Abstract
A highly sensitive fluorescent carbon quantum dots (CDs) was designed to measure the interaction of antidepressant drugs and serum albumins (SA). In present investigation the interaction of bovine serum albumin (BSA) and human serum albumin (HSA) with antidepressant drugs viz. amitryptiline hydrochloride (AMT), chlorpromazine hydrochloride (CPZ) and desipramine hydrochloride (DSP) bioconjugated on CDs have been studied by different spectroscopic techniques i.e., Fluorescence, UV-Visible, Dynamic light scattering (DLS) and FT-IR. The CDs were prepared by one-pot method using glucose and PEG-200. The developed CDs showed blue luminescence under irradiation with ultra-violet. The Stern-Volmer quenching constant (Ksv) indicates the presence of static quenching mechanism. The apparent binding constant Ka between antidepressant drugs with complex of SA-CDs have been determined. These results illustrated that CPZ shows strong binding with HSA. As further analyzed by FT-IR spectroscopy and DLS technique, the results suggested induced conformational changes on SA, thus confirming the experimental and theoretical results. Thus, a thorough knowledge of the energetics of drug-protein affinities in presence of CDs as attempted in this work is vital in giving way for appropriate drug delivery.
Keywords: Physical chemistry, Materials chemistry
1. Introduction
Fluorescent carbon nanodots have received increasing attention in a wide variety of analytical and biomedical applications owing to their outstanding properties such as minimal toxicity, superior biocompatibility, tunable photoluminescence and good water solubility [1, 2, 3, 4]. The binding mechanism of proteins with ligands from last few years have shed light on different areas of research and its important applications in numerous fields of science, such as the development of new biomaterials, biochemistry, food chemistry or pharmaceutical sciences [5, 6, 7, 8, 9, 10]. The nature of interaction between the drug molecule and protein gives new opportunity for the development of new drugs. Since drugs are the compounds which are carried by albumin, it is necessary to study the interaction of new drug with protein [11, 12, 13, 14, 15]. Serum albumins are a model globular protein which play a key role for acting as a carrier for several endogenous compounds. It has proved the most valuable invented globular proteins, used for transportation and metabolism of many biologically active compounds in the body [16, 17, 18].
Serum albumin has a well-established structure, having physicochemical properties, a versatile binding capacity stability and water solubility [19, 20]. In the present scenario bovine serum albumin (BSA) has created an extensive area of research because of its presence in blood plasma of animals. It consist a single polypeptide chain of 583 amino acids and contains 17 cysteine residues (eight disulfide bonds and one free thiol). It is divided into three specific binding sites (I, II and III) for high-affinity of drugs. Every sites is consists of two subdomains (A and B). Human serum albumin (HSA) contributes about 80% of the osmotic pressure of blood [21, 22]. It bears residue of 585 amino acids with molar mass of 66,411 g mol-1 having 17 disulfide bridges and free thiol (SH) group. Moreover, HSA consist of single tryptophan (TRP 214) present in subdomain IIA [23, 24, 25]. The theme has a better insight for research fields such as clinical medicine, chemistry and life sciences.
A tricyclic antidepressant drug (TCA) category of drug amitryptiline hydrocloride (N,N-dimethyl-3-(2-tricyclo [9.4.0.03,8]pentadeca-1 (15),3,5,7,11,13-hexaenylidene)propan-1-amine; hydrochloride), desipramine hydrochloride (3-(10,11-dihydro-5H-dibenzo [b,f]azepin-5-yl)-N-methylpropan-1-amine; hydrochloride) achlorpromazine hydrochloride (3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine; hydrochloride) has been widely used to treat major depression sickness, manic disorder and also for schizophrenia [26, 27, 28, 29]. The outcomes of the study of interaction of drug with SA and plasma of human or living tissue for diagnosing disease, controlling the amount of drug, obtaining optimum therapeutic concentration, controlling its side effects, evaluating the stability as well as the toxicology of the drug and complications of drug abuse.
Shahabadi et al. [29] observed the interaction of venlafaxin hydrochloride with bovine serum albumin (VEN-BSA) and revealed that the Stern-Volmer quenching constant Ksv is inversely correlated together with temperature and concentration. This suggests the probable quenching mechanism of binding reaction which is initiated due to complex formation. Field et al. [30] studied a development of quantum dots (QD) based-protein bioconjugate that provides for extracellular control of multicomponent drug release system. These results confirmed by time-resolved measurements of the QD donor excited state lifetime before and after maltose-induced conjugate release. Chamani and his co-workers [31] reported the binding of ciprofloxacin to human serum albumin (HSA) in the presence and absence of silver nanoparticles of three sizes as well as their various behaviors in drug delivery. These consists of, for example, the use of incident excitation light for the photocleavage of labile linkers and the application of magnetic, ultrasonic, or radiofrequency fields to facilitate drug release from properly sensitive nanocarriers. However, these drug release modalities are not without their margins [32, 33, 34]. The current researchers established that control intracellular release of drug cargos from nanobioconjugate carrier is critical for the successful implementation of nanoparticle (NP)-mediated drug delivery (NMDD) [35, 36].
In biophysical research, the most important idea is the interaction of drug molecule with biological molecule. Studies on drug-protein interaction are important in pharmacology and pharmacokinetics. Generally, the binding nature of drug-albumins is dynamic and reversible [37, 38, 39, 40]. We have already reported in previous paper [41, 42, 43] the interaction of serum albumins with fluorescent carbon quantum dots, surfactants and antidepressant drugs by surface tensiometer, conductivity meter and spectroscopic techniques. Herein, the present investigation focused on the interaction of serum albumins (Scheme 1) with antidepressant drugs (Scheme 2) in absence and presence of carbon quantum dots (CDs). The carbon quantum dots (CDs) synthesized by one-pot method using PEG (Polyethylene glycol-200) and glucose. The analysis of binding of different ligands with protein using multispectroscopic techniques such as fluorescence measurement, UV-Vis absorption, dynamic light scattering (DLS) and Fourier transform infrared spectroscopy (FT-IR), which have high sensitivity, reproducibility, cost effective and convenience. It plays emphasis role on the special binding site and the effect of antidepressant drugs on the secondary structure of serum albumins.
