Abstract
Abstract
Three poly(organosiloxanes) (hydromethyl-, dimethyl-, and epoxymethylsiloxane) of different chain lengths and pendant groups and their mixtures of dimethyl (DMC) or diethyl carbonates (DEC) were applied in the modification of fumed silica nanoparticles (FSNs). The resulting modified silicas were studied in depth using 29Si, 1H, and 13C solid-state NMR spectroscopy, elemental analysis, and nitrogen adsorption-desorption (BET) analysis. The obtained results reveal that the type of grafting, grafting density, and structure of the grafted species at the silica surface depend strongly on the length of organosiloxane polymer and on the nature of the “green” additive, DMC or DEC. The spectral changes observed by solid-state NMR spectroscopy suggest that the major products of the reaction of various organosiloxanes and their DMC or DEC mixtures with the surface are D (RR’Si(O0.5)2) and T (RSi(O0.5)3) organosiloxane units. It was found that shorter methylhydro (PMHS) and dimethylsiloxane (PDMS) and their mixtures with DMC or DEC form a denser coverage at the silica surface since SBET diminution is larger and grafting density is higher than the longest epoxymethylsiloxane (CPDMS) used for FSNs modification. Additionally, for FSNs modified with short organosiloxane PMHS/DEC and also medium organosiloxane PDMS/DMC, the dense coverage formation is accompanied by a greater reduction of isolated silanols, as shown by solid-state 29Si NMR spectroscopy, in contrast to reactions with neat organosiloxanes. The surface coverage at FSNs with the longest siloxane (CPDMS) greatly improves with the addition of DMC or DEC. The data on grafting density suggest that molecules in the attached layers of FSNs modified with short PMHS and its mixture of DMC or DEC and medium PDMS and its mixture of DMC form a “vertical” orientation of the grafted methylhydrosiloxane and dimethylsiloxane chains, in contrast to the reaction with PDMS/DEC and epoxide methylsiloxane in the presence of DMC or DEC, which indicates a “horizontal” chain orientation of the grafted methyl and epoxysiloxane molecules. This study highlights the major role of solid-state NMR spectroscopy for comprehensive characterization of solid surfaces.
Graphical abstract
Electronic supplementary material
The online version of this article (10.1186/s11671-019-2982-2) contains supplementary material, which is available to authorized users.
Keywords: Silicones, Dialkyl carbonates, 1H solid-state NMR spectroscopy, 29Si solid-state NMR spectroscopy, 13C solid-state NMR spectroscopy, Surface modification, Fumed nanosilica, Bonding density
Introduction
Hydrophobized fumed silica nanoparticles (FSNs) are of interest from a practical point of view because these materials can be better fillers of nonpolar or weakly polar polymers or more appropriate hydrophobic materials for other practical applications than unmodified hydrophilic silica [1–4]. Functionalization of FSNs can be performed using various traditional types of modifying agents such as alkoxy-, halo-, and aminosilanes and organosilazanes [3–8]. However, due to the high reactivity and moisture sensitivity of the modifying agents, purification is often critical for these hydrolyzable precursors. Organosiloxanes with methyl-terminated groups provide a viable and environmentally benign alternative to the chemical functionalization of oxides, taking into account three aspects of their structure that set it apart from carbon-based polymers: the bond lengths of Si–O and Si–C (1.63 and 1.90 Å) in organosiloxane are longer than the C–C (1.53 Å) bonds of most polymers; the S–O–Si bond angle (143°) is significantly greater than the C–C–C bond angles (109°) in the main chain of carbon-based polymers; and the differences in Pauling electronegativity values between silicon (1.8) and oxygen (3.5) and between silicon (1.8) and carbon (2.5) impart ionic character to both the Si–O backbone bonds (51% ionic) and the Si–C bonds (12% ionic). These three structural differences allow rotational and vibrational degrees of freedom in organosiloxane that are not available to carbon-based polymers and are the basis for unusual and unique properties: high thermal stability; excellent dielectric properties; and resistance to oxygen, water, and UV irradiation and so on [5, 8–11]. Linear organosiloxanes are generally not considered to be reactive with inorganic oxide surfaces, and an enormous research effort has been made over the last 50 years to develop other silicon-containing reagents with reactive functional groups [12]. One of the likely ways to increase the reactivity of a silicone polymer is partial depolymerization of high molecular poly(organosiloxanes), followed by grafting formed oligomers (with terminated alkoxy groups) on silica surfaces. Complete depolymerization of poly(dimethylsiloxanes) can be achieved by treatment of siloxanes with such toxic agents as various amines [13, 14]; thermal degradation (300–400 °C); and treatment with sulfuric acids, thionyl chloride, and mixtures of alkali (NaOH, KOH) or with alcohols (methanol, ethanol) [15–18]. In our previous work, we found that dimethyl carbonate, which is an environmentally friendly reagent [19, 20] that meets all the requirements of green chemistry, promotes partial depolymerization of organosiloxanes, making the resultant oligomers a candidate for surface functionalization [21]. However, no systematic characterization on the surface species of various depolymerized organosiloxanes on silica surface has been performed. Useful but limited information on the bonded species of silylated silica surfaces can be obtained through zeta potential, infrared spectroscopy, scanning, and transmission electron microscopy. One of the problems often met with these methods concern the difficulty in the identification of different OH and Si–O bonds. More specific information can be obtained by high-resolution 13C and 29Si cross-polarization magic-angle spinning NMR (CP-MAS NMR) and 1H MAS NMR. Indeed, only the use of abovementioned method allows a full characterization of the surface species on silylated silica. Some solid-state NMR studies have been already performed on gel and fumed silicas modified with different alkoxysilanes [22–28], mesoporous silica modified with cetyltrimethylammonium bromide [29], and 3-metacryloxypropyltrimethoxysilane (MPS) deposited in various solvents onto porous silica [30].
Therefore, the aim of this work is to study the surface species of various organosiloxanes and their mixtures with dimethyl or diethyl carbonate at a fumed silica surface depending on the polymer chain length of siloxane used as a modifying agent and on the nature of dimethyl or diethyl carbonate applied as an initiator for partial organosiloxane deoligomerization.
Experimental Methods
Chemical Reagents
For preparation of the modified silica surfaces, poly(methylhydrosiloxane) (code name PMHS, linear, –CH3 terminated, viscosity of ca. 3 cSt at 25 °C), poly(dimethylsiloxane) (code name PDMS, linear, –CH3 terminated, viscosity of ca. 100 cSt at 25 °C), and poly[dimethylsiloxane-co-(2-(3,4-epoxycyclohexyl)ethyl)methylsiloxane] (code name CPDMS, linear, –CH3 terminated, viscosity of ca. 3,300 cSt at 25 °C) were purchased from Sigma Aldrich, USA. Commercial, dimethyl carbonate (DMC), diethyl carbonate (DEC), and fumed silica (SiO2, SBET = 278 m2/g) were purchased from Aladdin Reagents, China. The purity of the reagents, as reported by the manufacturers, was ≥ 99.0 %. The reagents were used as received.
Modification of Fumed Silica Surfaces
Organosiloxanes were chosen as non-toxic and environmentally benign modifying reagents with high carbon content. FSNs were applied as a matrix for modification because of the high regularity hydroxyl groups on the surface and good dispersibility. In addition, the main advantage of these FSNs over larger monodisperse particles is the fact that they provide a large surface area and thus high sensitivity for solid-state NMR. The modification of the fumed silica surface was performed with PMHS, PDMS, and CPDMS at 180–200 °C for 2 h with or without addition of DMC or DEC, which does not contribute to the weight of modified silica due to the reaction mechanism in gaseous (nitrogen) dispersion media (i.e., without a solvent). The amount of modifier agent was determined to be 17 wt% of silica weight. The modification process was performed in a glass reactor with a stirrer with a rotational speed of 350–500 rpm. The modifying agent was added by means of aerosol-nozzle spray. The samples were subsequently cooled to room temperature after the synthesis.
Elemental Analysis
The content of grafted organic groups in the synthesized samples was measured a couple of times by a vario MACRO cube analyzer (Elementar, Germany), and average values for carbon content and relative deviations were calculated (Table 1). The anchored layer was oxidized to produce H2O and CO2 during heating of the samples in the oxygen flow at 750 °C.
Table 1.
Carbon content, bonding density, and surface area of grafted neat organosiloxanes and their mixtures with DMC or DEC at the SiO2 surface
| Sample | Carbon content, wt% | Bonding density ([Si(CH3R1O)), groups/nm2 where R1 = CH3, H, CH2CH2C6H9 | SBET, m2/g |
|---|---|---|---|
| SiO2 (A–300) | 0 | 0 | 278 |
| SiO2/PMHS | 2.42 ± 0.08 | 5.5 | 266 |
| SiO2/PMHS+DMC | 2.60 ± 0.04 | 6.0 | 253 |
| SiO2/PMHS+DEC | 2.17 ± 0.11 | 5.0 | 254 |
| SiO2/PDMS | 5.96 ± 1.17 | 7.2 | 220 |
| SiO2/PDMS+DMC | 6.04 ± 0.01 | 7.4 | 167 |
| SiO2/PDMS+DEC | 2.28 ± 0.20 | 2.5 | 235 |
| SiO2/CPDMS | 0.57 ± 0.04 | 0.1 | 274 |
| SiO2/CPDMS+DMC | 1.77 ± 0.01 | 0.4 | 248 |
| SiO2/CPDMS+DEC | 2.98 ± 0.28 | 0.7 | 261 |
The bonding density of the attached layers was calculated using the formula [11, 12]
| 1 |
where Mw is the molecular weight of the grafted group, %C is the carbon weight percentage of the modified silica, S(BET) is the surface area of the original silica (m2/g), and nс is the number of carbon atoms in the grafted group in each silicone used for modification. Equation 1 gives the number of [–Si(RR1)O–] repeat units per 1 nm2 of the surface (ρ) (where R is methyl group (CH3); R1 is hydro (H) or methyl (CH3) or epoxy(cyclohexylethyl) group (CH2CH2C6H9)).
