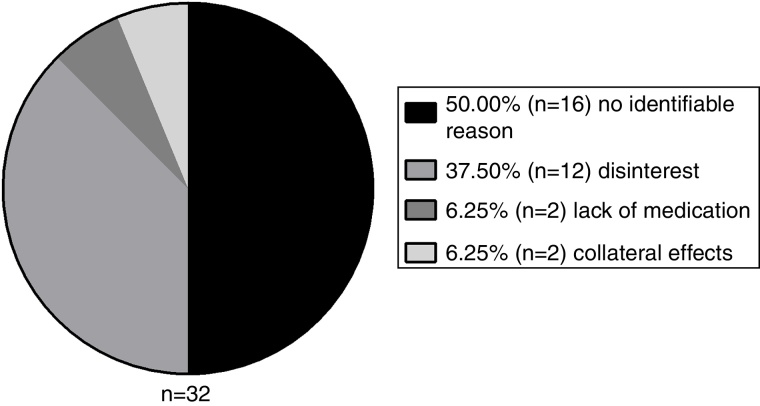Abstract
Background
There has been a revolution in the treatment of Chronic Myeloid Leukemia since imatinib's introduction. However, patient adherence has a great impact on the response obtained with medical treatment. This study's objective was to analyze the drug adherence and the factors that influenced it in patients with Chronic Myeloid Leukemia in a referral hospital in the Brazilian Amazon.
Method
This was a retrospective study including 120 patients with Chronic Myeloid Leukemia from January 2002 to December 2014. The adherence was estimated by the Proportion of Days Covered and the persistence by Kaplan–Meier analysis. The data was analyzed in Epi Info 7® software and the relationship between the variables was analyzed by Fisher's exact test. A p-value lower than 0.05 was considered significant.
Results
Twenty-seven patients (22.5%) were considered non-adherent. There has been irregular medication use and disinterest in the treatment in 20.83% (n = 25), of which 13 were considered non-adherent (p < 0.001). A total of 26.67% (n = 32) abandoned the treatment for a period. Of those, 56.25% (n = 18) were non-adherent (p < 0.001). Distance to the hospital, lack of medication and side-effects were all non-significant to low adherence. At the end of a 360-day follow-up, 44.16% (n = 53) of patients presented a break in persistence, whose average was 255 days.
Conclusion
The adherence found in this study was similar to that found in others of its kind. The only factors that negatively influenced the adherence were disinterest and abandonment of treatment, which can reflect the need to individually educate Chronic Myeloid Leukemia patients.
Keywords: Leukemia, Myelogenous, Chronic, Adherence, Imatinib, Amazon
Introduction
For more than a decade, with the introduction of imatinib, there has been a revolution in the treatment of Chronic Myeloid Leukemia.1, 2 It was the first drug of the Tyrosine-Kinase Inhibitors (TKI) class to be used in CML treatment.2 Its introduction produced a significant increase in patients’ survival.
Various recent studies have shown that the lack of adherence to imatinib is frequent and that this has a strong impact on the treatment's response, and even a reduction in survival.1, 3 There are diverse methods previously described to verify adherence and several formulas that can indirectly calculate this. The most common are: the Medication Possession Ratio (MPR), the Continuous Measure of Adherence (CMA) and the Proportion of Days Covered (PDC).4
Since the lack of adherence can be influenced by several factors, such as social status and demography, and as Brazil is a country historically marked by social and educational inequalities, studies to describe this correlation are necessary, especially in the Brazilian Amazon. This region's population is traditionally considered to be mainly composed of indigenous native people, riverside communities, and some rural workers who subsist from extractivism and agriculture.3, 5, 6
Studies that correlate the social and demographic issues of the Brazilian Amazon and the possible different adherence outcomes are almost nonexistent. Hence, this study analyzes the factors that can influence the adherence in patients diagnosed with CML in a hospital in the same region.
