Abstract
Background
Percutaneous vertebroplasty remains widely used to treat osteoporotic vertebral fractures although our 2015 Cochrane review did not support its role in routine practice.
Objectives
To update the available evidence of the benefits and harms of vertebroplasty for treatment of osteoporotic vertebral fractures.
Search methods
We updated the search of CENTRAL, MEDLINE and Embase and trial registries to 15 November 2017.
Selection criteria
We included randomised and quasi‐randomised controlled trials (RCTs) of adults with painful osteoporotic vertebral fractures, comparing vertebroplasty with placebo (sham), usual care, or another intervention. As it is least prone to bias, vertebroplasty compared with placebo was the primary comparison. Major outcomes were mean overall pain, disability, disease‐specific and overall health‐related quality of life, patient‐reported treatment success, new symptomatic vertebral fractures and number of other serious adverse events.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane.
Main results
Twenty‐one trials were included: five compared vertebroplasty with placebo (541 randomised participants), eight with usual care (1136 randomised participants), seven with kyphoplasty (968 randomised participants) and one compared vertebroplasty with facet joint glucocorticoid injection (217 randomised participants). Trial size varied from 46 to 404 participants, most participants were female, mean age ranged between 62.6 and 81 years, and mean symptom duration varied from a week to more than six months.
Four placebo‐controlled trials were at low risk of bias and one was possibly susceptible to performance and detection bias. Other trials were at risk of bias for several criteria, most notably due to lack of participant and personnel blinding.
Compared with placebo, high‐ to moderate‐quality evidence from five trials indicates that vertebroplasty provides no clinically important benefits with respect to pain, disability, disease‐specific or overall quality of life or treatment success at one month. Evidence for quality of life and treatment success was downgraded due to possible imprecision. Evidence was not downgraded for potential publication bias as only one placebo‐controlled trial remains unreported. Mean pain (on a scale zero to 10, higher scores indicate more pain) was five points with placebo and 0.7 points better (0.3 better to 1.2 better) with vertebroplasty, an absolute pain reduction of 7% (3% better to 12% better, minimal clinical important difference is 15%) and relative reduction of 10% (4% better to 17% better) (five trials, 535 participants). Mean disability measured by the Roland‐Morris Disability Questionnaire (scale range zero to 23, higher scores indicate worse disability) was 14.2 points in the placebo group and 1.5 points better (0.4 better to 2.6 better) in the vertebroplasty group, absolute improvement 7% (2% to 11% better), relative improvement 9% better (2% to 15% better) (four trials, 472 participants).
Disease‐specific quality of life measured by the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) (scale zero to 100, higher scores indicating worse quality of life) was 62 points in the placebo group and 2.3 points better (1.4 points worse to 6.7 points better), an absolute imrovement of 2% (1% worse to 6% better); relative improvement 4% better (2% worse to 10% better) (three trials, 351 participants). Overall quality of life (European Quality of Life (EQ5D), zero = death to 1 = perfect health, higher scores indicate greater quality of life) was 0.38 points in the placebo group and 0.05 points better (0.01 better to 0.09 better) in the vertebroplasty group, absolute improvement: 5% (1% to 9% better), relative improvement: 18% (4% to 32% better) (three trials, 285 participants). In one trial (78 participants), 9/40 (or 225 per 1000) people perceived that treatment was successful in the placebo group compared with 12/38 (or 315 per 1000; 95% CI 150 to 664) in the vertebroplasty group, RR 1.40 (95% CI 0.67 to 2.95), absolute difference: 9% more reported success (11% fewer to 29% more); relative change: 40% more reported success (33% fewer to 195% more).
Low‐quality evidence (downgraded due to imprecision and potential for bias from the usual‐care controlled trials) indicates uncertainty around the risk estimates of harms with vertebroplasty. The incidence of new symptomatic vertebral fractures (from six trials) was 48/418 (95 per 1000; range 34 to 264)) in the vertebroplasty group compared with 31/422 (73 per 1000) in the control group; RR 1.29 (95% CI 0.46 to 3.62)). The incidence of other serious adverse events (five trials) was 16/408 (34 per 1000, range 18 to 62) in the vertebroplasty group compared with 23/413 (56 per 1000) in the control group; RR 0.61 (95% CI 0.33 to 1.10). Notably, serious adverse events reported with vertebroplasty included osteomyelitis, cord compression, thecal sac injury and respiratory failure.
Our subgroup analyses indicate that the effects did not differ according to duration of pain (acute versus subacute). Including data from the eight trials that compared vertebroplasty with usual care in a sensitivity analyses altered the primary results, with all combined analyses displaying considerable heterogeneity.
Authors' conclusions
We found high‐ to moderate‐quality evidence that vertebroplasty has no important benefit in terms of pain, disability, quality of life or treatment success in the treatment of acute or subacute osteoporotic vertebral fractures in routine practice when compared with a sham procedure. Results were consistent across the studies irrespective of the average duration of pain.
Sensitivity analyses confirmed that open trials comparing vertebroplasty with usual care are likely to have overestimated any benefit of vertebroplasty. Correcting for these biases would likely drive any benefits observed with vertebroplasty towards the null, in keeping with findings from the placebo‐controlled trials.
Numerous serious adverse events have been observed following vertebroplasty. However due to the small number of events, we cannot be certain about whether or not vertebroplasty results in a clinically important increased risk of new symptomatic vertebral fractures and/or other serious adverse events. Patients should be informed about both the high‐ to moderate‐quality evidence that shows no important benefit of vertebroplasty and its potential for harm.
Plain language summary
Vertebroplasty for treating spinal fractures due to osteoporosis
Background
Osteoporosis is characterised by thin, fragile bones and may result in minimal trauma fractures of the spine bones (vertebrae). They can cause severe pain and disability.
Vertebroplasty involves injecting medical‐grade cement into a fractured vertebra, under light sedation or general anaesthesia. The cement hardens in the bone space to form an internal cast.
Study characteristics
This Cochrane review is current to November 2017. Studies compared vertebroplasty versus placebo (no cement injected) (five studies, 541 participants); usual care (eight studies, 1136 participants); kyphoplasty (similar, but before cement is injected a balloon is expanded in the fractured vertebra; seven studies, 968 participants) and facet joint steroid injection (one study, 217 participants). Trials were performed in hospitals in 15 countries, the majority of participants were female, mean age ranged between 63.3 and 80 years, and symptom duration ranged from a week to six months or more. Eight trials received at least some funding from medical device manufacturers and only two reported that they had no role in the trial.
Key results
Compared with a placebo (fake) procedure, vertebroplasty resulted in little benefit at one month:
Pain (lower scores mean less pain)
Improved by 7% (3% better to 12% better), or 0.7 points (0.3 better to 1.2 better) on a zero‐0 to 10‐point scale.
• People who had vertebroplasty rated their pain as 4.3 points.
• People who had placebo rated their pain as 5 points.
Disability (lower scores mean less disability)
Improved by 7% (2% better to 11% better), or 1.5 points (0.4 better to 2.6 better) on a zero to 23‐point scale.
• People who had vertebroplasty rated their disability as 12.7 points.
• People who had placebo rated their disability as 14.2 points.
Vertebral fracture or osteoporosis‐specific quality of life (lower scores mean better quality of life)
Better by 2% (1% worse to 6% better), or 2.33 points better (1.41 worse to 6.06 better) on a zero to 100‐point scale.
• People who had vertebroplasty rated their quality of life related to their fracture as 59.7 points.
• People who had placebo rated their quality of life related to their fracture as 62 points.
Overall quality of life (higher scores mean better quality of life)
Improved by 5% (1% better to 9% better), or 0.05 units (0.01 better to 0.09 better) on a zero = death to one = perfect health scale.
• People who had vertebroplasty rated their general quality of life as 0.43 points.
• People who had placebo rated their general quality of life as 0.38 points.
Treatment success (defined as pain moderately or a great deal better)
9% more people rated their treatment a success (11% fewer to 29% more), or nine more people out of 100.
• 32 out of 100 people reported treatment success with vertebroplasty.
• 23 out of 100 people reported treatment success with placebo.
Compared with a placebo or usual care:
New symptomatic vertebral fractures (at 12 to 24 months)
3% more new fractures with vertebroplasty (8% fewer to 13% more), or one more person out of 100.
• 10 out of 100 people had a new fracture with vertebroplasty.
• 7 out of 100 people had a new fracture with placebo or usual care.
Other serious adverse events:
1% fewer people (6% fewer to 4% more), had other serious adverse events with vertebroplasty; relative change 39% fewer (67% fewer to 10% more).
• 4 out of 100 people reported other serious adverse events with vertebroplasty.
• 5 out of 100 people reported other serious adverse events with placebo.
Quality of the evidence
High‐quality evidence shows that vertebroplasty does not provide more clinically important benefits than placebo. We are less certain of the risk of new vertebral fractures or other serious effects; quality was moderate due to the small number of events.
Serious adverse events that may occur include spinal cord or nerve root compression due to cement leaking out from the bone, cement leaking into the blood stream, rib fractures, infection in the bone, fat leaking into the bloodstream, damage to the covering of the spinal cord that could result in leakage of cerebrospinal fluid, anaesthetic complications and death.
Summary of findings
Summary of findings for the main comparison. Vertebroplasty for osteoporotic vertebral compression fracture.
| Vertebroplasty for osteoporotic vertebral compression fracture | ||||||
| Patient or population: people with osteoporotic vertebral compression fracture Settings: hospital, various countries including Australia, USA, UK, Canada, several European countries, Iran, China, Taiwan Intervention: vertebroplasty versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo1 | Vertebroplasty | |||||
| Pain Scale from: 0 to 10, 0 is no pain. Follow‐up: 1 month | The mean pain in the control groups was 5 points | The mean pain in the intervention groups was 0.7 points better (0.3 better to 1.2 better) | 535 (5 studies) | ⊕⊕⊕⊕ high2 | Absolute change 7% better (3% better to 12% better); relative change 10% better (4% better to 17% better)3 | |
| Disability (Roland‐Morris Disability Questionnaire) Scale from: 0 to 23; 0 is no disability. Follow‐up: 1 month | The mean disability in the control groups was 14.2 points | The mean disability in the intervention groups was 1.5 points better (0.4 better to 2.6 better) | 472 (4 studies) | ⊕⊕⊕⊕ high2 | Absolute change 7% better (2% better to 11% better); relative change 9% better (2% better to 15% better)3 | |
| Disease‐specific quality of Life (QUALEFFO) Scale from: 0 to 100; 0 is best. Follow‐up: 1 month | The mean quality of life (QUALEFFO) in the control groups was 62 points | The mean quality of life in the intervention groups was 2.33 points better (1.41 worse to 6.06 better) | 351 (3 studies) | ⊕⊕⊕⊕ high2 | Absolute change 2% better (1% worse to 6% better); relative change 4% better (2% worse to 10% better)3 | |
| Overall quality of Life (EQ5D) Scale from: 0 to 1; 1 is best. Follow‐up: 1 month | The mean quality of life (EQ‐5D) in the control groups was 0.38 points | The mean quality of life in the intervention groups was 0.05 points better (0.01 better to 0.09 better) | 285 (3 studies) | ⊕⊕⊕⊝ moderate4 | Absolute change 5% better (1% better to 9% better); relative change 18% improvement (4% better to 32% better)3 | |
|
Participant global assessment of success (People perceived their pain as better) Follow‐up: 1 month |
225 per 1000 | 315 per 1000 (150 to 664) | RR 1.40 (0.67 to 2.95) | 78 (1 study) | ⊕⊕⊕⊝ moderate4 | Absolute difference 9% more reported success (11% fewer to 29% more); relative change 40% more reported success (33% fewer to 195% more) |
|
Incident symptomatic vertebral fractures Follow‐up: 12 ‐24 months |
73 per 1000 |
95 per 1000 (34 to 264) |
RR 1.29 (0.46 to 3.62) | 840 (6 studies)5 | ⊕⊕⊝⊝ low4,6 | Absolute difference 3% more fractures with vertebroplasty (8% fewer to 13% more); relative difference 29% more (54% fewer to 262% more) |
| Other serious adverse events Follow‐up: 12‐24 months | 56 per 1000 | 34 per 1000 (18 to 62) | RR 0.61 (0.33 to 1.10) | 821 (5 studies)5 | ⊕⊕⊝⊝ low4,6 | Absolute difference 1% fewer events with vertebroplasty (6% fewer to 4% more); relative change 39% fewer (67% fewer to 10% more) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: Standardised mean difference; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 For incident vertebral fractures the comparison includes two placebo (sham)‐controlled trials and three trials that compared vertebroplasty versus usual care.
2 The internal validity of the five placebo‐controlled trials that have full or some results available is high. Four trials have published their results in peer‐reviewed journals (Buchbinder 2009; Clark 2016; Firanescu 2018; Kallmes 2009) while a fifth trial (VOPE 2015), completed in April 2014, was published as a thesis (http://www.forskningsdatabasen.dk/en/catalog/2371744560), and reported at a conference. Therefore we did not downgrade the evidence due to suspected publication bias although the results of one additional placebo‐controlled trial (VERTOS V) remain unpublished. This trial was previously reported as completed in June 2015, but its status has been changed to 'enrolling by invitation', at: https://clinicaltrials.gov/ct2/show/NCT01963039. While publication bias is possible, it is unlikely the conclusions will change when data from this trial become available.
3 Relative changes calculated as absolute change (mean difference) divided by mean at baseline in the placebo group from Buchbinder 2009 (values were: 7.1 points on 0 to 10 point VAS pain; 17.3 points on 0 to 23 point Roland‐Morris Disability questionnaire; 0.28 points on EQ‐5D quality of life scale; 59.6 points on the QUALEFFO scale).
4 Downgraded due to imprecision: the 95% confidence intervals do not exclude a clinically important change (defined as 1.5 points on the 0 to 10 pain scale; 2 to 3 points on the 0 to 23 point RMDQ scale; 0.074 on the 0 to 1 EQ‐5D quality of life scale, and 10 points on the 0 to 100 QUALEFFO scale); or for dichotomous outcomes the total number of participants was small, or number of events was small (<200); or data were from a single trial only
5 Pooled both placebo and usual care comparisons in the safety analyses.
6 Downgraded due to the possibility of detection bias in the studies with a usual care control group.
Background
Description of the condition
Vertebral compression fractures are among the most common type of fracture in patients with osteoporosis (Ström 2011). The estimated incidence of osteoporotic vertebral compression fractures in individuals 50 years or older has been reported to be 307 per 100,000 year based upon a German study, with the rate in women aged between 85 and 89 years almost eight‐fold higher that in women aged 60 to 64 years (Hernlund 2013). The same study estimated that the direct costs associated with a new osteoporotic vertebral fracture in the first year after its occurrence is about 6490 Euros, signifying that these fractures are costly. A Swedish study estimated that the lifetime risk for a symptomatic osteoporotic vertebral fracture for a person of the age of 45 is 15% for a woman and 8% for a man (Kanis 2000). In the USA, approximately 750,000 new osteoporotic vertebral fractures occur each year (Melton 1997).
A recent population‐based study examining trends in fracture incidence over time in Olmstead County, Minnesota, observed a dramatic increase in the incidence of vertebral fractures, from 1989 to 1991 to 2009 to 2011, and this was associated with an apparent earlier onset of vertebral fracture over time (Amin 2014). The vast majority of these (83.4%) were considered osteoporotic, defined in the study as resulting from no more than moderate trauma (by convention, equivalent to a fall from standing height or less). While some of the observed increase could have been partially attributable to incidentally diagnosed vertebral fractures, the findings are in keeping with a Dutch study that observed a rise in the number of emergency department visits due to osteoporotic vertebral fractures between 1986 and 2008, due to an increase in falls among the most elderly (Oudshoorn 2012). The results are also consistent with a Canadian study that observed a decline in the rate of all low‐trauma osteoporotic fractures over 20 years from 1986 to 2006 in the Province of Manitoba, except for vertebral fractures, which did not decline significantly in either sex (Leslie 2011).
Osteoporotic vertebral compression fractures are a common cause of both acute and chronic back pain in older populations, although only about a third of radiographic osteoporotic vertebral compression deformities present with acute pain. Both symptomatic and asymptomatic osteoporotic vertebral fractures can lead to substantial spinal deformity, functional limitation, pulmonary compromise and lowered quality of life. They are associated with an increased risk of further vertebral fractures and increased mortality (Lau 2008).
Management options for treating acutely painful osteoporotic vertebral fractures are limited and include provision of adequate analgesia, bed rest and physical therapy, as well as assessment and appropriate management of osteoporosis and risk factors for further fractures such as falls prevention. While most fractures generally heal within a few months, some people have persistent pain and disability, and require hospitalisation, long‐term care, or both (Kanis 1999).
Description of the intervention
Percutaneous vertebroplasty was first described in 1987 as a treatment for vertebral angioma (Galibert 1987), and subsequently it has been used to treat both benign and malignant vertebral fractures. The procedure may be performed under intravenous sedation or general anaesthesia by interventional radiologists, neurosurgeons or orthopaedic surgeons. Under imaging guidance, most often fluoroscopy, a large calibre needle is inserted into the affected vertebral body usually via a transpedicular route and bone cement, usually polymethylmethacrylate (PMMA), is injected (Hide 2004).
Early open‐label, and uncontrolled studies consistently reported dramatic immediate improvements in pain, and adverse events were reportedly uncommon (Hochmuth 2006). Despite the absence of evidence from high‐quality randomised controlled trials (RCTs) confirming its benefits, over the last 20 years the procedure has become incorporated into standard care in many parts of the world, most commonly reserved for patients who fail a period of conservative therapy. In the USA, dramatic increases in the use of vertebroplasty have been observed over the last two decades (Gray 2007a; Lad 2009; Leake 2011).
Documented adverse events occurring either during or after the procedure have included cord compression due to extension of the cement outside of the vertebral body, cement pulmonary embolism, infection, rib fractures and new adjacent or non‐adjacent vertebral fractures, osteolysis in the bone surrounding the injected material and death (Leake 2011).
How the intervention might work
The mechanism by which percutaneous vertebroplasty is purported to reduce pain is not known. At least three possible mechanisms have been proposed: (1) mechanical stabilisation of the fractured bone; (2) thermal destruction of nerve endings due to the high temperature reached during polymerisation of the injected cement; and (3) chemical destruction of the nerve endings due to the chemical composition of the cement (Belkoff 2001). The semisolid mixture of PMMA has been shown to restore strength and stiffness of vertebral bodies in post‐mortem studies (Belkoff 2001).
Why it is important to do this review
Percutaneous vertebroplasty has been widely adopted into clinical practice to treat painful osteoporotic vertebral compression fractures prior to supporting evidence of its efficacy and safety from high‐quality RCTs. The first RCT of vertebroplasty compared with usual care was published in 2007 (Voormolen 2007), while the first two placebo‐controlled trials were published in 2009 (Buchbinder 2009; Kallmes 2009).
Numerous systematic reviews and/or meta‐analyses of vertebroplasty for osteoporotic spinal fractures have been published (Anderson 2013; Bliemel 2012; Eck 2008; Gill 2007; Han 2011; Hochmuth 2006; Hulme 2006; Lee 2009; Liu 2013; Ma 2012; McGirt 2009; Papanastassiou 2012; Ploeg 2006; Robinson 2012; Shi 2012; Stevenson 2014; Taylor 2006; Trout 2006; Wang 2012; Xing 2013; Zhang 2013; Zou 2012). These have varied in inclusion criteria and methodologic rigour, and have reported conflicting results.
In 2015, we published a Cochrane systematic review that synthesised the best available evidence for the efficacy and safety of this procedure including 11 RCTs and one quasi‐RCT (1320 participants) (Buchbinder 2015). Based upon moderate‐quality evidence, our review did not support a role for vertebroplasty for treating osteoporotic vertebral fractures in routine practice. Since then the results of further trials have been published and/or presented including two additional placebo‐controlled trials. It is important to determine whether or not incorporation of the findings of the additional trials in an updated review alters our previous conclusions.
Objectives
To assess the benefits and harms of percutaneous vertebroplasty for treating patients with osteoporotic vertebral compression fractures.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any design (e.g. parallel, cross‐over, factorial) and controlled clinical trials using a quasi‐randomised method of allocation, such as by alternation or date of birth. Reports of trials were eligible regardless of the language or date of publication. Only trials published as full articles or available as a full trial report were considered for inclusion.
Types of participants
We included trials that enrolled adults with a diagnosis of osteoporotic vertebral compression fracture/s of any duration. The diagnosis of osteoporosis could have been based upon bone mineral densitometry or explicit clinical diagnostic criteria as defined by the studies. Trials enrolling participants with vertebral fractures due to another cause such as major trauma and malignancy were excluded.
Types of interventions
We included trials that evaluated percutaneous vertebroplasty, defined as percutaneous injection of bone cement (usually polymethylmethacrylate (PMMA)) or similar substances into a vertebral body under imaging guidance.
Comparators could be any of the following.
Placebo or sham procedure
Usual care (best supportive care)
Balloon kyphoplasty (similar to a percutaneous vertebroplasty, but prior to injection of bone cement a balloon is inserted into the vertebral body and expanded), or other similar procedures
Pharmacologic treatment (e.g. calcitonin, bisphosphonates, complementary medicine)
Non‐pharmacologic interventions (e.g. bracing, physical therapy or surgery)
Types of outcome measures
Major outcomes
The following outcomes were selected as the most important.
Mean overall pain measured on a visual analogue scale (VAS) or numerical rating scale (NRS)
Disability measured by the Roland‐Morris Disability Questionnaire (RMDQ) or other back‐specific disability measure
Vertebral fracture and/or osteoporosis‐specific health‐related quality of life, e.g. the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO)
Overall health‐related quality of life, e.g. European Quality of Life with 5 Dimensions (EQ‐5D) or Assessment of Quality of Life (AQoL) questionnaire
Treatment success measured by a participant‐reported global impression of clinical change (much or very much improved), or similar measure
New (incident) symptomatic vertebral fractures (the denominator was the number of participants, but the numerator could include more than one new fracture per participant)
Number of serious other adverse events judged to be due to the procedure (e.g. infection, clinical complications arising from cement leakage)
Minor outcomes
Proportion of participants with pain improved by a clinically relevant amount, for example improvement of at least 2.5 units or 30% on a zero or one to 10 scale
New (incident) radiographic vertebral fractures (the denominator was the number of participants, but the numerator could include more than one new fracture per participant)
Other adverse events
Timing of outcome assessment
We extracted outcome measures that assessed benefits of treatment (e.g. pain or function) at the following time points:
one to two weeks;
one month;
two to three months;
six months;
12 months;
24 months.
If data were available in a trial at multiple time points within each of the above periods (e.g. at one and two weeks), we only extracted data at the latest possible time point of each period. We extracted new vertebral fractures at 12 and 24 months, where available. We extracted other adverse events at all time points.
We collated the main results of the review into a 'Summary of findings' table which provides key information concerning the quality of evidence and the magnitude and precision of the effect of the interventions. We included the major outcomes (see above) in the 'Summary of findings' table at one month for outcomes assessing potential benefits of treatment (pain, disability, vertebral fracture and/or osteoporosis and overall quality of life and treatment success), 12 months for new symptomatic vertebral fractures (the most data available), and one month for serious other adverse events (judged to be related to the procedure).
Search methods for identification of studies
Electronic searches
We searched the following databases on 15 November 2017; updated from the search in the earlier version of this review, which was conducted on 12 November 2014.
The Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochane Library to Issue 11 of 12, 2017)
MEDLINE (via Ovid from January 2014 to 15 November 2017)
Embase (via Ovid from January 2014 to 15 November 2017)
The search strategies are shown in Appendix 1; Appendix 2; Appendix 3).
We also searched for ongoing trials and protocols of published trials in the clinical trials register that is maintained by the US National Institute of Health (http://clinicaltrials.gov) and the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organization (http://www.who.int/ictrp/en/) on 18 November 2017 (see Appendix 4).
No language restrictions were applied.
Searching other resources
We also reviewed the reference lists of the included trials and any relevant review articles retrieved from the electronic searches, to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
Two review authors (SD and RO (for previous version of the review) or RB and RJ or KR and RJ) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups:
possibly relevant ‐ studies that met the inclusion criteria and studies from which it was not possible to determine whether they met the criteria either from their title or abstract;
excluded ‐ those clearly not meeting the inclusion criteria.
If a title or abstract suggested that the study was eligible for inclusion, or we could not tell, we obtained a full‐text version of the article and two review authors independently assessed it to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a third author.
Data extraction and management
At least two review authors in various combinations (JH, AJ, RJ, KR) independently extracted data using a standard data extraction form developed for this review. The authors resolved any discrepancies through discussion or adjudication by a third author (RB). For each included study we recorded the following:
trial characteristics, including type (e.g. parallel or cross‐over), country, source of funding, and trial registration status (with registration number recorded if available);
participant characteristics, including age, sex, duration of symptoms, and inclusion/exclusion criteria;
intervention characteristics for each treatment group, and use of co‐interventions;
outcomes reported, including the measurement instrument used and timing of outcome assessment.
Five review authors (JH, AJ, RB, RJ and KR) in various combinations independently extracted all outcome data.
For a particular systematic review outcome there may be a multiplicity of results available in the trial reports (e.g. multiple scales, time points and analyses). To prevent selective inclusion of data based on the results, we used the following a priori defined decision rules to select data from trials.
Where trialists reported both final values and change from baseline values for the same outcome, we extracted final values.
Where trialists reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values.
Where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
For cross‐over RCTs, we preferentially extracted data from the first period only.
Where trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of combining data for the primary analysis of overall pain, we planned to combine overall pain with other types of pain in the following hierarchy: unspecified pain; pain with activity; or daytime pain. For trials that included more than one back‐specific measure of disability we preferentially extracted data for the RMDQ if reported.
Review authors who were also authors of relevant trials had no involvement in determining whether or not their trials met the inclusion criteria for this review, and also had no involvement in data extraction or 'Risk of bias' assessment of their own trials.
Assessment of risk of bias in included studies
At least two review authors (JH, AJ, RJ, KR) in various combinations independently assessed the risk of selection, performance, detection, attrition and reporting biases for all included RCTs by evaluating the following domains: random sequence generation, allocation concealment, blinding of participants, care provider, and outcome assessor for each outcome measure, incomplete outcome data and other biases, conforming to the methods recommended by Cochrane (Higgins 2011).
Each criterion was rated as low risk of bias, high risk of bias or unclear risk (either lack of information or uncertainty over the potential for bias). Disagreements among the review authors were discussed and resolved.
Measures of treatment effect
We used Cochrane's statistical software, Review Manager 5.3, to perform data analysis. We expressed dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous outcomes as mean differences (MDs) with 95% CIs if different trials used the same measurement instrument to measure the same outcome. Alternatively, we analysed continuous outcomes using the standardised mean difference (SMD) when trials measured the same outcome but employed different measurement instruments to measure the same conceptual outcome (e.g. disability).
To enhance interpretability of continuous outcomes, pooled SMDs of disability were back‐transformed to an original zero to 23 scale RMDQ, by multiplying the SMD and 95% CIs by a representative pooled standard deviation (SD) at baseline of the trial with the highest weight in the meta‐analysis and the least susceptibility to bias (Schünemann 2011b). We assumed VAS and NRS were comparable scales, and combined them in a meta‐analysis using the MD statistic (Bijur 2003; Birnbaumer 2003).
For studies comparing vertebroplasty with placebo, we used SD 2.9 for RMDQ (Buchbinder 2009).
For analyses comparing vertebroplasty with usual care that included Farrokhi 2011,
we used SMD for pain as (Farrokhi 2011 used a one to 10 pain scale in comparison to all other trials that used a zero to 10 pain scale), but back‐transformed the SMD to MD on a zero to 10 scale by multiplying the SMD and 95% CIs by the SD of pain at baseline from the control group of the Klazen 2010 trial (SD 1.6). For disability (vertebroplasty versus usual care), which included data for the RMDQ and Oswestry Disability Index (ODI), we back‐transformed the SMD to MD on the RMDQ (zero to 23 scale) by multiplying the SMD and 95% CIs by the SD of disability at baseline also from the control group of the Klazen 2010 trial (SD 4.2).
For analyses comparing vertebroplasty with kyphoplasty that included Evans 2015, we used SMD for disability (as Evans 2015 used the RMDQ compared to all other trials that used ODI), but back‐transformed the SMD to MD on the RMDQ (zero to 23 scale) by multiplying the SMD and 95% CIs by the standard deviation (SD) of pain at baseline from the control group of the Evans 2015 trial (SD 6.6).
Unit of analysis issues
The unit of analysis was the participant, rather than the number of fractures treated. For studies containing more than two intervention groups, making multiple pair‐wise comparisons between all possible pairs of intervention groups possible, we planned to include the same group of participants only once in the meta‐analysis.
Dealing with missing data
Where important data were missing or incomplete, we sought further information from the trial authors.
In cases where individuals were missing from the reported results, we assumed the missing values to have a poor outcome. For dichotomous outcomes that measured serious and other adverse events (e.g. incident fractures), we calculated the adverse event rate using the number of patients that received treatment as the denominator (worst‐case analysis). For dichotomous outcomes that measured benefits (e.g. proportion of participants reporting pain relief of 30% or greater), we calculated the worst‐case analysis using the number of randomised participants as the denominator. For continuous outcomes (e.g. pain), we calculated the MD or SMD based on the number of patients analysed at that time point. If the number of patients analysed was not presented for each time point, we used the number of randomised patients in each group at baseline.
For continuous outcomes with no SD reported, we calculated SDs from standard errors (SEs), 95% CIs or P values. If no measures of variation were reported and SDs could not be calculated, we planned to impute SDs from other trials in the same meta‐analysis, using the median of the other SDs available (Ebrahim 2013). For continuous outcomes presented only graphically, we extracted the mean and 95% CIs from the graphs using plotdigitizer (http://plotdigitizer.sourceforge.net/). For dichotomous outcomes, we used percentages to estimate the number of events or the number of people assessed for an outcome.
Where data were imputed or calculated (e.g. SDs calculated from SEs, 95% CIs or P values, or imputed from graphs or from SDs in other trials), we reported this in the Characteristics of included studies tables.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials.
We assessed statistical heterogeneity by visually assessing the scatter of effect estimates on the forest plots, and by using the Chi2 statistic and the I2 statistic (Higgins 2003). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% may not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity (Deeks 2011).
In cases of considerable heterogeneity, we intended to explore the data further by comparing the characteristics of individual studies and any subgroup analyses.
Assessment of reporting biases
To assess publication bias, we planned to generate funnel plots if at least 10 trials examining the same intervention comparison were included in the review, and comment on whether any asymmetry in the funnel plot was due to publication bias, or methodological or clinical heterogeneity of the trials (Sterne 2011).
To assess for potential small‐study effects in meta‐analyses (i.e. the intervention effect is more beneficial in smaller studies), we planned to compare effect estimates derived from a random‐effects model and a fixed‐effect model of meta‐analysis. In the presence of small‐study effects, the random‐effects model will give a more beneficial estimate of the intervention than the fixed‐effect estimate (Sterne 2011). However, as no statistically significant results were found, we could not perform this analysis.
To assess outcome reporting bias, we compared the outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications.
Data synthesis
Included studies were grouped based on whether percutaneous vertebroplasty was compared with a placebo or sham procedure, usual care or another active intervention.
For benefit, we considered the first comparison, vertebroplasty versus placebo, to be the least prone to bias and it was therefore the primary comparison for addressing the objectives of this review.
For safety (new vertebral fractures and other serious and other adverse events), as the results were similar for both placebo and usual care comparisons, to simplify the presentation we presented safety data in the same analyses.
We combined results of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of benefits and harms. To combine results we used a random‐effects meta‐analysis model based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results.
'Summary of findings' table
We presented the major outcomes for the primary comparison of vertebroplasty of the review in a 'Summary of findings' table (mean pain, disability, disease‐specific quality of life, overall quality of life, treatment success, incident fractures, and total number of serious adverse events). This table reports the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data for each major outcome as recommended by Cochrane (Schünemann 2011a), and was produced using GRADEpro software. The overall quality of the evidence was graded for each of the major outcomes using the GRADE approach (Schünemann 2011b). We included evidence only from trials with adequate treatment allocation concealment and blinding of participants and outcome assessment.
In the comments column, we calculated the absolute percentage change and the relative percentage change. Relative changes were calculated as absolute change (MD) divided by mean at baseline in the placebo group from Buchbinder 2009 (values were: 7.1 points on zero‐ to 10‐point VAS pain; 17.3 points on zero‐ to 23‐point Roland‐Morris Disability questionnaire; 0.28 points on EQ‐5D quality of life scale; 59.6 points on the QUALEFFO scale). In interpreting results, we assumed a minimal clinically important difference of 1.5 points on a 10‐point pain scale (Grotle 2004); two to three points on the zero‐ to 23‐point RMDQ (Trout 2005), 10 points on the zero to 100 QUALEFFO scale (Lips 1999), and 0.074 points on the EQ‐5D quality of life scale, zero = death to one = perfect health (Walters 2005).
Subgroup analysis and investigation of heterogeneity
Where sufficient data were available, the following subgroup analysis was performed.
Duration of symptoms ('acute' fractures versus 'subacute fractures')
Sensitivity analysis
Sensitivity analyses were performed to investigate the robustness of the treatment effect (of main outcomes) by performing an analysis that included all trials combined, i.e. trials with placebo or usual care comparator groups, to see if inclusion of trials that did not blind participants changed the overall treatment effect.
Results
Description of studies
Results of the search
We updated the search for the current review on 15 November 2017, searching for studies published since 2014 (the date of the search in the first version of the review).
The results of the search are presented in Figure 1. The updated search strategy identified 776 new citations (426 after de‐duplication). Of these, 37 were assessed in full text and nine new studies were included (Clark 2016; Evans 2015; Leali 2016; Sun 2016; Firanescu 2018; VOPE 2015; Wang 2015; Wang 2016; Yang 2016). Six of these were identified as ongoing or completed studies in the 2015 version of this review and have since reported results (Clark 2016; Evans 2015; VOPE 2015; Firanescu 2018; Wang 2015), (Firanescu 2018), and are included in this review update. The partially reported results of Firanescu 2018 were presented at a conference, and were identified in the last full search (15 November 2017). The results of the trial were published subsequent to the search and were available on 9 May 2018; thus we included information from this publication as a minor amendment to the review.
1.
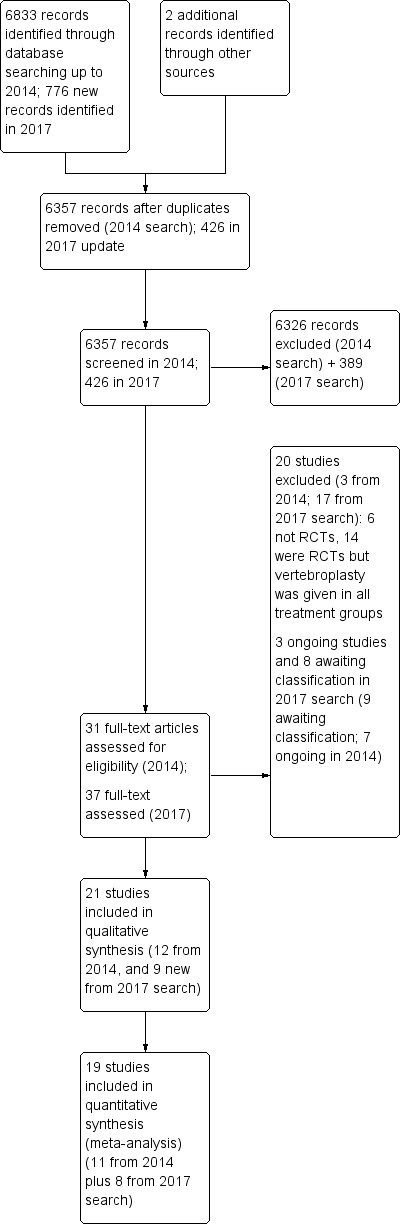
Study flow diagram.
Thus, in total in this update we included 21 trials (Blasco 2012; Buchbinder 2009; Chen 2014a; Clark 2016; Dohm 2014; Endres 2012; Evans 2015; Farrokhi 2011; Kallmes 2009; Klazen 2010; Leali 2016; Liu 2010; Rousing 2009; Sun 2016; Firanescu 2018; Vogl 2013; Voormolen 2007; VOPE 2015; Wang 2015; Wang 2016; Yang 2016).
Ten randomised controlled trials (RCTs) were registered in a trial registry (Blasco 2012; Buchbinder 2009; Clark 2016; Dohm 2014; Evans 2015; Farrokhi 2011; Kallmes 2009; Klazen 2010; Firanescu 2018; VOPE 2015), although one was registered retrospectively (Farrokhi 2011). One RCT reports that it is registered on clinicaltrials.gov (NCT00576546) (Vogl 2013), but this could not be verified. No trial registration was found for the other trials (Chen 2014a; Endres 2012; Leali 2016; Liu 2010; Rousing 2009; Sun 2016; Voormolen 2007; Wang 2015; Wang 2016; Yang 2016).
In total, 20 studies were excluded after full‐text assessment (see table of Characteristics of excluded studies): 17 studies identified in the updated search were excluded (Cai 2015; Chen 2014b; Chun‐lei 2015; Du 2014; Gu 2015; Li 2015a; Liu 2015; Min 2015; Son 2014; Xiao‐nan 2014; Yang 2014; Yi 2016; Ying 2017; Yokoyama 2016; Zhang 2015a; Zhang 2015b; Zhang 2015c), in addition to the three studies excluded in the earlier version of this review (Gilula 2013; Huang 2014; Yi 2014).
Three trials are classified as ongoing, although it is unclear if the trials are completed. We could not find any trial registration details for one study (Longo 2010), while a second trial is registered at ClinicalTrials.gov with an expected completion date of December 2014 (Sun 2012), but the current recruitment status is unknown. The third trial (VERTOS V), was recorded as completed in June 2015 at clinicaltrials.gov, but the trialists subsequently altered the recruitment status in January 2017 to state that the study is now 'enrolling by invitation', with a new estimated completion date of July 2018 (see table of Characteristics of ongoing studies).
Based upon a search of trial registries, we identified an additional four registered RCTs that are reported to have been completed but we were unable to find results (Dolin 2003; Laredo (OSTEO‐6); Laredo (STIC2); Sorensen 2005) (see table of Characteristics of studies awaiting classification). We also identified four completed RCTs that likely meet inclusion criteria of the review, but results are published in Chinese and we did not translate these prior to publication of this review update: two trials compare vertebroplasty with kyphoplasty (Tan 2016; Zhou 2015), another compared vertebroplasty with kyphoplasty and bone‐filling mesh container (Li 2015b) and the third vertebroplasty with usual care (Chen 2015).
Since the last version of the review, we have removed three ongoing trials (Damaskinos 2015 NCT02489825; Nieuwenhuijse 2012 NTR3282); and Zhao 2014 ChiCTR‐TRC‐14004835), as percutaneous vertebroplasty was given to participants in both treatment arms and thus, when trial results become available, they will not be eligible for inclusion in this review. An additional trial that was reportedly suspended due to difficulty recruiting participants prior to completion and which was included as a study awaiting classification in the last version of the review (Nakstad 2008 NCT00635297) has also been removed from this current version of the review as if the trial had been completed it would not be eligible for inclusion.
Detailed descriptions of all unpublished trials that are either completed, suspended or ongoing are provided in either the table of Characteristics of studies awaiting classification or table of Characteristics of ongoing studies and a summary of all unpublished trials is provided in Table 2.
1. Study characteristics of unpublished, ongoing and suspended or terminated trials.
| Trial registration number | Principle Investigator/s and Country | Comparator/s | Main selection criteria | Registration date | Recruitment commenced | Status 8 January 2018 | Planned sample size | Final sample size |
|
NCT00749060 ‘OSTEO‐6’ |
Laredo JD France |
Kyphoplasty; Usual care with or without brace | Age ≥ 50 years Fracture < 6 weeks |
8 Sept 2008 | Dec 2007 | Completed June 2012; results unpublished | 300 | 48 |
|
NCT00749086 ‘STIC2’ |
Laredo JD France |
Kyphoplasty | Age ≥ 50 years Fracture > 6 weeks |
8 Sept 2008 | Dec 2007 | Completed June 2012; results unpublished | 200 | 97 |
| NCT00203554 | Sorensen L Denmark |
Usual care | Fracture < 6 months | 16/09/2005 | Mar 2004 | Completed Jan 2008; results unpublished | 27 | 27 |
| ISRCTN14442024 (Also N0213112414) |
Dolin, S UK |
Usual care | Fracture > 4 weeks | 12 Sep 2003 | Nov 28 2005 | Completed (last updated 6 Feb 2014); results unpublished | Not provided | Not provided |
| NCT01677806 | Sun G China |
Usual care | Age ≥ 50 years Fracture < 6 weeks |
23 Aug 2012 | Oct 2012 | Recruitment status unknown (last updated 11 Sep 2014) | 114 | ‐ |
| Registration details not found. | Longo UG Italy |
3 weeks bed rest, rigid hyperextension corset, followed by 2‐3 months in a Cheneau brace (called ‘double‐blind) | Age ≥ 50 years | Trial registration not found | Unknown | Unknown (protocol published) | 200 | ‐ |
|
NCT01963039 ‘VERTOS V’ |
Carli D the Netherlands |
Sham | Age ≥ 50 years Fracture ≥ 12 weeks |
28 Aug 2013 | May 2013 | Previously reported as completed (Nov 2015) then recruiting again (Feb 2017) (protocol published) | 94 | ‐ |
| Record of trial registration not found | Chen JP China |
Usual care | Not available | Trial registration not found | Unknown | Completed; study awaiting translation | Unknown | 84 |
| Record of trial registration not found | Li DH China |
Kyphoplasty Bone filling mesh container |
Not available | Trial registration not found | Unknown | Completed; study awaiting translation | Unknown | 90 |
| Record of trial registration not found | Tan B China |
Kyphoplasty | Not available | Trial registration not found | Unknown | Completed; study awaiting translation | Unknown | 106 |
| Record of trial registration not found | Zhou W China |
Kyphoplasty | Not available | Trial registration not found | Unknown | Completed; study awaiting translation | Unknown | 80 |
* Abstract reported that analysis favoured vertebroplasty at 1 day and 1 week for pain, and disability measured by RMDQ and ODI (data not provided), but no evidence of important differences between groups at 1, 3, 6, 12 months for pain, RMDQ, ODI and SF‐36 function and SF‐36 physical and mental component scores. After 12 months follow‐up, there were 13 new fractures in the percutaneous vertebroplasty group and 11 new fractures in the facet joint block group. Abstract did not report method of randomisation, whether or not treatment allocation was concealed and whether or not participants and investigators were blinded to treatment allocation.
Included studies
A full description of all included trials is provided in the table of Characteristics of included studies and a summary of trial and participant characteristics is provided in Table 3.
