Abstract
A nickel-catalyzed reductive cross-coupling of alkylpyridinium salts and aryl bromides has been developed using Mn as the reductant. Both primary and secondary alkylpyridinium salts can be used, and high functional group and heterocycle tolerance is observed, including for protic groups. Mechanistic studies indicate formation of an alkyl radical, and controlling its fate was key to the success of this reaction.
Graphical Abstract
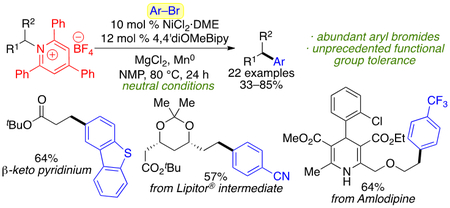
Authors are required to submit a graphic entry for the Table of Contents (TOC) that, in conjunction with the manuscript title, should give the reader a representative idea of one of the following: A key structure, reaction, equation, concept, or theorem, etc., that is discussed in the manuscript. Consult the journal’s Instructions for Authors for TOC graphic specifications.
The increasing prevalence of sp3-hybridized carbons in pharmaceuticals, along with their presence in natural products and other targets, has spurred exciting innovations in the development of reagents and methods for the incorporation of alkyl groups.1,2 Methods that harness ubiquitous functional groups in cross-couplings provide opportunities to exploit previously untapped feedstocks and for late-stage functionalization.3 Our efforts focus on methods that enable alkyl primary amines to be transformed into alkyl electrophiles. Alkyl amines are prevalent in molecules ranging from simple starting materials to complex natural products and drug candidates.1b, 4 They are easy to prepare, stable, and broadly compatible with many functional groups, allowing them to be carried deep into a synthetic sequence particularly when protected.4c, 4d From the perspective of medicinal discovery, alkyl amines are present in pharmaceutical libraries in vast numbers and offer considerable diversity. For example, Pfizer’s internal chemical inventory has >47,000 alkyl primary amines (vs. <30,000 primary and secondary alkyl halides).
We identified that alkyl amines, including those with unactivated alkyl groups, can become effective reagents for cross-couplings via conversion to Katritzky pyridinium salts.5,6,7,5c, 5d, 8,9 These Katritzky pyridinium salts are easily prepared in a single step from the primary amine, are air- and moisture-stable, and are convenient to purify. Their formation is selective for primary amines, allowing other Lewis basic groups to be tolerated in the substrate. We demonstrated a nickel-catalyzed Suzuki–Miyaura arylation of alkylpyridinium salts to form C(sp3)–C(sp2) bonds, the first example of using an unactivated alkyl amine derivative in a cross-coupling.5a Since our discovery, we and others have continued to develop this underappreciated class of reagents.5,10
Although the use of aryl boronic acids presents control over the arylation regioselectivity , it also presents limitations. Due to the basic conditions required for boronic acid activation, pyridinium salts with β-electron-withdrawing groups undergo elimination, and other protic groups are also problematic. An excess (3.0 equiv) of the arylboronic acid is required.5a Thirdly, aryl boronic acids must be prepared from aryl halides, adding steps and limiting opportunities for efficient parallel synthesis. Finally, the availability of aryl boronic acids is much more limited than aryl bromides. In contrast to only 6,200 (het)aryl boronic acids and esters, there are >56,000 (het)aryl bromides in Pfizer’s inventory. Similar constraints limit the potential of any organometallic coupling partner. Thus, a method for direct use of aryl bromides would be of high value.
Despite the exquisite methods developed for reductive couplings of other alkyl electrophiles,3d, 3g, 11 it was unclear if the reduction of the pyridinium cation and potential side reactions of the alkyl radical intermediate could be controlled in the presence of a stoichiometric reductant. Overcoming these challenges, we report conditions for the efficient coupling of alkyl pyridinium salts with aryl bromides (Scheme 1). This method uses abundant and diverse aryl bromides, approximately equimolar amounts of alkyl pyridinium salt and aryl bromide, and the neutral conditions allow exceptional scope of pyridinium salts, particularly those with base-sensitive functional groups. During the preparation of this manuscript, Han and Rueping reported couplings of alkylpyridinium salts and aryl iodides.12 These reports demonstrate feasibility of a reductive coupling. However, it is important to note that aryl iodides are less available than arylboronic acids and esters; for example, less than 300 (het)aryl iodides are in Pfizer’s internal inventory, making these methods of limited utility for the incorporation of diverse and readily available aryl groups.
Scheme 1.