29Si, 1H, and 13C CP/MAS NMR Measurements
Solid-state 1H MAS NMR spectra were recorded on a Bruker Avance 400 III HD spectrometer (Bruker, USA, magnetic field strength of 9.3947 T) at resonance frequency of 79.49 MHz. The powder samples were placed in a pencil-type zirconia rotor of 4.0 mm o.d. The spectra were obtained at a spinning speed of 10 kHz, with a recycle delay of 1 s. The adamantane was used as the reference of 1H chemical shift.
Solid-state 29Si CP/MAS NMR spectra were recorded on a Bruker Avance 400 III HD spectrometer (Bruker, USA, magnetic field strength of 9.3947 T) at resonance frequency of 79.49 MHz for 29Si using the cross-polarization (CP), magic-angle spinning (MAS), and a high-power 1H decoupling. The powder samples were placed in a pencil-type zirconia rotor of 4.0 mm o.d. The spectra were obtained at a spinning speed of 8 kHz (4 μs 90° pulses), a 8-ms CP pulse, and a recycle delay of 4 s. The Si signal of tetramethylsilane (TMS) at 0 ppm was used as the reference of 29Si chemical shift.
Solid-state 13C CP/MAS NMR spectra were recorded on a spectrometer (Bruker, USA, with a magic field strength of 9.3947 T) at a resonance frequency of 100.61 MHz for 13C using the cross-polarization (CP), magic-angle spinning (MAS), and a high-power 1H decoupling. The powder samples were placed in a pencil-type zirconia rotor of 4.0 mm o.d. The spectra were obtained at a spinning speed of 5 kHz (4 μs 90° pulses), a 2-ms CP pulse, and a recycle delay of 4 s. The methylene signal of glycine at 176.03 ppm was used as the reference of 13C chemical shift.
1H Liquid NMR Spectroscopy
1H NMR spectra of each initial organosiloxane (PMHS (Additional file 1: Figure S1), PDMS (Additional file 1: Figure S2), CPDMS (Additional file 1: Figure S3); see Additional file 1) were recorded at 90 MHz with an Anasazi Eft–90 spectrometer (Anasazi Instruments, USA). Each polymer was dissolved in deuterated chloroform CDCl3, and the resulting solution was analyzed by 1H NMR spectroscopy.
BET Measurements
To analyze the surface area (SBET, m2/g) of the silicas, the samples were degassed at 150 °C for 300 min. Low-temperature (77.4 K) nitrogen adsorption–desorption isotherms were recorded using a Micromeritics ASAP 2420 adsorption analyzer (Micromeritics Instrument Corp., USA). The specific surface area (Table 1, SBET) was calculated according to the standard BET method.
Results and Discussion
1H MAS NMR spectrum of neat fumed silica, Fig. 1 a, represents three main contributing peak lines at 1.1, 3.5, and 4.8 ppm. The peak at 1.1 ppm is assigned to isolated silanols at the SiO2 surface. Note that they were not detected directly at their usual position around 1.8 ppm in the spectrum of neat silica, but it is known that protons in isolated silanol groups also produce lines between 0.5 and 1.5 ppm. Similar spectral features were also observed in previous studies [29]. Chemical-shift lines between 3.5–5.0 ppm are assigned to weakly bound, relatively mobile water, and hydrogen bonded silanols (Figs. 1 and 2) [26, 27, 29].
Fig. 1.
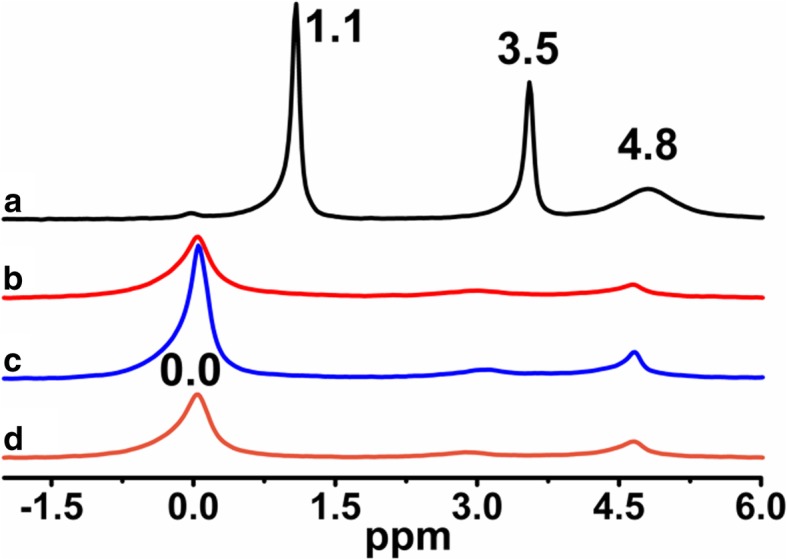
1H MAS NMR spectra of (a) neat fumed silica, (b) modified fumed silica with PMHS, modified with (c) mixtures of PMHS and dimethyl carbonate and (d) mixtures of PMHS and diethyl carbonate
Fig. 2.
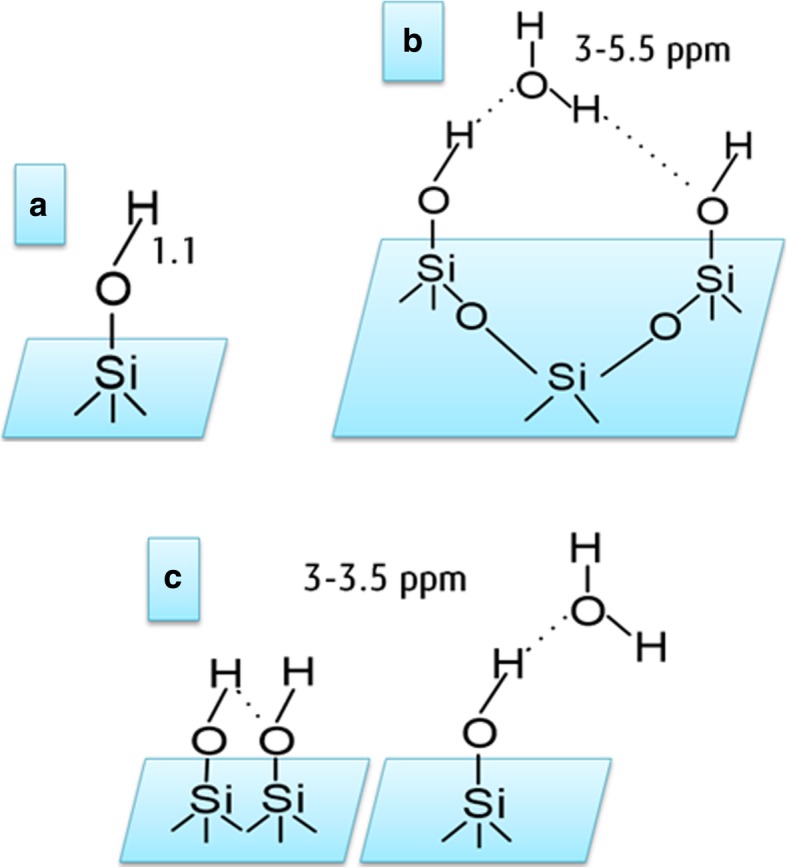
Schematic representation of (a) single silanol groups and (b, c) possible structures involving the silanol groups and physisorbed water at the silica surface. The values of chemical shift are assigned to these structures according to refs [26, 27, 29]
The intense resonance in the 3.5–5.0 ppm range has been studied widely by different researchers. Liu and Maciel [26], for example, by using CRAMPS observed a peak at 4.1 ppm in humidified fumed silica (Cab–O–Sil) which they reported as intermediate between that of liquid water protons (4.9 ppm) and that of the physisorbed water peak (3.5 ppm). According to their studies, a resonance at 3.5 ppm assigned to physisorbed water could easily be desorbed by evacuation at 25 °C. Moreover, evacuation at 100 or 225 °C led to further decrease in the intensity of this resonance, and it was attributed to “rapidly exchanging weakly hydrogen bonded hydroxyls, including those of both water and silanols” [25, 29]. On the other hand, the 1H MAS NMR investigation of silicas by Turov et al. [31, 32] reported the chemical shift of water at around 5 ppm at 25 °C. Several other studies of silicas have also attributed the resonances at 4.5–5.0 ppm to water on strongly hydrated surfaces and chemical shift near 3 ppm to water on significantly dehydrated surfaces, as reported by Turov et al. [32].
