Key Points
Despite adjusting for short stature, low BMD remains highly prevalent in adolescents with SCD.
Low BMD independently associates with chronic pain and hip osteonecrosis in pediatric SCD.
Abstract
Low bone mineral density (BMD) disproportionately affects people with sickle cell disease (SCD). Growth faltering is common in SCD, but most BMD studies in pediatric SCD cohorts fail to adjust for short stature. We examined low BMD prevalence in 6- to 18-year-olds enrolled in the Sickle Cell Clinical Research and Intervention Program (SCCRIP), an ongoing multicenter life span SCD cohort study initiated in 2014. We calculated areal BMD for chronological age and height-adjusted areal BMD (Ht-aBMD) z scores for the SCCRIP cohort, using reference data from healthy African American children and adolescents enrolled in the Bone Mineral Density in Childhood Study. We defined low BMD as Ht-aBMD z scores less than or equal to –2 and evaluated its associations with demographic and clinical characteristics by using logistic regression analyses. Of the 306 children and adolescents in our study cohort (mean age, 12.5 years; 50% female; 64% HbSS/Sβ0-thalassemia genotype; 99% African American), 31% had low areal BMD for chronological age z scores and 18% had low Ht-aBMD z scores. In multivariate analyses, low Ht-aBMD z scores associated with adolescence (odds ratio [OR], 7.7; 95% confidence interval [CI], 1.94-30.20), hip osteonecrosis (OR, 4.0; 95% CI, 1.02-15.63), chronic pain (OR, 10.4; 95% CI, 1.51-71.24), and hemoglobin (OR, 0.74; 95% CI, 0.57-0.96). Despite adjusting for height, nearly 20% of this pediatric SCD cohort still had very low BMD. As the SCCRIP cohort matures, we plan to prospectively evaluate the longitudinal relationship between Ht-aBMD z scores and markers of SCD severity and morbidity.
Visual Abstract
Introduction
Sickle cell disease (SCD) affects ∼100 000 people in the United States1 and millions in the developing world.2 SCD is characterized by recurrent vaso-occlusive episodes (VOEs), which occur when dense aggregates of lysis-prone sickled red blood cells, activated leukocytes, and platelets adhere to the microvascular endothelium and impair perfusion to tissues and organs downstream from the obstruction.3 In preclinical studies of sickle cell mice exposed to repeated hypoxia-reperfusion stress (a proxy for recurrent VOEs), osteoclast-driven bone resorption markers increased while osteoblast-mediated bone formation markers decreased.4 Adults with SCD also exhibited increased plasma and urine markers of bone resorption at baseline, compared with age-matched non-SCD control subjects.5,6 Urinary bone resorption markers increased during painful VOEs in adults with SCD, above already elevated baseline levels.7 This ongoing perturbation in bone homeostasis predisposes, in part, to low bone mineral density (BMD) in SCD.
The natural history of low BMD and osteoporosis in SCD has not been described. Low BMD reportedly affects 30% to 40% of children and adolescents with SCD,8-10 compared with an estimated 2.3% prevalence in age-specific reference populations.11 However, low BMD prevalence rates in pediatric SCD were primarily derived from small, cross-sectional studies, and contributing disease-related factors are not well established. The potential impact on bone density from clinical complications of SCD, including chronic hemolysis, red blood cell transfusion-associated iron overload, pubertal delay, low lean muscle mass, and vitamin D deficiency or behavioral factors such as low dietary calcium intake and physical inactivity due to chronic pain,12-14 require further investigation in a large SCD cohort.
Dual-energy X-ray absorptiometry (DXA) scans estimate areal bone mineral density (aBMD) from 2-dimensional projections of bone; aBMD does not account for bone depth and is therefore subject to size-related artifacts. In children, aBMD is converted to age- and sex-specific z scores indicating the number of standard deviations above or below reference population means. Height-adjusted aBMD z scores can correct the aforementioned size-related artifacts in aBMD assessment and further estimate the degree to which a low aBMD z score may be attributed to short stature.15 Adjusting aBMD z scores for height also accounts, in part, for pubertal timing through the effects of delayed or advanced puberty on height (ie, children with delayed puberty are typically short for age and vice versa).16
Growth delay commonly affects children and adolescents with SCD.9,13,17-20 The goal of the present study was to estimate low aBMD prevalence in a large longitudinal pediatric SCD cohort, determine the degree to which growth faltering affects low aBMD prevalence, and evaluate its association with clinical features of SCD. We analyzed aBMD from DXA scans of children and adolescents with SCD enrolled in the Sickle Cell Clinical Research and Intervention Program (SCCRIP), a lifetime cohort study of SCD maintained at St. Jude Children’s Research Hospital.21 We hypothesized a high prevalence of low bone density in our study participants, even after adjusting for low height.9,10,22-24 We also report on the clinical characteristics and laboratory abnormalities associated with low aBMD z scores in this pediatric SCD cohort.
Methods
This study included all pediatric SCCRIP participants who had undergone a screening DXA scan at steady state. Briefly, SCCRIP was initiated in April 2014 and comprises retrospective and longitudinal data on clinical, neurocognitive, geospatial, psychosocial, and health outcomes from all children, adolescents, and adults with SCD recruited from the 5 participating centers.21 Biological samples are banked for future genomics and proteomics studies. SCD-related outcomes, such as frequency of painful VOEs, emergency department visits or hospitalizations, exposure to disease-modifying therapy (eg, hydroxyurea, erythrocyte transfusions), end-organ function assessment (brain and skeletal imaging, transthoracic echocardiogram, and urinalysis), and overall mortality data are retroactively collected from time of enrollment and at prespecified intervals thereafter. St. Jude Children’s Research Hospital’s institutional review board approved of SCCRIP, and this study was conducted in accordance with the Declaration of Helsinki.
SCCRIP BMD cohort selection
Asymptomatic SCCRIP pediatric subjects are screened for low aBMD with DXA scans of the posterior anterior spine and whole body from age ≥6 years, which is when most children can comply with the positioning required for the study. Per protocol, children and adolescents not meeting the International Society for Clinical Densitometry criteria for osteoporosis at baseline continue with surveillance DXA scans every 6 years thereafter. We selected our study cohort from SCCRIP participants aged 6 to18 years at the time of their first screening DXA study and stratified them, according to chronological age, into 2 predefined developmental stages: early school-age (6 to <12 years) and adolescents (12-18 years).21 For those with multiple DXA scans, only the initial screening DXA scan was included in the analysis.