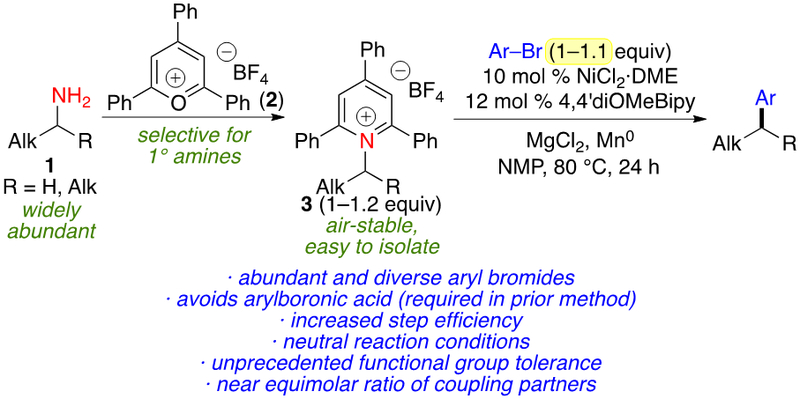
Reductive coupling of alkyl pyridinium salts and aryl bromides
In an effort to optimize conditions for the incorporation of heteroaryls, we selected the reductive coupling of pyridinium salt 3a and 3-bromoquinoline for optimization. To maximize the utility of this reaction for the use of precious alkyl amines and/or precious aryl bromides, we prioritized conditions that would allow approximately equimolar amounts of alkyl pyridinium salt and aryl halide to be used. Using NiBr2·DME and 4,4’-ditBuBipy, a promising 9% yield was observed with Zn as the reductant (Table 1, entry 1). However, dihyropyridine 5 was a major competing product, likely arising from addition of the alkyl radical intermediate to another equivalent of 3a. Changing the reductant to Mno gave increased yield (entry 2). Further improvement was observed with the addition of LiCl or MgCl2, by changing the solvent to NMP, and by reducing concentration (entries 3–5). These additives may accelerate reduction of the NiII intermediates or assist in activating the surface of the Mno.13 Under these conditions, the equivalents of Mno could be reduced (entry 6). Hypothesizing that an electron-rich ligand was necessary to promote single-electron transfer (SET) to the pyridinium cation, we found that 4,4’-diOMeBipy provided an even higher yield of 74% (entry 7). Because the aryl halide is often the precious component, we investigated conditions with aryl bromide as the limiting reagent, and observed 85% isolated yield (1.2 equiv pyridinium 3a, entry 8). Control experiments confirmed that NiCl2·DME, ligand, and Mn0 are required (entries 9–11, 13) and that MgCl2 still provides a beneficial effect (entry 12). With minimal precaution taken to exclude air and moisture (set up under air, no drying of glassware or solvents), 53% yield is observed (entry 14).
Table 1.
Optimization of primary alkyl pyridinium salta
 | |||||
|---|---|---|---|---|---|
| yield (%)b | |||||
| entry | ligand | additive | 4 | 5 | Ar–Ar |
| 1c,d | 4,4’-ditBuBipy | – | 9 | 24 | 5 |
| 2d | 4,4’-ditBuBipy | – | 26 | 25 | 4 |
| 3d | 4,4’-ditBuBipy | LiCl | 54 | 14 | 4 |
| 4 | 4,4’-ditBuBipy | LiCl | 60 | 12 | 10 |
| 5 | 4,4’-ditBuBipy | MgCl2 | 65 | 8 | 15 |
| 6e | 4,4’-ditBuBipy | MgCl2 | 67 | 6 | 17 |
| 7e | 4,4’-diOMeBipy | MgCl2 | 74 | 7 | 15 |
| 8e,f | 4,4’-diOMeBipy | MgCl2 | 85g | n.d. | n.d. |
| 9 e,f,h | 4,4’-diOMeBipy | MgCl2 | 0 | 36 | 0 |
| 10 e,f | none | MgCl2 | 0 | 16 | 14 |
| 11e,f,h | none | MgCl2 | 0 | 30 | 0 |
| 12 e,f | 4,4’-diOMeBipy | – | 50 | 24 | 12 |
| 13f,i | 4,4’-diOMeBipy | MgCl2 | 0 | 0 | 0 |
| 14e,f, j | 4,4’-diOMeBipy | MgCl2 | 53g | n.d. | n.d. |
Conditions: pyridinium salt 3a (0.10 mmol), 3-bromoquinoline (1.1 equiv), NiCl2·DME (10 mol %), ligand (12 mol %), Mn° (3.0 equiv), additive (0 or 1.0 equiv), NMP (0.17 M), 80 °C, 24 h, unless noted otherwise.
Determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
Zn° instead of Mn°.
NiBr2·DME (10 mol %), DMA (0.33 M).
Mn° (2.0 equiv).
3a (1.2 equiv), 3-bromoquinoline (1.0 equiv).
1.0 mmol scale. Isolated yield.
No NiCl2·DME.
No Mn°.
Minimal precautions to protect from air and moisture. n.d. = not determined.
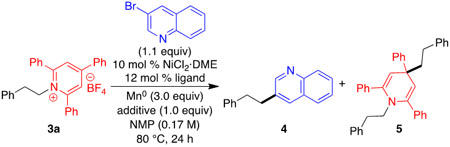
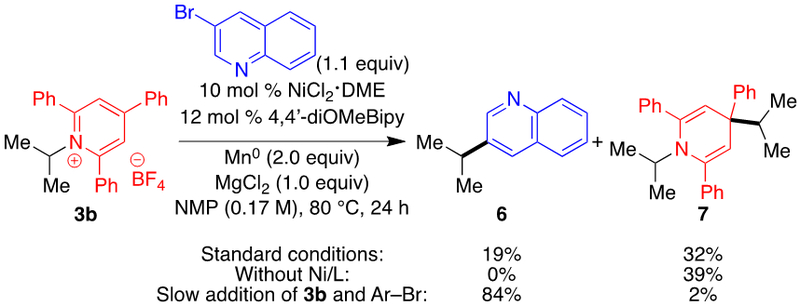
With these conditions in hand, we turned to the reductive coupling of secondary alkyl pyridinium salts. Disappointingly, only 19% yield of product 6 was observed, and dihydropyridine 7 was formed in 32% yield (Scheme 2). In the absence of Ni catalyst, product 7 is formed in 39% yield, indicating a non-catalyzed competitive pathway for the production of the isopropyl radical via direct reduction by Mn0. Hypothesizing that 7 forms from addition of the isopropyl radical to pyridinium cation 3b instead of combination with a Ni(II) intermediate (see mechanism discussion below), we proposed that maintaining a low concentration of pyridinium cation vs Ni catalyst would prevent formation of 7 and give higher yield of 6. Indeed, slow addition of a solution of 3b and aryl bromide resulted in a much improved 84% yield.14
Scheme 2.
Optimization of secondary alkyl pyridinium salta
a Conditions: pyridinium salt 3b (0.50 mmol), 3-bromoquinoline (1.1 equiv), NiCl2·DME (10 mol %), 4,4’-diOMeBipy (12 mol %), Mn° (2.0 equiv), MgCl2 (1.0 equiv), NMP (0.17 M), 80 °C, 24 h, unless noted otherwise. Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
These optimized conditions proved general for a wide range of alkyl pyridinium salts and aryl bromides (Scheme 3). Primary and secondary alkyl pyridinium salts underwent coupling in good yield, as did a benzylic pyridinium salt (15). Even a quaternary carbon can be formed via cross-coupling of a tertiary alkyl pyridinium salt, albeit in 35% yield (20). Pyridinium salts with more bulky tertiary alkyls cannot be prepared due to hindrance of the 2,6-diphenyls. In addition to general exploration of functional group tolerance, we were particularly interested in substrates that failed under the basic conditions of our Suzuki–Miyaura arylation.5a Excitingly, pyridinium salts with β-electron-withdrawing groups are effective substrates (11, 12); elimination is not observed under these mild, neutral conditions. Protic functional groups (primary amide, indole, alcohol, and dihydropyridine) were also well tolerated (16–18, 23, 27). Successful pyridinium formation and arylation of a number of natural product and pharmaceutically relevant alkyl amines highlights another advantage of using alkyl amines as precursors for late-stage functionalization. For example, pyridinium salts derived from amino acids (21, 22) and amino alcohols (23) can be utilized. Amine intermediates used in the synthesis of the pharmaceuticals mosapride and Lipitor® can also be arylated (24–26).15 Notably, the arylation to form 25 was successful on gram scale, demonstrating the scalability of this chemistry. Finally, arylation of the pharmaceutical amlodipine provides a dramatic example of the functional groups tolerated under these mild reaction conditions (27).16
Scheme 3.
