To the Editor:
The maximum symptom day (MSD) is a composite measure of asthma symptomatology that integrates 3 questions related to daytime and nighttime asthma symptoms. It is the largest value of the number of days in the previous 2 weeks that a participant reports (1) cough, wheezing, or shortness of breath, (2) slowed activities due to these symptoms, or (3) nocturnal awakening due to these symptoms. Originally defined in the pivotal National Cooperative Inner City Asthma Study, the MSD has been used as a primary end point in numerous studies of inner-city asthma (some of which are mentioned in the “Studies Using the MSD” section in this article’s Online Repository at www.jacionline.org). Surprisingly, since the first publication citing this outcome almost 20 years ago, it has never been validated.1
The standardization of outcomes is important in conducting comparable and reproducible research. A necessary requirement is the use of outcomes that have been contextually validated. Because this has not performed for the MSD, it was classified as ‘‘emerging’’ by the 2010 NIH/AHRQ Asthma Outcomes Workshop, where its core use in asthma research was not supported. Additional evidence reviews have criticized outcomes such as the MSD on this basis.2,3
Because a large body of research relies on the MSD, its validation is important, not only to support inferences in these past and future studies but also to address the disparity that arises by the prioritization of, and reliance on, an unvalidated outcome for research within minority populations. The unintended consequence is that the strength of evidence from these studies, which are specifically aimed at understanding and intervening upon asthma within especially vulnerable populations, may be downgraded, stifling development and dissemination of effective interventions.
Our objective was to assess the reliability, validity, responsiveness, and predictive characteristics of the MSD. We analyzed the Mouse Allergen and Asthma Intervention Trial, a parallel-design randomized clinical trial testing a pest management intervention for allergic asthma.4 A total of 350 participants recruited from Baltimore, Maryland, and Boston, Massachusetts, were followed every 3 months for 1 year (5 encounters). Outcomes recorded at each encounter included the MSD, days of rescue inhaler use in the previous 2 weeks, unanticipated asthma-related health care utilization and oral steroid use in the previous 3 months, and the Childhood Asthma Control Test (C-ACT, ages 5–11 years), Asthma Control Test (ACT, ages 12–17 years), and Pediatric Asthma Therapy Assessment Questionnaire-Control Subscale (ATAQ-Control).5 Prebronchodilator spirometry for FEV1percent-predicted (pp) and FEV1/forced vital capacity (FVC) was also performed. Spirometry and ACT/C-ACT were measured only at baseline and at 6 and 9 months.
Full details on methodology (Methods section) and participant characteristics (Table E1) are included in this article’s Online Repository at www.jacionline.org. Internal consistency reliability was assessed by Cronbach alpha at all visits. These ranged from to 0.82, indicating satisfactory reliability (see Table E2 in this article’s Online Repository at www.jacionline.org). The MSD was equal to all 3 of its constituent symptom responses in 39.5% of instances and to 2 of the 3 responses in 19.1% of instances. Otherwise, the MSD was determined by the global symptoms response in 27.4%, slowed activity in 9.8%, and nocturnal wakening in 4.2% of instances. Test-retest reliability was assessed by the intraclass correlation coefficient among individuals who had stable control on the ACT/C-ACT. This was 0.57 between the baseline and 6-month visits (n = 111) and 0.55 between the 6-month and 12-month visits (n = 120), reflecting moderate test-retest reliability.
TABLE E1.
Participant characteristics (n = 350)
| Characteristic | Value |
|---|---|
| Demographic characteristics, n (%) | |
| Age (y), mean ± SD | 9.8 ± 3.2 |
| Sex: male | 218 (62) |
| African-American race | 278 (79) |
| Hispanic ethnicity | 75 (21) |
| Annual household income, US $ | |
| <30,000 | 232 (66) |
| 30,000–50,000 | 57 (16) |
| >50,000 | 31 (9) |
| Refused or unknown | 30 (9) |
| Asthma medication characteristics, n (%) | |
| Use of controller | 309 (88) |
| Treatment step* | |
| SABA only | 38 (12) |
| Low-dose ICS or LRA | 61 (19) |
| Low-dose ICS + LABA, or medium-dose ICS | 49 (15) |
| Medium-dose ICS + LABA | 16 (5) |
| High-dose ICS ± LABA | 162 (50) |
| Asthma characteristics, median (IQR) | |
| MSD | 3 (0–7) |
| Days of rescue inhaler use/2 wk | 3 (1–8) |
| Asthma control scales | |
| ACT score | 19 (16–22) |
| C-ACT score | 19 (16–21) |
| ATAQ-Control score | 5 (3–6) |
| Event in previous 3 mo, n (%) | |
| Acute care need due to asthma | 234 (67) |
| Use of oral corticosteroids | 165 (47) |
| Prebronchodilator spirometry, mean ± SD† | |
| FEV1pp | 88 ± 17 |
| FVC pp | 98 ± 15 |
| FEV1/FVC ratio | 0.78 ± 0.09 |
ICS, Inhaled corticosteroid; LABA, long-acting beta agonist; LRA, leukotriene receptor antagonist; SABA, short-acting beta agonist.
