Abstract
Background
Moxibustion, a common treatment in traditional Chinese medicine, involves burning herbal preparations containing Artemisia vulgaris on or above the skin at acupuncture points. Its intended effect is to enhance body function, and it could reduce the side effects of chemotherapy or radiotherapy and improve quality of life (QoL) in people with cancer.
Objectives
To assess the effects of moxibustion for alleviating side effects associated with chemotherapy, radiotherapy or both in people with cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE via Ovid, Embase via Ovid and AMED (Allied and Complementary Medicine Database) from their inception to February 2018. We also searched databases in China including the Chinese BioMedical Literature Database (CBM), Chinese Medical Current Contents (CMCC), TCMonline, Chinese Dissertation Database (CDDB), China Medical Academic Conference (CMAC) and Index to Chinese Periodical Literature from inception to August 2017. Registries for clinical trials and other resources were also searched.
Selection criteria
We included randomised controlled trials (RCTs) comparing moxibustion treatment, including moxa cone and moxa stick, versus sham, no treatment or conventional treatment.
Data collection and analysis
Two review authors (HWZ and FC) independently extracted data on study design, participants, treatment and control intervention, and outcome measures, and they also assessed risk of bias in the included studies. We performed meta‐analyses, expressing dichotomous outcomes as risk ratios (RR) and continuous outcomes as mean differences (MD), with 95% confidence intervals (CI).
Main results
We included 29 RCTs involving 2569 participants. Five RCTs compared moxibustion versus no treatment, 15 compared moxibustion plus conventional treatment versus conventional treatment, one compared moxibustion versus sham moxibustion, and eight compared moxibustion versus conventional medicine. The overall risk of bias was high in 18 studies and unclear in 11 studies. Studies measured outcomes in various ways, and we could rarely pool data.
Moxibustion versus no treatment: low‐certainty evidence from single small studies suggested that moxibustion was associated with higher white blood cell counts (MD 1.77 × 109/L; 95% CI 0.76 to 2.78; 80 participants, low‐certainty evidence) and higher serum haemoglobin concentrations (MD 1.33 g/L; 95% CI 0.59 to 2.07; 66 participants, low‐certainty evidence) in people with cancer, during or after chemotherapy/radiotherapy, compared with no treatment. There was no evidence of an effect on leukopenia (RR 0.50, 95% CI 0.10 to 2.56; 72 participants, low‐certainty evidence) between study groups. The effects on immune function (CD3, CD4, and CD8 counts) were inconsistent.
Moxibustion versus sham moxibustion: low‐certainty evidence from one study (50 participants) suggested that moxibustion improved QoL (measured as the score on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐C30 (EORTC QLQ‐C30)) compared with sham treatment (MD 14.88 points; 95% CI 4.83 to 24.93). Low‐certainty evidence from this study also showed reductions in symptom scores for nausea and vomiting (MD −38.57 points, 95% CI −48.67 to −28.47) and diarrhoea (MD −13.81, 95% CI −27.52 to −0.10), and higher mean white blood cell count (MD 1.72 × 109/L, 95% CI 0.97 to 2.47), serum haemoglobin (MD 2.06 g/L, 95% CI 1.26 to 2.86) and platelets (MD 210.79 × 109/L, 95% CI 167.02 to 254.56) when compared with sham moxibustion.
Moxibustion versus conventional medicines: low‐certainty evidence from one study (90 participants) suggested that moxibustion improved WBC count eight days after treatment ended compared with conventional medicines (MD 0.40 × 109/L; 95% CI 0.15 to 0.65). Low‐certainty evidence from two studies (235 participants) suggested moxibustion improved serum haemoglobin concentrations compared with conventional medicines (MD 10.28 g/L; 95% CI 4.51 to 16.05).
Moxibustion plus conventional treatment versus conventional treatment alone: low‐certainty evidence showed that moxibustion plus conventional treatment was associated with lower incidence and severity of leukopenia (WHO grade 3 to 4) (RR 0.14, 95% CI 0.01 to 2.64; 1 study, 56 participants), higher QoL scores on the EORTC QLQ‐C30 (MD 8.85 points, 95% CI 4.25 to 13.46; 3 studies, 134 participants, I² = 26%), lower symptom scores for nausea and vomiting (RR 0.43, 95% CI 0.25 to 0.74; 7 studies, 801participants; I² = 19%), higher white blood cell counts (data not pooled due to heterogeneity), higher serum haemoglobin (MD 3.97 g/L, 95% CI 1.40 to 6.53; 2 studies, 142 participants, I² = 0%). There was no difference in platelet counts between the two groups (MD 13.48 × 109/L; 95% CI −16.00 to 42.95; 2 studies, 142 participants; I² = 34%).
Most included studies did not report related adverse events, such as burning or allergic reactions.
Authors' conclusions
Limited, low‐certainty evidence suggests that moxibustion treatment may help to reduce the haematological and gastrointestinal toxicities of chemotherapy or radiotherapy, improving QoL in people with cancer; however, the evidence is not conclusive, and we cannot rule out benefits or risks with this treatment. High‐quality studies that report adverse effects are needed.
Plain language summary
Moxibustion for alleviating side effects of chemotherapy or radiotherapy in people with cancer
The issue Moxibustion is used in traditional Chinese medicine to enhance quality of life and relieve the side effects of conventional treatments for a variety of diseases. As its application involves the burning of a herbal preparation, it can also cause some undesirable side effects itself, such as allergic reactions, burns and infection.
The aim of the review We conducted this systematic review to understand whether moxibustion can reduce common side effects of chemotherapy and radiotherapy and improve well‐being in people with cancer.
Selection criteria We reviewed 29 studies involving 2569 people with different types of cancer, receiving chemotherapy, radiotherapy or both.
What are the main findings? We found some small single studies showing various beneficial effects of moxibustion on increasing blood cells and promoting immunological function, decreasing gastrointestinal symptoms caused by toxicity of chemotherapy or radiotherapy (such as nausea and vomiting), and improving quality of life. However, the poor reporting and high risk of bias in study methods reduced the certainty of the evidence.
What is the certainty of the evidence? The evidence was of low or very low‐certainty.
What are the conclusions? There is presently no good evidence to support or oppose the use of moxibustion in people receiving treatment for cancer. High‐quality studies are needed, which should include reporting of adverse effects.
Summary of findings
Background
Description of the condition
Cancer rates have steadily increased over the past few decades, placing a huge burden on health systems worldwide. The global economic burden of cancer has more than doubled over the past 30 years. Cancer is the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases reported in 2012, and it is expected that annual cancer cases will rise to 22 million within the next two decades. The five most common sites of cancer diagnosed in men in 2012 were the lung, prostate, colon/rectum, stomach and liver, and in women they were the breast, colon/rectum, lung, cervix and stomach (WHO 2014).
Conventional cancer treatments include surgery, radiotherapy, chemotherapy and psychosocial support (WHO 2002). Advances in chemotherapy and radiotherapy in recent years have greatly improved treatment results (WHO 2014). However, cytotoxic drugs and ionising radiation also cause many distressing side effects. Some of these are serious enough to prompt discontinuation of treatment (Redmond 1996; Robbins 2002; WHO 2014). The side effects most commonly associated with chemotherapy and radiotherapy include fatigue, pain, and nausea and vomiting (Henry 2008; Stasi 2003). Other side effects include bone marrow suppression leading to anaemia; hair follicle cell damage leading to alopecia; gastrointestinal damage leading to diarrhoea and oral ulceration and skin reactions to radiation (Robbins 2002; WHO 2014). Although new drug development programmes have been undertaken to reduce the side effects of cancer therapy, satisfactory treatment still is not readily available to a large proportion of patients receiving chemotherapy and/or radiotherapy. New, effective treatments that can reduce chemotherapy‐ and radiotherapy‐associated adverse effects are needed, especially non‐pharmacological strategies with minimum harm (Cho 2010; Ellebaek 2008; Herrstedt 2007; Jordan 2007; Lotfi‐Jam 2008; Redmond 1996).
Description of the intervention
Moxibustion is a common treatment in traditional Chinese medicine and has been used in China and other Asian countries for millenia (Cho 2009). Moxibustion involves burning herbal preparations containing Artemisia vulgaris (mugwort) on or above the skin at acupuncture points. Moxibustion techniques commonly used in clinical practice to treat side effects of conventional cancer treatment involve either direct moxibustion with a traditional moxa stick (stick‐on moxa) (Yu 2003), or indirect moxibustion, achieved by placing insulating materials such as salt, monkshood cake, sliced ginger or garlic between the skin and a burning moxa cone (Chen 2000; Zhao 2007). The leaves of A vulgaris or mugwort, in Chinese called ai ye, are the main material used for moxibustion. Other Chinese herbs may be sometimes used in combination with mugwort. Mugwort is considered to be warm, acidic and bitter. It has the ability to warm the body's meridians, thereby promoting better circulation. According to Chinese medicine theory, the meridians are the channels inside the human body that circulate vital energy (in Chinese called qi and blood). Besides promoting the flow of vital energy through meridians, moxibustion, which stimulates some specific acupoints located along the meridians upon burning, is considered to have some specific treatment effects, such as strengthening the body's vital energy or facilitating digestion. Although practiced widely in East Asia, it is also associated with some adverse effects, such as allergic reactions, burns and infections (Chan 2014).
How the intervention might work
A systematic review demonstrated that acupuncture point stimulation, as performed through electro acupuncture and acupressure, may reduce chemotherapy‐induced nausea or vomiting (Ezzo 2006). Moxibustion is widely used in China and in other East Asian countries to reduce cancer pain and fever in people with cancer and to lessen the adverse effects of radiotherapy and chemotherapy (Lee 2010; Zhang 2008). Many clinical studies of moxibustion for people with cancer receiving chemotherapy or radiotherapy have indicated that it could alleviate some of the adverse effects of treatment, such as fatigue, nausea and vomiting, diarrhoea, alopecia and pain, as well as improving quality of life (Chen 2000; Chen 2008; Gao 2010; Jiang 2002; Kim 2010; Kuai 2008; Qiu 2008; Song 2003; Shen 2008; Zhang 2008; Zhao 2007).
The clinical effects of moxibustion may be attributable to the actions of enhancing immunity, relieving bone marrow suppression and producing an anti‐oxidative effect (Chen 2000; Cui 2007; Huang 1999; Jiang 2002; Pei 2007; Xu 2003a; Yu 2002a; Yu 2003; Zhao 2007). The infrared radiation peak (around 7.5 μm) of traditional indirect moxibustion with monkshood cake, ginger slices and garlic slices as the medium matches that of infrared radiation on human skin at some acupoints such as LI 4 (hegu), indicating involvement of a sympathetic vibration of infrared radiation from indirect moxibustion and the acupoints. These mechanisms of action (including thermal action, infrared radiation and sympathetic vibration) and their pharmacological effects may contribute to the therapeutic efficacy of moxibustion (Shen 2006). In addition, actions exerted on the acupoints by moxibustion may elicit systemic effects through transmission along meridians.
Why it is important to do this review
Given its potential effect, low cost and simplicity of application, moxibustion may be a valuable adjuvant treatment option for many people with cancer. However, practitioners should also consider the possible side effects related to moxibustion. A recently published systematic review on moxibustion for cancer care found limited evidence supporting the effectiveness of moxibustion for reducing cancer‐related nausea and vomiting (Lee 2010). Review authors evaluated moxibustion as the sole treatment for cancer, or as an adjunct to chemotherapy or radiotherapy; however, they did not clearly specify outcome measurements. Our systematic review focuses primarily on the effects of moxibustion for alleviating the side effects of chemotherapy, radiotherapy or both in people with cancer. We used a transparent and clearly defined systematic method to comprehensively evaluate the evidence. Findings from this systematic review should help to inform medical practitioners, patients and researchers about the effectiveness and safety of moxibustion for people with cancer receiving radiotherapy, chemotherapy or both.
Objectives
To assess the effects of moxibustion for alleviating side effects associated with chemotherapy, radiotherapy or both in people with cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). For randomised cross‐over trials, we included only phase 1 data because treatment carryover effects were likely.
Types of participants
We included participants of any age with any kind of malignant disease receiving chemotherapy, radiotherapy or both.
Types of interventions
The intervention was any type of moxibustion treatment, defined as burning moxa on or above any acupoint or at some specified region of the body. Commonly used techniques include direct and indirect moxibustion with a moxa cone or moxa stick. Direct moxibustion with a moxa cone consists of placing a small cone‐shaped moxa directly on the skin and burning it; in indirect moxibustion, a medium (salt, garlic, ginger, monkshood cake or any other herbs) separates the skin and the burning cone. In moxibustion with moxa stick, a practitioner lights one end of the moxa stick, which is roughly similar to a cigar in shape and size, and holds it for several minutes or even one hour close to the area being treated until the area turns red. We also included moxibustion treatments that involve burning materials made of moxa and/or other medicinal herbs, with or without the aid of an instrument, because these approaches are considered traditional moxibustion treatments. We excluded moxa needle therapy, which consists of inserting a needle into an acupoint and wrapping the end of the needle in an ignited moxa, because this treatment method also involves acupuncture. The acupuncture treatment combined with moxibustion makes it impossible to evaluate whether the treatment effect is due only to moxibustion.
The intervention in the control group may include a sham, no treatment or other conventional treatments that are currently accepted and widely used for patients receiving chemotherapy, radiotherapy or both, and may include treatments for raising white or red blood cell counts and haemoglobin levels, or for enhancing immunity. We did not accept other herbal or complementary medicines as a control intervention when there was no validated evidence about their effectiveness.
Basic oncological treatment (chemotherapy, radiotherapy) or supportive care should be identical in the intervention and control groups. We excluded the studies with Chinese medicines as the co‐administered treatment between groups because they may vary individually.
Types of outcome measures
Primary outcomes
Incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities, as reported according to internationally accepted criteria for common toxicities (e.g. World Health Organization (WHO) (Miller 1981), Eastern Cooperative Oncology Group (ECOG), or National Institutes of Health (NIH) criteria for adverse effects)
Secondary outcomes
Quality of life (QoL) as measured by a validated instrument (e.g. the 36‐Item Short Form Health Survey (SF‐36), the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐C30 (EORTC QLQ‐C30), or the World Health Organization QoL (WHOQOL)
Patient‐reported physical and psychological indices of symptom distress using a validated scale (e.g. visual analogues scale (VAS))
Other objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy (e.g. blood cell counts, measures of immunological function)
Modification or cessation of cancer treatments as the result of side effects or adverse effects, which may be measured as continuous or dichotomous data
Adverse events in the treatment and control groups (including serious and moderate ones), which may or may not be related to moxibustion treatment. We compared the possible occurrence of adverse events between the moxibustion group and the control group
The above outcome measurements were collected immediately after treatment and at the end of follow‐up.
Search methods for identification of studies
We searched for articles in all languages, applying no date restrictions.
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2), in the Cochrane Library;
MEDLINE via Ovid (1946 to January week 4 2018);
Embase via Ovid (1980 to 2018 week 6);
AMED (Allied and Complementary Medicine Database) (1985 to January 2018).
We also searched trials registries and Chinese databases, including Chinese BioMedical Literature Database (CBM), Chinese Medical Current Contents (CMCC), TCMonline, Chinese Dissertation Database (CDDB), China Medical Academic Conference (CMAC) and Index to Chinese Periodical Literature from their inception time to August 2017.
The search strategies in CENTRAL, MEDLINE and Embase are in Appendix 1,Appendix 2 and Appendix 3.
We identified all relevant articles on PubMed and used the 'related articles' feature to carry out further searches for newly published articles.
Searching other resources
We searched the following registries for ongoing trials: metaregister (www.controlled‐trials.com/mrct), Physicians Data Query (/www.ncbi.nlm.nih.gov), www.clinicaltrials.gov and www.cancer.gov/clinicaltrials.
To identify ongoing studies and grey literature, we searched USA CenterWatch Clinical Trials Listing Service (www.CenterWatch.com) and OpenSIGLE (System for Information on Grey Literature in Europe).
We checked the references of all included studies and relevant reviews to find further relevant articles.
Data collection and analysis
Selection of studies
We used the search strategy described above to obtain titles and abstracts of studies that may be relevant to the review. We entered all references from electronic databases into NoteExpress and removed duplicates. Two review authors (HWZ and FC) independently reviewed these titles and abstracts, discarding studies that were not eligible for the review and retaining those with potentially relevant data or information. We retrieved full texts of potentially eligible articles for further assessment, labelling each as 'include', 'exclude' or 'unclear' on full‐text review. We resolved disagreements by discussion and consensus. When the article fell into the unclear category due to unclear information or missing data, we contacted the trial authors for clarification, recording all communications.
Data extraction and management
Two review authors (HWZ and FC) independently carried out data extraction, using a pre‐tested data extraction form. When we found more than one publication of a study, we grouped reports together, using the publication with the most recent and complete data to extract outcomes. When earlier reports were the only ones to publish relevant outcomes, we used these data, noting any discrepancies between published versions. A third review author (ZXL) resolved disagreements between the two review authors in consultation with them.
For included trials, HWZ abstracted the following data as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
General information: published or unpublished, author, country, publication language, publication year, journal citation.
Trial design.
Participants: inclusion and exclusion criteria, total number enrolled and number in each comparison group, baseline characteristics, setting.
Interventions: administration route, timing of intervention, comparison intervention and any co‐intervention, expertise of practitioner.
Risk of bias in trials (see Assessment of risk of bias in included studies).
Follow‐up: length of follow‐up, reason for and number of dropouts and withdrawals, method of analysis.
Outcomes reported: the incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities, QoL, patient‐reported physical and psychological indices of symptom distress based on a validated scale, any other objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy, modification or cessation of cancer treatments as the result of side effects or adverse effects, and incidence and types of adverse events resulting from moxibustion.