Scheme 1.
Structure of serum albumins (Source – http://www.rcsb.org/).
Scheme 2.
Structure of drugs.
2. Experimental
2.1. Materials
Human serum albumin (HSA) and bovine serum albumin (BSA) (purity 99.96%) and antidepressant drugs amitryptiline hydrochloride (AMT), desipramine hydrochloride (DSP) and chlorpromazine hydrochloride (CPZ) (purity 99.88%) were procured from Sigma Aldrich Pvt. Ltd. Bangalore. Glucose and polyethyleneglycol-200 (PEG-200) procured from Sigma Aldrich Pvt. Ltd. Bangalore. All chemicals were employed as purchased and their standard solutions were prepared using Millipore water. All serum albumin solutions were prepared by phosphate buffer solution.
2.2. Methods
2.2.1. Synthesis of carbon quantum dots (CDs)
The CDs was synthesized by one-pot method [41]. In a beaker 1.0 g glucose and 15 ml PEG-200 was added, and dissolve it in 30 ml Millipore water followed by stirring for 10 minutes, till a clear solution was obtained. Now the solution was heated in a hot air oven for 1 hr at 160 °C. A pale yellow color solution was obtained. This solution was kept for dialysis by dialysis membrane 500 cutoffs for one day and again dialysis by dialysis membrane 1000 cutoffs. The resulted CDs stored at 4 °C.
2.2.2. Fluorescence
The fluorescence quenching mechanisms were investigated on a Cary Eclipse Fluorescence Spectrophotometer using a quartz cell with 1.0 cm path length in a thermostatically controlled cell holder at 300K. The excitation wavelength kept for BSA and HSA fluorescence was 280nm and its emission spectra were scanned in the range of 290–450 nm. Both excitation and emission slit widths were fixed at 5 nm. The fluorescence quenching measurements have been also performed for determining the binding constant (Ka) and Stern- Volmer constant (Ksv).
2.2.3. UV-visible spectroscopic measurement
The absorption measurements of solutions were carried out by Carry-50 UV-Vis spectrophotometer using a matched pair of quartz cells (path length: 1 cm) at 27 °C. The absorption spectra were scanned in the range of 240–450 nm. The absorbance measurements were performed keeping the concentration of serum albumins and varying the concentration of drugs from 0 to 0.55 mM and different concentration of CDs.
2.2.4. FT-IR spectroscopy
Using diffuse reflectance method FT-IR spectra were taken on a Nicolet iS10 Fourier transform infrared spectroscope (Thermofisher) by KBr pellets as a background. The FT-IR spectra of HSA and BSA in the presence and absence of drug at 27 °C were recorded in the range of 1500–1700 cm−1.
2.2.5. Dynamic light scattering (DLS)
The average hydrodynamic diameter and zeta potential of the formulated quantum dots were determined by Dynamic Light Scattering (DLS) analysis using ZetaSizer Nano ZS90 (Malvern Instruments Limited, Japan) equipped with a 4.0 mW He-Ne laser operating at 630 nm. For the particle size measurements 1 ml sample of quantum dots dispersion were taken in disposable cuvettes. All measurements were carried out after dispersing the quantum dots in appropriate volume of water at 300 K, at detection angle 120 °C for zeta potential.
3. Results and discussion
3.1. Characterization of CDs
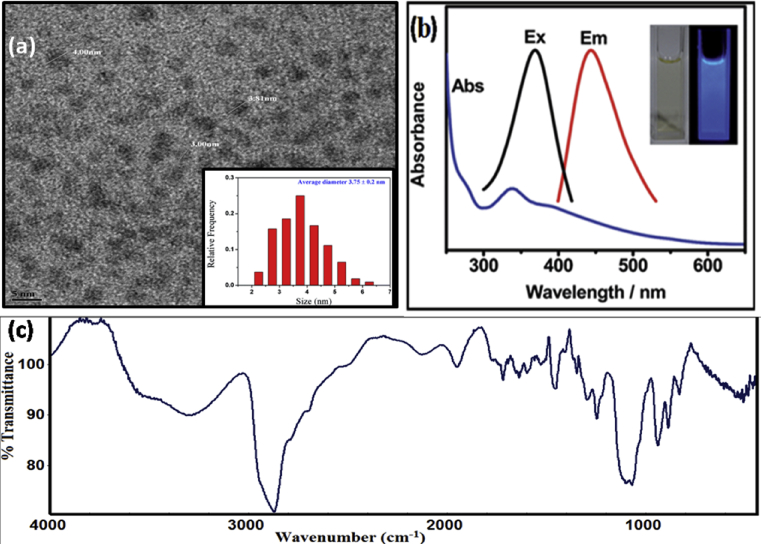
The TEM images of the prepared CDs was measured by JEOL, JEM-2100 F operated at accelerating voltage 200 kV. Monodispersed spherical shape CDs have been observed (Fig. 1a). FT-IR spectroscopy was used to determine the functional groups on the surface of the found CDs. As shown in Fig. 1c, the strong and broad absorption band 3397 cm−1 ascribed to the stretching vibrations of the O–H/N–H. UV-Vis absorption spectrum of the synthesized CDs comprising an absorption peak at 340 nm corresponding to the π-π∗transition of the aromatic sp2 domain. The CDs exhibited an obvious, narrow and symmetrical emission spectrum with a strong emission, maximum at 437 nm shown in Fig. 1b.
Fig. 1.
(a) TEM image of CDs (b) Absorption, excitation and emission spectra of CDs and (c) FTIR spectra of CDs.