2. Baseline demographic and clinical characteristics of the trial participants.
| Study | Country | Treatment Groups | Mean age, yrs | Mean symptom duration | Mean (SD) baseline pain (0‐10 scale$) | Mean (SD) baseline RMDQ+ (0‐24 scale†) | Mean (SD) baseline QUALEFFO (0‐100 scale) | Procedures performed by | Mean (range) volume cement injected (mL) | Follow‐up |
| Blasco 2012 | Spain | Vertebroplasty | 71.3 | 140.3 days | 7.2 (0.3) | ‐ | 65.2 (2.2) | Interventional radiologists | Not specified | 2 weeks, 2, 6, 12 months |
| Usual care | 71.3 | 143.1 days | 6.3 (0.4) | ‐ | 59.2 (2.2) | |||||
| Buchbinder 2009 | Australia | Vertebroplasty | 74.2 | 9 weeks^ | 7.4 (2.1) | 17.3 (2.8) | 56.9 (13.4) | Interventional radiologists | 2.8 (1.2 ‐ 5.5) | 1 week, 1, 3, 6, 12, 24 months |
| Placebo | 78.9 | 9.5 weeks^ | 7.1 (2.3) | 17.3 (2.9) | 59.6 (17.1) | |||||
| Clark 2016 | Australia | Vertebroplasty | 80 | 2.8 weeks | 8.1 (1.8) | 19.5 (3.5) | 65.4 (11.4) | Interventional radiologists | 7.5 (4.7 ‐ 10.3) | 3 days, 14 days, 1, 3 and 6 months |
| Placebo | 81 | 2.4 weeks | 8.2 (1.5) | 19.8 (3.7) | 67.7 (11.2) | |||||
| Chen 2014a | China | Vertebroplasty | 64.6 | 31 weeks | 6.5 (0.9)& | 18.6 (1.8)#& | ‐ | Orthopaedic surgeons | 3.6 (3 ‐ 6) | 1 day, 1 week, 1, 3, 6, 12 months |
| Usual care and brace | 66.5 | 29.5 weeks | 6.4 (0.9)& | 16.7 (1.3)#& | ‐ | |||||
| Dohm 2014 | USA and Canada | Vertebroplasty | 75.7 | ‐¤ | ˜7.6µ | ‐ | ‐ | Interventional radiologists and neuroradiologists, orthopaedic surgeons, neuroradiologists | 4.0 (3.0 to 6.0)¢ | 7 days, 1, 3, 12 and 24 months |
| Balloon kyphoplasty | 75.5 | ‐¤ | ˜7.6µ | ‐ | ‐ | Not stated | 4.6 (3.4 to 6.0)¢ | |||
| Endres 2012 | Germany | Vertebroplasty | 71.3 | ‐§ | 7.8 (0.9) | ‐ | ‐ | Orthopaedic surgeon | 3.1 (2 – 4) | Immediately, mean 5.8 months (range: 4 to 7) |
| Balloon kyphoplasty | 63.3 | ‐§ | 9.0 (0.7) | ‐ | ‐ | Orthopaedic surgeon | 3.9 (3 – 5) | |||
| Shield kyphoplasty | 67.1 | ‐§ | 8.8 (1.5) | ‐ | ‐ | Orthopaedic surgeon | 4.6 (3 – 6) | |||
| Evans 2015 | USA | Vertebroplasty | 76.1 | ‐ | 7.9 (2.0) | 16.3 (7.4) | ‐ | Not reported | Not reported | 3 days, 1, 6 and 12 months |
| Kyphoplasty | 75.1 | ‐ | 7.4 (1.9) | 17.3 (6.6) | ‐ | Not reported | Not reported | |||
| Farrokhi 2011 | Iran | Vertebroplasty | 72 | 27 weeks | 8.4 (1.6) | ‐ | ‐ | Neurosurgeons | 3.5 (1 ‐ 5.5) | 1 week, 2, 6, 12, 24, 36 months |
| Usual care | 74 | 30 weeks | 7.2 (1.7) | ‐ | ‐ | |||||
| Firanescu 2018 | the Netherlands | Vertebroplasty | 74.7 | 29.2 days | 7.7 (1.4) | 18 (4.5) | 68.4 (17.1) | Interventional radiologists | 5.11 (1 ‐ 11) | 1 day, 1 week, 1, 3, 6, 12 months |
| Placebo | 76.8 | 25.9 days | 7.9 (1.6) | 17.8 (4.7) | 69.7 (17.9) | |||||
| Kallmes 2009 | US, UK, Australia | Vertebroplasty | 73.4 | 16 weeks | 6.9 (2.0) | 16.6 (3.8) | ‐ | Interventional radiologists | 2.8 (1 ‐ 5.5)* | 3 days, 2 weeks, 1 month |
| Placebo | 73.3 | 20 weeks | 7.2 (2.0) | 17.5 (4.1) | ‐ | |||||
| Klazen 2010 | the Netherlands, Belgium | Vertebroplasty | 75.2 | 29.3 days | 7.8 (1.5) | 18.6 (3.6)# | 58.7 (13.5) | Interventional radiologists | 4.1 (1 ‐ 9) | 1 day, 1 week, 1, 3, 6, 12 months |
| Usual care | 75.4 | 26.8 days | 7.5 (1.6) | 17.2 (4.2)# | 54.7 (14.4) | |||||
| Leali 2016 | Italy | Vertebroplasty | ‐ | ‐§ | 4.8 (‐) | 53.6 (‐) | ‐ | Not reported | 4 (‐) | 1 and 2 days, 6 weeks, 3 and 6 months |
| Usual care | ‐ | ‐§ | ‐§ | ‐ | ‐ | Not reported | ||||
| Liu 2010 | Taiwan | Vertebroplasty | 74.3 | 15.8 days | 7.9 (0.7) | ‐ | ‐ | Not reported | 4.9 (0.7) | 3 days, 6 months, 1, 3 and 5 years |
| Balloon kyphoplasty | 72.3 | 17.0 days | 8.0 (0.8) | ‐ | ‐ | Not reported | 5.6 (0.6) | |||
| Rousing 2009 | Denmark | Vertebroplasty | 80 | 8.4 days | 7.5 (2.0) | ‐ | ‐ | Orthopaedic surgeons | Not reported | 3 months |
| Usual care and brace | 80 | 6.7 days | 8.8 (1.2) | ‐ | ‐ | |||||
| Sun 2016 | China | Vertebroplasty | 65.4 | ‐ | 8.5 (1.1) | 70.6 (8.6)× | ‐ | Not reported | 3.4 (0.3) | 2 days, 12 months |
| Kyphoplasty | 65.2 | ‐ | 8.2 (0.9) | 71.7(8.5)× | ‐ | Not reported | 4.2 (0.2) | |||
| Vogl 2013 | Germany and USA | Vertebroplasty | 74 | ‐¥ | 8.5 (1.2) | ‐ | ‐ | Not reported | 4.0 (1.1) | 1 day, 3 and 12 months |
| Shield kyphoplasty | 80 | ‐¥ | 8.3 (1.1) | ‐ | ‐ | Not reported | 3.8 (0.7) | |||
| Voormolen 2007 | the Netherlands | Vertebroplasty | 72 | 85 days | 7.1 (5 ‐ 9)+ | 15.7 (8‐24) | 60.0 (37 to 86) | Interventional radiologists | 3.2 (1.0 ‐ 5.0) | 2 weeks |
| Usual care | 74 | 76 days | 7.6 (5‐10) | 17.8 (8‐22) | 60.7 (38 to 86) | |||||
| VOPE 2015 | Denmark | Vertebroplasty | 70.6 | ‐ª | 7.47 () | ‐ | ‐ | Orthopaedic surgeons | Not reported˜ | 6 hours, weekly to 3 months, 12 months |
| Placebo (lidocaine injected) | 69.3 | ‐ª | 7.61 () | ‐ | ‐ | Orthopaedic surgeons | ||||
| Wang 2015 | China | Vertebroplasty | 69.43 | ‐ | 8.1 (1.2) | 71.22 (10.56)× | ‐ | Not reported | 3.31 (0.77) | 1 day, 3 and 12 months |
| Balloon kyphoplasty | 68.63 | ‐ | 8.0 (1.1) | 71.30 (10.22)× | ‐ | Not reported | 4.22 (1.29) | |||
| Wang 2016 | China | Vertebroplasty | 63.7 | ‐ª | 7.65 (1.11) | 18.3 (1.0) | ‐ | Spine surgeon | 5.5 (3.0 ‐ 9.0) | |
| Facet joint injection | 62.6 | ‐ª | 7.76 (1.06) | 18.45 (0.98) | ‐ | Spine surgeon | ||||
| Yang 2016 | China | Vertebroplasty | 77.1 | Not reported | 7.5 (1.1) | 80.2 (9.9)× | 78.1 (8.1) | Not stated | 4.5 (3‐6.5) | 1 week, 3, 6 and 12 months |
| Usual care | 76.2 | Not reported | 7.7 (1.1) | 81.5 (9.7)× | 77.5 (8.6) |
$1‐10 point scale used by Farrokhi 2011, 0 to 100 scale used by VOPE 2015 and we report pain with forward bending for this trial as overall pain not reported and have converted SE to SD; +RMDQ: Roland‐Morris Disability Questionnaire; †modified RMDQ (0‐23 scale) used by Buchbinder 2009, Kallmes 2009 and Firanescu 2018; ×Oswestry Disability Index (0 to 100) used by Leali 2016, Wang 2015, Yang 2016; ^ median duration of symptoms; ¤Not reported but symptom duration 6 months or less; µMean symptom duration reported graphically only; ¢Median and interquartile range;§Not reported but symptom duration 6 weeks or less; ªNot reported but symptom duration 8 weeks or less; &Data only included for the 42/46 in VP group and 43/50 in the usual care group who completed 12‐month follow‐up in groups assigned to at baseline; #Disability significantly higher in the vertebroplasty group; *from n = 20 treated at Mayo (personal communication); ¥Not reported but at least 6 weeks of conservative treatment; +Only range provided; ˜up to 2 mL.
Trial design, setting and characteristics
Five trials compared vertebroplasty with a placebo (a sham vertebroplasty procedure) (Buchbinder 2009; Clark 2016; Kallmes 2009; Firanescu 2018; VOPE 2015), eight trials compared vertebroplasty with usual care/optimum pain management (Blasco 2012; Chen 2014a; Farrokhi 2011; Klazen 2010; Leali 2016; Rousing 2009; Voormolen 2007; Yang 2016), one trial compared vertebroplasty with injection of local anaesthetic and glucocorticosteroid into the facet joint of the fractured vertebra in the spine (facet joint injection) (Wang 2016), while seven trials compared vertebroplasty with different kyphoplasty techniques (Dohm 2014; Endres 2012; Evans 2015; Liu 2010; Sun 2016; Vogl 2013; Wang 2015).
Trials were conducted in 15 different countries including: Australia (Buchbinder 2009; Clark 2016), USA (Evans 2015), USA, Australia and UK (Kallmes 2009), the Netherlands (Firanescu 2018; Voormolen 2007), the Netherlands and Belgium Klazen 2010), Denmark (Rousing 2009; VOPE 2015), Iran (Farrokhi 2011), Italy, France and Switzerland (Leali 2016), Spain (Blasco 2012), China (Chen 2014a; Sun 2016; Wang 2015; Wang 2016; Yang 2016), Taiwan (Liu 2010), Germany (Endres 2012), USA and Canada (Dohm 2014), and Germany and the USA (Vogl 2013). Eight trials received at least some funding from medical device companies who manufacture vertebral augmentation systems (Buchbinder 2009; Clark 2016; Dohm 2014; Evans 2015; Farrokhi 2011; Klazen 2010; Firanescu 2018; Vogl 2013); two clearly reported that they had no role in the trial (design, data collection, data analysis, preparation of the manuscript)(Buchbinder 2009; Clark 2016); while five trials did not report source of funding (Chen 2014a; Leali 2016; Sun 2016; Voormolen 2007; Wang 2015).
Trial duration varied from two weeks (Voormolen 2007) to three years (Farrokhi 2011). Seven trials allowed cross‐over: Kallmes 2009 allowed blinded cross‐over to the alternate procedure at one month or later if adequate pain relief was not achieved; both Voormolen 2007 and Farrokhi 2011 allowed participants assigned to the control arm still in severe pain after two weeks to undergo vertebroplasty; Blasco 2012, Chen 2014a and Yang 2016 allowed participants in the conservative therapy group to be considered for vertebroplasty if there was no improvement in pain but the timing of this decision was not provided; and in VOPE 2015 cross‐over between groups was allowed after three months although no details about who could cross over were not provided.
Trial participants
The 21 trials included 2852 randomised participants with trial sizes varying from 46 participants (Voormolen 2007) to 404 participants (Dohm 2014). In general, inclusion criteria for all trials were similar requiring a clinical history and imaging findings consistent with one or more acute osteoporotic vertebral fractures (see Characteristics of included studies table). Across all trials the majority of participants were female and Evans 2015 only included women (see Table 3). Mean age of participants ranged between 62.6 and 81 years.
Symptom duration varied across trials with mean duration ranging from around a week (Rousing 2009) to more than six months (Farrokhi 2011). For the placebo‐controlled trials, mean duration of symptoms ranged from less than three weeks to 20 weeks across treatment groups in the individual trials (vertebroplasty and placebo: Buchbinder 2009 nine and 9.5 weeks; Clark 2016 2.8 and 2.4 weeks; Kallmes 2009 16 and 20 weeks; Firanescu 2018 29.2 and 25.9 days; and VOPE 2015 did not report mean duration but trial inclusion specified a symptom duration of eight weeks or less).
Mean baseline pain and disability scores were similar across trials. Mean pain at baseline was greater than seven out of 10 (where 10 is the worst pain) in most trials, except the usual care group of Blasco 2012 (mean 6.3), both arms of Chen 2014a (means 6.5 and 6.4 in the vertebroplasty and usual care arms, respectively), the vertebroplasty arm of Kallmes 2009 (mean 6.9), and was low in Leali 2016 (mean 4.8 in vertebroplasty, not reported in usual care) and Leali 2016 (mean pain score 4.8 out of 25 and Oswestry Disability Index (ODI) 53.6% in the vertebroplasty group and not reported in the conservative care group). In VOPE 2015, the mean baseline pain was greater than 70 out of 100 for activity pain( 74.68 in the vertebroplasty group and 76.08 in the placebo group), but not for rest pain (40.55 in the vertebroplasty group and 53.04 in the placebo group). Sun 2016 did not report baseline pain or disability scores.
Interventions
Details of interventions in each trial are presented in the Characteristics of included studies table. Vertebroplasty was performed by different specialists in the included trials: interventional radiologists in seven trials (Blasco 2012; Buchbinder 2009; Clark 2016; Firanescu 2018; Kallmes 2009; Klazen 2010; Voormolen 2007), orthopaedic surgeons in four trials (Chen 2014a; Endres 2012; Rousing 2009; VOPE 2015), neurosurgeons in one trial (Farrokhi 2011), spine surgeons in one trial (Wang 2016), a combination of interventional radiologists and neuroradiologists, orthopaedic surgeons and neurosurgeons in one trial (Dohm 2014), and the background of the interventionalist was not reported in seven trials (Evans 2015; Leali 2016; Liu 2010; Sun 2016; Vogl 2013; Wang 2015; Yang 2016).
Vertebroplasty appeared to have been performed in a similar way across all trials. However in Dohm 2014, the majority of participants in both treatment arms (75.1% in the vertebroplasty group and 80.6% in the kyphoplasty group) also had perioperative postural reduction in an attempt to correct vertebral deformity, and in Wang 2016 postural reduction was performed before surgery in both groups.
Mean vertebroplasty cement volume was not specified in four trials (Blasco 2012; Evans 2015; Rousing 2009; VOPE 2015) (see Characteristics of included studies table and Table 2). VOPE 2015 reported that up to 2 mL of cement was inserted but it was not clear if a uni‐ or bipedicular approach was used (which may mean that up to 4 mL was inserted). For the remaining trials, the mean cement volume ranged from 2.8 mL (Buchbinder 2009; Kallmes 2009) to 7.5 mL (Clark 2016). Mean (range) cement volume in the four placebo‐controlled trials was 2.8 mL (1.2 ml to 5.5 mL) (Buchbinder 2009), 7.5 mL (4.7 mL to 10.3 mL) (Clark 2016), 2.8 mL (1 mL to 5.5 mL) (based upon a subset of 20 participants) (Kallmes 2009) and 5.11 mL (1 mL to 11 mL) (Firanescu 2018).
There was diversity in the way that the sham vertebroplasty procedure was delivered in the five placebo‐controlled trials. Buchbinder 2009 most closely simulated vertebroplasty using an identical procedure to that performed in the vertebroplasty group up to the insertion of the needle into the bone, at which point the vertebral body was gently tapped with a blunt stylet and bone cement was prepared to permeate the strong smell of the polymethylmethacrylate (PMMA) in the room. The sham procedure in the second placebo‐controlled trial was similar except that the vertebral body was not tapped with a blunt stylet (Kallmes 2009). For Clark 2016, both the trial registry and protocol paper reported that the placebo involved a 4 mm skin incision and light tapping of the skin, while the results paper states that a short needle was passed into the skin incision but not as far as the periosteum and that manual skin pressure and regular tapping on the needle was performed. The published protocol of Firanescu 2018 indicates that no needles would be placed periosteally in the placebo group but the methacrylate monomer would be opened to simulate the odour of mixing the bone cement and participants would receive verbal and physical cues (e.g. pressure on the back) to simulate the procedure.. The published reported noted that bone biopsy needles were positioned periosteally bilaterally with preparation of the cement occurring in close proximity to the participants (so that the mixing sound could be heard and the polymethylmethacrylate smelt by everyone in the room). In VOPE 2015, PMMA was also opened for the odour but instead of cement, 2 mL of local anaesthetic (lidocaine) was injected into the fractured vertebrae.
Eight trials used variations on usual care as the comparator group (Blasco 2012; Chen 2014a; Farrokhi 2011; Klazen 2010; Leali 2016; Rousing 2009; Voormolen 2007; Yang 2016) (see Characteristics of included studies table). This included analgesics including acetaminophen, codeine, tramadol and/or opioids in all instances, and these could be adjusted as needed. Five trials specified the use of non‐steroidal anti‐inflammatory drugs (NSAIDs), which could have been in addition to analgesics, when simple analgesia was ineffective or for those intolerant to opioid derivatives (Farrokhi 2011; Klazen 2010; Leali 2016; Voormolen 2007; Yang 2016). Three trials also prescribed calcitonin (Blasco 2012; Farrokhi 2011; Yang 2016) and three also offered brace treatment (Chen 2014a; Rousing 2009; Yang 2016).
Seven trials compared vertebroplasty with kyphoplasty (Dohm 2014; Endres 2012; Evans 2015; Liu 2010; Sun 2016; Vogl 2013; Wang 2015). Dohm 2014, Endres 2012, Liu 2010 and Wang 2015 compared vertebroplasty with balloon kyphoplasty. Endres 2012 also compared vertebroplasty with a shield kyphoplasty which instead of a balloon, uses specialised instrumentation to create a central cavity in the vertebral body and inserts a self‐expanding implant that controls the cement flow. Endres 2012 compared vertebroplasty with the same shield kyphoplasty. Evans 2015 compared vertebroplasty with kyphoplasty, however it did not report the kyphoplasty technique used. Sun 2016 compared high‐viscosity cement vertebroplasty with low‐viscosity cement kyphoplasty.
A single trial injected a mixture of prednisolone (125 mg:5 mL) and lidocaine (100 mg:5 mL) under fluoroscopic monitoring into the facet joint of the fractured vertebra as the control intervention (Wang 2016).
Outcomes
Pain
All trials included at least one measure of pain, but its measurement varied across trials. Three trials specified pain over the preceding 24 hours (Clark 2016; Farrokhi 2011; Kallmes 2009), one trial specified pain over the course of the previous week (Buchbinder 2009), and 16 trials did not specify a time period (Blasco 2012; Chen 2014a; Dohm 2014; Endres 2012; Evans 2015; Klazen 2010; Leali 2016; Liu 2010; Rousing 2009; Sun 2016; Firanescu 2018, Voormolen 2007; VOPE 2015; Wang 2015; Wang 2016; Yang 2016). Vogl 2013 only reported baseline pain in their published trial report but measurement of pain (and disability assessed by the Oswestry Disability Index (ODI)) was referred to in a congress abstract of the same trial published in German; whether or not a time period was specified is not known.
Buchbinder 2009 measured overall pain, pain at rest and pain in bed at night, while Kallmes 2009 measured average back pain intensity. Clark 2016 specified the measurement of pain at rest and pain with standing or activity in addition to average pain intensity but these results were not reported. Leali 2016 measured pain on a visual analogue scale (VAS) (zero ‐ no pain to five ‐ maximum pain) during walking, sitting and rising from a chair, bathing, dressing, and at rest, and summed all five scores to derive a total score out of 25. VOPE 2015 measured rest pain and pain during forward bending resembling a patient in activity (both on a zero to 100 VAS). All remaining trials referred to pain or mean pain unqualified by additional descriptors.
All but two trials, included a measure of pain using either a zero to 10 VAS or zero to 10 numerical rating scale, although the descriptor for a score of 10 differed across trials (e.g. maximum pain (Blasco 2012; Clark 2016), maximal imaginable pain (Buchbinder 2009), worst pain imaginable (Chen 2014a), worst possible (Dohm 2014), pain as bad as could be (Kallmes 2009), worst pain ever (Klazen 2010;Firanescu 2018), worse pain possible (Rousing 2009), worst pain in the patient's life (Voormolen 2007), and no descriptors specified (Evans 2015; Liu 2010; Wang 2015; Yang 2016)). The pain scale investigated by Farrokhi 2011 measured pain on a one (no pain) to 10 (excruciating pain) VAS, while Endres 2012 did not specify the pain scale explicitly although it was likely to have been on a zero to 100‐point scale (as mean baseline scores varied between 78.2 and 90). As described above, Leali 2016 used a zero to five VAS to measure pain during five activities and summed them for a score out of 25. Sun 2016 used a VAS to measure pain, but the scale range and descriptors (if any), were not reported. VOPE 2015 used a zero to 100 VAS and the descriptor (if any), were not reported.
Four trials also included a dichotomous measure of pain. Blasco 2012 measured the number of participants with moderate (pain ≥ 4) or severe (pain ≥ 7) pain at 12 months. Three trials included the proportion of participants with pain improved by a clinically relevant amount although the definitions varied. Buchbinder 2009 reported the proportion of people with improvement of overall pain, pain at rest and pain in bed at night of ≥ 2.5 units as post‐hoc analyses performed at the request of the publishing journal (external reviewer request). Clark 2016 reported the proportion of participants achieving an NRS pain score of <4 out of 10 (from a baseline score of ≥ 7 out of 10). Kallmes 2009 measured the proportion of participants with clinically important improvement in pain defined as at least 30% improvement.
Four trials also included other measures of pain. Evans 2015 and Kallmes 2009 included the Pain Frequency and Pain Bothersomeness Indices (each measured on a zero to four‐point scale, with higher scores indicating more severe pain). Klazen 2010 measured the number of pain‐free days (defined as days with a VAS score of three or lower) and Rousing 2009 included the Dallas Pain Questionnaire (DPQ), a 16‐item instrument that assesses four aspects of daily living affected by chronic back pain (day‐to‐day activities, work and leisure activities, anxiety and depression and social interest), measured as a percentage of pain interference in each of the four aspects (0% is no pain and 100% is pain all the time).
Disability
All except four trials (Blasco 2012; Liu 2010; Rousing 2009; VOPE 2015), included a back‐specific measure of disability or function and two trials included two back‐specific measures (Chen 2014a; Wang 2016). Nine trials included the Roland‐Morris Disability Questionnaire (RMDQ) (Buchbinder 2009; Chen 2014a; Clark 2016; Evans 2015; Kallmes 2009; Klazen 2010; Firanescu 2018; Voormolen 2007; Wang 2016) and 10 trials included the ODI (Chen 2014a; Dohm 2014; Endres 2012; Farrokhi 2011; Leali 2016; Sun 2016; Vogl 2013; Wang 2015; Wang 2016; Yang 2016) (although no data were presented in Vogl 2013). In Dohm 2014, section eight, regarding sexual activity was removed from the ODI. Three trials also included the physical function dimension component of the SF‐36 (Dohm 2014; Evans 2015; Kallmes 2009). Two trials only included the physical function dimension component of the SF‐36 as a measure of disability (Rousing 2009; VOPE 2015) and two trials included no measures of disability (Blasco 2012; Liu 2010).
Evans 2015 and Kallmes 2009 also included the Study of Osteoporotic Fractures‐Activities of Daily Living (SOF‐ADL) scale and Kallmes 2009 measured the proportion with clinically important improvement in disability (at least 30% improvement), while Rousing 2009 also included the Barthel Index and Farrokhi 2011 included ability to walk after one day.
Health‐related quality of life
Eight trials did not include a measure of health‐related quality of life (Chen 2014a; Endres 2012; Farrokhi 2011; Leali 2016; Liu 2010; Sun 2016; Vogl 2013; Wang 2015).
Seven trials included the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) (Blasco 2012; Buchbinder 2009; Clark 2016; Klazen 2010; Firanescu 2018; Voormolen 2007; Yang 2016) as a vertebral fracture and/or osteoporosis‐specific measure.
Six trials included an overall measure of health‐related quality of life: five trials included the Mental Component Summary (MCS) subscale of the SF‐36 (Evans 2015; Kallmes 2009; Rousing 2009; VOPE 2015; Wang 2016), eight trials included the European Quality of Life with 5 Dimensions (EQ‐5D) (Buchbinder 2009; Clark 2016; Dohm 2014; Evans 2015; Kallmes 2009; Klazen 2010; Rousing 2009; VOPE 2015), one trial included the Assessment of Quality of Life (AQoL) (Buchbinder 2009), and one trial included the Study of Osteoporotic Fractures‐Activities of Daily Living (SOF‐ADL6), Modified Deyo Patrick Pain Frequency and Bothersomeness Scale and the Osteoporotic Assessment Questionnaire (OPAQ) Body Image Scale (Evans 2015).
Treatment success
Two trials included a specific patient‐reported measure of treatment success. Buchbinder 2009 defined treatment success as 'moderately better' or 'a great deal better' for pain, fatigue and overall health on seven‐point ordinal scales, ranging from a 'great deal worse' to a 'great deal better'. Yang 2016 measured patient satisfaction as 'very satisfied' 'satisfied' or 'unsatisfied'.
As described above, three trials included one or more investigator‐specified measures of treatment success as determined by the number of participants who achieved various thresholds of pain and/or disability improvement (Buchbinder 2009; Clark 2016; Kallmes 2009).
Incident symptomatic and/or radiographically apparent vertebral fractures
Most trials recorded the occurrence of new symptomatic and/or radiologically apparent vertebral fractures.
Three trials reported the occurrence of both (Blasco 2012 up to 12 months; Buchbinder 2009 up to 24 months; Dohm 2014 up to 24 months).
Four trials reported new symptomatic vertebral fractures (Chen 2014a up to one year; Farrokhi 2011 up to 24 months; Leali 2016 up to six months; Voormolen 2007 up to two weeks) and five trials only reported occurrence of incident radiographic vertebral fractures (Clark 2016 at six months; Klazen 2010 at one, three and 12 months; Rousing 2009 at three and 12 months; VOPE 2015 at 12 months; Wang 2016 at 12 months; Yang 2016 at one, three, six and 12 months).
Liu 2010, Wang 2015 and Firanescu 2018 reported new vertebral fractures but did not specify if they were symptomatic or only detected on imaging, and Vogl 2013 reported radiographic refractures and adjacent level fractures up to 12 months and whether or not these were symptomatic. Endres 2012 only reported upon new adjacent fractures up to six months, while Evans 2015, Kallmes 2009 and Sun 2016 did not report occurrence of new vertebral fractures during the period of follow‐up.
Other serious adverse events
Adverse events, other than reporting of new symptomatic or asymptomatic vertebral fractures were variably reported across trials. Ten trials made specific reference to presence/absence of other adverse events in both treated groups (Buchbinder 2009; Clark 2016; Dohm 2014; Endres 2012; Kallmes 2009; Leali 2016; Sun 2016; Firanescu 2018; VOPE 2015; Yang 2016).
Blasco 2012, Chen 2014a, Wang 2015 and Yang 2016 reported on the presence/absence of clinical complications from cement leakage in the vertebroplasty‐treated group but did not report whether or not other adverse events occurred in either group; Farrokhi 2011, Klazen 2010 and Voormolen 2007 reported adverse events that occurred in the vertebroplasty‐treated group but did not report whether or not adverse events occurred in the usual care group. Evans 2015, Liu 2010, Rousing 2009 and Wang 2016 did not report the presence or absence of other adverse events.
Excluded studies
One controlled trial was excluded because participants were not assigned treatment at random but rather the authors stated that a surgeon at the outpatient ward blindly chose one of three different treatment modalities (vertebroplasty, kyphoplasty or usual care) to ensure similar pre‐treatment age, symptoms, grade and level of spinal diseases among the patients (Yi 2014). In addition, the vertebroplasty and kyphoplasty groups were combined in the data analysis and separate data for the vertebroplasty group were not provided. Another three studies were also excluded because participants were not assigned treatment at random (Du 2014; Yang 2014; Yokoyama 2016), and one study excluded was a retrospective study (Son 2014).
Other trials were excluded as vertebroplasty was administered to both treatment groups: with a different cement type in each group (Cai 2015; Chen 2014b; Gilula 2013; Gu 2015; Huang 2014; Li 2015a; Liu 2015; Min 2015; Xiao‐nan 2014; Ying 2017; Zhang 2015a), or trials compared unipedicular vertebroplasty with bipedicular vertebroplasty (Chun‐lei 2015; Zhang 2015b), or unilateral and bilateral vertebroplasty (Zhang 2015c).
Risk of bias in included studies
Risk of bias assessment for each study is reported in the Characteristics of included studies table and summarised in Figure 2 and Figure 3.
2.
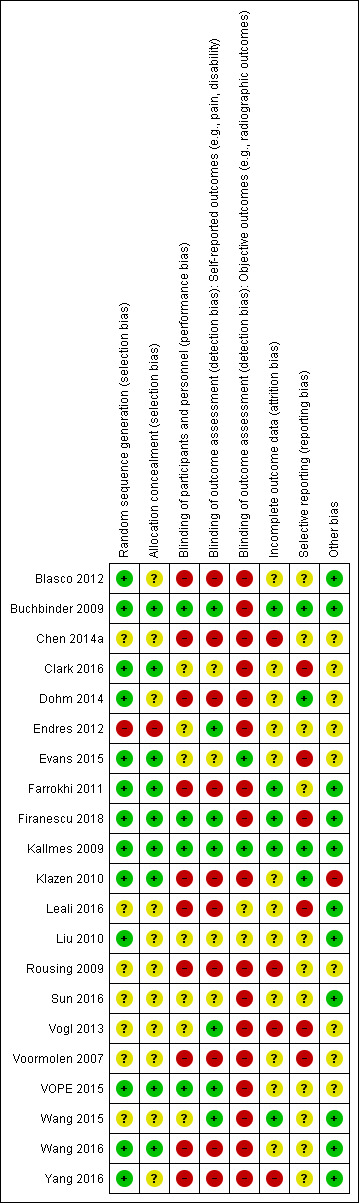
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
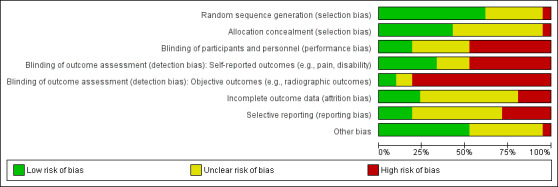
'Risk of bias summary': review authors' judgements about the risk of bias of the available evidence presented as percentages across all included studies.
Allocation
Six trials described adequate sequence generation and allocation concealment and were assessed as being at low risk of selection bias (Buchbinder 2009; Clark 2016; Evans 2015; Kallmes 2009; Firanescu 2018; Wang 2016). Dohm 2014 reported that randomisation was prepared by computer using a dynamic minimisation technique stratified by the number of prevalent vertebral fractures, aetiology and study centre, but treatment allocation was not concealed. Stratification by aetiology was unexplained as the selection criteria indicated that participants were included on the basis of osteoporotic fractures while fractures due to cancer and high‐energy trauma were excluded.
Three additional trials were also assessed as being at low risk of selection bias even though randomisation or allocation concealment was not explicitly reported (Farrokhi 2011; Klazen 2010; VOPE 2015). Farrokhi 2011 reported that the treatment assignment was kept in sealed envelopes. It is not clearly reported who prepared and opened the envelopes, but it is likely that allocation was concealed as they reported that neither the neurosurgeon (performing vertebroplasty) nor the physician (administering usual care) knew about the other study group and had no role in allocation. Klazen 2010 reported that an independent telephone operator allocated participants by telephone, therefore the allocation was likely concealed from the investigators. VOPE 2015 reported block randomisation design using 52 envelopes but no further details were provided. However participants and the single outcome assessor, as well as the biostatistician who performed the analysis were reportedly blinded to treatment allocation.
Blasco 2012, Liu 2010 and Yang 2016 reported that they prepared a computer‐generated random list but no information is provided regarding concealment of treatment allocation. In Chen 2014a, Leali 2016; Sun 2016; Vogl 2013 and Wang 2015, participants were "randomised" into treatment groups, however the trialists did not report the method of randomisation or whether concealment of treatment allocation was attempted. In Rousing 2009, sealed envelopes containing the treatment allocation were prepared beforehand by the investigating surgeon and 'sorted randomly' and type of treatment was unknown to the patient and the investigators until after the patient had provided written consent. In Voormolen 2007, the patients were randomised in two groups by an independent central operator but no further information is provided regarding concealment of treatment allocation. Endres 2012 was assessed as being at high risk of selection bias with respect to random sequence generation as participants were reported to have been distributed quasi‐randomly into three groups and the method was not reported. It was judged to be at unclear risk of selection bias as the single investigator was not blinded to treatment allocation although participants were reported to be blinded and all procedures were performed under general anaesthesia.
Blinding
Two trials were judged to be at low risk of performance and detection bias for all clinical outcomes as they blinded participants and all study personnel other than the person performing the intervention, and success of participant blinding was assessed to be successful (Buchbinder 2009; Kallmes 2009). However, after the one‐month follow‐up, Kallmes 2009 was considered to be at high risk of performance bias because more participants in the placebo group crossed over (27/63, 36%) compared with the vertebroplasty group (8/68, 12%) by the three‐month follow‐up. Buchbinder 2009 was judged to be at high risk of bias for the one‐ and two‐year assessment of radiographically‐apparent incident fractures as it was not possible to blind radiologists to treatment allocation due to the opacity of the cement. Firanescu 2018 was also judged to be at low risk of performance and detection bias for all clinical outcomes as they blinded participants although they did not explicitly report blinding of study personnel. Success of blinding was also assessed to be successful. In Firanescu 2018 it was not reported if incident fractures were detected clinically or radiographically. VOPE 2015 was also judged to be at low risk of performance and detection bias for all clinical outcomes as it appeared participants were blinded and the single assessor was blinded for all clinical outcomes, although blinding success was not evaluated. It was judged to be at high risk of bias for the assessment of radiographically‐apparent incident fractures as it was not possible to blind radiologists to treatment allocation due to the opacity of the cement.
In Clark 2016, success of blinding was only reported for a subset of participants (35/55 (60%) and 35/57 (61.4%) in the vertebroplasty and placebo groups, respectively at 14 days, and not reported at three days. In those in whom this outcome was reported, results differed with 80% (28/35) of the of the vertebroplasty group correctly guessing that they had undergone vertebroplasty compared with 54% (19/35) of the control group correctly guessing that they had received a placebo. Thus, we judged this trial as having an unclear risk of performance bias and detection bias for participant‐reported endpoints. Clark 2016 was judged to be at high risk of bias for radiographic outcomes as radiologists were not blinded due to the opacity of cement on imaging.
Ten trials were judged to be at high risk of performance and detection bias as participants and study personnel were likely aware of the treatment received (Blasco 2012; Chen 2014a; Dohm 2014; Farrokhi 2011; Klazen 2010; Leali 2016; Rousing 2009; Voormolen 2007; Wang 2016; Yang 2016). Evans 2015 did not report clearly if participants and personnel were blinded to treatment; the trial registry record reports the study was single‐blinded, in that participants were masked, but the results paper reports that site co‐ordinators and other personnel who collected trial participant data were unaware of treatment assignment. Two trials (Liu 2010; Sun 2016) were judged to be at unclear risk of detection bias for participant‐reported endpoints as it was unclear whether or not participants were blinded to treatment allocation. Liu 2010 was judged to be at unclear risk of bias for radiographic outcomes of vertebral height and kyphotic angle as while these were measured by technicians who were blinded to treatment allocation, it was not clear how variability of assessment was 'controlled via inter‐ and intra‐observer comparisons' (these outcomes were not reported in this review).
Vogl 2013 and Wang 2015 were judged to be at unclear risk of performance bias and low risk of detection bias for participant‐reported outcomes as participants were blinded to treatment allocation. However both, Vogl 2013 and Wang 2015 were judged to be at high risk of detection bias for investigator‐reported outcomes (In Vogl 2013, investigators were aware of treatment allocation and in Wang 2015, radiologists would have been able to tell the difference between vertebroplasty and kyphoplasty on imaging). Endres 2012 was assessed as being at unclear risk of performance bias as participants, but not the single investigator, were blinded to treatment allocation, low risk of bias for self‐assessed outcomes as participants were blinded and another orthopaedic surgeon not involved in the primary surgery performed the final follow‐up, and high risk of bias for investigator‐reported outcomes as radiologic outcomes were analysed by the unblinded orthopaedic surgeon who performed all procedures as well as another radiologist (status of radiologist with respect to blinding not reported).
Incomplete outcome data
Five trials were assessed as at low risk for attrition bias (Buchbinder 2009; Farrokhi 2011; Kallmes 2009; Firanescu 2018; Wang 2015), while the other trials were either considered to be at unclear (Blasco 2012; Clark 2016; Dohm 2014; Endres 2012; Evans 2015; Klazen 2010; Leali 2016; Liu 2010; Sun 2016; Voormolen 2007; Wang 2016) or high risk (Chen 2014a; Rousing 2009; Vogl 2013; Yang 2016).
Buchbinder 2009 had small and equal loss to follow‐up across treatment groups for shorter‐term benefit and safety outcomes although loss to follow‐up was greater when considering longer‐term outcomes. At two years, 29/38 (76%) and 28/40 (70%) had completed follow‐up in the vertebroplasty and sham groups, respectively.
Kallmes 2009 had a small and balanced loss to follow‐up across treatment groups up to one month.
Blasco 2012 was at unclear risk for attrition bias because while the proportion lost to follow‐up at 12 months was similar between groups (17/64 (27%) from the vertebroplasty group and 13/61 (21%) from the usual care group), the authors reported that the losses may not have been random, but related to worse pain in the usual care group.
Chen 2014a was judged to be at high risk of attrition bias as they performed a completers' analysis and excluded 7/50 participants allocated to receive conservative care on the basis that four refused conservative treatment and decided to have vertebroplasty at the three‐month follow‐up and an additional three were lost to follow‐up. However, while they stated that four participants in the vertebroplasty group were lost to follow‐up, they appeared to include all 46 participants allocated to receive vertebroplasty in the analysis to 12 months.
Clark 2016 was at unclear risk for attrition bias because while the proportion lost to follow‐up at three and six months was similar between groups (i.e. 8/61 (13.1%) from the vertebroplasty group and 7/59 (11.9%) from the placebo group at three months, and 10/61 (16.7%) from the vertebroplasty group and 8/59 (13.6%) from the placebo group at six months), the proportion lost to follow‐up at 14 days and one month were different (6/61 (9.8%) from the vertebroplasty group and 2/59 (3.4%) from the control group at both 14 days and one month). Reasons for withdrawal were also not reported. In addition, some data were missing for some outcomes reportedly due to inability to complete questionnaires by sick elderly participants. Most data were missing for the outcome of success of blinding at 14 days although the proportion missing was equal across treatment groups (data reported for 35/55 (60%) in the vertebroplasty group and 35/57 (61.4%) in the placebo group).
Dohm 2014 had small and equal loss to follow‐up across treatment groups for shorter‐term efficacy and safety outcomes. However, for the primary endpoints of new radiographic vertebral fracture at 12 and 24 months, loss to follow‐up was much greater. At 12 months 130/190 (68%) and 143/191 (75%) had completed follow‐up in the vertebroplasty and kyphoplasty groups, respectively, while at 24 months complete follow‐up was 91/190 (48%) and 100/191 (52%) in the vertebroplasty and kyphoplasty groups, respectively. While the reasons for loss to follow‐up were similar between groups, a higher proportion of those assigned to vertebroplasty (20/190; 11%) withdrew compared to the kyphoplasty group (11/191; 6%) and it is unclear if the reasons for withdrawal were systematically different.
In addition, seven participants who received vertebroplasty and four who received kyphoplasty underwent the alternate treatment for a subsequent vertebral fracture but the timing was not stated. An additional 70/88 (79.5%) participants with a new clinically recognised fracture underwent a subsequent vertebral augmentation during the trial ‐ it is implied that these participants received the same type of procedure as they had received as part of the trial. In all instances of spinal augmentation for a new vertebral fracture, the last observation before surgery was carried forward to later visits.
Endres 2012 was judged to be at unclear risk of attrition bias as data were unavailable for seven participants (two deaths and five participants who refused follow‐up although the treatment groups of these seven participants were not explicitly reported).
Evans 2015 was judged to be at unclear risk of attrition bias as a quarter of the participants did not complete follow‐up (21.8% of vertebroplasty group, 29.3% of kyphoplasty group). The authors report that the pattern of loss to follow‐up appeared to be missing at random.
Klazen 2010 was judged to be at unclear risk of attrition bias as a greater number of participants completed one‐year follow‐up in the vertebroplasty group (86/101, 85%) compared with 77/101 (76%) in the usual care group and 15 (15%) participants in the usual care group received vertebroplasty.
Leali 2016; Liu 2010; Sun 2016; Wang 2016 were judged to be at unclear risk for attrition bias because completeness of follow‐up was not explicitly reported.
Rousing 2009 was judged to be at high risk of attrition bias for several irregularities including failure to report baseline and follow‐up data for all participants.
Sun 2016 was judged to be at unclear risk of attrition bias as the numbers who were followed up were not reported.
In Firanescu 2018, follow‐up was nearly complete, with a small proportion lost in each group, for reasons that were likely unrelated to treatment.
Vogl 2013 was judged to be at high risk of attrition bias because of significant loss to follow‐up in both treatment arms (follow‐up complete at 12 months for 19 (68%) and 28 (57%) in the vertebroplasty and kyphoplasty groups, respectively), and the reasons for missing data were not reported.
Voormolen 2007 was judged to be at unclear risk of bias as the treatment group of four participants excluded from the analysis due to refusal to complete two‐week follow‐up was not reported.
VOPE 2015 was judged to be at unclear risk of bias because no data were presented for six participants who had been randomised. Two participants were excluded due to malignancy on biopsy and four were excluded due to receipt of further surgery during follow‐up. The type of surgery was not specified and the treatment allocation of these exclusions was not reported.
Wang 2015 had small and similar loss to follow‐up across treatment groups at three and 12 months.
Wang 2016 reported that 8/108 (7.4%) in the vertebroplasty group and 3/109 (2.7%) in the facet joint injection group were lost to follow‐up.
Yang 2016 was judged to be at high risk of attrition bias as a greater number of participants were missing from the conservative treatment group compared with the vertebroplasty group (8/64 missing (13%) from vertebroplasty group and 15/66 missing (23%) from conservative treatment group). eight (12.1%) participants in the conservative treatment group received vertebroplasty, and two 2 (3%) had open surgery.
Selective reporting
Four trials were assessed as at low risk of reporting bias (Buchbinder 2009; Dohm 2014; Kallmes 2009; Klazen 2010), although Dohm 2014 included an additional outcome of time (in days) to new clinical vertebral fracture that was not pre‐specified.
Six trials were judged to be at unclear risk of reporting bias because they did not appear to have been registered in a trial registry and did not publish a trial protocol (Chen 2014a; Endres 2012; Liu 2010; Rousing 2009; Sun 2016; Wang 2015; Wang 2016; Yang 2016). In addition Chen 2014a, only reported one‐day outcomes for mean pain.
Blasco 2012 was judged to be at unclear risk of reporting bias because adverse events were only reported for the vertebroplasty group and mean pain and quality of life and confidence intervals were reported graphically only.
Leali 2016 was judged to be at high risk as the trialists did not report summary statistics for pain and function (only the P value to indicate if there were statistical differences between treatments). Further, the trial did not appear to have been registered and no protocol was published.
Farrokhi 2011 was judged to be at unclear risk of reporting bis as it was unclear if any additional outcomes were measured and not reported.
Clark 2016 was judged to be at high risk of reporting bias for several reasons. The primary outcome was inconsistently reported in the published protocol as both the proportion of patients whose numerical rating scale (NRS) pain score reduced from 7/10 (or more) to 4/10 (or less) at 14 days, and the proportion of patients achieving a 14‐day pain score of less than four out of 10. The results paper states that the primary endpoint is the proportion of patients achieving an NRS pain score of less than four out of 10. In addition, several outcomes were pre‐specified in the published protocol but not reported in the results paper and several outcomes reported in the results paper were not pre‐specified.
Evans 2015 was judged to be at high risk of reported bias as adverse events were to be measured but were not reported.
Sun 2016 was judged to be at unclear risk of reporting bias as no registration or protocol were found although all outcomes reported in the methods were presented in the results.
Firanescu 2018 was judged to be at high risk of reporting bias as additional outcomes that were not pre‐planned according to the trial protocol were reported (proportion with pain VAS score ≥ five at 12 months follow up, progressive loss of vertebral body height of treated fractures (defined as ≥4 mm), and incident fractures (not defined).
Vogl 2013 was judged to be at high risk of report bias because in a published congress abstract of the same trial it was reported that pain intensity on a visual analogue scale (VAS) and disability assessed by the Oswestry Disability Index (ODI) were measured, but only baseline pain was presented in the published paper. In addition, the trial registration information could not be located.
Voormolen 2007 was judged to be at high risk of bias because the number of participants with an incident clinical vertebral fracture was only reported for the vertebroplasty group and measures of variance were not reported for continuous outcomes.
VOPE 2015 was judged to be at unclear risk of bias because pain on a NRS and spirometry were not reported.
Other potential sources of bias
No other sources of bias were detected for 11 trials (Blasco 2012; Buchbinder 2009; Farrokhi 2011; Kallmes 2009; Leali 2016; Liu 2010; Sun 2016; Firanescu 2018; Wang 2015; Wang 2016; Yang 2016).
Chen 2014a did not report on the number of 'prophylactic' vertebroplasties that were performed in the vertebroplasty group and did not specify a source of funding.
In Clark 2016, the description of the placebo procedure differed between the published protocol and results papers. The protocol states that a 4‐mm skin incision will be made with light tapping on the skin. The results paper states that a short needle was passed into the skin incision but not as far as the periosteum and that manual skin pressure and regular tapping on the needle was done to mimic vertebroplasty needle advance.
Dohm 2014 was sponsored by a device company which also contributed to study design, data monitoring, statistical analysis and reporting of results including manuscript authorship, paid for independent core laboratory and data safety‐monitoring board services, and terminated the study early.
Although Endres 2012 reported that there were no significant differences in baseline characteristics or planned vertebral treatment levels between treatment groups at baseline, participants in the vertebroplasty group appeared to be older on average than participants in the two other groups (71.3 versus 63.3 and 67.1 years in the balloon and shield vertebroplasty groups, respectively). In addition, participants in the kyphoplasty groups also appeared to have worse pain and disability scores at baseline compared to the vertebroplasty group (vertebroplasty: 78.2 and 68.2; balloon kyphoplasty: 90.0 and 77.0; and shield kyphoplasty 88.16 and 75.7, respectively). They also did not state whether BioMedEs had any role in the study other than funding translation and copy‐editing.
In Evans 2015 the approach, device, and cement used for both the vertebroplasty and kyphoplasty procedures were at the operators’ discretion.
Quality of life and disability were worse at baseline in the vertebroplasty group in Klazen 2010, which may have biased the results in favour of the vertebroplasty group.
In the trial by Rousing 2009, baseline pain was higher in the usual care group (8.8 versus 7.5) and it was only measured in 17/24 and 19/25 participants in the usual care and vertebroplasty‐treated groups, respectively. In addition, participants receiving usual care were hospitalised for longer (11.7 days versus 7.6 days); it is unclear if more pain medication and physiotherapy was offered, and how this would affect outcomes.
Vogl 2013 reported that Soteira Inc. (Natick, MA) funded the trial and provided the Cement Directed Kyphoplasty Systems but whether or not it had any other role in the trial was not explicitly reported.
In Voormolen 2007, eight participants withdrew after randomisation as they were not assigned to their preferred treatment (two in the vertebroplasty group and six in the usual care group).
