Abstract
Modulating inflammation by targeting IL-1β reduces recurrent athero-thrombotic cardiovascular events without lipid lowering. This presents an opportunity to explore other pathways associated with the IL-1β signaling cascade to modulate the inflammatory response post-myocardial infarction (MI). IL-7 is a mediator of the inflammatory pathway involved in monocyte trafficking into atherosclerotic plaques and levels of IL-7 have been shown to be elevated in patients with acute MI. Recurrent athero-thrombotic events are believed to be mediated in part by index MI-induced exacerbation of inflammation in atherosclerotic plaques. The objective of the study was to assess the feasibility of IL-7R blockade to modulate atherosclerotic plaque inflammation following acute MI in ApoE−/- mice. Mice were fed Western diet for 12 weeks and then subjected to coronary occlusion to induce an acute MI. IL-7 expression was determined using qRT-PCR and immuno-staining, and IL-7R was assessed using flow cytometry. Plaque inflammation was evaluated using immunohistochemistry. IL-7R blockade was accomplished with monoclonal antibody to IL-7R. IL-7 mRNA expression was significantly increased in the cardiac tissue of mice subjected to MI but not in controls. IL-7 staining was observed in the coronary artery. Plaque macrophage and lipid content were significantly increased after MI. IL-7R antibody treatment but not control IgG significantly reduced macrophage and lipid content in atherosclerotic plaques. The results show that IL-7R antibody treatment reduces monocyte/macrophage and lipid content in the atherosclerotic plaque following MI suggesting a potential new target to mitigate increased plaque inflammation post-MI.
Keywords: IL-7R, Plaque inflammation, Myocardial infarction
Highlights
-
•
Myocardial infarction increases inflammation in atherosclerotic plaques.
-
•
IL-7 is a mediator of inflammatory cell recruitment and is expressed in the ischemic myocardium.
-
•
Monoclonal antibody blockade of IL-7Rα reduced plaque lipid and macrophage content.
1. Introduction
A second athero-thrombotic event within one year of index event is an important complication of myocardial infarction (MI), the acute manifestation of coronary heart disease [1]. This increased risk of recurrence of MI has been linked to increased inflammation with increased metabolic activity in the atherosclerotic plaque following the initial ischemic insult [2]. Until recently, the accumulated evidence for this inflammatory hypothesis has mostly been pre-clinical in nature yet the evidence supporting a role of increased systemic and atherosclerotic plaque inflammation following myocardial infarction is compelling. The initial ischemic event is associated with a systemic inflammatory reaction [3] with increased metabolic activity in the atherosclerotic plaque observed following the initial ischemic insult [4]. The CANTOS trial using anti-inflammatory therapy with canakinumab, an IL-1β blocking monoclonal antibody, decreased the incidence of recurrent MI [5] representing a pivotal confirmation of the importance of inflammation in atherosclerotic disease following MI. Although anti-inflammatory therapy with canakinumab showed efficacy, discovering new pathways of modulating the post-MI inflammation could provide added benefit in terms of more selective targeting of the inflammation occurring in atherosclerotic plaques following ischemic insult to the myocardium. One such possible downstream mediator is IL-7, which has been shown to be reduced following IL-1β blockade [6]. Plasma levels of IL-7 are elevated in acute coronary syndrome patients and IL-7 enhances the expression of inflammatory chemokines in monocytes and peripheral blood mononuclear cells in these patients [7]. It was suggested to have predictive value for mortality of infarct-related cardiogenic shock patients [8]. IL-7 up-regulated cell adhesion molecules and monocyte chemoattractants in endothelial cells and promoted monocyte adhesion and trafficking of macrophages into aortas of ApoE−/- mice [9]. Blocking of IL-7R on monocytes of rheumatoid arthritis patients decreased in vitro chemotaxis of these cells [10] suggesting the possibility of modulation of monocyte infiltration and inflammation in atherosclerotic plaques. We therefore hypothesized that IL-7 is involved in post-MI atherosclerotic plaque inflammation, and modulation of the IL-7 response would yield beneficial results in terms of plaque inflammation. To this end we chose a combined ApoE−/- mouse model of atherosclerosis and myocardial infarction that has been shown to induce monocyte recruitment and adhesion into atherosclerotic plaques [11]. We characterized the atherosclerotic plaque of these animals and designed an intervention with IL-7R blockade to assess the effect on atherosclerotic plaque inflammation.