Based on the Composite Asthma Severity Index (n = 326).
Reported for ages ≥8 y (n = 305).
TABLE E2.
Internal consistency reliability and criterion validity
| Reliability |
Criterion validity Spearman correlation between MSD and criteria (n) |
||||||
|---|---|---|---|---|---|---|---|
| Encounter | Cronbach α | C-ACT | ACT | ATAQ-Control | SABA days | FEV1pp | FEV1/FVC |
| Baseline | 0.74 | −0.51 | −0.68 | 0.35 | 0.50 | −0.13* | −0.11* |
| 3 mo | 0.81 | 0.60 | 0.64 | ||||
| 6 mo | 0.82 | −0.57 | −0.75 | 0.56 | 0.59 | −0.20† | −0.23 |
| 9 mo | 0.74 | 0.60 | 0.74 | ||||
| 12 mo | 0.82 | −0.49 | −0.69 | 0.56 | 0.64 | −0.20† | −0.30 |
| Average | 0.79 | −0.52 | −0.71 | 0.53 | 0.62 | −0.18 | −0.21 |
All cells are statistically significant at a P value of < .001 unless noted otherwise.
Not statistically significant (P > .05).
P < .05.
Criterion validity was demonstrated by identifying significant Spearman correlations between MSD and ACT/C-ACT scores, ATAQ-Control scores, and days of rescue inhaler use at all visits (Table I). Absolute mean correlation with ACT score was 0.71, C-ACT score was 0.52, ATAQ-Control score was 0.53, and rescue inhaler use was 0.62. Low correlations between MSD and spirometric parameters were evident only at 6-and 12-month clinic visits.
TABLE I.
Internal consistency reliability and criterion validity
| Reliability |
Criterion validity Spearman correlation between MSD and criteria |
||||||
|---|---|---|---|---|---|---|---|
| Encounter | Cronbach α | C-ACT | ACT | ATAQ-Control | SABA days | FEV1pp | FEV1/FVC |
| Baseline | 0.74 | −0.51 | −0.68 | 0.35 | 0.50 | −0.13* | −0.11* |
| 3 mo | 0.81 | 0.60 | 0.64 | ||||
| 6 mo | 0.82 | −0.57 | −0.75 | 0.56 | 0.59 | −0.20† | −0.23 |
| 9 mo | 0.74 | 0.60 | 0.74 | ||||
| 12 mo | 0.82 | −0.49 | −0.69 | 0.56 | 0.64 | −0.20† | −0.30 |
| Average | 0.79 | −0.52 | −0.71 | 0.53 | 0.62 | −0.18 | −0.21 |
SABA, Short-acting beta agonist.
All cells are statistically significant at a P value of < .001 unless noted otherwise.
Not statistically significant (P > .05).
P < .05.
Discriminant validity was confirmed by comparing MSD distributions using Wilcoxon rank-sum tests between groups expected to have meaningful differences in asthma symptoms. At all visits, high MSD strongly identified participants who had poor control on ACT/C-ACT and ATAQ-Control scores, an asthma-related acute care visit, corticosteroid need, and rescue inhaler need (see Table E3 in this article’s Online Repository at www.jacionline.org).
TABLE E3.
