Abstract
Background
Familial Mediterranean fever, a hereditary auto‐inflammatory disease, mainly affects ethnic groups living in the Mediterranean region. Early studies reported colchicine as a potential drug for preventing attacks of familial Mediterranean fever. For those people who are colchicine‐resistant or intolerant, drugs such as rilonacept, anakinra, canakinumab, etanercept, infliximab, thalidomide and interferon‐alpha might be beneficial. This is an updated version of the review.
Objectives
To evaluate the efficacy and safety of interventions for reducing inflammation in people with familial Mediterranean fever.
Search methods
We used detailed search strategies to search the following databases: CENTRAL; MEDLINE; Embase; Chinese Biomedical Literature Database (CBM); China National Knowledge Infrastructure Database (CNKI); Wan Fang; and VIP. In addition, we also searched the clinical trials registries including ClinicalTrials.gov, the International Standard Randomized Controlled Trial Number Register, the WHO International Clinical Trials Registry Platform and the Chinese Clinical Trial Registry, as well as references listed in relevant reports.
Date of last search: 21 August 2018.
Selection criteria
Randomized controlled studies (RCTs) of people diagnosed with familial Mediterranean fever, comparing active interventions (including colchicine, anakinra, rilonacept, canakinumab, etanercept, infliximab, thalidomide, interferon‐alpha, ImmunoGuard™ (a herbal dietary supplement) and non‐steroidal anti‐inflammatory drugs) with placebo or no treatment, or comparing active drugs to each other.
Data collection and analysis
The authors independently selected studies, extracted data and assessed risk of bias. We pooled data to present the risk ratio or mean difference with their 95% confidence intervals. We assessed overall evidence quality according to the GRADE approach.
Main results
We included nine RCTs with a total of 249 participants (aged three to 53 years); five were of cross‐over and four of parallel design. Six studies used oral colchicine, one used oral ImmunoGuard™ and the remaining two used rilonacept or anakinra as a subcutaneous injection. The duration of each study arm ranged from one to eight months.
The three studies of ImmunoGuard™, rilonacept and anakinra were generally well‐designed, except for an unclear risk of detection bias in one of these. However, some inadequacy existed in the four older studies on colchicine, which had an unclear risk of selection bias, detection bias and reporting bias, and also a high risk of attrition bias and other potential bias. Neither of the two studies comparing a single to a divided dose of colchicine were adequately blinded, furthermore one study had an unclear risk of selection bias and reporting bias, a high risk of attrition bias and other potential bias.
We aimed to report on the number of participants experiencing an attack, the timing of attacks, the prevention of amyloid A amyloidosis, any adverse drug reactions and the response of a number of biochemical markers from the acute phase of an attack, but data were not available for all outcomes across all comparisons.
One study (15 participants) reported a significant reduction in the number of people experiencing attacks at three months with 0.6 mg colchicine three times daily (14% versus 100%), risk ratio 0.21 (95% confidence interval 0.05 to 0.95) (low‐quality evidence). A further study (22 participants) of 0.5 mg colchicine twice daily showed no significant reduction in the number of participants experiencing attacks at two months (low‐quality evidence). A study of rilonacept in individuals who were colchicine‐resistant or intolerant (14 participants) also showed no reduction at three months (moderate‐quality evidence). Likewise, a study of anakinra given to colchicine‐resistant people (25 participants) showed no reduction in the number of participants experiencing an attack at four months (moderate‐quality evidence).
Three studies reported no significant differences in duration of attacks: one comparing colchicine to placebo (15 participants) (very low‐quality evidence); one comparing single‐dose colchicine to divided‐dose colchicine (90 participants) (moderate‐quality evidence); and one comparing rilonacept to placebo (14 participants) (low‐quality evidence). Three studies reported no significant differences in the number of days between attacks: two comparing colchicine to placebo (24 participants in total) (very low‐quality evidence); and one comparing rilonacept to placebo (14 participants) (low‐quality evidence).
No study reported on the prevention of amyloid A amyloidosis.
One study of colchicine reported loose stools and frequent bowel movements (very low‐quality evidence) and a second reported diarrhoea (very low‐quality evidence). The rilonacept study reported no significant differences in gastrointestinal symptoms, hypertension, headache, respiratory tract infections, injection site reactions and herpes, compared to placebo (low‐quality evidence). The ImmunoGuard study observed no side effects (moderate‐quality evidence). The anakinra study reported no significant differences between intervention and placebo, including injection site reaction, headache, presyncope, dyspnea and itching (moderate‐quality evidence). When comparing single and divided doses of colchicine, one study reported no difference in adverse events (including anorexia, nausea, diarrhoea, abdominal pain, vomiting and elevated liver enzymes) between groups (moderate‐quality evidence) and the second study reported no adverse effects were detected.
The rilonacept study reported no significant reduction in acute phase response indicators after three months (low‐quality evidence). In the ImmunoGuard™ study, these indicators were not reduced after one month of treatment (moderate‐quality evidence). The anakinra study, reported that C‐reactive protein was significantly reduced after four months (moderate‐quality evidence). One of the single dose versus divided dose colchicine studies reported no significant reduction in acute phase response indicators after eight months (low‐quality evidence), while the second study reported no significant reduction in serum amyloid A concentration after six months (moderate‐quality evidence).
Authors' conclusions
There were limited RCTs assessing interventions for people with familial Mediterranean fever. Based on the evidence, three times daily colchicine appears to reduce the number of people experiencing attacks, colchicine single dose and divided dose might not be different for children with familial Mediterranean fever and anakinra might reduce C‐reactive protein in colchicine‐resistant participants; however, only a few RCTs contributed data for analysis. Further RCTs examining active interventions, not only colchicine, are necessary before a comprehensive conclusion regarding the efficacy and safety of interventions for reducing inflammation in familial Mediterranean fever can be drawn.
Plain language summary
Drugs for reducing inflammation in people with familial Mediterranean fever
Review question
We reviewed the evidence about the effect of treatments (e.g. colchicine, anakinra, rilonacept, canakinumab, etanercept, infliximab, thalidomide, interferon‐alpha, ImmunoGuard™ (a herbal supplement) and non‐steroidal anti‐inflammatory drugs) on people with familial Mediterranean fever (FMF).
Background
FMF is a hereditary inflammatory disease, with symptoms of an attack often including fever over 38℃, pain and inflammation of the membrane surrounding the chest cavity, the joints or the lungs. We wanted to discover whether these drugs were better for reducing inflammation for people with FMF than placebo (a dummy treatment containing no active medicine) or no treatment, and also to compare these drugs with each other.
Search date
The evidence is current to: 21 August 2018.
Study characteristics
The review includes nine studies including 249 people with FMF aged between three and 53 years old. Seven studies compared four of the drugs, colchicine, rilonacept, ImmunoGuard™ and anakinra, with placebo. Participants were chosen to receive one drug or placebo at random over a period ranging from one to four months. The remaining two studies compared colchicine 1 mg per day once daily with two to three times daily in children for six to eight months.
Key results
We aimed to report on the number of participants experiencing an attack, the timing of attacks, prevention of amyloid A amyloidosis, any side effects of treatment and the levels of a number of markers of inflammation during an attack. Not all studies reported on these outcomes. Given the differences in treatments and study design, it was not possible to combine any of the results that we did obtain from these studies. One study (15 participants) found that oral colchicine at a dose of 0.6 mg three times a day could help to reduce the numbers of people with attacks of FMF. However, oral colchicine administrated at a dose of 0.5 mg twice a day (22 participants), rilonacept (14 participants) or anakinra (25 participants) did not reduce the numbers of people with attacks. ImmunoGuard™ (24 participants) did not reduce levels of the markers of inflammation in the blood which are raised during the attack phase of FMF; these include the rate of fall of red blood cells when placed in a test tube, the white blood cell count and the presence of C‐reactive protein (a protein which is produced in the liver). Anakinra reduced C‐reactive protein level. Colchicine once daily and two to three times daily might not result in different outcomes including the timing of attacks, adverse drug reactions and acute phase response indicators.
Quality of the evidence
Three studies were well‐designed, while the others had some design problems which might affect the results. Four studies did not report clearly how the people were assigned to each treatment group. Four studies did not report whether researchers, who assessed the study outcomes, knew which individuals were assigned to which treatment. Four studies did not clearly explain the reasons for people withdrawing from a study and one study had a high percentage of participants who did not complete study. We could not confirm whether each planned outcome was reported in five studies. Five studies did not report the severity of FMF in groups at the beginning of treatment. We judged the evidence for the reported outcomes to be of moderate‐ to very low‐quality.
Summary of findings
Background
Please see the glossary for an explanation of terminology (Appendix 1).
Description of the condition
Familial Mediterranean fever (FMF) is an autosomal recessive, hereditary auto‐inflammatory disease and has a reference in the Online Mendelian Inheritance in Man database (OMIM) ID: 249100. The database catalogues all the known diseases with a genetic component and, when possible, links the diseases to the relevant genes in the human genome and provides references for further research and tools for genomic analysis of a catalogued gene. The primary characteristic of FMF is recurrent fever and serositis, which results in pain in the abdomen, chest, joints, muscles, etc. This condition mainly affects ethnic groups with Mediterranean ancestry, such as those of Jewish, Armenian, Turkish and Arabic origin, with a high prevalence of 1 in 200 to 1 in 1000 people affected in these ethnic groups (Shohat 2011; Soriano 2012). Regarding the rest of world, FMF is also not considered to be a rare disease in Italy, Spain, Greece and Japan (Konstantopoulos 2003; La Regina 2003; Migita 2012). Most people with FMF (approximately 90%) are diagnosed before the age of 20 years (Koné‐Paut 2011).
FMF occurs as a result of mutations in the MEditerranean FeVer (MEFV gene). This is the only gene currently known to be associated with FMF and is located on chromosome 16 (Centola 2000). The MEFV gene is comprised of 10 exons encoding for a protein called pyrin by the International FMF Consortium (The International FMF Consortium 1997) or marenostrin by the French FMF Consortium (French FMF Consortium 1997). Pyrin consists of 781 amino acids, expressed in neutrophils, eosinophils, monocytes, dendritic cells and fibroblasts, and plays a key role in the regulation of inflammation and apoptosis (Chae 2009; Mansfield 2001). Human pyrin contains four domains; the pyrin domain (PYD), the zinc‐finger domain (Bbox), the coiled coil domain (CC) and the B30.2 domain (Heilig 2018). The role of pyrin in the regulation of inflammation is not completely understood; however, the pyrin inflammasome and its role in the FMF has been studied (Park 2016). Inflammasomes are multiprotein signaling complexes, which play a major role in immune systems. The inflammasome is formed by a pattern recognition receptor (PRR), the adaptor protein (ASC (apoptosis‐associated speck‐like protein)) and pro‐caspase‐1 (Heilig 2018). Pyrin, a PRR, can bind to the ASC domain to form a pyrin inflammasome, resulting in caspase‐1 activation and further interleukin‐1β (IL‐1β) activation. The interleukin‐1 (IL‐1) family, a group of 11 cytokines, plays a central role in the regulation of immune and inflammatory responses. The pyrin inflammasome activation could be suppressed by the RhoA (a GTPase protein) activity (Park 2016; Xu 2014). RhoA GTPase can be activated by the RhoA activator that is released from depolymerized microtubules (Ozen 2017), suggesting a rationale for colchicine treatment.
There are mainly two phenotypes in FMF. Type 1 is commonly associated with recurrent short episodes of inflammation and serositis, including fever, peritonitis, synovitis, pleuritis and rarely pericarditis and meningitis (Shohat 2011). These symptoms and severity vary from one person to another. The typical clinical manifestations of FMF type 1 usually last from 12 to 72 hours and include the following typical attacks (Shohat 2011; Soriano 2012):
recurrent fever, characterized by a temperature ranging from 38℃ to 40℃;
abdominal attacks, featuring abdominal pain (usually the entire abdomen is involved);
arthritic attacks, frequently featuring as monoarthritis localized in the large joints of the leg (hip, knee, ankle);
chest attacks, including pleuritis and pericarditis;
pre‐attack symptoms, occurring 12 to 24 hours before any FMF attacks, usually including discomfort, abnormal taste sensation, dizziness, increased appetite, irritability, etc. (Lidar 2006).
The most severe complication of FMF is AA (amyloid A) amyloidosis leading to renal failure. Type 2 FMF is characterized by amyloidosis as the first clinical manifestation of the disease, in otherwise asymptomatic individuals (Livneh 2006). However, the existence of this phenotype is still controversial. Melikoğlu failed to prove the existence of type 2 FMF in their prospective designed study, even in siblings with significant proteinuria (Melikoğlu 2000). Furthermore, the common MEFV mutations are not significantly different between people who present with the typical phenotype and those have clinical type 2 disease (Balci 2002).
Description of the intervention
During the FMF attack period, it is reported that febrile and inflammatory episodes are usually treated with non‐steroidal anti‐inflammatory drugs (NSAIDs) (Ozen 2016; Shohat 2011; Soriano 2012).
Colchicine is an anti‐inflammatory drug and the most widely‐chosen treatment option for preventing inflammatory attacks and the deposition of amyloid (Ozen 2016; Shohat 2011). It is an alkaloid which can be extracted from two plants of the lily family: Colchicum autumnale and Gloriosa superba and has been used for centuries in acute gout arthritis, but its anti‐inflammatory efficacy has been demonstrated in other diseases as well. Colchicine was reported as an effective drug for preventing FMF attacks in the early 1970s (Goldfinger 1972). To prevent FMF attacks, it is mainly given orally, usually 1 mg to 2 mg per day in adults and 0.5 mg to 1 mg per day according to age and weight in children (Shohat 2011). After oral administration, colchicine is absorbed in the jejunum and ileum with a zero‐order rate process, with a half‐life of about four hours. Colchicine is mainly metabolised by the cytochrome P450 system in the liver and predominantly eliminated by biliary excretion with enterohepatic circulation (Cerquaglia 2005; Terkeltaub 2009).
For those people with FMF who are colchicine‐resistant or colchicine‐intolerant, a number of other drugs for treating FMF have been studied in clinical studies such as: anakinra (100 mg per day or every other day as a subcutaneous injection) (Ozen 2011); rilonacept (2.2 mg/kg (maximum, 160 mg) as a weekly, subcutaneous injection) (Hashkes 2012); canakinumab (150 mg every four weeks, subcutaneous injection) (Gül 2015); etanercept (25 mg twice a week as a subcutaneous injection) (Bilgen 2011); infliximab (4 mg/kg to 5 mg/kg at zero, two and six weeks and then every eight weeks by infusion) (Özçakar 2012); thalidomide (100 mg per day, orally) (Seyahi 2006); and interferon‐alpha (IFN‐α) (3 million international units (IU) per attack by subcutaneous injection) (Tweezer‐Zaks 2008).
How the intervention might work
Colchicine produces its anti‐inflammatory activity through different pharmacologic effects (Ben‐Chetrit 2006; Cerquaglia 2005; Cronstein 2006) such as:
preventing activation of neutrophils by binding β‐tubulin to make β‐tubulin‐colchicine complexes, then inhibiting the assembly of microtubules and mitotic spindle formation;
inhibiting the synthesis of tumor necrosis factor alpha (TNF‐α) and down‐regulating the surface expression of TNF‐α receptor;
inhibiting leukotriene B4 synthesis;
blocking cyclooxygenase‐2 (COX‐2) activity;
inhibiting tyrosine phosphorylation and superoxide anion production;
inhibiting arachidonate release and 5‐lipoxygenase;
suppressing delayed hypersensitivity reactions, histamine, insulin and parathormone release.
inhibiting pyrin inflammasome through RhoA activation.
Anakinra, rilonacept and canakinumab are IL‐1 inhibitors. Anakinra competitively inhibits the binding of IL‐1α and IL‐1β to the IL‐1 receptor (Alpay 2012). Rilonacept, known as IL‐1 Trap (Economides 2003), is a soluble decoy receptor fusion protein that binds IL‐1α and IL‐1β, and as a result prevents IL‐1 activation of cell surface receptors (Terkeltaub 2013). Canakinumab, a fully human anti‐IL‐1β monoclonal antibody with high selectivity binds to IL‐1β and inhibits its interaction with the IL‐1 receptor (Ozdogan 2017).
Etanercept, infliximab and thalidomide are tumor necrosis factor (TNF) antagonists (Sampaio 1991; Seyahi 2006). The role of TNF antagonists in FMF has not been clarified exactly. However, the level of serum TNF‐α increases during FMF attacks (Baykal 2003) and decreases with regular colchicine treatment (Kiraz 1998).
Finally, IFN‐α is a natural species‐specific immunomodulatory glycoprotein produced mainly by T and B lymphocytes. It increases macrophage and natural killer cell phagocytic activity as well as augmenting lymphocyte‐specific cytotoxicity (Tweezer‐Zaks 2008).
Why it is important to do this review
While there has been an evidence‐based peer review of the use of colchicine for the treatment of FMF (WHO 2013), this important topic has not yet been systematically evaluated. Moreover, there are no evidence‐based reviews of any other interventions for people with FMF. Therefore, we are performing a Cochrane Review of available clinical evidence to evaluate the efficacy and safety of interventions for reducing inflammation in FMF. This is an updated version of a previously published review (Wu 2015).
Objectives
To assess the efficacy and safety of interventions for reducing inflammation in FMF.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) of both parallel and cross‐over design. There was no restriction on publication status or language.
Types of participants
People of any age, gender, and in any care setting, who were diagnosed with FMF, were considered eligible for inclusion. For adults, diagnosis was based on the Tel Hashomer criteria (Livneh 1997; Soriano 2012) and for children on the Yalçinkaya criteria (Yalçinkaya 2009).