For each outcome: outcome definition (with diagnostic criteria if relevant).
Unit of measurement (if relevant).
For scales: upper and lower limits, and whether high or low score is good.
Results: number of participants allocated to each intervention group.
For each outcome of interest: sample size and missing participants.
Data on outcomes were extracted as follows:
For dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each treatment arm who experience the outcome of interest and the number of participants assessed at endpoint to estimate a risk ratio (RR).
For continuous outcomes (e.g. QoL), we extracted the final value and the standard deviation of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up to estimate the mean difference (MD) (if trials measured outcomes on the same scale) or standardised mean differences (SMD) (if trials measured outcomes on different scales) between treatment arms and standard error.
Assessment of risk of bias in included studies
Two review authors (HWZ and FC) independently assessed the risk of bias in the included studies, resolving any discrepancies by discussion and reaching conclusions by consensus. If disagreements persisted, a third review author (ZXL) helped to make the final decision.
To detect potential selection bias, performance bias, detection bias, attrition bias and reporting bias, we addressed the following six domains in the assessment of risk of bias.
-
Selection bias.
Random sequence generation.
Allocation concealment.
-
Performance bias.
Blinding of participants and personnel (participants and treatment providers) on subjective and objective outcomes.
-
Detection bias.
Blinding of outcome assessment.
-
Attrition bias.
-
Incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study, coding a satisfactory level of loss to follow‐up for each outcome, such as:
Low risk of bias, if fewer than 20% of participants were lost to follow‐up, and reasons were similar in both treatment arms.
High risk of bias, if more than 20% of participants were lost to follow‐up, or reasons for loss to follow‐up differed between treatment arms.
Unclear risk of bias, if authors did not report loss to follow‐up.
-
-
Reporting bias.
Selective reporting of outcomes.
-
Other possible sources of bias.
Baseline characteristics.
We categorised the risk of bias for each outcome, within and across included studies, into three levels: low, unclear and high risk of bias. On the basis of this assessment, we used the GRADE system to further evaluate the certainty of evidence for each individual outcome (Higgins 2009). This involved consideration not only of risk of bias (methodological quality) but also of directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias. The empirical evidence for each individual outcome was graded into four levels: high, moderate, low or very low certainty in accordance with the GRADE approach.
Measures of treatment effect
We used the following measures of the effect of treatment.
For dichotomous outcomes, we used the RR with 95% confidence interval (CI). To help determine the applicability of the results to individual participants, we planned to calculate the number needed to treat for a beneficial outcome (NNTB) across a range of assumed control risks if needed.
For continuous outcomes, we used the mean difference between treatment arms (with its 95% CI).
Unit of analysis issues
We analysed outcomes based on randomised participants. In the case of multiple intervention groups within a study, we performed pair‐wise comparisons relevant to the study objective. If necessary, we combined relevant groups to make a single comparison or split them to make multiple comparisons.
Dealing with missing data
Conducting available case analysis, we considered the potential impact of missing data in the 'Risk of bias' table and in interpretation of the results. We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies through visual inspection of forest plots, by estimation of the percentage of heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and, if possible, by subgroup analyses. When heterogeneity was present, we first reviewed study components such as participants, interventions and outcomes to decide whether the heterogeneity was substantial. If that were the case, we investigated and reported on possible reasons.
Assessment of reporting biases
Due to the widespread comparisons in the included studies, we did not undertake funnel plot analysis as planned.
Data synthesis
When clinically similar studies were available, we pooled their results in meta‐analyses.
For any dichotomous outcomes, we calculated the RR for each trial and then as a pooled effect estimate.
For continuous outcomes, we pooled the MDs between treatment arms at the end of follow‐up if all trials measured the outcome on the same scale.
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We undertook post hoc subgroup analysis based on the different conventional medicines in the control group. We did not conduct the planned subgroup analyses based on type of cancer, indirect or direct moxibustion, age of participants and duration of moxibustion treatment due to the widespread comparisons and limited number of included studies.
Sensitivity analysis
We planned a sensitivity analysis to explore the influence of adequate sequence generation and blinding as well as the possible influence of including data from the first period in a cross‐over study, but we were not able to carry this out due to the paucity of relevant included studies.
Summary of findings table
Based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we prepared a 'Summary of findings' table to present the review findings. We presented results for the following outcomes.
Incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities.
QoL (EORTC QLQ‐C30).
-
Patient‐reported physical and psychological indices of symptom distress.
Nausea/vomiting (EORTC QLQ‐C30, WHO grade 3 to 4).
Diarrhoea (EORTC QLQ‐C30).
-
Objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy.
Leukopenia (WHO grade 3 to 4).
WBC count (× 109/L).
Haemoglobin (g/L).
Platelets (× 109/L).
We used the GRADE system to rate the certainty of the evidence (Schünemann 2011), downgrading for inconsistency, design limitations (risk of bias), imprecision, indirectness and other factors, such as publication bias, where appropriate. Where the evidence was based on single studies, or where there was no evidence on a specific outcome, we included the pre‐specified outcome in the 'Summary of findings' tables and graded or explained accordingly. Two review authors (HWZ and FC) performed the grading, resolving differences by discussion and, if necessary, by involving a third review author (ZXL).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification.
Results of the search
Our searches yielded 1224 records. After removing duplicates, we screened the titles or abstracts of 823 records. We read the full texts of 202 records and finally included 29 RCTs in the review (Chen 2000; Chen 2015; Cheng 2005; Cheng 2016; Enkhtuya 2010; Fan 2001; Gao 2013; Hao 2014; Li 2011; Li 2012; Li 2014a; Li 2015; Li 2016; Liang 2002; Mo 2016; Ruan 2014; Tian 2015; Wang 2014; Wu 2013; Xu 2014a; Xu 2014b; Yang 2014; Yin 2013; Yu 2004; Yuan 2014; Zhang 2013Zhang 2016a; Zhang 2016b; Zhu 2017). Eight of the full texts contained insufficient or ambiguous information (Cui 2010; Lan 2013; Li 2014b; Liang 2012; Qiu 2015; Zhang 2014b; Zhang 2014c; Zhang 2014d), justifying their inclusion in the Characteristics of studies awaiting classification section, pending responses from the investigators (Figure 1). After careful comparison, we considered two records to pertain to the same study as Zhang 2013, while eight pertained to Yu 2004.
1.
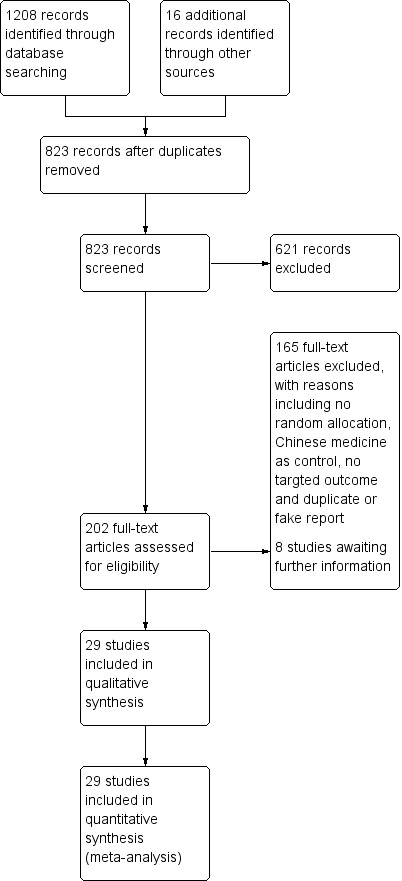
Study flow diagram.
Included studies
We included 29 studies involving 2569 participants. The sample sizes ranged from 24 to 332 participants. All studies were reported in Chinese; 28 were conducted in Chinese hospitals, whereas Enkhtuya 2010 took place in a Mongolian hospital. The participants had a variety of cancers, including nasopharyngeal carcinoma, gastric cancer, respiratory system cancer, primary non‐small cell lung cancer, breast cancer and cervical cancer. All but two studies, Fan 2001 and Liang 2002, reported participant gender, and overall, 1279 (49.8%) of participants in these studies were men.
In 2 studies, participants received simultaneous chemotherapy and radiotherapy plus moxibustion (Chen 2000; Cheng 2005); in 19 studies, participants received simultaneous moxibustion plus chemotherapy (Chen 2015; Cheng 2016; Enkhtuya 2010; Hao 2014; Li 2011; Li 2014a; Li 2015; Liang 2002; Ruan 2014; Wang 2014; Wu 2013; Xu 2014a; Xu 2014b; Yang 2014; Yin 2013; Yuan 2014; Zhang 2013; Zhang 2016b; Zhu 2017); in 1 study, participants received moxibustion before and after chemotherapy (Fan 2001); in 6 studies, participants received moxibustion after chemotherapy and/or radiotherapy (Gao 2013; Li 2012; Li 2016; Mo 2016; Tian 2015; Zhang 2016a); and in 1 study, participants received simultaneous moxibustion with radiotherapy (Yu 2004).
Fifteen studies compared moxibustion plus conventional treatment versus conventional treatment alone (Chen 2000; Chen 2015; Enkhtuya 2010; Gao 2013; Hao 2014; Li 2015; Li 2016; Ruan 2014; Xu 2014a; Yang 2014; Yin 2013; Yuan 2014; Zhang 2013; Zhang 2016b; Zhu 2017); eight compared moxibustion versus conventional medicines (Cheng 2005; Cheng 2016; Fan 2001; Li 2012; Li 2014a; Mo 2016; Tian 2015; Wang 2014); five compared moxibustion versus no treatment (Li 2011; Liang 2002; Wu 2013; Yu 2004; Zhang 2016a); and one compared moxibustion versus sham moxibustion (Xu 2014b).
In four studies (Enkhtuya 2010; Li 2011; Zhang 2013; Zhang 2016b), practitioners placed at least three continuous moxa cones directly on acupoints RN4 (guanyuan) or bilateral BL17 (geshu) and BL19 (danshu), and the treatment duration ranged from 5 to 10 days. Three studies used a direct grain‐sized moxa cone placed on the acupoints ST36 (zusanli), DU14 (dazhui), BL13 (feishu) or RN4 (guanyuan) with continuous 5, 9 or 18 cones, and the treatment duration ranged from 12 to 42 days (Gao 2013; Xu 2014a; Zhang 2016a). In two studies, a specially made direct moxa box on was used on acupoints RN13 (shangwan), RN12 (zhongwan), RN10 (xiawan), ST25 (tianshu), PC6 (neiguan), and ST36 (zusanli) (Cheng 2016; Li 2015). In seven studies, an indirect moxa cone was placed on salt on acupoint RN8 (shenque), or on ginger placed on the acupoints DU14 (dazhui), BL17 (geshu), BL20 (pishu), BL21 (weishu), ST36 (zusanli) or RN12 (zhongwan), with treatment duration ranging from 3 to 65 days (Chen 2000; Chen 2015; Cheng 2005; Li 2011; Li 2014a; Xu 2014b; Yuan 2014). Nine studies used a moxa stick (Fan 2001; Hao 2014; Liang 2002; Li 2016; Mo 2016; Tian 2015; Yang 2014; Yin 2013; Yu 2004), generally for about 10 to 30 minutes on acupoints ST36 (zusanli), SP6 (sanyinjiao) and RN8 (shenque) for 5 to 50 days. One study used moxa stick on acupoints RN8 (shenque), on a paste of grounded herbs (chaihu (Bupleuri Radix), chuanxiong (Chuanxiong Rhizoma), dangshen (Codonopsis Radix), maidong (Ophiopogonis Radix), wuweizi (Schisandrae Chinensis Fructus), danggui (Angelicae Sinensis Radix), huangqi (Astragali Radix) and shexiang (Moschus) for about 2 hours per treatment and 3 times per week, with duration of 126 days (Wu 2013). One study used indirect moxa box on ginger, which was placed on the bilateral acupoints ST36 (zusanli) and KI 1 (yongquan) (Zhu 2017). Only three studies used complementary acupoints based on syndrome differentiation, according to Chinese medicine theory (Hao 2014; Liang 2002; Yang 2014).
The conventional medicines used in the control group included leucogen, batilol, berbamine or recombinant human granulocyte colony‐stimulating factor (G‐CSF) injection. The conventional treatments administered in both groups were generally supportive and symptomatic ones, including granisetron for the prevention of vomiting.
In Xu 2014b, a moxa cone was placed on a slice of ginger in the treatment arm, and for the sham moxibustion, a piece of board in thickness of 0.6 mm was placed on a slice of ginger between the skin and moxa cone to insulate the heat of burned moxa cone.
Excluded studies
The main reasons for excluding studies were lack of random allocation (Chen 2010), moxibustion combined with other therapy (Chen 2006), Chinese medicine as the control intervention (Liu 2002), no targeted outcome (Chen 1991), varied conventional treatment depending on the symptoms in the control group or both groups (Liang 2014; Zhang 2014a; Zhong 2014), no chemotherapy or radiotherapy (Wang 2016), and duplicate or fake reports (Xu 2002a).
Most relevant studies took place in China, and many authors used self‐developed scales or national criteria to assess the treatment outcome (Zheng 2002).
Risk of bias in included studies
2.
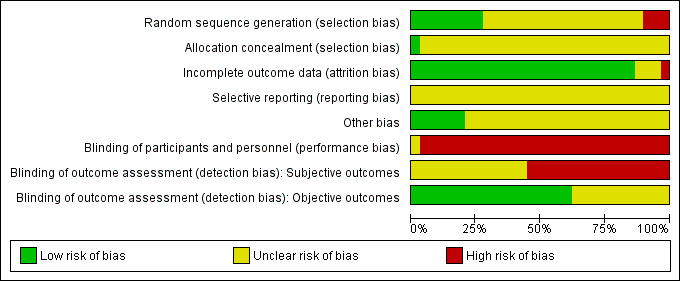
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
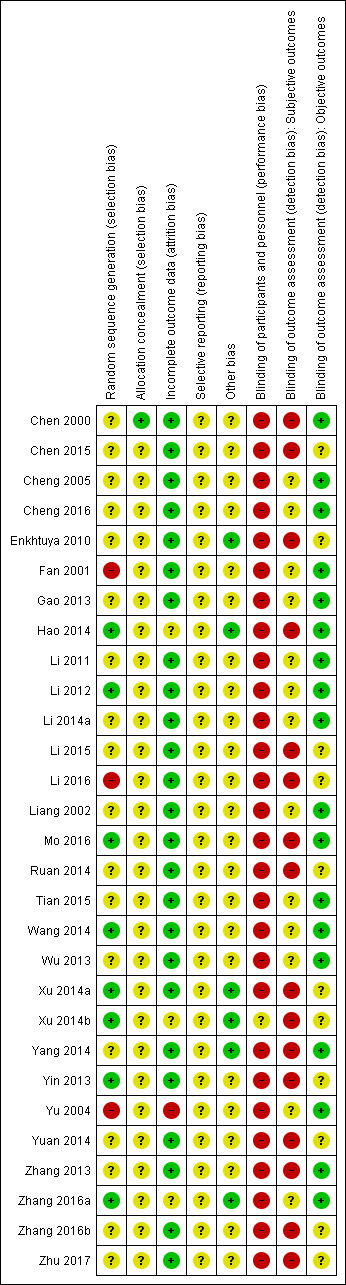
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only nine studies reported using a random number table or computer programme (Hao 2014; Li 2012; Li 2016; Mo 2016; Ruan 2014; Wang 2014; Xu 2014a; Xu 2014b; Zhang 2016a). Other studies mentioned only the random allocation without any further information. In three studies, there was a high imbalance in the number of cases between groups (Fan 2001; Li 2016; Yu 2004), which we considered conferred high risk of bias. One study reported using an envelop during random allocation. Only Chen 2000 reported adequate procedures for allocation concealment.
Blinding
No included study reported any procedure for undertaking blinding of participants or doctors, even the study using sham moxibustion. We considered all of the studies except Xu 2014b to be at high risk of performance bias. Xu 2014b used sham moxibustion, but there was no description of blinding measures. The risk of performance bias was unclear in this study. For detection bias, no study reported information on blinding. Because we thought the lack of blinding had less influence on the objective compared to subjective outcomes, we considered objective outcomes to be at unclear risk of bias and subjective ones to be at high risk.
Incomplete outcome data
All but three of the included studies reported complete data (Xu 2014b; Yu 2004; Zhang 2016a). In Xu 2014b, data were missing for 2/27 participants in both groups. In Yu 2004, data were missing for 2/38 and 9/30 participants, with no explanation for the reason. In Zhang 2016a, there were no data for 2/35 participants in the treatment group and 3/35 in the control group.
Selective reporting
There were no protocols available for included studies, but the review outcomes described in the Methods were generally reported, so we considered the risk of reporting bias to be unclear.
Other potential sources of bias
Eight studies presented baseline data, which were comparable between groups (Enkhtuya 2010; Hao 2014; Ruan 2014; Xu 2014a; Xu 2014b; Yang 2014; Zhang 2016a; Zhu 2017). The other studies reported only that some baseline data between groups were comparable without any detailed information, or they provided no information about comparability.
