Abstract
Yang Xiao, Ghulam Nabi, Jiwei Yang, Yujiang Hao, and Ding Wang (2018) Sex hormones play a crucial role in regulating testicular development and maintaining spermatogenesis in the male reproductive system. Knowledge of hormonal regulation in odontocetes is limited to captive species. In this study, the characteristics of hormonal regulation during the testicular development were assessed by histological and immunohistochemical methods in the East Asian finless porpoise (Neophocaena asiaeorientalis sunameri), native to the Chinese Yellow/Bohai Sea coast, China. The testes mass, seminiferous tubule cross section diameter, thickness of the tunica albuginea, and the level of testosterone (T) expression increased abruptly at the age of 3-3.5 years (body length 140-145 cm). However, the estradiol (E2) expression level decreased with age after 3 years. Therefore, we inferred that the male East Asian finless porpoise (EAFP) > 3 years old (body length > 140 cm) could be classified as the age of puberty onset. Immuno-localization with T was only observed in the interstitial fluid of all animals at all ages. In contrast, a positive reaction for E2 and its receptor could be observed in the Leydig, myoid, Sertoli, and germ cells at different developmental stages. T is presumed to maintain the tubular microenvironment for spermatogenesis while E2 may directly regulate spermatogenesis at the level of germ cells. Our findings provide useful information for understanding reproductive status and hormonal regulation in the male EAFP.
Keywords: Cetacean, Reproduction, Sex hormone, Immunolocalization, Testes
BACKGROUND
Gonadal development is regulated by sex hormones, including androgen and estrogen. In males, there is plenty of evidence indicating that both androgen and estrogen are essential in the regulation of reproductive physiology. Previous studies of mouse models have confirmed that the major androgen, namely testosterone (T), is required to maintain spermatogenesis (Singh et al. 1995; Yeh et al. 2002). Furthermore, estradiol (E2) can also affect proliferation, differentiation, and function of the cells in the male reproductive system (Odonnell et al. 2001). Although testosterone and estradiol levels in rodent testes are much higher than those present in serum (Comhaire and Vermeulen 1976; Hess 2000), hormonal regulation of testicular development is still not completely understood. Androgen and estrogen effects are mediated by their receptors, AR and ERs, respectively (ERα affects spermatogenesis and ERβ affects steroidogenesis) (Carreau et al. 2006). However, the sites of sex hormone action in the testes are different among mammals (Odonnell et al. 2001), which can lead to differential pathways to regulate spermatogenesis. Even in the same species, hormonal distribution and concentrations change with age from birth to adulthood (Bremner et al. 1994; Carreau et al. 1999). Therefore, a detailed understanding of the hormonal regulation in the testes is required to assess and manage male fertility.
In most cetacean species, reproductive hormone assays were conducted on blood samples collected from captive populations (Schroeder and Keller 1989; Robeck et al. 2005; Harrison and Ridgwa 2009). However, blood sampling can be quite difficult from wild cetaceans because they fully live in the ocean. Alternatively, the fresh carcasses, especially those collected as instant bycatch, could provide a unique opportunity to analyze sex hormone alteration in the testes. This may shed light on the reproductive endocrinology of wild cetaceans.
The EAFP is a recently recognized marine subspecies of the narrow-ridged finless porpoise. It has a wide range of distribution from the Taiwan Strait, north to the Yellow/Bohai Sea in China, and in the waters of Korea and Japan (Jefferson and Wang 2011). Due to the tremendous decline in population over the last decade, the IUCN Red List of Threatened Species updated its category from vulnerable to endangered (Wang and Reeves 2017). However, unlike its freshwater counterpart, the Critically Endangered Yangtze finless porpoise (N. a. asiaeorientalis, hereafter YFP), less attention has been given to understand the reproductive physiology of the EAFPs. In previous studies, only morphological and histological examination of the testes have been made in EAFP (Zhang 1992; Gao and Zhou 1993; Chang and Zhou 1995; Lee et al. 2013). Chen et al. (1997) did a preliminary study on serum T concentrations of the mature male YFP and Hao et al. (2007) was the first to report the relationship between serum T and E2 in male YFP. However, until now, little is known about the reproductive endocrinology of the wild male EAFPs due to difficulties in collecting blood samples.
The purpose of this study was to (1) describe the histological development of testes regulated by sex hormones (T and E2) with age, (2) investigate the distribution and expression levels of these two hormones and their receptors in testes from birth to adulthood, and (3) to understand the hormonal regulation of testicular development.
MATERIALS AND METHODS
Sampling
A total of 43 male EFPs were collected April- June in 2015 and 2016 from the Yellow/Bohai Sea, near the Penglai City, Shandong Province, China (Fig. 1). These animals were accidentally caught in gill nets and discovered within a few hours after death by local fishermen. Body length was measured from the tip of the beak to the notch in a fluke. Body weight was measured in the unit of 0.1 kg. Left testes removed from epididymis were weighed fresh in the unit of 0.01 g and preserved in 10% neutrally buffered formalin for histological development and steroid hormone expression examination. Teeth from the middle of the lower jaw were collected and stored at -20°C for age analysis.
Fig. 1.
Fig. 1. Sampling location (black dot) of the East Asian finless porpoise.
Laboratory analysis
Age was estimated by counting the dentinal growth layer groups (GLGs) on thin sections of each tooth (Hohn 1989; Read 1993; Fernandez and Hohn 1998). The sampled teeth were decalcified with EDTA (0.5M, pH, 8.0, Servicebio, China) and sectioned to 30 μm thicker through the middle of the pulp cavity by a cryoultramicrotome (Leica CM1950, Nussloch, Germany). The sections were then stained with Mayer’s hematoxylin and observed under a light microscope (ZEISS, Jena, Germany). Age estimates were made without reference to collaborative data, such as body length and body weight. It was assumed that one dentinal GLG in the teeth of the finless porpoises forms annually (Gao and Zhou 1993). Thus, a single GLG, which consists of an opaque and a translucent layer was counted as a 1-year. If there was only an opaque layer, we estimated it as a 0.5-year. Individuals under 1 year were estimated by the age-length curve (Gao and Zhou 1993).
