Abstract
Takatoshi Higuchi, Shun Watanabe, Ryotaro Manabe, Tsuyoshi Kaku, Akihiro Okamura, Yoshiaki Yamada, Michael J. Miller, and Katsumi Tsukamoto (2018) Japanese eels Anguilla japonica were tagged in order to understand their behavior in their spawning area. Three silver eels (EEL-A, B, C: TL792, 898, 992 mm) were tagged with pop-up satellite archival transmitting tags (PSATs) and released at different locations near/in their spawning area along the southern part of the West Mariana Ridge. EEL-A showed premature tag pop-up with mostly disordered records and the EEL-C tag did not pop up, while EEL-B showed stable diel vertical migrations during 31 of the 43 days it was tracked. EEL-B swam in shallower layers (411-182 m) during nighttime and deeper layers (563-885 m) during daytime. The mean nighttime swimming depth ± SD of EEL-B was significantly deeper during the full moon (342.4 ± 6.8 m) than the new moon (274.8 ± 16.9 m) and was positively correlated with the moon’s altitude. EEL-B reached its maximum depths (851.1 ± 22.8 m) and minimum water temperatures (4.9 ± 0.1°C) during the sun culmination (sun at its highest point in the sky) of each day. The daytime water temperature varied between 4.7 and 5.2°C, staying at an almost constant 5°C. The eel started to dive to deeper water around nautical twilight (sun altitude: -11.6 ± 4.6°) and rise shallower around sunset (sun altitude: -0.8 ± 1.4°); sun altitude and swimming depth were correlated during the dives at dawn and ascents up at dusk. These results suggest that the regular diel vertical migrations of Japanese eels are strictly regulated by both light intensity and the lower limit of water temperature.
Keywords: Japanese eel, Spawning area, Diel vertical migration, Light intensity, Pop-up tag
BACKGROUND
The 19 species and subspecies of freshwater eels (genus Anguilla) (Ege 1939; Castle and Williamson 1974; Watanabe et al. 2009) are catadromous fishes that spawn in low latitude tropical oceans and transport their larva to coastal waters before entering their estuarine and freshwater growth habitats (Tesch 2003; Aoyama 2009). They are classified as temperate and tropical anguillid species, depending on the geographic distribution of their growth habitats. Because the growth habitats of temperate eels are located at higher latitudes, the migration distances between the growth habitats and a species- specific spawning area are longer in temperate eels than in tropical ones. The temperate eels change into silver eels with black-pigmented backs and silvery undersides as their sexual maturation begins (Schmidt 1923; Tesch 2003; Okamura et al. 2002; Han et al. 2003; Tsukamoto 2009) and they begin their downstream migration between fall and winter.
The spawning area of the Japanese eel Anguilla japonica was found to be located along the southern part of the West Mariana Ridge by collecting newly hatched larvae, spawning- condition adults and fertilized eggs (Tsukamoto et al. 2003 2011; Tsukamoto 2006; Chow et al. 2009; Aoyama et al. 2014). Their spawning activities take place during a period of a few days before the new moon (Ishikawa et al. 2001; Tsukamoto et al. 2003 2011).
The behavior and routes used by Japanese eels during spawning migrations are not understood yet (Matsui 1972; Tsukamoto 1994; Yokose 2008), although Tsukamoto (2009) reviewed several hypotheses about possible oceanic migration routes. These hypotheses were tested using numerical simulations (Chang et al. 2016), and it was suggested that the most likely migration strategy is the “true navigation” method toward the exact location of the spawning area. However, there have been no direct observations of their spawning migrations across the whole region from the growth habitats to the spawning area.
Acoustic tags and pop-up satellite archival transmitting tags (PSATs) have been effective in understanding the behavior of anguillid eels during their spawning migrations. Swimming behaviors of Japanese eels and European eels (A. anguilla) have been traditionally examined in coastal areas using acoustic tags, and they were found to exhibit diel vertical migration during their marine migrations in coastal waters (Tesch 1978 1989; Aoyama et al. 2002; McCleave and Arnold 1999). The swimming behaviors of Japanese and European eels over deep water have also been tracked using ultrasonic transmitters in the North Pacific and Atlantic, respectively (Fricke and Kaese 1995; Aoyama et al. 1999; Chow et al. 2015). However, the observation durations of the studies were too short to cover the entire period of their oceanic migrations. PSAT tags can record data for longer periods than acoustic tags that have been used previously. Up until now, PSAT tagging studies have been conducted on 7 species of the genus Anguilla, including A. deffenbachii (Jellyman and Tsukamoto 2002 2005 2010), A. anguilla (Aarestrup et al. 2009; Wahlberg et al. 2014; Westerberg et al. 2014; Wysujack et al. 2015; Righton et al. 2016), A. japonica (Manabe et al. 2011), A. rostrata (Béguer-Pon et al. 2012 2015), A. marmorata, A. megastoma and A. obscura (Schabetsberger et al. 2013 2015 2016). All of those studies have shown that the eels show distinct diel vertical migration behavior and swim at shallow depths at night and much deeper depths during the day. The swimming depths during each period (day and night) vary among species or location. Japanese eel tracking using PSAT tags started in 2008, when their diel vertical migrations were observed (Manabe et al. 2011). Chow et al. (2015) used ultrasonic transmitters to report Japanese eels had similar vertical movements and a daily rhythm adjacent to Japan and along the West Mariana Ridge using ultrasonic transmitters.
However, almost all of the studies that used the two types of tags could only record fragmental behavior in coastal or nearby offshore areas during the first half of the entire spawning migration period, except for Fricke and Kaese (1995), Aoyama et al. (1999), Wysujack et al. (2015) and Chow et al. (2015), which released and tracked adult eels in the spawning area for the respective species. Furthermore, diel vertical migrations were not observed in some A. anguilla and A. japonica individuals tracked for short periods in the spawning area that stayed in the shallow warm water (Fricke and Kaese 1995; Aoyama et al. 1999), although eels released in the studies by Wysujack et al. (2015) and Chow et al. (2015) made diel vertical migrations. Thus, the behaviors of freshwater eels in each spawning area are still not clear. In addition, the mechanisms regulating the swimming behavior of anguillid eels during their spawning migration remain unknown.
Therefore, more information is needed on the oceanic migration behaviors of eels in/near their spawning areas in order to understand all aspects of their spawning migrations. The objective of this study was to examine the behavior of A. japonica silver eels tagged with PSATs and released in/ near their spawning area in the summer of 2014. In this paper, we report their swimming behaviors in the spawning area in relation to environmental factors and discuss the mechanisms of diel vertical migration using the long-term data that was obtained.