3.2. Binding constant and binding capacity
3.2.1. In absence of CDs
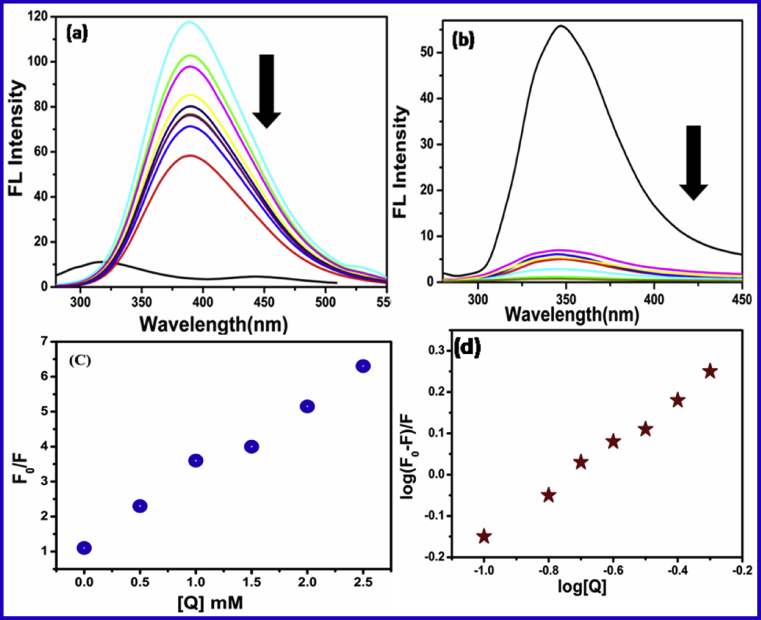
Fluorescence spectroscopy has been mostly used in the investigation of molecular interactions between ligands and proteins. The variety of parameters related to binding mechanism of antidepressant drugs with serum albumins have been investigated by means of fluorescence measurements. Serum albumins have three intrinsic fluorophores residues tryptophan (Trp), tyrosine (Tyr) and phenylalanine (Phen) [43]. Generally, the Trp alone contribute to intrinsic fluorescence, because Phen has a very low quantum yield and the fluorescence of Tyr get completely quenched when ionized. Therefore, the fluorescence of BSA and HSA regularly decreases with increasing concentration of drugs but does not show any significant shift of the emission at maximum wavelength. The fluorescence spectra of BSA and HSA with varying concentration of antidepressant drugs are shown in Fig. 2 (a and b).
Fig. 2.
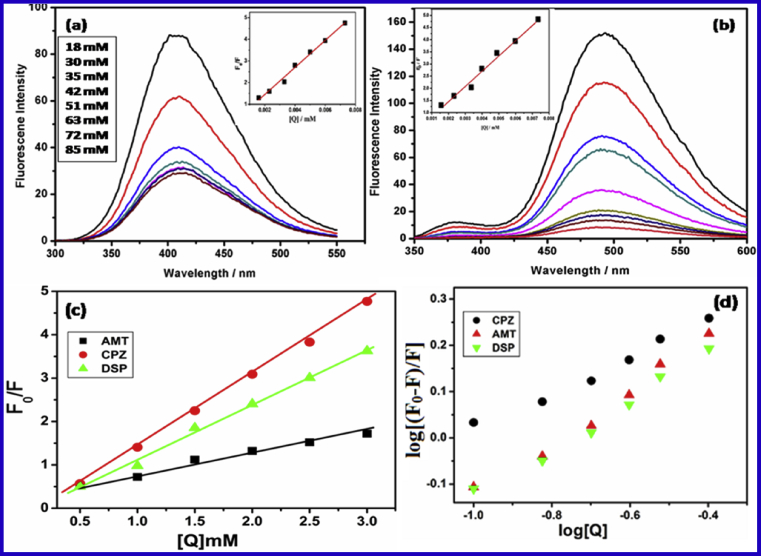
Emission spectra of (a) BSA and (b) HSA in the presence of CPZ at 300 K.(c) Stern-Volmer plots of fluorescence quenching of HSA by drugs (λmax = 280 nm) (d) Plots of vs. log [Q] for binding constant of HSA λmax = 280 nm at room temperature (pH 7.6).
In static quenching, when drug molecules bind independently to a set of equivalent sites on a protein, the relation between fluorescence quenching intensity of serum albumins and concentration of drugs can be calculated using following Eq. (1).
| (1) |
On plotting graph, versus log [Q], the number of binding sites (n) and binding constant Ka have been obtained Fig. 2 (d). In Table 1, different values of binding constant Ka and binding sites have been listed for drug associated with serum albumins. The obtained data clearly indicated that there was a binding site on serum albumins for drugs. The values of n approximately equivalent to 1 which specify the existence of just a single binding site in serum albumins for drugs. Hence, drugs most likely bind to the hydrophobic pocket spotted in subdomain IIA. The value of correlation coefficient (R2 = 0.999) shows the interaction of drugs with serum albumins the site binding model underlying Eq. (1).
Table 1.
Binding constant (Ka), Stern-Volmer quenching constant (Ksv) and binding capacity (n) and correlation coefficient (R)of BSA & HSA with drug systems at temperature 27 °C (pH 7.6).
| S.No. | System | Ksv× 10−4M | Ka× 10−4M | N | R |
|---|---|---|---|---|---|
| 1. | HSA + AMT | 10.02 | 10.9 | 1.089 | 0.990 |
| 2. | HSA + DSP | 5.08 | 6.31 | 1.193 | 0.992 |
| 3. | HSA + CPZ | 13.38 | 13.5 | 1.351 | 0.996 |
| 4. | BSA + AMT | 5.93 | 5.98 | 0.536 | 0.995 |
| 5. | BSA + DSP | 1.37 | 1.44 | 0.724 | 0.989 |
| 6. | BSA + CPZ | 25.6 | 31.9 | 1.114 | 0.997 |
From Table 1, it is evident that serum albumins were bound strongly with CPZ as compared to AMT and DSP, due to the presence of phenothiazine ring. The fluorophore commonly excited from the ground state (S0) to the first singlet state (S1) due to more π character, which the electron density exchanged from sulfur atom of the center ring of phenothiazineto the benzene rings [39, 40]. Since drugs have been carried by albumin it becomes necessary to study the interaction of new drug with protein.
Moreover, in the event of proteins the positive deviations from the binding constant equation can be registered as well in case of large quenching extent or domination of fluorescence process by one single residue (distance-dependent quenching due to a single molecular interaction) [31]. Fig 2 (a and b) depicts the fluorescence spectra of BSA and HSA fluorescence quenched by the drug. The plot of SA-drug systems shows an upward curvature, notbly at higher drug concentrations, what could indicate or the combined fluorescence quenching, or the existence of more than one binding site with different accessibilities for drug in the proximity of the SA tryptophan residues [44, 45]. The received values of Stern-Volmer KSV are distinct compared with the values of binding constant Ka for the system studied. Such results could be the consequence of concurrent formation of a coordinate bond between groups on the protein surface.
