Abstract
Tein-Shun Tsai and Jean-Jay Mao (2017) Shed snake skins have many applications for humans and other animals, and can provide much useful information to a field survey. When properly prepared and identified, a shed snake skin can be used as an important voucher; the morphological descriptions of the shed skins may be critical for taxonomic research, as well as studies of snake ecology and conservation. However, few convenient/ expeditious methods or techniques to identify shed snake skins in specific areas have been developed. In this study, we collected and examined a total of 1,260 shed skin samples - including 322 samples from neonates/ juveniles and 938 from subadults/adults - from 53 snake species in Taiwan and adjacent islands, and developed the first guide to identify them. To the naked eye or from scanned images, the sheds of almost all species could be identified if most of the shed was collected. The key features that aided in identification included the patterns on the sheds and scale morphology. Ontogenetic differences and intraspecific variation in the patterns of sheds were evident in some snake species, and the proportion of young snakes with patterned shed skins was larger than that of adults. The retention of markings on the ventral side of the body (especially the ventral head) during sloughing was much lower than that on the dorsal side. We hope that this pioneering work will not only encourage other researchers to develop similar keys for their country, but also promote local schools, organizations, and citizen scientists to conduct snake inventories.
Keywords: Ecdysis, Snake monitoring technique, Ontogenetic differences, Intraspecific variations, Guide and key
BACKGROUND
Molting (shedding, ecdysis) in reptiles results from cyclical changes in the underlying skin structure. Snakes periodically molt their outermost layer of epidermis, permitting the replacement of skin that has been abraded or damaged, the disposal of parasites, and growth (Greene 1997). The process of ecdysis is therefore viewed to signify a renewing of vital forces or as a sign of immortality (Crump 2015). Shed snake skins have several practical applications. First, in folk and traditional medicine, snake sheds have been used for treatment of ailments like glaucoma, eczema, hemorrhoids, wound healing, psoriasis, and parturition problems (Lev 2003; Mukherjee et al. 2013). Second, shed skins can serve as a tissue - collected without harming the animal - for studies of contaminant exposure (Kaur 1988; Hopkins et al. 2001; Jones et al. 2005) or DNA (Bricker et al. 1996; Clark 1998). DNA in shed skins is stable for at least one month as long as the shed is kept dry (Fetzner 1999). Third, ethical considerations make using human skin a major problem in transdermal research (Ngawhirunpat et al. 2006), but shed snake skin may offer a model membrane because of its partial similarities to human skin (Itoh et al. 1990; Priprem et al. 2007 2008; Kumpugdee- Vollrath et al. 2013; Torri et al. 2014). Shed skins can be used in exhibits and employed in educational programs at places such as snake farms, museums, or nature centers, where they may engender less fear than a live snake. Snake sheds can also be very aesthetically pleasing; many people have taken to creating beautiful snake shed jewelry (https://is.gd/ZBeJwB). In addition, skin lipid pheromones have been shown to play important roles in snake behavior. Shed skin releases pheromones into the environment and informs conspecifics about the reproductive status of the newly shed snake (Kubie et al. 1978). Other animals, such as ground squirrels, exploit the scent of rattlesnake (Crotalus sp.) sheds to reduce the risk of rattlesnake predation (Clucas et al. 2008a b), and some birds use snake sheds in their nests, which may function to decrease predation by mammalian predators (Medlin and Risch 2006) or reveal female parental quality (Trnka and Prokop 2011). Wildlife detector dogs can also be trained to find hidden snake sheds during surveys of wild snakes (Stevenson et al. 2010).
In addition, a shed snake skin can provide much useful information to a field survey. When properly prepared and identified, a shed snake skin can be used as a voucher, documenting the presence of a species without sacrificing or even necessarily finding an individual (Gray 2009 2012). In some species, if the shed skin is complete, the sex of the snake that left it may be inferred by counting the ventral and/or subcaudal scales. The location where a shed skin is found can provide insight regarding species habitat preferences (Gray 2009 2012). During ecdysis, a snake’s stratum corneum is stretched, resulting in a shed skin that is usually slightly longer (on average 11% increase reported in Gray 2009) than the actual snake. Using a regression equation, the actual snout-vent length (SVL) can be estimated from the length of a shed skin (Gray 2009). The relative size, shape, and arrangement of scales from sheds, as well as the sex and size measurements of the actual snake, could potentially be used to identify individuals and aid in investigations of site fidelity during ecdysis (Gray 2008). Studies on the global decline of snakes and other reptiles (Gibbons et al. 2000; Reading et al. 2010) have revealed that it is necessary to monitor wild populations in the long term, which must be aided by the establishment of standard methods and techniques (McDiarmid et al. 2012). In snake monitoring or surveying, snake sheds are normally ignored or discarded, but they can be a useful voucher for a specimen if the methods or technique to identify them are well developed. Because keratin is slow to break down, sheds may persist in an arid environment for well over a month, assuming they are not consumed by fungi or invertebrates. Marks made by scale clippings are observable in cast skins, so sheds can also be of value in mark-recapture studies (Gray 2002). Researchers could also sequence DNA from shed skins to use in population genetics studies.
Several publications or guides address identifying actual snakes, yet these same works may not be useful in identifying sheds because many rely on coloration, which sheds lack. Few convenient/fast methods or techniques to identify species of snake sheds in specific areas, however, have been developed, except in Canada (Gray 2012) and some areas in the United States (ex. Gray 2002 2015). In this study, we collected and examined shed skins from 53 species of snakes found in Taiwan and adjacent islands in order to develop the first guide and key to identify them; we hope that this will facilitate related studies on the biology and conservation of snakes.
MATERIALS AND METHODS
We collected shed skin samples of snakes from Taiwan (for most species), Green Island (for Laticauda spp. and Oligodon formosanus), Orchid Island (for Elaphe carinata yonaguniensis, Laticauda spp., O. formosanus, and Psammodynastes pulverulentus papenfussi), Kinmen (for Python bivittatus bivittatus), and Mazu (for O. chinensis). Sheds were collected in the field or obtained from individual snakes in captivity. The study was approved (IACUC approval number: NPUST-104-029) by the Animal Care and Use Committee of the National Pingtung University of Science and Technology (NPUST) and the captive animals were mainly raised in the Reptile and Amphibian Facility at NPUST, which has been accredited by the International Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). The sheds were processed and prepared before identification. The sheds were first immersed in clean water and lightly squeezed to soften them. They were then cut using dissecting scissors underneath the water from the angle of the mouth to the center of the tenth ventral; once this point was reached the cutting continued down the middle of the ventrals in the direction of the tail, until the tenth ventral anterior to the anal plate. The sheds were then cut at an angle towards the boundary line between the ventrals and the dorsals, and cutting continued along this line towards the tail tip. During the above procedure, any debris on the sheds was removed by hand or with forceps, and the sheds were simultaneously and carefully spread out. After that, the sheds were carefully spread out under the water onto mosquito nets, and the nets with sheds were lifted out of the water and placed on shelves to dry. After the sheds dried, they were separated carefully from the net, and scanned (Epson Perfection V37, Seiko Epson Corp., Japan) to obtain images for identification. The images were displayed using the auto-contrast function in Adobe Photoshop 7.0.1. Finally, the individually prepared shed skin samples were sealed in transparent bags, which were put into A4 size ring binders and stored in dry boxes. The shed samples were deposited as voucher specimens in 1) Department of Biological Science and Technology, NPUST; and 2) Department of Forestry and Natural Resources, National Ilan University. The scientific names of snakes in this study followed the Reptile Database (http://www.reptile-database.org), except for the undescribed endemic species (“Paiwan Keelback”; Hebius sp.) found in southern Taiwan and the Formosan Tiger Snake (Rhabdophis formosanus; Takeuchi et al. 2012 2014). The validity of subspecies in Trimeresurus stejnegeri (Guo et al. 2016), Hebius sauteri, and Sinonatrix percarinata (Zhao 2006) was questionable, and subspecies names for these species were not used in this study.
RESULTS
A total of 1,260 shed samples (one sample per individual, including 322 samples from neonates/juveniles and 938 samples from subadults/adults) from 53 species of snakes (including one undescribed species, Hebius sp.) were collected and examined in this study. Two samples of O. chinensis were collected from Hangzhou, China. Sheds were collected in the field (10%) or obtained from individual snakes in captivity (90%). Representative images of dorsal head, anterior body (about two head lengths posterior to the head), mid-body, and posterior body (about two head lengths anterior to the vent) of shed skins from each species are shown n figures 1-8. At least one complete set of shed images for each species was available, except for O. chinensis, which lacked the head region in all four shed samples. The ranges in the number of dorsal scale rows, ventrals, subcaudals, and other scale characters for each species were mainly summarized from previous reports (Maki 1931; Pope 1935; Wang and Wang 1956; Kuntz 1963; Ota and Toyama 1989; Tu et al. 1990; Zhao et al. 1998; Ota et al. 1999; Zhao 2006; You et al. 2013 2015). The sample sizes of sheds for each snake species are listed in table 1, where the summarized ranges of dorsal scale row, numbers of ventrals and subcaudals are also shown. The catalogue numbers of vouchers are listed in appendix table A1. We used “type I” and “type II” to designate different pattern forms (defined below) when inter- individual or ontogenetic variations in shed skins existed within a species. The key features (except for those summarized above) of a complete shed skin for each species are described as follows.
