Abstract
Acute lower respiratory tract infections (ALRTI) such as pneumonia and bronchiolitis are major causes of mortality and morbidity in children under 5 years of age. The main microbial agents responsible for ALRTI are either bacterial agents (Streptococcus pneumoniae, Haemophilus influenzae type b, Mycoplasma pneumoniae) or viruses (respiratory syncytial virus (RSV, also known as human orthopneumovirus), Myxovirus influenzae, Myxovirus parainfluenzae, adenovirus) [1]. More recently, other viruses (rhinovirus, metapneumovirus, coronavirus, bocavirus) have been implicated in ALRTI; their identification has been facilitated by new molecular biology techniques such as real-time PCR. To our knowledge, these emerging viruses have never been the subject of epidemiologic studies in our country.
Keywords: Algeria, ALRT, children, hMPv, PCR, RSV
Résumé
Les infections aiguës des voies respiratoires comptent parmi les principales causes de morbidité et de mortalité chez les enfants. Elles se produisent dans le monde entier et constituent l'un des principaux fardeaux mondiaux des maladies chez les enfants. Le but de cette étude était de déterminer l'étiologie virale des infections respiratoires chez les enfants hospitalisés et âgés de moins de 02 ans.
Sur une période d'un an, des aspirations nasales été effectuées chez 117 enfants âgés de 15 jours à 02 ans et hospitalisés pour une maladie respiratoire aiguë. Les échantillons prélevés ont été testés pour la présence de 12 virus respiratoires à l'aide d'une RT-PCR multiplex en temps réel. Des agents pathogènes ont été identifiés chez 97 enfants (82,9 %) et ont été fréquemment observés à l'automne et en hiver. Une co-infection a été observée dans 21,4 % des échantillons. Les pathogènes les plus fréquemment détectés étaient le Virus respiratoire syncytial (VRS, 47,9 %), le rhinovirus humain (hRV, 23,1 %), le métapneumovirus humain (hMPV, 22,2 %). Cette étude fournit des faits pertinents sur la circulation des virus respiratoires en Algérie et sur l'importance de l'utilisation de la PCR multiplexe comme outil intéressant pour la détection des virus. Un diagnostic précoce au moment de l'hospitalisation initiale peut réduire la propagation des virus dans les services de pédiatrie et améliorer la prise en charge.
Mots clés: Algérie, Infections aiguës des voies respiratoires, Co-infections virales, Diagnostic de laboratoire, Tests moléculaires, Virus respiratoires
Introduction
In 2008, 1.8 million children under 5 years of age died from ALRTI, accounting for 17% of all deaths among children in this age group. The majority (98%) of these preventable deaths occurred in developing countries [1]. Pneumonia dominates the causes of death of children under 5 years of age [2]. Acute respiratory infections are also associated with high morbidity. The incidence is estimated at 0.29 cases per child per year in developing countries, or about 151 million new ALRTI cases each year in developing countries. Among these ALRTI, acute viral bronchiolitis remains a major public health problem, affecting millions of children every year. Many viruses are involved and responsible for clinical pictures of variable severity. Knowledge of the epidemiology of viral respiratory infections is becoming increasingly clear because previously unidentified new viruses are being isolated.
In Algeria, lower respiratory tract infections are also an important cause of morbidity and are responsible for many emergency room visits and hospitalizations. They account for 25% of hospitalizations of children younger than 5 years. In 2009, there were 61536 hospitalizations for ALRTI out of a total of 252028 hospitalizations for all causes. ALRTI are the leading causes of mortality of children under 5 years: 11% of <5 years deaths (Table 1) amount to 1188 deaths by ALRTI of a total of 10684 deaths from all causes in 2009 [3]. The majority of these deaths are due to pneumonia [4].
Table 1.
Positivity rate by age
| Age (months) | % |
|---|---|
| 0–2 | 84.0 |
| 3–5 | 78.8 |
| 6–8 | 86.7 |
| 9–11 | 85.7 |
| 12–17 | 80.0 |
| 18–24 | 100.0 |
| Unknown | 65.1 |
| Total | 82.9 |
The objectives of this study were to identify respiratory viruses circulating in infants hospitalized with ALRTI; and to describe the epidemiologic and clinical characteristics associated with each virus.
Patients and Methods
We performed an exhaustive descriptive prospective cross-sectional study of children aged 0 and 24 months admitted with ALRTI to the paediatric department of the Blida University Hospital. The study was carried out between 21 December 2010 and 1 April 2011. The province of Blida is located 50 km west of Algiers; its population is estimated at 1085000 inhabitants, including 140000 children younger than 5 years, living in rural or urban areas.
