Abstract
Rice false smut (RFS), caused by Villosiclava virens, is an emerging devastating disease of rice panicles worldwide and produces yield loss and mycotoxin residues in rice. In this study, 18 plant essential oils (PEOs) were selected to evaluate antifungal activity via fumigation and contact methods against the mycelial growth and conidial germination of V. virens. The primary compositions of PEOs with stronger fungistatic activity were analyzed using gas chromatography (GC)-mass spectrometry (MS), and the changes in the mycelial morphology were observed using scanning electron microscopy (SEM). Antifungal tests showed that cinnamon bark oil and cinnamon oil had stronger fumigation and contact effects on V. virens than the other oils tested. The primary active composition in both cinnamon bark oil and cinnamon oil was trans-cinnamaldehyde, which exhibited contact activities with EC50 values of 2.13 and 35.9 μg/mL against mycelial growth and conidial germination, respectively. The hyphae surface morphological alterations caused by cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde included shriveling, vacuolation and exfoliation. In conclusion, cinnamon bark oil and cinnamon oil have the potential to prevent and control RFS, and trans-cinnamaldehyde is a promising natural lead compound for new fungicide discoveries to control RFS contamination and mycotoxin residues in rice.
Subject terms: Chemical biology, Natural products
Introduction
Rice false smut (RFS) caused by Villosiclava virens is becoming one of the most economically important grain diseases in China with its incidence growing to 76% due to the expanded planting of hybrid rice cultivars, overuse of nitrogenous fertilizers and an apparent change in climate1–3. RFS can reduce the yield and quality of rice by converting the grains to false smut balls4,5 and produce a variety of mycotoxins, including ustiloxins and ustilaginoidins, which may be deleterious to human and animal health. Ustiloxins inhibit cell microtubule assembly and skeletal formation6,7. In addition, Institute of Cancer Research (ICR) mice showed a variety of lesions of the liver, heart and kidney after eating rice grains and feed contaminated with RFS balls8. Ustilaginoidins are bis-naphtho-γ-pyrone mycotoxins, some of which exhibit teratogenicity against ICR mouse embryo limb buds and midbrain cells and inhibitory activities against adenosine triphosphate synthesis in mitochondria9,10.
Chemical fungicides are currently a major approach to control RFS in China. Several fungicides, including demethylation inhibitors (DMI), such as tebuconazole, prochloraz, and propiconazole, are commonly applied4,11. However, the overuse and high frequency use of chemical pesticides could induce resistance, residue and environmental pollution risk12. Thus, the potential alternative of plant essential oils (PEOs) rather than chemical fungicides has been receiving increasing amounts of attention with the goal of developing green fungicides and techniques to control RFS.
Currently, many types of PEOs with potent and broad-spectrum antifungal activities are considered to be the candidates to guard agri-foods, herbal medicines and wood13–15. Previous studies demonstrated that peppermint oil showed strong antifungal activity on seven genera of fungi16–18 and thyme oil had inhibitory effect on fruit rot, toxigenic and wood-rot fungi, including Colletotrichum, Fusarium, Phytophthora, Botryosphaeria, Aspergillus, Penicillium, Trametes and Laetiporus15,19,20. Many small molecular compounds are the natural primary constituents of PEOs, account for their antifungal activity and have been registered as botanical fungicides, such as carvacrol, allicin, and eugenol14,21.
Some plant extracts have fungitoxic effects on V. virens. Awuah reported that steam distillate from the leaves of Cymbopogon citratus completely inhibited the mycelial growth of V. virens, followed by a hot water extract from the fresh leaves of Ocimum gratissimum with a reduction in mycelial growth of 60%, and hot water extracts from the dry fruits of Monodera myrstica, Xylopia aethiopica and Chromoleana odorata reduced radial growth by 1.9–12.6%22. Jin et al. demonstrated that 30% ethanol extracts of Mentha arvensis at a 10% concentration completely inhibited the germination of chlamdospores and conidiospores, and the inhibition by Euphorbia helioswpia was 14.79% and 7.23, respectively23. However, there is limited information about the control of RFS by PEOs.
Fumigation and contact activity assays are important choices to comprehensively evaluate the antifungal activity of PEOs16,24. PEOs are complex natural mixtures, which contain volatile components and other chemicals at different contents18. The fumigation activity assay is useful to test the antifungal activity of volatile chemicals, and a contact activity experiment can assess the other components with the exception of comparing the antifungal activity with the general fungicides16,25. Through scanning electron microscopic (SEM) observation, the PEOs resulted in the formation of vacuolation, shriveling, collapse and fracture in the pathogenic fungus in both fumigation and contact activity experiments26,27.
Thus, the specific contents of this study are (1) to evaluate the antifungal activity of 18 PEOs using fumigation and contact methods (2) to explore the primary active components of the PEOs with higher fungistatic activity using GC-MS analysis, and (3) to observe the morphological alteration of V. virens hyphae after treatment with PEOs with higher antifungal activity and their primary compounds using SEM.
Results
Antifungal evaluation of 18 PEOs in vitro
Antifungal effect of 18 PEOs on mycelial growth
The fumigation and contact effects of 18 PEOs against the mycelial growth of V. virens are shown in Table 1. In the fumigation activity assay, all the 18 PEOs showed inhibitory effects on the mycelial growth of V. virens at the concentration of 10 µL/L air Acorus tatarinowii rhizome oil, Angelia dahurica oil, cinnamon bark oil, cinnamon oil, Litsea cubeba oil, myrrh oil and thyme oil inhibited the mycelial growth completely, followed by peppermint oil, clove oil, holly oil and Forsythia suspansa oil that inhibited 74.1%, 72.1%, 67.7% and 60.4%, respectively. In contrast, Artemisia argyi oil, camphor oil, Ligusticum wallichii oil, myristica oil, Platycladus orientalis oil and tea seed oil showed less than 50% inhibition against mycelial growth of V. virens.
Table 1.