Testicular development was based on histological examination. Tissue taken from the center of 17 testes were embedded in paraffin, sectioned to 5 μm by a microtome (Leica RM2235, Nussloch, Germany), and stained with hematoxylin-eosin for microscopic analysis at magnifications of ×100, ×200 and ×400. Parameters were measured by Images Pro Plus 6.0 (Media Cybernetics, USA). Ten round seminiferous tubules were randomly identified for each testis. The seminiferous tubule cross section diameter (STD) was measured twice for each tubule at 90° angles to one another across the tubule based on a basal lamina (Clarke 1956). The thickness of tunica albuginea (TTA) was measured in 16 testes for one losing its tunica albuginea. Reproductive status was classified into three stages. Individuals with small testes mass, narrow seminiferous tubules, and no spermatozoa were considered immature. Pubertal individuals were determined to be bigger testis and tubules, and spermatocytes could be identified. Mature individuals had remarkably increased testes and tubules sizes and small interstitial tissue; the entire process of spermatogenesis was present in the tubules (Akin et al. 1993).
T and E2 expression were detected using the Streptavidin-Biotin Complex kit (Boster, Wuhan, China). Left testes sections were dried for 60 min at 60°C, dewaxed in xylene and rehydrated with gradual ethanol to water. To demask antigen epitopes, the sections in sodium citrate buffer (pH 6.0) were boiled in a microwave at 120°C for 2 × 5 min. The sections were then soaked in 3% H2O2 in methanol for 10 min at room temperature (RT) to inactivate the endogenous peroxidase. Following rinsing in phosphate-buffered saline (PBS, pH 7.4) and blocking in goat non-immune serum for 20 min at RT, the sections were incubated with T antibody (GeneTex, USA, Monoclonal, 1:100 in PBS), E2 antibody (Biorbyt, UK, Monoclonal, 1:80 in PBS), AR antibody (Boster, Wuhan, China, polyclone, 1:50 in PBS), and ERβ antibody (Boster, Wuhan, China, polyclone, 1:80 in PBS) at 4°C overnight. After washing with PBS, the sections were incubated with biotinylated second antibody to goat for rabbit IgG (Boster, Wuhan, China, polyclone) for 30 min at RT, and washed with PBS. Following incubation with biotin-avidin horseradish peroxidase complex for 20 min at RT and washed with PBS, the sections combined with antibodies were reacted with 0.02% diaminobenzidine tetrahydrochloride (DAB, Boster, Wuhan, China) and then counterstained with hematoxylin. Two tissue sections in which the primary antibody was replaced with PBS were as negative controls in each run. Images were collected under a light microscope (ZEISS, Jena, Germany) by a CCD camera (TUCSEN, Fujian, China), and integrated optical density (IOD) was analyzed by Images Pro Plus 6.0 (Media Cybernetics, USA).
Statistical analysis
The data were analyzed using the software SPSS19.0 for Windows (SPSS Inc., Chicago, IL, USA). The STD, TTA, and testis mass (TM) at different reproductive statuses were compared using the Student’s independent t-tests. Scatter plots of STD, TTA, and TM against age as well as body length were used to analyze testis developmental trends. The Pearson correlation coefficient was calculated between T expression and E2 expression. Data are presented as mean ± SD. Differences were considered significant at P < 0.05.
RESULTS
Age estimation
Figure 2 shows a longitudinal slice of a tooth consisting of translucent and opaque zones under the transmitted light. The tooth is clearly divided into two parts by a highlighted neonatal line. The part inside of the neonatal line is dentine, and the outside is cementum. Ages were counted by GLGs in dentine, and basic data are shown in table 1. The oldest individual was 13 years old, while the youngest ones died about a month after birth.
Fig. 2.
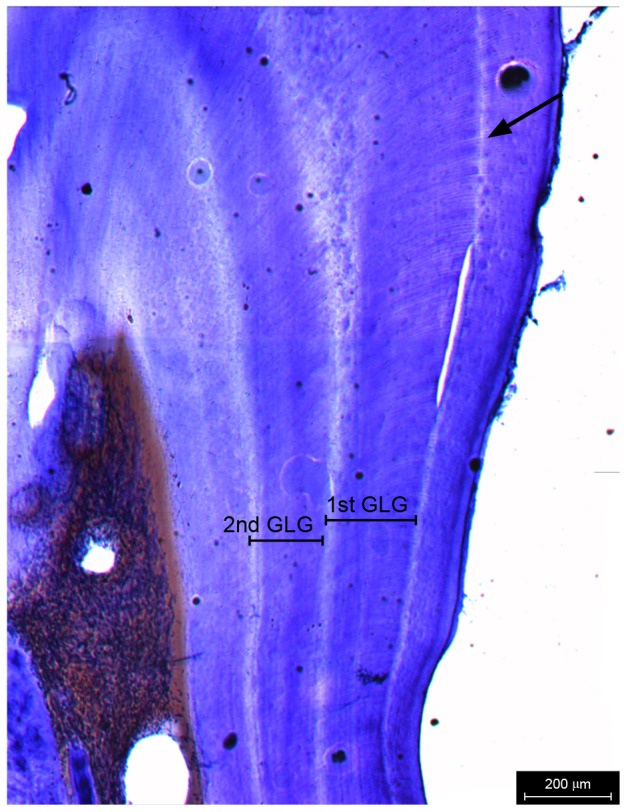
Fig. 2. Growth layer groups (GLGs) in the thin section of a tooth. One GLG consists of an opaque layer and a translucent layer. The arrow represents the neonatal line. Scale bar = 200 μm.
