Abstract
Background
Cardiopulmonary arrest in paediatric patients often results in death or survival with severe brain injury. Therapeutic hypothermia, lowering of the core body temperature to 32 °C to 34 °C, may reduce injury to the brain in the period after the circulation has been restored. This therapy has been effective in neonates with hypoxic ischaemic encephalopathy and adults after witnessed ventricular fibrillation cardiopulmonary arrest. The effect of therapeutic hypothermia after cardiopulmonary arrest in paediatric patients is unknown.
Objectives
To assess the clinical effectiveness of therapeutic hypothermia after paediatric cardiopulmonary arrest.
Search methods
We searched the Cochrane Anaesthesia Review Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 11); Ovid MEDLINE (1966 to December 2011); Ovid EMBASE (1980 to December 2011); Ovid CINAHL (1982 to December 2011); Ovid BIOSIS (1923 to December 2011); and Web of Science (1945 to December 2011). We searched the trials registry databases for ongoing trials. We also contacted international experts in therapeutic hypothermia and paediatric critical care to locate further published and unpublished studies.
Selection criteria
We planned to include randomized and quasi‐randomized controlled trials comparing therapeutic hypothermia with normothermia or standard care in children, aged 24 hours to 18 years, after paediatric cardiopulmonary arrest.
Data collection and analysis
Two authors independently assessed articles for inclusion.
Main results
We found no studies that satisfied the inclusion criteria. We found four on‐going randomized controlled trials which may be available for analysis in the future. We excluded 18 non‐randomized studies. Of these 18 non‐randomized studies, three compared therapeutic hypothermia with standard therapy and demonstrated no difference in mortality or the proportion of children with a good neurological outcome; a narrative report was presented.
Authors' conclusions
Based on this review, we are unable to make any recommendations for clinical practice. Randomized controlled trials are needed and the results of on‐going trials will be assessed when available.
Plain language summary
Therapeutic hypothermia as a neuroprotective therapy after cardiopulmonary arrest in children
Cardiopulmonary arrest in children is uncommon however the numbers of children who survive are very low. Resulting brain injury in the survivors can be devastating for the child and family. Cooling the patient to a temperature of 32 °C to 34 °C, which is 3 °C to 4 °C below normal (therapeutic hypothermia), has previously been found to improve survival and reduce brain injury in newborn infants who were deprived of oxygen during birth, and also in adults following cardiopulmonary arrest. The causes of cardiopulmonary arrest are different in children than in adults, and asphyxia at birth is also different, so the effect of therapeutic hypothermia on the proportion of children who survive or who have brain injury is unclear.
We therefore conducted a Cochrane systematic review of the literature, searching medical databases (CENTRAL, MEDLINE, EMBASE) until December 2011 and contacting international experts for high quality published and unpublished evidence. Our searches failed to find any randomized controlled studies that met our inclusion criteria. However, we found four on‐going trials which, when completed, may contribute to our review.
At present there is no evidence from randomized controlled trials to support or refute the use of therapeutic hypothermia within a few hours after return of spontaneous blood flow following cardiopulmonary arrest in children. International resuscitation guidelines currently recommend that doctors consider using the therapy in infants and children although more research is needed to be sure this is the correct recommendation with the lack of treatment options other than supportive care in an intensive care unit that are available.
Background
Therapeutic hypothermia has been shown clinically to be neuroprotective in neonates with hypoxic ischaemic encephalopathy secondary to birth asphyxia (Jacobs 2007) and in adults (greater than 18 years old) after witnessed ventricular fibrillation cardiopulmonary arrest (Arrich 2012; Holzer 2005; Walters 2011). Its role after cardiopulmonary arrest in children has not been established although the International Liaison Committee on Resuscitation guidelines recommend consideration of its use in this setting (de Caen 2010). This systematic review of the literature investigates the neuroprotective effects of therapeutic hypothermia in children after cardiopulmonary arrest.
Description of the condition
Cardiopulmonary arrest in children is a devastating event. The incidence of out‐of‐hospital cardiopulmonary arrest (OHCA) has been estimated to be 8 to 20/100,000 children/year with in‐hospital cardiopulmonary arrest (IHCA) 100 times more frequent (Donoghue 2005; Nadkarni 2006); 2% to 6% of children admitted to a paediatric intensive care unit (PICU) have cardiac arrest (Nadkarni 2006). Survival rates to discharge from hospital for children who suffer an IHCA are 15% to 30%, whilst those who suffer an OHCA have a worse survival rate (5% to 12%) (Donoghue 2005; Nadkarni 2006). Only 0.3% to 4% of children who suffer an OHCA survive neurologically intact (Donoghue 2005).
Neurological consequences of hypoxic ischaemic damage to the brain range from mild concentration, attention and short‐term memory problems to much more severe damage to the cerebral cortex, hippocampus, basal ganglia and cerebellum. Severe damage can result in significant long‐term loss of function with development of cerebral palsy, blindness, seizures and hypothalamic and pituitary insufficiency. Very severe damage can produce a persistent vegetative state or be fatal. Those children who do survive often have significant neurological disability with resultant emotional, time and financial impacts on themselves, their families, their educational and care needs, rehabilitation and society as a whole (Duncan 2009; Morris 1993; Ronco 1995).
Neurological outcomes are often assessed by the use of the Paediatric Cerebral Performance Category (PCPC) score (Fiser 1992) (Appendix 1). The PCPC scores can classically be combined into a good outcome (PCPC 1 to 3) or poor outcome (PCPC 4 to 6). If children with preceding disability are included then a good outcome may be recorded as no change from baseline. Other neurological outcome scores, for example the Vineland Adaptive Behaviour Scales (Sparrow 1984), have also been validated for use in assessing neurological outcomes in children.
The aetiology for paediatric cardiopulmonary arrest is different to that of adults, with respiratory disorders leading to hypoxia often preceding cardiopulmonary arrest in children (Young 2004). The most common causes of OHCA in children are sudden infant death syndrome, drowning and trauma (Atkins 2009). IHCA is predominantly secondary to respiratory insufficiency, hypotension, hypoperfusion, congestive cardiac failure or infection (Nadkarni 2006). The neonates studied while receiving therapeutic hypothermia after hypoxic ischaemic encephalopathy often did not have a cardiopulmonary arrest and therefore retained some cerebral blood flow in comparison to the absent cerebral blood flow during cardiopulmonary arrest (Azzopardi 2009). Therefore, the pattern of neurological injury in adults and neonates may be different to children and the efficacy of therapeutic hypothermia may be altered.
Description of the intervention
Therapeutic hypothermia is defined as the process of lowering core body temperature to between 32 °C and 34 °C. Therapeutic hypothermia can be administered through systemic cooling (by surface or invasive methods) and selective surface head cooling. Sedation and often neuromuscular blockade are required to tolerate the intervention and avoid shivering.
How the intervention might work
Hypothermia therapy for brain protection, applied before cerebral ischaemia, has allowed cardiac surgery to be performed in circulatory arrest states (Bigelow 1950). Clinical and experimental studies have demonstrated neuroprotective properties of therapeutic hypothermia when commenced during and after an hypoxic Ischaemic insult or ischaemic reperfusion injury (Azzopardi 2009; Bernard 2002; Busto 1987; Colbourne 1994; Colbourne 1995; Colbourne 1999;Fink 2004; Gluckman 2005; Gunn 1997; Hypothermia After Cardiac Arrest Study Group 2002; Shankaran 2005; Shankaran 2008). However, the pathophysiological mechanism by which therapeutic hypothermia may exert its neuroprotective effect is complex. There is evidence that it modulates a number of the key biochemical, metabolic and pathophysiological events in the brain which occur after cerebral ischaemia and reperfusion. These include reduction of cerebral metabolism and balancing out of energy failure by the protection of adenosine triphosphate stores (ATP) during cessation of cerebral blood flow (Steen 1983; Takasu 1996); attenuation of the biosynthesis, release and uptake of excitotoxic compounds such as glutamate and dopamine (Busto 1989; Suehiro 1999); and attenuation of the production of proteins important in apoptosis (Bax and Bcl‐2) (Xu 2002; Yenari 2002). It has also been shown to reduce free radical production (Kil 1996), improve delayed hypoperfusion (Karibe 1994), and is involved in neuronal anti‐inflammatory effects (Sutcliffe 2001).
Hyperthermia (fever) has been shown to be neurologically damaging if occurring after paediatric (Bembea 2010; Hickey 2000) and adult cardiopulmonary arrest (Suffoletto 2009; Takasu 2001; Takino 1991; Zeiner 2001). The risk of neurological damage increases for each degree of core body temperature greater than 37 °C (Zeiner 2001). Therefore, the neuroprotective effect of therapeutic hypothermia may also be exerted by actively avoiding the secondary insult of hyperthermia. Current international paediatric recommendations include aggressively treating fever after paediatric cardiopulmonary arrest (Biarent 2010). Extrapolation of neonatal and adult evidence has also led to recommendations to consider the use of therapeutic hypothermia for infants and children who remain comatose following resuscitation, and the suggestion that therapeutic hypothermia may be beneficial in adolescents who remain comatose following witnessed out‐of‐hospital ventricular fibrillation cardiac arrest (de Caen 2010).
