Abstract
Background
Eczema is a common chronic skin condition. Probiotics have been proposed as an effective treatment for eczema; their use is increasing, as numerous clinical trials are under way. This is an update of a Cochrane Review first published in 2008, which suggested that probiotics may not be an effective treatment for eczema but identified areas in which evidence was lacking.
Objectives
To assess the effects of probiotics for treating patients of all ages with eczema.
Search methods
We updated our searches of the following databases to January 2017: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, the Global Resource of Eczema Trials (GREAT) database, MEDLINE, Embase, PsycINFO, the Allied and Complementary Medicine Database (AMED), and Latin American Caribbean Health Sciences Literature (LILACS). We searched five trials registers and checked the reference lists of included studies and relevant reviews for further references to relevant randomised controlled trials (RCTs). We also handsearched a number of conference proceedings. We updated the searches of the main databases in January 2018 and of trials registries in March 2018, but we have not yet incorporated these results into the review.
Selection criteria
Randomised controlled trials of probiotics (live orally ingested micro‐organisms) compared with no treatment, placebo, or other active intervention with no probiotics for the treatment of eczema diagnosed by a doctor.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane. We recorded adverse events from the included studies and from a separate adverse events search conducted for the first review. We formally assessed reporting bias by preparing funnel plots, and we performed trial sequential analysis for the first primary outcome ‐ eczema symptoms at the end of active treatment.
We used GRADE to assess the quality of the evidence for each outcome (in italic font).
Main results
We included 39 randomised controlled trials involving 2599 randomised participants. We included participants of either gender, aged from the first year of life through to 55 years (only six studies assessed adults), who had mild to severe eczema. Trials were undertaken in primary and secondary healthcare settings, mainly in Europe or Asia. Duration of treatment ranged from four weeks to six months, and duration of follow‐up after end of treatment ranged from zero to 36 months. We selected no standard dose: researchers used a variety of doses and concentrations of probiotics. The probiotics used were bacteria of the Lactobacillus and Bifidobacteria species, which were taken alone or combined with other probiotics, and were given with or without prebiotics. Comparators were no treatment, placebo, and other treatments with no probiotics.
For all results described in this abstract, the comparator was no probiotics. Active treatment ranged from six weeks to three months for all of the following results, apart from the investigator‐rated eczema severity outcome, for which the upper limit of active treatment was 16 weeks. With regard to score, the higher the score, the more severe were the symptoms. All key results reported in this abstract were measured at the end of active treatment, except for adverse events, which were measured during the active treatment period.
Probiotics probably make little or no difference in participant‐ or parent‐rated symptoms of eczema (13 trials; 754 participants): symptom severity on a scale from 0 to 20 was 0.44 points lower after probiotic treatment (95% confidence interval (CI) ‐1.22 to 0.33; moderate‐quality evidence). Trial sequential analysis shows that target sample sizes of 258 and 456, which are necessary to demonstrate a minimum mean difference of ‐2 and ‐1.5, respectively, with 90% power, have been exceeded, suggesting that further trials with similar probiotic strains for this outcome at the end of active treatment may be futile.
We found no evidence suggesting that probiotics make a difference in QoL for patients with eczema (six studies; 552 participants; standardised mean difference (SMD) 0.03, 95% CI ‐0.36 to 0.42; low‐quality evidence) when measured by the participant or the parent using validated disease‐specific QoL instruments.
Probiotics may slightly reduce investigator‐rated eczema severity scores (24 trials; 1596 participants). On a scale of 0 to 103 for total Severity Scoring of Atopic Dermatitis (SCORAD), a score combining investigator‐rated eczema severity score and participant scoring for eczema symptoms of itch and sleep loss was 3.91 points lower after probiotic treatment than after no probiotic treatment (95% CI ‐5.86 to ‐1.96; low‐quality evidence). The minimum clinically important difference for SCORAD has been estimated to be 8.7 points.
We noted significant to extreme levels of unexplainable heterogeneity between the results of individual studies. We judged most studies to be at unclear risk of bias; six studies had high attrition bias, and nine were at low risk of bias overall.
We found no evidence to show that probiotics make a difference in the risk of adverse events during active treatment (risk ratio (RR) 1.54, 95% CI 0.90 to 2.63; seven trials; 402 participants; low‐quality evidence). Studies in our review that reported adverse effects described gastrointestinal symptoms.
Authors' conclusions
Evidence suggests that, compared with no probiotic, currently available probiotic strains probably make little or no difference in improving patient‐rated eczema symptoms. Probiotics may make little or no difference in QoL for people with eczema nor in investigator‐rated eczema severity score (combined with participant scoring for eczema symptoms of itch and sleep loss); for the latter, the observed effect was small and of uncertain clinical significance. Therefore, use of probiotics for the treatment of eczema is currently not evidence‐based. This update found no evidence of increased adverse effects with probiotic use during studies, but a separate adverse events search from the first review revealed that probiotic treatment carries a small risk of adverse events.
Results show significant, unexplainable heterogeneity between individual trial results. Only a small number of studies measured some outcomes.
Future studies should better measure QoL scores and adverse events, and should report on new probiotics. Researchers should also consider studying subgroups of patients (e.g. patients with atopy or food allergies, adults) and standardising doses/concentrations of probiotics given.
Keywords: Adolescent; Adult; Child; Child, Preschool; Female; Humans; Infant; Male; Middle Aged; Young Adult; Eczema; Eczema/therapy; Probiotics; Probiotics/therapeutic use; Randomized Controlled Trials as Topic; Symptom Assessment; Treatment Outcome
Plain language summary
Probiotics for treating eczema
Review question
This Cochrane Review aimed to find out, by analysing data from randomised controlled trials (RCTs), if probiotics (bacteria, fungi, or yeasts) are effective in treating eczema of any severity in people of all ages when compared with placebo (an identical but inactive treatment), no treatment, or another treatment that does not include probiotics. We wanted to find out if treatment with probiotics improves the symptoms, quality of life, or severity of eczema in patients at the end of active treatment and during follow‐up after the active treatment has finished.
Background
Eczema is an itchy, non‐contagious, inflamed skin condition that affects between 5% and 20% of people at some time in their life. People with eczema have different bacteria in their gut compared to people without eczema, and sometimes they have inflammation in their gut. It has been suggested that eczema symptoms may be treated by changing the mix of gut bacteria or by reducing inflammation in the gut. Probiotics, which are live micro‐organisms taken by mouth, such as the Lactobacillus bacteria found in unpasteurised milk and yoghurt, might achieve this.
This is an update of a previous Cochrane Review published in 2008; this update is important because more trials have been done since publication of the first review, and because use of probiotics is increasing and new treatments for eczema are needed.
Study characteristics
We included 39 randomised controlled clinical trials (RCTs) with 2599 participants, which we identified in searches up to January 2017.
These studies included people of either gender and of all ages, although most studies assessed children who had been told by a healthcare professional that they had eczema. Participants had eczema ranging from mild to severe, and RCTs compared treatment with live micro‐organisms (probiotics) of varying dose and concentration, taken by mouth, versus no treatment, placebo, or another treatment with no probiotics.
The probiotics included were bacteria of the Lactobacillus and Bifidobacteria species taken alone or in combination with other probiotics for a period ranging from four weeks up to six months. We did not look at studies that were seeking to prevent eczema. Most studies were done in Europe, and some were done in Asia, Australia, and New Zealand ‐ all in a medical setting. Most studies were conducted at a single centre. Reviewers applied no language restrictions on study selection. Ten studies were funded by companies supplying the probiotics, and another four studies did not declare the source of funding.
Key results
Please note that results in this summary are based on the following: a comparison of probiotic against no probiotic; treatment over six weeks to three months, except for the investigator‐rated eczema severity outcome, for which participants were treated longer (16 weeks); and outcomes measured at the end of the treatment period, apart from adverse events, which were assessed throughout treatment. Unless otherwise stated, outcomes were measured by participants or parents. The included studies assessed a variety of probiotics of differing concentrations or doses. With regard to score, the higher the score, the more severe were the symptoms.
We found that currently available probiotics probably make little or no difference in reducing eczema symptoms, such as itching and sleep loss (moderate‐quality evidence).
However, we did find that these probiotics may slightly reduce the severity of eczema scored by patients and their healthcare professionals in combination (low‐quality evidence), although it is uncertain if such a change is meaningful for patients.
In terms of patient quality of life, we found no evidence that probiotics make a difference (low‐quality evidence).
We found no evidence of an increase in adverse events; those reported in included studies that were related to treatment were tummy and gut upset with diarrhoea, constipation, vomiting, and colic pains (low‐quality evidence).
Analysis suggests that further probiotic studies assessing the effects of eczema symptoms may not be worthwhile, as they are unlikely to change the outcome at the end of active treatment.
Quality of the evidence
The quality of evidence supporting our key findings was low, apart from one moderate rating for participant‐rated symptoms of eczema. Reasons for this include variability between studies, which could not be explained, and not enough available data.
Summary of findings
for the main comparison.
| Comparison: probiotics vs no probiotics for treating eczema | ||||||
|
Patient or population: male and female patients 0 to 55 years of age with physician‐diagnosed eczema Settings: primary or secondary care. Europe: 22 studies with 1390 participants. Asia: 8 studies with 500 participants. Australasia: 2 studies with 116 participants Intervention: probiotics ± prebiotics Comparison: no probiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No probiotics | Probiotics | |||||
|
Primary outcome 1: participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of active treatment Visual analogue scale for itch and sleep disturbance ranging from 0 to 10 for each symptom and combined ranging from 0 to 20. The higher the score, the more severe the symptoms Duration of follow‐up from baseline until end of active treatment from 6 weeks to 3 months |
Mean SCORAD part C score ranged across control groups from 2 to 7.9 | Mean SCORAD part C score in the intervention groups was 0.44 points lower (1.22 lower to 0.33 higher) | ‐ | 754 (13) | ⊕⊕⊕⊝ moderatea | Two cross‐over studies included. Significant heterogeneity between studies Post hoc trial sequential analysis showed no effects of probiotics over control and suggests that further studies of currently available probiotic strains for this outcome may be futile |
|
Primary outcome 1: participant‐ or parent‐rated global change in eczema symptoms at the end of active treatment (binary outcome) Change in risk for worsened/unchanged eczema Duration of follow‐up from baseline until end of active treatment from 6 weeks to 3 months |
Low‐risk population | OR 0.40 (0.14 to 1.15) | 135 (3) | ⊕⊕⊝⊝ lowb | One cross‐over study included. Number of studies for this outcome was small. Moderate heterogeneity between studies | |
| 300 per 1000 | 146 per 1000 (57 to 330) | |||||
| Medium‐risk population | ||||||
| 400 per 1000 | 210 per 1000 (85 to 434) | |||||
| High‐risk population | ||||||
| 500 per 1000 | 286 per 1000 (123 to 535) | |||||
|
Primary outcome 2: participant‐ or parent‐rated participant quality of life score at the end of active treatment Scales used: DLQI, IDQoL, Skindex‐29, CDLQI. On those scales, the higher the score, the more severely the quality of life is affected Duration of follow‐up from baseline until end of active treatment from 8 weeks to 3 months |
Mean DLQI score ranged across control groups from 5.3 to 8.5 | Mean participant quality of life score in the intervention groups was 0.03 standard deviations higher (0.36 lower to 0.42 higher) | ‐ | 552 (6) |
⊕⊕⊝⊝ lowc | Small number of studies for this outcome. Significant heterogeneity |
|
Primary outcome 2: participant‐ or parent‐rated family quality of life score at the end of active treatment Scale used: DFI, FDLQI. On those scales, the higher the score, the more severely the quality of life is affected Duration of follow‐up from baseline until end of active treatment from 8 weeks to 3 months |
Mean change in DFI score during treatment ranged across control groups from ‐2 points to ‐3 points | Mean family quality of life score in the intervention groups was 0.19 standard deviations lower (0.56 lower to 0.18 higher) | ‐ | 358 (3) | ⊕⊝⊝⊝ very lowd | Very small number of studies for this outcome. Significant heterogeneity |
|
Secondary outcome 4: global eczema severity score (total SCORAD) at the end of active treatment (Investigator‐rated eczema severity) Scale used: total SCORAD ranging from 0 to 103. The higher the score, the more severe the disease Duration of follow‐up from baseline until end of active treatment from 8 weeks to 16 weeks |
Mean total SCORAD ranged across control groups from 8.5 to 40.21 points | Mean total SCORAD score in the intervention groups was 3.91 points lower (5.86 to 1.96 points lower) | ‐ | 1596 (24) | ⊕⊕⊝⊝ lowe | Two cross‐over studies included. Extreme levels of heterogeneity for this outcome. Evidence of reporting bias |
|
Secondary outcome 6: adverse events (gastrointestinal symptoms) during active treatment Duration of follow‐up from baseline until end of active treatment from 8 weeks to 3 months |
Low‐risk population | RR 1.54 (0.90 to 2.63) | 402 (7) | ⊕⊕⊝⊝ lowf | Small number of studies reported adverse events. Small number of events were included in this analysis | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Medium‐risk population | ||||||
| 100 per 1000 | 154 per 1000 (90 to 263) | |||||
| High‐risk population | ||||||
| 200 per 1000 | 308 per 1000 (180 to 526) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDLQI: Children's Dermatology Life Quality Index; CI: confidence interval; DFI: Dermatitis Family Impact; DLQI: Dermatology Life Quality Index; FDLQI: Family Dermatology Life Quality Index; IDQoL: Infant Dermatitis Quality of Life; OR: odds ratio; RR: risk ratio; SCORAD: Severity Scoring of Atopic Dermatitis. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level due to inconsistency as there was significant heterogeneity among studies (I² = 57%).
bDowngraded by two levels due to small number of studies for this outcome (imprecision) and because of moderate levels of heterogeneity among studies (I² = 48%).
cDowngraded by two levels due to small number of studies for this outcome (imprecision) and because of significant levels of heterogeneity among studies (I² = 68%).
dDowngraded by three levels due to inconsistency (one level) as there was significant heterogeneity among studies (I² = 57%) and because of very small number of studies (imprecision) for this outcome (two levels).
eDowngraded by two levels because of extreme levels of heterogeneity among studies (I² = 79%) and because of evidence of reporting bias.
fDowngraded by two levels because of small number of studies reporting adverse events and small number of events in the meta‐analysis for this outcome (imprecision).
Background
Description of the condition
Disease definition
Eczema is a non‐infective chronic inflammatory skin disease characterised by an itchy and usually red rash. The terms 'eczema' and 'dermatitis' have been used synonymously, and eczema is associated with atopy. 'Atopy' is defined as a genetic predisposition to become sensitised and produce immunoglobulin (Ig)E antibodies in response to ordinary exposure to allergens (Johansson 2004). Despite the association between eczema and atopy, up to 40% of children with eczema do not have atopy when defined according to allergy tests such as skin prick tests (Bohme 2001; Flohr 2004). A revised nomenclature for allergy provided in Johansson 2001 has been updated by the World Allergy Organization (Johansson 2004). The new nomenclature is based on the mechanisms that initiate and mediate allergic reactions. The term 'eczema' is proposed to replace the previous term 'atopic eczema/dermatitis syndrome'. What was termed atopic eczema dermatitis syndrome in 2001 is now thought to be not one single disease, but rather an aggregation of several diseases with certain characteristics in common. The term 'atopy' cannot be used until IgE sensitisation has been confirmed by IgE antibodies in the blood or by a positive skin prick test (SPT) to common environmental or dietary allergens such as pollen, house dust mite, cow's milk, or egg. If this is done, the term 'eczema' can be split into 'atopic eczema' and 'non‐atopic eczema'.
For the purpose of this review, we will use the term 'eczema' and will include eczema when IgE sensitisation has been confirmed, eczema when IgE sensitisation is absent, and eczema when IgE sensitisation has not been assessed.
Epidemiology and causes
Eczema is the most common inflammatory skin disease of childhood, affecting 5% to 20% of children at any one time (Nankervis 2016; Williams 1999). The International Study of Asthma and Allergies in Childhood (ISAAC) Phase III revealed the prevalence of current eczema for children six to seven years of age as ranging from 0.9% in India to 22.5% in Equador, and for adolescents 13 to 14 years of age, from 0.2% in China to 24.6% in Columbia (Odhiambo 2009). The same study showed prevalence of symptoms of severe eczema ranging from 0.0% to 4.9% for children aged six to seven years, and from 0.0% to 5.8% for adolescents aged 13 to 14 years. Around 2% of adults have eczema, and many of them have a more chronic and severe form (Charman 2002). One‐year prevalence of eczema in adults in the United States was estimated to be 10.2% (Silverberg 2013). Eczema is often associated with other atopic diseases such as asthma, allergic rhinitis, or food allergies (Beck 2000), and sufferers often have a family history of allergic disease. Wide variation in the prevalence of eczema has been noted between different countries, and studies suggest that prevalence is increasing in developing countries (Odhiambo 2009; Williams 2008).
The cause of eczema is not clearly understood. The finding that loss‐of‐function variants of the skin barrier protein filaggrin are a predisposing factor for atopic dermatitis in Western Europeans was an important one in research on the etiopathogenesis of atopic dermatitis (Palmer 2006). The same or other variants were found in other populations such as Japanese ‐ reported in Enomoto 2008 and Nemoto‐Hasebe 2009 ‐ and Han Chinese ‐ reported in Zhang 2011. The pathogenesis of eczema is complex and involves a combination of factors: skin barrier defects, innate and adaptive immunity, and exposure to environmental allergens and microbes (Bieber 2008). The innate and adaptive immune system products have an effect on major proteins of the epidermal barrier function and on defence against pathogens (Malik 2017). Research has also pointed to the possible role of gut microbes (Abrahamsson 2012; Bjorksten 2001; Ismail 2012; Song 2016; Watanabe 2003).
Clinical features
Eczema is an itchy, chronic, non‐contagious, and relapsing condition. In infancy, it is predominantly localised on the face, in the nappy area, and on extensor surfaces of the knees and elbows; in childhood, it involves mainly the flexures, the face, and the neck and continues similarly in adulthood. It can be generalised. Involvement of the hands and feet is more common in adulthood. In infancy, the rash of eczema consists of red, edematous papules and vesicles, and it later shows erythematous patches with papules, vesicles, exudate, crusting, lichenification, and hyperpigmentation or hypopigmentation depending on the skin type. It can be complicated by bacterial and viral infections and lymphadenopathy. The severity of eczema is variable, ranging from localised mild dryness and redness with little impact on quality of life to generalised involvement with severe limitation of everyday activity and sleep loss. Itch is the predominant symptom; it can be exacerbated by warmth, sweating, bathing, exercise, woollen clothes, and emotional upset (Rook 2016).
Natural history
For 45% of patients, eczema starts within the first six months of life, and by one and five years, 60% and 85%, respectively, of those likely to develop it will have done so. Up to 70% of these cases will have spontaneous remission before adolescence (Bieber 2008). Emerging evidence suggests that eczema may have similar prevalence in adolescence and early adulthood as in childhood (Abuabara 2018).
Impact
Eczema varies in severity, which can be measured in several ways. A systematic review of instruments measuring signs of eczema included 16 different scales used in validation studies. Two of them ‐ the Eczema Area and Severity Index (EASI) and the Severity Scoring of Atopic Dermatitis (SCORAD) ‐ are considered the best for assessing severity of signs of atopic dermatitis based on validity, responsiveness, internal consistency, interobserver and intraobserver reliability, interpretability, and feasibility (Schmitt 2013). The HOME initiative (Harmonizing Outcome Measures for Eczema) comprises an international group that is working to reach agreement on core outcome measures that should be reported in all clinical trials for eczema. Its goal is to enable comparison of data across trials for eczema (www.homeforeczema.org).
The intense itch and scratching can lead to severe sleep disturbance in children and adults with eczema, resulting in tiredness and lack of concentration. Sleep loss, as well as systemic inflammation and impaired quality of life, may contribute to mental health disorders associated with eczema, such as depression and attention deficit/hyperactivity disorder (Silverberg 2017). A study comparing the effect on quality of life of children with chronic skin disease shows that for children and parents, atopic dermatitis caused the greatest impairment, scoring worse than chronic diseases such as epilepsy, enuresis, and diabetes (Beattie 2006). Eczema has a significant impact on the quality of life of family or parents of the patient. Sleep loss, time spent caring for the patient, and time taken off work to look after the affected child have a significant impact on the quality of life and finances of the parents and family of the patient with eczema (Lewis‐Jones 2006).
Eczema also brings considerable costs to the community as a whole. For example, the cost of childhood eczema to the Australian community was estimated at AUD316.7 million (USD239.3 million; Euro195.9 million) per year in 1999 (Kemp 1999). In the United States, the estimated national cost of atopic dermatitis ranged between USD364 million and USD3.8 billion (Mancini 2008). The healthcare costs of eczema in adults are comparable to those of epilepsy, emphysema, and other chronic diseases (Ellis 2002). Direct costs to the family are incurred for purchasing treatments, special clothing, and bedding, and for extra laundry expenses; indirect costs are associated with lost working days when parents are looking after an unwell child. The wider economic implications are seen in the costs of healthcare professionals; the lost opportunities for parents of sick children who do not have the option of seeking employment; and employment limitations faced by the child as a result of missed schooling.
Description of the intervention
No cure is currently known for eczema; however, a wide range of treatments are available to control and reduce the symptoms (Fennessy 2000; Lamb 2002; Nankervis 2016). Health professionals assist people in management of their disease through a variety of treatment methods, including emollients, topical steroids, topical tars, and topical tacrolimus and pimecrolimus. Other treatments such as wet wrap dressings, phototherapy, avoidance of triggers such as food allergens, and complementary therapies are also used (Ernst 2000). Many treatments are of unknown effectiveness (Nankervis 2016). Emollients, topical corticosteroids, and topical calcineurin inhibitors are universally recommended (Nankervis 2016; Smethurst 2002). With deeper knowledge of the immunopathogenesis of atopic dermatitis, new treatments have emerged such as dupilumab, an interleukin (IL)‐4 receptor alpha subunit inhibitor, and inhibitors of the phosphodiesterase enzyme (Eichenfield 2017). Treatment regimens can be time‐consuming and expensive for patients and their families, and new treatments that are effective, cheap, and simple to administer are needed.
Probiotics are live micro‐organisms (e.g. Lactobacillus species) that when administered in adequate amounts confer a health benefit on the host (FAO/WHO 2002). Minimum requirements for probiotic status have been suggested to include assessment of strain identity, in vitro tests to screen potential probiotics, assessment of safety, and in vivo studies for substantiation of effects (Pineiro 2007). Micro‐organisms considered probiotics that are used in food and pharmaceutical preparations are predominantly lactic acid bacteria, and of those, mainly Lactobacillus and Bifidobacteria species, but also non‐lactic acid bacteria such as Saccharomyces boulardii (Holzapfel 2001). Probiotics are not the same as prebiotics, which are non‐digestible sugars found in some foods that encourage the growth of certain types of bacteria in the intestine.
How the intervention might work
Rationale for using probiotics to treat eczema
The intestinal microflora (or intestinal microbiota) is a large collection of micro‐organisms that live in the human intestine and confer intestinal, immune, and nutritional benefits on the host. The composition of the intestinal microflora has been found to be different in those with eczema, and such differences may precede the development of active eczema. One consistent finding in relevant studies is a reduced proportion of Bifidobacteria species in the faeces of infants with eczema (Bjorksten 2001; Kalliomaki 2001; Murray 2005), as well as in older children and young adults with atopic dermatitis (Watanabe 2003). In the latter study, lower numbers of Bifidobacteria species also correlated with greater severity of the disease. Later studies have shown that low microbial diversity in the neonatal period is associated with the development of eczema in the first year of life (Ismail 2012), eczema with atopy in the first two years of life (Abrahamsson 2012), and IgE‐associated eczema in the first 18 months (Wang 2008). Another study found that patients with atopic dermatitis had increased numbers of Faecalibacterium prausnitzii associated with low levels of short‐chain fatty acids, possibly leading to aberrant T‐helper type 2 cell (Th2) responses (Song 2016).
An intervention that has been proposed to influence the gut microbiome is the use of probiotics (Simonyte Sjödin 2016). Probiotics may alter the intestinal microbiota of people with eczema and may improve symptoms and signs of eczema. They are effective treatments for some gastrointestinal disorders characterised by a disturbed intestinal microbiota, such as diarrhoea (Guarino 2015). Some evidence suggests that they may prevent the development of eczema when given during pregnancy or in infancy (Dang 2013; Doege 2012; Mansfield 2014; Zhu 2010). Their precise mode of action is not well established. Current research is focused on the immunomodulatory effects of probiotics. Evidence indicates that several probiotic species stimulate regulatory T cells, which produce IL‐10 and tumour growth factor (TGF)‐β, and control T‐helper type 1 cell (Th1) and inhibition of Th2 responses (Vitaliti 2014). Th2 responses are particularly predominant in acute eczema and are increased in chronic eczema (Malik 2017).
Probiotics are widely consumed worldwide in the form of fermented milk, and they are potentially a cheap and accessible treatment for eczema. Although all probiotics have certain properties in common (low pathogenicity, resistance to gastric acid and bile salt digestion, and adherence to intestinal mucosa), the clinical and laboratory effects of probiotics can vary markedly between species (Allen 2003; Christensen 2002).
Why it is important to do this review
Probiotics have been marketed in infant formula and are recommended by some practitioners for treatment of eczema. They are increasingly used by consumers for treatment and prevention of a range of disorders, and they have been formally investigated in several clinical trials for treatment of eczema. Their role in treating eczema is nevertheless controversial (Williams 2005), and the first Cochrane Review on probiotics for treating eczema suggested that probiotics may not be an effective treatment for eczema but identified areas in which evidence was lacking (Boyle 2008). Moreover, reports suggest that probiotics can occasionally cause serious adverse effects (Besselink 2008; De Groote 2005; Hennequin 2000; Land 2005). It is therefore important to formally reassess the evidence for the efficacy of probiotics in treating eczema. Since the first Cochrane Review on probiotics for treating eczema was published (Boyle 2008), clinical trials have continued to investigate the use of probiotics for treating eczema, and more data are now available to assess their efficacy. The first review included trials conducted only in children and mainly in Europe; data are now available from trials conducted in adults and in Asian countries.
The rationale for this review comprises the following.
Eczema is a common disease with a negative impact on the individual, the family, and the community.
New treatments for eczema are needed.
Probiotics are increasingly used for treatment of eczema.
Cases of probiotic sepsis have been reported.
New clinical trials have been completed since publication of the first Cochrane Review on probiotics for treating eczema, and it is necessary to reassess the evidence on use of probiotics for eczema treatment.
Plans for this review were published as a protocol (Boyle 2006a), This Cochrane Review is an update of Boyle 2008.
Objectives
To assess the effects of probiotics for treating patients of all ages with eczema.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of probiotics for the treatment of eczema.
Types of participants
We included participants of any age or gender with eczema diagnosed by a doctor. We did not include participants with other specific forms of eczema such as contact eczema.
The National Health Service Technology Assessment systematic review of treatments for eczema used specific terms to identify trial participants (Hoare 2000). We used a modified classification of these terms as listed in Table 2. This list classifies forms of eczema included in this review and specific forms of eczema not included in this review. One of the review authors (RB) scrutinized identified studies that used terms in the 'possible atopic eczema' category (such as 'childhood eczema') and included the study only if the description of participants indicated the absence of such specific forms as 'allergic contact eczema'.
1. Terms used to categorise trial participants with eczema.
| Forms of eczema included | Forms of eczema excluded |
| Atopic eczema | Seborrhoeic eczema |
| Atopic dermatitis | Contact eczema |
| Besnier's prurigo | Allergic contact eczema |
| Neurodermatitis atopica (German) | Irritant contact eczema |
| Flexural eczema/dermatitis | Discoid/nummular eczema |
| Periorbital eczema | Asteatotic eczema |
| Childhood eczema | Varicose/stasis eczema |
| Infantile eczema | Photo‐/light‐sensitive eczema |
| 'Eczema' unspecified | Chronic actinic dermatitis |
| Constitutional eczema | Dyshidrotic eczema |
| Endogenous eczema | Pompholyx eczema |
| Chronic eczema | Hand eczema |
| Neurodermatitis | Frictional lichenoid dermatitis |
| Neurodermatitis (German) | Lichen simplex |
| Occupational dermatitis | |
| Prurigo |
Types of interventions
We included interventions involving ingested live micro‐organisms, including bacteria, fungi, or yeasts, ingested singly or in combination. We placed no restrictions on the duration of the intervention.
Comparators could consist of no treatment, placebo, or another active intervention with no probiotics. We excluded studies using other micro‐organisms or microbial products as the sole comparator. We did not exclude from this review studies that included an adjunct to the active treatment (such as antibiotics, other dietary management (e.g. allergen avoidance, prebiotic supplementation), or standard eczema treatments such as topical corticosteroids).
Types of outcome measures
Primary outcomes
Changes in participant‐rated, parent‐rated, or principal carer‐rated symptoms of eczema at the end of active treatment
Changes in quality of life at the end of active treatment
Secondary outcomes
Changes in participant‐rated, parent‐rated, or principal carer‐rated symptoms of eczema during the six‐month period after active treatment has ceased
Changes in quality of life within the six‐month period after active treatment has ceased
Changes in the need for other eczema treatment during active treatment or within the six‐month period after active treatment has ceased
-
Investigator‐rated eczema severity
Changes in global eczema severity as measured by a trained investigator or a medical practitioner at the end of active treatment
Changes in global eczema severity or change in the number of eczema flares as measured by participants, parents, principal carers, or a medical practitioner in the six‐month period after active treatment has ceased
Changes in the number of days lost from school or work due to eczema symptoms during active treatment
Adverse events during the active treatment period
For the above outcome measures:
parent‐rated or principal carer‐rated measurements of eczema symptoms and quality of life questionnaires refer to outcomes reported by the parent or the principal carer when the patient could not complete the scores (e.g. because the patient is an infant or a small child);
when available, we used changes in participant‐rated, parent‐rated, or principal carer‐rated global eczema severity in preference to assessments of specific eczema symptoms;
we assessed quality of life changes as measured by participants, their parent, or their principal carer on a published scale (e.g. Chren 1997; Finlay 1996); and
in addition to assessment of global symptom or disease severity, when available, we assessed changes in a composite rating scale using a published named scale (e.g. Severity Scoring of Atopic Dermatitis (SCORAD) (Kunz 1997)). When this was not available, we attempted to assess trial authors' modification of such a scale, or their own composite rating scale.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised all our search strategies in keeping with current Cochrane Skin practices. We have provided details of the previous search strategies in Boyle 2008. This review fully incorporates the results of searches conducted up to 26 January 2017.
Cochrane Skin Group Specialised Register, using the search strategy in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11), in the Cochrane Library, using the strategy presented in Appendix 2.
Global Resource of EczemA Trials (GREAT) database (Centre of Evidence Based Dermatology; accessed at www.greatdatabase.org.uk), using the browse function → Dietary interventions → Probiotics.
MEDLINE via Ovid (from 1946), using the strategy provided in Appendix 3.
Embase via Ovid (from 1974), using the strategy delineated in Appendix 4.
PsycINFO via Ovid (from 1806), using the strategy shown in Appendix 5.
Allied and Complementary Medicine Database (AMED) via Ovid (from 1985), using the strategy described in Appendix 6.
Latin American and Caribbean Health Science Information database (LILACS) (from 1982), using the strategy presented in Appendix 7.
We identified three additional reports of relevant trials through an update search conducted on 30 January 2018. We have added those three results to Studies awaiting classification and will incorporate them into the review at the next update.
Trials registers
We searched the following trials registers up to 10 March 2018, using the terms "eczema", "probiotic", and "probiotics".
International Standard Randomized Controlled Trials Number (ISRCTN) registry (www.isrctn.com).
ClinicalTrials.gov (www.clinicaltrials.gov).
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch).
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References lists
We checked the bibliographies of included studies and some reviews for further references to relevant RCTs.
Unpublished literature
When possible, we contacted trial authors and investigators for further information regarding the nature and status of identified studies.
Adverse events
We did not perform a separate search for adverse effects of probiotics for this update, but review authors conducted such a search for the first publication of this review. For this update, we considered adverse effects described in the included RCTs, and we reported the findings from the original additional adverse events search.
Handsearching
For this update, we handsearched the following conference proceedings.
European Academy of Allergology and Clinical Immunology Annual Meeting 2013 and 2014.
American Academy of Asthma, Allergy and Immunology Annual Meeting 2013 and 2014.
American Association of Immunologists Annual Meeting 2013, 2014, and 2015.
International Congress of Immunology 2013.
American Association of Dermatologists Annual Meeting 2013 and 2014.
International Investigative Dermatology Congress 2013.
International Symposium for Atopic Dermatitis 2014.
Data collection and analysis
Selection of studies
Two review authors (RB and AM) independently checked titles and abstracts identified through the searches. We excluded studies that did not refer to a randomised controlled trial of orally ingested probiotics for treating eczema. The same two review authors (RB and AM) independently assessed each study to determine whether it met the predefined selection criteria. When necessary, we contacted the authors of studies in deciding their eligibility for inclusion in the review. No major differences of opinion arose between the review authors. One study was published in Russian (Ivankhnenko 2013), one in Chinese (Guo 2015), and another in Polish (Cukrowska 2008). We assessed these studies after translation.
Data extraction and management
Two review authors (RB and AM) independently extracted study data. No major differences of opinion arose, and it did not prove necessary for a third review author to arbitrate over data extraction. We contacted trial authors for all included studies, some excluded studies, and ongoing studies by email or by post to obtain complete data sets.
We piloted a data collection form and used this information to summarise the trials. Two review authors (JL and AM) checked and entered the data. When complete data sets were available from trial authors, we used these data to calculate summary statistics such as mean and standard deviation before performing data entry.
Assessment of risk of bias in included studies
Two review authors (RB and AM) independently assessed studies for risk of bias using the Cochrane 'Risk of bias' tool (Chapter 8.5, in Higgins 2011), rating them as having 'low', 'unclear', or 'high' risk of bias. No major differences of opinion arose, and it was not necessary for a third review author to arbitrate over risk of bias assessment. Assessment of risk of bias included the following.
Method of generation of the randomisation sequence (selection bias): considered low risk of bias if the randomisation sequence resulted in unbiased allocation to any of the study groups by investigators and to comparable study groups.
Method of allocation concealment (selection bias): considered 'low risk' if it was clear from publications or correspondence with trial authors that the treatment assignment of each consecutive study participant could not be anticipated by investigators. For example, if treatment allocation was done by a third party such as a pharmacy department, we considered allocation concealment to have low risk of bias.
Blinding of participants and personnel (performance bias): judged as low risk if we found adequate information to ensure that study personnel and participants could not have knowledge of the allocated intervention.
Blinding of outcome assessor (detection bias): judged as low risk if we found adequate information to exclude knowledge of the allocated intervention by outcome assessors.
Incomplete outcome data (attrition bias): considered rates of loss to follow‐up in total and in each study group, along with reasons for these, and whether participants were analysed in the groups to which they were originally randomised (available case analysis), whether any participants were excluded after randomisation, and whether data were imputed for participants lost to follow‐up. We judged low risk of bias when data were missing and reasons for missing data could not have a clinically relevant impact on the effect size.
Selective reporting: considered low risk when all predefined outcomes of the study have been reported.
Other bias: considered low risk if we could detect no other sources of bias.
We defined studies with overall low risk of bias as studies when the randomisation process was clear; allocation concealment was clear and done; participants, clinicians, or outcome assessors were blinded; and we detected no attrition bias
Quality assessment
We also assessed factors contributing to the quality of the included trials.
Whether or not study aims, interventions (including doses of viable probiotic used, mode of administration, and duration of treatment), and outcome measures were clearly defined.
Whether treatment compliance was assessed.
Whether non‐study probiotics were adequately excluded from participants' diets.
Measures of treatment effect
We calculated a weighted pooled treatment effect across studies using a random‐effects model.
For dichotomous outcomes, we expressed the results as risk ratios (RRs) and 95% confidence intervals (CIs) for analyses containing only parallel‐group trials, and we used odds ratios (ORs) when we included in the meta‐analysis data from both cross‐over and parallel‐group studies, because the method used for combining parallel‐group and cross‐over study findings in meta‐analysis did not allow findings to be expressed as RRs (Elbourne 2002). For analyses that included both cross‐over and parallel‐group studies, we combined conditional (paired) ORs from cross‐over studies with ORs from parallel‐group studies to estimate pooled ORs. We used conditional ORs because they can be used to pool data from cross‐over studies with data from parallel‐group studies (Duffy 1989).
We used mean differences (MDs) and 95% CIs or standardised mean differences (SMDs) and 95% CIs to express results for continuous outcomes. When studies reported participant‐ or investigator‐rated symptoms on categorical scales (e.g. Passeron 2006), we made the data dichotomous by defining a cutoff at good improvement in eczema versus mild improvement, no change, or worsening of eczema.
Trial sequential analysis
For this review update, we used post hoc retrospective trial sequential analysis (TSA) for our first primary outcome.
Meta‐analyses carry risk of type I errors (false significant results) due to limited data from few and small trials and repetitive testing on updates as data from new trials accumulate (Brok 2008; Wetterslev 2008). TSA is a method that quantifies the statistical reliability of data within a meta‐analysis (Brok 2009; Wetterslev 2009). We estimated information size (IS, i.e. the least number of participants needed for a statistically significant result) based on the mean difference derived through clinical consensus, using a two‐sided 5% significance level and 90% power, and we diversity‐adjusted the data to reflect the quantity of heterogeneity by performing a random‐effects meta‐analysis. For estimation of the mean in the control group, which is a necessary step during TSA, we pooled control event rates for any low risk of bias trials contributing to the relevant meta‐analysis. In TSA, when the cumulative z‐curve crosses the trial sequential monitoring boundary, sufficient evidence of an association can be concluded and no further trials are needed. However, if the cumulative z‐curve does not cross the boundary and the IS is not reached, evidence is insufficient to reach a conclusion and further trials are required.
Other Cochrane groups have used TSA in their reviews (e.g. Allingstrup 2016 ‐ Cochrane Anaesthesia, Critical and Emergency Care Group). We used post hoc TSA for our first primary outcome ‐ changes in participant‐, parent‐, or principal carer‐rated symptoms of eczema at the end of treatment (TSA software).
Unit of analysis issues
We followed guidance from the Cochrane Handbook for Systematic Reviews of Interventions in addressing unit of analysis issues (Chapters 9 and 16, in Higgins 2011).
Cross‐over trials
When possible, we initially analysed cross‐over trials using appropriate paired analyses to estimate paired MDs (continuous outcomes) and paired ORs (dichotomous outcomes) with standard errors. We then combined outcome data from cross‐over trials and parallel‐group trials using the generic inverse variance method. We also analysed data from parallel‐group trials and cross‐over trials as separate subgroups, because cross‐over studies may not be appropriate for probiotic studies, as the duration of treatment effect is not well established.
Studies with multiple treatment groups
When studies reported more than one active intervention arm, we combined the two active interventions and analysed them together. We also analysed data from these studies in a separate stratified analysis to assess the effects of different strains of the probiotics.
Trials reporting non‐parametric statistics
When trials reported non‐parametric summary statistics, we attempted to convert data to parametric summary statistics by assuming that the reported median was the mean, and we estimated the standard error as interquartile range (IQR)/1.35 (Chapter 7.7.3.5, in Higgins 2011); however, we acknowledge that these are strong assumptions because many of the included trials did not include large sample sizes. Therefore we have added cautionary notes when we believe the impact of these assumptions could have strongly influenced the overall findings of the meta‐analysis. When non‐parametric statistics could not be converted to parametric statistics, we presented the data in an additional table (Table 3).
2. Non‐parametric analyses of SCORAD scores.
| Isolauri 2mo LGG | Isolauri 2mo Bb12 | Isolauri 2mo placebo | |
| N | 9 | 9 | 9 |
| Median | 1 | 0 | 13.4 |
| IQR | 0.1 to 8.7 | 0 to 3.8 | 4.5 to 18.2 |
IQR: interquartile range. SCORAD: Severity Scoring of Atopic Dermatitis.
Dealing with missing data
We assessed pooled data using available case analysis rather than intention‐to‐treat analysis with imputation. When the nature of missing data was not clear, we contacted study authors for clarification. When studies failed to report summary statistics such as standard deviations, we contacted trial authors for further information.
Assessment of heterogeneity
We assessed statistical heterogeneity using I². When we found substantial statistical heterogeneity between studies (I² > 50%), we explored possible reasons for this heterogeneity, including participant factors such as disease severity, treatment factors such as probiotic strain or dose, and study factors such as methodological quality criteria as described above. When we detected extreme levels of statistical heterogeneity between trials (e.g. I² > 85%), we considered whether it was appropriate to pool studies by considering their clinical and methodological differences.
Assessment of reporting biases
We performed formal assessment of reporting bias using a funnel plot for continuous outcomes when the number of studies with data available for inclusion in primary analyses was greater than 10, and we performed statistical assessment using Egger's test.
Data synthesis
When studies employed different tools to measure the same outcome, we calculated a pooled estimate of effect across studies using standardised mean differences (SMDs) and 95% CIs. When it was not possible to perform a meta‐analysis, we described the findings narratively.
Subgroup analysis and investigation of heterogeneity
We planned the following stratified analyses for this review.
Analysis by age (under 2 years vs 2 to 12 years vs over 12 years).
Concurrent treatment with antibiotics versus no concurrent treatment with antibiotics.
Atopic versus non‐atopic study participants, with atopy defined as at least one positive skin prick test (SPT) or radioallergosorbent test (RAST) to a common allergen.
Participants with a formally diagnosed (i.e. double‐blind placebo‐controlled food challenge) food allergy versus those without a formally diagnosed food allergy.
Participants with evidence of intestinal inflammation versus those without such evidence.
Participants with mild eczema (SCORAD < 15) versus moderate eczema (SCORAD 15 to 40) versus severe eczema (SCORAD > 40) at baseline.
We performed stratified analysis rather than subgroup analysis for the following reasons.
Some strata included small numbers of studies.
When differences were present, they were clearer to the observer.
Subgroup analysis assumes a fixed‐effect model, and the high heterogeneity seen even with subgroups suggests that this is not appropriate.
This approach is consistent with the approach used in the previous version of this review.
Use of multiple stratified analyses means that interpretation of 'significant' subgroup tests would be problematic due to the risk of chance spurious findings.
