Abstract
Background
Death is common in systemic inflammatory response syndrome (SIRS) or sepsis‐induced multisystem organ failure and it has been thought that antioxidants such as N‐acetylcysteine could be beneficial.
Objectives
We assessed the clinical effectiveness of intravenous N‐acetylcysteine for the treatment of patients with SIRS or sepsis.
Search methods
We searched the following databases: Cochrane Central Register of Clinical Trials (CENTRAL) (The Cochrane Library 2011, Issue 12); MEDLINE (January 1950 to January 2012); EMBASE (January 1980 to January 2012); CINAHL (1982 to January 2012); the NHS Trusts Clinical Trials Register and Current Controlled Trials (www.controlled‐trials.com); LILACS; KoreaMED; MEDCARIB; INDMED; PANTELEIMON; Ingenta; ISI Web of Knowledge and the National Trials Register to identify all relevant randomized controlled trials available for review.
Selection criteria
We included only randomized controlled trials (RCTs) in the meta‐analysis.
Data collection and analysis
We independently performed study selection, quality assessment and data extraction. We estimated risk ratios (RR) for dichotomous outcomes. We measured statistical heterogeneity using the I2 statistic.
Main results
We included 41 fully published studies (2768 patients). Mortality was similar in the N‐acetylcysteine group and the placebo group (RR 1.06, 95% CI 0.79 to 1.42; I2 = 0%). Neither did N‐acetylcysteine show any significant effect on length of stay, duration of mechanical ventilation or incidence of new organ failure. Early application of N‐acetylcysteine to prevent the development of an oxidato‐inflammatory response did not affect the outcome, nor did late application that is after 24 hours of developing symptoms. Late application was associated with cardiovascular instability.
Authors' conclusions
Overall, this meta‐analysis puts doubt on the safety and utility of intravenous N‐acetylcysteine as an adjuvant therapy in SIRS and sepsis. At best, N‐acetylcysteine is ineffective in reducing mortality and complications in this patient population. At worst, it can be harmful, especially when administered later than 24 hours after the onset of symptoms, by causing cardiovascular depression. Unless future RCTs provide evidence of treatment effect, clinicians should not routinely use intravenous N‐acetylcysteine in SIRS or sepsis and academics should not promote its use.
Plain language summary
Intravenous N‐acetylcysteine compared to placebo for treatment of systemic inflammatory response syndrome and sepsis in seriously ill adults
Systemic inflammatory response syndrome (SIRS) is a complex response to an insult such as major surgery or trauma. It is called sepsis syndrome, or simply sepsis, when infection is present. The generalized inflammatory reaction involves activation of leukocytes and endothelial cells and the release of inflammatory mediators and toxic oxygen free radicals. Diffuse microthrombosis can result in localized tissue perfusion abnormalities and low oxygenation (hypoxia). Both SIRS and sepsis can be difficult to treat and are major causes of multiple organ failure and the death of patients in the intensive care unit. SIRS and sepsis both lead to a drop in the level of antioxidants normally present in the body.
N‐acetylcysteine is an antioxidant with strong anti‐inflammatory effects that is used in treating endotoxaemia and overdoses of acetaminophen. This Cochrane review of 41 randomized controlled trials with 2768 critically ill adult patients found no evidence to support the theory that N‐acetylcysteine might reduce the risk of death in adults with SIRS or sepsis. Intravenous N‐acetylcysteine did not affect the length of stay in the intensive care unit, duration of mechanical ventilation, duration of support for the cardiovascular system or incidence of new organ failure. There is currently insufficient evidence to support the use of N‐acetylcysteine in SIRS or sepsis. We also found that when N‐acetylcysteine was administered more than 24 hours after the development of clinical signs of SIRS or sepsis it may even be harmful, by causing cardiovascular depression.
Twenty of the trials used N‐acetylcysteine in patients around the time of surgery, including cardiac, vascular and major abdominal surgery, and liver transplantation. Eight studies evaluated N‐acetylcysteine in patients with severe sepsis or septic shock associated with the medical conditions acute respiratory distress syndrome (ARDS), multiple organ failure, liver failure, malaria or burns. The dosing of N‐acetylcysteine, timing and duration of treatment, from a single intravenous dose to infusions up to seven days, varied. Twenty papers had a low risk of bias.
Summary of findings
for the main comparison.
| N‐acetylcysteine compared with placebo for SIRS or sepsis | ||||||
|
Patient or population: Adult patients with SIRS or sepsis Settings: Intensive care unit Intervention: N‐acetylcysteine Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | N‐acetylcysetine | |||||
| Mortality 30‐day |
136 per 1000 | 144 per 1000 (100 to 185) | RR 1.07 (0.80 to 1.43) | 930 (11) | +++O moderate | |
| Length of stay on the intensive care unit (days) | The mean Length of stay on the intensive care unit ranged across control groups from 1 to 33 days | The mean difference (95%CI) Length of stay on the intensive care unit in the intervention groups was ‐0.22 (‐0.86 to 0.43) | 1309 (19) | +++O moderate | ||
| Duration of mechanical ventilation (days) |
The mean Duration of mechanical ventilation ranged across control groups from 1 to 25 days | The mean difference (95% CI) Duration of mechanical ventilation in the intervention groups was ‐0.00 (‐0.04 to 0.04) | 423 (9) | +++O moderate | ||
| Incidence of new organ failure | 158 per 1000 | 165 per 1000 (123 to 241) | RR 1.01 (0.80 to 1.27) | 1224 (12) | +++O moderate | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; GRADE: GRADE Working Group grades of evidence (see explanations) | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
Background
There is an increasing incidence of systemic inflammatory response syndrome (SIRS) and sepsis, and consequent multiple organ failure, especially in the developed world. Despite recent advances in antibacterial and other therapies the mortality rate only shows a slow decline (Engel 2007).
Description of the condition
Since the discovery of oxygen free radicals about 30 years ago there has been a growing interest in their role in inflammatory diseases (Crimi 2006). Although the pathophysiology of systemic inflammatory response syndrome (SIRS) and sepsis is complex, and only partially understood, it has been proposed that following an insult, such as trauma, systemic infection or major surgery, the development of multiple organ failure is due to a generalized inflammatory reaction involving activation of leukocytes and endothelial cells and the release of inflammatory mediators and toxic oxygen free radicals of both intracellular and extracellular origin (Pinsky 2003). Another important mechanism of sepsis‐induced organ failure is diffuse microthrombosis, resulting in locoregional abnormalities of tissue perfusion and tissue hypoxia. In patients affected by mild‐to‐moderate organ dysfunction there is an imbalance between oxidant and antioxidant forces, and it has been suggested that in multiple organ failure there is an overwhelming production of oxygen free radicals that results in tissue destruction (Goode 1995). Several studies have demonstrated decreased concentrations of plasma antioxidants in septic patients and those in multiple organ failure as they try to combat the increased production of free radicals (Cowley 1996; Goode 1995; Ogilvie 1991).
Description of the intervention
Similar protection against free radical mediated injury to the endogenous mechanisms might be achieved by using exogenous agents such as N‐acetylcysteine, which has been used clinically for nearly 40 years. Historically it has been administered for chronic bronchitis, cystic fibrosis and paracetamol intoxication (Kiefer 2000; Pinsky 2003).
How the intervention might work
N‐acetylcysteine acts as a powerful oxygen free radical scavenger and also replenishes depleted glutathione stores, enhancing the endogenous antioxidant defence (Aruoma 1989; Repine 1992). It also has a powerful anti‐inflammatory effect when given as a pre‐ and post‐treatment in endotoxaemia (Schmidt 1997; Schmidt 1998). In experimental animal studies N‐acetylcysteine was administered before induction of sepsis with endotoxin infusion (Hsu 2004; Zhang 1994a). These studies suggested that timing of the treatment was important. In previous in vitro and in vivo studies N‐acetylcysteine treatment attenuated TNF and IL‐8 production and enhanced oxygen extraction in septic shock, severe multiple organ failure and acute respiratory distress syndrome (ARDS) (Paterson 2003; Peristeris 1992; Spapen 1998). Many authors have proposed the use of exogenous agents such as N‐acetylcysteine to prevent oxygen free radical damage in patients suffering from septic shock (Spies 1994; Zhang 1994b). Despite the theoretical advantages, the benefit of N‐acetylcysteine remains controversial in critically ill patients (Henderson 1994; Pinsky 2003). Molnar found that early treatment with N‐acetylcysteine (within 24 hours of hospital admission) may improve mortality in a subgroup of patients from a heterogeneous intensive care population (Molnar 1999). On the other hand, recent randomized placebo‐controlled studies in patients with ARDS and early septic shock or SIRS found no significant differences in gas exchange, development of ARDS, inflammatory response and mortality in patients treated with N‐acetylcysteine or placebo (Domenighetti 1997; Spapen 2005; Szakmany 2003). Furthermore, several papers reported conflicting and undesirable effects of N‐acetylcysteine administration. Depressed cardiac performance was found in septic patients and impaired bacterial killing due to augmented neutrophil phagocytosis was reported in animals and humans (Heller 2001; Koch 1996; Peake 1996). Late administration of N‐acetylcysteine was associated with an adverse outcome in experimental and clinical sepsis (Molnar 1999; Vassilev 2004).
Why it is important to do this review
Although many clinical trials have investigated its effect, the value of N‐acetylcysteine administration in the critically ill has not been assessed in a systematic review.
Objectives
Our aim was to critically appraise and summarize all randomized clinical trials involving intravenous N‐acetylcysteine administration in adult patients suffering from sepsis and SIRS.
We wanted to answer the question: does N‐acetylcysteine influence the outcome in sepsis or SIRS?
Our primary objective was to investigate whether intravenous N‐acetylcysteine has any effect on the 30‐day all‐cause mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in the meta‐analysis. No cross‐over or quasi‐randomization designs were allowed.
Types of participants
We included trials involving participants with SIRS and sepsis according to the Bone criteria (Bone 1992), regardless of the underlying cause. If the authors of the study did not specify that they used the Bone criteria, we have assessed the study to determine if the inclusion criteria were in line with the published SIRS or sepsis definition.
We only included trials conducted in adult patient populations. There are at least three important differences between adult and paediatric sepsis, which would make grouping of the data confusing. First, the causes of sepsis may be substantially different, with viral infections contributing a large proportion of the sepsis cases. Second, the risk for paediatric sepsis is almost exclusively in children with chronic comorbid medical conditions, such as chronic lung disease. Third, case‐fatality rates are substantially lower in children than in adults, although the risk of death is similar between children and young adults.
Types of interventions
We included studies where one of the groups was treated with intravenous N‐acetylcysteine in bolus intravenous doses or as a continuous infusion, or a combination of the two. We included studies if intravenous N‐acetylcysteine was compared with placebo or standard treatment. Placebo was considered if a similar composition and volume of fluid was administered without the active ingredient; standard treatment was considered if the patient received standard care without the administration of N‐acetylcysteine.
Types of outcome measures
Primary outcomes
Our principal outcome measure was 30‐day all‐cause mortality.
Overall mortality: we used the longest follow‐up data from each trial regardless of the period of follow up.
Secondary outcomes
1. Length of stay in the critical care unit. 2. Duration of mechanical ventilation. 3. Duration of inotropic support. 4. Incidence of new organ failures, such as: a) acute renal failure; b) ARDS; c) liver failure; d) circulatory failure; 5. quality of life; 6. cost.
Search methods for identification of studies
We used several techniques to identify published and ongoing studies for this review. We included all relevant published studies regardless of the language.
Electronic searches
We searched the following databases: Cochrane Central Register of Clinical Trials (CENTRAL) (The Cochrane Library 2011, Issue 12); MEDLINE (January 1950 to January 2012); EMBASE (January 1980 to January 2012); CINAHL (1982 to January 2012); the NHS Trusts Clinical Trials Register and Current Controlled Trials (www.controlled‐trials.com); LILACS; KoreaMED; MEDCARIB; INDMED; PANTELEIMON; Ingenta; ISI Web of Knowledge and the National Trials Register to identify all relevant randomized controlled trials available for review. We used the SilverPlatter MEDLINE and the Ovid version of all other databases.
We searched MEDLINE using the strategy detailed in Appendix 1. We modified this search strategy as necessary and applied it to the CENTRAL (Appendix 2), CINAHL (Appendix 3) and EMBASE databases as well. We applied the search strategy detailed in Appendix 1 to the LILACS, KoreaMED, MEDCARIB, INDMED, PANTELEIMON, CCT, Ingenta, ISI Web of Knowledge and National Trials Register databases.
We utilized the Cochrane highly sensitive RCT filter for MEDLINE from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We identified relevant studies initially by title then by abstract and finally by full text. Two authors (TSZ and BH) independently performed the electronic searches.
Searching other resources
We searched the bibliographies of reports of randomized trials and any identified reviews.
We also searched for abstracts from conference proceedings published from 1994 to December 2011 in the American Journal of Respiratory and Critical Care Medicine, Chest, Critical Care Medicine and Intensive Care Medicine.
We contacted the authors of all identified trials to ask them about any other published or unpublished trials that may have been conducted.
Data collection and analysis
Selection of studies
Selection of trials
Two authors (BH, TSZ) independently reviewed the titles and abstracts identified from the searches. We obtained full copies of potentially relevant trials and assessed all full copies according to the parameters outlined in 'Criteria for considering studies for this review'. Only eligible trials were considered for inclusion in the meta‐analysis. The authors resolved any discrepancies by discussion, if necessary.
Data extraction and management
Data collection
Two authors (BH, TSZ) independently extracted data using a form modified from one developed by the Cochrane Anaesthesia Review Group. We (as far as possible) extracted data on the basis of an intention‐to‐treat analysis. Two authors (BH and TSZ) entered all data independently into Review Manager (RevMan 5.1) after checking for differences.
Assessment of risk of bias in included studies
Assessment of methodological quality
We evaluated the validity and design characteristics of each trial. To draw conclusions about the overall risk of bias for an outcome it was necessary to evaluate the trials for major sources of bias, also defined as domains (random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias). The Cochrane Collaboration’s recommended tool for assessing risk of bias is neither a scale nor a checklist, but rather the domain‐based evaluation. Any assessment of the overall risk of bias involves consideration of the relative importance of the different domains (Higgins 2011).
We assessed the methodological quality of the included studies according to the Cochrane Collaboration's tool for assessing risk of bias.
We assessed the studies for bias based on the following criteria:
random sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessors;
incomplete outcome data;
selective reporting;
other bias.
We have generated a risk of bias tables for each included trial (Characteristics of included studies).
We defined the trials as having low risk of bias only if they adequately fulfilled the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions. We performed summary assessments of the risk of bias for each important outcome (across domains) within and across studies. We applied a 'Risk of bias' graph (Figure 1) and a 'Risk of bias' summary figure (Figure 2).
1.
