Abstract
Vasoactive Intestinal Peptide (VIP) is an important immunomodulator of CD4+ cells in normal and pathological conditions, which exerts its anti-inflammatory and immunomodulatory actions through VPAC receptors, VPAC1 and VPAC2. Only a decrease in the expression of VPAC1 mRNA on Th cells upon activation has been reported. Thus, the deepening in the knowledge of the behavior of these receptors may contribute to the design of new therapies based on their activation and/or blockade. In this study, we describe the expression pattern, cellular location and functional role of VIP receptors during the activation of human Th cells in healthy conditions and in early arthritis (EA). The protein expression pattern of VPAC1 did not change with the activation of Th lymphocytes, whereas VPAC2 was up-regulated. In resting cells, VPAC1 was located on the plasma membrane and nucleus, whereas it only appeared in the nucleus in activated cells. VPAC2 was always found in plasma membrane location. VIP receptors signaled through a PKA-dependent pathway in both conditions, and also by a PKA-independent pathway in activated cells. Both receptors exhibit a potent immunomodulatory capacity by controlling the pathogenic profile and the activation markers of Th cells. These results highlight a novel translational view in inflammatory/autoimmune diseases.
Subject terms: CD4-positive T cells, Neuroimmunology
Introduction
CD4+ T helper (Th) cells are decisive in the struggle against pathogens and in the maintenance of immune homeostasis. The activation and differentiation of Th cells can take many forms and generate immune memory. Anyhow, the extracellular environment has particular importance in the control of these processes both in homeostatic and pathological conditions. Over the last few years, several studies have shed light on the important role of neuroendocrine milieu in both lymphoid cells activation and differentiation. In this sense, vasoactive intestinal peptide (VIP) is one of the best studied neuropeptides that exerts a wide spectrum of actions on the immune system, from innate to adaptive immunity. Overall, VIP can be considered as an anti-inflammatory and immunomodulatory agent1–3. VIP modulates the differentiation of the different subsets of Th cells, increasing Th2 and T regulatory (Treg) subsets and decreasing Th1 and pathogenic Th17 cells1,2,4–9. This neuropeptide also regulates the heterogeneity and plasticity of Th17 cells in physiological and pathological conditions9–11. Moreover, healing effects of VIP in animal models of inflammatory/autoimmune diseases have been reported1–5. VIP is able to modulate all the stages comprised between the arrival of pathogens and Th cell differentiation in rheumatoid arthritis (RA) through its known anti-inflammatory and immunomodulatory actions6,10–15. Furthermore, early RA (eRA) or early spondyloarthritis patients with low VIP serum levels present a worse clinical course despite receiving more intense treatment16,17.
VIP exerts its actions through its specific receptors, VPAC1 and VPAC218. Th lymphocytes from mouse and human express both receptors1,19–21. Their mRNA expression pattern in Th cells changes in human pathologies, such as eRA or HIV infection9–11,22. Human and mouse in vitro T cell activation results in a loss of VPAC1 mRNA expression20,21. Moreover, VPAC1 might be useful as an activity marker since eRA patients with more severe inflammation and higher disease activity show lower levels of this receptor23. VPAC2 mRNA is induced after in vitro activation of mouse lymphocytes19, whereas low levels of VPAC2 have been described in resting human Th cells20.
The deepening in the knowledge of the behavior of these receptors may contribute to the design of new therapies based on their activation and/or blockade. Therefore, the aim of the present work is the study of protein/mRNA expression pattern, cellular location, signaling pathways and functionality of VIP receptors during the activation of human memory Th cells in healthy conditions and in one rheumatic pathology, EA. The understanding of protein expression and localization of these receptors can give the scientific community more significant information than the mRNA expression known so far. Moreover, changes in their localization and signaling pathway following the activation of the cells can arise in different therapeutic approaches.
Results
The expression pattern and cellular location of VPAC receptors change with the activation of human memory Th lymphocytes from healthy donors
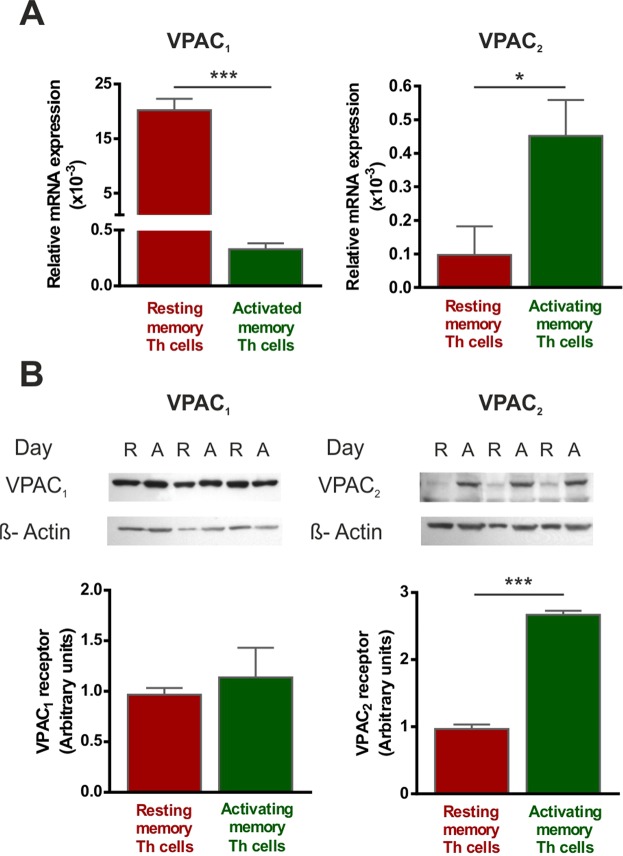
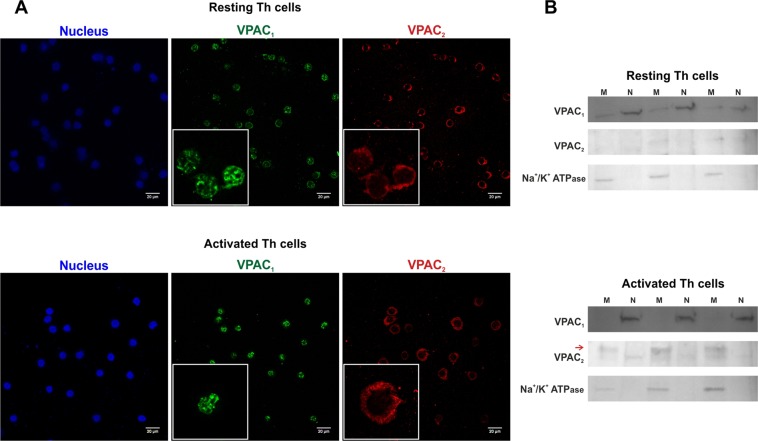
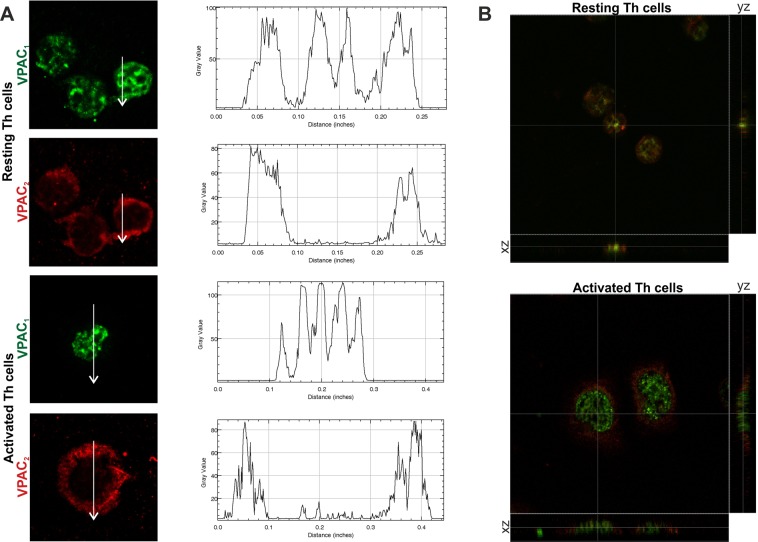
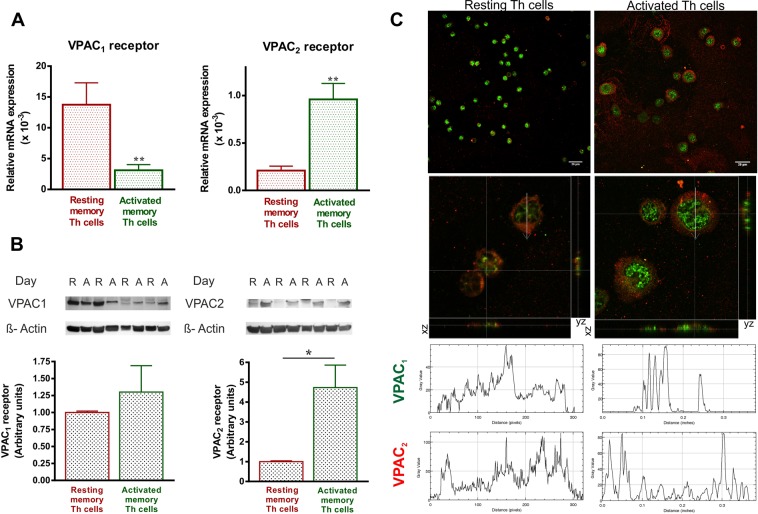
