outcomes were all addressed in the report |
| Other bias | Unclear risk | COIs were not provided |
Gragert 1990.
| Methods | Design: 2‐arm, parallel RCT Settings: an 11‐bed coronary care unit, single bedrooms, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number randomized: 40 (20 in each group) Total number analysed: 40 Sex: 20 men and 20 women Ages: ≥ 65 years of age, mean age = 72.9 ± 7.09 years |
|
| Interventions |
Study duration: 1 night |
|
| Outcomes |
Primary outcomes
|
|
| Notes | The author used 2‐way ANOVAs with noise, gender, and sleep outcomes as factors to analyse the effects and their interactions, without providing the detail of mean and SD of sleep outcomes in each group; we were unable to make contact with the author to acquire the additional data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized by drawing a random number |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measures were used for subjective sleep quality (non‐blinded) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgement of risk of bias |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Hu 2010.
| Methods | Design: 2‐arm, parallel RCT Settings: a cardiac surgical intensive care unit with 17 beds, open room, China |
|
| Participants | Adult cardiac surgical participants Inclusion criteria
Exclusion criteria
Number randomized: 50 Number analysed: 45 (25 in the intervention group, 20 in the control group) Mean age: 56.7 years Sex: 27 men, 18 women |
|
| Interventions |
Study duration: 3 days |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used block randomization; a random number table was used to select the blocks |
| Allocation concealment (selection bias) | High risk | The trial used the closed‐envelope technique, but the researcher was also the person responsible for recruitment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report measures were used for subjective sleep quality |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 15% of participants were excluded (N = 5) |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | There were no conflicts of interest |
Jaber 2007.
| Methods | Design: cross‐over design RCT Setting: ICU, medicosurgical department of anaesthesia and resuscitation, 16 beds, France |
|
| Participants | 30 participants were included: 15 non‐intubated participants and 15 intubated participants during weaning from mechanical ventilation Inclusion criteria
Inclusion criteria specific for the intubated group
Number randomized: 35 Number analysed: 30 Ages: 57.5 ± 12 years Sex: 17 men, 13 women |
|
| Interventions |
Music therapy was not performed in 5 participants (5/35 = 14%) |
|
| Outcomes |
Primary outcomes
|
|
| Notes | Only the whole period of the cross‐over study was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There were insufficient details |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel and participants to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants did not have music therapy (5/35 = 14%) |
| Selective reporting (reporting bias) | Unclear risk | Results were reported for all stated outcomes |
| Other bias | Low risk | There were no conflicts of interest |
Le Guen 2014.
| Methods | Design: 2‐arm, parallel design RCT Setting: Paris, France, a 1200‐bed university‐based teaching hospital, postanaesthesia care units (PACUs), an L‐shaped open ward |
|
| Participants | "46 patients without any neurological or respiratory failure undergoing major non‐cardiac surgery were included" Inclusion criteria
Exclusion criteria
Number randomized: 46 Number analysed: 41 (20 in the intervention group, 20 in the control group) Mean age: 60.5 years Sex: 34 men, 7 women |
|
| Interventions |
Study duration: 1 night of using earplugs, 1 night without earplugs |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Sample analysis was used; no difference was shown in participant characteristics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was a randomized study. There were insufficient details of sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measures were used for subjective sleep scores. Participants were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind the participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 participants were included; 5 participants were excluded from the final analysis (3 in the intervention group, 2 in the control group) |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | No COIs were declared |
Li 2011.
| Methods | Design: 2‐arm, parallel RCT Settings: coronary care unit, China |
|
| Participants |
Inclusion criteria
Number randomized: 52 (26 in both groups) Mean age: 64 years Sex: 29 men, 23 women |
|
| Interventions |
Study duration: 2 weeks |
|
| Outcomes |
|
|
| Notes | There was no provided information about general characteristics before randomization between the groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial was randomized (used a table of random numbers) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measures were used for subjective sleep quality. Participants were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind the personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant flow was not described |
| Selective reporting (reporting bias) | High risk | The trial did not provide information about general characteristics before randomization between the groups or the baseline sleep scores |
| Other bias | Unclear risk | Baseline sleep scores were not reported |
Martin 2008.
| Methods | Design: randomized, cross‐over trial Settings: ICU and telemetry unit at St. Vincent Healthcare in Billings, Montana, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Number randomized: 14 Number analysed: 10 Mean age: 66 ± 11.29 years Sex: 6 men, 4 women |
|
| Interventions |
Study duration: 1 night of using earplugs, 1 night without earplugs |
|
| Outcomes |
|
|
| Notes | Sample size calculation was used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to earplug use on either the first or second night. The researcher selected 1 of 2 folded pieces of paper. 1 piece read control and 1 read earplugs |
| Allocation concealment (selection bias) | High risk | The researcher generated the random sequence and did the research by herself. See above ‐ the paper was folded but not sealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher generated the random sequence and administered the VSH Sleep Scale questionnaire by herself |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 of the 14 participants were able to complete the 2 nights of study |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Namba 2012.
| Methods | Design: randomized, cross‐over trial to examine the effects of foot baths on sleep outcomes Settings: ICU, Okayama University Hospital, Japan |
|
| Participants | 6 ICU patients Exclusion criteria
Mean age: 65 years Sex: 3 women and 3 men |
|
| Interventions |
Study duration: a foot bath night and a non‐foot bath night Washout duration: at least 1 non‐foot bath day was provided between foot bath days |
|
| Outcomes |
Primary outcomes
PSG was performed from 9 p.m. to 6 a.m. on both days Secondary outcomes
|
|
| Notes | "Two patients had been prescribed sedatives which affected sleep directly. Three patients had been prescribed sleep medications" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was generated using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Unclear risk | No COIs were declared |
Parthasarathy 2002.
| Methods | Design: randomized, cross‐over clinical trial to assess the quality and quantity of sleep during ACV, PSV alone, and PSV with dead space Setting: pulmonary and critical care unit, USA |
|
| Participants | 11 male mechanically ventilated participants (ventilated through an endotracheal tube or tracheostomy) were recruited, aged 49 to 90 years All were receiving sedatives Exclusion criteria
Total number randomized: 11 Total number analysed: 11 Mean age: 67 years Sex: all men |
|
| Interventions |
"Patients were randomized to receive at least 2 hours each of the following three modes: assist‐control ventilation, pressure support alone, and pressure support with dead space" ACV: the ventilator was initially set in the assist‐control mode with a backup rate of 4 breaths per minute and tidal volume (Vt) of 8 ml/kg. The backup rate on assist‐control ventilation was then set at 4 breaths below the participant’s respiratory rate and kept at that setting for the rest of the study PSV: pressure support was adjusted to achieve a Vt equivalent to that during assist‐control ventilation, namely 8 ml/kg Study duration: at least 2 hours for each period between 10:00 p.m. and 06:00 a.m. There was no washout between cross‐over periods |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | The 2 groups were similar in terms of anthropometric data, pulmonary function tests, and arterial blood gas data sampled before randomization ACV: assist‐control mode with a backup rate of 4 breaths per minute and tidal volume (Vt) of 8 ml/kg PSV: pressure support was adjusted to achieve a Vt equivalent to that during assist‐control ventilation, namely 8 ml/kg Only the whole period of the cross‐over study was analysed We attempted to contact author Dr Tobin via email; however, we received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomized, but sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses were reported |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes. The authors did not report the number of participants in each period |
| Other bias | Low risk | Funds from medical groups/charities only (i.e., not manufacturers) supported the trial |
Richards 1998.
| Methods | Design: 3‐arm, parallel group design RCT Setting: medical CCU, single‐bed rooms, veterans medical centre in America |
|
| Participants |
Inclusion criteria
Total numbers randomized: 71 Total numbers analysed: 69 (24 in group 1, 28 in group 2, and 17 in group 3) Age: 55 to 79 years old; the mean age was 65.8 years Sex: all were men |
|
| Interventions |
Study duration: 1 night |
|
| Outcomes |
Primary outcomes
|
|
| Notes | Power analysis was used to determine the numbers of participants Intention‐to‐treat analysis was not performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a random number generator (author Richards KC provided the detail via email) |
| Allocation concealment (selection bias) | Unclear risk | No details about allocation concealment were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of scoring of sleep studies was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 15% of participants were excluded Quote: "Seventy‐one patients were randomized, one subject did not complete the study because his condition became unstable, one member of the back‐massage group was excluded because he met the criteria for an outlier (3 or more standard deviations from the group means for sleep efficiency index), 69 patients were analyzed" |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Richardson 2003.
