Abstract
Beta power suppression in the basal ganglia is stronger during movements that require high force levels and high movement effort but it has been difficult to dissociate the two. We recorded scalp EEG and basal ganglia local field potentials in Parkinson's disease patients (11 STN, 7 GPi) ON and OFF dopaminergic medication while they performed a visually-guided force matching task using a pen on a force-sensitive graphics tablet. Force adjustments were accompanied by beta power suppression irrespective of whether the force was increased or reduced. Before the adjustment was completed, beta activity returned. High beta power was specifically associated with slowing of the force adjustment. ON medication, the peak force rate was faster and cortico-basal ganglia beta phase coupling was more readily modulated. In particular, phase decoupling was stronger during faster adjustments. The results suggest that beta power in the basal ganglia does not covary with force per se, but rather with a related factor, the absolute force rate, or a more general concept of movement effort. The results also highlight that beta activity reappears during stabilization of isometric contractions, and that dopaminerelated suppression of cortico-basal ganglia beta coupling is linked to faster force adjustments.
Keywords: Force control, Isometric contraction, Cortico-basal ganglia coupling, Beta oscillations, Beta power, Beta coupling
1. Introduction
Mounting evidence suggests the basal ganglia are involved in regulating movement vigour (Da Silva et al., 2018; Mazzoni et al., 2007; Turner and Desmurget, 2010; Yttri and Dudman, 2016). Direct recordings from basal ganglia targets in patients have shown that reciprocal changes in beta and gamma band activities in the local field potential (LFP) covary with the production of different force levels (Tan et al., 2013) as well as with movement size and speed (Brücke et al., 2012; Joundi et al., 2012). Beta power in motor cortex and the basal ganglia is suppressed during ballistic movements (Brücke et al., 2008; Kilavik et al., 2013; Kühn et al., 2004), and especially so during vigorous movements (Tan et al., 2015, Tan et al., 2013), but during sustained, stable contractions, motor cortical beta power and corticomuscular coherence is increased (reviewed in Kilavik et al., 2013). One prominent hypothesis is that beta oscillations promote the status quo (Engel and Fries, 2010; Gilbertson et al., 2005) – or, in other words, the stability of the current state. Interestingly, beta oscillations reappearing after a movement are also modulated by sensory feedback (Tan et al., 2014a; Tan et al., 2014b; Torrecillos et al., 2015). Most motor assessments involve either ballistic movements or sustained force and thus to date it is unknown how basal ganglia beta oscillations relate to small adjustments of otherwise sustained, isometric forces. Yet small movements are impaired early in Parkinson's disease, as evinced by the deterioration in handwriting that is often the first symptom of the condition (Pinto and Velay, 2015; Rosenblum et al., 2013). Here we recorded the LFP in the subthalamic nucleus (STN) or globus pallidus interna (GPi) whilst Parkinsonian patients made visually-cued, low-force isometric force adjustments with a hand-held pen on a force-sensitive tablet. This allowed us to contrast spectral changes during isometric force increases and reductions. In particular, the paradigm enabled us to explore two contrasting hypotheses. The first is that beta oscillations are inversely related to force levels, in which case we should find decreased power during force increases, but increased power during force reductions. Alternatively, beta oscillations may be related to a directionless measure, such as the rate of any changing muscle contraction, or the effort made, rather than the generated force per se (Tan et al., 2015). In this case we should find that beta power is suppressed irrespective of the direction of the force change. In addition, by using the same paradigm both off and on dopaminergic medication, we were able to test the hypothesis that the pattern of basal ganglia activity associated with finely controlled motor adjustments differs according to dopaminergic status. Here, we were particularly interested in the balance between beta reactivity locally in the STN and GPi and in the long-range coupling between the STN/GPi and cortex (Cassidy et al., 2002; Litvak et al., 2011; van Wijk et al., 2017).
2. Methods
2.1. Participants
Eighteen patients with idiopathic Parkinson's disease (mean disease duration of 10 ± (STD) 5 years, mean age 62 ± 7 years, 16 males) provided written informed consent to take part in this study, which was approved by the local ethics committees. Clinical details of the patients are given in Supplementary Table 1. The patients showed 58 ± (STD) 14% improvement in the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS) on treatment with levodopa, indicating good responsiveness to medication.
All patients were implanted with bilateral DBS electrodes as a prelude to therapeutic high-frequency stimulation for advanced Parkinson's disease with motor fluctuations and/or dyskinesia 3–6 days prior to the recording. The target for the electrodes were the STN in eleven patients and the GPi in seven patients.
DBS electrode extension cables were externalized through the scalp to enable recordings before they were connected to a subcutaneous DBS pacemaker implanted in a second operation up to 7 days later. The electrode implantation procedure has been described previously (Foltynie and Hariz, 2010). The macroelectrodes implanted were Model 3389™ from Medtronic Neurologic Division and Model DB-2201™ and DB-2202™ from Boston Scientific. Surgeries and recordings were performed at one of the following three sites: King's College Hospital, London, University College Hospital, London, or the John Radcliffe Hospital, Oxford, UK. Several cases have been previously reported in other studies (Fischer et al., 2017a, 2017b).
2.2. Experimental paradigm
LFPs were recorded while patients performed a visually-guided force matching task. Each experimental run lasted 120 s, in which the size of a blue target box (contour width = 8 pixels), which denoted the target force, randomly changed on average every 3.7s ± 2.2s (Fig. 1A). The target force was on average 1.58 N ± 0.13 N, ranging from 0.31 to 2.79 N.
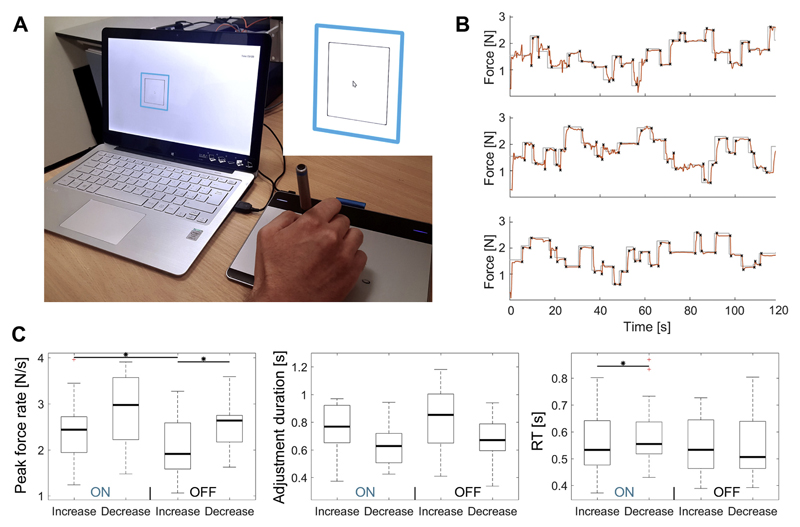
Fig. 1.
Examples of the pen force recordings. A Patients controlled the size of a black square on the screen to match a blue square by varying the force of a pen on a graphics tablet. In the example the force needs to be further increased to expand the black square. B The grey line with sharp jumps shows the target force and the red line shows the force applied by regulating the force of a pen on a graphics tablet. The black crosses show the start and end points of an adjustment. C Behavioural results: Peak (absolute) force rate, adjustment durations and reaction times (RT). For all measures, four pairwise comparisons were performed: ON force increase vs. ON decrease, OFF increase vs. OFF decrease, ON increase vs. OFF increase, ON decrease vs. OFF decrease. P-values are FDR-corrected to correct for multiple comparisons. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Patients were asked to control the size of a black box (contour width = 2 pixels) that was centered on the same point of the screen as the target box by varying the force of a pen on a graphic tablet (Wacom Intuos CTL-480, small). The box expanded with increasing force, and patients were instructed to match the size of the target box as precisely as possible. A similar setup has previously been introduced to assist rehabilitation protocols and assess motor performance (Confalonieri et al., 2012; Kirchner et al., 2011). The task was performed with the dominant hand, but if patients had severe tremor or rigidity in this hand, it was performed with the non-dominant one (in 6 of 18 patients). All patients were recorded ON medication, and a subset of 11 patients were also recorded OFF medication (5 STN, 6 GPi). 6 of the 11 patients were recorded first in the OFF medication condition to limit an order effect.