DXA scans and reference data
DXA scans of the total body less head (TBLH) were obtained by using a Hologic QDR-4500A device (Hologic Inc., Bedford, MA). All DXA scans included in this study were acquired between 20 May 2014 and 31 December 2017. Our reference population comprised healthy, typically developing African American children and young adults, aged 5 to 20 years, enrolled in the Bone Mineral Density in Childhood Study (BMDCS), a multicenter study that generated BMD reference curves from African American and non–African American control subjects across the United States.25
Clinical and laboratory variables
Pertinent clinical variables extracted from birth up to the DXA scan date included frequency of VOEs (defined as painful episodes, dactylitis, acute chest syndrome, or priapism requiring administration of IV analgesia in the clinic, emergency department, or inpatient setting), hydroxyurea exposure, duration of hydroxyurea treatment, chronic erythrocyte transfusions (defined as ≥4 erythrocyte transfusions per year), symptomatic hip osteonecrosis (confirmed on centrally reviewed magnetic resonance imaging of the hip joint), severity of hip osteonecrosis (based on the percentage of femoral head volume occupied by the necrotic lesion26), and chronic pain (defined as pain that recurs or persists for >6 months, partly based on consensus guidelines for chronic SCD pain).27
We included laboratory data obtained at steady state and within 30 days (often on the same day) of the DXA scan dates such as complete blood cell counts, quantitative fetal hemoglobin, serum creatinine, blood urea nitrogen, calcium, lactate dehydrogenase, liver function tests, high-sensitivity C-reactive protein, serum ferritin, 25-hydroxyvitamin D (25[OH]D), and parathyroid hormone levels. Because there is lack of consensus on optimal circulating concentrations of 25(OH)D, we defined vitamin D status as follows: normal, 25(OH)D ≥30 ng/mL; insufficient, 20 ng/mL ≤25(OH)D <30 ng/mL; and deficient, 25(OH)D <20 ng/mL. These definitions were based on thresholds recommended by the Endocrine Society28,29 and the Society for Adolescent Health and Medicine.30
Statistical analyses
We calculated age-, sex-, and ancestry-specific aBMD z scores, as well as height-adjusted aBMD z scores (Ht-aBMD z scores) using formulas generated from BMDCS reference data. TBLH aBMD z scores were used as the primary bone density assessment for the study cohort. Per the International Society for Clinical Densitometry criteria, aBMD was categorized as low (aBMD-for-age or Ht-aBMD z scores less than or equal to −2) or normal (aBMD-for-age or Ht-aBMD z scores greater than −2).31 Summary statistics were reported for all patients by using count and frequency for categorical variables, and mean, standard deviation, median, and range for continuous variables. Results of the analyses were stratified according to age category, sex, and BMD status. Differences were compared between groups by using Fisher’s exact test or χ2 test for categorical variables, and a 2-sample Student t test or Wilcoxon rank sum test for continuous variables. We assessed data normality on continuous variables by using the Shapiro Wilk test. The McNemar test was used to compare proportions of patients with low BMD by using the aforementioned categorical values for aBMD z scores and Ht-aBMD z scores.
Univariate logistic regression models were used to evaluate the association between low aBMD vs normal aBMD and covariates of interest. We then conducted multivariate logistic regression analyses on those covariates with a nominal association level of 0.1 from the univariate analyses, based on a step-wise model selection strategy. The same analyses were repeated on a subset of children and adolescents with HbSS/HbSβ0-thalassemia genotype, who typically have a more severe disease course. All data were analyzed by using R 3.2.5 software (R Foundation for Statistical Computing, Vienna, Austria), and P < .05 was considered statistically significant throughout the study.
Results
Of the 1084 people enrolled in SCCRIP as of 31 December 2017, a total of 332 had undergone at least 1 routine DXA scan. To reduce sample heterogeneity, we excluded people without TBLH BMD results, those who were aged >18 years at the time of their first DXA scan, and those with rare sickle genotypes. The final BMD study cohort comprised 306 school-age children and adolescents with 323 DXA scans. Fourteen subjects had >1 DXA scan, but we only included their initial studies to ensure all study participants were measured at baseline.
Participant characteristics
Table 1 shows the characteristics of the entire SCCRIP BMD cohort (N = 306), stratified according to age category and sex. SCD genotype, race/ethnicity, and exposure to disease-modifying therapy did not differ between children and adolescents. Ninety-six (31%) of the 306 subjects had low BMD, based on standard aBMD z scores. Using Ht-aBMD z scores, however, 56 (18%) of the 306 met low BMD criteria. Differences in Ht-aBMD z scores between school-age children and adolescents persisted after stratification according to sex. Low BMD prevalence using Ht-aBMD z scores was statistically significantly lower compared with estimates from standard aBMD z scores (18% vs 31%; P = 2 × 10−8) according to the McNemar test.
Table 1.
Baseline demographic characteristics and DXA results for the SCCRIP BMD Cohort
| Variable | All (N = 306) | Female (n = 153) | Male (n = 153) | |||
|---|---|---|---|---|---|---|
| School-age (n = 96 [31.4%]) | Adolescent (n = 210 [68.6%]) | School-age (n = 46 [30.1%]) | Adolescent (n = 107 [69.9%]) | School-age (n = 50 [32.7%) | Adolescent (n = 103 [67.3%]) | |
| SCD genotype | ||||||
| HbSS/Sβ0-thalassemia | 61 (63.5) | 136 (64.8) | 27 (58.7) | 67 (62.6) | 34 (68.0) | 69 (67.0) |
| HbSC | 28 (29.2) | 52 (24.8) | 16 (34.8) | 30 (28.0) | 12 (24.0) | 22 (21.4) |
| HbSβ+-thalassemia | 7 (7.3) | 22 (10.5) | 3 (6.5) | 10 (9.4) | 4 (8.0) | 12 (11.6) |
| Age at DXA, y | 7.2 ± 1.13* | 14.8 ± 2.16* | 7.0 ± 0.78* | 14.9 ± 2.16* | 7.4 ± 1.37* | 14.7 ± 2.17* |
| Race/ethnicity | ||||||
| African American | 94 (97.9) | 210 (100.0) | 44 (95.6) | 107 (100) | 50 (100) | 103(100) |
| Other | 2 (2.1) | 0 (0) | 2 (4.4) | 0 (0) | 0 (0) | 0 (0) |
| Hydroxyurea use | ||||||
| No | 42 (43.8) | 83 (39.5) | 21 (45.6) | 44 (41.1) | 21 (42.0) | 39 (37.9) |
| Yes | 54 (56.2) | 127 (60.5) | 25 (54.4) | 63 (58.9) | 29 (58.0) | 64 (62.1) |
| Chronic RBC transfusions† | ||||||
| No | 95 (99.0) | 202 (96.2) | 46 (100) | 101 (94.4) | 49 (98.0) | 101 (98.1) |
| Yes | 1 (1.0) | 8 (3.8) | 0 (0) | 6 (5.6) | 1 (2.0) | 2 (1.9) |
| Height, cm | 122.2 ± 9.74* | 160.0 ± 11.53* | 120.7 ± 7.97* | 158.1 ± 8.51* | 123.5 ± 11.03* | 161.9 ± 13.77* |
| Height-for-age z scores | −0.12 ± 1.07 | −0.21 ± 1.11 | −0.19 ± 0.96 | −0.14 ± 1.09 | −0.05 ± 1.17 | −0.29 ± 1.13 |
| TBLH aBMD, g/cm2 | 0.58 ± 0.07* | 0.86 ± 0.11* | 0.57 ± 0.06* | 0.85 ± 0.09* | 0.59 ± 0.08* | 0.86 ± 0.14* |
| TBLH aBMD z score | ||||||
| Normal | 73 (76.0) | 137 (65.2) | 35 (76.1) | 73 (68.2) | 38 (76.0) | 64 (62.1) |
| Low | 23 (24.0) | 73 (34.8) | 11 (23.9) | 34 (31.8) | 12 (24.0) | 39 (37.9) |
| TBLH aBMD z score | −1.14 ± 1.03* | −1.60 ± 1.25* | −1.07 ± 1.06* | −1.54 ± 1.20* | −1.20 ± 1.01* | −1.65 ± 1.30* |
| TBLH Ht-aBMD z score | ||||||
| Normal | 92 (95.8)* | 158 (75.2)* | 44 (95.6)* | 84 (78.5)* | 48 (96.0)* | 74 (71.8)* |
| Low | 4 (4.2)* | 52 (24.8)* | 2 (4.4)* | 23 (21.5)* | 2 (4.0)* | 29 (28.2)* |
| TBLH Ht-aBMD z score | −0.80 ± 0.69* | −1.36 ± 1.05* | −0.81 ± 0.72* | −1.34 ± 1.02* | −0.80 ± 0.67* | −1.37 ± 1.08* |
Data are presented as n (%) or mean ± standard deviation.