To determine whether the activation of human memory Th lymphocytes induces changes in the expression pattern of VPAC receptors, we measured mRNA levels of both receptors by semiquantitative real-time RT-PCR (Fig. 1A). After seven days of activation/expansion, memory Th lymphocytes showed decreased VPAC1 but increased VPAC2 mRNA expression. These changes were observed even after 24 h of activation (Table 1). Protein expression of VIP receptors was analyzed by western-blot (WB) and immunofluorescence staining. WB studies indicated that VPAC2 protein expression is higher in activated memory Th cells, however no differences were found in VPAC1 protein expression between resting and activated Th cells (Fig. 1B). These data indicate that changes in VPAC1 receptor transcripts were not found at protein level. The immunofluorescence staining studies corroborated the presence of both VPAC receptors in resting and activated memory human Th cells, however, it should be noted that intracellular location of both receptors showed a different pattern (Fig. 2A). In resting Th cells, VPAC1 receptor seems to appear in plasma membrane and nuclear regions, whereas in activated Th cells it is only found in nuclear location. To support this idea, we performed the subcellular fractionation into nuclear region and plasma membrane, and subsequent WB analysis (Fig. 2B). VPAC2 receptor appeared only in plasma membrane location in both resting- and activated- Th cells, although the presence of this receptor was greater in activated than resting cells. To verify, we performed a distribution analysis by fluorescence intensity and 3D vision which confirms our previous thought (Fig. 3).
Figure 1.
The expression pattern of VPAC receptors changes with the activation of memory Th lymphocytes from HD. (A) mRNA expression of VPAC1 and VPAC2 receptors was determined by semiquantitative real-time PCR analysis in resting- and seven days activated- Th cells. Results are expressed as relative mRNA levels (normalized to ACTB mRNA levels, 2−∆Ct). The mean ± SEM of triplicate determination of seven different HD samples are shown (*p < 0.05, ***p < 0.001). (B) Protein levels of VPAC1 and VPAC2 receptors in lysates of resting- and seven days activated- memory Th cells were measured by Western blotting. β-actin protein levels were determined as a loading control. Protein bands were analyzed by densitometric analysis and normalized against the intensity of β-actin. Results represent the mean ± SEM of seven different donors (***p < 0.001).
Table 1.
Time-course expression of VPAC receptors during the activation of memory Th cells from healthy donors.
| Activation/expansion days | VPAC1 receptor | VPAC2 receptor |
|---|---|---|
| 0 (Resting cells) | 20.1 ± 2.09 | 0.11 ± 0.09 |
| 1 | 1.13 ± 0.14*** | 0.42 ± 0.08* |
| 4 | 0.26 ± 0.04*** | 0.40 ± 0.16 |
| 7 | 0.33 ± 0.54*** | 0.45 ± 0.11* |
mRNA expression of VPAC1 and VPAC2 receptors was determined by real-time PCR analysis in resting and after 1, 4 or 7 days of activation/expansion. Results are expressed as relative mRNA levels (relative to ACTB mRNA levels, 2−∆Ct). The mean ± SEM of triplicate determination of five independent experiments are shown (*p < 0.05, ***p < 0.001). Relative mRNA expression (x 10−3).
Figure 2.
Cellular localization of VPAC receptors during the activation of memory Th lymphocytes from HD. (A) Immunofluorescence analysis on resting- (upper) and seven days activated- Th cells (lower) using specific antibodies for VPAC1 (Alexa Fluor 488, green), and VPAC2 (Alexa Fluor 594, red). Nuclei were counterstained with Hoechst (Blue). Fluorescence was examined on Leica SP2 AOBS confocal microscope (63X). Original scale bars, 20 µm. Boxed areas show higher magnification views of individual cells (3,17x zoom). Results are representative of five different donors. (B) VPAC1 and VPAC2 receptors in plasma membranes and nuclear fraction of resting- and seven days activated- memory Th cells were measured by Western blotting. Na+/K+ ATPase protein levels were determined as plasma membrane marker. Three of five different donors were shown.
Figure 3.
Distribution analysis of VPAC receptors across the Th lymphocytes from HD. Zoomed 63x images were imported into ImageJ to analyze the distribution of VIP receptors across the cell. (A) Representative graphs of intensity profiles plotted as a function of distance (measured in pixels) versus intensity (measured in gray-scale values) showed a distinctive distribution of receptors on each cell states. (B) Orthogonal projections of merged images from resting (up) and activated cells (down). In the lower side: XZ plane; in the right side: YZ plane.
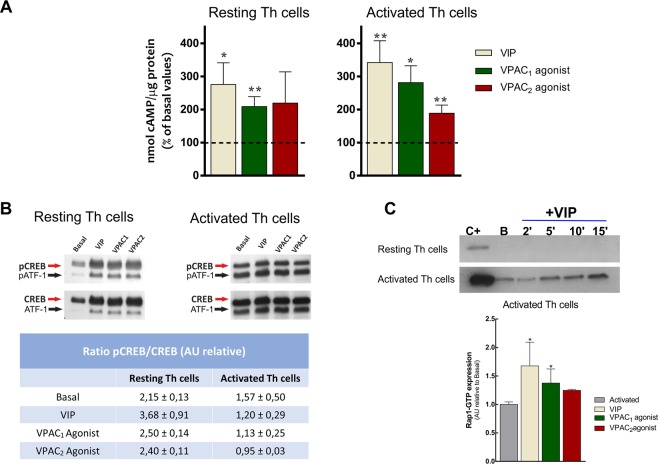
To check the functionality of these receptors in resting- and activated- Th cells we studied the signaling pathways triggered by cAMP, the main second messenger induced by both receptors. VIP, VPAC1 agonist and VPAC2 agonist increased cAMP levels in resting- and activated- Th cells (Fig. 4A). Next, we tested the specific pathways downstream this second messenger, namely PKA-dependent or canonical pathway and PKA-independent or non-canonical pathway. As the phosphorylation of transcription factor CREB is downstream PKA signalling, we measured it to assess the PKA-dependent pathway. VPAC agonists increased the ratio pCREB/CREB mainly in resting cells (Fig. 4B). Besides, the GTPase Rap1 is downstream of cAMP-activated guanine nucleotide exchange factor (EPAC), mediator of PKA-independent pathway. The presence of Rap1-GTP was only detected in activated Th cells after VIP treatment, gradually increasing from 2 min to 15 min of treatment (Fig. 4C, upper). After 15 min of stimulation, VIP and the two VPAC agonists induced the activation of Rap1 (Fig. 4C, down). Therefore, VPAC receptors mainly signal through the PKA-dependent pathway in resting Th cells, whereas the PKA-independent pathway is triggered in activated Th cells.
Figure 4.
