Abstract
Background
With the increased demand for whiter teeth, home‐based bleaching products, either dentist‐prescribed or over‐the‐counter products have been exponentially increasing in the past few decades. This is an update of a Cochrane Review first published in 2006.
Objectives
To evaluate the effects of home‐based tooth whitening products with chemical bleaching action, dispensed by a dentist or over‐the‐counter.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 12 June 2018), the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library (searched 12 June 2018), MEDLINE Ovid (1946 to 12 June 2018), and Embase Ovid (1980 to 12 June 2018). The US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (12 June 2018) and the World Health Organization International Clinical Trials Registry Platform (12 June 2018) were searched for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included in our review randomised controlled trials (RCTs) which involved adults who were 18 years and above, and compared dentist‐dispensed or over‐the‐counter tooth whitening (bleaching) products with placebo or other comparable products.
Quasi‐randomised trials, combination of in‐office and home‐based treatments, and home‐based products having physical removal of stains were excluded.
Data collection and analysis
Two review authors independently selected trials. Two pairs of review authors independently extracted data and assessed risk of bias. We estimated risk ratios (RRs) for dichotomous data, and mean differences (MDs) or standardised mean difference (SMD) for continuous data, with 95% confidence intervals (CIs). We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 71 trials in the review with 26 studies (1398 participants) comparing a bleaching agent to placebo and 51 studies (2382 participants) comparing a bleaching agent to another bleaching agent. Two studies were at low overall risk of bias; two at high overall risk of bias; and the remaining 67 at unclear overall risk of bias.
The bleaching agents (carbamide peroxide (CP) gel in tray, hydrogen peroxide (HP) gel in tray, HP strips, CP paint‐on gel, HP paint‐on gel, sodium hexametaphosphate (SHMP) chewing gum, sodium tripolyphosphate (STPP) chewing gum, and HP mouthwash) at different concentrations with varying application times whitened teeth compared to placebo over a short time period (from 2 weeks to 6 months), however the certainty of the evidence is low to very low.
In trials comparing one bleaching agent to another, concentrations, application method and application times, and duration of use varied widely. Most of the comparisons were reported in single trials with small sample sizes and event rates and certainty of the evidence was assessed as low to very low. Therefore the evidence currently available is insufficient to draw reliable conclusions regarding the superiority of home‐based bleaching compositions or any particular method of application or concentration or application time or duration of use.
Tooth sensitivity and oral irritation were the most common side effects which were more prevalent with higher concentrations of active agents though the effects were mild and transient. Tooth whitening did not have any effect on oral health‐related quality of life.
Authors' conclusions
We found low to very low‐certainty evidence over short time periods to support the effectiveness of home‐based chemically‐induced bleaching methods compared to placebo for all the outcomes tested.
We were unable to draw any conclusions regarding the superiority of home‐based bleaching compositions or any particular method of application or concentration or application time or duration of use, as the overall evidence generated was of very low certainty. Well‐planned RCTs need to be conducted by standardising methods of application, concentrations, application times, and duration of treatment.
Plain language summary
Home‐based chemical bleaching of teeth in adults
Review question What evidence is available regarding the different home‐based chemically‐induced bleaching agents in whitening teeth?
Background There has been an increasing demand for whiter teeth. Home‐based whitening products with a bleaching action have become popular and are prescribed to people by the dentist or purchased over‐the‐counter. A variety of whitening products are available which include hydrogen peroxide, carbamide peroxide, sodium percarbonate, sodium hexametaphosphate, sodium tripolyphosphate, and calcium peroxide. These agents are supplied in different concentrations and are used with different methods of application (gel in tray, strips, paint‐on gel, chewing gum, and mouthwash), which have varying application times and duration of treatment.
Study characteristics Authors from Cochrane Oral Health carried out this review of existing studies and the evidence is current up to 12 June 2018. We included 71 trials that involved 3780 adults who underwent tooth whitening procedures with various bleaching agents using different methods of application, length of application and duration of treatment. 26 studies compared a bleaching agent to placebo and 51 studies compared one bleaching agent to another bleaching agent.
Key results
The bleaching agents whitened teeth compared to placebo over a short time period (from 2 weeks to 6 months), however the certainty of the evidence is low to very low.
The evidence currently available is insufficient to draw reliable conclusions regarding the superiority of home‐based bleaching compositions or any particular method of application or concentration or application time or duration of use.
The most common adverse events were tooth sensitivity and oral irritation, which were reported with higher concentrations of active agents, although the effects were mild and transient.
Well‐planned randomised controlled trials need to be conducted by standardising methods of application, concentrations, application times and duration of treatment.
Certainty of evidence The overall certainty of the evidence was low to very low for all comparisons. This was because most of the comparisons were reported in single trials with small sample sizes and event rates. There was an unclear risk of bias in most of the trials.
Summary of findings
Background
Aesthetic dentistry has received increased attention in recent years, especially because people are more concerned about the aesthetic appearance of their smile (Demarco 2009). Technological innovations in dentistry have been added due to patients' desire to improve aesthetics of their teeth, which is an important aspect of quality of life (Pinto 2014). A survey conducted in the UK revealed that 28% of adults were dissatisfied with their smile and among 3215 subjects examined, 50% had some kind of tooth discolouration (Joiner 2006). Another survey performed in the UK in 2004 suggested that the public is concerned about dental appearance in terms of tooth colours (Alkhatib 2004). Based on the survey conducted by the American Academy of Cosmetic Dentistry in 2012, discoloured, stained or yellow teeth were the main reason for an unattractive smile. The same survey reported that there was a 29% increase in patients receiving tooth whitening in a span of 1 year and was expected to increase to 45% and more in the years to come. 70% of patients who opted for bleaching were females. 75% of respondents reported the use of at‐home or over‐the‐counter whitening products. 18% of dentists in the US recommended a home‐based bleaching method (Whitening survey 2012).
With this increased demand for whiter teeth, tooth whitening products have been exponentially increasing in the past few decades. Presently, tooth whitening products are the most popularly marketed oral care products (Whitening survey 2012).
Description of the condition
Tooth discolouration can be described as any change in the colour or translucency of a tooth and can be classified based on aetiology as extrinsic or intrinsic discolourations. Extrinsic discolourations adhere to the tooth surface (superficial stains), while intrinsic discolourations are integrated in the structure of teeth (Demarco 2009). However, in some cases, both intrinsic and extrinsic discolourations may affect tooth enamel, dentine or pulp.
Extrinsic staining
This usually results from accumulation of chromatogenic substances on the external tooth surface. These include smoking, pigments in foods and beverages, and metals such as iron or copper which lead to dark, brownish discolourations. These stains are localised mainly in the pellicle and are either generated by the reaction between sugars and amino acids or acquired from the retention of exogenous chromophores in the pellicle (Viscio 2000). This reaction is called Maillard reaction or the non‐enzymatic browning reaction. Most extrinsic stains can be removed by routine prophylactic procedures. With time, these stains darken and become more persistent but they are highly responsive to bleaching.
Intrinsic staining
This can result from genetic disorders such as dentinogenesis imperfecta, amelogenesis imperfecta, thalassaemia, sickle cell anaemia, antibiotics such as tetracyclines, high levels of fluoride intake, dental caries, pulpal haemorrhage, pulpal necrosis, pulp tissue remnants, root filling materials/endodontic irrigation, or amalgam restorations (Nathoo 1997; Kim 2010; Belobrov 2011; Carey 2014; Kolosowski 2014). Likewise, high fevers during the time of a tooth development may cause enamel hypoplasia that leads to banding‐type discolourations on the affected tooth surface. Aging is another common cause of discolouration. Over time, dentine tends to darken due to the formation of secondary dentine and the overlying enamel becomes thinner. Intrinsic stains cannot be removed by regular prophylactic procedures. However, they can be reduced by bleaching with agents penetrating the enamel and dentine to oxidize the chromogens, in some conditions.
Description of the intervention
Tooth whitening or bleaching is a procedure most commonly employed by professionals and patients. It is considered the least invasive aesthetic treatment for improving the appearance of discoloured teeth (Pinto 2014). It may be accomplished by physical removal of the stain or a chemical reaction (bleaching) to lighten the tooth colour. The active ingredient in most chemically‐induced whitening products is hydrogen peroxide (H2O2) which is delivered as hydrogen peroxide (HP) or carbamide peroxide (CP). CP is a stable complex, which will break down to HP and urea, once in contact with water. The basic bleaching action is due to the HP, which can be explained in three phases (Joshi 2016):
initial phase: diffusion of HP through the inter‐prismatic spaces and circulation within the tooth for 2 weeks;
second phase: interaction of HP with organic chromophores which can be influenced by temperature, pH, light and metal cations (Kwon 2015);
third phase: colour change through an altered tooth surface.
Bleaching action reaches an end point which is known as inherent lightness potential for that tooth (Matis 2000). Usually if there is no improvement in the shade after 6 weeks of bleaching, irrespective of the bleaching agent and concentration, then bleaching is assumed to have reached its end point (Joshi 2016).
Types of dental bleaching procedures
Tooth discolourations can be improved by several methods such as internal bleaching for non‐vital teeth, or external bleaching for vital teeth (Joiner 2006) or a combination of techniques.
Internal bleaching/non‐vital bleaching
It consists of walking bleach and thermocatalytic bleaching techniques and is done after endodontic treatment by the dentist and comprises of in‐office techniques, which are not in the scope of this review.
External bleaching methods/vital bleaching
There are three fundamental approaches for bleaching vital teeth.
In‐office or power bleaching (not in the scope of this review).
At‐home or dentist‐supervised bleaching.
Consumer‐purchased or over‐the‐counter (OTC) products which are available in pharmacies or supermarkets without any prescription or professional monitoring.
(Other non‐dental products like malic acid found in juice of green apples or do‐it‐yourself whitening with strawberry and baking soda were reported (Kwon 2015) but are not in the scope of this review.)
This review includes only at‐home or dentist‐supervised bleaching and consumer‐purchased or OTC products.
Home‐based bleaching methods (dentist‐supervised and OTC)
A variety of peroxide compounds, including hydrogen peroxide (HP), carbamide peroxide (CP) or urea peroxide, sodium percarbonate (SPC), sodium hexametaphosphate (SHMP), sodium tripolyphosphate (STPP), and calcium peroxide have been used as active ingredients in home‐based bleaching methods. These agents are supplied in different concentrations, used with varying application times and duration of treatment (Alqahtani 2014), and delivered in various forms.
Gels in trays
Dentist‐supervised home‐use tooth bleaching with custom trays is the most common bleaching procedure dispensed by dentists to their patients. Usually, this treatment modality consists of fabrication of a custom tray with and without reservoirs (Javaheri 2000; Caballero 2006; Baroudi 2014).
Whitening strips
These strips mainly contain hydrogen peroxide as the active agent in different concentrations. They are applied directly to the tooth surfaces and are thin flexible polyethylene strips coated with the bleaching gel. Continued research led to the development of strip‐based whitening with very thin peroxide gels less than 0.20 mm in thickness (Perdigão 2004; Duschner 2006).
Disadvantages of the strip system are that it can reach only a finite number of teeth, cannot adapt well on malposed teeth, may interfere with speech patterns and can impinge on gingiva.
Paint‐on gels
Paint‐on gels or varnishes are barrier‐free whitening products that present hydrogen or carbamide peroxide in a suspension that is brushed by an applicator over the tooth surface and which adheres to enamel. Some paint‐on gels have sodium percarbonate as their active ingredient. This method has gained popularity since the consumer just needs to paint a thin layer of whitening gel on their teeth (similar to nail polish application on finger nails). The added advantage is that the users can scallop the product around the gingiva and apply it to an unlimited number of teeth, regardless of the position in the arch.
A disadvantage of this method is that lesser contact time of these agents to the tooth surface may result in reduced whitening of teeth. In addition, the applicator brush is re‐used and stored in the gel product which might lead to microbial contamination. Hence, some manufacturers supply disposable cotton bud applicators for this purpose (Goldstein 1995; Carey 2014).
Whitening mouthrinses
Whitening mouthrinses prevent stains and fight plaque build‐up. Generally, a low concentration of hydrogen peroxide (1.5%), sodium hexametaphosphate have been included in the formulation to protect the teeth surface from new stains (Lima 2012).
Whitening chewing gums
These are well accepted and enjoyed by many as a frequent activity among children and adults, therefore, may be a means for local drug administration into the oral cavity (Barabolak 1991). Chewing gum based products possess a number of therapeutic benefits, including increased saliva flow and removal of food debris, plaque and surface stains (Walters 2004). Baking soda (Mankodi 2001; Soparkar 2001), sodium hexametaphosphate (White 2002; Walters 2004), and sodium tripolyphosphate (Shellis 2005; Porciani 2010) have been reported as the active ingredients in chewing gums.
Whitening dentifrices
The active components of tooth whitening dentifrices include hydrogen peroxide or carbamide peroxide which break down the organic molecules of biological film (Horn 2014). Additionally, abrasives such as alumina, dicalcium phosphate dihydrate and silica are also present in the formulation to promote stain removal (Demarco 2009). Their stain removal ability is related to the large quantity of abrasives in their formulation, which remove superficial extrinsic stains. However, the toothpaste abrasiveness needs to be moderated in order to prevent excessive wear to the underlying enamel and dentine. Toothpastes containing blue covarine, a pigment, which increases the perception of tooth whiteness are available in the market as bleaching toothpastes (Dantas 2015). Whitening dentifrices with desensitizing agents (Po 2014) to reduce the adverse event of sensitivity or dentifrices with casein phosphopeptide‐amorphous calcium phosphate (CPP‐ACP) to remineralize the enamel have been in use (de Vasconcelos 2012).
Whitening dental floss
Whitening dental floss has been introduced to promote stain reduction around the interproximal and subgingival areas. The stain removal properties are associated with the presence of silica in the composition, which promotes a superficial surface abrasion during application in the interdental region (Demarco 2009).
Whitening dentifrices, floss and toothbrushes which involve an abrasive action are not included in this review.
All the above products are either prescribed by the dentist for use at home or purchased by the consumer over‐the‐counter without professional consultation. The permitted concentration of HP varies between countries.
The at‐home technique offers many advantages:
self‐administration by the patient
less chair‐side time
high degree of safety
fewer adverse effects
low cost.
However, there are certain disadvantages:
results dependent on active patient compliance and diligence of use
high dropout rates (Leonard 2003)
excessive use by overzealous patients leads to thermal sensitivity, reported to be as high as 67% (Haywood 1992).
How the intervention might work
General mechanism of action
Hydrogen peroxide and carbamide peroxide formulations used as gels in trays/strips/paint‐on gels and mouthrinses
Tooth stains consist of compounds that have colour or darker shades called chromogens. Bleaching mainly constitutes removal of stains by chemical degradation of these chromogens. It is hypothesized that the basic chemistry of peroxide‐based whitening agents is attributed to reaction of hydrogen peroxide with the chromogens. Carbamide peroxide is also an active ingredient in many whitening products. It is a stable complex which breaks down in contact with water to release hydrogen peroxide (10% CP on contact with water, gets converted to 3% HP and 7% urea).
Chromogens fall into two categories: large organic compounds that have conjugated double bonds in their chemical structure and metal containing compounds. Bleaching of the organic compounds by hydrogen peroxide involves reacting with the double bonds to oxidize the double bond.This causes the chromogen to become a lighter coloured compound. Other hypothesized mechanisms include oxidation of proteins within the tooth matrix.
Bleaching of the metallic compounds is much more difficult. There are some professional products that contain sodium hypochlorite (NaOCl) which react with the double bonds of the chromogen in much the same way as peroxide (Carey 2014).
Sodium percarbonate used in paint‐on gel
19% sodium percarbonate has been developed to deliver peroxide over a sustained period without a fixed barrier. The anhydrous system has peroxide bound in a silicone polymer suspension. Applied with a brush to the dried tooth surface, the suspension is designed to form an enamel adherent substantive film that hydrates overnight to slowly release peroxide into the tooth.
Sodium hexametaphosphate used in chewing gums
This active ingredient is a high molecular weight condensed phosphate analogue which inhibits stain chromogen adsorption reducing overall extrinsic staining.
Sodium tripolyphosphate (STPP) used in chewing gums
The inhibitory action of hydroxy apatite bound STPP on adsorption of salivary proteins, makes it an effective agent for inhibiting and removing dental stain.
Why it is important to do this review
The first published version of this Cochrane Review concluded that there was evidence that whitening products work when compared with placebo/no treatment (Hasson 2006). However, all trials in that review were short term and the majority of the included studies had low methodological quality (i.e. high risk of bias). Moreover, in the past 12 years there may have been additional randomised controlled trials published, which needed to be considered in the review. Therefore, there was a need to update this review to identify new evidence from pragmatic long‐term clinical trials and also to re‐look at the outcomes and comparisons used in the previous version.
Objectives
To evaluate the effects of home‐based tooth whitening products with chemical bleaching action, dispensed by a dentist or over‐the‐counter.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Randomised controlled clinical trials comparing dentist‐dispensed or over‐the‐counter (OTC) tooth whitening products (with chemical, bleaching action) with placebo or other comparable products.
Full reports (either published or obtained from the investigators) had to be available for inclusion in the review.
The application of tooth whitening products had to be exclusively carried out at home and outcome data had to be presented for tooth whitening, irrespective of the application time.
Exclusion criteria
Quasi‐randomised trials.
Types of participants
Home‐based whitening involving adults who were 18 years old and above were included in our review regardless of gender, race, profession, geographical location or baseline tooth shade. Because of issues related to compliance and ingestion of a bleaching agent in children and young adults, we decided to include trials including adults who were 18 years old and above.
We included participants with teeth stained because of tetracycline use and smoking.
Types of interventions
Inclusion criteria
We considered any intervention including home‐based chemically‐induced whitening with the following comparisons:
comparisons of different interventions (e.g. professional monitored versus over‐the‐counter; over‐the‐counter product A versus B; professional monitored technique A versus B);
intervention versus placebo or no treatment;
comparisons between different concentrations;
comparisons between different time periods of application.
Exclusion criteria
Combination of in‐office and home‐based treatments were excluded.
Home‐based products having an abrasive action or physical removal of stains were excluded from the review (e.g. whitening dentifrices, whitening dental floss).
Types of outcome measures
Primary outcomes
Two primary outcomes were of interest.
1. Tooth whitening ‐ assessed by the dentist using any relevant tool
The American Dental Association (ADA) acceptance programme guidelines for home‐based tooth whitening products specify the use of two main methods to measure tooth colour in bleaching studies:
value‐oriented shade guides (subjective measurement); and
electronic devices/colour measurement devices (objective measurement).
Value‐oriented shade guides
Traditionally, visual colour determination is used based on visual comparison of tooth with colour standards (also called shade guides). The most common shade guides are Vitapan classical and its derivatives like Vitapan 3D master, tooth guide, bleached guide and linear guide. Ordinal scale ranging from 1 to 16 has been suggested by the manufacturer with 1 representing the lightest shade and 16 representing the darker shade. However, some authors considered 1 as the darkest and 16 as the lighter shade. Some studies reported a scale below 1 and beyond 16 when the tooth shade was lighter than the lightest shade tab and darker than the darkest shade.
Trubyte Bioform shade guide has also been used by some authors using a scale from 1 to 24.
Electronic devices/colour measurement devices
Instruments for clinical shade matching encompass spectrophotometers, colorimeters and imaging systems. They provide a more objective measurement of whiteness compared to shade guides. For instrumental colour assessment of teeth the issue of suitable metric that corresponds to perpetual whiteness is important. Colourimeters, spectrophotometers, spectroradiometer and camera systems can allow computation of CIExyz or CIEL*a*b* values described by commission international deLE clariage (CIE 1978) an international standard for three dimensional colour space.
Using a calibration standard, red‐green‐blue values are determined and converted to L* a* b* values where L* represents the degree of lightness and b* represents degree of yellowness. Tooth whitening is characterised by negative or decreased b* values (reduction in yellowness) and positive or increased L* values (increased lightness). A composite colour W is used by some and derived from individual L* a* b* changes from baseline values. Some clinicians use E* which indicates composite colour change irrespective of the direction of change.
In our review we considered the composite score represented by W* or E* values wherever provided. In the absence of both, the value of L* is considered as it indicates a positive change towards increased lightness.
2. Tooth whitening ‐ reported by the patient using any relevant tool
Improvement in tooth whitening as reported by the patient using any tool was considered in this review. Some authors reported visual analogue scale (VAS) from 0 to 10 to record patient acceptance with 0 indicating best acceptance and 10 indicating no acceptance, while some others used the scale where 0 represents least satisfaction and 10 represents most satisfaction.
Other scores used were on an ordinal scale of 0 to 3 where 0 = no improvement in whiteness, 1 and 2 = moderate improvement, and 3 = improvement. In such cases, we combined 1, 2, and 3 as improvement in shade and counted them as events.
Secondary outcomes
Patient satisfaction or acceptability of the tooth whitening procedure (patient comfort).
Adverse effects: any side effects reported due to the bleaching procedure were considered in this review and described qualitatively.
Oral health‐related quality of life.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 12 June 2018) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library (searched 12 June 2018) (Appendix 2);
MEDLINE Ovid (1946 to 12 June 2018) (Appendix 3);
Embase Ovid (1980 to 12 June 2018) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 12 June 2018) (Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 12 June 2018) (Appendix 6).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (Eachempati Prashanti (EP), Salian Kiran (SK)) independently screened the titles and abstracts from the electronic searches to identify potentially eligible studies. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. We obtained full‐text copies of all potentially eligible studies and two pairs of review authors (EP and Ibrahim Ethem (IE), SK and Puneet Gupta (PG)) further evaluated in detail the studies for inclusion. We recorded the reasons why studies did not meet the inclusion criteria in the 'Characteristics of excluded studies' table. We resolved any disagreements by discussion. When resolution was not possible, we consulted the arbiter (Sumanth Kumbargere Nagraj (SKN)). Articles in languages other than English were assessed by their abstracts where possible and if they appeared to be potentially eligible, we obtained and translated the full‐text article.
Data extraction and management
Two pairs of review authors (EP and IE, SK and PG) extracted the data independently, using a data extraction form specifically designed for this Cochrane Review. We resolved any disagreements by discussion. The two review authors independently checked data extraction forms obtained from translators and cross checked any doubtful aspects using Google translator. We entered all study details in the 'Characteristics of included studies' table in Review Manager 5 software (Review Manager 2014). We recorded the following details for each included trial.
Publication details like year of publication and language.
Demographic details of the report.
Inclusion and exclusion criteria.
Sample size.
Method of randomisation.
Allocation concealment.
Blinding.
Type of trial.
Method of assessing the outcome, and dropouts if any.
Type of intervention.
Details of the outcome reported.
Duration of follow‐up.
Results of the intervention.
Funding details.
Details about trials registration.
For obtaining additional data and clarifications, we contacted the authors of the included and excluded trials via email.
Assessment of risk of bias in included studies
We independently assessed the risk of bias in the included trials for seven domains: sequence generation; allocation concealment; performance bias and detection bias; incomplete outcome data; selective outcome reporting; and other biases. For each of these components, we assigned a judgement regarding the risk of bias as either 'high', 'low' or 'unclear', based on guidance in section 8.5.d of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We contacted the trial authors if details were missing in the publications or were unclear. We resolved disagreements through consensus. We recorded our judgements and justifications in 'Risk of bias' tables for each included study and generated a 'Risk of bias' summary figure. We used these judgements while grading of the overall quality of evidence for outcomes in the 'Summary of findings' tables for each comparison.
We summarised the risk of bias according to Higgins 2011 as follows:
| Risk of bias | Interpretation | In outcome | In included studies |
| Low | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
For dichotomous data, we used risk ratios (RRs), and for continuous data, we assessed the mean difference (MD) and presented results with 95% confidence intervals (CIs). For continuous data using different scales to measure the same primary outcome (improvement in tooth shade), we used the standardised mean difference (SMD). Change score and final score were combined in meta‐analysis according to Higgins 2011 section 7.7.3.1. When multiple time points were given, longest follow‐up time was considered (Higgins 2011, section 9.3.4).
Unit of analysis issues
For parallel‐group and cluster‐randomised studies, we used the individual as the unit of analysis. If clustered data were provided, we planned to adjust the standard errors of the estimates to take clustering into account (as outlined in section 16.3.4 of the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011)). For split‐mouth studies, we used the quadrant of the mouth within an individual as a unit of analysis. For studies that have used a split‐mouth design but reported data as a parallel‐group study, we calculated the odds ratios using the Becker‐Balagtas method, as outlined in Curtin 2002, using Stata software.
Dealing with missing data
We attempted to obtain missing data by contacting trial authors. If both mean and standard deviation were reported as graphs, we derived the data from the graphs by magnifying them and approximating the measures of mean and standard deviation. When mean and standard error (SE) were given, we calculated the standard deviation (SD) as given in Higgins 2011, section 7.7.3.3. When adjusted mean was given, we considered it in the analysis (Higgins 2011, section 9.2.3.2). When median and inter quartile range were given we used the data to calculate mean and SD. When mean and P value were given, SD was calculated.
When data were presented as median (skewed data), we qualitatively described the results in the review.
Assessment of heterogeneity
We assessed heterogeneity by examining the forest plot to check for overlapping CIs, using the Chi2 test for heterogeneity with a 10% level of significance to detect inconsistency in study results that were not due to random error (chance), and the I2 statistic to denote the percentage of inconsistency in results due to inter‐trial variability that exceeded chance. We used the guidance given by the Cochrane Handbook for Systematic Reviews of Interventions to interpret the I2 statistic: 0% to 40% as possibly insignificant, 30% to 60% as moderate heterogeneity, 50% to 90% as possibly substantial, and 75% to 100% as considerable heterogeneity depending on two factors: 1. inconsistency in results was due to differences in the direction of effect estimates between trials rather than due to differences in the magnitude of effect estimates favouring an intervention; 2. based on the strength of the evidence for heterogeneity from the P value for the Chi2 test for heterogeneity (Deeks 2011).
Assessment of reporting biases
We tested for publication bias using funnel plots and a formal test investigation of the degree of asymmetry using the method proposed by Egger 1997 wherever possible.
Data synthesis
We analysed the data using Review Manager 5 software (Review Manager 2014). We combined data available from trials with similar comparisons (same concentration and duration of application) and outcomes in the meta‐analysis. We used standardised mean differences to combine continuous data as some trials used different scales. We used the random‐effects model in the meta‐analysis. If data were presented as adjusted mean and SE, we calculated SD from SE and considered the adjusted mean for analysis. If data were described in the form of ordinal outcomes, we converted the scale into dichotomous data by combining relevant adjacent categories and analysed using risk ratios and 95% CI. When data were presented as odds ratios, log (odds ratio) was calculated based on the odds ratios and 95% CI given in the trial and the generic inverse variance method was applied. We calculated mean difference and standard error in split‐mouth and cross‐over trials and analysed the data using the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses where there was heterogeneity.
To identify the reasons for clinical or methodological heterogeneity in meta‐analyses, we carried out subgroup analyses, based on.
Population
Age group of the patient.
Baseline tooth shade.
Method
Different concentrations.
Varying application times and duration of treatment.
Outcome measures
Subjective measurement involving shade guide.
Objective measurements using electronic devices/instrument.
Sensitivity analysis
A sensitivity analysis is a repeat of the primary analysis or meta‐analysis, substituting alternative decisions or ranges of values for decisions that were arbitrary or unclear. It involves undertaking the meta‐analysis twice: first, including all studies and second, only including those that are definitely known to be eligible. Wherever feasible we did sensitivity analyses to assess the robustness of our findings by excluding data from trials at high risk and at unclear risk of bias.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2011). We used GRADEpro GDT 2015 (GRADEpro GDT 2015) and imported data from Review Manager 5 (Review Manager 2014) to create 'Summary of findings' tables for the comparisons included in this review. The tables provide information concerning the overall certainty of the evidence at the outcome level, the magnitude of effect of the intervention examined and the sum of available data on the primary and secondary outcomes. The GRADE approach (Schünemann 2011) considers 'certainty' to be a judgement of the extent to which we can be confident that the estimates of effect are correct. Evidence from randomised controlled studies is initially graded as high and downgraded on each of five domains after full consideration of risk of bias, indirectness, imprecision, inconsistency and publication bias. A GRADE certainty level of 'high' reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of 'moderate' certainty indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different. 'Low' and 'very low' certainty evidence limit our confidence in the effect estimate (Balshem 2011).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification.
Results of the search
We included 71 trials (78 reports) in the review. (If the same study (one population) was separated into multiple reports we included the primary study and considered the rest as reports as per Higgins 2011.)
See Figure 1 for the selection process of search results.
1.
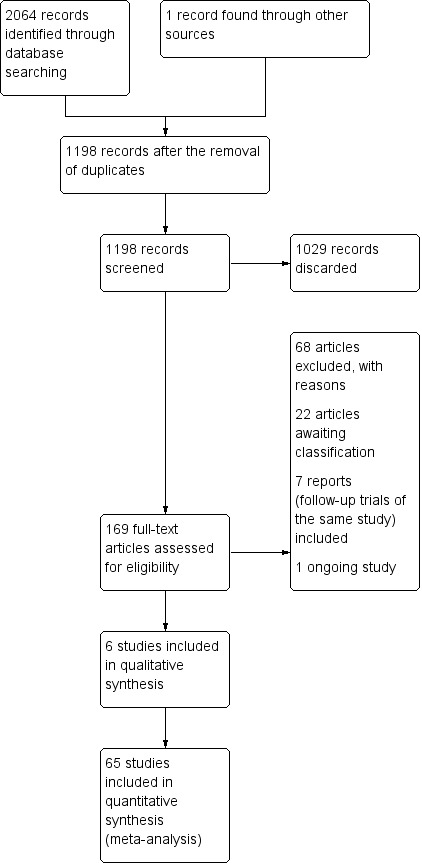
Study flow diagram.
Included studies
See Characteristics of included studies for further details.
Characteristics of trial settings and investigators
Seventy studies were in the English language, one was in German (Auschill 2007).
The countries of origin for the included studies were.
Forty‐two from the USA (Kowitz 1994; Nathoo 1994; Russell 1996; Matis 1998; Cibirka 1999; Gerlach 2000; Kihn 2000; Kugel 2000; Matis 2000; Mokhlis 2000; Nathoo 2001; Gerlach 2002; Gerlach 2002a; Gerlach 2002b; Karpinia 2002; Kugel 2002; Nathoo 2002; Barlow 2003; Gerlach 2003; Li 2003; Myers 2003; Nathoo 2003; Biesbrock 2004; Garcia‐Godoy 2004; Gerlach 2004; Gerlach 2004e; Hasturk 2004; Li 2004; Swift 2004; Cronin 2005; Gerlach 2005; Giniger 2005; Shahidi 2005; Matis 2006; Delgado 2007; Browning 2008; Gallo 2009; Papas 2009; Swift 2009; Bruhn 2012; Costa 2012; Oliveira 2013).
Three trials were from Brazil (Mederios 2008; Meireles 2010; Kose 2011); five from Germany (Auschill 2007; Bizhang 2007; Krause 2008; Ziebolz 2008; Auschill 2012); four from Italy (Porciani 2006; Ferrari 2007; Porciani 2010; Navarra 2014); four from the UK (Brunton 2004; Collins 2004a; Walters 2004; Mohan 2008); three from Spain (Alonso 2006; Berga‐Caballero 2006; Alonso 2014); two from Switzerland (Hannig 2007; Ziebolz 2007); two from Hong Kong (Wong 2004; Botelho 2017); one from China (Xu 2007); two from Turkey (Turkun 2010; Aka 2017); one from Japan (Tsubura 2005); one from Canada (Tam 2001); and one from Ireland (Hyland 2015).
Sixty‐one trials were parallel‐design trials and seven were split‐mouth (Matis 2000; Mokhlis 2000; Tsubura 2005; Alonso 2006; Matis 2006; Auschill 2007; Costa 2012); and three had a cross‐over design (Biesbrock 2004; Walters 2004; Porciani 2006).
Twelve of the included trials had more than two groups for comparison. One trial had five groups (Browning 2008). Two trials, Alonso 2014 and Gerlach 2000, had four groups. The remaining nine trials had three groups for comparison (Li 2003; Li 2004; Wong 2004; Matis 2006; Bizhang 2007; Xu 2007; Krause 2008; Hyland 2015; Aka 2017). Seven trials included placebo in their comparisons (Wong 2004; Bizhang 2007; Xu 2007; Browning 2008; Krause 2008; Hyland 2015; Aka 2017). We included these seven trials separately in both the analysis testing bleaching agent versus placebo and bleaching agent versus bleaching agent. See Additional Table 20 for details.
1. Details of analyses performed in multiarm trials.
| Trial | Interventions reported in the trials | Interventions considered in analyses | Reason |
| Aka 2017 |
|
Bleaching agent vs placebo
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Alonso 2014 |
|
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Bizhang 2007 |
|
Bleaching agent vs placebo
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Browning 2008 |
|
Bleaching agent vs placebo
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Gerlach 2000 |
|
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Hyland 2015 |
|
Bleaching agent vs placebo
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Krause 2008 |
|
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Li 2003 |
|
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Li 2004 |
|
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Matis 2006 |
|
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Wong 2004 |
|
Bleaching vs placebo
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
| Xu 2007 |
|
Bleaching vs placebo
Bleaching agent vs bleaching agent
|
Most commonly used concentrations |
vs = versus; 2x = twice; 3x = 3 times; 4x = 4 times.
Out of the 71 trials, 54 provided grant information and out of these one was government funded (Navarra 2014). 53 were funded by a pharmaceutical company (Nathoo 1994; Cibirka 1999; Gerlach 2000; Kihn 2000; Kugel 2000; Matis 2000; Mokhlis 2000; Nathoo 2001; Gerlach 2002; Gerlach 2002a; Gerlach 2002b; Karpinia 2002; Kugel 2002; Nathoo 2002; Barlow 2003; Gerlach 2003; Li 2003; Myers 2003; Nathoo 2003; Biesbrock 2004; Brunton 2004; Collins 2004a; Garcia‐Godoy 2004; Gerlach 2004; Gerlach 2004e; Hasturk 2004; Li 2004; Swift 2004; Walters 2004; Wong 2004; Cronin 2005; Gerlach 2005; Giniger 2005; Shahidi 2005; Tsubura 2005; Matis 2006; Porciani 2006; Bizhang 2007; Delgado 2007; Ferrari 2007; Hannig 2007; Xu 2007; Ziebolz 2007; Ziebolz 2008; Gallo 2009; Papas 2009; Swift 2009; Porciani 2010; Kose 2011; Auschill 2012; Costa 2012; Oliveira 2013; Hyland 2015).
All the trials were carried out in a single centre.
Characteristics of participants
Forty‐seven trials reported on both genders. The remaining 24 trials did not report on distribution of gender (Kowitz 1994; Nathoo 1994; Matis 1998; Cibirka 1999; Kihn 2000; Mokhlis 2000; Nathoo 2001; Nathoo 2002; Gerlach 2003; Myers 2003; Brunton 2004; Garcia‐Godoy 2004; Walters 2004; Berga‐Caballero 2006; Auschill 2007; Browning 2008; Krause 2008; Gallo 2009; Turkun 2010; Kose 2011; Bruhn 2012; Alonso 2014; Navarra 2014; Hyland 2015). The minimum age included in a study was 18 years and the maximum age included in a study was 79 years (Russell 1996). The minimum sample size was six (Berga‐Caballero 2006) and the maximum sample size was 117 (Collins 2004a) with a median value of 58.
Thirty‐one trials reported on the minimum baseline shade for inclusion in the trial. Twelve reported A2 as the baseline shade (Kugel 2000; Karpinia 2002; Gerlach 2003; Garcia‐Godoy 2004; Wong 2004; Cronin 2005; Hannig 2007; Ziebolz 2007; Ziebolz 2008; Papas 2009; Kose 2011; Oliveira 2013), 14 reported A3 as the baseline shade (Nathoo 1994; Kihn 2000; Nathoo 2001; Nathoo 2002; Nathoo 2003; Li 2003; Brunton 2004; Li 2004; Giniger 2005; Auschill 2007; Delgado 2007; Browning 2008; Mohan 2008; Auschill 2012), and three reported C1 (Matis 2006; Meireles 2010; Turkun 2010) based on Vita shade guide. Two studies used Trubyte shade guide; one used B85 as the baseline shade (Mokhlis 2000) while B65 was used in the other (Gallo 2009).
Two trials compared the effects of bleaching agent on participants with tetracycline stains (Kugel 2002; Matis 2006). One trial discussed the effect of dietary habits of participants on tooth whitening (Meireles 2010). One trial compared the effect of tooth whitening between smokers and non‐smokers (Porciani 2006).
Characteristics of interventions
We divided the 71 trials included in our review into two categories.
1. Bleaching agent versus placebo
Twenty‐six trials were included in this group. Among the 26 studies, the following comparisons were identified.
Six trials comparing carbamide peroxide (CP) gel in tray versus placebo.
Two trials comparing hydrogen peroxide (HP) gel in tray versus placebo.
Ten trials comparing HP strips versus placebo.
One trial comparing CP paint‐on gel versus placebo.
Two trials comparing HP paint‐on gel versus placebo.
Three trials comparing sodium hexametaphosphate (SHMP) chewing gum versus placebo.
One trial comparing sodium tripolyphosphate (STPP) chewing gum versus placebo.
One trial comparing HP mouthwash versus placebo.
1a. CP gel in tray versus placebo
Six trials were included in this comparison.
Hyland 2015, a three‐arm study compared 10% CP and 5% CP applied 2 hours a day for 2 weeks to the placebo gel. The CP formulation in this trial contained sodium tripolyphosphate. We compared 5% CP to placebo gel.
Browning 2008 used five interventions in his multiarm study. We compared 10% CP + 0.5% potassium nitrate (KNO3) + 0.25% sodium fluoride (NaF) to a placebo gel, applied for 11 weeks.
A three‐arm trial conducted by Aka 2017 used 6% HP, 10% CP and placebo. Analysis in this trial was done at tooth level. The study authors divided the groups based on the tooth shade as light, medium dark and dark. 10% CP gel was compared to placebo in our meta‐analysis, which was applied for 8 hours to 10 hours daily for 14 days. For the control group, no bleaching agent was applied.
Russell 1996 and Matis 1998 used 10% CP and used it overnight for 2 weeks with a follow‐up of 6 months.
Mederios 2008 used 10% CP overnight for 3 weeks.
1b. HP gel in tray versus placebo
Mohan 2008 and Myers 2003 studied the effect of 6% and 3% HP gel respectively which was applied twice daily for 14 days.
1c. HP strips versus placebo
Ten trials, studied the effect of HP strips versus placebo. All trials included HP strips with varying concentrations.
Gerlach 2004e used 10% HP, twice a day for 30 minutes for 1 week and 2 weeks respectively.
6% HP was used twice a day for 30 minutes in one multiarm trial Bizhang 2007.
Kugel 2000 used 5.3% HP, twice daily for 30 minutes each time for 2 weeks.
6% HP was used twice a day for 30 minutes in Swift 2009 for 2 weeks and 6 weeks.
Wong 2004 a multiarm trial, compared 6% HP strip, 18% CP paint‐on gel and placebo. Data for 6% HP strip versus placebo were used in this meta‐analysis. Strips were used twice a day for 30 minutes, for 14 days.
Papas 2009 used 10% HP, twice a day for 30 minutes for 1 week and 2 weeks.
Swift 2004 and Garcia‐Godoy 2004 compared 14% HP strips applied twice daily for 30 minutes for 3 weeks and 6 weeks respectively.
Gerlach 2002 used 5.3% HP, twice daily for 30 minutes each time for 2 weeks with a follow‐up of 6 months.
Bruhn 2012 used a HP gel twice a day for 3 weeks and reported on patient‐reported satisfaction and oral health‐related quality of life.
1d. CP paint‐on gel versus placebo
One trial Nathoo 2002 compared the effect of CP paint‐on gel with placebo.
Nathoo 2002 studied 18% CP versus placebo gel, which was applied immediately after brushing and subjects were instructed to keep their mouth open for 30 seconds after application. They refrained from eating and drinking for 30 minutes after application.
1e. HP paint‐on gel versus placebo
Two trials compared the effect of HP paint‐on gel with control gel.
Xu 2007, a multiarm trial, and Collins 2004a compared 6% and 5.9% HP gel applied twice daily for 2 weeks respectively. Collins also evaluated colour change at 1 week.
1f. SHMP chewing gum versus placebo
Three trials compared SHMP chewing gum to placebo.
Biesbrock 2004, a cross‐over study used 7.5% SHMP on 19 subjects each (period A) and 18 subjects each (period B) for 5 minutes daily for 2 days followed by 60 seconds rinse with tea. This was repeated approximately 8 times a day. Outcomes looked into were stain prevention and removal. Placebo group were given a negative control chewing gum.
Porciani 2006 was another cross‐over study among smokers and non‐smokers, using 4% SHMP on 54 subjects each in placebo and experimental groups. The subjects chewed chewing gum 4 times a day for 12 weeks. Placebo group received no gum.
Walters 2004 compared 5.6% SHMP to a negative control chewing gum in a 3‐day cross‐over trial separated by a 10‐day wash out period including 10 subjects in each group.
1g. STPP chewing gum versus placebo
Sodium tripolyphosphate was used in a study by Porciani 2010, including smokers and habitual tea users in each group. They were asked to chew the gum 3 times a day for 10 minutes for 6 weeks and compared to the placebo group who received a control chewing gum.
1h. HP mouthwash versus placebo
One trial (Hasturk 2004) compared 1.5% fluoridated HP‐based mouthrinse to placebo. Mouthrinse was used twice daily for 30 seconds for 6 months.
2. Bleaching agent versus bleaching agent
Fifty‐one trials were included in this group among which six are multiarm trials which were also included in the comparison of bleaching agent versus placebo.
Among these, the following comparisons were made.
Eighteen trials compared CP tray versus CP tray.
Seven trials compared CP tray versus HP tray.
Ten trials compared HP strips versus CP tray.
Two trials compared HP strips versus HP tray.
Two trials compared HP strips versus HP strips.
One trial compared HP strip versus HP mouthwash.
Two trials compared CP paint‐on versus HP strip.
Two trials compared HP paint‐on versus HP strip.
One trial compared SPC paint‐on versus HP strip.
Two trials compared CP paint‐on versus CP paint‐on.
One trial compared CP paint‐on versus HP paint‐on.
One trial compared HP paint‐on versus HP paint‐on.
One trial compared sodium percarbonate (SPC) paint‐on versus CP paint‐on.
One trials compared SPC paint‐on versus HP paint‐on.
2a. CP tray versus CP tray
A total of 18 trials are included comparing CP in a tray versus CP in a tray.
Nathoo 2001 used an overnight application of 5% and 10% CP (6 to 8 hours per day) for 1 week. Hyland 2015 used the same concentrations of bleaching agent for 2 hours daily for 2 weeks.
Four trials (Kowitz 1994; Nathoo 1994; Cibirka 1999; Tsubura 2005) reported on the effect of 10% CP of different brands. Tsubura 2005 and Cibirka 1999 used the gel in tray overnight for 2 weeks while Kowitz 1994 used it for 3 hours or more for 2 weeks. Nathoo 1994 applied the gel twice daily for 30 minutes, for a duration of 2 weeks.
Turkun 2010 reported on effects of 10% CP and 28% CP with an application time of 8 hours overnight and 20 minutes per day over a 2‐week period.
Meireles 2010 had 46 and 45 subjects in experimental and control groups respectively and applied the whitening agents 2 hours a day for 2 weeks. He followed the same regimen for 3 weeks with 45 and 44 participants in 10% and 16% CP groups respectively. He did a follow‐up study including 45 and 44 participants for 1 year and 42 and 39 participants for 2 years respectively, following the same application protocol.
Gallo 2009 reported a trial of 10 days duration with 30% CP with and without potassium nitrate. The application time was 1 hour per day.
Kose 2011 reported the effect of bleaching with 16% CP with and without potassium nitrate and sodium fluoride. The bleaching was done for 2 weeks with 6 hours application time.
Giniger 2005 is a 5‐year follow‐up trial comparing the effect of 16% CP (n = 13) versus 16% CP with amorphous calcium phosphate (n = 14). The application time was 3 hours per day over 2‐week period.
Matis 2006 is a follow‐up trial for 180 days continued from a split‐mouth trial with 40 and 39 participants in experimental and control groups on tetracycline stained teeth with overnight application using 10% CP and 15% CP over 6 months.
Krause 2008 conducted a trial on 10% CP and 17% CP with 2 hours a day application over a period of 2 weeks.
Matis 2000 and Kihn 2000 reported the effect of overnight application of 10% CP and 16% CP for 2 weeks.
Two trials, Navarra 2014 (n = 10 per group) and Browning 2008 (n = 19 per group), reported on the effect of 10% CP with and without potassium nitrate and sodium fluoride applied overnight for 2 weeks.
Tam 2001 reported a trial with 10% CP and 10% CP without potassium nitrate with overnight application time for 2 weeks.
2b. CP tray versus HP tray
Ziebolz 2007 studied the effect of 20% CP versus 7.5% HP. He used the bleaching gel for 4 hours a day for 20% CP group and 30 minutes a day for 7.5% HP group for 12 days. Mokhlis 2000 used the same concentration but applied the gel twice daily for an hour over a period of 12 weeks.
Alonso 2014 reported a 2‐week trial with an application time of 1 hour per day using 10% CP and 7.5% HP.
Delgado 2007 reported on application time of 30 minutes per day over 2 weeks for 20% CP and 9% HP.
Aka 2017 reported a multiarm trial at tooth level, comparing 10% CP and 6% HP. The application time was 8 hours to 10 hours per day over 2 weeks for CP group.
Berga‐Caballero 2006 reported a trial with 3.5% HP and 10% CP. The application time was 3 hours daily for 24 days for 3.5% HP group and 2 hours daily for 28 days in 10% CP group.