Table 1. Data on individual finless porpoises investigated in this study.
| Animal ID | Age (yr) | Body length (cm) | Body weight (kg) | Animal ID | Age (yr) | Body length (cm) | Body weight (kg) |
| M1 | 3 | 145 | 54 | M23 | 0.1 | 82 | 8 |
| M2 | 0.7 | 125 | 40 | M24 | 0.7 | 124 | 34.4 |
| M3 | 0.5 | 118 | 28.4 | M25 | 0.5 | 115.4 | 26.4 |
| M4 | 0.5 | 122.5 | 31.5 | M26 | 4.5 | 141 | 42.8 |
| M5 | 6 | 172 | 55.3 | M27 | 4.5 | 164 | 56.7 |
| M6 | 2.5 | 135.5 | 35 | M28 | 7 | 194 | 77.7 |
| M7 | 0.1 | 84 | 8.5 | M29 | 2 | 133 | 31.5 |
| M8 | 0.7 | 124 | 28.5 | M30 | 7.5 | 192 | 71.5 |
| M9 | 0.5 | 113.5 | 22 | M31 | 1 | 125.4 | 30 |
| M10 | 1.5 | 127 | 35.5 | M32 | 0.5 | 117.8 | 33.4 |
| M11 | 8 | 200.5 | 62 | M33 | 0.5 | 112.7 | 27 |
| M12 | 1.5 | 119 | 26 | M34 | 2.5 | 130 | 34 |
| M13 | 10 | 195 | 74.5 | M35 | 1.5 | 130.2 | 27.8 |
| M14 | 3.5 | 148 | 35.5 | M36 | 0.5 | 116.6 | 28.7 |
| M15 | 13 | 215 | 78 | M37 | 5 | 163 | 48.3 |
| M16 | 0.5 | 123.5 | 32.3 | M38 | 1 | 127 | 31.3 |
| M17 | 5 | 160 | 49.4 | M39 | 12 | 198 | 67.1 |
| M18 | 5.5 | 175 | 58.1 | M40 | 4 | 148.2 | 47.1 |
| M19 | 3 | 144 | 36.1 | M41 | 1 | 118 | 27.8 |
| M20 | 6 | 186 | 63.4 | M42 | 2 | 133 | 35.1 |
| M21 | 0.5 | 122 | 27.3 | M43 | 12 | 205 | 76.1 |
| M22 | 0.5 | 122 | 29.1 |
Testicular development
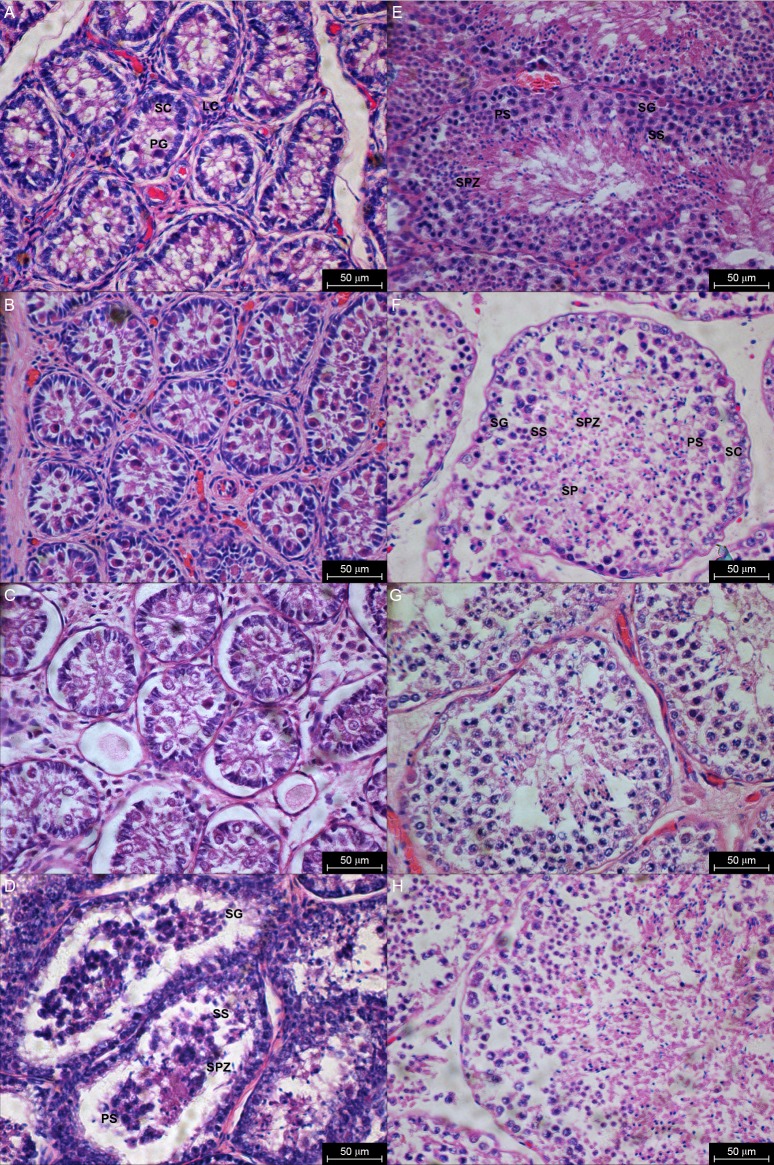
The histological sections of 17 porpoises were examined. Animals aged 0 to 3 years had narrow seminiferous tubules with no spermatozoa. The tubules were lined with a layer of germinal epithelium comprising the Sertoli cells and a few primordial germ cells. Interstitial tissue can be observed between the seminiferous tubules. The Leydig cells were abundant among the tubules and the dominant component of the interstitial tissue (Fig. 3 A, B, C). The STD increased when the animal reached 3.5 years. The spermatogonia, primary spermatocytes and secondary spermatocytes were observed, and a few spermatids or spermatozoa could be identified (Fig. 3D). All stages of spermatogenesis were present in a 5-year-old animal with a large sum of spermatozoa appeared in the luminal part of the seminiferous tubules (Fig. 3E). The interstitial tissue of animals over 6 years old occupied very little space between the seminiferous tubules and was much larger than the previous stage in younger animals. There were relatively few spermatogonia and spermatocytes, but many spermatids and spermatozoa (Fig. 3 F, G, H).
