Abstract
Background
Inflammatory bowel disease (IBD) is a chronic, relapsing disease of the gastrointestinal tract that is thought to be associated with a complex interplay between microbes and the immune system, leading to an abnormal inflammatory response in genetically susceptible individuals. Dysbiosis, characterized by the alteration of the composition of the resident commensal bacteria in a host compared to healthy individuals, is thought to play a major role in the pathogenesis of ulcerative colitis (UC) and Crohn's disease (CD), two subtypes of IBD. There is growing interest to correct the underlying dysbiosis through the use of fecal microbiota transplantation (FMT) for the treatment of IBD.
Objectives
The objective of this systematic review was to assess the efficacy and safety of FMT for the treatment of IBD.
Search methods
We searched the MEDLINE, Embase, Cochrane Library, and Cochrane IBD Group Specialized Register databases from inception to 19 March 2018. We also searched ClinicalTrials.gov, ISRCTN metaRegister of Controlled Trials, and the Conference Proceedings Citation Index.
Selection criteria
Only randomized trials or non‐randomized studies with a control arm were considered for inclusion. Adults or pediatric participants with UC or CD were eligible for inclusion. Eligible interventions were FMT defined as the administration of fecal material containing distal gut microbiota from a healthy donor to the gastrointestinal tract of a someone with UC or CD. The comparison group included participants who did not receive FMT and were given placebo, autologous FMT, or no intervention.
Data collection and analysis
Two authors independently screened the titles and extracted data from the included studies. We used the Cochrane risk of bias tool to assess study bias. The primary outcomes were induction of clinical remission, clinical relapse, and serious adverse events. Secondary outcomes included clinical response, endoscopic remission and endoscopic response, quality of life scores, laboratory measures of inflammation, withdrawals, and microbiome outcomes. We calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for dichotomous outcomes and the mean difference and 95% CI for continuous outcomes. Random‐effects meta‐analysis models were used to synthesize effect sizes across trials. The overall certainty of the evidence supporting the primary and selected secondary outcomes was rated using the GRADE criteria.
Main results
Four studies with a total of 277 participants were included. These studies assessed the efficacy of FMT for treatment of UC in adults; no eligible trials were found for the treatment of CD. Most participants had mild to moderate UC. Two studies were conducted in Australia, one study was conducted in Canada, and another in the Netherlands. Three of the included studies administered FMT via the rectal route and one study administered FMT via the nasoduodenal route. Three studies were rated as low risk of bias. One study (abstract publication) was rated as unclear risk of bias. Combined results from four studies (277 participants) suggest that FMT increases rates of clinical remission by two‐fold in patients with UC compared to controls. At 8 weeks, 37% (52/140) of FMT participants achieved remission compared to 18% (24/137) of control participants (RR 2.03, 95 % CI, 1.07 to 3.86; I² = 50%; low certainty evidence). One study reported data on relapse at 12 weeks among participants who achieved remission. None of the FMT participants (0/7) relapsed at 12 weeks compared to 20% of control participants (RR 0.28, 95% CI 0.02 to 4.98, 17 participants, very low certainty evidence). It is unclear whether there is a difference in serious adverse event rates between the intervention and control groups. Seven per cent (10/140) of FMT participants had a serious adverse event compared to 5% (7/137) of control participants (RR 1.40, 95% CI 0.55 to 3.58; 4 studies; I² = 0%; low certainty evidence). Serious adverse events included worsening of UC necessitating intravenous steroids or surgery; infection such as Clostridium difficile and cytomegalovirus, small bowel perforation and pneumonia. Adverse events were reported by two studies and the pooled data did not show any difference between the study groups. Seventy‐eight per cent (50/64) of FMT participants had an adverse event compared to 75% (49/65) of control participants (RR 1.03, 95% CI 0.81 to 1.31; I² = 31%; moderate certainty evidence). Common adverse events included abdominal pain, nausea, flatulence, bloating, upper respiratory tract infection, headaches, dizziness, and fever. Four studies reported on clinical response at 8 weeks. Forty‐nine per cent (68/140) of FMT participants had a clinical response compared to 28% (38/137) of control participants (RR 1.70, 95% CI 0.98 to 2.95, I² = 50%, low certainty evidence). Endoscopic remission at 8 weeks was reported by three studies and the combined results favored FMT over the control group. Thirty per cent (35/117) of FMT participants achieved endoscopic remission compared to 10% (11/112) of control participants (RR 2.96, 95 % CI 1.60 to 5.48, I² = 0%; low certainty evidence).
Authors' conclusions
Fecal microbiota transplantation may increase the proportion of participants achieving clinical remission in UC. However, the number of identified studies was small and the quality of evidence was low. There is uncertainty about the rate of serious adverse events. As a result, no solid conclusions can be drawn at this time. Additional high‐quality studies are needed to further define the optimal parameters of FMT in terms of route, frequency, volume, preparation, type of donor and the type and disease severity. No studies assessed efficacy of FMT for induction of remission in CD or in pediatric participants. In addition, no studies assessed long‐term maintenance of remission in UC or CD. Future studies are needed to address the therapeutic benefit of FMT in CD and the long‐term FMT‐mediated maintenance of remission in UC or CD.
Plain language summary
Stool transplantation for treatment of inflammatory bowel disease
Background
Ulcerative colitis (UC) and Crohn's disease (CD) are two types of inflammatory bowel disease (IBD) that lead to chronic inflammation in the digestive tract. The mechanism leading to inflammation in IBD is poorly understood, yet it is thought to involve a complex interaction between the immune system, the gut and gut microbes. New evidence suggests that the composition of gut microbes in a patient with IBD is different and possibly abnormal, and that correction of this abnormality might help control the inflammation seen in patients with UC and CD. Stool administration from healthy donors to patients with UC or CD is an intervention that seeks to restore a more healthy balance of gut microbes, and control IBD.
Review question
To assess the effectiveness of stool transplantation for the treatment of UC and CD.
Review methods
We searched multiple databases for randomized studies. A randomized study is a type of study where participants are allocated to an intervention or a control group in a random manner and is considered to be the most superior research design. We pooled data from different studies to obtain overall estimates of the effect of stool transplantation for the treatment of UC and CD. The literature search is current to 19 March 2018.
Study characteristics
We found four studies (277 participants) that assessed the effectiveness of stool transplantation for the treatment of adults with active UC. We did not find any randomized studies that assessed stool transplantation in participants with CD or in children. In addition, we did not find any studies that assessed maintenance of remission in participants with inactive IBD. Two of the identified studies were conducted in Australia, one in Canada, and one in the Netherlands. The dose, route, frequency, volume, type of donor, and severity of disease of recipients varied among the studies.
Key results
Combined results from four studies including 277 participants indicated that stool transplantation increased rates of resolution of symptoms (also termed clinical remission) of UC patients by two‐fold compared to controls. At 8 weeks after transplantation, 37% (52/140) of participants in the stool transplant group were in remission compared to 18% (24/137) of participants in the control group. Combined data from the same four studies showed similar rates of serious side effects. Seven per cent (10/140) of the stool transplantation group had a serious side effect compared to 5% (7/137) of the control group. Serious side effects included worsening of ulcerative colitis that required intravenous steroids or surgery; infections such as Clostridium difficile and cytomegalovirus, small bowel perforation, and pneumonia. The incidence of side effects were similar in both stool transplant and control groups and included abdominal pain, nausea, flatulence, bloating, upper respiratory tract infection, headaches, dizziness, and fever. Data from three included studies showed that stool transplantation helped improve UC when the assessment of disease resolution was made by the appearance of the intestinal lining when visualized with an endoscope.
Quality of evidence
We rated the overall quality of the evidence using the GRADE approach, which takes into account the type of studies, methodological flaws within studies, the consistency in reporting of results across studies, method of measurement of effect of intervention and statistical confidence in the summary estimates. Based on these criteria, we judged the overall quality of the evidence for most of the outcomes to be low based on a small number of events and participants and inconsistency of results.
Conclusions
Fecal microbiota transplantation may increase the proportion of participants achieving clinical remission in UC. However, the number of identified studies was small and the quality of evidence was low. There is uncertainty about the rate of serious side effects. Thus, no firm conclusions can be drawn regarding the benefits and harms of stool transplantation in people with active UC. We did not find any studies that addressed treatment of CD with stool transplantation or studies that assessed stool transplantation in children with IBD. In addition, we did not find any studies that assessed long‐term maintenance of remission in participants with inactive IBD. More studies are needed to enhance the knowledge about use of stool transplantation for treatment of IBD.
Summary of findings
Summary of findings for the main comparison. Fecal microbiota transplantation compared to control for treatment of ulcerative colitis.
| Fecal microbiota transplantation compared to control for treatment of ulcerative colitis | ||||||
| Patient or population: Participants with active ulcerative colitis inadequately controlled with medication Setting: Outpatient Intervention: Fecal microbiota transplantation via rectal or nasoduodenal routes Comparison: Autologous fecal administration or normal saline placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Control for Ulcerative Colitis | Risk with Fecal Microbiota Transplantation | |||||
| Clinical remission at 8 weeks | 175 per 1,000 | 356 per 1,000 (187 to 676) | RR 2.03 (1.07 to 3.86) | 277 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Clinical remission was defined by the included studies ‐ see characteristics of included studies |
| Serious adverse events Follow up: 8‐12 weeks | 51 per 1,000 | 72 per 1,000 (28 to 183) | RR 1.40 (0.55 to 3.58) | 277 (4 RCTs) | ⊕⊕⊝⊝ LOW 3 | Serious adverse events included worsening ulcerative colitis, Clostridium difficile infection, cytomegalovirus infection, small bowel perforation, cervix carcinoma and pneumonia |
| Adverse events Follow‐up: 8 weeks |
754 per 1,000 | 776 per 1,000 (611 to 988) | RR 1.03 (0.81 to 1.31) | 129 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | Common adverse events included abdominal pain, nausea, diarrhea, vomiting, colitis, flatulence, bloating, upper respiratory tract infection, headache, dizziness, fever and transient borborygmus |
| Clinical response at 8 weeks | 277 per 1,000 | 472 per 1,000 (272 to 818) | RR 1.70 (0.98 to 2.95) | 277 (4 RCTs) | ⊕⊕⊝⊝ LOW 5, 6 | Clinical response was defined by the included studies ‐ see characteristics of included studies |
| Endoscopic remission at 8 weeks | 98 per 1,000 | 291 per 1,000 (157 to 538) | RR 2.96 (1.60 to 5.48) | 229 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 7 | Endoscopic remission was defined by the included studies ‐ see characteristics of included studies |
| Clinical relapse at 12 weeks | 200 per 1,000 | 56 per 1,000 (4 to 996) | RR 0.28 (0.02 to 4.98) | 17 (1 RCT) | ⊕⊕⊝⊝ LOW 8 | |
| Endoscopic response at 8 weeks | 200 per 1,000 | 272 per 1,000 (52 to 1,000) | RR 1.36 (0.26 to 7.02) | 129 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 9 10 | Clinical response was defined by the included studies ‐ see characteristics of included studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to serious inconsistency (I² = 50%)
2 Downgraded one level due to serious imprecision (76 events) and wide confidence interval
3 Downgraded two levels due to very serious imprecision (17 events) and wide confidence interval
4 Downgraded one level due to serious imprecision (99 events)
5 Downgraded one level due to serious inconsistency (I² = 64%)
6 Dowgraded one level due to serious imprecision (106 events)
7 Downgraded one level due to serious imprecision (46 events) and wide confidence interval.
8 Downgraded two levels due to very serious imprecision (2 events) and very wide confidence interval
9 Downgraded two levels due to very serious inconsistency (I² = 82%)
10 Downgraded one level due to serious imprecision (31 events) and wide confidence interval
Background
Description of the condition
Ulcerative colitis (UC), and Crohn's disease (CD), subtypes of Inflammatory Bowel Disease (IBD), are chronic, relapsing diseases of the gastrointestinal (GI) tract. One of the proposed mechanisms for the development of IBD involves the interplay between microbes and the immune system, which may then lead to an abnormal inflammatory response in genetically susceptible individuals (Abraham 2009; Cleynen 2016). UC is characterized by inflammation of the colonic mucosa and can affect variable lengths of the colon (Abraham 2009; Ananthakrishnan 2015). CD can cause transmural inflammation and affect any part of the GI tract from mouth to anus, with a particular predilection for the terminal ileum (Abraham 2009; Ananthakrishnan 2015). The prevalence of UC and CD is increasing in both developing and developed countries (Ahuja 2010; Dahlhamer 2016; Molodecky 2012; Weintraub 2014). IBD is associated with poor quality of life, significant economic burden, and increased morbidity including the need for hospitalizations and surgical procedures (Abraham 2009; Abraham 2012; Mehta 2016). Most current treatment strategies for IBD focus on the control of inflammation with medications, including corticosteroids, 5‐aminosalicylic acid (5‐ASA) preparations, immune‐modulating drugs such as azathioprine, 6‐mercaptopurine and methotrexate, and biologic therapies such as infliximab, adalimumab, vedolizumab and ustekinumab (Abraham 2009; Vindigni 2016). Unfortunately, these medical therapies have the potential for significant adverse effects. Moreover, while these therapies provide some benefit in many cases (Abraham 2009; Vindigni 2016), there remain a significant number of patients who either do not respond to any of these treatment modalities or become refractory over time. Ultimately, some patients may require a surgical bowel resection (Vindigni 2016). This supports the need for alternative treatment strategies that target known pathogenic factors to supplement or replace existing treatment strategies.
Description of the intervention
There is growing evidence to suggest that 'dysbiosis' is one of the key elements in the pathogenesis of IBD and could be a potential therapeutic target (Assa 2016; Bejaoui 2015; Kostic 2014; Vindigni 2016). Dysbiosis is defined as any alteration in the composition of resident commensal bacteria communities relative to the communities found in healthy individuals (Petersen 2014). In IBD, a decrease in alpha biodiversity, an increase in certain pathogenic species, and an altered functional core of gut microbiota have been reported (De Preter 2012; Kostic 2014; Vindigni 2016).
Fecal microbiota transplantation (FMT) from healthy donors is one of the interventions used to correct dysbiosis (Cammarota 2017). While FMT is an increasingly studied intervention, most of the published literature on this intervention relates to the treatment of recurrent Clostridium difficile (C. difficile) infection (rCDI), where its efficacy is reported to be greater than 90% (Austin 2014; Cammarota 2015; Kassam 2013; Kelly 2016; Lee 2016; Leffler 2015; van Nood 2013; Youngster 2014). The Food and Drug Administration (FDA) of the United States of America considers FMT to be a 'biologic product' and a 'drug' under its regulations, and labelled it as an investigational new drug, with exceptions for the treatment for rCDI where the FDA exercises enforcement discretion (FDA 2016; Moore 2014). Even though methods to perform FMT are evolving, a typical FMT procedure involves selection and screening of the donor, collection and preparation of the donor stool for infusion, preparation of the patient to receive the stool infusion and administration of the stool via the upper or lower gastrointestinal tract (Cammarota 2017). There is no universally agreed upon tool for donor screening; however, most studies have adopted a screening strategy similar to that used for a human tissue donor (Austin 2014; Cammarota 2017; Moore 2014; Owens 2013). The donor is screened with an interview and blood and stool studies to rule out chronic diseases and active infections. After a donor is screened, the stool is collected to be used either immediately for infusion or frozen for later use. At least 30 to 50 g of feces are typically collected and mixed with normal saline or sterile water in preparation for infusion. The patient is usually prepared with a lavage prior to the infusion. The donor feces can be administered via colonoscopy, enemas, an upper gastrointestinal delivery route such as duodenal or gastric tubes, or through orally‐ingested frozen capsules. All modalities have been studied with overall comparable efficacy, even though the colonic route is thought to be the most efficacious (Cammarota 2017; Lee 2016;van Nood 2013;Youngster 2014). Per published international standards, infection control precautions should be adopted during FMT (Cammarota 2017).
How the intervention might work
The exact mechanism by which FMT might work for inducing remission in IBD is not well established at this time. However, the prevailing hypothesis is that FMT might correct the 'dysbiosis' associated with IBD, leading to a reversal or improvement of the associated inflammation (Moayyedi 2015; Paramsothy 2017a; Rossen 2015; Shi 2016; Sun 2016; Vindigni 2016). Knowledge around the use of FMT for treatment of IBD is evolving. Currently, there is no consensus on the volume, timing, route, and frequency of fecal administration necessary to achieve remission (Cammarota 2017; Kelly 2015; Moore 2014). While a single infusion of feces is enough to treat rCDI in most cases (Austin 2014; Cammarota 2015; Kassam 2013), multiple infusions might be required for the induction of remission in IBD as suggested by the recent FOCUS trial from Australia (Paramsothy 2017a). Similarly, response to FMT for rCDI may not vary much with the choice of donor (Osman 2016). However, donor selection might have a significant impact on the induction of remission in UC as reported by Moayyedi 2015, where seven out of nine patients who went into clinical remission received stool from a single donor.