Hb, hemoglobin; RBC, red blood cell.
P < .05 between school-age vs adolescent values.
Defined as ≥4 RBC transfusions per year.
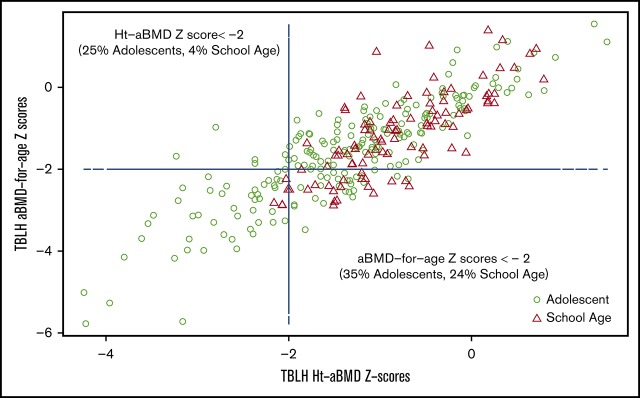
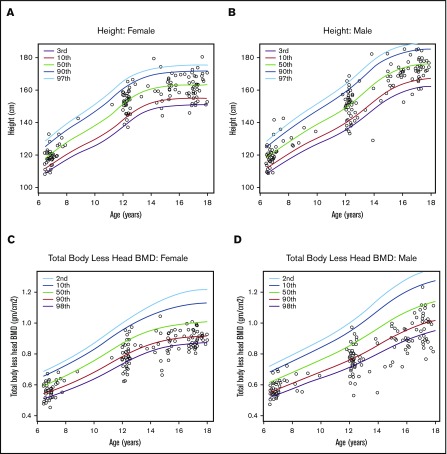
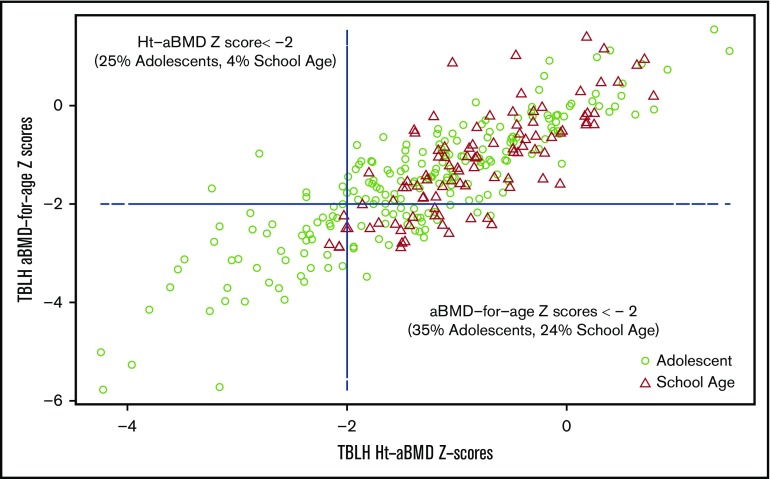
Low BMD was more prevalent in male subjects than in female subjects according to either standard aBMD z scores (33% male vs 29% female) or Ht-aBMD z scores (20% male vs 16% female). Overall, adolescent male subjects had the highest prevalence of low BMD according to both standard aBMD z scores (38%) and Ht-aBMD z scores (28%) (Table 1). Figure 1A-B illustrates the height distribution of the study cohort, stratified according to sex, compared with stature-for-age growth charts from the US National Center for Health Statistics (race/ethnicity nonspecific). Approximately 58% of the SCCRIP study cohort fell below the 50th percentile (z score = 0) curve for stature-for-age (P = .007). Figure 1C-D illustrates the TBLH aBMD distribution of the study cohort (stratified according to sex), compared with healthy African American control subjects from BMDCS. TBLH aBMD curves are not corrected for height because the BMDCS height adjustment formulas apply to aBMD z scores only. Figure 2 juxtaposes standard aBMD z scores with Ht-aBMD z scores to illustrate how adjusting for height in children and adolescents with SCD significantly decreases low BMD prevalence.
Figure 1.
Sex differences in height-for-age and TBLH aBMD-for-age curves in the SCCRIP pediatric cohort. Stature-for-age curves for female subjects (A) and male subjects (B) in the SCCRIP pediatric cohort, compared with reference data from healthy children and adolescents from the US National Center for Health Statistics. TBLH aBMD vs chronological age for female subjects (C) and male subjects (D) in the SCCRIP pediatric cohort. The TBLH aBMD reference curves were obtained from healthy African American control subjects, ages 6 to 18 years, enrolled in the BMDCS. The 50th percentile (green line) represents the mean value-for-age (z score = 0) of the reference populations.
Figure 2.
TBLH aBMD-for-age z scores vs Ht-aBMD z scores in the SCCRIP pediatric cohort.
Supplemental Table 1 summarizes the baseline characteristics of children and adolescents with HbSS/HbSβ0-thalassemia genotype only (n = 196 [64% of the entire study cohort]). We found similar results in the HbSS/HbSβ0-thalassemia subgroup, compared with the entire BMD cohort. However, the differences between those with normal vs low BMD in this subgroup analysis were more marked: 49% adolescent male subjects with HbSS/HbSβ0-thalassemia met low BMD criteria per standard aBMD z scores, compared with 35% using Ht-aBMD z scores (P = .003). Supplemental Figure 1 compares the age distribution of aBMD z scores (panels A-B) and Ht-aBMD z scores (panels C-D), stratified according to sex, for the HbSS/HbSβ0-thalassemia subgroup.
Clinical correlates of height-adjusted BMD
Table 2 compares clinical differences between study subjects with and without low Ht-aBMD z scores, stratified according to sex. HbSS/HbSβ0/+-thalassemia genotype, older age, adolescence, duration of hydroxyurea treatment, chronic pain, and hip osteonecrosis (both severe and less severe grades) statistically significantly associated with low Ht-aBMD z scores. School age category and HbSC genotype inversely associated with low Ht-aBMD z scores. The dose-dependent association between vitamin D status and low BMD in female subjects approached statistical significance (52% vitamin D deficient, 40% vitamin D insufficient, and 8% vitamin D replete) (P = .053).
Table 2.