Why it is important to do this review
Cardiopulmonary arrest in children is an important condition with a poor survival rate and a high chance of neurological injury leading to significant long‐term impact on individuals, families and society. There are currently no interventions available to decrease neurological injury other than supportive care in the intensive care unit, which is why this evaluation is important. Paediatric‐specific data are needed regarding the effect of therapeutic hypothermia due to the different aetiology and resultant pathophysiology compared to adults and neonates. Therapeutic hypothermia is used by some paediatric critical care units after cardiopulmonary arrest (Haque 2006; Scholefield 2010) and a meta‐analysis of the clinical trial data is therefore highly important.
Objectives
To systematically review the literature and, if feasible, perform a meta‐analysis concerning the neuroprotective effects of therapeutic hypothermia after cardiopulmonary arrest in children.
To determine whether therapeutic hypothermia is effective in improving the primary outcome of good neurological survival after cardiopulmonary arrest in children and the secondary outcome of improving overall survival.
To determine the extent of adverse effects and effects on quality of life in this context.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs) and quasi‐randomized controlled trials evaluating therapeutic hypothermia as a neuroprotective intervention after cardiopulmonary arrest in children. We excluded non‐randomized studies from the meta‐analysis but provided a narrative summary of these studies in the review’s Discussion section.
Types of participants
We planned to include all studies with children who were successfully resuscitated after a cardiopulmonary arrest in any setting. We planned to include neonates older than 24 hours of age and with a corrected gestational age of greater than or equal to 35 weeks, children and adolescents up to their 18th birthday. We planned to exclude neonates whose cardiopulmonary arrest occurred at the time of birth and adults greater than 18 years of age as these have been studied separately (Arrich 2012; Jacobs 2007) and the presumed cause of the cardiopulmonary arrest is different to children.
Types of interventions
Therapeutic hypothermia, regardless of how body temperature was reduced, applied after return of spontaneous circulation after cardiopulmonary arrest. We defined therapeutic hypothermia as a target temperature of 32 °C to 34 °C. We defined the control intervention as treatment according to the standard treatment after cardiopulmonary arrest at the time of the trial.
Types of outcome measures
Primary outcomes
Best neurological outcome at hospital discharge and within the first year as assessed by the Paediatric Cerebral Performance Category score and other validated outcome scores for use in children (e.g. Vineland Adaptive Behaviour Scales)
Survival up to six months and long‐term survival (long term defined as greater than one year)
Secondary outcomes
Survival to intensive care discharge
Survival to hospital discharge
Adverse event rates as reported by authors
Quality of life indicators at six months and long term
Search methods for identification of studies
Electronic searches
We searched the Cochrane Anaesthesia Review Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 11); Ovid MEDLINE (1966 to December 2011); Ovid EMBASE (1980 to December 2011); Ovid CINAHL (1982 to December 2011); Ovid BIOSIS (1923 to December 2011); and Web of Science (1945 to December 2011).
The Ovid MEDLINE specific search terms are described in Appendix 2. We based the search strategies for the other databases on the one for MEDLINE (CENTRAL, Appendix 3; EMBASE, Appendix 4; CINAHL, Appendix 5; Web of Science, Appendix 6). We combined the Ovid MEDLINE and EMBASE searches with the sensitive strategies described in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search for RCTs.
BIOSIS and Web of Science contain book articles and abstracts of conference presentations published in a wide range of relevant specialist journals.
We did not use language or publication type restrictions.
Searching other resources
We searched the bibliographies of all retrieved and relevant publications identified by these strategies for further studies. We searched the Current Controlled Trials (http://www.controlled‐trials.com/), Clinical Trials (http://clinicaltrials.gov/), Trials Central (http://www.trialscentral.org), Chinese Clinical Trial Registry (http://www.chictr.org/en/proj/search.aspx), World Health Organization (WHO) International Clinical Trials Registry (http://apps.who.int/trialsearch/) databases of ongoing trials. We searched Zetoc (http://zetoc.mimas.ac.uk/) and OpenSIGLE (http://opensigle.inist.fr/) and contacted experts in the field to search for any other published or unpublished literature and on‐going research.
Data collection and analysis
Selection of studies
Potentially eligible published studies were located based on screening of titles and abstracts by two authors (BS and HD). Potentially eligible on‐going trials were located on screening the title and trial description by two authors (BS and KM). We obtained full copies of potentially eligible studies. There was no blinding to the journal, the authors or the institution. Three authors (BS, KM and HD), acting independently, decided on inclusion or exclusion of studies based on predefined inclusion and exclusion forms. We resolved disagreements by discussion.
Data extraction and management
We planned to extract data from eligible studies and summarized them in a data extraction sheet (Appendix 7).
We planned to include baseline data on the demographics of study and control group participants. This included age and gender. In addition, we planned to extract the following information regarding the actual cardiopulmonary arrest: location, aetiology, duration of arrest, and time to return of spontaneous circulation for each group. We planned to record data on the intervention, time to implement the intervention, duration, and type of temperature control method. The temperature of the study group and control group at the start of a study, during intervention and after the intervention were recorded. We also planned to record the healthcare setting in which the interventions were performed. In addition, duration of follow up and numbers lost to follow up were planned to be extracted as well as outcomes.
All data regarding the interventions studied were independently extracted by two authors (BS and KM or HD). We resolved disagreements by discussion. We planned to contact primary authors to obtain missing data or to gain clarification.
Assessment of risk of bias in included studies
After we included all available eligible studies in the review, we planned to assign two authors (BS and KM or HD) to independently assess each study using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). We planned to assess six domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias (Appendix 7); completing a risk of bias table for each eligible study. Any disagreement was planned to be discussed amongst authors and consensus reached. We planned to present our assessment of risk of bias using a 'risk of bias summary figure', which presents all of the judgments in a cross‐tabulation of study by entry.
Measures of treatment effect
For dichotomous outcomes, we planned to express the estimate of effect of an intervention as the risk ratio (RR) together with the 95% confidence interval (CI). For continuous outcomes, we planned to use mean differences (MD) and standard deviations and summarize the data for each group using mean differences and 95% CIs.
Unit of analysis issues
We did not anticipate unit of analysis issues.
Dealing with missing data
If data were missing from trial reports we attempted to contact the original investigator for additional data. Where there were missing data, we planned to 'impute' the data and carry out a sensitivity analysis between studies in which data were 'imputed' for an intention‐to‐treat (ITT) analysis assuming that all missing participants experienced the event or that all missing participants did not experience the event.
Assessment of heterogeneity
We judged the degree of clinical heterogeneity, in particular in the application of therapeutic hypothermia. If significant clinical heterogeneity existed, pooling of data was not done; the data from individual studies was presented in a tabular format. We planned to test for statistical heterogeneity using visible inspection of the forest plot and the I2 statistic (Higgins 2011). We considered an I2 statistic > 50% as significant statistical heterogeneity and a value < 25% was considered ignorable statistical heterogeneity.
Assessment of reporting biases
We planned to assess risk of reporting bias by producing a funnel plot if there was a sufficient number of included studies (more than 10). We took the following steps to reduce reporting bias.
Searching of multiple databases, trial registries and conference proceedings, as described above.
Not applying any language restriction.
Excluding duplicate reports of the same study to avoid duplication bias.
Data synthesis
We planned to summarize the aims, methods and outcome measures of interest (neurological outcome, mortality, adverse events and quality of life indicators) for each included study. We expressed the outcome measures of interest for survivors relative to non‐survivors as the risk ratio (RR).
For both dichotomous and continuous data we planned to undertake a meta‐analysis using a random‐effects method with inverse variance. We planned to perform a sensitivity analysis by comparing this with a meta‐analysis using a fixed‐effect method with inverse variance.
We planned to use data at the aggregate (study) level.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis on the following variables if the data could be extracted from included studies.
Age and sex.
Method of inducing therapeutic hypothermia (systemic cooling versus selective head cooling).
Location of arrest (in‐hospital versus out‐of‐hospital arrest).
Duration of intervention.
Delay to induction of therapeutic hypothermia (less than six hours versus greater than six hours).
Rate of rewarming after therapeutic hypothermia.
First presenting cardiac rhythm (ventricular fibrillation or pulseless ventricular tachycardia versus asystole or pulseless electrical activity).
Meta‐regression was not performed due to the small number of included trials..
Sensitivity analysis
We planned to undertake sensitivity analysis for studies in which data were 'imputed' for ITT analysis by assuming that all missing participants experienced the event or that all missing participants did not experience the event.
Summary of findings table
We planned to use the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes:
best neurological outcome,
survival to intensive care discharge,
survival to hospital discharge,
survival up to six months,
long‐term survival.
In our review we planned to construct a 'Summary of findings' (SoF) table using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence takes into consideration within study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See: Characteristics of excluded studies and Characteristics of ongoing studies
Results of the search
Our sensitive search strategy resulted in 6229 hits with the database searches and 615 by other searches (see Figure 1). Following removal of duplicates (2931), the remaining 3913 were screened; 3649 were excluded on reviewing the title or abstract resulting in 264 for full paper review. The majority (188) were then excluded because they were not relevant or because the age of patients was outside the specified age for inclusion in this review (older than 24 hours of age and with a corrected gestational age of greater than or equal to 35 weeks, children and adolescents up to their 18th birthday); 54 on‐going studies were excluded of which 38 only included adults greater than 18 years old and 16 only included neonates less than 24 hours old. Finally, 18 studies were evaluated for inclusion, and four ongoing paediatric RCTs (NCT00754481; NCT00797680; NCT00878644; NCT00880087).