The study population was composed of children aged 15 days to 24 months with an ALRTI that required hospitalization in the pzaediatric department. Infants younger than 15 days old and children older than 2 years were excluded from the study. Acuteness of infection was defined as the presence of respiratory symptoms for less than 2 weeks. ALRTI is diagnosed in the presence of fever or hypothermia associated with at least one of the following symptoms: polypnoea, wheezing, abnormalities in pulmonary listening or friction. Hospitalization criteria are based on the presence of at least one of the following signs: rapid breathing (>60 breaths per minute), chest recession, cyanosis, disturbance of consciousness and eating disorders. Respiratory distress was considered as mild, moderate or severe depending on the intensity of the following parameters: respiratory rate, labored breathing, cyanosis, wheezing or crackles. Associated risk factors (very young age, prematurity) or comorbidity (heart disease, neuromuscular disease, severe malnutrition) may have constituted hospitalization criteria.
We sought to identify the viruses responsible for ALRTI. All children hospitalized during this period were systematically sampled by nasal or nasopharyngeal aspiration for the following respiratory viruses: influenza virus, RSV, coronavirus, adenovirus, rhinovirus, metapneumovirus and parainfluenza viruses 1, 2 and 3. The sample was taken at the time of hospitalization and stored at 4°C until it was sent to the influenza and respiratory virus laboratory of the Pasteur Institute in Sidi Fredj. Each swab was collected and placed in a tube containing 2 mL of virus transport medium (Becton Dickinson, USA). All samples were tested by real-time PCR and conventional PCR. An aliquot of 450 μL was tested for the presence of the influenza virus, then for RSV and HMPV. Another aliquot was used for conventional multiplex PCR for rhinovirus, coronavirus (OC43, 229E) and parainfluenza virus 1, 2 and 3. The rest of the sample was stored at −80°C for 1 year. The virus identification techniques used are provided in Supplementary Material S1.
For each child under 2 years of age hospitalized for ALRTI, a preestablished standard questionnaire was completed (Supplementary Material S2). The infant's identification and the data necessary to meet the study objectives were reported. Then a nasal or nasopharyngeal sample was taken via swab (Supplementary Material S1). Each swab was labelled with the child's full name and was accompanied by the clinical information sheet. Samples were sent to the reference laboratory within 1 week from the day of sampling to be stored at 4°C. Parental consent was obtained before nasal or nasopharyngeal sampling was performed.
We studied two main criteria. The first criterion was the proportion of children with identified respiratory tropism virus, both overall and according to different age groups. The second criterion was the proportion of children in whom the following were identified: influenza virus, RSV, rhinovirus, coronavirus, adenovirus, HMPV and parainfluenza virus. For the secondary criterion, for each virus identified, we studied the average positivity rate and its evolution over the study period depending on age and sex.
Results
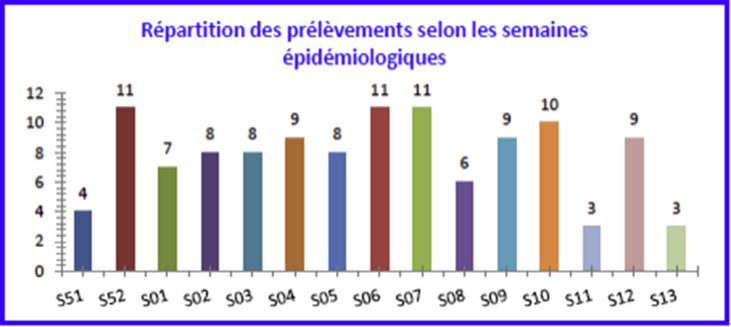
A total of 117 children aged 15 days to 24 months hospitalized for ALRTI were recruited for the study between 21 December 2010 and 1 April 2011, which represented 23% of children under 2 years of age hospitalized during the same period. The majority of patients (92.3%) came from the province of Blida. The maximum number of samples was taken during weeks 52, 06 and 07, and 10, corresponding respectively to the last week of December and mid-February, and the first week of March. These 4 weeks represent more than one third (36.7%) of the samples (Fig. 1). Most children underwent nasal swabbing (59%); just over one third (39%) underwent nasopharyngeal swabbing.
Fig. 1.
Sample breakdown by epidemiologic week.
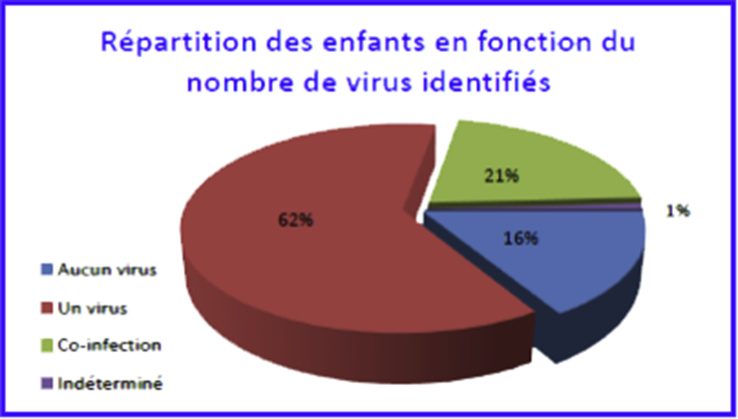
One or more respiratory tropism viruses were identified in 97 children (82.9%) (Fig. 2). In 19 children (16.2%), no respiratory virus among those tested was detected. In 25 children (21.4%), several respiratory viruses were identified as virus coinfection. In 72 children (62%), the viral infection was attributable to a single virus (Fig. 2).
