Abstract
Mechanisms of lung squamous cell carcinoma (LSCC) development are poorly understood. Here, we report that JNK1/2 activities attenuate Lkb1-deficiency-driven LSCC initiation and progression through repressing ΔNp63 signaling. In vivo Lkb1 ablation alone is sufficient to induce LSCC development by reducing MKK7 levels and JNK1/2 activities, independent of the AMPKα and mTOR pathways. JNK1/2 activities is positively regulated by MKK7 during LSCC development. Pharmaceutically elevated JNK1/2 activities abates Lkb1 dependent LSCC formation while compound mutations of Jnk1/2 and Lkb1 further accelerate LSCC progression. JNK1/2 is inactivated in a substantial proportion of human LSCC and JNK1/2 activities positively correlates with survival rates of lung, cervical and head and neck squamous cell carcinoma patients. These findings not only determine a suppressive role of the stress response regulators JNK1/2 on LSCC development by acting downstream of the key LSCC suppresser Lkb1, but also demonstrate activating JNK1/2 activities as a therapeutic approach against LSCC.
Subject terms: Non-small-cell lung cancer, Stress signalling, Cancer genomics, Prognostic markers, Cancer models
LKB1 is frequently mutated in lung squamous cell carcinomas. Here, the authors show that sole LKB1 depletion is sufficient to drive the development of this cancer, where downstream defective MKK7-JNK1/2 signalling activates the ∆Np63/p63 pathway to induce subsequent epithelial cells transformation and tumour progression.
Introduction
Precision medicine has been enjoying successes in recent years, especially with the application of genomics-driven cancer therapy1. For example, the discovery of key drivers (e.g. EGFR and ALK) of human lung adenocarcinoma using genetic mouse models has led to the development of targeted therapies2. Therefore, identifying cancer driver mutations in human cancers is valuable for targeted therapy2,3.
Lung squamous cell carcinomas (LSCCs) comprise about 25–30% of all lung cancers, which is the leading cause of cancer-related death in the United States and worldwide4. Human LSCC develops from normal airway epithelium and progresses through hyperplasia, squamous metaplasia, dysplasia, and carcinoma5. SCC characteristics emerge at the squamous metaplasia stage in the nests of neoplastic squamous cells with an increase in marker expression (e.g. p63)6. Genomic analysis of human LSCC tumors has provided a comprehensive landscape of the molecular alterations, including frequently mutated genes (e.g., TP53, PTEN and LKB1) and altered pathways (e.g., SOX2, PI(3)K/LKB1, EGFR/FGFR/RAS) associated with this disease7,8. Several preclinical models show that mice carrying compound mutations develop LSCC, as evidenced by studies on KrasG12D/Lkb1loss, Sox2OX/Lkb1loss, Ptenloss/Lkb1loss, and Cdkn2aloss/Ptenloss/Sox2OX mice8–11. In contrast, mutations in one single gene lead to lung adenocarcinomas (ADs), as shown by Trp53 deficiency, Pten ablation, KrasG12D or EgfrL858R activation and Sox2 overexpression (Sox2OX)10,12–14. To date, the question on whether LSCC can be initiated by a single gene mutation remains outstanding and the underlying mechanism of LSCC formation and progression remains unclear4,15.
In addition to genetic causes, LSCC development is frequently impacted by environmental stresses16, as documented in exposures to smoking17, air pollution18 and radon19. Upon environmental challenges, stress signaling, such as the MKK7-JNK1/2 pathway, participates in induction of inflammation responses and changes of expression of cancer-related genes20–22. The JNK1/2 signaling has been linked to cancers in lung, breast and colon and ovary, mainly related to adenocarcinoma cells23. However, the JNK1/2 pathway could act as either a positive or a negative regulator in tumorigenesis24–26. It is not clear what is the role of JNK1/2 signaling in SCC development despite that JNK1/2 is related to LSCC-associated environmental challenges.
LKB1 (also called STK11, serine/threonine kinase 11) is a frequently mutated gene in human LSCCs8,9. LKB1 has been shown as an important suppressor of LSCC, supported by observations that Lkb1 deficiency in mouse lungs drives LSCC development in conjunction with additional mutations8–10. However, ablation of Lkb1 alone in mouse lung using adenovirus-Cre (Ad-Cre) via intranasal delivery does not cause pulmonary neoplasia8,9. This may be due to the limitation on efficiency of intranasal delivery of Cre into large airways where human LSCC is frequently initiated5,27. Using a previously generated codon-optimized Cre recombinase under the control of the Club Cell Secretory Protein promoter (CCSPiCre) for all airway epithelial cells, including cells of large airways, we found that Lkb1 deficiency by itself is sufficient to induce LSCC. In contrast, no LSCC was induced after manipulation of other five candidate genes that have frequent mutations in human LSCCs, including TP53, PTEN, ERRFI1, SMAD4 and KRAS7. We further identified the stress response pathway MKK7-JNK1/2 as downstream effectors of Lkb1 in suppressing ΔNp63 signaling during LSCC development. The observations that LSCC patients with higher JNK1/2 activities have better survival rates and activating JNK1/2 activities attenuate LSCC progression suggest targeting the JNK1/2-mediated stress response pathway as a way to combat against LSCC.
Results
Lkb1 deficiency alone is sufficient to induce LSCC
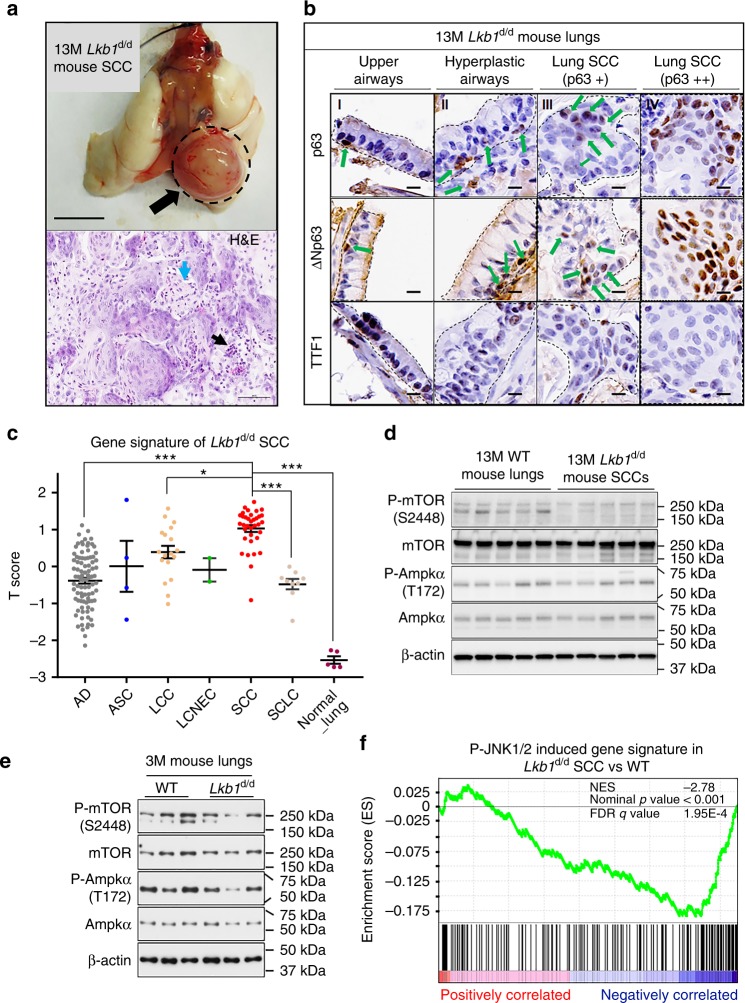
Ablation of Lkb1 in mouse airway was achieved by crossing Lkb1 floxed mice with CCSPiCre mice (Lkb1d/d: CCSPiCreLkb1f/f) (Supplementary Fig. 1a–b). Strikingly, LSCCs were found in Lkb1d/d mice, as early as, 11 months old in addition to AD (Fig. 1a and Supplementary Fig. 2a–b; Table 1 and Supplementary Data 1−2). LSCC lesions in Lkb1d/d mice displayed typical nests of neoplastic squamous cells, some with keratinization and most with infiltrates of neutrophils (Fig. 1a). Cells in the lesions exhibited positive staining of LSCC clinical markers (p63, ΔNp63 and CK5) and lack of lung AD marker TTF1 expression (Fig. 1b and Supplementary Fig. 1c–d)28. Two subpopulations of SCC cells are observed at lesions sites based on levels of LSCC markers p63 and ΔNp63. Cells in nests of neoplastic squamous cells (Fig. 1b, region IV and Supplementary Fig. 1c–d) exhibited higher levels of both p63 and ΔNp63. In contrast, SCC cells adjacent to large airways (Fig. 1b, region III and Supplementary Fig. 1c–d) have relatively lower expression of p63 and ΔNp63, which could be cells at the early phase of LSCC. Meanwhile, progressive lung SCC developmental stages (SCC-DSs) were also observed after Lkb1 ablation (Supplementary Data 1-2), including epithelial hyperplasia (5.4%) (Supplementary Fig. 1e), squamous metaplasia (1.8%) (Supplementary Fig. 1e), adenocarcinoma with squamous differentiation (10.7%) (Supplementary Fig. 1f) and adenosquamous carcinoma (ASC) (5.4%) (Supplementary Fig. 1g). These lung SCC-DSs showed the focal or diffuse positive staining of p63 and CK5, respectively, as well as the focal weak or negative staining of TTF1 (Supplementary Fig. 1e–g). On the other hand, unlike Lkb1 ablation, no lung SCCs and SCC-DSs were induced after individual manipulation of five known SCC-associated genes Pten, p53, Errfi1 (Mig6), Kras and Smad4 (Table 1 and Supplementary Data 1−2). Instead, lungs of Pten deletion, P53 ablation, Errfi1 knockout or KrasG12D activation had AD tumors, adenomas and atypical adenomatous hyperplasia that showed opposite profiles of marker expression compared with lung SCCs in Lkb1d/d mice (Table 1 and Supplementary Fig. 2b−f)8,29. Moreover, loss of Smad4 exhibited no histomorphologic change in lungs (Supplementary Fig. 2g–h), consistent with a previous report13. In summary, these findings reveal a unique capacity of Lkb1 deficiency in the induction of lung SCCs and SCC-DSs.
Fig. 1.
Lkb1 ablation alone induces LSCC development with negative JNK1/2 activities. a Upper panel: Gross appearance of lung tumors in 13-month-old (13M) CCSPiCreLkb1f/f (Lkb1d/d) mice. SCC squamous cell carcinoma; SCC was labeled by a dash cycle and an arrow (upper panel). Scale bar: 0.5 cm. Lower panel: Representative 13M Lkb1d/d mouse lung SCC (H&E). Infiltrates of neutrophils were commonly associated with SCC nests (black arrow, lower panel). Keratinization was frequently present in SCCs (blue arrow, lower panel). Scale bar: 60 μm. b Representative immunohistochemistry (IHC) staining of 13M Lkb1d/d mouse lung SCC. SCC markers: p63 and ΔNp63; AD marker: TTF1. “+” means the expression is mild or moderate, and “++” means the expression is strong. Dashes outline: Regions of interest. Positive staining is indicated in some panels by green arrows. Scale bar: 10 μm. c Alignment of the gene signature of 13-month-old Lkb1d/d mouse lung SCC tumors with Takeuchi human lung cancer database (GSE11969). A score greater than zero indicates a positive correlation with the murine gene signature of 13-month-old Lkb1d/d mouse. AD adenocarcinoma, ASC adenosquamous carcinoma, LCC large-cell carcinoma, LCNEC large-cell neuroendocrine carcinoma, SCC squamous cell carcinoma, SCLC small cell lung cancer. ANOVA (Tukey−Kramer Multiple Comparisons (TKMC) Test) was performed and error bar is SEM; *P < 0.05; ***P < 0.001. d Western blot (WB) analysis (n = 5) of protein expression in 13-month-old (13M) mice. e WB analysis (n = 3) of protein expression in 3-month-old (3M) mice. f Gene set enrichment analysis (GSEA) of pre-ranked genes detected in microarrays analysis of 13-month-old Lkb1d/d mouse lung SCCs. All detected genes (Supplementary Data 3) were ranked based on their fold changes (Lkb1d/d SCC vs. WT) from high (the left terminal of X-axis) to low (the right terminal of X-axis)
Table 1.
