Abstract
Background
Pre‐eclampsia and eclampsia are common causes of serious morbidity and death. Calcium supplementation may reduce the risk of pre‐eclampsia, and may help to prevent preterm birth. This is an update of a review last published in 2014.
Objectives
To assess the effects of calcium supplementation during pregnancy on hypertensive disorders of pregnancy and related maternal and child outcomes.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (18 September 2017), and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials (RCTs), including cluster‐randomised trials, comparing high‐dose calcium supplementation (at least 1 g daily of calcium) during pregnancy with placebo. For low‐dose calcium we included quasi‐randomised trials, trials without placebo, trials with cointerventions and dose comparison trials.
Data collection and analysis
Two researchers independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. Two researchers assessed the evidence using the GRADE approach.
Main results
We included 27 studies (18,064 women). We assessed the included studies as being at low risk of bias, although bias was frequently difficult to assess due to poor reporting and inadequate information on methods.
High‐dose calcium supplementation (≥ 1 g/day) versus placebo
Fourteen studies examined this comparison, however one study contributed no data. The 13 studies contributed data from 15,730 women to our meta‐analyses. The average risk of high blood pressure (BP) was reduced with calcium supplementation compared with placebo (12 trials, 15,470 women: risk ratio (RR) 0.65, 95% confidence interval (CI) 0.53 to 0.81; I² = 74%). There was also a reduction in the risk of pre‐eclampsia associated with calcium supplementation (13 trials, 15,730 women: average RR 0.45, 95% CI 0.31 to 0.65; I² = 70%; low‐quality evidence). This effect was clear for women with low calcium diets (eight trials, 10,678 women: average RR 0.36, 95% CI 0.20 to 0.65; I² = 76%) but not those with adequate calcium diets. The effect appeared to be greater for women at higher risk of pre‐eclampsia, though this may be due to small‐study effects (five trials, 587 women: average RR 0.22, 95% CI 0.12 to 0.42). These data should be interpreted with caution because of the possibility of small‐study effects or publication bias. In the largest trial, the reduction in pre‐eclampsia was modest (8%) and the CI included the possibility of no effect.
The composite outcome maternal death or serious morbidity was reduced with calcium supplementation (four trials, 9732 women; RR 0.80, 95% CI 0.66 to 0.98). Maternal deaths were no different (one trial of 8312 women: one death in the calcium group versus six in the placebo group). There was an anomalous increase in the risk of HELLP syndrome in the calcium group (two trials, 12,901 women: RR 2.67, 95% CI 1.05 to 6.82, high‐quality evidence), however, the absolute number of events was low (16 versus six).
The average risk of preterm birth was reduced in the calcium supplementation group (11 trials, 15,275 women: RR 0.76, 95% CI 0.60 to 0.97; I² = 60%; low‐quality evidence); this reduction was greatest amongst women at higher risk of developing pre‐eclampsia (four trials, 568 women: average RR 0.45, 95% CI 0.24 to 0.83; I² = 60%). Again, these data should be interpreted with caution because of the possibility of small‐study effects or publication bias. There was no clear effect on admission to neonatal intensive care. There was also no clear effect on the risk of stillbirth or infant death before discharge from hospital (11 trials, 15,665 babies: RR 0.90, 95% CI 0.74 to 1.09).
One study showed a reduction in childhood systolic BP greater than 95th percentile among children exposed to calcium supplementation in utero (514 children: RR 0.59, 95% CI 0.39 to 0.91). In a subset of these children, dental caries at 12 years old was also reduced (195 children, RR 0.73, 95% CI 0.62 to 0.87).
Low‐dose calcium supplementation (< 1 g/day) versus placebo or no treatment
Twelve trials (2334 women) evaluated low‐dose (usually 500 mg daily) supplementation with calcium alone (four trials) or in association with vitamin D (five trials), linoleic acid (two trials), or antioxidants (one trial). Most studies recruited women at high risk for pre‐eclampsia, and were at high risk of bias, thus the results should be interpreted with caution. Supplementation with low doses of calcium reduced the risk of pre‐eclampsia (nine trials, 2234 women: RR 0.38, 95% CI 0.28 to 0.52). There was also a reduction in high BP (five trials, 665 women: RR 0.53, 95% CI 0.38 to 0.74), admission to neonatal intensive care unit (one trial, 422 women, RR 0.44, 95% CI 0.20 to 0.99), but not preterm birth (six trials, 1290 women, average RR 0.83, 95% CI 0.34 to 2.03), or stillbirth or death before discharge (five trials, 1025 babies, RR 0.48, 95% CI 0.14 to 1.67).
High‐dose (=/> 1 g) versus low‐dose (< 1 g) calcium supplementation
We included one trial with 262 women, the results of which should be interpreted with caution due to unclear risk of bias. Risk of pre‐eclampsia appeared to be reduced in the high‐dose group (RR 0.42, 95% CI 0.18 to 0.96). No other differences were found (preterm birth: RR 0.31, 95% CI 0.09 to 1.08; eclampsia: RR 0.32, 95% CI 0.07 to 1.53; stillbirth: RR 0.48, 95% CI 0.13 to 1.83).
Authors' conclusions
High‐dose calcium supplementation (≥ 1 g/day) may reduce the risk of pre‐eclampsia and preterm birth, particularly for women with low calcium diets (low‐quality evidence). The treatment effect may be overestimated due to small‐study effects or publication bias. It reduces the occurrence of the composite outcome 'maternal death or serious morbidity', but not stillbirth or neonatal high care admission. There was an increased risk of HELLP syndrome with calcium supplementation, which was small in absolute numbers.
The limited evidence on low‐dose calcium supplementation suggests a reduction in pre‐eclampsia, hypertension and admission to neonatal high care, but needs to be confirmed by larger, high‐quality trials.
Plain language summary
Calcium supplementation during pregnancy for preventing blood pressure disorders and related problems
What is the issue?
Pre‐eclampsia is evident as high blood pressure and protein in the urine. It is a major cause of death in pregnant women and newborn babies worldwide. Preterm birth (birth before 37 weeks) is often caused by high blood pressure and is the leading cause of newborn deaths, particularly in low‐income countries.
Why is this important?
Evidence from randomised controlled trials shows that calcium supplements help prevent pre‐eclampsia and preterm birth and lower the risk of a woman dying or having serious problems related to high blood pressure in pregnancy. This is particularly for women on low calcium diets.
What evidence did we find?
We searched for evidence on 18 September 2017, and found 27 trials. We found evidence from 13 studies (involving 15,730 women) that calcium supplementation in high doses (at least 1 gram (g) daily) during pregnancy may be a safe way of reducing the risk of pre‐eclampsia, especially in women from communities with low dietary calcium and those at increased risk of pre‐eclampsia. Women receiving calcium supplements may also be less likely to die or have serious problems related to pre‐eclampsia (low‐quality evidence) and high blood pressure. Babies may be less likely to be born preterm (low‐quality evidence). The syndrome of haemolysis, elevated liver enzymes and low platelets was increased with calcium, but the absolute numbers were small (high‐quality evidence). High‐dose calcium did not have a clear effect on babies admitted to neonatal intensive care, or the number of stillbirths or deaths before discharge from hospital.
Further research is needed into the ideal dosage of supplementation. Limited evidence from 12 trials (2334 women) suggested that a relatively low dose of calcium may be effective in reducing pre‐eclampsia, high blood pressure, and babies admitted to intensive care (however, the quality of the evidence on calcium alone was reduced because eight of the included trials gave other medicines alongside calcium, such as vitamin D, linoleic acid or antioxidants). Low‐dose calcium did not have a clear effect on preterm birth, stillbirth or death before discharge from hospital.
One small study compared high‐dose calcium with low‐dose calcium. Pre‐eclampsia appeared to be reduced in the high‐dose group, but no other differences were found in preterm birth, or stillbirth.
What does this mean?
In settings where dietary calcium is low, supplementation is an important strategy to reduce the serious consequences of pre‐eclampsia. Where high‐dose supplementation is not feasible, the option of lower dose supplements (500 milligrams (mg) to 600 mg daily) might be considered in preference to no supplementation.
Summary of findings
for the main comparison.
| Calcium supplementation compared with placebo for preventing hypertensive disorders and related problems in pregnancy | ||||||
|
Patient or population: pregnant women Settings: outpatient Intervention: high‐dose calcium (≥ 1 g/day) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No calcium | Calcium | |||||
| Pre‐eclampsia | Overall |
RR 0.45 (0.31 to 0.65) RR 0.36 (0.20 to 0.65) RR 0.22 (0.12 to 0.42) |
15,730
(13) 10,678 (8) 587 (5) |
⊕⊕⊝⊝
low1 ⊕⊕⊝⊝ low1 ⊕⊕⊝⊝ low1 |
||
| 65 per 1000 | 29 per 1000 (20 to 42) | |||||
| Low‐calcium diet | ||||||
| 57 per 1000 | 21 per 1000 (11 to 37) | |||||
| High‐risk women | ||||||
| 176 per 1000 | 38 per 1000 (21 to 74) | |||||
| Preterm birth | Overall | RR 0.76 (0.60 to 0.97) | 15,275 (11) | ⊕⊕⊝⊝ low1 | ||
| 104 per 1000 | 79 per 1000 (62 to 101) | |||||
| HELLP syndrome | 1 per 1000 | 3 per 1000 | RR 2.67 (1.05 to 6.82) | 12,901 (2) |
⊕⊕⊕⊕ high | |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded two levels due to heterogeneity and small study effects (‐2)
CI: confidence interval HELLP: haemolysis, elevated liver enzymes and low platelets RR: risk ratio
Background
Description of the condition
High blood pressure, with or without proteinuria, is a major cause of maternal death and morbidity (Betrán 2005; Clark 2008; HMSO 1994; Khan 2006; NHMRC 1993), and perinatal morbidity and mortality (Langenveld 2011; Ozkan 2011), worldwide. Hypertension has been estimated to complicate 5% of all pregnancies and 11% of first pregnancies, half associated with pre‐eclampsia, and to account for up to 40,000 maternal deaths annually (Villar 2004). For this reason, strategies to reduce the risk of hypertensive disorders of pregnancy have received considerable attention (Bucher 1996; Carroli 1994; CLASP 1994; ECCPA 1996).
Spontaneous and medically induced preterm birth is commonly associated with hypertensive disorders. It is the leading cause of early neonatal death and infant mortality, particularly in low‐income countries (Villar 1994). Preterm survivors are at high risk of significant morbidity, especially respiratory disease and its sequelae, and long‐term neurological morbidity (Johnson 1993). Interventions to reduce preterm birth have been reviewed by Villar and colleagues (Villar 1998).
During early pregnancy, blood pressure normally falls; it then climbs slowly in later pregnancy to reach pre‐pregnancy levels at term (Villar 1989). These normal changes in blood pressure make the diagnosis of hypertension during pregnancy difficult. Clinical methods of measuring blood pressure are also subject to considerable inaccuracy (Villar 2004). A widely accepted definition, however, is a diastolic blood pressure equal to or greater than 90 millimetres of mercury (mmHg) or systolic pressure equal to or greater than 140 mmHg before the onset of labour (NHBPEP 2000). The consequences of high blood pressure are more serious if there is associated proteinuria. Hypertension and significant proteinuria (1+ by dipstick testing, equal to or greater than 300 mg per 24 hours, or equal to or greater than 30 mg per decilitre (dL)) (NHBPEP 2000), usually indicate the presence of pre‐eclampsia. The urine protein to creatinine ratio has been used increasingly as a measure of proteinuria (Yamasmit 2004). Predictors of poor outcome include low gestational age and high levels of proteinuria (von Dadelszen 2004).
Description of the intervention
Calcium supplementation is an oral dietary supplement, usually in the form of calcium carbonate or calcium gluconate. The dose is expressed in terms of the amount of elemental calcium in the preparation.
How the intervention might work
An inverse relationship between calcium intake and hypertensive disorders of pregnancy was first described in 1980 (Belizan 1980). This was based on the observation that Mayan Indians in Guatemala, who traditionally soak their corn in lime before cooking, had a high calcium intake and a low incidence of pre‐eclampsia and eclampsia. A very low prevalence of pre‐eclampsia had been reported from Ethiopia where the diet, among other features, contained high levels of calcium (Hamlin 1962). These observations were supported by other epidemiological and clinical studies (Belizan 1988; Hamlin 1952; Repke 1991; Villar 1983; Villar 1987; Villar 1993), and led to the hypothesis that an increase in calcium intake during pregnancy might reduce the incidence of high blood pressure and pre‐eclampsia among women with low calcium intake. An association has been found between pre‐eclampsia and hypocalciuria (Segovia 2004); lower urine calcium to creatinine ratio (Kazerooni 2003); hypocalcaemia (Kumru 2003); lower plasma and higher membranous calcium (Kisters 2000); lower dietary milk intake (Duvekot 2002); and between eclampsia and hypocalcaemia (Isezuo 2004).
Low calcium intake may cause high blood pressure by stimulating either parathyroid hormone or renin release, thereby increasing intracellular calcium in vascular smooth muscle (Belizan 1988), and leading to vasoconstriction. A possible mode of action for calcium supplementation is that it reduces parathyroid release and intracellular calcium, and so reduces smooth muscle contractility. By a similar mechanism, calcium supplementation could also reduce uterine smooth muscle contractility and prevent preterm labour and delivery (Villar 1990). Calcium might also have an indirect effect on smooth muscle function by increasing magnesium levels (Repke 1989). Recent evidence indicates that calcium supplementation affects uteroplacental blood flow (it lowers the resistance index in uterine and umbilical arteries) (Carroli 2010). Supplementation in the second half of pregnancy appears to reduce blood pressure directly, rather than preventing the endothelial damage associated with pre‐eclampsia (Hofmeyr 2008).
Calcium supplementation is attractive as a potential intervention to reduce the risk of a woman developing pre‐eclampsia as it is readily available, and is likely to be safe for the woman and her child. In addition, there is a possibility that it may have a preventative effect on the risk of hypertension in offspring (Belizan 1997). A theoretical risk of increased renal tract stone formation, or the occurrence of other adverse effects associated with calcium supplementation, has not been substantiated.
Why it is important to do this review
Calcium supplementation was tested in several randomised trials, commencing in the late 1980s, which suggested a promising beneficial effect on hypertensive disorders and related problems. The first systematic reviews highlighted the need for larger trials to assess the effects on important clinical outcomes in addition to pre‐eclampsia and preterm delivery, such as perinatal mortality (Carroli 1994; Duley 1995). A subsequent systematic review came to more promising conclusions (Bucher 1996), but these findings were not confirmed by a large trial in the USA (CPEP 1997), and the discrepancy elicited discussion (Villar 2000). Subsequently, a large trial conducted in communities with low dietary calcium intake has been reported (WHO 2006). In 2012 the World Health Organization (WHO) published guidelines recommending calcium supplementation with 1.5 g to 2 g elemental calcium daily for pregnant women with low dietary calcium. This recommendation has raised questions regarding the optimum dosage of calcium.
The WHO recommendation was based on available data from randomised trials. Most of the high‐quality trials reviewed used 1.5 g to 2 g of calcium daily, and there was little robust evidence regarding smaller dosages.
The dosage of 1.5 g to 2 g calcium daily is well above the daily recommended dietary calcium of 1 g to 1.2 g.
Logistically, calcium in this dosage is heavy to transport. Calcium carbonate plus glycine tablets containing 1.5 g elemental calcium and glycine daily (= 3750 mg calcium carbonate plus glycine) weigh about 200 g for a four‐week supply (84 tablets). This would amount to about 1 kg of tablets for 20 weeks, therefore, a clinic seeing 1000 pregnant women per year would need to receive 1000 kg of tablets each year.
The cost of calcium is moderately high (compared with supplements such as iron and folate), and the dosage thus has important cost implications.
A 2010 report from the Gambia study (Jarjou 2004) has suggested that calcium at the dosage of 1.5 g daily during pregnancy may impair the mother's ability to conserve calcium, causing rebound bone demineralisation following pregnancy. Although there are limitations to this study (conclusions were based on a sub‐set of women from the original trial; the hypothesis was not prespecified; multiple end‐point testing), the possibility of adverse effects due to the interruption of high‐dose calcium supplementation in women who have previously adapted to low dietary calcium intake is reason for caution.
For these reasons, when updating this review, we considered it important to systematically review the evidence on lower dosages of calcium supplementation in pregnancy. Originally, we had specified that randomised controlled trials with dosages below 1 g daily would be reviewed in subsequent updates of this review. However, in view of the lack of high‐quality trials of lower dosages, we revised the review to include lower quality studies (e.g. quasi‐randomised trials) of lower dosage studies only.
This is an update of a review last published in 2014.
Objectives
To assess the effects of calcium supplementation during pregnancy on hypertensive disorders of pregnancy and related maternal and child outcomes.
Subgroup analyses tested whether these effects were influenced by whether:
women had low or adequate dietary calcium intake prior to trial entry;
women were at low or average risk of hypertensive disorders, or at high risk.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials. We included trials that were presented only as abstracts if there was sufficient detail (published and unpublished) to confirm eligibility. For the original review of high‐dose calcium supplementation we excluded quasi‐random designs. However, for the 2012 update we included trials employing these weaker study designs (e.g. quasi‐randomisation by alternation, unstated or other methods), only for the subgroup of trials of calcium supplementation less than 1 g daily (low dose), with appropriate caution in the interpretation of the results. The reason for the discrepancy between high‐dose and low‐dose trials is that there are adequate data from appropriately randomised, placebo controlled trials of high‐dose but not low‐dose calcium supplementation for review. Cluster‐randomised trials were also eligible for inclusion.
Because of the addition of a comparison of trials of low‐dose calcium supplementation, it was also of interest to include trials comparing high‐ with low‐dose supplementation. Please see Differences between protocol and review for details.
Types of participants
Pregnant women, regardless of the risk of hypertensive disorders of pregnancy. We excluded women with diagnosed hypertensive disorders of pregnancy. We did not exclude women with multiple pregnancy.
Prespecified subgroups to be compared were as follows.
Women at low or average risk of hypertensive disorders of pregnancy (unselected)
Women at above average risk of hypertensive disorders of pregnancy. These included women selected by the trial authors on the basis of an increased risk of hypertensive disorders of pregnancy (e.g. teenagers or women older than 40 years, women with previous pre‐eclampsia, women with increased sensitivity to angiotensin II, women with pre‐existing hypertension). Primiparity alone was not regarded as a high‐risk factor.
Women or populations with low baseline dietary calcium intake (as defined by trial authors, or if not defined, mean intake of less than 900 mg per day)
Women or populations with adequate dietary calcium intake (as defined by trial authors, or if not defined, mean intake equal to or greater than 900 mg per day)
Types of interventions
1. High‐dose supplementation (> 1 g elemental calcium daily)
Supplementation with calcium from 34 weeks of pregnancy at the latest, compared with placebo treatment. We excluded studies with no placebo.
2. Low‐dose supplementation (< 1 g elemental calcium daily)
Supplementation with calcium from 34 weeks of pregnancy at the latest, with or without cointerventions (e.g. vitamin D, linoleic acid, anti‐oxidants or anti‐platelet agents), compared with placebo or no treatment. We prespecified the inclusion criteria to be less restrictive than for the high‐dose calcium comparisons because of the relatively small number of studies of low‐dose calcium supplementation; we subgrouped the outcomes by the cointerventions. This was added in the 2013 revision of this review.
3. Comparison of different dosages of calcium, added in the 2018 revision of this review.
Types of outcome measures
In the original protocol we prespecified 15 clinical measures of maternal and fetal or neonatal morbidity and mortality. In October 2004 we added seven additional outcomes (marked * below). For the 2013 update we added two outcome measures, marked ** below, in order to include newly published data. As such, these should be regarded as post‐hoc analyses, and interpreted with caution.
Primary outcomes
For the woman
High blood pressure as defined by trial authors, with or without proteinuria. Ideally, high blood pressure would be defined as diastolic blood pressure equal to or greater than 90 mmHg, or an increase in systolic blood pressure of 30 mmHg or more, or in diastolic blood pressure of 15 mmHg or more.
High blood pressure with significant proteinuria, as defined by trial authors. Ideally, proteinuria would be defined as 2+ by dipstick testing, equal to or greater than 300 mg per 24 hours, or equal to or greater than 500 mg per litre. Although the strict definition of pre‐eclampsia includes confirmation of no hypertension or proteinuria outside pregnancy, for convenience the above definition is referred to in this review as pre‐eclampsia.
For the child
Preterm birth (birth before 37 weeks of estimated gestation)
Admission to a neonatal intensive care unit
Stillbirth or death before discharge from hospital
Secondary outcomes
For the woman
Maternal death or serious morbidity. Serious morbidity includes eclampsia; renal failure; syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP syndrome); and admission to intensive care. This is a composite outcome of death or at least one measure of serious morbidity; in addition we planned to present each individual outcome.
Placental abruption
Caesarean section
*Proteinuria
*Severe pre‐eclampsia as defined by trial authors
*Eclampsia
*HELLP syndrome
*Intensive care unit admission
*Maternal death
Mother's hospital stay of seven days or more
**Miscarriage
For the child
Low birthweight (the first weight obtained after birth less than 2500 g)
Neonate small‐for‐gestational age as defined by trial authors
Neonate in intensive care unit for seven days or more
*Death or severe neonatal morbidity
Childhood disability
Systolic blood pressure greater than 95th percentile during childhood
Diastolic blood pressure greater than 95th percentile during childhood
**Dental caries in childhood (one or more decayed, missing or filled teeth, or as defined by trial authors)
Only those outcomes with data appear in the analysis table.
Outcomes for 'Summary of findings' table
We included the following GRADE outcomes in the 'Summary of findings' table.
Pre‐eclampsia
Preterm birth
HELLP syndrome
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (18 September 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the 'Specialized Register' section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (18 September 2017) for unpublished, planned and ongoing trial reports using the terms given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Hofmeyr 2014.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager 5 software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the quality of the body of evidence relating to the following outcomes for our main comparison.
Pre‐eclampsia
Preterm birth
HELLP syndrome
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014), in order to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. In future updates, as appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We have not identified any cluster‐randomised trials to date. We will include any future cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify cluster‐randomised trials in addition to the individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not relevant to this review.
Other unit of analysis issues
For multiple‐arm trials we have included only the two arms relevant to the review comparisons. We expect inclusion of multiple pregnancies to be rare. If multiple pregnancies are included in future updates of this review, we will analyse the neonates as individual participants. We will make adjustments for cluster effects only if the numbers of multiples are sufficient to justify statistical adjustment.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will use sensitivity analyses to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we planned not to combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
High‐dose calcium supplementation
We carried out the following subgroup analyses.
Trials in populations with low versus adequate dietary calcium intake
Trials in participants with low/average versus high hypertensive risk
Trials with small versus larger sample size
We also subgrouped the trials by both dietary calcium intake and trial size, to distinguish the differences between the two subgroups more clearly (not prespecified in the original protocol).
We used only primary outcomes in subgroup analyses 2, 3 and 4 above.
Low‐dose calcium supplementation
We carried out subgroup analysis by cointerventions (comparison 6). This was not done for the high‐dose calcium comparisons because cointerventions were not included.
We assessed subgroup differences by interaction tests available within Review Manager (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out a planned sensitivity analyses to explore the effect of risk of bias assessed by concealment of allocation, high attrition rates, or both, with studies at high risk of bias being excluded from the analyses in order to assess whether this makes any difference to the overall result. This sensitivity analysis was only possible for the comparison of high‐dose calcium versus placebo (there were too few studies in the comparison of low‐dose calcium versus no supplementation).
Results
Description of studies
Results of the search
See: Figure 1.
1.
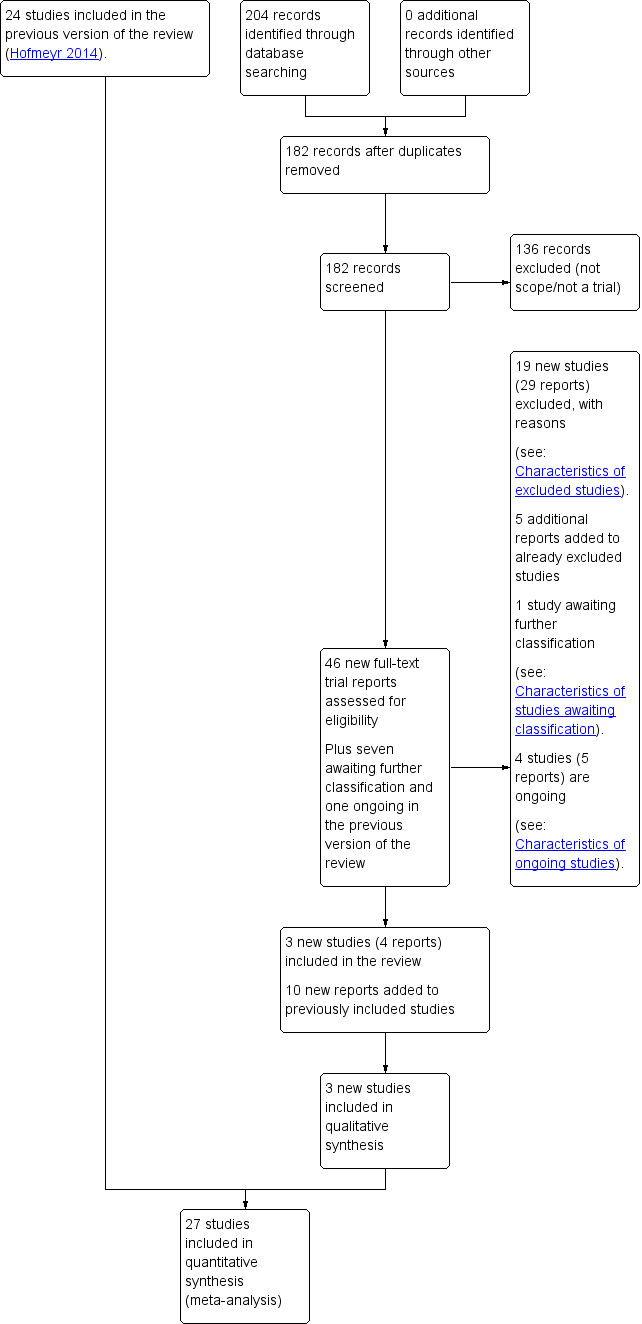
Study flow diagram.