1.
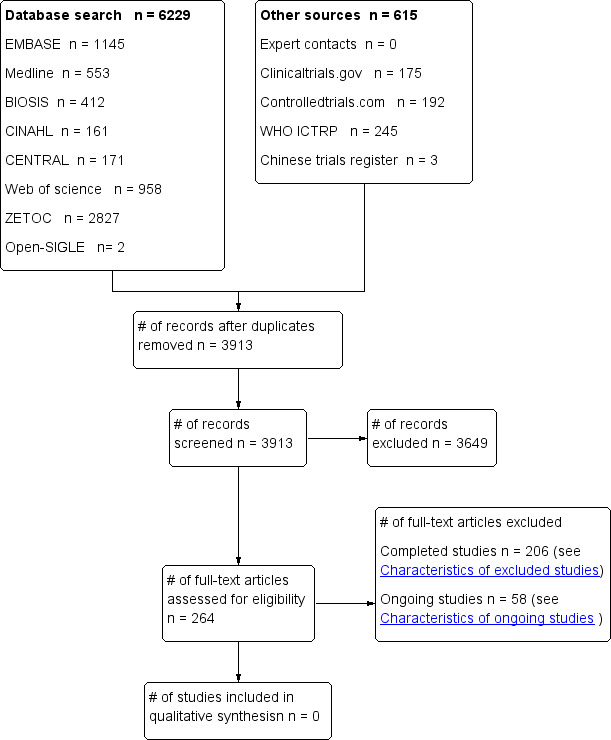
Study flow diagram.
After contacting experts about possible additional unpublished or on‐going relevant studies, none were found. The chief investigators of the four ongoing paediatric RCTs were all contacted and they confirmed that no results were available.
Included studies
There were no RCTs included from our systematic search of the medical databases.
Excluded studies
The remaining 18 studies were excluded for being non‐randomized prospective (five) or retrospective (six) cohort studies, a database registry study (one), case studies (three), a review (one), commentary (one), and protocol outline for a future study (one). See Characteristics of excluded studies for a full description of all excluded studies.
The four ongoing RCTs may potentially be assessed in the future and fulfil this review’s inclusion criteria (see Characteristics of ongoing studies).
Risk of bias in included studies
No RCTs were identified (see Characteristics of excluded studies and Characteristics of ongoing studies).
Allocation
Not relevant
Blinding
Not relevant
Incomplete outcome data
Not relevant
Selective reporting
Not relevant
Other potential sources of bias
Not relevant
Effects of interventions
No RCTs were identified (see Characteristics of excluded studies and Characteristics of ongoing studies).
Discussion
We did not find any high quality evidence, in the form of randomized or quasi‐randomized controlled trials, for or against the use of therapeutic hypothermia in children after cardiopulmonary arrest.
Excluded studies
We excluded three cohort studies comparing the use of therapeutic hypothermia and normothermia or standard care in the paediatric population: two retrospective published studies (Doherty 2009; Fink 2010) and one prospective study in abstract form only (Buttram 2009). A narrative review is given below.
Doherty 2009 retrospectively investigated the survival and neurological outcome of children by comparing the use of therapeutic hypothermia (n = 29) versus normothermia (n = 50). Included patients had predominantly suffered an in‐hospital cardiac arrest (IHCA) (95%) often with associated chronic cardiac conditions (71%) and after surgery (59%). In patients with an a priori defined cardiopulmonary arrest duration of at least three minutes and who survived to 12 hours post‐return of spontaneous circulation, the use of therapeutic hypothermia was associated with increased 30 day mortality (unadjusted odds ratio (OR) 2.5, 95% confidence interval (CI) 0.99 to 6.45; P = 0.054), increased six month mortality (unadjusted OR 3.62, 95% CI 1.37 to 9.62; P = 0.009) and an unfavourable neurological outcome (PCPC score 4 to 6) (unadjusted OR 2.92, 95% CI 1.1 to 7.69; P = 0.031). However, patients receiving therapeutic hypothermia were sicker due to a number of factors including longer duration of cardiopulmonary arrest, more pharmacological interventions during resuscitation, greater post‐resuscitation serum lactate levels, higher multi‐organ dysfunction score and more renal replacement therapies. More patients receiving therapeutic hypothermia also received extracorporeal life support. When logistic regression modelling was performed to account for these confounding factors, the use of therapeutic hypothermia did not statistically increase the risk of 30 day mortality (adjusted OR 2.5, 95% CI 0.55 to 11.49; P = 0.238), six month mortality (adjusted OR 1.99, 95% CI 0.45 to 8.85; P = 0.502) or unfavourable neurological outcome (adjusted OR 2.0, 95% CI 0.45 to 9.01; P = 0.364).
Fink 2010 in a single‐centre retrospective study also showed no significant difference in mortality and gross neurological outcomes for patients treated with either therapeutic hypothermia or normothermia after cardiopulmonary arrest. The patient population differed from the study by Doherty 2009 and included IHCA (55%) and out‐of‐hospital cardiac arrest (OHCA) (45%) patients with predominately an asphyxial aetiology (91%) causing the arrest. Excluded from the population were all patients with congenital heart disease. Patients received either therapeutic hypothermia (n = 40) or standard normothermia therapy (n = 141). Similar to Doherty 2009, patients receiving therapeutic hypothermia had a longer duration of cardiopulmonary arrest, more doses of epinephrine, and fewer were witnessed arrests and therefore they had an increased baseline risk of mortality. In patients who were normothermic at the start of therapy, the therapeutic hypothermia target temperature was reached within a median eight hours (IQR 5 to 7). Mortality at hospital discharge was similar for patients receiving therapeutic hypothermia (55.0%) and standard therapy (55.3%) (P = 1.0). In a multivariate logistic regression analysis, there remained no association between therapeutic hypothermia and hospital survival (adjusted OR for mortality 0.47, 95% CI 0.15 to 1.45; P = 1.0). This study also reported no difference in the proportion of survivors discharged home after therapeutic hypothermia compared to standard therapy (78% versus 68% respectively; P = 0.46).
The third study was only available in abridged abstract form (Buttram 2009). This prospective study compared therapeutic hypothermia (n = 33) and standard therapy (n = 13) in paediatric patients surviving to paediatric intensive care unit (PICU) admission after IHCA and OHCA. Mortality at hospital discharge was not significantly different (therapeutic hypothermia 61% versus standard therapy 77%; P = 0.49). However, no data were presented to compare the demographics of the two groups.
These three excluded studies were unable to demonstrate a significant difference in survival or a good neurological outcome at various time points after the use of therapeutic hypothermia compared with normothermia. Methodological problems including the non‐randomized design of the studies are a significant problem. In both Doherty 2009 and Fink 2010, therapeutic hypothermia was used in patients already at higher risk of mortality with associated risk factors known to exist after paediatric cardiopulmonary arrest. In addition, it is difficult to compare these two studies due to significant differences in the patient demographics between each study. The high proportion of IHCA patients with chronic cardiac conditions in the Doherty 2009 study will have had an increased chance of survival compared with the higher proportion of asphyxial arrest patients and OHCA patients in Fink 2010.
These studies do focus on important safety issues regarding the use of therapeutic hypothermia, which can usefully inform further prospective randomized studies. Excess hypothermia (defined as a core temperature less than 32 °C) was only reported in patients receiving therapeutic hypothermia, and occurred in 17.2% (Doherty 2009) and 15% (Fink 2010) of patients. Fink 2010 identified that excess hypothermia was associated with increased mortality in this group. Hyperthermia (defined as a temperature greater than 38 °C) in post‐cardiopulmonary arrest patients is known to increase mortality and neurological injury (Bembea 2010), and international recommendations advocate avoidance (Biarent 2010). However, it occurred in 38% of standard therapy and 17% of therapeutic hypothermia treated patients, although it was not associated with increased mortality in this study (Fink 2010). Neither Doherty 2009 or Fink 2010 reported an increase in post‐cardiopulmonary arrest infections, arrhythmias or bleeding in patients receiving therapeutic hypothermia. In view of these findings, we recommend that future randomized controlled trials ensure that any interventions controlling body temperature actively avoid excess hypothermia or hyperthermia, with continued surveillance of potential adverse effects of therapeutic hypothermia treatment.
We excluded two prospective, non‐randomized studies of paediatric patients who all received therapeutic hypothermia after cardiopulmonary arrest where there was no comparison group (Abend 2009; Topjian 2011). We excluded an additional three retrospective, non‐randomized studies of paediatric patients after cardiopulmonary arrest where factors associated with survival, including therapeutic hypothermia, were reported (Deasy 2010; Meert 2009; Moler 2011). Deasy 2010 reported that 23/46 (46%) of patients admitted to PICU after cardiopulmonary arrest were treated with therapeutic hypothermia. However, its use was not associated with survival (unadjusted OR 2.54, 95% CI 0.64 to 9.9] P = 0.184). Two retrospective studies of IHCA (Meert 2009) and OHCA (Moler 2011) separately reported the proportion of patients receiving therapeutic hypothermia, although the proportions were so small (4% and 2% respectively) that a comparison between groups was inappropriate.