The Tel Hashomer criteria include major and minor criteria (Livneh 2000). The diagnosis of FMF is at least one major criteria or at least two minor criteria.
| Tel Hashomer criteria (Livneh 2000) | |
| Major criteria | Peritonitis (generalized) |
| Pleuritis (unilateral) or pericarditis | |
| Monarthritis (hip, knee, ankle) | |
| Fever alone | |
| Incomplete abdominal attack | |
| Minor criteria | Chest |
| Joint | |
| Exertional leg pain | |
| Favorable response to colchicine | |
| Yalçinkaya criteria (Yalçinkaya 2009) | |
| Criteria | Description |
| Fever | Axillary temperature of ≧ 38℃; 6 ‐ 72 hours of duration; ≧ 3 attacks |
| Abdominal pain | 6 ‐ 72 hours of duration; ≧ 3 attacks |
| Chest pain | 6 ‐ 72 hours of duration; ≧ 3 attacks |
| Arthritis | 6 ‐ 72 hours of duration; ≧ 3 attacks; oligoarthritis |
| Family history of FMF | |
Types of interventions
We compared active interventions (including colchicine, anakinra, rilonacept, canakinumab (a post hoc addition), etanercept, infliximab, thalidomide, IFN‐α, ImmunoGuard™ (a post hoc addition) and NSAIDs) with placebo or no treatment. We also planned to include comparisons of these drugs with each other. There were no restrictions on drug administration dose, frequency, intensity or duration.
Types of outcome measures
Primary outcomes
Number of participants experiencing an attack
-
Timing of FMF attacks
duration of FMF attacks (days or hours)
interval time between attacks (days)
Prevention of AA amyloidosis
Secondary outcomes
Adverse drug reactions (ADRs)
-
Acute phase response
erythrocyte sedimentation rate (ESR)
white blood cell (WBC) count
fibrinogen concentration
C‐reactive protein (CRP)
serum amyloid A protein (SAA) concentration
Search methods for identification of studies
There are no restrictions in the searches regarding language or publication status.
Electronic searches
We searched relevant studies from the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (2018 issue 8), Ovid MEDLINE (1950 to August 2018), Ovid Embase (1980 to August 2018), Chinese Biomedical Literature Database (CBM) (1978 to August 2018), China National Knowledge Infrastructure Database (CNKI) (1979 to August 2018), Wan Fang database (1986 to August 2018) and the VIP database (1989 to August 2018). We also searched the following clinical studies registries for any ongoing studies: ClinicalTrials.gov (clinicaltrials.gov/), International Standard Randomized Controlled Trial Number Register (ISRCTN) (www.isrctn.com/), WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) and Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn/).
We have detailed the search strategy for CENTRAL, MEDLINE and Embase in the appendices (Appendix 2; Appendix 3; Appendix 4). The search strategy was modified and translated appropriately for each Chinese database search.
Date of the most recent searches: 21 August 2018.
Searching other resources
We searched references listed in relevant studies and reviews to identify any further relevant RCTs.
Data collection and analysis
Selection of studies
We used EndNote X6 software to merge retrieved reports from each database and to remove duplicate records of the same study (Endnote X6 2012). Two review authors (BW, TX) independently assessed the titles and abstracts of studies to exclude obviously irrelevant reports. We retrieved the full text copies of all potentially eligible reports, and reviewed them in the light of the inclusion criteria. Two review authors (BW, XY) made final decisions on the included studies by cross‐checking the results; we consulted a third review author (TX) when there were any disagreements. Where we identified multiple reports of the same study, we extracted the maximum amount of data from the multiple reports and identified one report as the primary reference.
Data extraction and management
We based data extraction on guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), using a data extraction form piloted by the Cochrane Cystic Fibrosis and Genetic Disorders Review Group, and included the following information: general data (authors, publication year, contact information, etc.); baseline data (number of participants, age, gender, etc.); risk of bias assessment information (details of randomisation, allocation concealment, blinding, incomplete outcome data, etc.); interventions; duration of follow up; outcome measures; and results. Two review authors (BW, XY) independently extracted and managed data from all included studies and attempted to resolve disagreements by discussion. When authors failed to reach an agreement, we involved a third review author (TX) as arbiter.
We did not combine different drugs in a single comparison (e.g. any drug versus placebo) or different duration of treatment (e.g. up to and including one month, over one month and up to three months, over three months and up to 12 months, 12 months and over), instead we presented separate comparisons at different time points.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies using the methods recommended in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Two review authors (BW, XY) independently evaluated the following seven items for each study: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other potential sources of bias. We judged the risk of bias for each item as 'low risk', 'high risk' or 'unclear risk' following the assessment criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Appendix 5). Finally, we produced a 'Risk of bias summary' and a 'Risk of bias' figure to present a visual assessment of the risk of bias.
Measures of treatment effect
For dichotomous outcomes (number of participants experiencing an attack, adverse drug reactions), we presented the risk ratios (RRs) with their 95% confidence intervals (CIs) for each individual study where data were available. For continuous outcomes (duration of FMF attacks, time between attacks, markers in the acute phase response), we presented the mean differences (MDs) with their 95% CIs for individual studies where data were available. If the time to the next attack was reported as the median (range) number of days, we reported these narratively. We planned to use the standardised mean differences (SMDs) where the same outcome was measured in a variety of ways among studies, however, only one RCT reported continuous outcomes based on the established inclusion criteria.
Unit of analysis issues
We included both parallel and cross‐over designed studies. We considered individual participants as the unit of analysis. We planned to re‐analyse any cluster‐randomized studies identified by calculating the effective sample sizes with the intra‐cluster coefficient (ICC) estimated externally from similar studies (Deeks 2011); however, we did not include any cluster‐randomized studies in this version of the review. We included five cross‐over studies in the review. For all of these, data from the first period only were available and where possible we analysed the data at the relevant time points as if the studies were of parallel design as we had originally planned (Elbourne 2002). We have reported other information from both arms of the cross‐over studies narratively.
Dealing with missing data
We attempted to contact the original study investigators when essential data were missing from the study reports; however, we failed to find any contact details for the contact authors of four studies published in 1974 and 1977 (Dinarello 1974; Goldstein 1974; Wright 1977; Zemer 1974). We planned to assume firstly that the missing participants experienced an attack and secondly that they did not experience an attack and would undertake an analysis based on each of these assumptions respectively. We examined the effects of these assumptions by performing a sensitivity analysis (Higgins 2011c).
Assessment of heterogeneity
Firstly, if clinical diversity existed between the studies (e.g. different drugs, or different treatment durations), we planned not to combine data from those studies. Secondly, for clinically homogeneous studies, we planned to perform a Chi² test, with P values less than 0.1 indicating significant statistical heterogeneity. If we had combined any studies, in order to identify any heterogeneity, we would have attempted to visually assess the forest plots to identify any aberrant results. Furthermore, we planned to quantify heterogeneity not due to chance by using the I² statistic (Higgins 2003). A rough guide for the interpretation of I² which we planned to use is as follows: 0% to 40% represented heterogeneity that might not be important; 30% to 60% might represent moderate heterogeneity; 50% to 90% might represent substantial heterogeneity; 75% to 100% represented considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We performed a comprehensive search for eligible RCTs to minimise reporting bias. We attempted to use funnel plots to assess publication bias (Sterne 2011); however, there were insufficient studies (less than 10 studies) to conduct this analysis. To evaluate selective reporting of outcomes, we compared the study protocols with the final study reports. When study protocols were not available, we compared the 'Methods' section of the published studies with the 'Results' section to identify any outcomes that were measured but not reported. We also used clinical judgement with respect to which outcomes we would expect to be reported given the intervention and study design.
Data synthesis
We used Review Manager software provided by Cochrane to conduct the statistical analysis (Review Manager 2014). We used a fixed‐effect model for the meta‐analysis in the absence of clinical, methodological and statistical heterogeneity. If we had combined data and the I² statistic had been greater than zero, we also planned to apply a random‐effects model to see whether the conclusions differed, and would have noted any difference. When analysis was not possible or appropriate, we presented a narrative summary (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
We would have performed a subgroup analysis for different age groups (18 years and under old versus above 18 years of age) or different duration of treatment (e.g. up to and including one month, over one month and up to three months, over three months and up to 12 months, 12 months and over); however, each analysis only included one study, so we were unable to conduct any subgroup analyses.
Sensitivity analysis
We intended to perform a sensitivity analysis for the primary outcomes to investigate the robustness of findings. We planned to conduct sensitivity analyses by comparing meta‐analysis results of:
removing cross‐over studies compared with all included studies;
removing studies at high risk of bias (e.g. one or more of the following items were at high risk: random sequence generation; allocation concealment; or selective reporting) compared with all included studies;
assuming that missing participants had a positive outcome versus a negative one for the outcome of 'number of participants experiencing an attack'.
We did undertake the third planned sensitivity analysis for one of the studies comparing colchicine to placebo (Zemer 1974).
Summary of findings table
We used GRADE Profiler (GRADE 2013) to import data from the Review Manager software to create 'Summary of findings tables' for each comparison evaluated in this review (Review Manager 2014). Summary of findings tables evaluated the overall quality of evidence on the primary and secondary outcomes. The GRADE system classified the quality of evidence in the following four grades: high; moderate; low; and very low (Schünemann 2011).
For each comparison we reported the following outcomes:
number of participants experiencing an attack;
duration of attacks;
number of days between attacks;
prevention of AA amyloidosis;
adverse drug reactions;
acute phase response.
Results
Description of studies
Results of the search
A total of 211 articles were identified from the search strategy, 56 of these remained after title and abstract screening; nine studies (14 references) met the inclusion criteria after the screening of the full texts (Amaryan 2003; Ben‐Zvi 2017; Dinarello 1974; Goldstein 1974; Hashkes 2012; Kosan 2004; Polat 2016; Wright 1977; Zemer 1974). One study is ongoing (NCT03446209); and one study (with one full published article and four conference abstracts has been listed as 'Awaiting classification' (De Benedetti 2018). A total of 36 articles were excluded.
The screening process is shown in the flow diagram (Figure 1) as recommended by the PRISMA statement (Moher 2009).
1.
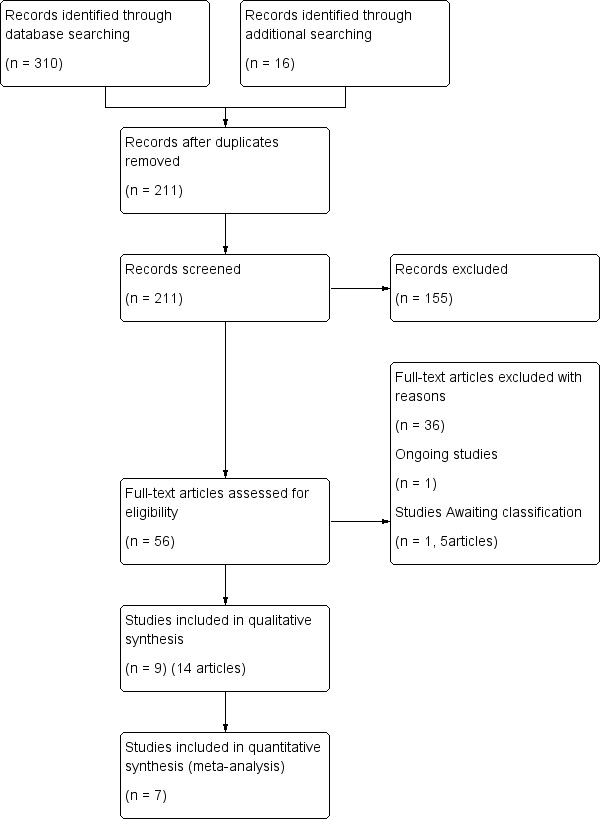
PRISMA flow diagram of study selection process
Included studies
Study design
Nine RCTs were included in this review. Five studies were of cross‐over design (Dinarello 1974; Goldstein 1974; Hashkes 2012; Wright 1977; Zemer 1974), four were parallel (Amaryan 2003; Ben‐Zvi 2017; Kosan 2004; Polat 2016). Four studies were conducted in the USA (Dinarello 1974; Goldstein 1974; Hashkes 2012; Wright 1977), two in Israel (Ben‐Zvi 2017; Zemer 1974), two in Turkey (Kosan 2004; Polat 2016) and one in Armenia (Amaryan 2003). Seven studies were conducted in a single centre (Amaryan 2003; Ben‐Zvi 2017; Dinarello 1974; Goldstein 1974; Kosan 2004; Wright 1977; Zemer 1974), one was conducted in six separate settings across the USA (Hashkes 2012) and one was in 10 centres in Turkey (Polat 2016). Sample sizes ranged from 10 participants (Goldstein 1974) to 90 participants (Polat 2016), but only two studies described a sample size calculation (Ben‐Zvi 2017; Polat 2016). One of the studies had three full publications (Hashkes 2012), three had two full publications (Amaryan 2003; Ben‐Zvi 2017; Dinarello 1974) and five had single full publications (Goldstein 1974; Kosan 2004; Polat 2016; Wright 1977; Zemer 1974).
Participants
A total of 249 people with FMF were randomized in the nine included studies. Of these, 160 participants completed the parallel studies (Amaryan 2003; Ben‐Zvi 2017; Kosan 2004; Polat 2016) and 51 completed the first phase of the five cross‐over studies (Dinarello 1974; Goldstein 1974; Hashkes 2012; Wright 1977; Zemer 1974). Seven studies reported the age of participants (Amaryan 2003; Ben‐Zvi 2017; Goldstein 1974; Hashkes 2012; Kosan 2004; Polat 2016; Wright 1977); the minimum age reported was three years old (Amaryan 2003) and the maximum was 53 years (Goldstein 1974). Seven studies reported participant gender at randomization (Amaryan 2003; Ben‐Zvi 2017; Hashkes 2012; Kosan 2004; Polat 2016; Wright 1977; Zemer 1974) and one after the study was completed (Goldstein 1974); a total of 107 participants were female and 115 were male. Five studies included FMF participants who suffered at least one attack per month (Ben‐Zvi 2017; Dinarello 1974; Goldstein 1974; Hashkes 2012; Wright 1977), but the remaining four did not report FMF severity (Amaryan 2003; Kosan 2004; Polat 2016; Zemer 1974).
Interventions
The nine studies evaluated four different interventions.
Four studies compared colchicine to placebo in people with FMF (Dinarello 1974; Goldstein 1974; Wright 1977; Zemer 1974). In first two studies, colchicine was given at a dose of 0.6 mg orally three times daily to participants who suffered at least one attack per month (Dinarello 1974; Goldstein 1974); in the third study in participants with a history of frequent FMF attacks, colchicine was given 3.6 mg orally for the first day (0.6 mg every hour for four hours, then every two hours for four hours) then 1.2 mg for the following two days (0.6 mg every 12 hours) (Wright 1977); and in the fourth study in for people with FMF not currently on any type of maintenance treatment, colchicine was given at a dose of 0.5 mg orally twice daily (Zemer 1974). Two studies in children with FMF compared colchicine given as a single dose (1 mg/day, once daily) to when it was given as a divided dose (1 mg/day, divided into two or three times in a day) (Kosan 2004; Polat 2016).
One study evaluated ImmunoGuard™ (a compound consisting of Andrographis paniculata Nees., Eleutherococcus senticosus Maxim., Schizandra chinensis Bail. and Glycyrrhiza glabra) compared to placebo in people with FMF who had never previously been treated with colchicine; treatment was given in the form of four tablets three times daily, with the total daily dose of the andrographolide being 48 mg (Amaryan 2003).
One study compared rilonacept (2.2 mg/kg/week to a maximum of 160 mg/week) given as a subcutaneous injection to placebo for colchicine‐resistant or colchicine‐intolerant people with FMF, in addition to oral colchicine administered in both groups (Hashkes 2012).
The final study compared anakinra (100 mg/day) given as a subcutaneous injection to placebo for people with FMF who were colchicine‐resistant (Ben‐Zvi 2017).
Outcomes
Four studies reported the number of participants experiencing an attack (Ben‐Zvi 2017; Goldstein 1974; Hashkes 2012; Zemer 1974) and three studies reported the timing of FMF attacks (two as the duration of FMF attacks (Hashkes 2012; Polat 2016) and one as the interval time between attacks (Wright 1977)); these are primary outcomes for this review. However, outcome data from the first phase or course could not be distinguished from the reports of two of the studies (Hashkes 2012; Wright 1977). Seven studies assessed adverse events (Amaryan 2003; Ben‐Zvi 2017; Dinarello 1974; Hashkes 2012; Kosan 2004; Polat 2016; Wright 1977). Five studies reported the acute phase response; in one study these measurements included CRP, WBC and ESR (Amaryan 2003), in one study CRP and SAA (Ben‐Zvi 2017), in one study ESR, WBC, CRP and fibrinogen (Kosan 2004), in one study ESR, CRP and SAA (Polat 2016) and in the fifth study CRP, ESR, SAA and fibrinogen, but again first‐phase outcome data could not be distinguished (Hashkes 2012).
Excluded studies
A total of 56 full texts were screened; of these, 36 studies were listed as excluded. There were 12 case reports (Alpay 2012; Bakkaloglu 2009; Belkhir 2007; Calligaris 2008; Gattringer 2007; Kuijk 2007; Mor 2007; Moser 2009; Roldan 2008; Sakallioglu 2006; Seyahi 2002; Stankovic Stojanovic 2012) and eight case series (Burstein 1997; Brik 2014; Dinarello 1976; Gül 2015; Hashkes 2014; Seyahi 2006; Zemer 1986; Zemer 1991). Six reports were not RCTs (Lidar 2004; Ofir 2008; Tunca 2004; Tweezer‐Zaks 2008; Yenokyan 2012; Uguztemur 2017); three were editorials (Anonymous 1977; Anonymous 1983; Ben‐Chetrit 2008), five were reviews (Adler 1998; Demirkaya 2016; Haviv 2016; Ozdogan 2017; Ter Haar 2013) and one was a letter (Sarkissian 2000). One excluded study was an RCT, but without pre‐specified disease (Hoffman 2008).
