Key Points
Unrelated-donor 7/8 BM and UCB HCT led to similar GRFS, CRFS, relapse, and OS in children with acute leukemia.
The risk of grade III-IV aGVHD was significantly higher in the 7/8 BM group than in the UCB group.
Abstract
We report graft-versus-host disease (GVHD)-free relapse-free survival (GRFS) (a composite end point of survival without grade III-IV acute GVHD [aGVHD], systemic therapy–requiring chronic GVHD [cGVHD], or relapse) and cGVHD-free relapse-free survival (CRFS) among pediatric patients with acute leukemia (n = 1613) who underwent transplantation with 1 antigen–mismatched (7/8) bone marrow (BM; n = 172) or umbilical cord blood (UCB; n = 1441). Multivariate analysis was performed using Cox proportional hazards models. To account for multiple testing, P < .01 for the donor/graft variable was considered statistically significant. Clinical characteristics were similar between UCB and 7/8 BM recipients, because most had acute lymphoblastic leukemia (62%), 64% received total body irradiation–based conditioning, and 60% received anti-thymocyte globulin or alemtuzumab. Methotrexate-based GVHD prophylaxis was more common with 7/8 BM (79%) than with UCB (15%), in which mycophenolate mofetil was commonly used. The univariate estimates of GRFS and CRFS were 22% (95% confidence interval [CI], 16-29) and 27% (95% CI, 20-34), respectively, with 7/8 BM and 33% (95% CI, 31-36) and 38% (95% CI, 35-40), respectively, with UCB (P < .001). In multivariate analysis, 7/8 BM vs UCB had similar GRFS (hazard ratio [HR], 1.12; 95% CI, 0.87-1.45; P = .39), CRFS (HR, 1.06; 95% CI, 0.82-1.38; P = .66), overall survival (HR, 1.07; 95% CI, 0.80-1.44; P = .66), and relapse (HR, 1.44; 95% CI, 1.03-2.02; P = .03). However, the 7/8 BM group had a significantly higher risk for grade III-IV aGVHD (HR, 1.70; 95% CI, 1.16-2.48; P = .006) compared with the UCB group. UCB and 7/8 BM groups had similar outcomes, as measured by GRFS and CRFS. However, given the higher risk for grade III-IV aGVHD, UCB might be preferred for patients lacking matched donors.
Visual Abstract
Introduction
The probability of finding an HLA-matched donor for hematopoietic cell transplantation (HCT) varies from 75% among whites with European backgrounds to 16% among blacks of South or Central American descent.1 In the absence of a matched related donor (MRD) or unrelated donor (URD), options for alternative donor HCT include umbilical cord blood transplantation (UCBT), haploidentical HCT, or partially HLA–matched (7/8) bone marrow (BM) or 7/8 peripheral blood (PB) HCT from a URD. In pediatric patients, PB grafts are rarely used because of the higher risks of chronic graft-versus-host disease (cGVHD), treatment failure (relapse or death), nonrelapse mortality (NRM), and overall mortality compared with BM.2
We compared the mortality and morbidity after pediatric alternative donor HCT using data from the Center for International Blood and Marrow Transplant Research (CIBMTR). We evaluated 2 novel composite end points: GVHD-free relapse-free survival (GRFS) and cGVHD-free relapse-free survival (CRFS). GRFS is defined as the absence of grade III-IV acute GVHD (aGVHD), systemic therapy–requiring cGVHD, relapse, or death. CRFS is defined as the absence of systemic therapy–requiring cGVHD, relapse, or death. We previously reported that BM grafts from MRDs led to superior GRFS at 1 and 2 years compared with other graft/donor types.3,4 Here, we analyzed GRFS and CRFS among alternative (nonmatched) donor HCT for children with no available MRD or matched URD.
Materials and methods
Objectives
The primary objective of the study was to compare GRFS and CRFS among pediatric patients (age ≤18 years) with acute leukemia who underwent an alternative donor HCT. Secondary objectives were to describe the distribution and incidence of events contributing to GRFS and CRFS.
Patient population
We included patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in complete remission (CR) who received a first alternative donor HCT (umbilical cord blood [UCB] or 7/8 BM from an URD) with myeloablative conditioning between 2000 and 2014, as reported to the CIBMTR. Exclusion criteria were the receipt of reduced-intensity conditioning, prior autologous or allogeneic HCT, ex vivo T-cell depletion (TCD) or CD34 selected graft, or UCB with <4/6 HLA-matched units. 7/8 PB (n = 48) and haploidentical HCT (n = 61) were excluded because of their small numbers. Data on minimal residual disease to define the quality of pre-HCT CR were not available. HLA matching for the UCB group was determined using intermediate-resolution typing, and high-resolution typing when available, for HLA-A and HLA-B loci and high-resolution typing for HLA-DRB1 loci. Roughly half (47%; n = 680) had allele-level matching data available. The matching for 7/8 BM was done using high-resolution typing for HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci.
Definitions and statistical analysis
Disease risk was stratified as early or intermediate per the CIBMTR standard criteria.5 Early disease was defined as AML/ALL in CR1, and intermediate-risk disease was defined as AML/ALL beyond CR1. Relapse was defined as the time from HCT to the clinical (not molecular or minimal disease) recurrence of the underlying leukemia. Death without relapse (NRM) was treated as a competing risk. Leukemia-free survival (LFS) was defined as the time from HCT to treatment failure (death or relapse). Overall survival (OS) was the time from HCT to death from any cause. Patients with graft failure (failure to engraft prior to subsequent HCT or death) were censored, with the exception of NRM, which was not censored at any point; if a patient died without relapse, they were classified as NRM, regardless of subsequent HCT. aGVHD6 and cGVHD7,8 were diagnosed according to standard criteria, although National Institutes of Health criteria9 for cGVHD were not prospectively used in reports to the CIBMTR during most of the study period.
