Highlights
-
•
External beam radiotherapy provides good symptom control for esophageal cancer.
-
•
A higher dose schedule is related to a longer time to second intervention.
-
•
Life expectancy is valuable in selecting the optimal palliative treatment schedule.
Keywords: Palliation, External beam radiotherapy, Esophageal cancer, Effectiveness, Second intervention, Survival
Abstract
Background and purpose
Although external beam radiotherapy (EBRT) is frequently used for palliative treatment of patients with incurable esophageal cancer, the optimal schedule for symptom control is unknown. This retrospective study evaluated three EBRT schedules for symptom control and investigated possible prognostic factors associated with second intervention and overall survival (OS).
Material and methods
Patients with esophageal cancer treated with EBRT with palliative intent between January 2009 and December 2015 were evaluated. Univariate and multivariate Cox regression models estimated the effect of treatment schedule (20 Gy in 5 fractions, 30 Gy in 10 fractions or 39 Gy in 13 fractions) on OS. To study the effect of prognostic factors on time to second intervention (repeat EBRT, intraluminal brachytherapy or stent placement) a competing risk model with death as competing event was used.
Results
205 patients received 20 Gy (31%), 30 Gy (38%) or 39 Gy (32%). Improvement of symptoms was observed in 72% with no differences between schedules. Median OS after 20 Gy, 30 Gy and 39 Gy was 4.6 months (95%CI 2.6–6.6), 5.2 months (95%CI 3.7–6.7) and 9.7 months (95%CI 6.9–12.5), respectively. Poor performance status (HR 2.25 (95%CI 1.53–3.29)), recurrent esophageal cancer (HR 1.69 (95%CI 1.15–2.47)) and distant metastasis (HR 1.73 (95%CI 1.27–2.35)) were significantly related to worse OS. Treatment with 30 Gy and 39 Gy was related to longer time to second intervention compared to 20 Gy (adjusted cause specific HR 0.50 (95%CI 0.25–0.99) and 0.27 (95%CI 0.13–0.56), respectively).
Conclusions
Palliative EBRT provides good symptom control in patients with symptomatic esophageal cancer. A higher dose schedule was related to a longer time to second intervention. Hence, selection based on life expectancy is vital to prevent unnecessary long treatment schedules in patients with expected short survival, and limit the chance of second intervention when life expectancy is longer.
1. Introduction
Esophageal cancer is the eighth most common cancer worldwide. Every year 400.000 people die from this disease, making it the sixth most common cause of death in the world [1]. In the Netherlands, the overall 5-year survival is approximately 19%, with most patients presenting at an advanced stage [2]. For these patients, palliative treatment often is the only option. Dysphagia is an important symptom, found in >70% of patients with esophageal cancer [3]. Since swallowing and eating problems have a large impact on the quality of life of patients, the most important aim of palliation is to relieve dysphagia symptoms.
Treatment options used for the palliation of dysphagia include local interventions such as stent placement [4], intraluminal brachytherapy (ILBT) [4], [5], [6], external beam radiotherapy (EBRT) [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], and systemic treatment with chemotherapy, or even, combined chemoradiation [18], [19]. A randomised trial [4] compared stent placement with ILBT for the treatment of incurable esophageal cancer. Stent placement gives more rapid improvement of dysphagia, but with shorter duration than ILBT, and is advised when life expectancy is less than three months. When long-term relief of dysphagia is desirable because of a longer life expectancy, ILBT or EBRT is more suitable [20]. In the Netherlands, palliative treatment of incurable esophageal cancer varies widely. Opstelten et al. reported that choice for treatment is not only related to patient and disease characteristics, but to hospital of diagnosis as well, suggesting a lack of therapeutic guidance [21]. Despite the evidence supporting ILBT [5], EBRT is generally more often applied [21].
Only a few studies have examined the effect of EBRT alone, using a great variety of radiotherapy schedules [7], [9], [13], [14], [15], [16]. Consequently, the optimal EBRT schedule for symptom control is still unknown. The aim of this retrospective study was to evaluate three EBRT schedules for symptom control and to investigate possible prognostic factors associated with second intervention and overall survival (OS).
2. Material and methods
2.1. Patient selection
We conducted a retrospective analysis of consecutive patients with cancer of the esophagus treated with palliative intent at the department of Radiotherapy of the Leiden University Medical Center in Leiden, the Netherlands. Patients were included when they received their first radiotherapy treatment with palliative intent between January 2009 and December 2015. Medical files were screened for data collection. The study protocol was approved by the Medical Ethical Committee of the Leiden University Medical Center, the Netherlands. Due to the retrospective aspect of the study, Dutch regulations do not require to obtain informed consent.