2. Materials and methods
2.1. Animals
The experimental protocols comply with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and were approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center. Male ApoE−/- mice on a C57BL/6 background were purchased from Jackson Laboratory. All mice were housed in a fully accredited animal facility, kept on a 12-h day/night cycle, and had unrestricted access to water and food. The mice were continuously fed a high fat diet [0.15% cholesterol, 21% fat (TD.88137, Envigo)] starting at 7 weeks of age until euthanasia at 19 weeks of age.
2.2. Myocardial infarction
At 13 weeks of age, the mice were anesthetized by a cocktail of Ketamine and Dexmedetomidine injected intra-peritoneally and subcutaneous NSAID for analgesia. Endotracheal intubation was performed by fiber optic intubation and mice were placed on mechanical ventilation for the duration of the surgery. MI surgery was performed by permanent ligation of the left descending coronary artery [12], confirmed by blanching of the myocardium. Sham procedure and non-surgical control mice were used as controls. Sham procedure followed the protocol for myocardial infarction surgery without pericardial manipulation or cardiac suture placement. Anesthesia reversal was accomplished with Atipamezol. Buprenorphine was administered immediately after surgical procedure and 12 h later for post-surgery analgesia. Group assignment (MI, Sham procedure, no procedure) was random. Echocardiography was performed using a VEVO 770 ultrasound apparatus. Ejection fraction was measured for evaluation of myocardial contractility 24 h before MI procedure and 1 day prior to euthanasia. Total number of animals used per group: Control N = 8; Sham N = 8; MI N = 16. Half of each group was used for a 1-week post-MI study and the other half for a 6-week post-MI study. The MI procedure resulted in approximately 35% attrition rate.
2.3. Tissue harvesting and preparation
At euthanasia the mouse hearts were harvested and embedded in OCT compound (Tissue-Tek) for cryo-sectioning. Spleens were harvested, underwent mechanical dissociation and processing with red blood cell lysis buffer (RBC Lysis Buffer 10x, eBioscience). Some tissue samples were flash-frozen for mRNA analysis.
2.4. IL-7 mRNA
Bone marrow, lymph nodes and spleen were collected and flash-frozen for RNA extraction. The mouse heart was cut transversely below the suture line to include ischemic myocardium as well as the rest of viable myocardium of the right ventricle. The overlying pericardium with pericardial fat was removed to avoid confounding from the immunologically active pericardial adipocytes. The same was performed for the non-surgical and sham control hearts. The tissue samples then underwent mechanical dissociation in TRIzol for RNA extraction. Q RT-PCR was performed using IL-7 primers (forward primer -TCTGCTGCCTGTCACATCATC, reverse primer -GGACATTGAATTCTTCACTGATATTCA) with GAPDH as reference gene. Results are expressed as fold-change relative to control samples as calibrator using the ΔΔCt method.
2.5. Immunohistochemical and lipid staining
Ten micrometer sections from aortic sinus were stained with Oil-red-o, MOMA-2 antibody (BioRad), CD3, and Ly6G (Biolegend) antibodies to identify lipids, macrophages, T cells, and neutrophils, respectively using standard immunohistochemical protocol [13]. Ig isotype was used as control. Three sections from each heart in 0.03 mm intervals were used for each stain. Secondary antibody conjugated to HRP with AEC as color substrate was used for detection and sections were counter-stained with hematoxylin. Computer assisted morphometric analysis was performed using ImagePro software (Media Cybernetics) averaging the measurements from 3 sections per mouse. The person performing the computer-assisted analysis was blinded toward mouse treatment groups.
IL-7 protein expression was assessed using an IL-7 monoclonal antibody (Boster Bio) in 2 slides from each heart at 0.1 mm intervals with 2 sections of 0.01 mm thickness per slide. Sections were collected from heart tissue distal from the aortic sinus, proximal to the ischemic myocardium. Secondary antibody, color detection, and analysis were same as above.
2.6. Flow cytometry
Following RBC lysis, the cells were washed in PBS and stained for viability (LIVE/DEAD Fixable Violet stain) and surface markers listed in the Supplemental Material. Flow cytometry was performed using a BD LSR Fortessa apparatus. General gating for analysis excluded cell doublets and non-viable cells.
2.7. Serum cholesterol
Cholesterol levels were measured using a commercially available kit according to manufacturer's instruction (Wako).
2.8. IL-7 receptor blocking
Blocking of IL-7R was performed following the mouse surgical and diet protocol described above. The treatment included intra-peritoneal twice-a-week injections of IL-7Rα blocking antibody (BioXCell: A7R34 monoclonal antibody) [14] with a loading dose of 500 μg of IL-7R mAb the first week and followed with twice-a-week dose of 125 μg IL-7R mAb until euthanasia 6 weeks post-MI [14,15]. The dose of IL-7R mAb was chosen to block peripheral effects on IL-7 as opposed to large central immune inhibition of IL-7 pathways. Controls received rat IgG purified from normal rat serum (EMD Millipore) in the same dose regimen.