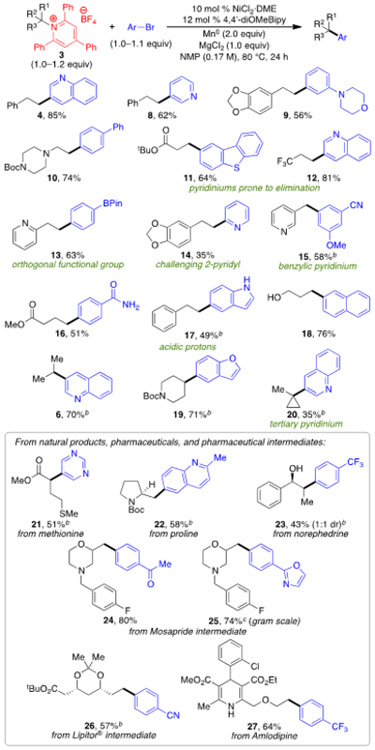
Substrate Scopea
a Conditions: pyridinium salt 3 (1.2 mmol), aryl bromide (1.0 mmol), NiCl2·DME (10 mol %), 4,4’-dimethoxy-2,2’-bipyridine (12 mol %), Mn° (2.0 equiv), MgCl2 (1.0 equiv), NMP (0.17 M) 80 °C, 24 h. Average isolated yields (±5%) from duplicate experiments. b Slow addition of pyridinium salt 3 (1.0 mmol) and aryl bromide (1.1 mmol). c 4.0 mmol scale, single experiment.
We also observed excellent scope with respect to the aryl bromide. Aryl boronate esters (13) can be incorporated into the products. The use of 2-pyridyl bromide enabled the challenging 2-pyridyl group to be installed (14), highlighting another advantage over using an aryl boronic acid.17 A range of other heteroaryl bromides was also effective, including quinolyl (4, 6, 12, 20, 22), 3-pyridyl (8), dibenzothiophenyl (11), benzofuranyl (19), and pyrimidyl (21). 3-Pyridyl iodide and chloride provided <10% yield.18 Aryl bromides with ortho substitution, electron-donating groups, and some complex heteroaryls provided low yields.19 Saturated heterocycles, including morpholines 9, 24, and 25, piperazine 10, piperidine 19, pyrrolidine 22, ketal 26, and dihydropyridine 27, were also tolerated. Additional functional groups compatible with this method include dioxalane (9, 14), Boc-protected amines (10, 19, 22), esters (11, 16, 21, 26, 27), trifluoromethyls (12, 23, 27), nitriles (15, 26), ethers (15, 27), thioethers (21), isoxazoles (25), and aryl chlorides (27). However, these conditions fail for alkyl or alkynyl bromides.
We also investigated one-pot transformations of amine to arylalkane. Although simultaneous addition of pyrilium salt, arylbromide, catalyst, and other reagents failed, telescoping pyridinium formation and cross-coupling provided 50% yield (Scheme 4). This yield is lower than when pyridinium salt 3a is isolated (75%), but this protocol may be advantageous in certain cases, such as parallel synthesis.
Scheme 4.
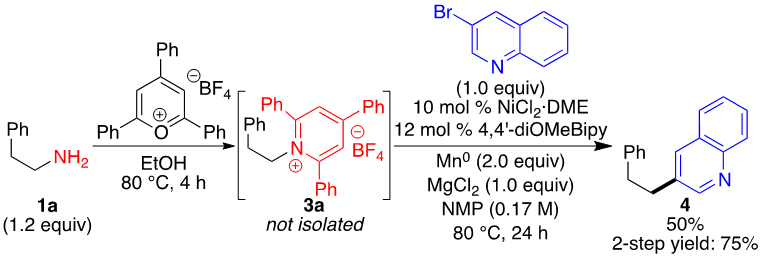
Telescoped pyridinium formation and cross-coupling
This reaction likely proceeds via a mechanism analogous to reductive cross-couplings of alkyl halides with aryl halides (Scheme 5A).20 Oxidative addition of aryl bromide to a Nio intermediate generates an arylnickel(II) bromide. Single-electron transfer (SET) from a NiI intermediate to the pyridinium salt generates a neutral pyridyl radical, which then fragments to give alkyl radical 28. Alkyl radical 28 may also form via reduction by Mno, particularly for secondary alkylpyridinium salts. Combination of radical 28 with an arylnickel(II) intermediate delivers NiIII species 29. Reductive elimination then releases product 30 and regenerates a NiI intermediate. In support of the oxidative addition of the aryl bromide, 24% yield of product 4 is observed when tetrakis(dimethylamino)ethylene (TDAE) is used in place of Mn0, suggesting an arylmanganese intermediate is not required (Scheme 5B). The intermediacy of alkyl radical 28 is consistent with the observed opening of cyclopropylmethyl pyridinium 3s, and the formation of TEMPO-trapped adduct 32 when TEMPO is added to the reaction. However, we cannot yet distinguish between a radical-chain bimetallic pathway and a radical-rebound pathway.21
Scheme 5.