3.2.2. In presence of CDs
In the presence of CDs, there are two types binding sites with various affinities that depend on the size of quantum dots. It is revealed that the interaction between SA and drugs in the presence of carbon dots have different behaviors; therefore CDs have various applications in protein solution that can be related to their different hydration layers. Ka is a measure of the binding of the proteins to the drugs, suggesting that the Ka value for SA-drug increasing in the presence of CDs at λex = 278 nm. These results led to the conclusion that the interaction between drug and SA changed after addition of CDs to the SA-drug complex. Consequently, the CDs caused a stronger complex to be established. The interaction behavior of SA-drug changed with the presence of CDs in the ternary systems at λex = 280 nm was higher than at λex = 295 nm, because at λex = 280 nm both Tyr and Trp were excited, whereas at λex = 295 nm only Trp was excited. The values of Ka and n are listed in Tables 1 and 2. As can be seen, the interaction between SA and drug in the absence and presence of CDs respectively. However, to the best of our knowledge, so many works reported in related to drug-protein interaction, but drug –protein interaction in presence of nanoparticles a least research articles published.
Table 2.
Binding constant (Ka), Stern-Volmer quenching constant (Ksv) and binding capacity (n) of BSA & HSA with Drug-CDs systems at temperature 300 K.
| S.No. | System | Ksv× 10−4M | Ka × 10−4M | N | R2 |
|---|---|---|---|---|---|
| 1. | HSA + AMT + CDs | 12.07 | 11.1 | 1.032 | 0.993 |
| 2. | HSA + DSP + CDs | 5.69 | 7.22 | 1.251 | 0.996 |
| 3. | HSA + CPZ + CDs | 15.03 | 15.2 | 1.353 | 0.998 |
| 4. | BSA + AMT + CDs | 5.00 | 5.54 | 0.810 | 0.982 |
| 5. | BSA + DSP + CDs | 2.78 | 2.36 | 0.824 | 0.979 |
| 6. | BSA + CPZ + CDs | 29.1 | 35.3 | 1.222 | 0.998 |
3.3. Fluorescence quenching mechanism
3.3.1. In absence of CDs
The fluorescence quenching basically divided into dynamic quenching and static quenching. Dynamic quenching represents from collision encounters between fluorophore and quencher, whereas quenching by formation of a complex between quencher and fluorophore predating excitation shows the static quenching. The quenching mechanism of serum albumins with drugs is static in nature. It shows the fluorescence intensity of BSA and HSA remains constant with increasing drug concentration because drug can bind to the HSA and BSA [42, 43, 44]. The quenching process of BSA and HSA with drugs have been studied and determined by the following equation [45].
| (2) |
Where and are the fluorescence intensities in the presence and absence of quencher, respectively. is the Stern-Volmer quenching constant obtained from linear Stern-Volmer plot of against [Q] (equation.2) at 300 K temperature. The Stern-Volmer plots of the quenching of HSA and BSA tryptophan residue by drug have been represented Fig. 2 (c). KSV is the Stern-Volmer static quenching constant, based on the experimental data. AMT, DSP and CPZ, the quenching constant KSV were obtained and listed in Table 1.
3.3.2. In presence of CDs
Serum albumins have the capability to restore the CDs intensity by interaction with the quencher absorbed on CDs surfaces. As shown in Fig.3a and 3b drug quenched the serum albumins fluorescence intensity then increased almost proportionally with the increase of CDs concentration added in the SA-Drug system. Studies of binding site with warfarin and ibuprofen by fluorescence spectroscopy reported in our previous article [41].
Fig. 3.
Fluorescence spectra represent the (a) CDs-CPZ and (b) CDs- AMT system in the presence of different serum albumins; (c) Stern-Volmer plots of fluorescence quenching of HSA by drugs (λmax = 280 nm) (d) Plots of vs. log [Q] for binding constant of HSA λmax = 280 nm at room temperature (pH 7.6).
The HSA/BSA concentration-dependent fluorescence intensity can therefore be utilized to develop the CDs fluorescence intensity based method for the determination of equilibrium parameters for HSA/BSA- Drug interaction. The binding constant (K) of drug molecule binding with HSA/BSA can be determined simultaneously shown in Fig. 3d. According to previous paper [46, 47, 48], we assume that a drug molecule bind with a binding site on the serum albumins to form a complex. The linear regression of a plot of F0/F against [Q] shown in Fig. 3c. The results obtained by all method were comparable with the previous by spectrophotometric methods [48, 49]. Table 2 shows the binding constant (Ka), Stern-Volmer quenching constant (Ksv) and binding capacity (n) and correlation coefficient (R2) of SA-drug-CDs systems at temperature 300 K.
3.4. UV-visible studies
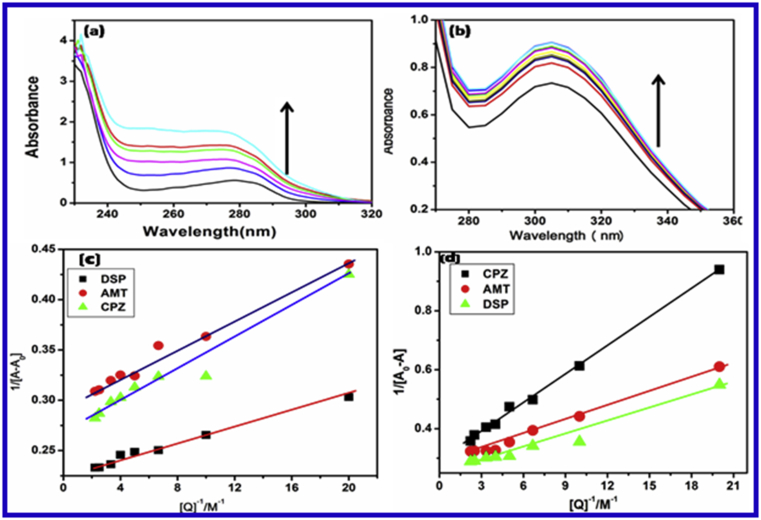
UV-Vis spectra of HSA-Drug and BSA-Drug systems have been recorded to investigated complex formation between protein and drug molecules and to explore the structural changes. UV absorption spectra of HSA and BSA proteins in the absence and presence of drugs have been shown in Fig. 4 (a and b). The absorbance of HSA-Drug and BSA-Drug system decreases with increasing concentration of drug, furthermore, the spectra found to be deshifted from 300nm to 350 nm at the maximum absorption wavelength of protein. The absorption peak shows around 278 nmwhich caused by the transition π-π∗ of aromatic residues in BSA and HSA are raised. The absorption maxima corresponding to drug-protein complex instead maximum peak position of a complex between protein and drug and the change in polarity around one tryptophan residue [20, 47].