Antifungal evaluation of 18 PEOs on the mycelial growth and conidial germination of V. virens using two methods.
| Plant essential oil | Inhibition of mycelial growth (%) | Inhibition of conidial germination (%) | ||
|---|---|---|---|---|
| 10 μL/L air Fumigation | 20 μg/mL Contact | 7.5 μL/L air Fumigation | 40 μg/mL Contact | |
| Acorus tatarinowii rhizome oil | 100 ± 0.00a | 3.60 ± 2.41b | 100 ± 0.00a | 0.00 ± 0.00b |
| Angelica dahurica oil | 100 ± 0.00a | 24.5 ± 4.12b | 100 ± 0.00a | 0.00 ± 0.00b |
| Artemisia argyi oil | 21.2 ± 2.58 cd | 5.74 ± 2.45b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Camphor oil | 24.9 ± 2.77 cd | 13.2 ± 2.27b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Cinnamon bark oil | 100 ± 0.00a | 66.1 ± 7.94a | 100 ± 0.0a | 100.00 ± 0.00a |
| Cinnamon oil | 100 ± 0.00a | 71.9 ± 0.875a | 100 ± 0.00a | 100.00 ± 0.00a |
| Clove oil | 72.1 ± 14.0ab | 12.4 ± 0.963b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Dalbergia wood oil | 45.8 ± 2.21bc | 26.9 ± 2.48b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Forsythia suspansa oil | 60.4 ± 1.96b | 13.9 ± 8.34b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Holly oil | 67.7 ± 1.87b | 13.4 ± 1.57b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Ligusticum wallichii oil | 46.6 ± 1.84bc | 32.6 ± 1.35b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Litsea cubeba oil | 100 ± 0.00a | 14.5 ± 7.17b | 100 ± 0.00a | 0.00 ± 0.00b |
| Myristica oil | 13.1 ± 3.30d | 9.74 ± 1.69b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Myrrh oil | 100 ± 0.00a | 17.0 ± 2.12b | 100 ± 0.00a | 0.00 ± 0.00b |
| Peppermint oil | 74.1 ± 3.81ab | 14.5 ± 2.85b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Platycladus orientalis oil | 12.2 ± 2.08d | 16.4 ± 0.808b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Tea seed oil | 4.37 ± 0.833d | 10.5 ± 0.851b | 0.00 ± 0.00c | 0.00 ± 0.00b |
| Thyme oil | 100 ± 0.00a | 19.8 ± 0.450b | 86.5 ± 1.01b | 0.00 ± 0.00b |
Data presented in table are mean ± SE. a–dSignificant differences at P < 0.05 level according to Scheffe’s multiple range test.
Alternatively, the inhibition of mycelial growth was 66.1% and 71.9%, respectively, after cinnamon bark oil and cinnamon oil treatment in the contact activity assay. L. wallichii oil, dalbergia wood oil and A. dahurica oil reduced only 32.6%, 26.9% and 24.5% of the mycelial growth, respectively. The other 13 PEOs showed lower than 20% inhibition.
According to the results of the antifungal evaluation of the 18 PEOs, the EC50 values of the PEOs with higher antifungal activity were estimated using probit analyses. Table 2 showed that both cinnamon bark and cinnamon oils exhibited the strongest antifungal activity by fumigation and contact methods with EC50 values less than 0.5 µL/L air and 4.28 and 4.47 µg/mL, respectively. Myrrh oil and L. cubeba oil showed strong fumigation activity with the EC50 values less than 0.5 µL/L air but no obvious contact activity. In contrast, dalbergia wood oil and L. wallichii oil exhibited moderate contact activity with EC50 values of 24.7 and 35.4 µg/mL, respectively, but not good fumigation activity. A. dahurica oil also showed fumigation activity with an EC50 value of 4.17 µL/L air, and thyme oil exhibited fumigation and contact activity with EC50 values of 20.8 µL/L air and 78.7 µg/mL, respectively.
Table 2.
EC50 value of ten substances tested on.
| Plant essential oils | Fumigation | Contact | |||
|---|---|---|---|---|---|
| Mycelial growth | Germination | Mycelial growth | Germination | ||
| Acorus tatarinowii rhizome oil | EC50 | <0.5 | <0.5 | — | 78.6 |
| χ 2 | — | — | — | 2.50 | |
| Angelica dahurica oil | EC50 | 4.17 | 1.46 | — | 78.9 |
| χ 2 | 2.39 | 1.28 | — | 7.52 | |
| Cinnamon bark oil | EC50 | <0.5 | <0.5 | 4.28 | 33.1 |
| χ 2 | — | — | 2.19 | 9.47 | |
| Cinnamon oil | EC50 | <0.5 | <0.5 | 4.47 | 30.9 |
| χ 2 | — | — | 2.99 | 9.37 | |
| Dalbergia wood oil | EC50 | — | — | 24.7 | — |
| χ 2 | — | — | 0.629 | — | |
| Ligusticum wallichii oil | EC50 | — | — | 35.4 | 234 |
| χ 2 | — | — | 0.215 | 15.5 | |
| Litsea cubeba oil | EC50 | <0.5 | <0.5 | — | — |
| χ 2 | — | — | — | — | |
| Myrrh oil | EC50 | <0.5 | <0.5 | — | 64.2 |
| χ 2 | — | — | — | 3.81 | |
| Thyme oil | EC50 | 20.8 | 6.96 | 78.7 | — |
| χ 2 | 0.220 | 6.78 | 12.8 | — | |
| Trans-cinnamaldehyde | EC50 | <0.5 | <0.5 | 2.13 | 35.9 |
| χ 2 | — | — | 4.19 | 12.4 | |
V. Virens under two conditions. Pearson χ2 statistic with P values indicating the goodness-of-fit for data to the expected probit response mode. “—” indicates that this essential oil has such low or high activity that the EC50 value cannot calculated or χ2 cannot be calculated with this method.