Multivariate analysis was performed using Cox proportional hazards models. All clinical variables were tested for the proportional hazards assumption. The graft/donor variable violated the proportional hazards assumption for LFS and cGVHD. An optimal cut point of 14 months was determined based on the maximum likelihood method, and separate hazard ratios (HRs) were estimated before and after 14 months. Other covariates that violated the proportional hazards assumption were adjusted through stratification. A stepwise modeling procedure selected factors using a threshold of 0.05 for entry and retention for model building. No 2-way interactions between donor/graft and the adjusted clinical variables in the models were detected at a 0.01 significance level. To account for multiple testing, P < .01 for the donor/graft variable was considered statistically significant. Analysis was done using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
A total of 1613 patients was analyzed (UCB, n = 1441 and 7/8 BM, n = 172; Table 1). The median age was 7 years (interquartile range, 3-11) in the UCB group and 10 years (interquartile range, 6-14) in the 7/8 BM group. Overall, more than half (58%) were males. The majority had ALL (62%), mostly in CR2; the rest had AML (38%), with similar numbers in CR1 and later. A majority (64%) received total body irradiation–based conditioning. In vivo TCD, with anti-thymocyte globulin (ATG) or alemtuzumab, was used in ∼60% of patients. Methotrexate-based GVHD prophylaxis was used more commonly in the 7/8 BM group (79%) than in the UCB group (15%). Other characteristics were similar in both groups. Among those who underwent UCBT, most (n = 1240; 86%) received a single UCB unit: 40% were 4/6 HLA matched, 44% were 5/6 HLA matched, and 16% were 6/6 HLA matched to the recipient. In the UCB group, the median precryopreservation total nucleated cell (TNC) dose was 6 × 107 per kilogram (range, 1-28 × 107); few patients (6%) had a TNC dose <3 × 107 per kilogram. In the 7/8 BM group, an almost equal number of patients had an A-locus mismatch (n = 61; 36%) or a C-locus mismatch (n = 63; 37%), followed by B-locus mismatch (n = 30; 18%), whereas DRB1 mismatch (n = 16; 9%) was infrequent. The median follow-up was 71 months (range, 3-198) in the UCB group and 96 months (range, 6-196) in the 7/8 BM group.
Table 1.
Baseline characteristics
| UCB (n = 1441) | 7/8 BM (n = 172) | |
|---|---|---|
| Recipient age, median (interquartile range), y | 7 (3-11) | 10 (6-14) |
| Males | 831 (58) | 109 (63) |
| Female donor–to–male recipient | 400 (28) | 53 (31) |
| Disease status prior to HCT | ||
| ALL CR1 | 288 (20) | 30 (17.5) |
| ≥CR2 | 606 (42) | 78 (45) |
| AML CR1 | 281 (20) | 30 (17.5) |
| ≥CR2 | 266 (18) | 34 (20) |
| Time from diagnosis to HCT, median (interquartile range), mo | 14 (5-32) | 18 (6-37) |
| ATG/alemtuzumab used | 862 (60) | 91 (53) |
| ATG* | 851 (99) | 63 (69) |
| Alemtuzumab | 11 (1) | 28 (31) |
| Conditioning regimen | ||
| Cy + TBI ± other | 920 (64) | 122 (71) |
| Bu + Cy/Mel ± other | 353 (24) | 25 (14) |
| Others† | 168 (12) | 25 (15) |
| GVHD prophylaxis | ||
| CNI + MMF ± others (not Cy, MTX) | 582 (40) | 20 (11) |
| CNI + MTX ± others (not Cy, MMF) | 209 (15) | 135 (79) |
| CNI ± steroids | 557 (39) | 10 (6) |
| Others ± CNI | 72 (5) | 7 (4) |
| Missing | 21 (1) | 0 |
| Karnofsky/Lansky performance score‡ | ||
| ≥90 | 1166 (81) | 139 (81) |
| <90 | 225 (16) | 18 (10) |
| Donor-recipient CMV serostatus | ||
| −/− | 672 (47) | 49 (29) |
| −/+ | 747 (52) | 47 (27) |
| +/− | 0 | 25 (15) |
| +/+ | 0 | 40 (23) |
| Missing | 22 (1) | 11 (6) |
| Recipient race, ethnicity | ||
| White, non-Hispanic | 843 (59) | 101 (59) |
| White, Hispanic | 242 (17) | 28 (16) |
| Nonwhite, non-Hispanic | 245 (17) | 32 (19) |
| Nonwhite, Hispanic | 21 (1) | 1 (<1) |
| Missing | 90 (6) | 10 (6) |
| Year of HCT | ||
| 2000-2003 | 296 (20) | 50 (29) |
| 2004-2007 | 398 (28) | 86 (50) |
| 2008-2011 | 533 (37) | 26 (15) |
| 2012-2014 | 214 (15) | 10 (6) |
| Follow-up, median (range), mo | 71 (3-198) | 96 (6-196) |
Unless otherwise indicated, data are n (%).
