Graphical abstract
Method name: Isolation of Cornu aspersum (brown garden snail) cellulase enzyme for saccharification of the cellulose component of waste paper materials
Keywords: Reducing sugars, Enzyme purification, Protein, Waste cellulose, Glucose, Biomass, Renewable resources, Dialysis
Abstract
Garden snails (Cornu aspersum) have been sacrificed by drowning the snails overnight in water. The visceral organs (inside the shell organs) have been separated from the foot as well as the shell and homogenized using tris−HCl buffer, pH 5. The homogenate of visceral organs was dialysed in distilled water at 4 °C for 18 h where after the dialysed material was used to bio-convert the cellulose component of various waste paper materials into fermentable sugars such as glucose. Saccharification of the waste cellulose materials was performed with the extracted snail cellulase during ten consecutive incubation periods of 2 h each. The amount of sugars produced during cellulase action on waste cellulose was determined by the dinitrosalicylic acid (DNS) method. All incubations were performed in triplicate and the percent saccharification of each paper material was determined as a fraction of the paper material exposed to cellulase action.
-
•
Cellulase extracted from brown garden snail
-
•
Saccharification of waste paper using garden snail cellulase
Specifications Table
| Subject Area: | Environmental Science |
| More specific subject area: | Biotechnology and renewable bioresources |
| Method name: | Isolation of Cornu aspersum (brown garden snail) cellulase enzyme for saccharification of the cellulose component of waste paper materials. |
| Name and reference of original method: | Designed and validated in our laboratories. |
| Resource availability: | https://doi.org/10.1007/s13762-018-1934-1 |
Method details
Lignocellulosic biomass is one of the most abundant, low cost and renewable sustainable feedstock available for the production of bio-chemicals due to the rich cellulose content of this biopolymer. Cellulose is a polymerized form of glucose molecules with β-1,4-linkages consisting of composite forms of highly crystalized microfibrils among amorphous matrixes, thus refusing access to hydrolyzing enzymes [1]. Much of the cellulosic waste is often disposed of by biomass burning which is a process that is not environmentally friendly. Cellulolysis can be applied to convert cellulose to glucose which is a multi-utility product for bioenergy and polyesters. The conversion of cellulose to glucose can be done in a much cheaper and biologically favourable process. The best cellulase systems used in cellulolysis are from the microbial system found in the gut of organisms living on cellulosic biomass as their major energy source, for example garden snails [2].
Method
Preparation of solutions
An amount of 0.6 g of tris−HCl buffer (Merck) was weighed and transferred into a 1000 ml volumetric flask and filled with distilled water to prepare a solution with concentration of 0.005 M. The pH of this solution was adjusted to pH 5 using a 32% hydrochloric acid (HCl), (Minema) and a 0.5 M potassium hydroxide (KOH), (Rochelle chemicals). Methanol (10 ml, Scienceworld) was added to the tris−HCl buffer solution to prevent the growth of micro-organisms and stored at 4 °C. The tris−HCl buffer (pH 5) solution was used during all incubation procedures when the various paper materials (filter paper, office paper, Woolworths paper, newspaper, foolscap paper, Pick ń Pay paper, kraft or brown envelope paper) were saccharified with the snail cellulase.
Glucose standard solutions with concentrations of 0.5 mg ml−1, 2 mg ml−1, 4 mg ml−1, 6 mg ml−1 and 8 mg ml−1 were used to construct a standard curve that was used to conclude the concentration of the sugars released by the cellulase action. Protein (albumin) standard solutions of concentrations 5 mg ml−1, 10 mg ml−1, 15 mg ml−1, 20 mg ml−1 and 25 mg ml−1 were to construct a protein standard curve that was used to calculation the amount of protein from the dialysed snail cellulase enzyme.
Garden snail cellulase extraction
Garden snails, represented in Fig. 5 were collected from the garden and sacrificed by drowning them in water for a period of 24 h as represented in Fig. 6. The sacrification of the garden snails was performed without the use of any chemical to prevent chemical interference with the cellulase action on waste paper materials. The shell of the snails as well as the feet were removed. Fig. 8 represents the foot and the visceral organs of the snail. The weighed visceral organs were cut into very small pieces (Fig. 9) and homogenised in tris−HCl buffer with a blender and thereafter stirred for a period of 1 h. The homogenate, represented in figure 10 was transferred into a test tube and centrifuged using Beckman, GP Centrifuge (UK, Marca) 3750RPM 1-98-345369 (figure 7) for 30 min and the collected supernatant transferred into a pre-soaked dialysis tube. A dialysis tube (30 cm × 3 cm) was soaked in water at 4 °C for 4 h before it was filled with the supernatant. The dialysis tube filled with the supernatant was dialysed in distilled water at 4 °C for 18 h where after the protein content of the dialysed solution was determined by the Buiret test method [3]. The dialysed extract of the cellulase enzyme was used to biodegrade the cellulose component of waste paper materials (filter paper, office paper, Woolworths paper, newspaper, foolscap paper, Pick ń Pay paper, kraft or brown envelope paper) into fermentable sugars such as glucose.