Fig. 3.
Fig. 3. Histological sections of testes from birth to adulthood. LC: Leydig cells; SC: Sertoli cells; PG: Primordial germ cells; SG: Spermatogonia; PS: Primary spermatocytes; SS: Secondary spermatocytes; SPZ: Spermatozoa. (A) 0.1yr. (B) 2.5yr. (C) 3yr. (D) 3.5yr. (E) 4.5yr. (F) 6yr. (G) 8yr. (H) 13yr. Scale bar = 50 μm.
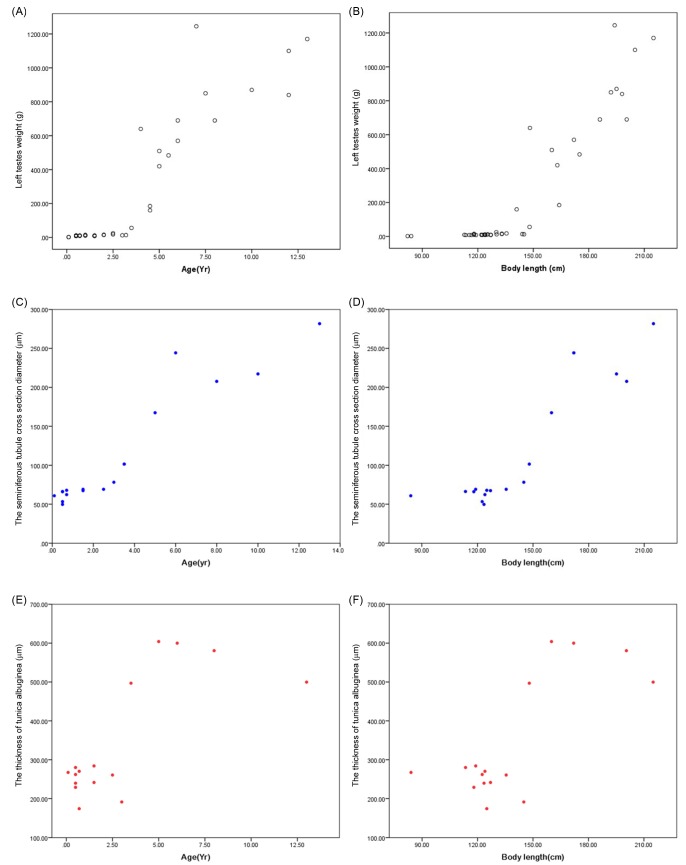
Based on the testicular features, 11 specimens were classified as immature and 5 as mature. One porpoise was recognized as pubertal. The result of STD, TTA, and TM are shown in table 2. Due to the small sample size (n = 1), the measurements of the pubertal individual were not used for statistical analysis. The mean STD, TTA, and TM for mature porpoises were significantly higher than for immature ones (i < 0.01). All these parameters showed an abrupt increase at the age of 3-3.5 years, and 140-145 cm body length (Fig. 4).
Table 2. Parameter estimates (mean ± SD) in the development of testes.
| Reproductive status | N | Seminiferous tubule cross section diameter (µm) | Thickness of tunica albuginea (µm) | Testes mass (g) |
| Immature | 11 | 64.48 ± 7.91 | 245.38 ± 35.53 | 9.87 ± 3.76 |
| Pubertal* | 01 | 101.53 ± 0 | 496.84 ± 0 | 56.33 ± 0 |
| Mature | 05 | 223.59 ± 42.61 | 585.00 ± 59.15 | 762.00 ± 2 66.31 |
N: Number of individuals examined. (*): Excluded from statistical analysis.
Fig. 4.
Fig. 4. Scatter plots of TM against age as well as body length (A, B); STD against age as well as body length (C, D); TTA against age as well as body length (E, F).