In each condition, four consecutive runs were recorded with breaks in-between ranging between several seconds to minutes depending on fatigue. Thus, patients performed the task for eight minutes in total, or twice this if both medication states were tested. In half of all runs, a red distractor box was present, which randomly differed in size from the target box. Patients were instructed to ignore the red box and focus on matching the size of the black box to the target box, which they successfully did. A demo of the task including the distractor box is shown in a video available on YouTube (https://www.youtube.com/watch?v=v7EbPjZB-dM). As we focus on motor dynamics in this study, we pooled the data across all runs.
2.3. Data recording
LFP and EEG signals were recorded with a TMSi Porti amplifier (2048 Hz sampling rate, TMS International, Netherlands). The timings of target changes were registered with a light-sensitive sensor attached to the corner of the presentation laptop. The software was programmed to produce a brightness pulse under the sensor at the onset of changes in the target size. The behavioural data (target force and applied force, sampled at 5 ms) were written to a separate text file stored on the presentation laptop and later merged with the LFP/EEG recordings in MATLAB (RRID:SCR_001622, v. 2016a, The MathWorks Inc., Natick, Massachusetts) by interpolating and resampling the behavioural data. EEG electrodes were placed over (or close to if sutures had to be avoided) Fz, Cz, Pz, Oz, C3 and C4 according to the international 10–20 system. For one patient, the electrode over ipsilateral motor cortex was intermittently inactive and thus had to be excluded.
2.4. Behavioural analysis
The force matching task required continuous control over hand and arm muscles. It consisted of stable periods and isometric force modulations for which a precise adjustment of muscle activity was required. Examples of the pen force recordings are shown in Fig. 1B. The start and end points of each force adjustment were tagged manually to ensure they were correctly identified. Sometimes these adjustments involved over- or undershoots into the opposite direction of the instructed force change. These were also tagged so they could be analysed separately. To get robust estimates of the central tendency of reaction times or the peak force rate, the median across trials was computed.
2.5. Analysis of EEG and LFP recordings
2.5.1. Frequency-band and channel selection
The data were first downsampled to 1000 Hz and artefacts in the LFP or EEG recordings were excluded by visual inspection (16% of the data). EEG electrodes over the contra- and ipsilateral motor cortex (C3 and C4) were re-referenced to the average of all recorded EEG channels (Fz, Cz, Pz, Oz, C3 and C4). To obtain spatially focal bipolar signals of the recorded LFPs, the difference between the raw signal of two neighbouring DBS electrode contacts was computed. If single channels saturated or were inactive (in 6 of 36 electrodes), the remaining surrounding contacts were subtracted instead. We included an additional bipolar configuration by calculating the difference between the lowest and highest contacts, as in some cases beta modulation across contiguous bipolar contacts was indistinct. For 8 of 36 electrodes the strongest modulation was in the configuration spanning the widest distance. To assess if modulation was present during force adjustments, we first computed the power for frequencies ranging from 5 to 45 Hz (in 1 Hz steps) and 55 to 100 Hz (in 5 Hz steps) using continuous Morlet wavelet transforms (fieldtrip-function ft_freqanalysis, RRID:SCR_004849, Oostenveld et al., 2011). The number of wavelet-cycles was 6 for frequencies below 50 Hz and 12 for frequencies above 50 Hz. To investigate in which frequency range the power modulation was strongest, for each subject the median across all force adjustments was computed, averaged across all bipolar signals for each electrode, and smoothed with a 0.1s sliding window. Significance tests based on a cluster-based permutation procedure for multiple comparison correction (see below) showed strongest modulation between 15 and 30 Hz just before the mid-point of the force adjustment (Fig. 2). To select one bipolar signal from each electrode for further analyses, only ON medication trials were considered as not all patients were recorded OFF medication and as beta modulation is stronger ON medication (Doyle et al., 2005). For each electrode, the channel with the strongest force adjustment-related 15–30 Hz beta decrease in this range was pre-selected. This was based on all trials in the ON medication condition irrespective of whether the force had to be increased or reduced. The channel with the highest modulation was identified by computing t-scores (across all adjustments) of 15–30 Hz beta power averaged across a −300:100 ms window around the mid-points of the adjustments, according to the significant cluster shown in Fig. 2. The instantaneous phase and power of 15–30 Hz beta oscillations was then extracted with a Hilbert-transform of the filtered data (Butterworth filter, filter order = 4, passed forwards and backwards, fieldtrip-function ft_preproc_highpassfilter and ft_preproc_lowpassfilter) to perform further analyses.
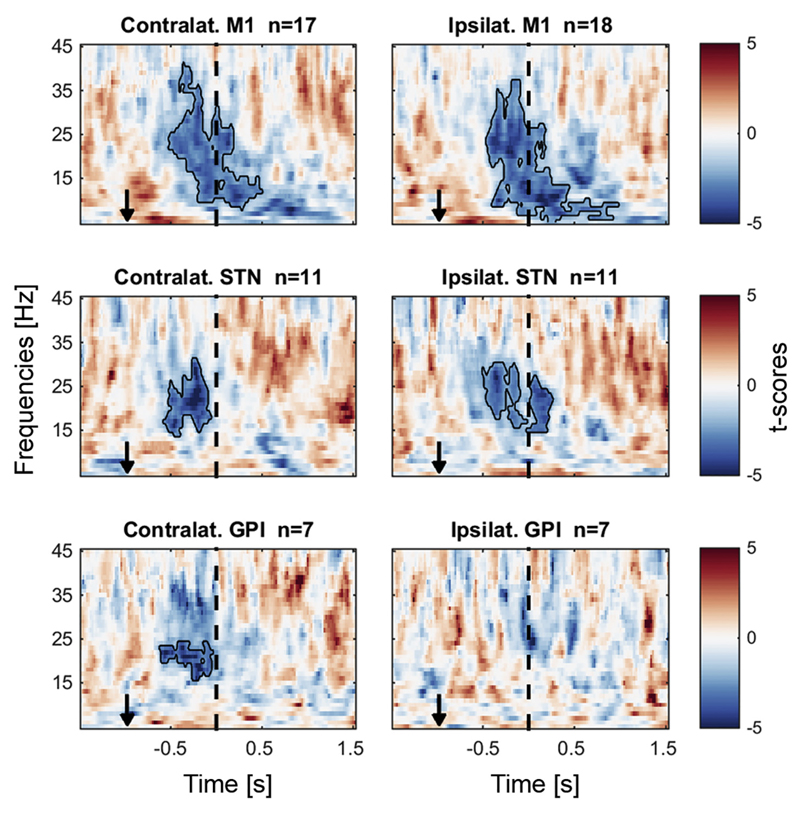
Fig. 2.
Beta power decreased at the time of a force adjustment. The data are aligned to the midpoint of each force adjustment, averaged across all bipolar channels in the ON medication condition. Power in each frequency band was normalized by the median of each session. The black outlines show significant clusters obtained with a cluster-based permutation procedure for multiple comparison correction (p < 0.05). Black arrows indicate the average time of the visual cue change.
2.5.2. Assessment of force adjustment-related power changes
To assess how power changed from the beginning to the end of a force adjustment despite variable durations, we subdivided each adjustment period into three segments: 1) 1 s before the adjustment started until the start, 2) from the start until the end, 3) from the end of the adjustment until 1 s afterwards. Each of these three segments was divided into 30 equidistant points, and then the median of beta power at these points was computed across all adjustments to get a robust estimate. Thus, for adjustments that took relatively long, the distances between the 30 points were larger than when they were short. This way, we evaluated how beta changed throughout different phases of the force adjustments, independent of how long they took. Before computing cluster-based permutation statistics (see below) smoothing was applied (moving average window size: 5 samples, MATLAB function smooth).
To test if beta increased significantly before completion of the adjustment, we computed Pearson's linear correlation coefficients for each patient based on the 30 points between the start and end of the force adjustment (x = bin number, y = beta power). The resulting coefficients were Fisher's Z-transformed and then tested against zero to find out if a relationship existed at the group level.