The short and long term safety of FMT in patients with IBD is not well established (Cammarota 2017; Kelly 2015; Moore 2014). Some studies report relatively minor adverse effects such as diarrhea, abdominal bloating, abdominal cramping, and fever in the immediate post‐procedure period (Khoruts 2016; Kunde 2013). In addition, FMT may increase the risk of a flare in IBD patients (Kelly 2014; Khoruts 2016). Concerns remain that feces may have microorganisms that can be pathogenic to the recipient, and that the change in the functional core of bacteria may confer an undesirable and unanticipated outcome (Alang 2015; Cammarota 2017). Animal models of FMT have demonstrated undesired weight changes that accompanied changes in the microbiome (Blanton 2016; Ridaura 2013). Serious adverse events have been reported in individual cases, including mortality (Kelly 2014), septic shock and toxic megacolon (Solari 2014), and aspiration pneumonia (Link 2016).
Why it is important to do this review
As an increasing body of evidence associates 'dysbiosis' with the pathogenesis of IBD, there have been efforts to correct the dysbiosis and assess if this can improve IBD‐associated outcomes (Chassaing 2011; Fuentes 2017; Morgan 2012; Nagao‐Kitamoto 2016; Rapozo 2017; Schulberg 2016; Vindigni 2016). Some of the interventions that might target gut microbiota include the use of probiotics, prebiotics, synbiotics, nutrition therapy, and FMT (Vindigni 2016). Most of these interventions have been the subject of Cochrane reviews (Mallon 2007; Naidoo 2011; Singh 2015). However, FMT for treatment of IBD has not been evaluated in a Cochrane review. Moreover, available non‐Cochrane reviews have not included some of the more recent studies (Colman 2014), only assessed a limited number of outcomes (Shi 2016), and meta‐analysed cohort studies and randomized trials in the same analysis (Sun 2016). Furthermore, additional evidence has recently become available since the publication of previous reviews (Costello 2017a). The most recent reviews have included both cohort studies and randomized trials but analyzed limited outcomes (Costello 2017b; Paramsothy 2017b). None of the previous reviews systematically assessed the overall quality of the evidence supporting the use of FMT for treatment of IBD with the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) criteria. Collectively, these observations make this an appropriate and opportune time to conduct a Cochrane systematic review.
Objectives
The objective of this systematic review was to assess the efficacy and safety of FMT for the treatment of IBD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials and non‐randomized studies with a comparator arm. Case reports, case series, case‐control, and single‐arm cohort studies were excluded.
Types of participants
Studies were included if the participants were diagnosed with UC or CD based on their history, physical examination, and gross endoscopic and histologic evaluations. We excluded studies where the diagnosis was made without endoscopic or histologic evaluation as these two measures were considered key initial diagnostic studies for IBD (Mowat 2011). There were no age restrictions for participants, and we included both pediatric and adult patients. We excluded studies using FMT for the treatment of pouchitis. We only included studies that followed participants for at least six weeks post‐FMT (Feakins 2013). We excluded studies where participants had active enteric infections such as C. difficile, as these conditions may mimic IBD. We excluded studies in which FMT was performed for recurrent C.difficile infection in patients with IBD and not for induction of remission.
Types of interventions
We included studies that evaluated FMT for the treatment of IBD. FMT for this review was defined as, "the administration of fecal material containing distal gut microbiota from a healthy individual (donor) to a patient with a disease or condition related to dysbiosis, or an alteration in their normal gut microbiota (Kelly 2015)." Comparator groups included standard medication, placebo, other control, or no intervention. We included studies irrespective of the type of stool (liquid or frozen), the volume of stool, routes of administration, frequency (i.e. single versus multiple infusions) and timing of the transplant (at initial diagnosis or to treat a flare). We excluded studies that used selective microbes rather than whole stool from the donor, as this intervention did not fulfil the definition of FMT.
Types of outcome measures
Primary outcomes
The following primary outcomes were considered:
1) Induction of clinical remission (as defined by the included studies);
2) Relapse (as defined by the included studies); and
3) Serious adverse events as defined by the authors.
We measured the primary outcomes as the number of patients achieving clinical remission, relapsing, or having serious adverse events, expressed as a proportion of the number of patients randomized. Further details on how data were abstracted for primary outcomes is given in the section Measures of treatment effect.
Secondary outcomes
Secondary outcomes included:
1) Clinical response (as defined by the included studies);
2) Endoscopic remission (as defined by the included studies);
3) Endoscopic response (as defined by the included studies);
4) Lab measures of inflammation including erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), and fecal calprotectin at the time of measurement of the primary outcome;
5) Quality of life (scores) at the time of the measurement of the primary outcome;
6) Any adverse events;
7) Withdrawals; and
8) Change in the alpha diversity of the fecal microbiome in the recipient.
Search methods for identification of studies
Electronic searches
We searched the MEDLINE, Embase, Cochrane Library, and Cochrane IBD Group Specialized Register databases from inception to 19 March 2018. Please see Appendix 1 for the detailed search strategy.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the ISRCTN metaRegister of Controlled Trials (mRCT; www.isrctn.com/page/mrct) for ongoing trials. We searched the reference sections of previously published randomized trials and meta‐analyses on this topic. We searched the Conference Proceedings Citation Index database to search for conference abstracts. We specifically searched the abstracts from the last 10 years from major conferences, such as Digestive Disease Week, Infectious Diseases Week, United European Gastroenterology Week, European Crohn's and Colitis Organization, North American Society of Pediatric Gastroenterology, Hepatology and Nutrition, and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Finally, we contacted authors of published and ongoing studies to seek new or additional data when needed.
Data collection and analysis
Selection of studies
Two authors (AI and MN) conducted the initial screening to select potentially eligible studies by reviewing titles and abstracts. Any potential discrepancies were resolved by discussion. After initial title and abstract screening, selected studies were further assessed by two authors (AI and MN) by review of full text and a final decision was made about inclusion or exclusion. Any discrepancies were resolved by discussion and consensus between two authors (AI and MN). If any conflict about the inclusion of a study persisted, a senior author (SA) was consulted to resolve the conflict.
Data extraction and management
Two authors (AI and MN) independently extracted data to a pre‐tested Microsoft Excel sheet (available on request). We extracted information on the characteristics of included studies such as study authors, date of publication, journal, site of the study, type of study, age of participants, definition of study population (inclusion/exclusion criteria), details of intervention (type, volume, frequency, route of administration of fecal transplant, source), outcomes (primary and secondary outcomes), and risk of bias. We extracted the raw values of events (numerators) in cases and controls along with a total number of subjects allocated (denominators) to intervention and control groups. For studies using randomized control trial designs, we extracted data on an intention‐to‐treat basis, which considers the initial allocation of participants to an intervention or control group irrespective of whether or not they received the intervention or completed the follow‐up (Gupta 2011). When data for continuous outcomes were reported as medians with ranges, we converted it to means with standard deviations by methods given in Hozo 2005.
Assessment of risk of bias in included studies
We used the Cochrane risk‐of‐bias tool to assess the risk of bias in included randomized trials (Higgins 2011). Briefly, risk of bias assessments were based on six criteria: sequence generation, allocation concealment, masking, incomplete outcome data, publication bias, and other bias. Each category was assessed as 'Low', 'High' or 'Unclear' risk of bias.
For observational studies, we planned to use the Ottawa‐Newcastle Scale to assess risk of bias (Wells 2017). Briefly, this scale assesses the risk of bias in observational studies based on three criteria: selection of the study groups, comparability of the groups, and ascertainment of either the exposure or outcome of interest for case‐control or cohort studies, respectively.
Measures of treatment effect
We expected that the authors of included studies would report a range of clinical, endoscopic, and histologic outcomes in response to treatment with FMT. The most important of these outcomes were 'induction of clinical remission' and 'clinical relapse,' which were the primary outcomes of our systematic review. We used the definitions of clinical remission and clinical relapse as defined by the included studies (e.g. Mayo score for UC studies and the Crohn's disease activity index for CD studies). If the primary outcome reported in the trial was a combination of clinical and endoscopic or histologic assessment, we tried to include clinical remission or relapse data only, if available. If disaggregated data were not available, we corresponded with the authors to obtain clinical remission or relapse data. If these data were not available from the authors of the original studies, we included the data for the combined outcome. All analyses from randomized trials were conducted on an intention‐to‐treat analysis basis.
We considered the primary outcome at 8 weeks and 12 weeks post‐FMT. If a study did not report a primary outcome exactly at 8 weeks but between 6 to 10 weeks post‐FMT, it was included as an outcome at 8 weeks. Similarly, if a study did not report the primary outcome exactly at 12 weeks but between > 10 weeks and 16 weeks, it was included as an outcome at 12 weeks post‐FMT. We planned to perform sensitivity analysis if there were a number of studies that did not report outcomes exactly at 8 or 12 weeks.
We calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for all dichotomous outcomes. We calculated the mean difference (MD) and corresponding 95% CI for continuous outcomes. For continuous outcomes that used different scales to measure the same underlying construct (e.g. quality of life scores), we planned to calculate the standardized mean difference (SMD) and 95% CI. For observational studies with a comparison group, we planned to calculate the odds ratio (OR) and corresponding 95% CI for dichotomous outcomes.
Unit of analysis issues
For studies that had multiple intervention groups (e.g. factorial design), the data were included in such a way that the only difference between the two groups was FMT. Co‐interventions were permitted if the co‐interventions were uniformly applied to both intervention and control groups. We considered outcomes at fixed intervals of follow‐up (e.g. 8 weeks, 12 weeks) irrespective of how often the same outcome was measured before or after that time interval. We only considered the effect of the first treatment attempt as defined by the authors. The treatment may have included multiple infusions of FMT; however, if a patient received a standard study treatment (intervention or placebo) more than once, the subsequent attempts were ignored. Such a scenario might occur if authors decide to treat all the patients allocated to placebo with the study intervention at the end of a randomized study. Adverse events were considered as reported by authors, and we assumed that each adverse event was an independent event unless the published report indicated otherwise.
Dealing with missing data
Attrition is an important factor that may affect the validity of studies, and differential dropout rates between the two study groups can lead to biased estimates of effect size (Dumville 2006). We described missing data, including dropouts and reasons for dropout as reported by authors. We contacted authors if data were missing and no reasons were provided for missing data. When authors report data for completers as well as controlling for dropouts (for example, imputed using regression methods), we extracted the latter. If data were not available for the primary outcome of this review, we contacted the authors for additional information. All data from randomized trials were analyzed on an intention‐to‐treat basis. As such, patients with missing values for the primary outcomes were assumed to be treatment failures.
Assessment of heterogeneity
We assessed clinical, methodological and statistical heterogeneity among included studies. Clinical heterogeneity was assessed by comparing the distribution of important factors such as study participants, dose, and frequency of FMT. Methodological heterogeneity was assessed by comparing data included in the 'Risk‐of‐bias' tables. Statistical heterogeneity was assessed based on visual inspection of forest plots, the I² statistic and the P value for the Chi² test. If the forest plot was indicative of a heterogeneous effect (opposite direction or prominent difference in magnitude of effect), while I² values were greater than 50% and P values for the Chi² test were less than 0.1, statistical heterogeneity was considered to be substantial. We explored potential explanations for heterogeneity using subgroup analyses as described below.
Assessment of reporting biases
We aimed to assess the potential publication bias based on the symmetry of the funnel plot. We planned to construct funnel plots if at least 10 studies were included in the pooled analysis.
Data synthesis
We synthesized data from individual trials using meta‐analysis when the interventions, patient groups, and outcomes were sufficiently similar (as determined by consensus) using the software Review Manager version 5.3 (RevMan 2014). We planned to conduct separate meta‐analyses for patients with UC and CD. For dichotomous outcomes, we calculated the pooled RR and corresponding 95% CI. We combined risk ratios (events per participant) and rate ratios (events per participant‐days/months/year) for two reasons: Studies were expected to be completed in a relatively short duration, and the primary outcome (induction of remission) was not expected to be a recurrent event. All meta‐analyses were conducted using the log risk ratio, with all reported results transformed back into the risk ratio metric for ease of interpretability.
For continuous outcomes, data were combined to get a pooled MD and corresponding 95% CI. When different scales were used to measure the same underlying construct, we calculated the standardized mean difference (SMD; Hedges’ g value) and corresponding 95% CI. We used a random‐effects model to conduct all the meta‐analyses. The rationale for using a random‐effects model was that we expected that there might be heterogeneity in the effect of FMT due to factors such as dose, frequency, or donor source (e.g. single donor or multi‐donor), as noted in the results of published studies (Moayyedi 2015; Paramsothy 2017a; Rossen 2015).
The overall quality of the evidence supporting the primary outcomes and selected secondary outcomes was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (Guyatt 2011). This method of evidence evaluation takes into consideration the impact of the type of studies (i.e. randomized versus observational), risk of bias, indirectness, inconsistency (i.e. unexplained heterogeneity), imprecision, and potential publication bias. The overall quality of the evidence was rated as 'high', 'moderate', 'low', or 'very low'. We presented the results of the GRADE evaluation in a 'Summary of Findings' table for all primary outcomes and selected secondary outcomes (e.g. clinical response, endoscopic remission).
Subgroup analysis and investigation of heterogeneity
We planned the following a priori subgroup analyses:
1) Route of administration: upper gastrointestinal tract (i.e. nasogastric, nasoduodenal, nasojejunal, gastric tube, capsulated) versus colonic (i.e. rectal or beyond);
2) Type of donor: single donor versus multiple donors;
We also planned subgroup analysis based on age of participants and frequency of administration; however, there were not enough studies to perform these subgroup analyses.
Sensitivity analysis
The following sensitivity analyses were performed:
1) Definition of clinical remission: studies that used clinical criteria only to define clinical remission or relapse versus studies that used a combination of clinical and endoscopic or histologic criteria to define remission or relapse and disaggregated data are not available; and
2) Choice of statistical model: random versus fixed‐effect models for primary outcomes.
Results
Description of studies
Results of the search
A search conducted on 19 March 2018 identified 1020 studies (See Figure 1). After removal of duplicates, 665 studies were retained for title and abstract screening. Thirty‐four studies met criteria for full‐text review. Eleven studies were excluded for reasons outlined in the Characteristics of excluded studies table. Eight reports of four studies were included in this systematic review (Costello 2017a; Moayyedi 2015; Paramsothy 2017a; Rossen 2015). Thirteen ongoing studies were identified (NCT01790061; NCT01793831; NCT01961492; NCT02272868; NCT02291523; NCT02335281; NCT02390726; NCT02734589; NCT02998112; NCT03006809; NCT03016780; NCT03104036; NCT02487238).
1.
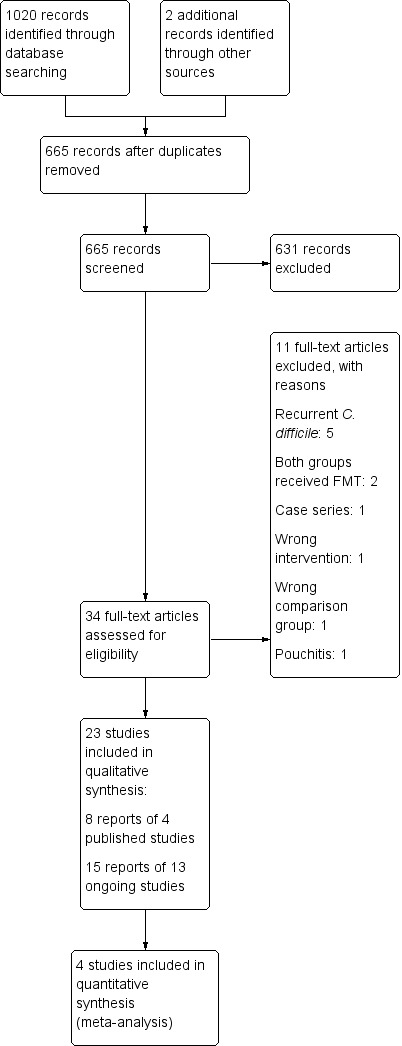
Study flow diagram
Included studies
Four randomized controlled trials assessed fecal microbiota transplantation for induction of remission in UC (Costello 2017a; Moayyedi 2015; Paramsothy 2017a; Rossen 2015). Three of the studies were published in peer‐reviewed journals (Moayyedi 2015; Paramsothy 2017a; Rossen 2015), while one study was reported in abstract form (Costello 2017a). Please see complete details of these studies in the Characteristics of included studies tables. No randomized controlled studies were available that addressed the use of FMT for treatment of CD. No observational studies were included.