Studies awaiting classification
One study is listed as 'Awaiting classification' (De Benedetti 2018). This is a placebo‐controlled and double‐blind parallel 16‐week study of canakinumab in participants with hereditary periodic fevers, including colchicine resistant or intolerant FMF. A total of 63 participants with familial Mediterranean fever were randomized. Canakinumab was given at a dose of 150 mg (or 2 mg/kg for participants weighing up to 40 kg) every four weeks for 16 weeks. The primary outcome is the proportion of participants who had a complete response by the end of the study. The secondary outcome is the proportion of participants who had a physician's global assessment score below two, a level of C reactive protein of 10 mg/L or less, or a level of SAA level of 10 mg/L or less at week 16.
Ongoing studies
One study of intravenous tocilizumab is ongoing (NCT03446209). This is a placebo‐controlled and double‐blind parallel 28‐week study in adults with FMF comparing intravenous tocilizumab once every four weeks for 28 weeks to placebo (0.9% saline). The primary outcome measure is the change in physician's global assessment score and the secondary outcomes are adverse events and a range of laboratory markers.
Risk of bias in included studies
Details are described in the risk of bias section of the Characteristics of included studies, and shown by the risk of bias graph (Figure 2) and the risk of bias summary (Figure 3).
2.
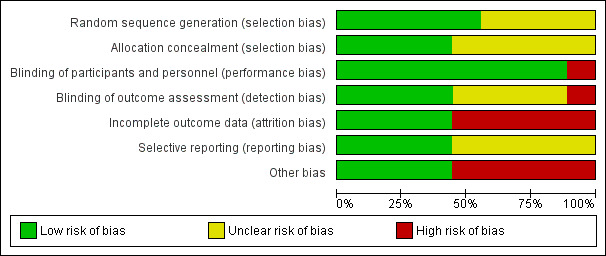
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
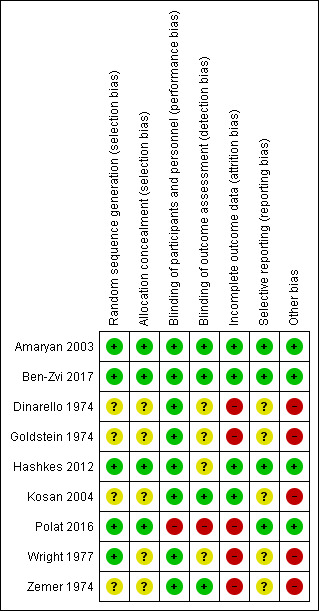
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Five studies adequately described sequence generation and we judged these to have a low risk of bias (Amaryan 2003; Ben‐Zvi 2017; Hashkes 2012; Polat 2016; Wright 1977). Amaryan stated that the sequence was derived using a simple randomization procedure (Amaryan 2003), Hashkes described using a computer‐generated code (Hashkes 2012), Ben‐Zvi reported using a predetermined key that was established by an external company (Ben‐Zvi 2017), Polat reported a computer‐based block randomization algorithm (Polat 2016) and Wright stated the randomization followed a method reported by Bradley Efron in 1971 named "Forcing a sequential experiment to be balanced" (Wright 1977). The remaining four RCTs did not describe sequence generation, and we judged that there was an unclear risk of bias (Dinarello 1974; Goldstein 1974; Kosan 2004; Zemer 1974).
Allocation concealment
Four studies adequately described the concealment of the treatment allocation and we judged these to have a low risk of bias (Amaryan 2003; Ben‐Zvi 2017; Hashkes 2012; Polat 2016). One study described using sequentially numbered drug containers of identical appearance (Amaryan 2003), the other three used a central allocation process (Ben‐Zvi 2017; Hashkes 2012; Polat 2016). The remaining five studies did not provide a sufficient description of the allocation concealment process and we judged the risk of bias as unclear (Dinarello 1974; Goldstein 1974; Kosan 2004; Wright 1977; Zemer 1974).
Blinding
Five RCTs reported using a double‐blind procedure for participants and personnel, so the risk of performance bias was low (Amaryan 2003; Ben‐Zvi 2017; Goldstein 1974; Hashkes 2012; Zemer 1974). Two studies reported that colchicine and placebo tablets were bottled, coded and dispensed by the Pharmaceutical Development Service, we also judged the the risk of performance bias was low (Dinarello 1974; Wright 1977). The two remaining RCTs, comparing different frequencies of colchicine administration, did not use a blinded procedure (Kosan 2004; Polat 2016). One of these two RCTs only reported our secondary outcomes which could not be influenced by blinding (or lack of it), so we judged this study to have a low risk of bias (Kosan 2004). Polat reported the primary outcome (duration of attacks) which could be influenced by blinding (or lack of it), so we judged this study to have a high risk of bias (Polat 2016).
One study reported outcome assessment was blinded, so we judged this study to have a low risk of detection bias (Zemer 1974). A further study reported that the investigators were blinded (Ben‐Zvi 2017). However, it was not clear if the blinding of outcome assessment was performed in the remaining three studies. Two studies only reported on one of our secondary outcomes which could not be influenced by blinding (or lack of it), so we judged these studies to also have a low risk of bias (Amaryan 2003; Kosan 2004). Again, Polat reported the primary outcome (duration of attacks) which could be influenced by blinding (or lack of it), so we judged this study to have a high risk of bias (Polat 2016). For the remaining four studies, the primary outcome of FMF attack measurement was likely to be influenced by lack of blinding, so we judged the risk of bias with respect to blinding of outcome assessment to be unclear (Dinarello 1974; Goldstein 1974; Hashkes 2012; Wright 1977).
Incomplete outcome data
Only one study reported all participants completed the follow‐up, we judged the study to have a low risk of bias (Kosan 2004).
The remaining eight included studies reported that there were participants lost to follow‐up. Of these, we judged three studies to have a low risk of bias (Amaryan 2003; Ben‐Zvi 2017; Hashkes 2012). Amaryan reported only one participant (less than 5%) in the control group was lost to follow‐up (Amaryan 2003). Hashkes reported that three participants withdrew, but an ITT analysis was performed and reasons given for the withdrawals (Hashkes 2012). Finally, Ben‐Zvi reported that seven participants (all in the placebo group) discontinued the study because of treatment failure in five participants and adverse events in two, again the ITT analysis was performed (Ben‐Zvi 2017).
Conversely, we judged the risk of bias to be high in five studies (Dinarello 1974; Goldstein 1974; Polat 2016; Wright 1977; Zemer 1974). Five out of 11 participants failed to complete the Dinarello study, with no indication if they had received one of the interventions or both, and no ITT analysis was reported (Dinarello 1974). Similarly, five out of 15 participants dropped out of the Goldstein study (Goldstein 1974), four out of nine participants failed to complete the Wright study (Wright 1977), nine out of 22 participants failed to complete the Zemer study and no ITT analysis was performed (Zemer 1974). In the Polat study, 11 out of 90 participants (eight in single‐dose group (17.78%) and three in the divided‐dose group (6.67%)) were lost to follow‐up and no ITT analysis was performed (Polat 2016).
Selective reporting
Four studies reported all of their pre‐specified outcomes according to the protocol or methods section of the full published paper (low risk of bias) (Amaryan 2003; Ben‐Zvi 2017; Hashkes 2012; Polat 2016). The remaining five studies failed to provide sufficient information to permit a judgement of risk, so the risk of bias for this domain was unclear (Dinarello 1974; Goldstein 1974; Kosan 2004; Wright 1977; Zemer 1974).
Other potential sources of bias
Five studies did not report the baseline characteristics of participants in each treatment group, so we could not evaluate baseline differences between groups in terms of e.g. mutation status, duration and frequency of FMF attacks; we therefore judged the risk of bias for this domain to be high in these five studies (Dinarello 1974; Goldstein 1974; Kosan 2004; Wright 1977; Zemer 1974). Furthermore, because of the difficulties in defining the severity of FMF and also of "colchicine‐resistance", there might be a potential risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Colchicine (oral) versus placebo for reducing inflammation in familial Mediterranean fever.
| Colchicine (oral) versus placebo for reducing inflammation in familial Mediterranean fever | ||||||
|
Participant or population: people with familial Mediterranean fever
Settings: outpatient (Israel and the USA)
Intervention: colchicine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Colchicine | |||||
| Number of participants experiencing an attack1,2 Follow‐up: 2 to 3 months | 1000 per 1000 | 210 per 1000 (50 to 950) | RR 0.21 (0.05 to 0.95) | 10 (1 studies) | ⊕⊕⊝⊝ low5,6 | Colchicine 0.6 mg orally 3x daily. |
| 900 per 1000 | 702 per 1000 (441 to 1000) | RR 0.78 (0.49 to 1.23) | 20 (1 studies) | ⊕⊕⊝⊝ low5,6 | Colchicine 0.5 mg orally 2x daily. | |
| Duration of attacks3,4 Follow‐up: 6 to 10 months | Wright 1977 reported that the duration of aborted attacks was less than 8 h, while all but 1 of the 18 unaborted attacks lasted more than 24 h and symptoms persisted more than 48 h in 15 of these 18 attacks. | 9 (1 studies) |
⊕⊝⊝⊝5,6,7 very low |
Data for separate treatment courses were unavailable and not analysed. | ||
| Goldstein 1974 stated there was no obvious difference in duration between 2 participants after colchicine prophylaxis. | 10 (1 studies) |
⊕⊝⊝⊝5,6,7 very low |
||||
| Number of days between attacks3,4 Follow‐up: 10 to 11 months | Dinarello 1974 reported the mean time between attacks was 15.1 days in the colchicine group versus 20.1 days in the placebo group. | 11 (1 studies) |
⊕⊝⊝⊝5,6,7 very low |
Data for separate treatment courses were unavailable and not analysed. No significant difference. |
||
| Wright 1977 reported that the mean duration of an attack after beginning a course of placebo was 10.4 days when the preceding course was colchicine versus 11.4 days when the preceding course was placebo. | 9 (1 studies) |
⊕⊝⊝⊝5,6,7 very low |
||||
| Prevention of AA amyloidosis | Not reported. | NA | ||||
|
Adverse drug reactions Follow‐up: 10 to 11 months |
Dinarello 1974 reported loose stools or frequent bowel movements, but no data were provided. | 11 (1 studies) |
⊕⊝⊝⊝5,6,7 very low |
|||
| Wright 1977 stated that 2 out of 9 participants experienced diarrhoea while taking colchicine (3.6 mg for the first day and 1.2 mg for the following 2 days), but symptoms disappeared when the dose was reduced 2.4 mg for the first day and 0.6 mg for the next 2 days in the subsequent treatment course. | 9 (1 studies) |
⊕⊝⊝⊝5,6,7 very low |
||||
| Acute phase response | Not reported. | NA | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AA: amyloid A; CI: confidence interval; NA: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
- Attack definition: any episode of fever and serositis reported by the participants during the study period.
- Attack definition: fever (above 38 ℃).
- Attack definition: acute, short‐lived episodes of peritonitis or pleuritis, usually with fever.
- Attack definition: symptoms of serosal inflammation accompanied by a temperature elevation to 37.8 ℃ or higher.
- Downgraded once for high risk due to incomplete outcome data and other bias, and unclear risk due to random sequence generation, allocation concealment, blinding of outcome and selective reporting.
- Downgraded once for the small sample size.
- Downgraded once for unavailable outcome data from each separate phase.
Summary of findings 2. Rilonacept versus placebo for reducing inflammation in familial Mediterranean fever.
| Rilonacept versus placebo for reducing inflammation in familial Mediterranean fever | ||||||
|
Participant or population: people with familial Mediterranean fever
Settings: outpatient (USA)
Intervention: rilonacept Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Rilonacept | |||||
| Number of participants experiencing an attack1 Follow‐up: 3 months | 1000 per 1000 | 870 per 1000 (590 to 1000) | RR 0.87 (0.59 to 1.26) | 14 (1 study) | ⊕⊕⊕⊝ moderate2 | RR < 1 indicates an advantage to rilonacept, no significant difference. |
| Duration of attacks1 Follow‐up: 12 months | The median duration was 3.2 days. | The median duration was 2.8 days. | NA | 14 (1 study) |
⊕⊕⊝⊝ low2,3 | First‐arm data were not reported separately. |
| Number of days between attacks1 Follow‐up: 12 months | The median time was 15 days to the first attack and 36 days to the second attack. | The median time was20 days to the first attack and 90 days to the second attack. | NA | 14 (1 study) |
⊕⊕⊝⊝ low2,3 | First‐arm data were not reported separately. |
| Prevention of AA amyloidosis | Not reported. | NA | ||||
| Adverse drug reactions | 1 participant reported gastrointestinal symptoms in the placebo group. | 3 participants reported gastrointestinal symptoms in the rilonacept group. | NA | 14 (1 study) |
⊕⊕⊝⊝ low2,3 | First‐arm data were not reported separately, the reported data was at the end of the study. |
| No participant reported hypertension in the placebo group. | 1 participant reported hypertension in the rilonacept group. | NA | ||||
| 1 participant reported headache in the placebo group. | 1 participant reportedheadache in the rilonacept group. | NA | ||||
| 7 participants reported respiratory tract infections in the placebo group as follows: respiratory infection (n = 1), upper respiratory tract infection or otitis (n = 4), sinusitis (n = 1) and other respiratory infection (n = 1). | 4 participants reported respiratory tract infections in the rilonacept group as follows: pneumonia (n = 1), upper respiratory tract infection or otitis (n = 1), sinusitis (n = 1), other respiratory infection (n = 1). | NA | ||||
| 5 participants reported injection site reactions in the placebo group. | 7 participants reportedinjection site reactions in the rilonacept group. | NA | ||||
| 2 participants reported herpes in the placebo group. | 1 participant reported herpes in the rilonacept group. | NA | ||||
| Acute phase response | The median ESR was 14 mm/h in the placebo group. | The median ESR was 5.8 mm/h in the rilonacept group. | NA | 14 (1 study) |
⊕⊕⊝⊝ low2,3 | First‐arm data were not reported separately, the reported data was at the end of the study. |
| The median fibrinogen was 9.56 μmol/L in the placebo group. | The median fibrinogen was 6.56 μmol/L in the rilonacept group. | NA | ||||
| The median CRP was4 mg/L in the placebo group. | The median CRP was2 mg/L in the rilonacept group. | NA | ||||
| The median SAA concentration was 15 mg/L in the placebo group. | The median SAA concentration was 13 mg/L in the rilonacept group. | NA | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AA: amyloid A; CI: confidence interval; CRP: C‐reactive protein; ESR: erythrocyte sedimentation rate; RR: risk ratio; SAA: serum amyloid A protein. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
- Attack definition: episodes of fever, serositis, acute arthritis, or an erysipelas‐like rash.
- Downgraded once for the small sample size.
- Downgraded once for unavailable outcome data from each separate phase.
Summary of findings 3. ImmunoGuardTM versus placebo for reducing inflammation in familial Mediterranean fever.
| ImmunoGuardTM versus placebo for reducing inflammation in familial Mediterranean fever | ||||||
|
Participant or population: participants with familial Mediterranean fever
Settings: outpatient (Armenia)
Intervention: ImmunoGuardTM Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ImmunoGuardTM | |||||
| Number of participants experiencing an attack1 | Not reported. | NA | ||||
| Duration of attacks1 | Not reported. | NA | ||||
| Number of days1 between attacks | Not reported. | NA | ||||
| Prevention of AA amyloidosis | Not reported. | NA | ||||
| Adverse drug reactions | The study reported that no side effects were observed. | 23 (1 study) |
⊕⊕⊕⊝ moderate2 | |||
|
Acute phase response Follow‐up: 1 month |
The mean CRP was 2.9 mg/L in the placebo group. | The mean CRP was 2.5 mg/L in the ImmunoGuardTM group. | NA | 23 (1 study) | ⊕⊕⊕⊝ moderate2 | The P values for the CRP, WBC and ESR were 0.45, 0.64 and 0.48, respectively, no significant difference. |
| The mean WBC was 11.2×10^9/L in the placebo group. | The mean WBC was 10.3×10^9/L in the ImmunoGuardTM group. | NA | ||||
| The mean ESR was 23.3mm/h in the placebo group. | The mean ESR was 20.4 mm/h in the ImmunoGuardTM group. | NA | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AA: amyloid A; CI: confidence interval; CRP: C‐reactive protein; ESR: erythrocyte sedimentation rate; NA: not applicable; WBC: white blood cell count. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
- Attack definition: fever ≥ 38℃, abdominal pain, chest pain, arthropathy, myalgia and erysipelas‐like erythema.
- Downgraded once for the small sample size.
Summary of findings 4. Anakinra versus placebo for reducing inflammation in familial Mediterranean fever.
| Anakinra compared with placebo for familial Mediterranean fever | ||||||
|
Patient or population: participants with familial Mediterranean fever Settings: outpatient (Israel) Intervention: anakinra Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Anakinra | |||||
|
Number of participants experiencing an attack1 Follow‐up: 4 months |
1000 per 1000 | 760 per 1000 (540 to 1000) | RR 0.76 (0.54 to 1.07) | 25 (1 study) | ⊕⊕⊕⊝ moderate2 | RR < 1 indicates an advantage to anakinra. Number of participants experiencing an attack at 1 and 2 months follow‐up were analysed; the difference between anakinra and placebo were not significant at either time‐point, RR 0.72 (95% CI 0.47 to 1.11) and RR 0.76 (95% CI 0.54 to 1.07), respectively. |
| Duration of attacks | Not reported. | NA | ||||
| Number of days between attacks | Not reported. | NA | ||||
| Prevention of AA amyloidosis | Not reported. | NA | ||||
| Adverse drug reactions | 308 per 1000 | 166 per 1000 (37 to 751) | RR 0.54 (0.12 to 2.44) | 25 (1 study) | ⊕⊕⊕⊝ moderate2 | Information from main text states: "The study reported that drug‐related adverse events were experienced by 16.7% of people in the anakinra group and 30.8% in the control group, including injection site reaction, headache, presyncope, dyspnea and itching" (Ben‐Zvi 2017). |
|
Acute phase response Follow‐up: 4 months |
The mean CRP was 19.9 mg/L in the placebo group. | The mean CRP was 3.9 mg/L in the anakinra group. | NA | 20 (1 study) | ⊕⊕⊕⊝ moderate2 | The P value was 0.006 for the CRP, significant difference, and 0.07 for the SAA, no significant difference. |
| The mean SAA was 110.3 mg/L in the placebo group. | The mean SAA was 11.1 mg/L in the anakinra group. | NA | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AA: amyloid A; CI: confidence interval; CRP: C‐reactive protein;NA: not applicable; RR: risk ratio; SAA: serum amyloid A. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
- Attack definition: fever ≥38℃ lasting 6 hours to 7 days and accompanied by painful in either the abdomen, the chest, the joints, or the skin.