| Methods | Design: 2‐arm, parallel group design RCT Setting: 3 intensive care units, single‐bed rooms in 2 teaching hospitals, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total numbers randomized: 36 Total numbers analysed: 29 (17 in the control group and 12 in the experimental group) Sex: 17 men, 19 women Mean age: 58.4 years |
|
| Interventions |
Study duration: 2 days |
|
| Outcomes |
Primary outcome
|
|
| Notes | There were no significant differences between groups for any demographic variable There was no pattern of administration of medications that could have affected sleep over time for any participant The paper did not report the results of sleep scores on day 1, day 2, and day 3 The mean sleep scores of the baseline night were as follows: control group was 57.85 ± 44.1, intervention group was 82 ± 44.6, showing a significant difference between them We attempted to contact author Dr S Richardson via email; however, we received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was generated using a coin toss |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The investigator was responsible for recruitment and was the individual who presented the tool to the subject on day 1. The research assistant, without knowing group membership of the subject, presented the tool to the subject on days 2 and 3" Comment: however, self‐report measures were used for subjective sleep data, and participants were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants and personnel to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The refusal rate for participation was 16% (n = 6); 36 were randomized; 29 were analysed 19.4% of participants dropped out |
| Selective reporting (reporting bias) | High risk | The results of sleep scores on day 1, day 2, and day 3 in both groups were not reported |
| Other bias | High risk | The mean sleep scores of the first night (namely baseline) were significantly different between the 2 groups |
Roche‐Campo 2013.
| Methods | Design: randomized, cross‐over clinical trial to evaluate the direct impact of mechanical ventilation on sleep quantity and quality Setting: a 24‐bed medical ICU, Henri Mondor teaching hospital, France |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total numbers randomized: 16 Total numbers analysed: 16 Sex: 11 men, 5 women Median age: 68 years, from 25 to 86 years old |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Only the whole period of the cross‐over study was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The sleep scorer was blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses were reported |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | There were no conflicts of interest |
Ruan 2006.
| Methods | Design: 2‐arm, parallel RCT Settings: ICU, China |
|
| Participants | A total of 73 ICU patientswere divided into 2 groups, chronic hypercapnic respiratory failure participants in stable conditions | |
| Interventions |
Study duration: 1 day |
|
| Outcomes |
Primary outcomes
|
|
| Notes | The general characteristics of the 2 groups before randomization were not different | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The "randomized order" was mentioned, but there was a lack of description about the randomization procedure or method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nurses (unblinded) measured subjective sleep quantity and quality |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data were not described |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgement of risk of bias |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Ryu 2012.
| Methods | Design: 2‐arm, parallel group design RCT Setting: cardiac care unit (CCU), K University D hospital, South Korea |
|
| Participants |
Inclusion criteria
Exclusion criteria
Numbers randomized: 60 Numbers analysed: 58 (29 in both group) |
|
| Interventions |
Study duration: 1 night |
|
| Outcomes |
Primary outcomes
The quantity and quality of sleep were measured using questionnaires at 7 a.m. the next morning |
|
| Notes | Power analysis was used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned using card number, the participants having an even number were assigned to experimental group, and those with odd number were assigned to control group" Comment: but there was a lack of description of the methods of card number generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although blinding of outcome assessor was performed, self‐reported measures were used for subjective sleep quality, and participants were unblinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants dropped out: 1 for having taken a sleep‐inducing drug, and 1 transferred to another unit |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | There were no COIs |
Scotto 2009.
| Methods | Design: 2‐arm, parallel group design RCT Settings: 2 critical care units of a Midwestern US teaching hospital, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Number randomized: 100 Number analysed: 88 (39 in the control group, 49 in the intervention group) Mean age: 63.1 years Sex: 53 men, 35 women |
|
| Interventions |
The duration of intervention: 1 night |
|
| Outcomes |
|
|
| Notes | We attempted to contact author CJ Scotto via email; however, we received no response Only t‐scores were provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper mentioned "randomly assigned", but there was a lack of description about the randomization procedure or method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measures were used for subjective sleep quality, and participants were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 15% of participants were excluded (N = 12) "100 participants were randomly assigned to earplug intervention or control, with 88 completing the study" |
| Selective reporting (reporting bias) | Unclear risk | The authors did not report the value of mean sleep scores in both groups, even though they stated 'statistically significant difference' |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Sha 2013.
| Methods | Design: 2‐arm, parallel group design RCT Settings: ICU, cancer hospital of Tianjin Medical University, China |
|
| Participants | Lung cancer participants in ICU after thoracotomy Inclusion criteria
Exclusion criteria
Number randomized: 240 (120 in each group) Number analysed: 112 in the control group, 107 in the intervention group Mean age: 55.5 years Sex: 146 men, 73 women |
|
| Interventions |
Study of duration: more than 7 days |
|
| Outcomes |
|
|
| Notes | The general characteristic of the 2 groups before randomization was not different | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper mentioned "randomly assigned", but there was a lack of description about the randomization procedure or method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measures were used for subjective sleep quality, and participants were not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 15% of participants were excluded (N = 21 ‐ 13 in the intervention group, 8 in the control group) |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | High risk | The PSQI scores before admission to the ICU were not provided |
Su 2013.
| Methods | Design: 2‐arm, parallel group RCT to test the effects of non‐commercial music on quality of sleep and relaxation indices Settings: a 45‐bed medical ICU, a 650‐bed multispecialty teaching hospital located in Taipei, single‐bed rooms, Taiwan |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number randomized: 28 (14 in both groups) Total number analysed: 28 Mean age: 61.68 ± 9.82 Sex: 17 men, 11 women |
|
| Interventions |
Music intervention consisted of 4 pieces of sedating piano music composed by 2 of the authors; the music was played on a Sony (CFD‐S07CP) CD player |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Power analysis was used There were no statistically significant pretest differences between the music and control groups in terms of participants' demographics and diagnoses and also no statistically significant differences in baseline subjective and objective PSG sleep parameters and heart rate, mean arterial pressure, and respiratory rate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lots were drawn to determine the group |
| Allocation concealment (selection bias) | Low risk | Quote: "All lots (labels) were packed in a jar that was prepared by another person" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers responsible for statistical analysis were not aware of which group participants were assigned, and the sleep technician scored blindly The authors who composed the music were blind to the procedures and not involved in data collection |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | There were no conflicts of interest |
Toublanc 2007.
| Methods | Design: randomized, cross‐over study to compare the impact of assist‐control ventilation (ACV) and pressure support ventilation with 6 cm H₂O inspiratory pressure (low PSV) on sleep quality Setting: respiratory ICU, single‐bed rooms, France |
|
| Participants | "Adult patients with chronic lung disease, intubated, and mechanically ventilated for an episode of acute respiratory failure of their chronic condition (I. e., chronic obstructive or restrictive pulmonary diseases). Near‐to‐wean ICU patients with acute on chronic respiratory failure. Patients were invited to participate in this study at the end of their weaning period, during the last night preceding the planned extubation, when the cause of respiratory failure was controlled, and when patients were able to sustain low levels of PSV. Patients also needed to be haemodynamically stable without any sedative, narcotic, or analeptic drugs administered for the previous 48 hour" Total number included: 22 Total number analysed: 20 Numbers of assessable participants in ACV first/low PSV first arms: 10/10 Sex: 15 men, 5 women Mean age: 65 ± 10.9 years |
|
| Interventions |
Study duration: 4 hours for each period (from 10 p.m. to 6 a.m.) There was no washout between cross‐over periods. |
|
| Outcomes |
|
|
| Notes | The 2 groups were similar in terms of anthropometric data, pulmonary function tests, and arterial blood gas data sampled before randomization The whole period, the first period, and the second period of the cross‐over study were analysed Considering the whole night, no significant differences in sleep architecture were observed. There was significantly lower wakefulness with ACV than in low PSV (30.8 ± 28.2% versus 69.0 ± 26.2%, P value < 0.05) In the first 4‐hour period and significant increases in stages 3 and 4 NREM sleep in the second part of the night |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer randomization method was used |
| Allocation concealment (selection bias) | Low risk | The trial used the closed‐envelope method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor was blinded; a neurologist blinded to the study manually scored sleep recordings |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The personnel were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients were not analyzed, one because of excessive electrical artefacts on polysomnographic records and the other because of onset of respiratory distress during the sleep study" |
| Selective reporting (reporting bias) | Low risk | Although the author did not report the mean scores of self‐perceived sleep quality in both groups, the author tested the correlations between self‐perceived sleep quality and sleep stages |
| Other bias | Unclear risk | The study report did not provide funding information or a conflict of interest statement |
Van Rompaey 2012.