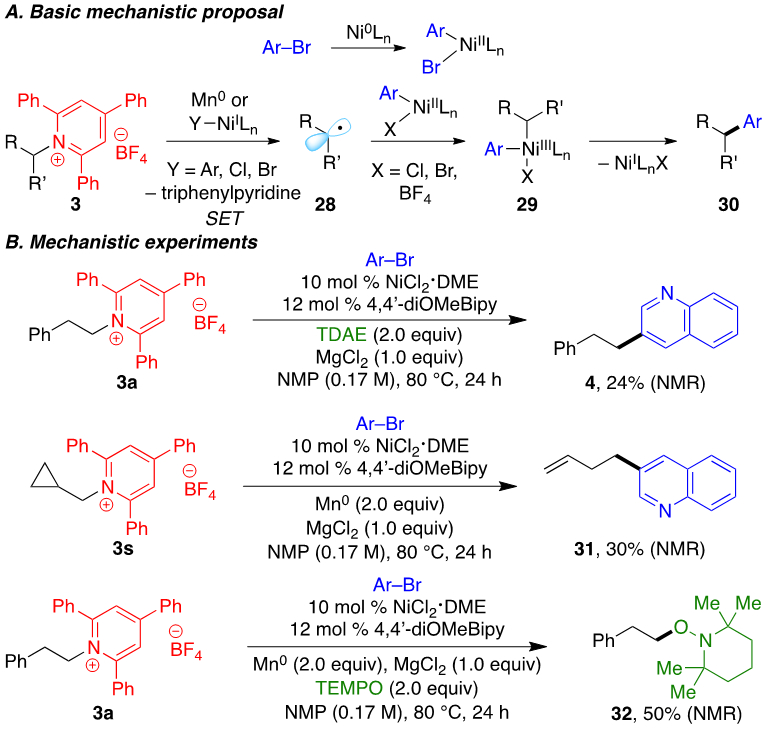
Mechanism and supporting experiments
In summary, we have developed a nickel-catalyzed reductive cross-coupling of alkyl pyridinium salts and aryl bromides. Given the abundance and diversity of alkyl amines and (het)aryl bromides available and the mild conditions, this method enables access to an exceptional variety of products, including those with protic and other base-sensitive functional groups. The success of this method, and the ability to use primary, secondary, and one tertiary alkyl pyridinium salts, relied on controlling the relative concentration of pyridinium salt to shuttle the alkyl radical intermediate along the desired catalytic cycle.
Supplementary Material
ACKNOWLEDGMENT
We thank NIH (R01 GM111820), Pfizer, Inc., and University of Delaware (UD) for University Graduate Fellowships (J. L., C.H.B.). We thank the Martin and Molander groups for helpful discussions. Data were acquired at UD on instruments obtained with assistance of NSF and NIH funding (NSF CHE0421224, CHE1229234, CHE0840401, and CHE1048367; NIH P20 GM104316, P20 GM103541, and S10 OD016267). We thank Lotus Separations, LLC, for assistance with SFC.
Footnotes
The authors declare no competing financial interest.
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website.
Experimental details and data (PDF)
REFERENCES
- 1. (a).Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756; [DOI] [PubMed] [Google Scholar]; (b) McGrath NA; Brichacek M; Njardarson JT A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar]
- 2. (a).Tasker SZ; Standley EA; Jamison TF Recent advances in homogeneous nickel catalysis. Nature 2014, 509, 299–309; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yan M; Lo JC; Edwards JT; Baran PS Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander G; Milligan JA; Phelan JP; Badir SO Recent Advances in Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem., Int. Ed. 0, doi: 10.1002/anie.201809431; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhou JS; Fu GC Cross-Couplings of Unactivated Secondary Alkyl Halides: Room-Temperature Nickel-Catalyzed Negishi Reactions of Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003, 125, 14726–14727. [DOI] [PubMed] [Google Scholar]
- 3. (a).Johnston CP; Smith RT; Allmendinger S; MacMillan DW Metallaphotoredox-catalysed sp(3)-sp(3) cross-coupling of carboxylic acids with alkyl halides. Nature 2016, 536, 322–325; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gutiérrez-Bonet Á; Tellis JC; Matsui JK; Vara BA; Molander GA 1,4-Dihydropyridines as Alkyl Radical Precursors: Introducing the Aldehyde Feedstock to Nickel/Photoredox Dual Catalysis. ACS Catal. 2016, 6, 8004–8008; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qin T; Cornella J; Li C; Malins LR; Edwards JT; Kawamura S; Maxwell BD; Eastgate MD; Baran PS A General Alkyl-Alkyl Cross-Coupling Enabled by Redox-Active Esters and Alkylzinc Reagents. Science 2016, 352, 801–805; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huihui KM; Caputo JA; Melchor Z; Olivares AM; Spiewak AM; Johnson KA; DiBenedetto TA; Kim S; Ackerman LK; Weix DJ Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides. J. Am. Chem. Soc. 2016, 138, 5016–5019; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cornella J; Edwards JT; Qin T; Kawamura S; Wang J; Pan CM; Gianatassio R; Schmidt M; Eastgate MD; Baran PS Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters. J. Am. Chem. Soc. 2016, 138, 2174–2177; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhang X; MacMillan DW Alcohols as Latent Coupling Fragments for Metallaphotoredox Catalysis: sp3-sp2 Cross-Coupling of Oxalates with Aryl Halides. J. Am. Chem. Soc. 2016, 138, 13862–13865; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Molander GA; Traister KM; O’Neill BT Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem. 2015, 80, 2907–2911. [DOI] [PubMed] [Google Scholar]