- Downgraded once for the small sample size.
Summary of findings 5. Colchicine single dose versus divided dose for reducing inflammation in familial Mediterranean fever.
| Colchicine single dose versus divided dose for reducing inflammation in familial Mediterranean fever | ||||||
| Patient or population: pediatric participants with familial Mediterranean fever Settings: outpatient (Turkey) Intervention: colchicine single dose versus divided dose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Colchicine divided dose | Colchicine single dose | |||||
| Number of participants experiencing an attack | Not reported. | NA | ||||
| Duration of attacks1 Follow‐up: 3 and 6 months | The mean duration of attacks in the divided‐dose group was 12.35 hours during 3 months follow up. | The mean duration of attacks in the single‐dose group was 0.04 lower (10.91 lower to 10.83 higher). | NA | 79 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| The mean duration of attacks in the divided‐dose group was 5.6 hours during 6 months follow up. | The mean duration of attacks in the single‐dose group was 2.8 higher (5.39 lower to 10.99 higher). | NA | ||||
| Number of days between attacks | Not reported. | NA | ||||
| Prevention of AA amyloidosis | Not reported. | NA | ||||
| Adverse drug reactions Follow‐up: 3 and 6 months | The study reported adverse drug reactions of both 3 months and 6 months as following, anorexia, nausea, diarrhoea, abdominal pain, vomiting, elevated ALT and AST, and none of the reported adverse drug reactions between single or split doses of colchicine groups were significant. | NA | 79 (1 study) |
⊕⊕⊕⊝ moderate2 | ||
| Acute phase response Follow‐up: 8 months | The mean ESR was 27 mm/h in the divided‐dose group. | The mean ESR was 25 mm/h in the single‐dose group. | NA | 39 (1 studies) | ⊕⊕⊝⊝ low3,4 | |
| The mean WBC was 7.9×10^9/L in the divided‐dose group. | The mean WBC was 8.5×10^9/L in the single‐dose group. | NA | 39 (1 studies) | ⊕⊕⊝⊝ low3,4 | ||
| The mean fibrinogen was 414 mg/dL in the divided‐dose group. | The mean fibrinogen was 387 mg/dL in the single‐dose group (P = 0.09). | NA | 39 (1 studies) | ⊕⊕⊝⊝ low3,4 | ||
| The mean CRP was 4 mg/L in the divided‐dose group. | The mean CRP was 5 mg/L in the single‐dose group. | NA | 39 (1 studies) | ⊕⊕⊝⊝ low3,4 | ||
| The mean SAA was3.28 mg/L in the divided‐dose group. | The mean SAA was3.28 mg/L in the single‐dose group. | NA | 79 (1 studies) | ⊕⊕⊕⊝ moderate2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AA: amyloid A; CI: confidence interval; OR: odds ratio; NA: not applicable; ESR: erythrocyte sedimentation rate; WBC: white blood cell count; CRP: C‐reactive protein; SAA: serum amyloid A. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
- Attack definition: fever ≥ 38℃ lasting less than 72 h and accompanied by abdominal pain, chest pain, erysipelas such as erythema and/or swelling in the joints, and laboratory findings demonstrating an acute phase response.
- Downgraded once for high risk due to lack of blinding and incomplete outcome data.
- Downgraded once for high risk due to other bias and and unclear risk due to random sequence generation, allocation concealment and selective reporting.
- Downgraded once for the small sample size.
The quality of the evidence has been graded for those outcomes included in the summary of findings tables, one table for each comparison for reducing inflammation in FMF. For the definitions of these gradings, please refer to the relevant tables; please see colchicine versus placebo (Table 1), rilonacept versus placebo (Table 2), ImmunoGuard™ versus placebo (Table 3), anakinra versus placebo (Table 4), and single‐dose colchicine versus divided‐dose colchicine (Table 5).
Colchicine versus placebo
Three of the studies in this comparison reported on the use of colchicine compared to placebo for preventing attacks (Dinarello 1974; Goldstein 1974; Zemer 1974) and one study on the effect of colchicine and placebo on an attack once it occurred (Wright 1977). The judgements on the quality of the evidence can be found in the tables (Table 1).
All four studies were of cross‐over design; two studies randomized 37 participants and reported data from the end of the first phase for 29 participants (Goldstein 1974; Zemer 1974). The first of these randomized 15 participants with 10 completing the study; however, the number of participants in each group at initial randomization were not known (Goldstein 1974). The second study randomized 22 participants and 19 completed phase Ⅰ treatment; one participant dropped out from the colchicine group and two from the placebo group (Zemer 1974). The remaining two studies randomized a total of 20 participants in a study of 59 or 60 courses but did not provide data for each separate treatment course (Dinarello 1974; Wright 1977); one of these studies randomized 11 participants of whom six completed the study (Dinarello 1974), and the final study randomized nine participants with five completing the study (Wright 1977).
Primary outcomes
1. Number of participants experiencing an attack
Two studies reported on this outcome and administered colchicine with different doses and frequency so we were not able to combine the data (Goldstein 1974; Zemer 1974). One study used 0.6 mg orally three times daily for three months (first period of the cross‐over study) (Goldstein 1974); and the second study used 0.5 mg orally twice daily for two months (first period of the cross‐over study) (Zemer 1974). The data from the Goldstein study showed a significant difference between colchicine 0.6 mg orally three times daily and placebo, RR 0.21 (95% CI 0.05 to 0.95) (low‐quality evidence), but the data from the Zemer study showed no significant difference between colchicine 0.5 mg orally twice daily and placebo, RR 0.78 (95% CI 0.49 to 1.23) (low‐quality evidence) (Analysis 1.1).
1.1. Analysis.
Comparison 1 Colchicine versus placebo, Outcome 1 Number of participants experiencing an attack.
In a sensitivity analysis for one study (Zemer 1974), we assumed firstly the missing participants experienced an attack, this analysis showed no significant difference between groups, RR 0.74 (95% CI 0.50 to 1.08); we assumed secondly the missing participants were free of attacks, this analysis also showed no significant difference between groups, RR 0.78 (95% CI 0.46 to 1.32) (Analysis 1.1).
2. Timing of FMF attacks
a. duration of attacks
One study gave either colchicine or placebo at the start of an attack (Wright 1977). The paper reported that in the aborted attacks symptoms lasted less than eight hours; an attack was considered to have been aborted only if symptoms lasted less than eight hours and fever did not occur. In 17 out of 18 unaborted attacks symptoms lasted more than 24 hours, and indeed persisted for more than 48 hours in 15 attacks. The "mild" unaborted attack which lasted less than 24 hours was the only unaborted attack in a participant receiving colchicine (Wright 1977).
Goldstein did not report data, but stated that for the attacks that occurred in the colchicine group there was no obvious difference in duration (Goldstein 1974).
We judged the quality of the evidence for this outcome to be very low.
b. number of days between attacks
Two cross‐over studies reported on the timing of attacks; however, we were not able to extract data from the first treatment course for analysis (Dinarello 1974; Wright 1977). Dinarello reported the mean (standard error (SE)) time until the next attacks after the beginning of the placebo period was 10.4 (1.4) days when the preceding course was colchicine, compared to 11.4 (1.7) days when the preceding course was also placebo (very low‐quality evidence) (Dinarello 1974). Wright reported the mean interval between attacks after colchicine treatment was 15.1 days and after placebo was 20.1 days, with no significant differences (very low‐quality evidence) (Wright 1977). Furthermore, Wright stated "The latter (placebo) group of intervals included a single large value (129 days) from Patient I, who experienced only two attacks during the trial and hence did not contribute any intervals after a course of colchicine to the combined data. If this long interval is eliminated, the mean interval length becomes 15.4 days" (Wright 1977).
3. Prevention of AA amyloidosis
No included study reported on this outcome.
Secondary outcomes
1. Adverse drug reactions
Two cross‐over studies reported adverse drug reactions (very low‐quality evidence); however, data from the first treatment period were not reported separately (Dinarello 1974; Wright 1977). Dinarello reported that participants taking 0.6 mg colchicine three times daily suffered no major side effects except loose stools or frequent bowel movements, but did not report the exact number (Dinarello 1974). Wright reported that two participants experienced diarrhoea, and the symptoms disappeared after a reduction in the colchicine dose (Wright 1977).
2. Acute‐phase response
No included study reported on this outcome.
Rilonacept versus placebo
One cross‐over study randomized 14 participants and compared rilonacept to placebo for people with FMF who were colchicine‐resistant or colchicine‐intolerant (Hashkes 2012). One participant was lost to follow‐up in the first phase of treatment after experiencing an attack therefore 13 participants completed the first arm of treatment. The judgements on the quality of the evidence can be found in the tables (Table 2).
Primary outcomes
1. Number of participants experiencing an attack
We were able to obtain first‐arm outcome data. Outcome data indicated that the participant lost to follow‐up in the first phase experienced an FMF attack (Hashkes 2012). The analysis showed no significant difference between rilonacept or placebo, RR 0.87 (95% CI 0.59 to 1.26) (moderate‐quality evidence) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Rilonacept versus placebo, Outcome 1 Number of participants experiencing an attack.
2. Timing of FMF attacks
a. duration of attacks
The study reported both the duration of FMF attacks and the time of the first and the second attack; however, first‐arm outcome data were not reported separately (Hashkes 2012). The reported median duration of attacks was 2.8 versus 3.2 days (P = 0.32) in the rilonacept and the placebo group, respectively (low‐quality evidence).
b. number of days between attacks
The median amount of time to the first attack was 20 versus 15 days (P = 0.066), and to the second attack 90 versus 36 days (P = 0.009) in the rilonacept and the placebo group, respectively (low‐quality evidence).
3. Prevention of AA amyloidosis
No included study reported on this outcome.
Secondary outcomes
1. Adverse drug reactions
The study reported total adverse events occurring during the study, but first‐arm outcome data could not be separated from the total outcome data (Hashkes 2012) (low‐quality evidence).
a. digestive system
The study reported that gastrointestinal symptoms occurred in three participants (four events) in the rilonacept group and one participant (one event) in the placebo group (Hashkes 2012).
b. motor system
The included study did not report on this outcome.
c. circulatory system
Only one participant experienced hypertension (two events) in the rilonacept group (Hashkes 2012).
d. urogenital system
The included study did not report on this outcome.
e. nervous system
One participant experienced headache (one event) in the rilonacept group, and one participant (one event) in the placebo group (Hashkes 2012).
f. respiratory system
In the rilonacept group, four participants experienced respiratory tract infections (pneumonia (n = 1), upper respiratory tract infection or otitis (n = 1), sinusitis (n = 1) and other respiratory infection (n = 1)). In the placebo group, seven participants had respiratory tract infections (respiratory infection (n = 1), upper respiratory tract infection or otitis (n = 4), sinusitis (n = 1) and other respiratory infection (n = 1)) (Hashkes 2012).
g. reproductive system
The included study did not report on this outcome.
h. endocrine system
The included study did not report on this outcome.
i. others
Injection site reactions occurred in seven participants (53 events) with rilonacept and five participants (13 events) with placebo. Herpes occurred in one participant (one event) with rilonacept and two participants (two events) with placebo (Hashkes 2012).
2. Acute‐phase response
The study reported acute‐phase responses during the study; however, first‐arm data were not reported separately for this outcome (Hashkes 2012) (low‐quality evidence).
a. ESR
The reported median ESR was 5.8 mm per hour versus 14 mm per hour (P = 0.156) in the rilonacept and placebo groups, respectively (Hashkes 2012).
b. WBC
The included study did not report on this outcome.
c. fibrinogen concentration
The reported median fibrinogen concentration was 6.56 μmol/L in the rilonacept group versus 9.56 μmol/L in the placebo group (P = 0.063) (Hashkes 2012).
d. CRP
The reported median CRP was 2 mg/L in the rilonacept group versus 4 mg/L in the placebo group (P = 0.22) (Hashkes 2012).
e. SAA concentration
The reported median SAA concentration was 13 mg/L in the rilonacept group versus 15 mg/L in the placebo group (P = 0.50) (Hashkes 2012).
ImmunoGuard™ versus placebo
One parallel RCT with 24 randomized participants (of whom 23 completed the laboratory results assessment) reported on ImmunoGuard™ versus placebo for people with FMF who had not previously been treated with colchicine therapy (Amaryan 2003). Information on the quality of the evidence is presented in the tables (Table 3).
Primary outcomes
1. Number of participants experiencing an attack
The included study did not report on this outcome.
2. Timing of FMF attacks
The included study did not report on this outcome.
3.Prevention of AA amyloidosis
No included study reported on this outcome.
Secondary outcomes
1. Adverse drug reactions
The study reported that no side effects were observed (moderate‐quality evidence).
2. Acute phase response
a. ESR
The study reported ESR during the attack phase and the analysis showed no significant difference between ImmunoGuard™ or placebo, MD ‐2.90 (95% CI ‐10.86 to 5.06) (moderate‐quality evidence) (Analysis 3.1).
3.1. Analysis.
Comparison 3 ImmunoGuard™ versus placebo, Outcome 1 Acute phase response.
b. WBC
The study reported WBC count during the attack phase and the analysis showed no significant difference between ImmunoGuard™ or placebo, MD ‐0.90 (95% CI ‐4.66 to 2.86) (moderate‐quality evidence) (Analysis 3.1).
c. fibrinogen concentration
The included study did not report on this outcome.
d. CRP
The study reported C‐reactive protein concentration during the attack phase, the analysis showed no significant difference between ImmunoGuard™ or placebo, MD ‐0.36 (95% CI ‐1.29 to 0.57) (moderate‐quality evidence) (Analysis 3.1).
e. SAA concentration
The included study did not report on this outcome.
Anakinra versus placebo
One parallel RCT with 25 participants reported on this comparison (Ben‐Zvi 2017); information on the quality of the evidence is presented in the tables (Table 4).
Primary outcomes
1. Number of participants experiencing an attack
The published paper of this included study did not report on this outcome (Ben‐Zvi 2017); however we contacted Professor Avi Livneh who was an author on the paper and he provided us with data for this outcome at one to four months follow‐up. The data showed no significant difference between anakinra and placebo at one month, RR 0.72 (95% CI 0.47 to 1.11); at two months, RR 0.76 (95% CI 0.54 to 1.07); or at four months, RR 0.76 (95% CI 0.54 to 1.07) (moderate‐quality evidence) (Analysis 4.1).
4.1. Analysis.
Comparison 4 Anakinra versus placebo, Outcome 1 Number of participants experiencing an attack.
2. Timing of FMF attacks
The included study did not report on this outcome.
3. Prevention of AA amyloidosis
No included study reported on this outcome.
Secondary outcomes
1. Adverse drug reactions
The study reported that drug‐related adverse events were experienced by 16.7% of people in the anakinra group and 30.8% in the control group, including injection site reaction, headache, presyncope, dyspnea and itching (Ben‐Zvi 2017). Differences between groups were not significant, RR 0.54 (95% CI 0.12 to 2.44) (moderate‐quality evidence) (Analysis 4.2).
4.2. Analysis.
Comparison 4 Anakinra versus placebo, Outcome 2 Drug‐related adverse events.
2. Acute phase response
a. ESR
The included study did not report on this outcome.
b. WBC
The included study did not report on this outcome.
c. fibrinogen concentration
The included study did not report on this outcome.
d. CRP
The study reported C‐reactive protein concentration during the attack phase, the analysis showed significant difference between anakinra and placebo, MD ‐16.00 (95% CI ‐27.38 to ‐4.62) (moderate‐quality evidence) (Analysis 4.3).
4.3. Analysis.
Comparison 4 Anakinra versus placebo, Outcome 3 Acute phase response.
e. SAA concentration
The study reported SAA concentration during the attack phase, the analysis showed no significant difference between anakinra or placebo, MD ‐99.20 (95% CI ‐204.69 to 6.29) (moderate‐quality evidence) (Analysis 4.3).
Colchicine single dose versus divided dose
Two parallel RCTs with 129 participants reported on this comparison (Kosan 2004; Polat 2016). The first study randomized 39 children with FMF to the mean (SD) single‐dose (colchicine 0.97 (0.35) mg/day once daily) or mean (SD) divided‐dose group (colchicine 0.95 (0.30) mg/day, with the dose divided across two or three times per day) (Kosan 2004). The second study randomized 90 children with FMF to the single‐dose group (colchicine 1 mg/day once daily) or the divided‐dose group (colchicine 1 mg/day divided into two doses per day) (Polat 2016). Information about the quality of the evidence can be found in the tables (Table 5).
Primary outcomes
1. Number of participants experiencing an attack
Neither study reported this outcome. We have tried to contact the authors but received no reply.
2. Timing of FMF attacks
a. duration of attacks
One study reported the duration of attacks at three months and six months; the analysis showed no significant difference between groups at either three months, MD ‐0.04 (95% CI ‐10.91 to 10.83) or at six months, MD 2.80 (95% CI ‐5.39 to 10.99) (moderate‐quality evidence) (Analysis 5.1).