The 2017 search retrieved 46 reports; we also reassessed seven reports awaiting further classification and one ongoing study from the previous version of the review (Hofmeyr 2014). In this version of the review, one study is awaiting further assessment and there are now four ongoing studies (five reports). There were five additional reports relating to two studies already excluded from the review (Diogenes 2011; Ettinger 2011), and 10 additional reports relating to three studies already included in the review. We have included three new studies (four reports) in the review (Asemi 2012; Asemi 2016; Khan 2013) and have excluded a further 19 further studies (29 reports). In total, the review now includes 27 studies and 44 are excluded.
Included studies
The studies were carried out in countries across the world: Philippines (Almirante 1998); Iran (Asemi 2012; Asemi 2016; Niromanesh 2001; Taherian 2002); Trinidad (Bassaw 1998); Argentina (Belizan 1991); China (Cong 1995; Li 2000); Australia (Crowther 1999); Colombia (Herrera 1998); Gambia (Jarjou 2004); India (Khan 2013; Kumar 2009; Marya 1987; Purwar 1996); Ecuador (L‐Jaramillo 1989; L‐Jaramillo 1990; L‐Jaramillo 1997); Hong Kong (Rogers 1999); Indonesia (Rumiris 2006); and the USA (CPEP 1997; S‐Ramos 1994; Villar 1990). In addition there were multicentre studies carried out in more than one country (Herrera 2006; Villar 1987; WHO 2006).
In six of the studies the dates of recruitment were not clear or not stated (Almirante 1998; Cong 1995; CPEP 1997; L‐Jaramillo 1990; Marya 1987; Niromanesh 2001). Recruitment started in the 1980s in five studies (Belizan 1991; L‐Jaramillo 1989; S‐Ramos 1994; Villar 1987; Villar 1990); in the 1990s in nine studies (Bassaw 1998; Crowther 1999; Herrera 1998; Jarjou 2004; Li 2000; L‐Jaramillo 1997; Purwar 1996; Rogers 1999; Taherian 2002); and in the 2000s in seven studies (Asemi 2012; Asemi 2016; Herrera 2006; Khan 2013; Kumar 2009; Rumiris 2006; WHO 2006).
Many of these studies were conducted before it was required for sources of funding and conflict of interest declarations to be reported in published reports. Ten studies did not mention sources of funding. In two studies trial funding was not reported, but it was stated that study drugs were provided by pharmaceutical companies (Niromanesh 2001; Purwar 1996); pharmaceutical companies also provided drugs for other trials that were otherwise government, health service or university funded (Asemi 2012; Asemi 2016; CPEP 1997; Jarjou 2004). Other trialists also stated that studies were supported by university, government or hospital funding (Crowther 1999; Herrera 1998; Herrera 2006; Jarjou 2004; Kumar 2009; L‐Jaramillo 1989; Taherian 2002). Two studies were WHO funded (L‐Jaramillo 1997; WHO 2006) and two were funded by national dairy food organisations (Villar 1987; Villar 1990).
Only two of the studies explicitly stated that authors had no known conflict of interest (Asemi 2016; Jarjou 2004).
Compliance, where reported, was generally more than 80% (84% and 86% for calcium and placebo in Belizan 1991; 84.5% and 86.2% in WHO 2006; 79% and 81% in S‐Ramos 1994). However, in one study compliance was 64% and 67% in the respective groups (CPEP 1997), and in another, 31% and 24% of women from each group stopped taking the tablets before the end of the trial (Crowther 1999). In L‐Jaramillo 1997, two women were withdrawn for non‐compliance.
High‐dose calcium supplementation (1 g/day or more)
We included 14 studies examining high‐dose supplements in the review. One study contributed no data, although authors have been contacted and data may be available for the next update (Jarjou 2004). Of the remaining 13 studies, four were multicentre studies: one was conducted in Argentina (Belizan 1991), one in the USA (CPEP 1997), another in Australia (Crowther 1999), and the fourth was international (WHO 2006). Most of the 15,730 women recruited to these studies were low risk (15,143 women) and had a low dietary intake of calcium (10,678). Most studies only recruited women who were nulliparous or primiparous. One study did not state the parity of women recruited (Niromanesh 2001), and another commented that most women were nulliparous (Villar 1990). For most studies the intervention was 1.5 g to 2 g per day of calcium. All of these studies compared high‐dose supplementation with a placebo.
Five studies enrolled women considered to be at high risk of pre‐eclampsia. The definitions of high risk and the actual risk (rate of pre‐eclampsia in the placebo group) were variable: positive 'roll‐over' test at 28 to 30 weeks (8/34) (L‐Jaramillo 1990); teenagers 17 years or younger (3/88) (Villar 1990); positive 'roll‐over' test at 28 to 32 weeks plus one clinical risk factor (7/15) (Niromanesh 2001); positive 'roll‐over' and positive angiotensin II infusion test (15/34) (S‐Ramos 1994); and nulliparous teenagers 17.5 years or younger (21/135) (L‐Jaramillo 1997). The clinical usefulness of the pooled results in this subgroup is therefore limited.
Two included studies conducted long‐term follow‐up of the children whose mothers were recruited to these trials (Belizan 1991; Hiller 2007). In Belizan 1991, only the subset of women recruited in private clinics were contacted; and in Hiller 2007, the outcomes reported differed from this review (but unpublished data may be made available by the authors at a later date).
Other studies have reported outcomes for small subsets of women (CPEP 1997: Hatton 2003; WHO 2006: Zhang 2007), but these data did not meet the inclusion criteria for this review.
Low‐dose calcium supplementation (less than 1 g/day)
We included 12 studies: four investigated calcium supplementation alone (Almirante 1998; Bassaw 1998; Cong 1995; Rogers 1999); five investigated calcium plus vitamin D (Asemi 2012; Asemi 2016; Li 2000; Marya 1987; Taherian 2002); two studies from the same group investigated calcium plus linoleic acid (Herrera 1998; Herrera 2006); and one investigated calcium plus antioxidants (Rumiris 2006).
Comparisons varied in the studies: five used placebo (Asemi 2012; Asemi 2016; Herrera 1998; Herrera 2006; Rumiris 2006); one used aspirin tablets (Bassaw 1998); four compared with no treatment (Cong 1995; Li 2000; Marya 1987; Taherian 2002); and two did not clearly describe their control group (Almirante 1998; Rogers 1999).
High‐dose versus low‐dose calcium supplementation (1 g/day or more versus less than 1 g/day)
One study compared high‐dose (2 g) with low‐dose (500 mg) calcium supplementation in unselected, normotensive pregnant women from a low socio‐economic status population in West Begal, India, from 34 weeks of pregnancy at the latest (Khan 2013) . A discrepancy between the numbers in each group was not accounted for, and we assessed the risk of bias as unclear.
Please see Characteristics of included studies for further details.
Excluded studies
We excluded 44 studies from the review (Characteristics of excluded studies).
There were a number of reasons for exclusion, and we excluded some studies for more than one reason. In five studies it was not clear how participants were allocated to groups and in some cases the method of allocation was non‐random (Felix 1991; Karandish 2003; Kawasaki 1985; Raman 1978; Salzano 2001). We excluded two other studies because of methodological problems; either the specified outcomes were not reported (Boggess 1997), or there were unexplained group discrepancies (Dizavandy 1998). In seven studies, reported in abstracts, there was too little information on methods and results for us to judge risk of bias and include data (Aghamohammady 2010; August 2002; Belizan 1983; Chames 2002; Prada 2001; Prada 2002; Repke 1989). There were four studies reported in trial registrations where it was not clear that studies had started or been completed (Anumba 2006; Bhatia 2010; Fung 2010; Lavin 1986). In one study the intervention was not calcium but rather dairy foods (Chan 2006), and in eight studies the intervention was not calcium alone but rather calcium plus other vitamins and minerals which could not be meaningfully included (Asemi 2017; Azami 2017 (unclear trial procedures); de Souza 2006 (high‐dose calcium plus aspirin); Diogenes 2011 (no hypertension data); MacDonald 1986 (no methods or results); Mosalanejad 2016 (calcium in both groups, vitamin D dose varied); Nooripour 2016 (calcium in both groups, vitamin D varied); Souza 2014 (high‐dose calcium plus aspirin)). In seven studies there was no placebo and calcium may have been compared with other active interventions (Anu 2017; Knight 1992; Montanaro 1990; Subprabha 2017; Suzuki 1996; Wanchu 2001; Zheng 2000). In three studies the aim of the intervention did not relate to maternal hypertension (Ettinger 2011; Herrera 2006a; Karamali 2016), and in a further three the participants were not eligible (e.g. they already had hypertension at recruitment) (NCT00000543; S‐Ramos 1995; Tamas 1997). In Hofmeyr 2015, women were not pregnant at the time of recruitment. One study was a dose comparison (Martin 2017), one examined high‐dose calcium (1 g) with vitamin D (Samimi 2016), and another examined the effects of enteric coating of calcium supplements on absorption (Roth 2014).
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3.
2.
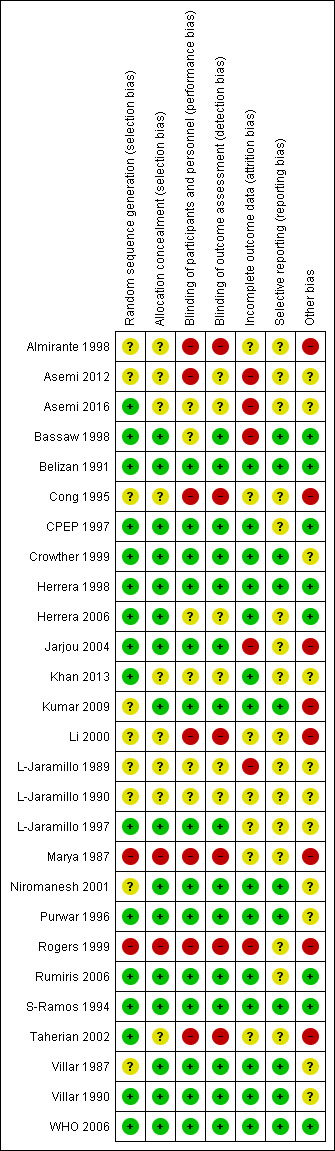
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
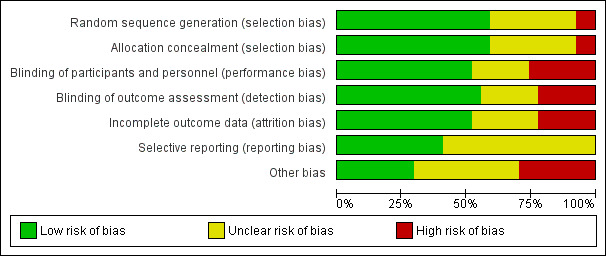
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The majority of studies included in the review used methods of sequence generation and allocation that we assessed as having low risk of bias. Sixteen trials used random number tables, computer generation or central randomisation methods to generate the randomisation sequence. Nine trials did not provide clear information about how the sequence was generated and two studies used methods that we assessed as being at high risk of bias (Marya 1987; Rogers 1999). The methods to conceal allocation at the point of randomisation were assessed as having low risk of bias in 16 trials, unclear risk of bias in nine trials, and high risk of bias in two trials.
Blinding
Where trials were placebo controlled, and it was clear that women and staff providing care were blind to treatment allocation, we assessed the studies as having low risk of performance bias; this applied to 14 trials. In six trials the methods to achieve blinding were not clearly described, and we assessed seven trials to have high risk of performance bias as women or staff were aware of the treatment group. For detection bias, we assessed 15 studies to have low risk of bias, six to have unclear risk of bias, and six to be at high risk of bias.
Incomplete outcome data
In six studies there were post‐randomisation exclusions or loss to follow‐up that meant we considered the studies to be at high risk of attrition bias. In seven studies, sample loss was either not described or we were uncertain about the impact of loss to follow‐up on results. In the remaining 14 trials, loss to follow‐up was low and balanced across groups and so we assessed these studies as being at low risk of bias.
Selective reporting
For 16 trials we had insufficient information to assess risk of outcome reporting bias; many trials did not have published protocols or did not report expected outcomes fully. We assessed 11 trials as being at low risk of outcome reporting bias.
Other potential sources of bias
We assessed eight trials as being at high risk of other bias, mainly because the methods were very poorly described. We assessed another eight trials as being unlikely to be at high risk of other bias; and for 11 studies we were uncertain, again often because of limited reporting.
Overall risk of bias in our main comparisons
High‐dose calcium supplementation
For overall risk of bias, all were double‐blind, placebo‐controlled trials. Pre‐specified outcome data were not available from all trials. Not all outcomes were consistently reported, therefore there is a possibility of reporting bias in some trials.
In L‐Jaramillo 1990, a large discrepancy in numbers allocated to each group was not explained. For Kumar 2009, we contacted the authors to clarify the imbalance in group size that occurred in their study. They have provided the explanation (see notes in Characteristics of included studies), but the imbalance does increase the potential for bias.
In some trials, individual denominators were not given for specific outcomes. Where it was clear that the outcomes were not measured in the entire group, we have adjusted the denominators accordingly. In other respects, the methodology of the studies included appears sound.
Low‐dose calcium supplementation
We considered four of these studies to be at low risk of bias (Bassaw 1998; Herrera 1998; Herrera 2006; Rumiris 2006), and six to be at high risk of bias because no placebo was used and either because random sequence generation and allocation concealment were not reported (Almirante 1998; Cong 1995; Li 2000); a consecutive series was used (Marya 1987); an open envelope method was used (Rogers 1999); or a 'table of random numbers' was reported to be used but there was no report of allocation concealment (Taherian 2002).
Effects of interventions
See: Table 1
High‐dose calcium supplementation versus placebo
In the 13 studies included in the meta‐analysis, significant heterogeneity of results occurred for four outcomes: pre‐eclampsia; high blood pressure; preterm birth and birthweight less than 2500 g. Factors accounting for the heterogeneity appeared to be maternal risk at trial entry, dietary calcium and trial size. The small trials have bigger effect sizes than the large trials; but as all the small trials recruited high‐risk women, this could also be related to risk status. In view of the heterogeneity, we used a random‐effects model for these four outcomes. As part of our sensitivity analysis, we removed the data from a study assessed to be at high risk of attrition bias (L‐Jaramillo 1989).
Data were not available for the following secondary outcomes: mother's hospital stay seven days or more; miscarriage; neonate in intensive care unit seven days or more; death or severe neonatal morbidity; and childhood disability.
Primary outcomes
(1) High blood pressure with or without proteinuria
The results follow a similar pattern to those for pre‐eclampsia (see below). Overall, there were fewer women with high blood pressure with calcium supplementation compared with placebo (12 trials, 15,470 women: average risk ratio (RR) 0.65, 95% confidence interval (CI) 0.53 to 0.81; heterogeneity: Tau² = 0.06; Chi² = 42.40, df = 11, P < 0.0001; I² = 74%; Analysis 1.1). The reduction in RR was greatest for the small trials, i.e. trials with fewer than 400 women (seven trials, 675 women: average RR 0.38, 95% CI 0.21 to 0.68; heterogeneity: Tau² = 0.38; Chi² = 18.26, df = 6, P = 0.006; I² = 67%; test for subgroup differences: Chi² = 6.20, df = 1 (P = 0.01), I² = 83.9%; Analysis 3.1.1), and for those with low baseline dietary calcium (seven trials, 10,418 women: average RR 0.44, 95% CI 0.28 to 0.70; heterogeneity: Tau² = 0.26; Chi² = 39.35, df = 6; test for subgroup differences: Chi² = 8.78, df = 2 (P = 0.01), I² = 77.2%; Analysis 1.1.2). Asymmetric funnel plots for these analyses suggest that the treatment effect may be overestimated due to small‐study effects or publication bias (Figure 4, Figure 5, Figure 6).
1.1. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 1 High blood pressure (with or without proteinuria).
3.1. Analysis.
Comparison 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, Outcome 1 High blood pressure (with or without proteinuria).
4.
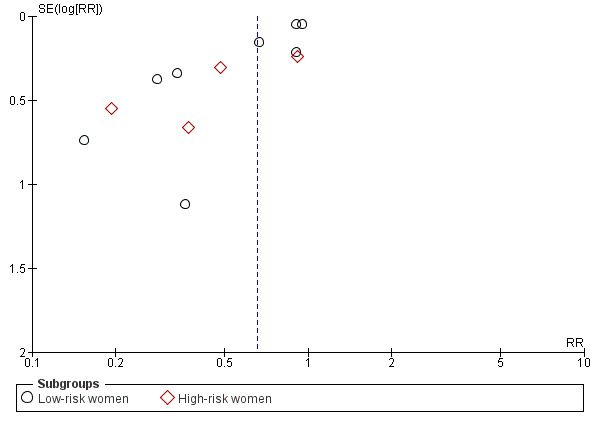
Funnel plot of comparison: 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, outcome: 2.1 High blood pressure (with or without proteinuria).
5.
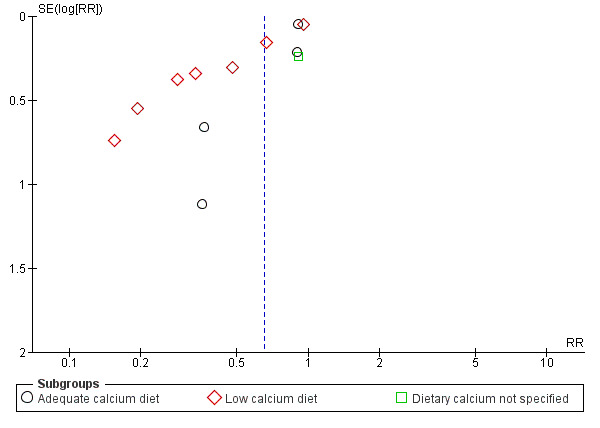
Funnel plot of comparison: 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, outcome: 1.1 High blood pressure (with or without proteinuria).
6.
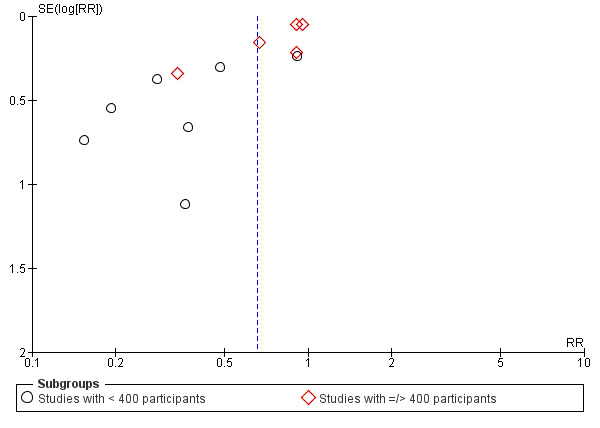
Funnel plot of comparison: 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, outcome: 3.1 High blood pressure (with or without proteinuria).
(2) Pre‐eclampsia
Overall, there was a reduction in the average risk of pre‐eclampsia (13 trials, 15,730 women: RR 0.45, 95% CI 0.31 to 0.65, low‐quality evidence; heterogeneity: Tau² = 0.20; Chi² = 40.31, df = 12 (P < 0.0001; I² = 70%); Analysis 1.2. This reduction in RR was greatest for women at high risk of pre‐eclampsia (five trials, 587 women: average RR 0.22, 95% CI 0.12 to 0.42; test for subgroup differences: Chi² = 6.81, df = 1, P = 0.009, I² = 85.3%; Analysis 2.2). Pre‐eclampsia was not reduced in the subgroup with adequate dietary calcium, but was for those with low baseline calcium intake (eight trials, 10,678 women: average RR 0.36, 95% CI 0.20 to 0.65; heterogeneity: Tau² = 0.44; Chi² = 29.35, df = 7, P = 0.0001; I² = 76%; Analysis 1.2). Assymetric funnel plots for these analyses suggest that the treatment effect may be overestimated due to small‐study effects or publication bias (Figure 7, Figure 8). There was also evidence of a subgroup difference between studies with small and larger samples sizes (test for subgroup differences: Chi² = 15.20, df = 1 (P < 0.0001), I² = 93.4%), Analysis 3.2.
1.2. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 2 Pre‐eclampsia.
2.2. Analysis.
Comparison 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, Outcome 2 Pre‐eclampsia.
7.
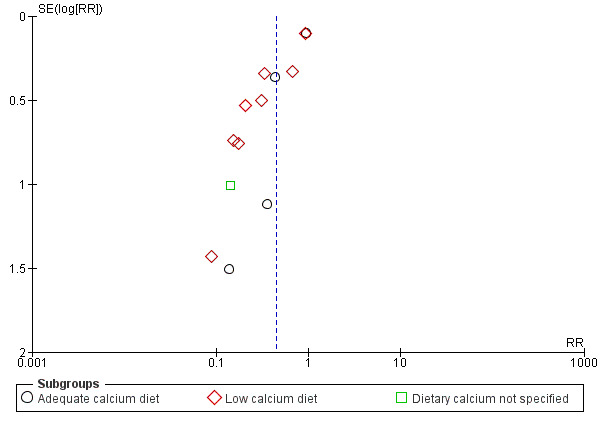
Funnel plot of comparison: 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, outcome: 1.2 Pre‐eclampsia.
8.
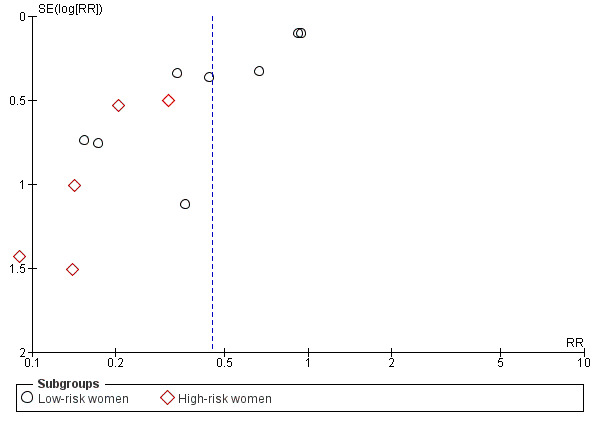
Funnel plot of comparison: 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, outcome: 2.2 Pre‐eclampsia.
3.2. Analysis.
Comparison 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, Outcome 2 Pre‐eclampsia.
When subgrouped by both dietary calcium intake and study size, the effect size appeared to be associated most strongly with study size (in the small studies, RR 0.21 for the low‐calcium trials and RR 0.26 for the adequate‐calcium trials; and in the large studies RR 0.63 and RR 0.70 respectively; Analysis 4.1; test for subgroup differences: Chi² = 10.28, df = 4 (P = 0.04), I² = 61.1%); Figure 9.
4.1. Analysis.
Comparison 4 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium and study sample size (not prespecified), Outcome 1 Pre‐eclampsia.
9.
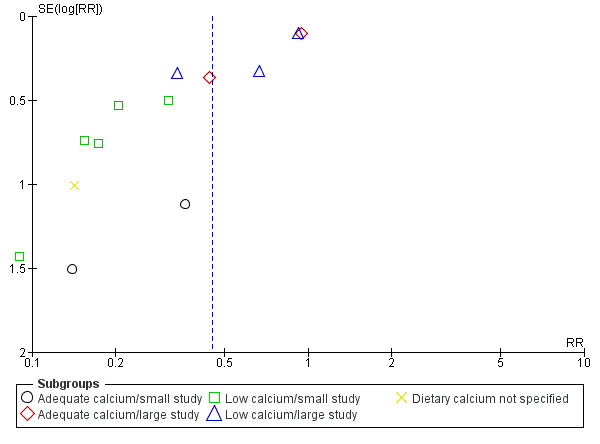
Funnel plot of comparison: 4 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium and study sample size (not pre‐specified), outcome: 4.1 Pre‐eclampsia.
Only one study included women with high risk of pre‐eclampsia and adequate dietary calcium (Villar 1990). The numbers were too small for meaningful statistical analysis (pre‐eclampsia in 0/90 participants with calcium, versus 3/88 participants with placebo).
(3) Preterm birth
Calcium supplementation reduced the average risk of preterm birth overall (11 trials 15,275 women: RR 0.76, 95% CI 0.60 to 0.97; low‐quality evidence; heterogeneity: Tau² = 0.05; Chi² = 20.04, df = 8 (P = 0.01); I² = 60%; Analysis 1.3), and amongst women at high risk of developing pre‐eclampsia recruited to four small trials (568 women: average RR 0.45, 95% CI 0.24 to 0.83; heterogeneity: Tau² = 0.00; Chi² = 1.73, df = 2, P = 0.42; I² = 0%; test for subgroup differences: Chi² = 3.48, df = 1 (P = 0.06), I² = 71.3%; Analysis 2.3). However, this reduction did not translate to a reduction in neonatal high care admissions of babies born < 2500 g. Asymmetric funnel plots for these analyses suggest that the treatment effect may be overestimated due to small‐study effects or publication bias (Figure 10, Figure 11, Figure 12). There was also evidence of a subgroup difference between studies with small and larger samples sizes (test for subgroup differences: Chi² = 4.90, df = 1 (P = 0.03), I² = 79.6%, Analysis 3.3).
1.3. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 3 Preterm birth.
2.3. Analysis.
Comparison 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, Outcome 3 Preterm birth.
10.
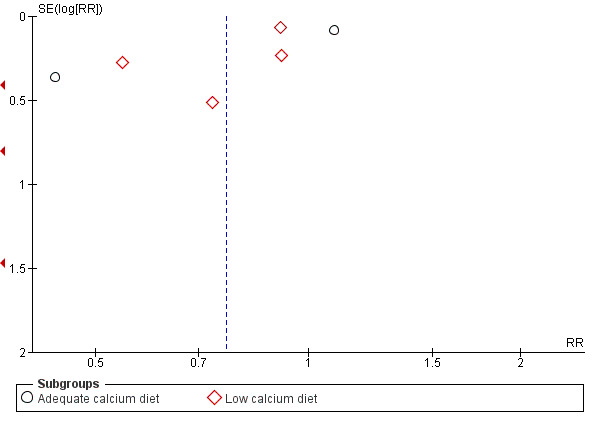
Funnel plot of comparison: 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, outcome: 1.3 Preterm birth.
11.