MATERIALS AND METHODS
Eels and release sites
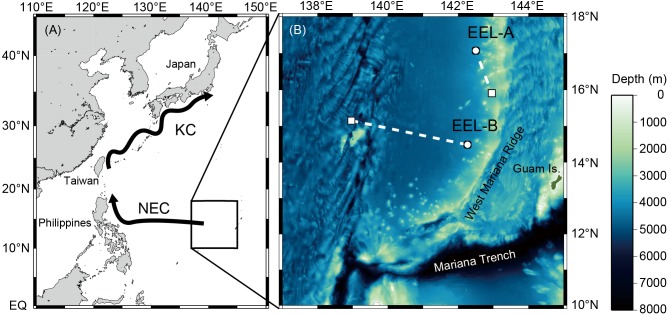
Three silver eels (EEL-A, B, C) were selected from eels captured by set nets in Mikawa Bay in November and December, 2013. The silvering stages of EEL-A, B and C were determined to be in the S1, S2 and S1 stages, respectively, according to the morphological index reported in Okamura et al. (2007). Eels were held in a 1000 L black plastic tank with seawater maintained at ~10°C at the IRAGO Institute, Aichi, Japan, until they were transported to the R/V Natsushima that was going to conduct a research cruise (NT14- 09) along the West Mariana Ridge from 14 May to 4 June, 2014. The purpose of the survey was to study the spawning ecology of Japanese eels and also release EEL-A, B, and C. The silver eels were transported from Japan to the release sites onboard the ship in a 1000 L plastic tank constantly supplied with surface seawater that was adjusted to temperatures of 20 to 22°C. The 3 eels were released at different sites along the West Mariana Ridge. EEL-A and B were released at 17°00'N, 142°31'E and 14°30'N, 142°14'E, respectively, at night on 19 May 2014. EEL-C was released at 11°50'N, 141°00'E at night on 30 May 2014 (Fig. 1).
Fig. 1.
Fig. 1. (A) Spawning area of the Japanese eel in the western North Pacific region (square) and the larval migration route to East Asia, using the North Equatorial Current (NEC) and the Kuroshio Current (KC). (B) Release (open circle) and pop-up locations (open square) of the tagged eels and bathymetric structure of the West Mariana Ridge.
Tag set up and attachment
Three PSAT tags (MiniPAT, Wildlife Computers Inc.) were used in the study. Each MiniPAT measures 124 mm in length (excluding the antenna), has a maximum diameter of 38 mm and weighs 60 g in air. The 3 tags can record depth (0 to 1700 m, resolution ± 0.5 m), water temperature (-20 to 50°C, resolution ± 0.05°C) and light intensity (5 × 10-12 to 5 × 10-2 W cm-2). The EEL-A and B tags were scheduled to pop up on 30 June 2014 (42 days after release) and the EEL-C tag was scheduled to pop up on 29 July 2014 (60 days after release). We set the MiniPATs to record the time series data every 2.5 minutes. The 3 tags were pre-programmed to interrupt the observations, detach, and initiate transmitting the data to the Argos satellite-based system in case of the death of the eel, the premature detachment of the tag after 3 consecutive days of constant depth readings (± 2.5 m) or the tag reaching 1800 m depth.
Before surgical attachment of the tag harnesses, eels were anaesthetized in a 2‰ eugenol seawater solution and their total lengths, body weights and silvering condition were determined based on Okamura et al. (2007) (Table 1). The harnesses to which the tags were later attached were surgically attached to the eels at the IRAGO Institute following the method of Manabe et al. (2011) 39 days before they were transported to the ship. In summary, a curved surgical needle (3/8 of a 9.3 cm diameter circle) with one end of the monofilament line attached was inserted through the dorsal side of the lateral musculature at two locations 3 cm anterior to the origin of the dorsal fin and pushed upward to emerge on the other side of the eel. The tags were attached to the harness bases with a plastic clamping band just before releasing onboard the ship. Eels were gently released from large plastic buckets of water lowered to the sea surface.
Table 1. Biological data on eels used in the study and eel release and tracking information. TL: total length; BW: body weight; SI: silvering index.
| Eel no. | SI | TL(mm) | BW (g) | Released | Surfaced | Tracking duration (d) | Travel speed (km/d) |
| EEL-A | S1 | 898 | 1100 | 19-May-14 01:02 | 13-Jun-14 23:47 | 27 | 4.93 |
| EEL-B | S2 | 992 | 1545 | 19-May-14 19:49 | 01-Jul-14 06:18 | 43 | 8.4 |
| EEL-C | S1 | 792 | 913 | 30-May-14 20:42 | - | - | - |
Data analysis
The release points and PSAT surfacing locations were plotted over a bathymetric chart (SRTM15_PLUS, Olson et al. 2014) made using the Generic Mapping Tools program (Wessel at al. 2013; Fig. 1). For detailed analysis of the data obtained from EEL-B, a 31-day period was selected starting on 31 May that corresponded to when the eel maintained a stable pattern of daytime depths and temperatures experienced that extended until the tag was released from the eel (Fig. 2). The swimming behavior of the eel was divided into four typical phases of daytime, nighttime, ascent and descent phases based on the methods reported in Westerberg et al. (2014) and Chow et al. (2015). To compare differences in swimming depth between daytime and nighttime periods, we used Mann- Whitney U tests with the package ‘exactRankTests’ in the statistical analysis software R (R Core Team 2015; Hothorn and Hornik 2015). The swimming depth during nighttime was compared with moon age and altitude, which were calculated in Keisan Online Calculator (http://keisan.casio. jp/exec/system/1239785915, Casio Computer Co., Ltd). The correlation between moon altitude and swimming depth was examined by Pearson correlation tests when the moon appeared and disappeared in the sky. Correlation analysis was applied to test the association between swimming depth during daytime and sun altitude, which was calculated using the method described in Campbell and Norman (2012). To assess the correlation between depth and temperature, and sunlight, the swimming depth and experienced water temperature were examined for correlation with sun altitude using the Spearman’s rank correlation coefficient. The correlation between sun altitude and swimming depths during diving and ascending periods was also tested using the Spearman’s rank correlation coefficient.
Fig. 2.
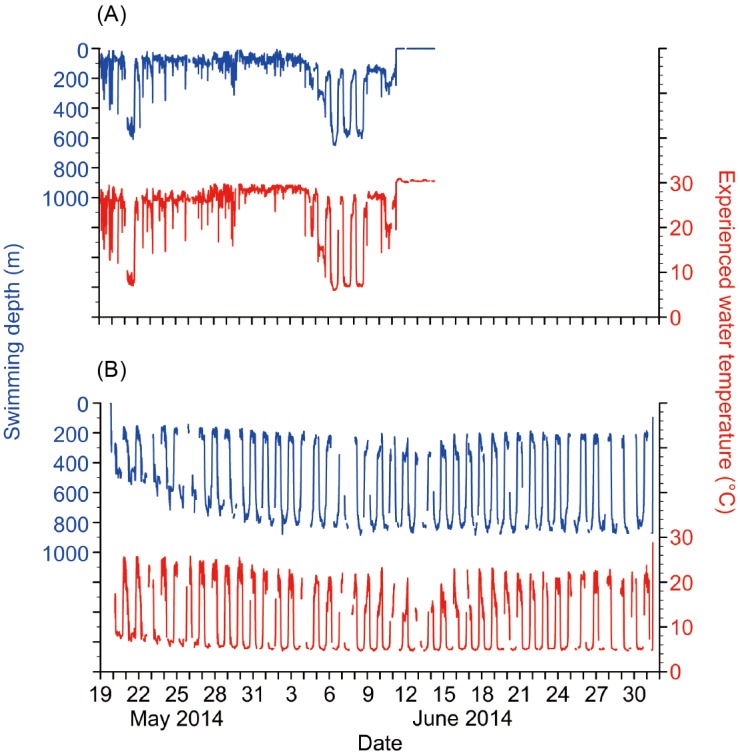
Fig. 2. Depth and temperature records of EEL-A (A) and EEL-B (B), which were released in or near the Japanese eel spawning area along the West Mariana Ridge. The swimming depths (blue) and experienced water temperatures (red) are shown in each panel.