Fig. 2.
Child breakdown by number of identified viruses.
Positivity rates exceeded 80% in all age groups and reached 100% in those over 18 months of age. However, it should be noted that the numbers were very low in these age groups (Table 1). There was no difference by sex (p 0.99).
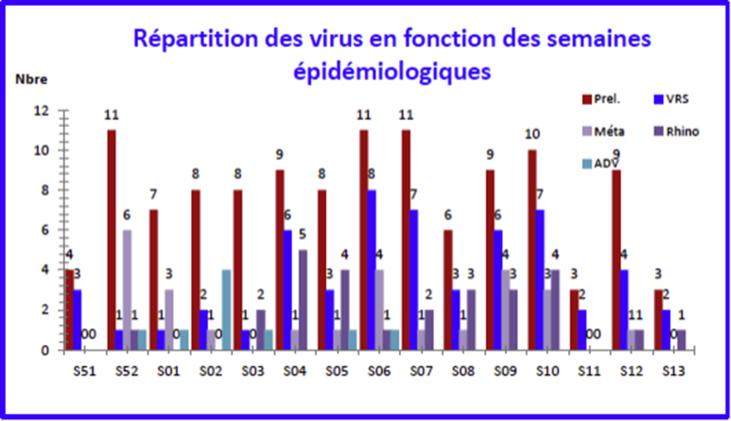
Of the nine viruses searched for, only six were identified: RSV (n = 56), rhinovirus (n = 27), metapneumovirus (n = 26), adenovirus (n = 9), influenza virus (n = 6) and parainfluenza type 3 (n = 3). No parainfluenza viruses types 1, 2 and 4 were identified; and no coronavirus was isolated (Fig. 3).
Fig. 3.
Virus breakdown by epidemiologic week.
Respiratory Syncytial Virus
RSV was isolated in 56 samples, or almost half of the children (47.9%). The majority of RSV samples were positive for type B (46 samples); only ten were positive for type A.
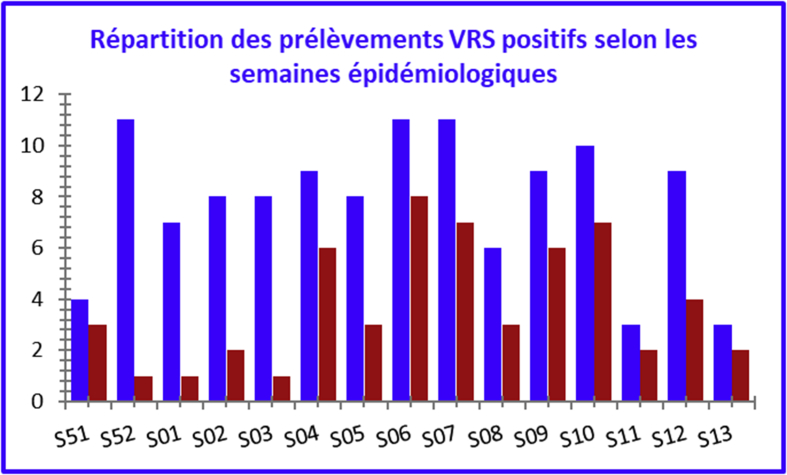
RSV was isolated throughout the study period. In terms of the number of samples taken, the maximum positivity rate was observed at week S51 (75%), followed by weeks S06 (72.7%), S04, S09, S11 and S13 (66.7%). These figures reflect a significant circulation of the virus during this period. Maximum positivity rates were observed between late January and late March (Fig. 4, Fig. 5).
Fig. 4.
Respiratory syncytial virus (human orthopneumovirus)-positive sample breakdown by epidemiologic week.
Fig. 5.
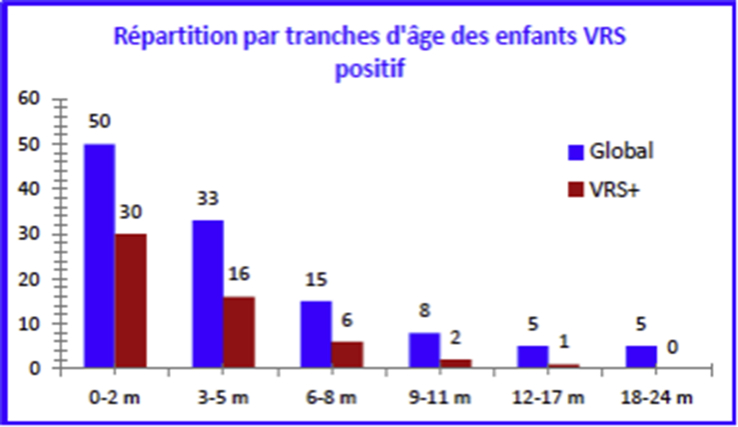
Respiratory syncytial virus (human orthopneumovirus)-positive child breakdown by age.