Inhibitory effects of 18 PEOs on conidial germination
Effects of the fumigation and contact of the 18 PEOs on the conidial germination of V. virens are shown in Table 1. In the fumigation activity test, A. dahurica oil, A. tatarinowii rhizome oil, cinnamon bark oil, cinnamon oil L. cubeba oil and myrrh oil exhibited 100% inhibition on conidial germination, and thyme oil inhibited 86.5% of the conidial germination of V. virens at the concentration of 7.5 μL/L air. In the contact activity tests, only cinnamon bark and cinnamon oils completely inhibited conidial germination. However, the other 16 PEOs had no significant inhibitory effect on the conidial germination of V. virens.
The results of the EC50 values of the PEOs with strong antifungal activity against the conidial germination of V. virens are shown in Table 2. Cinnamon bark and cinnamon oils showed the strongest inhibitory effect on the conidial germination in both the fumigation and contact activity assays with EC50 values less than 0.5 µL/L air and 33.1 and 30.9 µg/mL, respectively. L. cubeba oil exhibited strong fumigant activity with EC50 values less than 0.5 µL/L air but not good contact activity. In contrast, L. wallichii oil had moderate contact activity with an EC50 value of 234 µg/mL but little fumigation activity.
The major constituent of PEOs with higher antifungal activities and its contact activity against V. virens
Major constituent of PEOs with higher inhibitory activity
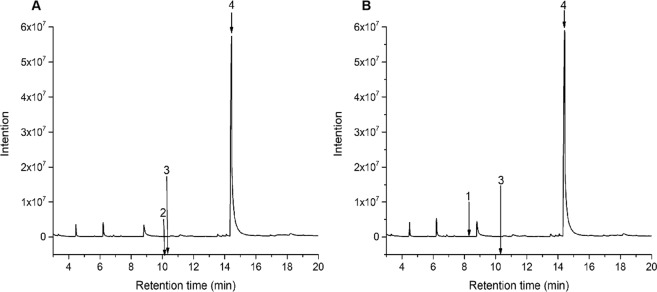
The major compounds of the cinnamon bark and cinnamon oils were analyzed using GC-MS and were listed in Table 3, along with their retention indices, compound names and relative contents. As presented in Fig. 1, the chemical components in cinnamon (Fig. 1A) and cinnamon bark (Fig. 1B) oils were satisfactorily separated under the chromatographic and MS conditions. The natural components identified from the cinnamon bark and cinnamon oils represented 71.47% and 71.46% of the total oils, respectively. The most abundant compound in both the cinnamon bark and cinnamon oils was trans-cinnamaldehyde. Its relative contents were 71.32% and 71.27%, respectively, in the two oils. These results were consistent with previous studies15,28,29. In addition, eucalyptol was found in the two oils, while o-cymene was identified solely in the cinnamon oil, and camphene was detected in the cinnamon bark oil.
Table 3.
Natural major compositions of cinnamon bark oil and cinnamon oil.
| Identified peaks | Retention index | Compound | Relative content (%) | |
|---|---|---|---|---|
| Cinnamon oil | Cinnamon bark oil | |||
| 1 | 943 | Camphene | — | 0.12 |
| 2 | 1042 | o-Cymene | 0.07 | — |
| 3 | 1059 | Eucalyptol | 0.07 | 0.08 |
| 4 | 1189 | Trans-cinnamaldehyde | 71.32 | 71.27 |
| Total | 71.46 | 71.47 | ||
Figure 1.
GC-MS chromatograms of the PEOs from (A) cinnamon oil and (B) cinnamon bark oil.
Antifungal activity of trans-cinnamaldehyde on V. virens
The fumigation and contact activity of trans-cinnamaldehyde on V. virens was shown in Table 2. The EC50 values of trans-cinnamaldehyde against mycelial growth and conidial germination were both less than 0.5 µL/L air in the fumigation activity assay, and 2.13 µg/mL and 35.9 µg/mL in the contact activity assay, respectively.
Mycelial morphology alteration of V. virens
SEM micrographs were used to determine the effect of cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde on the surface morphology of the mycelia after seven days treatment at the concentrations of 0.5 μL/L air and 2 µg/mL by fumigation and contact activity methods, respectively. As shown in Fig. 2, the hyphae exposed to the chosen concentrations of the vapor of the PEOs or grown on toxic media showed degenerative changes in the hyphal morphology in comparison to the thick, elongated, smooth surfaced hyphae in the control plates (Fig. 2A,a). The abnormal phenomena caused by fumigation included: exfoliated flakes (Fig. 2B), applanate (Fig. 2C), shriveling (Fig. 2C,D) and vacuolation (Fig. 2D). Contact effect on the mycelial surface exhibited more serious exfoliated flakes damage (Fig. 2b), membrane injury (Fig. 2c), collapse and blistering (Fig. 2d).
Figure 2.
Scanning electron micrograph of V. virens hyphae. The pictures on the top are treated at 0.5 μL/L air in the fumigation activity assay. (A) Hyphae without PEOs or trans-cinnamaldehyde (control). (B–D) Hyphae treated with cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde. On the bottom are treatments from the contact activity experiment at a concentration of 2 μg/mL. (a) Hyphae treated with acetone (CK). (b–d) Hyphae treated with cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde. All of the magnifications are ×10,000.
From the aspects of the substances tested, the cinnamon bark oil induced the mycelia surface rough and ruptured, but cinnamon oil caused the mycelial shriveling, applanate and membrane injury. As shown in Fig. 2(D,d), trans-cinnamaldehyde aggravated the mycelial abnormalities of collapsing and blistering.