Patients and methods
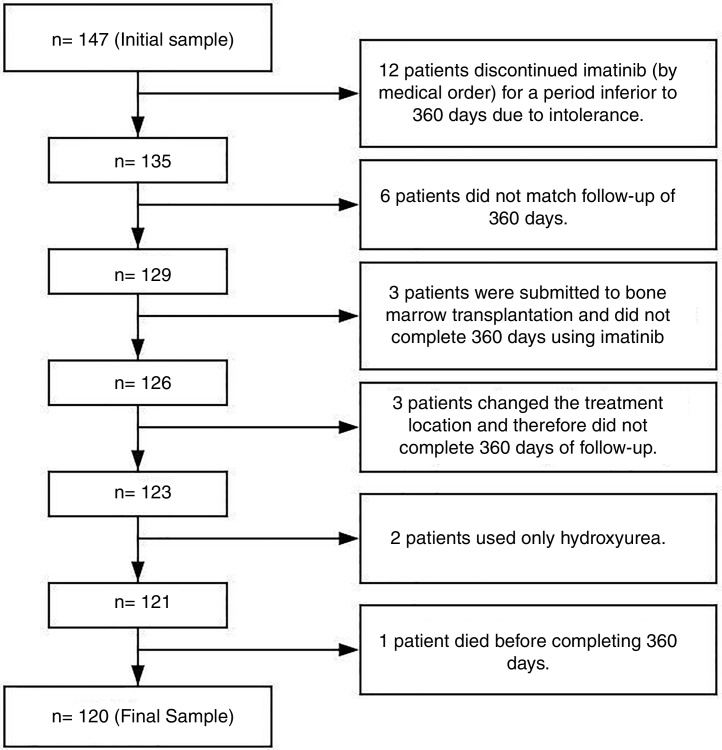
This was a descriptive, observational and retrospective study performed at Ophir Loyola Hospital (HOL) in the city of Belém, the Pará state capital. This hospital is considered a referral center for cancer treatment in the Brazilian Amazon region. The initial sample consisted of 147 patients, however only 120 were considered eligible (Figure 1).
Figure 1.
Illustrative diagram of the process of composing the studied sample.
We measured the imatinib adherence for 360 days (the first year of treatment) for 120 patients from the years 2002 to 2014 in non-electronic medical records. The records were sequentially selected to include the hospital patients over this period who were eligible, according to the inclusion and exclusion criteria. In this manner, there was no direct contact between the researchers and the patients, which precluded the need for a Term of Agreement document required by the hospital ethical committee norm.
The inclusion criteria included patients diagnosed with Chronic Myeloid Leukemia (CML) BCR/ABL-positive regardless of gender, age or race, for whom imatinib had been prescribed and who were followed for at least 360 days for a more trustworthy observation of adherence.7, 8, 9 The exclusion criteria included patients who had been in treatment with imatinib for less than 360 days due to important toxicity and had discontinued treatment with imatinib and changed to another TKI by medical order; patients that for any reasons did not match the follow-up of 360 days, and; patients who had had bone marrow transplantation early in the treatment (Figure 1). Although patients had more than one exclusion criteria, in Figure 1 they were grouped only under one for better understanding.
For the quantitative evaluation of adherence, we preferred the Proportion of Days Counted (PDC) method, calculated by the ratio of the days supplied by medication, divided by the total days of observation, as demonstrated below:
In order to homogenize the calculation, a fixed observation period of 360 days was used, following the first day of the prescription of imatinib. The group that reached a PDC ≥ 80% was defined as adherent, and as non-adherent, the group with PDC < 80%. This cutoff value was established by its utilization in several other studies.10, 11, 12
The variables that influenced the PDC value, and consequently the adherence, were further investigated.
Of the variables collected, the socio-demographic data included gender, age, place of origin, and educational level. The educational level was classified by years of study, stratified by the Brazilian national education guidelines laws and bases, which comprises: 0–1 years (analphabet), 2–9 years (complete and incomplete elementary), 10–13 years (complete and incomplete secondary), and ≥ 14 years (complete and incomplete tertiary). Missing data about this variable was referred as “not informed” and was not used or considered during analysis.
The sample was grouped according to the distance, in a straight line, from the place of origin to the city of Belem, where the hospital is located. The use of a straight line was preferred over the road distance because of the region's geographical characteristics, considering that many people use boats and, in some cases, airplanes, to go from one city to the other. Thus, the distance stratification was made according to the most geopolitically important cities in the surroundings of Belem. The intervals were the following: 0 km (Belem), 0–70 km, 70–150 km, 150–370 km, 370–600 km and greater than 600 km.