Table 1. The sample sizes of neonate/juvenile (N/J) and adult (A) sheds, ranges of number of dorsal scale rows (at anterior-middle-posterior body), ventrals, and subcaudals, and geographic distribution for 53 snake species. See the text for detailed descriptions of the classification of body patterns .
| Species | Body patterns | N/J | A | No. of dorsal scale rowsa | No. of ventralsa | Pairs of subcaudalsa | Occurrence in Taiwan or adjacent islandsb |
| Achalinus formosanus formosanus | Not-patterned | 0 | 1 | 27(29)-27(25)-25 | 158-184 | 61-83 (single row) | Mountain areas of Taiwan, at altitudes of 1000-2000 m |
| Achalinus niger | Not-patterned | 0 | 4 | 25-25-25 | 169-185 | 51-72 (single row) | Mountain areas of Taiwan, at altitudes of 1000-2000 m |
| Amphiesma stolatum | Patterned | 2 | 27 | 19-19-17 | 142-165 | 41-87 | Throughout Taiwan, Matsu, and Orchid Is., up to 500 m altitude |
| Boiga kraepelini | Patterned | 0 | 6 | 23(21,25)-21(19,23)-17(15) | 212-250 | 115-158 | Throughout Taiwan, up to 1500 m altitude |
| Not-patterned | 0 | 4 | |||||
| Bungarus multicinctus multicinctus | Patterned | 13 | 11 | 15(16,17)-15(16)-15 | 198-250 | 26-65 (single row) | Throughout Taiwan, Penghu, Kinmen, Matsu, Xiaoliuqiu, and Gueishan Is., up to 1000 m |
| Not-patterned | 0 | 51 | |||||
| Calamaria pavimentata pavimentata | Not-patterned | 0 | 2 | 13-13-13 | 167-192 | 13-23 | Throughout Taiwan and Orchid Is., up to 1500 m altitude |
| Cyclophiops major | Patterned | 14 | 0 | 15-15-15 | 155-189 | 61-97 | Throughout Taiwan, up to 1000 m altitude |
| Not-patterned | 0 | 18 | |||||
| Daboia siamensis | Patterned | 4 | 57 | 29(27,31)-29(27,31,33)-21(23) | 151-169 | 40-54 | Southern and south-eastern Taiwan, up to 500 m altitude |
| Deinagkistrodon acutus | Patterned | 1 | 8 | 21(22,23)-21(23)-17(18,19) | 152-176 | 41-63 | Throughout Taiwan, at altitudes of 150-1500 m |
| Elaphe carinata yonaguniensis | Patterned-type I | 12 | 0 | 23(21,25)-23(21,25)-19(17) | 209-230 | 78-99 | Throughout Taiwan and adjacent islands, up to 2000 m altitude |
| Patterned-type II | 1 | 7 | |||||
| Elaphe taeniurus friesi | Patterned-type I | 90 | 17 | 25(23)-23(21,25)-19(17) | 243-262 | 101-122 | Throughout Taiwan, up to 2000 m altitude |
| Patterned-type II | 0 | 102 | |||||
| Euprepiophis mandarinus | Patterned-type I | 0 | 11 | 23(21,25)-23(21)-19(17,21) | 181-238 | 49-76 | Northern, Central and Eastern Taiwan, at altitudes of 1000-2500 m |
| Patterned-type II | 0 | 3 | |||||
| Gonyosoma frenatum | Patterned | 3 | 0 | 19(21)-19(17)-15(13) | 200-227 | 108-151 | Southern and Southeastern Taiwan, up to 1500 meters altitude |
| Not-patterned | 0 | 2 | |||||
| Hebius miyajimae | Patterned | 0 | 2 | 19-19-17 | 141-152 | 87-92 | Mainly in northern Taiwan, at altitudes of 500-1600 m |
| Hebius sauteri | Not-patterned | 1 | 4 | 17-17-17 | 120-147 | 65-92 | Throughout Taiwan, up to 1500 m altitude |
| Hebius sp. (“Paiwan Keelback”) | Not-patterned | 1 | 0 | 19-19-17? | - | - | Limited areas of southern or eastern Taiwan, between 500 and 1300 meters altitude |
| Hypsiscopus plumbea | Not-patterned | 3 | 8 | 19-19-17(15) | 122-136 | 23-47 | Western and northern Taiwan, including Gueishan Is., up to 500 m altitude |
| Indotyphlops braminus | Not-patterned | 0 | 8 | 20-20-20 | 300-303 | 8-14 | Throughout Taiwan and adjacent islands, up to 500 m altitude |
| Laticauda colubrina | Patterned | 0 | 4 | 25-23,25-21 | 225-245 | 33-44 | Orchid Is., Green Is., eastern or southern coast of Taiwan |
| Laticauda laticaudata | Patterned | 0 | 8 | 19-19(21)-17 | 215-252 | 33-47 | Orchid Is., Green Is., eastern or southern coast of Taiwan |
| Laticauda semifasciata | Patterned | 0 | 10 | 23-23-19(21) | 178-203 | 32-43 | Orchid Is., Green Is., eastern or southern coast of Taiwan |
| Lycodon rufozonatus rufozonatus | Patterned | 5 | 12 | 17(19,21)-17(19)-15(17) | 184-225 | 45-95 | Throughout Taiwan and Matsu, up to 2000 m altitude |
| Not-patterned | 0 | 34 | |||||
| Lycodon ruhstrati ruhstrati | Patterned | 38 | 7 | 17(19)-17-15 | 193-233 | 64-116 | Throughout Taiwan, up to 1500 m altitude |
| Not-patterned | 0 | 7 | |||||
| Macropisthodon rudis rudis | Patterned | 3 | 3 | 23-23(21,25)-19 | 123-156 | 37-65 | Throughout Taiwan, at altitudes of 500-1500 m |
| Not-patterned | 0 | 2 | |||||
| Myrrophis chinensis | Type I | 2 | 0 | 23(25)-23-21(17,19) | 131-155 | 35-52 | Limited areas of Taipei, Taoyuan, Nantou, Kenting, and Kinmen, at altitudes up to 500 m |
| Type II | 0 | 9 | |||||
| Naja atra | Type I | 54 | 0 | 23(21,25,27)-21(19,20)-15(14,13) | 158-185 | 38-53 | Throughout Taiwan and Matsu, up to 1000 m altitude |
| Type II | 0 | 138 | |||||
| Oligodon chinensis | Patterned-type I | 2 | 0 | 17-17-15 | 158-206 | 40-73 | Matsu |
| Patterned-type II | 0 | 2 | |||||
| Oligodon formosanus | Patterned-type I | 7 | 0 | 19-19(17)-17(15) | 154-189 | 39-60 | Throughout Taiwan and adjacent islands, up to 1000 m altitude |
| Patterned-type II | 2 | 19 | |||||
| Oligodon ornatus | Patterned | 0 | 3 | 15-15-15 | 156-182 | 27-44 | Northern and Central Taiwan, at altitudes of 500-1500 m |
| Oreocryptophis porphyraceus kawakamii | Patterned-type I | 2 | 0 | 19(18,17)-19(17)-17(15) | 194-214 | 47-75 | Throughout Taiwan, up to 2200 m altitude |
| Patterned-type II | 0 | 7 | |||||
| Ovophis makazayazaya | Patterned | 0 | 7 | 25~31-25~29- 21 | 144-155 | 39-54 | Northern and western Taiwan, at altitudes of 500-2200 m |
| Not-patterned | 0 | 3 | |||||
| Pareas atayal | Not-patterned | 0 | 11 | 15-15-15 | 174-188 | 71-79 | Northern Taiwan, up to 1500 m altitudes |
| Pareas formosensis | Not-patterned | 0 | 10 | 15-15-15 | 170-180 | 69-82 | Throughout Taiwan except for the north-eastern tip of the island, up to 2000 m altitudes |
| Pareas komaii | Not-patterned | 0 | 7 | 15-15-15 | 162-182 | 60-76 | Central, southern, and eastern Taiwan, up to 2000 m altitudes |
| Plagiopholis styani | Patterned | 0 | 5 | 15-15-15 | 102-126 | 21-32 | Very limited areas around Taipei and the Northern Cross-Island Highway, at altitudes of 500-1100 m |