2.2. Data collection
Recorded pretreatment variables were age; sex; Karnofsky performance status (KPS) (if not registered, estimated according to the description of performance status within the patient chart or recorded as unknown if no interpretation was possible); comorbidity (according to the Adult Comorbidity Evaluation-27 [22]); dysphagia score (as stated by Knyrim [23]); weight loss; pain; hematemesis; other symptoms; primary or recurrent presentation; need for tube feeding; tumour location (according to the AJCC Cancer Staging Manual [24]); tumour length (if the tumour length on endoscopy was not reported, the tumour length on CT scan was used instead); histological type; TNM stage (according to the 7th edition [25], based on available imaging, such as endoscopy, ultrasound of the neck, CT scan and/or a whole body PET-CT scan); and indication(s) for palliative treatment (irresectable tumour, metastasis, performance status, patient preference or other). For both weight loss and tumour length, patients were divided into two categories based on the median. Follow-up was generally done at three to six weeks after treatment and continued according to the individual needs of the patient. Measured outcomes were clinical response, second intervention (repeat EBRT, ILBT and/or stent), acute toxicity and OS. Clinical response was defined as subjective improvement of symptoms, such as relief of pain, reduction of esophageal bleeding or improvement of dysphagia. The clinical response was interpreted from the medical files by the first author and, in case of doubt, reviewed by the last author. Time to second intervention was calculated from start of treatment to start of second intervention. Acute toxicity was defined as adverse effects reported in the patient chart during and within 6 weeks after treatment and was graded using the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [26]. OS was calculated from start of radiotherapy to death from any cause or last confirmed date of survival. When a patient was lost to follow-up, the municipal personal records database and general practitioner were consulted to obtain information about survival status.
2.3. External beam radiotherapy
Treatment schedules of 20 Gy in 5 fractions, 30 Gy in 10 fractions or 39 Gy in 13 fractions were used. In the departmental protocol, choice for any of the treatment schedules was based on expected survival, performance score, metastatic status, and patient preference. Treatment schedule was suggested by the responsible physician and approved by the department at daily team meetings. Patients were treated with one fraction a day, 4 days a week. Patients receiving 24 Gy in 6 fractions were included in the 20 Gy group, since we don't expect it to have a major impact on the outcomes in accordance with literature on palliative radiotherapy for bone metastases ([27], [28]).
A CT scan was performed for planning of the EBRT. The gross tumour volume (GTV) consisted of the tumour and nearby pathological lymph nodes. The clinical target volume (CTV) was formed by the GTV with a 1.0 cm margin in all directions, excluding other organs. For the planning target volume (PTV), the CTV was expanded with a 1.0 cm margin in all directions. A 2 or 4-field planning was used.
2.4. Statistical analysis
Recorded variables were reported with descriptive statistics. Chi-square test was used to test for significant differences between categorical baseline variables between groups based on the different treatment schedules.
OS was estimated with Kaplan-Meier’s methodology, using log-rank to test for significant difference between the treatment schedules. A Cox regression model was used to study the association between prognostic factors and OS. We selected possible prognostic factors based on clinical relevance and previous literature [17], [29], [30].
A competing risk model with death as competing event was employed to estimate the cumulative incidence of second intervention. To estimate the effect of prognostic factors on time to second intervention, a multivariate regression Cox model was fitted. Results are reported as cause specific hazard ratio along with 95% confidence interval [31].
Statistical analysis was performed using IBM SPSS software version 23, with a level of significance p value <0.05, unless otherwise mentioned. All analysis concerning the competing risk model were performed in R environment with the mstate library [32].
3. Results
218 patients were treated with radiotherapy with palliative intent between January 2009 and December 2015. Nine patients were excluded from analysis due to receiving ILBT (N = 4), ILBT in combination with EBRT (N = 2), or a deviant palliative EBRT schedule (two patients with a single dose of 6 or 8 Gy and one patient with 30 Gy in 10 fractions followed by a boost of 15 Gy in 5 fractions). Four patients were excluded because a stent was placed before starting with EBRT. The remaining 205 patients were divided into three groups according to their intended EBRT schedule; 20 Gy in 5 fractions for 63 patients (of whom 4 patients were treated with 24 Gy in 6 fractions) (30.7%), 30 Gy in 10 fractions for 77 patients (37.6%) and 39 Gy in 13 fractions for 65 patients (31.7%) (Table 1).