Fig. 4.
Absorption spectra of (a) BSA and (b) HSA in the presence of various concentration of drug (λmax = 280 nm) at 300 K. (c) Benesi-Hildebrand plot using changes in absorption spectra of HSA and (d) BSA [measured at 280 nm for AMT, CPZ and DSP] at room temperature (pH 7.6).
The binding constant of the drug-protein complex were also determined using UV-vis spectroscopic method by the equation given below [47].
| (3) |
where, A0, A, Amax are the absorbance in the absence of and at intermediate concentration of drug, and at saturation point, respectively. K is the binding constant. The plot of 1/[] gives straight lines (Fig.4 (c and d)) which further indicates the formation of 1:1 complex between proteins (BSA and HSA) and drugs (antidepressant). The values of the binding constant obtained from the intercept-to-slope ratio of the Bensei-Hildebrand plot for protein-drug complex shows that the CPZ shows more binding affinity towards serum albumins than other drugs as they readily interact. The interpretation of the latter band was supported by the presence of the lone pairs of electrons on sulfur atom in the phenothiazine ring. Table 3 reveals Binding constant (K) and correlation coefficient (R2) of BSA and HSA with antidepressant drugs at room temperature and pH 7.6.
Table 3.
Binding constant (K) and correlation coefficient (R2) of BSA and HSA with antidepressant drugs at 300 K (pH 7.6).
| S. No. | System | K x 10−4 M | R2 | |
|---|---|---|---|---|
| 1. | AMT | BSA | 3.24 | 0.982 |
| HSA | 0.64 | 0.980 | ||
| 2. | CPZ | BSA | 6.84 | 0.996 |
| HSA | 1.08 | 0.991 | ||
| 3. | DSP | BSA | 3.60 | 0.994 |
| HSA | 0.80 | 0.998 | ||
3.5. FTIR spectra
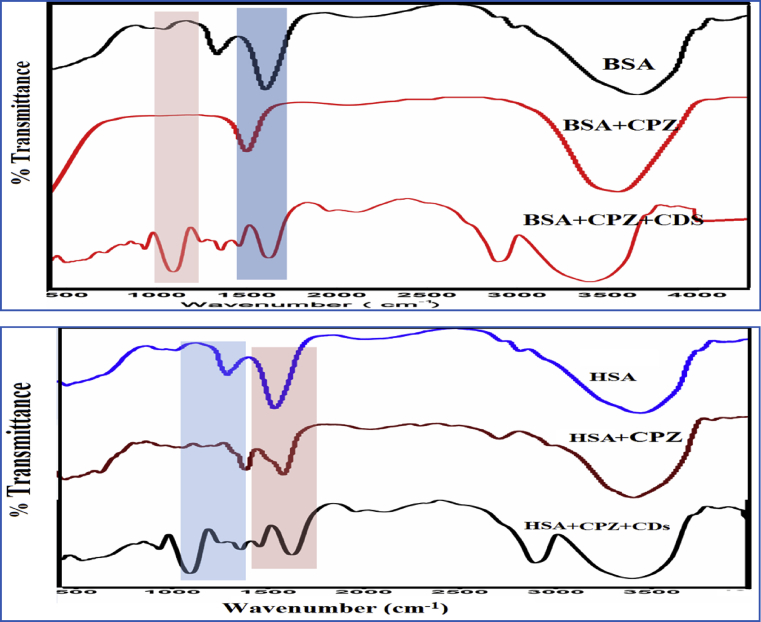
The Drug-Protein interaction and conformational changes can be interpreted from FTIR spectra as depicted in Fig. 5. Infrared spectra were taken employing diffuse reflectance accessory with Deuterium triglycine sulphate (DTGS) detector at resolution of 4 cm−1. The infrared spectrum of pure BSA and HSA and their interaction with one of the drug, AMT has been shown in Fig. 5. The protein structures have been studied and interpreted through the various amide groups in peptide group, however, of all the amide modes the amide I is most widely studied. The I and II peak of amide have been occurred at the range of 1500–1600 cm−1 and 1600-1700 cm−1 respectively. For the significant study of secondary structure of proteins enlighten more usefulness of amide band I. Hence amide bond is more sensitive to the changes in the secondary structure of protein [50, 51].
Fig. 5.
FTIR spectra of (a) BSA and (b) HSA with antidepressant drug AMT and CDs at pH 7.4.
Due to the stretching of the C=O bonds, the protein amide I bond is observed at 1655 cm−1 and the amide II bond, due to the coupled interaction of C–N stretching and N–H bending is observed at 1549 cm−1. It is evident from the figure that the proteins amide I band appeared at FTIR spectrum of free HSA and BSA in phosphate buffer solution and the difference absorption spectra upon binding with antidepressant drug are given in Fig. 5 for (A) BSA and for (B) HSA respectively. It shows that the peak position of amide I was shifted from 1670 to 1643.1 cm−1 in HSA-Drug system, while that of amide II was shifted from 1554.4 to 1567.8 cm−1 in HSA-Drug systems. These data shows the antidepressants drug has interacted with both serum albumins and the secondary structural changes leading to conformational changes.