2.5.3. Correlation between beta power and force
In examples of individual trials, we noticed plateaus in the force adjustment when beta power was high, indicating slowing (Supplementary Fig. 1). To assess at the group level if beta power correlates with the absolute force rate (and as control analysis also with the force level) irrespective of where the increase in power occurred within an adjustment, we again divided each adjustment into 30 equal bins. This resulted in [nr. of adjustments*30] values for both variables, which were pooled to compute one within-subject correlation coefficient for each patient. The resulting correlation coefficients were Fisher's Z-transformed to test if they differed significantly from zero at the group level.
As we observed a beta power increase towards the end of an adjustment, just before the adjustment comes to a halt (thus coinciding more likely with a lower force rate), this correlation may be mainly driven by beta power being higher and force rates being lower towards the end. To show that this alone could not explain the correlation, we tested if the correlation is also significant compared to a permutation distribution, which was created by keeping the order of the 30 bins intact for each trial but permuting the association between beta power and the force measurement across trials. This ensured that beta power values from late parts of an adjustment were always paired with absolute force rates from another late part of an adjustment. After performing this permutation 500 times we tested if the original correlation coefficient was more extreme than the permuted ones (computing two-tailed p-values according to Ernst (2004)).
2.5.4. Assessment of cortico-basal ganglia coupling
Coupling strength was evaluated by computing inter-site phase-clustering values (ISPC, Lachaux et al., 2000) based on the differences in beta phase between the concomitant EEG and LFP recordings (LFPφt − EEGφt). The phase was obtained by computing the Hilbert-transform of the 15–30 Hz filtered signals. ISPC values correspond to the length of the average vector of phase differences represented as vectors with length one on a unit circle (n = number of time points):
Note that the amplitude of the signal does not contribute to the ISPC estimate. However, if beta power drops too low in one or both of the signals, phase is poorly defined and coupling will necessarily drop.
2.5.5. Probability of beta bursts during movements and average amplitude
We also investigated whether movement-related power changes were associated with modified beta burst probabilities or differences in beta burst peak amplitudes. Beta bursts were classified as periods where the 15–30 Hz amplitude exceeded a threshold defined as the 75% percentile of the resting baseline recorded ON medication (Tinkhauser et al., 2017). Only suprathreshold events exceeding a duration of 100 ms were classified as bursts because anything shorter would contain less than two beta cycles. We tested specifically how the probability and peak amplitude varied in the first and second half of the movement.
2.6. Statistical analyses
2.6.1. Cluster-based permutation test
All statistical tests for which multiple time bins or multiple time and frequency bins (as in Fig. 2) were tested were corrected for multiple comparisons by using a cluster-based permutation correction approach (Maris and Oostenveld, 2007): A permutation distribution was generated by swapping the sign of a random subset of pairwise differences 2000 times. Suprathreshold-clusters (pre-cluster threshold: P < 0.05) were obtained for the original unpermuted data and for each permutation sample by computing the z-scores relative to the permutation distribution. If the sum of the absolute z-scores within any original suprathreshold-cluster exceeded the 95th percentile of the sums of absolute z-scores from the 2000 largest suprathreshold-clusters obtained from the permutation distribution, it was considered statistically significant. The procedure corrects for multiple comparisons by comparing not each point individually but by comparing the largest number of contingent significant points, which should be lower in the permuted data if the cluster did not occur just by chance. Any other tests that required multiple comparison correction but did not contain contingent time- or frequency bins were corrected using false discovery rate (FDR) correction.
2.6.2. ANOVAs and pairwise comparisons
To ensure that pooling of the STN and GPi recordings was justified, we computed the following ANOVAs to assess if beta power was modulated similarly in recordings from the STN and the GPi: The first ANOVA only included data from the ON medication condition (n = 18) with nucleus as between-subjects factor and hemisphere (contra−/ipsilateral) and adjustment type (force increase or reduction) as within-subjects factor.
The second ANOVA only included the subset of patients for which both ON and OFF medication conditions were recorded (n = 11). It contained again the between-subjects factor nucleus (STN/GPi) and two factors (medication: ON/OFF, and adjustment: increase/reduction). If the sphericity assumption was violated, Greenhouse-Geisser correction was applied. Pairwise comparisons were performed with paired t-tests or Wilcoxon signed-rank tests (denoted as WSR-test) if the normality assumption was violated (assessed with a Lilliefors test).
2.6.3. Correlations between peak force rates, power and coupling
To investigate the relationship between peak force rates versus beta power or beta phase coupling ON and OFF medication, we computed robust Spearman's correlation coefficients. The resulting coefficients were Fisher's Z-transformed before testing if they were significantly different from zero on a group level. Additionally, we controlled for the relative contribution of power changes by computing partial correlations between ISPC values and peak force rates.
3. Results
3.1. Behavioural results
The peak force rate was higher when the force was reduced than when it was increased. This was found to be statistically significant when patients were OFF medication (p = 0.011, t10 = −3.1). It was not strictly statistically significant, but a trend was also present ON medication (p = 0.065, t17 = −2.0, Fig. 1C). Comparing peak force rates between the ON and OFF medication condition, we found that the peak rate during force increases was significantly higher when patients were medicated (p = 0.017, t10 = 2.9). The peak rate during force decreases did not differ significantly between medication states (p = 0.278, t10 = −1.1).
ON medication, the average duration of the adjustment was 835 ms and 645 ms for force increases and reductions respectively. OFF medication, it was 824 ms and 676 ms (no significant differences after FDR correction for multiple comparisons, Fig. 1C). Reaction times were significantly faster for force increases compared to reductions when patients were ON medication (ON: p = 0.005, t17 = −3.3; OFF: p = 0.895, t10 = −0.1, Fig. 1C).
The average force preceding force increases was 1.30 ± 0.12 and 1.83 ± 0.17 N when the adjustment was completed, while for force reductions it was 1.81 ± 0.16 and 1.26 ± 0.12 N. The average amount of force change was similar for force increases and reductions (increases: 0.51 ± 0.05 N, reductions: 0.50 ± 0.05 N).
3.2. Electrophysiology
3.2.1. 15–30 Hz beta power (averaged across all channels) decreased during force adjustments
As a first step, before focussing on the bipolar signals with the strongest beta modulation, we investigated LFP power changes in the ON medication condition averaged across all bipolar channels and all adjustments (force increases and reductions). We aligned the data to the midpoint of each force adjustment (= the mid-point between two black crosses in Fig. 1B) and found a significant power decrease relative to the median power of each session in all contralateral structures, and even in the ipsilateral STN/GPi and M1 (Fig. 2). As the power decrease in subcortical structures was most pronounced between 15 and 30 Hz just before the midpoint of the force adjustment, we focussed all further analyses on channels showing the strongest 15–30 Hz beta decrease within a −300:100 ms window around the midpoint of the force adjustments. These contacts are presumed to have picked up activity related to motor control, and thereby to be closest to the dorsolateral sensorimotor region of the STN (Horn et al., 2017). In the OFF medication condition recorded in a subgroup, a similar beta power decrease occurred (Supplementary Fig. 2). Also when the data is aligned to the cue change, the beta decrease looks similar (Supplementary Fig. 3). No significant power modulation was observed in the gamma frequency range (Supplementary Fig. 4).
3.2.2. During force adjustments, beta power was lower than the resting baseline activity
We tested whether beta power during force adjustments was suppressed relative to a baseline activity at rest (recorded in both ON and OFF medication states). Power obtained from the −300:100 ms window around the midpoint of the force adjustments (as used for channel pre-selection and regardless of adjustment direction) was significantly suppressed in all channels in the ON medication condition (contra LFP: −14%, p = 0.013, t17 = −2.8; ipsi LFP: −13%, p = 0.007, t17 = −3.1; contra M1: −12%, p = 0.037, t17 = −2.3; ipsi M1: −17%, p = 0.007, t16 = −3.1) and in all channels apart from the ipsilateral STN/GPi in the OFF medication condition (contra LFP: −12%, p = 0.014, WSR-test (n = 11); ipsi LFP: −3.0%, p = 0.102, WSR-test (n = 11); contra M1: −20%, p < 0.001, t10 = −5.8; ipsi M1: −21%, p = 0.003, t9 = −4.0).
