Abstract
Yong Kit Samuel Chan, Tai Chong Toh, and Danwei Huang (2018) Archaster typicus is a microphagous sea star ubiquitous throughout sandy shoals of the Indo-Pacific. Along highly urbanised coasts, loss of sandy habitats through land reclamation and degradation of adjacent mangrove forests and seagrass meadows, which serve as nurseries for A. typicus, could lead to local extinction of this species. To determine the population status of A. typicus in Singapore, we performed belt-transect surveys at three modified shores, then compared size structure, clustering patterns and ontogenetic shifts within the Central Indo-Pacific region. We found that A. typicus individuals were, among other things, larger in Singapore (79.2 ± 14.2 mm) than the rest of the Central Indo-Pacific region with further differences amongst Singapore’s sites. Sea stars of this species were also greatly clustered in smaller areas within the transects, with most transects presenting small Nearest Neighbour Index values of < 1. While ontogenetic shifts were noted in previous studies, no juveniles have been recorded in the nursery habitats of mangroves and seagrasses, with limited size and mating seasonalities. Although A. typicus appears to have grown in size considerably on reclaimed beaches in Singapore, the lack of any apparent ontogenetic connectivity here may threaten the sea star populations in the near future, particularly in the context of growing coastal development in Southeast Asia.
Keywords: Asteroidea, Population ecology, Artificial shores, Intertidal ecology, Archaster typicus
BACKGROUND
Some species of sea stars (Echinodermata: Asteroidea) have important functions in marine ecosystems, principally influencing community assemblages via top-down selection pressure on prey populations (Paine 1974; Menge et al. 1994). Outbreaks or busts in these sea star populations potentially affect communities down the food chain, resulting in dramatic effects at the ecosystem level (Adjeroud et al. 2018). Other sea stars are microphagous grazers feeding on detritus, burrowing into the top layer of sediment and acting as bioturbators in the process to effect change on bacterial communities and landscapes (Lohrer et al. 2004; Jackson et al. 2009; Pillay et al. 2010). Sea stars as predators and bioturbators ultimately shape the community assembly and heterogeneity of landscape; the extent of which depends on their size and spatial patterns within their populations (Paine 1976; Mueller et al. 2011). In recent years, the importance of sea stars has been revealed because of the negative impacts they are facing in many ecosystems and the cascading effects from their loss on community structure. The sea star wasting syndrome devastated North American coasts between 2013 and 2014, reducing multi- species populations drastically; these declines reverberated through the food web (Montecino- Latorre et al. 2016; Schultz et al. 2016). Alongside disease, sea stars are also vulnerable to the effects of climate change (Przeslawski et al. 2008 2015; Wood et al. 2008). However, most research has focussed on important predatory species, like the corallivorous crown-of-thorns (Acanthaster planci), studied for its devastating impacts on coral reefs globally (Fabricius 2013), and little is known about other more inconspicuous species, despite their diversity (Lawrence 2013).
Species of the Archasteridae family are amongst the most ubiquitous and abundant sea stars in tropical coastal regions across the Indian and western Pacific Ocean (Sukarno and Jangoux 1977; Janssen et al. 1984; Vandenspiegel et al. 1998). There are three valid species found in different tropical regions, with some overlap - Archaster angulatus Müller and Troschel, 1842, found generally along the western coasts of Australia with some records in the central Indo- Pacific region; Archaster lorioli Sukarno and Jangoux, 1977, found in the Indian Ocean; and Archaster typicus Müller and Troschel, 1840, found across the Indo-Pacific (Sukarno and Jangoux 1977). The biology of A. typicus, and to a lesser extent A. angulatus, has been examined most notably for their pseudocopulatory behaviour, where males mount females to form mating pairs prior to the release of gametes (Komatsu 1983; Janssen et al. 1984; Run et al. 1988; Keesing et al. 2011). Archaster typicus has also been observed for its reproduction (Komatsu 1983; Run et al. 1988; Bos et al. 2013), ontogeny (Bos et al. 2011) and aspects of its feeding and behaviour (Bos et al. 2011; Mueller et al. 2011). However, few studies have examined its population ecology and ontogenetic shifts (Mukai et al. 1986; Bos et al. 2008a b 2011), especially within human- modified habitats which are increasingly common in the region. Population studies are necessary to determine sea star population status across geographic regions and time (Uthicke et al. 2009), while ontogenetic shifts highlight important nursery and adult habitats (Nakazawa 2014), aiding in the understanding of the species’ ecology and conservation trajectories in light of increasing coastal development in Southeast Asia.
In highly urbanised marine environments such as Singapore, A. typicus is vulnerable to local extinction from habitat loss due to heavy land reclamation of many coastal areas and offshore islands where they are commonly found (Vandenspiegel et al. 1998; Lane 2008). In particular, the loss of important mangrove and seagrass habitats known to be nursery grounds for this species (Bos et al. 2011) has likely impacted A. typicus populations (Bos et al. 2011; Tan et al. 2016). Reduction in the population of the once- abundant detritivore and burrower will likely impact the sediment structure and infaunal community (Lane 2008; Pillay et al. 2010). Interestingly, A. typicus has also been documented to grow to a much larger size in Singapore compared to other regions, though the exact size difference has never been quantified (Vandenspiegel et al. 1998; Lane 2008). The construction of artificial swimming lagoons in the 1970s and 2000s (Teh et al. 2010) may have created potential new habitats for adult populations and enabled sea stars with larger body sizes to thrive (Tan et al. 2016). A detailed study of these extraordinary A. typicus populations would allow us to compare size and distributional patterns between these highly-modified sandy shores and less disturbed environments elsewhere.