On‐going studies
We found four on‐going studies (NCT00754481; NCT00797680; NCT00878644; NCT00880087). Two phase III studies (NCT00878644; NCT00880087) using therapeutic hypothermia (32 ⁰C to 34 ⁰C) for 48 hours with gradual rewarming and further control of temperature to normothermia (36.0 ⁰C to 37.5 ⁰C) for a further three days are separately comparing children after IHCA and OHCA. These two studies have projected sample sizes of 350 OHCA (NCT00878644) and 500 IHCA patients (NCT00880087) to be powered to detect a 15% absolute treatment increase in survival with a good neurological outcome at 12 months. One phase II (pilot) study (NCT00754481) is comparing therapeutic hypothermia (33 ⁰C to 34 ⁰C) for 48 hours with gradual rewarming to normothermia (36.5 ⁰C to 37.5 ⁰C); and a fourth phase II (pilot) study (NCT00797680) is comparing 24 hours versus 72 hours of therapeutic hypothermia (32 ⁰C to 34 ⁰C) with gradual rewarming.
All four studies are using a similar therapeutic hypothermia target temperature (33 °C ± 1 °C), which is the target temperature demonstrated to be efficacious in neonatal and adult RCTs. Three studies will maintain the target temperature for 48 hours and compare this with a therapeutic active normothermia group (NCT00754481; NCT00878644; NCT00880087). The use of an actively controlled normothermia group aims to eliminate the confounding effect of hyperthermia (temperature greater than 38 ⁰C) in the control group. This was noted in the adult European Hypothermia After Cardiac Arrest trial (Hypothermia After Cardiac Arrest Study Group 2002) with approximately 25% of the patients in the control group experiencing hyperthermia and potentially increasing their risk of neurological injury (Hypothermia After Cardiac Arrest Study Group 2002).
Duration of therapy may be an important variable. The fourth study is comparing 24 and 72 hours of therapeutic hypothermia (NCT00797680). The other three studies are using 48 hours of therapeutic hypothermia as the treatment duration. This compares with the two adult studies which used 12 hours (Bernard 2002) and 24 hours (Hypothermia After Cardiac Arrest Study Group 2002) of therapy and the neonatal trials which used 72 hours of therapy (Azzopardi 2009; Jacobs 2007). Despite the wide variation in duration of therapy between studies, beneficial effects were still seen and therefore further investigation of this treatment variable is important. The advantages of a longer duration of therapy include the potential for greater beneficial effect from the intervention and this is supported in experimental animal studies, particularly with an increased delay from return of spontaneous circulation to commencement of therapy (Clark 2008; Colbourne 1999). However, prolonged duration may increase the risk of infection and associated morbidity. The increased duration may also have greater financial implications associated with prolonged ventilation and a longer stay in PICU, which should be taken into account.
Important functional and neurological outcomes are being assessed in all on‐going studies. The use of simple functional outcome scores (for example Paediatric Cerebral Performance Category (PCPC) (Fiser 1992)) will give some useful information regarding crude functioning. The PCPC score was designed and validated in the post‐cardiopulmonary arrest population but will not be able to demonstrate subtle functional and developmental problems. The use of multiple, in‐depth neurodevelopmental and neuropsychological tests, as planned in two studies, will hopefully give a much clearer and more accurate assessment of subtle effects of therapeutic hypothermia at different stages of brain development (NCT00878644; NCT00880087). The role of biomarkers (serological and radiological) as early indicators of neurodevelopmental outcome also provides a potentially useful tool, both in predicting short‐term survival and also in the levels of developmental outcome over the longer term (NCT00797680).
In a future Cochrane systematic review we hope to assess these four studies together with any additional randomized controlled studies and to perform a meta‐analysis as detailed above. In addition, we will use individual patient data, if possible.
Summary of main results
We did not find any studies that satisfied the inclusion criteria. We found four on‐going RCTs, which may be available for analysis in the future. Eighteen non‐randomized studies were excluded. Of these, three compared therapeutic hypothermia with standard therapy demonstrating no difference in mortality or the proportion with good neurological outcome.
Overall completeness and applicability of evidence
Currently the available evidence neither supports nor refutes the use of therapeutic hypothermia in the paediatric population. However, this lack of evidence justifies further randomized controlled trials and the encouragement of researchers and clinicians to continue investigation of this therapy. The on‐going studies will hopefully provide valuable information regarding this therapy and be available for future systematic assessment, as described in this review.
Quality of the evidence
We found no high quality evidence for or against the use of therapeutic hypothermia in children after cardiopulmonary arrest. Formal quality assessment and meta‐analysis of non‐randomized studies were not performed.
Potential biases in the review process
We undertook a systematic search for evidence using a sensitive search strategy to find RCTs, as described in Section 6.4 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), in order to limit the risk of missing published studies. Non‐randomized trials have been discussed in this review due to the absence of high quality RCTs. However, due to the design of the systematic search focusing on randomized studies there is a possibility that we missed non‐randomized studies. Contacting experts in paediatric critical care and hypothermia research did not uncover further randomized or non‐randomized studies and we confirmed that the on‐going studies identified were either still recruiting or undergoing analysis. The possibility of additional unpublished trials does exist, although we feel this is small.
Agreements and disagreements with other studies or reviews
The use of therapeutic hypothermia in children after cardiopulmonary arrest is outlined in the International Liaison Committee on Resuscitation (ILCOR) guidance (de Caen 2010). The use of therapeutic hypothermia (32 °C to 34 °C) may be beneficial in adolescent children presenting in ventricular fibrillation cardiopulmonary arrest. In infants and children who remain comatose after resuscitation from paediatric cardiopulmonary arrest, therapeutic hypothermia (32 °C to 34 °C) may be considered.
Support for these recommendations is extrapolated from the neonatal evidence of 72 hours of therapeutic hypothermia after hypoxic ischaemic encephalopathy within six hours of birth (Azzopardi 2009; Jacobs 2007) and the two RCTs of 12 to 24 hours of therapeutic hypothermia in adults after ventricular fibrillation cardiopulmonary arrest (Bernard 2002; Hypothermia After Cardiac Arrest Study Group 2002). The findings and limitations of the two retrospective cohort studies (Doherty 2009; Fink 2010) and the lack of paediatric high quality evidence are acknowledged. The recommendations are given with the caution that the benefits or harm of therapeutic hypothermia in this population is not known.
We found no further published randomized or non‐randomized paediatric studies since the publication of the ILCOR guidelines. Reassessment of the paediatric recommendations will be necessary when the on‐going studies identified in this review have been completed.
Authors' conclusions
Implications for practice.
We found no randomized controlled studies, so this review concludes that there is no evidence of effect of therapeutic hypothermia in paediatric cardiopulmonary arrest rather than evidence of no effect. Therefore, we can not give a recommendation for or against the use of therapeutic hypothermia in clinical practice. Current guidance from the International Liaison Committee on Resuscitation recommends consideration of therapeutic hypothermia in infants and children and that its use may be beneficial in adolescents with ventricular fibrillation cardiopulmonary arrest (de Caen 2010). These recommendations are extrapolated from neonatal and adult clinical trials. Further research is needed to confirm these recommendations in the paediatric population.
Implications for research.
Continued support for the on‐going in‐hospital and out‐of‐hospital cardiopulmonary arrest studies identified in this review is essential to enable assessment of any effect of therapeutic hypothermia after paediatric cardiopulmonary arrest (NCT00754481; NCT00797680; NCT00878644; NCT00880087). The important differences which exist in cardiopulmonary arrest aetiology and neurological development in the paediatric population, compared with the studied neonatal and adult populations, support this.
Further adequately sized studies will be required to investigate the effect of therapeutic hypothermia and to define the duration, temperature depth and rewarming rates during administration. It is highly likely that international collaboration will be required to recruit sufficient numbers of patients to ensure adequately powered studies and findings that are generally applicable.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Jane Cracknell and Karen Hovhannisyan from the Cochrane Anaesthetic Review Group. We would like to thank Mathew Zacharias (content editor), Nathan Pace (statistical editor), Jasmin Arrich, Ronan O'Sullivan, Alexis A Topjian (peer reviewers) and Janet Wale (consumer editor) for their help and editorial advice during the preparation of the protocol for the systematic review.
Appendices
Appendix 1. Paediatric Cerebral Performance Category
The Paediatric Cerebral Performance Categories (PCPC) are as follows (Fiser 1992).
PCPC 1: Normal cerebral performance. Normal at age appropriate level, school age child attends regular school classroom.
PCPC 2: Mild Disability. Conscious alert and able to interact at an age appropriate level, school age child attending school classroom but grade perhaps not appropriate for age. May have mild neurological deficit.
PCPC 3: Moderate disability. Conscious, sufficient cerebral function for age appropriate independent activities of daily life. School age child attending special education classroom. May have learning disability.
PCPC 4: Severe disability. Conscious, dependent on others for daily support because of impaired brain function.
PCPC 5: Coma or vegetative state. Any degree of coma without any of the criteria for brain death. Unawareness even if awake in appearance without interaction with the environment. Cerebral unresponsiveness. No evidence of cortical activity and not aroused by verbal stimuli. Possibly some reflexive responses spontaneous eye opening and/or sleep‐wake cycles.
PCPC 6: Brain death.
