Abstract
Background
The diagnosis of death using neurological criteria (brain death) has profound social, legal and ethical implications. The diagnosis can be made using standard clinical tests examining for brain function, but in some patient populations and in some countries additional tests may be required. Computed tomography (CT) angiography, which is currently in wide clinical use, has been identified as one such test.
Objectives
To assess from the current literature the sensitivity of CT cerebral angiography as an additional confirmatory test for diagnosing death using neurological criteria, following satisfaction of clinical neurological criteria for brain death.
Search methods
We performed comprehensive literature searches to identify studies that would assess the diagnostic accuracy of CT angiography (the index test) in cohorts of adult patients, using the diagnosis of brain death according to neurological criteria as the target condition. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 5) and the following databases from January 1992 to August 2012: MEDLINE; EMBASE; BNI; CINAHL; ISI Web of Science; BioMed Central. We also conducted searches in regional electronic bibliographic databases and subject‐specific databases (MEDION; IndMed; African Index Medicus). A search was also conducted in Google Scholar where we reviewed the first 100 results only. We handsearched reference lists and conference proceedings to identify primary studies and review articles. Abstracts were identified by two authors. Methodological assessment of studies using the QUADAS‐2 tool and further data extraction for re‐analysis were performed by three authors.
Selection criteria
We included in this review all large case series and cohort studies that compared the results of CT angiography with the diagnosis of brain death according to neurological criteria. Uniquely, the reference standard was the same as the target condition in this review.
Data collection and analysis
We reviewed all included studies for methodological quality according to the QUADAS‐2 criteria. We encountered significant heterogeneity in methods used to interpret CT angiography studies and therefore, where possible, we re‐analysed the published data to conform to a standard radiological interpretation model. The majority of studies (with one exception) were not designed to include patients who were not brain dead, and therefore overall specificity was not estimable as part of a meta‐analysis. Sensitivity, confidence and prediction intervals were calculated for both as‐published data and as re‐analysed to a standardized interpretation model.
Main results
Ten studies were found including 366 patients in total. We included eight studies in the as‐published data analysis, comprising 337 patients . The methodological quality of the studies was overall satisfactory, however there was potential for introduction of significant bias in several specific areas relating to performance of the index test and to the timing of index versus reference tests. Results demonstrated a sensitivity estimate of 0.84 (95% confidence interval (CI) 0.69 to 0.93). The 95% approximate prediction interval was very wide (0.34 to 0.98). Data in three studies were available as a four‐vessel interpretation model and the data could be re‐analysed to a four‐vessel interpretation model in a further five studies, comprising 314 patient events. Results demonstrated a similar sensitivity estimate of 0.85 (95% CI 0.77 to 0.91) but with an improved 95% approximate prediction interval (0.56 to 0.96).
Authors' conclusions
The available evidence cannot support the use of CT angiography as a mandatory test, or as a complete replacement for neurological testing, in the management pathway of patients who are suspected to be clinically brain dead. CT angiography may be useful as a confirmatory or add‐on test following a clinical diagnosis of death, assuming that clinicians are aware of the relatively low overall sensitivity. Consensus on a standard radiological interpretation protocol for future published studies would facilitate further meta‐analysis.
Plain language summary
The use of computed tomography angiography (CTA) to confirm the clinical diagnosis of brain death
This Cochrane diagnostic test accuracy review looked at the evidence for the radiology test computed tomography angiography (CTA), which demonstrates blood flow in the main vessels of the brain, to support the results of clinical tests of brain function performed in unconscious patients on mechanical breathing machines who are thought by their doctors to have died.
Establishing a correct diagnosis is very important as the diagnosis confirms the death of the patient, which will have profound legal and societal implications including making organs available for transplantation. The diagnosis of death using neurological criteria, or brain death, is usually made by performing a highly specific set of clinical tests on the patient. However in some cases, for example when patients are anaesthetized or heavily sedated, performing these tests may not be possible and additional tests are required such as CTA. In some countries it is a statutory requirement for doctors to always carry out an additional test and recently some clinicians have called for these additional tests to be mandatory even when there is no statutory requirement. It is important for doctors to know how useful CTA is when compared to, or added to, the usual clinical tests.
Ten studies were found, including 366 patients in total. Most of the studies were performed in intensive care departments but involved only small numbers of patients. In most studies it would be possible for the doctors performing the CTA test to already know the results of the clinical test. This might affect the study results, however this situation would also be the case in normal medical practice. Methods used to report the CTA study also varied from study to study and so the published results were re‐analysed to take this into account.
When compared to clinical testing for brain death, the CTA test had a sensitivity of 0.85. This means that in 100 cases of patients satisfying the clinical tests for death, the CTA test will correctly identify 85 of the cases. The data also showed that this might be as few as 77 cases per 100 and as many as 91 cases per 100. Our review was unable to tell us how many patients the CTA might falsely give a diagnosis of death for, when the patient was not dead. Based on these results, it appears that CTA is not good enough to be a compulsory test.
Summary of findings
Summary of findings'. 'What is the sensitivity of CT angiography as an additional confirmatory test for diagnosing death using neurological criteria, following satisfaction of clinical neurological criteria for brain death?
| What is the sensitivity of cerebral CT angiography as an additional confirmatory test for diagnosing death using neurological criteria, following satisfaction of clinical neurological criteria for brain death? | |||||
| Patients/population | Adult patients in whom a clinical diagnosis of brain death has been made | ||||
| Prior testing | Not described | ||||
| Index test | Cerebral CT angiography, performed and reported according to defined criteria | ||||
| Importance | Knowledge of the sensitivity of CT angiography would help answer the clinical question of what impact there might be if CT angiography became a mandatory confirmatory test in this patient cohort | ||||
| Reference standard | The reference standard is the diagnosis of brain death according to clinical criteria, which uniquely is the same as the target condition | ||||
| Studies | Predominantly small case series (single cases or very small case review groups were discounted). One case‐control series identified but the control data were discounted for meta‐analysis. Studies had to describe method of performing the reference standard and index test in sufficient detail to be reproducible, and for inclusion in the re‐analysis subgroup the method of radiological review of CT angiographic image series needed to be described in sufficient granularity for retrospective re‐synthesis to be possible. | ||||
| Test/subgroup | No. of participants (studies) | Sensitivity (95% CI) | 95% prediction interval | Implications | Comments |
| CT angiography (data as published) | 337 (8) | 0.84 (0.69‐0.93) | 0.34‐0.98 | With a sensitivity of 0.84, if CT cerebral angiography were to become mandatory, diagnostic doubt would be introduced in 16% of patients who currently satisfy clinical criteria for brain death. The prediction interval is very wide: that is, in future studies, 95% of true outcomes will report sensitivity between 0.34 and 0.98. Due to the nature of the published data, meta‐analysis of specificity could not be performed. | Small number of identified studies containing small numbers of cases, with only one study containing any control data (small numbers therefore not included for specificity meta‐analysis). The studies contained a representative spectrum of patients in a clinical setting. All studies were necessarily retrospective. In all studies, patients underwent the reference standard prior to undergoing the index test, and blinding between tests was considered likely to be very poor. There was significant heterogeneity specific to the CT angiography criteria used to review CT images. |
| CT angiography (retrospective re‐analysis to a four‐vessel radiological review methodology) | 314 (8) | 0.85 (0.77‐0.91) | 0.56‐0.96 | With a sensitivity of 0.85, if CT cerebral angiography with a standardised (and pragmatically selected) four‐vessel review methodology were to become mandatory, diagnostic doubt would be introduced in 15% of patients who currently satisfy clinical criteria for brain death. The prediction interval is reduced by retrospectively standardising vessel review methodology, but remains wide. Based on available data, no recommendation of one review methodology over another can be offered. Due to the nature of the published data, meta‐analysis of specificity could not be performed. | CT angiography review data were retrospectively synthesised to a minimum set of common review points, in order to try and limit heterogeneity identified in the main group. Published data in one study was not of sufficient granularity for this to be possible, leading to overall reduction in numbers of participants. The studies contained a representative spectrum of patients in a clinical setting. All studies were necessarily retrospective. In all studies, patients underwent the reference standard prior to undergoing the index test, and blinding between tests was considered likely to be very poor. |
| CAUTION: The results in this table should not be interpreted in isolation from the results of the individual included studies contributing to each summary test statistical measure. These are reported in the main body of the text of the review. | |||||
Background
The diagnosis of death using neurological criteria (brain death) is performed on patients with catastrophic brain injury who are comatosed and receiving mechanical ventilation. The patient will usually be in an intensive care unit. The diagnostic process is an important and potentially emotive clinical task which carries profound legal and societal implications, including making organs available for transplantation. Therefore, it is critical that the diagnosis is made in a safe, defensible and timely fashion.
There is widespread international acceptance and legal support for criteria to determine death on neurological grounds. The history of these criteria is generally dated from 1968 when the Ad Hoc Committee of the Harvard Medical School defined "irreversible coma as a new criterion for death" (JAMA 1968). The specific criteria themselves have been refined since 1968 and vary internationally (ANZICS 2010; Wijdicks 2002). In the USA the Uniform Declaration of Death Act codifies a whole‐brain formulation of brain death as "an individual who has sustained... irreversible cessation of all functions of the entire brain, including the brainstem, is dead" (President's Commission 1981). Additionally, in some areas of the USA the use of 'complementary diagnostic tests' is recommended (Canadian Council 2003).
United Kingdom (UK) guidance was first published in 1976 (Conference of Medical Royal Colleges 1976). In 2008 the UK Academy of Medical Royal Colleges published an updated Code of Practice for the Diagnosis and Confirmation of Death (Academy of Medical Royal Colleges 2008). The use of clinical neurological criteria to diagnose death was supported as standard practice. This process, carried out by two senior doctors, involves the examination of the patient to demonstrate irreversible loss of brainstem function, which if present will diagnose the patient as deceased. Additional confirmatory tests, including a variety of neuroimaging techniques, were only recommended where clinical criteria could not be fully satisfied. In this minority circumstance, confirmatory tests were suggested as having a role in reducing any element of uncertainty. However, no firm recommendation was made in the report as to which test should be used.
Consensus statements comparing confirmatory tests have been published in other countries, notably from the Canadian Council for Donation and Transplantation (2006) (Heran 2008; Young 2006). The Canadian Council recommended either radionuclide angiography or cerebral computed tomography angiography (CTA), to demonstrate the absence of brain blood flow, as the preferred imaging tests. No ratified international guidelines or current systematic reviews exist which review the combined diagnostic performance of these tests relative to the current reference standard of clinical testing. Despite this paucity in the literature, in some countries it is a statutory requirement for doctors to always carry out a confirmatory test to diagnose death using neurological criteria in addition to the clinical examination for brain death (Wijdicks 2002). Recent controversial case reports have highlighted physician uncertainty in this complex and challenging area of medical practice and are likely to promote an increased use of brain blood flow radiological investigations, with some clinicians calling for these confirmatory tests to be mandatory for diagnosing death using neurological criteria even when there is no statutory requirement (Roberts 2010; Webb 2011).
Target condition being diagnosed
The target condition is the clinical diagnosis of brain death, that is death diagnosed by the use of clinical neurological criteria. Fulfilment of the above minimum clinical criteria for the neurological determination of death, combined with clinician belief that death has been diagnosed using neurological criteria acceptable in that jurisdiction, acts as the target condition for this Cochrane review. Hereafter the target condition of death diagnosed by using clinical neurological criteria will be referred to as clinical brain death.
Index test(s)
The use of confirmatory tests to diagnose brain death varies between centres and across countries, depending to a great extent on consensus guidance, local availability and local experience with techniques. A detailed discussion of the relative merits of current tests that are available is beyond the scope of this review but is elegantly covered by Heran et al (Heran 2008). It is, however, relevant to note that the currently available tests measure different aspects of cerebral function or blood supply.
Electroencephalogram (EEG) and somatosensory evoked potentials look for the loss of bioelectrical activity in the brain, as evidenced by neuronal electrical activity or response to a presented stimulus. Other tests look for the cessation of the cerebral circulation. Transcranial Doppler demonstrates absolute flow velocity within interrogated cerebral vessels. Scintigraphic, CT and magnetic resonance imaging (MRI) perfusion studies directly calculate the relative or absolute perfusion of cerebral parenchyma (brain tissue) over time.
Cerebral CTA indirectly demonstrates flow or vessel patency within the cerebral circulation by monitoring the presence or absence of a radiodense contrast medium within the intracranial vessels. As such it is similar in nature to the historic reference standard, cerebral catheter angiography. Cerebral CTA differs because contrast is injected intravenously, for example into a peripheral vein, whereas in catheter angiography the contrast is injected intra‐arterially, usually by selectively manoeuvring a catheter into an extracranial artery in the neck.