Table 1.
Baseline characteristics.
| Characteristics | No. of patients (%) |
||||
|---|---|---|---|---|---|
| All patients (N = 205) | 20 Gy EBRT (N = 63) | 30 Gy EBRT (N = 77) | 39 Gy EBRT (N = 65) | P value | |
| Gender | |||||
| Male | 146 (71.2) | 48 (76.2) | 58 (75.3) | 40 (61.5) | 0.11 |
| Female | 59 (28.8) | 15 (23.8) | 19 (24.7) | 25 (38.5) | |
| Age | |||||
| ≤70 | 90 (43.9) | 33 (52.4) | 40 (51.9) | 17 (26.2) | <0.01 |
| >70 | 115 (56.1) | 30 (47.6) | 37 (48.1) | 48 (73.8) | |
| KPS | |||||
| 90–100 | 40 (19.5) | 13 (20.6) | 17 (22.1) | 10 (15.4) | 0.54 |
| 60–80 | 140 (68.3) | 40 (63.5) | 52 (67.5) | 48 (73.8) | |
| Unknown* | 25 (12.2) | 10 (15.9) | 8 (10.4) | 7 (10.8) | |
| Comorbidity | |||||
| None to mild | 93 (45.4) | 28 (44.4) | 40 (51.9) | 25 (38.5) | 0.27 |
| Moderate to severe | 112 (54.6) | 35 (55.6) | 37 (48.1) | 40 (61.5) | |
| Current presentation | |||||
| Primary presentation | 171 (83.4) | 50 (79.4) | 64 (83.1) | 57 (87.7) | 0.45 |
| Recurrent presentation | 34 (16.6) | 13 (20.6) | 13 (16.9) | 8 (12.3) | |
| Symptoms at presentation** | |||||
| Dysphagia | 184 (89.8) | 59 (93.7) | 73 (94.8) | 52 (80.0) | |
| Pain | 57 (27.8) | 22 (34.9) | 17 (22.1) | 18 (27.7) | |
| Weight loss | 149 (72.7) | 53 (84.1) | 53 (68.8) | 43 (66.2) | |
| Hematemesis | 5 (2.4) | 3 (4.8) | 0 (0) | 2 (3.1) | |
| Other | 53 (25.9) | 15 (23.8) | 22 (28.6) | 16 (24.6) | |
| No symptoms | 3 (1.5) | 0 (0) | 0 (0) | 3 (4.6) | |
| Dysphagia score before treatment*** | |||||
| 0–1 | 77 (37.6) | 21 (33.3) | 26 (33.8) | 30 (46.2) | 0.25 |
| 2–4 | 126 (61.4) | 41 (65.1) | 50 (64.9) | 35 (53.8) | |
| Unknown* | 2 (1.0) | 1 (1.6) | 1 (1.3) | 0 (0) | |
| Weight loss | |||||
| ≤6 kg | 103 (50.2) | 26 (41.3) | 38 (49.4) | 39 (60.0) | 0.17 |
| >6 kg | 89 (43.4) | 33 (52.4) | 33 (42.9) | 23 (35.4) | |
| Unknown* | 13 (6.3) | 4 (6.3) | 6 (7.8) | 3 (4.6) | |
| Tube feeding before treatment | |||||
| No | 159 (77.6) | 52 (82.5) | 55 (71.4) | 52 (80.0) | 0.18 |
| Yes | 44 (21.5) | 11 (17.5) | 22 (28.6) | 11 (16.9) | |
| Unknown* | 2 (1.0) | 0 (0) | 0 (0) | 2 (3.1) | |
| Tumour length | |||||
| ≤6 cm | 119 (58.0) | 32 (50.8) | 38 (49.4) | 49 (75.4) | <0.01 |
| >6 cm | 85 (41.5) | 31 (49.2) | 38 (49.4) | 16 (24.6) | |
| Unknown* | 1 (0.5) | 0 (0) | 1 (1.3) | 0 (0) | |
| Tumour location | |||||
| High – middle | 39 (19.0) | 6 (9.5) | 18 (23.4) | 15 (23.1) | 0.27 |
| Low – GEJ | 142 (69.3) | 46 (73.0) | 51 (66.2) | 45 (69.2) | |
| Anastomosis | 10 (4.9) | 4 (6.3) | 4 (5.2) | 2 (3.1) | |
| Unknown* | 14 (6.8) | 7 (11.1) | 4 (5.2) | 3 (4.6) | |
| Histology | |||||
| Adenocarcinoma | 120 (58.5) | 40 (63.5) | 48 (62.3) | 32 (49.2) | 0.03 |
| Squamous cell carcinoma | 66 (32.2) | 14 (22.2) | 26 (33.8) | 26 (40.0) | |
| Other | 15 (7.3) | 7 (11.1) | 1 (1.3) | 7 (10.8) | |
| Unknown* | 4 (2.0) | 2 (3.2) | 2 (2.6) | 0 (0) | |
| T stage | |||||
| T0–T3 | 61 (29.8) | 15 (23.8) | 21 (27.3) | 25 (38.5) | 0.99 |
| T4 | 28 (13.7) | 7 (11.1) | 10 (13.0) | 11 (16.9) | |
| Unknown* | 116 (56.6) | 41 (65.1) | 46 (59.7) | 29 (44.6) | |
| N stage | |||||
| N0 | 31 (15.1) | 4 (6.3) | 5 (6.5) | 22 (33.8) | <0.01 |
| N+ | 147 (71.7) | 49 (77.8) | 62 (80.5) | 36 (55.4) | |
| Unknown* | 27 (13.2) | 10 (15.9) | 10 (13.0) | 7 (10.8) | |
| M stage | |||||
| M0 | 69 (33.7) | 7 (11.1) | 15 (19.5) | 47 (72.3) | <0.01 |
| M1 | 125 (61.0) | 51 (81.0) | 61 (79.2) | 13 (20.0) | |
| Unknown* | 11 (5.4) | 5 (7.9) | 1 (1.3) | 5 (7.7) | |
Abbreviations: EBRT, External beam radiotherapy; KPS, Karnofsky performance status; GEJ, Gastroesophageal junction.
The ‘unknown’ categories were not included in calculation of p values.
Total >100% due to possibility of having more than one symptom at presentation. Calculation p value not possible.
Dysphagia score as stated by Knyrim [23]: 0 = no dysphagia/able to eat normal diet, 1 = able to swallow some solid foods, 2 = able to swallow only semi solid foods, 3 = able to swallow liquids only, 4 = total dysphagia.
Six patients, three in the 39 Gy and three in the 30 Gy group, stopped treatment prematurely (three on patient’s request, one due to disease progression, two because of intercurrent disease). In one patient, treatment was interrupted due to hospitalization for COPD exacerbation, after which the patient resumed treatment and received one additional fraction for compensation. All patients were included in the analysis.
3.1. Patient characteristics
Patient characteristics are summarized in Table 1. Most patients had symptoms of dysphagia (89.8%, of whom 61.4% severe dysphagia (score2-4)) and weight loss (72.7%). In the 20, 30 and 39 Gy group, respectively 47.6%, 48.1% and 73.8% of patients were over 70 years old (p = 0.002). Pathological lymph nodes were present in 77.8%, 80.5% and 55.4% in the 20 Gy, 30 Gy and 39 Gy group (p < 0.001). Distant metastasis at baseline were found in 81.0%, 79.2% and 20.0% in the 20 Gy, 30 Gy and 39 Gy group (p < 0.001).