Appendix 2. Ovid MEDLINE search strategy
Search strategy:
exp Hypothermia/ or exp Hypothermia, Induced/ or hypothermia.mp. or (cooling or temperature regulat*).ti,ab.
exp Heart Arrest/ or Ventricular Fibrillation/ or Cardiopulmonary Resuscitation/ or Tachycardia, Ventricular/ or neuroprotection.mp. or asystole.ti,ab. or dysrhythmia.mp. or ((heart or cardiac or ventricular or circulatory) adj3 arrest).mp. or (ventricular adj5 (fibrillation or tachycardia or arrhythmia)).mp. or pulseless electrical activity.mp. or electromechanical dissociation.mp. or cardiopulmonary resuscitation.mp. or (PEA or EMD or OOHCA).ti,ab. or non‐perfusing rhythm.mp.
1 and 2
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
3 and 4
Appendix 3. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Hypothermia explode all trees #2 MeSH descriptor Hypothermia, Induced explode all trees #3 hypothermia or (cooling or (temperature near (low or regulat*)) or hibernat* or cryotherapy or refrigeration):ti,ab #4 (#1 OR #2 OR #3) #5 MeSH descriptor Heart Arrest explode all trees #6 MeSH descriptor Ventricular Fibrillation explode all trees #7 MeSH descriptor Cardiopulmonary Resuscitation explode all trees #8 MeSH descriptor Tachycardia, Ventricular explode all trees #9 neuroprotection or asystole:ti,ab or distrithmia or ((hearth or cardiac or ventricular or circulat*) near arrest) or (ventricular near (fibrillation or tachycardia or arrhythmia)) or pulseless electrical activity or electromechanical dissociation or cardiopulmonary resuscitation or (PEA or EMD or OOHCA):ti,ab or non‐perfusing rhythm #10 (#5 OR #6 OR #7 OR #8 OR #9) #11 (#4 AND #10)
Appendix 4. Search strategy for EMBASE (OvidSP)
1. exp hypothermia/ or exp induced hypothermia/ or exp cryotherapy/ or hypothermia.mp. or (cooling or (temperature adj3 (low or regulat*)) or hibernat* or cryotherapy or refrigeration).ti,ab. 2. heart arrest/ or heart ventricle fibrillation/ or resuscitation/ or heart ventricle tachycardia/ or neuroprotection.mp. or asystole.ti,ab. or distrithmia.mp. or ((hearth or cardiac or ventricular or circulat*) adj3 arrest).mp. or (ventricular adj5 (fibrillation or tachycardia or arrhythmia)).mp. or pulseless electrical activity.mp. or electromechanical dissociation.mp. or cardiopulmonary resuscitation.mp. or (PEA or EMD or OOHCA).ti,ab. or non‐perfusing rhythm.mp. 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 5. Search strategy for CINAHL (EBSCOhost)
S1 ( (MH "Hypothermia") OR (MH "Hypothermia, Induced") OR (MH "Hypothermia (NANDA)") OR (MH "Cryotherapy") ) OR ( hypothermia or (cooling or (temperature and (low or regulat*)) or hibernat* or cryotherapy or refrigeration) ) S2 ( (MH "Heart Arrest") OR (MH "Ventricular Fibrillation") OR (MH "Tachycardia, Ventricular") OR (MH "Resuscitation, Cardiopulmonary") ) OR ( neuroprotection or asystole or distrithmia or ((hearth or cardiac or ventricular or circulat*) and arrest) or (ventricular and (fibrillation or tachycardia or arrhythmia)) or pulseless electrical activity or electromechanical dissociation or cardiopulmonary resuscitation or (PEA or EMD or OOHCA) or non‐perfusing rhythm ) S3 S1 and S2 S4 ( (MH "Randomized Controlled Trials") OR (MH "Random Assignment") OR (MH "Prospective Studies") OR (MH "Multicenter Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Double‐Blind Studies") OR (MH "Clinical Trials") ) OR ( random* or controlled clinical trial or placebo ) S5 S3 and S4
Appendix 6. Search strategy for ISI Web of Science and BIOSIS Citation Index
#1 TS=(hypothermia or (cooling or (temperature SAME (low or regulat*)) or hibernat* or cryotherapy or refrigeration)) #2 TS=(neuroprotection or asystole or distrithmia or ((hearth or cardiac or ventricular or circulat*) and arrest) or (ventricular SAME (fibrillation or tachycardia or arrhythmia)) or pulseless electrical activity or electromechanical dissociation or cardiopulmonary resuscitation or (PEA or EMD or OOHCA) or non‐perfusing rhythm) #3 #2 AND #1 #4 TS=(random* or (controlled SAME trial*) or prospective or multicenter) or TS=((blind* or mask*) SAME (single or double or triple)) #5 #4 AND #3
Appendix 7. Data Extraction Sheet
Data Extraction Form
| Study ID |
| Authors |
| Medline Journal ID |
| Year of Publication |
| Language |
Type of study: RCT Cohort Case‐control
(Circle relevant study type)
| Comment on study design: |
Tool for Assessing Risk of Bias
| Domain | Description | Review Authors Judgement |
| Random sequence Generation |
LOW RISK / HIGH RISK / UNCLEAR | |
| Allocation concealment |
LOW RISK / HIGH RISK / UNCLEAR | |
| Blinding of participants, personnel and outcome assessors. Outcome: |
LOW RISK / HIGH RISK / UNCLEAR | |
| Blinding of participants, personnel and outcome assessors. Outcome: |
LOW RISK / HIGH RISK / UNCLEAR | |
| Incomplete outcome data Outcome: |
LOW RISK / HIGH RISK / UNCLEAR | |
| Selective reporting Outcome: |
LOW RISK / HIGH RISK / UNCLEAR | |
| Other bias |
LOW RISK / HIGH RISK / UNCLEAR |
INCLUSION/EXCLUSION CRITERIA
Yes No
| Clearly defined |
||
| Inclusion criteria |
||
| Exclusion criteria |
||
PARTICIPANTS
| Number of eligible participants |
Number enrolled in study | ||
| Number of males |
Number of females | ||
| Number of children <18 |
Number in treatment group |
Treatment Group Control Group
| Witnessed arrest |
||
| Time to ROSC |
INTERVENTION
Control Group Treatment Group
| Method of cooling |
||
| Time to Randomization |
||
| Time to start of cooling |
||
| Time to target temperature |
||
| Depth of cooling |
||
| Length of cooling |
||
| COMMENTS ON TREATMENT | ||
Yes No Unclear
| Withdrawals |
OUTCOME Control Group Treatment Group
| Days in hospital |
||
| Ventilator Free days |
||
| Survival to admission |
||
| Survival to intensive care discharge |
||
| Survival to hospital discharge |
||
| 6 months survival |
||
| 12 months survival |
||
| Long term survival > 12 months |
||
| Neurological outcome at Hospital discharge | ||
| Neurological outcome at 6 months | ||
| Neurological outcome at 12 months | ||
| Long Term Neuro outcome > 12months | ||
| Adverse Events |
||
| Cost Effectiveness Evaluation |
|
CHANGES IN PROTOCOL |
|
CONTACT WITH AUTHOR |
|
COMMENTS ON THIS STUDY |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abend 2009 | Non‐randomized study Prospective, cohort study of paediatric post‐cardiopulmonary arrest patients treated with therapeutic hypothermia receiving continuous electroencephalography. Single centre, March 2007‐September 2008. 14/19 (74%) survived, four with severe neurological morbidity. No comparative normothermia group. |
| Bembea 2010 | Non‐randomized study Database registry of in‐hospital paediatric cardiopulmonary arrest patients (January 2005‐December 2007). Relationship of temperature in first 24 hours with survival and neurological outcome. 86/547 (16%) patients reported to have received; therapeutic hypothermia, extracorporeal membrane oxygenation or “ice‐packs to head”. No survival breakdown for this subgroup. |
| Buttram 2009 | Non‐randomized study (abstract only) Prospective, cohort study comparing therapeutic hypothermia with normothermia after paediatric out‐of‐hospital cardiopulmonary arrest. Age 1 day to 18 years. 33/46 (77%) received therapeutic hypothermia. No difference in survival to hospital discharge between patients treated with hypothermia (39%) and standard therapy (23%), P = 0.49. |
| Deasy 2010 | Non‐randomized study Retrospective, cohort study of paediatric out‐of hospital cardiopulmonary arrest patients in Melbourne, Australia (Oct 1999‐2007). Therapeutic hypothermia applied to 23/49 (46%) admitted to PICU with three survivors (OR 2.535, 95% CI 0.64‐9.9, P = 0.184). |
| Doherty 2009 | Non‐randomized study Retrospective, cohort study comparing therapeutic hypothermia with normothermia over a two year period (September 2001 ‐ August 2003) in five centres in Canada (4) and the UK (1). 29/79 (36.7%) received therapeutic hypothermia. 95% in‐hospital cardiopulmonary arrest. Median duration of therapeutic hypothermia 20.8 hours (IQR 12‐69 hours) at a mean temperature of 33.7±1.3°C. Hypothermia use was associated with higher mortality, more resuscitative interventions, higher post resuscitative lactate level and the use of ECMO. Non‐significant association between therapeutic hypothermia after adjustment for duration of cardiopulmonary arrest, use of ECMO and propensity score (adjusted OR 1.99, 95% CI 0.45 to 8.85, P = 0.502). |