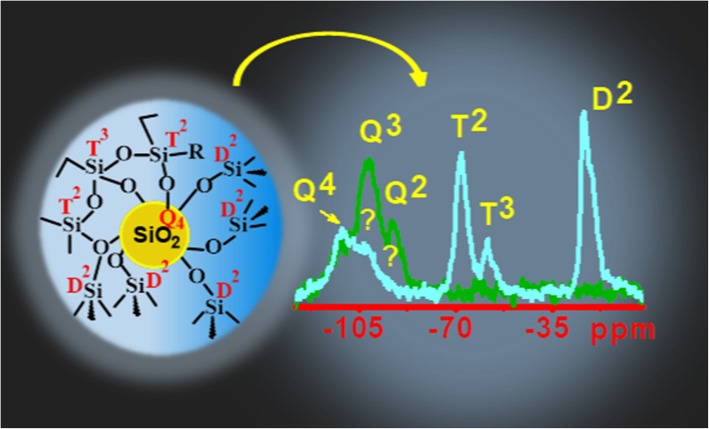
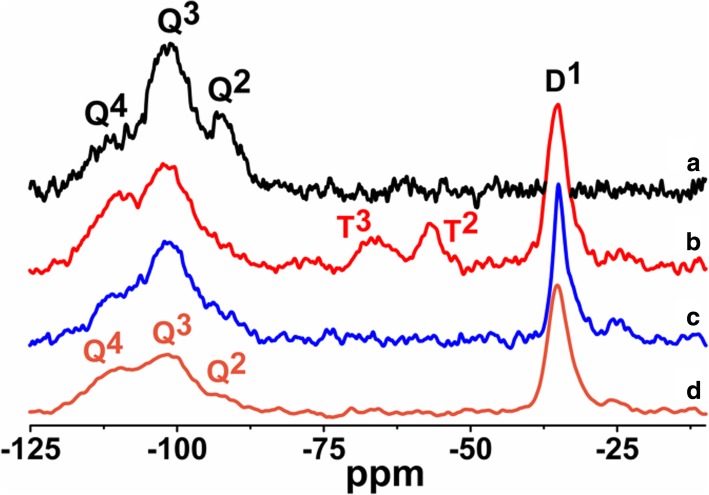
The 1H MAS NMR spectra of modified silicas (Fig. 1) with neat poly(methylhydro)siloxane (curve b) and its mixture of DMC or DEC (curves c and d) were similar to each other and displayed a broad peak (centered at 0.0 ppm) which confirms the grafting of alkyl siloxane species. All the spectra of presented samples show the intensity reduction of adsorbed water and hydrogen bonded silanols (3.5–5.0 ppm) and do not show the isolated silanols (1.1 ppm) presence, confirming that silicas were well modified. The appearance of the peak around 4.7 ppm can be assigned also to the proton in the Si-H group which was attached to the SiO2 surface, as well as alkyls during SiO2 functionalization. The presence of the adsorbed water in the modified samples can be explained by the fact that water molecules are much smaller than the cross-section of organosiloxane. Water can therefore penetrate the narrow nanovoids in the contact zones between adjacent nanoparticles in the aggregates, but polymer macromolecules cannot penetrate these voids. Nevertheless, the 1H MAS NMR spectra of modified silicas provide much less structural information than the 29Si CP/MAS NMR spectra of these composites in which is possible to see unique resonances of different grafted species [33]. The nomenclature used to define siloxane surface species grafted at the silica surface incorporates the use of the letters M, D, T, and Q of the organosiloxane units which represent R3SiO0.5, R2Si(O0.5)2, RSi(O0.5)3, and Si(O0,5)4 units, respectively, where R represents aliphatic and/or aromatic substituents or H [34]. The CP/MAS 29Si NMR spectrum of unreacted silica (Fig. 3 a) shows three signals with resolved peaks at − 91, − 100, and − 109 ppm. These peaks are assigned to silicon atoms in the silanediol groups, silanol groups, and silicon-oxygen tetrahedra of the SiO2 framework, respectively (Fig. 4a–c and Table 2), or, in other words, to silicon-oxygen tetrahedra Q2, Q3, and Q4 where the superscript indicates the number of siloxane bonds [34]. Appropriately, an assignment was made on the basis of the small difference between 29Si chemical shifts in solids and the corresponding shifts in a liquid, and data on soluble silicates were used for identification. The formation of an additional siloxane bond has been found to lead to an upfield signal shift of about 9 ppm [28].
Fig. 3.
29Si CP/MAS NMR spectra of (a) neat fumed silica, (b) modified fumed silica with neat PMHS, modified with (c) mixtures of PMHS and dimethyl carbonate and (d) mixtures of PMHS and diethyl carbonate
Fig. 4.
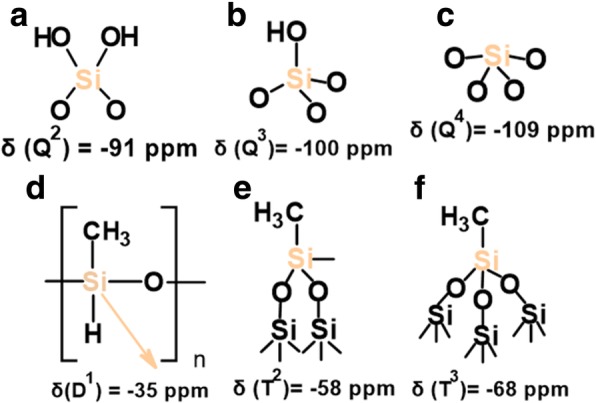
Various grafted PHMS species (a–f)
Table 2.
29Si Chemical shifts (δ) of grafted neat and depolymerizied organosiloxane species at SiO2 surface
| Species | δ, ppm |
|---|---|
| Si(OH)2(O–)2 | − 91 |
| Si(OH)(O–)3 | − 100 |
| Si(O–)4 | − 109 |
| Si(CH3)(H)(O–) | − 35 |
| (≡SiO)2SiR, R-attached polymer chain | − 58 |
| (≡SiO)3SiR, R-attached polymer chain | − 68 |
| Si(CH3)(R)(O–)2 in D4, R = CH3, C2H4C6H8 | − 19.5 |
| Si(CH3)(O–)2 in linear MD4M | − 23 |
| Si(CH3)2(O–)2, D1 | − 21 |
| Si(CH3)(C2H4C6H8), D2 | − 23 |
As can be seen in Fig. 3, after silica surface modification with low viscous poly(methylhydrosiloxane) and DEC (curve d), a significant decrease in the signals Q3 and Q2 is accompanied by an increase in the intensity growth of the signal Q4. Additionally, the signal at − 35 ppm appears, and this can be identified with the methylhydrosiloxane species (D1), (Fig. 4d and Table 2). This implies that a reaction has occurred between the silica surface and the PHMS/DEC mixture.
The surface of SiO2/PMHS+DEC also shows high grafting density of 5.0 groups/nm2 (Table 1) and larger diminution of SBET (Table 1) as compared with SiO2 modified with neat PMHS suggesting closely packed methylhydrosiloxane network. Close values of grafting densities have been reported for the self-assembled monolayers (SAMs) of C18H37SiH3, C18H37SiCl3, and C18H37P(O)(OH)2 on metals and metal oxides [35–37] and C18H37SH on gold [38]. The silicas modified with neat PMHS and its mixture of DMC show a grafting density even slightly higher around 5.5–6.0 groups/nm2 (Table 1). Nevertheless, the appearance of the chemical shifts at − 35, − 58, − 68 ppm of the D1, T2, and T3 units (Fig. 4d–f) for SiO2/PMHS (Fig. 3 b) and only the D1 unit for SiO2/PMHS+DMC (Fig. 3 c) is not accompanied by a significant reduction of the peak which corresponds to free silanols (− 100 ppm, Q3) as is the case for SiO2/PMHS+DEC (Fig. 3 d). The 13C CP/MAS NMR spectra of these modified FSNs (Fig. 5) show one prominent peak at about 43–46 ppm due siloxane alkyl chains grafted at their SiO2 surfaces. The sharp peak in the CP/MAS 13C NMR spectrum of SiO2/PMHS+DMC (Fig. 5 c) indicates well-ordered surface structures at the SiO2 surface. On the contrary, in the CP/MAS 13C NMR spectra of SiO2/PMHS (Fig. 5 a) and SiO2/PMHS+DMC (Fig. 5 b), the signals are relatively broad, indicating a restricted mobility of the functional groups attached to the siloxane framework. Additionally, a higher relative intensity of this signal 43–46 ppm for SiO2/PMHS+DEC may suggest a greater number of attached surface species at the SiO2 surface as compared with SiO2/PMHS and SiO2/PMHS+DMC.
Fig. 5.