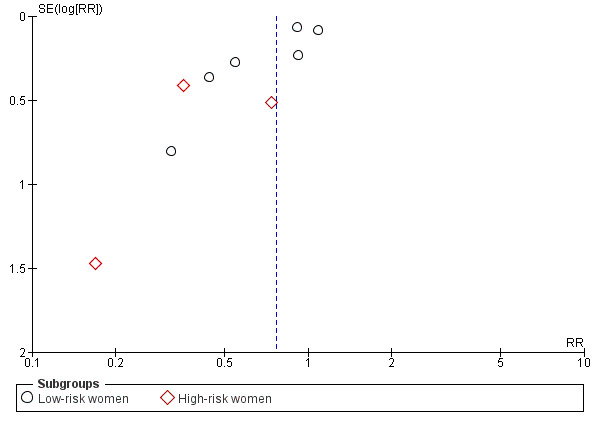
Funnel plot of comparison: 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, outcome: 2.3 Preterm birth.
12.
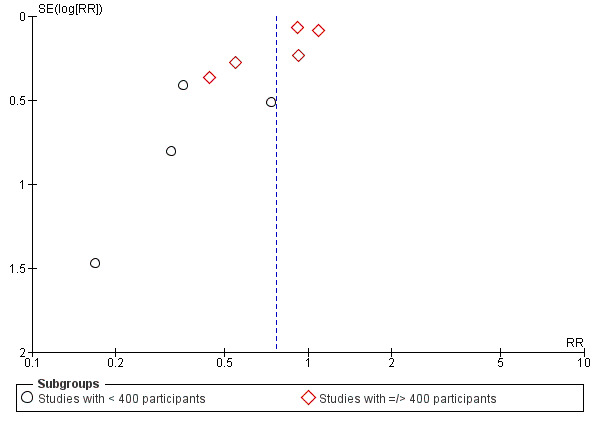
Funnel plot of comparison: 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, outcome: 3.3 Preterm birth.
3.3. Analysis.
Comparison 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, Outcome 3 Preterm birth.
(4) Admission to neonatal intensive care unit
There was no overall effect on the RR of admission to a neonatal intensive care unit (four trials, 13,406 women: RR 1.05, 95% CI 0.94 to 1.18; heterogeneity: Chi² = 2.83, df = 3 (P = 0.42); I² = 0%; Analysis 1.4).
1.4. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 4 Admission to neonatal intensive care unit.
(5) Stillbirth or death before discharge from hospital
There was no overall effect on the RR of a stillbirth or the baby dying before discharge from hospital (11 trials, 15,665 women: RR 0.90, 95% CI 0.74 to 1.09; heterogeneity: Chi² = 1.46, df = 5 (P = 0.92); I² = 0%; Analysis 1.5).
1.5. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 5 Stillbirth or death before discharge from hospital.
Secondary outcomes
(6) Maternal death or serious morbidity
The risk of maternal death or serious morbidity was reduced for women allocated to calcium supplementation compared with placebo (four trials, 9732 women: RR 0.80, 95% CI 0.66 to 0.98; Analysis 1.6). It should be noted that all events were restricted to one trial (WHO 2006) as the other three trials did not have any events.
1.6. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 6 Maternal death/serious morbidity.
(7) Placental abruption
In the five trials reporting this outcome, there was no clear difference between the groups (14,336 women: RR 0.86, 95% CI 0.55 to 1.34; heterogeneity: Chi² = 0.91, df = 2 (P = 0.63); I² = 0%; Analysis 1.7).
1.7. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 7 Placental abruption.
(8) Caesarean section
There was a reduction in caesarean section for women in the calcium group (eight trials, 15,234 women: RR 0.95, 95% CI 0.89 to 1.02; heterogeneity: Chi² = 5.21, df = 7 (P = 0.63); I² = 0%; Analysis 1.8), although the upper confidence limit just crossed the line of no effect.
1.8. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 8 Caesarean section.
(9) *Proteinuria
Only one trial reported proteinuria (WHO 2006), and there was no overall difference between the groups (8312 women: RR 1.04, 95% CI 0.86 to 1.26; Analysis 1.9).
1.9. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 9 Proteinuria (gestational with no proteinuria).
(10) *Severe pre‐eclampsia as defined by trial authors
Only one trial reported severe pre‐eclampsia (WHO 2006). Again, there was no clear difference between the groups (one trial, 8302 women: RR 0.74, 95% CI 0.48 to 1.15; Analysis 1.10).
1.10. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 10 Severe pre‐eclampsia.
(11) *Eclampsia
The two largest trials reported eclampsia (CPEP 1997; WHO 2006), as well as Kumar 2009. There was no clear difference between the groups (three trials, 13,425 women: RR 0.73, 95% CI 0.41 to 1.27; Analysis 1.11).
1.11. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 11 Eclampsia.
(12) *HELLP syndrome
Only the two largest studies reported HELLP syndrome (CPEP 1997; WHO 2006). The RR was higher for women allocated calcium supplementation, compared with placebo (two trials, 12,901 women: RR 2.67, 95% CI 1.05 to 6.82; high‐quality evidence; heterogeneity: Chi² = 0.19, df = 1 (P = 0.66); I² = 0%; Analysis 1.12).
1.12. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 12 HELLP syndrome.
(13) *Maternal intensive care unit admission
Only one trial reported admission to intensive care (WHO 2006). There was no clear difference between the groups (one trial, 8312 women: RR 0.84, 95% CI 0.66 to 1.07; Analysis 1.13).
1.13. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 13 Intensive care unit admission.
(14) *Maternal death
Only one trial reported maternal deaths (WHO 2006). There were few events in any group, with only one death in the calcium group and six in the placebo group (one trial, 8312 women: RR 0.17, 95% CI 0.02 to 1.39; Analysis 1.14).
1.14. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 14 Maternal death.
(15) Low birthweight (birthweight less than 2500 g)
Women in the calcium group were at reduced risk of having a baby with birthweight less than 2500 g (nine trials, 14,883 women: average RR 0.85, 95% CI 0.72 to 1.01; heterogeneity: Tau² = 0.02; Chi² = 9.93, df = 5 (P = 0.08); I² = 50%; Analysis 1.15), although the overall effect estimate just crossed the line of no effect.
1.15. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 15 Low birthweight (birthweight < 2500 g).
(16) Neonate small‐for‐gestational age
There was no overall effect on the RR of the baby being born small‐for‐gestational age (four trials, 13,615 women: RR 1.05, 95% CI 0.86 to 1.29; heterogeneity: Chi² = 2.74, df = 3 (P = 0.43); I² = 0%; Analysis 1.16).
1.16. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 16 Neonate small‐for‐gestational age as defined by trial authors.
(17) Childhood systolic blood pressure > 95th percentile
One trial assessed during childhood a subset of the children recruited whilst in utero (Belizan 1991). At about seven years of age, diastolic blood pressure greater than 95th percentile was reduced (514 women: RR 0.59, 95% CI 0.39 to 0.91; Analysis 1.17). While the baseline calcium intake in the original study was low (mean 646 mg, standard deviation (SD) 396 in the calcium group; and mean 642 mg, SD 448 in the placebo group, in a sample assessed during the first four months of the study), the group followed up were only from among the 614 women from the private hospital, not the 580 from the public hospitals. Their dietary calcium intake may have differed from the mean (i.e. it is more likely to be higher in more affluent women). The baseline calcium status of the women in this part of the study therefore cannot be classified.
1.17. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 17 Systolic blood pressure > 95th percentile during childhood.
In Crowther 1999, a follow‐up of mothers and offspring was conducted four to seven years later (45% of the original participants) and reported in Hiller 2007. Childhood blood pressure was reported as a continuous variable. It was concluded that calcium supplementation during pregnancy may lower the mean blood pressure of the children of women with hypertension in pregnancy. We have sought additional unpublished data from the authors which may be available/suitable for inclusion in the next update.
A limited follow‐up of mothers and infants from CPEP 1997, found reduced systolic blood pressure at two years of age in the calcium supplementation group (mean 95.4 mmHg, SD 7.6, n = 35 versus 100.2, 7.9, n = 18). We have not included the data in this review because the low and unequal follow‐up rate (35 and 18 from 497 invited to participate) limits the reliability of the results. In another report of CPEP 1997 (Hatton 2003), reduced systolic blood pressure was found in the offspring of the calcium supplementation group at two years of age. We have not included these data either because of the high losses to follow‐up.
A subsequent report of the Gambian trial (Jarjou 2004) found no significant difference in systolic blood pressure in 64% of the original trial offspring at between five and 10 years of age. This analysis was restricted to children born at term and the relevant data were not available for our meta‐analysis.
(18) Childhood diastolic blood pressure > 95th percentile
Data were available only from one study (Belizan 1991). There was no difference between groups (Analysis 1.18).
1.18. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 18 Diastolic blood pressure > 95th percentile during childhood.
(19) Dental caries in childhood
In one study (Belizan 1991), dental caries was assessed at 12 years of age in a subset of those enrolled. It is was not specified how this subset was randomly selected. As this was a post hoc outcome for this review, the data should be interpreted with caution. The study found a significant reduction in dental caries, defined as at least one decayed, filled or missing tooth (one trial, 195 children: RR 0.73, 95% CI 0.62 to 0.87; Analysis 1.19).
1.19. Analysis.
Comparison 1 Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, Outcome 19 Dental caries in childhood.
(20) Non‐prespecified outcomes (comparison 5)
Sub‐studies of WHO 2006 found no effect of calcium supplementation on uterine or umbilical artery resistance index or ultrasound estimates of fetal growth at 32 weeks; or platelet count, uric acid or urine protein/creatinine ratio at 35 weeks.
Sensitivity analysis
One study contributing data to this analysis was assessed to be at high risk of attrition bias (L‐Jaramillo 1989). Removing these study data from the analyses for high blood pressure (with or without proteinuria) (Analysis 1.1; Analysis 2.1; Analysis 3.1); pre‐eclampsia (Analysis 1.2; Analysis 2.2; Analysis 3.2; Analysis 4.1); stillbirth or death before discharge from hospital (Analysis 1.5; Analysis 2.5; Analysis 3.5); and low birthweight (Analysis 1.15) made little or no difference to the overall effect estimates.
2.1. Analysis.
Comparison 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, Outcome 1 High blood pressure (with or without proteinuria).
2.5. Analysis.
Comparison 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, Outcome 5 Stillbirth or death before discharge from hospital.
3.5. Analysis.
Comparison 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, Outcome 5 Stillbirth or death before discharge from hospital.
Low‐dose calcium supplementation with or without cointerventions, versus no calcium supplementation
We included nine studies with 2234 participants. The risk of bias was variable, and studies included those with and without co‐interventions.
Data were not available for the following secondary outcomes: maternal death or serious morbidity; proteinuria; HELLP syndrome; intensive care unit admission; mother's hospital stay seven days or more: neonate in intensive care unit seven days or more; death or severe neonatal morbidity; childhood disability; systolic blood pressure greater than 95th percentile during childhood; diastolic blood pressure greater than 95th percentile during childhood; dental caries in childhood.
Primary outcomes
(1) High blood pressure with or without proteinuria
Calcium supplementation was associated with a reduction in high blood pressure in five studies (665 women, RR 0.53, 95% CI 0.38 to 0.74; heterogeneity: Chi² = 2.55, df = 4 (P = 0.64); I² = 0%; test for subgroup differences: Chi² = 2.11, df = 2 (P = 0.35), I² = 5.2%; Analysis 6.1), including three studies of calcium supplementation alone (558 women, RR 0.57, 95% CI 0.39 to 0.82) and one of calcium plus linoleic acid (48 women, RR 0.20, 95% CI 0.05 to 0.82).
6.1. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 1 High blood pressure (with or without pre‐eclampsia).
(2) Pre‐eclampsia
Pre‐eclampsia was reduced with low‐dose calcium supplementation (nine studies, 2234 women, RR 0.38, 95% CI 0.28 to 0.52; I² = 0%; Analysis 6.2). The reduction was also consistent across the following subgroups: calcium alone (four studies, 980 women, RR 0.36, 95% CI 0.23 to 0.57); calcium plus linoleic acid (two studies, 134 women, RR 0.23, 95% CI 0.09 to 0.60); calcium plus vitamin D, (two studies, 1060 women: RR 0.49, 95% CI 0.31 to 0.78; 1060 women; I² = 17%) and in one trial of calcium plus antioxidants with low risk of bias (60 women, RR 0.24, 95% CI 0.06 to 1.01). Test for subgroup differences: Chi² = 2.55, df = 3 (P = 0.47), I² = 0%.
6.2. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 2 Pre‐eclampsia.
(3) Preterm birth
Overall, there was little or no effect on preterm birth for calcium with other supplements (six studies, 1290 women: average RR 0.83, 95% CI 0.34 to 2.03; I² = 64%; Analysis 6.3). Preterm birth was reduced in the experimental arm of one study of calcium supplementation alone (422 women, average RR 0.40, 95% CI 0.21 to 0.75; Analysis 6.3), but as it was not reported in the other three studies of calcium supplementation alone, the possibility of publication bias needs to be considered. For calcium plus vitamin D, the risk of preterm birth seemed to be increased with supplements (three studies, 760 women: RR 1.59, 95% CI 1.03 to 2.45; I² = 0%; Analysis 6.3).
6.3. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 3 Preterm birth.
(4) Admission to neonatal intensive care unit (ICU)
Admission to neonatal ICU was reported in only one trial of calcium supplementation alone, so the reduction in the calcium group may be due to publication bias (422 women: RR 0.44; 95% CI 0.20 to 0.99; Analysis 6.4).
6.4. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 4 Admission to neonatal intensive care unit.
(5) Stillbirth or death before discharge from hospital
There was no overall effect on the RR of a stillbirth or the baby dying before discharge from hospital (five trials, 1025 women: RR 0.48, 95% CI 0.14 to 1.67; heterogeneity: Chi² = 0.99, df = 4 (P = 0.91); I² = 0%; Analysis 6.5).
6.5. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 5 Stillbirth or death before discharge.
Secondary outcomes
(6) Placental abruption
Three studies reported this outcome and the event rates were too small for meaningful analysis (160 participants: RR 1.00, 95% CI 0.14 to 6.90; Analysis 6.6).
6.6. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 6 Placental abruption.
(7) Caesarean section
Caesarean section was reduced in two studies of calcium plus linoleic acid (134 women: average RR 0.55; 95% CI 0.35 to 0.87; heterogeneity: Tau² = 0.00; Chi² = 0.03, df = 1 (P = 0.86); I² = 0%), but not overall (four studies, 521 women, average RR 0.73; 95% CI 0.46 to 1.15; heterogeneity: Tau² = 0.13; Chi² = 7.48, df = 3 (P = 0.06); I² = 60%); Analysis 6.7.
6.7. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 7 Caesarean section.
(8) Severe pre‐eclampsia
Four trials reported severe pre‐eclampsia and there was no clear difference between the groups (246 participants: RR 0.40, 95% CI 0.14 to 1.15; I2 = 0%; Analysis 6.8).
6.8. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 8 Severe pre‐eclampsia.
(9) Eclampsia
One trial of calcium supplementation alone reported eclampsia. There was no clear difference between the groups (168 women: RR 0.17, 95% CI 0.01 to 4.06; Analysis 6.9).
6.9. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 9 Eclampsia.
(10) Miscarriage (non‐prespecified)
An unexpected finding in one small trial of calcium plus antioxidants, commencing at eight to 12 weeks of pregnancy, was a reduction in miscarriage in the calcium plus antioxidant group (60 women: RR 0.06, 95% CI 0.00 to 1.04; Analysis 6.10).
6.10. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 10 Miscarriage.
(11) Low birthweight (birthweight less than 2500 g)
The risk of having a baby with birthweight less than 2500 g was reduced with calcium supplementation plus linoleic acid (two studies, 134 women: RR 0.20, 95% CI 0.05 to 0.88; heterogeneity: Chi² = 0.00, df = 1 (P = 1.00); I² = 0%; Analysis 6.11).
6.11. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 11 Low birthweight (birthweight < 2500 g).
(12) Neonate small‐for‐gestational age
There was no overall effect on the risk of the baby being born small‐for‐gestational age (four trials, 854 women: RR 0.81, 95% CI 0.54 to 1.21; heterogeneity: Chi² = 2.06, df = 3 (P = 0.56); I² = 0%; Analysis 6.12).
6.12. Analysis.
Comparison 6 Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment, Outcome 12 Neonate small‐for‐gestational age.
Sensitivity analysis
We had planned to exclude studies assessed to have inadequate allocation concealment or high risk of attrition bias, or both. However, five studies either did not conceal allocation (Marya 1987), had high levels of attrition (Asemi 2012; Asemi 2016; Bassaw 1998), or both (Rogers 1999) and we did not feel that removing these would result in a meaningful analysis. If further studies are added in future updates we will perform the sensitivity analysis.
High‐dose (=/> 1 g) versus low‐dose (< 1 g) calcium supplements (one trial with 262 women)
Only a single trial with 262 women is included in this dose comparison and results were reported for only four of our prespecified outcomes. The risk of pre‐eclampsia appeared to be reduced in the high‐dose group compared to the low‐dose group (RR 0.42, 95% CI 0.18 to 0.96; Analysis 7.1); for other outcomes, although results were in the same direction, there was insufficient evidence to demonstrate any clear difference between groups (preterm birth: RR 0.31, 95% CI 0.09 to 1.08, Analysis 7.2; eclampsia: RR 0.32, 95% CI 0.07 to 1.53, Analysis 7.3; stillbirth: RR 0.48, 95% CI 0.13 to 1.83, Analysis 7.4).
7.1. Analysis.
Comparison 7 High‐dose (=/> 1 g) vs low‐dose (< 1 g) calcium supplements, Outcome 1 Pre‐eclampsia.
7.2. Analysis.
Comparison 7 High‐dose (=/> 1 g) vs low‐dose (< 1 g) calcium supplements, Outcome 2 Preterm birth.
7.3. Analysis.
Comparison 7 High‐dose (=/> 1 g) vs low‐dose (< 1 g) calcium supplements, Outcome 3 Eclampsia.
7.4. Analysis.
Comparison 7 High‐dose (=/> 1 g) vs low‐dose (< 1 g) calcium supplements, Outcome 4 Stillbirth.
Discussion
Summary of main results
High‐dose calcium supplementation
Our meta‐analysis showed that calcium supplementation with at least 1 g of calcium approximately halved the risk of pre‐eclampsia compared to placebo, with the confidence intervals estimating the true effect to be a risk reduction of between 35% and 69%. Women with an adequate dietary intake of calcium were the only subgroup where the confidence interval crossed the line of no effect. Nevertheless, the point estimate for this subgroup of women was a 38% risk reduction. The greatest risk reduction was for women at high risk of pre‐eclampsia (variably defined; 78% reduction). The meta‐analysis may be an over‐estimate, as funnel plot asymmetry suggested a small‐study effect or publication bias. In the largest study, the 8% reduction in pre‐eclampsia had a confidence interval that crossed the line of no effect.
There was also a 35% reduction in the risk of gestational hypertension, with the greatest effect also amongst women with low calcium diets. These data should be interpreted with caution because of the possibility of small‐study effects or publication bias.
The risk of having the composite outcome 'maternal death or severe morbidity' was reduced by 20% with calcium supplementation and there was a 24% reduction in the risk of preterm birth.
The risk of haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome was increased; however the absolute number of events was low (2.5/1000 versus 0.9/1000). There were no clear effects on other relevant outcomes at discharge from hospital. There were no clear differences between calcium supplementation and placebo for any other outcomes, although for several outcomes the confidence intervals approach statistical significance, e.g. for caesarean section a small (5%) reduction in risk associated with calcium supplementation is possible. For stillbirth and death before discharge from hospital, the point estimate is for a reduction of 10%, although no effect or a small increase in risk has not been excluded.
Low‐dose calcium supplementation
The results with low‐dose calcium supplementation alone are similar to those of the smaller studies with high‐dose supplementation, showing a reduction in pre‐eclampsia of about 60% which was consistent between studies with low and high risk of bias. High blood pressure with or without proteinuria was reduced by about 50%. Results for calcium plus other interventions were similar to those for calcium alone, but the possibility that the cointerventions contributed to the effect on pre‐eclampsia needs to be considered. For antioxidants, this is unlikely as antioxidants have not been found to reduce the risk of pre‐eclampsia (Rumbold 2008). For vitamin D there is as yet inadequate evidence regarding its effect on pre‐eclampsia (De‐Regil 2012); for linoleic acid there is also insufficient evidence. There were no other differences, other than a reduction in neonatal intensive care admission and low birthweight in the low‐dose calcium supplementation groups. These outcomes were reported only by one or two studies, suggesting reporting bias.
High‐dose versus low‐dose calcium supplementation
One small study, which had limitations, found a reduction in pre‐eclampsia with 2 g calcium daily versus 500 mg daily.
Overall completeness and applicability of evidence
We consider the evidence in favour of high‐dose calcium supplementation with respect to reducing the risk of pre‐eclampsia to be complete, particularly in women with low calcium diets and those at high risk. Although there were not individually significant reductions in the risk of death, severe pre‐eclampsia, eclampsia, or admission to intensive care, the point estimates for these outcomes favoured calcium supplementation, and so moderate reductions in these outcomes remain possible. The compound outcome death or severe morbidity was significantly reduced by 20%.
Few side effects were recorded in the included trials. In two trials, the risk of HELLP syndrome was increased with calcium supplementation. A possible explanation for this apparently anomalous finding is that calcium supplementation in the second half of pregnancy may only reduce blood pressure rather than the underlying pre‐eclamptic process. Lower blood pressures in the calcium group may have reduced the diagnosis of pre‐eclampsia and, thus, medical interventions to curtail pregnancy, allowing more time for the pre‐eclampsia to progress to HELLP syndrome (Hofmeyr 2007).
There remains little information about the long‐term follow‐up of children exposed to calcium in utero. One study evaluated childhood systolic hypertension and dental caries. The risk of both of these outcomes was significantly reduced, however, the latter effect was observed in a small subset of the children and the study was not originally designed to assess this outcome. These effects therefore need corroboration.
There is no information about any possible changes in the use of healthcare resources associated with calcium supplementation. It would seem plausible that a reduction in gestational hypertension and pre‐eclampsia might lead to fewer antenatal visits, less antepartum hospital admissions and fewer inductions of labour. However, the included trials do not provide data on these outcomes.
This 2018 update has included data from trials using less than 1 g calcium daily (mostly 500 mg to 600 mg). Over half of these studies were at high risk of bias and combined calcium with other supplements. However, the evidence seems to indicate that lower doses of calcium may be effective in reducing hypertensive disorders of pregnancy. The results of the low‐dose studies is therefore incomplete and need corroboration by larger high‐quality studies.
Quality of the evidence
We consider the evidence for the effect of high‐dose calcium supplementation on pre‐eclampsia and preterm birth to be low, due to heterogeneity and small study effects. We deemed the evidence for HELLP syndrome to be of high quality (see Table 1). The quality of evidence for the large studies subgroup was also high.
In general, heterogeneity of findings seemed to be largely associated with study size, with the small studies having the most positive results (see Figure 4 to Figure 12). These small‐study effects may indicate publication bias or other biases, or be caused by differences between small and large studies. As the small studies tended to recruit high‐risk women, at least some of the heterogeneity may be explained by calcium having a greater effect for high‐risk women. These data on heterogeneity related to sample size should be interpreted with caution however, as the sensitivity analysis was post‐hoc, and the cut‐off point for sample size (400) was arbitrary.
Potential biases in the review process
We sought to minimise potential biases in the review process by having clearly‐defined criteria for inclusion of studies, and by excluding members of the review team from decisions regarding studies in which they were involved. Post hoc inclusions are clearly identified, and acknowledged as a potential source of bias.
Agreements and disagreements with other studies or reviews
This evidence of a modest risk reduction in gestational hypertension and 'maternal deaths and serious morbidity' contrasts with the large epidemiological differences previously identified between populations with adequate and low dietary calcium intake (Belizan 1980; Hamlin 1952; Hamlin 1962). Possible explanations include the following.
Dietary calcium may be a marker for other aetiological factors.
Starting supplementation in the middle trimester of pregnancy may be too late to be fully effective.
The finding of reduced childhood hypertension needs replication but, if corroborated, has far‐reaching implications for public health. Although based on only a partial follow‐up in one study (Belizan 1991), this finding is supported by a very limited follow‐up in two other studies (CPEP 1997; Crowther 1999), as well as observational (McGarvey 1991), and animal (Bergel 2002) studies.
There are concerns regarding possible adverse effects of calcium supplementation, which may be dose‐related. Long‐term calcium use in later life has been associated with myocardial infarction, however the association may not be causal (Li 2012). In addition, in a 2010 publication of the Gambia study in which women received calcium supplementation of 1.5 g during pregnancy (Jarjou 2004), investigators reported reduced bone density in the women postpartum. They suggest that high‐dose calcium during pregnancy might reverse metabolic adaptation to long‐term low calcium diets, resulting in a rebound effect when withdrawn. This finding was based on a selected follow‐up and was opposite to the prior hypothesis and therefore needs confirmation in a prospective study.
Authors' conclusions
Implications for practice.
The possible reduction in hypertension, pre‐eclampsia and preterm birth (low‐quality evidence), and the reduction in the composite outcome 'maternal death or severe morbidity' (high‐quality evidence) with high‐dose calcium supplementation should be considered when making decisions about the use of calcium supplementation during pregnancy, particularly for those with low dietary intake or high risk of pre‐eclampsia. Based on evidence included in the previous version of this review, which was limited to high‐dose calcium supplementation, the World Health Organization recommends a calcium dose of 1.5 g to 2 g during pregnancy for women with low dietary calcium intake (WHO 2011). However, this recommendation may be associated with logistical difficulties in low‐income countries: calcium is relatively expensive, and the tablets are bulky and heavy (about 1 kg for a 20‐week supply of calcium carbonate and glycine, providing 1.5 g elemental calcium daily). Implementation may be subject to competing priorities in low‐resource settings. The current updated review adds limited data on the effects of lower calcium dosage, which may guide pragmatic decisions in settings where high‐dose supplementation is unachievable.
The increase in the risk of haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome was small in terms of absolute numbers. Therefore, we consider this limitation to be outweighed by the overall reduction in death or severe morbidity which is associated with calcium supplementation.
The one study which enrolled women with high risk of pre‐eclampsia and adequate dietary calcium was too small to guide practice.
Evidence on the effects of calcium supplementation combined with low‐dose aspirin (not included in this review) should also be considered.
Implications for research.