Forty patients (19.5%) had undergone previous treatment for esophageal cancer; treatment with curative intent (chemoradiation and/or surgery) (N = 9 in 20 Gy, N = 13 in 30 Gy, N = 7 in 39 Gy group) or palliative chemotherapy (N = 9 in 20 Gy, N = 4 in 30 Gy, N = 2 in 39 Gy group).
3.2. Clinical response
Before treatment, 89.8% of patients had symptoms of dysphagia. A clinical response (improvement of dysphagia, relief of pain and/or reduction of esophageal bleeding) was experienced by 72.2% of patients without significant differences between the three groups (p = 0.45). During the first three months, dysphagia score improved at least one point in 41.0% of patients (47.6% in the 20 Gy, 40.3% in the 30 Gy and 35.4% in the 39 Gy group).
3.3. Toxicity
Frequent adverse events were fatigue, esophageal pain, esophagitis and nausea. Eight (3.9%) patients (N = 5 in the 20 Gy, N = 2 in the 30 Gy, N = 1 in the 39 Gy group) experienced an acute adverse effect of grade ≥ 3, in particular esophageal stenosis (N = 4), fatigue (N = 3), esophagitis (N = 1) or melena (N = 1). Four patients had severe pain requiring opioid medication. In total, 20.5% of patients had no acute toxicity reported in the charts. Occurrence of toxicity was unknown in 10.7% of patients.
3.4. Survival
Median OS for the whole group was 6.2 months (95%CI 4.8–7.6 months), with OS at one year and two years equal to 26.3 ± 3.1% and 6.2 ± 1.7% respectively. Kaplan-Meier curves for OS in the different treatment schedules are shown in Fig. 1 (p = 0.020 computed with the log rank test). Median OS in the 20 Gy, 30 Gy and 39 Gy groups was 4.6 months 95%CI 2.6–6.6), 5.2 months (95%CI 3.7–6.7) and 9.7 months (95%CI 6.9–12.5), respectively. At the last date of data entry for this study, eight patients were still alive, with a median follow-up time of 4.9 months (range 1.1 - 60.2 months). Two of these patients survived at least six years after treatment. Both patients had no distant metastasis and were treated with 39 Gy.
Fig. 1.
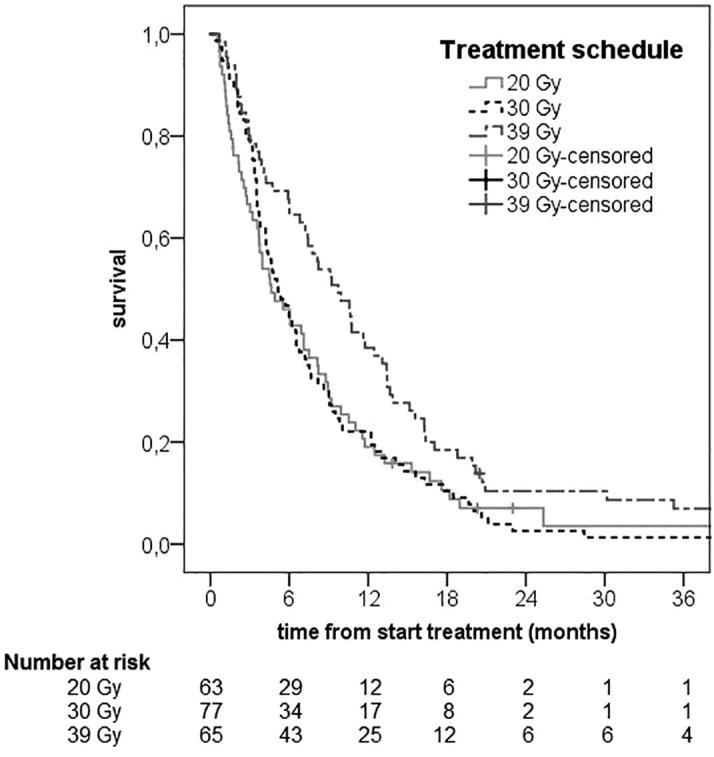
Overall survival by EBRT treatment schedules.
3.5. Univariate and multivariate analysis for overall survival
The univariate and multivariate analysis are shown in Table 2. In univariate analysis, treatment with the 39 Gy schedule showed a better OS compared to the 20 Gy schedule (HR 0.65 (95%CI 0.45–0.93)). Furthermore, in univariate analysis worse performance status (KPS), recurrent presentation of esophageal cancer, high dysphagia score, weight loss >6 kg, tumour length >6 cm, tube feeding before start of treatment, T stage and presence of distant metastasis were associated with worse OS. Patients older than 70 years showed a better OS in univariate analysis compared to patients aged 70 years or younger.
Table 2.