Fungal sporulation difference of V. virens
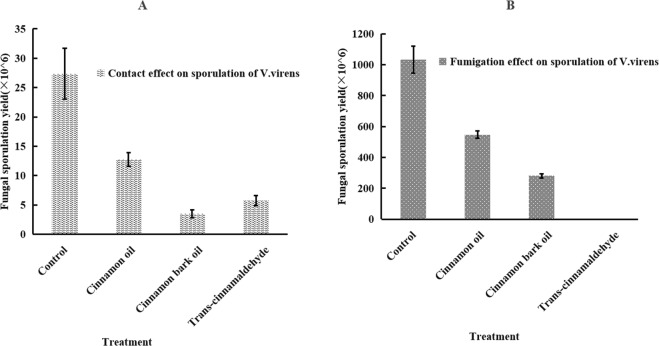
Sporulation of V. virens was significantly affected by the tested substances (cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde) under both fumigation and contact conditions. The results of fungal sporulation difference was shown in Table 4. Compared to the traditional contact method, fumigation method exhibited stronger inhibitory effect on the conidium sporulation. The fumigation treatment of trans-cinnamaldehyde completely inhibited the conidium sporulation of V. virens. Among the three tested substances, trans-cinnamaldehyde had the highest sporulation inhibition in the fumigation treatment method and cinnamon oil had the lowest inhibition under both treatment methods.
Table 4.
Fungal sporulation difference of cinnamon oil, cinnamon bark oil and trans-cinnamaldehyde by two method at 35 µg/mL and 0.5 µL/L air, respectively.
| Treatment | Fungal sporulation (×105/mL) | |
|---|---|---|
| Contact activity 35 µg/mL | Fumigation activity 0.5 µL/L air | |
| Control | 27.3 ± 4.33c | 1033.3 ± 88.2d |
| Cinnamon oil | 12.7 ± 1.19b | 546.67 ± 24.0c |
| Cinnamon bark oil | 3.49 ± 0.65a | 280 ± 15.3b |
| Trans-cinnamaldehyde | 5.73 ± 0.87ab | 0.00 ± 0.00a |
(Values are expressed as the means ± SE of three replicates, P < 0.05).
Discussion
PEOs are one of the important sources of novel fungicides30,31. In this study, we evaluated the fumigation and contact activities of 18 PEOs against V. viren. Among the 18 PEOs, cinnamon bark and cinnamon oils showed the strongest fumigation and contact activities against the mycelial growth and conidial germination of V. virens. In the fumigation assay, 6 PEOs, including A. dahurica oil, A. tatarinowii rhizome oil, cinnamon bark oil, cinnamon oil, L. cubeba oil and myrrh oil completely inhibited the mycelial growth and conidial germination of this pathogen, and the other 12 PEOs had moderate inhibitory effects. In the contact activity test, cinnamon bark and cinnamon oils showed stronger antifungal activity on the mycelial growth and conidial germination than the other 16 PEOs. It is interesting that L. cubeba oil had stronger fumigation activity while L. wallichii oil showed stronger contact activity against mycelial growth and conidial germination of V. virens. Similar researches have been reported that volatile phase effects of oregano, rosemary and lavender essential oils were more effective on fungal growth of P. infestans and B. cinerea than contact phase effect29,32. However, some other PEOs showed the contrary antifungal activity by two methods. For example, thyme, clove and cinnamon proved to be better contact effect than fumigation activity against P. roqueforti, P. corylophilum, Eurotium. repens and A. flavus. The components of PEOs and species of fungi are the main influence factors on the inhibitory effect33. The results of this study indicated that cinnamon bark and cinnamon oils had the strongest antifungal effects on V. virens, with EC50 values of 4.28 μg/mL and 33.1 μg/mL, respectively, against mycelial growth and 4.47 μg/mL and 34.6 μg/mL, respectively, against conidial germination, which were lower than the values generally reported14,32. In previous studies, cinnamon bark oil exhibited strong contact toxicity against toxigenic and foodborne fungi, and cinnamon oil had contact inhibitory effect on wood-rot fungi19,20,34,35. Alternatively, since cinnamon bark and cinnamon oils are both from the C. cassia plant, which is commonly known as cinnamon, consumer acceptance is less of a problem because the US Food and Drug Administration (US FDA) lists cinnamon as a generally recognized as safe substance GRAS), and it is used in the fields of food, Chinese medicine, cosmetic and other materials around the world17,35,36. A comparison of these results revealed that cinnamon bark and cinnamon oils have the potential to prevent and control RFS.
To explore the possible active constituent of cinnamon bark oil and cinnamon oil, the major components of cinnamon bark oil and cinnamon oil were analyzed, and its contact activity against mycelial growth and conidial germination was evaluated. The photographs of cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde with light microscope of V. virens hyphae can be seen in Supplementary Information in Figs S1 and S2. The results revealed that trans-cinnamaldehyde was the most abundant compound in both cinnamon bark and cinnamon oils and had a higher inhibitory effect on the mycelial growth than that of cinnamon bark and cinnamon oils. Similarly, several research papers reported that the fungicidal activity of cinnamon oil on wood-rot fungi and plant pathogenic fungi was attributed to its primary component, trans-cinnamaldehyde14,19. Many studies indicated that cinnmaldehyde could be used as an excellent candidate to control plant diseases caused by Rhizoctonia. solani, F. oxysporum, A. flavus, C. gloeosporioides, A. citrii and B. cinerea14,20,37. The results indicated that trans-cinnamaldehyde could be studied further to develop an eco-friendly and acceptable fungicide to protect rice from RFS and mycotoxin contamination.
The SEM micrographs indicated that the mycelia shrank and became applanate, exuvial and blistery after treatment with cinnamon bark oil and cinnamon oil. Soylu et al. found that Origanum essential oil caused the hyphal morphology of B. cinerea to degenerate, resulting in cytoplasmic coagulation, vacuolations, hyphal shriveling and protoplast leakage16, and Xu et al. also reported that trans-cinnamaldehyde induced the hyphae collapse and fracture of A. alternata27. As shown in the SEM graphs, cinnamon bark oil was more toxic than cinnamon oil, which might be related to the contents of camphene, eucalyptol, and o-cymene, since different chemical compounds stimulated or inhibited the mycelial growth alone or together38. The most serious disruption to the hyphae caused by trans-cinnamaldehyde indicated that it was the primary active compound in the cinnamon bark and cinnamon oils. Different abnormal phenomena might be responsible for the different treatments. As the previous study, fumigation impacts of PEOs on fungal structures might reflect effects of the volatiles emitted by oils on surface mycelial development and/or the perception/transduction of signals involved in the switch from vegetative to reproductive development16. The contact activity of PEOs may be impacted by interactions with the matrix components, which resulted in mycelial injury39. This result further confirmed that cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde inhibited mycelial growth by destroying the surface structure of the mycelia and provided some reference and a basis to further explore the action mechanism of PEOs against V. virens. The specific mechanism on gene expressions and protein activities of cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde against hyphae surface morphological alterations of V. virens remained unclear and needs to be studied.