| Methods | Design: 3‐arm, parallel group RCT Setting: ICU, Antwerp University Hospital, Belgium |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number randomized: 136 (69 in the experimental group, 67 in the control group) Total number analysed: 136 in night 1, 71 in night 2, 27 in night 3, and12 in night 4 Mean age: 59 years (range = 18 to 84) Sex: 66% were men |
|
| Interventions |
Study duration: 4 days |
|
| Outcomes |
|
|
| Notes | Sample size calculation was used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was achieved using a computer program |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research nurse and the critical care nurse scoring the NEECHAM scale had no information on the use of earplugs |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 136 participants were included and randomized and included in the analyses |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Low risk | There was no COI |
Wallace 1998.
| Methods | Design: randomized, cross‐over study to measure the effect of earplugs during the night‐time hours on the quality and quantity of sleep in ICU patients Settings: ICU with 60 beds, private rooms, tertiary care hospital providing level I trauma services and comprehensive heart services, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Number enrolled: 17 Number randomized: 13 Number analysed: 13 Numbers of assessable participants in treatment first/control first arms: 7/6 Sex: 5 men and 8 women Mean ages: 56.9 ± 20 years |
|
| Interventions |
Study duration: 1 night for each period, 1 night for washout. |
|
| Outcomes |
Primary outcome
Secondary outcome
Other outcomes
|
|
| Notes | The general characteristics of the 2 groups before randomization were not different Sound pressure levels, intensity of care, the verbal pain assessment scale or morphine equivalents, sensory alteration scores, the skin temperature data, and lighting levels were not significantly different between the 2 nights Only the whole period of the cross‐over study was analysed Days in ICU at enrolment = 12.6 ± 8.3 Sample size calculation was used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper mentioned "randomly assigned", but there was a lack of description about the randomization method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The sleep scorer was blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Thirty‐three eligible subjects were approached for consent, 16 declined to participate, 17 were enrolled, and 13 completed the study" |
| Selective reporting (reporting bias) | Low risk | Results were reported for all stated outcomes |
| Other bias | Unclear risk | There was insufficient information to permit judgement of risk of bias |
Wang 2012.
| Methods | Design: 2‐arm, parallel RCT Setting: cardiac care unit, China |
|
| Participants |
Inclusion criteria
Numbers randomized: 104 (52 in both group) Mean ages: 56 ± 0.5 years Sex: 56 men, 48 women |
|
| Interventions |
Study duration: 7 days |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This was a quasi‐RCT; participants were allocated to the intervention group based on the hospital orders |
| Allocation concealment (selection bias) | High risk | Allocation concealment was inadequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant flow was not described |
| Selective reporting (reporting bias) | Unclear risk | Results were reported for all stated outcomes, but the trialists did not provide measures of statistical significance for sleep outcomes, even though they stated "statistically significant difference in sleep quality between the control and experimental groups" |
| Other bias | Unclear risk | The trialists provided the preparation |
Xie 2011.
| Methods | Design: 2‐arm, parallel quasi‐RCT Settings: medical intensive care unit, China |
|
| Participants |
Inclusion criteria
Exclusion criteria
Numbers randomized: 75 (42 in the experimental group, 33 in the control group) Mean ages: 56.4 ± 10.2 years Sex: 43 men, 32 women |
|
| Interventions |
Study duration: 3 days |
|
| Outcomes |
Objective sleep outcomes
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This was a quasi‐RCT; participants were allocated to the intervention group based on the hospital orders |
| Allocation concealment (selection bias) | High risk | Allocation concealment was inadequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind personnel or participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data were not described |
| Selective reporting (reporting bias) | Unclear risk | The authors did not report the test statistic value for sleep outcomes between the 2 groups, even though they stated "statistically significant difference" |
| Other bias | Unclear risk | Information about general characteristics before randomization between the groups was not provided |
ACV = assist ‐control ventilation. AIS = Athens Insomnia Scale. AHRF = acute hypercapnic respiratory failure ANOVA = analysis of variance. APACHE II = Acute Physiology and Chronic Health Evaluation. APS = Acute Physiology Score. aPSV = automatically adjusted pressure support ventilation. BIS = Bispectral Index. BWV = Bach Werke Verzeichnis. CCU = critical care unit. COI = conflict of interest. cPSV = clinically adjusted pressure support ventilation. EEG = electroencephalogram. Hr = hour. I/E = inspiration/expiration. ICU = intensive care unit. MI = myocardial infarction. MOSS = medical outcomes study sleep. NEECHAM = Neelon/Champagne Confusion Scale. NIV = non‐invasive ventilation. NIVD = dedicated non‐invasive ventilator NIVICU = non‐invasive ICU ventilator NREM = non‐rapid eye movement. PACUs = postanaesthesia care units. PAV = proportional assist ventilation. PAV+base = proportional assist ventilation with baseline level of assist. PAV+high = proportional assist ventilation with level of assist. PB = Puritan Bennett. PCA = patient‐controlled analgesia. PCV = pressure‐controlled ventilation. PEEP = positive end‐expiratory pressure. pH = potential hydrogen. PS = pressure support. PShigh = pressure support ventilation with high pressure support. PSbase = pressure support ventilation with baseline pressure support. PSG = polysomnography. PSQI = Pittsburgh Sleep Quality Index. PSV = pressure support ventilation. PTCA = percutaneous coronary intervention. R/O = rule out. RASS = Richmond Agitation‐Sedation Scale. RCSQ = Richards‐Campbell Sleep Questionnaire. RCT = randomized controlled trial. REM = rapid eye movement. SD = standard deviation. SE = standard error. SF = short form. SME= seep maintenance efficiency. SSS = Stanford Sleepiness Scale. STAI = Speilberger State‐Trait Anxiety Inventory. SWS = slow‐wave sleep. Te = expiratory time. Ti = time. TSP = total sleep period. TST = total seep time. Ttot = total respiratory cycle time. VAS = visual analogue scale. VSH = Verran/Snyder‐Halpern. Vt = tidal volume.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnason 1995 | The outcomes were not relevant (blood pressure and heart rate, anxiety level (using Spielberger's State‐Trait Anxiety Inventory)) |
| Chen 2009 | There was no randomization |
| Cho 2013 | This was a non‐equivalent control group, non‐synchronized quasi experiment designed to test the effects of a lavender, roman chamomile, and neroli oil blend aromatherapy on anxiety, sleep, and blood pressure in coronary artery disease participants with ischaemic heart diseases in ICU |
| Cox 1999 | This was a 1‐group pretest‐post‐test experimental study, with a time series design |
| Diby 2008 | This was 1‐group pretest‐post‐test experimental study, with a quasi‐experimental study design |
| Dunn 1995 | The outcomes were not relevant: physiological variables (systolic and diastolic blood pressure, heart rate and rhythm, and respiratory rates) and participants' evaluation of their anxiety levels, mood, and ability to cope with their intensive care experience |
| Elliott 1994 | The outcomes were not relevant (anxiety level and physiologic variables (systolic and diastolic blood pressure and heart rate)) |
| Fang 2006 | This was a case report study |
| Fietze 2008 | The participants were not relevant. Participants with stable, pharmacologically treated CHF in the sleep centre |
| Figueroa‐Ramos 2010 | This was a non‐randomized trial, with a historical control. The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) identifier for the study is NCT00714194 |
| Gardner 2009 | The participants were not relevant. The participants were admitted to orthopaedic wards |
| Gunnarsdottir 2007 | The outcomes were not relevant (anxiety and physiological variables) |
| House 2003 | There was no randomization; this was a before‐after study in 2 participants |
| Kamdar 2013 | There was no randomization; this was a before‐after study in different participants |
| Koo 2008 | This was a non‐equivalent control group, non‐synchronized quasi‐experiment trial |
| Nunes 2008 | The intervention was not relevant (participants received 3 mg melatonin or placebo), and the participants were not relevant (COPD participants attending the respiratory outpatient clinic) |
| Olson 2001 | There was no randomization; this was a before‐after study in different participants |
| Richards 2000a | This was a systematic review |
| Richards 2003 | This was a systematic review |
| Richardson 2007 | This was a non‐randomized trial; participants were self selected into either an intervention or non‐intervention group |
| Robinson 2005 | This was described as a study, but there was no control group |
| Shilo 2000 | The intervention was not relevant. Participants received either 3 mg of controlled‐release melatonin or a placebo |
| Walder 2000 | The outcomes were not relevant (light level and noise level in an ICU) |
| Williamson 1992 | The participants were not ICU patients; they were postoperative coronary artery bypass graft (CABG) participants after transfer from an intensive care unit |
| Winck 2004 | The participants were not relevant; they had COPD and CVF and were admitted for pulmonary rehabilitation |
| Young 2008 | The participants were not relevant. Participants were recruited from a cross‐sectional study of 60 consecutive adult CF outpatients with mild to severe lung disease |
| Zimmerman 1996 | The participants were not relevant; all participants were moved out of the critical care unit before the sessions were initiated |
CABG = coronary artery bypass graft. CF = cystic fibrosis. CHF = congestive heart failure. COPD = chronic obstructive pulmonary disease. CVF = chronic ventilatory failure. ICU = intensive care unit.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01061242.