Discriminant validity: Difference in MSD by presence or absence of clinical factors at all visits
| Absence of factor |
Presence of factor |
|||||
|---|---|---|---|---|---|---|
| Factor | Visit | N | MSD, median (IQR) | N | MSD, median (IQR) | P value |
| Poor control on ACT/C-ACT | Baseline | 147 | 1 (0–3) | 199 | 4 (2–10) | <.001 |
| 6 mo | 189 | 0 (0–2) | 132 | 4 (2–7.5) | <.001 | |
| 12 mo | 193 | 0 (0–2) | 113 | 4 (2–12) | <.001 | |
| Poor control on ATAQ-Control | Baseline | 123 | 1 (0–3) | 277 | 4 (2–8) | <.001 |
| 3 mo | 201 | 1 (0–3) | 142 | 4 (3–8) | <.001 | |
| 6 mo | 203 | 0 (0–2) | 121 | 4 (2–8) | <.001 | |
| 9 mo | 201 | 0 (0–3) | 125 | 4 (3–10) | <.001 | |
| 12 mo | 196 | 0 (0–3) | 110 | 4 (2–8) | <.001 | |
| Need for acute care in previous 3 mo | Baseline | 116 | 2 (0–4) | 234 | 3 (1–8) | <.001 |
| 3 mo | 248 | 2 (0–4) | 98 | 3 (1–7) | <.001 | |
| 6 mo | 223 | 1 (0–4) | 101 | 3 (0–7) | <.001 | |
| 9 mo | 239 | 2 (0–3) | 87 | 4 (2–14) | <.001 | |
| 12 mo | 224 | 1 (0–3) | 84 | 4 (1–8.5) | <.001 | |
| Need for oral steroids in previous 3 mo | Baseline | 185 | 2 (0–5) | 165 | 3 (1–8) | .005 |
| 3 mo | 264 | 2 (0–4) | 82 | 3 (1–7) | <.001 | |
| 6 mo | 246 | 1 (0–4) | 78 | 3.5 (1–7) | .005 | |
| 9 mo | 249 | 2 (0–3) | 77 | 3 (2–7) | .002 | |
| 12 mo | 254 | 1 (0–4) | 54 | 4 (1–10) | <.001 | |
| Need for rescue inhaler in previous 2 wk | Baseline | 83 | 0 (0–3) | 267 | 3 (1–7) | <.001 |
| 3 mo | 104 | 0 (0–2) | 240 | 3 (2–7) | <.001 | |
| 6 mo | 115 | 0 (0–2) | 209 | 3 (1–6) | <.001 | |
| 9 mo | 107 | 0 (0–0) | 77 | 3 (2–7) | <.001 | |
| 12 mo | 107 | 0 (0–1) | 54 | 3 (1–7) | <.001 | |
Responsiveness was confirmed by 2 approaches. First, clinical trajectories of asthma control were defined by ACT/C-ACT. Using ANOVA, we found that the MSD distributions differed among these strata; the MSD decreased in those with improved control and increased in those with worsened control (see Table E4 in this article’s Online Repository at www.jacionline.org). Second, change in MSD was significantly correlated to changes in ATAQ-Control score and days of rescue inhaler use between all adjoining visits (see Table E5 in this article’s Online Repository at www.jacionline.org). There were no consistent correlations between change in MSD and change in FEV1pp and FEV1/FVC.
TABLE E4.
Comparison of change in MSD across change in asthma control as defined by ACT/C-ACT*
| Worsened |
No change |
Improved |
|||||
|---|---|---|---|---|---|---|---|
| Comparison | n | ΔMSD | n | ΔMSD | n | ΔMSD | P value |
| 6 mo vs baseline | 59 | 2.2 ± 4.2 | 111 | −0.9 ± 3.9 | 142 | −2. 6 ± 4.6 | <.001 |
| 12 mo vs 6 mo | 79 | 1.8 ± 4.7 | 120 | −0.1 ± 3.4 | 89 | −0. 6 ± 4.7 | <.001 |
Change in asthma control defined as at least 1 MID between visits, for ACT score ≥3, for C-ACT score ≥2.
TABLE E5.
Responsiveness
| Spearman correlation between ΔMSD and ΔCriteria measures | ||||
|---|---|---|---|---|
| Comparison | ΔATAQ-Control | ΔSABA days | ΔFEV1pp | ΔFEV1/FVC |
| 3 mo vs baseline | 0.35 | 0.23 | ||
| 6 mo vs 3 mo | 0.36 | 0.36 | ||
| 6 mo vs baseline | −0.13* | −0.16† | ||
| 9 mo vs 6 mo | 0.41 | 0.32 | ||
| 12 mo vs 9 mo | 0.59 | 0.42 | ||
| 12 mo vs 6 mo | −0.06* | −0.08* | ||
All cells are statistically significant at a P value of < .001 unless noted otherwise.
Not statistically significant (P > .05).
P = .03.
Predictive validity was confirmed by demonstrating significant associations between MSD and risk of acute health care or corticosteroid need in the following 3 months. A 1-day increase in MSD was associated with a 4% increase in risk of an acute care encounter (risk ratio, 1.04; 95% CI, 1.02–1.06; P < .01; Fig 1) and a 3% increase in risk of oral steroid utilization (risk ratio, 1.03; 95% CI, 1.01–1.05; P 5 .02; see Fig E1 in this article’s Online Repository at www.jacionline.org). There was no evidence of nonlinearity in the association between MSD and either outcome.