Hormonal expression
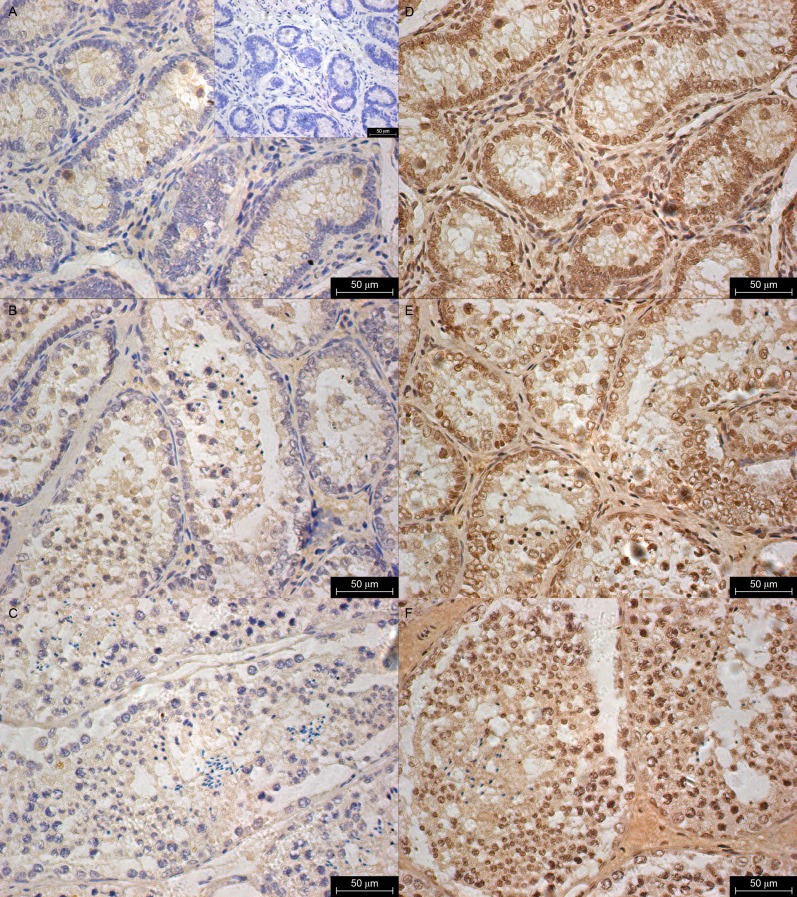
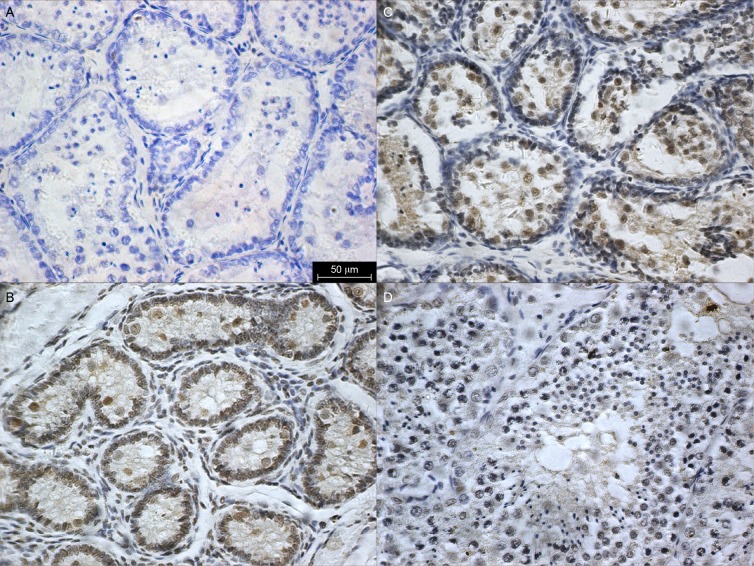
A total of 12 porpoises were examined for immunohistochemistry. Immunolocalization with T was only observed in the interstitial fluid of all animals at all ages (Fig. 5 A, B, C). In contrast, a positive reaction for E2 could be observed in both testicular interstitium and seminiferous epithelium (Fig. 5 D, E, F). In the interstitial compartment, specific labeling of E2 was found in the Leydig cells. Staining was variable in myoid cells at different ages. In the tubular compartment, strong E2 immunoreactivity was localized in the Sertoli cells and germ cells, including primordial germ cells, spermatogonia, spermatocytes, and spermatids. However, E2 was not detected in the spermatozoa.
Fig. 5.
Fig. 5. Immunoexpression of testosterone (A: 0.1yr, B: 3.5yr and C: 8yr) and estradiol (D: 0.1yr, E: 3.5yr and F: 8yr) in testes from birth to adulthood. Upper insert on panel A: negative control. Black arrows represent spermatozoa. Scale bar = 50 μm.
AR was not detected in the testes (Fig. 6A). A positive ERβ immunolocalization was observed in primordial germ cells from birth to adulthood. However, other germ cells did not express ERβ. In addition, a strong immunopositive signal for ERβ was only found in the Leydig cells and Sertoli cells, when the animal was immature (Fig. 6B, C, D).
Fig. 6.
Fig. 6. Immuno-expression of AR (A:3.5yr.) and ERβ (B: 0.1yr., C: 3.5yr. and D: 8yr.) in testes. Scale bar = 50 μm.
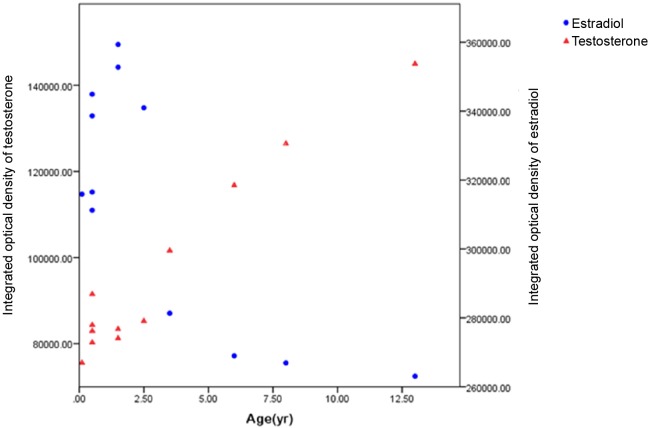
There was a remarkably negative correlation between the IOD of T and E2 (Fig.7,r=0.8,P< 0.01). The level of T expression was significantly lower than E2 when the animal was younger than 3 years. Furthermore, with increasing age, the IOD of T showed an abrupt increase at 3-3.5 years. However, the intensity of E2 expression exhibited an opposite trend.
Fig. 7.
Fig. 7. IOD of testosterone and estradiol with respect to age.
DISCUSSION
The present results compare testicular development of the EAFP from birth to adulthood. The developmental changes were determined by alterations in the TM and steroid hormone levels along with the evolution of histological parameters such as STD and TTA. Previous data on testes obtained from the finless porpoise showed a growth spurt in TM and STD at 4-5 years, which then was regarded as the age of sexual maturity (Zhang 1992; Lee et al. 2013). However, testicular enlargement is the first physical manifestation of puberty in males (Weiner et al. 2003). Puberty is a process to attain sexual maturity that is essential for reproduction and the existence of a species (Ebling 2003). In our study, all the testicular parameters (TM, STD, and TTA) increased rapidly at 3-3.5 years, which may signal the onset of a division of the seminiferous tubules and the gradual formation of more germ cell types (Jiang 1998). In addition, we found few spermatozoa in a 3.5-year-old EAFP, which was classified as puberty. Although the presence of spermatozoa in testis has been used as a criterion of maturity in many delphinid males (Kasuya et al. 1974; Murphy et al. 2005), we consider that EAFP may not be fully mature as testicular parameters were all less than mature porpoises but much greater than immature porpoises.