| Methods | 2‐arm, parallel trial |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes | This study has been completed. No data are presently available |
NCT01343095.
| Methods | 3‐arm, parallel trial |
| Participants | Adult participants who are admitted to the MICU for at least 24 hours with at least 72 hours' additional expected stay in the ICU, and who are mechanically ventilated |
| Interventions |
|
| Outcomes |
|
| Notes | This study was suspended because of a lack of study staff (last status update May 2014) |
NCT01580956.
| Methods | Cross‐over trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes | The study was completed in August 2012. No results are presently available |
NCT01607723.
| Methods | Cross‐over trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes | The study was completed in March 2014. No results are presently available |
Nerbass 2011.
| Methods | 2‐arm, parallel trial |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes | This study has been completed; data are published (Nerbass 2011) |
ICU = intensive care unit. JHH = Johns Hopkins Hospital. MICU = Medical Intensive Care Unit. NAVA = neurally adjusted ventilatory assist. PAV+ = proportional assist ventilation+. PSV = pressure support ventilation. RASS = Richmond Agitation‐Sedation Score.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐14004405.
| Trial name or title | Study on the affection of sleeping quality with non‐pharmacological intervention for cardiac valve voice improvement |
| Methods | 2‐arm parallel trial |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | January 2014 |
| Contact information | ChiCTR‐TRC‐14004405 |
| Notes | This study is currently recruiting participants |
IRCT2013030912749N1.
| Trial name or title | The effect of foot massage on the quality of sleep in patients with ischemic heart hospitalized in intensive care unit at the Ekbatan Hospital |
| Methods | 2‐arm parallel trial |
| Participants | Participants with ischaemic heart hospitalized in the intensive care unit Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2013 |
| Contact information | Dr Khodayar Oshvandi Email: oshvandi2004@yahoo.com |
| Notes | This study is currently recruiting participants |
NCT00638339.
| Trial name or title | Effects of invasive and non‐invasive mechanical ventilation on sleep In the ICU |
| Methods | Prospective case control |
| Participants | 18 years and older, critically ill participants undergoing invasive or non‐invasive mechanical ventilation in the medical ICU and CCU at Tufts New England Medical Center |
| Interventions |
|
| Outcomes |
Primary outcome
|
| Starting date | November 2006 |
| Contact information | Nicholas S Hill, MD Email: nhill@tufts‐nemc.org |
| Notes | This study is currently recruiting participants |
NCT01082016.
| Trial name or title | Sleep promotion in critically ill and injured patients cared for in the intensive care unit |
| Methods | Interventional study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | April 2010 |
| Contact information | Randall S Friese, MD University of Arizona College of Medicine |
| Notes | "Status unknown" (last update 2010) |
NCT01276652.
| Trial name or title | Sleep and circadian rhythms in mechanically ventilated patients: a feasibility and mechanistic study |
| Methods | Randomized controlled trial (parallel design, phase I) |
| Participants | 18 years and older, undergoing mechanical ventilation in the medical intensive care unit; estimated enrolment is 25 |
| Interventions |
|
| Outcomes |
Primary outcome
|
| Starting date | November 2011 |
| Contact information | ‐ |
| Notes | This study is currently recruiting participants |
NCT01284140.
| Trial name or title | Improving the sleep and circadian rhythms of mechanically ventilated patients |
| Methods | 2‐arm parallel trial |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | January 2011 |
| Contact information | The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) identifier: NCT01284140 |
| Notes | This study is currently recruiting participants |
NCT01523938.
| Trial name or title | Influence of perioperative hypnotherapy on postoperative improvement in cognitive performance. A randomized‐controlled open clinical monocentric interventional study |
| Methods | 2‐arm parallel trial |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | March 2012 |
| Contact information | The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) Identifier: NCT01523938 |
| Notes | This study is active and not currently recruiting participants |
NCT01727375.
| Trial name or title | Prevention of delirium with light in the intensive care unit |
| Methods | Interventional, parallel RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | July 2013 |
| Contact information | Claudia Spies, MD, Prof Email: claudia.spies@charite.de |
| Notes | The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) identifier: NCT01727375 This study is currently recruiting |
NCT02095496.
| Trial name or title | Contribution to the understanding of the involvement of mechanical ventilation in ICU patients sleep disorders |
| Methods | Interventional cross‐over RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2014 |
| Contact information | Emilie Bialais, PhD student Email: bialais@uclouvain.Be |
| Notes | The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) identifier: NCT02095496 This study is not yet open for participant recruitment |
Qureshi 2014.
| Trial name or title | A study to assess the effectiveness of using earplugs and eye masks during night on perceived quality of sleep among participants in intensive care units of AIIMS |
| Methods | Randomized cross‐over trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | January, 2014 |
| Contact information | Ms Ashia Qureshi Email: qureshi.ashia@gmail.com Koushal Dave Email: qureshi.ashia@gmail.com |
| Notes | CTRI/2014/01/004320 This study is open to recruitment |
AIIMS = All India Institute of Medical Sciences. ASA = American Society of Anesthesiologists. ASV = adaptive support ventilation. CAM‐ICU = confusion assessment method for ICU. CCU = critical care unit. MVR = mitral valve replacement. PEEP = positive end‐expiratory pressure. PEG = percutaneous endoscopic gastrostomy. PO = oral administration. RCT = randomized controlled trial. REM = rapid eye movement. SAS = sedation‐agitation scale. SBP = systolic blood pressure.
Differences between protocol and review
In the protocol (Hu 2010), we planned to synthesize data using Review Manager software (RevMan 5); in the review, we used RevMan 5.3.
At least one o utcome listed under 'Criteria for considering studies for this review' was a criteria for including studies; this was not pre‐specified in the protocol.
In the protocol, we originally specified the inclusion of studies of critically ill adult patients who were admitted to the intensive or critical care units, where the length of intensive care unit (ICU) stay was more than 48 hours, having stable hemodynamic status without any sedative, narcotic drugs administered in the 24 hours prior to recruitment. During the review, we did not restrict the length of ICU stay because several trials included participants where the expected length of stay in the ICU was more than 24 hours.
We added two additional authors to the team: Xin Huining was responsible for entering data, and David Evans was responsible for checking the review against peer review comments and revising the review for language.
We changed the Allied and Complementary Medicine Database (AMED) to Alt HealthWatch from May 2011, because we were then unable to access the AMED database.
We had planned to include the following additional treatment comparisons, but there were insufficient trials to do so, or the available trials had important clinical heterogeneity among them: acupressure versus other interventions or placebo, aromatherapy versus other interventions or placebo, back massage versus other interventions or placebo, foot baths versus other interventions or placebo, relaxation and imagery versus other interventions or placebo, foot massage versus other interventions or placebo, using sound masking versus other interventions or placebo, and social support intervention versus other interventions or placebo. Therefore, we included trials comparing these interventions with other therapies or placebo in the narrative but not the meta‐analysis of this review.
Considering the presence of carry‐over, we had planned to analyse the data from only the first period in cross‐over RCTs. However, only two cross‐over RCTs reported data from the first period and the cross‐over period, whereas the remaining studies only reported the whole period data. Thus, we took the decision to exclude cross‐over studies from the meta‐analyses.
We planned to test the robustness of the evidence by sensitivity analyses according to sequence generation, allocation concealment (adequate or unclear or inadequate), and blinding (adequate or unclear or inadequate or not performed). We intended to compare the fixed‐effect model results with the random‐effects model results. We planned to undertake sensitivity analyses to examine the effects of excluding study subgroups. None of these actions were performed due to the heterogenous nature of the included studies.