To evaluate the inhibitory effect on reproductivity of V. virens. The sporulation difference of V. virens was conducted. As shown in the Fig. 3, cinnamon bark oil, cinnamon oil and trans-cinnamaldehyde reduced the fungal sporulation yields significantly compared with the normal sporulation yield (control). Among the three tested substances, cinnamon bark oil possessed strongest contact effect (Fig. 3B) and trans-cinnamaldehyde showed the strongest fumigation effect (Fig. 3A) on the sporulation of V. virens, which is not accordance with the antifungal effect of the tested substances against the conidial germination of V. virens. The reason for different antifungal effect on germination and sporulation haven’t been reported before and the different compounds in both PEOs and the interaction between chemicals and conidium might be the important factors and need further study.
Figure 3.
Histogram of three tested substances on fungal sporulation yield of V. virens by two methods. (A) Is the sporulation yield of V. virens treated with acetone (Control) and cinnamon oil, cinnamon bark oil and trans-cinnamaldehyde at the concentration of 35 µg/mL by contact method, (B) is the sporulation yields of V. virens treated without PEOs (Control) and with cinnamon oil, cinnamon bark oil and trans-cinnamaldehyde at the concentration of 0.5 µL/L air by fumigation method. Data are represented means ± SE of 3 replications.
In summary, cinnamon bark oil and cinnamon oil showed obvious fumigation and contact activity against the mycelial growth, conidial germination and sporulation of V. virens, and their primary active compound was trans-cinnamaldehyde. The SEM observations revealed that the hyphae were shriveled and exfoliated after trans-cinnamaldehyde treatment. The sporulation was significantly decreased after fumigation with trans-cinnamaldehyde. This study demonstrated that cinnamon bark and cinnamon oils possess the potential to be used as a bio-fungicide to prevent and control RFS, and trans-cinnamaldehyde is a very promising natural lead compound for the discovery of high efficacy and low risk fungicides. Up till now it has not been reported that any plant essential oils can be used to prevent and control RFS in the greenhouse or fields. It is an important topic to evaluate the efficay of cinnamon bark oil and cinnamon oil against this disease in fields in further studies.
Materials and Methods
Plant essential oils
In the study, thirteen PEOs were purchased from the Jiangxi Xinsen Natural Vegetable Oil Co. Ltd. (label a). Four PEOs and trans-cinnamaldehyde (98.3%) were obtained from the Jiangxi Cedar Natural Medicinal Oil Co. Ltd. (label b), and one PEO was purchased from the Jiangxi Hengcheng Natural Flavor Oil Co. Ltd. (label c). (Jiangxi, China). Detailed information about the PEOs is listed in Table 5. All the PEOs were stored in sealed vials at 4 °C for further analysis.
Table 5.
Detailed information of 18 PEOs.
| Local name | Plant Latin name | Plant part used | Family | Genus |
|---|---|---|---|---|
| Acorus tatarinowii rhizome oila | Acorus tatarinowii | Root | Araceae | Acorus Linn. |
| Angelica dahurica oila | Angelica dahurica | Stem, leaf and root | Umbelliferae | Angelica Linn |
| Artemisia argyi oila | Artemisia argyi | Leaf | Compositae | Artemisia Linn. |
| Camphor oila | Cinnamonum camphora | Trunk and branch | Lauraceae | Cinnamomum Trew |
| Cinnamon bark oila | Cinnamomum cassia | Bark | Lauraceae | Cinnamomum Trew |
| Cinnamon oila | Cinnamomum cassia | Branch | Lauraceae | Cinnamomum Trew |
| Clove oila | Syringa oblata Lindl | Bud | Oleaceae | Syringa Linn. |
| Dalbergia wood oila | Dalbergia odorifera | Trunk and root | Leguminosae | Dalbergia |
| Forsythia suspansa oila | Forsythia suspensa | Fruit | Oleaceae | Forsythia Vahl |
| Holly oila | Ilex chinensis Sims | Leaf | Aquifoliaceae | Ilex Linn |
| Ligusticum wallichii oila | Ligusticum chuanxiong | Rootstock | Umbelliferae | Ligusticum Linn. |
| Litsea cubeba oilb | Litsea cubeba | Seed | Lauraceae | Lindera |
| Myristica oilb | Myristica ragrans | Aril | Myristicaceae | Myristica Gronov. |
| Myrrh oilb | Opopanax chrionium | Resin | Umbelliferae | Pastinaca Linn. |
| Peppermint oila | Mentha haplocalyx | Whole plant | Lamiaceae | Mentha Linn. |
| Tea seed oila | Camellia japonica L | Fruit | Camelliaceae | Camellia Linn. |
| Thujol oilc | Platycladus orientalis | Branch and leaf | Cupressaceae | Platycladus Spach |
| Thyme oilb | Thymus vulgaris L. | Above ground part | Lamiaceae | Thymus Linn. |
a,b,cMeans the PEOs were purchased from the Jiangxi Xinsen Natural Vegetable Oil Co. Ltd, Jiangxi Cedar Natural Medicinal Oil Co. Ltd and the Jiangxi Hengcheng Natural Flavor Oil Co. Ltd, respectively.