| Protobothrops mucrosquamatus | Patterned | 3 | 37 | 25~29-25(21,27,29)-17~21 | 194-233 | 70-108 | Throughout Taiwan, up to 1500 m altitude |
| Psammodynastes pulverulentus papenfussi | Patterned | 0 | 11 | 17-17-15(13) | 161-177 | 58-79 | Throughout Taiwan and Orchid Is., up to 1500 m altitude |
| Pseudoxenodon stejnegeri stejnegeri | Patterned | 0 | 2 | 19(17,21)-17-15(14) | 150-162 | 42-66 | Throughout Taiwan, at altitudes of 800-2500 m |
| Not-patterned | 0 | 3 | |||||
| Ptyas dhumnades | Patterned-type I | 2 | 0 | 16-16(14)-14 | 186-217 | 91-188 | Throughout Taiwan, up to 2000 m altitude |
| Patterned-type II | 0 | 3 | |||||
| Ptyas korros | Patterned | 2 | 0 | 15-15(13,14)-11 | 156-184 | 100-154 | Throughout Taiwan, up to 1000 m altitude |
| Not-patterned | 0 | 2 | |||||
| Ptyas mucosa | Patterned-type I | 25 | 0 | 19(17,21)-17(16)-14(13,15) | 170-213 | 94-143 | Throughout Taiwan, Kinmen, and Matsu, up to 1500 m altitude |
| Patterned-type II | 0 | 43 | |||||
| Python bivittatus bivittatus | Patterned | 1 | 18 | 53~64-64~72-40~44 | 255-263 | 63-71 | Kinmen |
| Rhabdophis formosanus | Patterned | 1 | 15 | 19-19-17(15) | 161-171 | 74-89 | Mountain areas of Taiwan, at altitudes of 1500-3000 m |
| Rhabdophis swinhonis | Patterned | 1 | 20 | 15(17)-15-15 | 124-165 | 49-74 | Throughout Taiwan, up to 1800 m altitude |
| Sibynophis chinensis chinensis | Patterned | 0 | 4 | 17-17-17 | 164-187 | 171-208 | Throughout Taiwan and Gueishan Is., up to 1500 m altitude |
| Sinomicrurus hatori | Patterned | 0 | 3 | 13-13-13 (occasionally 15) | 223-245 | 30-34 | Northern and eastern Taiwan, up to 2200 m altitude |
| Sinomicrurus macclellandi swinhoei | Patterned | 0 | 1 | 13-13-13 | 207-240 | 32-41 | Northern and western Taiwan, at altitudes up to 1000 m |
| Sinomicrurus sauteri | Patterned | 0 | 2 | 13-13-13 (occasionally 15) | 234-268 | 27-36 | South and central Taiwan, up to 2500 m altitude |
| Sinonatrix annularis | Patterned | 10 | 0 | 19-19-17 | 136-167 | 47-74 | Very limited areas of Taipei, and Taoyuan, at altitudes up to 1000 m |
| Not-patterned | 0 | 9 | |||||
| Sinonatrix percarinata | Patterned | 4 | 3 | 19-19-17 | 131-160 | 44-87 | Throughout Taiwan, at altitudes up to 1500 m |
| Not-patterned | 0 | 2 | |||||
| Trimeresurus gracilis | Patterned | 7 | 33 | 25~27-21~19-15~17 | 144-149 | 43-53 | Central mountains in Taiwan, at elevations of 2000-3500 m |
| Not-patterned | 0 | 5 | |||||
| Trimeresurus stejnegeri | Not-patterned | 4 | 52 | 21(22~25)-21(23)-15 | 154-178 | 43-80 | Throughout Taiwan, Green Is. and Orchid Is., up to 2000 m altitude |
| Xenochrophis piscator | Not-patterned | 2 | 9 | 19-19(17)-17(15) | 121-152 | 42-90 | Throughout Taiwan and Kinmen, up to 500 m altitude |
aThe numbers of dorsal scale rows, ventrals, and subcaudals are mainly summarized from previous reports (see the text). The data of Hebius sp. are measured from a uid-preserved specimen (voucher number: NIU-HE-001). (Maki 1931; Pope 1935; Wang and Wang 1956; Kuntz 1963; Ota and Toyama 1989; Tu et al. 1990; Zhao et al. 1998; Ota et al. 1999; Zhao 2006; You et al. 2013, 2015). bThe geographical distributions of snakes in Taiwan and adjacent islands are mainly according to Shang et al. (2009).
Achalinus formosanus formosanus
Whole body without markings (Fig. 1A); dorsal scales keeled (except for the outermost row); anal scale entire and subcaudals not divided; the parietal contacted posterolaterally by an enlarged paraparietal scale; the last supralabial very slender.
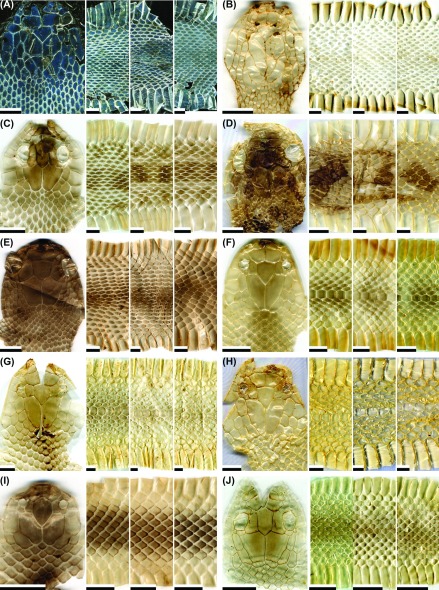
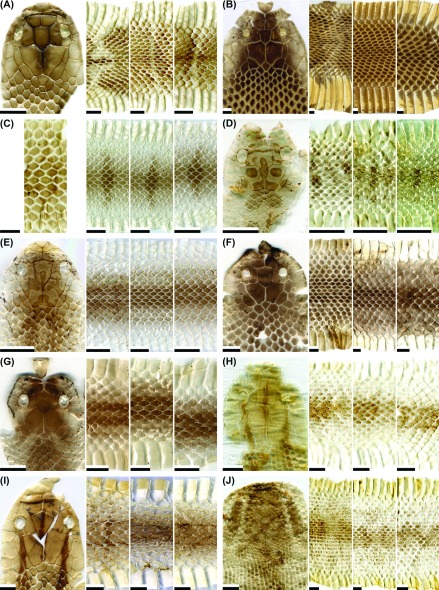
Fig. 1.
Fig. 1. Representative images of the dorsal head, anterior body (at a position about two head lengths posterior to the head), middle body, and posterior body (at a position about two head lengths anterior to the vent) of potential types of shed skins for the snake species: (A) Achalinus formosanus formosanus (not patterned; adult); (B) A. niger (not patterned; adult); (C) Amphiesma stolatum (patterned; adult); (D) Boiga kraepelini (patterned; adult); (E) B. kraepelini (not patterned; adult); (F) Bungarus multicinctus multicinctus (patterned; juvenile); (G) B. m. multicinctus (not-patterned but tinted with gray; adult); (H) B. m. multicinctus (not patterned; adult); (I) Calamaria pavimentata pavimentata (not patterned; adult); (J) Cyclophiops major (patterned; neonate). Scale bar = 0.5 cm.
Achalinus niger
Whole body without markings (Fig. 1B); dorsal scales at least on anterior part of body smooth; anal scale entire and subcaudals not divided; the parietal contacted posterolaterally by an enlarged paraparietal scale; the last supralabial very slender.
Amphiesma stolatum
Two light-colored stripes extending along the dorsal body (or at least the posterior part of body) and occasionally crossed by blackish bars anteriorly (Fig. 1C); dorsal body scales keeled (except for the outermost row); anal scale divided.
Boiga kraepelini
Dorsal body with (Fig. 1D) or without (Fig. 1E) cross bands; body scales smooth; dorsolateral body scales strongly oblique, at least on anterior part of body; temporals small and similar to nuchal scales; anal scale divided.
Bungarus multicinctus multicinctus
Dorsal body of neonates/juveniles and 18% of adults with cross bands (Fig. 1F); body of other adults without obvious markings (Figs. 1G, H), but the scales around the mid-dorsal line on 51% of these adults grayish (Fig. 1G) or grayish green; some sheds with grayish green or even light orange ventrals; body scales smooth; vertebral scales enlarged and hexagonal; anal scale entire and subcaudals not divided.
Calamaria pavimentata pavimentata
Whole body without markings (or with very faint, light stripes along the dorsal flanks) (Fig. 1I); body scales smooth; parietals in contact with supralabials; internasals absent; anal scale entire.
Cyclophiops major
Dorsal body with scattered, dark small spots in neonates (Fig. 1J) and without markings (Fig. 2A) in juveniles and adults; dorsal scales of at least anterior part of body smooth; anal scale divided.
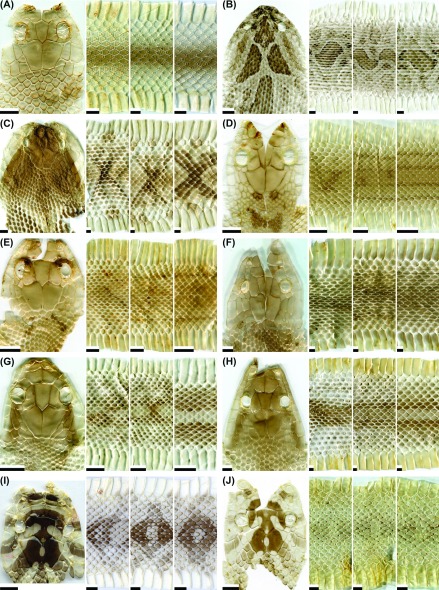
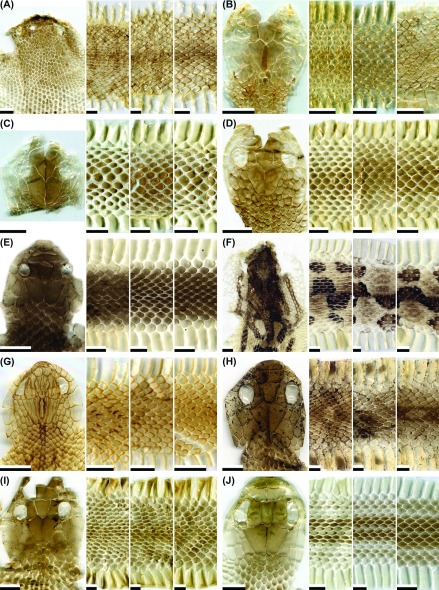
Fig. 2.