VOPE 2015 was completed in April 2014 and results have not been published in a peer‐reviewed journal. Baseline pain at rest in the vertebroplasty group was lower than in the placebo group (40.55 (SE 4.55) compared with 53.04 (SE 4.35), P = 0.0476). This may have biased the results in favour of the placebo group.
Effects of interventions
See: Table 1
Benefits
1. Vertebroplasty versus placebo (sham)
We judged the five placebo‐controlled trials to be clinically similar with respect to baseline participant characteristics of mean pain, disability and quality of life, facilitating pooling of data in a meta‐analysis (see Table 3). Mean duration of symptoms ranged from less than three weeks to 20 weeks across treatment groups in the individual trials, however the influence of symptoms duration on treatment effect was investigated in a planned subgroup analysis (see below). The major outcomes for the primary comparison of vertebroplasty versus placebo (sham procedure) is shown in Table 1.
Statistical heterogeneity was moderate in the pooled analysis for pain at one to two weeks (I2 = 35%), driven largely by the data from Clark 2016, the only placebo‐controlled trial to have been judged as having unclear risk of performance bias and detection bias for participant‐reported endpoints. Clark 2016 found a slight benefit in terms of pain relief with vertebroplasty, while the other studies found no evidence of an important difference with treatment. Heterogeneity was unimportant at the other time points.
There was no evidence of important differences between groups with respect to mean pain at one to two weeks (five studies, 539 participants) and a small, clinically unimportant improvement with vertebroplasty at one month (five studies, 535 participants), (mean difference (MD) and 95% confidence interval (95% CI) (MD ‐0.25 0 to 10, 95% CI ‐0.82 to 0.33; I2 = 35%) at one to two weeks and MD ‐0.73 0 to 10, 95% CI ‐1.18 to ‐0.28; I2 = 0%) at one month, on a 0 to 10 point pain scale) (Analysis 1.1).
1.1. Analysis.
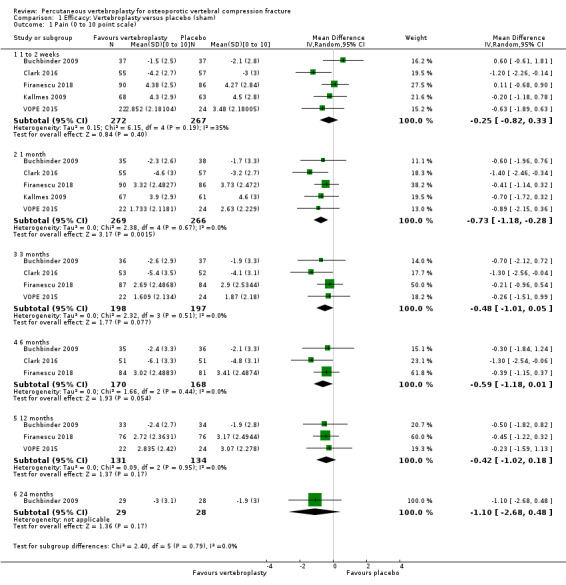
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 1 Pain (0 to 10 point scale).
At one month, mean pain was five points on a zero to 10 scale with placebo and 0.7 points lower (0.3 lower to 1.2 lower) with vertebroplasty, an absolute pain reduction of 7% (3% better to 12% better) and relative reduction of 10% (4% better to 17% better) (Table 1).
Based upon four trials (Buchbinder 2009; Clark 2016; Firanescu 2018; VOPE 2015), there was no evidence of important differences between groups in pain at three months (SMD ‐0.19, 95% CI ‐0.39 to 0.00, 395 participants), which translates to a mean reduction of 0.44 points (95% CI 0.90 to 0) on a 10‐point VAS scale.
Based upon three trials (Buchbinder 2009; Clark 2016; Firanescu 2018), there was no evidence of important differences between groups in pain at six months (SMD ‐0.22 (‐0.43 to ‐0.00), 338 participants), which translates to a mean reduction of 0.51 points (95% CI ‐0.99 to 0.00) on a 10‐point VAS scale. Based upon data from three trials (Buchbinder 2009; Firanescu 2018; VOPE 2015), there was no evidence of important differences between groups in pain at 12 months (SMD ‐0.17 (‐0.41 to 0.07, 265 participants), which translates to a mean reduction of 0.37 points (95% CI ‐0.94 to 0.16) on a 10‐point VAS scale; and based on data from a single trial (Buchbinder 2009), no differences at 24 months were observed (MD ‐1.10 points on a zero to 10 VAS scale, 95% CI ‐2.68 to 0.48, 57 participants), although the lower limit of the 95% CI means that an important difference can neither be confirmed or excluded.
We combined data from three trials that included a measure of the proportion of participants whose pain improved from baseline by a 'clinically important' amount although the definitions varied by trial (> 2.5 units from baseline (Buchbinder 2009), 30% or more from baseline (Kallmes 2009), four or less out of 10 (Clark 2016; Firanescu 2018)). Statistical heterogeneity was moderate to substantial at one to two weeks (I2 = 51%), and one month (I2 = 61%), but was unimportant at other time points that included combined data. Similar to the data for mean pain, this appeared to be driven by data from Clark 2016, which showed a benefit in terms of this outcome, while the other studies found no evidence of important differences with treatment. There was a benefit of vertebroplasty only at three, six and 12 months although the effect estimates were all greater than one: one to two weeks (data from two trials (Buchbinder 2009; Clark 2016): vertebroplasty: 38/99 versus placebo: 26/99, RR 1.43, 95% CI 0.78 to 2.60); one month (data from three trials (Buchbinder 2009; Clark 2016; Kallmes 2009): vertebroplasty: 89/166 versus placebo: 56/160, RR 1.53, 95% CI 0.99 to 2.36); three months (data from two trials (Buchbinder 2009; Clark 2016): vertebroplasty: 48/99 versus placebo: 30/99, RR 1.60, 95% CI 1.12 to 2.30); six months (data from two trials (Buchbinder 2009; Clark 2016): vertebroplasty: 54/99 versus placebo: 39/99, RR 1.38, 95% CI 1.02 to 1.87); 12 months (data from two trials (Buchbinder 2009; Firanescu 2018): vertebroplasty: 75/114 versus placebo: 59/116, RR 1.29, 95% CI 1.06 to 1.58); and 24 months (data from one trial (Buchbinder 2009): vertebroplasty: 19/38 versus placebo: 14/40, RR 1.43, 95% CI 0.84 to 2.42)(Analysis 1.2). These results may be sensitive to different cut‐off values (e.g. if Clark 2016 had utilised a cut‐off pain score of four or less out of 10 for this outcome as also stated in their protocol, rather than < 4 out of 10).
1.2. Analysis.
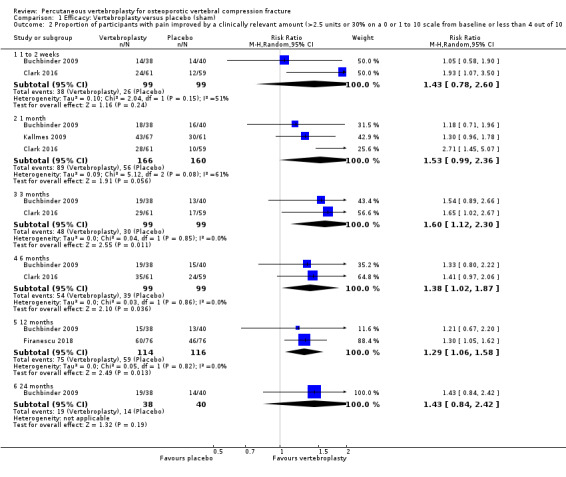
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 2 Proportion of participants with pain improved by a clinically relevant amount (>2.5 units or 30% on a 0 or 1 to 10 scale from baseline or less than 4 out of 10.
We could combine data for up to four trials at any time point for back‐related disability (Buchbinder 2009; Clark 2016; Kallmes 2009; Firanescu 2018). Statistical heterogeneity for disability was moderate to substantial in the pooled analysis at one to two weeks (I2 = 41%) and three months (I2 = 59%), and substantial to considerable six months (I2 = 62%) but was zero at one month.
With respect to disability (measured with the RMDQ [zero to 23 scale]), there was a small, clinically unimportant improvement with vertebroplasty at one month (4 studies, 472 participants, MD ‐1.50, 95% CI ‐2.61 to ‐0.38).
At one to two weeks (four studies, 475 participants), the MD 0.21 points (95% CI ‐1.23 to 1.65); at three months (three studies, 333 participants) the MD was ‐0.81 points (95% CI ‐3.15 to 1.52); and at six months (three studies, 324 participants), the MD was ‐1.82 points (95% CI ‐4.12 to 0.47) (Analysis 1.3). While the lower 95% CIs in the analyses at three and six mohts means that an important difference between groups can neither be confirmed or refuted, these results were largely driven by the data from Clark 2016, which had unclear risk of performance bias and detection bias for participant‐reported endpoints.
1.3. Analysis.
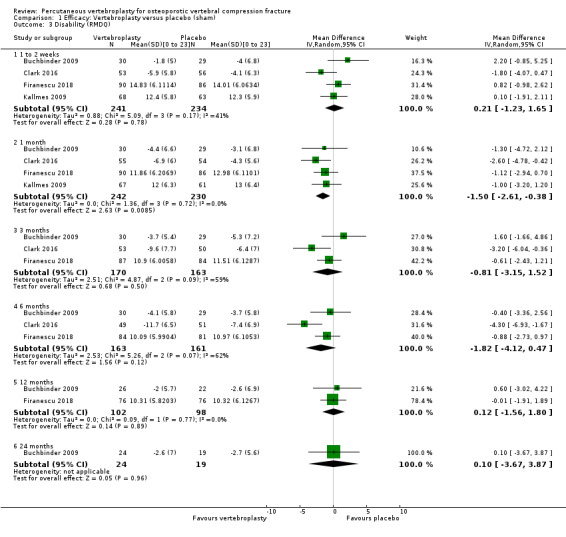
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 3 Disability (RMDQ).
There was no evidence of important between‐group differences in RMDQ scores at 12 months, based opon data from two trials (Buchbinder 2009; Firanescu 2018) (MD 0.12, 95% CI ‐1.56 to 1.80; 200 participants) and 24 months based upon data from one trial (Buchbinder 2009; Analysis 1.3). Data from the fifth trial did not measure disability by the RMDQ, but did report SF‐36 physical function data for three and 12 months and also observed no important differences between groups (data not shown) (VOPE 2015).
At one month, the mean RMDQ was 14 points with placebo group and 1.5 points lower (0.4 lower to 2.6 lower) in the vertebroplasty group, an absolute improvement in disability of 7% (1% better to 11% better) and relative improvement of 9% (2% better to 15% better) (Table 1).
We could combine data from up to three trials at any time point for vertebral fracture or osteoporosis‐specific health‐related quality of life (measured by the QUALEFFO). Statistical heterogeneity was unimportant at one month, six months and 12 months (I2 < 40%) and moderate at one to two weeks follow‐up (I2 = 49%) and six three months (I2 = 48%) There was no difference in vertebral fracture or osteoporosis‐specific health‐related quality of life (measured by the QUALEFFO) between vertebroplasty compared with placebo at one to two weeks (three studies, 352 participants): MD ‐3.10 (95% CI ‐6.96 to 0.75); at one month (three studies, 351 participants): MD ‐2.33, 95% CI ‐6.06 to 1.41); at three months (two studies, 244 participants): MD ‐0.42 (95% CI ‐4.23 to 3.39); at six months (three studies, 330 participants): MD ‐1.88 (95% CI ‐6.66 to 2.91); at 12 months (two studies, 219 participants): MD was ‐1.32 (95% CI ‐5.41 to 2.77); and at 24 months (one study, 57 participants), the MD was 1.30 (95% CI ‐5.48 to 8.08) at (Analysis 1.4).
1.4. Analysis.
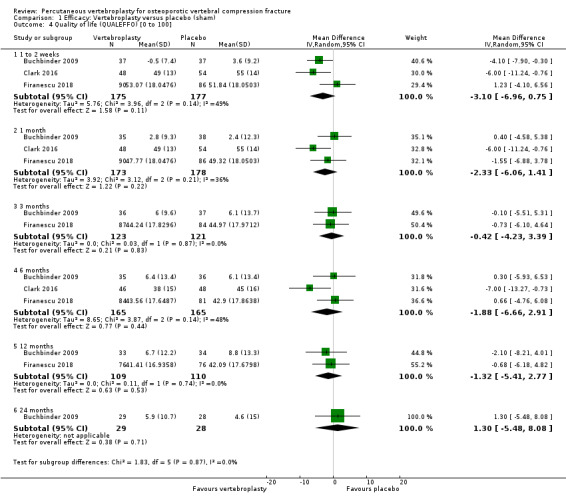
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 4 Quality of life (QUALEFFO) [0 to 100].
At one month, mean QUALEFFO was 62 points (0 to 100 point scale, where 0 is best quality of life) with placebo and 2.3 points better (1.4 points worse to 6.7 points), an absolute imrovement of 2% (1% worse to 6% better); relative improvement 4% better (2% worse to 10% better (Table 1).
One trial found no evidence of important differences between groups in treatment success up to 24 months (Analysis 1.5).
1.5. Analysis.
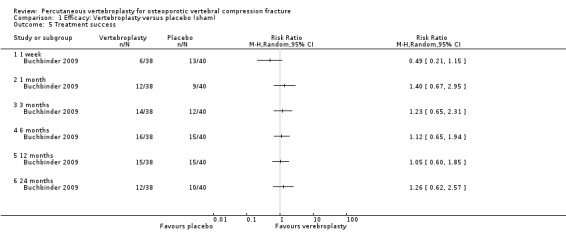
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 5 Treatment success.
There were no important differences between groups with respect to overall quality of life (measured with the EQ5D): MD 0.01 (95% CI ‐0.03 to 0.05) at one to two weeks (two studies, 164 participants); MD 0.05 (95% CI 0.01 to 0.09); at one month (three studies, 285 participants); MD 0.04 (95% CI ‐0.00 to 0.08); at three months (three studies, 203 participants); MD 0.06 (95% CI 0.01 to 0.10); at six months (two studies, 156 participants); MD ‐0.05 (95% CI ‐0.17 to 0.07); at 12 months (two studies, 93 participants); MD 0.00 (95% CI ‐0.24 to 0.24); at 24 months (one study, 44 participants) (Analysis 1.6).
1.6. Analysis.
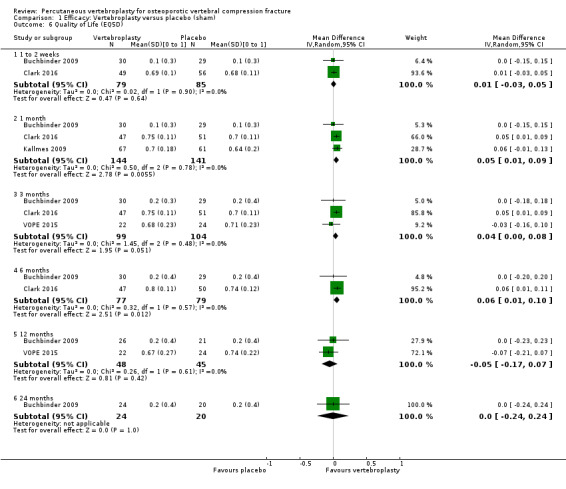
Comparison 1 Efficacy: Vertebroplasty versus placebo (sham), Outcome 6 Quality of Life (EQ5D).
2. Vertebroplasty versus usual care
The eight trials that compared vertebroplasty with usual care included participants with similar levels of baseline pain and disability and gender distribution was also similar across trials (see Table 3). Although one trial included younger participants (Chen 2014a), and the duration of symptoms varied from one week to six months, we judged that the trials were sufficiently clinically homogenous to allow data to be pooled. Data for pain and function from Leali 2016 could not be included in meta‐analyses as means and variance were not reported. In instances where analyses within a single data plot required a mix of MD and SMD analyses, we have shown the SMD in the plots and present both the SMD and MD in the results. For clarity, we have indicated where the MD was back‐transformed from the SMD to MD (either because Farrokhi 2011 was included together with other trials in the same meta‐analysis for mean pain or because both RMDQ and ODI were included in the same meta‐analysis for disability).
Based upon data from up to six trials, participants in the vertebroplasty group had greater improvement in mean pain compared with those in the usual care group at one to two weeks (six trials, 627 participants, SMD ‐1.33 (95% CI ‐2.26 to ‐0.39), back‐transformed MD ‐2.13 (95% CI ‐3.62 to ‐0.62); one month (three trials, 384 participants), SMD ‐2.06 (95% CI ‐3.35 to ‐0.76), back‐transformed MD ‐3.30 (95% CI ‐5.36 to ‐1.22); three months (six trials, 627 participants, SMD ‐1.18 (95% CI ‐1.95 to ‐0.40), back‐transformed MD ‐1.89 (95% CI ‐3.12 to ‐0.64); six months (five trials, 573 participants, SMD ‐1.05 95% CI ‐1.82 to ‐0.28), back‐transformed MD ‐1.68 (95% CI ‐2.91 to ‐0.45); and 12 months (six trials, 612 participants, SMD ‐1.02 (95% CI ‐1.74 to ‐0.30), back‐transformed MD ‐1.63 (95% CI ‐2.78 to ‐0.48) (Analysis 2.1). However there was considerable statistical heterogeneity across all pooled pain analyses with the I2 varying between 94% and 96%. Removing single trials from each analysis did not appreciably alter the results. At 24 months there was no evidence of important differences between groups in mean pain based upon one trial (77 participants, SMD ‐0.45 (95% CI ‐0.90 to 0.01) or MD ‐0.90 (95% CI ‐1.79 to ‐0.01).
2.1. Analysis.
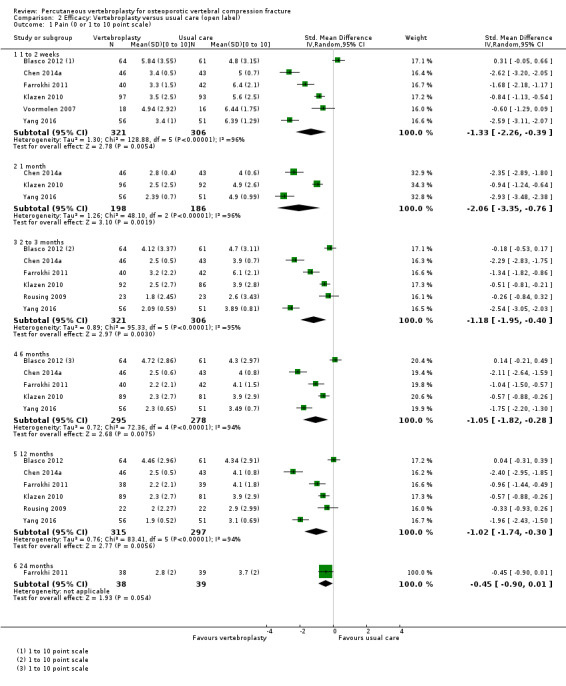
Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 1 Pain (0 or 1 to 10 point scale).
There was no evidence of important differences between groups in the proportion of participants who reported moderate or severe residual pain at 12 months (vertebroplasty: 36% and 19%; usual care 34% and 18%, respectively) in one trial (Blasco 2012).
Based upon data from up to five trials, improvement in disability also favoured the vertebroplasty group at one to two weeks (five trials, 494 participants) SMD ‐2.06 (95% CI ‐3.28 to ‐0.83), back‐transformed (0 to 23 RMDQ scale) MD ‐8.65 (95% CI ‐13.78 to ‐3.49); one month (three trials, 378 participants) SMD ‐1.52 (95% CI ‐3.00 to ‐0.04), back‐transformed MD ‐6.38 (95% CI ‐12.60 to ‐0.17); three months (four trials, 460 participants) SMD ‐2.76 (95% CI ‐4.65 to ‐0.87), back‐transformed MD ‐11.59 (95% CI ‐19.53 to ‐2.09); six months (four trials, 461 participants) SMD ‐1.84 (95% CI ‐3.37 to ‐0.30), back‐transformed MD ‐7.73 (95% CI ‐14.15 to ‐1.26); 12 months (four trials, 455 participants) SMD ‐1.59 (95% CI ‐2.79 to ‐0.38), back transformed MD ‐6.68 (95% CI ‐11.72 to ‐0.91); and 24 months (one trial, 77 participants) SMD ‐5.65 (95% CI ‐6.67 to ‐4.63) and MD ‐12.00 points on 0 to 100 ODI scale (95% CI ‐12.94 to ‐11.06) (Analysis 2.2). Considerable statistical heterogeneity was also present for all analyses (I2 ranging from 97% to 98%). Data from the sixth trial did not measure disability by the RMDQ or ODI, but did report SF‐36 physical function data for three and 12 months and observed no important differences between groups (data not shown) (Rousing 2009).
2.2. Analysis.
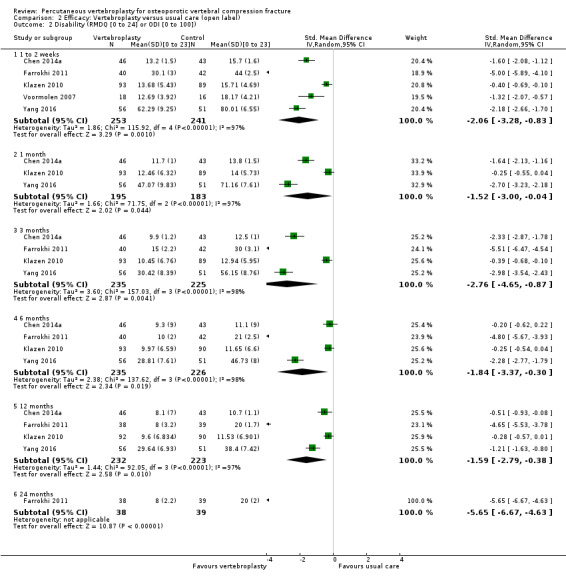
Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]).
There was no significant between‐group differences with respect to vertebral fracture or osteoporosis‐specific quality of life at any time point measured by the QUALEFFO, based upon data from up to four trials (one to two weeks: four trials, 448 participants, MD ‐5.67 (95% CI ‐11.65 to 0.32); one month: two trials, 289 participants, MD ‐10.18 (95% CI ‐21.49 to 1.13); three months: three trials, 415 participants, MD ‐5.83 (95% CI ‐15.41 to 3.75); six months: three trials, 415 participants, (MD ‐5.14 (95% CI ‐15.02 to 4.74); and 12 months: three trials, 415 participants, MD ‐3.40 (95% CI ‐9.90 to 3.11)) (Analysis 2.3). Considerable statistical heterogeneity was also present for all analyses (I2 varying between 83% and 95%).
2.3. Analysis.
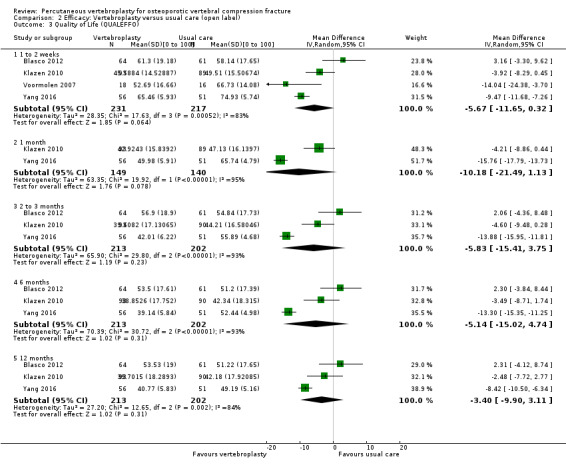
Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 3 Quality of Life (QUALEFFO).
Overall quality of life measured by the EQ‐5D marginally favoured the vertebroplasty group at one to two weeks (one trial, 183 participants, MD 0.08 (95% CI 0.00 to 0.15)); one month (one trial, 183 participants, MD 0.09 (95% CI 0.01 to 0.16)); and three months (two trials, 215 participants, MD 0.10 (95% CI 0.00 to 0.20)), but not six months (one trial, 183 participants, MD 0.07 (95% CI ‐0.02 to 0.15)) or 12 months (two trials, 215 participants, MD 0.07 (95% CI ‐0.00 to 0.14))(Analysis 2.4). Statistical heterogeneity was unimportant for the pooled analyses (I2 0% to 22%).
2.4. Analysis.
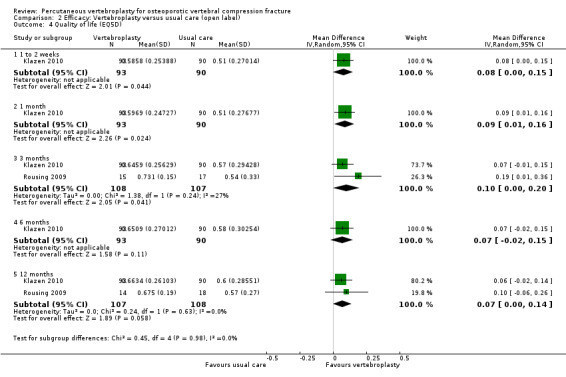
Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 4 Quality of life (EQ5D).
Treatment success was reported in one trial (Yang 2016) at 12 months (RR 1.43, 95% CI 1.03 to 1.98).
3. Vertebroplasty versus kyphoplasty
No efficacy data relevant to this review could be extracted from Vogl 2013. The aim of this trial was to compare leakage rates between treatment groups and only vertebral height and wedge angle were measured as efficacy outcomes. Only one of the four trials reported an a priori sample size calculation (Dohm 2014). However this trial was terminated after recruiting 404 of the planned sample size of 1234 participants.
Based upon data from up to four trials, there was no evidence of important differences between groups in pain at all time points: MD ‐0.06 (95% CI ‐0.37 to 0.25) at one to two weeks (two trials, 462 participants); MD ‐0.05 (95% CI ‐0.59 to 0.48) at one month (two trials, 441 participants); MD 0.14 (95% CI ‐0.11 to 0.39) at three months (two trials, 419 participants); MD ‐0.04 (95% CI ‐0.31 to 0.22) at six months (three trials, 230 participants); MD 0.16 (95% CI ‐0.07 to 0.40) at 12 months (four trials, 558 participants); MD ‐0.15 (95% CI ‐0.56 to 0.27) at 24 months (two trials, 320 participants) (Analysis 3.1). Statistical heterogeneity was unimportant for all analyses (I2 varying between 0% and 7%).
3.1. Analysis.
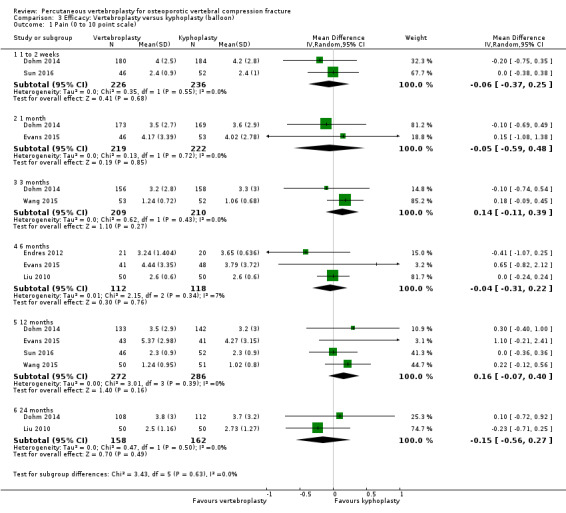
Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 1 Pain (0 to 10 point scale).
Based upon data from up to four trials, there was no evidence of important differences between groups in degree of improvement in disability at all time points: one to two weeks (one trial, 98 participants) MD (0 to 100 ODI scale) 0.10 95% CI ‐2.12 to 2.32); one month (two trials, 425 participants) SMD ‐0.11 (95% CI ‐0.30 to 0.08), back‐transformed to 0 to 23 RMDQ scale: MD ‐0.73 (95% CI ‐1.98 to 0.53); three months (two trials, 399 participants) MD 0.52 (95% CI ‐1.53 to 2.58); six months (one trial, 93 participants) SMD ‐0.06 (95% CI ‐0.46 to 0.35), back‐transformed MD ‐0.40 (95% CI ‐3.03 to 2.31); 12 months (four trials, 542 participants) SMD 0.00 (95% CI ‐0.16 to 0.17), back‐transformed MD 0.00 (95% CI ‐1.06 to 1.12); and 24 months (one trial, 201 participants) MD ‐1.30 (95% CI ‐6.45 to 3.85) (Analysis 3.2). Statistical heterogeneity was unimportant for all analyses (I2 = 0%).
3.2. Analysis.
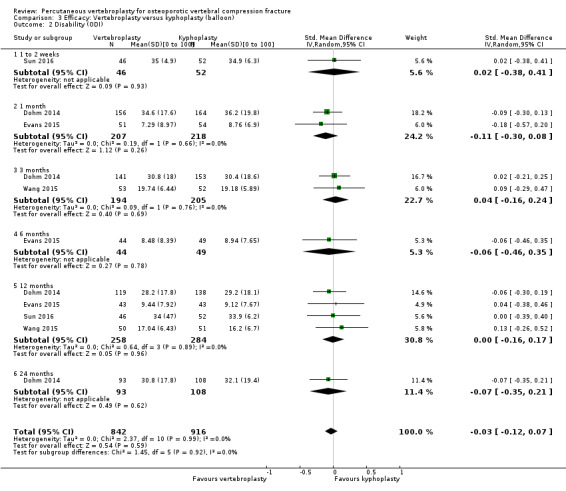
Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 2 Disability (ODI).
Based upon up to two trials, there was also no evidence of important differences between groups in degree of improvement in overall quality of life at one, three, six, 12 or 24 months (Analysis 3.3).
3.3. Analysis.
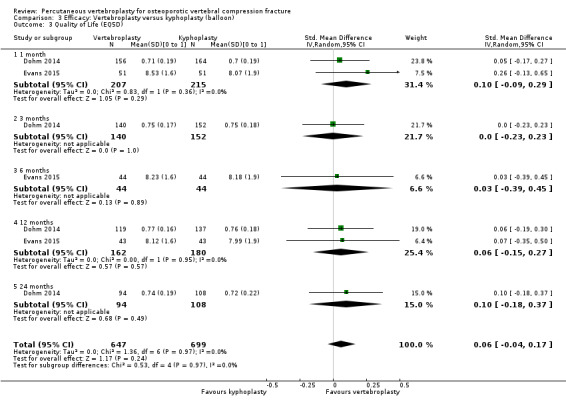
Comparison 3 Efficacy: Vertebroplasty versus kyphoplasty (balloon), Outcome 3 Quality of Life (EQ5D).
4. Vertebroplasty versus facet joint injection
Based upon one trial that compared vertebroplasty with facet joint injection with glucocorticosteroid and local anaesthetic, vertebroplasty was reported to be superior to facet joint injection with respect to improvement in pain on a zero‐ to 10‐point scale (MD ‐1.61, 95% CI ‐1.84 to ‐1.38, 206 participants) and disability on the RMDQ 0 to 23‐point scale (MD ‐3.42, 95%CI ‐3.72 to ‐3.12, 206 participants) at one week (Analysis 4.1; Analysis 4.2). There was no evidence of important differences between groups at other time points up to 12 months in pain or disability or quality of life (not reported at the one‐ to two‐week time point).
4.1. Analysis.
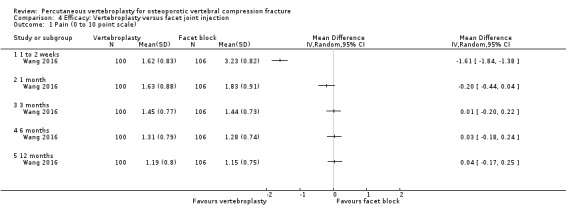
Comparison 4 Efficacy: Vertebroplasty versus facet joint injection, Outcome 1 Pain (0 to 10 point scale).
4.2. Analysis.
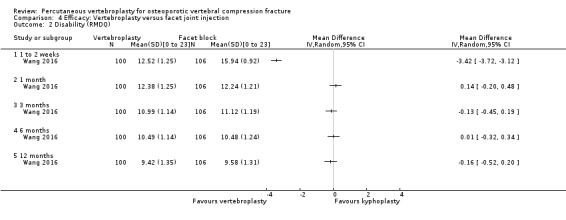
Comparison 4 Efficacy: Vertebroplasty versus facet joint injection, Outcome 2 Disability (RMDQ).
Harms
1. Vertebroplasty versus placebo (sham) or usual care
New clinically apparent and new radiographic fractures
Based upon six trials (control group was placebo for one trial and usual care for the other trials), there were slightly more new clinically apparent vertebral fractures up to 12 to 24 months in the vertebroplasty group (48 fractures in 418 participants (pooled incidence 95 per 1000) in comparison with the control group (31 fractures in 422 participants (73 per 1000)), but this was not statistically significant (RR 1.29 (95% CI 0.46 to 3.62)) (Analysis 5.1). There was substantial statistical heterogeneity (I2 70%), and clinical diversity: apart from different comparators, the trials also varied in terms of the age of participants (average age was younger in one trial), duration of follow up, and symptom duration which varied from a mean of nine to 20 weeks.
5.1. Analysis.
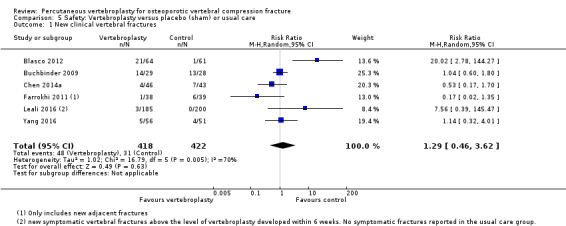
Comparison 5 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 1 New clinical vertebral fractures.
There were no evidence of important differences between groups in the number of new radiographic vertebral fractures up to 12 to 24 months based upon eight trials (vertebroplasty: 110 fractures in 411 participants (26.8%), control: 93 fractures in 393 participants (23.7%); RR 1.14 (95% CI 0.71 to 1.84) (control was placebo in four trials and usual care in four trials) (Analysis 5.2). However there was substantial statistical heterogeneity in the pooled analysis (I2 67%). Similar to the above analyses, other than the comparators, participants in the individual trials differed mainly with respect to symptom duration (ranging from mean duration of less than a week to 20 weeks).
5.2. Analysis.
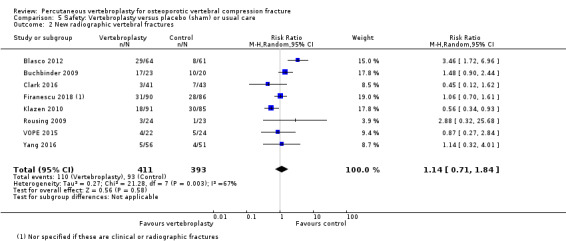
Comparison 5 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 2 New radiographic vertebral fractures.
Other serious adverse events
Data from the three placebo‐controlled trials (Buchbinder 2009; Clark 2016; Kallmes 2009) and two usual care‐controlled trials (Leali 2016; Yang 2016) could be pooled. Based upon these trials, there were no significant between‐group differences in the number of other serious adverse events (vertebroplasty: 16/408, placebo: 23/413; RR 0.61 (95% CI 0.33 to 1.10)(Analysis 5.3). Excluding Leali 2016, who reported serious adverse events that appeared unrelated to the treatment (e.g. depression, pneumonia, sleep disturbance), the results trended towards favouring the control group RR 1.26 (95% CI 0.41 to 3.88). However, several trials reported serious adverse events related to vertebroplasty.
5.3. Analysis.
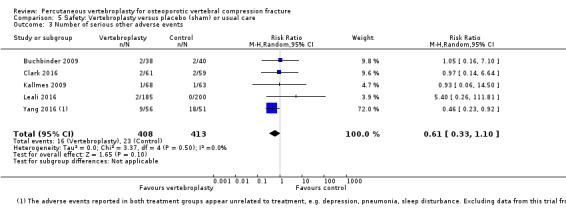
Comparison 5 Safety: Vertebroplasty versus placebo (sham) or usual care, Outcome 3 Number of serious other adverse events.
Buchbinder 2009 reported that three participants reported new rib fractures (one in the vertebroplasty group and two in the placebo group) and one participant who received vertebroplasty developed an adjacent new fracture and osteomyelitis requiring surgical drainage and antibiotic therapy. Nine other adverse events were reported in the vertebroplasty group up to one month (chest pain (three participants), pain or burning in thigh or leg (three participants), tightness in the back or rib cage (one participant), stomach pain (one participant), increased pain or muscle cramping around puncture site (one participant)) compared to one in the placebo group (pain or burning in thigh or leg (one participant)).
Clark 2016 reported that one patient had a respiratory arrest after administration of sedation, before starting the procedure. The patient was resuscitated and underwent the trial procedure two days later without incident. Another patient sustained a supracondylar humerus fracture in a paretic arm during transfer onto the radiology table. The fracture healed with a plaster cast.
Kallmes 2009 reported that one participant in the vertebroplasty group had an injury to the thecal sac during the procedure requiring hospitalisation while one participant in the placebo group was also hospitalised overnight due to tachycardia and rigours of unknown cause.
Klazen 2010 reported that two participants required atropine because of pain‐induced vasovagal reactions during the vertebroplasty procedure and a third participant developed an acute asthma exacerbation that led to stopping the procedure, although it was successfully completed one week later. These data could not be included in the pooled analysis as no adverse event data were reported for the usual care group. They also reported that asymptomatic cement leakage occurred in 97 of the 134 treated vertebrae (72%); most leakages were discal or into segmental veins and in one participant there was asymptomatic cement migration towards the lungs.
Voormolen 2007 reported that one participant in the usual care group who crossed over and received vertebroplasty after two weeks developed acute pain following the procedure due to an intrapedicular cement spur that broke upon manipulation by the bone biopsy needle causing a small cortical chip fracture at the medial border of the pedicle.
Farrokhi 2011 reported that one participant who received vertebroplasty developed severe right lower extremity pain and weakness due to epidural cement leakage which required immediate decompression through a bilateral laminectomy and evacuation of bone cement. After two months there was no radicular pain and the participant could walk unassisted. They reported that there were no instances of venous emboli or infection.
Blasco 2012 reported that asymptomatic cement leakage into the veins/discs during vertebroplasty occurred in 49% of procedures.
Chen 2014a reported that asymptomatic cement leakage occurred in 36 out of 69 (52%) treated vertebrae: intervertebral disc in eight participants (22%), puncture path in seven participants (19%), paravertebral space in nine participants (25%) and venous leakage in 12 participants (33%). There was asymptomatic cement migration into the venous system towards the lungs in two participants (2.9%).
2. Vertebroplasty versus kyphoplasty
New clinically apparent and radiographic fractures
Dohm 2014 reported 50/190 new clinically apparent vertebral fractures among participants who received vertebroplasty and 38/191 among those who received kyphoplasty over the course of the trial (RR 1.32 (95% CI 0.91 to 1.92)) (Analysis 6.1). Seventeen new clinical fractures were reported to have occurred within 30 days of vertebroplasty and nine within 30 days of kyphoplasty. One participant developed a new symptomatic vertebral fracture within two days of receiving vertebroplasty at the level below the procedure associated with inferior cement leakage, and it was considered to be possibly bone cement‐related.
6.1. Analysis.
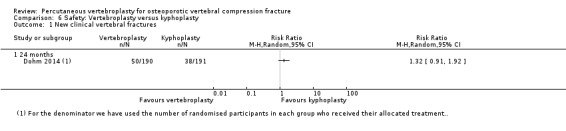
Comparison 6 Safety: Vertebroplasty versus kyphoplasty, Outcome 1 New clinical vertebral fractures.
There was no evidence of important differences between groups in the number of new radiographic vertebral fractures at 12 months based upon two trials (vertebroplasty: 58 fractures in 181 participants (32.0%), control: 54 fractures in 191 participants (28.3%); RR 0.81 (95% CI 0.21 to 3.17)(Analysis 6.2). At 24 months, based upon data from one trial (Dohm 2014) there were reported to be 64/111 new radiographic vertebral fractures in the vertebroplasty group and 54/110 in the kyphoplasty group (RR 1.17 (95% CI 0.92 to 1.51)). The denominators reported in both these analyses differed from the numbers reported to have completed 12‐ and 24‐month follow‐up in both groups according to the flow diagram in the published reports.
6.2. Analysis.
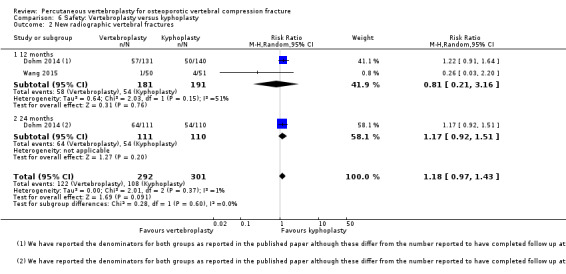
Comparison 6 Safety: Vertebroplasty versus kyphoplasty, Outcome 2 New radiographic vertebral fractures.
Endres 2012 reported that there were no adjacent vertebral fractures in any of the three treatment groups (vertebroplasty, balloon or shield kyphoplasty) up to six months.
Vogl 2013 reported that one participant in the shield kyphoplasty group experienced a symptomatic refracture at the treated level (and was retreated with vertebroplasty), while there were no refractures in the vertebroplasty group up to 12 months. They also reported that three adjacent level fractures were detected in one participant in the vertebroplasty group (3/39 levels treated, 7.7%) compared with two adjacent fractures in two participants (2/65 levels treated, 3.1%) in the kyphoplasty group up to 12 months.
Other serious adverse events
Dohm 2014 reported device/procedure/anaesthesia‐related (or possibly related) adverse events among 11 participants in the vertebroplasty group and 12 in the kyphoplasty group (RR 0.91, 95% CI 0.42 to 1.97, Analysis 6.3). Most of these occurred within 30 days of the procedures. Adverse events considered to be serious and related (or possibly related) to the anaesthesia included constipation (one kyphoplasty), procedural hypotension (one kyphoplasty), nausea/vomiting (one kyphoplasty), hypersensitivity (one vertebroplasty), postoperative change in mental status (one vertebroplasty), hallucination (one kyphoplasty), exacerbation chronic airways disease (one kyphoplasty), hypoxia (one vertebroplasty), and respiratory failure (one vertebroplasty). Adverse events considered serious and bone cement‐related (or possibly related) included symptomatic cement embolism (one in each group). Three participants in each group had procedural pain considered to be device‐related (or possible related) adverse events (4/4 and 2/3 were judged as serious events in the vertebroplasty and kyphoplasty groups, respectively). Other adverse events reported to be considered serious and device‐related (or possible related) included spinal fracture (one kyphoplasty), symptomatic vertebral fracture (two vertebroplasty, one kyphoplasty) (the distinction between these two adverse events was not clear), back pain (three vertebroplasty, two kyphoplasty), arthralgia (one kyphoplasty) and muscle spasm (one kyphoplasty).
6.3. Analysis.
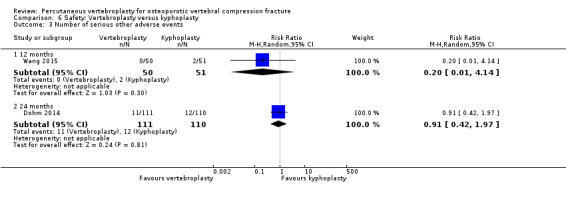
Comparison 6 Safety: Vertebroplasty versus kyphoplasty, Outcome 3 Number of serious other adverse events.
Additionally, one participant who received vertebroplasty developed implant site extravasation into the spinal canal considered non serious. A computed tomography (CT) scan demonstrated no significant canal stenosis and the participant required no medical intervention. One participant who received vertebroplasty also developed a haematoma, considered non serious and possibly related to the procedure as a result of lying prone on the operating table. No deaths were noted as device‐ or procedure‐related.
Wang 2015 reported that there were no other serious adverse events in the vertebroplasty group but two in the kyphoplasty group (RR 0.20, 95% CI 0.01 to 4.14, Analysis 6.3). One patient experienced severe discogenic back pain related to a disk leak and finally underwent discectomy with posterior spinal fusion. Another patient experienced an asymptomatic cement emboli in the right lung related to venous leakage.
Endres 2012 reported that no clinically relevant adverse events were observed in any of the three treatment groups. No clinically relevant complications arose from cement leakage in any of the three treatment groups and there were no significant differences in cement leakage between groups (vertebroplasty: four lateral and four disc leakages; balloon kyphoplasty: three lateral and one disc; shield kyphoplasty: one disc).
Sun 2016 reported cement leakage in 9/54 (17%) vertebrae treated with vertebroplasty and 11/60 (18%) vertebrae treated with kyphoplasty.
Vogl 2013 reported that two participants treated with vertebroplasty and four treated with shield kyphoplasty died during the course of the trial from causes unrelated to the interventions. No clinically relevant complications (or symptoms) were reported to have arisen from cement leakage in either treatment group. Shield kyphoplasty was reported to have resulted in significantly fewer cement leaks overall (six levels had multiple leaks with 42 total leaks compared with 12 levels with multiple leaks, total 54 leaks in the vertebroplasty group, P value reported 0.0132) and cement leaks per level (data only showed graphically in percentages, P value 0.0012), compared with the vertebroplasty group.
4. Vertebroplasty versus facet joint injection
New fractures
One trial that evaluated vertebroplasty versus facet joint injection reported no difference in incident radiographic vertebral fractures at 12 months (13/100 in vertebroplasty group versus 11/106 in the facet joint injection group (RR 1.25; 95% CI 0.59 to 2.67; Analysis 7.1).
7.1. Analysis.
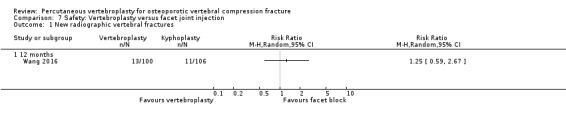
Comparison 7 Safety: Vertebroplasty versus facet joint injection, Outcome 1 New radiographic vertebral fractures.
