Abstract
Emmeline A. Jamodiong, Elizaldy A. Maboloc, Ronald D. Villanueva, and Patrick C. Cabaitan (2018) Acropora hyacinthus is a fast-growing tabular coral that dominates the shallow water coral assemblage in the Magsaysay reef at the Bolinao-Anda Reef Complex (BARC), northwestern Philippines. The timing of gamete development was investigated for A. hyacinthus through dissection and histological analyses of coral fragments that were collected monthly from February 2014 to April 2015 from the 22 tagged colonies. The spawning time was identified by the presence of oocytes in the sampled A. hyacinthus colonies through rapid sampling from January to March 2014, 2015 and 2016. Results show that A. hyacinthus is a hermaphroditic broadcast spawning coral with an annual gametogenic cycle. Acropora hyacinthus exhibits an extended release of gametes across 2 to 3 months, from February to April. Major release of gametes occurred in March. Two types of extended spawning patterns that are unique in this region were observed in this species (i.e., asynchronous spawning amongst colonies and split spawning of individual colonies). This study contributes to the increasing knowledge on the coral reproductive strategies in northwestern Philippines and provides information on availability of coral materials for coral reef restoration efforts and management.
Keywords: Corals, Acropora, Reproduction, Spawning pattern, Philippines
BACKGROUND
Coral reefs are among the most diverse and economically important marine ecosystems that support various biological components and provide resources and livelihood to people (Chou et al. 2002). The persistence and maintenance of coral communities are dependent on the presence of sexually reproducing coral individuals (Kaniewska et al. 2015). Sexual reproduction in corals has been demonstrated to be significantly influenced by annual temperature changes (Dai et al. 1993; Van Veghel 1994; Hagman et al. 1998; Mendes and Woodley 2002; Guest et al. 2005; Lueg et al. 2012; Nozawa 2012). However, with the changing environments brought about by global climate change, corals are exposed to temperature anomalies that sub-lethally impact coral reproduction (Ward et al. 2000). Thermal stress lessens reproductive output of reef corals (Jones and Berkelmans 2011). With the prediction that thermal stress events will frequently reoccur, it is imminent to understand how corals maintain their fecundity (Victor et al. 2009). Corals may have developed different spawning strategies that will allow propagules to persist and survive.
Reproductive strategies of Acropora species have been studied in different geographical areas (Babcock et al. 1986; Baird et al. 2002; Guest et al. 2005; Carroll et al. 2006; Mangubhai and Harrison 2008; Kenyon 2008; Hanafy et al. 2010; Bouwmeester et al. 2015; Sola et al. 2016). These acroporid species usually participate in a synchronous mass spawning event (Willis et al. 1985; Hanafy et al. 2010; Bouwmeester and Berumen 2015; Chelliah et al. 2015; Jamodiong et al. 2017), despite extended spawning characteristics (Baird et al. 2009). Extended spawning occurs when coral colonies spawn over a series of consecutive lunar cycles or months (Baird et al. 2009). In northwestern Philippines, broadcast spawning of corals, represented by species from the families Faviidae and Acroporidae, occur March to May (Bermas et al. 1992; Vicentuan et al. 2008). A detailed study on Favites species reproduction showed brief synchronous spawning (Maboloc et al. 2016), which supports the hypothesis that extended spawning may be a characteristic of Acropora species. However, comprehensive studies on the reproductive timing of Acropora corals in the Philippines are very scarce. Depending on geographic location, the length of spawning durations varies between genus and species (Baird et al. 2009).
Acropora hyacinthus (Dana 1846) is a fast-growing tabular coral that is widespread in the Indo-Pacific region (Veron 2000). They dominate the Magsaysay reef (c. 60% of the coral cover, Jamodiong personal observation) in the Bolinao- Anda Reef Complex (BARC) of the Philippines. This area is regarded as an important source of coral larvae for the neighboring reefs because of its high coral coverage (coral cover 80%, Cabaitan unpublished data) and Acropora corals’ synchronous mass spawning event (Jamodiong et al. 2017). However, despite the dominance of A. hyacinthus, no detailed study on its reproduction has been done in the Philippines. Studying the reproductive behavior of this particular coral species, which dominates the reef assemblage, will provide knowledge on coral spawning along this area and implications on the reproductive phenology of corals. Furthermore, the majority of the studies previously conducted were from the randomly sampled colonies per species (Harrison et al. 1984; Hanafy et al. 2010; Bouwmeester et al. 2015; Chelliah et al. 2015; Sola et al. 2016). Repeated sampling of the same colonies provides an opportunity to see the changes in the reproductive timing throughout the months. This study aimed to elucidate the reproductive biology of A. hyacinthus, specifically to determine gametogenesis, spawning synchrony among colonies, and spawning time variability of A. hyacinthus in the Magsaysay reef in northwestern Philippines. Insights on coral spawning patterns and knowledge on the availability of populations that may settle are essential in understanding how coral communities may be maintained (Bouwmeester et al. 2015).