We assessed the overall risk of bias as unclear in 11 studies (Cheng 2005; Cheng 2016; Gao 2013; Li 2011; Li 2012; Li 2014a; Liang 2002; Tian 2015; Wang 2014; Wu 2013; Zhang 2016a) and high in 18 others (Chen 2000; Chen 2015; Enkhtuya 2010; Fan 2001; Hao 2014; Li 2015; Li 2016; Mo 2016; Ruan 2014; Xu 2014a; Xu 2014b; Yang 2014; Yin 2013;Yu 2004; Yuan 2014; Zhang 2013; Zhang 2016b; Zhu 2017).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Moxibustion versus no treatment for side effects of chemotherapy or radiotherapy in cancer patients.
| Moxibustion versus no treatment for side effects of chemotherapy or radiotherapy in cancer patients | |||||
|
Patient or population: patients receiving chemotherapy or radiotherapy for cancer treatment Settings: hospital Intervention: moxibustion Comparison: no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No treatment | Moxibustion treatment | ||||
| The incidence and severity of toxicities: leukopenia (WHO grade 3 to 4) | 111 per 1000 | 56 per 1000 (11 to 284) | RR 0.50 (0.10 to 2.56) | 72 (1 study) | ⊕⊕⊝⊝ Lowa |
| QoL | No evidence | ||||
| Patient‐reported symptom: nausea/vomiting | No evidence | ||||
| Patient‐reported symptom: diarrhoea | No evidence | ||||
| Objective outcome measure: WBC count (× 109/L) | Mean WBC counts (× 109/L) in the control group was 3.60 | Mean WBC counts (×109/L) in the intervention group was 5.37 (4.36 to 6.38) | MD 1.77 (0.76 to 2.78) | 80 (1 study) | ⊕⊕⊝⊝ Lowa |
| Objective outcome measure: haemoglobin (g/L) | Mean haemoglobin (g/L) in the control group was 10.24 | Mean haemoglobin (g/L) in the intervention groups was 11.57 (11.44 to 11.7) | MD 1.33 (1.20 to 1.46) | 66 (1 study) | ⊕⊕⊝⊝ Lowa |
| Objective outcome measure: platelets (× 109/L) | No evidence | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; WBC: white blood cells; WHO: World Health Organization. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aDowngraded one level due to design limitations (high risk of bias) and one level due to imprecision (1 RCT of 66 to 80 participants).
Summary of findings 2. Moxibustion versus sham treatment for side effects of chemotherapy or radiotherapy in cancer patients.
| Moxibustion versus sham treatment for side effects of chemotherapy or radiotherapy in cancer patients | |||||
|
Patient or population: patients receiving chemotherapy or radiotherapy for cancer treatment Settings: hospital Intervention: moxibustion treatment Comparison: sham | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Sham | Moxibustion treatment | ||||
| The incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities | No evidence | ||||
| QoL (EORTC QLQ‐C30) | Mean QoL (EORTC QLQ‐C30) in the control group was 62.5 | Mean QoL (EORTC QLQ‐C30) in the intervention group was 77.38 (67.33 to 87.43) | MD 14.88 (4.83 to 24.93) | 50 (1 study) | ⊕⊕⊝⊝ Lowa |
| Patient‐reported symptom: nausea/vomiting (EORTC QLQ‐C30) | Mean nausea/vomiting score (EORTC QLQ‐C30) in the control groups was 46.67 | Mean nausea/vomiting score (EORTC QLQ‐C30) in the intervention group was 8.10 (−0.2 to 18.2) | MD −38.57 (−48.67 to −28.47) | 50 (1 study) | ⊕⊕⊝⊝ Lowa |
| Patient‐reported symptom: diarrhoea (EORTC QLQ‐C30) | Mean diarrhoea score (EORTC QLQ‐C30) in the control group was 30 | Mean diarrhoea score (EORTC QLQ‐C30) in the intervention group was 16.19 (2.48 to 29.9) | MD −13.81 (−27.52 to −0.1) | 50 (1 study) | ⊕⊕⊝⊝ Lowa |
| Objective outcome measure: WBC count (× 109/L) | Mean WBC count (× 109/L) in the control group was 4.1 | Mean WBC count (× 109/L) in the intervention group was 5.82 (5.07 to 6.57) |
MD 1.72 (0.97 to 2.47) | 50 (1 study) | ⊕⊕⊝⊝ Lowa |
| Objective outcome measure: haemoglobin (g/L) | Mean haemoglobin (g/L) in the control group was 9.67 | Mean haemoglobin (g/L) in the intervention group was 11.73 (10.93 to 12.53) | MD 2.06 (1.26 to 2.86) | 50 (1 study) | ⊕⊕⊝⊝ Lowa |
| Objective outcome measure: platelets (× 109/L) | Mean platelet count (× 109/L) in the control group was 172.9 |
Mean platelet count (× 109/L) in the intervention group was 383.69 (339.92 to 427.46) | MD 210.79 (167.02 to 254.56) | 50 (1 study) | ⊕⊕⊝⊝ Lowa |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QoL: quality of life. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aDowngraded one level due to design limitations (high risk of bias) and one level due to imprecision (1 RCT of 50 participants).
Summary of findings 3. Moxibustion versus conventional medicines for side effects of chemotherapy or radiotherapy in cancer patients.
| Moxibustion versus conventional medicines for side effects of chemotherapy or radiotherapy in cancer patients | |||||
|
Patient or population: patients receiving chemotherapy or radiotherapy for cancer treatment Settings: hospital Intervention: moxibustion treatment Comparison: conventional medicinesa | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| conventionalmedicine | Moxibustion treatment | ||||
| The incidence and severity of toxicities: haematological toxicity | 143 per 1000 | 81 per 1000 (21 to 315) |
RR 0.57 (0.15 to 2.20) | 72 (1 study) |
⊕⊕⊝⊝ Lowb |
| QoL (Karnofsky score) | The mean Karnofsky score in the control group was 80.5 | The mean Karnofsky score in the moxibustion group was 87.2 (82.9 to 91.5) | MD 6.70 (2.37 to 11.03) | 82 (1 study) | ⊕⊕⊝⊝ Lowb |
| Patient‐reported symptom: nausea/vomiting | No evidence | ||||
| Patient‐reported symptom: diarrhoea | No evidence | ||||
| Objective outcome measure: WBC count (× 109/L) | The mean WBC counts (× 109/L) in the control group was 5.7 | The mean WBC counts (× 109/L) in the intervention group was 6.10 (5.85 to 6.35) | MD 0.40 (0.15 to 0.65) | 90 (1 study) |
⊕⊕⊝⊝ Lowb |
| Objective outcome measure: haemoglobin (g/L) | The mean haemoglobin (g/L) in the control groups was 118 | The mean haemoglobin (g/L) in the intervention groups was 128.28 (122.51 to 134.05) |
MD 10.28 (4.51 to 16.05) | 235 (2 studies) | ⊕⊕⊝⊝ Lowb |
| Objective outcome measure: platelets (× 109/L) | One study reported that moxibustion was associated with a higher platelets counts compared with ondansetron and batilol (163 participants: MD 31.99 × 109/L; 95% CI 16.33 to 47.65) and another found no difference in platelets counts compared with batilol, leucogen and optional G‐CSF (47 participants: MD 6 × 109/L; 95% CI −4.86 to 16.86) | Not pooled due to high heterogeneity | 210 (2 studies) | ⊕⊕⊝⊝ Lowb | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; G‐CSF: granulocyte‐colony stimulating factor; MD: mean difference; QoL: quality of life; RR: risk ratio; WBC: white blood cells. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aConventional medication: batilol, leucogen, berbamine, G‐CSF and etc. bDowngraded one level due to design limitations (high risk of bias) and one level due to imprecision.
Summary of findings 4. Moxibustion + conventional treatment versus conventional medicine alone for side effects of chemotherapy or radiotherapy in cancer patients.
| Moxibustion + conventional treatment versus conventional medicine alone for side effects of chemotherapy or radiotherapy in cancer patients | |||||
|
Patient or population: patients receiving chemotherapy or radiotherapy for cancer treatment Settings: hospital Intervention: moxibustion plus conventional treatment Comparison: conventional treatmenta | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Conventional treatment | Moxibustion plus conventional treatment | ||||
| The incidence and severity of toxicities: leukopenia (WHO grade 3 to 4) | 107 per 1000 | 15 per 1000 (1 to 283) | RR 0.14 (0.01 to 2.64) | 56 (1 study) | ⊕⊕⊝⊝ Lowb |
| QoL (EORTC QLQ‐c30) | The mean QoL (EORTC QLQ‐c30) in the control groups was 65 | The mean QoL (EORTC QLQ‐c30) in the intervention groups was 73.85 (69.25 to 78.46) |
MD 8.85 (4.25 to 13.46) | 134 (3 studies) | ⊕⊕⊝⊝ Lowb |
| Patient‐reported symptom: nausea/vomiting (WHO grade 3 to 4) | 152 per 1000 | 65 per 1000 (38 to 112) | RR 0.43 (0.25 to 0.74) | 801 (7 studies) | ⊕⊕⊝⊝ Lowb |
| Patient‐reported symptom: diarrhoea | 33 per 1000 | 6 per 1000 (0 to 128) |
RR 0.19 (0.01 to 3.88) | 61 (1 study) | ⊕⊕⊝⊝ Lowb |
| Objective outcome measure: WBC count (× 109/L) | 2 studies (N = 200) both reported that moxibustion was associated with a slightly higher mean white blood cell count compared with control (MD 0.5 × 109/L; 95% CI 0.12 to 0.88; MD 1.5 × 109/L; 95% CI 1.14 to 1.86). One (N = 62) found no evidence of a difference compared with control (MD 0.41 × 109/L; 95% CI −0.22 to 1.04). | Not pooled due to high heterogeneity | 262 (3 studies) | ⊕⊕⊝⊝ Lowa | |
| Objective outcome measure: haemoglobin (g/L) | The mean haemoglobin (g/L) in the control groups was 108 | The mean haemoglobin (g/L) in the intervention groups was 111.97 (109.4 to 114.53) | MD 3.97 (1.4 to 6.53) | 142 (2 studies) | ⊕⊕⊝⊝ Lowb |
| Objective outcome measure: platelet (× 109/L) | The mean platelet (× 109/L) in the control group was 170 |
The mean platelet (× 109/L) in the intervention group was 183.48 (154 to 212.95) |
MD 13.48 (−16.00 to 42.95) |
142 (2 studies) | ⊕⊕⊝⊝ Lowb |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; QoL: quality of life; RR: risk ratio; WBC: white blood cells; WHO: World Health Organization. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aConventional medication: batilol, leucogen, berbamine, G‐CSF, etc. bDowngraded one level due to design limitations (high risk of bias) and one level due to imprecision (1 to 7 RCTs of 56 to 801 participants).
Moxibustion versus no treatment
Five trials contributed data to this comparison (Liang 2002; Li 2011; Yu 2004; Wu 2013; Zhang 2016a), but most analyses comprised only one or two trials.
Incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities: leukopenia
Liang 2002 found no difference between intervention and control groups in the incidence of WHO grade 3 or 4 leukopenia (RR 0.50; 95% CI 0.10 to 2.56; 1 study, 80 participants; Analysis 1.1; low‐certainty evidence, downgraded due to design limitations and imprecision).
1.1. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 1 Leukopenia (WHO grade 3 to 4).
Other objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy
WBC counts
In Li 2011, mean WBC count was higher in the moxibustion group compared with the control group (MD 1.77 × 109/L; 95% CI 0.76 to 2.78; 1 study, 80 participants; Analysis 1.2; low‐certainty evidence, downgraded due to design limitations and imprecision).
1.2. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 2 WBC count (× 109/L).
Haemoglobin concentration
One trial reported this outcome (Yu 2004). Mean serum haemoglobin concentration was higher in the moxibustion group compared with the control group (MD 1.33 g/L; 95% CI 0.59 to 2.07; 1 study, 66 participants; Analysis 1.3; low‐certainty evidence, downgraded due to design limitations and imprecision).
1.3. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 3 Haemoglobin (g/L).
Lymphocyte counts
In Yu 2004, moxibustion increased total lymphocyte count (CD3) compared with control (MD 5.30 g/L; 95% CI 1.46 to 9.14; 1 study, 57 participants; Analysis 1.5; low‐certainty evidence, downgraded due to design limitations and imprecision).
1.5. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 5 CD3 (g/L).
Meta‐analysis of Wu 2013 and Yu 2004 showed that moxibustion increased T‐helper cell (CD4) counts (MD 5.42 g/L; 95% CI 3.01 to 7.82; Analysis 1.6; 2 studies, 113 participants; I² = 0; low‐certainty evidence, downgraded due to design limitations and imprecision), but the results in cytotoxic T cell (CD8) counts were inconsistent (Analysis 1.7).
1.6. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 6 CD4 (g/L).
1.7. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 7 CD8 (g/L).
Platelets
Mean platelet count was slightly higher with moxibustion than no treatment (MD 30.80 × 109/L; 95% CI 8.03 to 53.57; Analysis 1.4; 1 study, 65 participants; low‐certainty evidence, downgraded due to design limitations and imprecision).
1.4. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 4 Platelets (× 109/L).
Immunoglobulin (Ig) count
Results of Wu 2013 and Yu 2004 in IgA (Analysis 1.8), IgM (Analysis 1.9) and IgG (Analysis 1.10) were all inconsistent. We did not perform meta‐analysis due to the high heterogeneity.
1.8. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 8 IgA (g/L).
1.9. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 9 IgM (g/L).
1.10. Analysis.
Comparison 1 Moxibustion treatment vs no treatment, Outcome 10 IgG (g/L).
The differences in moxibustion duration and participants between Wu 2013 and Yu 2004 may have contributed substantially to the high heterogeneity found in the meta‐analyses involving data from these trials.
Moxibustion versus sham moxibustion
Only one trial contributed data to this comparison (Xu 2014b). We graded all evidence as being of low certainty due to design limitations and imprecision.
Quality of life
Karnofsky score
A Karnofsky score is based on a performance index of physical ability; higher scores indicate better health and well‐being. Moxibustion was associated with a higher mean Karnofsky score compared with sham one (MD 10.86 points; 95% CI 5.1 to 16.62; 1 study, 50 participants; Analysis 2.1).
2.1. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 1 Karnofsky score.
EORTC QLQ‐C30
Moxibustion was associated with higher QoL scores, assessed by EORTC QLQ‐C30 (version 3.0), compared with sham one (MD 14.88 points; 95% CI 4.83 to 24.93; 1 study, 50 participants; Analysis 2.2).
2.2. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 2 QoL (EORTC QLQ‐C30) (version 3.0).
Patient‐reported physical and psychological indices of symptom distress
Nausea/vomiting
Moxibustion was associated with lower nausea and vomiting scores than the sham treatment, as assessed by EORTC QLQ‐C30 (version 3.0) (Analysis 2.3; 1 study, 50 participants: MD −38.57 points; 95% CI −48.67 to −28.47).
2.3. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 3 Nausea/vomiting (EORTC QLQ‐C30) (version 3.0).
Diarrhoea
Similarly, in Xu 2014b, moxibustion was associated with lower scores for diarrhoea only of borderline significance, assessed by EORTC QLQ‐C30 (version 3.0), compared with sham one (MD −13.81; 95% CI −27.52 to −0.1; 1 study, 50 participants; Analysis 2.4).
2.4. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 4 Diarrhoea (EORTC QLQ‐C30) (version 3.0).
Other objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy
WBC count
Moxibustion was associated with a higher mean white blood cell count compared with sham control (MD 1.72 × 109/L; 95% CI 0.97 to 2.47; 1 study, 50 participants; Analysis 2.5).
2.5. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 5 WBC count (× 109/L).
Haemoglobin
The mean serum haemoglobin concentration was higher with moxibustion than with sham control (MD 2.06 g/L; 95% CI 1.26 to 2.86; 1 study, 50 participants; Analysis 2.6).
2.6. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 6 Haemoglobin (g/L).
Platelets
Mean platelet count was higher with moxibustion than sham treatment (MD 210.79 × 109/L; 95% CI 167.02 to 254.56; 1 study, 50 participants; Analysis 2.7).
2.7. Analysis.
Comparison 2 Moxibustion treatment vs sham moxibustion, Outcome 7 Platelets (× 109/L).
Moxibustion versus conventional medicines
Eight trials contributed data to this comparison. We graded all evidence as being of low certainty due to design limitations (high risk of bias) and inconsistency (heterogeneity) (Figure 4). The different conventional medicines used as controls and heterogeneity of participant populations contributed to inconsistent findings.
4.
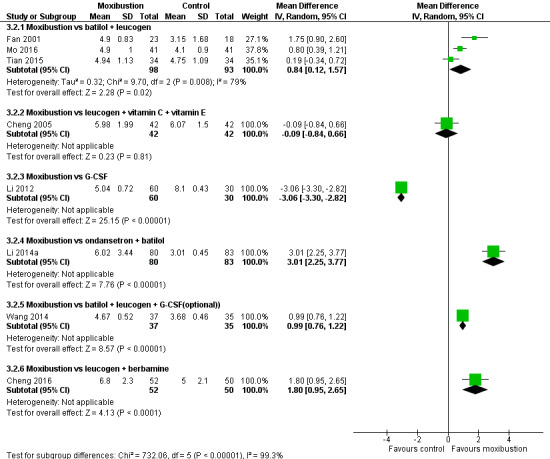
Forest plot of comparison: 3 Moxibustion treatment vs conventional medicine, outcome: 3.1 WBC counts (× 109/L) after treatment.
Incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities: haematological toxicity
Wang 2014 found no clear difference in the risk of haematological toxicity (assessed by the WHO grading system) due to chemotherapy when comparing moxibustion versus batilol plus leucogen plus optional G‐CSF, which was administered to those with neutropenia (RR 0.57; 95% CI 0.15 to 2.20; 1 study, 72 participants; Analysis 3.1).
3.1. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 1 Haematologic (adults) (WHO grade 3 to 4).
Quality of life: Karnofsky score
Mo 2016 reported that moxibustion was associated with a higher Karnofsky score compared with oral batilol plus legucogen (MD 6.70 points; 95% CI 2.37 to 11.03; 1 study, 82 participants; Analysis 3.6).
3.6. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 6 Karnofsky score.