| Fink 2010 | Non‐randomized study Retrospective, cohort study comparing therapeutic hypothermia with normothermia over a seven year period (July 2000‐August 2006) in a single centre (Pittsburgh, USA). 40/181 (22%) received therapeutic hypothermia. 55% in‐hospital cardiopulmonary arrest. Median duration of therapeutic hypothermia 24.0 hours (IQR 16‐48) at a median temperature of 34.0°C (33.5°C to 34.8°C). No difference in hospital mortality; 55% therapeutic hypothermia group versus 55.3% standard therapy group (P = 1.0). Hypothermia not associated with survival in univariate (OR 0.99, 95% CI 0.49 to 2.0, P = 1.0) or multivariate (OR 0.47, 95% CI 0.15 to 1.45, P = 0.2) analysis. |
| Hein 2004 | Case study |
| Kessler 2011 | Non‐randomized study Prospective, cohort study of paediatric post‐cardiopulmonary arrest patients treated with therapeutic hypothermia (24 hours, 34°C) and monitored with electroencephalography. 21/35 (60%) patients survived. No comparative normothermia group. |
| Kobr 2011 | Review |
| Le Guen 2011 | Non‐randomized study Prospective, cohort study of extracorporeal support following out‐of hospital refractory cardiopulmonary arrest. Inclusion of patients from 13 years to 70 years (mean (SD) 45 years (15)). All patients received therapeutic hypothermia. No breakdown of paediatric patient population. |
| Meert 2009 | Non‐randomized study Retrospective, cohort study of in‐hospital cardiopulmonary arrest patients. Conducted in 15 centres in the USA over 18 months (July 2003‐December 2004). Only 7/162 (4.1%) survivors and 8/181 (3.3%) non‐survivors received therapeutic hypothermia. |
| Meert 2010 | Comment |
| Moler 2009 | Non‐randomized study Retrospective, cohort study comparing in‐hospital and out‐of hospital paediatric cardiopulmonary patients in the USA over 18 months (July 2003‐December 2004). No outcome data for patients receiving or not receiving therapeutic hypothermia. |
| Moler 2011 | Non‐randomized study Retrospective, study of out‐of‐hospital cardiopulmonary arrest patients. Conducted in 15 centres in the USA over 18 months (July 2003–December 2004). Only 1/53 (2%) survivors and 2/85 (2%) non‐survivors received therapeutic hypothermia. |
| Sanada 1998 | Case study |
| Silfvast 2003 | Case study |
| Takeda 2009 | Protocol for a randomized controlled trial of pharyngeal cooling system during cardiopulmonary arrest. Age inclusion 16‐89 years. |
| Topjian 2011 | Non‐randomized study Prospective, intervention study of therapeutic hypothermia after paediatric cardiopulmonary arrest. Assessing the feasibility and safety of a standardized treatment protocol. Single centre, 6/12 (50%) of patients survived to discharge. No comparative normothermia group. |
ECMO: extracorporeal membrane oxygenation, IQR: interquartile range, OR: odds ratio, CI: confidence interval, SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
NCT00754481.
| Trial name or title | Hypothermia for Cardiac Arrest in Paediatrics (HypCAP) |
| Methods | Randomized, single blind (outcome assessor), parallel assignment efficacy Phase II study |
| Participants | ≥ 38 weeks gestation up to and including 17 yrs Chest compressions ≥3 minutes In‐hospital and out‐of‐hospital arrest GCS ≤ 10 at 1 hour post‐cardiopulmonary arrest Invasive mechanical ventilation Randomized within six hours |
| Interventions | 1) Therapeutic hypothermia: 48 hours at 33°C to 34°C with rewarming 0.5ºC every 2 hours to 36.5ºC 2) Therapeutic normothermia: 48 hours at 36.5°C to 37.5ºC |
| Outcomes | 1) Percentage of children achieving a "good outcome" (PCPC score of 1‐3) at 12 months 2) Cognitive and motor measures*, mortality (assessed at 1, 3, 6, and 12 months post‐arrest), cerebral oedema*, adverse effects of hypothermia therapy* (* measured at 12 months post‐arrest) |
| Starting date | January 2005 |
| Contact information | Dr Jamie Hutchison, The Hospital for Sick Children, Toronto, Canada. jamie.hutchison@sickkids.ca |
| Notes | Planned enrolment 40 Study finished recruiting |
NCT00797680.
| Trial name or title | Duration of Hypothermia for Neuroprotection After Paediatric Cardiac Arrest |
| Methods | Randomized, open label, parallel assignment, safety and efficacy Phase II study |
| Participants | 1 week ‐ 17 years Chest compressions by a healthcare worker In‐hospital and out‐of‐hospital arrest GCS ≤ 8 PICU physician decision to use therapeutic hypothermia Central venous or arterial catheter in situ |
| Interventions | 1) Therapeutic hypothermia: 72 hours at 33 ± 1ºC 2) Therapeutic hypothermia: 24 hours at 33 ± 1ºC |
| Outcomes | 1) Degree of brain injury as measured by serum and urine biomarkers and magnetic resonance spectroscopy at hospital discharge 2 ) Frequency of adverse events at 30 days |
| Starting date | October 2008 |
| Contact information | Dr Ericka Fink Children's Hospital of Pittsburgh, Pennsylvania, USA. finkel@ccm.upmc.edu |
| Notes | Planned enrolment 40 Planned end date April 2014 |
NCT00878644.
| Trial name or title | Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Paediatric Patients‐(THAPCA‐OH) (Out of Hospital) Trial |
| Methods | Randomized, single blind (outcome assessor), parallel assignment, safety and efficacy Phase III study |
| Participants | > 48 hours (with a corrected gestational age ≥ 38 weeks) and < 18 years chest compressions ≥ 2 minutes out‐of‐hospital cardiopulmonary arrest only mechanical ventilation Randomized within six hours |
| Interventions | 1) Therapeutic hypothermia: 48 hours at 33ºC±1ºC with gradual rewarm to 36.75ºC±0.75ºC maintained until 120 hours 2) Therapeutic normothermia: 120 hours at 36.75ºC±0.75ºC |
| Outcomes | 1) Survival with good neurobehavioral outcome (assessed at 12months) 2) Survival*, change in neurobehavioral function from pre‐cardiac arrest to 12 months post‐cardiac arrest, neuropsychological scores* (for participants who survive), neurological abnormality scores* (for participants who survive) (* measured at 12 months post‐arrest) |
| Starting date | September 2009 |
| Contact information | Prof Frank W Moler, University of Michigan, USA. fmoler@umich.edu |
| Notes | Planned enrolment 350 Planned study end date September 2015 |
NCT00880087.
| Trial name or title | Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Paediatric Patients‐(THAPCA‐IH) (In Hospital) Trial |
| Methods | Randomized, single blind (outcome assessor), parallel assignment safety and efficacy Phase III study |
| Participants | > 48 hours (with a corrected gestational age ≥ 38 weeks) and < 18 years chest compressions ≥ 2 minutes in‐hospital cardiopulmonary arrest only mechanical ventilation Randomized within six hours |
| Interventions | 1) Therapeutic hypothermia: 48 hours at 33ºC±1ºC with gradual re‐warm to 36.75ºC±0.75ºC maintained until 120 hours 2) Therapeutic normothermia: 120 hours at 36.75ºC±0.75ºC |
| Outcomes | 1) Survival with good neurobehavioral outcome (assessed at 12 months) 2) Survival*, change in neurobehavioral function from pre‐cardiac arrest to 12 months post‐cardiac arrest, neuropsychological scores* (for participants who survive), neurological abnormality scores* (for participants who survive) (* measured at 12months post‐arrest) |
| Starting date | September 2009 |
| Contact information | Prof Frank W Moler, University of Michigan, USA. fmoler@umich.edu |
| Notes | Planned enrolment 500 Planned end date September 2015 |
GCS: Glasgow Coma Score, PCPC: Paediatric Cerebral Performance Category
Differences between protocol and review
Addition of the trials databases from the World Health Organization and Chinese Trials Registry after discussion with international experts.
Contributions of authors
Conceiving the review: Barnaby Scholefield (BS), Heather Duncan (HD), Kevin Morris (KM)
Co‐ordinating the review: BS
Undertaking manual searches: BS, HD, KM
Screening search results: BS, HD, KM
Organizing retrieval of papers: BS
Screening retrieved papers against inclusion criteria: BS, HD, KM
Appraising quality of papers: BS, HD, KM
Abstracting data from papers: BS, HD, KM
Writing to authors of papers for additional information: BS
Providing additional data about papers: BS
Obtaining and screening data on unpublished studies: BS, HD, KM
Data management for the review: BS
Entering data into Review Manager (RevMan 5.1): BS
RevMan statistical data: BS, Paul Davies (PD)
Other statistical analysis not using RevMan 5.1: BS, PD
Double entry of data: (data entered by person one: BS; data entered by person two: KM)
Interpretation of data: BS, HD, KM, Khalid Khan (KK), Fang Gao Smith (FGS), Gavin Perkins (GP)
Statistical inferences: BS, PD, KK, FGS
Writing the review: BS, HD, KM, GP
Securing funding for the review: HD
Performing previous work that was the foundation of the present study: BS
Guarantor for the review (one author): BS
Person responsible for reading and checking review before submission: BS, KM, GP
Sources of support
Internal sources
Birmingham Children's Hospital NHS Trust, UK.