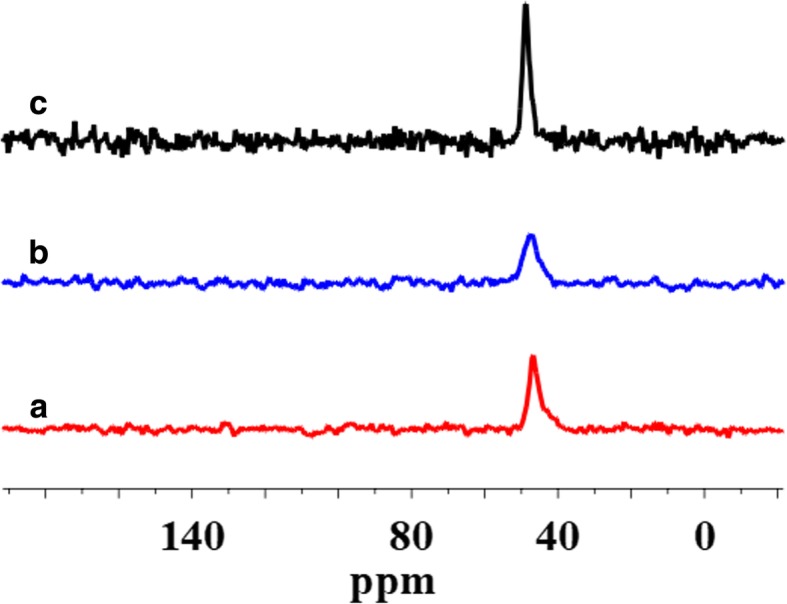
13C CP/MAS NMR spectra of (a) modified fumed silica with neat PMHS, modified with (b) mixtures of PMHS and dimethyl carbonate and (c) mixtures of PMHS and diethyl carbonate
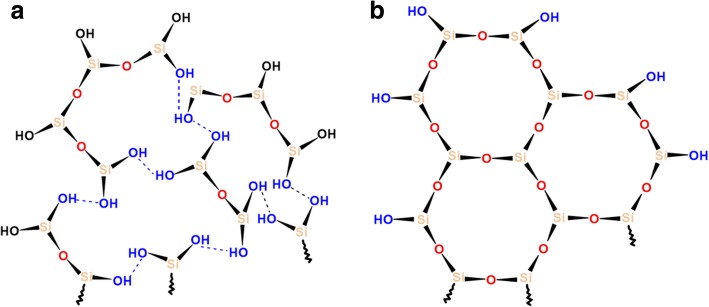
The abovementioned differences of modified silicas could be explained by several factors: (1) a type of organosiloxane bonding (physical or chemical) with SiO2 surface and (2) changes in the length of initial organosiloxane and its fragments after reactions with alkyl carbonate (as shorter polymer fragments will react intensively with silica surface sites due to the lower of steric hindrance of side polymer chains). It is therefore more likely that neat PHMS and the mixture of PHMS/DMC absorb at the SiO2 surface through the formation of adsorption complexes by the binding of hydrogen in the surface silanol group with siloxane oxygen of organosiloxane, while the chemical reaction between the SiO2 surface and PMHS/DEC could be carried out through the formation of chemical bond by the electrophilic substitution of the proton in the silanol group (see Scheme 1 below). The latter explains the significant reduction of free silanols peak at − 100 ppm (Q3) for SiO2/PHMS+DEC (Fig. 3 d). That fact that the resonance of isolated silanols (-100 ppm) is significantly decreased for SiO2/PHMS+DEC in comparison to unreacted SiO2 but does not disappear completely (even with close packing of methylhyrosiloxane groups of 4.0 group/nm2) indicates that some of the OH groups were inaccessible to the modifier reagent. These silanols could be located inside SiO2 nanoparticles. Note that these intra-particle silanols and water molecules can be removed upon heating at 550–700 °C, and only a very small amount of residual silanols remains upon heating even at 1000 °C [11]. The existence of intracrystalline hydroxyl groups is typical for layered silicates [28]. According to Iler [1], their formation is possible in an aerosil structure by the aggregation of SiO2 primary particles with a size of 1–2 nm into a finite globule with a diameter of 10–20 nm. In addition, one cannot rule out the possibility of internal hydroxy group formation in the course of diffusion of the water molecules into the SiO2 globules. On the other hand, unreacted silanols play an important role in the stabilization of alkylsilanes layers at the SiO2 surface as considered by other researchers [11, 35–43]. In the opinion of the authors [11], grafted silane layers form a closely packed monolayer film with an ordered amorphous structure with a significant number of the uncoupled silanols that interact with neighboring Si–OH groups via hydrogen bonding, while the alkyl chains (not shown in Fig. 6) are directed perpendicular to the plane of the siloxane network (Fig. 6a). The presence of uncoupled silanols supports enough space for the presence of alkyl chains grafted at SiO2 after the modification, as the maximal length of the Si–OH·····HO–Si sequence of bonds is ≈ 0.6 nm, which is notably higher than the Van de Waals diameter of the alkyl chains (≈ 0.46 nm). In the case of the absence of the uncoupled silanols, the attached monolayers form a hexagonal array (Fig. 6b) where Si atoms are connected via the siloxane network [39]. However, as was reported by Helmy et al. [11], such a structure is too constrained by steric repulsion between the grafted alkyl chains, as the maximum length of Si–O–Si bond is 0.32 nm, which is very much smaller than the Van der Waals diameter of the alkyl chain (0.46 nm).
Scheme 1.
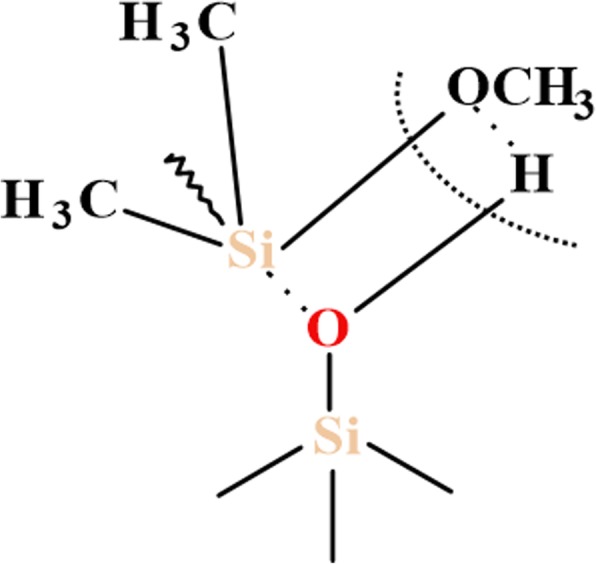
Attack by methoxysilane of silica silanol group
Fig. 6.
The amorphous-like structure (a) consists of the molecules bonded via Si-O-Si and Si-OH·····HO-Si bonds, proposed in [11] and (b) the crystalline-like structure has “extended” Si-O-Si bonds, proposed in [11, 38–41]
29Si CP/MAS NMR spectra of SiO2 modified with organosiloxane of medium chain length (PDMS) and its mixture with DMC or DEC are shown in Fig. 7. The chemical shifts, which appeared at − 19 and − 23 ppm for all the samples (Table 2), are assigned to D1 and D2 units in cyclotetrasiloxane and dimethylsiloxane species in linear MD4M, respectively (Fig. 8 a, b). Notice that the shift of dimethylsiloxane species (− 23 ppm) for silicas modified with PDMS (Fig. 7) is shifted to higher frequency ranges in comparison to SiO2 modified with PMHS (− 35 ppm, Fig. 3), which is explained by the fact that hydrides appear at relatively low frequency compared with their alkyl analogs [34]. The abovementioned resonances result from “capping” of the silica surfaces with modifier agent which is in a good agreement with earlier reports [5]. The sites denoted as T2 and T3, observed around − 58 and − 68 ppm for SiO2/PDMS and SiO2/PDMS+DMC (Fig. 7 b, c) are assigned to (≡SiO)2SiR and (≡SiO)3SiR functionalities (Fig. 8 c, d) where R represents the attached polymer chain. The presence of D as well as T sites for these samples indicates that functionalization of the SiO2 surfaces has occurred. Note that the appearance of the chemical shifts of the D and T units for SiO2 modified with mixture of PDMS/DMC (Fig. 7 c) is accompanied by a significant decrease in the resonances of free and geminal silanols and also SBET value (167 m2/g, Table 1), which may suggest that the reaction of the SiO2 with depolymerized PDMS occurred through the chemical bonding, as for SiO2/PMHS+DEC (Fig. 3 d). The surface also shows the highest grafting density (ρmax) – 7.4 groups/nm2 and the lowest surface area in comparison with other silicas presented in this work (Table 1). The ρmax value obtained for SiO2/PDMS+DMC is similar to those reported for the best quality monolayers derived from chloro- and aminosilanes [35, 44]. The only difference is that the modification of SiO2 with mixture of PDMS/DMC occurs with noncorrosive reagents, thus providing a cleaner and less hazardous environment than amino- and chlorosilanes. The high value of the grafting density for this surface indicates the formation of closely packed grafted organic layers. On the contrary, the resonances of free and geminal silanols are not shown to be greatly diminished in intensity for the surfaces, which were obtained by SiO2 modification with neat PDMS (Fig. 7 b) and its mixture of DEC (Fig. 7 d) but showing grafting density 7.2 and 2.5 groups/nm2. This could be explained by the partial adsorption of the modifier reagent at the SiO2 surface as was mentioned in the previous section. 29Si CP/MAS NMR data are in a good agreement with the BET data (Table 1), as surface areas for both samples are higher compared with SiO2/PDMS+DMC, confirming the smaller degree of chemisorption of the modifier agents at the SiO2 surfaces. In addition, the 13C CP/MAS NMR data (Fig. 9) are in excellent agreement with the data on grafting density (Table 1) and 29Si CP/MAS NMR data, and the relative intensities of the signals which correspond to organosiloxane chains (44–50 ppm, Fig. 9) attached to the SiO2 surface are higher for SiO2/PDMS+DMC (curve b) and SiO2/PDMS (curve a) as compared with SiO2/PDMS+DEC (curve c). All the signals in the 13C CP/MAS NMR spectra (Fig. 9) are relatively sharp, indicating well-ordered surface structures on the silica surface.
Fig. 7.