RESULTS
Data acquisition
Two PSATs attached to EEL-A and B successfully recorded, popped up to the surface and transmitted the data through the Argos satellite system. The EEL-C tag did not transmit any data. The PSAT of EEL-A surfaced at 15°54'N, 143°00'E on 14 June 2014 after the eel had moved 133 km to the south-southeast for 27 days at an average travel speed of 4.9 km d-1 (5.7 cm s-1) (Table 1, Fig. 1B). On the 21st day after release (11 June), the tag prematurely came off the eel and popped up to the sea surface and started transmitting data on 14 June. The premature pop-off of the Eel-A tag did not appear to result from predation because no unusual movements, abrupt deep dives or high temperature records were detected in a detailed examination of the data before the tag reached the surface. The PSAT of EEL-B surfaced at 15°11'N, 138°58'E on the scheduled date, 1 July 2014, after the eel moved 361 km to the west for 43 days at an average travel speed of 8.4 km d-1 (10 cm s-1) (Table 1, Fig. 1B).
Vertical movements
Eel-A only showed a regular daily rhythm of vertical movements for 3 days of its migration (6 to 8 June: 19-21 days after release, Fig. 2A). However, it made a deep dive to 600 m during the daytime of the 3rd day of its release (May 21); it made another deep dive (> 500 m) the next morning, but ascended to 60 m before noon. During the 3 days of regular vertical movements, the eel stayed at a shallow layer of 150-240 m during nighttime and a deep layer of 600-650 m during daytime. This pattern of diving and temperatures experienced by Eel-A was about the same as seen for Eel-B during its 4th to 7th day after release when it was swimming at shallower daytime depths; but due to the short duration the vertical migration of Eel-A, those data were not analyzed further.
Eel-B showed distinct daily vertical movements during the entire tracking period by staying at deeper layers during daytime and shallower layers during nighttime (Fig. 2B). The amplitude of vertical movements increased from the 2nd to 13th day after release (20-31 May) as the eel gradually moved to deeper depths. During this period, the depth of deeper layers gradually increased over time from 500 to 800 m, while the depth of shallower layers remained almost constant at about 200 m. From 31 May to the end of tracking (the detailed analysis period), EEL-B showed constant vertical movements between depths of about 200 m during nighttime and about 800 m during daytime. (Fig. 3). There was a significant difference between the swimming depths during nighttime (267.3 ± 52.6 m) and daytime (787.6 ± 54.9 m) (Mann-Whitney U test, p < 0.001). According to the daily change in swimming depth, the water temperature that the eel experienced varied widely from 18.2 ± 3.0°C in nighttime to 5.2 ± 0.3°C in daytime, with a statistically significant difference between day and night (p < 0.001).
Fig. 3.
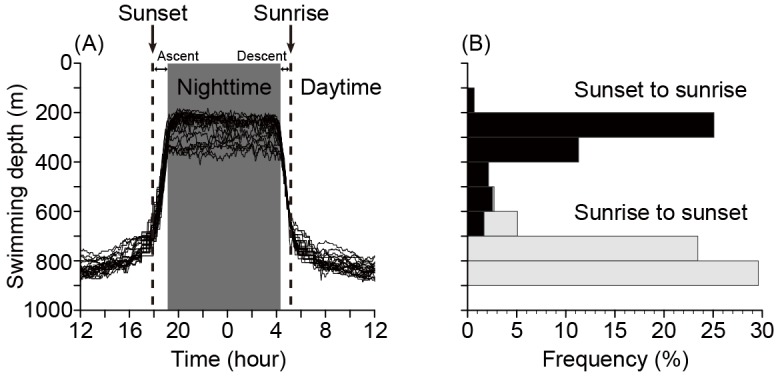
Fig. 3. (A) Swimming depths of EEL-B with all daily data superimposed. The vertical migrations were separated into 4 phases by sunset, sunrise, end point of ascending and start point of descending for the analyses of this study. (B) Frequency of EEL-B depth records separated into daytime (gray) and nighttime (black) periods.
Nighttime behavior
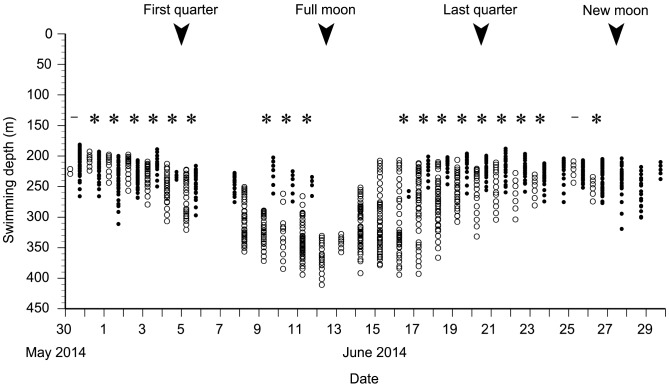
Data from 31 May to 1 July when there was a constant diel vertical migration of EEL-B were used to test the relationship between swimming depth and moon age, and swimming depth and moon altitude. There was a significant difference in swimming depths between when the moon was above or below the horizon in 18 of 20 nights (Mann-Whitney U tests, p < 0.05), but the several days around the full moon and new moon were not tested because these nights did not have both data with and without moonlight (Fig. 4). EEL-B swam at deeper layers (300.4 ± 50.5 m) when the moon was in the sky and at shallow layers (230.2 ± 20.0 m) when the moon was not present. There was a clear negative linear relationship between swimming depth with the moon in the sky and the lunar age (Spearman’s rank correlation coefficient, rho = -0.67, p < 0.001, Fig. 5A), suggesting correlation between the eel ascending to shallower layers and the waning moon. In contrast, there was no significant relationship between the swimming depth and lunar age for nights without the moon in the sky (Spearman rank correlation test, p > 0.05) (Fig. 5B). A strong negative linear relationship between swimming depth and moon altitude was detected (Pearson’s correlation test, r = -0.82, p < 0.001, Fig. 6), suggesting that the lower the moon altitude was, the shallower the eel swam.
Fig. 4.
Fig. 4. Swimming depths of EEL-B during nighttime periods for the 31-day observation period. The swimming depths were separated into nighttime with moon (open circles) and without moon (black circles) periods. Asterisks shows significant differences between nighttime with moon and without moon, and bars indicate no significant differences (Mann-Whitney U test, p = 0.06, 0.81).
Fig. 5.
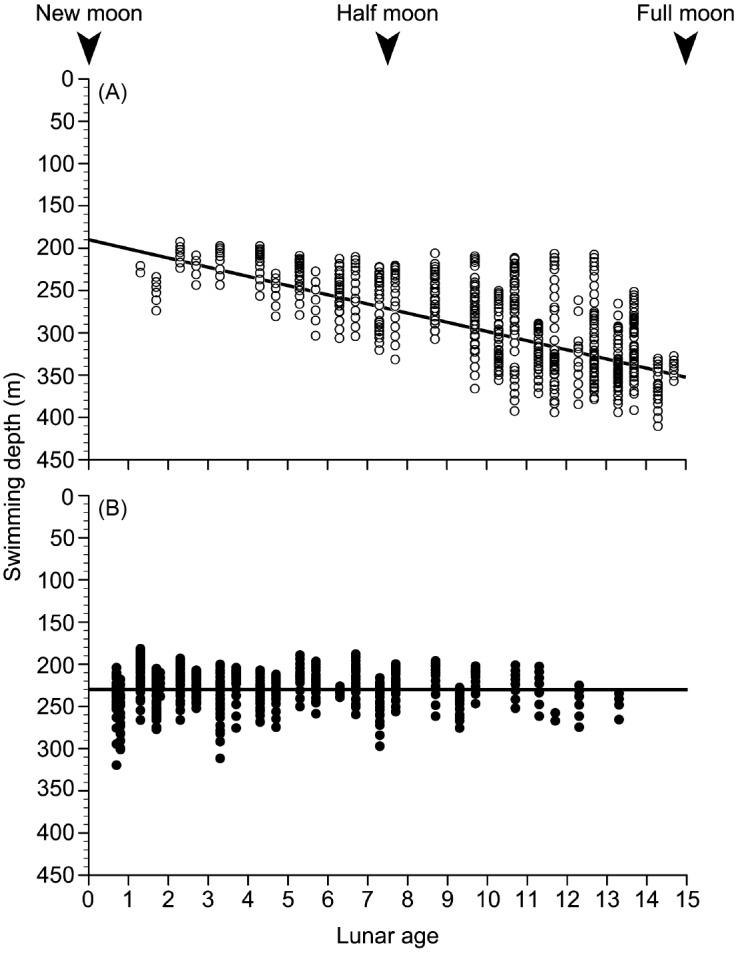
Fig. 5. Relationships between lunar ages and swimming depths during nighttime periods with (A) and without (B) the moon in the sky. The two halves of the lunar month (0-30) are superimposed in inverse directions (0-15; new moon to full moon, and full moon to new moon).
