Abstract Abstract
Background
Attention to the deep-sea environment has increased dramatically in the last decade due to the rising interest in natural resource exploitation. Although Colombia holds a large submerged territory, knowledge of the seabed and its biodiversity beyond 1,000 m depth is very limited. During 2015–2017, Anadarko Colombia Company (ACC) carried out hydrocarbon exploratory activities in the South-western Colombian Caribbean, at depths between 375 m and 2,565 m.
New information
Capitalising on available data resources from these activities, several cnidarian species were observed in ROV and towed camera surveys. We analysed over nine hours of video and 5,066 still images from these surveys, identifying organisms to the lowest possible taxonomic level. The images and associated data presented here correspond to 108 observations of deep-sea cnidarians, including seven new records for the Colombian Caribbean. Given the paucity of research and funding to explore the deep-sea in Colombia, the present dataset comprises the largest deep-sea Cnidaria imagery inventory to date for the Colombian Caribbean.
Keywords: Biodiversity, Benthic, DwC-A, Marine, Remote Operated Vehicle-ROV, Tow Camera, Soft Bottom
Introduction
Interest in the deep seas has increased dramatically in the last decade, due to the potential presence of natural resources (oil, gas, precious and rare metals, fishery resources, pharmaceuticals, biodiversity etc.). Likewise, there is an increasing concern about the health of this environment, which calls for the acquisition of baseline data (Mengerink et al. 2014). Although Colombia holds a large submerged territory and there has been extensive research on cnidarians from the continental margin and upper slope (Florez and Santodomingo 2010, Santodomingo et al. 2012), knowledge of the seabed below 1,000 m depth is sparse. All previous investigations addressing the characterisation of deep-water ecosystems below 1,000 m have relied on samples taken by box corer or benthic sleds (Polanco F et al. 2017), but no in-situ visual confirmations have been provided.
Visual observation of the seabed and its inhabitants is possible using vessels and submersible equipment that can be remote (ROV), autonomous (AUV) or manned. This is a non-invasive in-situ procedure that can reach depths and environments that are normally out of human reach (Huvenne et al. 2018). Therefore, visual data (photographs and videos) provide valuable information on the geological characteristics, physical structure and biological components of a benthic habitat (Pawson et al. 2015, Zawada et al. 2007). However, this sampling method is limited only to the identification of large organisms, i.e. megafauna, with no evasive behaviour (Zawada et al. 2007). Furthermore, the quality of the images is highly dependent on the equipment and experience and qualifications of the operator (Solem 2017). In addition, in instances such as the identification some soft-bodied cnidarians like anemones and cerianthids, image data provide limited information allowing the identification at high taxonomic ranks only (i.e. family and genus in some cases). Species-level identification for these particular cases requires a detailed description of internal and microanatomical characters (e.g. Carlgren 1949, England 1987, Häussermann 2004, Rodriguez and López-González 2013), all of which requires specimen sampling and examination.
Video and still photographs are useful for documenting biological and ecological information, species identification, public outreach and scientific publications (Etnoyer et al. 2006). It provides a useful resource that can be made readily available to other scientists, as it was in this case. However, the use of images for organism identification may impose some technical limitations, in particular regarding the quality of the images. High-quality images depend on the camera used during the survey (digital quality, the angle of the setup and zoom), water transparency conditions, the lighting setup (type and angle of the lights) and the file format used to store the image data. Quality also depends on the skill of the engineer who operates the equipment and his/her expertise on the observed organisms. This will determine whether essential morphological features for accurate identification of the organisms (diagnostic characters) could be targeted for close-ups stills. Finally, for some groups of organisms (especially those relying on internal characters for identification, e.g. sea anemones), this type of data provides limited information for reliable identifications (even at high taxonomic levels) and detailed taxonomic descriptions, requiring sampling and morphological observations in order to provide species-level identifications. Nonetheless, such “first-pass” biodiversity surveys provide an invaluable source of information, especially from previously unexplored environments and constitute the foundation for further, more thorough initiatives.
Several types of surveys are essential for the deep-sea hydrocarbon exploratory activities in Colombia. Exploratory drilling activities normally include ROV video surveys before and after drilling. These surveys are critical to evaluate the seabed conditions around the well to minimise potential impacts to sensitive ecosystems (e.g. chemosynthetic communities, deep-sea coral reefs) or archaeological sites. These vehicles are also essential to carry out inspections of the subsea infrastructure to keep the integrity of the well (Gates 2016, Tena 2011). In addition to ROV, detailed characterisation of seabed features and fauna can be obtained from towed camera surveys if needed. Given the paucity of research and funding to explore the deep-sea in Colombia, the present dataset comprises the largest deep-sea Cnidaria imagery inventory to date for the deep sea of Colombian Caribbean.
Cnidarians (jellyfish, corals, sea anemones, amongst others) are one of the most ancient invertebrate groups that keeps a simple body structure with two cell layers and a blind gut (Cairns et al. 2009, Daly et al. 2007). They are found in all aquatic (marine and freshwater) environments, being more diverse in marine habitats. Although simple in body organisation, cnidarians have evolved as specialised carnivores, catching their prey aided by cnidocytes, which are specialised cells with stinging structures, a phylum-defining trait (Technau and Steele 2011). In oceanic waters, cnidarians can be found in nearly all ecosystems from shallow waters to abyssal depths and from polar regions to tropical latitudes. Benthic cnidarians, such as corals colonising both soft and hard substrates, are efficient suspension feeders that provide a tridimensional habitat in the deep-sea (Etnoyer and Morgan 2005), which sustains high numbers of associated invertebrates (Buhl-Mortensen and Mortensen 2005). Cnidarians, particularly anthozoans, are now recognised as major contributors of biogenic environments in the deep-sea (Hourigan et al. 2017). In addition, even the rarest types of pelagic or benthic cnidarians are also found in the deep-sea (Osborn et al. 2007, Miranda et al. 2018). Given the low density of deep-sea fauna in general, numerous surveys are usually required to recognise common vs. rare species. Hydrocarbon drilling activities have provided some of the few opportunities to perform extensive observations in the deep-sea (Gates et al. 2017).