Epidemiological studies have found a difference in dietary calcium intake between high‐ and low‐income settings of about 500 mg. Doses of 1.5 g/day and higher are well above daily recommended dietary calcium intake. Some women find it difficult to swallow or chew three to four large tablets daily, which may affect adherence. Furthermore, doses in excess of 800 mg daily may inhibit iron absorption. Therefore, further research is necessary to determine the optimal dose of calcium supplements in pregnancy. It would also be relevant to assess whether supplementation via dietary modification, for women with low calcium intake, has the same benefits as the tablets administered in these trials.
Further research is needed to determine the effectiveness of calcium supplementation in women with high risk of pre‐eclampsia and adequate dietary calcium.
Further research is also needed to provide reassurance that calcium supplementation during pregnancy does not have any adverse effects for the children exposed whilst in utero, and to verify whether it reduces childhood hypertension.
The increase in the risk of HELLP syndrome identified by this review requires further investigation. Any future trials should also collect information about the use of health service resources, as well as other clinical outcomes.
In most of the studies reviewed, supplementation was commenced around 20 weeks of pregnancy. In one small trial of low‐dose calcium supplementation, commencing at eight to 12 weeks in high‐risk women, there was an unanticipated trend to reduced miscarriage. This interesting observation needs to be explored further in prospective research.
We have hypothesised, based on the finding in this review of no effect of calcium supplementation on proteinuria, that the benefits of calcium supplementation in the second half of pregnancy may be the result of a direct lowering effect on blood pressure, and that supplementation may be needed from before pregnancy to affect the genesis of pre‐eclampsia during placental development. This hypothesis is addressed in a separate review (Hofmeyr 2017).
Feedback
Stones, 7 December 2010
Summary
Noting that public health programs are now starting to include calcium supplementation, I wonder if the statements in the abstract and plain language summary that "there were no other clear benefits, or harms"/"No adverse effects have been found" should be revised to include mention of the increased risk of HELLP syndrome associated with calcium supplementation. At the very least it would prompt programmers to include surveillance and reporting for this life threatening complication and would help to clarify whether this is a real association.
(Feedback submitted by William Stones, December 2010)
Reply
We agree with the above feedback. We have added emphasis to the effect on HELLP syndrome to the discussion, and added to "Implications for practice": "..........The increase in the risk of HELLP syndrome was small in terms of absolute numbers, and therefore we considered it to be outweighed by the overall reduction in death or severe morbidity; and to "Implications for research": "The increase in the risk of HELLP syndrome identified by this review requires further investigation."
To the abstract results we have added "There was an anomalous increase in the risk of HELLP syndrome (two trials, 12,901 women: RR 2.67, 95% CI 1.05 to 6.82)."; and to the abstract conclusions we have added "We considered the latter benefit to outweigh the increase in HELLP syndrome, which was small in absolute numbers".
Contributors
Feedback: William Stones
Reply: G Justus Hofmeyr
Walkinshaw, 2 November 2010
Summary
I feel that the conclusion drawn for high‐risk women go beyond the data. Five trials are cited for high‐risk women. Of these one trial assessed risk by roll over test, another by roll over test plus angiotensin II infusion, and a third by roll over test plus at least one risk factor. All three of these trials excluded chronic medical conditions. For the two other trials, data for high‐risk women come either from a subgroup analysis or are unpublished data. Villar 1990 includes mainly nulliparous women and excluded medical disease; L‐Jaramillo 1990 includes nulliparous women and also excludes underlying medical disease. Thus three of the five trials do not describe high risk in any meaningfully clinically translatable way, and exclude the highest risk women (such as those with previous pre‐eclampsia, chronic hypertension, or renal disease). The two additional studies also largely exclude clinical high‐risk factors.
To draw a broad conclusion using the very impressive risk reduction in 'high risk' from this is not really translatable to clinical high risk. I think it will confuse clinicians, who will not look at the detail of the trials used and assume that high risk means the usual suspects, when it manifestly does not. The authors should consider some caveat to their conclusion. I actually think the current conclusion misleads. During the genesis of the NICE guidance we looked in some detail at this to determine if there was evidence of benefit for clinically high‐risk women, and concluded that at present those studies had not been performed. I do not feel that it is enough to rely on studies selecting women using research techniques to assess risk.
The issue in low‐risk women is more contentious and I make no comment on that part.
(Summary of comment from Stephen Walkinshaw, Obstetrician and Chair of NICE guideline development group for Hypertension in Pregnancy, November 2010)
Reply
We agree with the points made, and have added the following to the results section: "Five studies enrolled women considered to be at high risk of pre‐eclampsia. The definitions of high risk and the actual risk (rate of pre‐eclampsia in the placebo group) were variable: positive 'roll‐over test at 28‐30 weeks (8/34) (L‐Jaramillo 1990); teenagers 17 years or younger (3/88) (Villar 1990); positive 'roll‐over' test at 28‐32 weeks plus one clinical risk factor (7/15) (Niromanesh 2001); positive 'roll‐over' and positive angiotensin II infusion test (15/34) (S‐Ramos 1994); and nulliparous teenagers 17.5 years or younger (21/135) (L‐Jaramillo 1997). The clinical usefulness of the pooled results in this subgroup is therefore limited." To the abstract we have added: "The varied methods of selecting women as being at high‐risk limit the clinical usefulness of these pooled results."
Contributors
Feedback: Stephen Walkinshaw
Reply: G Justus Hofmeyr
What's new
| Date | Event | Description |
|---|---|---|
| 18 September 2017 | New search has been performed | The search was updated and three new trials included (Asemi 2012; Asemi 2016; Khan 2013). A new comparison of high‐dose versus low‐dose calcium has been incorporated into this update. |
| 18 September 2017 | New citation required but conclusions have not changed | The review includes a total of 27 trials. The conclusions remain unchanged. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 24 May 2013 | New citation required and conclusions have changed | Eleven studies have been included for this update (Almirante 1998; Bassaw 1998; Cong 1995; Herrera 1998; Herrera 2006; Jarjou 2004; Li 2000; Marya 1987; Rogers 1999; Rumiris 2006; Taherian 2002).Ten studies of low‐dose calcium added. New meta‐analyses performed. Substantially changed conclusions. Search updated in May 2014, six reports added to Studies awaiting classification (Asemi 2012b; Diogenes 2013; Goldberg 2013; Herrera 2006b; Jarjou 2013; Sulovic 2013a). |
| 28 March 2013 | New search has been performed | Search updated. Methods updated. |
| 6 January 2011 | Feedback has been incorporated | Feedback from William Stones and Stephen Walkinshaw added with replies from the authors. |
| 5 July 2010 | New citation required but conclusions have not changed | New author helped to update the review. |
| 31 May 2010 | New search has been performed | Search updated. Fifteen new reports identified: one new study (Kumar 2009) included and four new trials excluded (de Souza 2006; Dizavandy 1998; Herrera 1998a; Karandish 2003). |
| 31 October 2009 | Amended | Search updated. Fourteen new reports added to Studies awaiting classification. |
| 1 September 2008 | Amended | Converted to new review format. |
| 2 March 2006 | New search has been performed | Search updated. |
| 2 March 2006 | New citation required and conclusions have changed | A large trial of calcium supplementation in communities with low dietary calcium intake has been added (WHO 2006). |
Acknowledgements
We thank the trial authors who have contributed additional data for this review. We also thank Maria Kalousi and Oliver Boothroyd for their assistance with the translation of certain articles to English, and the Cochrane Pregnancy and Childbirth team for administrative support. We particularly thank Therese Dowswell for considerable assistance with data extraction and analysis for the 2018 revision. We thank Lelia Duley for her contribution to previous versions of this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
ICTRP
antenatal AND calcium
prenatal OR calcium
pregnancy AND calcium
ClinicalTrials.gov
(Advanced search)
Intervention studies
calcium = intervention
pregnancy = condition
Data and analyses
Comparison 1. Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 High blood pressure (with or without proteinuria) | 12 | 15470 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.53, 0.81] |
| 1.1 Adequate calcium diet | 4 | 5022 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 1.2 Low calcium diet | 7 | 10418 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.70] |
| 1.3 Dietary calcium not specified | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.45] |
| 2 Pre‐eclampsia | 13 | 15730 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.31, 0.65] |
| 2.1 Adequate calcium diet | 4 | 5022 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.32, 1.20] |
| 2.2 Low calcium diet | 8 | 10678 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.65] |
| 2.3 Dietary calcium not specified | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.02] |
| 3 Preterm birth | 11 | 15275 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.97] |
| 3.1 Adequate calcium diet | 4 | 5033 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.33] |
| 3.2 Low calcium diet | 7 | 10242 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.02] |
| 4 Admission to neonatal intensive care unit | 4 | 13406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.18] |
| 4.1 Adequate calcium diet | 1 | 4336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 4.2 Low calcium diet | 3 | 9070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 5 Stillbirth or death before discharge from hospital | 11 | 15665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 5.1 Adequate calcium diet | 4 | 5033 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.66, 1.90] |
| 5.2 Low calcium diet | 7 | 10632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.07] |
| 6 Maternal death/serious morbidity | 4 | 9732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.98] |
| 6.1 Low calcium diet | 4 | 9732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.98] |
| 6.2 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Placental abruption | 5 | 14336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.55, 1.34] |
| 7.1 Adequate calcium diet | 3 | 4830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.39, 1.68] |
| 7.2 Low calcium diet | 2 | 9506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.55] |
| 8 Caesarean section | 8 | 15234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.02] |
| 8.1 Adequate calcium diet | 3 | 4981 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 8.2 Low calcium diet | 5 | 10253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.04] |
| 9 Proteinuria (gestational with no proteinuria) | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 9.1 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Low calcium diet | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 10 Severe pre‐eclampsia | 1 | 8302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.15] |
| 10.1 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Low calcium diet | 1 | 8302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.15] |
| 11 Eclampsia | 3 | 13425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.27] |
| 11.1 Adequate calcium diet | 1 | 4589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 3.99] |
| 11.2 Low calcium diet | 2 | 8836 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.37, 1.26] |
| 12 HELLP syndrome | 2 | 12901 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.05, 6.82] |
| 12.1 Adequate calcium diet | 1 | 4589 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.73, 16.82] |
| 12.2 Low calcium diet | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.70, 7.32] |
| 13 Intensive care unit admission | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 13.1 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Low calcium diet | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 14 Maternal death | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.39] |
| 14.1 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Low calcium diet | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.39] |
| 15 Low birthweight (birthweight < 2500 g) | 9 | 14883 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 15.1 Adequate calcium diet | 4 | 5033 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.31, 1.13] |
| 15.2 Low calcium diet | 5 | 9850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.05] |
| 16 Neonate small‐for‐gestational age as defined by trial authors | 4 | 13615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.29] |
| 16.1 Adequate calcium diet | 1 | 4589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.92, 1.52] |
| 16.2 Low calcium diet | 3 | 9026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 17 Systolic blood pressure > 95th percentile during childhood | 1 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.91] |
| 17.1 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Low calcium diet | 1 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.91] |
| 18 Diastolic blood pressure > 95th percentile during childhood | 1 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 18.1 Adequate calcium diet | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Low calcium diet | 1 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 19 Dental caries in childhood | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.87] |
| 19.1 Low calcium diet | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.87] |
Comparison 2. Routine high‐dose calcium supplementation in pregnancy by hypertension risk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 High blood pressure (with or without proteinuria) | 12 | 15470 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.53, 0.81] |
| 1.1 Low‐risk women | 8 | 15143 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.89] |
| 1.2 High‐risk women | 4 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 0.97] |
| 2 Pre‐eclampsia | 13 | 15730 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.31, 0.65] |
| 2.1 Low‐risk women | 8 | 15143 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.83] |
| 2.2 High‐risk women | 5 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.42] |
| 3 Preterm birth | 11 | 15275 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.97] |
| 3.1 Low‐risk women | 7 | 14707 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.05] |
| 3.2 High‐risk women | 4 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.83] |
| 4 Admission to neonatal intensive care unit | 4 | 13406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.18] |
| 4.1 Low‐risk women | 3 | 13343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.19] |
| 4.2 High‐risk women | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.48] |
| 5 Stillbirth or death before discharge from hospital | 11 | 15665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 5.1 Low‐risk women | 8 | 15153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 5.2 High‐risk women | 3 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 9.20] |
2.4. Analysis.
Comparison 2 Routine high‐dose calcium supplementation in pregnancy by hypertension risk, Outcome 4 Admission to neonatal intensive care unit.
Comparison 3. Routine high‐dose calcium supplementation in pregnancy by study sample size.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 High blood pressure (with or without proteinuria) | 12 | 15470 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.53, 0.81] |
| 1.1 Studies with < 400 participants | 7 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.68] |
| 1.2 Studies with =/> 400 participants | 5 | 14795 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 2 Pre‐eclampsia | 13 | 15730 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.31, 0.65] |
| 2.1 Studies with < 400 participants | 8 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.36] |
| 2.2 Studies with =/> 400 participants | 5 | 14795 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.97] |
| 3 Preterm birth | 11 | 15275 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.60, 0.97] |
| 3.1 Studies with < 400 participants | 6 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.24, 0.76] |
| 3.2 Studies with =/> 400 participants | 5 | 14465 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 4 Admission to neonatal intensive care unit | 4 | 13406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.18] |
| 4.1 Studies with < 400 participants | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.48] |
| 4.2 Studies with =/> 400 participants | 3 | 13343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.19] |
| 5 Stillbirth or death before discharge from hospital | 11 | 15665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 5.1 Studies with < 400 participants | 6 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 9.20] |
| 5.2 Studies with =/> 400 participants | 5 | 14819 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
3.4. Analysis.
Comparison 3 Routine high‐dose calcium supplementation in pregnancy by study sample size, Outcome 4 Admission to neonatal intensive care unit.
Comparison 4. Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium and study sample size (not prespecified).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 13 | 15730 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.31, 0.65] |
| 1.1 Adequate calcium/small study | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.04, 1.50] |
| 1.2 Adequate calcium/large study | 2 | 4792 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.33, 1.46] |
| 1.3 Low calcium/small study | 5 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.38] |
| 1.4 Low calcium/large study | 3 | 10003 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.35, 1.14] |
| 1.5 Dietary calcium not specified | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.02] |
Comparison 5. Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine artery RI at 32 weeks | 1 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 2 Umbilical artery RI at 32 weeks | 1 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 3 Low platelet count at 35 weeks | 1 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.63, 2.18] |
| 4 High serum uric acid at 35 weeks | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.64, 1.57] |
| 5 High urine protein/creatinine ratio at 35 weeks | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 6 Ultrasound estimate of fetal growth at 32 weeks: femur length (cm)* | 1 | 377 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7 Ultrasound estimate of fetal growth at 32 weeks: biparietal diameter (cm)* | 1 | 377 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8 Ultrasound estimate of fetal growth at 32 weeks: abdominal circumference (cm)* | 1 | 377 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
5.1. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 1 Uterine artery RI at 32 weeks.
5.2. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 2 Umbilical artery RI at 32 weeks.
5.3. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 3 Low platelet count at 35 weeks.
5.4. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 4 High serum uric acid at 35 weeks.
5.5. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 5 High urine protein/creatinine ratio at 35 weeks.
5.6. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 6 Ultrasound estimate of fetal growth at 32 weeks: femur length (cm)*.
5.7. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 7 Ultrasound estimate of fetal growth at 32 weeks: biparietal diameter (cm)*.
5.8. Analysis.
Comparison 5 Routine high‐dose calcium supplementation in pregnancy by other outcomes (not prespecified), Outcome 8 Ultrasound estimate of fetal growth at 32 weeks: abdominal circumference (cm)*.
Comparison 6. Low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements vs placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 High blood pressure (with or without pre‐eclampsia) | 5 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.74] |
| 1.1 Calcium supplementation alone | 3 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.82] |
| 1.2 Calcium plus vitamin D | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.24, 1.75] |
| 1.3 Calcium plus linoleic acid | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.82] |
| 2 Pre‐eclampsia | 9 | 2234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.52] |
| 2.1 Calcium supplementation alone | 4 | 980 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.23, 0.57] |
| 2.2 Calcium plus vitamin D | 2 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.31, 0.78] |
| 2.3 Calcium plus linoleic acid | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.60] |
| 2.4 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.06, 1.01] |
| 3 Preterm birth | 6 | 1290 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.34, 2.03] |
| 3.1 Calcium supplementation alone | 1 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.75] |
| 3.2 Calcium plus vitamin D | 3 | 760 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.03, 2.45] |
| 3.3 Calcium plus linoleic acid | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.15] |
| 3.4 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.23] |
| 4 Admission to neonatal intensive care unit | 1 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.99] |
| 4.1 Calcium supplementation alone | 1 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.99] |
| 4.2 Calcium plus vitamin D | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcium plus linoleic acid | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Stillbirth or death before discharge | 5 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.67] |
| 5.1 Calcium supplementation alone | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.29] |
| 5.2 Calcium plus vitamin D | 1 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.15] |
| 5.3 Calcium plus linoleic acid | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.08, 4.41] |
| 5.4 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.39] |
| 6 Placental abruption | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.90] |
| 6.1 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Calcium plus vitamin D | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.90] |
| 7 Caesarean section | 4 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.15] |
| 7.1 Calcium supplementation alone | 2 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.40, 2.22] |
| 7.2 Calcium plus vitamin D | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcium plus linoleic acid | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.35, 0.87] |
| 8 Severe pre‐eclampsia | 4 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.14, 1.15] |
| 8.1 Calcium supplementation alone | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Calcium plus vitamin D | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.08, 4.39] |
| 8.3 Calcium plus linoleic acid | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.56] |
| 8.4 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.23] |
| 9 Eclampsia | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 9.1 Calcium supplementation alone | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 9.2 Calcium plus vitamin D | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Calcium plus linoleic acid | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Miscarriage | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.04] |
| 10.1 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.04] |
| 11 Low birthweight (birthweight < 2500 g) | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.88] |
| 11.1 Calcium supplementation alone | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Calcium plus vitamin D | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Calcium plus linoleic acid | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.88] |
| 12 Neonate small‐for‐gestational age | 4 | 854 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.21] |
| 12.1 Calcium supplementation alone | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Calcium plus vitamin D | 1 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.58, 1.38] |
| 12.3 Calcium plus linoleic acid | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.32] |
| 12.4 Calcium plus antioxidants | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.31] |
Comparison 7. High‐dose (=/> 1 g) vs low‐dose (< 1 g) calcium supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.18, 0.96] |
| 2 Preterm birth | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.08] |
| 3 Eclampsia | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.53] |
| 4 Stillbirth | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.13, 1.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almirante 1998.
| Methods | “divided into two groups and followed up until delivery.” | |
| Participants | 430 pregnant women who were nulliparas, adolescents and elderly. Dates of recruitment not stated. Setting not stated, Phillipines | |
| Interventions | Group B: 212 women received 500 mg elemental calcium from 16‐20 weeks till delivery. Group A: 210 women served as controls. |
|
| Outcomes | Pre‐eclampsia, preterm birth, admission to NICU | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No record of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Abstract only, no details, no placebo |
Asemi 2012.
| Methods | RCT described as single‐blind controlled clinical trial Individual randomisation |
|
| Participants | Setting: maternity clinics in Kashan Iran. Dates of recruitment: April 2011 to February 2012. Inclusion criteria: pregnant nulliparous women at risk of pre‐eclampsia, singleton pregnancy, 18‐35 years in third trimester. High risk was defined as nulliparous, with environmental, socioeconomic factors or obesity (not clear). 54 women recruited. Exclusion criteria: women with severe pre‐eclampsia, intrauterine fetal death, placental abruption, preterm birth or GDM. |
|
| Interventions | Experimental intervention: calcium‐vitamin D supplements for 9 weeks (500 mg calcium carbonate plus 200 IU vitamin D per day). Total number randomised: n = 27 women (24 analysed). Control/comparison intervention: placebo. Total number randomised: n = 27 women (25 analysed). |
|
| Outcomes | Compliance monitored weekly. Dietary intake (from diaries). Maternal weight, BMI, blood biochemical outcomes (fasting plasma glucose, cholesterol, high and low density lipoprotein‐cholesterol). | |
| Notes | Funding: the study was supported from a grant (university or government). Grant from the Vice‐chancellor for Research, Kashan University of Medical Sciences (KUMS). Study placebo provided by Share Darou Co, Tehran, Iran and supplements by Darou Pakhsh Co. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | This was a placebo controlled study but it was not clear how women were assigned to groups. Although the supplements and placebo packs were described as identical it was not clear whether or not staff were aware of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as a single‐blind trial. It was not clear what this meant. Women were provided with active or placebo drugs in identical packs. Only participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear if outcome assessment was blind but as most outcomes were biochemical lack of blinding may not have impacted on outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 54 women were recruited, 49 included in the analyses (25 in placebo group, 24 in calcium/vitamin D group). Women with severe pre‐eclampsia, preterm birth and GDM were excluded — it was not clear if these exclusions were after randomisation — these outcomes may have related to the intervention. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. It was not clear if all outcome data collected was reported. |
| Other bias | Unclear risk | Women in the placebo group had slightly lower weights pre‐pregnancy (and throughout pregnancy). |
Asemi 2016.
| Methods | Double‐blind parallel‐arm RCT, individual randomisation, placebo controlled | |
| Participants | Setting: clinics in Kashan, Iran. Dates of recruitment: March‐September 2012. Inclusion criteria: 46 women, 18‐40 with singleton pregnancy. Exclusion criteria: women with pre‐eclampsia, placental abruption and GDM (it appeared that women were excluded after randomisation). |
|
| Interventions | Experimental intervention: calcium‐vitamin D for 9 weeks from 25 weeks (500 mg calcium, 200 IU vitamin D per day). Total number randomised: n = 23. Control/comparison intervention: placebo pills of similar appearance. Total number randomised: n = 23. Women in both groups took iron and folic acid supplements. |
|
| Outcomes | Fasting plasma glucose, insulin metabolism, biomarkers of oxidative stress, vitamin D levels, BP, birth size, gestational age, mode of birth. | |
| Notes | Author contacted re information about women excluded after randomisation. Funding: reported to be funded by a grant from the vice‐chancellor for research, Kashan university of medical sciences. Reported that supplements and placebo were provided by Shahre Daru Co, Tehran. CoI: reported no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was reported that randomisation was by random numbers “taken from a computer”. |
| Allocation concealment (selection bias) | Unclear risk | Placebo controlled but method at point of randomisation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear if staff blind. Placebo controlled but it was not clear if staff were aware. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if outcome assessors blind, some outcomes may not be affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 women were excluded post‐randomisation for reasons that may have related to the intervention (participants that developed complications during the trial were excluded – we have added these back in and collected these data for our analysis). |
| Selective reporting (reporting bias) | Unclear risk | We had no protocol. Women were excluded post‐randomisation and no ITT. |
| Other bias | Unclear risk | Author contacted re post‐randomisation exclusions. |
Bassaw 1998.
| Methods | Randomised clinical trial. Participants were alternately allocated to either the supplemented or to the control groups in order to match for age, parity, ethnic group and BMI. Data from the 'control' group were not used in this analysis. Randomisation was conducted by the pharmacist using a table of random numbers, and supplements were distributed to the participants in sealed envelopes. Clinicians were unaware whether the participants were in the supplemented or control groups, and which supplementation was administered. | |
| Participants | Women attending a hospital in Trinidad between 1992 and 1995. Pregnant women recruited into the study before 20 weeks’ gestation primigravidae, or multigravidae with obstetric history of pre‐eclampsia. Participants were normotensive and urinalysis was negative for albuminuria. None had any underlying medical disorders such as chronic hypertension, renal disease, diabetes mellitus and collagen vascular disorders. |
|
| Interventions | 2 calcium tablets (1200 mg elemental calcium), a combination of 1 calcium tablet and 1 baby Cafenol (80 mg aspirin) or 1 baby Cafenol daily. All participants, including the controls, received the routine haematinics which were ferrous sulphate (200 mg) and folic acid (5 mg) daily. There were 114 primigravidae amongst the controls. Of the supplemented groups, 45 primigravidae received aspirin, 36 had calcium and aspirin, and calcium tablets were administered to 42 primigravidae. All of these women were less than 24 years of age. For this review we have used only data for calcium (600 mg) and Cafenol (80 mg aspirin) vs Cafenol daily (80 mg aspirin). |
|
| Outcomes | DBP (measured by the same observer with the participants in a sitting position, recorded at the onset of muffing ‐phase 4 Korotkoff sound), PIH (BP ≥ 140/90 mmHg), pre‐eclampsia (hypertension plus proteinuria). | |
| Notes | 8 participants were unavailable for analysis. Source of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The supplement vs control group were allocated by alternation, but it was clear that various supplemented groups were randomised by the pharmacist using random number tables. In this review we use only data for calcium plus aspirin vs aspirin, which were randomised. |
| Allocation concealment (selection bias) | Low risk | Supplements were distributed in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians were unaware whether the participants were in the supplemented or control groups, and which supplementation was administered. Participants were not blinded as placebos were not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported that clinicians recording outcomes were unaware of treatment group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants were unavailable for analysis. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | None noted |
Belizan 1991.