Other variables included information relevant to the adherence and were grouped into unintentional factors and intentional ones, according to those proposed by Dos Reis et al.7 in his study. Unintentional factors included distance from the place of origin, low educational level, adverse reactions and lack of medication at the hospital. Intentional factors were composed of the suspension of imatinib due to pregnancy, abandon without justification and the disinterest factor.
Abandon was defined in this study as the non-attendance to three or more consecutive medical appointments without justification. Disinterest was defined as irregular appointments or non-recommended change in dose, all without plausible explanation, despite adequate medical orientations. Persistence was defined as the time interval in days following the first use of imatinib to the first day of treatment discontinuation (the cessation of medication use without medical orientation).
Given the high prevalence in this region, we also investigated the relation between the malaise induced by parasitism and its association with the adherence.
Furthermore, we plotted a Kaplan–Meier curve using persistence as a studied phenomenon, as done by Santoleri et al.,4 and similar to Rego et al.5 The time of observation in the plot was 360 days.
For data analysis tabulation, we created an electronic form with the input of the variables of interest mentioned early in this text into the Epi Info 7® software. Subsequently, the databank was exported as a Microsoft Excel® file for better data visualization and workability. For graphics plotting, the Prism® software was used. For variable analysis, the continuous ones were analyzed using the Mann–Whitney test. The chosen statistic test for categorical variables was Fisher's exact. The confidence interval of 95% was used, and p-values inferior to 0.05 were considered significant.
Results
The study age average was 46 ± 17.4 years. Data concerning gender distribution, educational level and distance range analysis can be found in Table 1.
Table 1.
Sociodemographic and clinical characteristics of CML patients stratified by adherent and non-adherent groups.
| Socio-demographic data | Adherent | Non-adherent | p-value |
|---|---|---|---|
| 1. Gender | |||
| Male | 55.91% (n = 52) | 37.03% (n = 10) | – |
| Female | 44.09% (n = 41) | 62.96% (n = 17) | 0.124 |
| 2. Age | 46 ± 17.40 | 46 ± 17.46 | 0.111a |
| 3. Scholarship level | |||
| 0–1 year | 6.45% (n = 6) | 3.70 (n = 1) | 1.0 |
| 2–9 years | 39.78% (n = 37) | 29.63% (n = 8) | 1.0 |
| 10–13 years | 26.88% (n = 25) | 18.51% (n = 5) | 1.0 |
| ≥14 years | 8.60% (n = 8) | 7.41% (n = 2) | – |
| Not informed | 18.28% (n = 17) | 40.74% (n = 11) | – |
| 4. Distance range | |||
| Zero | 45.16% (n = 42) | 40.74% (n = 11) | – |
| Up until 70 km | 20.43% (n = 19) | 22.22% (n = 6) | 0.826 |
| 70–150 km | 7.53% (n = 7) | 7.41% (n = 2) | 0.821 |
| 150–370 km | 16.13% (n = 15) | 25.93% (n = 7) | 0.808 |
| 370–600 km | 8.60% (n = 8) | 0 | 0.425 |
| More than 600 km | 2.15% (n = 2) | 3.70% (n = 1) | 0.537 |
| Total | 100% (n = 93) | 100% (n = 27) | |
For this variable, it was used Mann–Whitney U Test. For the remaining variables it was chosen Fisher's exact.
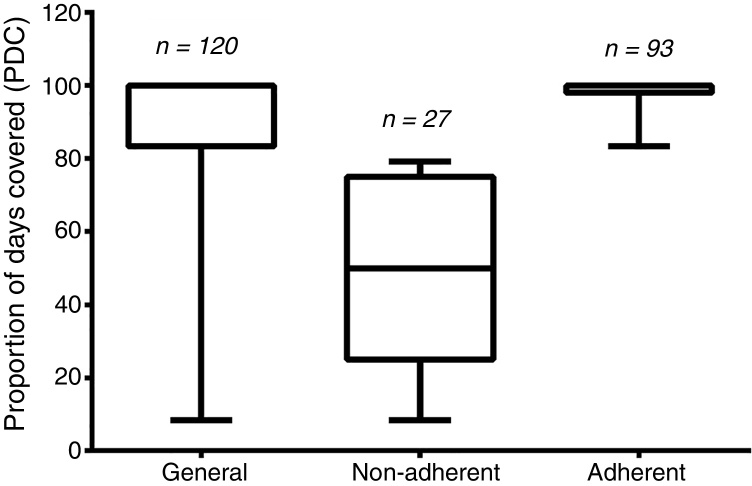
The PDC average was 86.51 ± 23.92, and 22.5% of the patients (n = 27) were considered non-adherents (i.e., having their PDC values under 80%). The remaining 77.5% (n = 93) were considered adherents. The general PDC value and those from the two groups are demonstrated in Figure 2.