Human rhinovirus
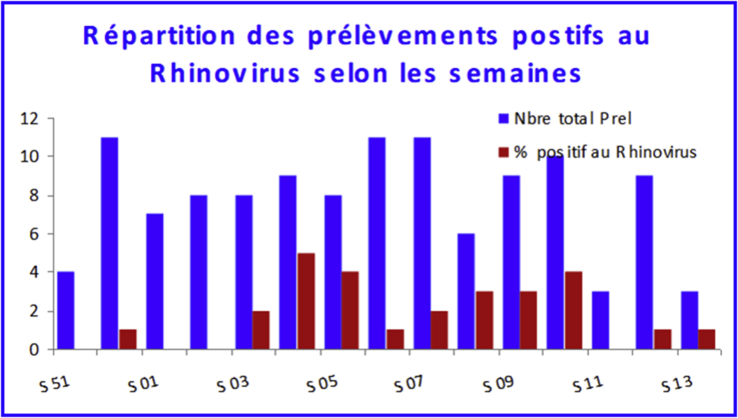
After RSV, human rhinovirus (HRV) was most commonly found. It was isolated in 27 samples, or nearly a quarter of the children (23.1%). HRV was isolated during the period from S52 to S13, although the maximum circulation phase was observed between S03 and S10. The highest positivity rate was recorded between weeks S04 (55.6%), S05 and S08 (50.0%) (Fig. 6).
Fig. 6.
Rhinovirus-positive sample breakdown by epidemiologic week.
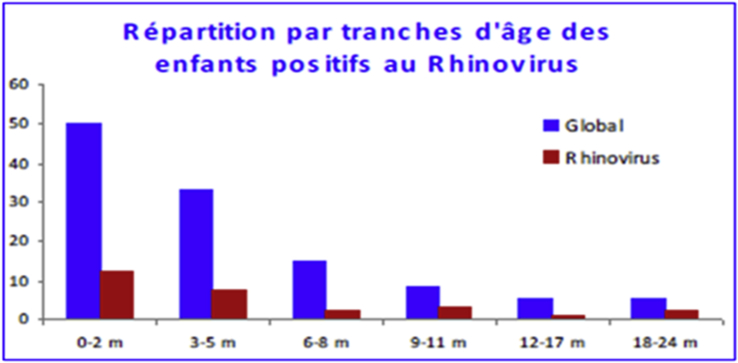
The average age of HRV-positive children was 5.3 ± 2.4 months—slightly older than all children in the study. The median age was 3 months, with extremes ranging from 0.1 to 24 months. The male/female sex ratio was 1.25 (Fig. 7).
Fig. 7.
Rhinovirus-positive child breakdown by age.
Human metapneumovirus
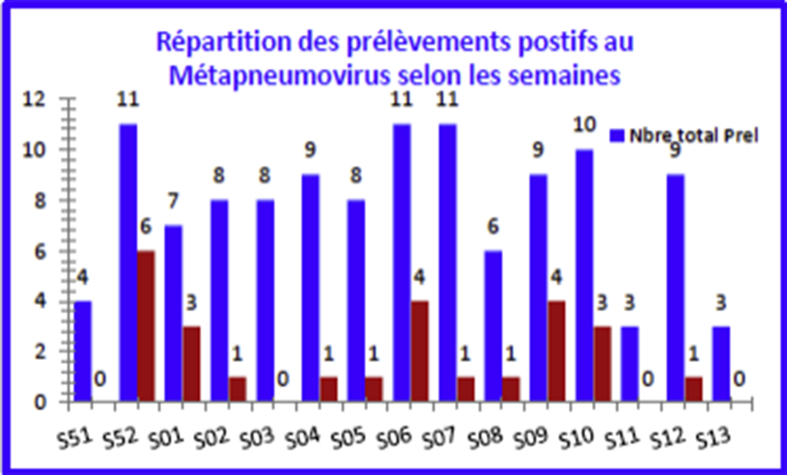
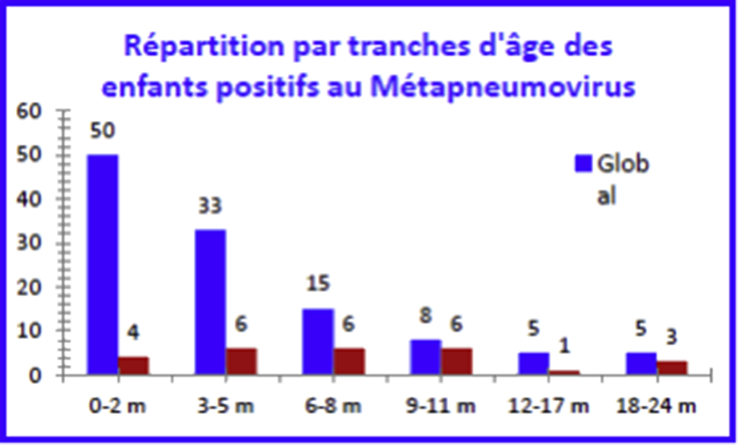
Human metapneumovirus (HMPV) was isolated from 26 samples (22.2%) of all children and during the period from S52 to S12 (Fig. 8). The maximum positivity rate was recorded between week S52 (54.5%) and week S01 (42.9%) (Fig. 9).