5.1. Analysis.
Comparison 5 Colchicine single dose versus divided dose, Outcome 1 Timing of FMF attacks.
b. number of days between attacks
The included study did not report on this outcome.
3. Prevention of AA amyloidosis
No included study reported on this outcome.
Secondary outcomes
1. Adverse drug reactions
Both studies reported adverse drug reactions (Kosan 2004; Polat 2016). Kosan reported no adverse effect was detected (Kosan 2004). Polat reported anorexia, nausea, diarrhoea, abdominal pain, vomiting, elevated ALT and elevated AST at both three and six months visit (Polat 2016). Analyses showed no significant difference between the single‐dose colchicine group and the divided‐dose colchicine group for any adverse event at either three months (moderate‐quality evidence) (Analysis 5.2) or six months (moderate‐quality evidence) (Analysis 5.3).
5.2. Analysis.
Comparison 5 Colchicine single dose versus divided dose, Outcome 2 Adverse drug reactions at three months.
5.3. Analysis.
Comparison 5 Colchicine single dose versus divided dose, Outcome 3 Adverse drug reactions at six months.
2. Acute phase response
a. ESR
The Kosan study reported ESR during the attack phase (Kosan 2004). The analysis showed no significant difference between colchicine single‐dose and divided‐dose groups, MD 2.00 (95% CI ‐4.33 to 8.33) (low‐quality evidence) (Analysis 5.4).
5.4. Analysis.
Comparison 5 Colchicine single dose versus divided dose, Outcome 4 Acute phase response.
b. WBC
The Kosan study also reported WBC during the attack phase (Kosan 2004); the analysis showed no significant difference between colchicine single‐dose and divided‐dose groups, MD ‐0.60 (95% CI ‐4.06 to 2.86) (low‐quality evidence) (Analysis 5.4).
c. fibrinogen concentration
The Kosan study reported fibrinogen concentration during the attack phase (Kosan 2004). Analysis showed no significant difference between colchicine single‐dose and divided‐dose groups, MD 27.00 (95% CI ‐4.45 to 58.45) (low‐quality evidence) (Analysis 5.4).
d. CRP
The Kosan study reported CRP during the attack phase (Kosan 2004); the analysis showed no significant difference between colchicine single‐dose and divided‐dose groups, MD ‐1.00 (95% CI ‐2.59 to 0.59) (low‐quality evidence) (Analysis 5.4).
e. SAA concentration
The Polat study reported SAA during the attack phase (Polat 2016); the analysis showed no significant difference between colchicine single‐dose and divided‐dose groups, MD 0.00 (95% CI ‐1.52 to 1.52) (moderate‐quality evidence) (Analysis 5.4).
Discussion
Summary of main results
There were very few RCTs investigating the effects and safety of interventions for treating FMF. The nine included studies assessed different interventions using varying study designs.
Four cross‐over studies and two parallel RCTs administered oral colchicine in different dosages and frequencies. The colchicine administration of 0.6 mg three times daily had a significant beneficial effect on the primary outcome measure of the number of people experiencing an attack but with low‐quality evidence (Goldstein 1974). However, the evidence showed no significant beneficial effect on the same outcome when 0.5 mg colchicine was administered twice daily (Zemer 1974). The mean number of days between FMF attacks was not significantly different between colchicine and placebo (Dinarello 1974; Wright 1977). The reported adverse drug reactions to colchicine were loose stools or frequent bowel movements (Dinarello 1974) and dose‐related diarrhoea (Wright 1977). No study comparing colchicine to placebo reported on acute phase response (Table 1). When comparing oral colchicine 1 mg once daily to colchicine 1 mg divided into two or three times daily for children with FMF, the differences in duration of FMF attacks, adverse drug reactions and acute phase response were not significant; the number of people experiencing attacks or the time intervals between attacks were not reported (Table 5).
The study comparing rilonacept to placebo reported no significant beneficial effect on the primary outcome measure of the number of people experiencing an attack, with moderate‐quality evidence (Table 2). There was no evidence of a beneficial effect of the other outcome measures in this review, including the duration and frequency of FMF attacks, adverse drug reactions or acute phase response.
The single parallel study comparing ImmunoGuard™ to placebo demonstrated no significant benefit on the review's secondary outcome measures of CRP, WBC and ESR with moderate‐quality evidence (Table 3). There were no reported adverse effects; the study did not report on the number of people experiencing an attack, the duration and frequency of FMF attacks, SAA protein and fibrinogen concentration.
One parallel study compared anakinra to placebo and demonstrated no significant difference on the review's primary outcome measure of the number of people experiencing an attack and also on total adverse drug reactions, with moderate‐quality evidence (Table 4). There was significant benefit on the review's secondary outcome measure of CRP, but no significant difference on SAA levels, both with moderate‐quality evidence (Table 4). The other outcome measures, including the frequency and duration of FMF attacks, ESR, WBC count and fibrinogen concentration were not reported.
Amyloidosis is the most significant complication of FMF. Unfortunately, we found none of the included studies reported the primary outcome of "prevention of AA amyloidosis".
Overall completeness and applicability of evidence
We were not able to review all the interventions we expected to, e.g. interventions such as canakinumab, etanercept, infliximab, thalidomide, interferon‐alpha (IFN‐α) and NSAIDs. The most common reason for this was that these interventions were evaluated in case reports rather than RCTs.
Furthermore, not all outcome measures, which we had defined a priori, were assessed. Four out of nine studies reported on the number of participants experiencing an attack, five out of nine studies reported on the timing (four of duration and two of frequency) of FMF attacks, none of the included RCTs reported prevention of AA amyloidosis, seven out of nine RCTs reported adverse drug reactions and five out of nine RCTs reported acute‐phase response. The two cross‐over RCTs published in 1974 both reported on the number of people experiencing an attack and Goldstein made a statement on the duration of the attacks, but they did not report on any of our other outcomes, including frequency of FMF attacks, adverse drug reactions and acute phase response (Goldstein 1974; Zemer 1974). In the remaining two cross‐over RCTs, outcome data were not reported separately for each treatment arm (Dinarello 1974; Wright 1977). We regarded the single study in which participants alternated treatment as a cross‐over RCT for the first two treatment phases; however, few data were reported after the first treatment phase (Hashkes 2012). Three included parallel RCTs did not report on the number of participants experiencing an attack or the duration or frequency of FMF attacks (Amaryan 2003; Kosan 2004; Polat 2016). No included study reported on all the outcome measures in this review.
Quality of the evidence
It may be premature to draw robust conclusions regarding FMF treatment given the small number of included studies with varying quality of evidence. A total of nine RCTs with 249 randomized participants were included in the review. With regards to the generation of allocation sequence, the concealment of treatment allocation and other potential sources of bias, such as baseline consistency of FMF severity, the three cross‐over RCTs published in 1974 (Dinarello 1974; Goldstein 1974; Zemer 1974) were methodologically poorer than the three more recent parallel RCTs (Amaryan 2003; Ben‐Zvi 2017; Hashkes 2012). The key limitation for most included RCTs was incomplete reporting of outcome data (Dinarello 1974; Goldstein 1974; Polat 2016; Wright 1977; Zemer 1974) and other sources of bias as baseline consistency of FMF severity (Dinarello 1974; Goldstein 1974; Kosan 2004; Wright 1977; Zemer 1974).
We present the evaluation of the quality of evidence for each outcome reviewed in the summary of findings tables. There was low‐quality evidence for the number of participants experiencing an attack who were treated with colchicine; the reasons for downgrading the quality were unclear risks for random sequence generation, for allocation concealment, for selective reporting and a high risk for incomplete outcome data reporting (Table 1). There was moderate‐quality evidence for the number of participants experiencing an attack with rilonacept and anakinra treatment, and for the acute‐phase response with ImmunoGuard™ and anakinra treatment, the reason for downgrading quality was the small sample size (Table 2; Table 3; Table 4). For the comparison of a single dose of colchicine versus divided doses, we judged the evidence to be of moderate quality for the duration of FMF attacks and adverse drug reactions, the reason for downgrading quality was the high risk of bias for blinding and incomplete outcome data; and the evidence was of low quality for the acute‐phase response, the reason for downgrading quality was unclear risks for random sequence generation, for allocation concealment, for selective reporting, for other existed bias and small sample size (Table 5).
Potential biases in the review process
We intended to include adults with FMF based on diagnosis by the 1997 Tel‐Hashomer criteria and children with FMF based on diagnosis by the 2009 Yalçinkaya criteria (Livneh 1997;Yalçinkaya 2009). However, we also included studies with participants described as having a diagnosis of FMF published before 1997. One study identified FMF participants mainly according to manifestations of attacks of fever, painful, and free of any known causative factor (Goldstein 1974). A second study simply reported that individuals with FMF were included (Zemer 1974). Two studies included adults with a history of frequent FMF attacks (Dinarello 1974; Wright 1977). Thus, there might be potential bias in the selection of participants.
The primary outcome measures included number of people experiencing an attack and the timing (frequency and duration) of FMF attacks. Attack definition varied slightly among studies. Zemer treated attacks as fever with a temperature exceeding 38℃ (Zemer 1974). Goldstein defined an attack as any episode of fever and serositis reported by the participants during the study period (Goldstein 1974). Dinarello treated attacks as serosal inflammation with fever (at least 37.8℃) (Dinarello 1974). Wright defined attack as peritonitis or pleuritis with fever (Wright 1977). Hashkes treated attacks as episodes of fever, serositis, acute arthritis, or an erysipelas‐like rash (Hashkes 2012). In the most recent study, attacks were defined as fever of above 38℃ lasting from six hours to seven days and accompanied by pain in either the abdomen, the chest, the joints, or the skin (Ben‐Zvi 2017).
Agreements and disagreements with other studies or reviews
Another systematic review of treatment for FMF has been performed (Demirkaya 2016); however, RCTs on this topic were rare. Demirkaya included six RCTs that are included in this Cochrane Review (Amaryan 2003; Dinarello 1974; Goldstein 1974; Hashkes 2012; Wright 1977; Zemer 1974) and a controlled clinical trial (CCT) (Tunca 2004). The review evaluated therapies as follows: colchicine (Dinarello 1974; Goldstein 1974; Wright 1977; Zemer 1974); rilonacept (Hashkes 2012); ImmunoGuard™ (Amaryan 2003); and interferon (Tunca 2004). Numerous non‐RCTs, such as case series and case reports, were identified. Colchicine was reported to effectively reduce FMF attacks (Dinarello 1976; Zemer 1991); moreover, "favourable response to colchicine" has been included in the Tel‐Hashomer criteria for FMF diagnosis (Livneh 1997). There were no other further studies reported on rilonacept, ImmunoGuard™ or anakinra for FMF to date.
Authors' conclusions
Implications for practice.
Based on the results of the current review, colchicine could be considered as a potential therapy for reducing the number of people with familial Mediterranean fever (FMF) experiencing attacks. The administration of oral colchicine at a dose of 0.6 mg three times daily might be effective; although in children with FMF the effects of a single 1 mg daily dose of oral colchicine 1 mg may not differ from the same dose divided into two or three times per day. For people with FMF who are colchicine‐resistant, anakinra might be effective. It would not be appropriate to give any practical advice for the use of rilonacept or ImmunoGuard™, since further studies are needed.
Implications for research.
This review is based on only four cross‐over and two parallel randomized controlled trials (RCTs) for colchicine and one study each for rilonacept, ImmunoGuard™ or anakinra. No included study reported on prevention of amyloid A amyloidosis. The four cross‐over studies of colchicine each only reported on one of the review's outcomes; moreover, outcome data from each treatment phase were not clearly and separately reported. Only four potential interventions for FMF were evaluated in an RCT setting and furthermore, the sample size of most included studies was too small. It is important to conduct further studies on other potential drugs using a randomized design, especially parallel randomized studies, based on the CONSORT guidelines (Moher 2012). With regards to outcome reporting, AA amyloidosis and unabridged outcomes with more detail should be reported. Further studies in this area should also define FMF and attacks according to universal criteria, such as the Tel‐Hashomer and the Yalçinkaya criteria, rather than various differing criteria.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2018 | New citation required and conclusions have changed | For people with familial Mediterranean fever who are colchicine‐resistant anakinra might be effective. For children with familial Mediterranean fever, there does not seem to be any difference between single or split doses of colchicine. |
| 16 October 2018 | New search has been performed | A new intervention of canakinumab was added to the review and consequently the search strategy was amended; a new search was performed. A total of 76 new reports were identified (after duplicates removed). One new study was included (Polat 2016). Six new studies (with one reference each) were added to 'Excluded studies' (Brik 2014; Demirkaya 2016; Gül 2015; Haviv 2016; Ozdogan 2017; Uguztemur 2017). One study previously listed as an 'Ongoing study' was included in the current review version (Ben‐Zvi 2017). Two studies previously listed as 'Awaiting classification' have now been included (Dinarello 1974; Wright 1977). One study of colchicine dose frequency which was previously listed as 'Excluded studies' has now been included after clarification that such comparisons are eligible (Kosan 2004). One new study has been listed as ongoing (NCT03446209). One study, with one full published article and four conference abstracts, has been listed as 'Awaiting classification' (De Benedetti 2018). "Prevention of AA amyloidosis" was added as a primary outcome and 'Adverse events' moved to secondary outcomes. |
History
Protocol first published: Issue 1, 2014 Review first published: Issue 3, 2015
| Date | Event | Description |
|---|---|---|
| 29 September 2015 | Amended | Comparator title added to summary of findings tables. |
Acknowledgements
We would like to acknowledge the Cochrane Cystic Fibrosis and Genetic Disorders Group, especially Managing Editor Nikki Jahnke and the Information Specialist, for their help in preparing the protocol and full review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Glossary
| amyloidosis | a variety of conditions where normally soluble proteins become insoluble and are deposited in various organs or tissues disrupting normal function |
| apoptosis | a process of programmed cell death |
| colocalize | to occur together in the same cell |
| cytotoxicity | process which results in cell damage or cell death |
| enterohepatic circulation | the circulation of drugs or other substances from the liver to the bile, followed by entry into the small intestine, absorption by the enterocyte and transport back to the liver |
| exon | a sequence of DNA that codes information for protein synthesis that is transcribed to messenger RNA |
| homotypic | of the same type or form |
| ileum | the final section of the small intestine |
| jejunum | the middle section of the small intestine |
| macrophage | a type of white blood cell that removes dying or dead cells and cellular debris |
| microtubule | fibrous, hollow rods, that function primarily to help support and shape the cell |
| oligomerize | to form a molecular complex that consists of a few monomer units |
| pericarditis | inflammation of the thin sac‐like membrane that surrounds the heart |
| peritonitis | inflammation of the peritoneum, the thin tissue that lines the inner wall of the abdomen and covers most of the abdominal organs |
| phagocytic activity | when a cell, such as a white blood cell, engulfs and absorbs waste material, harmful microorganisms, or other foreign bodies in the bloodstream and tissues |
| pleuritis | inflammation of the membrane that covers the lungs and lines the chest cavity |
| proteolytic | breakdown of proteins into smaller polypeptides or amino acids |
| serositis | inflammation of the tissues lining the lungs, heart, inner lining of the abdomen and organs within |
| synovitis | inflammation of the membrane surrounding a joint |
| tubulin | globular proteins that make up microtubules |
Appendix 2. CENTRAL search strategy
| Search strategy |
| #1 MeSH descriptor: [Familial Mediterranean Fever] explode all trees #2 ((familial mediterranean fever) or (familial paroxysmal polyserositi*) or (FMF)):ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor: [Colchicine] explode all trees #5 colchicine:ti,ab,kw #6 MeSH descriptor: [Interleukin 1 Receptor Antagonist Protein] explode all trees #7 (anakinra or rilonacept or canakinumab):ti,ab,kw #8 (etanercept or infliximab):ti,ab,kw #9 MeSH descriptor: [Interferon‐alpha] explode all trees #10 (interferon‐alpha or INF‐alpha or IFN‐α):ti,ab,kw #11 MeSH descriptor: [Thalidomide] explode all trees #12 thalidomide:ti,ab,kw #13 ImmunoGuard or Immuno‐Guard:ti,ab,kw #14 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 (#3 AND #14) |
Appendix 3. Ovid MEDLINE search strategy
| Search strategy |
| #1 exp Familial Mediterranean Fever/ #2 familial mediterranean fever.ab,ti,tw. #3 familial paroxysmal polyserositi*.ab,ti,tw. #4 1 or 2 or 3 #5 exp Colchicine/ #6 colchicine.ab,ti,tw. #7 exp Interleukin 1 Receptor Antagonist Protein/ #8 (anakinra or rilonacept or canakinumab).ab,ti,tw. #9 (etanercept or infliximab).ab,ti,tw. #10 exp Interferon‐alpha/ #11 interferon‐alpha.ab,ti,tw. #12 INF‐alpha.ab,ti,tw. #13 "IFN‐α".ab,ti,tw. #14 exp Thalidomide/ #15 thalidomide.ab,ti,tw. #16 ImmunoGuard.ab,ti,tw. #17 "Immuno‐Guard".ab,ti,tw. #18 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 #19 4 and 18 #20 randomized controlled trial.pt. #21 controlled clinical trial.pt. #22 randomized.ab. #23 placebo.ab. #24 clinical trials as topic/ #25 randomly.ab. #26 (crossover or cross‐over).tw. #27 trial.ti. #28 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 #29 humans/ #30 28 and 29 #31 19 and 30 |
Appendix 4. Ovid Embase search strategy
| Search strategy |
| #1 exp Familial Mediterranean Fever/ #2 familial mediterranean fever.ab,ti,tw. #3 familial paroxysmal polyserositi*.ab,ti,tw. #4 1 or 2 or 3 #5 exp Colchicine/ #6 colchicine.ab,ti,tw. #7 exp Interleukin 1 Receptor Antagonist Protein/ #8 (anakinra or rilonacept or canakinumab).ab,ti,tw. #9 (etanercept or infliximab).ab,ti,tw. #10 exp Interferon‐alpha/ #11 interferon‐alpha.ab,ti,tw. #12 INF‐alpha.ab,ti,tw. #13 "IFN‐α".ab,ti,tw. #14 exp Thalidomide/ #15 thalidomide.ab,ti,tw. #16 ImmunoGuard.ab,ti,tw. #17 "Immuno‐Guard".ab,ti,tw. #18 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 #19 4 and 18 #20 randomized controlled trial/ #21 crossover procedure/ #22 double‐blind procedure/ #23 single‐blind procedure/ #24 random$.tw. #25 factorial$.tw #26 (crossover$ or cross‐over$).tw. #27 placebo$.tw. #28 (double$ adj blind$).tw. #29 (singl$ adj blind$).tw. #30 assign$.tw. #31 allocat$.tw. #32 volunteer$.tw. #33 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 #34 19 and 33 |
Appendix 5. Criteria for judging risk of bias
Random sequence generation
'Low risk' of bias
The investigators describe a random component in the sequence generation process such as:
referring to a random number table;
using a computer random number generator;
coin tossing;
shuffling cards or envelopes;
throwing dice;
drawing of lots;
minimization.