External sources
No sources of support supplied
Declarations of interest
Barnaby Scholefield has received a travel grant to attend a hypothermia conference from the Paediatric Intensive Care Society, UK and financial support through employment to perform the systematic review from Birmingham Children's Hospital, UK. He also organised a trainee intensive care educational symposium with small (less than £700) corporate sponsorship from companies selling hypothermia cooling devices.
Gavin Perkins has received reimbursement of expenses to attend and speak at CPR conferences and to develop national and international CPR guidelines. He has received payment from Elsevier Publishing for his role as an editor for the journal Resuscitation.
Professor Khalid Khan's research is largely funded by public bodies in the UK and European Union. He has participated in research projects where pharmaceutical companies (for example Ferring Pharmaceuticals) have contributed a grant. He has received honoraria and has had travel and accommodation expenses covered or reimbursed for speaking at meetings from pharmaceutical companies (for example Ferring Pharmaceuticals) and from various Colleges, Universities and Societies. He receives royalties on his books from publishers (Hodder Arnold; Huber). His institution has received sponsorship for organising educational meetings (Alere; Ethicon; Ferring Pharmaceuticals; Hologic; Leo‐Pharma; Preglem/Quintiles; Viforpharma). He is in a Partnership where he offers advice on several matters including medical research and negligence. He provides expert reports in medical negligence cases for which a fee is paid by instructing solicitors.
All other authors: none known.
Edited (no change to conclusions)
References
References to studies excluded from this review
Abend 2009 {published data only}
- Abend NS, Topjian A, Ichord R, Herman ST, Helfaer M, Donnelly M, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009;72(22):1931‐40. [PUBMED: 19487651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bembea 2010 {published data only}
- Bembea MM, Nadkarni VM, Diener‐West M, Venugopal V, Carey SM, Berg RA, et al. Temperature patterns in the early postresuscitation period after pediatric inhospital cardiac arrest. Pediatric Critical Care Medicine 2010;11(6):723‐30. [DOI] [PubMed] [Google Scholar]
Buttram 2009 {published data only}
- Buttram S, Large B, Yeakel K, Williams K, Bobrow B, Dalton H. Therapeutic hypothermia after pediatric out of hospital cardiac arrest ‐ A single center experience. Critical Care Medicine 2009;37(12 Suppl):480. [Google Scholar]
Deasy 2010 {published data only}
- Deasy C, Bernard SA, Cameron P, Jaison A, Smith K, Harriss L, et al. Epidemiology of paediatric out‐of‐hospital cardiac arrest in Melbourne, Australia. Resuscitation 2010;81(9):1095‐100. [PUBMED: 20627518] [DOI] [PubMed] [Google Scholar]
Doherty 2009 {published data only}
- Doherty DR, Parshuram CS, Gaboury I, Hoskote A, Lacroix J, Tucci M, et al. Hypothermia therapy after pediatric cardiac arrest. Circulation 2009;119(11):1492‐500. [PUBMED: 19273725] [DOI] [PubMed] [Google Scholar]
Fink 2010 {published data only}
- Fink EL, Clark RS, Kochanek PM, Bell MJ, Watson RS. A tertiary care center's experience with therapeutic hypothermia after pediatric cardiac arrest. PediatricCritical Care Medicine 2010;11(1):66‐74. [PUBMED: 19935440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hein 2004 {published data only}
- Hein OV, Triltsch A, Buch C, Kox WJ, Spies C. Mild hypothermia after near drowning in twin toddlers. Critical Care 2004;8(5):R353‐7. [PUBMED: 15469580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2011 {published data only}
- Kessler SK, Topjian AA, Gutierrez‐Colina AM, Ichord RN, Donnelly M, Nadkarni VM, et al. Short‐term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocritical Care 2011;14(1):37‐43. [PUBMED: 20890677] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobr 2011 {published data only}
- Kobr J, Pizingerova K, Sasek L, Fremuth J, Fikrlova S. Induced therapeutic hypothermia following cardiac arrest in children. Bratislavske Lekarske Listy 2011;112(2):92‐6. [PUBMED: 21456509] [PubMed] [Google Scholar]
Le Guen 2011 {published data only}
- Guen M, Nicolas‐Robin A, Carreira S, Raux M, Leprince P, Riou B, et al. Extracorporeal life support following out‐of‐hospital refractory cardiac arrest. Critical Care 2011;15(1):R29. [PUBMED: 21244674] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meert 2009 {published data only}
- Meert KL, Donaldson A, Nadkarni V, Tieves KS, Schleien CL, Brilli RJ, et al. Multicenter cohort study of in‐hospital pediatric cardiac arrest. Pediatric Critical Care Medicine 2009;10(5):544‐53; 613‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meert 2010 {published data only}
- Meert K, Moler F, Dean JM. Study of hypothermia therapy after pediatric cardiac arrest reply. Pediatric Critical Care Medicine 2010;11(2):316‐7. [DOI] [PubMed] [Google Scholar]
Moler 2009 {published data only}
- Moler FW, Meert K, Donaldson AE, Nadkarni V, Brilli RJ, Dalton HJ, et al. In‐hospital versus out‐of‐hospital pediatric cardiac arrest: a multicenter cohort study. Critical Care Medicine 2009;37(7):2259‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moler 2011 {published data only}
- Moler FW, Donaldson AE, Meert K, Brilli RJ, Nadkarni V, Shaffner DH, et al. Multicenter cohort study of out‐of‐hospital pediatric cardiac arrest. Critical Care Medicine 2011;39(1):141‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanada 1998 {published data only}
- Sanada T, Ueki M, Tokudome M, Okamura T, Nishiki S, Niimura A, et al. [Recovery from out‐of‐hospital cardiac arrest after mild hypothermia: report of two cases]. Masui. The Japanese Journal of Anesthesiology 1998;47(6):742‐5. [PUBMED: 9691597] [PubMed] [Google Scholar]
Silfvast 2003 {published data only}
- Silfvast T, Tiainen M, Poutiainen E, Roine RO. Therapeutic hypothermia after prolonged cardiac arrest due to non‐coronary causes. Resuscitation 2003;57(1):109‐12. [PUBMED: 12668307] [DOI] [PubMed] [Google Scholar]
Takeda 2009 {published data only}
- Takeda Y, Shiraishi K, Naito H, Morimoto N, Morita K. A randomized controlled trial of pharyngeal cooling system during cardiopulmonary resuscitation. Intensive Care Medicine 2009;35:143. [Google Scholar]
Topjian 2011 {published data only}
- Topjian A, Hutchins L, DiLiberto MA, Abend NS, Ichord R, Helfaer M, et al. Induction and maintenance of therapeutic hypothermia after pediatric cardiac arrest: efficacy of a surface cooling protocol. Pediatric Critical Care Medicine 2011;12(3):e127‐35. [PUBMED: 20431502] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT00754481 {published data only}
- NCT00754481. Hypothermia for Cardiac Arrest in Paediatrics (HypCAP). www.clinicatrials.gov NCT00754481.
NCT00797680 {published data only}
- NCT00797680. Duration of Hypothermia for Neuroprotection After Paediatric Cardiac Arrest. www.clinicaltrials.gov NCT00797680.
NCT00878644 {published data only}
- NCT00878644. Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Paediatric Patients‐(THAPCA‐OH) (Out of Hospital) Trial. www.clinicaltrials.gov NCT00878644.
NCT00880087 {published data only}
- NCT00880087. Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Paediatric Patients‐(THAPCA‐IH) (In Hospital) Trial. www.clinicaltrials.gov NCT00880087.