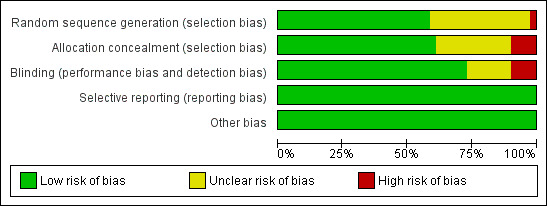
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
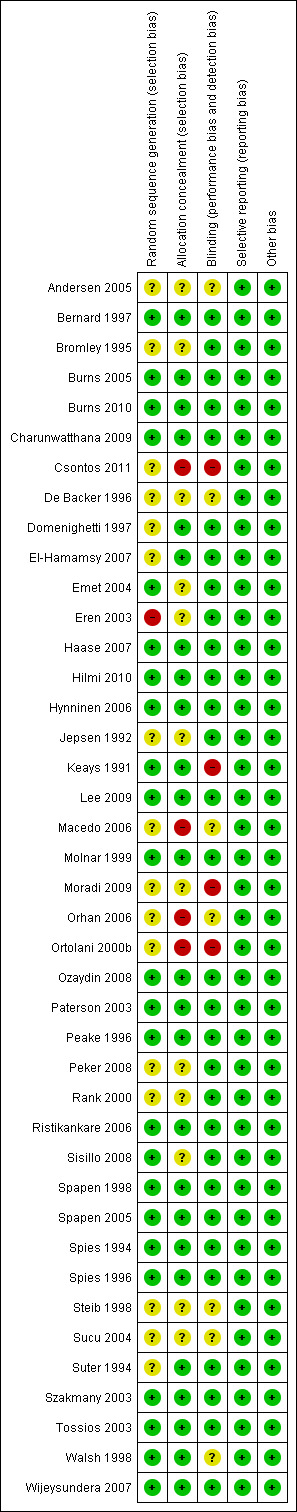
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
+: yes, ‐: no, ?: unclear
Measures of treatment effect
Dichotomous data
We summarized dichotomous data using risks ratio (RR) as the summary estimate of treatment effect with 95% confidence interval (CI).
Continuous data
We summarized continuous outcomes using the mean difference (MD). For data reported using different scales which cannot be converted to a uniform scale, we used the standardized mean difference (SMD). If the mean and SD of the continuous outcomes were not reported in the studies, we assigned the median as the mean if sample size was greater than 25 and estimated the SD from the range (that is, SD range 0.95/4 or interquartile range/1.35) as suggested by Hozo et al (Hozo 2005). If sample size was less than 25 we used formulaes suggested by Hozo et al to calculate the mean (Hozo 2005). If we could not calculate the mean and SD from the available data, we excluded the study from the analysis.
Unit of analysis issues
In trials where more than two treatment arms were present, using a different dosage of N‐acetylcysteine, we divided the control group into several parts so that the total numbers add up to the original size of the group.
Dealing with missing data
We contacted all the first authors and contact persons of the trials with missing data in order to retrieve the relevant data.
Intention‐to‐treat analysis is recommended in order to minimize bias in design, follow up, and analysis of the efficacy of randomized clinical trials. It gives a pragmatic estimate of the benefit of a change in treatment policy rather than of the potential benefit in patients who receive treatment exactly as planned (Hollis 1999). Full application of intention to treat is possible only when complete outcome data are available for all randomized participants. Despite the fact that about half of all published reports of randomized controlled trials state that Intention‐to‐treat analysis is used, handling of deviations from randomized allocation varies widely and many trials have missing data for the primary outcome variable. Methods used to deal with this are generally inadequate, potentially leading to bias (Hollis 1999).
Performing an Intention‐to‐treat analysis in a systematic review is not straightforward in practice since review authors must decide how to handle outcome data that are missing in the contributing trials. No consensus exists about how missing data should be handled in intention‐to‐treat analyses, and different approaches may be appropriate in different situations (Higgins 2011; Hollis 1999). In the case of missing data we chose 'complete‐case analysis' for our primary outcomes, which simply excludes from the analysis all participants with the outcome missing.
Selective outcome reporting occurs when non‐significant results are selectively withheld from publication. It is defined as selection on the basis of the results of a subset of the original variables recorded for inclusion in the publication of trials. The most important types of selective outcome reporting are: selective omission of outcomes from reports; selective choice of data for an outcome; selective reporting of analyses using the same data; selective reporting of subsets of the data; and selective under‐reporting of data (Higgins 2011). Statistical methods to detect within‐study selective reporting are still in their infant stage. We chose to explore selective outcome reporting by comparing publications with their protocols, if the latter were available.
Assessment of heterogeneity
Clinical heterogeneity
We used the term 'clinical heterogeneity' to describe clinical differences in the studies to do with the participants, interventions and outcomes. We based the decision to pool studies on the absence of clinical heterogeneity. We assessed clinical heterogeneity by judgment. We based these judgments on patient demographics, clinical circumstances and the comparability of the interventions applied.
Assessment of reporting biases
If sufficient studies were identified we constructed funnel plots (trial effect versus standard error) to assess for possible publication bias, expressed by asymmetry (Egger 1997). In the case of asymmetry we chose to apply the Arcsine‐Thompsen test, as proposed by Rücker (Rücker 2008).
Data synthesis
Patients admitted to the intensive care unit (ICU) are a heterogenous group in terms of admitting diagnosis, prognosis and resource use. This heterogeneity not only exist between individual patients in a single critical care unit but also in the case‐mix of patients admitted to different units. Whilst acknowledging this heterogeneity we decided to pool all studies together and perform individual sensitivity analyses to evaluate if there was any difference between the groups.
We pooled data using either the random‐effects model (REM) or fixed‐effect model (FEM) depending on the presence or absence of statistical heterogeneity. Risk ratios (RR) were pooled using the Mantel‐Hansen (M‐H) method. If there were many ‘zeros’ the Peto method was considered. The mean difference (MD) for continuous data was analysed using the inverse variance method. We used RevMan 5.1 for all analyses.
Subgroup analysis and investigation of heterogeneity
We performed stratified meta‐analyses, using all available mortality data only, to investigate the influence of: 1. sepsis versus SIRS; 2. aetiology of critical illness (medical versus surgical studies); 3. delay of administration of N‐acetylcysteine (less than 24 hours versus more than 24 hours from the first documented signs of SIRS or sepsis).
Conduct of the proposed subgroup analyses, defined a priori, was 'hypothesis generating'. We expected that all subgroup analyses would be underpowered and we viewed these as exploratory given their propensity to generate misleading conclusions (Oxman 1992).
Statistical heterogeneity
We conducted an evaluation of the heterogeneity of the data using the I2 statistic (Higgins 2002), which is a statistic that describes the proportion of variation in point estimates that is attributable to heterogeneity as opposed to sampling error. We decided a priori that in the case where the assumption of homogeneity was rejected for any endpoint (I2 > 50%) a random‐effects model would be applied to estimate the variance component associated with between‐study variation for that endpoint. According to this method, the variance for each individual study in the meta‐analysis is the sum of within‐ and between‐study components of the variance. We also decided a priori not to pool the data if the I2 statistic indicated significant heterogeneity (I2 > 80%).
Sensitivity analysis
1. Comparing estimates of the pooled intervention effect in trials with low risk of bias to estimates from trials with high risk of bias (i.e. trials having at least one inadequate risk of bias component).
2. Comparing estimates of the pooled intervention effect in trials based on the different components of risk of bias (random sequence generation, allocation concealment, blinding).
We calculated RR with 95% CI, if possible, for our sensitivity and subgroup analyses based on our primary outcome measure (mortality).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
We identified 6983 potential studies in the initial electronic search. One potential study was identified during a Google search. No additional studies were identified by contacting experts in the field or relevant pharmaceutical companies, or by searching personal reference databases of the authors or steering group. No additional studies were identified following screening of reference lists of potentially eligible studies and previously published systematic reviews (snowballing). See Figure 3 for our search results.
3.
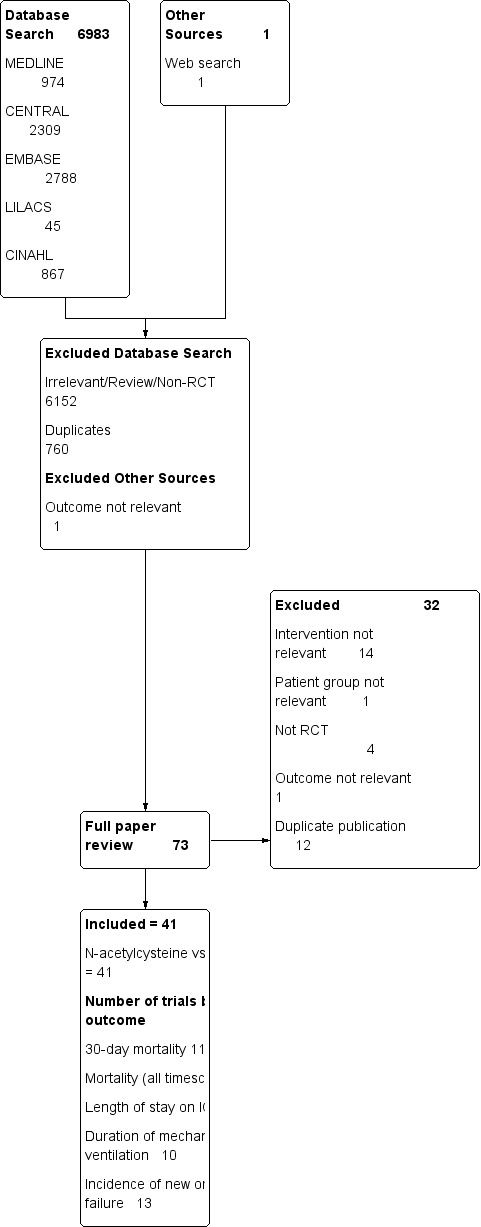
Study flow diagram.
Results of the search
We identified 73 potentially eligible studies following screening of abstracts of potential studies (BH, TSZ). We found no ongoing trials. The two review authors (BH, TSZ) completely agreed on the selection of included studies. We obtained no additional information from the primary authors.
Included studies
We included 41 fully published studies (including 2768 patients). A detailed summary of each included study, including patient characteristics and the N‐acetylcysteine dosing regimens used, can be found in Characteristics of included studies. The included studies were conducted between 1991 and 2009.
All studies used intravenous N‐acetylcysteine but two studies used oral N‐acetylcysteine preoperatively then intravenous N‐acetylcysteine intraoperatively (El‐Hamamsy 2007; Macedo 2006). Twenty studies evaluated the use of N‐acetylcysteine perioperatively, 15 during cardiac surgery (Andersen 2005; Burns 2005; De Backer 1996; El‐Hamamsy 2007; Eren 2003; Haase 2007; Orhan 2006; Ozaydin 2008; Peker 2008; Ristikankare 2006; Sisillo 2008; Spies 1996; Sucu 2004; Tossios 2003; Wijeysundera 2007); three during liver transplantation (Bromley 1995; Hilmi 2010; Steib 1998; Hilmi 2010); two in vascular surgery (Hynninen 2006; Macedo 2006) and one after major abdominal surgery (Szakmany 2003). Eight studies used N‐acetylcysteine in patients with severe sepsis or septic shock (Emet 2004; Ortolani 2000b; Paterson 2003; Peake 1996; Rank 2000; Spapen 1998; Spapen 2005; Spies 1994); five in ARDS (Bernard 1997; Domenighetti 1997; Jepsen 1992; Moradi 2009; Suter 1994); two in multiple organ failure (Burns 2010; Molnar 1999); three in fulminant liver failure (Keays 1991; Lee 2009; Walsh 1998), one in malaria (Charunwatthana 2009) and one in burns (Csontos 2011). There was a wide variety in the N‐acetylcysteine dose regime used, which ranged from 25 mg/kg to 150 mg/kg. Timing and duration of N‐acetylcysteine treatment varied considerably, from single bolus dose to infusions up to seven days. In one trial (Ortolani 2000b) N‐acetylcysteine was compared to rutin and placebo. We only report the data for the N‐acetylcysteine and placebo groups from this trial. Three trials compared N‐acetylcysteine with no treatment (Csontos 2011; Macedo 2006; Orhan 2006). Other clinical outcome variables in line with our defined primary and secondary outcomes were inconsistently reported.
Excluded studies
We excluded 32 potentially relevant publications (Adabag 2008; Agusti 1997; Barr 2008; Csontos 2011; Csontos 2011b; De Rosa 2000; Devlin 1997; Fischer 2005; Fraga 2011; Haase 2008; Heller 2001; Jepsen 1989; Koksal 2008; Komisarof 2007; Koramaz 2006; Kurian 2010; Laurent 1996; Lawlor 2007; Milewski 2006; Molnar 1998; Molnar 2003; Najafi 2009; Niemi 2006; Ortolani 2000a; Reinhart 1995; Sahib 2010; Saricaoglu 2005; Siriwardena 2007; Soltan‐Sharifi 2007; Spies 1993; Szakmany 2002; Wijeysundera 2009; Zingg 2007). The reasons for their exclusion are summarized in Characteristics of excluded studies.
Risk of bias in included studies
We have identified 20 papers with low risk of bias. For a more detailed description of individual trial qualities, see the table Characteristics of included studies. The various bias domains are presented in the 'Risk of bias' graph and a 'Risk of bias' summary figure (Figure 1; Figure 2).
Allocation
Random sequence generation was adequately reported in 24 trials (Bernard 1997; Burns 2005; Burns 2010; Charunwatthana 2009; Emet 2004; Haase 2007; Hilmi 2010; Hynninen 2006; Keays 1991; Lee 2009; Molnar 1999; Ozaydin 2008; Paterson 2003; Peake 1996; Ristikankare 2006; Sisillo 2008; Spapen 1998; Spapen 2005; Spies 1994; Spies 1996; Szakmany 2003; Tossios 2003; Walsh 1998; Wijeysundera 2007). Allocation concealment was adequately reported in 25 studies (Bernard 1997; Burns 2005; Burns 2010; Charunwatthana 2009; Domenighetti 1997; El‐Hamamsy 2007; Emet 2004; Haase 2007; Hilmi 2010; Hynninen 2006; Keays 1991; Lee 2009; Molnar 1999; Ozaydin 2008; Paterson 2003; Peake 1996; Ristikankare 2006; Spapen 1998; Spapen 2005; Spies 1994; Spies 1996; Suter 1994; Szakmany 2003; Tossios 2003; Walsh 1998; Wijeysundera 2007).
Blinding
Thirty trials provided sufficient data to be categorized as double blinded (Bernard 1997; Bromley 1995; Burns 2005; Burns 2010; Charunwatthana 2009; Domenighetti 1997; El‐Hamamsy 2007; Emet 2004; Eren 2003; Haase 2007; Hilmi 2010; Hynninen 2006; Jepsen 1992; Lee 2009; Molnar 1999; Ozaydin 2008; Paterson 2003; Peake 1996; Peker 2008; Rank 2000; Ristikankare 2006; Sisillo 2008; Spapen 1998; Spapen 2005; Spies 1994; Spies 1996; Suter 1994; Szakmany 2003; Tossios 2003; Wijeysundera 2007). The remaining 11 trials were either open label or did not provided sufficient information about how double blinding was achieved (Andersen 2005; Csontos 2011; De Backer 1996; Keays 1991; Macedo 2006; Moradi 2009; Orhan 2006; Ortolani 2000b; Steib 1998; Sucu 2004; Walsh 1998).
Incomplete outcome data
There was complete follow up in all trials except four for the primary outcome (De Backer 1996; Spapen 2005; Spies 1996; Walsh 1998). In these four trials the authors did not provide data on mortality nor on the length of follow up.
Selective reporting
In one study we found evidence of the possibility of selective reporting. In this study the number of patients recruited to the treatment (17 patients) and placebo (10 patients) groups was very different. Furthermore three patients in the N‐acetylcysteine group were excluded from the analysis, without reasonable explanation (Moradi 2009). We were unable to retrieve the original protocols of the trials and thus were unable to examine selective outcome reporting, as described above. However, all but four trials provided data on our primary outcome of mortality (De Backer 1996; Spapen 2005; Spies 1996; Walsh 1998).