Subgroup Analysis
Data from up to five placebo‐controlled trials were available for subgroup analysis comparing participants with pain duration 'acute' versus 'subacute'. Three trials only included acute fractures. This was defined as pain less than 6 weeks in Clark 2016, and 8 weeks or less in VOPE 2015. Firanescu 2018 altered their inclusion criteria from 6 weeks or less to 9 weeks or less after 6 months due to poor recruitment. Both Buchbinder 2009 and Kallmes 2009 performed post‐hoc subgroup analyses comparing data for participants with symptom duration of 6 weeks or less compared to more than 6 weeks.
The subgroup analysis found no important differences in outcomes for acute versus subacute pain with respect to pain at one to two weeks (Analysis 8.1) or one month (Analysis 8.2), disability at one to two weeks (Analysis 8.3) or one month (Analysis 8.4), or quality of life at one month (Analysis 8.5).
8.1. Analysis.
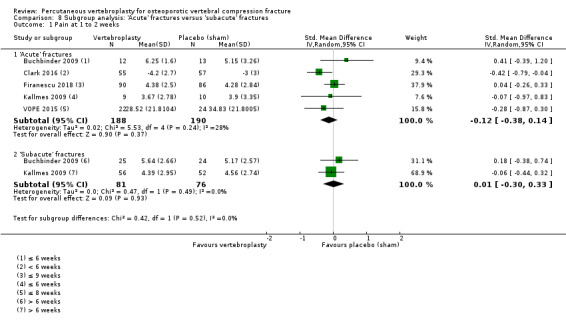
Comparison 8 Subgroup analysis: 'Acute' fractures versus 'subacute' fractures, Outcome 1 Pain at 1 to 2 weeks.
8.2. Analysis.
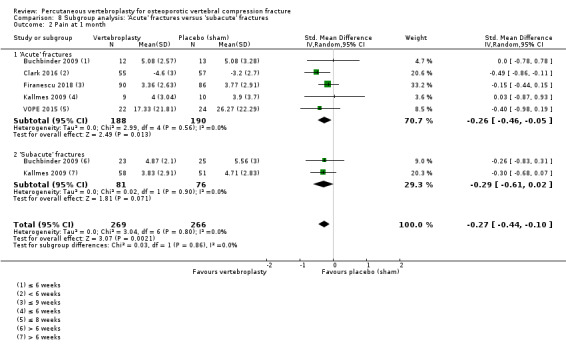
Comparison 8 Subgroup analysis: 'Acute' fractures versus 'subacute' fractures, Outcome 2 Pain at 1 month.
8.3. Analysis.
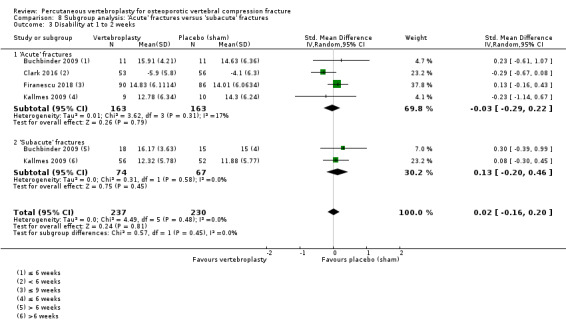
Comparison 8 Subgroup analysis: 'Acute' fractures versus 'subacute' fractures, Outcome 3 Disability at 1 to 2 weeks.
8.4. Analysis.
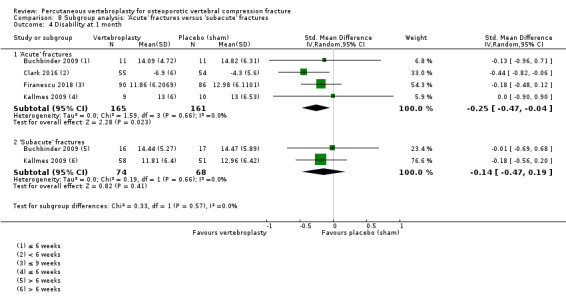
Comparison 8 Subgroup analysis: 'Acute' fractures versus 'subacute' fractures, Outcome 4 Disability at 1 month.
8.5. Analysis.
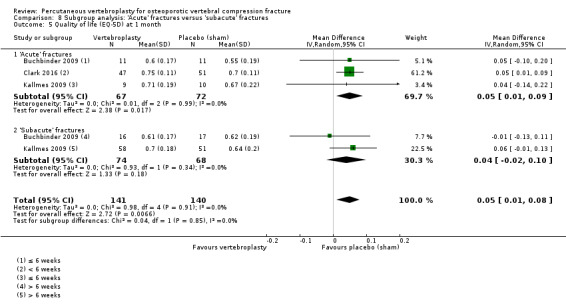
Comparison 8 Subgroup analysis: 'Acute' fractures versus 'subacute' fractures, Outcome 5 Quality of life (EQ‐5D) at 1 month.
If we make an assumption that 'acute' fractures could only be defined as pain for 6 weeks or less, and thus exclude data from Firanescu 2018 and VOPE 2015 from these analyses, this does not change the findings ‐ there are still no important differences in outcomes between participants with 'acute' fractures and those with 'subacute' fractures.
Sensitivity Analyses
Including open trials comparing vertebroplasty with usual care altered the results, and the considerable statistical heterogeneity makes interpretation of these results difficult. In comparison to the lack of important between‐group differences when only placebo‐controlled trials were included in analyses, the addition of trials with a usual care control resulted in the analysis favouring vertebroplasty for all endpoints assessed in the sensitivity analysis with some differences possibly reaching clinically importance (pain at one to two weeks (11 trials, 1166 participants, SMD ‐0.76 (‐1.30 to ‐0.22), back‐transformed MD ‐1.75 (95% CI ‐2.99 to ‐0.51), I2 95%); one month (eight trials, 919 participants, SMD ‐0.94 (‐1.55 to ‐0.34), back‐transformed MD ‐2.16 (95% CI ‐3.57 to ‐0.78), I2 95%); and three months (10 trials, 1021 participants, SMD ‐0.78 (‐1.28 to ‐0.29), back‐transformed MD ‐1.79 (95% CI ‐2.94 to ‐0.67), I2 93%); disability at one to two weeks (eight trials, 793 participants, SMD ‐1.25 (‐2.06 to ‐0.43), back‐transformed MD ‐3.63 (95% CI ‐5.97 to ‐1.25) and I2 96%); one month (six trials, 674 participants, SMD ‐0.88 (‐1.60 to ‐0.17), back‐transformed MD ‐2.55 (95% CI ‐4.64 to 0.0.49), I2 95%) and three months (six trials, 622 participants, SMD ‐1.85 (‐3.08 to ‐0.61), back‐transformed MD ‐5.37 (95% CI ‐8.93 to ‐1.77), I2 98%).
Discussion
Summary of main results
Vertebroplasty versus placebo (sham procedure)
Compared with placebo (sham vertebroplasty), high‐quality evidence based upon five trials (541 randomised participants, one trial with incomplete data reported) indicates that vertebroplasty provides no clinically important benefits with respect to pain, disability or disease‐specific quality of life, and moderate‐quality evidence of no benefits in terms of overall quality of life or treatment success up to one month (Table 1).
We did consider downgrading the evidence for all outcomes due to potential publication bias, however there is only one additional placebo‐controlled trial for which results are unavailable. We suspect that if the evidence from this trial becomes available, the conclusion is unlikely to change substantially. The evidence for quality of life and treatment success was downgraded from high to moderate due to imprecision but it is also unlikely that further data will alter our conclusions for these outcomes.
At one month, mean pain (on a scale zero to 10, higher scores indicate more pain) was five points with placebo and 0.6 points better (0.2 better to 1 better) with vertebroplasty, an absolute pain reduction of 6% (2% better to 10% better, minimal clinical important difference is 15%) and relative reduction of 8% (3% better to 14% better) (five trials, 535 participants). At one month, mean disability measured by the Roland‐Morris Disability Questionnaire (RMDQ) (scale range zero to 23, higher scores indicate worse disability) was 14.2 points in the placebo group and 1.7 points better (0.3 better to 3.1 better) in the vertebroplasty group, absolute improvement in disability 7% (1% to 14% better), relative change 10% better (3% to 18% better) (four trials, 472 participants).
At one month, disease‐specific quality of life measured by the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) (scale zero to 100, higher scores indicating worse quality of life) was 62 points in the placebo group and 2.3 points better (1.4 points worse to 6.7 points better), an absolute imrovement of 2% (1% worse to 6% better); relative improvement 4% better (2% worse to 10% better) (three trials, 351 participants). At one month, overall quality of life measured by the EQ5D (0 = death to 1 = perfect health, higher scores indicate greater quality of life at one month was 0.38 points in the placebo group and 0.05 points better (0.01 better to 0.09 better) in the vertebroplasty group, absolute improvement in quality of life 5% (1% to 9% better), relative change 18% better (4% to 32% better) (three trials, 285 participants). Based upon one trial (78 participants) at one month, 9/40 (or 225 per 1000) people perceived that treatment was successful in the placebo group compared with 12/38 (or 315 per 1000; 95% CI 150 to 664) in the vertebroplasty group, RR 1.40 (95% CI 0.67 to 2.95), absolute risk difference 9% more reported success (11% fewer to 29% more); relative change 40% more reported success (33% fewer to 195% more).
Based upon low‐quality evidence from six trials (one placebo, five usual care, 804 participants), with up to 24 months follow‐up, we are uncertain whether or not vertebroplasty increases the risk of new symptomatic vertebral fractures (95 per 1000; range 34 to 264) observed in the vertebroplasty group compared with 73 per 1000 in the control group; RR 1.29 (95% CI 0.46 to 3.62)). The evidence was downgraded due to imprecision and possibility of detection bias as most of the included trials for this comparison include a usual care comparator.
Similarly, based upon low‐quality evidence from five trials (three placebo, two usual care, 821 participants), the risk of serious other adverse events may not differ (34 per 1000, range 18 to 62) were observed in the vertebroplasty group compared with 56 per 1000) in the control group; RR 0.61 (95% CI 0.33 to 1.10)). The evidence was downgraded due to imprecision and possibility of detection bias from two trials and one of these trials that reported serious adverse events probably unrelated to treatment. Notably, serious adverse events reported with vertebroplasty included osteomyelitis, cord compression, thecal sac injury and respiratory failure.
Our subgroup analyses provided limited evidence that the effects did not differ according to 'acute' duration of pain (described variously amongst trials as < 6 weeks, ≤ 6 weeks, ≤ 8 weeks, or ≤ 9 weeks) versus 'subacute' duration of pain (> 6 weeks).
pain duration 'acute' versus 'subacute'. Three trials only included acute fractures. This was defined as pain less than 6 weeks in Clark 2016, and 8 weeks or less in VOPE 2015. Firanescu 2018 altered their inclusion criteria from 6 weeks or less to 9 weeks or less after 6 months due to poor recruitment. Both Buchbinder 2009 and Kallmes 2009 performed post‐hoc subgroup analyses comparing data for participants with symptom duration of 6 weeks or less
Including data from the eight trials that compared vertebroplasty with usual care in a sensitivity analyses altered the primary results towards favouring vertebroplasty, with all combined analyses displaying considerable heterogeneity, largely driven by the variation in results amongst the studies with a usual care comparison. These data are explainable on the basis that trials that used a usual care comparator are likely to have provided a biased overestimate of the benefits of vertebroplasty due to the lack of participant blinding. At all analysed time points (one to two weeks, one and three months), sensitivity analyses indicated significant differences in outcome for pain and disability outcomes for trials that included a placebo group in comparison to a usual care control.
Vertebroplasty versus other comparators
Consistent with the sensitivity analysis, synthesis of the available data from up to six of the eight trials that compared vertebroplasty with usual care in an open design, generally favoured vertebroplasty with respect to pain and disability at all time points up to 24 months although there was considerable statistical heterogeneity across all pooled pain analyses with I2 varying between 94% and 98%. There was no significant between‐group differences with respect to vertebral fracture or osteoporosis‐specific quality of life at any time point based upon data from up to four trials and these pooled data also displayed considerable statistical heterogeneity (I2 varying between 83% and 95%). However, overall quality of life marginally favoured the vertebroplasty group up to three months but not six months and statistical heterogeneity was unimportant for these pooled analyses (I2 0% to 22%).
Synthesis of the available data from up to three of the seven trials that compared vertebroplasty with kyphoplasty, indicate that there was no important differences between groups with respect to pain, disability or overall quality of life at any time point. Statistical heterogeneity was unimportant for all analyses (I2 varying between 0% and 7%). There were also no detectable between‐group differences with respect to incident radiographic or symptomatic vertebral fractures, adjacent fractures or other serious adverse events.
Based upon one trial that compared vertebroplasty with facet joint injection with glucocorticosteroid and local anaesthetic, vertebroplasty was reported to be superior to facet joint injection with respect to pain and disability at one week, and not superior for any outcomes at other time points up to 12 months. There was also no evidence of important differences between groups with respect to incident radiographic vertebral fractures at 12 months.
Overall completeness and applicability of evidence
This review included evidence provided from 20 randomised controlled trials (RCTs) and one quasi‐RCT undertaken across 15 countries, to assess the effects of vertebroplasty compared with either placebo (sham) (five trials), usual care (eight trials), balloon or shield kyphoplasty (seven trials) and facet joint injection of glucocorticoid and local anaesthetic (one trial). Eight trials reported at least some funding from medical device companies who manufacture vertebral augmentation systems, and, of these, only two reported that they had no other role in the trial.
Data from one placebo‐controlled trial has only been published as a thesis (VOPE 2015). A second placebo‐controlled trial (VERTOS V), was recorded as completed in June 2015 at clinicaltrials.gov, but the trialists subsequently altered the recruitment status in January 2017 to state that the study is now 'enrolling by invitation', with a new estimated completion date of July 2018.
With respect to trials that have used comparators other than placebo, we classified two as ongoing, although it is unclear if the trials are completed; an additional four have been reported to have been completed but we were unable to find published results; and another four completed RCTs that likely meet inclusion criteria have only been published in Chinese and are awaiting assessment. Inclusion of the results of these trials may alter the conclusions of our second and third comparisons (vertebroplasty compared with usual care or kyphoplasty, respectively).
Vertebroplasties were performed by interventional radiologists or neuroradiologists, orthopaedic surgeons or neurosurgeons across the different trials, however the procedure itself appeared similar. Mean cement injected varied across the 17 trials that reported these data. The study populations in all included trials appeared to be representative of patients seen in routine care. The age and gender ratio were similar across all trials apart from Chen 2014a, who included a younger study population. However symptom duration did vary widely across trials reflecting different philosophies on when vertebroplasty should be implemented. Some authors have suggested that vertebroplasty only be offered to patients with recent onset of pain (< 6 weeks) (Bono 2010). Our subgroup analysis, as well as the result of an individual patient data meta‐analysis from the same two trials (Staples 2011), suggests that treatment outcome is not influenced by duration of pain ≤ 6 weeks versus > 6 weeks. This supports our decision to include all trials in pooled analyses irrespective of symptom duration.
Trials varied in how they measured pain with respect to the scale used (although most used a continuous measure), time period specified and descriptors used. Only four trials included a dichotomous measure of pain. All except two trials included a measure of disability or function, with the RMDQ used most commonly (9/21 trials). Seven trials included the QUALEFFO and six included an overall generic measure of quality of life. Only two trials included a measure of patient‐reported treatment success although three trials did report the number who achieved various thresholds of pain and/or disability improvement. Most trials reported on the presence of new symptomatic and/or radiologically apparent vertebral fractures (although two trials only considered adjacent fractures (Endres 2012; Vogl 2013) and one of these also considered refracture of the treated vertebra (Vogl 2013). However other adverse events were less well‐reported across trials with three trials only reporting on adverse events that occurred in the vertebroplasty group.
The RCTs included in this review also varied in terms of duration of follow‐up (from two weeks to three years). For one placebo‐controlled trial we only included data up to one month, despite the availability of one‐year data, because of an a priori decision that for cross‐over RCTs, we would preferentially extract data only from period before cross‐over. Comstock 2013 reported that after one month, significantly fewer participants in the vertebroplasty group crossed over into the alternate group (11 of 68 participants (16%)) compared with the placebo (sham) group (38 of 63 participants (60%), P = 0.001). At one year, difference in pain favoured the vertebroplasty group (MD 1.02 (95% CI: 0.04 to 2.01); P = 0.042), but there was no evidence of an important difference in disability (MD in RMDQ 1.37 points (95% CI: 3.62 to 20.88), P = .231). In the as‐treated analyses, participants treated with vertebroplasty did not differ from the placebo (sham) group in terms of either mean pain (MD 0.85 (95% CI: 2.05 to 20.35), P = 0.166) or disability (RMDQ, MD 0.66 (95% CI: 3.30 to 21.98); P = 0.625). Therefore, including data from the one‐year intention‐to‐treat (ITT) analysis from this trial would not have appreciably altered our results. Based upon (ITT) pooled data from the two trials, at 12 months, there was no significant between‐group differences with respect to mean pain (MD 0.77 (95% CI ‐1.85 to 3.39) (data not shown).
While the lack of significant benefits of vertebroplasty in terms of pain and disability shown in the main analysis of this review would appear to indicate that any other clinical benefits are unlikely, some (Chen 2013; Edidin 2011; Lange 2014; Zampini 2010), but not all (McCullough 2013; McDonald 2011), population or clinic‐based observational studies have reported a reduction in mortality associated with spinal augmentation (vertebroplasty or kyphoplasty), compared to those who were untreated. However McCullough 2013 demonstrated that the survival benefit observed with traditional covariate adjustments was no longer in evidence after using propensity score matching to better account for selection bias (McCullough 2013), suggesting that selection bias from unrecognised confounding explains the observed survival benefit of spinal augmentation seen in other studies. Using propensity score matching McCullough 2013 also reported that one‐year major medical complications were similar between the groups, while rates of health‐care utilisation, including hospital and intensive care unit admissions and discharges to skilled nursing facilities, were higher among those treated with spinal augmentation.
Although this review did not demonstrate an increased risk of incident vertebral fractures associated with vertebroplasty, a clinically important increased risk cannot be excluded based upon our review due to the small number of events. Data from the RCTs that compared risk with vertebroplasty compared with placebo or usual care were also inconsistent as evident by the substantial statistical heterogeneity observed in the pooled analyses.
Observational studies that have compared the incidence of new vertebral fractures following vertebroplasty to the incidence in a cohort who did not receive the procedure have also reported conflicting results (Álvarez 2006; Chosa 2011; Mundano 2009; Tang 2011). To conclusively establish whether or not there is an increased risk of further vertebral fracture, particularly adjacent fracture associated with vertebroplasty will require additional large randomised trials with extended follow‐up and/or population‐based studies analysed carefully to account for differences in patient selection as outlined by McCullough 2013.
Based upon the randomised controlled data that we reviewed, cement leakage occurs frequently. While in most cases it does not result in symptoms, serious sequelae may occur. In our review, one trial reported one instance of cement leakage into the epidural space requiring immediate decompression. It is not possible to determine the rate of significant sequelae arising from cement leakage or embolism from our review due to the small number of events. However numerous cases of local cement leakage requiring decompression have been reported, as have reports of cement emboli requiring surgical removal from the inferior vena cava (Baumann 2006; Seo 2005), right heart chambers (Caynak 2009; Kim 2014), and the right pulmonary artery (François 2003). Cement embolism has also been reported to cause transient respiratory symptoms and death (Monticelli 2005), and deaths from fatal fat embolism after vertebroplasty have also been reported (Syed 2006). In a post‐hoc analysis of data from Buchbinder 2009, there was an increased odds of cement leakage with higher volumes of injected cement (Odds Ratio (OR) 2.8; 95% CI 1.3 to 6.1), but they found no relationship between cement leakage and efficacy outcome (Kroon 2014).
Complications from vertebroplasty reported in the included trials were osteomyelitis requiring surgical drainage, rib fractures, thecal sac injury requiring hospitalisation, pain‐induced vasovagal reactions an acute asthma exacerbation, and fracture of a cement spur. However, as event rates were low in included trials, we cannot be certain if there are, or are not, any between‐group differences for reported adverse events among the trials we reviewed. We also note that severe adverse events such as deaths due to pulmonary thromboembolism, fat embolism, extensive local cement leakage, infection and complications of anaesthesia and infection requiring surgery have all been reported in the literature (Al‐Nakshabandi 2011).
Quality of the evidence
The overall quality of evidence for our primary comparison (vertebroplasty versus placebo) was high for the pain, disability and osteoporosis‐specific quality of life outcomes according to the GRADE approach (Schünemann 2011b). According to the GRADE Working Group grades of evidence, a grading of high quality of evidence indicates that further research is unlikely to change the effect estimates substantially. For the remaining outcomes, the evidence was downgraded from high to moderate due to either imprecision (i.e. 95% CIs do not exclude clinically important differences (defined as 0.074 on the 0 to 1 EQ‐5D quality of life scale), or because the total number of events was small (for other outcomes). According to the GRADE Working Group grades of evidence, a grading of moderate quality of evidence indicates that further research may have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
While the available data from eight trials that compared vertebroplasty with usual care generally favoured vertebroplasty for some, but not all outcomes, they were all rated as having a high risk of performance and detection bias for self‐reported outcomes due to lack of participant and personnel blinding. While recognising that blinding of participants and personnel is difficult to achieve in procedural trials, failure to blind studies of this nature is problematic as trials with unblinded assessment of subjective outcomes (such as pain and function) are estimated to exaggerate the treatment benefit by 22% on average (ratio of OR 0.78; 95% credible interval 0.65 to 0.92) (Savović 2012). Correcting for these biases would likely drive the improvements seen with vertebroplasty over usual care, towards the null, in keeping with the findings from the placebo‐controlled trials.
Potential biases in the review process
We believe that we identified all relevant trials following a thorough search of all major databases with no language restrictions.
Six review authors independently assessed the trials for inclusion in the review, extracted data and assessed the risk of bias, and a third review author adjudicated whenever there was any discrepancy. Two of the review authors (RB and DK) are authors of three of the trials included in this review (Buchbinder 2009, and Kallmes 2009 and Evans 2015, respectively). To avoid any bias, these papers were assessed by an independent review author to assess whether they met the inclusion criteria for this review. Neither author was involved in the data extraction or 'Risk of bias' assessment of their own trials.
In view of the potential for bias of open studies when the main outcomes are self‐reported, we chose vertebroplasty compared with placebo (sham) as our primary analysis. This decision was vindicated based upon the results of our sensitivity analyses that were performed to determine whether our primary results were robust to the inclusion of the open trials.
Agreements and disagreements with other studies or reviews
Numerous systematic reviews and/or meta‐analyses investigating the effects of vertebroplasty for osteoporotic spinal fractures have been published, e.g. Anderson 2013; Bliemel 2012; Eck 2008; Gill 2007; Han 2011; Hochmuth 2006; Hulme 2006; Lee 2009; Liu 2013; Ma 2012; McGirt 2009; Papanastassiou 2012; Ploeg 2006; Robinson 2012; Shi 2012; Stevenson 2014; Taylor 2006; Trout 2006; Wang 2012; Zhang 2013; Zou 2012). These have differed in terms of focus.
Reviews that have synthesised the effectiveness of vertebroplasty have reported conflicting conclusions explainable on the basis of their inclusion criteria and/or methodological rigour (Anderson 2013; Hochmuth 2006; Hulme 2006; Liu 2013; McGirt 2009; Papanastassiou 2012; Ploeg 2006; Robinson 2012; Shi 2012; Stevenson 2014; Taylor 2006). Our results are consistent with systematic reviews that only included RCTs and performed separate analyses according to comparator (e.g. Liu 2013; Robinson 2012; Shi 2012; Stevenson 2014), while differing conclusions in other reviews are explainable on the basis that these reviews included pooled analyses combining placebo‐controlled and usual care comparators, included non‐randomised studies and/or combined vertebroplasty and kyphoplasty data (e.g. Anderson 2013; Papanastassiou 2012).
Four previous reviews that have focused upon the available evidence with respect to incident vertebral fractures (Bliemel 2012; Trout 2006; Zhang 2013; Zou 2012), have reported conclusions broadly consistent with ours that it is not possible to definitely establish whether or not there is an increased risk of further vertebral fracture associated with vertebroplasty. Trout 2006a has suggested that adjacent fractures may occur significantly sooner.
In contrast to our review, previous reviews that have evaluated the comparative efficacy and safety of vertebroplasty compared with kyphoplasty have all combined data from observational and experimental studies (Eck 2008; Gill 2007; Han 2011; Ma 2012), making comparison with our results problematic. More recent reviews (Han 2011; Ma 2012), included one of the four RCTs in our review (Liu 2010). In keeping with the findings of our review based upon only RCT data, previous reviews have generally concluded that the two treatments were of comparable longer‐term effectiveness and safety.
Dohm 2014 considered average kyphosis correction as a secondary radiographic endpoint and reported no between‐group difference in kyphotic angulation correction post‐operatively or at three or 12 months. At 24 months, the authors reported a mean difference of 1.43 degrees (95% CI 0.10 to 2.74 degrees) favouring the kyphosis group although the clinical importance of this finding was not discussed. Some previous reviews have suggested that kyphoplasty may be superior with respect to improving kyphosis angle and vertebral height compared with vertebroplasty, but have acknowledged that this remains to be proven in rigorous RCTs (Ma 2012).
Authors' conclusions
Implications for practice.
Based upon the currently available high‐ to moderate‐quality evidence, vertebroplasty has little meaningful effect on pain, disability, quality of life or treatment success for the treatment of acute or subacute osteoporotic vertebral fractures in routine clinical care. There were no demonstrable clinically important benefits compared with a placebo (sham procedure) and the results were consistent across the studies irrespective of the duration of fracture pain. Sensitivity analyses confirmed that open trials that compared vertebroplasty with usual care are likely to have overestimated any benefit of vertebroplasty. Correcting for these biases would likely drive any observed improvements seen with vertebroplasty over usual care towards the null, in keeping with the findings from the placebo‐controlled trials.
Although we did not demonstrate an increased risk of incident symptomatic vertebral fractures or other serious adverse events associated with vertebroplasty, a clinically important increased risk cannot be excluded based upon our review due to the small number of events. Furthermore, serious adverse events related to vertebroplasty were reported in several trials. Patients should be informed about the lack of high‐quality evidence supporting benefits of vertebroplasty and its potential for harm.
Implications for research.
Further high‐quality research is unlikely to substantially change the conclusions of this review regarding the potential benefits of vertebroplasty but may resolve whether or not it increases the risk of further vertebral fracture and serious adverse events. Current literature does not support the likelihood of identifying subsets of patients who would benefit from vertebroplasty. It is questionable whether further trials testing the efficacy of vertebroplasty are necessary. Any future review updates will restrict inclusion to only placebo‐controlled trials.
Feedback
Dr. Clark complain, 3 June 2018
Summary
We wish to complain about the Cochrane vertebroplasty review (CVR) (1) and ask that it be withdrawn from the Cochrane library. We believe that the report provides a biased analysis of the VAPOUR trial, contains inappropriate statistical analysis and errors in data presentation.
The VAPOUR trial (2) is a blinded randomised trial of vertebroplasty for acute painful osteoporotic fractures which showed clinical benefits for vertebroplasty over placebo in managing the most severely affected patient group. We believe that under‐reporting the positive VAPOUR results and the unjustified criticisms of VAPOUR data in the CVR has led to the negative finding on the benefits of vertebroplasty. The VAPOUR trial is very different to the negative trials conducted by the Cochrane authors. Readers of the CVR are not aware of these vital facts.
The Cochrane author group has significant potential conflict of interest, some of which is not declared. The first author (RB) is author of a key trial (3) in the review, wrote the first draft, provided the funding, is co‐ordinating editor of Cochrane MSK section and a member of the Medicare Services Advisory Committee (MSAC) which is currently assessing the application for Medicare funding of vertebroplasty in Australia. Another author (JH) is an editor of Cochrane MSK. DK and RB are first authors of twin trials (3,10), that were published together, and form a central part of the evidence being analysed by this review.
• MSAC assessment of vertebroplasty
The Interventional Radiology Society of Australasia (IRSA) has applied to MSAC (4) for Medicare funding of vertebroplasty in a patient group like the VAPOUR Trial. MSAC is a statutory body advising the government on Medicare funding. The timing of the Cochrane review is extraordinary and has impacted the MSAC review. The outcome of the application was to be decided by the ultimate committee, the MSAC Committee, on March 28. The first author of this CVR (RB) is a member of that Committee. The unpublished CVR was directly accepted for inclusion in MSAC committee deliberation. Cochrane reports, even if unpublished at the time, are historically granted direct passage to the ultimate and decisive MSAC committee due to their reputation of objectivity and quality. IRSA complained to the Australian Federal Health Minister about the inclusion of this Cochrane review, citing errors in the data and analysis, and potential conflicts of interest in the author group. MSAC Application 1466 has been delayed, pending review.
The objective of two authors (RB, DK) in the “No” case for vertebroplasty is evident by observing their negative Editorials in refereed journals (5). Their strong negative opinion of vertebroplasty should preclude them from writing an independent review e.g. Cochrane report. The CVR should allow a balanced perspective to emerge.
• Errors in data and meta‐analysis
There are critical problems with data content and meta‐analysis in the CVR. Meta‐analysis 8.1, 8.2 wrongly included all 180 patients from the VERTOS4 trial (6) in the <6/52 sub‐group, and none in the >6/52 group despite a median fracture duration of 6.1 weeks in that trial. This erroneous data provides dominant weighting in the analysis. Another methodologic problem with meta‐analyses 1.1, 8.1, 8.2, 8.3 and 8.4 is inclusion of different outcome measures (final values in some trials and change from baseline values in others). These were then blended together and expressed as a standardised mean difference, which contravenes Cochrane meta‐analysis guidelines. CVR used conference proceedings of VERTOS 4 to quote the mean duration of fracture in VERTOS4 as 29 and 26 days rather than 40 and 43 days as was correct. Including incorrect data, the wrong patients and faulty methodology in meta‐analysis renders the conclusions of the CVR as meaningless.
CVR under‐reports the positive outcomes in the VAPOUR trial. Readers of the Cochrane review could be excused for not realising that the VAPOUR trial showed clinically significant benefits from vertebroplasty at all timepoints, from 3 days to 6 months, in a severely affected, elderly patient group suffering severe fracture related disability. The review states (p25) “Clark 2016 found a slight benefit in terms of pain relief with vertebroplasty”, a misleading statement that under‐reports the positive findings of VAPOUR in a high‐risk patient cohort which has never been studied in this setting.
CVR misuses minimum clinically important difference (MCID) to dismiss the positive outcomes from vertebroplasty. It uses MCID directly to assess mean group scores. MCID is an individual measure, not a group measure, and it should be applied to individual patients, not to mean group scores, which is a fundamental, methodologic error (7). Using MCID to compare group outcomes requires that the trial measure this directly from individual patients (how many patients in each group obtained MCID benefit) and then compare the proportion of patients who had derived MCID benefit in each group. This analysis has not been done in any vertebroplasty trial. When applied directly to mean group scores, as per the CVR, a reduction in mean population pain of 1.5 units indicates clinical efficacy but a reduction of 1.4 does not. The between‐group difference in mean NRS pain in VAPOUR at 4 weeks is 1.4 units. According to CVR, this excludes clinically significant pain relief, despite 31% more patients in the vertebroplasty group reporting substantial pain relief at the same time (primary outcome). How can these apparently opposite outcomes co‐exist? The answer is that MCID pain is an individual patient measure, not a group measure and is not related to changes in mean group scores. Applying it in this fashion is statistically invalid, causing absurd arguments such as this one.
Analysis of the role of vertebroplasty in hospitalised patients is conspicuous by its absence in the CVR. There is not a single reference or mention of hospitalisation in the entire report. Unplanned hospital admission of patients with acute osteoporotic vertebral fractures is a common cause of hospitalisation in the elderly. These patients are often much worse affected than those who are ambulatory outpatients. It would be an error of judgement to suggest that these cohorts are similar and should be managed equally. The negative blinded trials all excluded hospitalised inpatients from enrolment. This hole in the evidence is filled by the VAPOUR trial. Hospitalised inpatients comprised 58% of VAPOUR’s enrolment.
• Unfair targeting of VAPOUR in risk of bias assessment
CVR applies risk of bias (ROB) tools unevenly, with a more critical, forensic style of assessment applied to the VAPOUR trial in comparison to other blinded trials. It makes unsubstantiated allegations of bias risk in procedural blinding, selective reporting, placebo design, study attrition which do not bear scrutiny. The faulty assessment is used as reason to ignore the clear benefits from vertebroplasty that were shown in the VAPOUR trial.
CVR questions the validity of blinding in VAPOUR, based upon the statistical responses to a “perception of treatment questionnaire”. More patients in the vertebroplasty group correctly guessed their assigned intervention than in the placebo group. 88% of vertebroplasty patients who guessed correctly stated pain relief as the motivating reason for their guess, but the Cochrane review ignores this fact.
The Cochrane collaboration tool for assessing risk of bias in randomised trials (http://www.bmj.com/content/343/bmj.d5928) states in its appendix:
“When blinding is employed, some have suggested that it would be sensible to ask trial participants at the end of the trial to guess which treatment they had been receiving. Evidence of correct guesses exceeding 50% would seem to suggest that blinding may have been broken, but in fact can simply reflect the patients’ experiences in the trial: a good outcome, or a marked side effect, will tend to be more often attributed to an active treatment, and a poor outcome to a placebo. Therefore, evaluations of the success of blinding may need to be interpreted carefully.”
These facts are exemplified in VAPOUR, with up to 31% (17‐50%) more patients in the vertebroplasty group experiencing strong pain relief (primary outcome) and correctly guessing vertebroplasty.
CVR grades VAPOUR at “high‐risk” of reporting bias because of typographic error in the published protocol where ≥7/10 is mistakenly written as >7/10. The VAPOUR trial published its protocol on ClinicalTrials.gov in 2011(8), before the trial commenced. This described the primary end‐point as:
• “The primary effectiveness analysis will be based on the number of patients whose numeric rating pain score drops from above 7 out of 10 at baseline to below 4 out of 10 at two weeks post‐intervention.
• “From above 7 out of 10 “should have been written “from 7 or above out of 10”.
Pain≥7/10 was an enrolment requirement for all patients as described in all publications of the protocol [ClinicalTrials.gov (8), Trials journal (9), The Lancet (2)]. The intention to treat analysis must include all patients enrolled in the trial, which is NRS pain ≥7/10, the inclusion criterion.
Allowing for the obviously typographic error, the analysis of the trial, published in the Lancet, is the same as the protocol published before trial commencement.
The patients in VAPOUR included a very high‐risk cohort and were the oldest, most fragile patient group ever enrolled into a vertebroplasty RCT. Mean age 80, 58% hospitalized at time of enrolment, 90% using strong opiates, mean T‐score lumbar spine ‐4.2. and multiple pre‐existing fractures. This is the only blinded RCT to include hospitalized inpatients. Following funding withdrawal for vertebroplasty in Australia, this therapeutic procedure was unavailable outside of the trial and allowed for enrolment of 78% of all eligible patients, including the severely affected and disabled ones, into the study. The long questionnaires were noted by data collectors to be onerous for this sick patient group, who complained about the volume of questions. Consequently, data collectors prioritized the primary questions of pain and disability, then quality of life, medications, and then, perception of treatment at the end. Those questions completed toward the end of the questionnaire have incomplete data due to patient fatigue.
CVR criticises VAPOUR’s incomplete reporting of the “perception of treatment” questionnaire. VAPOUR adopted the blinding questionnaire from Kallmes 2009 (10). This included questions for both patients and blinded outcome assessors. VAPOUR recorded this at days 3 and 14 and reported results from patients and data assessors at day 14 – time of primary endpoint. Kallmes 2009 did not list the perception of treatment questionnaire in the published protocol but the clinical protocol (which we have, as we were investigators in that trial) includes the questionnaire at days 3 and 14, and months 1, 3, 6 and 12. It reported (10) patient responses at 14 days, (not the primary endpoint) and not at the other 5 time‐points. It did not report the data collectors’ responses at all. It would have been interesting to know the outcome at 1 month, (time of primary endpoint), where more placebo patients inexplicably crossed‐over to vertebroplasty than vice versa. Buchbinder2009 (3) conducted the perception of treatment questionnaire at 2 years post vertebroplasty which is retrospective and of doubtful meaning. This questionnaire may have been added to the clinical protocol after trial commencement, as the first protocol publication (11) occurred when the trial was registered, retrospectively, and did not mention this questionnaire. It was first described in the next protocol publication (12), which was not published until 2 months after enrolment was terminated (3). Late addition to protocol may explain why it was administered retrospectively. Kallmes 2009 and Buchbinder 2009 have been cleared for inclusion in the Cochrane report but not VAPOUR. It would be reasonable to expect the same critique should be applied to all trials by the Cochrane report.
Allegation of placebo bias (“Other potential sources of bias” page 24), is unfair and unsubstantiated. The placebo in VAPOUR involved conscious sedation (IVI midazolam/fentanyl), mask oxygen and pulse oximetry and replication of the experience of a vertebroplasty. A skin incision of 5mm (as for vertebroplasty) was made and then a short, 25G needle (local anaesthetic needle) passed into the incision, but not as far as the bone. This was held in one hand while the other hand tapped on it and pressed onto the skin to suggest a vertebroplasty was being done. One can’t tap on a short 25G local anaesthetic needle without supporting the needle with the other hand – which means tapping on the second hand and indirectly on the back. The back has the least tactile sensitivity of any part of the body surface. The tactile sensations were designed to replicate the feeling of vertebroplasty needle entry. The initial (2011) published protocol description of placebo in ClinicalTrials.gov (8), and the final (2016) description in trial publication (2), are the same.
• Staples 2011 meta‐analysis.
CVR uses Staples meta‐analysis (13) of Buchbinder 2009 and Kallmes 2009 trials to argue that fracture duration is irrelevant to outcome from vertebroplasty. Staples 2011 answered criticism (14) that Buchbinder 2009 and Kallmes 2009 failed to assess patients with fractures <6/52 duration who had severe pain and did not inject sufficient PMMA to control the symptoms of such patients. Staples found 33 patients who had fractures ≤6/52 and severe pain, (like VAPOUR), but did not assess this group separately due to an insufficient number of patients. They assessed the ≤6/52 group (57 patients) and the “severe pain group” separately. These selection criteria are not “either/or” selection requirements for successful vertebroplasty outcome. Correct patient selection includes the combination of severe pain and fracture duration < 6 weeks duration ‐ the VAPOUR trial required both. VAPOUR required a severe pain score (7/10) for enrolment, (versus no specified pain threshold in Buchbinder 2009 and 3/10 in Kallmes2009). There were no inpatients in Staples meta‐analysis versus 58% in VAPOUR. PMMA dose was not reported in Staples 2011, so presumably was low as in the parent trials (mean PMMA volume 2.6cc and 2.8 cc versus 7.5 cc in VAPOUR). The clinical heterogeneity between Staples 2011 and VAPOUR is high, but this is not discussed in CVR.
The small Staples sub‐group meta‐analysis is claimed to be adequately powered (13):
“The original sample size calculations for the Australian trial indicated that 24 participants would be needed in each treatment group to show a 2.5 unit advantage in pain scores, assuming a standard deviation of 3.0, a significance level of 5%, and 80% power. The observed standard deviation for pain was about 2.0 rather than 3.0 as assumed for study planning and therefore the power calculations are conservative. From the combined trials 25 participants in the control group and 32 in the vertebroplasty group had recent onset pain and 50 in the control group and 49 in the vertebroplasty group had severe pain at baseline. Thus the combined data provided greater than 80% power to assess whether vertebroplasty had a 2.5 unit advantage over control for patients with acute fractures or severe pain. (Staples et al 2011, page 3)”
In fact, there are 25 (not 32 patients as quoted above) in the vertebroplasty group according to Tables 1, 2 and 3 in Staples. A between group difference of 2.5 units mean pain, in a blinded vertebroplasty trial, is inappropriate and unrealistic for power determination. There is no difference of this magnitude observed in the VAPOUR trial at any time point, in a patient group much more likely to benefit from vertebroplasty. A more appropriate analysis would have calculated power for a difference of 1 unit or 1.5 units, but this would have revealed insufficient patients in the sample. Staples 2011 is not adequately powered to draw conclusions about vertebroplasty efficacy in recent fractures.
There are multiple inconsistencies in Staples’ fracture duration data (Table 1, Staples 2011). It reports mean fracture duration in Kallmes 2009 as 22.5 weeks versus 16 and 20 weeks in the trial publication (10) and Buchbinder 2009 as 11.7 weeks versus 9‐9.5 weeks as reported in the CVR (p16). The contradictory fracture duration data between the parent trials and the Staples meta‐analysis, cast doubt over the veracity of fracture duration data of patients included in the Staples 2011 sub‐group analysis.
The only blinded trial to have been correctly reported for fracture duration in the CVR (p16) is the VAPOUR trial (2.8, 2.4 weeks). Buchbinder 2009 and Kallmes 2009 are reported with reduced duration, compared to Staples 2011, and VERTOS 4 mean duration is reduced to 29 and 26 days rather than 43 and 40 days, (as reported in the VERTOS 4 conference proceedings sourced by the CVR), or median duration 43(v) and 36 (p) days as reported in the trial publication (6). Each error acts to reduce the apparent difference between VAPOUR and the other blinded trials. A central dispute regarding vertebroplasty efficacy relates to the timing of the intervention, so these data reporting errors in the CVR are important.
• Clinical heterogeneity
The interpretation of meta‐analysis depends upon the degree on clinical heterogeneity of included trials. Cochrane teaching handbook states “clinical variation will lead to heterogeneity if the intervention effect is affected by the factors that vary across studies; most obviously, the specific interventions or patient characteristics. In other words, the true intervention effect will be different in different studies.” (http://training.cochrane.org/resource/exploring‐heterogeneity). The more the clinical variation, the less relevant is the meta‐analysis to individual studies. The trial most clinically different to the other blinded trials in the meta‐analyses is the VAPOUR trial – the only trial to include inpatients, worse osteoporosis, much earlier time of intervention, most severely affected patient group, and different technical approach.
CVR (p12) states “we assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, [...] were similar across trials.” It found (p16) that “trial participants in general inclusion criteria for all trials were similar”. The VAPOUR trial deliberately targeted a different patient group and used a different technique to Buchbinder 2009 and Kallmes 2009. CVR makes no attempt to assess these clinical differences in VAPOUR compared to their own trials.
CVR (p16) states that “vertebroplasty appears to be performed in a similar way across all trials.“ The VAPOUR trial described “vertebral fill” technique utilising high volumes of high viscosity PMMA for soft, fresh vertebral fractures resulting in three times the PMMA dose delivered compared to Kallmes2009 and Buchbinder2009.
The blinded trials have different patient selection, procedural technique and duration of fracture. The mean duration of fracture in VAPOUR (2.6 weeks) compares to VERTOS4 (6.1 weeks), Buchbinder 2009 (11.7weeks) (13), Kallmes 2009 (22.5 weeks) (13) and VOPE (unknown). The trials had different pain threshold (from no threshold in Buchbinder 2009, 3/10 in Kallmes 2009, 5/10 in VERTOS4 to 7/10 in VAPOUR). There are no hospitalised inpatients in any blinded trials except for VAPOUR‐where they constitute majority of enrolment. The trials used different volumes of PMMA from 2.6cc in Kallmes 2009 to 7.5 cc in VAPOUR. As has been described, the meta‐analyses in Cochrane vertebroplasty review contain critical error, using false data and methodology. Even if performed correctly, they would have had little meaning for the patients in the VAPOUR trial, given the high clinical heterogeneity of VAPOUR with the other blinded trials.
VAPOUR is the first blinded trial to verify benefit from vertebroplasty. It targets a different patient group to the other blinded trials; patients with severe pain, earlier time of intervention in the natural history of the fracture, use of a larger PMMA volume as required to stabilise soft, pliable, collapsing fractures, and the inclusion of hospitalised inpatients. Diluting the positive findings of the VAPOUR trial by incorrectly mingling them with the results of dissimilar trials has created a negative bias in CVR.
• Conclusion
We have established that the Cochrane vertebroplasty report is written by an author group unlikely to be objective, multiple author conflicts of interest have not been disclosed, the report contains critical errors in both data and meta‐analysis and the included trials have not been analysed appropriately. These facts should be carefully considered. We recommend that the publication be considered for withdrawal from the Cochrane library.
Dr William Clark
Director Interventional Radiology
St George Private Hospital, Sydney
Australia
Conjoint Associate Professor Terry Diamond
St George and Sutherland Clinical School
University of New South Wales
Sydney, Australia
Conjoint Associate Professor Paul Bird
St George and Sutherland Clinical School
University of New South Wales
Sydney, Australia
Conjoint Associate Professor Peter Gonski
School of Public Health and Community Medicine
University of New South Wales,
Sydney, Australia
(Editorial note: the version of the review Dr. Clark commented on is available at https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006349.pub3)
Reply
We believe this updated Cochrane review is the most rigorous and up to date synthesis of the evidence of percutaneous vertebroplasty for the treatment of painful osteoporotic vertebral fractures. By conforming to the recommendations for the conduct and reporting of Cochrane reviews we have minimised the potential for a biased review. We have responded to each of the relevant criticisms of Clark et al below (and also shown our responses within the text of their feedback to show that we have covered all important issues for convenience of the feedback editor).
The VAPOUR trial was one of five placebo‐controlled trials included in our review. Just as for the other trials, we included all the available data relating to the outcomes of interest from the VAPOUR trial. A systematic review and meta‐analysis combines data from all relevant trials to provide an estimate of the true effect of a treatment with more precision than can be obtained from a single trial. The results of VAPOUR were mostly consistent with the results from other trials as can be shown by an examination of statistical heterogeneity when data from VAPOUR were pooled with data from other trials. For example, there was no statistical heterogeneity (I2 = 0%) for mean pain at at 1, 3 and 4 to 6 months, disability at 1 month, QUALEFFO at 1‐2 weeks, or generic quality of life at any time point.