| Methods | Multicentre trial. Numbered, sealed opaque envelopes, containing randomisation codes. Of 593 (calcium) vs 601 (placebo) enrolled, 14 vs 13 were lost before starting treatment and excluded from analysis; 577 vs 588 had at least partial follow‐up. Follow‐up was incomplete for 52 vs 46, but delivery data were available in 17 vs 12 of these, giving delivery data for 544 vs 554. | |
| Participants | Nulliparous women, < 20 weeks' pregnant; BP < 140/90 mmHg (mean of 5 measurements); no present or past disease; not taking medication; normal oral glucose tolerance tests. Recruitment between 1987 and 1989. |
|
| Interventions | 2 g calcium as 500 mg calcium carbonate tablets, vs identical looking placebo tablets. Compliance was 84% (calcium) and 86% (placebo). | |
| Outcomes | Gestational hypertension (DBP 90 or more; SBP 140 or more mmHg, on 2 occasions 6 hours apart); pre‐eclampsia (gestational hypertension + proteinuria > 0.3 g/L on 2 random urine samples 6 hours apart); BP measured with random‐zero sphygmomanometers, Korotkoff sound 5. Perinatal death. Follow‐up: BP > 95th percentile for sex, age and height for children 5‐9 years. | |
| Notes | 3 hospitals in Rosario, Argentina. Data for preterm birth given as percentages, not clear what the denominators were. Assumed to be the numbers with complete follow‐up (527 vs 542) as these were the numbers which were divisible by the percentages to give whole numbers. Unpublished placental abruption data obtained from authors. Babies born in the private hospitals followed up at 7 years. Of 614 randomised (calcium 309/placebo 305), 301/299 completed the first study, 2/6 infant deaths and 1/0 maternal deaths had occurred, leaving 298/293 eligible for follow‐up. 289/285 were contacted, 10/5 refused to participate, 22/19 lived outside the country, and 257/261 were assessed (88% of those eligible). Funded by a research grant from The International Development Research Centre, Canada. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence ‐ Epistats Statistical Package |
| Allocation concealment (selection bias) | Low risk | Complete set of numbered sealed opaque envelopes was sent to each of 3 hospitals. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation code was held centrally such that the woman and her healthcare providers were blind to her trial group. Tablets were identical in appearance, weight, colour, taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation code was held centrally such that the woman and her healthcare providers were blind to her trial group. Tablets were identical in appearance, weight, colour, taste. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All or partial data available for 579/593 (Ca) and 588/601 (Pl) respectively. Delivery data were available for 544 and 554 respectively. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes addressed |
| Other bias | Low risk | Balanced group sizes, baseline characteristics including dietary calcium similar in both groups |
Cong 1995.
| Methods | “Healthy antepartum cases were randomized and divided into 3 groups.” | |
| Participants | Healthy primipara. Setting and dates of recruitment not stated (China) | |
| Interventions | A: 120 mg calcium daily; B: 240 mg calcium daily; C: no calcium (D: 1 g calcium; E: 2 g calcium; E: no calcium: not included as trials with high risk of bias not included in high calcium dose review). | |
| Outcomes | Biochemical studies; hypertension, pre‐eclampsia, birthweight, gestational age, method of delivery. | |
| Notes | First period (low dose) April 1987 to June 1988 (groups A, B, C); second period (high dose) April 1989 to June 1990 (groups C, D, F). Similar results for groups A and B, which were combined in this meta‐analysis Source of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Limited information |
| Other bias | High risk | Very limited description of methods |
CPEP 1997.
| Methods | Numbered treatment packs in computer‐generated simple randomisation sequence. Loss to follow‐up: calcium 132/2295 vs placebo 121/2294. | |
| Participants | Setting: medical centres in the USA. Dates of recruitment not clear. Pregnant nulliparas (45% black, 35% non‐Hispanic white, 17% Hispanic white). Passed compliance test (took 75% of placebo over 6‐14 days); BP 134/84 mmHg or less; urine protein dipstick negative or trace; 13‐21 weeks' pregnant. Exclusion criteria: taking medications; obstetric or pre‐existing diseases or personal characteristics which could influence study end points, absorption or metabolism of calcium; any risk associated with calcium supplementation, or compliance; elevated serum creatinine (1.0 mg per dL or more) or calcium (10.6 mg per dL or more); renal disease; haematuria; history or family history of urolithiasis; frequent use of calcium supplements or antacids. Of 11,959 women screened, 5703 were excluded initially and a further 1667 after the compliance test. The remaining 4589 women were enrolled. |
|
| Interventions | 2 g/day elemental calcium as calcium carbonate, or placebo. Taken until delivery, development of pre‐eclampsia or suspicion of urolithiasis. All women took 50 mg calcium per day as normal supplementation and were asked to drink 6 glasses of water per day. Compliance was 64% in the calcium group and 67% in the placebo group. 20% of women took > 90% of the allocated treatment. |
|
| Outcomes | Gestational hypertension (DBP sitting, fifth Korotkoff sound unless zero, 90 mmHg or more on 2 occasions, 4 hours to 1 week apart); severe gestational hypertension (DBP 110 mmHg twice or treated, or complications); proteinuria (300 mg/24 hours or more, 1+ on 2 occasions 4 hours to 1 week apart, 2+ or more, or protein/creatinine ratio 0.35 or more); pre‐eclampsia (gestational hypertension + proteinuria within 7 days of each other); severe pre‐eclampsia (50/2163 vs 59/2173); renal insufficiency (21/2163 vs 23/2173); urolithiasis (1/2163 vs 3/2173); prematurity (< 37 weeks); baby small‐for‐gestational age (124/2163 vs 105/2173); perinatal death. A limited follow‐up of mothers and infants found to have reduced SBP at 2 years of age in the calcium supplementation group (mean 95.4 mmHg, SD 7.6, n = 35 vs 100.2, 7.9, n = 18). The data have not been included in this review because of the low and unequal follow‐up rate (35 and 18 from 497 invited to participate) limit the reliability of the results. | |
| Notes | Multicentre trial, 5 US university centres. Maternal outcomes reported as percentages of the whole number enrolled. In this review, denominators of 2163 (calcium) and 2173 (placebo) have been used. Neonatal outcomes in the report are based on live births (2134 and 2139). Addition of abortions and fetal deaths brings these numbers to 2156 and 2166. It is not clear why a discrepancy in numbers remains. Sources of funding: supported by The National Institute of Child Health and Human Development and the National Heart, Lung and Blood Institute. Study medications were industry provided. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Packages of study tablets were prepared and numbered by pharmaceutical manufacturer according to a computer‐generated simple randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | On enrolment, each woman was assigned the next numbered package of medication at 1 of 5 centres. The blister‐packed tablets were identical in appearance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. The code was held centrally. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. The code was held centrally. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 10% |
| Selective reporting (reporting bias) | Unclear risk | Authors used total number of women enrolled to each group as denominator instead of total number minus those lost to follow‐up. Also small discrepancy in overall numbers but unlikely to affect results substantially. |
| Other bias | Low risk | Baseline characteristics similar |
Crowther 1999.
| Methods | Central telephone randomisation, stratified by centre using variable blocks. Double‐blind. | |
| Participants | Inclusion criteria: nulliparous women; singleton pregnancy; < 24 weeks' gestation; BP < 140/90 mmHg; expected to give birth at a collaborating centre. Exclusion criteria: antihypertensive therapy; medical contraindication to calcium supplementation. | |
| Interventions | Calcium carbonate 1.8 g daily or lactose placebo tablets, from 20‐24 weeks until birth. | |
| Outcomes | Primary: PIH (DBP 90 mmHg or more on 2 consecutive occasions 4 hours apart, or 110 mmHg once; pre‐eclampsia (as above plus proteinuria 0.3 g or more per 24 hours or 2+ protein or more on 2 random clean‐catch urine samples); preterm birth (< 37 weeks). Secondary: severe PIH (DBP 110 or more on 2 occasions 4 hours apart, or 120 or more once); severe pre‐eclampsia (as above plus proteinuria); very preterm birth (< 32 weeks; extremely preterm birth (< 28 weeks); maternal fetal and infant events after trial entry. | |
| Notes | 5 hospitals in Australia. August 1992 to December 1996.
Estimated sample size 948. Trial stopped prematurely for financial reasons.
31% in the calcium group and 24% in the placebo group stopped taking the tablets during the trial. Analysis was by ITT Sources of funding: a grant from the Queen Victoria Hospital Foundation and The Women's and Children's Hospital Foundation, Australia. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation centrally co‐ordinated using variable blocks |
| Allocation concealment (selection bias) | Low risk | Identical sealed treatment packs prepared by drug company |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Tablets identical in size, colour and consistency. Code held centrally and only broken after trial closure and exploratory data analyses. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Tablets identical in size, colour and consistency. Code held centrally and only broken after trial closure and exploratory data analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 10% |
| Selective reporting (reporting bias) | Low risk | ITT analysis. 227 in calcium group and 229 in placebo group. Baseline characteristics similar. |
| Other bias | Unclear risk | Only achieved 48% of recruitment target (456 instead of 948) due to lack of funds. |
Herrera 1998.
| Methods | Allocation sequence was generated using random number tables, and prepared by an administrative staff member. | |
| Participants | Recruitment May 1995 to May 1996, 3 hospital outpatient clinics in Cali, Colombia. 676 healthy primigravid women screened. Primigravidas with risk factors for pre‐eclampsia (high biopsychosocial risk, positive roll‐over test and high mean BP (> 85 mmHg) selected). |
|
| Interventions | 450 mg linoleic acid plus 600 mg calcium (n = 44) vs identical looking placebos (n = 45) in the third trimester. | |
| Outcomes | Biochemical studies; maternal and neonatal clinical outcomes. | |
| Notes | 1 study group excluded for taking ASA; 2 from control group lost to follow‐up Source of funding: the study was supported by a grant from the National Institute of Science and Technology of Colombia. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “ allocated randomly.” |
| Allocation concealment (selection bias) | Low risk | “ allocated randomly.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Double blind, placebo controlled trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Double blind, placebo controlled trial.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 from study group excluded for taking ASA; 2 from control group lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | “Double blind, placebo controlled trial.” |
Herrera 2006.
| Methods | "The participants were allocated in two random groups." | |
| Participants | March 2001 to March 2003; 4 outpatient clinics in Bangladesh and Colombia. 220 primigravid women screened for abnormal Doppler ultrasound in uterine or arcuate arteries (diastolic notch) from week 18 to 22 of gestation. Primigravidas < 19 years or > 35 years, 18 to 22 weeks with risk factors for pre‐eclampsia including reliable family history of PE were included. Those with DBP of 85 mm Hg or more at the first antenatal visit, cardiovascular or renal disease, or hypertensive or taking any medication at the time were excluded. Mean daily calcium intake was also similar at study entry (601.5 mg [range, 310—1101 mg] vs 576.0 mg [314—936 mg]; P = 0.94). |
|
| Interventions | 450 mg conjugated linoleic acid plus 600 mg calcium (n = 25) vs placebo (n = 25) from 18 to 22 weeks. | |
| Outcomes | Biochemical studies. | |
| Notes | 220 women screened; eco‐Doppler ultrasound positive in 53 women; 3 eligible women refused to participate 1 woman from the control group was lost during the follow‐up (change of residence) and 1 woman from the supplemented group withdrew. Source of funding: Columbia Institute for Science and Technology and Partners in Population and Development Institute, Dhaka, Bangladesh. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “random cards were prepared and sealed by an independent administrative staff member using a random number table prepared with the True Epistat statistical package version 5.0.” |
| Allocation concealment (selection bias) | Low risk | “allocated in two random groups….sequentially numbered, sealed, opaque envelope containing a card that indicated the study allocation.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “allocated in two random groups….sequentially numbered, sealed, opaque envelope containing a card that indicated the study allocation.” ‐ it appears the study was not double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “allocated in two random groups….sequentially numbered, sealed, opaque envelope containing a card that indicated the study allocation.” ‐ it appears the study was not double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 loss to follow‐up in each group |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Low risk | Low to moderate (appears not double‐blind) |
Jarjou 2004.
| Methods | Randomised double‐blind placebo‐controlled trial conducted in The Gambia between 1995 and 2000. | |
| Participants | 662 pregnant women were randomised; 452 of 546 live born children were followed up. | |
| Interventions | 1500 mg calcium (Ca) orally per day or placebo from 20 weeks' gestation until delivery. | |
| Outcomes | 1. Maternal BP at 36‐38 weeks' gestation
2. Breast‐milk calcium concentration during lactation
3. Postpartum bone mineral content of mother and baby 4. Cardiovascular risk in offspring 5. BP in offspring (Hawkesworth 2011). Follow‐up of 350 children (64%). There were no differences in mean BP measurements 6. Maternal plasma 25 hydroxyvitamin D, birthweight and infant growth and bone mineral accretion (Prentice 2009) |
|
| Notes | Source of funding: UK Medical Research Council. (Supplements and placebos were provided by a pharmaceutical company.) CoI: none of the authors reported a personal or financial conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% missing data |
| Selective reporting (reporting bias) | Unclear risk | Maternal hypertension outcomes have not yet been reported |
| Other bias | High risk | Of 155 women randomised, 125 who had normal pregnancy were selected for the sub‐studies. It's not clear whether bias could have been introduced by this selection. |
Khan 2013.
| Methods | Described as prospective randomised clinical trial | |
| Participants | Setting: women attending the antenatal clinic of a tertiary care hospital in West Bengal, India (described as serving a population with low socio‐economic status). Dates of recruitment: May 2010–April 2011. Inclusion criteria: 272 women. Healthy nulliparous women (18‐30 years) with singleton pregnancy, with BP < 140/90 mmHg and no proteinuria at first antenatal visit before 20 weeks. |
|
| Interventions | Experimental intervention: 127 women. High‐dose calcium supplements (2 g oral elemental calcium, 4 tablets daily) from 20 weeks’ gestation. Comparison intervention: 145 women. Low‐dose calcium (500 mg plus placebo, 1 active and 3 placebo tablets of similar appearance daily). |
|
| Outcomes | (Prespecified outcomes were not clear; it was stated what was recorded at subsequent antenatal visits but it was not clear if these were study outcomes). Reported: eclampsia and pre‐eclampsia, preterm birth, gestational age at birth, birthweight, IUGR, stillbirth, mean systolic and mean BP (not clear when the reported values were measured). | |
| Notes | Funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported using a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | This was reported to be a placebo controlled trial but it was not clear staff were aware of allocation at the point of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was supplied so women may have been blind to which arm they were in. It was not clear whether staff were aware of the treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear if it was recorded in notes whether women were in the intervention or placebo groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/272 were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | We had no trial protocol and it was not clear what the study outcomes were (much data that was collected were not reported). Several outcomes were not well defined (e.g. not clear when mean BP values were recorded). |
| Other bias | Unclear risk | No baseline characteristics reported |
Kumar 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Women were recruited between January 2005 and December 2007 at a hospital in New Delhi, India. Healthy normotensive primigravid women with uncomplicated single pregnancy; pregnancy 12 to 25 weeks' gestation, known date of the last menstrual period, and intention to deliver at Lok Nayak Hospital, New Delhi. Study population had a low dietary calcium. Exclusions: multiple pregnancy, polyhydramnios, fetal malformations, diabetes, chronic hypertension, renal disease, cardiovascular disease, urolithiasis, or BP of 140/90 mmHg or higher at first visit or at enrolment. |
|
| Interventions | 4 tablets (2 g calcium or placebo) were taken daily. | |
| Outcomes | Pre‐eclampsia (SBP > 140 mmHg and DBP > 90 mmHg on 2 occasions 4 hours apart after 20 weeks' pregnancy in women normotensive previously, together with proteinuria > 300 mg/24 h or 1+ on a clean‐catch dipstick in the absence of urinary infection); eclampsia; preterm delivery; caesarean section. Baseline characteristics comparable. |
|
| Notes | Imbalanced groups: 290 allocated to calcium, 262 to placebo group. 17 and 11 lost to follow‐up so 273 and 251 analysed respectively. See below. Source of funding: University Grant Commission, New Delhi, India. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple randomisation sequence developed manually |
| Allocation concealment (selection bias) | Low risk | Coded numbers assigned to treatment packets and distributed to participants using the random number sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Calcium and placebo tablets were identical. Randomisation code broken after completion of the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Calcium and placebo tablets were identical. Randomisation code broken after completion of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 10% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Other bias | High risk | Imbalance in size of groups. The authors were contacted regarding the imbalance and they explained that a random sequence was generated for 600 participants (unblocked) but recruiting was stopped at 552 participants and so 48 numbers remained unallocated. |
L‐Jaramillo 1989.
| Methods | Assigned independently in sequence using a table of random numbers. All 106 women enrolled completed the study (calcium 55, placebo 51), 14 women who delivered at 36‐38 weeks excluded (calcium 6, placebo 8), none developed gestational hypertension. These women are included in this review. | |
| Participants | Women recruited at a hospital antenatal clinic in Quito, Ecuador. Dates of recruitment 1984‐1986. Inclusion criteria: nulliparity; age 25 years or less; certain menstrual dates; clinic attendance before 24 weeks' gestation; residence in Quito; normotensive; no medical disorders; not taking medication or vitamin/mineral preparations. |
|
| Interventions | Calcium supplementation with 4 calcium gluconate tablets daily, each containing 500 mg elemental calcium, from after 23 weeks' gestation till delivery, vs identical placebo tablets. | |
| Outcomes | Gestational hypertension (BP 140/90 mmHg or more, or rise of 30 mmHg systolic or 15 mmHg diastolic, on 2 occasions 6 hours apart); weekly weight gain, mean (SEM) (calcium 412 (26) vs placebo 452 (28) g); birthweight (3097 (40) vs 2832 (50) g); length of gestation (39.3 (0.08) vs 38.7 (0.07) weeks). | |
| Notes | Quito, Ecuador (altitude 2800 m). 1984 to 1986. An earlier report of apparently the same study gave an incidence of gestational hypertension of calcium 3/46 vs placebo 13/46 (Lopez‐Jaramillo 1987). Source of funding: the study was supported by the Consejo Nacional de Ciencia y Technologia and Consejo Nacional de Universidades y Escuclas Polieenicas, Ecuador. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind. Identical containers and tablets prepared by the Faculty of Chemistry and Pharmacy, Central University of Ecuador. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind. Identical containers and tablets prepared by the Faculty of Chemistry and Pharmacy, Central University of Ecuador. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 women excluded from the report because they delivered before 38 weeks leaving 43/49 women in the calcium and placebo groups respectively. Data from the 14 excluded women are included in this review. |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Unclear risk | Not clear |
L‐Jaramillo 1990.
| Methods | Randomised, double‐blind trial. Stated "Each patient was assigned independently in sequence", and "All women completed the study". | |
| Participants | Women attending a hospital in Quito, Ecuador. Dates of recruitment not stated. Healthy nulliparous women with positive roll‐over test at 28‐30 weeks' gestational age ‐ judged at high risk for gestational hypertension. | |
| Interventions | 2000 mg elemental calcium daily, from 28‐32 weeks to delivery, vs placebo starch tablets. | |
| Outcomes | Gestational hypertension (BP > 140/90 mmHg on 2 occasions 6 hours apart); proteinuria (300 mg/L); duration of pregnancy (calcium mean 39.2 (SD 1.2) vs placebo 37.4 (2.3) weeks); birthweight (2936 (396) vs 2685 (427) g). | |
| Notes | Quito, Ecuador (altitude 2800 m). 22 in calcium group, 34 in placebo group. Source of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state that this was a RCT but no details of sequence generation are provided. |
| Allocation concealment (selection bias) | Unclear risk | No details given about how concealment was achieved or whether tablets looked identical |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated double‐blind but no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated double‐blind but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Unclear risk | Large discrepancy in size of groups not accounted for |
L‐Jaramillo 1997.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Hospital setting in Quito Ecuador, women recruited 1990‐1995. Inclusion criteria: age < 17.5 years; nulliparous; first prenatal visit before 20 weeks' gestation; certain menstrual dates; residency in Quito for at least 1 year; BP =/< 120/80 mmHg; no underlying medical disorders; no drug, mineral or vitamin therapy. Average daily calcium intake in this population is 51% of the recommended dietary allowance. |
|
| Interventions | Elemental calcium 2 g daily as calcium carbonate from 20 weeks (n = 134), vs placebo tablets (n = 140). | |
| Outcomes | Pre‐eclampsia (BP > 140/90 mmHg on 2 occasions > 6 hours apart, and proteinuria > 300 mg/L (> 1+ on dipstick on 2 occasions 4‐24 hours apart)). BP recorded as mean of 2 measurements, 2 minutes apart, in the right arm, in the sitting position (1st and 5th Korotkoff sounds). Maternal serum ionised calcium at 38 weeks was calcium group mean 1.23, SD 0.02 mM vs placebo 1.16, 0.02; umbilical cord serum ionised calcium levels were calcium 1.44, 0.04 vs placebo 1,37, 0.03; gestational length was calcium 39.6, 0.4 vs placebo 38.7, 0.3. |
|
| Notes | Quito, Ecuador (altitude 2800 m). 1990 to 1995. Source of funding: supported by the Safe Motherhood Program, World Health Organization. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used to assign each participant independently in sequence to calcium or placebo regimen |
| Allocation concealment (selection bias) | Low risk | Adequate. Tablets similar in weight, colour, size. Containers and tablets prepared by the Department of Chemistry and Pharmacy, Central University of Ecuador |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 274 recruited, 260 analysed |
| Selective reporting (reporting bias) | Unclear risk | Only participants with no missing values were included in the analyses (125 in calcium group and 135 in placebo group). |
| Other bias | Unclear risk | 14 withdrawals after randomisation: 12 by change to another hospital or private medical doctor, 2 by non‐compliance. 9/134 (6.7%) were from the calcium group and 5/140 (3.6%) from the placebo group. |
Li 2000.
| Methods | "Patients were divided into 3 groups." | |
| Participants | Women recruited at the outpatient clinic and labour ward of the First Afifliated Hospital of Xi’an Medical University between Aug 1996 to Dec 1998. High‐risk women with a predisposition to PIH. Participants were required to be at 20‐24 weeks' gestation when entering the study, with a BMI index of < 24, and an arterial pressure of < 11.3 kPa. Study states only that participants were "selected from our hospital outpatient clinic and labour ward". |
|
| Interventions | The first group (n = 29) received a daily dose of 600 mg of Calictrate‐D, the second group (n = 29) received 1200 mg if Calcitrate‐D daily, and the third group (n = 30), the control group, received nothing. From 20‐24 weeks till birth | |
| Outcomes | Hypertension; biochemical studies | |
| Notes | The outpatient clinic and labour ward of the First Afifliated Hospital of Xi’an Medical University. Aug 1996 to Dec 1998. No information on consent or ethical approval Source of funding: not clear (original article not in English). CoI: not clear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Methods not reported |
Marya 1987.
| Methods | "Randomly selected." Information from authors indicated that it was actually a consecutive series. | |
| Participants | Women attending the antenatal hospital of a hospital in India. Dates of recruitment not stated. 400 pregnant women 20 to 35 years old attending antenatal clinic. Dietary intake about 500 mg calcium and 40 IU vitamin D daily. |
|
| Interventions | 200 women daily supplement calcium 375 mg plus vitamin D 1200 IU from 20 to 24 weeks of pregnancy onwards, vs 200 women no supplement. | |
| Outcomes | "Toxaemia", biochemical studies, mean BP | |
| Notes | Medical College Hospital, Rohtak, India Souce of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not specified. Information from authors indicated that it was actually a consecutive series. |
| Allocation concealment (selection bias) | High risk | Not specified. Information from authors indicated that it was actually a consecutive series. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No record of losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No report of registered protocol |
| Other bias | High risk | Very limited reporting of methods |
Niromanesh 2001.
| Methods | Double‐blind, placebo‐controlled clinical trial. | |
| Participants | Women attending a hospital in Tehran, Iran. Dates of recruitment not stated Women at high risk for pre‐eclampsia: positive 'roll‐over' test and at least 1 risk factor for pre‐eclampsia; 28‐32 weeks' pregnant; BP < 140/90 mmHg. Exclusion criteria: chronic medical conditions. Not defined as low or adequate calcium intake (from table 1 dairy intake appears to be about 200 mL + 400 g per day) |
|
| Interventions | Elemental calcium 2 g daily (500 mg 6‐hourly) or placebo, coded by the pharmacy | |
| Outcomes | Pre‐eclampsia: an increase (30 mmHg) of SBP above 14 mmHg and an increase (15 mmHg) of DBP above 90 mmHg, twice 4‐6 hours apart, with proteinuria 1+; duration of pregnancy (39.5 SD 0.8 vs 37.7 SD 2.5 weeks); birthweight (3316 SD 308 vs 2764 SD 761 g); weekly maternal weight increase (no difference). | |
| Notes | No loss to follow‐up Source of funding: not stated; supplements and placebo tablets were provided by a pharmaceutical company. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were "randomly assigned". |
| Allocation concealment (selection bias) | Low risk | Adequate. Manufacturer coded the tablets which had same packaging and physical characteristics. A pharmacy dispensed the tablets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data (sample size = 30) |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | No incomplete data or loss to follow‐up |
Purwar 1996.
| Methods | Prospective, randomised, double‐blind, placebo‐controlled trial. Allocated by means of a computer‐generated randomisation list. After randomisation, 11/201 (5.5%) women were lost to follow‐up (calcium 6, placebo 5). | |
| Participants | Women attending a hospital clinic in Nagpur, India. Recruitment between 1993 and 1994. Calcium intake mean 336 mg (calcium) and 352 mg (placebo group) per day. Inclusion criteria: nulliparity; normal single viable pregnancy; known dates; antenatal clinic before 20 weeks; intending to deliver in the same institute; normal glucose tolerance test; no hypertension; no underlying medical disorders. Exclusion criteria: renal disease; collagen vascular disease; chronic hypertension; endocrinological disease; taking medication. |
|
| Interventions | Oral calcium containing 2 g elemental calcium daily (n = 103), compared with identical placebo tablets (n = 98), taken from 20 weeks | |
| Outcomes | Gestational hypertension (SBP > 140 mmHg and DBP > 90 mmHg, twice 6 hours apart) and pre‐eclampsia (hypertension + proteinuria =/> 0.3 g/24 hours) | |
| Notes | Nagpur, India Source of funding: not stated. Supplements and placebos were provided by a pharmaceutical company. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Containers and tablets prepared by a pharmaceutical firm in Nagpur. Tablets were the same size, weight and colour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Containers and tablets prepared by a pharmaceutical firm in Nagpur. Tablets were the same size, weight and colour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 10% |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Apart from 11 women lost to follow‐up, there are no missing data. Otherwise baseline characteristics and compliance were similar; balanced loss to follow‐up. |
Rogers 1999.
| Methods | Randomised to control vs aspirin vs calcium in ratio 1; 2; 2, using 5 unsealed envelopes, selected by participants. Imbalance suggested that ‘something went wrong’, perhaps a tendency for participants to select from a certain part of the pile of envelopes. | |
| Participants | Women attending a clinic in Hong Kong. Recruitment: July 1992 to Dec 1994. 500 primiparous Chinese women in 2nd trimester with sitting MAP 80 to 106 mmHg screened at 22‐24 weeks with rested left lateral automated BP (cut‐off MAP 60 mmHg). 369 selected: calcium 154, aspirin 132, control 83. 32 delivered elsewhere and were excluded, leaving 337. |
|
| Interventions | Aspirin 80 mg daily from 22 weeks vs calcium 600 mg daily from 22 to 32 weeks, then 1200 mg daily vs control | |
| Outcomes | Hypertension, pre‐eclampsia, mean BP | |
| Notes | Source of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 5 open envelopes |
| Allocation concealment (selection bias) | High risk | Unsealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | See above |
Rumiris 2006.