2.9. In vivo monocyte recruitment assay
To assess the role of IL-7 in monocyte recruitment in vivo, ApoE−/- mice were fed high fat diet for 6 weeks and implanted in the flanks with Matrigel solution (Fisher Scientific) containing IL-7 (15μg/ml) or Matrigel with PBS as control [16]. Mice were euthanized after 3 days and the Matrigel plugs were collected for recovery of inflammatory cell infiltrates using Cell Recovery Solution (Thermo Fisher). The recovered cells were stained with fluorescent antibodies for flow cytometric analysis.
2.10. Statistics
All data are reported as mean ± SD. Normality test was performed for data sets and analyzed using ANOVA followed by Tukey test for group comparisons, unless indicated otherwise. A p value < 0.05 was considered significant.
3. Results
3.1. Myocardial infarction
Echocardiographic assessment showed significantly increased LVIDs after MI (Supplemental Fig. 1A), with significantly reduced ejection fraction and fractional shortening following MI procedure (Supplemental Fig. 1B).
3.2. IL-7 mRNA expression is up-regulated locally in the cardiac tissue of MI mice
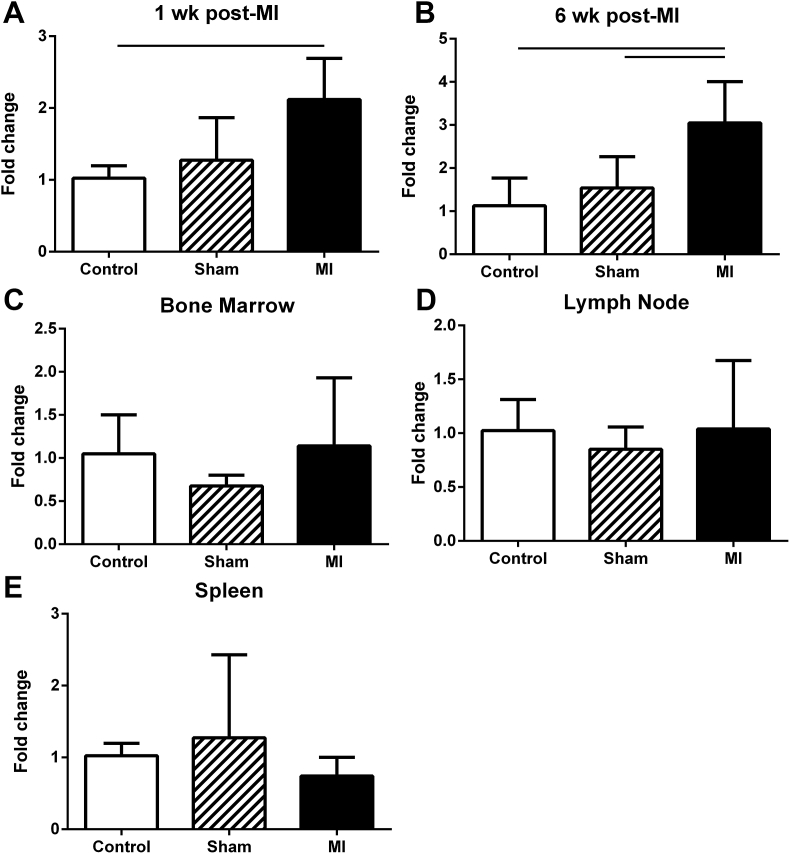
IL-7 mRNA expression was analyzed in the local cardiac tissue of the heart apex. There was a significant increase in the MI mouse group compared to non-surgical control 1 week post-MI (Fig. 1A) and the increased expression persisted up to 6 weeks post-MI compared to controls (Fig. 1B), suggesting IL-7 production is up-regulated locally in the myocardium following ischemic injury. IL-7 mRNA expression in lymphoid tissue and bone marrow did not differ among the three groups of mice (Fig. 1C–E).
Fig. 1.
IL-7 mRNA expression. IL-7 mRNA was assessed in myocardial tissue 1 week (A) and 6 weeks (B) after MI surgery. Bone marrow (C), lymph node (D), and spleen (E) were also assessed for IL-7 mRNA expression 1 week after MI. Bar over columns indicate statistical significance. N = 4–5 each group. (A) p = 0.02; (B) Control vs MI p = 0.015, Sham vs MI p = 0.04.