FIG 1.
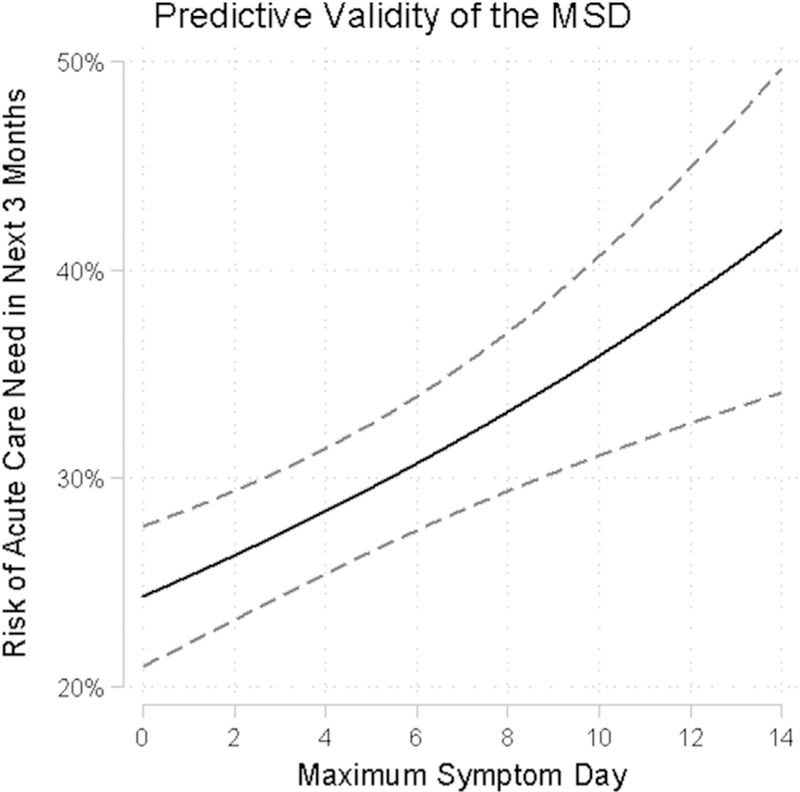
Risk of acute care encounter in subsequent 3 months by MSDs.
FIG E1.

Risk of corticosteroid use in subsequent 3 months by MSDs.
Finally, we performed an exploratory analysis assessing the correlation of spirometry to ACT/C-ACT and ATAQ-Control scores. Similar to the MSD, we found no to low correlations with FEV1pp and FEV1/FVC (see Table E6 in this article’s Online Repository at www.jacionline.org). No measures were consistently responsive to changes in spirometry (see Table E7 in this article’s Online Repository at www.jacionline.org).
TABLE E6.
Correlation between composite measures of asthma control and spirometry*
| Measure | Visit | FEV1pp | P value | FEV1/FVC | P value |
|---|---|---|---|---|---|
| ACT | Baseline | 0.09 | .44 | 0.03 | .77 |
| 6 mo | 0.29 | .01 | 0.25 | .03 | |
| 12 mo | 0.20 | .07 | 0.03 | .82 | |
| C-ACT | Baseline | 0.10 | .26 | 0.16 | .08 |
| 6 mo | 0.06 | .55 | 0.22 | .03 | |
| 12 mo | 0.19 | .06 | 0.44 | <.01 | |
| ATAQ-Control | Baseline | −0.11 | .12 | −0.16 | .02 |
| 6 mo | −0.09 | .25 | −0.11 | .13 | |
| 12 mo | −0.18 | .02 | −0.16 | .04 | |
| MSD | Baseline | −0.13 | .07 | −0.11 | .12 |
| 6 mo | −0.20 | .01 | −0.23 | <.01 | |
| 12 mo | −0.20 | .01 | −0.30 | <.01 |
Reported for ages ≥8 y; MSD included for comparison; bolded values are statistically significant at P < .05.
TABLE E7.