We also compared if the holding period, within which a stable force had to be applied, differed from the resting baseline. A 400 ms long window starting 400 ms before the cue changed was examined. No significant differences between the stable period and the resting baseline were found (ON: contra LFP: −0.5%, p = 0.939; ipsi LFP: −7.1%, p = 0.286; contra M1: −3.3%, p = 0.521; ipsi M1: −9.2%, p = 0.112; OFF: contra LFP: 5%, p = 0.831; ipsi LFP: 3%, p = 0.520; contra M1: −5.5%, p = 0.285; ipsi M1: −9.3%, p = 0.215).
For all further analyses, for example contrasting force increases and decreases, we obtained relative within-condition power changes by normalizing the data to the median of each recording session to minimize heteroscedasticity between conditions.
3.2.3. Beta power decreased more when the force was reduced than when it was increased, and behaved similarly in the two nuclei
Next, we assessed if power changes differed when the force was increased or reduced. We computed a 2 × 2 repeated-measures ANOVA with the adjustment-related beta power change as dependent variable (averaged within the −300:100 ms window around the midpoint of force adjustments, normalized within each session), and subcortical nucleus (STN/GPi) as between-subjects factor (ON medication only). The two within-subjects factors were change direction (CD: force increase/reduction) and hemisphere (HS: contra/ipsilateral). The ANOVA resulted only in a significant main effect of hemisphere. An interaction between change direction and hemisphere only came close to being significant (HS: p = 0.025, CD: p = 0.204, CD *nucl: p = 0.325, CD*HS: p = 0.080, HS*nucl: p = 0.683, CD*HS*nucl: p = 0.853, nucl: p = 0.819, see Supplementary Table 2 for F-statistics). When directly comparing the two beta power time courses using a cluster-based permutation procedure to correct for multiple comparisons over time, we saw a difference between force increases and reductions in the contralateral LFP (Fig. 3, red shaded areas): Beta power stayed more strongly suppressed throughout the adjustment when the force was reduced compared to when it was increased. Considering that the peak force rate during force reductions was faster than during increases (Fig. 1C), the observed power difference may be related to differences in adjustment speed, which will be investigated in more detail below.
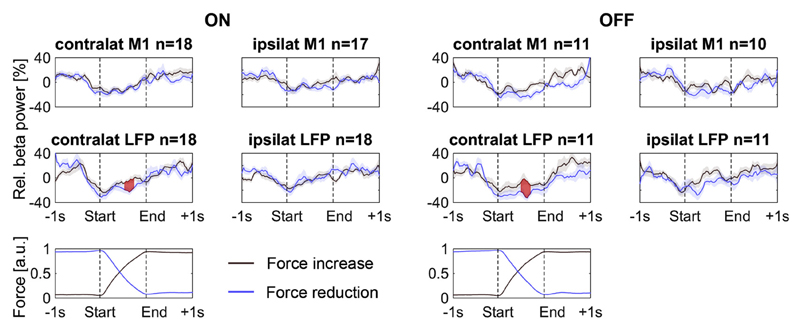
Fig. 3.
Baseline-normalized beta power decreased at the beginning of a force adjustment, regardless of direction, but gradually increased already before the adjustment was fully completed. Force increases and reductions were compared with a cluster-based permutation procedure to correct for multiple comparisons over the full time period and significant differences in beta power are shown as shaded areas in red. Relative beta power was suppressed by both force increases and reductions, but in the contralateral STN/GPi it stayed significantly more suppressed throughout the adjustment when the force was reduced. Note that the actual time that passed between the start and end of the adjustments was less than one second and varied across trials (see Methods on the temporal subdivision of the force adjustments). The force traces have been normalized between 0 and 1 before averaging. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A second ANOVA was computed to compare if the relative beta power suppression differed between the two medication states in the reduced subset of patients for which both ON and OFF medication conditions were recorded (n = 11). It contained again two factors (MED: ON/OFF, and CD: increase/reduction) and the between-subjects factor nucleus (STN/GPi). The dependent variable was the amount of beta suppression in the contralateral STN/GPi. Only the main effect CD was significant (CD: p = 0.007; MED: p = 0.908, MED*nucl: p = 0.947, CD*nucl: p = 0.402, CD*MED: p = 0.436, CD*MED*nucl: p = 0.105, nucl: p = 0.646). The fact that none of the effects involving the between-subjects factor nucleus were significant suggests that beta modulation related to the force adjustment was similar in the STN and the GPi. This similarity is also shown in Supplementary Fig. 5, which depicts the beta power time courses separately for the two subsets of STN and GPi recordings.
3.2.4. Trial-averaged beta power gradually began recovering from suppression already before the force adjustment was completed
Fig. 3 shows that beta power dropped already about 300 ms before the force adjustment started. Beta power suppression was maximal at the beginning of the force adjustment and began to recover gradually immediately afterwards – already before the change in force was completed.
To assess if beta significantly began recovering before the force change was completed, we computed Pearson's correlation coefficients for each patient based on the power at the 30 points between the start and end of the adjustment (x: bin number, y: beta power). We first tested if the Fisher's Z-transformed correlation coefficients differed from zero at the group level in the ON medication condition and included all adjustments of the isometric contraction irrespective of whether the force was increased or reduced. The coefficients were positive and significantly different from zero, showing a significant gradual increase (contra LFP: Z(R) = 1.6, p = 0.006, t17 = 3.1, ipsi LFP: Z(R) = 2.3, p < 0.001, t17 = 4.6, contra M1: Z(R) = 1.7, p = 0.002, t17 = 3.6, ipsi M1: Z(R) = 1.2, p = 0.029, t16 = 2.4). This demonstrates that in all regions, beta power began recovering before the force adjustment was completed.
To test if the gradual beta recovery differed between change directions, hemispheres and nuclei, we performed again a 2 × 2 ANOVA with GPi/STN as between-subjects factor. No significant main effects or interactions were found (see Supplementary Table 2). The 2*2 ANOVA with the reduced subset of patients and factors medication (ON/OFF) and change direction (increase/reduced) also showed no significant effects (see Supplementary Table 2). This suggests that the gradual beta power recovery before the adjustment was completed was generally present during force adjustments, irrespective of direction. As the adjustment slows down before reaching the correct force level, this also indicates that high beta power may be related to the absolute change in force – the absolute force rate.
3.2.5. Beta power recovery before the force adjustment was completed was associated with an increased burst probability
Fig. 3 shows that the average beta power was higher towards the end of a pressure change compared to the beginning but it is not clear if the average increase is due to an increased probability of high-amplitude beta events (= beta bursts) or an increased peak amplitude of these events. To help distinguish between these two options, we investigated how beta burst probability and peak amplitude varied from the beginning to the end of each pressure change in the contralateral LFP. We found that the probability of beta bursts was significantly higher in the second half compared to the first (ONH2-H1: 3.0%, p = 0.034, t17 = 2.3; OFFH2-H1: 6.7%, p = 0.008, WSR-test (n = 11)) and that the peak amplitude of these events did not differ (ONH2-H1: 47%, p = 0.227, t16 = 1.3; OFFH2-H1: 57%, p = 0.224, t8 = 1.3; dfs were reduced here as some halves did not contain any bursts).
3.2.6. Low force rates coincided with high beta power
Examples of single trials illustrated that high beta power could coincide with lower force rates not just towards the end but also midway through a force adjustment (Supplementary Fig. 1). Correlations between beta power and the simultaneously measured absolute force rate (see Methods section on Correlation between beta power and force) were highly significant at the group level (ON: mean rho = −0.06, p < 0.001, t17 = −4.7; OFF: mean rho = −0.08, p = 0.009, t10 = −3.3). The negative correlation shows that when beta power was high, the absolute force rate tended to be low.