We planned to perform subgroup analyses. However, as we only pooled two studies for each meta‐analysis in this review, subgroup analyses were not performed,
We replaced the outcome of arousal index with the sleep fragmentation index in the 'Summary of findings' table as the majority of trials reported the sleep fragmentation index and did not report the arousal index.
We originally included the following statements in the protocol but did not implement these plans during the review owing to the types of data available; we removed these methods from the current methods section, but they may be relevant to future updates.
Effect measures for dichotomous outcomes: we will calculate odds ratios (ORs) and 95% confidence intervals (CIs) for dichotomous outcomes.
Dealing with missing data: we will extract data regarding intention‐to‐treat (ITT). If the researchers did not perform ITT and participants lost to follow up are less than 20%, but sufficient raw data are available, we will conduct an ITT analysis prior to data entry into RevMan 5.0. If more than 20% of the data are missing from the study, we will exclude the study from the meta‐analysis and perform an available case analysis.
'Summary of findings' tables: we intend to include seven outcomes, such as sleep efficiency index; rapid eye movement (REM) sleep time; REM sleep latency; arousal index; mortality; length of ICU stay; and risk of delirium during ICU stay.
Contributions of authors
Conceiving the review: Rong‐Fang Hu (HRF). Co‐ordinating the review: HRF. Undertaking manual searches: HRF. Screening search results: HRF and Xiao Y Chen (CXY). Organizing retrieval of papers: HRF and CXY. Screening retrieved papers against inclusion criteria: HRF. Appraising quality of papers: HRF and Yueping Li (LYP). Extracting data from papers: HRF and CXY. Writing to authors of papers for additional information: HRF. Providing additional data about papers: HRF. Obtaining and screening data on unpublished studies: Xiao‐Ying Jiang (JXY). Data management for the review: HRF and JXY. Entering data into Review Manager (1): HRF. RevMan statistical data: HRF and Junmin Chen (CJM). Other statistical analysis not using RevMan: LYP. Double entry of data: (data entered by person one: HRF; data entered by person two: Xin Huining (XHN)). Interpretation of data: HRF, CJM, and Zhiyong Zeng (ZZY). Statistical inferences: LYP. Writing the review: HRF and David Evans (DE). Guarantor for the review (one author): HRF. Person responsible for reading and checking review before submission: CJM, HRF, and DE
Sources of support
Internal sources
School of Nursing, Fujian Medical University, China.
External sources
National Natural Science Funds of China (Grant No. 81201500) and Ministry of Education's Humanities and Social Sciences Project (No.11YJC190008), China.
Declarations of interest
Rong‐Fang Hu: nothing to declare. Xiao‐Ying Jiang: nothing to declare. Junmin Chen: nothing to declare. Zhiyong Zeng: nothing to declare. Xiao Y Chen: nothing to declare. Yueping Li: nothing to declare. Xin Huining: nothing to declare. David Evans: nothing to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Alexopoulou 2007 {published data only}
- Alexopoulou C, Kondili E, Vakouti E, Klimathianaki M, Prinianakis G, Georgopoulos D. Sleep during proportional‐assist ventilation with load‐adjustable gain factors in critically ill patients. Intensive Care Medicine 2007;33(7):1139‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Andréjak 2013 {published data only}
- Andréjak C, Monconduit J, Rose D, Toublanc B, Mayeux I, Rodenstein D, et al. Does using pressure‐controlled ventilation to rest respiratory muscles improve sleep in ICU patients?. Respiratory Medicine 2013;107(4):534‐41. [PUBMED: 23391488] [DOI] [PubMed] [Google Scholar]
Borromeo 1998 {unpublished data only}
- Borromeo AR. The effect of aromatherapy on the patient outcomes of anxiety and sleep quality in coronary care unit patients [PhD thesis]. USA: Texas Woman's University 1998. [ProQuest document ID: 732929911]
Bosma 2007 {published data only}
- Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, et al. Patient‐ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Critical Care Medicine 2007;35(4):1048‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cabello 2008 {published data only}
- Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, d'Ortho M, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Critical Care Medicine 2008;36(6):1749‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen JH, Chao YH, Lu SF, Shiung TF, Chao YF. The effectiveness of valerian acupressure on the sleep of ICU patients: a randomized clinical trial. International Journal of Nursing Studies 2012;49(8):913‐20. [PUBMED: 22391336] [DOI] [PubMed] [Google Scholar]
Córdoba‐Izquierdo 2013 {published data only}
- Córdoba‐Izquierdo A, Drouot X, Thille AW, Galia F, Roche‐Campo F, Schortgen F, et al. Sleep in hypercapnic critical care patients under noninvasive ventilation: conventional versus dedicated ventilators. Critical Care Medicine 2013;41(1):60‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foreman 2013 {published data only}
- Foreman B, Claassen J, Bazil C. Melatonin, light & noise reduction to improve sleep in the neurological intensive care unit (Abstract).. Poster session presented at 65th annual meeting of the American Academy of Neurology (AAN).2013 March 16‐23; San Diego, California. [EMBASE: 71129510]
Gao 2008 {published data only}
- Gao L, Li D, Wei LP, Xu D, Zhao D. Effect of controlled periods for family member accompany on psychology and sleep quality of patients in coronary care unit. Nursing Journal of Chinese People's Liberation Army 2008;25(5):9‐13. [Google Scholar]
Gragert 1990 {unpublished data only}
- Gragert MD. The use of a masking signal to enhance the sleep of men and women 65 years of age and older in the critical care environment [PhD thesis]. USA: The University of Texas at Austin 1990. [CINAHL Accession Number:1992137420]
Hu 2010 {unpublished data only}
- Hu RF. Study on sleep, melatonin and non‐pharmacological intervention for sleep promotion in ICU patients [PhD thesis]. China: Fujian Medical University 2010.
Jaber 2007 {published data only}
- Jaber S, Bahloul H, Guétin S, Chanques G, Sebbane M, Eledjam J. Effects of music therapy in intensive care unit without sedation in weaning patients versus non‐ventilated patients. Annales Françaises d'Anesthésie et de Réanimation 2007;26(1):30‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Le Guen 2014 {published data only}
- Guen M, Nicolas‐Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post‐anaesthesia care unit: a randomized study. British Journal of Anaesthesia 2014;112(1):89‐95. [PUBMED: 24172057] [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li Y, Shan Y, Zhang WP. Clinical nursing with Roy adaptation model in CCU of patients with sleep disorders. Chinese Journal of Practical Nervous Diseases 2011;14(6):1‐2. [Google Scholar]
Martin 2008 {unpublished data only}
- Martin KA. The effect of earplugs on perceived sleep quality of acute care patients [masters dissertation]. Montana State university 2008.
Namba 2012 {published data only}
- Namba S, Shimoyama I, Kiguchi T, Ujike Y. Effects of foot baths on sleep in ICU patients. Chiba Medical Journal 2012;88(6):59‐64. [EMBASE: 2013278557] [Google Scholar]
Parthasarathy 2002 {published data only}
- Parthasarathy S, Tobin M. Effect of ventilator mode on sleep quality in critically ill patients. American Journal of Respiratory and Critical Care Medicine 2002;166(11):1423‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richards 1998 {published data only}
- Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. American Journal of Critical Care 1998;7(4):288‐99. [MEDLINE: ] [PubMed] [Google Scholar]
- Richards KC. The effect of a muscle relaxation, imagery, and relaxing music intervention and a back massage on the sleep and psychophysiological arousal of elderly males hospitalized in the critical care environment [doctoral dissertation]. Austin: University of Texas 1993. [UMI Order PUZ9323533]
Richardson 2003 {published data only}
- Richardson SJ. Effects of relaxation and imagery on the sleep of critically ill adults. Dimensions of Critical Care Nursing 2003;22(4):182‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Richardson SJ. The effects of autogenic relaxation and guided imagery on insomnia in the critically ill adult [dissertation]. USA: University of Utah 1997.