Fecal microbiota transplantation for induction of remission in ulcerative colitis
Country
Two studies were conducted in Australia (Costello 2017a; Paramsothy 2017a), one study was conducted in Canada (Moayyedi 2015, and one in the Netherlands (Rossen 2015). The study conducted in the Netherlands was a single center study (Rossen 2015), whereas the other three studies were conducted in multiple centers (Costello 2017a; Moayyedi 2015; Paramsothy 2017a).
Study Population:
Age and gender
The percentage of male participants in the included studies ranged from 55% (Costello 2017a), to 59% (Moayyedi 2015). The mean age of participants ranged from 35 years in the Paramsothy 2017a study to 40 years in the Costello 2017a study.
Primary diagnosis and severity of disease
All four studies included adult patients with active UC. The diagnosis of UC was based on the Mayo Clinic Score and the Endoscopic Mayo Clinic score in three studies (Costello 2017a; Moayyedi 2015; Paramsothy 2017a), whereas one study used the Lennard‐Jones criteria (Rossen 2015). Disease location was reported in three studies (Moayyedi 2015; Paramsothy 2017a; Rossen 2015), and the proportions of patients who had pancolitis were as follows: 42% (Moayyedi 2015), 43% (Rossen 2015), and 23% (Paramsothy 2017a).
Concomitant Medications
Concomitant medications were allowed for participants in all of the included studies. The most commonly used medication was mesalamine and the proportion of patients who used mesalamine was: 54% (Moayyedi 2015), 62% (Rossen 2015) and 66% (Paramsothy 2017a).
Indications for FMT
FMT was used as a rescue therapy rather than primary induction therapy in all of the studies, meaning that patients had a known history of UC and their disease remained inadequately controlled with current medications. The median disease duration ranged from 5.8 years in the Paramsothy 2017a study to 8 years in the Rossen 2015 study.
Intervention
Donors
All four studies used feces produced by apparently healthy donors. Appendix 2 reports the inclusion and exclusion criteria for donors in the included studies.
Route
The route of administration was nasoduodenal in one study (Rossen 2015), and rectal enema in another study (Moayyedi 2015). Two studies administered the first dose in the cecum via colonoscopy, while the subsequent treatments were given via rectal enemas (Costello 2017a; Paramsothy 2017a).
Frequency
The frequency of administration varied across the included studies. Rossen 2015 administered FMT once every three weeks for a total of two doses. Costello 2017a administered a total of three doses within a week. Paramsothy 2017a administered FMT five times a week for eight weeks for a total of 40 doses.
Volume
The volume of FMT administered ranged from 50 ml in the Moayyedi 2015 study to 500 ml in the Rossen 2015 study.
Single vs. multidonor
Two studies used feces from a single donor to perform FMT (Moayyedi 2015; Rossen 2015), while the other two studies pooled feces from multiple donors and administered them as a single FMT per treatment (Costello 2017a; Paramsothy 2017a). The weight of administered feces varied from 25 g in the Costello 2017a study to 60 g in the Rossen 2015 study.
Colon preparation
One study used colon preparation before each dose of the transplant (Rossen 2015), while another study used no colon preparation (Moayyedi 2015). Two studies used colon preparation at the time of first administration via colonoscopy, but not for the subsequent enemas (Costello 2017a; Paramsothy 2017a). None of the included studies used antibiotics in the participants prior to FMT.
Stool preparation for transplant
The donor feces for transplant were prepared in aerobic conditions in three studies (Moayyedi 2015; Paramsothy 2017a; Rossen 2015), while one study prepared the feces in anaerobic conditions (Costello 2017a). One study used fresh fecal specimens from donors (Rossen 2015), while the other studies used either fresh or frozen specimens (Moayyedi 2015), or only frozen specimens (Costello 2017a; Paramsothy 2017a).
Comparison:
Two studies used autologous fecal administration as the comparison group (Costello 2017a; Rossen 2015), and the other two studies used placebo in the form of normal saline (Moayyedi 2015; Paramsothy 2017a). The volume, frequency of administration, and any colon preparation were similar between the control and intervention groups in the respective studies.
Outcomes
All four studies reported data on clinical and endoscopic outcomes. Please see the Effects of interventions section for details.
Follow‐up
The follow‐up time for measurement of primary outcomes ranged from 7 weeks in the Moayyedi 2015 study to 12 weeks in the Rossen 2015 study. Paramsothy 2017a followed participants for an additional eight weeks and offered the FMT to patients who were initially randomized to the placebo group, thus this part of the study was open label. Moayyedi 2015 conducted an open label follow‐up at 12 months after the initial trial was completed.
Excluded studies
The characteristics of excluded studies are reported in the Characteristics of excluded studies table. In summary, five studies were excluded because FMT was performed for treatment of recurrent C‐difficile rather than treatment of IBD (Angelberger 2016; Chin 2017; Fischer 2016; Mandalia 2016; Mintz 2016). Three studies were excluded because the comparison group did not include a group without FMT or the comparison groups included patients without IBD (Hourigan 2015; Ishikawa 2017; Wei 2016). One study each was excluded because it did not fulfil the criteria based on study design (Borody 2003), study population (Landy 2013) or study intervention (Gionchetti 2000).
Risk of bias in included studies
A summary of the risk of bias assessments is reported in Figure 2.
2.
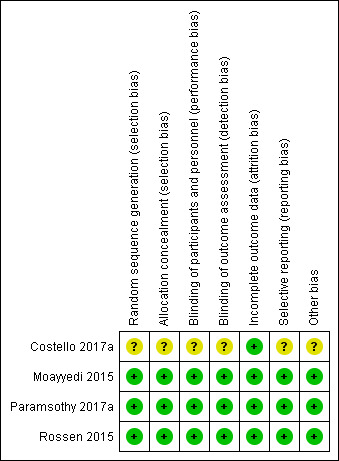
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three of the included studies adequately described the methods for random sequence generation and were rated as 'low' risk of bias (Moayyedi 2015; Paramsothy 2017a; Rossen 2015). Information from one of the studies was available in abstract form only, and a complete assessment could not be made (Costello 2017a). Allocation was successfully concealed in three of the included studies and these studies were rated as low risk of bias for this item (Moayyedi 2015; Paramsothy 2017a; Rossen 2015). One study was rated as unclear risk of bias (Costello 2017a).
Blinding
Blinding was assessed as low risk of bias in three studies (Moayyedi 2015; Paramsothy 2017a; Rossen 2015), and unclear in one study (Costello 2017a).
Incomplete outcome data
Two studies had attrition rates that were greater than 20% (Paramsothy 2017a; Rossen 2015). However, the drop‐out rates and reasons for dropping out were balanced across groups so we judged these two studies to be at low risk of bias. Drop‐out rates in the other two studies were low and we rated these studies as low risk of bias due to attrition (Costello 2017a; Moayyedi 2015).
Selective reporting
Selective reporting was assessed as low risk of bias for three studies (Moayyedi 2015; Paramsothy 2017a; Rossen 2015), while it was unclear in one study (Costello 2017a).
Other potential sources of bias
We did not identify any other major risk of bias in three of the included studies (Moayyedi 2015; Paramsothy 2017a; Rossen 2015), whereas the full text was not available to make such an assessment for one study (Costello 2017a).
Effects of interventions
See: Table 1
Induction of clinical remission for ulcerative colitis at eight weeks
Four randomized studies with a total of 277 participants contributed data for induction of clinical remission at 8 weeks. Thirty‐seven per cent (52/140) of FMT participants achieved remission at week 8 compared to 18% (24/137) of the control group. The pooled results showed that the FMT group had two‐fold higher rates of clinical remission compared to the control (RR 2.03, 95 % CI 1.07 to 3.86; I² = 50%; See Figure 3). The GRADE rating for the certainty of evidence was low.
3.
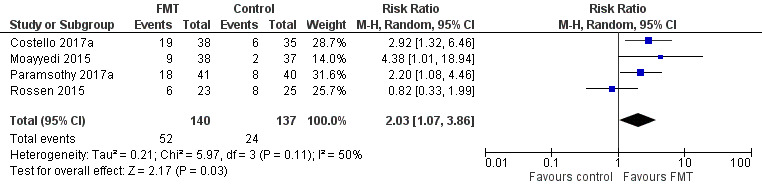
Forest plot of comparison: 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, outcome: 1.1 Clinical remission at 8 weeks.
Subgroup analyses
Planned subgroup analyses included 'route of administration' and 'type of donor' (see Table 2). FMT given via the colonic route showed a homogenous effect for induction of remission at 8 weeks (RR 2.66, 95 % CI 1.62 to 4.37, 3 studies, 229 participants, I² = 0%) compared to upper gastrointestinal tract administration (RR 0.82, 95 % CI 0.33 to 1.99, 1 study, 48 participants). For 'type of donor' the test for subgroup difference showed no difference between the multi‐donor and single donor subgroups (test for subgroup differences Chi² = 0.17, df = 1, P = 0.68, I² = 0%), although there was not enough information to determine whether there was a difference between subgroups.
1. Subgroup Analyses.
| Outcome | Subgroup Analyses | Number of Studies | Number of events/participants | Heterogeneity | Risk Ratio (95 % CI) | Test for Subgroup Difference: |
| Clinical Remission at 8 weeks | Route of Administration: Upper Gastrointestinal | 1 | FMT = 6/23 Control = 8/25 |
NA | 0.82 [0.33 to 1.99] | Chi² = 5.12, df = 1 (P = 0.02), I² = 80.5% |
| Route of Administration: Colonic/Enema | 3 | FMT = 46/117 Control = 16/112 |
Tau² = 0.00; Chi² = 0.79, df = 2 (P = 0.67); I² = 0% | 2.66 [1.62 to 4.37] | ||
| Clinical Remission at 8 weeks | Donor: Single donor | 2 | FMT = 15/61 Control = 10/62 |
Tau² = 1.12; Chi² = 3.94, df = 1 (P = 0.05); I² = 75% | 1.71 [0.32 to 9.29] | Chi² = 0.17, df = 1 (P = 0.68), I² = 0% |
| Donor: Multidonor | 2 | FMT = 52/140 Control = 24/137 |
Tau² = 0.21; Chi² = 5.97, df = 3 (P = 0.11); I² = 50% | 2.03 [1.07 to 3.86] | ||
| Serious Adverse Events | Route of Administration: Upper Gastrointestinal | 1 | FMT = 2/23 Control = 2/25 |
NA | 1.09 [0.17 to 7.10] | Chi² = 0.09, df = 1 (P = 0.76), I² = 0% |
| Route of Administration: Colonic/Enema | 3 | FMT = 8/117 Control = 5/112 |
Tau² = 0.00; Chi² = 0.06, df = 2 (P = 0.97); I² = 0% | 1.52 [0.51 to 4.50] | ||
| Serious Adverse Events | Donor: Single donor | 2 | FMT = 5/61 Control = 4/62 |
Tau² = 0.00; Chi² = 0.05, df = 1 (P = 0.82); I² = 0% | 1.28 [0.36 to 4.55] | Chi² = 0.04, df = 1 (P = 0.83), I² = 0% |
| Donor: Multidonor | 2 | FMT = 5/79 Control = 3/75 |
Tau² = 0.00; Chi² = 0.15, df = 3 (P = 0.99); I² = 0% | 1.40 [0.55 to 3.58] |
Abbreviations:
FMT: fecal microbiota transplantation
NA: Not applicable
Sensitivity analyses
Sensitivity analyses were performed based on choice of model for meta‐analysis and definition of clinical remission. A fixed‐effect model showed similar results (RR 2.13, 95 % CI 1.39 to 3.25, I² = 50%) compared to the primary random‐effects model used in this review. We decided a priori that the primary outcome 'clinical remission' would be based on a clinical score such (e.g. Mayo score or the simple clinical colitis activity index). Three of the included studies defined clinical remission based on clinical scores. Remission in the Moayyedi 2015 study was defined by the Mayo clinical score and the Mayo endoscopic score. The exclusion of this study resulted in decrease in the overall effect (RR 1.79, 95 % CI 0.88 to 3.63, I² = 58%).
All studies reported on a composite primary outcome where improvement in both clinical and endoscopic scores were considered as part of the definition of remission. We performed a post‐hoc sensitivity analysis where we pooled the primary outcomes based on the composite definition as reported by the primary studies. The pooled results from four studies showed similar results (RR 2.77, 95 % CI 1.54 to 4.98, I² = 0%) to those reported in our primary analysis (See Figure 3).
Induction of clinical remission 12 weeks
One study (48 participants) reported on clinical remission at 12 weeks (Rossen 2015). Thirty per cent (7/23) of FMT participants achieved remission at week 12 compared to 32% (8/25) of the control group (RR 0.95, 95 % CI 0.41 to 2.21; Analysis 1.2).
1.2. Analysis.
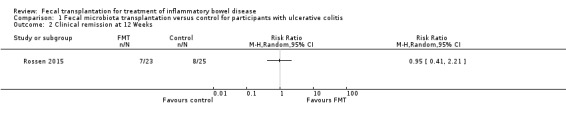
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 2 Clinical remission at 12 Weeks.
Clinical relapse at 12 weeks
This outcome was reported by one study (Rossen 2015). Few patients relapsed after achieving clinical remission. None (0/7) of the FMT participants who achieved clinical remission relapsed at 12 weeks compared to 20% (2/10) of participants in the control group (RR 0.28, 95 % CI 0.02, 4.98; Analysis 1.7). The overall certainty of the evidence according to the GRADE criteria was 'low'.
1.7. Analysis.
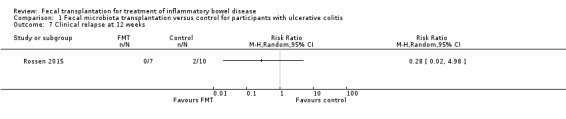
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 7 Clinical relapse at 12 weeks.
Serious adverse events
Four studies including 277 participants contributed data for this outcome. Serious adverse event rates were similar in both groups. Seven per cent of (10/140) the FMT group had a serious adverse event compared to 5% (7/137) of the control group (RR 1.40, 95 % CI 0.55 to 3.58, I² = 0%], see Figure 4. The overall certainty of the evidence was rated according to the GRADE criteria as 'low'.
4.
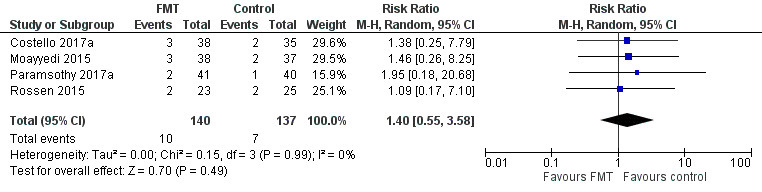
Forest plot of comparison: 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, outcome: 1.8 Serious adverse events.
Paramsothy 2017a reported two serious adverse events in FMT participants and both of these individuals had worsening ulcerative colitis. One participants required intravenous corticosteroids and other required colectomy. There was one serious adverse event in the control group. This individual required hospitalization for worsening ulcerative colitis. Moayyedi 2015 reported three serious adverse events in patients who received FMT; two of these individuals had worsening ulcerative colitis and one developed C. difficile infection. Two control participants had serious adverse events. Both of these individuals had worsening ulcerative colitis and one of them required colectomy. Rossen 2015 reported two serious adverse events in UC patients who received FMT. One of these individuals was found to have small bowel perforation and was ultimately diagnosed with CD and the other had a cytomegalovirus infection that was thought to be unrelated to the FMT. Two control participants had serious adverse events with one participants admitted to hospital with UC exacerbation and abdominal pain and the other was diagnosed with cervix carcinoma. Costello 2017a reported three serious adverse events in participants who received FMT. One of these participants had worsening ulcerative colitis, one had C. difficile colitis requiring colectomy, and one had pneumonia. Two serious adverse events occurred in the control group and both of these individuals had worsening ulcerative colitis. No mortality was reported in any of the included studies.
Subgroup analysis
Table 2 shows subgroup analysis for 'Route of Administration' and 'Type of Donor' with results similar to the primary analysis.
Sensitivity analysis
The use of a fixed‐effect statistical model showed results similar to the primary random‐effects analysis (RR 1.41, 95 % CI 0.55 to 3.59, I² = 0%).
Adverse events
Ttwo studies including 129 participants reported on the proportion of participants who developed adverse events (Paramsothy 2017a; Rossen 2015). Adverse event rates were similar in both groups. Seventy‐eight per cent (50/64) of the FMT group had an adverse event compared to 75% (49/65) of the control group (RR 1.03, 95% CI 0.81 to 1.31, I² = 31%, Analysis 1.12).
1.12. Analysis.
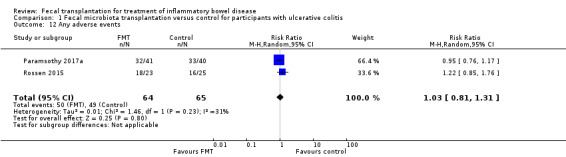
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 12 Any adverse events.