Summary of mouse phenotypes of lung tumors and lungs
| Genotype | No. | Age (Month) | The percent (%) of phenotypes in mouse lungs | |||||
|---|---|---|---|---|---|---|---|---|
| SCC-DS | SCC | SCC-DS + SCC | AD-DS | AD | AD-DS + AD | |||
| Lkb1 d/d | 56 | 11–14 | 25.0 | 16.1 | 32.8 | 16.1 | 37.5 | 53.6 |
| Pten d/d | 12 | 12–13 | 0 | 0 | 0 | 16.7 | 8.3 | 25.0 |
| P53 d/d | 10 | 12–13 | 0 | 0 | 0 | 10.0 | 10.0 | 20.0 |
| Mig6 d/d | 12 | 9 | 0 | 0 | 0 | 8.3 | 0 | 8.3 |
| Kras G12D | 8 | 1 | 0 | 0 | 0 | 100 | 37.5 | 100 |
| Smad4 d/d | 9 | 11–13 | 0 | 0 | 0 | 0 | 0 | 0 |
| WT | 25 | 1–14 | 0 | 0 | 0 | 4.0 | 0 | 4.0 |
Lkb1d/d: CCSPiCre Lkb1f/f; Ptend/d: CCSPiCrePtenf/f; P53d/d: CCSPiCrep53f/f; Mig6d/d: CCSPiCreMig6f/f; KrasG12D: CCSPiCreKrasG12D; Smad4d/d: CCSPiCreSmad4f/f; WT wild type. SCC development stages include epithelial hyperplasia, epithelial hyperplasia with squamous metaplasia, adenocarcinoma with squamous differentiation and adenosquamous carcinoma
Adenocarcinoma development stages include epithelial hyperplasia and adenoma
SCC squamous cell carcinoma, DS development stage, AD adenocarcinoma, No. number
Transcriptome profiling demonstrates that the Lkb1-deficient mouse LSCC exhibited a high degree of positive correlation with human LSCC, especially with LSCC Subtypes 2a and 2b, compared to other subtypes of human lung cancers (Fig. 1c and Supplementary Fig. 3a)30. These findings support that Lkb1d/d mouse LSCCs closely resemble human LSCCs at the transcriptome level. Functionally, Lkb1 deficiency altered expression of genes that are enriched in functions of cell movement, morphology, death and survival (Supplementary Table 1), reflecting the histological observations. Interestingly, the known LKB1 downstream target AMPKα31 did not change its phosphorylation levels in the absence of LKB1 at the SCC stage (Fig. 1d). Another LKB1 target mTOR even showed reduced levels of phosphorylation, in contrary to the previously reported role of LKB1 in suppressing mTOR activity (Fig. 1d)32. Like the observations in SCCs, no significant changes of AMPKα and mTOR phosphorylation levels were found in 1- and 3-month old mouse lungs at the pretumor stage in response to Lkb1 deficiency (Fig. 1e and Supplementary Fig. 3b–c). These results suggest that LKB1 utilizes pathways other than those of known cancer-associated ones for LSCC development. Close examination of genes altered by Lkb1 deficiency via ingenuity pathway analysis (IPA) revealed JNK1/2, P38, NFĸB and ERK1/2 as the common downstream pathways for candidate upstream regulators of Lkb1 responsive genes (Table 2 and Supplementary Data 3). Gene set enrichment analysis (GSEA) further showed that the P-JNK1/2 induced pathway, as the top enriched pathway, negatively correlated with the Lkb1 deficiency gene signature (Fig. 1f and Supplementary Table 4). These observations establish an association between Lkb1 deficiency and reduced JNK1/2 activities in LSCC.
Table 2.
Analysis of enriched upstream regulators and its core downstream pathways
| Top upstream regulators | P value of overlap | Core downstream pathways of TNF and IL1B |
|---|---|---|
| TGFB1 | 4.32E-41 | JNK1/2 pathway |
| TNF | 7.04E-39 | p38 pathway |
| TP53 | 2.13E-28 | NFĸB pathway |
| ERBB2 | 3.54E-26 | ERK1/2 pathway |
| IL1B | 7.85E-22 |
Top enriched upstream regulators were identified from these differentially expressed genes identified in the microarray (13-month-old (13M) Lkb1d/d mouse lung SCCs vs. 13M Lkb1f/f mouse lungs; Supplementary Data 3) using ingenuity pathway analysis (IPA)
SCC squamous cell carcinoma
JNK1/2 is inactivated in Lkb1-null induced mouse LSCCs
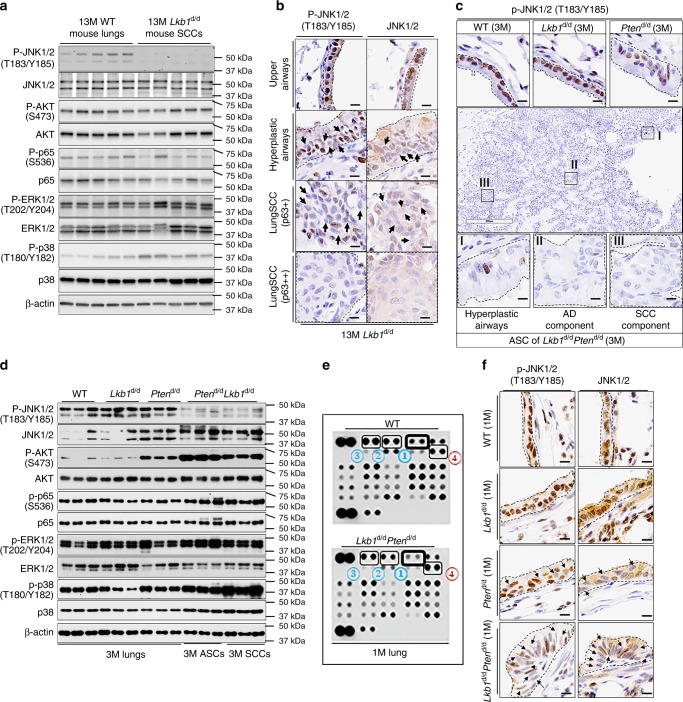
To further investigate the association between reduced JNK1/2 activities and LSCC development, we examined the phosphorylation of JNK1/2, P38, NFĸB and ERK1/2. Indeed, JNK1/2 phosphorylation was almost abolished in Lkb1d/d mouse LSCCs with the most robust change among all tested candidates (Fig. 2a). Reduced JNK1/2 phosphorylation is also seen in LSCC compared to normal upper airway tissues by immunohistochemistry (IHC) assay (Fig. 2b), consistent with western blot results. Moreover, JNK1/2 phosphorylation has an opposite expression pattern to that of ΔNp63 and p63 (Fig. 1b), which further supports a negative correlation between JNK1/2 activities and LSCC in situ.
Fig. 2.
p-JNK1/2 are reduced in Lkb1-deficiency-driven mouse LSCC development. a Western blot (WB) analysis (n = 5) of protein expression in 13-month-old (13M) Lkb1d/d mice. b Representative P-JNK1/2 and JNK1/2 IHC staining of mouse lungs (n = 6). Dashes outline regions of interest. P-JNK1/2 staining is usually nuclear while JNK1/2 staining is usually cytoplasmic and nuclear. c Representative IHC staining results of P-JNK1/2 in 3-month-old mouse lungs and lung tumors (n = 6). Black dashes point to the areas of interest. Box I: hyperplastic airway; Box II: adenocarcinoma (AD) component; Box III: peripheral squamous cell carcinoma (SCC) component. Scale bar: 300 μm (black box) and 10 μm (dark bar). d WB analysis of 3-month-old (3M) mouse lungs and lung tumors. e Kinome array was performed in 1-month-old (1M) mouse lungs (n = 6) (see Supplementary Fig. 4b for a full summary of kinome array). The dots in the membranes are anti-phosphorylated (P)-proteins and the numbers pointing out the top changed proteins were listed in Table 3. f Representative IHC staining of P-JNK1/2 and JNK1/2 in 1-month-old mouse lungs (n = 5). Arrows point to the cells with weak nuclear staining. Scale bar: 300 μm (Black box) and 10 μm (dark bar)
Next, the timing of change of JNK1/2 activities in response to Lkb1 deficiency was examined to determine the sequence of events between JNK1/2 inactivation and LSCC development. We took advantage of the CCSPiCreLkb1f/fPtenf/f (Lkb1d/dPtend/d) mouse model that has accelerated LSCC development in 3 months (Supplementary Fig. 3d–e; Supplementary Table 2; Supplementary Data 1−2 and 4), an incidence rate of lung adenosquamous cell carcinomas (ASCs) at 88.9% (Supplementary Table 2 and Supplementary Data 2), nearly identical molecular profiles between lung SCC and ASC (Supplementary Fig. 3d−g), close resemblance of the combined SCC/ASC transcriptome profile to that of human lung SCCs (Supplementary Fig. 3h and Supplementary Data 3) and similar enriched upstream regulators (Supplementary Table 3). In addition, epithelial hyperplasia (11.1%) and epithelial hyperplasia with squamous metaplasia (11.1%) were observed in the Lkb1d/dPtend/d mouse model (Supplementary Data 1-2). Most importantly, JNK1/2 activities were reduced in SCCs/ASCs (Fig. 2c, d and Supplementary Fig. 4a). The pretumor stage of Lkb1d/dPtend/d mice was defined at 1 month old of age because at this time only hyperplasia was found in the large airways (Supplementary Fig. 5a). A kinome array assay revealed that reduced phosphorylation levels of JNK1/2, ERK1/2 and p38α as well as increased AKT phosphorylation occur in Lkb1d/dPtend/d lungs at the pretumor stage (Fig. 2e; Table 3; Supplementary Fig. 4b–c and Supplementary Table 5). The western blot assay validated that only P-JNK1/2 is consistently downregulated in both SCCs and ASCs and in the pretumor stages (Fig. 2d and Supplementary Fig. 4c). The reduced JNK1/2 phosphorylation in Lkb1d/dPtend/d mouse hyperplastic epithelial cells was confirmed by the IHC assay (Fig. 2f). Histologically normal Lkb1-deficient airways have uniform p-JNK1/2, whereas hyperplastic lesions start to lose p-JNK1/2 positivity (Fig. 2b, f). This suggests that the impact of Lkb1 deletion on p-JNK1/2 levels does not appear to be entirely direct, implying that proliferation induced by Lkb1 loss occurs prior to the decline in p-JNK1/2 levels. The declined P-JNK1/2 levels may contribute to the development of hyperplastic lesions. As a feedback regulation mechanism, these hyperplastic lesions may facilitate the further decline of P-JNK1/2 by providing an oncogenic environment. These findings show that JNK1/2 inactivation occurs before tumor formation.
Table 3.