Alonso 2006 conducted a split‐mouth study on the effect of 3.5% HP and 5% potassium nitrate over 10% CP for 4 weeks. The application time was 3 hours per day.
2c. HP strips versus CP tray
Ten trials compared HP strips to CP gel in tray in varying concentrations and application times.
Gerlach 2002b used 5% CP gel with 5% potassium nitrate in tray and compared it to 6% HP strips used for 30 minutes, twice daily for 7 days.
Gerlach 2000 a multiarm trial; Gerlach 2002a; Hannig 2007; and Karpinia 2002 compared 10% CP gel in tray versus 5.3% or 6% or 6.5% HP strips for 2 weeks. Gerlach 2002b applied the strips twice daily for 30 minutes and gel in tray for 2 hours a day, both for a duration of 2 weeks. Gerlach 2002a applied strips twice a day for 1 hour and the tray for 2 hours once daily for 2 weeks. Hannig 2007 followed the same application protocol for strips as mentioned above, however, the tray was used once daily for 1 hour over 2 weeks. Karpinia 2002 applied whitening strips for 30 minutes, twice daily and whitening gel in tray for 2 hours daily.
Costa 2012 compared 35% CP gel in tray to 14% HP strips in a split‐mouth trial. Both interventions were applied simultaneously for 30 minutes, twice a day for 2 weeks and the application times were separated by 3 hours.
Ferrari 2007 used 6% HP versus 10% CP for 6 weeks. He applied the strips for 30 minutes, twice daily and gel in tray for 2 hours daily.
Kugel 2002 also followed the same regimen as Ferrari 2007 for a duration of 2 months.
Botelho 2017 used 6.5% HP strips versus 16% CP in tray with 13 and 11 participants allocated to each group respectively. The subjects wore tray with the gel for up to 2 hours or overnight during the 3‐month trial. Strip group applied the strips onto the labial surfaces of the teeth twice daily for 30 minutes for 3 months.
Li 2003 a multiarm trial compared 6.5% HP strips to 7.5% HP in tray and 16% CP in tray. Strips were applied twice a day for 30 minutes and tray was used overnight, both for 21 days.
2d. HP strips versus HP tray
Two trials compared HP strips to HP gel in tray.
Gerlach 2004 compared 14% HP strips used for 21 days and 9.5% HP in custom tray used for 9 days. Both the groups applied the bleaching agent twice a day for 30 minutes.
Auschill 2012 studied 5% HP in tray and 5.3% HP strips for 30 minutes, twice daily for 14 consecutive days.
2e. HP strip versus HP strip
Oliveira 2013 compared 9.5% high adhesion HP strips to marketed 10% control strip for 8 days. 9.5% HP strips were applied for 2 hours once daily and control strips were applied for 30 minutes once a day.
Shahidi 2005 studied 10% HP strip with very thin 6% HP gel (0.12 mm strip) versus 6% HP gel (0.2 mm) applied for 30 minutes, twice daily for 14 days.
2f. HP strip versus HP mouthwash
One trial (Gerlach 2005) compared two HP tooth whitening systems including 2% HP pre‐rinse and 10% HP strips. Pre‐rinse group was instructed to rinse twice daily with 15 ml solution for 60 seconds before brushing. The strip group were specified twice daily application for 30 minutes.
2g. CP paint‐on gel versus HP strip
Two trials compared HP strips to CP paint‐on gel (Wong 2004; Cronin 2005).
Both trials compared 6% HP strips to 18% CP paint‐on gel. In both trials strips were used twice daily for 2 weeks. Paint‐on was used twice daily for 30 minutes for 2 weeks in Cronin 2005 and 15 minutes in Wong 2004.
2h. HP paint‐on gel versus HP strip
Two trials compared 5.9% or 6% HP strips to 5.9% HP paint‐on gel (Auschill 2007; Xu 2007). In Xu 2007 a multiarm trial, both the groups applied the bleaching agent twice daily for 1 week. Auschill 2007 used strips twice daily for 30 minutes and paint‐on gel twice daily for 15 minutes.
2i. SPC paint‐on versus HP strip
One trial compared HP strips to sodium percarbonate (Bizhang 2007).
Bizhang 2007 compared 6% HP strips to 19% SPC. Strips were instructed to be applied twice daily for 30 minutes over a 14‐day period. Paint‐on gel was applied to the facial surfaces of the teeth for 14 days.
2j. CP paint‐on versus CP paint‐on
Two trials were included in this comparison.
Li 2004 a multiarm trial with 120 participants balanced equally into three groups, used 18% CP with different application times (twice 2x, thrice 3x, 4 times 4x per day). In the 2x group, no air drying was used and participants were asked not to eat and drink for 15 minutes after the gel was applied. In the 4x group, 30 seconds air drying and 30 minutes refraining from eating and drinking was advocated.
Brunton 2004 compared 18% CP gel and 16.4% CP gel, applied twice a day for 30 seconds each for 2 weeks. Subjects were asked to refrain from eating and drinking for 30 minutes.
2k. CP paint‐on versus HP paint‐on
One trial (Nathoo 2003) compared 8.7% HP versus 25% CP, where a thin layer of gel was applied one tooth at a time and subjects were instructed not to rinse, eat or drink for 15 minutes. This was repeated 3 times a day for 2 weeks.
2l. HP paint‐on versus HP paint‐on
One trial (Ziebolz 2008) studied 6% HP after potassium fluoride application versus 6% HP without desensitiser application. Application was done twice a day for 10 minutes for 7 days.
2m. SPC paint‐on versus CP paint‐on
Barlow 2003 compared 19% SPC and 18% CP gel twice a day for 2 weeks.
2n. SPC paint‐on versus HP paint‐on
Gerlach 2003 compared 19% SPC and 8.7% HP. Both groups advocated application of a thin layer of gel after drying the tooth at night. They were instructed not to eat or drink after application and to brush normally the next morning.
Outcomes reported in the trials
Primary outcomes
Sixty‐nine trials studied improvement in tooth whitening as assessed by the dentist using any relevant scale. 11 trials reported improvement in tooth whitening based on patient's satisfaction levels (Kowitz 1994; Tam 2001; Wong 2004; Matis 2006; Hannig 2007; Krause 2008; Mederios 2008; Meireles 2010; Bruhn 2012; Costa 2012; Aka 2017).
Secondary outcomes
Eight trials gave patient‐reported level of comfort with the treatment (Kugel 2002; Nathoo 2002; Wong 2004; Ziebolz 2007; Ziebolz 2008; Meireles 2010; Auschill 2012; Costa 2012).
Fourteen trials did not report on any adverse reaction after tooth whitening (Nathoo 1994; Cibirka 1999; Russell 1996; Nathoo 2002; Gerlach 2003; Nathoo 2003; Biesbrock 2004; Collins 2004a; Hasturk 2004; Walters 2004; Porciani 2006; Mohan 2008; Meireles 2010; Porciani 2010).
Meireles 2010 in one of his 2‐year follow‐up reports studied only oral health‐related quality of life as an outcome. Two trials have reported oral health‐related quality of life along with other outcomes (Wong 2004; Bruhn 2012).
Excluded studies
See Characteristics of excluded studies tables for further details. 22 articles were excluded as they were abstracts of conference presentations (Andreana 2000; Dickinson 2000; Browning 2001; Donly 2001; Godson 2001; Sagel 2001; Smith 2001; Swift 2001; Gerlach 2002d; Lee 2003; Amini 2009; Auschill 2009; Lisante 2009; Anastasia 2010; Archila 2010; Amini 2011; Majeed 2011; Simon 2011; Walter 2011; Garcia‐Godoy 2012; Mazur 2013; Perdigao 2013).
We procured 46 full‐text articles and excluded them for the following reasons.
Six trials included studies with in‐office bleaching (Burgio 2001; Matis 2005; Zantner 2006; Martin 2015; NCT02603354; NCT02682329).
Nine trials included children or adolescents in their study (Tam 1999; Donly 2002; Donly 2002a; Loyola‐Rodriguez 2003; Gerlach 2004d; Cardoso 2010; Corby 2014; Pinto 2014; Pinto 2017).
Five trials included studies, which used mechanical method of stain removal like toothbrushing, and whitening dentifrices (Simon 2001; Gerlach 2002c; Gerlach 2003a; Karpinia 2003; Gerlach 2004a).
One trial reported on home bleaching in which the agent was applied by a professional (Farrell 2006).
One trial reported the use of a non‐whitening chewing gum without an active ingredient (Yankell 1997).
Of the remaining 24 trials.
Nine trials reported only on effects on oral tissues or associated tooth sensitivity or both or were controlled clinical trials (Schulte 1993; Curtis 1996; Jorgensen 2002; Leonard 2002; Collins 2004; Leonard 2007; Farrell 2008; de Geus 2015a; de Geus 2015b).
Three trials reported the effects of whitening agents on pulp (Schulte 1994; Fugaro 2004; Fugaro 2005).
Four trials reported on the dilution kinetics of whitening agents (Matis 1999; Matis 2002; Gerlach 2004c; Marques 2012).
Two studies reported on plaque retention post‐bleaching (Schiff 1994; Gursoy 2008).
One study reported on oral microflora (Alkmin 2005).
One study reported on the effect of a desensitising agent (Leonard 2004).
One study reported on the effect of coffee exposure on bleaching (Rezende 2013).
One study reported the effect of bleaching on orthodontic brackets (Jadad 2011).
One study reported the effect of two different tray designs used during bleaching (Matis 2002a).
One study reported the efficacy of using a chromameter to assess bleaching (Gerlach 2002e).
Studies awaiting classification
See Characteristics of Characteristics of studies awaiting classification tables for further details.
Twelve studies had incomplete or missing data; hence, we could not use them for analysis (Gegauff 1993; Reinhardt 1993; Rosenstiel 1996; Barnes 1998; Pohjola 2002; Ozcan 2003; Browning 2004; Ferrari 2004; Gambarini 2004; Braun 2007; Shin 2010; Simon 2014).
We are awaiting full texts for eight published trials (Heymann 1998; Sielski 2003; Gerlach 2004b; Guerrero 2007; Bizhang 2017; Kim 2018; Maran 2018; Rossi 2018).
Two studies were protocol registration of completed studies, but we could not access the full text (NCT02151058; NCT03217994).
Ongoing studies
One clinical trial has not yet published the results and is ongoing (NCT03026725).
Risk of bias in included studies
See Figure 2 for details.
2.
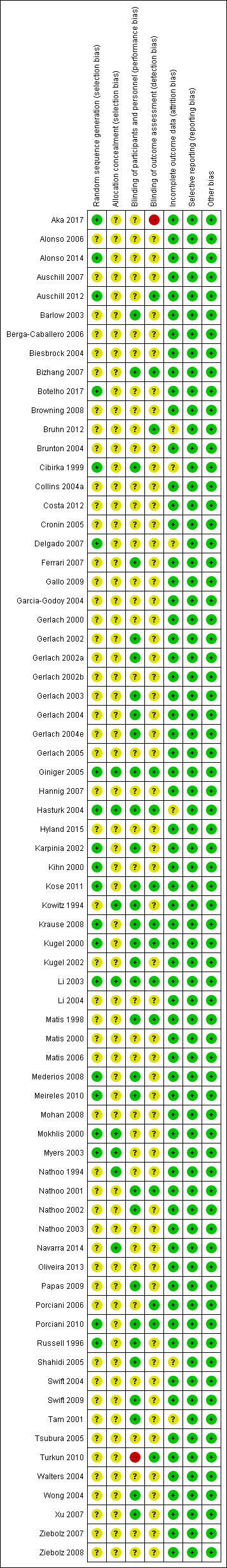
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twenty of the included trials reported the method of sequence generation and were at a low risk of bias (Russell 1996; Cibirka 1999; Kihn 2000; Mokhlis 2000; Karpinia 2002; Kugel 2002; Li 2003; Myers 2003; Hasturk 2004; Giniger 2005; Delgado 2007; Krause 2008; Mederios 2008; Meireles 2010; Porciani 2010; Kose 2011; Auschill 2012; Alonso 2014; Aka 2017; Botelho 2017); the remaining trials were at an unclear risk of bias. Eight of the included studies reported concealment of allocation (Kowitz 1994; Nathoo 1994; Mokhlis 2000; Li 2003; Myers 2003; Hasturk 2004; Giniger 2005; Navarra 2014). The rest of the trials were marked unclear.
Blinding
Out of 71 included trials, blinding of participants and personnel was unclear in 39 trials (Nathoo 1994; Gerlach 2000; Kihn 2000; Matis 2000; Mokhlis 2000; Gerlach 2002b; Myers 2003; Nathoo 2003; Biesbrock 2004; Brunton 2004; Collins 2004a; Garcia‐Godoy 2004; Li 2004; Swift 2004; Walters 2004; Cronin 2005; Gerlach 2005; Tsubura 2005; Alonso 2006; Berga‐Caballero 2006; Matis 2006; Porciani 2006; Auschill 2007; Delgado 2007; Hannig 2007; Ziebolz 2007; Browning 2008; Mohan 2008; Ziebolz 2008; Gallo 2009; Auschill 2012; Bruhn 2012; Costa 2012; Oliveira 2013; Alonso 2014; Navarra 2014; Hyland 2015; Aka 2017; Botelho 2017) and high in one (Turkun 2010); 31 trials had reported satisfactory blinding of participants and personnel.
Blinding of assessors was reported in 14 trials and were at a low risk of detection bias (Matis 1998; Kugel 2000; Nathoo 2001; Kugel 2002; Li 2003; Hasturk 2004; Giniger 2005; Porciani 2006; Bizhang 2007; Krause 2008; Porciani 2010; Turkun 2010; Auschill 2012; Bruhn 2012).
Aka 2017 was at a high risk of detection bias due to lack of clarity of how teeth were selected for outcome assessment.
The other 56 were marked unclear as the trials described their studies as 'double‐blinded', but no details of assessor blinding were given, or were single‐blinded studies (Kowitz 1994; Nathoo 1994; Russell 1996; Cibirka 1999; Gerlach 2000; Kihn 2000; Matis 2000; Mokhlis 2000; Tam 2001; Gerlach 2002; Gerlach 2002a; Gerlach 2002b; Karpinia 2002; Nathoo 2002; Barlow 2003; Gerlach 2003; Myers 2003; Nathoo 2003; Biesbrock 2004; Brunton 2004; Collins 2004a; Garcia‐Godoy 2004; Gerlach 2004; Gerlach 2004e; Li 2004; Swift 2004; Walters 2004; Wong 2004; Cronin 2005; Gerlach 2005; Shahidi 2005; Tsubura 2005; Alonso 2006; Berga‐Caballero 2006; Matis 2006; Auschill 2007; Delgado 2007; Hannig 2007; Ferrari 2007; Xu 2007; Ziebolz 2007; Browning 2008; Mederios 2008; Mohan 2008; Ziebolz 2008; Gallo 2009; Papas 2009; Swift 2009; Meireles 2010; Kose 2011; Costa 2012; Oliveira 2013; Alonso 2014; Navarra 2014; Hyland 2015; Botelho 2017).
Incomplete outcome data
Six trials (Cibirka 1999; Tam 2001; Hasturk 2004; Shahidi 2005; Delgado 2007; Bruhn 2012) were marked unclear for risk of attrition bias as the trials did not mention any details about dropouts and there was a mismatch between the number randomised and number analysed.
Selective reporting
All 71 trials were at a low risk of bias for selective reporting.
Other potential sources of bias
All 71 trials were at a low risk for other bias.
Overall risk of bias
Two studies were at low overall risk of bias (Li 2003; Giniger 2005); two at high overall risk of bias (Turkun 2010; Aka 2017); and the remaining 67 at unclear overall risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19
Summary of findings for the main comparison. CP gel in tray versus placebo for whitening teeth.
| Carbamide peroxide (CP) gel in tray compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP gel in tray Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with CP gel in tray | |||||
| 10% CP gel in tray versus placebo ‐ 6 months (higher RR indicates gel whiter) | Study population | RR 6.74 (3.15 to 14.40) | 109 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ | |
| 107 per 1000 | 722 per 1000 (337 to 1000) |
|||||
| 5% CP gel in tray versus placebo ‐ 2 weeks (higher shade indicates whiter) | The mean change in shade in the placebo group was 71.852 | Mean difference in shade change is 4.56 units higher in the CP gel group (1.52 higher to 7.59 higher) | ‐ | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 10% CP gel with desensitiser versus placebo ‐ 2 weeks (higher shade indicates whiter) | The mean change in shade in the placebo group was 9.40 | Mean difference in shade change is 4.70 units higher in the CP gel group (3.28 higher to 6.12 higher) | ‐ | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 10% CP gel (lighter shade) versus placebo ‐ 2 weeks (higher shade indicates whiter) | The mean change in shade in the placebo group was 1.40 | Mean difference in shade change is 4.50 units higher in the CP gel group (4.04 higher to 4.96 higher) | ‐ | 179 teeth (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Analysis done at tooth level |
| 10% CP gel (medium dark shade) versus placebo ‐ 2 weeks (higher shade indicates whiter) | The mean change in shade in the placebo group was was 1.20 | Mean difference in shade change is 9.30 units higher in the CP gel group (8.75 higher to 9.85 higher) | ‐ | 172 teeth (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Analysis done at tooth level |
| 10% CP gel (dark shade) versus placebo ‐ 2 weeks (higher shade indicates whiter) | The mean change in shade in the placebo group was 1.10 | Mean difference in shade change is 10 units higher in the CP gel group (9.44 higher to 10.56 higher) | ‐ | 176 teeth (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Analysis done at tooth level |
| Adverse effects | ||||||
| Main adverse events reported in majority of trials were mild and transient tooth sensitivity and oral irritation which occurred more in the intervention group compared to placebo | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of allocation concealment, performance and detection bias. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 2. HP gel in tray versus placebo for whitening teeth.
| Hydrogen peroxide (HP) gel in tray compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP gel in tray Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with HP gel in tray | |||||
| 6% HP gel versus placebo ‐ 14 days (higher shade indicates whiter) |
The mean change in shade in placebo group was 0.48 | Mean difference in shade change is 3.08 units higher in the HP gel group (2.28 higher to 3.88 higher) | ‐ | 49 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to selection, performance and detection bias. 2Downgraded for imprecision ‐ single trial and low sample and event rate.
Summary of findings 3. HP strips versus placebo for whitening teeth.
| Hydrogen peroxide (HP) strips compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP strips Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with HP strips | |||||
| 10% HP strip versus placebo ‐ day 8 (higher shade indicates whiter) | The mean change in shade in placebo group was 0.21 | Mean difference in shade change is 2.24 units higher in the HP strip group (1.72 higher to 2.76 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 6% HP strip versus placebo ‐ 2 weeks (higher shade indicates whiter) | ‐ | Mean difference in shade change is 2.24 units higher in the HP strip group (1.83 higher to 2.66 higher) | ‐ | 195 (4 RCTs) | ⊕⊕⊝⊝ LOW1, 2 | ‐ |
| 10% HP strip versus placebo ‐ 15 days (higher shade indicates whiter) | The mean change in shade in placebo group was 0.90 | Mean difference in shade change is 1.93 units higher in the HP strip group (1.34 higher to 2.52 higher) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 14% HP strip versus placebo ‐ 3 weeks (higher shade indicates whiter) | The mean change in shade in placebo group was 1.90 | Mean difference in shade change is 7.60 units higher in the HP strip group (6.18 higher to 9.02 higher) | ‐ | 28 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 6% HP strip versus placebo ‐ 6 weeks (higher shade indicates whiter) | The mean change in shade in placebo group was 1.82 | Mean difference in shade change is 2.90 units higher in the HP strip group (1.73 higher to 4.07 higher) | ‐ | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 14% HP strip versus placebo ‐ 6 weeks (higher shade indicates whiter) | The mean change in shade in placebo group was 0.45 | Mean difference in shade change is 5.16 units higher in the HP strip group (4.21 higher to 6.11 higher) | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 5.3% HP strip versus placebo ‐ 6 months (higher shade indicates whiter) | The mean change in shade in placebo group was 1.02 | Mean difference in shade change is 1.21 units higher in the HP strip group (0.67 higher to 1.75 higher) | ‐ | 52 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Main adverse events reported in majority of trials were mild and transient tooth sensitivity and oral irritation which occurred more in the intervention group compared to placebo | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to selection, performance, detection bias. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 4. CP paint‐on gel versus placebo for whitening teeth.
| Carbamide peroxide (CP) paint‐on gel compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP paint‐on gel Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with CP paint‐on | |||||
| 18% CP paint‐on gel versus placebo ‐ 3 weeks (higher shade indicates whiter) | The mean shade change in the placebo group was 0.34 | Mean difference in the shade change was 3.50 higher in the CP paint‐on gel group (3.12 higher to 3.88 higher) | ‐ | 77 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to selection and detection bias. 2Downgraded for imprecision ‐ single trial and low sample and event rate.
Summary of findings 5. HP paint‐on gel versus placebo for whitening teeth.
| Hydrogen peroxide (HP) paint‐on gel compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP paint‐on gel Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with HP paint‐on | |||||
| 6% HP paint‐on gel versus placebo ‐ 2 weeks (higher shade indicates whiter) | ‐ | SMD was 0.67 higher in HP paint‐on gel group (0.19 higher to 1.14 higher) | ‐ | 148 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Main adverse events reported in majority of trials were mild and transient tooth sensitivity and oral irritation which occurred more in the intervention group compared to placebo | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to selection, performance, and detection bias. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 6. SHMP chewing gum versus placebo for whitening teeth.
| Sodium hexametaphosphate (SHMP) chewing gum compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: SHMP chewing gum Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with SHMP chewing gum | |||||
| 7.5% SHMP chewing gum versus placebo ‐ 2 days (higher shade indicates whiter) | The mean shade change in placebo gum was ‐4.28 | Mean difference in the shade change was 0.89 higher in the SHMP chewing gum group (0.77 higher to 1.01 higher) | ‐ | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 5.6% SHMP chewing gum versus placebo ‐ 3 days (higher shade indicates whiter) | The mean shade change in placebo gum was ‐7.27 | Mean difference in the shade change was 2.60 higher in the SHMP chewing gum group (1.45 higher to 3.75 higher) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 4% SHMP chewing gum versus placebo ‐ 12 weeks (higher shade indicates whiter) | The mean shade change in placebo gum was ‐1.27 | Mean difference in the shade change was 0.14 lower in the SHMP chewing gum group (0.38 lower to 0.10 higher) | ‐ | 108 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to selection, performance, and detection bias. 2Downgraded for imprecision ‐ single trial and low sample and event rate.
Summary of findings 7. STPP chewing gum versus placebo for whitening teeth.
| Sodium tripolyphosphate (STPP) chewing gum compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: STPP chewing gum Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with STPP chewing gum | |||||
| 1% STPP chewing gum versus placebo ‐ 6 weeks (higher shade indicates whiter) | The mean shade change in placebo gum was ‐0.09 | Mean difference in the shade change was 0.18 higher in the STPP chewing gum group (0.10 higher to 0.26 higher) |
‐ | 108 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of allocation concealment. 2Downgraded for imprecision ‐ single trial and low sample and event rate.
Summary of findings 8. HP mouthwash versus placebo for whitening teeth.
| Hydrogen peroxide (HP) mouthwash compared to placebo for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP mouthwash Comparison: placebo | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with HP mouthwash | |||||
| 1.5% HP + 0.05% HF mouthwash versus placebo ‐ 6 months (higher OR indicates whiter) | Study population | OR 10.89 (5.08 to 23.35) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Log of odds ratio and SE were calculated and data analyzed using generic inverse variance method | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; F: fluoride; OR: odds ratio; RCT: randomised controlled trial; SE: standard error | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to attrition bias. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 9. CP gel in tray versus CP gel in tray for whitening teeth.
| Carbamide peroxide (CP) gel in tray compared to CP gel in tray for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP gel in tray Comparison: CP gel in tray | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CP gel in tray | Risk with CP gel in tray | |||||
| 10% CP versus 10% CP ‐ 2 weeks (higher RR indicates whiter) | Study population | RR 1.03 (0.90 to 1.18) | 66 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ | |
| 912 per 1000 | 937 per 1000 (704 to 990) | |||||
| 10% CP versus 16% CP ‐ 2‐year follow‐up (higher shade indicates whiter) | The mean after intervention in 10% CP group was ‐81 | Mean difference in shade change was 1.20 higher in 16% CP group (0.35 lower to 2.75 higher) | ‐ | 81 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 16% CP versus 16% CP + ACP ‐ 6 months (higher shade indicates whiter) | The mean change in 16% CP group was ‐5.45 | Mean difference in shade change was 0.78 higher in 16% CP + ACP group (0.37 higher to 1.19 higher) | ‐ | 27 (1 RCT) | ⊕⊕⊝⊝ LOW2 | ‐ |
| 5% CP versus 10% CP ‐ 2 weeks (higher shade indicates whiter) | The mean after intervention for 10% CP group was ‐76.813 | Mean difference in shade change was 0.41 higher in 5% CP group (2.17 lower to 2.98 higher) | ‐ | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 10% CP versus 15% CP ‐ 2 weeks (higher shade indicates whiter) | ‐ | Mean difference was 2.22 higher in 15% CP group (1.29 higher to 3.15 higher) | ‐ | 25 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 10% CP versus 10% CP + KN + NaF ‐ 2 weeks | ‐ | Standardised mean difference was 0.32 higher in 10% CP + KN + NaF group (0.20 lower to 0.84 higher) | ‐ | 58 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Tooth whitening ‐ reported by the patient | ||||||
| 10% CP versus 17% CP ‐ 3 weeks (higher shade indicates whiter) | The mean change in shade for 10% CP group was ‐14.10 | Mean difference in patient contentment was 2.6 higher in 17% CP group (2.57 higher to 2.63 higher) | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Higher concentrations of CP in tray led to more tooth sensitivity and gingival irritation. However, the symptoms were mild and transient. CP in tray with desensitiser showed significantly less sensitivity compared to the groups without the desensitiser | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) ACP: amorphous calcium phosphate; CI: confidence interval; KN: potassium nitrate; NaF: sodium fluoride; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of allocation concealment. 2 Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 10. CP gel in tray versus HP gel in tray for whitening teeth.
| Carbamide peroxide (CP) gel in tray compared to hydrogen peroxide (HP) gel in tray for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP gel in tray Comparison: HP gel in tray | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CP gel in tray | Risk with HP gel in tray | |||||
| 10% CP versus 7.5% HP ‐ 2 weeks (higher shade indicates whiter) | The mean shade change in the CP gel in tray group was 3.40 | Mean difference in shade change was 1 lower in the HP group (2.86 lower to 0.86 higher) | ‐ | 48 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 20% CP versus 9% HP ‐ 2 weeks (higher shade indicates whiter) | The mean shade change in the CP gel in tray group was ‐6.97 | Mean difference in shade change was 0.58 lower in the HP group (8.01 lower to 6.85 higher) | ‐ | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 20% CP versus 7.5% HP ‐ 12 days (higher shade indicates whiter) | The mean shade change in the CP gel in tray group was ‐2.59 | Mean difference in shade change was 0.99 lower in the HP group (2.32 lower to 0.34 higher) | ‐ | 56 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 20% CP versus 7.5% HP ‐ 12 weeks (higher shade indicates whiter) | The mean shade change in the CP gel in tray group was ‐2 | Mean difference in shade change was 0.25 lower in the HP group (0.40 lower to 0.10 lower) | ‐ | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 10% CP versus 6% HP (darker shade) ‐ 2 weeks (higher shade indicates whiter) | The mean shade change in the CP gel in tray group was ‐11.10 | Mean difference in shade change was 4.30 lower in the HP group (5.02 lower to 3.58 lower) | ‐ | 164 teeth (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Analysis done at tooth level |
| 10% CP versus 6% HP (medium dark and lighter shade) ‐ 2 weeks (higher shade indicates whiter) | ‐ | Mean difference in shade change was 2.22 lower in the HP group (2.63 lower to 1.81 lower) | ‐ | 349 teeth (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Analysis done at tooth level |
| Adverse effects | ||||||
| No difference was found between HP and CP in tray groups in relation to tooth sensitivity and oral irritation | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of allocation concealment. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 11. HP strips versus CP gel in tray for whitening teeth.
| Hydrogen peroxide (HP) strips compared to carbamide peroxide (CP) gel in tray for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP strips Comparison: CP gel in tray | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CP gel in tray | Risk with HP strips | |||||
| 6% HP versus 5% CP + 5% KN ‐ 1 week (higher shade indicates whiter) | The mean change in shade for the CP gel in tray was ‐1.20 | Mean difference was 0.71 higher for the strip group (1.35 lower to 0.07 lower) | ‐ | 32 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 6% HP versus 10% CP ‐ 2 weeks (higher shade indicates whiter) | ‐ | Mean difference was 0.42 higher for the strip group (0.92 lower to 0.09 higher) | ‐ | 149 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 14% HP versus 35% CP ‐ 30 days (higher shade indicates whiter) | The mean change in shade for the CP gel in tray was ‐4 | Mean difference was 0.58 lower for the strip group (0.61 lower to 1.77 higher) | ‐ | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 6% HP versus 10% CP ‐ 6 weeks (higher shade indicates whiter) | The mean change in shade for the CP gel in tray was ‐0.90 | Mean difference was 0.30 higher for the strip group (0.95 lower to 0.35 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 6.5% HP versus 15% CP ‐ 3 months (higher shade indicates whiter) | The mean change in shade for the CP gel in tray was ‐8.76 | Mean difference was 3.15 lower for the strip group (0.15 lower to 6.45 lower) | ‐ | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 6.5% HP versus 16% CP ‐ 21 days (higher shade indicates whiter) | The mean change in shade for the CP gel in tray was 2.10 | Mean difference was 2.10 lower for the strip group (1.16 lower to 3.04 lower) |
‐ | 55 (1 RCT) | ⊕⊕⊝⊝ LOW2 | ‐ |
| Adverse effects | ||||||
| When HP strips were compared to CP gel in tray, results were variable for adverse reactions (tooth sensitivity and oral irritation) with some trials favouring the strip group, some favouring the tray group and some showing no differences between the groups | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; KN: potassium nitrate; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 12. HP strips versus HP gel in tray for whitening teeth.
| Hydrogen peroxide (HP) strips compared to HP gel in tray for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP strips Comparison: HP gel in tray | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with HP gel in tray | Risk with HP strips | |||||
| 14% HP strip versus 9.5% HP gel in tray ‐ 22 days (higher shade indicates whiter) | The mean shade change for HP gel in tray was ‐1.75 | Mean difference was 1.40 higher for the strip group (2.35 lower to 0.45 lower) | ‐ | 29 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 5.3% HP strip versus 5% HP gel in tray ‐ 18 months (higher shade indicates whiter) | The mean shade change for HP gel in tray was ‐ 6.35 | Mean difference was 0.06 higher for the strip group (2.36 lower to 2.24 higher) | ‐ | 28 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Patient comfort | ||||||
| 5.3% HP strip versus 5% HP gel in tray | The mean patient acceptance for HP gel in tray was 2.23 | Mean difference in patient acceptance was 1.27 lower for the strip group (0.13 higher to 2.41 higher) | ‐ | 28 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Tooth sensitivity and oral irritation were mild and transient and did not differ between the groups | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ single trials, low sample and event rate.
Summary of findings 13. HP strips versus HP strips (different concentrations) for whitening teeth.
| Hydrogen peroxide (HP) strips compared to HP strips for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP strips Comparison: HP strips | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 10% HP strips | Risk with HP strips | |||||
| 6% HP versus 10% HP thin gel ‐ 15 days (higher shade indicates whiter) | The mean shade change in 10% thin HP gel strip group was ‐3.03 | Mean difference in 6% HP strip group was 0.68 lower (0.16 higher to 1.20 higher) | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 9.5% adhesion HP strips versus 10% HP strips ‐ day 9 (higher shade indicates whiter) | The mean shade change in 10% HP strip group was ‐2.30 | Mean difference in 9.5% adhesion strip group was 1.50 higher (2.33 lower to 0.67 lower) | ‐ | 29 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| When HP strips were compared to HP strips , very thin gel had lesser tooth sensitivity compared to thicker gel even though the concentration of HP was higher. Strips applied for 2 hours had greater symptoms of sensitivity compared with 30‐minute group. However, these results were not significant | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance, detection and attrition bias. 2Downgraded for imprecision ‐ single trials, low sample and event rate.
Summary of findings 14. CP paint‐on gel versus HP strips for whitening teeth.
| Carbamide peroxide (CP) paint‐on gel compared to hydrogen peroxide (HP) strips for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP paint‐on Comparison: HP strips | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CP paint‐on | Risk with HP strips | |||||
| 18% CP paint‐on gel versus 6% HP strips (higher shade indicates whiter) | ‐ | Standardised mean difference for HP strip group was 1.50 higher (1.06 higher to 1.94 higher) | ‐ | 102 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ low sample size and event rate.
Summary of findings 15. HP paint‐on gel versus HP strips for whitening teeth.
| Hydrogen peroxide (HP) paint‐on gel compared to HP strips for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP paint‐on Comparison: HP strips | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 5.9% HP paint‐on | Risk with 5.9% HP strip | |||||
| 5.9% HP paint‐on versus 5.9% HP strip (higher shade indicates whiter) | ‐ | Mean difference in 5.90% HP strip group was 2.70 higher (2.08 higher to 3.32 higher) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Tooth whitening ‐ reported by the patient | ||||||
| 5.9% HP paint‐on versus 5.9% HP strip (higher shade indicates whiter) | The mean patient satisfaction for 5.90% HP paint on gel group was ‐4 | Mean difference in patient satisfaction was 0.25 lower for 5.90% HP strip group (1.88 lower to 1.38 higher) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | |
| Adverse effects | ||||||
| Adverse events were mild in severity, and did not contribute to any treatment modification or early withdrawal. Slightly higher tooth hypersensitivity and gingival irritation in the strip group was found although there was no evidence of a difference between the groups | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ single trial, low sample and event rate.
Summary of findings 16. CP paint‐on versus CP paint‐on (different concentrations) for whitening teeth.
| Carbamide peroxide (CP) paint‐on compared to CP paint‐on for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP paint‐on Comparison: CP paint‐on | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparisons | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 18% CP paint‐on | Risk with CP paint‐on | |||||
| 18% CP paint‐on 2x versus 18% CP paint‐on 4x ‐ 1 week (higher shade indicates whiter) | The mean shade change in the 18% CP paint‐on 2x group was ‐2.79 | Mean difference in shade change was 1.39 higher in 18% CP paint‐on 4x group (0.50 higher to 2.28 higher) | ‐ | 69 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| 18% CP paint‐on versus 16.40% CP paint‐on ‐ 2 weeks (higher shade indicates whiter) | The mean shade change in the 18% CP paint‐on group was 8.20 | Mean difference in shade change was 0.70 lower in the 16.40% CP paint‐on group (2.21 lower to 0.81 higher) | ‐ | 93 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Very mild tooth sensitivity was found in the 4 times daily application group. Both gingival and tooth sensitivity were reported to be transient and caused none of the subjects to withdraw from the study | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; 2x: twice; 4x: 4 times | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ single trials, low sample and event rate.
Summary of findings 17. CP paint‐on versus HP paint‐on for whitening teeth.
| Carbamide peroxide (CP) paint‐on compared to hydrogen peroxide (HP) paint‐on for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: CP paint‐on Comparison: HP paint‐on | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CP paint‐on | Risk with HP paint‐on | |||||
| 25% CP paint‐on versus 8.75% HP paint‐on (higher shade indicates whiter) | The mean change in shade in 25% CP paint‐on group was 6.54 | Mean difference in shade change was 0.16 lower in 8.75% HP paint‐on group (1.39 lower to 1.07 higher) | ‐ | 59 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ single trial, low sample and event rate.
Summary of findings 18. HP paint‐on versus HP paint‐on for whitening teeth.
| Hydrogen peroxide (HP) paint‐on compared to HP paint‐on for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: HP paint‐on Comparison: HP paint‐on | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6% HP paint‐on | Risk with 6% HP paint‐on with KF | |||||
| 6% HP paint‐on versus 6% HP paint‐on with KF (higher shade indicates whiter) | The mean shade change in the 6% HP paint‐on group was 2.70 | Mean difference in shade change was 0.10 lower in 6% HP paint‐on with KF (0.56 lower to 0.36 higher) | ‐ | 67 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| Tooth sensitivity was noted in both groups with no evidence of a difference | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; KF: potassium fluoride; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection, performance and detection bias. 2Downgraded for imprecision ‐ single trial, low sample and event rate.
Summary of findings 19. SPC paint‐on versus CP paint‐on for whitening teeth.
| Sodium percarbonate (SPC) paint‐on compared to CP paint‐on for whitening teeth | ||||||
| Patient or population: adults undergoing bleaching Setting: home‐based Intervention: SPC paint‐on Comparison: CP paint‐on | ||||||
| Tooth whitening ‐ assessed by the dentist | ||||||
| Comparison | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CP paint‐on | Risk with SPC paint‐on | |||||
| 19% SPC paint‐on versus 18% CP ‐ 14 days (higher shade indicates whiter) | The mean shade change in 18% CP paint‐on group was ‐0.55 | Mean difference in shade change was 0.58 higher in the SPC paint‐on group (0.95 lower to 0.21 lower) | ‐ | 38 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | ‐ |
| Adverse effects | ||||||
| 1 subject in 19% SPC paint‐on group reported oral sensitivity. All adverse events were symptomatic and mild in severity | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded for risk of bias ‐ unclear risk of bias due to lack of selection and detection bias. 2Downgraded for imprecision ‐ single trial, low sample and event rate.
In our review, we tried to do the analyses based on duration as early effects, intermediate effects and long‐term effects. However due to high heterogeneity, many trials could not be combined in the individual categories. Also not many trials reported long‐term effects of bleaching agents. We also felt that combining varying methods of application and concentrations would not give a clearer picture to the customers who would want to choose among the different products available over‐the‐counter. With these considerations in mind, we chose to conduct the analyses based on the concentration of active agents and method of application. Only those trials having all the variables (concentration, active agent, method and timing of application and duration) similar were combined in meta‐analyses. The rest of the comparisons were reported independently.
As most of the trials reported data at multiple time points, the longest follow‐up time was considered in the meta‐analysis as described by the Cochrane Handbook for Systematic Reviews of Interventions section 9.3.4 (Higgins 2011). However, the other time points have also been discussed descriptively wherever relevant, as bleaching effect at the shorter time duration may have clinical implications. We discussed all the outcomes separately in the individual comparisons for ease of understanding.
1. Bleaching agent versus placebo
1a. CP gel in tray versus placebo
Tooth whitening ‐ assessed by the dentist
Six trials studied carbamide peroxide (CP) versus placebo (Russell 1996; Matis 1998; Browning 2008; Mederios 2008; Hyland 2015; Aka 2017) with Russell 1996 and Matis 1998 expressing dichotomous data. Browning 2008; Hyland 2015; and Aka 2017 were the multiarm trials from which we analysed data comparing CP gel in tray to placebo. Mederios 2008 was described qualitatively and not included in meta‐analyses as the data were represented as median scores.
Continuous outcome
Hyland 2015 and Aka 2017 used the digital images with CIEL*a*b* scoring while Browning 2008 and Mederios 2008 used the Vita shade guide. Aka 2017 analysed the groups based on baseline tooth shade as light shade, medium dark and dark shades. In all categories, the test group showed favourable results for whitening of teeth compared to placebo gel.
Hyland 2015 compared 5% CP formulation containing sodium tripolyphosphate for 2 weeks and showed L* values significantly higher compared to placebo group (mean difference (MD) 4.56, 95% confidence interval (CI) 1.52 to 7.59; 1 trial, 21 participants; Analysis 1.1).
1.1. Analysis.
Comparison 1 CP gel in tray versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Browning 2008 used 10% CP with a desensitiser versus placebo gel for 2 weeks with the test group showing better Vita shade scores for lightness compared to placebo (MD 4.70, 95% CI 3.28 to 6.12; 1 trial, 37 participants; Analysis 1.1).
Irrespective of the original shade, the CP group showed significantly higher E values compared to placebo in Aka 2017 (light shade: MD 4.50, 95% CI 4.04 to 4.96; 1 trial, 179 teeth; medium dark shade: MD 6.90, 95% CI 6.35 to 7.45; 1 trial, 172 teeth; darker shade: MD 10, 95% CI 9.44 to 10.56; 1 trial, 176 teeth) (Analysis 1.1).
Mederios 2008 compared 10% CP to placebo for 21 days and presented data as a medium score and interquartile range. The median increase in lightness of the teeth in the test group was 3 units based on the value‐ordered Vitapan shade guide. This improvement in lightness was maintained for 6 months in 88% of this group. In the placebo group, 8% has a 2‐unit reduction in tooth colour at day 21.
Dichotomous outcome
Russell 1996 and Matis 1998 studied the effect of 10% CP and the data at 2 weeks and 6 months for these trials were combined in the meta‐analysis. Russell 1996 used the Vita shade guide an categorised the scale for measurement as darker, same or lighter. The lighter shade score was considered as events. Matis 1998 used an ordinal scale where 0 represented no change; 1 represented slight change; 2 was moderate change and 3 represented a large colour change. Scores 2 and 3 were combined and considered as events.
A significant lightening effect was shown at 2 weeks. In addition, the lightening effect lasted when tested at 6 months for the majority of the subjects (risk ratio (RR) 6.74, 95% CI 3.15 to 14.40; 2 trials, 109 participants; Analysis 2.1). Russell 1996 showed similar results at 1 week, 6 weeks and 3 months interval. Matis 1998 also showed similar results at 4 weeks.
2.1. Analysis.
Comparison 2 CP gel in tray versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ reported by the patient
Mederios 2008 recorded volunteers satisfaction by administering a questionnaire at the end of 21 days of treatment and Aka 2017 reported patient satisfaction after 10 days, 14 days, 2 weeks and 6 months of bleaching, which was self‐assessed on a 7‐point scale, with 1 correlating to no satisfaction and 7 to maximum satisfaction. Patients in the CP group were more satisfied by the bleaching effect in both the trials.
Adverse effects
Most common adverse effects were gingival sensitivity, tooth sensitivity, gastrointestinal sensitivity in both the groups although they were transient and mild (Matis 1998). Sensitivity to hot and cold, gingival sensitivity, tongue and throat sensitivity was reported by Myers 2003 which was more for the test group. Mederios 2008 also reported tooth sensitivity, which was more for the CP group compared to the placebo group.
Other trials did not report any adverse reactions. No other secondary outcomes were reported.
1b. HP gel in tray versus placebo
Tooth whitening ‐ assessed by the dentist
Two trials studied the effect of hydrogen peroxide (HP) gel versus placebo (Myers 2003; Mohan 2008). Mohan 2008 reported significantly greater L* values at 3, 7 and 14 days in the 6% HP gel group indicating greater lightness (14 days: MD 3.08, 95% CI 2.28 to 3.88; 1 trial, 49 participants; Analysis 3.1).
3.1. Analysis.
Comparison 3 HP gel in tray versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Myers 2003 used Vita shade guide and found the 3% HP group with significantly lighter shades at 2, 12 and 26 weeks compared to the placebo group. Mean shade change was 4.2 Vita shade tabs at 2 weeks. At 26 weeks (6 months), the degree of whitening was 4.1 tabs.
No other outcomes were reported.
1c. HP strips versus placebo
See Figure 3.
3.
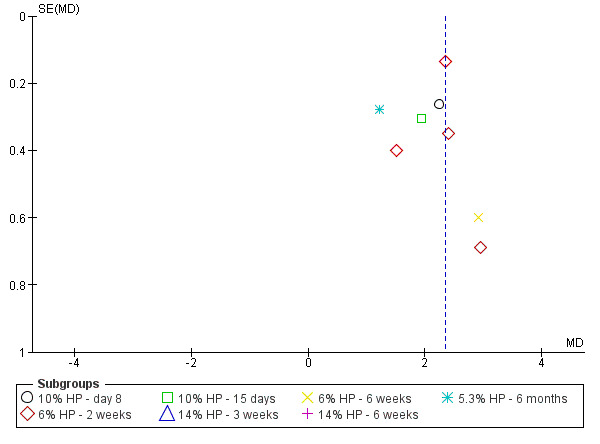
Funnel plot of comparison: 4 HP strip versus placebo, outcome: 4.1 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ assessed by the dentist
Ten trials studied the effect of HP strips against placebo (Kugel 2000; Gerlach 2002; Garcia‐Godoy 2004; Gerlach 2004e; Swift 2004; Wong 2004; Bizhang 2007; Papas 2009; Swift 2009; Bruhn 2012).
Gerlach 2004e who studied 10% HP for 8 days against a placebo showed greater L* value for the test group indicating better lightness (MD 2.24, 95% CI 1.72 to 2.76; 1 trial, 36 participants; Analysis 4.1).
4.1. Analysis.
Comparison 4 HP strip versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
The Kugel 2000; Wong 2004; Bizhang 2007; Swift 2009 trials were combined to analyse the effect of 6% / 5.3% HP versus placebo for 2 weeks. L* values significantly higher in the HP group indicated greater lightness compared to placebo (MD 2.24, 95% CI 1.83 to 2.66; 4 trials, 195 participants; Analysis 4.1).
Papas 2009 showed similar results as Gerlach 2004e at 15 days (MD 1.93, 95% CI 1.34 to 2.52; 1 trial, 40 participants; Analysis 4.1).
Swift 2004 and Garcia‐Godoy 2004 studied 14% HP versus placebo for 3 weeks and 6 weeks, using Vita shade guide and CIEL*a*b* scoring respectively. The 14% HP group demonstrated improvement in shades relative to the placebo strip group with a difference between treatment groups favouring the peroxide group in both trials (Swift 2004: MD 7.60, 95% CI 6.18 to 9.02; 1 trial, 28 participants; Garcia‐Godoy 2004: MD 5.16, 95% CI 4.21 to 6.11; 1 trial, 35 participants (Analysis 4.1)).