Figure 2.
Boxplot of the proportion of days covered by groups.
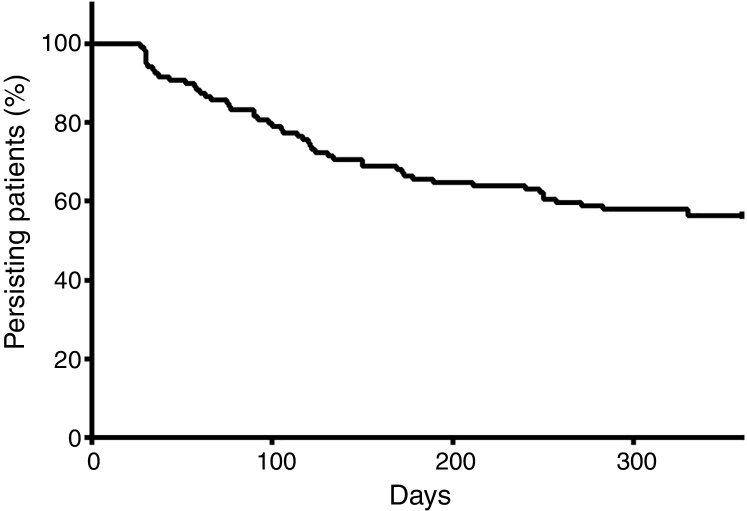
At the end of a 360-day follow-up, 44.16% (n = 53) patients demonstrated a break in persistence, as shown in Figure 3. It is noteworthy that more than 40% of the patients presented a break in persistence at the end of 360 days, and that approximately half of these cases occurred before 100 days. The average of persistence was 255 ± 130 days.
Figure 3.
Kaplan–Meier plot considering the break in persistence as the study phenomenon in follow-up.
A considerable amount of the population has suffered from parasitism during treatment (23.33%; n = 28), the highest incidence in the sample being Entamoeba histolytica, in 6.89% (n = 6) of the cases, followed by Ascaris lumbricoides in 4.17% (n = 5). Overall, the presence of parasitism was not a significant factor interfering in adherence (p = 0.22).
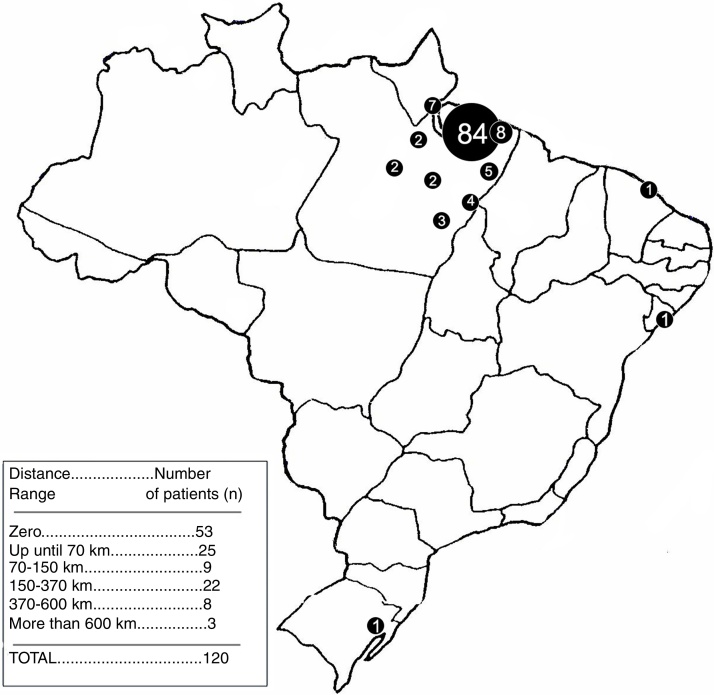
The relation of the distances from the respective patient's distance to the Ophir Loyola Hospital (HOL) for a medical appointment can be found in Figure 4.