This study thus seeks to examine spatial and temporal trends in A. typicus size and clustering in Singapore in the context of previous studies in the Central Indo-Pacific region. We look speci- fically at variation in Singapore and regional A. typicus populations in terms of (i) arm length, (ii) density and clustering and (iii) mating seasonality and ontogenetic habitat shifts. Findings will examine the claims that Singapore’s A. typicus are larger, drawing attention to regional size variations in Asteroidea species between localities. In the face of declining habitat diversity and availability of ontogenetic shifts to sustain local populations, we also highlight possible effects of coastal development on intertidal detritivorous sea stars in the region.
MATERIALS AND METHODS
Study site
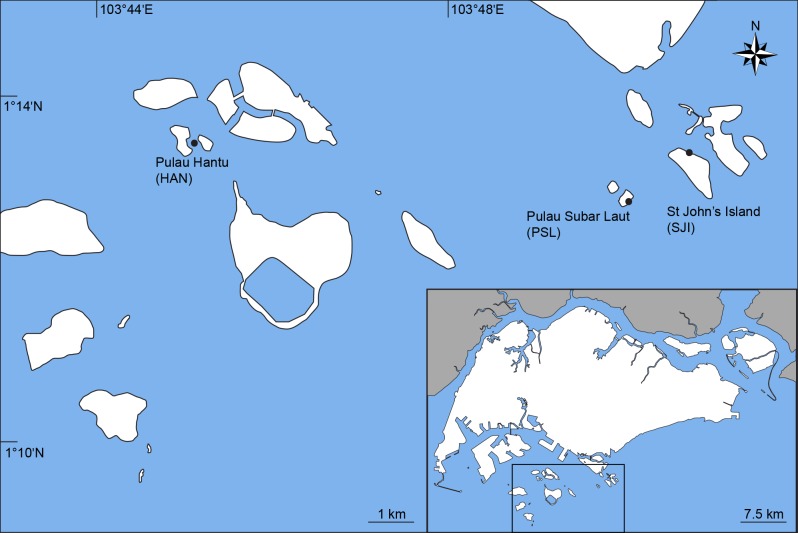
Three sites south of mainland Singapore were selected for the study - (i) Pulau Hantu (HAN; 1°13'31.6"N, 103°44'58.32"E), (ii) Pulau Subar Laut or Big Sisters’ Island (PSL; 1°12'50.6"N, 103°50'05.2"E), and (iii) St John’s Island (SJI; 1°13'09.4"N, 103°50'55.0"E) (Fig. 1). Reclamation was conducted around each of these islands to expand outwards from the original land area, creating lagoons partially bordered by cement- filled revetments (Teh et al. 2010; Tan et al. 2016). Overall, the sites have similar coastal habitats, consisting mainly of sandy shoals with some coral rubble and seagrass patches. A small mangrove patch is also present at Pulau Hantu.
Fig. 1.
Fig. 1. Map of Singapore with enlarged portion highlighting three study sites off the southern coast of mainland Singapore (HAN: Pulau Hantu, PSL: Pulau Subar Laut, and SJI: St John’s Island) (adapted from https://commons.wikimedia.org/wiki/File:Singapore_Outline. svg).
Field surveys
Field surveys were conducted monthly during low spring tides (0 to 0.4 m above chart datum; Maritime Port Authority, 2015) from June 2015 to May 2016. As no sea stars were observed in mangroves, two permanent belt transects (50 m by 5 m) were laid parallel to the seafront at the sandy lagoons with some seagrass cover where Archaster typicus were present for every survey. For each sea star recorded, the microhabitat (i.e. seagrass, sand) it was found in, as well as distances along and to the transects from the central disc position of each sea star (to the nearest 0.1 m), were recorded. Distances were converted to x and y coordinates and used to plot the location of each sea star along the transect to examine density. Nearest neighbour distances were also calculated and used to evaluate clustering. The length of the arm (R) directly opposite the madreporite was also measured along with all shorter damaged arms. Sea star arm lengths were measured to determine size and estimate age, as previous studies found arm length to be a good proxy for relative age in this species (Bos et al. 2011). Mating sea stars, observed with their central disc above each other (Run et al. 1988), were noted along with their positions. Proportions of mating sea stars were then used to identify mating seasonality.
To determine if sediment size is related to differences in animal size and clustering between sites, we collected substrate samples mid-way during the study (October and December 2015) to characterise the top-layer sediment profile at each site. A 4-cm-diameter container was pushed into sediment to a depth of about 8 cm. Sediment samples were then dried, sieved and weighted for each fraction according to the Krumbein phi scale - silt (< 63 μm), fine sand (63-250 μm), medium sand (250-500 μm), coarse sand (500-2000 μm) and gravel (> 2 mm).