During 2015–2017, Anadarko Colombia Company (ACC), a subsidiary of Anadarko Petroleum Corporation, carried out hydrocarbon exploratory activities in the deep sea South-western Colombian Caribbean. The activities included both ROV and towed camera surveys at depths between 375 m and 2,565 m. Capitalising on the availability of the images obtained from these activities, several organisms from different phyla were spotted and identified to the lowest possible taxonomic status. This is the first of a series of datasets reporting visual confirmations on the occurrences of deep-sea organisms, in this case, 108 cnidarians.
Sampling methods
Sampling description
ROV surveys
ROV video surveys were performed in a cross pattern. From a central point that could be a transponder (tool for positioning the drilling vessel) or the drilling location, surveys were executed in an 80 metre-long transect with a north trajectory, then an 80 metre-long transect with a south trajectory, an 80 metre-long transect with an east trajectory and finally an 80 metre-long transect with a west trajectory (Fig. 1). Video-transects included soft bottom images, where only one species of a pelagic cnidarian was encountered. Videos were taken before and after drilling for all the exploratory wells, or even during drilling for two of the wells.
Methodology for ROV video surveys.
Figure 1a.
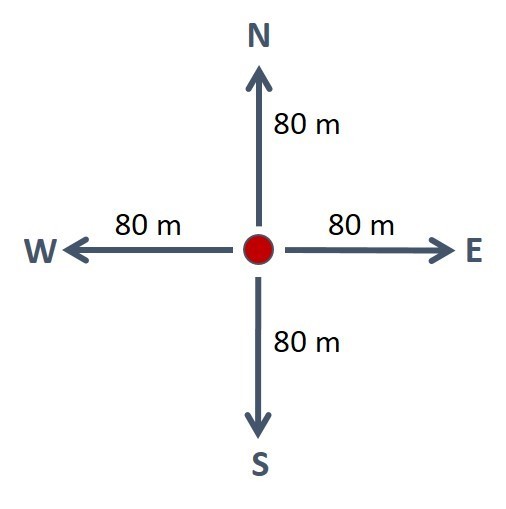
Schematic representation of the ROV transects is cross-fashion. Red circle denotes the location of the well or transponder.
Figure 1b.

Example of a snapshot taken from the ROV video, depicting a specimen of Poralia rufescens.
Towed camera surveys
Survey areas of interest (i.e. Chamana, Chamai, Yaduli, Cana Norte and Cawa) were assessed using 25 towed camera transects. These transects registered seafloor features, during 3-hour surveys, taking still images every 20 seconds. Benthic and pelagic megafauna specimens were recorded in the images.
Quality control
Videos were analysed twice for the presence of cnidarians by two different experts. This methodology allowed us to ensure all cnidarians were registered. Still images were also analysed twice by the same expert, who did preliminary identification to phylum and class. All cnidarian images were then identified to the lowest possible taxonomic level. When in doubt about the identification, additional experts were contacted and the images were sent to them in jpg format for towed camera photographs and as a snapshot (also in jpg format) for ROV videos.
Given that the acquisition of the images used in this paper was carried out for other purposes and objectives, they do not have the best quality for species identification. Nevertheless, the images were useful, and represent the first visual confirmation of these deep-sea organisms for the Colombian Caribbean.
Step description
For ROV surveys, we analysed a total of 48 video transects (duration: 9 h 9 min 26 sec), looking for benthic and pelagic cnidarians. We took snapshots of each cnidarian, registered coordinates and depth and identified them to the lowest possible taxonomic level. On the other hand, for towed camera images, we analysed a total of 5,066 photographs, looking for benthic and pelagic cnidarians. For each cnidarian, coordinates and depth were registered and identified to the lowest possible taxonomic level. We cropped the photographs to include only the organism and performed image correction to reduce the bluish colour cast with the Auto Tone function in Adobe Photoshop CC 2018 (Fig. 2).
Workflow of image correction in Adobe Photoshop CC 2018.
Figure 2a.
Original photograph of a cerianthid (subclass Ceriantharia) obtained from the tow camera.
Figure 2b.
Cropped photograph that includes only the organism and colour cast corrected using the Auto Tone function.
Using photographs and video footage, we highlighted the occurrences for Cnidaria here. Cnidarians were further identified to the lowest possible taxonomic level, based on comparison with literature (Fautin 2016, Molodtsova 2006, Williams 2011, Kramp 1961, Thuesen 2003, Burton and Lundsten 2008), databases (Board, WoRMS Editorial 2017, Fautin 2013) and with the help of taxonomic experts of each group. Taxonomic experts that helped in the identification of the cnidarians include Dennis Opresko (Smithsonian National Museum of Natural History), Mercer Brugler (New York City College of Technology), Tina Molodtsova (P.P. Shirshov Institute of Oceanology), Frederic Sinniger (University of the Ryukyus) and Steven Haddock (Monterey Bay Aquarium Research Institute). The taxonomy presented here is in accordance with the World Register of Marine Species – WORMS (http://www.marinespecies.org/).