VPAC receptors signal through canonical and non-canonical pathways in memory Th lymphocytes from HD. (A) Intracellular levels of cAMP under basal conditions and after 1 h treatment with 10 nM of VIP, VPAC1 agonist or VPAC2 agonist was determined in resting- and seven days activated- Th cells by ELISA analysis. Data are the mean ± SEM of duplicate determination of five different HD samples (*p < 0.05, **p < 0.01). Dashed lines represent the basal condition. (B) Western blot analysis of CREB and phosphorylated CREB (p-CREB) under basal conditions and after 1 h treatment with 10 nM of VIP, VPAC1 agonist or VPAC2 agonist was determined in resting- and seven days activated- Th cells. Activating transcription factor-1 (p-ATF1), the other CREB family member, is also shown. Table show the ratio p-CREB/CREB of relative densitometry units. Values are the mean ± SEM of relative densitometry units for each band of five different donors. (C) Upper: Resting- and seven days activated- Th cells were treated with VIP for different times. Rap1 activation was measured by GST-RSD pulldown followed by western blot with anti-Rap1 antibody. Positive control was obtained by treatment of lysates with GTPγS. A representative experiment of five other is shown. Down: Seven days activated Th lymphocytes were treated with VIP, VPAC1 agonist and VPAC2 agonist during 15 min. Rap1 activation was measured by GST-RSD pulldown followed by western blot with anti-Rap1 antibody. Data are the mean ± SEM of duplicate determination of five different donors (*p < 0.05).
Involvement of VPAC receptors in the immunomodulatory role of VIP during the activation of human memory Th cells from healthy donors
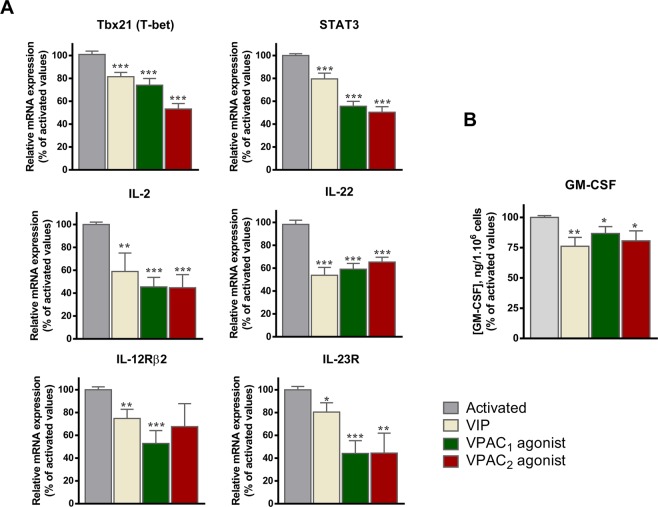
VIP modulates several molecules associated with a pathogenic profile during the activation of human memory Th cells10. Thus, we next determined the VPAC receptor subtype involved in this action, checking mRNA or protein expression of cytokines (IL-2, IL-22, GM-CSF), cytokine receptors (IL-12Rβ2, IL-23R) and transcription factors (STAT3, T-bet) associated with a pathogenic profile in these cells. Time-course response during the activation/expansion of cells showed that all molecules peaked at 1 day of activation (data not shown). As change in VPAC receptor expression is induced after 1 day of cell activation (Table 2), we tested the effect of VIP as well as VPAC agonists on these molecules (Fig. 5). Both VPAC1 and VPAC2 receptors modulated the pathogenic profile of memory Th cells after 1 day of activation, decreasing the expression of transcription factors, cytokines and cytokine receptors related to the pathogenic profile of memory Th cells.
Table 2.
Genes analyzed by semiquantitative real-time polymerase chain reaction.
| Gene | GeneBank accession no. | Sequence position/assay location (TaqMan®) | Sequence/assay ID (TaqMan®) |
|---|---|---|---|
| IL-22 | NM_020525.4 | 445 | Hs01574154_m1 |
| IL-2 | NM_000586.3 | 267 | Hs00174114_m1 |
| Tbx21 (T-bet) | NM_013351.1 | 707 | Hs00203436_m1 |
| STAT3 | NM_003150.3 | 488 | Hs01047580_m1 |
| IL-12Rβ2 | NM_001559.2 | 1,900 | Hs00155486_m1 |
| IL-23R | NM_144701.2 | 1,037 | Hs00332759_m1 |
| VPAC1 | NM_004624.3 | 306 | Hs00270351_m1 |
| VPAC2 | NM_003382.4 | 644 | Hs00173643_m1 |
| β-Actin | NM_001101.3 | 54 | Hs03023943_g1 |
Gene, genebank accession number, sequence position or assay location, and sequence or assay ID for each primer used in the study are shown.
Figure 5.
VPAC1 and VPAC2 receptors are involved in the immunomodulatory role of VIP during the activation of human memory Th cells from healthy donors. (A) mRNA expression of the transcription factors (STAT3 and Tbx21), cytokines (IL-2 and IL-22) and receptor cytokines (IL-12Rβ2 and IL-23R) was determined by real-time PCR analysis in resting- and one day activated- Th cells. Data were normalized with ACTB mRNA expression and are shown as the percentage of activated Th cells values. The mean ± SEM of triplicate determination of five different HD samples are shown (*p < 0.05, **p < 0.01, ***p < 0.001). (B) Protein expression of GM-CSF was analysed in culture supernatants by ELISA. Data are the mean ± SEM of duplicate determination of five different HD samples (*p < 0.05, **p < 0.01).
Contribution of VPAC receptors in the activation/proliferation/chemotaxis of human memory Th cells from healthy donors
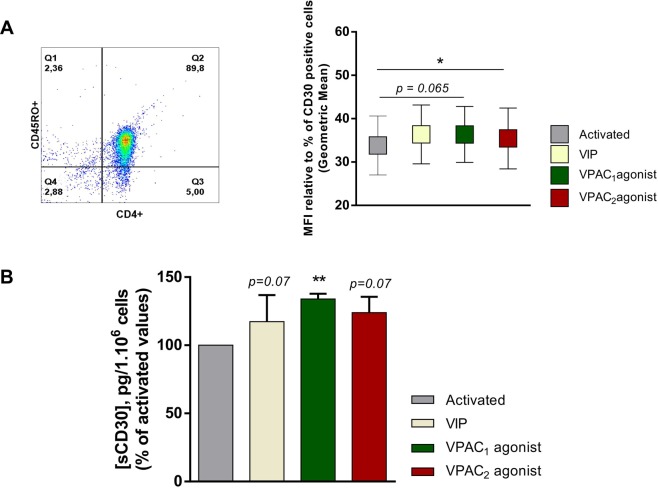
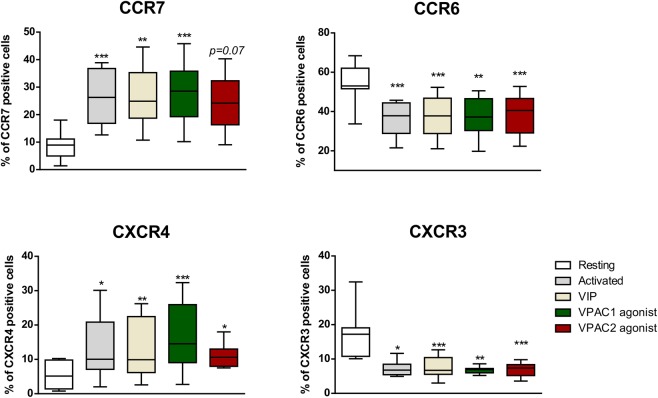
To study the involvement of each VPAC receptor in the activation of Th cells, we tested CD69, CD25, CD62L, CD154 and CD30 as activation markers. During the activation, the highest expression levels for all these molecules was at day 1, except for CD30 that was at day 4 (data not shown). No changes were observed in the presence of VIP or the VPAC agonists in the number of CD69+, CD25+, CD62L+ or CD154+ cells during the activation (data not shown); however, VIP increased the percentage of CD30+ cells and the secretion of this activation marker, probably signaling through both receptors (Fig. 6). Then, we decided to check the effect of each receptor subtype in the cell growth/survival during cell activation/expansion. No changes were observed in cell number in the presence of VIP, VPAC1 agonist or VPAC2 agonist (data not shown). To test the migration capacity, we studied the expression of several chemokine receptors. After one day of activation, Th cells increased the CCR7 and CXCR4 percentage of positive cells with respect to resting cells, whereas the number of CCR6- and CXCR3-positive cells was diminished (Fig. 7). To assess whether VIP or its receptors alter the functionality of these chemokines receptors during the activation, we performed a cell migration assay in the presence of suboptimal concentrations of their specific ligands, CCL19, CXL12, CCL20 and CXCL10, respectively in resting and one day activated Th cells. Th cells activation increased cell migration in response to CCL19, CXCL10 and CXCL12, but not to CCL20, a chemokine for CCR6 receptor. The presence of VIP during the activation maintained the capacity of migration of these cells to all chemokine tested (data not shown). In summary, VIP and its receptors increased the expression of CD30 and maintained the chemotactic capacity of Th cells during their activation.