Fig. 6.
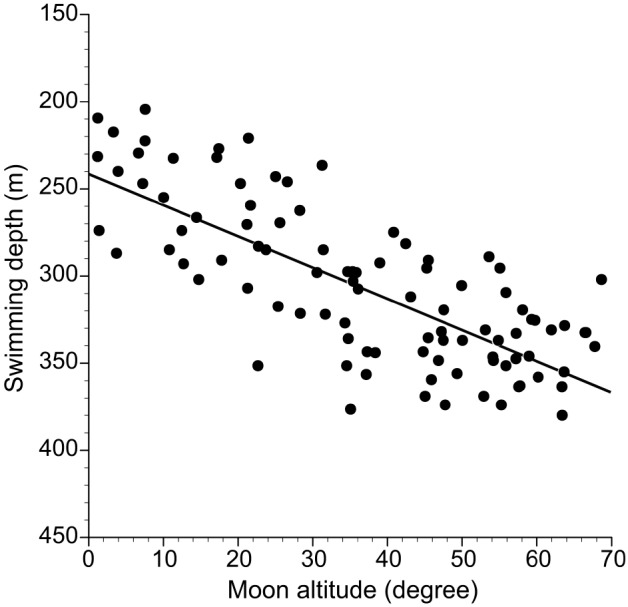
Fig. 6. Relationship between moon altitude and swimming depth of EEL-B during nighttime periods with the moon in the sky.
Daytime behavior
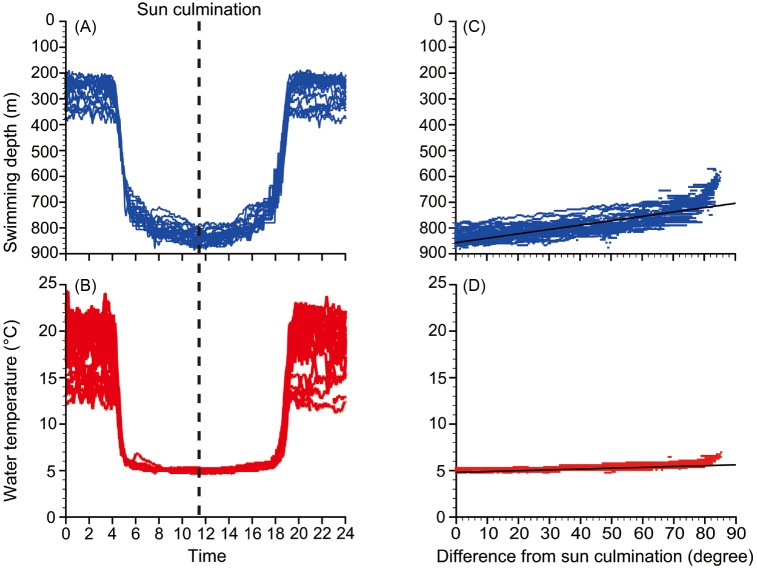
During daytime, EEL-B swam in deep water (787.6 ± 54.9 m) and experienced cold water temperatures (5.2 ± 0.3°C) (Fig. 7A). There was a strong positive linear relationship between swimming depth and the difference in sun altitudes from the sun culmination (sun at its highest point in the sky) during each day (Fig. 7B, Spearman’s rank correlation coefficient, rho = 0.81, p < 0.001). The relationship between the experienced temperature and the difference in sun altitude from culmination also showed a similar but less distinct tendency (Fig. 7D, Spearman’s rank correlation coefficient, rho = 0.77, p < 0.001). These correlations indicate that EEL-B reached the deepest depths and experienced the lowest temperatures during the sun culmination of each day (Fig. 7). The maximum swimming depth during daytime was 851.1 ± 22.8 m with a range of 797 to 885 m (Fig. 8). The minimum daytime water temperature was 4.9 ± 0.1°C with a range of 4.7 to 5.3°C.
Fig. 7.
Fig. 7. Swimming depth (A) and experienced water temperature (C) of EEL-B with all daily records superimposed from the analyzed period (31 days). The dotted line represents the averaged time of sun culminations during the analyzed period. Relationships between the differences in sun altitude from culmination and the swimming depth (B) and experienced water temperature (D) during daytime.
Fig. 8.
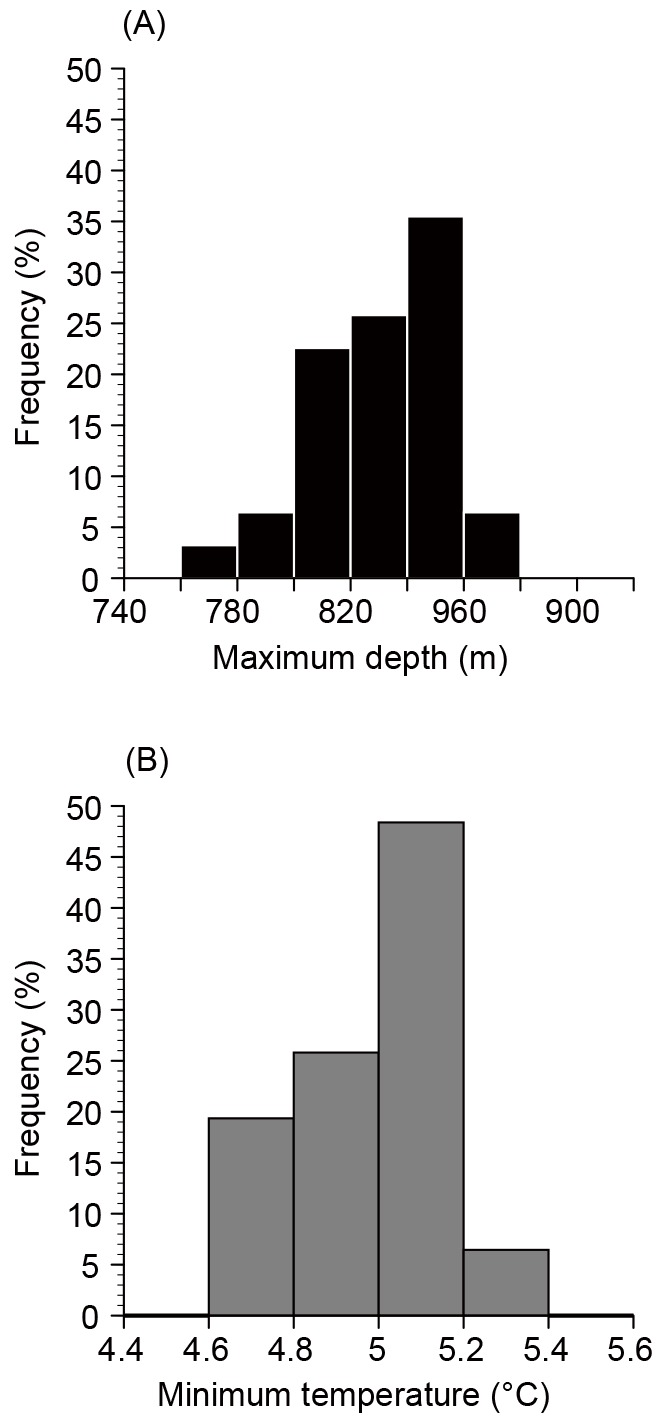
Fig. 8. Maximum swimming depths (A) and minimum experienced temperatures (B) of EEL-B each day for the 31 days.