Geographic coverage
Description
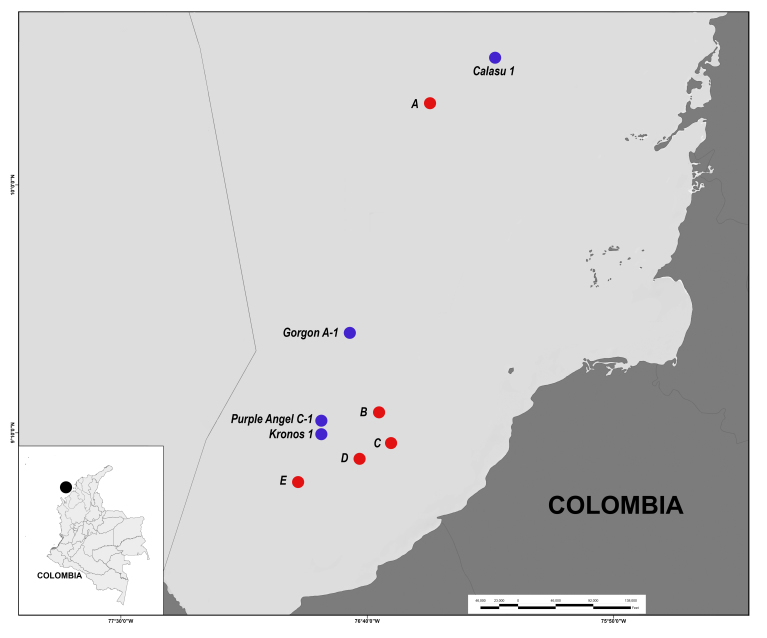
ACC’s deep sea hydrocarbon exploratory activities in the South-western Colombian Caribbean included four exploratory wells (Calasu 1, Kronos 1, Gorgon A-1 and Purple Angel C-1) and five other survey areas of interest (A-E), at depths between 375 m and 2,565 m (Fig. 3). Wells and areas of interest are approximately 54 to 74 km offshore from the nearest point in the Caribbean coast of Colombia.
Figure 3.
Geographical location of the sites surveyed at the South-western Colombian Caribbean. Blue circles depict the wells and red circles correspond to other surveyed areas.
Coordinates
9.27 and 10.369 Latitude; -76.481 and -76.932 Longitude.
Taxonomic coverage
Description
The data presented here correspond to 108 occurrences of deep-sea cnidarians from the South-western Colombian Caribbean, spotted over soft bottoms at depths between 375 to 2,565 m. The dataset contains the original data of depth, geographical coordinates, date and hour of the event, for each image that registered a cnidarian. Additional information includes the methodology used to obtain the images (see Methods section), taxonomic identification to the lowest possible taxonomic level, the name of the expert who identified the organism and the number of individuals of the species per image. The dataset also contains an extension with links to the images supporting the occurrences.
Seven occurrences were obtained through the ROV surveys, all corresponding to the pelagic jellyfish Poralia cf. rufescens Vanhöffen, 1902. Tow camera occurrences (n = 101) registered members of the classes Scyphozoa (n = 1), Hydrozoa (n = 13) and Anthozoa (n = 87). Only two orders of jellyfish, Trachymedusae (Crossota millsae Thuesen, 2003 and Voragonema pedunculata (Bigelow, 1913)) and Narcomedusae represent Hydrozoa, while members of seven orders represented Anthozoa. Within Anthozoa, organisms from the three subclasses (Hexacorallia, Octocorallia and Ceriantharia) were spotted. Hexacorallia was represented by anemones (order Actiniaria), the zoanthid Epizoanthus cf. stellaris Hertwig, 1888 (order Zoantharia), the black coral Bathypathes cf. patula Brook, 1889 (order Antipatharia) and cerianthids (order Ceriantharia). Octocorals were represented by the orders Alcyonacea and Pennatulacea, the latter with seven organisms belonging to the genus Umbellula Gray, 1870. Finally, corallimorphs (order Corallimorpharia) were represented by the genus Corallimorphus Moseley, 1877 (Table 1).
Table 1.
Cnidarians found in the deep waters of the South-western Colombian Caribbean. * New reports for the Colombian Caribbean.
| Species | Sampling Protocol | Depth range (m) |
| Poralia cf. rufescens Vanhöffen, 1902 | ROV | 1,565–1,818 |
| Hydrozoa | Towed Camera | 1,839–2,367 |
| Semaeostomeae | Towed Camera | 2,289 |
| Crossota millsae Thuesen, 2003* | Towed Camera | 1,165–1,189 |
| Voragonema pedunculata (Bigelow, 1913)* | Towed Camera | 2,340–2,562 |
| Narcomedusae | Towed Camera | 1,717 |
| Actiniaria | Towed Camera | 539–2,523 |
| Ceriantharia | Towed Camera | 428–2,058 |
| Corallimorphus Moseley, 1877* | Towed Camera | 2,259–2,526 |
| Hormathiidae Carlgren, 1932 | Towed Camera | 375–2,524 |
| Kadosactinidae Riemann-Zürneck, 1991 | Towed Camera | 2,352–2,430 |
| Bathypathes cf. patula Brook, 1889* | Towed Camera | 2,359–2,564 |
| Virgulariidae Verrill, 1868 | Towed Camera | 654–2,565 |
| Trichogorgia lyra Bayer & Muzik, 1976 | Towed Camera | 577–650 |
| Umbellula Gray, 1870* | Towed Camera | 1,633–2,525 |
| Chrysogorgiidae Verrill, 1883 | Towed Camera | 490 |
| Epizoanthus cf. stellaris Hertwig, 1888* | Towed Camera | 502–1,196 |
| Adamsia Forbes, 1840* | Towed Camera | 486–2,563 |
| Phelliactis Simon, 1842* | Towed Camera | 2,561 |
Based on the 101 occurrences from towed camera surveys, we registered eight new reports of cnidarians for the Colombian Caribbean. The new records for the area comprised two sea anemones, one zoanthid, one corallimorpharian, one octocoral, one black coral (Fig. 4) and two jellyfish species (Fig. 5).