As technology has developed over the last 20 years, cerebral CTA has now become a readily available, widely used technique, which is routinely used in the diagnosis of many intracranial vascular abnormalities. The ability to obtain high quality dynamic images of the cerebral vasculature in clinically unstable or ventilated patients in a short space of time (several seconds), and the subsequent ease of image review and lack of operator dependence, confers several practical advantages to cerebral CTA over cerebral catheter angiography, with supportive publications demonstrating high sensitivity and specificity (Escudero 2009a; Frampas 2009).
Methodologically, cerebral CTA is a straightforward investigation with recordable and reproducible imaging techniques, contrast volumes, concentrations, injection rates and other technical imaging factors (kV, mAs, rotation, timing) that are useful for any robust analysis. Moreover, and in contrast to cerebral catheter angiography, cerebral CTA can be performed at a hospital site without on‐site neuroradiological support. This allows the obtained images to be reviewed at a regional centre by an experienced neuroradiologist distant to the patient. In the UK there are significantly more hospitals with intensive care units managing the anticipated patient population that require a diagnosis of death using neurological criteria to be made than there are tertiary neuroradiological centres with catheter angiographic facilities, and therefore this is an important practical consideration when considering the role of CTA versus a traditionally favoured (and older) cerebral catheter angiographic study.
Clinical pathway
The neurological determination of death represents a set of criteria for diagnosing death when usual cardio‐respiratory criteria for determining death cannot be used. This is usually because cardio‐respiratory function, including the heart beat, is being maintained artificially in an intensive care unit. All patients undergoing this diagnostic pathway have catastrophic brain injury, are deeply comatosed, unresponsive and apnoeic (not breathing), with his or her lungs being mechanically ventilated. Fifteen hundred patients fulfil these conditions each year in the UK (NHS Blood and Transplant 2012/13).
Despite differences in conceptual understanding of brain death, in all countries which recognize the diagnosis of death using neurological criteria (brain death) a clinical examination of the brainstem that demonstrates absent brainstem reflexes and a clinical demonstration of apnoea, demonstrating the loss of the brainstem mediated capacity to breathe, form part of the diagnostic pathway. Thus the clinical criteria outlined by the UK Academy of Medical Royal Colleges in 2008 represent the minimum clinical criteria, which all countries recognize, for the determination of brain death.
It is not possible in any jurisdiction for a person to be diagnosed as deceased using neurological criteria without first attempting to satisfy minimum clinical criteria. These minimum clinical criteria are the following.
1. An established aetiology capable of causing structural damage to the brain, which could lead to the irreversible loss of the capacity for consciousness combined with the irreversible loss of the capacity to breathe.
2. An exclusion of reversible conditions capable of mimicking or confounding the diagnosis of death using clinical neurological criteria.
3. A clinical examination of the patient, which demonstrates:
a. profound coma,
b. absent brainstem reflexes,
c. apnoea.
When it is not possible to use clinical neurological criteria alone to make the diagnosis, for example where the ongoing effects of sedative agents cannot be excluded or trauma prevents a full clinical examination of the brainstem reflexes, another confirmatory test, such as a test for the absence of blood brain flow, may be used to make the diagnosis. As stated above, in some jurisdictions these confirmatory tests are statutorily required additional tests in order to make the diagnosis of death using neurological criteria.
Rationale
The authors of this review are aware of current controversies in the diagnosis of death using clinical neurological criteria without the use of additional confirmatory tests. Each of the currently available confirmatory tests has proponents and detractors, and there remains significant heterogeneity in the clinical application of all of these tests, the test methodology and the analysis of the findings. If the use of tests to confirm the diagnosis of death using neurological criteria becomes more widespread, then a greater understanding of each test becomes essential. Any change in current practice would have significant legal, ethical and societal implications.
The aim of this review is to assess cerebral CTA as an additional confirmatory test for the diagnosis of death following satisfaction of clinical neurological criteria. Knowledge of the sensitivity of CTA would help answer the clinically relevant question of what impact there would be if cerebral CTA became a mandatory confirmatory test for the diagnosis of brain death.
Cerebral CTA can also potentially be used to replace or supplement the clinical criteria where the clinical criteria cannot be fully satisfied. However, a preliminary review of the literature showed that studies usually had no negatives in the reference standard as included patients already fully satisfied the clinical neurological criteria for brain death before CTA testing. An example of patient flow through the testing procedure in a representative study is demonstrated as a flow chart (Figure 1). In such circumstances there are no true negatives (patients without a diagnosis of clinical brain death who have had CTA testing demonstrating flow) or false positives (patients without a diagnosis of clinical brain death who have had CTA testing demonstrating no flow). Hence specificity (true negatives/(true negatives + false positives)) is not estimable and it is not possible to make any claims regarding the use of cerebral CTA as a replacement or supplement for the clinical criteria where the clinical criteria cannot be fully satisfied. For clarity, the definitions of these terms as they relate to patient flow through the testing process are described in Table 2 in general terms, and in Table 3 as they relate to the representative study in Figure 1.
1.
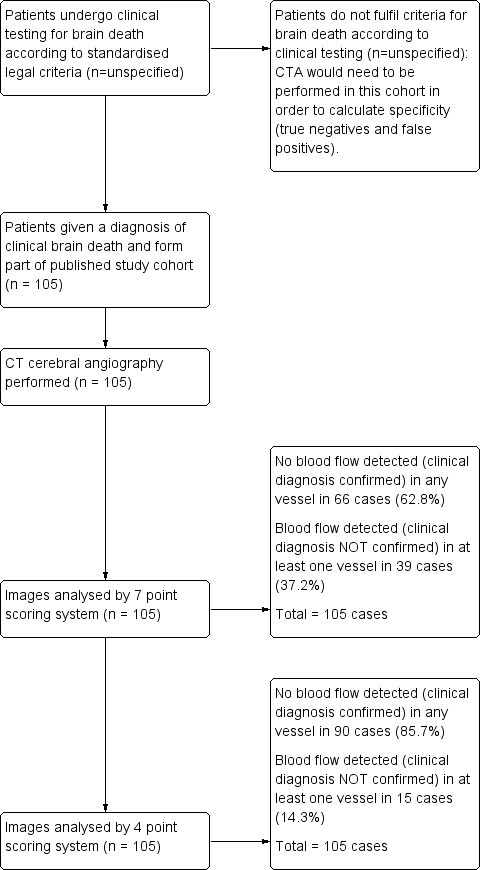
Example study flow diagram demonstrating typical patient progression through an included study (Frampas E et al).
1. Two by two table demonstrating definitions of true positive, false negative, false positive and false negative used in this review.
| Clinically Brain Dead (+) | Clinically Not Brain Dead (‐) | |
| No blood flow seen on CTA (+) | True Positive (TP) | False Positive (FP) |
| Blood flow seen on CTA (‐) | False Negative (FN) | True Negative (TN) |
2. Example of two by two table completed for the representative study in Figure 1 (Frampas E et al).
| Frampas E et al (n = 105) | Clinically Brain Dead (+) | Clinically Not Brain Dead (‐) |
| No blood flow seen on CTA (+) | 66 | unknown ‐ no published data |
| Blood flow seen on CTA (‐) | 39 | unknown ‐ no published data |
Some of the included studies in this review suggest that the specificity for CTA is 100%. However, apart from in one seminal paper which included healthy volunteers (Dupas 1998), these studies calculate their specificity by comparing patients to an assumed gold standard such as EEG or cerebral angiography (for example Frampas 2009) and have not investigated a cohort of patient who do not fully satisfy the clinical neurological criteria for brain death.
For this reason, this review is limited to investigating the sensitivity of CT angiography as an additional confirmatory test for the diagnosis of death using neurological criteria, after clinical brain death has been diagnosed.
CTA was chosen to study for the reasons listed in the 'Index test' section above. Should such a study be considered technically appropriate as a diagnostic test accuracy (DTA) test, this would in the future then allow comparison of the other confirmatory tests using a similar framework.
Objectives
To assess from the current literature the sensitivity of CT cerebral angiography as an additional confirmatory test for diagnosing death using neurological criteria, following satisfaction of clinical neurological criteria for brain death.
Secondary objectives
No secondary objective was examined.
Methods
Criteria for considering studies for this review
Types of studies
We included studies of patient populations, published in all languages. If relevant non‐English articles were identified but full‐text translations could not be obtained in press, we placed them in the 'Studies awaiting assessment' section and sought help from colleagues within The Cochrane Collaboration to obtain accurate translations.
We considered studies to be acceptable if:
the studies compared cerebral CTA (with or without other imaging or ancillary tests) with a clinical diagnosis of brain death, that is satisfaction of accepted clinical neurological criteria to diagnose death;
the participant population was defined as those in whom either a clinical diagnosis of brain death had already been made or a clinical diagnosis of brain death was established as part of the study;
the absolute numbers of true and false positives, and true and false negatives, were either available directly from the published study data, secondarily derivable from data within the published study or as supplementary published material, or available directly from the original study authors. Although at the outset we sought to collect data regarding numbers of true negatives and false positives from studies, it rapidly became apparent from a preliminary review of the literature that all but one of the published studies included no control group.
Other comparator tests were not reviewed as part of this study. We anticipated that many studies would include at least one other imaging or investigative comparator test; if these studies fulfilled our criteria for acceptance they were included.
In some studies, cerebral CTA may have been considered as a reference test and a further clinical test compared against it. Our search strategy was tailored to include these studies, however they were not included unless they provided a statement to the effect that 'all included patients satisfied a clinical diagnosis of brain death', or similar, allowing cerebral CTA to be viewed as an index test against the reference test of clinical testing. We planned to contact authors of any such studies to obtain relevant supplementary data.
Studies ideally defined clinical and imaging variables that were employed as part of their methodology. In particular, if details of the following were not specifically included we contacted the author(s) for further details.
Methodological variables
Time interval from reference standard to CTA (or vice versa)
Evidence of blinding of clinicians, reporters of CT angiography, reference standard findings
Clinical variables
Criteria used for diagnosis of brain death (including country of origin, name of approving regulatory body if applicable)
Experience of clinician(s)
Imaging acquisition
Contrast dose, contrast volume, acquisition volume, scanner type (single spiral or multislice), study time
Imaging reporting
Single, double, consensus reporting
Experience of reporter(s)
We excluded study reports that were written to focus on specific technical aspects of either the clinical or imaging technique. We also excluded commentary or opinion‐forming articles that did not have a clinical study component.
In the case of multiple publications by the same author or group of authors with similar data, we contacted the author(s) to establish the degree of similarity within their described patient populations. If this was not possible, we included only the most recent or complete study.
We considered it possible that we might encounter two patient subgroups in reviewed articles. That is:
patients who had already received a diagnosis of clinical brain death prior to undergoing CTA;
patients who did not have a diagnosis of clinical brain death but were suspected to be in such a state at the time of CTA and who subsequently went on to receive a diagnosis of clinical brain death.
It is possible that this temporal relationship may be of significance when looking for sources of heterogeneity, particularly as this review aimed to assess the role of CTA as an additional, confirmatory test rather than as a replacement, supplementary, triage or screening test. If sufficient data were available, we planned to perform a subgroup analysis for these two cohorts. However, this was not possible given sparse data.
Participants
We included patients in whom a diagnosis of brain death was suspected or in whom a clinical diagnosis of brain death had been made. We identified both adult and paediatric populations; this is consistent with the recommendations of the working party of the Academy of Medical Royal Colleges 2008 that standard adult clinical criteria and testing can be used in those over two months of age to diagnose brain death. However, there are several physiological and procedural differences in the performance of CTA in the paediatric population, namely the volume of contrast administered (which is related to weight) and cardiac output. These factors may significantly affect the utility of CTA in the paediatric population and, therefore, we planned to analyse data for adult (over 16 years) and paediatric populations separately. No paediatric data were identified.
Index tests
The index test was cerebral CTA. Image acquisition and reporting variables were defined as listed above.
Target conditions
The target condition was the clinical diagnosis of brain death. Included studies needed to define the clinical method used to establish a diagnosis of brain death.
Reference standards
The reference standard was the diagnosis of brain death, which is in effect the same as the target condition.
In order to reduce heterogeneity, we recorded the clinical method used to diagnose brain death. We contacted authors or established the legal requirements in the country of article authorship, or both, to establish this. We originally considered performing subgroup analysis between cohorts diagnosed according to a 'no brainstem function' clinical method and those diagnosed according to a 'no brain or brainstem function' clinical method, but subsequent paucity of data precluded this possibility.
Search methods for identification of studies
Electronic searches
In August 2012 we performed a comprehensive literature search in various electronic databases to identify relevant studies. As 'diagnostic test accuracy' is not a commonly used keyword or phrase in the same way that 'randomized trial' is, a simple search was not possible. Free text word searches rather than the use of subject headings or exploded terms from electronic database thesauri were required. Our search strategies cast as wide a net as possible in order to have the best possible chance of returning all relevant information for our review. Although labour intensive and time consuming, our search strategy was as detailed and complete as possible.
Electronic database searches used free text words in all fields with limitations only of time (1992 onwards, corresponding to the advent of CTA as a clinically useful technique) and human participants. Our searches were limited to papers studying patients over two months of age. Otherwise the searches were not limited by language or study design.