Several of the stratified analyses included more than one group with the same participant count, for example, in the 'any Lactobacillus species' group and in the 'other specific Lactobacillus species' groups.
Sensitivity analysis
When appropriate, we performed sensitivity analyses to examine the effects of excluding poor quality studies, defined as studies for which the randomisation process is unclear; allocation concealment is not clear or was not done; participants, clinicians, or outcome assessors were not blinded; no intention‐to‐treat analysis was performed; or risk of attrition bias is high.
We also performed, when appropriate, sensitivity analyses based on changes in scores from baseline to end of treatment to examine the effects of studies with baseline differences in eczema severity.
Assessment of quality of evidence
We applied the GRADE approach for the main comparisons to rate the quality of evidence for the prespecified outcomes included in Table 1 (Atkins 2004). We selected our primary outcomes; the secondary outcome 'Changes in global eczema severity as measured by a trained investigator or a medical practitioner at the end of active treatment'; and adverse events for inclusion in the Table 1.
Other
For this update, our consumer co‐author (AR) contributed to enhance the readability and clarity of the completed review.
Results
Description of studies
Results of the search
We updated the Electronic searches and fully incorporated the results to 26 January 2017. We identified 477 records from eight databases, five trials registers, and other sources. After removing duplicates, we (AM and RB) screened 472 records. We excluded 423 based on titles and abstracts, leaving a total of 49. Of these, six are ongoing studies (see Characteristics of ongoing studies), and five studies are awaiting classification (see Characteristics of studies awaiting classification).
We screened the remaining 38 records in full text when available. We excluded 11 records (see Characteristics of excluded studies). Combined with the eight studies excluded from the previous version of this review, the total of excluded studies is 19. We included 27 new studies. We identified 12 included studies in the earlier version of this review, for a total of 39 included studies overall (see Characteristics of included studies).
We identified three of the studies awaiting classification through an update search conducted on 30 January 2018 (Hulshof 2017; NCT02585986; Prakoeswa 2017). We have not fully assessed these, and we will incorporate them into the review at the next update.
We have presented the study flow diagram in Figure 1.
1.
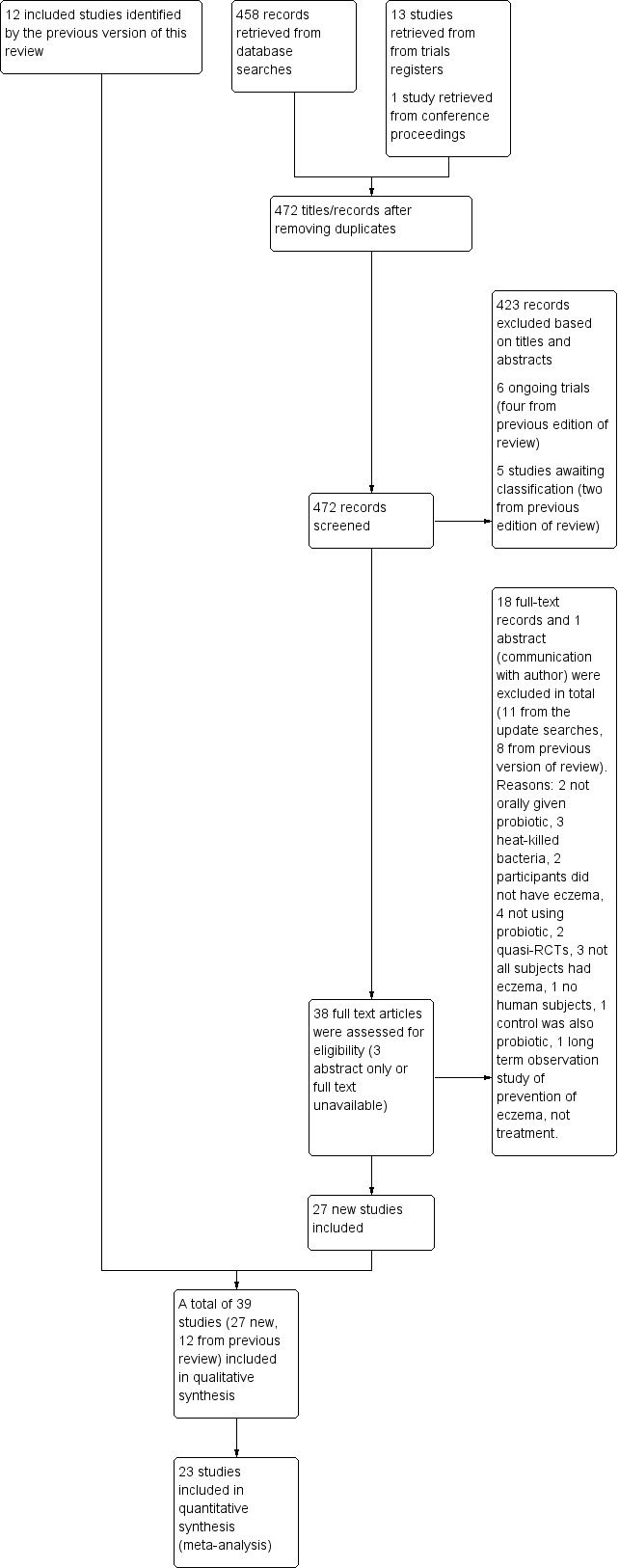
Study flow diagram.
Included studies
We included 39 studies with 2599 participants (12 studies with 781 participants from the first review, and 27 new studies with 1818 participants identified for this update) and have described all studies in the Characteristics of included studies section.
The authors of seven studies supplied complete data sets (Brouwer 2006; Goebel 2010; Han 2012; Passeron 2006; Rosenfeldt 2003; Sistek 2006; Weston 2005). The authors of five studies supplied summary data (Drago 2014; Flinterman 2007; Nermes 2010; Roessler 2007; Viljanen 2005). The authors of four studies responded to our requests by clarifying some questions relevant to their studies but did not provide additional data (Drago 2012; Iemoli 2012; Van der Aa 2010; Wang 2015). We received no response to requests for information from the authors of 17 studies (Cukrowska 2008; Farid 2011; Folster‐Holst 2006; Gruber 2007; Guo 2015; Isolauri 2000; Ivankhnenko 2013; Lin 2015; Majamaa 1997; Matsumoto 2014; Shafiei 2011; Taniuchi 2005; Woo 2010; Wu 2012; Wu 2015; Yang 2014; Yesilova 2012). The authors of one study responded that they were unable to supply their data for meta‐analysis (Kirjavainen 2003). We could not contact the authors of four studies (Gerasimov 2010; Hol 2008: invalid contact details; Gromert 2009: no contact details found; Yoshida 2010: no contact details found and no response from Sponsor Tokiwa Pharmaceuticals).
Design
All studies were randomised controlled trials; 37 were parallel‐group trials, and two were cross‐over trials.
Sample sizes
Studies involved sample sizes ranging from 13 to 252 participants.
Setting
Studies took place in primary or secondary care settings at European (24 studies), Australian and New Zealand (two studies), and Asian (13 studies in Korea, China, Iran, Japan, and Taiwan) centres.
Participants
Studies evaluated probiotics in children and adults of both genders. We could not calculate an accurate male/female ratio because data from some studies are not available. Participants in 14 studies were under the age of 18 months, and overall 33 studies assessed children up to the age of 18. The remaining six studies assessed only adults. Study authors did not mention the skin type of participants, and particularly did not mention whether studies included participants with skin of colour. All studies included participants with doctor‐diagnosed eczema.
Nineteen studies stated that eczema was diagnosed based on the criteria provided by Hanifin and Rajka. In three studies, the diagnosis was based on the UK Working Party criteria. In two studies, the diagnosis was based on the Consensus Guidelines for Diagnosis and Management of Atopic Dermatitis (Eichenfield 2004). One study used the definition of atopic eczema dermatitis syndrome (AEDS) for diagnosis. Another study based the diagnosis on the Guidelines for Management of Atopic Dermatitis provided by the Japanese Dermatological Association. In one study, the diagnosis was based on Erlangen score > 10 (atopic score of Diepgen) (Diepgen 1996). One study stated that the diagnosis of eczema was based on diagnostic criteria but did not specify which ones, and 11 studies did not specify the diagnostic criteria applied.
The severity of participants' eczema ranged from mild to severe. Eighteen studies did not prespecify the severity of eczema among participants (Brouwer 2006; Cukrowska 2008; Drago 2014; Flinterman 2007; Folster‐Holst 2006; Goebel 2010; Guo 2015; Hol 2008; Isolauri 2000; Ivankhnenko 2013; Kirjavainen 2003; Lin 2015; Majamaa 1997; Nermes 2010; Rosenfeldt 2003; Taniuchi 2005; Viljanen 2005; Yoshida 2010). Nine studies recruited participants with moderate to severe eczema (Drago 2012; Gerasimov 2010; Iemoli 2012; Matsumoto 2014; Shafiei 2011; Wang 2015; Weston 2005; Wu 2012; Yesilova 2012). Another study recruited participants with mild to severe eczema (Farid 2011). Nine studies recruited participants with eczema scored above a minimum Severity Scoring of Atopic Dermatitis (SCORAD) value (Gore 2011; Gruber 2007; Han 2012; Passeron 2006; Roessler 2007; Sistek 2006; Van der Aa 2010; Woo 2010; Wu 2015; with minimum SCORAD ≥ 10, 15 to 40, 20 to 50, 5 to 30, > 15, ≥ 25, ≥ 15, ≥ 10, and ≥ 15, respectively). One study recruited participants with moderate eczema (Gromert 2009). Another study recruited participants with mild to moderate eczema (Yang 2014).
Three studies assessed only children who had atopic eczema (Flinterman 2007; Sistek 2006; Wang 2015), and one study assessed only children with low levels of Bifidobacteria in their faeces (Taniuchi 2005).
Interventions
Twenty‐three studies used a single strain of probiotic with or without prebiotic (Brouwer 2006; Drago 2012; Drago 2014; Folster‐Holst 2006; Goebel 2010; Gore 2011; Gromert 2009; Gruber 2007; Han 2012; Isolauri 2000; Kirjavainen 2003; Lin 2015; Majamaa 1997; Matsumoto 2014; Nermes 2010; Passeron 2006; Taniuchi 2005; Van der Aa 2010; Weston 2005; Woo 2010; Wu 2012; Wu 2015; Yoshida 2010): 15 of these used Lactobacillus (L) species (L rhamnosus, L salivarius,L reuteri,L GG,L plantarum,L fermentum,L sakei) (Brouwer 2006; Drago 2012; Drago 2014; Folster‐Holst 2006; Gromert 2009; Gruber 2007; Han 2012; Kirjavainen 2003; Majamaa 1997; Nermes 2010; Passeron 2006; Weston 2005; Woo 2010; Wu 2012; Wu 2015); five used Bifidobacterium species (B lactis, B bifidum, B breve) with or without prebiotic (Lin 2015; Matsumoto 2014; Taniuchi 2005; Van der Aa 2010; Yoshida 2010); and three included one arm treated with Lactobacillus species and one with Bifidobacterium species (Goebel 2010; Gore 2011; Isolauri 2000).
Fifteen studies used probiotic mixtures of mainly Lactobacillus and Bifidobacteria species with or without prebiotic (Cukrowska 2008; Farid 2011; Flinterman 2007; Gerasimov 2010; Hol 2008; Iemoli 2012; Ivankhnenko 2013; Roessler 2007; Rosenfeldt 2003; Shafiei 2011; Sistek 2006; Viljanen 2005; Wu 2015; Yang 2014; Yesilova 2012). One study had three arms, all treated with Lactobacillus species (L paracasei,L fermentum); two arms used a single strain, and the third arm used a combination of the two strains (Wang 2015).
Trials identified no standard dose, and researchers used a variety of doses and concentrations of probiotics. They measured the daily dose most often in colony‐forming units (CFUs)/d or CFU/dose or CFU/gr or 100 mL of formula. Concentrations of probiotic bacteria varied from 10⁷/gr formula to 7.8 × 10¹⁰/d for different strains. One study reported the dose of probiotics in mgr (Wu 2015), and two studies gave no information on the concentrations of probiotics used (Guo 2015; Lin 2015).
Co‐interventions included extensively hydrolysed infant formula and prebiotic (Taniuchi 2005; Van der Aa 2010), extensively hydrolysed formula and elimination diets (non‐dairy, cow’s milk, or egg elimination diet) (Brouwer 2006; Gore 2011; Majamaa 1997; Viljanen 2005), extensively hydrolysed formula only (Hol 2008; Isolauri 2000; Kirjavainen 2003; Nermes 2010), a prebiotic (Farid 2011; Passeron 2006; Shafiei 2011; Wu 2012), and elimination diet alone (Cukrowska 2008; Ivankhnenko 2013). Placebo groups received the co‐intervention alone (Hol 2008; Kirjavainen 2003; Nermes 2010; Taniuchi 2005; Van der Aa 2010; Wu 2012), or they were given microcrystalline cellulose alone or with the study’s formula (Folster‐Holst 2006; Sistek 2006; Viljanen 2005; Wang 2015; Woo 2010), maltodextrin alone or with rice starch or anhydrous glucose or cellulose and silicone dioxide (Drago 2012; Drago 2014; Flinterman 2007; Gerasimov 2010; Goebel 2010; Gore 2011; Han 2012; Iemoli 2012; Weston 2005; Wu 2015), hydrolysed casein (Cukrowska 2008), skim milk powder with either dextrose or potato starch and lactose and prebiotic, sucrose, skim milk with glucose, inulin, dextrin, and silicon dioxide (Matsumoto 2014; Rosenfeldt 2003; Yesilova 2012), or glucose anhydrous crystalline powder (Yang 2014).
Seven studies did not specify the placebo (Brouwer 2006; Farid 2011; Gromert 2009; Gruber 2007; Ivankhnenko 2013; Roessler 2007; Yoshida 2010). One study provided no placebo, and the control group received no treatment (Lin 2015), and another study provided no placebo but participants in the control arm used the same topical treatment as those in the intervention arm (Guo 2015).
Outcomes
From 13 studies (Goebel 2010; Gruber 2007; Han 2012; Nermes 2010; Passeron 2006; Roessler 2007; Rosenfeldt 2003; Sistek 2006; Weston 2005; Woo 2010; Wu 2012; Yang 2014; Yoshida 2010), we obtained data for the first primary outcome of the review: changes in participant‐, parent‐, or principal carer‐rated symptoms of eczema at the end of active treatment. Five studies reported participant‐ or parent‐rated changes from baseline in eczema symptom scores at the end of active treatment (SCORAD part C or visual analogue scale (VAS) scores for pruritus and sleep loss) (Gerasimov 2010; Gruber 2007; Weston 2005; Wu 2015; Yang 2014), and the authors of four trials provided unpublished data for that outcome (Goebel 2010; Han 2012; Passeron 2006; Rosenfeldt 2003). Three of these studies reported parent‐ or participant‐rated overall change in eczema severity during study treatment (Passeron 2006; Rosenfeldt 2003; Weston 2005). One study (abstract only) reported this change narratively (Gromert 2009).
For the second primary outcome ‐ changes in quality of life at the end of active treatment ‐ 10 studies reported quality of life measures (Dermatology Life Quality Index (DLQI), Infant's Dermatology Quality of Life Index (IDQoL), Children's Dermatology Life Quality Index (CDLQI), Dermatitis Family Impact Scale (DFI), Skindex‐29) (Drago 2012; Gerasimov 2010; Gore 2011; Folster‐Holst 2006; Iemoli 2012; Wang 2015; Weston 2005; Wu 2012; Wu 2015; Yoshida 2010), and one study reported quality of life changes using a non‐validated questionnaire (Matsumoto 2014).
Three studies reported outcomes relevant to the first secondary outcome of the review ‐ changes in participant‐, parent‐, or principal carer‐rated symptoms of eczema within six months after active treatment had ceased (Han 2012; Sistek 2006; Weston 2005).
Three studies reported data relevant to the secondary outcome ‐ changes in quality of life within six months after active treatment has ceased (Iemoli 2012; Wang 2015; Weston 2005).
Eleven studies reported assessments of the need for other eczema treatment during the study intervention (Folster‐Holst 2006; Gerasimov 2010; Gore 2011; Gruber 2007; Han 2012; Rosenfeldt 2003; Van der Aa 2010; Weston 2005; Woo 2010; Wu 2012; Wu 2015), and two studies (one abstract only) reported this outcome narratively (Gromert 2009; Wang 2015).
For the fourth secondary outcome of the review, investigator‐rated eczema severity, 32 studies reported global eczema severity scores (total SCORAD index as absolute score or change from baseline) (Brouwer 2006; Cukrowska 2008; Drago 2012; Drago 2014; Farid 2011; Folster‐Holst 2006; Gerasimov 2010; Goebel 2010; Gore 2011; Gruber 2007; Han 2012; Hol 2008; Iemoli 2012; Ivankhnenko 2013; Lin 2015; Majamaa 1997; Nermes 2010; Passeron 2006; Roessler 2007; Rosenfeldt 2003; Shafiei 2011; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Weston 2005; Woo 2010; Wu 2012; Wu 2015; Yang 2014; Yesilova 2012; Yoshida 2010), and one study provided unpublished data (Flinterman 2007). Eight studies reported investigator‐rated eczema severity scores (EASI, SCORAD part A/B, categorical presentation of total SCORAD changes) (Cukrowska 2008; Majamaa 1997; Passeron 2006; Shafiei 2011; Weston 2005; Woo 2010; Yang 2014; Yoshida 2010), and the authors of four trials provided unpublished data on this outcome (Brouwer 2006; Goebel 2010; Han 2012; Sistek 2006). One study (abstract only) reported investigator‐rated changes in eczema severity narratively only (Gromert 2009). Twelve studies reported outcomes for changes in eczema severity within six months after treatment had ceased (Cukrowska 2008; Folster‐Holst 2006; Han 2012; Iemoli 2012; Isolauri 2000; Ivankhnenko 2013; Majamaa 1997; Roessler 2007; Sistek 2006; Viljanen 2005; Wang 2015; Weston 2005). One study reported the rate of recurrence within three months after the end of treatment (Guo 2015).
Eleven studies reported adverse events (Folster‐Holst 2006; Gerasimov 2010; Gore 2011; Gruber 2007; Matsumoto 2014; Passeron 2006; Sistek 2006; Wang 2015; Weston 2005; Wu 2012; Wu 2015), and four studies mentioned them (Drago 2012; Farid 2011; Iemoli 2012; Shafiei 2011).
Excluded studies
We excluded from the review 19 publications reporting RCTs; we have described these in the Characteristics of excluded studies section.
For three studies (Burk 2013; Ou 2012; Rose 2010), we could not ascertain whether all participants had eczema.
In two studies (Arkwright 2003; Gueniche 2008), interventions were given topically, not orally as defined in the protocol of this review.
In four studies, the intervention was not a probiotic, but this was not clear from the published abstracts (Foekel 2009; Ikezawa 2004; Leung 2004; Shibata 2009).
Two studies were quasi‐RCTs (Aryayev 2006; Chernysov 2009).
In one study, the control was also a probiotic (Matsumoto 2007), and three studies used heat‐killed bacteria (Moroi 2011; Murosaki 2006; Torii 2011); we excluded these studies because they did not fulfil the criteria set in the review protocol for included studies.
One study did not study probiotics in humans (Ogawa 2006).
One study was a follow‐up study of probiotics used for prevention, not treatment, of eczema (Laitinen 2005).
In two studies (Arvola 2006; Kalliomaki 2003), participants did not have eczema.
Ongoing studies
Among the "ongoing studies" identified for the first review, the Land study (NCT00378300) was withdrawn before recruitment started because of lack of funding.
We identified four ongoing trials for this update: one examining a probiotic (IRT5) for the treatment of atopic dermatitis conducted in Korea and currently recruiting (KCT0000914; which started in November 2013); one conducted in Brazil to study a mixture of probiotics for atopic dermatitis in children (NCT02519556; which is recruiting); one undertaken in Spain to study probiotics in children (NCT02585986a; which started in January 2016 and has completed recruitment); and one reported from Italy to study Lactobacillus reuteri and vitamin D in children with atopic dermatitis (NCT02945683; which is currently recruiting) (see Characteristics of ongoing studies).
Studies awaiting classification
We have identified four trials awaiting classification. One study from Australia studied probiotics in the management of eczema with a start year of 2004 (ACTRN12605000615684). The current status is unknown. We contacted the investigators but have received no response. One trial from the Netherlands studied the use of amino acid‐based formula with synbiotics in infants with non‐IgE‐mediated cow's milk allergy (Candy 2016). Some of the participants have eczema, and SCORAD measurement is one of the secondary outcomes. Researchers have not yet presented results for clinical outcomes of the study (see Characteristics of studies awaiting classification).
Risk of bias in included studies
We have presented review authors' judgement for each risk of bias item across all studies in the 'Risk of bias' graph (Figure 2), and for each study in the 'Risk of bias' summary (Figure 3). We have presented in Table 4 the review authors' quality assessment of other parameters of the included studies (clarity of statement of aims, interventions and outcomes, compliance assessment, exclusion of non‐study probiotics).
2.
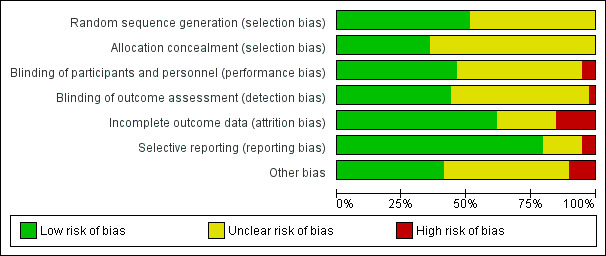
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
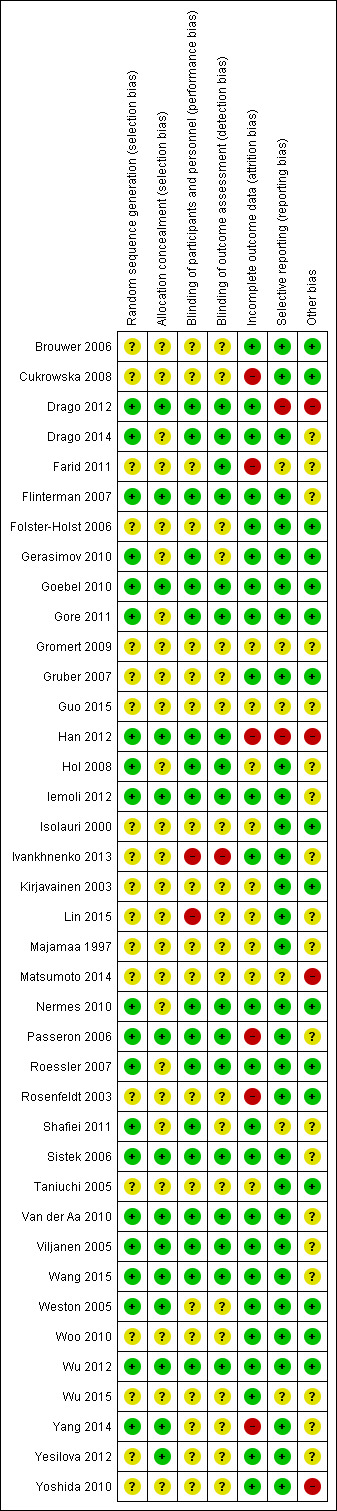
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3. Other parameters for quality assessment of included studies.
| Study | Clarity of methods | Compliance | Dietary management |
| Brouwer 2006 | Clear | No compliance measures described | Adequate exclusion of other probiotics during the study |
| Cukrowska 2008 | Total daily dose of intervention clear, but individual dose, frequency, and mode of administration not given | No compliance measures reported | Not stated |
| Drago 2012 | Clear | Dose count (returned sachet packets counted by clinical investigator). Compliance measured for the 2 groups: 84.5% and 84.7%. No significant difference | Clear instructions given: no change in usual diet but avoid any type of fermented food containing live micro‐organisms |
| Drago 2014 | Clear | No information provided | No information provided |
| Farid 2011 | Aims and Interventions clear. Outcomes not clear and baseline severity (SCORAD) not given | No information given | Inadequate information |
| Flinterman 2007 | Aims, interventions clear | Inadequate information | Inadequate information |
| Folster‐Holst 2006 | Clear | No compliance measures reported | Not stated |
| Gerasimov 2010 | Total daily dose of probiotics not clear. Remaining methods clear | Compliance checked from the parental report and the weight of remaining powder. Reported that there were no differences in compliance between the 2 groups | No information on adequate exclusion of other probiotics from the diet. Participants with challenge proved milk or egg allergy followed milk or egg elimination diet, respectively |
| Goebel 2010 | Clear | Compliance based on count of remaining capsules: average 94% for all groups and 93.6%, 95%, and 93.3% for Bifidobacterium, Lactobacillus, and placebo groups, respectively. No significant difference in compliance between the 3 groups (P = 0.6). No participating child had compliance lower than 72% | No information given |
| Gore 2011 | All methods clear Reporting of adverse events suggests that these were the result of the change in formula but the numbers are totals from intervention and control groups, and it is not certain whether the AEs are associated with the formula or the probiotics |
No compliance measures reported | Instructions given that other fermented or probiotic‐containing products were to be avoided |
| Gromert 2009 | Inadequate information available | No information | No information |
| Gruber 2007 | Unclear what the placebo was; otherwise clear | 92.5% of doses taken by probiotic group; 94.4% by placebo group | Not stated, other than encouragement to avoid allergens |
| Guo 2015 | Dose and exact consistency of probiotics unclear | No information | No information |
| Han 2012 | Preparation of the intervention not clear. Otherwise clear | No compliance measure described | Clear instructions not to consume fermented food and products containing live micro‐organisms |
| Hol 2008 | Trial designed to study effects of probiotics in participants with cow's milk allergy. Effects of probiotics on eczema ‐ secondary outcome. Aims, interventions, and outcome measures clear | Compliance measure not presented. "To optimise compliance, participants were supplied with study formula through the study team and batches were delivered at home" | No information provided |
| Iemoli 2012 | Clear | Method: count of return sachets. Reported that compliance was similar in the 2 groups but no measures reported | Instructions given so that participants do not change their diet during trial but should avoid fermented food products containing live micro‐organisms |
| Isolauri 2000 | Unclear ‐ dose and duration of probiotic treatment received not clearly described. Severity of participant eczema at baseline not described | No compliance measures reported | Not stated |
| Ivankhnenko 2013 | Placebo not described. Otherwise methods clear | No compliance measures reported | Not stated |
| Kirjavainen 2003 | Unclear ‐ intended duration of study treatment not stated | No compliance measures reported | Not stated |
| Lin 2015 | Exact dose of probiotics not given | No information provided | No information provided |
| Majamaa 1997 | Unclear ‐ precise dose of probiotic received by participants not stated | No compliance measures described | Not stated |
| Matsumoto 2014 | Clear | No information provided | Clearly stated: "All patients were asked to avoid probiotic supplements, fermented milk, lactic acid bacterial drinks and fermented soybean (natto) during the experimental period…" |
| Nermes 2010 | Clear | No compliance measures reported | Not stated |
| Passeron 2006 | Clear | No compliance measures described | Not stated |
| Roessler 2007 | Clear | No compliance measures described | Adequate exclusion of prebiotics and probiotics 3 weeks before the start and during the 20 weeks of the intervention |
| Rosenfeldt 2003 | Clear | No compliance measures described | Adequate exclusion of other probiotics during study |
| Shafiei 2011 | Intervention type not clear: synbiotic mixture of 7 strains of probiotics and fructo‐oligosaccharide. Dose, frequency of intake, and preparation clear Baseline characteristics given only for participants who completed the trial |
No compliance measures reported | Unclear. Stated that participants did not change diet before or during the trial |
| Sistek 2006 | Clear | Assessed by 2 telephone calls | One participant noted to have taken non‐study probiotic |
| Taniuchi 2005 | Clear | No compliance measures described | Not stated |
| Van der Aa 2010 | Clear | No compliance measures reported. Participants' parents were keeping diary for formula intake and adverse events. Formula with intervention was given on demand and at the end of intervention. No significant differences in formula intake were noted between the 2 groups | Unclear |
| Viljanen 2005 | Method for diagnosing eczema not described | No compliance measures described | Not stated |
| Wang 2015 | All clear. In the publication, not clear what the placebo was, but this was clarified by the study author | Yes: capsule count performed | Yes Stated: "During the study…and other probiotics were not permitted" |
| Weston 2005 | Clear | Sachet counts and parent‐completed sachet administration chart. Good compliance (94%) ‐ no differences between the 2 groups | Adequate exclusion of other probiotics during study |
| Woo 2010 | Clear | No measure of compliance was reported, but it was stated that the 2 groups had no difference in compliance | No information provided |
| Wu 2012 | Aims, interventions, and outcome measures clear. Exclusion criteria not given | Patients and parents were to return to investigators all unused intervention. No measure was reported | Instruction given to parents not to feed their children other probiotic preparations during the intervention |
| Wu 2015 | Aims, interventions, and outcome measures clear. Dose of probiotic not given in colony‐forming units, or similar measure of bacterial numbers | Compliance recorded: assessed based on a count of returned medication | No information provided |
| Yang 2014 | All clear | No information given | Instructions given to avoid any commercial probiotic‐containing products 2 weeks before study initiation. No comment about diet during the trial |
| Yesilova 2012 | All clear | No information provided | No information provided |
| Yoshida 2010 | Placebo not described. Otherwise clear | No compliance measures reported | No information given |
SCORAD: Severity Scoring of Atopic Dermatitis.
Allocation
Random sequence generation
For 20 studies, we judged the method used in generating the randomisation sequence as having low risk of bias, and for 17 of those (Drago 2012; Drago 2014; Gerasimov 2010; Goebel 2010; Han 2012; Hol 2008; Iemoli 2012; Passeron 2006; Roessler 2007; Shafiei 2011; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Weston 2005; Wu 2012; Yang 2014), the randomisation sequence was computer generated. For 19 studies, trial authors did not describe the method used in generating the randomisation sequence, so we judged these studies as having unclear risk of bias; this group included one trial in which trial authors provided no information on the randomisation sequence generation method used, although we judged treatment allocation as adequate (Yesilova 2012). We found no trials to be at high risk of bias for random sequence generation.
Allocation concealment
Authors of 25 studies did not describe concealment of treatment allocation, and we judged risk of bias as unclear for this domain (Brouwer 2006; Cukrowska 2008; Drago 2014; Farid 2011; Folster‐Holst 2006; Gerasimov 2010; Gore 2011; Gromert 2009; Gruber 2007; Guo 2015; Hol 2008; Isolauri 2000; Ivankhnenko 2013; Kirjavainen 2003; Lin 2015; Majamaa 1997; Matsumoto 2014; Nermes 2010; Roessler 2007; Rosenfeldt 2003; Shafiei 2011; Taniuchi 2005; Woo 2010; Wu 2015; Yoshida 2010).
We considered 14 trials to have low risk of selection bias due to allocation concealment. One trial described treatment allocation by the "closed envelope method", which we judged as adequate (Yesilova 2012). For the remaining 13 included studies, we did not consider treatment allocation concealment adequate because the allocating process excluded access to the randomisation sequence and knowledge of the treatment given (Drago 2012; Flinterman 2007; Goebel 2010; Han 2012; Iemoli 2012; Passeron 2006; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Weston 2005; Wu 2012; Yang 2014). For 10 of these studies, a pharmacy department or a blinded investigator provided treatment using a computer‐generated randomisation sequence (Drago 2012; Han 2012; Iemoli 2012; Passeron 2006; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Weston 2005; Yang 2014). These studies adequately concealed treatment allocation ‐ the third party was not involved in screening or enrolling participants, and the clinical trial staff enrolling participants did not have access to the randomisation sequence. One study gave sealed boxes with allocation numbers to participants using a randomisation table (Flinterman 2007). Two studies packed treatment in numbered sealed boxes and gave them to participants using a computer‐generated randomisation sequence (Goebel 2010; Wu 2012).
We found no studies at high risk of bias for allocation concealment.
Blinding
We judged studies to be at low risk of performance bias when trial authors provided enough information to exclude knowledge of the allocated intervention by participants or parents and personnel involved in the trial. We judged studies to be at low risk of detection bias when trial authors provided enough information to exclude knowledge of the allocated intervention by outcome assessors, but also by participants or parents for participant‐ or parent‐rated outcomes.
One study was not blinded (open‐label), so we judged it to be at high risk of bias in both domains (Ivankhnenko 2013). We judged another study to be at high risk of performance bias because trial authors did not mention blinding and the control group received no treatment and no placebo; hence we determined it was unlikely that blinding was done (Lin 2015). The same study provided inadequate information on blinding of the outcome assessor, and so we judged this study to be at unclear risk of detection bias.
We judged 16 studies to be at low risk for both performance and detection bias (Drago 2012; Drago 2014; Flinterman 2007; Goebel 2010; Gore 2011; Han 2012; Hol 2008; Iemoli 2012; Nermes 2010; Passeron 2006; Roessler 2007; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Wu 2012). Authors of eight of these studies stated that participants, clinicians, and outcome assessors were all blinded (Drago 2012; Gore 2011; Hol 2008; Roessler 2007; Sistek 2006; Van der Aa 2010; Wang 2015; Wu 2012). For another eight studies, we confirmed blinding of participants, clinicians, and outcome assessors through communication with trial authors (Drago 2014; Flinterman 2007; Goebel 2010; Han 2012; Iemoli 2012; Nermes 2010; Passeron 2006; Viljanen 2005).
We judged 18 studies to be at unclear risk of both performance and detection bias. Of these, trial authors described 14 studies as "double‐blind" without further clarification or provided no information on blinding (Brouwer 2006; Cukrowska 2008; Gromert 2009; Gruber 2007; Guo 2015; Isolauri 2000; Kirjavainen 2003; Majamaa 1997; Matsumoto 2014; Rosenfeldt 2003; Taniuchi 2005; Woo 2010; Wu 2015; Yoshida 2010). The remaining four studies provided information on some parts of the study but not on blinding of all participants/parents, clinicians, and outcome assessors (Folster‐Holst 2006; Weston 2005; Yang 2014; Yesilova 2012).
We judged Farid 2011 as having unclear risk of performance bias, as study authors did not provide enough information, but low risk of detection bias. Two studies provided enough information, and we judged them to be at low risk of performance bias, but information on outcome assessment was inadequate, and we judged them to be at unclear risk of detection bias (Gerasimov 2010; Shafiei 2011).
Incomplete outcome data
Follow‐up and exclusions
In this domain, we assessed rates of and reasons for losses to follow‐up for overall participants and for each intervention group, as well as exclusions from analysis for all outcomes.
We judged 24 studies to be at low risk of attrition bias with low rates of loss to follow‐up overall (ranging from 0 to 14%) and for each intervention group, and low exclusion rates (Brouwer 2006; Drago 2012; Drago 2014; Flinterman 2007; Folster‐Holst 2006; Gerasimov 2010; Goebel 2010; Gore 2011; Gruber 2007; Iemoli 2012; Ivankhnenko 2013; Nermes 2010; Roessler 2007; Shafiei 2011; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Weston 2005; Woo 2010; Wu 2012; Wu 2015; Yesilova 2012; Yoshida 2010). We judged losses to follow‐up under 20% to show low risk of attrition bias and 20% or over to show high risk. Of these, one study used imputation for missing data (Gore 2011). Another study reported different rates of loss to follow‐up in the two groups (8.9% in the probiotic group and 21% in the placebo group, with overall loss to follow‐up of 14%) and found the difference to be statistically insignificant (P = 0.11) (Woo 2010).
Overall losses to follow‐up when reported were low, with the exception of four studies, which reported losses to follow‐up ranging from 23% to 30% (Cukrowska 2008; Farid 2011; Han 2012; Yang 2014). We judged all of these studies to be at high risk of attrition bias. Another study had high rates of exclusion from analysis (25.9%), and we judged it to be at high risk of attrition bias (Rosenfeldt 2003). Passeron 2006 reported overall low rates of loss to follow‐up, but these varied significantly between the two groups (8% in the placebo group and 29% in the probiotic group). We judged this difference and the reasons for it as likely to affect all outcomes, and we judged this study to be at high risk of attrition bias.
For the nine remaining studies, we judged risk of attrition bias as unclear because trial authors provided inadequate information on losses to follow‐up and exclusions (Gromert 2009; Guo 2015; Hol 2008; Isolauri 2000; Kirjavainen 2003; Lin 2015; Majamaa 1997; Matsumoto 2014; Taniuchi 2005).
Selective reporting
We found little evidence of reporting bias in the included studies. However, we judged two studies to be at high risk of reporting bias (Drago 2012; Han 2012). One of these did not report scores for eczema symptoms (pruritus and sleep loss) after treatment, but study authors provided this information to us after communication (Han 2012). The other study reported and discussed only outcomes that were significant for the probiotic group (Drago 2012).
We judged six studies to be at unclear risk of reporting bias (Farid 2011; Gromert 2009; Guo 2015; Matsumoto 2014; Shafiei 2011; Wu 2015). Two of these reported baseline characteristics only for participants who completed the study (Farid 2011; Shafiei 2011). One study provided inadequate information to permit a judgement (Gromert 2009). We found this report only as a conference abstract and could not find registration of the trial to determine whether all outcomes had been reported. One study described all outcomes but did not provide most of the data numerically and did not provide information on trial registration (Wu 2015). Another study reported all outcomes but not numerically (Matsumoto 2014). However, study authors particularly analysed any favourable information/outcome for probiotics even if it was not statistically significant. Another study did not provide information on the dose of the probiotic given and reported results only narratively (Guo 2015).
The other 31 studies provided no evidence of selective reporting, and we judged them as having low risk of bias in this domain. Trial authors reported all outcomes as described in the publication and/or the trial registration. One study did not report the SCORAD score for eczema, which was a secondary outcome, but provided this information on our request (Flinterman 2007).
Other potential sources of bias
For 16 studies, we identified no sources of other bias, and we judged studies to be at low risk of other bias (Brouwer 2006; Cukrowska 2008; Folster‐Holst 2006; Gerasimov 2010; Goebel 2010; Gore 2011; Gruber 2007; Isolauri 2000; Kirjavainen 2003; Nermes 2010; Roessler 2007; Rosenfeldt 2003; Taniuchi 2005; Weston 2005; Woo 2010; Wu 2012). In particular, study authors declared that they received funding for the study and stated "no conflicts of interest".
The probiotic supplier sponsored or co‐sponsored 12 studies (Drago 2012; Flinterman 2007; Han 2012; Hol 2008; Iemoli 2012; Matsumoto 2014; Van der Aa 2010; Viljanen 2005; Wang 2015; Wu 2015; Yang 2014; Yoshida 2010). For four of these studies (Drago 2012; Han 2012; Matsumoto 2014; Yoshida 2010), we judged that the sponsor was likely to have influenced the study outcome or reporting of the study outcome, and we judged these studies to be at high risk of other bias. In addition, for Han 2012, power calculations of the final numbers of participants suggest that the study did not meet target recruitment and was discontinued "after the second interim analysis showed statistically significant differences between the groups". In another of these studies (Yoshida 2010), researchers did not match probiotic and placebo groups for eczema severity (total SCORAD score) at baseline.
Overall we assessed 19 studies as having unclear risk of other bias (Drago 2014; Farid 2011; Flinterman 2007; Gromert 2009; Guo 2015; Hol 2008; Iemoli 2012; Ivankhnenko 2013; Lin 2015; Majamaa 1997; Passeron 2006; Shafiei 2011; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Wu 2015; Yang 2014; Yesilova 2012). Six of these studies did not declare sponsorship of the trial nor conflicts of interest (Guo 2015; Ivankhnenko 2013; Majamaa 1997; Passeron 2006; Shafiei 2011; Yesilova 2012). Investigators reported one study in a conference abstract only and provided inadequate information for risk of bias assessment (Gromert 2009). One of these studies did not match probiotic and placebo groups for eczema severity (total SCORAD score) at baseline (Sistek 2006). For five studies (Drago 2014; Farid 2011; Iemoli 2012; Lin 2015; Wu 2015), trial authors did not make clear what role the supplier of the probiotic played in the study and did not declare trial sponsorship. The other seven studies were sponsored by the supplier of the intervention and the role of the sponsor in data analysis and publication was unclear (Flinterman 2007; Hol 2008; Van der Aa 2010; Viljanen 2005; Wang 2015; Wu 2015; Yang 2014); therefore, we judged them to be at unclear risk of bias.
Effects of interventions
See: Table 1
Primary outcomes
Changes in participant‐rated, parent‐rated, or principal carer‐rated symptoms of eczema at the end of active treatment
The authors of 13 studies with 795 randomised participants supplied published or unpublished data on parent‐ or participant‐rated symptom scores at the end of study treatments (SCORAD part C or equivalent) (Goebel 2010; Gruber 2007; Han 2012; Nermes 2010; Passeron 2006; Roessler 2007; Rosenfeldt 2003; Sistek 2006; Weston 2005; Woo 2010; Wu 2012; Yang 2014; Yoshida 2010). Researchers measured symptoms using a VAS for itch and sleep disturbance ranging from 0 to 10 for each symptom, then a combined score ranging from 0 to 20. Pooled available data from 754 participants in these studies show no significant improvement in favour of probiotic treatment (mean difference (MD) ‐0.44, 95% confidence interval (CI) ‐1.22 to 0.33; Analysis 1.1). Results show significant statistical heterogeneity between studies for this outcome measure (I² = 57%). The reason for this heterogeneity is not clear. We note here that authors of the Yang 2014 study reported their results in non‐parametric statistics, which we converted to parametric ones (see Methods: Unit of analysis issues). Inclusion of data from this study in the meta‐analysis did not change the overall significance of the outcome, but data conversion is based on the assumption that the data are not skewed. Trial sequential analysis shows that target sample sizes of 258 and 456, which are necessary to demonstrate a minimum mean difference of ‐2 (Figure 4) and ‐1.5 (Figure 5), respectively, with 90% power have been exceeded, suggesting that further trials with similar probiotic strains for this outcome may be futile. The sample size of 1026, which is necessary to demonstrate a minimum difference of ‐1 at 90% power, has not been reached (Figure 6). This suggests that further studies to determine whether these probiotics change the outcome by at least 1.5 points may be futile.