Common adverse events reported in the Paramsothy 2017a study included abdominal pain, colitis, flatulence, bloating, upper respiratory tract infection, headache, dizziness, and fever. FMT and control group participants had similar rates of these adverse events. Common adverse events reported in the Rossen 2015 study included nausea, fever, diarrhea, vomiting, abdominal pain, headache and transient borborygmus. The rates for these events were similar in both groups.
Clinical response at eight weeks
Clinical response rates at eight weeks were higher in the FMT group compared to the control group. Forty‐eight per cent (68/140) of FMT participants had a clinical response at week 8 compared to 28% (38/137) of the control group (RR 1.70, 95 % CI 0.98 to 2.95, I² = 64%, Analysis 1.13). The overall GRADE rating for the certainty of the evidence for this outcome was 'low'.
1.13. Analysis.
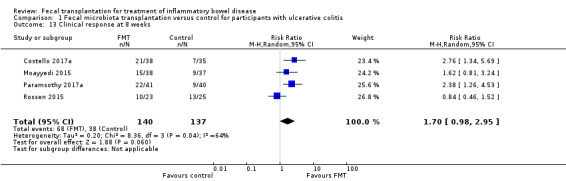
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 13 Clinical response at 8 weeks.
Clinical response at 12 weeks
One study with 46 participants reported on clinical response rates at 12 weeks (Rossen 2015). Clinical response rates at week 12 were similar in both groups. Forty‐eight per cent (11/23) of FMT participants had a clinical response at 12 weeks compared to 52% (13/25) of participants in the control group (RR 0.92, 95 % CI 0.52 to 1.63, Analysis 1.14).
1.14. Analysis.
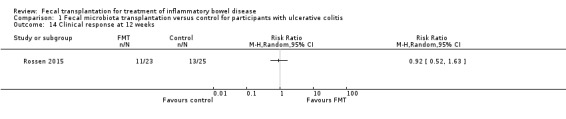
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 14 Clinical response at 12 weeks.
Endoscopic remission at eight weeks
Three studies including 229 participants reported on endoscopic remission at eight weeks (Costello 2017a; Paramsothy 2017a; Moayyedi 2015). Endoscopic remission rates were three times higher in the FMT group. Thirty per cent (35/117) of the FMT group achieved endoscopic remission at 8 weeks compared to 10% (11/112) of the control group (RR 2.96, 95 % CI 1.60 to 5.48, I² = 0%, Analysis 1.15). The overall certainty of the evidence for this outcome was 'moderate'.
1.15. Analysis.
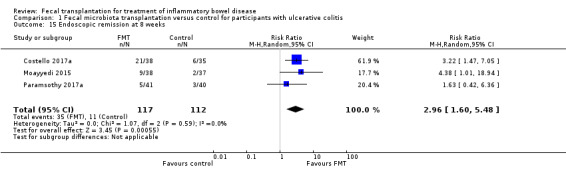
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 15 Endoscopic remission at 8 weeks.
Endoscopic remission at 12 weeks
Rossen 2015 reported data for endoscopic remission at 12 weeks. Endoscopic remission rates were similar in both groups. Nine per cent (2/23) of FMT participants achieved endoscopic remission at 12 weeks compared to 8% (2/25) of the control participants (RR 1.09, 95 % CI 0.17 to 7.10, Analysis 1.16).
1.16. Analysis.
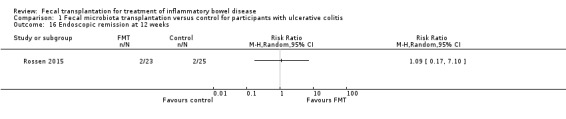
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 16 Endoscopic remission at 12 weeks.
Endoscopic response at eight weeks
Data for endoscopic response at 8 weeks were available from two studies including 129 participants (Paramsothy 2017a; Rossen 2015). Twenty‐eight per cent (18/64) of FMT participants achieved an endoscopic response at 8 weeks compared to 20% (13/65) of control participants (RR 1.36, 95 % CI 0.26 to 7.02, I² = 82%, Analysis 1.17). The overall certainty of the evidence for this outcome was 'very low'.
1.17. Analysis.
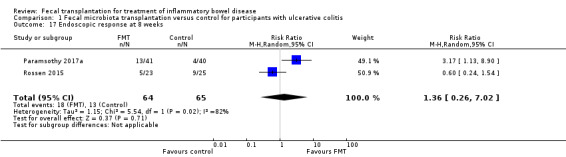
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 17 Endoscopic response at 8 weeks.
Endoscopic response at 12 weeks
One study including 48 participants reported data for endoscopic response at 12 weeks (Rossen 2015). Endoscopic response rates at 12 weeks were similar in both groups. Thirty‐five per cent (8/23) of FMT participants had an endoscopic response at 12 weeks compared to 36% (9/25) of the control participants (RR 0.97, 95 % CI 0.45 to 2.08, Analysis 1.18).
1.18. Analysis.
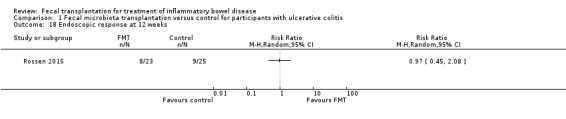
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 18 Endoscopic response at 12 weeks.
Withdrawals
Four studies with a total of 277 participants reported data on withdrawals for any reason (Costello 2017a; Moayyedi 2015; Paramsothy 2017a; Rossen 2015). Withdrawal rates were similar in both study groups. Thirteen per cent (18/140) of FMT participants withdrew before study completion compared to 14% (19/137) of control participants (RR 0.91, 95 CI 0.50 to 1.64, I² = 0%, Analysis 1.19).
1.19. Analysis.
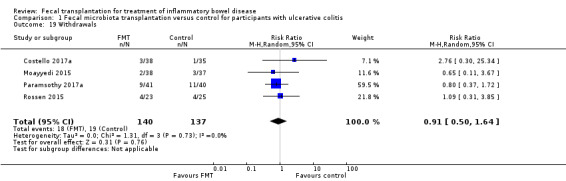
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 19 Withdrawals.
ESR at the longest follow‐up
Two studies including 113 participants reported data for ESR (Moayyedi 2015; Paramsothy 2017a). Mean ESR levels at longest follow‐up were similar in both study groups (MD ‐0.85, 95 % CI ‐4.86 to 3.17, I² = 0%, Analysis 1.20).
1.20. Analysis.
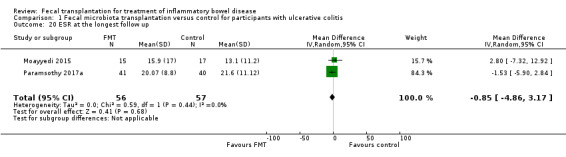
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 20 ESR at the longest follow up.
CRP at the longest follow‐up
Data on CRP levels at longest follow‐up were available from two studies including 113 participants (Moayyedi 2015; Paramsothy 2017a). The pooled analysis showed similar mean CRP levels at longest follow‐up (MD ‐0.85, 95 % CI ‐4.86 to 3.17, I² = 80%, Analysis 1.21). A high level of heterogeneity was detected for this comparison.
1.21. Analysis.
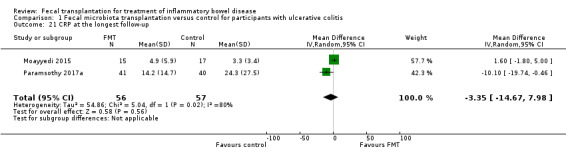
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 21 CRP at the longest follow‐up.
Fecal calprotectin at the longest follow‐up
One study including 81 participants reported data for fecal calprotectin at longest follow‐up (Paramsothy 2017a). Fecal calprotectin levels were lower in FMT participants compared to control participants (MD ‐156.00, 95 % CI ‐535.39 to 223.39).
Quality of life scores
Two studies including 156 participants reported inflammatory bowel disease quality of life (IBDQ) scores (Moayyedi 2015; Paramsothy 2017a). Although mean IBDQ scores were higher in FMT participants the difference does not appear to be clinically meaningful (MD 16.00, 95% CI 0.09 to 31.91, I² = 60%). One study reported quality of life scores between responders and non‐responders and thus could not be included in the meta‐analysis (Rossen 2015).
Microbiome outcomes
Data on microbiome outcomes were reported by three studies(Moayyedi 2015; Paramsothy 2017a; Rossen 2015). We planned a priori to do meta‐analysis for alpha diversity outcomes; however, data were presented in a way that we could not perform the meta‐analysis. A summary of methods used and outcomes reported from included studies is given below.
Moayyedi 2015 sequenced the V3 region of the 16S rRNA gene using MiSeq Illumina technology. QIIME and the Phyloseq R package was used for curation of data and in‐depth microbiota analyses. This study compared the microbiota of several different donors. Moreover, the authors compared the microbiota of FMT recipients during the time course of the study following FMT. Finally, responders and non‐responders microbiota were compared. Microbiota structure analyses utilizing the Bray‐Curtis dissimilarity metric demonstrated that patients receiving FMT showed a change in their microbiota following FMT. This shift lead to microbiota that was more similar to the donor microbiota over time. Moreover, the authors observed a difference in the microbiota between responders and non‐responders. Interestingly, two donors were associated with more successful FMTs and these individuals harbored increased Ruminococcus and Lachnospiraceae and decreased abundance of Streptococci and Escherichia.
Paramsothy 2017a sequenced the V1 through V3 region of 16S rRNA gene using MiSeq Illumina technology. Microbiota analysis and curation were performed utilizing mothur, and altered members of the microbiota were identified using the biomarker discovery algorithm linear discriminant analysis Effect Size (LEfSe). Shotgun metagenomics sequencing was also performed in subsequent follow‐up studies. The authors performed RNA extraction to ensure that bacteria detected in their analyses were live and active bacteria. Microbiota analyses were done on 70 patients (314 fecal samples) and 113 donor fecal samples; 55 individual donors were used and 58 batched donor samples. The microbiota of donors was analyzed along with patients receiving individual or batched donor FMTs. For recipients, the microbiota composition was analyzed prior to and following FMT, and patients were binned into responders and non‐responders. The microbiota of batched donor samples showed higher phylogenetic diversity than individual donors, and overall donor samples had higher diversity than baseline samples from patients with IBD. After four and eight weeks, patients receiving FMT saw an increase in phylogenetic diversity in the microbiota compared to baseline. LEfSe analysis determined that 295 microbial taxa were differentially altered following transplant and 78 of these members showed high linear discriminant analysis scores (>3). Interestingly, regardless of clinical outcome, the authors observed decreased abundance of operational taxonomic units (OTUs) affiliated with the Bacteroides genera and increased abundance of OTUs affiliated with the Prevotella genera. The authors further describe that FMT was associated with increased diversity in all patients. Importantly, recipients who achieved a successful primary outcome had greater richness in OTUs at baseline, during fecal microbiota transplantation and at 8 weeks. Finally, the authors performed LEfSe analysis comparing patients who responded to non‐responders; 87 Taxa were associated with primary outcomes in masked patients and 46 were associated with open‐label patients. Remission was associated with taxa with Barnsiella, Parabacteroides, Clostridium cluster IV and Ruminococcus. Moreover, Fusobacterium and Sutterella were associated with a lack of remission in all patients.
Rossen 2015 used the Human Intestinal Tract chip (HITchip) phylogenetic microarray, to perform microbiome diversity analysis. The study compared microbiota profiles of donors and patients with UC. Moreover, this study characterized microbiota profiles prior to and following FMT with donor stool or FMT with autologous stool. Finally, responders and non‐responders for each FMT group were compared. Microbiome analysis with HITchip showed that microbiota profiles and diversity indexes of patients with UC was different than healthy donors. This was highlighted by enrichment in taxa belonging to the Bacteroidetes, Proteobacteria, Bacilli, and Clostridium clusters IX and XI and decreased levels of Clostridium IV, IXIVa, and XVIII compared to donors. Following FMT with both donor stool and autologous stool, diversity increased in responders and did not increase in non‐responders. Shifts in the community taxa of responders could be observed in both donor and autologous FMTs. However, the shift between these two responder groups was distinct. Microbiota of FMT responders from donors were highlighted by increases in taxa belonging to the Clostridium IV, XIVa, and IVIII groups. Alternatively, the microbiota of responders that received autologous FMT was highlighted by an increase in Bacilli, Proteobacteria, and Bacteroidetes. Correlation analysis further revealed that microbiota of responding recipients showed increased similarity to donor microbiota following FMT. Importantly, no shifts in microbiota were observed in non‐responders.
Discussion
Summary of main results
This review synthesized findings from four studies that assessed the efficacy of FMT in 277 adult participants who had active UC. The pooled results showed that FMT may increase rates of clinical remission by two‐fold compared to the control group. The rate of serious adverse events is uncertain due to the wide confidence interval around the pooled effect estimate, which includes the possibility of more than a three‐fold increase in harms. The use of FMT may increase rates of endoscopic remission at eight weeks and increase rates of clinical response in patients with UC. Three of these studies sought to define the impact of FMT on the gut microbiota. In each of these three studies, FMT was shown to be effective in altering the microbiota by shifting the community structure to one similar to the donor community. However, only one study was able to establish a correlation between specific members of the gut microbiota and clinical response. We did not find any randomized or cohort study with a control arm that assessed the efficacy of FMT for treatment of CD. We did not find any studies that assessed FMT in pediatric participants. We did not find any studies that assessed long‐term maintenance of remission in participants with quiescent IBD.
Overall completeness and applicability of evidence
This review systematically assessed the efficacy and safety of FMT for treatment of IBD. Our objective was to assess the efficacy of FMT for induction and maintenance of remission in patients with UC and CD. No randomized or cohort studies with a control arm assessed FMT as a treatment of CD. Four randomized studies assessed treatment of UC with FMT and the main objective of these studies was to assess the efficacy of FMT for induction of remission. These studies did not assess FMT as maintenance therapy. The included studies reported data on clinical and endoscopic remission and serious adverse events. Other secondary outcomes such as quality of life and microbiome outcomes were also reported.
The successful use of FMT for the treatment of UC seems to be biologically plausible. This is based on earlier observations that suggest that UC patients have microbiota dysbiosis (Bejaoui 2015; Kostic 2014; Vindigni 2016). In addition, other therapeutics agents targeting the microbiome, such as probiotics, have demonstrated efficacy for maintenance of remission in UC (Mallon 2007). A causal association between dysbiosis and UC seems to be further supported by the studies in this review, as microbiome analyses suggest differential response in the microbiota of responders compared to non‐responders, highlighted by a shift towards the donor community microbiota in FMT responders (Moayyedi 2015; Paramsothy 2017a; Rossen 2015). Notably, Paramsothy 2017a was also able to identify several taxa that were associated with induction of remission and presence of other taxa that were associated with lack of effect. Similarly, increased alpha‐diversity was associated with an increased likelihood of a positive response. It is important to note that composition of human microbiota is highly heterogeneous with high inter‐individual variability. Thus, more in‐depth analyses exploring the functional impact of FMT on the microbiota of IBD patients are needed. Furthermore, these studies did not explore other components of the microbiome such as the virome or fungome, which have been recently appreciated as important factors for health and disease (Carding 2017; Witherden 2017). Finally, it is not clear if non‐microbial components of stool, such as bile acids, have any impact on treatment outcomes.
The treatment effect of FMT for induction of remission in UC was observed to be modest compared to use of FMT for treatment of recurrent C. difficile infection (Austin 2014; Cammarota 2015; Kassam 2013; Kelly 2016; Lee 2016; Leffler 2015; van Nood 2013; Youngster 2014). The reported efficacy of FMT for prevention of recurrent C.difficle is approximately 90% compared to a remission rate of approximately 37% for UC (Kassam 2013; Kelly 2016; Youngster 2014). Based on this observation, it can be deduced that the included studies were probably underpowered to detect the effect of FMT for induction of remission and future studies should calculate the required sample size based on expected clinical remission rates of around 35% to 40%. Also, the logistics of the intervention might be different for the treatment of UC compared to recurrent C. difficile. For example, a single administration of FMT might not be adequate to induce remission in people with active UC and multiple administrations might be required (Costello 2017a; Paramsothy 2017a). Also, donor characteristics may be critical, and the use of pooled donors may be preferred (Costello 2017a; Paramsothy 2017a). The route of administration might also play a role, with lower GI tract administration of greater benefit than upper GI tract administration (Costello 2017a; Paramsothy 2017a; Moayyedi 2015; Rossen 2015).
We are uncertain about the rate of serious adverse events due to the wide confidence interval around the pooled effect estimate, which includes the possibility of an increase in harms. The overall occurrence of serious adverse events was low with 10 events in 140 patients who underwent FMT and 7/137 events in patients receiving placebo. The most common serious adverse event was worsening of ulcerative colitis that necessitated the use of intravenous corticosteroids or surgery. However, worsening of ulcerative colitis seems to occur in similar rates among control group participants (Costello 2017a; Paramsothy 2017a; Moayyedi 2015; Rossen 2015). None of the included studies reported on long‐term adverse effects so future studies are needed to assess the long‐term safety of stool transplantation in patients with IBD. Thus, no conclusive statement can be made about the safety of FMT at this time.