In this study, characteristics of hormonal regulation during testicular development were assessed through T and E2 expression in the testes by immunohistochemistry. We found that the positive reaction for T was only observed in the interstitial fluid. The T is synthesized by the Leydig cells in the interstitial compartment of the testis and secreted into the seminiferous tubules (Dohle et al. 2003). Androgen effects are mediated by AR, which is present in the peritubular myoid cells, Leydig cells, and Sertoli cells (Sar et al. 1990). However, whether germ cells express AR has remained controversial. Several studies have demonstrated AR-positive staining in the germ cells among mammals (Vornberger et al. 1994; Zhou et al. 1996; Merlet et al. 2007), while other studies reported no immunostaining in these cells (Bremner et al. 1994; Suarezquian et al. 1999). Therefore, it is assumed that T may not act directly on the germ cells. However, it may function through the Sertoli cells by expressing AR and supporting germ cell development (Sar et al. 1990; Johnston et al. 2001). Furthermore, it has also been suggested that T can act directly upon germ cells and is transported into these cells by androgen binding protein (Larriba et al. 1995; Joseph et al. 1997). Due to the specificity of the antibody to human hormones, we did not detect AR in the EAFPs. Observations of T in our study suggest that it may help to maintain the tubular microenvironment for spermatogenesis.
T within the testis can be metabolized into E2 by aromatase. Aromatase activity has been measured in rat Leydig cells and Sertoli cells (Papadopoulos et al. 1986). However, in humans, the aromatase is mainly present in germ cells, which means a new source of E2 (Carreau S 2002). The role of E2 in the development of the male reproductive tract is still under debate, although there is growing evidence suggesting that E2 may directly regulate spermatogenesis. The observations that an aromatase inhibitor reduces spermatid maturation in rats and dogs (Tsutsumi et al. 1987ab) indicate that E2 could play a role in germ cell transformation and sperm maturation. The fact that ERβ is present in mammalian germ cells of various stages of their development has been reported in several studies (Bilinska et al. 2001; Makinen et al. 2001; Lucas et al. 2008). Nevertheless, none of these previous studies reported the localization of E2 and ER in the testes in cetaceans. Our finding shows that E2 and ERβ are respectively present in germ cells in different developmental stages. It suggests that germ cells are both the synthesis and target cell for E2 in EAFP, and spermatogenesis may be regulated directly by E2 at the level of germ cells. In addition, the observations regarding the distribution of ERβ in somatic cells (Leydig and Sertoli cells) suggest that estrogen may be involved in proliferation and differentiation in these cells.
The quantitative relationship between local testosterone concentrations and spermatogenesis has been the subject of numerous studies and debates (Turner et al. 1984; Rommerts 1988; Sharpe et al. 1988). Zirkin et al. (1989) found that spermatogenesis does not proceed in the absence of relatively high levels of testosterone (more than 70 nM) in the rat. In the present study, testosterone expression intensity was relatively lower when EAFP was immature, and spermatozoa were not found in the seminiferous tubules. After 3-3.5 years of age, testosterone intensity increases significantly with age and varies in adult animals as other cetacean species (Desportes et al. 1994; Rolland et al. 2005). It has become well established that testosterone is essential to enabling the timely initiation of puberty (Gromoll et al. 2000). The observations of T level in our study indicate that in EAFP, the onset of puberty could begin at 3 years of age. Interestingly the intensity of E2 exhibited an opposite trend, declining rapidly. A previous study demonstrated that plasma testosterone concentrations were closely correlated with estradiol concentrations in male minke whales (Balaenoptera acutorostrata) during the breeding season (Suzuki et al. 2001). In the YFP, Hao et al. (2007) observed a weak positive correlation between serum T and E2 concentrations (r = 0.39, P = 0.08). In contrast, our observation shows that testicular T and E2 were negatively correlated. We considered that circulating steroid concentration might be influenced by metabolism when these hormones are released into the blood. It is reported that ERα-deficient mice displayed higher levels of testicular testosterone secretion than wild- type mice in early fetal and neonatal development (Delbes et al. 2005). This observation shows a clear negative effect of endogenous estrogens on the activity and differentiated functions of the Leydig cell. As a result, we infer that the high value of E2 in immature EAFP may physiologically inhibit the synthesis of T by acting directly on the testis.
CONCLUSIONS
This study characterizes puberty based on testicular development and a parallel increase in testicular testosterone level, which attests that maturational changes in spermatogenic and steroidogenic components occur simultaneously. Consequently, based on the histological and immunohistochemical evaluation, we infer that the male EAFPs > 3 years old (body length > 140 cm) can be classified as the age of puberty onset. It is expected that these findings will provide useful information for understanding reproductive status and hormonal regulation in male EAFP. However, because of the limitations imposed by the number of individuals, we are cautious about making a comprehensive conclusion regarding the reproductive physiology of the EAFP. Further work should attempt to obtain data from animals at puberty to provide baseline information for investigating the endocrine dynamics in wild EAFP.
Acknowledgments
Acknowledgments: We thank Shenglun Zhang, Mingen Yin, and other staff at Ocean World of Penglai for their assistance in carcass collection, and we thank our colleagues for carcass dissection. This study was financially supported by the National Natural Science Foundation of China (No. 31430080).
List of abbreviations
- EAFP,
East Asian finless porpoise.
- T,
Testosterone.
- E2,
Estradiol.
- AR,
Androgen receptor.
- ER,
Estrogen receptor.
- YFP,
Yangtze finless porpoise.