Fig. 2. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) Cyclophiops major (not patterned; adult); (B) Daboia siamensis (patterned; adult); (C) Deinagkistrodon acutus (patterned; adult); (D) Elaphe carinata yonaguniensis (patterned-type I; neonate); (E) E. c. yonaguniensis (patterned-type I; juvenile); (F) E. c. yonaguniensis (patterned-type II; adult); (G) E. taeniurus friesi (patterned-type I; juvenile); (H) E. t. friesi (patterned-type II; adult); (I) Euprepiophis mandarinus (patterned-type I; adult); (J) E. mandarinus (patterned-type II; adult). Scale bar = 0.5 cm
Daboia siamensis
Dorsal body with blotches or designs edged by black and/or light lines (Fig. 2B); dorsal body scales strongly keeled (except for the outermost row); top of head with three large, prominent, dark patches forming a light “Y” between them and covered only with small scales (except for supraoculars or nasals); anal scale entire.
Deinagkistrodon acutus
Dorsal body with a series of large, triangular designs laterally (Fig. 2C); upper head brownish, with light-colored sides; dorsal body scales strongly keeled (except for the outermost rows); anal scale entire.
Elaphe carinata yonaguniensis
Nape or anterior body of neonates/juveniles with pairs of dark marks or cross bands (Figs. 2D, E; type I); posterior body of neonates/juveniles sometimes with light brown longitudinal stripes extending onto tail (Figs. 2D, E); the posterior half of the body of adults with longitudinal intermittent lines of darkened scale keels (Fig. 2F; type II); sides of ventrals in adults and some juveniles darkened; dorsal body scales strongly keeled (except for the outermost row); anal scale divided.
Elaphe taeniurus friesi
Anterior dorsal body with 2 or 4 rows of dark blotches or bands in neonates/juveniles and subadults (Fig. 2G; type I), but with a faint median band in adults (Fig. 2H; type II); the designs turning into two lateral series of dark bands on the posterior body, most obvious on tail; head usually with a stripe extending posteriorly from the eye; dorsal body scales feebly keeled except those on the anks; anal scale divided.
Euprepiophis mandarinus
Dorsal head with 2-3 broad, black cross- bands; dorsal body with a series of light-centered saddles (Fig. 2I; type I) or a series of curved and paired spots (Fig. 2J; type II); sides of some ventrals usually darkened; body scales smooth (or occasionally feebly keeled); anal scale divided.
Gonyosoma frenatum
In neonates/juveniles, top of head with dark stripes, upper body may have crossbars (Fig. 3A); in adults, body without markings (Fig. 3B) and head occasionally with a stripe mainly extending posteriorly from the eye; dorsal body scales smooth or feebly keeled; prefrontals in contact with supralabials; anal scale divided.
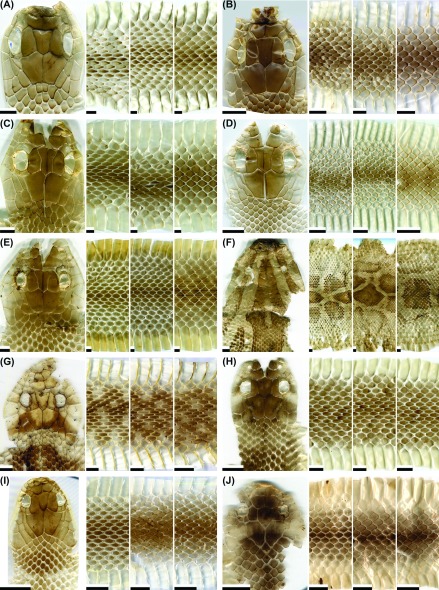
Fig. 3.
Fig. 3. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) Gonyosoma frenatum (patterned; juvenile); (B) G. frenatum (not patterned; adult); (C) Hebius miyajimae (patterned; adult); (D) H. sauteri (not patterned; adult); (E) Hebius sp. (not-patterned; juvenile); (F) Hypsiscopus plumbea (not patterned; adult); (G) Indotyphlops braminus (not patterned, with only part of head; adult); (H) Laticauda colubrina (patterned; adult); (I) L. laticaudata (patterned; adult); (J) L. semifasciata (patterned; adult). Scale bar = 0.5 cm, except for (G), where the scale bar = 0.1 cm.
Hebius miyajimae
Nape and dorsal body with a pair of light- colored longitudinal stripes (Fig. 3C); mid-dorsal and/or dorsolateral body scales keeled; anal scale divided.
Hebius sauteri
Body generally without markings (Fig. 3D); nape occasionally with a light line extending upward and posteriorly from corner of mouth; mid- dorsal and dorsolateral body scales keeled; anal scale divided.
Hebius sp.
(Fig. 3E) or occasionally with light cross lines anteriorly; nape with a light line extending upward and posteriorly from corner of mouth; mid-dorsal and dorsolateral body scales keeled; anal scale divided.
Hypsiscopus plumbea
Whole body without markings (Fig. 3F); body scales smooth; head with only one internasal scale; anal scale divided.
Indotyphlops braminus
Body very small (diameter less than 5 mm) and without designs; tail quite short, ending in a spine; scales all around body similar in size and smooth (Fig. 3G).
Laticauda colubrina
Body with bands that are narrower than interspaces (Fig. 3H); tail laterally compressed; body scales smooth; upper head distinctly marked; head with 3 prefrontals and no loreals, while the 2nd chin shields not in contact with the infralabials; anal scale divided.
Laticauda semifasciata
Body with bands of similar width to interspaces (Fig. 3I); tail laterally compressed; body scales smooth; upper head distinctly marked; head with 2 prefrontals and no loreals, while the 2nd chin shields not in contact with the infralabials; anal scale divided.
Laticauda semifasciata
Body with bands that are broadest at vertebral line, tapering off laterally (Fig. 3J); tail laterally compressed; body scales smooth; ventral scales on posterior half to two-thirds of body keeled; ventral scales on poseterior one-third of body notched in central region of posterior margin; upper head distinctly marked; head with 3 prefrontals and no loreals, while the 2nd chin shields not in contact with the infralabials; anal scale divided.
Lycodon rufozonatus rufozonatus
Back of upper head may have a light- colored “Λ” con guration; dorsal body of neonates, juveniles, and 26% of adults with dark or gray cross bands (Fig. 4A), while that of other adults without markings (Fig. 4B); body scales smooth; anal scale entire.
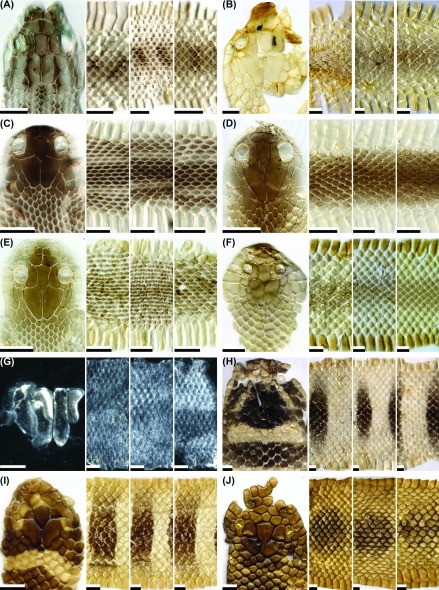
Fig. 4.
Fig. 4. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) Lycodon rufozonatus rufozonatus (patterned; neonate); (B) L. r. rufozonatus (not patterned; adult); (C) L. ruhstrati ruhstrati (patterned; adult); (D) L. r. ruhstrati (not patterned; adult); (E) Macropisthodon rudis rudis (patterned; juvenile); (F) M. r. rudis (not patterned; adult); (G) Myrrophis chinensis (type I; juvenile); (H) M. chinensis (type II; adult); (I) M. chinensis (type II; adult); (J) Naja atra (type I; juvenile). Scale bar = 0.5 cm.
Lycodon ruhstrati ruhstrati
Back of upper head with or without a light band; dorsal body of neonates, juveniles, and 50% of adults with dark or grey cross bands (Fig. 4C), while that of other adults without markings (Fig. 4D); usually greater than 6 rows of body scales (on the anks) smooth and others feebly keeled; anal scale entire.
Macropisthodon rudis rudis
Dorsal body of neonates/juveniles and 60% of adults with blotches or markings, variable in size, shape, and intensity of pigmentation (Fig. 4E), while that of other adults without obvious markings (Fig. 4F); upper head light brown in neonates/ juveniles or dark dirty brown in adults, may have a stripe extending posteriorly from the eye; sides of upper head light-colored; sides of ventrals of adults may be darkened; all dorsal body scales strongly keeled, keels darkened in adults; temporals keeled and spectacle separated from supralabials by suboculars; anal scale divided.