Other objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy
WBC count
Due to the high heterogeneity, we did not conduct a meta‐analysis of eight trials reporting WBC counts (Cheng 2005; Cheng 2016; Fan 2001; Li 2012; Li 2014a; Mo 2016; Tian 2015; Wang 2014). The subgroup analysis based on the conventional medicines in the control group is shown on the forest plot (Analysis 3.2). The pooled results of Fan 2001, Mo 2016 and Tian 2015 show that moxibustion was associated with higher WBC count compared with oral batilol and legucogen (MD 0.84 × 109/L; 95% CI 0.12 to 1.57; 3 studies, 191 participants; Analysis 3.2; I² = 79%). Cheng 2005 found no difference in WBC count when comparing moxibustion versus leucogen plus vitamin C plus vitamin E (MD −0.09 × 109/L; 95% CI −0.84 to 0.66; 1 study, 84 participants; Analysis 3.2). Li 2012 reported that G‐CSF increased WBC count more than moxibustion, but was associated with fever, sore muscle, fatigue and abnormally high WBC counts (MD −3.06 × 109/L; 95% CI −3.3 to −2.82; 1 study, 90 participants; Analysis 3.2) at the end of nine‐day treatment; however, eight days after the end of treatment, the moxibustion group had a higher mean WBC count than the G‐CSF group (MD 0.40 × 109/L; 95% CI 0.15 to 0.65; 1 study, 90 participants; Analysis 3.3). Li 2014a reported that moxibustion was associated with higher WBC count compared with ondansetron plus batilol (MD 3.01 × 109/L; 95% CI 2.25 to 3.77; 1 study, 163 participants; Analysis 3.2), and Wang 2014 reported that moxibustion was associated with higher WBC count compared with batilol plus leucogen plus optional G‐CSF (MD 0.99 × 109/L; 95% CI 0.76 to 1.22; 1 study, 72 participants; Analysis 3.2). Cheng 2016 reported that moxibustion was associated with a higher WBC count compared with leucogen plus berbamine (MD 1.8 × 109/L; 95% CI 0.95 to 2.65; 1 study, 102 participants; Analysis 3.2).
3.2. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 2 WBC count (× 109/L) at the end of treatment.
3.3. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 3 WBC count (× 109/L) after follow‐up (8 days).
Haemoglobin
In a meta‐analysis of two trials (Li 2014a; Wang 2014), moxibustion was associated with higher serum haemoglobin concentrations compared with conventional treatment (MD 10.28 g/L; 95% CI 4.51 to 16.05; 2 studies, 235 participants; Analysis 3.4; I² = 63%).
3.4. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 4 Haemoglobin (g/L).
Platelets
Li 2014a reported that moxibustion was associated with higher platelet counts compared with ondansetron plus batilol (MD 31.99 × 109/L; 95% CI 16.33 to 47.65; 1 study, 163 participants; Analysis 3.5). Wang 2014 found no difference in platelets counts compared with batilol plus leucogen plus optional G‐CSF (MD 6.00 × 109/L; 95% CI −4.86 to 16.86; 1 study, 47 participants; Analysis 3.5).
3.5. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 5 Platelets (× 109/L).
CD counts
Meta‐analysis of Cheng 2005 and Li 2014a found no clear difference in CD3 counts (MD 0.69 g/L; 95% CI −0.64 to 2.02; 2 studies, 247 participants; Analysis 3.7; I² = 0) without heterogeneity. However, their results on CD4 and CD8 varied, with high heterogeneity, so we did not pool these data. Cheng 2005 reported that moxibustion was associated with higher CD4 counts than leucogen plus vitamin C plus vitamin E (MD 15.18 g/L; 95% CI 13 to 17.36; 1 study, 84 participants; Analysis 3.8). Li 2014a reported that there was no difference in CD4 counts between moxibustion versus ondansetron plus batilol (MD 2.11 g/L; 95% CI −0.44 to 4.66; 1 study, 163 participants; Analysis 3.8).
3.7. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 7 CD3 (g/L).
3.8. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 8 CD4 (g/L).
Cheng 2005 reported that moxibustion was associated with higher CD8 counts than leucogen plus vitamin C plus vitamin E (MD 10.76 g/L; 95% CI 9.02 to 12.50; 1 study, 84 participants; Analysis 3.9). Li 2014a also reported that moxibustion was associated with higher CD8 counts compared with ondansetron and batilol (MD 4.06 g/L; 95% CI 1.85 to 6.27; 1 study, 163 participants; Analysis 3.9).
3.9. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 9 CD8 (g/L).
Immunoglobulin (Ig) count
Fan 2001 reported increases in IgA (MD 2.84 g/L; 95% CI 2.3 to 3.38; 41 participants; Analysis 3.10), IgG (MD 7.31 g/L; 95% CI 6.05 to 8.57; 41 participants; Analysis 3.11) and IgM (MD 2.06 g/L; 95% CI 1.66 to 2.46; 41 participants; Analysis 3.12) with moxibustion compared with conventional medicine.
3.10. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 10 IgA (g/L).
3.11. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 11 IgG (g/L).
3.12. Analysis.
Comparison 3 Moxibustion treatment vs conventional medicines, Outcome 12 IgM (g/L).
Moxibustion plus conventional medicine versus conventional medicine
Fifteen trials contributed data to this comparison. We graded all evidence as being of low certainty due to design limitations (high risk of bias) and imprecision.
Incidence and severity of chemotherapy‐ or radiotherapy‐related toxicities: leukopenia
Chen 2000 found no difference in the incidence of severe haematologic toxicity of chemotherapy as assessed by WHO grade 3 to 4 leukopenia between moxibustion plus conventional medicine versus conventional medicine alone (RR 0.14; 95% CI 0.01 to 2.64; 1 study, 56 participants; Analysis 4.1).
4.1. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 1 Leukopenia (WHO grade 3 to 4).
Quality of life
Karnofsky score
Meta‐analysis included four trials (Xu 2014a; Yang 2014; Zhang 2013; Zhang 2016b). Moxibustion combined with conventional medicine was associated with a higher mean Karnofsky score compared with conventional treatment (MD 7.21 points; 95% CI 5.74 to 8.68; 4 studies, 252 participants; Analysis 4.8; I² = 0%).
4.8. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 8 Karnofsky score.
EORTC QLQ‐C30, FACT‐G 4.0, FACT‐L 4.0
Meta‐analysis included three trials (Enkhtuya 2010; Xu 2014a; Zhu 2017). Moxibustion plus conventional medicine was associated with higher QoL (EORTC QLQ‐C30) scores compared with controls (MD 8.85 points; 95% CI 4.25 to 13.46; 3 studies, 134 participants; Analysis 4.9; I² = 26%).
4.9. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 9 QoL (EORTC QLQ‐c30).
Li 2016 reported that moxibustion plus conventional medicine increased QoL, as assessed by FACT‐G 4.0 (Functional Assessment of Cancer Therapy ‐ General) compared with the control group (MD 11.51 points; 95% CI 10.64 to 12.38; 1 study, 332 participants; Analysis 4.10). Zhang 2016b also reported increased QoL, as assessed by FACT‐L 4.0 (Functional Assessment of Cancer Therapy ‐ Lung) (MD 10.04 points; 95% CI 7.63 to 12.45; 1 study, 60 participants; Analysis 4.11).
4.10. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 10 QoL (FACT‐G).
4.11. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 11 QoL (FACT‐L).
Physical well‐being
Chen 2015 reported that moxibustion combined with conventional medicine increased physical well‐being compared with the control group, as assessed by FACT‐L 4.0 (MD −4.33 points; 95% CI −6.25 to −2.41; Analysis 4.12; 72 participants).
4.12. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 12 Physical well‐being (FACT‐L).
Patient‐reported physical and psychological indices of symptom distress
Nausea/vomiting
Meta‐analysis included seven trials (Chen 2000; Li 2016; Ruan 2014; Yang 2014; Yin 2013; Yuan 2014; Zhang 2016b). Moxibustion plus conventional medicine was associated with a reduced risk of severe nausea and vomiting (WHO grade 3 to 4) compared with the control group (RR 0.43; 95% CI 0.25 to 0.74; 7 studies, 801 participants; Analysis 4.3; I² = 19%; Figure 5).
4.3. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 3 Nausea/vomiting (WHO grade 3 to 4).
5.
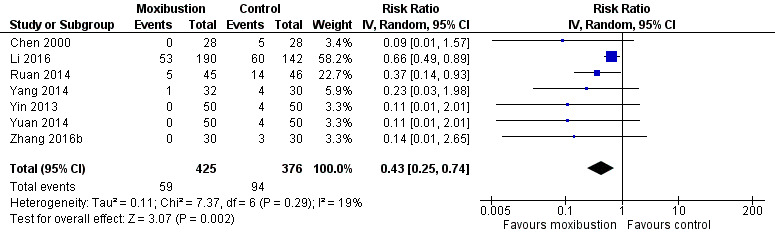
Forest plot of comparison: 4 Moxibustion treatment + conventional medicine vs conventional medicine, outcome: 4.3 Nausea/vomiting (WHO grade 3 to 4).
Li 2015 reported severe vomiting (Common Terminology Criteria for Adverse Events (CTCAE) v3.0 grade 3 to 5) and found no clear difference in this outcome between intervention and control groups (RR 0.07; 95% CI 0.00 to 1.14; 169 participants; Analysis 4.4).
4.4. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 4 Vomiting (CTCAE v3.0 grade 3 to 5).
Diarrhoea
Hao 2014 found no difference in the incidence of severe diarrhoea (WHO grade 3 to 4) with moxibustion plus conventional medicine compared with the control group (RR 0.19; 95% CI 0.01 to 3.88; 61 participants; Analysis 4.5).
4.5. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 5 Diarrhoea (WHO grade 3 to 4).
Other objective outcome measures aimed at assessing side effects of chemotherapy or radiotherapy
WBC count
The results on WBC count varied amongst three trials (Gao 2013; Yang 2014; Zhang 2013). Gao 2013 reported that moxibustion was associated with a slightly higher mean WBC count compared with control (MD 0.50 × 109/L; 95% CI 0.12 to 0.88; 1 study, 120 participants; Analysis 4.2). Yang 2014 found no difference between groups (MD 0.41 × 109/L; 95% CI −0.22 to 1.04; 1 study, 62 participants; Analysis 4.2). Zhang 2013 reported that moxibustion was associated with a higher mean white blood cell count compared with control (MD 1.5 × 109/L; 95% CI 1.14 to 1.86; 1 study, 80 participants; Analysis 4.2).
4.2. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 2 WBC count (× 109/L).
Haemoglobin
Meta‐analysis included two trials (Yang 2014; Zhang 2013). Mean haemoglobin concentration was higher with moxibustion plus conventional medicine than with conventional treatment alone (MD 3.97 g/L; 95% CI 1.4 to 6.53; 2 studies, 142 participants; Analysis 4.6; I² = 0%).
4.6. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 6 Haemoglobin (g/L).
Platelets
Meta‐analysis included two trials (Yang 2014; Zhang 2013). There was no clear difference in mean platelet counts between groups (MD 13.48 g/L; 95% CI −16.00 to 42.95; 2 studies, 142 participants; Analysis 4.7; I² = 34%).
4.7. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 7 Platelets (×109/L).
Immunoglobulin (Ig) count
Hao 2014 reported that moxibustion plus conventional medicine treatment increased IgA (MD 0.55 g/L; 95% CI 0.21 to 0.89; 61 participants; Analysis 4.13), IgG (MD 2.11 g/L; 95% CI 1.19 to 3.03; 61 participants; Analysis 4.14) and IgM (MD 0.40 g/L; 95% CI 0.19 to 0.61; 61 participants; Analysis 4.15) compared with conventional treatment.
4.13. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 13 IgA (g/L).
4.14. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 14 IgG (g/L).
4.15. Analysis.
Comparison 4 Moxibustion treatment + conventional medicine vs conventional medicine, Outcome 15 IgM (g/L).
Adverse effects
Only one study reported that a single participant with lung cancer presented fever and sore throat after receiving direct grain‐size moxibustion, but the symptoms resolved after 24 hours, and there was no relapse (Zhang 2016a). Only two studies reported that no participants experienced any obvious adverse effects of moxibustion during the study period (Hao 2014; Li 2014a). The other studies provided no information about any adverse events related to moxibustion.
Discussion
Summary of main results
This review includes 29 studies on moxibustion treatment for alleviating side effects of chemotherapy or radiotherapy in people with cancer. Five compared moxibustion versus no treatment, 15 compared moxibustion plus conventional treatment versus conventional treatment alone, 1 compared moxibustion versus sham treatment, and 8 compared moxibustion versus conventional medicines.
Single studies reported that compared with no treatment, moxibustion increased white blood cell count and haemoglobin in people with cancer receiving or after receiving chemotherapy/radiotherapy, but its effect on immunological function was inconsistent. A single study reported that moxibustion improved QoL; reduced the symptoms of nausea/vomiting and diarrhoea; and increased mean white blood cell count, mean haemoglobin concentration and mean platelet counts when compared with sham moxibustion.
When comparing moxibustion versus conventional medicines, there was no clear difference in mean white blood cell count, platelets, or CD count; however, moxibustion was associated with higher mean haemoglobin and immunoglobulin concentrations compared with conventional medicines. When moxibustion was added to conventional medicine, it helped decrease the symptoms of nausea/vomiting, improve QoL, increase white blood cell count and haemoglobin, and increase immunoglobulin. The overall risk of bias was high in 18 studies and unclear in 11 studies.
Overall, limited evidence suggests some promising effects of moxibustion in people undergoing chemotherapy or radiotherapy, such as improved haematological and immunological profiles, improved gastrointestinal symptom scores and improved QoL. However, due to the generally low quality and poor reporting of included studies, no high‐certainty evidence supports the use of moxibustion in people undergoing chemotherapy or radiotherapy.
Overall completeness and applicability of evidence
Some included studies assessed patient‐reported physical and psychological indices of symptom distress using self‐developed scales. They reported no modification or cessation of cancer treatment due to moxibustion. Most included studies provided no information on the adverse effects.
Twenty‐eight studies took place in China and one in Mongolia. The moxibustion treatment varied amongst included studies; furthermore, the proper procedures of moxibustion treatment were not adequately standardised. Although no studies reported the adverse events related to moxibustion, this treatment is well known to be related to some adverse effects such as allergic reactions, burns and infections (Chan 2014). These issues raise questions about the applicability of evidence in other countries or regions.
Quality of the evidence
The review included 29 studies involving 2569 people with cancer receiving chemotherapy or/and radiotherapy. The certainty of the included studies was generally low due to poor reporting and methodological design flaws. There were three main problems with study methodology. Firstly, most included studies provided no proper description on random number generation, allocation concealment or baseline characteristics. An imbalance between groups in three included studies introduced doubt on baseline comparability. Secondly, the lack of blinding measurement undertaken for participants or outcome assessors can introduce bias during the study period and outcome data collection, especially for subjective outcomes. Thirdly, the treatment outcomes were not assessed adequately. Some included studies used self‐developed scales to assess toxicity and participants' symptoms. We used the GRADE approach to assess certainty of evidence, downgrading once or twice for inconsistency or risk of publication bias. The different participants and chemotherapy or radiotherapy regimens may contribute much to the high heterogeneity among the included studies. Most studies reported positive results. Although we did not undertake funnel plot analysis due to the insufficient data, it is not possible to rule out the risk of publication bias (GRADE 2015).
Potential biases in the review process
We undertook a comprehensive search strategy with clear and rigid inclusion criteria to screen a large amount of articles. Some studies did not assess any of the reviewed outcomes. Other reviews might have included these studies; however, we considered that studies evaluating other outcomes were beyond the scope of this review, and we excluded them. Type I errors may also exist in the analysis of several subgroups when moxibustion is compared to conventional medicines.
Agreements and disagreements with other studies or reviews
Lee 2010 included five RCTs employing moxibustion as an adjuvant treatment for conventional medicine in people with any type of cancer. It found limited evidence to suggest moxibustion was an effective supportive therapy for nausea and vomiting in cancer. The findings remain similar to this review. Our study has a more comprehensive scope, but it was not possible to establish stronger evidence due to the sparse comparisons and low certainty in the included studies.
Authors' conclusions
Implications for practice.
Limited, low‐certainty evidence suggests that moxibustion may help to reduce the haematological and gastrointestinal toxicities of chemotherapy or radiotherapy and improve QoL in people with cancer; however, the evidence is not conclusive, and we cannot rule out benefits or risks with this treatment. High‐quality studies are needed, which should include reporting of adverse effects.
Implications for research.
Based on this review of current available studies, we suggest that future randomised controlled trials adhere to CONSORT guidelines, including:
1. proper description of random number generation and allocation concealment; 2. proper sample size to ensure sufficient power to detect difference between groups; 3. blinding outcome assessors, participants, and doctors by using reliable sham moxibustion (Zhao 2006); 4. clear description of any adverse effect observed during the study; 5. proper controlled intervention to examine the specific effect of moxibustion other than heat.
Acknowledgements
We would like to thank the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group for its support. Special thanks are given to Theresa Lawrie, Gail Quinn, Jo Morrison and Jo Platt for their valuable advice.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Moxibustion/
(moxa or moxibustion).mp.
1 or 2
exp Radiotherapy/
(radiotherap* or radiation).mp.
(chemoradi* or radiochemo*).mp.
radiotherapy.fs.
exp Antineoplastic Agents/
Antineoplastic Combined Chemotherapy Protocols/
chemotherap*.mp.
drug therapy.fs.
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
3 and 12
exp Neoplasms/
(cancer* or tumor* or tumour* or malignan*or carcinoma* or neoplas*).mp.
14 or 15
13 and 16
(animals not (humans and animals)).sh.