'High risk' of bias
The investigators describe a non‐random component in the sequence generation process, for example:
sequence generated by odd or even date of birth;
sequence generated by some rule based on date (or day) of admission;
sequence generated by some rule based on hospital or clinic record number;
allocation by judgement of the clinician;
allocation by preference of the participant;
allocation based on the results of a laboratory test or a series of tests;
allocation by availability of the intervention.
'Unclear risk' of bias
Insufficient information about the sequence generation process to permit judgement of low risk or high risk.
Allocation concealment
'Low risk' of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
central allocation (including telephone, web‐based and pharmacy‐controlled randomization);
sequentially numbered drug containers of identical appearance;
sequentially numbered, opaque, sealed envelopes.
'High risk' of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
using an open random allocation schedule (e.g. a list of random numbers);
assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered);
alternation or rotation;
date of birth;
case record number;
any other explicitly unconcealed procedure.
'Unclear risk' of bias Insufficient information to permit judgement of low risk or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
Blinding of participants and personnel
'Low risk' of bias
Any one of the following:
no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding;
blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
'High risk' of bias
Any one of the following:
no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding;
blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
'Unclear risk' of bias
Any one of the following:
insufficient information to permit judgement of low risk or high risk;
the study did not address this outcome.
Blinding of outcome assessment
'Low risk' of bias
Any one of the following:
no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding;
blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
'High risk' of bias
Any one of the following:
No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding;
Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
'Unclear risk' of bias
Any one of the following:
insufficient information to permit judgement of low risk or high risk;
the study did not address this outcome.
Incomplete outcome data
'Low risk' of bias
Any one of the following:
no missing outcome data;
reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size;
missing data have been imputed using appropriate methods.
'High risk' of bias
Any one of the following:
reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomization;
potentially inappropriate application of simple imputation.
'Unclear risk' of bias
Any one of the following:
insufficient reporting of attrition or exclusions to permit judgement of low risk or high risk (e.g. number randomized not stated, no reasons for missing data provided);
the study did not address this outcome.
Selective reporting
'Low risk' of bias
Any of the following:
the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way;
the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
'High risk' of bias
Any one of the following:
not all of the study's pre‐specified primary outcomes have been reported;
one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified;
one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis;
the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
'Unclear risk' of bias
Insufficient information to permit judgement of low risk or high risk. It is likely that the majority of studies will fall into this category.
Other potential sources of bias
'Low risk' of bias
The study appears to be free of other sources of bias.
'High risk' of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
'Unclear risk' of bias
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias
Data and analyses
Comparison 1. Colchicine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing an attack | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Colchicine 0.6 mg orally three times daily (at three months) | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.95] |
| 1.2 Colchicine 0.5 mg orally twice daily (at two months) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.49, 1.23] |
| 1.3 Sensitivity analysis for colchicine 0.5 mg orally twice daily (at two months) ‐ assumed with attack | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.50, 1.08] |
| 1.4 Sensitivity analysis for colchicine 0.5 mg orally twice daily (at two months) ‐ assumed without attack | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.32] |
Comparison 2. Rilonacept versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing an attack | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. ImmunoGuard™ versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute phase response | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 ESR (mm/h) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐10.86, 5.06] |
| 1.2 WBC (10^9/L) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐4.66, 2.86] |
| 1.3 CRP (mg/L) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐1.29, 0.57] |
Comparison 4. Anakinra versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing an attack | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At one month | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.11] |
| 1.2 At two months | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
| 1.3 At four months | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
| 2 Drug‐related adverse events | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.12, 2.44] |
| 3 Acute phase response | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 CRP (mg/L) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐14.00 [‐27.38, ‐4.62] |
| 3.2 SAA (mg/L) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐99.20 [‐204.69, 6.29] |
Comparison 5. Colchicine single dose versus divided dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Timing of FMF attacks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duration of attacks at three months (h) | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐10.91, 10.83] |
| 1.2 Duration of attacks at six months (h) | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐5.39, 10.99] |
| 2 Adverse drug reactions at three months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Anorexia | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.53, 7.07] |
| 2.2 Nausea | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.91] |
| 2.3 Diarrhea | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.53, 7.07] |
| 2.4 Abdominal pain | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.20, 3.75] |
| 2.5 Vomiting | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.09, 3.59] |
| 2.6 Elevated ALT | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.20, 3.75] |
| 2.7 Elevated AST | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.36] |
| 3 Adverse drug reactions at six months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Anorexia | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.31, 3.41] |
| 3.2 Nausea | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.91] |
| 3.3 Diarrhea | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.05, 14.55] |
| 3.4 Abdominal pain | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.34, 6.90] |
| 3.5 Vomiting | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.59] |
| 3.6 Elevated ALT | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.28, 27.84] |
| 3.7 Elevated AST | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.23, 3.26] |
| 4 Acute phase response | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 ESR (mm/h) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.33, 8.33] |
| 4.2 WBC (10^9/L) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.06, 2.86] |
| 4.3 Fibrinogen (mg/dL) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 27.0 [‐4.45, 58.45] |
| 4.4 CRP (mg/L) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.59, 0.59] |
| 4.5 SAA (mg/L) | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.52, 1.52] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amaryan 2003.
| Methods | RCT. Parallel design. Location: Armenia. Single centre. Carried out from January 2001 until January 2002. |
|
| Participants | 24 people with FMF, diagnosed according to the Tel‐Hashomer criteria, without prior colchicine therapy. 14 participants randomised to ImmunoGuard™ and 10 to placebo. Age: 3 ‐ 15 years. Gender: 10 females, 14 males. |
|
| Interventions | Intervention: ImmunoGuard™ (containing Andrographolide, Eleuteroside E, Schisandrins and Glycyrrhizin) Control: placebo (containing lactose 170 mg, calcium hydrophosphate, potato starch, microcristalline cellulose, magnesium stearate, silicagel) Administration: 4 tablets orally, 3 times daily for 1 month. | |
| Outcomes |
All outcomes measured at 1 month. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using simple randomization procedure. "Each jar of tablets was given a sequential number (1, 2, 3..) with the code concealed to the investigator. The sequential numbers were matched with the order of arrival of the participants". |
| Allocation concealment (selection bias) | Low risk | Quote: "Each jar was given a sequential number(1, 2, 3..) with the code concealed to the investigator. The sequential numbers were matched with the order of arrival of the participants." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The current study is a double blind placebo‐controlled trial." "Placebo tablets were organoleptically and visually identical to the verum ImmunoGuard." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double blind, but we do not know whether outcome assessment was blinded. The review's secondary outcome of acute phase response was not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 24 patients who completed the clinical trial, 23 patients had complete laboratory results." One (less than 5%) participant in the control group lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol could not be reviewed, however, comparison of methods section and results section indicated all outcome measurements were reported. |
| Other bias | Low risk | No other source of bias identified. |
Ben‐Zvi 2017.
| Methods | RCT. Parallel design. Location: Israel. Single centre. Carried out from January 2013 until August 2014. | |
| Participants | 25 people with colchicine‐resistant FMF, diagnosed according to the Tel‐Hashomer clinical criteria, with at least 2 MEFV mutations, suffered at least 1 attack per month in any of the 4 FMF sites (abdomen, chest, joints, skin) despite having received a maximal‐tolerated dose of colchicine (dosage ≥ 2 to ≤ 3 mg/day). 12 participants randomised to anakinra and 13 to placebo. Age, mean (SD): anakinra group 38.4 (10) years; placebo group 36.1(12.4) years. Gender: 14 females, 11 males. | |
| Interventions | Intervention: 100 mg/day anakinra subcutaneous injection for 4 months. Control: 100 mg/day placebo subcutaneous injection for 4 months. | |
| Outcomes |
Outcomes measured at 4 months. |
|
| Notes | Clinical Trials identifier: NCT01705756. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were recruited consecutively (by order of arrival) from our FMF‐dedicated clinic, and were randomly assigned, in a blinded manner, to receive treatment with either anakinra or placebo. Assignment to either the anakinra group or the placebo group was based on a predetermined key, unknown to both the investigators and the patients, that was established by an external company (TFS Trial Form Support, Lund, Sweden). The randomization was stratified by sex." |
| Allocation concealment (selection bias) | Low risk | Quote: "that (randomization) was established by an external company." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Assignment to either the anakinra group or the placebo group was based on a predetermined key, unknown to both the investigators and the patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assignment to either the anakinra group or the placebo group was based on a predetermined key, unknown to both the investigators and the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seven patients discontinued the study, all of whom were from the placebo group. The discontinuations were due to what was considered to be treatment failure in 5 patients and due to AEs (1 for pregnancy and 1 for drug allergy) in 2 patients." |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias according to the protocol. |
| Other bias | Low risk | No other source of bias identified. Sample size was calculated. |
Dinarello 1974.
| Methods | RCT: separate course of colchicine and placebo were administrated in random order, 28 days for a course with a total of 60 courses. Cross‐over design. Location: USA. 2 centres. | |
| Participants | 11 adults with a history of frequent attacks and characteristics of FMF. Age: unclear. Gender: unclear. | |
| Interventions | Intervention: 0.6 mg colchicine 3 times daily for 28 days (1 course). Control: matching placebo. | |
| Outcomes |
Outcomes measured at 11 months. |
|
| Notes | The outcome data could not be distinguished among each phase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Separate courses of colchicine, 0.6‐mg tablets, and placebo were administered in random order", however, the exactly randomization method was unclear. |
| Allocation concealment (selection bias) | Unclear risk | The exactly allocation method was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The tablets were bottled, coded and dispensed by the Pharmaceutical Development Service at the National Institutes of Health." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether outcome assessment was blinded was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six of the 11 patients had completed the study at the time it was terminated, whereas none of the remaining five patients had experienced a sufficient number of attacks for therapy to be considered either a success or a failure." |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be reviewed; moreover, the methods section did not predefine outcome measurements. |
| Other bias | High risk | The baseline characteristics of each participant were not described. |
Goldstein 1974.
| Methods | RCT. Cross‐over design (90 days for each course then switch to alternative; no reported washout period). Location: USA. Single centre. |
|
| Participants | 15 people with FMF and a high frequency of attacks (at least 1 attack a month for 1 year or more), absence of amyloidosis or concurrent disease, without chronic steroid or narcotic usage, and no evidence of pregnancy. Age: 16 ‐ 53 years. Gender: 8 females, 2 males (participants completed study). |
|
| Interventions | Intervention: 0.6 mg colchicine orally 3‐times daily for 90 days.
Control: matching placebo. No washout period or assessment of carryover effect was reported. |
|
| Outcomes |
Outcomes measured at 3 and 6 months. |
|
| Notes | The outcome data, except "number of participants experiencing an attack", could not be distinguished between phase I and II of the cross‐over study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind study". "Neither of the physicians involved in the patients' care was aware of the drug being administered". "A drug crossover was done by the pharmacist after 90 days of treatment, without the knowledge of the patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double blind; however, we do not know whether outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Ten of the 15 patients completed the 180‐day study. Five patients had to be eliminated from the study for failure to take the medication regularly or meet the follow‐up requirements, or both." No indication if the 5 participants who dropped out received 1 of the interventions or both, and no ITT analysis were reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be reviewed, moreover the methods section did not predefine outcome measurements. |
| Other bias | High risk | Differences of FMF severity between groups were not described. |
Hashkes 2012.
| Methods | RCT (single participant alternating treatment), treated as cross‐over design for the first 2 phases (no washout period). Location: USA. Carried out from October 2008 until January 2011. Randomization occurred at the beginning of the study to 1 of the 4 treatment sequences: rilonacept‐placebo‐rilonacept‐placebo, placebo‐rilonacept‐placebo‐rilonacept, rilonacept‐placebo‐placebo‐rilonacept, placebo‐rilonacept‐rilonacept‐placebo. So, we treat the first 2 courses as a cross‐over study. |
|
| Participants | 14 people with FMF diagnosed according to the Tel‐Hashomer clinical criteria, with at least 1 mutation on the MEFV gene, suffered an estimated mean of 1 or more attacks per month for 3 months before screening and 1 or more attacks per month during screening despite receiving adequate colchicine treatment. Age: 4 ‐ 47 years. Gender: 6 females, 8 males. |
|
| Interventions | Intervention: rilonacept 2.2 mg/kg/week subcutaneous injection (maximum, 160 mg/week) for 3 months Control: matching placebo. Administration: intervention for 3 months, then cross‐over for the other 3 courses, a total of 12 months. No washout period between each 2 treatment phase, nor assessment of carryover effect. | |
| Outcomes |
Outcomes measured at 12 months. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocked randomization, using computer‐generated code". |
| Allocation concealment (selection bias) | Low risk | Quote: "Blocked randomization not stratified by center was done at the study coordination center by the unblinded statistician using a computer‐generated code to ensure equal allocation of participants into treatment group sequences. After confirming eligibility, the unblinded statistician called the site pharmacist with the participant number and treatment assignments". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind", "Rilonacept and placebo vials were labelled by the pharmacist and were identical in appearance, including after preparation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double blind; however, we do not know whether outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the first treatment course: 1 participant in the control group lost to follow‐up. In the whole treatment process: 3 participants withdrew: lost to follow‐up (n = 1); travel difficulties (n = 1); lack of efficacy (n = 1). ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias according to the protocol. |
| Other bias | Low risk | No other source of bias identified. |
Kosan 2004.
| Methods | RCT. Parallel design. Location: Turkey. Single centre. |
|
| Participants | 39 pediatric outpatients with the diagnosis of FMF, diagnosed based on Tel Hashomer criteria. 20 participants randomised to colchicine 2 or 3 times per day (divided‐dose group) and 19 to colchicine once daily (single‐dose group). Age, mean (SD): single‐dose group 9.8 (4.3) years; divided‐dose group 10.2 (4.0) years. Gender: 21 females, 18 males. | |
| Interventions | Single‐dose group: colchicine 0.97 ± 0.35 mg/day once daily. Divided‐dose group: colchicine 0.95 ± 0.30 mg/day, dose divided into 2 or 3 times daily. NB not stated if mean and SD or mean and SE reported. |
|
| Outcomes |
Outcomes measured at 8 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided in two groups", however, the exactly randomization method was unclear. |
| Allocation concealment (selection bias) | Unclear risk | The exact method of allocation concealment was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, however, the review's secondary outcome of acute phase response was not influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, however, the review's secondary outcome of acute phase response was not influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be reviewed; moreover, the methods section did not predefine outcome measurements. |
| Other bias | High risk | Differences of FMF severity between groups were not described. |
Polat 2016.
| Methods | RCT. Parallel design. Location: Turkey. Multicentre. Carried out from October 2011 until April 2013. | |
| Participants | 90 children who were newly diagnosed with FMF according to the Yalçinkaya criteria or the Tel Hashomer criteria, and confirmed by genetic analysis with heterozygous or homozygous mutations. 45 participants each were randomised to colchicine twice daily (divided‐dose group) or once daily (single‐dose group). Age, mean (SD): single‐dose group 7.90 (1.96) years; divided dose group 7.78 (2.00) years. Gender: 40 females, 39 males (79 participants completed study). |
|
| Interventions | Single‐dose group: colchicine 1 mg/day once daily at 8:00 am. Divided‐dose group: colchicine 1 mg/day divided into 2 doses one at 8:00 am and one at 8:00 pm. |
|
| Outcomes |
Outcomes measured at 3 and 6 months. |
|
| Notes | Clinical Trials identifier: NCT02602028. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "It was a multicenter randomized controlled trial... The randomization was done at the baseline visit...Computer‐based block randomization algorithm was used with a block size of 2 and each patient was assigned to a treatment group with an equal chance of allocation." |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The review's secondary outcome of acute phase response was not influenced by lack of blinding, but the adverse events is likely to be influenced. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. The review's secondary outcome of acute phase response was not influenced by lack of blinding, but the adverse events is likely to be influenced. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 people lost to follow‐up in the Divided dose group (6.67%), and 3 participants refused the treatment and 5 lost to follow‐up in Single‐dose group (17.78%), and no ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias according to the protocol. |
| Other bias | Low risk | No other source of bias identified. Sample size was calculated. |
Wright 1977.
| Methods | RCT: the order of colchicine and placebo courses was determined by a randomization scheme, with a total of 59 courses (28 courses of colchicine and 31 courses of placebo). Cross‐over design. Location: USA. Single centre. | |
| Participants | 9 adults with a history of frequent FMF attacks. Age: 18 ‐ 54 years. Gender: 4 females, 5 males. | |
| Interventions | Intervention course: 3.6 mg oral colchicine for the first day (0.6 mg every hour for 4 hours; then every 2 hours for 4 hours), 1.2 mg for the following 2 days. Control course: matching placebo. | |
| Outcomes |
Outcomes measured at 10 months. |
|
| Notes | The outcome data could not be distinguished between each phase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of colchicine and placebo courses was determined by a randomization scheme", and the randomization followed the method reported by Bradley Efron in 1971 named "Forcing a sequential experiment to be balanced". |
| Allocation concealment (selection bias) | Unclear risk | The exact method of allocation concealment was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The tablets were bottled, coded, and dispensed by the Pharmaceutical Development Service at the National Institutes of Health". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether outcome assessment was blinded was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants completed the study and 3 dropped out. Quote: "Two of these patients had been attack‐free on chronic colchicine therapy before entering the trial, and they found that having attacks again was too disruptive to their lives to complete the trial. The other patient became discouraged and dropped out after four consecutive courses failed to alter his FMF attacks (three of the courses were placebo)." |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be reviewed; moreover, the methods section did not predefine outcome measurements. |
| Other bias | High risk | Differences of FMF severity between groups were not described. |
Zemer 1974.
| Methods | RCT. Cross‐over design, 2 months of first treatment and then crossed over to second arm with no washout period. Location: Israel. Single centre. |
|
| Participants | 22 participants with FMF. Gender: 4 females, 18 males. |
|
| Interventions | Intervention: 0.5 mg oral colchicine 2 times daily for 2 months.