Statistical analyses
Histograms of A. typicus arm lengths were plotted monthly for each site and the combined dataset to examine population structures for peaks and recruitment over time. Electronic Length Frequency Analysis (ELEFAN) was also conducted for each set of monthly length frequency classes by site to examine growth rates (following Pauly 1980; Mildenberger et al. 2017).
Selected variables - site, habitat, month and tide were used to fit an informed prior linear mixed- effects model for size data after checking for multicollinearity. Data collected in May had to be removed and months were grouped to allow for the modelling of the interaction between time and site. The model that best explained the results was then selected using the Akaike Information Criterion (AICc). All other descriptive graphs were plotted with all available data.
Clustering was examined using the Nearest Neighbour Index (NNI), calculated from the x and y coordinates of the sea stars on a two dimensional
plane using the following formula - NNI = , where r = nearest neighbour distance, n = number of observations and A = area (Clark and Evans 1954). Each mating pair was represented by one sea star, as more mating pairs would have reduced and confounded nearest neighbour distances. The NNI values compare the observed distribution to an expected distribution, with a value of 1 indicating a random distribution, values below 1 indicating the degree of clustering, and values above 1 indicating the degree of dispersion.
Statistical analyses were done in R version 3.3.3 (R Core Team, 2017) in RStudio version 1.0.136. with packages ‘AICcmodavg’ version 2.1- 0, ‘car’ version 2.1-1, ‘ggplot2’ version 2.2.1, ‘nlme’ version 3.1-131, ‘spatstat’ version 1.50-0 and ‘TropFishR’ version 1.2.1.
RESULTS
Size structure of Archaster typicus in Singapore
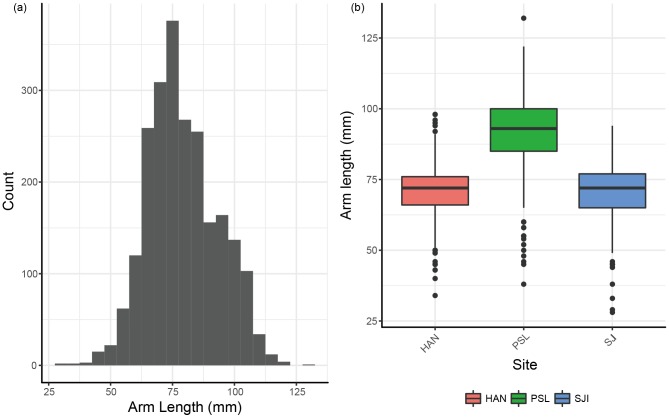
A total of 2304 Archaster typicus individuals were observed at all three study sites over 12 months. The pooled population of A. typicus arm lengths, R, shows a unimodal normal distribution with mean 79.16 ± 14.20 mm, modal class at 70- 80 mm and a range from 28 mm to 132 mm (Fig. 2a). Individual site-level histograms plotted monthly showed generally unimodal peaks with potentially small recruitment events in July to October 2015 and January 2016 (Fig. S1). The ELEFAN analysis also highlighted low growth rates of 0.1-0.15 mm year-1 with low mortality from the lack of an obvious decline in the main cohort numbers (Fig. S2).
The best mixed-effects model fitted sites, grouped months and habitats with an interaction between month and site (AICc = 16254.77), versus the model without the interaction term (∆AICc = +12.92). From the model, A. typicus was significantly larger in PSL (∆ = +17.3 mm; p = 0.0017) than HAN (67.42 mm) while SJI (∆ = -1.96 mm; p = 0.4688), and HAN were not significantly different (Fig. 2b). Similarly, mean values of arm lengths showed a similar pattern with PSL having much greater values than SJI and HAN, with similar values (Table 1). While months varied between significant and not significant (p = 0.0197 to 0.3464), all months showed a decrease in size compared to the first sampling, generally indicating a lack of distinct growth in the population (Table 2). Lastly, though habitat was not significant, sandy shoal sea stars were larger (∆ = +5.86 mm; p = 0.0602) than seagrass ones (Table 2). Though the interaction term between month and site improved the model, none of the interactions were significant, though PSL interactions generally resulted in larger or similarly-sized sea stars than SJI interactions (Table 2).
Fig. 2.
Fig. 2. (a) Arm length (mm) of all sea stars measured across all three Singapore sites. (b) Arm length (mm) of sea stars at each site (HAN: Pulau Hantu, PSL: Pulau Subar Laut, SJI: St John’s Island).
