Abstract
Sébastien Lavoué, Sahat Ratmuangkhwang, Hsuan-Ching Ho, Wei-Jen Chen, and Mohd Nor Siti Azizah (2018) Longfin herrings form a monophyletic, circumtropically distributed family of mostly marine teleost fishes, the Pristigasteridae (Clupeoidei), that includes 38 species classified into nine genera and three main lineages (the Pelloninae, Pristigasterinae, and the genus Ilisha). The external morphology and osteology of the Pristigasteridae provide only a few, sometimes conflicting, informative characters which makes it difficult to reconstruct their phylogeny, but their swimbladder (a visceral gas-filled chamber that has several important functions in the Teleostei) appears to be highly specialized and variable among species. In particular, the swimbladder of most Indo-West Pacific pristigasterid species exhibits one or paired post-coelomic extensions, whereas New World species do not. The presence of these extensions conflicts with the current systematic classification, as they are only found in subsets of different taxa. To examine this conflict, the most comprehensive molecular phylogenetic tree of the Pristigasteridae to date was built using six molecular markers and 21 species. This tree deeply disagreed with the current classification in that it indicated that the subfamilies Pelloninae and Pristigasterinae are not monophyletic and neither are the genera Ilisha, Pellona, and Opisthopterus. Using this tree to infer the evolution of the post-coelomic extensions, it was found that their absence is the ancestral condition in the Pristigasteridae. Indo-West Pacific species with post-coelomic extensions evolved later and form a monophyletic group, inside which species with only one extension form a monophyletic group. The consequences of our findings on the evolution and classification of Pristigasteridae are discussed. We suggest that only species of Pristigasteridae having one or paired post-coelomic extensions should be included in the genus Ilisha.
Keywords: Evolution, Phylogeny, Morphology, Molecules, Fish
BACKGROUND
The Pristigasteridae, collectively called longfin herrings, are a small family of ray-finned fishes belonging to the suborder Clupeoidei (Teleostei). The Clupeoidei include four additional families, the Chirocentridae, Engraulidae, Clupeidae, and Dussumieriidae, the last two of which are likely not reciprocally monophyletic (Grande 1985; Whitehead 1985; Whitehead et al. 1988; Lavoué et al. 2014). Pristigasterids are easily recognized by their laterally compressed body, full or almost full series of well-developed ventral scutes, large eyes, reduced or absent pelvic fins, a short dorsal fin (absent in the genus Raconda), and a long anal fin (with more than 30 fin-rays) (Whitehead 1985). The Pristigasteridae currently comprise 38 species classified into nine genera (Eschmeyer et al. 2018) and three main lineages: the subfamilies Pristigasterinae and Pelloninae and the genus Ilisha (minus I. africana, which is placed in the Pristigasterinae) (Grande 1985) (Fig. 1). Three genera include about three-quarters of the species diversity: Ilisha with 16 species, and Pellona and Opisthopterus each with six species (Eschmeyer et al. 2018). Two species of the Pristigasteridae - I. melastoma and I. elongata - are commonly found in the northern South China Sea (Shao et al. 2008), of which large (> 30 cm in standard length) specimens of I. elongata hold some economic importance in this region.
Fig. 1.
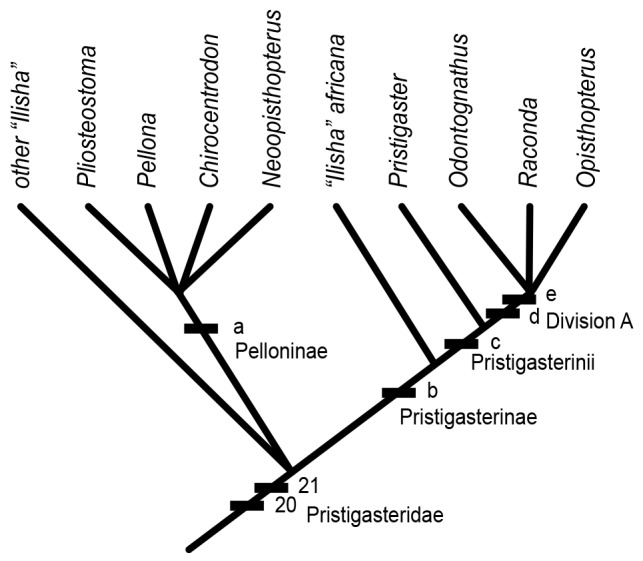
Fig. 1. Cladogram of pristigasterid fishes based on morphological characters (modified from Grande 1985). List of characters (reproduced from Grande, 1985): character 20: “Predorsal bones oriented either vertically or inclined anterodorsally”; character 21: “Loss of interlobar notch in third hypural of caudal skeleton”; character a: “Maxillary- premaxillary gap covered by bone”; character b: “Presence of a bony process on the first pleural rib which articulates with the shoulder girdle”; character c: “Loss of pelvic fin”; character d: “More than 23 predorsal bones”; character e: “More than 57 anal pterygiophores.”
The monophyly of the Pristigasteridae is supported by several morphological synapomorphies from the structures of the gill arches, pre-dorsal bones, and caudal fin (Nelson 1967; Grande 1985) and molecular characters (Bloom and Lovejoy 2014), but its phylogenetic position relative to the four other clupeoid families is still being debated (Grande 1985; Di Dario 2002; Li and Ortí 2007; Miyashita 2010; Lavoué et al. 2013; Bloom and Lovejoy 2014).