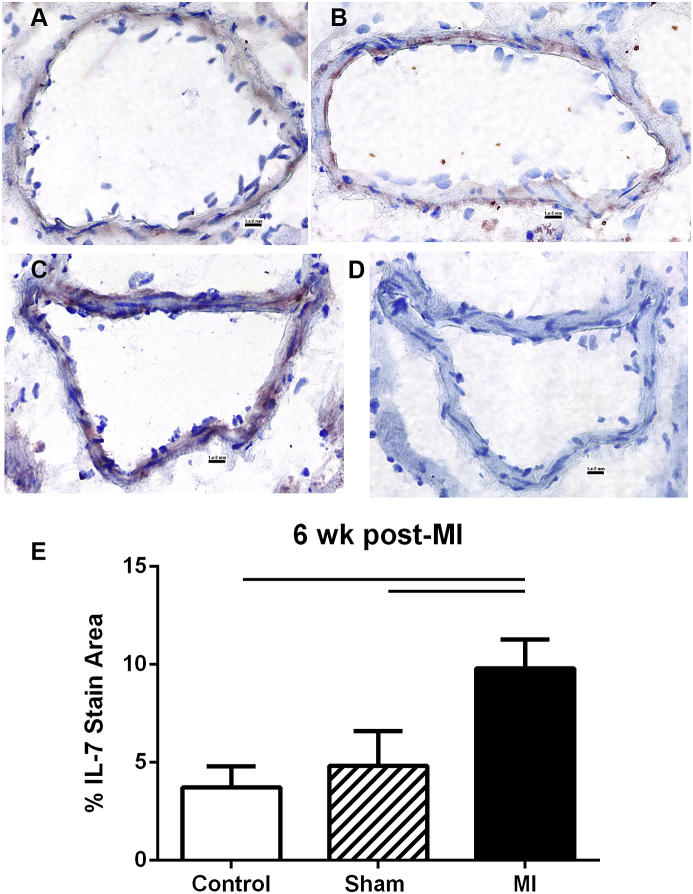
Immunostaining of cardiac tissue 6 weeks post-MI for IL-7 showed weak staining in the coronary artery in non-surgical control and sham (Fig. 2A and B), but focal staining distal to the infarct suture in MI group (Fig. 2C). Measurement of the stained area showed significantly increased IL-7 staining in the MI group compared to the controls (Fig. 2E).
Fig. 2.
IL-7 stain in coronary artery. Representative photos of IL-7 immuno-staining (reddish-brown) in the coronary arteries of control (A, N = 3), Sham (B, N = 3), and MI (C, N = 4) mice. Negative staining control (D) omitted the primary antibody. IL-7 stain area was measured and plotted (E). Scale bar = 0.01 mm. Bar over columns indicate statistical significance. Control vs MI p = 0.003; Sham vs MI p < 0.007.
3.3. Expression levels of IL-7R on monocytes are comparable among experimental groups
There were comparable percentages of IL-7R + splenic monocytes and comparable mean fluorescent intensity of IL-7R stained monocytes among the experimental groups 1 week after MI and 6 weeks after MI (Supplemental Fig. 2). Similar results were observed in splenic T cells (Supplemental Fig. 3). This suggested that IL-7R is present on a subgroup of monocytes and T cells and expression was not changed by the experimental conditions.
3.4. Increased inflammation in aortic sinus of MI mice
The lipid content of atherosclerotic plaques was significantly increased in the MI group (23.03 ± 8.36%; N = 5) compared to non-surgical control (8.62 ± 4.98%; N = 4) or sham control (9.67 ± 1.2%, N = 4), indicating increased lipid deposition following ischemic injury (Supplemental Figs. 4A–D). This was paralleled by significantly increased monocyte/macrophage (MOMA) staining in the aortic sinus plaque of MI mice (4.64 ± 1.82%) compared to non-surgical (0.68 ± 0.62%) or sham (1.79 ± 0.96%) controls (Supplemental Figs. 4E–H). The same was evident for CD3+ T cell content (21.62 ± 8.72 vs 6.31 ± 2.37 and 6.13 ± 2.03, respectively; p = 0.003, ANOVA) in the sinus plaque of MI mice compared to both non-surgical control and sham mice (Supplemental Fig. 4I-L). No differences were noted in the plaque neutrophil content (Supplemental Fig. 4M − P). Cholesterol levels were comparable among all experimental groups (MI: 1396 ± 399 mg/dL; non-surgical control: 1141 ± 411 mg/dL; sham control: 1290 ± 275 mg/dL).