Behavior during the transitional phases
EEL-B started to descend from 3:55 to 4:35 in the morning (4:12 ± 0:12) and ascend from 17:58 to 18:20 in the evening (18:06 ± 0:06) (Fig. 3A, Fig. 7). These times corresponded to sun altitudes of -12.98 ± 2.55° and -0.79 ± 1.33°, respectively, which were descents during the morning twilight and ascents at sunset. Negative linear relationships were detected between sun altitude and swimming depth at the start of diving (Fig. 9, rho = -0.80, p < 0.001, Spearman’s rank correlation test) and ascending (rho = -0.47, p < 0.05). There was a strong negative linear relationship between sun altitude and eel swimming depth during the vertical movements in both directions (Fig. 10, Spearman’s rank correlation coefficient, rho = -0.96, p < 0.001).
Fig. 9.
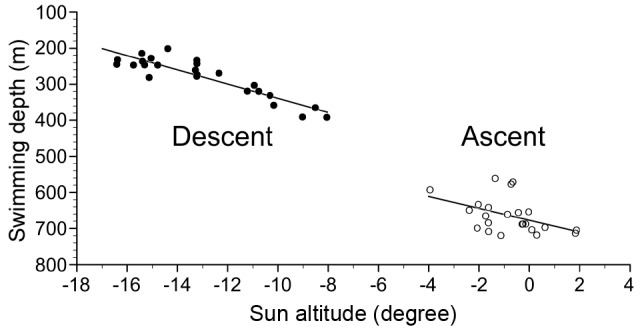
Fig. 9. Relationships between swimming depth and sun altitude at the starting points of descending and ascending.
Fig. 10.
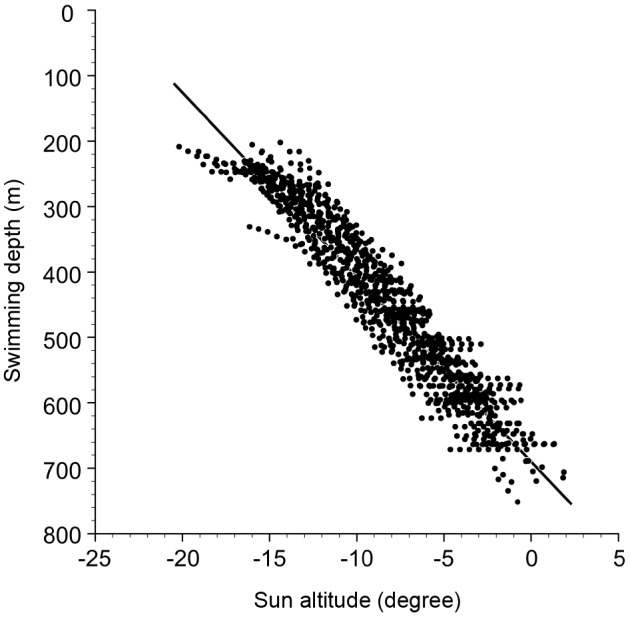
Fig. 10. Relationship between the sun altitude and the swimming depth for all records during the descending and ascending periods.
DISCUSSION
The present study obtained long-term data on open-ocean swimming behavior from 1 of 3 Anguilla japonica silver eels released within or near their spawning area. EEL-B showed a diel vertical migration behavior typical of migrating anguillid eels throughout the entire time the tag was attached; EEL-A only showed the regular large-scale vertical movements for about 3 of the 18 days that its tag remained attached. During that time however, it showed a similar behavior as was seen for EEL-B starting a few days after its release. EEL-B started to repeat the clear diel vertical migration from just after release and continued that behavior throughout the 43-day tracking period. EEL-A was an early stage silver eel while EEL-B was at the final continental water silvering stage (Okamura et al. 2007). It is unknown if that difference in silvering stage contributed to the different behavior, but the findings of this and the previous studies on this species (Manabe et al. 2011; Chow et al. 2015) indicate that Japanese eels likely make clear diel vertical migrations over the continental shelf and offshore in the western North Pacific during their spawning migrations. This same type of behavior has been seen in other temperate (Jellyman and Tsukamoto 2002 2005 2010; Aarestrup et al. 2009; Béguer-Pon et al. 2015; Righton et al. 2016) and tropical anguillid eels (Schabetsberger et al. 2013 2015) tagged with PSATs while migrating to their hydrographically variable spawning areas (Schabetsberger et al. 2016). There are variations in the swimming depths and experienced temperatures among the eel species (Table 2) but, similar to the diel vertical migrations of fishes and zooplankton (Iwasa 1982; Lampert 1989), these movements appear to avoid predators during the day by moving to deeper depths with lower light levels. We have observed several possible predators such as tunas along the West Mariana Ridge during research efforts at seamounts (Tsukamoto et al. 2003) that would be present in the upper few hundred meters during the day, so moving to deeper water during the day would be needed in their spawning area. (Béguer- Pon et al. 2012; Madigan et al. 2015)
Table 2. Examples of the mean swimming depths and experienced temperatures (mean ± SD) during day and night of migrating silver eels (Anguilla spp.) tagged with pop-up satellite archival transmitters in temperate and tropical areas.
| Species | Daytime Depth | Temperature | Nighttime Depth | Temperature | Reference |
| A. japonica | 787.6 ± 54.87 m | 5.2 ± 0.3°C | 267.3 ± 52.6 m | 18.2 ± 3.0°C | Thisstudy |
| A. anguilla | 564 ± 125 m | 10.12 ± 0.89°C | 282 ± 138 m | 11.68 ± 0.48°C | Arestrup et al. (2009) |
| A. rostrata | 618 ± 16 m | 12°C | 141 ± 14 m | 24°C | Béguer-Pon et al. (2015) |
| A. marmorata | 631 m | 6°C | 175 m | 23°C | Shcabetsberger et al. (2013) |
| A. megastoma | 743 m | 5.6°C | 186 m | 22.9°C | Shcabetsberger et al. (2013) |
The swimming depths and experienced temperatures of EEL-B during daytime suggests that the low temperature (from 4.6 to 5.4°C) influences the eel’s maximum depth, as shown in figures 7 and 8. Its maximum swimming depth appeared to be determined according to reaching a minimum temperature range of about 5°C, because the range of minimum temperatures it reached (< 1°C) was much more consistent than the range of its maximum depths (120 m) over time. A similar preference for a particular temperature (about 4 to 10°C) and not a maximum depth was also seen in an eel crossing the Kuroshio Current area south of Japan where the temperature structure varied greatly along its swimming route (Manabe et al. 2011). In the acoustic tracking study of Chow et al. (2015), one eel (WE6289) released in a similar area along the Kuroshio also showed evidence of following the depth of a similar minimum temperature range as it changed its daytime depths from about 450 m to almost 800 m while experiencing a similar low temperature close to 5°C each day. However, many of the natural eels tracked in that study (aquacultured eels were also tracked, but had irregular behavior) showed a general trend toward greater daytime depths over time, even if the temperature structure did not change much. Interestingly, the two natural eels released in the Japanese eel spawning area by Chow et al. (2015) showed vertical movements during the 2 days they were tracked that were about the same depths as EEL-B during its first 2 days after release. Japanese eels need to experience temperatures from 18 to 22°C for sexual maturation (Sato et al. 2006; Dou et al. 2008), and EEL-B experienced similar range of water temperature (18.2 ± 3.0°C during nighttime). Japanese eels in the present study experienced temperatures of around 5°C at their greatest daytime depths, which are also similar to the lowest temperatures of other species of eels in the Pacific (Jellyman and Tsukamoto 2010; Schabetsberger et al. 2013 2015), but lower than the coldest daytime temperatures of Atlantic eels (Aarestrup et al. 2009; Wysujack et al. 2015; Béguer-Pon et al. 2015).