Bu, busulfan; CMV, cytomegalovirus; CNI, calcineurin inhibitor; Cy, cyclophosphamide; Mel, melphalan; MMF, mycophenolate mofetil; MTX, methotrexate; TBI, total body irradiation.
One patient received ATG and alemtuzumab.
Five patients had unknown conditioning regimens.
The Lansky scale was used for 99% of the recipients younger than 16 years, whereas the Karnofsky scale was used for 94% of the recipients aged 16 to 17 years.
GRFS
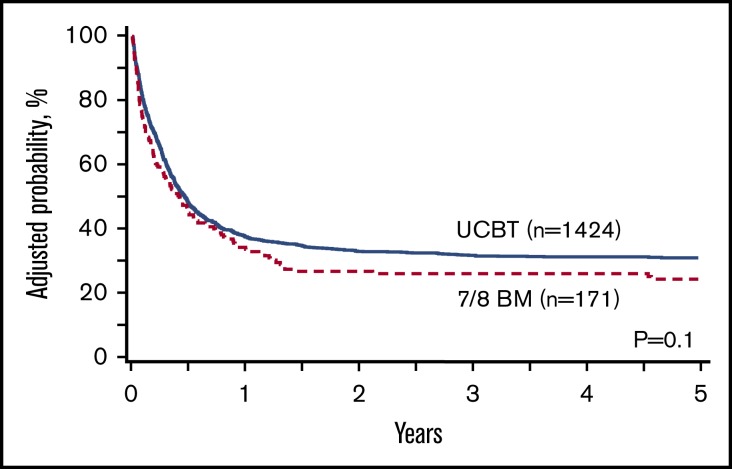
In univariate analysis, UCB had significantly higher GRFS compared with 7/8 BM (P < .001). One-year estimate of GRFS was 38% (95% confidence interval [CI], 36-41) in the UCB group vs 29% (95% CI, 22-36) in the 7/8 BM group; 2-year estimates were 33% (95% CI, 31-36) and 22% (95% CI, 16-29), respectively. However, in multivariate analysis stratified by GVHD prophylaxis (which was tied to the graft source and the conditioning regimen), GRFS was similar in both groups (Figure 1). Patients who received in vivo TCD had significantly superior GRFS compared with those who did not. Intermediate-risk disease status was associated with inferior GRFS compared with early disease. Compared with non-Hispanic whites, other racial and ethnic groups had worse GRFS (Table 2). When analyzed by the number of mismatches in the UCB group, GRFS was similar in groups that received HLA-matched UCB, 1 locus–mismatched UCB (hazard ratio [HR], 1.02; 95% CI, 0.82-1.25; P = .88), 2 loci–mismatched UCB (HR, 1.22; 95% CI, 0.99-1.51; P = .07), and 7/8 BM (HR, 1.23; 95% CI, 0.9-1.68; P = .2). After excluding patients who received double-unit UCB (dUCB), GRFS was similar in the UCB and 7/8 BM groups (HR, 1.06; 95% CI, 0.93-1.20; P = .39) (supplemental Table 1). After excluding patients who received 6/6 HLA–matched UCB, GRFS was similar in the UCB and 7/8 BM groups (HR, 1.13; 95% CI, 0.86-1.48; P = .38) (supplemental Table 2).
Figure 1.
Adjusted GRFS with UCBT and 7/8 BM HCT.
Table 2.
Multiple regression analysis
| Factor | GRFS* | CRFS* | aGVHD III-IV† | cGVHD | Relapse‡ | LFS§ | OS* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Donor/graft type | ||||||||||||||
| UCB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| 7/8 BM | 1.12 (0.87-1.45) | N.S. | 1.06 (0.82-1.38) | N.S. | 1.70 (1.16-2.48) | .006 | 6.17 (2.2-17.33)‖ | .0006 | 1.44 (1.03-2.02) | .03 | 2.14 (1.23-3.74)‖ | .007 | 1.07 (0.80-1.44) | N.S. |
| Disease status | ||||||||||||||
| Early | 1 | 1 | — | — | — | — | 1 | — | — | — | — | |||
| Intermediate | 1.20 (1.06-1.37) | .005 | 1.16 (1.01-1.33) | 0.03 | — | — | — | — | 1.22 (1.00-1.49) | .05 | — | — | — | — |
| ATG/alemtuzumab used | ||||||||||||||
| Yes | 1 | 1 | — | — | 1 | — | — | — | — | — | — | |||
| No | 1.24 (1.08-1.42) | .003 | 1.16 (1.01-1.34) | .04 | — | — | 1.57 (1.28-1.91) | <.0001 | — | — | — | — | — | — |
| Recipient race, ethnicity | ||||||||||||||
| White, Non-Hispanic | 1 | 1 | 1 | 1 | — | — | 1 | 1 | ||||||
| White, Hispanic | 1.30 (1.09-1.54) | .003 | 1.37 (1.15-1.63) | .0005 | 1.02 (0.73-1.42) | N.S. | 1.51 (1.17-1.94) | .001 | — | — | 1.12 (0.91-1.37) | N.S. | 1.13 (0.92-1.40) | N.S. |