MATERIALS AND METHODS
Rapid sampling and sample collection
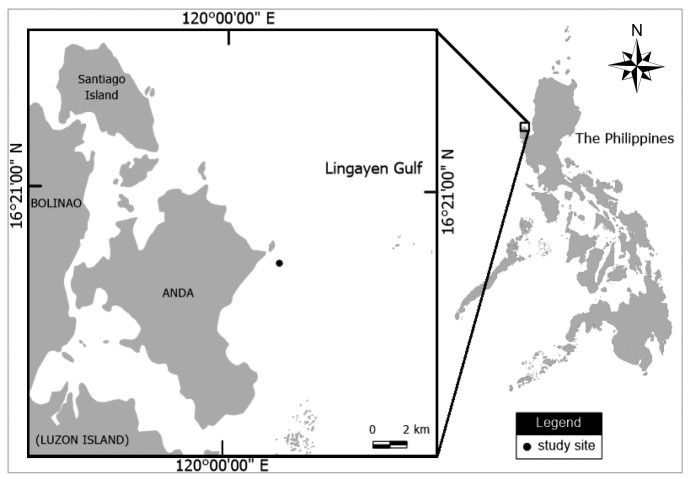
Fragments of Acropora hyacinthus were sampled from 22 tagged colonies (147 ± 48.81 cm in diameter) located at 2 to 3 m depth on the Magsaysay reef in the Bolinao-Anda Reef Complex (BARC), northwestern Philippines (16°18'38.3"N, 120°01'46.8"E) (Fig. 1). The tagged colonies were sampled monthly from February 2014 to April 2015 to determine the gametogenesis and fecundity. However, because of logistical constraints, no samples were collected in September 2014. A more intensive sampling, approximately every 1 to 2 weeks, were conducted during the expected spawning season to determine approximate spawning time, from February to April in 2014 and 2015 and from January to March in 2016. During each sampling, two to three fragments (2 to 3 cm in length) per colony were collected and the fragments were carefully checked for the presence or absence of oocytes underwater (after Baird et al. 2002). Samples were collected between the center and marginal area of the coral colonies. When oocytes were present, the color of oocytes was recorded. An HOBO Water Temperature Pro v2 Data Logger (Onset Computer Corp., Bourne, MA) was deployed in the sampling site from November 2014 to November 2016 to record seawater temperature.
Fig. 1.
Fig. 1. Study site location (Magsaysay reef) in the Bolinao - Anda Reef Complex, northwestern Philippines.
Histological examinations
The collected coral fragments were fixed in Zenkers solution for 24 hours, rinsed with running seawater for another 24 hours and decalcified by soaking the fragments to 10% buffered HCl for 2 to 3 days depending on the dissolution of the coral skeleton (Maboloc et al. 2016). Some of the decalcified tissue samples (n = 10) were used to determine the polyp fecundity and some were used to examine the gametogenesis (n = 10). For fecundity, decalcified fragments were dissected to determine the mean oocyte count per polyp for each individual colony. The tissue sample was placed on a clean petri dish and ten polyps per colony were haphazardly selected for examination. Each polyp was isolated from the tissue sample and dissected using dissecting needles under a stereomicroscope. In each polyp, the number of oocytes was counted and the minimum and maximum diameters of 10 haphazardly selected oocytes were photographed and measured using the MOTIC Images plus 2.0 software. For each oocyte, geometric mean diameter (GMD) was calculated using the minimum and maximum diameters and used as a metric for oocyte size (Maboloc et al. 2016). GMD is obtained by calculating the square root of the product of the maximum and minimum diameters. Mean oocyte size per month was calculated using the average of bimonthly and weekly oocyte sizes.
For gametogenesis, histological analysis was performed to see the different stages of the gametes using the monthly tissue samples from August 2014 to April 2015. Each sample was dehydrated in a series of alcohol solutions and cleared in xylene before embedding in paraffin wax. Cross and longitudinal sections at 5 μm thickness were cut within the fragments and mounted onto a glass slide. Slides were then stained with hematoxylin and counter stained with eosin and examined under a compound trinocular Zeiss microscope at different magnifications (from 100 x to 400 x magnifications). Oocyte development was recorded in different stages: stage I, when there was enlargement of the interstitial cells with large nuclei in the mesoglea of the mesentery; stage II, when cytoplasm started to accumulate around the nuclei; stage III when cytoplasm was larger such that the nucleus was centrally located; and stage IV for the mature level and when the oocyte achieved a full size with proliferation of yolk granules and indented nucleus. These stages were based on the criteria used by Szmant-Froelich et al. (1985). The developmental stages of the spermary were identified using criteria based on Goffredo et al. (2005): stage I was characterized by the formation of interstitial cells; stage II was characterized by the clustering of interstitial cells to form spermary; stage III was identified by the presence of spermary wall; stage IV when development of spermatocytes into spermatids are apparent by the presence of lumen in the spermary; and stage V with fully developed spermatozoans and tails forming ‘bouquet-like’ projections.