- 4. (a).Ruiz-Castillo P; Buchwald SL Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu Y; Ge H Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem. 2017, 9, 26–32; [Google Scholar]; (c) Lawrence SA, Amines: Synthesis, Properties and Applications. Cambridge University Press: New York, NY, 2004; [Google Scholar]; (d) Nugent TC, Chiral Amine Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2010. [Google Scholar]
- 5. (a).Basch CH; Liao J; Xu J; Piane JJ; Watson MP Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C–N Bond Activation. J. Am. Chem. Soc. 2017, 139, 5313–5316; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Plunkett S; Basch CH; Santana SO; Watson MP Harnessing Alkyl Pyridinium Salts as Electrophiles in De-aminative Alkyl-Alkyl Cross-Couplings. J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.1029b00111; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liao J; Guan W; Boscoe BP; Tucker JW; Tomlin JW; Garnsey MR; Watson MP Transforming Benzylic Amines into Diarylmethanes: Cross-Couplings of Benzylic Pyridinium Salts via C–N Bond Activation. Org. Lett. 2018, 20, 3030–3033; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Guan W; Liao J; Watson MP Vinylation of Benzylic Amines via C–N Bond Functionalization of Benzylic Pyridinium Salts. Synthesis 2018, 50, 3231–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. (a).Bapat JB; Blade RJ; Boulton AJ; Epsztajn J; Katrizky AR; Lewis J; Molina-Buendia P; Nie P-L; Ramsden CA Pyridines as Leaving Groups in Synthetic Transformations: Nucleophilic Displacements of Amino Groups, and Novel Preparations of Nitriles and Isocyanates. Tetrahedron Lett. 1976, 31, 2691–2694; [Google Scholar]; (b) Katrizky AR; Marson CM Pyrylium Mediated Transformations of Primary Amino Groups into Other Functional Groups. Angew. Chem., Int. Ed. 1984, 23, 420–429; [Google Scholar]; (c) Sowmiah S; Esperança JMSS; Rebelo LPN; Afonso CAM Pyridinium salts: from synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar]
- 7.Ouyang K; Hao W; Zhang WX; Xi Z Transition-Metal-Catalyzed Cleavage of C-N Single Bonds. Chem. Rev. 2015, 115, 12045–12090. [DOI] [PubMed] [Google Scholar]
- 8.For electronically or strain-activated alkyl groups, see:Huang C-Y; Doyle AG Nickel-Catalyzed Negishi Alkylations of Styrenyl Aziridines. J. Am. Chem. Soc. 2012, 134, 9541–9544;Li M-B; Wang Y; Tian S-K Regioselective and Stereospecific Cross-Coupling of Primary Allylic Amines with Boronic Acids and Boronates through Palladium-Catalyzed C–N Bond Cleavage. Angew. Chem., Int. Ed. 2012, 51, 2968–2971;Maity P; Shacklady-McAtee DM; Yap GPA; Sirianni ER; Watson MP Nickel-Catalyzed Cross Couplings of Benzylic Ammonium Salts and Boronic Acids: Stereospecific Formation of Diarylethanes via C–N Bond Activation. J. Am. Chem. Soc. 2013, 135, 280–285;Jensen KL; Standley EA; Jamison TF Highly Regioselective Nickel-Catalyzed Cross-Coupling of N-Tosylaziridines and Alkylzinc Reagents. J. Am. Chem. Soc. 2014, 136, 11145–11152;Shacklady-McAtee DM; Roberts KM; Basch CH; Song Y-G; Watson MP A general, simple catalyst for enantiospecific cross couplings of benzylic ammonium triflates and boronic acids: no phosphine ligand required. Tetrahedron 2014, 70, 4257–4263;Zhang H; Hagihara S; Itami K Making Dimethylamino a