Fig. 8.
Human metapneumovirus–positive sample breakdown by epidemiologic week.
Fig. 9.
Human metapneumovirus–positive child breakdown by age.
Virus coinfections
In 25 children (21.4%), several respiratory viruses were identified as virus coinfections. Among the viruses found in coinfection, RSV was the most common and accounted for almost three quarters of the coinfections diagnosed (19/25). It was associated with HRV or HMPV (Table 2). For 14.8% of patients, three viruses were isolated: HRV, RSV and HMPV. Thirty-four per cent of children for whom RSV was isolated had a virus coinfection.
Table 2.
Viral co-infection
| Positivity | N (%) |
|---|---|
| RSV positive (n = 56) | |
| No coinfection | 37 (66) |
| Coinfection | 19 (34) |
| HRV positive (n = 27) | |
| No coinfection | 12 (44) |
| Coinfection | 15 (44) |
| HMPV positive (n = 26) | |
| No coinfection | 12 (46) |
| Coinfection | 14 (54) |
HMPV, human metapneumovirus; HRV, human rhinovirus; RSV, respiratory syncytial virus (human orthopneumovirus).
Discussion
Viral respiratory tract infections are part of the paediatrician's routine. They most often occur in the form of an epidemic during the winter months. Many viruses may be involved and may be responsible for clinical pictures of different severity. Over the past 10 years, molecular biology techniques allowing the detection of the virus genome by PCR have made it possible to isolate many emerging viruses. Real-time PCR has revolutionized virologic diagnosis [5], [6]. In practice, ALRTI are rarely documented, yet the identification of respiratory viruses is useful and relevant because it could reduce the use of antibiotics, which are unnecessary in most cases but are widely prescribed. It should be possible to perform this virologic diagnosis, at least in cases of severe infections requiring hospitalization.
This study describes for the first time in our country the simultaneous detection of several respiratory viruses in children under 2 years of age hospitalized with ALRTI. It shows a high incidence of viral infections during ALRTI.
One virus was identified in the majority of children. The positivity rate observed (82.9%) was high—higher, in fact, than in many similar studies, which found it to range from 35% to 78% [7], [8], [9], [10], [11], [12], [13]. Bezerra et al. [14] found a comparable positivity rate (85.5%) in 407 children younger than 5 with acute upper or lower respiratory tract infection. The high rate observed in our study was partly due to the fact that this was a prospective study conducted by nasal sampling with good cost-effectiveness [15], most often in infants with severe ALRTI during the season corresponding to the peak of viral respiratory infections. This high virus detection rate may reflect the environment in which these children live, where viruses circulate in abundance as a result of family size and promiscuity. The viruses most frequently detected are RSV (48%), HRV (23%), HMPV (22%), adenovirus (7.5%) and influenza (5%). RSV is the most frequently implicated, regardless of the clinical picture, followed by HRV, then HMPV. The distribution of viruses observed in this survey is comparable to that already reported in the literature [11], [16], [17].
In a retrospective study conducted in France during the 2002–2003 season at the University Hospital of Caen, RSV was identified in 37% of a total of 556 nasal aspirations, HRV in 18% of cases and HMPV in 9.7% of cases [18]. We did not isolate coronavirus or parainfluenza viruses 1, 2 or 4, probably because of our sample size and the study period, which was focused on the winter period only. Our work is ongoing, however, and a virologic survey extending beyond the winter season is underway, covering the winter and spring of 2011–2012.
In our overall sample of children aged 15 days to 2 years, the average age of infants was 4.6 months, and 90% of them were younger than 12 months. RSV was responsible for respiratory infection in 47.9% of children. This rate is comparable to what has already been reported in other studies using the same method for detecting viruses by real-time PCR, with rates described as ranging from 44% to 49% [7], [11], [14], [19]. Even higher RSV positivity rates of about 50% have been reported [19]. This nonsegmented RNA virus of the family Paramyxoviridae is one of the main virus agents responsible for paediatric respiratory infections in the world [20] and is also the primary cause of paediatric hospitalization.
This virus can infect children despite the presence of maternal antibodies; natural infection offers only partial protection [21]. RSV type B was found to be the most common in our study; some publications have shown that group A was associated with more severe forms of bronchiolitis, which is not the case here. RSV was identified in 3-month-old infants in an average of 84.2% of cases; average age was under 6 months. Children infected with RSV are younger than those not infected with RSV at 3.1 months versus 5.9 months. Young age is identified as the main risk factor for severe bronchiolitis [22]. Acute viral bronchiolitis does not spare newborns [23].