Swift 2009 reported the HP group with better lightening effect compared to placebo at 4 weeks and 6 weeks (6 weeks: MD 2.90, 95% CI 1.73 to 4.07; 1 trial, 37 participants; Analysis 4.1).
5.3% HP versus placebo studied by Gerlach 2002e showed that most of the initial colour change remained at 6 months post‐treatment with the whitening strip group continuing to demonstrate highly significant improvements in tooth colour relative to the placebo group (MD 1.21, 95% CI 0.67 to 1.75; 1 trial, 52 participants; Analysis 4.1).
Tooth whitening ‐ reported by the patient
One trial (Bruhn 2012) compared 14% HP against placebo and reported the tooth colour satisfaction scale (TCSS). One trial (Wong 2004) reported patient satisfaction, rated using a product satisfaction questionnaire. Satisfaction regarding the whitening effect of the product was highest for the strip group compared to the placebo group in both trials.
Adverse effects
Treatment with HP whitening strips was generally well tolerated with adverse events confined to symptoms only. Mild and transient tooth sensitivity and oral irritation were the most common adverse events reported in most of the trials (Kugel 2000; Gerlach 2002e; Garcia‐Godoy 2004; Gerlach 2004e; Swift 2004; Papas 2009; Swift 2009).
Oral health‐related quality of life
One trial (Bruhn 2012) compared 14% HP strips against placebo and reported the oral health‐related quality of life (OHRQoL). Wong 2004 reported the standardised response mean for the oral health impact profile (OHIP) and its domains for subjects in each category of the global transition judgement on whether they were dissatisfied (‐1), neutral (0) or satisfied (1). No significant impact of tooth whitening was found in both trials on OHRQoL.
No trial reported on patient's level of comfort with treatment.
1d. CP paint‐on gel versus placebo
Tooth whitening ‐ assessed by the dentist
Nathoo 2002 compared 18% CP gel to placebo using Vita shade guide and the mean changes from baseline tooth shade rank score were compared across treatment groups. CP paint‐on gel exhibited a mean change score of 2.54 units greater that placebo at 2 weeks interval and 3.5 units higher at 3 weeks interval (3 weeks: MD 3.50, 95% CI 3.12 to 3.88; 1 trial, 77 participants; Analysis 5.1).
5.1. Analysis.
Comparison 5 CP paint‐on gel versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Patient comfort
In Nathoo 2002 participants anecdotally reported that the products were extremely easy to apply and did not interfere with speech or life style.
No other outcomes were reported.
1e. HP paint‐on gel versus placebo
Tooth whitening ‐ assessed by the dentist
Collins 2004a used Vita shade and Xu 2007 used digital image analysis with CIEL*a*b* scoring to test 6% HP paint‐on gel versus placebo. We combined the 2‐week data using standardised mean difference (SMD) and the paint‐on group exhibited greater whitening compared to placebo (SMD 0.67, 95% CI 0.19 to 1.14; 2 trials, 148 participants; Analysis 6.1). Similar results were found by Collins 2004a at 1 week interval.
6.1. Analysis.
Comparison 6 HP paint‐on gel versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Adverse effects
Collins 2004a reported the HP group participants with more gum irritation (reddening) compared to placebo although the symptoms were mild. One case of tooth sensitivity was noted.
No other outcomes were reported.
1f. SHMP chewing gum versus placebo
Tooth whitening ‐ assessed by the dentist
Among the three trials comparing sodium hexametaphosphate (SHMP) chewing gum to placebo, Biesbrock 2004 used digital image analysis with CIEL*a*b* scoring using 7.5% SHMP; Walters 2004 and Porciani 2006 used the Lobene stain index with 5.65% and 4% SHMP respectively. Accumulation of stain in all the trials was lower in the test group compared to placebo in Biesbrock 2004 at 1 day and 2 days (2 days: MD 0.89, 95% CI 0.77 to 1.01; 1 trial, 37 participants; Analysis 7.1); Walters 2004 at 3 days (MD 2.60, 95% CI 1.45 to 3.75; 1 trial, 20 participants; Analysis 7.1) also found the test group exhibiting lesser stains compared to placebo.
7.1. Analysis.
Comparison 7 Chewing gum SHMP versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Porciani 2006 reported 33% reduction in induced stain formation by the test group compared to placebo at 12 weeks (MD ‐0.14, 95% CI ‐0.38 to 0.10; 1 trial, 108 participants; Analysis 7.1).
No other outcomes were reported.
1g. STPP chewing gum versus placebo
Tooth whitening ‐ assessed by the dentist
Porciani 2010 studied 1% sodium tripolyphosphate (STPP) against a placebo using Lobene composite stain index which was reduced by 8.8% in the test group and increased by 8% in the control group (MD 0.18, 95% CI 0.10 to 0.26; 1 trial, 108 participants; Analysis 8.1).
8.1. Analysis.
Comparison 8 Chewing gum STPP versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
No other outcomes were reported.
1h. HP mouthwash versus placebo
Tooth whitening ‐ assessed by the dentist
Hasturk 2004 evaluated the tooth whitening effect of 1.5% HP fluoridated rinse by dichotomising the change in shades as measured by the Lobene stain index. Data were expressed in odds ratios (OR) at 1 month, 3 months, 6 months and overall tooth whitening. Generic inverse variance method was used and log of odds ratio and standard error were calculated for the analysis.
Compared with placebo, the HP mouthrinse group was more than 7 times as likely to show whitening at 1 month and 3 months. Greater whitening was also demonstrated by the mouthwash group at 6 months (OR 10.89, 95% CI 5.08 to 23.35; 1 trial, 78 participants; Analysis 9.1). Overall tooth whitening was almost 8.7 times more likely among the mouthwash group than the placebo group.
9.1. Analysis.
Comparison 9 HP mouthwash versus placebo, Outcome 1 Tooth whitening ‐ assessed by the dentist.
No other outcomes were reported.
2. Bleaching agent versus bleaching agent
2a. CP tray versus CP tray
See Figure 4.
4.
Funnel plot of comparison: 11 CP tray versus CP tray, outcome: 11.1 Tooth whitening ‐ assessed by the dentist.
Among the 18 trials, six used value‐oriented/Vita classical shade guide for measuring colour change (Cibirka 1999; Kihn 2000; Giniger 2005; Browning 2008; Gallo 2009; Kose 2011).
Seven trials used electronic instruments for colour change analysis (spectrophotometer, colorimeter, digital analysis) with CIEL*a*b* scoring system (Kowitz 1994; Matis 2000; Tsubura 2005; Matis 2006; Turkun 2010; Navarra 2014; Hyland 2015).
Three trials (Mokhlis 2000; Nathoo 2001; Meireles 2010) measured whitening using a combination of shade guide and electronic instruments. All these trials reported data in CIEL*a*b* scoring which was considered for analysis.
Krause 2008 reported patient contentment using inter‐personal modal intensity comparison where the patient pressed the bulb of a manometer in proportion to their objective concerning the bleaching outcome.
Meireles 2010 in one of his follow‐up reports used oral impact on daily performance (OHIP) scoring to assess oral health‐related quality of life.
Tam 2001 used photographic evaluation and visual analogue scale (VAS), which the patients marked daily in the evening before the next day's bleaching treatment.
Tooth whitening ‐ assessed by the dentist
Dichotomous outcome
Data were presented as an ordinal scale of darker, same or lighter in Cibirka 1999 trial. Number of participants in the lighter shade category were considered as events. A significant degree of lightening of tooth shade relative to baseline values at 2 weeks was demonstrated for both Opalescence and NiteWhite Excel (10% CP formulations), however no evidence of a difference was found between the groups (RR 1.03, 95% CI 0.90 to 1.18; 1 trial, 66 participants; Analysis 10.1).
10.1. Analysis.
Comparison 10 CP tray versus CP tray, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Continuous outcome
Nathoo 2001 which compared 5% and 10% CP found no evidence of a difference between the groups. Mean unit change after 7 days for 5% group was 6.7±1.9 while for the 10% group was 5.7±2 though the result was not statistically significant (MD ‐0.38, 95% CI ‐5.55 to 4.79; 1 trial, 58 participants; Analysis 11.1).
11.1. Analysis.
Comparison 11 CP tray versus CP tray, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Hyland 2015 a multiarm trial compared 5% and 10% CP with STPP formulations. It found no evidence of a difference between 55% and 10% CP in tooth whitening at 2 weeks following daily wear of tooth whitening trays for 2 hours a day (MD 0.41, 95% CI ‐2.17 to 2.98; 1 trial, 21 participants; Analysis 11.1).
Colgate Platinum (CLP) and Rembrandt Lighten (RL) (both 10% CP formulations) compared in Kowitz 1994; Nathoo 1994, showed significantly higher L* values for the CLP group compared to the RL group (MD ‐1.92, 95% CI ‐2.80 to ‐1.03; 2 trials, 88 participants; Analysis 11.1).
Both daytime at‐home bleaching system including 28% CP gel with a non‐custom tray (Meta tray) and conventional overnight at‐home 10% CP gel with a custom tray (Opalescence PF) in Turkun 2010 produced whitening compared to the baseline. Intergroup comparison showed 10% CP overnight bleaching group to be superior to the day time 20‐minute bleaching at 2 weeks and 1 year follow‐up (1 year follow‐up: MD ‐3.30, 95% CI ‐8.71 to 2.11; 1 trial, 20 participants; Analysis 11.1).
Follow‐up at 2 years (Meireles 2010) (MD 1.20, 95% CI ‐0.35 to 2.75; 1 trial, 81 participants; Analysis 11.1) revealed that the tooth shade remained significantly lighter compared to baseline in both the groups comparing 10% and 16% CP. However, tooth shade relapse showed no difference between the groups.
Gallo 2009 who used different concentrations of CP with and without desensitisers, showed no significant difference between the groups in terms of colour change at 10 days (MD ‐0.30, 95% CI ‐8.28 to 7.68; 1 trial, 40 participants; Analysis 11.1). Similar results were observed in Kose 2011 at 4 weeks (MD ‐0.20, 95% CI ‐0.44 to 0.04; 1 trial, 60 participants; Analysis 11.1).
At 180 days the amorphous calcium phosphate (ACP) group retained nearly 10% more of their original whitening treatment result compared to control in Giniger 2005 (MD 0.78, 95% CI 0.37 to 1.19; 1 trial, 27 participants; Analysis 11.1).
Polanight (PN) and Opalescence (OP) (both 10% CP formulations) compared in Tsubura 2005, showed significant difference in L* values for PN compared to OP. Bleaching with PN was considered more effective than that with OP in young patient group and in women (MD 1.46, 95% CI 0.13 to 2.79; 1 trial, 116 participants; Analysis 11.2).
11.2. Analysis.
Comparison 11 CP tray versus CP tray, Outcome 2 Tooth whitening ‐ assessed by the dentist.
Five‐year data of 10% CP versus 15% CP was analysed from Matis 2006. No evidence of a difference was found between the groups in relation to shade change (MD ‐1.47, 95% CI ‐3.56 to 0.62; 1 trial, 58 participants; Analysis 11.2).
Kihn 2000 showed the 15% CP group to have a larger amount of shade change than did the control group (MD 1.65, 95% CI 0.22 to 3.08; 1 trial, 52 participants; Analysis 12.1). Matis 2000 also showed similar results (MD 2.22, 95% CI 1.29 to 3.15; 1 trial, 25 participants; Analysis 12.2).
12.1. Analysis.
Comparison 12 CP tray versus CP tray, Outcome 1 Tooth whitening ‐ assessed by the dentist.
12.2. Analysis.
Comparison 12 CP tray versus CP tray, Outcome 2 Tooth whitening ‐ assessed by the dentist.
Data for 10% CP with and without potassium nitrate and sodium fluoride used in two trials (Browning 2008; Navarra 2014), was combined in the meta‐analysis and showed no evidence of a difference between the whitening systems (standardised mean difference (SMD) 0.32, 95% CI ‐0.20 to 0.84; 2 trials, 58 participants; Analysis 12.3).
12.3. Analysis.
Comparison 12 CP tray versus CP tray, Outcome 3 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ reported by the patient
Krause 2008 reported patient contentment with the bleaching outcome with an inter‐modal intensity comparison. Although the patient contentment score in the 17% group was higher, no evidence of a difference was found between both groups at 2 weeks and 3 weeks interval (3 weeks: MD 2.60, 95% CI 2.57 to 2.63; 1 trial, 20 participants; Analysis 11.3). All participants completing the 2‐year evaluation in Meireles 2010 reported no evidence of a difference between treatment groups regarding the patient‐reported satisfaction in relation to whitening and retention of whitening. Meireles used a questionnaire to rate patient satisfaction regarding whitening outcome in one of his follow‐up reports. No evidence of a difference in the whitening effect was found as reported by patients in both CP 10% and CP 16% groups.
11.3. Analysis.
Comparison 11 CP tray versus CP tray, Outcome 3 Tooth whitening ‐ reported by the patient.
In Matis 2006, at the 5th year evaluation appointment, seven were very pleased with how their teeth look at that time in both 10% and 15% groups. 14 of them were very pleased and seven reported they were not pleased with the appearance in both groups.
Tam 2001 presented data in range using the VAS scale reported by the patients. We did not use these values for analysis according to the Cochrane Handbook for Systematic Reviews of Interventions section 7.7.3.6 (Higgins 2011). The authors reported the lack of evidence of a difference in perceived whiteness between the groups with and without the desensitiser.
Patient comfort
One of Meireles 2010 follow‐up reports used a questionnaire to rate patient comfort on 1 to 5 scale (1 representing agree and 5 representing disagree). Participants from both whitening regimens reported positive opinions about the treatment. Despite this, the CP 10% group reported less interference with the tray when talking (P = 0.02) and less discomfort after application (P = 0.04) compared to the CP 16% group.
Adverse effects
Higher concentrations of CP had more sensitivity compared to lower concentrations (Nathoo 2001; Matis 2006; Krause 2008; Meireles 2010). Navarra 2014 and Browning 2008 reported bleaching agents with desensitiser with significantly lower sensitivity than the bleaching product that did not contain desensitising agents. Kihn 2000; Matis 2000; Gallo 2009 did not report any significant difference in the tooth and gingival irritation between the two groups. Tam 2001; Giniger 2005; Tsubura 2005; Kose 2011 reported mild irritation and sensitivity in both groups. Kowitz 1994; Nathoo 1994; Cibirka 1999; Turkun 2010; Hyland 2015 did not report any adverse events.
Oral health‐related quality of life
In Meireles 2010 2‐year follow‐up report the 16% CP group showed greater bleaching effect than 10% CP, however, OHRQoL was similar for both groups.
2b. CP tray versus HP tray
See Figure 5.
5.
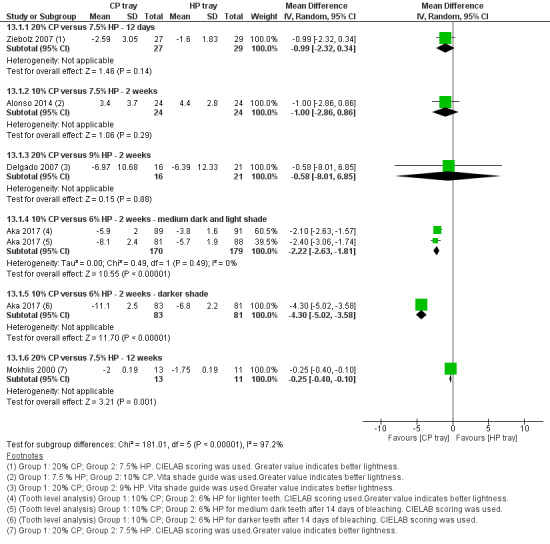
Forest plot of comparison: 13 CP tray versus HP tray, outcome: 13.1 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ assessed by the dentist
Among the seven trials, three used value‐oriented/Vita classical shade guide for measuring colour change (Alonso 2006; Berga‐Caballero 2006; Delgado 2007). Two trials used electronic instruments for colour change analysis (spectrophotometer, colorimeter, digital analysis) with CIEL*a*b* scoring system (Ziebolz 2007; Alonso 2014). Two trials (Mokhlis 2000; Aka 2017) measured whitening using a combination of shade guide and electronic instruments. All these trials reported data in CIEL*a*b* scoring which was considered for analysis.
Both 7.5% HP and 20% CP in Ziebolz 2007 resulted in significant colour improvements in all parameters compared to baseline. Though the reduction in yellowness was significantly more in the 20% CP group compared to 7.5% HP, improvement in lightness (L*) showed no evidence of a difference over the 12 days period (MD ‐0.99, 95% CI ‐2.32 to 0.34; 1 trial, 56 participants; Analysis 13.1).
13.1. Analysis.
Comparison 13 CP tray versus HP tray, Outcome 1 Tooth whitening ‐ assessed by the dentist.
We compared 10% CP to 7.5% HP used in a multiarm trial (Alonso 2014). There was no evidence of a difference between the groups though there was an increase in lightness compared to baseline in both groups (MD ‐1, 95% CI ‐2.86 to 0.86; 1 trial, 48 participants; Analysis 13.1).
Delgado 2007 9% HP group showed a statistically significant 1.54 greater shade rank score unit reduction in the mean tooth shade rank score after 5 days. However, after 7 and 14 days the rank greater score unit reductions were 1.18 and 0.83 for the 9% HP group compared to the 20% CP group, but not statistically significant (MD ‐0.58, 95% CI ‐8.01 to 6.85; 1 trial, 37 participants; Analysis 13.1).
Irrespective of the original shade, 10% CP bleaching groups in Aka 2017 multiarm trial showed significantly higher E values compared to 6% HP: medium dark and light shade: MD ‐2.22, 95% CI ‐2.63 to ‐1.81; 2 trials, 349 teeth; darker shade: MD ‐4.30, 95% CI ‐5.02 to ‐3.58; 1 trial, 164 teeth (Analysis 13.1).
Similar concentrations of CP and HP were used in another trial (Mokhlis 2000) which favoured the 20% CP group for better lightness in the first 14 days. But at the end of the study there was no evidence of a difference between the two groups (12 weeks: MD 0.25, 95% CI 0.10 to 0.40; 1 trial, 24 participants; Analysis 13.2).
13.2. Analysis.
Comparison 13 CP tray versus HP tray, Outcome 2 Tooth whitening ‐ assessed by the dentist.
Berga‐Caballero 2006 found that the changes in colour ranged from 1 to 10 shades of the Vita shade guide's brightness‐based classification; the whitening success percentage was between 315% and 100% on the Jane‐Roig scale, which is based on the greatest percentage of whitening that can be achieved in a tooth, depending on its initial colour. Both 10% CP applied for varying times and 3.5% HP were effective.
Alonso 2006 reported no evidence of a difference between the groups using 3.5% HP along with a desensitiser (potassium nitrate) versus 10% CP.
Tooth whitening ‐ reported by the patient
Aka 2017 reported that the 10% CP/PF (Opalescence PF) groups were more satisfied with the bleaching effect than those in the 6% HP groups.
Patient comfort
In Ziebolz 2007 both 7.5% HP and 10% CP groups showed a similar proportion of subjects with complaints regarding comfort of the bleaching treatment with no significant differences on a 4‐point ordinal scale.
Adverse effects
Mokhlis 2000; Ziebolz 2007; Aka 2017 did not report any significant difference in the tooth and gingival irritation between the two groups. Alonso 2006; Berga‐Caballero 2006; Delgado 2007; Alonso 2014 reported mild irritation and sensitivity in both groups.
No other outcomes were reported.
2c. HP strips versus CP tray
See Figure 6.
6.
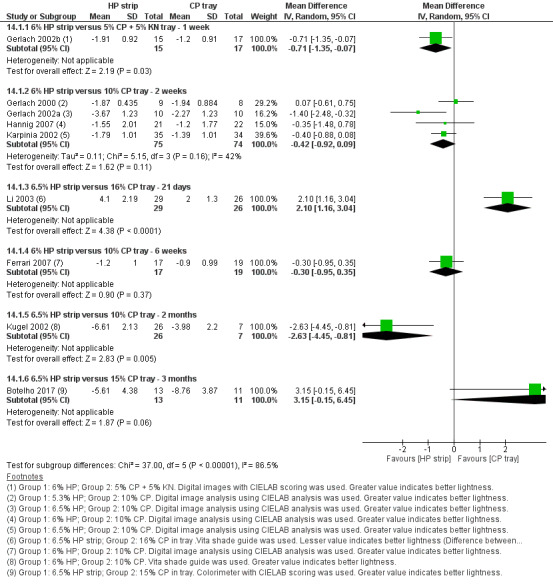
Forest plot of comparison: 14 HP strip versus CP tray, outcome: 14.1 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ assessed by the dentist
Among the 10 trials included in this group, seven trials used digital image analysis with CIEL*a*b* scoring (Gerlach 2000; Gerlach 2002b; Gerlach 2002a; Karpinia 2002; Ferrari 2007; Hannig 2007; Costa 2012). One trial (Botelho 2017) used a colorimeter with CIEL*a*b* scoring and two trials used the Vita shade guide (Kugel 2002; Li 2003).
Gerlach 2002b comparing 6% HP strips to 5% CP with desensitiser for 1 week, revealed 59% greater composite colour change for the strip group compared to the tray (MD ‐0.71, 95% CI ‐1.35 to ‐0.07; 1 trial, 32 participants; Analysis 14.1).
14.1. Analysis.
Comparison 14 HP strip versus CP tray, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Gerlach 2000; Gerlach 2002a; Karpinia 2002; and Hannig 2007 compared similar concentrations of HP strips and 10% CP in tray and were combined in a meta‐analysis. The combined analysis favoured the HP strip group in comparison to 10% CP in tray (MD ‐0.42, 95% CI ‐0.92 to 0.09; 4 trials, 149 participants; Analysis 14.1).
Li 2003 (who used 6.5% HP strips versus 16% CP in tray) favoured the tray group (MD 2.10, 95% CI 1.16 to 3.04; 1 trial, 55 participants; Analysis 14.1).
Ferrari 2007 at 6 weeks reported better whitening with HP strip group (L*) in comparison to 10% CP though the result was not statistically significant (MD ‐0.30, 95% CI ‐0.95 to 0.35; 1 study, 36 participants; Analysis 14.1). However, b values showed favourable results for the strip group at all intervals with statistical significance (P = 0.049).
Kugel 2002 reported that the 6.5% HP strip group experienced statistically significant superior reductions in shade compared to 10% CP in tray at both 1 and 2 month time points (2 months: MD ‐2.63, 95% CI ‐4.45 to ‐0.81; 1 trial, 33 participants; Analysis 14.1).
Intergroup comparison of 6.5% HP strips versus 15% CP in tray in Botelho 2017 showed that the tray group had greater overall colour changes (E) than the strip group at 3 months. However, no evidence of a difference was found between the groups at 3 months (MD 3.15, 95% CI ‐0.15 to 6.45; 1 trial, 24 participants; Analysis 14.1).
Twice daily use of 14% HP or 35% CP for 2 weeks in Costa 2012 split‐mouth trial resulted in significant improvement in tooth lightness relative to baseline. However, no difference was seen between the groups at 2 weeks and 1 month interval (1 month: MD 0.58, 95% CI ‐0.61 to 1.77; 1 trial, 24 participants; Analysis 14.2).
14.2. Analysis.
Comparison 14 HP strip versus CP tray, Outcome 2 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ reported by the patient
Costa 2012 used a patient satisfaction questionnaire to assess patient satisfaction of tooth whitening. 75% of participants could not see the difference in tooth whitening between the tray and strips. 255 of the patients who noted the difference felt the side that was whitened by the tray was whiter.
Hannig 2007 reported subjective colour score as reported by the patients. No difference between the groups was noted (MD ‐0.41, 95% CI ‐2.05 to 1.23; 1 trial, 43 participants; Analysis 14.3).
14.3. Analysis.
Comparison 14 HP strip versus CP tray, Outcome 3 Tooth whitening ‐ reported by the patient.
Patient comfort
Costa 2012 used the patient satisfaction questionnaire to assess patient comfort. 83% of participants in this split‐mouth trial preferred the tray treatment to the strips. 75% found it more comfortable to the teeth and only 38% found it more comfortable to soft tissues. 92% of the patients reported that it was easy to do the procedure twice a day. Kugel 2002 reported that two patients in the 10% CP tray group dropped out from the study after 1 month due to inconvenient regimen.
Adverse effects
Tooth sensitivity and oral irritation were the most common adverse events. Gerlach 2005 reported sensitivity was more common with the 20% tray system. In one trial (Karpinia 2002) tooth sensitivity was more common in the strip group and oral irritation was common in the tray group. Some trials did not show any difference in adverse events between the two groups (Gerlach 2002a; Gerlach 2002b; Kugel 2002; Li 2003; Ferrari 2007; Hannig 2007; Costa 2012).
No other outcomes were reported.
2d. HP strips versus HP tray
Among the two trials in this comparison, Auschill 2012 used Vita shade guide and Gerlach 2004 used digital image analysis using CIEL*a*b* scoring.
Tooth whitening ‐ assessed by the dentist
Gerlach 2004 which compared 14% HP strips and 9.5% HP in tray reported a superior 2‐fold increase in lightness in the strip group at the end of treatment (22 days) compared to the tray group (MD ‐1.40, 95% CI ‐2.35 to ‐0.45; 1 trial, 29 participants; Analysis 15.1). However, initial whitening at 10 days did not differ between the groups.
15.1. Analysis.
Comparison 15 HP strip versus HP tray, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Comparison of 5% HP strips to 5.3% HP gel in tray in Auschill 2012 showed no difference in whitening between the groups at 7 days, 2 weeks and 18 months intervals (18 months: MD 0.06, 95% CI ‐2.24 to 2.36; 1 trial, 28 participants; Analysis 15.1).
Patient comfort
One trial (Auschill 2012) reported patient acceptance graded based on VAS scale ranging from 0 to 10 (where 0 = no discomfort or best acceptance and 10 = severe discomfort or no acceptance). Statistical analysis of data demonstrated that the tray group showed statistically significantly more comfort (VAS 2.23 ± 1.49) than the strip group (VAS 3.50 ± 1.58) (MD 1.27, 95% CI 0.13 to 2.41; 1 study, 28 participants; Analysis 15.2).
15.2. Analysis.
Comparison 15 HP strip versus HP tray, Outcome 2 Patient comfort.
Adverse effects
Tooth sensitivity and oral irritation were mild and transient and did not differ between the groups (Gerlach 2004; Auschill 2012).
No other outcomes were reported.
2e. HP strip versus HP strip
Tooth whitening ‐ assessed by the dentist
Both trials (Shahidi 2005; Oliveira 2013) used digital image analysis with CIEL*a*b* scoring.
In Oliveira 2013 the 2‐hour 9.5% HP high adhesion strips group demonstrated statistically significant lightness improvement (L*) than the 30 minutes 10% HP group on 3, 5 and 9 days (9 days: MD ‐1.50, 95% CI ‐2.33 to ‐0.67; 1 trial, 29 participants; Analysis 16.1).
16.1. Analysis.
Comparison 16 HP strip versus HP strip, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Shahidi 2005 concluded that twice daily use of the very thin 10% HP gel strips resulted in significant tooth whitening after 7 days. Continued use of 10% strips at 15 days yielded significant incremental whitening greater than that seen with the lower concentration (6% HP strips) (MD 0.68, 95% CI 0.16 to 1.20; 1 trial, 35 participants; Analysis 16.1).
Adverse effects
Oliveira 2013 reported that nearly all adverse events were classified as mild in severity. Very thin gel group exhibited lower occurrence of oral irritation and higher tooth sensitivity compared to the 6% group (Shahidi 2005).
No other outcomes were reported.
2f. HP strip versus HP mouthwash
Tooth whitening ‐ assessed by the dentist
Gerlach 2005 used digital image analysis with CIEL*a*b* scoring comparing HP pre‐rinse to HP strips. Under the head‐to‐head testing conditions, 7‐day use of 10% HP whitening strips resulted in significant tooth colour improvement relative to the 2% HP rinse (MD ‐1.10, 95% CI ‐1.49 to ‐0.71; 1 trial, 28 participants; Analysis 17.1).
17.1. Analysis.
Comparison 17 HP strip versus HP mouthwash, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Adverse effects
In Gerlach 2005 tooth sensitivity and oral irritation was more common in the strip group. All adverse events were mild in severity and no subjects discontinued treatment because of these events.
No other outcomes were reported.
2g. CP paint‐on gel versus HP strips
Tooth whitening‐ assessed by the dentist
Two trials (Wong 2004; Cronin 2005) comparing 18% CP paint‐on gel with 6% HP strips and using digital images with CIEL*a*b* scoring were combined in a meta‐analysis. In both trials 6% HP strips were more effective in tooth whitening compared to the CP paint‐on gel (SMD 1.50, 95% CI 1.06 to 1.94; 2 trials, 102 participants; Analysis 18.1).
18.1. Analysis.
Comparison 18 CP paint‐on versus HP strip, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ reported by the patient
One trial (Wong 2004) reported patient satisfaction, rated using a product satisfaction questionnaire. Satisfaction regarding the whitening effect of the product was highest for the strip group (91%) compared to the paint‐on group (33%).
Oral health‐related quality of life
Wong 2004 reported the standardised response mean for the OHIP and its domains for subjects in each category of the global transition judgement on whether they were dissatisfied (‐1), neutral (0) or satisfied (1). The authors felt that OHIP was not a suitable instrument to determine impact of tooth whitening on quality of life.
No other outcomes were reported.
2h. HP paint‐on gel versus HP strips
Tooth whitening ‐ assessed by the dentist
Two trials, Auschill 2007 using Vita shade guide and Xu 2007 using digital image analysis and CIEL*a*b* scoring showed that HP strips provided superior whitening compared to the paint‐on gel group (Xu 2007: MD 1.28, 95% CI 0.77 to 1.79; 1 trial, 33 participants; Analysis 19.1); (Auschill 2007: MD 2.70, 95% CI 2.08 to 3.32; 1 trial, 40 participants; Analysis 19.2).
19.1. Analysis.
Comparison 19 HP paint‐on versus HP strip, Outcome 1 Tooth whitening ‐ assessed by the dentist.
19.2. Analysis.
Comparison 19 HP paint‐on versus HP strip, Outcome 2 Tooth whitening ‐ assessed by the dentist.
Tooth whitening ‐ reported by the patient
One trial (Auschill 2007) reported no difference in patient satisfaction score between the 5.9% HP strip and paint‐on groups (MD ‐0.25, 95% CI ‐1.88 to 1.38; 1 trial, 40 participants; Analysis 19.3).
19.3. Analysis.
Comparison 19 HP paint‐on versus HP strip, Outcome 3 Tooth whitening ‐ reported by the patient.
Adverse effects
Xu 2007 reported that adverse events were mild in severity, and did not contribute to any treatment modification or early withdrawal. Auschill 2007 found a slightly higher tooth hypersensitivity and a slightly higher gingival irritation in the strip group although there was no evidence of a difference between the groups.
No other outcomes were reported.
2i. SPC paint‐on versus HP strips
Tooth whitening ‐ assessed by the dentist
Bizhang 2007 reported that the 6% HP strips yielded significant (P < 0.02) initial whitening relative to 19% sodium percarbonate (SPC) paint‐on film when measured using digital image analysis and CIEL*a*b* scoring (MD 0.93, 95% CI 0.59 to 1.27; 1 trial, 47 participants; Analysis 20.1).
20.1. Analysis.
Comparison 20 SPC paint‐on versus HP strip, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Adverse effects
Bizhang 2007 reported tooth sensitivity and oral irritation as the most common adverse events, with strip use. These adverse events were typically symptomatic only, and confined to the treatment period.
No other outcomes were reported.
2j. CP paint‐on versus CP paint‐on
Tooth whitening ‐ assessed by the dentist
Two trials included in this comparison used Vita shade guide for measuring shade change.
Li 2004 trial used 18% CP with different application times. Between‐group analyses at 7, 14 and 21 days showed the means for groups 3x (3 times a day) and 4x (4 times a day) to be significantly higher than 2x (twice a day) group. However, 3x and 4x groups did not differ significantly (7 days; 2x versus 4x: MD 1.39, 95% CI 0.50 to 2.28; 1 trial, 69 participants; Analysis 21.1).
21.1. Analysis.
Comparison 21 CP paint‐on versus CP paint‐on, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Brunton 2004 comparing 18% and 16.4% CP found both groups with equally effective improvement in whiteness and the difference in whiteness between the two groups was neither statistically nor clinically significant (MD ‐0.70, 95% CI ‐2.21 to 0.81; 1 trial, 93 participants; Analysis 21.1).
Adverse effects
Li 2004 reported one subject to have very mild tooth sensitivity on the 7th day from the 4 times daily application group. Brunton 2004 reported that both gingival and tooth sensitivity were reported to be transient and caused none of the subjects to withdraw from the study.
No other outcomes were reported.
2k. CP paint‐on versus HP paint‐on
Tooth whitening ‐ assessed by the dentist
Nathoo 2003 trial results indicated no evidence of a difference between 25% CP and 8.7% HP paint‐on gels, though the shade improved in both groups compared to baseline measured according to the Vita shade scale (MD ‐0.16, 95% CI ‐1.39 to 1.07; 1 trial, 59 participants; Analysis 22.1).
22.1. Analysis.
Comparison 22 CP paint‐on versus HP paint‐on, Outcome 1 Tooth whitening ‐ assessed by the dentist.
No other outcomes were reported.
2l. HP paint‐on versus HP‐paint‐on
Tooth whitening ‐ assessed by the dentist
Ziebolz 2008 used Vita shade guide and reported significant improvement in tooth colour, in both groups compared to baseline. However no evidence of a difference was found between the groups (MD ‐0.10, 95% CI ‐0.56 to 0.36; 1 trial, 67 participants; Analysis 23.1).
23.1. Analysis.
Comparison 23 HP paint‐on versus HP paint‐on, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Patient comfort
Ziebolz 2008 reported that in both groups similar proportion of the subjects reported lack of comfort. Comfort rating on a 4‐point ordinal scale ranging from comfortable to very uncomfortable did not differ significantly.
No other outcomes were reported.
Adverse effects
Ziebolz 2008 reported tooth sensitivity in both groups with no evidence of a difference.
No other outcomes were reported.
2m. SPC paint‐on versus CP paint‐on
Tooth whitening ‐ assessed by the dentist
Barlow 2003 who used digital image analysis with CIEL*a*b* scoring, showed significant and meaningful improvement in tooth colour in 19% sodium percarbonate group used overnight compared to 18% CP used twice daily for 7 days and 14 days (14 days: MD ‐0.58, 95% CI ‐0.95 to ‐0.21; 1 trial, 38 participants; Analysis 24.1).
24.1. Analysis.
Comparison 24 SPC paint‐on versus CP paint‐on, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Adverse effects
Barlow 2003 reported adverse events related to gum irritation and lip irritation. All events were mild and only one subject in the 18% CP group discontinued the treatment.
No other outcomes were reported.
2n. SPC paint‐on versus HP paint‐on
Tooth whitening ‐ assessed by the dentist
Gerlach 2003 used digital image analysis with CIEL*a*b* scoring and reported uniform whitening for all individual and composite colour parameters for both 8.7% HP and 19% SPC groups. Head‐to‐head clinical testing of these two paint‐on gels demonstrated 4‐fold greater improvement in composite colour for the 19% SPC group (MD ‐0.36, 95% CI ‐0.71 to ‐0.01; 1 trial, 56 participants; Analysis 25.1).
25.1. Analysis.
Comparison 25 SPC paint‐on versus HP paint‐on, Outcome 1 Tooth whitening ‐ assessed by the dentist.
Adverse effects
Gerlach 2003 reported tooth sensitivity in one subject from both groups. One subject in the 19% SPC film group reported oral sensitivity. All adverse events were symptomatic and mild in severity.
No other outcomes were reported.
Discussion
Summary of main results
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19.
Bleaching agent versus placebo
Twenty‐six trials compared different whitening products with placebo or no treatment.
Key results for this comparison have been discussed below independently for each outcome.
Key results for the outcome: tooth whitening ‐ assessed by the dentist
Tray versus placebo:Table 1; Table 2
Carbamide peroxide (CP) gel in tray at 5% and 10% concentrations with varying application times and duration, was more effective than the placebo gel (very low‐certainty evidence).
Hydrogen peroxide (HP) gel in tray at 3% and 6% concentrations applied twice daily for 2 weeks, was more effective than the placebo gel (very low‐certainty evidence).
Strips versus placebo:Table 3
HP strips at 5.3%, 6%, 10% and 14% concentrations, with varying application times were more effective than the placebo gel (low‐ and very low‐certainty evidence).
Paint‐on versus placebo:Table 4; Table 5
CP paint‐on gel at 18% concentration and HP paint‐on gel at 6%, applied twice daily for 2 weeks, was more effective than the placebo gel (very low‐certainty evidence).
Chewing gum versus placebo:Table 6; Table 7
Sodium hexametaphosphate (SHMP) chewing gum at 4%, 5.6% and 7.5% concentrations, used 4 or 8 times a day showed reduction in stains compared to placebo gum (very low‐certainty evidence).
Sodium tripolyphosphate (STPP) chewing gum at 1% concentration used 3 times a day showed a reduction in stains compared to placebo gum (very low‐certainty evidence).
Mouthrinse versus placebo:Table 8
Fluoridated HP mouthwash, improved the shade of the teeth compared to placebo (very low‐certainty evidence).
Key results for the outcome: tooth whitening ‐ reported by the patient
Patient satisfaction regarding bleaching reported in two trials comparing CP gel in tray to placebo and HP strips to placebo showed more satisfaction in the intervention group.
Key results for the outcome: patient comfort
Patient comfort was more for the HP gel in tray group compared to HP strips.
Key results for the outcome: adverse effects
Main adverse events reported in most trials were mild and transient tooth sensitivity and oral irritation, which occurred more in the intervention group compared to placebo. However, the placebo group in some trials (tray versus placebo) reported adverse events, which could be due to irritation from the tray.
Key results for the outcome: oral health‐related quality of life
Oral health‐related quality of life (OHRQoL) reported in two trials comparing HP strips to placebo did not show any significant effect of tooth whitening on improvement in quality of life.
Bleaching agent versus bleaching agent
Fifty‐one trials compared one bleaching agent to another bleaching agent.
Key results for this comparison have been discussed below independently for each outcome.
Key results for the outcome: tooth whitening ‐ assessed by the dentist
Tray versus tray:Table 9; Table 10
Overnight application of two different brands of 10% CP formulations showed no difference between Opalescence and Nite White (very low‐certainty evidence). In another trial, Polanight was shown to have superior whitening compared to Opalescence (very low‐certainty evidence). A single trial with 48 participants compared Colgate Platinum and Rembrandt Lighten and showed improved lightening in the CLP group.
10% CP in tray compared to 15% / 16% CP in tray favoured the higher concentration group irrespective of duration of use and application time (very low‐certainty evidence). However, at the 2‐year follow‐up no evident difference was found between the groups.
10% CP with and without desensitisers (potassium nitrate and sodium fluoride) did not show any difference between the groups when used overnight for 2 weeks (very low‐certainty evidence). However, 16% CP + amorphous calcium phosphate (ACP) showed better whitening when used for 3 hours a day for 2 weeks (low‐certainty evidence).
Overnight application of 5% and 10% concentrations of CP did not show any difference in whitening between the groups (very low‐certainty evidence).
Varying concentrations of HP and CP in tray did not show any significant difference at 2 weeks or 12 weeks duration (very low‐certainty evidence). However, one trial showed superiority of 10% CP in tray over 6% HP in tray especially for the darkest shade teeth (very low‐certainty evidence).
Strip versus tray:Table 11; Table 12
6% HP strip was more effective in whitening effect compared to 10% CP group when tested for a duration of 2 weeks (very low‐certainty evidence). Similar concentrations tested at 6 weeks did not show any difference between the groups (very low‐certainty evidence).
6.5% HP strips compared to 16% CP in tray favoured the CP tray group when tested at 21 days and 3 months duration (low‐certainty evidence). However, one trial comparing 6.5% HP to 10% CP in tray tested for 2 months duration showed better whitening for the strip group.
Twice daily application of 14% HP and 35% CP did not show any differences in the intergroup comparison (very low‐certainty evidence).
6.5% HP strips compared to 15% CP did not show any difference between the groups (very low‐certainty evidence).
6% HP strips showed greater composite colour scores when compared to 5% CP with desensitiser (very low‐certainty evidence).
14% HP strips showed 2‐fold increase in lightness compared to 9.5% HP in tray (very low‐certainty evidence).
5% HP strips compared to 5.3% HP gel in tray showed no difference in whitening between groups (very low‐certainty evidence).
Strip versus strip:Table 13
9.5% high adhesion HP strips were more effective compared to 10% HP strips applied for 30 minutes (very low‐certainty evidence).
10% HP strips with a very thin gel were more effective compared to 6% HP strips (very low‐certainty evidence).
Strip versus mouthwash
10% HP whitening strips resulted in significant whitening of teeth compared to 2% HP mouthrinse used twice daily.
Paint‐on versus strips:Table 14; Table 15
6% or 5.9% HP strips showed more improvement in tooth whitening when compared to 18% CP or 5.9% HP paint‐on gels with varying application times and duration (very low‐certainty evidence). Similar results were obtained when HP strips were compared to 19% sodium percarbonate paint‐on gel.
Paint‐on versus paint‐on:Table 16; Table 17; Table 18; Table 19
No difference was found between 18% and 16.4% CP concentrations applied twice daily for 2 weeks (very low‐certainty evidence).
18% CP application 4 times a day was more effective compared to 2 times application (very low‐certainty evidence).
19% sodium percarbonate group was more effective in comparison to 18% CP used twice a day and 8.7% HP used overnight (very low‐certainty evidence).
HP paint‐on with and without desensitiser did not show any difference in tooth whitening. However, these results are from a single trial and cannot be considered with certainty.
No difference was found between 25% CP and 18.5% HP (very low‐certainty evidence).
Key results for the outcome: tooth whitening ‐ reported by the patient
Patient contentment for 17% CP in tray and 10% CP in tray groups were similar with no significant difference (very low‐certainty evidence).
Patient‐reported satisfaction was more for the 5% HP tray group compared to 5.3% HP strip group (very low‐certainty evidence).
No significant differences in patient satisfaction was observed for the paint‐on and strip groups (very low‐certainty evidence). Tooth hypersensitivity and oral irritation were more in the HP strip group with no statistical difference between the paint‐on group comparators.
Key results for the outcome: patient comfort
Patient comfort was better for the lower concentration group when CP in tray was tested against CP in tray.
Patient comfort did not vary for 7.5% HP in tray and 10% CP in tray groups.
Patient comfort was similar for 6% HP formulations with and without desensitiser. No significant difference was found in adverse events between any of the comparisons.
Key results for the outcome: adverse effects
Higher concentrations of CP in tray led to more tooth sensitivity and gingival irritation. However, the symptoms were mild and transient. CP in tray with desensitiser showed significantly less sensitivity compared to the groups without the desensitiser. No difference was found between HP and CP in tray groups in relation to tooth sensitivity and oral irritation.
When HP strips were compared to CP gel in tray, results were variable for adverse reactions (tooth sensitivity and oral irritation) with some trials favouring the strip group, some favouring the tray group and some showing no differences between the groups.
When HP strips were compared to HP strips, very thin gel had lesser tooth sensitivity compared to thicker gel even though the concentration of HP was higher. Strips applied for 2 hours had greater symptoms of sensitivity compared with the 30‐minute group. However, these results were not significant.
Adverse events occurred more in the strip group compared to mouthwash but were not significant.
Key results for the outcome: oral health‐related quality of life
No difference in OHRQoL was found in 10% CP in tray compared to 16% CP in tray.
Overall completeness and applicability of evidence
Completeness
We systematically searched for trials according to the methodology written in the protocol. We did an independent Google search and checked all cross references of included articles and other systematic reviews on home‐based bleaching to be sure that we did not miss any article. Two pairs of review authors did data extraction in duplicate. Trials, which were not included in the meta‐analysis were explained qualitatively. We selected trials with adult participants needing tooth whitening and included all types of interventions with different application methods and concentrations. We included comparisons with placebo as well as head‐to‐head comparisons. All clinically relevant outcomes of interest were analysed. We also included trials in which staining of teeth was due to tetracycline staining and smoking.
We did not exclude any trial due to missing data. For trials reporting data in graphs, we derived the data by magnifying them and approximating the measures of mean and standard deviation. When mean and standard error (SE) were given, we calculated the standard deviation (SD) as given in the Cochrane Handbook for Systematic Reviews of Interventions section 7.7.3.3 (Higgins 2011). When adjusted mean was given, we considered it in the analysis (Higgins 2011, section 9.2.3.2). When median and interquartile range were given we used the data to calculate mean and SD. When mean and P value were given, SD was calculated. When data were presented as median (skewed data), we qualitatively described the results in the review. When data were presented as odds ratios, log (odds ratio) was calculated based on the odds ratios and 95% confidence intervals given in the trial and the generic inverse variance method was applied.
Applicability
Although we had 71 trials (78 reports) included in this review, most of the comparisons were single trials and could not be combined in meta‐analyses due to varying methods of application, concentrations, application times, and duration of use. Bleaching agents in any mode of application were shown to be effective compared to placebo though the certainty of evidence is very low. The evidence generated is also of very low quality for most of the comparisons testing a bleaching agent versus another bleaching agent, and hence the results cannot be considered with certainty. Most of the trials report on short‐term improvement of shade (ranging from 1 day to 1 month) using home bleaching methods. As follow‐up has not been reported in most trials, the results cannot reflect the retention period for the whitening effect. However, the review encourages further high‐quality randomised controlled trials (RCTs) to be conducted by standardising methods of application, concentrations, application times, and duration of application.