Hazard ratios (HR) along with their 95% confidence interval (95%CI) for univariate and multivariate Cox regression model for overall survival.
| Variable | Univariate analysis |
Final multivariate analysis |
||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | ||||
| Male | 1.0 | |||
| Female | 0.85 (0.62–1.17) | NS | ||
| Age | ||||
| ≤70 | 1.0 | |||
| >70 | 0.71 (0.54–0.95) | 0.020 | ||
| KPS* | ||||
| 90–100 | 1.0 | 1.0 | ||
| 60–80 | 1.81 (1.3–2.6) | 0.002 | 2.25 (1.53–3.29) | <0.001 |
| Unknown | 2.2 (1.3–3.8) | 0.002 | 2.17 (1.28–3.66) | 0.004 |
| Current presentation | ||||
| First presentation | 1.0 | 1.0 | ||
| Recurrence | 1.66 (1.14–2.42) | 0.008 | 1.69 (1.15–2.47) | 0.007 |
| Dysphagia score before treatment | ||||
| 0–1 | 1.0 | |||
| 2–4 | 1.29 (0.96–1.72) | 0.087 | ||
| Weight loss | ||||
| ≤6 kg | 1.0 | |||
| >6 kg | 1.30 (0.97–1.73) | 0.081 | ||
| Tube feeding before treatment | ||||
| No | 1.0 | |||
| Yes | 1.54 (1.10–2.17) | 0.013 | ||
| Tumour length | ||||
| ≤6 cm | 1.0 | 1.0 | ||
| >6 cm | 1.44 (1.08–1.92) | 0.013 | 1.31 (0.97–1.76) | 0.081 |
| Tumour location | ||||
| High – middle | 1.0 | |||
| Low – GEJ | 1.00 (0.69–1.45) | NS | ||
| Anastomosis | 1.61 (0.79–3.26) | NS | ||
| Histology | ||||
| Adenocarcinoma | 1.0 | |||
| Squamous cell carcinoma | 0.85 (0.63–1.17) | NS | ||
| Other or unknown | 1.17 (0.71–1.93) | NS | ||
| T stage | ||||
| T0–T3 | 1.0 | |||
| T4 | 1.56 (0.99–2.45) | 0.056 | ||
| Unknown | 1.13 (0.83–1.55) | NS | ||
| N stage** | ||||
| N0 or unknown | 1.0 | |||
| N+ | 1.05 (0.77–1.44) | NS | ||
| M stage** | ||||
| M0 or unknown | 1.0 | 1.0 | ||
| M1 | 1.53 (1.14–2.05) | 0.004 | 1.73 (1.27–2.35) | 0.001 |
| Treatment schedule | ||||
| 20 Gy | 1.0 | |||
| 30 Gy | 0.99 (0.71–1.40) | NS | ||
| 39 Gy | 0.65 (0.45–0.93) | 0.018 | ||
Abbreviations: KPS, Karnofsky performance status; GEJ, Gastroesophageal junction.
For the KPS variable, a separate group ‘unknown’ was created and included in the model, because of the high amount of missing values.
The N0/M0 and unknown groups in the TNM N stage and M stage variables were combined, respectively, because of the low number of values for these variables in each treatment group.
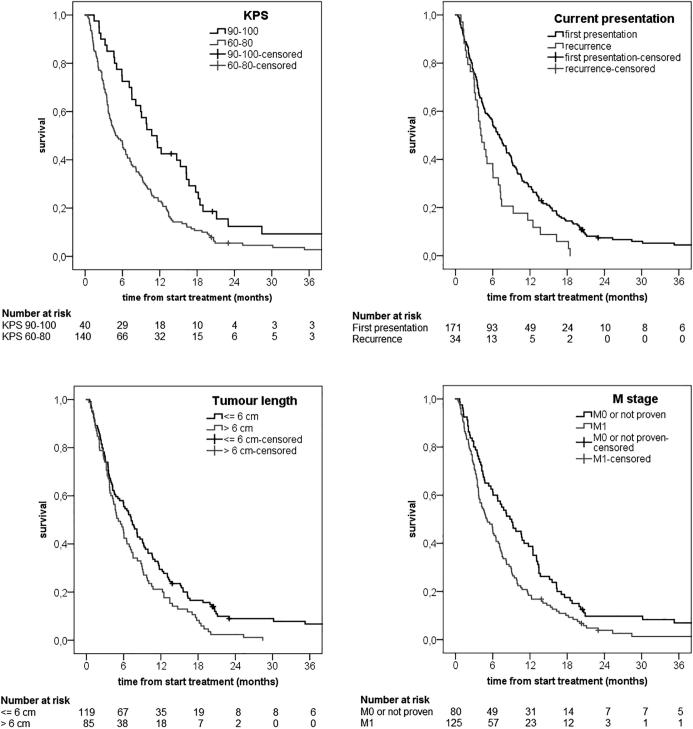
In the multivariate Cox regression model for OS the following prognostic factors were included: performance status (HR 2.25 (95%CI 1.53–3.29)), recurrent esophageal cancer (HR 1.69 (95%CI 1.15–2.47)), distant metastasis (HR 1.73 (95%CI 1.27–2.35)) and tumour length (HR 1.31 (95%CI 0.97–1.76)). Tumour length was statistically not significantly associated with OS, but was included in the final model because of significant association shown in previous literature [17]. Fig. 2 shows the estimated survival for the prognostic factors included in multivariate analysis.
Fig. 2.
Estimated overall survival by prognostic variables; Karnofsky performance status (KPS), current presentation, tumour length and distant metastasis present at baseline (M stage).
3.6. Second intervention
In total, 50 (24.4%) patients underwent a second intervention for dysphagia. Thirty-three patients had a stent placement, and 21 patients received reirradiation with EBRT, mostly 20 Gy (N = 12) or 30 Gy (N = 8). Five patients were reirradiated with ILBT, with a dose of 12 Gy (N = 2), 10 Gy (N = 2) or 8 Gy (N = 1).
No second intervention was given to 95 (46.3%) patients, 60 (29.3%) patients were lost to follow up. Retreatment occurred in 22 patients (34.9%) of the 20 Gy group, 17 patients (22.1%) of the 30 Gy group and 11 patients (16.9%) of the 39 Gy group.