Figure 6.
CD30 expression is increased by VIP by both receptors in activated memory Th cells from healthy donors. (A) Expression of CD30 was determined by flow cytometry in activated Th cells at day 4. Auto-fluorescence and isotype controls were set up to determine the non-specific fluorescence signal and percentage of total CD30 positive cells was quantified. The indicated proportion of positive cells was determined in the gate of CD4+CD45RO+ cells (a representative dot plot is shown). Comparison of CD30 Geometric Mean fluorescence intensity (gMFI) in the different conditions is shown. The values of gMFI was corrected by the percentage of CD30+ cells in each condition. Data are the mean ± SEM of five different donors performed by duplicated (*p < 0.05). (B) Soluble CD30 was analysed in culture supernatants by ELISA in activated Th cells at day 4. Results are the mean ± SEM of duplicate determinations of six different HD samples (**p < 0.01).
Figure 7.
VIP and its receptors maintain the migration capacity of activated memory Th cells from healthy donors. Expression of CCR6, CCR7, CXCR3 and CXCR4 was determined by flow cytometry in resting and one day activated Th cells. Auto-fluorescence and isotype controls were set up to determine the non-specific fluorescence signal and percentage of total positive cells was quantified. Results represent the mean ± SEM of duplicate determinations of five different HD samples (*p < 0.05, **p < 0.01, ***p < 0.001).
Comparative studies in human memory Th cells from EA patients: pattern expression and cellular localization of VIP receptors
To investigate if the observed changes in the expression pattern of VPAC receptors during the activation of memory Th cells also occur in pathological conditions, we tested their expression in memory Th lymphocytes from EA patients (Fig. 8). Transcripts of VPAC1 receptor decreased after seven days of activation; nevertheless, an increase of VPAC2 receptor mRNA expression was detected. Indeed, VPAC2/VPAC1 ratio was increased with the activation of Th lymphocytes from EA patients compared to resting cells (0.31 ± 0.05 and 0.01 ± 0.002, respectively). Regarding protein expression, we found no changes in VPAC1 expression and an upregulation of VPAC2 receptor. Distribution analysis by fluorescence intensity and 3D vision shown that VPAC1 appeared both in plasma membrane and nuclear localization in resting cells whereas after activation of Th cells from EA patients only nuclear location was found. Cellular location of VPAC2 receptor is limited to plasma membrane in both cell conditions.
Figure 8.
Changes in the expression pattern and cellular localization of VPAC receptors in EA patients. (A) mRNA expression of VPAC1 and VPAC2 receptors was determined by real-time PCR analysis in resting and seven days activated Th cells from EA patients. Results are expressed as relative mRNA levels (normalized to ACTB mRNA levels, 2−∆Ct). The mean ± SEM of triplicate determination of seven EA patients are shown (**p < 0.01). (B) Protein levels of VPAC1 and VPAC2 receptors in lysates of resting and seven days activated memory Th cells from EA patients were measured by Western blotting. β-actin protein levels were determined as a loading control. Protein bands were analyzed by densitometric analysis and normalized against the intensity of β-actin. Results represent the mean ± SEM of five different EA patients (*p < 0.05). (C) Immunofluorescence analysis on resting (left side) and seven days activated Th cells (right side) using specific antibodies for VPAC1 (Alexa Fluor 488, green), and VPAC2 receptors (Alexa Fluor 594, red). Upper: Fluorescence was examined on Leica SP2 AOBS confocal microscope (63X). Original scale bars, 20 µm. Medium: Orthogonal projections of magnified 63x images (3,17x zoom). In the lower side: XZ plane; in the right side: YZ plane. Lower: Representative graphs of intensity profiles plotted as a function of distance (measured in pixels) versus intensity (measured in gray-scale values). Results are representative of five different EA patients.
Discussion
The immunomodulatory role of VIP during the activation of Th cells has been clearly described in both healthy and pathological conditions, controlling their activation, polarization and plasticity1,6–11. Nevertheless, the implication of each VPAC receptor subtype has not yet been elucidated, especially taking into account previous studies indicating a change in the expression pattern of VPAC receptors in these cells20,21. The present study shows changes in the expression pattern, the cellular localization and the functional outcomes of these receptors during the activation of human Th cells. It is important to describe the first two items before trying to solve and understand the last one.
Concerning the expression pattern of VPAC1 in resting and activated memory Th cells from healthy donors (HD), we corroborate the down-regulation of VPAC1 mRNA expression with cell activation, previously described in cells of the immune network11,20,21. It has been demonstrated that the transcription factor Ikaros sets the threshold for TCR activation and undergoes an increase during T cell activation, engaging the VPAC1 promotor at Ikaros binding motifs and directly suppressing the transcription of VPAC1 gene24. As far as we know, this is the first study which reports the protein expression pattern of VPAC1 receptor during the activation of Th cells. Our data indicate that VPAC1 protein does not change with cells activation which may be caused by an increase in the mRNA stability, by a rise in protein translation rate, or by a decrease in the receptor destruction in the lysosomes, during the activation of Th cells.
Regarding the expression pattern of VPAC2, it has only been described that it is expressed at very low levels in human resting T cells, whereas in mouse T lymphocytes is detected only following stimulation with CD319,20. Our studies confirm that VPAC2 expression is very low in resting cells, but indicate a significant upregulation after Th activation in HD, both at mRNA and protein level, pointing out that VPAC2 is an inducible receptor during the activation of human Th cells. The overexpression of this receptor is not only related to lymphocytes activation, but also to their differentiation. In this regard, it has been described that the VIP/VPAC2 axis mediates the Th2 differentiation in mouse Th cells and the increase in VPAC2 expression after Th17 polarization of human naïve Th cells9,25.
When we compared the expression pattern of VPAC receptors during the activation of Th lymphocytes from healthy donors with that of EA patients, we found similar results at protein level, with unchanged VPAC1 expression and up-regulation of VPAC2 receptor. Data related to both VPAC transcripts were different to protein expression, probably due to a post-transcriptional regulation. In this sense, we observed a loss in mRNA expression of VPAC1 receptor, similar to data from HD, although to a lesser extent and starting with lower expression. These results are in agreement with our previous results which indicate that RA patients with severe inflammation and higher disease activity show lower VPAC1 mRNA levels which, in turn, is associated with the predominant proinflammatory Th1 profile23,26. In addition, the activation or Th17-differentiation of memory Th lymphocytes from eRA patients increases the mRNA ratio VPAC2/VPAC110,11. The observed increase in VPAC2 expression is greater in EA patients than in HD, which is also detected in other cells involved in this pathology, such as fibroblast-like synoviocytes or macrophage-like synoviocytes12,14. The lost in VPAC1 mRNA expression together with CD4+ T cells activation and VPAC2 up-regulation were also observed in other autoimmune diseases such as multiple sclerosis27. Besides, in a different immune disease like HIV, while VPAC1 protein expression remains unchanged, an increase in VPAC2 has been described in Th cells22.