Other potential sources of bias
The funnel plot of standard error versus risk ratio for 30‐day mortality (Figure 4) and the funnel plot for all reported mortality (Figure 5) showed a symmetrical distribution that indicated no bias or publication bias. Since there was no asymmetry or heterogeneity in the funnel plot, we found no need to apply the Arcsine‐Thompson test as proposed by Rücker (Rücker 2008).
4.
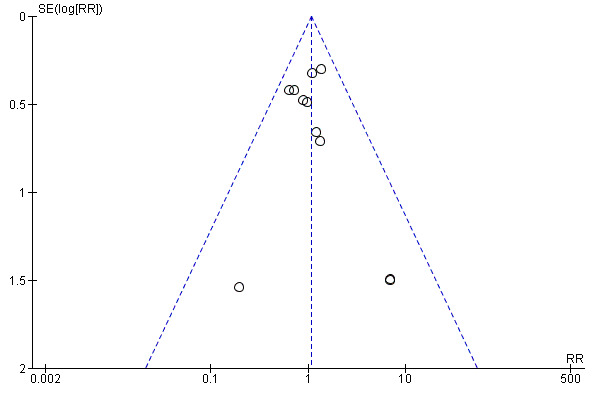
Funnel plot of comparison: 1 N‐acetylcysteine versus placebo, outcome: 1.1 30‐day all‐cause mortality.
5.
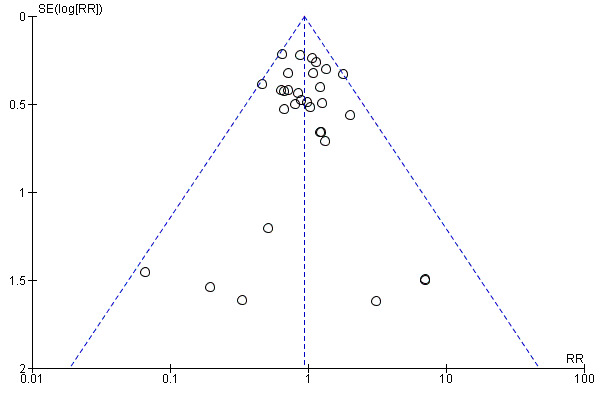
Funnel plot of comparison: 2 N‐acetylcysteine versus placebo ‐ subgroup analysis, outcome: 2.1 Mortality (all reported time scales).
Effects of interventions
See: Table 1
Eleven trials reported the primary outcome. Combining the data from these studies and applying complete‐case analysis showed no statistically significant effect of N‐acetylcysteine on 30‐day all‐cause mortality: 67/467 (14.35%) deaths in the N‐acetylcysteine group compared with 63/463 (13.60%) deaths in the control group (RR 1.07, 95% CI 0.80 to 1.43; I2 = 0%) (Analysis 1.1). In combining the data from the 37 studies reporting mortality, using the longest reported follow‐up data, mortality was similar: 223/1228 (18.16%) in the N‐acetylcysteine group and 241/1233 (19.55%) in the placebo group (RR 0.94, 95% CI 0.81 to 1.09; I2 = 0%) (Analysis 2.1).
1.1. Analysis.
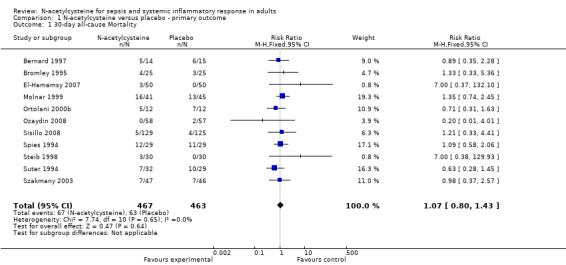
Comparison 1 N‐acetylcysteine versus placebo ‐ primary outcome, Outcome 1 30‐day all‐cause Mortality.
2.1. Analysis.
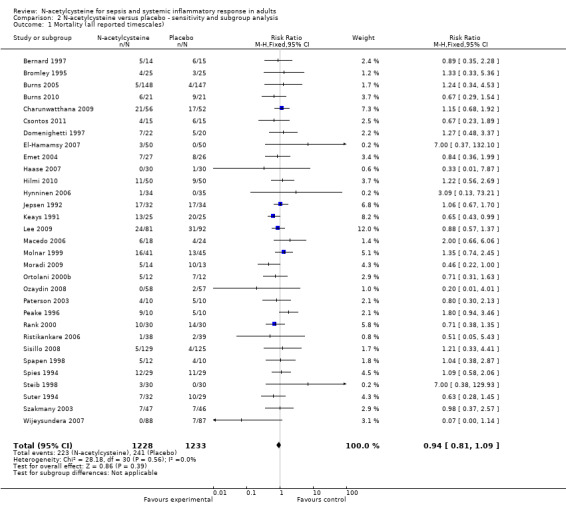
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 1 Mortality (all reported timescales).
Sensitivity analysis ‐ analysis of bias
Comparing estimates of the pooled intervention effect based on the overall risk of bias (Analysis 2.2) (high risk of bias: 12 studies, 919 patients; low risk of bias: 17 studies, 1524 patients); on random sequence generation (Analysis 2.3) (adequate sequence generation: 20 studies, 1899 patients; inadequate or unclear sequence generation: 12 studies, 602 patients); on allocation concealment (Analysis 2.4) (adequate allocation concealment: 22 studies, 1835 patients; inadequate or unclear allocation concealment: 11 studies, 706 patients) or on blinding (Analysis 2.5) (adequate blinding: 25 studies, 2228 patients; inadequate or unclear blinding: 6 studies, 233 patients) did not result in any statistically significant finding in any of the subgroups examined.
2.2. Analysis.
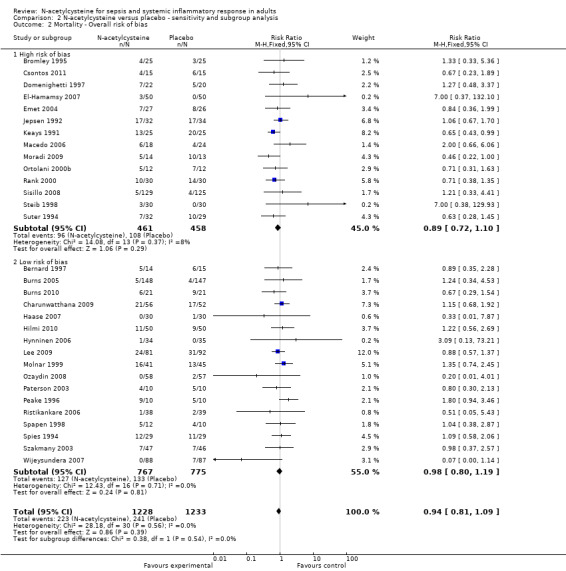
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 2 Mortality ‐ Overall risk of bias.
2.3. Analysis.
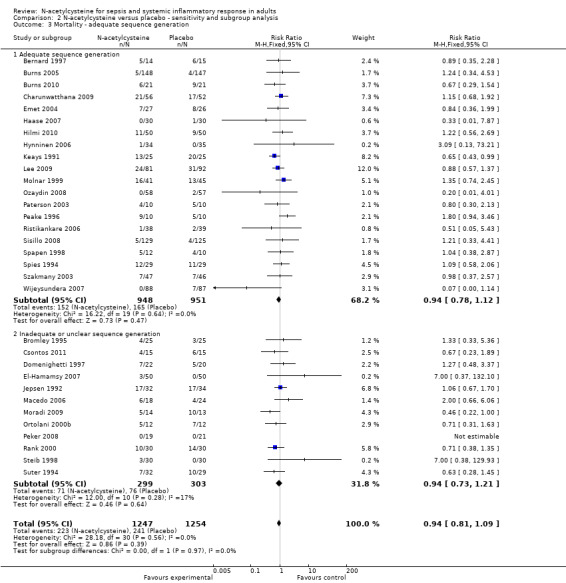
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 3 Mortality ‐ adequate sequence generation.
2.4. Analysis.

Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 4 Mortality ‐ Allocation concealment.
2.5. Analysis.
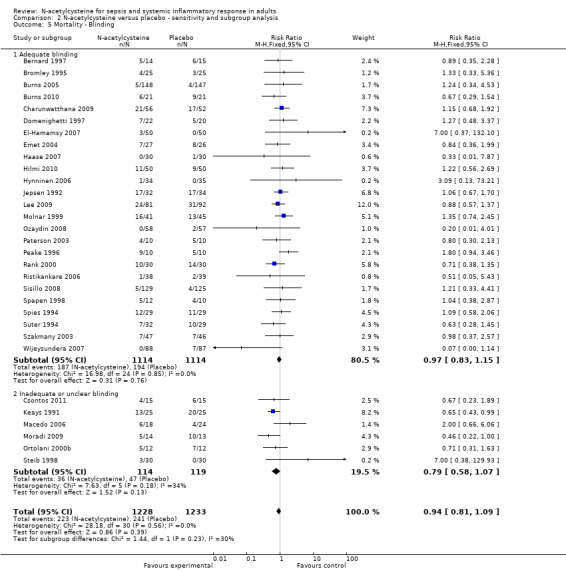
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 5 Mortality ‐ Blinding.
Sensitivity analysis ‐ analysis of follow‐up time
As the studies reported variable follow‐up times we have pooled the trials in two groups, one reporting short follow‐up time scales (less than 30 days) and one with a long follow‐up time scale. We could not find any significant difference in reported mortality between patients in the N‐acetylcysteine and placebo arms in any of these subgroups (Analysis 2.6) (short time scale: 14 studies, 1524 patients; long time scale: 18 studies, 957 patients).
2.6. Analysis.
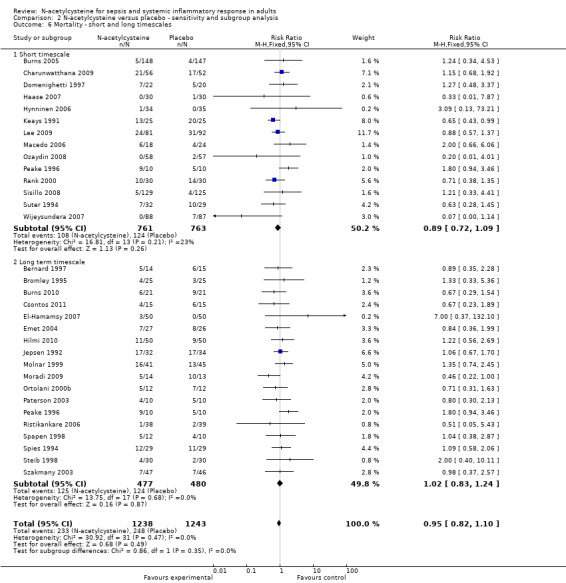
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 6 Mortality ‐ short and long timescales.
Subgroup analysis ‐ sepsis versus SIRS and medical versus surgical aetiology
None of the stratified meta‐analyses revealed any significant effect of N‐acetylcysteine. It did not affect mortality in patients admitted to the ICU with sepsis or SIRS (Analysis 2.7) (sepsis: eight studies, 369 patients; SIRS: nine studies, 527 patients), nor did it show any significant effect when we analysed the aetiology of the critical illness (Analysis 2.8) (medical background: 16 studies, 926 patients; surgical background: 13 studies, 1478 patients).
2.7. Analysis.
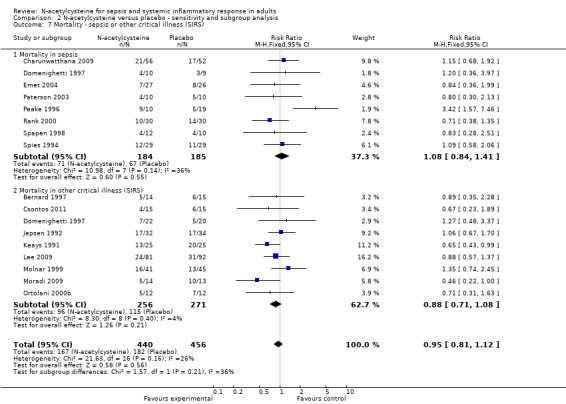
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 7 Mortality ‐ sepsis or other critical illness (SIRS).
2.8. Analysis.
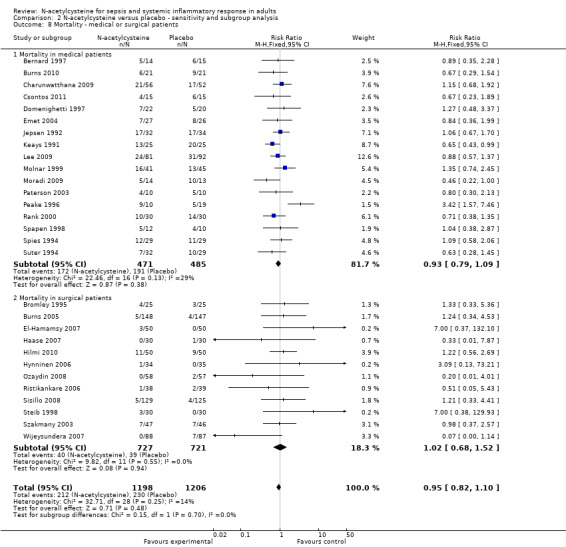
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 8 Mortality ‐ medical or surgical patients.
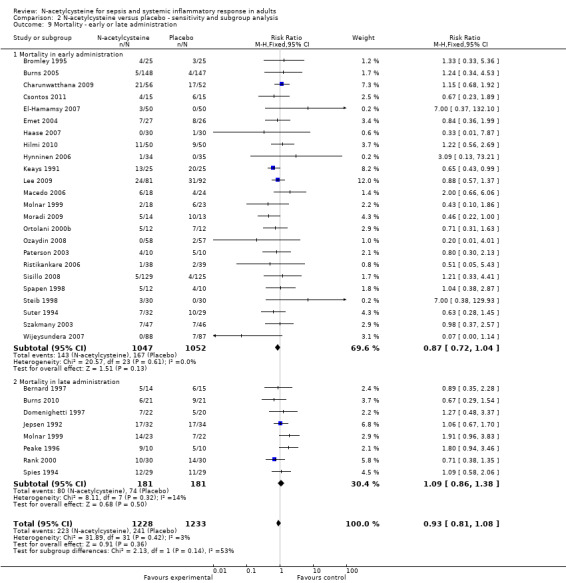
Subgroup analysis ‐ early versus late administration
Application of N‐acetylcysteine early to prevent the development of an oxidato‐inflammatory response did not affect outcome, nor did late application that is after 24 hours of developing symptoms (Analysis 2.9) (early administration: 24 studies, 2075 patients; late administration: eight studies, 362 patients). Three trials compared N‐acetylcysteine to no treatment and there was no significant difference between these two groups (10 deaths/43 patients versus 10 deaths/49 patients, respectively) (Csontos 2011; Macedo 2006; Orhan 2006).
2.9. Analysis.
Comparison 2 N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis, Outcome 9 Mortality ‐ early or late administration.
Secondary outcomes
N‐acetylcysteine did not show any significant effect on length of ICU stay, duration of mechanical ventilation or incidence of new organ failure (Analysis 3.1; Analysis 4.1; Analysis 5.1). When analysing length of stay there was significant heterogeneity that was not otherwise explained (I2 = 83%, P < 0.0001), therefore a summary estimate must be interpreted with great caution. Only five trials compared the duration of inotropic support, they reported that the difference was not significant between the N‐acetylcysteine and control groups (Burns 2005; Haase 2007; Hynninen 2006; Molnar 1999; Szakmany 2003). None of the trials reported healthcare costs and quality of life data.