New reports of cnidarians for the Colombian Caribbean.
Figure 4a.

Corallimorphus sp.
Figure 4b.

Phelliactis sp.
Figure 4c.

Adamsia sp. on a hermit crab (Sympagurus pictus)
Figure 4d.

Bathypathes cf. patula
Figure 4e.

Umbellula sp.
Figure 4f.

Epizoanthus cf. stellaris growing over a glass sponge (Hyalonema sp.) stalk
New reports of cnidarian medusae for the Colombian Caribbean.
Figure 5a.

Crossota millsae
Figure 5b.

Voragonema pedunculata
The genus Corallimorphus has a total of six valid species (Fautin 2016), present at all latitudes in deep waters (Fautin 2011), with the exception of C. profundus, which has been identified from shallow environments in Antarctica (Riemann-Zürneck and Iken 2003). Two species of the genus, C. ingens and C. rigidus, have been reported in the Atlantic Ocean (i.e. Den Hartog et al. 1993, Ocaña and Den Hartog 2002). Detailed records of members of the family Corallimorphidae are available for the greater Caribbean region and the Gulf of Mexico (Fautin and Daly 2009, González-Muñoz et al. 2013, González-Muñoz et al. 2016), but are limited to shallow water species. A study of the deep-sea communities off the east coast of Florida reports a record of Corallimorphus sp. based on still photographic data (Messing et al. 2012). With the available information, the authors were not able to classify the specimens beyond the genus level.
The genus Phelliactis (fam. Hormathiidae) is composed of 20 valid species distributed worldwide (Fautin 2016). In the Caribbean, records for species of the genus exist from hydrothermal vent communities in the Cayman Islands (Plouviez et al. 2015) and deep-sea benthos of Venezuela (Briggs et al. 1996). This last study reports the presence of P. michaelsarsi, effectively expanding the distribution range for the species, currently limited to the East and Mid Atlantic Ocean (Molodtsova et al. 2008). Unfortunately, this report does not provide enough information regarding the identification methodology employed by the authors in order to determine this species identity (i.e. geographical proximity, detailed morphological description), thus we cannot evaluate the validity of this record.
Anemones, belonging to the genus Adamsia, are known for holding symbiotic relationships with hermit crabs, most commonly with the species Sympagurus pictus (Daly et al. 2004), but reports also exist for the species Parapagurus pilosimanus and the gastropods of the genus Oocorys (Ammons and Daly 2008). There are a total of 6 valid species (Fautin 2016) with A. obvolva being the only species reported in the region, specifically in several localities from the Gulf of Mexico (Daly et al. 2004, Ammons and Daly 2008). The bathymetric range of the specimens reported here (Table 1) is larger than the range reported for A. obvolva (300 to 800 m). The records, presented here, would correspond to an expansion of the species’ geographic and bathymetric ranges; however, it was not possible to confirm its identity. Even the examination of diagnostic external anatomical characters, such as the presence of cinclides (small insertions occurring in the column of the anemone), requires detailed observation under the stereomicroscope.
Other hexacorallians, described as new reports for the Colombian Caribbean, include Bathypathes cf. patula and Epizoanthis cf. stellaris. Bathypathes patula is also a widespread, cosmopolitan species that has been described from the Pacific, the Atlantic, the Indian Ocean, the Gulf of México and Puerto Rico (Molodtsova 2006, Horowitz et al. 2018). It belongs to the Antipatharian genus Bathypathes Brook, 1889 (Anthozoa: Hexacorallia) which currently holds 19 species (Molodtsova and Opresko 2019). Bathypathes patula is characterised by a wide vertical distribution that ranges from 100 m to 5500 m in depth (Molodtsova 2006). On the other hand, the zoanthid genus Epizoanthus Gray, 1867 (Anthozoa: Hexacorallia) is a rich genus with 105 species and a global distribution (Reimer and Sinniger 2019, Kise and Reimer 2016). Particularly, Epizoanthis stellaris Hertwig, 1888 has been reported to grow over the siliceous spicules of glass sponges (Beaulieu 2001), as also seen in this study.
The pennatulacean genus Umbellula comprises 13 species (Cordeiro et al. 2019) and belongs to the monogeneric family Umbellulidae (Anthozoa: Octocorallia). This genus has a widespread to nearly worldwide distribution and is the deep-sea pennatulacean genus with the widest vertical distribution reaching depths of 6,000 m (Williams 2011). The most common species is U. lindahi that has a cosmopolitan distribution in all of the world's oceans (Tyler et al. 1995).
Finally, the two hydromedusae from the Rhopalonematidae family, Crossota millsae and Voragonema pedunculata (previously know as Benthocodon pedunculata), are bathypelagic and often found in groups drifting above the bottom; this is the reason why they are also described as benthopelagic. These two species have been reported in the Western Atlantic (the Bahamas and Dry Tortugas) (Larson et al. 1991, Larson et al. 1992) and the Gulf of Mexico (Valentine and Benfield 2013).
Temporal coverage
Data range: 2015-2-24 – 2017-4-26.
Usage rights
Use license
Other
IP rights notes
Creative Commons Attribution Non Commercial (CC-BY-NC) 4.0 License
Data resources
Data package title
First visual occurrence data for deep-sea cnidarians in the South-western Colombian Caribbean
Resource link
Alternative identifiers
https://ipt.biodiversidad.co/sibm/resource?r=anadarko_colombia_002
Number of data sets
1
Data set 1.
Data set name
First visual occurrence data for deep-sea cnidarians in the South-western Colombian Caribbean
Data format
Darwin Core Archive (DwC-A)
Number of columns
60
Character set
UTF-8
Description
The data presented here corresponds to occurrences of deep-sea cnidarians from the South-western Colombian Caribbean, spotted over soft bottoms at depths between 375 to 2,565 m.