RESULTS
Gametogenesis
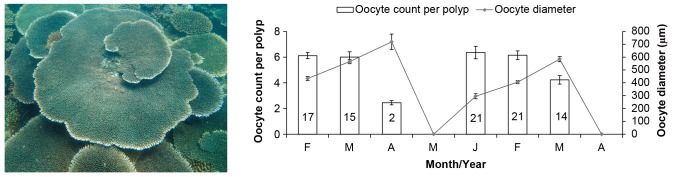
Acropora hyacinthus in northwestern Philippines is a hermaphroditic broadcast spawner, as demonstrated by the presence of both oocytes and spermaries in the same polyp. Staggered production of oocytes was observed in A. hyacinthus, with only few polyps and colonies containing eggs from August to December 2014; thus, only data for months with oocytes counts were presented in figure 2. Largest oocyte GMD was recorded in April 2014 with 718.77 ± 60.26 μm (mean ± SE) and March 2015 with 583.88 ± 19.44 μm (Fig. 2). Mean oocyte count polyp-1 was highest in January 2015 and lowest in April 2014 (Fig. 2). At the start of the monitoring, oocytes were recorded in 17 out of 22 tagged colonies in February 2014 with a mean oocyte count polyp-1 of 6.11 ± 0.24 (mean ± SE). In January 2015, 21 of the tagged colonies contained eggs (1 colony was not sampled because the tag was not found). Oocyte number was notably lower March to April 2014, from 6.00 ± 0.41 (mean ± SE) to 2.45 ± 0.17 (mean ± SE); and in February to March 2015, from 6.14 ± 0.33 (mean ± SE) to 4.23 ± 0.33 (mean ± SE) (Fig. 2).
Fig. 2.
Fig. 2. Mean monthly oocyte counts and diameter of Acropora hyacinthus from February - May (F - M) 2014 and January - April (J - A) 2015. Number inside the bars indicates the number of colonies with oocytes. Error bars are standard error of the means.
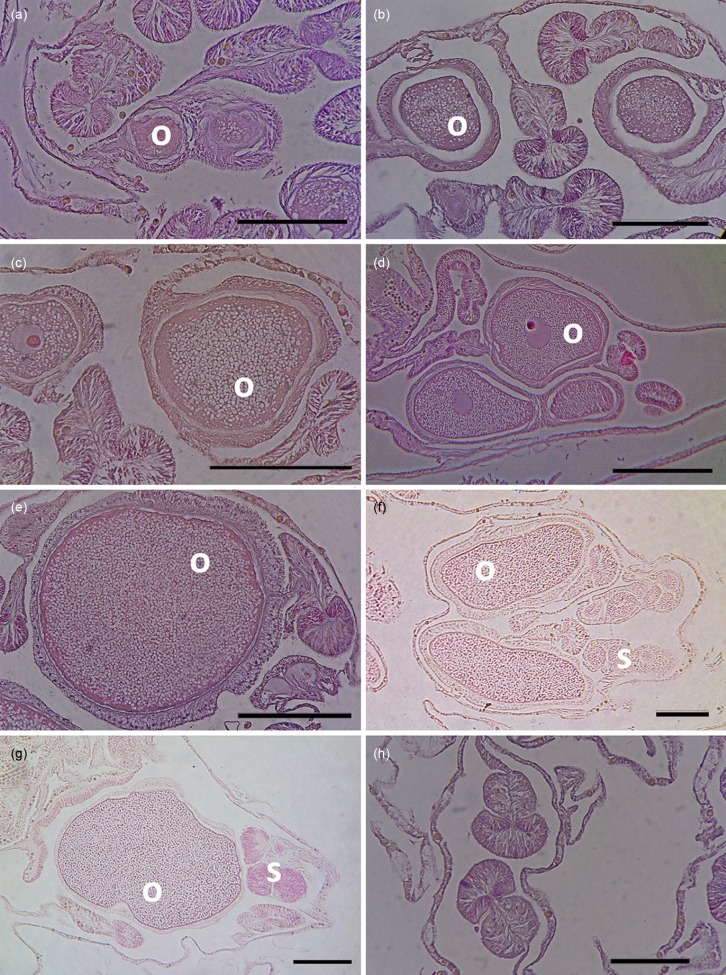
Histological analyses of tissue samples revealed a more defined pattern of variability in oocyte development. Early stage I oocytes were observed in some polyps in August 2014 (Fig. 3a). The size of the oocytes at early stage I measures < 100 μm, which was not noticeable using visual observation during dissection. Oocytes then continued to develop and increase in number in the succeeding months (Fig. 3b-c), which is consistent with the increase in fecundity per polyp observed through dissection from October to November 2014. More oocytes at stage II were noted on samples collected in December (Fig. 3d). In January, most oocytes were at stage III as characterized by a larger cytoplasm (Fig. 3e). The shape of the oocyte also started to change from round to elliptical. Elliptical oocytes were seen in February and March samples, which were mostly in stage IV. Oocytes at stage IV exhibited proliferation of yolk granules and large cytoplasmic area (Fig. 3g). On the other hand, spermaries were more difficult to recognize, especially at the early stages of development. Spermaries were noticed as dark stained oval structures clustering beside mature oocytes, as noted in February and March with stage II and V spermaries, respectively (Fig. 3f and 3g). Empty mesenteries were noted in April 2015 (Fig. 3h), indicative of spent colonies.