3.6. The stoichiometry of serum albumins attached carbon quautum dots
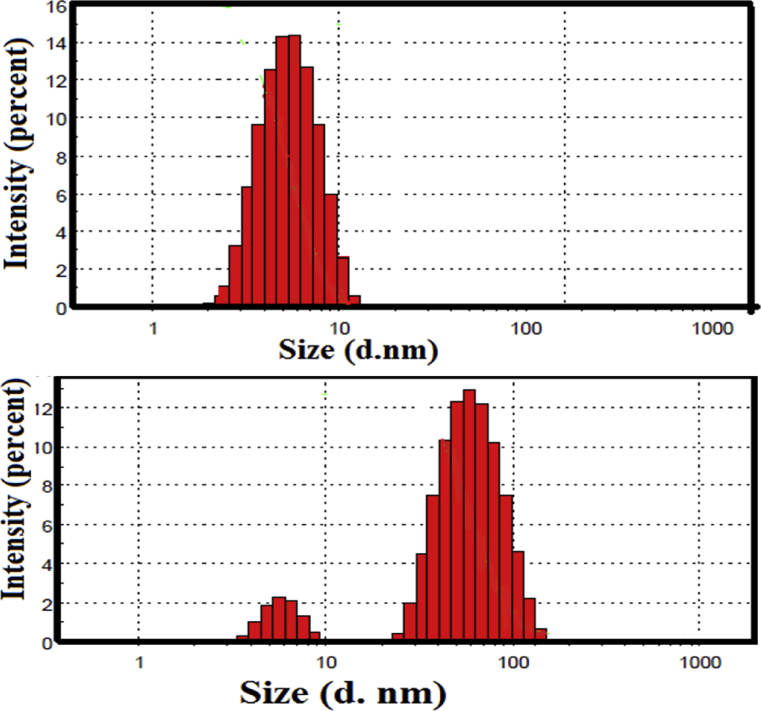
Dynamic light scattering (DLS) experiment was conducted with particles dispersed and size distribution during the assembly of CDs-SA-Drug. The variation in size, scattering intensities observed for antidepressant drugs and CDs. Particle size distribution was found in the range of 1–1000 nm. The results from DLS measurements showed that the size (diameter) of the nanohybrids did not change with the increasing number of serum albumins per CDs and antidepressant drugs, upon addition of various molar ratio of SA-antidepressant drugs. DLS determinations showed that the negative surface charge of the quantum dots neutralized upon adsorption of the serum albumin and drug. Generally, the ζ-potential of CDs was reduced when SA-drug were bond that the surface, further increase of the drug concentration did not lead to significant changes in the DLS as shown in Fig. 6. Moreover, the data revealed that the nanohybrid is a complex between CDs and protein which were formed due to electrostatic interactions of the negatively charged CDs and the cationic “hot spot” around the active site of SA antidepressant drugs. It also revealed the exact amount of SA-antidepressant drugs adsorbed over each CDs.
Fig. 6.
Particle size distribution of (A) HSA (B) BSA (C) HSA-CPZ-CDs and (D) BSA-CPZ-CDs.
4. Conclusion
In the present work, the binding of antidepressant drugs (AMT, CPZ, DSP) to serum albumins (BSA/HSA) were carried out by employing spectroscopic techniques under physiological conditions. The intrinsic tryptophan fluorescence of serum albumins turns out with a chance to be a vital tool for better understanding. The results enlighten the primary information on the binding of drugs to serum albumin protein. The experimental data showed that the Stern-Volmer quenching constant Ksv is inversely correlated; static quenching was confirmed to result in the fluorescence quenching. The results of UV-Visible spectra indicate that the conformational changes of BSA/HSA molecules are change significantly in the presence of antidepressant drugs. None of this method for enhancing fluorescence intensity of CDs by modifying CDs with serum albumins by virtue of the activated effect of antidepressant drugs was proposed. In this study firstly biconjugated quantum dots with serum albumins further studies the interaction of bioconjugated SA with antidepressant drugs. The drug-protein interaction based on quantum dots of biological significance is evident since albumin serves as a carrier of protein carrier for multiple drugs.
Declarations
Author contribution statement
Reshma Sahu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sandeep K Vaishanav, Toshikee Yadav, Srishti Sinha: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Swapnil Tiwari: Performed the experiments; Analyzed and interpreted the data.
Manmohan Satnami, Kallol K. Ghosh: Conceived and designed the experiments.
Funding statement
This work was supported by DST-FIST [no.SR/FST/CSI-259/2014(C)] and CCOST, Raipur (Chhattisgarh)[no.2744/CCOST/MRP/2015] and UGC New Delhi [no.43–183/2014 (SR)].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors are grateful to Prof. M. K. Deb, Head, School of Studies in Chemistry, Pt. Ravishankar Shukla University, Raipur (C.G.) for providing laboratory facility.