| Methods | Double‐blind, placebo‐controlled trial. Participants randomised according to a computer‐generated random number sequence by an independent third party who had no conflict of interest in the study. | |
| Participants | Women attending a university hospital in Indonesia between March 2003 and June 2004 60 pregnant women with low antioxidant status at 8 to 12 weeks of gestation Exclusion criteria: 1) history or current use of anti‐hypertensive medication or diuretics; 2) use of vitamin C > 150 mg and/or vitamin E > 75 IU per day; 3) known placental abnormalities; 4) current pregnancy as a result of in vitro fertilisation; 5) regular use of platelet active drugs or non‐steroidal anti‐inflammatory drugs (NSAIDs); 6) known fetal abnormalities; 7) documented uterine bleeding within a week of screening; 8) uterine malformations; 9) history of medical complications. |
|
| Interventions | Supplementation with calcium (800 mg), N‐acetylcysteine (200 mg), Cu (2 mg), Zn (15 mg), Mn (0.5 mg), and selenium (100 mcg) and vitamins A (1000 IU), B6 (2.2 mg), B12 (2.2 mcg), C (200 mg), and E (400 IU), from 8 to 12 weeks of gestation throughout pregnancy Both groups received Fe (30 mg) and folic acid (400 mcg). Placebo supplement’s size and appearance were matched with those of antioxidants. |
|
| Outcomes | Maternal: pre‐eclampsia, hypertension, proteinuria and abortion. Perinatal: IUGR, intrauterine fetal death, preterm delivery (before 37 weeks). |
|
| Notes | Source of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised according to a computer‐generated random number sequence by an independent third party who had no conflict of interest in the study. |
| Allocation concealment (selection bias) | Low risk | Participants were randomised according to a computer‐generated random number sequence by an independent third party who had no conflict of interest in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to comment |
| Other bias | Low risk | None noted |
S‐Ramos 1994.
| Methods | Double‐blind placebo‐controlled trial. 4/33 allocated calcium lost to follow‐up. | |
| Participants | Women attending a hospital in Jacksonville, Florida, USA between 1989 and 1993. University hospital serving low‐income population Normotensive nulliparas; positive roll‐over test (281/1065) and positive angiotensin II infusion test at 20‐24 weeks' gestation (67/281). 67 were allocated to calcium (33) or placebo (34). Exclusion criteria: factors increasing the risk of gestational hypertension, including renal disease, collagen vascular disease, diabetes mellitus, chronic hypertension, multifetal pregnancy. |
|
| Interventions | Calcium supplementation with 2 g per day elemental calcium as 500 mg calcium carbonate tablets, vs identical placebo tablets. Compliance checked with electronic pillboxes. Compliance was 79% vs 81%. | |
| Outcomes | Gestational hypertension (BP at least 140/90 mmHg on 2 occasions 4‐6 hours apart, on bedrest in hospital); pre‐eclampsia (gestational hypertension + proteinuria: 1+ or 300 mg/24 hours); severe pre‐eclampsia (pre‐eclampsia plus 1 of BP at least 160 mmHg systolic or 110 mmHg diastolic; proteinuria at least 5 g/24 hours; oliguria < 400 mL per day; elevated liver enzymes; thrombocytopenia < 100,000/microlitre; pulmonary oedema; severe epigastric pain). Birthweight (calcium 3245 (SD 414) vs placebo 3035 (542) g); mean gestational ages (35.6 vs 34.4 weeks); 5 minute Apgar < 7 (1/29 vs 1/34); cord arterial pH (7.25 (0.07) vs 7.20 (0.07)); fetal growth impairment (2/29 vs 4/34). |
|
| Notes | Jacksonville, Florida, USA. University hospital serving low‐income population Source of funding: not stated. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Tablets were prepared by a pharmaceutical company and were identical with respect to weight, size, flavour and appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Tablets were prepared by a pharmaceutical company and were identical with respect to weight, size, flavour and appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% attrition |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Data entered before breaking the code. ITT analysis. 4/33 in the calcium group lost to follow‐up so 29 in calcium and 34 in placebo, however even if the 4 lost to follow‐up had PIH, results would still have significantly favoured the calcium group. |
Taherian 2002.
| Methods | “Healthy antepartum cases were randomized and divided into 3 groups.” | |
| Participants | Women attending a hospital antenatal clinic in Iran between 1998 and 2001. 990 nulliparous women, single pregnancy, first prenatal visit before 20 weeks of gestation, SDP/DBP lower than 130/80 mmHg, and no proteinuria detectable by a dipstick. Participants were excluded if they had a history of cardiovascular, renal or endocrinologic problems, medical or obstetric complications; or if they had a known hazardous condition (multifetal gestation, hydatidiform mole). |
|
| Interventions | Group 1 received 75 mg aspirin each day from 20th week of pregnancy till delivery; group 2 were treated with 500 mg oral calcium‐D daily (calcium‐D = 500 mg calcium carbonate + 200 IU vitamin D); and the control group 3 received no medication at all. | |
| Outcomes | Participants were considered to have mild pre‐eclampsia if they demonstrated an increase of 30 mmHg in systolic or 15 mmHg in DBP above the standard pressure. In addition, they should have demonstrated equal or greater than 300 mg/24 hours in urine collection, or in 2 random urine specimens obtained 4 hours apart and containing at least 1+ protein by the dipstick method. Severe pre‐eclampsia was defined as BP equal or greater than 160/110 mmHg and 4+ protein by dipstick on 2 occasions 4 hours apart. | |
| Notes | April 1998 to March 2001. Antenatal outpatient clinics of Isfahan Health Centers Data presented as percentages with no individual n values. Have extrapolated n values from numbers and percentages given for main outcome PE (Aspirin 326, calcium 325, control 327) and calculated other numbers from percentages reported. Source of funding: Univesity Research Department. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a table of random number to assign each case independently to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | "randomly allocated to three equivalent groups." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No record of losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | There was limited information on methods, and no mention of loss to follow‐up. |
Villar 1987.
| Methods | Double‐blind, RCT | |
| Participants | Recruitment 1983‐1985. 34 black women from Johns Hopkins Hospital, Baltimore, USA, 18 white women from Rosario, Argentina Inclusion criteria: nulliparous or primiparous; known menstrual dates; age 18‐30 years; singleton pregnancy; negative roll‐over test. Exclusion criteria: underlying medical disorders. Mean calcium intake at 26 weeks was: calcium group: 1129 (SD 736) and placebo group 914 (478). |
|
| Interventions | Calcium carbonate 1.5 g (500 mg tablets) from 26 weeks' gestation vs placebo tablets. Women at Johns Hopkins Hospital also received vitamin preparations containing 200 mg calcium and 100 mg magnesium per day. | |
| Outcomes | Weight gain in last trimester of pregnancy; BP increase; gestational hypertension | |
| Notes | Source of funding: grants from the National Dairy Board and the National Dairy Council. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly assigned' ‐ no other details |
| Allocation concealment (selection bias) | Low risk | Random numbers in closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablets were the same weight, size and colour, prepared by The Johns Hopkins pharmacy and distributed to the 2 hospitals. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Tablets were the same weight, size and colour, prepared by The Johns Hopkins pharmacy and distributed to the 2 hospitals. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% attrition |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Women at Johns Hopkins Hospital only also received vitamin preparations containing 200 mg calcium/day. |
Villar 1990.
| Methods | Double‐blind, randomised trial | |
| Participants | Johns Hopkins Hospital, Baltimore, 1985‐1988 Pregnant women 17 years or younger; no underlying medical disorders; most were nulliparous with known last menstrual period and singleton pregnancy |
|
| Interventions | 2 g elemental calcium as 500 mg calcium carbonate tablets, vs placebo tablets. All women were prescribed prenatal vitamin tablets containing 200 mg calcium and 100 mg magnesium per day. | |
| Outcomes | Preterm labour; preterm delivery < 37 weeks (calcium 7.4 vs placebo 21.1%); delivery 30‐37 weeks; idiopathic prematurity; spontaneous prematurity; low birthweight (< 2500 g) (calcium 9.6% vs placebo 21.1%); postdates > 42 weeks (calcium 7.4 vs placebo 5.3%); impaired fetal growth (3.2 vs 3.2%); premature rupture of membranes (2.1 vs 1.0%); Apgar score < 8 at 5 minutes (4.4 vs 10.5%) | |
| Notes | Source of funding: grants from the National Dairy Board and the National Dairy Council. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes with bottle numbers; project co‐ordinator responsible for assigning treatment. Identical tablets and containers were prepared at The Johns Hopkins Hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% attrition |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Baseline characteristics similar except for maternal weight (higher in placebo group, P < 0.01) |
WHO 2006.
| Methods | Double‐blind, randomised trial. Randomisation stratified by centre, with computer‐generated blocks of 6‐8. Allocation by consecutively numbered treatment packs containing calcium tablets or identical placebo. Treatment packs were prepared centrally. | |
| Participants | Multicentre trial in Argentina, Egypt, India, Peru, South Africa and Vietnam. Enrolment from 2001‐2003 Populations with median daily calcium intake < 600 mg; primiparous women less than 20 weeks' pregnant Exclusion criteria: renal disease or urolithiasis; parathyroid disease; BP > 140 mmHg systolic or > 90 mmHg diastolic; history of hypertension; antihypertensive therapy; diuretic, digoxin, phenytoin or tetracycline treatment |
|
| Interventions | Chewable calcium carbonate tablets with 500 mg elemental calcium, 3 daily, or identical placebo, from enrolment till delivery. | |
| Outcomes | Primary outcomes: pre‐eclampsia (BP diastolic 90 mmHg or more, or systolic 140 mmHg or more, plus proteinuria 2+ on dipsticks or 300 mg per day; preterm birth (< 37 weeks). Secondary outcomes: severe pre‐eclampsia (diastolic 110 mmHg or more or systolic 160 mmHg or more); early onset pre‐eclampsia (< 32 weeks), PIH; eclampsia; placental abruption; birthweight < 2500 g; spontaneous preterm delivery; medically indicated preterm delivery; admission to neonatal ICU for > 2 days; fetal, neonatal and perinatal mortality (before discharge from hospital). |
|
| Notes | Multicentre trial in Argentina, Egypt, India, Peru, South Africa and Vietnam. Enrolment from 2001‐2003
14,362 women screened, 8325 randomised. Exclusions: 6 calcium (4 not pregnant, 2 lost before treatment started) and 7 placebo (5 not pregnant, 2 lost before treatment started). Loss to follow‐up: 143 and 155 in calcium and placebo group respectively (some data available on women not followed up to delivery). Treatment compliance 84.5% and 86.2% respectively. Baseline characteristics were well matched. An ancillary study in Argentina assessed 510 of the participants by Doppler ultrasound for RI, PI in uterine and umbilical arteries, and for bilateral uterine artery notching (Carroli 2010). Similarly, a group of 708 participants in South Africa were assessed for serum and urine parameters of endothelial damage (Hofmeyr 2008). Source of funding: the study was supported by UNDP/UNFPA/World Health Organization/World Bank Special Programme of Research, Development and Research Training. CoI: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation lists for each site with random blocks of 6 to 8 women |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered identical treatment boxes were allocated for each woman enrolled. Randomisation codes remained at the WHO Clinical Trial Unit until analysis. Boxes and tablet bottles were prepared and numbered by Magistra SA, Geneva and shipped to trial centres. The placebo and calcium tablets were identical. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 143/4151 and 155/4161 women in calcium and placebo groups respectively were missing delivery data but were included in other analyses. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | ITT principle. Baseline characteristics, compliance and dropout rates were similar. |
ASA: acetylsalicylic acid BMI: body mass index BP: blood pressure DBP: diastolic blood pressure dl: decilitre g: gram GDM: gestational diabetes mellitus ICU: intensive care unit ITT: intention to treat IU: international units IUGR: intrauterine growth restriction MAP: mean arterial pressure mcg: microgram mg: milligram mmHg: millimetre of mercury NICU: neonatal intensive care unit PE: pulmonary embolism PI: pulsatility index PIH: pregnancy‐induced hypertension RCT: randomised controlled trial RI: resistance index SBP: systolic blood pressure SD: standard deviation SEM: standard error of the mean vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghamohammady 2010 | No data given in abstract. 100 nulliparous women 35 years old or more randomly allocated to receive calcium 2000 g or placebo from 15‐20 weeks until term |
| Anu 2017 | This study compared calcium supplements with traditional herbal supplements, Shatavaryadi Choorna (there was no non‐active placebo). |
| Anumba 2006 | This is a trial registration. Last entry on trial registry 2011. Reported that trial stopped and recruitment stopped |
| Asemi 2017 | Clinical trial of the effect of multi mineral‐vitamin D supplementation compared with the placebo |
| August 2002 | Excluded pending full report of results. Inadequate data in abstracts for inclusion |
| Azami 2017 | This was not a placebo controlled trial and the effects of calcium as part of a broader intervention was not clear. In this study there were 3 intervention groups: 1. ferrous sulphate tablet plus multi‐mineral tablet containing calcium, magnesium, zinc and D3; 2. ferrous sulphate plus vitamin E and C; 3. ferrous sulphate only. |
| Belizan 1983 | Too little information on methods and results to include N = 36. No clinically important outcomes presented in format suitable for inclusion in this review Participants: healthy, 20‐35 years, singleton pregnancy. Intervention: calcium 1 g (n = 11), calcium 2 g (n = 11) or placebo (n = 14). Outcomes: DBP 20‐24 weeks, and in the third trimester. Study design: randomised, no further information. |
| Bhatia 2010 | This is a trial registration and there is no subsequent publication although the trial is reported to be complete. Calcium was compared with calcium plus vitamin D and these groups were compared with a non‐random control group. |
| Boggess 1997 | No specified outcomes reported. Primarily a study of cardiac output measurements. Mentioned 'hypertensive complications', but do not specify what these were in the placebo group N = 23. After randomisation, 5/23 (22%) were excluded. Participants: 18‐35 years. Excluded if BP > 140/90 mmHg at 24 weeks; smokers; illicit drug use; multiple pregnancy; cardiovascular renal or endocrine disease; hypertension in previous pregnancy; calcium supplementation > 200‐250 mg elemental calcium Intervention: oral calcium carbonate 1.5 g/day for 6 weeks from 28‐31 weeks, or placebo tablets. All had 200‐250 mg calcium in standard prenatal vitamin‐mineral preparations. Outcomes: gestational hypertension (BP at least 140.90 mmHg on 2 occasions, 6 hours apart); pre‐eclampsia (gestational hypertension plus at least 1+ proteinuria). Study design: randomised trial; randomisation schedule in balanced blocks of 10. |
| Chames 2002 | Excluded pending publication of full report. No relevant clinical outcomes reported in the abstract. No difference was found in blood lead levels between women receiving calcium 1000 mg daily from 13‐19 weeks (n = 24) or placebo (n = 26). |
| Chan 2006 | This is a trial registration for a study that is outside the scope of this review. The study examines dairy foods for pregnant adolescent women. The aim of the intervention is not primarily about the prevention of pre‐eclampsia or hypertensive disorders. |
| de Souza 2006 | Participants randomised to calcium 2 g/day AND aspirin (ASA) |
| Diogenes 2011 | Supplementation with calcium (600 mg) (n = 17) plus vitamin D vs placebo (n = 9). This was a placebo controlled trial looking at calcium supplements in pregnant adolescent young women (13‐19 years, mean age 17). The aim of the study was specifically looking at nutritional factors (bone mass in women and babies, etc.). The aim did not relate to hypertension and no such data are reported in any of the publications. |
| Dizavandy 1998 | Excluded due to the unexplained large and imbalanced loss to follow‐up (6/58 in calcium group and 24/85 in placebo group). Hypocalciuric women in Iran randomised to receive calcium (2 g) or identical placebo but method of randomisation is unclear. Attempts to contact authors for more details failed. |
| Ettinger 2011 | 670 women randomised to calcium 1.2 g vs placebo in first trimester of pregnancy (Mexico City, 2001‐2003). Calcium was associated with reduction in bone resorption during pregnancy. The purpose of this study was not related to the prevention of hypertension. |
| Felix 1991 | Excluded as allocation was by alternation, not random. 14 women received calcium supplementation 2 g/day and 11 received placebo. No women developed hypertension or pre‐eclampsia. The production of 6‐keto‐prostaglandin F1alpha by umbilical arteries was similar between groups. |
| Fung 2010 | This is a trial registration from 2010 – it has not been updated since 2013 and it is not clear whether or not the study has been completed. The aim of the study is to compare bone mass in black and white pregnant women receiving calcium supplements, starting in the 1st trimester of pregnancy. It is not about hypertension and is outside the scope of the review. |
| Herrera 2006a | This is a study looking at adolescent women. The aim of the study was not related to the prevention of hypertension; the study aimed to look at calcium levels 1 month after randomisation. Excluded as outside the scope of this review. |
| Hofmeyr 2015 | This study looks at calcium supplementation before pregnancy and is therefore outside the scope of this review. |
| Karamali 2016 | This is a placebo controlled trial recruiting women with gestational diabetes and outcomes relate to gestational diabetes (macrosomia, etc.). The intervention involved supplementation for only 6 weeks, starting at 24‐28 weeks of gestation. The main focus of this study is the treatment of women with diagnosed gestational diabetes and not the prevention of hypertensive disorders so this study is outside the scope of the review. |
| Karandish 2003 | No details of randomisation available (attempts were made to contact the author) and outcome assessed (birthweight) is not a review outcome. Study compared 1 g calcium vs placebo in 68 women from 28‐30 weeks' gestation. |
| Kawasaki 1985 | N = 94. Not a randomised trial. Interventions: calcium L‐aspartate 600 mg/day from 20 weeks to delivery (n = 22) vs no supplementation (n = 72). Outcomes: pregnancy‐induced hypertension. |
| Knight 1992 | Excluded because, placebo not used, and participants not followed till delivery. Normotensive (n = 30 and hypertensive (BP 140/85 mmHg or more, n = 20) nulliparous women "randomly allocated" to receive calcium 1 g from about 12 weeks to 32 weeks, or a control group. Follow‐up continued to 36 weeks. Mean DBP reduced in the hypertensive group receiving calcium. |
| Lavin 1986 | Planned trial of calcium vs placebo in women with a positive roll‐over test at 28‐32 weeks. Trial was apparently cancelled. |
| MacDonald 1986 | RCT of calcium AND vitamin D vs placebo in 55 Asian women. No method or results were provided in this personal communication from 1986. Attempts to contact the author for more details were unsuccessful. |
| Martin 2017 | This is a dose/regime comparison looking at adherence, so is outside the scope of the review. |
| Montanaro 1990 | N = 170. No placebo Participants: normotensive at 24 weeks' pregnancy. Interventions: calcium 2 g/day from 24 weeks to delivery. Outcomes: pregnancy‐induced hypertension, pre‐eclampsia. Study design: "randomised, single‐blinded trial". |
| Mosalanejad 2016 | In this trial both groups received calcium: calcium + 400 IU vitamin (usual care) plus 1000 IU D3 is compared with calcium + 400 IU vitamin D (i.e. difference between groups is the intervention group gets a large dose of vitamin D as well as usual care which includes calcium. |
| NCT00000543 | This is a trial registration from 1999 – the women recruited already had hypertension. |
| Nooripour 2016 | This is a trial registration looking at women with vitamin D deficiency. The intervention was vitamin D. Women in both groups received a multi‐vitamin/mineral tablet containing calcium and vitamin D, women in the intervention group received an additional vitamin D supplement. |
| Prada 2001 | Excluded pending publication of full report. Abstract does not include outcomes specified for this review. Mean BP was reduced in adolescents receiving calcium supplementation 1000 mg daily (n = 62) compared with placebo (n = 62). Not clear whether participants in this report include participants from Prada 2002 |
| Prada 2002 | Excluded pending publication of full report. Abstract does not include outcomes specified for this review. Mean BP was similar in adolescents and women with twin pregnancy receiving calcium supplementation 1000 mg daily (n = 94) compared with placebo (n = 93). Not clear whether participants in this report include participants from Prada 2001 |
| Raman 1978 | N = 273. Allocation was by strict rotation, a quasi‐randomised trial. Supplementation with 300 mg vs 600 mg vs placebo. No data given on pre‐eclampsia. There were biochemical data on only 87 women. |
| Repke 1989 | N = 255. Presented as abstract only. Little information on methods and results Interventions: calcium 2 g/day vs placebo, after 20 weeks of pregnancy. Study design: "randomised clinical trial". |
| Roth 2014 | This is a study looking at whether enteric coating enhances bioavailability of calcium and the outcomes were serum calcium soon after administration. This is outside the scope of this review. Pregnancy outcomes were not included. This was a cross‐over trial which is not suitable for measuring pregnancy outcomes. |
| S‐Ramos 1995 | N = 75. Excluded because calcium was used for treatment of women with pre‐eclampsia rather than prevention. Participants: nulliparous, gestation 24‐36 weeks; mild pre‐eclampsia (BP 140/90‐160/100, proteinuria at least 300 mg/day). Interventions: calcium 2 g/day elemental calcium (4 tablets of calcium carbonate 1250 mg), vs matching placebo. Outcomes: initial and last BP and biochemical markers; preterm delivery; caesarean section; severe pre‐eclampsia; gestation at delivery; birthweight; Apgar < 7 at 1 minute and 5 minutes; cord arterial pH < 7.16; fetal growth restriction; perinatal death. Study design: double‐blind, placebo‐controlled study using a computer‐generated random number list. |
| Salzano 2001 | Method of "randomisation" not described and no explanation given for discrepancy in group sizes (25 vs 40). |
| Samimi 2016 | N = 60. This study examined high‐dose calcium supplements (1 g) plus vitamin D. High‐dose supplements with additional vitamins or minerals are outside the scope of this review. |
| Souza 2014 | This study looked at women receiving aspirin plus calcium vs placebo. Women had chronic hypertension. The intervention was a combined intervention; the effect of calcium alone would not be apparent. |
| Subprabha 2017 | Trial registration. This is not a placebo controlled trial; rather, it looks at a traditional treatment vs calcium supplements. |
| Suzuki 1996 | N = 152. Not a randomised trial Interventions: calcium 1 g/day from 20 weeks vs no calcium. Outcomes: pre‐eclampsia, gestational hypertension. |
| Tamas 1997 | Study of treatment of gestational hypertension, not prevention, using the drug dobesilate calcium, not calcium supplementation. |
| Wanchu 2001 | No placebo used. 120 consecutive nulliparous women less than 20 weeks' pregnant "randomly assigned" to receive 2 g elemental calcium daily, or no treatment. Analysis restricted to 100 women who "completed the protocol". Mild pre‐eclampsia occurred in 9/50 vs 6/50 and severe pre‐eclampsia in 0/50 vs 2/50 study vs control groups respectively. |
| Zheng 2000 | This was not a placebo controlled trial. |
ASA: acetylsalicylic acid BP: blood pressure DBP: diastolic blood pressure g: gram IU: international units mg: milligram mmHg: millimetre of mercury RCT: randomised controlled trial vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Liu 2013.
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: nulliparity; age ≥ 35 years; previous pre‐eclampsia; family history of pre‐eclampsia; multiple pregnancy; time between pregnancies ≥ 10 years; body mass index ≥ 25; diastolic pressure ≥ 80 mmHg before 20 weeks' gestation; proteinuria ≥+ on more than 1 occasion or ≥ 300 mg/24 h before 20 weeks' gestation; underlying medical conditions (pre‐existing hypertension; pre‐existing renal disease; pre‐existing diabetes; presence of antiphospholipid antibodies; chronic autoimmune disease); male sex partner's predecessor wife has previous pre‐eclampsia. The inclusion criteria include women who already have hypertension, but it may be that women that are actually recruited may not have these specific risk factors. |
| Interventions | This is a calcium supplement dose comparison 600 mg versus 1200 mg; it is not a placebo controlled trial. |
| Outcomes | Pre‐eclampsia. |
| Notes | This is a trial registration — the study is specifically about prevention of pre‐eclampsia — recruitment should have ended (2015) but there are no subsequent updates of the registration or publications. Awaiting assessment pending further publications. |
mg: milligram mmHg: millimetre of mercury
Characteristics of ongoing studies [ordered by study ID]
Chaiarach 2017.
| Trial name or title | Combined therapy with low‐dose aspirin and calcium supplements during second trimester to reduce the risk of superimposed pre‐eclampsia in pregnant women with chronic hypertension: a randomised‐controlled trial |
| Methods | Placebo controlled randomised trial |
| Participants | 104 women with chronic hypertension Inclusion criteria
Exclusion criteria
|
| Interventions | Low‐dose aspirin (81 mg) and calcium carbonate 1500 mg per day compared with placebo |
| Outcomes | Pre‐eclampsia, neonatal outcomes |
| Starting date | 01 July 2017 (anticipated). Reported completion date: 31 May 2018. |
| Contact information | Sukanya Chaiarach, sukanyatanoorat@hotmail.com |
| Notes |
Mahomed 1998.
| Trial name or title | Calcium supplementation for the prevention of pregnancy‐induced hypertension and preterm labour in twin pregnancies |
| Methods | Randomised controlled trial |
| Participants | Women with twin pregnancy |
| Interventions | Calcium solution (1 g elemental calcium per 5 mL). |
| Outcomes | Pregnancy‐induced hypertension, preterm labour, perinatal mortality and short‐term morbidity, maternal morbidity |
| Starting date | Not stated |
| Contact information | Prof K Mahomed |
| Notes | Sample size 400 per group |
Sulovic 2013.
| Trial name or title | Did calcium management prevent pre‐eclampsia? |
| Methods | Randomised controlled trial |
| Participants | 9178 healthy nulliparous woman 14‐23 weeks' gestation |
| Interventions | 2 g calcium vs placebo until the end of pregnancy |
| Outcomes | Pre‐eclampsia, preterm birth |
| Starting date | Not clear |
| Contact information | Belgrade University (no email) |
| Notes | (Reported in abstract.) Although this study appears to have been completed we are awaiting publication of full trial results. There was insufficient information to assess risk of bias and extract data. |
Torloni 2015.
| Trial name or title | Low dose calcium to prevent pre‐eclampsia (AMCAL) |
| Methods | Randomised controlled trial |
| Participants | 1040 women 16‐20 weeks' gestation |
| Interventions | Dietary supplement: calcium: 1 chewable tablet daily, at bedtime, containing 500 mg elemental calcium (1250 mg calcium carbonate) plus educational sessions (women will participate in at least 2 interactive group educational sessions lasting 30 minutes each; session content to include importance of calcium during pregnancy and how to modify their diets to include calcium‐rich foods that are available locally) versus educational sessions alone. |
| Outcomes | Pre‐eclampsia, hypertensive disorders of pregnancy. Change in dietary calcium intake, hospital admission for hypertension, maternal mortality, severe maternal morbidity (eclampsia, HELLP), side effects, preterm birth, neonatal outcomes |
| Starting date | October 2014 (estimated completion October 2016) |
| Contact information | Professor da Silva Moura Souza, celsa22@hotmail.com |
| Notes | Last updated Feb 2016, still recruiting |
g: gram mg: milligram mL: millilitre vs: versus
Differences between protocol and review
The subgroup analysis for high‐dose calcium of both dietary calcium and study size was not prespecified in the original protocol. In October 2004 we added seven additional outcomes (marked * below). For the 2014 update we added two outcome measures, marked ** below, in order to include newly published data. As such, these should be regarded as post‐hoc analyses, and interpreted with caution.