Looking beyond the expression pattern, it is also interesting to identify the cellular situation of these receptors. As far as we know, this is the first study to describe that the cellular localization of the VPAC1 receptor changes with the activation of Th lymphocytes, which could be important as it might result in other different signaling pathways initiated by VIP in activated T lymphocytes, and may be used as a therapeutic strategy. In resting cells, the receptor is located on both the plasma membrane and inside the cell, in the nucleus, whereas this receptor is mainly restricted to a nuclear location in activated cells. VPAC2 receptor is always found in plasma membrane in both resting and activated Th cells. The intracellular position of functional G-Protein Coupled Receptors (GPCRs) in addition to plasma membranes is now broadly accepted by the scientific community28,29. In fact, VPAC1 receptor presence has been previously described in intracellular compartments such as the nucleus30,31. In human glioblastoma cell lines, there is a clear nuclear location for VPAC1 receptor, whereas VPAC2 receptor has a weak nuclear location31. Similar results related to VPAC1 receptor were observed in human breast cancer cells, where this receptor is functional in both plasma membrane and nuclear localization. Moreover, its translocation from plasma membrane to nucleus is induced by its own ligand, VIP30. In this sense, the palmitoylation of the N-terminal extracellular Cys37 of VPAC1 receptor, induced by VIP, mediates the nuclear translocation of this receptor32. The traffic of VPAC1 receptor from plasma membrane to nucleus could be related to the fact that the sequence of this receptor has a nuclear localization signal sequence in its intracytoplasmic C-terminal, not found in VPAC2 receptor31. Already in 2006, Goetzl hypothesized that VPAC receptors constitute a dynamic system for signaling in T cells, predicting that responses in the plasma membrane location would have a fast onset and brief duration, whereas receptors in the nuclear membrane would have responses with slow onset and sustained in time33. Moreover, some GPCRs such as the metabotropic glutamate receptor 5 are found mainly in the nucleus membrane and remain there via interactions with chromatin, exerting other functions related to transcription, chromosome remodeling and genomic integrity34. Thus we cannot rule out the possibility of other different signaling pathways and functions upon the VPAC1 receptor activation within the nucleus, which may arise in biological changes of T cells.
Different papers relating to GPCRs intracellular expression prove that these receptors are able to signal intracellularly. For instance, when endocytosed they can continue signaling as they are internalized together with their ligands, but they can also be activated in an autocrine manner, as ligands are synthesized in situ. Moreover, it has been described that some administrated ligands co-localize with their cognate receptors in the nucleus and are able to activate the downstream signaling pathways, speculating that ligands could be transported intracellularly using some active uptake mechanism35. More pathways can be used by the ligands to enter inside the cell and/or inside the nuclear envelope, such as endocytosis, channels or pores. Moreover, although VPAC1 is less expressed at mRNA level on activated T cells, its protein expression remains invariable; therefore, it is probably signaling from the inside.
Taking into account these facts, redistribution of GPCRs in cells changes the spatial and temporal compartmentalization of cAMP and could have differential functional actions on T cells36. cAMP has distinct effects on T cells, since it acts as a positive regulator of physiological T cell functions, whereas a short burst of cAMP induced by TCR is required for T cell activation. On the contrary, a sustained elevation in intracellular cAMP also acts as an immunosupressor. These differential effects of cAMP can be explained by different factors including the amount of cAMP produced, the cAMP effectors involved, and the spatial and temporal compartmentalization of cAMP37. Our results point to an upregulation of cAMP levels induced by VIP receptors in both resting and activated T cells. Once inside the cell, cAMP triggers various downstream pathways, mainly the canonical PKA pathway and the non-canonical EPAC pathway. Depending on the cell type, EPAC and PKA may act independently or synergistically or oppose to each other in regulating specific cellular functions38. In Th cells, the effects of the rise in cAMP, required for their activation, are mainly mediated by PKA whereas a sustained elevation of cAMP, necessary to activate EPAC1, leads to suppression of T cell activation, proliferation and chemotaxis37,39,40. Our results indicate that both receptors signal through cAMP-PKA-CREB pathway in resting cells, whereas the main signaling pathway in activated Th cells is cAMP-EPAC-Rap1.
The majority of studies related to the involvement of each VPAC receptor subtype on the immunomodulatory role of VIP, have been performed using receptor agonists and antagonists in different animal models, or VPAC-deficient mice. Although VPAC1 has been the main receptor involved in VIP action, more nuanced views suggest the participation of VPAC2 in immune regulation1,3,41,42. Focusing on human CD4+ T cells, there are few in vitro studies addressing this issue. During Th17 polarization from human naïve Th cells, VIP maintains a non-pathogenic profile through up-regulation of RORC, RORA, IL-17, IL-23R or STAT3. VPAC1 and VPAC2 are responsible for modulating the first three molecules; meanwhile VIP exerts upregulation of IL-23R through VPAC2 receptor and upregulation of STAT3 through VPAC1 receptor9. The pathogenic Th profile of human memory Th cells, after seven days of activation, is downregulated by VIP in HD and eRA patients, decreasing T-bet, STAT3, IL-2, IL-22, IL-12Rβ2, IL-23R or GM-CSF expression10. Our results indicate that all these molecules peak at day 1 of the Th cell activation, and VIP downregulates the abovementioned molecules through both VPAC receptors. Thus, the pathogenic profile of these cells can be modulated by both VIP receptors in our system.
Among the different activation markers of Th cell tested, we only found an up-modulatory effect of VIP on CD30 expression after four days of activation. CD30 antigen is a member of the TNF receptor superfamily that is expressed by activated memory Th cells but not by resting T cells. A soluble form of CD30 is released by CD30+ cells in vitro and in vivo, being sCD30 level closely related to cell CD30 expression43. CD30 induced signals mediated by NFκB and TRAF molecules cause cell proliferation or cell death. In addition several studies point to a role of sCD30 during Th differentiation and in the regulation of memory Th cell response44,45. CD30, as well as its soluble form sCD30, is an important costimulatory molecule in the regulation of the balance between Th1 and Th2 response43,44. Although in vitro studies have mainly associated the CD30 expression with a Th2/Th0 phenotype, in vivo studies suggest that CD30/CD30L signaling is involved in Th2 responses and Th2- associated diseases but also in Th1 and Th17 responses or pathologies such as RA44,45. In fact, CD30 T cells are proposed to exert an anti-inflammatory activity in RA. It has been described a high percentage of Treg cells in the synovial fluid of RA patients, presumably in order to control and block the disease progression. Almost 50% of these cells are expressing CD30, which enhances the idea that CD30 is expressed by cells with anti-inflammatory profiles45,46. As VIP is able to upregulate the expression of this marker in activated T cells, it may serve as an immunomodulatory agent. The mechanism by which VIP is able to upregulate CD30 and sCD30 expression could be by diminishing the IFNγ/IL4 ratio observed in activated T cells in vitro8 and in peripheral blood lymphocytes after polyclonal activation6, since it has been described that the expression of CD30 is controlled by the balance between IL-4 and IFNγ47. Moreover, VIP induce Th2 differentiation and a non-pathogenic Th17 polarization in human Th cells, which may explain the VIP effects observed on CD30 expression1,3,9,11.
An additional important characteristic of Th lymphocytes is their capacity to migrate from the bloodstream to inflamed tissues, where the expression pattern of chemokine receptors turns into essential to carry out this purpose. We tested CXCR4 and CCR7 as chemokine receptors that appear in naïve Th cells and could be modulated during Th activation and differentiation. CCR6 and CXCR3 where studied as two representative chemokine receptors linked to pathogenicity of Th cells, Th17 and Th1 subtypes48,49. Whereas the surface expression of CXCR4 and CCR7 were increased at day 1 after cell activation, CCR6 and CXCR3 were down-regulated. Chemokines and their receptors act in complex networks. Chemokine receptors form homo- and heterodimers, as well as higher order structures at the cell surface, in all cases dynamic structures50. Thus, we also checked the capacity of migration of the cells. This ability only increased in activated Th cells towards CCL19 and CXCL10, the ligands for CCR7 and CXCR3, respectively. That means that the activation of memory Th cells increases the chemokine receptors necessary for their homing and this is related to a Th1 profile. The presence of VIP or either VPAC agonist during the activation maintains the surface expression of all chemokine receptors studied and their functional migration capacity vs CCL19, CXCL12, CCL20 and CXCL10. Previous in vivo and in vitro studies have demonstrated that VIP down-regulates CXCL10/CXCR3, CXCL12/CXCR4, CCL20/CCR6, CCL19/CCR7 axis in different immune cells51–53. In other cases, VIP increases CCR6 surface expression during Th17 differentiation or polarization of Th cells towards a non-pathogenic phenotype9,10.