- GLGs,
Growth layer groups.
- STD,
Seminiferous tubule cross section diameter.
- TTA,
Thickness of tunica albuginea.
- TM,
Testes mass.
- RT,
Room temperature.
- PBS,
Phosphate-buffered saline.
- IOD,
Integrated optical density.
Footnotes
Authors’ contributions: YH conceived and designed the research, YX and JY performed the field work. YX performed the statistical analyses. YX, GN and YH wrote the paper. DW provided the resources and funds.
Competing interests: YX, GN, YH, JY and DW declare that they have no conflict of interest.
Availability of data and materials: All the data are provided within the manuscript.
Consent for publication: Not applicable.
Ethics approval consent to participate: All the procedures strictly adhered to Chinese law and ethical guidelines for wild animals.
References
- Akin P A, Peltier K M, Miller R B. Techniques for the preparation and examination of reproductive samples collected from dolphins in the eastern tropical Pacific. NOAA Tech Memo NMFS-SWFSC; 0192. [Google Scholar]
- Bilinska B, Schmalzfrączek B, Kotula M, Carreau S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol. 2001;178:427–427. doi: 10.1016/s0303-7207(01)00427-0. [DOI] [PubMed] [Google Scholar]
- Bremner W J, Millar M R, Sharpe R M, Saunders P T. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 135:1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- Carreau S. The testicular aromatase: From gene to physiological role. Reprod Biol. 2:5–12. [PubMed] [Google Scholar]
- Carreau S, Delalande C, Silandre D, Bourguiba S, Lambard S. Aromatase and estrogen receptors in male reproduction. Mol Cell Endocrinol. 246:65–68. doi: 10.1016/j.mce.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Carreau S, Genissel C, Bilinska B, Levallet J. Sources of oestrogen in the testis and reproductive tract of the male. Int J Androl. 22:211–223. doi: 10.1046/j.1365-2605.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Zhou K Y. The growth and reproduction of finless porpoise, Neophocaena phocaenoides, in the Yangtze River and Yellow/Bohai Sea. J Nanjing Normal Univ (Nat Sci) 18:114–124. [Google Scholar]
- Chen D Q, Zhao Q Z, Liu R J. Preliminary study on some serum hormones of Neophocaena phocaenoides in the Yangtze River. Acta Theriologica Sin. 17:43–47. [Google Scholar]
- Clarke R. Sperm whales of the Azores. 28:273–298. [Google Scholar]
- Comhaire F H, Vermeulen A. Testosterone concentration in the fluids of seminiferous tubules, the interstitium and the rete testis of the rat. J Endocrinol. 70:229–235. doi: 10.1677/joe.0.0700229. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor α. Endocrinology. 146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- Dohle G R, Smit M, Weber R F. Androgens and male fertility. World J Urol. 21:341–345. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- Desportes G M, Saboureau M, Lacroix A. Growth-related changes in testicular mass and plasma testosterone concentrations in long-finned pilot whales, Globicephala Melas. J Reprod Fertil. 102:237–244. doi: 10.1530/jrf.0.1020237. [DOI] [PubMed] [Google Scholar]
- Ebling F. Puberty: mind and body. J Neuroendocrinol. 15:323–324. doi: 10.1046/j.1365-2826.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Hohn A A. Age, growth, and calving season of bottlenose dolphins, Tursiops truncatus, off coastal Texas. Fish Bull. 96:357–365. [Google Scholar]
- Gao A L, Zhou K Y. Growth and reproduction of three populations of finless porpoise, Neophocaena phocaenoides, in Chinese waters. Aquat Mammals. 19:3–12. [Google Scholar]
- Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J Clin Endocrinol Metab. 85:2281–2286. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- Hao Y J, Chen D Q, Zhao Q Z, Wang D. Serum concentrations of gonadotropins and steroid hormones of Neophocaena phocaenoides asiaeorientalis in middle and lower regions of the Yangtze River. Theriogenology. 67:673–680. doi: 10.1016/j.theriogenology.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Harrison R J, Ridgwa S H. Gonadal activity in some Bottlenose dolphins (Tursiops truncatus) J Zool. 165:355–366. [Google Scholar]
- Hess R A. Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Rev Reprod. 5:84–92. doi: 10.1530/ror.0.0050084. [DOI] [PubMed] [Google Scholar]
- Hohn A A, Scott M D, Wells R S, Sweeney J C, Irvine A B. Growth layers in teeth from known-age, free-ranging bottlenose dolphins. Mar Mammal Sci. 5:315–342. [Google Scholar]
- Jefferson T A, Wang J Y. Revision of the taxonomy of finless porpoises (genus Neophocaena): the existence of two species. J Mar Anim Ecol. 4:3–16. [Google Scholar]
- Jiang X F. Studies on the development and histological characteristics of black finless porpoise, Neophocaena phocaenoides in the Yangtze River. Acta Hydrobiol Sin. 22:341–345. [Google Scholar]
- Johnston D S, Russell L D, Friel P J, Griswold M D. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology. 142:2405–2405. doi: 10.1210/endo.142.6.8317. [DOI] [PubMed] [Google Scholar]
- Joseph D R, O'brien D A, Sullivan P M, Becchis M, Tsuruta J K, Petrusz P. Overexpression of androgen-binding protein/sex hormone-binding globulin in male transgenic mice: tissue distribution and phenotypic disorders. Biol Reprod. 56:21–32. doi: 10.1095/biolreprod56.1.21. [DOI] [PubMed] [Google Scholar]
- Kasuya T, Miyazaki N, Dawbin W H. Growth and reproduction of Stenella attenuate in the Pacific coast of. Japan. Sci Reprod. 26:157–226. [Google Scholar]