Fig. 3.
Fig. 3. Histological sections of Acropora hyacinthus in different months: (a) August 2014, (b) October 2014, (c) November 2014, (d) December 2014, (e) January 2015, (f) February 2015, (g) March 2015, (h) April 2015, showing approximate duration of oogenesis of A. hyacinthus at the study location. The oocytes were first observed in some samples in August 2014 and disappeared in all tagged colonies in April 2015. Mature stages of oocytes and spermaries were observed in February and March 2015. o - oocytes; s - spermaries; scale bar = 100 μm.
The gametogenic cycle, inferred from histological examinations, was further confirmed through rapid sampling of colonies in the field for the presence of oocytes. In months (e.g., February and March) when histological samples contained oocytes at late stages III and IV (Fig. 3e and 3g), colonies sampled in the field contained eggs that were unpigmented or with cream white coloration. Moreover, when most of the oocytes were already mature (stage IV) based on histological analysis, eggs noted in the field were in different shades of pink than observed in March. In months (e.g., May to December) when oocytes were not present or too small in histological samples, colonies checked through rapid sampling were empty (without eggs).
Spawning pattern
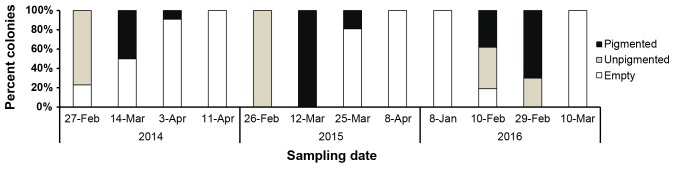
Spawning or release of gametes in A. hyacinthus was variable between years 2014, 2015 and 2016, according to the rapid sampling data that were corroborated with histological examinations. Spawning pattern was less synchronous during the 2014 spawning season, with 77% of the tagged colonies contained unpigmented oocytes in February (Fig. 4). By March, 50% of the colonies contained pigmented oocytes. On April 3, only 9% of the colonies remained gravid. All colonies were empty of oocytes by April 11, 2014. In 2015, all tagged colonies (n = 21) contained mature pigmented oocytes on March 12. The number of colonies with mature pigmented oocytes decreased to 19% on March 25 while all colonies were empty on April 8. In 2016, 30% of the colonies had unpigmented eggs while 70% have pigmented eggs on February 29. Further field examination by March 10 revealed that all tagged colonies were empty. Despite the observed asynchronous spawning between colonies, the release of gametes in the three spawning seasons coincided with the rising temperatures in the sampling site (see Jamodiong et al. 2017).
Fig. 4.
Fig. 4. Proportion of the tagged colonies (n = 22) with pigmented, unpigmented and empty eggs through rapid sampling of oocyte maturity in the field from 2014 to 2016.
DISCUSSION
Acropora hyacinthus displayed similar broadcast spawning patterns to conspecific corals in other regions (Willis et al. 1985; Nozawa 2012; Gilmour et al. 2016). The continuous production of oocytes exhibited by this species after the onset of oocyte production is a characteristic of most Acropora species (Shlesinger et al. 1998). Gametes showed variable characteristics as development proceeds. The oocyte was observed to change in shape from rounded to irregular ellipses as it shifted from immature to mature, which can be attributed to bundle-formation. Development of the spermaries may take place in a short duration; hence, only few stages of spermaries were observed in this study.
Coral reef species in the Indo-Pacific waters (Dai et al. 1993; Wilson and Harrison 2003; Bouwmester et al. 2015) and those located in low latitude areas (e.g., Great Barrier Reef, Caribbean, Singapore, Kenya, and Thailand) were known to exhibit extended spawning months (Willis et al. 1985; Bastidas et al. 2005; Guest et al. 2005; Mangubhai and Harrison 2008; Kongjandtre et al. 2010). In this study, the population of A. hyacinthus in the Magsaysay reef showed an extended spawning pattern as indicated in the variable proportions of gravid (pigmented) colonies on all the sampling dates during the three spawning seasons. For corals with high fecundity such as A. hyacinthus, this extended spawning pattern does not necessarily affect fertilization success (Mangubhai and Harrison 2008). Instead, split spawning helps coral gametes be released in a more favorable condition and have a greater chance of settlement and survival (Harrison et al. 1984; Wilson and Harrison 2003).