1.1. Analysis.
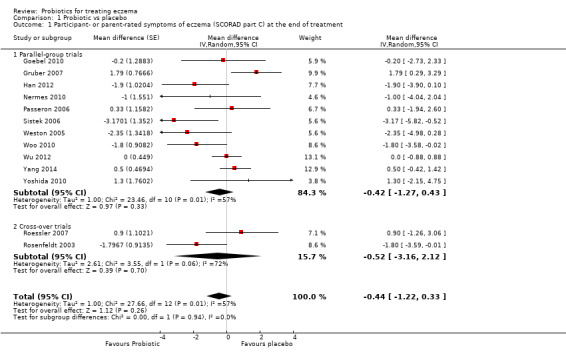
Comparison 1 Probiotic vs placebo, Outcome 1 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment.
4.
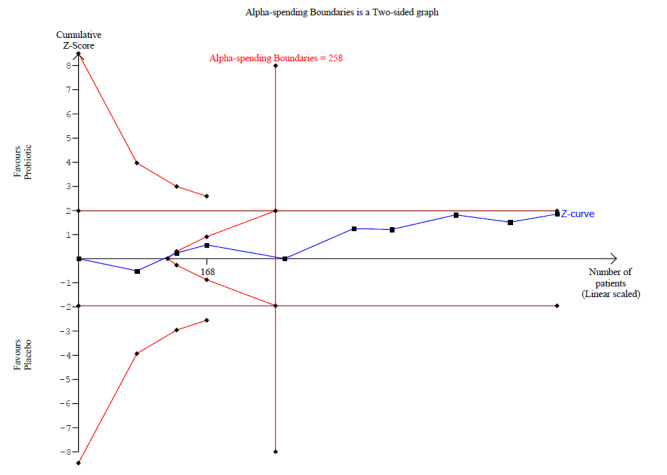
Trial sequential analysis for a minimum difference of ‐2 points difference in eczema symptoms (SCORAD part C; range 0 to 20) between probiotic and no probiotics at 90% power. The blue z‐curve of the meta‐analysis shows that the optimal heterogeneity‐adjusted information size of 258 has been reached. This suggests that future trials of similar interventions are unlikely to change the findings of no significant difference between probiotic and control for detection of at least a 2‐point difference.
5.
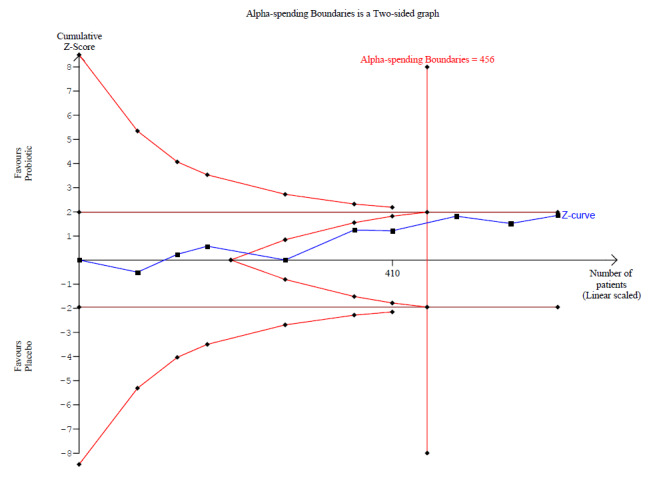
Trial sequential analysis for a minimum difference of ‐1.5 points difference in eczema symptoms (SCORAD part C; range 0 to 20) between probiotics and no probiotics at 90% power. The blue z‐curve of the meta‐analysis has crossed the red v‐shaped line of futility and has reached the optimal heterogeneity‐adjusted information size of 456. This suggests that future trials of similar interventions are unlikely to change the findings of no significant difference between probiotic and control for detection of at least a 1.5‐point difference.
6.
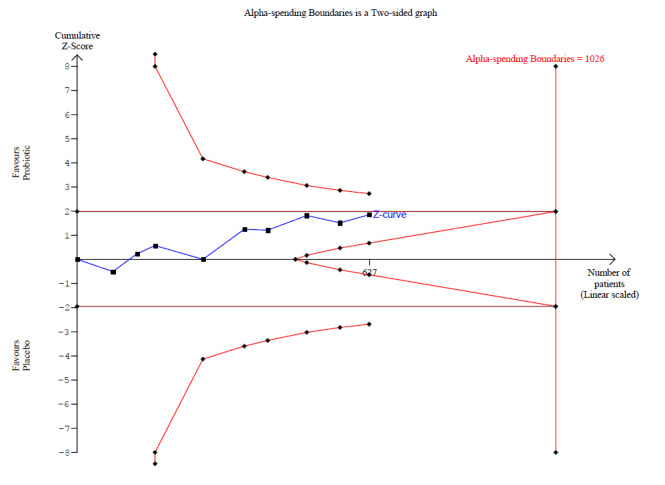
Trial sequential analysis for a minimum difference of ‐1 point difference in eczema symptoms (SCORAD part C; range 0 to 20) between probiotics and no probiotics at 90% power. The blue z‐curve of the meta‐analysis has not crossed the red v‐shaped line of futility and has not yet reached the optimal heterogeneity‐adjusted information size of 1026. This suggests that future trials of similar interventions may change the findings of no significant difference between probiotic and control for detection of at least a 1‐point difference.
Four trials reported parent‐rated overall evaluation of symptoms of eczema at the end of study treatment; however one trial did not provide any numerical data for this outcome and reported no significant differences between groups (Folster‐Holst 2006). Therefore, we included the remaining three trials with 150 randomised participants in the analysis, which comprised data for 135 participants (Passeron 2006; Rosenfeldt 2003; Weston 2005). We dichotomised the scale of two studies as 'better' versus 'worse' or 'the same' (Rosenfeldt 2003; Weston 2005), and the scale of one study measuring from 1 (worse) to 6 (much better) as '4 to 6' versus '1 to 3' (Passeron 2006). Pooling of data from these studies shows no significant reduction in the risk of worsened/unchanged eczema among probiotic‐treated individuals (odds ratio (OR) 0.40, 95% CI 0.14 to 1.15; Analysis 1.2). We detected moderate levels of statistical heterogeneity between trials (I² = 48%), which appeared to be related to the Rosenfeldt 2003 trial. Possible reasons for this heterogeneity include adequate exclusion of other probiotic sources from this cross‐over trial, or higher methodological quality of parallel‐group studies (defined as stating that an intention‐to‐treat analysis was performed, and that methods used for allocation concealment and randomisation sequence generation were adequate). Trial sequential analysis showed that the optimal information size for detecting a 30% difference in the probability of eczema improvement at 90% power is 1096, so that information is currently insufficient to conclude whether probiotics might have an impact on this outcome measure.
1.2. Analysis.
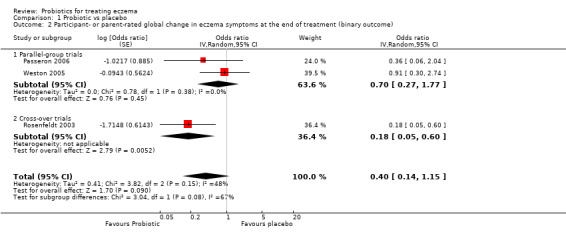
Comparison 1 Probiotic vs placebo, Outcome 2 Participant‐ or parent‐rated global change in eczema symptoms at the end of treatment (binary outcome).
One study (abstract publication only) reported significant reduction in symptoms of itching and loss of sleep in the probiotic group (P = 0.024) (Gromert 2009).
Another study did not use a validated score for participant‐rated symptoms of eczema (Matsumoto 2014). Study authors reported that Itch improvement level in the probiotic group was significantly higher than that in the placebo group at week 8 (end of intervention) (P < 0.05). The proportion of participants whose condition improved and whose scores were 0 (improved remarkably) and 1 (improved) by diagnosis was significantly greater in the LKM512 group than in the placebo group at week 8 (P < 0.05). However, results show no significant differences between groups with respect to other symptomatic scores.
Nine further studies with 688 participants and available data from 627 participants reported changes from baseline in parent‐ or participant‐reported eczema symptoms and found no significant differences between the two groups (MD ‐0.70, 95% CI ‐1.47 to 0.06; Analysis 1.3) (Gerasimov 2010; Goebel 2010; Gruber 2007; Han 2012; Passeron 2006; Rosenfeldt 2003; Weston 2005; Wu 2015; Yang 2014). Results show moderate statistical heterogeneity (I² = 33%) between studies for this outcome measure, which is significantly reduced when Gerasimov 2010 is removed, but the reason for this heterogeneity is not clear. Also we should note here that we converted non‐parametric statistics from the Yang 2014 study to parametric ones (see Methods: Unit of analysis issues), although inclusion of data from this study did not alter the significance of the outcome of this analysis.
1.3. Analysis.
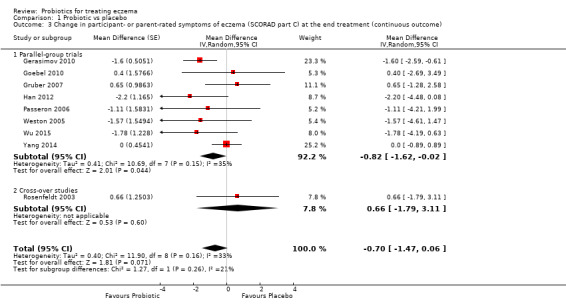
Comparison 1 Probiotic vs placebo, Outcome 3 Change in participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end treatment (continuous outcome).
Changes in quality of life at the end of active treatment
Ten studies provided quality of life (QoL) data (Drago 2012; Folster‐Holst 2006; Gerasimov 2010; Gore 2011; Iemoli 2012; Matsumoto 2014; Wang 2015; Weston 2005; Wu 2012; Yoshida 2010). We included data from six studies with 569 randomised participants and available data from 552 participants using four different scales in the meta‐analysis (Gerasimov 2010; Gore 2011; Iemoli 2012; Matsumoto 2014; Wang 2015; Yoshida 2010), which show no differences in quality of life between treatment groups (standardised mean difference (SMD) 0.03, 95% CI ‐0.36 to 0.42; I² = 68%; Analysis 1.4). We noted significant statistical heterogeneity, but this finding may be related to the different scales used.
1.4. Analysis.
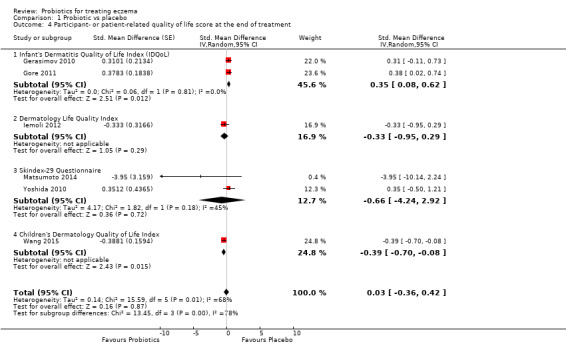
Comparison 1 Probiotic vs placebo, Outcome 4 Participant‐ or patient‐related quality of life score at the end of treatment.
Three studies with 372 randomised participants assessed family QoL by using the Dermatitis Family Impact Questionnaire as described in Lawson 1998, and the Family Dermatology Life Quality Index as discussed in Basra 2007 (Gerasimov 2010; Wang 2015; Weston 2005); pooled data from 358 participants show no significant differences (SMD ‐0.19, 95% CI ‐0.56 to 0.18; Analysis 1.5). Reasons for the significant statistical heterogeneity (I² = 59%) are not clear, but it is reduced when the Gerasimov 2010 study is removed.
1.5. Analysis.
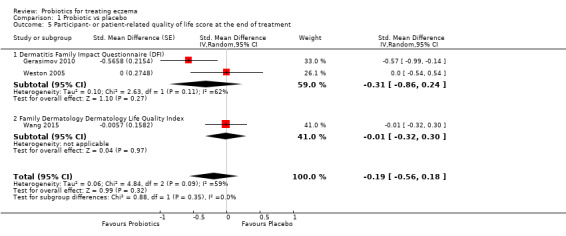
Comparison 1 Probiotic vs placebo, Outcome 5 Participant‐ or patient‐related quality of life score at the end of treatment.
Wu 2015 reported no statistically significant differences between groups in results of the Infant Dermatology Life Quality Index (P = 0.71) and the Dermatitis Family Impact Scale (P = 0.61). Three studies with 153 participants reported no significant differences in QoL between groups at the end of treatment using the Dermatology Life Quality Index, a different published scale ‐ as described in Ruden 1999 ‐ and an unpublished scale, respectively (Drago 2012; Folster‐Holst 2006; Wu 2012).
Secondary outcomes
Changes in participant‐rated, parent‐rated, or principal carer‐rated symptoms of eczema during the six‐month period after active treatment has ceased
Data from three studies comprising 195 participants showing changes in eczema symptoms after active treatment had ceased were available and could be pooled (Han 2012; Sistek 2006; Weston 2005). These three trials reported SCORAD part C scores at four weeks, eight weeks, and two weeks after cessation of study treatment, respectively. A pooled analysis of the data shows significant improvement in the participant‐/parent‐rated symptom score in favour of probiotic treatment (MD ‐1.81, 95% CI ‐3.13 to ‐0.49 points on SCORAD part C; I² = 0%; Analysis 1.6). One of these studies also reported a dichotomised global assessment by parents after cessation of probiotic treatment but no significant long‐term differences in the risk of worsened or unchanged eczema between probiotic and placebo interventions (OR 0.63, 95% CI 0.21 to 1.88; data not presented) (Weston 2005).
1.6. Analysis.
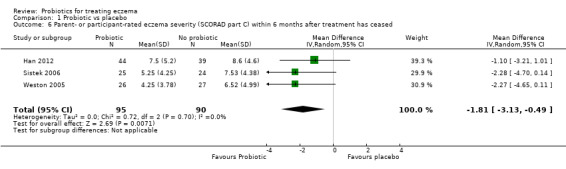
Comparison 1 Probiotic vs placebo, Outcome 6 Parent‐ or participant‐rated eczema severity (SCORAD part C) within 6 months after treatment has ceased.
Changes in quality of life within the six‐month period after active treatment has ceased
Three studies provided data for this outcome (Iemoli 2012; Wang 2015; Weston 2005). Two studies reported longest follow‐up of eight weeks (Iemoli 2012; Weston 2005), and one study described longest follow‐up of one month after cessation of treatment (Wang 2015). Weston 2005 reported that the median change in quality of life score eight weeks after the end of study treatment was ‐2.5 points for the probiotic group and ‐3.0 for the placebo group. Statistical comparison of available data was not possible due to lack of summary statistics. Pooled data from two studies with 261 participants show no significant differences between the two groups (SMD ‐0.08, 95% CI ‐0.35 to 0.20, I² = 0%; Analysis 1.7) (Iemoli 2012; Wang 2015).
1.7. Analysis.
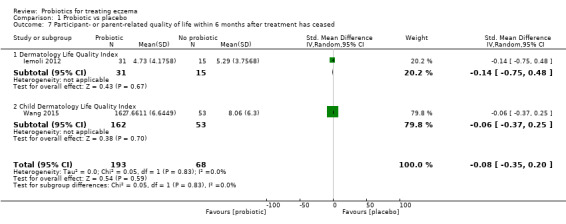
Comparison 1 Probiotic vs placebo, Outcome 7 Participant‐ or parent‐related quality of life within 6 months after treatment has ceased.
One study reported QoL measures for 12, 18, and 36 months post treatment, showing no significant differences between groups (Gore 2011).
Changes in the need for other eczema treatment during active treatment and within the six‐month period after active treatment has ceased
Eleven studies with 634 participants reported this outcome but only for the period of active treatment (Table 5) (Folster‐Holst 2006; Gerasimov 2010; Gore 2011; Gruber 2007; Han 2012; Rosenfeldt 2003; Van der Aa 2010; Weston 2005; Woo 2010; Wu 2012; Wu 2015). We could not pool the data due to differences in reporting of this outcome. For nine of these studies, differences between treatment groups were not statistically significant (Folster‐Holst 2006; Gore 2011; Gruber 2007; Han 2012; Rosenfeldt 2003; Van der Aa 2010; Woo 2010; Wu 2012; Wu 2015), and for one study (Weston 2005), trial authors reported no statistical analysis. The only study that reported significant differences was Gerasimov 2010, which shows a less cumulative quantity of topical corticosteroids used in the probiotic group during the study period (P = 0.006); however, study authors reported no significant differences in the frequency of use of topical corticosteroids at the final visit (P = 0.130).
4. Changes in the need for other eczema treatment during active treatment.
|
Rosenfeldt 2003 |
Gruber 2007 |
Weston 2005 |
Folster‐Holst 2006 |
Gerasimov 2010 |
Han 2012 |
Wu 2012 |
Woo 2010 |
Van der Aa 2010 |
Gore 2011 |
Wu 2015 |
|
| Median grams hydrocortisone butyrate applied (range) | Probiotic: 7.8 (0 to 67) Placebo: 6.0 (0 to 59) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean grams 1% hydrocortisone applied (SD) | ‐ | Probiotic: 0.8 (45.0) Placebo: 3.5 (29.8) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Median change in topical corticosteroid use score (IQR) | ‐ | ‐ | Probiotic: 0.25 (‐6.7 to 7.0) Placebo: ‐1.0 (‐8.0 to 0.7) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean applications per week (SD) | ‐ | ‐ | ‐ | Probiotic: 3.0 (0.6) Placebo: 3.2 (0.9) |
Probiotic: 0.8 (0.9) Placebo: 1.2 (1.4) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Number of participants using topical CS during study (%) | ‐ | ‐ | ‐ | ‐ | ‐ |
Baseline Probiotics: 13/58 (22.4%) Placebo: 14/60 (23.3%) At end of treatment Probiotics: 13/44 (29.5%) Placebo: 14/39 (36%) |
‐ | Probiotic: 22/45 (49%) Placebo: 20/43 (46%) |
Baseline Synbiotic: 25/45 (55.6%) Placebo: 22/44 (50%) At end of treatment Synbiotic: 22/41 (53.7%) Placebo: 24/42 (57.1%) |
‐ | ‐ |
| Mean grams 0.25% prednicarbate applied during study (SD) |
‐ | ‐ | ‐ | ‐ | ‐ | Probiotic:
1.6 g (6.5) Placebo: 1.5 g (4.0) |
‐ | ‐ | ‐ | ‐ | ‐ |
| Mean applications of CS or calcineurin inhibitor per month (SD) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: 23.5 (19.1) Placebo: 19.1 (19) |
‐ | ‐ | ‐ | ‐ |
| Median grams of 0.1% prednicarbate during Intervention (range) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic:
11 (0 to 63) Placebo: 13 (0 to 83) |
‐ | ‐ | ‐ |
| Median change in grams of 0.1% prednicarbate use during intervention (range) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: ‐0.5 (‐2.7 to 1.3) Placebo: ‐0.3 (‐1.9 to 2.5) |
‐ | ‐ | ‐ |
| Number of participants using standard skin care at end of intervention | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: 29/88 (33%) Placebo: 21/47 (45%) |
‐ |
| Number of participants using different potencies of TCS at end of intervention | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
Emollients only Probiotic: 31/88 (35%) Placebo: 18/47 (38%) Mild Probiotic: 54/88 (61%) Placebo: 29/47 (62%) ≥ moderate/potent Probiotic: 3/88 (3%) Placebo: 0 |
‐ |
| Mean grams of TCS (hydrocortisone 1% or mometasone 0.1%) used during study (SD) | ‐ | ‐ | ‐ | ‐ | Probiotic:
25.6 (14.5) Placebo: 33.3 (11.4) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Median grams of TCS (hydrocortisone 1% or mometasone 0.1%) used during study (range) | ‐ | ‐ | ‐ | ‐ | Probiotic: 25.0 (0.0 to 45.0) Placebo: 35.0 (15.0 to 50.0) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mean total amount (gr) of corticosteroid used during treatment period ± SD | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probiotic: 5.87 ± 7.48 Placebo: 4.73 ± 5.48 |
CS: corticosteroids. IQR: interquartile range. SD: standard deviation. TCS: topical corticosteroids.
Two studies reported no recorded differences in steroid consumption between groups (Gromert 2009 (abstract publication only); Wang 2015).
The sole study reporting changes in the need for other eczema treatment after treatment had ceased found no significant differences between placebo and probiotic groups in median topical corticosteroid scores eight weeks after cessation of the study intervention (Weston 2005).
Investigator‐rated eczema severity
Changes in eczema severity as measured by a trained investigator or a medical practitioner at the end of active treatment
All studies reported assessments relevant to this outcome. Twenty‐four studies with 1639 participants reported mean total SCORAD (SCORAD parts A, B, C) scores at the end of treatment (Drago 2012; Drago 2014; Flinterman 2007; Folster‐Holst 2006; Goebel 2010; Gore 2011; Gruber 2007; Han 2012; Hol 2008; Iemoli 2012; Ivankhnenko 2013; Lin 2015; Nermes 2010; Passeron 2006; Roessler 2007; Rosenfeldt 2003; Sistek 2006; Viljanen 2005; Wang 2015; Weston 2005; Woo 2010; Wu 2012; Yesilova 2012; Yoshida 2010). Trial authors provided data for one of these studies (Drago 2014); however, the P value for the difference between the two groups calculated via a two‐sided unpaired t test shows a slight difference (P < 0.001) from that reported in the manuscript (P = 0.015). Pooled analysis of available data on 1596 participants from these studies shows significant differences between probiotic and control treatments, with a mean difference of ‐3.91 points in favour of probiotic treatment (95% CI ‐5.86 to ‐1.96; Analysis 1.8). We detected extreme levels of statistical heterogeneity between trials (I² = 79%), which seemed to be related to parallel‐group trials (I² = 81%). The reasons for heterogeneity were not clear. Two studies reported significant baseline differences in eczema severity between placebo‐ and probiotic‐treated groups, which might have accounted for the increased difference in end of treatment SCORAD scores (Sistek 2006; Yoshida 2010). Schram 2011 assessed the minimal clinically important difference (MCID) in SCORAD, EASI, and Patient‐Oriented Eczema Measure (POEM) scores; researchers used as anchor points changes in Patient and Investigator Global Assessment (PGA and IGA). Schram suggested that the MCID in total SCORAD is 8.7, which is higher than the difference of 3.91 points shown in our Analysis 1.8 (i.e. lower than this cutoff point). Although the study data came from adult patients only, the mean difference of 3.91 points found in this analysis is of uncertain clinical significance. Schram also suggested that a minimum change in total SCORAD of 4.1 is the optimal cutoff change that can predict change in IGA, which is closer to the difference we found in Analysis 1.8.
1.8. Analysis.
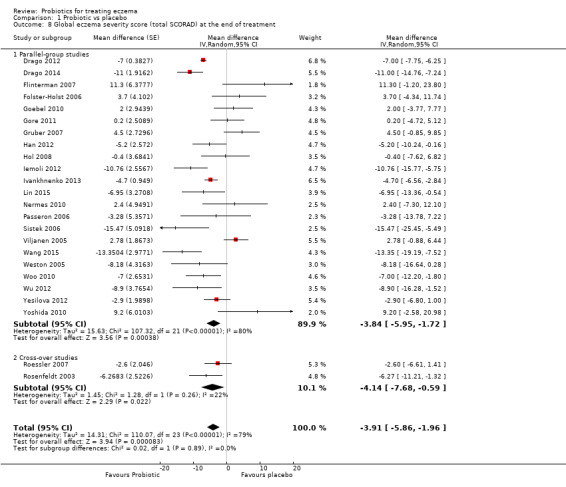
Comparison 1 Probiotic vs placebo, Outcome 8 Global eczema severity score (total SCORAD) at the end of treatment.
To explore this, we performed a sensitivity analysis using change scores (i.e. the difference in SCORAD score between start and end of treatment for each individual). Fourteen studies with 1086 randomised participants provided these data on 1035 participants (Farid 2011; Gerasimov 2010; Goebel 2010; Han 2012; Ivankhnenko 2013; Nermes 2010; Passeron 2006; Rosenfeldt 2003; Sistek 2006; Van der Aa 2010; Viljanen 2005; Weston 2005; Woo 2010; Wu 2015), which we have presented in Analysis 1.9. The data show a mean difference in SCORAD change of ‐4.46 points (95% CI ‐6.49 to ‐2.43) in favour of probiotic treatment, with substantial but diminished statistical heterogeneity between trials (I² = 51%). The reason for this is not clear, but it seems to be attributed most to the Farid 2011 study, which reported high rates of loss to follow‐up (23%), which researchers excluded from analysis.
1.9. Analysis.
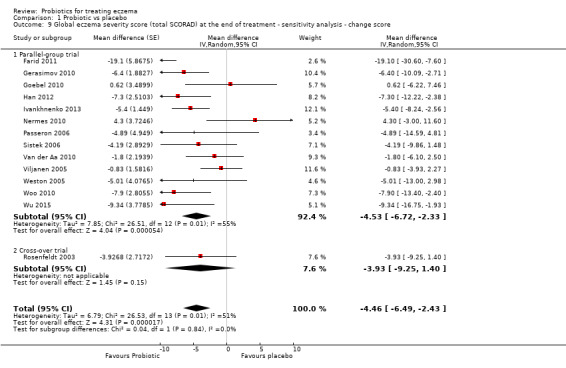
Comparison 1 Probiotic vs placebo, Outcome 9 Global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score.
We also performed a sensitivity analysis using total SCORAD scores at the end of treatment only for studies with low risk of bias (Analysis 1.10). We found nine studies with low risk of bias (Drago 2012; Flinterman 2007; Goebel 2010; Iemoli 2012; Sistek 2006; Van der Aa 2010; Viljanen 2005; Wang 2015; Wu 2012), but eight studies with 741 randomised participants provided data from 705 participants for analysis (Drago 2012; Flinterman 2007; Goebel 2010; Iemoli 2012; Sistek 2006; Viljanen 2005; Wang 2015; Wu 2012). Results show extreme levels of statistical heterogeneity (88%), and so we have pooled the data in the subtotal. The reasons for this heterogeneity were not clear, but it may be attributed to the use of different strains of probiotics.
1.10. Analysis.
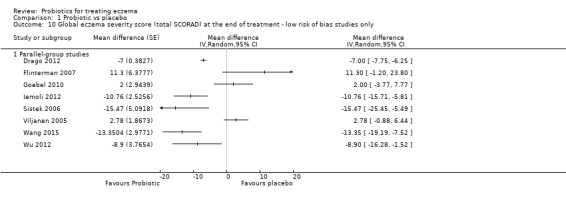
Comparison 1 Probiotic vs placebo, Outcome 10 Global eczema severity score (total SCORAD) at the end of treatment ‐ low risk of bias studies only.
The authors of 11 studies reported data for investigator‐rated eczema severity using objective SCORAD (parts A/B) scores (Brouwer 2006; Goebel 2010; Han 2012; Majamaa 1997; Passeron 2006; Shafiei 2011; Sistek 2006; Weston 2005; Woo 2010; Yang 2014; Yoshida 2010). Pooled data on 529 participants from 10 studies do not show a significant difference between groups (MD ‐2.24, 95% CI ‐4.69 to 0.20; I² = 54%; Analysis 1.11) (Brouwer 2006; Goebel 2010; Han 2012; Majamaa 1997; Passeron 2006; Sistek 2006; Weston 2005; Woo 2010; Yang 2014; Yoshida 2010). It is noted again that authors of the Yang 2014 and Majamaa 1997 studies reported their results using non‐parametric statistics, which we converted to parametric ones (see Methods; Unit of analysis issues). Inclusion of data from Yang 2014 in the meta‐analysis changed the overall significance of the outcome; this inclusion should be considered with caution.
1.11. Analysis.
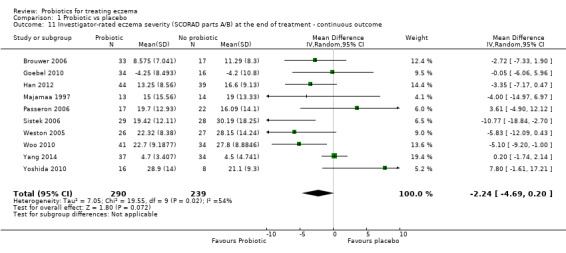
Comparison 1 Probiotic vs placebo, Outcome 11 Investigator‐rated eczema severity (SCORAD parts A/B) at the end of treatment ‐ continuous outcome.
In Cukrowska 2008, a categorical analysis of total SCORAD scores shows no significant differences between the two groups. Gromert 2009 (abstract publication only) reported that "the extension of the eczema was significantly decreased over time in the group given probiotic compared to the group given placebo (P = 0.024)".
We could not pool data from the other studies for this outcome measure. Taniuchi 2005 showed a significant reduction in symptom scores from baseline in both probiotic and placebo groups, but study authors did not present any statistical comparison between the two groups. Isolauri 2000 reported assessments as median scores with an interquartile range (shown in Table 3) and showed a statistically significant difference between end of treatment SCORAD scores for probiotic versus placebo groups. We could not pool the data from this study in Analysis 1.11, because SCORAD scores were so low that it could not be assumed that the median score approximates the mean. Kirjavainen 2003 reported mean total SCORAD score at the end of treatment (eight in the placebo group and five in the probiotic group) without statistical analysis of this difference; additionally, the duration of active treatment varied greatly between participants in this study. Passeron 2006 reported an investigator's global assessment scale of eczema improvement at the end of treatment for 39 participants; however, results show no significant differences in the risk of worse, unchanged, or mildly improved eczema between probiotic and no probiotic treatments (OR 0.41, 95% CI 0.07 to 2.38; data not presented as a forest plot). We could not include data from Shafiei 2011 in the meta‐analysis, but study authors reported no significant differences between groups. Matsumoto 2014 did not use a validated severity score and made no comment on study results in the publication. Guo 2015 did not use a validated score index to assess eczema severity but assessed improvement after interventions according to four grades: "complete resolution", "good response", "partial response", and "no response". Researchers considered participants who showed complete resolution and good response as responders, and those with partial and no response as non‐responders. At the end of treatment, study authors reported a response rate in the probiotic group of 91.1% and in the control arm of 76.7%, which they found to be statistically significant (P < 0.05).
Changes in global eczema severity or change in the number of eczema flares as measured by participants, parents, principal carers, or a medical practitioner in the six‐month period after active treatment has ceased
Seven studies with 581 participants and available data on 509 participants reported total SCORAD scores after active treatment had ceased for two (Han 2012; Roessler 2007), four (Ivankhnenko 2013; Sistek 2006; Wang 2015), and eight weeks post end of active treatment (Iemoli 2012; Weston 2005). Results show significant differences favouring probiotics over no probiotics (MD ‐7.72, 95% CI ‐11.85 to ‐3.59). We noted very high levels of statistical heterogeneity (83%) between studies of this analysis (Analysis 1.12), for which reasons are not clear but may involve differences in follow‐up times between studies. Pooling of data from parallel trials shows significant differences favouring only probiotics (MD ‐9.27, 95% CI ‐13.88 to ‐4.65), which exceeds the MCID of 8.7 in SCORAD demonstrated by Schram 2011. Even so, very high levels of heterogeneity (82%) may be due to differences in follow‐up duration between studies.
1.12. Analysis.
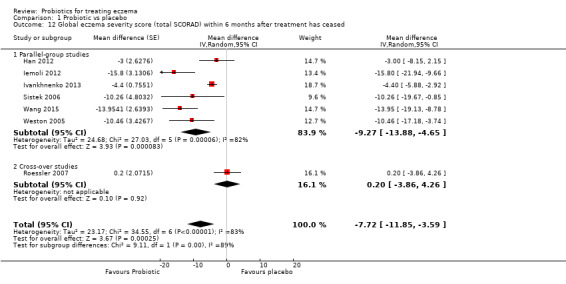
Comparison 1 Probiotic vs placebo, Outcome 12 Global eczema severity score (total SCORAD) within 6 months after treatment has ceased.
Majamaa 1997 reported median SCORAD scores of 16 (interquartile range (IQR) 6 to 25) in probiotic‐treated infants, and 14 (IQR 2 to 38) in placebo‐treated infants, one month after the study intervention had ceased with no statistical analysis presented. Isolauri 2000 reported median SCORAD scores of 0 in all active and placebo‐treated groups at six‐month follow‐up; however, the duration of study interventions provided in this study is not clear. Viljanen 2005 reported mean changes in SCORAD score four weeks after study interventions had ceased. Lactobacillus rhamnosus GG (LGG)‐treated infants had a mean reduction of 22.9 points, probiotic mix‐treated infants 20.4 points, and placebo‐treated infants 20.3 points, with no statistically significant differences between treatment groups at this time point. Finally, Folster‐Holst 2006 reported mean SCORAD scores four weeks after study interventions had ceased ‐ probiotic‐treated participants had a mean score of 32.8 versus 30.1 for placebo.
Pooled analysis of data from two studies with 102 participants show significant improvement in investigator‐rated eczema extent and severity (SCORAD parts A/B) in favour of probiotic treatment (MD ‐8.11, 95% CI ‐13.14 to ‐3.09) eight and four weeks, respectively, after cessation of treatment (Weston 2005; Sistek 2006; Analysis 1.13). We detected no statistical heterogeneity between these two studies (I² = 0%).
1.13. Analysis.
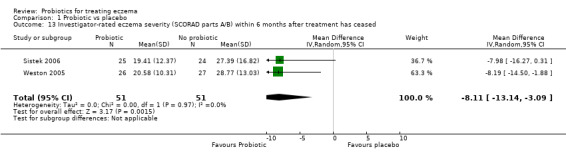
Comparison 1 Probiotic vs placebo, Outcome 13 Investigator‐rated eczema severity (SCORAD parts A/B) within 6 months after treatment has ceased.
Cukrowska 2008 presented changes in total SCORAD five months after the end of intervention as categorical data (improvement vs no improvement or exacerbation) and showed no significant differences between groups.
Gore 2011 and Van der Aa 2010 reported total SCORAD scores for longer than six months post treatment, hence longer than the time period defined in our review protocol. Gore 2011 provided data for total SCORAD 12, 18, and 36 months post treatment showing no significant differences between probiotic and placebo. Van der Aa 2010 showed total SCORAD scores at one year post treatment without statistical analysis, reporting slightly higher scores in the probiotic group than in the placebo group (mean ± SD: 35.4 ± 10.8 and 33.9 ± 10.6 in probiotic and placebo groups, respectively).
Guo 2015 reported recurrence rates over a period of three months after the end of treatment: 26.7% of participants in the probiotic arm had recurrence versus 68.9% of those in the control arm, which researchers found to be statistically significant (P < 0.05).
Changes in the number of days lost from school or work due to eczema symptoms during active treatment
No study reported this outcome.
Adverse events during the treatment period
Eight studies reported adverse events (AEs) in 105/624 participants (Folster‐Holst 2006; Gruber 2007; Matsumoto 2014; Passeron 2006; Sistek 2006; Wang 2015; Weston 2005; Wu 2012). One of these participants with vomiting withdrew from the study. Pooled data on gastrointestinal adverse events among 402 participants from seven of those trials with 427 randomised participants show no significant differences in adverse event rates between probiotic and control groups (RR 1.54, 95% CI 0.90 to 2.63; I² = 0%; Analysis 1.14) (Folster‐Holst 2006; Gruber 2007; Matsumoto 2014; Passeron 2006; Sistek 2006; Weston 2005; Wu 2012). We could not pool data from Wang 2015 because it was not clear which of the adverse events happened in each group. Trial authors stated that there were "no group differences in bowel cramps, fecal frequency, and gastroenteritis".
1.14. Analysis.
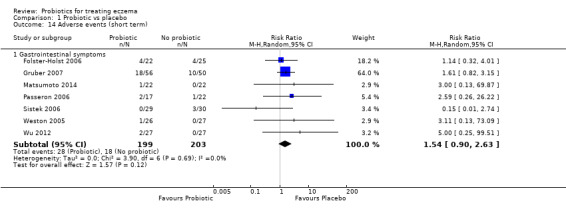
Comparison 1 Probiotic vs placebo, Outcome 14 Adverse events (short term).
Gerasimov 2010 found no significant differences in gastrointestinal and total adverse events. Investigators reported a total of 38 AEs in the probiotic group versus 35 in the control group, and a total of 14 gastrointestinal AEs (three diarrhoea, six constipation, five abdominal colic) in the probiotic group versus 12 (two diarrhoea, six constipation, four abdominal colic) in the control group. They considered no serious AEs (burn, croup, head injury, food poisoning) to be related to treatment.
Parents in Gore 2011 (42/137; 30.7%) reported some difficulties (e.g. green loose stools, increased vomiting, feed refusal, colic) that were considered related to changes in formula, and 24/137 (17.5%) stopped the formula. It is uncertain whether these difficulties were due only to the formula or to the probiotic, as researchers reported numbers from both groups.
Four studies reported no significant AEs during treatment (Drago 2012; Farid 2011; Iemoli 2012; Shafiei 2011). No other study provided any data on AEs.
Authors in Wu 2015 reported 35 AEs in the probiotic group and 37 in the control group but provided no details on the nature of these events or the statistical analysis. They stated that these events were not related to study products.
We did not update for this review update the separate search for adverse events that was done for the first review, which included non‐RCT data (Boyle 2006a). Please see Differences between protocol and review. This search revealed four case reports of sepsis related to probiotic use (Cherifi 2004; De Groote 2005; Lestin 2003; Riquelme 2003), including one death (Lestin 2003). It also revealed five reports of human safety assessments using probiotics (Burton 2006; Connolly 2005; Makelainen 2003; Srinivasan 2006; Wolf 1998), as well as four review articles on probiotic safety (Borriello 2003; Boyle 2006a; Ishibashi 2001; Salminen 1998). Safety assessments demonstrated no adverse effects of probiotics in humans, but case reports and review articles documented a total of 42 cases of suspected or proven probiotic sepsis (Boyle 2006a). Study authors did not definitively identify the probiotic origin of the infective organism in all of these 42 cases, and it is not possible to quantify the risk of such outcomes from available data. A subsequent report described increased risk of fatal bowel ischaemia in critically ill patients treated with one particular combination of probiotics (Besselink 2008). One review proposed some relative contraindications to probiotic use in view of the risk of sepsis (Boyle 2006a).
Stratified analyses
We undertook the following planned stratified analyses.
Analysis by age
We analysed global change in eczema symptoms and symptom scores (SCORAD part C) as well as global eczema severity scores from studies stratified by age (Analysis 1.15; Analysis 1.16; Analysis 1.17). Analysis of SCORAD part C scores shows no significant differences in symptom scores between probiotic and control treatments (age under 2 years: MD ‐0.39, 95% CI ‐2.20 to 1.42; I² = 58%; age 2 to 12 years: MD ‐0.63, 95% CI ‐2.04 to 0.78; I² = 64%; adults: MD 1.01, 95% CI ‐0.82 to 2.84; I² = 0%; Analysis 1.16).
1.15. Analysis.
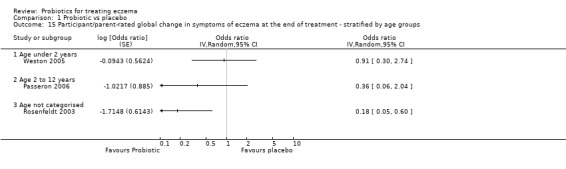
Comparison 1 Probiotic vs placebo, Outcome 15 Participant/parent‐rated global change in symptoms of eczema at the end of treatment ‐ stratified by age groups.
1.16. Analysis.
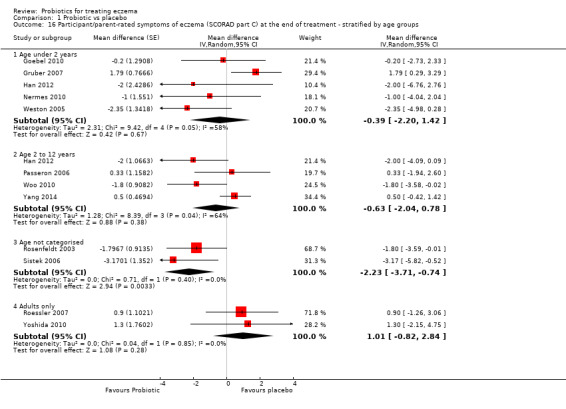
Comparison 1 Probiotic vs placebo, Outcome 16 Participant/parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by age groups.
1.17. Analysis.
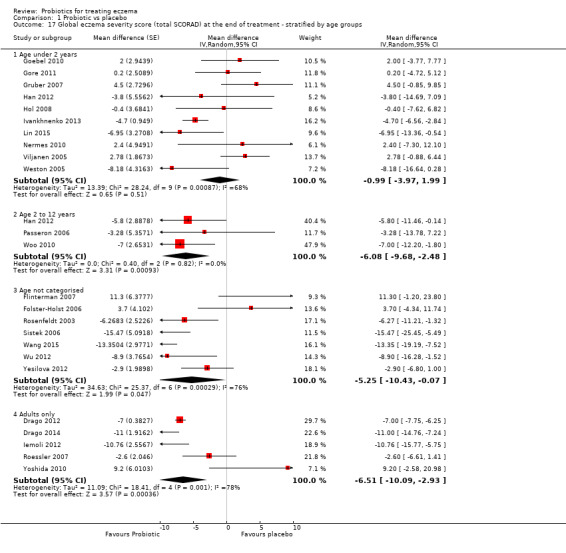
Comparison 1 Probiotic vs placebo, Outcome 17 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by age groups.
Analysis of total SCORAD scores by age group shows no significant differences in total SCORAD between probiotic and control treatments among those under two years of age (MD ‐0.99, 95% CI ‐3.97 to 1.99; I² = 68%); however, data from older age groups show a significant difference in favour of probiotics (age 2 to 12 years: MD ‐6.08, 95% CI ‐9.68 to ‐2.48; I² = 0%; adults: MD ‐6.51, 95% CI ‐10.09 to ‐0.07; I² = 78%; Analysis 1.17).
Analysis by antibiotic use during study intervention
Viljanen 2005 separately evaluated participants not exposed to antibiotics during the intervention period but did not report any end‐of‐treatment outcomes for this group.
Analysis by atopy
Three studies included only participants with proven atopy (Flinterman 2007; Sistek 2006; Wang 2015), and another study separately evaluated treatment effects in participants with atopy (Viljanen 2005). We could not pool SCORAD scores at the end of treatment for these studies due to extreme levels of statistical heterogeneity between trials (I² = 91%). We noted a significant difference in total SCORAD scores between probiotic and no probiotic groups when we analysed studies with a mix of atopic and non‐atopic participants (MD ‐4.15, 95% CI ‐6.02 to ‐2.27), and we detected high levels of heterogeneity (I² = 74%) (Analysis 1.18).