Figure 4.
Map of Brazil containing the demographic density of the sample.
None of the considered distance ranges have shown to be significant in relation to lesser distance ranges. Table 1 displays the p-values of the variables.
In 18.33% (n = 22) records, doctors recorded suspicion of the irregular use of medication and patient disinterest, and three more patients were classified in the disinterest group, owing to an irregular non-attendance to appointments during the treatment without evidence of a plausible cause, totaling 20.83% (n = 25) patients with this factor. Of these, 13 were considered non-adherents, which represented a significant factor in the lack of adherence (p < 0.001).
A total of 26.67% (n = 32) patients abandoned the treatment for a period and 56.25% of these showed a lack of adherence (n = 18), compared to 9.09% of the group that did not abandon. Therefore, this variable has also shown to be a significant interfering factor for non-adherence (p < 0.001). The abandon reasons are demonstrated in Figure 5.
Figure 5.
Distribution of abandonment causes of TKI use.
The lack of imatinib at the hospital affected 7.5% (n = 9) patients, however it was not a significant factor (p = 0.12).
According to the Common Terminology Criteria for Adverse Events (CTCAE), of the 2 patients that abandoned treatment due to collateral effects, one had Grade-3 pain in lower extremities and Grade-3 visual blurring, and the other had Grade-3 febrile neutropenia. These patients interrupted treatment without medical advice, and were not present at three or more medical appointments, thus being included in this study's definition of abandon.
Discussion
The low adherence to imatinib is a common problem in clinical practice, as described in various studies.2, 13, 14 It is related to a significant risk of therapeutic failure and imatinib resistance,2, 3, 14 as well as an elevation in costs for healthcare services.15, 16 Some studies verified that the MPR is inversely associated with costs and resources usage in the treatment of CML.3 These resources usage can be understood as a greater number of hospitalizations, higher out-patient costs and higher costs with pharmacological therapy not related to imatinib.3 Hence, the low adhesion to imatinib can be considered a public health problem.
This study's objectives were to describe and analyze the causes for the low adherence in the Amazon region, whereP a shortage of studies that correlate the clinical aspects with the sociodemographic characteristics has been verified, as well as to compare it with other Brazilian and worldwide studies.
The general PDC average was superior to 80%, indicating that the population is generally adherent, but it should be noted that the great variety of results found, as for the standard deviation, was relatively high. The adherence rate was 77.5%, which is similar to the findings of other international studies.2, 13 Nonetheless, it was considerably higher than a Brazilian study in the city of Fortaleza,7 in which only 53% of patients had satisfactory adherence. However, it is important to note that the adherence rate value is still far from the ideal in a serious disease like CML.2, 13
The persistence was demonstrated to be an important variable in this study: at the end of a short period (360 days), 44.16% of the patients presented at least one therapy discontinuation. Figure 4 shows that there is a great tendency to discontinue the therapy soon after the start, namely before 150 days. These findings contrast greatly with those of Santoreli et al.,4 who demonstrated persistence in more than 90% of patients using imatinib during the same period of observation. We speculate that this important discontinuation may be related to the patients’ lack of knowledge on the necessity for rigid control of the disease. In fact, a Brazilian study conducted by Hamerschlack et al.,17 in a sample from 8 Brazilian capital cities, concluded that despite patients being diagnosed rapidly and having good access to the treatment, they are lacking in comprehension of the importance of the control measures for CML.
Among the sociodemographic factors, the educational level did not significantly correlate with the low adherence, when compared to higher levels. However, a Brazilian study in the city of Teresina5 identified a positive correlation between the educational levels and the cytogenetic response. Nevertheless, a multicentric study18 did not report a positive correlation between these same variables. On the other hand, it is important to note that in the present study, there was no information on the educational level in 20.38% of the patients, which may have a direct impact on the results, impairing the analysis that has been performed only with informed data.