Clinical correlates of low BMD in the SCCRIP BMD cohort
| Variable | All (N = 306) | Female (n = 153) | Male (n = 153) | |||
|---|---|---|---|---|---|---|
| Normal BMD (n = 250 [81.7%]) | Low BMD (n = 56 [18.3%]) | Normal BMD (n = 128 [83.7%]) | Low BMD (n = 25 [16.3%]) | Normal BMD (n = 122 [79.7%]) | Low BMD (n = 31 [20.3%]) | |
| SCD genotype | ||||||
| HbSS/Sβ0-thalassemia | 152 (60.8)* | 45 (80.4)* | 75 (58.6)* | 19 (76.0)* | 77 (63.1)* | 26 (83.9)* |
| HbSC | 76 (30.4)* | 4 (7.1)* | 44 (34.4)* | 2 (8.0)* | 32 (26.3)* | 2(6.4)* |
| HbSβ+-thalassemia | 22 (8.8)* | 7 (12.5)* | 9 (7.1)* | 4 (16.0)* | 13 (10.7)* | 3 (9.7)* |
| Age at DXA, y | 11.9 ± 4.09* | 14.5 ± 2.75* | 12.2 ± 4.18 | 14.2 ± 2.77 | 11.7 ± 3.98* | 14.7 ± 2.77* |
| Age category | ||||||
| School-age | 92 (36.8)* | 4 (7.1)* | 44 (33.9)* | 2 (8.0)* | 48 (39.3)* | 2 (6.5)* |
| Adolescent | 158 (63.2)* | 52 (92.9)* | 84 (65.6)* | 23 (92.0)* | 74 (60.7)* | 29 (93.6)* |
| VOEs per year (total) | 0.25 ± 0.44 | 0.22 ± 0.48 | 0.21 ± 0.37 | 0.27 ± 0.59 | 0.29 ± 0.50 | 0.19 ± 0.39 |
| VOEs per year (2 y to DXA) | 0.58 ± 2.12 | 0.71 ± 1.70 | 0.47 ± 1.48 | 0.56 ± 1.23 | 0.69 ± 2.54 | 0.84 ± 2.57 |
| VOEs total (categorical) | ||||||
| <5 times | 141 (56.4) | 35 (62.5 | 67 (52.3) | 17 (68.0) | 74 (60.7) | 18 (58.1) |
| 5-10 times | 63 (25.2) | 11 (19.6) | 43 (33.6) | 3 (12.0) | 20 (16.4) | 8 (25.8) |
| >10 times | 46 (18.4) | 10 (17.9) | 18 (14.1) | 5 (20.0) | 28 (22.9) | 5 (16.1) |
| Hydroxyurea use | ||||||
| No | 109 (43.6) | 16 (28.6) | 57 (44.5) | 8 (32.0) | 52 (42.6) | 8 (25.8) |
| Yes | 141 (56.4) | 40 (71.4) | 71 (55.5) | 17 (68.0) | 70 (57.4) | 23 (74.2) |
| Hydroxyurea duration | ||||||
| <5 y | 187 (74.8) | 34 (60.7) | 97 (75.8) | 16 (64.0) | 90 (73.6) | 18 (58.1) |
| 5-10 y | 41 (16.4) | 15 (27.8) | 22 (17.2) | 7 (28.0) | 19 (15.6) | 8 (25.8) |
| >10 y | 22 (8.8) | 7 (12.5) | 9 (7.0) | 2 (8.0) | 13 (10.7) | 5 (16.1) |
| Hydroxyurea duration, y | 2.9 ± 3.95* | 4.1 ± 4.31* | 2.8 ± 3.88 | 3.6 ± 3.88 | 3.1 ± 4.03 | 4.4 ± 4.66 |
| Chronic RBC transfusions† | ||||||
| No | 245 (98.0) | 52 (92.9) | 125 (97.7) | 22 (88.0) | 120 (98.4) | 30 (96.8) |
| Yes | 5 (2.0) | 4 (7.1) | 3 (2.3) | 3 (12.0) | 2 (1.6) | 1 (3.2) |
| Chronic pain | ||||||
| No | 244 (97.6) | 51 (91.1) | 125 (97.7) | 24 (96.0) | 119 (97.5) | 27 (87.1) |
| Yes | 6 (2.4)* | 5 (8.9)* | 3 (2.3) | 1 (4.0) | 3 (2.5)* | 4 (12.9)* |
| Hip osteonecrosis | ||||||
| No | 242 (96.8) | 49 (87.5) | 126 (98.4) | 24 (96.0) | 116 (95.1) | 25 (80.7) |
| Yes | 8 (3.2)* | 7 (12.5)* | 2 (1.6) | 1 (4.0) | 6 (4.9)* | 6 (19.3)* |
| Hip osteonecrosis grade‡ | ||||||
| Less severe | 3 (1.2)* | 2 (3.6)* | 1 (0.8) | 0 (0) | 2 (1.6)* | 2 (6.5)* |
| Severe | 5 (2.0)* | 5 (8.9)* | 1 (0.8) | 1 (4.0) | 4 (3.3)* | 4 (12.9)* |
| None | 242 (96.8) | 49 (87.5) | 126 (98.4) | 24 (96.0) | 116 (95.1) | 25 (80.6) |
| Vitamin D status§ | ||||||
| Normal | 68 (28.8) | 9 (17.7) | 31 (25.4) | 2 (8.0) | 37 (32.5) | 7 (26.9) |
| Insufficient | 91 (38.6) | 23 (45.1) | 54 (44.3) | 10 (40.0) | 37 (32.5) | 13 (50.0) |
| Deficient | 77 (32.6) | 19 (37.3) | 37 (30.3) | 13 (52.0) | 40 (35.1) | 6 (23.1) |
Data are presented as n (%) or mean ± standard deviation.
P < .05 between low vs normal BMD per variable.
Defined as RBC transfusions ≥4 times/year.
Less severe, necrotic lesion ≤30% femoral head volume; severe, necrotic lesion >30% femoral head volume.
Normal, 25(OH)D, >30 ng/mL; insufficient, 25(OH)D 20 to 30 ng/mL; and deficient, 25(OH)D <20 ng/mL.
In the HbSS/HbSβ0-thalassemia subgroup, low BMD associated with older age, adolescent age category, fewer average VOEs per year, and hip osteonecrosis; only the association with age and adolescence in male subjects remained statistically significant after stratification according to sex (supplemental Table 2). In female subjects with HbSS/HbSβ0-thalassemia, low BMD correlated with vitamin D status at almost identical prevalence rates as those observed in the overall cohort: 53%, 37%, and 11% of female subjects with low BMD were vitamin D deficient, insufficient, and replete, respectively (P = .10).
Laboratory correlates of height-adjusted low bone density
Low Ht-aBMD z scores significantly associated with lower hemoglobin, higher total/indirect bilirubin, and lower calcium in the entire cohort; these associations only remained statistically significant in male subjects after stratification according to sex (Table 3). In female subjects, low Ht-aBMD z scores significantly associated with lower vitamin D levels (P = .039). In the HbSS/HbSβ0-thalassemia subgroup, hemoglobin no longer correlated with low Ht-aBMD z scores (supplemental Table 3). However, higher total and indirect bilirubin, lower fetal hemoglobin, and lower calcium associated with low Ht-aBMD z scores. Indirect bilirubin and fetal hemoglobin remained statistically significantly different in female subjects, whereas only calcium levels significantly differed in male subjects. Female subjects with HbSS/HbSβ0-thalassemia genotype and low Ht-aBMD z scores had significantly lower mean 25(OH)D concentrations, compared with those with normal height-adjusted bone density.
Table 3.