At the species level, reproductive synchrony among corals were hypothesized to break down from high-latitude areas to equatorial regions because of a narrower variation in environmental variables that influenced timing of reproduction (Oliver et al. 1988; Mangubhai and Harrison 2008). In the case of A. hyacinthus, previous studies showed synchronous spawning within and between colonies at the high-latitude environment, e.g., 32°N (Nozawa 2012); biannual spawning in the mid-latitude, e.g., 14°S (Gilmour et al. 2016); and a lesser degree of spawning synchrony in equatorial region, e.g., 5°S (Oliver et al. 1988). Observations in Pulau Tioman, Malaysia (2°N) on the same sampling year (Nozawa and Lin 2014) showed a distinct spawning pattern wherein only 5% of the colonies were pigmented in April (Chelliah et al. 2015), which appeared to be similar to the spawning pattern observed in the present study. The closer similarity to the spawning pattern in the latter study maybe driven by the exposure of similar biophysical conditions in the South China Sea (Dorman et al. 2016) and different genetic lineages from their northern counterparts (Suzuki et al. 2016). This information thus provides understanding on the reproductive behavior of this species across geographic locations.
Moreover, continuous sampling of the tagged colonies indicate that extended spawning did not only occur among individuals of A. hyacinthus, but within individual colonies as well. This feature is more prevalent during the higher synchronous spawning season of 2015, wherein before oocyte count per colonies reached zero, a slight decrease of the oocyte counts per colonies was observed. To our knowledge, this characteristic has never been recorded in this species. This finding may either be unique in this region or possibly a consequence of limited studies on the reproductive pattern and repeated sampling of tagged corals in other areas. There are only a few studies on gametogenesis in this species (Wallace 1985; Kenyon 2008), and most reproductive studies are based on spawning observations (Babcock et al. 1986; Hayashibara et al. 1993; Wilson and Harrison 2003; Carroll et al. 2006; Toh et al. 2012; Lin and Nozawa 2017). The decrease in the oocyte fecundity could not be the result of variable colony locations in the sampled polyps (Nozawa and Lin 2014) since fragment sampling was maintained in a single location within the colonies and majority of the colonies experienced a reduction in oocyte count. Nevertheless, since the A. hyacinthus has a large tabular colony structure, we suggest that this spawning variation within a colony may have resulted from the variable gamete maturity in each polyp, regardless of the polyp’s location. This disparity could be influenced by the varying distribution of physical factors (e.g., temperature, nutrient and other essential seawater components) in each polyp per colony (Monismith 2007; Nozawa 2012). The Magsaysay reef, where the samples were taken, is located near a sand bar, which exposes the A. hyacinthus colonies (and polyps) to variable wave actions. Acropora hyacinthus has a high-energy habitat preference (Celliers and Schleyer 2001), thus making this assumption plausible.
The reproductive biology of corals is highly influenced by temperature (Dai et al. 1993; Van Veghel 1994; Hagman et al. 1998; Mendes and Woodley 2002; Guest et al. 2005; Lueg et al. 2012; Nozawa 2012; Maboloc et al. 2016). In the present study, despite the variable spawning pattern observed among colonies, it was observed that the onset of temperature rise coincided with the release of egg bundles in February to April, which supports the contention that temperature only influences the latter part of gametogenesis until the spawning time (Nozawa 2012). During the periods when temperature (mean ± SD) started to rise [e.g., from January 2015 (26.80 ± 0.69°C) to February 2015 (26.98 ± 0.55°C); February 2015 (26.98 ± 0.55°C) to March 2015 (28.20 ± 0.54°C); and to April 2015 (29.40 ± 0.58°C)], oocyte count per colony simultaneously decreased. Rapid decrease of oocyte number in February to April coincided with rapid increase of temperature (1.40 ± 0.20°C), which was also exhibited by other Acropora species, particularly in the latter stage of gametogenic cycle (Gomez et al. 2018). Aside from temperature, the lunar phase was also inferred to influence spawning time. Although split-spawned and less synchronous, there is an overlap in spawning time between A. hyacinthus and with other acroporid species, where mass spawning of Acropora species in 2015 and 2016 were observed (Jamodiong et al. 2017). The mass spawning occurred 9 to 11 days after the last full moon and before the occurrence of first vernal equinox (Jamodiong et al. 2017).