1.18. Analysis.
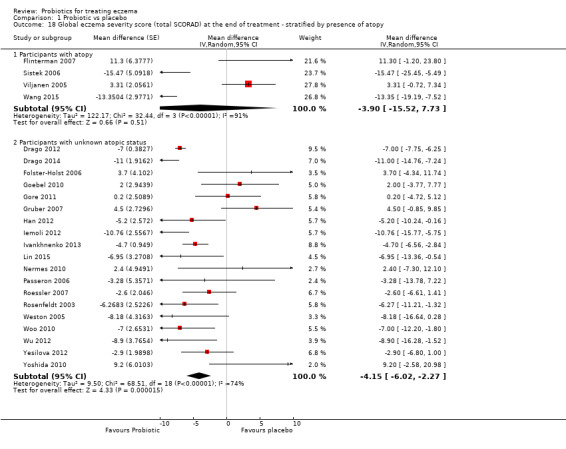
Comparison 1 Probiotic vs placebo, Outcome 18 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by presence of atopy.
Cukrowska 2008 presented results for IgE‐dependent and IgE‐independent eczema, defining IgE‐dependent eczema as raised total IgE or specific IgE to certain food allergens (not specified). Investigators reported data for total SCORAD as a dichotomous outcome ‐ "improvement versus no improvement/exacerbation" ‐ and noted a significant difference between groups favouring probiotics during the intervention period (P = 0.0329), but not during the five‐month post‐treatment period.
Van der Aa 2010, in a subgroup of participants with IgE‐associated eczema, found a significant difference in reduction of total SCORAD at the end of treatment compared with baseline, favouring probiotics (MD ‐4.6, 95% CI ‐9.1 to ‐0.1; P = 0.04). Researchers defined IgE‐associated atopic dermatitis as atopic dermatitis with raised total and/or specific serum IgE levels to house dust mites, cat, cow's milk, peanut, and egg at baseline.
In Gerasimov 2010, 53 out of 96 participants (25 in the probiotic group and 28 in the placebo group) had raised IgE (> 50 IU/mL). Data on the subgroup of participants with raised IgE show a significant difference in reduction of total SCORAD at the end of treatment favouring probiotics (P = 0.006). Data on the subgroup of participants without raised IgE (43 out of 96) show differences in the reduction of total SCORAD at the end of treatment that were not significant (P = 0.068). Study authors suggested that overall significant results for all participants favouring probiotics may be attributed to participants with IgE‐associated eczema, who constituted more than half of the study population.
Analysis by food allergy
Viljanen 2005 separately evaluated SCORAD scores in participants with proven cow's milk allergy. All participants in Ivankhnenko 2013 had proven cow's milk allergy. In Hol 2008, in which all participants had proven cow's milk allergy, data were available only for participants who had moderate to severe eczema. Results show no significant differences in end of treatment SCORAD scores between probiotic and placebo groups (MD ‐1.84, 95% CI ‐6.22 to 2.54) with extreme levels of statistical heterogeneity between trials (70%) (Analysis 1.19). We found significant differences in SCORAD scores favouring probiotics when we evaluated studies with a mix of food‐allergic and non‐food‐allergic participants (MD ‐3.21, 95% CI ‐5.63 to ‐0.79); however, we detected extreme levels of statistical heterogeneity between trials (I² = 76%) (Analysis 1.19)
1.19. Analysis.
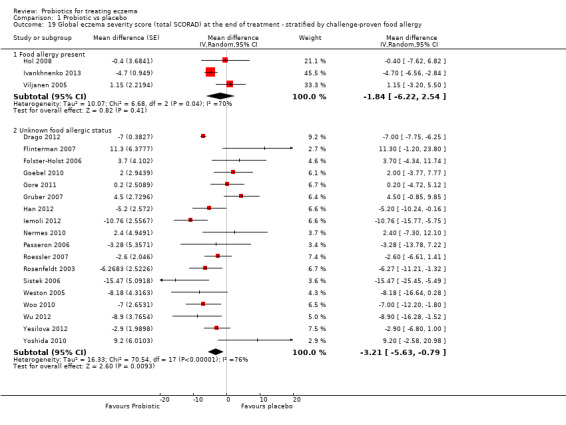
Comparison 1 Probiotic vs placebo, Outcome 19 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by challenge‐proven food allergy.
Analysis by intestinal inflammation
No study provided data for this subgroup analysis.
Analysis by disease severity
Six studies with 421 participants provided sufficient data to stratify end of treatment SCORAD scores by disease severity (Analysis 1.20) (Goebel 2010; Gruber 2007; Han 2012; Passeron 2006; Sistek 2006; Weston 2005). No evidence shows a difference in treatment efficacy according to disease severity (severe eczema: MD ‐3.71, 95% CI ‐10.05 to 2.64; I² = 0%; moderate eczema: MD ‐2.95, 95% CI ‐7.65 to 1.74; I² = 62%; mild eczema: MD ‐5.53, 95% CI ‐15.29 to 4.23).
1.20. Analysis.
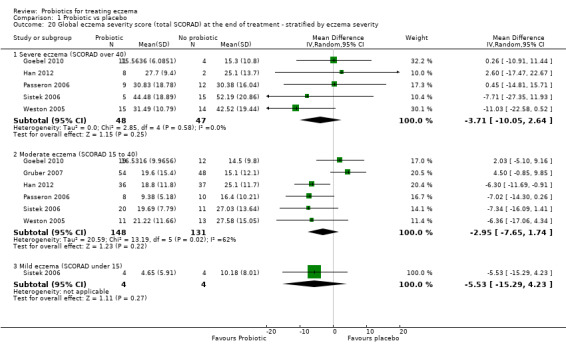
Comparison 1 Probiotic vs placebo, Outcome 20 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by eczema severity.
Gerasimov 2010 reported changes in total SCORAD scores by disease severity at the end of treatment. For both moderate and severe disease, the data favoured probiotics.
Analysis by probiotic species or strain
This stratified analysis was not a priori specified in the study protocol, but we undertook the analysis due to use of the same probiotic strain in some studies and heterogeneity between study results noted for some outcomes in this review. For this analysis, we analysed data for SCORAD part C and total SCORAD at end of treatment. We categorised studies as follows.
Lactobacillus GG, L rhamnosus, L salivarius, L casei and paracasei, and any Lactobacillus species alone or in combination with or without probiotics.
Bifidobacterium lactis, B breve, or any Bifidobacterium species alone or in combination with or without prebiotics.
Single versus multiple probiotics, with or without prebiotics
Probiotics without prebiotics.
Results show no significant differences in participant‐/parent‐rated symptoms of eczema (SCORAD part C) between groups for any of the probiotic subgroups (Analysis 1.21; Analysis 1.22; Analysis 1.23; Analysis 1.24).
1.21. Analysis.
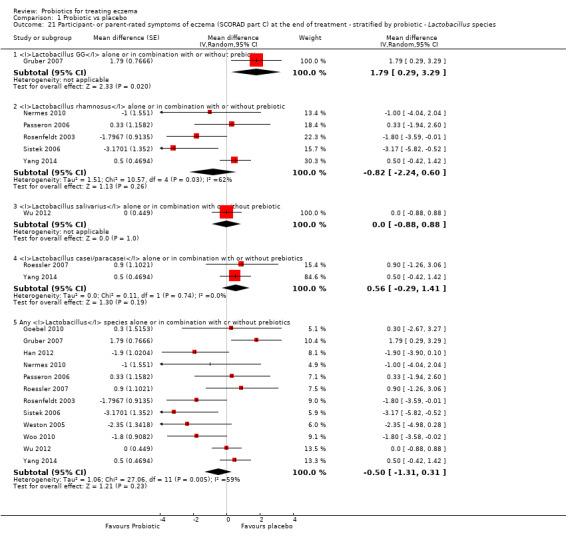
Comparison 1 Probiotic vs placebo, Outcome 21 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species.
1.22. Analysis.
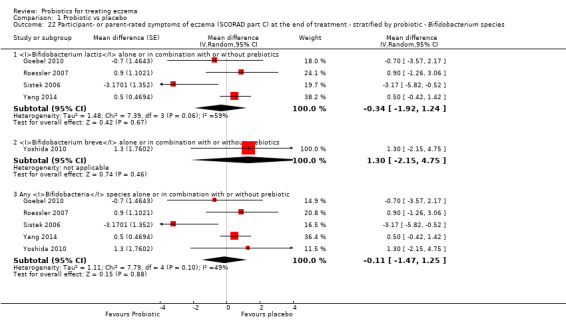
Comparison 1 Probiotic vs placebo, Outcome 22 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species.
1.23. Analysis.
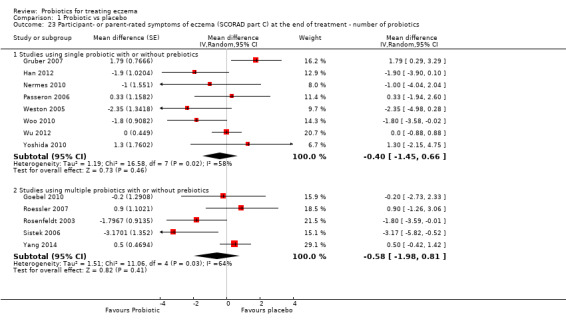
Comparison 1 Probiotic vs placebo, Outcome 23 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ number of probiotics.
1.24. Analysis.
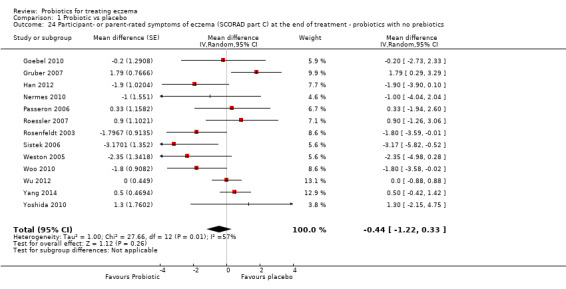
Comparison 1 Probiotic vs placebo, Outcome 24 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ probiotics with no prebiotics.
Pooled data (Analysis 1.25) show significantly higher total SCORAD scores after treatment with Lactobacillus GG compared with placebo (MD 3.37, 95% CI 0.55 to 6.20; I² = 0%). A significant difference in total SCORAD scores favoured probiotics compared with no probiotics after treatment with Lactobacillus salivarius (MD ‐6.86, 95% CI ‐10.08 to ‐3.63; I² = 74%; data from six studies: Drago 2012; Drago 2014; Flinterman 2007; Iemoli 2012; Wu 2012; Yesilova 2012), or with any Lactobacillus species (MD ‐3.80, 95% CI ‐6.06 to ‐1.54; I² = 79%; data from 21 studies: Drago 2012; Drago 2014; Flinterman 2007; Folster‐Holst 2006; Goebel 2010; Gore 2011; Gruber 2007; Han 2012; Hol 2008; Iemoli 2012; Nermes 2010; Passeron 2006; Roessler 2007; Rosenfeldt 2003; Sistek 2006; Viljanen 2005; Wang 2015; Weston 2005; Woo 2010; Wu 2012; Yesilova 2012), but with significant heterogeneity. Results show no significant differences in total SCORAD scores between probiotics and no probiotics after treatment with L rhamnosus, L casei, and L paracasei, and no significant differences in total SCORAD scores between probiotics and no probiotics after treatment with Bifidobacterium lactis, B breve, and any Bifiidobacterium species (Analysis 1.26). Data show a significant difference in total SCORAD scores between probiotics and no probiotics when treatment consisted of a single or multiple probiotic species (Analysis 1.27). A significant difference in total SCORAD scores favoured probiotics compared with no probiotics after treatment with any probiotic alone with no prebiotic (Analysis 1.28) (MD ‐3.83, 95% CI ‐5.81 to ‐1.86), but data show very high levels of heterogeneity (I² = 80%).
1.25. Analysis.
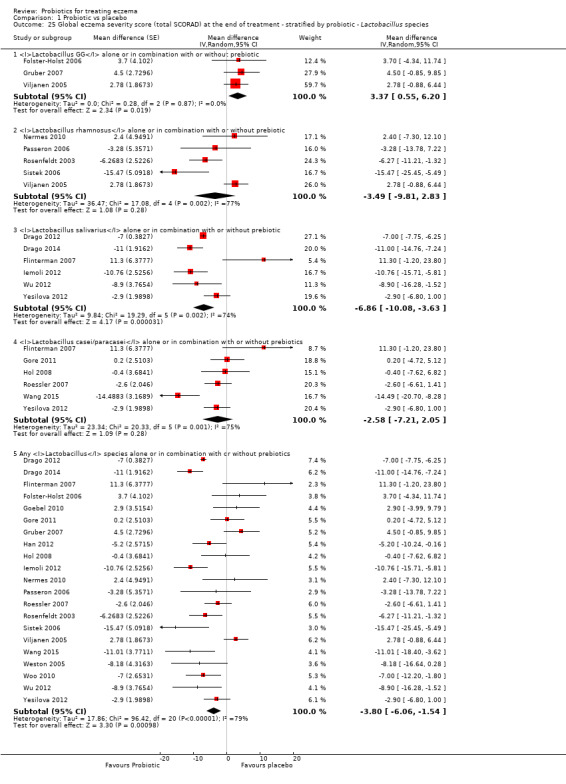
Comparison 1 Probiotic vs placebo, Outcome 25 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species.
1.26. Analysis.
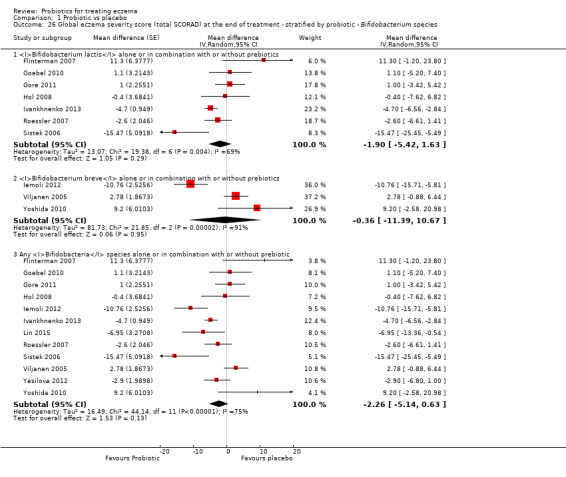
Comparison 1 Probiotic vs placebo, Outcome 26 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species.
1.27. Analysis.
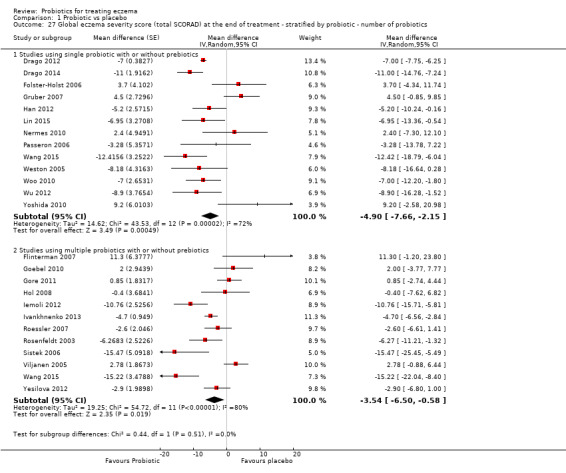
Comparison 1 Probiotic vs placebo, Outcome 27 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ number of probiotics.
1.28. Analysis.
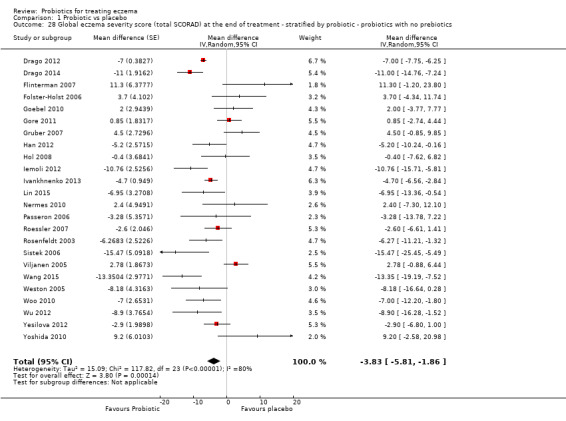
Comparison 1 Probiotic vs placebo, Outcome 28 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ probiotics with no prebiotics.
For the stratified analyses Analysis 1.16,Analysis 1.21,Analysis 1.22,Analysis 1.23, and Analysis 1.24, we used data from Yang 2014, which we converted from non‐parametric to parametric statistics (see Methods: Unit of analysis issues). Inclusion of these data should be considered with caution but did not change the overall significance of the findings of analyses.
Assessment of reporting bias in the meta‐analyses
We created funnel plots for continuous outcomes for which an adequate number of included studies provided data: participant‐ or parent‐rated symptoms of eczema at the end of treatment (Analysis 1.1), global eczema severity score at the end of treatment (Analysis 1.8), and global eczema severity score/sensitivity analysis/change score (Analysis 1.9). See Figure 7, Figure 8 and Figure 9, respectively.
7.
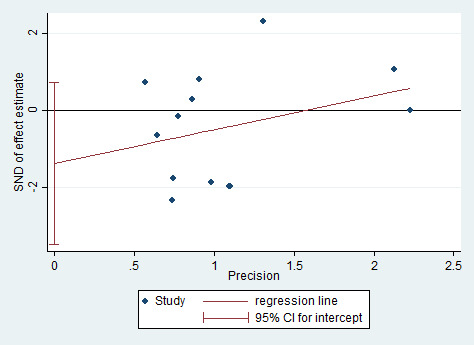
Egger's plot for Analysis 1.1: probiotic vs placebo for participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment.
8.
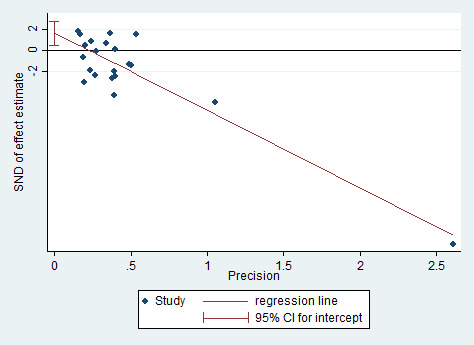
Egger's plot for Analysis 1.8: probiotic vs placebo for global eczema severity score (total SCORAD) at the end of treatment.
9.
Egger's plot for Analysis 1.9: probiotic vs placebo for global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score.
We performed Egger's test for asymmetry as a formal assessment of publication bias. For Analysis 1.1 and Analysis 1.9, P values from Egger's test show no significant asymmetry (P = 0.18 and P = 0.479, respectively; Figure 7 and Figure 9). However, for Analysis 1.8, the P value from Egger's test is significant, which indicates evidence of asymmetry in the plot for this outcome (P = 0.007) (Figure 8); however, the asymmetry appears to be related to an over‐influential study (Drago 2012). A sensitivity analysis excluding this study yielded a P value from Egger's test that was non‐significant (P = 0.357). Furthermore, we noted extreme levels of heterogeneity (I² = 79%) between studies for this outcome, which can lead to funnel plot asymmetry; therefore this interpretation should be considered with caution.
Discussion
Summary of main results
We included 39 randomised controlled trials (RCTs) with 2599 participants in the update of this review. Participants included both males and females and infants and adults, although most were children. The probiotics used in these studies were Lactobacillus and Bifidobacteria species, given as a single strain or in probiotic mixtures with or without prebiotics. Evidence shows significant heterogeneity between studies for most of the outcomes of the review (Table 1). Apart from the large variety of probiotics used, the variety of doses and concentrations of probiotics in the preparations used may have contributed to heterogeneity. For the following key results, the comparator was no probiotics, active treatment ranged from six weeks to three months (apart from the investigator‐rated eczema severity outcome, for which the upper limit of active treatment was 16 weeks), and outcome measurement occurred at the end of active treatment (except for adverse events, which were measured during the active treatment period).
Data from 13 studies with 754 participants contributed to our primary outcome ‐ changes in participant‐, parent‐, or principal carer‐rated symptoms of eczema at the end of active treatment ‐ and suggest that probiotics probably make little or no difference in eczema symptoms (moderate‐quality evidence). Post hoc trial sequential analysis shows that our analysis exceeded the sample size necessary to demonstrate a minimum difference of 1.5 points on a 20‐point scale in eczema symptoms between probiotics and placebo, and suggested that further trials of similar probiotic strains for this outcome at the end of active treatment may be futile.
Data from six studies with 552 participants were available for our other primary outcome ‐ changes in quality of life at the end of active treatment. We found no evidence that probiotics make a difference in quality of life for eczema sufferers (low‐quality evidence).
We found no data for our secondary outcome ‐ changes in the number of days lost from school or work due to eczema symptoms during treatment with probiotics.
For our fourth secondary outcome ‐ investigator‐rated eczema severity ‐ data from 24 studies with 1596 participants suggest that probiotics may slightly improve the composite severity score for Severity Scoring of Atopic Dermatitis (SCORAD). However, this difference is of uncertain clinical significance (low‐quality evidence).
Seven studies (402 participants) reported adverse effects during active treatment, and we found no evidence of a difference between probiotic use and use of no probiotics (low‐quality evidence). Adverse events related to treatment that were reported during the treatment period were gastrointestinal in nature (e.g. diarrhoea, vomiting).
For the update of this review, we did not perform a new search for adverse events. The adverse events search conducted for the first review found case reports of proven or suspected sepsis related to probiotic use (Boyle 2006a; Cherifi 2004; De Groote 2005; Lestin 2003; Riquelme 2003), including one death (Lestin 2003), and one report described increased risk of fatal bowel ischaemia in critically ill patients treated with one particular combination of probiotics.
Despite the large number of studies and participants included in this review, several outstanding uncertainties still surround the use of probiotics for treatment of eczema. These include the following: reasons for heterogeneity among trials; shortage of data on quality of life and other outcomes such as impact of probiotics on days lost from school/work or on use of other eczema treatments; use of non‐validated quality of life scores or recommended outcome measures by the HOME (Harmonizing Outcome Measures for Eczema) initiative; effects of probiotics on specific groups of patients (i.e. patients with atopy, food allergies, or skin of colour); effects of probiotics after the end of treatment; identification of the optimal dose/concentration or strain of probiotics; and adverse effects of probiotics.
Overall completeness and applicability of evidence
The numbers of studies and participants included after an updated search were larger than in the first review. Studies included all age groups, but only six studies looked at adults. Although the addition of studies to this update led to narrower confidence intervals, significant statistical heterogeneity between studies for primary and secondary outcomes remains evident.
Review results apply only to currently available and tested probiotic strains and at doses used in the included studies, which included Lactobacillus and Bifidobacteria species given as a single strain or in probiotic mixtures with or without prebiotics, and at varied doses and concentrations. We found no studies on non‐lactic acid bacteria.
Only 11 studies reported quality of life data at the end of active treatment, and of these, we could include in our analysis data from only six studies. The relatively small numbers of studies and participants reporting this outcome and the varied outcome assessment scales used mean that conclusions in relation to quality of life are limited.
Only eight studies clearly stated the presence and nature of any adverse events and the treatment groups in which they occurred.
Studies may be sufficient to show whether changes in participant‐ or parent‐reported symptoms of eczema at the end of active treatment are influenced by those probiotics studied in RCTs to date. However, for symptom changes during the six‐month period after active treatment has ceased and for other outcome measures (i.e. quality of life changes, days lost from school or work during active treatment, changes in the need for other eczema treatment during active treatment or within the six‐month period after active treatment has ceased, global eczema severity changes, and adverse events during treatment, as well as for other probiotics or the same probiotics but at different doses/concentration), studies are insufficient to provide clear conclusions. One limitation of many of the probiotic studies is that diverse concentrations and doses of bacteria are used without standardisation. We had to pool studies without assessing the rationale for strain selection or concentration. We performed stratified analysis by probiotic species post hoc because studies used so many different types of probiotics.
Studies have assessed a wide range of participants who met the review inclusion criteria: they included both genders and all age groups and individuals from differing countries of origin. Eczema ranged from mild to severe. This review includes data from a large number of trials showing the effects of probiotics on symptoms of eczema, but several uncertainties remain regarding the review's other outcomes of interest, as well as the optimal dose and strain of probiotics and the effects of probiotics in specific groups of patients (e.g. those with different skin types, atopic patients). This review reveals the uncertainty resulting from the suboptimal methodological quality of included studies and the need for use of validated outcome measures to facilitate standardisation of clinical trials and comparison of their outcomes.
Quality of the evidence
The overall quality of studies was mixed, largely due to missing information regarding randomisation procedures, blinding, and losses to follow‐up. We assessed only nine studies as being at low risk of bias because the randomisation process was clear; allocation concealment was clear and done; participants, clinicians, or outcome assessors were blinded; and we noted no attrition bias. One of these studies reported a chance imbalance in disease severity at baseline (Sistek 2006), and five were sponsored or co‐sponsored by the probiotic supplier (Drago 2012; Flinterman 2007; Van der Aa 2010; Viljanen 2005; Wang 2015).
We assessed the quality of evidence using the GRADE tool for key outcomes (Table 1). For 'changes in the participant‐ or parent‐/principal carer‐rated score of symptoms of eczema at the end of active treatment' (measured using SCORAD part C; continuous outcomes), we downgraded the quality of evidence to moderate because of significant heterogeneity among studies. For the 'participant‐ or parent‐/carer‐rated global change of eczema symptoms at the end of active treatment' (measured as 'worsened/unchanged or improved'; binary outcomes), we downgraded the quality of evidence to low because of the small number of studies reporting this outcome and the moderate heterogeneity between them. We downgraded the quality of evidence for 'changes in participant‐ or parent‐rated quality of life at the end of active treatment' to low because of the very small number of studies that reported this outcome and the significant heterogeneity noted between them. For 'participant‐ or parent‐rated family quality of life at the end of active treatment', we downgraded the quality of evidence to very low because of the very small number of studies that reported this outcome and significant heterogeneity. For 'global eczema severity score at the end of active treatment', we downgraded the quality of evidence to low because of extreme levels of heterogeneity between studies and evidence of reporting bias. For 'adverse events', we downgraded the quality of evidence to low due to the small number of studies reporting adverse events and the small number of events that could be included in the analysis.
Potential biases in the review process
Our inability to contact all trial authors for original data sets and clarifications may have introduced some uncertainty into our judgement on inclusion of studies and into our findings.
Analyses of SCORAD change scores and of SCORAD scores from studies with low risk of bias were post hoc analyses, justified by the imbalance in treatment severity at baseline in one included study. Any conclusions based on these data must therefore be guarded. The subgroup analysis by probiotic strain used was not predefined in the study protocol, and we undertook this due to the observation that some studies used the same probiotic strain. Analysis suggests that some probiotic strains may be more effective than others for the treatment of eczema; however, conclusions based on this post hoc analysis must also be guarded.
Also, investigators used variable concentrations and daily doses of different probiotics, which may have influenced study findings. Some of the heterogeneity noted in analyses of outcomes may be attributable to varying timing of outcome assessment; however, we performed no subgroup analysis by probiotic dose or concentration.
Agreements and disagreements with other studies or reviews
The data summarised in this review suggest that currently available probiotic strains that have been evaluated in RCTs probably are not effective for the treatment of symptoms of eczema and may not be clinically effective in changing eczema severity.
Previous studies found an association between the composition of the intestinal microbiota and eczema (Bjorksten 2001; Kalliomaki 2001), as well as increased gastrointestinal symptoms in children with eczema (Caffarelli 1998). These previous studies suggest that the composition of the intestinal microbiota is important in the pathophysiology of eczema, or that intestinal mucosal abnormalities associated with eczema lead to secondary changes in the resident intestinal microbiota. Evidence suggests that probiotics can lower the increased intestinal permeability associated with eczema (Rosenfeldt 2004), and studies such as Kalliomaki 2001,Kukkonen 2007, and Moro 2006, and systematic reviews including Dang 2013,Doege 2012,Mansfield 2014,Osborn 2007, and Zhu 2010, suggest that use of some probiotics or prebiotics during early infancy or pregnancy may prevent the development of eczema. Probiotics may therefore be ineffective for treating eczema, as shown in this current review, because the decrease in intestinal permeability associated with their use is insufficient to lead to resolution of established disease, or because the duration of treatment fails to allow sufficient modulation of intestinal microbiota composition or function to result in clinically meaningful change.
In this review, we found no significant differences in adverse events between probiotic and control during active treatment, and reported adverse events were of gastrointestinal upset. For the update of this review, we found three systematic reviews on the safety of probiotics, which included RCT and non‐RCT data. One systematic review did not show any statistically significant increased risk for adverse events associated with probiotics used for short periods in the setting of RCTs but noted lack of systematic reporting of adverse events in studies of probiotic interventions (Hempel 2011). Another systematic review described no significant adverse events in the setting of RCTs (Didari 2014). Both reviews found case reports of bacteraemia/fungaemia and sepsis and indicated that immunocompromised, critically ill, and postsurgical patients may be at greater risk (Didari 2014; Hempel 2011). Serious adverse events were rare. One systematic review of the safety of probiotics during pregnancy showed no difference in caesarean section rates, birth weight, and gestational age between probiotic and control groups (Dugoua 2009).
Three recent systematic reviews have examined studies of probiotics for treatment of eczema (Chang 2016a; Huang 2017; Kim 2014). All systematic reviews evaluated a primary outcome of total SCORAD score.
The most recent systematic review gathered data from 13 studies and 1070 child participants up to 18 years of age and showed a significant difference in SCORAD favouring probiotics over control, with mean reduction in SCORAD by 3.07 with a 95% confidence interval (CI) of ‐6.12 to ‐0.03 (Huang 2017). This review included studies published from 2000 to 2017 and only those published in English. Researchers did not find this favouring effect for probiotics among children under one year of age, only among children between 1 and 18 years of age (mean difference (MD) ‐4.50, 95% CI ‐7:45 to ‐1.54). These results are consistent with the data provided in our review, but in our review, we prioritised patient‐reported outcomes and noted that the size of effect on SCORAD is of uncertain clinical significance. Huang 2017 found greater differences favouring probiotics in subgroup analyses by continent (Europe: no difference; Asia: MD ‐5.39, 95% CI‐8.91 to ‐1.87; Australia: MD ‐11.20, 95% CI ‐13.76 to ‐8.64).
A second systematic review limited its focus to the use of probiotics in combination with prebiotics (synbiotics) (Chang 2016a), although review authors also included trials of synbiotics versus prebiotics. This review did not have a registered protocol and did not use GRADE to evaluate the quality of evidence. Review authors identified six trials with 369 child participants 0 to 14 years of age, studying the role of synbiotics for treating eczema. Treatment duration was between eight and 12 weeks, and review authors used pooled estimates for the change in SCORAD at eight weeks to assess clinical effects. A decrease in SCORAD score of 6.56 favoured synbiotics with a 95% CI of ‐11.43 to ‐1.68 and high statistical heterogeneity (I² = 77%). Results of this study are slightly more favourable towards probiotics compared with studies included in our review, with a high mean decrease in SCORAD, but they reflect a small subset of the studies identified in our systematic review. Differences in findings and conclusions may be due to different eligibility criteria, different outcome measures, and a different methodological approach.
An older systematic review with data from 25 studies and 1599 participants of all age groups reported a difference in SCORAD score of 4.51 points with a 95% CI of ‐6.78 to ‐2.24 favouring probiotics, and the same favourable effect of probiotics in individuals 1 to 18 years of age and in adults (Kim 2014). Results show no differences between probiotic and placebo in infants (< 1 year old).
Schram 2011 suggested that the minimally clinically important difference for SCORAD score is 8.7.
A World Allergy Organization position consensus statement did not recommend the use of probiotics for treatment of allergic diseases, including eczema (Fiocchi 2012). This qualitative, narrative review of evidence was available in the literature until the time of publication by an expert panel group.
Authors' conclusions
Implications for practice.
Data suggest that probiotics currently in use probably make little or no difference in patient‐rated eczema symptoms (moderate‐quality evidence) and may make no difference in quality of life for people with eczema (low‐quality evidence). Analysis of composite severity score (Severity Scoring of Atopic Dermatitis (SCORAD)) data suggests that any reduction in eczema severity from treatment with currently available probiotic strains is likely to be modest (< 5.86 points on the total SCORAD score) and therefore of uncertain clinical significance. Current use of probiotics for treatment of eczema is not evidence‐based.
We found no evidence to suggest that probiotic treatment is unsafe; however, reports from non‐randomised controlled trials indicate that it can lead to adverse events including sepsis and bowel ischaemia.
Implications for research.
Post hoc trial sequential analysis suggests that further studies of the effect on eczema symptoms of already available probiotic strains at varying concentrations at the end of treatment (up to three months) may be futile.
Future studies should report long‐term (i.e. six months after active treatment has ceased) data on eczema symptoms and quality of life, using validated quality of life scores, and should consider recommendations of the HOME initiative (Harmonizing Outcomes Measures for Eczema) for reporting outcome measures. Furthermore, future studies should ensure that they report methodological details regarding randomisation procedures, blinding, and loss to follow‐up, to ensure that a thorough risk of bias assessment can be done.
Further studies are needed to focus on the strain Lactobacillus salivarius versus placebo. Future studies should consider studying subgroups of patients (e.g. patients with atopy, patients with food allergies, adults, patients with different skin types) and testing new probiotic strains that have not yet been evaluated in randomised controlled trials versus no probiotic, at standardised doses and concentrations.
Future probiotic studies should provide thorough reporting of adverse events. In addition, investigators in future clinical studies should have a clearer understanding of the species and dosing (concentration of bacteria) used.
For future systematic reviews and meta‐analyses, researchers should consider stratified analyses based on dose/concentration of probiotics used because standardisation of dosing of probiotics is currently lacking.
What's new
| Date | Event | Description |
|---|---|---|
| 24 October 2018 | New search has been performed | A new search led to the addition of 27 new included studies, and we updated the review in line with MECIR standards. |
| 24 October 2018 | New citation required but conclusions have not changed | We incorporated GRADE into this update of the review, as well as including a trial sequential analysis. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 8 April 2008 | Amended | Converted to new review format |
| 12 May 2006 | New citation required and conclusions have changed | Made substantive amendments |
Acknowledgements
We are grateful to Judy Taylor and George Varigos for their contributions in writing the protocol for this review.
Cochrane Skin wishes to thank Gloria Sanclemente, who was the Cochrane Dermatology Editor for this review; Sally Wilkes, who was the Statistical Editor; Ching‐Chi Chi, who was the Methods Editor; the clinical referees, Nerys Roberts and Raja Sivamani; and the review's copy‐editor, Dolores Matthews. The review authors would like to thank the Australasian Cochrane Centre for expertise and advice regarding completion of this review, and Damian Jolly for statistical advice.
For the update of the review: we are grateful to Daniel Munblit and Gregor Harris for help with translation of Russian articles and communication with study authors, to Agata Dunsmore and Maciej Studzinski for help with translation of one Polish article and efforts for communication with its author, and to Ivy Ngu for help with translation of a Chinese article.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register (CRS) search strategy
(dermatitis or eczema or neurodermatitis or besnier*) AND (probiotic* or lactobacill* or bifidobacter* or lactococc* or saccharomyc* or microbiome* or microbiotica or "streptococcus thermophilus" or "bacillus subtilis" or "enterococcus faecalis" or "lactic acid bacteri*" or yoghurt or yoghourt or yogourt or yogurt or (gut or intestin* and (flora or microflora or microbiota)))
Appendix 2. CENTRAL (the Cochrane Library) search strategy
#1 probiotic*:ti,ab,kw OR lactobacill*:ti,ab,kw OR bifidobacter*:ti,ab,kw OR lactococc*:ti,ab,kw OR saccharomyc*:ti,ab,kw #2 streptococcus next thermophilus:ti,ab,kw OR bacillus next subtilis:ti,ab,kw OR enterococcus near/6 faec*:ti,ab,kw OR intestin* near/6 microflora:ti,ab,kw OR intestin* near/6 microbiotica:ti,ab,kw #3 lactic acid bacteri* #4 ((gut or intestinal) and (flora or microbiota or microflora)) #5 MeSH descriptor: [Probiotics] explode all trees #6 microbiome* or microbiotica #7 MeSH descriptor: [Lactobacillus] explode all trees #8 MeSH descriptor: [Lactococcus] explode all trees #9 MeSH descriptor: [Bifidobacterium] explode all trees #10 MeSH descriptor: [Saccharomyces] explode all trees #11 MeSH descriptor: [Streptococcus thermophilus] explode all trees #12 MeSH descriptor: [Bacillus subtilis] explode all trees #13 MeSH descriptor: [Enterococcus faecalis] explode all trees #14 (yoghurt or yoghourt or yogourt) .mp. #15 MeSH descriptor: [Yogurt] explode all trees #16 {or #1‐#15} #17 MeSH descriptor: [Eczema] explode all trees #18 MeSH descriptor: [Dermatitis, Atopic] explode all trees #19 MeSH descriptor: [Neurodermatitis] explode all trees #20 MeSH descriptor: [Dermatitis] explode all trees #21 eczema or dermatitis or neurodermatitis or besnier*:ti,ab,kw #22 #17 or #18 or #19 or #20 or #21 #23 #16 and #22
Appendix 3. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exp animals/ not humans.sh. 10. 8 not 9 11. exp Eczema/ or eczema.mp. 12. exp Dermatitis, Atopic/ 13. neurodermatitis.mp. or exp Neurodermatitis/ 14. exp Dermatitis/ or dermatitis.mp. 15. besnier$ prurigo.mp. 16. or/11‐15 17. exp Probiotics/ 18. probiotic$.mp. 19. exp Lactobacillus/ 20. lactobacill$.mp. 21. exp Bifidobacterium/ 22. bifidobacteri$.mp. 23. exp Lactococcus/ 24. lactococc$.mp. 25. exp Saccharomyces/ or saccharomyces.mp. 26. streptococcus thermophilus.mp. or exp Streptococcus thermophilus/ 27. lactic acid bacteri$.mp. 28. bacillus subtilis.mp. or exp Bacillus subtilis/ 29. enterococcus faecalis.mp. or exp Enterococcus faecalis/ 30. microbiome$.mp. 31. ((gut or intestinal) and (flora or microbiota or microflora)).mp. 32. microbiotica.mp. 33. (yoghurt or yoghourt or yogourt).mp. 34. exp Yogurt/ 35. or/17‐34 36. 10 and 16 and 35
[Lines 1‐10: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. crossover procedure.sh. 2. double‐blind procedure.sh. 3. single‐blind procedure.sh. 4. (crossover$ or cross over$).tw. 5. placebo$.tw. 6. (doubl$ adj blind$).tw. 7. allocat$.tw. 8. trial.ti. 9. randomized controlled trial.sh. 10. random$.tw. 11. or/1‐10 12. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 13. human/ or normal human/ 14. 12 and 13 15. 12 not 14 16. 11 not 15 17. eczema.mp. or exp ECZEMA/ 18. exp DERMATITIS/ or dermatitis.mp. 19. exp atopic dermatitis/ 20. neurodermatitis.mp. or exp NEURODERMATITIS/ 21. besnier$ prurigo.mp. 22. or/17‐21 23. exp probiotic agent/ 24. probiotic$.mp. 25. exp Lactobacillus/ 26. lactobacill$.mp. 27. exp Bifidobacterium/ 28. bifidobacteri$.mp. 29. exp Lactococcus/ 30. lactococc$.mp. 31. Saccharomyces.mp. or exp Saccharomyces/ 32. Streptococcus thermophilus.mp. or exp Streptococcus thermophilus/ 33. lactic acid bacteri$.mp. 34. Bacillus subtilis.mp. or exp Bacillus subtilis/ 35. Enterococcus faecalis.mp. or exp Enterococcus faecalis/ 36. microbiome$.mp. 37. ((gut or intestinal) and (flora or microbiota or microflora)).mp. 38. microbiotica.mp. 39. exp intestine flora/ 40. (yoghurt or yoghourt or yogourt).mp. 41. exp yoghurt/ 42. or/23‐41 43. 16 and 22 and 42
Appendix 5. PsycINFO (Ovid) search strategy
1. double‐blind.tw. 2. random$ assigned.tw. 3. control.tw. 4. 1 or 2 or 3 5. eczema.ti,ab. or exp Eczema/ 6. dermatitis.ti,ab. or exp Dermatitis/ 7. neurodermatitis.ti,ab. or exp Neurodermatitis/ 8. besnier$ prurigo.mp. 9. 5 or 6 or 7 or 8 10. probiotic$.mp. 11. (lactobacill$ or bifidobacteri$ or lactococc$ or saccharomyces or microbiome$ or microbiotica).mp. 12. ((gut or intestinal) and (flora or microbiota or microflora)).mp. 13. lactic acid bacteri$.mp. 14. bacillus subtilis.mp. 15. streptococcus thermophilus.mp. 16. enterococcus faecalis.mp. 17. (yoghurt or yoghourt or yogourt).mp. 18. or/10‐17 19. 4 and 9 and 18
[Lines 1‐3: therapy filter for PsycINFO (Ovid) created by the Health Information Research Unit at McMaster University].
Appendix 6. AMED (Ovid) search strategy
1. randomized controlled trial$/ 2. random allocation/ 3. double blind method/ 4. single blind method.mp. 5. exp Clinical trials/ 6. (clin$ adj25 trial$).mp. 7. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$ or dummy)).mp. 8. (placebo$ or random$).mp. 9. research design/ or clinical trials/ or comparative study/ or double blind method/ or random allocation/ 10. prospective studies.mp. 11. cross over studies.mp. 12. Follow up studies/ 13. control$.mp. 14. (multicent$ or multi‐cent$).mp. 15. ((stud or design$) adj25 (factorial or prospective or intervention or crossver or cross‐over or quasi‐experiment$)).mp. 16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp Eczema/ or eczema.mp. 18. dermatitis.mp. or exp Dermatitis/ 19. exp Dermatitis atopic/ 20. neurodermatitis.mp. or exp Neurodermatitis/ 21. besnier$ prurigo.mp. 22. or/17‐21 23. exp Probiotics/ 24. probiotic$.mp. 25. (lactobacill$ or bifidobacteri$ or lactococc$ or saccharomyces or microbiome$ or microbiotica).mp. 26. ((gut or intestinal) and (flora or microbiota or microflora)).mp. 27. lactic acid bacteri$.mp. 28. bacillus subtilis.mp. 29. streptococcus thermophilus.mp. 30. enterococcus faecalis.mp. 31. (yoghurt or yoghourt or yogourt).mp. 32. or/23‐31 33. 16 and 22 and 32
Appendix 7. LILACS search strategy
(dermatitis or eczema or neurodermatitis or eccema or besnier$) and (probiotic$ or lactobacill$ or bifidobacter$ or lactococc$ or saccharomyc$ or (streptococcus and thermophilus) or (bacillus and subtilis) or (enterococcus and faecalis) or (lactic and acid and bacteri$) or microbiome$ or microbiotica or yoghurt or yoghourt or yogourt)
The above terms were combined with the Controlled clinical trials topic‐specific query filter in LILACS.