Quality of the evidence
The overall certainty of the evidence was low to very low for all comparisons. When a bleaching agent was compared to placebo for the first outcome looking into improvement of shade as measured by the dentist, except for 6% HP strips compared to placebo, for which evidence was of low certainty, all the other comparisons had a very low‐certainty evidence. In the bleaching agent versus bleaching agent comparisons, the evidence for 16% CP in tray compared to 6.5% HP strip and 16% CP with amorphous calcium phosphate compared to 16% CP was graded as low certainty. The evidence from all remaining studies was graded as very low certainty. Three studies on patient contentment or satisfaction related to whitening treatment, which were included in the meta‐analysis were graded as of very low‐certainty evidence.
We downgraded the trials mainly for two reasons. Most of the trials were graded serious for risk of bias as they were at an unclear risk of bias and very serious to serious for imprecision as most of the trials were single with limited number of participants and low event rates. Few trials that were combined in meta‐analysis had high heterogeneity due to which we downgraded the certainty.
Potential biases in the review process
We have taken steps to minimise bias in every step of the review. We searched all the above mentioned databases, conference proceedings, and trial registries to include all relevant reports. We included foreign language reports in our review. We tried to contact trial authors for missing data through emails, peer contacts, Google search and university/hospital websites where they were previously affiliated. Nevertheless, there could be unpublished data which we could not trace with the above methods. We checked all cross‐references in the included articles and other systematic reviews conducted on home‐based bleaching and found articles which were missed in the search. Two review authors independently reviewed data extraction forms obtained from translators and cross‐checked doubtful areas using the Google translator.
Agreements and disagreements with other studies or reviews
We found three systematic reviews on home‐based bleaching and three conference abstracts.
Niederman 2000 studied only 10% CP tray‐based bleaching products, published between 1989 and 1999 and concluded on the superiority of the intervention over placebo. All the included seven trials have been reported in our review and interpreted similarly.
Gerlach 2007 and Gerlach 2009 studied the efficacy and safety of HP whitening strips against placebo and other controls concluding that strips exhibited superior whitening. All the trials included in these systematic reviews are included in our review and were in agreement with the two systematic reviews.
Three conference abstracts were identified which were systematic reviews of home‐based bleaching products. Brennan 2003 included trials with 19% sodium percarbonate and Gerlach 2013 did a meta‐analysis of trials using 10% HP strips. Gerlach 2010 reported the analysis of peroxide‐based self‐directed products. We did not compare these with our review due to the lack of complete details.
This Cochrane Review had a broader focus and included all types of bleaching agents and different methods of application compared to the other systematic reviews reported.
Authors' conclusions
Implications for practice.
We found low to very low‐certainty evidence over short time periods to support the effectiveness of home‐based chemically‐induced bleaching methods compared to placebo for all the outcomes tested.
We were unable to draw any conclusions regarding the superiority of home‐based bleaching compositions or any particular method of application or concentration or application time or duration of use, as the overall evidence generated was of very low certainty. Well‐planned randomised controlled trials (RCTs) need to be conducted by standardising methods of application, concentrations, application times, and duration of treatment.
Implications for research.
Further research should be undertaken to know the effectiveness of home‐based bleaching methods by conducting well‐planned RCTs with more clarity and uniformity in the variables. In designing such clinical trials, the following needs to be considered.
Evidence: the present evidence was insufficient to conclude that any of the comparisons of home‐based bleaching methods are effective. Trials should focus on testing similar concentrations with similar methods of application. Trials should focus on both short‐term and long‐term benefits of treatment. Studies should also focus on patient‐related outcomes and cost effectiveness. Furthermore, reports on clinical trials would be improved by following CONSORT recommendations.
Population: inclusion criteria for clinical trials should be well defined. Trials should include both genders in equal distribution.
Intervention: intervention should focus on similar concentrations used in earlier studies and similar application times with a longer follow‐up. This will add on to the existing evidence pool allowing us to make robust conclusions.
Comparison: various comparisons have been reported, but we found only single trials in most of the comparisons due to which the quality of evidence is very low. Hence, RCTs need to be conducted keeping in mind already published studies so that the number of trials for a particular comparison increase.
Outcome: patient‐reported outcomes were not considered in most of the trials. Cost effectiveness also needs to be added in the RCTs, which is of most interest to consumers.
What's new
| Date | Event | Description |
|---|---|---|
| 12 June 2018 | New citation required and conclusions have changed | New authors. Review update including 46 new studies bringing the total to 71 included studies. Methods updated. 'Summary of findings' tables included. Conclusions changed. |
| 12 June 2018 | New search has been performed | Searches updated to 12 June 2018. |
Acknowledgements
We are extremely thankful to Ms Anne Littlewood, Information Specialist; Ms Luisa M Fernandez Mauleffinch, Managing Editor; Dr Philip Riley, Editor; Ms Jo Weldon, Research Co‐ordinator; and Prof Helen Worthington, Co‐ordinating Editor, Cochrane Oral Health. We thank Prof Datuk Dr Abdul Razzak, Pro Vice Chancellor, Manipal Academy of Higher Education (MAHE), Melaka campus, for his constant encouragement to undertake Cochrane Reviews; Prof Dr Jaspal Singh Sahota, CEO Melaka‐Manipal campus for his support; Prof Dr Adinegara Lutfi Abas, Dean, Melaka‐Manipal Medical College; and Prof Dr Abdul Rashid Hj Ismail, Dean, Faculty of Dentistry, Melaka‐Manipal Medical College for all suggestions and help during the review preparation. The review authors wish to acknowledge the help of translator Dr Annette Blümle, Information Specialist and Researcher, Cochrane Germany; and peer reviewers Dr Alonso Carrasco‐Labra, Ms Malavika Tampi, Dr Laura Tam, Dr Adriana Manso and Ms Lesley McGovern Kupiec. We thank Prof Dr Sonal Bakul Joshi, KLE University, Belgaum, India for giving valuable inputs during our review preparation. The authors also wish to thank Prof Dr Pratap Tharyan, Mr Richard K and Mr Jabez Paul from the South Asian Cochrane Network, Vellore, India for their inputs during review completion. We are indebted to Ms Shazana Mohd Selva, Chief Librarian, Melaka‐Manipal Medical College; and Ms Janet Lear, School of Dentistry, The University of Manchester.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
1 (((tooth or teeth or dental) and (whiten* or bleach*)):ti,ab) AND (INREGISTER) 2 (((tooth or teeth or dental) and (stain* and remov*)):ti,ab) AND (INREGISTER) 3 (#1 or #2) AND (INREGISTER) 4 (("tooth whitening system" or "tooth bleaching system"):ti,ab) AND (INREGISTER) 5 ((tray* and (whiten* or bleach* or peroxide)):ti,ab) AND (INREGISTER) 6 ((strip* or dentifrice* or gel* or toothpaste* or paste* or rins* or mouthwash* or mouthrins* or "mouth wash*" or "mouth rins*"):ti,ab) AND (INREGISTER) 7 ("whitening kit*":ti,ab) AND (INREGISTER) 8 (#4 or #5 or #6 or 7) AND (INREGISTER) 9 (#3 and #8) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh "tooth bleaching"] #2 ((tooth or teeth or dental) near/5 (whiten* or bleach* or (stain* near/3 remov*))):ti,ab #3 #1 or #2 #4 ("tooth whitening system" or "tooth bleaching system"):ti,ab #5 (tray* and (whiten* or bleach* or peroxide)):ti,ab #6 [mh dentifrices] #7 ((strip* or dentifrice* or gel* or toothpaste* or paste* or rins* or mouthwash* or mouthrins* or "mouth wash*" or "mouth rins*") and (whiten* or bleach* or peroxide)):ti,ab #8 "whitening kit*":ti,ab #9 {or #4‐#8} #10 #3 and #9
Appendix 3. MEDLINE Ovid search strategy
1. Tooth bleaching/ 2. ((tooth or teeth or dental) adj5 (whiten$ or bleach$ or (stain$ adj3 remov$))).ti,ab. 3. 1 or 2 4. ("tooth whitening system" or "tooth bleaching system").ti,ab. 5. (tray$ and (whiten$ or bleach$ or peroxide)).ti,ab. 6. Dentifrices/ 7. ((strip$ or dentifrice$ or gel$ or toothpaste$ or paste$ or rins$ or mouthwash$ or mouthrins$ or "mouth wash$" or "mouth rins$") and (whiten$ or bleach$ or peroxide)).ti,ab. 8. "whitening kit$".ti,ab. 9. or/4‐8 10. 3 and 9
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐ maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011) (Lefebvre 2011).
1. randomised controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. tooth discoloration/ 2. ((tooth or teeth or dental) adj5 (whiten$ or bleach$ or (stain$ adj3 remov$))).ti,ab. 3. 1 or 2 4. tooth bleaching agent/ 5. ("tooth whitening system" or "tooth bleaching system").ti,ab. 6. (tray$ and (whiten$ or bleach$ or peroxide)).ti,ab. 7. toothpaste/ 8. (strip$ or dentifrice$ or gel$ or toothpaste$ or paste$ or rins$ or mouthwash$ or mouthrins$ or "mouth wash$" or "mouth rins$").ti,ab. 9. "whitening kit$".ti,ab. 10. or/4‐9 11. 3 and 10
This subject search was linked to an adapted version of the Cochrane Embase Project filter for identifying randomised controlled trials in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information).
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov search strategy
whiten and teeth bleach and teeth tooth and stain and removal
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
whiten and tooth or whiten and teeth bleach and tooth or bleach and teeth tooth and stain and removal
Data and analyses
Comparison 1. CP gel in tray versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 5% CP gel ‐ 2 weeks | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [1.52, 7.59] |
| 1.2 10% CP gel with desensitiser ‐ 2 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [3.28, 6.12] |
| 1.3 10% CP gel ‐ light shade ‐ 2 weeks | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | 4.5 [4.04, 4.96] |
| 1.4 10% CP gel ‐ medium dark shade ‐ 2 weeks | 1 | 172 | Mean Difference (IV, Fixed, 95% CI) | 6.90 [6.35, 7.45] |
| 1.5 10% CP gel ‐ darker shade ‐ 2 weeks | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [9.44, 10.56] |
Comparison 2. CP gel in tray versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 6.74 [3.15, 14.40] |
| 1.1 10% CP gel ‐ 6 months | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 6.74 [3.15, 14.40] |
Comparison 3. HP gel in tray versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6% HP gel ‐ 14 days | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 3.08 [2.28, 3.88] |
Comparison 4. HP strip versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 10% HP ‐ day 8 | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 2.24 [1.72, 2.76] |
| 1.2 6% HP ‐ 2 weeks | 4 | 195 | Mean Difference (IV, Random, 95% CI) | 2.24 [1.83, 2.66] |
| 1.3 10% HP ‐ 15 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.93 [1.34, 2.52] |
| 1.4 14% HP ‐ 3 weeks | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 7.6 [6.18, 9.02] |
| 1.5 6% HP ‐ 6 weeks | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 2.90 [1.73, 4.07] |
| 1.6 14% HP ‐ 6 weeks | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 5.16 [4.21, 6.11] |
| 1.7 5.3% HP ‐ 6 months | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 1.21 [0.67, 1.75] |
Comparison 5. CP paint‐on gel versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 18% CP paint‐on gel ‐ 3 weeks | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 3.50 [3.12, 3.88] |
Comparison 6. HP paint‐on gel versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6% HP ‐ 2 weeks | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.19, 1.14] |
Comparison 7. Chewing gum SHMP versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 3 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 7.5% SHMP gum ‐ 2 days | 1 | 37 | Mean Difference (Random, 95% CI) | 0.89 [0.77, 1.01] |
| 1.2 5.6% SHMP gum ‐ 3 days | 1 | 20 | Mean Difference (Random, 95% CI) | 2.6 [1.45, 3.75] |
| 1.3 4% SHMP gum ‐ 12 weeks | 1 | 108 | Mean Difference (Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
Comparison 8. Chewing gum STPP versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1% STPP gum ‐ 6 weeks | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.10, 0.26] |
Comparison 9. HP mouthwash versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | (Random, 95% CI) | Subtotals only | |
| 1.1 1.5% HP + 0.05% F ‐ 6 months | 1 | 78 | (Random, 95% CI) | 10.89 [5.08, 23.35] |
Comparison 10. CP tray versus CP tray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 10% CP tray versus 10% CP tray ‐ 2 weeks | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.18] |
Comparison 11. CP tray versus CP tray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 5% CP versus 10% CP ‐ 1 week | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐5.55, 4.79] |
| 1.2 5% CP versus 10% CP ‐ 2 weeks | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐2.17, 2.98] |
| 1.3 10% CP Colgate Platinum versus 10% CP Rembrandt Lighten ‐ 2 weeks | 2 | 88 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐2.80, ‐1.03] |
| 1.4 10% CP versus 28% CP ‐ 1 year follow‐up | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐8.71, 2.11] |
| 1.5 10% CP versus 16% CP ‐ 2‐year follow‐up | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.35, 2.75] |
| 1.6 30% CP versus 30% CP + KN ‐ 10 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐8.28, 7.68] |
| 1.7 16% CP versus 16% CP + KN+ NaF ‐ 4 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.44, 0.04] |
| 1.8 16% CP versus 16% CP + ACP ‐ 6 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.78 [0.37, 1.19] |
| 2 Tooth whitening ‐ assessed by the dentist | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 10% CP Polanight versus 10% CP Opalescence ‐ 2 weeks | 1 | 116 | Mean Difference (Fixed, 95% CI) | 1.46 [0.13, 2.79] |
| 2.2 10% CP versus 15% CP tetracycline‐stained teeth ‐ 5 years | 1 | 58 | Mean Difference (Fixed, 95% CI) | ‐1.47 [‐3.56, 0.62] |
| 3 Tooth whitening ‐ reported by the patient | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 10% CP versus 17% CP ‐ Patient‐contentment ‐ 3 weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [2.57, 2.63] |
Comparison 12. CP tray versus CP tray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 10% CP versus 15% CP ‐ 2 weeks | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 1.65 [0.22, 3.08] |
| 2 Tooth whitening ‐ assessed by the dentist | 1 | 25 | Mean Difference (Fixed, 95% CI) | 2.22 [1.29, 3.15] |
| 2.1 10% CP versus 15% CP ‐ 2 weeks | 1 | 25 | Mean Difference (Fixed, 95% CI) | 2.22 [1.29, 3.15] |
| 3 Tooth whitening ‐ assessed by the dentist | 2 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.20, 0.84] |
| 3.1 10% CP versus 10% CP + KN + NaF ‐ 2 weeks | 2 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.20, 0.84] |
Comparison 13. CP tray versus HP tray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 20% CP versus 7.5% HP ‐ 12 days | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐2.32, 0.34] |
| 1.2 10% CP versus 7.5% HP ‐ 2 weeks | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.86, 0.86] |
| 1.3 20% CP versus 9% HP ‐ 2 weeks | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐8.01, 6.85] |
| 1.4 10% CP versus 6% HP ‐ 2 weeks ‐ medium dark and light shade | 1 | 349 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐2.63, ‐1.81] |
| 1.5 10% CP versus 6% HP ‐ 2 weeks ‐ darker shade | 1 | 164 | Mean Difference (IV, Random, 95% CI) | ‐4.3 [‐5.02, ‐3.58] |
| 1.6 20% CP versus 7.5% HP ‐ 12 weeks | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.40, ‐0.10] |
| 2 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 20% CP versus 7.5% HP ‐ 12 weeks | 1 | 24 | Mean Difference (Fixed, 95% CI) | 0.25 [0.10, 0.40] |
Comparison 14. HP strip versus CP tray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6% HP strip versus 5% CP + 5% KN tray ‐ 1 week | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.35, ‐0.07] |
| 1.2 6% HP strip versus 10% CP tray ‐ 2 weeks | 4 | 149 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.92, 0.09] |
| 1.3 6.5% HP strip versus 16% CP tray ‐ 21 days | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 2.10 [1.16, 3.04] |
| 1.4 6% HP strip versus 10% CP tray ‐ 6 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.95, 0.35] |
| 1.5 6.5% HP strip versus 10% CP tray ‐ 2 months | 1 | 33 | Mean Difference (IV, Random, 95% CI) | ‐2.63 [‐4.45, ‐0.81] |
| 1.6 6.5% HP strip versus 15% CP tray ‐ 3 months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 3.15 [‐0.15, 6.45] |
| 2 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 14% HP strip versus 35% CP tray ‐ 30 days | 1 | 24 | Mean Difference (Fixed, 95% CI) | 0.58 [‐0.61, 1.77] |
| 3 Tooth whitening ‐ reported by the patient | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐2.05, 1.23] |
Comparison 15. HP strip versus HP tray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 14% HP strip versus 9.5% HP tray ‐ 22 days | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐1.4 [‐2.35, ‐0.45] |
| 1.2 5.3% HP strip versus 5% HP tray ‐ 18 months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐2.36, 2.24] |
| 2 Patient comfort | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 5.3% HP strip versus 5% HP tray | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [0.13, 2.41] |
Comparison 16. HP strip versus HP strip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 9.5% HP strip versus 10% HP strip ‐ day 9 | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.33, ‐0.67] |
| 1.2 6% HP strip versus 10% HP strip ‐ 15 days | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.16, 1.20] |
Comparison 17. HP strip versus HP mouthwash.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 10% HP strip versus 2% HP mouthwash | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.49, ‐0.71] |
Comparison 18. CP paint‐on versus HP strip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 18% CP paint‐on versus 6% HP strip | 2 | 102 | Std. Mean Difference (IV, Random, 95% CI) | 1.50 [1.06, 1.94] |
Comparison 19. HP paint‐on versus HP strip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 5.9% HP paint‐on versus 6% HP strip | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 1.28 [0.77, 1.79] |
| 2 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 5.9% HP paint‐on versus 5.9% HP strip | 1 | 40 | Mean Difference (Fixed, 95% CI) | 2.7 [2.08, 3.32] |
| 3 Tooth whitening ‐ reported by the patient | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 5.9% HP paint‐on versus 5.9% HP strip | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.88, 1.38] |
Comparison 20. SPC paint‐on versus HP strip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 19% SPC paint‐on versus 6% HP strip ‐ 6 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.59, 1.27] |
Comparison 21. CP paint‐on versus CP paint‐on.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 18% CP 2x versus 18% CP 4x ‐ 1 week | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 1.39 [0.50, 2.28] |
| 1.2 18% CP versus 16.4% CP ‐ 2 weeks | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.21, 0.81] |
Comparison 22. CP paint‐on versus HP paint‐on.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 25% CP paint‐on versus 8.75% HP paint‐on | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.39, 1.07] |
Comparison 23. HP paint‐on versus HP paint‐on.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6% HP paint‐on versus 6% HP + KF paint‐on | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.56, 0.36] |
Comparison 24. SPC paint‐on versus CP paint‐on.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 19% SPC paint‐on versus 18% CP paint‐on ‐ 14 days | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.95, ‐0.21] |
Comparison 25. SPC paint‐on versus HP paint‐on.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tooth whitening ‐ assessed by the dentist | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 19% SPC paint‐on versus 8.7% HP paint‐on | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.71, ‐0.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aka 2017.
| Methods | Title: evaluation of the efficacy and colour stability of 2 different at‐home bleaching systems on teeth of different shades Trial design: randomised, parallel‐group, controlled clinical trial Location: Izmir Katip Celebi University, Turkey Language: English Number of centres: 1 Recruitment period: January to July 2014 Funding source: not reported |
|
| Participants | Participants: 20 to 51 years old, mean age 26 years Total number: 200 Inclusion criteria:
Exclusion criteria:
Number randomised: 92 (all patients had similar number of light, medium dark and dark shaded teeth) Method of randomisation: randomisation table Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 90 |
|
| Interventions | Total number of intervention groups: 3 Group 1: placebo n = 31 Group 2: 10% carbamide peroxide gel (Opalescence PF) n = 30 Group 3: 6% hydrogen peroxide gel (Opalescence Go) n = 31 Duration of treatment: 14 days Each group was further divided based on the shade (tooth level analysis) Control group: light teeth n = 90, medium dark teeth n = 91, dark teeth n= 93 Experiment Group 2: light teeth n = 89, medium dark n = 81, dark teeth n = 83 Experiment Group 3: light teeth n = 91, medium teeth n = 88, dark teeth n = 81 |
|
| Outcomes | Shade evaluation (dental spectrophotometer); tooth sensitivity, gingival irritation and patient satisfaction (self‐assessed using a 7‐point scale: 1 correlating to no sensitivity, no problems, or no satisfaction; 7 correlating to severe sensitivity, problems, or satisfaction | |
| Notes | Sample size calculation: given Adverse effects: tooth sensitivity and irritation Key conclusions of the study authors: "A pre‐loaded tray system may be used for dental bleaching, but it is still less effective than conventional 10% carbamide peroxide system, irrespective of the initial shade. Bleaching was more effective with dark teeth compared to light teeth. Patient satisfaction was higher in 10% CP group compared to 6% HP" Correspondence required: no Contact: Department of Restorative Dentistry, Faculty of Dentistry, Izmir Katip Celebi University, Izmir, Turkey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned to one of the two treatment groups and a control group using a randomization table…" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessed as at high risk of bias due to the lack of clarity of how teeth were selected for outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 patients from 6% HP/Go groups with 6 light shades of teeth did not attend the 6 months visit" Comment: Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Alonso 2006.
| Methods | Title: comparison of the clinical efficacy and safety of carbamide peroxide and hydrogen peroxide in at‐home bleaching gels Trial design: split‐mouth randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 18 to 50 years, mean age: 31.8 years Total number: 16 Inclusion criteria:
Exclusion criteria:
Number randomised: 16 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 16 |
|
| Interventions | Total number of intervention groups: 2 3.5% hydrogen peroxide gel + 5% potassium nitrate 10% carbamide peroxide gel Duration of treatment: 3 hours a day on each arch, renew the gel every hour for 4 weeks |
|
| Outcomes | Change in tooth colour Vita shade guide: B1 lightest (1) – C4 darkest (16) Dental sensitivity: a scale of 4 levels, absence of sensitivity (grade 0), slight sensitivity not necessitating suspension of treatment (grade 1), sensitivity that forced suspension of treatment for 1 day (grade 2), sensitivity that led to suspension of treatment for more than 1 day (grade 3) Gingival irritation: present or absent |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Under the conditions of this study, no statistically significant differences were detected between 3.5% hydrogen peroxide containing 5% potassium nitrate (FKD) and the 10% carbamide peroxide‐based product (Opalescence)" Correspondence required: no Contact: Victor Alonso de la Peña; victorap@mundo‐r.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Both groups applied the products on a random basis" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 16 patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Alonso 2014.
| Methods | Title: randomised clinical trial on the efficacy and safety of 4 professional at home tooth whitening gels Trial design: randomised controlled clinical trial Location: Faculty of Medicine and Dentistry, Santiago de Compostela, Spain Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: mean age 25.9 years Total number: 96 Inclusion criteria:
Exclusion criteria:
Number randomised: 96 Method of randomisation: based on alphabetical order Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 96 |
|
| Interventions | Total number of intervention groups: 4 10% carbamide peroxide 15% carbamide peroxide 7.5% hydrogen peroxide 9.5% hydrogen peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Change in colour Vita shade guide arranged from lightest to darkest B1 (1) to C4 (16). And change in score was recorded ΔL, a*, b* were recorded (b*: decreased b indicates reduced yellowness; ΔL: increased ΔL is increased brightness) Sensitivity: 0 = none, 1 = mild, 2 = moderate, 3 = considerable, 4 = severe |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "There were no differences in the degree of whitening among the different products. With all of the products there was an increase in L*, a decrease in chromatic intensity (C*), and an increase in the value (tone) or hue (h*)" Contact: Dr Teijeiro; victorap@mundo‐r.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...they were randomly divided into 4 groups of 24 individuals by alphabetical order" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "this was controlled, parallel, randomized one centre…" However, method of blinding is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "this was controlled, parallel, randomized one centre…" However, method of blinding is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 96 participants completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Auschill 2007.
| Methods | Title: a clinical comparison of 2 over‐the‐counter bleaching systems Trial design: examiner‐blinded, split‐mouth randomised controlled trial Location: Freiburg, Germany Language: German (translates to English using Google translate) Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 21 to 36 years old. Mean age: 25.5 years Total number: 26 Inclusion criteria:
Number randomised: 26 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 20 |
|
| Interventions | Total number of intervention groups: 2 5.9% hydrogen peroxide gel 5.9% hydrogen peroxide strips Duration of treatment: 32 days |
|
| Outcomes | Improvement in tooth shade Vita shade guide: C1 lightest (1) to B4 darkest (16) |
|
| Notes | Sample size calculation: not reported Adverse effects: hypersensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "The subjects teeth treated with the strips‐system exhibited a 6.0 +/‐ 0.0 mean shade scores improvement compared to baseline (53.7 cycles; 1610.3 min), and the subjects teeth treated with the gel‐system exhibited a 3.3 +/‐ 1.4 mean shade scores improvement (64.6 cycles; 969.0 min). However, both treatments were able to whiten teeth statistically significantly compared to baseline. Side effects caused by the 2 systems were minimal and reversible. None of the teeth studied showed detectible enamel surface changes in the subsequent SEM analysis. Both methods were well accepted" Contact: Thorsten.auschill@uniklini‐freiburg.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"...subjects were randomly assigned to one of two groups." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Examiner‐blinded split‐mouth randomised controlled trial." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "6 out of 26 patients no longer appeared for follow‐up" Comment: as each group had 20 participants (split‐mouth trial) we rated it as low risk according to Higgins 2011 Section 8.5.d. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Auschill 2012.
| Methods | Title: randomised clinical trial of the efficacy, tolerability, and long‐term colour stability of 2 bleaching techniques: 18‐month follow‐up Trial design: 2‐cell, parallel, examiner‐blinded, randomised controlled trial Location: Albert‐Ludwigs‐University, Freiburg, Germany Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: 18 to 56 years old, mean age: 33.08 years Total number: 30 Inclusion criteria:
Exclusion criteria:
Number randomised: 30 Method of randomisation: a computer‐based randomisation Method of allocation concealment: not reported Method of blinding: personal examiner blinding done by coding which was only known to statistician Number evaluated: 28. 2 dropouts at follow‐up |
|
| Interventions | Total number of intervention groups: 2 Tray group: 5% hydrogen peroxide Strip group: 5.3% hydrogen peroxide Duration of treatment: 14 days |
|
| Outcomes | Improvement in tooth shade Vita shade guide: ranked from lightest B1 (1) to darkest C4 (16); mean score for anterior teeth was calculated Adverse effects. Patient recorded: mild, moderate and severe Patient acceptance, gingival irritation, sensitivity VAS scale: 0 (best acceptance) to 10 (no acceptance) |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Key conclusions of the study authors: "Both bleaching techniques (the tray technique with 5.0% hydrogen peroxide and the strip technique with 5.3% hydrogen peroxide) demonstrated similar success. Although a significant relapse in tooth shade was observed over an 18‐month post bleaching period, treated teeth were still significantly lighter compared to baseline. Adverse effects were minimal and reversible. Patient acceptance was statistically significantly higher in the tray group compared with the strip group" Correspondence required: no Contact: Dr Thorsten M Auschill; auschill@med.uni‐marburg.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐based randomisation scheme (generated before starting the study) allocated patients…" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "2‐cell, parallel, examiner‐blinded, randomised controlled trial." However, method is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "..examiners, who were blinded to the treatment modality and period, subjectively measured.. Personal blinding done by coding which was only known to statistician" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 had to be classified as dropouts for that single visit. Thus, 28 subjects were available at the 18‐month follow‐up" Comment: as each group had 14 participants each we considered it as low risk according to Higgins 2011 Section 8.5.d. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Barlow 2003.
| Methods | Title: clinical response of 2 brush‐applied peroxide whitening systems Trial design: double‐blinded, randomised, controlled, parallel‐group trial Location: university Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: 18 to 48 years old, mean age: 23 years Total number: 38 Inclusion criteria:
Exclusion criteria:
Number randomised: 38 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: similar packet kits Number evaluated: 38 |
|
| Interventions | Total number of intervention groups: 2 18% carbamide peroxide 19% sodium percarbonate (2 brush‐applied whitening kits) Duration of treatment: 14 days |
|
| Outcomes | Improvement in tooth colour b*: decreased b* indicates reduced yellowness ΔL: increased ΔL is increased brightness ΔW: negative ΔW indicates colour closer to white |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity and gingival irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Crest Night Effect provided significant and meaningful improvement in colour after 14 days... Both products were tolerated" Correspondence required: no Contact: A Barlow; barlow.ap@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"double‐blind, randomised, controlled, parallel‐group clinical trial." However, method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were supplied with blinded study kit boxes" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind, randomised, controlled, parallel‐group clinical trial." However, method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Berga‐Caballero 2006.
| Methods | Title: at‐home vital bleaching: a comparison of hydrogen peroxide and carbamide peroxide treatments Trial design: randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: age not reported Total number: 6 Inclusion criteria: not reported Exclusion criteria: not reported Number randomised: 6 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 6 |
|
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide: 20 to 28 days 3.5% hydrogen peroxide: 28 days Duration of treatment: 20 to 28 days |
|
| Outcomes | Improvement in tooth shade | |
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "In carbamide peroxide group, the daily length of application was 2 hours. In the first case the treatment lasted for 24 days, there was no pre‐ or post‐operative sensitivity and the tooth shade changed from A4 (canines) – A3.5 (incisors) to A2 (canines) – A1 (incisors). In hydrogen peroxide the duration of treatment was similar (28 days), no sensitivity was shown during treatment and the shade changed from A3.5 (canines) – A3 (incisors) to A1. Case 3 presented a change in shade from A4 (canines) – A3 (incisors) to A2 (canines) – A1 (incisors) after only 20 days; in this case the patient did mention slight sensitivity in the anterior mandibular teeth throughout the treatment" Correspondence required: no Contact: Professor Dr Forner Navarro; forner@uv.es |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly assigned to the two treatment groups." However, the method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Biesbrock 2004.
| Methods | Title: a chewing gum containing 7.5% sodium hexametaphosphate inhibits stain deposition compared with a placebo chewing gum Trial design: cross‐over randomised controlled trial Location: USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 18 to 70 years old. Mean age: 39.1 years Total number: 20 Inclusion criteria:
Exclusion criteria:
Number randomised: 19 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 18 |
|
| Interventions | Total number of intervention groups: 2 (cross‐over) 7.5% sodium hexametaphosphate chewing gum Placebo Duration of treatment: 2 weeks with 78 hours washout time |
|
| Outcomes | Improvement in tooth shade: reduction in stain ΔL, a*, b* values were recorded. Increase in L and reduction in b indicated whitening |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Sodium hexametaphosphate delivered from a chewing gum prevents dental stain formation and facilitates stain removal, which leads to a perceptible whitening benefit" Correspondence required: no Contact: Dr Aron RB, Procter and Gamble Company, Healthcare Research Center, Manson, Ohio, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to one of two treatment groups." However, method was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Randomised, double‐blinded cross‐over trial." However, method was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Randomised, double‐blinded cross‐over trial." However, method was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 woman abandoned the study" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Bizhang 2007.
| Methods | Title: clinical trial of long‐term colour stability of hydrogen peroxide strips and sodium percarbonate film Trial design: randomised, placebo‐controlled trial Location: Berlin, Germany Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 18 to 60 years old, mean age: 30 years Total number: 72 Inclusion criteria:
Exclusion criteria:
Number randomised: 72 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: blinded test kits Number evaluated: 70 |
|
| Interventions | Total number of intervention groups: 3 6% hydrogen peroxide whitening strips 19% sodium percarbonate brush‐applied gel that dries to a film Placebo brush‐applied gel without peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade Tooth whitening was characterized by decreased b* (reduction in yellowness) and increased ΔL* (increased brightness) |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "6% hydrogen peroxide whitening strips yielded significant (P < 0.02) initial whitening relative to baseline, placebo and a 19% sodium percarbonate, brush‐applied film. All treatments were well‐tolerated, both peroxide‐containing systems exhibited appreciable color retention throughout the 18‐month post‐treatment period, and here were no meaningful, persistent adverse events seen with long‐term follow‐up" Correspondence required: no Contact: Dr Mozhgan Bizhang; mozhgan.bizhang@med.uni‐duesseldorf.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 72 subjects from the greater Berlin metropolitan area were randomised equally to the strip, film and placebo groups." However, the method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Products were dispensed in a blinded subject kit box" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "With the examiners still blinded as to treatment, these subjects (strip and film groups) were evaluated at post‐treatment months 12, 15, 16, and 18" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "With respect to subject disposition, 71 subjects completed the end‐of‐treatment visit, and 70 (97%) completed the month 6 …" Comment: 2 dropouts were noted. Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Botelho 2017.
| Methods | Title: a randomised controlled trial of home bleaching of tetracycline‐stained teeth Trial design: randomised, examiner‐blinded controlled trial Location: University of Hong Kong Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: tray group: mean age 28.7 years; strip group: mean age 30.4 years Total number: 36 Inclusion criteria:
Exclusion criteria:
Number randomised: 26 Method of randomisation: coin toss Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 24 (2 dropouts (1 for each group) at follow‐up) |
|
| Interventions | Total number of intervention groups: 2 Tray: 15% carbamide peroxide Strip: 6.5% hydrogen peroxide Duration of treatment: 3 months |
|
| Outcomes | Improvement in tooth colour a*, b* and ΔL were recorded Whitening benefit was represented by negative b* (yellowness reduction), and positive ΔL (increasing lightness) |
|
| Notes | Sample size calculation: done Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Both groups experienced noticeable and significant ΔL*a*b* improvement at the end of the trial in comparison to the baseline. Significant improvement was observed in the first month for the tray group and in the first 2 months for the strip group (P < 0.05). While greater lightness improvement was observed in the tray group over the strip group in the first month, the opposite was noticed in the second month. There was no difference between 2 groups at the end of this trial and no adverse reactions were observed" Correspondence required: no Contact: Dr Botelho MG; botelho@hku.hk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to either group….. tossing coin" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Participants were clinically review by one reviewer who was blinded to their treatment." However, method of blinding is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Participants were clinically review by one reviewer who was blinded to their treatment." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "..each group had 1 participant that did not attend 2 months review" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Browning 2008.
| Methods | Title: comparison of traditional and low sensitivity whiteners Trial design: double‐blinded, placebo‐controlled clinical trial Location: Department of Restorative Dentistry, Indiana University School of Dentistry, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: adults Total number: 91 Inclusion criteria:
Exclusion criteria:
Number randomised: 91 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 91 |
|
| Interventions | Total number of intervention groups: 5 Experimental 1: 10% carbamide peroxide Experimental 2: 10% carbamide peroxide, 3% potassium nitrate Experimental 3: 10% carbamide peroxide, 0.5% potassium nitrate Experimental 4: 10% carbamide peroxide, 0.5% potassium nitrate, 0.25% sodium fluoride Group 5: placebo Duration of treatment: 11 weeks |
|
| Outcomes | Change in tooth shade. Vita shade guide lightest C1 (16) to B4 (1) | |
| Notes | Sample size calculation: not reported Adverse effects: tooth, hard and soft tissue sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Participants using one of the two whiteners with 0.5% potassium nitrate had sensitivity levels equivalent to those using the placebo. Relative to the whitening agent with no desensitising agent, the addition of 0.5% potassium nitrate resulted in a significant reduction in the number of days of sensitivity experienced by participants. When compared to the whitener without any potassium nitrate, the addition of 3% potassium nitrate did not result in a significant reduction in the number of days of sensitivity. The addition of potassium nitrate did not result in any significant change in bleaching efficacy" Correspondence required: no Contact: William D Browning; lewis46@iupui.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "placebo‐controlled, double‐blind randomised clinical trial compared..." However, method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Neither the participant nor the operator was aware which material was received." However, method is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Neither the participant nor the operator was aware which material was received." However, method is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts are mentioned |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Bruhn 2012.
| Methods | Title: vital tooth whitening effects on oral health–related quality of life in older adults Trial design: single‐blinded, randomised, pre–test, multiple post–test design Location: Hampton Roads area of Virginia, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 50 years old and above Total number: 62 Inclusion criteria:
Exclusion criteria:
Number randomised: 62 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: participants were assigned a number after being randomly assigned to a group Number evaluated: 53 |
|
| Interventions | Total number of intervention groups: 2 Control: placebo Experimental: 14% hydrogen peroxide Duration of treatment: 3 weeks |
|
| Outcomes | Trubyte New Hue Vitality Scale: 12 shades numbered from 1 to 12, with 1 being the lightest and 12 being the darkest Tooth Colour Satisfaction Scale (TCSS): very satisfied (5 points), satisfied (4 points), neither satisfied nor dissatisfied (3 points), dissatisfied (2 points) and very dissatisfied (1 point) Oral Health Impact Profile (OHIP): 5‐point Likert scale: very often (5 points), fairly often (4 points), occasionally (3 points), hardly ever (2 points), never (1 point) Additional Questions Survey (AQS): none (1 point), 1 to 2 (2 points), 3 to 4 (3 points), 5 to 6 (4 points) and 7 or more (5 points) |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: reported Key conclusions of the study authors: "The older adults who whitened their teeth experienced an increased satisfaction with their tooth colour as evidenced by the TCSS. Tooth whitening was not associated with improvements in overall OHRQoL, or its functional factors, psychological disabilities, psychological discomforts, physical disabilities and social disabilities subscales. Tooth whitening did affect the handicap subscale, which demonstrated that persons who experienced tooth whitening were more willing to work due to a perceived increase in health. Tooth whitening did affect the physical pain subscale, which demonstrated a lower OHRQoL for participants. Older adults who whitened their teeth reported fewer social activities 3 months after the initial post–testing. Regression analysis relating tooth colour satisfaction with overall OHRQoL revealed a significant correlation between tooth colour satisfaction and overall OHIP for the experimental group" Correspondence required: no Contact: Ann M Bruhn; abruhn@odu.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "62 participants 50 years of age and older were enrolled and randomly assigned to 1 of 2 groups by research assistants." However, the method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinicians collecting data were unaware of participant group status, since the participants were assigned a number after being randomly assigned to" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 53 participants completed the study" Comment: 9 dropouts were noted. Reason for dropouts not mentioned |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Brunton 2004.
| Methods | Title: a 6‐month study of 2 self‐applied tooth whitening products containing carbamide peroxide Trial design: double‐blinded, randomised, controlled, parallel‐group clinical trial Location: not reported Language: English Number of centres: not reported Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: 18 to 70 years old Total number: 95 Inclusion criteria:
Exclusion criteria:
Number randomised: 95 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 93, 2 dropouts |
|
| Interventions | Total number of intervention groups: 2 18% (Group 1) carbamide peroxide 16.4% (Group 2) carbamide peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade The shade guide was arranged with the 16‐shade tabs in order from B1 (1) to C4 (16) Gingival score: Loë and Silness Gingival Index Gingival and teeth sensitivity score: 0 none ‐ 5 severe |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Both products effectively whitened teeth with a treatment time of 2‐weeks. The different concentrations tested were equally effective in improving whiteness. The whitening systems tested produced little tooth or gingival sensitivity. Some whitening benefit is sustained for at least 6 months after cessation of treatment" Correspondence required: no Contact: Paul A Brunton; paul.brunton@man.ac.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 subjects failed to complete the 2‐week study (1 from each group) for reasons unrelated to the study" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Cibirka 1999.
| Methods | Title: clinical study of tooth shade lightening from dentist‐supervised, patient‐applied treatment with 2 10% carbamide peroxide gels Trial design: randomised double‐blinded study Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Ultradent |
|
| Participants | Participants: 18 years old and above Total number: not reported Inclusion criteria: not reported Exclusion criteria:
Number randomised: not reported Method of randomisation: randomisation table Method of allocation concealment: not mentioned Method of blinding: not reported Number evaluated: 66 |
|
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide from 2 different brands Duration of treatment: 2 weeks |
|
| Outcomes | Change in tooth colour Evaluation of colour for the 6 maxillary anterior teeth was done using a Vita shade guide at baseline, 1, 2, and 4 weeks Vita shade guide arranged in the order of lightness: C1 (1) to B4 (16) |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "The test revealed no statistically significant difference between group for lightening the teeth. The colour change was still significant after 2 weeks without further bleaching activity. The baseline evaluation of the maxillary incisors and canines for all subjects, regardless of group, demonstrated a significant shade difference, with the canines being darker. This difference was not seen after 2 weeks of active bleaching or at the 4‐week evaluation" Correspondence required: no Contact: Roman M Cibirka; rcibirka@mail.mcg.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician created a randomization table for all subjects and maintained subject identification" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "..outside of the box and the individual syringes were labelled with the subject number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised double‐blinded study." However, the method is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of subjects randomised to both groups (n = 32 in both groups) explained in the methodology does not match the numbers shown in the table (n = 32 and n = 34) |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Collins 2004a.
| Methods | Title: clinical evaluation of a novel whitening gel, containing 6% hydrogen peroxide and a standard fluoride toothpaste Trial design: examiner‐blinded, stratified, parallel‐design clinical trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported |
|
| Participants | Participants: 18 to 63 years old. Mean age 39.45 years Total number: 128 Inclusion criteria:
Exclusion criteria:
Number randomised: 128 Method of randomisation: stratified randomisation Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 117 |
|
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide paint‐on gel Placebo: non‐whitening toothpaste Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth colour Vita shade guide: C1: lightest (1 rank) – B4: darkest (16 rank) |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "The self‐applied tooth‐whitening gel containing 6% hydrogen peroxide has been shown to significantly improve the whiteness of teeth after 1 and 2 weeks of product use, compared to the baseline and the toothpaste only group" Correspondence required: no Contact: Dr Luisa Collins; luisa.z.collins@unilever.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were stratified based on their gender and age and randomly assigned." However, the method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "examiner‐blinded, stratified, parallel‐design clinical trial." However, method is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "examiner‐blinded, stratified, parallel‐design clinical trial." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ".. from the 11 subjects that failed to complete the clinical trial" Comment: missing of outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Costa 2012.
| Methods | Title: comparison of 2 at‐home whitening products of similar peroxide concentration and different delivery methods Trial design: randomised, single‐blinded, split‐mouth design Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Ultradent |
|
| Participants | Participants: 21 to 75 years old Total number: 25 Inclusion criteria:
Exclusion criteria:
Number randomised: 25 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 24 |
|
| Interventions | Total number of intervention groups: 2 35% carbamide peroxide 14% hydrogen peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade: a*. b* and L were recorded. Whitening benefit was represented by negative b (yellowness reduction), and positive ΔL (increasing lightness) Sensitivity: VAS scale: 1 no pain, 10 severe pain |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "There was no significant difference in tooth colour change between carbamide peroxide and hydrogen peroxide at either time point. By the end of the study no participants reported tooth and gingival sensitivity. Participants preferred CP over HP" Contact: Dr D Costa; dacostaj@ohsu.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised, single‐blinded, split‐mouth design clinical study." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomised, single‐blinded, split‐mouth design clinical study." However, method is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised, single‐blinded, split‐mouth design clinical study." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 25 participants enrolled and 24 completed the study" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Cronin 2005.
| Methods | Title: comparison of 2 over‐the‐counter tooth whitening products using a novel system Trial design: observer‐blinded, parallel‐group randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: sponsored by Pfizer |
|
| Participants | Participants: 18 years old and above Total number: 60 Inclusion criteria:
Exclusion criteria:
Number randomised: 60 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 59 |
|
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide 18% carbamide peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade: shade assessment by VITAPAN C4 ‐1 to B1 – 16 ΔL, a*, b* were recorded (b: decreased b* indicates reduced yellowness; ΔL: increased ΔL is increased brightness) |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Both the treatment showed significant improvement in tooth shade. 6% hydrogen peroxide showed better improvement when compared to carbamide peroxide" Correspondence required: no Contact: Martin J Cronin, Director Dental Research, New Institutional Service Co, Northfield, New Jersey, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Qualifying subjects were stratified and randomly assigned to 1 of the 2 test products." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "examiner‐blinded, randomised, parallel‐group study." However, method is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "examiner‐blinded, randomised, parallel‐group study." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 60 enrolled in study with 59 completing the study" Comment: plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Delgado 2007.
| Methods | Title: tooth‐whitening efficacy of custom tray‐delivered 9% hydrogen peroxide and 20% carbamide peroxide during daytime use: a 14‐day clinical trial Trial design: double‐blinded, randomised controlled clinical trial Location: University of Puerto Rico Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate |
|
| Participants | Participants: 25 to 64 years of age Total number: 46 Inclusion criteria:
Exclusion criteria:
Number randomised: 46 Method of randomisation: random list Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 37 |
|
| Interventions | Total number of intervention groups: 2 Group I: Colgate Visible White containing 9% hydrogen peroxide (9% HP) Group II: Opalescence containing 20% carbamide peroxide (20% CP) Duration of treatment: 30 minutes for 2 weeks |
|
| Outcomes | Improvement in tooth shade The shade guide tabs were arranged B1 to C4 representing the 1 to 16 scale |
|
| Notes | Sample size calculation: not reported Adverse effects: gingival irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Both 9% hydrogen peroxide and 20% carbamide peroxide products effectively whitened teeth after 5, 7 and 14 days of once‐a day 30‐minute applications. 9% hydrogen peroxide produced a statistically significant tooth shade improvement compared to the tooth whitening effect of 20% carbamide peroxide after 5 days of product use. Colgate Visible White 9% hydrogen peroxide and Opalescence (20% carbamide peroxide) had a similar whitening effect after 7 and 14 days of use. Both tooth whitening products tested produced little tooth sensitivity or gingival irritation" Contact: Evaristo Delgado; edelgadoc@yahoo.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were assigned following a random list to 1 of the 2 treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomised, single‐centre, parallel‐group, double‐blinded clinical trial." However, method is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised, single‐centre, parallel‐group, double‐blinded clinical trial." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "9 dropouts noted" Comment: 7 failed to keep up with study visits. Unbalanced number in both groups (20% CP: n=16 and 9% HP: n = 21). We are not sure if plausible effect size (difference in means ) among missing outcomes may have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Ferrari 2007.