Additional references
Arrich 2012
- Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004128.pub3] [DOI] [PubMed] [Google Scholar]
Atkins 2009
- Atkins DL, Everson‐Stewart S, Sears GK, Daya M, Osmond MH, Warden CR, et al. Epidemiology and outcomes from out‐of‐hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry‐Cardiac Arrest. Circulation 2009;119(11):1484‐91. [PUBMED: 19273724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azzopardi 2009
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England Journal of Medicine 2009;361(14):1349‐58. [PUBMED: 19797281] [DOI] [PubMed] [Google Scholar]
Bernard 2002
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. The New England Journal of Medicine 2002;346(8):557‐63. [PUBMED: 11856794] [DOI] [PubMed] [Google Scholar]
Biarent 2010
- Biarent D, Bingham R, Eich C, Lopez‐Herce J, Maconochie I, Rodriguez‐Nunez A, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 6. Paediatric life support. Resuscitation 2010;81(10):1364‐88. [PUBMED: 20956047] [DOI] [PubMed] [Google Scholar]
Bigelow 1950
- Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Annals of Surgery 1950;132(5):849‐66. [PUBMED: 14771796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Busto 1987
- Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. Journal of Cerebral Blood Flow and Metabolism 1987;7(6):729‐38. [PUBMED: 3693428] [DOI] [PubMed] [Google Scholar]
Busto 1989
- Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neuroscience Letters 1989;101(3):299‐304. [PUBMED: 2771174] [DOI] [PubMed] [Google Scholar]
Clark 2008
- Clark DL, Penner M, Orellana‐Jordan IM, Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Experimental Neurology 2008;212(2):386‐92. [PUBMED: 18538766] [DOI] [PubMed] [Google Scholar]
Colbourne 1994
- Colbourne F, Corbett D. Delayed and prolonged post‐ischemic hypothermia is neuroprotective in the gerbil. Brain Research 1994;654(2):265‐72. [PUBMED: 7987676] [DOI] [PubMed] [Google Scholar]
Colbourne 1995
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. The Journal of Neuroscience 1995;15(11):7250‐60. [PUBMED: 7472479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colbourne 1999
- Colbourne F, Li H, Buchan AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. Journal of Cerebral Blood Flow and Metabolism 1999;19(7):742‐9. [PUBMED: 10413028] [DOI] [PubMed] [Google Scholar]
de Caen 2010
- Caen AR, Kleinman ME, Chameides L, Atkins DL, Berg RA, Berg MD, et al. Part 10: Paediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2010;81 Suppl 1:e213‐59. [PUBMED: 20956041] [DOI] [PubMed] [Google Scholar]
Donoghue 2005
- Donoghue AJ, Nadkarni V, Berg RA, Osmond MH, Wells G, Nesbitt L, et al. Out‐of‐hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Annals of Emergency Medicine 2005;46(6):512‐22. [PUBMED: 16308066] [DOI] [PubMed] [Google Scholar]
Duncan 2009
- Duncan HP, Frew E. Short‐term health system costs of paediatric in‐hospital acute life‐threatening events including cardiac arrest. Resuscitation 2009;80(5):529‐34. [PUBMED: 19339101] [DOI] [PubMed] [Google Scholar]
Fink 2004
- Fink EL, Alexander H, Marco CD, Dixon CE, Kochanek PM, Jenkins LW, et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatric Critical Care Medicine 2004;5(2):139‐44. [PUBMED: 14987343] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiser 1992
- Fiser DH. Assessing the outcome of pediatric intensive care. The Journal of Pediatrics 1992;121(1):68‐74. [PUBMED: 1625096] [DOI] [PubMed] [Google Scholar]
Gluckman 2005
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365(9460):663‐70. [PUBMED: 15721471] [DOI] [PubMed] [Google Scholar]
Gunn 1997
- Gunn AJ, Gunn TR, Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. The Journal of Clinical Investigation 1997;99(2):248‐56. [PUBMED: 9005993] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ (Clinical research edition) 2008;336(7652):1049‐51. [PUBMED: 18467413] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haque 2006
- Haque IU, Latour MC, Zaritsky AL. Pediatric critical care community survey of knowledge and attitudes toward therapeutic hypothermia in comatose children after cardiac arrest. Pediatric Critical Care Medicine 2006;7(1):7‐14. [PUBMED: 16395067] [DOI] [PubMed] [Google Scholar]
Hickey 2000
- Hickey RW, Kochanek PM, Ferimer H, Graham SH, Safar P. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics 2000;106(1 Pt 1):118‐22. [PUBMED: 10878160] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011}. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holzer 2005
- Holzer M, Bernard SA, Hachimi‐Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta‐analysis. Critical Care Medicine 2005;33(2):414‐8. [PUBMED: 15699847] [DOI] [PubMed] [Google Scholar]
Hypothermia After Cardiac Arrest Study Group 2002
- The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England Journal of Medicine 2002;346(8):549‐56. [PUBMED: 11856793] [DOI] [PubMed] [Google Scholar]
Jacobs 2007
- Jacobs S, Hunt R, Tarnow‐Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003311.pub2] [DOI] [PubMed] [Google Scholar]
Karibe 1994
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood‐brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism 1994;14(4):620‐7. [PUBMED: 8014209] [DOI] [PubMed] [Google Scholar]
Kil 1996
- Kil HY, Zhang J, Piantadosi CA. Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. Journal of Cerebral Blood Flow and Metabolism 1996;16(1):100‐6. [PUBMED: 8530542] [DOI] [PubMed] [Google Scholar]
Morris 1993
- Morris RD, Krawiecki NS, Wright JA, Walter LW. Neuropsychological, academic, and adaptive functioning in children who survive in‐hospital cardiac arrest and resuscitation. Journal of Learning Disabilities 1993;26(1):46‐51. [PUBMED: 8418189] [DOI] [PubMed] [Google Scholar]
Nadkarni 2006
- Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in‐hospital cardiac arrest among children and adults. JAMA 2006;295(1):50‐7. [PUBMED: 16391216] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ronco 1995
- Ronco R, King W, Donley DK, Tilden SJ. Outcome and cost at a children's hospital following resuscitation for out‐of‐hospital cardiopulmonary arrest. Archives of Pediatrics and Adolescent Medicine 1995;149(2):210‐4. [PUBMED: 7849887] [DOI] [PubMed] [Google Scholar]
Scholefield 2010
- Scholefield BR, Duncan HP, Morris KP. Survey of the use of therapeutic hypothermia post cardiac arrest. Archives of Disease in Childhood 2010;95(10):796‐9. [PUBMED: 20736398] [DOI] [PubMed] [Google Scholar]
Shankaran 2005
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole‐body hypothermia for neonates with hypoxic‐ischemic encephalopathy. The New England Journal of Medicine 2005;353(15):1574‐84. [PUBMED: 16221780] [DOI] [PubMed] [Google Scholar]
Shankaran 2008
- Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole‐body hypothermia for neonatal hypoxic‐ischemic encephalopathy. Pediatrics 2008;122(4):e791‐8. [PUBMED: 18829776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparrow 1984
- Sparrow S, Balla D, Cicchetti D. Vineland Adaptive Behavoural Scale. Bloomington, MN: Pearson Assessments, 1984. [Google Scholar]
Steen 1983
- Steen PA, Newberg L, Milde JH, Michenfelder JD. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology 1983;58(6):527‐32. [PUBMED: 6859582] [PubMed] [Google Scholar]
Suehiro 1999
- Suehiro E, Fujisawa H, Ito H, Ishikawa T, Maekawa T. Brain temperature modifies glutamate neurotoxicity in vivo. Journal of Neurotrauma 1999;16(4):285‐97. [PUBMED: 10225215] [DOI] [PubMed] [Google Scholar]
Suffoletto 2009
- Suffoletto B, Peberdy MA, Hoek T, Callaway C. Body temperature changes are associated with outcomes following in‐hospital cardiac arrest and return of spontaneous circulation. Resuscitation 2009;80(12):1365‐70. [PUBMED: 19804929] [DOI] [PubMed] [Google Scholar]
Sutcliffe 2001
- Sutcliffe IT, Smith HA, Stanimirovic D, Hutchison JS. Effects of moderate hypothermia on IL‐1 beta‐induced leukocyte rolling and adhesion in pial microcirculation of mice and on proinflammatory gene expression in human cerebral endothelial cells. Journal of Cerebral Blood Flow and Metabolism 2001;21(11):1310‐9. [PUBMED: 11702046] [DOI] [PubMed] [Google Scholar]
Takasu 1996
- Takasu A, Yagi K, Okada Y. Effect of mild hypothermia on ischemia‐induced release of endothelin‐1 in dog brain. Resuscitation 1996;31(1):59‐64. [PUBMED: 8701110] [DOI] [PubMed] [Google Scholar]
Takasu 2001
- Takasu A, Saitoh D, Kaneko N, Sakamoto T, Okada Y. Hyperthermia: is it an ominous sign after cardiac arrest?. Resuscitation 2001;49(3):273‐7. [PUBMED: 11719121] [DOI] [PubMed] [Google Scholar]
Takino 1991
- Takino M, Okada Y. Hyperthermia following cardiopulmonary resuscitation. Intensive care medicine 1991;17(7):419‐20. [PUBMED: 1774396] [DOI] [PubMed] [Google Scholar]
Walters 2011
- Walters JH, Morley PT, Nolan JP. The role of hypothermia in post‐cardiac arrest patients with return of spontaneous circulation: a systematic review. Resuscitation 2011;82(5):508‐16. [PUBMED: 21367510] [DOI] [PubMed] [Google Scholar]
Xu 2002
- Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. Journal of Cerebral Blood Flow and Metabolism 2002;22(1):21‐8. [PUBMED: 11807390] [DOI] [PubMed] [Google Scholar]
Yenari 2002
- Yenari MA, Iwayama S, Cheng D, Sun GH, Fujimura M, Morita‐Fujimura Y, et al. Mild hypothermia attenuates cytochrome c release but does not alter Bcl‐2 expression or caspase activation after experimental stroke. Journal of Cerebral Blood Flow and Metabolism 2002;22(1):29‐38. [PUBMED: 11807391] [DOI] [PubMed] [Google Scholar]
Young 2004
- Young KD, Gausche‐Hill M, McClung CD, Lewis RJ. A prospective, population‐based study of the epidemiology and outcome of out‐of‐hospital pediatric cardiopulmonary arrest. Pediatrics 2004;114(1):157‐64. [PUBMED: 15231922] [DOI] [PubMed] [Google Scholar]
Zeiner 2001
- Zeiner A, Holzer M, Sterz F, Schorkhuber W, Eisenburger P, Havel C, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Archives of internal medicine 2001;161(16):2007‐12. [PUBMED: 11525703] [DOI] [PubMed] [Google Scholar]