17 not 18
Key:
mp = title, original title, abstract, name of substance word, subject heading word, unique identifier fs = floating subheading sh = subject heading
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Moxibustion] this term only #2 moxa or moxibustion #3 #1 or #2 #4 MeSH descriptor: [Radiotherapy] explode all trees #5 radiotherap* or radiation #6 chemoradi* or radiochemo* #7 Any MeSH descriptor with qualifier(s): [Radiotherapy ‐ RT] #8 MeSH descriptor: [Antineoplastic Agents] explode all trees #9 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] explode all trees #10 chemotherap* #11 Any MeSH descriptor with qualifier(s): [Drug therapy ‐ DT] #12 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13 #3 and #12 #14 MeSH descriptor: [Neoplasms] explode all trees #15 cancer* or tumor* or tumour* or malignan* or carcinoma* or neoplas* #16 #14 or #15 #17 #13 and #16
Appendix 3. Embase search strategy
1 moxibustion/ 2 (moxa or moxibustion).mp. 3 1 or 2 4 exp radiotherapy/ 5 (radiotherap* or radiation).mp. 6 (chemoradi* or radiochemo*).mp. 7 rt.fs. 8 exp chemotherapy/ 9 exp antineoplastic agent/ 10 dt.fs. 11 chemotherap*.mp. 12 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13 3 and 12 14 exp neoplasm/ 15 (cancer* or tumor* or tumour* or malignan* or carcinoma* or neoplas*).mp. 16 14 or 15 17 13 and 16 18 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 19 17 not 18
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword fs=floating subheading
Data and analyses
Comparison 1. Moxibustion treatment vs no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leukopenia (WHO grade 3 to 4) | 1 | 72 | Risk Ratio (IV, Random, 95% CI) | 0.5 [0.10, 2.56] |
| 2 WBC count (× 109/L) | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 1.77 [0.76, 2.78] |
| 3 Haemoglobin (g/L) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 1.33 [0.59, 2.07] |
| 4 Platelets (× 109/L) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 30.80 [8.03, 53.57] |
| 5 CD3 (g/L) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 5.30 [1.46, 9.14] |
| 6 CD4 (g/L) | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 5.42 [3.01, 7.82] |
| 7 CD8 (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 IgA (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 IgM (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 IgG (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 2. Moxibustion treatment vs sham moxibustion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Karnofsky score | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 10.86 [5.10, 16.62] |
| 2 QoL (EORTC QLQ‐C30) (version 3.0) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 14.88 [4.83, 24.93] |
| 3 Nausea/vomiting (EORTC QLQ‐C30) (version 3.0) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐38.57 [‐48.67, ‐28.47] |
| 4 Diarrhoea (EORTC QLQ‐C30) (version 3.0) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐13.81 [‐27.52, ‐0.10] |
| 5 WBC count (× 109/L) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.72 [0.97, 2.47] |
| 6 Haemoglobin (g/L) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.26, 2.86] |
| 7 Platelets (× 109/L) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 210.79 [167.02, 254.56] |
Comparison 3. Moxibustion treatment vs conventional medicines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haematologic (adults) (WHO grade 3 to 4) | 1 | 72 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.15, 2.20] |
| 2 WBC count (× 109/L) at the end of treatment | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Moxibustion vs batilol + leucogen | 3 | 191 | Mean Difference (IV, Random, 95% CI) | 0.84 [0.12, 1.57] |
| 2.2 Moxibustion vs leucogen + vitamin C + vitamin E | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.84, 0.66] |
| 2.3 Moxibustion vs G‐CSF | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.06 [‐3.30, ‐2.82] |
| 2.4 Moxibustion vs ondansetron + batilol | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 3.01 [2.25, 3.77] |
| 2.5 Moxibustion vs batilol + leucogen + G‐CSF(optional)) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.99 [0.76, 1.22] |
| 2.6 Moxibustion vs leucogen + berbamine | 1 | 102 | Mean Difference (IV, Random, 95% CI) | 1.80 [0.95, 2.65] |
| 3 WBC count (× 109/L) after follow‐up (8 days) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.15, 0.65] |
| 4 Haemoglobin (g/L) | 2 | 235 | Mean Difference (IV, Random, 95% CI) | 10.28 [4.51, 16.05] |
| 5 Platelets (× 109/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Karnofsky score | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 6.70 [2.37, 11.03] |
| 7 CD3 (g/L) | 2 | 247 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.64, 2.02] |
| 8 CD4 (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 CD8 (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 IgA (g/L) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 2.84 [2.30, 3.38] |
| 11 IgG (g/L) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 7.31 [6.05, 8.57] |
| 12 IgM (g/L) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 2.06 [1.66, 2.46] |
Comparison 4. Moxibustion treatment + conventional medicine vs conventional medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leukopenia (WHO grade 3 to 4) | 1 | 56 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.64] |
| 2 WBC count (× 109/L) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Nausea/vomiting (WHO grade 3 to 4) | 7 | 801 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.25, 0.74] |
| 4 Vomiting (CTCAE v3.0 grade 3 to 5) | 1 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.00, 1.14] |
| 5 Diarrhoea (WHO grade 3 to 4) | 1 | 61 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.01, 3.88] |
| 6 Haemoglobin (g/L) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 3.97 [1.40, 6.53] |
| 7 Platelets (×109/L) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 13.48 [‐16.00, 42.95] |
| 8 Karnofsky score | 4 | 252 | Mean Difference (IV, Random, 95% CI) | 7.21 [5.74, 8.68] |
| 9 QoL (EORTC QLQ‐c30) | 3 | 134 | Mean Difference (IV, Random, 95% CI) | 8.85 [4.25, 13.46] |
| 10 QoL (FACT‐G) | 1 | 332 | Mean Difference (IV, Random, 95% CI) | 11.51 [10.64, 12.38] |
| 11 QoL (FACT‐L) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 10.04 [7.63, 12.45] |
| 12 Physical well‐being (FACT‐L) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐4.33 [‐6.25, ‐2.41] |
| 13 IgA (g/L) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.21, 0.89] |
| 14 IgG (g/L) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 2.11 [1.19, 3.03] |
| 15 IgM (g/L) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.19, 0.61] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2000.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Patients with nasopharyngeal carcinoma (stage TNM III and IVa), Karnofsky > 60 Number (treatment/control): 56 (28/28) Mean age (range): 43.7 (18‐71) Gender (M/F): 30/26 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Indirect moxa cone on salt + conventional treatment vs conventional treatment Treatment group
Control group
Conventional treatment
|
|
| Outcomes | WHO Recommendations for Grading of Acute and Subacute Toxicity (1981) at the end of treatment
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Envelope was used for random allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Few outcome measures were reported |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, objective outcomes detected by machine were not influenced substantially |
Chen 2015.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with non‐small cell lung cancer who were receiving chemotherapy Number (treatment/control): 72 (36/36) Mean age (range): 58.53 (42‐72) Gender (M/F): 43/29 Country: Shanghai city, China Setting: hospital |
|
| Interventions | Indirect moxa cone on ginger + conventional medicine vs conventional medicine Treatment group
Control group
Conventional treatment
|
|
| Outcomes | FACT‐L4.0 (PWB) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was a statement about group similarity but without baseline characteristics data presented |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Cheng 2005.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with advanced nasopharyngeal carcinoma (stage III and IV), Karnofsky > 60 Number (treatment/control): 84 (42/42) Mean age (range): 45.9 (21‐78) Gender (M/F): 45/39 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Indirect moxa cone on salt vs conventional medicine Treatment group
Control group
|
|
| Outcomes | WBC count at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There were no baseline characteristics data presented and no statement of group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding However, the objective outcomes detected by machine were generally not influenced substantially. |
Cheng 2016.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients who were receiving chemotherapy Number (treatment/control): 102 (52/50) Mean age (range): not reported (37‐72) Gender (M/F): 71/31 Country: Zhejiang province, China Setting: hospital |
|
| Interventions | Direct moxa box vs conventional medicine Treatment group
Control group
Conventional treatment
|
|
| Outcomes | WBC count at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Enkhtuya 2010.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with primary gastric cancer (stage IIIA and IIIB), with expected survival time of more than 3 months and Karnofsky ≥ 60 Number (treatment/control): 24 (12/12) Mean age (range): 61.6 (36‐77) Gender (M/F): 13/11 Country: Mongolia Setting: hospital |
|
| Interventions | Direct moxa cone + conventional medicine vs conventional medicine Treatment group
Control group
Conventional medicine
|
|
| Outcomes | EORTC QLQ‐C30 at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Low risk | Baseline characteristics data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Fan 2001.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | The participants with respiratory and digestive system cancer receiving combination chemotherapy Number (treatment 1/treatment 2/control): 63 (23/22/18) Mean age (range): not reported Gender (M/F): not reported Country: Jiangsu province, China Setting: hospital |
|
| Interventions | Moxa stick vs acupoint injection vs conventional medicine Treatment group 1:
Treatment group 2:
Control group
|
|
| Outcomes | WBC count IgG IgA IgM Outcomes measured at the end of treatment |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described; there was imbalance on the number of participants between groups. |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was no baseline characteristics data presented and no statement of group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Gao 2013.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with cancer after chemotherapy or radiotherapy with leukopenia (WBC count < 4 × 109); expected survival time of more than 6 months; Karnofsky ≥ 70 Number (treatment/control): 120 (60/60) Mean age (range): 45.2 (19‐76) Gender (M/F): 58/62 Country: Jiangsu province, China Setting: hospital |
|
| Interventions | Direct grain‐sized moxa cone + conventional medicine vs conventional medicine Treatment group
Control group
Conventional treatment
|
|
| Outcomes | WBC count at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was no baseline characteristics data presented and no statement of group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Hao 2014.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | The participants with gastrointestinal tract and gynaecological cancer with expected survival time of more than 3 months; Karnofsky ≥ 60 Number (treatment/control): 61 (31/30) Mean age (range): 61.13 (35‐80) Gender (M/F): 37/24 Country: Jiangsu province, China Setting: hospital |
|
| Interventions | Moxa stick + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | IgA, IgG, IgM, diarrhoea (WHO grade 3 to 4) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in treatment, and 2 in control group were lost to follow‐up. Such attrition was not considered to bias the results substantially. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Low risk | Baseline characteristic data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Li 2011.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Patients with primary non‐small cell lung cancer (stage IIIa, IIIb and IV) with expected survival time of more than 3 months, Karnofsky > 60 Number (treatment/control): 80 (40/40) Mean age (range): not reported (41‐65) Gender (M/F): 51/29 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Direct moxa cone vs no treatment Treatment group
Control group
|
|
| Outcomes | WBC count at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There were no baseline characteristics data presented and no statement of group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Li 2012.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients with leukopenia after having received chemotherapy Number (treatment/control): 90 (60/30) Mean age: 63.8 Gender (M/F): 53/37 Country: Henan province, China Setting: hospital |
|
| Interventions | Indirect moxa cone on ginger vs conventional medicine Treatment group
Control group
|
|
| Outcomes | WBC count at the end of treatment and 8 days after the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was a statement about group similarity but without baseline characteristics data presented |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Li 2014a.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients receiving chemotherapy Number (treatment/control): 163 (80/83) Mean age (range): 67.83 (32‐75) Gender (M/F): 96/67 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Indirect moxa cone on ginger vs conventional medicine Treatment group
Control group
|
|
| Outcomes | WBC count, Hb, platelets, CD3, CD4, CD8 at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was a statement about group similarity but without baseline characteristics data presented |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Li 2015.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients who could accept the smell of moxibustion Number (treatment/control): 169 (85/84) Mean age (range): 53.8 (40‐88) Gender (M/F): 89/80 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Direct moxa box + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Vomiting (CTCAE 3.0 grade 3 to 5) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was no statement about group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Li 2016.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients with symptoms of nausea and vomiting after receiving chemotherapy. Number (treatment/control): 332 (190/142) Mean age (range): not reported (26‐87) Gender (M/F): 189/143 Country: Henan province, China Setting: hospital |
|
| Interventions | Moxa stick + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Nausea/vomiting (WHO grade 3 to 4), FACT‐G 4.0 (Functional Assessment of Cancer Therapy ‐ General) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number table was used, but there was high imbalance between groups |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | Baseline characteristic data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Liang 2002.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients receiving chemotherapy Number (treatment/control): 72 (36/36) Mean age (range): not reported Gender (M/F): not reported Country: Shandong province, China Setting: hospital |
|
| Interventions | Moxa stick vs no treatment Treatment group
Control group
|
|
| Outcomes | Leukocytes (WHO grade 3 to 4) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was no statement about group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Mo 2016.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients after chemotherapy with expected survival time of more than 6 months, Karnofsky ≥ 60, and WBC count less than 4 × 109/L; diagnosed with the Chinese medicine syndrome of qi and blood insufficiency Number (treatment/control): 82 (41/41) Mean age (range): 55.5 (34‐69) Gender (M/F): 48/34 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Moxa stick vs conventional medicine Treatment group
Control group
|
|
| Outcomes | WBC count and Karnofsky score at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was no statement about group similarity. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Ruan 2014.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with gastric cancer with expected survival time of more than 3 months; Karnofsky ≥ 60 Number (treatment/control): 91 (45/46) Mean age: 48.35 Gender (M/F): 52/39 Country: Zhejiang province and Shanghai city, China Setting: hospital |
|
| Interventions | Indirect moxa cone on herbal paste + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Vomiting (WHO grade 3 to 4) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | Baseline characteristics data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Tian 2015.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients after chemotherapy with expected survival time of > 3 months, Karnofsky ≥ 60, and WBC count less than 4 × 109/L Number (treatment/control): 68 (34/34) Mean age (range): 51.6 (32‐76) Gender (M/F): 41/27 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Moxa stick vs conventional medicine Treatment group
Control group
|
|
| Outcomes | WBC count at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | There was a statement about group similarity but without baseline characteristics data presented |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Wang 2014.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with gastric cancer with expected survival time of > 3 months; Karnofsky ≥ 60 They also needed to meet the criteria of Chinese medicine syndrome of insufficiency of heart and spleen. Number (treatment/control): 72 (37/35) Mean age: 52.7 Gender (M/F): 42/30 Country: Zhejiang province and Shanghai city, China Setting: hospital |
|
| Interventions | Indirect moxa cone on herbal paste vs conventional medicine Treatment group
Control group
Chemotherapy
|
|
| Outcomes | Hematologic (adults) (WHO grade 3 to 4), WBC count, Hb, platelets at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Wu 2013.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Patients with breast cancer receiving chemotherapy following radical mastectomy, diagnosed with syndrome of deficiency in spleen and kidney based on Chinese medicine theory Number (treatment/control): 60 (30/30) Mean age (range): 50.1 (36‐67) Gender (M/F): F Country: Shandong province, China Setting: hospital |
|
| Interventions | Indirect moxa stick on herbs vs no treatment Treatment group
Control group
|
|
| Outcomes | CD4, CD8, IgA, IgM, IgG at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Xu 2014a.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Patients with gynaecology malignancy who had received at least 2 courses of chemotherapy Number (treatment/control): 50 (25/25) Mean age (range): 53.8 (35‐73) Gender (M/F): 0/50 Country: Jiangsu province, China Setting: hospital |
|
| Interventions | Direct grain‐sized moxa cone + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Karnofsky score, EORTC QLQ‐C30 V3.0 (Chinese version) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Low risk | The baseline characteristics data were presented with good comparability. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Xu 2014b.
| Methods | Design: parallel RCT Randomisation method: random number table Blinding: unclear Power calculation: no Dropouts/withdrawals: yes (2 participants in each group) |
|
| Participants | Cancer patients with expected survival time of more than 3 months; Karnofsky ≥ 60 Number (treatment/control): 54 (27/27) Mean age (range): 48 (37‐67) Gender (M/F): 29/25 Country: Hong Kong, China Setting: clinic and hospital |
|
| Interventions | Indirect moxa cone on ginger vs sham moxibustion Treatment group
Control group
|
|
| Outcomes | Karnofsky score, EORTC QLQ‐C30 version 3.0 (QoL, nausea and vomiting, diarrhoea) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in each group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Low risk | The baseline characteristics data were presented with good comparability. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Sham moxibustion similar to the real one was used, but there was no assessment for the degree of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Yang 2014.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients with expected survival time of more than 3 months; Karnofsky ≥ 60 Number (treatment/control): 62 (32/30) Mean age (range): not reported Gender (M/F): 35/27 Country: Jiangsu province, China Setting: hospital |
|
| Interventions | Moxa stick + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | WBC count, Hb, platelet, vomiting (WHO grade 3 to 4), Karnofsky score at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Low risk | Baseline characteristic data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Yin 2013.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with non‐small lung cancer receiving chemotherapy, with Karnofsky ≥ 70 Number (treatment/control): 100 (50/50) Mean age (range): 55.5 (35‐67) Gender (M/F): 44/56 Country: Shanghai city, China Setting: hospital |
|
| Interventions | Moxa stick + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Vomiting (WHO grade 3 to 4) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Yu 2004.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: yes |
|
| Participants | The patients with cervical cancer (stage I, II and III) with Karnofsky score > 80 Number (treatment/control): 68 (38/30) Mean age (range): 58 (38‐81) Gender (M/F): F Country: Jiangsu province, China Setting: hospital |
|
| Interventions | Moxa stick vs no treatment Treatment group
Control group
|
|
| Outcomes | IgG, IgA, IgM, Hb, CD3, CD4, CD8 at the end of treatment | |
| Notes | The studies Yu 2011, Yuan 2003, Yu 2002, Yu 2003a, Yu 2003b, Xu 2002, Xu 2003 and Zhu 2003 were considered to be reports of the same study as Yu 2004. The additional data from these studies were incorporated into Yu 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described; there was an imbalance in the number of participants between groups |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Yuan 2014.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | Cancer patients receiving chemotherapy Number (treatment/control): 100 (50/50) Mean age (range): 54.3 Gender (M/F): 39/61 Country: Shanghai city, China Setting: hospital |
|
| Interventions | Indirect moxa cone on ginger + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Vomiting (WHO grade 3 to 4) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Zhang 2013.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | The patients with original non‐small cell lung cancer (stage III and IV) with expected survival time of more than 3 months, Karnofsky > 60 Number (treatment/control): 80 (40/40) Mean age (range): 57 (41‐65) Gender (M/F): 41/39 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Direct moxa cone + conventional medicine vs conventional medicine Treatment group
Control group
Conventional medicine
|
|
| Outcomes | Karnofsky score, WBC count, Hb, platelets at the end of treatment | |
| Notes | Some data from Lin 2012 were added into the study Zhang 2013. They were considered to be reports of the same study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Zhang 2016a.