The current phylogenetic hypothesis and classification of the family Pristigasteridae proposed by Grande (1985) (Fig. 1) need to be evaluated for two reasons. First, while the diagnoses for the six monospecific or species- poor (≤ 3 species) genera are straightforward (Norman 1923; Berry 1964; Whitehead 1985), the largest genus, Ilisha, which includes almost half of all pristigasterid species, is poorly diagnosed because a synapomorphy is absent. Some authors, including Grande (1985), cast doubt about the monophyly of Ilisha, arguing that it merely represents a grade (Whitehead 1985; de Pinna and Di Dario 2003). However, no recent taxonomic revision of this genus or new hypothesis on the Pristigasteridae phylogeny has been published. Second, some of the characters used by Grande (1985) to support clades above the level of genus either conflict with others or are absent (i.e., the absence of the pelvic fin conflicts with the presence of post-coelomic extensions) (Fig. 1).
Whereas the external morphology and osteology of species of Ilisha and several other pristigasterids offer limited information regarding their systematics, the morphology of their swimbladder is highly specialized and variable among species (Talwar and Whitehead 1971; Seshagiri Rao 1975, 1976; Whitehead and Blaxter 1989). The swimbladders of teleosts serve several important functions, such as regulating buoyancy or maintaining the hydrostatic position in the water column (Whitehead and Blaxter 1989; Pelster 2011). Specialization of Pristigasteridae swimbladders may have additional purposes such as sound production, reception, or amplification (Allen et al. 1976; Wahlberg and Westerberg 2003; Wilson et al. 2004; Yan 2004; Wang et al. 2017), although there is no direct evidence for such functions (Whitehead and Blaxter 1989).
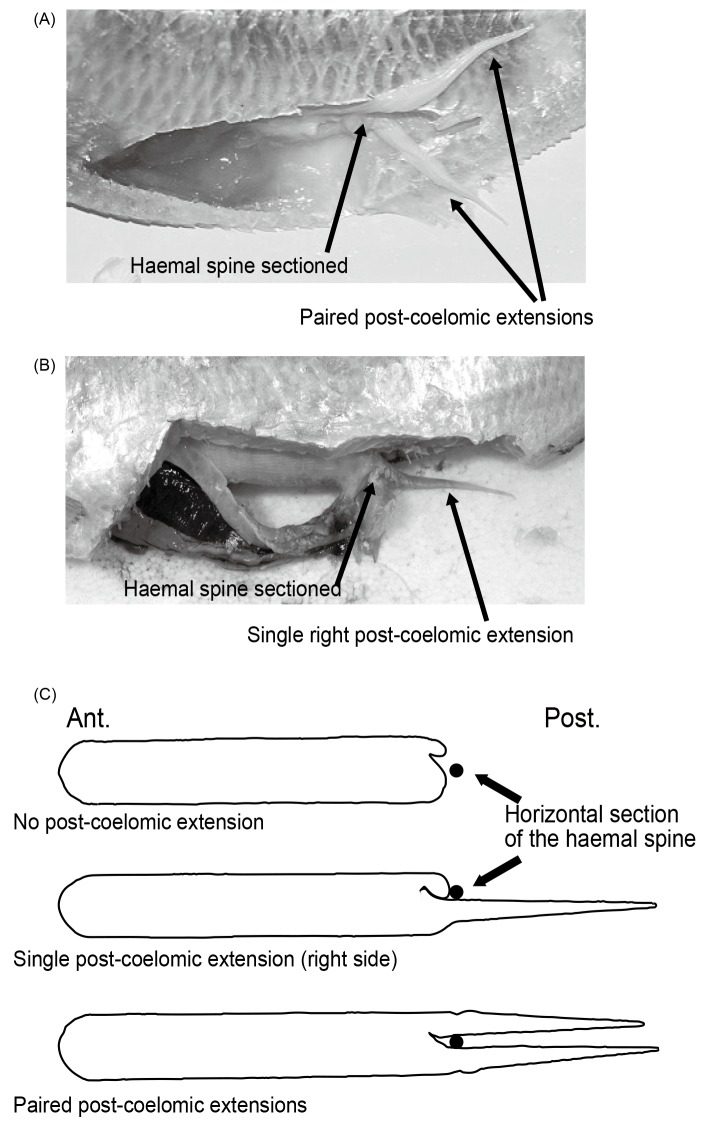
The most striking specialization in the swimbladders of most Indo-West Pacific (IWP) pristigasterid species is the one or two post- coelomic extensions (Valenciennes 1847; de Beaufort 1909), which is seen as a rare condition throughout the Teleostei (Poll 1969; Tominaga et al. 1996). In six IWP species of Ilisha (I. kampeni, I. lunula, I. compressa, I. melastoma, I. obfuscata, and I. striatula), IWP species of Opisthopterus and Raconda, and the West African I. africana, the posterior end of the swimbladder divides into two extensions (= diverticula) that pass backward on either side of the haemal spine and anal pterygiophores (Fig. 2A) (Kailola 1986; Whitehead and Blaxter 1989; Randall 1994). The diverticula lie just above the distal tips of the anal pterygiophores and extend to the anal fin. A second condition is found only in five IWP species of Ilisha (I. elongata, I. filigera, I. megaloptera, I. macrogaster, and I. pristigastroides). In these species, there is only one diverticulum, always on the right side of the body (Fig. 2B). In the third and last condition, which is the most common, the swimbladder has no post-coelomic diverticulum. This condition is found in Pellona, five endemic New World genera (Pliosteostoma, Chirocentrodon, Neoopisthopterus, Odontognathus, and Pristigaster), all New World representatives of Ilisha and Opisthopterus, as well as in two IWP species of Ilisha (I. sirishae and I. novacula).
Fig. 2.
Fig. 2. Posterior end of swimbladder showing post-coelomic diverticula. (A) Ilisha melastoma, dissected on the left side (12 cm standard length) showing the presence of two post-coelomic diverticula; (B) Ilisha elongata, dissected on the left side (42 cm standard length) showing the presence of one post-coelomic diverticulum; (C) Three diagrams (ventral view) summarizing the three conditions regarding to the presence (versus absence) and number (one or two) of post-coelomic extensions in Pristigasteridae.