Very young infants are more vulnerable to RSV infection; the fragility of their respiratory systems, combined with lower defenses at this age, lead to more severe bronchiolitis requiring hospitalization [1], [21]. The majority of positive RSV cases are concentrated in the period from late January to late March, which corresponds to the coldest months in our region. Thanks to improved molecular biology techniques, the detection of HRV infection has increased considerably, making it possible to link many ALRTI, including bronchiolitis, pneumonia, influenza syndromes and asthma exacerbations, to this virus. HRV (of the Picornaviridae family, like enteroviruses) was detected here only by multiplex PCR in 23.1% of cases, which is within the range found in several studies. The detection rate of HRV ranges from 24% to 48%, and it is generally associated with mild to moderate acute respiratory tract infections [24], [25], [26]. Therefore, it is a widespread viral infection and a frequent cause of care seeking. Miller et al. [27] showed that HRV is detected in nearly 26% of children younger than 5 years hospitalized for respiratory symptoms and fever. Its involvement in severe ALRTI in young children is also important [28].
Since the first identification of HRV in 1953, about 100 serotypes have been described, and others still appear, reflecting the high variability of this virus. This variability gives a definite advantage to molecular biology techniques versus cell culture [29], which made it possible to highlight the serotypes in this study. HRV group A and B are the most common groups. HRV group C seems to be responsible for more severe clinical forms [30]. The incidence of HRV in ALRTI in young children has never been studied in Algeria. In our study, HRV was isolated in 23% of children. The average age of the children was 5 months. The clinical picture was not different between HRV-positive and HRV-negative children, or with children in the overall study sample. Smuts et al. [31] found HRV in 58% of young South African children evaluated for wheezing and concluded that HRV is the first viral agent at this age in these children. They did not note a clinical difference between the HRV-positive and HRV-negative groups.
The detection of rhinoviruses using nasal aspiration may be associated with interpretation problems because this virus may persist 2 weeks or more after the acute period [32], and it may be detected in 9% to 36% of asymptomatic subjects [33]. Nevertheless, most authors have clearly demonstrated that HRV infection is much more frequent in sick children than in asymptomatic children [25], [34]. Metapneumovirus was the last paramyxovirus to be identified in the Netherlands in 2001 [35]. It belongs to the subfamily Pneumovirinae, which also includes RSV and had never been described before in Algeria; this finding is therefore novel. In this study, HMPV was responsible for infections in 22.2% of children. This rate of HMPV infection is high in our study population compared to what has been reported for hospitalized children in other studies. The incidence of HMPV infections is estimated at 5% to 10% of hospitalized ALRTI [36], [37]. In one Dutch study carried out over 17 months on samples from hospitalized patients of all ages, HMPV was found in 6.5% of patients with respiratory infections. Most HMPVs were detected in children under 5 years of age, with a peak observed in children between 4 and 6 months of age [38]. A Greek study found HMPV in 16.5% of samples, followed by HRV (14.3%) [39]. In the same country, a more recent study found HMPV infection in 6% of cases in 127 hospitalized infants under 12 months of age, all with a moderate clinical picture [40]. In our sample, the average age of children was 7.5 months. Van den Hoogen et al. [38] noted, as do we, that patients infected with HMPV are older than those infected with RSV.
The maximum positivity rate was observed between the end of December and January. The clinical characteristics were not significantly different from those of the overall sample or from those of HMPV-negative children. The percentage of children for whom the diagnosis of pneumonia was retained is higher (26.9%) than among children not infected with HMPV (21%). The search for HMPV was performed by multiplex and real-time PCR; the results were consistent in all cases.
Two or more viruses were identified in 25 children (21.4%), indicating the presence of virus coinfection. RSV was detected as a coinfection in 39% of cases; it is most often associated with HRV, then HMPV. PCR, which permitted the detection of several pathogens in the same patient, has highlighted the role of these virus coinfections. Their impact on the severity of the clinical picture remains unclear. Its frequency is estimated in different ways; the reported rates vary from 4% to 33%, depending on the study [13], [14], [19], [41], [42].
Bezerra et al. [14] obtained a particularly high rate of virus coinfection (39.6%); only one pathogen was identified in 46% of cases. Few studies have examined the correlation between virus coinfection and the clinical severity of ALRTI, and the results are contradictory. Semple et al. [43] and Greensill et al. [44] showed that HMPV and RSV coinfections correlate with the severity of clinical signs. Bezerra et al. [14] did not find a correlation between the severity of respiratory infection and bacterial coinfection. We did not analyse this correlation in our sample because of the small number of people coinfected with RSV/HMPV or RSV/HRV.