To sum up, this report dissects for the first time the role of VIP receptors, VPAC1 and VPAC2, during the activation of human memory Th cells. Both receptors exhibit high capacity of immunomodulation controlling the pathogenic profile, the activation markers and the migration ability. These results highlight the expression pattern and cellular localization of VPAC receptors, as well as their signaling pathways involved during the activation of Th cells. The change in VPAC expression pattern during the activation of memory Th cells is similar in HD and EA patients, which means that both receptors have immunomodulatory effects also in EA patients. The dissection of the behavior of VIP receptors can contribute to their use either as markers of activity in autoimmune diseases or to the development of new therapies based on their blocking and/or activation.
Methods
Healthy donors
Samples from 16 Healthy Donors (HD) were included in this study. The study was performed according to the recommendations of the Declaration of Helsinki and was approved by the Ethics Committees of the Transfusion Center of CAM. HD samples were obtained from buffy coats from the Transfusion Center. Following the Spanish Personal Data Protection law, the patients’ demographic information was confidential. All patients signed an informed consent form before sampling.
Patients
Samples from 14 patients on an EA register were analyzed (57% RA, 43% undifferentiated arthritis, and median age 49 years; mean disease duration at entry, 3.2 months). All patients belongs to PEARL’s cohort from Hospital de la Princesa, approved by Ethics Committees of La Princesa Hospital (Madrid, Spain) (PI-518). All patients signed an informed consent form before data were included in the register, and biological samples were stored at the local Biobank. Only data from patients fulfilling the 2010 American College of Rheumatology/European League Against Rheumatism criteria for RA within the 5-year follow-up (n = 7) or with chronic undifferentiated arthritis (n = 9) were analyzed54. Blood samples were collected before treatment prescription.
Isolation of human peripheral blood memory T cells
Memory Th cells were isolated from whole blood from HD or eRA patients. For mononuclear cell isolation, density gradient centrifugation by Ficoll–Hypaque (Sigma Aldrich, St Louis, MO, USA) was done. CD4+ T cells were isolated by negative selection using a CD4+ T Cell Isolation Kit II (Miltenyi Biotec, San Diego, CA, USA). CD4+CD45RO+ T cells were then isolated by negative selection using CD45RA+ MicroBeads (Miltenyi Biotec). The purity of CD4+CD45RO+ T cells was greater than 92%.
In vitro expansion of human memory T cells
CD4+CD45RO+ T cells were cultured in 96 U-well plates (0.1 × 106 cells/well) with RPMI-1640-GlutaMAX media (Life Technologies, Carlsbad, CA, USA) supplemented with 10% FBS (Lonza, Basel, Switzerland) and 1% penicillin/streptomycin (Life Technologies). Cells were activated/expanded with anti-CD3/anti-CD28 coated beads (Life Technologies). CD4+CD45RO+ T cells were cultured in the absence (activation condition) or presence of 10 nM of VIP (Polypeptide group, Strasbourg, France), VPAC1 agonist (K52R1,6L2,7VIP53,54 (1–7)/GRF (8–27)) or VPAC2 agonist (RO 25–1553) (Bachem A.G., Bubendorf, Switzerland) at different days (1, 4, 7). Cells without any treatment or activation and recollected at day 0 were considered resting cells.
Isolation of plasma membrane and nuclear fractions
Resting (day 0) and activated (day 7) cells were washed with ice-cold PBS and lysed by 40 strokes in 10 mM Tris-HCl (pH 7.4) containing 10 mM NaCl, 3 mM MgCl2, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 2 mM PMSF and 1 mM Na3VO4. The homogenates were centrifuged at 800 × g for 20 min at 4 °C. The supernatant was centrifuged at 100,000 × g for 20 min at 4 °C and the pellet, corresponding to the plasma membranes, was resuspended in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, ph 7.5, 150 mM NaCl, 30 mM NaF, 5 mM EDTA, 1% Triton X-100, 1% Nonidet P-40, 0,1% SDS, 1 mM DTT, 1 mM orthovanadate, proteinase inhibitor cocktail) and stored at −80 °C until use. The pellet from the first centrifugation was resuspended in 10 mM Tris-HCl (pH 7.4) containing 1.5 mM MgCl2, 10 mM KCl, 2 mM DTT, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 2 mM PMSF and 1 mM Na3VO4, incubated on ice for 10 min and centrifuged at 17,000 × g for 10 min at 4 °C. The supernatant was discarded and the pellet was resuspended in 20 mM Tris–HCl (pH 7.4) containing 0.5 M NaCl, 20% glycerol, 1.5 mM MgCl2, and 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 2 mM PMSF and 1 mM Na3VO4. After incubation on ice for 30 min and centrifugation at 20,000 × g for 20 min at 4 °C, the resulting supernatant corresponded to the nuclear fraction and was stored at −80 °C until use.
RNA extraction and semiquantitative real-time PCR
For total RNA extraction, we used TriReagent method (Sigma Aldrich). RNA (2 µg) were reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Life Technologies). Semiquantitative RT-PCR analysis for all molecules tested (Table 2) was performed using TaqMan Gene Expression Master Mix (Applied Biosystems, Waltham, MA, USA). We normalized each sample with β-actin, using the formula 2−ΔCt. Amplification was performed in a 7900HT Fast Real-Time PCR System apparatus (Applied Biosystems).
Immunocytochemistry staining
Cell suspensions (resting- and activated-cells at day 7) were seeded in PBS 1x and hold onto SuperFrost Plus slides (Thermofisher Scientific) 30 minutes at 37 °C, 5%CO2. Then slides were washed with PBS 1x and fixed. After rehydration and blocking, slides were incubated with rabbit polyclonal anti-human VPAC1 antibody (1:100, Thermo Fisher Scientific, Paisley, UK) and mouse monoclonal anti-human VPAC2 (1:50, Abnova) 1 hour at RT. After washing, Alexa Fluor 488 donkey anti-rabbit IgG and Alexa Fluor 594 goat anti-mouse IgG (1:500, Life Technologies) were used as secondary antibodies (1 hour at RT). Counterstaining was performed with 1 mg/ml Hoechst. Negative controls were performed in the absence of anti-VPAC1 and anti-VPAC2 antibodies. Fluorescence was examined on a Leica SP-2 Acousto-Optical Beam Splitter confocal microscope with inverted stand (Leica DM IRE2; objective, 63X; Leica, St. Gallen, Switzerland). Images were analyzed by ImageJ (Fiji).
Western blot
Protein extracts were obtained in ice-cold RIPA buffer. Protein extracts (10 and 40 µg for VPAC1 and VPAC2, respectively) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, France). After blocking, membranes were incubated overnight at 4 °C with rabbit polyclonal anti-human VPAC1 antibody (1:10000, Thermo Fisher Scientific) and mouse monoclonal anti-human VPAC2 antibody (1:1000, Abnova, Tapei, Taiwan). Mouse monoclonal anti-beta actin (ACTB) (1:10.000, Sigma Aldrich) was used as loading control. Appropriate horseradish peroxidase-conjugated secondary antibodies (1:10.000, Santa Cruz Biotechnology, Dallas, TX, USA) were applied and detected by Pierce SuperSignal West Pico (Thermo Fisher Scientific). Protein bands were analyzed using the Bio-Rad Quantity One program and normalized against β-actin.
Membrane and nuclear fraction extracts from resting and activated cells were subjected to SDS-PAGE and blotted on a nitrocellulose membrane (Bio-Rad). VPAC1 and VPAC2 antibodies were used as above mentioned. Rabbit anti-human Na+/K+ ATPase (1:1000, Cell Signaling Technology, Leiden, The Netherlands) was used as plasma membrane marker. Appropriate horseradish peroxidase-conjugated secondary antibodies (1:5000, Cell Signaling and 1:10000 Santa Cruz, respectively) were applied and detected by LumiGLO® (Cell Signaling). Protein bands were analyzed using with the Bio-Rad Quantity One program and normalized against fraction markers.