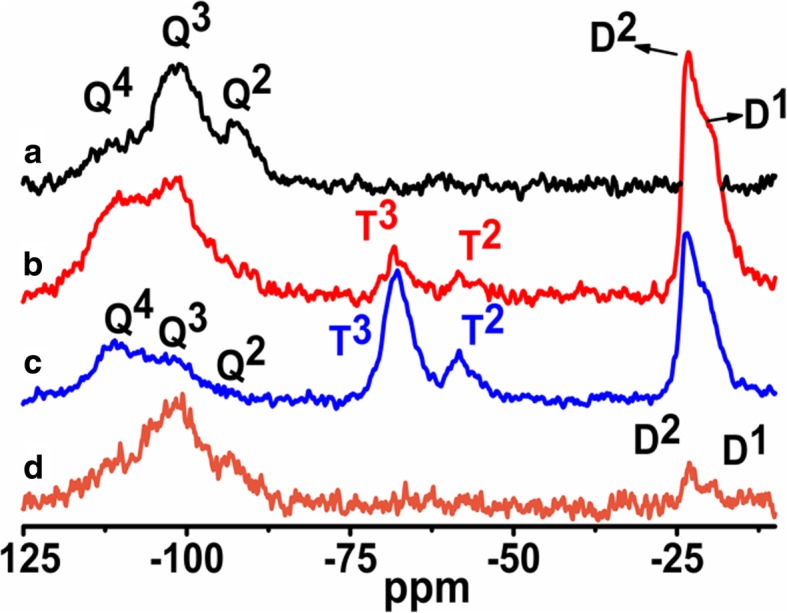
29Si CP/MAS NMR spectra of (a) neat fumed silica, (b) modified fumed silica with PDMS, modified with (c) mixtures of PDMS and dimethyl carbonate and (d) mixtures of PDMS and diethyl carbonate
Fig. 8.
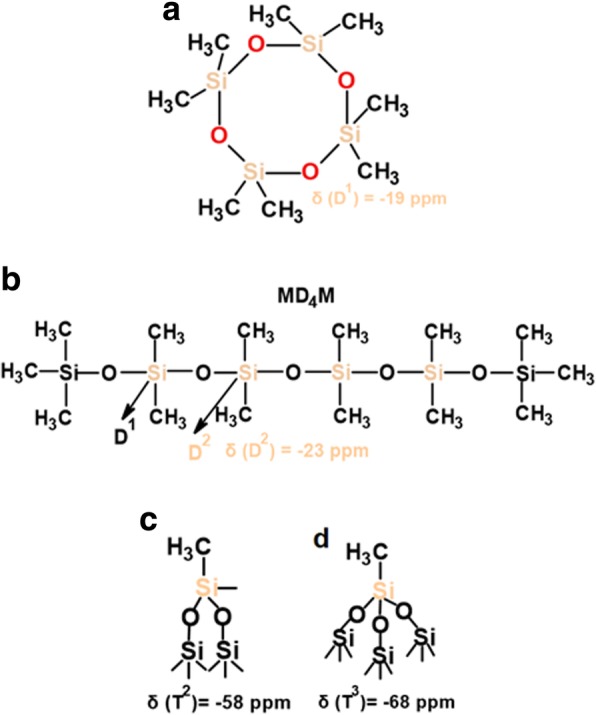
Various grafted PDMS species (a–d)
Fig. 9.
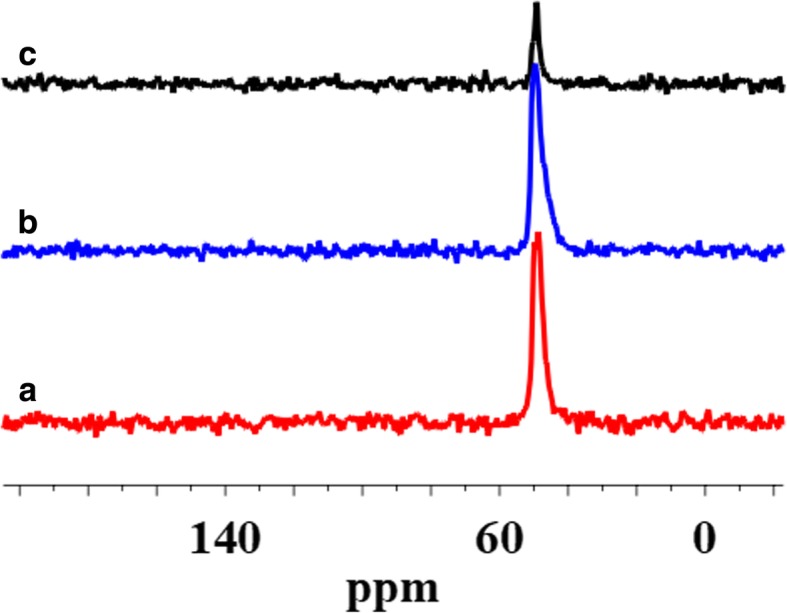
13C CP/MAS NMR spectra of (a) modified fumed silica with neat PDMS, modified with (b) mixtures of PDMS and dimethyl carbonate and (c) mixtures of PDMS and diethyl carbonate
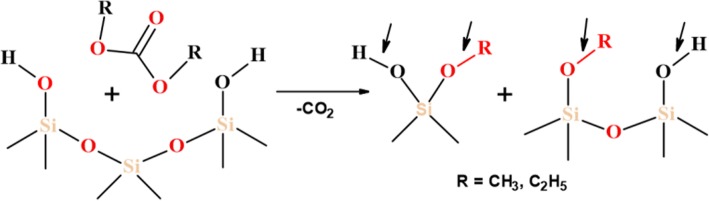
The denser coverage for SiO2/PMHS+DEC (discussed above) and SiO2/PDMS+DMC in comparison to other samples presented here can be explained also by the presence of additional reactive centers at the SiO2 surface, the attached methoxy groups (–OCH3 or OR), which can be formed by the reaction of DMC or DEC with the SiO2 surface (see Scheme 2 below).
Scheme 2.
The reaction of DMC or DEC with the SiO2 surface
1H MAS NMR spectra of FNSs modified with mixtures of PDMS/DMC or PDMS/DEC (Fig. 10 c, d) show the disappearance of the peaks of free and hydrogen-bonded silanols (δ = 1.1 ppm and δ = 4.8 ppm) as well as adsorbed water (δ = 3.5 ppm). The presence of grafted siloxane species is confirmed by the emergence of a chemical shift at 0.0 ppm for all the samples (Fig. 10 b–d). In spite of the methylsiloxane grafting presence for SiO2/PDMS (Fig. 10 b), its surface still contains free, hydrogen-bonded silanols and adsorbed water, which is in very good agreement with 29Si CP/MAS NMR data (Fig. 7 b). The values of the bonding density (Table 1) of the attached layers of FSNs modified with short PMHS and its mixture of DMC or DEC, as well as medium PDMS and its mixture of DMC, suggest a “vertical” orientation of the grafted dimethylsiloxane and methylhydrosiloxane molecules stabilized by lateral Si–O–Si bonds and Van der Waals interactions between the grafted alkyl chains [43–46], while FSNs modified with PDMS/DEC mixture show a grafting density of 1.9 groups/nm2, suggesting a “horizontal” orientation of the grafted dimethylsiloxane molecules [8, 35].
Fig. 10.
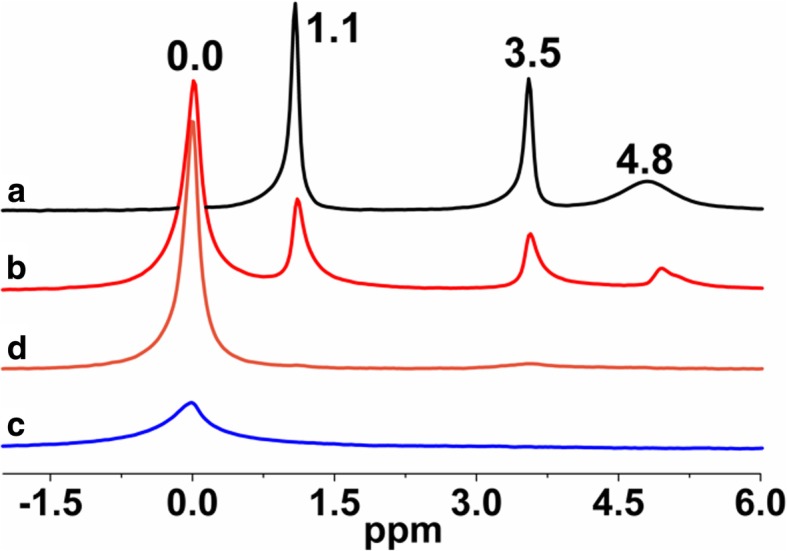
1H MAS NMR spectra of (a) neat fumed silica, (b) modified fumed silica with PDMS, modified with (c) mixtures of PDMS and dimethyl carbonate and (d) mixtures of PDMS and diethyl carbonate
Overall, from the solid-state NMR data obtained, it is evident that the addition of DMC to the modifying mixture has a significant effect on the chemical interaction of organosiloxane of a medium length of polymer chain (PDMS) used for modification at the silica surface, while DEC addition has practically no influence on the chemical interaction of SiO2 with PDMS. In contrast, the DEC has a great effect on the chemical interaction of short organosiloxane (PMHS) used for modification at the SiO2 surface, while DMC has minimal impact on the chemical interaction of SiO2 with PMHS.