Waste paper saccharification with Cornu aspersum cellulase
Rapid population increase, urbanization and industrialization have resulted in the generation of huge amounts of many types of wastes which necessitate proper management [[4], [5], [6], [7], [8]]. The paper materials were prepared as round discs (using a puncher) with a diameter of 6 mm and 20 pieces of each paper material (filter paper, office paper, Woolworths paper, newspaper, foolscap paper, Pick ń Pay paper, kraft or brown envelope paper) was weighed and the mass recorded as represented in Table 1 below.
Table 1.
The mass (mg) of 20 round discs pieces of each paper material.
| Paper | Mass (mg) |
|---|---|
| Filter paper | 31.2 |
| Office paper | 37.2 |
| Foolscap paper | 27.0 |
| News paper | 22.7 |
| Pick ń Pay advertising paper | 21.0 |
| Woolworths advertising paper | 43.6 |
| Kraft (brown envelope) paper | 53.5 |
The weighed pieces of different paper materials were transferred into labelled test tubes filled with 800 μl of tris−HCl buffer (pH 5) and 200 μl of dialysed snail cellulase enzyme. The cellulase-waste paper incubation was performed in triplicates at 50 °C for 2 h.
After incubation the tubes were centrifuged for 15 min using Thermo Scientific SL8 centrifuge (Germany), Rotor 75005701 and the supernatants were transferred into clean test tubes with the concentration of the produced sugars determined by the DNS method [9]. The precipitate of papers collected after centrifugation was kept at 4 °C for the next incubation period with fresh dialysed snail cellulase enzyme. Tris−HCl buffer (pH 5) of 800 μl and fresh dialysed snail cellulase of 200 μl were mixed with the precipitated paper material from the previous incubation, vortexed thoroughly and incubated at 50 °C for another 2 h. Re-incubation of precipitated papers was repeated for 10 consecutive incubation periods with the amount of sugars produced during each incubation period determined by the DNS method. All absorbance readings were taken using the Shimadzu UV Vis 1800 (Kyoto, Japan) spectrophotometer. For the concentration determination, the glucose standards and dialysed snail enzyme were read at 520 nm wavelength whereas the protein standards were read at 545 nm wavelength.
Results and discussion
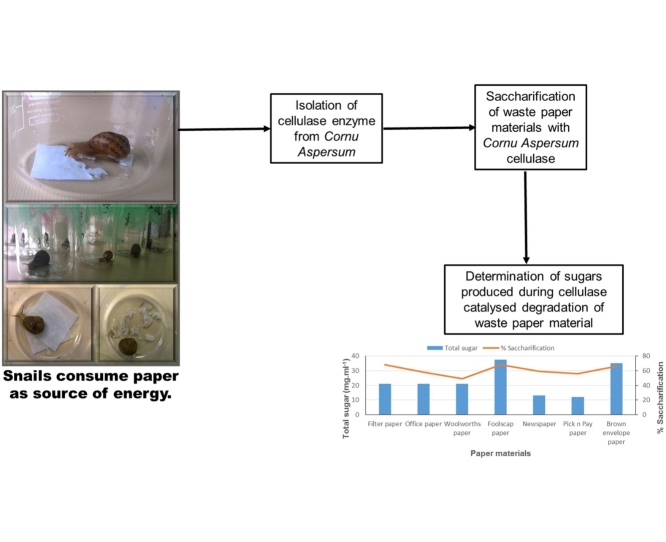
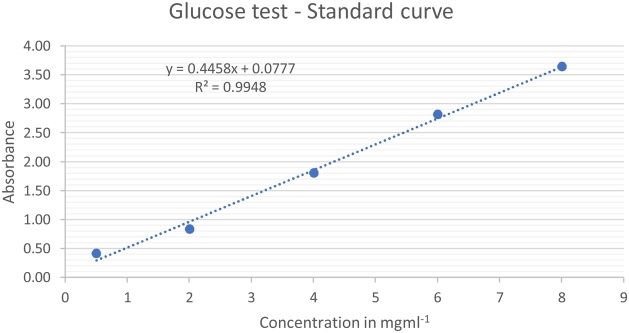
The amount of sugars present in the dialysed snail cellulase enzyme as well as the tris−HCl buffer used as a control, was calculated using the standard curve (Fig. 1) to be 0.23 mg ml−1 and 0.67 mg ml−1 respectively. This was evidence that during the dialysis method, sugars were removed from the snail material.
Fig. 1.
Glucose linear standard curve.
The amount of concentration of the dialysed snail enzyme and tris−HCl buffer is represented in Fig. 2 below.
Fig. 2.
Concentration of the dialysed snail enzyme and tris−HCl buffer in mg ml−1.
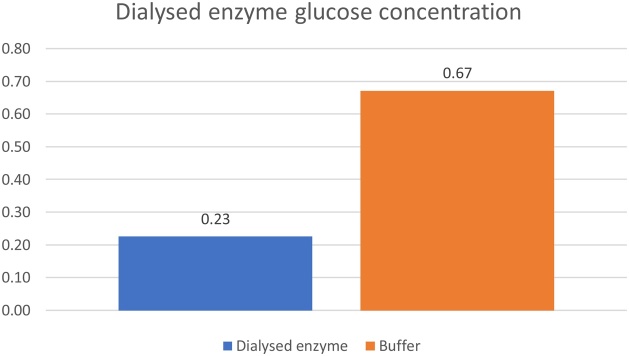
The amount of protein present in the dialysed snail enzyme was calculated using the standard curve (Fig. 3) to be 13.37 mg ml−1. This is the amount of protein retained in the snail material during the dialysis method.