Top changed phosphorylated (P)-proteins in Lkb1d/dPtend/d lungs (pretumor stage) compared to wild-type (WT) lungs at 1 month of age
| Top changed phosphorylated protein | Change direction | |
|---|---|---|
| ① | JNK pan (T183/Y185, T221/Y223) | Down |
| ② | Erk1/2 (T202/Y204, T185/Y187) | Down |
| ③ | P38α (T180/Y182) | Down |
| ④ | AKT (S473) | Up |
Decreased MKK7 reduced JNK1/2 phosphorylation in mouse LSCC
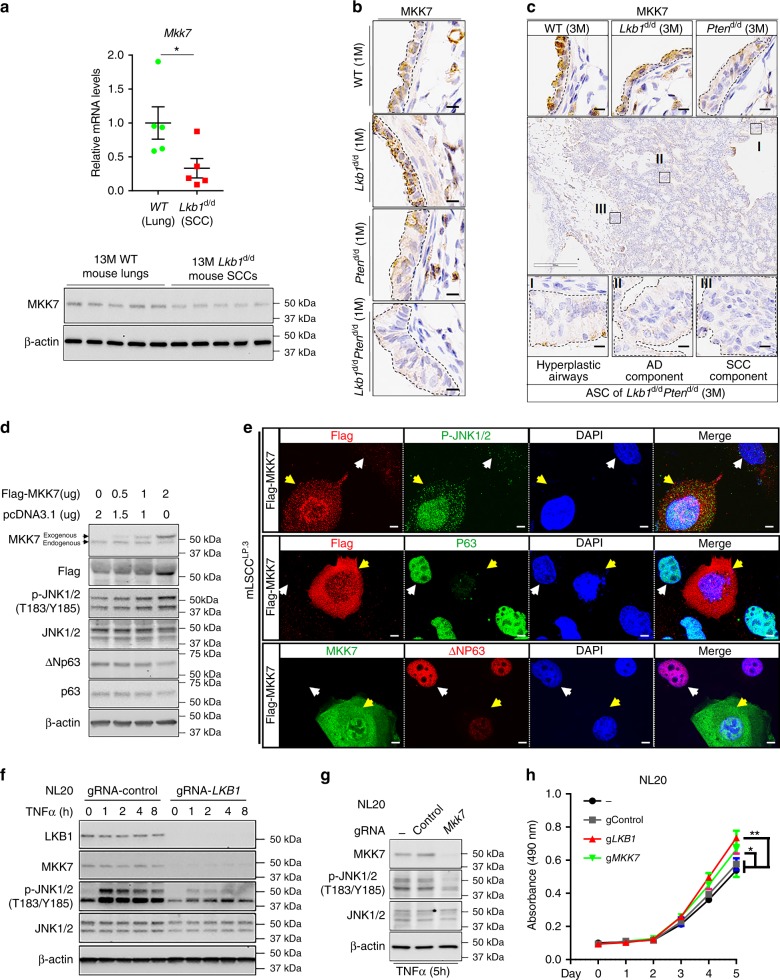
To investigate the underlying mechanism of JNK1/2 inactivation during LSCC development, we examined expression levels of MKK7, which phosphorylates JNK1/2 at T183/Y185 to regulate JNK1/2 activities in previous reports20,33,34. Both mRNA and protein expressions of MKK7 were indeed decreased in Lkb1d/d mouse LSCCs (Fig. 3a). The decrease of MKK7 expression occurs at the pretumor stage (Fig. 3b, c), sharing a similar pattern with that of the decreasing JNK1/2 phosphorylation along the course of LSCC development (Fig. 2c, f). Functionally, MKK7’s impact on JNK1/2 phosphorylation in LSCC development was assessed on a mouse LSCC cell line (mLSCC) that was generated from LSCC tumors of Lkb1d/dPtend/d mice. Exogenous expression of MKK7 in mLSCC by transfection restored JNK1/2 phosphorylation and decreased the levels of p63 and ΔNp63 (Fig. 3d, e). Similar results were observed in H157 cells, which are human LSCC cells with the mutations of LKB1 and PTEN (Supplementary Fig. 5c). Meanwhile, at the pretumor stage modeled by a nontumorigenic human bronchial epithelial cell line NL20 and Beas-2B, the MKK7 knockout also had reduced JNK1/2 phosphorylation levels (Fig. 3g and Supplementary Fig. 5e) while LKB1 ablation decreased levels of both MKK7 expression and phosphorylated JNK1/2 (Fig. 3f and Supplementary Fig. 5d). These results suggest that decreased MKK7 expression has a negative impact on JNK1/2 phosphorylation levels during LSCC development. Furthermore, ablation of LKB1 and MKK7 in NL20 and Beas-2b promoted cell growth (Fig. 3h and Supplementary Fig. 5f). These results not only delineate the LKB1-MKK7-JNK1/2 pathway at the early phase of LSCC development, but also establish a regulatory function of LKB1 on the MKK7-JNK1/2 signaling that is regarded as a stress response pathway in other systems.
Fig. 3.
Decreased MKK7 in response to Lkb1 deficiency reduces JNK1/2 activities. a qRT-PCR (upper panel) and WB (lower panel) analyses of the mRNA and protein expression of MKK7 in mouse lungs and lung SCCs, respectively (n = 5). Error bar is SEM; T test: *P < 0.05. b Representative IHC staining of MKK7 in 1-month-old mouse lungs (n = 5). Black dashes point to the areas of interest. Scale bar: 10 μm. c Representative IHC staining results of MKK7 in 3-month-old mouse lungs and lung tumors (n = 6). Black dashes point to the areas of interest. Box I: hyperplastic airway; Box II: adenocarcinoma (AD) component; Box III: peripheral squamous cell carcinoma (SCC) component. Scale bar: 300 μm (black box) and 10 μm (dark bar). d WB analysis of protein expressions in mouse lung SCC cells (mLSCCLP.3). e Co-staining of Flag/MKK7 and pJNK1/2 or p63 or ΔNp63 in mouse LSCC cells. Scale bar: 50 μm; Yellow arrows point out the cells expressing the exogenous Flag-MKK7; white arrows point out the cells without clear expression of the exogenous Flag-MKK7. f WB analysis of protein expressions in NL20 cells, an immortalized human bronchial epithelial cell line, after knockout of LKB1 or scramble (Control) using lentiviral Cas9/gRNA with the treatment of TNFα (20 ng/mL). g WB analysis of protein expressions in NL20 cells after knockout of MKK7 or scramble (Control) using lentiviral Cas9/gRNA under 5-h treatment of TNFα (20 ng/mL). h MTS assay analysis of cell viability of NL20. −: parental cells; gControl: gRNA targeting noncoding region; gLKB1: gRNA targeting LKB1; gMKK7: gRNA targeting MKK7
Jnk1/2 loss accelerates Lkb1-null induced LSCC development
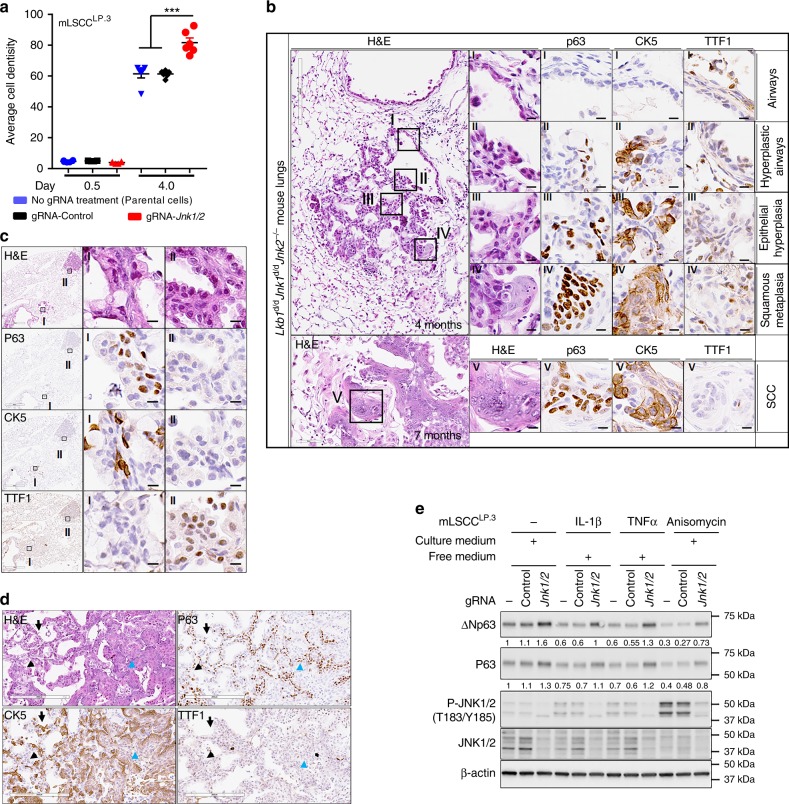
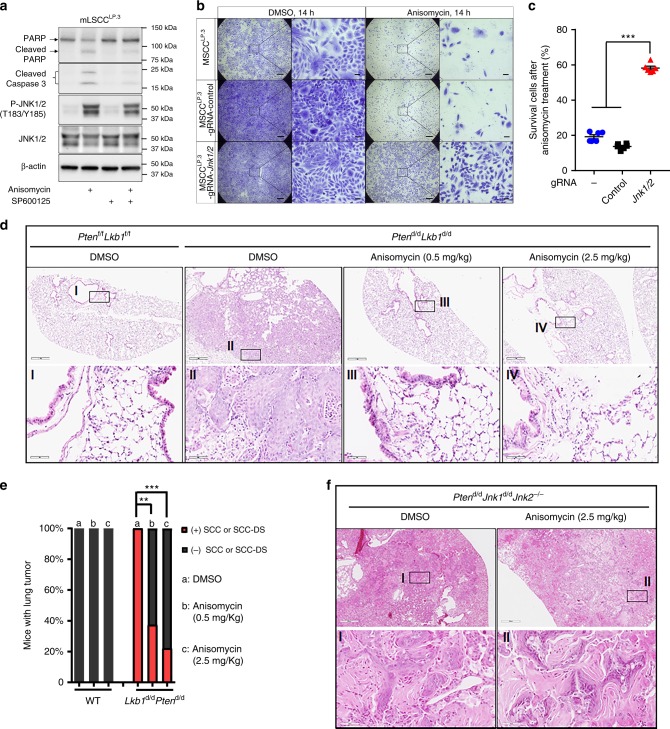
Since the Lkb1-deficient LSCC has a suppressed MKK7/JNK1/2 pathway and JNK1/2 have been indicated in tumor formation24,34,35, we hypothesize that JNK1/2 plays a role in the suppression of LSCC development. To test this, in vitro ablation of Jnk1/2 was first conducted in mLSCC cells to investigate its effect on cellular activities. As anticipated, Jnk1/2 ablation increased cell growth in mLSCC cells (Fig. 4a and Supplementary Fig. 6a–b). The in vivo role of JNK1/2 in LSCC development was next investigated by generating mice with the conditional ablation of both Lkb1 and Jnk1/2 (Lkb1d/dJnk1d/dJnk2−/−) (Supplementary Fig. 6c). Lkb1d/dJnk1d/dJnk2−/− mice started to develop squamous metaplasia in the lungs at 4 months old, followed by full penetrance of pathologic phenotypes (CK5+, P63+) at 7 months old and beyond (Fig. 4b and Supplementary Fig. 6d). The associated phenotypes include epithelial hyperplasia (66.7%) (Fig. 4c), epithelial hyperplasia with squamous metaplasia (25%) (Fig. 4b), and adenocarcinoma with squamous differentiation (16.7%) (Supplementary Fig. 6e), ASC (25%) (Fig. 4d) and SCC (33.3%) (Supplementary Fig. 6d) (Supplementary Data 1−2). Compared to 11−14-month-old Lkb1d/d mice that showed epithelial hyperplasia (5.4%) (Supplementary Fig. 1c–d), epithelial hyperplasia with squamous metaplasia (1.8%) (Supplementary Fig. 1e), squamous metaplasia (1.8%), adenocarcinoma with squamous differentiation (10.7%) (Supplementary Fig. 1f), ASC (5.4%) (Supplementary Fig. 1g), SCC (16.1%) (Fig. 1a, b) (Supplementary Data 1−2), and loss of Jnk1/2 in the Lkb1-deficient background accelerated LSCC development in vivo. Lkb1d/dJnk1d/dJnk2−/− mice also developed adenocarcinoma (Supplementary Fig. 6f). Although the ADs generally showed a papillary morphology microscopically, they resembled LSCCs in that they are positive for IHC expression of the SCC markers p63 and CK5 (Supplementary Fig. 6f). Interestingly, there is no LSCC formation in Jnk1d/dJnk2−/− mice, which only has adenoma/adenocarcinoma development (33.3%) (Supplementary Fig. 6g and Supplementary Data 1), concluding that the JNK1/2 pathway regulates LSCC development under a Lkb1-deficient background. These results suggest that Jnk1/2 knockout promotes Lkb1-deficiency-induced SCC formation by inducing SCC marker expression, such as p63. Taken together, these results reveal that compound mutations of Jnk1/2 and Lkb1 facilitate LSCC development and demonstrate that JNK1/2 act as major suppressors for Lkb1-dependent LSCC progression.