Data set 1.
| Column label | Column description |
|---|---|
| occurrenceID | An identifier for the Occurrence |
| basisOfRecord | The specific nature of the data record |
| institutionCode | The name in use by the institution having custody of the information referred to in the record |
| collectionCode | The name, acronym, coden or initialism identifying the collection or dataset from which the record was derived |
| catalogNumber | An identifier for the record within the dataset |
| type | The nature or genre of the resource |
| language | Thelanguage of the resource |
| rightsHolder | The organisation owning or managing rights over the resource |
| accessRights | Information about who can access the resource or an indication of its security status |
| institutionID | An identifier for the institution having custody of the information referred to in the record. |
| ownerInstitutionCode | The name in use by the institution having ownership of the information referred to in the record |
| recordedBy | People responsible for recording the original Occurrence |
| individualCount | The number of individuals represented present at the time of the Occurrence |
| lifeStage | The age class or life stage of the biological individual(s) at the time the Occurrence was recorded |
| occurrenceStatus | A statement about the presence or absence of a Taxon at a Location |
| preparations | A list of preparations and preservation methods for a specimen |
| samplingProtocol | Protocol used during an Event |
| samplingEffort | The amount of effort expended during an Event |
| eventDate | The date during which an Event occurred |
| eventTime | The time during which an Event occurred |
| year | The four-digit year in which the Event occurred |
| month | The ordinal month in which the Event occurred |
| day | The integer day of the month on which the Event occurred |
| verbatimEventDate | The verbatim original representation of the date and time information for an Event |
| habitat | A category or description of the habitat in which the Event occurred |
| higherGeography | Geographic location less specific than the information captured in the locality term |
| continent | The name of the continent in which the Location occurs |
| waterBody | The name of the water body in which the Location occurs |
| locationID | An identifier for the set of location information |
| country | The name of the country in which the Location occurs |
| countryCode | The standard code for the country in which the Location occurs |
| locality | The specific description of the place |
| verbatimDepth | The original description of the depth below the local surface |
| minimumDepthInMetres | The lesser depth of a range of depth below the local surface, in metres |
| maximumDepthInMetres | The greater depth of a range of depth below the local surface, in metres |
| verbatimLatitude | The verbatim original latitude of the Location |
| verbatimLongitude | The verbatim original longitude of the Location |
| verbatimCoordinateSystem | The spatial coordinate system for the verbatimLatitude and verbatimLongitude or the verbatimCoordinates of the Location |
| decimalLatitude | The geographic latitude of the geographic centre of a Location |
| decimalLongitude | The geographic longitude of the geographic centre of a Location |
| geodeticDatum | The ellipsoid, geodetic datum or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude as based |
| georeferencedBy | The organisation who determined the georeference for the Location |
| identifiedBy | A list of names of people who assigned the Taxon to the subject |
| dateIdentified | The date on which the subject was identified as representing the Taxon |
| identificationQualifier | A brief phrase or a standard term to express the determiner's doubts about the Identification |
| scientificNameID | An identifier for the nomenclatural details of a scientific name |
| nameAccordingToID | An identifier for the source in which the specific taxon concept circumscription is defined or implied |
| acceptedNameUsage | The full name, with authorship and date information if known, of the currently valid (zoological) or accepted (botanical) taxon |
| nameAccordingTo | The reference to the source in which the specific taxon concept circumscription is defined or implied - traditionally signified by the Latin "sensu" or "sec." |
| scientificName | The full scientific name, with authorship and date information if known |
| kingdom | The full scientific name of the kingdom in which the taxon is classified |
| phylum | The full scientific name of the phylum or division in which the taxon is classified |
| class | The full scientific name of the class in which the taxon is classified |
| order | The full scientific name of the order in which the taxon is classified |
| family | The full scientific name of the family in which the taxon is classified |
| genus | The full scientific name of the genus in which the taxon is classified |
| specificEpithet | The name of the first or species epithet of the scientificName |
| taxonRank | The taxonomic rank of the most specific name in the scientificName |
| scientificNameAuthorship | The authorship information for the scientificName |
| taxonomicStatus | The status of the use of the scientificName as a label for a taxon |
Acknowledgements
Thanks to Anadarko Colombia Company (ACC), a subsidiary of Anadarko Petroleum Company (APC) and Ecopetrol. Other companies involved in this oil & gas exploratory survey were Dolphin Drilling that operated the Mobile Operating Drilling Unit (MODU)and Subsea 7 (and its division I-TECH services) operated the ROV from the MODU. We thank their technical staff for conducting the ROV surveys and their willingness, time and patience that made possible the acquisition of the images we worked with. Towed camera images were provided by SERPORT and CSA Ocean Science Inc., during additional exploration studies of areas of interest. We want to thank Colciencias’ Post-Doctoral Program (Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación "Francisco José de Caldas" - COLCIENCIAS, Convocatoria 2017, Número 784) and additional taxonomic experts who contributed to the further identification of some organisms: Dennis Opresko (Smithsonian National Museum of Natural History), Mercer Brugler (New York City College of Technology), Tina Molodtsova (P.P. Shirshov Institute of Oceanology), Frederic Sinniger (University of the Ryukyus) and Steven Haddock (Monterey Bay Aquarium Research Institute). We would also like to thank the Instituto de Investigaciones Marinas y Costeras – INVEMAR for the funding and support during the analysis of the ROV videos and images, and the taxonomic identification. Contribution No. 1216 of INVEMAR. Additional thanks go to the SiB Colombia team (Dairo Escobar, Leonardo Buitrago, Camila Plata) who helped in the data and image publication process.