The present study also observed that the swimming depths used appeared to be influenced by light levels during both day and night. The behavior of EEL-B showed significant negative linear relationships between the swimming depth in each period of the day, daytime, ascending, nighttime and descending, and the environmental light conditions such as lunar age, moon altitude and sun altitude, indicating a negative phototaxis to natural light during both day and night. EEL-B swam deeper during the full moon period and when moonlight was present during any phase of the moon. Other Japanese eels farther away from the spawning area have also been shown to swim deeper during the full moon (Chow et al. 2015), as have migrating tropical eels in the western South Pacific (Schabetsberger et al. 2013 2015). Moonlight also affects the depth of other vertically migrating marine organisms in the open ocean, with deeper scattering layers of fishes and zooplankton being observed during the full moon (Prihartato et al. 2016). Predators such as tuna (Musyl et al. 2003; Kitagawa et al. 2007), swordfish (Dewar et al. 2011) and sharks (Campana et al. 2011; Musyl et al. 2011) also appear to swim deeper during full moon periods, presumably to follow their prey species. Other PSAT tagging studies suggest that sharks, tunas and marine mammals can be considered possible predators to freshwater eels during their oceanic migration (Béguer-Pon et al. 2012; Wahlberg et al. 2014)
Variations in temperature may be related to reproductive physiology of eels (Jellyman and Tsukamoto 2010). Sato et al. (2006) suggested that eels stop maturing at water temperatures < 10°C, so eels that experienced 5.2 ± 0.2°C water during daytime such as EEL-B likely slow down their maturation while at deep depths during the day. This has been hypothesized to be useful for slowing down the maturation process during the long migrations, but the eels also need to enter the warm water at night to allow maturation to proceed and to swim efficiently (Jellyman and Tsukamoto 2010).
In the present study, although EEL-A and EEL-B showed similarities in their patterns of regular vertical movements, they showed different directions of horizontal movements. EEL-A was released to the north of the typical spawning latitudes and moved horizontally a short distance southward along the West Mariana Ridge until it reached the northern edge of the spawning area. Its average speed to the south of 4.9 km d-1 (5.7 cm s-1) was slower than swimming speeds observed in previous PSAT research on Japanese eels closer to Japan, which were about 15.1 km d-1 (~ 17.5 cm s-1) (Manabe et al. 2011). EEL-B was released within the latitude and longitude of the spawning area and it moved horizontally a longer distance to the west at the latitudes of the North Equatorial Current (NEC), also at a slower average speed of 8.4 km d-1 (10 cm s-1) (Fig. 1B). Tsukamoto (1994) suggested that some Japanese eels migrate to their spawning area along the Izu- Bonin-Mariana Arc and West Mariana Ridge. The direction of EEL-A movement is consistent with that route, but the direction of EEL-B movement did not indicate any southward swimming. The PSAT of EEL-B popped up at its scheduled time 361 kilometers away from the released point 43 days after release. The eel was released within an area along the west side of the West Mariana Ridge where newly hatched preleptocephali were collected in previous years (Tsukamoto et al. 2011; Aoyama et al. 2014). Therefore, if EEL-B was not focused on oriented swimming within its spawning area then it would have been transported westward by the flow of the NEC. Anguillid eels have a geomagnetic sense (Durif et al. 2013), so it is possible EEL-B realized it was within its spawning area. The NEC generally has westward flow of about 20 to 40 cm s-1 (Tsukamoto 1990; Kimura et al. 1994; Hu and Hu 2014; Schönau and Rudnick 2015), so NEC transport may explain the eel’s movement. The westward movement of the eel was slower than expected from westward NEC transport, though, so it may have been making some effort to stay near the seamount ridge.
Another factor that could have affected the behavior of EEL-B is that it was not close to the level of gonadal maturation required for spawning. Freshwater eels transform into silver eels as the process of maturation begins before they start their long migrations (Aoyama and Miller 2003), but their gonad-somatic indexes (GSI: relative weight of gonad to body weight) are much lower than Japanese eels in spawning condition (Tsukamoto 2009). Their gonads are immature at the beginning of their downstream migration and the eggs of Japanese eels are at the primary yolk globule stage with GSI (≤ 3 - 4 %) (Sudo et al. 2011). Although EEL-B was at the final stage of silvering (S2), it was not in spawning condition because its belly was not remarkably large compared to eels ready to spawn (see Tsukamoto 2009). The GSI of Japanese eels captured within their spawning area is 18.8-40.3 for males (Chow et al. 2009; Tsukamoto et al. 2011) and 9.0-47.8 for females (Tsukamoto et al. 2011), so most of the gonadal maturation occurs during the offshore spawning migration while the eels are not feeding (Chow et al. 2010).
All of the previous studies that tracked the spawning migration behavior of Japanese eels were carried out in coastal waters, offshore near Japan, or for short durations within their spawning area (Aoyama et al. 1999 2002; Manabe et al. 2011; Chow et al. 2015). More PSAT tagging studies are needed to increase understanding of Japanese eel migration route and behavior as they swim from their growth habitats in East Asia to their spawning area west of the seamount ridge, and what they do in the spawning area. Numerical modelling experiments may also be useful to understand the possible factors affecting the migration route of Japanese eels, as was indicated in Chang et al. (2016), which used the average swimming depth reported in Manabe et al. (2011) to model the success of different types of migration strategies. The more detailed view of the diel vertical migration behavior obtained in the present study can guide future migration modelling studies and contribute to understanding the spawning migrations of Japanese eels.
Acknowledgments
Acknowledgments: We are grateful to the captain and crew of the R/V Natsushima of the Japan Marine Science and Technology Center (JAMSTEC), and to T. Miwa, T. Fukuba, A. Okino, A. Mizushina, K. Maruyama, Y. Sakamoto, A. Takeuchi, M. Miyao, Y. Sato and M. Toizumi for assistance onboard the ship. We also thank S. Iwamoto for assistance in part of the data analysis and members of the IRAGO Institute for the providing the eels used in the study. This study was partially supported by JSPS KAKENHI Grant Number JP26450268 and JP26252030.
Footnotes
Authors’ contributions: SW and KT contributed in designing the study and provided the pop- up tags, and TH and RM analyzed the data from the eels and examined the relationship between these data and the astronomical data. AO and YY collected the silver eels used in the experiment and made surgical operation on them for tagging. TH, SW, TK, MJM and KT released the tagged eels onboard the R/V Natsushima and collected the environmental data. TH, SW, MJ, and KT drafted the manuscript and other coauthors participated in revising it and approving the final manuscript.