Fig. 4.
Jnk1/2 ablation accelerated Lkb1-deficiency-induced LSCC progression. a Quantification of cell colony formation assay of mLSCCLP.3 cells after knockout of Jnk1/2 or scramble (Control) using Lentiviral Cas9/gRNA. Error bar is SEM; T test: ***P < 0.001. b Representative H&E and IHC staining of 4-month-old (4M) and 7-month-old (7M) CCSPiCreLkb1f/fJnk1f/fJnk2−/− (Lkb1d/dJnk1d/dJnk2−/−) mouse lungs. SCC markers: p63 and CK5; AD marker: TTF1. Scale bar: 600 μm (black box, 4 months), 60 μm (black box, 7 months), and 10 μm (solid black). c Representative H&E and IHC staining of 7-month-old Lkb1d/dJnk1d/dJnk2−/− mouse lung epithelial hyperplasia (I), and adenocarcinoma (II). Scale bar: 300 μm (black box, left panel), 60 μm (black box, middle and right panels). d The representative H&E and IHC staining of lung adenosquamous carcinoma in 7-month-old Lkb1d/dJnk1d/dJnk2−/− mice. Adenocarcinoma (AD) cells are indicated by black arrows, and squamous cell carcinoma (SCC) cells in the lower half of the image are indicated by the green arrowhead. A large portion of the AD component has positive staining for the typical SCC markers CK5 or p63 while having negative or weak staining for TTF1. The SCC component has positive staining for CK5 and p63 while having negative staining of TTF1. Scale bar: 200 μm. e WB analysis of protein expressions in mLSCCLP.3 cells under 5-h treatment of IL-1β (2 ng/mL), TNFα (20 ng/mL) and Anisomycin (10 μM). Free medium and culture medium are described in the Methods section
JNK1/2 inactivation activates mouse LSCC ΔNp63/p63 pathway
We next investigated the downstream effectors of JNK1/2 in regulation of lung SCC development. ΔNp63 is a squamous cell lineage marker promoting squamous cell stratification and tumor progression36,37. We had shown that ΔNp63 was induced in hyperplastic airways and lung SCCs (Fig. 1b and Supplementary Fig. 3f) and was not detected in mouse lung ADs (Supplementary Fig. 1h and 2b). Based on the observation that expression patterns were opposite between levels of ΔNp63 expression (Fig. 1b and Supplementary Fig. 3f) and JNK1/2 phosphorylation (Fig. 2b, f) during lung SCC development, we examined the effect of Jnk1/2 on the expression of ΔNp63. Jnk1/2 knockout mLSCC cells exhibited an increase in ΔNp63 levels (Fig. 4e). Conversely, treatment with stimuli of JNK1/2 pathway, IL-1β, TNFα or Anisomycin raised JNK1/2 phosphorylation levels and decreased the expression of ΔNp63 and p63 in parental or gRNA-Control mLSCC cells (Fig. 4e)38. Quantification of the protein bands of ΔNp63 and p63 showed JNK1/2 had a stronger effect in repressing ΔNp63 than inhibiting p63 expression (Fig. 4e and Supplementary Table 6). Global assessment of the impact of Jnk1/2 ablation on gene expression revealed increased P63 mRNA levels, altered expression of downstream targets of TP63, and changes in genes that are enriched for regulating cancer and cell proliferation (Table 4; Supplementary Table 7 and Supplementary Data 3). Downstream targets of TNF and IL-1β are also impacted by Jnk1/2 ablation (Table 4 and Supplementary Data 3), which is in accordance with the well-known role of JNK1/2 in mediating activities of these two cytokines38–40. In summary, these results indicate that JNK1/2 inactivation activates the ΔNp63/p63 pathway to promote LSCC development.
Table 4.
Ingenuity pathway analysis (IPA) of the differentially expressed genes after knockout Jnk1/2 in MSCCLP.3 cells (Supplementary Data 3)
| P value | |
| Top diseases | |
| Cancer | 2.25E-06–5.73E-19 |
| Top cellular function | |
| Cellular growth and proliferation | 2.25E-06–2.12E-28 |
| Cell death and survival | 2.25E-06–3.84E-26 |
| Enriched upstream regulators | |
| TNF | 1.26E-30 |
| TP63 | 8.77E-20 |
| IL1B | 2.33E-18 |
JNK1/2 activation attenuates mouse LSCC development
While JNK1/2 activities were reduced in LSCC, JNK1/2 proteins remain present in both tumor and pretumor stages of cells (Fig. 2a, b, d, f and Supplementary Fig. 4c). This observation raises the question whether manipulating JNK1/2 activities may have an impact on Lkb1-dependent tumor formation. JNK1/2 activator Anisomycin-treated mLSCC cells showed a significant increase of JNK1/2 phosphorylation levels and the expression of apoptosis markers, cleaved PARP and Caspase-3, whereas the JNK1/2 inhibitor SP600125 reduced levels of these apoptotic indicators (Fig. 5a)38,41. Less than 20% of mLSCC cells survived after Anisomycin treatment, in contrast to the Jnk1/2 knockout significantly maintaining cell survival (Fig. 5b, c). Consistent with the in vitro results, treatment of Lkb1d/dPtend/d mice with Anisomycin for 8 weeks significantly inhibited LSCC formation (Fig. 5d, e) and lung tumor development (Supplementary Fig. 7), with no obvious inhibition on lung adenoma/adenocarcinoma (Supplementary Data 1). In contrast, Anisomycin did not affect the development of LSCC and lung tumors in Lkb1d/dJnk1d/dJnk2−/− mice (Fig. 5f and Supplementary Data 1). These findings demonstrate that raising JNK1/2 activities can inhibit LSCC cell growth in vitro and tumor progression in vivo, suggesting that targeting JNK1/2 may benefit LSCC patients who have low JNK1/2 activities.
Fig. 5.
Pharmaceutical activation of JNK1/2 attenuates LSCC development. a WB analysis of protein levels in mLSCCLP.3 cells with the treatment of Anisomycin (10 μM, 5 h) and SP600125 (10 μM, 4.5 h). b Representative crystal staining of survival mLSCCLP.3 parental cells and stable cells with lentiviral Cas9/gRNA targeting Control or Jnk1/2 after treatment with Anisomycin or DMSO. Scale bar: 500 μm (large area) and 50 μm (small area). c Quantification of cell survival assay of survival mLSCCLP.3 parental cells and stable cells with lentiviral Cas9/gRNA targeting Control or Jnk1/2 was performed. Error bar is SEM; ANOVA-TKMC Test: ***P < 0.001. d Representative H&E staining of lungs or lung tumors of the mice treated with DMSO or Anisomycin. e Quantification of mice with lung SCC or SCC-DS at the endpoint of treatment (Fig. 5d). The b group of Lkb1d/dPtend/d had eight mice and the remainder of each group included nine mice. χ2 test (two-way): **P < 0.01; ***P < 0.001. f Representative H&E staining of lungs or lung tumors of the mice treated with DMSO (n = 9) or Anisomycin (n = 10)
JNK1/2 is inactivated in a large proportion of human LSCCs
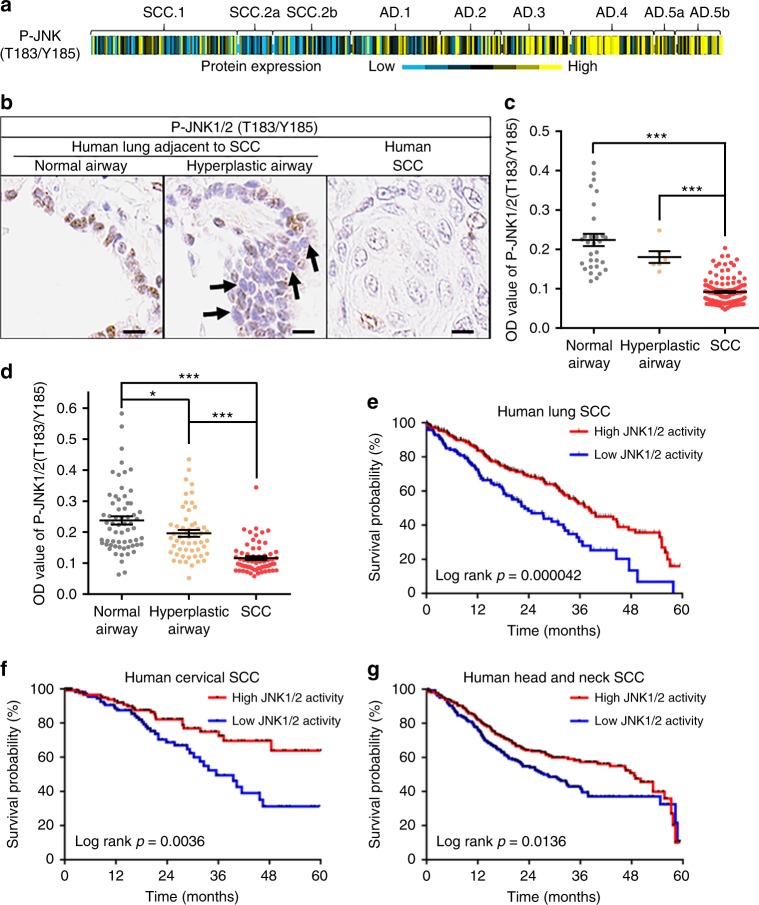
To further investigate the clinical relevance of JNK activities, JNK1/2 phosphorylation levels were surveyed in the TCGA protein array database30,42 and assayed using IHC in human lung cancer tissue arrays. JNK1/2 phosphorylation levels in a sizable proportion of human LSCCs was lower than the average level in the TCGA protein array database, especially when compared to human lung AD cases (Fig. 6a). Significantly lower levels of JNK1/2 phosphorylation in SCCs compared to normal airways were also observed in two independent sets of human LSCC tissue arrays (Fig. 6b–d and Supplementary Fig. 8a–b). In a subset of patients, hyperplastic airway cells also exhibited lower levels of JNK1/2 phosphorylation compared with normal airways (Fig. 6b and Supplementary Fig. 8a–b), resembling the mouse phenotype (Fig. 2b, c, f). In summary, JNK1/2 inactivation is present in a substantial proportion of human LSCCs.
Fig. 6.