Transformable Directing Group by Nickel-Catalyzed C–N Borylation. Chem. Eur. J. 2015, 21, 16796–16800;Basch CH; Cobb KM; Watson MP Nickel-Catalyzed Borylation of Benzylic Ammonium Salts: Stereospecific Synthesis of Enantioenriched Benzylic Boronates. Org. Lett. 2016, 18, 136–139;Hu J; Sun H; Cai W; Pu X; Zhang Y; Shi Z Nickel-Catalyzed Borylation of Aryl- and Benzyltrimethylammonium Salts via C-N Bond Cleavage. J. Org. Chem. 2016, 81, 14–24;Moragas T; Gaydou M; Martin R Nickel-Catalyzed Carboxylation of Benzylic C−N Bonds with CO2. Angew. Chem., Int. Ed. 2016, 55, 5053–5057;Yi Y-Q-Q; Yang W-C; Zhai D-D; Zhang X-Y; Li S-Q; Guan B-T Nickel-catalyzed C-N bond reduction of aromatic and benzylic quaternary ammonium triflates. Chem. Commun. 2016, 52, 10894–10897;Gui Y; Tian S-K Stereospecific Nucleophilic Substitution of Enantioenriched Tertiary Benzylic Amines via in Situ Activation with Benzyne. Org. Lett. 2017, 19, 1554–1557;Guisán-Ceinos M; Martín-Heras V; Tortosa M Regio- and Stereospecific Copper-Catalyzed Substitution Reaction of Propargylic Ammonium Salts with Aryl Grignard Reagents. J. Am. Chem. Soc. 2017, 139, 8448–8451.
- 9.For vinyl and aryl pyridinium salts, see:Buszek KR; Brown N N-Binylpyridinium and -ammonium Tetrafluoroborate Salts: New Electrophilic Coupling Partners for Pd(0)-Catalyzed Suzuki Cross-Coupling Reactions. Org. Lett. 2007, 9, 707–710;Moser D; Duan Y; Wang F; Ma Y; O’Neill Matthew J; Cornella J Selective Functionalization of Aminoheterocycles by a Pyrylium Salt. Angew. Chem., Int. Ed. 2018, 57, 11035–11039.
- 10. (a).Klauck FJR; James MJ; Glorius F Deaminative Strategy for the Visible‐Light‐Mediated Generation of Alkyl Radicals. Angew. Chem., Int. Ed. 2017, 56, 12336–12339; [DOI] [PubMed] [Google Scholar]; (b) Ociepa M; Turkowska J; Gryko D Redox-Activated Amines in C(sp3)–C(sp) and C(sp3)–C(sp2) Bond Formation Enabled by Metal-Free Photoredox Catalysis. ACS Catal. 2018, 8, 11362–11367; [Google Scholar]; (c) Wu J; He L; Noble A; Aggarwal VK Photoinduced Deaminative Borylation of Alkylamines. J. Am. Chem. Soc. 2018, 140, 10700–10704; [DOI] [PubMed] [Google Scholar]; (d) Sandfort F; Strieth-Kalthoff F; Klauck FJR; James MJ; Glorius F Deaminative Borylation of Aliphatic Amines Enabled by Visible Light Excitation of an Electron Donor–Acceptor Complex. Chem. Eur. J. 2018, 24, 17210–17214; [DOI] [PubMed] [Google Scholar]; (e) Hu J; Wang G; Li S; Shi Z Selective C−N Borylation of Alkyl Amines Promoted by Lewis Base. Angew. Chem., Int. Ed. 2018, 57, 15227–15231; [DOI] [PubMed] [Google Scholar]; (f) Zhang M-M; Liu F Visible-light-mediated allylation of alkyl radicals with allylic sulfones via a deaminative strategy. Organic Chemistry Frontiers 2018, 5, 3443–3446; [Google Scholar]; (g) For a recent three-component coupling of activated alkyl pyridinium salts, see: Klauck FJR; Yoon H; James MJ; Lautens M; Glorius F Visible-Light-Mediated Deaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal. 2019, 9, 236–241; [Google Scholar]; (h) Jiang X; Zhang MM; Xiong W; Lu LQ; Xiao WJ Deaminative (Carbonylative) Alkyl-Heck-type Reactions Enabled by Photocatalytic C-N Bond Activation. Angew. Chem., Int. Ed. 2019, 58, 2402–2406. [DOI] [PubMed] [Google Scholar]