We conducted electronic searches in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 5); MEDLINE (Ovid); EMBASE (Ovid); British Nursing Index (BNI) (Ovid); CINAHL (EBSCO); ISI Web of Science and BioMed Central. We also conducted searches in regional electronic bibliographic databases and subject‐specific databases (MEDION; IndMed; African Index Medicus). We searched ClinicalTrials.gov and also searched Google Scholar where we reviewed the first 100 results only. The dates of the searches were limited to January 1992 to August 2012 (by hand when databases did not allow electronic limits).
Our search strategies can be found in Appendix 1 and Appendix 2.
Searching other resources
We identified further studies for possible inclusion in our review by reviewing the reference lists from studies located by our initial search strategies. The Science Citation Index allowed the identification of other studies referencing already identified relevant work. A number of relevant conference proceedings were searched but no relevant articles or potential sources of data were identified. Finally we made contact with relevant experts in the field in order to identify other relevant studies not retrieved by our search strategies.
Data collection and analysis
Selection of studies
Two authors (AH, CB) were responsible for initially assessing studies identified by the search strategy, based on title and abstract. We retrieved potentially relevant studies in full, and the full‐text was reviewed and assessed by two of three other authors (TT, RD, DG). We resolved disagreements by the majority vote of the third author. We used proprietary reference manager software to manage the large number of studies at this stage. We documented study selection in a detailed flow chart.
Data extraction and management
Following identification of relevant studies, two authors (AH, CB) extracted the relevant information from each study. In addition to that information, detailed in the Methods section, we also recorded the following information:
journal name, Vancouver‐style reference, study design (e.g. systematic review, cross‐sectional study), method of recruitment (e.g. prospective or retrospective study), study setting, characteristics of patient population (including age, gender, underlying primary diagnosis leading to current clinical state, relevant medical history if available).
Assessment of methodological quality
We used the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) (QUADAS‐2 2011) tool, built on the original QUADAS (Whiting 2003) tool, to assess the methodological quality of each included study. The recommended QUADAS questions were used, which provide a structured set of questions each with a defined answer. These questions are designed to evaluate the presence of bias related to multiple aspects of study methodology. Each study was reviewed by two of three authors (TT, RD, DG). We resolved disagreement between the two reviewing authors by consensus. Should this not have been possible the third author was the arbiter, but such a scenario was not encountered. We combined the individual review author assessments and the agreed results of the QUADAS‐2 tool. The questions selected are listed in Appendix 3.
For the purposes of this review, we defined the following terms.
For QUADAS‐2 question 1d: 'A representative spectrum of patients' is defined as 'patients of > 2 months of age and either gender in whom there is a clinical suspicion of brain death, and who have undergone or are shortly to undergo clinical testing for the diagnosis of brain death'.
For QUADAS‐2 question 4a: a time period of two days is taken as the maximum reasonable interval between the reference standard and index test, or vice versa. This accounts for any delays built into the process of clinical testing; the possibility of residual effects of sedative drugs, which may require elimination; and practical delays in acquiring a CTA study.
For QUADAS‐2 questions 2a to 2e, and 5d to 5m: CT cerebral angiography is now a widespread and well‐understood technique among the radiology community worldwide. However, techniques vary considerably between centres and published protocols for CTA in this patient cohort differ significantly from standard CTA. 'Sufficient detail' is defined as 'standard whole brain CTA following intravenous injection of water‐soluble iodinated contrast media performed with a spiral or multidetector CT scanner'. Specific questions were designed to identify appropriate radiographic technique, reflecting elements of acquisition, patient factors affecting image quality, and radiographic reporting practice. When any study returned a negative or unclear response to these questions, two authors (TT, RD) further examined the imaging protocol description within the study and sought subsequent clarification from the study authors as to the exact nature of the imaging and reporting protocol as needed. Questions examining the expertise and blinding of reporting radiologists were also added.
For QUADAS‐2 questions 3a, 3f, 3g: studies containing a statement to the effect of 'all included patients already had a conclusive clinical diagnosis of brain death', or similar, would return a positive response to this question only if the criteria for clinical testing, the national protocol used or the regulatory body approving the testing was also identified, either in the published study or following correspondence. In addition, several further questions, which were relevant to this specific review, were added to this section to cover the expertise and blinding of the clinician(s) performing the reference study.
Where published data were insufficient for a methodological assessment to be made, we wrote to the lead authors of all selected studies to seek clarification in these areas. All authors were contacted twice, both in writing and (where possible) electronically. Replies were received from the majority of authors.
Three authors (TT, RD, DG) scored each item as ‘yes’ (positive, high quality), ‘no’ (negative, low quality) or ‘unclear’ (insufficient information). Following individual review of all selected articles, the three authors met and disagreements were resolved by consensus, if possible, at this stage.
We did not apply weights to the different items of the checklist or use a summary score to only incorporate studies with certain levels of quality in our analyses.
A preliminary reading of typical studies identified a significant potential source of heterogeneity relating to the index test, namely that with reference to the number of intracranial vessels examined as part of a CTA analysis, no globally accepted standardized technique is used throughout the current world literature. We felt that this could potentially introduce unacceptable heterogeneity into assessment of the index test. In order to attempt to correct for this, once we collected data 'as published' the CTA data were subsequently re‐analysed (if it was considered possible) in order to arrive at a standardized vessel analysis rating process. The second analysis was based on a reclassification of the results of the published studies according to the four‐point scale described by Frampas 2009, that is reporting opacification of the cortical segments of the middle cerebral arteries (MCAs) and the internal cerebral veins (ICVs) bilaterally. For this re‐analysis we considered a positive scan to be one in which there was ‘no contrast enhancement in the cortical branches of both the left and the right MCA, and no contrast enhancement in both the left and the right ICV, on a delayed venous CT study (that is one performed ≥ 60 sec following contrast injection)’. We selected this four‐point scale as it was likely to be the most inclusive published rating scale; specifically, it analyses the lowest number of vessels per case and therefore most other published data can be selectively re‐analysed to this scale, assuming that data were published (or available) on a per‐case basis. This step was performed only if there was consensus between three lead authors (TT, RD, DG) that the as‐published data, or data subsequently expanded and available from the original study author, could be re‐analysed in this manner.
Another potential source of heterogeneity in the world literature was the performance of the reference standard. As alluded to in the 'Clinical pathway' section above, the clinical criteria outlined by the UK (Academy of Medical Royal Colleges 2008) represent the minimum clinical criteria which all countries recognize for the determination of brain death. Consensus between three lead authors (TT, RD, DG) was used as the measure of achievement of these unifying clinical criteria, based on published information, supplementary information from the author, or national published legal requirements for the country in which the study was performed.
Statistical analysis and data synthesis
We generated a 2 x 2 cross‐classification table of brain death and the CTA angiography test result for each included study, using data either directly extracted from the study text (Table 4) or derived from presented data and re‐analysed to a four‐point rating scale (Table 5). Each table contained the tally of true positive, false positive, true negative and false negative cases. We entered the data into Review Manager 5.2 (Revman 5.2). Data entry was double checked by a second author.
3. Data extracted from included studies.
| Author and Year | Nature of test | Number of Patients | True Positives | False Negatives | Sensitivity (%) |
| Berenguer 2010 | "Absence of blood flow" | 25 | 19 | 6 | 76 |
| Bohatyrewicz 2010 | ?4 point score ‐ R+L M4 MCA, deep cerebral vv (how many?) | 24 | 24 | 0 | 100 |
| Combes 2007 | 10 point score ‐ A2 R+L ACA, M4 R+L MCA, P2 R+L PCA, basilar artery, R+L internal cerebral vv and great cerebral vein | 43 | 30 | 13 | 69.8 |
| Dupas 1998 | 18 vessels assessed: no specific scoring system used | 14 | ‐ | ‐ | not estimable |
| Escudero 2009a | "No intracerebral filling at the level of the carotid bifurcation or circle of Willis" + "contrast stop at foramen magnum". No venous component measured. | 27 | 24 | 3 | 88.9 |
| Frampas 2009 | 7 point score ‐ R+L pericallosal arteries, cortical segments of R+L MCAs, R+L ICVs and GCV | 105 | 66 | 39 | 62.9 |
| Frampas 2009 | 4 point score ‐ R+L MCA cortical segment and R+L ICV | 105 | 90 | 15 | 85.7 |
| Leclerc 2006 | 13 point score ‐ R+L m1, m2, m3 MCAs, R+L pericallosal arts, R+L PCAs, R+L ICVs and GCV. No per‐patient data available. | 15 | ?? | ?? | Not estimable |
| Quesnel 2007 | 7 point score ‐ R+L pericallosal arts, R+L terminal arts, R+L ICVs, GCV | 21 | 11 | 10 | 52.4 |
| Rieke 2011 | 7 point late score ‐ R+L A3 ACA, R+L M4 MCA, vein of Galen, R+L ICV (Dupas criteria) | 29 | 22 | 7 | 75.9 |
| Rieke 2011 | 4 point late score ‐ R+L M4 MCA, R+L ICV, i.e. Frampas, but late phase is 20 seconds later than Frampas. | 29 | 22 | 7 | 75.9 |
| Welschehold 2012a | Venous phase score ‐ R+L MCA‐M4, R+L ACA‐A3, R+L PCA‐P2, Basilar artery, R+L ICV | 63 | 41 | 22 | 65.1 |
| Welschehold 2012a | Arterial phase score ‐ R+L MCA‐M4, R+L ACA‐A3, R+L PCA‐P2, Basilar artery | 63 | 60 | 3 | 95.2 |
4. Data extracted from included studies and reclassified according to Frampas 2009 (four‐point assessment of cortical MCA branches and Internal cerebral veins only in venous phase).
| Author and year | Could available data be reclassified? If not, give reason/s | Number of Patients | True Positives | False Negatives | Sensitivity (%) |
| Berenguer 2010 | No. Authors acquired but excluded analysis of delayed/venous imaging, only analysed true arterial phase CTA |
||||
| Bohatyrewicz 2010 | Yes, already a 4 point score | 24 | 24 | 0 | 100 |
| Combes 2007 | Yes | 43 | 35 | 8 | 84.3 |
| Dupas 1998 | Yes ‐ a 4‐point score can be calculated from the available data | 14 | 14 | 0 | 100 |
| Escudero 2009a | No. CTA criteria as described by authors only review arterial phase and do not acquire any venous data |
||||
| Frampas 2009 | Data published as 4‐point score | 105 | 90 | 15 | 85.7 |
| Leclerc 2006 | Yes | 15 | 14 | 1 | 93.8 |
| Quesnel 2007 | Yes | 21 | 13 | 8 | 61.9 |
| Rieke 2011 | Yes, already a 4 point score. Acquired 20s later than Frampas but consensus from reviewing authors that this is acceptable |
29 | 22 | 7 | 75.9 |
| Welschehold 2012a | Yes, using venous phase data | 63 | 48 | 15 | 76.2 |
All included studies had structural zeros for false positives and true negatives; specificity was not estimable. The included studies were considered to be a sample of all possible hypothetical studies that could have, have been, and will be performed. It is considered an unconditional estimate of the true sensitivity. A logit (that is log odds) variance stabilizing transformation of the raw proportion (sensitivity) was used to constrain the 95% confidence intervals (CI) and prediction intervals to be contained within the parameter space (0, 1). Meta‐analysis was performed in the meta‐analysis package meta for version 1.9‐2 running in R version 3.0.2 (R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria) (Viechtbauer 2010). A generalized linear random‐effects model (binomial‐normal) was used to estimate summary statistics. The point estimate of sensitivity with a 95% CI and measure of heterogeneity (tau2) were reported. Approximate 95% prediction intervals of the point sensitivity estimate were generated. For a random‐effects model, a 95% prediction interval estimated where 95% of true sensitivities fall, including results of future studies.
Investigations of heterogeneity
No study‐level covariates were available; no modelling for reduction of heterogeneity by meta‐regression was attempted.
Sensitivity analyses
To check the robustness of estimation methods, models were generated using empirical Bayes and restricted maximum likelihood methods. Models were also estimated with an alternative variance stabilizing transformation, the arcsine transformed proportion. No modelling of sensitivity by study quality was performed.
Assessment of reporting bias
Assessment of reporting bias was performed by three review authors (TT, RD, DG). Given the patient population under investigation, we anticipated that it would be challenging for the researchers authoring the included studies to assess their data without any form of bias, particularly as clinical ‘blinding’ at many key stages throughout a study would pragmatically be very difficult. One key area in which bias could be introduced was considered to be the point of initial patient sampling, in particular whether exclusions were avoided wherever possible. We felt that such cases would be appropriately identified by the QUADAS‐2 tool.