Data and analyses
Comparison 1. Probiotic vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment | 13 | Mean difference (Random, 95% CI) | ‐0.44 [‐1.22, 0.33] | |
| 1.1 Parallel‐group trials | 11 | Mean difference (Random, 95% CI) | ‐0.42 [‐1.27, 0.43] | |
| 1.2 Cross‐over trials | 2 | Mean difference (Random, 95% CI) | ‐0.52 [‐3.16, 2.12] | |
| 2 Participant‐ or parent‐rated global change in eczema symptoms at the end of treatment (binary outcome) | 3 | Odds ratio (Random, 95% CI) | 0.40 [0.14, 1.15] | |
| 2.1 Parallel‐group trials | 2 | Odds ratio (Random, 95% CI) | 0.70 [0.27, 1.77] | |
| 2.2 Cross‐over trials | 1 | Odds ratio (Random, 95% CI) | 0.18 [0.05, 0.60] | |
| 3 Change in participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end treatment (continuous outcome) | 9 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.47, 0.06] | |
| 3.1 Parallel‐group trials | 8 | Mean Difference (Random, 95% CI) | ‐0.82 [‐1.62, ‐0.02] | |
| 3.2 Cross‐over studies | 1 | Mean Difference (Random, 95% CI) | 0.66 [‐1.79, 3.11] | |
| 4 Participant‐ or patient‐related quality of life score at the end of treatment | 6 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.36, 0.42] | |
| 4.1 Infant's Dermatitis Quality of Life Index (IDQoL) | 2 | Std. Mean Difference (Random, 95% CI) | 0.35 [0.08, 0.62] | |
| 4.2 Dermatology Life Quality Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.95, 0.29] | |
| 4.3 Skindex‐29 Questionnaire | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐4.24, 2.92] | |
| 4.4 Children's Dermatology Quality of Life Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.70, ‐0.08] | |
| 5 Participant‐ or patient‐related quality of life score at the end of treatment | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.56, 0.18] | |
| 5.1 Dermatitis Family Impact Questionnaire (DFI) | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.31 [‐0.86, 0.24] | |
| 5.2 Family Dermatology Dermatology Life Quality Index | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.32, 0.30] | |
| 6 Parent‐ or participant‐rated eczema severity (SCORAD part C) within 6 months after treatment has ceased | 3 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.13, ‐0.49] |
| 7 Participant‐ or parent‐related quality of life within 6 months after treatment has ceased | 2 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.20] |
| 7.1 Dermatology Life Quality Index | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.75, 0.48] |
| 7.2 Child Dermatology Life Quality Index | 1 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.25] |
| 8 Global eczema severity score (total SCORAD) at the end of treatment | 24 | Mean difference (Random, 95% CI) | ‐3.91 [‐5.86, ‐1.96] | |
| 8.1 Parallel‐group studies | 22 | Mean difference (Random, 95% CI) | ‐3.84 [‐5.95, ‐1.72] | |
| 8.2 Cross‐over studies | 2 | Mean difference (Random, 95% CI) | ‐4.14 [‐7.68, ‐0.59] | |
| 9 Global eczema severity score (total SCORAD) at the end of treatment ‐ sensitivity analysis ‐ change score | 14 | Mean difference (Random, 95% CI) | ‐4.46 [‐6.49, ‐2.43] | |
| 9.1 Parallel‐group trial | 13 | Mean difference (Random, 95% CI) | ‐4.53 [‐6.72, ‐2.33] | |
| 9.2 Cross‐over trial | 1 | Mean difference (Random, 95% CI) | ‐3.93 [‐9.25, 1.40] | |
| 10 Global eczema severity score (total SCORAD) at the end of treatment ‐ low risk of bias studies only | 8 | Mean difference (Random, 95% CI) | Totals not selected | |
| 10.1 Parallel‐group studies | 8 | Mean difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Investigator‐rated eczema severity (SCORAD parts A/B) at the end of treatment ‐ continuous outcome | 10 | 529 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐4.69, 0.20] |
| 12 Global eczema severity score (total SCORAD) within 6 months after treatment has ceased | 7 | Mean difference (Random, 95% CI) | ‐7.72 [‐11.85, ‐3.59] | |
| 12.1 Parallel‐group studies | 6 | Mean difference (Random, 95% CI) | ‐9.27 [‐13.88, ‐4.65] | |
| 12.2 Cross‐over studies | 1 | Mean difference (Random, 95% CI) | 0.2 [‐3.86, 4.26] | |
| 13 Investigator‐rated eczema severity (SCORAD parts A/B) within 6 months after treatment has ceased | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐8.11 [‐13.14, ‐3.09] |
| 14 Adverse events (short term) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Gastrointestinal symptoms | 7 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.90, 2.63] |
| 15 Participant/parent‐rated global change in symptoms of eczema at the end of treatment ‐ stratified by age groups | 3 | Odds ratio (Random, 95% CI) | Totals not selected | |
| 15.1 Age under 2 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Age 2 to 12 years | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Age not categorised | 1 | Odds ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Participant/parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by age groups | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| 16.1 Age under 2 years | 5 | Mean difference (Random, 95% CI) | ‐0.39 [‐2.20, 1.42] | |
| 16.2 Age 2 to 12 years | 4 | Mean difference (Random, 95% CI) | ‐0.63 [‐2.04, 0.78] | |
| 16.3 Age not categorised | 2 | Mean difference (Random, 95% CI) | ‐2.23 [‐3.71, ‐0.74] | |
| 16.4 Adults only | 2 | Mean difference (Random, 95% CI) | 1.01 [‐0.82, 2.84] | |
| 17 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by age groups | 24 | Mean difference (Random, 95% CI) | Subtotals only | |
| 17.1 Age under 2 years | 10 | Mean difference (Random, 95% CI) | ‐0.99 [‐3.97, 1.99] | |
| 17.2 Age 2 to 12 years | 3 | Mean difference (Random, 95% CI) | ‐6.08 [‐9.68, ‐2.48] | |
| 17.3 Age not categorised | 7 | Mean difference (Random, 95% CI) | ‐5.25 [‐10.43, ‐0.07] | |
| 17.4 Adults only | 5 | Mean difference (Random, 95% CI) | ‐6.51 [‐10.09, ‐2.93] | |
| 18 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by presence of atopy | 23 | Mean difference (Random, 95% CI) | Subtotals only | |
| 18.1 Participants with atopy | 4 | Mean difference (Random, 95% CI) | ‐3.90 [‐15.52, 7.73] | |
| 18.2 Participants with unknown atopic status | 19 | Mean difference (Random, 95% CI) | ‐4.15 [‐6.02, ‐2.27] | |
| 19 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by challenge‐proven food allergy | 21 | Mean difference (Random, 95% CI) | Subtotals only | |
| 19.1 Food allergy present | 3 | Mean difference (Random, 95% CI) | ‐1.84 [‐6.22, 2.54] | |
| 19.2 Unknown food allergic status | 18 | Mean difference (Random, 95% CI) | ‐3.21 [‐5.63, ‐0.79] | |
| 20 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by eczema severity | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Severe eczema (SCORAD over 40) | 5 | 95 | Mean Difference (IV, Random, 95% CI) | ‐3.71 [‐10.05, 2.64] |
| 20.2 Moderate eczema (SCORAD 15 to 40) | 6 | 279 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐7.65, 1.74] |
| 20.3 Mild eczema (SCORAD under 15) | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐5.53 [‐15.29, 4.23] |
| 21 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| 21.1 Lactobacillus GG alone or in combination with or without prebiotic | 1 | Mean difference (Random, 95% CI) | 1.79 [0.29, 3.29] | |
| 21.2 Lactobacillus rhamnosus alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐0.82 [‐2.24, 0.60] | |
| 21.3 Lactobacillus salivarius alone or in combination with or without prebiotic | 1 | Mean difference (Random, 95% CI) | 0.0 [‐0.88, 0.88] | |
| 21.4 Lactobacillus casei/paracasei alone or in combination with or without prebiotics | 2 | Mean difference (Random, 95% CI) | 0.56 [‐0.29, 1.41] | |
| 21.5 Any Lactobacillus species alone or in combination with or without prebiotics | 12 | Mean difference (Random, 95% CI) | ‐0.50 [‐1.31, 0.31] | |
| 22 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 22.1 Bifidobacterium lactis alone or in combination with or without prebiotics | 4 | Mean difference (Random, 95% CI) | ‐0.34 [‐1.92, 1.24] | |
| 22.2 Bifidobacterium breve alone or in combination with or without prebiotics | 1 | Mean difference (Random, 95% CI) | 1.3 [‐2.15, 4.75] | |
| 22.3 Any Bifidobacteria species alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐0.11 [‐1.47, 1.25] | |
| 23 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ number of probiotics | 13 | Mean difference (Random, 95% CI) | Subtotals only | |
| 23.1 Studies using single probiotic with or without prebiotics | 8 | Mean difference (Random, 95% CI) | ‐0.40 [‐1.45, 0.66] | |
| 23.2 Studies using multiple probiotics with or without prebiotics | 5 | Mean difference (Random, 95% CI) | ‐0.58 [‐1.98, 0.81] | |
| 24 Participant‐ or parent‐rated symptoms of eczema (SCORAD part C) at the end of treatment ‐ probiotics with no prebiotics | 13 | Mean difference (Random, 95% CI) | ‐0.44 [‐1.22, 0.33] | |
| 25 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Lactobacillus species | 21 | Mean difference (Random, 95% CI) | Subtotals only | |
| 25.1 Lactobacillus GG alone or in combination with or without prebiotic | 3 | Mean difference (Random, 95% CI) | 3.37 [0.55, 6.20] | |
| 25.2 Lactobacillus rhamnosus alone or in combination with or without prebiotic | 5 | Mean difference (Random, 95% CI) | ‐3.49 [‐9.81, 2.83] | |
| 25.3 Lactobacillus salivarius alone or in combination with or without prebiotic | 6 | Mean difference (Random, 95% CI) | ‐6.86 [‐10.08, ‐3.63] | |
| 25.4 Lactobacillus casei/paracasei alone or in combination with or without prebiotics | 6 | Mean difference (Random, 95% CI) | ‐2.58 [‐7.21, 2.05] | |
| 25.5 Any Lactobacillus species alone or in combination with or without prebiotics | 21 | Mean difference (Random, 95% CI) | ‐3.80 [‐6.06, ‐1.54] | |
| 26 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ Bifidobacterium species | 12 | Mean difference (Random, 95% CI) | Subtotals only | |
| 26.1 Bifidobacterium lactis alone or in combination with or without prebiotics | 7 | Mean difference (Random, 95% CI) | ‐1.90 [‐5.42, 1.63] | |
| 26.2 Bifidobacterium breve alone or in combination with or without prebiotics | 3 | Mean difference (Random, 95% CI) | ‐0.36 [‐11.39, 10.67] | |
| 26.3 Any Bifidobacteria species alone or in combination with or without prebiotic | 12 | Mean difference (Random, 95% CI) | ‐2.26 [‐5.14, 0.63] | |
| 27 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ number of probiotics | 24 | Mean difference (Random, 95% CI) | Subtotals only | |
| 27.1 Studies using single probiotic with or without prebiotics | 13 | Mean difference (Random, 95% CI) | ‐4.90 [‐7.66, ‐2.15] | |
| 27.2 Studies using multiple probiotics with or without prebiotics | 12 | Mean difference (Random, 95% CI) | ‐3.54 [‐6.50, ‐0.58] | |
| 28 Global eczema severity score (total SCORAD) at the end of treatment ‐ stratified by probiotic ‐ probiotics with no prebiotics | 24 | Mean difference (Random, 95% CI) | ‐3.83 [‐5.81, ‐1.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brouwer 2006.
| Methods | Three‐month parallel‐group randomised controlled trial | |
| Participants | Fifty‐one infants under 5 months age with mild/moderate eczema diagnosed using Hanifin and Rajka criteria, and a clinical history suggestive of cow's milk allergy. Randomisation was done at a 1:1 ratio: 17 participants were randomised in each arm (L rhamnosus, L GG, placebo). All participants were exclusively formula fed and received an extensively hydrolysed formula for 3 to 5 weeks before receiving the study intervention. Infants receiving antihistamines, oral corticosteroids, or any probiotic/antibiotic/antimycotic in the preceding 4 weeks were excluded, as were those with a congenital gastrointestinal malformation Setting: primary care in the Netherlands One participant lost to follow‐up |
|
| Interventions | Extensively hydrolysed whey‐based formula given alone, with Lactobacillus rhamnosus at 5 × 10⁹ CFUs/100 mL or with Lactobacillus GG at 5 × 10⁹ CFUs/100 mL. The study formula was offered at all feeds during the intervention period | |
| Outcomes | SCORAD assessed at baseline, and at 1, 2, and 3 months* Total IgE, specific IgE to food mix (cow's milk, egg white, soy, peanut, cod, and wheat) and cow's milk, and skin prick test for cow's milk *Denotes outcomes prespecified for this review |
|
| Notes | Study was funded by unrestricted grant from Numico Research, Wageningen, the Netherlands ‐ not related to probiotic. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all patients were randomised to either one of the study formulas..." Comment: no other information provided; method of randomisation not known |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "We conducted a randomised, double‐blind, placebo‐controlled study..." Comment: no further information provided; unclear whether blinding was adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "We conducted a randomised, double‐blind, placebo‐controlled study..." Comment: no further information provided; unclear whether blinding was adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant lost to follow‐up after randomisation; available case analysis without exclusions or imputation used |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found: all outcomes reported |
| Other bias | Low risk | No other bias found |
Cukrowska 2008.
| Methods | Eight‐month (3‐month intervention and 5‐month observation period) parallel‐group randomised double‐blind controlled trial | |
| Participants | Sixty children aged up to 24 months with atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka (3 out of 4 major criteria had to be met) and with symptoms of cow's milk allergy were recruited. Randomisation was done at a 1:1 ratio: 29 participants were randomised in the intervention arm and 31 in the placebo arm. Participants and mothers of breast‐fed children had to be on a non‐dairy diet. Participants who had used antibiotics and probiotics within 6 months before recruitment were excluded from the trial. Recruitment took place at a secondary paediatric centre in Poland | |
| Interventions | Probiotic mixture: Lactobacillus casei LOCK 0900, Lactobacillus casei LOCK 08, Lactobacillus paracasei LOCK 0919 at a total daily dose of 10⁹ CFUs/d given orally for 3 months Placebo: hydrolysed casein given orally for 3 months |
|
| Outcomes | Improvement vs exacerbation/no improvement based on SCORAD* • If SCORAD reduction > 2: improvement • If SCORAD reduction 0 to 2: lack of improvement • If SCORAD increased: deterioration *Denotes outcomes prespecified for this review |
|
| Notes | Study financed by Ministry of Education grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information provided on the random sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information given on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information given on blinding method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from translation from Polish: "The study carried out was randomised, double‐blinded..." Comment: no information given on blinding method |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29% of participants in probiotic group and 32% in placebo group lost to follow‐up at the end of the intervention Comment: losses to follow‐up similar in both groups but high in both and may have influenced the outcome of short‐term change in global eczema severity; no information provided on whether available case analysis was used. No reasons for losses to follow‐up given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as predefined |
| Other bias | Low risk | No other bias found |
Drago 2012.
| Methods | Twenty‐week parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | Thiirty‐eight adult participants between 18 and 46 years with moderate to severe atopic dermatitis diagnosed according to "Consensus guidelines in diagnosis and treatment of atopic dermatitis" (Eichenfield 2004). Randomisation was done at a 1:1 ratio: 19 participants were randomised in each arm. Patients who had received probiotics or antibiotics or who had used immunomodulators (tacrolimus or pimecrolimus) within 6 months from enrolment were excluded from the trial. Also excluded were patients with active allergic disease of the skin or the respiratory tract or chronic infectious disease, and pregnant or lactating patients. All participants completed the study Secondary care setting; recruiting from an Allergy and Immunology Unit in Italy |
|
| Interventions | Probiotic: Lactobacillus salivarius LDR0723 in maltodextrin, given in sachets dissolved in water or other cold liquid of preference twice daily at a dose of 1 × 10⁹ CFUs/g for 16 weeks. Placebo: maltodextrin alone given twice daily for 16 weeks | |
| Outcomes | • SCORAD at baseline and at end of treatment at 16 weeks* • DLQI at baseline, and at 4, 8, 16, and 20 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | Probiotics supplied by Probiotic Company. No information about conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerised randomisation schedule was prepared..." Comment: judged as low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocation and dispensing by a blind clinical investigator..." ‐ "the probiotic and placebo sachets were matched for size, shape and volume of contents" Comment: judged as adequate allocation concealment and hence at low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...allocation and dispensing by a blind clinical investigator..." ‐ "the probiotic and placebo sachets were matched for size, shape and volume of contents" Comment: judged as adequate and hence at low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote for participants who completion of the DLQI questionnaires: "the probiotic and placebo sachets were matched for size, shape and volume of contents" Quote for clinical assessor: "a single investigator who was blind to the treatment intervention performed all SCORAD assessment at the beginning.... and at the end of treatment..." Comment: judged as adequate and hence at low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the study" Comment: no losses to follow‐up; all participants analysed in the group to they were randomised; therefore, no incomplete data |
| Selective reporting (reporting bias) | High risk | All outcomes reported with numerical data or narratively Comment on SCORAD reporting: significance or non‐significance of difference in global eczema severity (SCORAD) between probiotic and placebo groups not reported. Only scores at baseline and end of treatment and at baseline and 4 weeks after treatment reported and commented on Quote: "the mean SCORAD score in the probiotic group was 27.57±3.4 versus 24.28±3.8 in the placebo group. After 4 months we observed a significant reduction in the SCORAD score in the probiotic‐treated group only (T0: 27.57±3.4 vs T16: 13.14±0.27, P<0.001) whereas no changes were reported in the placebo group (T0: 24.28±2.15 vs T16: 20.14±0.27, NS)" Comment on DLQI reporting: data presented at end of treatment and 4 weeks after end of treatment for the probiotic group only. Significant difference from baseline stated. Data for the placebo group not reported and given only narratively; no difference from baseline Quote: "DLQI progressively decreased in probiotic patients during treatment. This significant modification was observed after 8 weeks of treatment (T8) and was also maintained after 4 weeks after the end of the treatment (T20) (T0: 8.28±1.79 vs T8: 4.57±1.11, P=0.02; T0: 8.28±1.79 vs T16: 4.42±0.27, P=0.04; T0: 8.28±1.79 vs T20: 3.71±0.27, P=0.02). No differences were reported in the placebo group" Comment: only outcome data that were significant for the probiotic group reported. No comparison between probiotics and placebo. Report judged to be at high risk of reporting bias |
| Other bias | High risk | Commercial bias: probiotics supplied by Probiotic Company. Study double‐blind and randomised; unlikely that the commercial bias had an effect on the outcome. However, it had an impact on reporting because only positive outcomes for the probiotic group were reported Study assessed as having high risk of other bias |
Drago 2014.
| Methods | Thirty‐day randomised placebo‐controlled parallel pilot trial | |
| Participants | Twenty‐five adults 25 to 63 years of age with diagnosis of atopic dermatitis according to Hanifin and Rajka, with predominant rough fissured skin as well as pruritus for at least 2 months were recruited. Randomisation was done at a 1:2 ratio: 13 participants were randomised in the intervention arm and 12 in the control arm Pregnant and lactating women were excluded. Also excluded were patients with chronic dermatoses such as seborrhoeic dermatitis, contact dermatitis, nummular eczema, psoriasis, ichthyosis, immunodeficiency or any immunological disorder, scabies, cutaneous fungal infection, HIV‐associated skin disorders, malignant disease, T‐cell lymphoma, Letterer‐Siwe disease, progressive systemic disease, serious internal disease (e.g. serious decompensated diseases of the heart, liver, and/or kidneys, diabetes mellitus), or hypersensitivity toward one of the ingredients in the investigational product. Excluded were patients who had been taking part in another study or had taken an investigational product during the last 4 weeks before the start of treatment, or who had been receiving treatment with physical ultraviolet therapy, anti‐inflammatory medications used to treat atopic dermatitis, or immunomodulating medications 30 days before the start of the study, or non‐steroidal antirheumatic drugs, systemic glucocorticosteroids, tranquillisers, or antiemetic agents from the phenothiazine group 14 days before the start of the study, or antidepressants 7 days before the study All participants completed the study Country: Italy Setting: secondary Dermatology Unit |
|
| Interventions | Participants in the probiotic group (n = 13) received freeze‐dried mixture of 5 × 10⁹ CFUs/sachet of Lactobacillus salivarius LS01 (DSM 227775), 2 × 10⁹ CFUs/sachet of Streptococcus thermophilus ST10 (DSM 25246), and tara gum (125 mg) Participants in the placebo group (n = 12) received treatment with sachets containing gluten‐free maltodextrins Sachets were dissolved in water and were taken once daily for 30 days Study participants were allowed to continue to use any medication that they had been taking before the study at the same dose, unless the medication could be discontinued |
|
| Outcomes | Objective SCORAD index before treatment and after 30 days of treatment*
Staphylococcus aureus and clostridial faecal counts before treatment and after 30 days of treatment *Denotes outcomes prespecified for this review |
|
| Notes | The study has not been registered Probiotics were supplied by the manufacturer. Investigators declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised at a 1:1 ratio to receive treatment or placebo Quote from communication with study author: "Which was the method of randomisation? Patients were randomised to either probiotic or placebo groups with a 1:1 allocation according to computer‐generated random numbers" Judgement: probably low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from correspondence with study author: "Was there any blinding? Clinicians, microbiologists and participants were blinded" Judgement: probably adequate, i.e. low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from correspondence with study author: "Was there any blinding? Clinicians, microbiologists and participants were blinded" Judgement: probably adequate, i.e. low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the 25 patients who agreed to commence the protocol completed the study" Judgement: low risk of bias |
| Selective reporting (reporting bias) | Low risk | Quote: "The SCORAD index significantly diminished in the active group from T0 to T1 (P<0.0001, Fig. 1), whereas no variations were observed in the placebo group (P=0.274, Fig. 1). After 1 month of treatment, the SCORAD index in group A was significantly lower than in group B (P=0.015)" Judgement: SCORAD changes reported narratively and not numerically (P value only) between baseline and end of treatment for each group only and between the 2 groups at end of treatment All other outcomes reported |
| Other bias | Unclear risk | Probiotics supplied by the manufacturer. Investigators declared no conflicts of interest but did not report numerical results. Also inadequate information on allocation concealment, and not clear whether the manufacturer had any influence on this. Study not registered For these reasons, study judged to be at unclear risk of commercial bias |
Farid 2011.
| Methods | Eight‐week parallel‐group double‐blind placebo‐controlled randomised trial conducted between November 2007 and March 2009 | |
| Participants | Fifty‐two children from 3 months to 6 years of age with mild to severe atopic dermatitis. Randomisation was done at a 1:1 ratio, but no exact numbers were given for randomised participants in each arm. Patients who had prior exposure to probiotics, or who were at the time taking antibiotics, or who had major medical problems, were excluded from the trial. Twelve patients were lost to follow‐up Setting: secondary care, paediatric Allergy and Immunology Department in Iran |
|
| Interventions | Synbiotic mixture: Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus, and fructo‐oligo‐saccharide in 1‐gram sachets dissolved in water or breast milk, at a dose of 1 × 10⁹ CFUs/g twice daily for 8 weeks. Placebo not specified | |
| Outcomes | SCORAD change from baseline to 4 and 8 weeks and from 4 to 8 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | "No conflicts of interest" declared but funding not declared; probiotic provided by the manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly divided into two groups..." Comment: no other information provided; method of randomisation unknown; therefore unclear risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were randomly divided into two groups..." Comment: unclear whether allocation was concealed; no more information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant: "placebo powder was matched for size, shape and volume of contents" Clinicians: "this randomised, double blind, placebo controlled trial..." ‐ "SCORAD index assessment was performed by a single clinician who was blinded to intervention" Comment: risk of bias unclear regarding blinding of personnel allocating participants to treatment; no relevant information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "SCORAD index assessment was performed by a single clinician who was blinded to intervention" ‐ "placebo powder was matched for size, shape and volume of contents" ‐ "placebo powder was matched for size, shape and volume of contents" Comment: blinding of participants and investigators who assess the global eczema severity score (the only clinical outcome of this study) judged to be adequate; therefore study judged to be of low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of losses to follow‐up: 12 participants (23%) lost to follow‐up and excluded from analysis. We judged this to lead to high risk of attrition bias. Not clear whether rates of loss to follow‐up were similar in both groups. Reasons for loss to follow‐up given, but treatment group from which losses occurred not specified |
| Selective reporting (reporting bias) | Unclear risk | Twelve participants (23%) lost to follow‐up and excluded from analysis. Baseline characteristics and outcomes reported only for participants completing the trial. Unclear whether reporting only characteristics of participants can lead to reporting bias. Trial registered in the Iranian Registry for Clinical Trials retrospectively, and number of participants registered pertains to those who completed the study only. Unclear risk of bias, as not clear whether this could have influenced the outcome for global eczema severity |
| Other bias | Unclear risk | "No conflicts of interest" declared, but not the funding. Probiotic provided by the manufacturer |
Flinterman 2007.
| Methods | Three‐month parallel‐group randomised double‐blind placebo‐controlled trial | |
| Participants | Thirteen children from 0 to 3 years of age with atopic dermatitis, skin prick test ≥ 2+ for at least 2 food allergens, strong clinical history suggestive of food allergy, or positive placebo‐controlled challenge and IgE RAST. Randomisation was done at a 1:2 ratio: 7 participants were randomised in the intervention arm, and 6 in the control arm ≥ 0.7 KU/L for at least 2 food allergens were recruited in the trial. Patients on systemic immunomodulating drugs and those with other systemic diseases or immunodeficiency were excluded from the trial Recruitment took place at a secondary care setting in the Netherlands |
|
| Interventions | Probiotic mixture: Lactobacillus acidophilus W55, Lactobacillus casei W56, Lactobacillus salivarius W57, Lactobacillus lactis W58, Bifidobacterium infantis W52, Bifidobacterium lactis W18, Bifidobacterium longum W51 In rice starch and maltodextrin powder dissolved in warm water or infant formula before administration given once daily at a dose of 1 × 10⁹ CFUs for 3 months Placebo: rice starch and maltodextrin powder dissolved in warm water or infant formula before administration given once daily for 3 months |
|
| Outcomes | • Total SCORAD: secondary outcome for the trial ‐ not reported in relevant publication* • Allergen‐specific T‐ and B‐cell response in vivo and ex vivo • RAST (IgE levels) • Skin prick test *Denotes outcomes prespecified for this review |
|
| Notes | Study sponsored by probiotic manufacturer; no information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from communication with author: "Randomization was performed by the sponsor (Winclove Bio Industries BV), by using a randomisation table. They provided us with boxes with blinded sachets, with only study numbers on it. We anticipated to include 12 children (6 in each group), so Winclove made sure that the randomisation was performed as such that the first 12 numbers included 6 verum and 6 placebo" ‐ "We had 20 sets of blinded sachets at start of the study, to be able to include more children if there would be dropouts during the study" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | See above quote. Study author confirmed that the allocation sequence was concealed Comment: judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See above quote and: "The participants, clinician and outcome assessor were blinded until after the analysis of the results had been performed" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above quote and: "The participants, clinician and outcome assessor were blinded until after the analysis of the results had been performed" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/13 participants lost to follow‐up but no exclusions from analysis. Reason for exclusion given by study author: "one child had to stop during the study because of the use of antibiotics and high fever" Judged as low attrition bias |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported SCORAD ‐ secondary outcome ‐ not reported in the publication but provided by the study author |
| Other bias | Unclear risk | Study sponsored by probiotic manufacturer. Role of the sponsor in data analysis and publication unclear; hence study was judged to be at unclear risk of other bias |
Folster‐Holst 2006.
| Methods | Eight‐week parallel‐group randomised controlled trial from 2001 until 2002 | |
| Participants | Fifty‐four children 1 to 55 months of age with eczema diagnosed using Hanifin and Rajka criteria Setting: German Dermatology Centre Randomisation was done at a 1:1 ratio: 27 participants were randomised in each arm. Two‐centre trial. Six participants were lost to follow‐up, and 1 participant dropped out post randomisation but before treatment started |
|
| Interventions | Lactobacillus GG at 10¹º CFU/d as a twice‐daily dose, or microcrystalline cellulose placebo. Interventions given as capsules, which were mixed with milk if bottle fed, or mixed with water if not bottle fed | |
| Outcomes | • Parent global assessment of disease severity*
• Quality of life score (Ruden 1999)*
• SCORAD*
• Use of topical corticosteroid and systemic antihistamine treatment. Assessments made at 2, 4, 6, and 8 weeks after the start of the study* *Denotes outcomes prespecified for this review |
|
| Notes | Study was supported by InfectoPharm GmbH (Heppenheim, Germany) and Pharmacia GmbH (Freiburg, Germany) ‐ not related to probiotic "No conflicts of interest" declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated" Comment: no concrete information on randomisation method ‐ inadequate for a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Unable to assess the risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "double blind..." ‐ "...placebo preparation (microcrystalline cellulose) with identical appearance" Comment: inadequate information for a judgement on risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "double blind..." ‐ "...placebo preparation (microcrystalline cellulose) with identical appearance" Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven participants lost to follow‐up post randomisation (13%); of those, 1 after randomisation (not clear from which group) but before treatment started. Low rates of loss to follow‐up in both groups (15.4% in probiotic group and 7.4% in placebo). Available case analysis used without exclusions Comment: low incomplete data and judged as low risk for attrition bias for all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found. All outcomes reported |
| Other bias | Low risk | No other bias found |
Gerasimov 2010.
| Methods | Eight‐week parallel‐group randomised double‐blind placebo‐controlled trial held between June 2007 and June 2008 | |
| Participants | Ninety‐six children between 1 and 3 years of age with moderate to severe atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka were recruited Randomisation was done at a 1:1 ratio: 48 participants were randomised in each arm. Only patients whose parents or legal guardians had the ability to comprehend the study requirements and to provide informed consent and those with direct telephone access were recruited Patients with clinically evident bacterial skin lesions, chronic concomitant disease that would likely require use of immunosuppression or antihistamines during research period, presence of severe systemic disease or cancer at any site and stage, suspected or established primary/secondary immune deficiency, and food allergy other than egg or cow’s milk were excluded from the study. Also excluded were patients with mild disease and those currently taking systemic corticosteroids Six participants were lost to follow‐up Recruitment took place at a paediatric secondary care unit in Ukraine |
|
| Interventions | Synbiotic: mixture of Lactobacillus acidophilus DDS‐1 and Bifidobacterium lactis UABLA‐12 with fructo‐oligosaccharide in a rice maltodextrin powder given twice daily reconstituted in tepid water or juice or baby food and immediately fed for 8 weeks. Dose given: 5 × 10⁹ CFU/gr for the 2 probiotics and 50 mgr/gr of fructo‐oligosaccharide Placebo: rice maltodextrin powder only given twice daily reconstituted in tepid water or juice or baby food and immediately fed for 8 weeks Parents were given 140 doses of the intervention and were asked to give 112 doses in total Treatment of atopic dermatitis during intervention: skin hydration, emollients, avoidance of allergens and irritants according to PRACTALL (Practical Allergology) recommendations. Hydrocortisone 1% or Mometasone 0.1% ointment was allowed as rescue medication. Elimination diet for 2 months before and during trial period. Diet was cow's milk or egg free, depending on which food allergy the participant had. No elimination diet for participants without food allergies |
|
| Outcomes | • IDQoL changes at 4 and 8 weeks* • DFI at 4 and 8 weeks* • SCORAD parts A, B, and C at 2, 4, and 8 weeks* • Frequency of topical corticosteroid use (days per week) at 8 weeks* • Cumulative use of topical corticosteroids during intervention period* *Denotes outcomes prespecified for this review |
|
| Notes | Study was funded by the Lviv National Medical University of Ukraine. "No conflicts of interest" declared Twenty‐six participants in the probiotic group (60.5%) and 24 in the placebo group (51.1%) developed adverse effects: upper respiratory tract infection, lower respiratory tract infection, herpetic stomatitis, diarrhoea, constipation, abdominal colic. Two children in the probiotic group (4.7%) and 3 in the placebo group (6.4%) experienced severe adverse events (head injury and food poisoning) that were reported to be unrelated to the intervention under investigation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised using computer‐generated random codes to receive either probiotic or placebo treatment" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information; study judged to be at unclear risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "probiotic and placebo were identical in appearance, taste, smell, packing and manner of administration. All formulations were dispensed by a technician with investigator and patient blinded regarding the identity of the treatment" Comment: judged as adequate for low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "probiotic and placebo were identical in appearance, taste, smell, packing and manner of administration. All formulations were dispensed by a technician with investigator and patient blinded regarding the identity of the treatment" Comment: judged as having unclear risk of bias; no information on blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants lost to follow‐up (6.25%): 5/48 (10%) in the probiotic group and 1/48 (2%) in the placebo group. Reasons for losses to follow‐up given: intercurrent illness (2 in probiotic and 1 in placebo group, 1 protocol violation in probiotic group and 1 diet deviation in probiotic group). Participants analysed in the group to which they were randomised Comment: judged as low risk, as rates for follow up are low and were unlikely to have influenced outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
Goebel 2010.
| Methods | Eight‐week 3‐arm parallel‐group randomised double‐blind placebo‐controlled trial | |
| Participants | Fifty children from 7 to 24 months of age with atopic dermatitis diagnosed by general practitioner or dermatologist were recruited. Randomisation was done at a 1:1 ratio: 17 participants were randomised in the 2 intervention arms and 16 in the control arm. All participants completed the trial Setting: Danish primary care centre |
|
| Interventions | First arm/probiotic: Lactobacillus acidophilus NCFM in cellulose, silicon dioxide, and rice maltodextrin in a capsule given daily at a dose of 10¹º CFUs for 8 weeks Second arm/probiotic: Bifidobacterium animalis subsp lactis (B lactis Bi‐07) in cellulose, silicon dioxide, and rice maltodextrin in a capsule given daily at a dose of 10¹º CFUs for 8 weeks Third arm/placebo: cellulose, silicon dioxide, and rice maltodextrin in a capsule given daily |
|
| Outcomes | Total and subjective SCORAD at baseline and end of treatment at 8 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | Funding was by Danish Directorate of Food Fisheries and Agri Business Danish Dairy Research Foundation. "No conflicts of interest" declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from communication with author: "Randomization sequence was generated by the online available program http://www.randomizer.org/" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Low risk | Quote from communication with author: "The producer of the intervention product blinded the capsules as A, B and C before shipment" Comment: allocation sequence judged as adequate for low risk of bias after the study author's assurance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from communication with author: "The producer of the intervention product blinded the capsules as A, B and C before shipment, and blinding was maintained for participants, clinician and outcome assessor until finalized data analysis" Comment: judged as adequate for low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from communication with author: "The producer of the intervention product blinded the capsules as A, B and C before shipment, and blinding was maintained for participants, clinician and outcome assessor until finalized data analysis" Comment: judged as adequate for low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. All participants analysed in the group were randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
Gore 2011.
| Methods | Twelve‐week 3‐arm parallel‐group randomised double‐blind placebo‐controlled trial with follow‐up up to 36 months | |
| Participants | One hundred thirty‐seven infants between 3 and 6 months of age with physician‐diagnosed atopic dermatitis, in good general health, with normal growth, and consuming > 200 mL standard formula/d were recruited. Randomisation was done at a 1:2 ratio: 45 participants were randomised in the 2 intervention arms, and 45 in the control arm. SCORAD score at recruitment had to be ≥ 10 after standardised skin treatment for 2‐week run‐in period: 1% hydrocortisone ointment twice daily and emollients 2 to 4 times daily. Infants who were taking antibiotics or were on soya or extensively hydrolysed formula, those with congenital abnormalities or chronic disease, and those at less than 34 weeks' gestation were excluded from the trial Participants who were exclusively breastfed and those whose parents declined use of extensively hydrolysed formula were followed up as an open observational group Four participants were lost to follow‐up at 12 weeks Participants were recruited from primary care community clinics in the United Kingdom |
|
| Interventions | First probiotic arm: Bifidobacterium lactis in powder sachets at a dose of 10¹º CFUs/d given with meals for 12 weeks Second probiotic arm: Lactobacillus paracasei in powder sachets at a dose of 10¹º CFUs/d given with meals for 12 weeks Placebo arm: maltodextrin in powder sachets given with meals for 12 weeks All arms followed a dairy elimination diet and used an extensively hydrolysed whey formula |
|
| Outcomes | • Total SCORAD before 2‐week run‐in period, at baseline, at 4 and 12 weeks, and at 12, 18, and 36 months* • IDQoL before 2‐week run‐in period, at baseline, at 12 weeks, and at 12, 18, and 36 months* • Use of other eczema treatment at 12 weeks (end of intervention period)* • Number of infants receiving standard skin care: combination of topical steroids (TSs), emollients (≥ twice/d), and bath emollient* • Potency of TS* *Denotes outcomes prespecified for this review |
|
| Notes | Funding and "no conflict of interest" declared Forty‐two out of 137 (30.7%) parents reported some difficulties, e.g. green loose stools, increased vomiting, feed refusal, or colic thought to be related to change in formula), and 24 of 137 (17.5%) stopped the formula |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Minimization method was applied to allocate subjects to study groups applying breast feeding, family history and initial SCORAD as stratification factors (TrialBalance randomisation programme; Nestec, Lausanne, Switzerland)" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Unclear risk | No information to confirm whether treatment allocation could be predicted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All interventions identical Blinding of clinicians confirmed by study author |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Confirmed by study author |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/45 (4.4%) participants inL paracasei group, 1/45 (2.2%) in B lactis group, and 1/47 (2.1%) in placebo group lost to follow‐up. 10/45 (22.2%) participants in L paracasei group, 9/45 in B lactis group, and 9/47 (19.1%) in placebo group stopped the study diet as per protocol but continued the intervention. Available case analysis used without exclusions, with imputation for missing data Comment: judged as unlikely to have influenced outcomes on eczema severity and quality of life, as similar and low rates of loss to follow‐up in all groups Also similar rates of stopping study formula in all groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
Gromert 2009.
| Methods | Twelve‐month parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | Fifty children from 3 months to 4 years of age with moderate atopic dermatitis. No additional information provided on randomised numbers of participants in each arm | |
| Interventions | Probiotic:Lactobacillus reuteri at a dose of 1 × 10⁸ CFUs/d taken orally suspended in 5 drops of food oil for 12 months Placebo: not specified but also given for 12 months |
|
| Outcomes | • SCORAD* • Subjective symptoms of atopic dermatitis: itching and loss of sleep* • Use of steroid treatment* *Denotes outcomes prespecified for this review |
|
| Notes | All available information taken from a conference abstract. No publication found, and study authors could not be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this prospective, double blind, randomised study..." Inadequate information: information on this study taken only from a conference abstract |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In this prospective, double blind, randomised study..." Inadequate information: information on this study taken only from a conference abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In this prospective, double blind, randomised study..." Inadequate information: information on this study taken only from a conference abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Selective reporting (reporting bias) | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
| Other bias | Unclear risk | Inadequate information: information on this study taken only from a conference abstract |
Gruber 2007.
| Methods | Twelve‐week parallel‐group randomised controlled trial Last observation carried forward approach used for missing continuous data |
|
| Participants | 106 children 3 to 12 months of age with mild/moderate eczema and SCORAD 15 to 40, not receiving anti‐inflammatory treatment. Randomisation was done at a 1:1 ratio: 56 participants randomised in the intervention arm and 50 in the control arm. Four participants excluded from analysis after randomisation due to protocol breaches | |
| Interventions | Lactobacillus GG at 10¹º CFUs/d as a twice‐daily dose, or placebo | |
| Outcomes | • SCORAD*
• Use of 1% hydrocortisone ointment* *Denotes outcomes prespecified for this review |
|
| Notes | Funding declared and provided by InfectoPharm Arzneimittel und Consilium GmbH, Heppenheim, Germany (not linked with probiotics). No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information for a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind but no details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind but no details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. Four participants (3.9%) excluded from analysis after randomisation because of protocol breaches. Unlikely to have an impact on effect estimate |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found |
| Other bias | Low risk | No other bias found |
Guo 2015.
| Methods | Randomised trial | |
| Participants | 180 children with eczema, from 2 months to 3 years of age. No additional information provided on exact ratio of randomisation into 2 arms. Patients with other medical conditions excluded | |
| Interventions | Active: routine symptomatic treatment and combination of 4 living bacterium tablets (Bifidobacterium, Lactobacillus, Enterococcus, and Bacilus cereus) taken orally at a dose of 1 tablet twice a day for 1 month Control: routine symptomatic treatment Routine symptomatic treatment: mild disease – calamine lotion and zinc oxide ointment, 3 times daily for 2 weeks Severe disease – loratadine syrup, topical mometasone and topical mupirocin to stop after disease control All participants advised to avoid washing with hot water/spa products and scratching, and to look for potential allergens and avoidance advice |
|
| Outcomes | IL‐4, IL‐10, IgE, IFN‐γ, and Th1:Th2 ratio Relapse rate of the 2 groups in the 3‐month follow‐up visit. Relapse is defined as recurrence of rash within the 3 months Eczema improvement: • Complete resolution: ‐ complete clearance of rash and itch; and normal eating and sleeping patterns • Good response: > 70% eczema clearance, no obvious lichenification, almost complete resolution of itch/intermittent itch, eating and daily activities not affected • Partial response: 30% to 70% clearance, symptom improvement • No response: < 30% rash clearance, no obvious reduction in itch Complete resolution, good response, and partial response make up total percentage of responding participants |
|
| Notes | Contact: guoyangjie829@163.com Trial was conducted in a secondary care setting at a paediatric clinic No information provided on sponsorship/conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information for a judgement |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inadequate information for a judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Inadequate information for a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inadequate information for a judgement |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported; however dose of probiotics not given, and results reported only narratively |
| Other bias | Unclear risk | No information on trial registration and sponsorship |
Han 2012.