3.5. IL-7 receptor blocking decreased aortic sinus plaque lipid and macrophage content
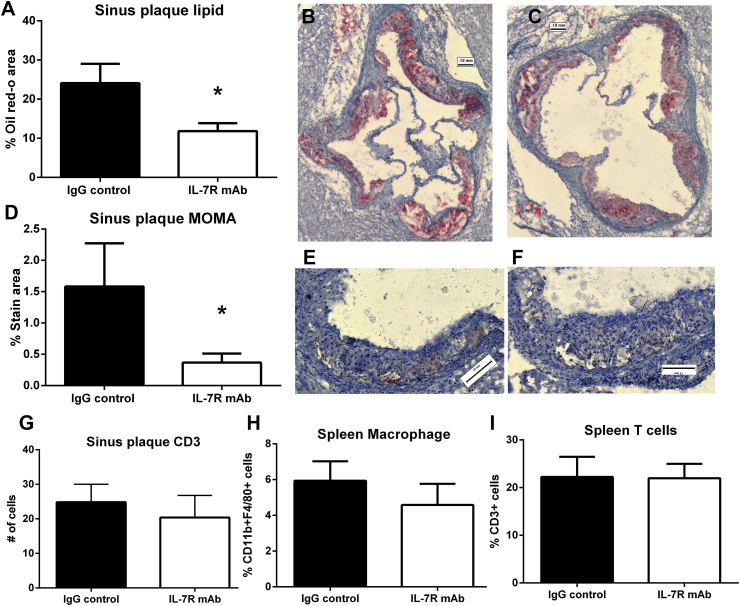
The results showed that MI increased IL-7 mRNA expression in cardiac tissue with concomitant increase in inflammation of aortic sinus plaques without significant alteration in IL-7R expression by monocytes and T cells. Thus, we postulated that targeting IL-7R using a monoclonal antibody to IL-7Rα would be a viable approach to alter IL-7 mediated inflammation. Compared to IgG antibody treated mice, anti-IL-7R mAb treated mice displayed significant reduction in lipid (24.08 ± 4.93% vs. 11.81 ± 2.02%, respectively; p = 0.004; N = 4 each; Fig. 3A–C) and macrophage content (1.58 ± 0.69% vs. 0.36 ± 0.14% respectively; p = 0.014; Fig. 3D–F). CD3+ T cell content in the atherosclerotic plaque of the aortic sinus was not affected by anti-IL-7R mAb blocking compared to IgG control (Fig. 3G). Serum cholesterol levels in IL-7R blocking or IgG control antibody injections were not significantly different (1183 ± 429 mg/dL IL-7R mAb vs 892 ± 173 mg/dL IgG control). Splenic macrophage and T cell populations were not affected by IL-7R mAb compared to IgG control (Fig. 3H and I).
Fig. 3.
Monoclonal antibody blocking of IL-7R post-MI. Aortic sinus plaque lipid (A), macrophage (D), or T cells stained area (G) in mice subjected to MI and injected with IgG control (B and E) or IL-7R mAb (C and F). Scale bar = 0.1 mm (A) *p = 0.004; (B) *p = 0.014 t-test; N = 4 each. Splenocytes were analyzed using fluorescent staining and flow cytometry for percentage of macrophage (H) or T cells (I). Gating for CD11b + F4/80 + macrophages as shown in Supplemental Fig. 2A. Gating for T cells as shown in Supplemental Fig. 3A.
3.6. IL-7 increased recruitment of monocytes in vivo
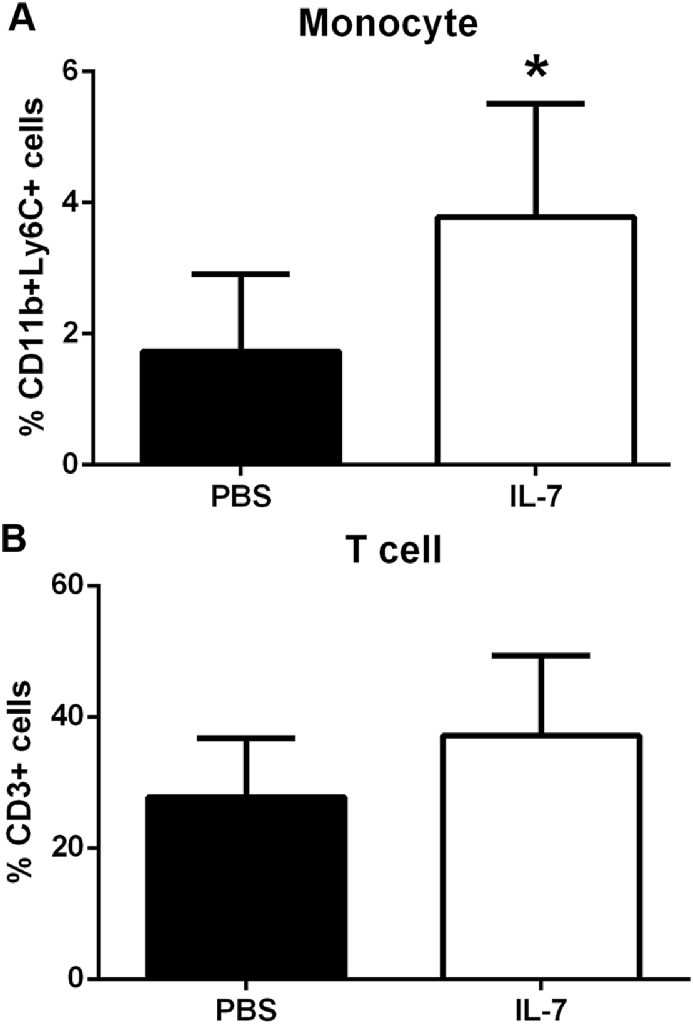
To assess the role of IL-7 in recruiting monocytes in vivo, Matrigel solution containing IL-7 was implanted into the flanks of mice fed a high fat diet. Three days after implantation, flow cytometric analysis of the digested IL-7 Matrigel plug showed increased monocyte infiltration compared to PBS Matrigel (Fig. 4A and B; N = 6 each; p = 0.04).
