Abstract
Background
Observational studies suggest higher pregnancy rates after the hysteroscopic removal of endometrial polyps, submucous fibroids, uterine septum or intrauterine adhesions, which are present in 10% to 15% of women seeking treatment for subfertility.
Objectives
To assess the effects of the hysteroscopic removal of endometrial polyps, submucous fibroids, uterine septum or intrauterine adhesions suspected on ultrasound, hysterosalpingography, diagnostic hysteroscopy or any combination of these methods in women with otherwise unexplained subfertility or prior to intrauterine insemination (IUI), in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Search methods
We searched the following databases from their inception to 16 April 2018; The Cochrane Gynaecology and Fertility Group Specialised Register, the Cochrane Central Register of Studies Online, ; MEDLINE, Embase , CINAHL , and other electronic sources of trials including trial registers, sources of unpublished literature, and reference lists. We handsearched the American Society for Reproductive Medicine (ASRM) conference abstracts and proceedings (from 1 January 2014 to 12 May 2018) and we contacted experts in the field.
Selection criteria
Randomised comparison between operative hysteroscopy versus control for unexplained subfertility associated with suspected major uterine cavity abnormalities.
Randomised comparison between operative hysteroscopy versus control for suspected major uterine cavity abnormalities prior to medically assisted reproduction.
Primary outcomes were live birth and hysteroscopy complications. Secondary outcomes were pregnancy and miscarriage.
Data collection and analysis
Two review authors independently assessed studies for inclusion and risk of bias, and extracted data. We contacted study authors for additional information.
Main results
Two studies met the inclusion criteria.
1. Randomised comparison between operative hysteroscopy versus control for unexplained subfertility associated with suspected major uterine cavity abnormalities.
In women with otherwise unexplained subfertility and submucous fibroids, we were uncertain whether hysteroscopic myomectomy improved the clinical pregnancy rate compared to expectant management (odds ratio (OR) 2.44, 95% confidence interval (CI) 0.97 to 6.17; P = 0.06, 94 women; very low‐quality evidence). We are uncertain whether hysteroscopic myomectomy improves the miscarriage rate compared to expectant management (OR 1.54, 95% CI 0.47 to 5.00; P = 0.47, 94 women; very low‐quality evidence). We found no data on live birth or hysteroscopy complication rates. We found no studies in women with endometrial polyps, intrauterine adhesions or uterine septum for this randomised comparison.
2. Randomised comparison between operative hysteroscopy versus control for suspected major uterine cavity abnormalities prior to medically assisted reproduction.
The hysteroscopic removal of polyps prior to IUI may have improved the clinical pregnancy rate compared to diagnostic hysteroscopy only: if 28% of women achieved a clinical pregnancy without polyp removal, the evidence suggested that 63% of women (95% CI 45% to 89%) achieved a clinical pregnancy after the hysteroscopic removal of the endometrial polyps (OR 4.41, 95% CI 2.45 to 7.96; P < 0.00001, 204 women; low‐quality evidence). We found no data on live birth, hysteroscopy complication or miscarriage rates in women with endometrial polyps prior to IUI. We found no studies in women with submucous fibroids, intrauterine adhesions or uterine septum prior to IUI or in women with all types of suspected uterine cavity abnormalities prior to IVF/ICSI.
Authors' conclusions
Uncertainty remains concerning an important benefit with the hysteroscopic removal of submucous fibroids for improving the clinical pregnancy rates in women with otherwise unexplained subfertility. The available low‐quality evidence suggests that the hysteroscopic removal of endometrial polyps suspected on ultrasound in women prior to IUI may improve the clinical pregnancy rate compared to simple diagnostic hysteroscopy. More research is needed to measure the effectiveness of the hysteroscopic treatment of suspected major uterine cavity abnormalities in women with unexplained subfertility or prior to IUI, IVF or ICSI.
Plain language summary
Hysteroscopy for treating suspected abnormalities of the cavity of the womb in women having difficulty becoming pregnant
Review question
Cochrane authors reviewed the evidence about the effect of the hysteroscopic treatment of suspected abnormalities of the cavity of the womb in women having difficulty becoming pregnant.
Background
Human life starts when a fertilised egg has successfully implanted in the inner layer of the cavity of the womb. It is believed that abnormalities originating from this site, such as polyps (abnormal growth of tissue), fibroids (non‐cancerous growth), septa (upside‐down, triangular‐shaped piece of tissue which divides the womb) or adhesions (scar tissue that sticks the walls of the womb together), may disturb this event. The removal of these abnormalities by doing a hysteroscopy using a very small diameter inspecting device might therefore increase the chance of becoming pregnant either spontaneously or after specialised fertility treatment, such as insemination or in vitro fertilisation.
Study characteristics
We found two studies. The first study compared the removal of fibroids versus no removal in 94 women wishing to become pregnant spontaneously from January 1998 to April 2005. The second study compared the removal of polyps versus simple hysteroscopy only in 204 women before insemination with husband's sperm from January 2000 to February 2004. The evidence is current to April 2018. Neither study reported funding sources.
Key results
In women with fibroids wishing to become pregnant spontaneously we were uncertain whether removal of the fibroids improved the pregnancy or miscarriage rate compared to usual management: uncertainty remains because the number of women (94) and the number of pregnancies (30) were too small and the quality of the evidence was very low. We found no data on live birth or complications due to surgery. We found no studies on women with polyps, septa or adhesions.
The hysteroscopic removal of polyps prior to intrauterine insemination (IUI; a fertility treatment where sperm is placed inside a woman's womb to fertilise the egg) is may improve the pregnancy rate compared to not removing polyps. If 28% of women become pregnant without surgery, the evidence suggests that about 63% of women will become pregnant following removal of polyps. We found no data on number of live births, hysteroscopy complications or miscarriage rates prior to IUI. We retrieved no studies in women before other fertility treatments.
More studies are needed before hysteroscopy can be proposed as a fertility‐enhancing procedure in the general population of women having difficulty becoming pregnant.
Quality of the evidence
The quality of the evidence retrieved was very low to low due to the limited number of participants and the poor design of the studies.
Summary of findings
Background
Description of the condition
Subfertility is "a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse" according to the International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of assisted reproductive technology (Zegers‐Hochschild 2017) (see: www.icmartivf.org/ivf‐glossary.html). It is estimated that 72.4 million women are subfertile and that 40.5 million of these women are currently seeking fertility treatment (Boivin 2007). Unexplained subfertility usually refers to a diagnosis (or lack of diagnosis) made in couples in whom all the standard investigations such as tests of ovulation, tubal patency and semen analysis are normal: it can be found in as many as 30% to 40% of subfertile couples (Ray 2012).
The evaluation of the uterine cavity seems a basic step in the investigation of all subfertile women since the uterine cavity and its inner layer, the endometrium, are assumed to be important for the implantation of the human embryo, called a blastocyst. Nevertheless, the complex mechanisms leading to successful implantation are still poorly understood (Taylor 2008). Despite the huge investment in research and developments of the technologies and biology involved in medically assisted reproduction (MAR), the maximum implantation rate per embryo transferred still remains only 30% (Andersen 2008). The different phases of the implantation process are established by the complex interchange between the blastocyst and the endometrium (Singh 2011).
Major uterine cavity abnormalities can be found in 10% to 15% of women seeking treatment for subfertility; they usually consist of the presence of excessive normal uterine tissue (Wallach 1972). The most common acquired uterine cavity abnormality is an endometrial polyp. This benign, endometrial stalk‐like mass protrudes into the uterine cavity and has its own vascular supply. Depending on the population under study and the applied diagnostic test, endometrial polyps can be found in 1% to 41% of the subfertile population (Silberstein 2006). A fibroid is an excessive growth originating from the muscular part of the uterine cavity. Fibroids are present in 2.4% of subfertile women without any other obvious cause of subfertility (Donnez 2002). A submucous fibroid is located underneath the endometrium and is thought to interfere with fertility by deforming the uterine cavity. Intrauterine adhesions are fibrous tissue strings connecting parts of the uterine wall. They are commonly caused by inflammation or iatrogenic tissue damage (meaning involuntarily caused by a physician's intervention, for example an aspiration curettage after miscarriage) and are present in 0.3% to 14% of subfertile women (Fatemi 2010).
A septate uterus is a congenital malformation in which the longitudinal band separating the left and right Müllerian ducts, which form the uterus in the human female foetus, has not been entirely resorbed. A uterine septum is present in 1% to 3.6% of women with otherwise unexplained subfertility (Saravelos 2008).
Ultrasonography (US), preferably transvaginally (TVS), is used to screen for possible endometrium or uterine cavity abnormalities in the work‐up of subfertile women. This evaluation can be expanded with hysterosalpingography (HSG), saline infusion/gel instillation sonography (SIS/GIS) and diagnostic hysteroscopy. Diagnostic hysteroscopy is generally considered as the gold standard procedure for the assessment of the uterine cavity since it enables direct visualisation; moreover, treatment of intrauterine pathology can be done in the same setting (Bettocchi 2004). Nevertheless, even for experienced gynaecologists, the hysteroscopic diagnosis of the major uterine cavity abnormalities may be problematic (Kasius 2011a).
Description of the intervention
Hysteroscopy is performed for the evaluation, or for the treatment of the uterine cavity, tubal ostia and endocervical canal in women with uterine bleeding disorders, Müllerian tract anomalies, retained intrauterine contraceptives or other foreign bodies, retained products of conception, desire for sterilisation, recurrent miscarriage and subfertility. If the procedure is intended for evaluating the uterine cavity only, it is called a diagnostic hysteroscopy. If the observed pathology requires further treatment, the procedure is called an operative hysteroscopy. In everyday practice, a diagnostic hysteroscopy confirming the presence of pathology will be followed by an operative hysteroscopy in a symptomatic woman.
Hysteroscopy allows the direct visualisation of the uterine cavity through a rigid, semi‐rigid or flexible endoscope. The hysteroscope consists of a rigid telescope with a proximal eyepiece and a distal objective lens that may be angled at 0° to allow direct viewing or offset at various angles to provide a fore‐oblique view. Advances in fibreoptic technology have led to the miniaturisation of the telescopes without compromising the image quality. The total working diameters of modern diagnostic hysteroscopes are typically 2.5 mm to 4.0 mm. Operative hysteroscopy requires adequate visualisation through a continuous fluid circulation using an inflow and an outflow channel. The outer diameters of modern operative hysteroscopes have been reduced to a diameter between 4.0 mm and 5.5 mm. The sheath system contains one or two 1.6 mm to 2.0 mm working channels for the insertion of small grasping or biopsy forceps, scissors, myoma fixation instruments, retraction loops, morcellators (surgical instruments used to divide and remove tissue during endoscopic surgery) and aspiration cannulae, or unipolar or bipolar electrodiathermy instruments.
Most diagnostic and many operative procedures can be done in a clinic setting using local anaesthesia and fluid distension media, while more complex procedures are generally performed as day surgery under general anaesthesia (Clark 2005). Operative hysteroscopic procedures require a complex instrumentation setup, special training of the surgeon, and appropriate knowledge and management of complications (Campo 1999).
Although complications from hysteroscopy are rare, they can be potentially life threatening. One multicentre study including 13,600 diagnostic and operative hysteroscopic procedures performed in 82 centres reported a complication rate of 0.28%. Diagnostic hysteroscopy had a significantly lower complication rate compared to operative hysteroscopy (0.13% with diagnostic versus 0.95% with operative). The most common complication of both types of hysteroscopy was uterine perforation (0.13% with diagnostic versus 0.76% with operative). Fluid intravasation occurred almost exclusively in operative procedures (0.02%). Intrauterine adhesiolysis was associated with the highest incidence of complications (4.5%); all of the other procedures had complication rates of less than 1% (Jansen 2000).
How the intervention might work
It is assumed that major uterine cavity abnormalities may interfere with factors that regulate the blastocyst‐endometrium interplay, for example hormones and cytokines, precluding the possibility of pregnancy. Many hypotheses have been formulated in the literature of how endometrial polyps (Shokeir 2004; Silberstein 2006; Taylor 2008; Yanaihara 2008), submucous fibroids (Pritts 2001; Somigliana 2007; Taylor 2008), intrauterine adhesions (Yu 2008), and uterine septum (Fedele 1996) are likely to disturb the implantation of the human embryo; nevertheless, the precise mechanisms of action through which each one of these major uterine cavity abnormalities affects this essential reproductive process are poorly understood. The foetal‐maternal conflict hypothesis tries to explain how a successful pregnancy may establish itself despite the intrinsic genomic instability of human embryos through the specialist functions of the endometrium, in particular its capacity for cyclic spontaneous decidualisation, shedding and regeneration. An excellent indepth review linking basic research of human implantation with clinical practice can be found elsewhere (Lucas 2013).
For endometrial polyps, submucous fibroids, intrauterine adhesions and uterine septum, observational studies have shown a clear improvement in the spontaneous pregnancy rate after the hysteroscopic removal of the abnormality (Taylor 2008). Two observational studies suggested a better reproductive outcome following hysteroscopic polypectomy in women prior to intrauterine insemination (IUI) (Kalampokas 2012; Shohayeb 2011). The chance for pregnancy is significantly lower in subfertile women with submucous fibroids compared to other causes of subfertility according to one systematic review and meta‐analysis of 11 observational studies (Pritts 2001; Pritts 2009). Three observational studies found a major benefit for removing a uterine septum by hysteroscopic metroplasty in subfertile women with a uterine septum (Mollo 2009; Shokeir 2011; Tomaževič 2010).
Why it is important to do this review
An updated National Institute for Health and Care Excellence (NICE) guideline on fertility assessment and treatment states that " Women should not be offered hysteroscopy on its own as part of the initial investigation unless clinically indicated because the effectiveness of surgical treatment of uterine abnormalities on improving pregnancy rates has not been established " (NICE 2013). However, there is a trend in reproductive medicine that is developing towards diagnosis and treatment of all major uterine cavity abnormalities prior to fertility treatment. This evolution can be explained by three reasons. First, diagnostic hysteroscopy is generally accepted in everyday clinical practice as the 'gold standard' for identifying uterine abnormalities because it allows direct visualisation of the uterine cavity (Golan 1996). Second, since 2004 several randomised controlled trials (RCTs) have demonstrated the technical feasibility and the high patient satisfaction rate in women undergoing both diagnostic and operative hysteroscopy for various reasons including subfertility (Campo 2005; De Placido 2007; Garbin 2006; Guida 2006; Kabli 2008; Marsh 2004; Sagiv 2006; Shankar 2004; Sharma 2005). Third, in a subfertile population screened systematically by diagnostic hysteroscopy, the incidence of newly detected intrauterine pathology may be as high as 50% (Campo 1999; De Placido 2007).
This review aimed to summarise and critically appraise the current evidence on the effectiveness of operative hysteroscopic interventions in subfertile women with major uterine cavity abnormalities, both in women with unexplained subfertility and women bound to undergo MAR. Since uterine cavity abnormalities may negatively affect the uterine environment, and therefore the likelihood of conceiving (Rogers 1986), it has been recommended that these abnormalities be diagnosed and treated by hysteroscopy to improve the cost‐effectiveness in subfertile women undergoing MAR, where recurrent implantation failure is inevitably associated with a higher economic burden to society.
The study of the association between subfertility and major uterine cavity abnormalities might increase our current understanding of the complex mechanisms of human embryo implantation. This could lead to the development of cost‐effective strategies in reproductive medicine with benefits for both the individual woman experiencing subfertility associated with major uterine cavity abnormalities as well as for society, in a broader perspective.
Objectives
To assess the effects of the hysteroscopic removal of endometrial polyps, submucous fibroids, uterine septum or intrauterine adhesions suspected on ultrasound, hysterosalpingography, diagnostic hysteroscopy or any combination of these methods in women with otherwise unexplained subfertility or prior to intrauterine insemination (IUI), in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Trials that were either clearly randomised or claimed to be randomised and did not have evidence of inadequate sequence generation such as date of birth or hospital number were eligible for inclusion.
Cluster trials were eligible if the individually randomised women were the unit of analysis.
Cross‐over trials were eligible for completeness but we planned to use only pre‐cross‐over data for meta‐analysis.
Exclusion criteria
Quasi‐randomised trials.
Types of participants
Inclusion criteria
Women of reproductive age with otherwise unexplained subfertility and endometrial polyps, submucous fibroids, septate uterus or intrauterine adhesions detected by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods. Besides unexplained subfertility as the main clinical problem, other gynaecological complaints, such as pain or bleeding, might or might not be present.
Women of reproductive age with subfertility, undergoing IUI, IVF or ICSI with endometrial polyps, submucous fibroids, septate uterus or intrauterine adhesions detected by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods.
Exclusion criteria
Women of reproductive age with no major uterine cavity abnormalities detected by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods.
Women of reproductive age with subfertility and intrauterine cavity abnormalities other than endometrial polyps, submucous fibroids, intrauterine adhesions and septate uterus, for example, subserous or intramural fibroids without cavity deformation on hysteroscopy, acute or chronic endometritis, adenomyosis or other so‐called 'subtle focal' lesions.
Women of reproductive age with endometrial polyps, submucous fibroids, intrauterine adhesions or septate uterus without subfertility.
Women of reproductive age with recurrent pregnancy loss.
Types of interventions
We addressed two types of randomised interventions; within both comparisons we stratified the suspected major uterine cavity abnormalities into endometrial polyps, submucous fibroids, uterine septum and intrauterine adhesions. For the second comparison, there was a stratification into IUI, IVF or ICSI.
Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities diagnosed by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods.
Operative hysteroscopy versus control in women undergoing IUI, IVF or ICSI with suspected major uterine cavity abnormalities diagnosed by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods.
Types of outcome measures
Primary outcomes
Effectiveness: live birth, defined as a delivery of a live foetus after 20 completed weeks of gestational age that resulted in at least one live baby born. The delivery of a singleton, twin or multiple pregnancy was counted as one live birth.
Adverse events: hysteroscopy complications, defined as any complication due to hysteroscopy.
Secondary outcomes
-
Pregnancy
Ongoing pregnancy, defined as a pregnancy surpassing the first trimester or 12 weeks of pregnancy.
Clinical pregnancy with foetal heartbeat, defined as a pregnancy diagnosed by US or clinical documentation of at least one foetus with a heartbeat (Zegers‐Hochschild 2017).
Clinical pregnancy, defined as a pregnancy diagnosed by US visualisation of one or more gestational sacs or definitive clinical signs of pregnancy (Zegers‐Hochschild 2017).
Adverse events: miscarriage, defined as the spontaneous loss of a clinical pregnancy before 20 completed weeks of gestation, or if gestational age was unknown a foetus with a weight of 400 g or less.
We planned to report the minimally important clinical difference (MICD) for the primary outcome of live birth. An MICD of 5% for the live birth rate was predefined as being relevant for the benefits. The imputation of this value was based on data from a clinical decision analysis on screening hysteroscopy prior to IVF (Kasius 2011b).
Search methods for identification of studies
An updated search was done in liaison with the Cochrane Gynaecology and Fertility (CGF) Group Information Specialist (Marian Showell).
Two review authors (JB and SVW) independently performed a comprehensive search of all published and unpublished reports that described hysteroscopy in subfertile women with endometrial polyps, submucous fibroids, intrauterine adhesions or septate uterus, or undergoing MAR. The search strategy was not limited by language, year of publication or document format. We merged all the retrieved citations from the CGF Specialised Register, the Cochrane Central Register of Studies Online (CENTRAL), MEDLINE, Embase, Web of Science, the BIOSIS PREVIEWS and handsearch‐related articles and removed duplicates using specialised software (EndNote Web 3.5; www.myendnoteweb.com/EndNoteWeb.html; last done: 12 May 2018).
Electronic searches
For the 2018 update of this Cochrane Review, we searched the following bibliographic databases, trial registers and websites:
The Cochrane Gynaecology and Fertility Group's (CGF) Specialised Register, searched on 16 April 2018, PROCITE platform (Appendix 1), CENTRAL Register of Studies Online (CRSO), searched on 16 April 2018, web platform (Appendix 2), MEDLINE OvidSP (searched from 1946 to 16 April 2018) (Appendix 3), and Embase OvidSP (searched from 1980 to 16 April 2018) (Appendix 4).
The search strategy combined both index and free‐text terms.
Our MEDLINE search included the Cochrane highly sensitive search strategy for identifying randomised trials using the PubMed format which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Section 6.4.11.1, box 6.4.a) (Higgins 2011).
Our Embase search included the SIGN trial filter developed by the Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/methodology/filters.html#random).
Other electronic sources of trials were:
Database of Abstracts of Reviews of Effectiveness (DARE) and the Health Technology Assessment Database (HTA Database) through the Centre for Reviews and Dissemination (searched 12 May 2018) (www.crd.york.ac.uk);
National Guideline Clearinghouse (www.guideline.gov) for evidence‐based guidelines (searched 12 May 2018);
BIOSIS previews through ISI Web of Knowledge (isiwebofknowledge.com) and CINAHL (www.cinahl.com) through EBSCOhost available at the Biomedical Library Gasthuisberg of the Catholic University of Leuven (from 1961 to 1 May 2018) (Appendix 5);
trial registers for ongoing and registered trials: Current Controlled Trials (www.controlled‐trials.com), ClinicalTrials.gov provided by the US National Institutes of Health (clinicaltrials.gov/ct2/home) and the WHO International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/) (searched 12 May 2018);
citation indexes: Science Citation Index through Web of Science (scientific.thomson.com/products/sci/) – SCI‐EXPANDED (2014 to 12 May 2018) and Conference Proceedings Citation Index – Science (CPCI‐S) (2014 to 12 May 2018) and Scopus available at the Biomedical Library Gasthuisberg of the Catholic University of Leuven) (from inception to 12 May 2018);
conference abstracts and proceedings on the ISI Web of Knowledge (isiwebofknowledge.com) applying 'SCI‐EXPANDED' (2014 to 12 May 2018) and 'CPCI‐S' (2014 to 12 May 2018) (Appendix 6);
LILACS database (searched 12 May 2018) (bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F) (searched 12 May 2018);
European grey literature through Open Grey database (searched to 12 May 2018) (www.opengrey.eu/subjects/).