Correlation between change in composite measures of asthma control and change in spirometry*
| Measure | Comparison | ΔFEV1pp | P value | ΔFEV1/FVC | P value |
|---|---|---|---|---|---|
| ACT | 6 mo vs baseline | 0.26 | .03 | 0.24 | .051 |
| 12 mo vs 6 mo | 0.10 | .40 | 0.05 | .70 | |
| C-ACT | 6 mo vs baseline | 0.12 | .24 | 0.10 | .30 |
| 12 mo vs 6 mo | 0.04 | .73 | 0.09 | .43 | |
| ATAQ-Control | 6 mo vs baseline | −0.01 | .89 | −0.04 | .60 |
| 12 mo vs 6 mo | 0.13 | .10 | 0.00 | .99 | |
| MSD | 6 mo vs baseline | −0.13 | .09 | −0.16 | .03 |
| 12 mo vs 6 mo | −0.06 | .46 | −0.08 | .30 |
MSD included for comparison; bolded values are statistically significant at P < .05.
Our results support the MSD as a valid asthma outcome within minority populations. In this dual-center interventional trial of inner-city asthma, the MSD was a reliable and responsive measure that consistently correlated with markers of asthma control. Increases in MSD were associated with increased risk of subsequent asthma-related acute health care utilization and use of corticosteroids, without floor or ceiling effects.
Development of patient-reported outcomes that are contextually meaningful are valuable for understanding the impact of diseases specific to underrepresented or disproportionately burdened populations. This is especially true of urban asthma, which is associated with more symptomatic, uncontrolled disease, driven by risk factors that are unique to these environments. However, if such outcomes are not validated, future public health impacts of studies that use them are fundamentally weakened. This study addresses this disparity, which has existed for the MSD for nearly 2 decades.
The MSD is simple to collect, easy to understand, and intrinsically meaningful. Community asthma stakeholders endorse symptom outcomes such as the MSD as more patient-centered and interpretable.6 The Composite Asthma Severity Index, the only other instrument developed in an inner-city population, includes symptoms with medication parameters, lung function, and exacerbations, necessarily diminishing the importance of symptoms within the overall instrument.7 In addition, MSD’s moderate correlation to the other patient-reported outcomes in the Mouse Allergen and Asthma Intervention Trial suggests that it captures unique information not fully represented by these other measures. The MSD therefore represents a valuable end point to be independently determined, especially in trials that do not actively modify participant medications.
There are 2 opportunities for further study. First, the moderate test-retest reliability at 6 months may have been influenced by the long time interval, which we note is substantially longer than the 4-to 12-week spans reported for other asthma questionnaires. Second, provider assessment of asthma control was unavailable from this study, which would be an additionally informative criterion.
It is notable that the MSD, ACT/C-ACT, and ATAQ-Control were all poorly correlated to lung function. This finding is compatible with literature reporting inconsistent relationships between spirometry and symptoms in children, and it underscores its complementary value in pediatric asthma.
In conclusion, the MSD is a reliable, responsive, and valid measure of asthma symptomatology that is consistently correlated with other measures of asthma control among inner-city populations. Our results strengthen the level of evidence of studies that have used this outcome and support its continued use in observational and interventional trials of inner-city asthma.
Acknowledgments
This work was supported by grants U01Al083238, K24 AI114769, and K24 AI106822 from the National Institute of Allergy and Infectious Diseases; grants F32 ES028578, P50 ES018176, R01 ES023447, and R01 ES026170 from the National Institute of Environmental Health Sciences; and EPA Agreement Number 83615201.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004;351:1068–80. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan JA, Lemanske RF Jr, Canino GJ, Elward KS, Kattan M, Matsui EC, et al. Asthma outcomes: symptoms. J Allergy Clin Immunol 2012;129:S124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leas BF, D’Anci KE, Apter AJ, Bryant-Stephens T, Lynch MP, Kaczmarek JL, et al. Effectiveness of indoor allergen reduction in asthma management: a systematic review. J Allergy Clin Immunol 2018;141:1854–69. [DOI] [PubMed] [Google Scholar]
- 4.Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: a randomized clinical trial. JAMA 2017;317:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner EA, Diette GB, Algatt-Bergstrom PJ, Nguyen TT, Clark RD, Markson LE, et al. The asthma therapy assessment questionnaire (ATAQ) for children and adolescents. Dis Manag 2004;7:305–13. [DOI] [PubMed] [Google Scholar]
- 6.Shelef DQ, Rand C, Streisand R, Horn IB, Yadav K, Stewart L, et al. Using stakeholder engagement to develop a patient-centered pediatric asthma intervention. J Allergy Clin Immunol 2016;138:1512–7. [DOI] [PubMed] [Google Scholar]
- 7.Wildfire JJ, Gergen PJ, Sorkness CA, Mitchell HE, Calatroni A, Kattan M, et al. Development and validation of the Composite Asthma Severity Index—an outcome measure for use in children and adolescents. JAllergy Clin Immunol 2012;129:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]