Myrrophis chinensis
Nape with a vertebral stripe; in neonates/ juveniles, upper body may have scattered dark spots, lateral body with an obvious longitudinal band of light color, involving first to fourth scale rows (Fig. 4G; type I); in adults, upper body may have scattered dark spots (Fig. 4H; type II) or a faint longitudinal band of light color on the lateral body (Fig. 4I; type II); body scales smooth; head with only one internasal scale; anal scale divided.
Naja atra
Upper body without markings (Fig. 4J) or with irregular or scattered crosslines of light color; nape may have a spectacle-like mark (Figs. 5A, B); upper body of 15% of neonates/juveniles and 96% of adults darkened; ventrals without dark color in neonates/juveniles (Fig. 4J and Fig. 5A; type I), but generally darkened and mottled with white in adults (Fig. 5B; type II); body scales smooth and dorsolateral scales strongly oblique; vertebral scales much smaller than lateral scales; loreals absent and temporals larger than nuchal scales; anal scale generally entire.
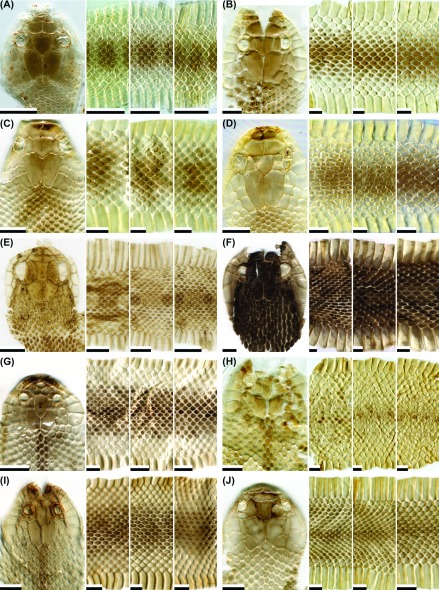
Fig. 5.
Fig. 5. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) Naja atra (type I; juvenile); (B) N. atra (type II; juvenile); (C) Oligodon chinensis (patterned-type I; juvenile; note that the leftmost panel [as patterned-type II] was from the posterior body region of an adult); (D) O. formosanus (patterned-type I; neonate); (E) O. formosanus (patterned-type II; adult); (F) O. formosanus (patterned-type II; adult; Orchid Is.); (G) O. ornatus (patterned; adult); (H) Oreocryptophis porphyraceus kawakamii (patterned-type I; juvenile); (I) O. p. kawakamii (patterned-type II; adult); (J) Ovophis makazayazaya (patterned; adult). Scale bar = 0.5 cm.
Oligodon chinensis
Upper body with a series of cross bars that are broader on the back than on the sides (Fig. 5C; type I); each cross bar having a light-colored center in adults (leftmost image in Fig. 5C; type II); dorsal head may have symmetrical brown designs, including a forward-pointing dark chevron at the back of the head; body scales smooth; anal scale entire.
Oligodon formosanus
Upper body with a series of paired spots along the vertebral line (Fig. 5D; type I) in neonates, or with longitudinal stripes and occasionally cross bands (Figs. 5E, F; type II) in juveniles or adults; dorsal head usually with symmetrical brown designs, including forward-pointing dark chevrons at the back of the head; body scales smooth; anal scale entire.
Oligodon ornatus
Dorsal body with longitudinal stripes (Fig. 5G); dorsal head usually with symmetrical brown designs, including forward-pointing dark chevrons at the back of the head; body scales smooth; prefrontals in contact with supralabials; anal scale divided.
Oreocryptophis porphyraceus kawakamii
Upper body of neonates/juveniles with prominent brown bands and occasionally longitudinal lines (Fig. 5H; type I), while upper body of adults with a pair of brown longitudinal lines and occasionally faint transverse bands or lines (Fig. 5I; type II); dorsal head with three distinct dark longitudinal stripes, including one middle stripe and two lateral stripes extending posteriorly from the eyes (Figs. 5H, I); body scales smooth; anal scale divided.
Ovophis makazayazaya
Upper body of 70% of adults with a mid-dorsal series of diffuse and irregular dark cross bands, and a faint, spotted pattern laterally (Fig. 5J), while that of other adults not patterned (Fig. 6A); upper head generally with a dark, thick band extending from eye to corner of mouth; body scales smooth or occasionally feebly keeled around the vertebral line posteriorly; upper head covered only with small scales (except supraoculars); anal scale entire.
Fig. 6.
Fig. 6. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) Ovophis makazayazaya (not patterned; adult); (B) Pareas atayal (not patterned; adult); (C) P. formosensis (not patterned; adult); (D) P. komaii (not patterned; adult); (E) Plagiopholis styani (patterned; adult); (F) Protobothrops mucrosquamatus (patterned; adult); (G) Psammodynastes pulverulentus papenfussi (patterned; adult); (H) Pseudoxenodon stejnegeri stejnegeri (patterned; adult); (I) P. s. stejnegeri (not patterned; adult); (J) Ptyas dhumnades (patterned-type I; juvenile). Scale bar = 0.5 cm.
Pareas atayal
Body without markings, or occasionally with irregular ladder-shaped markings dorsally; dorsal body with 3-9 keeled scale rows before vent (Fig. 6B); vertebral scales slightly larger than neighboring dorsal scales; head without mental groove and with one slender subocular; anal scale entire.
Pareas formosensis
Body generally without markings; body scales smooth (Fig. 6C); vertebral scales slightly larger than neighboring dorsal scales; head without mental groove and with one slender subocular; anal scale entire.
Pareas komaii
Body generally without markings; dorsal body with 9-13 keeled scale rows before vent (Fig. 6D); vertebral scales slightly larger than neighboring dorsal scales; head without mental groove and with one slender subocular; anal scale entire.
Plagiopholis styani
Upper body without markings or occasionally mottled; nape with a grayish or dark crossband (Fig. 6E); body scales smooth; loreal scales absent; anal scale entire.
Protobothrops mucrosquamatus
Upper body with a mid-dorsal series of dark, alternating, connected half-blotches, and a separate lateral blotch on each side of body tending to be in line with the mid-dorsal blotch on that side (Fig. 6F); head usually with a narrow, dark stripe extending posteriorly from the eye (Fig. 6F); body scales (heavily) keeled; upper head covered only with small scales (except for supraoculars) and with more than 10 scales between the supraoculars; anal scale entire.
Psammodynastes pulverulentus papenfussi
Upper body without markings or occasionally mottled or even with stripes; dorsal head with longitudinal con guration of dark wavy “Y” shapes (Fig. 6G); head with three pairs of chin shields; body scales smooth; anal scale entire.
Pseudoxenodon stejnegeri stejnegeri
Upper body with (alternating) squarish blotches or striped markings (Fig. 6H), or not patterned (Fig. 6I); a stripe extending posteriorly from eye or a chevron on nape with its apex pointing toward the head occasionally present; ventrals on anterior part of body usually with dark spots; upper body scales keeled and dorsolateral body scales strongly oblique, at least on anterior body; anal scale divided.
Ptyas dhumnades
Upper body with pair(s) of dark stripes from neck to tail (Fig. 6J; type I) in the young, which fade on the posterior body (Fig. 7A; type II) in adults; sides of ventrals in adults may be gray tinted; dorsal body scales in even-numbered rows; body scales keeled around the vertebral line and the keels darkened; anal scale divided.
Fig. 7.
Fig. 7. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) Ptyas dhumnades (patterned-type II; adult); (B) P. korros (patterned; juvenile; note the faint, light scales arranged in a transverse row on the anterior body); (C) P. korros (not patterned; adult); (D) P. mucosa (patterned-type I; juvenile); (E) P. mucosa (patterned-type II; adult); (F) Python bivittatus bivittatus (patterned; adult); (G) Rhabdophis formosanus (patterned; adult); (H) R. swinhonis (patterned; adult); (I) Sibynophis chinensis chinensis (patterned; adult); (J) Sinomicrurus hatori (patterned; adult). Scale bar = 0.5 cm.
Ptyas korros
Upper body of neonates/juveniles may have transverse rows of light spots across the anterior body (Fig. 7B); upper body of adults without markings (Fig. 7C), the tips of scales on the posterior body may be darkened; sides of ventrals of adults may be gray tinted; upper body scales smooth or feebly keeled posteriorly; each side of head with 2-4 loreal scales; anal scale divided.
Ptyas mucosa
In neonates/juveniles, anterior upper body with faint, light cross lines, and posterior upper body with dark cross bands or lines (Fig. 7D; type I); in adults, posterior upper body with longitudinal intermittent lines of darkened scale keels, and sides of ventrals darkened (Fig. 7E; type II); posterior body scales keeled around the vertebral line, and the keels darkened in adults; each side of head with 2-5 loreal scales; anal scale divided.
Python bivittatus bivittatus
Body with many large brown blotches; head with a large, light-colored “Λ” configuration consisting of a pair of stripes each extending from the temporal region, through the eye, and to the nose (Fig. 7F); anterior body scales in greater than 50 rows; ventrals small and width only about two times that of the neighboring dorsal scale; body scales smooth; anal scale entire.