The distance to the City of Belém was also not a significant factor interfering with the adherence, which is surprising since the displacement difficulty in certain towns in the Amazon region is still an issue. Moreover, even extremely great distances did not represent a significant factor, when compared with relatively smaller distances. Nonetheless, this corroborates with another Brazilian study by Rego et al. (2015).5
Parasitism was initially supposed to be an important factor interfering in the treatment adherence, taking into consideration the region's undeveloped reality and high parasitism rate. It was believed that clinical signs of certain diseases could be confused, especially by the patient, with imatinib's side effects, such as nausea and vomiting,17, 18, 19 and in that manner lead to intentional discontinuation by the patient. Despite not being significant, it is important to highlight the retrospective nature of this study, which does not invalidate the need for a prospective study in high parasitism rate areas.
The disinterest and abandon represent significant factors influencing low adherence. This strengthens the possibility that some patients can lack comprehension about the need for long-term control. The abandon causes are various, but the side effects and lack of medication at the hospital were associated in relatively few cases. Kekäle et al.,20 using questionnaire interviews, did not find a correlation between side effects and adherence, despite the fact that 97% ot the patients reported at least one side effect, half of them having a negative influence on life. However, there are reports of certain side effects being important factors for intentional non-adherence.18, 21
The disinterest was the most prevalent identifiable factor. However, half of the patients did not seem to have an identifiable reason to stop the medication. The 16 patients that presented abandon of treatment without an identifiable reason constitute a group that did not present disinterest, by the latter definition in this study. These patients abruptly abandoned treatment and we could not find any plausible explanation for this phenomenon in the medical records, hence their classification as an unknown cause.
This reinforces the results described by Efficace et al.,18 who in his study reported the imatinib non-adherent population as heterogeneous and questioned the possible efficacy of general measures to improve adherence. In another study, the same author highlighted the scarce literature on potential causes for the low adherence in anticancer treatment and the individual and personal factors of each patient.21 Multidisciplinary teams and individualized measures seem to be better choices for adherence improvement.15
Although not being an objective of this study, other factors were described as being capable of negatively influencing adherence: Major Depressive Disorder (MDD)2 and major imatinib costs to the patient.2, 22 The justification for the exclusion of these analyses was made based on the need for a population previously diagnosed with MDD and the fact that imatinib is dispensed by the public healthcare system (Sistema Único de Saúde – SUS) without additional cost to the patient.
The limitations of this study include its retrospective nature, which implies in some considerations, such as the missing data. In this study, some variables (e.g.: skin color, CML phase at diagnosis) were found to be absent in a series of records and hence, could not be taken into consideration. As described by Guérin et al.23 and Kappor et al.,2 these factors may not serve as a complete background for patients.
The choice of an indirect method to measure adherence (in this case, the PDC), while demonstrating that the patients are receiving the medication regularly, does not guarantee that they are really taking it. The study by Marin et al.24 used an interesting approach to solve this problem, using a microelectronic monitoring device in the cap of the medication bottle, which recorded each time the bottle was opened. Unfortunately, this method was not viable, considering the retrospective nature of this study. Despite these imprecisions, the strong points of an indirect method to measure adherence, such as the PDC, includes its costless use, wide application and the fact that it is a recognized method.10, 11, 22
Despite the latter discussion, it is believed that this study will make possible the obtaining of data that will enrich the history of the scarce literature about the theme and, possibly, compound the theoretical foundations for the development of actions destined to the attainment of full adherence indices, especially in the Brazilian Amazon region. However, it is important to emphasize the need for prospective studies regarding this theme in closely observed environments.