3.1. Analysis.
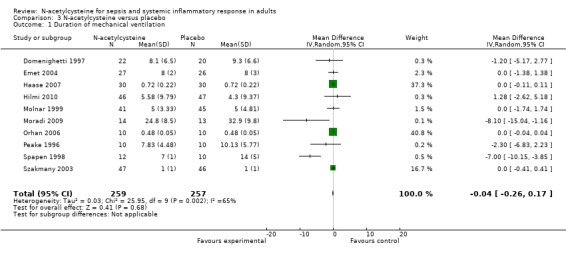
Comparison 3 N‐acetylcysteine versus placebo, Outcome 1 Duration of mechanical ventilation.
4.1. Analysis.
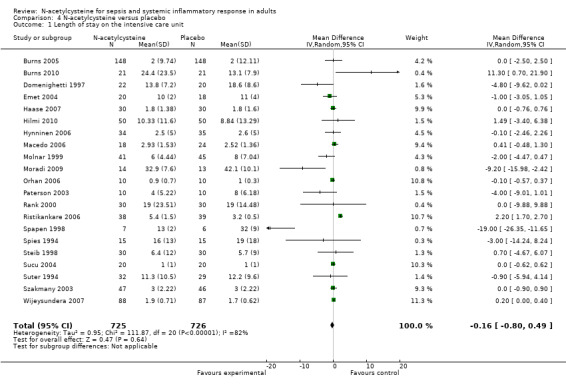
Comparison 4 N‐acetylcysteine versus placebo, Outcome 1 Length of stay on the intensive care unit.
5.1. Analysis.
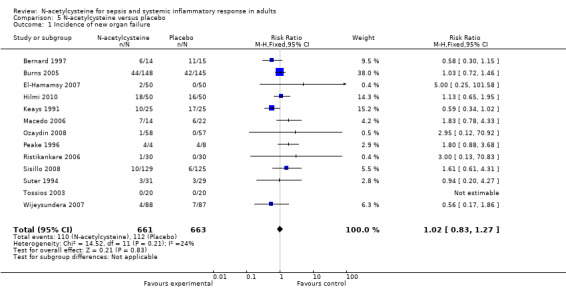
Comparison 5 N‐acetylcysteine versus placebo, Outcome 1 Incidence of new organ failure.
Discussion
In this systematic review of 41 trials with 2768 patients with sepsis and SIRS we found no benefits of N‐acetylcysteine on survival (See Table 1). The analysis on mortality showed no heterogeneity and was robust when performing different subgroup and sensitivity analyses. The sparse data on mortality are not promising but are not evidence of the absence of a beneficial effect; the data suggest that a potentially beneficial effect of N‐acetylcysteine must be very modest and the actual point estimate on the 30‐day all‐cause mortality suggests harm (Analysis 1.1; Analysis 2.1).
As the included studies reported the mortality over variable time periods, there was the possibility of over‐ or underestimating the mortality when pooling studies with long and short follow‐up times. Therefore we have performed a subgroup analysis, which showed no significant difference between short‐term, that is less than 30 days, and long‐term, that is more than 30 days up to one year, reported mortality (Analysis 2.6).
Despite the well‐defined inclusion criteria, there is considerable heterogeneity amongst the trials in terms of patient population, timing and dosage of N‐acetylcysteine. We included 20 studies in which N‐acetylcysteine was used to prevent the evolution of the oxido‐inflammatory response, where patients developed SIRS during the operation. We also identified 21 studies where patients were treated with N‐acetylcysteine after they had already developed symptoms of SIRS or sepsis. To address this heterogeneity we completed an a priory defined subgroup analysis. However, the analysis did not show any effect on mortality when patients with sepsis or with an established systemic inflammatory response were studied (Analysis 2.7).
To assess the impact of N‐acetycysteine on the various primary aetiologies, we have conducted subgroup analyses in patients admitted to the ICU with a 'medical' condition (16 studies of patients with conditions ranging from sepsis, multiorgan failure and ARDS to acute fulminant liver failure and burns) as opposed to a distinct surgical insult (13 studies, including patients undergoing cardiac, vascular and major abdominal surgery). None of these yielded significant results (Analysis 2.8).
Early or late administration of N‐acetylcysteine did not affect the outcome (Analysis 2.9). Although all of the methodologically sound trials involving this patient population observed that late administration might be harmful (Molnar 1999; Peake 1996; Spies 1994), our sensitivity analysis could not confirm this (Analysis 2.9) (eight studies, 362 patients). Peake and colleagues (Peake 1996) and later Spapen et al (Spapen 2005) also demonstrated that late administration of N‐acetylcysteine in patients with septic shock caused depression of cardiovascular performance, as indicated by a decrease in cardiac output and the mean arterial pressure. The possibility for excess mortality was attributed to depressed cardiovascular function with a higher need of vasopressors. This finding was corroborated by reports showing that delayed administration also failed to improve tissue oxygenation and partial pressure of arterial oxygen over fraction of inspired oxygen (PaO2/FiO2) ratio (Agusti 1997; Spapen 2005). This could not be confirmed when analysing the secondary outcome measures, mainly because of the lack of reported data on length of inotropic support. However, it has been mentioned in various trials that the infusion of N‐acetylcysteine caused temporary haemodynamic instability, but this was not quantified further (Emet 2004; Molnar 1999; Peake 1996; Rank 2000; Spapen 2005; Spies 1996).
Perhaps the failure to find a clinical role for therapies aimed at the reduction of oxygen free radicals is because the studies are based on the limited paradigm that oxygen free radicals only cause injury. An alternative view is that although oxygen free radicals are potentially highly toxic, redox reactions are also part of the basic chemical processes of life. Oxygen free radicals are an essential part of many metabolic pathways; they are part of the frameworkof basic energy producing processes. Organisms have had to evolve elaborate mechanisms to live with these reactive molecules and seem also to have evolved to use the reactive nature of these molecules for intracellular signal transduction (Magder 2006). Based on these results a key concept in dealing with oxygen free radicals must be to regulate but not eradicate them.
Early administration, on the other hand, whilst not showing a statistically significant effect did indicate a possible benefit (23 studies) (Analysis 2.9). Similar signal was detected when only the high quality trials were analysed (data not shown). Early administration of N‐acetylcysteine could beneficially affect the lipid peroxidation of cell membranes. It is becoming increasingly apparent that redox‐sensitive signalling pathways, known to be important in initiating inflammatory‐immune activation pathways, influence a broad array of cell signalling and gene pathways in the context of the transition metal milieu (Cross 2006). These can relate to antimicrobial host defence and to reparative and adaptive processes, sometimes involving production of anti‐inflammatory cytokines and wound healing (Cross 2006).
The dose of N‐acetylcysteine in the included trials showed great variability, from a single bolus of 50 mg/kg to continuous infusions delivering N‐acetylcysteine in doses in the region of 15,000 to 20,000 mg/day. The rationale for the different regimens in different studies has not been clarified and so their clinical relevance is questionable. It is possible that the applied dose was either excessive or inadequate in some of the studies. The majority of the studies based their dosing regimens on the protocol for patients receiving treatment for acetaminophen poisoning (150 mg/kg). It has been suggested that the initial concentration of 150 mg/kg N‐acetylcysteine should be modified since liver damage in acetaminophen poisoning was prevented just as effectively by the lowest as well as at the highest plasma concentrations of the drug (Prescott 1989). The possible disadvantage of excessive doses could be the formation of toxic intermediate molecules during N‐acetylcysteine metabolism, such as thiol and glutathionyl free radicals, glutathione disulfide and cysteine (Harman 1984).
The studies showed a great variability regarding the length of treatment, from one single bolus to six days of continuous treatment, with no evidence to support any of the applied methods. Interestingly, the most recent experimental data indicates that prolonged N‐acetylcysteine was deleterious even in acetaminophen intoxication, for which N‐acetylcysteine is normally the antidote (Yang 2009). Based on our data, we could not find any correlation between the dosage of N‐acetylcysteine and outcome in this patient population.
Summary of main results
In summary, we found that N‐acetylcysteine is ineffective in reducing mortality and morbidity in SIRS and sepsis.
Overall completeness and applicability of evidence
Our study pools data on the effect of intravenous N‐acetylcysteine from 41 studies involving 2768 patients. The trials were identified following a detailed systematic search of the literature. Study inclusion criteria were tightly defined and the meta‐analysis was rigorously conducted according to a predefined analysis plan addressing specific hypotheses. The meta‐analysis combined data from a group of predominantly underpowered single centre studies. However, the included studies reflect international practice, from North America to the Middle East and Australasia, although the majority of included studies are from major teaching centres.
Although there was minimal heterogeneity among trial results on mortality, we are aware that we pooled clinical trials that are typical of critical care and perioperative medicine in terms of age, patients, settings, and treatment regimens. Thus, the validity of our meta‐analysis may be criticized. However, all trials included critically ill patients where a common systemic inflammatory pathway was activated. Therefore, we think that there is a good biologic reason to perform a broad meta‐analysis, which also considerably increases the generalizability and usefulness of the review (Goetzsche 2000).
Quality of the evidence
The quality of the included studies varied and it was apparent from the result of the sensitivity analysis that internal flaws in the study design could result in significant bias. Only 20 out of 41 trials were scored as high quality and according to our power calculation the sample size of 1524 patients that was included in these trials is not enough to exclude malevolence. To ascertain the presence of a 2% relative risk reduction, according to our calculations 8548 patients would be needed in a well‐designed, transparently reported multicentre trial to clear the doubts about the use of N‐acetylcysteine in the critically ill.
Potential biases in the review process
Several possible sources of bias arise in this meta‐analysis. First, selection bias is possible with every review. To avoid this, we searched for studies from several databases without language restrictions. Second, the possibility of publication bias cannot be excluded. Visual inspection of the Funnel plot also suggested that publication bias is unlikely. Third, flaws in the original study designs are a significant potential source of bias. The studies included in this review are typical of studies in critical care research in general in that the vast majority of studies are underpowered and single centre. The meta‐analysis includes 2768 patients but the unit of analysis is the study (or study subgroup) and the sample size (41 studies) is relatively small. Although defined a priori, our stratified analysis (subgroup analysis) should be seen as hypothesis generating only. Fourth, while studies utilized different N‐acetylcysteine doses and schedules, we were unable to assess this heterogeneity on outcomes, thus we cannot discern the optimal N‐acetylcysteine dosing from our meta‐analysis.
Agreements and disagreements with other studies or reviews
This review represents the best up‐to‐date summary of the literature. We framed a tightly defined question and used explicit inclusion criteria for studies and a predefined analysis plan. Our primary result agrees with from those of recent reviews in this area, which have raised questions about the efficacy of N‐acetylcysteine in preventing renal dysfunction, other morbidity or death in the perioperative period (Adabag 2009; Baker 2009; Ho 2009; Naughton 2008; Nigwekar 2009).
Clinical application of N‐acetylcysteine in sepsis has been questioned in two recent editorials in leading journals (Molnar 2008; Spapen 2004). These voiced similar concerns to ours regarding the efficacy and safety of the drug.
Authors' conclusions
Implications for practice.
Overall, this meta‐analysis puts doubt on the safety and utility of intravenous N‐acetylcysteine as an adjuvant therapy in SIRS and sepsis. At best, N‐acetylcysteine is ineffective in reducing mortality and complications in this patient population. At worst, it can be harmful, especially when administered later than 24 hours after the onset of symptoms, by causing cardiovascular depression. Unless future RCTs provide evidence of treatment effect, clinicians should not routinely use intravenous N‐acetylcysteine in SIRS and sepsis and academics should not promote its use.
Implications for research.
Many issues regarding the use of N‐acetylcysteine in patients with SIRS and sepsis have received remarkably poor attention or remain unresolved. The pharmacokinetics and pharmacodynamics of the drug are virtually unknown. It remains to be determined whether and how N‐acetylcysteine influences basic cellular processes such as bacterial clearance, neutrophil‐endothelial cell interplay and apoptosis. More information is needed about possible drug interactions and toxic effects of N‐acetylcysteine or its metabolites. Until we have a better understanding of these questions, intravenous N‐acetylcysteine should only be used in adequately powered (and preferably multicentre) studies testing an explicitly framed hypothesis. These should be methodologically rigorous and blinded.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Harald Herkner (content editor), Marialena Trivella (statistical editor), William L. Baker, Mical Paul (peer reviewers), Ann E. Fonfa (CARG consumer panel representative) for their help and editorial advice during the preparation of the systematic review. We would also like to thank Hywel Roberts, Harald Herkner, Mitchell J Schwaber, Mical Paul, Herbert Spapen, Karen Hovhannisyan, Marialena Trivella and Jane Cracknell for their help and editorial advice during the preparation of the protocol for the systematic review.