Pathogenic fungi
V. virens (CICC 2710) was bought from the China Center of Industrial Culture Collection (CICC) and cultured on potato dextrose agar medium (PDA, Difco Company) at 4 °C. The pathogen has been identified with PCR by Beijing Majorbio Sanger Bio-pharm Technology Co., Ltd. (See the Supplementary Fig. S3).
Determination of the fumigation and contact activities of 18 PEOs on mycelial growth
The contact activity of the PEOs was determined using the toxic medium method. First, the stock solution was made by adding 0.04 g PEOs to 10 mL acetone. Second, a 100 μL stock solution of individual PEO was immediately added to the 20 mL sterile PDA medium at 45–50 °C and mixed thoroughly, which was designated toxic medium at the concentration of 20 μg/mL. When the EC50 values (the concentration of the tested substance causing a 50% inhibition against the mycelial growth compared with the control) of the PEOs with higher antifungal activity and individual components were determined, appropriate volumes of the stock solution of the PEOs and compounds were adjusted to a series of concentration gradients using acetone. The concentrations tested were 0.250 to 200 μg/mL. The control received the same quantity of acetone mixed with PDA. The 20 mL toxic medium was poured onto three aseptic 6-cm plastic Petri dishes. V. virens was inoculated immediately by plating a 5 mm diameter disc of the fungus cut with a sterile cork borer from the edge of actively growing cultures on PDA plates in the center of each plate. The Petri dishes were incubated in the dark at 28 °C.
The fumigation activity was determined as described by Soylu et al.40 with some modifications. Petri dishes (60 × 12 mm, which offer 28 mL air spaces after the addition of 6 mL PDA medium) were used and inoculated with a 5-mm diameter disc of V. virens in the center. A 0.28 μL aliquot of the PEOs was added to the inner surface of the lid of the Petri dishes, and the final concentration was 10 μL/L air (the EC50 values of PEOs with higher antifungal activity and chemicals were determined by pipetting different amounts of the substances tested (0.5 to 6 μL) into the inner surface of the lid of the Petri dishes). The control group was prepared without oil. The plate was immediately sealed with parafilm to prevent any leakage of the PEOs. Each concentration was tested in triplicate. The 5-mm diameter mycelial disc cut from a 15-day-old colony of V. virens grown on PDA plates and placed upside down in the center of the Petri dishes.
After 7 days of incubation at 28 °C in the dark, the colony growth diameter (mm) was measured using a 150-mm digital caliper. The growth inhibition was calculated using the formula41:
where: DC, DT – average diameter (mm) of the fungal colony of the control and the treatment, respectively. Each test was repeated three times.
Determination of the fumigation and contact activities of 18 PEOs on conidial germination
The fumigation and contact inhibitory activities of the PEOs on conidial germination were conducted as described by Soylu et al.42 with slight modification. A spore suspension (106 conidia/mL) of V. virens was prepared from fermentative potato sucrose broth (PS) as described by Lu et al.43. A suspension of 80 μL was spread onto the surface of the PDA medium or toxic medium as described in 2.3. The treated concentrations of the PEOs were 7.5 μL/L air for the fumigation activity experiment and 40 μg/mL for the contact activity test. Different concentrations of PEOs with higher antifungal activity (0.5 to 7.5 μL/L air in the fumigation activity test and 32 to 260 μg/mL in the contact activity assay) were applied for the determination of EC50 values. The plates were incubated at 28 °C until the conidial germination in the control reached >90% (10–12 h, depending on the rate of germination of V. virens). Conidial germination was defined as the point at which the germ tube length exceeds the short diameter of the spore. The percentage of conidial germination was estimated under a microscope (Olympus BX51, Tokyo, Japan).
Each test was repeated three times. The percent inhibition was calculated as follows:
where Gc and Gt represent the germination rate in control and treated Petri dishes, respectively.
Analysis of the primary natural components of PEOs with higher antifungal activity
The primary natural components of the strongest PEOs were determined using a gas chromatograph Agilent 7890 A interfaced with a QP2010 5975 C mass spectrometer (Shimadzu). An Rtx-5MS capillary column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness) was used. The column temperature was maintained at 40 °C for 3 min and programmed to 200 °C at a rate of 10 °C/min. The carrier gas was helium with a flow rate of 1 mL/min. The MS were collected at 70 eV and a mass range of 30–550 amu (scan time 0.3 s). Qualitative identification of the compounds of the PEOs was based on retention indices relative to n-alkanes and computer matching with the NIST14. LIB (National Institute of Standards and Technology) in combination with published data. Quantitative analysis of the individual natural major component was based on the peak area normalization measurement, and the relative content of each component was calculated using the % peak area.
Mycelial morphology observation of V. virens
Using a clean blade, the square fungal block (5 mm × 5 mm × 2 mm) was cut from the fungal colony incubated in 2.3. The fungal block was dipped into 3.5% glutaraldehyde in 0.1 mol/L phosphate buffer at room temperate for 48 h, and the mycelial block was washed with sterile water four times to rinse off the glutaraldehyde in 30 min. The mycelia were fixed with 1% OsO4 for 2 h. The samples were dehydrated in a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 100%) for a period of 20 min in each series and finally treated with 100% ethanol. The samples were dried in a drying apparatus (Leica EM CPD030, Germany) up to the critical point with CO2. The fixed material was mounted on stubs using double-sided carbon tape and coated with gold/palladium in a sputter coater system in a high-vacuum chamber (Hitachi MC1000, Japan) for 1 min at 6 mA. The samples were examined, and digital images were captured using an SU 8010 SEM at an accelerating voltage of 12 kV.