In 2014 we added a new comparison: low‐dose calcium supplementation, with or without cointerventions, versus no supplementation. In 2018 we added an additional comparison: high‐dose versus low‐dose calcium supplementation.
Secondary outcomes
For the woman
*Proteinuria.
*Severe pre‐eclampsia as defined by trial authors.
*Eclampsia.
*HELLP syndrome.
*Intensive care unit admission.
*Maternal death.
** Miscarriage.
For the child
*Death or severe neonatal morbidity.
**Dental caries in childhood (one or more decayed, missing or filled teeth, or as defined by trial authors).
The methods section for this review, Criteria for considering studies for this review, was updated in 2012.
Amendments 2014
We made the following amendments for the 2014 review.
We included a separate analysis for trials with less than 1 g of calcium daily.
If there were insufficient high‐quality randomised placebo‐controlled trials of low‐dose calcium alone to provide robust evidence of effectiveness, we separately reviewed additional evidence from lower quality studies, with appropriate caution in the interpretation of the results:
quasi‐randomised trials (by alternation, unstated method of allocation or other quasi‐random methods);
trials without placebo control;
trials of calcium plus additional supplements (e.g. vitamin D, linoleic acid, or anti‐platelet agents).
We included subgroup analysis by trial quality and cointerventions.
In 2018, we added in an additional search of ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP).
Contributions of authors
Álvaro Atallah and Justus Hofmeyr prepared the protocol for the initial Cochrane Review. Justus Hofmeyr prepared the data analysis for the initial Cochrane Review and is primarily responsible for maintaining the review, with input from the other authors. Tess Lawrie prepared the first draft of the 2010 update of the review with input from Justus Hofmeyr and Álvaro Atallah.
Justus Hofmeyr prepared the protocol revision and the first draft of the text for the 2013 update. Justus Hofmeyr and Regina Torloni performed the study selection and data extraction for the 2013 update. Justus Hofmeyr contributed to the analysis and writing of the 2018 revision. All authors gave input and approved the final version of the 2018 revision.
Sources of support
Internal sources
Universidade Federal de Sao Paulo/Escola Paulista de Medicina, Brazil.
Medical Research Council, UK.
Department for International Development, UK.
(GJH) Effective Care Research Unit, University of the Witwatersrand/Fort Hare, Eastern Cape Department of Health, South Africa.
External sources
UNDP/UNFPA/WHO/World Bank (HRP), Switzerland.
NHS Programme for Research and Development, UK.
Declarations of interest
G Justus Hofmeyr: Justus Hofmeyr was a collaborator in the WHO Calcium Trial (WHO 2006), which was included in this review and did not participate in the decision on inclusion and data extraction for this study
Theresa A Lawrie: none known.
Álvaro N Atallah: none known.
Maria Regina Torloni: Maria Regina Torloni is investigator for an ongoing study (Torloni 2015) which may potentially be included in future updates of this review
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Almirante 1998 {published data only}
- Almirante CY. Calcium supplementation during pregnancy in the prevention of EPH gestosis. Prenatal and Neonatal Medicine 1998;3 Suppl 1:24. [Google Scholar]
Asemi 2012 {published data only}
- Asemi Z, IRCT201212105623N3. Comparison of effectiveness of multivitamin, multivitamin‐mineral supplements, probiotic and normal Gaz on pregnancy outcomes in pregnant women. en.search.irct.ir/view/11730 (first received 26 December 2012).
- Asemi Z, Tabassi Z, Heidarzadeh Z, Khorammian H, Sabihi SS, Samimi M. Effect of calcium‐vitamin D supplementation on metabolic profiles in pregnant women at risk for pre‐eclampsia: a randomized placebo‐controlled trial. Pakistan Journal of Biological Sciences 2012;15(7):316‐24. [DOI] [PubMed] [Google Scholar]
Asemi 2016 {published data only}
- Asemi Z, Samimi M, Siavashani MA, Mazloomi M, Tabassi Z, Karamali M, et al. Calcium‐vitamin D co‐supplementation affects metabolic profiles, but not pregnancy outcomes, in healthy pregnant women. International Journal of Preventive Medicine 2016;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassaw 1998 {published data only}
- Bassaw B, Roopnarinesingh S, Roopnarinesingh A, Homer H. Prevention of hypertensive disorders of pregnancy. Journal of Obstetrics and Gynaecology 1998;18(2):123‐6. [DOI] [PubMed] [Google Scholar]
Belizan 1991 {published and unpublished data}
- Belizan JM. Prevention of hypertensive disorders of pregnancy with calcium supplementation. 8th World Congress on Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires. 1992:93.
- Belizan JM, Villar J, Bergel E, Pino A, Fulvio S, Galliano SV, et al. Long term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ 1997;315:281‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizan JM, Villar J, Gonzalez L, Campodonico L, Bergel E. Calcium supplementation to prevent hypertensive disorders of pregnancy. New England Journal of Medicine 1991;325:1399‐405. [DOI] [PubMed] [Google Scholar]
- Bergel E, Gibbons L, Rasines MG, Luetich A, Belizan JM. Maternal calcium supplementation during pregnancy and dental caries of children at 12 years of age: follow‐up of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2010;89(11):1396‐402. [DOI] [PubMed] [Google Scholar]
- Stephens IF. Effect of calcium supplementation during pregnancy on blood pressure of offspring. Authors cannot be sure of effect's generalisability to all children aged 5‐9 [letter; comment]. BMJ 1998;316(7126):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Belizan JM, Repke J. The effect of calcium supplementation on the incidence of hypertensive disorders of pregnancy and prematurity. 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:54.
- Villar J, Belizan JM, Repke JT. Does calcium supplementation reduce pregnancy‐induced hypertension and prematurity?. Proceedings of the International Symposium on advances in the prevention of low birthweight; 1988 May 8‐11; Cape Cod, Massachusetts. 1988:187‐95.
Cong 1995 {published data only}
- Cong KJ, Chi SL, Liu GR. Calcium and pregnancy induced hypertension. Chinese Journal of Obstetrics and Gynecology 1993;28:657‐9. [PubMed] [Google Scholar]
- Cong KJ, Chi SL, Liu GR. Calcium supplementation during pregnancy for reducing pregnancy induced hypertension. Chinese Medical Journal 1995;108:57‐9. [PubMed] [Google Scholar]
- Cong KJ, Chi SL, Liu GR. Calcium supplementation during pregnancy to reduce pregnancy induced hypertension. Beijing Medical Journal 1992;5:268. [Google Scholar]
CPEP 1997 {published data only}
- Habli M, Levine RJ, Qian C, Sibai B. Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. American Journal of Obstetrics and Gynecology 2007;197(4):406.e1‐406.e7. [DOI] [PubMed] [Google Scholar]
- Hatton DC, Harrison‐Hohner J, Coste S, Reller M, McCarron D. Gestational calcium supplementation and blood pressure in the offspring. American Journal of Hypertension 2003;16:801‐5. [DOI] [PubMed] [Google Scholar]
- Levine RJ for the CPEP Study Group. Calcium for preeclampsia prevention (CPEP): a double‐blind, placebo‐controlled trial in healthy nulliparas. American Journal of Obstetrics and Gynecology 1997;176:S2. [DOI] [PubMed] [Google Scholar]
- Levine RJ for the CPEP Study Group. The trial of calcium for preeclampsia prevention (CPEP). 8th World Congress on Hypertension in Pregnancy ‐ Protagonists and Presentations; 1992 November 8‐12; Buenos Aires, Argentina. 1992:94.
- Levine RJ, Esterlitz JR, Raymond EG, DerSimonian R, Hauth JC, Ben Curet L, et al. Trial of calcium for preeclampsia prevention (CPEP): rationale, design, and methods. Controlled Clinical Trials 1996;17:442‐69. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, et al. Trial of calcium to prevent preeclampsia. New England Journal of Medicine 1997;337(2):69‐76. [DOI] [PubMed] [Google Scholar]
- NCT00000534. Calcium for pre‐eclampsia prevention (CPEP). clinicaltrials.gov/ct2/show/record/NCT00000534 (first received 27 October 1999).
Crowther 1999 {published data only}
- Crowther C, Hiller J, Pridmore B, Bryce R, Duggan P, Hague W, et al. Calcium supplementation in nulliparous women for the prevention of pregnancy included hypertension, pre‐eclampsia and preterm birth: an Australian randomised trial. 2nd Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30‐April 4; Alice Springs, Australia. 1998:101.
- Crowther CA, Hiller JE, Pridmore B, Bryce R, Duggan P, Hague WM, et al. Calcium supplementation in nulliparous women for the prevention of pregnancy‐induced hypertension, preeclampsia and preterm birth: an Australian randomized trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(1):12‐8. [DOI] [PubMed] [Google Scholar]
- Hiller JE, Crowther CA, Moore VA, Willson K, Robinson JS. Calcium supplementation in pregnancy and its impact on blood pressure in children and women: follow up of a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(2):115‐21. [DOI] [PubMed] [Google Scholar]
Herrera 1998 {published data only}
- Herrera JA, Arevalo‐Herrera M, Herrera S. Prevention of preeclampsia by linoleic acid and calcium supplementation: a randomized controlled trial. Obstetrics & Gynecology 1998;91(4):585‐90. [DOI] [PubMed] [Google Scholar]
Herrera 2006 {published data only}
- Herrera JA, Arevalo‐Herrera M, Shahabuddin AK, Ersheng G, Herrera S, Garcia RG, et al. Calcium and conjugated linoleic acid reduces pregnancy‐induced hypertension and decreases intracellular calcium in lymphocytes. American Journal of Hypertension 2006;19(4):381‐7. [DOI] [PubMed] [Google Scholar]
- Herrera JA, Shahabuddin AK, Ersheng G, Wei Y, Garcia RG, Lopez‐Jaramillo P. Calcium plus linoleic acid therapy for pregnancy‐induced hypertension. International Journal of Gynecology & Obstetrics 2005;91(3):221‐7. [DOI] [PubMed] [Google Scholar]
- Herrera JA, Shahabuddin AKM, Faisal M, Ersheng G, Wei Y, Lixia D, et al. Effects of supplementation with oral calcium and linoleic acid in primigravidas at high risk [Efectos de la supplementacion oral con calcio y acido linoleico conjugado en primigravidas de alto riesgo]. Colombia Medica 2004;35(1):31‐7. [Google Scholar]
Jarjou 2004 {published data only}
- Goldberg G, Yin J, Jarjou L, Prentice A. Maternal calcium supplementation during pregnancy and blood pressure in rural Gambian children at 3 and 9 years old. Annals of Nutrition & Metabolism 2013;63(Suppl 1):728, Abstract no: PO982. [Google Scholar]
- Goldberg GR, Jarjou LM, Cole TJ, Prentice A. Randomized, placebo‐controlled, calcium supplementation trial in pregnant Gambian women accustomed to a low calcium intake: effects on maternal blood pressure and infant growth. American Journal of Clinical Nutrition 2013;98(4):972‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesworth S, Sawo Y, Fulford AJ, Goldberg GR, Jarjou LM, Prentice A, et al. Effect of maternal calcium supplementation on offspring blood pressure in 5‐ to 10‐y‐old rural Gambian children. American Journal of Clinical Nutrition 2010;92(4):741‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesworth S, Walker CG, Sawo Y, Fulford AJ, Jarjou LM, Goldberg GR, et al. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. American Journal of Clinical Nutrition 2011;94(6 Suppl):1853S‐1860S. [DOI] [PubMed] [Google Scholar]
- Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. American Journal of Clinical Nutrition 2010;92(2):450‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjou LM, Prentice A, Bennett J. Impact of calcium supplementation in the preceding pregnancy on the human milk calcium concentration of Gambian women. Advances in Experimental Medicine and Biology 2004;54:347‐9. [DOI] [PubMed] [Google Scholar]
- Jarjou LM, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, et al. Randomized, placebo‐controlled, calcium supplementation study in pregnant Gambian women: effects on breast‐milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. American Journal of Clinical Nutrition 2006;83(3):657‐66. [DOI] [PubMed] [Google Scholar]
- Jarjou LMA, Bennett J, Laidlaw A, Goldberg GR, Prentice A. Changes in bone turnover and calcitrophic hormones in lactating Gambian women supplemented with calcium during pregnancy. Journal of Human Lactation 2007;23(1):86‐7. [Google Scholar]
- Jarjou LMA, Sawo Y, Goldberg GR, Laskey MA, Cole TJ, Prentice A. Persistent effects of calcium supplementation during pregnancy on maternal bone mineral content: a follow‐up study in Gambian women. Proceedings of the 16th ISRHML Conference "Breastfeeding and the Use of Human Milk. Science and Practice"; 2012 September 27th ‐ October 1st; Trieste, Italy. 2012:Abstract no.:105.
- Jarjou MA, Yankuba S, Goldberg R, Laskey MA, Cole J, Prentice A. Unexpected long‐term effects of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake: a follow‐up study. American Journal of Clinical Nutrition 2013;98(3):723‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Goldberg GR, Jarjou LMA. The effects of pregnancy calcium supplementation on calcium metabolism after lactation in Gambian women with a low calcium intake. Breastfeeding Medicine 2016;11(2):A‐20‐A‐21, Abstract no: P‐8. [Google Scholar]
- Prentice A, ISRCTN72582014. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. isrctn.com/ISRCTN72582014 (first received 27 March 2013). [DOI] [PMC free article] [PubMed]
- Prentice A, ISRCTN96502494. Calcium requirements of pregnant women in The Gambia. isrctn.com/ISRCTN96502494 (first received 6 November 2007).
- Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25‐hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatrica 2009;98(8):1360‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KA, Jarjou L, Prentice A. Maternal calcium supplementation and offspring growth. Osteoporosis International 2016;27(1 Suppl 1):S327‐8. [Google Scholar]
- Ward KA, Prentice A, Jarjou L. Long‐term effects of maternal calcium supplementation on childhood growth differ between males and females in a population accustomed to a low calcium intake. Bone 2017;103:31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2013 {published data only}
- Khan A, Mandal SK, Pal A. Role of high dose calcium in the prevention of preeclampsia. Bangladesh Journal of Obstetrics and Gynecology 2013;28(2):66‐70. [Google Scholar]
Kumar 2009 {published and unpublished data}
- Kumar A, Devi SG, Batra S, Singh C, Shukla DK. Calcium supplementation for the prevention of pre‐eclampsia. International Journal of Gynecology & Obstetrics 2009;104(1):32‐6. [DOI] [PubMed] [Google Scholar]
Li 2000 {published data only}
- Li X, Gou W. Study on prevention of pregnancy induced hypertension and effect of platelet intracellular free ca˜(2+) by calcium supplementation. Journal of Xi'an Medical University 2000;21(1):46‐8. [Google Scholar]
L‐Jaramillo 1989 {published and unpublished data}
- Lopez‐Jaramillo P, Narvaez M, Weigel RM, Yepez R. Calcium supplementation reduces the risk of pregnancy‐induced hypertension in an Andes population. British Journal of Obstetrics and Gynaecology 1989;96:648‐55. [PubMed] [Google Scholar]
- Lopez‐Jaramillo P, Narvaez M, Yepez R. Effect of calcium supplementation on the vascular sensitivity to angiotensin II in pregnant women. American Journal of Obstetrics and Gynecology 1987;156:261‐2. [DOI] [PubMed] [Google Scholar]
- Narvaez M, Lopez‐Jaramillo P, Weigel M. Calcium (Ca++) supplementation reduces the risk for pregnancy induced hypertension (PIH). World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:180‐1.
L‐Jaramillo 1990 {published data only}
- Lopez‐Jaramillo P, Narvaez M, Felix C, Lopez A. Dietary calcium supplementation and prevention of pregnancy hypertension. Lancet 1990;335:293. [DOI] [PubMed] [Google Scholar]
- Narvaez M, Lopez‐Jaramillo P, Weigel M. Calcium (Ca++) supplementation reduces the risk for pregnancy induced hypertension (PIH). World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:180‐1.
L‐Jaramillo 1997 {published data only}
- Lopez‐Jaramillo P, Delgado F, Jacome P, Teran E, Ruano C, Rivera J. Calcium supplementation and the risk of preeclampsia in Ecuadorian pregnant teenagers. Obstetrics & Gynecology 1997;90:162‐7. [DOI] [PubMed] [Google Scholar]
Marya 1987 {published and unpublished data}
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecologic and Obstetric Investigation 1987;24:38‐42. [DOI] [PubMed] [Google Scholar]
Niromanesh 2001 {published data only}
- Niromanesh S, Laghaii S, Mosavi‐Jarrahi A. Supplementary calcium in prevention of pre‐eclampsia. International Journal of Gynecology & Obstetrics 2001;74:17‐21. [DOI] [PubMed] [Google Scholar]
Purwar 1996 {published data only}
- Purwar M, Kulkarni H, Motghare V, Dhole S. Calcium supplementation and prevention of pregnancy induced hypertension. Journal of Obstetrics and Gynaecology Research 1996;22(5):425‐30. [DOI] [PubMed] [Google Scholar]
- Purwar M, Motghare V, Kulkarni H. Calcium supplementation and prevention of pregnancy induced hypertension: randomized double blind controlled trial. Journal of Clinical Epidemiology 1996; Vol. 49, issue Suppl 1:28S. [DOI] [PubMed]
Rogers 1999 {published data only}
- Rogers MS, Fung HYM, Hung CY. Calcium and low‐dose aspirin prophylaxis in women at high risk of pregnancy‐induced hypertension. Hypertension in Pregnancy 1999;18(2):165‐72. [DOI] [PubMed] [Google Scholar]
Rumiris 2006 {published data only}
- Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertension in Pregnancy 2006;25:241–53. [DOI] [PubMed] [Google Scholar]
S‐Ramos 1994 {published data only}
- Sanchez‐Ramos L, Briones DK, Kaunitz AM, Delvalle GO, Gaudier FL, Walker KD. Prevention of pregnancy‐induced hypertension by calcium supplementation in angiotensin II‐sensitive patients. Obstetrics & Gynecology 1994;84:349‐53. [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Delvalle GO, Briones D, Walker C, Delke I, Gaudier F. Prevention of preeclampsia by calcium supplementation in angiotensin‐sensitive patients. American Journal of Obstetrics and Gynecology 1994;170:408. [PubMed] [Google Scholar]
Taherian 2002 {published data only}
- Taherian AA, Taherian A, Shirvani A. Prevention of pre‐eclampsia with low‐dose aspirin or calcium supplementation. Archives of Iranian Medicine 2002;5(3):151‐6. [Google Scholar]
Villar 1987 {published and unpublished data}
- Repke JT, Villar J, Anderson C, Pareja G, Dubin N, Belizan JM. Biochemical changes associated with blood pressure reduction induced by calcium supplementation during pregnancy. American Journal of Obstetrics and Gynecology 1989;160:684‐90. [DOI] [PubMed] [Google Scholar]
- Villar J, Repke J, Belizan JM, Pareja G. Calcium supplementation reduces blood pressure during pregnancy: results of a randomized controlled clinical trial. Obstetrics & Gynecology 1987;70:317‐22. [PubMed] [Google Scholar]
Villar 1990 {published and unpublished data}
- Villar J, Belizan JM, Repke J. The effect of calcium supplementation on the incidence of hypertensive disorders of pregnancy and prematurity. 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:54.
- Villar J, Belizan JM, Repke JT. Does calcium supplementation reduce pregnancy‐induced hypertension and prematurity?. Advances in the prevention of low birthweight; 1988 May 8‐11; Cape Cod, Massachusetts. 1998:187‐95.
- Villar J, Repke JT. Calcium supplementation during pregnancy may reduce preterm delivery in high‐risk populations. American Journal of Obstetrics and Gynecology 1990;163:1124‐31. [DOI] [PubMed] [Google Scholar]
WHO 2006 {published and unpublished data}
- Abalos E, Merialdi M, Wojdyla D, Carroli G, Campodónico L, Yao SE, et al. Effects of calcium supplementation on fetal growth in mothers with deficient calcium intake: a randomised controlled trial. Paediatric and Perinatal Epidemiology 2010;24(1):53‐62. [DOI] [PubMed] [Google Scholar]
- Abdel‐Aleem H, Merialdi M, Elsnosy ED, Elsedfy GO, Abdel‐Aleem MA, Villar J. The effect of calcium supplementation during pregnancy on fetal and infant growth: a nested randomized controlled trial within WHO calcium supplementation trial. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(2):94‐100. [DOI] [PubMed] [Google Scholar]
- Carroli G, Merialdi M, Wojdyla D, Abalos E, Campodonico L, Yao SE, et al. Effects of calcium supplementation on uteroplacental and fetoplacental blood flow in low‐calcium‐intake mothers: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(1):45.e1‐45.e9. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Mlokoti Z, Nikodem VC, Mangesi L, Ferreira S, Singata M, et al. Calcium supplementation during pregnancy for preventing hypertensive disorders is not associated with changes in platelet count, urate, and urinary protein: a randomized control trial. Hypertension in Pregnancy 2008;27(3):299‐304. [DOI] [PubMed] [Google Scholar]
- Villar J, Abdel‐Aleem H, Merialdi M, Mathai M, Ali M, Zavaleta N, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. American Journal of Obstetrics and Gynecology 2006;194:639‐49. [DOI] [PubMed] [Google Scholar]
- Villar J, Aleem HA, Merialdi M, Mathai M, Ali M, Zavaleta N, et al. WHO randomized trial of calcium supplementation among low calcium intake pregnant women [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S2. [DOI] [PubMed] [Google Scholar]
- Villar J, ISRCTN37214165. Calcium supplementation during pregnancy in low‐intake populations. isrctn.com/ISRCTN37214165 (first received 19 March 2014).
- Zhang J, Villar J, Sun W, Merialdi M, Abdel‐Aleem H, Mathai M, et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2007;197(2):162.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aghamohammady 2010 {published data only}
- Aghamohammadi A, Rajabi A. The effect of calcium supplementation during pregnancy on preterm delivery and preeclampsia in nulliparous beyond age 35. Acta Obstetricia et Gynecologica Scandinavica 2012;91 (Suppl 159):60. [Google Scholar]
- Aghamohammady A. The effect of calcium supplementation during pregnancy on preterm delivery in primiparous beyond age 35. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):100. [Google Scholar]
Anu 2017 {published data only}
- Anu MS, CTRI/2017/03/008259. Ayurvedic management in preventing intra uterine growth retardation. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=18028 (first received 30 March 2017).
Anumba 2006 {published data only}
- Anumba D, ISRCTN25268811. Randomised double blind placebo controlled trial of calcium supplements in teenage pregnancy. isrctn.com/ISRCTN25268811 (first received 29 September 2006).
Asemi 2017 {published data only}
- Asemi Z, IRCT201704225623N109. Clinical trial of the effect of multi mineral‐vitamin D supplementation compared with the placebo on pregnancy outcomes in women with gestational diabetes. en.search.irct.ir/view/37291 (first received 25 April 2017).
August 2002 {published data only}
- August P, Sison M, Helseth G. Identification of prognostic indices and impact of calcium supplementation in women at high risk for pre‐eclampsia: data from a randomized clinical trial [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):44. [Google Scholar]
- August P, Sison MC, Helseth G. Clinical outcomes of African Americans with chronic hypertension during pregnancy. Hypertension in Pregnancy 2002; Vol. 21, issue Suppl 1:55.
Azami 2017 {published data only}
- Azami M, Azadi T, Farhang S, Rahmati S, Pourtaghi K. The effects of multi mineral‐vitamin D and vitamins (C+E) supplementation in the prevention of preeclampsia: an RCT. International Journal of Reproductive Biomedicine 2017; Vol. 15, issue 5:273‐8. [PMC free article] [PubMed]
- Azami M, IRCT2016062528617N1. The effects of multi minerals (Zn, Mg and Ca) and vitamins (C and E) supplementation in the prevention of preeclampsia. en.search.irct.ir/view/31125 (first received 24 September 2016).
Belizan 1983 {published data only}
- Belizan JM, Villar J, Zalazar A, Rojas L, Chan D, Bryce GF. Preliminary evidence of the effect of calcium supplementation on blood pressure in normal pregnant women. American Journal of Obstetrics and Gynecology 1983;146:175‐80. [DOI] [PubMed] [Google Scholar]
Bhatia 2010 {published data only}
- Bhatia V, CTRI/2010/091/000499. Vitamin D and calcium nutrition: deficiency and evaluation of supplementation. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1674 (first received 19 May 2010).