JNK1/2 is inactivated in human LSCCs and predicts SCC patients’ survival. a The heatmap of the reverse phase protein array (RPPA) value of P-JNK1/2 in TCGA human lung cancer cohorts (SCC.1, n = 159; SCC.2a, n = 38; SCC.2b, n = 88; AD.1, n = 94; AD.2, n = 68; AD.3, n = 75; AD.4, n = 90; AD.5a, n = 25; AD.5b, n = 49). b, c Representative IHC staining (b) and the semi-quantification (c) of P-JNK1/2 in a human LSCC tissue array. The quantifications were calculated with DAB staining density (brown color) using ImageJ. Optical Density (OD) = log (max intensity/Mean intensity). Two random equal regions of each patient were quantified for SCC (70 patients), and 30 normal airway regions (eight patients) and eight hyperplastic airways (four patients) were quantified. ANOVA (TKMC test) was performed and the error bar is SEM; ***P < 0.001. Arrows (left panel): Weak nuclear staining; Scale bar: 10 μm. d Semi-quantification of a tissue array was calculated with DAB staining density (brown color) as described in (c); three random equal regions of each patient were quantified, including SCC (21 patients), the matched normal airway (21 patients) and the hyperplastic airways (18 patients). ANOVA (TKMC Test) was performed and the error bar is SEM; *P < 0.05; ***P < 0.001. e Prediction of human LSCC patient survival rate using Jnk1/2 knockout mouse signature. The gene signature was generated using the DEGs identified after knockout of Jnk1/2 in MSCCLP.3 cells (Supplementary Data 3). High JNK1/2 activities were defined as patient gene signature scores lower than 0 and vice versa. Each small bar of curve line represents one patient and T test was performed. If the score was below 0, JNK1/2 activities were considered high, and vice versa. f, g Higher JNK1/2 activities are associated with longer survival rate of cervical and head and neck SCC patients. The method was as described in (e) and Methods
JNK1/2 activities predict survival rates of SCC patients
Gene signature analysis of LSCC patients was then investigated to determine whether JNK1/2 activities could predict patient survival. JNK1/2 activities were defined by a gene signature that was generated using these DEGs induced by knocking out Jnk1/2 in mLSCC cells (Supplementary Data 3). Each TCGA human LSCC patient’s JNK1/2 activities were estimated by applying mouse Jnk1/2 knockout gene signatures to expression profiles of human tumors43. Survival analysis showed that patients with higher JNK1/2 activities in LSCC tumors had a better survival rate and longer relapse-free survival (RFS) than those with lower JNK1/2 activities (Fig. 6e and Supplementary Fig. 8c-–d). In contrast, JNK1/2 activities were unable to predict the survival of lung adenocarcinoma patients (Supplementary Fig. 8e–f). In conjunction with the findings on JNK1/2 activities’ antitumor function in mice (Fig. 5), these results suggest a beneficial effect of increasing JNK1/2 signaling for treatment of human LSCCs.
Considering that JNK1/2 activation is required to induce cell apoptosis in multiple SCC cells44–47, we generated the tissue-specific JNK1/2 gene signature to predict the survival of other human SCC patients. Notably, survival analysis showed that the patients whose cervical or head and neck SCC tumors had higher JNK1/2 activities had better survival rate than those with lower JNK1/2 activities (Fig. 6f, g). These results suggest that the JNK1/2 pathway is a conserved suppressor for SCC in multiple tissues.
Discussion
In this study, we generated a mouse lung SCC model in which the sole ablation of Lkb1 in mouse lung airways is sufficient to induce LSCC and have established Lkb1 as a key suppressor gene of LSCC. Lkb1 deficiency results in decreased expression of MKK7, a reduction of JNK1/2 phosphorylation, lower JNK1/2 activities and elevated ΔNp63 signaling, which subsequently leads to epithelial cells transformation and progression into LSCC (Fig. 7). Loss of Lkb1 however does not automatically cause loss of p-JNK1/2 in normal mouse airway epithelial cells. This would indicate that other events must occur to alter p-JNK1/2 levels. However, even though p-JNK1/2 is lost during tumor progression, JNK1/2 protein levels are maintained. Therefore, JNK1/2 activities can still be stimulated at the tumor stage (Figs. 4e and 5a) and activation of JNK1/2 signaling may serve as a tool against LSCC for both treatment and prevention.
Fig. 7.
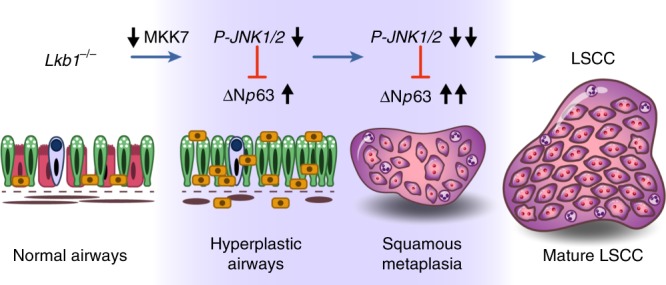
Working model. Lkb1 loss in airways decreases MKK7 expression levels, reduces JNK1/2 phosphorylation levels and activities, and de-represses oncogenic and squamous linage pathway(s) (e.g. ΔNp63 pathway), which prompts hyperplasia and squamous metaplasia in normal airway cells, breaks the barrier against LSCC formation, and promotes LSCC development
Previous studies showed that simulation of LSCC in vivo had only been successfully achieved by compound mutations, including KrasG12D/Lkb1loss, Sox2OX/Lkb1loss, Ptenloss/Lkb1loss, and Cdkn2aloss/Ptenloss/Sox2OX8–11. Although Lkb1 is involved in three of these mouse models, ablating Lkb1 alone using adenovirus-Cre (Ad-Cre) fails to cause pulmonary neoplasia9. As a result, Pten loss or Sox2 overexpression was given more weight as the major factors in driving LSCC development. Since the efficiency and accuracy of delivering Cre recombinase intranasally and intratracheally remain uncertain27,48, findings based on this method may be limited in scope due to technical challenges, especially for pathogenesis in the mouse large airways where human LSCC is frequently initiated5. On the contrary, CCSPiCre is a proven efficient Cre driver that mediates the loxP-dependent DNA recombination in all airway epithelial cells including the large airways13,49. Results based on the CCSPiCre system indeed revealed a previously underestimated role of Lkb1 lost-of-function mutation that by itself Lkb1 deficiency is sufficient to initiate LSCC, in comparison with any single mutation in either Trp53, Pten, Errfi1, Smad4 or KrasG12D (Table 1)10,12–14. Furthermore, the merit that Lkb1 ablation alone induces LSCC offers a simplified platform for screening other regulators of LSCC development. These observations not only place Lkb1 in its right place of the LSCC driving formula, but also show that the CCSPiCreLkb1f/f model is a valuable tool for further investigating additional players that contribute to LSCC development.
In the CCSPiCreLkb1f/f model, LSCC and lung adenocarcinoma were formed at different locations of mouse lung. LSCCs were usually located in the central part of the lung (Fig. 1a and Supplementary Fig. 1c), close to the trachea, after the deletion of Lkb1 in large airways (Supplementary Figs. 1a–b and 5a–b). In contrast, lung adenocarcinomas were often found in the distal parts of CCSPiCreLkb1f/f mouse lung (Supplementary Fig. 2a). This is consistent with human LSCC that belongs to central lung neoplasm50 and is frequently initiated from large airways5. Taken together, our CCSPiCre mouse can be an efficient model for the in vivo study of lung diseases initiated from large airway and of the de novo role of these frequently mutated genes.
JNK1/2 inactivation is associated with not only LSCC but also cervical and head and neck SCC (Fig. 6), suggesting a conserved role of JNK1/2 in various types of SCC development. JNK1/2 activation is required to induce cell apoptosis in multiple SCC cells, including esophageal, oral, head and neck and cervical SCC cells44–47. These results suggest that JNK1/2 inactivation in vivo may promote or lead to SCC formation and progression besides lung SCC. A recent in vivo example is the off-target actions of BRAF inhibitors. They were used for inhibiting melanoma growth, but at the same time also promoted squamous cell carcinoma development that has been reported due to JNK pathway inhibition51. In addition to lung SCCs, LKB1 and MKK7, as upstream regulators of P-JNK1/2, are also found to be frequently mutated in other human SCCs, such as cervical, cutaneous, head and neck and esophageal SCCs (cBioPortal databases)52,53. Meanwhile, we showed that the ΔNp63 pathway, a well-proven oncogenic and squamous linage pathway36, was activated after JNK1/2 inactivation. This further suggests that JNK1/2 inactivation promotes SCC development through a common downstream target p63 since TP63 was also amplified in human cervical and head and neck SCCs (cBioPortal databases). In summary, JNK1/2 pathway may act as a conserved gatekeeper preventing the development of SCCs.
Current targeted therapies for LSCC are limited because the key drivers have not been identified. Although the Ikka mutant (K44A) knock-in mice does develop spontaneous LSCCs54, K44A mutation has not been identified in human LSCC, and the copy number losses and other genomic mutations in IKKa are also rare in human LSCCs7. Here, we demonstrated the potential to target JNK1/2 for human SCC treatment in preclinical models in that activation of P-JNK1/2 using Anisomycin induced LSCC cell death and inhibited LSCC development (Fig. 5). Anisomycin, a natural product produced by some Streptomyces bacteria, stimulates JNK1/2 phosphorylation55. Clinically, Anisomycin has been successfully used for the treatment of amoebic dysentery and trichomoniasis55. It also sensitizes tumor cells to apoptosis induced by the TNF-related apoptosis-inducing ligand (TRAIL)56. The observation that JNK1/2 activities positively correlate with the survival rates of multiple types of SCC patients suggests application of JNK1/2 activators, such as Anisomycin or its derivatives could be an option for SCC patients with low JNK1/2 activities.
Drugs targeting JNK1/2 that are currently under clinical trial or FDA-approved for the treatment of other cancers could be investigated for their anticancer effects on LSCCs. For example, Genistein, which is an enzyme inhibitor and in clinical trial, has been showed to have antitumor effects in human hepatocellular carcinoma cells and to activate JNK1/2 57. In addition, 2-methoxyestradiol, which belongs to mitosis modulators and is also in clinical trial, induced apoptosis in prostate cancer cells through the activation of P-JNK1/258. Therefore, repurposing existing drugs to activate JNK1/2 for LSCC treatment is possible.
Although epithelial hyperplasia and squamous metaplasia are regarded as the initial stages of human LSCC5, not much was described in previous in vivo models4,11. Hence, the mechanism of LSCC formation and progression regulated by genetic alternations are largely unknown. Our work documented tumor progression at the stages of epithelial hyperplasia and squamous metaplasia with different clinical markers as well as provided underlying mechanisms that contribute to pathogenesis. First, we observed epithelial hyperplasia with squamous metaplasia had positive expression of p63 and CK5 in Lkb1d/d (Supplementary Fig. 1e) and in Lkb1d/dJnk1d/dJnk2−/− (Fig. 4b) mice, but both epithelial hyperplasia and adenomas had negative expression of p63 and CK5 in KrasG12D mice (Supplementary Fig. 2f). Consistent with the observations in human lung cancers, these mouse results show that epithelial hyperplasia can develop into SCC, AD or ASC, and the developmental direction of epithelial hyperplasia can be reflected by expression of these markers, such as p63 and CK5 for SCC and TTF1 for AD. In the ASCs, the portions of tumors consisting of AD cells also exhibited the same marker expression of SCC cells (p63 and CK5) in Lkb1d/d (Supplementary Fig. 1g), in Lkb1d/dPtend/d (Supplementary Fig. 3e) and in Lkb1d/dJnk1d/dJnk2−/− (Fig. 4d) mice. Meanwhile, ASC’s molecular profiles are almost identical to that of SCC (Supplementary Fig. 3g). Therefore, the clinical marker staining, such as p63 and CK559, can predict the developing tract before the formation of these typical pathological phenotypes. Second, Lkb1d/d, Lkb1d/dJnk1d/dJnk2−/− and Lkb1d/dPtend/d mouse models in our study can recapitulate the different LSCC developmental stages (DSs) (Fig. 4b–d; Supplementary Figs. 1d–g and 3d–e; Supplementary Data 1−2). This offers the possibility of systematically analyzing the dynamic changes of molecules during LSCC development and to identify these genetic “gatekeepers” of LSCC progression in vivo. We observed that the downregulation of P-JNK1/2 was associated with the progression of LSCC in Lkb1d/d and Lkb1d/dPtend/d mice. As we described in Table 5, Jnk1/2 knockout under Lkb1-loss background not only shortened the average developing time of LSCC-DSs from 13 months to 4 months, but also increased the incidence of LSCC-DSs from 25 to 100%, besides the promotion of LSCCs. Unlike Lkb1d/d mouse lungs in which only some of epithelial hyperplasia were positive for staining of p63 and CK5 (Supplementary Fig. 1c–d and Supplementary Data 2), they can be detected in all epithelial hyperplasia of Lkb1d/dJnk1d/dJnk2−/− mouse lungs (Fig. 4b, c and Supplementary Data 2). Furthermore, some of lung adenocarcinoma in Lkb1d/dJnk1d/dJnk2−/− mice showed p63 and CK5 positive staining (Supplementary Fig. 6f). These results conclude that JNK1/2 inactivation drives LSCC formation and development in response to Lkb1 deficiency. Therefore, evaluation of LSC-DSs can facilitate studying what and how genetic elements regulate LSCC development.