Rhabdophis formosanus
Upper body with a checkered appearance (Fig. 7G); nape with a light, anteriorly curved cross band, with a brown cross band adjoining in the front and rear; sides of ventrals may be darkened (Fig. 7G); upper body scales keeled, except for the outermost row and the four scale rows lateral to vertebral scales over nuchal glands; anal scale divided.
Rhabdophis swinhonis
Upper body without markings or occasionally mottled, except that nape has a dark chevron whose apex points toward the body, and anterior to which is a lighter area (Fig. 7H); upper body scales keeled, except for the outer two rows and the nape scales; anal scale divided.
Sibynophis chinensis chinensis
Uupper body without markings, except that nape has a dark collar bordered posteriorly by a light stripe (Fig. 7I); dorsal head dark except for the supralabials; body scales smooth; anal scale divided.
Sinomicrurus hatori
Dorsal body with a dark mid-dorsal stripe (Fig. 7J); dorsal head with an unpigmented crossband behind eyes; loreal scales absent, prefrontals not in contact with supralabials; body scales smooth; anal scale divided.
Sinomicrurus macclellandi swinhoei
Dorsal body with a series of narrow cross bands (Fig. 8A); dorsal head with an unpigmented, broad crossband behind the eyes; loreal scales absent, prefrontals not in contact with supralabials; body scales smooth; anal scale divided.
Fig. 8.
Fig. 8. Representative images of the dorsal head, anterior body, middle body, and posterior body of potential types of shed skins for the snake species: (A) S. macclellandi swinhoei (patterned; adult); (B) S. sauteri (patterned; adult); (C) Sinonatrix annularis (patterned; neonate); (D) S. annularis (not patterned; adult); (E) S. percarinata (patterned; juvenile); (F) S. percarinata (not patterned; adult); (G) Trimeresurus gracilis (patterned; adult); (H) T. gracilis (not patterned; adult); (I) T. stejnegeri (not patterned; adult); (J) Xenochrophis piscator (not patterned; adult). Scale bar = 0.5 cm.
Sinomicrurus sauteri
Dorsal body with a dark mid-dorsal stripe (Fig. 8B); dorsal head with an unpigmented crossband behind eyes; loreal scales absent, prefrontals not in contact with supralabials; body scales smooth; anal scale divided.
Sinonatrix annularis
Dorsal body with crossbands (Fig. 8C) or without markings (Fig. 8D); dorsal body scales feebly keeled in neonates or keeled in juveniles and adults, except those on the flanks (outer 1-2 rows of scales); internasals triangular and pointed anteriorly; usually one labial in contact with the eye; anal scale divided.
Sinonatrix percarinata
Dorsal body with crossbands (Fig. 8E) or without markings (Fig. 8F); dorsal body scales feebly keeled in neonates or keeled in juveniles and adults, except those on the flanks (outer 1-2 rows of scales); internasals triangular and pointed anteriorly; usually two labials in contact with the eye; anal scale divided.
Trimeresurus gracilis
Upper body of neonates/juveniles and 87% of adults brownish with a mid-dorsal series of dark blotches with lateral blotches on each side tending to be in line with the mid-dorsal one (Fig. 8G), while that of other adults without obvious markings (Fig. 8H); upper head of those with patterned body generally bearing a dark, thick band extending from eye to corner of mouth; upper dorsal body scales (feebly) keeled, except those on the anks; upper head covered only with small scales (except for supraoculars) and with fewer than 9 scales located between supraoculars; anal scale entire.
Trimeresurus stejnegeri
Whole body light-colored and without designs; upper dorsal body scales keeled; upper head covered only with small scales (except for supraoculars) and with 9-15 scales located between supraoculars (Fig. 8I); anal scale entire.
Xenochrophis piscator
Whole body without obvious markings; dorsal body scales feebly keeled, except those on the anks (outer 3 rows of scales); internasals triangular and pointed anteriorly (Fig. 8J); usually two labials in contact with the eye; anal scale divided.
In addition, for convenient and expeditious identification of complete shed skins of the 53 snake species in Taiwan and adjacent islands, we provide a key in appendix I.
DISCUSSION
The identification of shed snake skins can augment data collected for species inventories (Gray 2002). Investigations of shed skin patterns could enhance our understanding of the reproductive, behavioral, and physiological ecology of snakes, and help structure conservation strategies (Lillywhite and Sheehy 2016). Both accurate and precise morphological descriptions of shed snake skins are critical not only for taxonomic research, but also for forensic efforts, customs inspections, and the conservation of species (Baker 2006). Although the exact rate of decay for snake sheds in the wild is almost unknown, snake sheds either are quickly consumed by fungi/ animals or become fragmented and faded. In a series of eld experiments, Gray (2005) found that 10 cm sections of shed skins lasted an average of 7 days when covered by objects and 11.5 days in the open; one shed skin section lasted up to 46 days. When a shed is incomplete or faded, it will have fewer characters identifiable to the naked eye or from scanned images and the key for shed snake skins in this study may not be applicable. Therefore, to accurately identify snake sheds using the key in this study, one should endeavor to collect complete shed samples as much as possible and soon after ecdysis. The sheds of the 53 species of snakes in this study can be identified using the guide or the key, although it was not easy to differentiate between H. sauteri and Hebius sp., S. hatori and S. sauteri, or even S. percarinata and X. piscator. The differences between S. hatori and S. sauteri are minor even on the actual skins and their external morphology is similar to S. japonicus boettgeri, which is distributed on several islands of the Ryukyu Archipelago, Japan (Ota et al. 1999; Mochida et al. 2015; Kaito et al. 2017); the body coloration of this species probably works as crypsis through background matching or disruptive camou age rather than aposematism (Mochida et al. 2015). The differences between shed skins of Sinomicrurus snakes in Taiwan and on the Ryukyu Islands could be the presence or absence of a transverse light band on the head, the number of longitudinal stripes on the dorsal body, or the number and extent of transverse bands on the dorsal body. In addition, apical pits and an apical notch on a scale (Chiasson 1981; Chiasson and Lowe 1989; Gray 2002 2012 2015) could partly be examined from the scanned images, but examining such characters in neonates or some species (e.g., L. r. rufozonatus, L. r. ruhstrati, or P. mucosa) might be difficult and require the use of a microscope. When shed skins are incomplete and/or their markings have faded, species identification may instead depend on examination of micro-structure morphology on the surface of scales using light microscopy or scanning electron microscopy (Tsai et al. unpublished data). It has been shown that the microdermatoglyphic patterns of the dorsal scales are species-specific (e.g., Price 1982; Wang and Zhou 1998) and the scales of the original animals and sloughs of the same species showed identical microdermatoglyphic characters (Wang and Zhou 1998). Nevertheless, scanning electron microscopy may be expensive and of limited availability for some identifiers. In this study we develop a more convenient identification method, requiring only the naked eye or scanned images, which could easily be used in a snake inventory by a local school or other organization or by citizen scientists (Todd et al. 2017).
Ontogenetic differences and intraspecific variation in shed patterns were evident in about 23 snake species in this study. Gray (2002) mentioned that pattern is usually less visible on the sheds of younger snakes. We found that the markings on the shed skins of young snakes tended to be lighter than those on the shed skins of patterned adult individuals of the same species, such as D. siamensis, P. mucrosquamatus, P. b. bivittatus, and R. swinhonis. However, we also found that the proportion of young snakes with patterned shed skins was larger than that of adults, corresponding to ontogenetic loss of pattern in some individuals of species such as B. m. multicinctus, L. r. rufozonatus, L. r. ruhstrati, M. r. rudis, M. chinensis, S. annularis, S. percarinata, and T. gracilis. Ontogenetic differences in the distribution of melanocytes (Krey and Farajallah 2013), the quantity of melanin deposition in the skin tissues, and the growth and development of skin are likely underyling causes of the above trends. In addition, although we did not collect the sheds from young individuals of 22 snake species, this should not reduce the applicability of the key developed in this study. The patterns on the sheds of most young snakes could be inferred when conspecific adults have patterned sheds, because most ontogenetic shifts in snake patterns primarily involve a loss in pattern clarity. Most of the above 22 snake species (except Achalinus spp., I. braminus, C. p. pavimentata, and Pareas spp.) had patterned sheds as adults and should also have patterned sheds in young snakes, while the shed skins from young snakes of Achalinus spp. and I. braminus should not be patterned because these snakes have no patterns on their actual body. Moreover, we did not find distinct geographical variation in the patterns visible on shed skins. It has been shown that O. formosanus from different regions of Taiwan, Green Is. or Orchid Is. may have polymorphic markings or colorations on their body (https://goo.gl/WjsW2H), but a similar striped pattern was visible on the dorsal body of sheds of this species from above sites, with only occasional variation in the markings on the head (Figs. 5E, F). The distinct sexual dimorphism of T. stejnegeri’s actual skin color (Tsai and Tu 1998; Zhao 2006) was not visible in their sheds.