Fig. 3.
Protein linear standard curve.
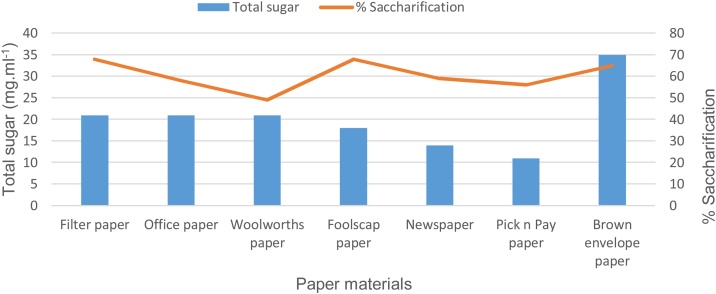
The total amount of sugar released from each paper material during the various successive incubation periods is illustrated in Fig. 4. These results indicate that the highest yield of sugar was produced from foolscap paper at a total concentration of 37 mg ml−1 and at a relative percentage saccharification of 65%. Kraft (brown envelope) paper released the second highest total amount of sugars (35 mg ml−1) and a percentage saccharification 65%. The total amount of sugar released from filter paper, office paper and Woolworths advertising paper were the same at 21 mg ml−1 and thus produced the third highest sugar concentration. The percentage saccharification of these three paper materials are however not identical as filter paper resulted in 68% saccharification followed by office paper at 55% and Woolworths paper at 50%.
Fig. 4.
Total sugar formation and % saccharification of various paper materials during successive treatment with dialysed snail enzyme cellulase.
Conclusion
Although the bioconversion of waste cellulose with garden snail (Cornu aspersum) cellulase produced fermentable sugars such as glucose, the process needs more investigation to make it commercially viable. The current investigation is at an initial stage of the development process and the cost effectiveness of the procedure needs to be analysed. Future prospect of the research will include:
-
1
Full characterization of the cellulase complex.
-
2
Pretreatment of cellulose substrate resulting in the higher susceptibility for the cellulase enzyme.
-
3
Resolve the cellulase complex into components and re-combination of these components in order to increase the extend of saccharification.
Acknowledgement
We would like to thank the department of Pharmacology and Therapeutics at the Sefako Makgatho Health Sciences, South Africa for allowing this research to be performed in the departmental laboratories.
Funding: Department of Higher Education. Research Development Grant. ID: RDG: TM Mamabolo. South Africa.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.mex.2019.04.019.
Contributor Information
T.M. Ndlovu, Email: Thabisile.Ndlovu@smu.ac.za.
J.P.H. Van Wyk, Email: bioenergy.res@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Watanabe H., Tokuda G. Review – animal cellulases. Cell. Mol. Life Sci. 2001;58:1167–1178. doi: 10.1007/PL00000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta P., Samant K., Sahu A. Isolation of cellulose-degrading bacteria and their cellulolytic potential. Int. J. Microbiol. 2011:1–5. doi: 10.1155/2012/578925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janairo G., Lindley S.Y., Yap L., LIanos-Lazaro N., Robles J. Determination of the sensitivity range of biuret test for undergraduate biochemistry experiments. e-J. Sci. Technol. 2011;5(6):77–83. [Google Scholar]
- 4.Najafpoor A.A., Zarei A., Jamali-Behnam F., Vahedian-Shahroudi M., Zarei A. A study identifying causes of construction waste production and applying safety management on construction site. Iran. J. Health Sci. 2014;2(3):49–54. [Google Scholar]
- 5.Qasemi M., Afsharnia M., Zarei A., Najafpoor A.A., Salari S., Shams M. 2018. Phenol Removal From Aqueous Solution Using Citrullus colocynthis Waste Ash.www.elsevier.com/locate/dib [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazloomi S., Zarei A., Alasvand S., Farhadi A., Nourmoradi H., Bonyadi Z. Analysis of quality and quantity of health-care wastes in clinical laboratories: a case study of Ilam city. Environ. Monit. Assess. 2019;191:207. doi: 10.1007/s10661-019-7345-z. [DOI] [PubMed] [Google Scholar]
- 7.Qasemi M., Zarei A., Afsharnia M., Salehi R., Allahdadi M., Farhang M. 2018. Data on Cadmium Removal From Synthetic Aqueous Solution Using Garbage Ash.www.elsevier.com/locate/dib [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alimohammadi M., Yousefi M., Mayvan F.A., Taghavimanesh V., Navaie H., Mohammadif A.A. 2018. Dataset on the Knowledge, Attitude and Practices of Biomedical Wastes Management Among Neyshabur Hospital’s Healthcare Personnel.http://www.elsevier.com/locate/dib [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.