Control: placebo 2 times daily for 2 months.
Treatment 1 for 2 months, then cross‐over to alternate treatment for a further 2 months. No washout period, but have used paired t‐test to account for cross‐over design for the outcome 'number of attacks'. |
|
| Outcomes |
Outcomes measured at 1, 2, 3 and 4 months. |
|
| Notes | The outcome data, except "number of patients experiencing an attack", could not be distinguished between phase I and II of the cross‐over study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". "They (participants) were not informed what drug was being tried or that administration of placebo was part of the program. None of them were known to be on any maintenance therapy or had taken part in a previous drug study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The physicians of the follow‐up clinic were responsible for the referral of patients for the study and tabulating their attacks. They had no knowledge of whether the patient was receiving drug or placebo, or of the randomization schedule." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the first treatment phase: 3 participants lost to follow‐up, 1 in the colchicine group and 2 in the control group, and no ITT analysis was performed In the whole treatment process: "Of the 22 patients who entered the study, nine failed to complete it." |
| Selective reporting (reporting bias) | Unclear risk | Protocol could not be reviewed; moreover, the methods section did not predefine outcome measurements. |
| Other bias | High risk | Difference in severity of FMF between groups were not described. |
CRP: C‐reactive protein ESR: erythrocyte sedimentation rate FMF: familial Mediterranean fever ITT: intention‐to‐treat MEFV: Mediterranean fever RCT: randomized controlled trial SAA: serum amyloid A protein concentration SD: standard deviation SE: standard error WBC: white blood cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 1998 | Review. |
| Alpay 2012 | Case report. |
| Anonymous 1977 | Editorial. |
| Anonymous 1983 | Editorial. |
| Bakkaloglu 2009 | Case report. |
| Belkhir 2007 | Case report. |
| Ben‐Chetrit 2008 | Editorial. |
| Brik 2014 | Case series. |
| Burstein 1997 | Case series. |
| Calligaris 2008 | Case report. |
| Demirkaya 2016 | A systematic review. |
| Dinarello 1976 | Case series. |
| Gattringer 2007 | Case report. |
| Gül 2015 | Case series. |
| Hashkes 2014 | Case series, abstract only. |
| Haviv 2016 | Review. |
| Hoffman 2008 | Not pre‐specified disease, not people with FMF. |
| Kuijk 2007 | Case report. |
| Lidar 2004 | Controlled clinical trial, not pre‐specified comparisons, colchicine unresponsive versus responsive people. |
| Mor 2007 | Case report. |
| Moser 2009 | Case report. |
| Ofir 2008 | Controlled clinical trial, not pre‐specified comparisons, pregnancies of women with versus without FMF. |
| Ozdogan 2017 | Review. |
| Roldan 2008 | Case report. |
| Sakallioglu 2006 | Case report. |
| Sarkissian 2000 | Letter to editor. |
| Seyahi 2002 | Case report. |
| Seyahi 2006 | Case series. |
| Stankovic Stojanovic 2012 | Case report. |
| Ter Haar 2013 | Review. |
| Tunca 2004 | Controlled clinical trial, not randomized allocation, interferon‐α versus placebo. |
| Tweezer‐Zaks 2008 | Participant self‐controlled trial, interferon‐α versus negative control. Historical case control where participants' previous episodes were the control. |
| Uguztemur 2017 | Controlled clinical trial, not randomly allocated. |
| Yenokyan 2012 | Case‐crossover study, precipitating factors in attacks versus attack‐free periods. |
| Zemer 1986 | Case series. |
| Zemer 1991 | Case series. |
FMF: familial Mediterranean fever
Characteristics of studies awaiting assessment [ordered by study ID]
De Benedetti 2018.
| Methods | RCT. Placebo‐controlled, double blind. Parallel design. Duration: 16 weeks. |
| Participants | Participants with hereditary periodic fevers, including crFMF, hyper‐immunoglobulin D Syndrome (also mevalonate kinase deficiency (HIDS/MKD), and tumor necrosis factor receptor associated periodic syndrome (TRAPS). 1 cohort per disease. crFMF was diagnosed with the Tel‐Hashomer criteria, and fulfil the following criteria: • at least 1 known MEFV exon 10 mutation, • at least 1 fever episode per month despite a standard dose of colchicine (1.5 mg to 3.0 mg/day or equivalent paediatric‐adjusted regimen) or at least 1 fever episode per month with unacceptable side effects to colchicine. 63 participants with crFMF randomized. |
| Interventions |
Intervention: canakinumab 150 mg (or 2 mg/kg for participants weighing ≤ 40 kg) every 4 weeks for 16 weeks. Control: placebo. |
| Outcomes |
Primary outcome measure Proportion of participants who had a complete response, defined as resolution of the baseline flare at day 15 (PGA score of <2 plus CRP level of ≤10 mg/L or a reduction by ≥70% from baseline) and no new flare (PGA score of ≥2 and CRP level of ≥30 mg/L) until week 16. Secondary outcome measure Proportion of participants who had a PGA score <2, a CRP level ≤10 mg/L, or a SAA level ≤10 mg/L at week 16. |
| Notes | ClinicalTrials.gov Identifier: NCT02059291. In the subsequent phase up to week 40, participants who had a complete response underwent a second randomization to receive canakinumab or placebo every 8 weeks. Participants who underwent a second randomization and had a subsequent flare and all other participants received open‐label canakinumab. |
crFMF: colchicine resistant/intolerant familial Mediterranean fever CRP: C‐reactive protein PGA: physician's global assessment SAA: serum amyloid A
Characteristics of ongoing studies [ordered by study ID]
NCT03446209.
| Trial name or title | Tocilizumab for the Treatment of Familial Mediterranean Fever |
| Methods | RCT. Placebo‐controlled and double‐blind phase 2 study. Parallel design. Duration: 28 weeks. Multicenter. |
| Participants | People with FMF diagnosed with the Tel‐Hashomer criteria, and fulfil the following criteria: • 18 to 64 years of both genders; • with at least one heterozygous or homozygous mutation of the MEFV gene; • inadequate response or intolerance to colchicine; • attack during the last 12 weeks. |
| Interventions |
Intervention: tocilizumab intravenously once every 4 weeks for 28 weeks. Control: placebo (0.9% physiological saline). |
| Outcomes | Primary outcome measure: measured change of PGA. Secondary outcome measure: adverse events, ESR, SAA, CRP, blood cell count, creatinine, uric acid, GFR, GGT, ALT, AST, bilirubin. |
| Starting date | 23 April 23 2018. |
| Contact information | Jörg Henes, PD Dr. med. +49 (0)7071‐29 80681, joerg.henes@med.uni‐tuebingen.de Theodoros Xenitidis, Dr. med. +49‐7071‐29 80681, theodoros.xenitidis@med.uni‐tuebingen.de |
| Notes | ClinicalTrials.gov Identifier: NCT03446209. |
ALT: alanine aminotransferase AST: aspartate aminotransferase CRP: C‐reactive protein ESR: erythrocyte sedimentation rate GFR: glomular filtration rate GGT: gamma‐glutamyl transferase PGA: physician's global assessment SAA: serum amyloid A
Differences between protocol and review
We intended to assess all active interventions for FMF treatment, however, the protocol did not specifically name ImmunoGuard™ and canakinumab, which were identified during the search process. We added ImmunoGuard™ and canakinumab as an active intervention in the "Types of interventions" section in a post hoc change.
Review Manager 5.2 software was updated to Review Manager 5.3 (Review Manager 2014).
Summary of findings tables were added in the 'Methods' section at the update in 2017.
We added "Prevention of AA amyloidosis" as a primary outcome.
Contributions of authors
Bin Wu developed and updated the review, co‐ordinated its development, completed the first draft, performed part of the writing and editing of the review, advised on the review and approved final version prior to submission.
Ting Xu developed and updated the review, co‐ordinated its development, performed part of the data collection and analysis, advised on the review and approved the final version prior to submission.
Xi Yin co‐ordinated the review development and update, performed part of the data collection and analysis, made an intellectual contribution, advised on part of the review and approved the final version prior to submission.
Youping Li conceived the review question, made an intellectual contribution, advised on the review and approved the final version prior to submission.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
All authors declare: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Amaryan 2003 {published data only}
- Amaryan G, Astvatsatryan V, Gabrielyan E, Panossian A, Panosyan V, Wikman G. Double‐blind, placebo‐controlled, randomized, pilot clinical trial of ImmunoGuard‐‐a standardized fixed combination of Andrographis paniculata Nees, with Eleutherococcus senticosus Maxim, Schizandra chinensis Bail. and Glycyrrhiza glabra L. extracts in patients with Familial Mediterranean Fever. Phytomedicine 2003;10(4):271‐85. [DOI] [PubMed] [Google Scholar]
- Panossian A, Hambartsumyan M, Panosyan L, Abrahamyan H, Mamikonyan G, Gabrielyan E, et al. Plasma nitric oxide level in familial Mediterranean fever and its modulations by Immuno‐Guard. Nitric Oxide: Biology and Chemistry 2003;9(2):103‐10. [DOI] [PubMed] [Google Scholar]
Ben‐Zvi 2017 {published data only}
- Ben‐Zvi I, Kukuy O, Giat E, Pras E, Feld O, Kivity S, et al. Anakinra for colchicine‐resistant familial Mediterranean fever: a randomized, double‐blind, placebo‐controlled trial. Arthritis & Rheumatology 2017;69(4):854‐62. [DOI] [PubMed] [Google Scholar]
- Ben‐Zvi I, Livneh A. Colchicine failure in familial Mediterranean fever and potential alternatives: embarking on the anakinra trial. Israel Medical Association Journal 2014;16(5):271‐3. [PubMed] [Google Scholar]
Dinarello 1974 {published data only}
- Dinarello CA, Wolff SM, Goldfinger SE, Dale DC, Alling DW. Colchicine therapy for familial Mediterranean fever. A double blind trial. New England Journal of Medicine 1974;291(18):934‐7. [DOI] [PubMed] [Google Scholar]
- Wolff SM, Dinarello CA, Dale DC, Goldfinger SE, Alling DW. Colchicine therapy of familial Mediterranean fever. Transactions of the Association of American Physicians 1974;87:186‐94. [PubMed] [Google Scholar]
Goldstein 1974 {published data only}
- Goldstein RC, Schwabe AD. Prophylactic colchicine therapy in familial Mediterranean fever. A controlled, double‐blind study. Annals of Internal Medicine 1974;81(6):792‐4. [DOI] [PubMed] [Google Scholar]
Hashkes 2012 {published data only}
- Hashkes PJ, Spalding SJ, Giannini EH, Huang B, Johnson A, Park G, et al. Rilonacept for colchicine‐resistant or ‐intolerant familial Mediterranean fever: a randomized trial. Annals of Internal Medicine 2012;157(8):533‐41. [DOI] [PubMed] [Google Scholar]
- Hashkes PJ, Spalding SJ, Giannini EH, Huang B, Park G, Barron KS, et al. Rilonacept (interleukin‐1 trap) for treatment of colchicine resistant familial Mediterranean fever: a randomized, multicenter double‐blinded, alternating treatment phase II trial. Pediatric Rheumatology 2011;9(Suppl 1):O38. [Google Scholar]
- Hashkes PJ, Spalding SJ, Hajj‐Ali R, Giannini EH, Johnson A, Barron KS, et al. The effect of rilonacept versus placebo on health‐related quality of life in patients with poorly controlled familial Mediterranean fever. BioMed Research International 2014;854842:1‐8. [DOI: 10.1155/2014/854842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosan 2004 {published data only}
- Kosan C, Ozkan B. Once‐daily use of colchicine in children with familial Mediterranean fever. Clinical Pediatrics 2004;43(7):605‐8. [DOI] [PubMed] [Google Scholar]
Polat 2016 {published data only}
- Polat A, Acikel C, Sozeri B, Dursun I, Kasapcopur O, Gulez N, et al. Comparison of the efficacy of once‐ and twice‐daily colchicine dosage in pediatric patients with familial Mediterranean fever‐‐a randomized controlled non‐inferiority trial. Arthritis Research & Therapy 2016;18(85):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1977 {published data only}
- Wright DG, Wolff SM, Fauci AS, Alling DW. Efficacy of intermittent colchicine therapy in familial Mediterranean fever. Annals of Internal Medicine 1977;86(2):162‐5. [DOI] [PubMed] [Google Scholar]
Zemer 1974 {published data only}
- Zemer D, Revach M, Pras M, Modan B, Schor S, Sohar E, et al. A controlled trial of colchicine in preventing attacks of familial Mediterranean fever. New England Journal of Medicine 1974;291(18):932‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adler 1998 {published data only}
- Adler Y, Finkelstein Y, Guindo J, Rodriguez de la Serna A, Shoenfeld Y, Bayes‐Genis A, et al. Colchicine treatment for recurrent pericarditis. A decade of experience. Circulation 1998;97(21):2183‐5. [DOI] [PubMed] [Google Scholar]
Alpay 2012 {published data only}
- Alpay N, Sumnu A, Calışkan Y, Yazıcı H, Türkmen A, Gül A. Efficacy of anakinra treatment in a patient with colchicine‐resistant familial Mediterranean fever. Rheumatology International 2012;32(10):3277‐9. [DOI] [PubMed] [Google Scholar]
Anonymous 1977 {published data only}
- Anonymous. Colchicine in familial Mediterranean fever. Lancet 1977;1(8022):1140‐1. [PubMed] [Google Scholar]
Anonymous 1983 {published data only}
- Anonymous. Colchicine for familial Mediterranean fever. FDA Drug Bulletin 1983;13(1):4. [PubMed] [Google Scholar]
Bakkaloglu 2009 {published data only}
- Bakkaloglu SA, Aksu T, Goker B, Unlusoy A, Peru H, Fidan K, et al. Sulphasalazine treatment in protracted familial Mediterranean fever arthritis. European Journal of Pediatrics 2009;168(8):1017‐9. [DOI] [PubMed] [Google Scholar]
Belkhir 2007 {published data only}
- Belkhir R, Moulonguet‐Doleris L, Hachulla E, Prinseau J, Baglin A, Hanslik T. Treatment of familial Mediterranean fever with anakinra. Annals of Internal Medicine 2007;146(11):825‐6. [DOI] [PubMed] [Google Scholar]
Ben‐Chetrit 2008 {published data only}
- Ben‐Chetrit E, Ozdogan H. Non‐response to colchicine in FMF‐definition, causes and suggested solutions. Clinical and Experimental Rheumatology 2008;26(4 Suppl 50):S49‐51. [PubMed] [Google Scholar]
Brik 2014 {published data only}
- Brik R, Butbul‐Aviel Y, Lubin S, Ben Dayan E, Rachmilewitz‐Minei T, Tseng L, et al. Canakinumab for the treatment of children with colchicine‐resistant familial Mediterranean fever: a 6‐month open‐label, single‐arm pilot study. Arthritis & Rheumatology 2014;66(11):3241‐3. [DOI] [PubMed] [Google Scholar]
Burstein 1997 {published data only}
- Burstein R, Seidman DS, Zemer D, Shpilberg O, Arnon R, Epstein Y, et al. Chronic colchicine treatment does not impair glucose tolerance in familial Mediterranean fever patients. European Journal of Clinical Pharmacology 1997;52(1):27‐30. [DOI] [PubMed] [Google Scholar]
Calligaris 2008 {published data only}
- Calligaris L, Marchetti F, Tommasini A, Ventura A. The efficacy of anakinra in an adolescent with colchicine‐resistant familial Mediterranean fever. European Journal of Pediatrics 2008;167(6):695‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demirkaya 2016 {published data only}
- Demirkaya E, Erer B, Ozen S, Ben‐Chetrit E. Efficacy and safety of treatments in familial Mediterranean fever: a systematic review. Rheumatology International 2016;36(3):325‐31. [DOI] [PubMed] [Google Scholar]
Dinarello 1976 {published data only}
- Dinarello CA, Chusid MJ, Fauci AS, Gallin JI, Dale DC, Wolff SM. Effect of prophylactic colchicine therapy on leukocyte function in patients with familial Mediterranean fever. Arthritis & Rheumatism 1976;19(3):618‐22. [DOI] [PubMed] [Google Scholar]
Gattringer 2007 {published data only}
- Gattringer R, Lagler H, Gattringer KB, Knapp S, Burgmann H, Winkler S, et al. Anakinra in two adolescent female patients suffering from colchicine‐resistant familial Mediterranean fever: effective but risky. European Journal of Clinical Investigation 2007;37(11):912‐4. [DOI] [PubMed] [Google Scholar]
Gül 2015 {published data only}
- Gül A, Ozdogan H, Erer B, Ugurlu S, Kasapcopur O, Davis N, et al. Efficacy and safety of canakinumab in adolescents and adults with colchicine‐resistant familial Mediterranean fever. Arthritis Research & Therapy 2015;17(243):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashkes 2014 {published data only}