- 11. (a).Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767–1775; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem. 2014, 79, 4793–4798; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Everson DA; Shrestha R; Weix DJ Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2010, 132, 920–921; [DOI] [PubMed] [Google Scholar]; (d) Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2012, 134, 6146–6159; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang S; Qian Q; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Halides with Secondary Alkyl Bromides and Allylic Acetate. Org. Lett. 2012, 14, 3352–3355; [DOI] [PubMed] [Google Scholar]; (f) Molander GA; Traister KM; O’Neill BT Reductive Cross-Coupling of Nonaromatic, Heterocyclic Bromides with Aryl and Heteroaryl Bromides. J. Org. Chem. 2014, 79, 5771–5780; [DOI] [PubMed] [Google Scholar]; (g) Wang X; Wang S; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2015, 137, 11562–11565; [DOI] [PubMed] [Google Scholar]; (h) Wang X; Ma G; Peng Y; Pitsch CE; Moll BJ; Ly TD; Wang X; Gong H Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 14490–14497; [DOI] [PubMed] [Google Scholar]; (i) Zhang P; Le CC; MacMillan DW Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc. 2016, 138, 8084–8087; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Amatore M; Gosmini C Direct Method for Carbon–Carbon Bond Formation: The Functional Group Tolerant Cobalt-Catalyzed Alkylation of Aryl Halides. Chem. Eur. J. 2010, 16, 5848–5852; [DOI] [PubMed] [Google Scholar]; (k) Sun S-Z; Martin R Nickel-Catalyzed Umpolung Arylation of Ambiphilic α-Bromoalkyl Boronic Esters. Angew. Chem., Int. Ed. 2018, 57, 3622–3625. [DOI] [PubMed] [Google Scholar]
- 12. (a).Han also reported reductive coupling with alkyl and alkynyl halides. See: Ni S; Li C; Mao Y; Pan Y; Han J Ni-Catalyzed Deamination Cross-Electrophile Coupling of Katritzky Salts with Halides via C–N Bond Activation. ChemRxiv 2019, DOI: 10.26434/chemrxiv.7638164.v7638161; [DOI] [Google Scholar]; (b) Yue H; Zhu C; Shen L; Geng Q; Hock KJ; Yuan T; Cavallo L; Rueping M Nickel-catalyzed C–N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling. Chem. Sci. 2019, DOI: 10.1039/c1039sc00783k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. (a).Feng C; Cunningham DW; Easter QT; Blum SA Role of LiCl in Generating Soluble Organozinc Reagents. J. Am. Chem. Soc. 2016, 138, 11156–11159; [DOI] [PubMed] [Google Scholar]; (b) Zhao C; Jia X; Wang X; Gong H Ni-Catalyzed Reductive Coupling of Alkyl Acids with Unactivated Tertiary Alkyl and Glycosyl Halides. J. Am. Chem. Soc. 2014, 136, 17645–17651. [DOI] [PubMed] [Google Scholar]
- 14.Aryl bromide was added via slow addition to prevent diaryl formation. See Supporting Information.
- 15. (a).Kato S; Morie T; Yoshida N Synthesis and Biological Activities of Metabolites of Mosapride, a New Gastroprokinetic Agent. Chem. Pharm. Bull. 1995, 43, 699–702; [DOI] [PubMed] [Google Scholar]; (b) Roth BD Trans-6-[2-(3- or 4-carboxamido-substituted pyrrol-1-yl)alkyl]-4-hydroxypyran-2-one Inhibitors of Cholesterol Synthesis US 4,681,893, 1987. [Google Scholar]
- 16.Arrowsmith JE; Campbell SF; Cross PE; Stubbs JK; Burges RA; Gardiner DG; Blackburn KJ Long-acting dihydropyridine calcium antagonists. 1. 2-Alkoxymethyl derivatives incorporating basic substituents. J. Med. Chem. 1986, 29, 1696–1702. [DOI] [PubMed] [Google Scholar]
- 17.Everson DA; Buonomo JA; Weix DJ Nickel-Catalyzed Cross-Electrophile Coupling of 2-Chloropyridines with Alkyl Bromides. Synlett 2014, 25, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.See Supporting Information.
- 19.For additional studies of heteroaryl bromides, see the Supporting Information.
- 20.Biswas S; Weix DJ Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc. 2013, 135, 16192–16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.For further description of these pathways and related experiments, please see the Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