As can be seen from the 29Si CP/MAS NMR spectrum of SiO2 modified with the longest polymer, poly[dimethylsiloxane-co-(2-(3,4-epoxycyclohexyl)ethyl)methylsiloxane (CPDMS) (Fig. 11 a), the resonances of grafted methyl-epoxy species around − 23 and − 19 ppm are very hardly detectable, which implies mostly an inert nature of this polymer in relation to the SiO2 surface. 29Si CP/MAS NMR spectra of silicas modified with the longest organosiloxane in the presence of additives—DMC or DEC (Fig. 11 c, d)—represent peaks of grafted siloxane species at − 22, − 21, and − 19 ppm (Table 2) which are assigned to a mixture of D2 and D1 units in linear MD4M siloxane (Fig. 12b) and D1 unit in cyclotetrasiloxane (Fig. 12a), respectively. The grafting density for these surfaces is not as high as for surfaces modified with short (PMHS) and medium siloxane (PDMS) and representing values of 0.4 and 0.7 group/nm2 (Table 1), which are closer to “horizontal” chain orientation at the SiO2 surfaces. However, these values are three to five times higher than SiO2 modified with the neat polymer—CPDMS (ρmax = 0.1 group/nm2, Table 1), and the data is in a good agreement with BET values (Table 1) which are lower for these samples than for SiO2/CPDMS one. A somewhat lower reactivity of neat CPDMS in relation to SiO2 surface could be attributed to the steric hindrance which could be caused by the long polymer chain units and epoxide groups which are present in this polymer, as long-chain organosiloxanes could form a helix structure [46, 47] due to the corresponding rotations around the Si–O bonds, which greatly limits the number of organosiloxane segments which are capable of interacting with active silica sites. On the other hand, in the concentrated solutions of organosiloxane in hexane, for example, the fraction of unfolded molecules increases [46], and this resulted in an increase in the density of contacts between the siloxane molecules and the SiO2 surface OH groups. Taking this into account, the use of an alkyl carbonate is beneficial, as under its influence the organosiloxane might change its structure, which in turn promotes the better accessibility of the formed polymer fragments to the silica surface silanols, and this promotes higher polymer adsorption at the SiO2 surface. The peaks broadening for SiO2/CPDMS+DMC (Fig. 11 c) and SiO2/CPDMS+DEC (Fig. 11 d) are due to a different steric orientation of the closely adjacent methyl and epoxy groups [34].
Fig. 11.
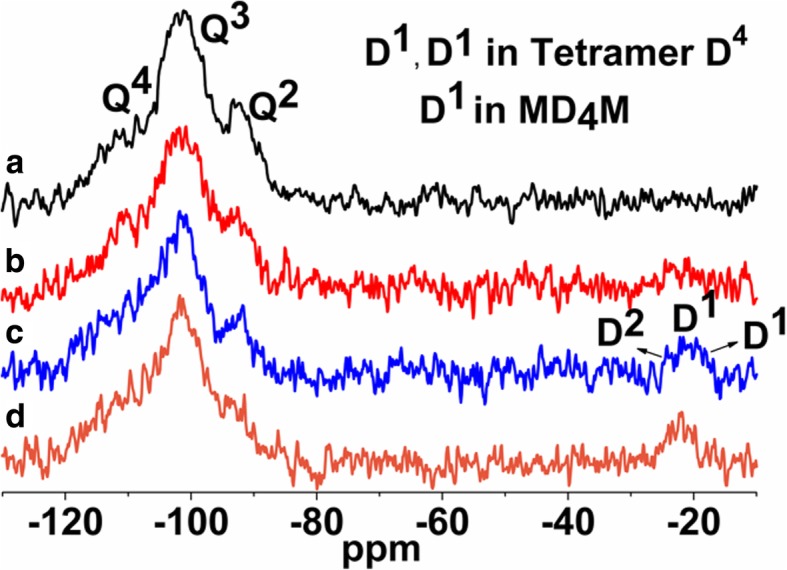
29Si CP/MAS NMR spectra of (a) neat fumed silica, (b) modified fumed silica with CPDMS, modified with (c) mixtures of CPDMS and dimethyl carbonate and (d) mixtures of CPDMS and diethyl carbonate
Fig. 12.
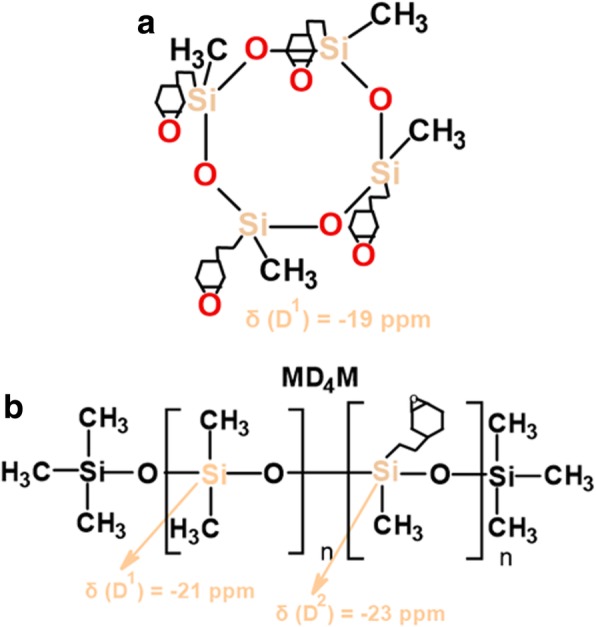
Various grafted CPDMS species (a, b)
According to 1H MAS NMR, the spectra of the silicas which were modified by methyl-epoxy siloxane in the presence of DMC (Fig. 13 c) or DEC (Fig. 13 d) are nearly identical and in excellent agreement with 29Si CP/MAS NMR (Fig. 11 c, d). Grafted methyl-epoxy siloxane on silica surfaces for both samples resulted in shifts of 0.0 ppm and 3.2 ppm. The chemical shift at 3.2 ppm confirms the presence of the characteristic methy/epoxy groups for all the samples. In contrast, the resonance at 0.0 ppm for SiO2, modified with neat CPDMS (Fig. 13 b) is hardly detectable, which in accordance with the grafting density data (Table 1) demonstrates the small amount of long CPDMS units grafted at the SiO2 surface. Additionally, the 13C CP/MAS NMR data (Fig. 14) support this conclusion because only a very faint peak due to the alkyl groups at 44–50 ppm can be observed for the resulting samples. Note that this signal in the 13C CP/MAS NMR spectra of the presenting samples (Fig. 14) is broad, indicating a restricted mobility of the functional groups attached to the siloxane framework as discussed above.
Fig. 13.
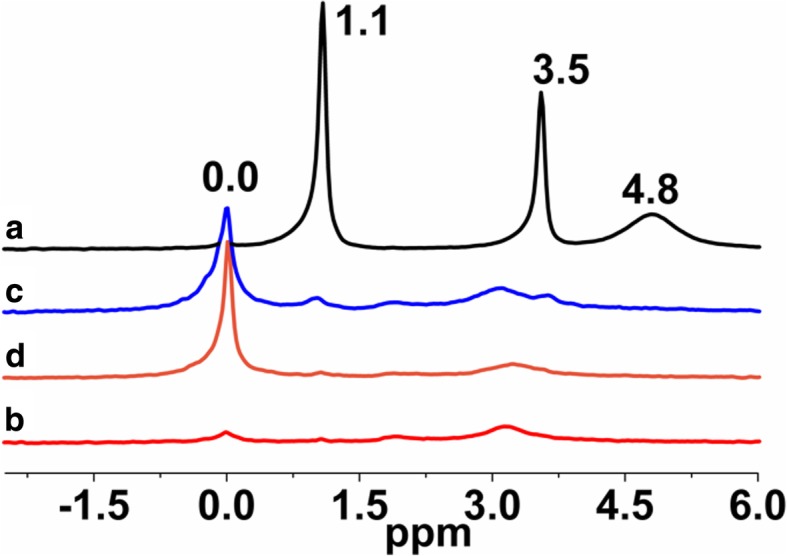
1H MAS NMR spectra of (a) neat fumed silica, (b) modified fumed silica with neat CPDMS, modified with (c) mixtures of CPDMS and dimethyl carbonate and (d) mixtures of CPDMS and diethyl carbonate
Fig. 14.
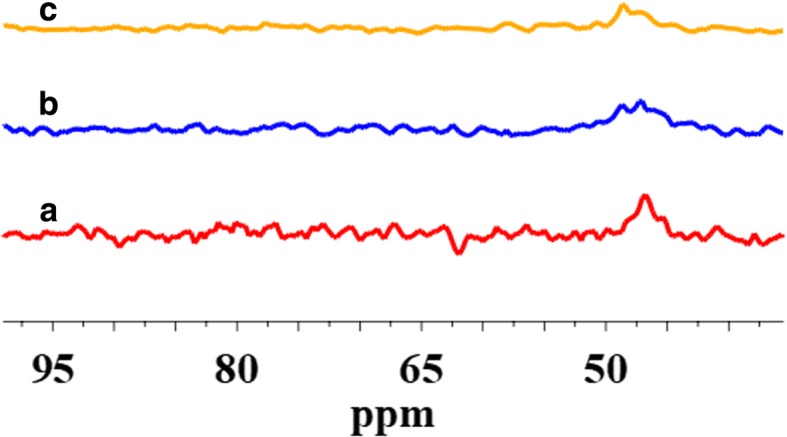
13C CP/MAS NMR spectra of (a) modified fumed silica with neat CPDMS, modified with (b) mixtures of CPDMS and dimethyl carbonate and (c) mixtures of CPDMS and diethyl carbonate
Note that all the presented surfaces generally exhibit the grafting density decrease as the size of the polymer increases, used for surfaces functionalization. Similar results were also presented for silicas functionalized by different bis-fluoroalkyl disiloxanes [12]. This can be due to steric hindrance from the long polymer chains on the macromolecule, as discussed above.