Searching other resources
Two review authors (JB and JK for the first published version, JB and SVW for this update) independently handsearched the reference lists of reviews, guidelines, included and excluded studies, and other related articles for additional eligible studies. One review author (JB) contacted the first or corresponding authors of included studies to ascertain if they were aware of any ongoing or unpublished trials.
Two review authors (JB and JK for the first published version, JB and SVW for this update) independently handsearched the American Society for Reproductive Medicine (ASRM) conference abstracts and proceedings (from 1 January 2014 to 12 May 2018).
One review author (JB) contacted European experts and opinion leaders in the field of hysteroscopic surgery to ascertain if these experts were aware of any relevant published or unpublished studies.
Data collection and analysis
Selection of studies
Two or three review authors were responsible for independently selecting the studies (FB and TD for the first published version, JB, SVW and MB for this update). We scanned titles and abstracts from the searches and obtained the full text of those articles that appeared to be eligible for inclusion. We linked multiple reports of the same study together while citing all the references and indicating the primary reference of the identified study. On assessment, we categorised the trials as 'included studies' (Characteristics of included studies table), 'excluded studies' (Characteristics of excluded studies table), 'ongoing studies' (Characteristics of ongoing studies table) or 'studies awaiting classification' (Characteristics of studies awaiting classification table). Any disagreements between both review authors who are content experts were resolved through consensus or by a third review author (BWM for the first published version, SW for this update). We contacted the first or corresponding authors of the primary study reports for further clarification when required. If disagreements between review authors were not resolved, we categorised the studies as 'awaiting classification' and reported the disagreement in the final review. We avoided the exclusion of studies on the basis of the reported outcome measures throughout the selection phase by searching all potential eligible studies that could have measured the primary or secondary outcomes even if these were not reported. We appraised studies in an unblinded fashion, as recommended by the CGF Group.
Data extraction and management
Two review authors, one methodologist (JB) and one topic area specialist (SW), independently assessed the studies that appeared to meet the inclusion criteria by using data extraction forms based on the items listed in the protocol of this Cochrane Review (Appendix 7). We pilot‐tested the data extraction form and process by reviewing 10 randomly chosen study reports. In the pilot phase, two review authors consistently identified one retracted record (Shokeir 2011) on the basis of finding duplicated parts from another study included in the present Cochrane review (Pérez‐Medina 2005). For studies with multiple publications, we used the main trial report as the primary data extraction source and additional details supplemented from secondary papers if applicable. One review author (JB) contacted the first or corresponding authors of the original studies to obtain clarification whenever additional information on trial methodology or original trial data was required. We sent reminder correspondence if a reply was not obtained within two weeks. The two review authors resolved any discrepancies in opinion by discussion; they searched for arbitration by a third review author if consensus was not reached (BWM). One review author (BWM) resolved disagreements which could not be resolved by the review authors after contacting the first or corresponding authors of the primary study reports. If this failed, we reported the disagreement in the review.
Assessment of risk of bias in included studies
Two review authors (JB and SW) independently assessed the risk of bias of the included studies using the Cochrane 'Risk of bias' assessment tool that considers the following criteria, listed in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, tables 8.5.a and 8.5.b) (Higgins 2011): random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, completeness of outcome data, selective outcome reporting and other potential sources of bias. We resolved any disagreements by consensus or by discussion with a third review author (BWM). We presented the conclusions in the 'Risk of bias' table (Characteristics of included studies table), with a full description of all judgements and incorporated them into the interpretation of review findings by means of sensitivity analyses.
We presented a narrative description of the quality of evidence, which is necessary for the interpretation of the results of the review and which is based on the review authors' judgements on the risk of bias of the included trials (Quality of the evidence).
Measures of treatment effect
For the dichotomous data for live birth, pregnancy, miscarriage and hysteroscopy complications, we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (OR). We presented 95% confidence intervals (CI) for all outcomes. The OR has mathematically sound properties that are consistent with benefit or harm and which work well in most RCTs on the effectiveness of reproductive surgery given that sample sizes are usually small and trial events are rare. Where data to calculate ORs were not available, we planned to utilise the most detailed numerical data available that might facilitate similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking account of legitimate differences. We contacted the corresponding or first authors of all included trials that reported data in a form that was not suitable for meta‐analysis, such as time‐to‐pregnancy (TTP) data. We planned to report the data of those reports that failed to present additional data that could be analysed under 'other data'; we did not included TTP data in any meta‐analyses.
Unit of analysis issues
All primary and secondary outcomes were expressed as per woman randomised. In addition, we reported narrative data on miscarriage expressed as per pregnancy as a secondary analysis. We planned to summarise reported data that did not allow a valid analysis, such as 'per cycle', in an additional table without any attempt at meta‐analysis. We counted multiple live births and multiple pregnancies as one live birth or one pregnancy event. We planned to include only first‐phase data from cross‐over trials, if available.
Dealing with missing data
We aimed to analyse the data on an intention‐to‐treat (ITT) basis. We tried to obtain as much missing data as possible from the original investigators. If this was not possible, we undertook imputation of individual values for the primary outcomes only. We assumed that live births would not have occurred in participants without a reported primary outcome. For all other outcomes, we analysed only the available data. We subjected any imputation of missing data for the primary outcomes to sensitivity analysis. If there were substantial differences in the analysis as compared to an available data analysis, we reported this in the review.
Assessment of heterogeneity
We planned to consider whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary, if more randomised studies were included. We planned to carry out a formal assessment of statistical heterogeneity using the I² statistic combined with the Q‐statistic. Cochrane's Q test, a type of Chi² statistic, is the classical measure to test significant heterogeneity. Cochrane's Q test is calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies. The Q‐statistic follows Chi² distribution with k‐1 degree of freedom where k is the number of studies. Q greater than k‐1 suggests statistical heterogeneity. A low P value of Cochrane's Q test means significant heterogeneous results among different studies; usually, the P value at 0.10 is used as the cut‐off. The Q‐statistic has low power as a comprehensive test of heterogeneity especially when the number of studies is small. The Q‐statistic informs us about the presence or absence of heterogeneity; it does not report on the extent of such heterogeneity. The I² statistic describes the percentage of variation across studies that is due to significant heterogeneity rather than random chance. It measures the extent of heterogeneity. An I² statistic greater than 50% was taken to indicate substantial heterogeneity (Higgins 2003). We planned to explore possible explanations for heterogeneity by performing sensitivity analyses in Review Manager 5, if there was evidence of substantial statistical heterogeneity (Review Manager 2014).
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias, reporting bias and within‐study reporting bias, we planned to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert in identifying duplication of data. We aimed to detect within‐trial selective reporting bias, such as trials failing to report obvious outcomes, or reporting them in insufficient detail to allow inclusion. We planned to seek published protocols and to compare the outcomes between the protocol and the final published study report. Where identified studies failed to report the primary outcomes (e.g. live birth), but did report interim outcomes (e.g. pregnancy), we would have undertaken informal assessment as to whether the interim values were similar to those reported in studies that also reported the primary outcomes. If there were outcomes defined in the protocol or the study report with insufficient data to allow inclusion, the review indicated this lack of data and suggested that further clinical trials need to be conducted to clarify these knowledge gaps. If there were 10 or more studies, we planned to create a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies). A gap on either side of the graph would have given a visual indication that some trials had not been identified. Given the low number of studies included in the final review, it was not possible to assess reporting bias formally.
Data synthesis
One review author (JB) entered the data and carried out the statistical analysis of the data using Review Manager 5 (Review Manager 2014). We considered the outcomes live birth and pregnancy to be positive and higher numbers as a benefit. We considered the outcomes miscarriage and hysteroscopy complications in the protocol as negative effects and higher numbers harmful. These aspects were taken into consideration when assessing the summary graphs. In the quantitative synthesis an increase in the odds of a particular outcome, either beneficial or harmful, was displayed graphically to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
We planned to combine data from primary studies in a meta‐analysis with Review Manager 5 using the Peto method and a fixed‐effect model (Higgins 2011) for the following comparisons, if more randomised studies could have been included and if significant clinical diversity and statistical heterogeneity could have been confidently ruled out.
Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities diagnosed by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods.
Operative hysteroscopy versus control in women undergoing MAR with suspected major uterine cavity abnormalities diagnosed by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods.
We planned to define analyses that were both comprehensive and mutually exclusive so that all eligible study results were slotted into one of the two predefined strata only. If there were no retrieved trials for some comparisons, the review indicated their absence identifying knowledge gaps which need further research. Since meta‐analysis was not possible due to the limited number of studies included in the review, we presented a narrative overview as prespecified in the protocol).
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses to determine the separate evidence within the following subgroups, if enough data were available.
Those studies that reported 'live birth' and 'ongoing or clinical pregnancy' to assess any overestimation of effect and reporting bias.
For the two types of randomised comparison, stratified according to the type of uterine abnormality, we planned to carry out subgroup analyses according to the extent or severity of the uterine abnormality. We used the length and diameter in centimetres or calculated volumes of endometrial polyps and submucous fibroids, the lengths and widths of uterine septa and the European Society of Gynaecological Endoscopy (ESGE) classification for intrauterine adhesions as references when applicable (Wamsteker 1998).
We planned to carry out subgroup analyses based on the modifier participant age if enough studies were available.
The interpretation of the statistical analysis for subgroups is not without problems. In the final review, we reported the interpretation of any subgroup analysis performed restrictively, if at all possible, and with utmost caution even if enough data were retrieved.
Sensitivity analysis
We aimed to perform sensitivity analyses for the primary outcomes to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether conclusions would have differed if:
eligibility was restricted to studies at low risk of bias in all domains;
alternative imputation strategies were adopted;
a random‐effects rather than a fixed‐effect model was adopted;
the summary effect measure was risk ratio rather than OR.
Overall quality of the body of evidence: 'summary of findings' table
We prepared 'summary of findings' tables using GRADE and Cochrane methods (GRADEpro GDT; Higgins 2011). These tables evaluated the overall quality of the body of evidence for the main review outcomes (primary outcome of effectiveness – live birth – as well as secondary outcome of effectiveness – clinical pregnancy – and the adverse events – hysteroscopy complication and miscarriage) for the two main review comparisons (randomised comparison between operative hysteroscopy versus control for unexplained subfertility associated with suspected major uterine cavity abnormalities and randomised comparison between operative hysteroscopy versus control for suspected major uterine cavity abnormalities prior to MAR). We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). Two review authors independently made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
For the present update, there were 108 records from the CGFG Specialised Register, 347 records from CRSO, 469 from MEDLINE, 160 from Embase and 33 from Web of Science. An electronic search in DARE produced 50 records; there were 13 guidelines from National Guideline Clearinghouse, 25 records from the ISRCTN register of controlled trials and 20 records from WHO ICTRP. We identified 538 additional references in Scopus. We identified 184 records in CINAHL and 59 records in LILACS but none in Open Grey. We handsearched 400 abstracts in the congress proceedings of the ASRM for the period 2014 to 2018; we identified no additional abstracts after contacting the experts of the European Society for Gynaecological Endoscopy (ESGE).
After combining 2006 records identified from electronic searches with 400 additional records through handsearching, we screened 2406 records for duplicates by using a specialised software program (EndNote Web). After the removal of 561 duplicate references, we screened the titles or abstracts, or both of 1845 records. We removed 1683 records that were obviously irrelevant. We assessed 162 full‐text articles for eligibility. We did not include additional RCTs in the updated version, so finally we included two RCTs addressing the research questions of this Cochrane Review (Characteristics of included studies table). We excluded 64 studies for various reasons (Characteristics of excluded studies table); 58 studies did not address the PICO research questions of this Cochrane Review, four studies were not randomised, one was a quasi‐randomised trial and one potentially eligible RCT was excluded (Shokeir 2010) because the study report had been retracted at the request of the publisher. Two trials are still ongoing (Characteristics of ongoing studies table).
See: PRISMA flow chart (Figure 1).
1.
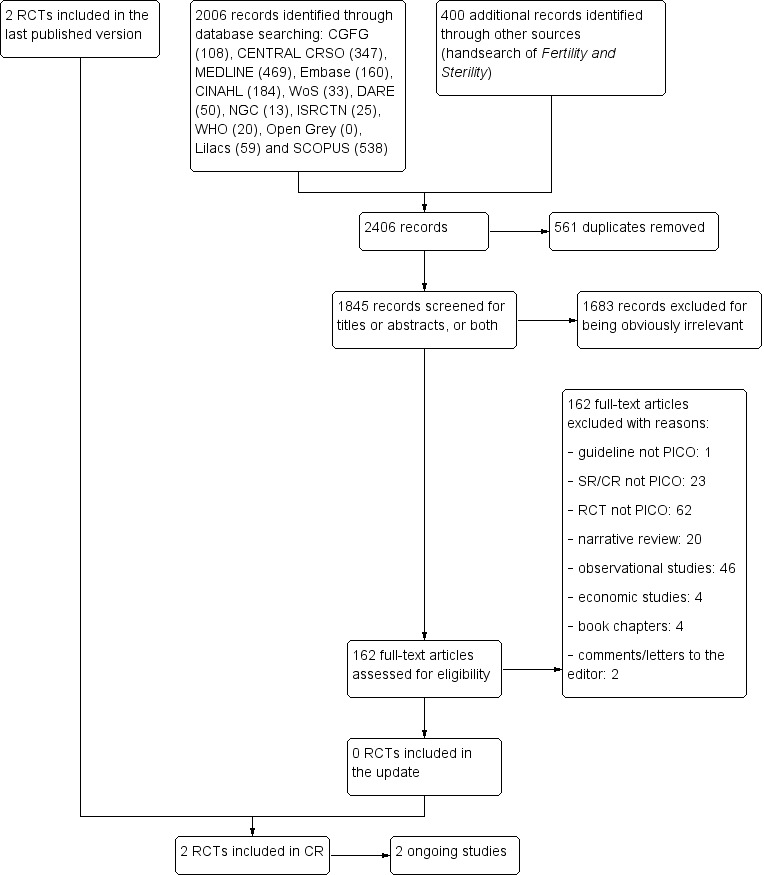
Study flow diagram: summary of searches since 2014. CR: Cochrane Review; PICO: Population, Intervention, Comparator, Outcome; RCT: randomised controlled trial; SR: systematic review.
Included studies
Study design and setting
The review included two parallel‐design RCTs. Both were single‐centre studies, one conducted in Italy (Casini 2006) and the other in Spain (Pérez‐Medina 2005).
Participants
One study included 94 women with submucous fibroids with or without intramural fibroids and otherwise unexplained subfertility (Casini 2006). There were 52 women in the intervention group and 42 women in the control group. The mean participant age was 31 years (range 29 to 34) in the subgroup of women with submucous fibroids only and 32 years (range 30 to 35) in the subgroup of women with mixed intramural‐submucous fibroids. All women underwent a complete fertility assessment. The presence of uterine fibroids was by TVS. All women who were affected by uterine fibroids excluding all other causes of infertility were asked to participate in the study. Only women aged 35 years or less with a problem of subfertility for at least one year and the presence of one fibroid of diameter 40 mm or less were selected for randomisation. Women older than 35 years or with other causes of infertility at the performed examinations were excluded. Other exclusion criteria were the presence of two or more fibroids of diameter greater than 40 mm; body weight greater than 20% of normal weight; and use of medication containing oestrogens, progestins or androgens in the eight weeks prior to the study.
The second study included 215 women with unexplained, male or female factor infertility for at least 24 months bound to undergo IUI with a sonographic diagnosis of endometrial polyps (Pérez‐Medina 2005). 11 women were lost to follow‐up, six in the intervention group and five in the control group. Data from 101 women in the intervention group and 103 women in the control group were available for analysis.The mean participant age was 31 years (range 27 to 35). All women experienced primary subfertility; they all underwent a complete fertility assessment. Unexplained infertility was diagnosed in women with normal ovulatory cycles, semen analysis, HSG and postcoital testing. Female factor infertility was diagnosed in women with ovulatory dysfunction, cervical factor or endometriosis. Male factor infertility was diagnosed if two semen analyses obtained at least one month apart were subnormal according to the WHO criteria. The sonographic diagnosis of endometrial polyps was established by the demonstration of the vascular stalk of the endometrial polyp by colour Doppler in a hyperechogenic formation with regular contours occupying the uterine cavity, surrounded by a small hypoechogenic halo. Women older than 39 years of age or with anovulation or uncorrected tubal disease or previous unsuccessful use of recombinant follicle‐stimulating hormone (FSH), as well as women with a male partner with azoospermia, were excluded from randomisation.
For details of the inclusion and exclusion criteria, see Characteristics of included studies table.
Interventions
One trial treated the intervention group with hysteroscopic surgery to remove the fibroids; TVS was done three months after the procedure (Casini 2006). Women in the intervention group were suggested to abstain from sexual intercourse for three months and then to start having regular fertility‐oriented intercourse. Women in the control group were asked to immediately start having fertility‐oriented intercourse. Both groups were monitored for up to 12 months after study commencement.
The second trial performed all hysteroscopic interventions in an outpatient clinic setting under local anaesthesia by one gynaecologist (Pérez‐Medina 2005). In the intervention group, the endometrial polyps suspected on Doppler US were extracted by means of rigid 1.5 mm scissors and forceps through the working channel of a 5.5 mm continuous flow hysteroscope. All removed polyps were submitted for histopathological examination. If resection was not possible during the outpatient hysteroscopy, the woman was scheduled for operative hysteroscopy under spinal anaesthesia in the operating theatre of the hospital. All the hysteroscopic interventions were done in the follicular phase of the menstrual cycle. The women of the intervention group were scheduled to receive four cycles of IUI, using subcutaneous injections of FSH 50 IU (international units) daily from the third day of the cycle. The first IUI treatment cycle was started three cycles after the operative hysteroscopy. In the control group, the endometrial polyps suspected on Doppler US were left in place during diagnostic hysteroscopy using a 5.5 mm continuous flow hysteroscope; polyp biopsy was performed to establish a histopathological diagnosis. All women in the control group were scheduled to receive four cycles of IUI, using subcutaneous injections of FSH 50 IU daily from the third day of the cycle. The first IUI treatment cycle was scheduled three cycles after the diagnostic hysteroscopy. Four IUI cycles were attempted before finishing the trial.
Outcomes
Neither of the two included studies reported data on the primary outcomes for this review, live birth and hysteroscopy complication rates.
The first trial measured two secondary outcomes, clinical pregnancy and miscarriage rate (Casini 2006). A clinical pregnancy was defined by the visualisation of an embryo with cardiac activity at six to seven weeks of pregnancy. Miscarriage was defined by the loss of an intrauterine pregnancy between the seventh and 12th weeks of gestation.
The second trial reported only one secondary outcome, the clinical pregnancy rate (Pérez‐Medina 2005). This was defined by a pregnancy diagnosed by US visualisation of one or more gestational sacs.
The authors of one study gave a plausible explanation for the failure to report on the live birth rate (Pérez‐Medina 2005). They failed to give an explanation for the lack of data on the other primary outcome, the hysteroscopy complication rate. The study authors of the other trial could not be contacted successfully for further clarification on the absence of reporting the primary outcomes (Casini 2006).
Excluded studies
We excluded 64 studies on hysteroscopic interventions for various reasons.
One randomised trial (Shokeir 2010) was excluded since the main published report was retracted at the request of the editor of the publishing journal as it duplicated parts of a paper on a different topic that had already appeared in another journal published years before (Pérez‐Medina 2005).
One trial was a quasi‐randomised trial (Pabuccu 2008).
Four trials were non‐randomised studies (De Angelis 2010; Gao 2013; Mohammed 2014; Trninić‐Pjević 2011).
-
Fifty‐eight randomised trials did not address the prespecified PICO (Participants, Interventions, Comparisons and Outcomes) research questions of this Cochrane Review.
Fourteen trials studied the effectiveness of hysteroscopy in subfertile women bound to undergo IVF or ICSI treatment with unsuspected or no uterine cavity abnormalities (Abiri 2014; Aghahosseini 2012; Aleyassin 2017; Basma 2013; Demirol 2004; Di Florio 2013; El‐Nashar 2011; Elsetohy 2015; El‐Toukhy 2009; Fatemi 2007; Rama Raju 2006; Shawki 2012; Smit 2016; Sohrabvand 2012).
One trial assessed the effectiveness of hysteroscopy prior to IUI in women with no previously detected uterine anomalies (Moramezi 2012).
Three trials assessed the effectiveness of endometrial injury (El‐Khayat 2015; Hare 2013; Weiss 2005).