Competing interests: The authors declare that they have no competing interests.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Consent for publication: Not applicable.
Ethics approval consent to participate: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The surgical attachments were done in an anesthetized condition.
References
- Aarestrup K, Økland F, Hansen M M, Righton D, Gargan P, Castonguay M, Bernatchez L, Howey P, Sparholt H, Pedersen M I, Mckinley R S. Oceanic Spawning Migration of the European Eel (Anguilla anguilla) Science; 2009. 325. [DOI] [PubMed] [Google Scholar]
- Aoyama J. Life history and evolution of migration in catadromous eels (genus Anguilla) Aqua-BioScience Monogr; 2009. 2 [Google Scholar]
- Aoyama J, Miller M J. The silver eel. Springer. pp. 107–117.
- Aoyama J, Hissmann K, Yoshinaga T, Sasai S, Uto T, Ueda H. Swimming depth of migrating silver eels Anguilla japonica released at seamounts of the West Mariana Ridge, their estimated spawning sites. Mar Ecol Prog Ser. 186:265–269. [Google Scholar]
- Aoyama J, Sasai S, Miller M J, Shinoda A, Nakamura A, Kawazu K, Tsukamoto K. A preliminary study of the movements of yellow and silver eels, Anguilla japonica, in the estuary of the Fukui River, Japan, as revealed by acoustic tracking. Hydrobiologia. 470:31–36. [Google Scholar]
- Aoyama J, Watanabe S, Miller M J, Mochioka N, Otake T, Yoshinaga T, Tsukamoto K. Spawning sites of the Japanese eel in relation to oceanographic structure and the West Mariana Ridge. PLoS ONE; 2014. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguer-Pon M, Benchetrit J, Castonguay M, Aarestrup K, Campana S E, Stokesbury Mjw, Dodson J J. Shark predation on migrating adult American Eels (Anguilla rostrata) in the Gulf of St. Lawrence. PLoS ONE; 2012. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguer-Pon M, Castonguay M, Shan S, Benchetrit J, Dodson J J. Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat Commun. 6:1–9. doi: 10.1038/ncomms9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana S E, Dorey A, Fowler M, Wang Joyce W, Wright Z, Yashayaev D. Migration pathways, behavioural thermoregulation and overwintering grounds of blue sharks in the Northwest Atlantic. PLoS ONE; 2011. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G S, Norman J M. An introduction to environmental biophysics. Springer Science & Business Media; 2012. [Google Scholar]
- Castle Phj, Williamson G R. On the validity of the freshwater eel species Anguilla ancestralis Ege from Celebes. Copeia. 2:569–570. [Google Scholar]
- Chang Y L, Miyazawa Y, Béguer-Pon M. Simulating the oceanic migration of silver Japanese eels. PLoS ONE; 2016. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S, Kurogi H, Mochioka N, Kaji S, Okazaki M, Tsukamoto K. Discovery of mature freshwater eels in the open ocean. Fish Sci. 75:257–259. [Google Scholar]
- Chow S, Kurogi H, Katayama S, Ambe D, Okazaki M, Watanabe T, Ichikawa T, Kodama M, Aoyama J, Shinoda A, Watanabe S, Tsukamoto K, Miyazaki S, Kimura S, Yamada Y, Nomura K, Tanaka H, Kazeto Y, Hata K, Handa T, Tawa A, Mochioka N. Japanese eel Anguilla japonica do not assimilate nutrition during the oceanic spawning migration: Evidence from stable isotope analysis. Mar Ecol Prog Ser. 402:233–238. [Google Scholar]
- Chow S, Okazaki M, Watanabe T, Segawa K, Yamamoto T, Kurogi H, Tanaka H, Kawai Ai K, Yamamoto M, Mochioka S, Manabe N, Miyake R. Light-sensitive vertical migration of the Japanese eel Anguilla japonica revealed by real-time tracking and its utilization for geolocation. PLoS ONE; 2015. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar H, Prince E D, Musyl M K, Brill R W, Sepulveda C, Luo J, Foley D, Orbesen E S, Domeier M L, Nasby-Lucas N, Derke Snodgrass, Michael L R, Hoolihan J P, Block B A, Mcnaughton L M. Movements and behaviors of swordfish in the Atlantic and Pacific Oceans examined using pop-up satellite archival tags. Fish Oceanogr. 20:219–241. [Google Scholar]
- Dou S Z, Yamada Y, Okamura A, Shinoda A, Tanaka S, Tsukamoto K. Temperature influence on the spawning performance of artificially-matured Japanese eel, Anguilla japonica, in captivity. Environ Biol Fishes. 82:151–164. [Google Scholar]
- Durif Cmf, Browman H I, Phillips J B, Skiftesvik A B, Vøllestad L A, Stockhausen H H. Magnetic Compass Orientation in the European Eel. PLoS ONE; 2013. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege V. A revision of the genus Anguilla Shaw: a systematic, phylogenetic and geographical study. Dana Report. 16:1–256. [Google Scholar]
- Fricke H, Kaese R. Tracking of artificially matured eels (Anguilla anguilla) in the Sargasso Sea and the problem of the eel's spawning site. Naturwissenschaften. 82:32–36. [Google Scholar]
- Han Y S, Liao I C, Huang Y S, He J T, Chang C W, Tzeng W N. Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture. 219:578–579. [Google Scholar]
- Hothorn T, Hornik K. exactRankTests: Exact distributions for rank and permutation tests. 2015.