While the VAPOUR trial reported favourable results and interpreted their findings as indicating a worthwhile benefit of vertebroplasty, a closer examination shows that while their primary outcome of proportion of participants with pain of <4 out of 10 favoured the vertebroplasty group at all time points, their point estimates of differences between groups with respect to mean pain as measured by NRS was only of clinical relevance at 2‐3 days and not different at all when measured by VAS in the subgroup who completed VAS assessments at 14 days and 6 months. These latter results were all in keeping with the other trials and also suggest that the results of the comparison of proportions of participants who improved could have varied if a different cut off (e.g. an NRS score of 4 or less had been used). We also do not consider that the VAPOUR trial was so different to the other trials in terms of the participants that were enrolled – this is evident by an examination of the data summarised in Additional Table 2. Where the trial characteristics (e.g. symptom duration, mean pain scores, volume of cement injected, etc), appeared to differ slightly we noted this in the text.
Potential for conflict of interest: We agree that potential conflicts of interest are important to declare. The review authors adhered to Cochrane's stringent policy on conflict of interest. The involvement of two of the seven authors in primary research in the subject area of this review was declared (see Declarations of Interest section). In addition, as noted in the methods, these authors had no involvement in deciding whether their trials met inclusion criteria for the review and no involvement in data extraction or risk of bias assessments for their own trials. There is no restriction on Cochrane editors and editorial staff authoring Cochrane reviews provided they are not involved in the editorial process. All editorial processes for this review occurred at the Ottawa Cochrane Musculoskeletal editorial base. As indicated in the review, there was no specific funding for updating the review. While Prof Buchbinder is a member of the Australian Medicare Services Advisory Committee (MSAC), it is not clear to us how this would pose a conflict on the review.
MSAC is an independent non‐statutory committee, which appraises new medical services proposed for public funding (under ‘Medicare’), and provides advice to Government on whether a new medical service should be publicly funded on an assessment of its comparative safety, clinical effectiveness, cost‐effectiveness, and total cost, using the best available evidence. As stated, MSAC is currently assessing the application for Medicare funding of vertebroplasty in Australia. And as MSAC uses the best available evidence to inform its decisions, the Cochrane review will likely be used to assist in making funding decisions (which is one of the intended purposes of Cochrane reviews). In view of her involvement in the subject area and her preference not to be involved in any MSAC decision on this topic, Prof Buchbinder had already declared her potential conflict and has been excluded from any of discussion of vertebroplasty at MSAC including the timing of those discussions.
Dr Clark reports no potential conflict of interest in submitting this ‘complaint’. However he was on the Advisory Panel that advised MSAC to first list vertebroplasty on an interim basis on the Medical Benefits Scheme in Australia in 2004‐5. He was also on the Advisory Panel that provided advice to MSAC in 2011. The MSAC decision in 2011 was that continued public funding for vertebroplasty was not supported based upon the strength of the available evidence and interim funding for the procedure was withdrawn. It was noted that Dr Clark did not agree that his views were reflected in the final MSAC Assessment Report.
Our published editorials and commentaries reflect our evidence‐based assessment of vertebroplasty at any one point in time. For example, prior to the completion of the Buchbinder and Kallmes trials, Prof Buchbinder published an editorial jointly with Prof Richard Osborne in the Medical Journal of Australia with the title ‘Vertebroplasty: a promising but as yet unproven intervention for painful osteoporotic spinal fractures’(Buchbinder R, Osborne RH. MJA 2006; 185:351‐2.) Other papers followed as the data emerged and our interpretation of the evidence changed.
Discrepancies between the conference proceedings and published data for Firanescu 2018 (Vertos IV): Our updated review was published prior to the full publication of VERTOS IV (Firanescu 2018), and, as stated, the available data were extracted from conference proceedings. The conference proceedings did not report a change in inclusion criteria from symptoms for less than 6 weeks to inclusion of participants with symptoms less than 9 weeks and thus we originally included this trial within the less than 6 weeks subgroup analysis.
Our review has now been updated to reflect the more recent data. We have relabelled the subgroup analysis to compare response to vertebroplasty of participants with ‘acute’ fractures as defined by the trials versus ‘subacute’ fractures. For the ‘acute’ fractures we included VAPOUR (symptoms less than 6 weeks), Firanescu 2018 (symptoms less than 9 weeks), VOPE (symptoms less than 8 weeks) and the subgroups of Buchbinder 2009 and Kallmes 2009 with symptoms for 6 weeks or less. In addition we performed a sensitivity analysis excluding Firanescu 2018 and VOPE from the subgroup analysis and this did not change the results.
Regarding the symptom duration of Vertos IV (Firanescu 2018), the conference proceedings reported a mean (SD) duration of pain of 29.2 (16.3) and 25.9 (13.8) in the vertebroplasty and placebo groups respectively. In their published paper they report a median (interquartile range) of number of days before the procedure of 43 (29 to 52) days and 36 (24 to 51) days in the vertebroplasty and placebo groups respectively as well as a median (interquartile range) of number of days from radiographic diagnosis to procedure of 13 (7 to 18) and 11 (7 to 17) days. They also report that only 20/90 and 12/86 participants in the vertebroplasty and placebo groups respectively had duration of pain for more than 6 weeks but less than 9 weeks at the time of randomisation, indicating that their duration of pain data may be negatively skewed since the mean values are lower than the medians.
Combining final and change scores and combining outcomes for the same domain that have been measured using different instruments: We consider that we have used appropriate methods for both combining final and change scores (see Section 7.7.3.1 and of The Cochrane Handbook), and for combining outcomes for the same domain that have been measured with different instruments (see Section 9.2.3.2 of The Cochrane Handbook). In all cases we have provided the SMD as well as a back transformation into units more easily understood by readers.
To address the criticism of using the SMD and combining both change and end scores, the current version of the Handbook – which is under revision ‐ does advise that final value and change scores not be combined together to calculate an SMD, since the difference in standard deviation does not reflect differences in measurement scale, but differences in the reliability of the measurements. However they can be combined when calculating mean differences (see Section 9.4.5.2 in The Cochrane Handbook). Advice from our Cochrane Musculoskeletal statistician indicates however that it probably does not change the meta‐analysis results (assuming the meta‐analysis is robust), if we do combine change and end of treatment scores using the SMD.
We also could have made the assumption that all studies measured pain using comparable pain scales (so assuming that NRS and VAS scores are comparable; VAPOUR was the only one to use the NRS) (see: https://www.jwatch.org/em200305140000005/2003/05/14/visual‐analog‐scale‐vs‐numerical‐rating‐scale), and calculated an MD instead of an SMD. We have made this change in the review. The results remain robust if we do this. For example for pain at 1 month and assuming all trials used a comparable scale, MD is ‐0.73 (95% CI ‐1.18 to ‐0.28), and this is similar to the back‐transformed MD reported in our review (MD ‐0.62 (95% CI ‐1.01 to ‐0.23)), and still indicates no clinically important between‐group difference.
If a reader was to decide that Clark's study should not be included in that analysis as it used the NRS, then sensitivity analysis with these data removed produces a similar result: MD ‐0.58 (95% CI ‐1.08 to ‐0.08) (still no important clinical difference between groups, and still no change in overall conclusion).
Minimally important differences: We stand by our interpretation of the VAPOUR trial. The review included five placebo‐controlled trials, of which the trial by Clark et al (VAPOUR trial) was one. As per Cochrane review standards (MECIR), we used minimally important differences (MID) between groups to aid in interpretation of the difference between treatment and the 95% confidence intervals (CI). For pain, we assumed a minimal clinically important difference of 1.5 points on a 10‐point pain scale (Dworkin et al; J Pain 2008; 9:105‐21). Thus, if a clinical difference was to exist between groups the mean reduction in pain and the 95% CI’s would have to be larger than ‐1.5 points on a 10 point pain scale. Thus, overall, the evidence shows no clinically important difference between vertebroplasty and control groups with regard to pain (applying the MID to the mean difference and the 95% CIs). Looking at the data for the Clark trial the 95% confidence intervals indicate that an important difference in pain between vertebroplasty and placebo can neither be confirmed nor excluded even though it is a statistically significant result (MD ‐1.40, 95% CI ‐2.46 to ‐0.34).
Combining data from five trials gives more certainty than focussing on the results from one study and looking for reasons why it might differ. This is especially important in the absence of any evidence of statistical heterogeneity between the studies as we can see here. When we combined data from three trials that included a measure of the proportion of participants whose pain improved from baseline by a 'clinically important' amount, there was still no important difference between groups.
Application of the Cochrane risk of bias tool to included trials: The authors applied the Cochrane risk of bias tool to all included studies. Each trial was reviewed by at least two, up to four review authors, which helps ensure that all included studies are assessed fairly.
Hospitalised patients: We agree that acutely painful osteoporotic spinal fractures may warrant hospitalisation. However, we consider that being hospitalised does not necessarily indicate more severe symptoms. In any case their assertion that all other placebo‐controlled trials excluded hospitalised patients is incorrect. Buchbinder 2009 included both outpatients and hospitalised patients as has been reported previously (e.g. see Buchbinder R, Osborne RO, Kallmes D. Invited editorial presents an accurate summary of the results of two randomised placebo‐controlled trials of vertebroplasty. MJA 2010; 192(6):338‐41)). In addition, hospitalisation does not appear to have been an exclusion criterion of either Firanescu 2018 or VOPE.
Interpreting success of blinding: We agree with Clark et al that success of blinding data need to be interpreted carefully due to bias that may arise when trial participants experience good or poor outcomes. It is also true that Buchbinder et al only measured blinding success at the end of the trial and with hindsight it would have made more sense for this to be captured much earlier for the reasons outlined. Success of blinding was in the study protocol from the outset (see trial registration).
The issue however is that unlike all the other trials that measured and reported blinding success, VAPOUR was the only one to have found a large difference between those in the placebo and active groups. While this could be due to better outcomes for those in the active group, another plausible interpretation is lack of success of blinding. In addition, success of blinding was only reported for a subset of patients and there was also a failure to report day 3 results of perception of treatment (which may or may not have found conflicting results), both of which may represent potential reporting bias.
We did not consider that the VAPOUR protocol paper made it clear that a needle was inserted. However, readers can decide for themselves as we report the wording used in both the protocol and results papers in the Table of Included Studies.
Staples 2011: The Cochrane review performed its own subgroup analysis to investigate the effect of symptom duration on outcome from vertebroplasty. It is correct that in Staples 2011 we performed separate analyses as outlined. However both trials included patients with severe pain. Baseline pain scores in these trials had comparable or greater baseline levels of pain compared with VAPOUR and combined individual level data indicated mean pain scores of 7.1 in both treatment groups.
We agree that Buchbinder 2009 was powered to look for a treatment benefit of 2.5 units in pain scores (SD 3.0) and this was based upon the existing data at the time (controlled before‐after studies and single arm trials only). However it also failed to detect even smaller but clinically important differences. We agree that VAPOUR failed to find a difference of this magnitude and neither did the other placebo‐controlled trials. They also failed to find smaller but still clinically important benefits.
The assertion that Staples 2011 had insufficient power to detect smaller between‐group differences is incorrect. As clearly reported in Staples 2011, combining data from the two trials (sample size 209), provided over 94% power to detect a 1.5 unit difference in pain (assuming SD 3.0) and 88% power to detect a 3 unit difference in the Roland Morris Disability Questionnaire (assuming SD 6.7). There is a typographical error in the text regarding numbers of participants in the vertebroplasty and control subgroups with pain 6 weeks or less but this does not change the interpretation of the data.
There are no inconsistencies between the data reported in Staples 2011 compared to Buchbinder 2009 and Kallmes 2009. The data reported in Staples report mean pain in both trials overall, compared with mean pain per treatment group in Kallmes 2009 and median pain per treatment group in Buchbinder. The data for the overall and subgroup analyses are correct.
Cement volume: We agree that Clark injected more cement compared with both Kallmes 2009 and Buchbinder 2009 and this is clearly reported in our review. Both trials however described filling the vertebrae. In addition Firanescu 2018 also injected a greater volume of cement (range 1 to 11 ml, mean of 5.11 ml), although they did not observed clinically important benefits. In a post‐hoc analysis of data from Buchbinder 2009, there was an increased odds of cement leakage with higher volumes of injected cement (Odds Ratio [OR] 2.8; 95% CI 1.3 to 6.1), but no relationship between cement volume and efficacy outcome (Kroon F, Staples MP, et al. Vertebroplasty for osteoporotic vertebral fractures: Two‐year results from a randomized controlled trial. Journal of Bone and Mineral Research 2014;29(6):1346‐55). The finding of no relationship between cement volume and efficacy outcomes has also been reported in other studies (e.g. Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2006;27(9):1933‐7. ; Al‐Ali F, Barrow T, Luke K. Vertebroplasty: what is important and what is not. AJNR Am J Neuroradiol. 2009;30(10):1835‐9.) In updating our review to include Firanescu 2018, we have reinserted and added to the discussion about volume of cement to make these points.
Contributors
Rachelle Buchbinder and Renea Johnston draft the response.
The rest of the authors commented and approved the draft.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2018 | Amended | Further clarifications from authors |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 4, 2015
| Date | Event | Description |
|---|---|---|
| 2 November 2018 | New citation required but conclusions have not changed | Feedback added and changes made in the review to better clarify some aspects. |
| 2 November 2018 | Feedback has been incorporated | Response Dr. Clark's feedback added |
| 29 October 2018 | Amended | Further publication available for VERTOS IV study: reference updated, outcome data added for two more outcomes (disability and disease‐specific quality of life). We had assumed new vertebral fractures were clinical in the conference proceedings but further information in the full trial report indicated they were probably new radiographic fractures and these data were updated. We updated the subgroup analysis to compare 'acute' versus 'subacute' fractures to include data from all five placebo‐controlled trials. |
| 30 April 2018 | Amended | Minor correction in the plain language summary |
| 31 January 2018 | New citation required but conclusions have not changed | Review updated, with nine new trials added; 21 trials included in total. |
| 15 November 2017 | New search has been performed | Review updated and nine new trials included (Clark 2016; Evans 2015; Leali 2016; Sun 2016; Firanescu 2018; VOPE 2015; Wang 2015; Wang 2016; Yang 2016). Three of the new trials included a placebo control (Clark 2016; Firanescu 2018; VOPE 2015). Full results of Firanescu 2018 are not yet published but this review includes results for pain, incident fractures and other adverse events from conference proceedings. One additional placebo‐controlled trial (VERTOS V) remains unpublished. |
| 24 April 2015 | New citation required and conclusions have changed | Review published; 12 studies included. |
| 16 October 2008 | Amended | Converted to RM5. CMSG ID C142‐P |
Acknowledgements
The original review was supported by grants from the University Hospital Foundation and the Canadian Radiology Foundation. The authors would like to thank Ms Louise Falzon, formerly from the Cochrane Musculoskeletal Group, for designing the search strategies for the review; Ms Tamara Rader, formerly Cochrane Musculoskeletal Group Knowledge Translation Specialist for conducting the electronic database searches in the first version of the review; Dr Mauritz Voormolen and Dr Caroline Klazen who provided additional information from their trials; Dr. Sean Crowther and Dr. Ken Ong for assistance with review of abstracts; and Dr. Kerry Siminoski and Dr. Sumit Majumdar for advice in the early stages of the first version of the review.
The authors of this update would like to thank the authors of the original protocol and previous published version of the review for their contribution: RGW Lambert, SR Majumdar, SS Dhillon, R Owen and K Siminoski.
The authors thank Emil Jesper Hansen from the University of Southern Denmark for providing us with his PhD thesis, which reported the results of the VOPE trial.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials
Ovid EBM Reviews ‐ Cochrane Central Register of Controlled Trials <November 2017>
1 exp Spine/ (4032)
2 (spine or spinal or vertebra$).tw. (20306)
3 exp Fractures, Bone/ (3949)
4 fractur$.ti. (6791)
5 1 or 2 (21641)
6 3 or 4 (8007)
7 5 and 6 (1504)
8 exp Spinal Fractures/ (561)
9 7 or 8 (1528)
10 exp Bone Cements/ (769)
11 exp Methylmethacrylates/ (389)
12 methacrylate$.tw. (251)
13 bone cement$.tw. (201)
14 exp Fracture Fixation, Internal/ (1077)
15 exp Vertebroplasty/ (112)
16 vertebroplast$.tw. (202)
17 cementoplast$.tw. (10)
18 sacroplast$.tw. (2)
19 or/10‐18 (2421)
20 9 and 19 (244)
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R) <1946 to 15 Nov 2017>
1 exp Spine/ (139498)
2 (spine or spinal or vertebra$).tw. (418563)
3 exp Fractures, Bone/ (178841)
4 fractur$.ti. (115672)
5 1 or 2 (468594)
6 3 or 4 (191239)
7 5 and 6 (26174)
8 exp Spinal Fractures/ (14540)
9 7 or 8 (27200)
10 exp Bone Cements/ (22211)
11 exp Methylmethacrylates/ (13907)
12 methacrylate$.tw. (14505)
13 bone cement$.tw. (5178)
14 exp Fracture Fixation, Internal/ (40925)
15 exp Vertebroplasty/ (2389)
16 vertebroplast$.tw. (2681)
17 cementoplast$.tw. (136)
18 sacroplast$.tw. (114)
19 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (80212)
20 9 and 19 (4910)
21 randomized controlled trial.pt. (504969)
22 controlled clinical trial.pt. (100398)
23 randomized.ab. (391361)
24 placebo.ab. (189112)
25 drug therapy.fs. (2146488)
26 randomly.ab. (265938)
27 trial.ab. (410806)
28 groups.ab. (1657653)
29 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (4140566)
30 exp animals/ not humans.sh. (4742004)
31 29 not 30 (3539516)
32 20 and 31 (999)
Appendix 3. Embase search strategy
Embase <1974 to 2017 November 13>
1 exp Spine/ (167882)
2 (spine or spinal or vertebra$).tw. (539379)
3 exp Fracture/ (261382)
4 fractur$.tw. (268168)
5 1 or 2 (583524)
6 3 or 4 (343636)
7 5 and 6 (52536)
8 exp Spine Fracture/ (23056)
9 7 or 8 (57421)
10 exp Bone Cement/ (13553)
11 exp Methacrylic Acid Methyl Ester/ (6622)
12 methacrylate$.tw. (19209)
13 bone cement$.tw. (6639)
14 exp Fracture Fixation/ (80116)
15 exp percutaneous vertebroplasty/ (5942)
16 vertebroplast$.tw. (4173)
17 cementoplast$.tw. (264)
18 sacroplast$.tw. (166)
19 or/10‐18 (119819)
20 9 and 19 (9183)
21 random$.tw. (1261919)
22 factorial$.tw. (31693)
23 crossover$.tw. (64719)
24 cross over.tw. (28196)
25 cross‐over.tw. (28196)
26 placebo$.tw. (265608)
27 (doubl$ adj blind$).tw. (184923)
28 (singl$ adj blind$).tw. (20450)
29 assign$.tw. (329237)
30 allocat$.tw. (123296)
31 volunteer$.tw. (228042)
32 crossover procedure/ (54127)
33 double blind procedure/ (145236)
34 randomized controlled trial/ (482169)
35 single blind procedure/ (30196)
36 or/21‐35 (1954138)
37 20 and 36 (905)
Appendix 4. Trial registries
ClinicalTrials.Gov
Intervention: Vertebroplasty
World Health Organization: International Clinical Trials Registry Platform Search Portal
Intervention: Vertebroplasty
Data and analyses
Comparison 1. Efficacy: Vertebroplasty versus placebo (sham).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (0 to 10 point scale) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 5 | 539 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.82, 0.33] |
| 1.2 1 month | 5 | 535 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.18, ‐0.28] |
| 1.3 3 months | 4 | 395 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.01, 0.05] |
| 1.4 6 months | 3 | 338 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.18, 0.01] |
| 1.5 12 months | 3 | 265 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.02, 0.18] |
| 1.6 24 months | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.68, 0.48] |
| 2 Proportion of participants with pain improved by a clinically relevant amount (>2.5 units or 30% on a 0 or 1 to 10 scale from baseline or less than 4 out of 10 | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 1 to 2 weeks | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.78, 2.60] |
| 2.2 1 month | 3 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.99, 2.36] |
| 2.3 3 months | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.12, 2.30] |
| 2.4 6 months | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.02, 1.87] |
| 2.5 12 months | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.06, 1.58] |
| 2.6 24 months | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.84, 2.42] |
| 3 Disability (RMDQ) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 to 2 weeks | 4 | 475 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐1.23, 1.65] |
| 3.2 1 month | 4 | 472 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.61, ‐0.38] |
| 3.3 3 months | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐3.15, 1.52] |
| 3.4 6 months | 3 | 324 | Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐4.12, 0.47] |
| 3.5 12 months | 2 | 200 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐1.56, 1.80] |
| 3.6 24 months | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐3.67, 3.87] |
| 4 Quality of life (QUALEFFO) [0 to 100] | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1 to 2 weeks | 3 | 352 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.96, 0.75] |
| 4.2 1 month | 3 | 351 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐6.06, 1.41] |
| 4.3 3 months | 2 | 244 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐4.23, 3.39] |
| 4.4 6 months | 3 | 330 | Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐6.66, 2.91] |
| 4.5 12 months | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐5.41, 2.77] |
| 4.6 24 months | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐5.48, 8.08] |
| 5 Treatment success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 1 week | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 1 month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of Life (EQ5D) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 1 to 2 weeks | 2 | 164 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 6.2 1 month | 3 | 285 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 6.3 3 months | 3 | 203 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 6.4 6 months | 2 | 156 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.10] |
| 6.5 12 months | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 6.6 24 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
Comparison 2. Efficacy: Vertebroplasty versus usual care (open label).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (0 or 1 to 10 point scale) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 6 | 627 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.26, ‐0.39] |
| 1.2 1 month | 3 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.35, ‐0.76] |
| 1.3 2 to 3 months | 6 | 627 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.95, ‐0.40] |
| 1.4 6 months | 5 | 573 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.82, ‐0.28] |
| 1.5 12 months | 6 | 612 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.74, ‐0.30] |
| 1.6 24 months | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, 0.01] |
| 2 Disability (RMDQ [0 to 24] or ODI [0 to 100]) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 to 2 weeks | 5 | 494 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.28, ‐0.83] |
| 2.2 1 month | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.00, ‐0.04] |
| 2.3 3 months | 4 | 460 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐4.65, ‐0.87] |
| 2.4 6 months | 4 | 461 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐3.37, ‐0.30] |
| 2.5 12 months | 4 | 455 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐2.79, ‐0.38] |
| 2.6 24 months | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.65 [‐6.67, ‐4.63] |
| 3 Quality of Life (QUALEFFO) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 to 2 weeks | 4 | 448 | Mean Difference (IV, Random, 95% CI) | ‐5.67 [‐11.65, 0.32] |
| 3.2 1 month | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐10.18 [‐21.49, 1.13] |
| 3.3 2 to 3 months | 3 | 415 | Mean Difference (IV, Random, 95% CI) | ‐5.83 [‐15.41, 3.75] |
| 3.4 6 months | 3 | 415 | Mean Difference (IV, Random, 95% CI) | ‐5.14 [‐15.02, 4.74] |
| 3.5 12 months | 3 | 415 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐9.90, 3.11] |
| 4 Quality of life (EQ5D) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1 to 2 weeks | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.00, 0.15] |
| 4.2 1 month | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.01, 0.16] |
| 4.3 3 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.00, 0.20] |
| 4.4 6 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.02, 0.15] |
| 4.5 12 months | 2 | 215 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] |
| 5 Treatment success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.5. Analysis.
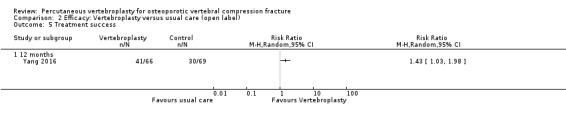
Comparison 2 Efficacy: Vertebroplasty versus usual care (open label), Outcome 5 Treatment success.
Comparison 3. Efficacy: Vertebroplasty versus kyphoplasty (balloon).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (0 to 10 point scale) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 weeks | 2 | 462 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.25] |
| 1.2 1 month | 2 | 441 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.59, 0.48] |
| 1.3 3 months | 2 | 419 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.11, 0.39] |
| 1.4 6 months | 3 | 230 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.31, 0.22] |
| 1.5 12 months | 4 | 558 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.07, 0.40] |
| 1.6 24 months | 2 | 320 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.56, 0.27] |
| 2 Disability (ODI) | 4 | 1758 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.12, 0.07] |
| 2.1 1 to 2 weeks | 1 | 98 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.38, 0.41] |
| 2.2 1 month | 2 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 2.3 3 months | 2 | 399 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.16, 0.24] |
| 2.4 6 months | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.46, 0.35] |
| 2.5 12 months | 4 | 542 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.16, 0.17] |
| 2.6 24 months | 1 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.35, 0.21] |
| 3 Quality of Life (EQ5D) | 2 | 1346 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.04, 0.17] |
| 3.1 1 month | 2 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.09, 0.29] |
| 3.2 3 months | 1 | 292 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 3.3 6 months | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.39, 0.45] |
| 3.4 12 months | 2 | 342 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.15, 0.27] |
| 3.5 24 months | 1 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.18, 0.37] |
Comparison 4. Efficacy: Vertebroplasty versus facet joint injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (0 to 10 point scale) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 1 to 2 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Disability (RMDQ) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 1 to 2 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of Life (SF‐36) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 1 month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.3. Analysis.
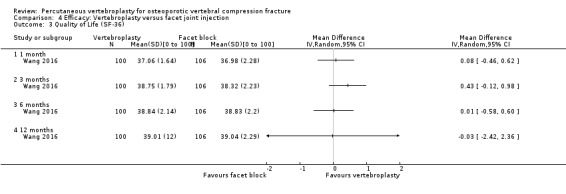
Comparison 4 Efficacy: Vertebroplasty versus facet joint injection, Outcome 3 Quality of Life (SF‐36).
Comparison 5. Safety: Vertebroplasty versus placebo (sham) or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New clinical vertebral fractures | 6 | 840 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.46, 3.62] |
| 2 New radiographic vertebral fractures | 8 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.71, 1.84] |
| 3 Number of serious other adverse events | 5 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.10] |
Comparison 6. Safety: Vertebroplasty versus kyphoplasty.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New clinical vertebral fractures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 New radiographic vertebral fractures | 2 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.97, 1.43] |
| 2.1 12 months | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.21, 3.16] |
| 2.2 24 months | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.92, 1.51] |
| 3 Number of serious other adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 12 months | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.14] |
| 3.2 24 months | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.42, 1.97] |
Comparison 7. Safety: Vertebroplasty versus facet joint injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New radiographic vertebral fractures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Subgroup analysis: 'Acute' fractures versus 'subacute' fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at 1 to 2 weeks | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 'Acute' fractures | 5 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.38, 0.14] |
| 1.2 'Subacute' fractures | 2 | 157 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.30, 0.33] |
| 2 Pain at 1 month | 5 | 535 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.44, ‐0.10] |
| 2.1 'Acute' fractures | 5 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.46, ‐0.05] |
| 2.2 'Subacute' fractures | 2 | 157 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.61, 0.02] |
| 3 Disability at 1 to 2 weeks | 4 | 467 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.16, 0.20] |
| 3.1 'Acute' fractures | 4 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.29, 0.22] |
| 3.2 'Subacute' fractures | 2 | 141 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.20, 0.46] |
| 4 Disability at 1 month | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 'Acute' fractures | 4 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.47, ‐0.04] |
| 4.2 'Subacute' fractures | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.47, 0.19] |
| 5 Quality of life (EQ‐5D) at 1 month | 3 | 281 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.08] |
| 5.1 'Acute' fractures | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 5.2 'Subacute' fractures | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
Comparison 9. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at 1 to 2 weeks (0 or 1 to 10 point scale) | 11 | 1166 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.30, ‐0.22] |
| 1.1 Sham (placebo) control | 5 | 539 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.30, 0.12] |
| 1.2 Usual care (open label) control | 6 | 627 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.26, ‐0.39] |
| 2 Pain at 1 month (0 or 1 to 10 point scale) | 8 | 919 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.55, ‐0.34] |
| 2.1 Sham (placebo) control | 5 | 535 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.44, ‐0.10] |
| 2.2 Usual care (open label) control | 3 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.35, ‐0.76] |
| 3 Pain at 3 months (0 or 1 to 10 point scale) | 10 | 1021 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.28, ‐0.29] |
| 3.1 Sham (placebo) control | 4 | 394 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.40, ‐0.00] |
| 3.2 Usual care (open label) control | 6 | 627 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.95, ‐0.40] |
| 4 Disability at 1 to 2 weeks (RMDQ [0 to 24] or ODI [0 to 100])) | 8 | 793 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐2.06, ‐0.43] |
| 4.1 Sham (placebo) control | 3 | 299 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.34, 0.34] |
| 4.2 Usual care (open label) control | 5 | 494 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.28, ‐0.83] |
| 5 Disability at 1 month (RMDQ [0 to 24] or ODI [0 to 100]) | 6 | 674 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.60, ‐0.17] |
| 5.1 Sham (placebo) control | 3 | 296 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.50, ‐0.04] |
| 5.2 Usual care (open label) control | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.00, ‐0.04] |
| 6 Disability at 3 months (RMDQ [0 to 24] or ODI [0 to 100]) | 6 | 622 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐3.08, ‐0.61] |
| 6.1 Sham (placebo) control | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.78, 0.55] |
| 6.2 Usual care (open label) control | 4 | 460 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐4.65, ‐0.87] |
9.1. Analysis.
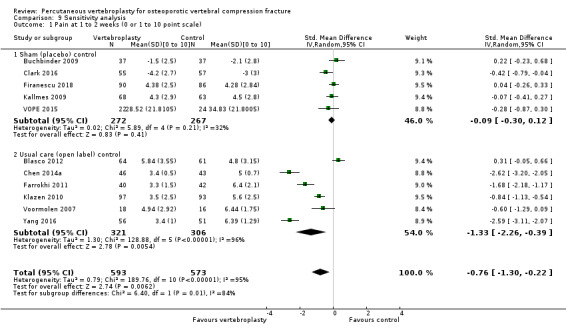
Comparison 9 Sensitivity analysis, Outcome 1 Pain at 1 to 2 weeks (0 or 1 to 10 point scale).
9.2. Analysis.
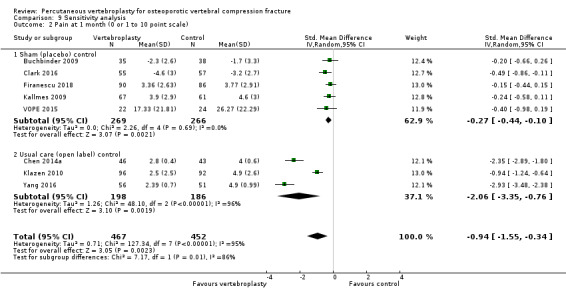
Comparison 9 Sensitivity analysis, Outcome 2 Pain at 1 month (0 or 1 to 10 point scale).
9.3. Analysis.
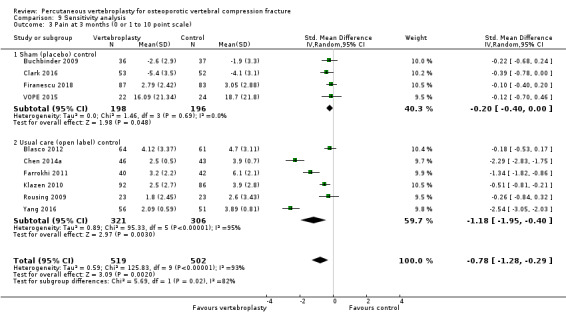
Comparison 9 Sensitivity analysis, Outcome 3 Pain at 3 months (0 or 1 to 10 point scale).
9.4. Analysis.
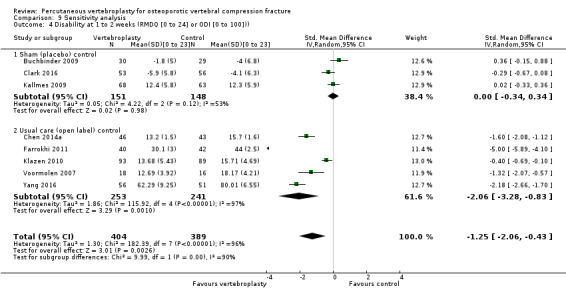
Comparison 9 Sensitivity analysis, Outcome 4 Disability at 1 to 2 weeks (RMDQ [0 to 24] or ODI [0 to 100])).
9.5. Analysis.
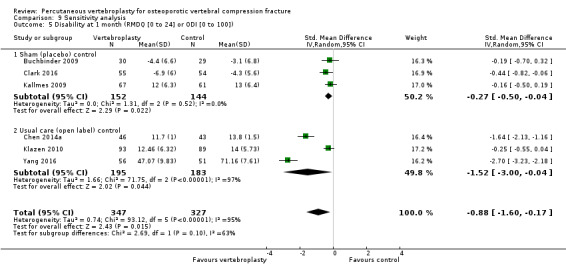
Comparison 9 Sensitivity analysis, Outcome 5 Disability at 1 month (RMDQ [0 to 24] or ODI [0 to 100]).
9.6. Analysis.
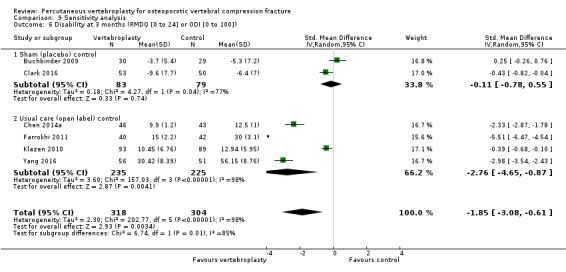
Comparison 9 Sensitivity analysis, Outcome 6 Disability at 3 months (RMDQ [0 to 24] or ODI [0 to 100]).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blasco 2012.
| Methods |
Design: single‐centre parallel group, two‐arm open‐label randomised controlled trial Setting: patients recruited from primary care centres, Spain Timing: April 2006 to Jan 2010 Intervention: percutaneous vertebroplasty and usual care versus usual care alone Sample size: 64 patients required per group to have 80% power to detect a difference of at least 1.5 units on a 0‐10 VAS between groups in primary pain endpoint; overall type‐1 error rate was set at 5% Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group (64 participants): Mean (SD) age: 71.33 (9.95); 47 female, 17 male Mean (SD) duration of back pain: 140.3 (96.09) days Number (%) participants with symptom onset < 6 weeks: 2 (3%) Number (%) participants with symptom onset < 4 months: 32 (50%) Mean (SD) number of vertebral fractures at baseline: 3.55 (2.82) Mean (SD) baseline pain score 7.21 (0.33) on a 0‐10 VAS (higher score indicates worse pain) Mean (SD) QUALEFFO‐41 score: 65.19 (2.23) on a 0 to 100 scale (higher score indicates worse quality of life) Usual care group (61 participants): Mean (SD) age: 75.27 (8.53); 50 female, 11 male Mean (SD) duration of back pain: 143.1 (130.33) days Number (%) participants with symptom onset < 6 weeks: 4 (7%) Number (%) participants with symptom onset < 4 months: 32 (52.5%) Mean (SD) number of vertebral fractures at baseline: 3.02 (2.14) Mean (SD) baseline pain score: 6.31 (0.35) Mean (SD) QUALEFFO‐41 score: 59.17 (2.17) |
|
| Interventions |
Vertebroplasty group Percutaneous vertebroplasty was performed by an experienced interventional neuroradiologist. A 25‐gauge needle was used to infiltrate the skin overlying the pedicle and to infiltrate the periosteum of the posterior lamina. Using a bilateral transpedicular approach a 10‐ or 13‐gauge needle was inserted posterolaterally relative to the eye of the pedicle, and through gentle tapping the needle penetrated the pedicle into the anterior two‐thirds of the fractured vertebrae and polymethylmethacrylate cement was injected. Following the procedure, participants were strictly rested in bed for 6 hours. Standardised analgesics were given as necessary and nasal calcitonin was given for the first month. Usual care group All participants received analgesics with a standardised format and nasal calcitonin for the first month. In case of no improvement in pain, the participant was considered for vertebroplasty (timing of decision not specified). Both groups When treatment in either group was ineffective (defined as pain 7 or more out of 10) or there was an intolerance to drug therapy, participants were offered rescue therapy consisting of an intrathecal infusion of 25 μg fentanyl and 1.5 mg bupivacaine. After one month, both treatment groups began or continued treatment with bisphosphonates (or teriparatide or strontium ranelate if there was an intolerance to bisphosphonates), prescribed by the attending physician. |
|
| Outcomes | Participants were assessed at baseline, 2 weeks, and at 2, 6 and 12 months. Outcomes
Outcomes included in this review
|
|
| Source of funding | Fundacio La Marato de TV3, the Spanish Society of Medical Radiology and the Catalan Society of Rheumatology | |
| Notes | Trial registered on 13 Oct 2009 at ClinicalTrials.gov, registration number NCT00994032. We extracted pain and quality of life data at 2 weeks, 2 months (pooled with the 3‐month outcome data from other trials), 6 and 12 months. These outcomes were reported in graphical format only; we extracted the mean and 95% confidence intervals from the graphs (http://plotdigitizer.sourceforge.net/) and converted 95% CI to SD. Adverse events were only reported for vertebroplasty. It is not reported if any adverse events occurred with usual care. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed using a computer‐generated random list. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants assessed their pain and quality of life and were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Rheumatologists who assessed radiographs and MRIs were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The proportion lost to follow‐up was similar between groups at the 12‐month follow‐up (17/64 or 27% from vertebroplasty and 13/61 or 21% from usual care), although the authors report that the losses may not have been random, but related to worse pain in the usual care group. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported for vertebroplasty group; it is unclear if any adverse events occurred in the usual care group; mean pain and quality of life and confidence intervals were reported graphically only. |
| Other bias | Low risk | None apparent. |
Buchbinder 2009.
| Methods |
Design: multicentre (four sites), parallel group, two‐arm double‐blind randomised placebo‐controlled trial Setting: Melbourne, Australia Timing: April 2004 to October 2010 Interventions: percutaneous vertebroplasty versus sham vertebroplasty (placebo) Sample size: 24 participants per group required to detect at least a 2.5‐unit (SD 3) advantage of vertebroplasty over placebo in terms of pain (0 to 10 point scale), based on a two‐sided type 1 error rate of 5% and power 80%. Additional sample size calculation of 82 participants per group needed to show an increase by a factor of three in the risk of further vertebral fractures at 24 months. Original sample size was increased to 200 to allow for potential dropout. Trial enrolment terminated before reaching planned sample size for long‐term outcomes because it became evident that this sample size would not be achieved within an acceptable period of time and that the study’s power was sufficient to address the primary aim. This decision was made without knowledge of any outcome results. Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 74.2 (14.0) years; 31 female: 7 male Median (interquartile range (IQR)) duration of back pain: 9.0 (3.8 to 13.0) weeks Duration of back pain < 6 weeks: N = 12 (32%) Mean (SD) baseline pain score: 7.4 (2.1) Mean (SD) baseline QUALEFFO score: 56.9 (13.4) Mean (SD) RMDQ: 17.3 (2.8) Any medication for osteoporosis: N = 35 (92%); bisphosphonates: N = 31 (82%) Previous vertebral fractures: 18 (47%) Opioid use at baseline: 30 (79%) T score for bone mineral density (BMD) 2.5 or less at lumbar spine: 21/34; at femoral neck: 13/34 Placebo group: Mean (SD) age: 78.9 (9.5) years; 31 female: 9 male Median (IQR) duration of back pain: 9.5 (3.0 to 17.0) weeks Duration of back pain < 6 weeks: N = 13 (32%) Mean (SD) baseline pain score: 7.1 (2.3) Mean (SD) baseline QUALEFFO score: 59.6 (17.1) Mean (SD) RMDQ: 17.3 (2.9) Any medication for osteoporosis: N = 37 (92%); bisphosphonates: N = 32 (80%) Previous vertebral fractures: 21 (52%) Opioid use at baseline: 34 (85%) T score for bone mineral density (BMD) 2.5 or less at lumbar spine: 21/28; at femoral neck: 15/28 |
|
| Interventions | Percutaneous vertebroplasty or placebo (sham) procedure, performed by experienced interventional radiologists, who had formal training in vertebroplasty and appropriate certification. Vertebroplasty The left pedicle of the fracture site was identified with the use of a metallic marker. A 25‐gauge needle was used to infiltrate the skin overlying the pedicle and a 23‐gauge needle was used to infiltrate the periosteum of the posterior lamina. An incision was made in the skin, and a 13‐gauge needle was placed posterolaterally relative to the eye of the pedicle. Gentle tapping guided the needle through the pedicle into the anterior two thirds of the fractured vertebral body. Anterior‐posterior and lateral images were recorded with the needle in the correct position. Prepared PMMA (approximately 3 mL) was slowly injected into the vertebral body, infiltration of the vertebral body was confirmed radiographically. A bipedicular approach was used only if there was inadequate instillation of cement with the unipedicular approach. Injection was stopped when substantial resistance was met or when the cement reached the posterior quarter of the vertebral body; injection was also stopped if cement leaked into extraosseous structures or veins. All participants in the vertebroplasty group received cephalothin, administered intravenously immediately after PMMA injection. Sham procedure (placebo) The same procedures as those in the vertebroplasty group up to the insertion of the 13‐gauge needle to rest on the lamina. The central sharp stylet was then replaced with a blunt stylet. To simulate vertebroplasty, the vertebral body was gently tapped, and PMMA was prepared so that its smell permeated the room. Follow‐up care After the intervention, all participants received usual care. Treatment decisions were made at the discretion of the treating physician, who received up‐to‐date guidelines on the management of osteoporosis. Analgesia was given according to standard practice, and its use was recorded. |
|
| Outcomes | Outcomes were reported at 1 week, and 1, 3, 6, 12 and 24 months. Primary endpoint was 3 months. Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | The study was supported by grants from the National Health and Medical Research Council of Australia (284354), Arthritis Australia, the Cabrini Education and Research Institute, and Cook Australia. Cook Australia had no role in the design of the trial, the collection or analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication. | |
| Notes | Trial registered at anzctr.org.au, number ACTRN012605000079640 on 5 August 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation (in permuted blocks of 4 and 6) were generated by computer‐generated random numbers; stratified according to treatment centre, sex and duration of symptoms (< 6 weeks or ≥ 6 weeks). |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure concealment of the assigned intervention, the treating radiologist received the opaque, sealed envelope containing the assigned intervention just prior to the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants and investigators, other than the treating radiologists, were unaware of treatment assignments. At 24 months 55 participants completed the assessment of blinding question. In the vertebroplasty group 16/29 believed they had received vertebroplasty, 2 believed they had received placebo and 11 were unsure; in the placebo group 8/26 believed they had received vertebroplasty, 7 believed they had received placebo and 11 were unsure. The Blinding Index was 0.59 (95% CI, 0.47–0.71), indicating adequate blinding at the end of the study. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | Participants (who assessed their pain, disability, quality of life and treatment success) were unaware of treatment assignment. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Assessment of the timed up‐and‐go test was performed by a blinded outcome assessor at 12 and 24 months. Radiologists who assessed follow‐up radiographs and MRIs after 24 months were aware of treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was small and equal across treatment groups for shorter‐term outcomes. There was complete follow‐up at one month for 35/38 from the vertebroplasty group (3 did not return questionnaire) and 38/40 from the sham group (2 did not return questionnaire); at 6 months this was 35/38 from the vertebroplasty group (2 died for reasons considered unrelated to the trial and 1 did not return their questionnaire), and 36/40 from the sham group (2 died for reasons considered unrelated to the trial and 2 did not return their questionnaires). Loss to follow‐up was greater for longer‐term outcomes. At two years, 29/38 (76%) participants in the vertebroplasty group had completed follow‐up (5 had died, 1 withdrew due to dementia and 3 did not return questionnaires), and 28/40 (70%) participants in the sham group completed follow‐up (7 had died, 1 withdrew due to illness and 4 did not return questionnaires). |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the published protocol were reported. |
| Other bias | Low risk | None apparent. |
Chen 2014a.
| Methods |
Design: single‐centre parallel group, two‐arm open‐label randomised controlled trial Setting: Shanghai, China Timing: Jan 2007 to Dec 2012 Interventions: percutaneous vertebroplasty or usual care Sample size:a priori sample size calculation not reported Analysis: completers' analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 64.6 (9.1) years; 32 female:14 male Duration (SD) of back pain: 7.07 (3.00) months Mean (SD) number of vertebral fractures at baseline 2.28 (1.00) Mean (SD) baseline pain score: 6.5 (0.9) N (%) use of osteoporotic drugs: 12 (26%) Bone density T score (SD): ‐3.02 (0.80) Usual care group: Mean (SD) age: 66.5 (9.1) years; 30 female:13 male Duration (SD) of back pain: 6.81 (2.51) months Mean (SD) number of vertebral fractures at baseline 2.00 (0.09) Mean (SD) baseline pain score: 6.4 (0.9) N (%) use of osteoporotic drugs: 18 (42%) Bone density T score (SD): ‐3.00 (0.44) |
|
| Interventions | Percutaneous vertebroplasty performed by orthopaedic surgeons. Vertebroplasty All procedures were performed under local anaesthesia and undertaken on a single plane angiography system under fluoroscopic guidance in the operating theatre. The patient was placed in a prone position on the operating table. After local anaesthesia, a small incision was made with a scalpel blade. Thereafter, a bone puncture needle (13 G, Cook Medical, Bloomington, IN, USA) was placed transpedicularly in the fractured vertebra. After removal of the inner needle, commercially available polymethyl methacrylate (PMMA) (Osteo‐Firm, Cook Medical) was carefully injected into the fractured vertebra under continuous fluoroscopic monitoring via lateral and anteroposterior (AP) projections in order to ensure adequate lesion filling and to avoid PMMA leakage or migration into the venous system Injection was ceased when substantial resistance was met or when the cement reached the cortical edge of the fractured vertebral body; injection was also stopped if cement leaked into extraosseous structures or veins. In general, a total of 3 mL to 5 mL of PMMA was injected into the fractured vertebral body. Post‐procedural fluoroscopic evaluation was obtained to show optimal filling of the lesion with no evidence of PMMA extravasation. Vertebroplasty was also performed with one or more procedures on other fractures seen on MRI at adjacent levels above and below the chronic osteoporotic compression fractures to prevent new fractures. After the procedure, a CT scan of the treated vertebral bodies was done with 2 mm slices to identify the distribution of cement in the lesion, cement leakage outside the vertebral body, or other local complications. Conservative care Participants in the conservative care group were hospitalised and offered brace treatment, analgesia, general mobilising physiotherapy and treatment for osteoporosis including calcitriol and alendronate. |
|
| Outcomes | Outcomes were reported at 1 day, 1 week, and 1, 3, 6 and 12 months after treatment. Primary outcomes
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | Not reported. | |
| Notes | There is no record of trial registration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to receive either vertebroplasty or conservative treatment". However, the method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided about whether or not treatment allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and investigators were aware of treatment allocation. It is not clear if participants in the percutaneous vertebroplasty group were offered treatment for osteoporosis. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants assessed their pain and function and were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | It is not stated who assessed the imaging outcomes. It is unlikely that they were blinded to treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No flow diagram is provided. Of the 50 participants allocated to receive conservative care, four refused conservative treatment and decided to have vertebroplasty at the 3‐month follow‐up and an additional three were lost to follow‐up. Results are presented for only 43 participants. The authors state that of the 46 participants allocated to receive vertebroplasty, four were lost to follow‐up but they appear to perform the analysis on all 46 participants to 12 months. |
| Selective reporting (reporting bias) | Unclear risk | The one‐day outcomes are only reported for mean pain. Hospital stay, outpatient visits and medical aids were not reported. The trial does not appear to have been registered and no trial protocol is published. |
| Other bias | Unclear risk | It is not clear how many 'prophylactic' procedures were performed in the vertebroplasty group. |
Clark 2016.