Conclusion
An in-depth solid-state NMR study of FSNs functionalized with organosiloxanes of various lengths of polymer chains and their mixtures of DMC or DEC is presented. For better analysis of the length of polymer chain effects, the organosiloxanes studied here are much longer and with a larger difference in the viscosity as well as pendant groups than the organosiloxanes studied before [12, 35, 48–54]. The obtained results reveal that the structure of the grafted species, type of grafting, and grafting density at the SiO2 surface depend strongly on the length of organosiloxane polymer and on the nature of the “green” additive, DMC or DEC. Spectral changes observed by solid-state NMR spectroscopy suggest that the major products of the reaction of various organosiloxanes and their DMC or DEC mixtures with the FSNs were D (RR’Si(O0.5)2) and T (RSi(O0.5)3) organosiloxane units. The appearance of grafted siloxane units at SiO2/PHMS+DEC and SiO2/PDMS+DMC surfaces is accompanied by a significant reduction of Q3 signals, while for neat organosiloxanes and some of their mixture with alkyl carbonate used for SiO2 modification, a reduction of Q3 is hardly observable. The small amounts of residual silanols (hardly accessible for modifier reagents used) and physisorbed water remain in all the samples of modified silicas (note that the crude silica was not preheated at high temperatures).
Addition of DMC to the modifying mixture facilitates the passage of chemical reaction between medium (PDMS) or long (CPDMS) polymer and the SiO2 surface. Diethyl carbonate addition somewhat worsens the chemical reaction between medium organosiloxane (PDMS) and SiO2 surface but greatly facilitates the reaction when organosiloxanes at short (PMHS) and long polymer chain (CPDMS) are applied for FSNs modification. Thus, from the technological point of view, for FSNs modification with short organosiloxanes, it is reasonable to use DEC; at medium organosiloxane, the application of DMC is necessary; and at long organosiloxane, it is beneficial to use both DMC and DEC.
The data for CP/MAS NMR, BET, and chemical analysis suggest the “vertical” orientation of grafted organosiloxane chains when short and medium polymer or its mixture with DMC (ρ = 7.2–7.4 groups/nm2) are applied for FSNs modification. The reaction of FSNs with medium and long polymer and its mixture with DEC (PDMS/DEC or CPDMS/DEC) leads to the formation of the “horizontal” chains at the surface (ρ = 0.1–2.5 groups/nm2). The findings open new ways for the preparation of similar materials of the same quality using different substrates such as various silicas—silica gels, porous silicas, and precipitated silica. The comparison of the influence of substrate nature on poly(organosiloxane)/alkyl carbonate modification is of undoubted interest for future study.
Additional file
Figure S1. 90 MHz 1H NMR spectrum of neat PMHS. Figure S2. 90 MHz 1H NMR spectrum of neat PDMS; the inset shows the methyl group shifts of parent PDMS. Figure S3. 90 MHz 1H NMR spectrum of neat CPDMS; the inset shows the methyl group shifts of parent CPDMS. (DOCX 1498 kb)
Acknowledgements
Not applicable
Funding
This research was supported by the Special Funding of the ‘Belt and Road’ International Cooperation of Zhejiang Province under grant 2015C04005 and China Postdoctoral Science Foundation grant Z741020001. Partly, this research was supported by the Center for Integrated Nanotechnologies, an Office of the Science User Facility operated for the US Department of Energy (DOE), Office of Science by Los Alamos National Laboratory (Contract DE-AC52-06NA25396), and Sandia National Laboratories (Contract DE-NA-0003525).
Availability of Data and Materials
The datasets supporting the conclusions of this work are included within the article. Any raw data generated and/or analyzed in the present study are available from the corresponding author on request.
Abbreviations
- %C
Carbon weight percentage
- CP/MAS NMR
Cross-polarization magic-angle spinning nuclear magnetic resonance
- CPDMS
Poly[dimethylsiloxane-co-(2-(3,4-epoxycyclohexyl)ethyl)methylsiloxane]
- DEC
Diethyl carbonate
- DMC
Dimethyl carbonate
- FSNs
Fumed silica nanoparticles
- PDMS
Poly(dimethysiloxane)
- PMHS
Poly(methylhydrosiloxane)
- SBET
Surface area
- SiO2
Silica
- δ
Chemical shift
- ρ
Bonding density
Authors’ Contributions
ISP, YMM, IMH, and DZ conceived and designed the experiments; ISP and YMM performed all the experiments; ISP, IMH, and YMM analyzed and interpreted the data; ISP wrote the manuscript; and ZL and WD contributed reagents/materials/analysis tools. All the authors revised and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iryna S. Protsak, Email: ips@zjut.edu.cn
Yevhenii M. Morozov, Email: mem@zjut.edu.cn
Wen Dong, Email: wendong@zjut.edu.cn.
Zichun Le, Email: lzc@zjut.edu.cn.
Dong Zhang, Email: zhanglouis@qq.com.
Ian M. Henderson, Email: imh@omphalosbiosci.com
References
- 1.Iler RK. The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. 1st ed. New York: Wiley; 1979. [Google Scholar]
- 2.Bergna HE, Roberts WO. Colloidal silica: fundamentals and applications. 1st ed. Boca Raton: Taylor and Francis; 2005. [Google Scholar]
- 3.Vansant EF, van der Voort P, Vrancken KC. Characterization and chemical modification of the silica surface. 1st ed. Amsterdam: Elsevier; 1997. [Google Scholar]
- 4.Bluemel J. Reactions of ethoxysilanes with silica: a solid-state NMR study. JACS. 1995;117:2112–2113. doi: 10.1021/ja00112a033. [DOI] [Google Scholar]
- 5.Litvinov VM, Barthel H, Weis J. Structure of a PDMS layer grafted onto a silica surface studied by means of DSC and solid-state NMR. Macromolecules. 2002;35:4356–4364. doi: 10.1021/ma0119124. [DOI] [Google Scholar]
- 6.Park SE, Prasetyanto EA. Morphosynthesis and catalysis by organofunctionalized mesoporous materials. In: Wyman EB, Skief MC, editors. Organosilanes, Properties, Performance, and Applications. UK ed. New York: Nova Science Publishers; 2010. pp. 101–131. [Google Scholar]
- 7.Daoud WA, Xin JH, Tao X. Synthesis and characterization of hydrophobic silica nanocomposites. Appl Surf Sci. 2006;252:5368–5371. doi: 10.1016/j.apsusc.2005.12.020. [DOI] [Google Scholar]
- 8.Bernardoni F, Kouba M, Fadeev AY. Effect of curvature on the packing and ordering of organosilane monolayers supported on solids. Chem Mater. 2008;20:382–387. doi: 10.1021/cm070842y. [DOI] [Google Scholar]
- 9.Chojnowski J, Rubinsztajn S, Fortuniak W, Kurjata J. Synthesis of highly branched alkoxysiloxane−dimethylsiloxane copolymers by nonhydrolytic dehydrocarbon polycondensation catalyzed by tris(pentafluorophenyl)borane. Macromolecules. 2008;41:7352–7358. doi: 10.1021/ma801130y. [DOI] [Google Scholar]
- 10.Gun’ko VM, Pakhlov EM, Goncharuk OV, Andriyko LS, Marynin AI, Ukrainets AI, Charmas B, Skubiszewska-Zięba J, Blitz JP. Influence of hydrophobization of fumed oxides on interactions with polar and nonpolar adsorbates. Appl Surf Sci. 2017;423:855–868. doi: 10.1016/j.apsusc.2017.06.207. [DOI] [Google Scholar]
- 11.Helmy R, Wenslow RW, Fadeev AY. Reaction of organosilicon hydrides with solid surfaces: an example of surface-catalyzed self-assembly. JACS. 2004;126:7595–7600. doi: 10.1021/ja0498336. [DOI] [PubMed] [Google Scholar]
- 12.Graffius G, Bernardoni F, Fadeev AY. Covalent functionalization of silica surface using “inert” poly(dimethylsiloxanes) Langmuir. 2014;30:14797–14807. doi: 10.1021/la5031763. [DOI] [PubMed] [Google Scholar]
- 13.Chang CL, Lee HS, Chen CK. Aminolysis of cured siloxane polymers. Polym Degrad Stab 1999; 65: 1–4. https://doi.org/10.1016/S0141-3910(98)00099-00098
- 14.Hsiao YC, Hill LW, Pappas SP. Reversible amine solubilization of cured siloxane polymers. J Appl Polym Sci. 1975;19:2817–2820. doi: 10.1002/app.1975.070191017. [DOI] [Google Scholar]
- 15.Clarson SJ, Semlyen JA. Studies of cyclic and linear poly(dimethylsiloxanes): 21 high temperature thermal behavior. Polymer. 1986;27:91–95. doi: 10.1016/0032-3861(86)90360-5. [DOI] [Google Scholar]
- 16.Thomas TH, Kendrick TC. Thermal Analysis of Polydimethylsiloxanes. I. Thermal Degradation in Controlled Atmospheres. J Polym Sci B. 1969;7:537–549. [Google Scholar]
- 17.Brook MA, Zhao S, Liu L, Chen Y. Surface etching of silicone elastomers by depolymerization. Can J Chem. 2012;90:153–160. doi: 10.1139/v11-145. [DOI] [Google Scholar]