Twelve trials had a study population that included women who were not of reproductive age experiencing gynaecological problems other than subfertility (Clark 2015; Hamerlynck 2015; Javidan 2017; Kamel 2014; Lara‐Dominguez 2016; Lieng 2010a; Muzii 2007; Muzii 2017; Nappi 2013; Rubino 2015; Smith 2014; van Dongen 2008).
Two trials had a study population that included only women with repeated miscarriage (Vercellini 1993) or recurrent implantation failure (Cao 2018).
Eighteen trials studied the effectiveness of adjunctive therapies (hyaluronic acid gel, amnion graft, balloon catheter, cyclical hormone replacement therapy alone or intrauterine device alone or both cotreatments combined, autologous platelet rich plasma) for the prevention of intrauterine adhesions following hysteroscopic adhesiolysis (Abu Rafea 2013; Acunzo 2003; Aghajanova 2018; Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Guo 2017; Hanstede 2016; Lin 2014; Liu 2016; Paz 2014; Revel 2011; Roy 2014; Tonguc 2010; Xiao 2015).
Six trials compared different surgical techniques for treating uterine septum in a mixed study population of women with subfertility or recurrent pregnancy loss (Colacurci 2007; Darwish 2009; Parsanezhad 2006; Roy 2015; Roy 2017; Youssef 2013).
One trials assessed the interobserver agreement of gynaecologists performing hysteroscopy for a septate uterus who received instructions on the assessment of the septum prior to the procedure versus gynaecologists who did not (Smit 2015).
One trial was an RCT investigating the effects of mindfulness‐based stress reduction on anxiety, depression and quality of life in women with intrauterine adhesions (Chen 2017).
See: Characteristics of excluded studies table.
Studies awaiting classification
We found no studies awaiting classification.
Ongoing studies
We retrieved two ongoing studies (SEPTUM trial; TRUST trial).
The TRUST trial is a multicentre RCT. The starting date was 1 October 2008. The sample size of this study is 68 women; on 4 May 2018 the 63rd participant was included.
The SEPTUM trial was designed as a pilot multicentre RCT to assess the feasibility for a larger adequately powered trial. The trial is closed to further recruitment due to feasibility issues with recruitment. Six participants were included and will be followed up for 24 months post intervention (personal correspondence with Dr Matthew Prior).
See: Characteristics of ongoing studies table.
Risk of bias in included studies
See the 'Risk of bias' summary for the review authors' judgements about each risk of bias item in the included study (Figure 2).
2.
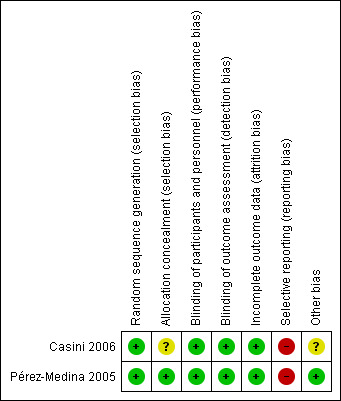
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
See the 'Risk of bias' graph for the review authors' judgements about each risk of bias item presented as percentages across the two included studies (Figure 3).
3.
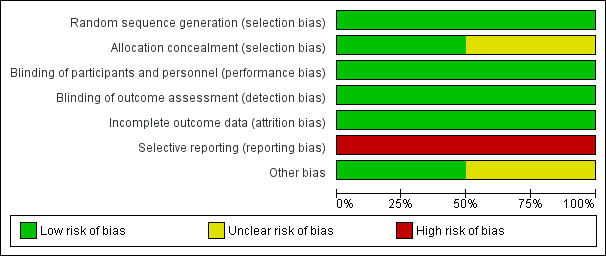
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged both studies at low risk of selection bias related to random sequence generation, as both used computerised random numbers tables (Casini 2006; Pérez‐Medina 2005).
We judged one study at low risk for selection bias related to allocation concealment, as they used sequentially numbered, opaque, sealed envelopes to conceal the random allocation of women to one of the comparison groups (Pérez‐Medina 2005). We judged the second trial at unclear risk for selection bias related to allocation concealment since the method used was not reported and no further clarification by the authors could be obtained (Casini 2006).
Blinding
We judged both studies at low risk of performance and detection bias since both surgical studies had unequivocal outcomes that are most likely not affected by unclear blinding of participants, personnel and outcome assessors (Casini 2006; Pérez‐Medina 2005). The methods were not reported and no further clarification could be obtained.
Incomplete outcome data
We judged both studies at low risk of attrition bias. One study reported outcome data of all randomised women (Casini 2006). The second study analysed the majority of women randomised (95%) (Pérez‐Medina 2005). The missing outcome data in the remaining 5% were balanced in numbers with similar reasons for missing data between the two comparison groups.
Selective reporting
We judged both studies at high risk of reporting bias (Casini 2006; Pérez‐Medina 2005). Both studies failed to include data for the primary outcome live birth, which could reasonably have been reported in studies conducted over a seven‐year (Casini 2006) and a four‐year (Pérez‐Medina 2005) period. Although a plausible explanation was given by the contact author of one study (Pérez‐Medina 2005), we judged that it could have been possible to obtain data on the live birth rates if the study authors had contacted the referring gynaecologists (see Characteristics of included studies table). Moreover, one trial reported no data on adverse outcomes such as miscarriage or hysteroscopy complications (Pérez‐Medina 2005), whereas the second study reported miscarriage rates only for the adverse events (Casini 2006).
Other potential sources of bias
We judged one study to be at unclear risk of other potential sources of bias (Casini 2006). The study did not report the mean ages and duration of infertility in the intervention and control group of women with submucous fibroids; we were unable to obtain these data from the study authors given that we were unsuccessful in contacting them. It is unclear whether this might have caused imbalance in the baseline characteristics between the comparison groups in this randomised trial (Casini 2006). Moreover, it was unclear whether hysteroscopy had been performed in all participants to confirm the position of the ultrasonically detected fibroids.
We judged the second study at low risk of other potential sources of bias since there was no evidence of baseline imbalance in the participant characteristics between the two comparison groups (Pérez‐Medina 2005).
Publication bias could not be formally assessed due to the very limited number of studies included in this Cochrane Review.
Effects of interventions
Summary of findings for the main comparison. Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities.
| Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities | ||||||
|
Patient or population: women with submucous fibroids and otherwise unexplained subfertility Settings: infertility centre in Rome, Italy Intervention: hysteroscopic removal of 1 submucous fibroid ≤ 40 mm Comparison: no surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No surgery | Myomectomy | |||||
| Live birth | No data reported. | |||||
| Adverse events: hysteroscopy complications | No data reported. | |||||
|
Clinical pregnancya Ultrasound 12 months |
214 per 1000 | 400 per 1000 (209 to 627) |
OR 2.44 (0.97 to 6.17) |
94 (1 study) | ⊕⊝⊝⊝ Very lowb,c | — |
|
Adverse events: miscarriaged Ultrasound 12 months |
119 per 1000 | 172 per 1000 (63 to 477) |
OR 1.54 (0.47 to 5.00) |
94 women (1 study) | ⊕⊝⊝⊝ Very lowb,c | — |
| *The basis for the assumed risk is the control group risk of the single included study (Casini 2006). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aClinical pregnancy defined by the visualisation of an embryo with cardiac activity at six to seven weeks' gestational age.
bDowngraded by two levels for very serious risk of bias (unclear allocation concealment, high risk of selective outcome reporting and unclear whether there is other bias caused by imbalance in the baseline characteristics).
cDowngraded by one level for serious imprecision (wide confidence interval of the effect size estimate).
dMiscarriage was defined by the clinical loss of an intrauterine pregnancy between the 7th and 12th weeks of gestation.
Summary of findings 2. Operative hysteroscopy versus control in women undergoing medically assisted reproduction with suspected major uterine cavity abnormalities.
| Operative hysteroscopy versus control in women undergoing medically assisted reproduction with suspected major uterine cavity abnormalities | ||||||
|
Patient or population: subfertile women with endometrial polyps diagnosed by ultrasonography prior to treatment with gonadotropin and intrauterine insemination Settings: infertility unit of a university tertiary hospital in Madrid, Spain Intervention: hysteroscopic polypectomy using a 5.5 mm continuous flow office hysteroscope with a 1.5 mm scissors and forceps Comparison: diagnostic hysteroscopy using a 5.5 mm continuous flow office hysteroscope and polyp biopsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Polypectomy | |||||
| Live birth | No data reported. | |||||
| Adverse events: hysteroscopy complications | No data reported. | |||||
|
Clinical pregnancya Ultrasound 4 intrauterine insemination cycles |
282 per 1000 | 634 per 1000 (451 to 894) |
OR 4.41 (2.45 to 7.96) |
204 (1 study) | ⊕⊕⊝⊝ Lowb,c | — |
| Adverse events: miscarriage | No data were reported for this secondary outcome. | |||||
| *The basis for the assumed risk is the control group risk of the single included study (Pérez‐Medina 2005). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aClinical pregnancy was defined by the presence of at least one gestational sac on ultrasound.
bDowngraded by one level for serious risk of bias (high risk for selective outcome reporting).
cDowngraded by one level for serious imprecision (wide confidence interval of the effect size estimate).
1. Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities
See: Table 1.
Endometrial polyps
The search identified no studies on endometrial polyps.
Submucous fibroids
One study compared hysteroscopic myomectomy versus no surgery in women with unexplained subfertility and submucous fibroids only or combined with intramural fibroids (Casini 2006).
Primary outcomes
Live birth
There were no data for live births.
Adverse events: hysteroscopy complications
There were no data for hysteroscopy complications.
Secondary outcomes
Clinical pregnancy
In women with otherwise unexplained subfertility and submucous fibroids, we were uncertain whether hysteroscopic myomectomy improved the clinical pregnancy rate compared to expectant management (OR 2.44, 95% CI 0.97 to 6.17; P = 0.06, 94 women; Analysis 1.1; Figure 4).
1.1. Analysis.
Comparison 1 Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities, Outcome 1 Clinical pregnancy.
4.
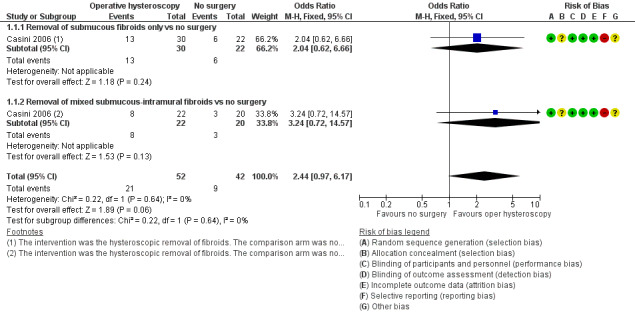
Forest plot of comparison: 1 Hysteroscopic myomectomy vs no surgery in women with unexplained subfertility and submucous fibroids. Outcome: 1.1 Clinical pregnancy per woman randomised.
Adverse events: miscarriage
There was insufficient evidence to determine whether there was a difference between the removal of one submucous fibroid of diameter 40 mm or less in women with otherwise unexplained subfertility for at least one year versus expectant management for the outcome of miscarriage per woman randomised (OR 1.54, 95% CI 0.47 to 5.00; P = 0.47, 94 women; Analysis 1.2; Figure 5). As a secondary analysis, we reported the miscarriage rate per clinical pregnancy. There was insufficient evidence to determine whether there was a difference between the groups (OR 0.58, 95% CI 0.12 to 2.85; P = 0.50, 30 clinical pregnancies in 94 women).
1.2. Analysis.
Comparison 1 Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities, Outcome 2 Adverse events: miscarriage.
5.
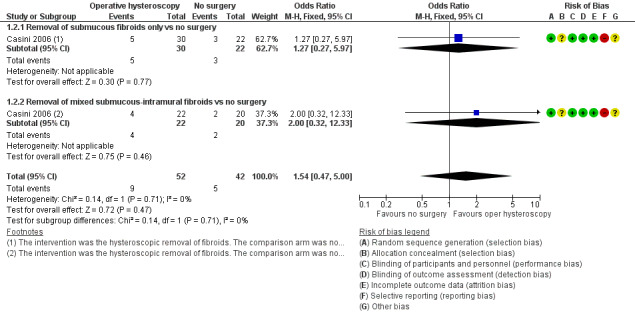
Forest plot of comparison: 1 Hysteroscopic myomectomy vs no surgery in women with unexplained subfertility and submucous fibroids. Outcome: 1.2 Miscarriage per clinical pregnancy.
Subgroup analyses
We performed no subgroup analyses across studies to assess any overestimation of treatment effect or reporting bias, due to the limited number of studies.
One prespecified subgroup analysis within the trial was done for the two secondary outcomes of clinical pregnancy and miscarriage according to whether submucous fibroids only or mixed submucous‐intramural fibroids were considered. There was insufficient evidence to determine whether there was a difference for the secondary outcome clinical pregnancy between the 'submucous only' subgroup (OR 2.04, 95% CI 0.62 to 6.66; P = 0.24, 1 RCT, 52 women) and the 'mixed submucous‐intramural' subgroup (OR 3.24, 95% CI 0.72 to 14.57; P = 0.13, 1 RCT, 42 women); the tests for subgroup differences demonstrated no statistical heterogeneity beyond chance (Chi² = 0.22, degrees of freedom (df) = 1; P = 0.64, I² = 0%). There was insufficient evidence to determine whether there was a difference for the secondary outcome miscarriage between the 'submucous only' subgroup (OR 0.63, 95% CI 0.09 to 4.40; P = 0.64, 1 RCT, 19 clinical pregnancies in 52 women) and the 'mixed submucous‐intramural' subgroup (OR 0.50, 95% CI 0.03 to 7.99; P = 0.62, 1 RCT, 11 clinical pregnancies in 42 women); the tests for subgroup differences demonstrated no statistical heterogeneity beyond chance (Chi² = 0.02, df = 1, P = 0.90, I² = 0%).
Uterine septum
There were no data for uterine septum.
Intrauterine adhesions
There were no data for intrauterine adhesions.
2. Operative hysteroscopy versus control in women undergoing medically assisted reproduction with suspected major uterine cavity abnormalities
See: Table 2.
Endometrial polyps prior to intrauterine insemination
One study compared hysteroscopic removal of polyps versus diagnostic hysteroscopy and polyp biopsy in women with endometrial polyps undergoing gonadotropin treatment and IUI (Pérez‐Medina 2005).
Primary outcomes
Live birth
There were no data for live births.
Adverse events: hysteroscopy complications
There were no data for hysteroscopy complications.
Secondary outcomes
Clinical pregnancy
The hysteroscopic removal of polyps prior to IUI may have improved the clinical pregnancy rate compared to diagnostic hysteroscopy only: if 28% of women achieved a clinical pregnancy without polyp removal, the evidence suggested that 63% of women (95% CI 45% to 89%) would have achieved a clinical pregnancy after the hysteroscopic removal of the endometrial polyps (OR 4.41, 95% CI 2.45 to 7.96; P < 0.00001, 204 women; Analysis 2.1; Figure 6). The number needed to treat for an additional beneficial outcome was 3 (95% CI 2 to 4).
2.1. Analysis.
Comparison 2 Operative hysteroscopy versus control in women undergoing medically assisted reproduction with suspected major uterine cavity abnormalities, Outcome 1 Clinical pregnancy.
6.
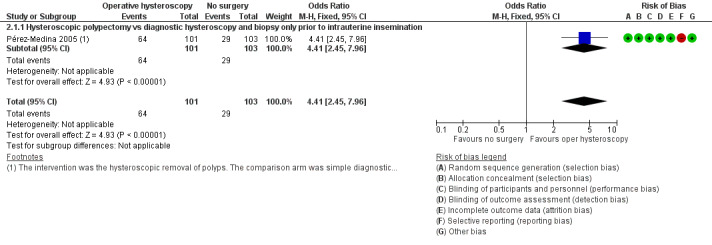
Forest plot of comparison: 2 Hysteroscopic removal of polyps vs diagnostic hysteroscopy and biopsy only prior to intrauterine insemination. Outcome: 2.1 Clinical pregnancy per woman randomised.
Adverse events: miscarriage
There were no data for miscarriage.
Subgroup analyses
We performed no subgroup analyses across studies to assess any overestimation of treatment effect or reporting bias given the limited number of studies.
We performed the following two subgroup analyses within the included study.
A first prespecified subgroup analysis studied the effect of polyp size on the secondary outcome of clinical pregnancy. On histopathological examination, the mean size of the polyps removed was 16 mm (range 3 mm to 24 mm). The primary study examined the effect of polyp size on the clinical pregnancy rate in the intervention group. They analysed the data based on the size of the removed polyps, subdivided into four groups based in their quartiles (less than 5 mm, 5 mm to 10 mm, 11 mm to 20 mm and greater than 20 mm); there was insufficient evidence to determine whether there was a difference between these four subgroups given the limited number of data (P = 0.32) (Table 3). There was no evidence of an effect of polyp size on the outcome of clinical pregnancy, but these results should be interpreted carefully given the limited numbers in only one included study. There were no data on the estimated size of the polyps in the control group.
1. Effect of polyp size on clinical pregnancy rates in the intervention group.
| Polyp size | Clinical pregnancya | Clinical pregnancy rate (95% CI)b |
| < 5 mm | 19/25 | 76% (72% to 80%) |
| 5–10 mm | 18/32 | 56% (53% to 59%) |
| 11–20 mm | 16/26 | 61% (58% to 65%) |
| > 20 mm | 11/18 | 61% (58% to 64%) |
CI: confidence interval.
aClinical pregnancy is defined by a pregnancy diagnosed by ultrasound visualisation of at least one gestational sac per woman randomised.
bNo significant difference was found for the clinical pregnancy rates between the 4 subgroups (P = 0.32).
The second subgroup analysis studied the effect of the timing of the IUI treatment after hysteroscopy on the secondary outcome clinical pregnancy. About 29% of women in the polypectomy group, compared to 3% in the diagnostic hysteroscopy group, became pregnant in the three‐month period after the hysteroscopy before the treatment with gonadotropin and IUI was started; this was calculated from the Kaplan‐Meier survival analysis in the published report of the primary study (Pérez‐Medina 2005). Hysteroscopic polypectomy may have improved the clinical pregnancy rate compared to diagnostic hysteroscopy and polyp biopsy in women waiting to be treated with gonadotropin and IUI (OR 13, 95% CI 3.9 to 46; P < 0.0001, 1 study, 204 women; available‐data analysis). The number needed to treat to for an additional beneficial outcome after hysteroscopic polypectomy while waiting for further treatment with gonadotropin and IUI was 4 (95% CI 3 to 6). In women who started gonadotropin and IUI treatment the pregnancy rates per woman were 49% in the intervention group and 26% in the control group, calculated from data in the published report of the primary study (Pérez‐Medina 2005). Hysteroscopic polypectomy may have improved the clinical pregnancy rate in women who started from three months after the surgical procedure with gonadotropin and IUI treatment (OR 2.7, 95% CI 1.4 to 5.1; P = 0.003, 1 RCT, 172 women; available‐data analysis). The number needed to treat for an additional beneficial outcome when treated with gonadotropin and IUI after a prior hysteroscopic polypectomy was 4 (95% CI 3 to 12). This was a post hoc subgroup analysis. Quoting from the primary study published report "A second important conclusion in our study is that pregnancies after polypectomy are frequently obtained spontaneously while waiting for the treatment, suggesting a strong cause–effect of the polyp in the implantation process. This led us to defer the first IUI to three menstrual cycles after the polypectomy is performed. Longer series are needed to verify these results". Data from this subgroup analysis should be treated with caution as subgroup analysis by itself is observational in nature, and statistical interpretation of its results is not without problems.
Sensitivity analyses
A sensitivity analysis comparing an ITT analysis assuming that clinical pregnancies would not have occurred in participants with missing data, rather than an available‐data analysis, did not affect the statistical significance of the main analysis for the secondary outcome clinical pregnancy (OR 4.0, 95% CI 2.3 to 7.2; P < 0.00001; 1 RCT, 215 women randomised). No other imputation strategies for dealing with the missing data were assumed given the limited number of studies.
Endometrial polyps prior to in vitro fertilisation or intracytoplasmic sperm injection
We found no studies on endometrial polyps prior to IVF or ICSI.
Submucous fibroids prior to intrauterine insemination, in vitro fertilisation or intracytoplasmic sperm injection
We found no studies on submucous fibroids prior to IUI, IVF or ICSI.
Uterine septum prior to intrauterine insemination, in vitro fertilisation or intracytoplasmic sperm injection
We found no studies on uterine septum prior to IUI, IVF or ICSI.
Intrauterine adhesions prior to intrauterine insemination, in vitro fertilisation or intracytoplasmic sperm injection
We found no studies on intrauterine adhesions prior to IUI, IVF or ICSI.
Other analyses
We planned to do sensitivity analyses for Analysis 1.1; Analysis 1.2; and Analysis 2.1 to consider whether conclusions would have differed if eligibility had been restricted to studies at low risk of bias in all domains. We retrieved only one study for each random comparison. Moreover for both studies there was at least one item at high risk of bias (Figure 2). Hence no sensitivity analyses were performed in the present updated Cochrane Review.
Discussion
Summary of main results
This systematic review aimed to investigate whether the hysteroscopic treatment of suspected major uterine cavity abnormalities made a difference to the main outcomes of live birth or pregnancy and the adverse events (hysteroscopy complications and miscarriage) in subfertile women with otherwise unexplained subfertility or before IUI, IVF or ICSI. We searched for studies on two randomised comparisons to study the effectiveness of operative hysteroscopy in the treatment of subfertility associated with major uterine cavity abnormalities.