Appendices
Appendix 1. Search strategy to identify eligible trials in MEDLINE
| Search terms |
| #52 #25 and #51 |
| #51 #34 or #50 |
| #50 #49 not #34 |
| #49 #47 not #48 |
| #48 (TG=ANIMALS) not ((TG=HUMAN) and (TG=ANIMALS)) |
| #47 #35 or #36 or #37 or #38 or #40 or #41 or #42 or #43 or #44 or #45 or #46 |
| #46 RESEARCH‐DESIGN |
| #45 random* in AB |
| #44 random* in TI |
| #43 placebo* in AB |
| #42 placebo* in TI |
| #41 PLACEBOS |
| #40 (#39 in TI) or (#39 in AB) |
| #39 (singl* or doubl* or trebl* or tripl*) near (blind* or mask*) |
| #38 (clin* near trial*) in AB |
| #37 (clin* near trial*) in TI |
| #36 explode CLINICAL‐TRIALS / all subheadings |
| #35 CLINICAL‐TRIAL in PT |
| #34 #32 not #33 |
| #33 (TG=ANIMALS) not ((TG=HUMAN) and (TG=ANIMALS)) |
| #32 #26 or #27 or #28 or #29 or #30 or #31 |
| #31 SINGLE‐BLIND‐METHOD |
| #30 DOUBLE‐BLIND‐METHOD |
| #29 RANDOM‐ALLOCATION |
| #28 RANDOMIZED‐CONTROLLED‐TRIALS |
| #27 CONTROLLED‐CLINICAL‐TRIAL in PT |
| #26 RANDOMIZED‐CONTROLLED‐TRIAL in PT |
| #25 #15 and #24 |
| #24 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 |
| #23 Free?Radical* Scavenger* |
| #22 explode "Free‐Radical‐Scavengers" / all SUBHEADINGS in MIME,MJME |
| #21 Antioxidant* |
| #20 explode "Antioxidants‐" / all SUBHEADINGS in MIME,MJME |
| #19 NAC |
| #18 N?acetylcystein* |
| #17 acetylcystein* |
| #16 explode Acetylcysteine/ all subheadings |
| #15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 |
| #14 peri?operat* |
| #13 "Perioperative‐Care" / all SUBHEADINGS in MIME,MJME |
| #12 ("Intensive‐Care" / all SUBHEADINGS in MIME,MJME) or ("Intensive‐Care‐Units" / all SUBHEADINGS in MIME,MJME) |
| #11 explode Septicemia/ all subheadings |
| #10 septi* or sepsis |
| #9 septic shock or ARDS or Multiple?Organ* Failure or Sepsis complication* or Sepsis Syndrome or organ dysfunction* or pancreatitis or surgery |
| #8 ("Surgical‐Procedures‐Operative" / all SUBHEADINGS in MIME,MJME) or ("Specialties‐Surgical" / all SUBHEADINGS in MIME,MJME) or ("Surgery‐" / all SUBHEADINGS in MIME,MJME) |
| #7 explode "Respiratory‐Distress‐Syndrome‐Adult" / all SUBHEADINGS in MIME,MJME |
| #6 (explode "Pancreatitis‐" / all SUBHEADINGS in MIME,MJME) or (explode "Pancreatitis‐Acute‐Necrotizing" / all SUBHEADINGS in MIME,MJME) |
| #5 explode "Multiple‐Organ‐Failure" / all SUBHEADINGS in MIME,MJME |
| #4 explode Shock‐Septic/ all subheadings |
| #3 system* infla?mator* response syndrome |
| #2 explode "Sepsis‐Syndrome" / all SUBHEADINGS in MIME,MJME |
| #1 explode "Sepsis‐" / all SUBHEADINGS in MIME,MJME |
Appendix 2. Search strategy to identify eligible trials from the CENTRAL database
| Search terms |
| #1 MeSH descriptor Sepsis explode all trees |
| #2 MeSH descriptor Sepsis Syndrome explode all trees |
| #3 system* infla?mator* response syndrome |
| #4 MeSH descriptor Shock, Septic explode all trees |
| #5 MeSH descriptor Multiple Organ Failure explode all trees |
| #6 MeSH descriptor Pancreatitis, this term only |
| #7 MeSH descriptor Respiratory Distress Syndrome, Adult, this term only |
| #8 MeSH descriptor Surgery explode all trees |
| #9 MeSH descriptor Surgical Procedures, Operative, this term only |
| #10 MeSH descriptor Specialties, Surgical explode all trees |
| #11 MeSH descriptor Intensive Care explode all trees |
| #12 MeSH descriptor Intensive Care Units explode all trees |
| #13 MeSH descriptor Perioperative Care explode all trees |
| #14 MeSH descriptor Perioperative Care explode all trees |
| #15 septic shock or ARDS or Multiple?Organ* Failure or Sepsis complication* or Sepsis Syndrome or organ dysfunction* or pancreatitis or surgery |
| #16 peri?operat* or septi* or sepsis |
| #17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 oR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) |
| #18 MeSH descriptor Acetylcysteine explode all trees |
| #19 MeSH descriptor Antioxidants explode all trees |
| #20 MeSH descriptor Free Radical Scavengers explode all trees |
| #21 (FreeRadical* Scavenger*) or (Free‐Radical* Scavenger*) or (Free Radica |
| #22 (18 OR 19 OR 20 OR 21) |
| #23 (17 AND 22) |
Appendix 3. Search strategy to identify eligible trials from the CINAHL database
| Search terms |
| #1 explode Sepsis‐ / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #2 explode Systemic‐Inflammatory‐Response‐Syndrome / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #3 explode Shock‐Septic / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #4 explode Multiple‐Organ‐Dysfunction‐Syndrome / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #5 Pancreatitis‐ / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #6 (explode Respiratory‐Distress‐Syndrome / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE) or (explode Respiratory‐Distress‐Syndrome‐Acute / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE) |
| #7 explode Surgery‐Operative / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #8 (Critical‐Care / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE) or (Intensive‐Care‐Units / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE) |
| #9 Perioperative‐Care / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #10 system* infla?mator* response syndrome |
| #11 septic shock or ARDS or Multiple?Organ* Failure or Sepsis complication* or Sepsis Syndrome or organ dysfunction* or pancreatitis or surgery or septi* or sepsis or peri?operat* |
| #12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 |
| #13 explode "Acetylcysteine‐" / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #14 explode "Antioxidants‐" / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #15 explode "Free‐Radical‐Scavengers" / all TOPICAL SUBHEADINGS / all AGE SUBHEADINGS in DE |
| #16 (Free?Radical* Scavenger*) or Antioxidant* or NAC or N?acetylcystein* or acetylcystein* |
| #17 #13 or #14 or #15 or #16 |
| #18 #12 and #17 |
Appendix 4. Search strategy to identify eligible trials in the EMBASE database
| Search terms |
| #40 #22 and #39 |
| #39 #34 not #38 |
| #38 #36 not #37 |
| #37 #35 and #36 |
| #36 (ANIMAL or NONHUMAN) in DER |
| #35 HUMAN in DER |
| #34 #31 or #32 or #33 |
| #33 (SINGL* or DOUBL* or TREBL* or TRIPL*) near ((BLIND* or MASK*) in TI,AB) |
| #33 (SINGL* or DOUBL* or TREBL* or TRIPL*) near ((BLIND* or MASK*) in TI,AB) |
| #32 (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI,AB |
| #31 #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 |
| #30 "SINGLE‐BLIND‐PROCEDURE"/ all subheadings |
| #29 "DOUBLE‐BLIND‐PROCEDURE"/ all subheadings |
| #28 "PHASE‐4‐CLINICAL‐TRIAL"/ all subheadings |
| #27 "PHASE‐3‐CLINICAL‐TRIAL"/ all subheadings |
| #26 "MULTICENTER‐STUDY"/ all subheadings |
| #25 "CONTROLLED‐STUDY"/ all subheadings |
| #24 "RANDOMIZATION"/ all subheadings |
| #23 "RANDOMIZED‐CONTROLLED‐TRIAL"/ all subheadings |
| #22 #15 and #21 |
| #21 #16 or #17 or #18 or #19 or #20 |
| #20 acetylcystein* or N?acetylcystein* or NAC or Antioxidant* or (Free?Radical* Scavenger*) |
| #19 explode "scavenger‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #18 explode "antioxidant‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #17 explode "antioxidant‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #16 explode "acetylcysteine‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 |
| #14 peri?operat* |
| #13 "perioperative‐period" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #12 "intensive‐care" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #11 explode "septicemia‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #10 septi* or sepsis |
| #9 septic shock or ARDS or (Multiple?Organ* Failure) or (Sepsis complication*) or (Sepsis Syndrome) or (organ dysfunction*) or pancreatit* or surg* |
| #8 explode "surgical‐technique" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #7 explode "surgery‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #6 "adult‐respiratory‐distress‐syndrome" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #5 ("acute‐pancreatitis" / all SUBHEADINGS in DEM,DER,DRM,DRR) or ("pancreatitis‐" / all SUBHEADINGS in DEM,DER,DRM,DRR) |
| #4 explode "multiple‐organ‐failure" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #3 system* infla?mator* response syndrome |
| #2 explode "septic‐shock" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| #1 explode "sepsis‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
Data and analyses
Comparison 1. N‐acetylcysteine versus placebo ‐ primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day all‐cause Mortality | 11 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.43] |
Comparison 2. N‐acetylcysteine versus placebo ‐ sensitivity and subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all reported timescales) | 31 | 2461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 2 Mortality ‐ Overall risk of bias | 31 | 2461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 2.1 High risk of bias | 14 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.10] |
| 2.2 Low risk of bias | 17 | 1542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.19] |
| 3 Mortality ‐ adequate sequence generation | 32 | 2501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 3.1 Adequate sequence generation | 20 | 1899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.12] |
| 3.2 Inadequate or unclear sequence generation | 12 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.21] |
| 4 Mortality ‐ Allocation concealment | 33 | 2541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 4.1 Adequate allocation concealment | 22 | 1835 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.13] |
| 4.2 Inadequate or unclear allocation concealment | 11 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.20] |
| 5 Mortality ‐ Blinding | 31 | 2461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 5.1 Adequate blinding | 25 | 2228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.15] |
| 5.2 Inadequate or unclear blinding | 6 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.07] |
| 6 Mortality ‐ short and long timescales | 31 | 2481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 6.1 Short timescale | 14 | 1524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.09] |
| 6.2 Long term timescale | 18 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.83, 1.24] |
| 7 Mortality ‐ sepsis or other critical illness (SIRS) | 16 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.12] |
| 7.1 Mortality in sepsis | 8 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.41] |
| 7.2 Mortality in other critical illness (SIRS) | 9 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.08] |
| 8 Mortality ‐ medical or surgical patients | 29 | 2404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 8.1 Mortality in medical patients | 17 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 8.2 Mortality in surgical patients | 12 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.52] |
| 9 Mortality ‐ early or late administration | 31 | 2461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.08] |
| 9.1 Mortality in early administration | 24 | 2099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.04] |
| 9.2 Mortality in late administration | 8 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.86, 1.38] |
Comparison 3. N‐acetylcysteine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation | 10 | 516 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.17] |
Comparison 4. N‐acetylcysteine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of stay on the intensive care unit | 21 | 1451 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.80, 0.49] |
Comparison 5. N‐acetylcysteine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of new organ failure | 13 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.83, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 2005.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | Cardiac surgery, high risk | |
| Interventions | iv N‐acetylcysteine 100mg/kg bolus on induction then 20mg/kg/hr during surgery versus placebo (not specified) | |
| Outcomes | Inflammatory response, mortality | |
| Notes | No side effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation to treatment group not clearly stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of treatment: not stated. Blinding of assessor: not stated. Blinding of data analysis: not stated. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Bernard 1997.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | ARDS | |
| Interventions | iv N‐acetylcysteine 210 mg daily for 10 days versus placebo (5% dextrose) | |
| Outcomes | 30‐day mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer permuted block generation. |
| Allocation concealment (selection bias) | Low risk | Ready made concealed bags supplied by pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators, medical and nursing team and patients were blinded. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Bromley 1995.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Orthotopic liver transplantation | |
| Interventions | iv N‐acetylcysteine 150 mg/kg bolus then 12.5mg/kg/hr for 4 hrs then 6.25 mg/kg/hr until the end of surgery versus placebo (not specified) | |
| Outcomes | haemodynamic variables, 30‐day mortality, morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated "randomized". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated: "double‐blind". |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Burns 2005.
| Methods | Randomized, quadruple blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | iv N‐acetylcysteine 4 times 600mg in 24 hours, two intraoperatively two postoperatively versus placebo (5% dextrose) | |
| Outcomes | renal function, mortality, morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated permuted blocks strategy. |
| Allocation concealment (selection bias) | Low risk | Centrally prepared premixed and preprepared bags. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated in manuscript: Patients, health care clinicians, and individuals participating in the data collection and data analysis were blinded to treatment assignment. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Burns 2010.
| Methods | Randomised controlled trial | |
| Participants | Critically ill patients undergoing contrast enhanced CT | |
| Interventions | iv N‐acetylcysteine 5000mg before CT, 2500mg 6 hours and 12 hours after CT versus placebo (dextrose 5%) | |
| Outcomes | increase in creatinine by 50 micromol/L by day 5, ICU length of stay | |
| Notes | No adverse reactions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number tables. |
| Allocation concealment (selection bias) | Low risk | Study medication centrally prepared and supplied in identical bags. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, medical and nursing staff were blinded to the intervention. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Charunwatthana 2009.
| Methods | Randomized double blinded trial | |
| Participants | Severe malaria | |
| Interventions | iv N‐acetylcysteine 150mg/kg bolus, 50mg/kg for 4 hrs then 100mg/kg for 16 hrs versus placebo (dextrose 5%) | |
| Outcomes | lactate clearance, mortality, morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer allocated blocks. |
| Allocation concealment (selection bias) | Low risk | Identical ampoules supplied centrally. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Doctors, nurses and patients were masked for the treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Csontos 2011.
| Methods | Randomised controlled trial | |
| Participants | Patients with severe burns | |
| Interventions | N‐acetylcysteine 150mg/kg bolus and 12mg/kg/hr for 5 days versus standard treatment (no placebo) | |
| Outcomes | oxidative stress, inflammatory markers, oedema formation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states randomised controlled trial, but no method given for randomization. |
| Allocation concealment (selection bias) | High risk | N‐acetylcysteine was compared to standard treatment (no placebo). |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
De Backer 1996.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery, low risk | |
| Interventions | iv N‐acetylcysteine 72mg/kg bolus preoperatively then 72 mg/kg for 12 hrs versus placebo (0.9% NaCl) | |
| Outcomes | inflammatory mediators in BAL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated: "randomized". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Only stated: "double‐blinded fashion". |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Domenighetti 1997.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | ARDS | |
| Interventions | iv N‐acetylcysteine 190 mg/kg/day for 3 days versus placebo (not specified) | |
| Outcomes | mortality, morbidity, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states: "randomized". |
| Allocation concealment (selection bias) | Low risk | Similar vials. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Researchers, medical, nursing staff and patients blinded. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
El‐Hamamsy 2007.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine oral 600 mg preoperatively then 150 mg/kg iv bolus on incision versus placebo (5% dextrose) | |
| Outcomes | mortality, organ dysfunction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states: "randomized". |
| Allocation concealment (selection bias) | Low risk | Similar preparation for N‐acetylcysteine and placebo. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States: "double‐blinded". |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Emet 2004.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Severe sepsis | |
| Interventions | iv N‐acetylcysteine 150 mg/kg bolus then 12 mg/kg/hr for 6 hrs versus placebo (dextrose 5%) | |
| Outcomes | Gastric tonometry, inflammatory response, mortality, length of mechanical ventilation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated blocks. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drug solution and infusion was administered to all patients by a nurse without any knowledge about the study protocol. Patients and medical staff was blinded to the treatment. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Eren 2003.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | Cardiac surgery (low risk) | |
| Interventions | iv N‐acetylcysteine 100 mg/kg bolus on induction and 40 mg/kg/day for 24 hrs versus placebo (0.9% NaCl) | |
| Outcomes | haemodynamics, oxygenation, time for extubation, lipid peroxidation, mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | States: The decision of which procedure should be performed was made independently by the surgeon. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medical, nursing staff and patients. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Haase 2007.
| Methods | Randomized, multi blind, placebo‐controlled trial | |
| Participants | Cardiac surgery (high risk for postoperative renal failure) | |
| Interventions | iv N‐acetylcysteine 150mg/kg bolus on induction, 50mg/kg for 4 hrs then 100mg/kg for 16 hrs versus placebo (dextrose 5%) | |
| Outcomes | Absolute change in serum creatinine from baseline, morbidity, LOS, mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator with permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Central randomization, premixed bags were used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and all staff were blinded to the treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hilmi 2010.
| Methods | Randomised controlled double blinded clinical trial | |
| Participants | Patients undergoing orthotopic liver transplantation | |
| Interventions | N‐acetylcysteine 140mg/kg bolus at the start of surgery, 70mg/kg every 4 hours for a total of 12 doses versus placebo (5% dextrose) | |
| Outcomes | incidence of post‐transplantation acute kidney injury, liver graft performance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation in blocks. |
| Allocation concealment (selection bias) | Low risk | Central randomization, premixed bags were used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and all staff were blinded to the treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hynninen 2006.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Vascular surgery | |
| Interventions | iv N‐acetylcysteine 150 mg/kg bolus on induction then 150 mg/kg for 24 hrs versus placebo (dextrose 5%) | |
| Outcomes | renal dysfunction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation in blocks. |
| Allocation concealment (selection bias) | Low risk | Central randomization, premixed bags. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and staff. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Jepsen 1992.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | ARDS | |
| Interventions | iv N‐acetylcysteine 150 mg/kg bolus and 20 mg/kg/hr for 7 days versus placebo (not stated) | |
| Outcomes | resolution of ARDS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated: patients were randomized. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and staff were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Keays 1991.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | Patients with fulminant liver failure | |
| Interventions | iv N‐acetylcysteine 150mg/kg bolus on induction, 50mg/kg for 4 hrs then 100mg/kg for 16 hrs versus placebo (dextrose 5%) until recovery from encephalopathy or death | |
| Outcomes | mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of treatment: no. Blinding of assessor: not stated. Blinding of data analysis: not stated. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Lee 2009.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Liver failure patients waiting for liver transplantation | |
| Interventions | iv N‐acetylcysteine 150 mg/kg/h over 1 hour, followed by 12.5 mg/kg/h for 4 hours, then continuous infusions of 6.25 mg/kg N‐acetylcysteine for the remaining 67 hours versus placebo (5% dextrose) | |
| Outcomes | 21‐day mortality, transplant‐free survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Central allocation, premixed bags prepared by pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, staff and investigators blinded for treatment intervention. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Macedo 2006.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Vascular surgery patients | |
| Interventions | N‐acetylcysteine 2400 mg oral before surgery then 1200 mg iv for 48 hrs postop versus no treatment | |
| Outcomes | occurrence of acute renal failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | High risk | N‐acetylcysteine versus no treatment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated "double‐blinded" but compared N‐acetylcysteine versus no treatment. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Molnar 1999.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Ventilated patients with two or more organ failures | |
| Interventions | iv N‐acetylcysteine 150 mg/kg bolus then 12 mg/kg/hr for 3 to 5 days versus placebo (dextrose 5%) | |
| Outcomes | mortality, resolution of organ dysfunction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, medical and nursing staff were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Moradi 2009.