| Methods |
Design: multicentre (nine sites), parallel group, double‐blind randomised placebo‐controlled trial Setting: Sydney, Australia Timing: November 2011 to December 2014 Interventions: percutaneous vertebroplasty versus sham vertebroplasty (placebo) Sample size: 60 patients required per group to have greater than 80% power and 95% confidence to detect a difference in the proportion of patients achieving a 14‐day pain score of less than 4 out of 10 (from a baseline score greater than or equal to 7 out of 10) from 35% in the control group to 65% in the active group (as stated in the results paper). The protocol paper presents different primary endpoints in different parts of the manuscript both consistent with the results paper as well as the proportion of patients whose pain score reduces to 4 or less out of 10 at 14 days. Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 80 (7) years; 48 female: 13 male Hospital inpatient: 34 (56%) Previous osteoporotic fractures: 36 (62%) Medications for osteoporosis:
Bone mineral density (T score)
Serum vitamin D (nmol/L): 70 (27) Duration of fracture:
Spinal segment of fracture:
Vertebral body height loss (percentage points): 47 (15) Genant grade (standing)
Number of vertebral bodies treated
Opioids for pain: 53 (87%) Baseline measures
Placebo group: Mean (SD) age: 81 (7) years; 40 female: 19 male Hospital inpatient: 34 (58%) Previous osteoporotic fractures: 32 (54%) Medications for osteoporosis:
Bone mineral density (T score)
Serum vitamin D (nmol/L): 73 (22) Duration of fracture:
Spinal segment of fracture:
Vertebral body height loss (percentage points): 46 (15) Genant grade (standing)
Number of vertebral bodies treated
Opioids for pain: 53 (90%) Baseline measures
|
|
| Interventions | Percutaneous vertebroplasty or placebo (sham) procedure, performed by interventional radiologists who had undergone vertebroplasty training and were actively providing a vertebroplasty service. Vertebroplasty Participants were positioned prone on the procedure table with oxygen mask applied. Pulse oximetry and rate were monitored continuously. IV midazolam and fentanyl were given to all patients before starting the procedure. Lidocaine was injected subcutaneously and a 4 mm skin incision was made. An 11‐gauge or 13‐gauge vertebroplasty needle was introduced into the vertebral body with either a unipedicular or bipedicular technique with fluoroscopic guidance. An AVAMAX kit (CafeFusion Corporation) according to the approved kit technique. The aim was to fill the vertebral body with sufficient PMMA to prevent future collapse – from superior to inferior endplate, mid‐pedicle in frontal projection, and from anterior cortex to posterior third of vertebral body. Injection was ceased when these endpoints were reached or if PMMA extravasated outside the bone. PMMA volume and extravasation were recorded. Sham procedure (placebo) The placebo procedure, designed to simulate vertebroplasty, included subcutaneous lidocaine but not periosteal numbing. It is unclear whether or not a needle was passed into the skin incision: the protocol states that a 4‐mm skin incision will be made with light tapping on the skin; the results paper states that a short needle was passed into the skin incision but not as far as the periosteum and that manual skin pressure and regular tapping on the needle was done to mimic vertebroplasty needle advance. Conversation about PMMA mixing and injection suggested that vertebroplasty was being done. The results paper states "that the vertebroplasty kit used in the trial is virtually odourless, with a closed mixing and delivery system, and was not opened during placebo procedures—patients could not smell, hear, or see it." Follow‐up care After the procedure, participants received the usual medical care directed by their attending physicians. |
|
| Outcomes | Outcomes were measured at baseline, 3 days, 14 days, and 1, 3, and 6 months. Baseline, 14 day, and 6 month interviews were done in person by research staff. The remaining interviews (day 3, month 1 and 3) were done by telephone by the same, masked research staff. An exception to this will be inpatients that remain in hospital at day 3 who may be interviewed at bedside. Patients unable to visit the research office at day 14 and 6 months were interviewed by telephone. The following outcomes were extracted from the protocol paper. Primary outcome
Secondary outcomes
The results paper included additional outcomes of participant rating of their confidence in the guess regarding treatment allocation at 2 weeks as a percentage, and nomination of the main reason for their guess; outcome assessor also guessed treatment allocation at 2 weeks, their confidence in the guess and main reason based on observed changes in patient mobility and apparent pain since baseline. The results paper also reported that the investigators had made changes to some outcome measures after trial commencement including removal of a planned SF‐36 Health Survey at all time points and QUALEFFO at 3 days and 3 months (SF‐36 not pre‐planned and QUALEFFO not planned to be measured at these time points according to the published protocol paper. They also stated that the questionnaire to assess masking efficacy was added after trial commencement (although success of blinding at 3 days and 14 days was pre‐specified in the published protocol) and that incomplete data collection occurred due to some sick, elderly patients having difficulty completing the long questionnaires. Outcomes included in this review
|
|
| Source of funding | The study was funded by an unrestricted educational grant from CareFusion Corporation (San Diego, CA, USA). Carefusion Corporation had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication. Study sponsor: Optimus Clinical Research |
|
| Notes | Clinicaltrials.gov trial identifier: NCT01482793 registered on 28 November 2011. For success of blinding, results were only presented for a subset of participants (35/55 (60%) and 35/57 (61.4%) in the vertebroplasty and placebo groups respectively at 14 days, and not reported at 3 days.80% of the vertebroplasty group correctly guessed their treatment assignment (vertebroplasty), compared with 58% of the placebo group (sham procedure) measured at 2 weeks. Success of blinding at 3 days, a pre‐specified outcome, was not reported. Three patients in each group died from causes judged unrelated to the procedure. In the vertebroplasty group there were two serious adverse events related to the procedure. One patient had a respiratory arrest after administration of sedation, before starting the procedure. The patient was resuscitated and underwent the trial procedure two days later without incident. One patient sustained a supracondylar humerus fracture in a paretic arm during transfer onto the radiology table. The fracture healed with a plaster cast. In the control group there were two serious adverse events related to the fracture. Both patients developed spinal cord compression due to interval collapse and retropulsion of the fracture several weeks after enrolment. Neither had substantial fracture retropulsion at time of study enrolment. One patient underwent spinal decompressive surgery with resolution of the neurological deficit. The other patient, not considered a surgical candidate, became paraplegic. A pre‐specified sub‐group analysis comparing outcomes for three different regions of the spine (thoracic (T4 to T10), thoraco‐lumbar (T11 to L2) and lumbar (L3‐L5).) excluding those with two acute fractures involving more than one of these regions, was reported in the results paper, comparing thoracic or lumbar fractures combined versus outcomes for thoraco‐lumbar fractures. Their logistic regression model showed strong evidence ( P = 0.0012) of different treatment outcomes between these spinal segments (i.e. benefit of vertebroplasty only for a thoraco‐lumbar fracture). Another subgroup analysis reported to be pre‐specified (but not reported in the protocol paper) found no significant difference ( P = 0.12) in treatment outcomes between fracture age ≤ 3 weeks versus > 3 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (1:1) to receive either vertebroplasty or placebo by the National Health and Medical Research Council (NHMRC) Clinical Trials Centre automated telephone service, which provided random computer‐generated numbers. |
| Allocation concealment (selection bias) | Low risk | The interventional radiologist called this system once participant was in the procedure room, immediately before the procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “The participants, investigators (other than the radiologists performing the procedure), and trial outcome assessors were masked to patient group assignments.” Unclear whether participants were successfully blinded due to incomplete outcome reporting (data for only 35/55 (60%) in the vertebroplasty group and 35/57 (61.4%) in the placebo group were reported for this outcome) and in those in whom success of blinding was reported there was a difference in the proportion who correctly guessed their assigned treatment. 28/35 (80%) of the vertebroplasty group correctly guessed that they had undergone vertebroplasty compared with 19/35 (54%) of the control group correctly guessing that they had received a placebo at 14 days [this difference is statistically significant, Chi‐square = 8.8112, P value < 0.01 as calculated by review authors]. Success of blinding was also not reported at the pre‐specified time point of 3 days. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Unclear risk | Unclear whether participants were successfully blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Radiologists who assessed follow‐up radiographs were aware of treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A greater number of participants were missing at the 14‐day follow‐up in the vertebroplasty group (6/61, 9.8%) compared with 2/59 (3.4%) in the placebo group. Data were unavailable for 8 (13.1%) and 7 (11.9%) in vertebroplasty and placebo groups respectively at the 3‐month follow‐up. Reasons for withdrawal were not reported. In addition, some data were missing for some outcomes reported to be due to inability to complete questionnaires by sick elderly participants. Most data were missing for the outcome of success of blinding at 14 days although the proportion missing was equal across treatment groups (data reported for 35/55 (60%) in the vertebroplasty group and 35/57 (61.4%) in the placebo group). |
| Selective reporting (reporting bias) | High risk | Primary outcome is inconsistently reported in the published protocol as both the proportion of patients whose NRS pain score reduces from 7/10 (or more) to 4/10 (or less) at 14 days, and the proportion of patients achieving a 14‐day pain score of less than 4 out of 10. The results paper states that the primary endpoint is the proportion of patients achieving an NRS pain score of less than 4 out of 10. The following outcomes were pre‐specified in the published protocol but not reported in the results paper:
The following outcomes were not pre‐specified in the published protocol but reported in the results paper:
|
| Other bias | Unclear risk | Placebo procedure differs between published protocol and results paper. The protocol reports that a 4‐mm skin incision will be made with light tapping on the skin. The results paper states that a short needle was passed into the skin incision but not as far as the periosteum and that manual skin pressure and regular tapping on the needle was done to mimic vertebroplasty needle advance. |
Dohm 2014.
| Methods |
Design: multicentre, parallel group, two‐arm open randomised controlled trial including 75 sites Setting: USA and Canada Timing: October 2006 to May 2011 Interventions: percutaneous vertebroplasty versus balloon kyphoplasty Sample size: 1234 participants required to detect an 8.7% difference in subsequent radiographic fracture (40% in vertebroplasty, 31.3% in kyphoplasty), 20% withdrawal, 80% power and 5% type I error rate. Analysis: modified intention‐to‐treat analysis using all data available from the 381 participants randomised and treated (23 participants withdrew before receiving treatment, 15 assigned to vertebroplasty and 8 assigned to kyphoplasty) |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 75.7 (10.5) years; 144 female: 46 male Duration of back pain: not stated Mean (SD) baseline pain score (from the trial registry website): 7.7 (1.8) out of 10 Mean (SD) baseline Oswestry Disability Index score (from the trial registry website): 57.8 (16) out of 100 Bisphosphonate use: N = 65 (34.2%) Opioid use at baseline: 126/169 (74.6%) T score for bone mineral density (BMD) less than ‐1: N = 133 (83.2%) Kyphoplasty group: Mean (SD) age: 75.5 (10.3) years; 151 female: 40 male Duration of back pain: not stated Mean (SD) baseline pain score (from the trial registry website): 7.8 (1.8) out of 10 Mean (SD) baseline Oswestry Disability Index score (from the trial registry website): 59 (17.5) out of 100 Bisphosphonate use: N = 75 (39.3%) Opioid use at baseline: 122/165 (73.9%) T score for bone mineral density (BMD) less than ‐1: N = 138 (83.6%) |
|
| Interventions | Specialist training of those performing the procedures was not reported. Investigator requirements were 50 lifetime procedures or 20 in the last year for each procedure. If an investigator only qualified for one of the procedures, they could participate as a team with an investigator qualified in the other technique. Tools and polymethylmethacrylate bone cement used were approved or cleared by the FDA for treating vertebral fractures by using kyphoplasty and vertebroplasty, respectively. Vertebroplasty Procedures were performed according to local practices and was not standardised across centres. Balloon kyphoplasty The procedure was performed by using a bilateral approach. Kyphon Osteo Introducer Systems, Inflatable Bone Tamps, HV‐R Bone Cement, Bone Filler Devices, and other balloon kyphoplasty devices were manufactured by Medtronic Spine, Sunnyvale, California. Both treatment groups In the results it is stated that investigators were to attempt vertebral deformity correction regardless of treatment; 142/189 (75.!%) participants in the vertebroplasty group and 154/191 (80.6%) participants in the kyphoplasty group had perioperative postural reduction. |
|
| Outcomes | Outcomes were reported at 7 days, 1, 3, 12 and 24 months after treatment Primary outcomes
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | Medtronic Spine sponsored the study and contributed to study design, data monitoring, statistical analysis and reporting of results and paid for independent core laboratory and data safety‐monitoring board services. | |
| Notes | Trial registered on 5 May 2006, registration number: NCT00323609. Known as ‘KAVIAR’ trial. Due to higher than anticipated withdrawal rate (38%), low patient enrolment, and patient/investigator willingness for randomisation, the sponsor terminated the study before reaching the planned sample size after enrolling 404 participants. This decision was made without knowledge of any outcome results. Enrolled participants left the study without additional follow‐up except that any not reaching the 1‐month visit were followed to collect 30‐day safety data. Outcomes reported in the published report differ from planned outcomes according to trial registration. Outcomes not reported in the published paper include SF‐36 Mental Component Summary, quality of life questionnaire (mini‐OQLQ), ambulatory status, change in vertebral body height; change in sagittal vertical axis; vertebral fracture‐related health care utilisation. Time to new clinical fracture reported in results but not listed as outcome in trial registration. We extracted the reported denominators for number of participants with new radiographic fractures at 12 and 24 months in each treatment group, which differed from the number of participants with complete follow‐up at these time points as reported in the flow diagram. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer using a dynamic minimisation technique stratified by the number of prevalent vertebral fractures, aetiology and study centre. It is not clear how participants were stratified by aetiology as the inclusion criteria specified that participants have osteoporotic fractures (and people with fractures due to cancer or high‐energy trauma were excluded). |
| Allocation concealment (selection bias) | Unclear risk | It is not reported whether or not the random sequence was concealed from investigators prior to allocating a participant to treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not concealed from participants or investigators. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants were told of their treatment group immediately following randomisation, this way may have influenced their assessment of self‐reported outcomes. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | It is not stated who assessed the imaging outcomes but it is unlikely that they were blinded to treatment assignment as both procedures will be detected on imaging. An independent radiologist determined cement volume and was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was small and equal across treatment groups for short‐term outcomes. Follow‐up was completed at 1 month for 181/190 who underwent vertebroplasty (5 withdrew, 1 lost, 1 other medical reason, 1 logistical reasons, 1 died) and 180/191 who underwent kyphoplasty (4 withdrew, 1 lost, 1 other medical reason, 4 other reason, 1 died). However loss to follow‐up was greater for the primary endpoints measured at 12 and 24 months. At 12 months there was complete follow‐up for 130/190 (68%) in the vertebroplasty group (20 withdrew, 4 lost, 3 logistical reason, 2 other medical reason, 3 other reason, 1 due to unrelenting pain, 14 died, 13 sponsor terminated study) and 143/191 (75%) in the kyphoplasty group (11 withdrew, 4 lost, 3 other medical reason, 4 logistical reasons, 1 due to unrelenting pain, 5 other reason, 10 died, 10 sponsor terminated study). At 24 months, 91/190 (48%) participants in the vertebroplasty group had completed follow‐up (23 withdrew, 10 lost, 5 logistical reason, 7 other medical reason, 3 other reason, 1 due to unrelenting pain, 21 died, 29 sponsor terminated study), and 100/191 (52%)participants in the kyphoplasty group completed follow‐up (13 withdrew, 9 lost, 3 other medical reason, 9 logistical reasons, 1 due to unrelenting pain, 11 other reason, 16 died, 29 sponsor terminated study). Overall, the reasons for the losses were similar except a higher proportion who received their assigned treatment withdrew from the vertebroplasty group (20/190; 11%) compared to the kyphoplasty group (11/191; 6%). It is unclear if the reasons for withdrawal were systematically different. In the methods it is stated that seven participants who received vertebroplasty and 4 who received kyphoplasty underwent the alternate treatment for a subsequent vertebral fracture but the timing was not stated. It is stated that for any participant having surgery for a new vertebral fracture, the last observation before surgery was carried forward to later visits. In the results it is stated that 70/88 (79.5%) participants with a new clinically recognised fracture underwent a subsequent vertebral augmentation but these data are not presented by treatment group and the timing of further vertebral augmentation is not specified. |
| Selective reporting (reporting bias) | Low risk | All outcomes planned at trial registration were reported although on the trial registration site primary and secondary outcomes were modified after the trial was completed. Some outcomes not reported in the published paper (e.g. SF‐36 Mental Component Summary, quality of life questionnaire (mini‐OQLQ), ambulatory status, change in vertebral body height; change in sagittal vertical axis; vertebral fracture‐related healthcare utilisation) were reported in the trial registration report but not the published paper. Time to clinical fracture (in days) was reported but was not listed as an outcome in the trial registration. |
| Other bias | Unclear risk | The trial was sponsored by a device company. The company also contributed to study design, data monitoring, statistical analysis and reporting of results including manuscript authorship, paid for independent core laboratory and data safety‐monitoring board services, and terminated the study early. |
Endres 2012.
| Methods |
Design: two‐arm open‐label single‐centre quasi‐randomised controlled trial Setting: Germany Timing: not stated Interventions: percutaneous vertebroplasty, balloon kyphoplasty or shield kyphoplasty Sample size:a priori sample size calculation not reported Analysis: completers' analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (range) age: 71.3 (63‐77) years; 12 female:8 male (gender of one participant not specified) Prevalent vertebral fractures at baseline: 1 Mean (SD) baseline pain score: 78.2 (9.36) Mean (SD) baseline Oswestry Disability Index: 68.2 (5.7) Balloon kyphoplasty: Mean (range) age: 63.3 (53‐77) years; 14 female:6 male Prevalent vertebral fractures at baseline: 1.25 (range 1 ‐ 3) Mean (SD) baseline pain score: 90.0 (7.07) Mean (SD) baseline Oswestry Disability Index: 77.0 (4.2) Shield kyphoplasty: Mean (range) age: 67.1 (47‐79) years; 14 female:4 male Prevalent vertebral fractures at baseline: 1.14 (range 1‐2) Mean (SD) baseline pain score: 88.16 (15.06) Mean (SD) baseline Oswestry Disability Index: 75.7 (9.1) |
|
| Interventions | All procedures were performed by the same orthopaedic surgeon under biplane fluoroscopy and general anaesthesia. Vertebroplasty This was performed through a unipedicular transpedicular approach with one 13‐gauge bone biopsy needle (Stryker) placed in the anterior third of the vertebral body. Once the needle was in place, liquid and powder PMMA (high viscosity SpinePlex, Stryker, Germany) were mixed to toothpaste consistency. Under biplane guidance, the cement was injected through the needle until the vertebral body was filled in the posterior 25% or until there was leakage. No postural manoeuvre was performed to retain alignment before or during the procedure. Balloon kyphoplasty This was also performed through a unipedicular approach with a unilateral working cannula and standard kyphoplasty equipment (high viscosity KyphX HV‐R, Medtronic, Germany). A drill passing through the cannula created a tract for the 20 mm balloon to be inserted in the centre of the vertebral body. Cement, mixed according to the manufacturer's recommendations, was injected as described for vertebroplasty. Injection was usually about 14 minutes after start of mixing. Shield kyphoplasty The Soteira shield kyphoplasty is a percutaneous minimally invasive system that enables a fractured vertebral body to be accessed through a unipedicular approach. The implant site was prepared by manually creating a cavity, and bone cement (Soteira, high viscosity) was delivered via an implantable cement director, the Shield Implant. This is a hollow, self‐expandable, coated implant that is marketed in a range of sizes and is attached to a disposable delivery system. Follow‐up All participants were discharged 2 days after surgery. All participants received daily standard doses of oral aminobisphosphonate, 1000 mg calcium and 1000 IU vitamin D3. In addition, physiotherapy and pain medication was prescribed as required. |
|
| Outcomes | Outcomes were reported immediately postoperatively and a mean of 5.8 months (range: 4 to 7 months) after treatment. Primary outcomes
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | BioMedEs funded translation and copyediting. It is not reported whether or not other funding was received. | |
| Notes | Trial registration: not found. The authors stated that there were no significant differences in baseline characteristics between groups but the review authors judged there to be significant pre‐treatment group differences: there were significant differences between groups with respect to age (mean age of participants in the vertebroplasty group was greater than the other groups) and participants allocated to the kyphoplasty groups appeared to have worse scores for pain and ODI at baseline compared with the vertebroplasty group. We extracted data from vertebroplasty and balloon kyphoplasty groups only. We converted the 0 to 100 pain scale to a 0 to 10 pain scale for the purposes of pooling data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The included participants were distributed quasi‐randomly into three groups." |
| Allocation concealment (selection bias) | High risk | Quasi‐random method of allocation likely precluded concealment of sequence to the single investigator who allocated the participants to treatment as it was predictable. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to treatment allocation. A single investigator who performed all procedures was not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | Pain and disability was self‐assessed by participants who were unaware of their treatment allocation. Another orthopaedic surgeon not involved in the primary surgery performed the final follow‐up. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Images were analysed by the (unblinded) orthopaedic surgeon who performed the procedures, as well as by a radiologist. It is not reported whether or not the radiologist was blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were unavailable for 7 participants (two deaths and five participants who refused follow‐up: treatment group not specified, however data were not reported for 1 participant in the vertebroplasty, 2 in the balloon kyphoplasty group and 4 in the shield kyphoplasty group). |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered and trial protocol was not published. All outcomes listed in the methods are reported. |
| Other bias | Unclear risk | Participants in the vertebroplasty group were older on average than participants in other groups (71.3 versus 63.3 and 67.1 years in the balloon and shield vertebroplasty groups respectively). Participants in the kyphoplasty groups also appeared to have worse pain and disability scores at baseline compared to the vertebroplasty group (vertebroplasty: 78.2 and 68.2; balloon kyphoplasty: 90.0 and 77.0; and shield kyphoplasty 88.16 and 75.7, respectively). It is not clear if BioMedEs had any role in the study other than funding translation and copyediting. |
Evans 2015.
| Methods |
Design: multicentre (nine sites) parallel group, two‐arm randomised controlled trial Setting: USA Timing: unclear Interventions: percutaneous vertebroplasty versus kyphoplasty Sample size: total sample size of 96 participants would provide 80% power to detect a ‘medium’ effect size of 0.58; planned to recruit a total of 120 participants assuming up to 20% loss to follow‐up at 12 months Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 76.1 (10.0) years; 77.6% female: 22.4% male Mean (SD) Charlson score: 0.43 (0.90) Comorbidities: 57.9% Neurological deficit: 4.7% Prior fracture history · Vertebral: 17.9% · Non‐vertebral: 3.6% · None listed: 78.6% Prior back surgery: 3.6% Osteoporosis: 45.0% Mean (SD) current fracture %: 25.2 (18.8) Current fracture level · L1 only: 12.5% · T11 only: 7.1% · T12 only 8.9% · Other locations: 50.0% · Not listed: 21.4% Narcotic medication at entry: 64.1% NSAID at entry: 22.2% Mean (SD) average pain (0 to 10): 7.9 (2.0) Mean (SD) SOF‐6 ADL score: 17.4 (3.1) Mean (SD) Roland‐Morris disability score: 16.3 (7.4) Mean (SD) EuroQOL score: 10.1 (1.6) Mean (SD) SF‐36 aggregate physical health: 26.6 (7.6) Mean (SD) SF‐36 aggregate mental health: 42.4 (12.7) Mean (SD) OPAQ body image score (1 to 5): 3.5 (1.4) Kyphoplasty group: Mean (SD) age: 75.1 (10.1) years; 64.8% female: 35.2% male Mean (SD) Charlson score: 0.49 (0.99) Comorbidities: 60.5% Neurological deficit: 8.7% Prior fracture history · Vertebral: 10.2% · Non‐vertebral: 11.9% · None listed: 78.0% Prior back surgery: 10.2% Osteoporosis: 37.2% Mean (SD) current fracture %: 23.1 (17.5) Current fracture level · L1 only: 17.0% · T11 only: 10.2% · T12 only 15.2% · Other locations: 35.6% · Not listed: 22.0% Narcotic medication at entry: 57.1% NSAID at entry: 26.2% Mean (SD) average pain (0 to 10): 7.4 (1.9) Mean (SD) SOF‐6 ADL score: 17.7 (4.0) Mean (SD) Roland‐Morris disability score: 17.3 (6.6) Mean (SD) EuroQOL score: 10.4 (1.9) Mean (SD) SF‐36 aggregate physical health: 26.1 (6.9) Mean (SD) SF‐36 aggregate mental health: 45.4 (14.2) Mean (SD) OPAQ body image score (1 to 5): 3.5 (1.4) |
|
| Interventions |
Vertebroplasty Vertebral augmentation was performed according to standard practice according to each practitioner’s preference. The approach, device, and cement used for the procedure were at the operators’ discretion. Kyphoplasty Also performed according to standard practice according to each practitioner’s preference. The approach, device, and cement used for the procedure were at the operators’ discretion. |
|
| Outcomes | Outcomes were measured at baseline, 3 days, 1 month, 6 months, and 12 months after treatment (telephone follow up at day 3, 1 month and 6 months and in person at 1 year). Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | Supported by Carefusion, Johnson and Johnson/DePuy Synthes Spine, Cardinal Health and Stryker. | |
| Notes | Clinicaltrials.gov trial identifier: NCT00279877 SD for EuroQOL EQ‐5 Health States Instrument imputed from baseline for both treatment groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial participants were randomly assigned to either the kyphoplasty intervention or the vertebroplasty intervention using a variable block randomisation scheme to ensure balance of assignment to both groups over time. The block sizes were randomly varied and of sufficient size to minimise the ability of any investigator or co‐ordinator to guess the next assigned treatment. |
| Allocation concealment (selection bias) | Low risk | The random treatment assignments were placed in sequentially numbered envelopes and distributed to the clinical sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is reported as 'single‐blind' in the clinical trial registry, but reports of who was blinded are contradictory: the site co‐ordinators and other personnel who collected trial participant data and administered the study questionnaires were blinded to the assigned treatment, according to the results report but participant blinding was reported in the trial registry. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Unclear risk | Unclear if the 'single‐blinded' study refers to blinding of participants or not. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | Low risk | No objective outcomes were measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A quarter of the participants did not complete follow‐up (21.8% of vertebroplasty group, 29.3% of kyphoplasty group). The authors report that the pattern of loss to follow‐up appeared to be missing at random. |
| Selective reporting (reporting bias) | High risk | Adverse events were to be measured but were not reported. |
| Other bias | Unclear risk | Quote: “Another limitation of our study is the lack of standardisation of the procedures. The approach, device, and cement used for the procedures were at the operators’ discretion.” |
Farrokhi 2011.
| Methods |
Design: two‐arm open‐label randomised controlled trial; control group allowed to cross‐over into vertebroplasty group from one month (cross‐overs < 2 months = 4, < 6 months = 3, <12 months = 3, < 36 months = 10) Setting: Iran Timing: Sept 2004 to Jan 2009 Interventions: percutaneous vertebroplasty or usual care Sample size:a priori sample size calculation not reported Analysis: reported an intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (range) age: 72 (59 to 90) years; 30 female:10 male Duration (range) of back pain: 30 (6 to 54) weeks Previous vertebral fractures: 50 Mean (SD) baseline pain score: 7.2 (1.7) Initial pain medication‐ paracetamol (acetaminophen) and codeine: 30 (75%); NSAIDs: 20 (50%) Usual care group: Mean (range) age: 74 (55 to 87) years; 30 female:12 male Duration (range) of back pain: 27 (4 to 50) weeks Previous vertebral fractures: 56 Mean (SD) baseline pain score: 8.4 (1.6) Initial pain medication‐ paracetamol (acetaminophen) and codeine: 30 (71%); NSAIDs: 32 (76%) |
|
| Interventions | Percutaneous vertebroplasty performed by neurosurgeons; usual care ('optimal medical therapy') delivered by a physician. Vertebroplasty Induction of conscious sedation (a combination of IV fentanyl and midazolam) in 10 (25%) participants and general anaesthesia in 30 (75%) participants. Patients were placed prone and single‐plane C‐arm equipment was used. Using sterile techniques, an 11‐gauge needle was inserted into the vertebral body via a unilateral parapedicular approach in 35 (87.5%) patients and via a bilateral transpedicular approach in 5 (12.5%) patients. A bilateral transpedicular approach was used only if there was inadequate instillation of cement with the unilateral approach under fluoroscopy. A polymethylmethacrylate mixture was injected into the vertebral body. Following the procedure, the patient remained supine in bed. During cement injection, fluoroscopic monitoring with a C‐arm unit was used in both planes. It is not stated whether or not participants in the vertebroplasty group could also receive analgesia and/or treatment of osteoporosis. Usual care The usual care group was prescribed 250 mg acetaminophen with codeine twice daily, 400 mg ibuprofen twice a day, 1000 mg calcium daily, 400 IU vitamin D daily, 70 mg alendronate orally once weekly, and 200 IU calcitonin daily. Analgesia could be increased by the treating physician as needed. Cross‐over to vertebroplasty was permitted after 1 month. Follow‐up care A change in lifestyle and physical treatment (undefined) was also suggested to participants in both groups. |
|
| Outcomes | Outcomes were reported at 1 week, and 2, 6, 12, 24 and 36 months after treatment Primary outcomes
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | The Vice‐chancellor for research affairs of Shiraz University of Medical Sciences and Apadana Tajhizgostar Co. (distributor of medical devices) provided grant support. | |
| Notes | Trial registered retrospectively on 11 Oct 2009 at www.irct.ir, number IRCT138804252193N1. All participants in the control group were permitted to undergo vertebroplasty after 1 month; 4 crossed over before 2 months, 3 before 6 months, 3 before 12 months and 10 before 36 months (total cross‐over 20/42 (47.6%)). We included the 2‐month follow‐up data in the 3‐month analyses/meta‐analyses. The trialists report epidural cement leakage in one participant receiving vertebroplasty, and no cases of venous emboli or infection. It is not reported if any adverse events occurred with usual care. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by computerised random number generator |
| Allocation concealment (selection bias) | Low risk | The treatment assignment was kept in sealed envelopes. It is not explicitly reported who prepared and opened the envelopes, but it is likely that allocation was concealed and the neurosurgeon (performing vertebroplasty) and the physician (administering usual care) had no role in allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded; the treating clinicians were unaware of the other treatment group |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants self‐assessed pain and disability and were unblinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | It is not stated who assessed the imaging outcomes. It is unlikely that they were blinded to treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was small and equal across groups (3/40 participants in the vertebroplasty group and 3/42 participants in the usual care group at the final 36 month follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | Unclear if any additional outcomes were measured and not reported; e.g., incident fracture is not listed in the methods as an outcome but data are reported in the results for the 2‐year (but not end of study) follow‐up. |
| Other bias | Low risk | None apparent |
Firanescu 2018.
| Methods |
Design: multicentre (four sites), parallel group, two‐arm randomised placebo‐controlled trial Setting: the Netherlands Timing: January 2011 to January 2014 Interventions: percutaneous vertebroplasty versus sham vertebroplasty (placebo) Sample size: 90 participants per group required to detect at least a 1.5 point difference in pain relief on a 0 to 10 Visual Analogue Scale (VAS) between vertebroplasty and placebo, based on a two‐sided type 1 error rate of 5% and a 20% withdrawal rate. Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 74.7 (10.7) years; 67 female: 23 male Mean (SD) duration of back pain: 29.2 (16.3) days Median (IQR) duration of back pain to treatment: 43 (29‐52) days Mean (SD) baseline pain score: 7.7 (1.4) Mean (SD) RMDQ: 18.0 (4.5) Mean (SD) baseline QUALEFFO score: 68.4 (17.1) Number of vertebral compression fractures at baseline: 115 Medication for osteoporosis: n = 42 (47%) Number of vertebral fractures at baseline: 115 Opioid use at baseline: n = 42 (47%) Mean (SD) T score for bone mineral density (BMD): ‐2.4 (1.0) Placebo group: Mean (SD) age: 76.9 (8.1) years; 66 female: 20 male Mean (SD) duration of back pain: 25.9 (13.8) days Median (IQR) duration of back pain to treatment: 36 (24‐51) days Mean (SD) baseline pain score: 7.9 (1.6) Mean (SD) RMDQ: 17.8 (4.7) Mean (SD) baseline QUALEFFO score: 69.7 (17.9) Number of vertebral compression fractures at baseline: 108 Medication for osteoporosis: n = 49 (57%) Opioid use at baseline: n = 25 (29%) Mean (SD) T score for bone mineral density (BMD): ‐2.4 (1.0) |
|
| Interventions | Percutaneous vertebroplasty or placebo (sham) procedure. IV cefazolin (2g) one hour prior to procedure. Under fluoroscopic guidance, the practitioner infiltrates the skin and subcutaneous tissues overlying the pedicle of the target vertebra or vertebrae with 1% lidocaine and infiltrates the periosteum of the pedicles with 0.25% bupivacaine (Marcaine). In both groups the cement (polymethylmethacrylate) was prepared in close proximity to the participants. Vertebroplasty Bone biopsy needles were positioned bilaterally, using standard transpedicular placement. 11‐gauge or 13‐gauge needles passed into the central aspect of the vertebrae, bone cement prepared on the bench and injected into the vertebral body under constant fluoroscopy, until the cement reaches the posterior aspect of the vertebral body or leaks into an extraosseal space. Sham procedure (placebo) Bone biopsy needles were positioned bilaterally periosteally and the procedure was simulated using verbal and physical cues. Follow‐up care Both study groups received the same patient care during hospital stay and at discharge. Pain medication (paracetamol, tramadol, tramadol and paracetamol, or morphine) was dispensed as needed, according to participant demand, and their use recorded. NSAIDs were allowed only for participants intolerant of opiate‐derived drugs, or if already used. Drugs for osteoporosis were prescribed according to the standard protocol of the participating institutions for patients with osteoporotic vertebral fractures. |
|
| Outcomes | Outcomes collected at 1‐day, 1 week, and 1, 3, 6 and 12 months after the procedure. Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | Grant (S‐I‐013) from Stryker | |
| Notes | Trial registration: NCT01200277 Sponsored by: St. Elisabeth Hospital, Tilburg, Netherlands Study completed as of 17 Nov 2014 at clinicaltrials.gov Adverse events: there were 8 deaths in the vertebroplasty group and 5 in the placebo group (all reported as unrelated to the procedures by the trialists); cement leakage reported in 103/159 treated vertebral bodies in the vertebroplasty group (number of participants with an event not reported); in vertebroplasty, n=1 had respiratory insufficiency and n =1 a vasovagal response due to the procedure; no adverse events reported for the placebo group. Incident fractures: 15/91 participants (31 fractures) in vertebroplasty and 19/89 (28 fractures) in placebo. It was not reported whether these were radiographic or clinically symptomatic. However, 12 participants (six in each group) underwent re‐intervention during follow‐up for one or more new symptomatic fractures and all were treated according to the original treatment assignment. Conference proceedings reported that 5 participants in the vertebroplasty group and 4 in the placebo group had 3 vertebral fractures and participants in the vertebroplasty group received vertebroplasty into all 3 levels. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation in a block size of sic, randomisation ratio 1:1, and a maxiumum sample size of 84 for each participating centre. operator. |
| Allocation concealment (selection bias) | Low risk | Protocol reports that randomisation occurred once the participant was prepped for surgery and sedated. It is probable that this process ensured that the treatment group was concealed from participants, internists and outcome assessors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, internists and outcome assessors were blinded as were nurses involved in patient care during hospital stay. It was not possible to mask the interventional and diagnostic radiologists. The day after treatment, 74 (82%) of participants in the vertebroplasty group and 70 (81%) in the placebo group believed they had undergone vertebroplasty The remainder were either unsure or though they had undergone the sham procedure. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | A similar proportion of participants in both groups believed they had received vertebroplasty suggesting blinding was successful. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Diagnostic radiologists were not blinded to treatment intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was nearly complete, with a small proportion lost in each group, for reasons that were likely unrelated to treatment. |
| Selective reporting (reporting bias) | High risk | Additional outcomes that were not pre‐planned according to the trial protocol were reported (height loss, incident fractures, proportion with pain VAS score ≥5 at 12 months). |
| Other bias | Low risk | No other biases apparent. |
Kallmes 2009.
| Methods |
Design: multicentre, two‐arm, randomised placebo‐controlled cross‐over trial; cross‐over to the other treatment group was allowed at 1 month or later if adequate pain relief was not achieved Setting: USA (5 centres), UK (5 centres) and Australia (1 centre) Timing: not reported Interventions: percutaneous vertebroplasty versus sham vertebroplasty (placebo) Sample size: initial sample size calculation of 250 participants was based upon an ability to detect a 2.5‐point difference on the Roland‐Morris Disability Questionnaire (RMDQ) and a 1‐point difference on the 0‐ to 10‐point pain intensity scale, with at least 80% power and a two‐sided type 1 error of 0.05. After early difficulty in recruitment and a planned interim analysis of the first 90 participants, target enrolment was reduced to 130 participants based upon accrual rates and revised power calculations. With 130 participants, there was more than 80% power to detect a 3‐point difference between groups on the RMDQ (assumed SD 6.7), and a 1.5‐point difference on the pain rating (assumed SD 2.7) at 1 month. Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group: Mean (SD) age: 73.4 (9.4) years; 53 female:15 male Mean (interquartile range; IQR) duration of back pain: 16 (10 to 36 weeks) Duration of back pain <14 weeks: 30 (44%) Mean (SD) baseline pain score: 6.9 (2.0) points Mean (SD) baseline RMDQ score: 16.6 (3.8) points Number with 1; 2; or 3 spinal levels treated: 48 (71%); 13 (19%); 7 (10%) Opioid use: 38 (56%) Placebo group: Mean (SD) age: 74.3 (9.6) years; 46 female:17 male Mean (interquartile range; IQR) duration of back pain: 20 (8 to 38 weeks) Duration of back pain <14 weeks: 24 (38%) Mean (SD) baseline pain score: 7.2 (1.8) points Mean (SD) baseline RMDQ score: 17.5 (4.1) points Number with 1; 2; or 3 spinal levels treated: 41 (65%); 14 (22%); 8 (13%) Opioid use: 40 (63%) |
|
| Interventions | Percutaneous vertebroplasty or the sham procedure was performed by highly experienced interventional radiologists having performed a mean of 250 procedures (range: 50 to 800). Procedures were performed in a fluoroscopy suite, under conscious sedation using sterile technique. Using fluoroscopic guidance, the practitioner infiltrated the skin and subcutaneous tissues overlying the pedicle of the target vertebra or vertebrae with 1% lidocaine and infiltrated the periosteum of the pedicles with 0.25% bupivacaine. Vertebroplasty 11‐gauge or 13‐gauge needles were passed into the central aspect of the target vertebra or vertebrae. Barium opacified PMMA was prepared on the bench and infused under constant lateral fluoroscopy into the vertebral body. Infusion was stopped when the PMMA reached the posterior aspect of the vertebral body or entered an extraosseous space. Sham procedure Verbal and physical cues, such as pressure on the patient’s back, were given, and the methacrylate monomer was opened to simulate the odour associated with mixing of PMMA, but the needle was not placed and PMMA was not infused. Follow‐up care Both groups of patients were monitored in the supine position for 1 to 2 hours before discharge. |
|
| Outcomes | Outcomes were reported 3 and 14 days, and 1 month (and 3 months for the primary outcomes) and at various times up to one year. Primary outcomes
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | Supported by a grant (R01‐AR49373) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. | |
| Notes | Trial registered at ClinicalTrials.gov, number NCT00068822. For this review, we only extracted outcomes at 2 weeks and 1 month (i.e. before cross‐over, and thus prior to the likely breaking of the randomisation schedule). After 1 month, significantly fewer participants in the vertebroplasty group crossed over into the alternate group (11 of 68 participants (16%)) compared with the placebo (sham) group (38 of 63 participants (60%), P = 0.001). At one year, difference in pain favoured the vertebroplasty group (MD 1.02 (95% CI 0.04 to 2.01); P = .042) but there was no evidence of an important difference in disability (MD in RMDQ 1.37 points (95% CI 3.62 to 20.88), P = .231). In the as‐treated analyses, participants treated with vertebroplasty did not differ from the placebo (sham) group in terms of either mean pain (MD 0.85 (95% CI 2.05 to 20.35), P = .166) or disability (RMDQ MD 0.66 (95% CI 3.30 to 21.98); P = .625). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation (sizes ranging from 4 to 12 participants), stratified by study centre; sequences were generated by a random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Allocation occurred just prior to the procedure using numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Group assignments were concealed from participants and study personnel. Before one month one participant in the vertebroplasty group and two participants in the sham group crossed over to the other treatment group. By three months more participants in the placebo group had crossed over (27/63, 36%) compared with the vertebroplasty group (8/68, 12%) and the reasons for the different cross‐over rate is unknown ‐ while it is possible that more participants in the control group had unsatisfactory pain outcomes, no difference in pain intensity was observed. At 14 days, 63% of patients in the control group correctly guessed that they had undergone the control intervention, and 51% of patients in the vertebroplasty group correctly guessed that they had undergone vertebroplasty. Patients in both the vertebroplasty group and the control group expressed a moderate degree of confidence, on a scale of 0 (not certain) to 10 (extremely certain), in their treatment guess (mean scores, 4.0 and 4.1, respectively; P = 0.78). In the control group, 18 of 33 patients (55%) who did not cross over to vertebroplasty correctly guessed at 14 days that they had undergone the control intervention, as compared with 20 of 27 patients (74%) who eventually crossed over (P = 0.12). Among the eight patients in the vertebroplasty group who crossed over to the control group, six (75%) guessed incorrectly at 1 month that they had received the control intervention. As we only considered outcomes to one month, we judged this trial to be at low risk of bias up until one‐month follow‐up. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | Participants were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | Low risk | No objective outcomes were assessed up to one‐month follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was small and balanced across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes measured to 3 months planned in the published protocol were reported. |
| Other bias | Low risk | None apparent. |
Klazen 2010.
| Methods |
Design: multicentre (6 centres), two‐arm open‐label randomised controlled trial; control group allowed to cross‐over to vertebroplasty at one week post intervention Setting: the Netherlands and Belgium Timing: not reported Interventions: percutaneous vertebroplasty or usual care Sample size:a priori sample size calculation based on ability to detect a difference of 25% in significant pain relief with vertebroplasty compared with usual care based on a two‐sided type 1 error rate of 5% and power 80%. Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty Group: Mean (SD) age: 75.2 (9.8) years; 70 female, 31 male Mean (SD) duration of back pain: 29.3 (17.1) days Number of VCFs at baseline: 2.4 (1.9) Mean (SD) pain at baseline: 7.8 (1.5) Mean (SD) RMDQ: 18.6 (3.6) Mean (SD) QUALEFFO: 58.7 (13.5) Bone density T score: ‐3.0 (1.17) Usual care group Mean (SD) age: 75.4 (8.4) years; 70 female, 31 male Mean (SD) duration of back pain: 26.8 (16.0) days Number of VCFs at baseline: 2.1 (1.5) Mean (SD) pain at baseline: 7.5 (1.6) Mean (SD) RMDQ: 17.2 (4.2) Mean (SD) QUALEFFO: 54.7 (14.4) Bone density T score: ‐3.0 (1.05) |
|
| Interventions |
Percutaneous vertebroplasty Percutaneous vertebroplasty was performed using a single or biplane angiography system under fluoroscopic guidance. After local analgesia, two 11‐ or 13‐gauge bone‐biopsy needles were placed transpedicularly in the fractured vertebral body. Polymethylmetacrylate bone cement (Osteo‐Firm, COOK Medical, Bloomington, IN, USA) was injected through bone‐biopsy needles under continuous fluoroscopic monitoring to identify local cement leakage or migration into the venous system towards the lung. In patients who had more than one fracture with bone oedema on MRI, all vertebral bodies were treated in one or more procedures. After the procedure, a CT scan of the treated vertebral bodies was performed with 2 mm slices to identify cement leakage or other possible local complications. Usual care 'Optimal Pain Management (OPM)' consisted of the use of analgesics in ascending order
Corrections in dose and classification of pain medication were made when necessary by the internist, and in most cases physiotherapy was prescribed. Follow‐up care All patients received osteoporosis medication, such as bisphosphonates together with supplemental calcium and vitamin D. |
|
| Outcomes | Outcomes were reported at baseline, 1 week, and 1, 3, 6 and 12 months. Pain diary ‐ pain VAS and use of analgesia recorded daily to 1 month. Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | The study was sponsored by ZonMw (Dutch organisation for health care research and innovation of care), project number 945‐06‐351 and an unrestricted grant from the COOK Medical (Bloomington, IN, USA). | |
| Notes | Trial registered at ClinicalTrials.gov. Registration number NCT00232466. "VERTOS II" Pre‐treatment group differences: participants allocated to vertebroplasty had worse scores for EQ5‐D; QUALEFFO and RMDQ at baseline. RMDQ and QUALEFFO means only shown graphically in the trial report. Dr Klazen provided mean (SD) data for the RMDQ, EQ5D and QUALEFFO at all time points to 12 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation codes with a block size of six. |
| Allocation concealment (selection bias) | Low risk | As an independent telephone operator allocated participants by telephone, the allocation was likely concealed from the investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and study personnel were aware of treatment assignment. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Radiologists were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A greater number of participants completed one‐year follow‐up in the vertebroplasty group (86/101, 85%) compared with 77/101 (76%) in the usual care group. Fifteen (15%) participants in the usual care group received vertebroplasty. |
| Selective reporting (reporting bias) | Low risk | The trial authors published the planned outcomes in a trial protocol and provided results for each planned outcome. |
| Other bias | High risk | Quality of life and disability were worse at baseline in the vertebroplasty group which may have biased the results favouring the vertebroplasty group. |
Leali 2016.