We also tested if this correlation was significant against a permutation distribution that controls for the tendency of beta power being high towards the end of an adjustment, where slowing was often observed. It was still significant (ON: perm. p = 0.002; OFF: perm. p = 0.002).
Finally, we repeated the same analysis using the force level instead of the absolute force rate. No significant correlation was present for the force level (ON: mean rho = 0.01, p = 0.184, WSR-test (n = 18), perm. p = 0.262; OFF: mean rho = 0, p = 0.996, t10 = 0, perm. p = 0.956).
3.2.7. Beta power was higher before the correct force level was reached when it was not followed by overshoot
The increased amplitude of beta oscillations before the end of a force adjustment may play a role in slowing down and stabilizing muscle activity just before the correct force level is reached. If this was the case, beta power should be relatively reduced when a force adjustment ended with an overshoot. Indeed, when we compared beta power within the final 400 ms before the force adjustment ended (when no overshoot was present, versus the 400 ms before the force change reversed when the target force was overshot), we found that beta power in the contralateral STN/GPi was significantly lower when the target was overshot in both medication conditions (ON: p = 0.001, t17 = −4.0, OFF: p = 0.016, t10 = −2.9, also see Supplementary Fig. 6). ON medication, this was also the case in the contralateral M1 and ipsilateral LFP (ON: contra M1: p = 0.021, t17 = −2.5, ipsi LFP: p = 0.006, t17 = −3.2). Note that adjustments that ended with an overshoot were less common than those that ended more accurately (% overshoot trials ON: 41%, OFF: 36%).
In a similar vein, we also assessed if trials, in which beta activity was high, resulted in less overshoot at the end of a force adjustment. We median-split trials into high and low beta power trials, and found that this was the case for the contralateral LFP and M1 (ON: contra LFP: p = 0.035, WSR-test (n = 18), ipsi LFP: p = 0.058, WSR-test (n = 18); contra M1: p = 0.008, t17 = 3.0, ipsi M1: p = 0.300, t16 = 1.1; OFF: contra LFP: p = 0.009, t10 = 3.2, ipsi LFP: p = 0.496, t10 = 0.7, contra M1: p = 0.100, t10 = 1.8, ipsi M1: p = 0.048, t9 = 2.3).
Note, though, that the force adjustments that ended with an overshoot were also distinct in other properties. Overshoots were stronger when the peak force rate was higher (both p < 0.001, ON t17 = 7.5, OFF t10 = 5.4, pooled across increases and reductions) and when the amount of force change was smaller (both p < 0.001, ON t17 = −4.3, OFF t10 = −6.4).
3.2.8. Beta M1-STN/GPi phase coupling decreased at the onset of force adjustments when dopamine levels were relatively restored
Several studies have shown significant coupling between motor cortical regions and the basal ganglia at beta frequencies (Hirschmann et al., 2011; Litvak et al., 2011; Oswal et al., 2016; Tinkhauser et al., 2018; van Wijk et al., 2017). Thus, the difference in peak force rate between the ON and OFF medication condition may also be accompanied by differences in coupling between M1 and the basal ganglia nuclei. Phase coupling strength was quantified by computing inter-site phase clustering (ISPC) values across time separately for each trial (see Methods).
First, we tested if coupling strength was significantly higher than that observed by chance by comparing the original ISPC values against a null-distribution created by shuffling the trial-to-trial-association between the LFP and EEG signals 500 times. For example, the LFP signal from trial 1 was paired with the EEG signal from trial 5. For each of the 500 permutations, the trial order of the EEG signal was shuffled before re-pairing it with the LFP signal. ISPC values were computed within 600 ms long windows centred around the following five points: 1 s before the start of the force adjustment, at the start, at the mid-point of the adjustment, at the end, and 1 s after the adjustment ended. 600 ms was chosen as length, because if beta oscillations in both sites are relatively regular for several cycles, permutation-ISPC values would also be relatively high (despite permuting the trials) when windows are small and contain only a small number of cycles.
Fig. 4A shows significant coupling 1 s before the force adjustments started and immediately afterwards in both the ON and OFF medication conditions. When dopamine levels were relatively restored (ON medication), beta coupling decreased at the onset of the adjustment to a level where coupling strength did not exceed the chance level (p-value above 0.05). When dopamine levels were low in the OFF medication condition, the reduction in coupling was less pronounced and that which did occur was delayed to around the mid-points of the force adjustments.
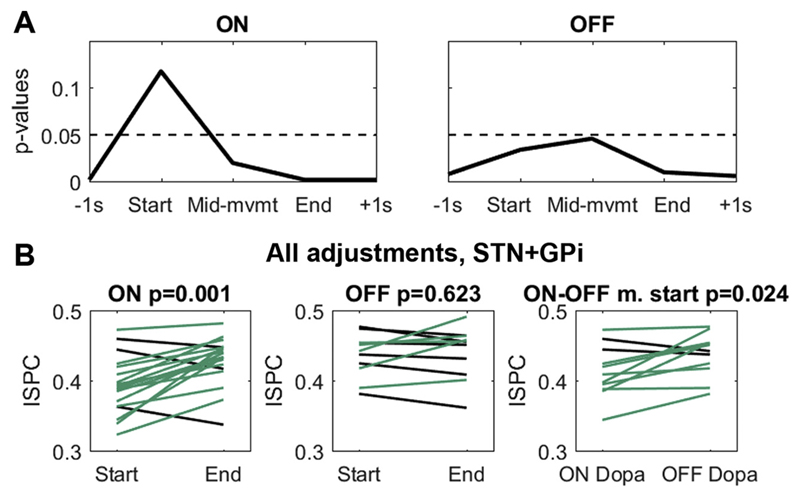
Fig. 4.
Contralateral M1-STN/GPi beta phase coupling. A P-values below 0.05 show that coupling was significant compared to a permutation distribution (also after FDR-correction). Note that ON medication (left), decoupling (where p > 0.1) took place earlier, already at the start of an adjustment, than OFF medication (right), where decoupling was weaker and most pronounced in the middle of the adjustments. B When patients were ON medication, phase coupling between the contralateral BG nuclei and M1 was significantly reduced in the first 200 ms of a force adjustment compared with the final 200 ms (left). Positive differences are plotted in green, negative differences in black. The degree of decoupling in these first 200 ms was significantly stronger ON medication compared with OFF medication (right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next, we tested directly if coupling was significantly lower at the beginning of force adjustments compared to the end (pooled across force increases and reductions) and if this reduction in coupling at the onset of adjustments, also further referred to as “decoupling”, was stronger ON medication. ISPCs were computed for each trial in a 200 ms window directly after the adjustment began and in a 200 ms window directly before it ended. We found that ON medication, coupling was significantly lower at the beginning of the adjustment compared to the end (Fig. 4B, ON: p = 0.001, t17 = −4.2; OFF: p = 0.623, t10 = −0.5). Additionally, when directly comparing ON versus OFF medication, the amount of decoupling at the beginning was significantly stronger ON medication (StartON-OFF: p = 0.024, t10 = −2.7). This difference was again present to a similar extent in both basal ganglia nuclei (two-sample t-test on the ON-OFF ISPC differences between the GPi and STN: p = 0.467, t9 = 0.76).
Beta decoupling at the onset of force adjustments was similar for increases and reductions (Supplementary Fig. 7), but the difference in coupling strength between ON and OFF medication seemed to be more pronounced for force increases, which was also where the behavioural difference in the peak force rate was found. The differences in coupling strength were specific for the contralateral hemisphere as no differences were present in coupling between beta from the contralateral LFP and ipsilateral M1 or the ipsilateral LFP and ipsilateral M1 (Supplementary Fig. 8 + 9).