| Methods | Sixteen‐week parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | One hundred eighteen children between 1 and 13 years of age with atopic dermatitis diagnosed according to Hanifin and Rajka criteria with SCORAD between 20 and 50 Randomisation was done at a 1:1 ratio: 58 participants were randomised in the intervention arm, and 60 in the control arm. After selection, participants went through a 2‐week washout period, when both groups were administered placebo only. Patients who had taken systemic corticosteroids, probiotics, or phototherapy within a month before enrolment, with systemic immunosuppression within 3 months before enrolment, with SCORAD < 20 after a 2‐week washout period, or with other concomitant skin disease or systemic illness were excluded from the study. One recruiting centre was located in Korea. Thirty‐five participants were lost to follow‐up | |
| Interventions | Probiotic: Lactobacillus plantarum CJLP 133 given orally twice daily at a dose of 0.5 × 10¹º CFUs for 12 weeks Placebo: maltodextrin and anhydrous glucose twice daily for 2 weeks as a washout period by both groups, and then for 12 weeks by the control group |
|
| Outcomes | • Total SCORAD at baseline, after 2‐week washout period, and at 8, 14, and 16 weeks, and changes from week 2 (start of intervention) to week 14 (end of intervention) and week 16 (2‐week follow‐up after end of intervention)* • Amount in weight of topical corticosteroids used during the whole study and during intervention only* • Number of participants using topical corticosteroids during the study* *Denotes outcomes prespecified for this review |
|
| Notes | Trial sponsored by probiotic supplier; 2 investigators are employed by this company. No other information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using a computer‐generated list of random numbers" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "The random number list was prepared and posted on the fronts of the bags containing the materials by an investigator who was not involved clinically in the trial", "...placebo preparation with identical appearance and taste" Comment: probably done and judged as adequate for low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo identical in appearance and taste Also quote from correspondence with study author: "clinicians and outcome assessors had been blinded during the study period" Comment: judged as adequate for low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from correspondence with study author: "clinicians and outcome assessors had been blinded during the study period" Comment: judged as adequate for low risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis used but high rates of losses to follow‐up (30%). 14/58 (24%) participants from probiotic group and 21/60 (35%) from placebo group lost to follow‐up. Reasons for losses to follow‐up given and similar in both groups Incomplete data likely to have an impact on the effect estimate; judged as high risk |
| Selective reporting (reporting bias) | High risk | Symptom scores (i.e. for pruritus, sleep loss, and SCORAD part C) after intervention not reported |
| Other bias | High risk | Commercial bias: trial sponsored by probiotic supplier, and 2 of the investigators employed by the company. It is likely that the sponsor had an influence on the outcome and on selective reporting Selection bias: power calculation and final numbers of participants suggest that study did not meet the target According to publication: "Study discontinued after the second interim analysis showed statistically significant differences between the groups" Study assessed to be at high risk of bias |
Hol 2008.
| Methods | Eighteen‐month multi‐centre double‐blind randomised controlled parallel‐group trial conducted from March 2004 until May 2007 | |
| Participants | One hundred nineteen infants younger than 6 months of age with documented cow's milk allergy, judged by an elimination challenge test (open) and re‐elimination, in conformity with guidelines of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Randomisation was done at a 1:1 ratio. Excluded from the study were infants breastfeeding during the study, infants older than 6 months of age, patients with chronic disease that may be relevant to this study, such as pre‐existing chest abnormalities (e.g. bronchopulmonary dysplasia (BPD), relevant congenital abnormalities), gastrointestinal disease (coeliac disease, enzyme disorders), and metabolic disease, premature infants at less than 32 weeks, infants with congenital abnormalities that may be relevant to this study, and infants using systemic drugs for allergy (corticosteroids and antihistamines) Multi‐centre trial recruiting in a paediatric secondary care setting in the Netherlands One hundred six infants completed the study (89%) |
|
| Interventions | Probiotic group: Lactobacillus casei CRL431 (Lactobacillus paracasei, subspparacasei) and Bifidobacterium lactis Bb‐12 (B animalis subsp lactis). For each probiotic, 10⁷ CFUs/gr of formula was provided. This was given for 6 months in extensively hydrolysed formula and was continued in standard formula if infants became cow's milk tolerant, or in the same extensively hydrolysed formula if not Control group: extensively hydrolysed formula for 6 months and continued in standard formula if infants became cow's milk tolerant, or in the same extensively hydrolysed formula if not |
|
| Outcomes | • Development of tolerance for cow's milk, observed by a challenge test (double‐blind) at 6 months • Eczema severity by SCORAD index at 6 and 12 months* • T‐ and B‐lymphocyte subsets at 12 months *Denotes outcomes prespecified for this review |
|
| Notes | Study co‐sponsored by probiotic/formula supplier. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized randomisation schedule was prepared by a statistician" ‐ "the groups were stratified and block randomised according to age at inclusion (<20 weeks and >20 weeks), birth weight (<2500gr or >2500gr) and (reported) atopic diseases in first‐degree relatives (yes or no)" Comment: judged as adequate for low risk |
| Allocation concealment (selection bias) | Unclear risk | No information available to allow a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Probiotics and formula were image and taste matched, and participants and researchers remained blinded to group assignment for the duration of the study" Comment: probably done for all parties |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Probiotics and formula were image and taste matched, and participants and researchers remained blinded to group assignment for the duration of the study" Comment: probably done for all parties |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall losses to follow‐up 11%. No data available for the 2 groups separately, and no data available for the subgroup of participants who had eczema Inadequate for a judgement for that subgroup only Low risk of attrition bias for the primary outcome of the study (development of tolerance to cow's milk) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. No detailed data reported on all eczema participants (only moderate to severe), but significance of the difference noted Quote: "The probiotic group (n=51) showed improvement at 6 and 12 months, and the placebo group (n=54) showed improvement only at 6 months. However, after adjusting for the baseline values there were no significant differences in the change from baseline between probiotics and placebo treatment at 6 months (P=.9) and at 12 months (P=.14)" |
| Other bias | Unclear risk | Commercial bias: formula and probiotics supplied by producer company. The role of the manufacturer in data analysis and publication is unknown; therefore we assessed this study to be at unclear risk of other bias |
Iemoli 2012.
| Methods | Twenty‐week parallel‐group double‐blind placebo‐controlled randomised trial from April until September 2010 | |
| Participants | 48 adults between 18 and 55 years of age with moderate to severe atopic dermatitis diagnosed according to "Consensus guidelines in diagnosis and treatment of atopic dermatitis" (Eichenfield 2004). Participants were randomised at a 2:1 intervention:control ratio (32 participants in intervention arm/16 participants in control arm). Patients who had taken probiotics in the 6 months before enrolment, or topical immunomodulators, corticosteroids, or antihistamines; who had chronic disease, congenital or acquired immunosuppression, acute or chronic infection, or allergic contact dermatitis; and pregnant or lactating patients were excluded from the trial. Also excluded were patients who were on elimination diets without a known food allergy and those with hypersensitivity to the components of the probiotics Participants were recruited at a secondary Immunology centre in Italy Two participants were lost to follow‐up |
|
| Interventions | Mixture of probiotics in maltodextrin: Lactobacillus salivarius LS01 DSM 2275, Bifidobacterium breve BR03 DSM 16604, given twice daily at a dose of 1 × 10⁹ CFUs/gr for each probiotic for 12 weeks Placebo: maltodextrin given only twice daily for 12 weeks |
|
| Outcomes | • DLQI at baseline and at 12 and 20 weeks* • SCORAD at baseline and at 12 and 20 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | "No conflicts of interest" declared. No information on funding. Probiotics provided by the manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised with a 2:1 ratio..." Quote from communication with study author: "A computerized randomisation schedule was prepared..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote from communication with study author: "allocation and dispensing by blind clinical investigator" Comment: judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Probiotic and placebo sachets were matched for size, shape and volume of contents" Quote from communication with study author: "allocation and dispensing by blind clinical investigator" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A single investigator blinded to the treatment arm, performed all SCORAD assessments..." Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of loss to follow‐up (4%) overall. 1 participant lost to follow‐up in each arm: 3% in probiotic group and 6% in placebo group. Different reasons for losses to follow‐up given but overall low rates unlikely to have a significant influence on effect estimates for all outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | "No conflicts of interest" declared. Unavailable information on funding. Probiotics provided by the manufacturer |
Isolauri 2000.
| Methods | Parallel‐group three‐arm randomised controlled trial. Duration of treatment unclear | |
| Participants | Twenty‐seven infants ‐ ages not stated ‐ with eczema diagnosed using the Hanifin and Rajka criteria. Randomisation was done at a 1:1 ratio: 9 participants were randomised in each arm. All infants were exclusively breastfed and were tolerant of the study formula without added probiotic Setting: paediatric service in Finland Unclear how many participants lost to follow‐up |
|
| Interventions | Extensively hydrolysed whey‐dominant cow's milk formula with no probiotic added, with Lactobacillus GG added at 3 × 10⁸ CFUs/g, or with Bifidobacterium lactis Bb‐12 added at 1 × 10⁹ CFUs/g | |
| Outcomes | SCORAD ‐ interval of assessment unclear* *Denotes outcomes prespecified for this review |
|
| Notes | Study funded by the Academy of Finland, European Union (FAIR CT96‐1028), and the Medical Research Funds of Tampere and Turku University Hospital "No conflict of interest" declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this randomised, double blind study, the patients were divided into three groups..." Comment: inadequate information to assess risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information given. No assessment of risk of bias can be done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In this randomised, double blind study, the patients were divided into three groups..." Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In this randomised, double blind study, the patients were divided into three groups..." Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information on losses to follow‐up not given. Inadequate information for a judgement on risk of bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found |
| Other bias | Low risk | No other bias found |
Ivankhnenko 2013.
| Methods | Eight‐week parallel‐group open‐label randomised placebo‐controlled trial | |
| Participants | Sixty full‐term infants 3 to 12 months of age with clinically diagnosed eczema and challenge proven cow's milk allergy, breast and formula fed. Randomisation was done at a 1:1 ratio: 30 participants were randomised in each arm. Excluded were patients who had received any probiotics within a month before recruitment and those with other allergies, severe comorbidities, and malformations Country: Ukraine 5 participants lost to follow‐up |
|
| Interventions | Probiotic: Bifidobacterium lactis BB‐12 and Streptococcus thermophilus TH‐4 for 4 weeks No information on placebo Daily dose of probiotics: 1 × 10⁹ CFUs for BB‐12 and 1 × 10⁸ CFUs for TH‐4. Both groups on cow's milk elimination diet |
|
| Outcomes | SCORAD at 4 and 8 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | No mention of sponsor. No declaration of conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translated Russian article: "In this open label randomised prospective clinical study..." Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for assessment, so study judged as having unclear risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants lost to follow‐up (8%), with 2 participants from probiotic group (6.6%) and 3 participants (10%) from placebo group. Reasons for losses to follow‐up not given for each group. Not clear whether losses to follow‐up were excluded from analysis. However overall rates of loss to follow‐up were low and were unlikely to have a significant influence on effect estimates for study outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No mention of sponsor. No declaration of conflicts of interest |
Kirjavainen 2003.
| Methods | Parallel‐group randomised controlled trial. Intended duration of treatment not clear | |
| Participants | Twenty‐seven infants (mean age 5.5 months) with eczema and suspected cow's milk allergy. Method for diagnosing eczema not described Setting: hospital paediatric department in Finland Unclear how many participants lost to follow‐up Participants randomised 2:1, probiotic:control |
|
| Interventions | Lactobacillus GG at 3 × 10¹º CFUs/kg/d, mixed with extensively hydrolysed whey formula, or the same formula without probiotic. A third treatment arm (excluded from this review) used heat‐inactivatedLGG at 3 × 10¹º CFUs/kg/d, mixed with extensively hydrolysed whey formula | |
| Outcomes | SCORAD* *Denotes outcomes prespecified for this review |
|
| Notes | Study terminated early due to adverse effects in a third treatment arm. Third treatment arm not included in this systematic review because it involved the use of killed bacteria Study funded by the Academy of Finland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These infants were randomly assigned into placebo, viable LGG..." Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information reported. Inadequate information for a judgement on risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "These infants were randomly assigned into placebo, viable LGG or heat‐inactivated LGG groups and accordingly given in a double blind manner..." Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "These infants were randomly assigned into placebo, viable LGG or heat‐inactivated LGG groups and accordingly given in a double blind manner..." Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data given on losses to follow‐up. Inadequate information for a judgement on risk of bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
Lin 2015.
| Methods | Four‐week randomised parallel controlled trial | |
| Participants | Forty‐four infants of both genders with newly diagnosed eczema based on the diagnostic criteria for eczema up to 3 years of age were recruited. Randomisation was done at 1:2 ratio: 22 participants were randomised in each group. Infants who had been treated with antibiotics, probiotics, or other drugs and food at least 2 weeks before the start of the study were excluded. Also excluded were children suffering from pneumonia, capillary bronchitis, and other diseases, or who had been treated with antibiotics or hormones during the experimental process Infants were treated with antiallergic therapy and dietary guidance Recruitment took place in a secondary paediatric setting in China between December 2010 and March 2011 |
|
| Interventions | Intervention group received triple viable capsules containing Bifidobacterium bifidum 3 times daily for 4 weeks Control group received no treatment |
|
| Outcomes | • Total SCORAD index at baseline and after 4 weeks of intervention • Bifidobacterium bifidum stool levels at baseline and after 4 weeks of treatment |
|
| Notes | Sponsorship of the trial not declared. Not clear what role the supplier of the probiotic played in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 40 infants were randomly divided into treatment and control groups" Judgement: inadequate information on randomisation method; risk of bias judged as unclear |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The control group … did not receive any special treatment and were not administered a placebo drug" Judgement: unlikely to be done adequately with no treatment and no placebo in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The control group … did not receive any special treatment and were not administered a placebo drug" Otherwise no information on blinding of outcome assessors; hence risk of detection bias judged to be unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on losses to follow‐up and missing outcome data Judgement: unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Not clear what role the supplier of the probiotic played in the study. Sponsorship of the trial not declared Judgement: unclear risk of commercial bias |
Majamaa 1997.
| Methods | One‐month parallel‐group randomised controlled trial | |
| Participants | Thirty‐one children 2 to 16 months of age with eczema diagnosed using the Hanifin and Rajka criteria, and a history suggestive of cow's milk allergy. Randomisation was done at a 1:1 ratio. Children currently receiving systemic corticosteroid treatment were excluded Setting: paediatric clinic in Finland Unclear how many participants were lost to follow‐up |
|
| Interventions | Cow's milk elimination diet, topical eczema treatment, and extensively hydrolysed cow's milk formula with or without addition of probiotic Lactobacillus GG. Probiotic given at 5 × 10⁸ CFUs/g formula | |
| Outcomes | SCORAD assessed at 1 and 2 months* *Denotes outcomes prespecified for this review |
|
| Notes | No information available on funding or conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients....participated in a randomised double blind study" Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients....participated in a randomised double blind study" Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The patients....participated in a randomised double blind study" Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on losses to follow‐up. Inadequate information for a judgement on risk of bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | No information on funding available |
Matsumoto 2014.
| Methods | Eight‐week multi‐centre randomised double‐blind placebo‐controlled parallel trial | |
| Participants | Forty‐four adults with moderate to severe atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka were recruited. Randomisation was done at a 1:1 ratio: 22 participants were randomised in each arm. Participants continued to use their medications as usual and did not change quantities or levels of corticosteroid medicine during the experimental period. Participants were asked to avoid probiotic supplements, fermented milk, lactic acid bacterial drinks, and fermented soybean (natto) during the experimental period Recruitment took place at 8 dermatology clinics in Japan |
|
| Interventions | Participants in the intervention group received capsules containing approximately 6 × 10⁹ CFUs of Bifidobacterium animalis subsp lactis and an excipient that consisted of skim milk, glucose, inulin, dextrin, and silicone dioxide for 8 weeks Control group received capsules containing the excipient only for 8 weeks |
|
| Outcomes | • Itch score by behavioural rating scales and by 100‐mm visual analogue scale (VAS) at baseline and at 4 and 8 weeks • Skin severity score using the reference proposed by the Research Group granted by the Japanese Ministry of Health, Labor and Welfare at baseline and at 4 and 8 weeks • Quality of life using Skindex‐29 (Japanese version) at baseline and at 4 and 8 weeks • Faecal levels of B lactis at baseline and at 4 and 8 weeks |
|
| Notes | Trial registration: UMIN000005695 Four of the investigators/study authors are employed by the supplier |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 44 participants eligible for the study were randomly assigned to receive LKM512 capsule or a placebo by the masked quota director" Judgement: inadequate information on randomisation method; hence judgement of unclear risk of selection bias |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" mentioned, but trial registration shows open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" mentioned, but trial registration shows open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported but not numerically, and any favourable information/outcome for probiotics particularly analysed, even if not statistically significant |
| Other bias | High risk | Commercial bias: 4 investigators/study authors employed by supplier; likely that this has influenced the design and outcome of the trial |
Nermes 2010.
| Methods | Three‐month parallel‐group randomised placebo‐controlled trial | |
| Participants | Thirty‐nine full‐term and otherwise healthy infants with atopic dermatitis diagnosed according to the Hanifin and Rajka criteria were recruited. Randomisation was done at a 1:1 ratio: 19 participants were randomised in the intervention arm, and 20 in the control arm. Patients with skin or other severe infections were excluded Recruitment was done at a paediatric secondary care setting in Finland Two participants were lost to follow‐up |
|
| Interventions | Probiotic: Lactobacillus rhamnosus GG in extensively hydrolysed casein formula given at a dose of 5.0 × 10⁷ CFUs/gr, achieving a daily dose of 3.4 × 10⁹ CFUs/d for 3 months Placebo: extensively hydrolysed casein formula for 3 months |
|
| Outcomes | • Total SCORAD* *Denotes outcomes prespecified for this review |
|
| Notes | Study was partially funded by the formula manufacturer and a grant from the Academy of Finland. No conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from correspondence with study author: "the randomisation was carried out by the formula supplier (Mead Johnson, Evansville, IN, USA). The method used was block randomisation..." Comment: probably done; judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information given; inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from correspondence with study author: "The study was a double‐blind, placebo controlled intervention study" ‐ "The information disclosing the codes of the study products was kept by a person not involved in the study and opened after the study was completed" Comment: probably done; judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from correspondence with study author: "The study was a double‐blind, placebo controlled intervention study" ‐ "The information disclosing the codes of the study products was kept by a person not involved in the study and opened after the study was completed" Comment: probably done; judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants lost to follow‐up, all from placebo group. Available case analysis unclear but very low rates of loss to follow‐up (5% in total and 10% in placebo group) unlikely to change the effect estimate. Reasons for losses to follow‐up given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
Passeron 2006.
| Methods | Three‐month parallel‐group randomised controlled trial | |
| Participants | Forty‐eight children 2 to 12 years of age with moderate/severe eczema diagnosed by UK Working Party Criteria and total SCORAD over 14. Randomisation was done at a 1:1 ratio: 24 participants were randomised in each arm. Exclusion criteria included current flare of eczema, exposure to systemic corticosteroids or immunosuppressants in the previous 3 months, and other known immune deficiency Setting: hospital dermatology clinic in France Nine participants lost to follow‐up |
|
| Interventions | Skim milk powder, potato starch, and lactose‐containing prebiotic, with or without Lactobacillus rhamnosus Lcr35 at 3.6 × 10⁹ CFUs/d, given as a 3‐times‐daily dose mixed with cold water or other liquid | |
| Outcomes | • Parent or participant global assessment of eczema severity*
• SCORAD*
• Investigator global assessment of eczema severity*
Assessments were done at baseline and at 1, 2, and 3 months *Denotes outcomes prespecified for this review |
|
| Notes | Three episodes of mild abdominal pain reported ‐ 2 in probiotic group, 1 in placebo (prebiotic alone) group Funding and conflict of interest not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence as confirmed by study authors. Judged as having low risk of bias |
| Allocation concealment (selection bias) | Low risk | Adequate as confirmed by study authors and judged as having low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients, their parents and the dermatologists were blinded to the treatment the patient was receiving" ‐ "Each patient was examined by the same dermatologist at each visit" Study authors confirmed blinding of all parties in the trial Comment: probably done and judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients, their parents and the dermatologists were blinded to the treatment the patient was receiving" "Each patient was examined by the same dermatologist at each visit" Study authors confirmed blinding of all parties in the trial Comment: probably done and judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Available case analysis used without exclusions after randomisation with low total rates of loss to follow‐up (18.7%). Losses to follow‐up per group: 29% in synbiotic group and 8% in placebo group. Reasons for losses in the synbiotic group were non‐attendance at follow‐up visits (5/24 participants) and withdrawal of consent (2/24). In the prebiotic group, 2/24 participants did not attend for follow‐up Significant differences in rates of loss to follow‐up in the 2 groups, which probably had an impact on all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Funding and conflict of interest not reported |
Roessler 2007.
| Methods | Twenty‐week cross‐over randomised double‐blind placebo‐controlled trial | |
| Participants | Sixteen adults between 18 and 40 years of age with eczema diagnosed based on Erlangen score > 10 (atopic score of Diepgen) and SCORAD severity score 5 to 30 were recruited. Only patients who were willing to apply cosmetic products and the Class II topical corticosteroid Advantan were recruited. Exclusion criteria were disease necessitating medication with systemic corticosteroids, immunosuppression, or cytostatics within 4 weeks before the start of the study; phototherapy or systemic treatments within 4 weeks before the start of the study; long‐acting antihistamines, antibiotics, long‐term systemic corticosteroids, depot steroids, tranquillisers, and psychopharmaceuticals with antihistamine effect or within 7 days from skin prick test; or astemizole intake within 4 weeks before prick testing. Also excluded were patients with active skin infection; asthma needing treatment with corticosteroids; indigestibility/allergy to milk components (including skin prick test); lactose intolerance; acute or chronic symptomatic heart disease or severe internistic disease; autoimmune disease; immune deficiency (including immune suppressive treatment); immune complex‐induced immunopathy or malignant tumour; or abuse of alcohol, drugs, or medicaments, as well as pregnant and breastfeeding women Recruitment was done at a secondary care centre in Germany One participant withdrew after randomisation and before treatment started |
|
| Interventions | Probiotic yoghurt drink containing Streptococcus thermophilus enriched with Lactobacillus paracasei Lpc‐37 (3.9 × 10⁸ CFUs/g), Lactobacillus acidophilus 74‐2 (2.9 × 10⁴ CFUs/g), Bifidobacterium lactis DGCC 420 (5.9 × 10⁴ CFUs/g) taken as 100 mL twice daily for 8 weeks Total daily dose was Lpc‐37: 7.8 × 10¹º CFUs/d, 74‐2: 5.8 × 10⁶ CFUs/d, and DGCC 420: 1.2 × 10⁷ CFUs/d Placebo drink not otherwise specified given as 100 mL twice daily Crossing over from one to the other intervention involved a washout period of 2 weeks Instructions were given for elimination of other probiotic and prebiotic products for 3 weeks before the start of treatment and during the 20 weeks of intervention |
|
| Outcomes | Total SCORAD and SCORAD part C at baseline and after 8 weeks of each intervention* *Denotes outcomes prespecified for this review |
|
| Notes | Study was sponsored by a grant from Zott Dairy GmbH (not the probiotic supplier). It was declared that Zott had no involvement in study design, data analysis, and publication, and study authors declared no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all subjects were randomly assigned to 2 treatment groups (1:1) according to a computer generated blocked randomisation list (blocked randomisation)" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear whether treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo drink had an identical composition except for the probiotic cultures and had the same appearance, taste and smell as the probiotic drink" ‐ "Enrollment and assignment to interventions were performed by the trial physician and AR. All involved persons (trial physician and scientific staff) were blinded. In addition study products were blinded and labelled with a numerical code by the production dairy" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The placebo drink had an identical composition except for the probiotic cultures and had the same appearance, taste and smell as the probiotic drink" ‐ "Enrollment and assignment to interventions were performed by the trial physician and AR. All involved persons (trial physician and scientific staff) were blinded. In addition study products were blinded and labelled with a numerical code by the production dairy" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant (6.6%) withdrew after randomisation and before the start of treatment and was excluded from analysis. All other participants were analysed in the group to which they had been randomised. Low rates of loss to follow‐up were unlikely to have a significant influence on effect estimates |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
Rosenfeldt 2003.
| Methods | Six‐week randomised controlled cross‐over trial | |
| Participants | Fifty‐eight children 1 to 13 years of age with eczema diagnosed using the UK Working Party Criteria. Children who had received systemic corticosteroids at any time were excluded Setting: hospital paediatric and dermatology departments in Denmark 15 participants lost to follow‐up |
|
| Interventions | Skimmed milk powder with dextrose anhydrate 2 g/d or a mix of Lactobacillus rhamnosus 19070‐2 and Lactobacillus reuteri DSM12246 at 2 × 10¹º CFUs/d of each strain. Both placebo and probiotic preparations administered twice daily with 2.5 to 5 mL water | |
| Outcomes | • Global self‐assessment by participant or parent*
• SCORAD*
• Need for other treatment ‐ topical corticosteroid* *Denotes outcomes prespecified for this review |
|
| Notes | Study was supported by Danish Research and Development Programme for Food Technology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised in a double‐blind crossover design...." ‐ "Blocked randomisation with 4 patients in each block was applied" Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Inadequate information for a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients were randomised in a double‐blind crossover design...." Comment: inadequate information for a judgement on risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The patients were randomised in a double‐blind crossover design...." Comment: inadequate information for a judgement on risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fifteen participants (25.9%) excluded from analysis. Five of these excluded during active treatment and 9 during placebo. Reasons for exclusion reported Comment: high rates of exclusion probably affecting all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
Shafiei 2011.
| Methods | Parallel‐group double‐blind placebo‐controlled randomised trial conducted between December 2008 and November 2009 | |
| Participants | Forty‐one children from 1 to 36 months of age with moderate to severe (SCORAD > 25) atopic dermatitis diagnosed according to Hanifin and Rajka criteria were recruited. Randomisation was done at a 1:1 ratio: 20 participants were randomised in the intervention arm, and 21 in the control arm. Exclusion criteria were administration of systemic steroids, recurrent infection, evidence of immunodeficiency, congenital abnormality, chronic disease, and problems in eating. Five participants were lost to follow‐up Setting: Iranian Paediatric Allergy and Immunology Department |
|
| Interventions | Synbiotic: 7‐strain probiotic and synbiotic in a sachet given daily at a dose of 1 × 10⁹ CFUs of probiotic and 990 mgr of fructo‐oligosaccharide for 2 months (59 days) Placebo: sucrose in a 1000‐mgr sachet given daily for 2 months (58 days) Both interventions were prepared by mixing with water, breast milk, formula, or solid food |
|
| Outcomes | Total and objective SCORAD changes from baseline to end of intervention after 2 months* *Denotes outcomes prespecified for this review |
|
| Notes | Sponsorship is unclear. Conflicts of interest are not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed according to a computer‐generated balanced block randomisation to synbiotic and placebo groups" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "At enrolment we assigned the study number and provided the participants with the appropriate sachet" Comment: not clear whether allocation to a treatment could have been predicted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated: "Partcipants and investigators blinded for the duration of the trial" ‐ "Placebo and intervention image‐matched and identical in appearance, taste and smell" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is stated: "Partcipants and investigators blinded for the duration of the trial", but no specific information provided on outcome assessor Comment: judged as unclear risk due to lack of specific information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants out of 41 (12%) were lost to follow‐up: 2 participants were from the probiotic group (10%) and 3 from the placebo group (14%). Reasons for loss to follow‐up were given and were similar in both groups. Case analysis was available. Low rates of loss to follow‐up unlikely to have a significant impact on the effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Baseline characteristics and outcomes reported only for participants who completed the trial. Unclear whether groups were matched at baseline |
| Other bias | Unclear risk | Sponsorship unclear |
Sistek 2006.
| Methods | Twelve‐week parallel‐group randomised controlled trial | |
| Participants | Sixty children 1 to 10 years of age with eczema diagnosed by UK Working Party criteria, SCORAD of at least 10 at recruitment, and a positive skin prick or RAST test to at least 1 common environmental or food allergen. Randomisation was done at a 1:1 ratio: 30 participants were randomised in each arm. Exclusion criteria were oral corticosteroid, immunosuppressant, or antibiotic in the previous month; previous immune deficiency or malignancy; and greater than 10‐point improvement in SCORAD during 2 weeks before the start of study treatment Setting: hospital clinic in New Zealand One participant lost to follow‐up |
|
| Interventions | Microcrystalline cellulose placebo or Lactobacillus rhamnosus and Bifidobacterium lactis given together once daily at a combined total dose of 2 × 10¹º CFUs/d. Treatment capsules administered as a powder mixed with food or drink, or taken in capsule form | |
| Outcomes | SCORAD assessed at 2 weeks before treatment, at the start of treatment, and 2, 12, and 16 weeks later* *Denotes outcomes prespecified for this review |
|
| Notes | One participant noted to be taking another non‐investigational probiotic Study was was funded by New Zealand Research Council. No conflicts of interest were declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomly assigned to treatment or placebo groups, using computer‐generated random numbers" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Adequate: treatment allocated by third party as confirmed by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "The control group received a placebo....that looked and tasted the same as the probiotic" ‐ "Both subjects and investigators were blind to the treatment groups" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The control group received a placebo....that looked and tasted the same as the probiotic" ‐ "Both subjects and investigators were blind to the treatment groups" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Available case analysis used without exclusions after randomisation and very low rates of loss to follow‐up (1/60 participants, 1.7%) Comment: low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Significant differences in baseline SCORAD in the 2 groups, with more severe mean SCORAD in the placebo group. Study authors state that this could have been avoided if randomisation had been blocked Comment: uncertain how this difference could have influenced the effect estimate |
Taniuchi 2005.
| Methods | Three‐month parallel‐group randomised controlled trial | |
| Participants | Seventeen children 3 to 18 months of age with eczema diagnosed by Hanifin and Rajka criteria, and with cow's milk hypersensitivity diagnosed by suggestive history plus evidence of cow's milk‐specific IgE. No information on the randomisation ratio was given. All participants had reduced levels of Bifidobacteria in their faeces (< 30% of total bacteria) and were receiving extensively hydrolysed cow's milk formula for at least 2 weeks before randomisation Setting unclear Unclear how many participants were lost to follow‐up |
|
| Interventions | Raffinose prebiotic containing extensively hydrolysed cow's milk formula with or without Bifidobacterium breve M‐16V at 5 to 15 × 10⁹ CFUs/d | |
| Outcomes | Investigator‐rated eczema scoring system* *Denotes outcomes prespecified for this review |
|
| Notes | No numerical outcome data available Study was supported by Grants‐in‐Aid from the Morianga Houshikai and the Mami Mitzutani Foundation. No conflicts of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 17 infants were divided into 2 groups at random" Comment: inadequate information; inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information; inadequate information for a judgement on risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on losses to follow‐up or data analyses |
| Selective reporting (reporting bias) | Low risk | No risk found |
| Other bias | Low risk | No other bias found |
Van der Aa 2010.
| Methods | Twelve‐week multi‐centre parallel‐group double‐blind placebo‐controlled randomised trial | |
| Participants | Ninety infants 0 to 7 months of age, exclusively formula fed, with a diagnosis of atopic dermatitis based on Hanifin and Rajka diagnostic criteria and SCORAD > 15 were recruited in the study. Randomisation was done at a 1:1 ratio: 46 participants were randomised in the intervention arm, and 44 in the control arm Infants who had received systemic corticosteroids, antibiotics, antimycotics, calcineurin inhibitors, or probiotics during 4 weeks or antihistamines during 2 weeks before enrolment were excluded from the study. Also excluded were infants needing systemic treatments other than antibiotics during the study and infants with major medical problems or GI or skin conditions other than atopic dermatitis Setting: participants recruited at 7 paediatric and dermatology secondary care centres in the Netherlands Eight participants were lost to follow‐up. Five participants (4 in the symbiotic group and 1 in the placebo group) were excluded from analysis because no SCORAD assessment was undertaken after baseline assessment |
|
| Interventions | Synbiotic: extensively hydrolysed whey‐based formula with Bifidobacterium breve M‐16V with 90% scGOS and 10% lcFOS given on demand at a dose of 1.3 × 10⁹ CFUs/100 mL of probiotic and 0.8 gr/100 mL of prebiotic for 12 weeks Placebo: formula given only on demand for 12 weeks |
|
| Outcomes | • Changes in total SCORAD from baseline to 4, 8, and 12 (end of treatment) weeks* • Total SCORAD in 1 year after the intervention • Frequency and mean class of topical steroids used at baseline and at end of treatment* *Denotes outcomes prespecified for this review |
|
| Notes | Two participants experienced severe adverse events: RSV bronchiolitis and severe cow's milk allergy. These were not related to the intervention Sponsored by a probiotic/formula company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised, using computer‐generated 4‐blocked design lists, drawn up by a statistician with stratification according to recruiting hospital and current use of topical steroids..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Formulas were prepared and coded by Danone Research and dispensed by the pharmacy of the Academic Medical Centre", "both formulas were identical with respect to smell, taste, texture, colour and packaging" Comment: third party involved; judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "both formulas were identical with respect to smell, taste, texture, colour and packaging. The investigator, participants' own physicians and parents were all blind to the treatment groups" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigator, participants' own physicians and parents were all blind to the treatment groups. Participants were clinically assessed by one investigator (L.B.A.), at weeks 0, 4, 8 and 12" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/46 (13%) participants from the probiotic group and 2/44 (4.5%) from the placebo group discontinued treatment but were not excluded from analysis. Five participants (5.5%) were excluded post randomisation because of unavailable assessment data after baseline. Four were from the probiotic group, and one from the placebo group. Losses to follow‐up included in analysis but not certain how missing data were handled Comment: overall low rates of missing data; judged to have low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sponsored by a probiotic/formula company. The role of the sponsor in data analysis is unclear; therefore the study was judged to be at unclear risk of bias |
Viljanen 2005.
| Methods | Four‐week parallel‐group randomised controlled trial | |
| Participants | 252 infants younger than 12 months of age with a clinical diagnosis of eczema and a clinical history suggestive of cow's milk allergy. Randomisation was done at a 1:1 ratio: 84 participants were randomised in each arm. Infants who had received a probiotic preparation for over a week in the preceding 6 weeks were excluded Participants were selected from primary care referrals to a hospital clinic in Finland Twenty‐two participants were lost to follow‐up |
|
| Interventions | Cow's milk elimination diet, extensively hydrolysed formula, and capsules of microcrystalline cellulose placebo, Lactobacillus GG (10¹º CFUs/d), or probiotic mix (Lactobacillus GG 10¹º CFUs/d, Bifidobacterium breve Bbi 99 4 × 10⁸ CFUs/d,Lactobacillus rhamnosus LC705 10¹º CFUs/d, and Propionibacterium JS 4 × 10⁹ CFUs/d). Capsules were mixed with food twice daily | |
| Outcomes | SCORAD assessed at end of treatment and 4 weeks later* *Denotes outcomes prespecified for this review |
|
| Notes | Study was supported by Finnish Research foundations and the probiotic supplier. No information on conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were randomised at the first visit according to computer‐generated block randomisation..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Adequate: treatment allocated by third party as confirmed by study author |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinician blinded as confirmed by study author |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded as confirmed by study author |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by "treatment received", and 4 participants (1.6%) were excluded from analysis because they did not tolerate the study formula. Twenty‐two (8.7% to 5.9% in LGG group, 10.6% in probiotic mix group, and 9.75% in placebo group) losses to follow‐up occurred. Unlikely to have an impact on effect estimate and judged as having low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Study was supported by Finnish Research foundations and the probiotic supplier. Not clear whether the probiotic supplier had any influence on data analysis |
Wang 2015.
| Methods | Four‐arm parallel‐group double‐blind randomised placebo‐controlled trial conducted from December 2011 until September 2013 | |
| Participants | 220 children and adolescents from 1 to 18 years of age with moderate to severe atopic dermatitis (SCORAD > 15) diagnosed according to the criteria of Hanifin and Rajka with symptoms present for at least 6 months before the study and with atopy as shown by at least 1 positive skin test (weal size ≧ 3 mm) or 1 positive RAST (IgE ≥ 0.7 kU/L) test to any common food or environmental allergens. Randomisation was done at a 1:1 ratio: 55 participants were randomised in each arm Excluded were patients on systemic corticosteroids, immunosuppressive therapy, or antibiotic or antimycotic treatment 4 weeks before the study, or using antihistamine 3 days before enrolment; and those who used probiotic preparations within 4 weeks before the study. Also excluded were patients with immune deficiency or other major medical problems Setting: secondary paediatric centre Country: Taiwan |
|
| Interventions | Four intervention arms: first arm received Lactobacillus paracasei GMNL‐133 (LP) at a dose of 2 × 10⁹ CFUs/d. Second arm received Lactobacillus fermentum GM‐090 (LF) at a dose of 2 × 10⁹ CFUs/d. Third arm received Lactobacillus paracasei GMNL‐133 (LP) and Lactobacillus fermentum GM‐090 (LF) at a dose of 4 × 10⁹ CFUs/d. Fourth arm received placebo. Interventions were given orally in capsule form for 3 months During the study, corticosteroids, antibiotics, calcineurin inhibitors, antihistamines, and other probiotics were not permitted, with the exception of topical corticosteroids (fluticasone propionate) in case of severe flares and itching. All patients applied emollients and were educated on skin care |
|
| Outcomes | For AD: SCORAD, Children's Dermatology Life Quality Index (CDLQI) and Family Dermatology Life Quality Index (FDLQI) at baseline and at 1, 2, 3, and 4 months Changes in total serum IgE and skin prick test reactivity, serum and urine biomarkers, and faecal probiotic species composition at baseline and at 3 months For asthma: GINA guideline asthma severity |
|
| Notes | Trial registration: NCT01635738. Approval by the Ethics Committee of the Taipei Hospital Ministry of Health and Welfare GenMont Biotec Inc.: probiotic manufacturer that provided study costs for probiotics. No information on conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised, using computer‐generated 4‐block design lists, drawn up by a statistician, with stratification according to age, gender, AD severity, and current use of topical steroids" |
| Allocation concealment (selection bias) | Low risk | Comment: probably done and judged as having low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were enrolled by the investigator and sequentially assigned a patient number connected to a code. Capsules were prepared and coded by GenMont Biotec Inc, with cGMP facilities and dispensed by the study nurse" ‐ "All investigators, study nurses and participants were blind to treatment assignment for the duration of the study" Judgement: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were enrolled by the investigator and sequentially assigned a patient number connected to a code. Capsules were prepared and coded by GenMont Biotec Inc, with cGMP facilities and dispensed by the study nurse" ‐ "All investigators, study nurses and participants were blind to treatment assignment for the duration of the study" Judgement: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "Finally analysed 100% of LP, 96% of LF, 98% of LP + LF group, and 96% of placebo" ‐ "Intention‐to‐treat analysis regardless of compliance" Judgement: low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | GenMont Biotec Inc.: probiotic manufacturer that provided study costs for probiotics. It is not clear what the role of the sponsor was in data analysis and publication; hence the study was judged to be at unclear risk of bias |
Weston 2005.
| Methods | Eight‐week parallel‐group block randomised controlled trial | |
| Participants | Fifty‐six children 6 to 18 months of age with moderate/severe eczema diagnosed by Hanifin and Rajka criteria and modified SCORAD score of at least 25 at enrolment Randomisation was done at a 1:1 ratio: 28 participants were randomised in each arm. Those previously exposed to probiotics, currently receiving antibiotics, or with other major medical problems were excluded Setting: community and hospital outpatient clinic in Australia Three participants lost to follow‐up |
|
| Interventions | Lactobacillus fermentum VR1‐003PCC 2 × 10⁹ CFUs/d as a sachet reconstituted by parents with 5 to 10 mL water twice daily, or maltodextrin placebo | |
| Outcomes | • Global self‐assessment by parent*
• Dermatitis Family Impact Questionnaire (Lawson 1998)*
• SCORAD*
• Need for other eczema treatment ‐ topical corticosteroid. Assessments made at baseline and at 2, 4, 8, and 16 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | One probiotic‐treated participant withdrew due to gastrointestinal illness (vomiting) Funding for principal investigator was provided by Research Fellowship by television channel and funding for IgE assay by VRI Biomedical. Probiotics and placebo supplied by manufacturer. No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerised randomisation schedule was prepared by the hospital biostatistician with allocation and dispensing of sachets by the pharmacy department" ‐ "The groups were stratified and block randomised according to following criteria: (a) modified SCORAD (25‐50; 50 and over), (b) current topical corticosteroid potency.... and (c) age..." Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "A computerised randomisation schedule was prepared by the hospital biostatistician with allocation and dispensing of sachets by the pharmacy department. The probiotic and placebo sachets were matched for size, shape and volume of contents" Comment: third party involved in the allocation process and interventions were identical. Judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: "The probiotic and placebo sachets were matched for size, shape and volume of contents" ‐ "A SCORAD assessment was also performed by a clinician blind to the intervention" ‐ "...a single investigator performed all SCORAD assessments at week 0, 8, and 16" Comment: outcome assessor clearly stated as blinded and interventions probably identical. However no other information provided to clarify whether other personnel and the parents of infants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "The probiotic and placebo sachets were matched for size, shape and volume of contents" ‐ "A SCORAD assessment was also performed by a clinician blind to the intervention" ‐ "...a single investigator performed all SCORAD assessments at week 0, 8, and 16" Comment: outcome assessor clearly stated as blinded and interventions probably identical. However no other information provided to clarify whether other personnel and the parents of infants were blinded, which may have affected patient/parent‐reported outcomes (DFI and global self‐assessment) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Available case analysis was used without exclusions after randomisation. Low rates of loss to follow‐up (5.4% overall; 7.1% in probiotic group and 3.6% in placebo group). Reasons for losses to follow‐up presented Low risk of attrition bias for all outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
Woo 2010.