| Methods | Randomized, single blinded, placebo‐controlled trial | |
| Participants | ARDS | |
| Interventions | iv N‐acetylcysteine 150 mg/kg bolus then 50 mg/kg/day for 3 days versus placebo (dextrose 5%) | |
| Outcomes | mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated; "simple randomization". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of treatment: patients only. Blinding of assessor: no. Blinding of data analysis: not stated. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Orhan 2006.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery (low risk) | |
| Interventions | iv N‐acetylcysteine 50 mg/kg bolus on induction vs no treatment | |
| Outcomes | oxidative stress levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | High risk | Method of allocation to treatment group not clearly stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated double blind, but investigated N‐acetylcysteine versus no treatment. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Ortolani 2000b.
| Methods | Randomised controlled trial | |
| Participants | ARDS patients | |
| Interventions | iv N‐acetylcysteine 50 mg/kg bolus 8 hourly for 9 days versus placebo (5% dextrose). Third group received 50 mg/kg N‐acetylcysteine plus rutin 5mg/kg, but this data is not included | |
| Outcomes | lipid peroxidation, glutathione levels | |
| Notes | Only iv N‐acetylcysteine and placebo group data are analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "patients were randomly assigned" but no method described. |
| Allocation concealment (selection bias) | High risk | No data. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blinded trial. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Ozaydin 2008.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine 50 mg/kg bolus preoperatively then 50 mg/kg/day for 48 hrs versus placebo (0.9% NaCl) | |
| Outcomes | incidence of postoperative AF | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment assignment. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Paterson 2003.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Patients with sepsis | |
| Interventions | N‐acetylcysteine 150mg/kg bolus then, 50mg/kg for 4 hrs then 50mg/kg for 16 hrs versus placebo (0.9% NaCl) for 72 hrs | |
| Outcomes | NF‐kB activation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope, premixed infusion. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for the treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Peake 1996.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Septic shock patients | |
| Interventions | N‐acetylcysteine 150mg/kg bolus then 50mg/kg for 4 hrs then 100mg/kg for 44 hrs versus placebo (dextrose 5%) | |
| Outcomes | improvement in haemodynamic variables, resolution of organ failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope, premixed bag of infusion by pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Peker 2008.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine 50 mg/kg bolus preoperatively then 50 mg/kg/day for 48 hrs versus placebo (0.9% NaCl) | |
| Outcomes | markers of myocardial injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated: patients were randomized. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, medical staff and investigators blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Rank 2000.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Septic shock | |
| Interventions | N‐acetylcysteine 150 mg/kg bolus then 12.5 mg/kg/hr versus placebo (dextrose 5%) for 90 minutes | |
| Outcomes | liver blood flow and liver function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states: "patients were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Ristikankare 2006.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine 150mg/kg bolus on induction, 50mg/kg for 4 hrs then 100mg/kg for 16 hrs versus placebo (0.9% NaCl) | |
| Outcomes | renal function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation. |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy prepared the study infusions. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, staff and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Sisillo 2008.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine 1200 mg bolus before induction then 1200 mg 12 hourly for 36 hrs versus placebo (0.9% NaCl) | |
| Outcomes | occurrence of acute renal failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocations. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Spapen 1998.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Septic shock patients | |
| Interventions | N‐acetylcysteine 150 mg/kg bolus then 50 mg/kg over 4 hours versus placebo (dextrose 5%) | |
| Outcomes | haemodynamic and oxygen transport variables, inflammatory markers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Infusions prepared by hospital pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocations. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Spapen 2005.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Severe sepsis | |
| Interventions | N‐acetylcysteine50 mg/kg/4 hrs followed by 100 mg/kg/24 hrs for 44 hrs versus placebo (5% dextrose) | |
| Outcomes | microalbuminuria, organ dysfunction scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Infusions prepared by hospital pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocations. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Spies 1994.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Septic shock patients | |
| Interventions | N‐acetylcysteine 150 mg/kg bolus then 12.5 mg/kg/hr for 90 minutes versus placebo (dextrose 5%) | |
| Outcomes | oxygen consumption, haemodynamic variables | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Spies 1996.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | High risk cardiac surgery patients | |
| Interventions | N‐acetylcysteine 150 mg/kg bolus versus placebo (dextrose 5%) | |
| Outcomes | VO2, tissue oxygenation | |
| Notes | No mortality or other clinical outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Steib 1998.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | Liver transplant patients | |
| Interventions | N‐acetylcysteine 150mg/kg bolus then, 50mg/kg for 4 hrs then 100mg/kg for 16 hrs versus placebo (dextrose 5%) | |
| Outcomes | intraoperative haemodynamics and postoperative graft function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not‐stated, only says "(patients) were allocated randomly to be administered either NAC or, ... placebo". |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation to treatment group not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of treatment: not stated.
Blinding of assessor: not stated.
Blinding of data analysis: not stated. Probably not, as no indication of blinding in the manuscript. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Sucu 2004.
| Methods | Randomized placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine 50 mg/kg on induction than daily for 3 days versus placebo (0.9% saline) | |
| Outcomes | Antioxidant capacity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of allocation sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation to treatment group not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of treatment: not stated. Blinding of assessor: not stated. Blinding of data analysis: not stated. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Suter 1994.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | ALI/ARDS | |
| Interventions | N‐acetylcysteine 40 mg/kg/day for 3 days versus placebo (not stated) | |
| Outcomes | development of ARDS, mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states: "patients were randomized". |
| Allocation concealment (selection bias) | Low risk | Similar vials for treatment and placebo. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Szakmany 2003.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Major abdominal surgery | |
| Interventions | N‐acetylcysteine 150 mg/kg bolus on induction then 12 mg/kg/hr intraoperatively versus placebo (dextrose 5%) | |
| Outcomes | organ dysfunction, mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, pre‐prepared infusions. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, staff and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Tossios 2003.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery | |
| Interventions | N‐acetylcysteine 100 mg/kg bolus on induction then 12 mg/kg/hr intraoperatively versus placebo (0.9% NaCl) | |
| Outcomes | antioxidant levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Identical appearance glass vials, supplied by pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, staff and investigators were blinded for treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Walsh 1998.
| Methods | Randomized controlled trial | |
| Participants | Fulminant liver failure patients | |
| Interventions | iv N‐acetycysteine 150 mg/kg body over 15 minutes, followed by 50 mg/kg over 4 hours, followed by 100mg/kg over 16 hours versus placebo (5% dextrose) | |
| Outcomes | haemodynamic variables, N‐acetycysteine levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, matching infusion bags. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Wijeysundera 2007.
| Methods | Randomized, double blinded, placebo‐controlled trial | |
| Participants | Cardiac surgery with moderate renal insufficiency | |
| Interventions | N‐acetylcysteine 100 mg/kg on induction then 20 mg/kg/hr for 4 hrs versus placebo (dextrose 5%) | |
| Outcomes | renal impairment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random tables. |
| Allocation concealment (selection bias) | Low risk | Central randomization and preparation of drugs by independent pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, clinicians and data‐collectors were blinded to treatment allocation. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adabag 2008 | Oral N‐acetylcysteine |
| Agusti 1997 | Cross‐over design |
| Barr 2008 | Oral N‐acetylcysteine |
| Csontos 2011b | Duplicate publication |
| De Rosa 2000 | Oral N‐acetylcysteine |
| Devlin 1997 | Non‐RCT |
| Fischer 2005 | Duplicate publication |
| Fraga 2011 | i.v. N‐acetylcysteine and deferoxamine combination |
| Haase 2008 | Duplicate publication |
| Heller 2001 | i.v. N‐acetylcysteine regime versus different N‐acetylcysteine regime (not placebo) |
| Jepsen 1989 | Oral N‐acetylcysteine |
| Koksal 2008 | Non‐intravenous N‐acetylcysteine |
| Komisarof 2007 | Oral N‐acetylcysteine |
| Koramaz 2006 | Oral N‐acetylcysteine |
| Kurian 2010 | Non‐intravenous N‐acetylcysteine |
| Laurent 1996 | Duplicate publication |
| Lawlor 2007 | Oral N‐acetylcysteine |
| Milewski 2006 | Oral N‐acetylcysteine |
| Molnar 1998 | Duplicate publication |
| Molnar 2003 | Duplicate publication |
| Najafi 2009 | Duplicate publication |
| Niemi 2006 | Duplicate publication |
| Ortolani 2000a | N‐acetylcysteine and glutathione (GSH) in treatment arm |
| Reinhart 1995 | Duplicate publication |
| Sahib 2010 | Non‐randomized trial, oral N‐acetylcysteine |
| Saricaoglu 2005 | No clinical outcome data |
| Siriwardena 2007 | N‐acetylcysteine, selenium and vitamin C in treatment arm |
| Soltan‐Sharifi 2007 | Duplicate publication |
| Spies 1993 | Duplicate publication |
| Szakmany 2002 | Duplicate publication |
| Wijeysundera 2009 | Duplicate publication |
| Zingg 2007 | Non‐randomized trial (before‐after design) |
Differences between protocol and review
In the original protocol Martin Matejovic was one of the authors, however due to personal circumstances he could not contribute to the study any further. The authors are grateful for his contribution and help.
The authors decided to omit the planned subgroup analysis comparing bolus and continuous infusion administration of N‐acetylcysteine.
The authors decided to introduce a further primary outcome variable: mortality in the longest reported follow‐up period.
Contributions of authors
Conceiving the review: Tamas Szakmany (TSZ) Co‐ordinating the review: TSZ Undertaking manual searches: Balázs Hauser (BH), TSZ Screening search results: BH, TSZ Organizing retrieval of papers: BH Screening retrieved papers against inclusion criteria: BH Appraising quality of papers: TSZ, BH Abstracting data from papers: TSZ, BH Writing to authors of papers for additional information: TSZ Providing additional data about papers: TSZ Obtaining and screening data on unpublished studies: BH Data management for the review: TSz Entering data into Review Manager (RevMan 5.1): TSz, BH RevMan statistical data: TSZ Other statistical analysis not using RevMan: TSZ Double entry of data: (data entered by person one: TSZ; data entered by person two: BH) Interpretation of data: TSZ, BH, Peter Radermacher (PR) Statistical inferences: TSZ Writing the review: TSZ, BH, PR Securing funding for the review: TSZ, BH, PR Performing previous work that was the foundation of the present study: TSZ, BH, PR Guarantor for the review (one author): TSZ Person responsible for reading and checking review before submission: TSZ
Sources of support
Internal sources
-
Cwm Taf LHB, Directorate of Anaesthetics, Critical Care and Theatres, UK.
Departmental funding
External sources
No sources of support supplied
Declarations of interest
Dr Tamas Szakmany: received a Clinical Research Fellowship from the Welsh Assembly Government and numerous travel grants and speaker fees from Edwards Lifesciences, Philips, GSK and B Braun. None of these funds were in relation to the work published here. He conducted clinical trials using N‐acetylcysteine postoperatively (Szakmany 2003).
Dr Balázs Hauser: Grants pending; Young Investigator Award of the European Society of Intensive Care Medicine 2001: Experimental study with N‐acetylcysteine in porcine endotoxaemia; Roman Herzog Research fellowship of the Alexander von Humboldt Stiftung and the Gemeinnützige Hertie Stiftung 2003 to 2005: Experimental studies in porcine endotoxaemia. He has no competing financial interest regarding this manuscript
Prof Peter Radermacher: Prof Radermacher's institution received a research grant from the Deutsche Forschungsgemeinschaft (DFG project Ra 396/3‐2 entitled “ROS, iNOS, and HO‐1 in endotoxic shock”) that allowed a research fellow from his institution to perform a study (Vassilev 2004) on N‐acetylcysteine in porcine endotoxaemia.
Tamas Szakmany, Balázs Hauser and Peter Radermacher conducted animal studies using N‐acetylcysteine.
None of the authors have received financial support from pharmaceutical companies marketing N‐acetylcysteine.