| Nonwhite, Non-Hispanic | 1.33 (1.13-1.57) | .0008 | 1.38 (1.16-1.64) | .0003 | 1.60 (1.20-2.13) | .001 | 1.42 (1.09-1.85) | .009 | — | — | 1.29 (1.07-1.55) | .009 | 1.39 (1.14-1.68) | .0008 |
| Nonwhite, Hispanic | 2.05 (1.26-3.32) | .004 | 1.63 (0.99-2.69) | .05 | 3.22 (1.69-6.13) | .0004 | 0.55 (0.14-2.22) | N.S. | — | — | 1.71 (0.99-2.95) | N.S. | 1.84 (1.08-3.14) | 0.02 |
| Missing | 1.23 (0.95-1.59) | N.S. | 1.34 (1.03-1.74) | .03 | 0.80 (0.46-1.38) | N.S. | 1.36 (0.90-2.04) | N.S. | — | — | 1.08 (0.80-1.45) | N.S. | 0.99 (0.72-1.35) | N.S. |
| Karnofsky/Lansky score | ||||||||||||||
| <90 | — | — | 1 | — | — | — | — | — | — | 1 | 1 | |||
| ≥90 | — | — | 0.78 (0.65-0.93) | .005 | — | — | — | — | — | — | 0.72 (0.60- 0.86) | .0005 | 0.70 (0.58-0.85) | .0003 |
| Missing | — | — | 0.76 (0.53-1.10) | N.S. | — | — | — | — | — | — | 0.82 (0.55-1.22) | N.S. | 0.82 (0.55- 1.23) | N.S. |
| Donor-recipient CMV status | ||||||||||||||
| −/− | — | — | — | — | 1 | — | — | — | — | 1 | 1 | |||
| −/+ | — | — | — | — | 1.22 (0.96-1.55) | N.S. | — | — | — | — | 1.33 (1.15-1.54) | .0001 | 1.44 (1.23-1.67) | <.0001 |
| Missing | — | — | — | — | 1.81 (0.98-3.33) | N.S. | — | — | — | — | 1.15 (0.71- 1.87) | N.S. | ||
| Year of HCT | ||||||||||||||
| 2000-2003 | 1 | — | — | — | — | — | — | 1 | 1 | 1 | ||||
| 2004-2007 | 0.91 (0.76-1.09) | N.S. | — | — | — | — | — | — | 0.90 (0.69-1.18) | N.S. | 0.85 (0.69-1.03) | N.S. | 0.84 (0.68-1.03) | N.S. |
| 2008-2011 | 0.78 (0.64-0.95) | .012 | — | — | — | — | — | — | 0.79 (0.60-1.03) | N.S. | 0.71 (0.57-0.88) | .002 | 0.71 (0.56-0.89) | .003 |
| 2012-2014 | 1.02 (0.81-1.29) | N.S. | — | — | — | — | — | — | 1.46 (1.08- 1.97) | .014 | 1.11 (0.86- 1.44) | N.S. | 0.97 (0.74-1.28) | N.S. |
Bold type denotes values with statistically significant P (<.01).
aGVHD III-IV, grade III-IV aGVHD; N.S., not significant (P > .05); —, not applicable.
Stratified variable: GVHD prophylaxis.
Stratified variable: patient age.
Stratified variables: disease, patient age.
Stratified variables: disease, GVHD prophylaxis.
Beyond 14 months after HCT.
CRFS
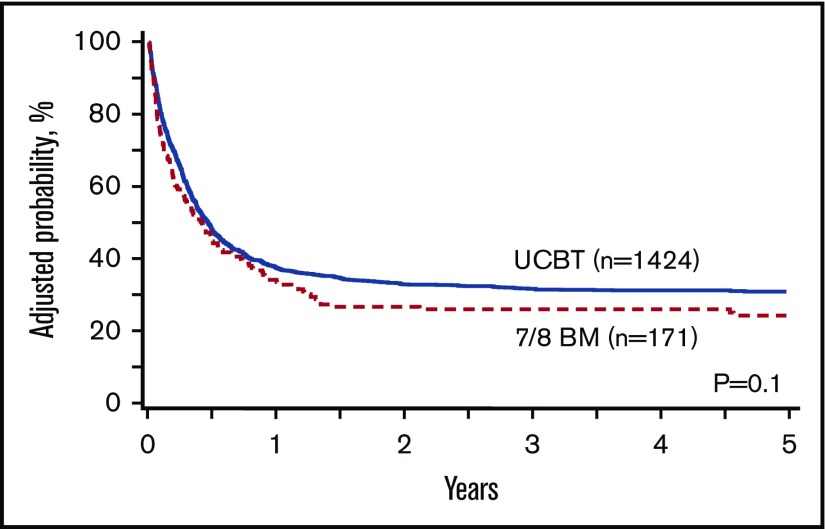
The univariate estimates of CRFS after UCB at 1 year (43%; 95% CI, 41-46) and 2 years (38%; 95% CI 35-40) were slightly higher than those for 7/8 BM at 1 year (38%; 95% CI, 30-45) and 2 years (27%; 95% CI, 20-34) (P = .05). In multivariate analysis stratified by GVHD prophylaxis, there was no difference in CRFS between the donor groups (Figure 2). Patients with a Karnofsky or Lansky performance score <90 had significantly worse CRFS. Compared with non-Hispanic whites, other racial and ethnic groups had inferior CRFS (Table 2). When analyzed by the number of mismatches in the UCB group, CRFS was similar in the groups that received HLA-matched UCB, 1 locus–mismatched UCB (HR, 0.97; 95% CI, 0.78-1.21; P = .8), 2 loci–mismatched UCB (HR, 1.15; 95% CI, 0.93-1.44; P = .2), and 7/8 BM (HR, 1.11; 95% CI, 0.8-1.52; P = .53). After excluding dUCB, CRFS was similar in the UCB and 7/8 BM groups (HR, 1.03; 95% CI, 0.90-1.17; P = .66) (supplemental Table 1). After excluding 6/6 HLA–matched UCB, CRFS was similar in the UCB and 7/8 BM groups (HR, 1.06; 95% CI, 0.81-1.40; P = .68) (supplemental Table 2).