Boggess 1997 {published data only}
- Boggess KA, Samuel L, Schmuckler BC, Waters J, Easterling TR. A randomised controlled trial of the effect of third trimester calcium supplementation on maternal hemodynamic function. Obstetrics & Gynecology 1997;90:157‐61. [DOI] [PubMed] [Google Scholar]
Chames 2002 {published data only}
- Chames M, Bendich A, Bogden J, Sibai B, Prada J. A randomized trial of calcium supplementation effects on blood lead levels in pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S137. [Google Scholar]
Chan 2006 {published data only}
- Chan G, NCT00320125. Effects of dairy foods on adolescent pregnant mothers and their newborns. https://clinicaltrials.gov/ct2/show/record/NCT00320125 (first received 28 April 2006). [NCT00320125]
de Souza 2006 {published data only}
- Souza EV. Aspirin and calcium to prevent preeclampsia in chronic hypertensive women with abnormal uterine artery Doppler ultrasound [abstract] [Acido acetilalicilico associado ao calcio na prevencao da pre‐eclampsia em gestantes hipertensas cronicas selecionadas pela dopplervelocimetria das arterias uterinas]. Revista Brasileira de Ginecologia y Obstetricia 2006;28(2):136. [Google Scholar]
- Souza EV, Sass N, Atallah AN, Kular L Jr. Aspirin and calcium to prevent pre eclampsia in chronic hypertension women with abnormal uterine artery doppler ultrasound [abstract]. Hypertension in Pregnancy 2006;25(Suppl 1):152. [Google Scholar]
Diogenes 2011 {published data only}
- Bezerra FF, NCT01732328. Calcium plus vitamin D supplementation during pregnancy of adolescent mothers: effects on maternal and infant bone mass, calcium and bone metabolism and breast milk composition. clinicaltrials.gov/show/NCT01732328 (first received 19 November 2012).
- Diogenes ME, Bezerra FF, Rezende EP, Donangelo CM. Calcium plus vitamin D supplementation during third trimester of pregnancy in adolescents accustomed to low calcium diets did not affect infant bone mass at early lactation in a randomized controlled trial. Journal of Nutrition 2015;145:1515‐23. [DOI] [PubMed] [Google Scholar]
- Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: a randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2013;98(1):82‐91. [DOI] [PubMed] [Google Scholar]
- Diogenes MEL, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Maternal vitamin D status of adolescent mothers at mid‐pregnancy influence bone mineral content of their newborns. FASEB Journal 2011;25:603.19. [Google Scholar]
- Normando P, Diogenes ME, Cabello PH, Cabello GM, Donangelo CM, Bezerra FF. Calcium plus vitamin D supplementation during pregnancy interacts with polymorphisms in the promoter region of the VDR gene to affect postpartum bone mass of Brazilian adolescent mothers: a randomized controlled trial. Nutrition 2016;32(10):1068‐74. [DOI] [PubMed] [Google Scholar]
Dizavandy 1998 {published data only}
- Dizavandy EB, Alavi GS, Cordi M. The effect of calcium supplementation in the prevention of hypertensive disorders of pregnancy in nulliparous women. Medical Journal of the Islamic Republic of Iran 1998;12(1):11‐4. [Google Scholar]
Ettinger 2011 {published data only}
- Ettinger AS, Lamadrid H, Mercado A, Kordas K, Peterson K, Hu H, et al. Effect of calcium on bone resorption and bone mineral density in pregnancy: A randomized control trial. American Journal of Epidemiology 2011;173(Suppl):S236. [Google Scholar]
- Hu H, Hernandez‐Avila M, NCT00558623. Controlled trial in pregnancy of dietary supplements for suppression of bone resorption and mobilization of lead into plasma. clinicaltrials.gov/show/NCT00558623 (first received 14 November 2007).
Felix 1991 {published data only}
- Felix C, Jacome P, Lopez A, Moya W, Narvaez M, Lopez‐Jaramillo P. The hypotensive effect of calcium supplementation during normal pregnancy in Andean women is not related to vascular production of prostacyclin by umbilical arteries. Journal of Obstetrics and Gynaecology 1991;11(2):93‐6. [Google Scholar]
Fung 2010 {published data only}
- Fung E, NCT01145573. Testing the calcium DRI during pregnancy: a study of bone health in black and white women. clinicaltrials.gov/show/NCT01145573 (first received 11 June 2010). [NCT01145573]
Herrera 2006a {published data only}
- Herrera JA, Arevalo‐Herrera M, Villegas A, Herrera S, Villalba M, Bromet A. Calcium oral supplementation in adolescent pregnant women. Colombia Medica 2006;37(2 Suppl 1):15‐20. [Google Scholar]
Hofmeyr 2015 {published data only}
- Hofmeyr GJ, PACTR201105000267371. WHO randomized trial of calcium supplementation before pregnancy to reduce recurrent pre‐eclampsia. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201105000267371 (first received 6 December 2010).
- Hofmeyr GJ, Seuc AH, Betran AP, Purnat TD, Ciganda A, Munjanja SP, et al. The effect of calcium supplementation on blood pressure in non‐pregnant women with previous pre‐eclampsia: an exploratory, randomized placebo controlled study. Pregnancy Hypertension 2015;5(4):273‐9. [DOI] [PubMed] [Google Scholar]
Karamali 2016 {published data only}
- Karamali M, Asemi Z, Ahmadi‐Dastjerdi M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double‐blind, placebo‐controlled trial. Public Health Nutrition 2016;19(1):156‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamali M, IRCT201407115623N23. Effect of vitamin D plus calcium co‐supplementation on pregnancy outcomes in gestational diabetes. en.search.irct.ir/view/19118 (first received 21 July 2014).
Karandish 2003 {published data only}
- Karandish M, Djazayery A, Mahmoudi M, Behrooz A. The effect of calcium supplementation during pregnancy on birth weight. Medical Journal of Reproduction and Infertility 2003;4(3):184. [Google Scholar]
Kawasaki 1985 {published data only}
- Kawasaki N, Matsui K, Ito M, Nakamura T, Yoshimura T, Ushijima H, et al. Effect of calcium supplementation on the vascular sensitivity to angiotensin II in pregnant women. American Journal of Obstetrics and Gynecology 1985;153:576‐82. [DOI] [PubMed] [Google Scholar]
Knight 1992 {published data only}
- Knight KB, Keith RE. Calcium supplementation on normotensive and hypertensive pregnant women. American Journal of Clinical Nutrition 1992;55:891‐5. [DOI] [PubMed] [Google Scholar]
Lavin 1986 {unpublished data only}
- Lavin JP. The effect of calcium supplementation on pregnancy induced hypertension. Personal communication 1986.
MacDonald 1986 {unpublished data only}
- MacDonald HN. Fetal and maternal benefits from calcium and vitamin D supplementation of pregnant Asians. Personal communication 1986.
Martin 2017 {published data only}
- Dickin K. A cluster‐randomized, non‐inferiority open‐label trial of the impact of supplementation regimen on consumption of prenatal calcium and iron/folic acid supplements and adherence to related recommendations. clinicaltrials.gov/ct2/show/record/NCT02238704 (first received 5 September 2014). [NCT02238704]
- Martin SL, Omotayo MO, Pelto GH, Chapleau GM, Stoltzfus RJ, Dickin KL. Adherence‐specific social support enhances adherence to calcium supplementation regimens among pregnant women. Journal of Nutrition 2017;147(4):688‐96. [DOI] [PubMed] [Google Scholar]
- Omotayo M, Stoltzfus R, Martin S, Kung'u J, Dickin K. WHO guidelines on calcium supplementation for prevention of preeclampsia: adoption, feasibility and acceptability in rural Kenya. FASEB Journal 2016;30:[abstract no: 1149.29]. [Google Scholar]
- Omotayo MO, Dickin KL, Chapleau GM, Martin SL, Chang C, Mwanga EO, et al. Cluster‐randomized non‐inferiority trial to compare supplement consumption and adherence to different dosing regimens for antenatal calcium and iron‐folic acid supplementation to prevent preeclampsia and anaemia: rationale and design of the micronutrient initiative study. Journal of Public Health Research 2015;4(3):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotayo MO, Dickin KL, Pelletier DL, Mwanga EO, Kung'u JK, Stoltzfus RJ. A simplified regimen compared with who guidelines decreases antenatal calcium supplement intake for prevention of preeclampsia in a cluster‐randomized noninferiority trial in rural kenya. Journal of Nutrition 2017 [epub ahead print]. [DOI] [PubMed]
Montanaro 1990 {published data only}
- Montanaro D, Boscutti G, Antonucci F, Messa P, Mioni G, Driul P, et al. Prevention of pregnancy‐induced hypertension (PIH) and pre‐eclampsia (PE) by oral calcium supplementation. Proceedings of the 10th International Congress of Nephrology; 1987 July 26‐31; London, UK. 1987:281.
- Montanaro D, Boscutti G, Mioni G, Driul P, Tosolini G. Calcium supplementation decreases the incidence of pregnancy‐induced hypertension (PIH) and pre‐eclampsia (PE). 7th World Congress of Hypertension in Pregnancy; 1990; Perugia, Italy. 1990:267.
Mosalanejad 2016 {published data only}
- Mosalanejad N, IRCT2016121430612N2. Compare the effect of vitamin D and calcium plus vitamin D on pregnancy outcomes in pregnant women. en.search.irct.ir/view/34642 (first received 26 December 2016).
NCT00000543 {published data only}
- NCT00000543. Oral calcium in pregnant women with hypertension. clinicaltrials.gov/ct2/show/record/NCT00000543 (first received 27 October 1999).
Nooripour 2016 {published data only}
- Nooripour S, IRCT2015041321736N1. Clinical trial on the evaluation of calcium and vitamin d in the cord serum of neonates, whose mothers were under vitamin d treatment during their pregnancy. en.search.irct.ir/view/22976 (first received 1 February 2016).
Prada 2001 {published data only}
- Prada J, Tsang R, Guo S. Reduction of blood pressure from calcium supplementation in adolescent pregnancy: a randomized trial [abstract]. American Journal of Hypertension 2001;14(4 Pt 2):179A. [Google Scholar]
Prada 2002 {published data only}
- Prada JA, Sibai BM, Guo S. Effect of calcium supplementation on the maternal blood pressure of adolescents and twins [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S217. [Google Scholar]
Raman 1978 {published data only}
- Raman L, Rajalakshmi K, Krishnamachari K, Gowrinath Sastry J. Effect of calcium supplementation to undernourished mothers during pregnancy on the bone density of neonates. American Journal of Clinical Nutrition 1978;31:466‐9. [DOI] [PubMed] [Google Scholar]
Repke 1989 {published data only}
- Repke J, Villar J, Bergel E, Belizan JM. The effect of iron absorption in patients receiving calcium supplementation. 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 February 1‐4; New Orleans, Louisiana, USA. 1989:512.
Roth 2014 {published data only}
- Roth D, Zlotkin S, NCT01678079. Bioavailability and acceptability of enteric‐coated microencapsulated calcium during pregnancy: a randomized crossover trial in Bangladesh (Encapsulated Calcium Absorption in Pregnancy). clinicaltrials.gov/show/NCT01678079 (first received 30 August 2012).
- Roth DE, Pezzack B, Al Mahmud A, Abrams SA, Islam M, Aimone Phillips A, et al. Bioavailability of enteric‐coated microencapsulated calcium during pregnancy: a randomized crossover trial in Bangladesh. American Journal of Clinical Nutrition 2014;100(6):1587‐95. [DOI] [PubMed] [Google Scholar]
Salzano 2001 {published data only}
- Salzano P, Felicetti M, Laboccetta A, Borrelli P, Domenico A, Borrelli A. Prevention of gestational hypertension with calcium, linoleic acid, mono and polyunsaturated fatty acid supplements. Minerva Ginecologica 2001;53(4):235‐8. [PubMed] [Google Scholar]
Samimi 2016 {published data only}
- Samimi M, IRCT201102135444N3. Effects of calcium and vitamin D co‐supplementation on pre‐eclampsia parameters, metabolic status and biomarkers of oxidative stress in pregnant women at risk for pre‐eclampsia. en.search.irct.ir/view/5288 (first received 31 January 2015).
- Samimi M, Kashi M, Foroozanfard F, Karamali M, Bahmani F, Asemi Z, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre‐eclampsia. Journal of Human Nutrition and Dietetics 2016;29(4):505‐16. [DOI] [PubMed] [Google Scholar]
Souza 2014 {published data only}
- Souza EV, Torloni MR, Atallah AN, Dos Santos GM, Kulay Jr L, Sass N. Aspirin plus calcium supplementation to prevent superimposed preeclampsia: a randomized trial. Brazilian Journal of Medical and Biological Research 2014;47(5):419‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
S‐Ramos 1995 {published data only}
- Sanchez‐Ramos L, Adair CD, Delvalle GO, Gaudier F, Delke I. Calcium supplementation in mild preeclampsia remote from term: a prospective randomized double‐blind clinical trial. American Journal of Obstetrics and Gynecology 1993;168:385. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Adair D, Kaunitz AM, Briones DK, Delvalle GO, Delke I. Calcium supplementation in mild pre‐eclampsia remote from term: a randomized double‐blind clinical trial. Obstetrics & Gynecology 1995;85:915‐8. [DOI] [PubMed] [Google Scholar]
Subprabha 2017 {published data only}
- Subprabha K, CTRI/2016/10/007373. Role of madhuraushadha siddha avaleha as garbhini rasayana in the 6th and 7th month of pregnancy. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=15659 (first received 22 May 2017).
Suzuki 1996 {published data only}
- Suzuki Y, Itoh Y, Hayashi Y, Murakami I, Yamaguchi K, Ohshima T, et al. Calcium supplementation to prevent gestational hypertension. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington. 1996:113.
Tamas 1997 {published data only}
- Tamas P, Szabo I, Szekely J, Csermely T, Prievara FT, Nemeth L, et al. Effects of Doxium 500 (R) in gestational hypertension [A doxium 500 (R) Hatasanak vizsgalata terhessegi Hypertoniaban (kettos vak, placebo‐kontrollalt tanulmany)]. Magyar Noorvosok Lapja 1997;60(3):181‐7. [Google Scholar]
Wanchu 2001 {published data only}
- Wanchu M, Malhotra S, Khullar M. Calcium supplementation in pre‐eclampsia. Journal of the Association of Physicians of India 2001;49:795‐8. [PubMed] [Google Scholar]
Zheng 2000 {published data only}
- Zheng QS, Zhang YP. Clinical experience with calcium supplementation in pregnancy. Journal of Practical Obstetrics and Gynecology 2000;16(2):102‐3. [Google Scholar]
References to studies awaiting assessment
Liu 2013 {published data only}
- Liu X, NCT01806454. Calcium supplementation to prevent preeclampsia in sichuan province of china: a multi‐center, prospective random trial. https://clinicaltrials.gov/ct2/show/NCT01806454. NCT01806454 (first received 1 March 2013) 2013.
References to ongoing studies
Chaiarach 2017 {published data only}
- Chaiarach S, TCTR20170629006. Combined therapy with low dose aspirin and calcium supplements during second trimester to reduce the risk of superimposed preeclampsia in pregnant women with chronic hypertension: a randomized‐controlled trial. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2628. TCTR20170629006 (first received 21 June 2017) 2017.
Mahomed 1998 {unpublished data only}
- Mahomed K, Marume A, Hammond N, Madzima M. Calcium supplementation for the prevention of pregnancy induced hypertension and preterm labour in twin pregnancies: a randomised controlled trial. Personal communication 1998.
Sulovic 2013 {published data only}
- Sulovic N, Kontic‐Vucinic O, Relic G, Sulovic L. Did calcium management prevent preeclampsia?. Pregnancy Hypertension 2011;1:287. [DOI] [PubMed] [Google Scholar]
- Sulovic N, Kontic‐Vucinic O, Sulovic L, Relic G, Nebojsa R. Did calcium management prevent preeclampsia?. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:454. [Google Scholar]
Torloni 2015 {published data only}
- Torloni MR, NCT02338687. Low dose calcium supplementation to prevent preeclampsia: a cluster randomized study. clinicaltrials.gov/show/NCT02338687 (first received 11 January 2015).
Additional references
Belizan 1980
- Belizan JM, Villar J. The relationship between calcium intake and edema, proteinuria, and hypertension‐gestosis: an hypothesis. American Journal of Clinical Nutrition 1980;33:2202‐10. [DOI] [PubMed] [Google Scholar]
Belizan 1988
- Belizan JM, Villar J, Repke J. The relationship between calcium intake and pregnancy‐induced hypertension: up‐to‐date evidence. American Journal of Obstetrics and Gynecology 1988;158:898‐902. [DOI] [PubMed] [Google Scholar]
Belizan 1997
- Belizan JM, Villar J, Bergel E, Pino A, Fulvio S, Galliano SV, et al. Long term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ 1997;315:281‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergel 2002
- Bergel E, Belizan JM. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. BJOG: an international journal of obstetrics and gynaecology 2002;109:540‐5. [PubMed] [Google Scholar]
Betrán 2005
- Betrán AP, Wojdyla D, Posner SF, Gülmezoglu AM. National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health 2005;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucher 1996
- Bucher HC, Guyatt GH, Cook RJ. Effect of calcium supplementation on pregnancy‐induced hypertension and preeclampsia: a meta‐analysis of randomized controlled trials. JAMA 1996;275:1113‐7. [DOI] [PubMed] [Google Scholar]
Carroli 1994
- Carroli G, Duley L, Belizan JM, Villar J. Calcium supplementation during pregnancy: a systematic review of randomised controlled trials. British Journal of Obstetrics and Gynaecology 1994;101(9):753‐8. [DOI] [PubMed] [Google Scholar]
Carroli 2010
- Carroli G, Merialdi M, Wojdyla D, Abalos E, Campodonico L, Yao SE, et al. Effects of calcium supplementation on uteroplacental and fetoplacental blood flow in low‐calcium‐intake mothers: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010; Vol. 202:45.e1‐45.e9. [DOI] [PubMed]
Clark 2008
- Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(1):36.e1‐e5. [DOI] [PubMed] [Google Scholar]
CLASP 1994
- CLASP (Collaborative Low‐dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low‐dose aspirin for the prevention and treatment of pre‐eclampsia among 9364 pregnant woman. Lancet 1994;343:619‐29. [PubMed] [Google Scholar]
De‐Regil 2012
- De‐Regil LM, Palacios C, Ansary A, Kulier R, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008873.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duvekot 2002
- Duvekot EJ, Groot CJ, Bloemenkamp KW, Oei SG. Pregnant women with a low milk intake have an increased risk of developing preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;105:11‐4. [DOI] [PubMed] [Google Scholar]
ECCPA 1996
- ECPPA (Estudo Collaborativo para Prevenção da Pre‐eclampsia com Aspirina) Collaborative Group. ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant woman. British Journal of Obstetrics and Gynaecology 1996;103:39‐47. [DOI] [PubMed] [Google Scholar]
Hamlin 1952
- Hamlin RHJ. The prevention of eclampsia and pre‐eclampsia. Lancet 1952;i:64‐8. [DOI] [PubMed] [Google Scholar]
Hamlin 1962
- Hamlin RHJ. Prevention of pre‐eclampsia. Lancet 1962;1:864‐5. [Google Scholar]
Hatton 2003
- Hatton DC, Harrison‐Hohner J, Coste S, Reller M, McCarron D. Gestational calcium supplementation and blood pressure in the offspring. American Journal of Hypertension 2003;16:801‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hiller 2007
- Hiller JE, Crowther CA, Moore VA, Willson K, Robinson JS. Calcium supplementation in pregnancy and its impact on blood pressure in children and women: follow up of a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(2):115‐21. [DOI] [PubMed] [Google Scholar]
HMSO 1994
- HMSO. Report on confidential enquiries into maternal deaths in the United Kingdom 1988‐1990. Department of Health Welsh Office, Scottish Office Home and Health Department, Department of Health and Social Security, Northern Ireland. London: HMSO, 1994. [Google Scholar]
Hofmeyr 2007
- Hofmeyr G, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre‐eclampsia and related problems: a systematic review and commentary. BJOG: an international journal of obstetrics and gynaecology 2007;114(8):933‐43. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2008
- Hofmeyr GJ, Mlokoti Z, Nikodem VC, Mangesi L, Ferreira S, Singata M, et al. Calcium supplementation during pregnancy for preventing hypertensive disorders is not associated with changes in platelet count, urate, and urinary protein: a randomized control trial. Hypertension in Pregnancy 2008;27(3):299‐304. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2017
- Hofmeyr GJ, Manyame S. Calcium supplementation commencing before or early in pregnancy, or food fortification with calcium, for preventing hypertensive disorders of pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD011192.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isezuo 2004
- Isezuo SA, Ekele BA. Eclampsia and abnormal QTc. West African Journal of Medicine 2004;23:123‐7. [DOI] [PubMed] [Google Scholar]
Johnson 1993
- Johnson A, Townshend P, Yudkin P, Bull D, Wilkinson AR. Functional abilities at age 4 years of children born before 29 weeks gestation. BMJ 1993;306:1715‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazerooni 2003
- Kazerooni T, Hamze‐Nejadi S, Kazerooni T, Hamze‐Nejadi S. Calcium to creatinine ratio in a spot sample of urine for early prediction of pre‐eclampsia. International Journal of Gynecology & Obstetrics 2003;80:279‐83. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74. [DOI] [PubMed] [Google Scholar]
Kisters 2000
- Kisters K, Barenbrock M, Louwen F, Hausberg M, Rahn KH, Kosch M. Membrane, intracellular, and plasma magnesium and calcium concentrations in preeclampsia. American Journal of Hypertension 2000;13:765‐9. [DOI] [PubMed] [Google Scholar]
Kumru 2003
- Kumru S, Aydin S, Simsek M, Sahin K, Yaman M, Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in preeclamptic and healthy pregnant women. Biological Trace Element Research 2003;94:105‐12. [DOI] [PubMed] [Google Scholar]
Langenveld 2011
- Langenveld J, Ravelli AC, Kaam AH, Ham DP, Pampus MG, Porath M, et al. Neonatal outcome of pregnancies complicated by hypertensive disorders between 34 and 37 weeks of gestation: a 7 year retrospective analysis of a national registry. American Journal of Obstetrics and Gynecology 2011;205(6):540.e1‐540.e7. [DOI] [PubMed] [Google Scholar]
Li 2012
- Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC‐Heidelberg). Heart 2012;98(12):920‐5. [DOI] [PubMed] [Google Scholar]
McGarvey 1991
- McGarvey ST, Zinner SH, Willett WC, Rosner B. Maternal prenatal dietary potassium, calcium, magnesium and infant blood pressure. Hypertension 1991;17:218‐24. [DOI] [PubMed] [Google Scholar]
NHBPEP 2000
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183:S1‐S22. [PubMed] [Google Scholar]
NHMRC 1993
- NHMRC. NHMRC Report on Maternal Deaths in Australia 1988‐1990. Canberra: Government Publishing Service, 1993. [Google Scholar]
Ozkan 2011
- Ozkan H, Cetinkaya M, Koksal N, Ozmen A, Yıldız M. Maternal preeclampsia is associated with an increased risk of retinopathy of prematurity. Journal of Perinatal Medicine 2011;39(5):523‐7. [DOI] [PubMed] [Google Scholar]
Repke 1991
- Repke JT, Villar J. Pregnancy‐induced hypertension and low birth weight: the role of calcium. American Journal of Clinical Nutrition 1991;54:237S‐241S. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rumbold 2008
- Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004227.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Segovia 2004
- Segovia BL, Vega IT, Villarreal EC, Licona NA. Hypocalciuria during pregnancy as a risk factor of preeclampsia. Ginecologia y Obstetricia de Mexico 2004;72:570‐4. [PubMed] [Google Scholar]
Villar 1983
- Villar J, Belizan JM, Fischer PJ. Epidemiologic observations on the relationship between calcium intake and eclampsia. International Journal of Gynecology & Obstetrics 1983;21:271‐8. [DOI] [PubMed] [Google Scholar]
Villar 1989
- Villar J, Repke J, Markush L, Calvert W, Rhoads G. The measuring of blood pressure during pregnancy. American Journal of Obstetrics and Gynecology 1989;161:1019‐24. [DOI] [PubMed] [Google Scholar]
Villar 1993
- Villar J, Belizan JM, Fisher PJ. Epidemiologic observation on the relationship between calcium intake and eclampsia. International Journal of Gynecology & Obstetrics 1993;21:271‐8. [DOI] [PubMed] [Google Scholar]
Villar 1994
- Villar J, Ezcurra EJ, Gurtner de la Fuente V, Campodonico L. Preterm delivery syndrome: the unmet need. Research and Clinical Forums 1994;16:9‐39. [Google Scholar]
Villar 1998
- Villar J, Gulmezoglu AM, Onis M. Nutritional and antimicrobial interventions to prevent preterm birth: an overview of randomized controlled trials. Obstetrical and Gynecological Survey 1998;53(9):575‐85. [DOI] [PubMed] [Google Scholar]
Villar 2000
- Villar J, Belizan JM. Same nutrient, different hypotheses: disparities in trials of calcium supplementation during pregnancy. American Journal of Clinical Nutrition 2000;71 Suppl:1375S‐1379S. [PubMed] [Google Scholar]
Villar 2004
- Villar J, Say L, Shennan A, Lindheimer M, Duley L, Conde‐Agudelo A, et al. Methodological and technical issues related to the diagnosis, screening, prevention and treatment of pre‐eclampsia and eclampsia. International Journal of Gynecology & Obstetrics 2004;85(Suppl 1):S28‐S41. [DOI] [PubMed] [Google Scholar]
von Dadelszen 2004
- Dadelszen P, Magee LA, Devarakonda RM, Hamilton T, Ainsworth LM, Yin R, et al. The prediction of adverse maternal outcomes in pre‐eclampsia. Journal of Obstetrics and Gynaecology Canada: JOGC 2004;26:871‐9. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO recommendations for prevention and treatment of pre‐eclampsia and eclampsia. whqlibdoc.who.int/publications/2011/9789241548335_eng.pdf 2011. [PubMed]
Yamasmit 2004
- Yamasmit W, Chaithongwongwatthana S, Charoenvidhya D, Uerpairojkit B, Tolosa J. Random urinary protein‐to‐creatinine ratio for prediction of significant proteinuria in women with preeclampsia. Journal of Maternal‐Fetal & Neonatal Medicine 2004;16:275‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2007
- Zhang J, Villar J, Sun W, Merialdi M, Abdel‐Aleem H, Mathai M, et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2007;197(2):162.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Duley 1995
- Duley L. Routine calcium supplementation in pregnancy. [revised 23 June 1993] In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hofmeyr 2002
- Hofmeyr GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001059] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2006
- Hofmeyr GJ, Atallah ÁN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001059.pub2] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD001059.pub3] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2014
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001059.pub4] [DOI] [PubMed] [Google Scholar]