References
- 1.Lim S.Y., Shen W., Gao Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015;44:362–381. doi: 10.1039/c4cs00269e. [DOI] [PubMed] [Google Scholar]
- 2.Zhao A.D., Chen Z.W., Zhao C.Q., Gao N., Ren J.S., Qu X.G. Recent advances in bioapplications of C-dots. Carbon. 2015;85:309–327. [Google Scholar]
- 3.Freire R.M., Le N.D.B., Jiang Z., SooKim C., Rotello V.M., Fechine P.B.A. Carbon quantum dot-based nanoprobes for metal ion detection. Sensor. Actuator. B. 2018;255:2725–2732. [Google Scholar]
- 4.Elzoghby A.O., Samy W.M., Elgindy N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release. 2012;161:38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Baker S.N., Baker G.A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem. Int. Ed. 2010;49:6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y., Yu F., Park Y.S., Wang J., Shin M.C., Chung H.S., Yang V.C. Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. Biomaterials. 2010;31:9086–9091. doi: 10.1016/j.biomaterials.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu B., Zhu R., Wang M., Liang P., Qian Y., Wang S. Fluorescent carbon dots from antineoplastic drug etoposide for bioimaging in vitro and in vivo. J. Mater. Chem. B. 2017;5:7796–7800. doi: 10.1039/c7tb01628j. http://pubs.rsc.org/en/results?searchtext=Author%3ABin%20Wu [DOI] [PubMed] [Google Scholar]
- 8.Cincotto F.H., Golinellia D.L.C., Machadob S.A.S., Moraes F.C. Electrochemical sensor based on reduced graphene oxide modified with palladium nanoparticles for determination of desipramine in urine samples. Sens. Actuators, B. 2017;239:488–493. [Google Scholar]
- 9.Field L.D., Walper S.A., Susumu K., Lasarte-Aragones G., Oh E., Medintz I.L., Delehanty J.B. A quantum dot-protein bioconjugate that provides for extracellular control of intracellular drug release. Bioconjug. Chem. 2018;29:2455–2467. doi: 10.1021/acs.bioconjchem.8b00357. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya A.A., Grune T., Curry S. Analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 2000;303:721–732. doi: 10.1006/jmbi.2000.4158. [DOI] [PubMed] [Google Scholar]
- 11.Petitpas I., Grune T., Battacharya A.A., Curry S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J. Mol. Biol. 2001;314:955–960. doi: 10.1006/jmbi.2000.5208. [DOI] [PubMed] [Google Scholar]
- 12.Ghuman J., Zunszain P.A., Petitpas I., Bhattacharya A.A., Otagiri M., Curry S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005;353:38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 13.Beauchemin R., Soukpoe-Kossi C.N.N., Thomas T.J., Thomas T., Carpentier R., Tajmir-Riahi H.A. Polyamine analogues bind human serum albumin. Biomacromolecules. 2007;8:3177–3183. doi: 10.1021/bm700697a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanakis C.D., Tarantilis P.A., Tajmir-Riahi H.A., Polissiou M.G. Dimethylcrocetin, and safranal bind human serum albumin: stability and antioxidative properties. J. Agric. Food Chem. 2007;55:970–977. doi: 10.1021/jf062638l. [DOI] [PubMed] [Google Scholar]
- 15.Soukpoe-Kossi C.N.N., Sedaghat-Herati R., Ragi C., Hotchandani S., Tajmir-Riahi H.A. Retinol and retinoic acid bind human serum albumin: stability and structural features. Int. J. Biol. Macromol. 2007;40:484–490. doi: 10.1016/j.ijbiomac.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Rub M.A., Khan J.M., Azum N., Asiri A.M. Aggregation and conformational stability evaluation of myoglobin in the presence of ionic surfactant. J. Mol. Liq. 2017;241:91–98. [Google Scholar]
- 17.Cheema M.A., Taboada P., Barbosa S., Castro E., Siddiq M., Mosquera V. Energetics and conformational changes upon complexation of a phenothiazine drug with human serum albumin. Biomacromolecules. 2007;8:2576–2585. doi: 10.1021/bm070354j. [DOI] [PubMed] [Google Scholar]
- 18.Alam M.S., ud-Din Kabir. Light scattering studies of amphiphilic drugs promethazine hydrochloride and imipramine hydrochloride in aqueous electrolyte solutions. J. Phys. Chem. B. 2008;112:12962–12967. doi: 10.1021/jp804238k. [DOI] [PubMed] [Google Scholar]
- 19.Alam M.S., ud-Din Kabir, Mandal A.B. Thermodynamics at the cloud point of phenothiazine drug chlorpromazine hydrochloride-additive systems. J. Chem. Eng. Data. 2010;55:1893–1896. [Google Scholar]
- 20.Khan A.B., Khan J.M., Ali M.S., Khan R.H., ud-Din Kabir. Interaction of amphiphilic drugs with human and bovine serum albumins. Spectrochim. Acta Mol. Biomol. Spectrosc. 2012;97:119–124. doi: 10.1016/j.saa.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 21.Neis V.B., Moretti M., Bettio L.E.B., Ribeiro C.M., Rosa P.B., Goncalves F.M., Lopes M.W., Leal R.B., Rodrigues A.L.S. Agmatine produces antidepressant-like effects by activating ampa receptors and mTor signaling. J. Euroneurol. 2016;26:959–971. doi: 10.1016/j.euroneuro.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Arias H.R., T Duda K.M., Feuerbach D., Sullivan C.J., Maciejewski R., Jozwiak K. Tricyclic antidepressants and mecamylamine bind to different sites in the human α4β2 nicotinic receptor ion channel. Int. J. Biochem. Cell Biol. 2010;42:1007–1018. doi: 10.1016/j.biocel.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludka F.K., Constantino L.C., Cim T.D., Binder L.B., Zomkowski A., Rodrigues A.L.S., Tasca C.I. Involvement of PI3K/Akt/GSK-3b and mTOR in the antidepressant-like effect of atorvastatin in mice. J. Psychiatr. Res. 2016;82:9450–9457. doi: 10.1016/j.jpsychires.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Santos M.G., Tavares I.M.C., Barbosa A.F., Bettini J., Figueiredo E.C. Analysis of tricyclic antidepressants in human plasma using online-restricted access molecularly imprinted solid phase extraction followed by direct mass spectrometry identification/quantification. J. Talanta. 2017;163:8–16. doi: 10.1016/j.talanta.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 25.Hunsela F.V., Wautersb A., Vandoolaeghe E., Neelsb H.P., Demedts M. Lower total serum protein, albumin, and beta- and gamma-globulin in major and treatment-resistant depression: effects of antidepressant treatments. J. Psychiatr. Res. 1996;65:159–169. doi: 10.1016/s0165-1781(96)03010-7. [DOI] [PubMed] [Google Scholar]