Funding Statement
Funding agencies include Anadarko Colombia Company (ACC), a subsidiary of Anadarko Petroleum Company (APC), Ecopetrol, and Colciencias’ Post-Doctoral Program (Convocatoria 2017, Número 784).
References
- Ammons Archie W., Daly Marymegan. Distribution, habitat use and ecology of deepwater Anemones (Actiniaria) in the Gulf of Mexico. 10.1016/j.dsr2.2008.07.015. Deep Sea Research Part II: Topical Studies in Oceanography. 2008;55:2657–2666. doi: 10.1016/j.dsr2.2008.07.015. [DOI] [Google Scholar]
- Beaulieu S. E. Life on glass houses: sponge stalk communities in the deep sea. Marine Biology. 2001;138(4):803–817. doi: 10.1007/s002270000500. [DOI] [Google Scholar]
- Board WoRMS Editorial. World Register of Marine Species. Available from http://www.marinespecies.org at VLIZ. Accessed 2019-02-24. VLIZ. 2017 doi: 10.14284/170. [DOI]
- Briggs Kevin B., Richardson Michael D., Young David K. The classification and structure of megafaunal assemblages in the Venezuela Basin, Caribbean Sea. Journal of Marine Research. 1996;54(4):705–730. doi: 10.1357/0022240963213736. [DOI] [Google Scholar]
- Buhl-Mortensen L., Mortensen P. B. Cold-water corals and ecosystems. Springer; Berlin, Heidelberg: 2005. Distribution and diversity of species associated with deep-sea gorgonian corals off Atlantic Canada.849-879. [DOI] [Google Scholar]
- Burton E. J., Lundsten L. Davidson Seamount Taxonomic Guide. Marine Sanctuaries Conservation Series ONMS-08-08. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries; Silver Spring, MD: 2008. 145 pp [Google Scholar]
- Cairns S. D., Gershwin L. A., Brook F. J., Pugh P., Dawson E. W., O. Ocaña V. ,, Vervoort W., Williams G., Watson J. E., Opresko D. M., Schuchert P., Hine P. M., Gordon D. P., Campbell H. J., Wright A. J., Sánchez J. A., Fautin D. G. Phylum Cnidaria: corals, medusae, hydroids, Myxozoa. New Zealand inventory of biodiversity; 2009. 43 [Google Scholar]
- Carlgren Oskar H. A survey of the Ptychodactiaria, Corallimorpharia and Actiniaria. Kungliga Svenska Vetenskapsakademiens Handlingar. 1949;1:1–121. [Google Scholar]
- Cordeiro R., van Ofwegen L., Williams G. World List of Octocorallia. Umbellula Gray, 1870. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=128499. [2019-02-24T00:00:00+02:00];
- Daly M, Ardelean A, Cha H-R, Campbell AC, Fautin DG. A new species, Adamsia obvolva (Cnidaria: Anthozoa: Actiniaria), from the Gulf of Mexico, and a discussion of the taxonomy of carcinoecium-forming sea anemones. Bulletin of Marine Science. 2004;74:385–399. [Google Scholar]
- Daly Marymegan, Brugler Mercer R., Cartwright Paulyn, Collins Allen G., Dawson Michael N., Fautin Daphne G., France Scott C., Mcfadden Catherine S., Opresko Dennis M., Rodriguez Estefania, Romano Sandra L., Stake Joel L. The Phylum Cnidaria: A Review Of Phylogenetic Patterns And Diversity 300 Years After Linnaeus *. Zenodo. 2007 doi: 10.5281/ZENODO.180149. [DOI]
- Den Hartog J. C., Ocaña O., Brito A. Corallimorpharia collected during the CANCAP expeditions (1976-1986) in the south-eastern part of the North Atlantic*. Zoologische Verhandelingen. 1993;282(1):1–76. [Google Scholar]
- England K. W. Certain Actiniaria (Cnidaria, Anthozoa) from the Red Sea and tropical Indo-Pacific Ocean. Bulletin of the British Museum (Natural History) Zoology. 1987;53:205–205. [Google Scholar]
- Etnoyer P., Morgan L. E. Cold-water corals and ecosystems. Springer; Berlin, Heidelberg: 2005. Habitat-forming deep-sea corals in the Northeast Pacific Ocean.331-343. [DOI] [Google Scholar]
- Etnoyer P. J., Cairns S. D., Sanchez J. A., Reed J. K., Lopez J. V., Schroeder W. W., Brooke S. D., Watling L., Baco-Taylor A. R., Williams G. C., Lindner A., France S. C., Bruckner A. W. Deep-Sea Coral Collection Protocols: a Synthesis of Field Experience from Deep-Sea Coral Researchers, Designed to Build Our National Capacity to Document Deep-Sea Coral Diversity. National Oceanic and Atmospheric Administration,Technical Memorandum NMFS-OPR-28; Silver Spring: 2006. 53 [Google Scholar]
- Fautin Daphne G. Hexacorallians of the World. http://hercules.kgs.ku.edu/Hexacoral/Anemone2/
- Fautin D. G., Daly M. Actiniaria, Corallimorpharia, and Zoanthidea (Cnidaria) of the Gulf of Mexico Gulf of. In: Felder D. L., Camp D. K., editors. Gulf of Mexico–Origins, Waters, and Biota. Biodiversity. A&M University Press, College; Texas: 2009. [Google Scholar]
- Fautin Daphne Gail. Corallimorphus Niwa New Species (Cnidaria: Anthozoa), New Zealand Members Of Corallimorphus, And Redefinition Of Corallimorphidae And Its Members. Zenodo. 2011 doi: 10.5281/ZENODO.202799. [DOI]
- Fautin Daphne Gail. Catalog to families, genera, and species of orders Actiniaria and Corallimorpharia (Cnidaria: Anthozoa). Zootaxa. 2016;4145(1):1–449. doi: 10.11646/zootaxa.4145.1.1. [DOI] [PubMed] [Google Scholar]