Edited (no change to conclusions)
References
References to studies included in this review
Andersen 2005 {published data only}
- Andersen LW, Thiis J, Kharazmi A, Rygg I. The role of N‐acetylcystein administration on the oxidative response of neutrophils during cardiopulmonary bypass. Perfusion 2005;10:21‐6. [DOI: 10.1177/026765919501000105] [DOI] [PubMed] [Google Scholar]
Bernard 1997 {published data only}
- Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, et al. A trial of antioxidants N‐acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997;112:164‐72. [PUBMED: 9228372] [DOI] [PubMed] [Google Scholar]
Bromley 1995 {published data only}
- Bromley PN, Cottam SJ, Hilmi I, Tan KC, Heaton N, Ginsburg R, et al. Effects of intraoperative N‐acetylcysteine in orthotopic liver transplantation. British Journal of Anaesthesia 1995;75(3):352‐4. [PUBMED: 7547057] [DOI] [PubMed] [Google Scholar]
Burns 2005 {published data only}
- Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, et al. Perioperative N‐acetylcysteine to prevent renal dysfunction in high‐risk patients undergoing cabg surgery: a randomized controlled trial. JAMA 2005;294(3):342‐50. [PUBMED: 16030279] [DOI] [PubMed] [Google Scholar]
Burns 2010 {published data only}
- Burns KE, Priestap F, Martin C. N‐acetylcysteine in critically ill patients undergoing contrast‐enhanced computed tomography: a randomized trial. Clinical Nephrology 2010;74(4):323‐6. [DOI] [PubMed] [Google Scholar]
Charunwatthana 2009 {published data only}
- Charunwatthana P, Abul Faiz M, Ruangveerayut R, Maude RJ, Rahman MR, Roberts LJ 2nd, et al. N‐acetylcysteine as adjunctive treatment in severe malaria: a randomized, double‐blinded placebo‐controlled clinical trial. Critical Care Medicine 2009;37(2):516‐22. [DOI: 10.1097/CCM.0b013e3181958dfd] [DOI] [PMC free article] [PubMed] [Google Scholar]
Csontos 2011 {published data only}
- Csontos C, Rezman B, Foldi V, Bogar L, Drenkovics L, Röth E, et al. Effect of N‐acetylcysteine treatment on oxidative stress and inflammation after severe burn. Burns 2011;38(3):428‐37. [DOI: ; PUBMED: 21978796] [DOI] [PubMed] [Google Scholar]
De Backer 1996 {published data only}
- Backer WA, Amsel B, Jorens PG, Bossaert L, Hiemstra PS, Noort P, et al. N‐acetylcysteine pretreatment of cardiac surgery patients influences plasma neutrophil elastase and neutrophil influx in bronchoalveolar lavage fluid. Intensive Care Medicine 1996;22(9):900‐8. [PUBMED: 8905424] [DOI] [PubMed] [Google Scholar]
Domenighetti 1997 {published data only}
- Domenighetti G, Suter PM, Schaller MD, Ritz R, Perret C. Treatment with N‐acetylcysteine during acute respiratory distress syndrome: a randomized, double‐blind, placebo‐controlled clinical study. Journal of Critical Care 1997;12(4):177‐82. [PUBMED: 9459113 ] [DOI] [PubMed] [Google Scholar]
El‐Hamamsy 2007 {published data only}
- El‐Hamamsy I, Stevens LM, Carrier M, Pellerin M, Bouchard D, Demers P, et al. Effect of intravenous N‐acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double‐blind, placebo‐controlled clinical trial. The Journal of Thoracic and Cardiovascular Surgery 2007;133:7‐12. [PUBMED: 17198774] [DOI] [PubMed] [Google Scholar]
Emet 2004 {published data only}
- Emet S, Memis D, Pamukçu Z. The influence of N‐acetyl‐L‐cystein infusion on cytokine levels and gastric intramucosal pH during severe sepsis. Critical Care 2004;8(4):229‐30. [DOI: 10.1186/cc2866] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eren 2003 {published data only}
- Eren N, Cakir O, Oruc A, Kaya Z, Erdinc L. Effects of N‐acetylcysteine on pulmonary function in patients undergoing coronary artery bypass surgery with cardiopulmonary bypass. Perfusion 2003;18(6):345‐50. [DOI: 10.1191/0267659103pf696oa] [DOI] [PubMed] [Google Scholar]
Haase 2007 {published data only}
- Haase M, Haase‐Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, et al. Phase II, randomized, controlled trial of high‐dose N‐acetylcysteine in high‐risk cardiac surgery patients. Critical Care Medicine 2007;35(5):1324‐31. [DOI: 10.1097/01.CCM.0000261887.69976.12] [DOI] [PubMed] [Google Scholar]
Hilmi 2010 {published data only}
- Hilmi IA, Peng, Z, Planinsic RM, Damian D, Dai F, Tyurina YY, Kagan VE, Kellum JA. N‐acetylcysteine does not prevent hepatorenal ischaemia–reperfusion injury in patients undergoing orthotopic liver transplantation. Nephrology, Dialysis, Transplantation 2010;25:2328‐2333. [DOI: 10.1093/ndt/gfq077] [DOI] [PubMed] [Google Scholar]
Hynninen 2006 {published data only}
- Hynninen MS, Niemi TT, Pöyhiä R, Raininko EI, Salmenperä MT, Lepäntalo MJ, et al. N‐acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: a randomized, double‐blind, placebo‐controlled trial. Anesthesia and Analgesia 2006;102(6):1638‐45. [DOI: 10.1213/01.ANE.0000219590.79796.66] [DOI] [PubMed] [Google Scholar]
Jepsen 1992 {published data only}
- Jepsen S, Herlevsen P, Knudsen P, Bud MI, Klausen NO. Antioxidant treatment with N‐acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo‐controlled study. Critical Care Medicine 1992;20(7):918‐23. [PUBMED: 1617983] [DOI] [PubMed] [Google Scholar]
Keays 1991 {published data only}
- Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ 1991;303(6809):1026‐9. [PUBMED: 1954453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N‐acetylcysteine improves transplant‐free survival in early stage non‐acetaminophen acute liver failure. Gastroenterology 2009;137(3):856‐64. [DOI: 10.1053/j.gastro.2009.06.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macedo 2006 {published data only}
- Macedo E, Abdulkader R, Castro I, Sobrinho AC, Yu L, Vieira JM Jr. Lack of protection of N‐acetylcysteine (NAC) in acute renal failure related to elective aortic aneurysm repair‐a randomized controlled trial. Nephrology, Dialysis, Transplantation 2006;21(7):1863‐9. [DOI: 10.1093/ndt/gfl079; PUBMED: 16522657] [DOI] [PubMed] [Google Scholar]
Molnar 1999 {published data only}
- Molnár Z, Shearer E, Lowe D. N‐Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo‐controlled study. Critical Care Medicine 1999;27(6):1100‐4. [PUBMED: 10397212] [DOI] [PubMed] [Google Scholar]
Moradi 2009 {published data only}
- Moradi M, Mojtahedzadeh M, Mandegari A, Soltan‐Sharifi MS, Najafi A, Khajavi MR, et al. The role of glutathione‐S‐transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N‐acetylcysteine. Respiratory Medicine 2009;103(3):434‐41. [PUBMED: 18993042] [DOI] [PubMed] [Google Scholar]
Orhan 2006 {published data only}
- Orhan G, Yapici N, Yuksel M, Sargin M, Senay S, Yalcin AS, et al. Effects of N‐acetylcysteine on myocardial ischemia–reperfusion injury in bypass surgery. Heart and Vessels 2006;21:42‐7. [PUBMED: 16440148] [DOI] [PubMed] [Google Scholar]
Ortolani 2000b {published data only}
- Ortolani O, Conti A, Gaudio AR, Masoni M, Novelli G. Protective effects of N‐acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock 2000;13(1):14‐8. [PUBMED: 10638663] [DOI] [PubMed] [Google Scholar]
Ozaydin 2008 {published data only}
- Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, et al. N‐acetylcysteine for prevention of postoperative atrial fibrillation: a prospective, randomized, placebo‐controlled pilot study. European Heart Journal 2008;29:625‐31. [DOI: 10.1093/eurheartj/ehn011; PUBMED: 18263874] [DOI] [PubMed] [Google Scholar]
Paterson 2003 {published data only}
- Paterson RL, Galley HF, Webster NR. The effect of N‐acetylcysteine on nuclear factor‐kappa B activation, interleukin‐6, interleukin‐8, and intercellular adhesion molecule‐1 expression in patients with sepsis. Critical Care Medicine 2003;31(11):2474‐8. [DOI: 10.1097/01.CCM.0000089945.69588.18; PUBMED: 14605526] [DOI] [PubMed] [Google Scholar]
Peake 1996 {published data only}
- Peake SL, Moran JL, Leppard PI. N‐acetyl‐L‐cysteine depresses cardiac performance in patients with septic shock. Critical Care Medicine 1996;24(8):1302‐10. [PUBMED: 8706483] [DOI] [PubMed] [Google Scholar]
Peker 2008 {published data only}
- Peker O, Peker T, Erdogan D, Ozaydin M, Kapan S, Sutcu R, et al. Effects of intravenous N‐acetylcysteine on periprocedural myocardial injury after on‐pump coronary artery by‐pass grafting. The Journal of Cardiovascular Surgery 2008;49(4):527‐31. [PUBMED: 18665117] [PubMed] [Google Scholar]
Rank 2000 {published data only}
- Rank N, Michel C, Haertel C, Lenhart A, Welte M, Meier‐Hellmann A, et al. N‐acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double‐blind study. Critical Care Medicine 2000;28(12):3799‐807. [PUBMED: 11153617] [DOI] [PubMed] [Google Scholar]
Ristikankare 2006 {published data only}
- Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta‐Ylinen R, et al. Lack of renoprotective effect of i.v. N‐acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. British Journal of Anaesthesia 2006;97(5):611‐6. [DOI: 10.1093/bja/ael224; PUBMED: 16914459] [DOI] [PubMed] [Google Scholar]
Sisillo 2008 {published data only}
- Sisillo E, Ceriani R, Bortone F, Juliano G, Salvi L, Veglia F, et al. N‐acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Critical Care Medicine 2008;36(1):81‐6. [PUBMED: 18090169] [DOI] [PubMed] [Google Scholar]
Spapen 1998 {published data only}
- Spapen H, Zhang H, Demanet C, Vleminckx W, Vincent JL, Huyghens L. Does N‐acetyl‐L‐cysteine influence cytokine response during early human septic shock?. Chest 1998;113(6):1616‐24. [PUBMED: 9631802] [DOI] [PubMed] [Google Scholar]
Spapen 2005 {published data only}
- Spapen HD, Diltoer MW, Nguyen DN, Hendrickx I, Huyghens LP. Effects of N‐acetylcysteine on microalbuminuria and organ failure in acute severe sepsis: results of a pilot study. Chest 2005;127(4):1413‐9. [PUBMED: 15821223] [DOI] [PubMed] [Google Scholar]
Spies 1994 {published data only}
- Spies CD, Reinhart K, Witt I, Meier‐Hellmann A, Hannemann L, Bredle DL, et al. Influence of N‐acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: results from a prospective, randomized, double‐blind study. Critical Care Medicine 1994;22(11):1738‐46. [PUBMED: 7956276] [PubMed] [Google Scholar]
Spies 1996 {published data only}
- Spies C, Giese C, Meier‐Hellmann A, Specht M, Hannemann L, Schaffartzik W, et al. [The effect of prophylactically administered n‐acetylcysteine on clinical indicators for tissue oxygenation during hyperoxic ventilation in cardiac risk patients] [Einflus der prophylaktischen gabe von N‐Azetylzystein auf klinische indikatoren der gewebeoxygenierung unter hyperoxie bei kardialen rizikopatienten]. Anaesthesist 1996;45(4):343‐50. [PUBMED: 8702052] [DOI] [PubMed] [Google Scholar]
Steib 1998 {published data only}
- Steib A, Freys G, Collin F, Launoy A, Mark G, Boudjema K. Does N‐acetylcysteine improve hemodynamics and graft function in liver transplantation?. Liver Transplantation and Surgery 1998;4(2):152‐7. [PUBMED: 9516568] [DOI] [PubMed] [Google Scholar]
Sucu 2004 {published data only}
- Sucu N, Cinel I, Unlu A, Aytacoglu B, Tamer L, Kocak Z, et al. N‐acetylcysteine for preventing pump‐induced oxidoinflammatory response during cardiopulmonary bypass. Surgery Today 2004;34:237‐42. [DOI: 10.1007/s00595-003-2699-8; PUBMED: 14999536] [DOI] [PubMed] [Google Scholar]
Suter 1994 {published data only}
- Suter PM, Domenighetti G, Schaller MD, Laverrière MC, Ritz R, Perret C. N‐acetylcysteine enhances recovery from acute lung injury in man. A randomized, double‐blind, placebo‐controlled clinical study. Chest 1994;105(1):190‐4. [PUBMED: 8275731 ] [DOI] [PubMed] [Google Scholar]
Szakmany 2003 {published data only}
- Szakmany T, Marton S, Molnar Z. Lack of effect of prophylactic N‐acetylcysteine on postoperative organ dysfunction following major abdominal tumour surgery: a randomized, placebo‐controlled, double‐blinded clinical trial. Anaesthesia and Intensive Care 2003;31(3):267‐71. [PUBMED: 12879670] [DOI] [PubMed] [Google Scholar]
Tossios 2003 {published data only}
- Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, et al. N‐acetylcysteine prevents reactive oxygen species‐mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double‐blind, placebo‐controlled clinical trial. The Journal of Thoracic and Cardiovascular Surgery 2003;126(5):1513‐20. [DOI: 10.1016/S0022-5223(03)00968-1; PUBMED: 14666027] [DOI] [PubMed] [Google Scholar]
Walsh 1998 {published data only}
- Walsh TS, Hopton P, Philips BJ, Mackenzie SJ, Lee A. The effect of N‐acetylcysteine on oxygen transport and uptake in patients with fulminant hepatic failure. Hepatology 1998;27(5):1332‐40. [PUBMED: 9581688] [DOI] [PubMed] [Google Scholar]
Wijeysundera 2007 {published data only}
- Wijeysundera DN, Beattie WS, Rao V, Granton JT, Chan CT. N‐acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre‐existing moderate renal insufficiency. Canadian Journal of Anaesthesia 2007;54(11):872‐81. [PUBMED: 17975231] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adabag 2008 {published data only}
- Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, et al. Utility of N‐acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. American Heart Journal 2008;155(6):1143‐9. [DOI: 10.1016/j.ahj.2008.01.013; NCT00211653; PUBMED: 18513531] [DOI] [PubMed] [Google Scholar]
Agusti 1997 {published data only}
- Agustí AG, Togores B, Ibañez J, Raurich JM, Maimó A, Bergada J, et al. Effects of N‐acetylcysteine on tissue oxygenation in patients with multiple organ failure and evidence of tissue hypoxia. The European Respiratory Journal 1997;10(9):1962‐6. [DOI: 10.1183/09031936.97.10091962] [DOI] [PubMed] [Google Scholar]
Barr 2008 {published data only}
- Barr LF, Kolodner K. N‐acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Critical Care Medicine 2008;36(5):1427‐35. [PUBMED: 18434903] [DOI] [PubMed] [Google Scholar]
Csontos 2011b {published data only}
- Csontos C, Rezman B, Foldi V, Bogar L, Bognar Z, Drenkovics L, Röth E, Weber G, Lantos J. Effect of N‐acetylcysteine treatment on the expression of leukocyte surface markers after burn injury. Burns 2011;37(3):453‐64. [DOI: 10.1016/j.burns.2010.10.008; PUBMED: 21131132] [DOI] [PubMed] [Google Scholar]
De Rosa 2000 {published data only}
- Rosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, et al. N‐acetylcysteine replenishes glutathione in HIV infection. European Journal of Clinical Investigation 2000;30(10):915‐29. [PUBMED: 11029607] [DOI] [PubMed] [Google Scholar]
Devlin 1997 {published data only}
- Devlin J, Ellis AE, McPeake J, Heaton N, Wendon JA, Williams R. N‐acetylcysteine improves indocyanine green extraction and oxygen transport during hepatic dysfunction. Critical Care Medicine 1997;25(2):236‐42. [PUBMED: 9034257 ] [DOI] [PubMed] [Google Scholar]
Fischer 2005 {published data only}
- Fischer UM, Tossios P, Mehlhorn U. Renal protection by radical scavenging in cardiac surgery patients. Current Medical Research and Opinion 2005;21(8):1161‐4. [PUBMED: 16083524] [DOI] [PubMed] [Google Scholar]
Fraga 2011 {published data only}
- Fraga CM, Tomasi CD, Biff D, Topanotti MFL, Felisberto F, Vuolo F, et al. The effects of N‐acetylcysteine and deferoxamine on plasma cytokine and oxidative damage parameters in critically ill patients with prolonged hypotension: A randomized controlled trial. Journal of Clinical Pharmacology 2011;Nov 1:Epub ahead of print. [PUBMED: 22045831] [DOI] [PubMed] [Google Scholar]
Haase 2008 {published data only}