To detect cyclic AMP response element binding (CREB) phosphorylation, memory Th cells were cultured 2 hours without any stimuli (resting) or 7 days with anti-CD3/anti-CD28 coated beads (activated). In both conditions, cells were cultured in serum-free medium 30 min before adding VIP, VPAC1 or VPAC2 agonist for 15 minutes. Cells were washed in ice-cold tris-buffered saline (TBS) and lysed in RIPA buffer. After centrifugation, supernatants were quantified and frozen until western blot was performed. Protein extracts (10 µg) were subjected to SDS-PAGE and blotted on a PVDF membrane (Bio-Rad). After blocking, membranes were incubated overnight at 4 °C with rabbit anti-human pCREB antibody and rabbit anti-human CREB antibody (both 1:1000, Cell Signaling Technology) using the later as loading control. Horseradish peroxidase-conjugated anti-rabbit antibody (1:5000, Cell Signaling) was applied and detected by LumiGLO® (Cell Signaling). Protein bands were analyzed using the Bio-Rad Quantity One program and normalized against total CREB.
Pull down assays for detection of activated Rap1
To quantify activated Rap1, we performed active Rap1 pull-down assays (Thermo Scientific) following the manufacturer’s instructions. In brief, memory Th cells were cultured 7 days with anti-CD3/anti-CD28 coated beads (activated), and then 15 minutes in presence or absence of VIP or VPAC1 and VPAC2 agonists. After treatment, cells were washed with ice-cold TBS and lysed in lysis buffer. Positive control was obtained by treatment of lysates with GTPγS for 30 minutes. Cell lysates were incubated with a Rap1 binding domain-GST fusion protein and subjected to a glutathione-agarose resin, resulting in precipitation of activated GTP bond Rap1. The precipitates were subjected to standard SDS-PAGE and blotted on a nitrocellulose membrane (Bio-Rad). Primary and secondary antibodies from the manufacturer kit were used and detected by Pierce SuperSignal West Pico (Thermo Fisher Scientific). Protein bands were analyzed using the Bio-Rad Quantity One program.
Measurement of cytokines
Levels of Granolocyte-Macrophage Colony Stimulation Factor (GM-CSF) and soluble CD-30 (sCD30) were measured from cell culture supernatants by Enzyme-Linked Immuno Sorbent Assay (ELISA) test (eBioscience, San Diego, CA, USA), according to the manufacturer’s instructions.
Measurements of cAMP levels
Intracelullar cyclic adenosilmonophosphate (cAMP) levels were measured after 1 hour of stimulation with 10 nM of VIP or VPAC agonists by Enzyme-Linked Immuno Sorbent Assay (ELISA) test (Enzo Life Sciences, NY, USA) from cell lysate (0,1 M HCL solution), according to the manufacturer’s instructions. cAMP levels were corrected by protein content of cell lysate measured by QuantiProTM BCA Assay Kit (QBCA).
Flow cytometry analysis
After one day of culture, cells were collected and labeled with APC-conjugated CD30 (clone BerH8), PE-conjugated CCR6 (clone 11A9), FITC-conjugated CCR7 (Clone 150503), APC-conjugated CXCR3 (clone 1C61) or PE-Cy5-conjugated CXCR4 (clone 12G5), all from BD Biosciences. Autofluorescence and isotype controls were set up to define non-specific fluorescence. Cytometric analysis was performed using a BD FACScalibur flow cytometer (BD Biosciences, using BD FACSDiva software). Data analysis was performed using FCS Express v3 (De Novo Software, Glendale, CA, USA).
Cell migration assay
Cell migration was evaluated using 6.5 mm Transwell plates with 5.0 µm Pore Polycarbonate Membrane Insert (NY, USA). 300.000 cells (100 µl), previously resuspended in chemotaxis medium (RPMI 1640, 0.5% FBS, 10 Mm HEPES), were placed in the upper chamber of the Transwell. Different concentrations of CXCL12 (20 nM), CXCL10 (50 nM), CCL19 (100 nM and 50 nM) and CCL20 (100 nM and 50 nM) in chemotaxis medium was added to lower chamber of Transwell. Chemotaxis medium without stimuli was added to the lower chamber as a control. Plates were incubated 2 hours at 37 °C, 5% CO2. Cells which had migrated to the lower chamber were counted in a EPICS XL flow cytometer (Beckman Coulter), and expressed as a percentage of input cells
Statistical analysis
Parametric test (t test and one way-ANOVA) were used to compare different cell populations. Statistical tests were done using GraphPad Prism Version 6.0 software (GradphPad Software).
Supplementary information
Acknowledgements
The authors are grateful to the Centro de Transfusiones de la Comunidad de Madrid for the recruiting of healthy donors, to the patients of EA Register, to the Servicio de Reumatología del Hospital Universitario de la Princesa for collecting EA samples and to the Centro de Citometría de la UCM for the provided services. This work was supported by funding of Instituto de Salud Carlos III, Spain, co-financed by FEDER, European Union: RETICS program, Red de Investigación en Inflamación y Enfermedades Reumáticas (RIER) (RD16/0012/0008; RD16/0012/0006; RD16/0012/0011), the projects (PI12/00758, PI14/00477 and PI17/0027),and grant from PI12/00758.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors read and approved the final manuscript. Y.J., R.P.G., I.G.C. and R.V.R. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Y.J., R.V.R., I.G.C., R.P.G., I.G.A. and M.M. provided the study conception and design. R.V.R., I.G.C., M.C. and J.M.R.F. acquired the data. R.V.R., Y.J., I.G.C., R.P.G. and M.M. analyzed and interpreted the data. Y.J., R.V.R., I.G.C. and R.P.G. prepared the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
R. Villanueva-Romero and I. Gutiérrez-Cañas contributed equally.
R. P. Gomariz and Y. Juarranz jointly supervised this work.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43717-2.
References
- 1.Gomariz RP, et al. VIP-PACAP system in immunity: new insights for multitarget therapy. Ann. N. Y. Acad. Sci. 2006;1070:51–74. doi: 10.1196/annals.1317.031. [DOI] [PubMed] [Google Scholar]
- 2.González-Rey E, Anderson P, Delgado M. Emerging roles of vasoactive intestinal peptide: a new approach for autoimmune therapy. Ann. Rheum. Dis. 2007;66:iii70–iii76. doi: 10.1136/ard.2007.078519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganea D, Hooper KM, Kong W. The neuropeptide VIP: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. (Oxf) 2015;213:442–452. doi: 10.1111/apha.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado M, Abad C, Martínez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 5.Abad C, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 6.Gutiérrez-Cañas I, et al. Immunoregulatory properties of vasoactive intestinal peptide in human T cell subsets: implications for rheumatoid arthritis. Brain Behav. Immun. 2008;22:312–317. doi: 10.1016/j.bbi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Yadav M, Rosenbaum J, Goetzl EJ. Cutting edge: vasoactive intestinal peptide (VIP) induces differentiation of Th17 cells with a distinctive cytokine profile. J. Immunol. 2008;180:2772–2778. doi: 10.4049/jimmunol.180.5.2772. [DOI] [PubMed] [Google Scholar]
- 8.Jimeno R, et al. Effect of VIP on the balance between cytokines and master regulators of activated helper T cells. Immunol. Cell. Biol. 2012;90:178–86. doi: 10.1038/icb.2011.23. [DOI] [PubMed] [Google Scholar]
- 9.Jimeno R, et al. Vasoactive intestinal peptide maintains the nonpathogenic profile of human Th17-polarized cells. J. Mol. Neurosci. 2014;54:512–525. doi: 10.1007/s12031-014-0318-3. [DOI] [PubMed] [Google Scholar]
- 10.Jimeno R, et al. The pathogenic Th profile of human activated memory Th cells in early rheumatoid arthritis can be modulated by VIP. J. Mol. Med. 2015;93:457–467. doi: 10.1007/s00109-014-1232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimeno R, et al. Th17 polarization of memory Th cells in early arthritis: the vasoactive intestinal peptide effect. J. Leukoc. Biol. 2015;98:257–269. doi: 10.1189/jlb.3A0714-327R. [DOI] [PubMed] [Google Scholar]
- 12.Juarranz Y, et al. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- 13.Carrión M, et al. Inflammatory mediators alter interleukin-17 receptor, interleukin-12 and -23 expression in human osteoarthritic and rheumatoid arthritis synovial fibroblasts: immunomodulation by vasoactive intestinal Peptide. Neuroimmunomodulation. 2013;20:274–84. doi: 10.1159/000350892. [DOI] [PubMed] [Google Scholar]