Table 1. Arm length of Archaster typicus across sites(HAN: Pulau Hantu, PSL: Pulau Subar Laut, SJI: St John’s Island).
| Site | Total | HAN | PSL | SJI |
| Count | 2304 | 864 | 877 | 563 |
| Mean | 79.162 ± 14.195 | 71.295 ± 8.471 | 92.140 ± 11.301 | 71.018 ± 9.593 |
| Min | 28 | 34 | 38 | 28 |
| Max | 132 | 98 | 132 | 94 |
Table 2. Linear mixed-effects model of A. typicus Size against Month, Site and Habitat with random effect of Transect (HAN: Pulau Hantu, PSL: Pulau Subar Laut, SJI: St John’s Island).
| Size ~ Month + Site + Habitat + Month:Site, random = ~ 1 | Transect | ||||
| Value | Std Error | t-value | p-value | |
| (Intercept) | 67.42126 | 3.601800 | 18.718769 | 0.0000 |
| Month Aug/Sep | -2.45030 | 1.401070 | -1.748874 | 0.0805 |
| Month Oct/Nov | -1.28368 | 1.363050 | -0.941768 | 0.3464 |
| Month Dec/Jan | -2.65203 | 1.136333 | -2.333846 | 0.0197 |
| Site PSL | 17.31711 | 2.296627 | 7.540237 | 0.0017 |
| Site SJI | -1.95746 | 2.448444 | -0.799469 | 0.4688 |
| Habitat Shoal | 5.86055 | 3.117338 | 1.879986 | 0.0602 |
| Month Aug/Sep : Site PSL | 4.51784 | 1.837096 | 2.459231 | 0.7716 |
| Month Aug/Sep : Site SJI | 2.97494 | 1.800943 | 1.651881 | 0.0987 |
| Month Oct/Nov : Site PSL | 0.61489 | 1.760722 | 0.349223 | 0.7270 |
| Month Oct/Nov : Site SJI | -0.58030 | 1.841946 | -0.315047 | 0.7528 |
| Month Dec/Jan : Site PSL | 2.35179 | 1.593758 | 1.475625 | 0.1402 |
| Month Dec/Jan : Site SJI | 2.51851 | 1.613408 | 1.560985 | 0.1187 |
Clustering in Archaster typicus in Singapore
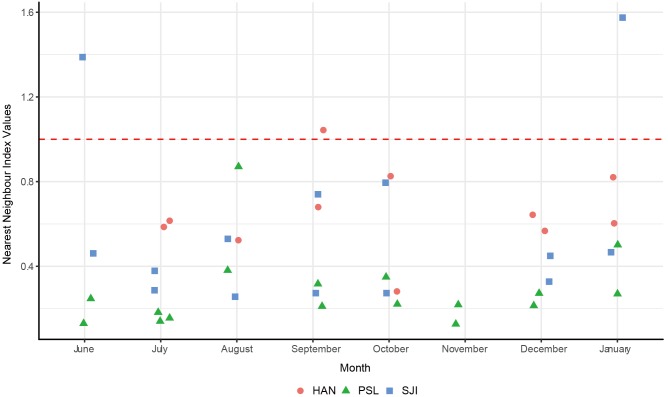
Across all 44 transects, most NNI values were below 1 (n = 40; 90.9%), highlighting a clustered distribution, with only 4 transects (9.1%) having NNI values above 1 (Fig. 3). The NNI values depicted greater clustering at PSL - with most values lower than 0.3 - and weaker clustering at HAN - with most values around 0.6 (Fig. 3). Clustering did not seem to vary according to month, with no clear pattern through time (Fig. 3).
Fig. 3.
Fig. 3. Nearest Neighbour Index (NNI) values of transects across months showing generally clustered populations of A. typicus at the transect level. Red dashed line (NNI = 1) indicates a random distribution, with values above 1 indicating dispersion and values below 1 indicating clustering (HAN: Pulau Hantu, PSL: Pulau Subar Laut, SJI: St John’s Island).
Ontogenetic shifts and mating seasonality in Singapore
There was a clear absence of sea stars within the Pulau Hantu mangrove during all sampling trips. The number of sea stars within the seagrass patches were also very low (n = 10) compared to within sandy shoals (n = 2294). Though there were no significant differences between sea star size on sandy shoals and seagrasses, sea stars were still slightly larger at sandy shoals (∆ = +5.86 mm; p = 0.0602) (Table 2). Additionally, the smallest individuals on seagrasses were 28 mm compared to the 33 mm ones on the shoals. No individuals smaller than this arm length had been observed at other habitats in Singapore.
Counts of mating sea stars did not seem to indicate mating seasonality, although mating proportions seemed to increase in December and January, with sporadic high mating proportions in individual transects across sites and months with no clear trend such as in one transect in HAN in October and one transect each in PSL and SJI in August.
Sediment analysis
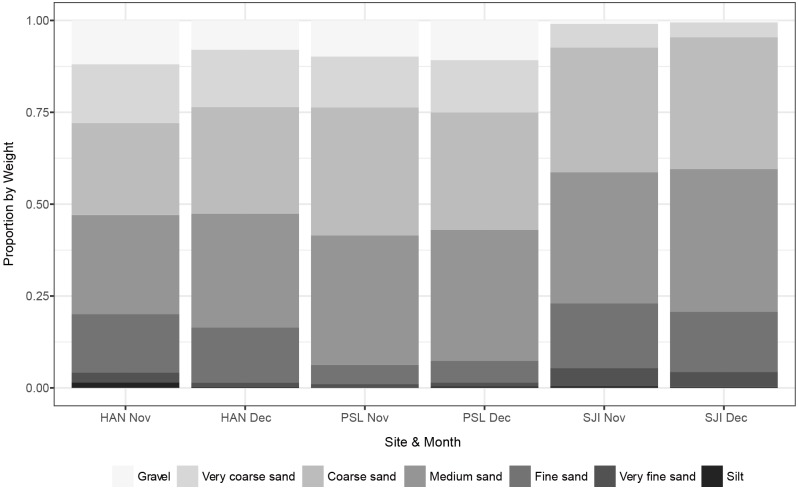
Sediment fractions across months did not vary considerably, though the sediment profile of the different sites differed, being a possible explanation for the differences in sea stars across sites (Fig. 4). In general, most of the sediments found consisted mostly of medium to coarse sediments, with fine sediments least common in PSL and very coarse sediments least common in SJI (Fig. 4).