Treatment schedule was the only significant prognostic factor for time to second intervention, also after correction for possible confounding factors as tumour length, dysphagia score and histology. The adjusted cause specific hazard ratio was 0.50 (95%CI 0.25–0.99) and 0.27 (95%CI 0.13–0.56) for 30 and 39 Gy respectively (reference category 20 Gy). The cumulative incidence for time to second intervention is shown in Fig. 3. At six months, the cumulative incidence was 30.8% (95%CI 18.4–43.3), 18.2% (95%CI 7.7–28.6) and 4.1% (95%CI 0–9.8) in the 20 Gy, 30 Gy and 39 Gy treatment groups, respectively.
Fig. 3.
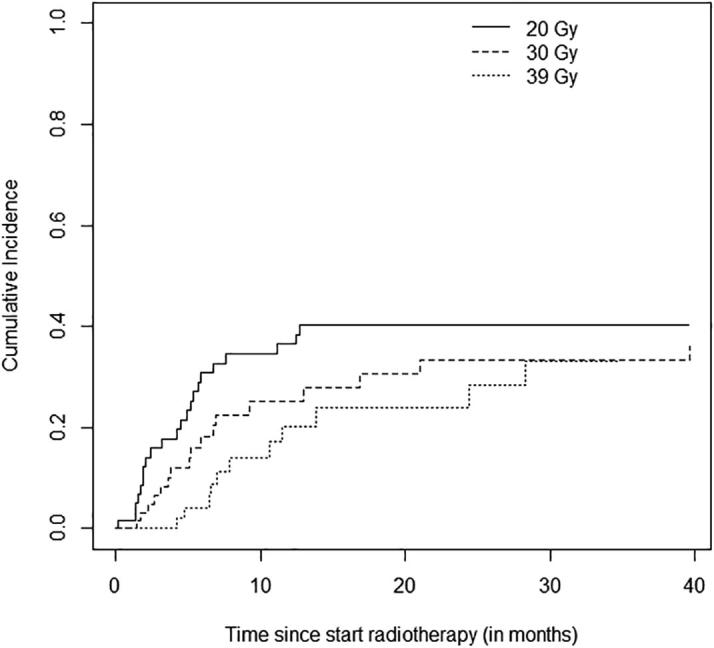
Cumulative incidence of second intervention after palliative EBRT in patients with esophageal cancer.
After the palliative EBRT, twenty-five patients (12.2%) were treated with chemotherapy (14 in the 20 Gy, 8 in the 30 Gy and 3 patients in the 39 Gy group).
4. Discussion
In our retrospective analysis investigating the effectiveness of EBRT for symptomatic esophageal cancer, a clinically significant improvement of symptoms was seen, with comparable outcomes in effect between 20, 30 and 39 Gy. Performance status, recurrent esophageal cancer, tumour length and distant metastasis were prognostic factors for overall survival. A longer time to second intervention was observed in patients treated with the 30 Gy or 39 Gy treatment schedule, compared to the 20 Gy schedule.
We found an improvement of symptoms in 72.2% of patients. Few studies examined the effect of EBRT alone [7], [9], [13], [15]. Murray et al. [7] performed a retrospective analysis of 132 patients treated with 20 Gy in 5 fractions, finding a 75% improvement of dysphagia. Thirty-one patients treated with 40 Gy in 20 fractions with accelerated fractionation (two fractions a day) were analysed by Kassam et al. [15]. These patients showed a dysphagia improvement of 69%. Homs et al. examined the effect of 12 Gy ILBT in 95 patients with inoperable esophageal carcinoma and found an improvement of 74% [6]. Thirty inoperable and previously irradiated patients with recurrent esophageal cancer, treated with 4-6 fractions of 5-7 Gy high-dose-rate ILBT, were evaluated by Wong Hee Kam et al [33]. After 1 month, complete response was found in 53%, with a median local progression-free survival of 9.8 months.
Most patients in our cohort experienced fatigue, esophageal pain, esophagitis or nausea, as seen in other studies as well [7], [15]. Severe acute toxicity observed in our cohort is comparable to other literature [7], [15]. In our 20 Gy group alone, a few more patients had severe adverse effects (7.9%) than patients in the other subgroups (2.6% and 1.5%) and the patients of Murray et al. (3%)[7]. Treatment with ILBT resulted in 12% of severe toxicity in Homs et al. [6].
We found a median OS of 6.2 months (95%CI 4.8–7.6) and a one year survival of 26.3% ± 3.1%. In the analysis of Murray et al. [7], median OS was 6.1 months, slightly better than the 20 Gy group in our cohort (4.6 months). This could be explained by the high percentage of distant metastasis in our treatment group (81.0%) compared to Murray et al. (28%). Albertsson et al. [13] found a median OS of 29 weeks and a one year survival of 22%, in patients treated with 24–45 Gy in fractions of 2 Gy. A median OS of 8 months was found in analysis by Kassam et al. [15]. The better survival observed by Kassam et al. could be related to the difference in exclusion criteria, the lower dysphagia score at baseline or the accelerated fractionation treatment. Homs et al. [6] examined the effect of 12 Gy ILBT and found a median OS of 155 days, comparable to our results.