- 14.Carrión M, et al. VIP impairs acquisition of the macrophage proinflammatory polarization profile. J. Leuk. Biol. 2016;100:1385–1393. doi: 10.1189/jlb.3A0116-032RR. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva-Romero R, et al. The anti-Inflammatory mediator, Vasoactive Intestinal Peptide, modulates the differentiation and function of Th subsets in Rheumatoid Arthritis. J. Immunol. Res . 2018;2018:6043710. doi: 10.1155/2018/6043710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez C, et al. Serum levels of vasoactive intestinal peptide as a prognostic marker in early arthritis. PLoS One. 2014;9:e85248. doi: 10.1371/journal.pone.0085248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seoane IV, et al. Vasoactive Intestinal Peptide in Early Spondyloarthritis: Low Serum Levels as a Potential Biomarker for Disease Severity. J. Mol. Neurosci. 2015;56:577–584. doi: 10.1007/s12031-015-0517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmar AJ, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado M, et al. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J. Neuroimmunol. 1996;68:27–38. doi: 10.1016/0165-5728(96)00063-X. [DOI] [PubMed] [Google Scholar]
- 20.Lara-Marquez ML, et al. Selective gene expression and activation-dependent regulation of Vasoactive Intestinal Peptide receptor Type 1 and Type 2 in human T cells. J. Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 21.Vomhof-DeKrey EE, Dorsam GP. Stimulatory and suppressive signal transduction regulates vasoactive intestinal peptide receptor-1 (VPAC-1) in primary mouse CD4 T cells. Brain Behav. Immun. 2008;22:1024–1031. doi: 10.1016/j.bbi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ipp H, et al. CD4+T cells in HIV infection show increased levels of expression of a receptor for vasoactive intestinal peptide, VPAC2. Immunol. Res. 2014;60:11–15. doi: 10.1007/s12026-014-8487-2. [DOI] [PubMed] [Google Scholar]
- 23.Seoane IV, et al. Clinical Relevance of VPAC1 Receptor Expression in Early Arthritis: Association with IL-6 and Disease Activity. PLoS One. 2016;11:e0149141. doi: 10.1371/journal.pone.0149141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorsam G, Goetzl EJ. Vasoactive intestinal peptide receptor-1 (VPAC-1) is a novel gene target of the hemolymphopoietic transcription factor Ikaros. J. Biol. Chem. 2002;277:13488–13493. doi: 10.1074/jbc.M107922200. [DOI] [PubMed] [Google Scholar]
- 25.Voice J, et al. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J. Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- 26.Delgado M, et al. Genetic association of vasoactive intestinal peptide receptor with rheumatoid arthritis. Altered expression and signal in immune cells. Arthritis. Rheum. 2008;58:1010–1019. doi: 10.1002/art.23482. [DOI] [PubMed] [Google Scholar]
- 27.Sun W, Hong J, Zang YCQ, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int. Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- 28.Bhosle VK, Rivera JC, Chemtob S. New insights into mechanisms of nuclear translocation of G-protein coupled receptors. Small GTPases, Jan. 2017;26:1–10. doi: 10.1080/21541248.2017.1282402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magalhaes AC, Dunn H, Feguson SSG. Regulation of GPCR activity trafficking and localization by GPCR-interact proteins. Br. J. Pharmacol. 2012;165:1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdehita A, et al. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cáncer. Peptides. 2010;31:2035–2045. doi: 10.1016/j.peptides.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Barbarin A, et al. Atypical nuclear localization of VIP receptors in glioma cell lines and patients. Biochem. Biophys. Res. Commun. 2014;454:524–530. doi: 10.1016/j.bbrc.2014.10.113. [DOI] [PubMed] [Google Scholar]
- 32.Yu R, et al. The palmitoylation of the N-terminal extracellular Cys37 mediates the nuclear translocation of VPAC1 contributing to its anti-apoptotic activity. Oncotarget. 2017;8:42728–42741. doi: 10.18632/oncotarget.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goetzl EJ. Hypothesis: VPAC G protein-coupled receptors for vasoactive intestinal peptide constitute a dynamic system for signaling T cells from plasma membrane and nuclear membrane complexes. Reg. Pep. 2006;137:75–78. doi: 10.1016/j.regpep.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Sergin I, et al. Sequences within the C Terminus of the Metabotropic Glutamate Receptor 5 (mGluR5) Are Responsible for Inner Nuclear Membrane Localization. J Biol Chem. 2017;292:3637–3655. doi: 10.1074/jbc.M116.757724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jong YI, et al. GPCR signalling from within the cell. Br J Pharmacol. 2018;175:4026–4035. doi: 10.1111/bph.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolaev VO, et al. Beta2- adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;37:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 37.Arumugham VB, Baldari CT. cAMP: a multifaceted modulator of immune synapse assembly and T cell activation. J. Leuk. Biol. 2017;101:1–16. doi: 10.1189/jlb.2RU1116-474R. [DOI] [PubMed] [Google Scholar]
- 38.Rueda CM, Jackson CM, Chougnet CA. Regulatory T-cell-mediated suppression of conventional T-cells and dendritic cells by different cAMP intracellular pathways. Front. Immunol. 2016;7:216. doi: 10.3389/fimmu.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conche C, Boulla G, Trautmann A, Randriamampita C. T cell adhesion primes antigen receptor-induced calcium responses through a transient rise in adenosine 3’,5’-cyclic monophosphate. Immunity. 2009;30:33–43. doi: 10.1016/j.immuni.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Almahariq M, et al. Exchange protein directly activated by cAMP modulates regulatory T-cell-mediated immunosuppression. Biochem. J. 2015;465:295–303. doi: 10.1042/BJ20140952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan YV, Abad C, Wang Y, Lopez R, Waschek JA. VPAC2 (vasoactive intestinal peptide receptor type 2) receptor deficient mice develop exacerbated experimental autoimmune encephalomyelitis with increased Th1/Th17 and reduced Th2/Treg responses. Brain Behav. Immun. 2015;44:167–175. doi: 10.1016/j.bbi.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abad C, et al. VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage. J Neuroinflammation. 2016;13:169. doi: 10.1186/s12974-016-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellegrini P, Berghella AM, Contasta I, Adorno D. CD30 antigen:not a physilogical marker for Th2 cells but an important costimulator molecule in the regulation of the balance between Th1/Th2 response. Transpl. Immunol. 2003;12:49–61. doi: 10.1016/S0966-3274(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 44.Tinazzi E, et al. In rheumatoid arthritis soluble CD30 ligand is present at high levels and induces apoptosis of CD30+T. cells. Immunol. Let. 2014;161:236–240. doi: 10.1016/j.imlet.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Barbieri A, et al. Characterization of CD30/CD30L+ cells in peripheral blood and sinovial fluid of patients with rheumatoid arthritis. J. Immunol. Res. 2015;2015:729654. doi: 10.1155/2015/729654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tur BS, et al. Soluble CD30 Levels in Patients with Rheumatoid Arthritis. Turk. J. Rheumatol. 2009;24:131–5. [Google Scholar]
- 47.Nakamura T, et al. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-gamma. J. Immunol. 1997;158:2090–8. [PubMed] [Google Scholar]
- 48.Mellado M, et al. T cell migration in rheumatoid arthritis. Front. Immunol. 2015;6:384. doi: 10.3389/fimmu.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagafuchi Y, et al. Immunophenotyping of rheumatoid arthritis reveals a linkage between HLA-DRB1 genotype, CXCR4 expression on memory CD4(+) T cells, and disease activity. Sci. Rep. 2016;6:29338. doi: 10.1038/srep29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muñoz LM, et al. Chemokine receptor oligomerization: a further step toward chemokine function. Immunol. Lett. 2012;145:23–29. doi: 10.1016/j.imlet.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Abad C, et al. cDNA array analysis of cytokines, chemokines, and receptor involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm. Bowel. Dis. 2005;11:674–684. doi: 10.1097/01.MIB.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X, Jing H, Ganea D. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J. Neuroimmunol. 2002;133:81–94. doi: 10.1016/S0165-5728(02)00365-X. [DOI] [PubMed] [Google Scholar]
- 53.Weng Y, Sun J, Wu Q, Pan J. Regulatory effects of vasoactive intestinal peptide on the migration of mature dendritic cells. J. Neuroimmunol. 2007;182:48–54. doi: 10.1016/j.jneuroim.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Aletaha D, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.