Fungal sporulation difference of V. virens
The sporulation of V. virens was tested according to the method described by Sun et al.44 with some modification. A spore suspension (107 conidia/mL) of V. virens was prepared from fermentative potato sucrose broth (PS) as described in 4.4. A suspension of 50 μL was spread onto the surface of the PDA medium or toxic medium as described in 2.3. The treated concentrations of the PEOs were 0.5 μL/L air for the fumigation activity experiment and 35 μg/mL for the contact activity test. Plates were sealed with parafilm and incubated at 28 °C and cultured for 10d for sporulation. Fungal spores of each Perti-dish were dislodged with appropriate amount of distilled water and suspended by vigorous mixing for 30 s. The number of spores was determined with a hemacytometer under a microscope (magnification 100×). Each combination of medium and conidial suspension was replicated three times.
Statistical analysis
The data were analyzed by a one-way analysis of variance (ANOVA) using the SPSS program (IBM SPSS Statistics 22.0). The measurement of the difference was determined using Scheffe’s multiple range test. Values of P < 0.05 were considered statistically significant. Assays were performed in triplicate, and the results were expressed as the mean (±SE). The EC50 values were calculated using probit analysis.
Supplementary information
Acknowledgements
This research was supported by National Key Research and Development Program of China for financial supports (2016YFD0200500). We are grateful to Professor Hanwen Ni of China Agricultural University providing assistance in revising the first draft. We thank Beijing Majorbio Sanger Bio-pharm Technology Co., Ltd for identifying the pathogen using PCR. We also thank American Journal Experts (AJE) for English language editing.
Author Contributions
J.G.Z. and H.Y.J. designed the study, J.G.Z. and Z.X.G. performed the experiments, J.G.Z. and T.T.L. analyzed the results, J.G.Z. wrote the manuscript. T.-T.L., L.Z., L.-G.M. and H.-Y.J. edited the manuscript. Y.-N.Z. and L.-G.M. reviewed the manuscript. H.Y.J. supervised the study.
Data Availability
Te datasets generated and analysed during the current study are not publicly available due to possible valorisation of the data but are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43433-x.
References
- 1.Rush MC, Shahjahan AKM, Jones JP, Groth DE. Outbreak of false smut of rice in Louisiana. Plant dis. 2000;84:100. doi: 10.1094/PDIS.2000.84.1.100D. [DOI] [PubMed] [Google Scholar]
- 2.Lu MH, Liu WC, Zhu F. Study on the epidemic regularity and control strategy of rice false smut in recent years. China plant prot. 2018;38:44–47. [Google Scholar]
- 3.Shi NN, et al. Development and application of an allele-specific PCR assay for detecting T409C mutation of cyp51 gene linked with tebuconazole resistance in Villosiclava virens (rice false smut) Can. J. Plant. Pathol. 2017;39:318–324. doi: 10.1080/07060661.2017.1356873. [DOI] [Google Scholar]
- 4.Fan J, et al. Current understanding on Villosiclava virens, a unique flower‐infecting fungus causing rice false smut disease. Mol. Plant. Pathol. 2016;17:1321–1330. doi: 10.1111/mpp.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhargava P, Kumar A, Kumar S, Azad CS. Impact of fungicides and nanoparticles on Ustilaginoidea virens causing false smut disease of rice. J. Pharmacogn. Phytochem. 2018;7:1541–1544. [Google Scholar]
- 6.Koiso Y, et al. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994;47:765–773. doi: 10.7164/antibiotics.47.765. [DOI] [PubMed] [Google Scholar]
- 7.Koiso Y, et al. Isolation and structure of an antimitotic cyclic peptide, ustiloxin F. J. Antibiot. 1998;51:418–422. doi: 10.7164/antibiotics.51.418. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura KI, et al. Lupinosis-Like lesions in mice caused by ustiloxin, produced by Ustilaginoieda virens: A morphological study. Natural toxins. 1994;2:22–28. doi: 10.1002/nt.2620020106. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya T, Sekita S, Koyama K, Natori S, Takahashi A. Effect of chaetochromin A, chaetochromin D and ustilaginoidin A, bis (naphtho‐γ‐pyrone) derivatives, on the mouse embryo limb bud and midbrain cells in culture. Congenital Anomalies. 1987;27:245–250. doi: 10.1111/j.1741-4520.1987.tb00707.x. [DOI] [Google Scholar]
- 10.Sun WB, et al. New ustilaginoidins from rice false smut balls caused by Villosiclava virens and their phytotoxic and cytotoxic activities. J. Agr. Food. Chem. 2017;65:5151–5160. doi: 10.1021/acs.jafc.7b01791. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Frequency distribution of sensitivity of Ustilaginoidea virens to four EBI fungicides, prochloraz, difenoconazole, propiconazole and tebuconazole, and their efficacy in controlling rice false smut in Anhui Province of China. Phytoparasitica. 2013;41:277–284. doi: 10.1007/s12600-013-0288-y. [DOI] [Google Scholar]
- 12.Wang, F. Study on genetic diversity and DMI fungicide resistance molecular mechanism of Villosiclava virens. Huazhong Agricultural University, China (Ph D thesis (in Chinese)) (2015).
- 13.Božik M, et al. Selected essential oil vapours inhibit growth of Aspergillus spp. in oats with improved consumer acceptability. Ind. Crops. Prod. 2017;98:146–152. doi: 10.1016/j.indcrop.2016.11.044. [DOI] [Google Scholar]
- 14.Xie Y, Huang Q, Wang Z, Cao H, Zhang D. Structure -activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops. Prod. 2017;97:388–394. doi: 10.1016/j.indcrop.2016.12.043. [DOI] [Google Scholar]
- 15.Sarkhosh A, et al. In vitro evaluation of eight plant essential oils for controlling Colletotrichum, Botryosphaeria, Fusarium and Phytophthora fruit rots of avocado, mango and papaya. Plant. Protect. Sci. 2018;54:153–162. doi: 10.17221/49/2017-PPS. [DOI] [Google Scholar]
- 16.Soylu EM, Kurt S, Soylu S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food. Microbiol. 2010;143:183–189. doi: 10.1016/j.ijfoodmicro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Baker, B. P. & Grant, J. A. Cinnamon and cinnamon oil profile, https://hdl.handle.net/1813/56117/ (2018).