The less synchronous spawning within and among the colonies of A. hyacinthus differ from the Favites species (e.g., F. colemani and F. abdita) in the study area. These faviid species showed synchronized gametogenesis despite the distance of individual colonies; e.g., even up to 3 km away (Maboloc et al. 2016). Apparently, the study by Nozawa (2012) at a higher latitude reported a high synchrony in Acropora species and low spawning synchrony among faviid species. Differences between reproductive patterns in the same family across geographic locations can be a biological adaptation to local environmental features (Lin and Nozawa 2017). In the Bolinao-Anda Reef Complex, spawning patterns may depend on how the colonies may response to associated spawning cues. Unlike with the faviid’s massive growth forms and large polyp sizes, A. hyacinthus exhibits large colony sizes with tiny and numerous polyps, allowing differences in reproduction among individual polyps that could result in the asynchronous spawning seen in this species (Willis et al. 1985).
It is not clear why some colonies in the 2014 reproductive season (5 out of 22 tagged colonies) appeared to have empty mesenteries since the start of the monitoring. It could be possible that these colonies with empty mesenteries previously experienced bleaching events and/or crown-of- thorns infestation (Arceo et al. 2001; Vergara et al. 2010), thus affecting its reproductive behavior. Other studies also reported that bleaching resulted in physiological stress and energy reduction, thus affecting the fecundity of A. hyacinthus (Armoza- Zvuloni et al. 2011; Nozawa 2012). These colonies may also have spawned earlier during the spawning season in 2014 given that a less synchronous spawning pattern occurred during this year. Lastly, these colonies did not reproduce during this year. Mendes and Woodley (2002) proposed that, unlike in the GBR, spawning may not be always annual in other locations. Some corals miss one spawning season in preparation for a more successful gamete release on the next spawning season (Hughes et al. 2002). The observed lesser synchronous spawning pattern in 2014 and 2016, compared to a higher degree of synchronous spawning in 2015, supports this idea and warrants a long-term investigation of the reproductive patterns of A. hyacinthus colonies.
CONCLUSIONS
In summary, this is the first detailed repro- ductive study of the coral Acropora hyacinthus in northwestern Philippines. The spawning pattern varied annually with spawning duration from February to April. Major release of gametes occurred in March during the mass spawning of Acropora. This finding suggests that this dominant coral species has the potential to continuously produce larvae and recruit in this area. Information on the timing of this species can also provide insights for future coral reef conservation efforts in this region. Further investigation will not only clarify the long-term spawning behavior of this species, but also verify the effect of environmental stresses (e.g., bleaching) on the reproductive biology of this species.
Acknowledgments
Acknowledgments: We would like to thank Ronaldo De Guzman, Renato Uriarte, Marcos Ponce, Fernando Castrence, Christine Angelito and Charlon Ligson for their logistical and technical support. Tracy Tabalanza provided the map of the study site. Thanks to Dr. Yoko Nozawa for comments on the initial draft of this paper. The study was funded by a grant from the Department of Science and Technology-Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development to RDV and PCC.
Footnotes
Author’s Contributions: EAJ, EAM, RDV conceived and designed the research; EAJ, EAM performed the experiments; EAJ, EAM, PCC analyzed the data; RDV, PCC provided materials and funds; EAJ, EAM, PCC wrote and edited the manuscript. Competing Interests: The authors declare that they have no competing interests.
Availability of data and materials: The data and materials that support the findings of this study are available from the corresponding author and University of the Philippines, The Marine Science Institute - Bolinao Marine Laboratory upon reasonable request.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Arceo H O, Quibilan M C, Aliño P M, Lim G, Licuanan W Y. Coral bleaching in Philippine reefs: Coincident evidences with mesoscale thermal anomalies. Bull Mar Sci. 69:579–593. [Google Scholar]
- Armoza-Zvuloni R, Segal R, Kramarsky-Winter E, Loya Y. Repeated bleaching events may result in high tolerance and notable gametogenesis in stony corals: Oculina patagonica as a model. Mar Ecol Prog Ser. 426:149–159. [Google Scholar]
- Babcock R C, Bull G D, Harrison P L, Heyward A J, Oliver J K, Wallace C C, Willis B L. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol. 90:379–394. [Google Scholar]
- Baird A H, Guest J R, Willis B L. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Syst. 40:551–571. [Google Scholar]
- Baird A H, Marshall P A, Wolstenholme J. Latitudinal variation in the reproduction of Acropora in the Coral Sea. Proc 9th Int Coral Reef Symp. 1:385–389. [Google Scholar]
- Bastidas C, Croquer A, Zubillaga A L, Ramos R, Kortnik V, Weinberger C, Marquez L M. Coral mass-and split-spawning at a coastal and an offshore Venezuelan reef, southern Caribbean. Hydrobiologia. 541:101–106. [Google Scholar]
- Bermas N A, Aliño P M, Atrigenio M P, Uychiaoco A. Observations on the reproduction of scleractinian and soft corals in the Philippines. Proc 7th Int Coral Reef Symp. 1:443–447. [Google Scholar]
- Bouwmeester J, Baird A H, Chen C J, Guest J R, Vicentuan K C, Berumen M L. Multi-species spawning synchrony within scleractinian coral assemblages in the Red Sea. Coral Reefs. 34:65–77. [Google Scholar]
- Bouwmeester J, Berumen M L. High reproductive synchrony of Acropora (Anthozoa: Scleractinia) in the Gulf of Aqaba, Red Sea. . F1000Res. 2015. [DOI] [PMC free article] [PubMed]
- Carroll A, Harrison P, Adjeroud M. Sexual reproduction of Acropora reef corals at Moorea, French Polynesia. Coral Reefs. 31:93–97. [Google Scholar]
- Celliers L, Schleyer M H. Acropora hyacinthus and Acropora austera dominance on a high-energy reef top at Kosi Bay, South Africa. Coral Reefs; 2001. 20 [Google Scholar]
- Chelliah A, Amar H B, Hyde J, Yewdall K, Steinberg P D, Guest J R. First record of multi-species synchronous coral spawning from Malaysia. PeerJ; 2015. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou L M, Tuan V S, Yeemin T, Cabanban A, Kessna I. Status of Southeast Asia coral reefs. Wilkinson C (ed) Status of the coral reefs of the world: 2002. Australian Institute of Marine Science. pp. 123–152.