| Methods | Design: parallel RCT Randomisation method: computer programme Blinding: no Power calculation: no Dropouts/withdrawals: yes (2 in treatment group/3 in control group) |
|
| Participants | People with non‐small cell lung cancer who had received chemotherapy after pulmonary lobectomy Number (treatment/control): 70 (35/35) Mean age: 55.55 (available participants) Gender (M/F): 42/23 (available participants) Country: Beijing city, China Setting: hospital |
|
| Interventions | Direct grain‐sized moxa cone vs no treatment Treatment group
Control group
|
|
| Outcomes | Platelet at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer programme |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants dropped out in the treatment group and 3 in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Low risk | Baseline characteristic data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcome was reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding; however, machine‐measured objective outcomes were not influenced substantially |
Zhang 2016b.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | The patients with original non‐small cell lung cancer (stage IIIa, IIIb and IV) with expected survival time of > 3 months, Karnofsky > 60 Number (treatment/control): 60 (30/30) Mean age (range): 57.22 (42‐70) Gender (M/F): 31/29 Country: Guangdong province, China Setting: hospital |
|
| Interventions | Direct moxa cone + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | FACT‐L, Karnofsky score, nausea/vomiting (WHO grade 3 to 4) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | It was mentioned that the groups were comparable, but no baseline characteristics data were presented. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
Zhu 2017.
| Methods | Design: parallel RCT Randomisation method: not reported Blinding: no Power calculation: no Dropouts/withdrawals: no |
|
| Participants | People with primary liver cancer who were eligible for receiving transarterial chemoembolization (TACE), and were diagnosed with Chinese medicine syndrome of stagnation of liver qi and spleen deficiency Number (treatment/control): 60 (30/30) Mean age: 47.84 Gender (M/F): 44/16 Country: Guangxi province, China Setting: hospital |
|
| Interventions | Indirect moxa box on ginger + conventional medicine vs conventional medicine Treatment group
Control group
|
|
| Outcomes | Qol (EORTC QLQ‐c30) at the end of treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Limited outcome measures were reported. |
| Other bias | Unclear risk | Baseline characteristic data were comparable. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No objective outcome was reported. |
5‐FU: fluorouracil; CD3: lymphocyte count; CD4: T‐helper cell; CD8: cytotoxic T cell; CTCAE: Common Terminology Criteria for Adverse Events; EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer QoL Questionnaire; FACT‐G 4.0: Functional Assessment of Cancer Therapy ‐ General; G‐CSF: granulocyte‐colony stimulating factor; Hb: haemoglobin; Ig: immunoglobulin; im: intramuscular; iv: intravenous; NVB: vinorelbine; PDD: cis‐platinum (II) diamminedichloride; PWB: physical well‐being; QoL: quality of life; RCT: randomised controlled trial; WBC: white blood cells; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 1991 | No relevant outcome measure was available in the study report |
| Chen 2006 | Moxibustion plus acupuncture treatment was used as the treatment intervention. |
| Chen 2010 | There was no random allocation for intervention assignment. |
| Chen 2012 | No relevant outcome measure was available in the study report. |
| Ding 2008 | Although random allocation was mentioned in the abstract, sequence of admission to hospital determined group assignment. |
| Fan 2011 | Although random allocation was mentioned in the abstract, visiting order determined group assignment. |
| Ge 2011 | No relevant outcome measure was available in the study report. |
| Guo 2011 | No relevant outcome measure was available in the study report. |
| Huang 2015 | No relevant outcome measure was available in the study report. |
| Jiang 2002 | Acupressure was applied after moxibustion in the treatment group. |
| Jin 2003 | Acupuncure was used together with moxibustion in the treatment group. |
| Li 2007 | No relevant outcome measure was available in the study report. |
| Liang 2014 | The conventional medicine in 2 groups varied depending on the symptoms. |
| Liu 2001 | The treatment duration was different between the comparison groups. |
| Liu 2002 | The combination treatment of chemotherapy and Chinese herbal medicine was used as the control intervention. |
| Liu 2006 | The treatment group assignment was determined by the patient preference. |
| Liu 2013 | The treatment information about chemotherapy was not provided. |
| Long 2012 | It was a cross‐over study, but no phase I study data were available. |
| Ou 1992 | No information about the outcome data was provided. |
| Qiu 2008 | Acupressure was administered with moxibustion in the treatment group. |
| Shao 2012 | No relevant outcome measure was available in the study report. |
| Shen 2002 | No relevant outcome measure was available in the study report. |
| Shen 2010 | It has the same results on some outcomes as Fan 2001. It was possibly a fake article. |
| Shen 2011 | No relevant outcome measure was available in the study report. |
| Song 2003 | No relevant outcome measure was available in the study report. |
| Tang 2011 | Warm needle moxibustion was used as the treatment intervention. |
| Wang 2016 | The patients with cancer pain might not receive chemotherapy or radiotherapy. |
| Xiang 2011 | Acupressure was administered with moxibustion in the treatment group. |
| Xu 2008 | No random allocation |
| Yao 1998 | The sequential balanced coefficient method was used for intervention assignment. |
| Zhang 2014a | The dose of G‐CSF in the control group varied. |
| Zhong 2011 | Acupressure was administered with moxibustion in the treatment group. |
| Zhong 2014 | The conventional medicine in the control group varied according to the side effects of chemotherapy. |
G‐CSF: granulocyte‐colony stimulating factor.
Characteristics of studies awaiting assessment [ordered by study ID]
Cui 2010.
| Methods | Cross‐over RCT |
| Participants | Patients with lung cancer, Karnofsky > 60 |
| Interventions | Moxa stick + conventional treatment vs conventional treatment |
| Outcomes | WHO Recommendations for Grading of Acute and Subacute Toxicity (Vomiting) (Miller 1981) |
| Notes | Information on the number of participants in the treatment and control group in phase I and II trial study was not reported. No response has been received after sending enquiry mail at the time of drafting the review. |
Lan 2013.
| Methods | RCT |
| Participants | Cancer patients receiving chemotherapy |
| Interventions | Indirect moxibustion on ginger + conventional treatment vs conventional treatment |
| Outcomes | Nause and vomiting assessed by a problematic grading criteria |
| Notes | Description on the outcome assessment time was unclear. No response has been received to enquiry mail at the time of drafting the review. |
Li 2014b.
| Methods | RCT |
| Participants | Cancer patients receiving chemotherapy |
| Interventions | Moxa stick treatment + chemotherapy vs chemotherapy |
| Outcomes | Incidence and grading of chemotherapy‐induced diarrhoea |
| Notes | The treatment duration was not reported. No response has been received after sending enquiry mail at the time of drafting the review. |
Liang 2012.
| Methods | RCT |
| Participants | The digestive people with cancer receiving chemotherapy |
| Interventions | Moxa stick treatment on herbal paste + chemotherapy vs chemotherapy |
| Outcomes | Grading of toxic effect, WBC, blood platelet |
| Notes | The treatment duration was not reported. No response has been received after sending enquiry mail at the time of drafting the review. |
Qiu 2015.
| Methods | RCT |
| Participants | People with cervical cancer receiving radiotherapy |
| Interventions | Moxa stick treatment + conventional medicine vs conventional medicine |
| Outcomes | Radiation Therapy Oncology Group (lower GI) |
| Notes | The moxibustion treatment lasted during the whole period of radiotherapy; however, no information about the treatment duration was reported. No response has been received to enquiry mail at the time of drafting the review. |
Zhang 2014b.
| Methods | RCT |
| Participants | Cancer patients receiving radiotherapy |
| Interventions | Indirect moxa cone on ginger + conventional medicine vs conventional medicine |
| Outcomes | WBC count, Hb, platelets |
| Notes | The moxibustion treatment duration was not provided. No response has been received to enquiry mail at the time of drafting the review. |
Zhang 2014c.
| Methods | RCT |
| Participants | Cancer patients receiving chemotherapy |
| Interventions | Direct grain‐size moxibustion + chemotherapy vs chemotherapy |
| Outcomes | WBC count |
| Notes | The information about treatment duration was unclear. No response has been received to enquiry mail at the time of drafting the review. |
Zhang 2014d.
| Methods | RCT |
| Participants | Cancer patients receiving chemotherapy with expected survival time of more than 3 months and Karnofsky > 60 |
| Interventions | Indirect moxa cone on ginger vs conventional medicine |
| Outcomes | WBC count, Hb, platelets, Karnofsky score |
| Notes | The information about treatment duration was unclear. No response has been received to enquiry mail at the time of drafting the review. |
GI: gastrointestinal; Hb: haemoglobin; RCT: randomised controlled trial; WBC: white blood cells; WHO: World Health Organization.
Differences between protocol and review
We did not conduct the planned subgroup, sensitivity analyses and reporting bias assessment due to the heterogeneous comparisons and limited number of included studies. We undertook post hoc subgroup analysis based on the different conventional medicines in the control group.
With regard to study selection, we excluded studies with Chinese medicines as the co‐administered treatment between groups because they may vary individually, which may bias the comparison results between groups.
We changed the outcome 'quality of life' from a primary outcome to a secondary outcome.
We added the plan for a 'Summary of findings' table was added to the review.
Contributions of authors
Drafting of the protocol: HWZ, ZXL, WCC
Study selection: HWZ, FC
Extraction of data from studies: HWZ, FC
Entry of data into RevMan/check of data entry: HWZ, FC
Carrying out the analysis: HWZ, FC
Interpreting the analysis: HWZ, JLT, ZXL
Drafting of the final review: HWZ, ZXL, WCC, JLT
Disagreement resolution: ZXL
Updating of the review: HWZ, FC, ZXL
Sources of support
Internal sources
The Chinese University of Hong Kong, Hong Kong.
External sources
No sources of support supplied
Declarations of interest
Hong Wei Zhang: none known. Zhi Xiu Lin: none known. Fan Cheung: none known. William Chi‐Shing Cho: none known. Jin‐Ling Tang: none known.
New
References
References to studies included in this review
Chen 2000 {published data only}
- Chen K, Jiang Y, Wen HP, Lv XZ, Lu L, Wang H, et al. Clinical study on treatment of nasopharyngeal carcinoma by radio and chemotherapy with supplementary moxibustion on Shenque Point [Ai jiu shen que xue fu zhu fang hua liao zhi liao bi yan ai de lin chuang yan jiu]. Chinese Journal of Integrated Chinese and Western Medicine 2000;20(10):733‐5. [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen FR, Wang M. Observation on curative effect of ginger moxibustion for improving adverse gastrointestinal reactions of non‐small cell lung cancer patients receiving chemotherapy [Ge jiang ai jiu gai shan fei xiao xi bao fei ai hua liao bing ren wei chang dao du fu fan ying de liao xiao guan cha]. Chinese Nursing Research 2015;29(3B):990‐2. [Google Scholar]
Cheng 2005 {published data only}
- Cheng Z, Jiang Y, Chen K. Observation on the short‐term treatment effect of moxibustion on acupoint Shenque with combination of radio‐chemo‐therapy for the treatment of 42 cases of advanced nasopharyngeal carcinoma [Fang hua liao fa pei he ai jiu shen que xue zhi liao wan qi bi yan ai 42 li jin qi liao xiao guan cha]. New Journal of Traditional Chinese Medicine 2005;37(4):58‐9. [Google Scholar]
Cheng 2016 {published data only}
- Cheng L, Xu GY. The observation on the prevention and treatment effects of moxibustion on the chemotherapy induced leukopenia [Ai jiu liao fa fang zhi hua liao suo zhi bai xi bao jian shao guan cha]. Zhejiang Zhong Yi Za Zhi [Zhejiang Journal of Traditional Chinese Medicine] 2016;51(8):600. [Google Scholar]
Enkhtuya 2010 {published data only}
- Vankhuu E, Chai TQ. Clinical and Experimental Studies of Moxa at Guanyuan on Effects of the Chemotherapy Patients Vital Signs [Ai jiu guan yuan dui hua liao qi sheng ming ti zheng ying xiang de lin chuang ji shi yan yan jiu] [PhD thesis]. Guangzhou: Guangzhou University of Chinese Medicine, 2010. [Google Scholar]
Fan 2001 {published data only}
- Fan Y, Yang ZM, Wan M, Wu XF, Yan JL. Clinical study on preventing the virulent and side‐effect of malignant tumor due to chemotherapy by Acu‐mox [Zhen jiu fang zhi e xing zhong liu hua liao du fu fan ying de lin chuang yan jiu]. Shanghai Zhen Jiu Za Zhi [Shanghai Journal of Acupuncture and Moxibustion] 2001;20(1):12‐4. [Google Scholar]
Gao 2013 {published data only}
- Gao F. Observation on the treatment of direct grain‐sized moxa cone for leucopenia caused by chemotherapy [Mai li jiu zhi liao zhong liu fang hua liao hou bai xi bao jian shao zheng de liao xiao guan cha]. Zhong Yi Yao Dao Bao [Guiding Journal of Traditional Medicine and Pharmacy] 2013;19(12):81‐2. [Google Scholar]
Hao 2014 {published data only}
- Hao ZH, Xu LF. Clinical Observation on Immune Function in Patients with Mild Moxibustion Cancer Chemotherapy [Wen he jiu dui zhong liu hua liao huan zhe mian yi gong neng de lin chuang guan cha] [Masters thesis]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2014. [Google Scholar]
Li 2011 {published data only}
- Li LX, Wang T. Clinical observation on the direct moxibustion on Acupoint Sihua to prevent the WBC decrease caused by chemotherapy for lung cancer [Zhi jie jiu si hua xue yu fang fei ai hua liao yao wu suo zhi bai xi bao jian shao de lin chuang guan cha]. The 11th Conference Proceedings of Guangdong Acupuncture Association. Conference title; date the conference took place in the format YYYY MM DD‐DD; place of conference. ADD: ADD, 2011:232‐3.