Figure 2.
Adjusted CRFS with UCBT and 7/8 BM HCT.
GVHD
The incidence of grade III-IV aGVHD at day 100 was 18% (95% CI, 16-21) in the UCB group and 29% (95% CI, 23-36) in the 7/8 BM group (P < .001). In multivariate analysis stratified by age, the 7/8 BM group had a 70% higher risk of grade III-IV aGVHD compared with the UCB group. Nonwhites had a significantly higher risk than whites (Table 2). Similar findings were noted after excluding dUCB (supplemental Table 1) and after excluding 6/6 HLA–matched UCB (supplemental Table 2). Compared with HLA matched UCB, the risk of aGVHD III-IV was slightly, but not significantly higher in 1 locus mismatched UCB (HR 1.94, 95% CI 1.15-3.26, P = .012), but was significantly higher both in 2 loci mismatched UCB (HR 2.6, 95% CI 1.55-4.36, P = .0003) and in 7/8 BM (HR 3.6, 95% CI 1.98-6.57, P < .0001).
The estimates of cGVHD at 1- and 2 years were 22% (95% CI, 19-24) and 23% (95% CI, 20-25), respectively with UCB, but significantly higher with 7/8 BM at 28% (95% CI, 22-35) and 34% (95% CI, 27-41), respectively, P = .004. In multivariate analysis, the covariate of donor type violated the proportional hazards assumption for cGVHD; therefore, a cutoff point of 14 months was used for analysis. There was no difference in the risk of cGVHD with UCB and 7/8 BM during the first 14 months post-HCT. However, beyond 14 months, 7/8 BM had a sixfold higher risk for cGVHD than UCB, although there were only a few events (5 of 37 at risk) in the BM group beyond 14 months. Similar findings were noted after excluding dUCB (supplemental Table 1) and 6/6 HLA–matched UCB (supplemental Table 2). Moreover, when analyzed by the number of mismatches in the UCB group, the risk of cGVHD (before or after 14 months) was similar in all UCB groups and the 7/8 BM group (data not shown). The risk of cGVHD was significantly higher in those who did not receive in vivo TCD. Compared with non-Hispanic whites, Hispanics and nonwhites had higher risks for cGVHD (Table 2).
Relapse
Relapse risk was similar at 1 year (20% in both groups) or 2 years (26% with UCB and 28% with 7/8 BM) (P = .22). In multivariate analysis stratified by disease and age and adjusted for covariates, including disease status, there was a trend toward a higher risk for relapse in the 7/8 BM group (HR, 1.44; 95% CI, 1.03-2.02; P = .03) (Table 2). Results remained unchanged after excluding dUCB (supplemental Table 1) and 6/6 HLA–matched UCB (supplemental Table 2). There was no difference in the risk of relapse among the HLA-matched UCB, 1 locus–mismatched UCB (HR, 0.92; 95% CI, 0.69-1.23; P = .6), 2 loci–mismatched UCB (HR, 0.76; 95% CI, 0.56-1.04; P = .09), and 7/8 BM (HR, 1.24; 95% CI, 0.82-1.88; P = .3) groups.
LFS/OS
The univariate estimates of LFS were similar at 1 year (60% in both groups) and at 2 years (52% with UCB and 49% with 7/8 BM) (P = .10). In multivariate analysis stratified by disease and GVHD prophylaxis, UCB and 7/8 BM had similar LFS within the first 14 months of HCT (cutoff period chosen due to violation of proportional hazard assumption). However, beyond 14 months, 7/8 BM had twofold inferior LFS compared with UCB. Again, this should be interpreted with caution because of the relatively small number of events (20 of 56 at risk) in the 7/8 BM group beyond 14 months. Exclusion of dUCB (supplemental Table 1) or 6/6 HLA–matched UCB (supplemental Table 2) yielded similar findings. Moreover, when analyzed by the number of mismatches in the UCB group, LFS was similar in all UCB groups and the 7/8 BM group, before and after 14 months (data not shown). LFS was significantly inferior in the CMV D−/R+ (seronegative donor/seropositive recipient) group compared with the CMV−/− group. Factors associated with better LFS were Karnofsky or Lansky performance score >90 and HCT performed between 2008 and 2011 (Table 2).
The univariate estimates of OS were similar in the UCB (66%; 95% CI, 64-68) and 7/8 BM (65%; 95% CI, 58-72) groups at 1 and 2 years (59% in both groups). Multivariate analysis stratified by GVHD prophylaxis showed similar OS with 7/8 BM and UCB (Table 2) and when restricted to single UCB only (supplemental Table 2) or when restricted to mismatched-UCB only (supplemental Table 2). The CMV D−/R+ group had a significantly higher risk for mortality compared with the CMV−/− group. Factors associated with superior OS were Karnofsky or Lansky performance score >90, white race, and HCT performed between 2008 and 2011 (Table 2). There was no difference in OS between HLA-matched or -mismatched UCB and 7/8 BM (data not shown).