- 26.Chang J.C., Tomlinson I.D., Warnement M.R., Iwamoto H., DeFecile L.J., Blakely R.D., Rosenthal S.J. A fluorescence displacement assay for antidepressant drug discovery based on ligand-conjugated quantum dots. J. Am. Chem. Soc. 2011;133:17528–17531. doi: 10.1021/ja204301g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manosso L.M., Moretti M., Ribeiro C.M., Goncalves F.M., Leal R.B., Lucia A., Rodrigues S. Antidepressant-like effect of zinc is dependent on signaling pathways implicated in bdnf modulation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;59:59–67. doi: 10.1016/j.pnpbp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Elakovic I., Brkljacic J., Matic G. Gender-related differences in the effects of antidepressant imipramine on glucocorticoid receptor binding properties and association with heat shock proteins in the rat liver and kidney. Eur. J. Pharmacol. 2009;608:7–13. doi: 10.1016/j.ejphar.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Shahabadi N., Hadidi S. Molecular modeling and spectroscopic studies on the interaction of the chiral drug venlaflaxinne hydrochloride with bovine serum albumin. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014;122:100–108. doi: 10.1016/j.saa.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Field L.D., Walper S.A., Susumu K., Lasarte-Aragones G., Oh E., Medintz I.L., Delehanty J.B. A quantum dot-protein bioconjugate that provides for extracellular control of intracellular drug release. Bioconjug. Chem. 2018;29:2455–2467. doi: 10.1021/acs.bioconjchem.8b00357. [DOI] [PubMed] [Google Scholar]
- 31.Iranfar H., Rajabi O., Salari R., Chamani J. Probing the interaction of human serum albumin with ciprofloxacin in the presence of silver nanoparticles of three sizes: multispectroscopic and ζ potential investigation. J. Phys. Chem. B. 2012;116:1951–1964. doi: 10.1021/jp210685q. [DOI] [PubMed] [Google Scholar]
- 32.Bi S., Song D., Tian Y., Zhou X., Liu Z., Zhang H. Molecular spectroscopic study on the interaction of tetracyclines with serum albumins. Spectrochim. Acta Mol. Biomol. Spectrosc. 2005;61:629–636. doi: 10.1016/j.saa.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Soenen S.J., De Cuyper M., De Smedt S.C., Braeckmans K. Investigating the toxic effects of iron oxide nanoparticles. Methods Enzymol. 2012;509:195–224. doi: 10.1016/B978-0-12-391858-1.00011-3. [DOI] [PubMed] [Google Scholar]
- 34.Ninomiya K., Yamashita T., Kawabata S., Shimizu N. Targeted and ultrasound-triggered drug delivery using liposomes co-modified with cancer cell-targeting aptamers and a thermosensitive polymer. Ultrason. Sonochem. 2014;21:1482–1488. doi: 10.1016/j.ultsonch.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Stanisavljevic M., Krizkova S., Vaculovicova M., Kizek R., Adam V. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens. Bioelectron. 2015;74:562–574. doi: 10.1016/j.bios.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 36.Zhao D., Li J., Yang T., He Z. "Turn off-on" fluorescent sensor for platinum drugs-DNA interactions based on quantum dots. Biosens. Bioelectron. 2014;52:29–35. doi: 10.1016/j.bios.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Iwao Y., Tomiguchia I., Domura A., Mantaira Y., Minami A., Suzuki T., Ikawa T., Kimura Shin-ichiro, Itai S. Inflamed site-specific drug delivery system based on the interaction of human serum albumin nanoparticles with myeloperoxidase in a murine model of experimental colitis. Eur. J. Pharm. Biopharm. 2018;125:141–147. doi: 10.1016/j.ejpb.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee T., Singh S.K., Kishore N. Binding of naproxen and amitriptyline to bovine serum albumin: biophysical aspects. J. Phys. Chem. B. 2006;110:24147–24156. doi: 10.1021/jp062734p. [DOI] [PubMed] [Google Scholar]
- 39.Rub M.A., Khan J.M., Yaseen Z., Khan R.H., ud-Din Kabir. Conformational changes of serum albumin upon complexation with amphiphilic drug imipramine hydrochloride. J. Proteins Proteomics. 2012;3:207–215. [Google Scholar]
- 40.Geng F., Zheng L., Yu L., Li G., Tung C. Interaction of bovine serum albumi with two alkylimidazolium-based ionic liquids investigated by microcalorimetry and circular dichroism. Process Biochem. 2010;45:306–311. [Google Scholar]
- 41.Reshma S., Vaishnav K., Karbhal I., Satnami M.L., Ghosh K.K. Spectroscopic studies on in vitro molecular interaction of highly fluorescent carbon dots with different serum albumins. J. Mol. Liq. 2018;255:279–287. [Google Scholar]
- 42.Yadav T., Tikariha D., Lakra J., Tiwari A.K., Saha S.K., Ghosh K.K. Surface properties of amphiphilic drugs in presence of cationic surfactants. J. Surface Sci.Technol. 2014;30:93–110. [Google Scholar]
- 43.Sinha S., Tikariha D., Lakra J., Yadav T., Kumari S., Saha S.K., Ghosh K.K. Interaction of bovine serum albumin with cationic monomeric and dimeric surfactants: A comparative study. J. Mol. Liq. 2016;218:421–428. [Google Scholar]
- 44.Tesseromatis C., Alevizou A. The role of the protein-binding on the mode of drug action as well the interactions with other drugs. Eur. J. Drug Metabol. Pharmacokinet. 2008;33:225–230. doi: 10.1007/BF03190876. [DOI] [PubMed] [Google Scholar]
- 45.Jin J., Zhu J., Yao X., Wu L. Study on the binding of farrerol to human serum albumin. J. Photochem. Photobiol. A: Chemistry. 2007;191:59–65. [Google Scholar]
- 46.Khramov A.N., Stenken J.A. Enhanced microdialysis recovery of some tricyclic antidepressants and structurally related drugs by cyclodextrin mediated transport. Analyst. 1999;124:1027–1033. doi: 10.1039/a901236b. [DOI] [PubMed] [Google Scholar]
- 47.Sulkowska A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002;614:227–232. [Google Scholar]
- 48.Choudhary S., Kishore N. Drug- protein interactions in micellar media: Thermodynamic aspects. J. Colloid Interface Sci. 2014;431:118–126. doi: 10.1016/j.jcis.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 49.Mahajan S., Mahajan R.K. Interactions of phenothiazine drugs with bile salts: Micellization and binding studies. J. Colloid Interface Sci. 2012;387:194–204. doi: 10.1016/j.jcis.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 50.Katrahalli U., Jaldappagari S., Kalanur S.S. Probing the binding of fluoxetine hydrochloride to human serum albumin by multispectroscopic. Spectrochim. Acta Mol. Biomol. Spectrosc. 2010;75:314–319. doi: 10.1016/j.saa.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Galantini L., Leggio C. Human serum albumin unfolding: a small-angle x-ray scattering and light scattering study. J. Phys. Chem. 2008;B112:15460–15469. doi: 10.1021/jp806821e. [DOI] [PubMed] [Google Scholar]