The first randomised comparison was operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities – stratified into endometrial polyps, submucous fibroids, intrauterine adhesions or septate uterus – diagnosed by US, SIS/GIS, HSG, diagnostic hysteroscopy or any combination of these methods (Table 1). We retrieved and critically appraised only one trial in this category comparing the hysteroscopic removal of one submucous fibroid with a diameter of 40 mm or less in women aged 35 years or less with otherwise unexplained subfertility versus expectant management (no surgery followed by fertility‐oriented intercourse) (Casini 2006). There was insufficient evidence to determine whether there was a difference between groups. This may have been due to a type II error: we calculated that a sample size of 91 participants was needed to detect a difference of 19% for the outcome of clinical pregnancy between groups with a statistical power of 80% at a confidence level of 95% (α = 0.05 and β = 0.20). In other words, a study population of at least 182 participants is needed to detect any statistically significant difference if present; compared to only 94 women in the study (Casini 2006). There was insufficient evidence to determine whether there was a difference between groups for the miscarriage rate. We found no data on live birth or hysteroscopy complication rates. We found no studies in women with endometrial polyps, intrauterine adhesions or septate uterus for this randomised comparison.
The second randomised comparison was operative hysteroscopy versus control in women undergoing MAR – stratified into IUI, IVF or ICSI – with suspected major uterine cavity abnormalities – stratified into endometrial polyps, submucous fibroids, intrauterine adhesions or septate uterus – diagnosed by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods (Table 2). We retrieved and critically appraised one trial in this category randomly comparing hysteroscopic polypectomy versus diagnostic hysteroscopy comparison in subfertile women with suspected endometrial polyps bound to undergo IUI (Pérez‐Medina 2005). The hysteroscopic removal of polyps with a mean size of 16 mm (range 3 mm to 24 mm) may have improved clinical pregnancy rates compared to simple diagnostic hysteroscopy. A sensitivity analysis on the choice to use an ITT analysis by making the imputation that clinical pregnancies would not have occurred in participants with missing data rather than an available‐data analysis did not demonstrate an impact on the overall results. We found no data for live birth, hysteroscopy complication or miscarriage rates. The increase in clinical pregnancy rates following hysteroscopic polypectomy was due to a higher proportion of spontaneous conceptions before starting IUI and a higher chance of conceiving after starting gonadotropin treatment and IUI. The results of this post hoc subgroup analysis should be interpreted with caution. We found no studies in women with submucous fibroids, intrauterine adhesions or septate uterus prior to IUI or in women with all types of suspected uterine cavity abnormalities prior to IVF or ICSI.
Due to the lack of studies, there was no formal assessment of publication bias.
Overall completeness and applicability of evidence
Evidence on the effectiveness of treating suspected major uterine cavity abnormalities by operative hysteroscopy compared to a control intervention in women with otherwise unexplained subfertility was insufficient. We found no trials on the hysteroscopic treatment of endometrial polyps, intrauterine adhesions or uterine septum compared to a control intervention in women with otherwise unexplained subfertility. The only included study in this category failed to report on the primary outcomes for this review. Evidence on the effectiveness of operative hysteroscopy compared to control in subfertile women with associated major uterine cavity abnormalities prior to MAR was incomplete since data had been found only for subfertile women with suspected endometrial polyps prior to IUI. The search found no data on the effectiveness of operative hysteroscopy versus control in subfertile women with other suspected major cavity abnormalities such as submucous fibroids, intrauterine adhesions or uterine septum prior to IUI or other techniques such as IVF or ICSI for all outcomes. Moreover, for the randomised comparison of hysteroscopic polypectomy versus diagnostic hysteroscopy prior to IUI, there were no data available for the primary outcomes. The evidence retrieved was by consequence insufficient to address all the objectives of the present Cochrane Review.
The uncertainty of whether there were differences between the comparison groups in the trial of hysteroscopic myomectomy in women with submucous fibroids and otherwise unexplained subfertility did not exclude a clinically relevant benefit with the hysteroscopic removal of fibroids. It is generally accepted that submucous fibroids are very likely to interfere with normal fertility (Pritts 2001; Pritts 2009). In everyday practice, most skilled hysteroscopic surgeons will counsel women with submucous fibroids associated with otherwise unexplained subfertility or bound to be treated with IUI, IVF or ICSI to have the submucous fibroids removed before further expectant management or MAR; besides offering participation in a pragmatic RCT on this topic there seems no other sound clinical alternative.
Although the results of the trial on hysteroscopic polypectomy are relevant for everyday practice (Pérez‐Medina 2005), one‐third of the randomised women treated by IUI had an ovulatory disorder other than anovulation. In everyday clinical practice, ovulatory disorder is by itself not an indication for IUI as opposed to male factor (Bensdorp 2007) and unexplained subfertility (Veltman‐Verhulst 2012). We have considered doing a sensitivity analysis to determine if the inclusion and exclusion of women with ovulatory disorders could have influenced the magnitude of the treatment effect but failed to obtain the data from the study authors.
Quality of the evidence
See Table 4; Table 5; Table 1; Table 2.
2. GRADE evidence profile – unexplained subfertility and submucous fibroids.
|
Quality assessment Submucous fibroids and unexplained subfertility | ||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations |
| Clinical pregnancy (follow‐up 1 year; ultrasounda) | ||||||
| 1 | RCT | Very seriousb | Not serious | Not indirectness | Seriousc | None |
| Miscarriage (follow‐up 1 year; ultrasoundd) | ||||||
| 1 | RCT | Very seriousb | Not serious | Not serious | Seriousc | None |
aA clinical pregnancy was defined by the visualisation of an embryo with cardiac activity at six to seven weeks' gestational age.
bUnclear allocation concealment and high risk of selective outcome reporting.
cWide confidence intervals.
dMiscarriage was defined by the clinical loss of an intrauterine pregnancy between the 7th and 12th weeks of gestation.
3. GRADE evidence profile – endometrial polyps prior to intrauterine insemination.
|
Quality assessment Endometrial polyps prior to gonadotropin and IUI treatment | ||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations |
| Clinical pregnancy (follow‐up 4 IUI cycles; ultrasounda) | ||||||
| 1 | RCT | Seriousb | Not serious | Not serious | Not serious | None |
IUI: intrauterine insemination; RCT: randomised controlled trial.
aClinical pregnancy was defined by the presence of at least one gestational sac on ultrasound.
bThere was high risk for selective outcome reporting bias.
The present review included only two trials; neither reported the primary outcomes live birth or hysteroscopy complications.
We graded the evidence using the GRADE tool (GRADEpro GDT) of the trial on hysteroscopic myomectomy as very low (Casini 2006). It was a small study with few events. We downgraded by two levels for very serious risk of bias: there was uncertainty about allocation concealment, it was unclear whether there was imbalance in the baseline characteristics of the study groups and there was a high risk of selective outcome reporting. Moreover, we downgraded by one level for serious imprecision given the wide CIs of the point estimate of the treatment effect.
We graded the evidence of the trial on hysteroscopic polypectomy as low (Pérez‐Medina 2005): we downgraded by one level for serious risk of bias related to a high risk of selective outcome reporting (see Assessment of risk of bias in included studies). We downgraded by one level for serious imprecision given the wide CIs of the point estimate of the treatment effect.
Potential biases in the review process
There is an earlier published version of this review (Bosteels 2010). Given our prior knowledge of potentially eligible studies for this clinical research topic, there might have been some potential for detection bias. We carried out a comprehensive literature search using a search strategy that was more extensive than the one used in the earlier published systematic review. This enabled us to identify a far greater number of randomised studies on hysteroscopic surgery in subfertile women, many of which did not address the particular research questions prespecified in the protocol (see Characteristics of excluded studies table).
Agreements and disagreements with other studies or reviews
According to one systematic review and critical appraisal, robust and high‐quality RCTs are needed before hysteroscopy can be regarded as a first‐line procedure in all infertile women, especially during the basal clinical assessment of the couple, when assisted reproductive treatment is not indicated yet (Di Spiezio Sardo 2016).
The findings and conclusions on the evidence for the removal of fibroids by hysteroscopy are in accordance with the findings of one Cochrane Review (Metwally 2012). The authors concluded that there was currently no definitive evidence from RCTs regarding the effect of hysteroscopic myomectomy on fertility outcomes. In contrast, according to the findings and conclusions of two systematic reviews of observational studies on fibroids and subfertility, fertility outcomes are decreased in women with submucosal fibroids, and removal is likely to benefit the reproductive outcome (Pritts 2001; Pritts 2009).
One Cochrane Review concluded that additional well‐designed RCTs on the effectiveness of hysteroscopic polypectomy for improving reproductive outcome in subfertile women are needed, which is in accordance with our findings (Jayaprakasan 2014). One systematic review of mainly very‐low quality observational studies concluded that polypectomy appeared to have a favourable outcome in infertile women (Lieng 2010b). Nevertheless, the body of evidence supporting the removal of endometrial polyps is limited, and future research evaluating the outcome of this common procedure is required.
One Cochrane Review concluded that there was no evidence to support hysteroscopic septum resection in women of reproductive age with a septate uterus to improve reproductive outcomes, although this procedure is often performed worldwide for this indication (Rikken 2017). RCTs are urgently needed (Rikken 2016): two studies are ongoing (SEPTUM trial; TRUST trial).
Authors' conclusions
Implications for practice.
In women with otherwise unexplained subfertility and submucous fibroids, we are uncertain whether clinical pregnancy rates improved after hysteroscopic myomectomy compared to expectant management because there was only one small single‐centre study of very low quality.
Hysteroscopic polypectomy may improve the chance of conceiving compared to diagnostic hysteroscopy only in subfertile women with a sonographic diagnosis of endometrial polyps prior to intrauterine insemination (IUI) for unexplained, male or female factor infertility for at least 24 months. The quality of the single‐centre study retrieved was low.
Implications for research.
The evidence retrieved from only two randomised studies was insufficient to address all the objectives of the present Cochrane Review.
More well‐designed randomised controlled trials are needed to assess whether the hysteroscopic removal of endometrial polyps, submucous fibroids, septa or intrauterine adhesions is likely to benefit women with otherwise unexplained subfertility associated with these suspected uterine pathologies compared to a control intervention. Equally, more clinical research is needed on the effectiveness of treating endometrial polyps, submucous fibroids, septa or intrauterine adhesions in subfertile women bound to undergo IUI, in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
There are knowledge gaps concerning the effects of the number, size or extent and the localisation of the major uterine cavity abnormalities on the main outcomes in women with otherwise unexplained subfertility or prior to medically assisted reproduction.
Well‐designed randomised studies are needed to assess the relationship between the timing of the hysteroscopic intervention and subsequent IUI, IVF or ICSI treatment.
Future randomised studies should report on primary outcomes such as live birth and adverse events such as miscarriage and hysteroscopic complications.
What's new
| Date | Event | Description |
|---|---|---|
| 20 June 2018 | New citation required but conclusions have not changed | The conclusions of this review have not changed |
| 17 June 2018 | New search has been performed | Updated literature search from 1 January 2014 until 16 April 2018. No new studies included. |
History
Protocol first published: Issue 11, 2011 Review first published: Issue 1, 2013
| Date | Event | Description |
|---|---|---|
| 29 October 2014 | New search has been performed | This review has been updated but no new studies were eligible for inclusion. |
| 29 October 2014 | New citation required but conclusions have not changed | There was no change to our conclusions. |
| 29 August 2014 | Feedback has been incorporated | Feedback on clinical diversity in this review, received from Professor Hossam Shawki. |
Acknowledgements
Cochrane Gynaecology and Fertility (CGF) Group: we wish to thank Prof Cindy Farquhar, CGF Co‐ordinating Editor; Ms Jane Clarke, former CGF Managing Editor; Ms Helen Nagels, CGF Managing Editor and Ms Jane Marjoribanks, CGF Assistant Managing Editor for their advice and support. We have used the Cochrane Consumers and Communication Group (CCCG) supplementary author advice for describing the results of the present updated Cochrane Review (Ryan 2016). We thank Ms Marian Showell, CGF Information Specialist for assistance in searching the CGF Specialised Register and the handsearch.
Biomedical Library Gasthuisberg, Catholic University, Leuven, Belgium. Many thanks to Mr Jens De Groot for skilful assistance in developing the literature search strategy.
Prof Tirso Pérez‐Medina, head of the department of Gynaecology at the University Hospital Puerta de Hierro, Madrid, Spain, has answered all the queries concerning the randomised controlled trial on the effectiveness of hysteroscopic polypectomy prior to IUI.
The Board of the European Society of Gynaecological Endoscopy (ESGE). Prof Hans Brolmann (Past ESGE President) and Dr Rudi Campo (Former ESGE Secretary) have been very helpful in contacting a group of experts in hysteroscopy in the field of Reproductive Medicine. Dr Rudi Campo (ZOL Genk, Belgium), Dr Dick Schoot (Catharina Hospital, Eindhoven, the Netherlands), Prof Attilio Di Spiezio Sardo (University of Naples 'Frederico II', Naples, Italy), Prof Hervé Fernandez (Hôpital Bicêtre, Le Kremin‐Bicêtre, France), Prof Kristine Juul Hare ( Gynækologisk‐Obstetrisk afdeling, Hvidovre Hospital, Hvidovre, Denmark) and Dr Matthew Prior (Newcastle Fertility Centre, Newcastle, UK) have provided data on published or ongoing randomised trials relevant to the research questions.
Dr Ben Cohlen (Fertility Centre Isala, Zwolle, the Netherlands), Prof Willem Ombelet (ZOL, Genk, Belgium) and Prof Carl Spiessens (Leuven University Fertility Centre, Leuven, Belgium) have provided useful data on the clinical pregnancy rates after gonadotropin stimulation and IUI. Dr Mariette Goddijn (AMC Amsterdam, the Netherlands) has given valuable feedback on the risk of bias assessment for one of the included trials at the occasion of the oral opposition and defence of the PhD thesis of the first author.
Ms Elizabeth Bosselaers (Managing Secretary CEBAM, Cochrane Belgium) has assisted in improving the plain language summary.
We acknowledge comments sent by Prof Hossam Eldin Shawki Abdalla MD of the Obstetrics & Gynecology Department, Faculty of Medicine, El‐Minia University, Egypt. Our formal response was published in October 2014 and the points made were taken into account in this update.
The authors of the 2018 update thank Dr Jenneke Kasius for her contributions to previous versions of this review.
Appendices
Appendix 1. The Cochrane Gynaecology and Fertility Group (CGFG) specialised register search strategy
Searched 16 April 2018
PROCITE Platform
Keywords CONTAINS "hysteroscopic "or "hysteroscopy" or "hysteroscope" or "endoscopy" or Title CONTAINS "hysteroscopic "or "hysteroscopy" or "hysteroscope" or "endoscopy" AND Keywords CONTAINS "subfertility" or "subfertility‐Female" or "infertility" or "IVF" or "ICSI" or "IUI" or "in vitro fertilisation" or "in vitro fertilization" or "Intrauterine Insemination" or "artificial insemination" or "assisted conception" or "assisted reproduction techniques" or "myoma" or "myomas" or "myomectomy" or "septate uterus" or "polypectomy" or "polyp removal" or "polyps" or "adhesiolysis" or "adhesion" or "adhesions" or "synechiotomy" or "Leiomyoma" or "leiomyomata" or "fibroids" or "Asherman's Syndrome" or "uterine septa" or "uterine septum" or "uterine disease" or "uterine leiomyomas" or "uterine malformation" or "Uterine Neoplasms" or "uterine polyps" or "metroplasty" or "intrauterine adhesions" or "fibroid" or "fibroids" or Title CONTAINS "subfertility" or "assisted reproduction techniques" or "myoma" or "myomas" or "myomectomy" or "septate uterus" or "polypectomy" or "metroplasty" or "intrauterine adhesions" or "fibroid" or "fibroids"
272 records
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 16 April 2018
Web Platform
#1 MESH DESCRIPTOR Hysteroscopy EXPLODE ALL TREES 349
#2 Hysteroscop*:TI,AB,KY 902
#3 endoscop*:TI,AB,KY 17242
#4 #1 OR #2 OR #3 18070
#5 MESH DESCRIPTOR Infertility, Female EXPLODE ALL TREES 1127
#6 (subfertil* or infertil*):TI,AB,KY 5049
#7 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2919
#8 (IVF or ICSI):TI,AB,KY 4175
#9 (artificial insemination):TI,AB,KY 182
#10 (assisted conception):TI,AB,KY 86
#11 (in vitro fertili*):TI,AB,KY 2233
#12 (intracytoplasmic sperm):TI,AB,KY 1373
#13 (intrauterine insemination):TI,AB,KY 734
#14 MESH DESCRIPTOR Leiomyoma EXPLODE ALL TREES 449
#15 MESH DESCRIPTOR myoma EXPLODE ALL TREES 21
#16 (myoma* or myomectom*):TI,AB,KY 946
#17 Leiomyoma*:TI,AB,KY 631
#18 (sept* uter*):TI,AB,KY 19
#19 (?Uter* adj4 polyp*):TI,AB,KY 38
#20 (Endometri* adj4 polyp*):TI,AB,KY 172
#21 (adhesiolysis or septoplast* or metroplast*):TI,AB,KY 381
#22 MESH DESCRIPTOR Tissue Adhesions EXPLODE ALL TREES 342
#23 (remov* adj2 adhesion*):TI,AB,KY or (?intrauter* adj2 adhesion*):TI,AB,KY 62
#24 synech*:TI,AB,KY 270
#25 (uter* adj2 malform*):TI,AB,KY 38
#26 (Uter* adj4 Neoplasm*):TI,AB,KY 2161
#27 (uter* adj4 abnormalit*):TI,AB,KY 99
#28 fibroid*:TI,AB,KY 557
#29 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 12761
#30 #4 AND #29 623
Appendix 3. MEDLINE search strategy
Searched from 1946 to 16 April 2018
OVID Platform
1 exp Hysteroscopy/ (4330) 2 Hysteroscop$.tw. (6123) 3 Uteroscop$.tw. (13) 4 endoscop$.tw. (179897) 5 Endoscopy/ (47569) 6 or/1‐5 (201815) 7 exp Infertility/ (60956) 8 subfertil$.tw. (4522) 9 Infertilit$.tw. (44291) 10 exp Sperm Injections, Intracytoplasmic/ or exp Reproductive Techniques/ or exp Reproductive Techniques, Assisted/ or exp Fertilization in Vitro/ (137421) 11 (in vitro fertili$ or intracytoplasmic sperm).tw. (25251) 12 (IVF or ICSI).tw. (24282) 13 IUI.tw. (1544) 14 artificial insemination.tw. (5969) 15 (intrauter* adj2 inseminat*).tw. (2287) 16 assisted conception.tw. (1110) 17 assisted reproduct*.tw. (12595) 18 exp Myoma/ or exp Leiomyoma/ (21709) 19 (myoma or myomectomy).tw. (5800) 20 septoplast*.tw. (1522) 21 Asherman* syndrome.tw. (251) 22 metroplast*.tw. (363) 23 (sept* adj2 resect*).tw. (382) 24 septate uterus.tw. (392) 25 polypectomy.tw. (4158) 26 (remov$ adj2 polyp*).tw. (1326) 27 adhesiolysis.tw. (1346) 28 exp Tissue Adhesions/ (11844) 29 (?intrauter* adj2 adhesion$).tw. (445) 30 (remov$ adj4 adhesion$).tw. (414) 31 polyp$.tw. (254390) 32 uterine septa.tw. (92) 33 uterine septum.tw. (231) 34 synech*.tw. (9278) 35 Leiomyoma$.tw. (12730) 36 (uter$ adj2 malform$).tw. (775) 37 (Uter$ adj4 Neoplasm$).tw. (842) 38 (uter$ adj4 abnormalit$).tw. (1035) 39 fibroid$.tw. (5706) 40 or/7‐39 (512038) 41 randomized controlled trial.pt. (458772) 42 controlled clinical trial.pt. (92329) 43 randomized.ab. (408806) 44 placebo.tw. (193271) 45 clinical trials as topic.sh. (183298) 46 randomly.ab. (288659) 47 trial.ti. (180948) 48 (crossover or cross‐over or cross over).tw. (76033) 49 or/41‐48 (1172279) 50 exp animals/ not humans.sh. (4446637) 51 49 not 50 (1078473)
Appendix 4. Embase search strategy
Searched from 1980 to 16 April 2018
OVID Platform
1 exp hysteroscopy/ (10442) 2 Hysteroscop$.tw. (10344) 3 endoscop$.tw. (272107) 4 exp endoscopy/ or exp urogenital endoscopy/ (538246) 5 or/1‐4 (654400) 6 exp female infertility/ or exp infertility therapy/ (117458) 7 subfertil$.tw. (6209) 8 Infertil$.tw. (73959) 9 artificial insemination/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ or exp intrauterine insemination/ (71950) 10 (in vitro fertili$ or intracytoplasmic sperm).tw. (32834) 11 (IVF or ICSI or IUI).tw. (43015) 12 artificial insemination.tw. (5647) 13 (intrauter* adj2 inseminat*).tw. (3417) 14 assisted conception.tw. (1601) 15 assisted reproduct*.tw. (19314) 16 or/6‐15 (181531) 17 exp uterus myoma/ or exp myoma/ (34252) 18 exp leiomyoma/ (17459) 19 (myoma or myomectomy).tw. (8654) 20 septate uterus.tw. (626) 21 polypectomy.tw. (7240) 22 adhesiolysis.tw. (2445) 23 (remov$ adj2 adhesion$).tw. (253) 24 (?intrauter* adj2 adhesion$).tw. (679) 25 (septoplast* or metroplast*).tw. (2357) 26 (sept* adj2 resect*).tw. (560) 27 Asherman* syndrome.tw. (411) 28 polyp$.tw. (286769) 29 uterine septa.tw. (154) 30 uterine septum.tw. (469) 31 synech$.tw. (9682) 32 Leiomyoma$.tw. (15738) 33 (uter$ adj4 malform$).tw. (1402) 34 (Uter$ adj4 Neoplasm$).tw. (926) 35 (uter$ adj4 abnormalit$).tw. (1420) 36 fibroid$.tw. (9428) 37 or/17‐36 (342555) 38 5 and 16 and 37 (2475) 39 Clinical Trial/ (964209) 40 Randomized Controlled Trial/ (495134) 41 exp randomisation/ (77876) 42 Single Blind Procedure/ (31024) 43 Double Blind Procedure/ (145965) 44 Crossover Procedure/ (54970) 45 Placebo/ (309585) 46 Randomi?ed controlled trial$.tw. (179133) 47 Rct.tw. (28028) 48 random allocation.tw. (1767) 49 randomly allocated.tw. (29351) 50 allocated randomly.tw. (2307) 51 (allocated adj2 random).tw. (794) 52 Single blind$.tw. (20651) 53 Double blind$.tw. (181005) 54 ((treble or triple) adj blind$).tw. (765) 55 placebo$.tw. (266160) 56 prospective study/ (441083) 57 or/39‐56 (1888652) 58 case study/ (53662) 59 case report.tw. (350881) 60 abstract report/ or letter/ (1032536) 61 or/58‐60 (1428530)