Data on long‐term maintenance of remission was not reported on by the included studies. The primary focus of the included studies was to assess the efficacy and short‐term safety of FMT for induction of clinical remission in UC. Rossen 2015 reported on clinical relapse at 12 weeks among participants who achieved clinical remission. There were no relapses in the FMT group (0/7) compared to a 20% (2/10) relapse rate in control participants. Moayyedi 2015 also assessed 37 participants who received FMT in an open label fashion at 12 months from study onset. Eight of the nine participants who went into remission during the double blind phase of the trial were in remission at 52 weeks without relapsing. Four of these individuals were able to stop all of their medications and remained in remission. Three of these eight participants had been receiving FMT once a month. The one participant out of nine who eventually relapsed, did so after a course of antibiotics. Eleven participants who did not go into remission in the double blind stage of the study chose to have further open label treatment with FMT for 6 to 12 weeks and four of these individuals went into remission. Of all the participants that had FMT and were followed for 52 weeks, one required a colectomy during this period. Paramsothy 2017a planned a follow‐up at 8 weeks after double blind or open label allocation to FMT and this assessment included 63 participants. Of the 35 participants who achieved remission after double blind or open label allocation to FMT, 23 (66%) were in remission at 8 week follow‐up. Five patients who were not in clinical remission after eight weeks of FMT therapy (open label or double blind), went into clinical remission during the next 8 weeks. Twenty out of 63 patients who underwent FMT required escalation of therapy during the eight week follow‐up after completion of therapy. Therefore, overall, no conclusive statement can be made about long‐term maintenance of remission and future double blind studies are needed to further define the role of FMT for maintenance of remission in both UC and CD.
Quality of the evidence
The overall certainty of the evidence was low for most of the outcomes. The GRADE criteria considers type of studies, risk of bias, indirectness, inconsistency (i.e. unexplained heterogeneity), imprecision, and potential publication bias. Most of our assessments were downgraded to lower levels due to inconsistency and imprecision. The overall number of events for the primary outcome was small (76/277) and the confidence interval around the pooled effect estimate was wide, thus yielding an imprecise summary effect estimate. Two of the included studies were stopped early due to futility issues (Moayyedi 2015; Rossen 2015). However, the Cochrane Handbook guidelines do not consider these studies to be at high risk of bias (Higgins 2011). We graded evidence for serious side effect as 'low'. We downgraded the evidence for this outcome based on very serious imprecision (i.e. 17 events). In summary, the results for the outcomes assessed in this review are uncertain and no firm conclusions regarding the efficacy and safety of FMT in active UC can be drawn.
Potential biases in the review process
This review was conducted following the standardized methods of the Cochrane Collaboration. We searched for both published, and ongoing studies. However, the number of included studies was small so the subgroup analyses lacked adequate power to provide definitive conclusions. The number of included studies was less than 10, so we could not perform analyses to assess potential publication bias. We initially aimed to include single arm cohort studies. However, it was decided post‐hoc that we would only include randomized trials and observational studies with a control arm. This decision was related to our concern that single arm studies may not provide comparative evidence and that two recent reviews performed meta‐analysis of single arm cohort studies and are discussed below in the section on Agreements and disagreements with other studies or reviews (Costello 2017b; Paramsothy 2017b).
Some of the pooled analyses (i.e. Analysis 1.1; Analysis 1.13; Analysis 1.17 ) had a high level of statistical heterogeneity and it can be argued in some cases that data should not be pooled for these outcomes. However, we think that role of FMT for treatment of UC is biologically plausible and the variation in the direction and magnitude of effect (two main contributors to statistically heterogeneity) might be related to the conduct of the FMT intervention. For example, exclusion of the study by Rossen 2015 that used a nasoduodenal route for administration of FMT reduced the statistical heterogeneity to zero in Analysis 1.1 and Analysis 1.4. The same was shown for the subgroup analysis for the primary outcome based on route of administration, which indicated that all three studies that used rectal route for administration of FMT had a homogeneous effect in favor of treatment (Analysis 1.3). Based on this observation, it can be suggested that the rectal route might be better than administration via the upper GI tract; however, more studies are needed before a more conclusive statement can be made.
1.1. Analysis.
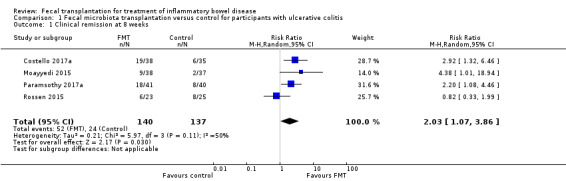
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 1 Clinical remission at 8 weeks.
1.4. Analysis.
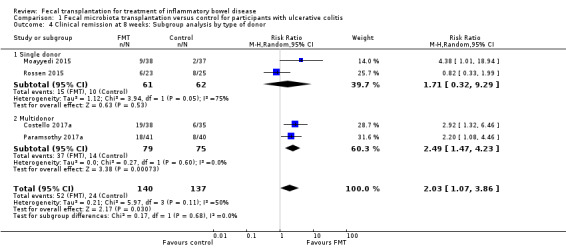
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 4 Clinical remission at 8 weeks: Subgroup analysis by type of donor.
1.3. Analysis.
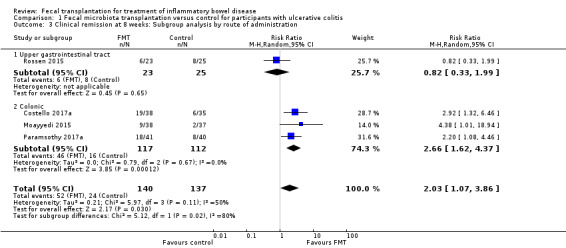
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 3 Clinical remission at 8 weeks: Subgroup analysis by route of administration.
Recent literature has suggested the need to assess mucosal healing as part of the assessment of response to therapy for IBD as it might better predict long term outcomes, including risk of surgery (Auzoux 2016; De Preter 2012; Dulai 2015). We decided a priori that the primary outcome of 'clinical remission' would be based on definitions derived from clinical scores. This decision was based on a lack of a standardized definition of mucosal healing as there is currently no consensus on the method of assessment of mucosal healing. There is also no consensus on the extent of mucosal healing that would be associated with desired long‐term outcomes (Armuzzi 2012; Auzoux 2016; Dave 2012; Dulai 2015; Peyrin‐Biroulet 2012). In any case, we conducted a sensitivity analysis and a post‐hoc analysis of a composite outcome of clinical and endoscopic remission as reported by the primary studies. Data on clinical remission defined by improvement in clinical scores were available from all studies except Moayyedi 2015. When this study was excluded from the analysis, the direction of the effect remained in favor of FMT; however, the effect size decreased (RR 1.79, 95 % CI 0.88 to 3.63, I² = 58%). This attenuated effect was likely related to the small number of studies and small number of events in the remaining three studies. A post‐hoc analysis of data from the four studies with definition of clinical remission based on clinical scores and endoscopic scores showed similar results to the primary analysis (RR 2.77, 95 % CI 1.54 to 4.98, I² = 0%). Interestingly, there was no statistical heterogeneity in this analysis. Thus, overall it seems likely that FMT might have a biologically plausible effect in favor of FMT irrespective of how clinical remission was defined.
Agreements and disagreements with other studies or reviews
Two recent reviews have assessed the effect of FMT on the treatment of IBD and included both observational and randomized studies (Costello 2017b; Paramsothy 2017b). Both of these reviews meta‐analyzed observational and randomized studies separately. The summary estimates for randomized studies were reported as odds ratios (OR) compared to risk ratios in our review.
Costello 2017b included 14 cohort studies and 4 randomized trials and assessed efficacy of FMT for UC only. The overall results from RCTs showed that FMT was associated with a beneficial effect for clinical remission (OR 3.67, 95 % CI 1.82 to 7.39) and clinical response (OR 2.48, 95 % CI 1.18 to 5.21). The pooled data from single‐arm cohort studies showed that 24% of the UC patients went into clinical remission after FMT. No meta‐analysis of safety outcomes or endoscopic or laboratory outcomes was performed and no overall assessment was made for the quality of the evidence. The follow‐up time for the primary outcome was also not reported.
Paramsothy 2017b included 53 studies and assessed the efficacy of FMT for the treatment of UC, CD, and pouchitis. This review included case reports, case series, single arm cohort studies and RCTs. Pooled data from four RCTs for UC showed that use of FMT was associated with an improvement in clinical remission (OR 2.89, 95 % CI 1.36 to 6.13) and clinical response rates (OR 2.48, 95 % CI 1.18 to 5.21). Pooled data from 14 observational studies showed that 33% of UC patients achieved clinical remission after FMT. Pooled data from 6 cohort studies showed that 52% of CD patients achieved clinical remission after FMT. Pooled data from 4 cohort studies showed 63% of CD patients had a clinical response after FMT. Data on the effect of FMT on pouchitis were reported by 4 studies; however, no meta‐analyses were performed for this outcome. Data on safety outcomes were described but again no meta‐analysis was performed. Moreover, no overall quality assessment was made for the combined data.
Authors' conclusions
Implications for practice.
Fecal microbiota transplantation may increase the proportion of participants achieving clinical remission in UC. However, the number of identified studies was small and the quality of evidence was low. There is uncertainty about the rate of serious adverse events. As a result, no solid conclusions can be drawn at this time.
Implications for research.
More studies are needed to further delineate some aspects of the intervention in terms of route (rectal versus upper GI tract), frequency, type of donor (single versus pooled donor), timing (primary induction versus rescue therapy), and preparation of stool (aerobic versus anaerobic; frozen versus fresh). Most of the patients in the included studies had mild to moderate UC and it is not clear if the efficacy will be similar, better or worse in patients with severe disease. Also, it is not clear if a combination of interventions, such as the use of antibiotics before the transplant or use of nutritional therapy or probiotics have advantages over FMT alone. No data were available for efficacy of FMT in paediatric patients with IBD so future studies are needed in this population. Future clinical trials are also needed to assess the efficacy of FMT for induction of remission in CD and to assess the efficacy of FMT for maintenance of remission in UC and CD. Further, additional evaluations of the microbiome and metabolome are needed to establish the exact mechanism of action of FMT for the treatment of IBD. Currently, there are 13 ongoing studies (estimated enrolment of 1170 participants). This review will be updated when the results of these studies are available.
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search strategies
MEDLINE
1. Exp Inflammatory bowel disease/
2. Crohn*.mp.
3. Ulcerative colitis.mp
4. IBD.mp.
5. Inflammatory bowel disease*.mp.
6. Or/1‐5
7. Fecal microbiota transplant*.mp.
8. Faecal microbiota transplant*.mp.
9. fecal microbiome transplant*.mp.
10. Stool transplant*.mp.
11. FMT.mp.
12. Fecal transfusion*.mp.
13. Fecal bacteriotherap*.mp.
14. Or/7‐13
15. 6 and 14
Embase
1. Exp Inflammatory bowel disease/
2. Crohn*.mp.
3. Ulcerative colitis*.mp
4. IBD.mp.
5. Inflammatory bowel disease*.mp.
6. Or/1‐5
7. Fecal microbiota transplant*.mp.
8. Faecal microbiota transplant*.mp.
9. fecal microbiome transplant*.mp.
10. Stool transplant*.mp.
11. FMT.mp.
12. Fecal transfusion*.mp.
13. Fecal bacteriotherap*.mp.
14. Or/7‐13
15. 6 and 14
Cochrane CENTRAL
#1 MeSH: [Inflammatory bowel disease] explode all trees
#2 Crohn
#3 Ulcerative colitis
#4 IBD
#5 #1 or #2 or #3 or #4
#6 MeSH: [Fecal transplant] explode all trees
#7 Fecal microbiota transplant*
#8 Faecal microbiota transplant*
#9 Fecal microbiome transplant*
#10 Stool transplant*
#11 FMT
#12 Fecal transfusion*
#13 Fecal bacteriotherap*
#14 Fecal bacteriotherap*
#15 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #5 and #15
Clinical Trials. Gov
1. Fecal transplantation and Inflammatory Bowel Disease
2. Fecal transplant and Inflammatory Bowel Disease
3. Fecal microbiota transplant and Inflammatory Bowel Disease
Appendix 2. Characteristics of donors in the included studies
Donor inclusion criteria:
‐ Volunteers
‐ Age between 18 and 60 years of age
‐ otherwise healthy, as assessed by a screening questionnaire
Donor exclusion criteria: ‐ Use of antibiotics in the previous 3 months
Lab testing for donors
Blood studies: human immunodeficiency virus (HIV) 1/2, hepatitis A immunoglobulin M, hepatitis B surface antigen, hepatitis C antibody, syphilis, human T‐ lymphotrophic virus 1/II, vancomycin‐resistant enterococcus, or methicillin‐resistant Staphylococcus aureus
Stool studies: Salmonella, Shigella, Campylobacter, Escherichia coli O157 H7, Yersinia, ova, cysts, and parasites, or C. difficile toxin.
Donors eligibility criteria: Males and females aged 18 to 65 years, No history or current symptoms of gastrointestinal disease including but not limited to IBD and irritable bowel syndrome (IBS), No other major active medical co‐morbidities, Minimal regular medications with no medications that may interfere with stool viability including no antimicrobials (antibiotics, antivirals, antifungals), probiotics and proton pump inhibitors in the preceding three months, provide written informed consent to participate as shown by a signature on the consent form.
Donors exclusion criteria: Known HIV, hepatitis B or hepatitis C infection, known exposure to HIV or viral hepatitis within the previous 12 months, high risk sexual behavior (e.g. sexual contact with anyone with HIV/AIDS or viral hepatitis, men who have sex with men, sex for drugs or money), use of illicit drugs, tattoo or body piercing within the preceding 6 months, incarceration or history of incarceration, known current communicable disease (e.g. upper respiratory tract infection), risk factors for variant Creutzfeldt‐Jakob disease, travel within last two weeks to areas of the world where diarrhoeal illnesses are endemic or risk of traveller’s diarrhea is high, history of or current IBS, chronic constipation, chronic diarrhea or other intrinsic gastrointestinal illness or condition, history of or current gastrointestinal malignancy or known polyposis or strong family history of colorectal cancer, history of major gastrointestinal surgery (e.g. gastric bypass, partial colectomy), antimicrobials (antibiotics, antivirals, antifungals), probiotics or proton pump inhibitors (PPIs) within the preceding three months, major immunosuppressive medications (e.g. calcineurin inhibitors, biological agents, exogenous glucocorticoids), systemic anti‐neoplastic agents, household members with active GI infection, systemic autoimmunity (e.g. multiple sclerosis, connective tissue disease), atopic disease (e.g. moderate ‐ severe asthma, eosinophilic disorders of the gastrointestinal tract), metabolic syndrome, obesity (body mass index > 30) or moderate to severe under‐nutrition or malnutrition, chronic pain syndromes (e.g. chronic fatigue syndrome, fibromyalgia) or neurologic or neurodevelopmental disorders, history of malignant illness or ongoing oncologic therapy.
Donors: Lab Testing
Stool testing: Clostridium difficile toxin polymerase chain reaction, fecal microscopy/culture/sensitivity with routine bacterial culture for enteric pathogens, fecal Giardia antigen, fecal Cryptosporidium antigen, fecal ova, cysts or parasites (including Blastocystis hominis and Dientamoeba fragilis), norovirus enzyme immunoassay.
Blood testing: Complete blood count, electrolytes, urea and creatinine, liver function tests, erythrocyte sedimentation rate, C‐reactive protein, HIV type 1 and 2, hepatitis A virus immunoglobulin M, hepatitis B virus surface antigen, hepatitis B virus core antibody (immunoglobulin M and imunoglobulin G), hepatitis B virus surface antibody, hepatitis C virus antibody, rapid plasma reagin or fluorescent treponemal antibody‐absorbed, human T‐cell lymphotropic virus 1 and 2.
Donors eligibility criteria:
‐ Healthy partners, relatives, or volunteers (18 years of age) were screened for fecal donation using the Dutch Red Cross Questionnaire addressing risk factors for potential transmissible diseases used for screening of blood donors in the Netherlands.
Donors lab testing:
Blood: Lues serologie (syphilis), cytomegalovirus, Epstein‐Barr virus, hepatitis A virus (total antibody), hepatitis B surface antigen, hepatitis C virus antibody, HIV (1þ2 antibodies/antigen), human T‐cell lymphotropic virus (I + II antibodies), antibodies against Entamoeba histolytica, Strongyloides (strongyloides enzyme‐linked immunosorbent assay).
Stool:Salmonella, Shigella, Yersinia, Campylobacter bacteria, Clostridium difficile toxin, multiplex polymerase chain reaction containing probes against enteral viruses (rotavirus, norovirus, enterovirus parechovirus, sapovirus, adenovirus 40/41/52, astrovirus), feces test plus triple feces test: Giardia polymerase chain reaction.