Results
Results of the search
Searches were carried out in August 2012. We identified 2630 citations from our initial electronic database searches. Exclusion of citations was mainly due to papers not matching our inclusion criteria. Of the papers evaluated which were felt not to be appropriate for this review, these were made up of case reports or very small (two or three patients) case series and consensus articles or opinion pieces without additional patient data. One further potentially large cohort study was identified (Musacchio 2010) but this was in the form of unpublished data in summary form (conference PowerPoint presentation, see reference). After discussion, these data were not considered by any review author to have been demonstrably subject to a robust peer review process; the data were therefore excluded from this review. After evaluation of citation title and abstract or full article, we identified 11 full papers for consensus methodological assessment. Two papers by the same author (Welschehold 2012a; Welschehold 2012b) seemed to report on similar cohorts of patients. We contacted the lead author who was able to confirm that the latter study included the previously reported on cohort of patients and we therefore only included the second of these two studies in our review. A flow chart describing the search process is shown in Figure 2. After removal of duplicates the final number of studies identified by the electronic searches was 10.
2.
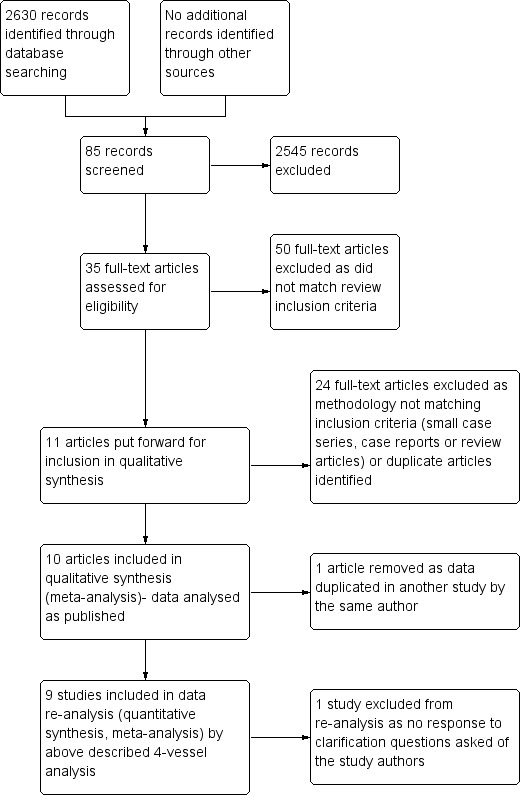
Search and analysis flow diagram.
Handsearches of the references of those studies considered for our review were also carried but we did not identify any further papers for review.
In addition, we contacted international experts in the field but no further suggested studies were identified.
Searches of ongoing studies (Current Controlled Trials and ClinicalTrials.gov) and conference proceedings did not identify any further work that may have been worthy of inclusion in this review.
As elaborated further on below (Summary of main results: 'Factors affecting interpretation', 'Index test'), due to significant heterogeneity in relation to the specific CTA radiological analyses used a decision was made to perform a second statistical analysis, where possible, following reclassification of as‐published data to a standardized four‐vessel analysis model. Decisions regarding suitability of published data for this reclassification, and the subsequent reclassification process itself, were undertaken by the three lead authors (TT, RD, DG). There was complete consensus in all cases.
After detailed review of the short list of potential studies for inclusion, and following multiple attempts to contact relevant authors, one paper (Dupas 1998) was excluded from our analysis as we had originally planned it as the as‐published data did not use any specific scoring system. This gave us nine papers for inclusion in the original as‐published review. However, there was consensus that the data from this paper could be reinterpreted into a four‐point analysis and therefore it was included in a second meta analysis.
Methodological quality of included studies
Assessment of methodological quality
The results of the quality assessment are presented in Figure 3 and Figure 4. The majority of included studies provided sufficient detail with regard to patient characteristics and clinical setting for us to be satisfied that the included patient groups were well matched to our clinical question. Most studies also scored well in their descriptions of both the index test and the reference standard, with the majority of studies referring or alluding to national legal frameworks particularly with regard to the reference standard.
3.
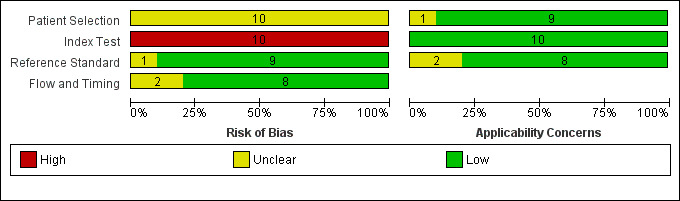
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
4.
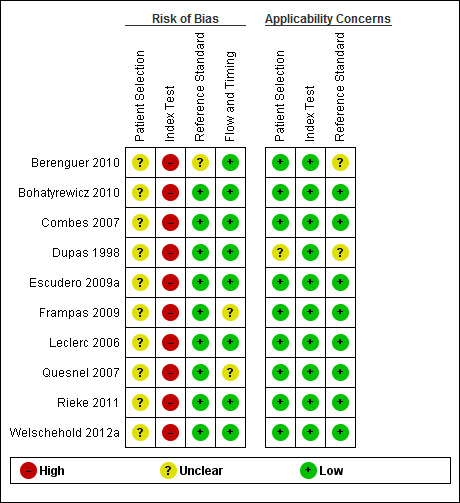
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Initial disagreement between review authors mainly concerned the more specialist domain questions, for example 5d to 5h regarding details of the CT scanning protocol, but these were resolved with discussion. All three authors agreed that items 5k and 5l, assessing hypovolaemia and cardiovascular insufficiency at time of CTA, were effectively duplicate questions; they also proved challenging to score reliably.
Based on published data alone, studies performed poorly overall when describing specific elements of the performance of the index test, particularly 2a ('Was the radiologist blinded to results of clinical testing?'), 5j (single or double review?) and 5m ('Were all studies reported by the same person?'). In all studies, patients underwent the reference standard (clinical testing for brain death) prior to undergoing the index test (CTA) and therefore it was considered likely by all three authors that high risk of bias was being introduced at this point.
Findings
With one exception, all studies reported CTA investigation in patients already declared clinically brain dead. One included study, representing the seminal work by Dupas 1998, proposed the use of CTA as a diagnostic test for brain death. However, it included 11 normal control patients not suspected of clinical brain death and 14 patients already diagnosed as clinically brain dead. As case‐control studies are likely to provide a biased and inflated estimate of specificity, the control arm data were not used (Whiting 2004). Of the 14 brain dead reported by Dupas 1998. no specific scoring system was used. Additionally, Leclerc 2006 did not provide interpretable tallies of TP and FN in the original report. Thus neither of these studies was included in the meta analysis of originally reported data.
The second analysis was based on a reclassification of the results of the published studies according to the four‐point scale described by Frampas 2009, This was not possible for Berenguer 2010 and Escudero 2009a.
Our meta‐analysis was a univariate generalized linear random‐effects modelling of sensitivity. The sample sizes in the included studies were small to modest. The logit transformation of raw proportions was used; the summary estimates of logit sensitivity were back transformed by an inverse logit function to sensitivity as a proportion.
The data and the R code used for meta‐analysis are listed in Appendix 4 and Appendix 5. Statistical output is detailed in Appendix 6.
The CTA original data (eight studies, 337 patients) had a sensitivity point estimate of 0.84 with 95% CI (0.69 to 0.93). The 95% approximate prediction interval was wide (0.34 to 0.98).
Using the CTA four‐vessel data (eight studies, 314 patients) , the estimate of sensitivity was 0.85 (95% CI 0.77 to 0.91). The 95% approximate prediction interval was 0.56 to 0.96; in future studies, 95% of true outcomes will report sensitivity in the range 0.56 to 0.96. For both the four‐vessel and original data, the value of estimates by empirical Bayes and restricted maximum likelihood and using an arcsine transformation were very similar to those reported by generalized linear mixed estimation. The results are demonstrated graphically as forest plots in Figure 5 and Figure 6.
5.
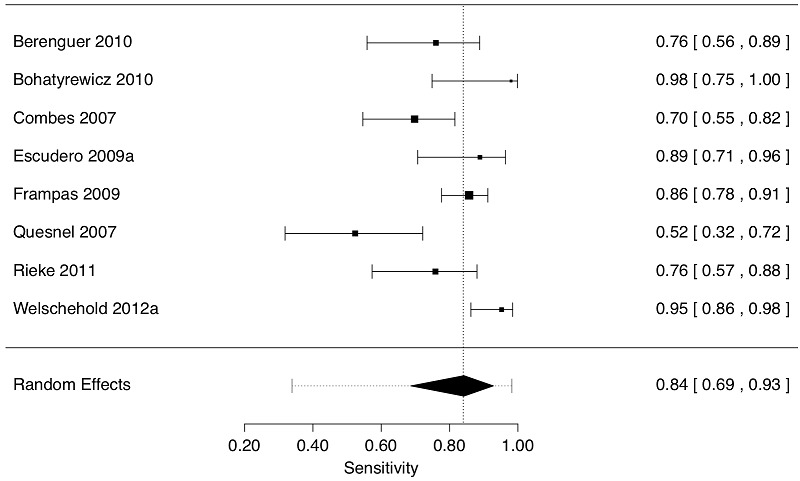
Forest plot of 8 studies displaying sensitivity (decimal fractions) by the original grading in the studies. The observed effect size (sensitivity) of each study is indicated by a square with corresponding 95% Confidence Interval; larger squares reflect greater precision of the estimate. The diamond is the summary value of sensitivity; the edges of the diamond are the 95% Confidence Interval of the summary sensitivity. Confidence Intervals are asymmetric. Numeric values are shown in the right column. The dotted vertical line is the summary sensitivity value. The dotted horizontal line enclosing the diamond is the 95% Prediction Interval (0.34, 0.98) of the summary sensitivity value. There is a large degree of statistical heterogeneity (I2 = 81%).
6.
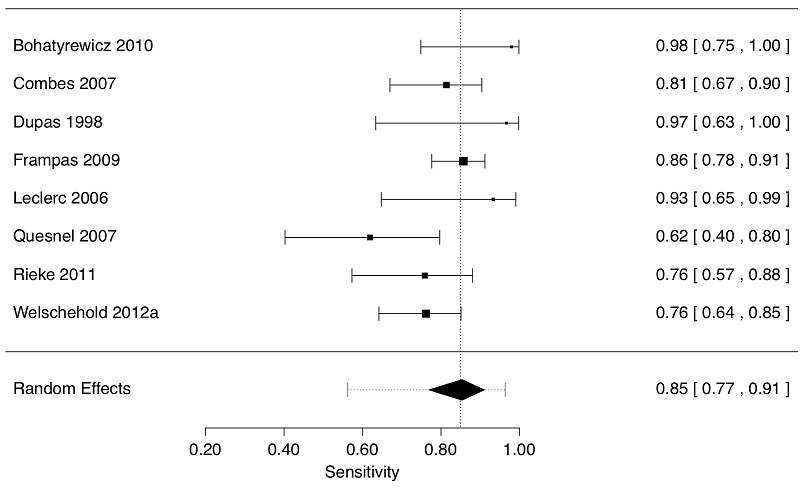
Forest plot of 8 studies displaying sensitivity (decimal fractions) by 4 vessel grading in the studies. The observed effect size (sensitivity) of each study is indicated by a square with corresponding 95% Confidence Interval; larger squares reflect greater precision of the estimate. The diamond is the summary value of sensitivity; the edges of the diamond are the 95% Confidence Interval of the summary sensitivity. Confidence Intervals are asymmetric. Numeric values are shown in the right column. The dotted vertical line is the summary sensitivity value. The dotted horizontal line enclosing the diamond is the 95% Prediction Interval (0.56, 0.96) of the summary sensitivity value. There is a large degree of statistical heterogeneity (I2 = 71%)
Discussion
Summary of main results
The aim of this review was to identify and summarize the available evidence for the sensitivity of a widely available imaging test (CTA) in the management pathway of patients who are, or are suspected to be, brain dead. The results show that when compared with the current reference standard of clinical testing for brain death, CTA can be considered a moderately sensitive test but, based on published data, with very wide confidence and prediction intervals. A key finding of this review is that despite relatively standardized image acquisition techniques, there are multiple models of image interpretation in current use and this has limited the meta‐analysis to such a degree that it has been necessary to re‐analyse published data to a minimum inclusive data set, where possible, in order to proceed.
Factors affecting interpretation
Population and setting
Due to the nature of the clinical condition and the index test, a case‐control design was successfully avoided in all studies. It might be considered that the risk of selection bias would be high in such a small and specialist cohort; however, the majority of studies described a consecutive sampling approach that might minimise selection bias. What is not described in any study is the percentage of patients undergoing clinical testing for brain death but then not proceeding to CTA for any reason (for example lack of consent from next of kin). Studies are necessarily retrospective and the risk of bias is high.
Reference standard
As described earlier, in this review the reference standard and the target condition are the same, that is, a clinical diagnosis of brain death. As described earlier, the clinical criteria outlined by the UK Academy of Medical Royal Colleges in 2008 represent the minimum clinical criteria that all countries recognize for the determination of brain death, and hence were used as the reference standard for this review.