- Hashkes P, Butbul Aviel Y, Lubin S, Ben‐Dayan E, Tseng L, Brik R. Long‐term efficacy of canakinumab in childhood colchicine resistant familial Mediterranean Fever. Arthritis & Rheumatism 2014;66(Suppl 11):S108. [Abstract no.: A76] [DOI] [PubMed] [Google Scholar]
Haviv 2016 {published data only}
- Haviv R, Hashkes PJ. Canakinumab investigated for treating familial Mediterranean fever. Expert Opinion on Biological Therapy 2016;16(11):1425‐34. [DOI] [PubMed] [Google Scholar]
Hoffman 2008 {published data only}
- Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin‐1 Trap) in patients with cryopyrin‐associated periodic syndromes: results from two sequential placebo‐controlled studies. Arthritis & Rheumatism 2008;58(8):2443‐52. [DOI] [PubMed] [Google Scholar]
Kuijk 2007 {published data only}
- Kuijk LM, Govers AM, Frenkel J, Hofhuis WJ. Effective treatment of a colchicine‐resistant familial Mediterranean fever patient with anakinra. Annals of the Rheumatic Diseases 2007;66(11):1545‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lidar 2004 {published data only}
- Lidar M, Scherrmann JM, Shinar Y, Chetrit A, Niel E, Gershoni Baruch R, et al. Colchicine non‐responsiveness in familial Mediterranean fever: clinical, genetic, pharmacokinetic, and socioeconomic characterization. Seminars in Arthritis and Rheumatism 2004;33(4):273‐82. [DOI] [PubMed] [Google Scholar]
Mor 2007 {published data only}
- Mor A, Pillinger MH, Kishimoto M, Abeles AM, Livneh A. Familial Mediterranean fever successfully treated with etanercept. Journal of Clinical Rheumatology 2007;13(1):38‐40. [DOI] [PubMed] [Google Scholar]
Moser 2009 {published data only}
- Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, et al. Successful treatment of familial Mediterranean fever with Anakinra and outcome after renal transplantation. Nephrology, Dialysis, Transplantation 2009;24(2):676‐8. [DOI] [PubMed] [Google Scholar]
Ofir 2008 {published data only}
- Ofir D, Levy A, Wiznitzer A, Mazor M, Sheiner E. Familial Mediterranean fever during pregnancy: an independent risk factor for preterm delivery. European Journal of Obstetrics, Gynaecology and Reproductive Biology 2008;141(2):115‐8. [DOI] [PubMed] [Google Scholar]
Ozdogan 2017 {published data only}
- Ozdogan H, Ugurlu S. Canakinumab for the treatment of familial Mediterranean fever. Expert Review of Clinical Immunology 2017;13(5):393‐404. [DOI] [PubMed] [Google Scholar]
Roldan 2008 {published data only}
- Roldan R, Ruiz AM, Miranda MD, Collantes E. Anakinra: new therapeutic approach in children with Familial Mediterranean Fever resistant to colchicine. Joint Bone Spine 2008;75(4):504‐5. [DOI] [PubMed] [Google Scholar]
Sakallioglu 2006 {published data only}
- Sakallioglu O, Duzova A, Ozen S. Etanercept in the treatment of arthritis in a patient with familial Mediterranean fever. Clinical and Experimental Rheumatology 2006;24(4):435‐7. [PubMed] [Google Scholar]
Sarkissian 2000 {published data only}
- Sarkissian A, Papazian M, Sanamyan A, Leumann E. Colchicine in the treatment of renal amyloidosis secondary to familial Mediterranean fever. Nephrology, Dialysis, Transplantation 2000;15(7):1098. [DOI] [PubMed] [Google Scholar]
Seyahi 2002 {published data only}
- Seyahi E, Ozdogan H, Masatlioglu S, Yazici H. Successful treatment of familial Mediterranean fever attacks with thalidomide in a colchicine resistant patient. clinical and Experimental Rheumatology 2002;20(4 Suppl 26):S43‐4. [PubMed] [Google Scholar]
Seyahi 2006 {published data only}
- Seyahi E, Ozdogan H, Celik S, Ugurlu S, Yazici H. Treatment options in colchicine resistant familial Mediterranean fever patients: thalidomide and etanercept as adjunctive agents. Clinical and Experimental Rheumatology 2006;24(5 Suppl 42):S99‐103. [PubMed] [Google Scholar]
Stankovic Stojanovic 2012 {published data only}
- Stankovic Stojanovic K, Delmas Y, Torres PU, Peltier J, Pelle G, Jéru I, et al. Dramatic beneficial effect of interleukin‐1 inhibitor treatment in patients with familial Mediterranean fever complicated with amyloidosis and renal failure. Nephrology, Dialysis, Transplantation 2012;27(5):1898‐901. [DOI] [PubMed] [Google Scholar]
Ter Haar 2013 {published data only}
- Ter Haar N, Lachmann H, Özen S, Woo P, Uziel Y, Modesto C, et al. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Annals of the Rheumatic Diseases 2013;72(5):678‐85. [DOI] [PubMed] [Google Scholar]
Tunca 2004 {published data only}
- Tunca M, Akar S, Soyturk M, Kirkali G, Resmi H, Akhunlar H, et al. The effect of interferon alpha administration on acute attacks of familial Mediterranean fever: A double‐blind, placebo‐controlled trial. Clinical and Experimental Rheumatology 2004;22(4 Suppl 24):S37‐S40. [PubMed] [Google Scholar]
Tweezer‐Zaks 2008 {published data only}
- Tweezer‐Zaks N, Rabinovich E, Lidar M, Livneh A. Interferon‐alpha as a treatment modality for colchicine‐resistant familial Mediterranean fever. Journal of Rheumatology 2008;35(7):1362‐5. [PubMed] [Google Scholar]
Uguztemur 2017 {published data only}
- Uguztemur E, Celik S, Sadri S, Ergun G, Asci E, Velipasalar O, et al. The efficacy of different colchicine doses in treatment of familial Mediterranean fever patients. Annals of Rheumatic Diseases 2017;76(Supplement 2):716. [Google Scholar]
Yenokyan 2012 {published data only}
- Yenokyan G, Armenian HK. Triggers for attacks in familial Mediterranean fever: application of the case‐crossover design. American Journal of Epidemiology 2012;175(10):1054‐61. [DOI] [PubMed] [Google Scholar]
Zemer 1986 {published data only}
- Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni, J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. New England Journal of Medicine 1986;314(16):1001‐5. [DOI] [PubMed] [Google Scholar]
Zemer 1991 {published data only}
- Zemer D, Livneh A, Danon YL, Pras M, Sohar E. Long‐term colchicine treatment in children with familial Mediterranean fever. Arthritis & Rheumatism 1991;34(8):973‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
De Benedetti 2018 {published data only}
- Benedetti F, Anton J, Gattorno M, Lachmann H, Kone‐Paut I, Ozen S, et al. Efficacy and safety of Canakinumab in patients with periodic fever syndromes (colchicine‐resistant fmf, hids/mkd and traps): results from a phase 3, pivotal, umbrella trial. Pediatric Rheumatology 2017;15(Supplement 1):no pagination. [Google Scholar]
- Benedetti F, Gattorno M, Anton J, Ben‐Chetrit E, Frenkel J, Hoffman HM, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. New England Journal of Medicine 2018;378(20):1908‐19. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Miettunen P, Kallinich T, Horneff G, Brik R, Tomassini A, et al. Genetic phenotypes impacting efficacy and safety of canakinumab in patients with colchicine‐resistant FMF, traps and HIDS/MKD: results from cluster study. Arthritis & Rheumatology 2017;69(S10):no pagination. [Google Scholar]
- Kone‐Paut I, Hofer M, Benseler S, Kuemmerle‐Deschner JB, Jansson A, Rosner I, et al. Improvement of disease activity in patients with colchicine‐resistant FMF, HIDS/MKD and traps assessed by autoinflammatory disease activity index (AIDAI): Results from a randomized phase iii trial. Arthritis & Rheumatology 2017;69(S10):398‐9. [Google Scholar]
- Lachmann H, Simon A, Anton J, Gattorno M, Kone‐Paut I, Ozen S, et al. Effect of canakinumab on health‐related quality of life in patients with periodic fever syndromes. Pediatric Rheumatology. Conference: 23rd Paediatric Rheumatology European Society Congress. 2017; Vol. 15:no pagination.
References to ongoing studies
NCT03446209 {published data only}
- NCT03446209. Tocilizumab for the treatment of familial Mediterranean fever. clinicaltrials.gov/ct2/show/NCT03446209 (first received 26 February 2018).
Additional references
Balci 2002
- Balci B, Tinaztepe K, Yilmaz E, Guçer S, Ozen S, Topaloğlu R, et al. MEFV gene mutations in familial Mediterranean fever phenotype II patients with renal amyloidosis in childhood: a retrospective clinicopathological and molecular study. Nephrology Dialysis Transplantation 2002;17(11):1921‐3. [DOI] [PubMed] [Google Scholar]
Baykal 2003
- Baykal Y, Saglam K, Yilmaz MI, Taslipinar A, Akinci SB, Inal A. Serum sIL‐2r, IL‐6, IL‐10 and TNF‐alpha level in familial Mediterranean fever patients. Clinical Rheumatology 2003;22(2):99‐101. [DOI] [PubMed] [Google Scholar]
Ben‐Chetrit 2006
- Ben‐Chetrit E, Bergmann S, Sood R. Mechanism of the anti‐inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology (Oxford) 2006;45(3):274‐82. [DOI] [PubMed] [Google Scholar]
Bilgen 2011
- Bilgen SA, Kilic L, Akdogan A, Kiraz S, Kalyoncu U, Karadag O, et al. Effects of anti‐tumor necrosis factor agents for familial Mediterranean fever patients with chronic arthritis and/or sacroiliitis who were resistant to colchicine treatment. Journal of Clinical Rheumatology 2011;17(7):358‐62. [DOI] [PubMed] [Google Scholar]
Centola 2000
- Centola M, Wood G, Frucht DM, Galon J, Aringer M, Farrell C, et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood 2000;95(10):3223‐31. [PubMed] [Google Scholar]
Cerquaglia 2005
- Cerquaglia C, Diaco M, Nucera G, La Regina M, Montalto M, Manna R. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: an update. Current Drug Targets. Inflammation and Allergy 2005;4(1):117‐24. [DOI] [PubMed] [Google Scholar]
Chae 2009
- Chae JJ, Aksentijevich I, Kastner DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. British Journal of Haematology 2009;146(5):467‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cronstein 2006
- Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Research and Therapy 2006;8 Suppl 1:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DGonbehalfoftheCSMG, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org.
Economides 2003
- Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler‐Stec EM, Hartnett C, et al. Cytokine traps: multi‐component, high‐affinity blockers of cytokine action. Nature Medicine 2003;9(1):47‐52. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Endnote X6 2012 [Computer program]
- Thomson Reuters. Endnote X6. Version 6.0. San Francisco: Thomson Reuters, 2012.
French FMF Consortium 1997
- French FMF Consortium. A candidate gene for familial Mediterranean fever. Nature Genetics 1997;17(1):25‐31. [DOI] [PubMed] [Google Scholar]
Goldfinger 1972
- Goldfinger SE. Colchicine for familial Mediterranean fever. The New England Journal of Medicine 1972;287(25):1302. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- The GRADE Working Group. GRADE Profiler 3.6 for Windows. http://tech.cochrane.org/revman/gradepro 2013.
Heilig 2018
- Heilig R, Broz P. Function and mechanism of the pyrin inflammasome. European Journal of Immunology 2018;48(2):230‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC on behalf of the CSMG and the CBMG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kiraz 1998
- Kiraz S, Ertenli I, Arici M, Calgüneri M, Haznedaroglu I, Celik I, et al. Effects of colchicine on inflammatory cytokines and selectins in familial Mediterranean fever. Clinical and Experimental Rheumatology 1998;16(6):721‐4. [PubMed] [Google Scholar]
Konstantopoulos 2003
- Konstantopoulos K, Kanta A, Deltas C, Atamian V, Mavrogianni D, Tzioufas AG, et al. Familial Mediterranean fever associated pyrin mutations in Greece. Annals of the Rheumatic Diseases 2003;62(5):479‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koné‐Paut 2011
- Koné‐Paut I, Hentgen V, Touitou I. Current data on familial Mediterranean fever. Joint Bone Spine 2011;78(2):111‐4. [DOI] [PubMed] [Google Scholar]
La Regina 2003
- Regina M, Nucera G, Diaco M, Procopio A, Gasbarrini G, Notarnicola C, et al. Familial Mediterranean fever is no longer a rare disease in Italy. European Journal of Human Genetics 2003;11(1):50‐6. [DOI] [PubMed] [Google Scholar]
Lidar 2006
- Lidar M, Yaqubov M, Zaks N, Ben‐Horin S, Langevitz P, Livneh A. The prodrome: a prominent yet overlooked pre‐attack manifestation of familial Mediterranean fever. Journal of Rheumatology 2006;33(6):1089‐92. [PubMed] [Google Scholar]
Livneh 1997
- Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis and Rheumatism 1997;40(10):1879‐85. [DOI] [PubMed] [Google Scholar]
Livneh 2000
- Livneh A, Langevitz P. Diagnostic and treatment concerns in familial Mediterranean fever. Baillière's Best Practice & Research. Clinical Rheumatology 2010;14(3):477‐98. [DOI] [PubMed] [Google Scholar]
Livneh 2006
- Livneh A. Amyloidosis of familial Mediterranean fever (FMF)‐‐insights to FMF phenotype II. Harefuah 2006;145(10):743‐5, 782. [PubMed] [Google Scholar]
Mansfield 2001
- Mansfield E, Chae JJ, Komarow HD, Brotz TM, Frucht DM, Aksentijevich I, et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood 2001;98(3):851‐9. [DOI] [PubMed] [Google Scholar]
Melikoğlu 2000
- Melikoğlu M, Ozdoğan H, Korkmaz C, Kasapçopur O, Arisoy N, Akkuş S, et al. A survey of phenotype II in familial Mediterranean fever. Annals of the Rheumatic Diseases 2000;59(11):910‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Migita 2012
- Migita K, Uehara R, Nakamura Y, Yasunami M, Tsuchiya‐Suzuki A, Yazaki M, et al. Familial Mediterranean fever in Japan. Medicine (Baltimore) 2012;91(6):337‐43. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Journal of Clinical Epidemiology 2009;62:1006‐12. [DOI] [PubMed] [Google Scholar]
Moher 2012
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2012;10(1):28‐55. [DOI] [PubMed] [Google Scholar]
Ozen 2011
- Ozen S, Bilginer Y, Aktay Ayaz N, Calguneri M. Anti‐interleukin 1 treatment for patients with familial Mediterranean fever resistant to colchicine. Journal of Rheumatology 2011;38(3):516‐8. [DOI] [PubMed] [Google Scholar]
Ozen 2016
- Ozen S, Demirkaya E, Erer B, Livneh A, Ben‐Chetrit E, Giancane G, et al. EULAR recommendations for the management of familial Mediterranean fever. Annals of the Rheumatic Diseases 2016;75(4):644‐51. [DOI] [PubMed] [Google Scholar]
Ozen 2017
- Ozen S, Batu ED, Demir S. Familial Mediterranean fever: recent developments in pathogenesis and new recommendations for management. Frontiers in Immunology 2017;8(253):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2016
- Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nature Immunology 2016;17(8):914‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sampaio 1991
- Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. Journal of Experimental Medicine 1991;173(3):699‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shohat 2011
- Shohat M, Halpern GJ. Familial Mediterranean fever‐‐a review. Genetics in Medicine 2011;13(6):487‐98. [DOI] [PubMed] [Google Scholar]
Soriano 2012
- Soriano A, Manna R. Familial Mediterranean fever: new phenotypes. Autoimmunity Reviews 2012;12(1):31‐7. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D on behalf of the CBMG, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Terkeltaub 2009
- Terkeltaub RA. Colchicine update: 2008. Seminars in Arthritis and Rheumatism 2009;38(6):411‐9. [DOI] [PubMed] [Google Scholar]
Terkeltaub 2013
- Terkeltaub RA, Schumacher HR, Carter JD, Baraf HS, Evans RR, Wang J, et al. Rilonacept in the treatment of acute gouty arthritis: a randomized, controlled clinical trial using indomethacin as the active comparator. Arthritis Research and Therapy 2013;15(1):R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
The International FMF Consortium 1997
- The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 1997;90(4):797‐807. [DOI] [PubMed] [Google Scholar]
WHO 2013
- WHO. 19th Expert Committee: Expert reviews. www.who.int/selection_medicines/committees/expert/19/reviews/en/index.html (accessed 13 June 2013).
Xu 2014
- Xu H, Yang J, Gao W, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 2014;513(7517):237‐41. [DOI] [PubMed] [Google Scholar]
Yalçinkaya 2009
- Yalçinkaya F, Ozen S, Ozçakar ZB, Aktay N, Cakar N, Düzova A, et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology (Oxford) 2009;48(4):395‐8. [DOI] [PubMed] [Google Scholar]
Özçakar 2012
- Özçakar ZB, Yüksel S, Ekim M, Yalçınkaya F. Infliximab therapy for familial Mediterranean fever‐related amyloidosis: case series with long term follow‐up. Clinical Rheumatology 2012;31(8):1267‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wu 2015
- Wu B, Xu T, Li Y, Yin X. Interventions for reducing inflammation in familial Mediterranean fever. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010893.pub2] [DOI] [PubMed] [Google Scholar]