| Methods |
Design: multicentre (four centres) two‐arm randomised controlled trial Setting: Italy, France, Switzerland Timing: not reported Interventions: percutaneous vertebroplasty or usual care Sample size: sample size calculation not reported Analysis: not explicitly reported |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics: characteristics were reportedly similar but as data were reported sparsely and not reported for both groups, this could not be verified Overall: age range: 56 to 82 years Vertebroplasty group: mean baseline pain score 0 to 10 VAS: 4.8, no measure of variance reported mean baseline ODI score: 53.6, no measure of variance reported Usual care: not reported |
|
| Interventions |
Vertebroplasty
Vertebroplasty was performed using transpedicular approach under local anaesthesia with mepivacaine 2% and naropin 10%. A mean volume of 4 ml of PMMA was injected into each fractured vertebral body with supervision of fluoroscopy. All the patients were subjected to analgesia after surgery, according to individual needs. According to increasing analgesic power, the patients were treated with acetaminophen, non‐steroidal drugs (NSAIDs), or derivatives of morphine. Usual care Conservative care consisting of pain medication, osteoporosis medication, physiotherapy or bracing. |
|
| Outcomes | Outcomes were evaluated at 24 and 48 hours, 1 month later, 3 months, and 6 months Study outcomes
Outcomes included in this review
|
|
| Source of funding | Not reported | |
| Notes | No record of trial registration identified. Pain and disability could not be included in analyses: the trialists did not report summary statistics for each group. The trialists report that pain and disability were improved from baseline with vertebroplasty but not with usual care. Between‐group analyses were not reported, except to say that clinical results were 'similar in both groups' at 6 weeks and 3 to 6 months. Adverse events: vertebroplasty: N=2, fracture of a transverse process, bleeding of psoas muscles; incident symptomatic vertebral fractures within 6 weeks (n = 3). Usual care: no incident fractures reported. It was reported that 1 participant in the vertebroplasty group and 3 in the usual care group died 'after 6 months from their fracture'. Withdrawals: n = 15 from vertebroplasty (7 for 'technical reasons', 8 could not maintain the prone position); none reported for usual care. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel unlikely due to the nature of the interventions. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | As participants were unblinded, there is a risk of bias in the measurement of pain and function, and adverse events. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | Unclear risk | Blinding of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on withdrawals or loss to follow‐up given. |
| Selective reporting (reporting bias) | High risk | Pain and function could not be extracted as summary data for each treatment group were not reported. No trial registration and no published protocol. |
| Other bias | Low risk | None apparent. |
Liu 2010.
| Methods |
Design: single‐centre, two‐arm, randomised controlled trial Setting: Taichung, Taiwan Timing: not stated Interventions: percutaneous vertebroplasty versus kyphoplasty Sample size:a priori sample size calculation not reported Analysis: not reported |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty Group Mean (SD) age: 74.3 (6.4) years; 38 female, 12 male Mean (SD) duration between 'injury' and treatment: 15.8 (6.7) days Location: 12 T12, 38 L1 Mean (SD) pain at baseline: 7.9 (0.7) Kyphoplasty group Mean (SD) age: 72.3 (7.6) years; 39 female, 11 male Mean (SD) duration between 'injury' and treatment: 17.0 (7.7) days Location: 11 T12, 39 L1 Mean (SD) pain at baseline: 8.0 (0.8) |
|
| Interventions |
Percutaneous vertebroplasty The surgical procedures involved IV general anaesthesia (Propofol) and 2% xylocaine injected locally. A special bone biopsy needle (Angiotech, USA) was passed percutaneously and slowly through each side of the pedicle into the vertebral body. The bone filler PMMA (Zimmer) was prepared and mixed with both gentamicin, to reduce risk of infection, and powder containing barium, allowing X‐ray visualisation. An optimal amount of bone filler was injected into the vertebral body via the needles on both sides. All procedures were performed under a mobile C‐arm X‐ray. Balloon kyphoplasty The same anaesthesia was employed. Using image guidance, two small incisions were made, a probe was placed into the vertebral space at the fracture site. The bone was drilled and a balloon (VCF‐X CentralMedical Tech., Taiwan), called a bone tamp, was inserted on each side. The balloons were then inflated with contrast medium (to facilitate image guidance X‐rays) and expanded to the desired height and removed. The spaces created by the balloons were then filled with PMMA (prepared as for vertebroplasty) to bind the fracture. Follow‐up All participants undertook an orally administered treatment regimen to protect their bone density after surgery (details not reported). |
|
| Outcomes | Outcomes were reported at baseline, 3 days, 6 months, 1, 2 and 5 years. Primary outcomes
Outcomes included in this review
|
|
| Source of funding | Grant from Chung‐Shan Medical University Hospital (CS08110). | |
| Notes | Trial registration: not found. We extracted outcomes at 6 months, 1 and 2 years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned according to permuted block randomisation which was likely to be adequate. |
| Allocation concealment (selection bias) | Unclear risk | Whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Whether or not participants and investigators were blinded to treatment allocation is not reported. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Unclear risk | Whether or not participants were blinded to treatment allocation is not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | Unclear risk | Quote: "Radiographic measurement was made by technicians 'blind' to treatment group status, with variability controlled via inter‐ and intra‐observer comparisons". No details of the inter‐ and intra‐observer comparisons are reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of follow‐up was not explicitly reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered and trial protocol was not published. All outcomes listed in the methods are reported. |
| Other bias | Low risk | None apparent. |
Rousing 2009.
| Methods |
Design: single‐centre, parallel group, two‐arm open‐label randomised controlled trial Setting: Denmark Interventions: percutaneous vertebroplasty or usual care Timing: not reported Sample size:a priori sample size of 16 participants per group was calculated, based on being able to detect a difference in pain of 2 points on a 0‐10 VAS (SD set to 2 points) with 80% power and type 1 error rate of 0.05; increased to 20/group to allow for unpredictable patient exclusions and other unexpected events Analysis: completers' analysis used |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion Criteria
Baseline characteristics Vertebroplasty Group Mean (range) age: 80 (65 to 96) years; 19 female, 6 male Mean (95% CI) duration of fracture: 8.4 (3.7 to 13.0) days Mean (95% CI) pain at baseline: 7.5 (60.6 to 8.4) based upon 19 participants Mean (95% CI) physical function at baseline (SF‐36 PCS): 36.7 (30.0 to 43.4) based upon 17 participants Mean (95%) CI) EQ‐5D: 0.356 (0.196 to 0.516) based upon 17 participants Usual care group (n = 24) Mean (range) age: 80 (71 to 93) years; 21 female, 3 male Mean (95% CI) duration of fracture: 6.7 (21. to 11.4) days Mean (95% CI) pain at baseline: 8.8 (8.2 to 9.3) based upon 17 participants Mean (95% CI) physical function at baseline (SF‐36 PCS): 33.4 (26.2 to 40.7) based upon 17 participants Mean (95%) CI) EQ‐5D: 0.083 (‐0.151 to 0.317) based upon 16 participants |
|
| Interventions |
Vertebroplasty Percutaneous vertebroplasty was performed by orthopaedic surgeons specialised in spine surgery. Most patients were mildly sedated and all patients were prepared for general anaesthetic in case of complications. Under biplane fluoroscopic control and with the patients in a prone position 11‐ to 13‐gauge needles were placed using a uni or bilateral transpedicular approach. Bone cement (PMMA) was injected under continuous fluoroscopy. In cases of extra vertebral cement leakage, the injection was terminated. Monitoring during the procedure included electrocardiogram, oxygen saturation, and blood pressure. After the procedure, the patients remained in a prone position for 30 minutes and then lay supine for a further 90 minutes. Usual care Patients offered brace treatment in addition to pain medication and physiotherapy. Follow‐up care Both groups were hospitalised and offered pain medication and physiotherapy until discharge. |
|
| Outcomes | Outcomes were reported at 3 and 12 months. Outcomes
The following additional measures were added after commencement of the trial and only measured in 17 participants in each group.
Outcomes included in this review
|
|
| Source of funding | Foundation and Danish government funds. The authors stated that no commercial party received benefits from the study. | |
| Notes | Trial registration: not found. Baseline pain score was higher in the usual care group (8.8 versus 7.5, P = 0.02) and mean stay in hospital was significantly longer in the usual care group (11.7 days vs 7.6 days, P = 0.01). We calculated the SD for pain, SF‐36 Physical Function and EQ‐5D outcomes from the reported 95% CIs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: sealed envelopes containing the treatment assignment "were prepared beforehand by the investigating surgeon and sorted randomly". |
| Allocation concealment (selection bias) | Unclear risk | It is not reported whether or not envelopes were opaque and whether steps were taken to ensure use of consecutive envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and investigators were likely aware of treatment, given the nature of the interventions |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants were aware of treatment assignment. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Radiologists were aware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Baseline data are not included for one participant in the vertebroplasty group as they did not contribute follow‐up data (refused to attend for 3‐month follow‐up). An additional single participant in each group did not contribute outcome data as they died before the 3‐month follow‐up and an additional single participant in each group did not contribute 12‐month outcome data as they died sometime after the 3‐month follow‐up. Additional outcomes were added after the trial started (EQ5D, Barthel, MMSE, three physical tests), and were not collected for all participants. Baseline pain data were also either not collected and/or not reported for 6 (24%) participants in the vertebroplasty group and 7 (29%) participants in the usual care group and other baseline data were not collected and/or not reported for up to 13/25 participants in the vertebroplasty group and up to14/24 participants in the usual care group depending upon outcome. Three‐month follow‐up outcome data were either not collected and/or not reported for up to 14/24 participants in the vertebroplasty‐treated group and 9/23 participants in the usual care group depending upon outcome. Twelve‐month follow‐up outcome data were either not collected and/or not reported for up to 10/22 participants in the vertebroplasty‐treated group and 9/22 participants in the usual care group depending upon outcome. |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered and trial protocol was not published. All outcomes listed in the methods are reported. |
| Other bias | Unclear risk | Baseline pain was higher in the usual care group (8.8 versus 7.5) (although it was only measured in 17/24 and 19/25 participants in the usual care and vertebroplasty‐treated groups respectively. Participants receiving usual care were hospitalised for longer (11.7 days versus 7.6 days); it is unclear if more pain medication and physiotherapy was offered, and how this would affect outcomes. |
Sun 2016.
| Methods |
Design: single‐centre, parallel group, two‐arm randomised controlled trial Setting: China Interventions: high‐viscosity cement percutaneous vertebroplasty or low‐viscosity cement percutaneous kyphoplasty Timing: June 2010 to August 2013 Sample size: sample size calculation not reported Analysis: completers' analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria None reported Baseline characteristics Vertebroplasty Group Mean (SD) age: 65.4 (2.6) years No. female/male: 34/12 Mean (SD) follow‐up (months):22.7 (3.3) No. vertebra bodies: 54 Mean (SD) Injected cement volume (ml): 3.4(0.3) Mean (SD) operative time (min): 45.2 (4.7) Mean (SD) VAS: 8.5 (1.1) Mean (SD) ODI: 70.6 (8.6) Kyphoplasty group (n = 24) Mean (SD) age: 65.2 (3.3) years No. female/male: 38/14 Mean (SD) follow‐up (months):21.7 (2.3) No. vertebra bodies: 60 Mean (SD) Injected cement volume (ml): 4.2 (0.2) Mean (SD) operative time (min): 64 (5) Mean (SD) VAS: 8.2 (0.9) Mean (SD) ODI: 71.7 (8.5) |
|
| Interventions |
High‐viscosity cement percutaneous vertebroplasty Patients were placed in a prone position and treated with local anaesthesia. The position of fractured vertebra was fixed by using C‐arm fluoroscopy. A scalpel was used to make a 0.5 cm incision on the skin, after which the PVP needles were used to access the back muscle to reach the vertebral body. When the tip of the needle entered the posterior, middle and anterior of vertebral body, the frontal imaging displayed in C‐arm fluoroscopy screen showed the position of the needle to be at the paries lateralis, middle line, and paries medialis of radix arcus vertebrae. When the tip of needle reached the anterior and middle 3/4 of the vertebral body, the position of the needle was adjusted to enable injection of the high‐viscosity cement into the vertebral body. The volume of cement used was about 3 mLto 4 mL per vertebra. The entire surgery proceeding was guided by C‐arm fluoroscopy. Low‐viscosity cement percutaneous kyphoplasty The anaesthesia and puncture techniques were the same as those of high‐viscosity cement PVP. Through the working tunnel made by the needle, cannula, and drill, the balloon was inserted into the centre of the vertebral body and contrast medium was injected using a high pressure pump. Then the balloon was extracted and approximately 4 mL of cement was injected into each vertebral body. |
|
| Outcomes | Outcomes were measured at 2 days and 12 months after surgery Study outcomes
Outcomes used in this review
|
|
| Source of funding | Not reported | |
| Notes | No trial registry record found Adverse events Vertebroplasty: serious adverse events: none reported; other adverse events: cement leakage 9/54 (16.7%) Kyphoplasty: serious adverse events: none reported; other adverse events: cement leakage 11/60 (18.3%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear whether there was blinding of participants and study personnel. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Unclear risk | Since blinding of participants is not confirmed there could be a risk of bias in the measurement of self‐reported outcomes of pain and functional activity. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Not reported but likely that radiologists would be able to tell the difference between vertebroplasty and kyphoplasty on a radiograph. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found. Trial not registered. The results of all outcomes specified in the methods are reported. |
| Other bias | Low risk | No other bias apparent. |
Vogl 2013.
| Methods |
Design: multicentre, two‐arm, randomised controlled trial Setting: Germany (3 centres) USA (1 centre) Timing: March 2008 to Sept 2009 Interventions: percutaneous vertebroplasty versus kyphoplasty Sample size:a priori sample size calculation not reported Analysis: completers' analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty Mean (SD) age: 74 (11.5) years; 19 female, 9 male Mean (SD) pain at baseline: 8.49 (1.18) Usual care Mean (range) age: 80 (71 to 93) years; 21 female, 3 male Mean (SD) pain at baseline: 8.31 (1.12) |
|
| Interventions |
Percutaneous vertebroplasty The procedure was performed using a bipedicular cement injection in accordance with each participating physician's standard technique. The same cement was used for both procedures (Spineplex, Stryker Instruments, Kalamazoo, MI). Cement directed kyphoplasty system The kyphoplasty system was provided by Soteira Inc, Natick, MA. The surgical procedure began with access gained through a unilateral intrapedicular or extrapedicular approach. The curved design of the cavity creation instrument allowed the physician to drill a curved path from one pedicle, crossing the sagittal midline and stopping with the contralateral anterior quadrant of the vertebral body. The drill converted to a cavity cutting reamer in situ, which created a 10‐mm diameter cylindrical cavity. The cement directing implant consisted of a non load‐bearing, hollow, passively self‐expanding cylindrical device manufactured from a textile composite of nitinol wire, polyethylene teraphthalate fibre, and polycarbonate urethane. The implant was 10 mm in diameter and 15 mm, 20 mm or 25 mm long. The size of the implant was chosen to match the length of the cavity. The implant was designed to contain initially‐injected cement then regulate and direct cement flow into surrounding cancellous bone. Because the cavity was created by cutting, in contrast to bone compaction as in a balloon kyphoplasty, cement was able to penetrate into the bone beyond the boundaries of the cavity. Cement injection into the implant and directed through openings in its wall created a cement mantle in the anterior vertebral body, which extended towards the endplates and stabilised the fracture by filling cracks and voids, interdigitating with viable cancellous bone. Device placement in a centrally‐located cavity provided bilateral cement flow with the vertebral body, crossing both sides of the sagittal midline using a unipedicular approach. The nitinol‐based implant is expanded prior to cement injection to create a barrier to limit posterior cement flow into the basivertebral vein and spinal canal, while still allowing cement to permeate the vertebral body. |
|
| Outcomes | Patient follow‐up occurred at 3 and 12 months. Plain radiographs and CT scans were taken within 24 hours of the procedure and at 3 months, and plain radiographs were also taken at 12 months. Outcomes
Outcomes included in this review
|
|
| Source of funding | Funding provided by Soteira Inc. (Natick, MA) | |
| Notes | Trial reported to be registered with ClinicalTrials.gov with ID: NCT00576546 but this could not be verified. No efficacy outcomes were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised into vertebroplasty or cement‐directed kyphoplasty in a ratio of 1:2 but the method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded but investigators were not blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | Participants were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Cement leakage, changes in vertebral body height and the incidence of new fractures measured by investigators using radiographs were assessed by the investigators who were aware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A completers' analysis was performed. Data were available for 23 (82%) and 37 (76%) in vertebroplasty and kyphoplasty groups, respectively at the 3‐month follow‐up and 19 (68%) and 28 (57%) at the final 12‐month follow‐up. It is unclear it this is significantly different and the reasons for missing data are not reported. |
| Selective reporting (reporting bias) | High risk | A Congress abstract of the same trial is reported in German (https://www.thieme‐connect.com/products/ejournals/abstract/10.1055/s‐0031‐1279397), and indicates that pain intensity on a visual analogue scale (VAS) and disability assessed by the Oswestry Disability Index (ODI) were measured but these data are not presented in this paper. |
| Other bias | Unclear risk | The role of Soteira Inc. (Natick, MA) in the trial, other than supply of the Cement Directed Kyphoplasty System, is not explicitly reported. |
Voormolen 2007.
| Methods |
Design: multicentre (three hospitals), two‐arm open‐label randomised controlled trial; control group allowed to cross‐over to vertebroplasty at two weeks Setting: the Netherlands and Belgium Timing: not reported Interventions: percutaneous vertebroplasty or usual care Sample size:a priori sample size calculation not reported Analysis: completers' analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty Mean (range) age: 72 (59 to 84) years; 14 females, 4 males Mean (range) duration of back pain (units not reported, assumed as days): 85 (47 to 138) days Mean (range) number of pre‐existing vertebral compression fractures: 3.3 (1 to 8) at T5 to L5 Mean (range) baseline pain: 7.1 (5 to 9) Mean (range) baseline disability, RMDQ: 15.7 (8 to 22) Mean (range) baseline quality of life, QUALEFFO: 60 (37 to 86) No pain medication: 2 (11%) Paracetamol: 4 (22%) NSAIDs: 6 (33%) Opioids: 6 (33%) Usual care Mean (range) age: 74 (55 to 88) years; 14 females, 2 males Mean (range) duration of back pain (units not reported, assumed as days): 76 (46 to 141) days Mean (range) number of pre‐existing vertebral compression fractures: 3.1 (1 to 8) at T5 to L5 Mean (range) baseline pain: 7.6 (5 to 10) Mean (range) baseline disability,RMDQ: 17.8 (9 to 24) Mean (range) baseline quality of life, QUALEFFO: 67 (38 to 86) No pain medication: 1 (6%) Paracetamol: 7 (44%) NSAIDs: 3 (19%) Opioids: 5 (31%) |
|
| Interventions |
Vertebroplasty Percutaneous vertebroplasty was performed under local anaesthesia on a biplane (in 2 hospital departments) or monoplane (in 1 hospital department) angiographic unit. In most cases, a bilateral transpedicular approach was used. Under continuous fluoroscopy, PMMA bone cement (Osteopal V; Biomet Merck, Ried B. Kerzers, Switzerland) was injected manually using 1 mL syringes and 11‐ or 13‐gauge bone biopsy needles (Cook Europe Bjaeverskov, Denmark). Immediately after the percutaneous vertebroplasty, a CT scan with multiplanar reconstructions of the treated levels was performed to assess the cement deposition and to identify possible extra cement leakage or other local complications that might not have been noted under fluoroscopy. Usual care Participants were treated with the following medications, in ascending order.
The dose per day of prescribed analgesics was regulated, and the class of pain medication was adjusted as needed. |
|
| Outcomes | Outcomes were reported at 1 day and 2 weeks Outcomes
Outcomes included in this review
|
|
| Source of funding | None reported. | |
| Notes | Trial registration: not found. Standard deviations not reported for pain, disability or quality of life in the trial report but were provided by Dr Voormolen. The trial authors report that the original protocol was to follow participants for up to 12 months with outcome assessments at 1 day, 2 weeks and 3, 6, and 12 months. Participants randomised to usual care who still had severe pain after two weeks could cross‐over to receive vertebroplasty. As the majority of participants receiving usual care crossed over to vertebroplasty after two weeks, the authors stopped the study early, and did not collect outcome data beyond two weeks. There were two adjacent incident vertebral fractures in the vertebroplasty group within the two‐week follow‐up period, but it is unclear if there were any incident fractures in the usual care group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | An independent central operator allocated participants to treatment but whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and investigators were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Radiologists were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four participants were excluded from the analysis (refused to complete 2‐week follow‐up). The treatment group of these participants is not reported. |
| Selective reporting (reporting bias) | High risk | Trial protocol is not available. The number of participants with an incident clinical vertebral fracture is only reported for the vertebroplasty group. Measures of variance were not reported for continuous outcomes. |
| Other bias | Unclear risk | Eight participants withdrew after randomisation as they were not assigned to their preferred treatment (2 in the vertebroplasty group and 6 in the usual care group). The source of funding is not reported. |
VOPE 2015.
| Methods |
Design: single centre two‐arm double‐blind randomised placebo‐controlled trial Setting: Denmark Timing: May 2011 to April 2014 Interventions: percutaneous vertebroplasty versus sham vertebroplasty (placebo). The thesis stated that cross‐over between groups was allowed after 3 months. Sample size:a priori sample size calculation of 80 participants based upon requiring 26 participants per group to detect a difference of 20 units in improvement in pain on a 0 to 100 VAS (SD 25 units), based on a two‐sided type 1 error rate of 5% and power 80% and allowing for dropouts. Sample size was reduced to 52 (26 per group) due to difficulties with recruitment (low willingness to participate). (NB unpublished manuscript in thesis differs to thesis text in sample size estimation. Unpublished manuscript indicates that sample size based upon requiring 23 participants per group to detect 20 units difference, SD 20 units). Analysis: completers' analysis (excluded 4 participants due to need for further spine surgery and 2 participants who were excluded prior to receipt of intervention due to malignant biopsies, treatment allocation not stated but based upon numbers four were from the vertebroplasty group and 2 were from the placebo group) |
|
| Participants |
Number of participants
Inclusion criteria (from trial registry)
Exclusion criteria (from trial registry)
Inclusion criteria (from thesis report)
Exclusion criteria (from thesis report)
Baseline characteristics Vertebroplasty group Mean (range) age: 70.59 (54 to 90) years; 18 female: 4 male Mean (SE) baseline pain score at rest: 40.55 (4.55) Mean (SE) baseline pain score with activity: 74.68 (4.55) BMD T‐score: ‐2.7 (0.25) Vertebral levels: T6 to L5, 27 levels treated Placebo group Mean (range) age: 69.33 (53 to 84) years; 22 female: 2 male Mean (SE) baseline pain score at rest: 53.04 (4.35) Mean (SE) baseline pain score with activity: 76.08 (4.35) BMD T‐score: ‐2.2 (0.254) Vertebral levels: T7 to L5, 28 levels treated |
|
| Interventions |
Vertebroplasty All procedures were performed by spinal surgeons in the operating room. All vertebroplasties were performed under local anaesthesia using the V‐Max Mixing and Delivery System (DePuy Acromed, Leeds, England. Participants were placed prone on a Jackson table and lidocaine was used to anaesthetise the entry points. The 11‐gauge needles (Jamshidi needles) were then inserted in the fractured vertebral body via the pedicles under fluoroscopic guidance and a biopsy specimen was taken (not specified if uni‐ or bilateral pedicular approach). The PMMA cement was mixed and 2 mL of cement was injected into the pedicle under constant fluoroscopic guidance. The injection was stopped if the cement reached the posterior border of the vertebrae, showed signs of disc infiltration or the patient complained of leg symptoms. Placebo (sham vertebroplasty) The same procedure was performed for the placebo except that 2 mL of lidocaine (10 mg/mL) was injected into each Jamshidi needle (placed in the pedicle of the vertebral fracture as per the vertebroplasty group). The PMMA cement was mixed to create the odour similar to the vertebroplasty procedure. Both groups At inclusion, after a DEXA scan, all participants were commenced on treatment for osteoporosis. Prior to injecting either lidocaine of the PMMA, a needle biopsy was obtained (standard procedure at this site). Through the procedure, the staff in the operation room communicated minimally to ensure patients were unaware of the procedure performed. |
|
| Outcomes | Outcomes were reported at baseline, 6 hours, every week to 3 months, and 12 months The primary outcome was specified to be pain relief at 1 day, 1‐12 weeks, and 12 months. Both VAS and NRS were specified in the clinical trial registry. The thesis specifies both pain at rest and pain with activity on a VAS but not which was the primary endpoint. Outcomes (from the published thesis)
Additional outcomes in the trial registration: Lung capacity (spirometer) at 3 and 12 months Outcomes used in the review
|
|
| Source of funding | The primary sponsor was Sygehus Lillebaelt, Denmark (Hospital). The secondary sponsor was Odense University Hospital. | |
| Notes | Trial registration: NCT01537770 and EUCTR2010‐024050‐10‐DK Abstract (no numerical data reported): http://journals.sagepub.com/doi/abs/10.1055/s‐0036‐1582763, Global Spine J 2016; 06 ‐ GO106 Thesis cited: http://www.forskningsdatabasen.dk/en/catalog/2371744560; full text copy of the thesis provided by the author. No participants crossed over between treatment groups during this study. As SF‐36 physical function may be conceptually different to RMDQ and other back‐specific disability measures we did not combine it with other disability measures in our meta‐analysis. However no between‐groups differences were reported for SF‐36 at any time point. The amount and frequency of opioid use also did not differ between groups over time. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was a block randomisation design using 52 envelopes". No further details were reported. |
| Allocation concealment (selection bias) | Low risk | The randomisation envelope was opened after the bone biopsy had been taken. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to treatment allocation. Throughout the procedure there was minimal communication with the participants and operating room staff. The primary investigator of the trial (EH) performed all screening procedures and follow‐up examinations and was blinded to the participant's assigned treatment arm throughout the study period. Success of blinding was not assessed. The biostatistician was also blinded to treatment allocation when performing the analysis. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | Participants were unaware of treatment assignment. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Radiologists who assessed follow‐up radiographs at 3 and 12 months were aware of treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six of the 52 participants were not included in the analysis (4/26 (6.5%) in the vertebroplasty group and 2/26 (3.3%)in the placebo group. Of these, 2 were excluded due to malignant biopsies and 4 were excluded due to the need for further spinal surgery during the period of follow‐up but their treatment allocation was not reported. |
| Selective reporting (reporting bias) | Unclear risk | NRS pain data and spirometry were not reported. It is not clear whether pain at rest or with forward bending to resemble a patient in activity was the primary endpoint. |
| Other bias | Unclear risk | Thesis published in 2015 and includes an unpublished manuscript. Baseline pain at rest in the vertebroplasty group was lower than in the placebo group (40.55 (SE 4.55) compared with 53.04 (SE 4.35), P = 0.0476, although pain with activity was similar between groups (vertebroplasty 74.68 (SE 4.55), placebo 76.08 (SE 4.35)). |
Wang 2015.
| Methods |
Design: single‐centre, parallel‐group, two‐arm randomised controlled trial Setting: China Timing: 1 January 2012 to 12 February 2014 Interventions: high‐viscosity cement vertebroplasty versus balloon kyphoplasty Sample size:a priori sample size calculation not reported Analysis: intention‐to‐treat analysis planned |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group Mean (SD) age: 69.43 (8.94) years; 77.4% female: 22.6% male Mean (SD) VAS pain score: 8.10 (1.23) Mean (SD) ODI score: 71.22 (10.56) Mean (SD) compression rate: 29.98 (18.12) Balloon kyphoplasty group Mean (SD) age: 68.63 (8.39) years; 74.1% female: 25.9% male Mean (SD) VAS pain score: 8.04 (1.13) Mean (SD) ODI score: 71.30 (10.22) Mean (SD) compression rate: 28.67 (19.31) |
|
| Interventions | A unipedicular approach was adopted in all patients in this study. Injected cement volume was recorded for patients in both treatment groups. The endpoint of cement injection for both techniques was the presence of radiologically‐adequate filling, the start of leakage, and/or significantly increased pressure during injection. High‐viscosity cement vertebroplasty Vertebroplasty consists of injecting cement into a collapsed vertebra in order to reinforce the fractured vertebra and gain pain relief. The Confidence Spinal Cement System (DePuy Spine Inc, USA) was used. Balloon kyphoplasty An inflatable balloon is inserted into a collapsed vertebral body. Once inflated, the balloon elevates the endplates and creates a cavity. Then the cement is injected at a low pressure into the cavity of the collapsed vertebral body. Injecting cement into a cavity under low pressure reduces the risk of cement leakage. The Kyphon system (USA) was used. A low‐viscosity cement was used (OSTEOPAL V, Heraeus Medican GmbH, Germany) Follow‐up care After the procedure all patients remained supine in bed for 24 hours, and were referred for treatment with calcium and vitamin D supplements, and anti‐resorptive or anabolic agents. |
|
| Outcomes | Outcomes were measured at baseline, 1 day, 3 months, and 1 year after treatment. A primary outcome was not specified. Outcomes reported
Outcomes included in this review
|
|
| Source of funding | Funding source not stated. | |
| Notes | Clinicaltrials.gov trial identifier: not reported. No published protocol found. Triallists reported leakage in 9/68 vertebral joints in the vertebroplasty group and 22/72 joints in the kyphoplasty group, but did not report the number of participants with leakage. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were reportedly randomised into treatment groups, however randomisation method is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Whether or not treatment allocation was concealed is not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “Patients were blinded to which group they were assigned.” Not reported whether personnel were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | Low risk | Quote: “Patients were blinded to which group they were assigned.” |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Not reported whether radiographers were aware of treatment. However, they would be able to tell the difference between vertebroplasty and balloon kyphoplasty on a radiograph. It is stated that blinded data about cement leakage and vertebral body height were collected by radiologists. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were unavailable for 0 (0%) and 2 (3.3%) participants in the vertebroplasty and balloon kyphoplasty groups respectively at the 3‐month follow‐up. Data were unavailable for 3 (5.7%) and 3 (5.6%) participants in the vertebroplasty and balloon kyphoplasty groups, respectively at the 12‐month follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found. Pain, ODI and vertebral body height data were excluded from participants who had a further vertebral fracture during follow‐up. |
| Other bias | Low risk | None apparent. |
Wang 2016.
| Methods |
Design: two‐arm single‐centre parallel‐group randomised controlled trial Setting: China Timing: January 2009 to January 2013 Interventions: percutaneous vertebroplasty or facet joint injection with steroid and local anaesthetic Sample size:a priori sample size calculation not reported Analysis: intention‐to‐treat analysis |
|
| Participants |
Number of participants
Inclusion criteria Severe pain caused by acute (fracture occurred within 2 weeks) or subacute (fracture occurred within 2–8 weeks) osteoporotic vertebral fracture. Exclusion criteria
Baseline characteristics Percutaneous vertebroplasty Mean (SD) age (years): 63.7 (5.7) No. male/female: 19/81 No. acute fractures: 87 No. Subacute fractures: 13 Mean (SD) Bone density T score: ‐3.06 (0.38) No. (%) use of osteoporotic drugs: 23 (23) No. (%) new fracture patients: 13 (13) Facet joint injection Mean (SD) age (years): 62.9 (5.3) No. male/female: 22/84 No. acute fractures: 90 No. Subacute fractures: 16 Mean (SD) Bone density T score: ‐3.03 (0.41) No. (%) use of osteoporotic drugs: 19 (18) No. (%) new fracture patients: 11 (10.4) |
|
| Interventions | Both vertebroplasty and facet joint injection were performed under local anaesthesia using a plane angiography system under fluoroscopic guidance by spine surgeons in the hospital. Postural reduction was performed before surgery. Percutaneous vertebroplasty Vertebroplasty was performed using a bilateral or unilateral transpedicular approach. The patient was placed in the prone position on the table. After positioning of the fractured vertebrae and administration of local anaesthesia, an incision (approximately 5 mm long) was made using a scalpel. A bone puncture needle was then placed in the fractured vertebral body, transpedicularly. After removal of the inner needle, 3 mL to 9 mL of a poly methyl methacrylate (PMMA) bone cement was injected into the fractured vertebrae under continuous fluoroscopic visualization via lateral and antero‐posterior projections in order to ensure adequate filling and to avoid cement extravasation posteriorly into the spinal canal or migration into the venous system to prevent pulmonary embolism, which is a significant complication. The injection was stopped when the surgeon met substantial resistance or when the cement reached the cortical edge of the fractured vertebral body; the injection was also stopped if cement was near the spinal canal or leaked into extraosseous structures. Facet joint injection Injection was performed using a bilateral posterior approach with the participant in the prone position. After positioning of the fractured vertebral body and administration of local anaesthesia, a spinal needle was inserted into the facet joint capsule of the fractured vertebral body. Thereafter, a mixture of prednisolone (125 mg:5 mL) and lidocaine (100 mg:5 mL) was injected under fluoroscopic monitoring. Follow‐up care Participants in both groups wore a brace for 3 months after treatment. |
|
| Outcomes | Outcomes were reported at 1 day, 1 week, 1, 3, 6, and 12 months after treatment A primary outcome was not specified. Outcomes reported
Outcomes included in this review
|
|
| Source of funding | The authors reported that no funds were received in support of this study. | |
| Notes | Clinicaltrials.gov trial identifier, or other trial registration record: none found. Protocol publication not found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Not clearly reported, but as allocation was performed by an independent observer, bias may have been minimised. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, appears unblinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | The article did not report blinding of participants. The primary and secondary outcomes were self‐reported questionnaires, hence there is risk of bias in measurement of pain, physical functioning and quality of life. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | Outcome assessors were unblinded thus there is a risk of bias in the assessment of incident radiographic vertebral fractures. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/108 (7.4%) in the vertebroplasty group and 3/109 (2.7%) in the facet joint injection group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The results of all study outcomes were reported. Study protocol was not found. |
| Other bias | Low risk | No other biases apparent. |
Yang 2016.
| Methods |
Design: single‐centre, parallel‐group, two‐arm randomised controlled trial Setting: Shanghai, China Timing: January 2009 – December 2011 Interventions: high‐viscosity cement vertebroplasty versus conservative treatment Sample size: sample of 48 participants per group would be required for 90% power to show at least a two‐unit (on a 0 to 10 scale) advantage of vertebroplasty over conservative treatment in respect to pain with a SD of 3.0, based on a two‐sided type 1 error of 5% Analysis: not reported |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Vertebroplasty group Mean (SD) age: 77.1 (6.0) years; 64.3% female: 35.7% male Mean (SD) bone density T score: ‐3.3 (0.6) Mean (SD) VAS score: 7.5 (1.1) Mean (SD) ODI score: 80.2 (9.9) Mean (SD) QUALEFFO: 78.1 (8.1) Usual care group Mean (SD) age: 76.2 (5.6) years; 64.7% female: 35.3% male Mean (SD) bone density T score: ‐3.2 (0.7) Mean (SD) VAS score: 7.7 (1.1) Mean (SD) ODI score: 81.5 (9.7) Mean (SD) QUALEFFO: 77.5 (8.6) |
|
| Interventions |
Vertebroplasty All vertebroplasties were performed by two experienced surgeons. The patient was placed in prone position on the operating table. After local infiltration anaesthesia, a bone puncture needle was placed transpedicularly in the fractured vertebral body under a C‐arm fluoroscopic monitoring. Then, polymethylmethacrylate (PMMA) was carefully injected into the fractured vertebra with the fluoroscopic control. Injection ceased when cement reached the cortical edge of the vertebral body or leaked into the extraosseous structures or veins. If the cement did not reach the midline on the anterior‐posterior fluoroscopic film, another side of injection was performed. In patients who had more than one fracture, all the fractured bodies were treated in a single procedure. After the treatment, a CT scan of the treated vertebral bodies was completed to identify cement distribution and leakage. Usual care Patients were confined to horizontal bed rest for the initial 2 weeks after diagnosis. Then, they were encouraged to stand up and walk with brace and assistance. The bed rest time was extended if the back pain worsened when they stood up and walked. For pain medication, nonsteroidal anti‐inflammatory drugs (NSAIDs) were prescribed for every patient. Additional analgesics, such as tramadol and morphine, would be added in case NSAIDs were not effective. Two weeks after diagnosis, physical therapy was started. Follow‐up care All patients in both groups were prescribed treatment for osteoporosis including bisphosphonates, calcium supplementation, and vitamin D. |
|
| Outcomes | Outcomes were measured at 1 day, 1 week, 1, 3 and 6 months and 1 year. Primary outcome
Secondary outcomes
Outcomes included in this review
|
|
| Source of funding | No commercial entity paid for any materials used in the study. Costs of the vertebroplasty procedure, medication and physical therapy were covered by insurance. | |
| Notes | Trial registration: not found. Not reported how many participants contributed data at 1 week, 1 month, 3 month and 6 month so we used number of participants for 12 months for all time points. Cement leakage was seen in 22 out of the 65 treated vertebral bodies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Every patient was given a serial number according to the consecutive sequence of recruitment, and randomly assigned to PVP or conservative treatment group using computer‐generated randomised codes, according to the serial number. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided about whether or not treatment allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and investigators were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (e.g., pain, disability) | High risk | Participants assessed their pain and function and were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes (e.g., radiographic outcomes) | High risk | It is not stated who assessed the imaging outcomes. It is unlikely that they were blinded to treatment assignment as vertebroplasty cement is opaque and will be detected on imaging. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A greater number of participants completed one‐year follow‐up in the vertebroplasty group (8/64 missing, 13%) compared with 15/66 missing (23%) in the conservative treatment group. 8 (12.1%) participants in the conservative treatment group received vertebroplasty, and 2 (3%) had open surgery. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol found. |
| Other bias | Low risk | No other biases apparent. |
BMD: bone mineral density CI: confidence interval CT: computed tomography IU: international unit IV: intravenous MD: mean difference MMSE: Mini‐Mental Status Examination MRI: magnetic resonance imaging NRS: numerical rating scale NSAID: non‐steroidal anti‐inflammatory drugs ODI: Oswestry Disability Index PMMA: polymethyl methacrylate QUALY:quality‐adjusted life year RCT: randomised controlled trial RMDQ: Roland‐Morris Disability Questionnaire SD: standard deviation SE: standard error SF‐36: Short form 36 VAS: visual analogue scale VCF: vertebral compression fracture
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cai 2015 | RCT comparing two different types of vertebroplasty. |
| Chen 2014b | RCT comparing two different types of vertebroplasty. |
| Chun‐lei 2015 | RCT comparing unipedicular vertebroplasty and bipedicular vertebroplasty, vertebroplasty given to both treatment groups. |
| Du 2014 | Treatment allocation not random, "Patients were divided according to the surgeon they had been referred to." |
| Gilula 2013 | RCT comparing different cement types, vertebroplasty given to both treatment groups. Trial registration: NCT00290862. |
| Gu 2015 | RCT comparing screw fixation and vertebroplasty to vertebroplasty. |
| Huang 2014 | RCT comparing different cement types, vertebroplasty given to both treatment groups. |
| Li 2015a | RCT comparing two different types of vertebroplasty. |
| Liu 2015 | RCT comparing two different types of vertebroplasty. |
| Min 2015 | RCT comparing vertebroplasty in both groups. |
| Son 2014 | Not an RCT (retrospective cohort). |
| Xiao‐nan 2014 | RCT comparing different cement types, vertebroplasty given to both treatment groups. |
| Yang 2014 | Non‐randomised study. |
| Yi 2014 | Non‐randomised study. |
| Yi 2016 | Non‐randomised study. |
| Ying 2017 | RCT comparing low and high viscosity cement types, vertebroplasty given to both treatment groups. |
| Yokoyama 2016 | Non‐randomised study. |
| Zhang 2015a | RCT comparing different cement types, vertebroplasty given to both treatment groups. |
| Zhang 2015b | RCT comparing unipedicular vertebroplasty and bipedicular vertebroplasty, vertebroplasty given to both treatment groups. |
| Zhang 2015c | RCT comparing unilateral and bilateral vertebroplasty, vertebroplasty given to both treatment groups. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2015.
| Methods | Two‐arm randomised controlled trial. |
| Participants | Sample size = 84 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Follow‐up time was 3 months and at 12‐54 months (average of 34.7 months for final follow‐up) Study outcomes VAS score ODI and vertebral height |
| Study name | Bone cement injection as vertebral augmentation therapy for osteoporotic vertebral compression fractures. [Chinese] |
| Starting date | Jan 2010 |
| Contact information | Chen Junping, Department of Orthopedics, the Fifth Affiliated Hospital of Zunyi Medical College, Zhuhai 519100, Guangdong Province, China |
| Notes | Trial not registered at clinical trials.gov website Published in Chinese, awaiting translation. |
Dolin 2003.
| Methods | Randomised controlled trial, open‐label. Sample size not specified. |
| Participants |
Inclusion criteria
|
| Interventions |
|
| Outcomes | Duration of follow‐up and outcomes measured not specified. |
| Study name | A randomised controlled trial of vertebroplasty for the treatment of osteoporotic vertebral crush fractures. |
| Starting date | November 2005. |
| Contact information | Principal Investigator: Simon Dolin |
| Notes | Trial Registration: ISRCTN14442024 (Also N0213112414); http://www.isrctn.com/ISRCTN14442024. Primary Sponsor: Record provided by the NHS Trusts Clinical Trials Register ‐ Department of Health (UK). Completed, but no results available. |
Laredo (OSTEO‐6).
| Methods | Randomised controlled trial, open‐label |
| Participants | N = 48 (planned sample size 300) Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Follow‐up to one year Primary outcome
Secondary outcomes
|
| Study name | Prospective randomized comparative study of balloon kyphoplasty, vertebroplasty and conservative management in acute osteoporotic vertebral fractures of less than 6 weeks |
| Starting date | December 2007 |
| Contact information | Principal Investigator: Jean‐Denis Laredo |
| Notes | Trial Registration: NCT0749060 ('OSTEO‐6') Primary sponsor: Assistance Publique ‐ Hôpitaux de Paris; Secondary sponsor: Ministry of Health, France Completed June 2012. |
Laredo (STIC2).
| Methods | Randomised controlled trial, open‐label |
| Participants | N = 97 (planned sample size 200) Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Follow‐up to one year Primary outcome
Secondary outcomes
|
| Study name | Prospective randomized study of balloon kyphoplasty and vertebroplasty in subacute (older than 6 weeks) osteoporotic vertebral fractures (STIC2) |
| Starting date | December 2007 |
| Contact information | Principal Investigator: Jean‐Denis Laredo |
| Notes | Trial Registration: NCT0749086 ('STIC2') Primary sponsor: Assistance Publique ‐ Hôpitaux de Paris, France; Secondary sponsor: Ministry of Health, France Completed June 2012 |
Li 2015b.
| Methods | Three‐arm randomised controlled trial |
| Participants | Sample size = 90 Inclusion and exclusion criteria not available |
| Interventions |
|
| Outcomes | Duration of follow‐up not given Study outcomes
|
| Study name | Bone filling mesh container for treatment of vertebral compression fractures can reduce the leakage of bone cement |
| Starting date | Not given |
| Contact information | Not given |
| Notes | Trial not registered at clinicaltrials.gov website Publication in Chinese, awaiting translation |
Sorensen 2005.
| Methods | Randomised controlled trial, open‐label |
| Participants | N = 27 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Follow‐up to 12 months Primary outcome
Secondary outcomes
|
| Study name | Percutaneous vertebroplasty versus conservative treatment of pain: a prospective, randomized controlled study of osteoporotic fractures in the spine |
| Starting date | March 2004 |
| Contact information | Principal Investigator: Leif Sorensen |
| Notes | Trial Registration: NCT00203554 Primary sponsor: University of Aarhus, Denmark Completed January 2008, no results available |
Tan 2016.
| Methods | Two‐arm randomised controlled trial |
| Participants | Sample size = 106 53 randomised to percutaneous vertebroplasty group and 53 randomised to percutaneous kyphoplasty group Inclusion and exclusion criteria unable to read as the full text is in Chinese |
| Interventions |
|
| Outcomes | Follow‐up to 6 months Study outcomes
|
| Study name | Percutaneous kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral compression fractures: A randomized comparison. [Chinese] |
| Starting date | Not given |
| Contact information | Not available |
| Notes | Trial not registered in Clinicaltrials.gov website |
Zhou 2015.
| Methods | Two‐arm randomised controlled trial |
| Participants | Sample size = 80 Inclusion and exclusion criteria not provided |
| Interventions |
|
| Outcomes | Follow‐up at 3 months Study outcomes
|
| Study name | Percutaneous vertebroplasty with high‐viscosity bone cement for treatment of severe osteoporotic thoracolumbar vertebral compression fractures. [Chinese] |
| Starting date | Not available |
| Contact information | Zhou Wei, Zhongxiang City, Hubei Province, 2005 Huazhong University of Science and Technology |
| Notes | Trial not registered in Clinical trials.gov website |
ADL: activities of daily living CT: computed tomography MRI: magnetic resonance imaging ODI: Oswestry disability index TDM: tomodensitometry VAS: visual analogue scale VCF: vertebral compression fracture
Characteristics of ongoing studies [ordered by study ID]
Longo 2010.
| Trial name or title | The effectiveness and safety of vertebroplasty for osteoporotic vertebral compression fractures. A double blind, prospective, randomised, controlled study |
| Methods | Randomised controlled trial, participant blinded |
| Participants | Planned sample size = 164 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Duration of follow‐up to 24 months Primary outcome
Secondary outcomes
|
| Starting date | Not specified |
| Contact information | Principal Investigator: Umile Giuseppe Longo, MD, Italy Phone: +39 06 22 54 11 E‐mail: g.longo@unicampus.it |
| Notes | Unclear if registered in a trial registry Primary sponsor: Not specified Status of trial unknown |
Sun 2012.
| Trial name or title | Investigational percutaneous vertebroplasty efficacy and safety trial |
| Methods | Randomised controlled trial, open‐label |
| Participants | Planned sample size = 140 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Follow‐up duration 12 months Primary outcome
Secondary outcomes
|
| Starting date | October 2012 |
| Contact information | Principal Investigator: Gang Sun, The Jinan Military General Hospital |
| Notes | Trial registration: NCT01677806 Primary sponsor: Jinan Military General Hospital, China; Secondary sponsors: Beijing Friendship Hospital China Medical University; Shanghai 10th People's Hospital; Shanghai 6th People's Hospital; The Second Affiliated Hospital of Chongqing Medical University Recruitment status unknown at last date of verification (11 Sep 2014), although expected completion date was December 2014, as reported at clinicaltrials.gov (accessed 20 Dec 2017). |
VERTOS V.
| Trial name or title | VERTOS V |
| Methods | Randomised controlled trial, participant blinded |
| Participants | Planned sample size = 94 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Duration of follow‐up to 12 months Primary outcome
Secondary outcomes
|
| Starting date | May 2013 |
| Contact information | Dr Dennis Carli |
| Notes | Trial registration: NCT01963039 Primary sponsor: St. Elisabeth Hospital, Tilburg, the Netherlands Study completed June 2015 (as reported at clinicaltrials.gov in September 2016); no study results posted. Status changed 12 January 2017: study now 'enrolling by invitation', with a new estimated completion date of July 2018 |
MRI: magnetic resonance imaging ODI: Oswestry disability index OVCF: osteoporotic vertebral compression fracture RMDQ: Roland‐Morris Disability Questionnaire QUALEFFO: Quality of Life Questionnaire of the European Foundation for Osteoporosis VAS: visual analogue scale VCF: vertebral compression fracture
Differences between protocol and review
For the original review the protocol was extensively updated to conform with updated conduct and reporting standards of systematic reviews as recommended by Cochrane and the MECIR project.