Fig. 4.
Increased monocyte recruitment in vivo by IL-7. Matrigel plugs containing 15μg/ml IL-7 or PBS injected into flanks of mice fed high fat diet were collected after 3 days and subjected to digestion for cell recovery. The recovered cells were analyzed for fluorescent staining and flow cytometry. Gating for monocytes as depicted in Supplemental Fig. 2A. Gating for T cells as depicted in Supplemental Fig. 3A. Results are expressed as mean percentage of total cell infiltrates of each Matrigel plug. *p = 0.04 t-test; N = 6 each.
4. Discussion
Myocardial infarction is associated with increased levels of inflammatory cytokines as a result of coronary occlusion and myocardial necrosis [2]. Residual inflammation in spite of optimal medical therapy highlights the importance of the inflammation in post-MI atherosclerotic plaque dynamics. As a targeted aproach to modulation of the inflammatory response post-MI, the IL-7 pathway is interesting for several reasons. It is associated with the IL-1β pathway [6] which has been shown to be important in the context of post-MI plaque inflammation. IL-7 levels are increased in patients with acute coronary syndrome [7] suggesting potential involvement of the pathway in plaque inflammation in this disease setting. IL-7 works as a chemoattractant for monocytes and causes increased pro-inflammatory cytokine and adhesion molecule production [9,17]. We determined the expression of IL-7 mRNA in multiple mouse tissues following MI. Interestingly, IL-7 mRNA was only increased locally in the myocardium of the MI mice, but not in either of the controls. This hints at its localized effect in the heart. Immunostaining showed IL-7 expression in the coronary arteries of the MI mice. A limitation of this method of analysis is that the exact cellular source of IL-7 remains uncharacterized. Endothelial cells present in the myocardium are able to express IL-7, mediated in part by IL-1β signaling [6]. We also assessed the monocyte population in the spleens of the experimental mice to determine the presence of IL-7R on their surface. Flow cytometric analysis revealed that the fraction of monocytes expressing the IL-7R and the expression level as shown by mean fluorescent intensity was not significantly different among the experimental groups. This is consistent with previous reports on IL-7R expression on monocytes [10].
Both in animal models and in humans, inflammatory monocyte subsets have been shown to be present in the atherosclerotic plaque and are associated with coronary plaque vulnerability in coronary artery disease patients with well regulated lipid levels [18]. Furthermore, inflammatory monocytes are an independent predictor of cardiovascular events in patients with atherosclerosis [19]. Our study showed, consistent with previously published findings [11], that the monocyte/macrophage fraction in the aortic sinus plaques was significantly increased following myocardial infarction thus presenting a good target for immune modulation. This increase in monocyte/macrophage infiltration in the plaque was paralleled by increased T cell infiltration and lipid deposition indicating increased immune inflammatory activity in the atherosclerotic plaque following MI. A possible mechanism for this are increased leukocyte endothelial adhesive interactions following acute MI leading to increased plaque inflammation [20]. This is observed indirectly in human atherosclerotic lesions following an ischemic cardiac event showing increased metabolism post-MI as determined by PET-CT scans and FDG labeling as discussed above [4].