Many markings on the actual skins were not visible on the shed skins. In appendix table A2, we summarize the visibility of markings on the actual and shed skin of all 53 species of snakes in this study, separated by body region. We found that most of these snake species have markings on the dorsal side of their actual body (81% of species with markings on the head, 91% on the body, and 91% on the tail); in contrast, markings were visible on the shed skins of only 55% (dorsal head), 75% (dorsal body), and 68% (dorsal tail). Fewer species have markings on ventral side of the actual body (43% on the ventral head, 75% on the ventral body, and 77% on the ventral tail), and even fewer have markings on the ventral side of the shed skins (6% ventral head; 17% ventral body; 19% ventral tail). Thus, 67% of snake species with markings on the actual skin of the dorsal head, 83% with markings on the dorsal body, and 75% with markings on the dorsal tail also had some markings on their shed skins, whereas only 13% (ventral head), 23% (ventral body), and 24% (ventral tail) of snake species with markings on the ventral side also had some markings on the sheds. The degree of retention for the markings on the ventral side of the body (especially on the ventral side of the head) during sloughing was much lower than that on the dorsal side. Pattern retention in shed snake skins is due to deposition of melanin from epidermal melanophores. It can be hypothesized that there is a lack or reduction in epidermal melanophores in the ventral epidermis. The causes of these differences and their potential biological significance, if any, remain to be investigated in future studies.
The sheds of some species bear distinct traits that are useful for species identification. For example, 1) the sheds of many viperids (such as D. siamensis, D. acutus, P. mucrosquamatus, and T. gracilis) and sea snakes (such as L. colubrina and L. laticaudata) had deeply darkened, apparent, and unfaded patterns even on adults; 2) the sheds of some species (such as E. t. friesi, O. p. kawakamii, P. p. papenfussi, P. b. bivittatus, S. c. chinensis, and Sinomicrurus spp.) had distinctive markings on the head; 3) the sheds of some species (such as E. mandarinus, O. chinensis, and R. formosanus) had unique markings on the body; 4) the sheds of some species (such as B. kraepelini, B. m. multicinctus, M. r. rudis, N. atra, and Pareas spp.) had special scutellation on the head or body; 5) the sheds of some species (such as E. c. yonaguniensis, E. mandarinus, M. r. rudis, N. atra, P. s. stejnegeri, Ptyas spp., and R. formosanus) sometimes had darkened markings on the sides of ventrals, which could be a good character for species identification. In addition, the geographic location and habitat where sheds are found in the wild could also be used as auxiliary information for species identification (Gray 2012 2015). Mainly according to Shang et al. (2009), we listed the geographical distribution of the 53 species in Taiwan and the adjacent islands in table 1 to help make shed identification faster and more accurate. Also, ventral scales and smooth (or feebly keeled) dorsal scales on the shed skins of most species in this study may produce iridescence when observed at a certain angle through the light, except for those of I. braminus, Laticauda spp., and/or P. b. bivittatus. Therefore, checking for the presence of iridescence (see also Monroe and Monroe 1967; Verveen and Rouwkema 2007) on shed skins might also be considered an applicable method for species identification.
CONCLUSIONS
A shed snake skin found during a field survey can provide much useful information. Investigations on the use of shed skins for species identification can advance our understanding of the geographic distribution, activity patterns, and reproductive, behavioral, and physiological ecology of snakes, with important implications for conservation. In this study, we present a pioneering guide to the identification of local snake sheds in Taiwan, which may also be useful in other parts of Asia. The sheds of almost all species can be identified to the naked eye or from scanned images if a complete shed is collected. The key features used include the patterns on sheds and scale morphology. Ontogenetic differences and intraspecific variation in the patterns on the sheds were evident in some snake species, and the proportion of young snakes with patterned shed skins was higher than that of adults. The degree of retention for the markings on the ventral side of body (especially the ventral head) during sloughing was much lower than that on the dorsal side. We hope this pioneer work will not only encourage other researchers to develop similar keys for their country, but also promote local schools, organizations, and citizen scientists to conduct snake inventories.
Supplementary materials
Key to the complete shed skins of 53 snake species in Taiwan and adjacent islands. (download)
The catalogue numbers of shed vouchers for 53 species of snakes. See the text for detailed descriptions on the classification of body patterns. (download)
Comparisons of the actual skin and shed skin in 53 snakes bearing banding, stripes, spots, or other markings on dorsal/ ventral head, body, and tail. Cases with markings are denoted by a check mark and those with some exceptions are denoted by a checkmark in parentheses. Note that “Y” means neonates or juveniles and “A” means adults. The counts and ratios are listed at the end of the table. (download)
Acknowledgments
Acknowledgments: We thank Bo-Shen Wang, Shih-Hao Wang, Wei-Chieh Hsu, Chao-An Tu, Zuo-Ming Cai, En-Dao Liang, Pi-Hang Chen, Chin- Hsiu Lin, Kai-Xiang Zhang, Chun-Yu Chen, Kai- Cing Liou, Qing-Guo Ji, Miaoju Lin, Ya-Chi Yang, Zi-You Fan, Yi-Jie Lee, Meng-Qun Zhou, Chih-Chi Liu, Ruo-Cian Hu, and other colleagues and friends for their great help and assistance in collecting samples and preparing specimens. We thank Chung-Wei You and Dr. Juan Lei for offering shed samples of O. chinensis collected from Mazu and Hangzhou, respectively. We thank Shih-Ping Chou and Dr. Si-Min Lin for offering some shed samples of P. b. bivittatus collected from Kinmen. We also thank Dr. Ming-Chung Tu for assistance with the study. Special thanks go to Dr. Andrew M. Durso for reviewing a previous manuscript of this paper. This work was funded by the National Science Council (NSC 100-2621-B-020-001), Council of Agriculture, and Kaohsiung City Government.
Footnotes
Authors’ contributions: TST and JJM collected samples. TST designed the study, wrote the manuscript, prepared the tables and figures, and provided the identification key. Both authors participated in revising the manuscript and approved the final manuscript.
Competing interests: The authors have no competing interests to declare.
Availability of data and materials: The supporting data will be provided by the corresponding author on request.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Baker B W. Forensic implications of dorsal scale row counts on puff-faced water snakes (Colubridae: Homalopsinae: Homalopsis buccata) Herp Rev. 37:171–173. [Google Scholar]
- Bricker J, Bushar L M, Reinert H K, Gelbert L. Purification of high quality DNA from shed skin. Herp Rev. 27:133–134. [Google Scholar]
- Chiasson R B. The apical pits of Agkistrodon (Reptilia: Serpentes) J Arizona-Nevada Acad Sci. 16:69–73. [Google Scholar]
- Chiasson R B, Lowe C H. Ultrastructural scale patterns in Nerodia and Thamnophis. J Herpetol. 23:109–118. [Google Scholar]
- Clark A. Reptile sheds yield high quality DNA. Herp Rev. 29:16–18. [Google Scholar]
- Clucas B, Owings D H, Rowe M P. Donning your enemy's cloak: Ground squirrels exploit rattlesnake scent to reduce predation risk. Proc R Soc Lond B Biol Sci. 275:847–852. doi: 10.1098/rspb.2007.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clucas B, Rowe M P, Owings D H, Arrowood P C. Snake scent application in ground squirrels, Spermophilus spp.: A novel form of antipredator behaviour? Anim Behav. 75:299–307. [Google Scholar]
- Crump M. Eye of Newt and Toe of Frog, Adder's Fork and Lizard's Leg. Chicago, Illinois, USA): University of Chicago Press; 2015. [Google Scholar]
- Fetzner JW Jr. Extracting high-quality DNA from shed reptile skins: A simplified method. BioTechniques. 26:1052–1054. doi: 10.2144/99266bm09. [DOI] [PubMed] [Google Scholar]
- Gibbons J W, Scott D E, Ryan T J, Buhlmann K A, Tuberville T D, Metts B S, Greene J L, Mills T, Leiden Y, Poppy S, Winne C T. The global decline of reptiles. Bioscience. 50:653–666. [Google Scholar]
- Gray B S. A key to the shed skins of northeastern snakes. Bull Chicago Herp Soc. 37:121–128. [Google Scholar]
- Gray B S. The Serpent's Cast: A Guide to the Identification of Shed Skins from Snakes of the Northeast and Mid-Atlantic States. The Center for North American Herpetology Monograph Series Number; 2005. 1 [Google Scholar]