Laboratory parameters in the SCCRIP BMD cohort
| Variable | All (N = 306) | Female (n = 153) | Male (n = 153) | |||
|---|---|---|---|---|---|---|
| Normal BMD (n = 250 [81.7%]) | Low BMD (n = 56 [18.3%]) | Normal BMD (n = 128 [83.7%]) | Low BMD (n = 25 [16.3%]) | Normal BMD (n = 122 [79.7%]) | Low BMD (n = 31 [20.3%]) | |
| WBC, ×109/L | 9.3 ± 4.03 | 9.6 ± 3.71 | 9.0 ± 3.92 | 9.5 ± 4.37 | 9.7 ± 4.13 | 9.6 ± 3.14 |
| Hemoglobin, g/dL | 10.2 ± 1.60* | 9.5 ± 1.45* | 10.0 ± 1.45 | 9.5 ± 1.31 | 10.3 ± 1.74* | 9.5 ± 1.58* |
| Platelets, ×109/L | 343.5 ± 174.97 | 383.0 ± 182.02 | 323.4 ± 158.85 | 388.0 ± 196.77 | 363.9 ± 188.42 | 378.8 ± 172.07 |
| LDH, U/L | 411.0 ± 177.51 | 420.9 ± 164.17 | 374.8 ± 149.96 | 369.9 ± 143.80 | 449.9 ± 196.30 | 461.6 ± 170.24 |
| Total bilirubin, mg/dL | 1.9 ± 1.28* | 2.6 ± 1.62* | 1.7 ± 1.09 | 2.4 ± 1.72 | 2.0 ± 1.44* | 2.7 ± 1.55* |
| Indirect bilirubin, mg/dL | 2.1 ± 1.21* | 2.6 ± 1.48* | 2.0 ± 0.96 | 2.6 ± 1.58 | 2.1 ± 1.41* | 2.6 ± 1.44* |
| Fetal hemoglobin, % | 15.3 ± 10.64 | 13.1 ± 7.82 | 15.0 ± 10.85 | 11.7 ± 7.64 | 15.6 ± 10.46 | 14.2 ± 7.95 |
| Albumin, g/dL | 4.4 ± 0.27 | 4.4 ± 0.31 | 4.4 ± 0.27 | 4.5 ± 0.25 | 4.5 ± 0.28 | 4.4 ± 0.35 |
| ALP, U/L | 158.4 ± 72.09 | 142.5 ± 59.77 | 140.4 ± 72.30 | 128.1 ± 62.48 | 176.4 ± 67.50 | 154.3 ± 55.81 |
| AST/SGOT, U/L | 34.8 ± 15.17 | 34.8 ± 12.29 | 32.1 ± 15.14 | 31.4 ± 11.22 | 37.5 ± 14.78 | 37.6 ± 12.62 |
| Calcium, mg/dL | 9.5 ± 0.37* | 9.4 ± 0.31* | 9.4 ± 0.38 | 9.4 ± 0.28 | 9.5 ± 0.35* | 9.3 ± 0.34* |
| Creatinine, mg/dL | 0.5 ± 0.16 | 0.5 ± 0.13 | 0.5 ± 0.15 | 0.5 ± 0.11 | 0.5 ± 0.18 | 0.5 ± 0.15 |
| BUN, mg/dL | 7.5 ± 2.67 | 7.0 ± 2.07 | 7.2 ± 2.16 | 6.7 ± 2.26 | 7.9 ± 3.08 | 7.1 ± 1.93 |
| Vitamin D, ng/mL | 25.0 ± 11.13 | 22.5 ± 8.35 | 24.8 ± 10.85* | 19.6 ± 8.17* | 25.3 ± 11.46 | 25.2 ± 7.70 |
Data are presented as mean ± standard deviation.
ALP, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; SGOT, serum glutamic-oxaloacetic transaminase; WBC, white blood cell count.
P < .05 for normal vs low BMD values, per variable.
Univariate and multivariate analyses for correlates of height-adjusted low bone density
HbSS/HbSβ0-thalassemia genotype, older age, adolescent age category, chronic pain, hip osteonecrosis, hydroxyurea use (ever), and hydroxyurea exposure for 5 to 10 years were all statistically significantly associated with low Ht-aBMD z scores in the entire study cohort (supplemental Table 4). In the HbSS/HbSβ0-thalassemia subgroup, only older age, adolescence, and hip osteonecrosis significantly associated with low Ht-aBMD z score. Lower hemoglobin, higher total and indirect bilirubin, and lower serum calcium significantly correlated with low Ht-aBMD z scores in the entire study cohort. In the HbSS/HbSβ0-thalassemia subgroup, higher total and indirect bilirubin, lower fetal hemoglobin, and lower serum calcium were significantly associated with low Ht-aBMD z scores.
In multivariate logistic regression analysis, low Ht-aBMD z scores significantly associated with adolescence, hip osteonecrosis, chronic pain, and low hemoglobin concentration (Table 4). In the HbSS/HbSβ0-thalassemia subgroup, low Ht-aBMD z scores only associated with adolescent age category, although its association with chronic pain and indirect hyperbilirubinemia approached significance. In our entire study cohort, children and adolescents with chronic pain, compared with those without chronic pain, had 10 times increased odds (95% confidence interval, 1.51-71.24) of having low Ht-aBMD z scores. Similarly, those with hip osteonecrosis had 4 times increased odds of having low Ht-aBMD z scores, compared with those without hip osteonecrosis (95% confidence interval, 1.02-15.63). Within the HbSS/HbSβ0-thalassemia subgroup, low BMD prevalence was not statistically significantly different between those with and without hip osteonecrosis.
Table 4.
Multivariate regression of low BMD correlates in the SCCRIP cohort
| Variable | Total cohort | HbSS/Sβ0-thalassemia subgroup | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | |
| Adolescent category | 7.7 (1.94-30.20) | .0037 | 4.7 (1.23-18.17) | .023 |
| Hip osteonecrosis | 4.0 (1.02-15.63) | .046 | 3.7 (0.69-20.11) | .13 |
| Chronic pain | 10.4 (1.51-71.24) | .017 | 9.3 (0.97-88.70) | .053 |
| Hemoglobin | 0.74 (0.57-0.96) | .022 | NA | NA |
| Indirect bilirubin | NA | NA | 1.4 (0.99-1.90) | .055 |
CI, confidence interval; NA, variable not selected in final model.
Discussion
Rationale for height-adjusted BMD in pediatric SCD
Low BMD is prevalent in SCD, but many studies fail to account for low height when assessing BMD in children with SCD. Meeuwes et al24 compared aBMD z scores in 27 children and young adults with HbSS genotype in northeast Brazil (56% female; ages 7-28 years) vs a healthy reference population of similar chronological age, ethnicity, and sex.32 They found low BMD in 41% of their subjects and reported an association between low BMD and low height-for-age, weight-for-age, and body-mass-index-for-age. The authors surmised that delayed growth in children and adolescents with sickle cell anemia increased risk for low BMD. Others criticized the study by Meeuwes et al for failing to correct aBMD z scores for short stature because growth failure and delayed puberty are well-recognized complications of SCD.9,33 In a similar study from the United States, Lal et al10 reported on low BMD prevalence in 25 children with the HbSS genotype (52% female; ages 10.2-19.8 years) recruited from Children’s Hospital (Oakland, CA) compared with age-, sex-, and ethnicity-specific normal reference curves.34 The authors found low BMD in the proximal femur in 8 (33%) and lumbar spine in 14 (56%) study participants.