Fulfilment of the minimum clinical criteria for the neurological determination of death, combined with clinician belief that death has been diagnosed using neurological criteria acceptable in that jurisdiction, was achieved in every paper included in this review.
Index test
The review protocol (Taylor 2012) anticipated that heterogeneity may be introduced due to the interpretation of the imaging results. One problematic example of this was the inter‐study difference in selection of intracranial vessels for evaluation of opacification, which varied from relatively limited such as the four‐vessel evaluation of Frampas et al (cortical segments of the MCAs and the ICVs) (Frampas 2009) to more extensive such as in the study by Welschehold et al (supraclinoid ICAs, horizontal MCAs, cortical segments of the MCAs, pericallosal arteries, ambient segment of PCAs, intradural segment of vertebral arteries, basilar artery, straight sinus and ICVs) (Welschehold 2012a). While the selected studies were able to classify the CTAs as positive or negative depending on the criteria applied in the study, it was clear that the definition of ‘positivity’ varied with the variability of the number and location of the intracranial vessels evaluated and the positivity criteria applied.
To account for this heterogeneity we made a decision to analyse the data from the studies in two ways. The first analysis was conducted as per protocol, using the ‘as‐published’ sensitivity for each of the selected studies; these data are presented in Table 4. The second analysis was based on a reclassification of the results of the published studies according to the four‐point scale described by Frampas et al, that is reporting opacification of the cortical segments of the MCAs and the ICVs bilaterally. For this re‐analysis we considered a positive scan to be one in which there is ‘no contrast enhancement in the cortical branches of both the left and the right MCA, and no contrast enhancement in both the left and the right ICV, on a delayed venous CT study (that is one performed ≥ 60 sec following contrast injection)’. This reclassification and re‐analysis was possible because these vessels were commonly evaluated across the studies, and we were able to successfully extract the four‐point scale data from eight studies: in two studies because the four‐point scale was directly reported (Frampas 2009; Rieke 2011) and in six studies because we were able to derive the four‐point scale from the text of the results, from published individual patient data (Bohatyrewicz 2010; Combes 2007; Dupas 1998; Leclerc 2006; Quesnel 2007; Welschehold 2012a). Two of the selected studies (Berenguer 2010; Escudero 2009a) could not be included in the four‐point scale re‐analysis because neither study reported opacification of the ICVs and we were unable to obtain this data directly from the authors.
The meta‐analysis of the four‐point scale data thus gives a synthesized result from 314 patients with a consistent CTA evaluation in terms of assessment of vessel opacification; the four‐point scale data are presented in Table 5.
Reliability
This review focused on the validity of CTA in patients already considered to be brain dead by established clinical criteria. An assessment of reliability has not been formally performed. Although it was agreed by all review authors that the included studies met the criteria for CTA (index test), in only a few cases would it be possible to directly reproduce the exact CTA protocol that was selected or performed based on the information within the studies. In addition, neither inter nor intra‐observer variability figures were available, and it was often unclear whether the observers had been specifically trained or were performing studies as part of their normal working practice.
Strengths and weaknesses of the review
The major weakness of this review must be the relatively small number of identified studies, containing small numbers of patients. We specifically excluded case studies or very small case series, but the world literature is small in this field and therefore several case studies were identified and rejected.
Another significant weakness is the significant heterogeneity we encountered specific to the CTA criteria for diagnosing brain death. We have attempted to compensate by effectively re‐analysing the published (source) data, but in doing so we have excluded 46 patients from an already small sample.
Our search identified one large data set (Musacchio 2010) which appears to match our target population; however the data remains (as of May 2013) unpublished beyond a conference presentation and therefore presumed not yet subject to peer review. Following contact, one other author also indicated that their group is in the process of expanding their data set. It is therefore likely that, should this review be re‐performed in 18 months, further larger patient cohorts may be available to include in this meta‐analysis. However, given the nature of the subject matter and the potential implications of the findings of this Cochrane diagnostic test accuracy (DTA) review in clinical practice, all three review authors felt that only published data which had been subject to peer review should be included in this incarnation of the review.
As only one paper (Dupas 1998) included patients who were not clinically brain dead, specificity, and thus positive predictive values, could not be meaningfully statistically calculated as part of this meta‐analysis. One might consider that a healthy patient will necessarily have flow within their intracranial arteries, and that this would be demonstrated as the presence of contrast on a CTA. However this consideration requires several assumptions to be true (namely that patient health equals intracranial flow equals contrast enhancement) and cannot be considered to hold true for all cases in the absence of a demonstrable study ‘control’ population. This is a significant limitation of almost all included studies and thus of this meta‐analysis, and is a function of the patient cohort and study environments.
Given that CTA involves exposure to both ionising radiation and an intravenous contrast bolus, it would likely be difficult to ethically justify the scanning of an age‐matched healthy cohort for future studies. However, it might be possible to recruit or create age‐matched cohort groups from patients undergoing assessment for (for example) intracranial aneurysm in order to allow future studies to calculate true negative, false positive, positive predictive value (PPV) and specificity data.
A high risk of bias was identified for the interpretation of the index test due to the fact that the radiologists interpreting the CTA scans appeared not to have been blinded to the clinical status of the patients being studied. However, all three reviewer authors who assessed the methodological quality of the included studies acknowledge that this reflects the real‐world situation when such patients undergo CTA as an ancillary test for the diagnosis of brain death. For future studies, such bias could be minimized if authors of studies in this area arrange and report the results of independent blinded review of CTA data that include data from carefully constructed control groups and, if built into the independent blinded review, could allow assessment of intra and inter‐observer reliability of CTA interpretation in the context of brain death.
It is important to note that although this review has re‐analysed published data according to a four‐point interpretation model, this model was pragmatically selected from the current published models as the one most likely to include as many studies as possible in our second analysis, that is to create as large a cohort as possible. Based on available data, the recommendation of one interpretation model over another on statistical grounds alone cannot be performed and is indeed beyond the scope of this review.
Given the high risk of bias and significant heterogeneity, an argument could be made for not developing a meta‐analysis for this clinical question at all. However, we suggest that there is additional value in so doing despite the clear limitations of the subsequent results. The process of meta‐analysis has highlighted the relative scarcity of high quality large cohort studies in this important clinical area. It also hopefully highlights to future authors that consideration of how they might deal with the areas of bias and index test heterogeneity that we have encountered would be useful.
Applicability of findings to the review question
See below
Authors' conclusions
Implications for practice.
Given the results of this review cerebral CT angiography (CTA) should not become a mandatory or routine confirmatory test in the care pathway for the diagnosis of death using neurological criteria owing to its relatively low sensitivity. Even selecting a standardized interpretation model, cerebral CT angiography has a sensitivity of 0.85 (95% CI 0.77 to 0.91). Were cerebral CTA to become mandatory, around 15% of patients who currently satisfy long established neurological criteria for death might no longer be considered deceased. This would have implications for patient care, preventing or delaying the withdrawal of mechanical ventilation and end of life care, increasing the demand on intensive care resources, and prolonging the suffering of families without necessarily improving the safety of the clinical diagnosis. This would pose a significant legal, ethical and societal challenge. Such a change would be particularly impactful in countries that currently do not have a statutory requirement for an additional confirmatory test, for example the UK, Canada and some areas in the US.
This is an important conclusion given that following three recent reversal of brain death cases in North America, recommendations were made to require the use of confirmatory tests, such as validated ancillary radiological studies, in the routine declaration of neurological death (Roberts 2010; Webb 2011). Our review would not advise the use of cerebral CTA to support this recommendation.
Our inability to estimate specificity, on account of the lack of true negatives and false positives in our study population, prevents us making any recommendation regarding the use of cerebral CTA as an add‐on or replacement test for clinical neurological criteria for death. We acknowledge that this remains an unresolved, important clinical question.
However, this review did demonstrate that the reporting criteria utilized for CTA analysis need to be selected with care. We suggest that in centres considering including CTA as part of the management pathway of this patient cohort, it is important to describe the CTA imaging protocol used, the reporting expertise expected, and the imaging interpretation model utilized following acquisition of the CTA in order to ensure that the test is as robust as possible.
Implications for research.
There is a clear need for good quality, larger scale prospective studies of CTA in patients with clinical testing confirming brain death. The current world literature suggests that such large scale studies are unlikely to be possible in single‐centre institutions and as such we would encourage co‐operation between centres performing CTA in order that larger cohorts can be examined.
We also suggest that further meta‐analyses on this subject, or updates to this review, would be greatly facilitated by either publication of, or access to, tables reporting the opacification of specific vessels (particularly those included in the four‐point scale) in order that a standardized meta‐analysis can be performed on CTA data to facilitate formation of large cohorts in this select patient population. Consensus on a standard radiological interpretation protocol for future published studies would greatly facilitate further meta‐analysis.
Future research on this topic must include a true negative control population so that specificity can be estimated.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2012 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 19 June 2014 | Amended | Bohatyrewicz 2010 ‐ spelling errors in citation corrected |
Acknowledgements
We would like to thank Harald Herkner (Cochrane Anaesthesia Review Group (CARG) content editor), the DTA editorial team, Stephan Langevin, Bryan Young (CARG peer reviewers) and Ann Fonfa (consumer) for their help and editorial advice during the preparation of the systematic review.
We would also like to thank Jane Cracknell (CARG Managing Editor), Mathew Zacharias (CARG content editor), Constantine Gatsonis (DTA contact editor), G Bryan Young, Stephan Langevin, Thomas Sycha and Stephen Streat (CARG peer reviewers) and the DTA peer reviewers for their help and editorial advice during the preparation of the protocol for the systematic review.
Appendices
Appendix 1. EMBASE and MEDLINE strategies via OvidSP
EMBASE and MEDLINE
("brain dea*" OR "brain stem dea*" OR "coma depasse" OR "irreversible coma").any field [Limit to: Publication Year 1992‐Current and Human]
("CT" OR "CTA" OR "CTCA" OR "comput* tomograph*" OR "comput* aided tomograph*" OR "comput* tomograph* angio*" OR "comput* aided tomograph* angio*").any field [Limit to: Publication Year 1992‐Current and Human]
1 AND 2 [Limit to: Publication Year 1992‐Current and Human]
Appendix 2. Other database strategies
These databases do not allow combining search steps as in step 3 above. All results of the searches below will therefore be dealt with individually.