Appendix 5. CINAHL search strategy
Searched from 1961 to 16 April 2018
EBSCO Platform
S43 S30 AND S42 184 S42 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 1,224,457 S41 TX allocat* random* 8,624 S40 (MH "Quantitative Studies") 19,276 S39 (MH "Placebos") 10,763 S38 TX placebo* 50,564 S37 TX random* allocat* 8,624 S36 (MH "Random Assignment") 46,918 S35 TX randomi* control* trial* 146,875 S34 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) 950,353 S33 TX clinic* n1 trial* 223,176 S32 PT Clinical trial 86,215 S31 (MH "Clinical Trials+") 238,828 S30 S6 AND S29 1,027 S29 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 24,606 S28 TX fibroid* 1,459 S27 TX (uter* N2 abnormalit*) 644 S26 TX (uter* N2 neoplasm*) 2,615 S25 TX (uter* malform*) 642 S24 TX Leiomyoma 3,066 S23 TX (uter* N4 polyp*)or TX (endometri* N4 poly*) 371 S22 TX(remov* N2 adhesion*) 28 S21 (MM "Adhesions") 637 S20 TX adhesiolysis 212 S19 TX (sept* uter*) 727 S18 TX (myoma or myomectomy) 1,107 S17 (MM "Leiomyoma") 2,182 S16 (MM "Myoma+") 184 S15 TX (assisted conception) 827 S14 TX (artificial insemination) 538 S13 (MM "Insemination, Artificial") 277 S12 TX (IVF or ICSI) 2,283 S11 TX (Reproducti* Technique*) 5,749 S10 MH reproduction techniques 3,361 S9 (MM "Fertilization in Vitro") 1,923 S8 TX subfertil* or TX infertil* 10,820 S7 (MM "Infertility") 4,677 S6 S1 OR S2 OR S3 OR S4 OR S5 56,419 S5 (MM "Endoscopy") 8,846 S4 TX endoscop* 55,025 S3 TX Uteroscop* 1 S2 TX Hysteroscop* 1,501 S1 (MM "Hysteroscopy") 659
Appendix 6. Web of Science search strategy
Searched 16 April 2018
Web Platform
TOPIC: (((((Hysteroscopy OR Uterine Endoscop* OR Uteroscop* OR Hysteroscopic Surg* OR (hysteroscopic AND (polypectom* OR myomectom* OR synechiolysis OR adhesiolysis OR metroplast* OR septoplast* OR septum resection*))) AND (female AND (Subfertility OR Infertility OR Sterility)) AND ((Endometri* AND (polyp OR polyps)) OR Leiomyoma* OR Fibromyoma* OR Fibroid* OR fibromas OR Myoma* OR Synechiae OR ((Intrauterine OR uterine) AND adhesion*) OR (Asherman* AND Syndrome*) OR ((septa OR septum) AND (uterine OR intrauterine)) OR uterine diseases OR uterine neoplasms OR ((uterine OR intrauterine) AND (congenital abnormalities)) OR (Fertilization SAME "in Vitro") OR IVF OR ICSI OR reproductive techniques OR embryo transfer Or zygote intrafallopian transfer OR ((intrauterine OR artificial) AND insemination)))))) DocType=All document types; Language=All languages Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=2014‐2018
Appendix 7. Items of data extraction
1. Source
Study ID
Report ID
Review author ID
Citation and contact details
2. Eligibility
Confirm eligibility for review
Reason for exclusion
3. Trial characteristics
-
Study design
Random sequence generation
Participant recruitment
Participant inclusion and exclusion criteria
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessors
Completeness of outcome data
Selective outcome reporting
Other potential sources of bias
-
Follow‐up
Duration of follow‐up
Type of follow‐up
-
Size of study
Number of women recruited
Number of women randomised
Number of women excluded
Number of women withdrawn and lost to follow‐up
Number of women analysed
-
Study setting
Single‐centre or multicentre
Location
Timing and duration
-
Diagnostic criteria
Screening by transvaginal ultrasound (TVS)
Screening by hysterosalpingography (HSG)
Screening by TVS and HSG
Screening by other ultrasound diagnostic procedures, e.g. saline infusion sonography or gel instillation sonography
Screening by hysteroscopy
Diagnosis confirmed by hysteroscopy and biopsy
4. Characteristics of the study participants
-
Baseline characteristics
Age
Primary or secondary subfertility
Duration of subfertility
Diagnostic work‐up: baseline follicle stimulating hormone, semen analysis, diagnosis of tubal pathology, confirmatory test of ovulation
Other contributory causes to subfertility than uterine factor
Previous treatments: in vitro fertilisation (IVF), intrauterine insemination (IUI) or other treatments
-
2. Treatment characteristics
IUI natural cycle
IUI controlled ovarian stimulation with antioestrogens or gonadotropins
IVF protocol and number of embryos transferred
Intracytoplasmic sperm injection protocol and number of embryos transferred
Detailed description of the hysteroscopic procedure
5. Interventions
Total number of intervention groups
Absence of other interventions in the treatment and control group
For each intervention and comparison group of interest
Specific intervention
Intervention details
Timing of the intervention
6. Outcomes
Outcomes and time points collected
Outcomes and time points reported
Definition and unit of measurement for each of the following outcomes:
Primary outcomes
Live birth delivery rate
Hysteroscopy complication rate
Secondary outcomes
Ongoing pregnancy rate
Clinical pregnancy with foetal heartbeat
Clinical pregnancy rate
Miscarriage rate
For each outcome of interest:
Sample size
Missing participants
Summary data for each intervention group in 2 × 2 table
Estimate of effect with 95% confidence interval
Subgroup analyses
7. Miscellaneous
Funding source
Key conclusions of the study authors
Miscellaneous comments from the study authors
References to other relevant studies
Correspondence required
Miscellaneous comments by the review authors
Data and analyses
Comparison 1. Operative hysteroscopy versus control in women with otherwise unexplained subfertility and suspected major uterine cavity abnormalities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 1 | 94 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.97, 6.17] |
| 1.1 Removal of submucous fibroids only vs no surgery | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.62, 6.66] |
| 1.2 Removal of mixed submucous‐intramural fibroids vs no surgery | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.72, 14.57] |
| 2 Adverse events: miscarriage | 1 | 94 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.47, 5.00] |
| 2.1 Removal of submucous fibroids only vs no surgery | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.27, 5.97] |
| 2.2 Removal of mixed submucous‐intramural fibroids vs no surgery | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.32, 12.33] |
Comparison 2. Operative hysteroscopy versus control in women undergoing medically assisted reproduction with suspected major uterine cavity abnormalities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 1 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.41 [2.45, 7.96] |
| 1.1 Hysteroscopic polypectomy vs diagnostic hysteroscopy and biopsy only prior to intrauterine insemination | 1 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.41 [2.45, 7.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Casini 2006.
| Methods | Parallel‐group, randomised, controlled, single‐centre trial Power calculation not reported Approved by the hospital's ethics committee No source of funding or conflict of interest reported |
|
| Participants | Country: Italy Setting: AGUNCO Obstetrics and Gynecology Centre, Rome Population: women referred to the centre from January 1998 to April 2005 for fertility problems were examined for inclusion. All women underwent routine examinations including the study of ovarian function (FSH, luteinising hormone, oestradiol and progesterone concentrations); prolactin, free triiodothyronine, free thyroxine and thyroid‐stimulating hormone concentrations; postcoital test; TVUS; HSG and analysis of the partner's semen. The TVUS was performed to diagnose the presence of uterine fibroids. After these examinations, all women found to be affected by uterine fibroids excluding all other causes of infertility were asked to participate in the study. Type of subfertility: infertility for ≥ 1 year (range: 1 to 5 years); no further clarification on primary versus secondary subfertility Mean age: women with submucous fibroids alone: 31.4 ± 2.5 years; women with mixed submucous‐intramural fibroids: 32.2 ± 2.5 years Number recruited: 193 women Number participants: 181 women Number participants with submucous fibroids only: 52 women Number participants with mixed submucous‐intramural fibroids: 42 women Inclusion criteria: aged ≤ 35 years; infertility for ≥ 1 year; presence of 1 knot or fibroid of diameter ≤ 40 mm (or both) and absence of other causes of infertility at the performed examinations Exclusion criteria: presence of ≥ 2 knots or fibroids of diameter > 40 mm (or both); body weight > 20% of normal weight; and use of medication containing oestrogens, progestins or androgens within 8 weeks prior to study Duration of study: 86 months; conducted January 1998 to April 2005 |
|
| Interventions |
Participants were examined by TVUS 3 months after surgery. Participants who underwent surgery were suggested to abstain from having sexual intercourse for 3 months and then to start having regular fertility‐oriented intercourse. Participants who did not undergo surgery were asked to immediately start having regular fertility‐oriented intercourse (intercourse during the 6‐day fertile interval ending on the day of ovulation). Participants were monitored for up to 12 months after study commencement. |
|
| Outcomes | Clinical pregnancy defined by the visualisation of an embryo with cardiac activity at 6–7 weeks of pregnancy Miscarriage classified as clinical loss of an intrauterine pregnancy between the 7th and 12th weeks of gestation |
|
| Notes | Authors stated that the differences in pregnancy rates between the comparison groups were statistically significant for the women with submucous fibroids (P < 0.05), which is in contrast with the calculation of the results in Review Manager 5. The definition of knot was unclear: it could not be clarified since we were unable to contact the study authors. Unclear whether a hysteroscopy was done in all women to confirm the exact position of the ultrasonically detected fibroids. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subsequently, women of each group were randomized into two subgroups, according to a randomisation table." Comment: low risk of selection bias related to random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated: no further clarification obtained from the study authors. Comment: unclear risk of selection bias related to allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Method not stated: no further clarification obtained from the study authors. Comment: not applicable as this is a surgical study with unequivocal outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Method not stated: no further clarification obtained from the study authors. Comment: not applicable as this is a surgical study with unequivocal outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One hundred and ninety‐three patients were diagnosed as affected by uterine fibroid excluding all other causes of infertility and met the requirements of the inclusion and exclusion criteria. Of these, 181 decided to participate in the study. Among the 181 patients, 52 had submucosal fibroids (SM group) while 45 had intramural fibroids (IM group), 11 had subserosal fibroids (SS group), 42 had a mix of submucosal–intramural (SM‐IM group) and 31 patients had a mix of intramural–subserosal fibroids (IM‐SS group)." Quote: "Out of 181 women, 68 become pregnant." Comment: low risk for attrition bias. |
| Selective reporting (reporting bias) | High risk | All specified outcomes reported in the results section. Nevertheless, the published report failed to include results for the live birth rate, which was the primary outcome of interest that would be expected to have been reported for a trial on fertility treatment conducted over a 7‐year period. |
| Other bias | Unclear risk | The mean ages and duration of infertility in the intervention and control group of women with submucous fibroids were not reported. No further clarification by the authors was obtained. It was unclear whether there might have been imbalance in the baseline characteristics between the comparison groups. Failure to do a hysteroscopy in all women to confirm the position of the ultrasonically detected fibroids could have caused information bias. |
Pérez‐Medina 2005.
| Methods | Parallel‐group, randomised, controlled, single‐centre trial Power analysis performed. To detect an expected difference in pregnancy rate between the intervention and control group of 15% at a level of 0.05 with a power of 80%, a sample size of 200 women (i.e. 100 women per group) was required. From 2800 women attending the centre, 452 women fulfilling the inclusion criteria were selected; 215 women were randomised (107 women in the intervention group and 108 women in the control group). Data on outcomes of 204 women were available for analysis (101 in the intervention group and 103 in the control group). This study had therefore adequate statistical power to detect a difference between the comparison groups if really present. Approved by the hospital's ethics committee. No source of funding or conflict of interest reported. |
|
| Participants | Country: Spain Setting: infertility unit of an university tertiary hospital in the Spanish capital Madrid Population: women with unexplained, male or female factor infertility for ≥ 24 months bound to undergo IUI with a sonographic diagnosis of endometrial polyps Unexplained infertility was diagnosed in women with normal ovulatory cycles, semen analysis, HSG and postcoital testing. Male factor infertility was diagnosed if 2 semen analyses obtained ≥ 1 month apart were subnormal according to the WHO criteria. Female factor infertility was diagnosed in women with ovulatory dysfunction, cervical factor or endometriosis. Type of subfertility: primary subfertility (correspondence with study authors) Mean age: treatment group: 30.8 years (range 26.7 to 34.9); control group: 30.9 years (range 26.5 to 35.3) Number recruited: 452 women Number randomised: 215 women Inclusion criteria: women with ≥ 24 months of subfertility with a sonographic diagnosis of endometrial polyps bound to undergo IUI for unexplained, male or female factor infertility Exclusion criteria: women aged > 39 years, anovulation, azoospermia, uncorrected tubal disease or previous unsuccessful use of recombinant FSH Duration of the study: 50 months; conducted January 2000 to February 2004 |
|
| Interventions | 1 surgeon (the first author of the study) performed all hysteroscopic procedures by intention in an outpatient clinic setting under local anaesthesia
Duration: women were scheduled to receive 4 cycles of IUI with subcutaneous injection of recombinant FSH 50 IU daily from the 3rd day, and the first IUI was planned for 3 cycles after hysteroscopy in both groups. 4 IUI cycles were attempted before finishing the trial. |
|
| Outcomes | Primary: quote: "We studied the crude pregnancy rate in both groups" Comment: clinical pregnancy; crude pregnancy was defined by the study authors as follows: "the presence of a gestational sac on ultrasound" (correspondence with the study authors) Secondary: time‐to‐pregnancy and influence of the size of the endometrial polyps on the pregnancy rate |
|
| Notes | All study data were obtained in personal communication from the study authors. 1. Quote: "Patients underwent a complete infertility evaluation that included TVUS in the early proliferative phase, basal body temperature recording to assess ovulation, postcoital test (PCT), HSG, semen analysis and, in some patients, diagnostic laparoscopy." Comment: according to correspondence with the first author, the aim of the laparoscopy was exclusively diagnostic in the evaluation of cases of unexplained infertility of unknown origin. If tubal pathology was detected by laparoscopy, the participant was excluded from randomisation. The numbers of women undergoing a laparoscopy were balanced between the 2 comparison groups. 2. This study performed IUI for various indications: male factor (21%), cervical factor (11%), endometriosis (11%), or unexplained subfertility (49%) and ovulation disorder (33%). Anovulation was reported in the methods section as an exclusion criterion. The study authors defined ovulation disorder as follows: quote: "A combination of irregular menstrual cycles with multicystic ovaries on TVUS and basal gonadotrophin measurements within the normal range" (correspondence with the first study author). Comment: in everyday clinical practice ovulation disorder is not an indication for IUI by itself. 3. Data on the number or the localisation of the polyps could not be retrieved since the first author no longer works in the university hospital. 4. Data on the size of the polyps in the control group could not be obtained for similar reasons as note 3. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised to one of the two groups with use of an opaque envelope technique, with assignment determined by a computerized random number table." Quote: "Subjects were randomised into one of two groups in a 1:1 ratio using a restricted randomisation." Comment: probably done, but using simple randomisation, with an equal allocation ratio, by referring to a table of random numbers generated by a computer. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised to one of the two groups with use of an opaque envelope technique, with assignment determined by a computerized random number table." Comment: sequentially numbered, opaque, sealed envelopes were used according to correspondence with the first author; probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Method not stated: no further clarification obtained from the study authors. Comment: not applicable as this is a surgical study with unequivocal outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Method not stated: no further clarification obtained from the study authors. Comment: not applicable as this is a surgical study with unequivocal outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "11 patients were lost from the study, 6 in the study group (3 lost to follow‐up, 2 pathologic reports of submucosal myoma and 1 in whom the polyp was not confirmed) and 5 in the control group (1 lost to follow‐up, 2 in whom the polyp was not confirmed and 2 pathologic reports of myoma)." Comment: missing outcome data were balanced in numbers across the comparison groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | High risk | All specified outcomes reported in the results section. The final study report nevertheless failed to include results for the live birth rate, which is the primary outcome of interest expected for a trial on fertility treatment conducted over a 4‐year period. Data on the outcomes live birth and miscarriage were not available since most the majority of randomised women were referred by gynaecologists from outside the tertiary university hospital and were referred back when pregnant for further follow‐up by the referring gynaecologist. No clarification could be obtained for the lack of data on hysteroscopic complications. |
| Other bias | Low risk | No evidence for imbalance in the baseline characteristics. |
CI: confidence interval; FSH: follicle‐stimulating hormone; HSG: hysterosalpingography; IU: international units; IUI: intrauterine insemination; TVUS: transvaginal ultrasound; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abiri 2014 | Not addressing the research questions described in the protocol. RCT in women with no suspected major uterine cavity abnormalities undergoing a first IVF treatment cycle. |
| Abu Rafea 2013 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing intrauterine balloon stenting vs no stenting following hysteroscopic treatment for septate uterus. |
| Acunzo 2003 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial studying the efficacy of hyaluronic acid gel in preventing the development of intrauterine adhesions following hysteroscopic adhesiolysis. Mixed population of women with intrauterine adhesions, presenting with subfertility or other gynaecological complaints. Primary outcome: adhesion scores. |
| Aghahosseini 2012 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing hysteroscopy prior to a subsequent IVF attempt vs immediate IVF without prior hysteroscopy conducted in women with ≥ 2 failed IVF cycles with unsuspected or no uterine cavity abnormalities. Main outcomes: biochemical pregnancy, clinical pregnancy and delivery rates. |
| Aghajanova 2018 | Not addressing the research questions described in the protocol. Randomised controlled open pilot clinical trial studying the efficacy of the intrauterine infusion of autologous platelet rich plasma vs saline infusion after operative hysteroscopy for the management of moderate‐to‐severe Asherman's syndrome. |
| Aleyassin 2017 | Not addressing the research questions described in the protocol. RCT studying the effectiveness of routine hysteroscopy compared to no prior hysteroscopy in women with normal transvaginal ultrasound and HSG before the first ICSI treatment cycle. |
| Amer 2010 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial in subfertile women comparing the application of amnion graft, either fresh or dried to an intrauterine balloon vs the application of an intrauterine balloon without amnion graft as an adjunctive procedure after the hysteroscopic lysis of severe intrauterine adhesions, diagnosed at clinic hysteroscopy in women with infertility with or without menstrual disorders as the primary symptom. Outcomes: improvement in adhesion grade, improvement in menstruation, increased uterine length at sounding, complications and reproductive outcome. |
| Basma 2013 | Not addressing the research questions described in the protocol. Women with detectable uterine pathology by ultrasound were excluded from participating in the trial aiming to study the effectiveness of hysteroscopy before a first trial ICSI. |
| Cao 2018 | Not addressing the research questions described in the protocol. Systematic review including clinical trials of hysteroscopy with or without endometrial biopsy in women with recurrent implantation failure and no suspected intrauterine lesions. |
| Chen 2017 | Not addressing the research questions described in the protocol. RCT investigating the effects of mindfulness‐based stress reduction on anxiety, depression and quality of life in women with intrauterine adhesions. |
| Clark 2015 | Not addressing the research questions described in the protocol. The target population included women with abnormal uterine bleeding and hysteroscopically diagnosed uterine polyps. |
| Colacurci 2007 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing 2 different surgical techniques for metroplasty: operative hysteroscopy using the resectoscope with a unipolar knife vs the Versapoint device. Mixed population of women with septate uterus and a history of recurrent miscarriage or primary subfertility. Outcomes: operative parameters, complications, need for a second intervention and reproductive outcome parameters. |