- Larriba S, Esteban C, Toran N, Gerard A, Gerard Audi L, Reventos H. Androgen binding protein is tissue-specifically expressed and biologically active in transgenic mice. J Steroid Biochem Mol Biol. 53:573–578. doi: 10.1016/0960-0760(95)00103-7. [DOI] [PubMed] [Google Scholar]
- Lee Y R, An Y R, Park K J, Sohn H, An D H, Kim S A. Age and reproduction of the finless porpoises, Neophocaena asiaeorientalis, in the Yellow Sea. Korea. Anim Cells Syst. 17:366–373. [Google Scholar]
- Lucas T F, Siu E R, Esteves C A, Monteiro H P, Oliveira C A, Porto C S, Lazari M F. 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod. 78:101–114. doi: 10.1095/biolreprod.107.063909. [DOI] [PubMed] [Google Scholar]
- Makinen S, Makela S, Weihua Z, Warner M, Rosenlund B, Salmi S, Hovatta O, Gustafsson J. Localization of oestrogen receptors alpha and beta in human testis. Mol Hum Reprod. 7:497–503. doi: 10.1093/molehr/7.6.497. [DOI] [PubMed] [Google Scholar]
- Merlet J, Racine C, Moreau E, Moreno S G, Habert R. Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc Natl Acad Sci. 104:3615–3620. doi: 10.1073/pnas.0611421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Collet A, Rogan E. Mating strategy in the male common dolphin (Delphinus delphis): What gonadal analysis tells us. J Mammal. 86:1247–1258. [Google Scholar]
- Odonnell L, Robertson K M, Jones M, Simpson E R. Estrogen andspermatogenesis. Endocr Rev. 22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Carreau S, Szerman-Joly E, Drosdowsky M A, Dehennin L, Scholler R. Rat testis 17-beta estradiol: identification by gas chromatogarphy-mass spectrometry and age-related cellular distribution. J Steroid Biochem. 24:1211–1216. doi: 10.1016/0022-4731(86)90385-7. [DOI] [PubMed] [Google Scholar]
- Read A J, Wells R S, Hohn A A, Scott M D. Patterns of growth in wild bottlenose dolphins, Tursiops truncatus. J Zool. 231:107–123. [Google Scholar]
- Robeck T R, Monfort S L, Calle P P, Dunn J L, Jensen E, Boehm J R, Young S, St Clark. Reproduction, growth and development in captive beluga (Delphinapterus leucas) Zoo Biol. 24:29–49. [Google Scholar]
- Rolland R M, Hunt K E, Kraus S D, Wasser S K. Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen Comp Endocrinol. 142:308–317. doi: 10.1016/j.ygcen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Rommerts Ffg. How much androgen is required for maintenance of spermatogenesis. J Endocrinol. 116:7–9. doi: 10.1677/joe.0.1160007. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn D B, French F S, Wilson E M. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 127:3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Schroeder J P, Keller K V. Seasonality of serum testosterone levels and sperm density in Tursiops truncatus. J Exp Zool. 249:316–321. doi: 10.1002/jez.1402490310. [DOI] [PubMed] [Google Scholar]
- Sharpe R M, Donachie K, Cooper I. Re-evaluation of the intratesticular level of testosterone required for quantitative maintenance of spermatogenesis in the rat. J Endocrinol. 117:19–26. doi: 10.1677/joe.0.1170019. [DOI] [PubMed] [Google Scholar]
- Singh J, O'neill C, Handelsman D J. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 136:5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- Suarezquian C A, Martinezgarcia F, Nistal M, Regadera J. Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab. 84:350–358. doi: 10.1210/jcem.84.1.5410. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mogoe T, Asada M, Miyamoto A, Tetsuka M, Ishikawa H, Ohsumi S, Fukui Y. Plasma and pituitary concentrations of gonadotropins (FSH and LH) in minke whales (Balaenoptera acutorostrata) during the feeding season. Theriogenology. 55:1127–1141. doi: 10.1016/s0093-691x(01)00472-1. [DOI] [PubMed] [Google Scholar]
- Tsutsumi I, Fugimori K, Nakamura R M, Mather J P, Ono T, Dizerega G S. Disruption of seminiferous epithelial function in the rat by ovarian protein. Biol Reprod. 36:451–461. doi: 10.1095/biolreprod36.2.451. [DOI] [PubMed] [Google Scholar]
- Tsutsumi I, Toppari J, Campeau J D, Dizerega G S. Reduction of fertility in the male rat by systemic treatment with follicle regulatory protein. Fertil Steril. 47:689–695. doi: 10.1016/s0015-0282(16)59123-7. [DOI] [PubMed] [Google Scholar]
- Turner T T, Jones C E, Howards S S, Ewing L L, Zegeye B, Gunsalus G L. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- Vornberger W, Prins G, Musto N A, Suarezquian C A. Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. 1994. [DOI] [PubMed]
- Wang J Y, Reeves R. Neophocaena asiaeorientalis. The IUCN Red List of Threatened Species. pp. 2017–2025.
- Weiner I B, Freedheim D K, Schinka J A, Velicer W F, Lerner R M. Handbook of Psychology. Hoboken, New Jersey): John Wiley and Sons; 2003. [Google Scholar]
- Yeh S, Tsai M Y, Xu Q, Mu X M, Lardy H, Huang K, Lin H, Yeh S, Altuwaijri S, Zhou X, Xing L, Boyce B F, Hung M C, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci. 99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X F. Studies on the age determination, growth and reproduction of finless porpoise Neophocaena phocaenoides. Chinese. 16:289–298. [Google Scholar]
- Zhou X, Kudo A, Kawakami H, Hirano H. Immunohistochemical localization of androgen receptor in mouse testicular germ cells during fetal and postnatal development. Anat Rec. 245:509–518. doi: 10.1002/(SICI)1097-0185(199607)245:3<509::AID-AR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Zirkin B R, Santulli R, Awoniyi C A, Ewing L L. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 124:3043–3049. doi: 10.1210/endo-124-6-3043. [DOI] [PubMed] [Google Scholar]