The presence or absence of these post- coelomic diverticula contradict the current classification because species with diverticula are found both within and outside of the genera Ilisha and Opisthopterus. Whitehead and Blaxter (1989) noted this disagreement and admitted that these two genera could be split based on features of their swimbladders, regardless of their overall external similarity. Those authors, however, did not modify the classification. Whitehead and Blaxter (1989) observed that all New World species have swimbladders that lack post-coelomic diverticula, while species with one or paired post-coelomic diverticula are only found in the IWP region along with the West African species I. africana (Table 1).
Table 1. List of species of Pristigasteridae (21), specimens (45) and molecular markers (with GenBank accession numbers) examined in this study (abbreviations: GB, GenBank; COI, cytochrome oxidase I; cytb, cytochrome b; rag1, recombination activating gene 1; rag2 recombination activating gene 2). GenBank accession numbers of newly determined sequences in this study are in bold. “/” indicates that the respective sequence is not determined. Generic classification of Pristigasteridae follows Whitehead (1985).
In this study, we examined the evolution of the swimbladder structure in the Pristigasteridae to determine whether the presence of post-coelomic diverticula is derived. For that, we reconstructed the most comprehensive, molecular-based phylogenetic tree of the Pristigasteridae to date, paying special attention to IWP species. We then used this tree to reconstruct and discuss the origin and evolution of post-coelomic extensions and, finally, we comment on the classification of the Pristigasteridae.
MATERIALS AND METHODS
Taxonomic and character samplings
For this study, we obtained novel nucleotide sequences from 33 specimens of longfin herrings (family Pristigasteridae) of 11 IWP species which were collected from local fish markets in Taiwan, Malaysia, Thailand, and Sri Lanka (Table 1). We dissected most specimens to examine the morphology of the swimbladder and report on the presence or absence of post-coelomic diverticula as described by Whitehead and Blaxter (1989). For each specimen, a muscle sample was taken and preserved in 95% ethanol.
To build our character sampling, we selected the following four mitochondrial genes as markers to reconstruct the phylogeny of the Pristigasteridae: the partial 12S ribosomal (r)RNA (about 600 base pairs [bp]) and 16S rRNA (about 600 bp), the partial cytochrome oxidase I (COI; 650 bp), and the complete cytochrome b (1140 bp). Our character sampling was completed with the addition of the partial nuclear genes, Rag1 (about 1200 bp) and Rag2 (about 1100 bp).
We combined our data with previously published data from 12 specimens of ten species, mostly from the New World (Lavoué et al. 2007; Lavoué et al. 2013; Bloom and Lovejoy 2014). In total, our dataset included 5296 characters and 45 specimens from 21 species belonging to seven genera (only the New World genera Pliosteostoma and Neoopisthopterus are missing). Coilia nasus, an anchovy, was used to root the phylogenetic trees.
DNA extraction, amplification, and sequencing
Total genomic DNA of each tissue sample was extracted using the LabTurbo DNA Mini Kit LGD480-220 (TAIGEN Bioscience, Taipei, Taiwan), a semi-automatic method, following the manufacturer’s protocol. We then used the polymerase chain reaction (PCR) technique to amplify the targeted nucleotide markers from the extracted DNA. For each marker, the following PCR primer pairs were used (the names and sequences of the reverse primers are indicated in italics): 12S_229F (5’-GYCGGTAAAAYTCGTGCCAG-3’) and 12S_954R (5’-YCCAAGYGCACCTTCCGGTA-3’) (Li and Ortí 2007); 16S_135F (5’-GCAATAGAVAWA GTACCGCAAGG-3’) and 16S_1072R (5’-CCTTYGCACGGTYARAATAC-3’) (Li and Ortí 2007); Cytb_AnchF (5’-TGACTTGAAA AACCACCGTTGTTATTCAAC-3’) and Cytb_ AnchR (5’-CTAGCTTTGGGAGYTAGDGGTG GRAGTT-3’) (Bloom and Lovejoy 2012); COI_ FishF1 (5’-TCAACCAACCACAAAGACATTGG CAC-3’) and COI_FishR2 (5’-ACTTCAGGGTG ACCGAAGAATCAGAA-3’) (Ward et al 2005); newly designed primer pair RAG1_PrisF (5’- CAGATCTTCCAGCCCCTGCACACC-3’) and RAG1_PrisR (5’- AGTTGGTGATCTTGCCGTCGT AGCGG-3’); and newly designed primer pair RAG2_PrisF (5’-TCAAGCTGCGCCCCATCTCCTT CTCCA-3’) and RAG2_PrisR (5’-CTTCATGCACT GGGCGTGGACCCA-3’).
PCR products were purified using the AMPure magnetic bead cleanup protocol (Agencourt Bioscience, Taipei, Taiwan) before being sent for sequencing in both directions (forward and reverse) at the Center of Biotechnology (National Taiwan University), using the Sanger sequencing method (Sanger et al. 1977).
To check the quality of the chromatograms, we used the software 4Peaks vers. 1.7 (available at http://nucleobytes.com/4peaks/). The consensus sequence of each gene and each specimen was built from the forward and reverse sequences using the software CodonAligner 3.7.2 (Codoncode, Dedham, MA, USA).
For each marker (i.e., 12S, 16S, COI, cytochrome b, Rag1 and Rag2), we built a matrix “specimens*characters” that was used for subsequent phylogenetic analyses. Alignments of the protein-coding genes, cytochrome b, COI, rag1 and rag2, were straightforward. The 12S and 16S rRNA genes were separately aligned with the software Proalign vers. 0.5 (Löytynoja and Milinkovitch 2003) using default parameter settings. Regions with posterior probabilities of ≤ 90% were excluded from the alignments.