| Darwish 2009 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing extended sectioning by resectoscopy vs sequential cold knife excision for treating a complete utero‐cervicovaginal septum in a mixed population of women with infertility or pregnancy loss. Main outcomes: operating time, perioperative bleeding, complications, reproductive outcome, and participant and husband satisfaction. |
| De Angelis 2010 | Study on the effectiveness of hysteroscopic metroplasty for small septate uterus in women with repeated IVF implantation failure. Although denoted by the authors as the first prospective randomised controlled study on this subject, the trial did not use a valid random sequence generation. Quote: "These patients, once informed about the situation, were randomly allocated, depending on their personal decision ..." |
| De Iaco 2003 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing the application of hyaluronan derivative gel (Hyalobarrier gel) after hysteroscopic surgery vs surgical treatment alone in women aged 18–65 years, with gynaecological conditions other than subfertility. Primary outcome: adhesion score at second‐look hysteroscopy. |
| Demirol 2004 | Not addressing the research questions described in the protocol. Parallel‐group randomised comparison between clinic hysteroscopy prior to a subsequent IVF attempt or immediate IVF without prior clinic hysteroscopy conducted in women with ≥ 2 failed IVF cycles with unsuspected or no uterine cavity abnormalities. Outcomes: number of oocytes retrieved, fertilisation rate, number of embryos transferred, first trimester miscarriage and clinical pregnancy rates. |
| Di Florio 2013 | Not addressing the research questions described in the protocol. RCT comparing the new 16 Fr mini‐resectoscope with the traditional 22 Fr resectoscope and Bettocchi 15 Fr hysteroscope for the treatment of uterine cavitary lesions. |
| Di Spiezio Sardo 2011 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing the use of Intercoat absorbable adhesion barrier gel vs no adhesion barrier after hysteroscopic synechiolysis in a mixed population of women with infertility or other gynaecological conditions. Primary outcomes: incidence of de novo intrauterine adhesions, adhesion scores and patency of the internal uterine ostium. |
| El‐Khayat 2015 | Not addressing the research questions described in the protocol. RCT evaluating the role of endometrial injury in the cycle preceding ovarian stimulation for IUI cycle on the clinical pregnancy rate. |
| El‐Nashar 2011 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing diagnostic hysteroscopy with directed biopsy or hysteroscopic treatment of unsuspected uterine cavity abnormalities (or both) vs no hysteroscopy in women with primary infertility treated with ICSI. Primary outcome: clinical pregnancy. |
| El‐Toukhy 2009 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing hysteroscopy vs no hysteroscopy in women with recurrent implantation failure with IVF. Status: completed. |
| Elsetohy 2015 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial aimed at assessing the role of using clinic hysteroscopy as a routine investigation in improving ICSI pregnancy rates in 2 groups of infertile women with no abnormality detected on transvaginal ultrasonographic examination. |
| Fatemi 2007 | Not addressing the PICO research question of this Cochrane Review. |
| Fuchs 2014 | Not addressing the PICO research question of this Cochrane review. RCT evaluating the safety and effectiveness of Oxiplex/AP gel (Intercoat) in reducing intrauterine adhesion formation after hysteroscopic treatment because of retained products of conception. |
| Gan 2017 | Not addressing the PICO research question of this Cochrane Review. RCT studying the efficacy of freeze‐dried amnion graft for prevention of intrauterine adhesion reformation after hysteroscopic adhesiolysis. |
| Gao 2013 | Observational non‐randomised study on the effectiveness of hysteroscopy in women with repeated implantation failure. |
| Guida 2004 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing hysteroscopic surgery for the removal of polyps, fibroids or septa followed by the application of auto‐cross linked hyaluronic acid gel vs hysteroscopic surgery without the adhesion barrier in a mixed population of women with subfertility and other gynaecological symptoms associated with endometrial polyps, submucous fibroids or septa. Main outcomes: rates of adhesion formation and adhesion scores. |
| Guo 2017 | Not addressing the research questions described in the protocol. Prospective, randomised, controlled trial to evaluate the efficacy of different doses of oestrogen treatment (2 mg and 6 mg daily) after hysteroscopic adhesiolysis in women with moderate‐to‐severe adhesion according to the American Fertility Society classification of intrauterine adhesions. |
| Hamerlynck 2015 | Not addressing the research questions described in the protocol. Multicentre, open‐label, randomised, controlled trial comparing hysteroscopic morcellation with bipolar resectoscopy for removal of endometrial polyps, in terms of procedure time, peri‐ and postoperative adverse events, tissue availability and short‐term effectiveness. |
| Hanstede 2016 | Not addressing the research questions described in the protocol. Single‐blind RCT assessing whether exogenous hormone administration starting immediately after a successful hysteroscopic adhesiolysis, in women with Asherman's syndrome may reduce the incidence of spontaneous recurrence of adhesions more than the endogenic production of hormones. |
| Hare 2013 | Not addressing the research questions described in the protocol. Randomised controlled study examining the effect of scratching in a normal visually and histologically endometrium. Women with uterine pathology were excluded before or during clinic hysteroscopy. |
| Javidan 2017 | Not addressing the research questions described in the protocol. Single‐blind RCT to assess the outcomes of surgery in a group of women who were randomly submitted to preoperative gonadotropin‐releasing hormone agonists in comparison with women who received no medication. |
| Kamel 2014 | Not addressing the research questions described in the protocol. Randomised controlled study to compare mechanical (cold scissor) vs electrosurgical metroplasty (bipolar twizzle) in terms of feasibility and pain scoring during ambulatory‐based hysteroscopic metroplasty for short, narrow‐based uterine septa. |
| Lara‐Dominguez 2016 | Not addressing the research questions described in the protocol. RCT comparing the resection of endometrial polyps with 2 different devices: the Versapoint bipolar electrode and the diode laser. |
| Lieng 2010a | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing transcervical resection by hysteroscopy of endometrial polyps suspected on TVUS and SIS vs observation for 6 months. The study population included premenopausal women with bleeding problems associated with endometrial polyps. The aim of the trial was to study the clinical effectiveness of transcervical resection of endometrial polyps for the outcome periodic blood loss. Women wishing to become pregnant were excluded from the trial. Primary outcome: periodic blood loss measured by the Pictorial Blood Assessment Chart. |
| Lin 2014 | Not addressing the research questions described in the protocol. Randomised trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation following hysteroscopic adhesiolysis. |
| Liu 2016 | Not addressing the research questions described in the protocol. RCT randomly comparing oestradiol valerate (Progynova) 3 mg or 9 mg per diet before surgery or no hormonal treatment before transcervical resection of adhesions. |
| Mohammed 2014 | Comparative non‐randomised study on the value of hysteroscopy prior to IVF/ICSI. |
| Moramezi 2012 | Not addressing the research questions described in the protocol. RCT studying the effectiveness of hysteroscopy before IUI on reproductive outcome in infertile women with no suspected intrauterine lesions during baseline fertility assessment. |
| Muzii 2007 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial in women aged 18–75 years comparing operative hysteroscopy using the monopolar resectoscope vs hysteroscopic bipolar electrode excision for the treatment of endometrial polyps. Outcomes: operating times, difficulty of the operation, surgeon satisfaction with the procedure, complications, postoperative pain and participant satisfaction. |
| Muzii 2017 | Not addressing the research questions described in the protocol. Multicentric randomised trial in 180 women affected by endometrial hyperplasia, myomas or endometrial polyps undergoing operative hysteroscopy comparing cefazolin 2 g intravenously 30 minutes prior to the procedure vs no antibiotic treatment. |
| Nappi 2013 | Not addressing the research questions described in the protocol. Double‐blind, randomised, placebo‐controlled study to assess the incidence of infectious complications and the protective effect of antibiotic administration during operative hysteroscopic procedures in a clinic setting. 1046 consecutively enrolled women with intrauterine lesions were randomly allocated to the intervention group (523 participants administered cefazolin 1 g intramuscularly) and the control group (523 participants administered with 10 mL of isotonic sodium chloride solution), and treated in clinic setting by operative hysteroscopy for endometrial polypectomy, uterine septa, submucosal myomas and intrauterine adhesions. |
| Pabuccu 2008 | Quasi‐randomised trial comparing early second‐look clinic hysteroscopic adhesiolysis after hysteroscopic adhesiolysis and IUD insertion vs no early second‐look operative hysteroscopy in subfertile women with intrauterine adhesions. The method of sequence generation was based on alternation: women were allocated to the intervention or control groups based on their study entry. Main outcomes: pregnancy and live birth rate. |
| Parsanezhad 2006 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial in a mixed study population of women with a history of pregnancy wastage or infertility and an associated complete uterine septum comparing metroplasty with complete section of the cervical septum vs metroplasty with preservation of the cervical septum. Outcomes: operating time, distending media deficit, total distending media used, intraoperative bleeding, complications and reproductive outcome. |
| Paz 2014 | Not addressing the research questions described in the protocol. Randomised, double‐blind, controlled trial to evaluate the efficacy and safety of Intercoat (Oxiplex/AP Gel) in preventing intrauterine adhesions after operative hysteroscopy. |
| Rama Raju 2006 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial conducted in women with ≥ 2 failed IVF cycles with unsuspected or no uterine cavity abnormalities comparing clinic hysteroscopy prior to a subsequent IVF attempt or immediate IVF without prior hysteroscopy. Outcomes: number of oocytes retrieved, fertilisation rate, number of embryos transferred and clinical pregnancy rates. |
| Revel 2011 | Not addressing the research questions described in the protocol. Parallel‐group RCT assessing the safety of hyaluronic acid gel to prevent intrauterine adhesions in hysteroscopic surgery |
| Roy 2014 | Not addressing the research questions described in the protocol. RCT to evaluate the efficacy of oestrogen in preventing intrauterine adhesions following hysteroscopic septal resection and to investigate its effect on reproductive outcome. |
| Roy 2015 | Not addressing the research questions described in the protocol. RCT to compare the operation and reproductive outcome of hysteroscopic septal resection using unipolar resectoscope vs bipolar resectoscope. |
| Roy 2017 | Not addressing the research questions described in the protocol. Randomised, prospective, parallel, comparative, single‐blinded study comparing the operative and reproductive outcome of hysteroscopic myomectomy using unipolar resectoscope vs bipolar resectoscope in women with infertility and menorrhagia. |
| Rubino 2015 | Not addressing the research questions described in the protocol. Randomised, prospective, comparative setting clinical trial to examine efficacy of hysteroscopic removal of polyps and myomas on health‐related quality of life and symptom severity at 1‐year postprocedure in a clinic vs ambulatory surgical centre. |
| Shawki 2012 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial conducted to determine the incidence of unsuspected uterine cavity abnormalities detected by clinic hysteroscopy in women before ICSI treatment compared to ICSI without prior hysteroscopy. Main outcomes: incidence of unsuspected uterine abnormalities, and implantation and clinical pregnancy rates. |
| Shokeir 2010 | Published report describing a parallel‐group randomised trial comparing hysteroscopic myomectomy vs diagnostic hysteroscopy and biopsy in women with otherwise unexplained primary infertility and submucous fibroids. Primary outcome: clinical pregnancy rates. Quote from Fertility and Sterility searched on 16 January 2012: "This article has been retracted at the request of the editor as it duplicates parts of a paper that had already appeared in Hum. Reprod., 20 (2005) 1632–1635, DOI:10.1093/humrep/deh822." |
| Smit 2015 | Not addressing the research questions described in the protocol. RCT assessing the interobserver agreement among gynaecologists who were randomised into 2 groups: 1 group received diagnostic criteria for a septate uterus before assessment of videos, whereas the other group assessed the recordings without instruction. |
| Smit 2016 | Not addressing the research questions described in the protocol. Pragmatic, multicentre, RCT in women with a normal TVUS of the uterine cavity and no previous hysteroscopy who were scheduled for their first IVF treatment randomly comparing treatment with hysteroscopy of detected intracavitary abnormalities before starting IVF vs immediate start of the IVF treatment. |
| Smith 2014 | Not addressing the research questions described in the protocol. Multicentre, single‐blind, randomised, controlled trial to evaluate whether hysteroscopic morcellation or bipolar electrosurgical resection was more favourable for removing endometrial polyps in a clinic setting in terms of feasibility, speed, pain and acceptability. |
| Sohrabvand 2012 | Not addressing the research questions described in the protocol. Parallel‐group RCT to assess the effectiveness of diagnostic hysteroscopy in women prior to IVF/IICSI. The study population included women with a normal hysterosonography and normal vaginal ultrasound during the past 12 months. |
| Tonguc 2010 | Not addressing the research questions described in the protocol. Parallel‐group randomised study comparing hysteroscopic lysis of intrauterine adhesions with or without adjunctive therapy (cyclical hormone replacement therapy alone or intrauterine device alone or both cotreatments combined) after hysteroscopic metroplasty in a mixed population of women with subfertility or recurrent miscarriage (or both). Main outcomes: incidence of de novo adhesion formation and ongoing pregnancy rate. |
| Trninić‐Pjević 2011 | Clinical controlled trial on the effectiveness of hysteroscopy prior to IVF; no random sequence generation. |
| van Dongen 2008 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing the hysteroscopic removal of polyps or fibroids by conventional hysteroscopy using a resectoscope vs hysteroscopic morcellation in a mixed population of women with infertility or other gynaecological conditions. Outcomes: mean number of insertions into the uterine cavity and mean operating time. |
| Vercellini 1993 | Not addressing the research questions described in the protocol. Parallel‐group randomised comparing metroplasty using the resectoscope vs microscissors for treating uterine septum in women with repeated miscarriage. Outcomes: mean operating time, mean amount of distension medium used and complications. |
| Weiss 2005 | Not addressing the research questions described in the protocol. Parallel‐group RCT to determine whether performing curettage the month prior to embryo transfer increases the chance of embryo implantation. |
| Xiao 2015 | Not addressing the research questions described in the protocol. Prospective, randomised, double‐blind, controlled clinical trial assessing the efficacy and safety of auto‐crosslinked hyaluronic acid gel for preventing intrauterine adhesions after hysteroscopic adhesiolysis. |
| Youssef 2013 | Not addressing the research questions described in the protocol. Parallel‐group randomised trial comparing 2 different surgical techniques for metroplasty: resectoscopy with monopolar knife vs small‐diameter hysteroscopy fitted with a 5 Fr reusable bipolar electrode. Outcomes: pregnancy, miscarriage and live birth rates. |
HSG: hysterosalpingography; ICSI: intracytoplasmic sperm injection; IUD: intrauterine device; IUI: intrauterine insemination; IVF: in vitro fertilisation; PICO: Participants, Interventions, Comparisons and Outcomes; RCT: randomised controlled trial; SIS: saline infusion sonography; TVUS: transvaginal ultrasound.
Characteristics of ongoing studies [ordered by study ID]
SEPTUM trial.
| Trial name or title | Assessment of hysteroscopic metroplasty in women with a uterine septum and a history of miscarriage: a randomised controlled trial – SEPTUM. |
| Methods | Pilot multicentre randomised controlled trial to assess feasibility for a larger adequately powered trial. |
| Participants | Women with septate uteri, history of miscarriage or preterm birth, or infertility |
| Interventions | Intervention: hysteroscopic septal resection Comparator: no intervention |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 9 December 2014 |
| Contact information | Dr Matthew Prior Subspecialty Registrar in Reproductive Medicine and Surgery Newcastle Fertility Centre email: matt@matthewprior.com mobile: +44 7817 627 712 |
| Notes | Quote: "The trial is ongoing but closed to recruitment due to feasibility issues with recruitment. Six participants were recruited and will be followed up for 24 months post intervention." |
TRUST trial.
| Trial name or title | TRUST – The Randomised Uterine Septum Transsection trial |
| Methods | Multicentre, parallel‐group, randomised controlled trial. No masking/blinding |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention: hysteroscopic metroplasty Comparator: no surgical resection |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 1 October 2008 |
| Contact information | Dr JFW Rikken |
| Notes | 4 May 2018: inclusion of 63rd participant; targeted sample size = 68. Trial website: www.studies‐obsgyn.nl/trust/ |
Differences between protocol and review
At the 2014 update:
As a result of further peer review, the objectives of the review have been rephrased. The descriptions in the Types of interventions and Data synthesis sections were modified accordingly. For both comparisons, we made a stratification according to the types of uterine pathology; for the second comparison, we made a clear distinction between IUI, IVF or ICSI.
'Summary of findings' tables using the GRADE approach were added.
In the Assessment of risk of bias in included studies section of the review, the items 'blinding of participants and personnel' and 'blinding of outcome assessors' were reinserted as requested by the editorial reviewers. We assessed all six items including blinding of participants, personnel and outcome assessors in the final review as opposed to the protocol.
In the Assessment of heterogeneity section of the review, we added the Q‐statistic.
In the Subgroup analysis and investigation of heterogeneity section of the review, we planned to conduct a further subgroup analysis based on the women's age.
At the 2018 update:
We added a fourth exclusion criterion in the section Types of participants: women of reproductive age with no major uterine cavity abnormalities detected by US, SIS, GIS, HSG, diagnostic hysteroscopy or any combination of these methods. The objective of this review was to assess the effectiveness of operative hysteroscopy in women with suspected uterine cavity abnormalities. The effectiveness of hysteroscopy in women without suspected uterine cavity abnormalities is the objective of a second Cochrane review. The addition of this fourth exclusion criterion is to emphasise the difference between the reviews since they include different study populations.
Contributions of authors
JB: co‐ordinated the writing of the protocol and review and its update.
SVW and MB: assisted in the literature search and study selection.
FB and TD: independently assessed the retrieved published reports for inclusion of potentially eligible studies.
JB and SW: independently extracted study data.
FB and BWM: gave advice on review methodology and content and critically appraised the Cochrane Review.
Sources of support
Internal sources
-
CEBAM, Belgium.
Research grant was obtained through CEBAM, the Centre for Evidence‐based Medicine, Belgian Branch of Cochrane
External sources
No sources of support supplied
Declarations of interest
FB (principal investigator) and BWM (co‐investigator) were involved in the design and conduct of the 'inSIGHT trial' (SIGnificance of Routine Hysteroscopy Prior to a First 'in Vitro Fertilization' Treatment Cycle: NCT 01242852), which was financially supported by ZonMw, a Dutch government operated consortium responsible for granting funds in the field of clinical practice research.
Thomas D'Hooghe is paid full time by Merck KGaA, Darmstadt, Germany as Vice‐President and Head of Global Medical Affairs Fertility since October 1st 2015. He is also part time employed by KU Leuven (University of Leuven, Belgium) as Professor in Reproductive Medicine and by Yale University as Adjunct Professor Obstetrics and Gynecology. His co‐authorship on the current paper is part of his academic activity, and Merck KGaA does not have marketed products related to hysteroscopy. Professor D'Hooghe's employment by Merck is not in breach of Cochrane's Commercial Sponsorship Policy (clause 2) as he does not have a real or potential financial interest in the outcome of this review. This matter was referred to Cochrane's Funding Arbiter for advice.
FB has received monetary compensation for the following: member of the external advisory board for Merck Serono and Ferring, the Netherlands; educational activities for Ferring BV, the Netherlands; consultancy work for Gedeon Richter, Belgium; strategic co‐operation with Roche on automated anti‐Müllerian hormone (AMH) assay development; and research co‐operation with Ansh Labs.
BWM has received consultancy from ObsEva Geneva, Guerbet, and Merck; payment for review preparation from European Journal of Obstetrics and Gynecology and Reproductive Biology; and travel/accommodation/meeting expenses for various non‐commercial scientific meetings.
JB, SW, SVW and MB do not have conflicts of interest for the research presented in this updated Cochrane Review.