Two more recent case-control studies reported on low BMD prevalence in children with and without SCD, using standard reference curves from the National Health and Nutrition Examination Survey, corrected for chronological age and sex. Bordbar et al35 compared aBMD z scores from DXA scans of the lumbar spine and femoral neck in 70 Iranian children with SCD (51% male; mean age, 11.4 ± 5.8 years; 59% HbSS genotype) vs 70 age- and sex-matched healthy control subjects. The authors found low BMD in 33% to 34% of the SCD group, compared with 13% to 15% of the control group. Mokhtar et al36 studied low BMD prevalence in a cohort of Egyptian children with HbSS/HbSβ0-thalassemia genotype (n = 30; 40% female; mean age, 10.7 ± 3.9 years) and their siblings with sickle cell trait (n = 17; 41% female; mean age, 10.2 ± 3.0 years) compared with age- and sex-matched healthy control subjects (n = 32; 50% female; mean age, 11.8 ± 3.2 years). Using aBMD z scores from DXA scans of the lumbar spine and femoral neck, the authors found that low BMD affected ∼37% of children with sickle cell anemia and 12% with sickle cell trait, compared with none of the healthy control subjects.
We addressed some of the limitations of previous studies by comparing the height and TBLH aBMD of our large study cohort vs a well-described national reference group of the same age, sex, and race11,15 by using standard aBMD z scores and Ht-aBMD z scores (Figure 1). Similar to previously published studies, we found low aBMD z scores in 24% of children and 35% of adolescents in our SCCRIP study cohort. After height correction, low BMD prevalence rates decreased significantly to 4% in children and 26% in adolescents (Figure 2). In the HbSS/HbSβ0-thalassemia subgroup, 28% of children and 43% of adolescents had low aBMD z scores, compared with 7% and 30% with low Ht-aBMD z scores. In our pediatric SCD cohort, low BMD prevalence in children markedly decreased after correcting for short stature; low BMD remained highly prevalent in adolescents, although at a significantly lower rate than previously described.
Low BMD correlates with older age, hemolysis, and chronic pain
In our study, low BMD prevalence increased from early childhood to adolescence, as previously reported.10,24,35,36 The association between adolescent age category and low BMD likely reflects the length of time required for bone deficits to arise from the cumulative vascular insults of SCD. In the univariate logistic regression, any hydroxyurea exposure or hydroxyurea exposure for 5 to 10 years significantly associated with low BMD (supplemental Table 4). Despite this finding, those with low BMD had lower fetal hemoglobin levels, which may be due to poorer hydroxyurea adherence and/or decreased fetal hemoglobin response in older children and adolescents. All hydroxyurea variables fell out of the multivariate models. However, this finding may possibly be due to confounding; that is, hydroxyurea improves hemoglobin concentrations, decreases VOE frequency, and may reduce progression to chronic pain.
The association of anemia and indirect hyperbilirubinemia with low BMD supports the hypothesis that chronic hemolysis increases erythropoietic drive, bone marrow expansion, and cortical thinning, which subsequently decrease bone mass.37 Recurrent acute painful episodes can coexist with chronic sickle cell pain, typically reported in older adolescents and adults.38,39 Six of the 15 imaging-confirmed cases of hip osteonecrosis, a major cause of chronic SCD pain,27,40 also met our criteria for chronic pain. In our multivariate regression analysis, however, both hip osteonecrosis and chronic pain independently associated with low BMD. The estimated high magnitude of effect between low BMD and chronic pain has not been previously reported. Mechanical loading from muscle mass and physical activity aid bone accrual and skeletal strength.41,42 Chronic pain and hip osteonecrosis both can limit physical activity in children and adolescents with SCD. Because physical activity data are not currently collected in SCCRIP, we lack sufficient evidence to assert that physical inactivity impaired bone accretion in our study cohort. Opioids, the main treatment of chronic pain in SCCRIP, may suppress bone formation by osteoblasts43 and increase the risk for low BMD in people who chronically use opioids.44,45 Although SCCRIP data are collected retrospectively and prospectively, our study was a cross-sectional examination of existing BMD data; therefore, we cannot infer causality between low BMD and chronic pain or hip osteonecrosis at this time. Chronic pain in this pediatric SCD cohort significantly associated with low Ht-aBMD z scores in those with HbSS/HbSβ0-thalassemia, not HbSC and HbSβ+-thalassemia, suggesting that low BMD further complicates the more severe SCD genotypes.
Sex differences with vitamin D deficiency and low BMD
Vitamin D deficiency in SCD has been well described46-55; some studies have further investigated its association with low BMD.56-60 Bordbar et al35 found that 60% of their HbSS/HbSβ0-thalassemia cases had vitamin D deficiency, compared with 83% of the healthy age- and sex-matched control cases. Based on the clinical practice at St. Jude Children’s Research Hospital, all infants with SCD were screened for vitamin D deficiency; those with low vitamin D levels were prescribed cholecalciferol 2000 IU by mouth daily, and those with normal vitamin D levels received cholecalciferol 1000 IU by mouth daily for maintenance. Universal vitamin D supplementation may have precluded us from finding an association between vitamin D deficiency and low BMD in the overall study cohort.
We found a nonsignificant association between hypovitaminosis D and low BMD in female subjects (Table 2). Concurrent vitamin D insufficiency or deficiency could plausibly increase female subjects’ susceptibility to low BMD. At St. Jude Children’s Research Hospital, preferred contraceptive methods include an intrauterine device with progesterone and abstinence. None of the female subjects in our study cohort were on oral contraceptive pills, and only 2% were exposed to depot medroxyprogesterone, a parenteral contraceptive known to impair bone accrual in growing children. Because so few girls received depot medroxyprogesterone, we cannot assert an association between exposure to this drug and low BMD, regardless of vitamin D status.
Strengths and limitations
Our study highlights the importance of height adjustment when assessing BMD in children and adolescents with SCD or other chronic illnesses, which can adversely affect bone accrual and skeletal maturation. Although our definition of chronic pain was similar to that used in other chronic pain studies in children and adolescents,61,62 the chronic pain prevalence rate in our cohort was much lower than those reported in SCD pain diary studies.38,39 The retrospective ascertainment of chronic pain likely limited our ability to detect all cases. Despite the paucity of chronic pain and hip osteonecrosis in our cohort, the large sample size allowed us to measure associations between low BMD and both chronic SCD complications that had not been previously described. We were unable, however, to retrospectively verify a key criterion of the chronic SCD pain consensus that required physical and/or radiographic studies of affected areas.27 Although the chronic SCD pain consensus criteria stem from the International Association for the Study of Pain’s well-established definitions of chronic pain,63-67 they still require prospective validation.
Delayed puberty and low lean muscle mass are well-recognized risk factors for low BMD in children and adolescents with SCD.9,19 Nutritional status, physical activity, comorbid asthma, and concurrent systemic steroid use also affect pediatric bone health. None of these variables factored into our BMD analyses because they are not currently collected in SCCRIP. Because we have not yet performed repeated DXA scans in our study cohort, we cannot determine if low BMD preceded or coincided with the onset of chronic pain or hip osteonecrosis.