Phylogenetic analyses
We inferred partitioned maximum likelihood (ML) phylogenetic trees using the software RAxML (Stamatakis 2006) from the previously built combined six-marker matrix. PartitionFinder vers. 1.1.1 (Lanfear et al. 2012) was used to calculate the best partition scheme from seven basic partitions (i.e., the first, second, and third codon positions of the two combined mitochondrial coding-protein genes plus the first, second, and third positions of the two combined nuclear coding- protein genes plus the concatenated 12S/16S rRNAs). A four-partition scheme was inferred. We then performed ML heuristic phylogenetic searches under the general time-reversible model with discrete gamma-distributed rate heterogeneity [GTR + Γ] applied to each of the four partitions. We executed 100 searches and found the best ML tree by comparing the final likelihoods among the 100 inferred trees. To evaluate the robustness of the internal branches of the ML tree, 1000 bootstrap replicates were calculated for each matrix using the GTR + Γ model.
Statistical tests for alternative topologies
The likelihoods of different topologies, in which the monophyly of different taxonomic groups is separately constrained, were evaluated using the Shimodaira-Hasegawa (SH) and approximately unbiased (AU) tests as implemented in the program CONSEL vers. 0.1j (Shimodaira and Hasegawa 2001; Shimodaira 2002). First, a four-partition ML tree search of the combined dataset was performed using RAxML and the GTR + Γ model, in which we a priori constrained the monophyly of different groups (the constrained topology tree was first built using Mesquite vers. 3.31; Maddison and Maddison 2009). Site-wise log-likelihoods of the constrained and unconstrained topologies were calculated with RAxML (-f g option in RAxML), and p values were finally calculated with CONSEL.
Reconstruction of swimbladder evolution
We reconstructed the evolution of the presence and number (one or two) post- coelomic diverticula of the swimbladder onto our ML phylogenetic tree, using a symmetrical one-rate (“Mk1”) model of character evolution. This model assumes that transitions between each morphological condition occurred at the same rate. Character state reconstructions were performed using Mesquite vers. 3.31 (Maddison and Maddison 2009). Data on the presence and number of post-coelomic diverticula of the swimbladder of the Pristigasteridae at the species level are summarized in appendix 1 and were assembled from our direct observations and from Whitehead and Blaxter (1989) (see their Table 3, page 353). We coded three conditions: the absence of a diverticulum (coded “0”), the presence of only one diverticulum that always lies on the right side (coded “1”), and the presence of two diverticula (coded “2”).
RESULTS
Phylogenetic structure
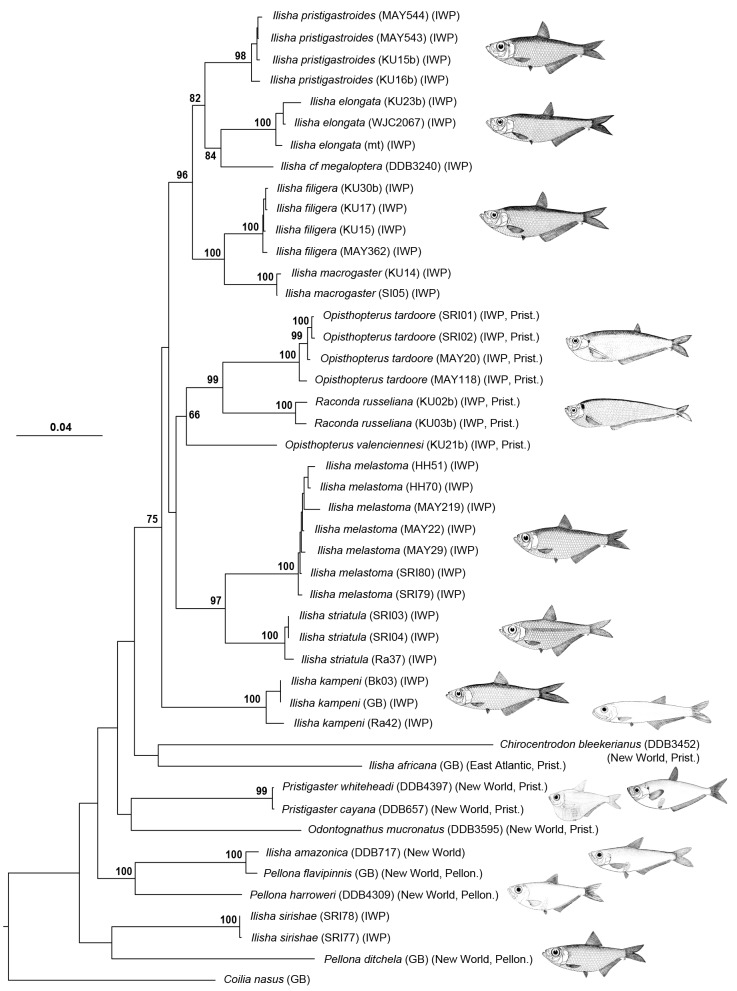
The ML phylogenetic tree is shown in figure 3. This tree was fully resolved, but several relationships - mostly at the base of the tree, among New World taxa - were not well supported by bootstrap proportions (BPs) of > 75%. Each species represented by more than one specimen was monophyletic. This tree conflicted with the current classification of the Pristigasteridae in several aspects. None of the three main lineages identified by Grande (1985) (Fig. 1), i.e., the Pelloninae, Pristigasterinae, and Ilisha (minus I. africana), were monophyletic. Our results showed that the genus Ilisha did not form a monophyletic group because 1) I. sirishae, I. africana and I. amazonica were only distantly related to other species of Ilisha and 2) Raconda russelliana and the two species of Opisthopterus were nested within the IWP species of Ilisha (minus I. sirishae). The genera Pellona and Opisthopterus were also not respectively monophyletic relative to the remaining pristigasterids or to R. russelliana.
Fig. 3.