3.2.9. When the peak force rate was high, cortico-basal ganglia beta phase-coupling was reduced – but only ON medication
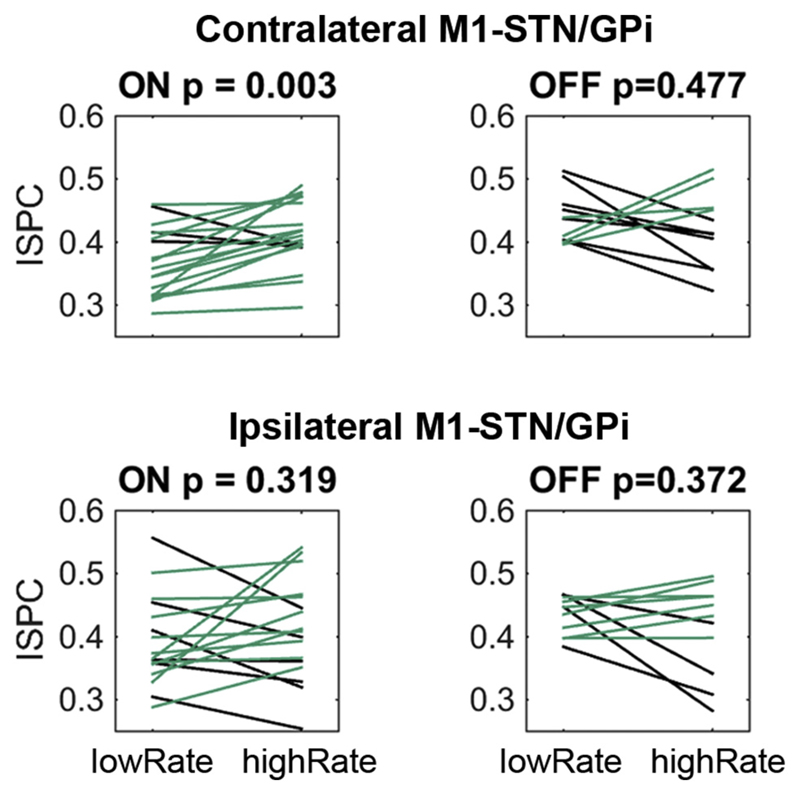
To test if the peak force rate itself was related to coupling strength, we median-split trials into slow and fast force adjustments. The median-split was performed separately for the ON and OFF medication condition. We only included trials in which the force was changed into the correct direction without any inadvertent initial changes into the wrong direction. Coupling was much reduced during faster compared to slower force adjustments, which was again specific to the ON medication condition and the contralateral hemisphere (Fig. 5, ON p = 0.003, t17 = −3.4, OFF p = 0.477, t10 = 0.7). This difference was not just due to the reduced sample size in the OFF medication condition, because for a reduced set of patients the difference still was significant in the ON medication recordings (subset ON: p = 0.042, t10 = −2.3).
Fig. 5.
Low and high peak force rates relate to differences in coupling strength ON medication, and to differences in local beta power OFF medication. Only contralateral M1-STN/GPi coupling was significantly lower when force adjustments were rapid and patients were medicated (top left). Positive differences are plotted in green, negative differences in black. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Note that the difference in peak force rate between fast and slow trials also was significantly higher ON medication (mean difference ON = 2.0 N/s, OFF = 1.7 N/s, p = 0.012, t10 = 3.1), indicating a broader range of force rates.
When comparing beta power instead of beta coupling between trials with low and high peak force rates, a power difference in the contralateral STN/GPi was only observed OFF medication: Local beta power was earlier and more strongly suppressed when the adjustment was faster (Supplementary Fig. 10).
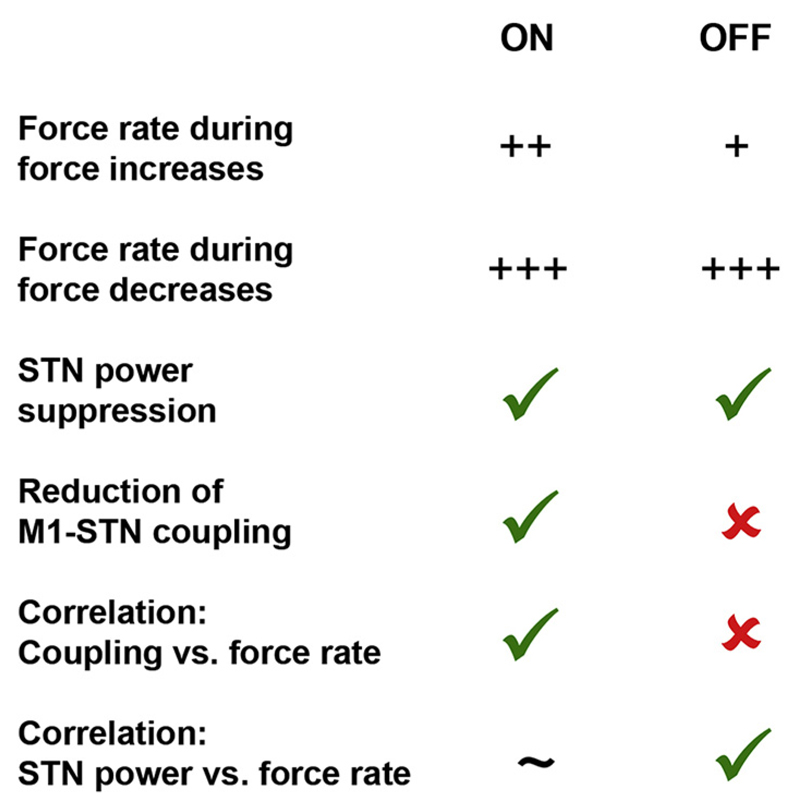
To corroborate our finding of a relationship between the absolute rate of the force adjustment and inter-regional coupling, we computed within-subjects correlations between the peak force rates and ISPCs as well as local power (in a 0.2s long window starting at the onset of the force adjustment). In line with the above findings, the second-level test to see if the correlations significantly differed from zero showed that ON medication, the peak force rate was significantly correlated with the coupling strength (ON: mean rho = −0.07, p = 0.028, t17 = −2.4), which was not the case OFF medication (OFF: mean rho = 0.05, p = 0.364, t10 = 1.0). Instead, OFF medication, the peak force rate correlated consistently with power in the contralateral STN/GPi (mean rho = −0.14, p = 0.003, t10 = −3.9) and slightly less with power in contralateral M1 (mean rho = −0.12, p = 0.045, t10 = −2.3). These correlations with beta power were not significant when patients were ON medication (contra LFP: mean rho = −0.07, p = 0.054, t17 = −2.1, contra M1: mean rho = −0.04, p = 0.329, t17 = −1.0). A summary of all key findings is shown in Fig. 6.
Fig. 6.
Summary of the key findings. The force rate during force increases, but not decreases, was significantly higher ON versus OFF medication (see also Fig. 1C). STN beta power was significantly suppressed at the onset of the adjustment in both conditions but M1-STN beta phase coupling was only reduced ON medication. The correlation between beta phase coupling and absolute force rate also was only significant ON medication. Conversely, the correlation between STN beta power and force rate was significant OFF medication, with only a trend in this direction ON medication.
Finally, to test if the ON-medication correlation between the peak force rate and ISPCs would be diminished when controlling for local power, we computed partial correlations. The effect diminished only slightly when controlling for power from the contralateral M1 (mean rho = −0.06, p = 0.037, t17 = −2.3) and the contralateral STN/GPi (mean rho = −0.06, p = 0.064, t17 = −2.0).
These findings suggest distinct effects of dopamine depletion on cortico-basal ganglia beta coupling and local beta power. Altogether, this indicates that when dopamine levels are low, beta coupling between the contralateral M1 and the basal ganglia is less flexibly modulated, associated with a reduced dynamic range of how fast the adjustments were performed. When patients were off medication, long-range coupling was not significantly modulated, which was not merely related to the reduced sample size. Instead, low and high peak force rates were accompanied by differences in local beta synchronization, particularly in the contralateral STN/GPi.
Taken together, beta power and beta coupling were relatively suppressed at the onset of force adjustments but already began recovering before they ended and postural stabilization set in. Power suppression was strongest when the force was reduced, which was performed faster. Cortico-basal ganglia beta phase decoupling at the onset of force adjustments was significant only ON medication, thus after dopamine withdrawal the flexibility of cortico-basal ganglia coupling was strongly reduced.