Future directions
Because SCCRIP was initiated in 2014 and DXA scans are scheduled for every 6 years, we anticipate the next follow-up BMD assessment in our study cohort in 2020. As the SCCRIP cohort matures, we plan to study the between-incident low BMD (at various anatomic sites) and other SCD end points (eg, osteonecrosis of the femoral or humeral heads, onset of chronic pain). Our collaborators in the SCCRIP Pain Working Group plan to validate the chronic SCD pain consensus criteria using SCCRIP data. We also plan to assess longitudinal bone health outcomes in the SCCRIP cohort, stratified according to sex, SCD genotype, age at hydroxyurea initiation, and hydroxyurea adherence. Using self-reported fracture histories and vertebral fracture analyses obtained at the time of DXA scans, we can also estimate the prevalence and cumulative incidence of fractures as the SCCRIP cohort matures into adulthood.
In conclusion, height adjustment results in a more conservative estimate of low BMD prevalence in children with SCD. Despite correcting for short stature, however, low BMD remains highly prevalent in adolescents. The association between low BMD and hip osteonecrosis in this pediatric cohort warrants further investigation into the role that physical activity plays in bone accrual in children with SCD. Access to the SCCRIP data set offers investigators a unique opportunity to longitudinally assess BMD as the study cohort matures and to definitively study the effect of low BMD on clinically relevant end points such as hip osteonecrosis, chronic pain, and fractures in people with SCD.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are indebted to SCCRIP Investigators for their input on this project.
O.O.A. was supported by a K12 Benign Hematology Clinical Research grant (National Institutes of Health, National Heart, Lung, and Blood Institute 4K12HL087165-10) and the National Heart, Lung, and Blood Institute’s Programs to Increase Diversity Among Individuals Engaged in Health-Related Research (5R25HL106365-09). SCCRIP is partly supported by the American Lebanese Syrian Associated Charities at St. Jude Children’s Research Hospital.
Authorship
Contribution: O.O.A. and B.S.Z. proposed the study concept; O.O.A., J.G.G., G.K., B.S.Z. and J.S.H. wrote the manuscript; and O.O.A., J.G.G., G.K., M.V., J.R.H., W.C., S.C.K., B.S.Z., and J.S.H. agreed to the study design, edited the manuscript, and approved the final submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oyebimpe O. Adesina, University of Washington, Seattle Cancer Care Alliance, 825 Eastlake Ave E, MS CE3-300, Seattle, WA 98109; e-mail: adesina@uw.edu.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(suppl 4):S512-S521. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg MH. Sickle cell anemia, the first molecular disease: overview of molecular etiology, pathophysiology, and therapeutic approaches. ScientificWorldJournal. 2008;8:1295-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalle Carbonare L, Matte’ A, Valenti MT, et al. . Hypoxia-reperfusion affects osteogenic lineage and promotes sickle cell bone disease. Blood. 2015;126(20):2320-2328. [DOI] [PubMed] [Google Scholar]
- 5.Bolarin DM, Swerdlow P, Wallace AM, Littsey L. Type I collagen as a marker of bone metabolism in sickle cell hemoglobinopathies. J Natl Med Assoc. 1998;90(1):41-45. [PMC free article] [PubMed] [Google Scholar]
- 6.Bolarin DM, Azinge EC. Osteocalcin and specific markers of bone resorption in sickle cell disease. Acta Physiol Hung. 2010;97(3):290-296. [DOI] [PubMed] [Google Scholar]
- 7.Nur E, Mairuhu W, Biemond BJ, et al. ; CURAMA study group. Urinary markers of bone resorption, pyridinoline and deoxypyridinoline, are increased in sickle cell patients with further increments during painful crisis. Am J Hematol. 2010;85(11):902-904. [DOI] [PubMed] [Google Scholar]
- 8.Brinker MR, Thomas KA, Meyers SJ, et al. . Bone mineral density of the lumbar spine and proximal femur is decreased in children with sickle cell anemia. Am J Orthop (Belle Mead NJ). 1998;27(1):43-49. [PubMed] [Google Scholar]
- 9.Buison AM, Kawchak DA, Schall JI, et al. . Bone area and bone mineral content deficits in children with sickle cell disease. Pediatrics. 2005;116(4):943-949. [DOI] [PubMed] [Google Scholar]
- 10.Lal A, Fung EB, Pakbaz Z, Hackney-Stephens E, Vichinsky EP. Bone mineral density in children with sickle cell anemia. Pediatr Blood Cancer. 2006;47(7):901-906. [DOI] [PubMed] [Google Scholar]
- 11.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. . Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung EB, Kawchak DA, Zemel BS, Rovner AJ, Ohene-Frempong K, Stallings VA. Markers of bone turnover are associated with growth and development in young subjects with sickle cell anemia. Pediatr Blood Cancer. 2008;50(3):620-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61(5 pt 1):607-613. [DOI] [PubMed] [Google Scholar]
- 14.Ballas SK, Kesen MR, Goldberg MF, et al. . Beyond the definitions of the phenotypic complications of sickle cell disease: an update on management. ScientificWorldJournal. 2012;2012:949535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zemel BS, Leonard MB, Kelly A, et al. . Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wren TA, Kalkwarf HJ, Zemel BS, et al. ; Bone Mineral Density in Childhood Study Group. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164(6):1280-1285.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard MB, Zemel BS, Kawchak DA, Ohene-Frempong K, Stallings VA. Plasma zinc status, growth, and maturation in children with sickle cell disease. J Pediatr. 1998;132(3 pt 1):467-471. [DOI] [PubMed] [Google Scholar]
- 18.Barden EM, Kawchak DA, Ohene-Frempong K, Stallings VA, Zemel BS. Body composition in children with sickle cell disease. Am J Clin Nutr. 2002;76(1):218-225. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, Sprinz PG, Welch J, et al. . Weight status of children with sickle cell disease. Pediatrics. 2013;131(4):e1168-e1173. [DOI] [PubMed] [Google Scholar]
- 20.Adegoke SA, Braga JAP, D Adekile A, Figueiredo MS. Impact of hydroxyurea on anthropometry and serum 25-hydroxyvitamin d among children with sickle cell disease. J Pediatr Hematol Oncol. 2018;40(4):e243-e247. [DOI] [PubMed] [Google Scholar]
- 21.Hankins JS, Estepp JH, Hodges JR, et al. . Sickle Cell Clinical Research and Intervention Program (SCCRIP): a lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. 2018;65(9):e27228. [DOI] [PubMed] [Google Scholar]
- 22.Brinker MR, Thomas KA, Meyers SJ, et al. . Bone mineral density of the lumbar spine and proximal femur is decreased in children with sickle cell anemia. Am J Orthop. 1998;27(1):43-49. [PubMed] [Google Scholar]
- 23.Lal A, Fung E, Kammen B, et al. . Evaluation of bone mineral density in children with sickle cell anemia. Blood. 2004;104(11):34A. [DOI] [PubMed] [Google Scholar]
- 24.Meeuwes M, Souza de Carvalho TF, Cipolotti R, et al. . Bone mineral density, growth, pubertal development and other parameters in Brazilian children and young adults with sickle cell anaemia. Trop Med Int Health. 2013;18(12):1539-1546. [DOI] [PubMed] [Google Scholar]