We next investigated the involvement of IL-7 in the inflammatory cascade following ischemic injury to the myocardium. We tested the hypothesis that blocking IL-7R on circulating leukocytes would result in decreased inflammatory activity in the atherosclerotic plaque. In our experiments, IL-7R blocking had no effect on splenic monocyte, macrophage, and T cell populations. The lack of systemic effect of IL-7R mAb injections on immune cell populations is consistent with a previous report that showed an age-dependent effect of IL-7R blockade on lymphocytes [21]. In the report, IL-7R antibody treatment reduced splenic lymphocytes in young mice but not in middle-aged mice. In our study, although the intervention did not change the splenic inflammatory cell populations, the atherosclerotic plaque of these animals displayed a significant reduction in monocyte/macrophage cell infiltration and in lipid content, both of which have been implicated in the formation of vulnerable unstable plaques and are independent predictive factors for cardiovascular events [22]. The reduced macrophage infiltration may be mediated by the blocking of the chemotactic property of IL-7 [9]. The reduced lipid content by IL-7R antibody treatment may be consequential to reduced inflammation, or to altered lipid metabolism after IL-7R blockade, as reported in IL-7R deficient mice [23]. However, our results show that the effects were local, not systemic. The involvement of IL-7 in monocyte recruitment is supported by the increased monocyte presence in the Matrigel plugs containing IL-7.
In conclusion, our report identifies the IL-7 pathway as a potential target of intervention to mitigate the inflammatory cascade increased in atherosclerotic plaques during the post-MI response.
Funding
Funding was provided by The Eleanor and Harold Foonberg Chair in Cardiac Intensive Care Fund; The Heart Foundation; The Petersen Foundation; Steinberg Foundation; Annenberg Foundation; Spielberg Fund.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100647.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kikkert W.J., Hoebers L.P., Damman P., Lieve K.V., Claessen B.E., Vis M.M., Baan J., Jr., Koch K.T., de Winter R.J., Piek J.J., Tijssen J.G., Henriques J.P. Recurrent myocardial infarction after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am. J. Cardiol. 2014;113(2):229–235. doi: 10.1016/j.amjcard.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Fang L., Moore X.L., Dart A.M., Wang L.M. Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. 2015;12(3):305–312. doi: 10.11909/j.issn.1671-5411.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remskar M., Horvat M., Hojker S., Noc M. Procalcitonin in patients with acute myocardial infarction Wien. Klin. Wochenschr. 2002;114(5–6):205–210. [PubMed] [Google Scholar]
- 4.Joshi N.V., Toor I., Shah A.S., Carruthers K., Vesey A.T., Alam S.R., Sills A., Hoo T.Y., Melville A.J., Langlands S.P., Jenkins W.S., Uren N.G., Mills N.L., Fletcher A.M., van Beek E.J., Rudd J.H., Fox K.A., Dweck M.R., Newby D.E. Systemic atherosclerotic inflammation following acute myocardial infarction: myocardial infarction begets myocardial infarction. J. Am. Heart Assoc. 2015;4(9):e001956. doi: 10.1161/JAHA.115.001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., Kastelein J.J.P., Cornel J.H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida-Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P.R.F., Troquay R.P.T., Libby P., Glynn R.J. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar V., Yin J., Mirza A.M., Phan D., Vanegas S., Issafras H., Michelson K., Hunter J.J., Kantak S.S. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216(2):313–320. doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Damas J.K., Waehre T., Yndestad A., Otterdal K., Hognestad A., Solum N.O., Gullestad L., Froland S.S., Aukrust P. Interleukin-7-mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003;107(21):2670–2676. doi: 10.1161/01.CIR.0000070542.18001.87. [DOI] [PubMed] [Google Scholar]
- 8.Prondzinsky R., Unverzagt S., Lemm H., Wegener N.A., Schlitt A., Heinroth K.M., Dietz S., Buerke U., Kellner P., Loppnow H., Fiedler M.G., Thiery J., Werdan K., Buerke M. Interleukin-6, -7, -8 and -10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin. Res. Cardiol. 2012;101(5):375–384. doi: 10.1007/s00392-011-0403-3. [DOI] [PubMed] [Google Scholar]