Li 2012 {published data only}
- Li PH. Clinical study on ginger moxibustion for the treatment of leukopenia after chemotherapy of cancer [Ge jiang jiu zhi liao ai zheng hua liao hou bai xi bao jian shao zheng lin chuang yan jiu]. China Journal of Chinese Medicine 2012;27(173):1244‐5. [Google Scholar]
Li 2014a {published data only}
- Li X, Tian HQ, Liang GW, Li HL, Huang ZQ, Chen XZ. Clinical observation of ginger moxibustion on cellular immune function of 80 chemotherapy patients [Ge jiang jiu dui hua liao huan zhe xi bao mian yi gong neng ying xiang 80 li lin chuang guan cha]. Yunnan Zhong Yi Zhong Yao Za Zhi [Yunnan Journal of Traditional Chinese Medicine and Materia Medica ] 2014;35(4):50‐2. [Google Scholar]
Li 2015 {published data only}
- Li LQ, Zhong MW, Ye HQ, Tang RD, Tan XL, Li RA, et al. Investigation of vomit ‐ stopping efficacy of moxa box moxibustion in chemotherapy of platinum‐based drugs [Ai xiang jiu zai han bo lei yao wu hua liao zhong zhi tu de liao xiao tan tao]. Clinical Journal of Chinese Medicine 2015;7(6):120‐1. [Google Scholar]
Li 2016 {published data only}
- Li L, Wang P, He W, Yuan HL, Jiao YW, Yang QH, et al. The treatment effect of complementary moxibustion for nausea and vomiting on patients with advanced cancer [Xue wei ai jiu fa fu zhu zhi liao zhong liu zhong mo qi huan zhe e xin ou tu de liao xiao]. Lishizhen Medicine and Materia Medica Research 2016;27(6):1417‐8. [Google Scholar]
Liang 2002 {published data only}
- Liang X, Xiao J. Moxibustion treatment of toxic reaction produced in malignant tumour chemotherapy: 36 cases. International Journal of Clinical Acupuncture 2002;13(2):145‐7. [Google Scholar]
Mo 2016 {published data only}
- Mo T, Tian H, Yue SB, Fan ZN, Zhang ZL. Clinical observation of acupoint moxibustion on leukocytopenia caused by tumor chemotherapy [Xue wei ai jiu zhi liao zhong liu hua liao suo zhi bai xi bao jian shao lin chuang guan cha]. Shijie Zhong Yi Yao [World Chinese Medicine] 2016;11(10):2120‐2. [Google Scholar]
Ruan 2014 {published data only}
- Ruan Y, Zhang WX, Wang XA, Gu QH, Jiao JP, Liu L. Effect of herb‐partitioned moxibustion on chemotherapy‐induced nausea and vomiting of gastric cancer [Ge yao jiu yu fang wei ai hua liao xiang guan xing ou tu lin chuang guan cha]. Chinese Archives of Traditional Chinese Medicine 2014;32(11):2664‐6. [Google Scholar]
Tian 2015 {published data only}
- Tian H, Lin H, Zhang L, Fan ZN, Zhang ZL. Effective research on treating leukopenia following chemotherapy by moxibustion [Ai jiu zhi liao hua liao hou bai xi bao jian shao zheng de liao xiao yan jiu]. Clinical Journal of Chinese Medicine 2015;7(10):35‐6. [Google Scholar]
Wang 2014 {published data only}
- Wang JN, Zhang WX, Gu HQ, Jiao JP, Liu L, Wei PK. Protection of herb‐partitioned moxibustion on bone marrow suppression of gastric cancer patients in chemotherapy period [Ge yao jiu dui wei ai hu aliao qi jian gu sui yi zhi bao hu zuo yong de lin chuang guan cha]. Chinese Archives of Traditional Chinese Medicine 2014;32(12):2922‐5. [Google Scholar]
Wu 2013 {published data only}
- Wu HQ, Su XZ, Su QD, Zhang YG, Guo YQ. Effect on the immunity in breast cancer patients treated with medicine‐isolated moxibustion on the umbilicus [Ge yao jiu qi fa dui ru xian ai huan zhe mian yi gong neng de ying xiang]. Shi Jie Zhong Xi Yi Jie He Za Zhi [World Journal of Integrated Traditional and Western Medicine] 2013;8(6):588‐90,93. [Google Scholar]
Xu 2014a {published data only}
- Xu Y, Dong Q. Clinical Observation of Therapeutic Effect on Chemotherapy‐induced Vomiting and Hepatorenal Function with Grain‐moxibustion in Gynecologic Malignancy Patients [Mai li jiu dui fu ke e xing zhong liu hua liao hou ou tu fan ying ji gan shen gong neng gan yu zuo yong de lin chuang guan cha] [Masters thesis]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2014. [Google Scholar]
Xu 2014b {published data only}
- Xu SA, Yang J, Zhao BX. Possible therapeutic effect of ginger‐partitioned moxibustion on chemotherapy induced toxic side effects and quality of life scale: a clinical observation [Ge jiang jiu dui hua liao qi du fu fan ying ji sheng cun zhi liang ying xiang de lin chuang guan cha]. Huanqiu Zhong Yi Yao [Global Traditional Chinese Medicine] 2014;7(12):901‐5. [Google Scholar]
Yang 2014 {published data only}
- Yang Q, Xu LF. Clinical Observation on the Patient's Bone Marrow Suppression Chemotherapy Treatment of Mild Moxibustion [Wen he jiu zhi liao zhong liu hua liao huan zhe gu sui yi zhi de lin chuang guan cha] [Masters thesis]. Nanjjing: Nanjjing University of Traditional Chinese Medicine, 2014. [Google Scholar]
Yin 2013 {published data only}
- Yin Q. Moxibustion combined with acupoint injection to prevent nausea and vomiting in 50 patients [Ai jiu lian he xue wei zhu she yu fang fei ai hua liao suo zhi e xin ou tu 50 li]. Chinese Medicine Modern Distance Education of China 2013;11(20):62‐3. [Google Scholar]
Yu 2004 {published data only}
- Xu LF, Yu ZC, Cheng HZ, Dong Q, Yuan HX, Song YG, et al. Effect of moxibustion on hemogram of cervix carcinoma patients under chemotherapy [Ai jiu dui gong jing ai fang liao huan zhe xue xiang ying xiang de guan cha]. Journal of Nanjing TCM University (Natural Science) 2002;18(4):238‐40. [Google Scholar]
- Xu LF, Yu ZC, Zhan Z, Cheng HZ, Dong Q, Yuan HX, et al. Effects of moxibustion on immunoregulatory factors in the patient of cervical carcinoma in radiotherapy [Ai jiu dui gong jing ai fang liao huan zhe mian yi tiao jie yin zi de ying xiang]. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2003;23(1):41‐3. [Google Scholar]
- Yu L, Xu LF. The influence of moxibustion on the immune function of patients with cervical cancer receiving radiotherapy [Ai jiu dui gong jing ai fang liao huan zhe mian yi gong neng de ying xiang]. Zhen Jiu Lin Chuang Za Zhi [Journal of Clinical Acupuncture and Moxibustion] 2004;20(3):47‐9. [Google Scholar]
- Yu YZ. The influence of moxibustion on the immunoglobulin of patients with cervical cancer receiving radiotherapy [Ai jiu dui gong jing ai fang liao huan zhe mian yi qiu dan bai de ying xiang]. Zhonghua Xian Dai Zhong Xi Yi Za Zhi [Chinese Journal of Current Traditional and Western Medicine] 2003;1(8):719‐20. [Google Scholar]
- Yu ZC, Wang HF, Xu LF. Effect of moxibustion on immunologic function in patients with cervical carcinoma in radiotherapy [Jiu liao dui gong jing ai fang liao huan zhe mian yi gong neng de ying xiang]. Xian Dai Zhong Xi Yi Jie He Za Zhi [Modern Journal of Integrated Traditional Chinese and Western Medicine] 2003;12(24):2629‐30,44. [Google Scholar]
- Yu ZC, Xu LF, Zhan Z, Cheng HZ, Yuan HX, Song YG. The influence of moxibustion on immunoglobulin in cervical carcinoma patients receiving radiotherapy [Ai jiu dui gong jing ai fang liao huan zhe mian yi qiu dan bai de ying xiang]. Shanghai Zhen Jiu Za Zhi [Shanghai Journal of Acupuncture and Moxibustion] 2002;21(6):15‐6. [Google Scholar]
- Yu ZC, Yuan HX, Xu LF, Zhang Z, Cheng HZ, Song YG. Effects of moxibustion on hemoglobin and immunoglobulin in cervical cancer patients undergoing radiotherapy [Jiu fa dui gong jing ai fang liao huan zhe xue hong dan bai ji mian yi qiu dan bai de ying xiang]. Zhen Jiu Tui Na Yi Xue [Journal of Acupuncture and Tuina Science] 2011;9(6):359‐61. [Google Scholar]
- Yuan HX, Yu ZC, Cheng HZ, Xu LF, Song YG. Influence of moxibustion on hemoglobin during radiotherapy in patients with cervical cancer [Ai jiu dui gong jing ai fang liao huan zhe xue hong dan bai de ying xiang]. Shanghai Zhen Jiu Za Zhi [Shanghai Journal of Acupuncture and Moxibustion] 2003;22(7):33‐4. [Google Scholar]
- Zhu MH, Xu LF, Yu ZC, Zhan Z, Cheng HZ, Yuan HX, Song YG. Effect of moxibustion on peripheral T‐cell subgroups of patients with cancer of uterine cervix [Ai jiu dui gong jing ai fang liao huan zhe wai zhou xue t xi bao ya qun de ying xiang]. Nanjing Zhong Yi Yao Da Xue Xue Bao [Journal of Nanjing TCM University] 2003;19(1):44‐6. [Google Scholar]
Yuan 2014 {published data only}
- Yuan XH, Yao Z, Dong HJ. Observation on the preventive effect of indirect moxibustion on ginger combined with antiemetics for 30 cases of indigestive reactions to chemotherapy [Ge jiang jiu lian he zhi tu yao yu fang hua liao zhi wei chang dao fan ying 50 li xiao guo guan cha]. Qilu Hu Li Za Zhi [Journal of Qilu Nursing] 2014;20(3):121‐2. [Google Scholar]
Zhang 2013 {published data only}
- Lin GH, Li LX, Zhang QF, Lin LZ. The influence of direct moxibustion on Acupoint Sihua on the marrow inhibitation caused by chemotherapy for lung cancer [Zhi jie jiu si hua xue dui fei ai hua liao gu sui yi zhi de ying xiang]. The 12th Conference Proceedings of Guandong Acupuncture Association. Conference title; date the conference took place in the format YYYY MM DD‐DD; place of conference. Add: Add, 2012:30‐3.
- Zhang QF, Li LX, Lin GH, Lin LZ. Effect of direct moxibustion at Sihua points on cytokine of chemotherapy patients with lung cancer [Zhi jie jiu si hua xue dui fei ai hua liao huan zhe xi bao yin zi de ying xiang]. Zhongguo Zhen Jiu [Chinese Acupuncure & Moxibustion] 2013;33(3):207‐10. [PubMed] [Google Scholar]
Zhang 2016a {published data only}
- Zhang MX, Guan L. Effect of scarring moxibustion at acupoints of Zusanli (ST36) and Feishu (BL13) on neutrophil‐to‐lymphocyte ratio and platelet count in patients with non‐small‐cell lung cancer after chemotherapy. A randomised controlled trial [Mai li jiu zu san li fei shu xue dui hua liao hou fei xiao xi bao fei ai huan zhe de zhong xing li lin ba xi bao bi lv ji xue xiao ban shu liang de ying xiang: sui ji dui zhao yan jiu]. Zhongguo Lin Chuang Yi Sheng Za Zhi [Chinese Journal for Clinicians] 2016;44(1):42‐4. [Google Scholar]
Zhang 2016b {published data only}
- Zhang QF, Li LX, Fan DY. The influence of direct moxibustion on the acupoint sihua on the quality of life of lung cancer patients [Zhi jie jiu si hua xue dui fei ai hua liao huan zhe sheng cun zhi liang de ying xiang]. Journal of Practical Traditional Chinese Internal Medicine 2016;32(10):1006‐7. [Google Scholar]
Zhu 2017 {published data only}
- Zhu JY, Xu W, Chen C, Ou J, Mao SF, Mo XL, Lu DL. Clinical effect of ginger‐partitioned moxibustion combined with transarterial chemoembolization in treatment of primary liver cancer with stagnation of liver qi and spleen deficiency [Ge jiang jiu lian he jing dong mai hua liao shuan se shu zhi liao gan yu pi xu xing yuan fa xing gan ai de xiao guo guan cha]. Journal of Clinical Hepatology 2017;33(1):87‐90. [Google Scholar]
References to studies excluded from this review
Chen 1991 {published data only}
- Chen HL, Wang L, Shao MY, Zhou HB, Guo XM, Wang SZ, et al. Observations on the treatment of chemotherapy‐induced leukocytopenia with acupuncture and moxibustion [Zhen jiu zhi liao hu aliao yin qi bai xi bao jian shao zheng 376 li liao xiao guan cha]. Chinese Journal of Integrated Traditional and Western Medicine 1991;11(6):350‐2. [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen J, Zhang DM, Chen L. Clinical observation on the acupuncture and moxibustion treatment for chemotherapy‐induced vomitting [Zhen jiu zhi liao hua liao xing ou tu de lin chuang guan cha]. Journal of Qiqihar Medical College 2006;27(14):1705‐6. [Google Scholar]
Chen 2010 {published data only}
- Chen ZJ, Li LN. Observation on the treatment effect of thunder and fire moxibustion for nausea and vomiting caused by platinum‐based chemotherapy [Lei huo jiu dui han bo lei yao wu hua liao suo zhi e xin ou tu de liao xiao guan cha]. Xin Zhong Yi [Journal of New Chinese Medicine] 2010;42(12):88‐9. [Google Scholar]
Chen 2012 {published data only}
- Chen CF. Moxibustion treatment for 18 cases of patients with diarrhea related to cancer [Ai jiu fa zhi liao ai xiang guan xing fu xie 18 li]. Zhongguo Zhong Xi Yi Jie He Xiao Hua Za Zhi [Chinese Journal of Integrated Traditional and Western Medicine on Digestion] 2012;20(11):514‐5. [Google Scholar]
Ding 2008 {published data only}
- Ding QN, Xu LF. Observe of Moxibustion on the Clinical Symptom and Hemogram During Radiotherapy in Patients with Esophageal Cancer [Ai jiu dui 60 li shi guan ai fang liao du fu fan ying ji xue xiao de guan cha] [Masters thesis]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2008. [Google Scholar]
Fan 2011 {published data only}
- Fan Y, Yang ZM, Wan M, Wu XF, Yan JL. Effects of moxibustion therapy on preventing and treating side effects from chemotherapy of maligant tumor patients [Jiu fa fang zhi e xing zhong liu huan zhe hua liao du fu fan ying liao xiao guan cha]. Journal of Acupuncture and Tuina 2011;9(6):351‐3. [Google Scholar]
Ge 2011 {published data only}
- Ge M, Niu GS, Liu FQ, Guo JG, Hou XF. The hot sensitive moxibustion gathers rises the white medicinal powder after the stomach cancer chemotherapy the white blood cell reduction sickness curative effect observation [Re min jiu he sheng bai yao dui wei ai hua liao hou bai xi bao jian shao zheng de liao xiao guan cha]. An Mo Yu Kang Fu Yi Xue [Chinese Manipulation & Rehabilitation Medicine] 2011;2(59):53‐4. [Google Scholar]
Guo 2011 {published data only}
- Guo JF, Lin LY, Li QJ, Guo ZT. Moxibustion to chemothreapy medicine result hangfire disgusting vomit nursing observation [Jiu fa dui hua liao yao wu suo zhi de chi fa xing er xin ou tu de hu li guan cha]. An Mo Yu Kang Fu Yi Xue [Chinese Manipulation & Rehabilitation Medicine] 2011;2(2):61‐2. [Google Scholar]
Huang 2015 {published data only}
- Huang QT, Chen L, Jiang YF. Observation on the treatment of moxibustion with ondansetron to prevent the reactions of digestive tract caused by chemotherapy [Lei huo jiu lian he ang dan si qiong yu fang hua liao xiao hua dao fan ying de xiao guo guan cha]. Guangxi Zhong Yi Yao [ Guangxi Journal of Traditional Chinese Medicine] 2015;38(5):27‐9. [Google Scholar]
Jiang 2002 {published data only}
- Jiang GM, Shan QH, Tan QW. Clinical study of moxibustion on apoptosis of peripherical blood lymphocyte in patients with maliganant tumor after chemotherapy [Ai jiu dui zhong liu hua liao hou wai zhou xue lin ba xi bao diao wang ying xiang de yan jiu]. Shandong Zhong Yi Yao Da Xue Xue Bao [Journal of Shandong University of TCM] 2002;26(4):290‐3. [Google Scholar]
Jin 2003 {published data only}
- Jin ZX. The operation method of loess cake moxibustion and its clinical observation on treatment of mastocarcinoma [Ge huang tu bing jiu de cao zuo fang fa ji qi zhi liao ru xian ai de lin chuang guan cha]. Beijing Zhong Yi Yao Da Xue Xue Bao [Journal of Beijing University of Traditional Chinese Medicine (Clinical Medicine)] 2003;10(1):33‐4. [Google Scholar]
Li 2007 {published data only}
- Li QJ, Pei LY, Guo YC. Clinical observation on the treatment of mild moxibustion for 87 cases of leukopenia after chemotherapy for tumor [Wen he jiu zhi liao zhong liu fang hua liao hou bai xi bao jian shao zheng 87 li lin chuang guan cha]. Jiangsu Zhong Yi Yao [Jiangsu Journal of Traditional Chinese Medicine] 2007;39(1):41. [Google Scholar]
Liang 2014 {published data only}
- Liang JJ, Tang L, Wang EC, Chen XX. Clinial study on the treatment of indirect moxa cone on ginger for the adverse effects of chemotherapy for lung cancer [Ge jiang jiu jian qing fei ai hua liao zhong fu fan ying de lin chuang yan jiu]. Guangming Zhong Yi [Guangming Journal of Chinese Medicine] 2014;29(9):1841‐2. [Google Scholar]
Liu 2001 {published data only}
- Liu J, Yu RC, Rao XQ, Wang YT, Wang XM, Zhang Q, Tang WJ. Study on effect of moxibustion and Guben Yiliu III combined with chemotherapy in treating middle‐late stage malignant tumor [Jiu fa he gu ben yi liu san hao jie he hua liao zhi liao zhong wan qi e xing zhong liu de lin chuang guan cha]. Zhongguo Zhong Xi Yi Jie He Za Zhi [Chinese Journal of Integrative Medicine] 2001;21(4):262‐4. [PubMed] [Google Scholar]
Liu 2002 {published data only}
- Liu J, Yu RC, Tang WJ, Zhao WS, Xu YM, Yang GW, et al. Influence of combined therapy of Guben Yiliu III, moxibustion and chemotherapy on immune function and blood coagulation mechanism in patients with mid‐late stage malignant tumor [Jiu fa he gu ben yi liu san hao jie he hua liao dui zhong wan qi e xing zhong liu huan zhe mian yi gong neng ji ning xue ji zhi de ying xiang]. Zhongguo Zhong Xi Yi Jie He Za Zhi [Chinese Journal of Integrated Medicine] 2002;22(2):104‐6. [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu LB, Le J, Xu JY, Lv HB, Feng ZY, Guo J. Clinical observation on the moxa cone moxibustion on Acupoint Zusanli for the treatment of gastrointestinal function after chemotherapy [Ai zhu jiu zu san li zhi liao hua liao hou wei chang gong neng ying xiang de lin chuang guan cha]. Jilin Zhong Yi Yao [Jilin Journal of Traditional Chinese Medicine] 2006;26(8):47. [Google Scholar]
Liu 2013 {published data only}
- Liu Q, Xing JY, Cheng Y, Zhang YY. The application of moxibustion on the nursing of cancer patients [Ai jiu liao fa zai zhong liu hu li zhong de ying yong]. Lin Chuang Hu Li [Guide of China Medicine] 2013;11(14):752‐4. [Google Scholar]
Long 2012 {published data only}
- Long SQ, Xiao SJ, Zhou YZ, Deng H, Pan ZQ, He WF, et al. Moxa box moxibustion on Acupint Shenque and Zhongwan for the prevention and treatment of digestive system side effects caused by the platinum‐based chemotherapy [Ai xiang jiu shen que zhong wan xue fang zhi han bo lei hua liao fang an suo zhi xiao hua dao fu fan ying]. Xin Zhong Yi [Journal of New Chinese Medicine] 2012;44(7):138‐9. [Google Scholar]
Ou 1992 {published data only}
- Ou YQ, Jiang Y, Ma DF, Wei MJ, Cao QL, Cao H, et al. The influence on the immune function of the indirect moxibustion on salt on acupoint Shenque [Ge yan zhuang jiu shen que xue dui ji ti mian yi gong neng de ying xiang dong wu shi yan ji lin chuang yan zheng]. Xin Zhong Yi [New Journal of Traditional Chinese Medicine] 1992;24(2):32‐3. [Google Scholar]
Qiu 2008 {published data only}
- Qiu CQ, Chen YH, Yang LQ. Clinical observation of moxibustion treatment for gastrointestinal reactions caused by chemotherapy for malignant hematopathy [Ai jiu zhi liao e xing xue ye bing hua liao suo zhi wei chang dao fan ying de lin chuang guan cha]. Xian Dai Hu Li [Modern Nursing] 2008;14(3):352‐3. [Google Scholar]
Shao 2012 {published data only}
- Shao KW, Kong YM. Indirect moxibustion treatment on herbs for the treatment of 82 cases of patients with leukopenia after chemotherapy [Ge yao bing jiu zhi liao hua liao hou bai xi bao jian shao zheng 82 li]. Hunan Zhong Yi Za Zhi [Hunan Journal of Traditional Chinese Medicine] 2012;28(3):86‐7. [Google Scholar]
Shen 2002 {published data only}
- Shen MH, Xu LF, Zhan Z, Yu ZC, Cheng HZ, Yuan HX, et al. The influence of moxibustion on the red cell immune adherence and TNF on patients with cervix carcinoma receiving radiotherapy [Ai jiu dui fang liao gong jing ai huan zhe de hong xi bao mian yi nian fu gong neng he TCN de ying xiang]. Anhui Zhong Yi Lin Chuang Za Zhi [Clinical Journal of Anhui Traditional Chinese Medicinei] 2002;15(5):352‐3. [Google Scholar]
Shen 2010 {published data only}
- Shen GW. Clinical study on different moxibustion treatment methods for side effects of chemotherapy [Bu tong zhen jiu fang fa fang zhi hua liao du fu fan ying de lin chuang yan jiu]. Shi Yong Lin Chuang Yi Yao Za Zhi [Journal of Clinical Medicine in Practice] 2010;14(9):57‐9. [Google Scholar]
Shen 2011 {published data only}
- Shen GW, Zhao JS. Acupuncture and moxibustion regulation for post‐chemotherapy gastric motility [Bu tong zhen jiu liao fa dui hua liao hou wei dong li de tiao jie zuo yong]. Journal of Acupuncture and Tuina Science 2011;9(6):362‐6. [Google Scholar]
Song 2003 {published data only}
- Song YG, Yuan H, Xu LF. Clinical observation on effect of moxibustion at Shenque on recent diarrhea in patients treated with radiotherapy for cervical carcinoma [Ai jiu shen que deng dui gong jing ai fang liao huan zhe jin qi fu xie de lin chuang guan cha]. Nanjing Zhong Yi Yao Da Xue Xue Bao [Journal of Nanjing TCM University] 2003;19(2):107‐8. [Google Scholar]
Tang 2011 {published data only}
- Tang SM, Nie QM, Xiang N. Clinical observation on the warm needle moxibustion with tonify spleen and stomach for the treatment of 30 cases of leukopenia after chemotherapy for cancer [Wen zhen jiu jian pi yi wei fa zhi liao zhong liu fang hua liao hou bai xi bao jian shao zheng 30 li lin chuang guan cha]. Annual National Conference of Chinese Medicine for Cancer. 2011:353.