Fig. 4.
Fig. 4. Sediment proportions across sites and between months showing greater proportions of coarse material at PSL (HAN: Pulau Hantu, PSL: Pulau Subar Laut, SJI: St John’s Island).
DISCUSSION
Archaster typicus populations have been studied in their natural habitat (Komatsu 1983; Mukai et al. 1986; Run et al. 1988; Bos et al. 2008a 2011 2013), though no attempts have been made to examine these populations in human-modified habitats, despite them being ubiquitous in Indo-Pacific intertidal ecosystems facing an increasing number of stressors. This study characterises populations in these artificial sandy shoals and finds local and geographic scale variations in body size and distributions, possibly reflecting differences in growth between sites and the expansion of suitable habitats. A relatively large population can still be found, but this may be maintained by low mortality rates. The lack of juveniles within nursery habitats, distinct mating seasonality and recruitment events could cause trouble for future populations, and presents a possible trajectory for this species in other regions currently undergoing coastal development with increasing numbers of artificial shores.
Mean sea star arm lengths, R, were the largest in Singapore at 79 mm, as compared to a mean R of 65 mm in the Philippines (Bos et al. 2011) and 49 mm in Japan (Mukai et al. 1986). The modal class of 70-80 mm also supports that this is the most common size range of sea stars encountered. Aside from regional differences, sea star sizes amongst sites within Singapore also differed significantly. Lawrence et al. (2011) also found significant size differences of about 30 mm in arm length for Archaster angulatus in Australia between two different sites; they attributed the differences to differential growth rates. This was rationalised through the similar maximum sizes and structuring around a unimodal mean, indicative of a single cohort as opposed to different age groups (Lawrence et al. 2011). Similarly, in Singapore, the similar maximum sizes between sites and structuring around a unimodal mean for each site likely indicate that the differences in Singapore are due to differential growth rates and not different ages, though indeterminate growth is generally observed in Asteroidea species (Sebens 1987; Menge and Sanford 2013). This is one explanation for the regional size differences as well, identifying possible finer scale differences within different regions.
Bos et al. (2011) noted a relatively slow growth rate of A. typicus adults in the Philippines while Keesing (2017) found relatively higher growth rates in A. angulatus adults in Australia. We applied the sizes found in Singapore to growth rates provided by the previous two studies and found that the growth rates indicate different ages (Keesing (2017) - ~2 years and Bos et al. (2011) - ~4 years). Similarly, ELEFAN indicated that Singapore’s sea stars grew slowly, though this pattern will need to be further verified through growth experiments as sea stars can exhibit reduction in size over time as well due to their water vascular system and a lack of nutrition (Pauly 1980; Mildenberger et al. 2017). Growth and size are also related to habitat and substrate through food availability (Feder and Christensen 1966; Sebens 1987; Lawrence et al. 2011). A. typicus is likely a detritivore (Mukai et al. 1986) and has been observed feeding with the eversion of its stomach over substrate. Lawrence et al. (2011) found that A. angulatus were larger in habitats with greater proportions of finer sediment, while Mukai et al. (1986) did not examine the relation between food and size but noted that food was not thought to be limiting abundance. The same trend between size and finer sediment proportion was not found here, as the largest sea stars were found on the Sisters’ Island with the lowest proportions of finer sediments of the three sites examined. Bos et al. (2011) also examined ontogenetic shifts with growth and habitats, finding that sea stars experienced ontogenetic shifts from mangroves to seagrasses to sandy shoals and organic material decreased correspondingly. This warrants further investigation into the detrital content in the different sediment fractions and possibly the difference in quality across sites and regions, as these may have been influenced by the creation of artificial shores.
Relatedly, ontogenetic shifts in which sea star size increases from structurally complex habitats to more open ones have been observed in the Philippines, with larger sea stars using size as a defence mechanism against predation (Scheibling and Metaxas 2008; Mueller et al. 2011; Rogers and Elliott 2013). The larger size can also confer greater movement speed, allowing for more efficient foraging across the habitat (Mueller et al. 2011). Nevertheless, we did not observe an ontogenetic shift in A. typicus of Singapore, despite extensive searches in nearby mangrove forests and seagrass meadows from 2013 to 2016 (pers. obs.). The surveys reported here reveal only a few adult sea stars that are associated with seagrasses, while juveniles of similar sizes to those in the Indo-Pacific region have not been found in Singapore. This may have been the result of extensive reclamation efforts over mangroves and seagrasses, limiting the connectivity and preventing ontogenetic shifts. Keesing (2017) found a similar absence of A. angulatus juveniles, though cohorts of smaller individuals were still found through their multi-year study that grew and joined the adult cohorts in terms of size. The lack of smaller individuals and juveniles could indicate lack of successful recruitment or the existence of nursery sites yet to be discovered.