Table 5.
Summary of Lkb1d/dJnk1d/dJnk2−/− mouse lung tumor phenotypes in comparison to Lkb1d/d mouse lung tumor phenotypes
| Genotype | No. | Age (Month) | The percent (%) of phenotypes in mouse lungs | |||||
|---|---|---|---|---|---|---|---|---|
| SCC-DS | SCC | SCC-DS + SCC | AD-DS | AD | AD-DS + AD | |||
| Lkb1 d/d | 56 | 11–14 | 25.0 | 16.1 | 32.8 | 16.1 | 37.5 | 53.6 |
| Lkb1 d/d Jnk1 d/d Jnk2 −/− | 12 | 7–8 | 100 | 33.3 | 100 | 8.3 | 33.3 | 33.3 |
SCC squamous cell carcinoma, DS development stage, AD adenocarcinoma, No. number
Investigation of how JNK1/2 signaling inhibits ΔNp63 expression helps further dissect the mechanism of JNK1/2 repressing LSCC development. One potential link between JNK1/2 and ΔNp63 is c-Jun. ΔNp63 has been reported to be negatively regulated by c-Jun in response to Amyloid-β-Induced cell stress60 while JNK1/2 is a typical activator of c-Jun34. Interestingly, our microarray analysis of SCCs in Lkb1d/d and Lkb1d/dPtend/d mice showed that c-Jun expression is significantly decreased (Supplementary Data 3). We also observed the significant reduction of c-Jun in a Jnk1/2 knockout array (gRNA-Jnk1/2 vs. gRNA-Control) (Supplementary Data 3). These results suggest inactivation of the JNK1/2 pathway induces ΔNp63 expression partially due to the decrease of c-Jun. Continued identification of the pathways regulated by JNK1/2 will serve to better target and customize therapy for SCC.
The LSCC model resulting from Lkb1 loss in the pulmonary epithelium represents a model to study the cellular origins of SCCs. Anatomic location precursors suggest that SCCs in our mouse model arise de novo (Figs. 1b and 4b; Supplementary Fig. 1c-e and Supplementary Data 2). Our CCSPiCre has been shown to have Cre activity in the basal cells of the proximal airway13, which are p63-positive and are generally thought to be one of the main cellular origins11. This suggests that some of SCC cells were developed from these p63-positive basal cells. The observation of SCC only in Lkb1d/d (8.9%), Lkb1d/dPtend/d (11.1%) and Lkb1d/dJnk1d/dJnk2−/− (16.7%) mice also supports this idea (Supplementary Data 1-2). Here, we defined the mice of SCC only as the ones that have only SCC lesions and nonadenocarcinoma precursors. Meanwhile, SCCs may also transdifferentiate from adenocarcinoma or its precursors. This idea was indicated by the SCC marker staining (CK5 and P63), which was positive in SCC-DSs of these models (Supplementary Figs. 1f–g, 3e and 6e–f; Fig. 4c, d and Supplementary Data 2), especially for these adenocarcinoma precursors. Considering the reports of the trans-differentiation from Lkb1-deficient adenocarcinoma (AD) into SCC in Ad-Cre-KrasG12DLkb1f/f mice37,61,62, this possibility also exists. The use of single-cell RNA-Seq tumors for this model at varying stages of development will help identify the changes in cell types and the transcriptome of these cells during SCC progression. This will aid in understanding the cellular origins of SCC.
Interestingly, the COSMIC database (https://cancer.sanger.ac.uk/cosmic), which includes 1588 SCC and 36 ASC samples among 8018 patients, shows that the frequency of LKB1 mutations in pure human SCC and ASC is 1.89% and 11.11%, respectively. In other words, the mutation rate in ASC is much higher (5.89 fold) than SCC. Therefore, LKB1 may require secondary mutations, such as PTEN or alterations in JNK1/2 signaling to direct the progression of the de novo tumors to SCC vs. ASC or to promote the progression of ASC to SCC. Meanwhile, we observed one common mutation (CDS: c.1062C > G; AA: p.F354L) between SCC and ASC, which may indicate the potential for transdifferentiating.
In summary, we exploited the experimental merits of the mouse to establish Lkb1 as a key driver gene from those frequently mutated genes in human LSCCs, and to understand the formation and progression of LSCC by unveiling the de novo role of JNK1/2 inactivation. Extending the finding of JNK1/2 from mice to human shows that JNK1/2 is inactivated in a large population of LSCCs and its activity is positively associated with longer survival rate of multiple human SCC patients, including LSCC. Our model-informed progression analysis, together with genetic, functional and clinical relevance studies, establishes JNK1/2 as a key regulator of LSCC progression in mice and humans, providing a promising target for the therapy of SCC patients with low JNK1/2 activities, including LSCCs.
Methods
Mice
CCSPiCre and Mig6f/f mouse strains were made by Dr. DeMayo’s lab and Jnk1f/fJnk2−/− mouse strain was made by Dr. Roger Davis’s lab and the generation methods of these mice were in previous publications13,63,64. Lkb1f/f (FVB;129S6-Stk11tm1Rdp/Nci), KrasG12D (B6.129-Krastm4Tyj/Nci) and p53f/f (FVB.129P2-Trp53tm1Brn/Nci) mouse strains were from the NCI Frederick Mouse Repository. Ptenf/f (C;129S4-Ptentm1Hwu/J) strain were from Jackson Lab. Smad4f/f (Smad4RobCA) stain is from Dr. Elizabeth J. Robertson’s lab. The deletion or overexpression of those genes in mouse lung was achieved by breeding conditional genetic mice with CCSPiCre mice. All animal protocols were approved by the National Institute of Environmental Health Sciences and Baylor College of Medicine. All experiments were conducted in accordance with relevant guidelines and regulations of both institutions. All strains were B6;129 and all mice were genotyped by Transnetyx. Similar numbers of female and male mice were purposely included in all the mouse experiments, and no histopathologic differences were observed between female mice and male mice during lung tumor development.
Anisomycin treatment
WT (Lkb1f/fPtenf/f) and Lkb1d/dPtend/d (CCSPiCreLkb1f/fPtenf/f) mice at 6 weeks old received Anisomycin (SIGMA, A9789) or vehicle through intraperitoneal (i.p.) injection for 8 weeks65,66. Anisomycin was dissolved in DMSO and diluted in PBS, and the final dissolved reagent was constituted by 7.5% DMSO and 92.5% PBS, which served as the vehicle. Two concentrations (0.5 mg/kg and 2.5 mg/kg) of Anisomycin were used for treatment three times per week67. Similarly, Lkb1d/dJnk1d/d Jnk2−/− (CCSPiCreLkb1f/fJnk1f/fJnk2−/−) mice at 6–7 months old received Anisomycin (2.5 mg/kg) or vehicle through intraperitoneal (i.p.) injection for 8 weeks. Fifty microliters was used per injection. Two hours after the last treatment, mice were euthanized for tissue collection and examination of lung.
Cell derivation and culture
mLSCCLP.3 cells were isolated from Lkb1d/dPtend/d mouse lung SCC tumors using a cancer cell isolation kit as per the protocol. NL-20, Beas-2B and H157 cells were purchased from ATCC. The culture medium for MSCCLP.3 cells included RPMI, 10% FBS, penicillin (100 IU/mL) and streptomycin (100 μg/mL). NL20 was cultured using complete growth medium: Ham’s F12 medium, 2.7 g/L glucose, 0.005 mg/mL insulin, 10 ng/mL epidermal growth factor, 0.001 mg/mL transferrin, 500 ng/mL hydrocortisone and 4% FBS. Beas-2B was cultured using the BEGM kit (Lonza, No. CC-3170). H157 cells was cultured using DMEM and 10% FBS, penicillin (100 IU/mL) and streptomycin (100 μg/mL). Given that P-JNK1/2 in lung SCC tumors of Lkb1d/dPtend/d mice was low (Fig. 2c, d), TNFα or IL-1β was used to treat mLSCC cells. Before the treatment of TNFα or IL-1β, the cells were starved with free medium overnight. The free medium means RPMI for mLSCCLP.3 cells or Ham’s F12 medium for NL20 cells or BEBM medium for Beas-2B cells. As described in the American Type Culture Collection (ATCC), an NL20 cell is an immortalized and nontumorigenic human bronchial epithelial cell line that was established by expression of replication-defective SV40 large T antigen, and Beas-2B cells were isolated from normal human bronchial epithelium obtained from the autopsy of noncancerous individuals and infected with an adenovirus 12-SV40 virus hybrid (Ad12SV40) and cloned. H157 cells are human LSCC cells with mutations of LKB1 and PTEN.
Plasmid transfection
Flag-MKK7 and Flag-pcDNA3.1 were purchased from Addgene. Both plasmids were transfected into mLSCCLP.3 and H157 cells following the manufacturer protocol of Lipofectamine 2000. Western blot and immunofluorescence (IF) were performed to examine the transfected cells after transfection for 48 h using the similar methods68. Details of IF staining process: 1. Place cells on 12 mm coverslips in 24-well plates (Fisher-12-545-82-12cir-1D); 2. Wash cells twice using 1× PBS (pH 7.4); 3. Fix cells in 0.5 mL 4% paraformaldehyde (10 mL 16% in 30 mL PBS) (polysciences, Inc. # 18814) for 10 min at RT; 4. Wash cells twice using 1× PBS (pH 7.4); 5. Incubate cells in 0.5 mL 0.5% Triton-X-100 in PBS for 5 min at RT; 6. Wash cells twice using 1× PBST (PBS with 0.2% Tween 20, pH 7.4); 7. Prepare blocking buffer using 1× PBST with 5% normal donkey serum (Jackson Immune Research 017-000-121) plus 0.2% Fish Stain Gelatin (leave at 37 °C to dissolve), and incubate cells in blocking buffer 60 min at room temperature (RT); 8. Incubate cells using primary antibodies for overnight at 4 °C, which is diluted in blocking buffer (1:100–1:1000); 9. Wash cells three times using 1× PBST 5 min each at RT; 10. Incubate cells using secondary Ab for 60 min at RT, which was diluted in blocking buffer (1:200); 11. Wash cells three times using 1× PBST 5 min each at RT; 12. Place cells on slide using Vectashield Mounting Media (H1500, Hardset); 13. IF pictures were taken using confocal microscopy.
Cell proliferation, colony and survival assay
For cell proliferation assay, ~2000 NL20 and Beas-2B cells were seeded in one well in a 96-well plate at day 0, respectively. Each group has four replicates. Each assay was performed by adding 20 μL MTS reagent (Promega, #G3582) into each well containing 200 μL cell culture medium, incubating for 1 h and then recording absorbance at 490 nm with a 96-well plate reader. mLSCCLP.3 cells were cultured in six-well plates and each experiment group included six wells for colony and survival assay. For cell colony assay, ~10,000 cells were seeded at day 0. For the survival assay, ~1 × 105 cells were seeded at day 0. At the endpoint of experiments, cells were washed one time using 1× PBS and then fixed by 3.7% paraformaldehyde (PFA) for 15 min at room temperature (RT). PFA was removed and the cells were stained for 30 min with 0.05% Crystal Violet69. Cells were gently washed five times using tap water. Plates were drained by inverting them overnight, and each well was photographed. The stained cells were counted based on the photo using ImageJ method70.