Roche‐Campo 2013 {published data only}
- Roche‐Campo F, Thille AW, Drouot X, Galia F, Margarit L, Córdoba‐Izquierdo A, et al. Comparison of sleep quality with mechanical versus spontaneous ventilation during weaning of critically III tracheostomized patients. Critical Care Medicine 2013;41(7):1637‐44. [PUBMED: 23507721] [DOI] [PubMed] [Google Scholar]
Ruan 2006 {published data only}
- Ruan GR, Wang B. The effect of psychologic intervention in improving severe patient's sleep. Liaoning Medicine Journal (Chinese) 2006;20(6):329‐30. [Google Scholar]
Ryu 2012 {published data only}
- Ryu MJ, Park JS, Park H. Effect of sleep‐inducing music on sleep in persons with percutaneous transluminal coronary angiography in the cardiac care unit. Journal of Clinical Nursing 2012;21(5‐6):728–35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Scotto 2009 {published data only}
- Scotto CJ, McClusky C, Spillan S, Kimmel J. Earplugs improve patients' subjective experience of sleep in critical care. Nursing in Critical Care 2009;14(4):180‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sha 2013 {published data only}
- Sha Y, Kong QQ, He H, Luo HY, Li JL. Effect of individualized music intervention on sleep quality for patients in ICU after thoracotomy. Journal of Nurses Training 2013;28(16):1451‐3. [Google Scholar]
Su 2013 {published data only}
- Su CP, Lai HL, Chang ET, Yiin LM, Perng SJ, Chen PW. A randomized controlled trial of the effects of listening to non‐commercial music on quality of nocturnal sleep and relaxation indices in patients in medical intensive care unit. Journal of Advanced Nursing 2013;69(6):1377‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toublanc 2007 {published data only}
- Toublanc B, Rose D, Glérant JC, Francois G, Mayeux I, Rodenstein D, et al. Assist‐control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Medicine 2007;33(7):1148‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Rompaey 2012 {published data only}
- Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Critical Care 2012;16(3):R73. [PUBMED: 22559080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 1998 {published data only}
- Wallace CJ. The effect of earplugs on polysomnographic sleep and description of sleep‐disturbing factors in critically ill subjects [PhD Thesis]. The University of Utah 1998. [AAT 9911402]
Wang 2012 {published data only}
- Wang YL, Wu YX. Effect of foot massage combined with the use of a Chinese herbal sleep pillow on sleep in coronary care unit patients. Journal of Qilu Nursing (Chinese) 2012;18(1):38‐9. [Google Scholar]
Xie 2011 {published data only}
- Xie JL, Diao JD, Ma ZJ, Feng WL, Feng WS. The effect of audio‐visual segregation on the sleep disorder of patient in ICU. Journal of Clinical Medicine in Practice (Chinese) 2011;15(16):42‐4. [Google Scholar]
References to studies excluded from this review
Barnason 1995 {published data only}
- Barnason S, Zimmerman L, Nieveen J. The effects of music interventions on anxiety in the patient after coronary artery bypass grafting. Heart & Lung 1995;24(2):124‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen H. The Impact of nursing intervention on sleep quality of ICU patients. Chinese Primary Health Care 2009;23(10):123‐4. [Google Scholar]
Cho 2013 {published data only}
- Cho MY, Min ES, Hur MH, Lee MS. Effects of aromatherapy on the anxiety, vital signs, and sleep quality of percutaneous coronary intervention patients in intensive care units. Evidence‐Based Complementary and Alternative Medicine 2013 Feb 17 [Epub ahead of print]. [PUBMED: 23476690] [DOI] [PMC free article] [PubMed]
Cox 1999 {published data only}
- Cox C, Hayes J. Physiologic and psychodynamic responses to the administration of therapeutic touch in critical care. Complementary Therapies in Nursing & Midwifery 1999;5(3):87‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diby 2008 {published data only}
- Diby M, Romand JA, Frick S, Heidegger C, Walder B. Reducing pain in patients undergoing cardiac surgery after implementation of a quality improvement post‐operative pain treatment program. Journal of Critical Care 2008;23(3):359–71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dunn 1995 {published data only}
- Dunn C, Sleep J, Collett D. Sensing an improvement: an experimental study to evaluate the use of aromatherapy, massage and periods of rest in an intensive care unit. Journal of Advanced Nursing 1995;21(1):34‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elliott 1994 {published data only}
- Elliott D. The effects of music and muscle relaxation on patient anxiety in a coronary care unit. Heart & Lung 1994;23(1):27‐35. [MEDLINE: ] [PubMed] [Google Scholar]
Fang 2006 {published data only}
- Fang CS, Liu CF. Applying back massage protocol to promote an Intensive care unit patient's quality of sleep. Hu Li Za Zhi (Taiwan) 2006;53(6):78‐84. [PUBMED: 17160874] [PubMed] [Google Scholar]
Fietze 2008 {published data only}
- Fietze I, Blau A, Glos M, Theres H, Baumann G, Penzel T. Bi‐level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne‐Stokes respiration. Sleep Medicine 2008;9(6):652–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Figueroa‐Ramos 2010 {published and unpublished data}
- Figueroa‐Ramos MI. The effect of a sedation wake‐up trial and spontaneous breathing trial on the occurrence of delirium and perception of sleep in critically ill trauma patients [PhD thesis]. USA: University of California 2010.
- Figueroa‐Ramos MI, Arroyo‐Novoa CM, Padilla G, Rodríguez‐Ortiz P, Cooper BA, Puntillo KA. Feasibility of a sedation wake‐up trial and spontaneous breathing trial in critically ill trauma patients: a secondary analysis. Intensive and Critical Care Nursing 2013;29(1):20‐7. [PUBMED: 22705052] [DOI] [PubMed] [Google Scholar]
- NCT00714194. Effect of daily interruption of continuous sedation on delirium, sleep perception in intensive care unit (ICU) patients (ICU delirium). clinicaltrials.gov/ct2/show/NCT00714194 (accessed 2008).
Gardner 2009 {published data only}
- Gardner G, Collins C, Osborne S, Henderson A, Eastwood M. Creating a therapeutic environment: a non‐randomised controlled trial of a quiet time intervention for patients in acute care. International Journal of Nursing Studies 2009;46(6):778–86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gunnarsdottir 2007 {published data only}
- Gunnarsdottir TJ, Jonsdottir H. Does the experimental design capture the effects of complementary therapy? A study using reflexology for patients undergoing coronary artery bypass graft surgery. Journal of Clinical Nursing 2007;16(4):777‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
House 2003 {unpublished data only}
- House MA. Testing the effect of earplug use on sleep in critically ill [masters dissertation]. The University of British Columbia 2003.
Kamdar 2013 {published data only}
- Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Critical Care Medicine 2013;41(3):800‐9. [PUBMED: 23314584] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koo 2008 {published data only}
- Koo YJ, Koh HJ. Effects of eye protective device and ear protective device application on sleep disorder with coronary disease patients in CCU. Taehan Kanho Hakhoe Chi 2008;38(4):582‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nunes 2008 {published data only}
- Nunes DM, Mota RMS, Machado MO, Pereira ED, Bruin VMS, Bruin PF. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Brazilian Journal of Medical and Biological Research 2008;41(10):926‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olson 2001 {published data only}
- Olson DM, Borel CO, Laskowitz DT, Moore DT, McConnell ES. Quiet time: a nursing interventions to promote sleep in neurocritical care units. American Journal of Critical Care 2001;10(2):74‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Richards 2000a {published data only}
- Richards KC, Gibson R, Overton‐McCoy AL. Effects of massage in acute and critical care. AACN Clinical Issues: Advanced Practice in Acute & Critical Care 2000;11(1):77‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richards 2003 {published data only}
- Richards K, Nagel C, Markie M, Elwell J, Barone C. Use of complementary and alternative therapies to promote sleep in critically ill patients. Critical Care Nursing Clinics of North America 2003;15(3):329‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richardson 2007 {published data only}
- Richardson A, Allsop M, Coghill E, Turnock C. Earplugs and eye masks: do they improve critical care patients' sleep?. Nursing in Critical Care 2007;12(6):278‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robinson 2005 {published data only}
- Robinson SB, Weitzel T, Henderson L. The Sh‐h‐h‐h Project: non‐pharmacological interventions. Holistical Nursing Practice 2005;19(6):263‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shilo 2000 {published data only}
- Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, et al. Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiology International 2000;17(1):71‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walder 2000 {published data only}
- Walder B, Francioli D, Meyer JJ, Lançon M, Romand JA. Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels. Critical Care Medicine 2000;28(7):2242‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williamson 1992 {published data only}
- Williamson JW. The effects of ocean sounds on sleep after coronary artery bypass graft surgery. American Journal of Critical Care 1992;1(1):91‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Winck 2004 {published data only}
- Winck JC, Vitacca M, Morais A, Barbano L, Porta R, Teixeira‐Pinto A, et al. Tolerance and physiologic effects of nocturnal mask pressure support vs proportional assist ventilation in chronic ventilatory failure. Chest 2004;126(2):382‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Young 2008 {published data only}
- Young AC, Wilson JW, Kotsimbos TC, Naughton MT. Randomised placebo controlled trial of non‐invasive ventilation for hypercapnia in cystic fibrosis. Thorax 2008;63(1):72‐7. [PUBMED: 17675317] [DOI] [PubMed] [Google Scholar]
Zimmerman 1996 {published data only}
- Zimmerman L, Nieveen J, Barnason S, Schmaderer M. The effects of music interventions on postoperative pain and sleep in coronary artery bypass graft (CABG) patients. Scholarly Inquiry for Nursing Practice: an Inernational Journal 1996;10(2):153‐70. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01061242 {unpublished data only}
- NCT01061242. Post‐ICU neurocognitive performance and sleep quality ratings following exposure to a medical ICU sleep quality improvement project. clinicaltrials.gov/ct2/show/NCT01061242.