We did not assess the prevalence of these viruses in asymptomatic infants from the same population sampled over the same period. Other authors have identified respiratory viruses, in particular HRV and coronavirus, in 9% to 36% of asymptomatic children [45], but it has been pointed out that HRV infection is much more frequent in sick children than in asymptomatic children [25], [34]. It is unlikely that the identification of HRV is a simple coincidence in our population because we targeted the most severe manifestation of ALRTI. We did not evaluate the role of bacterial agents; this will have to be the subject of further studies.
Conclusions
This study describes for the first time in our country the simultaneous detection of several respiratory viruses in children under 2 years of age hospitalized for ALRTI. We found a high incidence of virus infections in ALRTI. RSV is the most frequently identified viral agent, particularly in infants under 6 months of age. For the first time in Algeria, this study identified emerging viruses, highlighted the high incidence of HRV and HMPV, and emphasized the important role these two viruses play as a frequent reason to seek care. The identification of viruses in ALRTI may help avoid the misuse of antibiotics.
This study confirms the necessity of identifying the virus to determine the local epidemiology of viral ALRTI. This knowledge is essential to predict epidemics, organize healthcare structures and plan prevention measures for groups at risk. The organization of health monitoring networks for viral ALRTI, as is the case for influenza, would make it possible to optimize the quality of care, and to better organize hospitalization and collaboration between hospital paediatricians and city paediatricians in order to reduce the use of hospital emergency services. This study was performed over a long period during the winter and spring of 2011–2012 and will need to be expanded to confirm and refine these initial observations. This study may also provide a starting point for other studies, particularly those searching for risk factors for diseases such as asthma, because certain viral infections have been implicated in the genesis of this prevalent disease.
Conflict of Interest
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2019.100536.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw T.M., Johansson E.W., Hodge M., World Health Organization, United Nation Children’s Fund (UNICEF) World Health Organization; Geneva: 2006. Pneumonia: the forgotten killer of children.https://www.who.int/maternal_child_adolescent/documents/9280640489/en/ Available at: [Google Scholar]
- 3.Bilan d’évaluation du PNL I.R.A. Ministry of Health, Population and Hospital Reform (MSPRH); 2010. Algiers, Algeria. [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2009. World health statistics. [Google Scholar]
- 5.Bellau-Pujol S., Vabret A., Legrand L., Dina J., Gouarin S., Petitjean-Lecherbonnier J. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126:53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkins S.M., Webb D.L., Torrance S.A., El Saleeby C., Harrison L.M., Aitken J.A. Comparison of real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol. 2005;50:2356–2362. doi: 10.1128/JCM.43.5.2356-2362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scagnolari M.F., Perangeli B.E., De Angelis A.G., Moretti B.R. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Chil. 2010;95:35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 8.Niang M.N., Diop O.M., Sarr F.D., Goudiaby D., Malou-Sompy H., Ndiaye K. Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal: the EVIRA project. J Med Virol. 2010;82:866–872. doi: 10.1002/jmv.21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malekshahi S.S., Azad T.M., Yavarian J., Shahmahmoodi S., Naseri M., Rezaei F. Molecular detection of respiratory viruses in clinical specimens from children with acute respiratory disease in Iran. Pediatr Infect Dis J. 2010;29:931–933. doi: 10.1097/inf.0b013e3181e2062e. [DOI] [PubMed] [Google Scholar]
- 10.Don M., Fasoli L., Paldanius M., Vainionpää R., Kleemola M., Räty R. Aetiology of community acquired pneumonia: serological results of a paediatric survey. Scand J Infect Dis. 2005;37:806–812. doi: 10.1080/00365540500262435. [DOI] [PubMed] [Google Scholar]
- 11.Manoha C., Espinosa S., Aho S.L., Huet F., Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221–226. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Freymuth F., Quibriac M., Petitjean J., Daon F., Amiel M.L. [Viruses responsible for respiratory infections in pediatrics. Evaluation of 3480 nasal aspirates performed in children over a 6-year period] Ann Pediatr (Paris) 1987;34:493–501. [PubMed] [Google Scholar]
- 13.Kaplan N.M., Dove W., Abd-Eldayem S.A., Abu-Zeid A.F., Shamoon H.E., Hart C.A. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol. 2008;80:168–174. doi: 10.1002/jmv.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezerra P.G., Britto M.C., Correia J.B., Duarte Mdo C., Fonceca A.M., Rose K. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heikkinen T., Marttila J., Salmi A.A., Ruuskanen O. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J Clin Microbiol. 2002;40:4337–4339. doi: 10.1128/JCM.40.11.4337-4339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali S.A., Williams J.V., Chen Q., Faouri S., Shehabi A., Jundi E.A. Human metapneumovirus in hospitalized children in Amman, Jordan. J Med Virol. 2010;82:1012–1016. doi: 10.1002/jmv.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Woensel J.B., van Aalderen W.M., Kimpen J.L. Viral lower respiratory tract infection in infants and young children. BMJ. 2003;327:36–40. doi: 10.1136/bmj.327.7405.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freymuth F., Vabret A., Legrand L., Dina J., Gouarin S., Cuvillon-Nimal D., Brouard J. Human metapneumovirus. Pathol Biol (Paris) 2009;57:133–141. doi: 10.1016/j.patbio.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonzel L., Tenenbaum T., Schroten H., Schildgen O., Schweitzer-Krantz S., Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27:589–594. doi: 10.1097/INF.0b013e3181694fb9. [DOI] [PubMed] [Google Scholar]