ISI Web of Science
Topic=("brain dea*" OR "irreversible coma" OR "coma depasse") AND Topic=("CT" OR "CTA" OR "CTCA" OR "comput* tomograph*" OR "comput* aided tomograph*" OR "comput* tomograph* angio*" OR "comput* aided tomograph* angio*") Timespan=1992‐2012. Databases=SCI‐EXPANDED
MEDION
1. "ICPC_code N (Neurological)"
2. "Signs_code I (Medical Imaging)"
3. Hand search to select only those citations published after January 1992 and only human studies
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
1. "brain dead" or "brain death" or "coma depasse" or "irreversible coma" [Title, Abstract, Keyword]
2. "CT" or "CTA" or "CTCA" or "CAT" or "computer tomograph" or "computer aided tomograph" or "computer tomograph angiography" or "computer tomograph angiogram" or "computer aided tomograph angiography" or "computer aided tomograph angiogram" or "computer tomograph cerebral angiography" or "computer tomograph cerebral angiogram" [Title, Abstract, Keyword]
3. #1 and #2
4. Hand search to select only those citations published after January 1992 and only human studies
BNI (Ovid)
As the search strategy for MEDLINE and EMBASE above
CINAHL (EBSCO)
As the search strategy for MEDLINE and EMBASE above
BioMed Central
1. ("brain dea*" OR "brain stem dea*" OR "coma depasse" OR "irreversible coma") AND ("CT" OR "CTA" OR "CTCA" OR "comput* tomograph*" OR "comput* aided tomograph*" OR "comput* tomograph* angio*" OR "comput* aided tomograph* angio*") (All words) in All fields (full text) from 1997 to 2012
2. Hand search to identify only human studies
African Index Medicus
IndMed
1. "brain dead"
2. "brain death"
3. "coma depasse"
4. "irreversible coma"
5. "CT"
6. "CTA"
7. "CTCA"
8. "CAT"
9. "computer tomograph"
10. "computer aided tomograph"
11. "computer tomograph angiography"
12. "computer tomograph angiogram"
13. "computer aided tomograph angiography"
14. "computer aided tomograph angiogram"
15. "computer tomograph cerebral angiography"
16. "computer tomograph cerebral angiogram"
Google Scholar (first 100 results only)
1. ("brain dead" OR "brain death" OR "coma depasse" OR "irreversible coma") AND ("CT" OR "CTA" OR "CTCA" OR "CAT" OR "computer tomograph" OR "computer aided tomograph" OR "computer tomograph angiography" OR "computer tomograph angiogram" OR "computer aided tomograph angiography" OR "computer aided tomograph angiogram" OR "computer tomograph cerebral angiography" OR "computer tomograph cerebral angiogram") [Limits set to 1992‐2012, anywhere in document]
2. Hand search to include only human studies
Appendix 3. QUADAS‐2 checklist
| Item | Yes | No | Unclear |
| 1a ‐ Was a consecutive or random sample of patients enrolled? | |||
| 1b ‐ Was a case‐control design avoided? | |||
| 1c ‐ Did the study avoid inappropriate exclusions? | |||
| 1d ‐ Was the spectrum of pts representative of the pts who will receive the test in practice? | |||
| 1e ‐ Were selection criteria clearly described? | |||
| 1f ‐ Could the selection of patients have introduced bias? | |||
| 1g‐ Summary of patient characteristics and setting | |||
| 1h‐ Are there concerns that the included patients and setting do not match the review question? | |||
| 2a ‐ Was the radiologist blinded to results of clinical testing? | |||
| 2b ‐ Was the method of the index test described in enough detail to make it replicable? | |||
| 2c ‐ Index test ‐ was the expertise of the reporting radiologist recorded? | |||
| 2d ‐ Could the conduct or interpretation of the index test have introduced bias? | |||
| 2e ‐ CTA criteria for diagnosing brain death | |||
| 2f ‐ Are there concerns that the index test, its conduct, or interpretation differ from the review question? | |||
| 2g ‐ Were index test results interpreted without knowledge of the ref standard results? | |||
| 3a ‐ Is the ref standard likely to correctly classify the target condition? | |||
| 3b ‐ Were ref standard results interpreted without knowledge of the index test results? | |||
| 3c ‐ Did the whole or a selection of the study population receive the reference standard of diagnosis? | |||
| 3d ‐ Was the ref standard independent of the index test? | |||
| 3e ‐ Was the method of the ref standard described in enough detail to make it replicable? | |||
| 3f ‐ Was the clinician performing the ref standard examination blinded to results of the index test? | |||
| 3g ‐ Reference standard ‐ was the expertise of the interpreting clinicians recorded? | |||
| 3h ‐ Could the reference standard, its conduct, or its interpretation have introduced bias? | |||
| 3i ‐ Are there concerns that the target condition as defined by the reference standard does not match the question? | |||
| 4a ‐ Is the time period short enough to be reasonably sure the target condition did not change? | |||
| 4b ‐ Did all pts receive the same ref standard regardless of the index test result? | |||
| 4c ‐ Were all pts included in the analysis? | |||
| 4d ‐ Could pt flow have introduced bias? | |||
| 5a ‐ Were the same clinical data available when test results were interpreted as would be in clinical practice? | |||
| 5b ‐ Were un‐interpretable or indeterminate results reported? | |||
| 5c ‐ Were study withdrawals explained? | |||
| 5d ‐ CT timing specified? | |||
| 5e ‐ CT ‐ contrast dose specified? | |||
| 5f ‐ CT ‐ was any objective/subjective assessment of study quality performed? | |||
| 5g ‐ CT ‐ volume of contrast specified? | |||
| 5h ‐ CT ‐ volume of acquisition specified? | |||
| 5i ‐ CT ‐ scanner type specified? | |||
| 5j ‐ CT ‐ report ‐ single or double review specified? | |||
| 5k ‐ CT‐ was an assessment of hypovolaemia made prior to scanning? | |||
| 5l ‐ CT‐ was cardiovascular status assessed as sufficient prior to scanning? | |||
| 5m ‐ CT ‐ were all studies reported by the same person/s? |
Appendix 4. Data as used for analyses
CTA.dt Test Study.ID Year.of.study TP FN 1: CTA‐ data as published Berenguer 2010 2010 19 6 2: CTA‐ data as published Bohatyrewicz 2010 2010 2010 24 0 3: CTA‐ data as published Combes 2007 2007 30 13 4: CTA‐ data as published Escudero 2009a 2009 24 3 5: CTA‐ data as published Frampas 2009 2009 90 15 6: CTA‐ data as published Quesnel 2007 2007 11 10 7: CTA‐ data as published Rieke 2011 2011 22 7 8: CTA‐ data as published Welschehold 2012a 2012 60 3
CTA4.dt Test Study.ID Year.of.study TP FN 1: CTA‐ 4 vessel reanalysis Bohatyrewicz 2010 2010 2010 24 0 2: CTA‐ 4 vessel reanalysis Combes 2007 2007 35 8 3: CTA‐ 4 vessel reanalysis Dupas 1998 1998 14 0
4: CTA‐ 4 vessel reanalysis Frampas 2009 2009 90 15
5: CTA‐ 4 vessel reanalysis Leclerc 2006 2006 14 1 6: CTA‐ 4 vessel reanalysis Quesnel 2007 2007 13 8 7: CTA‐ 4 vessel reanalysis Rieke 2011 2011 22 7 8: CTA‐ 4 vessel reanalysis Welschehold 2012a 2012 48 15
Appendix 5. R code for analysis
require(metafor) # published analysis CTA #estimate effect measures CTAPAS <‐ escalc(measure = 'PAS', xi = TP, mi = FN, add = 1/2, to = 'only0', vtype = 'UB', data = CTA.dt) CTAPLO <‐ escalc(measure = 'PLO', xi = TP, mi = FN, add = 1/2, to = 'only0', vtype = 'LS', data = CTA.dt) #model CTAPAS.REML.rma <‐ rma.uni(yi = yi, vi = vi, data = CTAPAS, method = 'REML', knha = T) CTAPLO.REML.rma <‐ rma.uni(yi = yi, vi = vi, data = CTAPLO, method = 'REML', knha = T) CTAPAS.EB.rma <‐ rma.uni(yi = yi, vi = vi, data = CTAPAS, method = 'EB', knha = T) CTAPLO.EB.rma <‐ rma.uni(yi = yi, vi = vi, data = CTAPLO, method = 'EB', knha = T) # estimates predict(CTAPAS.REML.rma, transf = transf.iarcsin) predict(CTAPLO.REML.rma, transf = transf.ilogit ) predict(CTAPAS.EB.rma, transf = transf.iarcsin) predict(CTAPLO.EB.rma, transf = transf.ilogit ) #glmm model CTAPLO.glmm.rma <‐ rma.glmm(xi = TP, mi = FN, measure = 'PLO', data = CTA.dt, model = 'UM.RS', tdist = T, slab = Study.ID) predict(CTAPLO.glmm.rma, transf = transf.ilogit ) # glmm forest plot forest(CTAPLO.glmm.rma, transf = transf.ilogit, addcred = T, refline = 0.84, xlab = 'Sensitivity', mlab = 'Random Effects', efac = 2) # 4 vessel reanalysis CTA #estimate effect measures CTA4PAS <‐ escalc(measure = 'PAS', xi = TP, mi = FN, add = 1/2, to = 'only0', vtype = 'UB', data = CTA4.dt) CTA4PLO <‐ escalc(measure = 'PLO', xi = TP, mi = FN, add = 1/2, to = 'only0', vtype = 'LS', data = CTA4.dt) #model CTA4PAS.REML.rma <‐ rma.uni(yi = yi, vi = vi, data = CTA4PAS, method = 'REML', knha = T) CTA4PLO.REML.rma <‐ rma.uni(yi = yi, vi = vi, data = CTA4PLO, method = 'REML', knha = T) CTA4PAS.EB.rma <‐ rma.uni(yi = yi, vi = vi, data = CTA4PAS, method = 'EB', knha = T) CTA4PLO.EB.rma <‐ rma.uni(yi = yi, vi = vi, data = CTA4PLO, method = 'EB', knha = T) # estimates predict(CTA4PAS.REML.rma, transf = transf.iarcsin) predict(CTA4PLO.REML.rma, transf = transf.ilogit ) predict(CTA4PAS.EB.rma, transf = transf.iarcsin) predict(CTA4PLO.EB.rma, transf = transf.ilogit ) #glmm model CTA4PLO.glmm.rma <‐ rma.glmm(xi = TP, mi = FN, measure = 'PLO', data = CTA4.dt, model = 'CM.EL', tdist = F, slab = Study.ID) predict(CTA4PLO.glmm.rma, transf = transf.ilogit ) # glmm forest plot forest(CTA4PLO.glmm.rma, transf = transf.ilogit, addcred = T, refline = 0.85, xlab = 'Sensitivity', mlab = 'Random Effects', efac = 2, alim = c(0.2, 1))
Appendix 6. Full output of statistical analysis
[1] "summary(CTAPLO.REML.rma)" Random‐Effects Model (k = 8; tau^2 estimator: REML) logLik deviance AIC BIC AICc ‐10.4447 20.8895 24.8895 24.7813 27.8895 tau^2 (estimated amount of total heterogeneity): 0.6467 (SE = 0.4846) tau (square root of estimated tau^2 value): 0.8042 I^2 (total heterogeneity / total variability): 76.46% H^2 (total variability / sampling variability): 4.25 Test for Heterogeneity: Q(df = 7) = 25.8697, P‐val = 0.0005 Model Results: estimate se tval pval ci.lb ci.ub 1.4977 0.3656 4.0969 0.0046 0.6333 2.3622 ** ‐‐‐ Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 [1] "predict(CTAPLO.REML.rma, transf = transf.ilogit)" pred se ci.lb ci.ub cr.lb cr.ub 0.8172 NA 0.6532 0.9139 0.3564 0.9731 [1] "summary(CTAPLO.EB.rma)" Random‐Effects Model (k = 8; tau^2 estimator: EB) logLik deviance AIC BIC AICc ‐11.4512 19.1184 26.9024 27.0612 29.3024 tau^2 (estimated amount of total heterogeneity): 0.8111 (SE = 0.5911) tau (square root of estimated tau^2 value): 0.9006 I^2 (total heterogeneity / total variability): 80.29% H^2 (total variability / sampling variability): 5.07 Test for Heterogeneity: Q(df = 7) = 25.8697, P‐val = 0.0005 Model Results: estimate se tval pval ci.lb ci.ub 1.5179 0.3718 4.0827 0.0047 0.6387 2.3970 ** ‐‐‐ Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 [1] "predict(CTAPLO.EB.rma, transf = transf.ilogit)" pred se ci.lb ci.ub cr.lb cr.ub 0.8202 NA 0.6545 0.9166 0.3130 0.9786 [1] "summary(CTAPLO.glmm.rma)" Random‐Effects Model (k = 8; tau^2 estimator: ML) logLik deviance AIC BIC AICc ‐23.5819 22.4493 51.1638 53.5638 51.3226 tau^2 (estimated amount of total heterogeneity): 0.8390 tau (square root of estimated tau^2 value): 0.9160 I^2 (total heterogeneity / total variability): 80.82% H^2 (total variability / sampling variability): 5.21 Tests for Heterogeneity: Wld(df = 7) = 22.6880, P‐val = 0.0019 LRT(df = 7) = 35.8868, P‐val < .0001 Model Results: estimate se tval pval ci.lb ci.ub 1.6730 0.3763 4.4460 0.0030 0.7832 2.5628 ** ‐‐‐ Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 [1] "predict(CTAPLO.glmm.rma, transf = transf.ilogit)" pred se ci.lb ci.ub cr.lb cr.ub 0.8420 NA 0.6864 0.9284 0.3388 0.9823 [1] "summary(CTA4PLO.REML.rma)" Random‐Effects Model (k = 8; tau^2 estimator: REML) logLik deviance AIC BIC AICc ‐9.2908 18.5817 22.5817 22.4735 25.5817 tau^2 (estimated amount of total heterogeneity): 0.1145 (SE = 0.1708) tau (square root of estimated tau^2 value): 0.3384 I^2 (total heterogeneity / total variability): 35.64% H^2 (total variability / sampling variability): 1.55 Test for Heterogeneity: Q(df = 7) = 13.4828, P‐val = 0.0612 Model Results: estimate se tval pval ci.lb ci.ub 1.4235 0.2528 5.6305 0.0008 0.8257 2.0213 *** ‐‐‐ Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 [1] "predict(CTA4PLO.REML.rma, transf = transf.ilogit)" pred se ci.lb ci.ub cr.lb cr.ub 0.8059 NA 0.6954 0.8830 0.6046 0.9185 [1] "summary(CTA4PLO.EB.rma)" Random‐Effects Model (k = 8; tau^2 estimator: EB) logLik deviance AIC BIC AICc ‐10.3759 14.6725 24.7518 24.9107 27.1518 tau^2 (estimated amount of total heterogeneity): 0.3957 (SE = 0.3928) tau (square root of estimated tau^2 value): 0.6290 I^2 (total heterogeneity / total variability): 65.66% H^2 (total variability / sampling variability): 2.91 Test for Heterogeneity: Q(df = 7) = 13.4828, P‐val = 0.0612 Model Results: estimate se tval pval ci.lb ci.ub 1.5079 0.3031 4.9751 0.0016 0.7912 2.2245 ** ‐‐‐ Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 [1] "predict(CTA4PLO.EB.rma, transf = transf.ilogit)" pred se ci.lb ci.ub cr.lb cr.ub 0.8187 NA 0.6881 0.9024 0.4643 0.9593 [1] "summary(CTA4PLO.glmm.rma)" Random‐Effects Model (k = 8; tau^2 estimator: ML) logLik deviance AIC BIC AICc ‐21.3784 21.4062 46.7569 49.1569 46.9157 tau^2 (estimated amount of total heterogeneity): 0.5161 tau (square root of estimated tau^2 value): 0.7184 I^2 (total heterogeneity / total variability): 71.39% H^2 (total variability / sampling variability): 3.49 Tests for Heterogeneity: Wld(df = 7) = 8.4853, P‐val = 0.2917 LRT(df = 7) = 24.3410, P‐val = 0.0010 Model Results: estimate se zval pval ci.lb ci.ub 1.7642 0.2849 6.1919 <.0001 1.2058 2.3227 *** ‐‐‐ Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 [1] "predict(CTA4PLO.glmm.rma, transf = transf.ilogit)" pred se ci.lb ci.ub cr.lb cr.ub 0.8537 NA 0.7696 0.9107 0.5620 0.9637
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berenguer 2010.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | |||
| Target condition and reference standard(s) | Acceptable reference standard? Yes | ||
| Flow and timing | Withdrawals explained? Unclear Partial verification avoided? Unclear Uninterpretable results reported? Unclear | ||
| Comparative | |||
| Notes | Apnea test performed in only 13 patients‐ i.e. a subset of the whole group. Uninterpretable or indeterminate results are not reported. It is not stated if the radiologist is blinded. Author has responded, awaiting data from them‐ Feb 2013 |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | No | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | No | ||
| CTA: timings described | No | ||
| CTA: contrast dose described | No | ||
| CTA: image quality described or accounted for | Yes | ||
| CTA: volume of contrast described | No | ||
| CTA: volume of acquisition described | No | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Unclear | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Unclear | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Unclear | ||
| CTA: all series reported by same person/s? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | No | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | No | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Unclear | ||
| Low | |||
Bohatyrewicz 2010.