- Florez P., Santodomingo N. Cnidaria: Corales escleractinios, antipatarios, anémonas, zoantídeos, octocorales e hidroides. In: INVEMAR (Eds.) Biodiversidad del margen continental del Caribe colombiano. Serie de Publicaciones Especiales, Invemar No. 2010;20:4588. [Google Scholar]
- Gates Andrew. Deep-sea life of Tanzania. National Oceanography Centre; Southampton: 2016. [Google Scholar]
- Gates Andrew R., Benfield Mark C., Booth David J., Fowler Ashley M., Skropeta Danielle, Jones Daniel O. B. Deep-sea observations at hydrocarbon drilling locations: Contributions from the SERPENT Project after 120 field visits. 10.1016/j.dsr2.2016.07.011. Deep Sea Research Part II: Topical Studies in Oceanography. 2017;137:463–479. doi: 10.1016/j.dsr2.2016.07.011. [DOI] [Google Scholar]
- González-Muñoz Ricardo, Simões Nuno, Tello-Musi José Luis, Rodríguez Estefanía. Sea anemones (Cnidaria, Anthozoa, Actiniaria) from coral reefs in the southern Gulf of Mexico. ZooKeys. 2013;341:77–106. doi: 10.3897/zookeys.341.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Muñoz Ricardo, Simões Nuno, Guerra-Castro Edlin J., Hernández-Ortíz Carlos, Carrasquel Gabriela, Mendez Enio, Lira Carlos, Rada Martin, Hernández Iván, Pauls Sheila M., Croquer Aldo, Cruz-Motta Juan J. Sea anemones (Cnidaria: Actiniaria, Corallimorpharia, Ceriantharia, Zoanthidea) from marine shallow-water environments in Venezuela: new records and an updated inventory. 10.1186/s41200-016-0016-7. Marine Biodiversity Records. 2016;9(1) doi: 10.1186/s41200-016-0016-7. [DOI] [Google Scholar]
- Häussermann Verena. Identification and taxonomy of soft-bodied hexacorals exemplified by Chilean sea anemones; including guidelines for sampling, preservation and examination. Journal of the Marine Biological Association of the UK. 2004;84(5):931–936. doi: 10.1017/s0025315404010215h. [DOI] [Google Scholar]
- Horowitz J., Opresko D. M., Bridge T. C.L. Black corals (Anthozoa: Antipatharia) from the deep (916 m–2542 m) Coral Sea, north-eastern Australia. Zootaxa. 2018;4472(2):307. doi: 10.11646/zootaxa.4472.2.5. [DOI] [PubMed] [Google Scholar]
- Hourigan T. F., Etnoyer P. J., Cairns S. D. Introduction to the State of Deep-Sea Coral and Sponge Ecosystems of the United States. The State of Deep-Sea Coral and Sponge Ecosystems of the United States 2017
- Huvenne V. A. I., Robert Katleen, Marsh Leigh, Lo Iacono Claudio, Le Bas Tim, Wynn R. B. Submarine Geomorphology. 2018. ROVs and AUVs. [DOI]
- Kise Hiroki, Reimer James. Unexpected diversity and a new species of Epizoanthus (Anthozoa, Hexacorallia) attached to eunicid worm tubes from the Pacific Ocean. ZooKeys. 2016;562:49–71. doi: 10.3897/zookeys.562.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramp P. L. Synopsis of the Medusae of the World. Journal of the Marine Biological Association of the United Kingdom. 1961;40:7. doi: 10.1017/s0025315400007347. [DOI] [Google Scholar]
- Larson R. J., Mills C. E., Harbison G. R. Western Atlantic midwater hydrozoan and scyphozoan medusae: in situ studies using manned submersibles. Coelenterate Biology: Recent Research on Cnidaria and Ctenophora. 1991 doi: 10.1007/978-94-011-3240-4_45. [DOI]
- Larson R. J., Matsumoto G. I., Madin L. P., Lewis L. M. Deep-sea benthic and benthopelagic medusae, recent observations from submersibles and a remotely operated vehicle. Bulletin of Marine Science. 1992;51:277–277. [Google Scholar]
- Mengerink Kathryn J., Dover Cindy L. Van, Ardron Jeff, Baker Maria, Escobar-Briones Elva, Gjerde Kristina, Koslow J. Anthony, Ramirez-Llodra Eva, Lara-Lopez Ana, Squires Dale, Sutton Tracey, Sweetman Andrew K., Levin Lisa A. A call for deep-ocean stewardship. Science. 2014;344(6185):696–698. doi: 10.1126/science.1251458. [DOI] [PubMed] [Google Scholar]
- Messing C., Walker B. K., Reed J. K. Deep-Water Benthic Habitat Characterization and Cable Impact Assessment for the South Florida Ocean Measurement Facility (SFOMF) Naval Surface Warfare Center, Carderock Division; 2012. [Google Scholar]
- Miranda Lucília S., Mills Claudia E., Hirano Yayoi M., Collins Allen G., Marques Antonio C. A review of the global diversity and natural history of stalked jellyfishes (Cnidaria, Staurozoa) 10.1007/s12526-017-0721-4. Marine Biodiversity. 2018;48(4):1695–1714. doi: 10.1007/s12526-017-0721-4. [DOI] [Google Scholar]
- Molodtsova Tina N., Sanamyan Nadezhda P., Keller Natalya B. Anthozoa from the northern Mid-Atlantic Ridge and Charlie-Gibbs Fracture Zone. 10.1080/17451000701821744. Marine Biology Research. 2008;4:112–130. doi: 10.1080/17451000701821744. [DOI] [Google Scholar]