Histopathology and immunohistochemistry
The experiments were performed following the similar methods8,17. Mouse lungs were fixed in 4% paraformaldehyde and paraffin-embedded according to standard protocols. H&E and IHC analyses were performed on 5 μm lung sections. Sections underwent dewaxing using CitriSolv clearing agent followed by antigen retrieval and peroxide quenching with 3% H2O2 in methanol for 15 min and blocked with 5% normal goat serum plus Avidin (four drops of Avidin from Avidin/Biotin Blocking Kit/mL) or MOM blocking reagents at RT for 1 h. Subsequently, the slides were incubated with primary antibodies diluted in 5% normal goat serum plus Biotin (four drops of Biotin from Avidin/Biotin Blocking Kit/mL) at 4 °C overnight. Slides were washed using 1× PBS five times (5 min per time), and then incubated with 1% BSA to block at RT for 30 min and then followed with secondary antibodies diluted in 1% BSA at RT for 30 min. The slides were then developed using the Vectastain ABC kit and DAB reagents. The subgroups of different proteins from the same staining slide (e.g., four H&E groups (Supplementary Fig. 1d) are indicated in the large area (Supplementary Fig. 1c). All H&E and IHC results were scanned at the NIEHS Image Core, including the tissue array slides. Scanning method: 1. Slides were cleaned with an isopropanol solution prior to scanning. 2. Slides were then scanned using the Aperio AT2 Scanner, (Aperio: Leica Biosystems Inc., Buffalo Grove, IL). This machine uses line scanning technology to capture high-resolution, seamless digital images of glass slides. 3. After scanning, slides were viewed and captured using Aperio ImageScope v. 12.3.0.5056 (Aperio: Leica Biosystems Inc., Buffalo Grove, IL), a digital slide viewing program. Measurements were taken from these images using a measurement scale that was internally calibrated for each image as it was scanned. p63 and ΔNp63 and TTF1 are nuclear staining and multifocal. Squamous cell carcinomas showed positive staining of both P63 and ΔNp63. Within the SCCs, the compact arrangements of basal-appearing cells with scant cytoplasm tended to show nuclear staining of p63.
Human tissue array
The array samples shown in Fig. 6b, c and Supplementary Fig. 8a were purchased from US Biolab. The detailed patient information can be downloaded from US Biolab website. And the other batches of array (Fig. 6d and Supplementary Fig. 8b) were from the Pathology and Histology Core at Baylor College of Medicine. Histological examinations were confirmed by Dr. Patricia D. Castro and Dr. Michael M. Ittmann. The detailed patient information can be found in Supplementary Table 8. The full staining slides were scanned at the NIEHS Image Core as described in the paragraph of Histopathology and IHC, and random regions of each patient were quantified using the optical density (OD) values, which were calculated based on the DAB staining density (brown color) using ImageJ17. OD = log (max intensity/mean intensity). ANOVA (TKMC Test) was performed.
Microarray and ingenuity pathway analysis
RNA isolation: Following the same RNA isolation protocol8, total RNAs were isolated from mouse lungs or lung tumors using TRIzol reagent and cleaned by RNeasy kit. For mouse cells, total RNAs were isolated by RNeasy kit. All mRNAs were reversely transcribed into cDNA with the M-MLV kit. RNA quality was assessed by using the Agilent Model 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Experiment processes: (1) GSE111313 and GSE111314 arrays were performed at NIEHS. Gene expression analysis was conducted using Agilent Whole Mouse Genome 4 × 44 multiplex format oligo arrays (Agilent Technologies, 014868) following the Agilent one-color microarray-based gene expression analysis protocol. Starting with 400 ng of total RNA, Cy3-labeled cRNA was produced following the manufacturer’s protocol. For each sample, 1.65 μg of Cy3-labeled cRNAs was fragmented and hybridized for 17 h in a rotating hybridization oven. Slides were washed and then scanned with an Agilent Scanner. Data were obtained using the Agilent Feature Extraction software (v12), using the one-color defaults for all parameters. The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. The resulting data were processed using OmicSoft Array Studio (Version 10) software. (2) GSE111335 was performed at BCM using SurePrint G3 Mouse GE 8 × 60 K Microarray Kit: The Genomic and RNA Profiling Core first conducted Sample Quality checks using the NanoDrop ND-1000 and Agilent Bioanalyzer. Agilent One-Color Expression-Low Input Quick Amp v6.6 (Sept 2012) protocol was followed.
Labeling protocol: Fifty nanograms of total RNA, combined with RNA spike mix, were reverse transcribed using a T7 Primer Mix to produce cDNA. The cDNA product was transcribed using T7 RNA Polymerase, producing cyanine-3-labeled cRNA. The labeled cRNA was purified using a Qiagen RNeasy Mini Kit. Purified products were quantified using the NanoDrop spectrophotometer for yield and dye incorporation and tested for integrity on the Agilent Bioanalyzer. Six hundred nanograms of the labeled cRNA was fragmented.
Hybridization protocol: Approximately 480 ng of fragmented cRNA samples was loaded onto each of the Mouse G3 8 × 60 K Expression arrays. The arrays were hybridized in an Agilent Hybridization Chamber for 17 h at 65 °C with rotation at 10 rpm.
Wash protocol: The arrays were washed using the Agilent Expression Wash Buffers One and Two, followed by acetonitrile, as per the Agilent protocol.
Scan protocol: Once dry, the slides were scanned with the Agilent Scanner (G2565BA) using Scanner Version C and Scan Control software version A.8.3.1. Data extraction and quality assessment of the microarray data was completed using Agilent Feature Extraction Software Version 11.0.1.1.
Expression array analysis: The files with raw expression value were used to identify differentially expressed genes (DEGs) by the Genomics Suite Gene Expression workflow of Partek software package version 6.6 (Partek Inc., St. Louis, MO, USA). The quantile normalization and log2 transformation were applied to generate signal values of all samples. The expression profiles were compared among different groups using a one-way ANOVA model, and all the DEGs are listed in Supplementary Data 3.
Functional analysis: Functional analysis of DEGs was performed using the Ingenuity Pathway Analysis (IPA, www.ingenuity.com) based on the content of 2015-03-22. For a given biological category in IPA, Fisher’s exact test was used to calculate the P value. The categories with P values less than 0.05 were defined as significantly enriched. Microarray data were deposited in the Gene Expression Omnibus (GEO, accession GSE111313, GSE111314 and GSE111335).
Real-time quantitative PCR and western blotting (WB)
These experiments were done using the same conditions8,17. Briefly, lung tissues were stored at −80 °C and total RNA was extracted using the TRIzol method followed by purification using an RNeasy Mini Kit. RNA quality was assessed by using the Agilent Model 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Total RNAs were reverse transcribed into cDNA with the M-MLV kit following the protocol. The list of primers for SYBR® Green is shown in Supplementary Table 9. The Taqman probes for 18s (4310893E) and Lkb1 (Mm00488470_m1) were purchased from Applied Biosystems. Full blots of key figures can be found in Supplementary Fig. 9.
Kinome array
The kinome array was purchased from R&D Systems (Cat. no.: ARY003B), which can simultaneously detect the relative site-specific phosphorylation of 43 kinases and 2 related total proteins. Each experiment group (Supplementary Fig. 4b-c and Supplementary Table 5) included six mouse lungs (~15 mg per mouse). Lungs were combined and homogenized to extract proteins. The remainder of the steps followed the standard protocol of the array kit. Nonsaturated dots in the membrane were quantified using ImageJ13 and normalized in the other groups to WT group (Lkb1f/f Ptenf/f mouse lungs).
Cas9/gRNA experiments
The gRNA sequences are included in Supplementary Table 9. These gRNAs were cloned into the LentiCRISPRv2 vector63. CRISPR-Lenti nontargeting control plasmid was purchased from MilliporeSigma (Billerica, MA) (CRISPR12-1EA). MAX Efficiency DH5α (ThermoFisher Scientific, Cat# 18258012) were used to amplify plasmids. Lentiviruses were produced in the Viral Vector Core Laboratory of NIH/NIEHS or Baylor College of Medicine and were used to infect cells for the knockout of the specific gene. The pooled cells infected by gRNA were collected for western blot to confirm the knockout efficiency. Noninfected and gRNA-control-infected cells were used as controls.
Gene signature analysis
The gene expression array dataset GSE11969 (Takeuchi et al., 2006) was analyzed with the mouse lung cancer gene signature approach13. The publicly available datasets of human SCC (n = 553), AD (n = 576), head and neck SCC (n = 566), and cervical SCC (n = 308) cohorts were downloaded from the UCSC Xena webpage (https://xenabrowser.net/hub/). The mean-normalized gene and protein expression values were used for further analyses. The patient phenotype included survival time (OS) and longer relapse-free survival (RFS). The gene expression profile of each human sample was scored based on Jnk1/2 signature, which was generated from the mouse Jnk1/2 knockout model using our previous published method43. For the head and neck SCC, and cervical SCC datasets, only the genes passing expression cutoff filter of average FPKM (Fragments Per Kilobase Million) higher than 5 are included in the analysis. The gene signature score (also known as a “t-score”) was defined for each sample as the two-sided t statistic comparison of the high Jnk1/2-signature genes’ expression profile with the low Jnk1/2-signature genes’ profile. Samples with a gene signature score (t-score) higher than 0, which share a similar gene signature profile of Jnk1/2 knockout in mLSCCLP.3 cells, were classified as having low JNK1/2 activities and vice versa.
Gene set enrichment analysis (GSEA)
The oncogenic signatures (c6.all.v6.1.symbols.gmt) of the GSEA 3.0 version were used. All detected genes were pre-ranked based on their fold changes (SCC vs. WT) from high to low (Supplementary Data 3). The settings of GSEA were listed: Number of permutations (1000), Enrichment statistic (Classic), Normalization mode (Meandiv), Make detailed gene set report (True) and Seed for permutation (Timestamp).
Quantification and statistical analysis
Data are expressed as mean + SD or mean + SEM. The sample size (n) represents biological replicates. Student’s t test was used for comparison of two group averages. When there were more than two groups, one-way ANOVA Tukey−Kramer multiple comparisons (TKMC) test was performed. GraphPad Prism version 7.02 was used to generate the survival curves and calculate the P value of log-rank (Mantel−Cox) test. Spearman correlation between gene signature and protein expression and the P value was calculated using Partek Genomics Suite 6.6 software. Group size was determined based on the results of preliminary experiments and no statistical method was used to predetermine sample size in animal studies. All histopathological results were blinded to pathologists, and the evaluation reports, including the classification of different groups, are provided by pathologists. All the bioinformatics and statistical analyses were analyzed or confirmed by bioinformaticians. The genotyping of mice was conducted by Transnetyx. All the array experiments were unbiasedly conducted and analyzed by core facilities. All qRT-PCR experiments were performed in a blinded manner. The rest of group design and outcome analysis were not performed in a blinded manner. Statistical significance was considered for all the datasets when the P value was less than 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We appreciate Janet DeMayo for the help of proofreading the manuscript. We would like to thank Douglas Joubert, NIH Library Editing Service, for reviewing the manuscript. Many thanks to Linwood Koonce for his great mouse work in supporting this project. We appreciate Drs. Paul Wade, Steve Kleeberger, Kenneth S. Korach, Carmen J. Williams, Humphrey Yao, Guang Hu, and Robert C. Sills for their valuable suggestions. We appreciate the support from the NIEHS animal facility (Galo A. Defaz, Angela Dickerson, and Molly Comins), Knockout Mouse Core (Dr. Manas K. Ray, Dr. Artiom Gruzdev, and Gregory J. Scott), Digital Imaging Core (Eli Ney), Molecular Genomics Core (Drs. Kevin Gerrish and Liwen Liu) and Viral Vector Core (Drs. Negin Martin and Shih-Heng Chen). We also thank Image Associates, Inc. at NIEHS for the help in generating the cartoon of working model. We thank BCM Genetically Engineered Mouse Core, Biostatistics and Informatics Group, Tissue Culture, and Genomic Profiling Core. Some human samples were provided by the Pathology and Histology Core at Baylor College of Medicine with funding from the NIH (NCI P30-CA125123). This work was mainly supported by research grants to F.J.D. (NIEHS, Z1AES103311-01).
Author contributions
J.L. and F.J.D. designed experiments and research aims. J.L. conducted experiments with the help of M.R. and S.-N.C., and analyzed data with help from T.W. and J.-L.L.. J.L., S.-P.W. and F.J.D. wrote the manuscript with help from T.W., C.J.W. and R.J.D.. T.W., C.J.C. and J.-L.L. performed and confirmed all the bioinformatics and statistical analyses. K.S.J., C.J.W., P.D.C. and M.M.I. provided histopathological evaluations and confirmed the interpretation of tumor pathologic phenotypes. R.J.D. provided the Jnk1f/fJnk2−/− strain and contributed critical information and helpful discussions. F.J.D. supervised the whole project.