Multivariate analysis revealed poor performance status, recurrent esophageal cancer and distant metastasis to be significant prognostic factors for worse OS. A tumour length of >6 cm was associated with worse OS. Three other studies investigated prognostic factors associated with OS in patients with incurable esophageal cancer, all proving distant metastasis to be significantly related to OS. In multivariate analysis by Homs et al. [6] and Steyerberg et al. [17], performance status was a prognostic factor as well, both using the WHO performance scale. Bergquist et al. [29] used the KPS, which was, in contrast to our study, only significant in the univariate analysis. A tumour length of >10 cm was a prognostic factor in the studies of Homs et al. and Steyerberg et al. as well. Dysphagia score was only significant in the univariate analysis, similar to Steyerberg et al.
In our cohort the 20 and 30 Gy groups only show a slight difference in OS, indicating that the pre-treatment estimation of survival in our daily clinical practice was not optimal. A predictive model for survival, as shown in previous literature [30], is important to prevent unnecessary long treatment schedules.
In total, 24.4% of patients in our cohort underwent a second intervention, compared to 44% treated with ILBT by Homs et al. [6]. Murray et al. observed a number of EBRT patients requiring a second intervention similar to our cohort [7]. After adjusting for possible confounders, patients treated with the 30 Gy or 39 Gy in our cohort experienced a longer time to second intervention. This might be due to the higher total dosage, suggesting a dose-response effect of EBRT as observed with ILBT in a recent review by Fuccio et al. [5]. However, as shown in a study on radiation of bone metastasis by Van der Linden et al. [34], choice of retreatment is possibly biased. Physicians were more willing to retreat patients after a short treatment schedule, because expectations of effectiveness were less and additional treatment stayed within limits of radiation tolerance. We did not investigate the effect of chemotherapy after palliative EBRT on symptom control. A recent RCT showed there was no statistically significant difference in dysphagia relief between chemoradiation and radiotherapy alone [35].
The main limitation of our cohort study is the retrospective design, which resulted in a high percentage of missing data, and the necessity to interpret patient-doctor communication on symptoms from the patient charts.
5. Conclusions
Our study shows that palliative EBRT provides good symptom control in the majority of patients with symptomatic esophageal cancer. A higher dose schedule was related to a longer time to second intervention. Hence, life expectancy is valuable in selecting the optimal treatment schedule to prevent an unnecessary long treatment and limit the chance of second intervention when life expectancy is longer.
Declarations of interest
None.
Contributor Information
Natasja R. Walterbos, Email: n.r.walterbos@lumc.nl.
Marta Fiocco, Email: m.fiocco@lumc.nl.
Karen J. Neelis, Email: k.j.neelis@lumc.nl.
Yvette M. van der Linden, Email: ymvanderlinden@lumc.nl.
Alexandra M.J. Langers, Email: a.m.j.langers@lumc.nl.
Marije Slingerland, Email: m.slingerland@lumc.nl.
Wobbe O. de Steur, Email: w.o.de_steur@lumc.nl.
Femke P. Peters, Email: f.p.peters@lumc.nl.
Irene M. Lips, Email: i.m.lips@lumc.nl.
References
- 1.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. http://globocan.iarc.fr; 2013 [accessed 23-11-2016]. [DOI] [PubMed]
- 2.The Netherlands Cancer Registry. Incidence, Survival and Mortality Cancer Oesophagus. www.dutchcancerfigures.nl; 2017 [accessed 01-03-2017].
- 3.Dai Y., Li C., Xie Y., Liu X., Zhang J., Zhou J. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2014;10:Cd005048. doi: 10.1002/14651858.CD005048.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homs M.Y., Steyerberg E.W., Eijkenboom W.M., Tilanus H.W., Stalpers L.J., Bartelsman J.F. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364(9444):1497–1504. doi: 10.1016/S0140-6736(04)17272-3. [DOI] [PubMed] [Google Scholar]
- 5.Fuccio L., Mandolesi D., Farioli A., Hassan C., Frazzoni L., Guido A. Brachytherapy for the palliation of dysphagia owing to esophageal cancer: a systematic review and meta-analysis of prospective studies. Radiother Oncol. 2017;122(3):332–339. doi: 10.1016/j.radonc.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Homs M.Y., Steyerberg E.W., Eijkenboom W.M., Siersema P.D. Predictors of outcome of single-dose brachytherapy for the palliation of dysphagia from esophageal cancer. Brachytherapy. 2006;5(1):41–48. doi: 10.1016/j.brachy.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Murray L.J., Din O.S., Kumar V.S., Dixon L.M., Wadsley J.C. Palliative radiotherapy in patients with esophageal carcinoma: a retrospective review. Pract Radiat Oncol. 2012;2(4):257–264. doi: 10.1016/j.prro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V., Mahantshetty U., Dinshaw K.A., Deshpande R., Sharma S. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002;52(2):310–315. doi: 10.1016/s0360-3016(01)01822-3. [DOI] [PubMed] [Google Scholar]