- 20.Wertz G.W., Moudy R.M. Antigenic and genetic variation in human respiratory syncytial virus. Pediatr Infect Dis J. 2004;23:S19–S24. doi: 10.1097/01.inf.0000108189.87181.7c. [DOI] [PubMed] [Google Scholar]
- 21.El-Hajje M.J., Moulin F., de Suremain N., Marc E., Cosnes-Lambe C., Pons-Catalano C. Respiratory syncytial virus in hospitalized children. A 3-year study. Presse Med. 2008;37:37–43. doi: 10.1016/j.lpm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Fodha I., Landolsi N., Vabret A., Sboui H., Trabelsi A., Freymuth F. Epidemiology and clinical presentation of respiratory syncytial virus infection in a Tunisian neonatal unit from 2000 to 2002. Ann Trop Pediatr. 2004;24:219–225. doi: 10.1179/027249304225018966. [DOI] [PubMed] [Google Scholar]
- 23.Guittet V., Brouard J., Vabret A., Lafay F., Guillois B., Duhamel J.F. [Rhinovirus and acute respiratory infections in hospitalized children. Retrospective study, 1998–2000] Arch Pediatr. 2003;10:417–423. doi: 10.1016/S0929-693X(03)00090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piotrowska Z., Vázquez M., Shapiro E.D., Weibel C., Ferguson D., Landry M.L. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J. 2009;28:25–29. doi: 10.1097/INF.0b013e3181861da0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusel M.M.H., de Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 26.Blomqvist S., Roivainen M., Puhakka T., Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol. 2006;66:263–268. doi: 10.1002/jmv.2140. [DOI] [PubMed] [Google Scholar]
- 27.Miller E.K., Lu X., Erdman D.D., Poehling K.A., Zhu Y., Griffin M.R. New vaccine surveillance network. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltola V., Jartti T., Putto-Laurila A., Mertsola J., Vainionpää R., Waris M. Rhinovirus infection in children: a retrospective and prospective hospital-based study. J Med Virol. 2009;81:1831–1838. doi: 10.1002/jmv.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coiras M.T., Aguilar J.C., García M.L., Casas I., Pérez-Breña P. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested–PCR assays. J Med Virol. 2004;72:484–495. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwane M.K., Prill M.M., Lu X., Miller E.K., Edwards K.M., Hall C.B. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 31.Smuts H.E., Workman L.J., Zar H.J. Human rhinovirus infection in young African children with acute wheezing. BMC Infect Dis. 2011;11:65. doi: 10.1186/1471-2334-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay I.M. Human rhinovirus: the cold wars resume. J Clin Virol. 2008;42:297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Zalm M.M., van Ewijk B.E., Wilbrink B., Uiterwaal C.S., Wolfs T.F., van der Ent C.K. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154:396–400. doi: 10.1016/j.jpeds.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson N.M. Virus infections, wheeze and asthma. Paediatr Respir Rev. 2003;4:184–192. doi: 10.1016/S1526-0542(03)00052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams J.V., Edwards K.M., Weinberg G.A., Griffin M.R., Hall C.B., Zhu Y. Population-based incidence of human metapneumovirus in hospitalized children. J Infect Dis. 2010;201:1890–1898. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foulongne V., Guyon G., Rodière M., Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–359. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 38.van den Hoogen B.G., Osterhaus D.M., Fouchier R.A. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23(1 Suppl. l):S25–S32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 39.Xepapadaki P., Psarras S., Bossios A., Tsolia M., Gourgiotis D., Liapi-Adamidou G. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30:267–270. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammas I.N., Koutsaftiki C., Nika E., Vagia F., Zaravinos A., Priftis K.N. Detection of human metapneumovirus in infants with acute respiratory tract infection. Mol Med Rep. 2011;4:267–271. doi: 10.3892/mmr.2011.416. [DOI] [PubMed] [Google Scholar]
- 41.Regamey N., Kaiser L., Roiha H.L., Deffernez C., Kuehni C.E., Latzin P. Viral etiology of acute respiratory infections with cough in infancy: a community based birth cohort study. Pediatr Infect Dis J. 2008;27:100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 42.Akinloye O.M., Rönkkö E., Savolainen-Kopra C., Ziegler T., Iwalokun B.A., Deji-Agboola M.A. Specific viruses detected in Nigerian children in association with acute respiratory disease. J Trop Med. 2011;1–6 doi: 10.1155/2011/690286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greensill J., McNamara P., Dove W., Flanagan B., Smyth R. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkley J.A., Munywoki P., Ngama M., Kazungu S., Abwao J., Bett A. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.