Events contributing to GRFS and CRFS
In the UCB group, relapse (30%) and grade III-IV aGVHD (28%) were the leading events accounting for GRFS, followed by cGVHD (23%) and death (19%). In the 7/8 BM group, most events were related to grade III-IV aGVHD (38%), followed by relapse and cGVHD (24% each) and death (14%). With regard to the CRFS events, cGVHD (35%) and relapse (36%) contributed almost equally in the UCB group; in the 7/8 BM group, cGVHD was the most common event (45%), followed by relapse (31%) and death (24%).
In-patient hospital days and overall causes of death
The median number of days spent in the hospital within the first 100 days of HCT was similar in the UCB (46 days; interquartile range, 34-67) and the 7/8 BM (44 days; interquartile range, 32-60) groups.
Leukemia relapse was the principal cause of death in both groups (43% each). GVHD contributed to 12% and 20% of deaths in the UCB and 7/8 BM groups, respectively. Infection-related deaths were more common in the UCB group (13%) than in the 7/8 BM group (4%). Details about specific infections were not available. More deaths due to organ failure, idiopathic pneumonia syndrome, or acute respiratory distress syndrome occurred in the 7/8 BM group (27%) than in the UCB group (15%). Two percent of deaths in the UCB group were attributed to graft failure; none occurred in the 7/8 BM group (supplemental Table 3).
Discussion
We observed that myeloablative UCB and 7/8 BM HCT in children with acute leukemia led to similar survival without the morbidity events, represented by GRFS and CRFS, and similar relapse and OS, but with a 70% higher risk for grade III-IV aGVHD in the 7/8 BM group. The risks of cGVHD and LFS were also similar in both groups, at least up to 14 months, after which these outcomes favored the UCB group, although definitive conclusions cannot be made because of the limited numbers of late at-risk patients and events in the 7/8 BM group. Similar findings were noted when analyzed by the degree of mismatch (0, 1, or 2) in the UCB group, because we did not find any differences in GRFS, CRFS, OS, relapse, LFS, or cGVHD in the 7/8 BM group vs the UCB group, regardless of the number of mismatches, although the risk for grade III-IV aGVHD was significantly higher in the 7/8 BM group than in the HLA-matched UCB group. Because 2 randomized prospective trials showed a higher risk for grade III-IV aGVHD10 and/or cGVHD10,11 with dUCB vs single-unit UCB transplantation, we performed additional analyses after excluding dUCB and noted similar findings.
In addition to the donor/graft source, factors that had a significant impact on HCT outcomes included the use of in vivo TCD, recipient CMV serostatus, disease status, performance status, and the year of HCT. The effects of in vivo TCD in UCBT have been studied repeatedly with conflicting results reported,12-18 perhaps related to the different types of ATG, as well as differing doses and timing. In our study, in vivo TCD was associated with superior GRFS and a reduced risk for cGVHD, without any impact on grade III-IV aGVHD, CRFS, relapse, LFS, or OS. Also, although NRM was not 1 of the studied end points, exploratory analysis revealed no impact of in vivo TCD on NRM (data not shown). Because a vast majority of in vivo TCD was achieved using ATG (99% in the UCB group and 69% in the 7/8 BM group), subgroup analysis to investigate the impact of ATG vs alemtuzumab could not be performed. In our study, the risk of relapse was similar in patients with HLA-matched vs HLA-mismatched UCB, which is consistent with the findings of some studies19 and contrary to others.20,21 Although the precise reason for this discrepancy is unclear, it might be related to different study populations.
CMV donor/recipient serostatus also affected several HCT outcomes. Because all UCB units are CMV seronegative, we focused on seronegative donors, which constituted 94% of the entire study population. Among these, CMV-seropositive recipients had notably poorer LFS and OS compared with seronegative recipients. The contribution of CMV reactivation to these outcomes is unclear, a factor that is known to be associated with an increased risk for NRM and poor OS.22-24
Transplantations performed between 2008 and 2011 were associated with significantly higher LFS and OS and trended toward superior GRFS compared with those performed before 2008. These data might be explained by the somewhat greater number of patients with early disease (AML/CR1, 56% vs 46%; ALL/CR1, 37% vs 24%-28%) and Karnofsky or Lansky score > 90 (84% vs 76%-79%) compared with previous years. Furthermore, few patients received 7/8 BM grafts from 2008 onward (<5% vs 14%-18%), and fewer patients in the UCB group had a TNC dose <3 × 107 per kilogram (<2% vs 7%-10%) compared with previous years. These factors (ie, more patients undergoing UCB and with higher cell dose, early-risk disease, and better performance status) may have accounted for the findings noted. It is unclear why this trend was not sustained in the latest study period (2012-2014); however, it might be related to the relatively smaller number of transplantations performed in this era than in the preceding years.
In addition, we noted significant racial and ethnic disparities in outcomes. Whites, especially of non-Hispanic ethnicity, had a remarkably lower risk for grade III-IV aGVHD or cGVHD and superior GRFS, CRFS, LFS, and OS than other racial/ethnic groups. As highlighted by other investigators, further research is needed to decipher whether these differences are a surrogate marker of confounders, such as their socioeconomic status, access to health care, and education, or are directly related to differences in biological factors, such as disparities in metabolism of chemotherapy drugs or HLA matching.25,26
Previous studies showed comparable survival with UCB and HLA-matched or -mismatched BM grafts, with some advantages and no clear disadvantage of UCB.20,27 For instance, some studies showed a lower risk for relapse with UCBT than with HLA-matched BM HCT,20 and others showed lower aGVHD and cGVHD with UCBT than with mismatched-BM HCT,27 with similar LFS and OS.20,27 Our study, which is the largest and most recent of its kind, adds to these data and provides a global perspective of outcomes, as assessed by the novel composite end points (GRFS and CRFS), in addition to the individual outcomes.