At the time that the protocol was developed, we had planned to include controlled before and after studies (CBAs) and interrupted time series (ITS) in our efficacy analysis if there were no published randomised controlled trials (CTs) or quasi‐RCTs. Subsequent to the publication of the protocol, several RCTs were published and we therefore only included RCTs or quasi‐RCTs in this review.
Subsequent to publication of the protocol, we clarified the possible comparators eligible for inclusion, i.e. that we would include randomised controlled trials of vertebroplasty compared with any comparator, including sham, conservative treatment or other surgical procedures such as kyphoplasty, but would exclude trials that compared vertebroplasty to another type of vertebroplasty.
Differences between first version of the review and current updated version
We removed four trials that were classed as ongoing trials or awaiting classification in the first version of this review (Damaskinos 2015 NCT02489825; Nakstad 2008 NCT00635297; Nieuwenhuijse 2012 NTR3282; Zhao 2014 ChiCTR‐TRC‐14004835), as we subsequently discovered that percutaneous vertebroplasty was given to participants in both treatment arms and thus, when trial results become available, they will not be eligible for inclusion in this review.
We used mean differences for calculating effect sizes on pain scores as this enabled us to combine change with end of treatment scores.
Calculating number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH), an additional person: we had planned to calculate these for outcomes that showed a statistically significant difference between groups. However, in light of any differences we found being small and clinically unimportant, we decided that such statistics were difficult to interpret.
We updated the subgroup analysis based upon data available from the newly published placebo‐controlled trials to compare 'acute' to 'subacute' fractures. Previously we compared pain duration ≤ 6 weeks to > 6 weeks. However all three new placebo‐controlled trials only included participants with 'acute' fractures defined as pain less than 6 weeks in Clark 2016, 8 weeks or less in VOPE 2015, and Firanescu 2018 altered their inclusion criteria from 6 weeks or less to 9 weeks or less after 6 months due to poor recruitment. Both Buchbinder 2009 and Kallmes 2009 performed post‐hoc subgroup analyses comparing data for participants with symptom duration of 6 weeks or less compared to more than 6 weeks.
Contributions of authors
For this review update: R Buchbinder, R Johnston and KJ Rischin drafted the review update. R Buchbinder, R Johnston, KJ Rischin, K Golmohammadi, A Jones, J Homik, and D Kallmes conducted the updated search and/or independently selected trials for inclusion and/or extracted the data, and/or performed a 'Risk of bias' assessment and/or assessed the quality of the body of evidence for the main outcomes using the GRADE approach and/or provided critical comment on the manuscript. All authors approved the final manuscript.
Sources of support
Internal sources
Monash Department of Clinical Epidemiology, Cabrini Institute and Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Australia.
External sources
R Buchbinder is supported in part by an Australian National Health and Medical Research Council Practitioner Fellowship, Australia.
Declarations of interest
R Buchbinder was a principal investigator of Buchbinder 2009. D Kallmes was a principal investigator of Kallmes 2009 and Evans 2015. D Kallmes participated in IDE trial for Benvenue Medical spinal augmentation device. He is a stockholder, Marblehead Medical, LLC, Development of spine augmentation devices. He holds a spinal fusion patent license, unrelated to spinal augmentation/vertebroplasty. R Buchbinder and D Kallmes did not perform risk of bias assessments for their own or any other placebo‐controlled trials included in the review.
Edited (no change to conclusions)
References
References to studies included in this review
Blasco 2012 {published data only}
- Blasco J, Martinez‐Ferrer A, Macho J, San Roman L, Pomes J, Carrasco J, et al. Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: a 12‐month randomized follow‐up, controlled trial. Journal of Bone and Mineral Research 2012;27(5):1159‐66. [DOI] [PubMed] [Google Scholar]
- Blasco J, Martinez‐Ferrer A, San Roman J, Macho A, Lopez Rueda D, Campodonico C, et al. Risk factors for the development of chronic back pain after percutaneous vertebroplasty. European Society of Radiology. 2014.
- Peris P, Blasco J, Carrasco JL, Martinez‐Ferrer A, Macho J, San Roman L, et al. Risk factors for the development of chronic back pain after percutaneous vertebroplasty versus conservative treatment. Calcified Tissue International 2015;96(2):89‐96. [DOI] [PubMed] [Google Scholar]
Buchbinder 2009 {published data only}
- Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures.[see comment]. New England Journal of Medicine 2009;361(6):557‐68. [DOI] [PubMed] [Google Scholar]
- Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt CJ, et al. Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: a randomised controlled trial [ACTRN012605000079640]. BMC Musculoskeletal Disorders 2008;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon F, Staples MP, Ebeling P, Wark J, Osborne R, Mitchell P, et al. Vertebroplasty for osteoporotic vertebral fractures: Two‐year results from a randomized controlled trial. Journal of Bone and Mineral Research 2014;29(6):1346‐55. [DOI] [PubMed] [Google Scholar]
- Staples MP, Howe BM, Ringler M, Mitchell P, Wriedt C, Wark J, et al. Vertebroplasty for osteoporotic vertebral fractures: radiological outcomes from a 2 year randomized controlled trial. Archives of Osteoporosis 2015;10(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014a {published data only}
- Chen D, An ZQ, Song S, Tang JF, Qin H. Percutaneous vertebroplasty compared with conservative treatment in patients with chronic painful osteoporotic spinal fractures. Journal of Clinical Neuroscience 2014;21:473‐7. [DOI] [PubMed] [Google Scholar]
Clark 2016 {published data only}
- Bird P, Clark W, Diamond T, Schlaphoff G, Smerdely P, Gonski P, et al. A placebo controlled trial of vertebral fill technique vertebroplasty for acute painful osteoporotic fracture (VAPOUR trial). Arthritis and Rheumatology. Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting. 2016; Vol. 68:413‐4.
- Clark W, Bird P, Diamond T, Gonski P. Vertebroplasty for acute painful osteoporotic fractures (VAPOUR): study protocol for a randomized controlled trial. Trials 2015;16:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W, Bird P, Gonski P, Diamond T, Smerdely P, McNeil H, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2016;388(10052):1408‐16. [DOI] [PubMed] [Google Scholar]
Dohm 2014 {published data only}
- Dohm M, Black CM, Dacre A, Tillman JB, Fueredi G, KAVIAR investigators. A randomized trial comparing balloon kyphoplasty and vertebroplasty for vertebral compression fractures due to osteoporosis. AJNR: American Journal of Neuroradiology 2014;35(12):2227‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endres 2012 {published data only}
- Endres S, Badura A. Shield kyphoplasty through a unipedicular approach compared to vertebroplasty and balloon kyphoplasty in osteoporotic thoracolumbar fracture: A prospective randomized study. Orthopaedics & Traumatology: Surgery & Research 2012;98(3):334‐40. [DOI] [PubMed] [Google Scholar]
Evans 2015 {published data only}
- Evans A, Kip K, Brinjikji W, Layton K, Jensen M, Gaughen J, et al. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. Journal of Neurointerventional Surgery 2015;8(7):756‐63. [DOI] [PubMed] [Google Scholar]
Farrokhi 2011 {published data only}
- Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. Journal of Neurosurgery. Spine 2011;14(5):561‐9. [DOI] [PubMed] [Google Scholar]
Firanescu 2018 {published and unpublished data}
- Firanescu C, Lohle PN, Vries J, Klazen CA, Juttmann JR, Clark W, et al. VERTOS IV study group. A randomised sham controlled trial of vertebroplasty for painful acute osteoporotic vertebral fractures (VERTOS IV). Trials 2011;12(93):http://www.trialsjournal.com/content/12/1/93. [DOI: 10.1186/1745-6215-12-93] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firanescu C, Vries J, Lodder P, Schoemaker MC, Smeets AJ, Vemans A, et al. VERTOS IV study group. American Society Spine Radiology (ASSR) 2017 Annual Symposium. San Diego, 2017.
- Firanescu CE, Vries J, Lodder P, Venmans A, Shoemaker MC, Smeet AJ, Donga E, Juttmann JR, Klazen CAH, Elgersma OEH, Jansen FH, Tielbeek AV, Boukrab I, Schonenberg K, Rooij WJJ, Hirsch JA, Lohle PNM. Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial. BMJ 2018 2018;360:k1551. [DOI: 10.1136/bmj.k1551] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kallmes 2009 {published data only}
- Brinjikji W, Comstock BA, Heagerty PJ, Jarvik JG, Kallmes DF. Investigational Vertebroplasty Efficacy and Safety Trial: detailed analysis of blinding efficacy. Radiology 2010;257(1):219‐25. [DOI] [PubMed] [Google Scholar]
- Comstock BA, Sitlani CM, Jarvik JG, Heagerty PJ, Turner JA, Kallmes DF. Investigational vertebroplasty safety and efficacy trial (INVEST): patient‐reported outcomes through 1 year. Radiology 2013;269:224‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LA, Jarvik JG, Heagerty PJ, Hollingworth W, Stout L, Comstock BA, et al. INvestigational Vertebroplasty Efficacy and Safety Trial (INVEST): a randomized controlled trial of percutaneous vertebroplasty. BMC Musculoskelet Disorders 2007;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallmes DF, Comstock BA, Gray LA, Heagerty PJ, Hollingworth W, Turner JA, et al. Baseline pain and disability in the Investigational Vertebroplasty Efficacy and Safety Trial. AJNR: American Journal of Neuroradiology 2009;30(6):1203‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures.[see comment]. New England Journal of Medicine 2009;361(6):569‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Heagarty PJ, et al. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta‐analysis. BMJ 2011;342:d3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klazen 2010 {published data only}
- Klazen CA, Lohle PN, Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open‐label randomised trial. Lancet 2010;376(9746):1085‐92. [PUBMED: 20701962] [DOI] [PubMed] [Google Scholar]
- Klazen CA, Venmans A, Vries J, Rooij WJ, Jansen FH, Blonk MC, et al. Percutaneous vertebroplasty is not a risk factor for new osteoporotic compression fractures: results from VERTOS II. AJNR: American Journal of Neuroradiology 2010;31(8):1447‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klazen CA, Verhaar HJ, Lampmann LE, Juttmann JR, Blonk MC, Jansen FH, et al. VERTOS II: percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007;8:33. [PUBMED: 17973983] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leali 2016 {published and unpublished data}
- Leali PT, Solla F, Maestretti G, Balsano M, Doria C. Safety and efficacy of vertebroplasty in the treatment of osteoporotic vertebral compression fractures: A prospective multicenter international randomized controlled study. Clinical Cases in Mineral and Bone Metabolism 2016;13(3):234‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu JT, Li CS, Chang CS, Liao WJ. Long‐term follow‐up study of osteoporotic vertebral compression fracture treated using balloon kyphoplasty and vertebroplasty. Journal of Neurosurgery Spine 2015;23(1):94‐8. [DOI] [PubMed] [Google Scholar]
- Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporosis International 2010;21:359–64. [DOI] [PubMed] [Google Scholar]
Rousing 2009 {published data only}
- Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J, Rousing Rikke, et al. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three‐months follow‐up in a clinical randomized study. Spine 2009;34(13):1349‐54. [DOI] [PubMed] [Google Scholar]
- Rousing R, Hansen KL, Andersen MO, Jespersen SM, Thomsen K, Lauritsen JM. Twelve‐months follow‐up in forty‐nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine 2010;35(5):478‐82. [PUBMED: 20190623] [DOI] [PubMed] [Google Scholar]
Sun 2016 {published and unpublished data}
- Sun K, Liu Y, Peng H, Tan JF, Zhang M, Zheng XN, et al. A comparative study of high‐viscosity cement percutaneous vertebroplasty vs. low‐viscosity cement percutaneous kyphoplasty for treatment of osteoporotic vertebral compression fractures. Journal of Huazhong University of Science and Technology. Medical Sciences 2016;36(3):389‐94. [DOI] [PubMed] [Google Scholar]
Vogl 2013 {published data only}
- Vogl TJ, Pflugmacher R, Hierholzer J, Stender G, Gounis M, Wakhloo A, et al. Cement directed kyphoplasty reduces cement leakage as compared with vertebroplasty: results of a controlled, randomized trial. Spine 2013;38(20):1730‐6. [DOI] [PubMed] [Google Scholar]
Voormolen 2007 {published data only}
- Voormolen MH, Lohle PN, Fransen H, Juttmann JR, Waal Malefijt J, Lampmann LE. [Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: first short term results]. Nederlands Tijdschrift Voor Geneeskunde 2003;147(32):1549‐53. [PubMed] [Google Scholar]
- Voormolen MH, Lohle PN, Juttmann JR, Graaf Y, Fransen H, Lampmann LE. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. Journal of Vascular and Interventional Radiology 2006;17(1):71‐6. [DOI] [PubMed] [Google Scholar]
- Voormolen MH, Lohle PN, Lampmann LE, Wildenberg W, Juttmann JR, Diekerhof CH, et al. Prospective clinical follow‐up after percutaneous vertebroplasty in patients with painful osteoporotic vertebral compression fractures. Journal of Vascular and Interventional Radiology 2006;17(8):1313‐20. [DOI] [PubMed] [Google Scholar]
- Voormolen MH, Mali WP, Lohle PN, Fransen H, Lampmann LE, Graaf Y, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short‐term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. American Journal of Neuroradiology 2007;28(3):555‐60. [PMC free article] [PubMed] [Google Scholar]
- Voormolen MH, Rooij WJ, Sluzewski M, Graaf Y, Lampmann LE, Lohle PN, et al. Pain response in the first trimester after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures with or without bone marrow edema. AJNR: American Journal of Neuroradiology 2006;27(7):1579‐85. [PMC free article] [PubMed] [Google Scholar]
- Voormolen MH, Rooij WJ, Graaf Y, Lohle PN, Lampmann LE, Juttmann JR, et al. Bone marrow edema in osteoporotic vertebral compression fractures after percutaneous vertebroplasty and relation with clinical outcome. AJNR: American Journal of Neuroradiology 2006;27(5):983‐8. [PMC free article] [PubMed] [Google Scholar]
VOPE 2015 {unpublished data only}
- Hansen EJ. A double‐blind randomized clinical study comparing vertebroplasty with a SHAM procedure for acute osteoporotic vertebral compression fractures (VOPE). Thesis, University of Southern Denmark: Center for Spine Surgery and Research, Middelfart Hospital and Institute of Regional Health Research, University of Southern Denmark, Denmark 2015.
- Hansen EJ, Simony A, Rousing R, Carreon LY, Tropp H, Andersen MO. Double blind placebo‐controlled trial of percutaneous vertebroplasty (VOPE). Global Spine Journal. 2017; Vol. 6 Issue 1_suppl:s‐0036‐1582763. [DOI: ]
- NCT01537770. Vertebroplasty versus periost infiltration with lidocaine as pain treatment in osteoporotic fractures in the thoracic and lumbar spine. clinicaltrials.gov/show/NCT01537770 Trial registered: EUCTR 2010‐024050‐10 (Also NCT01537770) completed as of 11 September 2015.
Wang 2015 {published data only}
- Wang C, Ma J, Zhang C, Nie L. Comparison of high‐viscosity cement vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Pain Physician 2015;18(2):187‐94. [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Hao D, Guo H, Wang B, Wang X. Treatment for acute or subacute osteoporosis vertebral compression fractures: Percutaneous vertebroplasty versus facets blocking (a clinical randomized study). European Spine Journal; http://eposterlyon.eurospine.org/cm_data/eposter/P17.PDF. 2014; Vol. 23, Issue 5:Supplement, pp 527‐557.
- Hao D, Wang B, Guo H, Wang X. Treatment for acute or subacute osteoporosis vertebral compression fractures: Percutaneous vertebroplasty versus facets blocking (a clinical randomized study). European Spine Journal 2014;23(1):S533. [Google Scholar]
- Wang B, Guo H, Yuan L, Huang D, Zhang H, Hao D. A prospective randomized controlled study comparing the pain relief in patients with osteoporotic vertebral compression fractures with the use of vertebroplasty or facet blocking. European Spine Journal 2016;25:3486‐94. [DOI: 10.1007/s00586-016-4425-4] [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang EZ, Xu JG, Huang GZ, Xiao WZ, Liu XK, Zeng BF, et al. Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures. Spine 2016;41(8):653‐60. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cai 2015 {published data only}
- Cai J, Hao YW, Li C, Yang HQ. Percutaneous vertebroplasty with bone cement injection for osteoporotic vertebral compression fractures via transpedicular approach [Chinese]. Chinese Journal of Tissue Engineering Research 2015;19(30):4892‐7. [Google Scholar]
Chen 2014b {published data only}
- Chen C, Bian J, Zhang W, Zhao C, Wei H. Unilateral versus bilateral vertebroplasty for severe osteoporotic vertebral compression fractures. Journal of Spinal Disorders & Techniques 2014;27(8):E301‐4. [DOI] [PubMed] [Google Scholar]
Chun‐lei 2015 {published data only}
- Chen‐lei L, Yi‐he H, Gui‐qing W, Yong‐zhi T, Xiang‐jiang W, Han‐tao H. Curative effects of unipedicular and bipedicular vertebroplasty in treating osteoporotic vertebral compression fractures in the elderly population. Journal of Xi'an Jiatong University 2015;36:6. [Google Scholar]
Du 2014 {published data only}
- Du J, Li X, Lin X. Kyphoplasty versus vertebroplasty in the treatment of painful osteoporotic vertebral compression fractures: two‐year follow‐up in a prospective controlled study. Acta Orthopaedica Belgica 2014;80(4):477‐86. [PubMed] [Google Scholar]
Gilula 2013 {published data only}
- Gilula L, Persenaire M. Subsequent fractures post–vertebral augmentation: Analysis of a prospective randomized trial in osteoporotic vertebral compression fractures. AJNR: American Journal of Neuroradiology 2013;34(2):221‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gu 2015 {published data only}
- Gu YT, Zhu DH, Liu HF, Zhang F, McGuire R. Minimally invasive pedicle screw fixation combined with percutaneous vertebroplasty for preventing secondary fracture after vertebroplasty. Journal of Orthopaedic Surgery 2015;10:31 doi: 10.1186/s13018‐015‐0172‐1. [DOI: 10.1186/s13018-015-0172-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2014 {published data only}
- Huang XN. Percutaneous vertebroplasty for treatment of osteoporotic vertebral fractures: High‐viscosity versus low‐viscosity bone cement [Chinese]. Chinese Journal of Tissue Engineering Research 2014;18(16):2461‐7. [Google Scholar]
Li 2015a {published data only}
- Li J, Shen WL, Chang XH. Comparison of clinical effects between unilateral and bilateral percutaneous vertebroplasty for osteoporotic vertebral compression fractures. [Chinese]. Journal of Dalian Medical University 2015;37(2):145‐7. [Google Scholar]
Liu 2015 {published data only}
- Liu CL, Hu YH, Wang GQ, Tang YZ, Wang XJ, Hou HT. Curative effects of unipedicular and bipedicular vertebroplasty in treating osteoporotic vertebral compression fractures in the elderly population. [Chinese]. Journal of Xi'an Jiaotong University (Medical Sciences) 2015;36(6):857‐61. [Google Scholar]
Min 2015 {published data only}
- Min P, Zhang YP, Cao H. CT‐guided bone cement injection combined with artificial tiger bone meal to repair osteoporotic vertebral compression fractures: Callus growth and bone healing [Chinese]. Chinese Journal of Tissue Engineering Research 2015;19(3):335‐9. [Google Scholar]
Son 2014 {published data only}
- Son S, Lee SG, Kim WK, Park CW, Yoo CJ. Early vertebroplasty versus delayed vertebroplasty for acute osteoporotic compression fracture: Are the results of the two surgical strategies the same?. Journal of Korean Neurosurgical Society 2014;56(3):211‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiao‐nan 2014 {published data only}
- Xiao‐nan H. Percutaneous vertebroplasty for treatment of osteoporotic vertebral fractures: high viscosity versus low‐viscosity bone cement. Chinese Journal of Tissue Engineering Research 2014;18(16):2461‐7. [Google Scholar]
Yang 2014 {published data only}
- Yang DH, Cho KH, Chung YS, Kim YR. Effect of vertebroplasty with bone filler device and comparison with balloon kyphoplasty. European Spine Journal 2014;23(12):2718‐25. [DOI] [PubMed] [Google Scholar]
Yi 2014 {published data only}
- Yi X, Lu H, Tian F, Wang Y, Li C, Liu H, et al. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Archives of Orthopaedic and Trauma Surgery 2014;134(1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yi 2016 {published data only}
- Yi HJ, Jeong JH, Im SB, Lee JK. Percutaneous vertebroplasty versus conservative treatment for one level thoracolumbar osteoporotic compression fracture: results of an over 2‐year follow‐up. Pain Physician 2016;19(5):E743‐50. [PubMed] [Google Scholar]
Ying 2017 {published data only}
- Ying L. Chen Z, Yang G. Clinical study of high viscosity bone cement in the treatment of osteoporotic vertebral compression fracture. Biomedical Research (India) 2017;28(18):7837‐40. [Google Scholar]
Yokoyama 2016 {published data only}
- Yokoyama K, Kawanishi M, Yamada M, Tanaka H, Ito Y, Kawabata S, et al. Comparison of vertebroplasty versus vertebral perforation for the treatment of acute vertebral compression fractures. Turkish Neurosurgery 2016;26(3):423‐9. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Kawanishi M, Yamada M, Tanaka H, Ito Y, Kuroiwa T. Long‐term therapeutic effects of vertebroplasty for painful vertebral compression fracture: a retrospective comparative study. British Journal of Neurosurgery 2017;31(2):184‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2015a {published data only}
- Zhang L, Wang J, Feng X, Tao Y, Yang J, Wang Y, et al. A comparison of high viscosity bone cement and low viscosity bone cement vertebroplasty for severe osteoporotic vertebral compression fractures. Clinical Neurology and Neurosurgery 2015;129:10‐6. [DOI] [PubMed] [Google Scholar]
Zhang 2015b {published data only}
- Zhang L, Liu Z, Wang J, Feng X, Yang J, Tao Y, et al. Unipedicular versus bipedicular percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a prospective randomized study. BMC Musculoskeletal Disorders 2015;16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2015c {published data only}
References to studies awaiting assessment
Chen 2015 {published data only}
- Chen JP, Qi XW, Li SJ, Kuang LP, Yuan XH, Wang GS, et al. Bone cement injection as vertebral augmentation therapy for osteoporotic vertebral compression fractures. [Chinese]. Chinese Journal of Tissue Engineering Research 2015;19(21):3292‐6. [DOI: ] [Google Scholar]
Dolin 2003 {unpublished data only}
- Doin S. A randomised controlled trial of vertebroplasty for the treatment of osteoporotic vertebral crush fractures. http://www.isrctn.com/ISRCTN14442024 (first received 12 September 2003) Trial completed.
Laredo (OSTEO‐6) {unpublished data only}
- NCT00749060. Prospective randomized comparative study of balloon kyphoplasty, vertebroplasty and conservative management in acute osteoporotic vertebral fractures of less than 6 weeks. clinicaltrials.gov/show/NCT00749060 (first received September 8, 2008) Trial completed June 2012.
Laredo (STIC2) {unpublished data only}
- NCT00749086. Prospective randomized study of balloon kyphoplasty and vertebroplasty in subacute (older than 6 weeks) osteoporotic vertebral fractures (STIC2). clinicaltrials.gov/show/NCT00749086 Trial registered: NCT00749086. Recruitment commenced Dec 2007. Trial completed June 2012.
Li 2015b {published data only}
- Li DH, Liu XW, Peng XT, Wang ZG, Wang BC, Jin P, et al. Bone filling mesh container for treatment of vertebral compression fractures can reduce the leakage of bone cement. [Chinese]. Chinese Journal of Tissue Engineering Research 15 Jan 2015;19(3):358‐3. [Google Scholar]
Sorensen 2005 {unpublished data only}
- NCT00203554. Percutaneous vertebroplasty versus conservative treatment of pain: a prospective, randomized controlled study of osteoporotic fractures in the spine. clinicaltrials.gov/show/NCT00203554 (first received 16 September 2005).
Tan 2016 {published data only}
- Tan B, Liu XW, Liu G, Li YS, Qin ZJ, Yang CP. Percutaneous kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral compression fractures: A randomized comparison [Chinese]. Chinese Journal of Tissue Engineering Research 2016;20(4):539‐43. [Google Scholar]
Zhou 2015 {published data only}
- Zhou W. Percutaneous vertebroplasty with high‐viscosity bone cement for treatment of severe osteoporotic thoracolumbar vertebral compression fractures [Chinese]. Chinese Journal of Tissue Engineering Research 2015;19(46):7534‐8. [Google Scholar]
References to ongoing studies
Longo 2010 {unpublished data only}
- Longo UG, Loppini M, Denaro L, Brandi ML, Maffulli N, Denaro V. The effectiveness and safety of vertebroplasty for osteoporotic vertebral compression fractures. A double blind, prospective, randomized, controlled study. Clinical Cases in Mineral and Bone Metabolism : the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases 2010;7(2):109‐13. [PUBMED: 22460014] [PMC free article] [PubMed] [Google Scholar]
Sun 2012 {unpublished data only}
- NCT01677806. Investigational percutaneous vertebroplasty efficacy and safety trial. clinicaltrials.gov/show/NCT01677806 (first received 23 August 2012).
VERTOS V {unpublished data only}
- NCT01963039. A trial of vertebroplasty for painful chronic osteoporotic vertebral fractures (VERTOS V). clinicaltrials.gov/show/NCT01963039 First registered 28 August 2013.
Additional references
Al‐Nakshabandi 2011
- Al‐Nakshabandi NA. Percutaneous vertebroplasty complications. Annals of Saudi Medicine 2011;31(3):294‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amin 2014
- Amin S. Achenbach SJ. Atkinson EJ. Khosla S. Melton LJ 3rd. Trends in fracture incidence: a population‐based study over 20 years. Journal of Bone and Mineral Research 2014;29(3):581‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2013
- Anderson PA, Froyshteter AB, Tontz WL Jr. Meta‐analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. Journal of Bone and Mineral Research 2013;28(2):378‐32. [DOI] [PubMed] [Google Scholar]
Baumann 2006
- Baumann A, Tauss J, Baumann G, Tomka M, Hessinger M, Tiesenhausen K. Cement embolization into the vena cava and pulmonal arteries after vertebroplasty: interdisciplinary management. European Journal of Vascular and Endovascular Surgery 2006;31(5):558‐61. [DOI] [PubMed] [Google Scholar]
Belkoff 2001
- Belkoff S, Mathis JM, Jasper LE, Deramond H. The biomechanics of vertebroplasty: The effect of cement volume on mechanical behavior. Spine 2001;26(14):1537‐41. [DOI] [PubMed] [Google Scholar]
Bijur 2003
- Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Academic Emergency Medicine 2003;10:390‐2. [DOI] [PubMed] [Google Scholar]
Birnbaumer 2003
- Birnbaumer DM. Visual Analog Scale vs. Numerical Rating Scale: Much Ado About Nothing?. NEJM Journal Watch. Available from: https://www.jwatch.org/em200305140000005/2003/05/14/visual‐analog‐scale‐vs‐numerical‐rating‐scale 2013.
Bliemel 2012
- Bliemel C, Oberkircher L, Buecking B, Timmesfeld N, Ruchholtz S, Krueger A. Higher incidence of new vertebral fractures following percutaneous vertebroplasty and kyphoplasty‐‐fact or fiction?. Acta Orthopaedica Belgica 2012;78(2):220‐9. [PubMed] [Google Scholar]
Bono 2010
- Bono C, Heggeness M, Mick C, Resnick D, Watters, WC 3rd. North American SpineSociety: newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine Journal 2010;10(3):238‐40. [DOI] [PubMed] [Google Scholar]
Caynak 2009
- Caynak B, Onan B, Sagbas E, Duran C, Akpinar B. Cardiac tamponade and pulmonary embolism as a complication of percutaneous vertebroplasty. Annals of Thoracic Surgery 2009;87(1):299‐301. [DOI] [PubMed] [Google Scholar]
Chen 2013
- Chen AT, Cohen DB, Skolasky RL. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the Medicare population. Journal of Bone & Joint Surgery. American volume 2013;95(19):1729‐36. [DOI] [PubMed] [Google Scholar]
Chosa 2011
- Chosa K, Naito A, Awai K. Newly developed compression fractures after percutaneous vertebroplasty: comparison with conservative treatment. Japanese Journal of Radiology 2011;29(5):335‐41. [DOI] [PubMed] [Google Scholar]
Comstock 2013
- Comstock BA, Sitlani CM, Jarvik JG, Heagerty PJ, Turner JA, Kallmes DF. Investigational vertebroplasty safety and efficacy trial (INVEST): patient‐reported outcomes through 1 year. Radiology 2‐13;269:224‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Damaskinos 2015
- Damaskinos M. Study about the effect of preventive adjacent level cement augmentation after osteoporotic vertebral compression fractures. clinicaltrials.gov/show/NCT0248925 (first received 25 June 2015) 2015.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [DOI] [PubMed] [Google Scholar]
Eck 2008
- Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta‐analysis of the literature. Spine Journal 2008;8(3):488‐97. [DOI] [PubMed] [Google Scholar]
Edidin 2011
- Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the Medicare population. Journal of Bone and Mineral Research 2011;26(7):1617‐26. [DOI] [PubMed] [Google Scholar]
François 2003
- François K, Taeymans Y, Poffyn B, Nooten G. Successful management of a large pulmonary cement embolus after percutaneous vertebroplasty: a case report. Spine 2003;28(29):E424‐5. [DOI] [PubMed] [Google Scholar]
Galibert 1987
- Galibert P, Deramond H, Rosat P, Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty [Note preliminaire sur le traitement des angiomes vertebraux par vertebroplastie acrylique percutanee.]. Neuro‐Chirurgie 1987;33(2):166‐8. [PUBMED: 3600949] [PubMed] [Google Scholar]
Genant 1993
- Genant HK, Wu CY, Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. Journal of Bone and Mineral Research : the official journal of the American Society for Bone and Mineral Research 1993;8(9):1137‐48. [PUBMED: 8237484] [DOI] [PubMed] [Google Scholar]
Gill 2007
- Gill JB, Kuper M, Chin PC, Zhang Y, Schutt R Jr. Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician 2007;10(4):583‐90. [PubMed] [Google Scholar]
Gray 2007a
- Gray D, Hollingworth W, Onwudiwe N, Deyo RA, Jarvik JG. Thoracic and lumbar vertebroplasties performed in US Medicare enrollees, 2001‐2005. JAMA 2007;298(15):1760‐2. [DOI] [PubMed] [Google Scholar]
Grotle 2004
- Grotle M, Brox JI, Vøllestad NK. Concurrent comparison of responsiveness in pain and functional status measurements used for patients with low back pain. Spine 2004;29:E492‐E501. [DOI] [PubMed] [Google Scholar]
Han 2011
- Han S, Wan S, Ning L, Tong Y, Zhang J, Fan S. Percutaneous vertebroplasty versus balloon kyphoplasty for treatment of osteoporotic vertebral compression fracture: a meta‐analysis of randomised and non‐randomised controlled trials. International Orthopaedics 2011;35(9):1349‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernlund 2013
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Archives of Osteoporosis 2013;8(1‐2):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hide 2004
- Hide IG, Gangi A. Percutaneous vertebroplasty: history, technique and current perspectives. Clinical Radiology 2004;59(6):461‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003/09/06 2003; Vol. 327, issue 7414:557‐60. [1468‐5833: (Electronic)] [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).
Hochmuth 2006
- Hochmuth K, Proschek D, Schwarz W, Mack M, Kurth AA, Vogl TJ. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. European Radiology 2006;16(5):998‐1004. [PUBMED: 16395532] [DOI] [PubMed] [Google Scholar]
Hulme 2006
- Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine 2006;31(17):1983‐2001. [DOI] [PubMed] [Google Scholar]
Kanis 1999
- Kanis JA, Johnell O. The burden of osteoporosis. Journal of Endocrinological Investment 1999;22:583‐8. [DOI] [PubMed] [Google Scholar]
Kanis 2000
- Kanis JA, Johnell O, Oden A, Sernbo I, Redlund‐Johnell I, Dawson A, et al. Long‐term risk of osteoporotic fracture in Malmo. Osteoporosis International 2000;11(8):669‐774. [DOI] [PubMed] [Google Scholar]
Kim 2014
- Kim SP, Son BS, Lee SK, Kim DH. Cardiac perforation due to intracardiac bone cement after percutaneous vertebroplasty. Journal of Cardiac Surgery 2014;29(4):499‐500. [DOI] [PubMed] [Google Scholar]
Kroon 2014
- Kroon F, Staples MP, Ebeling P, Wark J, Osborne R, Mitchell P, et al. Vertebroplasty for osteoporotic vertebral fractures: Two‐year results from a randomized controlled trial. Journal of Bone and Mineral Research 2014;29(6):1346‐55. [DOI] [PubMed] [Google Scholar]
Lad 2009
- Lad SP, Patil CG, Lad EM, Hayden MG, Boakye M. National trends in vertebral augmentation procedures for the treatment of vertebral compression fractures. Surgical Neurology 2009;71(5):580‐4. [DOI] [PubMed] [Google Scholar]
Lange 2014
- Lange A, Kasperk C, Alvares L, Sauermann S, Braun S. Survival and cost comparison of kyphoplasty and percutaneous vertebroplasty using German claims data. Spine 2014;39(4):318‐26. [DOI] [PubMed] [Google Scholar]
Lau 2008
- Lau E, Ong KL, Kurtz SM, Schmier J, Edidin AA. Mortalityfollowing the diagnosis of a vertebral compression fracture in theMedicare population. Journal of Bone & Joint Surgery. American volume 2008;90(7):1479‐86. [DOI] [PubMed] [Google Scholar]
Leake 2011
- Leake CB, Brinjikji W, Cloft HJ, Kallmes DF. Trends of inpatient spine augmentation: 2001‐2008. AJNR: American Journal of Neuroradiology 2011;32:1464‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009
- Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta‐analysis of complications. Spine 2009;34(11):1228‐32. [DOI] [PubMed] [Google Scholar]
Leslie 2011
- Leslie WD, Sadatsafavi M, Lix LM, Azimaee M, Morin S, Metge CJ, et al. Secular decreases in fracture rates 1986–2006 for Manitoba, Canada: a population‐based analysis. Osteoporosis International 2011;22:2137‐43. [DOI] [PubMed] [Google Scholar]
Lips 1999
- Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, et al. Quality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Osteoporosis International 1999;10:150‐60. [DOI] [PubMed] [Google Scholar]
Liu 2013
- Liu J, Li X, Tang D, Cui X, Li X, Yao M, et al. Comparing pain reduction following vertebroplasty and conservative treatment for osteoporotic vertebral compression fractures: a meta‐analysis of randomized controlled trials. Pain Physician 2013;16(5):455‐64. [PubMed] [Google Scholar]
Ma 2012
- Ma XL, Xing D, Ma JX, Xu WG, Wang J, Chen Y. Balloon kyphoplasty versus percutaneous vertebroplasty in treating osteoporotic vertebral compression fracture: grading the evidence through a systematic review and meta‐analysis. European Spine Journal 2012;21(9):1844‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCullough 2013
- McCullough BJ, Comstock BA, Deyo RA, Kreuter W, Jarvik JG. Major medical outcomes with spinal augmentation vs conservative therapy. JAMA Internal Medicine 2013;173(16):1514‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2011
- McDonald RJ, Achenbach SJ, Atkinson EJ, Gray LA, Cloft HJ, Melton III LJ, et al. Mortality in the vertebroplasty population. AJNR: American Journal of Neuroradiology 2011;32:1818‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGirt 2009
- McGirt MJ, Parker SL, Wolinsky JP, Witham TF, Bydon A, Gokaslan ZL. Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: an evidenced‐based review of the literature. Spine Journal 2011;9(6):501‐8. [PUBMED: 19251485] [DOI] [PubMed] [Google Scholar]
Melton 1997
- Melton LJ 3rd, Thamer M, Ray NF, Chan JK, Chesnut CH 3rd, Einhorn TA, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. Journal of Bone and Mineral Research 1997;12(1):16‐23. [DOI] [PubMed] [Google Scholar]
Monticelli 2005
- Monticelli F, Meyer HJ, Tutsch‐Bauer E. Fatal pulmonary cement embolism following percutaneous vertebroplasty (PVP). Forensic Science International 2005;149(1):35‐8. [DOI] [PubMed] [Google Scholar]
Mundano 2009
- Mudano AS, Bian J, Cope JU, Curtis JR, Gross TP, Allison JJ, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population‐based cohort study. Osteoporosis International 2009;20:819‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakstad 2008
- NCT00635297. Percutaneous vertebroplasty. Study of refracture rates after prophylactic vertebroplasty of adjacent vertebrae. clinicaltrials.gov/show/NCT00635297 (first received 5 March 2008).
Nieuwenhuijse 2012
- NTR3282. Viscosity of PMMA bone cement in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a randomized controlled trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3282 14 Feb 2012.
Oudshoorn 2012
- Oudshoorn C, Hartholt KA, Zillikens MC, Panneman MJ, Velde N, Colin EM, et al. Emergency department visits due to vertebral fractures in the Netherlands, 1986–2008: steep increase in the oldest old, strong association with falls. Injury 2012;43(4):458‐61. [DOI] [PubMed] [Google Scholar]
Papanastassiou 2012
- Papanastassiou ID, Phillips FM, Meirhaeghe J, Berenson JR, Andersson GB, Chung G, et al. Comparing effects of kyphoplasty, vertebroplasty, and non‐surgical management in a systematic review of randomized and non‐randomized controlled studies. European Spine Journal 2012;21(9):1826‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ploeg 2006
- Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. European Spine Journal 2006;15(12):1749‐58. [DOI] [PubMed] [Google Scholar]
Robinson 2012
- Robinson Y, Olerud C. Vertebroplasty and kyphoplasty—a systematic review of cement augmentation techniques for osteoporotic vertebral compression fractures compared to standard medical therapy. Maturitas 2012;72:42‐9. [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seo 2005
- Seo JS, Kim YJ, Choi BW, Kim TH, Choe KO. MDCT of pulmonary embolism after percutaneous vertebroplasty. AJR. American Journal of Roentgenology 2005;184(4):1364‐5. [DOI] [PubMed] [Google Scholar]
Shi 2012
- Shi MM, Cai XZ, Lin T, Wang W, Yan SG. Is there really no benefit of vertebroplasty for osteoporotic vertebral fractures? A meta‐analysis. Clinical Orthopaedics & Related Research 2012;470(10):2785‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staples 2011
- Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Heagarty PJ, et al. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta‐analysis. BMJ 2011;342:d3952.. BMJ 2011;342:d3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stevenson 2014
- Stevenson M, Gomersall T, Lloyd Jones M, Rawdin A, Hernandez M, Dias S, et al. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost‐effectiveness analysis. Health Technology Assessment (Winchester, England) 2014;18(17):1‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ström 2011
- Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Archives of Osteoporosis 2011;6:59‐155. [DOI] [PubMed] [Google Scholar]
Syed 2006
- Syed MI, Jan S, Patel NA, Shaikh A, Marsh RA, Stewart RV. Fatal fat embolism after vertebroplasty: Identification of the high‐risk patient. AJNR: American Journal of Neuroradiology 2006;27:343‐5. [PMC free article] [PubMed] [Google Scholar]
Tang 2011
- Tang H, Zhao J, Hao C. Osteoporotic vertebral compression fractures: surgery versus non‐operative management. Journal of International Medical Research 2011;39(4):1438‐47. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine 2006;31(23):2747‐55. [DOI] [PubMed] [Google Scholar]
Trout 2005
- Trout AT, Kallmes DF, Gray LA, Goodnature BA, Everson SL, Comstock BA, et al. Evaluation of vertebroplasty with a validated outcome measure: the Roland‐Morris Disability Questionnaire. AJNR: American Journal of Neuroradiology 2005;26:2652‐7. [PMC free article] [PubMed] [Google Scholar]
Trout 2006
- Trout AT, Kallmes DF. Does vertebroplasty cause incident vertebral fractures? A review of available data. AJNR: American Journal of Neuroradiology 2006;27(7):1397‐403. [PMC free article] [PubMed] [Google Scholar]
Trout 2006a
- Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR: American Journal of Neuroradiology 2006;27:217‐223. [PMC free article] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE. Comparison ofthe minimally important difference for two health state utility measures: EQ‐5Dand SF‐6D. Quality of Life Research 2005;14(6):1523‐32. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang LJ, Yang HL, Shi YX, Jiang WM, Chen L. Pulmonary cement embolism associated with percutaneous vertebroplasty or kyphoplasty: a systematic review. Orthopaedic Audio‐Synopsis Continuing Medical Education 2012;4(3):182‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xing 2013
- Xing D, Ma JX, Ma XL, Wang J, Xu WG, Chen Y, et al. A meta‐analysis of balloon kyphoplasty compared to percutaneous vertebroplasty for treating osteoporotic vertebral compression fractures. Journal of Clinical Neuroscience 2013;20(6):795‐803. [DOI] [PubMed] [Google Scholar]
Zampini 2010
- Zampini JM, White AP, McGuire KJ. Comparison of 5766 vertebral compression fractures treated with or without kyphoplasty. Clinical Orthopaedics and Related Research 2010;468(7):1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang YZ, Kong LD, Cao JM, Ding WY, Shen Y. Incidence of subsequent vertebral body fractures after vertebroplasty. Journal of Clinical Neuroscience 2014;21(8):1292‐7. [DOI] [PubMed] [Google Scholar]
Zhao 2014
- Zhao J, Liu B. ChiCTR‐TRC‐14004835. A clinical trial of percutaneous vertebroplasty with high viscosity bone cement. 23 June 2014. Trial pending. chictr.org/en/proj/show.aspx?proj=8628.
Zou 2012
- Zou J, Mei X, Zhu X, Shi Q, Yang H. The long‐term incidence of subsequent vertebral body fracture after vertebral augmentation therapy: a systemic review and meta‐analysis. Pain Physician 2012;15(4):E515‐22. [PubMed] [Google Scholar]
Álvarez 2006
- Álvarez L, Alcarez M, Pérez‐Higueras A, Granizo JJ, Miguel I, Rossi RE, et al. Percutaneous vertebroplasty: Functional improvement in patients with osteoporotic compression fractures. Spine 2006;31:1113‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Buchbinder 2015
- Buchbinder R, Golmohammadi K, Johnston RV, Owen RJ, Homik J, Jones A, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD006349.pub2] [DOI] [PubMed] [Google Scholar]
Lambert 2007
- Lambert RGW, Golmohammadi K, Majumdar SR, Jones A, Buchbinder R, Dhillon SS, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006349] [DOI] [PubMed] [Google Scholar]