- 9.Welsch J., Kup P.G., Nieder C., Khosrawipour V., Buhler H., Adamietz I.A. Survival and symptom relief after palliative radiotherapy for esophageal cancer. J Cancer. 2016;7(2):125–130. doi: 10.7150/jca.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laskar S.G., Lewis S., Agarwal J.P., Mishra S., Mehta S., Patil P. Combined brachytherapy and external beam radiation: an effective approach for palliation in esophageal cancer. J Contemp Brachytherapy. 2015;7(6):453–461. doi: 10.5114/jcb.2015.56765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblatt E., Jones G., Sur R.K., Donde B., Salvajoli J.V., Ghosh-Laskar S. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol. 2010;97(3):488–494. doi: 10.1016/j.radonc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Sur R., Donde B., Falkson C., Ahmed S.N., Levin V., Nag S. Randomized prospective study comparing high-dose-rate intraluminal brachytherapy (HDRILBT) alone with HDRILBT and external beam radiotherapy in the palliation of advanced esophageal cancer. Brachytherapy. 2004;3(4):191–195. doi: 10.1016/j.brachy.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Albertsson M., Ewers S.B., Widmark H., Hambraeus G., Lillo-Gil R., Ranstam J. Evaluation of the palliative effect of radiotherapy for esophageal carcinoma. Acta Oncol. 1989;28(2):267–270. doi: 10.3109/02841868909111261. [DOI] [PubMed] [Google Scholar]
- 14.Prasad N.R., Karthigeyan M., Vikram K., Parthasarathy R., Reddy K.S. Palliative radiotherapy in esophageal cancer. Indian J Surg. 2015;77(1):34–38. doi: 10.1007/s12262-013-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassam Z., Wong R.K., Ringash J., Ung Y., Kamra J., DeBoer G. A phase I/II study to evaluate the toxicity and efficacy of accelerated fractionation radiotherapy for the palliation of dysphagia from carcinoma of the oesophagus. Clin Oncol (R Coll Radiol) 2008;20(1):53–60. doi: 10.1016/j.clon.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kellokumpu-Lehtinen P., Huovinen R., Nikkanen V. Survival and esophageal passage after radiotherapy of inoperable esophageal carcinoma. A retrospective study of 106 cases. Acta Oncol. 1990;29(2):175–178. doi: 10.3109/02841869009126541. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg E.W., Homs M.Y., Stokvis A., Essink-Bot M.L., Siersema P.D. Stent placement or brachytherapy for palliation of dysphagia from esophageal cancer: a prognostic model to guide treatment selection. Gastrointest Endosc. 2005;62(3):333–340. doi: 10.1016/s0016-5107(05)01587-7. [DOI] [PubMed] [Google Scholar]
- 18.Harvey J.A., Bessell J.R., Beller E., Thomas J., Gotley D.C., Burmeister B.H. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus. 2004;17(3):260–265. doi: 10.1111/j.1442-2050.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 19.van Ruler M.A., Peters F.P., Slingerland M., Fiocco M., Grootenboers D.A., Vulink A.J. Clinical outcomes of definitive chemoradiotherapy using carboplatin and paclitaxel in esophageal cancer. Dis Esophagus. 2017;30(4):1–9. doi: 10.1093/dote/dow033. [DOI] [PubMed] [Google Scholar]
- 20.Oncoline. Oesophageal cancer. http://www.oncoline.nl/oesofaguscarcinoom; 2015 [accessed 23-11-2016].
- 21.Opstelten J.L., de Wijkerslooth L.R., Leenders M., Bac D.J., Brink M.A., Loffeld B.C. Variation in palliative care of esophageal cancer in clinical practice: factors associated with treatment decisions. Dis Esophagus. 2016 doi: 10.1111/dote.12478. [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo J.F., Tierney R.M., Costas I., Grove L., Spitznagel E.L., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 23.Knyrim K., Wagner H.J., Bethge N., Keymling M., Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329(18):1302–1307. doi: 10.1056/NEJM199310283291803. [DOI] [PubMed] [Google Scholar]
- 24.Rice T.W., Blackstone E.H., Rusch V.W. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 25.Brierley J.D., Gospodarowicz M.K., Wittekind C. 8th ed. Wiley-Blackwell; Oxford: 2017. TNM Classification of malignant tumours. [Google Scholar]
- 26.U.S. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm; 2009 [accessed 21-11-2016].
- 27.Chow E., Harris K., Fan G., Tsao M., Sze W.M. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 28.Rich S.E., Chow R., Raman S., Liang Zeng K., Lutz S., Lam H. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126(3):547–557. doi: 10.1016/j.radonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Bergquist H., Johnsson A., Hammerlid E., Wenger U., Lundell L., Ruth M. Factors predicting survival in patients with advanced oesophageal cancer: a prospective multicentre evaluation. Aliment Pharmacol Ther. 2008;27(5):385–395. doi: 10.1111/j.1365-2036.2007.03589.x. [DOI] [PubMed] [Google Scholar]
- 30.Westhoff P.G., de Graeff A., Monninkhof E.M., Bollen L., Dijkstra S.P., van der Steen-Banasik E.M. An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2014;90(4):739–747. doi: 10.1016/j.ijrobp.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 31.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 32.de Wreede L.C., Fiocco M., Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Wong Hee Kam S., Rivera S., Hennequin C., Lourenco N., Chirica M., Munoz-Bongrand N. Salvage high-dose-rate brachytherapy for esophageal cancer in previously irradiated patients: A retrospective analysis. Brachytherapy. 2015;14(4):531–536. doi: 10.1016/j.brachy.2015.02.392. [DOI] [PubMed] [Google Scholar]
- 34.van der Linden Y.M., Lok J.J., Steenland E., Martijn H., van Houwelingen H., Marijnen C.A. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys. 2004;59(2):528–537. doi: 10.1016/j.ijrobp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Penniment M.G., De Ieso P.B., Harvey J.A., Stephens S., Au H.J., O'Callaghan C.J. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in advanced oesophageal cancer: a multicentre randomised controlled trial (TROG 03.01). Lancet. Gastroenterol Hepatol. 2018;3(2):114–124. doi: 10.1016/S2468-1253(17)30363-1. [DOI] [PubMed] [Google Scholar]