NCT01343095 {published data only}
- NCT01343095. A randomized controlled trial of direct noise reduction in the ICU using overnight application of in‐ear earplugs or in‐ear earplugs plus noise‐canceling headphones to reduce the incidence and duration of ICU delirium. clinicaltrials.gov/ct2/show/NCT01343095.
NCT01580956 {unpublished data only}
- NCT01580956. Evaluation a new ventilatory modes: VARIABLE‐PSV: a randomized controlled cross‐over study: the "VARIABLE‐PSV" study. clinicaltrials.gov/ct2/show/NCT01580956.
NCT01607723 {unpublished data only}
- NCT01607723. Comparison of two new ventilatory modes: NAVA vs PAV+: a randomized controlled cross‐over study: the "NAVA‐PAV" study. clinicaltrials.gov/ct2/show/NCT01607723.
Nerbass 2011 {published data only}
- Nerbass FB, Feltrim MI, Souza SA, Ykeda DS, Lorenzi‐Filho G. Effects of massage therapy on sleep quality after coronary artery bypass graft surgery. Clinics (Sao Paulo) 2011;65(11):1105‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐14004405 {unpublished data only}
- ChiCTR‐TRC‐14004405. Study on the affection of sleeping quality with non‐pharmacological intervention for cardiac valve voice improvement. www.chictr.org.cn/showprojen.aspx?proj=5165. [ChiCTR‐TRC‐14004405]
IRCT2013030912749N1 {unpublished data only}
- IRCT2013030912749N1. The effect of foot massage on the quality of sleep in patients with ischemic heart hospitalized in intensive care unit at the Ekbatan Hospital. www.irct.ir/searchresult.php?keyword=&id=12749&number=1&prt=4717&total=10&m=1.
NCT00638339 {unpublished data only}
- NCT00638339. Effects of invasive and noninvasive mechanical ventilation on sleep In the ICU. clinicaltrials.gov/ct2/show/NCT00638339.
NCT01082016 {unpublished data only}
- NCT01082016. Sleep promotion in critically ill and injured patients cared for in theintensive care unit. clinicaltrials.gov/ct2/show/NCT01082016.
NCT01276652 {unpublished data only}
- NCT01276652. Sleep and circadian rhythms in mechanically ventilated patients: a feasibility and mechanistic study. clinicaltrials.gov/ct2/show/NCT01276652.
NCT01284140 {unpublished data only}
- NCT01284140. Improving the sleep and circadian rhythms of mechanically ventilated patients. clinicaltrials.gov/ct2/show/NCT01284140.
NCT01523938 {unpublished data only}
- NCT01523938. Influence of perioperative hypnotherapy on postoperative improvement in cognitive performance. A randomized‐controlled open clinical monocentric interventional study. clinicaltrials.gov/ct2/show/NCT01523938.
NCT01727375 {unpublished data only}
- NCT01727375. Prevention of delirium with light in the intensive care unit. clinicaltrials.gov/ct2/show/NCT01727375.
NCT02095496 {unpublished data only}
- NCT02095496. Contribution to the understanding of the involvement of mechanical ventilation in ICU patients sleep disorders. clinicaltrials.gov/ct2/show/NCT02095496.
Qureshi 2014 {unpublished data only}
- Qureshi A, Dave K. A study to assess the effectiveness of using earplugs and eye masks during night on perceived quality of sleep among patients in intensive care units of AIIMS. CTRI.
Additional references
Abad 2015
- Abad VC, Guilleminault C. Pharmacological treatment of sleep disorders and its relationship with neuroplasticity. Curr Top Behav Neurosci 2015;25:503‐53. [DOI] [PubMed] [Google Scholar]
Achermann 1987
- Achermann P, Borbély AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Human Neurobiology 1987;6(3):203‐10. [PUBMED: 2896653] [PubMed] [Google Scholar]
Benca 1997
- Benca RM, Quintas J. Sleep and host defenses: a review. Sleep 1997;20(11):1027‐37. [PUBMED: 9456469] [PubMed] [Google Scholar]
Bent 2006
- Bent S, Padula A, Moore D, Patterson M, Mehling W. Valerian for sleep: a systematic review and meta‐analysis. The American Journal of Medicine 2006;119(12):1005‐12. [PUBMED: 17145239] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradt 2009
- Bradt J, Dileo C. Music for stress and anxiety reduction in coronary heart disease patients. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006577.pub2; PUBMED: 19370642] [DOI] [PubMed] [Google Scholar]
Bradt 2010
- Bradt J, Dileo C. Music therapy for end‐of‐life care. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007169.pub2; PUBMED: 20091619] [DOI] [PubMed] [Google Scholar]
Bradt 2011
- Bradt J, Dileo C, Grocke D, Magill L. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD006911; PUBMED: 21833957] [DOI] [PubMed] [Google Scholar]
Bradt 2014
- Bradt J, Dileo C. Music interventions for mechanically ventilated patients. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD006902.pub3; PUBMED: 25490233] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buscemi 2005
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta‐analysis. Journal of General Internal Medicine 2005;20(12):1151‐8. [PUBMED: 16423108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1989
- Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. The American Review of Respiratory Disease 1989;140(4):907‐9. [PUBMED: 2802378] [DOI] [PubMed] [Google Scholar]
Cheuk 2012
- Cheuk DK, Yeung WF, Chung KF, Wong V. Acupuncture for insomnia. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD005472.pub3; PUBMED: 22972087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2000
- Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest 2000;117(3):809‐18. [PUBMED: 10713011] [DOI] [PubMed] [Google Scholar]
Cronin 2001
- Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep 2001;24(1):39‐44. [PUBMED: 11204052] [DOI] [PubMed] [Google Scholar]
Czeisler 1986
- Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep‐wake cycle. Science 1986;233:667–671. [DOI] [PubMed] [Google Scholar]
de Niet 2009
- Niet G, Tiemens B, Lendemeijer B, Hutschemaekers G. Music‐assisted relaxation to improve sleep quality: meta‐analysis. Journal of Advanced Nursing 2009;65(7):1356‐64. [PUBMED: 19456998] [DOI] [PubMed] [Google Scholar]
Dignani 2015
- Dignani L, Toccaceli A, Lucertini C, Petrucci C, Lancia L. Sleep and Quality of Life in People With COPD: A Descriptive‐Correlational Study. Clinical Nursing Research 2015;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Drouot 2008
- Drouot X, Cabello B, D'Ortho MP, Brochard L. Sleep in the intensive care unit. Sleep Medicine Reviews 2008;12(5):391‐403. [PUBMED: 18502155] [DOI] [PubMed] [Google Scholar]
Eddleston 2000
- Eddleston JM, White P, Guthrie E. Survival, morbidity, and quality of life after discharge from intensive care. Critical Care Medicine 2000;28(7):2293‐9. [PUBMED: 10921555] [DOI] [PubMed] [Google Scholar]
Evans 2016
- Evans DJW, Butler A, Alderson P, Smith AF, Lewis SR. Pharmacological agents for the promotion of sleep in the intensive care unit. Cochrane Database of Systematic Reviews (In progress).