| Methods | Twelve‐week parallel‐group randomised double‐blind placebo‐controlled trial conducted between January 2007 and August 2008 | |
| Participants | Eighty‐eight children between 2 and 10 years of age with atopic eczema‐dermatitis syndrome (AEDS) defined as pruritic chronic or chronically relapsing non‐infectious dermatitis with features and distribution as suggested by Hanifin. Randomisation was done at a 1:1 ratio: 45 participants were randomised in the intervention arm, and 43 in the control arm. Participants had to have the disease for at least 6 months, with SCORAD ≥ 25 and change in SCORAD not more than 10% within the first 2 weeks Excluded from the trial were patients who had used cyclosporine, systemic corticosteroids, topical calcineurin inhibitors, or Chinese herbal medicine during the 3 months before recruitment Recruitment was carried out at a secondary care paediatric unit in Korea Thirteen participants were lost to follow‐up |
|
| Interventions | Probiotic: freeze‐driedLactobacillus sakei KCTC 10755BP in microcrystalline cellulose dissolved in 2.5 to 5 mL of water or any liquid preferred by the participant at a dose of 5 × 10⁹ CFUs not otherwise specified for 12 weeks Placebo: microcrystalline cellulose dissolved in 2.5 to 5 mL of water or any liquid preferred by the participant for 12 weeks Standardised topical treatment for all participants: take a bath once daily with warm water for 5 to 10 minutes and apply an emollient immediately after bathing. Permitted to use topical corticosteroids as required but only 0.1% prednicarbate |
|
| Outcomes | • Total SCORAD and SCORAD part C* • Amount of topical corticosteroid (TCS) used during the study, change in TCS use from baseline, and number of participants using TCS during the intervention* *Denotes outcomes prespecified for this review |
|
| Notes | Research supported by the Basic Science Research Program, National Research Foundation of Korea Funded by the Ministry of Education, Science and Technology, Korea. No conflicts of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised in a double‐blind design..." Comment: inadequate information for a judgement on risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "patients were randomised in a double‐blind design..." Comment: inadequate information on which part was blinded and the method of blinding interventions; inadequate information for a judgement on risk of bias for blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "patients were randomised in a double‐blind design..." Comment: inadequate information on which part was blinded and the method of blinding interventions; inadequate information for a judgement on risk of bias for blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed in the group to which they were initially randomised. 4/45 participants (8.9%) from the probiotic group and 9/43 (21%) from the placebo group were lost to follow‐up and were excluded from analysis. The difference in losses to follow‐up in the 2 groups was found to be statistically insignificant (P = 0.11). Overall losses to follow up were low (14%) Comment: judged as low risk for attrition bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Baseline characteristics presented only for participants who completed the study, but low rates of loss to follow‐up; hence the study was judged to be at low risk of bias |
Wu 2012.
| Methods | Ten‐week parallel‐group double‐blind randomised placebo‐controlled trial | |
| Participants | Sixty children from 2 to 14 years of age with eczema diagnosed by Hanifin and Rajka criteria and moderate to severe disease (SCORAD > 25) who had eczema symptoms for at least 4 days before diagnosis. Randomisation was done at a 1:1 ratio: 30 children were randomised in each arm Setting: secondary care with 1 recruiting centre in Taiwan Six participants were excluded post randomisation but before treatment started |
|
| Interventions | Synbiotic: Lactobacillus salivarius PM‐A0006 2 × 10⁹ CFUs/25 mgr and fructo‐oligosaccharide 475 mgr in a capsule preparation twice daily Control: corn starch 25 mgr and fructo‐oligosaccharide 475 mgr in a capsule twice daily |
|
| Outcomes | • SCORAD* • 13‐item quality of life daily diary • Global eczema severity • SCORAD parameters for pruritus and sleep loss* • Frequency of use of topical corticosteroid or calcineurin inhibitor (times/month)* *Denotes outcomes prespecified for this review |
|
| Notes | Two probiotic‐treated participants initially had mild diarrhoea but overall tolerated treatment well Sponsorship declared and no conflicts of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children were randomised into two groups...in a double‐blind manner using a computer‐generated blocked randomisation list provided by ProMD Biotech Co., Taiwan. A block size of four was used and stratified according to sex, age and diagnosis of moderate to severe AD" Comment: judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was opened only after all data were analysed." Comment: probably done, so judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The synbiotic and prebiotic products, which were identical in appearance, odour and taste, were delivered in numbered packages directly to the parents according to randomisation list. The code was opened only after all data were analysed" ‐ "the same investigator (KGW), who was blinded to group assignment, enrolled patients and performed all SCORAD assessments at weeks 0, 4, 8 and 10" Comment: judged as adequate for low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The synbiotic and prebiotic products, which were identical in appearance, odour and taste, were delivered in numbered packages directly to the parents according to randomisation list. The code was opened only after all data were analysed" ‐ "the same investigator (KGW), who was blinded to group assignment, enrolled patients and performed all SCORAD assessments at weeks 0, 4, 8 and 10" Comment: judged as adequate for low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants (10% in total and in each group) withdrew after randomisation but before treatment initiation and were excluded from analysis. Reasons for withdrawal given and similar in both groups Comment: low rates of excluded patients unlikely to have a significant impact on effect estimate; hence low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias found |
Wu 2015.
| Methods | Eight‐week parallel‐group 2‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | Sixty‐seven children 4 to 48 months of age with atopic dermatitis diagnosed using Hanifin‐Rajka criteria with SCORAD > 15 at enrolment. Randomisation was done at a 1:1 ratio: 34 participants were randomised in the intervention arm, and 33 in the control arm Exclusion criteria: clinically evident infection in skin lesions, severe asthma or acute asthma attack within 3 months, autoimmune disease, immunodeficiency, exposure to phototherapy, use of systemic corticosteroids within 1 month |
|
| Interventions | Active: 1 capsule of ComProbi containing 350 mg ofLactobacillus rhamnosus (MP108) and maltodextrin per day Control: 1 capsule of maltodextrin per day If capsule could not be swallowed, parents were instructed to mix the powder in water, breast milk, milk, or food heated to < 40°C Rescue medication: Cutivate cream (GlaxoSmithKline, Duhram, UK) in cases of uncontrolled symptoms |
|
| Outcomes | Primary • Change in SCORAD after 8‐week treatment Secondary • Change in SCORAD at post‐baseline visits • Frequency and total quantity of corticosteroids used during 8‐week treatment • Comparison of frequency of atopic dermatitis and symptom‐free duration • Comparisong of mean changes from baseline in IDQoL at weeks 4 and 8 • Comparisong of mean changes from baseline on Dermatitis Family Impact Questionnaire at weeks 4 and 8 |
|
| Notes | Contact: cshy095@csh.org.tw Country: Taiwan Study registration not given CY Biotech provided probiotic, but it is not clear whether the company had a role in study design, data analysis, or interpretation or other aspects of the study. However, study authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information provided; stated that the study was "randomised" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study stated as "double‐blind", but no information provided to explain who was blinded and how blinding was done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study stated as "double‐blind", but no information provided to explain who was blinded and how blinding was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (9%) from the probiotic arm and 1 (3%) from the control arm Study authors present data derived from intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | All predefined outcomes were at least described, but not all data were given. No clinical trial registration was given |
| Other bias | Unclear risk | CY Biotech provided probiotic, but it is not clear whether the company had a role in study design, data analysis or interpretation, or other aspects of the study. However study authors declared no conflict of interest Hence we judged the study to be at unclear risk of commercial bias |
Yang 2014.
| Methods | Six‐week parallel‐group double‐blind placebo‐controlled randomised trial conducted between November 2010 and October 2011 | |
| Participants | One hundred children from 2 to 9 years of age with mild to moderate (SCORAD < 40) atopic dermatitis diagnosed according to the criteria of Hanifin and Rajka were recruited. Randomisation was done at a 1:1 ratio: 50 participants were randomised in each arm Exposure to commercial probiotic products during the 4 weeks before the study Premature children and those receiving antibiotic, systemic corticosteroid, immunosuppressive, or Chinese herbal therapies within 4 weeks before enrolment were excluded form the study. Also excluded were patients with acute gastrointestinal infection, chronic underlying disease, or baseline factors predisposing to infection (e.g. neurological disease; metabolic disease; chronic respiratory disease; congenital anomaly of the heart, gastrointestinal system, or lung; known or suspected immunodeficiency) Recruitment took place at a tertiary paediatric centre in Korea Twenty‐nine participants were lost to follow‐up |
|
| Interventions | Probiotic mixture: Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Bifidobacterium lactis in glucose anhydrous crystalline powder derived from cornstarch prepared in warm water or juice given orally immediately after meals twice daily at a dose of 1 × 10⁹ CFUs of each probiotic strain for 6 weeks Control (placebo): glucose anhydrous crystalline powder prepared in warm water or juice given orally immediately after meals twice daily for 6 weeks Instructions given to stop topical corticosteroids and calcineurin inhibitors, oral antihistamines, and any commercial probiotic‐containing products 2 weeks before study initiation Parents were trained in appropriate bathing and skin care practices and were given instructions on application of emollients |
|
| Outcomes | • EASI score at baseline and end of treatment and change from baseline* • VASP score at baseline and end of treatment and change from baseline* *Denotes outcomes prespecified for this review |
|
| Notes | Study sponsored by the probiotic supplier; the supplier's role in data analysis and publication is unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation software was used to randomly allocate children..." Comment: computer‐generated and so judged as adequate for low risk of bias |
| Allocation concealment (selection bias) | Low risk | Comment: third party ‐ not involved in the trial ‐ allocated treatment. Study judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The probiotics mixture and placebo controls were identical in colour, taste, smell, packing and manner of administration. All formulations were dispensed by a pharmacist not associated with the study.", "both investigators and study subjects were blinded to the identity of the intervention" Comment: not clear if clinicians were blinded; patients were blinded; judged as having unclear risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The probiotics mixture and placebo controls were identical in colour, taste, smell, packing and manner of administration. All formulations were dispensed by a pharmacist not associated with the study" ‐ "both investigators and study subjects were blinded to the identity of the intervention" Comment: not clear if outcome assessor was blinded; judged as having unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Available case analysis but high rates of loss to follow‐up (29%: 26% in probiotic group and 32% in placebo group), which were excluded from analysis Comment: effect estimate for all outcomes may have been affected; judged as having high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Study sponsored by the probiotic supplier, and the supplier's role in data analysis and publication is unclear. Therefore the study was judged to be at unclear risk of bias |
Yesilova 2012.
| Methods | Eight‐week parallel‐group double‐blind randomised placebo‐controlled trial | |
| Participants | Forty children 1 to 13 years of age with atopic dermatitis diagnosed according to Hanifin and Rajka criteria and with moderate to severe disease. Randomisation was done at a 1:1 ratio: 20 participants were randomised in each arm. Excluded were patients on medication including antihistamines and corticosteroids for 14 days before recruitment, as well as those with malabsorption. One participant was lost to follow‐up | |
| Interventions | Probiotic mixture:Bifidobacterium bifidum,Lactobacillus acidophilus,Lactobacillus casei,Lactobacillus salivarius given orally at a dose of 2 × 10⁹ CFUs and a total daily dose of 4 × 10⁹ CFUs for 8 weeks Placebo: skim milk powder and dextrose |
|
| Outcomes | SCORAD at baseline and at 8 weeks* *Denotes outcomes prespecified for this review |
|
| Notes | Conflicts of interest/sponsorship not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were referred to a nurse who was involved in the study to receive either probiotic or placebo. The nurse randomised each patient to two different treatment groups using the closed‐envelope method" Comment: not clear how the "closed envelopes" had been created and whether the sequence generation had been random. Judged as having unclear risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Closed envelope method" Comment: probably done; judged as adequate for low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients were referred to a nurse who was involved in the study to receive either probiotic or placebo. The nurse randomised each patient to two different treatment groups using the closed‐envelope method. The authors had no role in the treatment decision and were blinded to the treatment groups" Comment: inadequate information provided on which parts were blinded and whether the interventions were identical. Judged as having unclear risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The patients were referred to a nurse who was involved in the study to receive either probiotic or placebo. The nurse randomised each patient to two different treatment groups using the closed‐envelope method. The authors had no role in the treatment decision and were blinded to the treatment groups" Comment: inadequate information on which parts were blinded and whether interventions were identical. Judged as having unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from the placebo group was lost to follow‐up (2.5%). Available case analysis was used. Very few losses to follow‐up were unlikely to affect the effect estimate for any outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. Differences between probiotic and placebo not given but reported narratively: "Our results demonstrated an improved SCORAD index in both groups, but with higher levels in the probiotic group (65%) than in the placebo group (46%). In the probiotic group, a greater decrease of SCORAD index scores was shown after treatment in patients with high SCORAD index scores. However this difference did not reach a statistically significant level" |
| Other bias | Unclear risk | Conflicts of interest/sponsorship not declared Baseline characteristics of 2 groups not given. Uncertain if they were matched Judged to be at uncertain risk of other bias |
Yoshida 2010.
| Methods | Eight‐week parallel‐group placebo‐controlled trial | |
| Participants | Twenty‐four adults with atopic dermatitis diagnosed according to the Guideline for Management of AD by the Japanese Dermatological Association were recruited and randomised at a 2:1 ratio of intervention:control (16 participants in intervention arm/8 participants in control arm) Recruitment took place in a centre in Japan No participants were lost to follow‐up |
|
| Interventions | Probiotic: Bifidobacterium breve (lyophilised powder of live B breve YY) in a capsule preparation at a dose of 1.0 × 10¹º CFUs/capsule taken twice daily after breakfast and after dinner for 8 weeks Placebo not described |
|
| Outcomes | • SCORAD: total, objective, subjective* • Japanese version of Skindex‐29* *Denotes outcomes prespecified for this review |
|
| Notes | Sponsorship was not declared, but 2 of the authors of the report are linked to the supplier of the probiotic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Test meals for 24 subjects were randomly allocated..." Comment: no other information provided; judged as having unclear risk |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. All participants analysed |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Selection bias: baseline SCORAD scores not matched between the 2 groups Commercial bias: sponsorship not declared but 2 of the authors of the report linked to the supplier of the probiotic; this may have affected the outcome Study was judged to have high risk of other bias |
AD: atopic dermatitis. AEDS: atopic eczema‐dermatitis syndrome. BPD: bronchopulmonary dysplasia. CDLQI: Children's Dermatology Life Quality Index. CFU: colony‐forming unit. cGMP: cyclic guanosine monophosphate. DFI: Dermatology Family Impact. DLQI: Dermatology Life Quality Index. EASI: Eczema Area Severity Index. FDLQI: Family Dermatology Life Quality Index. IDQoL: Infant Dermatitis Quality of Life. IFN: interferon. IgE: immunoglobulin E. IL: interleukin. LF: Lactobacillus fermentum. LP: Lactobacillus paracasei. RAST: radioallergosorbent test. RSV: respiratory syncytial virus. SCORAD: Severity Scoring of Atopic Dermatitis. TCS: topical corticosteroid. TS: topical steroid. VAS: visual analogue scale. VASP: Visual Analogue Scale of Pruritus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arkwright 2003 | Intradermally given probiotic |
| Arvola 2006 | Participants did not have eczema |
| Aryayev 2006 | Not an RCT. Only a quasi‐RCT |
| Burk 2013 | Not all participants had eczema |
| Chernysov 2009 | Not an RCT. Quasi‐RCT |
| Foekel 2009 | Not a trial on probiotics. Unpasteurised mare's milk trial |
| Gueniche 2008 | Topical intervention |
| Ikezawa 2004 | Intervention was not a probiotic |
| Kalliomaki 2003 | Participants did not have eczema |
| Laitinen 2005 | Not a study of changes in eczema symptoms or severity. Follow‐up of a study on probiotics for prevention, not treatment, of allergies |
| Leung 2004 | Intervention was not a probiotic |
| Matsumoto 2007 | Control is also a probiotic. Exclusion criteria in protocol |
| Moroi 2011 | Heat‐killed bacteria. Not live micro‐organisms; exclusion criteria in protocol |
| Murosaki 2006 | Heat‐killed bacteria. Not live micro‐organisms; exclusion criteria in protocol |
| Ogawa 2006 | Trial studied effects of probiotics in mice only. Human participants were healthy volunteers without eczema |
| Ou 2012 | Not all participants had eczema |
| Rose 2010 | Not all participants had eczema |
| Shibata 2009 | Not a probiotic ‐ a prebiotic only |
| Torii 2011 | Heat‐killed bacteria. Not live micro‐organisms; exclusion criteria in protocol |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12605000615684.
| Methods | Eight‐week parallel‐group randomised placebo‐controlled trial |
| Participants | Children from 6 months to 12 years of age with eczema and SCORAD > 15 Inclusion criteria: guardians proficient in English. Not previously actively treated with probiotics for eczema. No current chronic illness other than asthma, allergic rhinitis, or food allergy Exclusion criteria: none Country: Australia |
| Interventions | Probiotic: Lactobillus GG 3 × 10⁸ to 1 × 10⁹ CFUs twice daily for 8 weeks vs placebo |
| Outcomes | SCORAD at 0, 8, and 16 weeks. |
| Notes | ACTRN12605000615684. Unknown current status |
Candy 2016.
| Methods | Eight‐week prospective randomised multi‐centre double‐blind controlled study |
| Participants | Seventy‐one full‐term infants with suspected non‐IgE‐mediated cow's milk allergy (36 infants in control group, 35 in active group) Cow's milk allergy participants presented predominantly with gastrointestinal symptoms, and 10% with dermatological symptoms Country: Netherlands |
| Interventions | Active: amino acid‐based formula (AAF) with synbiotics designed for dietary management of cow's milk allergy (prebiotic: chicory‐derived neutral oligofructose, long‐chain inulin 9:1 ratio, and concentration 0.63 g/100 mL; probiotic: Bifidobacterium breve M‐16V at a concentration 1.47 × 10⁹ CFUs/100 mL formula) Control: commercially available formula with AAF only Intake/instructions: participants were instructed to consume a minimum, age‐specific, daily formula intake from the end of week 2 (infants 0 to 6 months of age, 500 mL; 6 to 8 months of age, 450 mL; and 49 months of age, 350 mL) Duration of intervention: 8 weeks |
| Outcomes | Primary
Secondary
|
| Notes | Trial acronym: ASSIGN‐1 Register: NTR3979 (Netherlands Trial Register) Funding/Sponsor: Nutricia Research BV Contact: Willemien Sinke; willemien.sinke@nutricia.com |
Hulshof 2017.
| Methods | Randomised double‐blind controlled trial |
| Participants | 31 infants with objective SCORAD (score > 20; moderate to severe atopic dermatitis) up to and including 11 months of age, with elevated total IgE or specific IgE levels, or both, were included (n = 31) Country: Netherlands |
| Interventions | Synbiotic: extensively hydrolysed whey‐based formula with mixture of short‐chain galacto‐, long‐chain fructo‐oligosaccharides (scGOS/lcFOS, ratio 9:1) and Bifidobacterium breve M‐16V (active) at a dose of 1.0 × 10⁹ CFUs/g Control: extensively hydrolysed whey‐based formula Duration of intervention: 4 months |
| Outcomes | Severity of atopic dermatitis and correlation to serum chemokines
|
| Notes | Study presented at Conference Registered in the Dutch Trial Register: NTR3447 |
NCT02585986.
| Methods | Randomised double‐blind placebo‐controlled clinical trial |
| Participants | Children of both genders between 4 and 17 years of age. Estimated enrolment: 50. Country: Spain Inclusion criteria:
Exclusion criteria
|
| Interventions |
Duration of intervention: 12 weeks |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | Current status: results published Registration: NCT02585986 (ClinicalTrials.gov). Sponsor: Biopolis SL |
Prakoeswa 2017.
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | 22 children 0 to 14 years of age (12 probiotic and 10 placebo) with mild to moderate atopic dermatitis meeting the Hanifin‐Rajka diagnostic criteria. New outpatients from the clinic at the Allergy Immunology Division of the Dermatology and Venereology Department, Faculty of Medicine, Indonesia. Participants had to have age‐related total serum IgE levels: 10 to 15 years > 200 IU/mL, 6 to 9 years > 90 IU/mL, 1 to 5 years > 60 IU/mL, < 1 year > 1.5 IU/mL. Participants had to be in apparent good health and willing to participate in the study, and had to sign informed consent Exclusion criteria: use of systemic corticosteroids or phototherapy in the previous month, systemic immunosuppressive drugs in the previous 3 months, probiotic use in the previous 4 weeks, use of topical medications such as corticosteroids or calcineurin inhibitors in the previous week, immunosuppressive conditions or other serious disease, clinical skin disease, and other systemic disease Country: Indonesia |
| Interventions | Intervention: microencapsulated L plantarum IS‐10506 at a dose of 10¹º CFUs/d Control: placebo; skim milk ‐ Avicel Duration: 12 weeks |
| Outcomes | SCORAD score at 0, 2, 8, and 14 weeks Total IgE, IL‐4, IFN‐γ, Foxp3=/IL‐10, IL‐17 at 0 and 14 weeks |
| Notes | ‐ |
AAF: amino acid‐based formula. CC: Clostridium coccidioides. CFU: colony‐forming unit. CGI: Clinical Global Impression. ER: Eubacterium rectale. IFN: interferon. IgE: immunoglobulin E. IL: interleukin. SCFA: short‐chain fatty acid. SCORAD: Severity Scoring of Atopic Dermatitis.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800015330.
| Trial name or title | Study on the effect and mechanism of probiotics on patients with atopic dermatitis |
| Methods | Randomised parallel controlled trial with 4 intervention groups |
| Participants | Participants randomised in 4 groups. Aim is to recruit 30 participants in each group Inclusion criteria: meet the diagnostic criteria of Hanifin and Rajka; from 7 to 60 years old; have not taken probiotics (such as lactic acid bacteria, bifidobacteria, etc.) yoghourt, beverages, etc. (in the past 2 months, adherence to requirement of taking probiotic productions for 2 months, co‐operation with survey and collection of blood samples, faeces, and other biological samples); volunteers who sign informed consent Exclusion criteria: pregnant or lactating women; short‐term or long‐term use of antibiotics, accompanied by diabetes, cardiovascular disease, history of malignant disease complications, and any other interference with results of tests evaluating skin disease, enteritis, etc.; accompanied by mental illness |
| Interventions |
|
| Outcomes | Primary
Secondary
|
| Starting date | Date of registration: 23/03/2018. Prospective registration |
| Contact information | Wenwei Lu; Tel: +8618762691080; email: luwenwei@jiagnan.edu.cn 1800 Lihu Avenue, Binhu District, Wuxi, Jiangsu, China |
| Notes | Open for recruitment Study sponsors: People's Hospital of Tinghu District of Yancheng, China, and Jiangnan University |
CTRI/2017/08/009236.
| Trial name or title | A study to observe if probiotics supplementation is helpful in atopic dermatitis in children |
| Methods | Phase III randomised placebo‐controlled parallel‐group trial. Blinded investigator. Randomisation computer generated |
| Participants | Children of both genders, from 6 months to 12 years of age who have atopic dermatitis of any severity diagnosed based on the Hanifin and Rajka criteria Exclusion criteria: immunocompromised children; children with severe kidney, liver, or systemic disease; other ages Target sample size = 114 |
| Interventions | Probiotics and placebo. No other information available |
| Outcomes | Primary outcomes
|
| Starting date | Date of first enrolment: 02/07/2016 |
| Contact information | Professor Sanjeev Handa, Professor of Dermatology, Venereology, and Leprology handa_sanjeev@yahoo.com Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh 160012, India |
| Notes | Study sponsored by PGIMER Currently recruiting |
CTRI/2017/10/010018.
| Trial name or title | Probiotics in treatment of atopic dermatitis in children |
| Methods | Double‐blind randomised placebo‐controlled parallel‐group trial. Computer‐generated randomisation Method of allocation concealment: sequentially numbered, sealed, opaque envelopes |
| Participants | Children with atopic dermatitis of both genders, from 6 months to 18 years of age, attending JIPMER Paediatrics or Dermatology. Target sample size = 108 Exclusion criteria: children with acute gastrointestinal infection; children with chronic underlying disease on immunosuppressive therapy; children with known or suspected immunodeficiency; children on prolonged antibiotic and antituberculous therapy; children who are included in other studies |
| Interventions | Probiotic: Lactobacillus rhamnosus GG. Dose: 10 billion CFUs/capsule or sachet/d for 3 months Control: placebo: anhydrous glucose powder Both groups receive also standard treatment |
| Outcomes | Primary outcome
Secondary outcomes
Time point for secondary outcomes: 1 year |
| Starting date | 01/11/2017. Registered as not recruiting yet |
| Contact information | Dhayalini RK; dhayaliniraj22@gmail.com Department of Pediatrics (OPD 165) and Department of Dermatology (OPD 72), JIPMER Hospital, Dhauranthri Nagar, Gorimedu, Pondicherry 605006, PONDICHERRY |
| Notes | Country: India Registered prospectively Ethics approval received Sponsorship: JIPMER Hospital and Research Institution |
KCT0000914.
| Trial name or title | IRT5 probiotics atopic dermatitis |
| Methods | Phase III 8‐week parallel‐group randomised double‐blind placebo‐controlled clinical trial |
| Participants | Children 5 to 12 years of age with atopic dermatitis, SCORAD score between 25 and 50, and continuous or intermittent symptoms of atopic dermatitis over 6 months. Target sample size = 110 participants; 55 in each group Other inclusion criterion: signs informed consent form with a legal representative before participating in the study Exclusion criteria
|
| Interventions | Probiotic: IRT5 probiotics, 1 × 10¹º CFUs/sachet, to be taken orally 1 sachet per day for 8 weeks Placebo group: lactose, to be taken orally 1 sachet per day for 8 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | Date of first enrolment: 06/11/2013 |
| Contact information | Professor Kim Beom Jun, CHUNG‐ANG University, Korea |
| Notes | Registration: KCT0000914. Current status: recruiting. No updates to the registry since 2013 Primary sponsor: KOREA YAKULT CORPORATION. Affiliation: CHUNG‐ANG University |
NCT02519556.
| Trial name or title | Trial on effectiveness of combined probiotics in atopic dermatitis in children |
| Methods | Phase IV randomised parallel‐group double‐blind placebo‐controlled clinical trial |
| Participants | Children of both genders older than 6 months up to 19 years of age Inclusion criteria
Exclusion criteria
|
| Interventions | Probiotic: probiotic comprising the mixture of strains: Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus paracasei, and Bifidobacterium lactis, at a dose of 1 gram sachet, once a day for 6 months (drug: Probiatop) Control: placebo or maltodextrin in sachet once a day for 6 months |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2015, with provisional completion date July 2017 |
| Contact information | Contact: Paula Albuquerque, MD; 5516981329192; paula_albuquerque@usp.br |
| Notes | Registration: NCT02519556 (ClinicalTrials.gov) Country: Brazil. Sponsor: Casa Espirita Terra de Ismael. Estimated primary completion date: July 2017. Currently enrolling by participant invitation only |
NCT02945683.
| Trial name or title | ATOPIA‐D3: effects of Lactobacillus reuteri plus vitamin D3 in children with atopic dermatitis |
| Methods | Randomised double‐blind placebo‐controlled clinical trial |
| Participants | Children of both genders, 1 to 4 years of age with diagnosis of atopic dermatitis. Recruitment target: 88 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | February 2015 |
| Contact information | Attilio Boner, Professor +390458124615 attilio.boner@univr.it |
| Notes | Study currently recruiting. Estimated completion date February 2018 |
AD: atopic dermatitis. CDLQI: Children's Dermatology Life Quality Index. CFU: colony‐forming unit. ELISA: enzyme‐linked immunosorbent assay. IDLQI: Infant's Dermatitis Life Quality Index. IFN: interferon. IgE: immunoglobulin E. IL: interleukin. QoL: quality of life. SCORAD: Severity Scoring of Atopic Dermatitis. TGF: transforming growth factor.
Differences between protocol and review
Changes between protocol and first or updated review
We changed the title, which was "Probiotics for atopic eczema" in the protocol to "Probiotics for treating eczema", on the advice of the referees.
We made small changes to the Background on the advice of referees of the first review.
We made small changes to the Criteria for considering studies for this review section to clarify issues.
In the protocol, we used the terms "short term" and "long term" for primary and secondary outcomes. We have replaced these terms with the phrases "at the end of active treatment" and "within six months after active treatment has ceased", respectively. We did this for clarity.
RB and FB completed data extraction in the first review, in place of RB and MT in the protocol, due to time availability.
In the review, when complete data sets were available from trial authors, we used these data to calculate summary statistics such as mean and standard deviation before data entry, but this was not stated in the protocol.
In the review, when studies reported participant‐ or investigator‐rated symptoms on categorical scales (e.g. Passeron 2006), we made the data dichotomous by defining a cutoff at good improvement in eczema versus mild improvement, no change, or worsening of eczema. This was not predefined in the protocol.
In the protocol, expressing numbers needed to treat was mentioned, but not in this review. We were going to estimate numbers needed to treat provided clinically positive results favoured probiotics; therefore this was not done.
In the review, we had to deal with data from studies with multiple treatment groups by combining the data from these groups and by converting non‐parametric statistics to parametric summary statistics, but this was not mentioned in the protocol.
In the protocol, it was not described that for analyses with extreme heterogeneity (e.g. I² statistic > 85%), we would consider not undertaking a meta‐analysis.
In the review, we calculated the pooled estimate using standardised mean differences when studies used different tools to measure the same outcome, which had not been stated in the protocol.
In the protocol, we did not mention that we would use available case analysis, rather than intention‐to‐treat analysis with imputation.
In the protocol, we had planned some subgroup analyses, but in the review, we presented the data in a stratified analysis. We have explained reasons for this in the Methods section and in the subsection Subgroup analysis and investigation of heterogeneity.
In the review, we performed analysis stratified by severity (mild, moderate, severe) of eczema based on the Severity Scoring of Atopic Dermatitis (SCORAD), which had not been clearly stated in the protocol. In the protocol, it was stated that we would split the eczema into mild, moderate, and severe if enough data were available, but not that a stratified analysis would take place.
Extra stratified analyses were undertaken in the review, and this had not been specified in the protocol ‐ outcome data were analysed according to the probiotic strain used (Analysis 1.21 to Analysis 1.28). We undertook these analyses because of unexplained heterogeneity between studies for primary and secondary outcomes and use of the same probiotic strain in several studies. As discussed above, conclusions from this analysis must be guarded due to its post hoc nature.
We edited the Objectives in line with MECIR reporting standards.
Selection of studies: for the protocol, RB and MT performed study selection; for the review update, RB and AM performed study selection. We encountered no differences that would require an arbitration.
For the update, RB and AM performed data extraction, but for the protocol, this was performed by RB and MT, and in the first review, by RB and FB.
In the protocol and in the first review, RB and FB checked the data, but in the updated review, it was JL and AM.
Assessment of risk of bias in the updated version of the review: we used the Cochrane 'Risk of bias' tool (Chapter 8.5, in Higgins 2011), which is not given in the protocol.
We reported dichotomous outcome data as odds ratios (ORs) in the first review, and risk ratios (RRs) were planned in the protocol. In the update for dichotomous outcomes, we expressed the results as RRs and 95% confidence intervals (CIs) for analyses containing only parallel‐group trials, and we used ORs when data from cross‐over studies were included in the meta‐analysis, in keeping with the methods stated in Elbourne 2002 and Duffy 1989.
We performed sensitivity analyses based on change in scores from baseline for both first and updated reviews and for studies with low risk of bias in the update. In the update, we defined studies with overall low risk of bias as those studies for which the randomisation process was clear; allocation concealment was clear and done; participants, clinicians, or outcome assessors were blinded; and there was no attrition bias.
We assessed the quality of evidence in the update of the review by using the GRADE tool, as is now recommended by Cochrane.
We used trial sequential analysis for our primary outcome in the update of this review (please see Methods).
For the update of the review, we performed assessments of reporting bias.
For the updated review, we revised the search methods in line with current Cochrane Skin practices. We included a search of the GREAT database (Global Resources of Eczema Trials) and of the following trials registers: the Australian New Zealand Clinical Trials Register (ANZCTR), the World Health Organization International Clinical Trials Registry platform, and the EU Clinical Trials Register. We did not update previous searches of ISI Web of Science, or of the Ongoing Skin Trials Register, whose content has now been migrated to ANZCTR. For full details of previous searches for the earlier review, see Boyle 2008.
Previously, we searched MEDLINE for adverse effects of probiotics. We did not perform an adverse events search for this update, but we recorded adverse events reported in included and excluded trials. Adverse events of probiotics have been well established, and we have referred only to relevant review articles.
We added a consumer (AR) to the review authors' group, as required by Cochrane for the review update. We also added a new review author (AM) to the review authors' group.
In this update, we presented 'Summary of findings' tables, but this was not stated in the protocol and was not done in the first review.
Contributions of authors
Linking with editorial base and co‐ordinating contributions from co‐reviewers (AM). Drafting protocol (RB with contributions from all co‐reviewers of the first review). Running the search (Cochrane trial search co‐ordinator and AM). Identifying relevant titles and abstracts from searches (AM and RB). Obtaining copies of trials (AM). Selecting trials (AM and RB). Extracting data from trials (RB and AM). Entering data into RevMan (JL and AM). Carrying out analyses (JL, AM, and RB). Interpreting data (RB, AM, and JL). Drafting final review (AM, RB, with contributions from all co‐reviewers). Checking readability and clarity of the review (AR).
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
Murdoch Children's Research Institute, Australia.
University of Melbourne, Australia.
Royal Children's Hospital, Australia.
Department of Paediatrics, Imperial College, London, UK.
-
Western Infirmary, Glasgow, UK.
Library Services
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Robert Boyle: "I have received speaker honoraria, support for travel to a conference, and a research grant from Danone Research, whose parent company market products containing probiotics. I undertook a consultancy project for an infant formula company (Dairy Goat Cooperative) in 2017, to help them design a robust and ethical clinical trial of an infant formula milk. The project related to prevention of eczema, but did not involve probiotics or eczema treatment". Mimi Tang: "My conflicts of interest are as follows:
past member of global scientific advisory board Danone Nutricia (resigned December 2016);
past member of medical advisory board for Oceania Nestle Nutrition Institute (resigned);
speaker fees at symposia sponsored by Danone Nutricia, Abbott, and Nestle Health Science;
consultant to Deerfield, GLG, and Bayer;
employee with shares/share options Prota Therapeutics;
inventor on a patent owned by MCRI (Murdoch Children's Research Institute);
grant, received from my institution, from Prota Therapeutics;
royalties from Wiley, as an author of a book Kids Food Allergies for Dummies; and
payment for development of educational presentations from MD Linx ‐ I developed a GP education module: 'Microbiota and Immune Development'".
Areti Makrygeorgou: "I have received speaker honorarium by Celgene and support to travel to a conference by Novartis". Jo Leonardi‐Bee: nothing to declare. Fiona J Bath‐Hextall: nothing to declare. Dedee F Murrell: "I run a clinical trials centre for a variety of skin diseases, including atopic dermatitis and give lectures on this topic. I am a Councillor (a voluntary position) representing Australia on the International Eczema Council. I have received travel expenses and payment for lectures from Sanofi. My institution has received grants for my role as investigator on atopic dermatitis clinical trials assessing crisaborole (Anacor Pharmaceuticals), dupilumab (Regeneron), tralokinumab (MedImmune), and nemolizumab (Galderma)." Amanda Roberts: nothing to declare. Nerys Roberts (clinical referee): "I am the Steering Committee chair of the Atopic Dermatitis Anti‐IgE Paediatric Trial (ADAPT)". Raja Sivamani (clinical referee): "I serve as a Scientific Advisor for Dermveda".