Fig. 3. Maximum likelihood phylogenetic tree of Pristigasteridae. Coilia nasus is used to root the tree. Bootstrap proportions are indicated at nodes when > 65%. Branch lengths are proportional to number of substitutions. Generic classification follows Whitehead (1985). The specimen code of each specimen is indicated after its species name, followed by its geographic distribution (Indo-West Pacific [IWP] or East Atlantic or New World) and its subfamily assignment as established by Grande (1985) (Pritigasterinae [Prist.] or Pelloninae [Pellon.] or no subfamily). Fish illustrations Source: Whitehead (1985).
In contrast to the current classification, we found support (BP = 88%) for the monophyly of a large group including all IWP pristigasterid species (except Ilisha sirishae and Pellona ditchela). This group was the sister group of a clade comprising the West African I. africana and New World Chirocentrodon bleekerianus. The phylogenetic position of C. bleekerianus should be considered with caution, because only 16S rRNA was sampled and its terminal branch was significantly longer than those of other species. When C. bleekerianus was excluded from the dataset (data not shown), I. africana was sister of the IWP pristigasterid species; this clade was supported by a BP of 60%.
The SH and AU tests statistically rejected the monophylies of Ilisha, Opisthopterus, Pellona, the Pelloninae, and Pristigasterinae, but they did not reject the hypothesis that the IWP Ilisha (minus I. sirishae), Raconda, and Opisthopterus along with I. africana form a monophyletic group.
Reconstruction of swimbladder morphology evolution
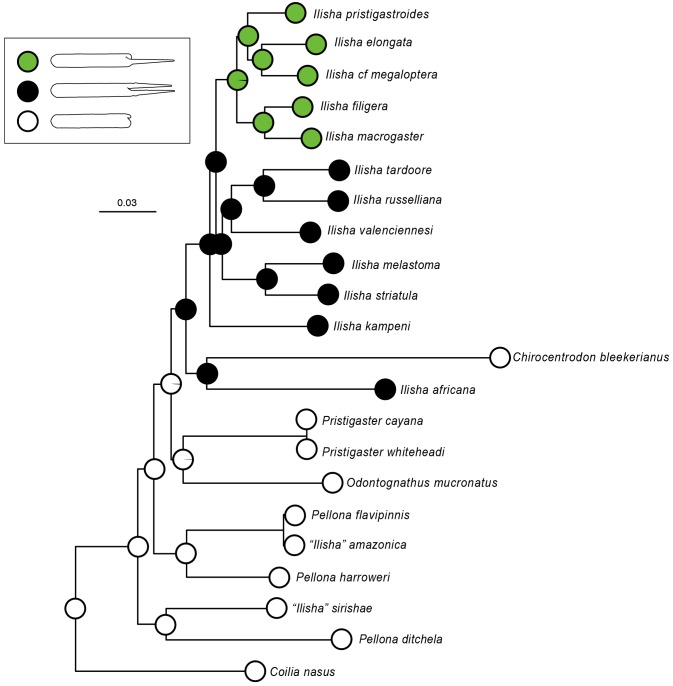
The ancestral state reconstruction of the swimbladder morphology onto our ML tree (Fig. 4) showed that the absence of post-coelomic diverticula was the ancestral condition in the Pristigasteridae. The presence of paired post- coelomic diverticula originated later, most likely once in the ancestor of the clade ((C. bleekerianus, I. africana), IWP taxa), followed by one complete reversal in C. bleekerianus. When C. bleekerianus was excluded from the dataset (see previous section), the reconstruction strongly supported the hypothesis that the presence of paired post- coelomic diverticula evolved only once with no reversion. Finally, the presence of only one diverticulum in some species of Ilisha evolved once from a condition of paired post-coelomic diverticula, after the loss of the left diverticula.
DISCUSSION
Phylogenetic relationships
We reconstructed the most comprehensive molecular-based phylogeny of Pristigasteridae (longfin herrings) in order to examine the evolution of its swimbladder and discuss previous phylogenetic hypotheses. Longfin herrings were early recognized as a natural subgroup (tribe or subfamily) of the family Clupeidae because of their distinctive long anal fin (Günther 1868; Norman 1923; Berry 1964; Nelson 1967 1970). Nelson (1967) noted that “in terms of gill-arch structure, the Dussumieriinae and Pristigasterinae appear to be the most primitive” and, consequently, Nelson (1970) removed the Pristigasterinae from the Clupeidae and elevated it to the family rank (i.e., the Pristigasteridae). The phylogenetic position of the Pristigasteridae relative to other clupeoid families was left unresolved by Nelson (1970), and it is still being debated (Di Dario 2002; Li and Ortí 2007; Miyashita 2010; Lavoué et al. 2013; Bloom and Lovejoy 2014). Since the recent taxonomic reassignment of the Late Cretaceous-Paleocene[?] fossil †Gasteroclupea branisae from Bolivia to the extinct order †Ellimmichthyiformes (Marramà and Carnevale 2017), there are no more pristigasterid fossils and, consequently, no direct paleontological information available on the evolution of these fishes.
Prior to this work, only one phylogenetic hypothesis of the Pristigasteridae based on morphological characters was published (Grande 1985) (Fig. 1), in which the family Pristigasteridae [= Pristigasteroidea in Grande (1985)] was divided into three groups: the Pelloninae [= Pellonidae in Grande (1985)], the Pristigasterinae [= Pristigasteridae in Grande (1985), which also includes I. africana], and the genus Ilisha (minus I. africana). According to Grande (1985), the Pelloninae was supported by one character, the “maxillary-premaxillary gap covered by bone”, whereas the subfamily Pristigasterinae was supported by the presence of a “bony process on the first pleural rib which articulates with the shoulder girdle”. No character was given for the genus Ilisha (minus I. africana), and relationships among these three groups were left unresolved. de Pinna and di Dario (2003) briefly commented on the phylogeny of the Pristigasteridae, and they mentioned that the Pelloninae and Pristigasterinae are likely not reciprocally monophyletic. Bloom and Lovejoy (2014) examined the molecular phylogeny of 11 pristigasterid species, eight of them from the New World. They found I. africana to be sister to the rest of the Pristigasteridae, and the two species of IWP Ilisha were deeply nested within the New World lineages, making this group paraphyletic.