- Hu S, Hu D. Variability of the Pacific North Equatorial Current from repeated shipboard acoustic doppler current profiler measurements. J Oceanogr. 70:559–571. [Google Scholar]
- Ishikawa S, Suzuki K, Inagaki T, Watanabe S, Kimura Y, Okamura A, Otake T, Mochioka N, Suzuki Y, Hasumoto H, Oya M, Miller M J, Lee T W, Fricke H, Tsukamoto K. Spawning time and place of the Japanese eel Anguilla japonica in the North Equatorial Current of the western North Pacific Ocean. Fish Sci. 67:1097–1103. [Google Scholar]
- Iwasa Y. Vertical migration of zooplankton: A game between predator and prey. Am Nat. 120:171–180. [Google Scholar]
- Jellyman D, Tsukamoto K. First use of archival transmitters to track migrating freshwater eels Anguilla dieffenbachii at sea. Mar Ecol Prog Ser. 233:207–215. [Google Scholar]
- Jellyman D, Tsukamoto K. Swimming depths of offshore migrating longfin eels Anguilla dieffenbachii. Mar Ecol Prog Ser. 286:261–267. [Google Scholar]
- Jellyman D, Tsukamoto K. Vertical migrations may control maturation in migrating female Anguilla dieffenbachii. Mar Ecol Prog Ser. 404:241–247. [Google Scholar]
- Kimura S, Tsukamoto K, Sugimoto T. A model for the larval migration of the Japanese eel: roles of the trade winds and salinity front. Mar Biol. 119:185–190. [Google Scholar]
- Kitagawa T, Boustany A M, Farwell C J, Williams T D, Castleton M R, Block B A. Horizontal and vertical movements of juvenile bluefin tuna (Thunnus orientalis) in relation to seasons and oceanographic conditions in the eastern Pacific Ocean. Fish Oceanogr. 16:409–421. [Google Scholar]
- Lampert W. The adaptive significance of diel vertical migration of zooplankton. Br Ecol Soc. 3:21–27. [Google Scholar]
- Madigan D J, Carlisle A B, Gardner L D, Jayasundara N, Micheli F, Schaefer K M, Fuller D W, Block B A. Assessing niche width of endothermic fish from genes to ecosystem. Proc Natl Acad Sci. 112:8350–8355. doi: 10.1073/pnas.1500524112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe R, Aoyama J, Watanabe K, Kawai M, Miller M J, Tsukamoto K. First observations of the oceanic migration of Japanese eel, from pop-up archival transmitting tags. Mar Ecol Prog Ser. 437:229–240. [Google Scholar]
- Matsui I. Eel biology-biological study. KouseishaKouseikaku; 1972. [Google Scholar]
- Mccleave J D, Arnold G P. Movements of yellow-and silver-phase European eels (Anguilla anguilla L.) tracked in the western North Sea. ICES J Mar Sci. 56:510–536. [Google Scholar]
- Musyl M K, Brill R W, Boggs C H, Curran D S, Kazama T K, Seki M P. Vertical movements of bigeye tuna (Thunnus obesus) associated with islands, buoys, and seamounts near the main Hawaiian Islands from archival tagging data. Fish Oceanogr. 12:152–169. [Google Scholar]
- Musyl M K, Brill R W, Curran D S, Fragoso N M, Mcnaughton L M, Nielsen A, Kikkawa B S, Moyes C D. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish Bull. 109:341–368. [Google Scholar]
- Okamura A, Yamada Y, Yokouchi K, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K. A silvering index for the Japanese eel Anguilla japonica. Environ Biol Fishes. 80:77–89. [Google Scholar]
- Okamura A, Yamada Y, Tanaka S, Horie N, Utoh T, Mikawa N, Akazawa A, Oka H P. Atmospheric depression as the final trigger for the seaward migration of the Japanese eel Anguilla japonica. Mar Ecol Prog Ser. 234:281–288. [Google Scholar]
- Olson C J, Becker J J, Sandwell D T. A new global bathymetry map at 15 arcsecond resolution for resolving seafloor fabric: SRTM15_PLUS. Abstracts of the Fall Meeting of American Geophysical Union, the Moscone Center. pp. 15–19.
- Prihartato P K, Irigoien X, Genton M G, Kaartvedt S. Global effects of moon phase on nocturnal acoustic scattering layers. Mar Ecol Prog Ser. 544:65–75. [Google Scholar]
- Righton D, Westerberg H, Feunteun E, Økland F, Gargan P, Amilhat E, Metcalfe J, Lobon-Cervia J, Sjo Berg N, Simon J, Acou A, Vedor M, Walker A, Trancart T, Bra Mick U, Aarestrup K. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci Adv; 2016. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. R Foundation for Statistical Computing(Vienna, Austria) 2015.
- Sato N, Kawazoe I, Suzuki Y. Effects of temperature on vitellogenesis in Japanese eel Anguilla japonica. Fish Sci. 72:961–966. [Google Scholar]
- Schabetsberger R, Miller M J, Dall'olmo G, Kaiser R, Økland F, Watanabe S, Aarestrup K, Tsukamoto K. Hydrographic features of anguillid spawning areas: Potential signposts for migrating eels. Mar Ecol Prog Ser. 554:141–155. doi: 10.3354/meps11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabetsberger R, Økland F, Aarestrup K, Kalfatak D, Sichrowsky U, Tambets M, Dall'olmo G, Kaiser R, Miller P I. Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser. 475:177–190. [Google Scholar]
- Schabetsberger R, Økland F, Kalfatak D, Sichrowsky U, Tambets M, Aarestrup K, Gubili C, Sarginson J, Boufana B, Jehle R, Dall'olmo G, Miller M J, Scheck A K, Quartly Roland. Genetic and migratory evidence for sympatric spawning of tropical Pacific eels from Vanuatu. Mar Ecol Prog Ser. 521:171–187. [Google Scholar]
- Schmidt J. The breeding places of the eel. Philos Trans R Soc Lond B Biol Sci. 211:179–208. [Google Scholar]
- Schönau M C, Rudnick D L. Glider observations of the North Equatorial Current in the western tropical Pacific. J Geophys Res Ocean. 120:3586–3605. [Google Scholar]
- Sudo R, Tosaka R, Ijiri S, Adachi S, Suetake H, Suzuki Y, Horie N, Tanaka S, Aoyama J, Tsukamoto K. Effect of temperature decrease on oocyte development, sex steroids, and gonadotropin β-subunit mRNA expression levels in female Japanese eel Anguilla japonica. Fish Sci. 77:575–582. [Google Scholar]
- Tesch F W. Telemetric observations on the spawning migration of the eel (Anguilla anguilla) west of the European continental shelf. Environ Biol Fishes. 3:203–209. [Google Scholar]
- Tesch F W. Changes in swimrning depth and direction of silver eels (Anguilla anguilla L.) from the continental shelf to the deep sea. Aquat Living Resour. 2:9–20. [Google Scholar]
- Tesch F W. Blackwell Science, Oxford. Tsukamoto K. 1990. Recruitment mechanism of the eel, Anguilla japonica, to the Japanese coast. J Fish Biol. 36:659–671. [Google Scholar]
- Tsukamoto K. Origin of diadromous fishes and mechanism of migration. Tokyo): Tokai University Press; 1994. [Google Scholar]
- Tsukamoto K. Spawning of eels near a seamount. Nature; 2006. 439. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K. Oceanic migration and spawning of anguillid eels. J Fish Biol. 74:1833–1852. doi: 10.1111/j.1095-8649.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Otake T, Mochioka N, Lee T W, Fricke H, Inagaki T, Aoyama J, Ishikawa S, Kimura S, Miller M J, Hasumoto H, Oya M, Suzuk Y. Seamounts, new moon and eel spawning: The search for the spawning site of the Japanese eel. Environ Biol Fishes. 66:221–229. [Google Scholar]
- Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller M J, Aoyama J, Kimura S, Watanabe S, Yoshinaga T, Shinoda A, Kuroki M, Oya M, Watanabe T, Hata K, Ijiri S, Kazeto Y, Nomura K, Tanaka H. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun; 2011. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg M, Westerberg H, Aarestrup K, Feunteun E, Gargan P, Righton D. Evidence of marine mammal predation of the European eel (Anguilla anguilla L.) on its marine migration. Deep Res Part I Oceanogr Res Pap. 86:32–38. [Google Scholar]
- Watanabe S, Aoyama J, Tsukamoto K. A new species of freshwater eel Anguilla luzonensis (Teleostei: Anguillidae) from Luzon Island of the Philippines. Fish Sci. 75:387–392. [Google Scholar]
- Wessel P, Smith Whf, Scharroo R, Luis J, Wobbe F. Generic Mapping Tools: Improved version released. Eos, Trans Am Geophys Union. 94:409–410. [Google Scholar]
- Westerberg H, Sjoberg N, Lagenfelt I, Aarestrup K, Righton D. Behaviour of stocked and naturally recruited European eels during migration. Mar Ecol Prog Ser. 496:145–157. [Google Scholar]
- Wysujack K, Westerberg H, Aarestrup K, Trautner J, Kurwie T, Nagel F, Hanel R. The migration behaviour of European silver eels (Anguilla anguilla) released in open ocean conditions. Mar Freshw Res. 66:145–157. [Google Scholar]
- Yokose H. Geological approach to the spawning sites of the Japanese eel. Kaiyo Monthly. 48:45–58. [Google Scholar]