Archaster typicus individuals have been known to live in large aggregations. In the Philippines, sea star density decreased from juvenile habitats of mangroves and seagrasses to sandy shoals for adults (Bos et al. 2011). Here, we found populations restricted to a few sandy lagoons, with extremely patchy distribution at each site. This study found more similar distributions to those in Janssen et al. (1984), which observed most sea stars to be within sandy patches close to seagrasses, and fewer within seagrasses. When we used the Nearest Neighbour Index (NNI) to quantify the fine-scale distribution of sea stars in situ, clustering was found in most of the transects surveyed, with most individuals aggregated in small patches of about 20 m2 within the transects. In the few studies that have quantified the spatial distribution of A. typicus, clustering patterns differed greatly across different scales, with greater aggregation at smaller scales of about 0.25 m2 to 4 m2 (Mukai et al. 1986). Absolute density per transect was generally higher in this study (0.20 ± 0.16 individuals m-2) than in similar sandy shoals in the transect-based study in the Philippines (0.05 individuals m-2; Bos et al. 2011), though lower than in quadrat based studies in the Philippines (4.12 individuals m-2; Janssen et al. 1984); Okinawa, Japan (3.5 to 35.2 individuals m-2; Mukai et al. 1986); and Taiwan (3 individuals m-2; Run et al. 1988). Keesing et al. (2011) found slightly higher densities of A. angulatus in Australia (1.11 individuals m-2).
This high level of clustering in Singapore, in spite of the muted mating seasonality, suggests high levels of detrital content in these patches that the sea stars are feeding on. Spatial clustering of asteroids is also thought to raise fertilization efficacy (Levitan 1998; Mercier and Hamel 2013). While mating proportions show no clear patterns, some sharp increases in mating proportions during December and January have been observed. Thus clustering could still enable greater ease of pseudocopulation and fertilization efficacy afterwards (Himmelman et al. 2008; Keesing et al. 2011). Mating seasons also differed among regions, with distinct seasons in higher latitudinal sites such as Japan (Mukai et al. 1986) and Taiwan (Run et al. 1988), while some studies in the same area in the Philippines found seasonality and others did not (Janssen 1991; Bos et al. 2011 2013). In Singapore, the tropical environment may present more ideal conditions year round for fertilization, possibly explaining the greater variance in mating pairs found over different months. However, the actual cues for spawning post-pseudocopulation is still unconfirmed (Bos et al. 2008b 2013).
CONCLUSIONS
Our investigation of the fine-scale distribution and density of Archaster typicus is useful in guiding spatial comparisons of sea stars across survey techniques and locales. Results show that, despite lower densities here compared to other sites in the region, the populations of sea stars examined are generally clustered, possibly due to food availability. The larger sizes of A. typicus in Singapore’s modified marine environment highlight that some sea stars may respond in interesting ways to coastal development. The creation of these new sandy lagoons has increased the area of available habitats and may provide more food resources for A. typicus. However, this will likely be offset by the lack of connectivity between their juvenile habitats of mangroves and seagrass beds, which have declined in spatial extents by 91.4% since 1922 (Hilton and Manning 1995; Lai et al. 2015) and 42.9% since the 1960s (Yaakub et al. 2014). A longer term study to examine intergenerational trends in sea star distribution and abundance could reveal if and how the adult populations are actually being sustained, especially with their indeterminate growth and low perceived mortality (Sebens 1987; Menge and Sanford 2013). Identifying juvenile populations and possible ontogenetic shifts and further confirming mating seasonality in Singapore will also aid in the conservation of this species locally. Further major disturbances, which can affect the population and connectivity, continue to pose a great threat to this species. As the coastal zones of this rapidly- urbanised region continues to be developed, it has become critical to understand how reduced habitat connectivity and altered habitat substrates can affect intertidal organisms such as the sand-sifting sea star.
Supplementary Materials
Histogram of sea star arm length (mm) by months (rows) and sites (columns) showing generally unimodal peaks and lack of recruitment events of juvenile sea stars. (download)
Electronic Length Frequency Analysis (ELEFAN) of sea star arm lengths by sites showing a small growth rate and low mortality. (download)
Acknowledgments
Acknowledgments: This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Marine Science R&D Programme (MSRDP-P03). We would like to thank Roman Carrasco for statistical advice and members of the Reef Ecology Lab for help with the surveys, as well as two anonymous reviewers for constructive comments that helped improve the manuscript.
Footnotes
Authors’ contributions: YKSC and DH conceived the study. YKSC collected and analysed the data. All authors interpreted the data and contributed to writing this paper.
Competing interests: YKSC, TCT and DH declare they have no competing interests.
Availability of data and materials: All datasets, R scripts and supplementary information are available at Zenodo.
Consent for publication: Not applicable.