Although our taxonomic sampling is still incomplete, with two missing New World genera, Pliosteostoma and Neoopisthopterus classified in the subfamily Pelloninae, along with some other New World species (such as those belonging to the genus Opisthopterus), our results (see ML tree on Fig. 3) refute the phylogenetic hypothesis of Grande (1985) and expand the results of Bloom and Lovejoy (2014) to support the non-monophyly of several taxa. We found that species of Opisthopterus and Raconda are nested within IWP species of Ilisha and do not form a monophyletic group with Odontognathus and Pristigaster. This means that the loss of pelvic fins, which supported the monophyly of these four genera in Grande’s (1985) phylogenetic hypothesis (Fig. 1), evolved independently, at least twice, in the family Pristigasteridae.
We confirm that I. africana forms a distinct lineage, only distantly related to other species of Ilisha. The other species of Ilisha, however, still do not form a monophyletic group (see Fig. 3) because: 1) I. amazonica is more closely related to some New World species of Pellona than to IWP species of Ilisha, 2) I. sirishae is more closely related to Pellona ditchela than to other IWP species of Ilisha, and 3) Raconda and Opisthopterus are nested within IWP species of Ilisha (minus I. sirishae), making this group paraphyletic.
Evolution of the swimbladder morphology
The swimbladder is a visceral gas-filled chamber that serves several important functions, such as buoyancy, floatability, and hydrostatic positioning. The morphology of the Clupeoidei’s swimbladder is overall quite uniform with only a few specializations, except in the family Pristigasteridae where the swimbladder is both specialized and variable among species. Whitehead and Blaxter (1989) wrote that “of the [five] clupeoid families, the pristigasterids show the most striking specializations of swimbladder form, with their post-coelomic diverticula, muscular tunica interna and pleural rib pockets”. Among these three specializations, the presence of post-coelomic diverticula in some species of the Pristigasteridae was noted as early as the mid- 19th Century (Valenciennes 1847), and this condition appears to be rare within the Teleostei (Poll 1969; Tominaga et al. 1996). The function of these diverticula is unknown. The presence of these extensions in only some species of the Pristigasteridae is especially intriguing, because the ecology and biology of all species of the Pristigasteridae seem similar (Blaber et al. 1998). So far, there is no reported ecological difference associated with the presence or absence of the diverticula, but more studies are needed.
Seshagiri Rao (1975 1976) [but see also Talwar and Whitehead (1971)] was among the first authors who considered the presence/absence and the number of post-coelomic diverticula in the Pristigasteridae as taxonomic characters. That author distinguished three groups among IWP Ilisha species, which are otherwise morphologically very similar. Seshagiri Rao (1975 1976), however, did not indicate whether this character, alongside its taxonomic value within Ilisha, could be used within the Pristigasteridae to define monophyletic groups. Later, Whitehead and Blaxter’s (1989) review of the morphology of the swimbladder of the Clupeoidei explicitly stated that this character potentially has some phylogenetic importance in the Pristigasteridae, but those authors did not reorganize the phylogeny and classification based on this.
We showed that the most ancestral condition in the Pristigasteridae is the absence of post- coelomic diverticula (Fig. 4); this is also the most common condition within the Clupeoidei and Teleostei (Poll 1969; Tominaga et al. 1996). Thus, our ancestral character state reconstruction supports the hypothesis of only one evolutionary origin for the presence of post-coelomic diverticula in the Pristigasteridae, challenging the current phylogeny and classification and confirming the hypothesis of Whitehead and Blaxter (1989). Whether the absence of diverticula in Chirocentrodon is a consequence of a reversal event should be further investigated for three reasons (which might not be independent of each other): 1) our data do not statistically reject the phylogenetic hypothesis that I. africana is sister to the IWP clade (instead of being sister to Chirocentrodon); 2) only the 16S rRNA gene is available for Chirocentrodon; and 3) the phylogenetic branch of Chirocentrodon is significantly longer than the others. Finally, one further specialization of the swimbladder occurred within the IWP clade with the loss of the left diverticulum in five species of Ilisha, making this condition the most derived one in the Pristigasteridae.
Fig. 4.
Fig. 4. Reconstruction of the evolution of post-coelomic diverticula within the Pristigasteridae. Ancestral conditions [no post coelomic extension (white), paired post-coelomic extensions (black) and single post coelomic extension (green)] at nodes reconstructed using a maximum likelihood method of ancestral character inference under the model “Mk1.” Pie charts show likelihood support for ancestral habitat states (sum = 1) for the corresponding nodes. Note that the phylogenetic position of Chirocentrodon bleekerianus sister to Ilisha africana is not supported and additional data are needed to confidently resolve it. Classification is revised according to our results (see DISCUSSION).
Implications for classification of the Pristigasteridae
Characters from the visceral morphology of the Teleostei are not frequently used in systematics, although they potentially contain as much phylogenetic information as other characters. This is particularly true in the Clupeoidei, where the visceral morphology has only been used to distinguish two closely related clupeid genera, Sprattus and Clupea (absence versus presence of a pterotic bulla) or in the taxonomy of the “gizzard” shads [gut anatomy; (Nelson and Rothman 1973)].