Data availability
Microarray data were deposited in the Gene Expression Omnibus (GEO, accession GSE111313, GSE111314 and GSE111335). Different detailed data are provided in Supplementary Data 1−5. All detailed information of key reagents, including the dilution of antibodies, was listed in Supplementary Data 5. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tianyuan Wang, Chad J. Creighton, San-Pin Wu.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09843-1.
References
- 1.Senft D, Leiserson MDM, Ruppin E, Ronai ZA. Precision oncology: the road ahead. Trends Mol. Med. 2017;23:874–898. doi: 10.1016/j.molmed.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3.Poole W, Leinonen K, Shmulevich I, Knijnenburg TA, Bernard B. Multiscale mutation clustering algorithm identifies pan-cancer mutational clusters associated with pathway-level changes in gene expression. PLoS Comput. Biol. 2017;13:e1005347. doi: 10.1371/journal.pcbi.1005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AP, Adrianzen Herrera D, Zhang Y, Perez-Soler R, Cheng H. Mouse models in squamous cell lung cancer: impact for drug discovery. Expert Opin. Drug Discov. 2018;13:347–358. doi: 10.1080/17460441.2018.1437137. [DOI] [PubMed] [Google Scholar]
- 5.Wistuba II. Molecular pathogenesis of non-small cell lung carcinomas. J. Lung Cancer. 2012;11:12–20. doi: 10.6058/jlc.2012.11.1.12. [DOI] [Google Scholar]
- 6.Nobre AR, Albergaria A, Schmitt F. p40: a p63 isoform useful for lung cancer diagnosis—a review of the physiological and pathological role of p63. Acta Cytol. 2013;57:1–p48. doi: 10.1159/000345245. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research, N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay A, et al. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep. 2014;8:40–49. doi: 10.1016/j.celrep.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferone G, et al. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell. 2016;30:519–532. doi: 10.1016/j.ccell.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong Y, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 2017;7:86–101. doi: 10.1158/2159-8290.CD-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Jian, Cho Sung-Nam, Akkanti Bindu, Jin Nili, Mao Jianqiang, Long Weiwen, Chen Tenghui, Zhang Yiqun, Tang Ximing, Wistub Ignacio I., Creighton Chad J., Kheradmand Farrah, DeMayo Francesco J. ErbB2 Pathway Activation upon Smad4 Loss Promotes Lung Tumor Growth and Metastasis. Cell Reports. 2015;10(9):1599–1613. doi: 10.1016/j.celrep.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellar A, Egan C, Morris D. Preclinical murine models for lung cancer: clinical trial applications. Biomed. Res. Int. 2015;2015:621324. doi: 10.1155/2015/621324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villaruz LC, Burns TF, Socinski MA. Do oncogenic drivers exist in squamous cell carcinoma of the lung? Oncology (Williston Park) 2013;27:913–904. [PMC free article] [PubMed] [Google Scholar]
- 16.Bender E. Epidemiology: the dominant malignancy. Nature. 2014;513:S2–S3. doi: 10.1038/513S2a. [DOI] [PubMed] [Google Scholar]
- 17.Delgado J, et al. Lung cancer pathogenesis associated with wood smoke exposure. Chest. 2005;128:124–131. doi: 10.1378/chest.128.1.124. [DOI] [PubMed] [Google Scholar]
- 18.Nafstad P, et al. Lung cancer and air pollution: a 27 year follow up of 16 209 Norwegian men. Thorax. 2003;58:1071–1076. doi: 10.1136/thorax.58.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat. Res. 2006;608:157–162. doi: 10.1016/j.mrgentox.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Tournier C, et al. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soberanes S, et al. Particulate matter air pollution induces hypermethylation of the p16 promoter Via a mitochondrial ROS-JNK-DNMT1 pathway. Sci. Rep. 2012;2:275. doi: 10.1038/srep00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gkouveris I, Nikitakis NG. Role of JNK signaling in oral cancer: a mini review. Tumour Biol. 2017;39:1010428317711659. doi: 10.1177/1010428317711659. [DOI] [PubMed] [Google Scholar]
- 24.Tournier C. The 2 faces of JNK signaling in cancer. Genes Cancer. 2013;4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cellurale C, et al. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol. Cell Biol. 2011;31:1565–1576. doi: 10.1128/MCB.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy NJ, et al. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 2003;17:629–637. doi: 10.1101/gad.1062903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travis WD, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch. Pathol. Lab. Med. 2013;137:668–684. doi: 10.5858/arpa.2012-0263-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, et al. Mig-6 deficiency cooperates with oncogenic Kras to promote mouse lung tumorigenesis. Lung Cancer. 2017;112:47–56. doi: 10.1016/j.lungcan.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F, et al. Multiplatform-based molecular subtypes of non-small-cell lung cancer. Oncogene. 2017;36:1384–1393. doi: 10.1038/onc.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. (Oxf.) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Bergami P, et al. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol. Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 35.Fosbrink M, Aye-Han NN, Cheong R, Levchenko A, Zhang J. Visualization of JNK activity dynamics with a genetically encoded fluorescent biosensor. Proc. Natl Acad. Sci. USA. 2010;107:5459–5464. doi: 10.1073/pnas.0909671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, et al. Lkb1 inactivation drives lung cancer lineage switching governed by Polycomb Repressive Complex 2. Nat. Commun. 2017;8:14922. doi: 10.1038/ncomms14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y, et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat. Commun. 2014;5:4629. doi: 10.1038/ncomms5629. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, et al. Complete inhibition of anisomycin and UV radiation but not cytokine induced JNK and p38 activation by an aryl-substituted dihydropyrrolopyrazole quinoline and mixed lineage kinase 7 small interfering RNA. J. Biol. Chem. 2005;280:19298–19305. doi: 10.1074/jbc.M413059200. [DOI] [PubMed] [Google Scholar]
- 39.Verma G, Datta M. IL-1beta induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;15:864–876. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- 40.Wicovsky A, et al. Sustained JNK activation in response to tumor necrosis factor is mediated by caspases in a cell type-specific manner. J. Biol. Chem. 2007;282:2174–2183. doi: 10.1074/jbc.M606167200. [DOI] [PubMed] [Google Scholar]
- 41.Bennett BL, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl Acad. Sci. USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbani R, et al. A pan-cancer proteomic perspective on The Cancer Genome Atlas. Nat. Commun. 2014;5:3887. doi: 10.1038/ncomms4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin J, et al. COUP-TFII inhibits TGF-beta-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493:236–240. doi: 10.1038/nature11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarapore RS, Yang Y, Katz JP. Restoring KLF5 in esophageal squamous cell cancer cells activates the JNK pathway leading to apoptosis and reduced cell survival. Neoplasia. 2013;15:472–480. doi: 10.1593/neo.122126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su CC, et al. Cantharidin induced oral squamous cell carcinoma cell apoptosis via the JNK-regulated mitochondria and endoplasmic reticulum stress-related signaling pathways. PLoS ONE. 2016;11:e0168095. doi: 10.1371/journal.pone.0168095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Song XM, Ji YY, Jiang H, Xu LG. The dual mTORC1 and mTORC2 inhibitor AZD8055 inhibits head and neck squamous cell carcinoma cell growth in vivo and in vitro. Biochem. Biophys. Res. Commun. 2013;440:701–706. doi: 10.1016/j.bbrc.2013.09.130. [DOI] [PubMed] [Google Scholar]
- 47.Gao LJ, et al. The role of globular heads of the C1q receptor in HPV 16 E2-induced human cervical squamous carcinoma cell apoptosis is associated with p38 MAPK/JNK activation. J. Transl. Med. 2013;11:118. doi: 10.1186/1479-5876-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAuley JL, et al. The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol. 2017;10:1581–1593. doi: 10.1038/mi.2017.16. [DOI] [PubMed] [Google Scholar]
- 49.You R, et al. IL17A regulates tumor latency and metastasis in lung adeno and squamous SQ.2b and AD.1 cancer. Cancer Immunol. Res. 2018 doi: 10.1158/2326-6066.CIR-17-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suarez E, Knollmann-Ritschel BEC. Squamous cell carcinoma of the lung. Acad. Pathol. 2017;4:2374289517705950. doi: 10.1177/2374289517705950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vin H, et al. BRAF inhibitors suppress apoptosis through off-target inhibition of JNK signaling. Elife. 2013;2:e00969. doi: 10.7554/eLife.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Z, et al. The pivotal role of IKKalpha in the development of spontaneous lung squamous cell carcinomas. Cancer Cell. 2013;23:527–540. doi: 10.1016/j.ccr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng X, et al. Biosynthesis of the pyrrolidine protein synthesis inhibitor anisomycin involves novel gene ensemble and cryptic biosynthetic steps. Proc. Natl Acad. Sci. USA. 2017;114:4135–4140. doi: 10.1073/pnas.1701361114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slipicevic A, et al. Low-dose anisomycin sensitizes melanoma cells to TRAIL induced apoptosis. Cancer Biol. Ther. 2013;14:146–154. doi: 10.4161/cbt.22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roh T, Kim SW, Moon SH, Nam MJ. Genistein induces apoptosis by down-regulating thioredoxin-1 in human hepatocellular carcinoma SNU-449 cells. Food Chem. Toxicol. 2016;97:127–134. doi: 10.1016/j.fct.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Davoodpour P, Landstrom M. 2-Methoxyestradiol-induced apoptosis in prostate cancer cells requires Smad7. J. Biol. Chem. 2005;280:14773–14779. doi: 10.1074/jbc.M414470200. [DOI] [PubMed] [Google Scholar]
- 59.Ma Y, et al. Expression of p63 and CK5/6 in early-stage lung squamous cell carcinoma is not only an early diagnostic indicator but also correlates with a good prognosis. Thorac. Cancer. 2015;6:288–295. doi: 10.1111/1759-7714.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fonseca MB, Nunes AF, Rodrigues CM. c-Jun regulates the stability of anti-apoptotic DeltaNp63 in amyloid-beta-induced apoptosis. J. Alzheimers Dis. 2012;28:685–694. doi: 10.3233/JAD-2011-111547. [DOI] [PubMed] [Google Scholar]
- 61.Han X, et al. Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat. Commun. 2014;5:3261. doi: 10.1038/ncomms4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li F, et al. LKB1 inactivation elicits a redox imbalance to modulate non-small cell lung cancer plasticity and therapeutic response. Cancer Cell. 2015;27:698–711. doi: 10.1016/j.ccell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Jian, Cho Sung-Nam, Wu San-Pin, Jin Nili, Moghaddam Seyed Javad, Gilbert Jennifer L., Wistuba Ignacio, DeMayo Francesco J. Mig-6 deficiency cooperates with oncogenic Kras to promote mouse lung tumorigenesis. Lung Cancer. 2017;112:47–56. doi: 10.1016/j.lungcan.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das M, et al. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc. Natl Acad. Sci. USA. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wanisch K, Wotjak CT. Time course and efficiency of protein synthesis inhibition following intracerebral and systemic anisomycin treatment. Neurobiol. Learn. Mem. 2008;90:485–494. doi: 10.1016/j.nlm.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Z, et al. In vivo toxicological evaluation of Anisomycin. Toxicol. Lett. 2012;208:1–11. doi: 10.1016/j.toxlet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, et al. REGgamma modulates p53 activity by regulating its cellular localization. J. Cell Sci. 2010;123:4076–4084. doi: 10.1242/jcs.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016;2016:prot087379. doi: 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 70.Rafehi, H. et al. Clonogenic assay: adherent cells. J. Vis. Exp., 10.3791/2573 (2011). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Microarray data were deposited in the Gene Expression Omnibus (GEO, accession GSE111313, GSE111314 and GSE111335). Different detailed data are provided in Supplementary Data 1−5. All detailed information of key reagents, including the dilution of antibodies, was listed in Supplementary Data 5. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.