- Dai C F, Soong K, Fan T Y. Sexual reproduction of corals in northern and southern Taiwan. Proc 7th Int Coral Reef Symp. 1:448–454. [Google Scholar]
- Dorman J G, Castruccio F S, Curchitser F N, Kleypas J A, Powell T M. Modelled connectivity of Acropora millepora populations from reefs of the Spratly Islands and the Greater South China Sea. Coral Reefs. 35:169–179. [Google Scholar]
- Gilmour J P, Underwood J N, Howells E J, Gates E, Heyward A J. Biannual spawning and temporal reproductive isolation in Acropora corals. PLoS ONE; 2016. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredo S, Radetić J, Airi V, Zaccanti F. Leptopsammia pruvoti (Scleractinia, Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis. 147:485–495. [Google Scholar]
- Gomez E J, Jamodiong E A, Maboloc E A, Ligson C A, Tabalanza T D, Villanueva R D, Cabaitan P C. Gametogenesis and reproductive pattern of the reef-building coral Acropora millepora in northwestern Philippines. Invertebr Reprod Dev; 2018. [Google Scholar]
- Guest J R, Baird A H, Goh Bpl, Chou L M. Reproductive seasonality in an equatorial assemblage of scleractinian corals. Coral Reefs. 24:112–116. [Google Scholar]
- Hagman D K, Gittings S R, Deslarzes J P. Timing, species participation and environmental factors influencing annual mass spawning at the Flower Garden Banks (Northwest Gulf of Mexico) Gulf Mex Sci. 2:170–179. [Google Scholar]
- Hanafy M H, Aamer M A, Habib M, Rouphael A B, Baird A H. Synchronous reproduction of corals in the Red Sea. Coral Reefs. 29:119–124. [Google Scholar]
- Harrison P L, Babcock R C, Bull G D, Oliver J K, Wallace C C, Willis B L. Mass spawning in tropical reef corals. Science. 223:1186–1189. doi: 10.1126/science.223.4641.1186. [DOI] [PubMed] [Google Scholar]
- Hayashibara T, Shimoike K, Kimura T, Hosaka S, Heyward A, Harrison P, Kudo K, Omori M. Patterns of coral spawning at Akajima Island. Mar Ecol Prog Ser. 10:253–262. [Google Scholar]
- Hughes T P, Baird A, Dinsdale E A, Harriott V J, Moltschaniwskyj N, Pratchett M S, Tanner J E, Willis B L. Detecting regional variation using meta-analysis and large-scale sampling: latitudinal patterns in recruitment. Ecology. 83:436–451. [Google Scholar]
- Jamodiong E A, Maboloc E A, Leriorato J C, Tanedo Mcs, Diaz L, Tabalanza T D, Cabaitan P C, Villanueva R D. Coral spawning and spawn-slick observation in the Philippines. Mar. Biodiv; 2017. [Google Scholar]
- Jones A M, Berkelmans R. Tradeoffs to thermal acclimation: energetics and reproduction of a reef coral with heat tolerant Symbiodinium type-D. Jour Mar Biol; 2011. [Google Scholar]
- Kaniewska P, Alon S, Karako-Lampert S, Hoegh-Guldberg O, Levy O. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. 2015. 4. [DOI] [PMC free article] [PubMed]
- Kenyon J C. Acropora (Anthozoa:Scleractinia) Reproductive synchrony and spawning phenology in the Northern Line Islands, Central Pacific, as inferred from size classes of developing oocytes. Pacific Science. 62:569–578. [Google Scholar]
- Kongjandtre N, Ridgway T, Ward S, Hoegh-Guldberg O. Broadcast spawning patterns of Favia species on the inshore reefs of Thailand. Coral Reefs. 29:227–234. [Google Scholar]
- Lin C H, Nozawa Y. Variability of spawning time (lunar day) in Acropora versus merulinid corals: a 7-yr record of in situ coral spawning in Taiwan. Coral Reefs. 36:1268–1278. [Google Scholar]
- Lueg J R, Moulding A L, Kosmynin V N, Gilliam D S. Gametogenesis and spawning of Solenastrea bournoni and Stephanocoenia intersepta in Southeast Florida, USA. Jour Mar Biol; 2012. [Google Scholar]