The first published version of the present Cochrane Review has been part of a PhD thesis entitled "Studies on the effectiveness of endoscopic surgery in reproductive medicine" (dare.uva.nl/record/497164), which has been successfully defended at the faculty of Medicine of the University of Amsterdam, the Netherlands on 2 September 2014 by the first author (JB).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Casini 2006 {published data only}
- Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecological Endocrinology 2006;22(2):106‐9. [DOI: 10.1080/09513590600604673; PMID: 16603437] [DOI] [PubMed] [Google Scholar]
Pérez‐Medina 2005 {published data only}
- Pérez‐Medina T, Bajo‐Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Human Reproduction 2005;20(6):1632‐5. [DOI: 10.1093/humrep/deh822; PMID: 5760959] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abiri 2014 {unpublished data only}
- Abiri A. The effect of hysteroscopy on successful pregnancy in IVF in the infertile women who are candidate for the first IVF cycle, 2014. en.irct.ir/trial/15689?revision=15689 Date first received: 1 August 2014. [IRCT: IRCT2014052616912N4]
Abu Rafea 2013 {published data only}
- Abu Rafea BF, Vilos GA, Oraif AM, Power SG, Cains JH, Vilos AG. Fertility and pregnancy outcomes following resectoscopic septum division with and without intrauterine balloon stenting: a randomized pilot study. Annals of Saudi Medicine 2013;33(1):34‐9. [DOI: 10.5144/0256-4947.2013.34] [DOI] [PMC free article] [PubMed] [Google Scholar]
Acunzo 2003 {published data only}
- Acunzo G, Guida M, Pellicano M, Tommaselli GA, Spiezio Sardo A, Bifulco G, et al. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective, randomized, controlled study. Human Reproduction 2003;18(9):1918‐21. [DOI: 10.1093/humrep/deg368; PMID: 12923149] [DOI] [PubMed] [Google Scholar]
Aghahosseini 2012 {published data only}
- Aghahosseini M, Ebrahimi N, Mahdavi A, Aleyasin A, Safdarian L, Sina S. Hysteroscopy prior to assisted reproductive technique in women with recurrent implantation failure improves pregnancy likelihood. Fertility and Sterility 2012;98(3 Suppl):S4, O‐13. [DOI: 10.1016/j.fertnstert.2012.07.015] [DOI] [Google Scholar]
Aghajanova 2018 {published data only}
- Aghajanova L, Letourneau J, Cedars MI, Huddleston HG. Autologous platelet rich plasma as a novel treatment for Asherman syndrome: results of a pilot randomized clinical trial. Fertility and Sterility 2018;109(3):e13. [Accession number: 621569300] [Google Scholar]
Aleyassin 2017 {published data only}
- Aleyassin A, Abiri A, Agha Hosseini M, Sarvi F. The value of routine hysteroscopy before the first intracytoplasmic sperm injection treatment cycle. Gynecologic and Obstetric Investigation 2017;82(2):125‐30. [DOI: 10.1159/000445801; PUBMED: 27160848] [DOI] [PubMed] [Google Scholar]
Amer 2010 {published data only}
- Amer MI, Abd‐El‐Maeboud KHI, Abdelfatah I, Salama FA, Abdallah AS. Human amnion as a temporary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. Journal of Minimally Invasive Gynecology 2010;17(5):605‐11. [DOI: 10.1016/j.jmig.2010.03.019; PMID: 20576472 ] [DOI] [PubMed] [Google Scholar]
Basma 2013 {unpublished data only}
- Basma E, Elmaghraby H. Role of hysteroscopy before first trial ICSI: a prospective randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201402000691997 Date of registration: 29 October 2013. [PACTR: PACTR201402000691997]
Cao 2018 {published data only}
- Cao HY, You D, Yuan M, Xi M. Hysteroscopy after repeated implantation failure of assisted reproductive technology: a meta‐analysis. Journal of Obstetrics and Gynaecology Research 2018;44(3):365‐73. [DOI: 10.1111/jog.13571; ISSN: 1341‐8076] [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen Y, Yang H, Liu L, Fang R. Effects of mindfulness‐based stress reduction on the anxiety, depression and quality of life of patients with intrauterine adhesion: a randomized controlled trial. International Journal of Clinical and Experimental Medicine 2017;10(2):2296‐305. [IJCEM: 0037301; ISSN: 1940‐5901] [Google Scholar]
Clark 2015 {unpublished data only}
- Clark TJ. A randomised controlled trial of Outpatient Polyp Treatment for abnormal uterine bleeding (OPT). www.isrctn.com/ISRCTN65868569 Date first registered: 11 October 2007. [ISRCTN: ISRCTN65868569]
- Clark TJ, Middleton LJ, Cooper NA, Diwakar L, Denny E, Smith P, et al. A randomised controlled trial of Outpatient versus inpatient Polyp Treatment (OPT) for abnormal uterine bleeding. Health Technology Assessment 2015;19(61):1‐194. [DOI: ; PUBMED: 26240949] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colacurci 2007 {published data only}
- Colacurci N, Franciscis P, Mollo A, Litta P, Perino A, Cobellis L, et al. Small‐diameter hysteroscopy with Versapoint versus resectoscopy with a unipolar knife for the treatment of septate uterus: a prospective randomized study. Journal of Minimally Invasive Gynecology 2007;14:622‐7. [DOI: 10.1016/j.jmig.2007.04.010; PMID: 17848325] [DOI] [PubMed] [Google Scholar]
Darwish 2009 {published data only}
- Darwish AM. Extended resectoscopic versus sequentional cold knife‐resectoscopic excision of the unclassified complete uterocervicovaginal septum: a randomized trial. Fertility and Sterility 2008;90(Suppl):S446. [DOI: 10.1016/j.fertnstert.2008.07.968; ISSN: 0015‐0282] [DOI] [PubMed] [Google Scholar]
- Darwish AM, Elsaman AM. Extended resectoscopic versus sequential cold knife‐resectoscopic excision of the unclassified complete uterocervicovaginal septum: a randomized trial. Fertility and Sterility 2009;92(2):722‐6. [DOI: 10.1016/j.fertnstert.2008.06.019; PMID: 18692837 ] [DOI] [PubMed] [Google Scholar]
De Angelis 2010 {published data only}
- Angelis C, Antinori M, Cerusico V, Antinori, S. Hysteroscopic surgery prior to IVF. Reproductive Biomedicine Online 2010;20 Suppl 3:S81. [Google Scholar]
De Iaco 2003 {published data only}
- Iaco PA, Muzzupapa G, Bovicelli A, Marconi S, Bitti SR, Sansovini M, et al. Hyaluronan derivative gel (Hyalobarrier gel®) in intrauterine adhesion prevention after operative hysteroscopy. Ellipse 2003;19(1):15‐8. [Google Scholar]
Demirol 2004 {published data only}
- Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reproductive BioMedicine Online 2004;8(5):590‐4. [Accession number: 13019241; PMID: 15151729] [DOI] [PubMed] [Google Scholar]
Di Florio 2013 {published data only}
- Florio C, Immediata V, Gagliano D, Zumpano A, Tartaglia C, Selvaggi L, et al. Use of a 16 French resectoscope as an alternative device in the treatment of uterine lesions: a randomized controlled trial. Human Reproduction (Oxford, England) 2013;28:i360‐i361. [DOI: 10.1093/humrep/det223] [DOI] [Google Scholar]
Di Spiezio Sardo 2011 {published data only}
- Spiezio Sardo A, Spinelli M, Bramante S, Scognamiglio M, Greco E, Guida M, et al. Efficacy of a polyethylene oxide‐sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. Journal of Minimally Invasive Gynecology 2011;18:462‐9. [DOI: 10.1016/j.jmig.2011.04.007; PMID: 21777835 ] [DOI] [PubMed] [Google Scholar]
El‐Khayat 2015 {published data only}
- El‐Khayat W. Office hysteroscopy and endometrial snip improve intrauterine insemination outcome. clinicaltrials.gov/ct2/show/NCT01544426 Date first registered: 6 March 2012. [CTG: NCT01544426]
- El‐Khayat W, Elsadek M, Saber W. Comparing the effect of office hysteroscopy with endometrial scratch versus office hysteroscopy on intrauterine insemination outcome: a randomized controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;194:96‐100. [Accession number: 26344351; CTG: NCT01544426; DOI: 10.1016/j.ejogrb.2015.08.025; PUBMED: 26344351] [DOI] [PubMed] [Google Scholar]
El‐Nashar 2011 {published data only}
- El‐Nashar IH, Nasr A. The role of hysteroscopy before intracytoplasmic sperm injection (ICSI): a randomized controlled trial. Fertility and Sterility 2011;96(3 Suppl):S266. [DOI: 10.1016/j.fertnstert.2011.07.1016; ISSN: 0015‐0282] [DOI] [Google Scholar]
Elsetohy 2015 {published data only}
- Elsetohy KA, Askalany AH, Hassan M, Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Archives of Gynecology and Obstetrics 2015;291(1):193‐9. [DOI: 10.1007/s00404-014-3397-z] [DOI] [PubMed] [Google Scholar]
El‐Toukhy 2009 {published and unpublished data}
- El‐Toukhy T. TRial of OutPatient HYsteroscopy in in‐vitro fertilisation (IVF) Trophy in IVF. www.isrctn.com/ISRCTN35859078 Date first registered: 5 February 2009. [ISRCTN: ISRCTN35859078]
- El‐Toukhy T, Campo R, Sunkara SK, Khalaf Y, Coomarasamy A. A multi‐centre randomised controlled study of pre‐IVF outpatient hysteroscopy in women with recurrent IVF implantation failure: Trial of Outpatient Hysteroscopy – [TROPHY] in IVF. Reproductive Health 2009;6:20. [DOI: 10.1186/1742-4755-6-20; PMCID: 2795733] [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Toukhy T, Khalaf Y, Coomarasamy A, Tabanelli C, Gordts SS, Gordts S, et al. A multicentre randomised study of pre‐IVF outpatient hysteroscopy in women with recurrent IVF‐ET failure – the TROPHY trial. Human Reproduction (Oxford, England) 2014;29(Supp 1):i36‐7. [Google Scholar]
Fatemi 2007 {unpublished data only}
- Fatemi HM, Devroey P, Fauser B, Broekmans FJ, Kasius JC. The impact of treating minor uterine cavity abnormalities diagnosed by office hysteroscopy in unselected in vitro fertilization (IVF) cases. clinicaltrials.gov/ct2/show/NCT00830401 Date first registered: 27 January 2009. [CTG: NCT00830401]
Fuchs 2014 {published data only}
- Fuchs N, Smorgick N, Ben Ami I, Vaknin Z, Tovbin Y, Halperin R, et al. Intercoat (Oxiplex/AP gel) for preventing intrauterine adhesions after operative hysteroscopy for suspected retained products of conception: double‐blind, prospective, randomized pilot study. Journal of Minimally Invasive Gynecology 2014;21(1):126‐30. [Accession number: 23954387; DOI: 10.1016/j.jmig.2013.07.019; PUBMED: 23954387] [DOI] [PubMed] [Google Scholar]
- Pansky M. Efficiency of Intercoat (Oxiplex/AP Gel) in decreasing intrauterine adhesions. clinicaltrials.gov/ct2/show/NCT01377779 21 June 2011. [CTG: NCT01377779]
Gan 2017 {published data only}
- Gan L, Duan H, Sun FQ, Xu Q, Tang YQ, Wang S. Efficacy of freeze‐dried amnion graft following hysteroscopic adhesiolysis of severe intrauterine adhesions. International Journal of Gynaecology and Obstetrics 2017;137(2):116‐22. [Accession number: 28170094; DOI: 10.1002/ijgo.12112; PUBMED: 28170094] [DOI] [PubMed] [Google Scholar]
Gao 2013 {unpublished data only}
- Gao M. Hysteroscopy assess and improve uterine receptivity for women with repeated implantation failure. www.chictr.org.cn/showprojen.aspx?proj=5684 Date first received: 6 August 2013. [ChiCTR: ChiCTR‐ONRC‐13003882]
Guida 2004 {published data only}
- Guida M, Acunzo G, Spiezio Sardo A, Bifulco G, Piccoli R, Pellicano M, et al. Effectiveness of auto‐crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Human Reproduction 2004;19(6):1461‐4. [DOI: 10.1093/humrep/deh238; PMID: 15105384] [DOI] [PubMed] [Google Scholar]
Guo 2017 {published data only}
- Guo J, Li TC, Liu Y, Xia E, Xiao Y, Zhou F, et al. A prospective, randomized, controlled trial comparing two doses of oestrogen therapy after hysteroscopic adhesiolysis to prevent intrauterine adhesion recurrence. Reproductive Biomedicine Online 2017;35(5):555‐61. [Accession number: 28784336; DOI: 10.1016/j.rbmo.2017.07.011; PUBMED: 28784336] [DOI] [PubMed] [Google Scholar]
Hamerlynck 2015 {published data only}
- Hamerlynck TW, Schoot BC, Vliet HA, Weyers S. Removal of endometrial polyps: hysteroscopic morcellation versus bipolar resectoscopy. A randomized trial. Journal of Minimally Invasive Gynecology 2015;22(7):1237‐43. [Accession number: 26192235; DOI: 10.1016/j.jmig.2015.07.006; PUBMED: 26192235] [DOI] [PubMed] [Google Scholar]
Hanstede 2016 {published data only}
- Hanstede M, Emanuel MH. Tertiary prevention of morbus Asherman: a randomized controlled trial. Journal of Minimally Invasive Gynecology. Netherlands: Elsevier Inc., 2016; Vol. 23 7 Suppl 1:S6‐S7. [Call number: CN‐01250529]
- Hanstede MM, Emanuel MH. Tertiary prevention of morbus Asherman. Evaluation of hormonal support with estrogen and gestagen post adhesiolysis. Gynecological Surgery 2016;13 Suppl 1(7):S6‐S7. [Google Scholar]
Hare 2013 {unpublished data only}
- Hare J. No significant effect of endometrial scratching before second IVF treatment. HFOG Congress Odense Danmark 2018.
- Hare KJ. Hysteroscopy before in vitro fertilization – does it improve the outcome?. clinicaltrials.gov/ct2/show/NCT01743391 Date first registered: 6 December 2012. [CTG: NCT01743391]
Javidan 2017 {published data only}
- Javidan AN, Jafarabadi M, Latifi S, Farhadkhani M, Gorginzadeh M. Uterine resectoscopic myomectomy with and without microrelin pretreatment: a single‐blinded randomized clinical trial. Biomedical Research (India) 2017;28(16):6963‐7. [Call number: CN‐01414851] [Google Scholar]
Kamel 2014 {published data only}
- Kamel MA, El‐Tawab SS, El‐Ashkar OS, Hassan MIA. Mini‐scissor versus bipolar twizzle in ambulatory hysteroscopic metroplasty: a prospective randomized study. Journal of Gynecologic Surgery 2014;30(3):147‐51. [Accession number: 373287640] [Google Scholar]
Lara‐Dominguez 2016 {published data only}
- Lara‐Dominguez MD, Arjona‐Berral JE, Dios‐Palomares R, Castelo‐Branco C. Outpatient hysteroscopic polypectomy: bipolar energy system (Versapoint) versus diode laser – randomized clinical trial. Gynecological Endocrinology 2016;32(3):196‐200. [Accession number: 26527251; DOI: 10.3109/09513590.2015.1105209; PUBMED: 26527251 ] [DOI] [PubMed] [Google Scholar]
Lieng 2010a {published data only}
- Lieng M, Istre O, Sandvik L, Engh V, Qvigstad E. Clinical effectiveness of transcervical polyp resection in women with endometrial polyps: randomized controlled trial. Journal of Minimally Invasive Gynecology 2010;17(3):351‐7. [DOI: 10.1016/j.jmig.2010.01.019; PMID: 20417427 ] [DOI] [PubMed] [Google Scholar]
Lin 2014 {unpublished data only}
- Lin X, Wei ML, Li TC, Zhou F, Zhang SY. A prospective randomized control trial to compare the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation following hysteroscopic adhesiolysis. Gynecological Surgery 2014;11(1):89‐90. [ISRCTN: ISRCTN6969027269690272] [Google Scholar]
Liu 2016 {published data only}
- Liu AZ, Zhao HG, Gao Y, Liu M, Guo BZ. Effectiveness of estrogen treatment before transcervical resection of adhesions on moderate and severe uterine adhesion patients. Gynecological Endocrinology 2016;32(9):737‐40. [Accession number: 26982384; DOI: 10.3109/09513590.2016.1160375; PUBMED: 26982384 ] [DOI] [PubMed] [Google Scholar]
Mohammed 2014 {unpublished data only}
- Mohammed AM, Gomaa MF. Value of routine hysteroscopy prior to IVF/ICSI cycles. clinicaltrials.gov/ct2/show/NCT02245750 Date first received: 22 September 2014. [CTG: NCT02245750]
Moramezi 2012 {published and unpublished data}
- Moramezi F. Effect of hysteroscopy before intra uterine insemination on fertility in infertile couples. en.irct.ir/trial/9340 Date first received: 9 April 2012. [IRCT: IRCT201201308867N1] [DOI] [PubMed]
- Moramezi F, Barati M, Mohammadjafari R, Barati S, Hemadi M. Effect of hysteroscopy before intra uterine insemination on fertility in infertile couples. Pakistan Journal of Biological Sciences 2012;15(19):942‐6. [PUBMED: 24159691] [DOI] [PubMed] [Google Scholar]
Muzii 2007 {published data only}
- Muzii L, Bellati F, Pernice M, Manci N, Angioli R, Panici PB. Resectoscopic versus bipolar electrode excision of endometrial polyps: a randomized study. Fertility and Sterility 2007;87(4):909‐17. [DOI: 10.1016/j.fertnstert.2006.08.113; PMID: 17239873] [DOI] [PubMed] [Google Scholar]
Muzii 2017 {published data only}
- Muzii L, Donato V, Boni T, Gaglione R, Marana R, Mazzon I, et al. Antibiotics prophylaxis for operative hysteroscopy: a multicenter randomized controlled clinical study. Reproductive Sciences (Thousand Oaks, Calif.) 2017;24(4):534‐8. [Call number: CN‐01374762; DOI: 10.1177/1933719116660848] [DOI] [PubMed] [Google Scholar]
Nappi 2013 {published data only}
- Nappi L, Spiezio Sardo A, Spinelli M, Guida M, Mencaglia L, Greco P, et al. A multicenter, double‐blind, randomized, placebo‐controlled study to assess whether antibiotic administration should be recommended during office operative hysteroscopy. Reproductive Sciences 2013;20(7):755‐61. [Accession number: 23232966; DOI: 10.1177/1933719112466308; PUBMED: 23232966 ] [DOI] [PubMed] [Google Scholar]
Pabuccu 2008 {published data only}
- Pabuccu R, Onalan G, Kaya C, Selam B, Ceyhan T, Ornek T, et al. Efficiency and pregnancy outcome of serial intrauterine device‐guided hysteroscopic adhesiolysis of intrauterine synechiae. Fertility and Sterility 2008;90(5):1973‐7. [DOI: 10.1016/j.fertnstert.2007.06.074; PMID: 18774563] [DOI] [PubMed] [Google Scholar]
Parsanezhad 2006 {published data only}
- Parsanezhad ME, Alborzi S, Zarei A, Dehbashi S, Shirazi LG, Rajaeefard A, et al. Hysteroscopic metroplasty of the complete uterine septum, duplicate cervix, and vaginal septum. Fertility and Sterility 2006;85(5):1473‐7. [DOI: 10.1016/j.fertnstert.2005.10.044; PMID: 16600229 ] [DOI] [PubMed] [Google Scholar]
Paz 2014 {published data only}
- Paz M, Kaufman Y, Brandes KO, Segev E, Rofe G, Auslender R, et al. Intercoat (oxiplex/AP gel) for preventing intrauterine adhesions after operative hysteroscopy: double blind prospective randomized pilot study. Journal of Minimally Invasive Gynecology 2014;21 Suppl 1(6):S96. [DOI: 10.1016/j.jmig.2013.07.019; PUBMED: 23954387] [DOI] [PubMed] [Google Scholar]
Rama Raju 2006 {published data only}
- Rama Raju GA, Shashi Kumari G, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Archives of Gynecology and Obstetrics 2006;274(3):160‐4. [DOI: 10.1007/s00404-006-0174-7; PMID: 16715289] [DOI] [PubMed] [Google Scholar]
Revel 2011 {unpublished data only}
- Revel A. Safety study of use of hyaluronic acid gel to prevent intrauterine adhesions in hysteroscopic surgery. clinicaltrials.gov/ct2/show/NCT01464528 Date first received: 3 November 2011. [CTG: NCT01464528]
Roy 2014 {published data only}
- Roy KK, Negi N, Subbaiah M, Kumar S, Sharma JB, Singh N. Effectiveness of estrogen in the prevention of intrauterine adhesions after hysteroscopic septal resection: a prospective, randomized study. Journal of Obstetrics & Gynaecology Research 2014;40(4):1085‐8. [Accession number: 24612233; DOI: 10.1111/jog.12297; PUBMED: 24612233] [DOI] [PubMed] [Google Scholar]
Roy 2015 {published data only}
- Roy KK, Kansal Y, Subbaiah M, Kumar S, Sharma JB, Singh N. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. Journal of Obstetrics & Gynaecology Research 2015;41(6):952‐6. [Accession number: 25491475; DOI: 10.1111/jog.12646; PUBMED: 25491475 ] [DOI] [PubMed] [Google Scholar]
Roy 2017 {published data only}
- Roy K, Metta S, Kansal Y, Kumar S, Singhal S, Vanamail P. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. Journal of Human Reproductive Sciences 2017;10(3):185‐93. [Accession number: 619005877; DOI: 10.4103/jhrs.JHRS_134_16; PUBMED: 29142447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubino 2015 {published data only}
- Rubino RJ, Lukes AS. Twelve‐month outcomes for patients undergoing hysteroscopic morcellation of uterine polyps and myomas in an office or ambulatory surgical center. Journal of Minimally Invasive Gynecology 2015;22(2):285‐90. [Accession number: 25446547; DOI: 10.1016/j.jmig.2014.10.015; PUBMED: 25446547] [DOI] [PubMed] [Google Scholar]
Shawki 2012 {published data only}
- Shawki HE, Elmorsy M, Eissa MK. Routine office hysteroscopy prior to ICSI and its impact on assisted reproduction program outcome: a randomized controlled trial. Middle East Fertility Society Journal 2012;17(1):14‐21. [DOI: 10.1016/j.mefs.2011.04.005] [DOI] [Google Scholar]
Shokeir 2010 {published data only}
- Shokeir T, El‐Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertility and Sterility 2010;94(2):724‐9. [DOI: 10.1016/j.fertnstert.2009.03.075; PMID: 19406399] [DOI] [PubMed] [Google Scholar]
Smit 2015 {published data only}
- Smit JG, Overdijkink S, Mol BW, Kasius JC, Torrance HL, Eijkemans MC, et al. The impact of diagnostic criteria on the reproducibility of the hysteroscopic diagnosis of the septate uterus: a randomized controlled trial. Human Reproduction 2015;30(6):1323‐30. [Accession number: 605712453; DOI: 10.1093/humrep/dev082; PUBMED: 25904634 ] [DOI] [PubMed] [Google Scholar]
Smit 2016 {published and unpublished data}
- Broekmans FJ. SIGnificance of Routine Hysteroscopy Prior to a First 'in Vitro Fertilization' (IVF) Treatment Cycle (inSIGHT). clinicaltrials.gov/ct2/show/NCT01242852 Date first received: 17 November 2010. [CinicalTrials.gov: NCT01242852]
- Smit JG, Kasius JC, Eijkemans MJ, Koks CA, Golde R, Oosterhuis JG, et al. The inSIGHT study: costs and effects of routine hysteroscopy prior to a first IVF treatment cycle. A randomised controlled trial. BMC Women's Health 2012;12:22. [DOI: 10.1186/1472-6874-12-22; http: www.biomedcentral.com/1472‐6874/12/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JG, Kasius JC, Eijkemans MJ, Koks CA, Golde R, Nap AW, et al. Hysteroscopy before in‐vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet 2016;387(10038):2622‐9. [Accession number: 27132052; DOI: 10.1016/S0140-6736(16)00231-2; PUBMED: 27132052] [DOI] [PubMed] [Google Scholar]
Smith 2014 {published data only}
- Smith PP, Middleton LJ, Connor M, Clark TJ. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstetrics & Gynecology 2014;123(4):745‐51. [Accession number: 24785600; DOI: 10.1097/AOG.0000000000000187; PUBMED: 24785600] [DOI] [PubMed] [Google Scholar]
Sohrabvand 2012 {unpublished data only}
- Sohrabvand F. Evaluation of diagnostic hysteroscopy findings in patients candidate ART (IVF, ICSI). en.irct.ir/trial/2316?revision=2316 Date first registered: 8 October 2012. [IRCT: IRCT201208152565N6]
Tonguc 2010 {published data only}
- Tonguc EA, Var T, Yilmaz N, Batioglu S. Intrauterine device or estrogen treatment after hysteroscopic uterine septum resection. International Journal of Gynaecology and Obstetrics 2010;109(3):226‐9. [DOI: 10.1016/j.ijgo.2009.12.015; PMID: 20152976] [DOI] [PubMed] [Google Scholar]
- Tonguc EA, Var T, Yilmaz N, Batioglu S. Management after hysteroscopic metroplasty: with or without intrauterine device (IUD) insertion and estrogen administration. Fertility and Sterility 2008;90(Suppl):S165. [DOI: 10.1016/j.fertnstert.2008.07.918] [DOI] [Google Scholar]
Trninić‐Pjević 2011 {published data only}
- Trninić‐Pjević A, Kopitović V, Pop‐Trajković S, Bjelica A, Bujas I, Tabs D, et al. Effect of hysteroscopic examination on the outcome of in vitro fertilization. Vojnosanit Pregl 2011;68(6):476‐80. [Accession number: 21818913] [DOI] [PubMed] [Google Scholar]
van Dongen 2008 {published data only}
- Emanuel MH, Dongen H, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled study among residents in training. Fertility and Sterility 2009;92(3 Suppl):S5. [DOI: 10.1016/j.fertnstert.2009.07.019] [DOI] [PubMed] [Google Scholar]
- Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. Journal of Minimally Invasive Gynecology 2008;15(4):466‐71. [DOI: 10.1016/j.jmig.2008.02.002; PMID: 18588849] [DOI] [PubMed] [Google Scholar]
Vercellini 1993 {published data only}
- Vercellini P, Vendola N, Colombo A, Passadore C, Trespidi L, Fedele L. Hysteroscopic metroplasty with resectoscope or microscissors for the correction of septate uterus. Surgery, Gynecology and Obstetrics 1993;176(5):439‐42. [PMID: 8480265] [PubMed] [Google Scholar]
Weiss 2005 {unpublished data only}
- Weiss A, Shalev E, Geslevich J. Endometrial curettage before embryo transfer. clinicaltrials.gov/ct2/show/NCT00367367 Date first registered: 22 August 2006. [CTG: NCT00367367]
Xiao 2015 {published data only}
- Xiao S, Wan Y, Zou F, Ye M, Deng H, Ma J, Wei Y, Tan C, Xue M. Prevention of intrauterine adhesion with auto‐crosslinked hyaluronic acid gel: a prospective, randomized, controlled clinical study. Zhonghua Fu Chan Ke za Zhi 2015;50(1):32‐6. [Accession number: 25877422; PUBMED: 25877422] [PubMed] [Google Scholar]
- Xiao S, Wan Y, Zou F, Ye M, Deng H, Ma J, et al. A randomized multi‐center controlled study on the efficacy and safety of a new crosslinked hyaluronan gel to prevent intrauterine adhesion following hysteroscopic adhesiolysis. Giornale Italiano di Ostetricia e Ginecologia 2015;37(4):216‐9. [Call number: CN‐01138402] [Google Scholar]
Youssef 2013 {published data only}
- Youssef HM. Uterine septum dissection using mini‐hysteroscopy with bipotrode 5 fr bipolar electrode versus monopolar resectoscopy with a unipolar knife: a randomized controlled study. Fertility and Sterility 2013;100(3 Suppl 1):S394. [Google Scholar]
References to ongoing studies
SEPTUM trial {published data only}
- Prior M. Pilot randomised controlled trial of hysteroscopic septal resection. www.isrctn.com/ISRCTN28960271 Date first received: 6 January 2015.