- Molodtsova T., Opresko D. World List of Antipatharia. Bathypathes Brook, 1889. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=103304. [2019-02-24T00:00:00+02:00];
- Molodtsova T. N. Black corals (Antipatharia: Anthozoa: Cnidaria) of the north-eastern Atlantic Biogeography of the North Atlantic Seamounts. In: Mironov A. N., Gebruk A. V., Southward A. J., editors. Biogeography of the North Atlantic Seamounts. KMK Press, Moscow; 2006. 141 - 151 [Google Scholar]
- Ocaña O., Den Hartog J. C. A catalogue of actiniaria and corallimorpharia from the Canary Islands and from Madeira. Universidade dos Açores; 2002. [Google Scholar]
- Osborn Dawn Alexandra, Silver Mary W., Castro Carmen G., Bros Shannon M., Chavez Francisco P. The habitat of mesopelagic scyphomedusae in Monterey Bay, California. 10.1016/j.dsr.2007.04.015. Deep Sea Research Part I: Oceanographic Research Papers. 2007;54(8):1241–1255. doi: 10.1016/j.dsr.2007.04.015. [DOI] [Google Scholar]
- Pawson D. L., Nizinski M. S., Ames C. L., Pawson D. J. Deep-sea echinoids and holothurians (Echinodermata) near cold seeps and coral communities in the northern Gulf of Mexico. Bulletin of Marine Science. 2015;91(2):167–204. doi: 10.5343/bms.2014.1064. [DOI] [Google Scholar]
- Plouviez Sophie, Jacobson Alixandra, Wu Mengyou, Van Dover Cindy L. Characterization of vent fauna at the Mid-Cayman Spreading Center. 10.1016/j.dsr.2014.11.011. Deep Sea Research Part I: Oceanographic Research Papers. 2015;97:124–133. doi: 10.1016/j.dsr.2014.11.011. [DOI] [Google Scholar]
- Polanco F Andrea, Cedeño-Posso Cristina, Montoya-Cadavid Erika, Borrero-Pérez Giomar Helena, Dorado-Roncancio Edgar Fernando, Garrido Manuel, Cárdenas-Oliva Adibe, Yepes-Gaurisas Daniela, Yepes-Narváez Vanesa, Escarria Eugenia, Mutis Maria, Arteaga-Flórez Catalina, Martínez Bibian, Ayala-Galán Karen, Caldera Sandy, Gutierrez-Salcedo J. M., Santodomingo Nadia, Gracia C. A., Flórez Paola, Benavides-Serrato M., Suárez-Mozo N. Y., Alonso Carvajar David. Twenty years of deep sea research in the Colombian Caribbean. Deep Sea Life. 2017;(10):16–17.
- Reimer J., Sinniger F. World List of Zoantharia. Epizoanthus Gray, 1867. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=100790. [2019-02-24T00:00:00+02:00];
- Riemann-Zürneck K., Iken K. Corallimorphus profundus in shallow Antarctic habitats: bionomics, histology, and systematics (Cnidaria: Hexacorallia) Zoologische Verhandelingen. 2003;345:367–367. [Google Scholar]
- Rodriguez E, López-González PJ. New records of Antarctic and Sub-Antarctic sea anemones (Cnidaria, Anthozoa, Actiniaria and Corallimorpharia) from the Weddell Sea, Antarctic Peninsula, and Scotia Arc. Zootaxa. 2013;3625(1):1. doi: 10.11646/zootaxa.3625.1.1. [DOI] [PubMed] [Google Scholar]
- Santodomingo Nadiezhda, Reyes Javier, Flórez Paola, Chacón-Gómez Isabel Cristina, van Ofwegen Leen P., Hoeksema Bert W. Diversity and distribution of azooxanthellate corals in the Colombian Caribbean. Marine Biodiversity. 2012;43(1):7–22. doi: 10.1007/s12526-012-0131-6. [DOI] [Google Scholar]
- Solem Anne Jieli Louise. Analysis of current ROV Operations in the Norwegian Aquaculture - Reducing Risk in exposed Aquaculture Operations. Norwegian University of Science and Technology 2017
- Technau U., Steele R. E. Evolutionary crossroads in developmental biology: Cnidaria. Development. 2011;138(8):1447–1458. doi: 10.1242/dev.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena Ioseba. Automating ROV Operations in aid of the Oil & Gas Offshore Industry. SeeByte Whitepaper. 2011:1–9.
- Thuesen Erik V. Crossota millsae (Cnidaria: Trachymedusae: Rhopalonematidae), a new species of viviparous hydromedusa from the deep sea off California and Hawaii. Zootaxa. 2003;309(1):1. doi: 10.11646/zootaxa.309.1.1. [DOI] [Google Scholar]
- Tyler P. A., Bronsdon S. K., Young C. M., Rice A. L. Ecology and Gametogenic Biology of the GenusUmbellula (Pennatulacea) in the North Atlantic Ocean. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 1995;80(2):187–199. doi: 10.1002/iroh.19950800207. [DOI] [Google Scholar]
- Valentine Marla M., Benfield Mark C. Characterization of epibenthic and demersal megafauna at Mississippi Canyon 252 shortly after the Deepwater Horizon Oil Spill. Marine Pollution Bulletin. 2013;77:196–209. doi: 10.1016/j.marpolbul.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Williams Gary C. The Global Diversity of Sea Pens (Cnidaria: Octocorallia: Pennatulacea) PLoS ONE. 2011;6(7):e22747. doi: 10.1371/journal.pone.0022747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawada D. G., Thompson P. R., Butcher J. A new towed platform for the unobtrusive surveying of benthic habitats and organisms. Revista de Biología Tropical. 2007;56:51–63. doi: 10.15517/rbt.v56i0.5577. [DOI] [Google Scholar]