- 9.Li R., Paul A., Ko K.W., Sheldon M., Rich B.E., Terashima T., Dieker C., Cormier S., Li L., Nour E.A., Chan L., Oka K. Interleukin-7 induces recruitment of monocytes/macrophages to endothelium. Eur. Heart J. 2012;33(24):3114–3123. doi: 10.1093/eurheartj/ehr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Kim S.J., Chamberlain N.D., Pickens S.R., Volin M.V., Volkov S., Arami S., Christman J.W., Prabhakar B.S., Swedler W., Mehta A., Sweiss N., Shahrara S. The novel role of IL-7 ligation to IL-7 receptor in myeloid cells of rheumatoid arthritis and collagen-induced arthritis. J. Immunol. 2013;190(10):5256–5266. doi: 10.4049/jimmunol.1201675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta P., Courties G., Wei Y., Leuschner F., Gorbatov R., Robbins C.S., Iwamoto Y., Thompson B., Carlson A.L., Heidt T., Majmudar M.D., Lasitschka F., Etzrodt M., Waterman P., Waring M.T., Chicoine A.T., van der Laan A.M., Niessen H.W., Piek J.J., Rubin B.B., Butany J., Stone J.R., Katus H.A., Murphy S.A., Morrow D.A., Sabatine M.S., Vinegoni C., Moskowitz M.A., Pittet M.J., Libby P., Lin C.P., Swirski F.K., Weissleder R., Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihailovic P.M., Lio W.M., Herscovici R., Chyu K.Y., Yano J., Zhao X., Zhou J., Zhou B., Freeman M.R., Yang W., Shah P.K., Cercek B., Dimayuga P.C. Keratin 8 is a potential self-antigen in the coronary artery disease immunopeptidome: a translational approach. PLoS One. 2019;14(2):e0213025. doi: 10.1371/journal.pone.0213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihailovic P.M., Lio W.M., Yano J., Zhao X., Zhou J., Chyu K.Y., Shah P.K., Cercek B., Dimayuga P.C. The cathelicidin protein CRAMP is a potential atherosclerosis self-antigen in ApoE(-/-) mice. PLoS One. 2017;12(11):e0187432. doi: 10.1371/journal.pone.0187432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratz I.K., Truong H.A., Yang S.H., Maurano M.M., Lee K., Abbas A.K., Rosenblum M.D. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 2013;190(9):4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis C.R., Seamons A., Maxwell J., Treuting P.M., Nelson L., Chen G., Phelps S., Smith C.L., Brabb T., Iritani B.M., Maggio-Price L. Interleukin-7 receptor blockade suppresses adaptive and innate inflammatory responses in experimental colitis. J. Inflamm. 2012;9(1):39. doi: 10.1186/1476-9255-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelbertsen D., Rattik S., Knutsson A., Bjorkbacka H., Bengtsson E., Nilsson J. Induction of T Helper 2 Responses against Human Apolipoprotein B100 Does Not Affect Atherosclerosis in ApoE-/- Mice. Cardiovasc. Res. 2014;103:304–312. doi: 10.1093/cvr/cvu131. 2. [DOI] [PubMed] [Google Scholar]
- 17.Alderson M.R., Tough T.W., Ziegler S.F., Grabstein K.H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J. Exp. Med. 1991;173(4):923–930. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto H., Yoshida N., Shinke T., Otake H., Kuroda M., Sakaguchi K., Hirota Y., Toba T., Takahashi H., Terashita D., Uzu K., Tahara N., Shinkura Y., Kuroda K., Nagasawa Y., Nagano Y., Tsukiyama Y., Yanaka K.I., Emoto T., Sasaki N., Yamashita T., Ogawa W., Hirata K.I. Impact of CD14(++)CD16(+) Monocytes on Coronary Plaque Vulnerability Assessed by Optical Coherence Tomography in Coronary Artery Disease Patients. Atherosclerosis. 2018;269:245–251. doi: 10.1016/j.atherosclerosis.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Rogacev K.S., Cremers B., Zawada A.M., Seiler S., Binder N., Ege P., Grosse-Dunker G., Heisel I., Hornof F., Jeken J., Rebling N.M., Ulrich C., Scheller B., Bohm M., Fliser D., Heine G.H. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012;60(16):1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Wright A.P., Ohman M.K., Hayasaki T., Luo W., Russo H.M., Guo C., Eitzman D.T. Atherosclerosis and Leukocyte-Endothelial Adhesive Interactions Are Increased Following Acute Myocardial Infarction in Apolipoprotein E Deficient Mice. Atherosclerosis. 2010;212:414–417. doi: 10.1016/j.atherosclerosis.2010.06.022. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chougnet C.A., Tripathi P., Lages C.S., Raynor J., Sholl A., Fink P., Plas D.R., Hildeman D.A. A major role for Bim in regulatory T cell homeostasis. J. Immunol. 2011;186(1):156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virmani R., Burke A.P., Farb A., Kolodgie F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006;47(8 Suppl):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 23.Lee M., Song S.J., Choi M.S., Yu R., Park T. IL-7 Receptor Deletion Ameliorates Diet-Induced Obesity and Insulin Resistance in Mice. Diabetologia. 2015;58:2361–2370. doi: 10.1007/s00125-015-3684-7. 10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.