| Study characteristics | |||
| Patient sampling | Authors attempted to enrol all eligible patients but could not do so due to clinical factors. In addition 25 cases were excluded from publication due to incorrect application of CTA protocol. | ||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | CTA, single review by experienced neuroradiologist, confirmed by author | ||
| Target condition and reference standard(s) | Clinical testing performed on each patient by three specialists, according to Polish law‐ anaesthesiology/ITU, neurology/neurosurgery, and anaesthesiology. Confirmed by author | ||
| Flow and timing | Withdrawals explained? 25 patients excluded rather than withdrawn, due to incorrect application of CTA protocol Uninterpretable results reported? yes | ||
| Comparative | |||
| Notes | Author responded Jan 2013 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Unclear | ||
| Were selection criteria clearly defined? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Yes | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | No | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Yes | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Combes 2007.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | Withdrawals explained? No withdrawals Uninterpretable results reported? Unclear | ||
| Comparative | |||
| Notes | No reply from author despite two letters (Feb 2013) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Unclear | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Unclear | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Unclear | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Dupas 1998.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | ITU setting, French hospital | ||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | This is a case‐control study with 14 cases and 11 controls. However the data as published, precludes analysis of specificity for any particular vessel scoring system (consensus, DG, TT, RD) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | No | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | No | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | No | ||
| CTA: report‐ at least double review? | Yes | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Unclear | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Unclear | ||
| CTA: all series reported by same person/s? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Unclear | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | No | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Escudero 2009a.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | Withdrawals explained? None listed Uninterpretable results reported? None listed | ||
| Comparative | |||
| Notes | Awaiting further details from author (has responded) ‐ Feb 2013 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Yes | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | No | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Unclear | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Unclear | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Frampas 2009.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | Withdrawals explained? Yes ‐ missing Uninterpretable results reported? Unclear | ||
| Comparative | |||
| Notes | 7 patients missing from the analysis ‐ representing study withdrawals. Scanner type described as 'multi detector' rather than specific make, model, number of detectors. No contact from author despite two letters (Feb 2013) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Unclear | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Yes | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Yes | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Unclear | |||
Leclerc 2006.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | Withdrawals explained? None listed Uninterpretable results reported? Yes | ||
| Comparative | |||
| Notes | Primary author has responded (Feb 2013). No further data, beyond that in the published article, is available from the author | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | No | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Unclear | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Unclear | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Yes | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Unclear | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Unclear | ||
| CTA: all series reported by same person/s? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Quesnel 2007.
| Study characteristics | |||
| Patient sampling | Consecutive sequential sample, confirmed by author | ||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | CTA, sequentially double reviewed by (unblinded) senior radiologist and (blinded) neuroradiologist ‐ confirmed by author | ||
| Target condition and reference standard(s) | Clinical testing performed by senior consultants in anaesthesiology and/or intensive care medicine ‐ confirmed by author | ||
| Flow and timing | Withdrawals explained? Unclear (equipoise between 3 reviewers DG TT RL) Uninterpretable results reported? Unclear (equipoise between 3 reviewers DG TT RL) | ||
| Comparative | |||
| Notes | Author responded Jan 2013 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Was the radiologist blinded to the results of clinical testing? | Yes | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Yes | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Yes | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Yes | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Unclear | |||
Rieke 2011.
| Study characteristics | |||
| Patient sampling | Retrospective. 8 patients excluded due to different (nonstandard) protocol usage. All patients were considered with no a priori exclusions | ||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | CTA performed by same radiologist/s | ||
| Target condition and reference standard(s) | |||
| Flow and timing | Withdrawals explained? Yes
Uninterpretable results reported? None (confirmed by author) Time interval between clinical testing and CTA = median 5hr 16min (data from author) |
||
| Comparative | |||
| Notes | 8 patients excluded. Author comments that exclusion was performed as their assessment was somewhat random and therefore to include them would have introduced potential source of error and methodological pitfall. The excluded group did not differ from the included group re clinical presentation/state etc (data from author) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Unclear | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Yes | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Yes | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Welschehold 2012a.
| Study characteristics | |||
| Patient sampling | All patients included if possible. Exclusion criteria (renal disease, <18yrs old) and in some cases consent not provided by relatives. No other exclusion criteria (data from author) | ||
| Patient characteristics and setting | Representative spectrum | ||
| Index tests | CTA, double reported by consultant radiologists, confirmed by author | ||
| Target condition and reference standard(s) | |||
| Flow and timing | Withdrawals explained? Two patients not included ‐ 1 had no venous imaging, 1 had delayed++ venous imaging. These patients have not been included in the published paper as data is incomplete (data from author) Uninterpretable results reported? None reported by author (data from author) | ||
| Comparative | |||
| Notes | Author responded, Jan 2013, confirming that this paper adds to the data originally published in previous work by same author | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes | ||
| Were selection criteria clearly defined? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index Test | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| Was the radiologist blinded to the results of clinical testing? | No | ||
| Was the expertise of the reporting radiologist recorded? | Yes | ||
| Are CTA criteria for diagnosing brain death described or referenced? | Yes | ||
| Is the method of the index test (CTA) described in enough detail to make it replicable? | Yes | ||
| CTA: timings described | Yes | ||
| CTA: contrast dose described | Yes | ||
| CTA: image quality described or accounted for | Yes | ||
| CTA: volume of contrast described | Yes | ||
| CTA: volume of acquisition described | Yes | ||
| CTA: scanner type described | Yes | ||
| CTA: report‐ at least double review? | Yes | ||
| CTA: hypovolaemia accounted for/ efforts to correct? | Yes | ||
| CTA: blood pressure/MAP accounted for/ efforts to correct? | Yes | ||
| CTA: all series reported by same person/s? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Did the whole of the study population receive the reference standard of diagnosis? | Yes | ||
| Was the reference standard independent of the index test? | Yes | ||
| Was the method of the reference standard described in enough detail to make it replicable? | Yes | ||
| Was the clinician performing the reference standard examination blinded to results of the index test? | Yes | ||
| With regard to reference test: was the expertise of the interpreting clinician/s recorded? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be in clinical practice? | Yes | ||
| Low | |||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bacigalupo 2007 | Discussion article. No new data |
| Badenes Quiles 2011 | Single case, not using index test |
| Bell 2010 | Letter. No new data |
| Beltramello 2010 | Review article. No new data |
| Bosnell 2011 | Review article. No new data |
| Busl 2009 | Review article. No new data |
| Cheng 2008 | Editorial piece. No new data |
| Dicocco 2011 | CTA versus cerebral angiography but different study population and different reference standard (trauma) |
| Escudero 2009b | Review article. No new data |
| Goila 2009 | Review article. No new data |
| Heran 2008 | Review article. No new data |
| Joffe 2007 | Letter only. No new data |
| Karakatsanis 2008 | Review article. No new data |
| Lang 2011 | Letter, review. No new data |
| Latronico 2008 | Letter. No new data |
| Latronico 2011 | Editorial. No new data |
| Leclerc 2007 | Consensus article. No new data |
| Machado 2010 | Review article. No new data |
| Pellon 2010 | Single case report |
| Qureshi 2004 | Narrative report only of two cases undergoing CTA, angiography and perfusion. Significant errors identified in the article (e.g. CT perfusion Figure 2). Rejected by two reviewers |
| Shemie 2008 | Consensus report. No new data |
| Sherrington 2011 | Abstract of subject review and current practice. No new patient data |
| Van der Lugt 2010 | Review article. No new data |
| Welschehold 2012b | Data in this publication is duplicated and expanded in Welschehold 2012 European Journal of Neurology ‐ confirmed by author |
| Wijdicks 2002 | Review and practice article. No new data |
Characteristics of ongoing studies [ordered by study ID]
Musacchio 2010.
| Trial name or title | |
| Target condition and reference standard(s) | |
| Index and comparator tests | |
| Starting date | |
| Contact information | |
| Notes | Summary data as part of a Powerpoint presentation. Methodologically incomplete: reference standard not described and results grouped and summarized. Cannot be used for either all‐comers or 4‐vessel analysis in current form. Search performed for abstract or published article by same author group but nothing identified. Potentially large study cohort therefore should be searched for when this review is next re‐run |
Differences between protocol and review
Significant methodological addition: addition of four‐vessel CTA re‐analysis to attempt to correct for otherwise substantial heterogeneity in reported studies.
Methodological modification: at the time of the original protocol publication (Taylor 2012), QUADAS was the preferred assessment process. By the time the review was performed, QUADAS‐2 2011 was the preferred assessment process: the original protocol questions were therefore migrated from QUADAS and were considered as additional questions to the QUADAS‐2 methodological assessment process. QUADAS additional questions were modified and expanded into new domains in QUADAS‐2 (Appendix 3).
Change to protocol: assessment of 'utility' has been removed as a nebulous term. The review concentrates on sensitivity alone. Specificity is not assessed for reasons detailed in the review. As a result, amendments have been made to the 'Objectives' and 'Background' sections for clarity.
Change to protocol: textual changes to 'Target condition' and 'Imaging reporting' sections for clarity.
Contributions of authors
Tim Taylor (TT), Rob A Dineen (RD), Dale C Gardiner (DG), Charmaine H Buss (CB), Allan Howatson (AH), Nathan Pace (NP)
Conceiving the review: TT, RD, DG
Co‐ordinating the review: TT
Undertaking manual searches: AH, CB
Screening search results: AH, CB
Organizing retrieval of papers: AH, CB
Screening retrieved papers against inclusion criteria: AH, CB
Appraising quality of papers: TT, RD, DG
Abstracting data from papers: AH, TT, RD, DG
Writing to authors of papers for additional information: TT, RD, DG
Providing additional data about papers: AH, CB, TT, RD, DG
Obtaining and screening data on unpublished studies: AH, CB, TT, RD, DG
Data management for the review: TT, AH, CB
Entering data into Review Manager (Revman 5.2): AH, CB, TT
RevMan statistical data: NP
Other statistical analysis not using RevMan: NP
Double entry of data: (data entered by person one: AH; data entered by person two: TT)
Interpretation of data: NP
Statistical inferences: NP
Writing the review: all authors
Securing funding for the review: DG
Performing previous work that was the foundation of the present study: not applicable
Guarantor for the review (one author): TT
Person responsible for reading and checking review before submission: all authors
Sources of support
Internal sources
None identified, Other.
External sources
None identified, Other.
Declarations of interest
Tim Taylor and Allan Howatson were fully funded to attend the Cochrane DTA training course in Amsterdam, September 2010. This was paid for by a grant from NHS Blood and Transplant.
Dale Gardiner is the Clinical Lead for Organ Donation, Nottingham University Hospitals NHS Trust and the Midlands Regional Clinical Lead for Organ Donation. Nottingham University Hospitals NHS Trust is reimbursed by NHS Blood and Transplant for his time.
Charmaine Buss is a Specialist Nurse for Organ Donation employed and managed by NHS Blood and Transplant.
Nathan Pace: none known.