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Brouwer 2006 {published and unpublished data}
- Brouwer ML, Wolt‐Plompen SA, Dubois AE, Heide S, Jansen DF, Hoijer MA, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo‐controlled trial. Clinical and Experimental Allergy 2006;36(7):899‐906. [CENTRAL: CN‐00571157; PUBMED: 16839405] [DOI] [PubMed] [Google Scholar]
Cukrowska 2008 {published data only}
- Cukrowska B, Ceregra A, Klewicka E, Slizewska K, Motyl I, Libudzisz Z. Probiotic lactobacillus casei and lactobacillus paracasei strains in treatment of food allergy in children [Probiotyczne szczepy lactobacillus casei i lactobacillus paracasei w leczeniu alergii pokarmowej u dzieci]. Przegląd Pediatryczny 2010;40(1):21‐5. [CENTRAL: CN‐00803280] [Google Scholar]
- Cukrowska B, Ceregra A, Rosiak I, Klewicka E, Slizewska K, Motyl I, et al. The influence of probiotic Lactobacillus casei and paracasei strains on clinical status of atopic eczema in children with food allergy on cow's milk proteins [Wpływ probiotycznych szczepów Lactobacillus casei i paracasei na przebieg kliniczny wyprysku atopowego u dzieci z alergią pokarmową na białka mleka krowiego]. Pediatria Wspolczesna 2008;10(2):67‐70. [CENTRAL: CN‐00707920] [Google Scholar]
Drago 2012 {published and unpublished data}
- Drago L, Iemoli E, Rodighiero V, Nicola L, Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo‐controlled study. International Journal of Immunopathology and Pharmacology 2011;24(4):1037‐48. [CENTRAL: CN‐00843712; PUBMED: 22230409] [DOI] [PubMed] [Google Scholar]
- Drago L, Toscano M, Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. Journal of Clinical Gastroenterology 2012;46(Suppl):S56‐S63. [CENTRAL: CN‐00967749; PUBMED: 22955359] [DOI] [PubMed] [Google Scholar]
Drago 2014 {published data only}
- Drago L, Vecchi E, Toscano M, Vassena C, Altomare G, Pigatto P. Treatment of atopic dermatitis eczema with a high concentration of Lactobacillus salivarius LS01 associated with an innovative gelling complex: a pilot study on adults. Journal of Clinical Gastroenterology 2014;48(Suppl 1):S47‐51. [CENTRAL: CN‐01113253] [DOI] [PubMed] [Google Scholar]
Farid 2011 {published data only}
- Farid R, Ahanchian H, Jabbari F, Moghiman T. Effect of a new synbiotic mixture on atopic dermatitis in children: a randomized‐controlled trial. Iranian Journal of Pediatrics 2011;21(2):225‐30. [CENTRAL: CN‐00894605] [PMC free article] [PubMed] [Google Scholar]
Flinterman 2007 {published and unpublished data}
- Flinterman AE, Knol EF, Ieperen‐van Dijk AG, Timmerman HM, Knulst AC, Bruijnzeel‐Koomen CA, et al. Probiotics have a different immunomodulatory potential in vitro versus ex vivo upon oral administration in children with food allergy. International Archives of Allergy and Immunology 2007;143(3):237‐44. [PUBMED: 17290150] [DOI] [PubMed] [Google Scholar]
Folster‐Holst 2006 {published data only}
- Folster‐Holst R, Muller F, Schnopp N, Abeck D, Kreiselmaier I, Lenz T, et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. British Journal of Dermatology 2006;155(6):1256‐61. [CENTRAL: CN‐00576925; PUBMED: 17107398] [DOI] [PubMed] [Google Scholar]
Gerasimov 2010 {published data only}
- Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double‐blind, placebo‐controlled, clinical trial. American Journal of Clinical Dermatology 2010;11(5):351‐61. [CENTRAL: CN‐00762545; PUBMED: 20642296] [DOI] [PubMed] [Google Scholar]
Goebel 2010 {published and unpublished data}
- Gobel R, Larsen, N, Molgaard C, Jakobsen M, Michaelsen KF. Probiotics to young children with atopic dermatitis: a randomized placebo‐controlled trial. International Journal of Probiotics and Prebiotics 2010;5(2):53‐60. [CENTRAL: CN‐00803376] [Google Scholar]
- Larsen N, Vogensen FK, Gøbel R, Michaelsen KF, Abu Al‐Soud W, Sørensen SJ, et al. Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp lactis Bi‐07. FEMS Microbiology Ecology 2011;75(3):482‐96. [CENTRAL: CN‐00771294; PUBMED: 21204871] [DOI] [PubMed] [Google Scholar]
Gore 2011 {published and unpublished data}
- Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow‐up until age 3 years. Clinical and Experimental Allergy 2011;42(1):112‐22. [CENTRAL: CN‐00843786; PUBMED: 22092692] [DOI] [PubMed] [Google Scholar]
Gromert 2009 {published data only}
- Gromert N, Axelsson I. Lactobacillus reuteri effect on atopic eczema in childhood. Journal of Pediatric Gastroenterology and Nutrition 2009;43(Suppl 3):E148‐9. [CENTRAL: CN‐00980761] [Google Scholar]
Gruber 2007 {published data only (unpublished sought but not used)}
- Gruber C, Wendt M, Sulser C, Lau S, Kulig M, Wahn U, et al. Randomised, placebo‐controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007;62(11):1270‐6. [CENTRAL: CN‐00619933; PUBMED: 17919141] [DOI] [PubMed] [Google Scholar]
Guo 2015 {published data only}
- Guo Y‐H, Mou Y‐D, Wang H‐S, Guo Y‐J. Clinical effect of microecologics as an adjuvant therapy on infants' eczema. Journal of Dalian Medical University 2015;37(6):571‐88. [DOI: 10.11724/jdmu.2015.06.13] [DOI] [Google Scholar]
Han 2012 {published and unpublished data}
- Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatric Allergy and Immunology 2012;23(7):667‐73. [CENTRAL: CN‐00871398; PUBMED: 23050557] [DOI] [PubMed] [Google Scholar]
Hol 2008 {published data only}
- Hol J, Leer EH, Elink Schuurman BE, Ruiter LF, Samsom JN, Hop W, et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. Journal of Allergy and Clinical Immunology 2008;121(6):1448‐54. [CENTRAL: CN‐00639187; PUBMED: 18436293] [DOI] [PubMed] [Google Scholar]
Iemoli 2012 {published and unpublished data}
- Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. Journal of Clinical Gastroenterology 2012;46(Suppl):S33‐40. [CENTRAL: CN‐00871399; PUBMED: 22955355] [DOI] [PubMed] [Google Scholar]
Isolauri 2000 {published data only}
- Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clinical and Experimental Allergy 2000;30(11):1604‐10. [CENTRAL: CN‐00330163; PUBMED: 11069570] [DOI] [PubMed] [Google Scholar]
Ivankhnenko 2013 {published data only}
- Ivakhnenko O, Niankovskyy S. Clinical effectiveness of probiotics in complex treatment of infants with cow's milk allergy. Georgian Medical News 2013;216:39‐45. [CENTRAL: CN‐01123797; PUBMED: 23567307] [PubMed] [Google Scholar]
Kirjavainen 2003 {published data only}
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. Journal of Pediatric Gastroenterology and Nutrition 2003;36(2):223‐7. [CENTRAL: CN‐00435079; PUBMED: 12548058] [DOI] [PubMed] [Google Scholar]
Lin 2015 {published data only}
- Lin R‐J, Qiu L‐H, Guan R‐Z, Hu S‐J, Liu Y‐Y, Wang G‐J. Protective effect of probiotics in the treatment of infantile eczema. Experimental and Therapeutic Medicine 2015;9(5):1593‐6. [CENTRAL: CN‐01069064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Majamaa 1997 {published data only}
- Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. Journal of Allergy and Clinical Immunology 1997;99(2):179‐85. [CENTRAL: CN‐00136911; PUBMED: 9042042] [DOI] [PubMed] [Google Scholar]
Matsumoto 2014 {published data only}
- Matsumoto M, Ebata T, Hirooka J, Hosoya R, Inoue N, Itami S, et al. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis. Annals of Allergy, Asthma and Immunology 2014;113(2):209‐16. [CENTRAL: CN‐00998958; PUBMED: 24893766] [DOI] [PubMed] [Google Scholar]
Nermes 2010 {published and unpublished data}
- Nermes M, Kantele JM, Atosuo TJ, Salminen S, Isolauri E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clinical and Experimental Allergy 2010;41(3):370‐7. [CENTRAL: CN‐00779496; PUBMED: 21121981] [DOI] [PubMed] [Google Scholar]
Passeron 2006 {published and unpublished data}
- Passeron T, Lacour J‐P, Fontas E, Ortonne JP. Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy 2006;61(4):431‐7. [CENTRAL: CN‐00563037; PUBMED: 16512804] [DOI] [PubMed] [Google Scholar]
Roessler 2007 {published and unpublished data}
- Roessler A, Forssten SD, Glei M, Ouwehand AC, Jahreis G. The effect of probiotics on faecal microbiota and genotoxic activity of faecal water in patients with atopic dermatitis: a randomized, placebo‐controlled study. Clinical Nutrition 2012;31(1):22‐9. [CENTRAL: CN‐00882332; PUBMED: 21963389] [DOI] [PubMed] [Google Scholar]
- Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clinical and Experimental Allergy 2008;38(1):93‐102. [CENTRAL: CN‐00621150; PUBMED: 18028460] [DOI] [PubMed] [Google Scholar]
Rosenfeldt 2003 {published and unpublished data}
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. Journal of Allergy and Clinical Immunology 2003;111(2):389‐95. [CENTRAL: CN‐00413280; PUBMED: 12589361] [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V, Benfeldt E, Valerius NH, Paeregaard A, Michaelsen KF. Effects of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. Journal of Pediatrics 2004;145(5):612‐6. [CENTRAL: CN‐00492535; CRSREF: 3073882; PUBMED: 15520759] [DOI] [PubMed] [Google Scholar]
Shafiei 2011 {published data only}
- Shafiei A, Moin M, Pourpak Z, Gharagozlou M, Aghamohammadi A, Aghamohamadi A, et al. Synbiotics could not reduce the scoring of childhood atopic dermatitis (SCORAD): a randomized double blind placebo‐controlled trial. Iranian Journal of Allergy, Asthma and Immunology 2011;10(1):21‐8. [CENTRAL: CN‐00801473; PUBMED: 21358011] [PubMed] [Google Scholar]
Sistek 2006 {published and unpublished data}
- Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, Crane J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children?. Clinical and Experimental Allergy 2006;36(5):629‐33. [CENTRAL: CN‐00564896; PUBMED: 16650048] [DOI] [PubMed] [Google Scholar]
Taniuchi 2005 {published data only}
- Hattori K, Yamamoto A, Sasai M, Taniuchi S, Kojima T, Kobayashi Y, et al. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi [Allergy] 2003;52(1):20‐30. [CENTRAL: CN‐00558320; PUBMED: 12598719] [PubMed] [Google Scholar]
- Taniuchi S, Hattori K, Yamamoto A, Sasai M, Hatano Y, Kojima T, et al. Administration of Bifidobacterium to infants with atopic dermatitis: changes in fecal microflora and clinical symptoms. Journal of Applied Research 2005;5(2):387‐96. [CENTRAL: CN‐00569597] [Google Scholar]
Van der Aa 2010 {published and unpublished data}
- Aa LB, Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, et al. Synbiotics prevent asthma‐like symptoms in infants with atopic dermatitis. Allergy 2011;66(2):170‐7. [CENTRAL: CN‐00779370; PUBMED: 20560907] [DOI] [PubMed] [Google Scholar]
- Aa LB, Heymans HS, Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, et al. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized‐controlled trial. Clinical and Experimental Allergy 2010;40(5):795‐804. [CENTRAL: CN‐00761390; PUBMED: 20184604] [DOI] [PubMed] [Google Scholar]
- Aa LB, Lutter R, Heymans HS, Smids BS, Dekker T, Aalderen WM, et al. No detectable beneficial systemic immunomodulatory effects of a specific synbiotic mixture in infants with atopic dermatitis. Clinical and Experimental Allergy 2012;42(4):531‐9. [CENTRAL: CN‐00839910; PUBMED: 22092915] [DOI] [PubMed] [Google Scholar]
Viljanen 2005 {published and unpublished data}
- Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, et al. Lactobacillus GG effect in increasing IFN‐gamma production in infants with cow's milk allergy. Journal of Allergy and Clinical Immunology 2004;114(1):131‐6. [CENTRAL: CN‐00480315; PUBMED: 15241356] [DOI] [PubMed] [Google Scholar]
- Viljanen M, Kuitunen M, Haahtela T, Juntunen‐Backman K, Korpela R, Savilahti E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatric Allergy and Immunology 2005;16(1):65‐71. [CENTRAL: CN‐00520986; PUBMED: 15693914] [DOI] [PubMed] [Google Scholar]
- Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, Sarnesto A, et al. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema‐dermatitis syndrome. Journal of Allergy & Clinical Immunology 2005;115(6):1254‐9. [CENTRAL: CN‐00515574; PUBMED: 15940143] [DOI] [PubMed] [Google Scholar]
- Viljanen M, Savilahti E, Haahtela T, Juntunen‐Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double‐blind placebo‐controlled trial. Allergy 2005;60(4):494‐500. [CENTRAL: CN‐00513132; PUBMED: 15727582] [DOI] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang IJ, Wang JY. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clinical & Experimental Allergy 2015;45(4):779‐87. [CENTRAL: CN‐01069031; PUBMED: 25600169] [DOI] [PubMed] [Google Scholar]
Weston 2005 {published and unpublished data}
- Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, et al. Clinical effects of probiotics are associated with increased interferon‐gamma responses in very young children with atopic dermatitis. Clinical and Experimental Allergy 2005;35(12):1557‐64. [PUBMED: 16393321] [DOI] [PubMed] [Google Scholar]
- Weston S, Dunstan J, Roper J, Breckler L, Halbert A, Richmond P, et al. Probiotics provide clinical benefit in moderate and severe atopic dermatitis: a randomised controlled trial. Journal of Investigative Dermatology 2005;125:596. [Google Scholar]
- Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Archives of Disease in Childhood 2005;90(9):892‐7. [CENTRAL: CN‐00521688; PUBMED: 15863468] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston S, Richmond P, Halbert A, Prescott SL. Effects of probiotics in infants with atopic dermatitis: a randomised double blind placebo controlled trial. Australasian Journal of Dermatology 2004;45:A13. [CENTRAL: CN‐00594250] [Google Scholar]
Woo 2010 {published data only}
- Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema‐dermatitis syndrome. Annals of Allergy, Asthma and Immunology 2010;104(4):343‐8. [CENTRAL: CN‐00742516; PUBMED: 20408346] [DOI] [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu KG, Li TH, Peng HJ. Lactobacillus salivarius plus fructo‐oligosaccharide is superior to fructo‐oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double‐blind, randomized, clinical trial of efficacy and safety. British Journal of Dermatology 2012;166(1):129‐36. [CENTRAL: CN‐00841293; PUBMED: 21895621] [DOI] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu Y‐J, Wu W‐F, Hung C‐W, Ku M‐S, Liao P‐F, Sun H‐L, et al. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4‐48 months with atopic dermatitis: an 8‐week, double‐blind, randomized, placebo‐controlled study. Journal of Microbiology, Immunology and Infection 2017;50(5):684‐92. [DOI: 10.1016/j.jmii.2015.10.003; PUBMED: 26733351] [DOI] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang H‐J, Min TK, Lee HW, Pyun BY. Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double‐blind, placebo‐controlled trial.. Allergy, Asthma & Immunology Research 2014;6(3):208‐15. [CENTRAL: CN‐00988082; EMBASE: 2014300281] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yesilova 2012 {published data only}
- Yesilova Y, Calka O, Akdeniz N, Berktas M. Effect of probiotics on the treatment of children with atopic dermatitis. Annals of Dermatology 2012;24(2):189‐93. [CENTRAL: CN‐00861289; PUBMED: 22577270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshida 2010 {published data only}
- Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, Yamada N, et al. Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Medica 2010;53(2):37‐45. [CENTRAL: CN‐00865095] [Google Scholar]
References to studies excluded from this review
Arkwright 2003 {published data only}
- Arkwright PD, David TJ. Effect of Mycobacterium vaccae on atopic dermatitis in children of different ages. British Journal of Dermatology 2003;149(5):1029‐34. [CENTRAL: CN‐00472817; PUBMED: 14632810] [DOI] [PubMed] [Google Scholar]
Arvola 2006 {published data only}
- Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 2006;117(4):e760‐8. [CENTRAL: CN‐00556020; PUBMED: 16585287] [DOI] [PubMed] [Google Scholar]
Aryayev 2006 {published and unpublished data}
- Aryayev ML, Kukushkin NV. Combined therapy with pimecrolimus cream 1% and probiotic in children with atopic dermatitis. European Journal of Pediatrics 2006;165(Suppl 1):53. [CENTRAL: CN‐00764255] [Google Scholar]
Burk 2013 {published and unpublished data}
- Burks AW, Harthoorn LF, Langford JE, Ampting MT, Goldberg SB, Ong PY, et al. Functional effects of an amino‐acid based formula with synbiotics in cow's milk allergic infants. Allergy 2013;68(Suppl 97):703. [CENTRAL: CN‐00985742; EMBASE: 71369861] [Google Scholar]
Chernysov 2009 {published data only}
- Chernyshov PV. Randomized, placebo‐controlled trial on clinical and immunologic effects of probiotic and emollient in children with atopic eczema and cow milk allergy. Allergo Journal 2010;19(5):336‐7. [EMBASE: 70284293] [Google Scholar]
- Chernysov PV. Randomized, placebo‐controlled trial on clinical and immunologic effects of probiotic containing Lactobacillus rhamnosus R0011 and L. helveticus R0052 in infants with atopic dermatitis. Microbial Ecology in Health and Disease 2009;21(3‐4):228‐32. [EMBASE: 2010028537] [Google Scholar]
Foekel 2009 {published data only}
- Foekel C, Schubert R, Kaatz M, Schmidt I, Bauer A, Hipler UC, et al. Dietetic effects of oral intervention with mare's milk on the Severity Scoring of Atopic Dermatitis, on faecal microbiota and on immunological parameters in patients with atopic dermatitis. International Journal of Food Sciences and Nutrition 2009;60(Suppl 7):41‐52. [CENTRAL: CN‐00751360; PUBMED: 19462320] [DOI] [PubMed] [Google Scholar]
Gueniche 2008 {published data only}
- Gueniche A, Breton L. Vitreoscilla filiformis lysate, a probiotic profile for topical skin care. Journal of Investigative Dermatology 2009;129:S15. [EMBASE: 70384507] [Google Scholar]
- Gueniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, et al. Effects of nonpathogenic gram‐negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double‐blind, placebo‐controlled clinical study. British Journal of Dermatology 2008;159(6):1357‐63. [CENTRAL: CN‐00665626; PUBMED: 18795916] [DOI] [PubMed] [Google Scholar]
- Gueniche AG, Breton L. Vitreoscilla filiformis lysate, a probiotic profile for topical skin care. European Journal of Immunology 2009;39:S658. [EMBASE: 70347441] [Google Scholar]
Ikezawa 2004 {published data only}
- Ikezawa Z, Kondo M, Okajima M, Nishimura Y, Kono M. Clinical usefulness of oral itraconazole, an antimycotic drug, for refractory atopic dermatitis. European Journal of Dermatology 2004;14(6):400‐6. [CENTRAL: CN‐00504055; PUBMED: 15564204] [PubMed] [Google Scholar]
Kalliomaki 2003 {published data only}
- Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4‐year follow‐up of a randomised placebo controlled trial. Lancet 2003;361(9372):1869‐71. [CENTRAL: CN‐00558789; PUBMED: 12788576] [DOI] [PubMed] [Google Scholar]
Laitinen 2005 {published data only}
- Laitinen K, Kalliomaki M, Poussa T, Lagstrom H, Isolauri E. Evaluation of diet and growth in children with and without atopic eczema: follow‐up study from birth to 4 years. British Journal of Nutrition 2005;94(4):565‐74. [CENTRAL: CN‐00838395] [DOI] [PubMed] [Google Scholar]
Leung 2004 {published data only}
- Leung TF, Ma KC, Cheung LT, Lam CW, Wong E, Wan H, et al. A randomized, single‐blind and crossover study of an amino acid‐based milk formula in treating young children with atopic dermatitis. Pediatric Allergy and Immunology 2004;15(6):558‐61. [CENTRAL: CN‐00511281; PUBMED: 15610371] [DOI] [PubMed] [Google Scholar]
Matsumoto 2007 {published data only}
- Matsumoto M, Aranami A, Ishige A, Watanabe K, Benno Y. LKM512 yogurt consumption improves the intestinal environment and induces the T‐helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clinical and Experimental Allergy 2007;37(3):358‐70. [CENTRAL: CN‐00641021; EMBASE: 2007128104] [DOI] [PubMed] [Google Scholar]
Moroi 2011 {published data only}
- Moroi M, Uchi S, Nakamura K, Sato S, Shimizu N, Fujii M, et al. Beneficial effect of a diet containing heat‐killed Lactobacillus paracasei K71 on adult type atopic dermatitis. Journal of Dermatology 2011;38(2):131‐9. [CENTRAL: CN‐00778680; PUBMED: 21269308] [DOI] [PubMed] [Google Scholar]
Murosaki 2006 {published data only}
- Murosaki S, Yamamoto Y, Yotsumoto H, Kubo H, Nomoto S, Usuki K, et al. Effects of intake of syrup supplemented with nigerooligosaccharides and heat‐killed Lactobacillus plantarum L‐137 on skin symptom and immune function in patients with atopic dermatitis. Japanese Pharmacology and Therapeutics 2006;34(10):1087‐96. [CENTRAL: CN‐00707197] [Google Scholar]
Ogawa 2006 {published data only}
- Ogawa T, Hashikawa S, Asai Y, Sakamoto H, Yasuda K, Makimura Y. A new synbiotic, Lactobacillus casei subsp. casei together with dextran, reduces murine and human allergic reaction. FEMS Immunology & Medical Microbiology 2006;46(3):400‐9. [CENTRAL: CN‐00563540; PUBMED: 16553814] [DOI] [PubMed] [Google Scholar]
Ou 2012 {published and unpublished data}
- Keet CA, Wood RA. Randomized, placebo‐controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Pediatrics 2008;122(Suppl 4):S198‐9. [EMBASE: 2009166612] [Google Scholar]
- Ou C‐Y, Kuo H‐C, Wang L, Hsu T‐Y, Chuang H, Liu C‐A, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double‐blind, placebo‐controlled trial. Clinical and Experimental Allergy 2012;42(9):1386‐96. [CENTRAL: CN‐00850372; EMBASE: 2012510284; PUBMED: 22925325] [DOI] [PubMed] [Google Scholar]
Rose 2010 {published and unpublished data}
- Rose MA, Schubert R, Schulze J, Zielen S. Follow‐up of probiotic Lactobacillus GG effects on allergic sensitization and asthma in infants at risk. Clinical and Experimental Allergy 2011;41(12):1819‐21. [CENTRAL: CN‐00834541] [DOI] [PubMed] [Google Scholar]
- Rose MA, Stieglitz F, Köksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clinical and Experimental Allergy 2010;40(9):1398‐405. [CENTRAL: CN‐00767900; PUBMED: 20604800] [DOI] [PubMed] [Google Scholar]
Shibata 2009 {published data only}
- Shibata R, Kimura M, Takahashi H, Mikami K, Aiba Y, Takeda H, et al. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clinical and Experimental Allergy 2009;39(9):1397‐403. [CENTRAL: CN‐00719173; PUBMED: 19508323] [DOI] [PubMed] [Google Scholar]
- Shibata R, Koga Y, Takeda H, Nishima S. Clinical effects of prebiotics with kestose, a fructo‐oligosaccharide, on atopic dermatitis in infants. Allergy 2009;64:447. [CENTRAL: CN‐01029517] [DOI] [PubMed] [Google Scholar]
Torii 2011 {published data only}
- Torii S, Torii A, Itoh K, Urisu A, Terada A, Fujisawa T, et al. Effects of oral administration of Lactobacillus acidophilus L‐92 on the symptoms and serum markers of atopic dermatitis in children. International Archives of Allergy and Immunology 2011;154(3):236‐45. [CENTRAL: CN‐00779204; PUBMED: 20861645] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12605000615684 {published data only}
- ACTRN12605000615684. Probiotics in the management of eczema [A double‐blind, randomised, placebo controlled study to identify markers for the differential response of children with eczema following treatment with probiotics]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=782 (first received 23 September 2005).
Candy 2016 {published data only}
- Candy DCA, Ampting MTJ, Oude Nijhuis MM, Wopereis H, Butt AM, Peroni DG, et al. Dietary management of non‐IgE mediated cow's milk allergic infants with a synbiotics‐supplemented amino acid‐based formula: effects on faecal microbiota and clinical symptoms. Journal of Pediatric Gastroenterology, Hepatology and Nutrition. Lippincott Williams and Wilkins, 2016:S402.
Hulshof 2017 {published data only}
- Hulshof L, Overbeek SA, Wyllie Al, Chu Mj, Bogaert D, Jager W, et al. Dietary intervention with a synbiotic mixture of scGOS/lcFOS with Bifidobacterium breve M‐16V in infants with atopic dermatitis shows beneficial immunological changes in chemokine profiles. Allergy: European Journal of Allergy and Clinical Immunology 2017;72:309‐10. [CENTRAL: CN‐01417584] [Google Scholar]
NCT02585986 {published data only}
- NCT02585986. Intervention study to evaluate a probiotic in mild atopic dermatitis young patients [Randomized, double blind, placebo‐controlled Intervention study to evaluate safety and efficiency of a probiotic in symptoms reduction and use of topic corticoids in mild atopic dermatitis patients aged 4 to 17 years]. clinicaltrials.gov/ct2/show/NCT02585986 (first received 13 October 2015).
Prakoeswa 2017 {published data only}
- Prakoeswa CRS, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, et al. Lactobacillus plantarum IS‐10506 supplementation reduced SCORAD in children with atopic dermatitis. Beneficial Microbes 2017;8(5):833‐40. [CENTRAL: CN‐01420752] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800015330 {published data only}
- ChiCTR1800015330. Effects of probiotics on inflammation and intestinal microflora in patients with atopic dermatitis. www.chictr.org.cn/showprojen.aspx?proj=24603 (first received 23 March 2018).
CTRI/2017/08/009236 {published data only}
- CTRI/2017/08/009236. A study to observe if probiotics supplementation is helpful in atopic dermatitis children. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/08/009236 (first received 2 August 2018).
CTRI/2017/10/010018 {published data only}
- CTRI/2017/10/010018. Probiotics in treatment of atopic dermatitis in children. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/010018 (first received 6 October 2017).
KCT0000914 {published data only}
- KCT0000914. A 8 week, randomized, double‐blind, placebo‐controlled clinical trial for the evaluation of the efficacy and safety of IRT5 probiotics on atopic dermatitis in children [IRT5 probiotics atopic dermatitis]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=3350 (accessed before 7 December 2016).
NCT02519556 {published data only}
- NCT02519556. Trial on effectiveness combined probiotics in atopic dermatitis in children [Randomized, double blind, controlled trial on effectiveness combined probiotics in the treatment of atopic dermatitis in children up to 6 months]. clinicaltrials.gov/ct2/show/NCT02519556 (first received 4 August 2015).
NCT02945683 {published data only}
- NCT02945683. Effects of Lactobacillus reuteri plus vitamin D3 in children with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT02945683 (first received 26 October 2016).
Additional references
Abrahamsson 2012
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. Journal of Allergy and Clinical Immunology 2012;129(2):434‐40. [PUBMED: 22153774] [DOI] [PubMed] [Google Scholar]
Abuabara 2018
- Abuabara K, Yu AM, Okhovat JP, Allen E, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta‐analysis of longitudinal studies. Allergy 2018;73(3):696‐704. [DOI: 10.1111/all.13320; PUBMED: 28960336] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2003
- Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003048.pub2] [DOI] [PubMed] [Google Scholar]
Allingstrup 2016
- Allingstrup M, Wetterslev J, Ravn FB, Moeller AM, Afshari A. Antithrombin III for critically ill patients. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD005370.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Priss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490‐4. [PUBMED: 15205295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Basra 2007
- Basra MK, Sue‐Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. British Journal of Dermatology 2007;156(4):791. [PUBMED: 17300244] [DOI] [PubMed] [Google Scholar]
Beattie 2006
- Beattie PE, Lewis‐Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. British Journal of Dermatology 2006;155(1):145‐51. [DOI: 10.1111/j.1365-2133.2006.07185.x; PUBMED: 16792766] [DOI] [PubMed] [Google Scholar]
Beck 2000
- Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. Journal of Allergy & Clinical Immunology 2000;106(5 Suppl):S258‐63. [PUBMED: 11080741] [DOI] [PubMed] [Google Scholar]
Besselink 2008
- Besselink MG, Santvoort HC, Buskens E, Boermeester MA, Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised double‐blind placebo‐controlled trial. Lancet 2008;371(9613):651‐9. [PUBMED: 18279948 ] [DOI] [PubMed] [Google Scholar]
Bieber 2008
- Bieber T. Atopic dermatitis. New England Journal of Medicine 2008;358(14):1483‐94. [PUBMED: 18385500] [DOI] [PubMed] [Google Scholar]
Bjorksten 2001
- Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. Journal of Allergy and Clinical Immunology 2001;108(4):516‐20. [PUBMED: 11590374] [DOI] [PubMed] [Google Scholar]
Bohme 2001
- Bohme M, Svensson A, Kull I, Nordvall SL, Wahlgren CF. Clinical features of atopic dermatitis at two years of age: a prospective, population‐based case‐control study. Acta Dermato‐Venereologica 2001;81(3):193‐7. [PUBMED: 11558876] [DOI] [PubMed] [Google Scholar]
Borriello 2003
- Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clinical Infectious Diseases 2003;36(6):775‐80. [PUBMED: 12627362] [DOI] [PubMed] [Google Scholar]
Boyle 2006a
- Boyle RJ, Robins‐Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks?. American Journal of Clinical Nutrition 2006;83(6):1256‐64. [PUBMED: 16762934] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Burton 2006
- Burton JP, Wescombe PA, Moore CJ, Chilcott CN, Tagg JR. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Applied & Environmental Microbiology 2006;72(4):3050‐3. [PUBMED: 16598017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caffarelli 1998
- Caffarelli C, Cavagni G, Deriu FM, Zanotti P, Atherton DJ. Gastrointestinal symptoms in atopic eczema. Archives of Disease in Childhood 1998;78(3):230‐4. [PUBMED: 9613352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2016a
- Chang YS, Trivedi MK, Jha A, Lin Y‐F, Dimaano L, Garcia‐Romero MT. Synbiotics for prevention and treatment of atopic dermatitis. JAMA Pediatrics 2016;170(3):236‐42. [DOI: 10.1001/jamapediatrics.2015.3943; PUBMED: 26810481] [DOI] [PubMed] [Google Scholar]
Charman 2002
- Charman C, Williams HC. Epidemiology. In: Thomas Bieber, Donald YM Leung editor(s). Atopic Dermatitis. 1st Edition. New York: Marcel Dekker Inc, 2002:21‐42. [Google Scholar]
Cherifi 2004
- Cherifi S, Robberecht J, Miendje Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta Clinica Belgica 2004;59(4):223‐4. [PUBMED: 15597730] [DOI] [PubMed] [Google Scholar]
Chren 1997
- Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of SKINDEX, a quality‐of‐life instrument for patients with skin diseases. Archives of Dermatology 1997;133(11):1433‐40. [PUBMED: 9371029] [PubMed] [Google Scholar]
Christensen 2002
- Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. Journal of Immunology 2002;168(1):171‐8. [PUBMED: 11751960] [DOI] [PubMed] [Google Scholar]
Connolly 2005
- Connolly E, Abrahamsson T, Bjorksten B. Safety of D(‐)‐lactic acid producing bacteria in the human infant. Journal of Pediatric Gastroenterology & Nutrition 2005;41(4):489‐92. [PUBMED: 16205524] [DOI] [PubMed] [Google Scholar]
Dang 2013
- Dang D, Zhou W, Lun ZJ, Mu X, Wang DX, Wu H. Meta‐analysis of probiotics and/or prebiotics for the prevention of eczema. Journal of International Medical Research 2013;41(5):1426‐36. [PUBMED: 23908398] [DOI] [PubMed] [Google Scholar]
De Groote 2005
- Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatric Infectious Disease Journal 2005;24(3):278‐80. [PUBMED: 15750472] [DOI] [PubMed] [Google Scholar]
Didari 2014
- Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opinion on Drug Safety 2014;13(2):227‐39. [PUBMED: 24405164] [DOI] [PubMed] [Google Scholar]
Diepgen 1996
- Diepgen TL, Sauerbrei W, Fartasch M. Development and validation of diagnostic scores of atopic dermatitis incorporating criteria of data quality and practical usefulness. Journal of Clinical Epidemiology 1996;49:1031‐8. [DOI] [PubMed] [Google Scholar]
Doege 2012
- Doege K, Grajecki D, Zyrax BC, Dentinika E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood ‐ a meta‐analysis. British Journal of Nutrition 2012;107(1):1‐6. [PUBMED: 21787448] [DOI] [PubMed] [Google Scholar]
Duffy 1989
- Duffy SW, Rohan TE, Altman DG. A method for combining matched and unmatched binary data. Application to randomized, controlled trials of photocoagulation in the treatment of diabetic retinopathy. American Journal of Epidemiology 1989;130(2):371‐8. [PUBMED: 2750732] [DOI] [PubMed] [Google Scholar]
Dugoua 2009
- Dugoua JJ, Machado MM, Zhu X, Chen X, Koren G, Einarson T. Probiotic safety in pregnancy: a systematic review and meta‐analysis of randomized controlled trials of Lactobacillus, Bifidobacterium and Saccharomyces spp. Journal of Obstetrics and Gynaecology Canada 2009;31(6):542‐52. [PUBMED: 19646321] [DOI] [PubMed] [Google Scholar]
Eichenfield 2004
- Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy 2004;59(Suppl 78):86‐92. [PUBMED: 15245365] [DOI] [PubMed] [Google Scholar]
Eichenfield 2017
- Eichenfield LF, Stein Gold LF. Addressing the immunopathogenesis of atopic dermatitis: advances in topical and systemic treatment. Seminars in Cutaneous Medicine and Surgery 2017;36(2 Suppl 2):S45‐8. [PUBMED: 28654711] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vaillancourt JM. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Ellis 2002
- Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. Journal of the American Academy of Dermatology 2002;46(3):361‐70. [PUBMED: 11862170] [DOI] [PubMed] [Google Scholar]
Enomoto 2008
- Enomoto H, Hirata K, Otsuka K, Kawai T, Takahashi T, Hirota T, et al. Filaggrin null mutations are associated with atopic dermatitis and elevated levels of IgE in the Japanese population: a family and case‐control study. Journal of Human Genetics 2008;53(7):615‐21. [PUBMED: 18521703] [DOI] [PubMed] [Google Scholar]
Ernst 2000
- Ernst E, Pittler MH, Stevinson C. Complementary/alternative medicine in dermatology: evidence‐assessed efficacy of two diseases and two treatments. American Journal of Clinical Dermatology 2000;3(5):341‐8. [PUBMED: 12069640] [DOI] [PubMed] [Google Scholar]
FAO/WHO 2002
- Joint FAO/WHO Working Group. Guidelines for the evaluation of probiotics in food 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf?ua=1. London, Ontario, Canada, (accessed before 8 December 2016).
Fennessy 2000
- Fennessy M, Coupland S, Popay J, Naysmith K. The epidemiology and experience of atopic eczema during childhood: a discussion paper on the implications of current knowledge for health care, public health policy and research. Journal of Epidemiology & Community Health 2000;54(8):581‐9. [PUBMED: 10890869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 1996
- Finlay AY. Measurement of disease activity and outcome in atopic dermatitis. British Journal of Dermatology 1996;135(4):509‐15. [PUBMED: 8915137] [PubMed] [Google Scholar]
Fiocchi 2012
- Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle RJ, Cocco R, et al. Clinical use of probiotics in pediatric allergy (CUPPA): a World Allergy Organization position paper. World Allergy Organisation Journal 2012;5(11):148‐67. [PUBMED: 23282383] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flohr 2004
- Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis?. Journal of Allergy and Clinical Immunology 2004;114(1):150‐8. [PUBMED: 15241359] [DOI] [PubMed] [Google Scholar]
Guarino 2015
- Guarino A, Guandalini S, Lo Vecchio A. Probiotics for the prevention and treatment for diarrhea. Journal of Clinical Gastroenterology 2015;49(Suppl 1):S37‐45. [PUBMED: 26447963] [DOI] [PubMed] [Google Scholar]
Hempel 2011
- Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JNV, Suttorp MJ, et al. Safety of probiotics to reduce risk and prevent or treat disease. Evidence Report/ Technology Assessment Number 200. AHRQ Publication No. 11‐E007. April 2011. www.ahrq.gov/sites/default/files/wysiwyg/research/findings/evidence‐based‐reports/probiotic‐evidence‐report.pdf (accessed before 8 December 2016).
Hennequin 2000
- Hennequin C, Kauffmann‐Lacroix C, Jobert A, Viard JP, Ricour C, Jacquemin JL, et al. Possible role of catheters in Saccharomyces boulardii fungemia. European Journal of Clinical Microbiology and Infectious Diseases 2000;19(1):16‐20. [PUBMED: 10706174] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Hoare 2000
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technology Assessment 2000;4(37):1‐191. [PUBMED: 11134919] [PMC free article] [PubMed] [Google Scholar]
Holzapfel 2001
- Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. American Journal of Clinical Nutrition 2001;73(2 Suppl):365S‐73S. [PUBMED: 11157343] [DOI] [PubMed] [Google Scholar]
Huang 2017
- Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta‐analysis of randomized controlled trials. Frontiers in Cellular and Infection Microbiology 2017;7:392. [DOI: 10.3389/fcimb.2017.00392; PUBMED: 28932705] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishibashi 2001
- Ishibashi N, Yamazaki S. Probiotics and safety. American Journal of Clinical Nutrition 2001;73(2 Suppl):S465‐70. [PUBMED: 11157359] [DOI] [PubMed] [Google Scholar]
Ismail 2012
- Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Licciardi PV, Robins‐Browne RM, et al. Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high risk infants. Paediatric Allergy and Immunology 2012;23(7):674‐81. [PUBMED: 22831283] [DOI] [PubMed] [Google Scholar]
Johansson 2001
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel‐Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56(9):813‐24. [PUBMED: 11551246] [DOI] [PubMed] [Google Scholar]
Johansson 2004
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832‐6. [PUBMED: 15131563] [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Assesing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [PUBMED: 11440947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalliomaki 2001
- Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. Journal of Allergy and Clinical Immunology 2001;107(1):129‐34. [PUBMED: 11150002] [DOI] [PubMed] [Google Scholar]
Kemp 1999
- Kemp AS. Atopic eczema: its social and financial costs. Journal of Paediatrics and Child Health 1999;35(3):229‐31. [PUBMED: 10404440] [DOI] [PubMed] [Google Scholar]
Kim 2014
- Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta‐analysis of randomized controlled trials. Annals of Allergy, Asthma, & Immunology 2014;113(2):217‐26. [PUBMED: 24954372] [DOI] [PubMed] [Google Scholar]
Kukkonen 2007
- Kukkonen K, Savilahti E, Haahtela T, Juntunen‐Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto‐oligosaccharides in the prevention of allergic diseases: a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2007;119(1):192‐8. [PUBMED: 17208601] [DOI] [PubMed] [Google Scholar]
Kunz 1997
- Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195(1):10‐9. [PUBMED: 9267730] [DOI] [PubMed] [Google Scholar]
Lamb 2002
- Lamb SR, Rademaker M. Pharmacoeconomics of drug therapy for atopic dermatitis. Expert Opinion on Pharmacotherapy 2002;3(3):249‐55. [PUBMED: 11866675] [DOI] [PubMed] [Google Scholar]
Land 2005
- Land MH, Rouster‐Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005;115(1):178‐81. [PUBMED: 15629999] [DOI] [PubMed] [Google Scholar]
Lawson 1998
- Lawson V, Lewis‐Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. British Journal of Dermatology 1998;138(1):107‐13. [PUBMED: 9536231] [DOI] [PubMed] [Google Scholar]
Lestin 2003
- Lestin F, Pertschy A, Rimek D. Fungemia after oral treatment with Saccharomyces boulardii in a patient with multiple comorbidities [Fungamie nach oraler Gabe]. Deutsche Medizinische Wochenschrift 2003;128(48):2531‐3. [PUBMED: 14648435] [DOI] [PubMed] [Google Scholar]
Lewis‐Jones 2006
- Lewis‐Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. International Journal of Clinical Practice 2006;60(8):984‐92. [DOI: 10.1111/j.1742-1241.2006.01047.x] [DOI] [PubMed] [Google Scholar]
Makelainen 2003
- Makelainen H, Tahvonen R, Salminen S, Ouwehand AC. In vivo safety assessment of two Bifidobacterium longum strains. Microbiology & Immunology 2003;47(12):911‐4. [PUBMED: 14695440] [DOI] [PubMed] [Google Scholar]
Malik 2017
- Malik K, Heitmiller KD, Czarnowski T. An update on the pathophysiology of atopic dermatitis. Dermatologic Clinics 2017;35(3):317‐26. [PUBMED: 28577801] [DOI] [PubMed] [Google Scholar]
Mancini 2008
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatric Dermatology 2008;25(1):1‐6. [PUBMED: 18304144] [DOI] [PubMed] [Google Scholar]
Mansfield 2014
- Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta‐analysis. Military Medicine 2014;179(6):580‐92. [PUBMED: 24902123] [DOI] [PubMed] [Google Scholar]
Moro 2006
- Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Archives of Disease in Childhood 2006;91(10):814‐9. [PUBMED: 16873437] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2005
- Murray CS, Tannock GW, Simon MA, Harmsen HJ, Welling GW, Custovic A, et al. Fecal microbiota in sensitized wheezy and non‐sensitized non‐wheezy children: a nested case‐control study. Clinical and Experimental Allergy 2005;35(6):741‐5. [PUBMED: 15969664] [DOI] [PubMed] [Google Scholar]
Nankervis 2016
- Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping Systematic Review of Treatments for Eczema. 7th Edition. Vol. 4, Programme Grants for Applied Research, NIHR Journals Library, 2016. [PUBMED: 27280278] [PubMed] [Google Scholar]
Nemoto‐Hasebe 2009
- Nemoto‐Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. FLG mutation p.Lys4021X in the C‐terminal imperfect filaggrin repeat in Japanese patients with atopic eczema. British Journal of Dermatology 2009;161(6):1387‐90. [PUBMED: 19663875] [DOI] [PubMed] [Google Scholar]
Odhiambo 2009
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher IM, ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. Journal of Allergy and Clinical Immunology 2009;124(6):1251‐8. [PUBMED: 20004783] [DOI] [PubMed] [Google Scholar]
Osborn 2007
- Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006475.pub2] [DOI] [PubMed] [Google Scholar]
Palmer 2006
- Palmer CN, Irvine AD, Terron‐Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss‐of‐function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics 2006;38(4):441‐6. [PUBMED: 16550169] [DOI] [PubMed] [Google Scholar]
Pineiro 2007
- Pineiro M, Stanton C. Probiotic bacteria: legislative framework ‐ requirements to evidence basis. Journal of Nutrition 2007;137(3 Suppl 2):850S‐3S. [PUBMED: 17311986] [DOI] [PubMed] [Google Scholar]
Riquelme 2003
- Riquelme AJ, Calvo MA, Guzman AM, Depix MS, Garcia P, Perez C, et al. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. Journal of Clinical Gastroenterology 2003;36(1):41‐3. [PUBMED: 12488707] [DOI] [PubMed] [Google Scholar]
Rook 2016
- Arden‐Jones MR, Flohr C, Reynolds NJ, Holden CA. Atopic eczema. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D editor(s). Rook's Textbook of Dermatology. 9th Edition. Vol. 2, New York, NY: John Wiley & Sons, 2016. [Google Scholar]
Rosenfeldt 2004
- Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. Journal of Pediatrics 2004;145(5):612‐6. [PUBMED: 15520759] [DOI] [PubMed] [Google Scholar]
Ruden 1999
- Ruden U, Staab D, Kehrt R, Wahn U. Development and validation of a disease‐specific questionnaire to measure quality of life in parents of children with atopic dermatitis [Entwicklung und Validierung eines krankheitsspezifischen Fragebogens zur Erfassung der Lebensqualitat von Eltern neurodermitiskranker Kinder]. Zeitschrift fur Gesundheitswissenschaften 1999;7:335‐50. [DOI: 10.1007/BF02966499] [DOI] [Google Scholar]
Salminen 1998
- Salminen S, Wright A, Morelli L, Marteau P, Brassart D, Vos WM, et al. Demonstration of safety of probiotics ‐ a review. International Journal of Food Microbiology 1998;44(1‐2):93‐106. [PUBMED: 9849787] [DOI] [PubMed] [Google Scholar]
Schmitt 2013
- Schmitt J, Langan S, Deckert S, Svensson A, Kobyletzki L, Thomas K, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. Journal of Allergy and Clinical Immunology 2013;132(6):1337‐47. [PUBMED: 24035157] [DOI] [PubMed] [Google Scholar]
Schram 2011
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67(1):99‐106. [PUBMED: 21951293] [DOI] [PubMed] [Google Scholar]
Silverberg 2013
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. Journal of Allergy and Clinical Immunology 2013;132(5):1132‐8. [PUBMED: 24094544] [DOI] [PubMed] [Google Scholar]
Silverberg 2017
- Silverberg J. Selected co‐morbidities of atopic dermatitis: atopy, neuropsychiatric and musculoskeletal disorders. Clinics in Dermatology 2017;35(4):360‐6. [PUBMED: 28709566] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simonyte Sjödin 2016
- Simonyte Sjödin K, Vidman L, Rydén P, West CE. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Current Opinion in Allergy and Clinical Immunology 2016;16(4):390‐5. [PUBMED: 27253486] [DOI] [PubMed] [Google Scholar]
Smethurst 2002
- Smethurst D. Atopic eczema. Clinical Evidence. 8th Edition. London: BMJ, 2002:1664‐82. [PubMed] [Google Scholar]
Song 2016
- Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies‐level dysbiosis in the human gut microbiome underlying atopic dermatitis. Journal of Allergy and Clinical Immunology 2016;137(3):852‐60. [PUBMED: 26431583] [DOI] [PubMed] [Google Scholar]
Srinivasan 2006
- Srinivasan R, Meyer R, Padmanabhan R, Britto J. Clinical safety of Lactobacillus casei shirota as a probiotic in critically ill children. Journal of Pediatric Gastroenterology & Nutrition 2006;42(2):171‐3. [PUBMED: 16456410] [DOI] [PubMed] [Google Scholar]
TSA software [Computer program]
- Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet. Trial Sequential Analysis (TSA). Version 0.9.5.10 Beta. Copenhagen, Denmark: Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, 2017.
Vitaliti 2014
- Vitaliti G, Pavone P, Guglielmo F, Spataro G, Falsaperla R. The immunomodulatory effect of probiotics beyond atopy: an update. Journal of Asthma 2014;51(3):320‐32. [PUBMED: 24256057] [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. Journal of Allergy and Clinical Immunology 2008;121(1):129‐34. [PUBMED: 18028995] [DOI] [PubMed] [Google Scholar]
Watanabe 2003
- Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. Journal of Allergy and Clinical Immunology 2003;111(3):587‐91. [PUBMED: 12642841] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1999
- Williams H, Robertson C, Stewart A, Ait‐Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. Journal of Allergy and Clinical Immunology 1999;103(1 Pt 1):125‐38. [PUBMED: 9893196] [DOI] [PubMed] [Google Scholar]
Williams 2005
- Williams HC. Clinical practice. Atopic dermatitis. New England Journal of Medicine 2005;352(22):2314‐24. [PUBMED: 15930422] [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams H, Stewart A, Mutius E, Cookson W, Anderson RH, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide. Journal of Allergy and Clinical Immunology 2008;121(4):947‐54. [PUBMED: 18155278] [DOI] [PubMed] [Google Scholar]
Wolf 1998
- Wolf BW, Wheeler KB, Ataya DG, Garleb KA. Safety and tolerance of Lactobacillus reuteri supplementation to a population infected with the human immunodeficiency virus. Food & Chemical Toxicology 1998;36(12):1085‐94. [PUBMED: 9862651] [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang H, Guo Y, Wang W, Shi M, Chen X, Yao Z. Mutations in the filaggrin gene in Han Chinese patients with atopic dermatitis. Allergy 2011;66(3):420‐7. [PUBMED: 21039602] [DOI] [PubMed] [Google Scholar]
Zhu 2010
- Zhu DL, Yang WX, Yang HM. Meta analysis of lactic acid bacteria as probiotics for the primary prevention of infantile eczema. Zhonqquo Dang Dai Er Ke Za Zhi 2010;12(9):734‐9. [PUBMED: 20849726] [PubMed] [Google Scholar]
References to other published versions of this review
Boyle 2006b
- Boyle RJ, Bath‐Hextall F, Donath S, Murrell D, Tang MLK, Taylor J, et al. Probiotics for atopic eczema. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006135] [DOI] [Google Scholar]
Boyle 2008
- Boyle RJ, Bath‐Hextall FJ, Leonardi‐Bee J, Murrell DF, Tang MLK. Probiotics for treating eczema. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006135.pub2] [DOI] [PubMed] [Google Scholar]