Data and analyses
Comparison 1. Fecal microbiota transplantation versus control for participants with ulcerative colitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical remission at 8 weeks | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.07, 3.86] |
| 2 Clinical remission at 12 Weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clinical remission at 8 weeks: Subgroup analysis by route of administration | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.07, 3.86] |
| 3.1 Upper gastrointestinal tract | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.33, 1.99] |
| 3.2 Colonic | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [1.62, 4.37] |
| 4 Clinical remission at 8 weeks: Subgroup analysis by type of donor | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.07, 3.86] |
| 4.1 Single donor | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.32, 9.29] |
| 4.2 Multidonor | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [1.47, 4.23] |
| 5 Clinical remission at 8 weeks: Sensitivity analysis using fixed‐effect model | 4 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.39, 3.25] |
| 6 Clinical remission: Composite of clinical score and endoscopic score | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [1.54, 4.98] |
| 7 Clinical relapse at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Serious adverse events | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.55, 3.58] |
| 9 Serious adverse events: Subgroup analysis by route of administration | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.55, 3.58] |
| 9.1 Upper Gastrointestinal | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.17, 7.10] |
| 9.2 Colonic | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.51, 4.50] |
| 10 Serious adverse events: Subgroup analysis by type of donor | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.55, 3.58] |
| 10.1 Single donor | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.36, 4.55] |
| 10.2 Multidonor | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.39, 6.29] |
| 11 Serious adverse events: Sensitivity analysis using fixed‐effect model | 4 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.55, 3.59] |
| 12 Any adverse events | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.31] |
| 13 Clinical response at 8 weeks | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.98, 2.95] |
| 14 Clinical response at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Endoscopic remission at 8 weeks | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [1.60, 5.48] |
| 16 Endoscopic remission at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 17 Endoscopic response at 8 weeks | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.26, 7.02] |
| 18 Endoscopic response at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 19 Withdrawals | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.50, 1.64] |
| 20 ESR at the longest follow up | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐4.86, 3.17] |
| 21 CRP at the longest follow‐up | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐3.35 [‐14.67, 7.98] |
| 22 Fecal calprotectin at the longest follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23 Quality of life score: IBDQ | 2 | 156 | Mean Difference (IV, Random, 95% CI) | 16.00 [0.09, 31.91] |
1.5. Analysis.
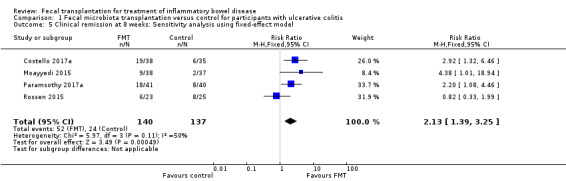
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 5 Clinical remission at 8 weeks: Sensitivity analysis using fixed‐effect model.
1.6. Analysis.
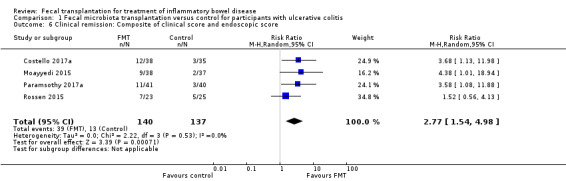
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 6 Clinical remission: Composite of clinical score and endoscopic score.
1.8. Analysis.
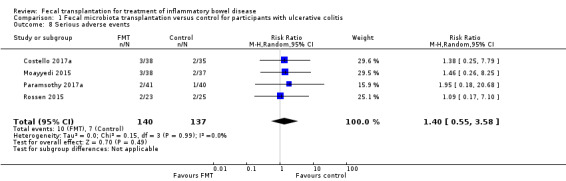
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 8 Serious adverse events.
1.9. Analysis.
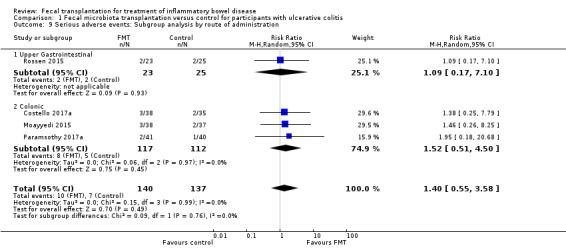
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 9 Serious adverse events: Subgroup analysis by route of administration.
1.10. Analysis.
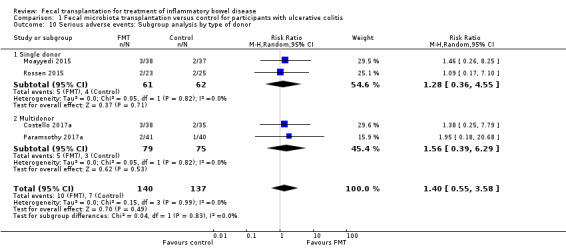
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 10 Serious adverse events: Subgroup analysis by type of donor.
1.11. Analysis.
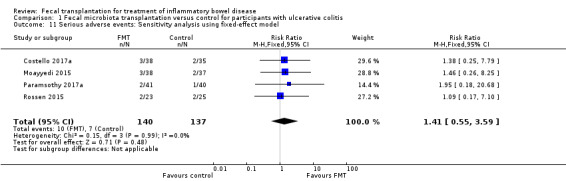
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 11 Serious adverse events: Sensitivity analysis using fixed‐effect model.
1.22. Analysis.
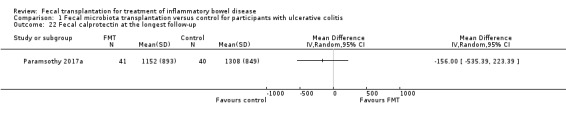
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 22 Fecal calprotectin at the longest follow‐up.
1.23. Analysis.
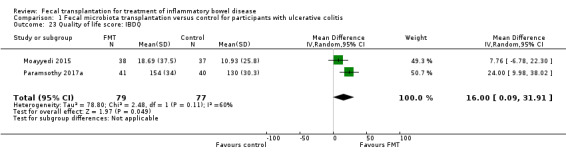
Comparison 1 Fecal microbiota transplantation versus control for participants with ulcerative colitis, Outcome 23 Quality of life score: IBDQ.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Costello 2017a.
| Methods | A multi‐center randomized, double‐blind, placebo‐controlled trial conducted in Australia | |
| Participants |
Inclusion Criteria Adults with active UC (total Mayo 3 to 10 with an endoscopic Mayo sub‐score ≥2) N = 73 |
|
| Interventions |
Intervention: Anaerobically prepared donor stool (pooled from 3 to 4 donors); n = 38 Route: First administration via colonoscopy followed by 2 enemas by day 7 Comparison: Autologous FMT (placebo) n = 35 |
|
| Outcomes |
Primary outcome Steroid‐free remission of UC as defined by a total Mayo score of ≤2 with an endoscopic Mayo score of ≤1 at week Secondary Outcomes: Clinical response (≥3 point reduction in Mayo score) Clinical remission (SCCAI ≤2) Endoscopic remission (Mayo ≤1) Adverse Events |
|
| Notes | Study information was available in the form of an abstract Further information was provided by the authors on request; however, this did not include enough information that a complete risk of bias assessment could be made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reported as an abstract and no further information was available |
| Allocation concealment (selection bias) | Unclear risk | Study reported as an abstract and no further information was available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reported as an abstract and no further information was available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study reported as an abstract and no further information was available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Overall attrition: 4% |
| Selective reporting (reporting bias) | Unclear risk | Study reported as an abstract and no further information was available |
| Other bias | Unclear risk | Study reported as an abstract and no further information was available |
Moayyedi 2015.
| Methods | Randomized, double blind trial conducted in Canada | |
| Participants |
The inclusion criteria Adult patients 18 years or older with UC (N = 75) Active UC defined as a Mayo Clinic score > 4 with an endoscopic Mayo Clinic score > 1 Concomitant treatments for UC, such as mesalamine, glucocorticoids, immunosuppressive therapy (e.g. azathioprine), or tumor necrosis factor antagonists were permitted, provided these had been used at a stable dose for at least 12 weeks (4 weeks for glucocorticoids) and disease remained active The Exclusion Criteria Use of antibiotics or probiotics in the last 30 days Concomitant Clostridium difficile infection or another enteric pathogen Disease severity that required hospitalization Pregnancy Unable to give informed consent |
|
| Interventions |
Study Intervention Single donor feces (multiple donors recruited for study but one patient received feces from one donor only); n = 38 Route: Retention enema Frequency: 1 times per weeks for 6 weeks, total 6 treatments Weight of stool: 50 g Volume administer per treatment: 50 ml Comparison: Placebo (water); n = 37 Route: Retention enema Frequency: 1 times per weeks for 6 weeks, total 6 treatments Volume administer per treatment: 50 ml |
|
| Outcomes |
Primary Outcome: UC remission at week 7, defined as a full Mayo score <3 and complete healing of the mucosa at flexible sigmoidoscopy (endoscopic Mayo score = 0) Secondary Outcomes: Clinical response: Improvement in UC symptoms (defined as 3 improvement in full Mayo score), change in Mayo score, IBDQ, EQ‐5D scores, ESR, CRP, adverse events |
|
| Notes | Study was stopped early due to futility Data on quality of life scores was reported as change from baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomized 1:1 according to a computer‐generated randomization" Comment: Most likely done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was held centrally at the McMaster Gastroenterology Clinical Trials Unit to ensure concealment of allocation" Comment: Most likely done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The treatment location was masked to the patient, health care workers caring for the patient, and investigators" Comment: Most likely done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The treatment location was masked to the patient, health care workers caring for the patient, and investigators" Comment: Most likely done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Overall loss to follow up was 6%, the reason for attrition was similar between the two groups |
| Selective reporting (reporting bias) | Low risk | Comment: Authors seem to report all the a priori outcomes The trial was registered with ClinicalTrials.gov, number: NCT01545908 |
| Other bias | Low risk | Comment: The trial was stopped early due to futility; however, the data was completely described for included patients |
Paramsothy 2017a.
| Methods | Randomized, double blind trial conducted in Australia | |
| Participants |
The inclusion Criteria Adult patients aged 18 to 75 years with UC for greater than 3 months (N = 81) Clinically and endoscopically active UC, with a total Mayo score of 4 to 10 and the Mayo endoscopy subscore to be 1 or greater and physician’s global assessment subscore 2 or less. Provide written informed consent to participate as shown by a signature on the consent form Exclusion criteria Consent not obtained or unavailable or inability to communicate with the investigators. Pregnancy, lack of contraception for non‐pregnant women Patient in remission, proctitis < 5 cm, non‐UC IBD, perianal disease, constipation‐predominant UC with < 3 bowel motions/day, mild UC (Mayo score < 4) Severe UC (Mayo score > 10), severe anaemia, leucopenia or granulocytopenia, toxic megacolon Active gastrointestinal infection, Irritable bowel syndrome, diverticulitis, neoplasm etc. Significant gastrointestinal surgery e.g. colon resection, colectomy Medications: antimicrobials (antibiotics, antifungals, antivirals),biologics e.g. infliximab, adalimumab; calcineurin inhibitors; mammalian target of rapamycin inhibitors, chemotherapeutic anti‐neoplastic agents, lymphocyte depleting biological agents; probiotic therapy; experimental drug Steroid dependency: requiring > 20 mg prednisone or > 9 mg budesonide daily Clinical evidence of any major, co‐morbid chronic disease Patients with food hypersensitivity |
|
| Interventions |
Study Intervention Donor: Multidonor feces; n = 41 Route: First infusion in terminal Ileum, then colonic enemas Frequency: 5 times per weeks for 8 weeks, total 40 treatments Weight of stool: 37.5 g Volume per treatment: 150 ml Comparison Placebo: Isotonic Saline; n = 40 Route: First infusion in terminal Ileum, then colonic enemas Frequency: 5 times per weeks for 8 weeks, total 40 treatments Volume per treatment: 150 ml |
|
| Outcomes |
Primary Outcome: composite of steroid‐free clinical remission at week 8 (defined as a total Mayo score of 2 or less) and endoscopic remission or response which (Mayo subscores of 1 or less, and at least a 1 point reduction from baseline in the endoscopy subscore) Secondary Outcomes Steroid‐free clinical remission at week 8 (defined as combined Mayo subscores of 1 or less for rectal bleeding plus stool frequency) Steroid‐free clinical response (defined as either a decrease of 3 points or more on the Mayo score, a 50% or greater reduction from baseline in combined rectal bleeding plus stool frequency Mayo subscores, or both) Steroid‐free endoscopic response at week 8 (defined as a Mayo endoscopy subscore of 1 or less, with a reduction of at least 1 point from baseline) Steroid‐free endoscopic remission at week 8 (defined as a Mayo endoscopy subscore of 0) Quality of life (assessed with the IBDQ) Adverse events |
|
| Notes | Data on quality of life scores was reported as medians and range and was converted to mean and standard deviation by methods given in Hozo 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a pre‐established computer‐generated randomisation list with permutated blocks of four and stratified for study site and concomitant corticosteroid use" Comment: Most likely done |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized centrally by the Centre for Digestive Diseases after screening in a 1:1 ratio" Comment Most likely done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and investigators were unaware of treatment allocation" Comment: Most likely done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study investigators who played a participants’ assessment did not see the investigational product at any time" Comment: Most likely done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition: 24% The dropout rate was similar in both groups and reason for drop outs were given and they were similar in both groups |
| Selective reporting (reporting bias) | Low risk | Comment: The authors seems to report all the a priori outcomes The trial was registered with ClinicalTrials.gov, number NCT01896635 |
| Other bias | Low risk | Comment: No other major risk of bias was noticed |
Rossen 2015.
| Methods | Randomized, double blind trial conducted in the Netherlands | |
| Participants |
The Inclusion criteria Adult patients with UC (N = 48) Established UC according to the Lennard‐Jones criteria, a patient‐reported SCCAI of between 4 and 11 and stable medication, which was continued during the study period Subjects were allowed stable doses of thiopurines, mesalamine, or corticosteroids 10 mg/day for the 8 weeks before inclusion An endoscopic Mayo score of 1 at baseline sigmoidoscopy The exclusion criteria An infectious cause of a UC disease flare A history of colectomy A current stoma A life expectancy of < 12 months Pregnancy Hospital admission Use antibiotics or probiotics within 6 weeks before inclusion. Use of antitumour necrosis factor or methotrexate treatment within 8 weeks before inclusion, or cyclosporine within 4 weeks before inclusion |
|
| Interventions |
Study Intervention Stool transplant: Single donor feces; n = 23 Route: Nasoduodenal Frequency: 1 times per 3 weeks, total 2 treatments Weight of stool: 120 g Volume administer per treatment: 500 ml Comparison: Placebo: autologous feces; n = 25 Route: Nasoduodenal Frequency: 1 times per 3 weeks, total 2 treatments Weight of stool: 120 g Volume administer per treatment: 500 ml |
|
| Outcomes |
Primary outcome: Combination of clinical remission (defined as a SCCAI score 2) and 1‐point improvement on the combined Mayo endoscopic score of the sigmoid and rectum, as compared with baseline sigmoidoscopy at week 12 Secondary Outcomes: Clinical response (defined as a reduction of 1.5 points on the SCCAI) at week 12 Clinical remission (defined as a SCCAI of 2) at week 12 Endoscopic response at week 12 Change in median IBDQ score at week 12 Adverse events |
|
| Notes | Study was stopped early due to futility Multiple donors recruited for study but one patient received feces from one donor only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from study protocol: "Alea software will be used to perform randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote from study protocol: "Randomisation and preparation of the feces will be performed by one of the research nurses, she is the only person who will know which treatment the patient will be given and will have no role in further part of the study" Comment: Most likely done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from study protocol: "Patients will be blinded until Follow‐up data until the end of the study (t:12 months) is collected" Comment: Most likely done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from study protocol: "Blinding of participants and trial members was guaranteed by collecting both donor and recipient feces on both treatment days" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 25% attrition The dropout rate was similar in both groups. Reason for drop outs were given and they were similar in both groups |
| Selective reporting (reporting bias) | Low risk | Comment: The authors seems to report all the a priori outcomes The trial was registered at ClinicalTrials.gov Number: NCT01650038 Study protocol was available for review |
| Other bias | Low risk | Comment: The trial was stopped earlier due to futility; however, the data was completely described for included patients |
UC: ulcerative colitis
FMT: fecal microbiota transplantation
SCCAI: Simple Clinical Colitis Activity Index
IBDQ: Inflammatory Bowel Disease Questionnaire
EQ‐5D: EuroQoL Group Quality of Life Questionnaire
ESR: erythrocyte sedimentation rate
CRP: C‐reactive protein
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angelberger 2016 | Cohort study that included patients with recurrent Clostridium difficile infection |
| Borody 2003 | This was a case series |
| Chin 2017 | Study included patients with IBD who had recurrent Clostridium difficile infection FMT was done for treatment of recurrent Clostridium difficile infection |
| Fischer 2016 | Study included patients with IBD who had recurrent Clostridium difficile infection |
| Gionchetti 2000 | The intervention was probiotics (VSL#3) and not FMT |
| Hourigan 2015 | The comparison group included children without IBD |
| Ishikawa 2017 | Both the study groups received FMT, one group received FMT with antibiotics and the other group received FMT without antibiotics |
| Landy 2013 | This study included patients with pouchitis only |
| Mandalia 2016 | Study included IBD patients who had recurrent Clostridium difficile infection |
| Mintz 2016 | The study also did not have the control arm This study included patients with recurrent Clostridium difficile and UC |
| Wei 2016 | Both groups received FMT, one group received FMT plus pectin and the other group received FMT only |
IBD: inflammatory bowel disease
FMT: fecal microbiota transplantation
UC: ulcerative colitis
Characteristics of ongoing studies [ordered by study ID]
NCT01790061.