The presence of post-coelomic diverticula in the Pristigasteridae was noted a long time ago, but never considered a phylogenetic character until Whitehead and Blaxter (1989) suggested it. Considering the work of Whitehead and Blaxter (1989), our own phylogenetic results, and the necessity to establish a phylogenetic classification for the Pristigasteridae, we herein propose to diagnose the genus Ilisha Richardson 1846 (type species: I. elongata) by the presence of one or two post-coelomic diverticula in the swimbladder. Therefore, the new definition of Ilisha includes all IWP pristigasterid species from the genera Raconda, Opisthopterus, and Ilisha (except I. sirishae and I. novacula, which do not have such a post-coelomic extension), and the East Atlantic I. africana. Raconda and Opisthopterus are synonymized with Ilisha according to the new definition. The New World species of Ilisha (I. amazonica and I. furthii) and Opisthopterus (four species) along with IWP I. sirishae and I. novacula are left insertae sedis within the Pristigasteridae until a more-complete systematic study of the New World species can be undertaken.
This phylogenetic-based modification classifying the Pristigasteridae has several advantages: 1) it clarifies the taxonomic situation of the genus Ilisha, particularly the situation of IWP species of Ilisha; 2) it minimizes the number of changes without the need to erect several new genera for species-poor lineages that are problematic to diagnose; and 3) it simplifies the taxonomic revision of New World pristigasterid species of “Ilisha” and “Opisthopterus.”
Finally, we underscore the need to revise the classification of the genus Pellona, especially that of the two Indo-West Pacific species, P. ditchela and P. dayi. However, a denser taxonomic sampling among New World taxa is necessary to achieve this goal.
CONCLUSIONS
Our phylogenetic results show that the subfamilies Pristigasterinae and Pelloninae and the genera Ilisha, Pellona, and Opisthopterus are not monophyletic, leading to the conclusion that the classification of the family Pristigasteridae must be revised. We found that the presence of post- coelomic diverticula in the swimbladder represents a derived condition, and it is phylogenetically conserved with a unique origin. Furthermore, species of Ilisha with only one diverticulum in their swimbladder secondarily evolved from a common ancestor that had paired diverticula. Given the multiple functions of the swimbladder in the Teleostei, it is likely that the presence of one or two diverticula in the Pristigasteridae is associated with some function that remains to be determined. Further studies should expand taxonomic sampling within the Pristigasteridae and investigate the function of swimbladder specializations in these fishes, which otherwise exhibit similar life traits and histories. Finally, we formalized the taxonomic implication of our phylogenetic results in re- diagnosing the genus Ilisha by the presence of post-coelomic diverticula in the swimbladder.
Supplementary materials
List of all species of Pristigasteridae (38 species) along with corresponding information concerning their distribution and the absence (“No”) or presence of one (“1”) or two (“2”) post-coelomic diverticula. Data from Whitehead and Blaxter (1989) and Eschmeyer et al. (2018). Classification follows Grande (1985); see figure 1. (download)
Acknowledgments
Acknowledgments: This study was supported by research grants MOST103-2119-M-002-019- MY3 and MOST106-2119-M-002-032 from the Ministry of Science and Technology of Taiwan. We express our gratitude to Drs. Pei-Chun Lo and Jhen-Nien Chen (IONTU, Taipei) for sharing samples and their help with the molecular biology. Two anonymous reviewers and editor Hin-Kiu Mok provided constructive comments that improved the manuscript.
Footnotes
Authors’ contributions: SL designed the study. SR, H-CH, W-JC, SAMN performed the field work. SL performed molecular and analytical work and wrote the original draft. All authors contributed to revising the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no competing interests.
Availability of data and materials: Sequence data are available in GenBank (www.ncbi. nlm. nih.gov) under accession numbers MH325506- MH325649.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicabl
References
- Allen J M, Blaxter Jhs, Denton E J. The functional anatomy and development of the swimbladder-inner earlateral line system in herring and sprat. J Mar Biol Assoc Uk. 56:471–486. [Google Scholar]
- Berry F H. Review and emendation of: Family Clupeidae. Copeia. pp. 720–730.
- Blaber Sjm, Staunton-Smith J, Milton D A, Fry G, Van Der Velde T, Pang J, Wong P, Boon-Teck O. Bloom DD, Lovejoy NR. 2012. Molecular phylogenetics reveals a pattern of biome conservatism in New World anchovies (family Engraulidae) Estuar Coast Shelf S. 47:701–715. doi: 10.1111/j.1420-9101.2012.02464.x. [DOI] [PubMed] [Google Scholar]
- Bloom D D, Lovejoy N R. The evolutionary origins of diadromy inferred from a time-calibrated phylogeny for Clupeiformes (herring and allies) Proc R Soc B: Biol Sci; 2014. 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaufort L F. Die Schwimmblase der Malacopterygii. Morphologisches Jahrbuch. 39:526–644. [Google Scholar]
- D I D A R I O F M C C. 0 0 3 . P r i s t i g a s t e r i d a e (Pristigasterids) pp. 43–45.
- Dario Di. Evidence supporting a sister-group relationship between Clupeoidea and Engrauloidea (Clupeomorpha) Copeia. pp. 496–503.
- Eschmeyer W N, Fricke R, Van Der Laan R. Catalog of Fishes electronic version.
- Grande L. Recent and fossil clupeomorph fishes with materials for revision of the subgroups of clupeoids. Bull Amer Mus Nat Hist. 181:231–372. [Google Scholar]
- Günther A. Catalogue of the Fishes in the British Museum. London): Taylor and Francis; 1868. [Google Scholar]
- Kailola P J. A new species of clupeid fish (Pisces: Pristigasteridae) from Northern Australia and Papua. Beagle: Rec Mus Art Gal Northern Terr. 3:51–57. [Google Scholar]
- Lanfear R, Calcott B, Ho Syw, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Lavoué S, Konstantinidis P, Chen W-J. Progress in clupeiform systematics. In: Ganias K (ed) Biology and ecology of anchovies and sardines. Science Publishers. pp. 3–42.