- Maboloc EA, Jamodiong EA, Villanueva RD. Reproductive biology and larval development of the scleractinian corals Favites colemani and F. abdita (Faviidae) in northwestern Philippines. Invertebr Reprod Dev. 2016;60:1–11. [Google Scholar]
- Mangubhai S, Harrison P L. Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar Ecol Prog Ser. 360:85–96. [Google Scholar]
- Mendes J M, Woodley J D. Timing of reproduction in Montastrea annularis: relationship to environmental variables. Mar Ecol Prog Ser. 227:241–251. [Google Scholar]
- Monismith S G. Hydrodynamics of the coral reefs. Annu Rev Fluid Mech. 39:37–55. [Google Scholar]
- Nozawa Y. Annual variation in the timing of coral spawning in a high-latitude environment: influence of temperature. Biol Bull. 222:192–202. doi: 10.1086/BBLv222n3p192. [DOI] [PubMed] [Google Scholar]
- Nozawa Y, Lin C H. Effects of colony size and polyp position on polyp fecundity in the scleractinian coral genus Acropora. Coral Reefs. 33:1057–1066. [Google Scholar]
- Oliver J K, Babcock R C, Harrison P L, Willis B L. Geographic extent of mass coral spawning: clues to ultimate causal factors. Proc 6th Int Coral Reef Symp. 2:803–810. [Google Scholar]
- Shlesinger Y, Goulet T L, Loya Y. Reproductive patterns of scleractinian corals in the northern Red Sea. Mar Biol. 132:691–701. [Google Scholar]
- Sola E, Marques Da Silva I, Glassom D. Reproductive synchrony in a diverse Acropora assemblage at Vamizi Island, Mozambique. Mar Ecol. 37:1373–1385. [Google Scholar]
- Suzuki G, Keshavmurthy S, Wallace C, Shirayama Y. Genetic preference of peripheral isolation and low diversity in marginal populations of the Acropora hyacinthus complex. Coral Reefs. 35:1419–1432. [Google Scholar]
- Szmant-Froelich A, Reutter M, Riggs L. Sexual reproduction of Favia fragum (Esper): lunar patterns of gametogenesis, embryogenesis, embryogenesis and planulation in Puerto Rico. Bull Mar Sci. 37:880–892. [Google Scholar]
- Toh T C, Guest J, Chou L M. Coral larval rearing in Singapore: observations on spawning timing, larval development and settlement of two common scleractinian coral species. In: Tan KS (ed) Contributions to Marine Science, National University of Singapore, Republic of Singapore. pp. 81–87.
- Veghel Van. Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. Mar Ecol Prog Ser. 109:209–219. [Google Scholar]
- Veron Jen, Vergara Mwb, Geronimo R, Ticzon V S, Dizon R M, Villanueva R D, Baria M V, Vicentuan-Cabaitan K, Cruz Dela, Bollozos D W, Ravago-Gotangco I S. Building capacity in coral reef science: an anthology of CRTR scholars' research. The Coral Reef Targeted Research & Capacity Building for Management. 3:60–74. [Google Scholar]
- Vicentuan K C, Guest J R, Baria M V, Cabaitan P C, Dizon R M, Villanueva R D, Aliño P M, Edwards A J, Gomez E D, Heyward A J. Multi-species spawning of corals in north-western Philippines. Coral Reefs; 2008. 27 [Google Scholar]
- Victor S, Golbuu Y, Yukihara H, Van Woesik R. Acropora size-frequency distributions reflect spatially variable conditions on coral reefs of Palau. Bull Mar Sci. 85:149–157. [Google Scholar]
- Wallace C C. Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Mar Biol. 88:217–233. [Google Scholar]
- Ward S, Harrison P, Hoegh-Guldberg O. Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. Proc 9th Int Coral Reef Symp. 2:1123–1128. [Google Scholar]
- Willis B L, Babcock R C, Harrison P L, Oliver J K, Wallace C C. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proc 5th Int Coral Reef Cong. 4:343–348. [Google Scholar]
- Wilson J R, Harrison P L. Spawning patterns of scleractinian corals at the Solitary Islands -a high latitude coral community in eastern Australia. Mar Ecol Prog Ser. 260:115–123. [Google Scholar]