3.2.10. Control analyses
Finally, we performed several control analyses. Larger force adjustments tend to be performed faster, which was also the case in our task (correlation between the amount of force change and peak force rates: mean rho ON = 0.52, OFF = 0.56, both p < 0.001). But importantly, no beta power differences were found in the contralateral LFP when splitting trials into small and large force changes (Supplementary Fig. 11). The strength of cortico-basal ganglia beta coupling also was not significantly modulated by the amount of force change (Supplementary Fig. 12).
Larger force changes also tended to take longer (mean rho ON = 0.44, OFF = 0.39, both p < 0.001). Additionally, the rate of the force adjustments and their duration was anti-correlated: When force adjustments were performed slowly, they took longer (mean rho ON = −0.22, OFF = −0.25, both p < 0.001). When dividing them into adjustments with short and long durations, a difference similar to the one when median-splitting trials according to the peak force rate was found in the OFF medication condition: Beta power was higher when the adjustment took longer (and when they were slower, Supplementary Fig. 13). However, cortico-basal ganglia coupling was not significantly modulated by differences in duration (Supplementary Fig. 14).
Finally, we evaluated if the peak force rate differed between trials in which a visual distractor stimuli (a second box coloured red instead of blue) was present or not. No significant differences were found (ON increase: p = 0.173, t17 = 1.4; ON reduction: p = 0.616, WSR-test (n = 18), OFF increase: p = 0.753, t10 = −0.3; OFF reduction: p = 0.655, t10 = −0.5).
4. Discussion
Our task required patients to perform relatively small, finely controlled force adjustments while continuously maintaining an active muscle tone to hold the pen and apply the visually cued force level. We found that beta LFP power in the STN/GPi and cortico-basal ganglia phase coupling was suppressed at the onset of force adjustments and that the change was greater ON dopaminergic medication than OFF medication, in line with previous reports (Androulidakis et al., 2007; Devos et al., 2006; Doyle et al., 2005).
We also showed a gradual beta power increase before the force adjustment was completed, which may be linked to the controlled nature of the cued adjustments, as will be discussed in more detail below.
4.1. Beta power suppression does not relate to the absolute applied force but to the force rate
Beta power suppression occurs during movement and has been shown to be larger for actions that generate higher force levels (Fischer et al., 2017a; Tan et al., 2015, Tan et al., 2013). However, rather than being inversely related to the force levels, we found beta suppression to be related to the absolute force rate for both force increases and reductions. Thus, beta oscillations may be related to a directionless measure, such as the rate of any change in force or, more broadly, the movement effort – or subjective gain of a motor command (Tan et al., 2015). In addition, in our experiment the peak force rate was faster when the force was reduced compared to when it was increased. Beta power decreased more strongly when the force was reduced, consistent with the hypothesis that beta power suppression reflects the absolute force rate rather than the force level.
4.2. Beta activity returned before the force adjustments were completed and was associated with a reduced force rate and more precise completion
For both directions of force adjustments, we saw a gradual recovery of beta power before the adjustment was completed in the trial average. Our paradigm was remarkable for the controlled nature of isometric force adjustments – performance accuracy was stressed by the examiner, and visual feedback was continuously provided to enable patients to produce the cued target force with high accuracy. Accordingly, force adjustments took on average longer than 600 ms, despite the relatively small changes in force. The fact that an increase in beta activity occurred before the adjustment was completed raises two non-exclusive possibilities. First, it may play an active role in slowing down the adjustment and, in line with this, the elevated STN/GPi beta power coincided with a slowing of the force rate. Second, the increase in beta power occurring before the force adjustment was completed may play a role in the integration of visual and proprioceptive feedback to achieve accurate visuo-motor control (Tan et al., 2014b). The latter idea stems from studies on sensorimotor adaptation (Tan et al., 2016, Tan et al., 2014b; Torrecillos et al., 2015), which have led to the hypothesis that a post-movement increase in beta activity is linked to integration of sensory information and updating of an internal forward model (Cao and Hu, 2016). Both ideas are consistent with our observation that beta power was lower before patients overshot the target force, i.e. when they failed to stabilize the adjustment fast enough. In the periods of sustained, stable isometric contraction, beta power was not significantly suppressed relative to baseline beta activity recorded at rest. This again implies that suppression of beta oscillations does not merely occur when muscles are tonically contracted but only when the strength of the contraction is adjusted.
4.3. Cortico-basal ganglia beta phase coupling was more readily modulated ON medication
We also investigated changes in long-range motor-cortical-basal ganglia beta phase coupling and found evidence to suggest different organisation of processing ON and OFF medication. We demonstrated that beta phase coupling was significantly reduced at the start of the force adjustments when patients were ON medication. This was specific to M1-STN/GPi coupling in the contralateral hemisphere. Stronger decoupling was associated with a higher peak force rate when patients were ON medication. OFF medication, we only saw differences in local STN/GPi power. This suggests that cortico-basal ganglia beta coupling is more dynamic when dopamine levels are relatively restored. The improved flexibility in coupling in turn may underscore the larger dynamic range of force rates observed in the ON medication state.
Notably, ON medication, slow and fast force adjustments were not associated with pronounced differences in local beta power. This points towards the relative independence between long-range phase coupling and local beta activity, which may be unmasked in this study because of the special nature of the fine motor control task.
An interesting observation is that early symptoms of Parkinson's disease often include handwriting impairments (Pinto and Velay, 2015; Rosenblum et al., 2013). Handwriting requires fine control over the hand. Considering that we observed an impaired dynamic range of task-related cortico-basal ganglia beta phase decoupling when dopamine levels were low, an early feature of Parkinson's disease may be pathological alterations in the dynamic range of coupling that may cause early impairments of fine motor control in the absence of gross motor symptoms.
4.4. Limitations
Several limitations in our study are worth highlighting. First, we cannot establish causality in any of the relationships evidenced in this observational study. Second, we recorded motor cortical EEG activity and local field potentials from the STN or the GPi, depending on each patient's implantation target. As none of our comparisons indicated differences between the STN and the GPi, in agreement with previous reports of similar beta modulation between the two structures (Brücke et al., 2012; Joundi et al., 2012), we did not distinguish between the two sites. However, it could be argued that we were underpowered to detect any but the biggest differences in LFP reactivity between the two targets. Third, as we were primarily interested in the force dynamics, we pooled the data across all trials irrespective of the presence of a visual distractor. However, to be sure that this was not a confounding factor, we computed a control analysis and showed that the presence of a distractor did not result in significant differences in force rates. Fourth, we cannot categorically ascribe the beta band changes observed here to the STN or GPi alone. This is particularly the case for the eight wide-field bipolar signals that were included in a small group of subjects because beta modulation was more pronounced than in more focal contiguous bipolar contacts.
Finally, we did not detect any significant gamma power increase in our task, probably because the required force adjustments were too small (Tan et al., 2013). Past studies involving large, ballistic movements have related STN gamma and not beta power to movement speed (Joundi et al., 2012; Lofredi et al., 2018). Our continuous low-force tracking task may have made it possible to detect more subtle changes in beta activity while larger movements might quickly result in a floor effect of beta power suppression.
5. Conclusions
We have demonstrated that small, controlled force adjustments are accompanied by initial beta desynchronization followed by increased beta activity closer to completion of the adjustment. Cortico-basal ganglia beta phase coupling was significantly reduced at the start of an adjustment, but only ON and not OFF medication, suggesting that the dynamic range of cortico-basal ganglia coupling is impaired during dopamine withdrawal. Beta power suppression and phase decoupling was most closely linked to the rate of the force adjustments and not the force level per se. The appearance of beta synchronization instead may be linked to the timely and precise stabilization of force that is required to perform the present visuomotor force matching task.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2019.03.004.
Acknowledgements
This work was supported by the Medical Research Council [MC_UU_12024/1] and the EU [FP7-ICT 610391]. PB was further funded by the National Institute of Health Research Oxford Biomedical Research Centre. HT was additionally funded by Medical Research Council [MR/P012272/1]. The Unit of Functional Neurosurgery is supported by the Parkinson Appeal UK, and the Monument Trust.