We acknowledge other limitations of our study besides its retrospective nature. First, haploidentical HCTs were excluded because of the low number performed in children during this time period. Most of the earlier haploidentical HCTs were performed with CD34+ selected or T-cell–depleted grafts,28-34 which were associated with delayed immune reconstitution and high NRM because of viral or fungal infections,32,33,35 high risk for relapse, and poor LFS.30 These outcomes may improve with the use of novel GVHD prophylaxis techniques, including posttransplantation cyclophosphamide,36,37 selective depletion of αβ T cells,38 or T-cell–costimulation blockade.39 Next, the impact of the locus of mismatch in the 7/8 BM group could not be assessed as a result of the small subgroups. Moreover, the conditioning regimen of total body irradiation, cyclophosphamide, and fludarabine is associated with superior outcomes compared with other regimens in the UCBT setting.40 The inclusion of a variety of non–total body irradiation, cyclophosphamide, and fludarabine regimens may have diluted the outcomes of UCBT in our study. Last, the data on measurable residual disease pre- and post-HCT were unavailable.
We conclude that, in pediatric patients with acute leukemia, myeloablative UCBT and 7/8 BM HCT led to similar GRFS, CRFS, OS, and the relapse risk, although 7/8 BM group had significantly higher risk of aGVHD III-IV, suggesting that UCB may be preferred over 7/8 BM, especially if the UCB unit is >4/6 HLA matched. Inclusion of novel GVHD prophylaxis regimens may improve these outcomes for both approaches. As experience develops in pediatric HCT, these outcomes will need to be contrasted against haploidentical HCT to identify preferred alternatives for patients without available matched donors.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Institutes of Health, National Cancer Institute, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases; Grant/Cooperative Agreement 1U24HL138660 from the National Institutes of Health, National Heart, Lung, and Blood Institute and National Cancer Institute; contract HHSH250201700006C with the Health Resources and Services Administration/Department of Health and Human Services; and grants N00014‐17‐1‐2388, N00014‐17‐1‐2850, and N00014‐18‐1‐2045 from the Office of Naval Research; and grants from Adaptive Biotechnologies; *Amgen, Inc.; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol-Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; Medical College of Wisconsin; *Merck & Co., Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick's Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and University of Minnesota (*corporate members).
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: R.S.M. designed the study, analyzed results, and wrote the manuscript; S.G.H., S.R.S., M. Arora, and D.J.W. designed the study, analyzed results, and critically reviewed the manuscript; T.W. and M.T.H. provided the statistical support; D.R.C., A.M.A., J.P., H.A.-A., I.A., M. Aljurf, M. Askar, J.J.A., V.B., C.B., S.C., S.G., J.G., R.P.G., U.G., P.H., G.C.H., Y.I., C.K., P.K., M.L.M., N.M., D.I.M., P.M., T.N., R.F.O., A.P., M.A.D., T.P., M.Q., H.R., O.R., A. Saad, B.N.S., S.S., A. Shah, N.S., K.R.S., M.S., T.S., J.S., T.T., L.F.V., K.M.W., B.W., J.W., and J.A.Y. interpreted data and critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: G.C.H. has acted as a consultant or advisor for Pfizer, Kite Pharma, Incyte, and Jazz Pharmaceuticals; owns stock in Sangamo Therapeutics, Juno Therapeutics, Kite Pharma, Novartis, Insys Therapeutics, AbbVie, GW Pharmaceuticals, Cardinal Health, Immunomedics, Endocyte, Clovis Oncology, Cellectis, Aetna, CVS Health, Celgene, bluebird bio, Bristol-Myers Squibb/Medarex, CRISPR Therapeutics, IDEXX Laboratories, Johnson & Johnson, Pfizer, Procter & Gamble, Vertex Pharmaceuticals, and Jazz Pharmaceuticals; received research funding from Takeda, Jazz Pharmaceuticals, and Pharmacyclics; and received other remuneration (travel, expenses, accommodations) from Kite Pharma, Incyte, Pfizer, the Falk Foundation, Jazz Pharmaceuticals, and Astellas Pharma. The remaining authors declare no competing financial interests.
Correspondence: Rohtesh S. Mehta, Stem Cell Transplantation, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0423, Room FC5 2060, Houston, TX 77030; e-mail: rmehta1@mdanderson.org.
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eapen M, Horowitz MM, Klein JP, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol. 2004;22(24):4872-4880. [DOI] [PubMed] [Google Scholar]
- 3.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RS, Peffault de Latour R, DeFor TE, et al. Improved graft-versus-host disease-free, relapse-free survival associated with bone marrow as the stem cell source in adults. Haematologica. 2016;101(6):764-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Society for Blood and Marrow Transplantation. RFI Disease Classifications and Corresponding CIBMTR Classifications. https://www.asbmt.org/practice-resources/rfi-forms. Accessed 23 July 2018.