- Haase M, Haase‐Fielitz A, Ratnaike S, Reade MC, Bagshaw SM, Morgera S, et al. N‐Acetylcysteine does not artifactually lower plasma creatinine concentration. Nephrology, Dialysis, Transplantation 2008;23(5):1581‐7. [DOI: 10.1093/ndt/gfm818] [DOI] [PubMed] [Google Scholar]
Heller 2001 {published data only}
- Heller AR, Groth G, Heller SC, Breitkreutz R, Nebe T, Quintel M, et al. N‐acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Critical Care Medicine 2001;29(2):272‐6. [PUBMED: 11246305] [DOI] [PubMed] [Google Scholar]
Jepsen 1989 {published data only}
- Jepsen S, Klaerke A, Nielsen PH, Nielsen ST, Simonsen O. Systemic administration of N‐acetylcysteine has no effect on postoperative lung function following elective upper laparotomy in lung healthy patients. Acta Anaesthesiologica Scandinavica 1989;33(3):219‐22. [PUBMED: 2658458] [DOI] [PubMed] [Google Scholar]
Koksal 2008 {published data only}
- Köksal H, Rahman A, Burma O, Halifeoglu I, Bayar MK. The effects of low dose N‐acetylcysteine (NAC) as an adjunct to cardioplegia in coronary artery bypass surgery. Anadolu Kardiyoloji Dergisi: AKD = The Anatolian Journal of Cardiology 2008;8(6):437‐43. [PUBMED: 19103540] [PubMed] [Google Scholar]
Komisarof 2007 {published data only}
- Komisarof JA, Gilkey GM, Peters DM, Koudelka CW, Meyer MM, Smith SM. N‐acetylcysteine for patients with prolonged hypotension as prophylaxis for acute renal failure (NEPHRON). Critical Care Medicine 2007;35(2):435‐41. [DOI: 10.1097/01.CCM.0000253816.83011.DB] [DOI] [PubMed] [Google Scholar]
Koramaz 2006 {published data only}
- Koramaz I, Pulathan Z, Usta S, Karahan SC, Alver A, Yaris E, et al. Cardioprotective effect of cold‐blood cardioplegia enriched with N‐acetylcysteine during coronary artery bypass grafting. Annals of Thoracic Surgery 2006;81(2):613‐8. [DOI: 10.1016/j.athoracsur.2005.08.013] [DOI] [PubMed] [Google Scholar]
Kurian 2010 {published data only}
- Kurian GA, Paddikkala J. N‐acetylcysteine and magnesium improve biochemical abnormalities associated with myocardial ischaemic reperfusion in South Indian patients undergoing coronary artery bypass grafting: a comparative analysis. Singapore Medical Journal 2010;51(5):381‐8. [PubMed] [Google Scholar]
Laurent 1996 {published data only}
- Laurent T, Markert M, Feihl F, Schaller MD, Perret C. Oxidant‐antioxidant balance in granulocytes during ARDS. Effect of N‐acetylcysteine. Chest 1996;106(1):163‐6. [PUBMED: 8549180] [DOI] [PubMed] [Google Scholar]
Lawlor 2007 {published data only}
- Lawlor DK, Moist L, DeRose G, Harris KA, Lovell MB, Kribs SW, et al. Prevention of contrast‐induced nephropathy in vascular surgery patients. Annals of Vascular Surgery 2007;21(5):593‐7. [PUBMED: 17823041] [DOI] [PubMed] [Google Scholar]
Milewski 2006 {published data only}
- Milewski J, Rydzewska G, Degowska M, Kierzkiewicz M, Rydzewski A. N‐acetylcysteine does not prevent post‐endoscopic retrograde cholangiopancreatography hyperamylasemia and acute pancreatitis. World Journal of Gastroenterology : WJG 2006;12(23):3751‐5. [PUBMED: 16773694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molnar 1998 {published data only}
- Molnar Z, MacKinnon KL, Shearer E, Lowe D, Watson ID. The effect of N‐acetylcysteine on total serum anti‐oxidant potential and urinary albumin excretion in critically ill patients. Intensive Care Medicine 1998;24(3):230‐5. [PUBMED: 9565804] [DOI] [PubMed] [Google Scholar]
Molnar 2003 {published data only}
- Molnar Z, Szakmany T, Koszegi T. Prophylactic N‐acetylcysteine decreases serum CRP but not PCT levels and microalbuminuria following major abdominal surgery. A prospective, randomised, double‐blinded, placebo‐controlled clinical trial. Intensive Care Medicine 2003;29(5):749‐55. [PUBMED: 12682719] [DOI] [PubMed] [Google Scholar]
Najafi 2009 {published data only}
- Najafi A, MojtahedzadehM, Mahmoodpoor A, Aghamohammadi M, Ahmadi A, Nahreini S, et al. Effect of N‐acetylcysteine on microalbuminuria in patients with acute respiratory distress syndrome. Archives of Medical Science 2009;5(3):408‐14. [Google Scholar]
Niemi 2006 {published data only}
- Niemi TT, Munsterhjelm E, Pöyhiä R, Hynninen MS, Salmenperä MT. The effect of N‐acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm. Blood Coagulation & Fibrinolysis 2006;17(1):29‐34. [PUBMED: 16607076 ] [DOI] [PubMed] [Google Scholar]
Ortolani 2000a {published data only}
- Ortolani O, Conti A, Gaudio AR, Moraldi E, Cantini Q, Novelli G. The effect of glutathione and N‐acetylcysteine on lipoperoxidative damage in patients with early septic shock. American Journal of Respiratory Critical Care Medicine 2000;161(6):1907‐11. [PUBMED: 10852765] [DOI] [PubMed] [Google Scholar]
Reinhart 1995 {published data only}
- Reinhart K, Spies CD, Meier‐Hellmann A, Bredle DL, Hannemann L, Specht M, et al. N‐acetylcysteine preserves oxygen consumption and gastric mucosal pH during hyperoxic ventilation. American Journal of Respiratory and Critical Care Medicine 1995;151:773‐9. [PUBMED: 7881669] [DOI] [PubMed] [Google Scholar]
Sahib 2010 {published data only}
- Sahib AS, Al‐Jawad FH, Alkaisy AA. Effect of antioxidants on the incidence of wound infection in burn patients. Annals of Burns and Fire Disasters 2010;23(4):199‐205. [PUBMED: 21991225] [PMC free article] [PubMed] [Google Scholar]
Saricaoglu 2005 {published data only}
- Saricaoglu F, Dal D, Salman AE, Atay OA, Doral MN, Salman MA, et al. Effect of low‐dose N‐acetyl‐cysteine infusion on tourniquet‐induced ischaemia‐reperfusion injury in arthroscopic knee surgery. Acta Anaesthesiologica Scandinavica 2005;49(6):847‐51. [PUBMED: 15954970] [DOI] [PubMed] [Google Scholar]
Siriwardena 2007 {published data only}
- Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, et al. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n‐acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut 2007;56(10):1439‐44. [PUBMED: 17356040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soltan‐Sharifi 2007 {published data only}
- Soltan‐Sharifi MS, Mojtahedzadeh M, Najafi A, Reza Khajavi M, Reza Rouini M, Moradi M, et al. Improvement by N‐acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti‐oxidant power: evidence for underlying toxicological mechanisms. Human & Experimental Toxicology 2007;26(9):697‐703. [PUBMED: 17984140] [DOI] [PubMed] [Google Scholar]
Spies 1993 {published data only}
- Spies C, Reinhart K, Witt I, Walensky C, Hannemann L, Specht M, et al. Influence of N‐acetylcysteine on O2 consumption and gastric intramucosal pH in septic patients. Critical Care Medicine 1993;21 Suppl 4:183. [Google Scholar]
Szakmany 2002 {published data only}
- Szakmány T, Tóth I, Márton S, Molnár Z. [Effect of prophylactic N‐acetylcysteine on postoperative organ dysfunction and inflammatory markers after major abdominal surgery for cancer. Prospective, randomized, double‐blind, placebo‐controlled clinical trial] [Profilaktikus N‐acetylcysteine kezeles hatasa a posztoperativ szervdiszfunkciora es gyulladasos markerek szintjere kiterjesztett hasi tumorsebeszeti beavatkozasok utan. Prospektiv, randomizalt, kettos‐vak, placebo kontrolalt klinikai tanulmany]. Magyar Sebészet 2002;55(6):369‐74. [PUBMED: 12616822 ] [PubMed] [Google Scholar]
Wijeysundera 2009 {published data only}
- Wijeysundera DN, Karkouti K, Rao V, Granton JT, Chan CT, Raban R, et al. N‐acetylcysteine is associated with increased blood loss and blood product utilization during cardiac surgery. Critical Care Medicine 2009;37(6):1929‐34. [PUBMED: 19384218] [DOI] [PubMed] [Google Scholar]
Zingg 2007 {published data only}
- Zingg U, Hofer CK, Seifert B, Metzger U, Zollinger A. High dose N‐acetylcysteine to prevent pulmonary complications in partial or total transthoracic esophagectomy: results of a prospective observational study. Diseases of the Esophagus 2007;20(5):399‐405. [PUBMED: 17760653] [DOI] [PubMed] [Google Scholar]
Additional references
Adabag 2009
- Adabag AS, Ishani A, Bloomfield HE, Ngo AK, Wilt TJ. Efficacy of N‐acetylcysteine in preventing renal injury after heart surgery: a systematic review of randomized trials. European Heart Journal 2009;30(15):1910‐7. [PUBMED: 19282300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aruoma 1989
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N‐acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology & Medicine 1989;6:593‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baker 2009
- Baker WL, Anglade MW, Baker EL, White CM, Kluger J, Coleman CI. Use of N‐acetylcysteine to reduce post‐cardiothoracic surgery complications: a meta‐analysis. European Journal of Cardiothoracic Surgery 2009;35(3):521‐7. [PUBMED: 19147369] [DOI] [PubMed] [Google Scholar]
Bone 1992
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cowley 1996
- Cowley HC, Bacon PJ, Goode HF, Webster NR, Jones JG, Menon DK. Plasma antioxidant potential in severe sepsis: a comparison of survivors and nonsurvivors. Critical Care Medicine 1996;24:1179‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crimi 2006
- Crimi E, Sica V, Williams‐Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, Napoli C. The role of oxidative stress in adult critical care. Free Radical Biology & Medicine 2006;40(3):398‐406. [DOI: 10.1016/j.freeradbiomed.2005.10.054; PUBMED: 16443154] [DOI] [PubMed] [Google Scholar]
Cross 2006
- Cross CE, Asbeck BS, Halliwell B. More antioxidants in sepsis: still paved with uncertainties. Critical Care Medicine 2006;34(2):569‐571. [PUBMED: 16424756] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphic test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engel 2007
- Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Medicine 2007;33(4):606‐18. [DOI: 10.1007/s00134-006-0517-7; PUBMED: 17323051] [DOI] [PubMed] [Google Scholar]
Goetzsche 2000
- Gotzsche PC. Why we need a broad perspective on meta‐analysis.It may be crucially important for patients. BMJ 2000;321(7261):585‐6. [PUBMED: 10977820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goode 1995
- Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Critical Care Medicine 1995;23:646‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harman 1984
- Harman LS, Mottley C, Mason RP. Free radical metabolites of L‐cysteine oxidation. The Journal of Biological Chemistry 1984;259:5606‐11. [PUBMED: 6325443 ] [PubMed] [Google Scholar]
Henderson 1994
- Henderson A, Hayes P. Acetylcysteine as a cytoprotective antioxidant in patients with severe sepsis: potential new use for an old drug. The Annals of Pharmacotherapy 1994;28:1086‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statist Med 2002;21:1539‐58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from http://www.cochrane‐handbook.org: The Cochrane Collaboration, 2011. [Google Scholar]
Ho 2009
- Ho KM, Morgan DJ. Meta‐analysis of N‐acetylcysteine to prevent acute renal failure after major surgery. American Journal of Kidney Diseases 2009;53(1):33‐40. [DOI: 10.1053/j.ajkd.2008.05.019; PUBMED: 18649982] [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [PUBMED: 10480822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13‐23. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2004
- Hsu BG, Yang FL, Lee RP, Peng TC, Harn HJ, Chen HI. N‐acetylcysteine ameliorates lipopolysaccharide‐induced organ damage in conscious rats. Journal of Biomedical Science 2004;11:152‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kiefer 2000
- Kiefer P, Vogt J, Radermacher P. From mucolytic to antioxidant and liver protection: new aspects in the intensive care unit career of N‐acetylcysteine. Critical Care Medicine 2000;28:3935‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koch 1996
- Koch T, Heller S, Heissler S, Breil I, Schiefer HG, Ackern K, et al. Effects of N‐acetylcysteine on bacterial clearance. European Journal of Clinical Investigation 1996;26:884‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Magder 2006
- Magder S. Reactive oxygen species: toxic molecules or spark of life?. Critical Care 2006;10(1):208. [DOI: 10.1186/cc3992; PUBMED: 16469133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molnar 2008
- Molnár Z. N‐acetylcysteine as the magic bullet: too good to be true. Critical Care Medicine 2008;36(2):645‐6. [PUBMED: 18216629] [DOI] [PubMed] [Google Scholar]
Naughton 2008
- Naughton F, Wijeysundera D, Karkouti K, Tait G, Beattie WS. N‐acetylcysteine to reduce renal failure after cardiac surgery: a systematic review and meta‐analysis. Canadian Journal of Anaesthesia 2008;55(12):827‐35. [PUBMED: 19050086] [DOI] [PubMed] [Google Scholar]
Nigwekar 2009
- Nigwekar SU, Kandula P. N‐acetylcysteine in cardiovascular‐surgery‐associated renal failure: a meta‐analysis. Annals of Thoracic Surgery 2009;87(1):139‐47. [PUBMED: 19101287] [DOI] [PubMed] [Google Scholar]
Ogilvie 1991
- Ogilvie AC, Groeneveld AB, Straub JP, Thijs LG. Plasma lipid peroxides and antioxidants in human septic shock. Intensive Care Medicine 1991;17:40‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116:78‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peristeris 1992
- Peristeris P, Clark BD, Gatti S, Faggioni R, Mantovani A, Mengozzi M, et al. N‐acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cellular Immunology 1992;140:390‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pinsky 2003
- Pinsky MR. Antioxidant therapy for severe sepsis: promise and perspective. Critical Care Medicine 2003;31:2697‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prescott 1989
- Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N‐acetylcysteine in patients with paracetamol overdosage.. European Journal of Clinical Pharmacology 1989;37(5):501‐6. [PUBMED: 2598989] [DOI] [PubMed] [Google Scholar]
Repine 1992
- Repine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet 1992;339:466‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Schmidt 1997
- Schmidt H, Schmidt W, Müller T, Böhrer H, Gebhard MM, Martin E. N‐acetylcysteine attenuates endotoxin‐induced leukocyte‐endothelial cell adhesion and macromolecular leakage in vivo. Critical Care Medicine 1997;25:558‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmidt 1998
- Schmidt W, Walther A, Gebhard MM, Martin E, Schmidt H. Influence of N‐acetylcysteine treatment on endotoxin‐induced microcirculatory disturbances. Intensive Care Medicine 1998;24(9):967‐72. [DOI] [PubMed] [Google Scholar]
Spapen 2004
- Spapen H. N‐acetylcysteine in clinical sepsis: a difficult marriage. Critical Care 2004;8(4):229‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vassilev 2004
- Vassilev D, Hauser B, Bracht H, Iványi Z, Schoaff M, Asfar P, et al. Systemic, pulmonary, and hepatosplanchnic effects of N‐acetylcysteine during long‐term porcine endotoxemia. Critical Care Medicine 2004;32(2):525‐32. [PUBMED: 14758174] [DOI] [PubMed] [Google Scholar]
Yang 2009
- Yang R, Miki K, He X, Killeen ME, Fink MP. Prolonged treatment with N‐acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Critical Care 2009;13(2):R55. [DOI: 10.1186/cc7782; PUBMED: 19358737] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 1994a
- Zhang H, Spapen H, Benlabed M, Nguyen DN, Buurman WA, Vincent JL. Pentoxifylline improves the tissue oxygen extraction capabilities during endotoxic shock. Shock 1994;2:90‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 1994b
- Zhang H, Spapen H, Nguyen DN, Benlabed M, Buurman WA, Vincent JL. Protective effects of N‐acetyl‐L‐cysteine in endotoxemia. The American Journal of Physiology 1994;266:H1746‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