Abbreviation
- DBS
Deep brain stimulation
- EEG
Electroencephalogram
- GPi
Internal globus pallidus
- ISPC
Intersite phase clustering
- LFP
Local field potential
- STN
Subthalamic nucleus
Footnotes
Competing interests
The authors declare no competing interests
References
- Androulidakis AG, Kühn AA, Chen CC, Blomstedt P, Kempf F, Kupsch A, Schneider G-HH, Doyle L, Dowsey-Limousin P, Hariz MI, Brown P. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson's disease. Brain. 2007;130:457–468. doi: 10.1093/brain/awl358. [DOI] [PubMed] [Google Scholar]
- Brücke C, Kempf F, Kupsch A, Schneider GH, Krauss JK, Aziz T, Yarrow K, Pogosyan A, Brown P, Kühn AA. Movement-related synchronization of gamma activity is lateralized in patients with dystonia. Eur J Neurosci. 2008;27:2322–2329. doi: 10.1111/j.1460-9568.2008.06203.x. [DOI] [PubMed] [Google Scholar]
- Brücke C, Huebl J, Schonecker T, Neumann W-J, Yarrow K, Kupsch A, Blahak C, Lutjens G, Brown P, Krauss JK, Schneider G-H, et al. Scaling of movement is related to Pallidal oscillations in patients with dystonia. J Neurosci. 2012;32:1008–1019. doi: 10.1523/JNEUROSCI.3860-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Hu Y-M. Beta rebound in Visuomotor adaptation: still the status quo? J Neurosci. 2016;36:6365–6367. doi: 10.1523/JNEUROSCI.1007-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Confalonieri M, Guandalini G, Da Lio M, De Cecco M. Force and touch make video games “serious” for dexterity rehabilitation. Stud Health Technol Inform. 2012;177:139–144. [PubMed] [Google Scholar]
- Da Silva JA, Tecuapetla F, Paixão V, Costa RM. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature. 2018;554:244–248. doi: 10.1038/nature25457. [DOI] [PubMed] [Google Scholar]
- Devos D, Szurhaj W, Reyns N, Labyt E, Houdayer E, Bourriez JL, Cassim F, Krystkowiak P, Blond S, Destée A, Derambure P, et al. Predominance of the contralateral movement-related activity in the subthalamocortical loop. Clin Neurophysiol. 2006;117:2315–2327. doi: 10.1016/j.clinph.2006.06.719. [DOI] [PubMed] [Google Scholar]
- Doyle LMF, Kühn AA, Hariz M, Kupsch A, Schneider GH, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson's disease. Eur J Neurosci. 2005;21:1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: a basis for exact inference. Stat Sci. 2004;19:676–685. doi: 10.1214/088342304000000396. [DOI] [Google Scholar]
- Fischer P, Pogosyan A, Cheeran B, Green AL, Aziz TZ, Hyam J, Little S, Foltynie T, Limousin P, Zrinzo L, Hariz M, et al. Subthalamic nucleus beta and gamma activity is modulated depending on the level of imagined grip force. Exp Neurol. 2017a;293:53–61. doi: 10.1016/j.expneurol.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, Pogosyan A, Herz DM, Cheeran B, Green AL, Fitzgerald J, Aziz TZ, Hyam J, Little S, Foltynie T, Limousin P, et al. Subthalamic nucleus gamma activity increases not only during movement but also during movement inhibition. Elife. 2017b;6:1–21. doi: 10.7554/eLife.23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltynie T, Hariz MI. Surgical management of Parkinson's disease. Expert Rev Neurother. 2010;10:903–914. doi: 10.1586/ERN.10.68. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13-35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann J, Özkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson's disease. Neuroimage. 2011;55:1159–1168. doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Horn A, Neumann W-J, Degen K, Schneider G-H, Kühn AA. Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Brittain JS, Green AL, Aziz TZ, Brown P, Jenkinson N. Oscillatory activity in the subthalamic nucleus during arm reaching in Parkinson's disease. Exp Neurol. 2012;236:319–326. doi: 10.1016/j.expneurol.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A. The ups and downs of beta oscillations in sensorimotor cortex. Exp Neurol. 2013;245:15–26. doi: 10.1016/j.expneurol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Kirchner M, De Cecco M, Confalonieri M, Da Lio M. A joint force-position measurement system for neuromotor performances assessment. MeMeA 2011 - 2011. IEEE Int Symp Med Meas Appl Proc; 2011. pp. 583–586. [DOI] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Rodriguez E, Le van Quyen M, Lutz A, Martinerie J, Varela FJ. Studying single-trials of phase synchronous activity in the brain. International Journal of Bifurcation and Chaos. 2000;10:2429–2439. [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Lofredi R, Neumann WJ, Bock A, Horn A, Huebl J, Siegert S, Schneider GH, Krauss JK, Kuühn AA. Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson's disease. Elife. 2018;7:1–22. doi: 10.7554/eLife.31895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Beudel M, Zrinzo L, Limousin P, Hariz M, Foltynie T, Litvak V, Brown P. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson's disease. Brain. 2016;139:1482–1496. doi: 10.1093/brain/aww048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Velay J-L. Handwriting as a marker for PD progression: a shift in paradigm. Neurodegener Dis Manag. 2015;5:367–369. doi: 10.2217/nmt.15.29. [DOI] [PubMed] [Google Scholar]
- Rosenblum S, Samuel M, Zlotnik S, Erikh I, Schlesinger I. Handwriting as an objective tool for Parkinson's disease diagnosis. J Neurol. 2013;260:2357–2361. doi: 10.1007/s00415-013-6996-x. [DOI] [PubMed] [Google Scholar]
- Tan H, Pogosyan A, Anzak A, Ashkan K, Bogdanovic M, Green AL, Aziz T, Foltynie T, Limousin P, Zrinzo L, Brown P. Complementary roles of different oscillatory activities in the subthalamic nucleus in coding motor effort in parkinsonism. Exp Neurol. 2013;248:187–195. doi: 10.1016/j.expneurol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Jenkinson N, Brown P. Dynamic neural correlates of motor error monitoring and adaptation during trial-to-trial learning. J Neurosci. 2014a;34:5678–5688. doi: 10.1523/JNEUROSCI.4739-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Zavala B, Pogosyan A, Ashkan K, Zrinzo L, Foltynie T, Limousin P, Brown P. Human subthalamic nucleus in movement error detection and its evaluation during visuomotor adaptation. J Neurosci. 2014b;34:16744–16754. doi: 10.1523/JNEUROSCI.3414-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Pogosyan A, Ashkan K, Cheeran B, Fitzgerald JJ, Green AL, Aziz T, Foltynie T, Limousin P, Zrinzo L, Brown P. Subthalamic nucleus local field potential activity helps encode motor effort rather than force in parkinsonism. J Neurosci. 2015;35:5941–5949. doi: 10.1523/JNEUROSCI.4609-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Wade C, Brown P. Post-movement Beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. J Neurosci. 2016;36:1516–1528. doi: 10.1523/JNEUROSCI.3204-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkhauser G, Pogosyan A, Little S, Beudel M, Herz DM, Tan H, Brown P. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson's disease. Brain. 2017;140:1053–1067. doi: 10.1093/brain/awx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkhauser G, Torrecillos F, Duclos Y, Tan H, Pogosyan A, Fischer P, Carron R, Welter ML, Karachi C, Vandenberghe W, Nuttin B, et al. Beta burst coupling across the motor circuit in Parkinson's disease. Neurobiol Dis. 2018;117:217–225. doi: 10.1016/j.nbd.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecillos F, Alayrangues J, Kilavik BE, Malfait N. Distinct modulations in sensorimotor Postmovement and Foreperiod -band activities related to error salience processing and sensorimotor adaptation. J Neurosci. 2015;35:12753–12765. doi: 10.1523/JNEUROSCI.1090-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Neumann WJ, Schneider GH, Sander TH, Litvak V, Kühn AA. Low-beta cortico-pallidal coherence decreases during movement and correlates with overall reaction time. Neuroimage. 2017;159:1–8. doi: 10.1016/j.neuroimage.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yttri EA, Dudman JT. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature. 2016;533:402–406. doi: 10.1038/nature17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.