Ethics approval consent to participate: ANot applicable.
Compliance with ethical standards: The research was carried out according to the applicable international and/or institutional guidelines for the sampling of the species studied.
Conflict of interest: CYKS, TTC and HD declare that they have no conflict of interest.
References
- Adjeroud M, Kayal M, Peignon C, Juncker M, Mills S C, Beldade R, Dumas P. Ephemeral and Localized Outbreaks of the Coral Predator Acanathaster cf. solaris in the Southwestern Lagoon of New Caledonia. Zool Stud. 57:1–11. doi: 10.6620/ZS.2018.57-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos A R, Alipoyo Jce, Cardona L T, Gumanao G S, Salac F N. Population Structure of Common Indo-Pacific Sea Stars in the Davao Gulf. Philippines. UPV J Nat Sci. 13:11–24. [Google Scholar]
- Bos A R, Gumanao G S, Alipoyo Jce, Cardona L T. Population dynamics, reproduction and growth of the Indo-Pacific horned sea star, Protoreaster nodosus (Echinodermata; Asteroidea) Mar Biol. 156:55–63. [Google Scholar]
- Bos A R, Gumanao G S, Van Katwijk M M, Mueller B, Saceda M M, Tejada Rlp. Ontogenetic habitat shift, population growth, and burrowing behavior of the Indo- Pacific beach star, Archaster typicus (Echinodermata; Asteroidea). . Mar Biol. 2011;158:639–648. doi: 10.1007/s00227-010-1588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos A R, Gumanao G S, Mueller B, Saceda M M. Size at maturation , sex differences , and pair density during the mating season of the Indo-Pacific beach star Archaster typicus (Echinodermata: Asteroidea) in the Philippines. Invert Reprod Dev. 57:1–7. [Google Scholar]
- Clark P J, Evans F C. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology. 35:445–453. [Google Scholar]
- Fabricius K. Acanthaster planci. Johns Hopskins University Press. pp. 132–141.
- Feder H M, Christensen A M. Aspects of asteroid biology. In: Boolootian RA (ed) Physiology of Echinodermata. Interscience Publishers. pp. 87–127.
- Hilton M J, Manning S S. Conversion of Coastal Habitats in Singapore: Indications of Unsustainable Development. Environ Conserv. 22:307–322. [Google Scholar]
- Himmelman J H, Dumont C P, Gaymer C F, Vallières C, Drolet D. Spawning synchrony and aggregative behaviour of cold-water echinoderms during multi-species mass spawnings. Mar Ecol Prog Ser. 361:161–168. [Google Scholar]
- Jackson A C, Murphy R J, Underwood A J. Patiriella exigua: Grazing by a starfish in an overgrazed intertidal system. Mar Ecol Prog Ser. 376:153–163. [Google Scholar]
- Janssen H H. Surprising findings on the sea star Archaster typicus. Philipp Sci. 28:89–98. [Google Scholar]
- Janssen H H, Orosco C, Largo D, Ayson F, Uy W. Some recent findings on the seastar, Archaster typicus Müller et Troschel 1840. Philipp Sci. 21:51–74. [Google Scholar]
- Keesing J K. Population size structure, growth, arm number and damage in the sea star Archaster angulatus Müller and Troschel, 1842 (Echinodermata: Asteroidea) Invertebr Reprod Dev. 2017;61:1–9. [Google Scholar]
- Keesing J K, Graham F, Irvine T R, Crossing R. Synchronous aggregated pseudo-copulation of the sea star Archaster angulatus Müller & Troschel, 1842 (Echinodermata: Asteroidea) and its reproductive cycle in south-western Australia. Mar Biol. 158:1163–1173. [Google Scholar]
- Komatsu M. Development of the Sea-star, Archaster typicus, with a Note on Male-on-female Superposition. Annot Zool Jpn. 56:187–195. [Google Scholar]
- Lai S, Loke Lhl, Hilton M J, Bouma T J, Todd P A. The effects of urbanisation on coastal habitats and the potential for ecological engineering: A Singapore case study. Ocean Coast Manag. 103:78–85. [Google Scholar]
- Lane Djw. Echinodermata. In: Davison GWH (ed) The Singapore Red Data Book: Threatened Plants & Animals of Singapore, 2nd edn. Nature Society Singapore; 2008. [Google Scholar]
- Lawrence J M. Starfish: Biology and Ecology of the Asteroidea. Baltimore: Johns Hopskins University Press; 2013. [Google Scholar]
- Lawrence J M, Keesing J K, Irvine T R. Population characteristics and biology of two populations of Archaster angulatus (Echinodermata: Asteroidea) in different habitats off the central-western Australian coast. J Mar Biol Assoc United Kingdom. 91:1577–1585. [Google Scholar]
- Levitan D R. Sperm limitation, gamete competition, and sexual selection in external fertilizers. Academic Press. pp. 175–217.
- Lohrer A M, Thrush S F, Gibbs M M. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature. 431:1092–1095. doi: 10.1038/nature03042. [DOI] [PubMed] [Google Scholar]
- Menge B A, Berlow E L, Blanchette C A, Navarrete S A, Yamada S B. The Keystone Species Concept: Variation in interaction strength in a rocky intertidal habitat. Ecol Monogr. 64:249–286. [Google Scholar]
- Menge B A. Ecological Role of Sea Stars from Populations to Meta-ecosystems. Johns Hopskins University Press. pp. 67–80.