- 18.Diánez F, et al. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett Appl Microbiol. 2018;67:400–410. doi: 10.1111/lam.13053. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Wang Z, Huang Q, Zhang D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops. Prod. 2017;108:278–285. doi: 10.1016/j.indcrop.2017.06.041. [DOI] [Google Scholar]
- 20.Wang HW, et al. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind Crops. Prod. 2018;120:180–186. doi: 10.1016/j.indcrop.2018.04.053. [DOI] [Google Scholar]
- 21.Ferhout H, Bohatier J, Guillot J, Chalchat JC. Antifungal activity of selected essential oils, cinnamaldehyde and carvacrol against Malassezia furfur and Candida albicans. J. Essen Oil Res. 2011;11:119–129. doi: 10.1080/10412905.1999.9701086. [DOI] [Google Scholar]
- 22.Awuah RT. Fungitoxic effects of extracts from some west African plants. Ann. Appl. Biol. 1989;115:451–453. doi: 10.1111/j.1744-7348.1989.tb06564.x. [DOI] [Google Scholar]
- 23.Jin SX, Qian Q, Dai GH, Xue HW. Inhibition of extracts of Mentha arvensis and Euphorbia helioswpia to Ustilaginoidea virens. J Shanghai Jiaotong uni (Agr sci). 2005;23:92–94. [Google Scholar]
- 24.Puškárová A, Bučková M, Kraková L, Pangallo D, Kozics K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human hel 12469. cells. Sci. Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regnier T, Combrinck S, Veldman W, Plooy WD. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii, and other postharvest pathogens of citrus. Ind. Crops. Prod. 2014;61:151–159. doi: 10.1016/j.indcrop.2014.05.052. [DOI] [Google Scholar]
- 26.Castro JC, et al. Bioactivity of essential oils in the control of Alternaria alternata in dragon fruit (Hylocereus undatus Haw.) Ind. Crops. Prod. 2017;97:101–109. doi: 10.1016/j.indcrop.2016.12.007. [DOI] [Google Scholar]
- 27.Xu. L, Tao N, Yang W, Jing G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crops. Prod. 2018;112:427–433. doi: 10.1016/j.indcrop.2017.12.038. [DOI] [Google Scholar]
- 28.Rojas-Graü MA, et al. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate-apple puree edible films. J. Food. Eng. 2007;81:634–641. doi: 10.1016/j.jfoodeng.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Singh HB, Srivastava M, Singh AB, Srivastava AK. Cinnamon bark oil, a potent fungitoxicant against fungi causing respiratory tract mycoses. Allergy. 2010;50:995–999. doi: 10.1111/j.1398-9995.1995.tb02515.x. [DOI] [PubMed] [Google Scholar]
- 30.Nazzaro F, Fratianni F, Coppola R, Feo VD. Essential oils and antifungal activity. Pharmaceuticals. 2017;10:1–20. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparks TC, Hahn DR, Garizi NV. Natural products, their derivatives, mimics and synthetic equivalents: role in agrochemical discovery. Pest. Manag Sci. 2017;73:700–715. doi: 10.1002/ps.4458. [DOI] [PubMed] [Google Scholar]
- 32.Yilar M, Kadioglu I, Telci I. Chemical composition and antifungal activity of Salvia officinalis L., S. cryptantha (Montbret et Aucher ex Benth.) and S. tomentosa (Mill.) plant essential oils and extracts. Fresen. Environ. Bull. 2018;27:1695–1706. [Google Scholar]
- 33.Suhr KI, Nielsen PV. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J Appl Microbiol. 2003;94:4. doi: 10.1046/j.1365-2672.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- 34.Jham GN, Dhingra OD, Jardim CM, Valente VMM. Identification of the major fungitoxic component of cinnamon bark oil. Trop. Plant. Pathol. 2005;30:404–408. [Google Scholar]
- 35.Brnawi WI, et al. Comparison of cinnamon essential oils from leaf and bark with respect to antimicrobial activity and sensory acceptability in strawberry shake. J. Food. Sci. 2018;83:475–480. doi: 10.1111/1750-3841.14041. [DOI] [PubMed] [Google Scholar]
- 36.Khan A, Safdar M, Khan MMA, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 37.Combrinck S, Regnier T, Kamatou GPP. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crops. Prod. 2011;33:344–349. doi: 10.1016/j.indcrop.2010.11.011. [DOI] [Google Scholar]
- 38.Simas DL, et al. Citrus species essential oils and their components can inhibit or stimulate fungal growth in fruit. Ind. Crops. Prod. 2017;98:108–115. doi: 10.1016/j.indcrop.2017.01.026. [DOI] [Google Scholar]
- 39.Li YJ, et al. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops. Prod. 2016;80:186–193. doi: 10.1016/j.indcrop.2015.11.008. [DOI] [Google Scholar]
- 40.Soylu EM, Soylu S, Kurt S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia. 2006;161:119–128. doi: 10.1007/s11046-005-0206-z. [DOI] [PubMed] [Google Scholar]
- 41.Pandey DK, Tripathi NN, Tripathi RD, Dixit SN. Fungitoxic and phytotoxic properties of the essential oil of Hyptis suaveolens. J. Plant. Dis. Protect. 1982;89:344–349. [Google Scholar]
- 42.Soylu EM, et al. Chemical composition and antifungal activity of the essential oil of Artemisia annua L. against foliar and soil-borne fungal pathogens. J. Plant. Dis. Protect. 2005;112:229–239. [Google Scholar]
- 43.Lu S, et al. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agr. Food Chem. 2015;63:3501–3508. doi: 10.1021/acs.jafc.5b00694. [DOI] [PubMed] [Google Scholar]
- 44.Sun MH, Gao L, Liu XZ, Wang JL. Fungal sporulation in two-stage cultivation. Mycosystema. 2009;28:64–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Te datasets generated and analysed during the current study are not publicly available due to possible valorisation of the data but are available from the corresponding author on reasonable request.