- 25.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. . The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087-2099. [DOI] [PubMed] [Google Scholar]
- 26.Karimova EJ, Rai SN, Howard SC, et al. . Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25(12):1525-1531. [DOI] [PubMed] [Google Scholar]
- 27.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain. 2017;18(5):490-498. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. . Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153-1158. [DOI] [PubMed] [Google Scholar]
- 30.Society for Adolescent Health and Medicine. Recommended vitamin D intake and management of low vitamin D status in adolescents: a position statement of the society for adolescent health and medicine. J Adolesc Health. 2013;52(6):801-803. [DOI] [PubMed] [Google Scholar]
- 31.Crabtree NJ, Arabi A, Bachrach LK, et al. ; International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225-242. [DOI] [PubMed] [Google Scholar]
- 32.Khan AA, Bachrach L, Brown JP, et al. ; Canadian Panel of the International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64. [DOI] [PubMed] [Google Scholar]
- 33.Spinola-Castro AM, Siviero-Miachon AA. Low bone mineral density in patients with sickle cell anaemia (SCA) and short stature should be interpreted with caution. Trop Med Int Health. 2014;19(3):364-365. [DOI] [PubMed] [Google Scholar]
- 34.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84(12):4702-4712. [DOI] [PubMed] [Google Scholar]
- 35.Bordbar MR, Haghpanah S, Zarei T, Dabbaghmanesh MH, Omrani GR, Saki F. Evaluation of bone mineral density in children with sickle-cell anemia and its associated factors in the south of Iran: a case-control study. Arch Osteoporos. 2017;12(1):70. [DOI] [PubMed] [Google Scholar]
- 36.Mokhtar GM, Tantawy AAG, Hamed AA, Adly AAM, Ismail EAR, Makkeyah SM. Tartrate-resistant acid phosphatase 5b in young patients with sickle cell disease and trait siblings: relation to vasculopathy and bone mineral density. Clin Appl Thromb Hemost. 2017;23(1):64-71. [DOI] [PubMed] [Google Scholar]
- 37.Onuba O. Bone disorders in sickle-cell disease. Int Orthop. 1993;17(6):397-399. [DOI] [PubMed] [Google Scholar]
- 38.Smith WR, Penberthy LT, Bovbjerg VE, et al. . Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94-101. [DOI] [PubMed] [Google Scholar]
- 39.Jacob E, Duran J, Stinson J, Lewis MA, Zeltzer L. Remote monitoring of pain and symptoms using wireless technology in children and adolescents with sickle cell disease. J Am Assoc Nurse Pract. 2013;25(1):42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. . Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033-1048. [DOI] [PubMed] [Google Scholar]
- 41.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48(5):1103-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14-27. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Castrillón JL, Olmos JM, Gómez JJ, et al. . Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology. 2000;72(3):187-194. [DOI] [PubMed] [Google Scholar]
- 44.Mattia C, Di Bussolo E, Coluzzi F. Non-analgesic effects of opioids: the interaction of opioids with bone and joints. Curr Pharm Des. 2012;18(37):6005-6009. [DOI] [PubMed] [Google Scholar]
- 45.Daniell HW. Opioid osteoporosis. Arch Intern Med. 2004;164(3):338–, author reply 338.. [DOI] [PubMed] [Google Scholar]
- 46.Nolan VG, Nottage KA, Cole EW, Hankins JS, Gurney JG. Prevalence of vitamin D deficiency in sickle cell disease: a systematic review. PLoS One. 2015;10(3):e0119908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adewoye AH, Chen TC, Ma Q, et al. . Sickle cell bone disease: response to vitamin D and calcium. Am J Hematol. 2008;83(4):271-274. [DOI] [PubMed] [Google Scholar]
- 48.Busse JA, Seelaboyina KN, Malonga G, Setty MJ, Moulton T. Vitamin D level and its correlation with hemoglobin in pediatric sickle cell disease patients. Blood. 2013;122(21):4677. [Google Scholar]
- 49.Garrido C, Cela E, Beléndez C, Mata C, Huerta J. Status of vitamin D in children with sickle cell disease living in Madrid, Spain. Eur J Pediatr. 2012;171(12):1793-1798. [DOI] [PubMed] [Google Scholar]
- 50.Goodman BM III, Artz N, Radford B, Chen IA. Prevalence of vitamin D deficiency in adults with sickle cell disease. J Natl Med Assoc. 2010;102(4):332-335. [DOI] [PubMed] [Google Scholar]
- 51.Kundnani A, Sison V, Ortega PA, Neugebaurer R, Moulton T. Prevalence of vitamin D deficiency in children with sickle cell disease in inner city New York [abstract]. Am J Hematol. 2012;87(7):E31. Abstract 047. [Google Scholar]
- 52.Osunkwo I, Hodgman EI, Cherry K, et al. . Vitamin D deficiency and chronic pain in sickle cell disease. Br J Haematol. 2011;153(4):538-540. [DOI] [PubMed] [Google Scholar]
- 53.Soe HH, Abas AB, Than NN, et al. . Vitamin D supplementation for sickle cell disease. Cochrane Database Syst Rev. 2017;1:CD010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buison AM, Kawchak DA, Schall J, Ohene-Frempong K, Stallings VA, Zemel BS. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145(5):622-627. [DOI] [PubMed] [Google Scholar]
- 55.Rovner AJ, Stallings VA, Kawchak DA, Schall JI, Ohene-Frempong K, Zemel BS. High risk of vitamin D deficiency in children with sickle cell disease. J Am Diet Assoc. 2008;108(9):1512-1516. [DOI] [PubMed] [Google Scholar]
- 56.Adams-Graves P, Daniels AB, Womack CR, Freire AX. Bone mineral density patterns in vitamin D deficient African American men with sickle cell disease. Am J Med Sci. 2014;347(4):262-266. [DOI] [PubMed] [Google Scholar]
- 57.Arlet JB, Courbebaisse M, Chatellier G, et al. . Relationship between vitamin D deficiency and bone fragility in sickle cell disease: a cohort study of 56 adults. Blood. 2012;120(21):2103. [DOI] [PubMed] [Google Scholar]
- 58.Chapelon E, Garabedian M, Brousse V, Souberbielle JC, Bresson JL, de Montalembert M. Osteopenia and vitamin D deficiency in children with sickle cell disease. Eur J Haematol. 2009;83(6):572-578. [DOI] [PubMed] [Google Scholar]
- 59.Özdemir ZT, Özkan EA, Akkoca AO, et al. . Osteoporosis and vitamin D deficiency in patients with sickle cell disease. J Clin Anal Med. 2016;7(4):483-487. [Google Scholar]
- 60.Sadat-Ali M, Al-Elq A, Al-Turki H, Sultan O, Al-Ali A, AlMulhim F. Vitamin D level among patients with sickle cell anemia and its influence on bone mass. Am J Hematol. 2011;86(6):506-507. [DOI] [PubMed] [Google Scholar]
- 61.Fisher E, Law E, Dudeney J, Palermo TM, Stewart G, Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2018;9:CD003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roth-Isigkeit A, Thyen U, Stöven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):e152-e162. [DOI] [PubMed] [Google Scholar]
- 63.Treede RD, Rief W, Barke A, et al. . A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicholas M, Vlaeyen JWS, Rief W, et al. ; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28-37. [DOI] [PubMed] [Google Scholar]
- 65.Perrot S, Cohen M, Barke A, Korwisi B, Rief W, Treede RD; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77-82. [DOI] [PubMed] [Google Scholar]
- 66.Scholz J, Finnerup NB, Attal N, et al. ; Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Treede RD, Rief W, Barke A, et al. . Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19-27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.