Wang 2016 {published data only}
- Wang DF, Liu QM, Lu H. The influence of moxibustion on the Du meridian and nursing care on the quality of life of patients with cancer pain [Du mai jiu jia hu li gan yu dui ai zheng teng tong huan zhe sheng huo zhi liang de ying xiang]. Zhongguo Min Jian Liao Fa [China's Naturopathy] 2016;24(2):83‐4. [Google Scholar]
Xiang 2011 {published data only}
- Xiang P. Observation on the treatment effect of moxibustion to relieve nausea and vomiting caused by complementary chemotherapy after operation for colon cancer [Ai jiu jian qing jie chang ai shu hou fu zhu hua liao suo zhi e xin ou tu xiao guo guan cha]. Zhongguo Zhong Yi Ji Zheng [Journal of Emergency in Traditional Chinese Medicine] 2011;20(8):1327‐8. [Google Scholar]
Xu 2008 {published data only}
- Xu LF, Yu ZC, Shen MH, Tian DL, Dong Q, Song YG, et al. The influence of moxibustion on the immune function of 60 patients with esophageal cancer receiving radiotherapy [Ai jiu dui 60 li shi dao ai fang liao huan zhe mian yi gong neng de ying xiang]. Nanjing Zhong Yi Yao Da Xue Xue Bao [Journal of Nanjing TCM University] 2008;24(1):12‐4. [Google Scholar]
Yao 1998 {published data only}
- Yao JQ. Clinical observation on the indirect moxibustion treatment on ginger for leukopenia caused by chemotherapy [Ge jiang jiu zhi liao hua liao suo zhi bai xi bao jian shao zheng lin chuang guan cha]. Beijing Zhong Yi [Beijing Journal of Traditional Chinese Medicine] 1998;48(2):40‐1. [Google Scholar]
Zhang 2014a {published data only}
- Zhang J, Wang LL. The Clinical Research on Myelosuppression and Quality of Life after Chemotherapy Treated by Grain‐sized Moxibustion [Mai li jiu dui hua liao hou gu sui yi zhi ji sheng huo zhi liang ying xiang de lin chuang yan jiu] [Masters thesis]. Nanjing: Nanjing University of Traditional Chinese Medicine, 2014. [Google Scholar]
Zhong 2011 {published data only}
- Zhong SW, Xu SW, Xu B, Sun Y, Tian H. Clinical study on the treatment of moxibustion combined with Granisetron Hydrochloride Injection for nause and vomitting caused by chemothrapy for breast cancer [Ai jiu lian he yan suan ge la si qiong zhu she ye zhi liao ru xian ai hua liao suo zhi e xin ou tu de lin chuang yan jiu]. Zhong Yao Cai [Journal of Chinese Medicinal Materials] 2011;34(6):1007‐9. [Google Scholar]
Zhong 2014 {published data only}
- Zhong ZG, Tang CZ. Clinical Study of Treating Side Effects of Chemotherapy of Lung Cancer by Ginger Moxibustion on Sihua Acupoint [Sihua xue ge jiang jiu dui fei ai huan zhe hua liao hou fu fan ying ying xiang de lin chuang yan jiu] [Masters thesis]. Guangzhou: Guangzhou University of Traditional Chinese Medicine, 2014. [Google Scholar]
References to studies awaiting assessment
Cui 2010 {published data only}
- Cui DL, Wang LX, Fu CJ. Clinical observation on the moxibustion treatment combined with granisetron for the prevention of vomiting and nausea caused by chemotherapy for lung cancer [Ge la si qiong pei he ai jiu yu fang fei ai hua liao suo zhi e xin ou tu de lin chuang guan cha]. Changchun Zhong Yi Yao Da Xue Xue Bao [Journal of Changchun University of Traditional Chinese Medicine] 2010;26(3):390‐1. [Google Scholar]
Lan 2013 {published data only}
- Lan HX. Clinical observation on the treatment of ginger moxibustion with special device for prevention of vomiting after chemotherapy for cancer patients [Wen jiu qi xing ge jiang jiu yu fang zhong liu huan zhe hua liao hou ou tu de lin chuang guan cha]. Guangxi Zhong Yi Yao Da Xue Xue Bao [Journal of Guangxi University of Chinese Medicine] 2013;16(2):37‐9. [Google Scholar]
Li 2014b {published data only}
- Li Q, Cai XL. Clinical observation of moxibustion in prevention and treatment of chemotherapy‐induced diarrhea [Ai jiu fang zhi hua liao xiang guan xing fu xie de lin chuang guan cha]. Liaoning Zhong Yi Za Zhi [Liaoning Journal of Traditional Chinese Medicine] 2014;41(2):331‐2. [Google Scholar]
Liang 2012 {published data only}
- Liang JJ, Luo GY, Du ZP. Clinical observation on the prevention of herbal moxibustion on the marrow inhibition induced by chemotherapy for digestive cancer [[Yao wu jiu yu fang xiao hua dao zhong liu hua liao dao zhi gu sui yi zhi lin chuang guan cha]]. Zhongguo Zhong Yi Ji Zheng [Journal of Emergency in Traditional Chinese Medicine] 2012;21(8):1330‐1. [Google Scholar]
Qiu 2015 {published data only}
- Qiu SH, Kong YL, Liang ZX, Li QJ, Gui L, Yi MT, et al. Clinical effect of the prevention and treatment of moxibustion for acute radiation proctitis in patients with cervical cancer [Ai jiu fang zhi gong jing ai huan zhe ji xing fang she xing zhi chang yan de xiao guo]. Guangdong Yi Xue [Guangdong Medical Journal] 2015;36(6):958‐9. [Google Scholar]
Zhang 2014b {published data only}
- Zhang CY, Wu ZF. Clinical observation on the treatment effect of indirect moxa cone on ginger combined with [Ge jiang ai jiu lian he rui bai dui e xing zhong liu huan zhe hua liao hou gu sui yi zhi de xiao guo guan cha ji hu li]. Zhongguo Yi Yao Zhi Nan [Guide of China Medicine] 2014;12(4):213‐4. [Google Scholar]
Zhang 2014c {published data only}
- Zhang J, Wang LL. The clinical research on myelosuppression and quality of life after chemotherapy treated by grain‐sized moxibustion [Mai li jiu dui hua liao hou gu sui yi zhi ji sheng huo zhi liang ying xiang de lin chuang yan jiu]. Master Degree Thesis in Nanjing University of Taditional Chinese Medicine 2014.
Zhang 2014d {published data only}
- Zhang L, Chai TQ. The study about the clinical observation of the gastrointestinal reaction after the malignant tumor chemotherapy by the treatment of moxibustion on ginger [Ge jiang jiu zhi liao e xing zhong liu hua liao hou wei chang dao fan ying de lin chuang yan jiu]. Master Thesis Degree in Guangzhou University of Traditional Chinese Medicine 2014.
Additional references
Chan 2014
- Chan K, Zhang HW, Lin ZX. Chapter 48. Treatments used in complementary and alternative medicine. Side Effects of Drugs Annual. Vol. 36, Elsevier, 2015. [Google Scholar]
Chen 2008
- Chen ZJ, Guo YP, Wu ZC. Advances of clinical study on acupuncture and moxibustion for treatment of cancer pain [Zheng jiu zhi liao ai xing teng tong de lin chuang yan jiu jin zhan]. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2008;28(5):392‐4. [PubMed] [Google Scholar]
Cho 2009
- Cho WC. Chinese versus Western medicine. In: Schwab M editor(s). Encyclopedia of Cancer. 2nd Edition. Vol. 3, New York: Springer, 2009:652‐4. [Google Scholar]
Cho 2010
- Cho WC (editor). Supportive Cancer Care With Chinese Medicine. New York: Springer, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cui 2007
- Cui J, Yan J. Effect of moxibustion or acupuncture at Geshu acupoint on the granulocyte‐macrophage colony stimulating factor of cyclophosphamide induced leukopenic rats [Ai jiu huo zhen ci ge shu xue dui huan lin xian an suo zhi bai xi bao jian shao da shu you sheng li xi bao ‐ ju shi xi bao ji luo ci ji yin zi de zuo yong]. Zhongguo Zu Zhi Gong Cheng Yan jiu Yu Lin Chuang Kang Fu [Journal of Clinical Rehabilitative Tissue Engineering Research] 2007;11(28):5473‐6. [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Ellebaek 2008
- Ellebaek E, Herrstedt J. Optimizing antiemetic therapy in multiple‐day and multiple cycles of chemotherapy. Current Opinion in Supportive & Palliative Care 2008;2(1):28‐34. [DOI] [PubMed] [Google Scholar]
Ezzo 2006
- Ezzo J, Richardson MA, Vickers A, Allen C, Dibble S, Issell BF, et al. Acupuncture‐point stimulation for chemotherapy‐induced nausea or vomiting. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD002285.pub2] [DOI] [PubMed] [Google Scholar]
Gao 2010
- Gao CG. Clinical observation of ondansetron and moxibustion on zusanli point in treating delayed vomiting caused by cisplatin [En dai xi tong pei he zu san li xue wei ai jiu zhi liao shun bo suo zhi chi fa xing ou tu de lin chuang guan cha]. Lin Chuang He Li Yong Yao [Chinese Journal of Clinical Rational Drug Use] 2010;3(17):19‐20. [Google Scholar]
GRADE 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 19 September 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Henry 2008
- Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross‐sectional national survey in the U.S. Supportive Care in Cancer 2008;16(7):791‐801. [DOI] [PubMed] [Google Scholar]
Herrstedt 2007
- Herrstedt J, Dombernowsky P. Anti‐emetic therapy in cancer chemotherapy: current status. Basic & Clinical Pharmacology & Toxicology 2007;101(3):143‐50. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. The Cochrane Collaboration.
Huang 1999
- Huang X, Zhao CY. The influence of indirect moxibustion with herbal medicine on the hematopoietic system and cell factors after the treatment of radiotherapy for cancer [Ge yao bing jiu dui zhong liu fang liao hou zao xue xi tong ji xi bao yin zi de ying xiang]. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 1999;19(1):35‐7. [Google Scholar]
Jordan 2007
- Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy‐induced nausea and vomiting: past, present, and future recommendations. Oncologist 2007;12(9):1143‐50. [DOI] [PubMed] [Google Scholar]
Kim 2010
- Kim SY, Chae Y, Lee SM, Lee H, Park HJ. The effectiveness of moxibustion: an overview during 10 years. Evidence‐based Complementary and Alternative Medicine 2011;2011:306515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuai 2008
- Kuai L, Chen H, Yang HY. Current status and prospect of acupuncture‐moxibustion in treatment of cancer pain: a review [Ai zheng teng tong de zhen jiu zhi liao xian zhuang yu zhan wang]. Zhong Xi Yi Jie He Xue Bao [Journal of Chinese Integrative Medicine] 2008;6(2):197‐202. [DOI] [PubMed] [Google Scholar]
Lee 2010
- Lee MS, Choi TY, Park JE, Lee SS, Ernst E. Moxibustion for cancer care: a systematic review and meta‐analysis. BMC Cancer 2010;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lotfi‐Jam 2008
- Lotfi‐Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. Journal of Clinical Oncology 2008;26(34):5618‐29. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47(1):207‐14. [DOI] [PubMed] [Google Scholar]
Pei 2007
- Pei J, Yu YM, Wei H, Fang ZQ, Guan DY, Yu QW, et al. Experimental research on moxibustion in modulating tumor immunosuppression [Ai jiu tiao jie zhong liu mian yi yi zhi xiao ying de shi yan yan jiu]. Shanghai Zhong Yi Yao Za Zhi [Shanghai Journal of Traditional Chinese Medicine] 2007;41(8):1‐4. [Google Scholar]
Redmond 1996
- Redmond K. Advances in supportive care. European Journal of Cancer Care 1996;5 Suppl(2):1‐7. [DOI] [PubMed] [Google Scholar]
Robbins 2002
- Robbins MA, Gosselin TK. Symptom management in radiation oncology: acute and long‐term side effects. American Journal of Nursing 2002;102(Suppl 4):32‐6; quiz 49‐52. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Shen 2006
- Shen X, Ding G, Wei J, Zhao L, Zhou Y, Deng H, et al. An infrared radiation study of the biophysical characteristics of traditional moxibustion. Complementary Therapies in Medicine 2006;14(3):213‐9. [DOI] [PubMed] [Google Scholar]
Shen 2008
- Shen QP, Tian HQ. Acupuncture‐moxibustion in treatment of cancer pain: a review [Zhen jiu zhi liao ai tong de zhan wang]. Henan Zhong Yi [HenanTraditional Chinese Medicine] 2008;28(6):84‐6. [Google Scholar]
Stasi 2003
- Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer‐related fatigue: evolving concepts in evaluation and treatment. Cancer 2003;98(9):1786‐801. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. National Cancer Control Programmes: Policies and Managerial Guidelines. Geneva: World Health Organization, 2002. [Google Scholar]
WHO 2014
- Stewart BW, Wild CP, editors. World Cancer Report. Lyon: World Health Organization; International Agency for Research on Cancer, 2014. [Google Scholar]
Yu 2003
- Yu ZC, Wang HF, Xu LF. Effect of moxibustion on immunologic function in patients with cervical carcinoma in radiotherapy [Jiu liao dui gong jing ai fang liao huan zhe mian yi gong neng de ying xiang]. Xian Dai Zhong Xi Yi Jie He Za Zhi [Modern Journal of Integreated Traditional Chinese and Western Medicine] 2003;12(24):2629‐30,44. [Google Scholar]
Zhang 2008
- Zhang JL, Li SS, Luo WH, Zhang DF, Shu RG. Research of moxibustion therapy for cancer: a review [Jiu fa zai zhong liu zhi liao zhong de zuo yong yan jiu jin zhan]. Jiangxi Zhong Yi Yao [Jiangxi Journal of Traditional Chinese Medicine] 2008;39(1):59‐61. [Google Scholar]
Zhao 2006
- Zhao B, Wang X, Lin Z, Liu R, Lao L. A novel sham moxibustion device: a randomized, placebo‐controlled trial. Complementary Therapies in Medicine 2006;14(1):53‐60. [DOI] [PubMed] [Google Scholar]
Zhao 2007
- Zhao XX, Lu M, Zhu X, Gao P, LI YL, Wang XM, et al. Multi‐centre clinical evaluation of ginger‐partitioned moxibustion for treatment of leukopenia induced by chemotherapy [Ge jiang jiu zhi liao hua liao suo zhi bai xi bao jian shao zheng duo zhong xin sui ji dui zhao yan jiu]. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2007;27(10):715‐20. [PubMed] [Google Scholar]
Zheng 2002
- Zheng XY, editor. Guiding principles for clinical research on new drugs of traditional Chinese medicine. Beijing: Chinese Medical Science and Technology Press, 2002. [Google Scholar]