| Trial name or title | Standardized Fecal Microbiota Transplantation for Ulcerative Colitis |
| Methods | Interventional clinical trial, non‐randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: Standardized FMT FMT by gastroscopy administration of fresh or frozen bacteria from healthy donor to the mid‐gut or whole colon Comparison: Traditional treatments Traditional treatments according to associated guidelines |
| Outcomes |
Primary Outcome Measures
The efficacy and durability of clinical remission (days) after FMT procedure defined as Montreal score S0 (clinical remission) Secondary Outcome Measures
|
| Starting date | November 2012 |
| Contact information | Faming Zhang, Associate Professor, Gastroenterology, The Second Hospital of Nanjing Medical University |
| Notes | Estimated enrolment: 500 |
NCT01793831.
| Trial name or title | Standardized Fecal Microbiota Transplantation for Crohn’s Disease |
| Methods | Interventional (clinical trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Experimental: Fecal microbiota transplantation Standard FMT, administered once. Sham Comparator: Traditional treatments, e.g. 5‐ASA Traditional treatments according to associated guidelines, including 5‐ASA, immunomodulatory therapy, corticosteroids, and biologics |
| Outcomes |
Primary Outcome Measures
Secondary Outcome Measures :
|
| Starting date | November 2012 |
| Contact information | Faming Zhang, Associate Professor, Gastroenterology, The Second Hospital of Nanjing Medical University |
| Notes | Estimated enrolment: 30 |
NCT01961492.
| Trial name or title | Fecal Microbiota Transplantation in Patients With Ulcerative Colitis |
| Methods | Interventional (clinical trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: Fecal microbiota transplantation A single FMT via colonoscope as an adjunct therapy to standard medical treatment Comparison: Standard medical treatment Standard medical treatment as recommended by the ECCO Guidelines of UC therapy |
| Outcomes |
Primary Outcome Measures
Secondary Outcome Measures
|
| Starting date | October 2013 |
| Contact information | Marko Kalliomäki, Specialist, Turku University Hospital |
| Notes | Estimated enrolment: 40 |
NCT02272868.
| Trial name or title | Fecal Microbial Transplant in Pediatric Crohn's Disease (FMTCD) |
| Methods | Interventional clinical trial, randomized |
| Participants |
Inclusion criteria:
Exclusion Criteria
|
| Interventions |
intervention: Fecal microbiome transplant Pretransplant (Rifaximin 400 mg 3 times daily x 10 days + omeprazole 20 mg night before transplant and day of transplant + MiraLAX 17 g 3 times daily x 2 days) + FMT Comparison: Normal saline Pretransplant (Rifaximin 400 mg 3 times per day x 10 days + omeprazole 20 mg night before transplant and day of transplant + miralax 17 g 3 times per day x 2 days) + normal saline |
| Outcomes | Primary Outcome Measures
|
| Starting date | January 2015 |
| Contact information | David Suskind, Principal Investigator, Seattle Children's Hospital |
| Notes | Trial completed No results available 7 participants |
NCT02291523.
| Trial name or title | The Effect of Therapeutic Fecal Transplant on the Gut Microbiome in Children With ulcerative colitis (FMT_UC) |
| Methods | Interventional (clinical trial), randomized |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Intervention: FMT with healthy donor stool administered through colonoscopy and high dose 5‐ASA (Pentasa) Comparison: Placebo and high dose 5‐ASA (Pentasa) |
| Outcomes |
Primary Outcome
Secondary Outcomes:
|
| Starting date | November 14, 2016 |
| Contact information | Sonia Michail, MD, Professor of Clinical Pediatrics, Children's Hospital Los Angeles |
| Notes | Estimated enrolment: 101 |
NCT02335281.
| Trial name or title | Standardized Fecal Microbiota Transplantation for Inflammatory Bowel Disease (SFMT‐IBD) |
| Methods | Interventional (clinical trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: Standardized FMT Participants will receive standardized FMT administered once to the mid‐gut by nose‐jejunum nutrition tube Comparison: Mesalazine The patients will receive traditional medicine of mesalazine treatment |
| Outcomes |
Primary Outcome
Clinical remission defined as HBI score ≦ 4. The endpoint of follow‐up is the time of clinical recurrence Secondary Outcome
Hospitalization days from administration to discharge when at clinical remission |
| Starting date | January 2015 |
| Contact information | Yanling Wei, Department of Gastroenterology, Research Institute of Surgery, Da Ping Hospital, The Third Military Medical University, Third Military Medical University |
| Notes | Estimated enrolment: 40 |
NCT02390726.
| Trial name or title | Fecal Microbiota Transplant in the Treatment of ulcerative colitis (FMTUC) |
| Methods | Interventional (clinical trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention FMT and microbial maintenance plus standard therapy Comparison: Sham FMT and sham microbial maintenance plus standard therapy |
| Outcomes |
Primary Outcomes
Secondary Outcomes
|
| Starting date | December 2015 |
| Contact information | Peter L Moses, MD |
| Notes | 20 participants enrolled |
NCT02487238.
| Trial name or title | PediFETCh trial |
| Methods | Randomized, single‐blinded, placebo controlled trial |
| Participants | Pediatric patients aged 3 to 17 years with UC or IBD‐U |
| Interventions |
Intervention FMT given via retention enema, 2 times per week for 6 weeks Comparison Normal saline enema, 2 times per week for six weeks |
| Outcomes |
Outcomes
|
| Starting date | November 2015 |
| Contact information | Dr. Nikhil Pai; pain@ mcmaster.ca, Division of Pediatric Gastroenterology & Nutrition, Department of Pediatrics, McMaster University, Hamilton, Canada |
| Notes | Authors presented data on three patients in a recent conference; however, mainly secondary outcomes were described and no data were available on clinical remission We decided to not include data from 3 patients at this time Authors were contacted to ask if they plan to present further data and they replied that they have not complied the results as the trial is ongoing Estimated enrolment: 50 |
NCT02734589.
| Trial name or title | Pilot Study of Fecal Transplantation Using a Unique Diet for Donor and Recipient in Mild to Moderate Treatment Refractory Colitis in Inflammatory Bowel Disease |
| Methods | Interventional (Clinical Trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: FMT with Diet for the donor and for the recipient Undergo standard fecal transplantation by colonoscopy on day 1 and 60 ml rectal enemas on days 2 and 14 with dietary pre‐conditioning of the donor for 14 days and dietary treatment of the recipient immediately after transplantation and for the following 12 weeks. Comparison I: Fecal microbiota transplantation (FMT) without Diet Undergo standard fecal transplantation by colonoscopy on day 1 and 60 ml rectal enemas on days 2 and 14 from the same donor without dietary conditioning. Comparison II: Dietary therapy only The patient will receive detailed instructions regarding the UC diet to be used over 12 weeks without FMT. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | January 24, 2017 |
| Contact information | Prof. Arie Levine, Director, Pediatric Gastroenterology and Nutrition unit., Wolfson Medical Center |
| Notes | Estimated enrolment: 34 |
NCT02998112.
| Trial name or title | Fecal Microbiota Transplantation for ulcerative colitis Through Colonic Transendoscopic Enteral Tubing |
| Methods | Interventional (Clinical Trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention 5‐ASA 4 g/day enema through TET for 1 week; 200 ml fecal microbiota suspension infusion through TET for 3 times every other day in one week Comparison: Placebo 5‐ASA 4 g/day enema through TET for 1 week; 200 ml saline infusion through TET for 3 times every other day in one week |
| Outcomes |
Primary Outcomes
Secondary Outcomes:
The change of intestinal microbiota composition after FMT compared with subject original microbiota and donor's microbiota |
| Starting date | December 2016 |
| Contact information | Faming Zhang, Associate professor, Gastroenterology, The Second Hospital of Nanjing Medical University |
| Notes | Estimated enrolment: 188 |
NCT03006809.
| Trial name or title | Optimal Fecal Microbiota Transplant Dosing for Mild to Moderate Ulcerative Colitis |
| Methods | Interventional (Clinical Trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: 1 Pretreatment antibiotics + FMT delivered by colonoscopy + FMT capsules per week x 6 weeks Intervention: 2 No antibiotics, FMT delivered by colonoscopy + FMT capsules per week x 6 weeks Intervention: 3 Pretreatment antibiotics + FMT delivered by colonoscopy + FMT delivered by enema once per week x 6 weeks Intervention: 4 No antibiotics, FMT delivered by colonoscopy + FMT delivered by enema once per week x 6 weeks |
| Outcomes |
Primary Outcomes
Secondary Outcomes
|
| Starting date | March 2, 2017 |
| Contact information | Najwa Elnachef, Assistant Clinical Professor, University of California, San Francisco |
| Notes | Estimated enrolment: 40 |
NCT03016780.
| Trial name or title | Fecal Microbiota Transplantation for Ulcerative Colitis (FMTFUC) |
| Methods | Interventional (Clinical Trial), randomized |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Active Comparator: Treatment in part 1 Fecal microbiota transplantation and traditional treatments will be used in patients with ulcerative colitis in part 1 Placebo Comparator: Placebo in part 2 The traditional treatments and normal saline will be used in patients with ulcerative colitis in part 2 according to associated guidelines |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | July 2016 |
| Contact information | Xiaoan Li, Ph.D First Affiliated Hospital of Chengdu Medical College |
| Notes | Estimated enrolment: 60 |
NCT03104036.
| Trial name or title | Faecal Bacteriotherapy for Ulcerative Colitis (FACTU) |
| Methods | Interventional (Clinical Trial), randomized |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention: Faecal bacterial transplantation enema Will be applied enema prepared from 50 g of stool of examined donor dissolved in 150 ml of normal saline, the 1st week 5 times, than one time a week until the end of the 6th week Comparison: Mesalazine enema Will be treated with 4 g Mesalazine enema 1x daily for 2 weeks, then every other day until the end of the 6th week |
| Outcomes |
Primary outcome: Clinical remission [Time Frame: Week 12] Mayo score ≤ 2 with no subscore > 1 Secondary Outcome
|
| Starting date | April 2017 |
| Contact information | Jan Březina, MUDr. (principal investigator), Institute for Clinical and Experimental Medicine |
| Notes | Estimated enrolment: 60 |
UC: ulcerative colitis
FMT: fecal microbiota transplantation
CD: Crohn's disease
HBI: Harvey Bradshaw Index
5‐ASA: 5‐aminosalicylic acid
PUCAI: Pediatric Ulcerative Colitis Activity Index
ECCO: European Crohn's and Colitis Organisation
PCDAI: Pediatric Crohn's Disease Activity Index
HIV: human immunodeficiency virus
CRP: C‐reactive protein
ESR: erythrocyte sedimentation rate
IBD: Inflammatory Bowel Disease
PCR: Polymerase chain reaction
IBD‐U: Inflammatory Bowel Disease Unclassified
SF‐36: 36‐item Short Form Health Survey (quality of life)
CMV: cytomegalovirus
PSC: primary sclerosing cholangitis
UTI: urinary tract infection
BCC: basal cell carcinoma
TET: transendoscopic enteral tubing
ANC: absolute neutrophil count
GFR: glomerular filtration rate
NSAIDs: Non‐steroidal anti‐inflammatory drugs
AIDS: acquired immune deficiency syndrome
Differences between protocol and review
1) We decided to exclude single‐arm phase I trials because of the lack of a control arm and the inability to make a causal association from these studies.
2) A post‐hoc analysis was conducted to include data for primary outcome of clinical remission based on clinical score and endoscopic assessment.
Contributions of authors
AI and SA conceptualised the idea of the study. AI, MN, and SA wrote the manuscript. ETS assisted with formulating the methods for the protocol. SA, OG, JZ, DMBB and ETS helped with revisions and provided overall supervision for completion of the manuscript.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Aamer Imdad: None known
Maribeth R Nicholson: None known
Sari Acra: None known
Oscar Gomez‐Duarte: receives funding from the NIH for research on enteric pathogens. In addition to his employment at the University at Buffalo, he offers lectures at the University of Santander for which he receive honoraria. He is a member of the Revista Colombiana de Anestesiologia Editorial board which compensates him for time as an editor.
Emily E Tanner‐Smith: None known
Joseph P Zackular: None known
Dawn M Borromeo Beaulieu: a consultant for Abbvie, Takeda and Janssen
New
References
References to studies included in this review
Costello 2017a {published data only}
- Costello S, Waters O, Bryant R, Katsikeros R, Makanyanga J, Schoeman M, et al. Short duration, low intensity pooled faecal microbiota transplantation induces remission in patients with mild moderately active ulcerative colitis: a randomised controlled trial. Journal of Crohn's and Colitis 2017;11(Suppl 1):S23. [Google Scholar]
Moayyedi 2015 {published and unpublished data}
- Moayyedi P, Surette M, Wolfe M, Taraschi R, Kim P, Libertucci J, et al. A randomized, placebo controlled trial of fecal microbiota therapy in active ulcerative colitis. Gastroenterology 2014;146(5):S‐159. [Google Scholar]
- Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015;149(1):102‐9. [DOI] [PubMed] [Google Scholar]
Paramsothy 2017a {published data only}
- Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo‐controlled trial. Lancet 2017;389(10075):1218‐28. [DOI] [PubMed] [Google Scholar]
- Paramsothy S, Kamm MA, Walsh A, Bogaerde J, Samuel D, Leong RW, et al. Multi donor intense faecal microbiota transplantation is an effective treatment for resistant ulcerative colitis: A randomised placebo‐controlled trial. Gastroenterology 2016;150(4):S122‐3. [Google Scholar]
- Paramsothy S, Kamm MA, Walsh AJ, Bogaerde J, Samuel D, Leong RWL, et al. Multi donor intense faecal microbiota transplantation is an effective treatment for resistant ulcerative colitis: a randomised placebo controlled trial and microbiota analysis. Journal of Gastroenterology and Hepatology. 2016; Vol. 31:143.
Rossen 2015 {published data only}
- Fuentes S, Rossen NG, Spek MJ, Hartman JH, Huuskonen L, Korpela K, et al. Microbial shifts and signatures of long‐term remission in ulcerative colitis after faecal microbiota transplantation. ISME Journal 2017;11(8):1877‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen NG, Fuentes S, Spek MJ, Tissen JG, Duflou A, Lowenberg M, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149(1):110‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angelberger 2016 {published data only}
- Allegretti JR, Kassam Z, Smith M, Korzenik JR, Chan W. Irregular bowel movements following fecal microbiota transplantation (FMT) are associated with pre‐existing irritable bowel syndrome but not FMT‐related factors. Gastroenterology 2016;150(4):S742. [Google Scholar]
Borody 2003 {published data only}
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. Journal of Clinical Gastroenterology 2003;37(1):42‐7. [DOI] [PubMed] [Google Scholar]
Chin 2017 {published data only}
- Chin SM, Sauk J, Mahabamunuge J, Kaplan JL, Hohmann EL, Khalili H. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in Patients with Inflammatory Bowel Disease: A Single‐Center Experience. Clinical Gastroenterology & Hepatology 2017;15:597‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 2016 {published data only}
- Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri SM, Blumenkehl M, et al. Fecal Microbiota Transplantation is Safe and Efficacious for Recurrent or Refractory Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2016;22(10):2402‐9. [DOI] [PubMed] [Google Scholar]
Gionchetti 2000 {published data only}
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119(2):305‐9. [DOI] [PubMed] [Google Scholar]
Hourigan 2015 {published data only}
- Hourigan S, Ann Chen L, Grigoryan Z. Microbiome changes associated with sustained eradication of clostridium difficile after fecal microbiota transplantation in children with and without inflammatory bowel disease. Gastroenterology 2015:S45. [DOI] [PubMed] [Google Scholar]
Ishikawa 2017 {published data only}
- Ishikawa D, Sasaki T, Osada T, Kuwahara‐Arai K, Haga K, Shibuya T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflammatory Bowel Diseases 2017;23(1):116‐25. [DOI] [PubMed] [Google Scholar]
Landy 2013 {published data only}
- Landy J, Al‐Hassi HO, Mann ER, Peake ST, McLaughlin SD, Ciclitira PJ, et al. A prospective controlled pilot study of fecal microbiota transplantation for chronic refractory pouchitis. Gastroenterology 2013;144(5):S897. [Google Scholar]
Mandalia 2016 {published data only}
- Mandalia A, Ward A, Tauxe W, Kraft CS, Dhere T. Fecal transplant is as effective and safe in immunocompromised as non‐immunocompromised patients for Clostridium difficile. International Journal of Colorectal Disease 2016;31(5):1059‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mintz 2016 {published data only}
- Mintz M, Monzur F, Chowdhury T, Rowejl L, Grewal S, Li E, et al. Comparing fecal microbial transplant outcomes in patients with recurrent clostridium difficile or ulcerative colitis. Inflammatory Bowel Diseases 2016;22(1):S31. [Google Scholar]
Wei 2016 {published data only}
- Wei Y, Gong J, Zhu W, Tian H, Ding C, Gu L, et al. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiology 2016;16:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01790061 {published data only}
- NCT01790061. Standardized Fecal Microbiota Transplantation for Ulcerative Colitis [Efficacy, Durability and Safety of Standardized Fecal Microbiota Transplantation in Patients With Moderate to Severe Ulcerative Colitis]. clinicaltrials.gov/ct2/show/NCT01790061 (first received 13 February 2013).