- 6.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 7.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406-414. [DOI] [PubMed] [Google Scholar]
- 8.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. [DOI] [PubMed] [Google Scholar]
- 9.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389-401.e1. [DOI] [PMC free article] [PubMed]
- 10.Wagner JE Jr, Eapen M, Carter S, et al. ; Blood and Marrow Transplant Clinical Trials Network. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371(18):1685-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michel G, Galambrun C, Sirvent A, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127(26):3450-3457. [DOI] [PubMed] [Google Scholar]
- 12.Zheng C, Luan Z, Fang J, et al. Comparison of conditioning regimens with or without antithymocyte globulin for unrelated cord blood transplantation in children with high-risk or advanced hematological malignancies. Biol Blood Marrow Transplant. 2015;21(4):707-712. [DOI] [PubMed] [Google Scholar]
- 13.Willemsen L, Jol-van der Zijde CM, Admiraal R, et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant. 2015;21(3):473-482. [DOI] [PubMed] [Google Scholar]
- 14.Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126-132. [DOI] [PubMed] [Google Scholar]
- 15.Veys P, Wynn RF, Ahn KW, et al. Impact of immune modulation with in vivo T-cell depletion and myleoablative total body irradiation conditioning on outcomes after unrelated donor transplantation for childhood acute lymphoblastic leukemia. Blood. 2012;119(25):6155-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiesa R, Gilmour K, Qasim W, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156(5):656-666. [DOI] [PubMed] [Google Scholar]
- 17.Shah AJ, Kapoor N, Crooks GM, et al. The effects of Campath 1H upon graft-versus-host disease, infection, relapse, and immune reconstitution in recipients of pediatric unrelated transplants. Biol Blood Marrow Transplant. 2007;13(5):584-593. [DOI] [PubMed] [Google Scholar]
- 18.Myers GD, Krance RA, Weiss H, et al. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant. 2005;36(11):1001-1008. [DOI] [PubMed] [Google Scholar]
- 19.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947-1954. [DOI] [PubMed] [Google Scholar]
- 21.Gluckman E, Rocha V. Donor selection for unrelated cord blood transplants. Curr Opin Immunol. 2006;18(5):565-570. [DOI] [PubMed] [Google Scholar]
- 22.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka K, Nishida T, Asano-Mori Y, et al. Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant. 2015;21(11):2008-2016. [DOI] [PubMed] [Google Scholar]
- 24.Ramanathan M, Teira P, Battiwalla M, et al. Impact of early CMV reactivation in cord blood stem cell recipients in the current era. Bone Marrow Transplant. 2016;51(8):1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirtane K, Lee SJ. Racial and ethnic disparities in hematologic malignancies. Blood. 2017;130(15):1699-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majhail NS, Nayyar S, Santibañez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47(11):1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang MJ, Davies SM, Camitta BM, et al. Comparison of outcomes after HLA-matched sibling and unrelated donor transplantation for children with high-risk acute lymphoblastic leukemia [published correction appears in Biol Blood Marrow Transplant. 2012;18:1466]. Biol Blood Marrow Transplant. 2012;18(8):1204-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang P, Teltschik HM, Feuchtinger T, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165(5):688-698. [DOI] [PubMed] [Google Scholar]
- 29.Leung W, Campana D, Yang J, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118(2):223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingebiel T, Cornish J, Labopin M, et al. ; Pediatric Diseases and Acute Leukemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115(17):3437-3446. [DOI] [PubMed] [Google Scholar]
- 31.Marks DI, Khattry N, Cummins M, et al. Haploidentical stem cell transplantation for children with acute leukaemia. Br J Haematol. 2006;134(2):196-201. [DOI] [PubMed] [Google Scholar]
- 32.Ortín M, Raj R, Kinning E, Williams M, Darbyshire PJ. Partially matched related donor peripheral blood progenitor cell transplantation in paediatric patients adding fludarabine and anti-lymphocyte gamma-globulin. Bone Marrow Transplant. 2002;30(6):359-366. [DOI] [PubMed] [Google Scholar]
- 33.Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27(8):777-783. [DOI] [PubMed] [Google Scholar]
- 34.Goldman FD, Rumelhart SL, DeAlacron P, et al. Poor outcome in children with refractory/relapsed leukemia undergoing bone marrow transplantation with mismatched family member donors. Bone Marrow Transplant. 2000;25(9):943-948. [DOI] [PubMed] [Google Scholar]
- 35.Lang P, Greil J, Bader P, et al. Long-term outcome after haploidentical stem cell transplantation in children. Blood Cells Mol Dis. 2004;33(3):281-287. [DOI] [PubMed] [Google Scholar]
- 36.Jaiswal SR, Chakrabarti A, Chatterjee S, et al. Haploidentical peripheral blood stem cell transplantation with post-transplantation cyclophosphamide in children with advanced acute leukemia with fludarabine-, busulfan-, and melphalan-based conditioning. Biol Blood Marrow Transplant. 2016;22(3):499-504. [DOI] [PubMed] [Google Scholar]
- 37.Sawada A, Shimizu M, Isaka K, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for advanced pediatric malignancies. Pediatr Hematol Oncol. 2014;31(8):754-764. [DOI] [PubMed] [Google Scholar]
- 38.Locatelli F, Merli P, Pagliara D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood. 2017;130(5):677-685. [DOI] [PubMed] [Google Scholar]
- 39.Watkins B, Qayed M, Bratrude B, et al. T cell costimulation blockade with abatacept nearly eliminates early severe acute graft versus host disease after HLA-mismatched (7/8 HLA matched) unrelated donor transplant, with a favorable impact on disease-free and overall survival [abstract]. Blood. 2017;130(suppl 1). Abstract 212. [Google Scholar]
- 40.Eapen M, Kurtzberg J, Zhang MJ, et al. Umbilical cord blood transplantation in children with acute leukemia: impact of conditioning on transplantation outcomes. Biol Blood Marrow Transplant. 2017;23(10):1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.