Conclusion
In our study, the adherence was generally similar to that found in other similar works and, despite the peculiar characteristics of the Brazilian Amazon region, the only studied factors that negatively influenced adherence were two intentional variables (disinterest and abandonment of treatment), independently of the educational level or distance to the central city. In half of the cases, it was not possible to find justifiable reasons for the low adherence. In light of this scenario, it was verified that it is necessary to educate the patient with CML on what can be done for his or her health, through better communication between the healthcare team and the patient, with the intent to maximize the benefits of imatinib and reduce costs.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Trivedi D., Landsman-Blumberg P., Darkow T., Smith D., McMorrow D., Mullins C.D. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J Manag Care Pharm [Internet] 2014;20(10):1006–1015. doi: 10.18553/jmcp.2014.20.10.1006. Available from: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=18571 [accessed 15.02.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapoor J., Agrawal N., Ahmed R., Sharma S.K., Gupta A., Bhurani D. Factors influencing adherence to imatinib in indian chronic myeloid leukemia patients: a cross-sectional study. Mediterr J Hematol Infect Dis [Internet] 2015;7(1):e2015013. doi: 10.4084/MJHID.2015.013. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4344173/pdf/mjhid-7-1-e2015013.pdf [accessed 17.02.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour E.J., Kantarjian H., Eliasson L., Cornelison A.M., Marin D. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol [Internet] 2012;87:687–691. doi: 10.1002/ajh.23180. [accessed 05.03.17] [DOI] [PubMed] [Google Scholar]
- 4.Santoleri F., Sorice P., Lasala R., Rizzo R.C., Costantini A. Patient adherence and persistence with imatinib, nilotinib, dasatinib in clinical practice. PLOS ONE [Internet] 2013;8(2):e56813. doi: 10.1371/journal.pone.0056813. Available from: http://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0056813&type=printable [accessed 10.0.3.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rego M.N., Metze K., Lorand-Metze I. Low educational level but not low income impairs the achievement of cytogenetic remission in chronic myeloid leukemia patients treated with imatinib in Brazil. Clinics [Internet] 2015;70(5):322–325. doi: 10.6061/clinics/2015(05)03. Available from: http://www.scielo.br/pdf/clin/v70n5/1807-5932-clin-70-05-322.pdf [accessed 22.0.3.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Confalonieri U.E.C. Saúde na Amazônia: um modelo conceitual para a análise de paisagens e doenças. Estudos Avançados [Internet] 2005;19(53):221–236. Available from: http://www.scielo.br/pdf/ea/v19n53/24090.pdf [accessed 11.04.17] [Google Scholar]
- 7.Dos Reis S.R.C., Quixadá A.T.S., Nunes S.T., Camelo Cid D.M., Holanda de Souza J., Eloy da Costa C.M.B. Adherence to treatment with imatinib in chronic myeloid leukemia: a study of the first decade of responses obtained at a Brazilian hospital. Rev Bras Hematol Hemoter [Internet] 2013;35(3):174–179. doi: 10.5581/1516-8484.20130053. Available from: http://www.scielo.br/pdf/rbhh/v35n3/1516-8484-rbhh-35-3-0174.pdf [accessed 25.02.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Dewik N.I., Morsi H.M., Samara M.M., Ghasoub R.S., Gnanam C.C., Bhaskaran S.K. Is adherence to imatinib mesylate treatment among patients with chronic myeloid leukemia associated with better clinical outcomes in Qatar? Clin Med Insights: Oncol. 2016;10:95–104. doi: 10.4137/CMO.S32822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward M.A., Fang M.A., Richards K.L., Walko C.M., Earnshaw S.R., Happe L.E. Comparative evaluation of patients newly initiating first-generation versus secondgeneration tyrosine kinase inhibitors for chronic myeloid leukemia and medication adherence, health services utilization, and healthcare costs. Curr Med Res Opin. 2015;31(2):289–297. doi: 10.1185/03007995.2014.991440. [DOI] [PubMed] [Google Scholar]
- 10.Roebuck M.C., Kaestner R.J., Dougherty J.S. Impact of medication adherence on health services utilization in medicaid. Med Care. 2018;56(3):266–273. doi: 10.1097/MLR.0000000000000870. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande C.G., Kogut S., Laforge R., Willey C. Impact of medication adherence on risk of ischemic stroke, major bleeding, and deep vein thrombosis in atrial fibrillation patients using novel oral anticoagulants. Curr Med Res Opin. 2018;34(7):1285–1292. doi: 10.1080/03007995.2018.1428543. [DOI] [PubMed] [Google Scholar]