NCT01793831 {published data only}
- NCT01793831. Standardized Fecal Microbiota Transplantation for Crohn's Disease [Efficacy and Safety of Standardized Fecal Microbiota Transplantation for Moderate to Severe Crohn's Disease]. clinicaltrials.gov/ct2/show/NCT01793831 (first received 18 February 2013).
NCT01961492 {published data only}
- NCT01961492. Fecal Microbiota Transplantation in Patients with Ulcerative Colitis [Single Fecal Microbiota Transplantation Via Colonoscope as an Adjunct Therapy in the Treatment of Ulcerative Colitis]. clinicaltrials.gov/ct2/show/NCT01961492 (first received 11 October 2013). [Google Scholar]
NCT02272868 {published data only}
- NCT02272868. Fecal Microbial Transplant in Pediatric Crohn's Disease (FMTCD) [Fecal Microbial Transplant in Pediatric Crohn's Disease: A Double Blind Placebo Control Study]. clinicaltrials.gov/ct2/show/NCT02272868 (first received 23 October 2014).
NCT02291523 {published data only}
- NCT02291523. The Effect of Therapeutic Fecal Transplant on the Gut Microbiome in Children with Ulcerative Colitis (FMT_UC) [The Effect of Therapeutic Fecal Transplant on the Gut Microbiome in Children With Ulcerative Colitis]. clinicaltrials.gov/ct2/show/NCT02291523 (first received 14 November 2014).
NCT02335281 {published data only}
- NCT02335281. Standardized Fecal Microbiota Transplantation for Inflammatory Bowel Disease (SFMT‐IBD) [Efficacy, Durability and Safety of Standardized Fecal Microbiota Transplantation for Severe Inflammatory Bowel Disease]. clinicaltrials.gov/ct2/show/NCT02335281 (first received 9 January 2015).
NCT02390726 {published data only}
- NCT02390726. Fecal Microbiota Transplant in the Treatment of Ulcerative Colitis (FMTUC) [Fecal Microbiota Transplant in the Treatment of Ulcerative Colitis]. clinicaltrials.gov/ct2/show/NCT02390726 (first received 17 March 2015).
NCT02487238 {published data only}
- NCT02487238. Pediatric FEcal Microbiota Transplant for Ulcerative Colitis (PediFETCh) [A Single‐Blind, Randomized, Placebo‐Controlled Trial of Human Fecal Microbiota Transplantation for the Therapy of Pediatric Ulcerative Colitis and Inflammatory Bowel Disease Unclassified]. clinicaltrials.gov/ct2/show/NCT02487238 (first received 1 July 2015).
- Pai N, Popov J. Protocol for a randomised, placebo‐controlled pilot study for assessing feasibility and efficacy of faecal microbiota transplantation in a paediatric ulcerative colitis population: PediFETCh trial. BMJ Open 2017;7:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai N, Popov J, Lee C. A randomized, placebo‐controlled trial of fecal microbial transplantation for pediatric ulcerative colitis (pedifetch trial). Journal of Pediatric Gastroenterology and Nutrition 2016;63:S79‐80. [Google Scholar]
NCT02734589 {unpublished data only}
- NCT02734589. Pilot Study of Fecal Transplantation Using a Unique Diet for Donor and Recipient in Mild to Moderate Treatment Refractory Colitis in Inflammatory Bowel Disease [Fecal Transplantation Using a Novel Conditioning Method for Donor and Recipient in Mild to Moderate Treatment Refractory Colitis in Inflammatory Bowel Disease]. clinicaltrials.gov/ct2/show/NCT02734589 (first received 12 April 2016).
NCT02998112 {published data only}
- NCT02998112. Fecal Microbiota Transplantation for Ulcerative Colitis Through Colonic Transendoscopic Enteral Tubing [Fecal Microbiota Transplantation for Ulcerative Colitis Through Colonic Transendoscopic Enteral Tubing]. clinicaltrials.gov/ct2/show/NCT02998112 (first received 20 December 2016).
NCT03006809 {published data only}
- NCT03006809. Optimal Fecal Microbiota Transplant Dosing for Mild to Moderate Ulcerative Colitis [Optimal Fecal Microbiota Transplant Dosing for Mild to Moderate Ulcerative Colitis]. clinicaltrials.gov/ct2/show/NCT03006809 (first received 30 December 2016).
NCT03016780 {unpublished data only}
- NCT03016780. Fecal Microbiota Transplantation for Ulcerative Colitis (FMTFUC) [Evaluation of The Effect of Fecal Microbiota Transplantation on Ulcerative Colitis and Its Mechanism]. clinicaltrials.gov/ct2/show/NCT03016780 (first received 11 January 2017).
NCT03104036 {published data only}
- NCT03104036. Faecal Bacteriotherapy for Ulcerative Colitis (FACTU) [Faecal Bacteriotherapy for Ulcerative Colitis]. clinicaltrials.gov/ct2/show/NCT03104036 (first received 7 April 2017). [Google Scholar]
Additional references
Abraham 2009
- Abraham C, Cho JH. Inflammatory bowel disease. New England Journal of Medicine 2009;361(21):2066‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abraham 2012
- Abraham BP, Mehta S, El‐Serag HB. Natural history of pediatric‐onset inflammatory bowel disease: a systematic review. Journal of Clinical Gastroenterology 2012;46(7):581‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahuja 2010
- Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia‐Pacific area: a comparison with developed countries and regional differences. Journal of Digestive Diseases 2010;11(3):134‐47. [DOI] [PubMed] [Google Scholar]
Alang 2015
- Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infectious Diseases 2015;2(1):ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananthakrishnan 2015
- Ananthakrishnan A. Epidemiology and risk factors for IBD. Nature Reviews. Gastroenterology & Hepatology 2015;12(4):205‐17. [DOI] [PubMed] [Google Scholar]
Armuzzi 2012
- Armuzzi A, Assche G, Reinisch W, Pineton de Chambrun G, Griffiths A, Sladek M, et al. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. Journal of Crohn's & Colitis 2012;6(4):492‐502. [DOI] [PubMed] [Google Scholar]
Assa 2016
- Assa A, Butcher J, Li J, Elkadri A, Sherman PM, Muise AM, et al. Mucosa‐Associated Ileal Microbiota in New‐Onset Pediatric Crohn's Disease. Inflammatory Bowel Diseases 2016;22(7):1533‐9. [DOI] [PubMed] [Google Scholar]
Austin 2014
- Austin M, Mellow M, Tierney WM. Fecal microbiota transplantation in the treatment of Clostridium difficile infections. American Journal of Medicine 2014;127(6):479‐83. [DOI] [PubMed] [Google Scholar]
Auzoux 2016
- Auzoux J, Boschetti G, Lahlou W, Aubourg A, Girault A, Lecomte T, et al. Assessment of Mucosal Healing in Inflammatory Bowel Disease. Journal of Clinical Gastroenterology and Hepatology 2016;1(1):1‐8. [Google Scholar]
Bejaoui 2015
- Bejaoui M, Sokol H, Marteau P. Targeting the Microbiome in Inflammatory Bowel Disease: Critical Evaluation of Current Concepts and Moving to New Horizons. Digestive Diseases 2015;33(Suppl 1):105‐12. [DOI] [PubMed] [Google Scholar]
Blanton 2016
- Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016;351(6275):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cammarota 2015
- Cammarota G, Masucci L, Ianiro G, Bibbo S, Dinoi G, Costamagna G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology & Therapeutics 2015;41(9):835‐43. [DOI] [PubMed] [Google Scholar]
Cammarota 2017
- Cammarota G, Ianiro G, Tilg H, Rajilić‐Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; Vol. 66, issue 4:569‐80. [DOI] [PMC free article] [PubMed]
Carding 2017
- Carding SR, Davis N, Hoyles L. Review article: the human intestinal virome in health and disease. Alimentary Pharmacology & Therapeutics 2017;46(9):800‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chassaing 2011
- Chassaing B, Darfeuille‐Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140(6):1720‐28. [DOI] [PubMed] [Google Scholar]
Cleynen 2016
- Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet 2016; Vol. 387, issue 10014:156‐67. [DOI] [PMC free article] [PubMed]
Colman 2014
- Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta‐analysis. Journal of Crohn's & Colitis 2014;8(12):1569‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costello 2017b
- Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta‐analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Alimentary Pharmacology & Therapeutics 2017;46(3):213‐24. [DOI] [PubMed] [Google Scholar]
Dahlhamer 2016
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥ 18 Years ‐ United States, 2015. MMWR. Morbidity and Mortality Weekly Report 2016;65(42):1166‐9. [DOI] [PubMed] [Google Scholar]
Dave 2012
- Dave M, Loftus EV Jr. Mucosal healing in inflammatory bowel disease‐a true paradigm of success?. Gastroenterology and Hepatology 2012;8(1):29‐38. [PMC free article] [PubMed] [Google Scholar]
De Preter 2012
- Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F, et al. Decreased mucosal sulfide detoxification is related to an impaired butyrate oxidation in ulcerative colitis. Inflammatory Bowel Diseases 2012;18(12):2371‐80. [DOI] [PubMed] [Google Scholar]
Dulai 2015
- Dulai PS, Levesque BG, Feagan BG, D'Haens G, Sandborn WJ. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointestinal Endoscopy 2015;82(2):246‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2006
- Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ 2006;332(7547):969‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2016
- FDA. Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm488223.pdf (accessed 28 June 2017).
Feakins 2013
- Feakins RM, British Society of Gastroenterology. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. Journal of Clinical Pathology 2013;66(12):1005‐26. [DOI] [PubMed] [Google Scholar]
Fuentes 2017
- Fuentes S, Rossen NG, Spek MJ, Hartman JH, Huuskonen L, Korpela K, et al. Microbial shifts and signatures of long‐term remission in ulcerative colitis after faecal microbiota transplantation. ISME Journal 2017;11(8):1877‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2011
- Gupta SK. Intention‐to‐treat concept: A review. Perspectives in Clinical Research 2011;2(3):109‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;20(5):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassam 2013
- Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta‐analysis. American Journal of Gastroenterology 2013;108(4):500‐8. [DOI] [PubMed] [Google Scholar]
Kelly 2014
- Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. American Journal of Gastroenterology 2014;109(7):1065‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2015
- Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015;149(1):223‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2016
- Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Annals of Internal Medicine 2016; Vol. 165, issue 9:609‐16. [DOI] [PMC free article] [PubMed]
Khoruts 2016
- Khoruts A, Rank KM, Newman KM, Viskocil K, Vaughn BP, Hamilton MJ, et al. Inflammatory Bowel Disease Affects the Outcome of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clinical Gastroenterology & Hepatology 2016;14(10):1433‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kostic 2014
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146(6):1489‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunde 2013
- Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. Journal of Pediatric Gastroenterology & Nutrition 2013;56(6):597‐601. [DOI] [PubMed] [Google Scholar]
Lee 2016
Leffler 2015
- Leffler DA, Lamont JT. Clostridium difficile infection. New England Journal of Medicine 2015;372(16):1539‐48. [DOI] [PubMed] [Google Scholar]
Link 2016
- Link A, Lachmund T, Schulz C, Weigt J, Malfertheiner P. Endoscopic peroral jejunal fecal microbiota transplantation. Digestive & Liver Disease 2016;48(11):1336‐9. [DOI] [PubMed] [Google Scholar]
Mallon 2007
- Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005573.pub2] [DOI] [PubMed] [Google Scholar]
Mehta 2016
- Mehta F. Report: economic implications of inflammatory bowel disease and its management. American Journal of Managed Care 2016;22(3 Suppl):s51‐60. [PubMed] [Google Scholar]
Molodecky 2012
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46‐54. [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore T, Rodriguez A, Bakken JS. Fecal microbiota transplantation: a practical update for the infectious disease specialist. Clinical Infectious Diseases 2014;58(4):541‐5. [DOI] [PubMed] [Google Scholar]
Morgan 2012
- Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology 2012;13(9):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mowat 2011
Nagao‐Kitamoto 2016
- Nagao‐Kitamoto H, Shreiner A B, Gillilland MG 3rd, Kitamoto S, Ishii C, Hirayama A, et al. Functional Characterization of Inflammatory Bowel Disease‐Associated Gut Dysbiosis in Gnotobiotic Mice. Cellular and Molecular Gastroenterology and Hepatology 2016;2(4):468‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naidoo 2011
- Naidoo K, Gordon M, Fagbemi AO, Thomas AG, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007443.pub2] [DOI] [PubMed] [Google Scholar]
Osman 2016
- Osman M, Stoltzner Z, O'Brien K, Ling K, Koelsch E, Dubois N, et al. Donor Efficacy in Fecal Microbiota Transplantation for Recurrent Clostridium difficile: Evidence From a 1,999‐Patient Cohort. Open Forum Infectious Diseases 2016;3(Suppl 1):841. [Google Scholar]
Owens 2013
- Owens C, Broussard E, Surawicz C. Fecal microbiota transplantation and donor standardization. Trends in Microbiology 2013;21(9):443‐5. [DOI] [PubMed] [Google Scholar]
Paramsothy 2017b
- Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta‐analysis. Journal of Crohn's & Colitis 2017;11(10):1180‐99. [DOI] [PubMed] [Google Scholar]
Petersen 2014
- Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cellular Microbiology 2014;16(7):1024‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peyrin‐Biroulet 2012
- Peyrin‐Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. Journal of Crohn's & Colitis 2011 Oct;5(5):477‐83. [DOI] [PubMed] [Google Scholar]
Rapozo 2017
- Rapozo DC, Bernardazzi C, Souza HS. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World Journal of Gastroenterology 2017;23(12):2124‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ridaura 2013
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341(6150):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulberg 2016
- Schulberg J, Cruz P. Characterisation and therapeutic manipulation of the gut microbiome in inflammatory bowel disease. Internal Medicine Journal 2016;46(3):266‐73. [DOI] [PubMed] [Google Scholar]
Shi 2016
- Shi Y, Dong Y, Huang W, Zhu D, Mao H, Su P. Fecal Microbiota Transplantation for Ulcerative Colitis: A Systematic Review and Meta‐Analysis. PLoS One 2016;11(6):e0157259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2015
- Singh S, Stroud AM, Holubar SD, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch‐anal anastomosis for chronic ulcerative colitis. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD001176.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solari 2014
- Solari PR, Fairchild PG, Noa LJ, Wallace MR. Tempered enthusiasm for fecal transplant. Clinical Infectious Diseases 2014;59(2):319. [DOI] [PubMed] [Google Scholar]
Sun 2016
- Sun D, Li W, Li S, Cen Y, Xu Q, Li Y, et al. Fecal Microbiota Transplantation as a Novel Therapy for Ulcerative Colitis: A Systematic Review and Meta‐Analysis. Medicine 2016;95(23):e3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Nood 2013
Vindigni 2016
- Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therapeutic Advances in Gastroenterology 2016;9(4):606‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weintraub 2014
- Weintraub Y, Mimouni FB, Cohen S. Temporal trends in inflammatory bowel disease publications over a 19‐years period. World Journal of Gastroenterology 2014;20(44):16745‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2017
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 7 June 2017).
Witherden 2017
- Witherden EA, Shoaie S, Hall RA, Moyes DL. The Human Mucosal Mycobiome and Fungal Community Interactions. Journal of Fungi 2017;3(4):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Youngster 2014
- Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open‐label, controlled pilot study. Clinical Infectious Diseases 2014; Vol. 58, issue 11:1515‐22. [DOI] [PMC free article] [PubMed]