TRUST trial {published data only}
- Rikken JF, Kowalik CR, Emanuel MH, Mol BW, Veen F, Wely M, et al. TRUST study – the randomized uterine septum transsection trial. www.studies‐obsgyn.nl/trust/ 2014.
- Rikken JFW, Kowalik CR, Emanuel MH, Bongers MY, Spinder T, Kruif JH, et al. The randomised uterine septum transsection trial (TRUST): design and protocol. BMC Women's Health 2018;18:163. [DOI: 10.1186/s12905-018-0637-6; PUBMED: 30290803] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Andersen 2008
- Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Human Reproduction 2008;23:756‐71. [DOI: 10.1093/humrep/den014; PMID: 18281243] [DOI] [PubMed] [Google Scholar]
Bensdorp 2007
- Bensdorp A, Cohlen BJ, Heineman MJ, Vanderkerchove P. Intra‐uterine insemination for male subfertility. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000360.pub4] [DOI] [PubMed] [Google Scholar]
Bettocchi 2004
- Bettocchi S, Nappi L, Ceci O, Selvaggi L. Office hysteroscopy. Obstetrics and Gynecology Clinics of North America 2004;31:641‐54. [DOI: 10.1016/j.ogc.2004.05.007; PMID: 15450325] [DOI] [PubMed] [Google Scholar]
Boivin 2007
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Human Reproduction 2007;22(6):1506‐12. [DOI: 10.1093/humrep/dem046; PMID: 17376819] [DOI] [PubMed] [Google Scholar]
Campo 1999
- Campo R, Belle Y, Rombauts L, Brosens I, Gordts S. Office mini‐hysteroscopy. Human Reproduction Update 1999;5(1):73‐81. [DOI: 10.1093/humupd/5.1.73; PMID: 10333371] [DOI] [PubMed] [Google Scholar]
Campo 2005
- Campo R, Molinas CR, Rombauts L, Mestdagh G, Lauwers M, Braekmans P, et al. Prospective multicentre randomised controlled trial to evaluate factors influencing the success rate of office diagnostic hysteroscopy. Human Reproduction 2005;20(1):258‐63. [DOI: 10.1093/humrep/deh559; PMID: 15550496] [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark TJ, Gupta JK. Handbook of Outpatient Hysteroscopy. A Complete Guide to Diagnosis and Therapy. London: Hodder Education, 2005. [Google Scholar]
De Placido 2007
- Placido G, Clarizia R, Cadente C, Castaldo G, Romano C, Mollo A, et al. Compliance and diagnostic efficacy of mini‐hysteroscopy versus traditional hysteroscopy in infertility investigation. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;135(1):83‐7. [DOI: 10.1016/j.ejogrb.2007.02.028; PMID: 17481803] [DOI] [PubMed] [Google Scholar]
Di Spiezio Sardo 2016
- Spiezio Sardo A, Carlo C, Minozzi S, Spinelli ML, Pistotti V, Alviggi C, et al. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta‐analysis. Human Reproduction Update 2016;22(4):479‐96. [DOI: 10.1093/humupd/dmw008; PUBMED: 27008893] [DOI] [PubMed] [Google Scholar]
Donnez 2002
- Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for debate?. Human Reproduction 2002;17:1424‐30. [DOI: 10.1093/humrep/17.6.1424; PMID: 12042254] [DOI] [PubMed] [Google Scholar]
Fatemi 2010
- Fatemi HM, Kasius JC, Timmermans A, Disseldorp J, Fauser BC, Devroey P, et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Human Reproduction 2010;25(8):1959‐65. [DOI: 10.1093/humrep/deq150; PMID: 20570971] [DOI] [PubMed] [Google Scholar]
Fedele 1996
- Fedele L, Bianchi S, Marchini M, Franchi D, Tozzi L, Dorta M. Ultrastructural aspects of endometrium in infertile women with septate uterus. Fertility and Sterility 1996;65:750‐2. [PMID: 8654633] [PubMed] [Google Scholar]
Garbin 2006
- Garbin O, Kutnahorsky R, Göllner JL, Vayssiere C. Vaginoscopic versus conventional approaches to outpatient diagnostic hysteroscopy: a two‐centre randomised prospective study. Human Reproduction 2006;21(11):2996‐3000. [DOI: 10.1093/humrep/del276; PMID: 16845121] [DOI] [PubMed] [Google Scholar]
Golan 1996
- Golan A, Eilat E, Ron‐El R, Herman A, Soffer Y, Bukovsky I. Hysteroscopy is superior to hysterosalpingography in infertility investigation. Acta Obstetricia et Gynecologica Scandinavica 1996;75(7):654‐6. [DOI: 10.3109/00016349609054692; PMID: 8822660] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guida 2006
- Guida M, Spiezio Sardo A, Acunzo G, Sparice S, Bramante S, Piccoli R, et al. Vaginoscopic versus traditional office hysteroscopy: a randomised controlled study. Human Reproduction 2006;21(12):3253‐7. [DOI: 10.1093/humrep/del298; PMID: 16861744] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PMID: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jansen 2000
- Jansen FW, Vredevoogd CB, Ulzen K, Hermans J, Trimbos JB, Trimbos‐Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstetrics and Gynecology 2000;96(2):266‐70. [DOI: 10.1016/S0029-7844(00)00865-6; PMID: 10908775] [DOI] [PubMed] [Google Scholar]
Jayaprakasan 2014
- Jayaprakasan K, Polanski L, Sahu B, Thornton JG, Raine‐Fenning N. Surgical intervention versus expectant management for endometrial polyps in subfertile women. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009592] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabli 2008
- Kabli N, Tulandi T. A randomised trial of outpatient hysteroscopy with and without intrauterine anesthesia. Journal of Minimally Invasive Gynecology 2008;15(3):308‐10. [DOI: 10.1016/j.jmig.2008.01.013; PMID: 18439502] [DOI] [PubMed] [Google Scholar]
Kalampokas 2012
- Kalampokas T, Tzanakaki D, Konidaris S, Iavazzo C, Kalampokas E, Gregoriou O. Endometrial polyps and their relationship in the pregnancy rates of patients undergoing intrauterine insemination. Clinical and Experimental Obstetrics and Gynecology 2012;39(3):299‐302. [Accession number: WOS:000308510700006 ; ISSN: 0390‐6663 ] [PubMed] [Google Scholar]
Kasius 2011a
- Kasius JC, Broekmans FJ, Veersema S, Eijkemans MJ, Santbrink EJ, Devroey P, et al. Observer agreement in the evaluation of the uterine cavity by hysteroscopy prior to in vitro fertilization. Human Reproduction 2011;26(4):801‐7. [DOI: 10.1093/humrep/der003; PMID: 21310749] [DOI] [PubMed] [Google Scholar]
Kasius 2011b
- Kasius JC, Eijkemans MJ, Mol BW, Fauser BC, Broekmans FJM. Cost‐effectiveness of hysteroscopy screening for infertile women. Human Reproduction 2011;26 Suppl 1:i338‐9. [DOI] [PubMed] [Google Scholar]
Lieng 2010b
- Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2010;89:992‐1002. [DOI: 10.3109/00016349.2010.493196; PMID: 20528202] [DOI] [PubMed] [Google Scholar]
Lucas 2013
- Lucas ES, Salker MS, Brosens JJ. Uterine plasticity and reproductive fitness. Reproductive Biomedicine Online 2013;27:664‐72. [DOI: 10.1016/j.rbmo.2013.06.012] [DOI] [PubMed] [Google Scholar]
Marsh 2004
- Marsh F, Kremer C, Duffy S. Delivering an effective outpatient service in gynaecology. A randomised controlled trial analysing the cost of outpatient versus daycase hysteroscopy. British Journal of Obstetrics and Gynaecology 2004;111(3):243‐8. [DOI: 10.1111/j.1471-0528.2004.00064; PMID: 14961886] [DOI] [PubMed] [Google Scholar]
Metwally 2012
- Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD003857] [DOI] [PubMed] [Google Scholar]
Mollo 2009
- Mollo A, Franciscis P, Colacurci N, Cobellis L, Perino A, Venezia R, et al. Hysteroscopic resection of the septum improves the pregnancy rate of women with unexplained infertility: a prospective controlled trial. Fertility and Sterility 2009;91:2628‐31. [DOI: 10.1016/j.fertnstert.2008.04.011; PMID: 18571168] [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. nice.org.uk/guidance/cg156 20 February 2013.
Pritts 2001
- Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstetrical and Gynecological Survey 2001;56(8):483‐91. [Accession number: 00006254‐200108000‐00022; PMID: 11496160] [DOI] [PubMed] [Google Scholar]
Pritts 2009
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertility and Sterility 2009;91:1215‐23. [DOI: 10.1016/j.fertnstert.2008.01.051; PMID: 18339376] [DOI] [PubMed] [Google Scholar]
Ray 2012
- Ray A, Shah A, Gudi A, Homburg R. Unexplained infertility: an update and review of practice. Reproductive BioMedicine Online 2012;24:591‐602. [DOI: 10.1016/j.rbmo.2012.02.021] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rikken 2016
- Rikken JF, Kowalik CR, Emanuel MH, Mol BW, Veen F, Wel M, et al. Hysteroscopic septum resection versus expectant management for women with a septate uterus. Human Reproduction 2016;31(Suppl 1):i463. [Accession number: 615297756] [Google Scholar]
Rikken 2017
- Rikken JF, Kowalik CR, Emanuel MH, Mol BW, Veen F, Wely M, et al. Septum resection for women of reproductive age with a septate uterus. Cochrane Database of Systematic Reviews 2017, Issue 1. [Accession number: 614070938; DOI: 10.1002/14651858.CD008576.pub4; PUBMED: 28093720 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 1986
- Rogers PA, Milne BJ, Trounson AO. A model to show human uterine receptivity and embryo viability following ovarian stimulation for in vitro fertilization. Journal of In Vitro Fertilization and Embryo Transfer 1986;3:93‐8. [PMID: 3701185] [DOI] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Synnot A, Hill S. Describing results, version 2, 2016. cccrg.cochrane.org/author‐resources (accessed prior to 4 November 2018).
Sagiv 2006
- Sagiv R, Sadan O, Boaz M, Dishi M, Schechter E, Golan A. A new approach to office hysteroscopy compared with traditional hysteroscopy. Obstetrics and Gynecology 2006;108(2):387‐92. [DOI: 10.1097/01.AOG.0000227750.93984.06; PMID: 16880310] [DOI] [PubMed] [Google Scholar]
Saravelos 2008
- Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Human Reproduction Update 2008;14:415‐29. [DOI: 10.1093/humupd/dmn018; PMID: 18539641] [DOI] [PubMed] [Google Scholar]
Shankar 2004
- Shankar M, Davidson A, Taub N, Habiba M. Randomised comparison of distension media for outpatient hysteroscopy. British Journal of Obstetrics and Gynaecology 2004;111(1):57‐62. [DOI: 10.1046/j.1471-0528.2003.00004; PMID: 14687053] [DOI] [PubMed] [Google Scholar]
Sharma 2005
- Sharma M, Taylor A, Spiezio Sardo A, Buck L, Mastrogamvrakis G, Kosmas I, et al. Outpatient hysteroscopy: traditional versus the 'no‐touch' technique. British Journal of Obstetrics and Gynaecology 2005;112(7):963‐7. [DOI: 10.1111/j.1471-0528.2005.00425; PMID: 15958000] [DOI] [PubMed] [Google Scholar]
Shohayeb 2011
- Shohayeb A, Shaltout A. Persistent endometrial polyps may affect the pregnancy rate in patients undergoing intrauterine insemination. Middle East Fertility Society Journal 2011;16(4):259‐64. [DOI: 10.1016/j.mefs.2011.03.003] [DOI] [Google Scholar]
Shokeir 2004
- Shokeir TA, Shalan HM, El‐Shafei MM. Significance of endometrial polyps detected hysteroscopically in eumenorrheic infertile women. Journal of Obstetrics and Gynaecology Research 2004;30:84‐9. [DOI: 10.1111/j.1447-0756.2003.00163; PMID: 15009608] [DOI] [PubMed] [Google Scholar]
Shokeir 2011
- Shokeir T, Abdelshaheed M, El‐Shafei M, Sherif L, Badawy A. Determinants of fertility and reproductive success after hysteroscopic septoplasty for women with unexplained primary infertility: a prospective analysis of 88 cases. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;155:54‐7. [DOI: 10.1016/j.ejogrb.2010.11.015; PMID: 21185112] [DOI] [PubMed] [Google Scholar]
Silberstein 2006
- Silberstein T, Saphier O, Voorhis BJ, Plosker SM. Endometrial polyps in reproductive‐age fertile and infertile women. Israel Medical Association Journal 2006;8:192‐5. [PMID: 16599056] [PubMed] [Google Scholar]
Singh 2011
- Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation; network of hormones, cytokines, and growth factors. Journal of Endocrinology 2011;210:5‐14. [DOI: 10.1530/JOE-10-0461; PMID: 21372150] [DOI] [PubMed] [Google Scholar]
Somigliana 2007
- Somigliana E, Vercellini P, Daguati R, Pasin R, Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Human Reproduction Update 2007;13:465‐76. [DOI: 10.1093/humupd/dmm013; PMID: 17584819] [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor E, Gomel V. The uterus and fertility. Fertility and Sterility 2008;89(1):1‐15. [DOI: 10.1016/j.fertnstert.2007.09.069; PMID: 18155200] [DOI] [PubMed] [Google Scholar]
Tomaževič 2010
- Tomaževič T, Ban‐Frangež H, Virant‐Klun I, Verdenik I, Požlep B, Vrta nik‐Bokal E. Septate, subseptate and arcuate uterus decrease pregnancy and live birth rates in IVF/ICSI. Reproductive Biomedicine Online 2010;21(5):700‐5. [DOI: 10.1016/j.rbmo.2010.06.028; PMID: 20864409] [DOI] [PubMed] [Google Scholar]
Veltman‐Verhulst 2012
- Veltman‐Verhulst SM, Cohlen BJ, Hughes E, Heineman MJ. Intra‐uterine insemination for unexplained subfertility. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD001838.pub4] [DOI] [PubMed] [Google Scholar]
Wallach 1972
- Wallach EE. The uterine factor in infertility. Fertility and Sterility 1972;23(2):138‐58. [PMID: 4551503] [DOI] [PubMed] [Google Scholar]
Wamsteker 1998
- Wamsteker K, Block S. Diagnostic hysteroscopy: technique and documentation. Endoscopic Surgery for Gynecologists. London: Saunders, 1998:511‐24. [Google Scholar]
Yanaihara 2008
- Yanaihara A, Yorimitsu T, Motoyama H, Iwasaki S, Kawamura T. Location of endometrial polyp and pregnancy rate in infertility patients. Fertility and Sterility 2008;90:180‐2. [DOI: 10.1016/j.fertnstert.2007.05.072; PMID: 17889854] [DOI] [PubMed] [Google Scholar]
Yu 2008
- Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertility and Sterility 2008;89(3):715‐22. [DOI: 10.1016/j.fertnstert.2007.03.070; PMID: 17681324] [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers‐Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, Poel S. The International Glossary on Infertility and Fertility Care, 2017. Fertility and Sterility 2017;108(3):393‐406. [DOI: 10.1016/j.fertnstert.2017.06.005; PMID: 28760517 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bosteels 2010
- Bosteels J, Weyers S, Puttemans P, Panayotidis C, Herendael B, Gomel V, et al. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Human Reproduction Update 2010;16(1):1‐11. [DOI: 10.1093/humupd/dmp033; PMID: 19744944] [DOI] [PubMed] [Google Scholar]
Bosteels 2013
- Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BW, D'Hooghe TM. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD009461.pub2] [DOI] [PubMed] [Google Scholar]