| Methods | Title: daytime use of a custom bleaching tray or whitening strips: initial and sustained colour improvement Trial design: randomised, parallel, examiner‐blinded study Location: Livorno, Italy Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 19 to 56 years old. Mean age 32.8 years Total number: 43 Inclusion criteria:
Exclusion criteria: not reported Number randomised: not reported Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: blinded kit boxes Number evaluated: 36 |
|
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide strips 10% carbamide peroxide tray Duration of treatment: 2 weeks |
|
| Outcomes | Colour improvement a*. b* and ΔL were recorded Whitening benefit was represented by negative b* (yellowness reduction), and positive ΔL (increasing lightness) |
|
| Notes | Adverse effects: tooth sensitivity and gingival irritation Health‐related quality of life: not reported Key conclusions of the study authors: "The strip system yielded significant reduction in yellowness compared to the custom tray, at both end‐of‐treatment and post‐treatment monitoring. Compared to Week 2, the strip group retained 89%‐92% of the initial colour improvement at Week 6 (4 weeks post‐treatment), while the tray group had 80%‐90%. Both daytime treatments were well‐tolerated, with minor tooth sensitivity and oral irritation representing the most common findings" Correspondence required: no Contact: Professor Dr Marco Ferrari; ferrarimar@unisi.it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised, parallel, examiner‐blinded study." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Because of the dissimilar delivery systems, test products were supplied in blinded kit boxes" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised, parallel, examiner‐blinded study." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "6 subjects (4 in the strip group and 2 in the tray group) missed the 2‐week visit, while 1 additional subject (in the tray group) missed the.." Comment: 7 dropouts were reported. HP strips group n = 17 and CP tray group n = 19. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gallo 2009.
| Methods | Title: evaluation of 30% carbamide peroxide at‐home bleaching gels with and without potassium nitrate Trial design: double‐blinded, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Dent Mat |
|
| Participants | Participants: adults Total number: 40 Inclusion criteria:
Exclusion criteria:
Number randomised: 40 Method of randomisation: not mentioned Method of allocation concealment: not mentioned Method of blinding: not reported Number evaluated: 40 |
|
| Interventions | Total number of intervention groups: 2 30% carbamide peroxide with 5% potassium nitrate in tray 30% carbamide peroxide in tray Duration of treatment: 1 hour per day for 10 days |
|
| Outcomes | Change in tooth shade: Bioform Colour Ordered Shade Guide System: B85 darkest (1) to B59 lightest (24) Sensitivity of tooth: 0 to 10 scale (0: no pain, 10: severe) Gingival irritation: 0 normal, 1 mild, 2 moderate, 3 severe |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity and gingiva irritation Health‐related quality of life: not reported Key conclusions of the study authors: "30% carbamide bleaching gels effectively whiten teeth without causing a significant increase in tooth sensitivity or changes in gingival condition. Potassium nitrate has little effect in sensitivity when treatment time is short" Correspondence required: no Contact: Dr John Gallo; jgallo@lsuhsc.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "this double‐blinded, randomised clinical study evaluated the effectiveness..." However, method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Neither subjects nor evaluators knew which bleaching gel (treatment A….. was used.." However, method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Neither subjects nor evaluators knew which bleaching gel (treatment A….. was used.." However, method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Garcia‐Godoy 2004.
| Methods | Title: placebo‐controlled, 6‐week clinical trial on the safety and efficacy of a low‐gel, 14% hydrogen‐peroxide whitening strip Trial design: parallel‐group, double‐blinded, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: consenting adults, mean age: 32.1 years Total number: 39 Inclusion criteria:
Exclusion criteria:
Number randomised: 39 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 35. 4 dropouts, 2 from each group |
|
| Interventions | Total number of intervention groups: 2 Experiment: 14% hydrogen peroxide Control: placebo Duration of treatment: 3 weeks |
|
| Outcomes | Improvement in tooth colour b*: decreased b* indicates reduced yellowness ΔL: increased ΔL is increased brightness ΔW: negative W indicates colour closer to white |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Twice‐daily use of Crest Whitestrips Supreme resulted in a highly significant improvement in tooth colour after 3 weeks, with colour improvement continuing over 6 weeks" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised double‐blinded parallel‐group clinical trial. Eligible subjects were randomised to a low gel 14% ...or placebo group." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomised double‐blinded parallel‐group clinical trial." However, method of blinding is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised double‐blinded parallel‐group clinical trial." However, method of blinding is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 subjects 1 in each group discontinued treatment because of a treatment related adverse event" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2000.
| Methods | Title: a randomised clinical trial comparing a novel 5.3% hydrogen peroxide whitening strip to 10%, 15%, and 20% carbamide peroxide tray‐based bleaching system Trial design: randomised controlled, examiner‐blinded, parallel‐group clinical trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 24 to 57 years old. Mean age: 42.8 years Total number: 36 Inclusion criteria:
Exclusion criteria:
Number randomised: 36 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 32 |
|
| Interventions | Total number of intervention groups: 4 5.3% hydrogen peroxide strip 10% carbamide peroxide gel in tray 15% carbamide peroxide gel in tray 20% carbamide peroxide gel in tray Duration of treatment: 14 days, 2 hours per day |
|
| Outcomes | Improvement in tooth colour ΔL, a*, b* values were recorded. Increase in ΔL and reduction b* indicated whitening |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: ".. all groups experienced a greater than 1‐unit mean improvement in all parameters relative to baseline. For the primary study variable, reduction of yellow (delta b*) outcomes after 14 hours of using the experimental strip were comparable to those observed with the 10% tray group after 28 hours of use. These 2 treatment groups did not differ statistically with respect to any of the colour measurements used in this study. For the tray groups, there was a reasonable dose relationship for the primary endpoint, delta b*, with the 15% and 20% tray groups averaging 17% and 68% improvements in yellow, respectively, over the 10% group. Except for the 20% carbamide peroxide system, where sensitivity was relatively common, all test products were well tolerated" Correspondence required: no Contact: Dr Robert W Gerlach, 8700 Mason Montgomery Road, Mason OH, 45040 8006 USA; gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised controlled, examiner‐blinded, parallel group." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not Mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised controlled, examiner‐blinded, parallel group." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "4 participants missed day 14" Comment: missing outcome data balanced in numbers across intervention groups (n = 9 in HP strip and n = 8 in CP tray group) |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2002.
| Methods | Title: initial colour change and colour retention with a hydrogen peroxide bleaching strip Trial design: randomised double‐blinded design Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble. The authors are employees |
|
| Participants | Participants: 18 to 71 years old. Mean age: 40.9 years Total number: 57 Inclusion criteria:
Exclusion criteria:
Number randomised: 57 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: identically labelled and packaged, differing in appearance only as to a unique subject number Number evaluated: 52 completed study and 49 completed follow‐up |
|
| Interventions | Total number of intervention groups: 2 Experiment: 5.3% hydrogen peroxide – Crest white strips Control: placebo strips Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth colour b*: decreased b* indicates reduced yellowness ΔL: increased ΔL is increased brightness ΔE: overall whiteness |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and gingival irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Whitening strip group continuing to demonstrate improvements in tooth colour relative to baseline and placebo. Age was found to significantly contribute to initial colour improvement, with younger subjects experiencing a greater initial reduction in yellowness compared to older participants, but not to post‐treatment colour retention. The whitening strips were well tolerated, with minor tooth sensitivity and oral irritation representing the most common findings during treatment. There were no persistent or new treatment‐related adverse events during the 6‐month monitoring" Contact: Dr Robert W Gerlach, 8700 Mason Montgomery Road, Mason OH, 45040 8006 USA; gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised double‐blinded placebo‐controlled study.. subjects were randomised to either the whitening strip or placebo strip groups." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All products in this double‐blinded clinical trial were identically labelled and packaged, differing in appearance only as to a unique subject number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 57 enrolled subjects, 52 completed bleaching treatment, while 49 completed the 6‐month study" Comment: placebo group has 29 participants while hydrogen peroxide group has 23 participants. In spite of this difference, the results favour the intervention group. Hence we presume that plausible effect size (difference in means) among missing outcomes may not have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described are reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2002a.
| Methods | Title: comparative clinical efficacy of 2 professional bleaching systems Trial design: randomised, parallel, examiner‐blinded clinical trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 22 to 59 years old, mean age 38.25 years Total number: 20 Inclusion criteria: no history of previous bleaching Exclusion criteria: not reported Number randomised: 20 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: uniquely numbered subject identification label Number evaluated: 20 |
|
| Interventions | Total number of intervention groups: 2 (tray versus strip) Hydrogen peroxide 10%: 30 minutes twice daily Hydrogen peroxide 6.5% + carbamide peroxide 10%: 2 hours once daily Duration of treatment: 14 days |
|
| Outcomes | Improvement in tooth shade. Colour change (ΔL*, Δa*, and Δb*) was determined by comparing each post‐treatment visit to baseline. Negative Δb* (reduction in yellowness) and positive ΔL* (increased brightness) were considered to be indicative of a whitening benefit |
|
| Notes | Sample size calculation: not reported Adverse effects: 1 patient had mild irritation at the tip of tongue in strip group Health‐related quality of life: not reported Key conclusions of the study authors: "Under the conditions tested, this clinical trial demonstrates that the 14‐contact‐hour treatment with the strip system resulted in superior whitening efficacy compared with the 28‐contact‐hour treatment with the tray system" Contact: Dr Robert W Gerlach; gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised, examiner‐blinded, clinical trial." However, method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Test products were over labelled with a uniquely numbered subject identification label" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised, examiner‐blinded, clinical trial." However, method is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 20 subjects completed the 14‐ day treatment and were considered eligible for evaluation for all analyses" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2002b.
| Methods | Title: comparative response of whitening strips to a low peroxide and potassium nitrate bleaching gel Trial design: randomised, examiner‐blinded clinical trial. 2 arms Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: mean age of 37 years Total number: 34 Inclusion criteria: be willing to have their teeth whitened Exclusion criteria:
Number randomised: 34 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not repeated Number evaluated: 32 |
|
| Interventions | Total number of intervention groups: 2 5% carbamide peroxide bleaching gel + potassium nitrate in custom tray: once daily application 6% hydrogen peroxide bleaching strip: twice daily application Duration of treatment: 7 days |
|
| Outcomes | Improvement in tooth shade ΔL, a*, b* were recorded. Increase in ΔL and decrease in b* indicates whitening |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Twice daily application of 6% hydrogen peroxide strip resulted in better whitening compared to 1 daily application of 5% carbamide peroxide. Sensitivity was less with 6% hydrogen peroxide" Contact: gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study subjects were randomly assigned to 1 of 2 groups." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomised examiner‐blinded clinical study." However, method is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised examiner‐blinded clinical study." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 subjects missed the day 7 visit" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2003.
| Methods | Title: randomised clinical trial comparing overnight use of 2 self‐directed peroxide tooth whiteners Trial design: blinded randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: mean age 40.3 years Total number: 57 Inclusion criteria:
Exclusion criteria:
Number randomised: 57 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: test products were over packaged in kit boxes that were labelled only with a unique subject identification number to assure blinding Number evaluated: 56, 1 dropout |
|
| Interventions | Total number of intervention groups: 2 Crest Night Effects 19% sodium percarbonate Colgate Simply White Night 8.7% hydrogen peroxide Duration of treatment: 14 nights |
|
| Outcomes | Improvement in tooth colour b*: decreased b* indicates reduced yellowness ΔL: increased ΔL is increased brightness ΔW: negative ΔW indicates colour closer to white |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "After 14 nights treatment, adjusted mean (SE) change in yellowness (delta b*) was ‐0.95 (0.092) for the 19% sodium percarbonate film and ‐0.17 (0.096) for the 8.7% hydrogen peroxide gel, with these groups differing statistically (P < 0.0001). Other individual and composite colour parameters also demonstrated significantly greater whitening for the 19% sodium percarbonate film compared to the 8.7% hydrogen peroxide gel after 14 nights use. Only the 19% sodium percarbonate film exhibited significant (P < 0.0001) proximal colour improvement (delta b*) after 2 weeks, approximately 98% of that seen on the body of the tooth, providing evidence of proximal bleaching and uniform spatial whitening following use of this barrier‐free system. Both products were well‐tolerated, with no subjects discontinuing treatment early due to a causal adverse event PMID: 15055983 Contact: Dr Robert W Gerlach, Procter and Gamble Company, 8700 Mason Montgomery Road, Mason OH 45040 8006 USA; gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Blinded randomised controlled trial." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Test products were over packaged in kit boxes that were labelled only with a unique subject identification number to assure blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 subject discontinued the study" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2004.
| Methods | Title: clinical trial comparing 2 daytime hydrogen‐peroxide professional vital‐bleaching systems Trial design: randomised, examiner‐blinded clinical trial Location: USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: adults aged 18 to 64 Total number: 31 Inclusion criteria:
Exclusion criteria:
Number randomised: 31 Method of randomisation: not reported Method of allocation concealment: unclear Method of blinding: unclear Number evaluated: 29 |
|
| Interventions | Total number of intervention groups: 2. 14% hydrogen peroxide whitening strips 9.5% hydrogen peroxide custom‐tray–based system Duration of treatment: 22 days |
|
| Outcomes | Improvement in tooth colour: ΔL, a*, b* values were recorded. Increase in L and reduction b* indicated whitening | |
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritations were the most common side effects. 28% of the patients reported 1 or both. Occurrence of either sensitivity or irritation was 13% in strip group, 56% in tray group Health‐related quality of life: not reported Key conclusions of the study authors: "At the end of the treatment, the 14% hydrogen peroxide whitening strips caused 2‐fold reduction in yellowness and better in‐use tolerability when compared to 9.5% hydrogen peroxide custom‐tray system" Correspondence required: no Contact: gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomised, examiner‐blinded clinical trial..." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "... which had been packed into a blinded kit box labelled with a unique subject number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The randomised, examiner‐blinded clinical trial..." However, method of blinding is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 subject in each group did not return for the Day 22 visit" Comment: plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2004e.
| Methods | Title: placebo‐controlled clinical trial evaluating a 10% hydrogen peroxide whitening strip Trial design: randomised, double‐blinded, placebo‐controlled trial Location: University of Florida, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 18 to 50 years old. Mean age: 38.8 years Total number: 39 Inclusion criteria:
Exclusion criteria: not reported Number randomised: 39 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: similar white foil packing with unique ID Number evaluated: 34. 2 dropouts on 8th day of evaluation and 3 for all parts of study |
|
| Interventions | Total number of intervention groups: 2 10% hydrogen peroxide whitening strips Placebo Duration of treatment: 7 days |
|
| Outcomes | Improvement in tooth colour b*: decreased b* indicates reduced yellowness ΔL: increased ΔL is increased brightness |
|
| Notes | Sample size calculation: not reported Adverse effects: oral irritation and tooth sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Statistically significant tooth whitening was evident after 3 days' treatment with 10% hydrogen peroxide whitening strips, and colour improved with continued usage over 7 days" Contact: Dr Robert W Gerlach; gerlach.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised double‐blinded, placebo‐controlled clinical trial was conducted... Groups were randomly assigned to treatment based on age and baseline tooth colour." No further details are given |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each strip was packaged in an individual white foil pouch, with the subject identification number... Strips were over packaged in a kit box,.." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "3 subjects (1 in the 10% group and 2 in placebo group) failed all or pat of the day 8 evaluation" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned are reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Gerlach 2005.
| Methods | Title: clinical trial comparing 2 hydrogen peroxide tooth whitening systems: strips versus pre‐rinse Trial design: randomised, examiner‐blinded, parallel‐group trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 29 to 58 years old. Mean age 39.8 years Total number: 28 Inclusion criteria:
Exclusion criteria: not reported Number randomised: 28 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 28 |
|
| Interventions | Total number of intervention groups: 2 Mouthwash: 2% hydrogen peroxide Strip: 10% hydrogen peroxide Duration of treatment: 7 days |
|
| Outcomes | Improvement in tooth shade a*, b* and ΔL were recorded Whitening benefit was represented by negative b* (yellowness reduction), and positive ΔL (increasing lightness) |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Relative to baseline, the whitening strip group experienced colour improvement at day 3, continuing through day 8. The pre‐rinse group did not show any significant change at day 3, and had a significant increase in yellowness at day 8. The strip group exhibiting significantly greater whitening at day 8. Both products were well tolerated, with no participants discontinuing treatment early as the result of an adverse event. In head‐to‐head testing, 7‐day use of the 10% hydrogen peroxide whitening strips resulted in significant tooth colour improvement relative to a barrier‐free 2% hydrogen peroxide pre‐brushing mouthrinse" Correspondence required: no Contact: Dr Geralch; geralch.rw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...subjects were randomly assigned to 1 of 2 test groups." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Examiner‐blinded, parallel‐group, randomised controlled trial." However, method of blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Examiner‐blinded, parallel‐group, randomised controlled trial." However, method of blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the participants completed all the visits and were evaluated" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Giniger 2005.
| Methods | Title: a 180‐day clinical investigation of the tooth whitening efficacy of a bleaching gel with added amorphous calcium phosphate Trial design: double‐blinded randomised controlled trial Location: USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Discus Dental, Culver City, USA |
|
| Participants | Participants: continuation of a previously published study, 27 agreed to continue. Mean age 46.4 years Total number: 27 Inclusion criteria:
Exclusion criteria: not reported Number randomised: 27 Method of randomisation: stratified randomisation schedule Method of allocation concealment: secret coding Method of blinding: identical pack Number evaluated: 27 |
|
| Interventions | Number of groups: 2 Control: 16% carbamide peroxide Experimental: 16% carbamide peroxide with 0.5% soluble calcium phosphate derived in part from calcium nitrate and potassium pyrophosphate Duration of treatment: 19 days |
|
| Outcomes | Improvement in tooth shade: Vita shade guide C1 (1) to B4 (16) Gingival Index score: 0 (no gingivitis) to 3 (severe gingivitis) Dentinal hypersensitivity: 0 (no pain) to 3 (pain during application of stimuli and immediately there after) VAS scale: 0 (no pain) to 10 (highest level of pain) |
|
| Notes | Adverse effects: none Key conclusions of the study authors: "This study demonstrated that the ACP product offers 10% better long‐term (6‐months) whitening efficacy that the traditional bleaching gel. No significant adverse effect. Tooth sensitivity, soft tissue health, and gingival health remained similar to baseline levels" Contact: Dr Giniger; mginiger@mg‐co.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " ..we randomised subjects into test and control groups, according to a stratified randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "The evaluator of the teeth shades and sensitivity scores (MG) was blinded to this schedule.. other than a secret product code number known only to a co‐worker" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The evaluator of the teeth shades and sensitivity scores (MG) was blinded to this schedule, and the products looked identical to him and to the subjects" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluator of the teeth shades and sensitivity scores (MG) was blinded to this schedule, and the products looked identical to him and to the subjects" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 27 requalified subjects completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Hannig 2007.
| Methods | Title: efficacy and tolerability of 2 home bleaching systems having different peroxide delivery Trial design: randomised, single‐blinded, parallel‐group trial Location: University of Göttingen, Germany Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 18 to 60 years. Mean age 29.35 years Total number: 47 Inclusion criteria:
Exclusion criteria:
Number randomised: 47 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 43 (at 2 weeks) |
|
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide strips 10% carbamide peroxide gel in tray Duration of treatment: 14 days |
|
| Outcomes | Tooth shade improvement: ΔL, a*, b* values were recorded. Increase in L and reduction on b* Patient satisfaction: to 10 (0 = no whitening, 10 = maximal satisfying whitening effect) |
|
| Notes | Sample size calculation: not reported Adverse effects: transient tooth sensitivity and oral soft tissue irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Both whitening systems showed significant tooth colour improvement after 2 weeks of use.... No statistical significant difference was observed between the 2 systems..... Both systems were well tolerated and caused comparable levels of transient tooth sensitivity and oral soft tissue irritation" Correspondence required: no Contact: Christian Hannig, christian.hannig@uniklinik‐feriburg.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "this single‐blinded, randomised, parallel‐group study...... Subjects were stratified according to the baseline anterior maxillary tooth brightness (L*) as determined by digital image analysis system and by the criteria of smoker/non‐smoker. Randomisation to treatment was performed within each strata." However, method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "this single‐blinded, randomised, parallel‐group study." However, method not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "this single‐blinded, randomised, parallel‐group study." However, method not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "5 subjects dropped out of the study due to reasons not related to bleaching therapy at different stages. 2 subjects from the WS [strips] group withdrew after 5 days of treatment because of product‐related side effects" Comment: number of participants at 2 weeks: 22 in tray group and 21 in strip group. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Hasturk 2004.
| Methods | Title: efficacy of a fluoridated hydrogen peroxide‐based mouthrinse for the treatment of gingivitis Trial design: randomised, double‐blinded, placebo‐controlled, parallel‐group trial Location: Boston Medical Center, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Dent Mat |
|
| Participants | Participants: 18 to 50 years old Total number: 110 Inclusion criteria:
Exclusion criteria:
Number randomised: 99 Method of randomisation: random numbers chart Method of allocation concealment: reported Method of blinding: similar coded bottle Number evaluated: 78 |
|
| Interventions | Total number of intervention groups: 2 Hydrogen peroxide + 0.05% sodium fluoride mouthrinse Placebo Duration of treatment: 30 seconds twice daily for 6 months |
|
| Outcomes | Plaque Index Modified Gingival Index Bleeding on probing dichotomous (1 or 0) Intensity of stain: dichotomous (1 if lightened or if no change) |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Subjects using the test rinse were also 6 times more likely to exhibit an improvement in tooth colour after 6 months than were subjects using placebo. As a result of the clinical evaluations and microbial analysis, test mouthrinse was found to be safe during a 6‐month period" Contact: Thomas E Van Dyke, tvandyke@bu.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment was assigned by use of random number charts, and subject assignment was made by an individual who was not involved in the treatment or measurement procedures" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment was assigned by use of random number charts, and subject assignment was made by an individual who was not involved in the treatment or measurement procedures" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "The placebo rinse was an identical base formulation to the test mouthrinse...;" " Mouthrinses were labelled to conform with prescribing regulations and were coded to maintain double masking ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All clinical measurements were performed under the same conditions by the same investigator who was blinded to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "Each subject was considered as the unit of analysis. Therefore, subject who completed at least 1 post‐baseline clinical assessment visit were included in the intent‐to‐treat population;" "99 subjects were randomised, and 78 subjects completed the whole course of the study. 38 subjects were in placebo group and 40 in the test group" Comment: no details of reasons for dropouts are given |
| Selective reporting (reporting bias) | Low risk | All outcomes have been reported adequately. Conclusions conform to the results obtained |
| Other bias | Low risk | None |
Hyland 2015.
| Methods | Title: a new 3‐component formulation for the efficient whitening of teeth (Carbamide Plus) Trial design: randomised, double‐blinded, placebo‐controlled, clinical trial Location: Eastman Dental Hospital, UK Language: English Number of centres: 1 Recruitment period: not reported Funding source: SMT Ltd and DEL CAST studentship |
|
| Participants | Participants: adults Total number: 33 Inclusion criteria: not reported Exclusion criteria:
Number randomised: 32 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 32 |
|
| Interventions | Total number of intervention groups: 3 5% carbamide peroxide gel 10% carbamide peroxide gel Placebo Duration of treatment: 2 hours per day for 2 weeks |
|
| Outcomes | Improvement in tooth colour ΔL, a*, b*: increase in ΔL and reduction in b* indicates whitening |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "A new tooth‐whitening product Carbamide Plus containing urea, hydrogen peroxide, and STPP as active components containing 5% hydrogen peroxide has been shown to be as effective as the commercially available carbamide peroxide containing 10% hydrogen peroxide. There were no statistically significant differences between Carbamide Plus and 10% carbamide peroxide in tooth whitening at 2 weeks following daily wear of tooth whitening trays for 2 hours per day" Correspondence required: no Contact: BW Hyland; j.callan@ulster.ac.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The recruited subjects were randomly allocated to 1 of 3 study groupings: non‐active placebo gel..." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomised, double‐blinded, placebo‐controlled clinical trial." However, method is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised, double‐blinded, placebo‐controlled clinical trial." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Karpinia 2002.
| Methods | Title: vital bleaching with 2 at‐home professional systems Trial design: randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 18 to 65 years old. Mean age 37.2 years Total number: 69 Inclusion criteria:
Exclusion criteria:
Number randomised: 69 Method of randomisation: randomised in blocks of 4 Method of allocation concealment: not reported Method of blinding: unique coded label Number evaluated: 67 |
|
| Interventions | Total number of intervention groups: 2 6.5% hydrogen peroxide strips 10% carbamide peroxide in tray Duration of treatment: 30 minutes twice daily for 3 weeks |
|
| Outcomes | Improvement in tooth shade: ΔL, a*, b* values were recorded. Increase in ΔL and decrease in b* indicates whitening | |
| Notes | Sample size calculation: not reported Adverse effects: oral and gingival irritation Health‐related quality of life: not reported Key conclusions of the study authors: "For between group comparisons, strip subjects had a statistically significant or directionally favourable whitening response relative to the tray system at intermediary time points, while at the end of treatment, the strip group had highly statistically significant (P < or = 0.005), superior whitening response for all colour parameters measured in the study. Both treatments were generally well tolerated, with 35% to 40% of the subjects in each group reporting minor tooth sensitivity or gingival irritation" Correspondence required: no Contact: KA Karpinia, University of Florida, Gainesville, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study population was randomised in blocks of 4" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each product was over labelled with a unique subject identification number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 patients from tray group and 1 patient from tray and strip group missed the evaluation group at 7 days and 3 weeks respectively" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Kihn 2000.
| Methods | Title: a clinical evaluation of 10% versus 15% carbamide peroxide tooth whitening agents Trial design: randomised, double‐blinded study Location: University of Maryland Dental School, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Dentsply |
|
| Participants | Participants: 18 to 65 years old Total number: 57 Inclusion criteria: maxillary anterior teeth of shade A3 or darker Exclusion criteria: not reported Number randomised: 56 Method of randomisation: reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 52 |
|
| Interventions | Total number of intervention groups: 2 Experiment: 15% carbamide peroxide gel Control: 10% carbamide peroxide gel Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth colour Sensitivity: 0 (no pain) to 20 (severe) |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "There was a significant difference in colour change between the 10% CP and 15% CP groups at the end of the study period. There was no significant difference in level of tooth sensitivity between the 2 groups, and the incidence was equal; there was, however, a significant difference in variability of tooth sensitivity between the 2 groups" Correspondence required: no Contact: Patricia W Kihn; tkihn@caulk.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "paired list of names was supplied to the manufacturer, which then randomly assigned 1 member of each pair" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "authors conducted a double‐blinded study of human subjects to..." However, method of blinding is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "authors conducted a double‐blinded study of human subjects to..." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 56 subjects who began the study, 26 pairs of matched subjects (n = 52 individual subjects) completed the study" Commnet: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Kose 2011.
| Methods | Title: clinical effects of at‐home bleaching along with desensitizing agent application Trial design: randomised, double‐blinded, controlled trial Location: University Estadua, Brazil Language: English Number of centres: 1 Recruitment period: not reported Funding source: FGM Dental Products |
|
| Participants | Participants: 18 to 30 years old. Mean age 24 years Total number: 60 Inclusion criteria:
Exclusion criteria:
Number randomised: 60 Method of randomisation: coin toss Method of allocation concealment: not reported Method of blinding: similar syringes Number evaluated: 60 |
|
| Interventions | Total number of intervention groups: 2 16% carbamide peroxide in tray + placebo 16% carbamide peroxide in tray + desensitizer gel (DG; 5% potassium nitrate and 2% sodium fluoride) Duration of treatment: 4 weeks |
|
| Outcomes | Improvement in tooth shade: Vita shade guide B1 (highest) to C4 (lowest) Tooth sensitivity: 0 ‐ none, 1 – mild, 2 ‐ moderate, 3 ‐ considerable, 4 ‐ severe |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "The use of desensitizing agent did not affect the bleaching efficacy of the CP. The prevalence and intensity of tooth sensitivity was similar for both groups. However, participants from the placebo group had sensitivity in 33.6% of the bleaching days, which was significantly higher than the desensitizing agent experimental group (20.1%)" Correspondence required: no Contact: Dr Kose, School of Dentistry, State University of Ponda, Santa Catarina, Brazil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly divided into experimental or control group by coin toss" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the subjects nor the evaluators knew to which group the subjects were assigned" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the subjects nor the evaluators knew to which group the subjects were assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 60 participants who began study completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Kowitz 1994.
| Methods | Title: comparative clinical evaluation of 2 professional tooth whitening products Trial design: single‐blinded, parallel, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: adults Total number: 50 Inclusion criteria: not reported Exclusion criteria: not reported Number randomised: 50 Method of randomisation: not reported Method of allocation concealment: reported Method of blinding: coded packs Number evaluated: 48 |
|
| Interventions | Total number of intervention groups: 2 10% urea peroxide from 2 different brands 1 hour application twice daily Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade Sensitivity Patient‐reported satisfaction |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Colgate Platinum was 62% more effective at tooth whitening after 1 week and 83% more effective after 2 weeks of treatment versus Rembrandt. At the termination of the study, the mean colour difference (deltaE) for Colgate Platinum was 4.29 and 2.34 for Rembrandt. Statistical analysis demonstrated that the Colgate product is significantly superior at increasing tooth whiteness, increasing tooth lightness, reducing redness, and reducing yellowness. In this study, no adverse reactions were noted on clinical examination and none were reported by panelists with normal healthy dentition" Correspondence required: no Contact: Dr Kowitz, University of California, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "single‐blinded, randomised, 2‐celled parallel clinical trial." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither the subject no the investigator were informed to which group the individual belonged" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each subject was assigned a coded test product...." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 dropped out because of problems unrelated to study" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Krause 2008.
| Methods | Title: subjective intensities of pain and contentment with treatment outcomes during tray bleaching of vital teeth employing different carbamide peroxide concentrations Trial design: double‐blinded, randomised controlled trial Location: university Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: mean age 31 years Total number: 30 Inclusion criteria:
Exclusion criteria: not reported Number randomised: 30 Method of randomisation: computer generated numbers Method of allocation concealment: not reported Method of blinding: unlabelled similar packs Number evaluated: 30 |
|
| Interventions | Total number of intervention groups: 3 10% carbamide peroxide tray 17% carbamide peroxide tray 0% carbamide peroxide tray Duration of treatment: 7 days |
|
| Outcomes | Pain sensation Patient contentment with bleaching: 0 no contentment, 100 maximum contentment |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: yes, patient satisfaction Key conclusions of the study authors: "Application of carbamide peroxide–containing bleaching agents to vital teeth causes pain correlated with the agent's concentration. Since both highly and less concentrated gels might result in a similar contentment with the treatment outcome, the use of highly concentrated agents appears not to be justified to improve vital tooth colour" Contact: Felix Krause, fkrause@uni‐bonn.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "kits were assigned to the patients employing computer‐generated random numbers by a different operator" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Unlabelled treatment kits numbered consecutively. Thus, both the operator and the patient were unaware of the..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Unlabelled treatment kits numbered consecutively. Thus, both the operator and the patient were unaware of the..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Kugel 2000.
| Methods | Title: tooth‐whitening efficacy and safety: a randomised and controlled clinical trial Trial design: randomised, single‐centre, double‐blinded, parallel‐group, placebo‐controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 18 to 66 years old. Mean age 33 years Total number: 70 Inclusion criteria:
Exclusion criteria:
Number randomised: 70 Method of randomisation: block randomisation Method of allocation concealment: not reported Method of blinding: identical package and examiners were blinded Number evaluated: 66. 4 dropouts |
|
| Interventions | Total number of intervention groups: 2 5.3% hydrogen peroxide gel delivered with polyethylene gel Placebo Duration of treatment: 2 weeks |
|
| Outcomes | Change in tooth shade: Vita shade guide tabs arranged according to lightness (B1 light to C4 dark) Oral soft and hard tissue examination Dental hypersensitivity Gingival Index: Loë and Silness Index Plaque Index: Silness and Loë Index |
|
| Notes | Sample size calculation: not reported Adverse effects: soft tissue irritation and dental hypersensitivity Health‐related quality of life: not reported Key conclusions of the study: "Peroxide gel‐treated group showed significant whitening of teeth. Both treatments were generally well tolerated. The strips offer ease of use, comfort, and shorter duration of wear compared with other at‐home bleaching systems" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was a randomised, placebo‐controlled, double‐blinded, parallel‐group study. Block randomisation was done" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Except for the presence of peroxide, test products were identical in composition and packaging" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All examinations were performed blinded as to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts in each group at the end of the 2‐week trial. It is unlikely that 4 dropouts could affect the overall results. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes discussed were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Kugel 2002.
| Methods | Title: daily use of whitening strips on tetracycline‐stained teeth: comparative results after 2 months Trial design: randomised controlled trial Location: Tufts University, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 22 to 70 years old Total number: 40 Inclusion criteria: adult patients with tetracycline stains Exclusion criteria: not reported Number randomised: 40 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: identical packages Number evaluated: 33 |
|
| Interventions | Total number of intervention groups: 2 6.5% hydrogen peroxide strips 10% carbamide peroxide tray Duration of treatment: 2 months |
|
| Outcomes | Improvement with tooth shade. Vita shade guide arranged from darkest to lightest (B4 to C1) Plus 2 additional shades of B4+ and C1+ |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "6.5% carbamide peroxide strips provided similar benefit to 10% carbamide peroxide used over 2 months period" Correspondence required: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised clinical trial." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Products were packed in 1 month kits, and all labelling was identical except for unique.." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "7 subjects withdrew from the treatment in the first month" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Li 2003.
| Methods | Title: comparison of clinical efficacy and safety of 3 professional at‐home tooth whitening systems Trial design: randomised, 3‐cell, parallel‐group, investigator‐blinded trial Location: university Language: English Number of centres: 1 Recruitment period: not reported Funding source: Discuss Dental |
|
| Participants | Participants: 23 to 67 years old Total number: 90 Inclusion criteria:
Exclusion criteria: not reported Number randomised: not reported Method of randomisation: randomisation scheme generated by a computer Method of allocation concealment: reported Method of blinding: reported Number evaluated: 82 |
|
| Interventions | Total number of intervention groups: 3 groups 6.5% hydrogen peroxide: 21 days, twice daily ‐ 30‐minute application 7.5% hydrogen peroxide: 18 days, twice daily ‐ 30‐minute application 16% carbamide peroxide: 21 days, overnight ‐ 30‐minute application Duration of treatment: 18 to 21 days based on the intervention |
|
| Outcomes | Improvement in tooth shade: Vitapan shade guide: B1 to C4 (lightest to darkest shade ranking) ΔL, a*, b* and ΔW using chromometre. Increase in L and W indicated whitening. Reduction in b* indicated whitening |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "All 3 systems were effective and safe. Nite White Excel (16% CP) resulted in significant greater shade reductions in periods between days 7, 14, or 21 and baseline than did the other 2 systems. Tooth sensitivity and gingival irritation was seen in all groups, but it was lower in the Nite White Excel group" Correspondence required: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Qualified subjects were divided into ….. using randomisation scheme generated by computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure blinding, the group assignment and product distribution was done by project coordinator who was not involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To ensure blinding, the group assignment and product distribution was done by project coordinator who was not involved in the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To ensure blinding, the group assignment and product distribution was done by project coordinator who was not involved in the study... while clinical examiner performed examination without knowledge of treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 5 subjects withdrew….3 did not show up.." Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Li 2004.
| Methods | Title: comparative tooth whitening efficacy of 18% carbamide peroxide liquid gel using 3 different regimens Trial design: stratified, double‐blinded, parallel‐group, randomised controlled trial Location: Loma Linda University, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Pamolive |
|
| Participants | Participants: 18 to 65 years old. Mean age 39.06 years Total number: 120 Inclusion criteria:
Exclusion criteria:
Number randomised: 120 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 104. 16 dropouts |
|
| Interventions | Total number of intervention groups: 3 18% carbamide peroxide:
Duration of treatment: 21 days |
|
| Outcomes | Improvement in tooth shade Vita shade guide: lightest (C1) 1 to darkest (B4) 16 Oral tissue: normal/abnormal Gingival Index: Loë and Silness Gingival Index: 0 absence of inflation, 1 mild, 2 moderate, 3 severe Tooth sensitivity: 0 (no pain) to 10 (severe pain) Opinion survey: 1 (more positive) to 5 (least positive) |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: reported Key conclusions of the study authors: "Subjects who used 3x and 4x regimen achieved the greatest shade improvement. However those values were only 1 shade better than twice daily and no dry regimen. There was no significant difference between 3x and 4x regimens. Patients who used 2x and 3x regimens found it to be more convenient" Correspondence required: no Contact: Dr Yun Po Zhang; yun_zhzng@copal.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "120 qualifying subjects…. randomly assigned within strata to 1 of the 3..." However, the method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "stratified, double‐blinded, parallel‐group, randomised controlled trial." However, the method of blinding is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "stratified, double‐blinded, parallel‐group, randomised controlled trial." However, the method of blinding is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "104 subjects completed the clinical study: there were 16 dropouts…. The data revealed the number was well balanced" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Matis 1998.
| Methods | Title: efficacy and safety of a 10% carbamide peroxide bleaching gel Trial design: double‐blinded, randomised controlled trial Location: Indiana University, School of Detitistry, Indianapolis, Indiana, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: mean age 45.27 years Total number: 60 Inclusion criteria:
Exclusion criteria:
Number randomised: 60 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: dental assistant managed the grouping. All products were in identical syringes Number evaluated: 59. 1 patient dropped out from 4th week from active group |
|
| Interventions | Total number of intervention groups: 2 groups 10% carbamide peroxide gel in tray Placebo Duration of treatment: 2 weeks |
|
| Outcomes | Change in tooth colour: Truebyte shade guide: 0 lightest, 25 darkest Colourimetre: b*: decreased b* indicates reduced yellowness, L: increased L is increased brightness Gingival sensitivity Tooth sensitivity Gastrointestinal sensitivity Sensitivity scale: scale of 1 to 5: 1 = no sensitivity, 2 = slight sensitivity, 3 = moderate sensitivity, 4 = considerable sensitivity, 5 = severe sensitivity |
|
| Notes | Sample size calculation: not reported Adverse effects: gingival, tooth and gastrointestinal sensitivity was reported Health‐related quality of life: not reported Key conclusions of the study authors: "The product used in this study is an effective and physiologically acceptable tooth whitening agent. Initial colour regression occurred within the first month for incisors, and within 10 weeks for canines, but neither regressed back to baseline for the duration of this 6‐ month study" Contact: Dr BA Matis; bmatis@iusd.iupui.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "60 patients were randomised into 2 equal subgroups balanced by age, gender, and oral health status." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were given coded packages of Opalescence whitening gel. All products were in identical syringes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A dental assistant was responsible for group balancing, so that the evaluators could continue to be blind to which treatment group each patient was assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 2 weeks all 60 participants who were randomised were evaluated. However 1 dropout was noticed after 4 weeks and 22 weeks reporting. Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described are reported adequately. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Matis 2000.
| Methods | Title: clinical evaluation of bleaching agents of different concentrations Trial design: split‐mouth, randomised controlled trial Location: Indiana University‐Purdue University, Indianapolis, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Ultradent |
|
| Participants | Participants: 26 to 73 years old. Mean age 50.4 years Total number: 25 Inclusion criteria:
Exclusion criteria:
Number randomised: 25 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 25 |
|
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide 15% carbamide peroxide with 0.11% fluoride ion Duration of treatment: 14 days |
|
| Outcomes | Whitening of tooth. ΔL, a*, b*. Increase in ΔL and reduction in b* indicated whitening | |
| Notes | Sample size calculation: not reported Adverse effects: tooth and gingival irritation Key conclusions of the study authors: "All 3 methods of evaluation revealed a significant difference in the tooth lightness achieved by 10% and 15% products at 2 weeks but no significant difference at 6 weeks. No statistically significant difference was found in gingival or tooth sensitivity" Correspondence required: no Contact: Dr Bruce A Matis; Bmatis@ijsd.njpui.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a double‐blinded, 6‐week study in which participants were randomised into 2 groups by tooth shades." However, method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a double‐blinded, 6‐week study in which participants were randomised into 2 groups by tooth shades." However, method is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a double‐blinded, 6‐week study in which participants were randomised into 2 groups by tooth shades." However, method is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 25 subjects completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Matis 2006.
| Methods | Title: extended bleaching of tetracycline‐stained teeth: a 5‐year study Trial design: split‐mouth, randomised controlled trial Location: Wuhan University School of Stomatology, China Language: English Number of centres: 1 Recruitment period: not reported Funding source: Ultradent |
|
| Participants | Participants: not reported Total number: 59 Inclusion criteria:
Exclusion criteria:
Number randomised: 59 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 44 |
|
| Interventions | Total number of intervention groups: 3 10% carbamide peroxide 15% carbamide peroxide 20% carbamide peroxide Duration of treatment: 6 months |
|
| Outcomes | Improvement in tooth colour: colourimeter readings in CIELa*b* for Vitalescence Restorative Masters Shade Guide |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth and gingival sensitivity Key conclusions of the study authors: "The maximum lightening that occurred within 6 months happened during the first month of bleaching. Values increased the most during the bleaching of tetracycline stained teeth. There were small changes in the green red or blue‐yellow spectrums of colour throughout the study. At 4.5 years post‐bleaching, all 3 concentrations of bleaching agents had retained more than 65% of their original colour change. Increased tooth sensitivity occurs with higher concentrations of CP gels" Correspondence required: no Contact: Dr Bruce A Matis; Bmatis@ijsd.njpui.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At baseline evaluation, subjects were randomly assigned to 1 of 6 groups." However, method is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Subjects were not aware of the concentration of bleaching agent they were using." However, method is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 59 subjects who initially enrolled in the study, 44 completed the 5‐year evaluation. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Mederios 2008.
| Methods | Title: effectiveness of nightguard vital bleaching with 10% carbamide peroxide Trial design: randomised controlled trial Location: Universidade Federal do Rio Grande do Norte, Brazil Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: university students. 18 to 25 years old. Mean age 21.6 years Total number: 50 Inclusion criteria:
Exclusion criteria: not reported Number randomised: 50 Method of randomisation: raffle Method of allocation concealment: not reported. Patients were randomly allotted to either group Method of blinding: the placebo was placed in empty Opalescence PF packaging so that neither the volunteer nor the examiner knew which gel was being used Number evaluated: 49 |
|
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide gel in tray Placebo Duration of treatment: 21 days |
|
| Outcomes | Change in tooth shade: Vita shade guide ‐ arranged from lightest to dark (1 light and 16 darkest) Gingival Bleeding Index modified by Lang Tooth sensitivity: yes or no Patient satisfaction: satisfactory or non‐satisfactory |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and gingival bleeding Health‐related quality of life: reported Key conclusions of the study authors: "NGVB with 10% carbamide peroxide, when use in the current study, was effective for lightening tooth colour, both for the period immediately after treatment and for the 6‐month follow‐up period. Of the 2 main side effects assessed, tooth sensitivity was more prevalent than gingival irritation" Contact: Dr Medeiros; cristinamedeiros@digizap.com.br |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In a simple raffle, the 50 volunteers were randomly allocated to 1 of the 2 groups.." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo gel had the same physical characteristics as the experimental gel. The placebo was placed in empty Opalescence PF packaging so that neither the volunteer nor the examiner knew which gel was being used" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All analysis were done by the evaluator" Comment: but it is not mentioned whether the same evaluator dispensed the gel or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All volunteers completed the study.... The data of 1 volunteer from the placebo group were lost because of upper right lateral incisor anodontia" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described are reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Meireles 2010.
| Methods | Title: double‐blinded randomised clinical trial of 2 carbamide peroxide tooth bleaching agents: 2‐year follow‐up Trial design: double‐blinded, randomised clinical trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: not reported Total number: 183 Inclusion criteria:
Exclusion criteria:
Number randomised: 92 Method of randomisation: randomisation table Method of allocation concealment: not reported Method of blinding: labels were removed Number evaluated: 91 |
|
| Interventions | Total number of intervention groups: 2 Control: carbamide peroxide 10% in tray Experimental: carbamide peroxide 16% in tray Duration of treatment: 2 hours per day for 3 weeks |
|
| Outcomes | Improvement in tooth shade: ΔL, a*, b* values recorded Oral impact on daily performance (OIDP) 0 = no sensitivity; 1 = mild sensitivity; 2 = moderate sensitivity; 3 = considerable sensitivity and 4 = severe sensitivity The self‐reported general health was based on a Likert scale: excellent; very good; good; regular; bad (latter categorized in excellent/very good and good/regular) |
|
| Notes | Sample size calculation: mentioned Adverse effects: tooth sensitivity Health‐related quality of life: reported Key conclusions of the study authors: "The whitening effect evaluated by visual shade matching and digital spectrophotometer remained similar after 6 months of bleaching treatment using any of the carbamide peroxide concentrations tested. Additionally, the high consumption of staining beverage and food had no influence in the whitening effect longevity. Quality of life is complex and encompasses different domains. Although positive impact of the dental bleaching was detected, with patients showing more their teeth without embarrassment, difficult in dental hygiene and pain resulting from the treatment were also reported, and this can negatively impact daily performances. Dentists must consider these aspects when performing aesthetics procedures" This is a 2‐year follow‐up report of the previous study Correspondence required: no Contact: SS Meireles; soniasaeger@hotmail.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation table to allocate the participants in each study...." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The product concentration label was removed, therefore, the examiners and participants were blinded to the agent" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout. Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Mohan 2008.