- Mercier A, Hamel J-F. Reproduction in Asteroidea. Johns Hopskins University Press. pp. 37–50.
- Mildenberger T M, Taylor M H, Wolff M. TropFishR: an R package for fisheries analysis with length-frequency data. Methods Ecol Evol. 8:1520–1527. [Google Scholar]
- Montecino-Latorre D, Eisenlord M E, Turner M, Yoshioka R, Harvell Drew, Semmens Pattengill-, Nichols C V, Gaydos J D. Devastating transboundary impacts of sea starwasting disease on subtidal asteroids. PLoS ONE. 11:1–21. doi: 10.1371/journal.pone.0163190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B, Bos A R, Graf G, Gumanao G S. Size-specific locomotion rate and movement pattern of four common indo-pacific sea stars (Echinodermata; Asteroidea) Aquat Biol. 12:157–164. [Google Scholar]
- Mukai H, Nishihira M, Kamisato H, Fujimoto Y. Distribution and Abundance of the Sea-Star Archaster typicus in Kabira Cove. Bull Mar Sci. 38:366–383. [Google Scholar]
- Nakazawa T. Ontogenetic niche shifts matter in community ecology: a review and future perspectives. Popul Ecol. 2014;57:347–354. [Google Scholar]
- Paine R T. Intertidal community structure: Experimental studies on the relationship between an dominant competitor and its principal predator. Oecologia. 15:93–120. doi: 10.1007/BF00345739. [DOI] [PubMed] [Google Scholar]
- Paine R T. Size-Limited Predation: An Observational and Experimental Approach with the Mytilus-Pisaster Interaction. Ecology. 57:858–873. [Google Scholar]
- Pauly D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J Conseil. 39:175–192. [Google Scholar]
- Pillay D, Branch G M, Steyn A. Unexpected effects of starfish grazing on sandflat communities following an outbreak. Mar Ecol Prog Ser. 398:173–182. [Google Scholar]
- Przeslawski R, Ahyong S, Byrne M, Wörheide G, Hutchings P. Beyond corals and fish: The effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob Chang Biol. 14:2773–2795. [Google Scholar]
- Przeslawski R, Byrne M, Mellin C. A review and metaanalysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Chang Biol. 21:2122–2140. doi: 10.1111/gcb.12833. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2017. [Google Scholar]
- Rogers T L, Elliott J K. Puget Sound: Differences in relative abundance and size structure of the sea stars Pisaster ochraceus and Evasterias troschelii among habitat types in; 2013. [Google Scholar]
- Washington Usa. Mar Biol. 160:853–865. [Google Scholar]
- Run J Q, Chen C P, Chang K H, Chia F S. Mating behaviour and reproductive cycle of Archaster typicus (Echinodermata: Asteroidea) Mar Biol. 99:247–253. [Google Scholar]
- Scheibling R E, Metaxas A. Abundance, spatial distribution, and size structure of the sea star Protoreaster nodosus in Palau, with notes on feeding and reproduction. Bull Mar Sci. 82:221–235. [Google Scholar]
- Schultz J A, Cloutier R N, Côté I M. Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ; 2016. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebens K P. The Ecology of Indeterminate Growth in Animals. Annu Rev Ecol Syst. 18:371–407. [Google Scholar]
- Sukarno P, Jangoux M. Révision du genre Archaster Müller et. Troschel. Rev Zool africaine. 91:817–844. [Google Scholar]
- Tan K S, Acerbi E, Lauro F M. Marine habitats and biodiversity of Singapore's coastal waters: A review. 2016.
- Teh T S, Raju D K, Santosh K, Chandrasekar J. Future sea level rise implications on development of Lazarus Island. Singapore Southern Islands. WIT Trans Ecol Environ. 130:121–133. [Google Scholar]
- Uthicke S, Schaffelke B, Byrne M. A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol Monogr. 79:3–24. [Google Scholar]
- Vandenspiegel D, Lane Djw, Stampanato S, Jangoux M. The asteroid fauna (Echinodermata) of Singapore, with a distribution table and an illustrated identification to the species. Raffles Bull Zool. 46:431–470. [Google Scholar]
- Wood H L, Spicer J I, Widdicombe S. Ocean acidification may increase calcification rates, but at a cost. Proc R Soc London B. 275:1767–73. doi: 10.1098/rspb.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakub S M, Mckenzie L J, Erftemeijer Pla, Bouma T, Todd P A. Courage under fire: Seagrass persistence adjacent to a highly urbanised city-state. Mar Pollut Bull. 83:417–424. doi: 10.1016/j.marpolbul.2014.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histogram of sea star arm length (mm) by months (rows) and sites (columns) showing generally unimodal peaks and lack of recruitment events of juvenile sea stars. (download)
Electronic Length Frequency Analysis (ELEFAN) of sea star arm lengths by sites showing a small growth rate and low mortality. (download)