- Lavoué S, Miya M, Musikasinthorn P, Chen W-J, Nishida M. Mitogenomic evidence for an Indo-West Pacific origin of the Clupeoidei (Teleostei: Clupeiformes) PLoS ONE; 2013. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoué S, Miya M, Saitoh K, Ishiguro N B, Nishida M. Phylogenetic relationships among anchovies, sardines, herrings and their relatives (Clupeiformes), inferred from whole mitogenome sequences. Mol Phylogenet Evol. 43:1096–1105. doi: 10.1016/j.ympev.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Li C, Ortí G. Molecular phylogeny of Clupeiformes (Actinopterygii) inferred from nuclear and mitochondrial DNA sequences. Mol Phylogenet Evol. 44:386–398. doi: 10.1016/j.ympev.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Löytynoja A, Milinkovitch M C. A hidden Markov model for progressive multiple alignment. Bioinformatics. 19:1505–1513. doi: 10.1093/bioinformatics/btg193. [DOI] [PubMed] [Google Scholar]
- Maddison W P, Maddison D R. 6 4 , a freshwater double-armored herring (Clupeomorpha, Ellimmichthyiformes) from the Late Cretaceous-Paleocene of South America. Hist Biol. 29:904–917. [Google Scholar]
- Miyashita T. Unique occipital articulation with the first vertebra found in pristigasterids, chirocentrids, and clupeids (Teleostei: Clupeiformes: Clupeoidei) Ichthyol Res. 57:121–132. [Google Scholar]
- Nelson G J. Gill arches of teleostean fishes of the family Clupeidae. Copeia. pp. 389–399.
- Nelson G J. The hyobranchial apparatus of teleostean fishes of the families Engraulidae and Chirocentridae. Am Mus Novit. 2410:1–30. [Google Scholar]
- Nelson G J, Rothman M N. The species of gizzard shads (Dorosomatinae) with particular reference to the IndoPacific region. Bull Amer Mus Nat Hist. 150:131–206. [Google Scholar]
- Norman J R. A revision of the Clupeid fishes of the genus Ilisha and allied genera. Ann Mag Nat Hist. 11:1–22. [Google Scholar]
- Pelster B. Buoyancy, locomotion, and movement in fishes: Swimbladder function and buoyancy control in fishes. Academic Press. pp. 526–534.
- Poll M. Le prolongement caudal de la vessie hydrostatique des poissons actinoptérygiens. Bull Acad R. 55:486–505. [Google Scholar]
- Randall J E. Ilisha compressa, a new species of clupeid fish from the Persian Gulf. Raffles Bull Zool. 42:893–899. [Google Scholar]
- Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci. 74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Seshagiri. A new species of clupeid fish, Ilisha sirishai from Visakhapatnam. India. Hydrobiologia. 47:463–468. [Google Scholar]
- Rao Seshagiri. Notes on the Indo-West Pacific species of clupeid fish genus Ilisha, with a key to their identification. Copeia. 1976:503–509. [Google Scholar]
- Shao K T, Ho H C, Lin P L, Lee P F, Lee M Y, Tsai C Y, Liao Y C, Lin Y C, Chen J P, Yeh H M. A checklist of the fishes of Southern Taiwan. Raffles B Zool. 19:233–271. [Google Scholar]
- Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst Biol. 51:492–508. doi: 10.1080/10635150290069913. [DOI] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 17:1246–1247. doi: 10.1093/bioinformatics/17.12.1246. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihoodbased phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Talwar P K, Whitehead Pjp. The clupeoid fishes described by Francis Day. Nat Hist) Zool. 22:59–85. [Google Scholar]
- Tominaga Y, Sakamoto K, Matsuura H. Posterior extension of the swimbladder in percoid fishes, with a literature survey of other teleosts. The University Museum. The University of Tokyo Bulletin. 36:1–73. [Google Scholar]
- Valenciennes A. Histoire naturelle des poissons. P. Bertrand; 1847. [Google Scholar]
- Wahlberg M, Westerberg H. Sounds produced by herring (Clupea harengus) bubble release. Aqua Living Resour. 16:271–275. [Google Scholar]
- Wang Z T, Nowacek D P, Akamatsu T, Wang K X, Liu J C, Duan G Q, Cao H J, Wang D ;, Ward R D, Zemlak T S, Innes B H, Last P R, Hebert Pdn. Diversity of fish sound types in the Pearl River Estuary. Philos T R Soc B. 5:1847–1857. doi: 10.7717/peerj.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead Pjp. Clupeoid fishes of the World (Suborder Clupeoidei): An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf herrings. Part 1. Chirocentridae, Clupeidae and Pristigasteridae. FAO Fish Synop. 125:1–303. [Google Scholar]
- Whitehead Pjp, Blaxter Jhs. Swimbladder form in clupeoid fishes. Zool J Linn Soc B. pp. 299–372.
- Whitehead Pjp, Nelson G J, Wongratana T. Clupeoid fishes of the World (Suborder Clupeoidei): An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf herrings. Part 2. Engraulididae. FAO Fish Synop. 125:305–579. [Google Scholar]
- Wilson B, Batty R S, Dill L M. Pacific and Atlantic herring produce burst pulse sounds. Proc R Soc B (Supplement) 271:95–97. doi: 10.1098/rsbl.2003.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H Y. The role of gas-holding structures in fish hearing: an accoustically evoked potentials approach. Springer Science+Business Media. pp. 189–209.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of all species of Pristigasteridae (38 species) along with corresponding information concerning their distribution and the absence (“No”) or presence of one (“1”) or two (“2”) post-coelomic diverticula. Data from Whitehead and Blaxter (1989) and Eschmeyer et al. (2018). Classification follows Grande (1985); see figure 1. (download)