| Methods | Title: clinical study to evaluate the efficacy of a novel tray‐based tooth whitening system Trial design: parallel, examiner‐blinded, stratified, randomised controlled trial Location: Department of Fixed and Removable Prosthodontics, Leeds Dental Institute, UK Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 18 to 70 years old Total number: 50 Inclusion criteria:
Exclusion criteria:
Number randomised: 50 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 49. 1 dropout from control group |
|
| Interventions | Total number of intervention groups: 2 Experimental: tray‐based 6% hydrogen peroxide Control: no treatment Duration of treatment: 14 days |
|
| Outcomes | Change in tooth shade Oral irritation Vita shade guide arranged based on lightness (B1 lightest and C4 darkest). 1 is lightest ‐ 16 is darkest b*: decreased b* indicates reduced yellowness; ΔL: increased ΔL is increased brightness |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Significant tooth whitening was evident after 3 days treatment with the tray‐based whitening system and colour improved with continued usage over 14 days. It also supports our previous study results that the WIO index is appropriate for assessing changes in tooth whiteness" Contact: Dr Naveen Mohan, Dental Health Unit, 3A Skelton House, Lloyd Street North, Manchester Science Park, Manchester, England M15 6SH, UK; iain.pretty@manchester.ac.uk (IA Pretty) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Balancing the 2 groups on the basis of baseline tooth colour, subjects were randomly assigned to either a tray‐based bleaching system or a non‐treatment control group." However, the method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A parallel, examiner‐blinded, stratified 2‐group clinical study." No other details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A parallel, examiner‐blinded, stratified 2‐group clinical study." No other details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A parallel, examiner‐blinded, stratified 2‐group clinical study." No other details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 dropout due to ill health was withdrawn from the study" Comment: plausible effect size (difference in means ) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described are reported. The conclusion is in accordance with results |
| Other bias | Low risk | None |
Mokhlis 2000.
| Methods | Title: clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime Trial design: split‐mouth, double‐blinded, randomised controlled trial Location: University Purdue, Indianapolis, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: funded by Ultradent products Inc. |
|
| Participants | Participants: not reported Total number: 24 Inclusion criteria:
Exclusion criteria:
Number randomised: 24 Method of randomisation: reported Method of allocation concealment: reported Method of blinding: not reported Number evaluated: 24 |
|
| Interventions | Total number of intervention groups: 2 20% carbamide peroxide gel 7.5% hydrogen peroxide gel Duration of treatment: 2 weeks |
|
| Outcomes | Change in colour: ΔL, a*, b* values were recorded. Increase in L and reduction in b* indicated whitening Tooth and soft tissue sensitivity: 5‐point scale: 1 none; 2 mild; 3 moderate; 4 considerable; and 5 severe |
|
| Notes | Sample size calculation: no Adverse effects: tooth and soft tissue sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Use of the 20% CP resulted in significantly more lightness than the 7.5% HP during the first 14 days of the study, but at the end of the study, there was no significant difference between products with regard to tooth lightness. In addition, the authors found no statistically significant difference between products with regard to gingival or tooth sensitivity" Contact: GR Mokhlis, Indiana University School of Dentistry, Indianapolis, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomised according to the baseline shade guide into 2 groups by a study monitor not directly involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "A study monitor assigned side of the mouth and the other gel to the opposite side. The monitor then labelled each box of bleaching gel accordingly" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded study but method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinded study but method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 24 patients completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Myers 2003.
| Methods | Title: clinical evaluation of a 3% hydrogen peroxide tooth whitening gel Trial design: double‐blinded, placebo‐controlled, randomised trial Location: School of Dentistry, Medical College of Georgia, Augusta, Georgia, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Applied Dental Sciences |
|
| Participants | Participants: age not reported Total number: 65 Inclusion criteria:
Exclusion criteria:
Number randomised: 65 Method of randomisation: randomisation table by statistician Method of allocation concealment: syringes were labelled with the participant number. A single labelled syringe of gel was retained from each participant's box for later testing if needed Method of blinding: not reported Number evaluated: 65 |
|
| Interventions | Total number of intervention groups: 2 3% hydrogen peroxide Nightguard gel Placebo Duration of treatment: 2 weeks |
|
| Outcomes | Colour change: Vita shade guide arranged base on lightness: 1 lightest (C1) to 16 darkest (B4) Dental sensitivity Irritation to tongue, gingiva, and throat |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity in tongue and gingiva, dental sensitivity to hot and cold Health‐related quality of life: not reported Key conclusions of the study authors: "Patient‐applied NGVB with a 3% hydrogen peroxide gel for 30 minutes 3 times a day for 2 weeks was effective in whitening teeth an average of 4.2 Vita shade tabs. The lightening effect was maintained at 6 months, and the side effects with this agent were similar to other whitening agents. The use of this material could be considered for patients who cannot comply with the regimen" Contact: Michael L Myers, Department of Oral Rehabilitation, School of Dentistry, Medical College of Georgia, Augusta, Georgia 3091 USA; mmyers@mail.mcg.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation table was prepared by the lead statistician (CMR), who also maintained participant identification numbers used throughout the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomisation table was prepared by the lead statistician (CMR), who also maintained participant identification numbers used throughout the study..... The outside of the box and the individual syringes were labelled with the paticipant number" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded study but method of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinded study but method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Nathoo 1994.
| Methods | Title: clinical evaluation of Colgate Platinum professional tooth whitening system and Rembrandt lightening bleaching gel Trial design: single‐blinded, randomised, parallel‐group clinical trial Location: Colgate Palmolive Research Centre, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: not reported Total number: 40 Inclusion criteria: not mentioned Exclusion criteria: not mentioned Number randomised: 40 (n = 20 per group) Method of randomisation: not reported Method of allocation concealment: reported Method of blinding: not reported Number evaluated: 38 (1 dropout from each group: n = 19 in each group) |
|
| Interventions | Total number of intervention groups: 2 (tray versus tray) 10% urea peroxide, 30 minutes twice daily (Colgate Platinum) 10% urea peroxide, 30 minutes twice daily (Rembrandt lightening) Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade (objective assessment): increase in L and reduction in b* indicates whitening Improvement in tooth shade (subjective assessment): Vita shade guide arrange in order of lightness: percentage increase |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Colgate Platinum was 46% more effective at tooth whitening after 1 week, and 96% more effective after 2 weeks of treatment. The results demonstrated that the Colgate product was significantly superior versus Rembrandt at increasing tooth whiteness (increase in delta E), and tooth lightness (increase in delta L*). No adverse reactions were noted on clinical examination" Correspondence required: no Contact: Saleem A Nathoo, Colgate Palmolive Research Centre, Piscataway, New Jersey, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Single‐blinded, randomised, parallel‐group clinical trial." However, method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "The identity of the products was concealed neither the subjects nor investigator were informed about the identity of products or to which group the individual belonged" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The identity of the products was concealed neither the subjects nor investigator were informed about the identity of products nor to which group the individual belonged" but details of the blinding method are not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Single‐blinded, randomised, parallel‐group clinical trial." However, method is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 40 participants 38 participants completed the study (1 dropout from each group)" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Nathoo 2001.
| Methods | Title: comparative 7‐day clinical evaluation of 2 tooth whitening products Trial design: double‐blinded, parallel‐group, randomised controlled clinical trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: 18 to 65 years old Total number: 60 Inclusion criteria: adults with shade darker than A3 Exclusion criteria: not reported Number randomised: 60 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: syringes were coded and wrapped Number evaluated: 58 |
|
| Interventions | Total number of intervention groups: 2 5% carbamide peroxide in tray 10% carbamide peroxide in tray Duration of treatment: 1 week, 6 to 8 hours per day |
|
| Outcomes | Improvement in tooth shade: Vita shade guide: tabs arranged from dark to light ΔL, a*, b* values: increase in ΔL and decrease in b* indicates lightening of teeth |
|
| Notes | Sample size calculation: not reported Adverse effects: hypersensitivity: sensitivity as reported ‐ as yes or no questions Health‐related quality of life: not reported Key conclusions of the study authors: "...whitening data showed that there was no significant difference between the 2 products after 1 week. The data suggest that these products are clinically equivalent for tooth whitening. However, the subjective data collected on tooth hypersensitivity showed that the product containing 5% carbamide peroxide was associated with less discomfort. Of the group using the 5% carbamide peroxide product, 20% reported transient sensitivity of their teeth after product use for 1 week compared with 53% of the group using the product with 10% carbamide peroxide. The product containing 5% carbamide peroxide was associated with less tooth hypersensitivity after 1 week of application" Correspondence required: no Contact: Saleem A Nathoo, Colgate Palmolive Research Centre, Piscataway, New Jersey, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Double‐blinded, randomised, controlled, parallel‐group clinica trial." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The identity of the products were wrapped, neither the investigator no subjects were informed about.." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The identity of the products were wrapped, neither the investigator no subjects were informed about.." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "of the 60 participants who began the study 29 matched pairs (n = 58) remained throughout the study" Comment: plausible effect size (difference in means ) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Nathoo 2002.
| Methods | Title: efficacy of novel, non‐tray paint on 18% carbamide peroxide gel Trial design: double‐blinded, parallel‐group, randomised trial Location: Colgate Palmolive Research Centre, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: 18 to 58 years old. Mean age 39.79 years Total number: 80 Inclusion criteria:
Exclusion criteria:
Number randomised: not reported Method of randomisation: method not reported Method of allocation concealment: method not reported Method of blinding: non‐removable white packing with patient identification number Number evaluated: 77 |
|
| Interventions | Total number of intervention groups: 2 18% carbamide peroxide paint‐on gel Placebo Duration of treatment: 21 days |
|
| Outcomes | Change in tooth shade Vita shade guide arranged base on lightness: 1 lightest (C1) to 16 darkest (B4) |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Study showed that subjects' teeth in the liquid whitening gel‐treated group exhibited an overall improvement and a 3.5‐shade difference compared with teeth in the placebo gel group. No soft tissue adverse reaction were reported" Contact: Saleem A Nathoo, Colgate Palmolive Research Centre, Piscataway, New Jersey, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Qualifying subjects were then stratified... and randomly assigned within strata to one of the study treatment groups.." However, the method by which randomisation was done is not mentioned in the article |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "For blinding, all products were overwrapped with a non‐removable white label containing a unique subject identification number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of patients randomised were 80. However, there were 3 dropouts. Quote: "Subjects who did not complete the study dropped out for reasons unrelated to the use of the treatments or adverse events" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned are reported adequately. Conclusion conforms to results |
| Other bias | Low risk | None |
Nathoo 2003.
| Methods | Title: comparative clinical investigation of tooth whitening efficacy of 2 whitening gels Trial design: randomised, double‐blinded, parallel‐group trial Location: Oral Health Clinical Services, Piscataway, New Jersey, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Colgate Palmolive |
|
| Participants | Participants: 18 to 70 years old. Mean age 28.5 years Total number: 59 Inclusion criteria:
Exclusion criteria:
Number randomised: 59 Method of randomisation: not mentioned Method of allocation concealment: not mentioned Method of blinding: not mentioned Number evaluated: 59 |
|
| Interventions | Total number of intervention groups: 2 Gel I: 25% carbamide peroxide Gel II: 8.7% hydrogen peroxide Duration of treatment: 3 weeks |
|
| Outcomes | Improvement in tooth shade The shade guide was arranged with 16‐shade tabs in order from B1 (1) to C4 (16) |
|
| Notes | Sample size calculation: not reported Adverse effects: none reported Health‐related quality of life: not reported Key conclusions of the study authors: "The authors concluded that all subjects exhibited statically significant tooth shade lightening. There was no significant difference in the between both gels for one time night usage for 2/3 weeks application. No adverse reaction was noted" Correspondence required: no Contact: Dr William DeVizio, William_devizio@colpal.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the participants completed the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Navarra 2014.
| Methods | Title: effects of 2 10% carbamide peroxide Nightguard bleaching agents, with and without desensitizer, on enamel and sensitivity: an in vivo study Trial design: blinded, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: supported, in part, by grants from MIUR (Italy) |
|
| Participants | Participants: 20 to 50 years old. Mean age 25.3 years Total number: 80 Inclusion criteria: not reported Exclusion criteria:
Number randomised: 20 Method of randomisation: not reported Method of allocation concealment: reported Method of blinding: not reported Number evaluated: 20 |
|
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide with fluoride and potassium nitrate in tray 10% carbamide peroxide without desensitizing agents in tray Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth colour ΔL, a*, b*, ΔW values were recorded. Increase in ΔL and reduction in b* indicated whitening |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Key conclusions of the study authors: "The use of 10% carbamide peroxide gel with fluoride and potassium nitrate reduced the incidence of sensitivity during the bleaching treatment compared to a bleaching agent that did not contain desensitizing agents. The bleaching effectiveness of the tested products was comparable" Correspondence required: no Contact: M Cadenaro, m.cadenaro@fmc.units.it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "An operator not involved in the research protocol performed the randomisation." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "allocated groups were recorded on cards contained in sequentially numbered, sealed envelopes that were blindly assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "blinded randomised controlled trial." However, method of blinding is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "blinded randomised controlled trial." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Oliveira 2013.
| Methods | Title: safety and efficacy of a high‐adhesion whitening strip under extended wear regimen Trial design: randomised, blinded, parallel‐group trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 19 to 64 years old. Mean age 42 years Total number: 29 Inclusion criteria:
Exclusion criteria:
Number randomised: 29 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: blinded test kits Number evaluated: 28 |
|
| Interventions | Total number of intervention groups: 2 9.5% hydrogen peroxide gel strips 10% hydrogen peroxide gel strips Duration of treatment: 9 days |
|
| Outcomes | Improvement in tooth shade. ΔL, a*, b* values: increase in ΔL and decrease in b* indicates lightening of teeth |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "The 2‐hour regimen for the 9.5% hydrogen peroxide high‐adhesion whitening strip was more efficient for tooth whitening than the 30‐minute regimen of 10% hydrogen peroxide whitening strip. Both treatments were well tolerated and the use of the test products during the study time frame was considered safe" Contact: GM Oliveira, gmoliv03@louisville.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..subjects were randomly assigned to 1 of the 2 groups.... properly balanced for age and baseline tooth colour." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A randomised, blinded, parallel group, single centre..... All test products and related instructions for use were packaged in blinded test kits with appropriate research labeling for distribution." No other details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A randomised, blinded, parallel group, single centre..... oral examination was conducted by an examiner blinded to treatment assignment to identify possible changes in oral status..." No other details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 subject in the 2‐hour strip group missed the day 5 visit" Comment: plausible effect size (difference in means ) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Papas 2009.
| Methods | Title: placebo‐controlled clinical trial of use of 10% hydrogen peroxide whitening strips for medication‐induced xerostomia Trial design: randomised, double‐blinded, placebo‐controlled clinical trial Location: University School of Dental Medicine and New England Medical Center, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: supported, in part, by Procter & Gamble |
|
| Participants | Participants: mean age 50 years Total number: 42 Inclusion criteria:
Exclusion criteria:
Number randomised: 42 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: identical package and coded Number evaluated: 40 (1 withdrew and 1 did not report after 15 days) |
|
| Interventions | Total number of intervention groups: 2 Control: placebo Experimental: 10% hydrogen peroxide whitening strips Duration of treatment: 2 weeks |
|
| Outcomes | Tooth colour change Oral irritation and sensitivity b*: decreased b* indicates reduced yellowness; ΔL: increased ΔL is increased brightness |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and gingival irritation Key conclusions of the study authors: "At day 8, the peroxide group experienced colour improvement relative to baseline and placebo. Mild and transient tooth sensitivity represented the most common adverse events. No subject discontinued treatment due to a product‐related adverse event" Contact: Athena S Papas, Tufts School of Dental Medicine, 1 Kneeland Street, Boston, MA 02111, USA; athena.papas@tufts.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised double‐blinded placebo‐controlled clinical trial was conducted... eligible subjects were randomly assigned to .." However, the method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Except for the presence or absence of peroxide, the test strips were identical in appearance. The test strips (peroxide or placebo) were dispensed in a small, white, cardboard box with instructions specifying twice daily strip application for 30 minutes before toothbrushing. To further ensure blinding, each box was labelled only with a unique subject identification number and necessary contact information" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 subject from placebo group voluntarily withdrew from the study.." Comment: 1 participant withdrew and 1 did not report after 15 days. Plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned are reported adequately. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Porciani 2006.
| Methods | Title: whitening effect by stain inhibition from a chewing gum with sodium hexametaphosphate in a controlled 12‐week single‐blinded trial Trial design: randomised, single‐blinded, cross‐over trial Location: University of Siena, Dental School, Siena, Italy Language: English Number of centres: 1 Recruitment period: not reported Funding source: Perfetti Van Melle |
|
| Participants | Participants: mean age 30.6 years Total number: 54 Inclusion criteria:
Exclusion criteria:
Number randomised: 54 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 54 |
|
| Interventions | Total number of intervention groups: 2 4% sodium hexametaphosphate Placebo Duration of treatment: 12 weeks |
|
| Outcomes | Reduction in stain (0 = no stain, 1 = light stain, 2 = moderate stain, 3 = heavy stain) Stain area (0 = no stain, 1 = stain covering up to 1/3 of the region, 2 = stain covering from 1/3 to 2/3 of the region, and 3 = stain covering greater than 2/3 of the region) Stain intensity Smoker versus non‐smokers |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "The results indicated that chewing gum containing sodium hexametaphosphate reduced induced stain formation by 33% compared to no gum treatment" Correspondence required: no Contact: Francesco Porciani, piercateadsl@libero.it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned to the test gum or no‐gum group.." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the teeth were scored for stain deposits by the same examiner who was blinded to the product assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 54 subjects (27 females and 27 males) initially enrolled in the study completed it" |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Porciani 2010.
| Methods | Title: effect on dental stain occurrence by chewing gum containing sodium tripolyphosphate Trial design: double‐blinded, parallel‐group, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Perfetti van Melle |
|
| Participants | Participants: 18 to 54 years old. Mean age 28.9 years Total number: 111 Inclusion criteria:
Exclusion criteria:
Number randomised: 111 Method of randomisation: random table Method of allocation concealment: not reported Method of blinding: similar looking package, shape, flavour and weight of the chewing gum. Participants instructed not to discuss the treatment they were receiving Number evaluated: 108. 3 participants dropped out from experiment group |
|
| Interventions | Total number of intervention groups: 2 Experimental: sodium tripolyphosphate (1%) containing gum Control: placebo Duration of treatment: 6 weeks |
|
| Outcomes | Reduction in stain intensity and extent of stain Lobene modified index: smaller value shows improvement Stain composite index: smaller value shows improvement |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "This trial showed a reduction in dental stain by a chewing gum containing sodium tripolyphosphate after 6 weeks" Contact: PF Porciani, piercateadsl@libero.it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each subject entered in the test or control group using a random table .." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both chewing gums had the same flavour, weight, shape, colour, and packaging so that the participants were blinded as to the identity of the gum" Comment: additionally, participants were instructed not to tell other subjects to which group they were assigned in order to minimize inadvertent disclosure to the study participants and staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data were scored by the same blinded operator for all measurements" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "... 3 eventually dropped out, though the cause was unrelated to polyphosphates" Comment: plausible effect size (difference in means ) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned were reported adequately. Conclusion reflected study results (in the abstract) |
| Other bias | Low risk | None |
Russell 1996.
| Methods | Title: dentist‐supervised home bleaching with 10% carbamide peroxide gel Trial design: double‐blinded, randomised controlled trial Location: Medical College of Georgia, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 22 to 79 years. Mean age 43.9 years Total number: 50 Inclusion criteria:
Exclusion criteria:
Number randomised: 50 Method of randomisation: randomisation table Method of allocation concealment: not reported Method of blinding: both gels were provided by the manufacturer in small kits containing syringes Number evaluated: 50 |
|
| Interventions | Total number of intervention groups: 2 Experimental: carbamide peroxide 10% tray Control: placebo Duration of treatment: 2 weeks (follow‐up of 6 months) |
|
| Outcomes | Lightening of teeth Vita shade guide arrange in ascending order for lightness. Rank 1: darkest (B4), rank 16: lightness (C1) |
|
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "The 10% carbamide peroxide product and treatment regimen for vital bleaching used in this study have been shown to produce a significant lightening effect immediately after treatment consistent with other studies. In addition, this study shows that the lightening effect lasts at least 6 months for the majority of subjects" Contact: Carl M Russell, Office of Biostatistics, Medical College of Georgia, Augusta, GA 30912‐4900, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation table containing subject identification numbers and group assignment was prepared by the lead statistician" |
| Allocation concealment (selection bias) | Unclear risk | Not done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo treatment gel was the same as the active gel except the carbamide peroxide was omitted. Both gels were provided by the manufacturer in small kits containing syringes.." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported. The number randomised were the number assessed in both groups over the 6‐month period |
| Selective reporting (reporting bias) | Low risk | All outcomes described are reported adequately. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Shahidi 2005.
| Methods | Title: randomised controlled trial of 10% hydrogen peroxide whitening strips Trial design: randomised, double‐blinded clinical trial Location: Hill Top Research Inc, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 19 to 48 years old. Mean age 32.4 years Total number: 40 Inclusion criteria: not reported Exclusion criteria:
Number randomised: 40 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: reported Number evaluated: 35 |
|
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide 0.2 mm strip 10% hydrogen peroxide 0.13 mm strip Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth shade. ΔL, a*, b* values: increase in ΔL and decrease in b* indicates lightening of teeth |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Key conclusions of the study authors: "Vital bleaching with 10% hydrogen peroxide strips at 1 week was as effective as 6% hydrogen peroxide strips used for 2 weeks. At the end of 2 weeks 10% hydrogen peroxide was better than 6% hydrogen peroxide. Both treatments were generally well tolerated, with mild and transient tooth sensitivity or oral irritation representing the most common adverse events" Contact: Robert Geralch, geralchgw@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised, double‐blinded clinical trial.... Subjects were randomly assigned to 1 of the strip groups." However, the method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The test and experimental strips were identical in size... each strip was packaged in an individual white foil pouch, with subject identification number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 dropouts at the end of the trial. Reason for dropouts not mentioned. We are not sure if the plausible effect size (difference in means) among missing outcomes may have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcome described are reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Swift 2004.
| Methods | Title: 3‐week clinical trial of a 14% hydrogen peroxide, strip‐based bleaching system Trial design: blinded, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 19 to 70 years old. Mean age 50 years Total number: 29 Inclusion criteria:
Exclusion criteria:
Number randomised: 29 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 28 |
|
| Interventions | Total number of intervention groups: 2 14% hydrogen peroxide bleaching strips Placebo Duration of treatment: 3 weeks |
|
| Outcomes | Change in tooth colour Vita shade guide arranged base on lightness: 1 lightest (C1) to 16 darkest (B4) Plaque and Gingival Index |
|
| Notes | Sample size calculation: not reported Adverse effects: gingival irritation and tooth sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Professional hydrogen peroxide strips evaluated in this clinical trial proved effective for tooth whitening with minimal side effects. Only 2 of the 13 subjects in the experimental group reported any tooth sensitivity, and only 3 reported any soft tissue irritation. The symptoms were described as mild in each case. In addition, changes in the gingival index and plaque index were very minor" Contact: Edward Swift, ed_swift@dentistry.unc.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were stratified and randomised to treatment groups based on their VITA shade and age." However, the method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "29 subjects were enrolled in the study and received either the experimental or placebo product; 28 completed the clinical trial" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described are reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Swift 2009.
| Methods | Title: effects of duration of whitening strip treatment on tooth colour Trial design: randomised, double‐blinded, placebo‐controlled clinical trial Location: University of North Carolina, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble (authors are employed by this company) |
|
| Participants | Participants: 25 to 58 years Total number: 40 Inclusion criteria:
Exclusion criteria:
Number randomised: 40 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: blinded test kits with similar over packing Number evaluated: week 2 n = 39, week 4 n = 36, week 6 n = 37 (week 2: 1 dropout from peroxide group; week 4: 1 dropout from peroxide and 3 from placebo; and week 6: 1 dropout from peroxide and 2 dropouts from placebo group) |
|
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide gel strips Placebo Duration of treatment: 2 weeks |
|
| Outcomes | Change in tooth shade b*: decreased b* indicates reduced yellowness; ΔL: increased ΔL is increased brightness |
|
| Notes | Sample size calculation: not reported Adverse effects: tooth sensitivity and oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Twice‐daily use of 6% hydrogen peroxide whitening strips resulted in teeth becoming lighter and less yellow versus baseline and placebo during initial 2‐week use, with no evidence of placebo response during sustained (weeks 2‐6) use" Contact: Edward Swift, ed_swift@dentistry.unc.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised double‐blinded placebo‐controlled clinical trial was conducted... After balancing for starting tooth colour and age, subjects were randomly assigned to peroxide or placebo strips." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo strips were identical to the test strips. Quote: "All test products and instructions for use were packaged in a blinded test kit.." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 subject in peroxide group was dismissed early as a recall failure. 4 other subjects completed the study but were excluded from the statistical analysis because of missed visits or non‐compliance" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes discussed have been reported adequately. Conclusions are in accordance with the results |
| Other bias | Low risk | Outliers in the data sets are removed. It may have an effect on the results |
Tam 2001.
| Methods | Title: effect of potassium nitrate and fluoride on carbamide peroxide bleaching Trial design: split‐mouth, double‐blinded, randomised clinical trial Location: University of Toronto, Canada Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Participants: 20 to 53 years old with a mean age of 31 (10) years Total number: 42 Inclusion criteria: not reported Exclusion criteria: no previous history of desensitizing agents Number randomised: not reported Method of randomisation: not reported Method of allocation concealment: syringes were randomly numbered and selected for use Method of blinding: identical packs Number evaluated: 40 |
|
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide with 3% potassium nitrate and 0.11 fluoride ion wt/vol 10% carbamide peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Tooth sensitivity Tooth whitening: patient‐reported improvement |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Key conclusions of the study authors: "A 10% carbamide peroxide bleaching gel containing potassium nitrate and fluoride produced less tooth sensitivity than did the control bleaching gel during a 2‐week at‐home bleaching treatment" Correspondence required: no Contact: Dr Laura Tam, laura.tam@utotonto.ca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blinded randomised clinical trial." However, method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "syringes were randomly numbered and selected for use on either the left or right side of each patient's dental arch" Comment: we are not sure if the person allocating the participants and the person conducting the study are the same |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "formulations were manufactured specifically for this study (Ultradent) and were packaged identically for..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blinded randomised clinical trial." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "1 patient chose to discontinue treatment .... A total of 9 treatment days (out of a potential total of 294 treatment days) were missed by the patients, either because of general tooth sensitivity or for personal reasons" Comment: no clear mention of dropouts in the article |
| Selective reporting (reporting bias) | Low risk | All outcomes discussed have been reported adequately. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Tsubura 2005.
| Methods | Title: clinical evaluation of a new bleaching product Polanight in a Japanese population Trial design: split‐mouth, randomised controlled trial Location: private dental clinic Language: English Number of centres: 1 Recruitment period: not reported Funding source: SDI Ltd |
|
| Participants | Participants: 18 to 47 years old. Mean age 30 years Total number: 58 Inclusion criteria: not reported Exclusion criteria: not reported Number randomised: 58 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 58 |
|
| Interventions | Total number of intervention groups: 2 Polanight: 10% carbamide peroxide Opalescence: 10% carbamide peroxide Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth colour ΔL, a*, b* values: increase in ΔL and decrease in b* indicates lightening of teeth |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Treatment with either agent demonstrated significant bleaching effects produced by the treatment. Bleaching with PN was considered more effective than that with OP in the young patient group and in the women" Correspondence required: no Contact: R Yamaguchi, hshimo@ngt.ndu.ac.jp |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomly selected from the patients visiting." However, method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Turkun 2010.
| Methods | Title: 1‐year clinical evaluation of the efficacy of a new daytime at‐home bleaching technique Trial design: parallel group randomised controlled clinical trial Location: Ege University, Turkey Language: English Number of centres: 1 Recruitment period: not reported Funding source: no |
|
| Participants | Participants: 20 to 30 years old Total number: 20 Inclusion criteria:
Exclusion criteria:
Number randomised: 20 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 20 |
|
| Interventions | Total number of intervention groups: 2 28% carbamide peroxide gel in daytime non‐custom‐fit tray (Meta Tray) 10% carbamide peroxide gel in overnight custom‐fit tray (Opalescence PF) Duration of treatment: 10 days |
|
| Outcomes | Improvement in tooth shade: ΔL, a*, b* values: increase in ΔL and decrease in b* indicates improvement Sensitivity: 0 ‐ no changes noted, 1 ‐ mild sensitivity, 2 ‐ moderate sensitivity, and 3 ‐ severe sensitivity |
|
| Notes | Sample size calculation: not mentioned Adverse effects: sensitivity Health‐related quality of life: not mentioned Key conclusions of the study authors: "...daytime at‐home bleaching system tested (Meta Tray) produced significant bleaching effects. However, the clinical efficacy of the overnight bleaching system was found superior to this new daytime at‐home bleaching system. Al though the new bleaching system exhibited less tooth sensitivity probably because of the reduced contact time of the bleaching gel with tooth surfaces, the application of the bleaching agent with a non‐customized tray provoked more gingival sensitivity in this group. The whitening effect remained similar 1 year after the bleaching" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "20 adult subjects .....were selected to participate in this randomised, controlled clinical trial." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 1 group had a custom‐made tray while the other used a prefabricated tray. There is a high risk that participants get to know the difference |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...by an examiner who did not know the treatment details of the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 20 participants completed this study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described have been reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Walters 2004.
| Methods | Title: benefits of sodium hexametaphosphate‐containing chewing gum for extrinsic stain inhibition Trial design: placebo‐controlled, randomised, examiner‐blinded, 2‐period cross‐over trial Location: London, UK Language: English Number of centres: 1 Recruitment period: not reported Funding source: author is employed by Procter & Gamble |
|
| Participants | Participants: 22 to 58 years old. Mean age 30.4 years Total number: 11 Inclusion criteria:
Exclusion criteria:
Number randomised: 11 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 10. 1 dropout from the experimental (chewing gum first/negative control gum second) due to an adverse event |
|
| Interventions | Total number of intervention groups: 2 Experimental: 5.6% sodium hexametaphosphate chewing gum Control: non‐sodium hexametaphosphate chewing gum Duration of treatment: 3 days, 10 days washout period before cross‐over |
|
| Outcomes | Reduction in induced extrinsic stains | |
| Notes | Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Sodium hexametaphosphate‐containing chewing gum can significantly reduce induced extrinsic dental stain formation, compared to a non‐sodium hexametaphosphate chewing gum" Contact: Patricia A Walters, walters.pa@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a negative‐controlled, examiner‐blinded, randomised, 2‐period cross‐over design." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was a negative‐controlled, examiner‐blinded, randomised, 2‐period cross‐over design." However, method of blinding is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study was a negative‐controlled, examiner‐blinded, randomised, 2‐period cross‐over design." However, method of blinding is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 subject in the experimental sequence dropped from the study due to an adverse event, reported and diagnosed by the examiner" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned are reported adequately. Conclusions are in accordance with results |
| Other bias | Low risk | None |
Wong 2004.
| Methods | Title: randomised controlled trial of home tooth whitening products Trial design: randomised, double‐blinded, single‐centre clinical trial with 3 parallel groups Location: Hong Kong University Language: English Number of centres: 1 Recruitment period: not reported Funding source: none |
|
| Participants | Participants: 18 to 30 years old Total number: 157 Inclusion criteria:
Exclusion criteria:
Number randomised: 87 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: brands covered with foil Number evaluated: 63 |
|
| Interventions | Total number of intervention groups: 3 6% hydrogen peroxide strip. Twice daily for 2 weeks 18% carbamide peroxide paint‐on gel Placebo: non‐whitening toothpaste Duration of treatment: 2 weeks |
|
| Outcomes | Improvement in tooth whitening: ΔL, a*, b*: increase in ΔL and reduction in b* indicates whitening Satisfaction of tooth whitening: 9‐point Likert scale |
|
| Notes | Sample size calculation: none Adverse effects: irritation of gums or teeth Health‐related quality of life: 0 to 4 (never ‐ hardly ever ‐ occasionally ‐ fairly often ‐ often), Oral Health Impact Profile Key conclusions of the study authors: "Crest Whitestrips (6.5% hydrogen peroxide) and Colgate Simply White (18% carbamide peroxide) are both effective in tooth whitening with the former being more effective" Correspondence required: no Contact: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subject inclusion and exclusion criteria, and randomly allocated into 1 of the following 3 groups." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "brands of all study products were masked by covering the products with adhesive aluminium foils" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "randomised controlled, double‐blinded, single‐center clinical trial." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Each study group started with 29 subjects and the 3 groups W, S, and P ended with 22, 21 and 20 subjects respectively" Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Xu 2007.
| Methods | Title: randomised clinical trial comparing whitening strips, paint‐on gel and negative control Trial design: randomised, examiner‐blinded, placebo‐controlled study Location: Shanghai Second Medical University, Shanghai, China Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble |
|
| Participants | Participants: 18 to 45 years old. Mean age 21.85 years. 92% females. Total number: 52 Inclusion criteria: not reported Exclusion criteria: not reported Number randomised: 52 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: similar kits Number evaluated: 49 |
|
| Interventions | Total number of intervention groups: 3 Whitening strips 6% hydrogen peroxide (n = 18), Paint‐on gel 5.8% hydrogen peroxide (n = 17; 2 dropouts) Negative control (water rinse) (n = 17; 1 dropout) Duration of treatment: 8 days for whitening strip, 15 days for gel and water rinse |
|
| Outcomes | Change in tooth colour ΔL, a*, b* values were recorded. Increase in ΔL and reduction in b* indicates whitening of tooth |
|
| Notes | Sample size calculation: not reported Adverse effects: mild sensitivity in strip group Health‐related quality of life: not reported Key conclusions of the study authors: "Despite similarities in starting concentration (6% hydrogen peroxide), the strip and paint‐on gel differed significantly on improvement in yellowness, brightness, and redness, as well as overall colour improvement. These differences were achieved with one‐half the treatment duration (7 versus 14 days) for strips compared to the paint‐on gel. Since only 1 of the products used a barrier, differences in residence time of the peroxide gel under a strip versus the barrier‐free paint‐on gel may have contributed to the relative clinical response of these 2 peroxide‐containing products" Contact: Dr Xu, xuxiao@smmail.cn |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly assigned to peroxide whitening strips (the positive control), paint‐on peroxide whitening gel, or water (the negative control)." However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were provided an identically appearing kit box labelled only with the unique subject number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 18 subjects in the strip group completed the research, while 2 subjects in the paint‐on group and 1 subject in the water rinse group failed to complete the study" Comment: plausible effect size (difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Ziebolz 2007.
| Methods | Title: efficacy and oral side effects of 2 highly concentrated tray‐based bleaching systems Trial design: randomised, 2‐armed, parallel clinical study Location: University of Göttingen, Germany Number of centres: 1 Recruitment period: not reported Funding source: study supported by Kettenbach, Germany |
|
| Participants | Participants: 20 to 48 years old Total number: 60 Inclusion criteria:
Exclusion criteria:
Number randomised: 60 Method of randomisation: stratified, randomised distribution Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 56 (4 dropouts) ‐ three dropouts in VW due to therapy pain and one dropout in OP due to therapy pain. |
|
| Interventions | Total number of intervention groups: 2 7.5% hydrogen peroxide gel in tray (Visalys) 20% carbamide peroxide gel in tray (Opalescence) Duration of treatment: 12 days |
|
| Outcomes | Tooth colour change: L*, a*, b* values: increase in L* and decrease in b* indicated lightening of teeth Hypersensitivity: 0 to 10 (0 = no hypersensitivity, 10 = high hypersensitivity) Acceptability: comfortable, slightly disturbing, uncomfortable or very uncomfortable |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "The bleaching systems demonstrated significant tooth colour improvement for Δb* and ΔL*. They did produce significantly different whitening response for Δb*, with Opalescence showing significant higher Δb*. After bleaching therapy, the intensity of tooth hypersensitivity was increased significantly compared to baseline, with no significant difference between both groups" Contact: Dr Dirk Ziebolz, dirk.ziebolz@zm‐goettingen.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A stratified, randomised distribution of the subjects to the 2 treatment groups..." However, method is not reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "3 subjects from the Visalys group and 1 from the Opalescence group withdrew during bleaching therapy..." Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
Ziebolz 2008.
| Methods | Title: influence of a desensitizing agent on efficacy of a paint‐on bleaching agent Trial design: double‐blinded, randomised controlled trial Location: University of Göttingen, Germany Language: English Number of centres: 1 Recruitment period: not reported Funding source: Ivoclar Vivadent, Schaan, Liechtenstein |
|
| Participants | Participants: mean age 26.27 years Total number: 80 Inclusion criteria:
Exclusion criteria:
Number randomised: 80 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 67. 13 dropouts |
|
| Interventions | Total number of intervention groups: 2 Control: hydrogen peroxide 6% without desensitizers (n = 40, final 7 dropouts) Experimental: hydrogen peroxide 6% with desensitizer (n = 40, final 6 dropouts) Duration of treatment: 10 days |
|
| Outcomes | Tooth colour change: Vita shade guide Tooth hypersensitivity: 0 = no sensitivity, 10 = high sensitivity Acceptability: interview: comfortable, slightly disturbing, uncomfortable or very uncomfortable Tolerability: interview: comfortable, slightly disturbing, uncomfortable or very uncomfortable |
|
| Notes | Sample size calculation: not reported Adverse effects: sensitivity and irritation in both groups Key conclusions of the study authors: "treatment groups developed tooth hypersensitivities during bleaching therapy. The number of subjects exhibiting sensitivities after bleaching increased more in the group without application of VivaSens (plus 15.2%) than in the group with VivaSens (2.9%), but the difference between the groups was not significant. Lack of statistical significance might be due to the low increase of tooth sensitivities due to the bleaching. This relatively low incidence of tooth hypersensitivity might be explained by the low concentration of peroxide (6.0%) in the bleaching agent used in this study. It might be speculated that a distinct higher number of subjects with tooth hypersensitivities due the bleaching therapy in the control group (Group A) without VivaSens application might have better demonstrated the positive effect of the desensitizing agent" Contact: Dr Dirk Ziebolz, dirk.ziebolz@zm‐goettingen.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 80 subjects were distributed randomly among 2 groups" However, method of randomisation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blinded, randomised, controlled, parallel‐group clinical trial." However, method of blinding is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "13 subjects failed to complete the 2‐week study (1 from each group) for reasons unrelated to the study Comment: missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. Conclusions are in accordance with the results |
| Other bias | Low risk | None |
CP = carbamide peroxide; HP = hydrogen peroxide; NGVB = nightguard vital bleaching; SE = standard error; STPP = sodium tripolyphosphate; VAS = visual analogue scale; WIO = whiteness index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alkmin 2005 | Study compared the effects of 2 bleaching agents on oral microbiota. |
| Amini 2009 | Abstract: insufficient information to include. |
| Amini 2011 | Abstract: insufficient information to include. |
| Anastasia 2010 | Abstract: insufficient information to include. |
| Andreana 2000 | Abstract: insufficient information to include. |
| Archila 2010 | Abstract: insufficient information to include. |
| Auschill 2009 | Abstract: insufficient information to include. |
| Browning 2001 | Abstract: insufficient information to include. |
| Burgio 2001 | Study involved in‐office bleaching as intervention. |
| Cardoso 2010 | Study compared the clinical effectiveness and tooth sensitivity associated with different bleaching agents in children. |
| Collins 2004 | Study compared the effect of self‐applied tooth whitening gel on oral soft tissue. |
| Corby 2014 | Study compared the effect of hydrogen peroxide whitening strips in adolescent twins. |
| Curtis 1996 | Study compared the effect of carbamide peroxide whitening gel on oral soft tissue. |
| de Geus 2015a | Controlled clinical trial evaluating the genotoxicity and efficacy of at‐home bleaching in smokers and non‐smokers. |
| de Geus 2015b | Controlled clinical trial evaluating tooth sensitivity in smokers. |
| Dickinson 2000 | Abstract: insufficient information to include. |
| Donly 2001 | Abstract: insufficient information to include. |
| Donly 2002 | Study compared the use of whitening strips in children. |
| Donly 2002a | Study compared the use of whitening strips in children and adolescents. |
| Farrell 2006 | The home‐based bleach was applied professionally in the clinic. |
| Farrell 2008 | Study compared the effect of hydrogen peroxide whitening strips on tooth sensitivity and oral irritation. |
| Fugaro 2004 | Study evaluated the histological changes in dental pulp after nightguard vital bleaching. |
| Fugaro 2005 | Study assessed the expression of specific neuropeptides associated with inflammation. |
| Garcia‐Godoy 2012 | Abstract: insufficient information to include. |
| Gerlach 2002c | The intervention included toothbrushing. |
| Gerlach 2002d | Abstract: insufficient information to include. |
| Gerlach 2002e | Study compared 2 professional tooth whitening systems for shade change using chroma meter. |
| Gerlach 2003a | One of the interventions included a whitening dentifrice. |
| Gerlach 2004a | One of the interventions included a whitening dentifrice. |
| Gerlach 2004c | Study evaluated the peroxide degradation and dilution kinetics of a bleaching agent. |
| Gerlach 2004d | Trial included teenagers and younger age group participants. |
| Godson 2001 | Abstract: insufficient information to include. |
| Gursoy 2008 | Study compared the effect of external tooth bleaching on dental plaque accumulation and discolouration. |
| Jadad 2011 | Study evaluated the colour alterations with a new dental bleaching product in patients wearing orthodontic appliances. |
| Jorgensen 2002 | Study compared the incidence of tooth sensitivity after home whitening treatment. |
| Karpinia 2003 | One of the interventions included a whitening dentifrice. |
| Lee 2003 | Abstract: insufficient information to include. |
| Leonard 2002 | Study evaluated safety issues when using a 16% carbamide peroxide whitening solution. |
| Leonard 2004 | Study evaluated the efficacy of a desensitizing agent used along with a bleaching agent. |
| Leonard 2007 | Study evaluated effects on oral tissues and associated tooth sensitivity and patients' perceptions during tooth bleaching. |
| Lisante 2009 | Abstract: insufficient information to include. |
| Loyola‐Rodriguez 2003 | Study included adolescents. |
| Majeed 2011 | Abstract: insufficient information to include. |
| Marques 2012 | Study compared salivary hydrogen peroxide release kinetics and potential toxicity of systemic exposure of 4 different whitening products. |
| Martin 2015 | In‐office bleaching was used in intervention. |
| Matis 1999 | Study evaluated in vivo degradation rate of beaching gels in bleaching trays. |
| Matis 2002 | Study compared different degradation of 15% carbamide peroxide. |
| Matis 2002a | A clinical evaluation of a bleaching agent used with and without reservoirs. |
| Matis 2005 | Quasi‐randomised controlled trial. |
| Mazur 2013 | Abstract: insufficient information to include. |
| NCT02603354 | Study involved in‐office bleaching as intervention. |
| NCT02682329 | Study involved in‐office bleaching as intervention. |
| Perdigao 2013 | Abstract: insufficient information to include. |
| Pinto 2014 | Tooth whitening with hydrogen peroxide in adolescents. |
| Pinto 2017 | Study compared whitening with hydrogen peroxide in adolescents. |
| Rezende 2013 | Study compared the clinical effects of exposure to coffee during at‐home vital bleaching. |
| Sagel 2001 | Abstract: insufficient information to include. |
| Schiff 1994 | Study is not about home‐based bleaching. |
| Schulte 1993 | Study evaluated clinical changes in the gingiva as a result of at‐home bleaching. |
| Schulte 1994 | Study evaluated and compared pulpal responses of teeth exposed to a bleaching agent. |
| Simon 2001 | Trial comparing tooth whitening with peroxide‐containing strips to a marketed whitening dentifrice. |
| Simon 2011 | Abstract: insufficient information to include. |
| Smith 2001 | Abstract: insufficient information to include. |
| Swift 2001 | Abstract: insufficient information to include. |
| Tam 1999 | Study participants included adolescents. |
| Walter 2011 | Abstract: insufficient information to include. |
| Yankell 1997 | Intervention used in the study (chewing gum) is excluded from this review. |
| Zantner 2006 | In‐office application was done for 1 group. |
Characteristics of studies awaiting assessment [ordered by study ID]
Barnes 1998.
| Methods | Title: clinical evaluation of a new 10% carbamide peroxide tooth‐whitening agent Trial design: double‐blinded clinical trial Location: University of Maryland Dental School, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Dentsply |
| Participants | Participants: 18 to 65 years Total number: 61 Inclusion criteria: A3 or darker shade Exclusion criteria: not reported Number randomised: not reported Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 50 |
| Interventions | Total number of intervention groups: 2 groups 10% carbamide peroxide gel in tray Placebo Duration of treatment: 4 hours to overnight for 2 weeks |
| Outcomes | Improvement in tooth shade: 2 weeks, 3 months and 6 months Tooth sensitivity Gingival irritation |
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "The average shade change for the placebo users was less than 1 shade. The average shade change for the NUPRO Gold users was 6.96 shades. Tooth hypersensitivity varied from none to severe. Tissue irritation was minimal. The results of these evaluations indicate that NUPRO Gold is effective as a tooth whitening system, when administered properly under the supervision of a dentist, with commonly reported side effects of transient tooth sensitivity and minimal gingival sensitivity. Little or no change in tissue health was noted" Correspondence required: yes: unclear if it is a randomised controlled trial. Authors have been mailed requesting for the data Contact: Dr Douglas Barnes, University of Maryland, Maryland, USA |
Bizhang 2017.