Rob Dineen: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Berenguer 2010 {published data only}
- Berenguer CM, Davis FE, Howington JU. Brain death confirmation: Comparison of computed tomographic angiography with nuclear medicine perfusion scan. Journal of Trauma Injury, Infection and Critical Care March 2010;68(3):553‐9. [PUBMED: 20220416] [DOI] [PubMed] [Google Scholar]
Bohatyrewicz 2010 {published data only}
- Bohatyrewicz R, Sawicki M, Walecka A, Walecki J, Rowinski O, Bohatyrewicz A, et al. Computed tomographic angiography and perfusion in the diagnosis of brain death. Transplantation Proceedings 2010;42:3941‐6. [PUBMED: 21168593] [DOI] [PubMed] [Google Scholar]
Combes 2007 {published data only}
- Combes J‐C, Chomel A, Ricolfi F, d'Athis P, Freysz M. Reliability of computed tomographic angiography in the diagnosis of brain death. Transplantation Proceedings 2007;39:16‐20. [PUBMED: 17275466] [DOI] [PubMed] [Google Scholar]
Dupas 1998 {published data only}
- Dupas B, Gayet‐Delacroix M, Villers D, Antonioli D, Veccherini M, Soulillou J. Diagnosis of brain death using two‐phase spiral CT. American Journal of Neuroradiology April 1998;19:641‐7. [PUBMED: 9576648] [PMC free article] [PubMed] [Google Scholar]
Escudero 2009a {published data only}
- Escudero D, Otero J, Marques L, Parra D, Gonzalo JA, Albaiceta G, et al. Diagnosing brain death by CT perfusion and multislice CT angiography. Neurocritical Care 2009;11:261‐71. [PUBMED: 19565357] [DOI] [PubMed] [Google Scholar]
Frampas 2009 {published data only}
- Frampas E, Videcoq M, deKerviler E, Ricolfi F, Kuoch V, Mourey F, et al. CT angiography for brain death diagnosis. American Journal of Neuroradiology 2009;30:1566‐70. [PUBMED: 19406767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leclerc 2006 {published data only}
- Leclerc X, Taschner CA, Vidal A, Strecker G, Savage J, Gauvrit JY, et al. The role of spiral CT for the assessment of the intracranial circulation in suspected brain‐death. Journal of Neuroradiology 2006;33:90‐5. [PUBMED: 16733422] [DOI] [PubMed] [Google Scholar]
Quesnel 2007 {published data only}
- Quesnel C, Fulgencio J‐P, Adrie C, Marro B, Payen L, Lembert N, et al. Limitations of computed tomographic angiography in the diagnosis of brain death. Intensive Care Medicine 2007;33:2129‐35. [PUBMED: 17643226] [DOI] [PubMed] [Google Scholar]
Rieke 2011 {published data only}
- Rieke A, Regli B, Mattle HP, Brekenfeld C, Gralla J, Schroth G, et al. Computed tomography angiography (CTA) to prove circulatory arrest for the diagnosis of brain death in the context of organ transplantation. Swiss Medical Weekly 2011;141:w13261. [PUBMED: 21971739] [DOI] [PubMed] [Google Scholar]
Welschehold 2012a {published data only}
- Welschehold S, Kerz T, Boor S, Reuland K, Thomke F, Reuland A, et al. Detection of intracranial circulatory arrest in brain death using cranial CT‐angiography. European Journal of Neurology 2013;20(1):173‐9. [PUBMED: 22788547] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bacigalupo 2007 {published data only}
- Bacigalupo F, Huerta D, Montefusco‐siegmund R. The debate about death: an imperishable discussion?. Biological Research 2007;40:523‐34. [PUBMED: 18575685] [PubMed] [Google Scholar]
Badenes Quiles 2011 {published data only}
- Badenes Quieles R, Moreno J, Garcia‐Perez M, Pastor E, Maruenda A, et al. Cerebral blood flow by thermal diffusion during assessment of brain death. Presentation 0988 Berlin 2011.
Bell 2010 {published data only}
- Bell D, Murphy P. Letter: Barriers to brainstem death testing and organ donation can be addressed. Anaesthesia 2010;65:646‐56. [PUBMED: 20565396] [DOI] [PubMed] [Google Scholar]
Beltramello 2010 {published data only}
- Beltramello A, Riccardi G, Pizzini F, Piovan E. Updates in the determination of brain death. The Neuroradiology Journal 2010;23:145‐50. [DOI] [PubMed] [Google Scholar]
Bosnell 2011 {published data only}
- Bosnell R, Madder H. Concepts of brain death. Surgery 2011;29(7):289‐94. [Google Scholar]
Busl 2009 {published data only}
- Busl K, Greer D. Pitfalls in the diagnosis of brain death. Neurocritical Care 2009;11:276‐87. [DOI: 10.1007/s12028-009-9231-y; PUBMED: 19444652] [DOI] [PubMed] [Google Scholar]
Cheng 2008 {published data only}
- Cheng W, Lin K. Editorial: Supplementary tests for confirmation of brain death. Journal of Zhejiang University Science B 2008;9(11):921‐2. [DOI: 10.1631/jzus.B0870001; PUBMED: 18988312 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dicocco 2011 {published data only}
- DiCocco J, Emmett K, Fabian T, Zarzaur B, Williams J, Croce M. Multidetector Computed Tomography: more slices still don't cut it. Annals of Surgery March 2011;253(3):444‐50. [PUBMED: 21263309] [DOI] [PubMed] [Google Scholar]
Escudero 2009b {published data only}
- Escudero D. Diagnosing brain death [Diagnostico de muerte encefalica]. Medicina Intensiva 2009;33(4):185‐95. [PUBMED: 19558940] [DOI] [PubMed] [Google Scholar]
Goila 2009 {published data only}
- Golia A, Pawar M. The diagnosis of brain death. Indian Journal of Critical Care Medicine 2009;13(1):7‐11. [PUBMED: 19881172 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008 {published data only}
- Heran M, Heran N, Shemie S. A review of ancillary tests in evaluating brain death. The Canadian Journal of Neurological Sciences 2008;35:409‐19. [PUBMED: 18973057] [DOI] [PubMed] [Google Scholar]
Joffe 2007 {published data only}
- Joffe A. Letter: Limitations of brain death in the interpretation of computed tomography angiography. Intensive Care Medicine 2007;33:2218. [PUBMED: 17940745 ] [DOI] [PubMed] [Google Scholar]
Karakatsanis 2008 {published data only}
- Karakatsanis K. Review: 'Brain death': should it be reconsidered?. Spinal Cord 2008;46:396‐401. [PUBMED: 17700512] [DOI] [PubMed] [Google Scholar]
Lang 2011 {published data only}
- Lang C. Letter: There is no reversible brain death. Critical Care Medicine 2011;39(9):2205‐6. [PUBMED: 21849846 ] [DOI] [PubMed] [Google Scholar]
Latronico 2008 {published data only}
- Latronico N, Zamperetti N, Bellomo R, Defanti C. Letter: Brain death: what's in a name?. Intensive Care Medicine 2008;34:1352. [PUBMED: 18351321] [DOI] [PubMed] [Google Scholar]
Latronico 2011 {published data only}
- Latronico N, Zamperetti N, Rasulo F. Editorial: Brain death models: solutions in search of problems. Critical Care Medicine 2011;39(3):595‐6. [PUBMED: 21330863] [DOI] [PubMed] [Google Scholar]
Leclerc 2007 {published data only}
- Leclerc X. Editorial: CT angiography for the diagnosis of brain death: recommendations of the French Society of Neuroradiology (SFNR) [Diagnostic par angioscanner de lar mort encephalique: recommandations de la societe francaise de neuroradiologie (SFNR)]. Journal of Neuroradiology 2007;34:217‐9. [PUBMED: 17719630 ] [DOI] [PubMed] [Google Scholar]
Machado 2010 {published data only}
- Machado C, Perez J, Scherle C, Korein J. When are ancillary tests recommended in brain death confirmation?. The Internet Journal of Neurology ISSN 1531‐295X 2010;12(2):n/a. [Google Scholar]
Pellon 2010 {published data only}
- Pellon R, Lucas E, Fernandez C, Florez A, Piedra T. Usefulness of addition of CT perfusion to CT angiography for brain death diagnosis in a child. Neuropediatrics 2010;41(4):189‐92. [DOI] [PubMed] [Google Scholar]
Qureshi 2004 {published data only}
- Qureshi A, Kirmani J, Xavier A, Siddiqui A. Computed tomographic angiography for diagnosis of brain death. Neurology 2004;62:652‐3. [PUBMED: 14981190] [DOI] [PubMed] [Google Scholar]
Shemie 2008 {published data only}
- Shemie S, Lee D, Sharpe M, Tampieri D, Young B. Brain blood flow in the neurological determination of death: Canadian expert report. Canadian Journal of Neurological Sciences 2008;35:140‐5. [PUBMED: 18574925] [DOI] [PubMed] [Google Scholar]
Sherrington 2011 {published data only}
- Sherrington A, Barrass L, Mandersloot G, Healy M. Abstract: Survey of the use of ancillary tests in the diagnosis of brain stem death. Critical Care 2011;15 Suppl 1:P523. [Google Scholar]
Van der Lugt 2010 {published data only}
- Lugt A. Imaging tests in determination of brain death. Neuroradiology 2010;52:945‐7. [PUBMED: 20820765] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welschehold 2012b {published data only}
- Welschehold S, Boor S, Reuland K, Beyer C, Kerz T, Reuland A, Müller‐Forell W. CT Angiography as a Confirmatory Test in Brain Death. Intracranial Pressure and Brain Monitoring XIV, Acta Neurochirurgica Supplementum 2012;114:311‐6. [DOI: 10.1007/978-3-7091-0956-4_60] [DOI] [PubMed] [Google Scholar]
Wijdicks 2002 {published data only}
- Wijdicks E. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology 2002;58:20‐5. [PUBMED: 11781400] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Musacchio 2010 {unpublished data only}
- Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Academy of Medical Royal Colleges 2008
- A code of practice for the diagnosis and confirmation of brain death. www.collemergencymed.ac.uk/asp/document.asp?ID=4635. London.
ANZICS 2010
- ANZICS. The Anzics Statement on Death and Organ Donation. http://www.anzics.com.au/death‐and‐organ‐donation 2010; Vol. Edition 3.1.
Canadian Council 2003
- CCDT. A review of the literature on the determination of brain death. Canadian Council for Donation and Transplantation, Executive Summary Document, http://www.ccdt.ca/english/publications/lit‐pdfs/Brain‐Death‐Long‐Lit‐Review.pdf. 2003.
Conference of Medical Royal Colleges 1976
- No authors listed. Diagnosis of brain death. Statement issued by the honorary secretary of the Conference of Medical Royal Colleges and their Faculties in the United Kingdom on 11th October 1976. British Medical Journal 1976;2:1187‐8. [PUBMED: 990836] [DOI] [PMC free article] [PubMed] [Google Scholar]
JAMA 1968
- No authors listed. A Definition of Irreversible Coma: report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA 1968;205:337‐40. [PUBMED: 5694976] [PubMed] [Google Scholar]
NHS Blood and Transplant 2012/13
- NHS Blood and Transplant. Organ Donation and Transplantation Activity Report 2012/13. http://www.organdonation.nhs.uk/statistics/transplant_activity_report/current_activity_reports/ukt/activity_report_2012_13.pdf 2013.
President's Commission 1981
- Defining death: medical, legal and ethical issues in the determination of death. President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. http://bioethics.georgetown.edu/pcbe/reports/past_commissions/defining_death.pdf 1981.
QUADAS‐2 2011
- Whiting P, Jutjes A, Westwood M, Mallett S, Deeks J, Reitsma J, et al. QUADAS‐2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine October 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Revman 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.2. The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2010
- Roberts DJ, MacCulloch KA, Versnick EJ, Hall RL. Should ancillary brain blood flow analyses play a larger role in the neurological determination of death?. Canadian Journal of Anaesthesia 2010;57:927‐35. [PUBMED: 20706879] [DOI] [PubMed] [Google Scholar]
Viechtbauer 2010
- Viechtbauer W. Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software 2010;36(3):1‐48. [Google Scholar]
Webb 2011
- Webb AC, Samuels OB. Reversible brain death after cardiopulmonary arrest and induced hypothermia. Critical Care Medicine 2011;39(6):1‐5. [PUBMED: 21494112] [DOI] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;10(3):25. [PUBMED: 14606960] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2004
- Whiting P, Ruties AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Annals of Internal Medicine 2004;140:189‐202. [PUBMED: 14757617] [DOI] [PubMed] [Google Scholar]
Young 2006
- Young GB, Shemie SD, Doig CJ, Teitelbaum J. Brief review: the role of ancillary tests in the neurological determination of death. Canadian Journal of Anaesthesia 2006;53(6):620‐7. [PUBMED: 16738299] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Taylor 2012
- Taylor T, Dineen RA, Gardiner DC, Buss CH, Howatson A, Pace NL. Computed tomography (CT) angiography for confirmation of the clinical diagnosis of brain death. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009694] [DOI] [PMC free article] [PubMed] [Google Scholar]