Fichten 1995
- Fichten CS, Creti L, Amsel R, Brender W, Weinstein N Libman E. Poor sleepers who do not complain of insomnia: myths and realities about psychological and lifestyle characteristics of older good and poor sleepers. Journal of Behavioral Medicine 1995;18(2):189‐223. [PUBMED: 7563046] [DOI] [PubMed] [Google Scholar]
Freedman 1999
- Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. American Journal of Respiratory and Critical Care Medicine 1999;159(4 Pt 1):1155‐62. [PUBMED: 10194160] [DOI] [PubMed] [Google Scholar]
Freedman 2001
- Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. American Journal of Respiratory and Critical Care Medicine 2001;163(2):451‐7. [PUBMED: 11179121] [DOI] [PubMed] [Google Scholar]
Friese 2007
- Friese RS, Diaz‐Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?. The Journal of Trauma 2007;63(6):1210‐4. [PUBMED: 18212640] [DOI] [PubMed] [Google Scholar]
Friese 2008
- Friese RS. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Critical Care Medicine 2008;36(3):697‐705. [PUBMED: 18176314] [DOI] [PubMed] [Google Scholar]
Frisk 2003
- Frisk U, Nordström G. Patients' sleep in an intensive care unit‐‐patients' and nurses' perception. Intensive & Critical Care Nursing 2003;19(6):342‐9. [PUBMED: 14637294] [DOI] [PubMed] [Google Scholar]
Gabor 2001
- Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Current Opinion in Critical Care 2001;7(1):21‐7. [PUBMED: 11373507] [DOI] [PubMed] [Google Scholar]
Gabor 2003
- Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. American Journal of Respiratory and Critical Care Medicine 2003;167(5):708‐15. [PUBMED: 12598213] [DOI] [PubMed] [Google Scholar]
Gałuszko‐Węgielnik 2012
- Gałuszko‐Węgielnik M, Jakuszkowiak‐Wojten K, Wiglusz MS, Cubała WJ, Landowski J. The efficacy of Cognitive‐Behavioural Therapy (CBT) as related to sleep quality and hyperarousal level in the treatment of primary insomnia. Psychiatr Danub. 2012;24(Suppl 1):S51‐5. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ (Clinical research ed.) 2008;336(7651):995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardin 2006
- Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Chest 2006;129(6):1468‐77. [PUBMED: 16778263] [DOI] [PubMed] [Google Scholar]
Hauri 1997
- Hauri PJ. Can we mix behavioral therapy with hypnotics when treating insomniacs?. Sleep 1997;20(12):1111‐8. [MEDLINE: ; PUBMED: 9493920] [DOI] [PubMed] [Google Scholar]
Hays 2005
- Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Medicine 2005;6(1):41‐4. [PUBMED: 15680294] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jacobi 2002
- Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Critical Care Medicine 2002;30(1):119–41. [PUBMED: 11902253] [DOI] [PubMed] [Google Scholar]
Klimm 1987
- Klimm HD, Dreyfus JF, Delmotte M. Zopiclone versus nitrazepam: a double‐blind comparative study of efficacy and tolerance in elderly patients with chronic insomnia. Sleep 1987;10(Suppl 1):73‐8. [PUBMED: 3326118] [DOI] [PubMed] [Google Scholar]
Liu 1996
- Liu XC, Tang MQ, Hu L, Wang AZ, Wu HX, Zhao GF, Gao CL. Reliability and validity of the Pittsburgh sleep quality index. Chinese Journal of Psychiatry 1996;29(2):103‐8. [Google Scholar]
McClusky 1991
- McClusky HY, Milby JB, Switzer PK, Williams V, Wooten V. Efficacy of behavioral versus triazolam treatment in persistent sleep‐onset insomnia. The American Journal of Psychiatry 1991;148(1):121‐6. [MEDLINE: ; PUBMED: 1888345] [DOI] [PubMed] [Google Scholar]
Meyer 1994
- Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP. Adverse environmental conditions in the respiratory and medical ICU settings. Chest 1994;105(4):1211‐6. [PUBMED: 8162751] [DOI] [PubMed] [Google Scholar]
Milisen 2005
- Milisen K, Foreman MD, Hendrickx A, Godderis J, Abraham IL, Broos P, et al. Psychometric properties of the Flemish translation of the NEECHAM Confusion Scale. BMC Psychiatry 2005;25(5):16. [PUBMED: 15792498] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mistraletti 2008
- Mistraletti G, Carloni E, Cigada M, Zambrelli E, Taverna M, Sabbatici G, et al. Sleep and delirium in the intensive care unit. Minerva Anestesiologica 2008;74(6):329‐33. [PUBMED: 18500209] [PubMed] [Google Scholar]
Montgomery 2002
- Montgomery P, Dennis J. Bright light therapy for sleep problems in adults aged 60+. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003403; PUBMED: 12076478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2003
- Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003161; PUBMED: 12535460] [DOI] [PubMed] [Google Scholar]
Nieuwenhuijs 2006
- Nieuwenhuijs DJ. Processed EEG in natural sleep. Best Practice & Research. Clinical Anaesthesiology 2006;20:49‐56. [PUBMED: 16634413] [DOI] [PubMed] [Google Scholar]
Novaes 1999
- Novaes MA, Knobel E, Bork AM, Pavão OF, Nogueira‐Martins LA, Ferraz MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Medicine 1999;25(12):1421‐6. [PUBMED: 10660851] [DOI] [PubMed] [Google Scholar]
O'Donoghue 2012
- O'Donoghue FJ, Wellard RM, Rochford PD, Dawson A, Barnes M, Ruehland WR, Jackson ML, Howard ME, Pierce RJ, Jackson GD. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep 2012;35:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orwelius 2008
- Orwelius L, Nordlund A, Nordlund P, Edéll‐Gustafsson U, Sjöberg F. Prevalence of sleep disturbances and long‐term reduced health‐related quality of life after critical care: a prospective multicenter cohort study. Critical Care (London, England) 2008;12(4):R97. [MEDLINE: ; PUBMED: 18673569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozone 2000
- Ozone M, Itoh H, Yamadera W, Ohbuchi K, Hayashida K, Sasaki M, et al. Changes in subjective sleepiness, subjective fatigue and nocturnal sleep after anaesthesia with propofol. Psychiatry and Clinical Neurosciences 2000;54(3):317‐8. [PUBMED: 11186093] [DOI] [PubMed] [Google Scholar]
Pandharipande 2006
- Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Critical Care Clinics 2006;22(2):313‐27. [PUBMED: 16678002] [DOI] [PubMed] [Google Scholar]
Parthasarathy 2004
- Parthasarathy S, Tobin MJ. Sleep in the intensive care unit. Intensive Care Medicine 2004;30(2):197‐206. [PUBMED: 14564378] [DOI] [PubMed] [Google Scholar]
Pun 2007
- Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest 2007;132(2):624‐36. [PUBMED: 17699134] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2000
- Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. Journal of Nursing Measurement 2000;8(2):131‐44. [PUBMED: 11227580] [PubMed] [Google Scholar]
Shilo 1999
- Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. The American Journal of the Medical Sciences 1999;317(5):278‐81. [PUBMED: 10334113] [DOI] [PubMed] [Google Scholar]
SiIber 2007
- Silber MH, Ancoli‐Israel S, Bonnet MH, Chokroverty S, Grigg‐Damberger MM, Hirshkowitz M, et al. The visual scoring of sleep in adults. Journal of Clinical Sleep Medicine 2007;3(2):121–31. [MEDLINE: ] [PubMed] [Google Scholar]
Simini 1999
- Simini B. Patients's perceptions of intensive care. Lancet 1999;354(9178):571‐2. [PUBMED: 10470711] [DOI] [PubMed] [Google Scholar]
Snyder‐Halpern 1987
- Snyder‐Halpern R, Verran JA. Instrumentation to describe subjective sleep characteristics in healthy subjects. Research in Nursing and Health 1987;10(3):155‐163. [PUBMED: 3647537] [DOI] [PubMed] [Google Scholar]
Soldatos 2000
- Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD‐10 criteria. Journal of Psychosomatic Research 2000;48(6):555‐60. [PUBMED: 11033374] [DOI] [PubMed] [Google Scholar]
Tamrat 2014
- Tamrat R, Huynh‐Le MP, Goyal M. Non‐pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. Journal of General Internal Medicine 2014;29(5):788‐95. [PUBMED: 24113807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rompaey 2008
- Rompaey B, Schuurmans M, Shortridge‐Baggett L, Truijen S, Elseviers M, Bossaert L. A comparison of the CAM‐ICU and the NEECHAM Confusion Scale in intensive care delirium assessment: an observational study in non‐intubated patients. Critical Care (London, England) 2008;12(1):R16. [PUBMED: 18282269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinhouse 2006
- Weinhouse GL, Schwab RJ. Sleep in the Critically ill Patient. Sleep 2006;29(5):707‐16. [PUBMED: 16774162] [DOI] [PubMed] [Google Scholar]
Weinhouse 2009
- Weinhouse GL, Watson PL. Sedation and sleep disturbances in the ICU. Critical Care Clinics 2009;25(3):539‐49. [PUBMED: 19576529] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hu 2010
- Hu R‐F, Jiang X‐Y, Chen J, Zeng Z, Chen XY, Li Y, Huining X. Non‐pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008808] [DOI] [PMC free article] [PubMed] [Google Scholar]