- 18.Chang CL, Lee HSJ, Chen CK. Nucleophilic cleavage of crosslinked polysiloxanes to cyclic siloxane monomers: mild catalysis by a designed polar solvent system. J Polym Res. 2005;12:433–438. doi: 10.1007/s10965-004-1871-1. [DOI] [Google Scholar]
- 19.Selva M, Fabrisa M, Perosa A. Decarboxylation of dialkyl carbonates to dialkyl ethers over alkali metal-exchanged faujasites. Green Chem. 2011;13:863–872. doi: 10.1039/c0gc00536c. [DOI] [Google Scholar]
- 20.Ono Y. Dimethyl carbonate for environmentally benign reactions. Pure Appl Chem 1996; 68: 367–375. https://doi.org/10.1016/S0920-5861(96)00130-00137
- 21.Protsak I, Henderson IM, Tertykh V, Dong W, Le Z. Cleavage of organosiloxanes with dimethyl carbonate: a mild approach to graft-to-surface modification. Langmuir. 2018;34:9719–9730. doi: 10.1021/acs.langmuir.8b01580. [DOI] [PubMed] [Google Scholar]
- 22.Gun′ko VM, Turov VV. Nuclear magnetic resonance studies of interfacial phenomena. 1st ed. Boca Raton: CRC Press/Taylor & Francis Group; 2013. [Google Scholar]
- 23.Spataro G, Champouret Y, Florian P, Coppel Y, Kahn ML. Multinuclear solid-state NMR study: a powerful tool for understanding the structure of ZnO hybrid nanoparticles. Phys Chem Chem Phys. 2018;20:12413–12421. doi: 10.1039/C8CP01096J. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T, Singappuli-Arachchige D, Slowing II, Pruski M. Spatial distribution of organic functional groups supported on mesoporous silica nanoparticles (2): a study by 1H triple-quantum fast-MAS solid-state NMR. Phys Chem Chem Phys. 2018;20:22203–22209. doi: 10.1039/C8CP04425B. [DOI] [PubMed] [Google Scholar]
- 25.Bronnimann CE, Zeigler RC, Maciel GE. Proton NMR study of dehydration of the silica gel surface. JACS. 1988;110:2023–2026. doi: 10.1021/ja00215a001. [DOI] [Google Scholar]
- 26.Liu CC, Maciel GE. The fumed silica surface: a study by NMR. JACS. 1996;118:5103–5119. doi: 10.1021/ja954120w. [DOI] [Google Scholar]
- 27.Maciel GE, Sindorf DW. Silicon-29 NMR study of the surface of silica gel by cross polarization and magic-angle spinning. JACS. 1980;102:7606–7607. doi: 10.1021/ja00545a056. [DOI] [Google Scholar]
- 28.Brei VV. 29Si solid-state NMR study of the surface structure of aerosil silica. J Chem Soc Faraday Trans. 1994;90:2961–2964. doi: 10.1039/ft9949002961. [DOI] [Google Scholar]
- 29.Trebosc J, Wiench JW, Huh S, Lin VSY, Pruski M. Solid-state NMR study of MCM-41-type mesoporous silica nanoparticles. JACS. 2005;127:3057–3068. doi: 10.1021/ja043567e. [DOI] [PubMed] [Google Scholar]
- 30.De Haan JW, Van Den Bogaert HM, Ponjeé JJ, Van de Ven LJM. Characterization of modified silica powders by Fourier transform infrared spectroscopy and cross-polarization magic angle spinning NMR. J Colloid Interface Sci. 1986;110:591–600. doi: 10.1016/0021-9797(86)90411-X. [DOI] [Google Scholar]
- 31.Turov VV, Chodorowski S, Leboda R, Skubiszewska-Zie J, Brei VV. Thermogravimetric and 1H NMR spectroscopy studies of water on silicalites. Colloids Surf A Physicochem Eng Asp. 1999;158:363–373. doi: 10.1016/S0927-7757(99)00180-6. [DOI] [Google Scholar]
- 32.Turov VV, Leboda R. Application of 1H NMR spectroscopy method for determination of characteristics of thin layers of water adsorbed on the surface of dispersed and porous adsorbents. Adv Colloid Interface Sci. 1999;79:173–211. doi: 10.1016/S0001-8686(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 33.Williams EA. Recent advances in silicon-29 NMR spectroscopy. Annu Rep NMR Spectrosc. 1984;15:235–289. doi: 10.1016/S0066-4103(08)60209-4. [DOI] [Google Scholar]
- 34.Engelhardt G, Jancke H. Structure investigation of organosilicon polymers by silicon-29 NMR. Polym Bull. 1981;5:577–584. doi: 10.1007/BF00255295. [DOI] [Google Scholar]
- 35.Fadeev AY, Kazakevich YV. Covalently attached monolayers of oligo(dimethylsiloxane)s on silica: a siloxane chemistry approach for surface modification. Langmuir. 2002;18:2665–2672. doi: 10.1021/la011491j. [DOI] [Google Scholar]
- 36.Wasserman SR, Whitesides GM, Tidswell IM, Ocko BM, Pershan PS, Axe JD. The structure of self-assembled monolayers of alkylsiloxanes on silicon: a comparison of results from ellipsometry and low-angle x-ray reflectivity. JACS. 1989;111:5852–5861. doi: 10.1021/ja00197a054. [DOI] [Google Scholar]
- 37.Gao W, Dickinson L, Grozinger C, Morin FG, Reven L. Self-assembled monolayers of alkylphosphonic acids on metal oxides. Langmuir. 1996;12:6429–6435. doi: 10.1021/la9607621. [DOI] [Google Scholar]
- 38.Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. JACS. 1989;111:321–335. doi: 10.1021/ja00183a049. [DOI] [Google Scholar]
- 39.Stevens MJ. Thoughts on the structure of alkylsilane monolayers. Langmuir. 1999;15:2773–2778. doi: 10.1021/la981064e. [DOI] [Google Scholar]
- 40.Rye RR. Transition temperatures for n-alkyltrichlorosilane monolayers. Langmuir. 1997;13:2588–2590. doi: 10.1021/la960934u. [DOI] [Google Scholar]
- 41.Kessel CR. Formation and characterization of a highly ordered and well-anchored alkylsilane monolayer on mica by self-assembly. Langmuir. 1991;7:532–538. doi: 10.1021/la00051a020. [DOI] [Google Scholar]
- 42.Parikh AN, Allara DL, Azouz IB, Rondelez F. An intrinsic relationship between molecular structure in self-assembled n-alkylsiloxane monolayers and deposition temperature. J Phys Chem. 1998;31:7577–7590. [Google Scholar]
- 43.Fadeev AY. Hydrophobic monolayer surfaces: synthesis and wettability. In: Somasundaran P, editor. Encyclopedia for Surface and Colloid Science. 2nd ed. New York: Taylor & Francis; 2010. p. 2854. [Google Scholar]
- 44.Fadeev AY, McCarthy TJ. Self-assembly is not the only reaction possible between alkyltrichlorosilanes and surfaces: monomolecular and oligomeric covalently attached layers of dichloro- and trichloroalkylsilanes on silicon. Langmuir. 2000;16:7268–7274. doi: 10.1021/la000471z. [DOI] [Google Scholar]
- 45.Brzoska JB, Azouz IB, Rondelez F. Silanization of solid substrates: a step toward reproducibility. Langmuir. 1994;10:4367–4373. doi: 10.1021/la00023a072. [DOI] [Google Scholar]
- 46.Lipatov YS, Sergeeva LM, Kondor R, Slutzkin D. Adsorption of polymers. 1. New York: Wiley; 1974. [Google Scholar]
- 47.Gun’ko VM, Borysenko MV, Pissis P, Spanoudaki A, Shinyashiki N, Sulim IY, Kulik TV, Palyanytsy BB. Polydimethylsiloxane at the interfaces of fumed silica and zirconia/fumed silica. Appl Surf Sci. 2007;253:7143–7156. doi: 10.1016/j.apsusc.2007.02.185. [DOI] [Google Scholar]
- 48.Krumpfer JW, Fadeev AY. Displacement reactions of covalently attached organosilicon monolayers on Si. Langmuir. 2006;22:8271–8272. doi: 10.1021/la060969m. [DOI] [PubMed] [Google Scholar]
- 49.Kazakevich YV, Fadeev AY. Adsorption characterization of oligo(dimethylsiloxane)-modified silicas: an example of highly hydrophobic surfaces with non-aliphatic architecture. Langmuir. 2002;18:3117–3122. doi: 10.1021/la011490r. [DOI] [Google Scholar]
- 50.Li YF, Xia YX, Xu DP, Li GL. Surface reaction of particulate silica with polydimethylsiloxanes. J Polym Sci. 1981;19:3069–3079. [Google Scholar]
- 51.Guba GY, Bogillo VI, Chuiko AA. Kinetics and mechanism of the reaction of organosiloxanes with the surface of pyrogenic silica. Theor Exp Chem. 1993;28:146–150. doi: 10.1007/BF00573927. [DOI] [Google Scholar]
- 52.Barthel H, Nikitina E. INS and IR study of intermolecular interactions at the fumed silica-polydimethylsiloxane interphase, Part 3. Silica-siloxane adsorption complexes. Silicon Chem. 2004;1:261–279. doi: 10.1023/B:SILC.0000018353.32350.c9. [DOI] [Google Scholar]
- 53.Smith JS, Borodin O, Smith GD, Kober EM. A molecular dynamics simulation and quantum chemistry study of poly(dimethylsiloxane)-silica nanoparticle interactions. J Polym Sci B. 2007;45:1599–1615. doi: 10.1002/polb.21119. [DOI] [Google Scholar]
- 54.Sulym IY, Borysenko MV, Goncharuk OV, Terpilowski K, Sternik D, Chibowski E, Gun’ko VM. Structural and hydrophobic–hydrophilic properties of nanosilica/zirconia alone and with adsorbed PDMS. Appl Surf Sci. 2011;258:270–277. doi: 10.1016/j.apsusc.2011.08.045. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 90 MHz 1H NMR spectrum of neat PMHS. Figure S2. 90 MHz 1H NMR spectrum of neat PDMS; the inset shows the methyl group shifts of parent PDMS. Figure S3. 90 MHz 1H NMR spectrum of neat CPDMS; the inset shows the methyl group shifts of parent CPDMS. (DOCX 1498 kb)
Data Availability Statement
The datasets supporting the conclusions of this work are included within the article. Any raw data generated and/or analyzed in the present study are available from the corresponding author on request.