| Methods | Title: effectiveness of a new non‐hydrogen peroxide bleaching agent after single use ‐ a double‐blind placebo‐controlled short‐term study Trial design: double‐blinded randomised placebo‐controlled trial Location: not reported in abstract Number of centres: 1 Recruitment period: not reported in abstract Funding source: not reported in abstract |
| Participants | Participants: not reported Total number: 40 Inclusion criteria: not reported in abstract Exclusion criteria: not reported in abstract Number randomised: 40 Method of randomisation: not reported in abstract Method of allocation concealment: not reported in abstract Method of blinding: not reported in abstract Number evaluated: not reported in abstract |
| Interventions | Total number of intervention groups: 2 Over‐the‐counter product Placebo Duration of treatment: 1 day |
| Outcomes | Improvement in tooth shade |
| Notes | Yet to procure full text for this article |
Braun 2007.
| Methods | Title: spectrophotometric and visual evaluation of vital tooth bleaching employing different carbamide peroxide concentrations Trial design: double‐blinded randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
| Participants | Participants: not reported Total number: 30 Inclusion criteria: not reported Exclusion criteria: not reported Number randomised: 30 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: unmarked syringes Number evaluated: 30 |
| Interventions | Total number of intervention groups: 3 0% carbamide peroxide 10% carbamide peroxide 17% carbamide peroxide Duration of treatment: 1 week |
| Outcomes | Improvement in tooth shade |
| Notes | Sample size calculation: not reported Adverse effects: not reported Key conclusions of the study authors: "The study indicates that higher concentration bleaching agents might whiten teeth faster with major changes in lightness and chroma. However, by bleaching daily for 1 week, similar effects can be achieved with both a high and a low concentration agent. After treatment, a regression of the resultant shade has to be expected" Correspondence required: yes: authors have been mailed requesting for missing data Contact: Andreas Braun, andreas.braun@uni‐bonn.de |
Browning 2004.
| Methods | Title: safety and efficacy of a nightguard bleaching agent containing sodium fluoride and potassium nitrate Trial design: unknown |
| Participants | Participants: 22 Total number: 22 Number randomised: not clear Method of randomisation: not clear Method of allocation concealment: not clear Method of blinding: not clear Number evaluated: 22 |
| Interventions | 10% carbamide peroxide with potassium nitrate and sodium fluoride Placebo |
| Outcomes | Tooth whitening Sensitivity of teeth, gingiva, tongue and throat |
| Notes | Randomisation: not mentioned Sample size calculation: not reported Correspondence required: yes: authors have been mailed requesting for missing data Contact: Dr William Browning, wbrownin@mail.mcg.edu |
Ferrari 2004.
| Methods | Title: clinical trial evaluating the peroxide concentration response of whitening strips over 28 days Trial design: randomised, double‐blinded, parallel‐group clinical study Location: private practice, Italy Language: English Number of centres: 1 Recruitment period: not reported Funding source: Procter & Gamble and Fiji |
| Participants | Participants: not reported Total number: 37 Inclusion criteria: not reported Exclusion criteria: not Reported Number randomised: 37 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: similar packing Number evaluated: only 34 completed the study among them only 32 – day 7, 29 ‐ day 14, 28 – day 28 were present |
| Interventions | Total number of intervention groups: 3 1.8% hydrogen peroxide strips 3.3% hydrogen peroxide strips 5.3% hydrogen peroxide strips Duration of treatment: 28 days |
| Outcomes | Improvement in tooth shade Tooth whitening was characterized by decreased b* (reduction in yellowness) and increased ΔL* (increased brightness) |
| Notes | Sample size calculation: not reported Adverse effects: oral irritation Health‐related quality of life: not reported Key conclusions of the study authors: "Hydrogen peroxide at concentrations ranging from 1.8%‐5.3% resulted in significant colour improvement versus baseline as early as Day 7. There was a concentration‐response for reduction in yellowness (delta b*) and lightness improvement (deltaL*) at all time points, favouring the higher concentrations. While the concentration‐whitening relationship approached a linear response at Day 7, continued treatment resulted in incremental colour improvement. All 3 peroxide concentrations were well tolerated, and no subjects discontinued early due to a treatment‐related adverse event" Correspondence required: yes: number of participants per group is not reported, authors have been mailed requesting these data Contact: Marco Ferrari, Piazza Attias 19, Livorno 57120, Italy, ferrarimar@unisi.it |
Gambarini 2004.
| Methods | Title: efficacy and safety assessment of a new liquid tooth whitening gel containing 5.9% hydrogen peroxide Trial design: double‐blinded randomised controlled trial Location: University of Rome, Italy Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
| Participants | Participants: 21 to 52 years Total number: 30 Inclusion criteria: Vita shade score of A2 or darker; adults; staining in teeth Exclusion criteria: not reported Number randomised: 30 Method of randomisation: not mentioned Method of allocation concealment: not mentioned Method of blinding: not reported Performance/detection bias: not reported Number evaluated: 30 |
| Interventions | Total number of intervention groups: 2 Hydrogen peroxide gel 5.9% Placebo Duration of treatment: 2 weeks |
| Outcomes | Tooth colour change Sensitivity Bleeding on probing Gingival recession |
| Notes | Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "5.9% hydrogen peroxide was significantly effective in lightening tooth shade. After only 2 weeks, patients enrolled in the study exhibited an overall mean 4.48‐shade improvement from baseline, which was significantly greater than placebo group and far exceeded the ADA minimum requirements to claim "clinical efficacy". In the new Colgate Simply White Clear Whitening Gel group, periodontal health (PI and BOP) improved with time overall. Moreover, dentin hypersensitivity did not significantly increase, and all treatments were generally well tolerated" Correspondence required: yes: missing data, standard deviation cannot be calculated as P values have not been reported in the study. Author has been contacted Contact: Dr Gianluca Gambarini, ggambarini@tin.it |
Gegauff 1993.
| Methods | Title: evaluating tooth colour change from carbamide peroxide gel Trial design: double‐blinded randomised controlled trial Location: Ohio State University, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Ultradent Products, Inc |
| Participants | Participants: 20 to 27 years. Mean age 22 years Total number: 20 Inclusion criteria: no anterior restorations; history of anterior tooth pain or trauma; recurrent gingival lesions or abnormal variation in tooth colour; adults Exclusion criteria: not reported Number randomised: 20 Method of randomisation: not mentioned Method of allocation concealment: not reported Method of blinding: similar coded syringes Performance/detection bias: not reported Number evaluated: control: n = 17, (3 dropouts) |
| Interventions | Total number of intervention groups: 2 Carbamide peroxide 10% Placebo gel Duration of treatment: 8 weeks |
| Outcomes | Tooth colour change: b* (decreased b* indicates reduced yellowness); ΔL* (increased ΔL* is increased brightness) Gingival Index Effect on sulcus depth |
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "We found that maxillary canines had a higher lightness change than maxillary incisors. Additionally, the bleaching treatments significantly reduced the yellowness of all the maxillary anterior teeth. However, this reduction was partially reversed" Correspondence required: yes: missing data, mean and standard deviation not reported, authors have been contacted requesting data Contact: Dr Gegauff, College of Dentistry, Ohio State University, 305 West 12th Ave, Columbus 432101241, USA |
Gerlach 2004b.
| Methods | Title: vital bleaching with a thin peroxide gel: the safety and efficacy of a professional‐strength hydrogen peroxide whitening strip Trial design: double‐blinded, randomised controlled trial Location: not available Language: English Number of centres: not available Recruitment period: not available Funding source: not available |
| Participants | Participants: adults Total number: 38 Inclusion criteria: not available Exclusion criteria: not available Number randomised: not available Method of randomisation: not available Method of allocation concealment: not available Method of blinding: not available Number evaluated: not available |
| Interventions | Total number of intervention groups: 2 6% hydrogen peroxide 14% hydrogen peroxide Duration of treatment: 2 weeks |
| Outcomes | Improvement in tooth shade |
| Notes | Sample size calculation: not available Adverse effects: oral irritation Health‐related quality of life: not available Key conclusions of the study authors: "Use of the thin 14% hydrogen peroxide gel strip resulted in greater whitening, including 42% to 49% greater improvement in tooth colour and faster whitening onset than that seen with a 6% hydrogen peroxide whitening strip, without clinical evidence of increased oral‐tissue irritation" Not able to retrieve full text Contact: Dr Robert W Gerlach, Hill Top Research, West Palm Beach, Florida, USA, gerlach.rw@pg.com |
Guerrero 2007.
| Methods | Title: clinical response of a professional whitening strip system. A randomised, double‐blind, placebo‐controlled study |
| Participants | A total of 30 volunteer students and staff at the National Autonomous University of Mexico |
| Interventions | 6.5% hydrogen peroxide strips Placebo strips |
| Outcomes | Safety and efficacy of bleaching strips |
| Notes | Full text not available |
Heymann 1998.
| Methods | Title: clinical evaluation of 2 carbamide peroxide tooth‐whitening agents Trial design: blinded‐study Location: not available Language: English Number of centres: not available Recruitment period: not available Funding source: not available |
| Participants | Participants: not available Total number: 51 Inclusion criteria: not available Exclusion criteria: not available Number randomised: not available Method of randomisation: not available Method of allocation concealment: not available Method of blinding: not available Number evaluated: not available |
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide: 2 different brands Duration of treatment: 1 week |
| Outcomes | Improvement in tooth shade |
| Notes | Sample size calculation: not available Adverse effects: tooth sensitivity and gingival irritation Health‐related quality of life: not available Key conclusions of the study authors: "Not significant difference in the bleaching was noted between 2 groups. Mild gingival irritation and hypersensitivity was noticed between both the groups" Not able to retrieve full text |
Kim 2018.
| Methods | Title: bleaching effects on colour, chemical, and mechanical properties of white spot lesions Trial design: randomised, double‐blinded, placebo‐controlled trial Location: not given in abstract Language: English Number of centres: 1 Recruitment period: not given in abstract Funding source: not given in abstract |
| Participants | Participants: not reported Total number: 40 Inclusion criteria: not reported in abstract Exclusion criteria: not reported in abstract Number randomised: 40 Method of randomisation: not reported in abstract Method of allocation concealment: not reported in abstract Method of blinding: not reported in abstract Number evaluated: not reported in abstract |
| Interventions | Total number of intervention groups: 5 5 groups: 2 test groups (strip and paint‐on), 2 negative control groups and 1 positive control group (dentist‐supervised home bleaching) Duration of treatment: 4 weeks |
| Outcomes | Improvement in tooth shade |
| Notes | Yet to procure full text for this article |
Maran 2018.
| Methods | Title: tooth sensitivity with a desensitizing‐containing at‐home bleaching gel ‐ a randomised triple‐blind clinical trial Trial design: randomised, triple‐blinded, placebo‐controlled trial Location: not given in the abstract Language: English Number of centres: 1 Recruitment period: not given in the abstract Funding source: not given in the abstract |
| Participants | Participants: not reported Total number: 60 Inclusion criteria: not reported in abstract Exclusion criteria: not reported in abstract Number randomised: 60 Method of randomisation: not reported in abstract Method of allocation concealment: not reported in abstract Method of blinding: not reported in abstract Number evaluated: not reported in abstract |
| Interventions | Total number of intervention groups: 2 Desensitizing‐containing (3% potassium nitrate and 0.2% sodium fluoride) and desensitizing‐free 10% carbamide peroxide gel Duration of treatment: 21 days |
| Outcomes | Improvement in tooth shade |
| Notes | Yet to procure full text for this article |
NCT02151058.
| Methods | Title: a clinical trial to test the effect of a marketed mouthrinse on stain removal Trial design: single‐blinded, randomised controlled trial Location: not reported Language: English Number of centres: 1 Recruitment period: not reported Funding source: Johnson & Johnson Consumer and Personal Products Worldwide |
| Participants | Participants: 18 to 65 years Total number: 225 Inclusion criteria:
Exclusion criteria:
Number randomised: not reported Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: not reported |
| Interventions | Total number of intervention groups: 3 Negative control: Colgate® Regular Cavity Protection Experimental: experimental mouthrinse Active comparator: Crest® 3D White MultiCareWhitening Rinse |
| Outcomes | Reduction in stain |
| Notes | Sample size calculation: not reported A protocol for a randomised controlled trial. Full text not available Contact: not reported |
NCT03217994.
| Methods | Title: efficacy of 2 teeth whitening gels. A prospective, double‐blind, randomised clinical trial with split‐mouth design |
| Participants | Adults without prior tooth whitening treatments, tooth decay, or restorations of the upper front teeth. The patients had tooth colours of A3 or less according to the Vita classical scale |
| Interventions | 37.5% hydrogen peroxide gel to bleach teeth 6% hydrogen peroxide gel to bleach teeth |
| Outcomes | Change in tooth colour |
| Notes | A protocol for a randomised controlled trial. Full text not available |
Ozcan 2003.
| Methods | Title: the efficacy of 2 prototype chewing gums for the removal of extrinsic tooth stain Trial design: Double‐blinded, randomised controlled trail, parallel design Location: Marmra University, Istanbul, Turkey Language: English Number of centres: 1 Recruitment period: not reported Funding source: Dandy Sakiz ve Sekerleme Sanayi ve Ticaret AS, Istanbul |
| Participants | Participants: 18 to 24 years. Mean age 20.6 years Total number: 76 Inclusion criteria: healthy Individual; 12 score able anterior teeth; no orthodontic restoration Exclusion criteria: pregnant or lactating women Number randomised: 60 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: not reported |
| Interventions | Total number of intervention groups: 2 Group 1: sorbitol powder, maltilol syrup, mannitol, glycerine and flavour, no active ingredients Group 2: sorbitol powder, maltilol syrup, mannitol, glycerine and flavour, sodium tripolyposphate, dicalcium phosphate and sodium bicarbonate 1:4:5 Duration of treatment: 4 weeks |
| Outcomes | Reduction of stain in teeth and gingiva Gingiva and body were scored by 4‐point intensity scale ranging from no stain (0) to heavy stain (3) and a 4‐point area scale ranging from no stain (0) to stain covering greater than 2/3 of region (3) |
| Notes | Sample size calculation: not reported Adverse effects: reported Health‐related quality of life: reported Key conclusions of the study authors: "The overall difference between the stain scores after 4‐weeks' use of the chewing gums was statistically significant for both test Product A (without active ingredient) and Product B (with active ingredient) with regard to the mean baseline stain scores. This difference represented a 48% reduction in stain scores for those subjects using Product A, while the reduction was 64% for the subjects using Product B" Correspondence required: yes: missing data. Authors have been contacted Contact: Assistant Professor Dr Mutlu Ozcan, University of Groningen, Faculty of Medical Sciences, Oral Health Institute, Antonius Deusinglaan 1, 971 3 AV, Groningen, The Netherlands; mutluozcan@hotmail.com |
Pohjola 2002.
| Methods | Title: sensitivity and tooth whitening agents Trial design: randomised controlled trial Location: Medical College of Georgia Language: English Number of centres: 1 Recruitment period: not reported Funding source: not reported |
| Participants | Participants: 18 years old and above Total number: 12 Inclusion criteria: no medical condition; minimal gingival inflammation; no previous history of vital bleaching; A3 shade or above Exclusion criteria: active caries Number randomised: 12 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: not reported |
| Interventions | Total number of intervention groups: 3 3 brands of commercial product with 10% carbamide peroxide Rembrandt Xtra comfort Nite White Excel Fx product Duration of treatment: 2 weeks |
| Outcomes | Improvement in tooth shade Vita shade guide |
| Notes | Sample size calculation: not reported Adverse effects: sensitivity and gingival irritation Key conclusions of the study authors: "There was no significant difference between the products with respect to improvement in tooth whitening. All 3 products produced sensitivity. Thermal sensitivity was less with Rembrandt Xtra comfort and Nite White Excel" Correspondence required: yes: missing data, P‐value not reported. Authors have been contacted Contact: Dr Randall M Pohjola, rpobjola2mail.mcg.edu |
Reinhardt 1993.
| Methods | Title: a clinical study of nightguard vital bleaching Trial design: double‐blinded, randomised controlled clinical trial Location: University of Iowa, USA Language: English Number of centres: 1 Recruitment period: not reported Funding source: Dentmart and Omnii International |
| Participants | Participants: not reported Total number: 56 Inclusion criteria: not reported Exclusion criteria: patients with significant periodontal disease; internal tooth staining Number randomised: 56 Method of randomisation: not reported Method of allocation concealment: mentioned, but method not reported Method of blinding: not reported Number evaluated: 56 |
| Interventions | Total number of intervention groups: 4 (3 different tray‐based brands versus placebo). Intervention with 2 different application regimens for each Proxigei
White & Brite
Rembrandt Lighten
Control
Duration of treatment: 3 weeks |
| Outcomes | Improvement in shade: Vita shade guide: lightest to darkest using Munsell Value Gingival Index: 0 = no inflammation; I = inflammation, but no bleeding; 2 = bleeding on probing; and 3 = spontaneous bleeding Plaque Index: 0 = no plaque; 1= plaque detectable; 2 = plaque from interproximal surface to interproximal surface; 3 = plaque on more than half of tooth |
| Notes | Sample size calculation: not reported Adverse effects: sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "The overnight (1 application) method produced tooth‐lightening resulted at least equivalent to those of the multiple application (3‐application, 3‐hour) method. When Proxigei or Rembrandt was used, overnight and 3‐hour replenishment produced similar results. The use of White & Brite overnight also produced results similar to those of Rembrandt and Proxigei. The least effective treatment was White & Brite used with 3‐hour replenishment" Correspondence required: yes: the percentage of the active ingredient used in the trial is not clear. Authors contacted Contact: John W Reinhardt, Department of Operative Dentistry, University of Iowa College of Dentistry, Iowa City 52242, USA |
Rosenstiel 1996.
| Methods | Title: randomised clinical trial of the efficacy and safety of a home bleaching procedure Trial design: double‐blinded, randomised controlled trial Location: Ohio State University College, Columbus, Ohio, USA Language: English Number of centres: 1 Funding source: Ultradent |
| Participants | Participants: women: 28.4 years (range of 21 to 44 years), men: 30.1 years (range of 20 to 57 years) Total number: 52 Inclusion criteria: 6 maxillary anterior teeth; free of restoration/caries Exclusion criteria: intrinsic staining; hypoplasia; fluorosis Number randomised: 52 Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: 52 |
| Interventions | Total number of intervention groups: 2 10% carbamide peroxide Placebo Duration of treatment: 5 days |
| Outcomes | Colour change: b (decreased b* indicates reduced yellowness); ΔL* (increased ΔL* is increased brightness) Sulcus depth Vitality Gingival Index |
| Notes | Sample size calculation: not reported Adverse effects: sensitivity and gingival irritation Health‐related quality of life: not reported Key conclusions of the study authors: "The control group significant reduction in mean colour change at the end of 6 months. The mean colour change was more in canines compared to central incisor. Lightness was significantly increased for the active canines but less with central incisors. There was not change in vitality, sulcus depth or Gingival Index score" Correspondence required: yes: missing data, mean and standard deviation not reported. Authors have been mailed requesting for the data. Contact: Dr Stephen F Rosenstiel, Section of Restorative and Prosthetic Dentistry, Ohio State University, College of Dentistry, 305 West 12th Avenue, Columbus, Ohio 43210, USA |
Rossi 2018.
| Methods | Title: tooth colour changes and sensitivity in patients undergoing dental bleaching with 10% hydrogen peroxide using customized trays or strips: a randomised clinical trial Trial design: randomised, double‐blinded, placebo‐controlled trial Location: not given in the abstract Language: English Number of centres: 1 Recruitment period: not given in the abstract Funding source: not given in the abstract |
| Participants | Participants: not reported Total number: 50 Inclusion criteria: not reported in abstract Exclusion criteria: not reported in abstract Number randomised: 50 Method of randomisation: not reported in abstract Method of allocation concealment: not reported in abstract Method of blinding: not reported in abstract Number evaluated: not reported in abstract |
| Interventions | Total number of intervention groups: 2 10% hydrogen peroxide strip versus tray Placebo Duration of treatment: 14 days |
| Outcomes | Improvement in tooth shade |
| Notes | Yet to procure full text for this article |
Shin 2010.
| Methods | Title: the evaluation of clinical efficacy and longevity of home bleaching without combined application of in‐office bleaching Trial design: randomised controlled trial Location: not reported Language: Korean Number of centres: 1 Recruitment period: not reported Funding source: not reported |
| Participants | Participants: age 19 to 40 years Total number: 28 Inclusion criteria: mild tooth discolouration; 6 anterior teeth present Exclusion criteria: resin filling; porcelain restoration; dental caries; gingivitis and periodontitis Number randomised: not reported Method of randomisation: not reported Method of allocation concealment: not reported Method of blinding: not reported Number evaluated: not reported |
| Interventions | Total number of intervention groups: 2 15% carbamide peroxide tray Placebo Duration of treatment: 4 weeks |
| Outcomes | Change in tooth colour: b (decreased b* indicates reduced yellowness); ΔL* (increased ΔL* is increased brightness) |
| Notes | Sample size calculation: not reported Adverse effects: not reported Health‐related quality of life: not reported Key conclusions of the study authors: "Stronger colour change was observed for overall teeth samples in experimental group immediately after treatment (at 4 weeks) compared to ones in control group. There was also a significant difference between baseline and 8 weeks or 12 weeks as the tooth got darker with time) Correspondence required: yes: missing data, number of participants in control and experimental groups not reported. Authors have been mailed requesting for the data Contact: Sung Wun Yang, dentyun@catholic.ac.kr |
Sielski 2003.
| Methods | Randomised, controlled, examiner‐blinded, parallel‐group clinical study to determine efficacy of tooth‐whitening gel when used once daily at night, as compared with a commercially available dentifrice |
| Participants | 75 adults |
| Interventions | Non‐whitening dentifrice only A tooth‐whitening gel with a commercially available dentifrice |
| Outcomes | Change in tooth colour |
| Notes | Full text not available |
Simon 2014.
| Methods | Title: placebo‐controlled clinical trial evaluating 9.5% hydrogen peroxide high‐adhesion whitening strips Trial design: parallel, double‐blinded, randomised controlled trial Location: University of Tennessee, USA Language: English Number of centres: 1 Funding source: Procter & Gamble |
| Participants | Participants: 18 to 65 years Total number: 54 Inclusion criteria: 4 teeth which are A2 shade or darker; healthy adults Exclusion criteria: intrinsic staining; tooth sensitivity; orthodontic device Number randomised: not mentioned Method of randomisation: not mentioned Method of allocation concealment: not mention Method of blinding: not mentioned Number evaluated: 54 |
| Interventions | Total number of intervention groups: 2 9.5% hydrogen peroxide strips Placebo Duration of treatment: 3 weeks |
| Outcomes | Tooth colour change: b (decreased b* indicates reduced yellowness); ΔL* (increased ΔL* is increased brightness) Tooth sensitivity |
| Notes | Sample size calculation: not reported Adverse effects: oral irritation and tooth sensitivity Health‐related quality of life: not reported Key conclusions of the study authors: "Experimental 9.5% hydrogen peroxide strip yielded significant tooth whitening relative to a placebo strip as early as after three days of product use" Correspondence required: yes: authors have been contacted for missing data Contact: Dr James F Simon, farrells.2@pg.com |
Characteristics of ongoing studies [ordered by study ID]
NCT03026725.
| Trial name or title | Effect of tricalcium phosphate on efficacy and sensitivity with vital tooth whitening using 20% carbamide peroxide |
| Methods | Randomised controlled trial |
| Participants | Not given |
| Interventions | Not given |
| Outcomes | Improvement in shade |
| Starting date | 2017 |
| Contact information | clinicaltrials.gov/show/nct03026725 |
| Notes | Results not yet reported |
Differences between protocol and review
There are a few differences between the previous version of the review and this updated version.
Previous review considered outcome data for tooth whiteness immediately after 2 weeks of product applications whereas we considered trials with any duration of treatment.
This version excluded quasi‐randomised trials.
Trials addressing bleaching for tetracycline‐stained teeth were not included in the previous review. We included two trials comparing the effects of a bleaching agent on participants with tetracycline stains.
Some trials which were included in the previous review are not included in the present review as they consisted of whitening dentifrices which were part of our exclusion criteria (Additional Table 46).
Trials on whitening chewing gums and mouthrinses are included in our current review but absent in the previous review.
In the previous review analysis was not combined for trials with the same bleaching agent using different measurement methods (Vita shade guide and colorimeter). We used standardised mean difference (SMD) and combined data wherever relevant.
Report on examiner calibration and correlation analysis between concentration of peroxide and effect size was not included in our review but is mentioned in the previous review.
We updated the methods, used GRADE to assess the certainty of the evidence and included 'Summary of findings' tables.
2. Comparison of included articles: 2006 review version versus current version.
| The 2006 review included 25 trials of which 17 trials were included in our review, 3 are under awaiting classification, 2 articles were excluded with reason, 2 were discarded during the initial screening, and 1 did not appear in our search and we could not obtain a copy of: | |||
| 2006 review | Current review | ||
| Included records | Included records | Awaiting classification | No longer included |
|
Barnes 1998 Brunton 2004 Cronin 2005 Gerlach 2000 Gerlach 2001 Gerlach 2002 Gerlach 2002a Gerlach 2003 Gerlach 2004a Gerlach 2004b Karpinia 2002 Karpinia 2003 Kihn 2000 Kowitz 1994 Kowitz 1994a Kugel 2000 Li 2003 Li 2004 Matis 2000 Mokhlis 2000 Nathoo 1994 Nathoo 2002 Nathoo 2003 Panich 2001 Sielski 2003 |
Brunton 2004 Cronin 2005 Gerlach 2000 Gerlach 2002 Gerlach 2002a Gerlach 2003 Karpinia 2002 Kihn 2000 Kowitz 1994 Kugel 2000 Li 2003 Li 2004 Matis 2000 Mokhlis 2000 Nathoo 1994 Nathoo 2002 Nathoo 2003 |
Barnes 1998: unclear if the trial is a randomised controlled trial Gerlach 2004b: full‐text not available Sielski 2003: full‐text not available (We did not use the data from the previous review as we needed to be sure about the studies characteristics) |
Gerlach 2001: reported on an anticavity whitening dentifrice; discarded during initial screening Gerlach 2004a: 1 of the interventions included a whitening dentifrice; excluded with reason Karpinia 2003: 1 of the interventions included a whitening dentifrice; excluded with reason Kowitz 1994a: reported on whitening toothpaste; discarded during initial screening Panich 2001: an MSc thesis, did not appear in our search. We could neither procure the full text of the thesis nor the published version, hence we did not include in our review |
Contributions of authors
Prashanti Eachempati: protocol, selecting trials, analyses, final review, and updating review.
Sumanth Kumbargere Nagraj: arbiter, analyses, final review, and updating review.
Salian Kiran Kumar Krishanappa: obtaining copies of trials, selecting trials, data extraction, and entering data into Review Manager 5.
Puneet Gupta: selecting trials, data extraction, and entering data into Review Manager 5.
Ibrahim Ethem Yayali: selecting trials, data extraction, and entering data into Review Manager 5.
Sources of support
Internal sources
Faculty of Dentistry, Melaka‐Manipal Medical College, Manipal University, Melaka Campus, Malaysia.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
Prashanti Eachempati: none known. Sumanth Kumbargere Nagraj: none known. Salian Kiran Kumar Krishanappa: none known. Puneet Gupta: none known. Ibrahim Ethem Yaylali: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aka 2017 {published data only}
- Aka B, Celik EU. Evaluation of the efficacy and color stability of two different at‐home bleaching systems on teeth of different shades: a randomized controlled clinical trial. Journal of Esthetic and Restorative Dentistry 2017;29(5):325‐38. [PUBMED: 28322505] [DOI] [PubMed] [Google Scholar]
Alonso 2006 {published data only}
- Alonso de la Peña V, Balboa Cabrita O. Comparison of the clinical efficacy and safety of carbamide peroxide and hydrogen peroxide in at‐home bleaching gels. Quintessence International 2006;37(7):551‐6. [PUBMED: 16841603] [PubMed] [Google Scholar]
Alonso 2014 {published data only}
- Alonso de la Peña V, López Ratón M. Randomized clinical trial on the efficacy and safety of four professional at‐home tooth whitening gels. Operative Dentistry 2014;39(2):136‐43. [PUBMED: 23862715] [DOI] [PubMed] [Google Scholar]
Auschill 2007 {published data only}
- Auschill TM, Barcsay LA, Arweiler NB. Strips versus gel: a clinical comparison of two over‐the‐counter bleaching systems. Schweizer Monatsschrift fur Zahnmedizin 2007;117(8):843‐56. [PUBMED: 17879675] [PubMed] [Google Scholar]
Auschill 2012 {published data only}
- Auschill TM, Schneider‐Del Savio T, Hellwig E, Arweiler NB. Randomized clinical trial of the efficacy, tolerability, and long‐term color stability of two bleaching techniques: 18‐month follow‐up. Quintessence International 2012;43(8):683‐94. [PUBMED: 23034421] [PubMed] [Google Scholar]
Barlow 2003 {published data only}
- Barlow A, Gerlach RW, Date RF, Brennan K, Struzycka I, Kwiatkowska A, et al. Clinical response of two brush‐applied peroxide whitening systems. Journal of Clinical Dentistry 2003;14(3):59‐63. [PUBMED: 14520775] [PubMed] [Google Scholar]
Berga‐Caballero 2006 {published data only}
- Berga‐Caballero A, Forner‐Navarro L, Amengual‐Lorenzo J. At‐home vital bleaching: a comparison of hydrogen peroxide and carbamide peroxide treatments. Medicina Oral, Patologia Oral y Cirugia Bucal 2006;11(1):E94‐9. [PUBMED: 16388304] [PubMed] [Google Scholar]
Biesbrock 2004 {published data only}
- Biesbrock AR, Walters P, Bartizek RD. A chewing gum containing 7.5% sodium hexametaphosphate inhibits stain deposition compared with a placebo chewing gum. Compendium of Continuing Education in Dentistry 2004;25(4):253‐4. [PUBMED: 15645861] [PubMed] [Google Scholar]
Bizhang 2007 {published data only}
- Bizhang M, Muller M, Phark JH, Barker ML, Gerlach RW. Clinical trial of long‐term color stability of hydrogen peroxide strips and sodium percarbonate film. American Journal of Dentistry 2007;20(A):23A‐7A. [PUBMED: 19681255] [PubMed] [Google Scholar]
Botelho 2017 {published data only}
- Botelho MG, Chan AWK, Newsome PRH, McGrath CP, Lam WYH. A randomized controlled trial of home bleaching of tetracycline‐stained teeth. Journal of Dentistry 2017;67:29‐35. [DOI: 10.1016/j.jdent.2017.05.003; PUBMED: 28478214] [DOI] [PubMed] [Google Scholar]
Browning 2008 {published data only}
- Browning WD, Chan DC, Myers ML, Brackett WW, Brackett MG, Pashley DH. Comparison of traditional and low sensitivity whiteners. Operative Dentistry 2008;33(4):379‐85. [PUBMED: 18666494] [DOI] [PubMed] [Google Scholar]
Bruhn 2012 {published data only}
- Bruhn AM, Darby ML, McCombs GB, Lynch CM. Vital tooth whitening effects on oral health‐related quality of life in older adults. Journal of Dental Hygiene 2012;86(3):239‐47. [PUBMED: 22947847] [PubMed] [Google Scholar]
Brunton 2004 {published data only}
- Brunton PA, Ellwood R, Davies R. A six‐month study of two self‐applied tooth whitening products containing carbamide peroxide. Operative Dentistry 2004;29(6):623‐6. [PUBMED: 15646216] [PubMed] [Google Scholar]
Cibirka 1999 {published data only}
- Cibirka RM, Myers M, Downey MC, Nelson SK, Browning WD, Hawkins IK, et al. Clinical study of tooth shade lightening from dentist‐supervised, patient‐applied treatment with two 10% carbamide peroxide gels. Journal of Esthetic Dentistry 1999;11(6):325‐31. [PUBMED: 10825867] [DOI] [PubMed] [Google Scholar]
Collins 2004a {published data only}
- Collins LE, Maggio B, Liebman J, Blanck M, Lefort S, Waterfield P, et al. Clinical evaluation of a novel whitening gel, containing 6% hydrogen peroxide and a standard fluoride toothpaste. Journal of Dentistry 2004;32(Suppl 1):13‐7. [PUBMED: 14738830] [DOI] [PubMed] [Google Scholar]
Costa 2012 {published data only}
- Costa JB, McPharlin R, Hilton T, Ferracane JI, Wang M. Comparison of two at‐home whitening products of similar peroxide concentration and different delivery methods. Operative Dentistry 2012;37(4):333‐9. [PUBMED: 22433035] [DOI] [PubMed] [Google Scholar]
Cronin 2005 {published data only}
- Cronin MJ, Charles CA, Zhao Q, Dembling WZ. Comparison of two over‐the‐counter tooth whitening products using a novel system. Compendium of Continuing Education in Dentistry 2005;26(2):140, 142, 144‐8. [PUBMED: 15948518] [PubMed] [Google Scholar]
Delgado 2007 {published data only}
- Delgado E, Hernandez‐Cott PL, Stewart B, Collins M, Vizio W. Tooth‐whitening efficacy of custom tray‐delivered 9% hydrogen peroxide and 20% carbamide peroxide during daytime use: a 14‐day clinical trial. Puerto Rico Health Sciences Journal 2007;26(4):367‐72. [PUBMED: 18246965] [PubMed] [Google Scholar]
Ferrari 2007 {published data only}
- Ferrari M, Cagidiaco MC, Monticelli F, Kugel G, Barker ML, Gerlach RW. Daytime use of a custom bleaching tray or whitening strips: initial and sustained color improvement. American Journal of Dentistry 2007;20 Spec No A:19A‐22A. [PUBMED: 19681254] [PubMed] [Google Scholar]
Gallo 2009 {published data only}
- Gallo JR, Burgess JO, Ripps AH, Bell MJ, Mercante DE, Davidson JM. Evaluation of 30% carbamide peroxide at‐home bleaching gels with and without potassium nitrate ‐ a pilot study. Quintessence International 2009;40(4):1‐6. [PUBMED: 19417866] [PubMed] [Google Scholar]
Garcia‐Godoy 2004 {published data only}
- Garcia‐Godoy F, Villalta P, Barker ML, Gerlach RW. Placebo‐controlled, 6‐week clinical trial on the safety and efficacy of a low‐gel, 14% hydrogen‐peroxide whitening strip. Compendium of Continuing Education in Dentistry 2004;25(8 Suppl 2):21‐6. [PUBMED: 15645891] [PubMed] [Google Scholar]
Gerlach 2000 {published data only}
- Gerlach RW, Gibb RD, Sagel PA. A randomized clinical trial comparing a novel 5.3% hydrogen peroxide whitening strip to 10%, 15%, and 20% carbamide peroxide tray‐based bleaching systems. Compendium of Continuing Education in Dentistry 2000;29:S22‐8; S42‐3. [PUBMED: 11908406] [PubMed] [Google Scholar]
Gerlach 2002 {published data only}
- Gerlach RW, Gibb RD, Sagel PA. Initial color change and color retention with a hydrogen peroxide bleaching strip. American Journal of Dentistry 2002;15(1):3‐7. [PUBMED: 12074226] [PubMed] [Google Scholar]
Gerlach 2002a {published data only}
- Gerlach RW, Zhou X. Comparative clinical efficacy of two professional bleaching systems. Compendium of Continuing Education in Dentistry 2002;23(1A):35‐41. [PUBMED: 11913292] [PubMed] [Google Scholar]
Gerlach 2002b {published data only}
- Gerlach RW, Zhou X, McClanahan SF. Comparative response of whitening strips to a low peroxide and potassium nitrate bleaching gel. American Journal of Dentistry 2002;15:19A‐23A. [PUBMED: 12512987] [PubMed] [Google Scholar]
Gerlach 2003 {published data only}
- Gerlach RW, Barker ML. Randomized clinical trial comparing overnight use of two self‐directed peroxide tooth whiteners. American Journal of Dentistry 2003;16 Spec No:17B‐21B. [PUBMED: 15055983] [PubMed] [Google Scholar]
Gerlach 2004 {published data only}
- Gerlach RW, Zhou X. Clinical trial comparing two daytime hydrogen‐peroxide professional vital‐bleaching systems. Compendium of Continuing Education in Dentistry 2004;25(8 Suppl 2):33‐40. [PUBMED: 15645893] [PubMed] [Google Scholar]
Gerlach 2004e {published data only}
- Gerlach RW, Sagel PA, Barker ML, Karpinia KA, Magnusson I. Placebo‐controlled clinical trial evaluating a 10% hydrogen peroxide whitening strip. Journal of Clinical Dentistry 2004;15(4):118‐22. [PUBMED: 15794457] [PubMed] [Google Scholar]
Gerlach 2005 {published data only}
- Gerlach RW, Tucker HL, Anastasia MK, Barker ML. Clinical trial comparing 2 hydrogen peroxide tooth whitening systems: strips versus pre‐rinse. Compendium of Continuing Education in Dentistry 2005;26(12):874‐8. [PUBMED: 16389774] [PubMed] [Google Scholar]
Giniger 2005 {published data only}
- Giniger M, Macdonald J, Ziemba S, Felix H. The clinical performance of professionally dispensed bleaching gel with added amorphous calcium phosphate. Journal of the American Dental Association 2005;136(3):383‐92. [PUBMED: 15819354] [DOI] [PubMed] [Google Scholar]
- Giniger M, Spaid M, MacDonald J, Felix H. A 180‐day clinical investigation of the tooth whitening efficacy of a bleaching gel with added amorphous calcium phosphate. Journal of Clinical Dentistry 2005;16(1):11‐6. [PUBMED: 15974218] [PubMed] [Google Scholar]
Hannig 2007 {published data only}
- Hannig C, Lindner D, Attin T. Efficacy and tolerability of two home bleaching systems having different peroxide delivery. Clinical Oral Investigations 2007;11(4):321‐9. [PUBMED: 17593406] [DOI] [PubMed] [Google Scholar]
Hasturk 2004 {published data only}
- Hasturk H, Nunn M, Warbington M, Dyke TE. Efficacy of a fluoridated hydrogen peroxide‐based mouthrinse for the treatment of gingivitis: a randomised clinical trial. Journal of Periodontology 2004;75(1):57‐65. [PUBMED: 15025217 ] [DOI] [PubMed] [Google Scholar]
Hyland 2015 {published data only}
- Hyland BW, McDonald A, Lewis N, Tredwin C, Petrie A, Hall S, et al. A new three‐component formulation for the efficient whitening of teeth (Carbamide Plus). Clinical Oral Investigations 2015;19(6):1395‐404. [PUBMED: 25381018 ] [DOI] [PubMed] [Google Scholar]
Karpinia 2002 {published data only}
- Karpinia KA, Magnusson I, Sagel PA, Zhou X, Gerlach RW. Vital bleaching with two at‐home professional systems. American Journal of Dentistry 2002;15:13A‐18A. [PUBMED: 12512986] [PubMed] [Google Scholar]
Kihn 2000 {published data only}
- Kihn PW, Barnes DM, Romberg E, Peterson K. A clinical evaluation of 10 per cent versus 15 per cent carbamide peroxide tooth ‐ whitening agents. Journal of the American Dental Association 2000;131:1478‐84. [PUBMED: 11042989] [DOI] [PubMed] [Google Scholar]
Kose 2011 {published data only}
- Kose C, Reis A, Baratieri LN, Loguercio AD. Clinical effects of at‐home bleaching along with desensitizing agent application. American Journal of Dentistry 2011;24(6):379‐82. [PUBMED: 22263337] [PubMed] [Google Scholar]
Kowitz 1994 {published data only}
- Kowitz GM, Nathoo SA, Wong R. Comparative clinical evaluation of two professional tooth‐whitening products. Compendium (Newtown, Pa). Supplement 1994;15(17):S635‐9. [PUBMED: 8205580] [PubMed] [Google Scholar]
Krause 2008 {published data only}
- Krause F, Jepsen S, Braun A. Subjective intensities of pain and contentment with treatment outcomes during tray bleaching of vital teeth employing different carbamide peroxide concentrations. Quintessence International 2008;39(3):203‐9. [PUBMED: 18618034] [PubMed] [Google Scholar]
Kugel 2000 {published data only}
- Kugel G, Kastali S. Tooth‐whitening efficacy and safety: a randomized and controlled clinical trial. Compendium of Continuing Education in Dentistry 2000;21(29):S16‐21; S42. [PUBMED: 11908405] [PubMed] [Google Scholar]
Kugel 2002 {published data only}
- Kugel G, Aboushala A, Zhou X, Gerlach RW. Daily use of whitening strips on tetracycline‐stained teeth: comparative results after 2 months. Compendium of Continuing Education in Dentistry 2002;23(1A):29‐34. [PUBMED: 11913291] [PubMed] [Google Scholar]
Li 2003 {published data only}
- Li Y, Lee SS, Cartwright SL, Wilson AC. Comparison of clinical efficacy and safety of three professional at‐home tooth whitening systems. Compendium of Continuing Education in Dentistry 2003;24(5):357‐60. [PUBMED: 12793220] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li Y, Lee SS, Cartwright S, Wilson AC, DeVizio W, Petrone M, et al. Comparative tooth whitening efficacy of 18% carbamide peroxide liquid whitening gel using three different regimens. Journal of Clinical Dentistry 2004;15(1):11‐6. [PUBMED: 15218710] [PubMed] [Google Scholar]
Matis 1998 {published data only}
- Matis BA, Cochran MA, Eckert G, Carlson TJ. The efficacy and safety of a 10% carbamide peroxide bleaching gel. Quintessence International 1998;29(9):555‐65. [PUBMED: 9807138] [PubMed] [Google Scholar]
Matis 2000 {published data only}
- Matis BA, Mousa HN, Cochran MA, Eckert GJ. Clinical evaluation of bleaching agents of different concentrations. Quintessence International 2000;31(5):303‐10. [PUBMED: 11203940] [PubMed] [Google Scholar]
Matis 2006 {published data only}
- Matis BA, Wang Y, Eckert GJ, Cochran MA, Jiang T. Extended bleaching of tetracycline‐stained teeth: a 5‐year study. Operative Dentistry 2006;31(6):643‐51. [PUBMED: 17153971 ] [DOI] [PubMed] [Google Scholar]
- Matis BA, Wang Y, Jiang T, Eckert GJ. Extended at‐home bleaching of tetracycline‐stained teeth with different concentrations of carbamide peroxide. Quintessence International 2002;33(9):645‐55. [PUBMED: 12666888] [PubMed] [Google Scholar]
Mederios 2008 {published data only}
- dos Santos Medeiros MC, Lima KC. Effectiveness of nightguard vital bleaching with 10% carbamide peroxide ‐ a clinical study. Journal of the Canadian Dental Association 2008;74(2):163‐163e. [PUBMED: 18353201] [PubMed] [Google Scholar]
Meireles 2010 {published data only}
- Meireles SS, Goettems ML, Dantas RVF, Bona AD, Santos IS, Demarco FF. Changes in oral health related quality of life after dental bleaching in a double‐blind randomized clinical trial. Journal of Dentistry 2014;42:114‐21. [PUBMED: 24316342] [DOI] [PubMed] [Google Scholar]
- Meireles SS, Heckmann SS, Leida FL, Santos IS, Bona AD, Demarco FF. Efficacy and safety of 10% and 16% carbamide peroxide tooth‐whitening gels: a randomized clinical trial. Operative Dentistry 2008;33(6):606‐12. [PUBMED: 19051852 ] [DOI] [PubMed] [Google Scholar]
- Meireles SS, Heckmann SS, Santos IS, Bona AD, Demarco FF. A double blind randomized clinical trial of at‐home tooth bleaching using two carbamide peroxide concentrations: 6‐month follow‐up. Journal of Dentistry 2008;36(11):878‐84. [PUBMED: 18722039 ] [DOI] [PubMed] [Google Scholar]
- Meireles SS, Santos IS, Bona AD, Demarco FF. A double‐blind randomized clinical trial of two carbamide peroxide tooth bleaching agents: 2‐year follow‐up. Journal of Dentistry 2010;38(12):956‐63. [PUBMED: 20709137] [DOI] [PubMed] [Google Scholar]
- Meireles SS, Santos IS, Bona AD, Demarco FF. A double‐blind randomized controlled clinical trial of 10 percent versus 16 percent carbamide peroxide tooth‐bleaching agents. One‐year follow‐up. Journal of the American Dental Association 2009;140(9):1109‐17. [PUBMED: 19723943] [DOI] [PubMed] [Google Scholar]
- Meireles SS, Santos IS, Della BA, Demarco FF. Randomized clinical trial of two tooth bleaching agents: 2‐year follow‐up. IADR/AADR/CADR General Session; 2009 Apr 1‐4; Miami (Florida). 2009. [2367]
Mohan 2008 {published data only}
- Mohan N, Westland S, Brunton P, Ellwood R, Pretty IA, Luo W. A clinical study to evaluate the efficacy of a novel tray based tooth whitening system. Journal of Dentistry 2008;36(1):21‐6. [PUBMED: 18006206 ] [DOI] [PubMed] [Google Scholar]
Mokhlis 2000 {published data only}
- Mokhlis GR, Matis BA, Cochran MA, Eckert GJ. A clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime. Journal of the American Dental Association 2000;131(9):1269‐77. [PUBMED: 10986827] [DOI] [PubMed] [Google Scholar]
Myers 2003 {published data only}
- Myers ML, Browning WD, Downey MC, Hackman ST. Clinical evaluation of a 3% hydrogen peroxide tooth‐whitening gel. Journal of Esthetic and Restorative Dentistry 2003;15(1):50‐6. [PUBMED: 12638773] [DOI] [PubMed] [Google Scholar]
Nathoo 1994 {published data only}
- Nathoo SA, Chmielewski MB, Rustogi KN. Clinical evaluation of Colgate Platinum Professional Toothwhitening System and Rembrandt Lighten Bleaching Gel. Compendium (Newtown, Pa). Supplement 1994;14(17):S640‐5. [PUBMED: 8205581] [PubMed] [Google Scholar]
Nathoo 2001 {published data only}
- Nathoo S, Santana E 3rd, Zhang YP, Lin N, Collins M, Klimpel K, et al. Comparative seven‐day clinical evaluation of two tooth whitening products. Compendium of Continuing Education in Dentistry 2001;22(7):599‐604; 606; 608. [PUBMED: 11494621] [PubMed] [Google Scholar]
Nathoo 2002 {published data only}
- Nathoo S, Stewart B, Zhang YP, Chaknis P, Rustogi KN, Devizio W, et al. Efficacy of a novel, nontray, paint‐on 18% carbamide peroxide whitening gel. Compendium of Continuing Education in Dentistry 2002;23(11 Suppl 1):26‐31. [PUBMED: 12789994] [PubMed] [Google Scholar]
Nathoo 2003 {published data only}
- Nathoo S, Stewart B, Petrone ME, Chaknis P, Zhang YP, DeVizio W, et al. Comparative clinical investigation of the tooth whitening efficacy of two tooth whitening gels. Journal of Clinical Dentistry 2003;14(3):64‐9. [PUBMED: 14520776] [PubMed] [Google Scholar]
Navarra 2014 {published data only}
- Navarra CO, Reda B, Diolosa M, Casula I, Lenarda R, Breschi L, et al. The effects of two 10% carbamide peroxide nightguard bleaching agents, with and without desensitizer, on enamel and sensitivity: an in vivo study. International Journal of Dental Hygiene 2014;12(2):115‐20. [PUBMED: 24119064] [DOI] [PubMed] [Google Scholar]
Oliveira 2013 {published data only}
- Oliveira GM, Miguez PA, Oliveira GB, Swift EJ Jr, Farrell S, Anastasia MK, et al. Safety and efficacy of a high‐adhesion whitening strip under extended wear regimen. Journal of Dentistry 2013;41 Suppl 3:e46‐52. [PUBMED: 23228500] [DOI] [PubMed] [Google Scholar]
Papas 2009 {published data only}
- Papas AS, Kugel G, Singh M, Barker ML, Gerlach RW. Placebo‐controlled clinical trial of use of 10% hydrogen peroxide whitening strips for medication‐induced xerostomia. Gerontology 2009;55(5):511‐6. [PUBMED: 19707010] [DOI] [PubMed] [Google Scholar]
Porciani 2006 {published data only}
- Porciani PF, Grandini S, Perra C, Grandini R. Whitening effect by stain inhibition from a chewing gum with sodium hexametaphosphate in a controlled twelve‐week single‐blind trial. Journal of Clinical Dentistry 2006;17(1):14‐6. [PUBMED: 16838876] [PubMed] [Google Scholar]
Porciani 2010 {published data only}
- Porciani PF, Perra C, Grandini S. Effect on dental stain occurrence by chewing gum containing sodium tripolyphosphate ‐ A double‐blind six‐week trial. Journal of Clinical Dentistry 2010;21(1):4‐7. [PUBMED: 20527505] [PubMed] [Google Scholar]
Russell 1996 {published data only}
- Russell CM, Dickinson GL, Johnson MH, Curtis JW Jr, Downey MC, Haywood VB, et al. Dentist‐supervised home bleaching with ten percent carbamide peroxide gel: a six‐month study. Journal of Esthetic Dentistry 1996;8(4):177‐82. [PUBMED: 9468838] [DOI] [PubMed] [Google Scholar]
Shahidi 2005 {published data only}
- Shahidi H, Barker ML, Sagel PA, Gerlach RW. Randomized controlled trial of 10% hydrogen peroxide whitening strips. Journal of Clinical Dentistry 2005;16(3):91‐5. [PUBMED: 16305009] [PubMed] [Google Scholar]
Swift 2004 {published data only}
- Swift EJ Jr, Miguez PA, Barker ML, Gerlach RW. Three‐week clinical trial of a 14% hydrogen‐peroxide, strip‐based bleaching system. Compendium of Continuing Education in Dentistry 2004;25(8 Suppl 2):27‐32. [PUBMED: 15645892] [PubMed] [Google Scholar]
Swift 2009 {published data only}
- Swift EJ Jr, Heymann HO, Wilder AD Jr, Barker ML, Gerlach RW. Effects of duration of whitening strip treatment on tooth color: a randomized, placebo‐controlled clinical trial. Journal of Dentistry 2009;37 Suppl 1:e51‐6. [PUBMED: 19523738] [DOI] [PubMed] [Google Scholar]
Tam 2001 {published data only}
- Tam L. Effect of potassium nitrate and fluoride on carbamide peroxide bleaching. Quintessence International 2001;32(10):766‐70. [PUBMED: 11820045] [PubMed] [Google Scholar]
Tsubura 2005 {published data only}
- Tsubura S, Yamaguchi R. Clinical evaluation of a new bleaching product 'Polanight' in a Japanese population. Odontology 2005;93(1):52‐5. [PUBMED: 16170477] [DOI] [PubMed] [Google Scholar]
Turkun 2010 {published data only}
- Turkun M, Celik EU, Aladag A, Gokay N. One‐year clinical evaluation of the efficacy of a new daytime at‐home bleaching technique. Journal of Esthetic and Restorative Dentistry 2010;22(2):139‐48. [PUBMED: 20433566 ] [DOI] [PubMed] [Google Scholar]
Walters 2004 {published data only}
- Walters PA, Biesbrock AR, Bartizek RD. Benefits of sodium hexametaphosphate containing chewing gum for extrinsic stain inhibition. Journal of Dental Hygiene 2004;78(4):1‐7. [PUBMED: 16197748] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong AHH. A Randomized Controlled Trial of Home Tooth‐Whitening Products [Dissertation]. The University of Hong Kong 2004.