- 12.Sussman D., Yehoshua A., Kowalski J., Lee W., Kish J., Chaudhari S. Adherence and persistence of mirabegron and anticholinergic therapies in patients with overactive bladder: a real-world claims data analysis. Int J Clin Pract. 2017;71:e12824. doi: 10.1111/ijcp.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliasson L., Clifford S., Barber N., Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leukemia Research [Internet] 2011;35:626–630. doi: 10.1016/j.leukres.2010.10.017. Available from: http://www.lrjournal.com/article/S0145-2126(10)00510-2/pdf [accessed 23.04.17] [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim A.R., Eliasson L., Apperley J.F., Milojkovic D., Bua M., Szydlo R. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood [Internet] 2011;117(14):3733–3736. doi: 10.1182/blood-2010-10-309807. Available from: http://www.bloodjournal.org/content/bloodjournal/117/14/3733.full.pdf [accessed 15.05.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Almeida M.H., Pagnano K.B., Vigorito A.C., Lorand-Metze I., de Souza C.A. Adherence to tyrosine kinase inhibitor therapy for chronic myeloid leukemia: a Brazilian single-center cohort. Acta Haematol [Internet] 2013;130:16–22. doi: 10.1159/000345722. Available from: https://www.karger.com/Article/Abstract/345722 [accessed 18.05.17] [DOI] [PubMed] [Google Scholar]
- 16.Wu E.Q., Johnson S., Beaulieu N., Arana M., Bollu V., Guo A. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin [Internet] 2010;26:61–69. doi: 10.1185/03007990903396469. [accessed 20.05.17] [DOI] [PubMed] [Google Scholar]
- 17.Hamerschlak N., de Souza C., Cornacchioni A.L., Pasquini R., Tabak D., Spector N. Patients’ perceptions about diagnosis and treatment of chronic myeloid leukemia: a cross-sectional study among Brazilian patients. Sao Paulo Med J [Internet] 2015;133(6):471–479. doi: 10.1590/1516-3180.2014.0001306. Available from: http://www.scielo.br/pdf/spmj/v133n6/1516-3180-spmj-1516-3180-2014-0001306.pdf [accessed 27.05.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efficace F., Rosti G., Cottone F., Breccia M., Castagnetti F., Iurlo A. Profiling chronic myeloid leukemia patients reporting intentional and unintentional non-adherence to lifelong therapy with tyrosine kinase inhibitors. Leukemia Research [Internet] 2014;38:294–298. doi: 10.1016/j.leukres.2013.07.003. Available from: http://www.lrjournal.com/article/S0145-2126(13)00230-0/pdf [accessed 29.05.17] [DOI] [PubMed] [Google Scholar]
- 19.Flynn K.E., Atallah E. Quality of life and long term therapy in patients with chronic myeloid leukemia. Curr Hematol Malig Rep [Internet] 2016;11(2):80–85. doi: 10.1007/s11899-016-0306-5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4860261/pdf/nihms760565.pdf [accessed 30.05.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kekäle M., Peltoniemi M., Airaksinen M. Patient-reported adverse drug reactions and their influence on adherence and quality of life of chronic myeloid leukemia patients on per oral tyrosine kinase inhibitor treatment. Dovepress [Internet] 2015;9:1733–1740. doi: 10.2147/PPA.S92125. Available from: https://www.dovepress.com/patient-reported-adverse-drug-reactions-and-their-influence-on-adheren-peer-reviewed-fulltext-article-PPA [accessed 18.06.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efficace F., Baccarani M., Rosti G., Cottone F., Castagnetti F., Breccia F. Investigating factors associated with adherence behavior in patients with chronic myeloid leukemia: an observational patient-centered outcome study. Br J Cancer [Internet] 2012;107:904–909. doi: 10.1038/bjc.2012.348. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3464760/pdf/bjc2012348a.pdf [accessed 05.08.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dusetzina S.B., Winn A.N., Abel G.A., Huskamp H.A., Keating N.L. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol [Internet] 2014;32(4):306–311. doi: 10.1200/JCO.2013.52.9123. [accessed 10.09.17] [DOI] [PubMed] [Google Scholar]
- 23.Guérin A., Chen L., Wu E.Q., Leon D.P., Griffin J.D. A retrospective analysis of therapy adherence in imatinib resistant or intolerant patients with chronic myeloid leukemia receiving nilotinib or dasatinib in a real-world setting. Curr Med Res Opin. 2012;28(7):1–8. doi: 10.1185/03007995.2012.705264. [DOI] [PubMed] [Google Scholar]
- 24.Marin D., Bazeos A., Mahon F.X., Eliasson L., Milojkovic D., Bua M. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]