- Gray B S. A note on site fidelity for ecdysis in the northern brown snake, Storeria dekayi dekayi. Bull Chicago Herp Soc. 43:1–3. [Google Scholar]
- Gray B S. Estimating snout to vent length from data acquired from the shed skins of the northern brown snake, Storeria dekayi dekayi. J Kansas Herpetol. 32:17–19. [Google Scholar]
- Gray B S. Guide to the Identification of the Shed Skins of the Snakes of Canada. USA): 2012. [Google Scholar]
- Gray B S. Shed Snakeskin Identification: A Guide to Snakeskins Found in Pennsylvania. Pennsylvania Amphibian & Reptile Survey. USA): 2015. [Google Scholar]
- Greene H W. Snakes: the Evolution of Mystery in Nature. California, USA): University of California Press; 1997. [Google Scholar]
- Guo P, Liu Q, Chen X, Nguyen T. Complex longitudinal diversification across South China and Vietnam in Stejneger's pit viper, Viridovipera stejnegeri (Schmidt, 1925) (Reptilia: Serpentes: Viperidae) Mol Eco. 25:2920–2936. doi: 10.1111/mec.13658. [DOI] [PubMed] [Google Scholar]
- Hopkins W A, Roe J H, Snodgrass J W, Jackson B P, Kling D E, Rowe C L, Congdon J D. Nondestructive indices of trace element exposure in squamate reptiles. Environ Pollut. 115:1–7. doi: 10.1016/s0269-7491(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Itoh T, Xia J, Magavi R, Nishihata T, Rytting J H. Use of shed snake skin as a model membrane for in vitro percutaneous penetration studies: comparison with human skin. Pharm Res. 7:1042–1047. doi: 10.1023/a:1015943200982. [DOI] [PubMed] [Google Scholar]
- Jones D E, Nader P B, Holladay S D. Organochlorine detection in the shed skins of snakes. Ecotoxicol Environ Saf. 60:282–287. doi: 10.1016/j.ecoenv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kaur S. Lead in the scales of cobras and wall lizards from rural and urban areas of Punjab. India. Sci Total Environ. 77:289–290. doi: 10.1016/0048-9697(88)90064-2. [DOI] [PubMed] [Google Scholar]
- Kaito T, Ota H, Toda M. The evolutionary history and taxonomic reevaluation of the Japanese coral snake, Sinomicrurus japonicus (Serpentes, Elapidae), endemic to the Ryukyu Archipelago, Japan, by use of molecular and morphological analyses. J Zool Syst Evol Res. 55:156–166. [Google Scholar]
- Krey K, Farajallah A. Skin histology and microtopography of Papuan white snake (Micropechis ikaheka) in relation to their zoogeographical distribution. HAYATI J Biosci. 20:7–14. [Google Scholar]
- Kubie J L, Cohen J, Halpern M. Shedding enhances the sexual attractiveness of oestradiol treated garter snakes and their untreated penmates. Ani Behav. 26:562–570. [Google Scholar]
- Kumpugdee-Vollrath M, Subongkot T, Ngawhirunpat T. Model membrane from shed snake skins. Int J Med Heal Biomed Bioeng Pharm Eng. 7:669–676. [Google Scholar]
- Kuntz R E. Snakes of Taiwan. Quart J Taiwan Mus. 16:1–80. [Google Scholar]
- Lev E. Traditional healing with animals (zootherapy): Medieval to present-day Levantine practice. J Ethnopharmacol. 85:107–118. doi: 10.1016/s0378-8741(02)00377-x. [DOI] [PubMed] [Google Scholar]
- Lillywhite H B, Sheehy C M. Synchrony of ecdysis in snakes. Herpetol Conserv Biol. 11:286–292. [Google Scholar]
- Maki M. A Monograph of the Snakes of Japan. Tokyo, Japan): Dai-ichi Shobo, Publisher; 1931. [Google Scholar]
- Mcdiarmid R W, Foster M S, Guyer C, Gibbons J W, Chernoff N. Berkeley, California, USA): University of California Press; 2012. [Google Scholar]
- Medlin E C, Risch T S. An experimental test of snake skin use to deter nest predation. Condor. 108:963–965. [Google Scholar]
- Mochida K, Zhang W-Y, Toda M. The function of body coloration of the hai coral snake Sinomicrurus japonicus boettgeri. Zool Stud; 2015. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe E A, Monroe S E. Origins of iridescent colors on the indigo snake. Science. 159:97–98. doi: 10.1126/science.159.3810.97-a. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Dasgupta S C, Gomes A. Effect of Naja naja Laurenti shed skin extract on estrous cycle, hormone-cytokine profiles, histopathology of ovary and uterus of Swiss albino mice. Indian J Exp Biol. 51:235–240. [PubMed] [Google Scholar]
- Ngawhirunpat T, Panomsuk S, Opanasopit P, Rojanarata T, Hatanaka T. Comparison of the percutaneous absorption of hydrophilic and lipophilic compounds in shed snake skin and human skin. Pharmazie. 61:331–335. [PubMed] [Google Scholar]
- Ota H, Ito A, Lin J-T. Systematic review of the elapid snakes allied to Hemibungarus japonicus (Gunther, 1868) in the East Asian islands, with description of a new subspecies from the central Ryukyus. J Herpetol. 33:675–687. [Google Scholar]
- Ota H, Toyama M. Taxonomic re-definition of Achalinus formosanus Boulenger (Xenoderminae: Colubridae: Ophidia), with description of a new subspecies. Copeia. 0:597–602. [Google Scholar]
- Pope C H. The Reptiles of China: Turtles, Crocodilians. Snakes, Lizards. American Museum of Natural History; 1935. 10 [Google Scholar]
- Price R M. Dorsal snake scale microdermatoglyphics: ecological indicator or taxonomic tool? J Herpetol. 16:294–306. [Google Scholar]
- Priprem A, Khamlert C, Pongjanyakul T, Radapong S, Rittirod T, Chitropas P. Comparative permeation studies between scale region of shed snake skin and human skin in vitro. Am J Agri Biol Sci. 3:444–450. [Google Scholar]
- Priprem A, Pongjanyakul T, Khamlert C, Chitropas P, Kanla P, Sripanidkulchai K. Shed skin of Ophiophagus hannah: Structural topography and in vitro permeation of nicotine and phenol. Am J Anim Vet Sci. 2:84–88. [Google Scholar]
- Reading C J, Luiselli L M, Akani G C, Bonnet X, Amori G, Ballouard J M, Filippi E, Naulleau G, Pearson D, Rugiero L. Are snake populations in widespread decline? Biol Lett. 6:777–780. doi: 10.1098/rsbl.2010.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G, Li P X, Yang Y J. Guide to the Amphibians and Reptiles of Taiwan. Taipei, Taiwan.): OWL Publishing House; 2009. [Google Scholar]
- Stevenson D J, Ravenscroft K R, Zappalorti R T, Ravenscroft M D, Weigley S W, Jenkins C L. Using a wildlife detector dog for locating eastern indigo snakes (Drymarchon couperi) Herp Rev. 41:437–442. [Google Scholar]
- Takeuchi H, Ota H, Oh H-S, Hikida T. Serpentes: Colubridae), with special reference to prominent geographical differentiation of the mitochondrial cytochrome b gene in Japanese populations. Biol J Linn Soc. 105:395–408. [Google Scholar]
- Takeuchi H, Zhu G-X, Ding L, Tang Y, Ota H, Mori A, Oh H-S, Hikida T. Taxonomic validity and phylogeography of the East Eurasian natricine snake. Curr Herpetol. 33:148–153. [Google Scholar]
- Todd B D, Nowakowski A J, Rose J P, Price S J. Species traits explaining sensitivity of snakes to human land use estimated from citizen science data. Biol Cons. 206:31–36. [Google Scholar]
- Torri C, Mangoni A, Teta R, Fattorusso E, Alibardi L, Fermani S, Bonacini I, Gazzano M, Burghammer M, Fabbri D, Falini G. Skin lipid structure controls water permeability in snake molts. J Struct Biol. 185:99–106. doi: 10.1016/j.jsb.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Trnka A, Prokop P. The use and function of snake skins in the nests of great reed warblers Acrocephalus arundinaceus. Ibis. 153:627–630. [Google Scholar]
- Tsai T-S, Tu M-C. Sexual dimorphism of Chinese green tree viper, Trimereusrus s. stejnegeri. Biol Bull Nat Taiwan Normal Univ. 33:13–22. [Google Scholar]
- Tu M C, Fong S C, Lue K Y. Reproductive biology of the sea snake, Laticauda semifasciata, in Taiwan. J Herpetol. 24:119–126. [Google Scholar]
- Verveen A A, Rouwkema J. The iridescent epidermis of Boa constrictor. Litteratura Serpentium. 27:117–136. [Google Scholar]
- Wang C S, Wang Y M. The reptiles of Taiwan. Quart J Taiwan Mus. 9:1–86. [Google Scholar]
- Wang Y, Zhou K. A microdermatoglyphic analysis of 16 species of snakes. Chin J Appl Environ Biol. 4:152–158. [Google Scholar]
- You C-W, Li S-W, Lau A. Distribution and natural history of the ratsnakes of Taiwan: An annotated photographic review. Bushmaster Publications. 0:369–384. [Google Scholar]
- You C-W, Lin S-M. Diversity of the snaileating snakes Pareas (Serpentes, Pareatidae) from Taiwan. Zool Scripta. 44:349–361. [Google Scholar]
- Zhao E, Huang M, Zong Y. Beijing, China): Science Press; 1998. 3 [Google Scholar]
- Zhao E. Anhui Science and Technology Publishing House. Anhui, China): 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Key to the complete shed skins of 53 snake species in Taiwan and adjacent islands. (download)
The catalogue numbers of shed vouchers for 53 species of snakes. See the text for detailed descriptions on the classification of body patterns. (download)
Comparisons of the actual skin and shed skin in 53 snakes bearing banding, stripes, spots, or other markings on dorsal/ ventral head, body, and tail. Cases with markings are denoted by a check mark and those with some exceptions are denoted by a checkmark in parentheses. Note that “Y” means neonates or juveniles and “A” means adults. The counts and ratios are listed at the end of the table. (download)