Xu 2007 {published data only}
- Xu X, Zhu L, Tang Y, Wang Y, Zhang K, Li S, et al. Randomized clinical trial comparing whitening strips, paint‐on gel and negative control. American Journal of Dentistry 2007;20:28A‐31A. [PUBMED: 19681256] [PubMed] [Google Scholar]
Ziebolz 2007 {published data only}
- Ziebolz D, Helms K, Hannig C, Attin T. Efficacy and oral side effects of two highly concentrated tray‐based bleaching systems. Clinical Oral Investigations 2007;11(3):267–75. [PUBMED: 17333304 ] [DOI] [PubMed] [Google Scholar]
Ziebolz 2008 {published data only}
- Ziebolz D, Hannig C, Attin T. Influence of a desensitizing agent on efficacy of a paint‐on bleaching agent. American Journal of Dentistry 2008;21(2):77‐82. [PUBMED: 18578172] [PubMed] [Google Scholar]
References to studies excluded from this review
Alkmin 2005 {published data only}
- Alkmin YT, Sartorelli R, Florio FM, Basting RT. Comparative study of the effects of two bleaching agents on oral microbiota. Operative Dentistry 2005;30(4):417‐23. [PUBMED: 16130860] [PubMed] [Google Scholar]
Amini 2009 {published data only}
- Amini P, Qaqish J, Anastasia MK, Hamilton AJ, Gerlach RW, Farrell S. Clinical response of experimental whitening strips with 6‐weeks daily use. IADR/AADR/CADR General Session; 2009 Apr 1‐4; Miami (Florida). 2009. [2277 ]
Amini 2011 {published data only}
- Amini P, Qaqish J, Conde E, Anastasia MK, Widmeyer V, Farrell S. Immediate effects and color degradation profile of 2‐hour whitening strip. IADR/AADR/CADR General Session; 2011 March 16‐19; San Diego (California). 2011. [2539]
Anastasia 2010 {published data only}
- Anastasia MK, Conde E, Widmeyer V, Fiedler S, Farrell S. Clinical efficacy and safety of two marketed whitening treatments. AADR/CADR Annual Meeting; 2010 March 3‐6; Washington (DC). 2010. [447]
Andreana 2000 {published data only}
- Andreana S, Ciancio SG, Mather ML. Clinical evaluation of bleaching gels on patients with sensitive teeth. Journal of Dental Research 2000;79(1 Suppl):216 (Abs No 583). [Google Scholar]
Archila 2010 {published data only}
- Archila L, Ibanez E, Conde E, Widmeyer V, Anastasia MK, Farrell S. Short‐term whitening efficacy of marketed high‐adhesion whitening strips. AADR/CADR Annual Meeting; 2010 March 3‐6; Washington (DC). 2010. [833]
Auschill 2009 {published data only}
- Auschill TM, Schneider‐del ST, Hellwig E, Arweiler NB. Clinical comparison of two different bleaching systems: tray versus strips. IADR/AADR/CADR General Session; 2009 Apr 1‐4; Miami (Florida). 2009. [2832]
Browning 2001 {published data only}
- Browning WD, Myers ML, Downey MC, Pohjola RM, Hackman ST, Cliett BA. Safety and efficacy of an experimental tooth whitening agent. Journal of Dental Research 2001;80(1 Suppl):182 (Abs No 1176). [Google Scholar]
Burgio 2001 {published data only}
- Burgio PA, Donovan TE, Kugel G, Matranga LF. Multi‐center clinical study to compare the effectiveness of dental bleaching. Journal of Dental Research 2001;80(1 Suppl):150 (Abs No 918). [Google Scholar]
Cardoso 2010 {published data only}
- Cardoso PC, Reis A, Loguercio A, Vieira LC, Baratieri LN. Clinical effectiveness and tooth sensitivity associated with different bleaching times for a 10 percent carbamide peroxide gel. Journal of the American Dental Association 2010;141(10):1213‐20. [PUBMED: 20884923] [DOI] [PubMed] [Google Scholar]
Collins 2004 {published data only}
- Collins LZ, Maggio B, Gallagher A, York M, Schafer F. Safety evaluation of a novel whitening gel, containing 6% hydrogen peroxide and a commercially available whitening gel containing 18% carbamide peroxide in an exaggerated use clinical study. Journal of Dentistry 2004;32(1):47‐50. [PUBMED: 14738835] [DOI] [PubMed] [Google Scholar]
Corby 2014 {published data only}
- Corby PM, Biesbrock A, Gerlach R, Corby AL, Moreira A, Schork NJ, et al. Treatment responses to tooth whitening in twins. Twin Research and Human Genetics 2014;17(1):23‐6. [PUBMED: 24429255] [DOI] [PubMed] [Google Scholar]
Curtis 1996 {published data only}
- Curtis JW, Dickinson GL, Downey MC, Russell CM, Haywood VB, Myers ML, et al. Assessing the effects of 10 percent carbamide peroxide on oral soft tissues. Journal of the American Dental Association 1996;127(8):1218‐23. [PUBMED: 8803398] [DOI] [PubMed] [Google Scholar]
de Geus 2015a {published data only}
- Geus JL, Rezende M, Margraf LS, Bortoluzzi MC, Fernandez E, Loguercio AD, et al. Evaluation of genotoxicity and efficacy of at‐home bleaching in smokers: a single‐blind controlled clinical trial. Operative Dentistry 2015;40(2):E47‐55. [PUBMED: 25535783] [DOI] [PubMed] [Google Scholar]
de Geus 2015b {published data only}
- Geus JL, Bersezio C, Urrutia J, Yamada T, Fernandez E, Loguercio AD, et al. Effectiveness of and tooth sensitivity with at‐home bleaching in smokers: a multicenter clinical trial. Journal of the American Dental Association 2015;146(4):233‐40. [PUBMED: 25819654] [DOI] [PubMed] [Google Scholar]
Dickinson 2000 {published data only}
- Dickinson GL, Browning WD, Hawkins IK, Downey MC, Myers ML, Hackman ST, et al. Clinical evaluation of ADS tooth whitening gel. Journal of Dental Research 2000;79(1 Suppl):438 (Abs No 2359). [Google Scholar]
Donly 2001 {published data only}
- Donly KJ, Gerlach R, Segura A, Walters P, Zhou X. Post‐orthodontic tooth whitening. Journal of Dental Research 2001;80(1 Suppl):151 (Abs No 924). [Google Scholar]
Donly 2002 {published data only}
- Donly KJ, Donly AS, Baharloo L, Rojas‐Candelas E, Garcia‐Godoy F, Zhou X, et al. Tooth whitening in children. Compendium of Continuing Education in Dentistry 2002;23(1A):22‐8. [PUBMED: 11913290] [PubMed] [Google Scholar]
Donly 2002a {published data only}
- Donly KJ, Gerlach RW. Clinical trials on the use of whitening strips in children and adolescents. General Dentistry 2002;50(3):242‐5. [PUBMED: 12116511] [PubMed] [Google Scholar]
Farrell 2006 {published data only}
- Farrell S, Barker ML, Sagel PA, Gerlach RW. Use of a physical barrier to improve efficacy of a paint‐on whitening gel: a seven‐day randomized clinical trial. Journal of Clinical Dentistry 2006;17(5):117‐21. [PUBMED: 17240929] [PubMed] [Google Scholar]
Farrell 2008 {published data only}
- Farrell S, Barker ML, McMillan DA, Gerlach RW. Placebo‐controlled trial evaluating safety with 12‐months continuous use of 6% hydrogen peroxide whitening strips. Journal of Dentistry 2008;36(9):726‐30. [PUBMED: 18635304] [DOI] [PubMed] [Google Scholar]
Fugaro 2004 {published data only}
- Fugaro JO, Nordahl I, Fugaro OJ, Matis BA, Mjor IA. Pulp reaction to vital bleaching. Operative Dentistry 2004;29(4):363‐8. [PUBMED: 15279473] [PubMed] [Google Scholar]
Fugaro 2005 {published data only}
- Fugaro OJ, Fugaro JO, Matis B, Gregory RL, Cochran MA, Mjor I. The dental pulp: inflammatory markers and vital bleaching. American Journal of Dentistry 2005;18(4):229‐32. [PUBMED: 16296427] [PubMed] [Google Scholar]
Garcia‐Godoy 2012 {published data only}
- Garcia‐Godoy C, Truehart D, Rothrock JK, Farrell S, Anastasia MK, Conde E, et al. Whitening efficacy and color degradation profile of 2‐hour whitening strips. AADR/CADR Annual Meeting; 2012 March 21‐24; Tampa (Florida). 2012. [478]
Gerlach 2002c {published data only}
- Gerlach RW, Sagel PA, Jeffers ME, Zhou X. Effect of peroxide concentration and brushing on whitening clinical response. Compendium of Continuing Education in Dentistry 2002;23(1A):16‐21. [PUBMED: 11913289] [PubMed] [Google Scholar]
Gerlach 2002d {published data only}
- Gerlach RW, Gibb RD, Zhou X. Clinical response of maxillary and mandibular teeth following use of 6.5% hydrogen peroxide whitening strips. Journal of Dental Research 2002;81(1 Suppl):A‐253 (Abs No 1954). [Google Scholar]
Gerlach 2002e {published data only}
- Gerlach RW, Campolongo KL, Zhou X. Use of a chroma meter to compare the shade change for two professional tooth whitening systems. Journal of Dental Research 2002;81(1 Suppl):A‐253 (Abs No 1957). [Google Scholar]
Gerlach 2003a {published data only}
- Gerlach RW, Barker ML. Clinical response of three direct‐to‐consumer whitening products: strips, paint‐on gel, and dentifrice. Compendium of Continuing Education in Dentistry 2003;24(6):458, 461‐4, 466. [PUBMED: 14515596] [PubMed] [Google Scholar]
Gerlach 2004a {published data only}
- Gerlach RW, Barker ML, Tucker HL. Clinical response of three whitening products having different peroxide delivery: comparison of tray, paint‐on gel, and dentifrice. Journal of Clinical Dentistry 2004;15(4):112‐7. [PUBMED: 15794456] [PubMed] [Google Scholar]
Gerlach 2004c {published data only}
- Gerlach RW, Barker ML, McMillan DA, Sagel PA, Walden GL. In‐use comparative kinetics of professional whitening strips: peroxide recovery from strips, teeth, gingiva, and saliva. Compendium of Continuing Education in Dentistry 2004;25(8 Suppl 2):14‐20. [PUBMED: 15645890] [PubMed] [Google Scholar]
Gerlach 2004d {published data only}
- Gerlach RW, Barker ML. Professional vital bleaching using a thin and concentrated peroxide gel on whitening strips: an integrated clinical summary. Journal of Contemporary Dental Practice 2004;5(1):1‐17. [PUBMED: 14973556] [PubMed] [Google Scholar]
Godson 2001 {published data only}
- Goodson JM, Taveres M, Stutz J, Liao SS, Kent R, Newman M. Colorimeter measurement of tooth whitening. Journal of Dental Research 2001;80(1 Suppl):182 (Abs No 1170). [1170] [Google Scholar]
Gursoy 2008 {published data only}
- Gursoy UK, Eren DI, Bektas OO, Hurmuzlu F, Bostanci V, Ozdemir H. Effect of external tooth bleaching on dental plaque accumulation and tooth discoloration. Medicina Oral, Patologia Oral y Cirugia Bucal 2008;13(4):E266‐9. [PUBMED: 18379454] [PubMed] [Google Scholar]
Jadad 2011 {published data only}
- Jadad E, Montoya J, Arana G, Gordillo LA, Palo RM, Loguercio AD. Spectrophotometric evaluation of color alterations with a new dental bleaching product in patients wearing orthodontic appliances. American Journal of Orthodontics and Dentofacial Orthopedics 2011;140(1):e43‐7. [PUBMED: 21724070] [DOI] [PubMed] [Google Scholar]
Jorgensen 2002 {published data only}
- Jorgensen MG, Carroll WB. Incidence of tooth sensitivity after home whitening treatment. Journal of the American Dental Association 2002;133(8):1076‐82. [PUBMED: 12198987] [DOI] [PubMed] [Google Scholar]
Karpinia 2003 {published data only}
- Karpinia K, Magnusson I, Barker ML, Gerlach RW. Clinical comparison of two self‐directed bleaching systems. Journal of Prosthodontics 2003;12(4):242‐8. [PUBMED: 15061232] [DOI] [PubMed] [Google Scholar]
Lee 2003 {published data only}
- Lee BJ, Paik DI, Kim JB. Clinical evaluation of the tooth‐whitening effect of a 2.6% hydrogen peroxide tooth‐whitening strip. AADR/CADR Anual Meeting; 2003 March; San Antonio (Texas). 2003. [1876]
Leonard 2002 {published data only}
- Leonard RH Jr, Garland GE, Eagle JC, Caplan DJ. Safety issues when using a 16% carbamide peroxide whitening solution. Journal of Esthetic and Restorative Dentistry 2002;14(6):358‐67. [PUBMED: 12542101] [DOI] [PubMed] [Google Scholar]
Leonard 2004 {published data only}
- Leonard RH, Smith LR, Garland GE, Caplan DJ. Densensitizing Agent Efficacy during Whitening in an At Risk Population. Journal of Esthetic and Restorative Dentistry 2004;16(1):49‐56. [PUBMED: 15259543] [DOI] [PubMed] [Google Scholar]
Leonard 2007 {published data only}
- Leonard RH Jr, Smith LR, Garland GE, Tiwana KK, Zaidel LA, Pugh G Jr, et al. Evaluation of side effects and patients' perceptions during tooth bleaching. Journal of Esthetic and Restorative Dentistry 2007;19(6):355‐64; discussion 365‐6. [PUBMED: 18005286] [DOI] [PubMed] [Google Scholar]
Lisante 2009 {published data only}
- Lisante TA, Askinazi J, Simmons K, Charles C, Mcguire JT. Comparative whitening effectiveness of three hydrogen peroxide‐containing mouthrinses. IADR/AADR/CADR General Session; 2009 Apr 1‐4; Miami (Florida). 2009. [2597]
Loyola‐Rodriguez 2003 {published data only}
- Loyola‐Rodriguez JP, Pozos‐Guillen Ade J, Hernandez‐Hernandez F, Berumen‐Maldonado R, Patino‐Marin N. Effectiveness of treatment with carbamide peroxide and hydrogen peroxide in subjects affected by dental fluorosis: a clinical trial. Journal of Clinical Pediatric Dentistry 2003;28(1):63‐7. [PUBMED: 14604145] [DOI] [PubMed] [Google Scholar]
Majeed 2011 {published data only}
- Majeed A, Grobler SR, Moola MH. Clinical evaluation of two at‐home tooth‐whitening products using a spectrophotometer. 45th CED‐IADR/NOF Meeting; 2011 Aug 31; Budapest (Hungary). 2011. [231]
Marques 2012 {published data only}
- Marques DN, Mata AD, Silveira JM, Marques JR, Amaral JP, Guilherme NF. Hydrogen peroxide release kinetics into saliva from different whitening products: a double‐blind, randomized clinical trial. Clinical Oral Investigations 2012;16(1):155‐63. [PUBMED: 21221681] [DOI] [PubMed] [Google Scholar]
Martin 2015 {published data only}
- Martin J, Vildosola P, Bersezio C, Herrera A, Bortolatto J, Saad JR, et al. Effectiveness of 6% hydrogen peroxide concentration for tooth bleaching ‐ A double‐blind, randomized clinical trial. Journal of Dentistry 2015;43(8):965‐72. [PUBMED: 26057085] [DOI] [PubMed] [Google Scholar]
Matis 1999 {published data only}
- Matis BA, Gaiao U, Blackman D, Schultz FA, Eckert GJ. In vivo degradation of bleaching gel used in whitening teeth. Journal of the American Dental Association 1999;130(2):227‐35. [PUBMED: 10036846] [DOI] [PubMed] [Google Scholar]
Matis 2002 {published data only}
- Matis BA, Yousef M, Cochran MA, Eckert GJ. Degradation of bleaching gels in vivo as a function of tray design and carbamide peroxide concentration. Operative Dentistry 2002;27(1):12‐8. [PUBMED: 11817465] [PubMed] [Google Scholar]
Matis 2002a {published data only}
- Matis BA, Hamdan YS, Cochran MA, Eckert GJ. A clinical evaluation of a bleaching agent used with and without reservoirs. Operative Dentistry 2002;27(1):5‐11. [PUBMED: 11817468] [PubMed] [Google Scholar]
Matis 2005 {published data only}
- Matis BA, Cochran M, Wang G, Franco M, Eckert GJ, Carlotti RJ, et al. A clinical evaluation of bleaching using whitening wraps and strips. Operative Dentistry 2005;30(5):588‐92. [PUBMED: 16268392] [PubMed] [Google Scholar]
Mazur 2013 {published data only}
- Mazur RF, Busato P, Souza EM, Vieira S, Marini A, Rached R. Effect of aloe vera on the adverse effects of bleaching. IADR/AADR/CADR General Session; 2013 March 20‐23; Seattle (Washington). 2013. [1144]
NCT02603354 {published data only}
- NCT02603354. Efectiveness of a brief protocol by a low (6%) concentration gel peroxide hydrogen of teeth bleaching (brief 6%). clinicaltrials.gov/ct2/show/NCT02603354 (first received 11 November 2015).
NCT02682329 {unpublished data only}
- NCT02682329. Combination of dental bleaching techniques, randomized clinical trial. clinicaltrials.gov/ct2/show/NCT02682329 (first received 15 February 2016).
Perdigao 2013 {published data only}
- Perdigao J, Dutra‐Correa M, Saraceni CHC, Delazari MA, Kodama RM, Bergamini MR. Clinical evaluation of 10% carbamide peroxide with different desensitizers. IADR/AADR/CADR General Session; 2013 March 20‐23; Seattle (Washington). 2013. [603]
Pinto 2014 {published data only}
- Pinto MM, Godoy CH, Bortoletto CC, Olivan SR, Motta LJ, Altavista OM, et al. Tooth whitening with hydrogen peroxide in adolescents: study protocol for a randomized controlled trial. Trials 2014;15:395. [PUBMED: 25315893] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2017 {published data only}
- Pinto MM, Goncalves ML, Mota AC, Deana AM, Olivan SR, Bortoletto C, et al. Controlled clinical trial addressing teeth whitening with hydrogen peroxide in adolescents: a 12‐month follow‐up. Clinics (Sao Paulo, Brazil) 2017;72(3):161‐70. [PUBMED: 28355362] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rezende 2013 {published data only}
- Rezende M, Loguercio AD, Reis A, Kossatz S. Clinical effects of exposure to coffee during at‐home vital bleaching. Operative Dentistry 2013;38(6):E229‐36. [PUBMED: 23570297] [DOI] [PubMed] [Google Scholar]
Sagel 2001 {published data only}
- Sagel PA, Walters PA, Gibb RD, Gerlach RW. Duration of tooth whitening following 14 days treatment with peroxide‐containing whitening strips. Journal of Dental Research 2001;80(1 Suppl):182 (Abs No 1173). [Google Scholar]
Schiff 1994 {published data only}
- Schiff T, Borden LC. The effect of a new experimental prebrushing dental rinse on plaque removal. Journal of Clinical Dentistry 1994;4(4):107‐10. [PUBMED: 8031477] [PubMed] [Google Scholar]
Schulte 1993 {published data only}
- Schulte JR, Morrissette DB, Gasior EJ, Czajewski MV. Clinical changes in the gingiva as a result of at‐home bleaching. Compendium 1993;14(11):1362, 1364‐6. [PUBMED: 8620374] [PubMed] [Google Scholar]
Schulte 1994 {published data only}
- Schulte JR, Morrissette DB, Gasior EJ, Czajewski MV. The effects of bleaching application time on the dental pulp. Journal of the Americal Dental Association 1994;125(10):1330‐5. [PUBMED: 7844297] [DOI] [PubMed] [Google Scholar]
Simon 2001 {published data only}
- Simon JF, McClanahan SF, Gerlach RW. Clinical trial comparing tooth whitening with peroxide‐containing strips to a marketed whitening dentifrice. Journal of Dental Research 2001;80(1 Suppl):237 (Abs No 1616). [Google Scholar]
Simon 2011 {published data only}
- Simon JF, Lewis M, Powell L, Wilson N, Conde E, Anastasia MK, et al. Efficacy of 2‐hour whitening strip under extended use conditions. IADR/AADR/CADR General Session; 2011 March 16‐19; San Diego (California). 2011. [2540]
Smith 2001 {published data only}
- Smith LR, Leonard RH, Eagle JC, Murdoch WC, Garland GE, Caplan DJ. Efficacy of desensitizing gel in reducing tooth sensitivity during whitening. Journal of Dental Research 2001;80(1 Suppl):246 (Abs No 1683). [Google Scholar]
Swift 2001 {published data only}
- Swift EJ Jr, Heymann HO, Ritter AV, Rosa BT, Wilder AD Jr. Clinical evaluation of a novel 'trayless' tooth whitening system. Journal of Dental Research 2001;80(1 Suppl):151 (Abs No 921). [Google Scholar]
Tam 1999 {published data only}
- Tam L. Clinical trial of three 10% carbamide peroxide bleaching products. Journal of the Canadian Dental Association 1999;65(4):201‐5. [PUBMED: 10224721] [PubMed] [Google Scholar]
Walter 2011 {published data only}
- Walter R, Miguez PA, Oliveira GCB, Oliveira GMS, Farrell S, Anastasia MK, et al. Safety and efficacy of a 2‐hour whitening strip. IADR/AADR/CADR General Session; 2011 March 16‐19; San Diego (California). 2011. [555]
Yankell 1997 {published data only}
- Yankell SL. Efficacy of chewing gum in preventing extrinsic tooth staining. Journal of Clinical Dentistry 1997;8(6):169‐72. [PUBMED: 9586534] [PubMed] [Google Scholar]
Zantner 2006 {published data only}
- Zantner C, Derdilopoulou F, Martus P, Kielbassa AM. Randomized clinical trial on the efficacy of 2 over‐the‐counter whitening systems. Quintessence International 2006;37:695‐706. [PUBMED: 17017631] [PubMed] [Google Scholar]
References to studies awaiting assessment
Barnes 1998 {published data only}
- Barnes DM, Kihn PW, Romberg E, George D, DePaola L, Medina E. Clinical evaluation of a new 10% carbamide peroxide tooth‐whitening agent. Compendium of Continuing Education in Dentistry 1998;19(10):968‐72, 977‐8. [PUBMED: 10371880] [PubMed] [Google Scholar]
Bizhang 2017 {published data only}
- Bizhang M, Domin J, Danesh G, Zimmer S. Effectiveness of a new non‐hydrogen peroxide bleaching agent after single use ‐ a double‐blind placebo‐controlled short‐term study. Journal of Applied Oral Science 2017;25(5):575‐84. [PUBMED: 29069156] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2007 {published data only}
- Braun A, Jepsen S, Krause F. Spectrophotometric and visual evaluation of vital tooth bleaching employing different carbamide peroxide concentrations. Dental Materials 2007;23(2):165‐9. [PUBMED: 16504281] [DOI] [PubMed] [Google Scholar]
Browning 2004 {published data only}
- Browning WD, Chan DC, Frazier KB, Callan RS, Blalock JS. Safety and efficacy of a nightguard bleaching agent containing sodium fluoride and potassium nitrate. Quintessence International 2004;35(9):693‐8. [PUBMED: 15470992] [PubMed] [Google Scholar]
Ferrari 2004 {published data only}
- Ferrari M, Kugel G, Cagidiaco MC, Barker ML, Gerlach RW. Clinical trial evaluating the peroxide concentration response of whitening strips over 28 days. American Journal of Dentistry 2004;17(4):291‐4. [PUBMED: 15478494] [PubMed] [Google Scholar]
Gambarini 2004 {published data only}
- Gambarini G, Testarelli L, Luca M, Dolci G. Efficacy and safety assessment of a new liquid tooth whitening gel containing 5.9% hydrogen peroxide. American Journal of Dentistry 2004;17(2):75‐9. [PUBMED: 15151330] [PubMed] [Google Scholar]
Gegauff 1993 {published data only}
- Gegauff AG, Rosenstiel SF, Langhout KJ, Johnston WM. Evaluating tooth color change from carbamide peroxide gel. Journal of the American Dental Association 1993;124(6):65‐72. [PUBMED: 8505452] [DOI] [PubMed] [Google Scholar]
Gerlach 2004b {published data only}
- Gerlach RW, Sagel PA. Vital bleaching with a thin peroxide gel: the safety and efficacy of a professional‐strength hydrogen peroxide whitening strip. Journal of the American Dental Association 2004;135(1):98‐100. [PUBMED: 14959882] [DOI] [PubMed] [Google Scholar]
Guerrero 2007 {published data only}
- Guerrero JC, Jimenez‐Farfan MD, Lopez‐Salgado A, Barker ML, Gerlach RW. Professional whitening strips in a university population. American Journal of Dentistry 2007;20 Spec No A:15A‐8A. [PUBMED: 19681253] [PubMed] [Google Scholar]
Heymann 1998 {published data only}
- Heymann HO, Swift EJ Jr, Bayne SC, May KN Jr, Wilder AD Jr, Mann GB, et al. Clinical evaluation of two carbamide peroxide tooth‐whitening agents. Compendium of Continuing Education in Dentistry 1998;19(4):359‐62, 364‐6. [PUBMED: 9656849] [PubMed] [Google Scholar]
Kim 2018 {published data only}
- Kim YM, Ha AN, Kim JW, Kim SJ. Double‐blind randomized study to evaluate the safety and efficacy of over‐the‐counter tooth‐whitening agents containing 2.9% hydrogen peroxide. Operative Dentistry 2018;43(3):272‐81. [PUBMED: 29513635] [DOI] [PubMed] [Google Scholar]
Maran 2018 {published data only}
- Maran BM, Vochikovski L, Andrade Hortkoff DR, Stanislawczuk R, Loguercio AD, Reis A. Tooth sensitivity with a desensitizing‐containing at‐home bleaching gel ‐ a randomized triple‐blind clinical trial. Journal of Dentistry 2018;72:64‐70. [PUBMED: 29551346] [DOI] [PubMed] [Google Scholar]
NCT02151058 {published data only}
- NCT02151058. A clinical trial to test the effect of a marketed mouth rinse on stain removal [Whitening action of a hydrogen peroxide/sodium fluoride containing mouth rinse: a 2 week randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02151058 (first received 30 May 2014).
NCT03217994 {published data only}
- NCT03217994. Efficacy of a 6% hydrogen peroxide tooth bleaching agent [Efficacy of a 6% hydrogen peroxide tooth bleaching agent without ligth]. clinicaltrials.gov/ct2/show/NCT03217994 (first received 14 July 2017).
Ozcan 2003 {published data only}
- Ozcan M, Kulak Y, Kazazoglu E. The efficacy of two prototype chewing gums for the removal of extrinsic tooth stain. International Dental Journal 2003;53(2):62‐6. [PUBMED: 12731691] [DOI] [PubMed] [Google Scholar]
Pohjola 2002 {published data only}
- Pohjola RM, Browning WD, Hackman ST, Myers ML, Downey MC. Sensitivity and tooth whitening agents. Journal of Esthetic and Restorative Dentistry 2002;14(2):85‐91. [PUBMED: 12008806] [DOI] [PubMed] [Google Scholar]
Reinhardt 1993 {published data only}
- Reinhardt JW, Eivins SE, Swift EJ Jr, Denehy GE. A clinical study of nightguard vital bleaching. Quintessence International 1993;24(6):379‐84. [PUBMED: 8234642] [PubMed] [Google Scholar]
Rosenstiel 1996 {published data only}
- Rosenstiel SF, Gegauff AG, Johnston WM. Randomized clinical trial of the efficacy and safety of a home bleaching procedure. Quintessence International 1996;27(6):413‐24. [PUBMED: 8941836] [PubMed] [Google Scholar]
Rossi 2018 {published data only}
- Rossi B, Freitas PM, Tedesco TK, Goncalves F, Ferreira LS. Tooth color changes and sensitivity in patients undergoing dental bleaching with 10% hydrogen peroxide using customized trays or strips: a randomized clinical trial. Minerva Stomatologica 2018;67(2):55‐61. [PUBMED: 29446268] [DOI] [PubMed] [Google Scholar]
Shin 2010 {published data only}
- Shin BG, Yang SE. The evaluation of clinical efficacy and longevity of home bleaching without combined application of in office bleaching. Journal of Korean Academy of Conservative Dentistry 2010;35(5):387‐94. [Google Scholar]
Sielski 2003 {published data only}
- Sielski C, Conforti N, Stewart B, Chaknis P, Petrone ME, DeVizio W, et al. A clinical investigation of the efficacy of a tooth‐whitening gel. Compendium of Continuing Education in Dentistry 2003;24(8):612‐4, 616‐8. [PUBMED: 14692166] [PubMed] [Google Scholar]
Simon 2014 {published data only}
- Simon JF, Powell L, Hollis S, Anastasia MK, Gerlach RW, Farrell S. Placebo‐controlled clinical trial evaluating 9.5% hydrogen peroxide high‐adhesion whitening strips. Journal of Clinical Dentistry 2014;25(3):49‐52. [PUBMED: 26054177] [PubMed] [Google Scholar]
References to ongoing studies
NCT03026725 {published data only}
- NCT03026725. Effect of tri calcium phosphate on efficacy and sensitivity with Vital tooth whitening using 20% carbamide peroxide [Effect of tri calcium phosphate paste on efficacy and postoperative sensitivity associated with at‐home Vital tooth whitening using 20% carbamide peroxide ‐ A randomized controlled study]. clinicaltrials.gov/ct2/show/NCT03026725 (first received 20 January 2017).
Additional references
Alkhatib 2004
- Alkhatib MN, Holt R, Bedi R. Prevalence of self‐assessed tooth discolouration in the United Kingdom. Journal of Dentistry 2004;32(7):561‐6. [PUBMED: 15386863] [DOI] [PubMed] [Google Scholar]
Alqahtani 2014
- Alqahtani MQ. Tooth‐bleaching procedures and their controversial effects: a literature review. Saudi Dental Journal 2014;26(2):33‐46. [PUBMED: 25408594] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Barabolak 1991
- Barabolak R, Hoerman K, Kroll B, Record D. Gum chewing profiles in the US population. Community Dentistry and Oral Epidemiology 1991;19(2):125‐6. [PUBMED: 2049920] [DOI] [PubMed] [Google Scholar]
Baroudi 2014
- Baroudi K, Hassan NA. The effect of light‐activation sources on tooth bleaching. Nigerian Medical Journal 2014;55(5):363‐8. [PUBMED: 25298598] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belobrov 2011
- Belobrov I, Parashos P. Treatment of tooth discoloration after the use of white mineral trioxide aggregate. Journal of Endodontics 2011;37(7):1017‐20. [PUBMED: 21689563] [DOI] [PubMed] [Google Scholar]
Brennan 2003
- Brennan K, Barker ML, Zhou X, Barlow AP, Gerlach RW. Factors contributing to tooth whitening with a direct application of percarbonate bleaching film: meta‐analysis of 7 clinical trials. IADR/PER General Session; 2003 June; Goteborg (Sweden). 2003. [1047]
Caballero 2006
- Berga‐Caballero A, Forner‐Navarro L, Amengual‐Lorenzo J. At‐home vital bleaching: a comparison of hydrogen peroxide and carbamide peroxide treatments. Medicina Oral, Patologia Oral y Cirugia Bucal 2006;11(1):94‐9. [PUBMED: 16388304] [PubMed] [Google Scholar]
Carey 2014
- Carey CM. Tooth whitening: what we now know. Journal of Evidence‐based Dental Practice 2014;14:70‐6. [PUBMED: 24929591] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Dantas 2015
- Dantas AAR, Bortolatto JF, Roncolato A, Merchan H, Floros MC, Kuga MC, et al. Can a bleaching toothpaste containing Blue Covarine demonstrate the same bleaching as conventional techniques? An in vitro, randomized and blinded study. Journal of Applied Oral Science 2015;23(6):609‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vasconcelos 2012
- Vasconcelos AA, Cunha AG, Borges BC, Machado CT, dos Santos AJ. Tooth whitening with hydrogen/carbamide peroxides in association with a CPP‐ACP paste at different proportions. Australian Dental Journal 2012;57(2):213‐9. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Demarco 2009
- Demarco FF, Meireles SS, Masotti AS. Over‐the‐counter whitening agents: a concise review. Brazilian Oral Research 2009;23:64‐70. [PUBMED: 19838560] [DOI] [PubMed] [Google Scholar]
Duschner 2006
- Duschner H, Götz H, White DJ, Kozak KM, Zoladz JR. Effects of hydrogen peroxide bleaching strips on tooth surface color, surface microhardness, surface and subsurface ultrastructure, and microchemical (Raman spectroscopic) composition. Journal of Clinical Dentistry 2006;17(3):72‐8. [PUBMED: 17022369] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerlach 2001
- Gerlach RW, Barker ML, Sagel PA. Comparative efficacy and tolerability of two direct‐to‐consumer tooth whitening systems. American Journal of Dentistry 2001;14(5):267‐72. [PUBMED: 11803987] [PubMed] [Google Scholar]
Gerlach 2007
- Gerlach RW. Tooth whitening clinical trials: a global perspective. American Journal of Dentistry 2007;20 Spec No A:3A‐6A. [PUBMED: 19681251] [PubMed] [Google Scholar]
Gerlach 2009
- Gerlach RW, Barker ML, Karpinia K, Magnusson I. Single site meta‐analysis of 6% hydrogen peroxide whitening strip effectiveness and safety over 2 weeks. Journal of Dentistry 2009;37(5):360‐5. [PUBMED: 19233534] [DOI] [PubMed] [Google Scholar]
Gerlach 2010
- Gerlach RW, Anastasia MK, Farrell S, Barker ML, Sagel PA. Safety and efficacy meta‐analysis of self‐directed tooth whitening by delivery. AADR/CADR Annual Meeting; 2010 March 3‐6; Washington (DC). 2010. [828]
Gerlach 2013
- Gerlach RW, Barker ML, Anastasia MK, Farrell S, Sagel PA. Subject‐level meta‐analysis of 10% hydrogen peroxide whitening strip clinical response. IADR/AADR/CADR General Session; 2013 March 20‐23; Seattle (Washington). 2013. [27]
Goldstein 1995
- Goldstein RE, Graber DA. Complete Dental Bleaching. 1st Edition. Chicago: Quintessence Publishing, 1995:73‐4. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 12 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haywood 1992
- Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence International 1992;23(7):471‐88. [PUBMED: 1410249] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horn 2014
- Horn BA, Bittencourt BF, Gomes OM, Farhat PA. Clinical evaluation of the whitening effect of over‐the‐counter dentifrices on vital teeth. Brazilian Dental Journal 2014;25(3):203‐6. [PUBMED: 25252254] [DOI] [PubMed] [Google Scholar]
Javaheri 2000
- Javaheri DS, Janis JN. The efficacy of reservoirs in bleaching trays. Operative Dentistry 2000;25(3):149‐51. [PubMed] [Google Scholar]
Joiner 2006
- Joiner A. The bleaching of teeth: a review of the literature. Journal of Dentistry 2006;34(7):412‐9. [PUBMED: 16569473] [DOI] [PubMed] [Google Scholar]
Joshi 2016
- Joshi SB. An overview of vital teeth bleaching. Journal of Interdisciplinary Dentistry 2016;6(1):3‐13. [DOI: 10.4103/2229-5194.188155] [DOI] [Google Scholar]
Kim 2010
- Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. Journal of Endodontics 2010;36(6):1086‐91. [PUBMED: 20478471] [DOI] [PubMed] [Google Scholar]
Kolosowski 2014
- Kolosowski KP, Sodhi RN, Kishen A, Basrani BR. Qualitative analysis of precipitate formation on the surface and in the tubules of dentin irrigated with sodium hypochlorite and a final rinse of chlorhexidine or QMiX. Journal of Endodontics 2014;40(12):2036‐40. [PUBMED: 25305239] [DOI] [PubMed] [Google Scholar]
Kowitz 1994a
- Kowitz GM, Nathoo SA, Rustogi KN, Chmielewski MB, Liang LJ, Wong R. Clinical comparison of Colgate Platinum Tooth Whitening System and Rembrandt Gel Plus. Compendium (Newtown, Pa). Supplement 1994;15(17):S646‐51. [PUBMED: 8205582] [PubMed] [Google Scholar]
Kwon 2015
- Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. Journal of Esthetic and Restorative Dentistry 2015;27(5):240‐57. [PUBMED: 25969131] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leonard 2003
- Leonard RH Jr, Haywood B, Caplan DJ, Tart ND. Nightguard vital bleaching of tetracycline‐stained teeth: 90 months post treatment. Journal of Esthetic and Restorative Dentistry 2003;15(3):142‐52. [PUBMED: 12859112] [DOI] [PubMed] [Google Scholar]
Lima 2012
- Lima FG, Rotta TA, Penso S, Meireles SS, Demarco FF. In vitro evaluation of the whitening effect of mouth rinses containing hydrogen peroxide. Brazilian Oral Research 2012;26(3):269‐74. [PUBMED: 22641448] [DOI] [PubMed] [Google Scholar]
Mankodi 2001
- Mankodi SM, Conforti N, Berkowitz H. Efficacy of baking soda‐containing chewing gum in removing natural tooth stain. Compendium of Continuing Education in Dentistry 2001;22(7A):29‐32. [PUBMED: 11913307] [PubMed] [Google Scholar]
Nathoo 1997
- Nathoo SA. The chemistry and mechanisms of extrinsic and intrinsic discoloration. Journal of the American Dental Association 1997;128:6‐10. [PUBMED: 9120149] [DOI] [PubMed] [Google Scholar]
Niederman 2000
- Niederman R, Tantraphol MC, Slinin P, Hayes C, Conway S. Effectiveness of dentist‐prescribed, home‐applied tooth whitening. A meta‐analysis. Journal of Contemporary Dental Practice 2000;1(4):20‐36. [PUBMED: 12167948] [PubMed] [Google Scholar]
Panich 2001
- Panich M. In Vivo Evaluation of 15‐Percent Carbamide Peroxide and 5.5‐Percent Hydrogen Peroxide Whitening Agents During Daytime Use [Masters thesis]. Indiana (USA): School of Dentistry, Indiana University, 2001. [Google Scholar]
Perdigão 2004
- Perdigão J, Baratieri LN, Arcari GM. Contemporary trends and techniques in tooth whitening: a review. Practical Procedures and Aesthetic Dentistry 2004;16(3):185‐92. [PUBMED: 15199693] [PubMed] [Google Scholar]
Po 2014
- Po LH, Wilson NW. Effects of different desensitizing agents on bleaching treatments. European Journal of General Dentistry 2014;3(2):93‐9. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shellis 2005
- Shellis RP, Addy M, Rees GD. In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. Journal of Dentistry 2005;33(4):313‐24. [PUBMED: 15781139] [DOI] [PubMed] [Google Scholar]
Soparkar 2001
- Soparkar P, Newman MB. Effects of a baking soda gum on extrinsic dental stain: results of a longitudinal 4‐week assessment. Compendium of Continuing Education in Dentistry 2001;22(7A):25‐8. [PUBMED: 11913306] [PubMed] [Google Scholar]
Viscio 2000
- Viscio D, Gaffar A, Fakhry‐Smith S, Xu T. Present and future technologies of tooth whitening. Compendium of Continuing Education in Dentistry 2000;28:36‐43. [PUBMED: 11908346] [PubMed] [Google Scholar]
White 2002
- White DJ. A new and improved 'dual action' whitening dentifrice technology ‐ sodium hexametaphosphate. Journal of Clinical Dentistry 2002;13(1):1‐5. [PUBMED: 11507924] [PubMed] [Google Scholar]
Whitening survey 2012
- American Academy of Cosmetic Dentistry. Whitening survey. www.aacd.com/proxy/files/Publications%20and%20Resources/Whitening%20Survey_Aug12(1).pdf (accessed prior to 3 July 2018).
References to other published versions of this review
Hasson 2006
- Hasson H, Ismail A, Neiva G. Home‐based chemically‐induced whitening of teeth in adults (Review). Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006202] [DOI] [PubMed] [Google Scholar]


